- 1Innovative Drug Research Center, College of Life Sciences and Medicine, Key Laboratory of Plant Secondary Metabolism and Regulation of Zhejiang Province, Zhejiang Sci-Tech University, Hangzhou, China
- 2State Key Laboratory of Genetic Engineering, Collaborative Innovation Center of Genetics and Development, Department of Biochemistry, Institute of Plant Biology, School of Life Sciences, Fudan University, Shanghai, China
- 3Department of Pharmaceutical Botany, School of Pharmacy, Naval Medical University, Shanghai, China
Salvia officinalis is an important dietary supplement that is widely used as a flavor regulator and plays an important role in the prevention and treatment of neurodegenerative diseases. Salvia miltiorrhiza Bunge is a famous Chinese herbal medicine for treating cardiovascular diseases. Secondary metabolites with diverse structures endow the two species with various edible and medicinal values. However, the differences in secondary metabolites between the leaves of S. officinalis and S. miltiorrhiza are still unclear. Herein, FlavourSpec® combined with spatial metabolomics was used to explore the distribution patterns of secondary metabolites including volatile and non-volatile components. The results indicated that the chemical compositions of the two Salvia species were significantly different. Specifically, S. miltiorrhiza Bunge contained high levels of phenolic acid components with a furan ring that can hardly be detected in S. officinalis. The volatile small molecules as well as carnosic acid and its derivatives were found to be major components of S. officinalis leaves. Because of the long-term exposure of leaves to ultraviolet radiation and the same environmental stress, carnosic acid and its derivatives exhibit widespread distribution characteristics in S. officinalis leaves. The work explored the similarities and differences in secondary metabolites of S. officinalis and S. miltiorrhiza Bunge, providing not only the material basis to develop the application value in dietary nutrition, but also a theoretical foundation for the development and utilization of medicinal resources of Salvia.
Highlights
● The method to detect the spatial distribution of chemicals in-situ was developed.
● Carnosic acid and carnosol were widely distributed in S. officinalis leaves.
● Flavourspec® was first used to explore the aromatic components in Salvia species.
● S.officinalis was found as perfect material to extract plant essential oils.
● The leaves of S.militiorhiza were rich in phenolic acid polymer with furan rings.
1 Introduction
Salvia officinalis is an important dietary supplement that not only is widely used as a flavor regulator in food processing but also plays an important role in the prevention and treatment of neurodegenerative diseases (Ghorbani and Esmaeilizadeh, 2017; Uță et al., 2021). Carnosic acid and its derivatives are reported as main active compounds in S. officinalis (Birtić et al., 2015). Moreover, it was found that S. officinalis is also rich in volatile components and can be used to treat diseases such as epilepsy, ulcers, rheumatism, and hyperglycemia (Koubaa et al., 2019). S. miltiorrhiza is a traditional Chinese herbal medicine and plays an important role in the prevention and treatment of cardiovascular diseases (Zhang et al., 2019). Tanshinones and phenolic acids were reported to have medicinal values (Wang and Peters, 2022). In recent years, volatile components in S. miltiorrhiza have also attracted widespread attention due to their significant functions; however, no systematic study has yet been performed to explore the difference between the chemical components of the two species (Li and Wang, 2009; Liang et al., 2009; Luo et al., 2014).
Flavourspec® is a newly emerged gas-phase detection technology that combines gas chromatography (GC) with ion mobility spectrometry (IMS). It has high resolution and high sensitivity, is simple to operate, and performs rapid analysis compared with gas chromatography–mass spectrometry (GC-MS). The working principle of GC-MS is that helium or hydrogen gas carries volatile components through a chromatographic column under high-temperature conditions, and diverse interactions between chemicals and stationary phases lead to the separation of compositions (Beale et al., 2018). The mass spectrometer identifies the chemical composition and molecular structure of volatile components in the sample by measuring the mass-to-charge ratio (m/z) of atoms or molecules (Fiehn, 2016). However, an increasing number of studies indicated that temperature and other factors can obviously influence the chemical compositions of volatile components (D'Auria and Racioppi, 2015). For instance, as temperature increases, the content of 1,8-eucalyptus and camphor in the essential oils decreases, whereas the content of pinene increases (D'Auria and Racioppi, 2015). Flavourspec® detects volatile components at normal temperature and pressure and proves to be a promising platform in the optimization of food processing methods and the selection of preservation methods (Yang et al., 2024).
Plants synthesize secondary metabolites with diverse structures to cope with changes in external environment and their own growth. Plant secondary metabolites typically have a wide range of biological activities and are important resources for developing new drugs. It is reported that structural diversity leads to diverse biological activities (Deng et al., 2023; Liu et al., 2023). Secondary metabolites typically exhibit significant structural diversity and tissue-specific distribution patterns due to the differences in stress and gene expression (Ramakrishna and Ravishankar, 2011). It is of great significance for their development and utilization to explore active natural products and elucidate their tissue-specific distribution patterns. With the development of spatial metabolomics, the spatial and temporal distribution properties of secondary metabolites can be elucidated. For instance, it was found that tanshinone was mainly distributed in the periderm of S. miltiorrhiza roots (Tong et al., 2022). In addition, the transcriptional landscape of main cell types in Taxus wallichiana leaves was also analyzed (Zhan et al., 2023), which was crucial for revealing cell-type-specific regulation of secondary metabolism. It is important to elucidate the spatiotemporal distribution characteristics of chemical components, which contribute to revealing the functional genes that regulate their synthesis for the quality control and resource development of medicinal plants. S. miltiorrhiza and S. officinalis are famous medicinal plants; the differences and similarities of secondary metabolites in their roots have been clarified (Hu et al., 2023). However, the accumulation patterns of secondary metabolites in leaves are still unclear. In this work, FlavourSpec® was combined with spatial metabolomics to clarify the distribution pattern of volatile and non-volatile components in leaves. Desorption electrospray ionization mass spectrometry imaging (DESI-MSI) was further used to reveal the spatial distribution of secondary metabolites in S. officinalis to provide a basis for the secondary metabolism regulation network and functional food development of S. officinalis and S. miltiorrhiza.
2 Materials and methods
2.1 Materials and chemicals
Experimental materials were collected at the Shanghai National Forest Germplasm Resource Centre of Lamiaceae Plant (China). The samples were identified as S. officinalis and S. miltiorrhiza by associate professor Chenyi Li from the Institute of Plant Biology, School of Life Sciences, Fudan University. The Specimen Hall of Shanghai Botanical Garden was used to store the plant specimens.
Mature leaves with consistent physiological development stages and good phenotypic uniformity were selected as experimental materials. For the detection of volatile and non-volatile components, three biological replicates were independently set up for each treatment group. All the reference standards (purely analytical) including butanone, 2-butanone, 2-pentanone, 2-hexanone, 2-heptanone, 2-octanone, and 2-nonanone were purchased from Aladdin and used to calculate retention indices for the characterization of more volatile components. All the standards with purity above 98% including caffeic acid, rosmarinus acid, salvianolic acid B, lithospermic acid, and carnosic acid were acquired from BioBioPha (Yunnan, China). Formic acid was purchased from Fisher Scientific (Fair Lawn, NJ, USA), pure distilled water and leucine encephalin were obtained from Waters (Hong Kong, China), acetonitrile and methanol were purchased from Merck (Darmstadt, Germany), warfarin was purchased from Sigma-Aldrich (Madrid, Spain), and all reagents used in the spatial metabolome were LC-MS grade.
2.2 Sample preparation anddata collection for volatile compounds
The leaves of S. miltiorrhiza Bunge and S. officinalis (0.5 g) were taken into a 20-mL headspace injection bottle and incubated at a speed of 500 rpm for 20 min at 40°C. FlavourSpec® was used to detect the chemical composition of volatile components. Each sample was measured three times in parallel. The conditions were as follows: MXT-5 capillary chromatography column (15 m × 0.53 mm, 1.0 μm); nitrogen was used as carrier gas and migratory gas; the injection needle temperature and column temperature were 85°C and 60°C, respectively; gradient elution was run for 30 min; the carrier gas flow was as follows: 0–2 min, 2 mL/min; 2–10 min, 2–20 mL/min; 10–20 min, 20–100 mL/min; and 20–30 min, 100–150 mL/min; migratory gas flow was 150 mL/min; the column temperature and IMS temperature were 60°C and 45°C, respectively; injection volume was 500 μL; and sampling in the non-shunt mode was used to collect data.
2.3 Sample preparation and data collection of LC-MS and DESI-MSI analysis
The leaves of S. miltiorrhiza Bunge and S. officinalis were crushed into homogeneous powders and sieved by a 40-mesh sieve. The prepared powders (0.01 g) were dissolved in 1 mL of 70% methanol (v/v) that contained warfarin (2 μg/mL); ultrasonic (53 kHz, 350 W) extraction was carried out for 60 min at greenhouse temperatures. The sample was then centrifuged at 12,000 rpm for 15 min to collect the supernatant. The vise was used and compressed with high pressure for probably 1 min to transfer metabolites of S. officinalis leaves to the PTFE membranes for DESI-MSI analysis.
The ACQUITY UPLC system (Waters) equipped with a binary pump, an autosampler, and a column compartment was combined with a Xevo G2-XS QTOF mass spectrometer (Waters) equipped with an electrospray ionization source to collect the non-volatile components in both positive- and negative-ion modes. An ACQUITY UPLC T3 column (2.1 mm × 100 mm, 1.8 µm) was used to separate the sample components at constant temperature (40°C). The 0.1% formic acid in water (solvent A) and in acetonitrile (solvent B) was taken as the mobile phase for gradient elusion. The elution gradient was as follows: 2%–20% B over 0–7 min, 20%–22% B over 7–11 min, 22%–60% B over 11–20 min, 60%–65% B over 20–25 min, 65%–65% B over 25–28 min, 65%–95% B over 28–30 min, 95%–95% B over 30–33 min, and final re-equilibration at 2% B for 5 min. The injection volume was 1 µL with a flow rate of 0.40 mL/min. The instrument settings of the mass spectrometer were as follows: sample cone, 30 V; the source temperature and desolvation temperature were 150°C and 450°C, respectively; the cone gas flow and desolvation gas flow were 50 and 600 L/h, respectively; and capillary voltages in negative-ion mode and in positive-ion mode were 2.5 and 3 kV, respectively. The mass range was set to 100 to 1,200 Da with a scan time of 0.35 s. The low collision energy was 6 eV and steadily increased from 15 to 30 eV, used as the high collision energy. A sodium formate solution (0.5 mM) was used to calibrate the instrument, and leucine enkephalin (200 pg/μL) was used as an external standard for mass correction during data collection. The MassLynx v.4.2 (Waters) was used to view mass spectra.
DESI-MSI was performed to analyze the spatial distribution of non-volatile components in S. officinalis leaves. The parameters referred to in the literature (Tong et al., 2022) are as follows: In both negative-ion mode and positive-ion mode, 98% methanol (v/v) and 100% methanol were used as solvent, capillary voltage was 3.5 kV in negative-ion mode and 4.5 kV in positive-ion mode with a flow rate was 2.5 µL/min, mass range was set from 100 to 1,200 Da, sampling cone was 60 V, collection angle and incident spray angle were respectively set as 10° and 65°, sprayer-to-inlet distance and sprayer-to-surface distance were 4 and 1 mm, respectively, and pixel resolution of images was 100 × 100 µm and viewed using HDI Imaging v1.4.
2.4 Data analysis for the volatile compounds
The calibration curves for retention time and retention index were established using butanone, 2-butanone, 2-pentanone, 2-hexanone, 2-heptanone, 2-octanone, and 2-nonanone. The chemical composition of volatile components was characterized using the NIST databases. The Laboratory Analytical Viewer was performed to plot the GC-MSI fingerprints of S. miltiorrhiza Bunge and S. officinalis. The Dynamic PCA software was used to perform principal component analysis (PCA).
2.5 Data analysis of LC-MS and DESI-MSI
The raw mass spectrum data were subjected to format conversion (ABF Converter, https://www.reifycs.com/AbfConverter/), and then the processed data were imported into MS-DIAL v 4.60 (http://prime.psc.riken.jp/compms/msdial/main.html) for peak detection, alignment, and normalization to obtain peak tables. The parameters were as follows: MS1 and MS2 tolerance was 0.01 and 0.02 Da, respectively; retention time range was 1–28 min; retention time tolerance was set to 0.15 min; MS1 and MS2 mass ranged from 100 to 5,000 Da; mass slice width was 0.1 Da; the minimum peak height was 20,000; and MS2 abundance cutoff was 800 amplitudes. Adduct types such as [M+H]+, [M+Na]+, [M+K]+, and [2M+H]+ were selected for positive-ion mode, and [M-H]−, [M+HCOO]−, [M+Na-2H]−, [M+K-2H]−, and [2M-H]− were selected for negative-ion mode. The warfarin (5 μg/mL) in each data file was calculated in MS-DIAL for normalization processing.
2.6 Chemometric analysis
The normalized data were imported to SIMCA-P 14.1 (Umetrics AB, Umea, Sweden) to carry out the PCA. The differential metabolites between two species were screened by orthogonal partial least squares discriminate analysis (OPLS-DA), and compounds with variable importance in projection (VIP) of more than 1 were defined as potential chemical markers.
3 Results and discussion
3.1 Differential analysis of volatile components between S. miltiorrhiza Bunge and S. officinalis
FlavourSpec® was carried out to analyze the distribution pattern of volatile components in the leaves of S. miltiorrhiza Bunge and S. officinalis. GC-IMS and the fingerprints of volatile components indicated the significant differences in the chemical composition of the two species (Figure 1). The red dots in the GC-IMS suggested the intensity of the ion signal; it can be seen that the content of volatile components in S. officinalis was generally higher than that in S. miltiorrhiza Bunge; therefore, it can be used to extract essential oils. S. officinalis can be widely used in perfume manufacturing and the development of health products due to the strong aroma derived from the high concentration of volatile components. The NIST database was then used to identify the chemical compositions of the volatile components. A total of 99 components were detected (Figure 1; Supplementary Table 1), and 8 compounds were not characterized among the 99 compounds.
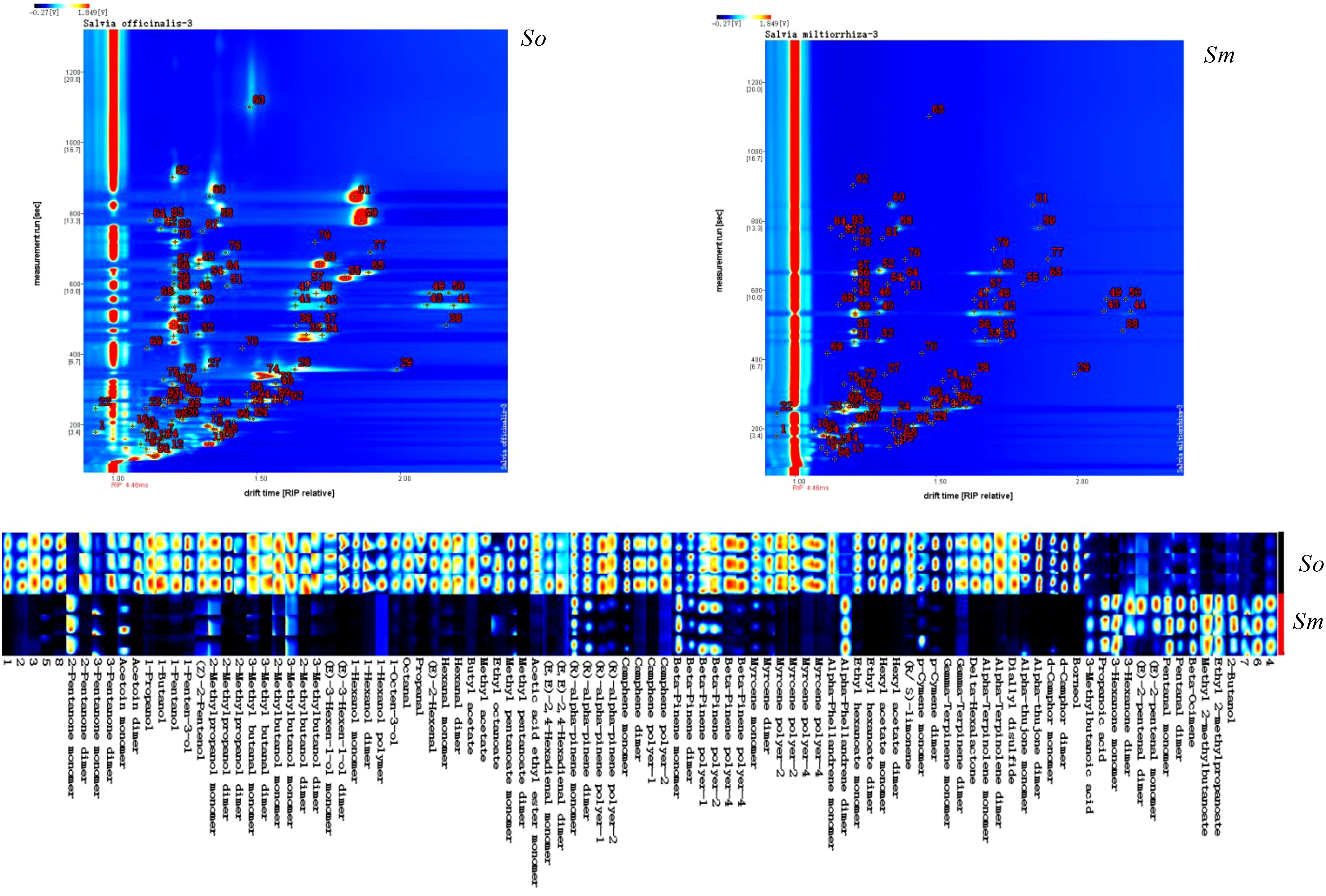
Figure 1. The volatile components detected from S. officinalis (So) and S. miltiorrhiza Bunge (Sm) using FlavourSpec® and fingerprints of volatile components of S. officinalis (So) and S. miltiorrhiza Bunge (Sm).
PubChem (https://pubchem.ncbi.nlm.nih.gov/) and ClassyFire (http://classyfire.wishartlab.com/) were used to classify the volatile components. The results indicated that volatile components in the two important herbs were composed of monoterpenes, carboxylic acids and its derivatives, fatty acyls, and organooxygen compounds. The significant differences in the relative content and number of different categories of components are shown in Figure 2. The relative content of monoterpenes in the volatile components of S. officinalis was 0.616% and that in S. miltiorrhiza Bunge was 0.493%. Fatty acyls and carboxylic acid derivatives also had a high proportion in S. officinalis and S. miltiorrhiza Bunge, respectively. The relative content of fatty acyls in the volatile components of S. officinalis was four times higher than that in S. officinalis based on the findings of this work. The relative content of fatty acyls in S. officinalis was 0.313%, whereas that in S. miltiorrhiza Bunge was 0.067%. The carboxylic acid derivatives in the volatile components of S. miltiorrhiza Bunge were 0.112%, whereas those in S. officinalis were 0.039%. The proportion of several unidentified components in the volatile components of S. miltiorrhiza Bunge was significantly higher than that of S. officinalis, indicating that relatively less research was conducted on volatile components in S. miltiorrhiza Bunge. The organic oxygen compounds were the most abundant components identified among volatile compounds (36), followed by monoterpenoids, with 35 components identified as monoterpenoids (Figure 2A). The number of carboxylic acids and derivatives was nine. The number of these kinds of components was relatively low (Figure 2B). As an important aromatic medicinal plant, the volatile components of S. officinalis leaves have been extensively reported in literature. GC-MS was the mainstream method used for measuring volatile components. The results reported from the literature indicated that plant organic volatile small molecules mainly consisted of monoterpenes, sesquiterpenes, and their oxygen-containing compounds, fatty acid compounds (Baj et al., 2013). To the best of our knowledge, in this study, FlavourSpec® was innovatively used to detect the volatile components of S. officinalis and S. miltiorrhiza Bunge. The results indicated that volatile components of S. officinalis and S. miltiorrhiza Bunge were mainly composed of monoterpenes and fatty acid compounds, consistent with previous literature reports (Doolaege et al., 2007). However, no compounds belonging to sesquiterpenes were detected. FlavourSpec® was mainly used for detecting the odor of volatile components, indicating that the odor of S. officinalis leaves may mainly come from monoterpenes and their oxygen-containing derivatives.
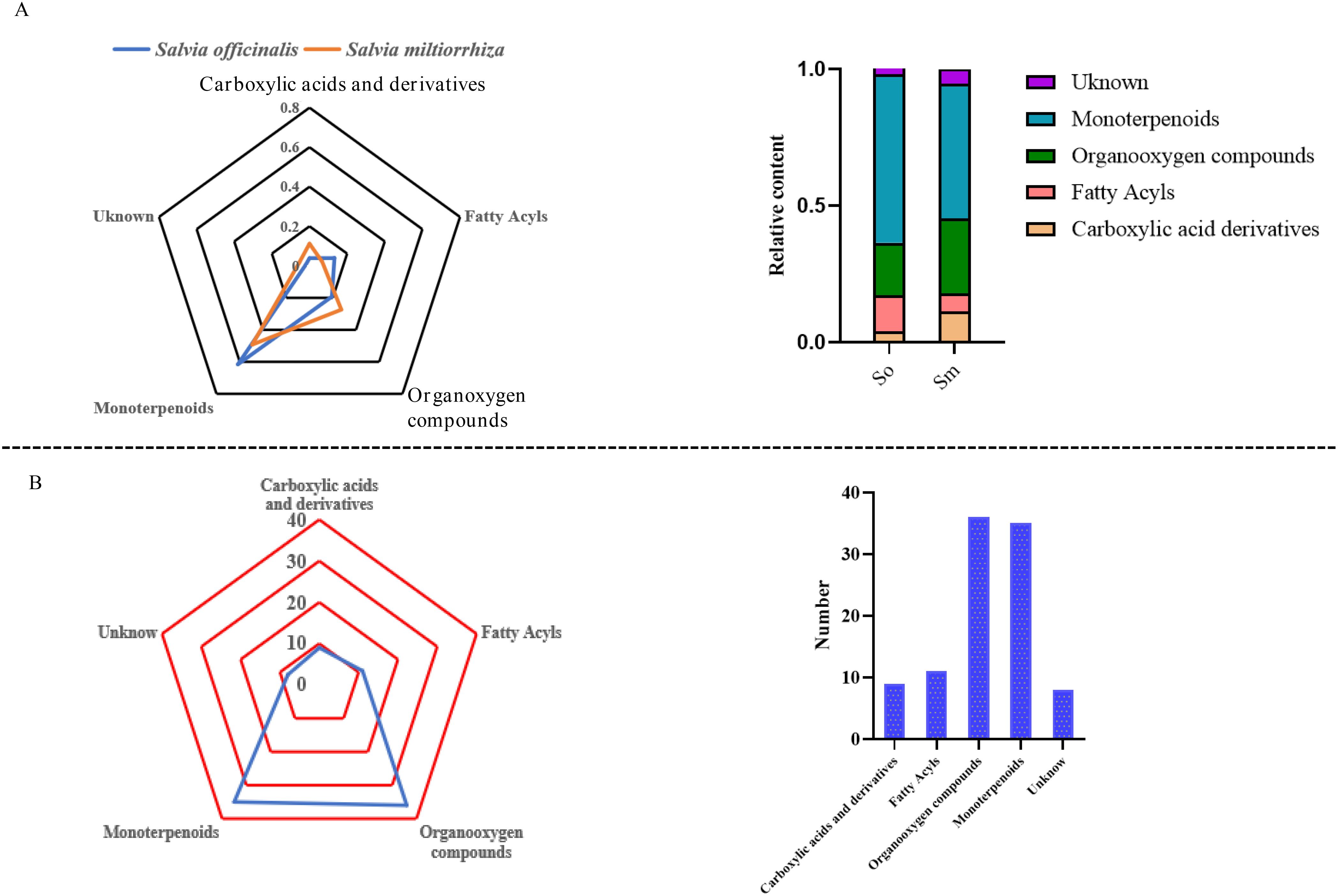
Figure 2. The relative content and number of different categories of volatile components in S. officinalis and S. miltiorrhiza Bunge. (A) The relative content of different categories of volatile components in S. officinalis and S. miltiorrhiza Bunge. (B) The number of different categories of volatile components in S. officinalis and S. miltiorrhiza Bunge.
Simca 14.0 was used to perform the PCA and OPLS-DA multivariate statistical analysis based on the distribution pattern of volatile components to explore the similarities and differences in the chemical compositions. The results indicated that S. miltiorrhiza Bunge and S. officinalis showed a significant trend of segregation based on PCA. S. miltiorrhiza Bunge and S. officinalis were grouped together separately. It was indicated that the chemical compositions of volatile components in the two medicinal species differed significantly (Figure 3A). In addition, it was found that the chemical composition of volatile components in S. officinalis was significantly richer than that in S. miltiorrhiza Bunge according to the fingerprint spectra of volatile components and showed significant differences in chemical composition. The OPLS-DA was performed to screen differential compounds, and a total of 22 compounds were regarded as significantly different compounds based on VIP > 1. The differential compounds were mainly composed of monoterpenes. It was indicated that the volatile components of aromatic plants mainly consist of monoterpenes, sesquiterpenes, and organic fatty acid compounds (Figure 3B). In this work, sesquiterpenes were almost not detected from the volatile components. Therefore, it was speculated that the odor of S. officinalis mainly comes from monoterpenes. The s-polt was used to show the distribution of differential compounds in two medicinal plants.
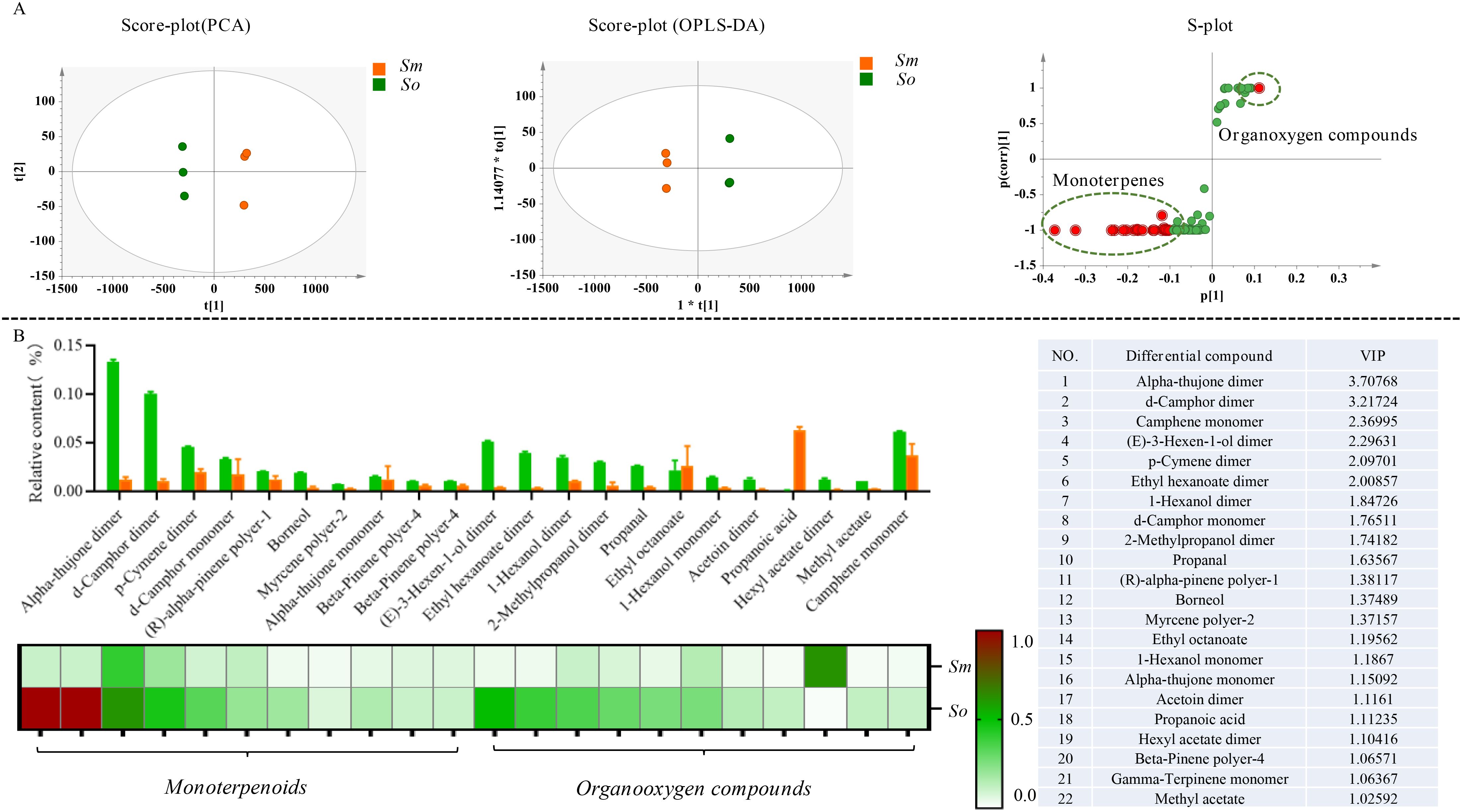
Figure 3. The significant difference in the volatile components was detected in S. officinalis and S. miltiorrhiza Bunge. (A) Multivariate statistical analysis of volatile components in S. officinalis and S. miltiorrhiza Bunge followed as PCA, OPLS-DA, and S-plot. (B) Distribution patterns of differential compounds in S. officinalis and S. miltiorrhiza Bunge.
3.2 Analysis of differences in non-volatile components between S. miltiorrhiza Bunge and S. officinalis
Tandem MS was performed to investigate the comprehensive metabolite profiles of S. officinalis and S. miltiorrhiza Bunge. It was performed in both positive- and negative-ion modes; the latter was more sensitive based on the total ion chromatogram. The differences and similarities in secondary metabolites between two herbs focused on the results in the negative-ion mode. The secondary metabolites in the leaves of S. miltiorrhiza were mainly concentrated within 18 min of elution, whereas secondary metabolites in the leaves of S. officinalis were mainly eluted within 25 min (Figure 4A). A total of 3,623 ions were detected, and MS-DIAL was performed to extract peak tables for the multivariate statistical analysis. The in-house database of Salvia genus was applied to characterize the chemicals, and 61 components were detected in the leaves of S. officinalis and S. miltiorrhiza Bunge, including organic acids, phenolic acids, and carnosic acid and its derivatives (Supplementary Table 2). The leaves of S. officinalis and S. miltiorrhiza were both detected with significant levels of rosmarinic acid. The content of danshensu and caffeic acid was relatively low, which was mainly used as a precursor for the synthesis of rosmarinic acid. It indicated the presence of active phenolic acid synthesis pathways in the leaves. Rosmarinic acid had significant antioxidant capacity and was important for leaves to prevent ultraviolet (UV) damage (Dahchour, 2022). Although the content of rosmarinic acid that was detected in the leaves was not significant, the content of rosmarinic acid in S. miltiorrhiza leaves was significantly higher than that in S. officinalis. The relative content of differential components in S. miltiorrhiza Bunge and S. officinalis is shown in Figure 4A. Salvianolic acid B, the significantly differential metabolite, was enriched with the leaves of S. miltiorrhiza but was almost undetectable in S. officinalis.
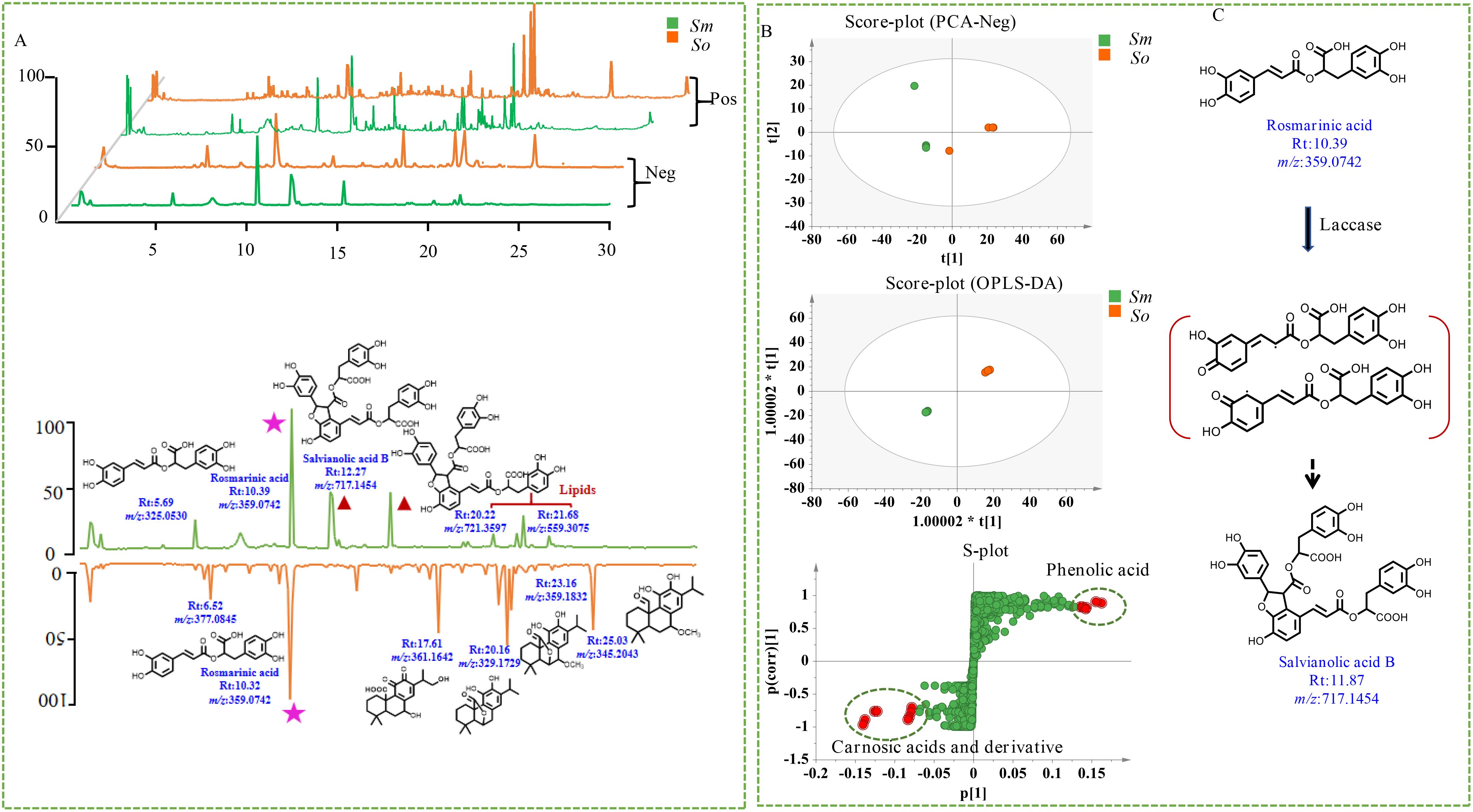
Figure 4. Multivariate statistical analysis of non-volatile components in S. officinalis and S. miltiorrhiza Bunge. (A) Total ion chromatogram of non-volatile components in S. officinalis and S. miltiorrhiza Bunge. (B) Chemical clustering analysis of S. officinalis and S. miltiorrhiza Bunge with different modeling methods. (C) Possible molecular mechanism of rosmarinic acid conversion to salvianolic acid B.
PCA was performed, and there was a clear separation trend indicating that the secondary metabolites in S. miltiorrhiza Bunge and S. officinalis were significantly different (Figure 4B). The OPLS-DA model was used to explore the differential compounds, and the compounds with a VIP of 4 were considered to be significantly differential compounds. A total of 20 structures were identified as differential compounds (Supplementary Table 3).
The chemical characterization of differential compounds was performed based on the fragmentation pathway in MS. The main differential compounds along with the corresponding product ions are shown in Figures 5A, B. Take the differential compounds with a retention time of 21.16 and m/z 331.1917[M-H]− as examples to explain structural characterization. The ESI source gave the [M-H-44]− ions as the base peak, and the molecular formula was determined to be C20H28O4 according to [M-H]−. The MS/MS prominent ion was [M-H-44]− at m/z 285.184 at 30% energy collision. The MS behavior and fragmentation pattern of neutral loss of the carboxyl group to produce fragment ions were consistent with those of carnosic acid reported in literature (Doolaege et al., 2007); thus, the ion was identified as carnosic acid. The retention of m/z 345.167[M-H]− in the reverse-phase chromatography column was shorter than that of carnosic acid, indicating that the polarity was greater compared with carnosic acid (Figure 5A). The ions at m/z 345.167[M-H]− was detected as base peak under the primary channel of mass spectrometry (MS1) (Doolaege et al., 2007). The two structures, as derivatives of carnosic acid, were mainly enriched in the leaves of S. officinalis. Two ions that significantly enriched leaves of S. miltiorrhiza Bunge were selected for chemical characterization, one ion at m/z 717.1417 [M-H]−, and another ion at m/z 537.1033 [M-H]− (Figure 5B). The polarity of the two ions was greater than carnosic acid derivatives and can be eluted within 15 min. The molecular formula of the ion at m/z 717.1417 [M-H]− was determined to be C36H30O16; the MS/MS ion was [M-H-198]− at m/z 519 at 30% energy collision, suggesting its neutral loss of danshensu. The MS/MS prominent ion was [M-H-198-198]− at m/z 321 at 30% energy collision, indicating that the loss of danshensu was easier than caffeic acid. The fragmentation pattern was consistent with salvianolic acid B that was reported in the literature (Liu et al., 2007). Similar to salvianolic acid B, the ion at m/z 537.1033 [M-H]− was determined to be C27H22O12, and loss of the carboxyl group at m/z 493 [M-H-44]− and danshensu yielded MS2 fragmentation ion at m/z 295 [M-H-44-198]−, which was identified as lithospermic acid (Liu et al., 2007). The two ions were significantly enriched in leaves of S. miltiorrhiza Bunge. It indicated that the differential compounds were mainly composed of phenolic acid and carnosic acid and its derivatives.
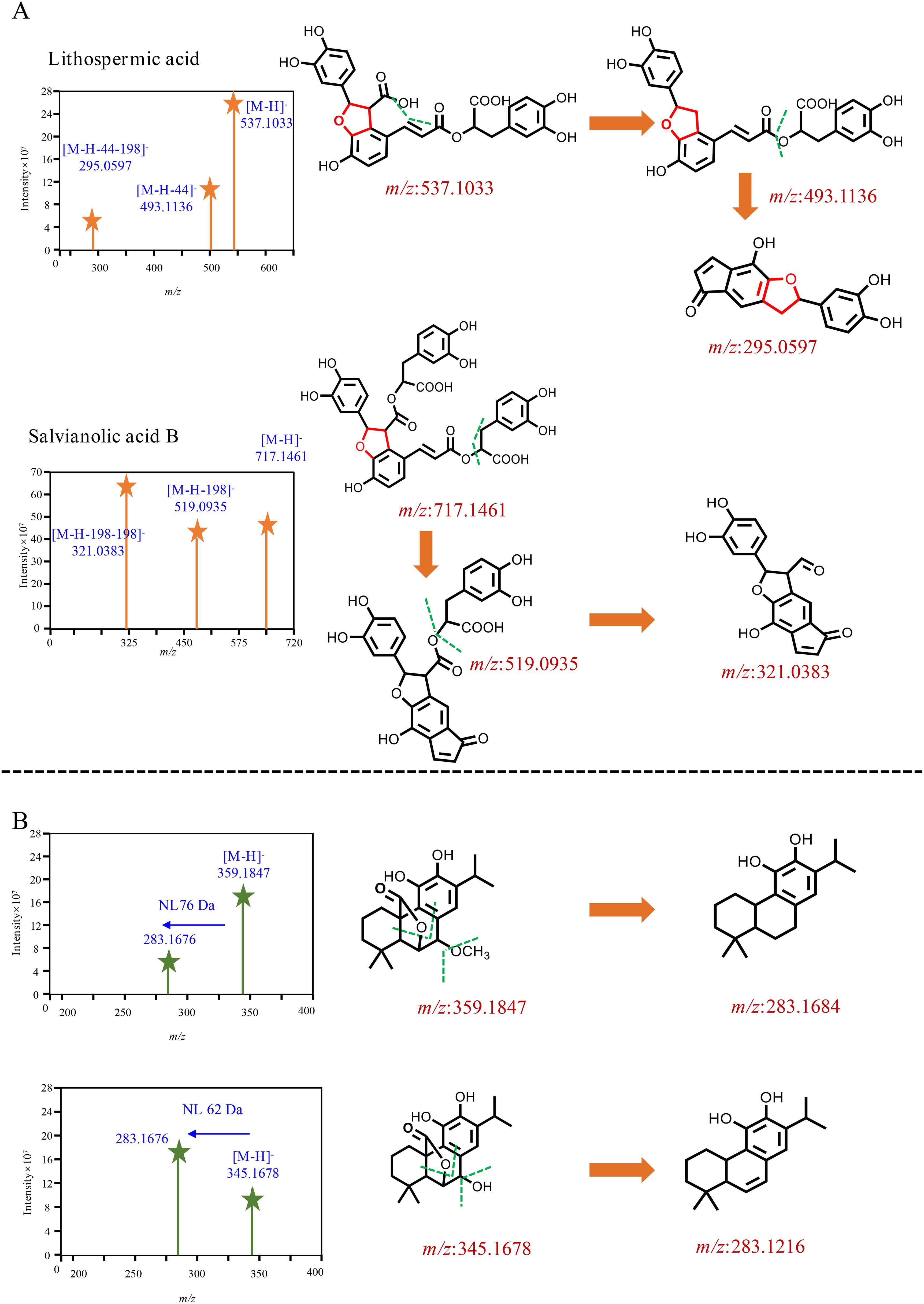
Figure 5. Mass spectrometry behavior of differential compounds. (A) The two components significantly enriched in S. miltiorrhiza, (B) The two components significantly enriched in S. officinalis.
Previous studies have investigated the distribution of abietane-type diterpenes in the roots and leaves of both S. miltiorrhiza and S. officinalis (Li et al., 2020). The abietane-type diterpenes in S. miltiorrhiza roots were mainly composed of tanshinones, while the abietane-type diterpenes in S. officinalis were mainly enriched in the leaves, with carnosic acid and its derivatives as the main component. Tanshinones were not detected in either the leaves or roots of S. officinalis (Li et al., 2020). This work systematically investigated the distribution patterns of secondary metabolites in S. miltiorrhiza and S. officinalis, filling the gap in previous research and providing direction for the resource utilization and molecular breeding of the two species. For example, we found high levels of rosmarinic acid in the leaves of S. officinalis, indicating that the phenolic acid biosynthesis pathway was quite active in S. officinalis leaves. However, no components containing the furan structure such as salvianolic acid B were detected. According to previous research (Di et al., 2013), tetramer salvianolic acid B was mainly synthesized from rosmarinic acid as a substrate under the catalysis of laccase (Figure 4C). It was indicated that part of the rosmarinic acid synthesized from S. miltiorrhiza was used for downstream synthesis of salvianolic acid B, and another part was used for self-accumulation; thus, the content was significantly higher than that of S. officinalis. Previous research indicated that metabolites with a furan ring were the main active compounds of S. miltiorrhiza; in this work, salvianolic acid B was also found in the leaves. S. officinalis is considered an important medicinal and edible plant and is widely used in the treatment of neurodegenerative diseases and in condiments in Europe. In this work, the high content of rosmarinic acid without phenolic acid components containing furan structures was detected in this species. S. officinalis is now widely cultivated in various regions. Therefore, establishing a genetic transformation system for S. officinalis and utilizing biotechnology to cultivate new varieties of S. officinalis that can simultaneously synthesize polyphenolic acids such as salvianolic acid B and carnosic acid derivatives are important to the resource development of Salvia species. External environmental stress is the main driving force for enzyme evolution (Lau and Bolin, 2024); carnosic acid and its derivatives have been reported to have strong antioxidant capacity, which may be closely related to the adaptation of S. officinalis to harsh climates in the European Mediterranean region.
3.3 The spatial distribution of carnosic acid and its derivatives in S. officinalis leaves
The spatial distribution of secondary metabolites in S. miltiorrhiza has been reported in the literature. However, the in situ distribution of carnosic acid and its derivatives in S. officinalis leaves is still unclear; thus, DESI was performed in this work to fill the research gap. The results indicated that carnosic acid and its derivatives showed wide distribution in the leaves (Figure 6); 11-hydroxyferruginol and 11,20-dihydroxyferruginol with a low content as precursors of carnosic acid and its derivatives only detected distribution at the leaves’ edge. Carnosic acid and its derivatives were reported with significant antioxidant effects (Birtić et al., 2015). S. officinalis was native to the Mediterranean region of Europe; the remarkable accumulation of carnosic acid and its derivatives is associated with the harsh climatic conditions. It was reported in the literature that carnosol was a spontaneous oxidation product of carnosic acid (Zhang et al., 2012). In this study, the two important metabolites were both found to be widely distributed in the leaves, indicating the relationship between carnosol and carnosic acid to some extent. Rosmanol was a structural analogue of carnosol that added a hydroxyl group at the C7 position and showed a weak ionic signal throughout the entire tissue range of the leaves. It suggested that rosmarinol and carnosol had an upstream–downstream relationship in their biosynthetic pathways. Spatial localization can also be used to analyze the biosynthetic pathways of secondary metabolites. The active compounds usually show a clear differential distribution in medicinal plants. For example, tanshinone was mainly distributed in the periderm of S. miltiorrhiza roots. Carnosic acid and its derivatives were widely distributed in the leaves of S. officinalis. The specific spatial distribution of secondary metabolites may be closely related to environmental pressure. Tanshinones have been reported to be secreted into the soil environment, inhibiting the growth of other plants and occupying ecological niches (Li et al., 2024), whereas the leaves were completely exposed to the environment and subjected to the UV radiation pressure caused by the wide distribution pattern of carnosic acid and its derivatives (Loussouarn et al., 2017).
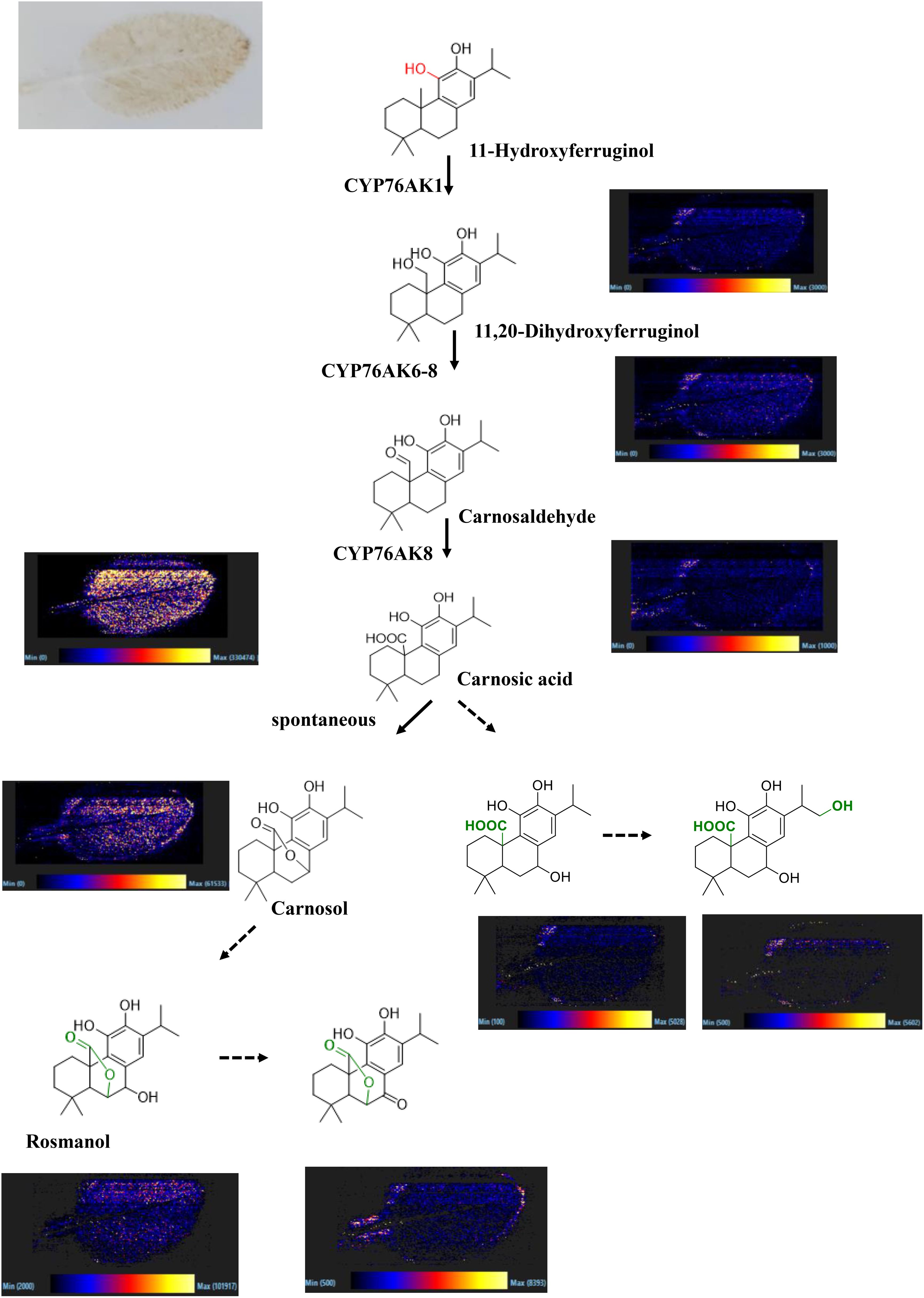
Figure 6. Spatial distribution of carnosic acid and its derivatives in the leaves of S. officinalis. (A) The two components significantly enriched in S. miltiorrhiza, (B) The two components significantly enriched in S. officinalis.
4 Conclusion
The structure of secondary metabolites leads to the biological activity of medicinal plants. S. miltiorrhiza Bunge and S. officinalis are famous medicinal herbs widely used to treat cardiovascular and neurodegenerative diseases. The structural diversity of diterpenoids and their formation mechanisms in the roots have been reported. To explore the chemical diversity of secondary metabolites in the leaves, FlavourSpec® combined with spatial metabolomics was conducted to analyze the accumulation patterns of metabolites that include volatile and non-volatile components. It was found that phenolic acid components such as rosmarinic acid and caffeic acid are widely distributed in S. officinalis and S. miltiorrhiza Bunge, and S. miltiorrhiza Bunge contained a high content of phenolic acid components with a furan ring structure; however, these kinds of compounds are hardly detected in S. officinalis. The compounds of S. officinalis leaves are mainly composed of carnosic acid and its derivatives, which exhibit widespread distribution characteristics in S. officinalis leaves. Therefore, it indicated that S. officinalis is an excellent raw material for extracting volatile components and developing functional foods due to its extensive antioxidant activity. In addition, the leaves of S. officinalis contained abundant volatile components compared with S. miltiorrhiza Bunge. This work analyzed the similarities and differences in secondary metabolites of S. officinalis and S. miltiorrhiza Bunge systematically, providing theoretical and material basis for the development and utilization of medicinal resources of Salvia spp.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
FW: Writing – original draft, Methodology, Supervision, Formal Analysis, Data curation, Funding acquisition. CL: Software, Writing – original draft, Visualization, Investigation. HY: Methodology, Writing – original draft, Funding acquisition. DY: Writing – review & editing. JY: Writing – review & editing. LZ: Conceptualization, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant nos. 82225047, 82170274, and 82304665), the National Key Research and Development Program of China (no. 2022YFC3501703), the China Postdoctoral Science Foundation funded project (11110032542402), the Natural Science Foundation of Zhejiang Province (LZ24H280003), and Department level project (23042213-Y).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1613313/full#supplementary-material.
References
Baj, T., Ludwiczuk, A., Sieniawska, E., Skalicka-Woźniak, K., Widelski, J., Zieba, K., et al. (2013). GC-MS analysis of essential oils from Salvia officinalis L.: comparison of extraction methods of the volatile components. Acta Poloniae Pharm. 70, 35–40.
Beale, D. J., Pinu, F. R., Kouremenos, K. A., Poojary, M. M., Narayana, V. K., Boughton, B. A., et al. (2018). Review of recent developments in GC-MS approaches to metabolomics-based research. Metabolomics 14, 152. doi: 10.1007/s11306-018-1449-2
Birtić, S., Dussort, P., Pierre, F. X., Bily, A. C., and Roller, M. (2015). Carnosic acid. Phytochemistry 115, 9–19. doi: 10.1016/j.phytochem.2014.12.026
D’Auria, M. and Racioppi, R. (2015). The effect of drying of the composition of volatile organic compounds in Rosmarinus officinalis, Laurus nobilis, Salvia officinalis and Thymus serpyllum. a hs-spme-gc-ms study. J. Essential Oil-Bearing Plants 18, 1209–1223. doi: 10.1080/0972060X.2014.895213
Dahchour, A. (2022). Anxiolytic and antidepressive potentials of rosmarinic acid: A review with a focus on antioxidant and anti-inflammatory effects. Pharmacol. Res. 184, 106421. doi: 10.1016/j.phrs.2022.106421
Deng, W., Chen, F., Zhao, Y., Zhou, M., and Guo, M. (2023). Anti-hepatitis B virus activities of natural products and their antiviral mechanisms. Chin. J. Natural Medicines 21, 803–811. doi: 10.1016/S1875-5364(23)60505-9
Di, P., Zhang, L., Chen, J., Tan, H., Xiao, Y., Dong, X., et al. (2013). ¹³C tracer reveals phenolic acids biosynthesis in hairy root cultures of Salvia miltiorrhiza. ACS Chem. Biol. 8, 1537–1548. doi: 10.1021/cb3006962
Doolaege, E. H., Raes, K., Smet, K., Andjelkovic, M., Van Poucke, C., De Smet, S., et al. (2007). Characterization of two unknown compounds in methanol extracts of Rosemary oil. J. Agric. Food Chem. 55, 7283–7287. doi: 10.1021/jf071101k
Fiehn, O. (2016). Metabolomics by gas chromatography-mass spectrometry: combined targeted and untargeted profiling. Curr. Protoc. Mol. Biol. 114, 30.4.1–30.4.32. doi: 10.1002/0471142727.mb3004s114
Ghorbani, A. and Esmaeilizadeh, M. (2017). Pharmacological properties of Salvia officinalis and its components. J. Traditional Complementary Med. 7, 433–440. doi: 10.1016/j.jtcme.2016.12.014
Hu, J., Qiu, S., Wang, F., Li, Q., Xiang, C. L., Di, P., et al. (2023). Functional divergence of CYP76AKs shapes the chemodiversity of abietane-type diterpenoids in genus Salvia. Nat. Commun. 14, 4696. doi: 10.1038/s41467-023-40401-y
Koubaa, F. G., Abdennabi, R., Salah, A. S. B., and El Feki, A. (2019). Microwave extraction of Salvia officinalis essential oil and assessment of its GC-MS identification and protective effects versus vanadium-induced nephrotoxicity in Wistar rats models. Arch. Physiol. Biochem. 125, 404–413. doi: 10.1080/13813455.2018.1478427
Lau, J. A. and Bolin, L. G. (2024). The tiny drivers behind plant ecology and evolution. Am. J. Bot. 111. doi: 10.1002/ajb2.16358
Li, Y., Chen, J., Zhi, J., Huang, D., Zhang, Y., Zhang, L., et al. (2024). The ABC transporter SmABCG1 mediates tanshinones export from the peridermic cells of Salvia miltiorrhiza root. J. Integr. Plant Biol. 67, 135-149. doi: 10.1111/jipb.13806
Li, X. and Wang, Z. (2009). Chemical composition, antimicrobial and antioxidant activities of the essential oil in leaves of Salvia miltiorrhiza bunge. J. Essential Oil Res. 21, 476–480. doi: 10.1080/10412905.2009.9700222
Li, S., Zhu, N., Tang, C., Duan, H., Wang, Y., Zhao, G., et al. (2020). Differential distribution of characteristic constituents in root, stem and leaf tissues of Salvia miltiorrhiza using MALDI mass spectrometry imaging. Fitoterapia 146, 104679. doi: 10.1016/j.fitote.2020.104679
Liang, Q., Liang, Z. S., Wang, J. R., and Xu, W. H. (2009). Essential oil composition of Salvia miltiorrhiza flower. Food Chem. 113, 592–594. doi: 10.1016/j.foodchem.2008.08.035
Liu, J., Ge, Z., Jiang, X., Zhang, J., Sun, J., and Mao, X. (2023). A comprehensive review of natural products with anti-hypoxic activity. Chin. J. Natural Medicines 21, 499–515. doi: 10.1016/S1875-5364(23)60410-8
Liu, A. H., Guo, H., Ye, M., Lin, Y. H., Sun, J. H., Xu, M., et al. (2007). Detection, characterization and identification of phenolic acids in Danshen using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. J. Chromatography. A 1161, 170–182. doi: 10.1016/j.chroma.2007.05.081
Lou, J., Mao, Z., Shan, T., Wang, Q., and Zhou, L. (2014). Chemical composition, antibacterial and antioxidant properties of the essential oils from the roots and cultures of Salvia miltiorrhiza. J. Essential Oil-Bearing Plants. 17, 380–384. doi: 10.1080/0972060x.2014.895199
Loussouarn, M., Krieger-Liszkay, A., Svilar, L., Bily, A., Birtić, S., and Havaux, M. (2017). Carnosic Acid and Carnosol, Two major antioxidants of rosemary, act through different mechanisms. Plant Physiol. 175, 1381–1394. doi: 10.1104/pp.17.01183
Ramakrishna, A. and Ravishankar, G. A. (2011). Influence of abiotic stress signals on secondary metabolites in plants. Plant Signaling Behav. 6, 1720–1731. doi: 10.4161/psb.6.11.17613
Tong, Q., Zhang, C., Tu, Y., Chen, J., Li, Q., Zeng, Z., et al. (2022). Biosynthesis-based spatial metabolome of Salvia miltiorrhiza Bunge by combining metabolomics approaches with mass spectrometry-imaging. Talanta 238, 123045. doi: 10.1016/j.talanta.2021.123045
Uţă, G., Manolescu, D. Ş., and Avram, S. (2021). Therapeutic properties of several chemical compounds of Salvia officinalis L. in Alzheimer’s Disease. Mini Rev. Medicinal Chem. 21, 1421–1430. doi: 10.2174/1389557521999201230200209
Wang, Z. and Peters, R. J. (2022). Tanshinones: Leading the way into Lamiaceae labdane-related diterpenoid biosynthesis. Curr. Opin. Plant Biol. 66, 102189. doi: 10.1016/j.pbi.2022.102189
Yang, Y., Xie, J., Wang, Q., Wang, L., Shang, Y., Jiang, Y., et al. (2024). Volatolomics-assisted characterization of the key odorants in green off-flavor black tea and their dynamic changes during processing. Food Chemistry: X 22, 101432. doi: 10.1016/j.fochx.2024.101432
Zhan, X., Qiu, T., Zhang, H., Hou, K., Liang, X., Chen, C., et al. (2023). Mass spectrometry imaging and single-cell transcriptional profiling reveal the tissue-specific regulation of bioactive ingredient biosynthesis in Taxus leaves. Plant Commun. 4, 100630. doi: 10.1016/j.xplc.2023.100630
Zhang, J., Liang, R., Wang, L., and Yang, B. (2019). Effects and mechanisms of Danshen-Shanzha herb-pair for atherosclerosis treatment using network pharmacology and experimental pharmacology. J. Ethnopharmacology 229, 104–114. doi: 10.1016/j.jep.2018.10.004
Keywords: spatial metabolomics, chemical diversity, volatile components, phenolic acids, carnosic acid, Salvia officinalis, Salvia miltiorrhiza Bunge
Citation: Wang F, Li C, Yu H, Yang D, Ye J and Zhang L (2025) Biosynthesis-based metabolomics analysis reveals chemical diversity between two Salvia species. Front. Plant Sci. 16:1613313. doi: 10.3389/fpls.2025.1613313
Received: 17 April 2025; Accepted: 29 May 2025;
Published: 04 July 2025.
Edited by:
Wei Sun, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Shuncang Zhang, Yangzhou University, ChinaZaibiao Zhu, Nanjing Agricultural University, China
Copyright © 2025 Wang, Li, Yu, Yang, Ye and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang, aWRyY0B6c3R1LmVkdS5jbg==
 Feiyan Wang1
Feiyan Wang1 Chenyi Li
Chenyi Li Dongfeng Yang
Dongfeng Yang Ji Ye
Ji Ye Lei Zhang
Lei Zhang