- 1College of Horticulture, Henan Agricultural University, Zhengzhou, Henan, China
- 2Henan International Joint Laboratory of Horticultural Plant Biology, Henan Agricultural University, Zhengzhou, Henan, China
- 3College of Plant Protection, Henan Agricultural University, Zhengzhou, Henan, China
- 4Plant Breeding, Wageningen University and Research, Wageningen, Netherlands
Cyclic nucleotide-gated ion channel (CNGC) genes play vital roles in plant growth, development, and responses to both biotic and abiotic stresses. However, the current research on CNGCs in potato (Solanum tuberosum) remain largely uncharacterized. Blackleg disease is one of the most devastating diseases worldwide, causing severe yield losses. Understanding the role of the StCNGC gene family in blackleg resistance is therefore of significant importance. In this study, we identified 11 StCNGC genes in the potato genome and conducted phylogenetic analysis, gene structure characterization, and conserved motif prediction. Expression patterns were examined in different tissues and under stress conditions. The identified StCNGCs were classified into five groups, and showed conserved gene structures and motifs within groups. Most StCNGCs were induced under biotic stress conditions. Notably, silencing StCNGC2 conferred resistance to blackleg disease and resulted in the upregulation the pathogenesis-related marker gene StPR1. Together, these findings suggest that StCNGC2 plays a crucial role in potato defense against blackleg disease and provide a foundation for further functional studies of the StCNGC gene family.
Introduction
Calcium ions (Ca2+) serve as critical secondary messengers in plant signaling pathways, mediating responses to developmental cues and environmental stimuli (He et al., 2018; Yang et al., 2021). Ca2+ transport across membranes is facilitated by channels and carriers, with channel proteins as the main path for rapid Ca2+ transport crossing the membrane. As such, calcium channels are essential for generating calcium signals (Wang et al., 2021a).
Cyclic nucleotide-gated channels (CNGCs) are ligand-gated Ca2+ channels that are essential for signal transduction and cellular homeostasis (Jarratt-Barnham et al., 2021; Wang et al., 2024). They possess distinctive structural features, comprising six transmembrane helices (S1–S6) with a pore-forming segment located between S5 and S6, and a cyclic nucleotide (cNMP)-binding domain (CNBD) and a calmodulin (CaM) binding domain (CaMBD) at the C-terminus (Kaplan et al., 2007; Jarratt-Barnham et al., 2021). CNGCs can be activated by cNMP binding and inhibited by Ca2+/CaM binding (Trudeau and Zagotta, 2002). CaM binds to isoleucine glutamine (IQ) motifs in a Ca2+-dependent manner (Fischer et al., 2013). The CNBD contains a hinge region and a phosphate-binding cassette (PBC) (Jarratt-Barnham et al., 2021). PBC interacts with cAMP ribose-phosphate, while the flexible helix, referred to as the “hinge”, interacts with the PBC (Young and Krougliak, 2004; James and Zagotta, 2018). Owing to their structural uniqueness, CNGCs are candidates for ligand-gated/voltage-independent/cation transporters, which are significant in both plant development/growth regulation and biotic/abiotic stress responses (Kaplan et al., 2007; Moeder et al., 2011).
Multiple CNGC genes have been identified across different plant species, for instance, 20 members in Arabidopsis thaliana (Mäser et al., 2001), 47 in Triticum aestivum (Guo et al., 2018), 18 in Solanum lycopersicum (Saand et al., 2015), 16 in Oryza sativa (Wang et al., 2023), 26 in Brassica oleracea (Kakar et al., 2017), 29 in Solanum melongena (Jiang et al., 2023), 29 in Brassica rapa (Baloch et al., 2021), 20 in Malus domestica (Qiu et al., 2024), 35 in Nicotiana tabacum (Nawaz et al., 2019), 15 in Ziziphus jujuba (Wang et al., 2020), 16 in Saccharum spontaneum (Zhang et al., 2023a), 43 in Mangifera indica (Zhang et al., 2023b), 12 in Zea mays (Hao and Qiao, 2018), and 21 in Pyrus spp (Chen et al., 2015).
In Arabidopsis, CNGC genes were divided into 5 groups. Members from each of the groups have been reported to be involved in developmental regulation and stress resistance (Mäser et al., 2001; Dietrich et al., 2020; Tipper et al., 2023). For instance, the AtCNGC2–AtCNGC4 channel complex is known to be negatively regulated by calmodulins (CaMs) and positively regulated by the immune kinase BOTRYTIS-INDUCED KINASE 1 (BIK1). In the resting state, CaMs suppress channel activity, while upon pathogen detection, BIK1 phosphorylates and activates the channel, promoting Ca2+ influx for pattern-triggered immunity activation (Zhang et al., 2024b). The AtCNGC2 (DND1) mutant is resistant to a variety of pathogens, including the bacterium P. syringae, the fungal pathogens B. cinerea and A. brassicicola, and the oomycete H. arabidopsidis (Jurkowski et al., 2004; Young and Krougliak, 2004; Su’udi et al., 2011). The chimeric gene Arabidopsis CNGC11/12 activates resistance responses toward multiple pathogens (such as Hyaloperonospora parasitica and Phytophthora parasitica) (Yoshioka et al., 2001, 2006). Similarly, AtCNGC19 is induced by P. indica infection, and it regulates the synthesis and accumulation of indole glucosinolates, and further increases plant pathogen resistance (Jogawat et al., 2020).
Recent studies in other plant species have further highlighted the role of CNGCs in stress resistance. For instance, knocking down CNGC2 orthologs in tomato and potato markedly reduced their vulnerability to fungal infections responsible for powdery mildew, late blight, and gray mold (Sun et al., 2016a, 2016b, 2017). Similarly, mutations in the apple MdCNGC2 gene resulted in constitutive accumulation of salicylic acid (SA) and pathogenesis-related protein 1 (MdPR1) in apple callus, thereby restricting the spread of B. dothidea (Zhou et al., 2020). In Z. jujuba, ZjCNGC2 was shown to interact with mitogen-activated protein kinase 4 (MAPK4), suggesting its involvement in cold stress responses via the MAPK signaling cascade (Wang et al., 2020).
Potato (Solanum tuberosum, auto-tetraploid, 2n=4x=48) is the fourth most important food crop worldwide (Hou et al., 2019; Fan et al., 2022), following maize (Zea mays), rice (Oryza sativa), and wheat (Triticum aestivum) in 2019 (https://www.fao.org/home/en/). Despite its significance, a substantial amount of potato yield is lost per year due to viral, bacterial, or fungal diseases and pest attacks (Czajkowski et al., 2011; Zhang et al., 2019; Wu et al., 2020). Among these, soft rot and blackleg, caused by the bacterial pathogen Pectobacterium carotovorum, are particularly devastating. These diseases present in distinct forms, with stem infections referred to as blackleg and tuber infections as soft rot (Pérombelon, 2002). Therefore, there is an urgent need for sustainable and environmentally friendly strategies to control crop diseases (Zhu et al., 2024). Susceptibility (S) genes are plant genes exploited by pathogens to promote disease development. Mutations in S genes are typically recessively inherited and are considered a durable strategy to confer broad-spectrum resistance in plants (Garcia-Ruiz et al., 2021; Koseoglou et al., 2022; Gao et al., 2024). As previously noted, CNGCs have been identified as potential S genes in several plant species. Despite their importance, studies on the CNGC gene family in potato remain limited, particularly regarding their role in biotic stress resistance.
In this study, we identified 11 StCNGC genes and analyzed their structural features, phylogenetic relationships, and expression patterns under stress conditions. Further functional validation through silencing of StCNGC2 revealed its involvement in enhancing resistance to P. carotovorum. These findings contribute to our understanding of StCNGCs in improving disease resistance in this critical crop.
Methods
Identification of StCNGC family genes
To identify CNGCs in potato, 20 Arabidopsis CNGC protein sequences were retrieved from TAIR (Mäser et al., 2001). We employed these sequences as queries to search against the potato genome sequences downloaded from the Potato Genome Sequencing Consortium database (PGSC, version 6.1). A cut-off of E value < 10–5 was applied to ensure the reliability of the sequences. Meanwhile, the HMM (Hidden Markov Model) files cNMP_binding.hmm (Cyclic nucleotide-binding domain, PF00027) and Ion_trans.hmm (Ion transport protein, PF00520) were downloaded from the Pfam database (Paysan-Lafosse et al., 2025). Simple hmmsearch (Potter et al., 2018) from TBtools (Chen et al., 2023) was carried out to search for CNGC proteins in potato. The protein sequences obtained from BLASTp and hmmsearch methods were then combined, with redundant sequences removed. To check the presence of CNBD and ion transport domains, we submitted these protein sequences to the SMART program (Schultz, 2000), InterPro (Blum et al., 2024), and NCBI-CDD database (Marchler-Bauer et al., 2017). Proteins without CNBD and ion transport domains were excluded from further analysis. The same approach was employed to identify CNGCs in four Gramineae crops (Z. mays, T. aestivum, O. sativa, and S. bicolor) and one nightshade member (S. lycopersicum).
Analysis of physicochemical properties and chromosomal location
ExPaSy (Duvaud et al., 2021) was used to calculate the molecular weight (MW) and isoelectric point (pI) of the proteins. Chromosomal distribution of StCNGCs and genetic sequence data were obtained from a BLASTn search of the EnsemblPlant database (Yates et al., 2022). The genomic locations of StCNGC genes were visualized on chromosomes using MapChart software based on their genome coordinates.
Gene structure, conserved motifs and promoter cis-acting elements analysis
The exon-intron structures were analyzed using the online program GSDS (Hu et al., 2015), utilizing the coding sequences (CDS) and whole gene sequences from the StCNGC genes. The conserved motifs were identified using the online program MEME (Bailey et al., 2009), with parameters set for motif widths ranging from 6 to 200 bp and a maximum of 12 motifs. The 1500 bp sequence upstream of the promoter region of each StCNGC was identified by searching EnsemblPlants (SolTub_3.0), and was then analyzed using PlantCARE (Lescot, 2002) for the cis-acting elements analysis. The obtained data were then visualized by using TBtools (v2.146) software (Chen et al., 2023).
Analysis of phylogenetic relationships, gene duplication events
A multiple sequence alignment of the amino acid sequences was performed using DNAMAN software (version 6.0). The whole amino acid sequences were aligned using the ClustalW algorithm, and a phylogenetic tree was generated with MEGA software (version 7) (Kumar et al., 2016), employing the neighbour-joining (NJ) approach with 1000 bootstrap iterations (Bi et al., 2024). Gene duplication analysis of the CNGC genes was explored by plotting intraspecies covariance maps using the TBtools (v2.146) software built-in Dual Synteny Plot program function (Chen et al., 2023).
Plant abiotic stress treatments
Three-week-old in vitro-propagated potato plantlets were transplanted into soil and grown in a greenhouse under controlled conditions (25 ± 2°C, 75% relative humidity, and a 16:8 h light/dark photoperiod). Potato derivatives cv. Désirée was used as a non-transgenic control. RNAi::StCNGC2 transgenic lines #5 and #17, corresponding to DND1A-5(+) and DND1A-17(+), were obtained from a previous study. They exhibit significantly reduced StCNGC2 expression (Sun et al., 2016a).
For abiotic stress treatments, potato plants grown in the greenhouse for 4–6 weeks were exposed to the following conditions: drought stress by withholding water for 8 days, heat stress by maintaining greenhouse temperature at 42°C for 2 d, and cold stress by incubating plants at 4°C for 5 d. Potato leaves were collected at the end of each treatment. All materials were snap-frozen in liquid nitrogen and stored at − 80°C for subsequent expression analyses. Three biological replicates (four plants per replicate) were examined for each treatment.
Pathogen infection assays
Four–six-week-old potato plants with fully developed composite leaves were infected with Phytophthora infestans, Botrytis cinerea, and Pectobacterium carotovorum to assess pathogen response. Detached leaves were infected with P. infestans strain 88069, following the method described by Vleeshouwers et al. (1999). Leaf samples were collected at 48 hours post-inoculation (hpi). For the B. cinerea infection, leaflets were inoculated with strain B05.10 at a density of 3×105 spores/ml, and the inoculation method was referred to Sun et al. (2017). Leaf samples were collected at 48 hpi. For the bacterial assay, P. carotovora subsp. brasiliense strain 212 was prepared at a concentration of 1×103 cfu/ml, and the inoculations were performed as described by Rietman et al. (2014). Stem samples were collected at 46 hpi. An equal volume of autoclave ddH2O was used as mock treatment following the same procedure as for pathogen inoculation. All plant samples were immediately frozen in liquid nitrogen and stored for the following gene expression analyses. For each treatment, three replicates (four plants per replicate) were examined.
Analysis of tissue-specific gene expression
Transcriptome data were acquired from the Spud DB website (http://solanaceae.plantbiology.msu.edu/index.shtml) to examine the expression patterns of StCNGC genes in different tissues. Five different organs were included in the analysis, namely, leaves, flowers, roots, stems, and tubers. Gene expression levels were quantified as fragments per kilobase per million mapped reads (FPKM) (Supplementary Table S6). Raw FPKM values were normalized by the logarithmic method, and a heat map was generated with TBtools software (Chen et al., 2023) to visualize the expression levels.
Additionally, leaves, flowers, roots, stems and tubers were also collected from potato cv. Désirée plants for total RNA extraction and validation of expression patterns via RT-qPCR analysis.
Anatomical analysis of stem structures
To examine the infection of P. carotovora, stem cross-sections were collected 1 cm above the inoculation site at 0, 12, 24, 36, and 46 hpi using sharp scalpels. Samples for each genotype were observed using a bright-field binocular microscope (Olympus SZX16, Japan). Images were captured using a microscope-mounted digital camera (TOUPCAM™, Germany) with consistent settings across all observations. For each time point, at least twelve plants were analyzed.
RNA isolation and gene expression analysis
Total RNA was extracted using an RNA isolation kit (Huayueyang, China), following the manufacturer’s protocol. The concentration and quality of RNA were assessed using a Nanodrop 2000 spectrophotometer (Thermo Scientific, China). First-strand cDNA was synthesized using a cDNA synthesis kit (Monad, MonScript™ RTIII AII-in-One Mix with dsDNase, China).
Quantitative real-time PCR (RT-qPCR) was conducted on a C1000™ Thermal Cycler PCR system (Bio-Rad) by using MonAmp™ SYBR® Green qPCR Mix (Monad, None ROX). The potato StEF1a (Sotub06g010680) served as an internal control. The comparative gene expression level was determined utilizing the 2 -ΔΔCT method (Livak and Schmittgen, 2001). Three technical replicates were included for each reaction, and three biological replicates were included for each sample. Primer sequences are listed in Supplementary Table S7.
Results
Identification of StCNGC gene family in potato
To identify the CNGC gene family of potato, BLASTP was performed based on the sequence of the 20 CNGC members of Arabidopsis thaliana. Meanwhile, Hidden Markov models (HMMs) for the cNMP_binding domain (PF00027) and the Ion_trans domain (PF00520) were applied using hmmsearch against the potato genome. Afterwards, SMART, InterPro, PROSITE conserved domain search tools, and NCBI Conserved Domain Data (CDD) were employed to confirm the presence of CNBD and ion transport domains in the putative proteins. Domain composition analyses revealed that some candidate genes carried potassium channel AKT/KAT domains (Shaker type) (Su et al., 2001). Therefore, the remaining 11 genes, including CNBD and ion transport domains, and no additional potassium channel domains, were designated as StCNGC genes.
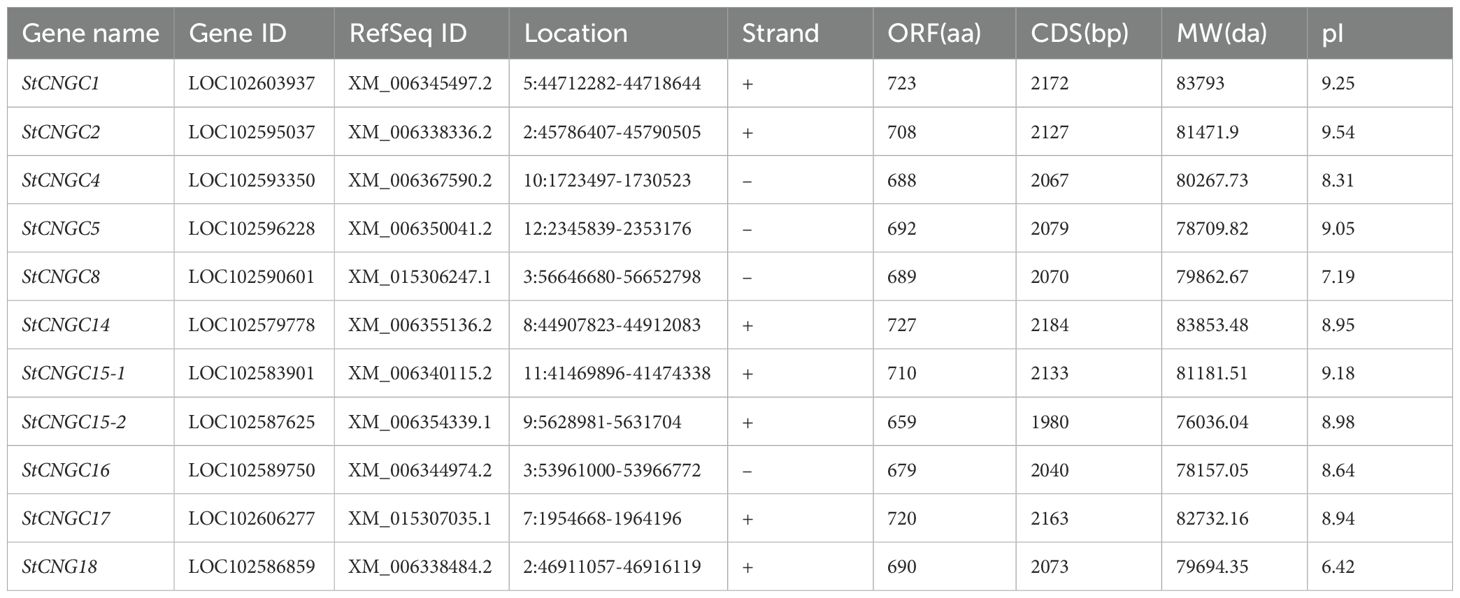
Chromosomal mapping revealed their distribution across nine of the 12 potato chromosomes (Figure 1). There were two StCNGC genes on Chr 2 and Chr 3 and one StCNGC gene on each of the other chromosomes. No genes were found on Chr 1, Chr 4 and Chr 6. The identified StCNGC protein length ranges from 659 (StCNGC15-2) to 727 (StCNGC14) amino acids (aa) in length, with molecular weights (MWs) of 76036.04 to 83853.48 Da. Most StCNGC proteins (except for StCNGC8 and StCNGC18) had Isoelectric point (pI) values above 8.0.
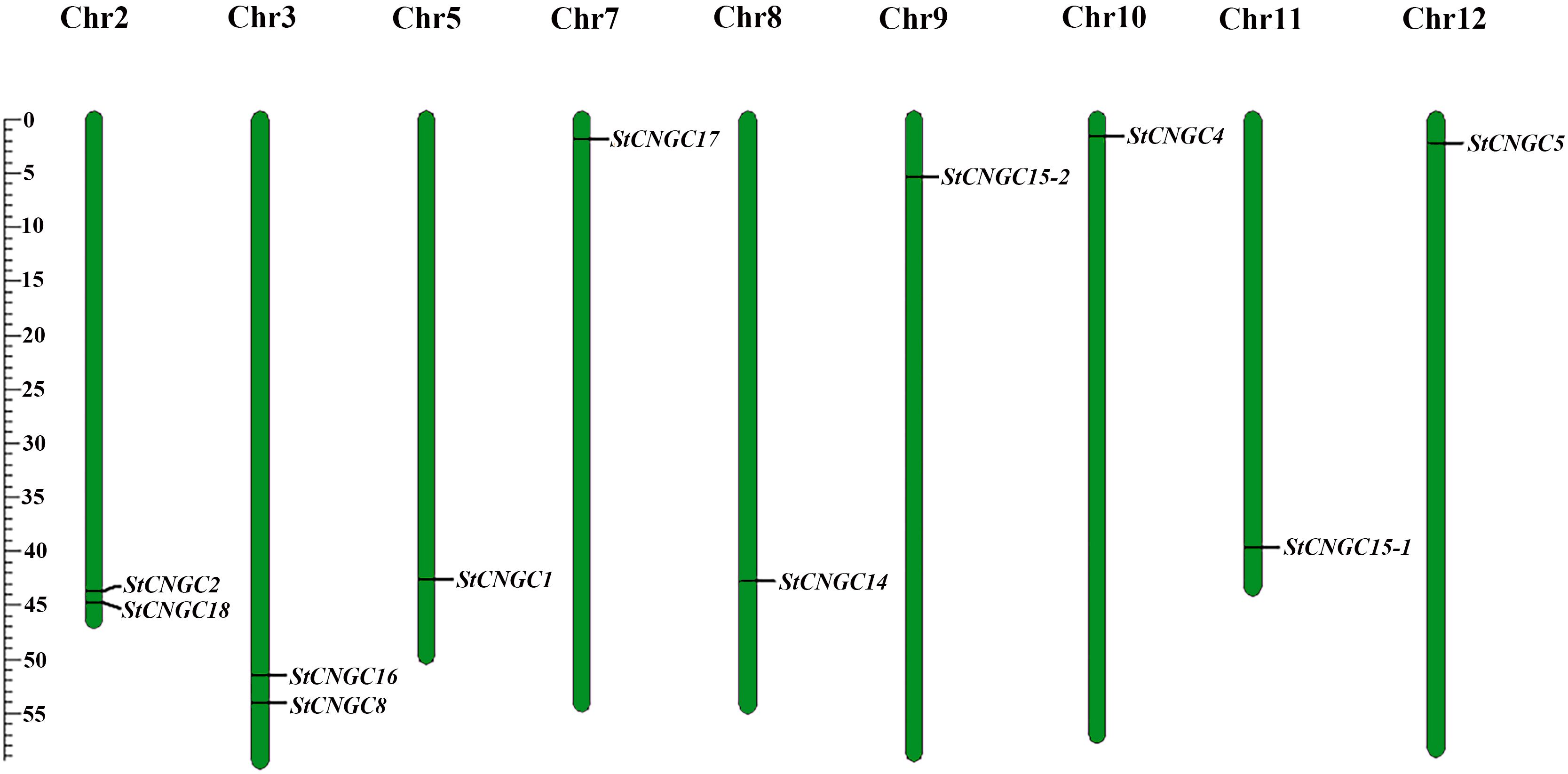
Figure 1. Chromosomal distribution of potato CNGCs. Each CNGC member was mapped on its physical chromosome location. The chromosome number (Chr02-Chr12) is indicated at the top. Only chromosomes containing StCNGC genes are represented in this figure.
Sequence alignment and phylogenetic analysis
Sequence alignment of StCNGCs has revealed a variable number of transmembrane helices, ranging from 3 to 7 (Supplementary Table S1), as well as highly conserved CNBD and CaMBD, and isoleucine–glutamine (IQ) motifs (Figure 2). Within the CNBD domain, the two most conserved regions are the PBC and the adjacent hinge region (Figure 2). The consensus PBC motif was identified to be: [L]-X(4)-[F]-X-[G]-[DE]-[E]-[L]-[L]-X-[W]-[AC]-[L]-X(6,7,8)-[L]-[P]-X-[S]-[TS]-X-[TS]-X(7)-[E]-[AS]-[F]-[AG]-[VL]-X-[A] (X denotes any residue). The CaMBD motif, [H]-[F]-[R]-[Y]-X-[F]-X-[N]-[E]-X(2)-[K]-[R]-X-[A]-[R], was not well conserved, while the IQ motif [I]-[Q]-X-[A]-[W]-[RF]-[R] was relatively conserved among the StCNGC proteins.
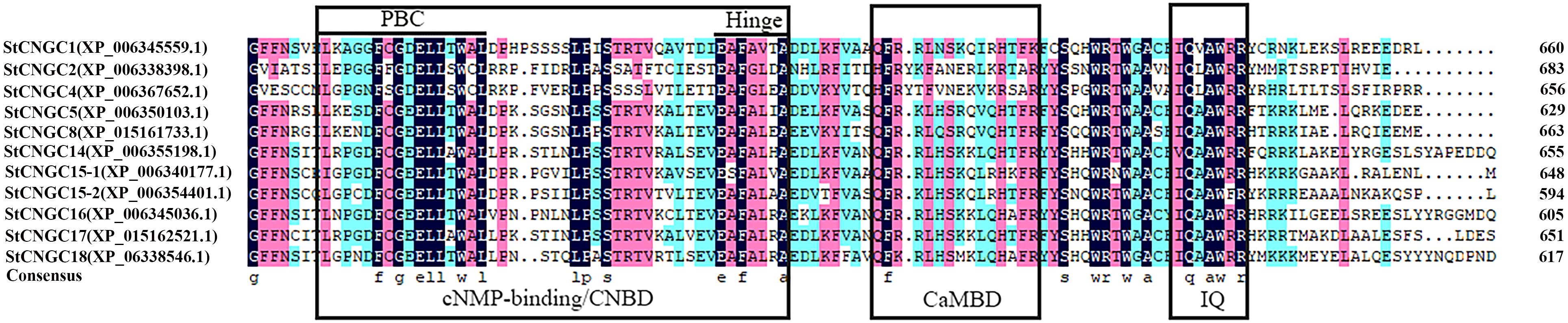
Figure 2. Conserved amino acids and multiple sequence alignment of the StCNGC proteins. Sequences were displayed by the DNAMAN software. The StCNGC conserved sequences were marked with a purple background for an amino acid identity greater than 75% and a light blue background for an amino acid identity greater than 50%. The family-specific conserved motifs and active sites are indicated.
To assess the evolutionary relationships of the StCNGC proteins, complete protein sequences of 91 CNGCs from seven angiosperm species were analyzed phylogenetically using MEGA software. These CNGC proteins included 20 sequences from Arabidopsis, 11 from potato, 13 from tomato, 13 from corn, 14 from rice, 8 from wheat, and 12 from sorghum (Supplementary Table S2). The resulting phylogenetic tree clustered the CNGC proteins into five distinct groups (Groups I, II, III, IVa, and IVb), following the established Arabidopsis CNGC classification. Group III emerged as the largest with 34 genes, including six potato CNGC genes (StCNGC14, StCNGC15-1, StCNGC16, StCNGC17, and StCNGC18). Group II and Group IVb contained 20 and 13 genes, respectively, each with two potato CNGC members. Group I contained only one potato CNGC gene (StCNGC1) and no StCNGC was assigned to Group IVa.
Gene structure analysis and motif identification
We also conducted exon-intron structure analyses based on the corresponding genome and coding sequences. The number of exons in the potato CNGC family varied from 6 to 8 (Figure 3). To further elucidate the structural and functional properties of the StCNGC proteins, conserved motifs were identified using MEME (Bailey et al., 2009). Twelve putative motifs, designated as motifs 1 to 12, were identified in the StCNGC family (Figure 3, Supplementary Table S3). The relative positions and arrangements of these motifs varied across the five phylogenetic groups, forming distinct motif patterns (Figure 3). All the StCNGCs contained motifs 1–8 and motif 11, representing the core conserved motifs of the StCNGC family. Motif 1 is the CNBD located at the C-terminal region, while motif 12 corresponds to the CaMBD and the IQ domain. Furthermore, motifs 2, and 4–8 correspond to the transmembrane domain in the N-terminal region. Interestingly, some motifs exhibited gene-specific losses. For instance, motif 9 was not found in StCNGC2, StCNGC4, and StCNGC8, while motif 10 was not found in StCNGC2, StCNGC4, StCNGC8, and StCNGC15-1. Motifs 3, 9, 10, and 11 are responsible for unknown functions.
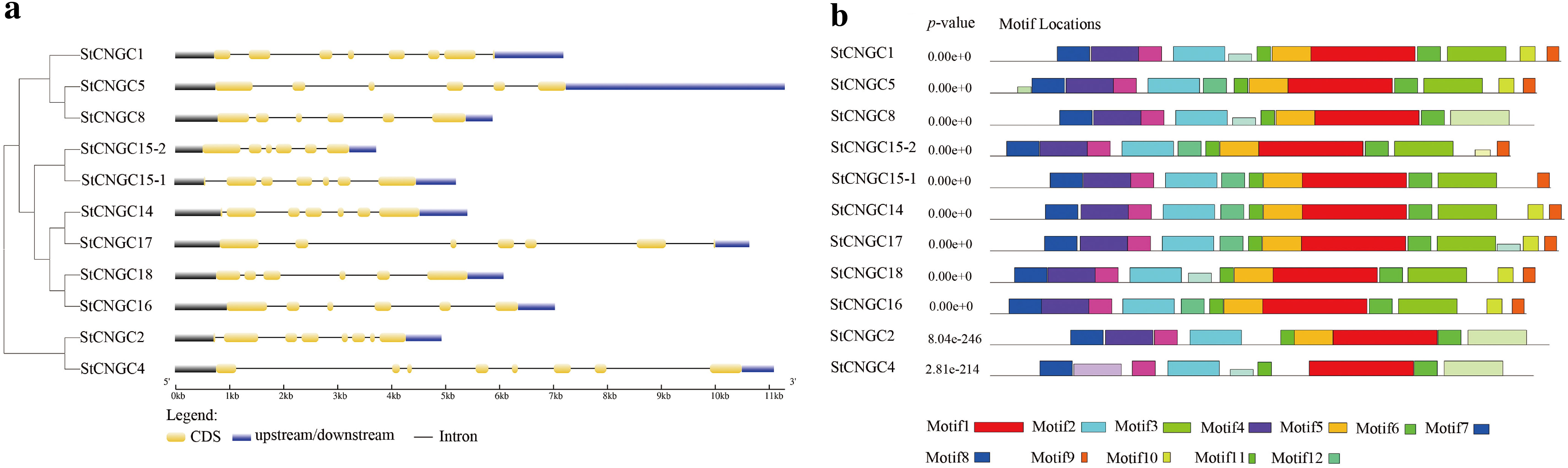
Figure 3. Schematic structure of CNGC genes in potato. (a) Gene structural analysis of potato CNGCs. Introns and exons are represented by black lines and yellow boxes, respectively. The untranslated regions (UTRs) are indicated by blue boxes. The sizes of the exons and introns can be estimated using the scale at the bottom. (b) Putative protein motifs of each potato StCNGCs by MEME. Conserved motifs (1–12) are represented by different colored boxes while non-conserved sequences are shown by gray lines.
Covariance analysis of the StCNGCs
Collinear analysis of potato and Arabidopsis CNGCs revealed that 28 segmental duplication gene pairs were detected between Arabidopsis and potato, with amino acid sequence similarity greater than 60%. The potato genome contained 8 segmental duplication gene pairs (Figure 4). The allocation of segmental duplication genes across chromosomes was unequal, with two genes located on chromosome 2, two on chromosome 3, and one gene present on chromosomes 5, 7, 8, 9, 10, 11, and 12. Overall, the CNGC gene family in potato exhibited notable collinearity with Arabidopsis.
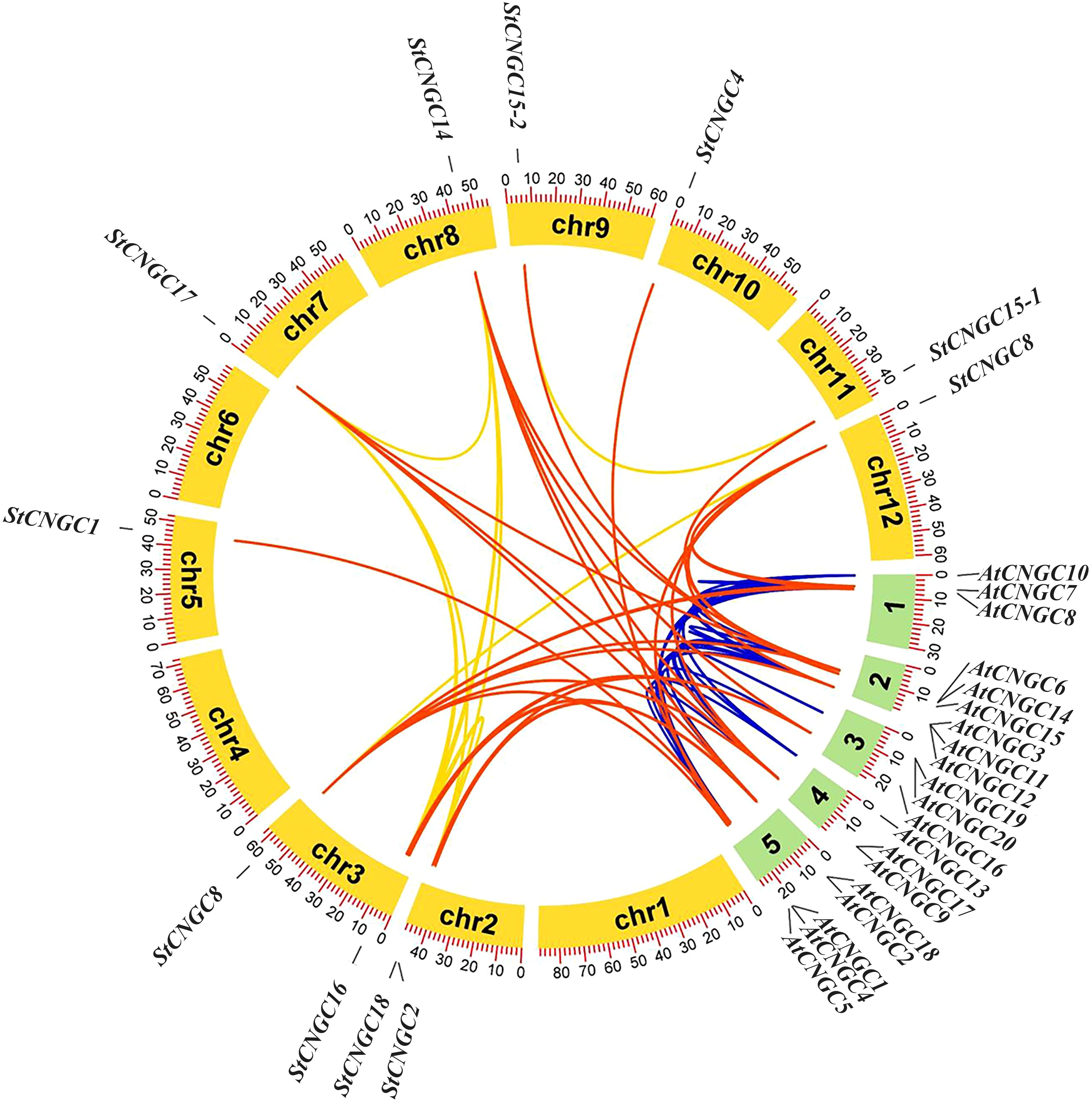
Figure 4. Homology analysis of CNGCs between potato and Arabidopsis genomes. Yellow arcs represent potato chromosomes and green arcs represent Arabidopsis chromosomes. Red, blue, and golden lines indicate homologous gene pairs between potato and Arabidopsis.
Cis-acting elements analysis of the StCNGCs
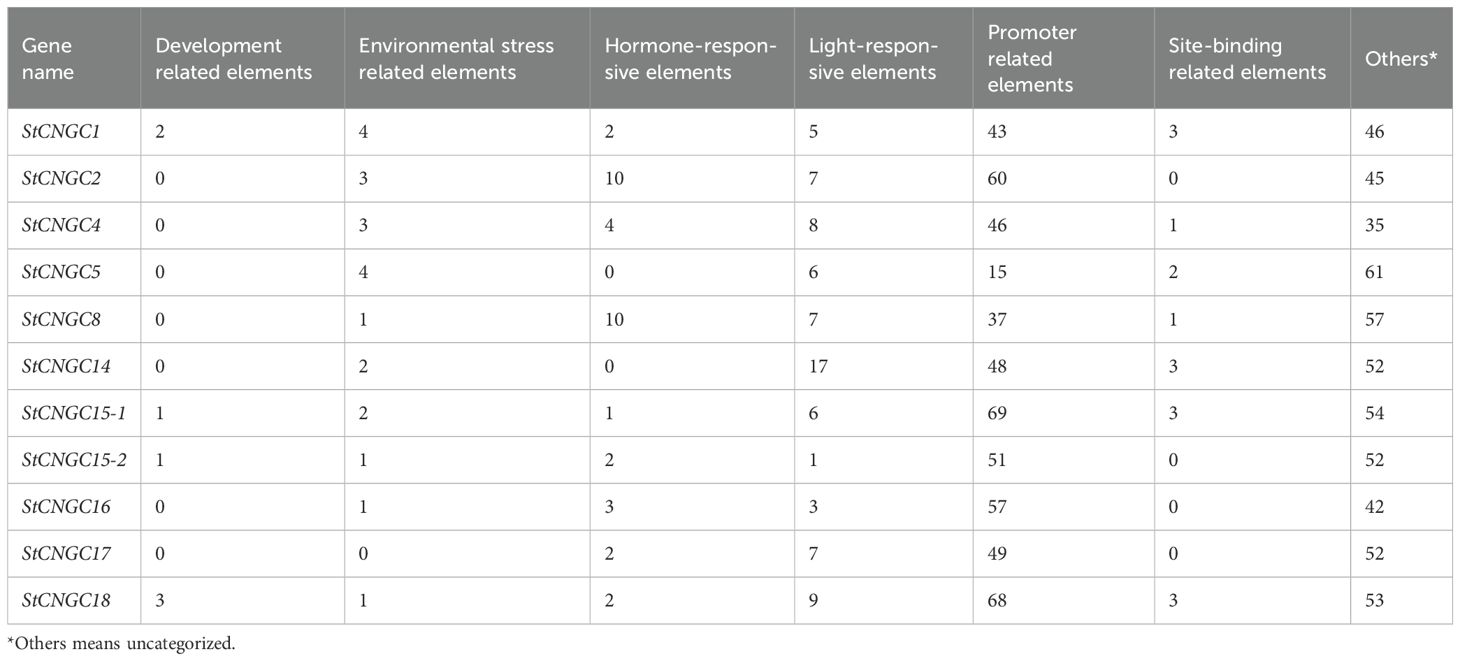
To examine the potential cis-elements participating in the transcriptional regulation of StCNGCs, a 1.5 kb promoter region upstream of each StCNGC coding sequence was investigated in PlantCARE database. As summarized in Table 1, the cis-acting elements in the promoter regions of plant StCNGCs were highly diverse. A total of 78 types of cis-acting elements were detected, including 33 with unknown functions. The predominant elements in the StCNGC promoter regions were light-responsive elements, comprising 16 distinct types (Supplementary Table S4). The number of cis-elements per promoter ranged from a minimum of 15 in StCNGC5 to a maximum of 68 in StCNGC18 (Table 1). Eight types of hormone response elements were detected across the promoter regions, corresponding to auxin, abscisic acid, SA, gibberellin and jasmonic acid. Notably, 10 corresponding hormone response elements were identified in StCNGC2 and StCNGC8, while StCNGC5 and StCNGC14 lacked hormone-responsive elements. Particularly, auxin cis-regulatory elements, including one auxin-responsive region (AuxRR) and a TGA box, were found in the promoter regions of StCNGC8, StCNGC15–2 and StCNGC17, which indicated potential auxin regulation (Supplementary Table S4).
In addition to hormonal regulation, environmental stress-related elements were identified in the promoter regions. Four types of stress-responsive elements were observed, with ARE elements (cis-acting regulatory element essential for the anaerobic induction) presented in 8 StCNGCs and TC-rich repeats (TC-rich repeats) detected in 5 StCNGCs (Supplementary Table S4).
Additionally, the GCN4 motif, associated with endosperm expression, was the predominant development-related element found in the promoters of three StCNGC genes. StCNGC1 was the only gene containing one circadian control element, while StCNGC18 promoter contained elements associated with palisade mesophyll cell differentiation (Supplementary Table S4).
Tissue-specific expression analysis of the StCNGC gene family
A hierarchical clustering heatmap was constructed to examine the expression patterns of StCNGC genes in different plant organs (root, stem, leaf, flower, and tuber) utilizing transcriptome data from the PGSC database. Results showed that StCNGC8, StCNGC16, and StCNGC18 had similar expression patterns, being highly expressed in flowers but nearly undetectable in other tissues. Although StCNGC14 was expressed in all checked tissues, its expression levels remained low. While StCNGC2, StCNGC4, StCNGC5, and StCNGC17 exhibited high expression across all analyzed organs, and StCNGC2 and StCNGC5 showed the highest expression in flowers and roots, respectively (Supplementary Figure S1).
To validate the transcriptome data, we further analyzed the expression of 11 StCNGCs in different organs of potato cv. Désirée by RT-qPCR. The results were largely consistent with the published transcriptome data (Figure 5, Supplementary Table S5): StCNGC5 and StCNGC17 were highly expressed in roots, with low expression in tubers and stems. Most StCNGCs were significantly expressed in flowers, except for StCNGC1, StCNGC2, and StCNGC4, which showed low expression levels, contrary to the transcriptome data findings. This discrepancy may result from the differences in sampling methods or developmental stages between this study and the previous studies.
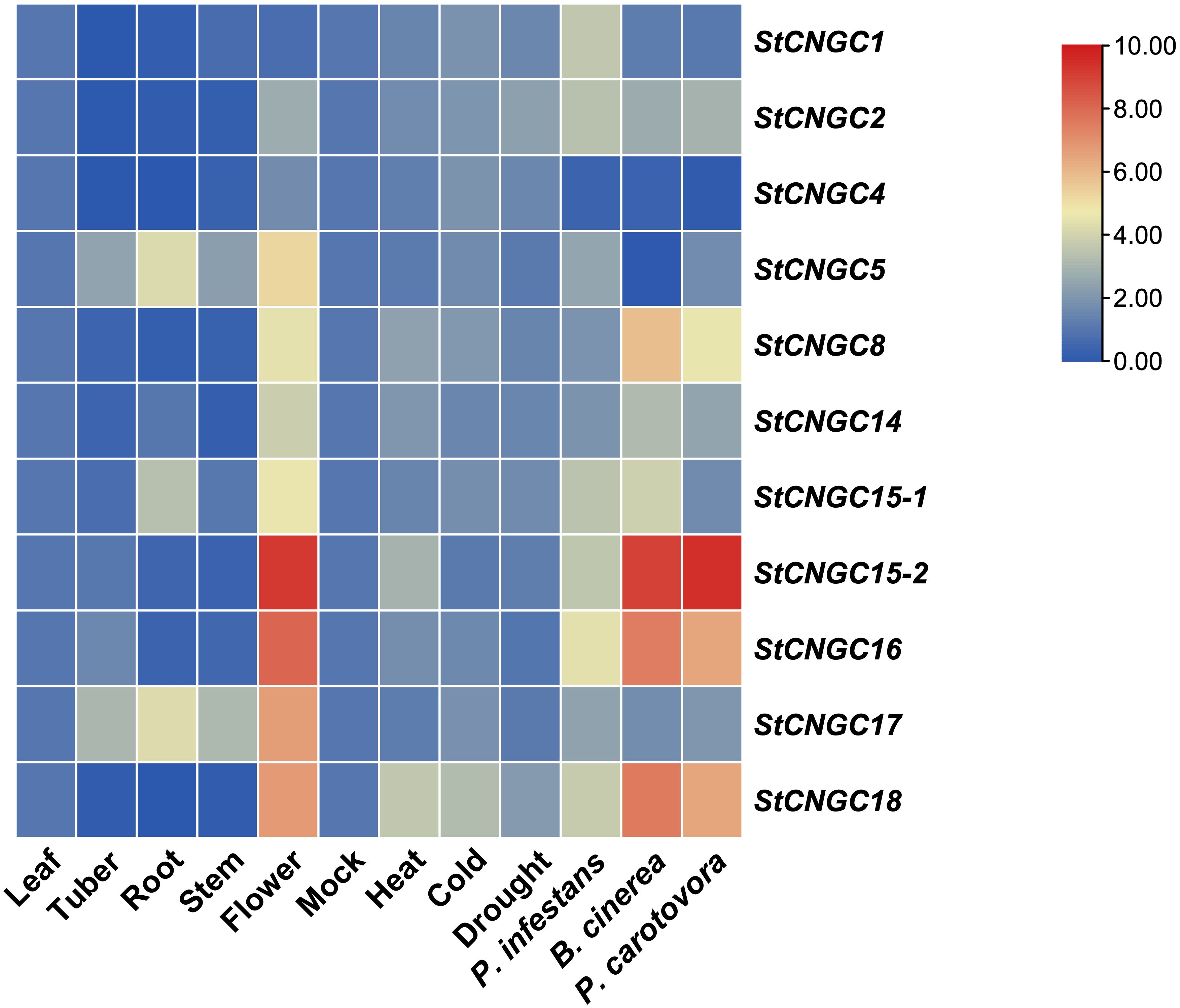
Figure 5. Relative expression analysis of StCNGCs in different tissues and under different stress conditions. For the tissue-specific analysis, leaf, tuber, root, stem, and flower samples were collected from mature cv. Désirée plants for RNA extraction and RT-qPCR analysis. For stress-response analysis, leaves (abiotic and biotic treatments) and stems (for blackleg infection only) were collected for RNA extraction and RT-qPCR analysis. The stress conditions are as follows: Heat (42°C for 2 days), Cold (4°C for 5 days), Drought (water withheld for 8 days), Phytophthora infestans infection for 48 h, Botrytis cinerea infection for 48 h (Bc/48), and Pectobacterium carotovorum infection for 46 h. The corresponding mock treatments included room temperature, normal watering, or ddH2O inoculation for the same duration as each stress condition. All stress-treated samples were compared to their respective mock controls. The mock shown in the heatmap is a collection of condition-specific controls, not a universal mock used across all experiments. The potato StEF1a gene was used as an internal control, and three independent samples were included in these experiments. Gene expression levels are represented as log2(Fold change), where red indicates high expression levels and blue indicates low expression levels.
To investigate the potential function of StCNGCs in response to stresses, we further analyzed their expression in potato leaf under control conditions and different stress treatments using RT-qPCR (Figure 5, Supplementary Table S5). None of the StCNGCs were induced under drought stress. For heat and cold stress, only StCNGC18 showed increased expression compared to control. Notably, some StCNGCs were significantly induced under biotic stress conditions. For instance, the expression levels of StCNGC1, StCNGC2, StCNGC15-1, StCNGC15-2, StCNGC16, and StCNGC18 were highly increased in P. infestans infected leaves with late blight disease. Additionally, StCNGC2, StCNGC8, StCNGC14, StCNGC15-2, StCNGC16, and StCNGC18 showed increased expression in leaves with B. cinerea-induced grey mold or P. carotovorum-induced blackleg infection compared to mock treatment. Given previous findings that silencing StCNGC2/StDND1 confers broad-spectrum resistance to pathogens such as powdery mildew, late blight, and gray mold (Sun et al., 2016a, 2017), we hypothesize that StCNGC2 also plays a role in blackleg resistance.
StCNGC2-silenced potato plants showed enhanced resistance to blackleg disease
Next, wild-type cv. Désirée and RNAi::StCNGC2 potato plants were inoculated with P. carotovorum. Disease symptoms were observed at five time points (0, 12, 24, 36, and 46 hours post-inoculation [hpi]). In cv. Désirée plants, blackening of vascular tissue, and pulp degradation were evident by 12 hpi, accompanied by softening of petioles near the inoculation sites (Figure 6a). Later on, necrosis in the petiole pith increased progressively, leading to visible tissue degeneration and vascular blackening. In contrast, RNAi::StCNGC2 plants did not show typical blackleg disease symptoms, such as wilting, soft rot, or vascular blackening, even after 46 dpi (Figure 6a).
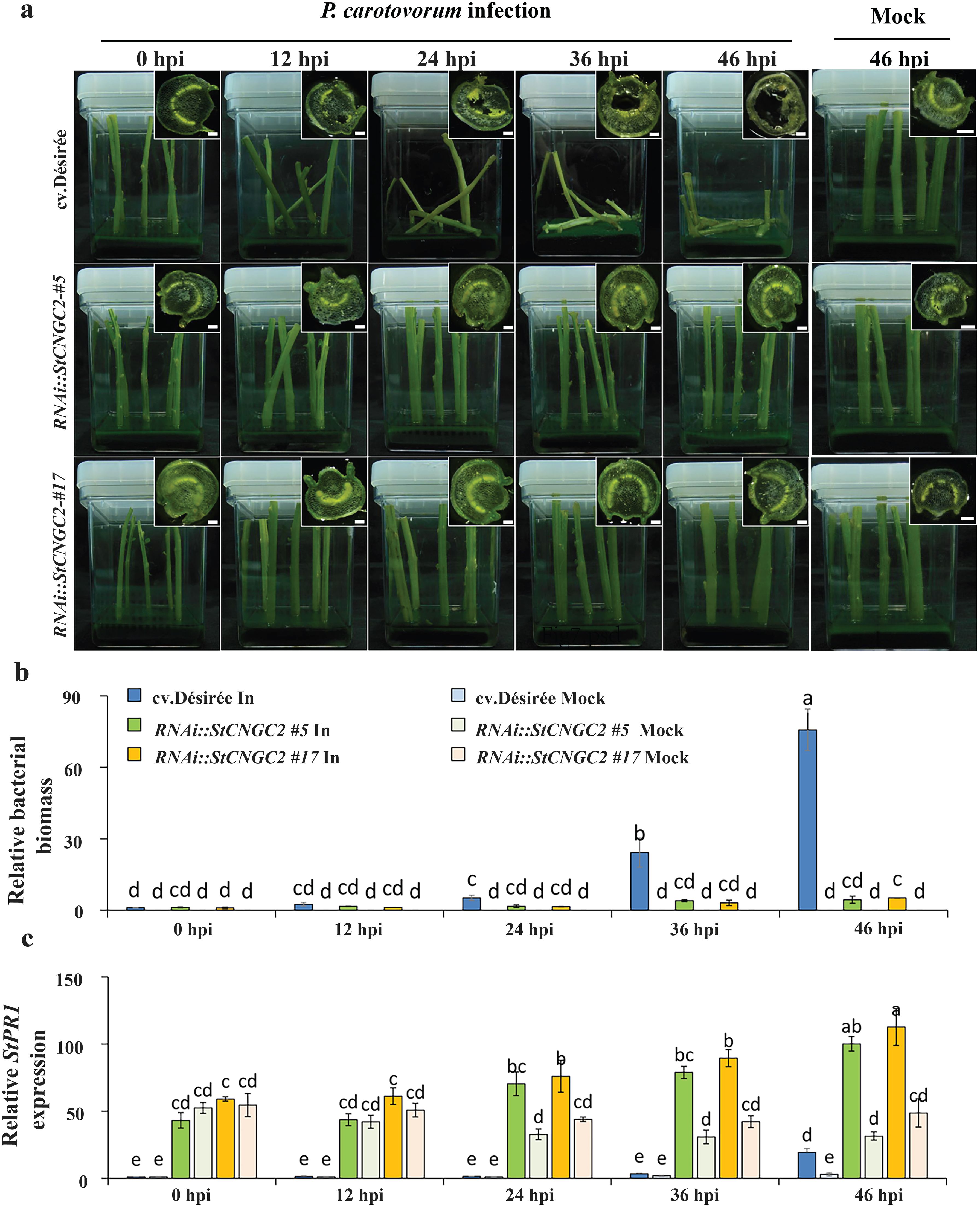
Figure 6. Analysis of potato blackleg inoculation in cv. Désirée- and StCNGC2-silenced potato plants. (a) Disease symptoms in potato petioles mock-treated or infected with Pectobacterium carotovorum. Pathogen-infected samples were imaged at 0, 12, 24, and 48 hours post-inoculation (hpi). The mock-treated sample was imaged at 48 hours post-inoculation (hpi) as a control. Preliminary observations indicated no visible changes in mock-treated samples throughout the time course. The insets show cross-section images of representative petioles at respective time points (Scale bar = 1 mm). (b) The relative bacterial content in mock treated and infected cv. Désirée- and StCNGC2-silenced potato plants. (c) The expression levels of StPR1 genes analyzed by RT-qPCR. The different letters indicate significant differences between time points according to Duncan’s multiple range test (P < 0.05; n = 3) performed in SPSS. In: petioles inoculated by P. carotovorum; Mock: petioles immersed in MES buffer as a control.
To confirm the abovementioned observations, we quantified bacterial biomass by RT-qPCR at various times after inoculation with P. carotovorum (Figure 6b). In cv. Désirée plants, there was a steady increase in bacterial biomass from 12 hpi. In contrast, in RNAi::StCNGC2 plants, the bacterial biomass remained restricted to a low level, confirming an increased resistance to P. carotovorum (Figure 6b).
Previously, elevated expression levels of PR1, a well-established marker of systemic acquired resistance (SAR), were reported in the respective mutants with reduced expression of CNGC2 in Arabidopsis, potato and tomato (Clough et al., 2000; Sun et al., 2016b, 2017). To investigate the mechanism underlying the reduced P. carotovorum susceptibility in StCNGC2-silenced potato plants, we measured the expression of StPR1 in a time-course infection (0, 12, 24, 36, and 46 hpi) by P. carotovorum. Noteworthy, a significantly higher StPR1 expression was observed in RNAi::StCNGC2 #5 and #17 than in cv. Désirée even under mock treatment. When challenged with P. carotovorum, StPR1 was significantly induced in cv. Désirée at 46 hpi, StCNGC2-silenced plants showed an earlier induction (at 24 hpi) (Figure 6c).
Discussion
Genome-wide studies of the CNGC family have been carried out in many plant species. In this study, we identified 11 CNGC family genes in potato. Additionally, 14, 8, 13, and 12 CNGCs were identified in rice, wheat, corn, and sorghum, respectively (Figure 7). The pI and charge of a protein are important for its solubility, subcellular localization, and interaction (Khaldi and Shields, 2011).
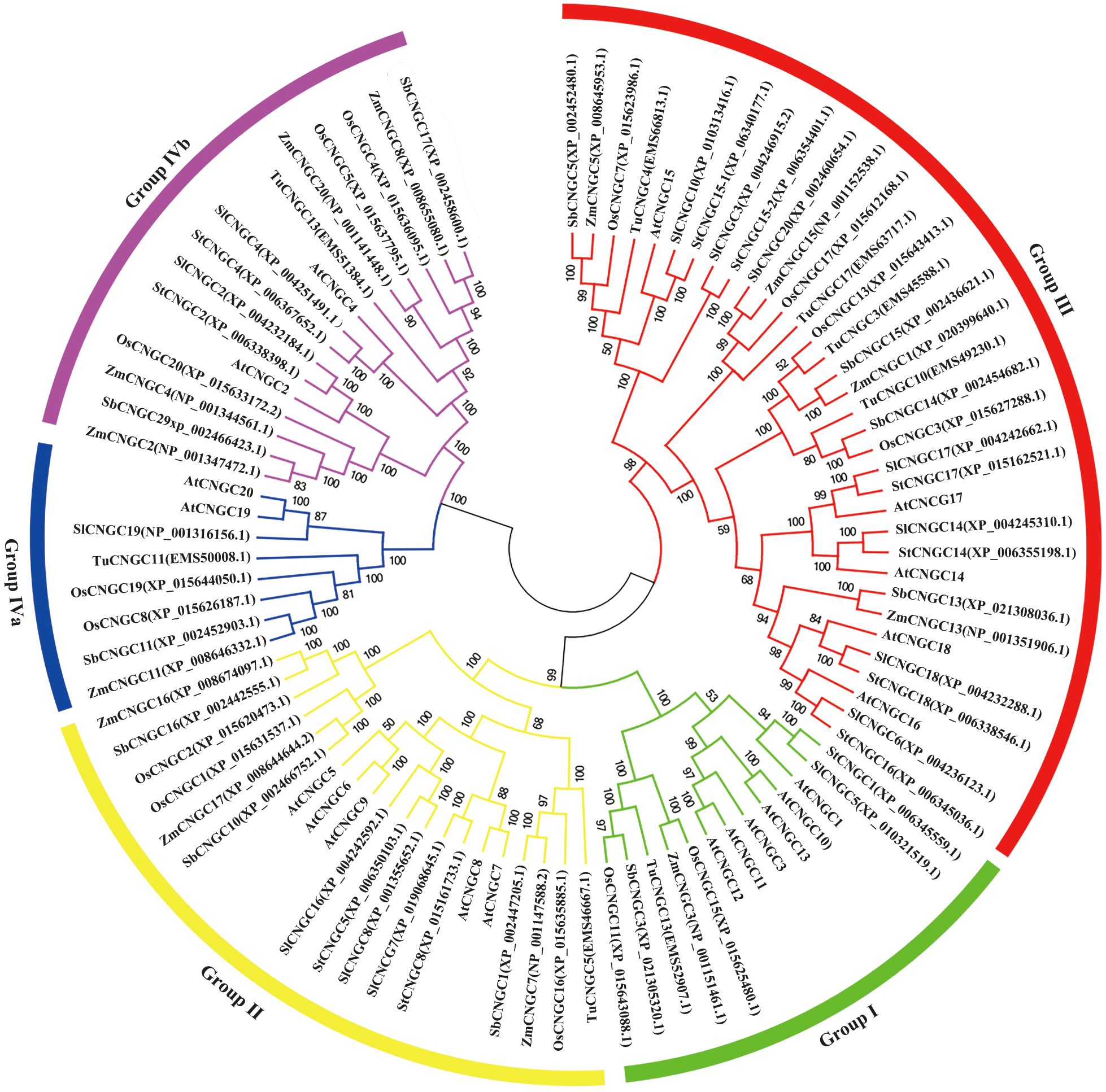
Figure 7. Phylogenetic analysis of the CNGC proteins in corn (ZmCMGC), rice (OsCNGC), wheat (TuCNGC), Sorghum (SbCNGC), Arabidopsis (AtCNGC), potato (StCNGC), and tomato (SlCNGC). The subgroups are marked by color bars. The phylogenetic tree was constructed using the neighbor-joining method with 1000 bootstrap replication.
Studies indicate that cytoplasmic proteins exhibit an acidic isoelectric point (pI < 7.4), nuclear proteins display a more neutral pI (7.4 < pI < 8.1), and membrane proteins frequently possess basic pI values (pI > 8.1), with basic residues adjacent to the membrane-spanning region enhancing protein stabilization within the membrane (Schwartz et al., 2001; Khaldi and Shields, 2011). According to our results, the 11 StCNGCs contain varying numbers of transmembrane domains (Supplementary Table S1), and most of them (except for StCNGC8 and StCNGC18) have high pI values (> 8.1) (Table 2), indicating that they could be membrane proteins. The StCNGC genes showed high similarity to their corresponding AtCNGC genes in terms of plant CNGC-specific domains, amino acid composition, gene structure, and phylogenetic classification. They were categorized into five distinct groups with high bootstrap support. Homologous genes within the same group are expected to share comparable structural, functional, and evolutionary characteristics (Huang et al., 2018; Cheng et al., 2021; Wang et al., 2021b). Our finding offers insights into the roles of CNGC genes in potato.
CNGCs play a crucial role in regulating various biological processes, including growth and development, responses to environmental stresses, and plant defense mechanisms (Qi et al., 2010). This study showed that the cis-elements in the promoter region of plant StCNGCs were highly diverse, including various types of light response elements, hormone-related elements and environmental stress elements. This diversity aligns with the expression profiles observed in different conditions (Supplementary Table S4). In-depth gene expression analyses in this study highlighted the critical roles of StCNGC genes in potato growth, development, and stress responses (Figure 5, Supplementary Table S5). We analyzed StCNGCs expression in different tissues and found that most StCNGCs were expressed at high levels in flower tissues, which is consistent with the findings of previous research on these genes in Arabidopsis (Gao et al., 2016; Pan et al., 2019). For instance, a mutation in AtCNGC18 causes a defect in pollen tube growth, resulting in curved, short, and often thin pollen tubes that are unable to enter the transport tube (Frietsch et al., 2007). In this study, the expression of StCNGCs in response to abiotic stresses (drought, heat, and cold) and biotic stresses (late blight, grey mold, and blackleg) was also explored (Figure 5). Notably, StCNGC18 showed a higher expression than other genes under heat and cold stress, indicating its putative role in extreme temperature stress response. StCNGC2 (StDND1), showed increased expression under various biotic stimuli, consistent with its proposed function in mediating Ca2+ influx during PTI activation. Noteworthy, StCNGC15-2, StCNGC16, and StCNGC18 were also highly expressed in leaf tissue undergoing late blight, grey mold and blackleg infections, suggesting their potential implications in broad-range disease resistance. While these findings provide new insights into the role of CNGC genes in blackleg disease, further studies utilizing gene editing approaches (Zhang et al., 2021) are required to validate the specific functions of StCNGC15-2, StCNGC16 and StCNGC18 in disease resistance.
The CNGC2 (DND1) mutation in Arabidopsis, tomato, potato, and apple improved plant resistance to resistant to a broad-spectrum of pathogens, including bacteria, fungi, and oomycetes (Govrin and Levine, 2000; Jurkowski et al., 2004; Young and Krougliak, 2004; Genger et al., 2008; Su’udi et al., 2011; Zhang et al., 2024b). In this study, we demonstrated that silencing StCNGC2 improves blackleg disease resistance (Figure 6). Interestingly, overexpressing onion CNGC2 homolog gene in Nicotiana benthamiana improves plant resistance to Phytophthora nicotianae by promoting ROS accumulation, suggesting a positive role against pathogens (Qin et al., 2024). This contradiction suggests that CNGC2 homologs may have different regulatory roles in plant immunity, depending on the plant species, developmental stage, and type of pathogen encountered. These differences highlight the complexity of calcium signaling in plant immunity and underscore the importance of species-specific functional validation of each gene family member.
PR1 is reported to be a gene marker for systemic acquired resistance and exogenous SA treatment induces PR gene expression in many dicotyledonous plant species (Gal-On, 2007; Qu et al., 2024; Zhang et al., 2024a). Many studies have shown that the accumulation of SA in plants increases resistance to pathogens. For example, blackleg-resistant Atcngc2 mutant accumulates higher levels of SA than wild-type Arabidopsis plants; overexpression of OsWRKY13 in rice increased SA accumulation and enhanced resistance to bacterial blight (Ahn, 2007; Qiu et al., 2007). In this study, we found the level of StPR1 gene expression in StCNGC2 silenced lines was significantly higher than that in cv. Désirée (Figure 6), indicating the elevated StPR1 levels are associated with improved plant resistance.
Disrupting plant S genes represents an alternative approach to achieve recessive, and potentially more durable, disease resistance (Garcia-Ruiz et al., 2021; Koseoglou et al., 2022). Our results confirmed StCNGC2 as an S gene contributing to susceptibility to P. carotovorum. Given that mutation of S genes has been shown to enhance disease resistance across multiple plant species (Zaidi et al., 2018), further genome editing of StCNGC2 to investigate its role in broader biotic stress responses and the potential crosstalk among these pathways represents a promising direction for future research.
Conclusion
In summary, 11 StCNGC genes were comprehensively identified in the potato genome and classified into four clades based on phylogenetic analysis with Arabidopsis CNGC groupings. The motif composition of StCNGC proteins and the cis-acting elements in their promoter regions were analyzed in detail. The expression profiles of StCNGC genes revealed their differential expression across various tissues, and under multiple stress conditions, indicating their involvement in plant growth and stress responses. Notably, silencing StCNGC2 enhanced resistance to blackleg disease, highlighting its role as a susceptibility (S) gene. Furthermore, our findings suggest that StCNGC genes play roles in both biotic and abiotic stress responses, highlighting their potential to enhance potato resistance to pathogens and environmental challenges. Collectively, this study provides a comprehensive overview of the CNGC gene family in the potato genome and establishes a foundation for further investigation into the mechanisms underlying StCNGC function and their applications in crop improvement.
Data availability statement
All relevant data are contained within the article. The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Ethics statement
This study was conducted in accordance with the People’s Republic of China and international authorities relevant guidelines and legislation, including the official website of the Committee on Publication Ethics (http://www.publicationethics.org/) and the European Association of Science Editors (EASE) and other institutions.
Author contributions
KS: Funding acquisition, Writing – review & editing, Conceptualization, Investigation, Project administration. SL: Investigation, Methodology, Writing – original draft. HM: Investigation, Writing – review & editing. QZ: Investigation, Writing – review & editing. HL: Writing – review & editing, Investigation. SS: Writing – review & editing. EJ: Writing – review & editing. RV: Writing – review & editing. YB: Writing – review & editing, Investigation. CL: Writing – review & editing. ZJ: Formal analysis, Methodology, Writing – review & editing. YS: Writing – review & editing. GM: Conceptualization, Investigation, Writing – review & editing, Funding acquisition, Visualization, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the NSFC (32172575, 32301848, 31801420), Youth Talent Promotion Project of Henan Province (2021HYTP037), Science and Technology Activities Foundation for Returned Scholars of Henan Province (30602137), Henan Provincial Colleges and Universities Youth Key Teacher Project (2024GGJS028), the Key Scientific Research And Development Project of Henan province (252102110184) and Teaching Reform Project of Henan Agricultural University (2023XJGLX008).
Acknowledgments
We would like to thank Yan Zhang, and Hongxu Chen for help in bioinformatic work and sampling. We thank Changhe Feng and Tianyou Meng for maintenance of the potato plant material. We thank Yongchun Shi and Xiaoyu Li for assisting in the microscopic studies. The authors are grateful to Professor Søren K. Rasmussen from Copenhagen University, for his help in manuscript revising and proofing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1614191/full#supplementary-material
Abbreviations
Bp, base pair; CDS, coding domain sequences; CNGC, cyclic nucleotide-gated ion channel; CNMPs, cyclic nucleotide monophosphates (3′,5′-cAMP and 3′,5′-cGMP); CNBD, cyclic nucleotide-binding domain; CaM, calmodulin; CaMB, CaM-binding;
cpr22, pathogenesis related genes 22; CNGC2 (DND1), defense no death 1; IQ, isoleucine–glutamine; MW, molecular weight; MAPK, mitogen-activated protein kinase; PBC, phosphate-binding cassette; SA, salicylic acid; pI, isoelectric point; PR1, pathogenesis related protein 1.
References
Ahn, I. P. (2007). Disturbance of the Ca2+/calmodulin-dependent signalling pathway is responsible for the resistance of Arabidopsis dnd1 against Pectobacterium carotovorum infection. Mol. Plant Pathol. 8, 747–759. doi: 10.1111/j.1364-3703.2007.00428.x
Bailey, T. L., Boden, M., Buske, F. A., Frith, M., Grant, C. E., Clementi, L., et al. (2009). MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 37, W202–W208. doi: 10.1093/nar/gkp335
Baloch, A. A., Raza, A. M., Rana, S. S. A., Ullah, S., Khan, S., Zaib-Un-Nisa, et al. (2021). BrCNGC gene family in field mustard: genome-wide identification, characterization, comparative synteny, evolution and expression profiling. Sci. Rep. 11, 24203. doi: 10.1038/s41598-021-03712-y
Bi, H., Liu, Z., Liu, S., Qiao, W., Zhang, K., Zhao, M., et al. (2024). Genome-wide analysis of wheat xyloglucan endotransglucosylase/hydrolase (XTH) gene family revealed TaXTH17 involved in abiotic stress responses. BMC Plant Biol. 24, 640. doi: 10.1186/s12870-024-05370-4
Blum, M., Andreeva, A., Florentino, L. C., Chuguransky, S. R., Grego, T., Hobbs, E., et al. (2024). InterPro: the protein sequence classification resource in 2025. Nucleic Acids Res. 6 (53), gkae1082. doi: 10.1093/nar/gkae1082
Chen, C., Wu, Y., Li, J., Wang, X., Zeng, Z., Xu, J., et al. (2023). TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 16, 1733–1742. doi: 10.1016/j.molp.2023.09.010
Chen, J., Yin, H., Gu, J., Li, L., Liu, Z., Jiang, X., et al. (2015). Genomic characterization, phylogenetic comparison and differential expression of the cyclic nucleotide-gated channels gene family in pear (Pyrus bretchneideri Rehd.). Genomics 105, 39–52. doi: 10.1016/j.ygeno.2014.11.006
Cheng, J., Ma, J., Zheng, X., Lv, H., Zhang, M., Tan, B., et al. (2021). Functional analysis of the gibberellin 2-oxidase gene family in peach. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.619158
Clough, S. J., Fengler, K. A., Yu, I., Lippok, B., Smith, R. K., and Bent, A. F. (2000). The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. 97, 9323–9328. doi: 10.1073/pnas.150005697
Czajkowski, R., Pérombelon, M. C. M., Van Veen, J. A., and van der Wolf, J. M. (2011). Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathol. 60, 999–1013. doi: 10.1111/j.1365-3059.2011.02470.x
Dietrich, P., Moeder, W., and Yoshioka, K. (2020). Plant cyclic nucleotide-gated channels: new insights on their functions and regulation. Plant Physiol. 184, 27–38. doi: 10.1104/pp.20.00425
Duvaud, S., Gabella, C., Lisacek, F., Stockinger, H., Ioannidis, V., and Durinx, C. (2021). Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 49, W216–W227. doi: 10.1093/nar/gkab225
Fan, Y., Feng, H., Jin, X., Yue, J., Liu, Y., Li, Z., et al. (2022). Estimation of the nitrogen content of potato plants based on morphological parameters and visible light vegetation indices. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1012070
Fischer, C., Kugler, A., Hoth, S., and Dietrich, P. (2013). An IQ domain mediates the interaction with calmodulin in a plant cyclic nucleotide-gated channel. Plant Cell Physiol. 54, 573–584. doi: 10.1093/pcp/pct021
Frietsch, S., Wang, Y., Sladek, C., Poulsen, L. R., Romanowsky, S. M., Schroeder, J. I., et al. (2007). A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc. Natl. Acad. Sci. 104, 14531–14536. doi: 10.1073/pnas.0701781104
Gal-On, A. (2007). Zucchini yellow mosaic virus: insect transmission and pathogenicity —the tails of two proteins. Mol. Plant Pathol. 8, 139–150. doi: 10.1111/j.1364-3703.2007.00381.x
Gao, Q., Gu, L., Wang, H., Fei, C., Fang, X., Hussain, J., et al. (2016). Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc. Natl. Acad. Sci. 113, 3096–3101. doi: 10.1073/pnas.1524629113
Gao, J., Zhang, N., Liu, G., Tian, J., Chen, M., Wang, Y., et al. (2024). Regulation of maize growth and immunity by ZmSKI3-mediated RNA decay and post-transcriptional gene silencing. J. Integr. Plant Biol. 66, 2561–2577. doi: 10.1111/jipb.13780
Garcia-Ruiz, H., Szurek, B., and Van den Ackerveken, G. (2021). Stop helping pathogens: engineering plant susceptibility genes for durable resistance. Curr. Opin. Biotechnol. 70, 187–195. doi: 10.1016/j.copbio.2021.05.005
Genger, R. K., Jurkowski, G. I., McDowell, J. M., Lu, H., Jung, H. W., Greenberg, J. T., et al. (2008). Signaling pathways that regulate the enhanced disease resistance of Arabidopsis “Defense, No Death” mutants. Mol. Plant Microbe Interact. 21, 1285–1296. doi: 10.1094/MPMI-21-10-1285
Govrin, E. M. and Levine, A. (2000). The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 10, 751–757. doi: 10.1016/S0960-9822(00)00560-1
Guo, J., Islam, M. A., Lin, H., Ji, C., Duan, Y., Liu, P., et al. (2018). Genome-wide identification of cyclic nucleotide-gated ion channel gene family in wheat and functional analyses of TaCNGC14 and TaCNGC16. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00018
Hao, L. and Qiao, X. (2018). Genome-wide identification and analysis of the CNGC gene family in maize. PeerJ 6, e5816. doi: 10.7717/peerj.5816
He, L., Yu, L., Li, B., Du, N., and Guo, S. (2018). The effect of exogenous calcium on cucumber fruit quality, photosynthesis, chlorophyll fluorescence, and fast chlorophyll fluorescence during the fruiting period under hypoxic stress. BMC Plant Biol. 18, 180. doi: 10.1186/s12870-018-1393-3
Hou, J., Liu, T., Reid, S., Zhang, H., Peng, X., Sun, K., et al. (2019). Silencing of α-amylase StAmy23 in potato tuber leads to delayed sprouting. Plant Physiol. Biochem. 139, 411–418. doi: 10.1016/j.plaphy.2019.03.044
Hu, B., Jin, J., Guo, A., Zhang, H., Luo, J., and Gao, G. (2015). GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31, 1296–1297. doi: 10.1093/bioinformatics/btu817
Huang, Q., Wang, M., and Xia, Z. (2018). The SULTR gene family in maize (Zea mays L.): Gene cloning and expression analyses under sulfate starvation and abiotic stress. J. Plant Physiol. 220, 24–33. doi: 10.1016/j.jplph.2017.10.010
James, Z. M. and Zagotta, W. N. (2018). Structural insights into the mechanisms of CNBD channel function. J. Gen. Physiol. 150, 225–244. doi: 10.1085/jgp.201711898
Jarratt-Barnham, E., Wang, L., Ning, Y., and Davies, J. M. (2021). The complex story of plant cyclic nucleotide-gated channels. Int. J. Mol. Sci. 22, 874. doi: 10.3390/ijms22020874
Jiang, Z., Du, L., Shen, L., He, J., Xia, X., Zhang, L., et al. (2023). Genome-Wide Exploration and Expression Analysis of the CNGC Gene Family in Eggplant (Solanum melongena L.) under Cold Stress, with Functional Characterization of SmCNGC1a. Int. J. Mol. Sci. 24, 13049. doi: 10.3390/ijms241713049
Jogawat, A., Meena, M. K., Kundu, A., Varma, M., and Vadassery, J. (2020). Calcium channel CNGC19 mediates basal defense signaling to regulate colonization by Piriformospora indica in Arabidopsis roots. J. Exp. Bot. 71, 2752–2768. doi: 10.1093/jxb/eraa028
Jurkowski, G. I., Smith, R. K., Yu, I., Ham, J. H., Sharma, S. B., Klessig, D. F., et al. (2004). Arabidopsis DND2, a second cyclic nucleotide-gated ion channel gene for which mutation causes the “Defense, No Death” phenotype. Mol. Plant Microbe Interact. 17, 511–520. doi: 10.1094/MPMI.2004.17.5.511
Kakar, K. U., Nawaz, Z., Kakar, K., Ali, E., Almoneafy, A. A., Ullah, R., et al. (2017). Comprehensive genomic analysis of the CNGC gene family in Brassica oleracea: novel insights into synteny, structures, and transcript profiles. BMC Genomics 18, 869. doi: 10.1186/s12864-017-4244-y
Kaplan, B., Sherman, T., and Fromm, H. (2007). Cyclic nucleotide-gated channels in plants. FEBS Lett. 581, 2237–2246. doi: 10.1016/j.febslet.2007.02.017
Khaldi, N. and Shields, D. C. (2011). Shift in the isoelectric-point of milk proteins as a consequence of adaptive divergence between the milks of mammalian species. Biol. Direct 6, 40. doi: 10.1186/1745-6150-6-40
Koseoglou, E., van der Wolf, J. M., Visser, R. G. F., and Bai, Y. (2022). Susceptibility reversed: modified plant susceptibility genes for resistance to bacteria. Trends Plant Sci. 27, 69–79. doi: 10.1016/j.tplants.2021.07.018
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Lescot, M. (2002). PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30, 325–327. doi: 10.1093/nar/30.1.325
Livak, K. J. and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Marchler-Bauer, A., Bo, Y., Han, L., He, J., Lanczycki, C. J., Lu, S., et al. (2017). CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 45, D200–D203. doi: 10.1093/nar/gkw1129
Mäser, P., Thomine, S., Schroeder, J. I., Ward, J. M., Hirschi, K., Sze, H., et al. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126, 1646–1667. doi: 10.1104/pp.126.4.1646
Moeder, W., Urquhart, W., Ung, H., and Yoshioka, K. (2011). The role of cyclic nucleotide-gated ion channels in plant immunity. Mol. Plant 4, 442–452. doi: 10.1093/mp/ssr018
Nawaz, Z., Kakar, K. U., Ullah, R., Yu, S., Zhang, J., Shu, Q. Y., et al. (2019). Genome-wide identification, evolution and expression analysis of cyclic nucleotide-gated channels in tobacco (Nicotiana tabacum L.). Genomics 111, 142–158. doi: 10.1016/j.ygeno.2018.01.010
Pan, Y., Chai, X., Gao, Q., Zhou, L., Zhang, S., Li, L., et al. (2019). Dynamic interactions of plant CNGC subunits and calmodulins drive oscillatory Ca2+ channel activities. Dev. Cell 48, 710–725.e5. doi: 10.1016/j.devcel.2018.12.025
Paysan-Lafosse, T., Andreeva, A., Blum, M., Chuguransky, S. R., Grego, T., Pinto, B. L., et al. (2025). The Pfam protein families database: embracing AI/ML. Nucleic Acids Res. 53, D523–D534. doi: 10.1093/nar/gkae997
Pérombelon, M. C. M. (2002). Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathol. 51, 1–12. doi: 10.1046/j.0032-0862.2001.Shorttitle.doc.x
Potter, S. C., Luciani, A., Eddy, S. R., Park, Y., Lopez, R., and Finn, R. D. (2018). HMMER web server: 2018 update. Nucleic Acids Res. 46, W200–W204. doi: 10.1093/nar/gky448
Qi, Z., Verma, R., Gehring, C., Yamaguchi, Y., Zhao, Y., Ryan, C. A., et al. (2010). Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc. Natl. Acad. Sci. 107, 21193–21198. doi: 10.1073/pnas.1000191107
Qin, L., Li, X., Ren, S., He, G., Zhang, X., Wang, A., et al. (2024). AcCNGC2, coding a cyclic nucleotide-gated channel protein from onion, enhances Phytophthora nicotianae resistance in transgenic Nicotiana benthamiana. Sci. Hortic. 326, 112779. doi: 10.1016/j.scienta.2023.112779
Qiu, L., Mei, C., Qi, Z., Yang, J., Li, N., Li, M., et al. (2024). Genome-wide analysis of apple CNGC family allows the identification of MdCNGC15A negatively regulating apple salt tolerance. Plant Stress 14, 100606. doi: 10.1016/j.stress.2024.100606
Qiu, D., Xiao, J., Ding, X., Xiong, M., Cai, M., Cao, Y., et al. (2007). OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol. Plant-Microbe Interactions® 20, 492–499. doi: 10.1094/MPMI-20-5-0492
Qu, K., Yin, Z., Gao, C., Song, G., Guo, Z., Yin, G., et al. (2024). Mutagenesis-derived resistance to black ooint in wheat. Plant Dis. 108, 899–907. doi: 10.1094/PDIS-07-23-1369-RE
Rietman, H., Finkers, R., Evers, L., van der Zouwen, P. S., van der Wolf, J. M., and Visser, R. G. F. (2014). A stringent and broad screen of Solanum spp. tolerance against Erwinia bacteria using a petiole test. Am. J. Potato Res. 91, 204–214. doi: 10.1007/s12230-013-9339-7
Saand, M. A., Xu, Y.-P., Li, W., Wang, J.-P., and Cai, X.-Z. (2015). Cyclic nucleotide gated channel gene family in tomato: genome-wide identification and functional analyses in disease resistance. Front. Plant Sci. 06. doi: 10.3389/fpls.2015.00303
Schultz, J. (2000). SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 28, 231–234. doi: 10.1093/nar/28.1.231
Schwartz, R., Ting, C. S., and King, J. (2001). Whole proteome pI values correlate with subcellular localizations of proteins for organisms within the three domains of life. Genome Res. 11, 703–709. doi: 10.1101/gr.158701
Su, H., Golldack, D., Katsuhara, M., Zhao, C., and Bohnert, H. J. (2001). Expression and stress-dependent induction of potassium channel transcripts in the common ice plant. Plant Physiol. 125, 604–614. doi: 10.1104/pp.125.2.604
Su’udi, M., Kim, M. G., Park, S. R., Hwang, D. J., Bae, S. C., and Ahn, I. P. (2011). Arabidopsis cell death in compatible and incompatible interactions with Alternaria brassicicola. Mol. Cells 31, 593–602. doi: 10.1007/s10059-011-2203-z
Sun, K., Van Tuinen, A., Van Kan, J. A. L., Wolters, A.-M. A., Jacobsen, E., Visser, R. G. F., et al. (2017). Silencing of DND1 in potato and tomato impedes conidial germination, attachment and hyphal growth of Botrytis cinerea. BMC Plant Biol. 17, 235. doi: 10.1186/s12870-017-1184-2
Sun, K., Wolters, A.-M. A., Loonen, A. E. H. M., Huibers, R. P., van der Vlugt, R., Goverse, A., et al. (2016a). Down-regulation of Arabidopsis DND1 orthologs in potato and tomato leads to broad-spectrum resistance to late blight and powdery mildew. Transgenic Res. 25, 123–138. doi: 10.1007/s11248-015-9921-5
Sun, K., Wolters, A.-M. A., Vossen, J. H., Rouwet, M. E., Loonen, A. E. H. M., Jacobsen, E., et al. (2016b). Silencing of six susceptibility genes results in potato late blight resistance. Transgenic Res. 25, 731–742. doi: 10.1007/s11248-016-9964-2
Tipper, E., Leitão, N., Dangeville, P., Lawson, D. M., and Charpentier, M. (2023). A novel mutant allele of AtCNGC15 reveals a dual function of nuclear calcium release in the root meristem. J. Exp. Bot. 74, 2572–2584. doi: 10.1093/jxb/erad041
Trudeau, M. C. and Zagotta, W. N. (2002). Mechanism of calcium/calmodulin inhibition of rod cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. 99, 8424–8429. doi: 10.1073/pnas.122015999
Vleeshouwers, V. G. A. A., van Dooijeweert, W., Paul Keizer, L. C., Sijpkes, L., Govers, F., and Colon, L. T. (1999). A laboratory assay for Phytophthora infestans resistance in various Solanum species reflects the field situation. Eur. J. Plant Pathol. 105, 241–250. doi: 10.1023/A:1008710700363
Wang, Y., Dai, X., Xu, G., Dai, Z., Chen, P., Zhang, T., et al. (2021a). The Ca2+-CaM signaling pathway mediates potassium uptake by regulating reactive oxygen species homeostasis in tobacco roots under low-K+ stress. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.658609
Wang, L., Li, M., Liu, Z., Dai, L., Zhang, M., Wang, L., et al. (2020). Genome-wide identification of CNGC genes in Chinese jujube (Ziziphus jujuba Mill.) and ZjCNGC2 mediated signalling cascades in response to cold stress. BMC Genomics 21, 191. doi: 10.1186/s12864-020-6601-5
Wang, X., Wu, F., Zhang, J., Bao, Y., Wang, N., Dou, G., et al. (2023). Identification of the CNGC gene family in rice and mining of alleles for application in rice improvement. Plants 12, 4089. doi: 10.3390/plants12244089
Wang, Q., Yang, G., Jia, R., Wang, F., Wang, G., Xu, Z., et al. (2024). Utilizing the mutant library to investigate the functional characterization of GhGLR3.4 regulating jasmonic acid to defense pest infestation. Plant J. 120, 2889–2903. doi: 10.1111/tpj.17152
Wang, Z., Zhang, L., Dong, C., Guo, J., Jin, L., Wei, P., et al. (2021b). Characterization and functional analysis of phytoene synthase gene family in tobacco. BMC Plant Biol. 21, 32. doi: 10.1186/s12870-020-02816-3
Wu, E., Wang, Y., Yahuza, L., He, M., Sun, D., Huang, Y., et al. (2020). Rapid adaptation of the Irish potato famine pathogen Phytophthora infestans to changing temperature. Evol. Appl. 13, 768–780. doi: 10.1111/eva.12899
Yang, H., You, C., Yang, S., Zhang, Y., Yang, F., Li, X., et al. (2021). The role of calcium/calcium-dependent protein kinases signal pathway in pollen tube growth. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.633293
Yates, A. D., Allen, J., Amode, R. M., Azov, A. G., Barba, M., Becerra, A., et al. (2022). Ensembl Genomes 2022: an expanding genome resource for non-vertebrates. Nucleic Acids Res. 50, D996–D1003. doi: 10.1093/nar/gkab1007
Yoshioka, K., Kachroo, P., Tsui, F., Sharma, S. B., Shah, J., and Klessig, D. F. (2001). Environmentally sensitive, SA-dependent defense responses in the cpr22 mutant of Arabidopsis. Plant J. 26, 447–459. doi: 10.1046/j.1365-313X.2001.2641039.x
Yoshioka, K., Moeder, W., Kang, H. G., Kachroo, P., Masmoudi, K., Berkowitz, G., et al. (2006). The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell 18, 747–763. doi: 10.1105/tpc.105.038786
Young, E. C. and Krougliak, N. (2004). Distinct structural determinants of efficacy and sensitivity in the ligand-binding domain of cyclic nucleotide-gated channels. J. Biol. Chem. 279, 3553–3562. doi: 10.1074/jbc.M310545200
Zaidi, S. S.-A., Mukhtar, M. S., and Mansoor, S. (2018). Genome editing: Targeting susceptibility genes for plant disease resistance. Trends Biotechnol. 36, 898–906. doi: 10.1016/j.tibtech.2018.04.005
Zhang, Y., Li, Y., Yang, J., Yang, X., Chen, S., Xie, Z., et al. (2023b). Genome-wide analysis and expression of cyclic nucleotide–gated ion channel (CNGC) family genes under cold stress in mango (Mangifera indica). Plants 12, 592. doi: 10.3390/plants12030592
Zhang, N., Lin, H., Zeng, Q., Fu, D., Gao, X., Wu, J., et al. (2023a). Genome-wide identification and expression analysis of the cyclic nucleotide-gated ion channel (CNGC) gene family in Saccharum spontaneum. BMC Genomics 24, 281. doi: 10.1186/s12864-023-09307-3
Zhang, X., Pan, Y., Hao, X., Guo, C., Wang, X., Yan, X., et al. (2024a). Overexpression of a grapevine VqWRKY2 transcription factor in Arabidopsis thaliana increases resistance to powdery mildew. Plant Cell Tissue Organ Cult. PCTOC 157, 16. doi: 10.1007/s11240-024-02746-0
Zhang, Z., Wang, Q., Yan, H., Cang, X., Li, W., He, J., et al. (2024b). Lighting-up wars: Stories of Ca2+ signaling in plant immunity. New Crops 1, 100027. doi: 10.1016/j.ncrops.2024.100027
Zhang, H., Yao, Y., Chen, S., Hou, J., Yu, Y., Liu, T., et al. (2019). SbRFP1 regulates cold-induced sweetening of potato tubers by inactivation of StBAM1. Plant Physiol. Biochem. 136, 215–221. doi: 10.1016/j.plaphy.2019.01.019
Zhang, D., Zhang, Z., Unver, T., and Zhang, B. (2021). CRISPR/Cas: A powerful tool for gene function study and crop improvement. J. Adv. Res. 29, 207–221. doi: 10.1016/j.jare.2020.10.003
Zhou, H., Bai, S., Wang, N., Sun, X., Zhang, Y., Zhu, J., et al. (2020). CRISPR/Cas9-mediated mutagenesis of MdCNGC2 in apple callus and VIGS-mediated silencing of MdCNGC2 in fruits improve resistance to Botryosphaeria dothidea. Front. Plant Sci. 11, 575477. doi: 10.3389/fpls.2020.575477
Keywords: calcium signaling, plant immunity, pathogenesis-related proteins, blackleg resistance, potato
Citation: Sun K, Liu S, Mao H, Zha Q, Liu H, Shen S, Jacobsen E, Visser RGF, Bai Y, Li C, Jia Z, Meng G and Shen Y (2025) Genome-wide identification of cyclic nucleotide-gated channel gene family in Solanum tuberosum and silencing of StCNGC2 provides resistance to Pectobacterium carotovorum. Front. Plant Sci. 16:1614191. doi: 10.3389/fpls.2025.1614191
Received: 18 April 2025; Accepted: 24 June 2025;
Published: 15 July 2025.
Edited by:
Katarzyna Otulak-Kozieł, Warsaw University of Life Sciences, PolandReviewed by:
Wanwan Liang, Chinese Academy of Agricultural Sciences, ChinaFarhan Goher, Guangdong Academy of Science (CAS), China
Copyright © 2025 Sun, Liu, Mao, Zha, Liu, Shen, Jacobsen, Visser, Bai, Li, Jia, Meng and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiqi Jia, amlhemhpcWkyMDE1QDE2My5jb20=; Geng Meng, bWVuZ2dlbmdAaGVuYXUuZWR1LmNu; Yawen Shen, eWF3ZW4uc2hlbkBoZW5hdS5lZHUuY24=
†These authors have contributed equally to this work
‡ORCID: Kaile Sun, orcid.org/0000-0002-2178-994X
Richard G. F. Visser, orcid.org/0000-0002-0213-4016
 Kaile Sun
Kaile Sun Shuai Liu1
Shuai Liu1 Richard G. F. Visser
Richard G. F. Visser Yuling Bai
Yuling Bai Geng Meng
Geng Meng Yawen Shen
Yawen Shen