- 1Bioprocess Engineering, AlgaePARC, Wageningen University, Wageningen, Netherlands
- 2Department of Botany and Microbiology, Faculty of Science, Tanta University, Tanta, Egypt
- 3Faculty of Biosciences and Aquaculture, Nord University, Bodø, Norway
Sterols are essential for eukaryotic cell membrane integrity and fluidity, and they demonstrate valuable pharmaceutical and nutraceutical benefits, including anti-inflammatory, antioxidant, anti-cancer, and cholesterol-lowering properties. Traditionally, animal, plant, and microbial fermentation sterols face sustainability, economic, and ethical challenges. Microalgae have emerged as a promising alternative due to their biochemical diversity, rapid growth, and controlled cultivation capabilities. This review explores microalgae’s potential for sterol production, highlighting their advantages, including sustainability and sterol profile diversity, while addressing key challenges of low sterol yields, species-dependent variations, and industrial scalability. We discuss how recent advancements in metabolic engineering, cultivation technologies, and process optimization could enhance sterol production. By integrating innovative biotechnological strategies, microalgae hold the potential to become a viable and sustainable sterol source for pharmaceutical, nutraceutical, and industrial applications.
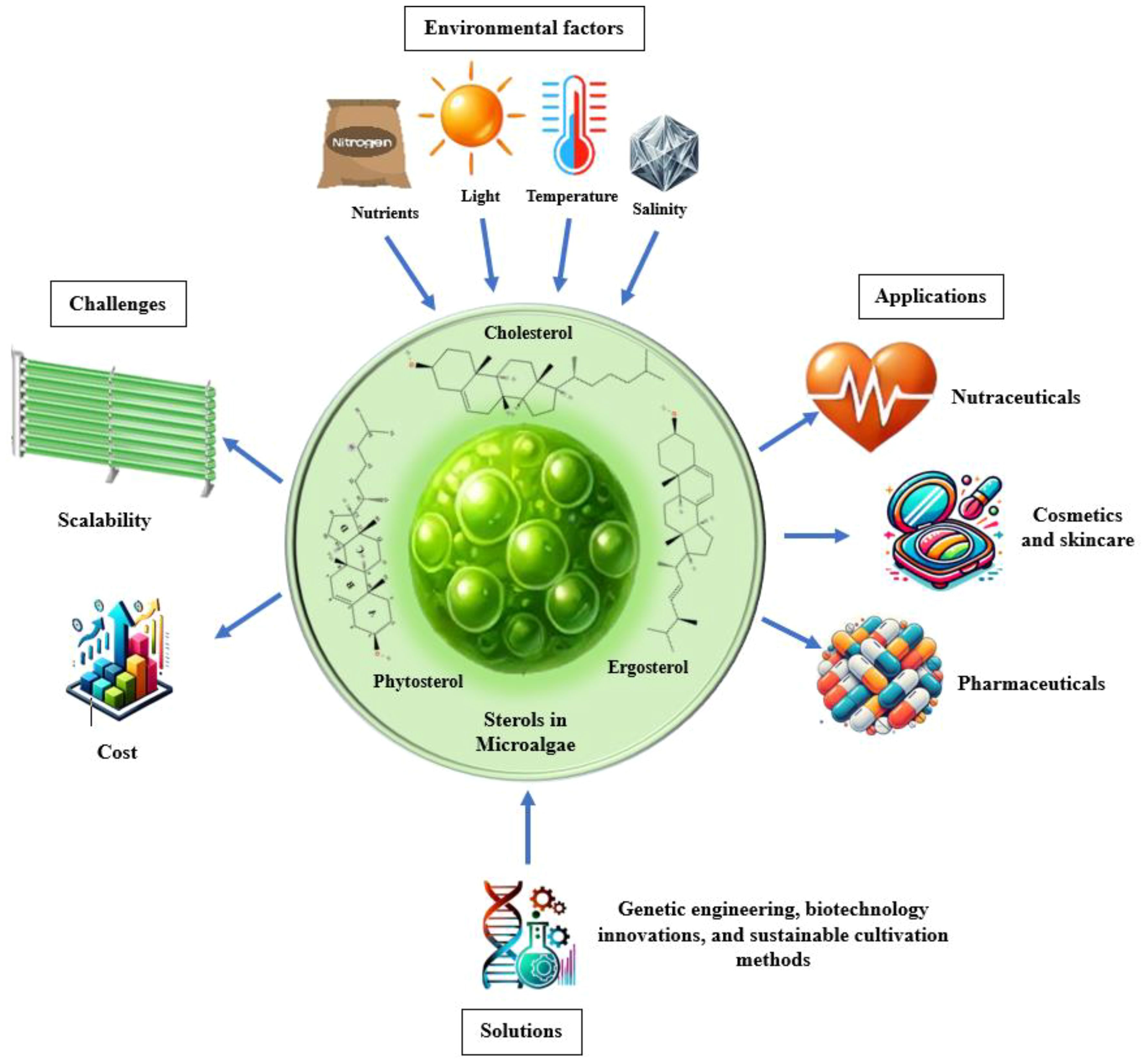
Graphical Abstract. Sterols in microalgae: influencing factors, challenges, solutions, and applications
Introduction
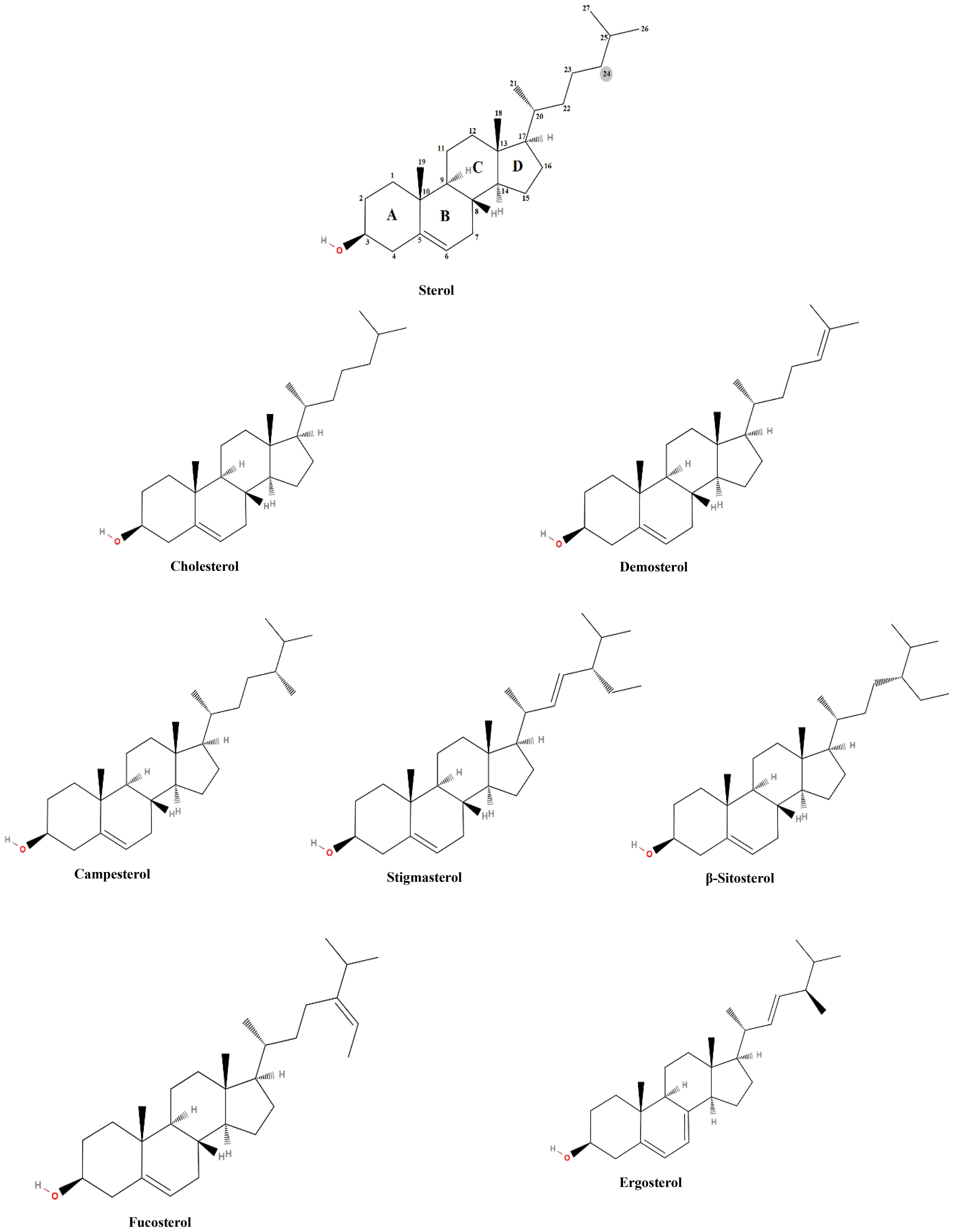
Sterols are vital lipid compounds that play critical roles in cellular structure and function, serving as integral components of eukaryotic cell membranes (Figure 1). They regulate membrane fluidity and integrity, influence intracellular signaling, and modulate enzyme and receptor activities, impacting fundamental biological processes such as gene expression, cell division, and apoptosis. Additionally, sterols are key intermediates in isoprenoid lipid biosynthesis, a pathway essential for synthesizing bioactive molecules involved in cellular homeostasis (Volkman, 2003). Structurally, sterols are characterized by a tetracyclic cyclopenta[α]phenanthrene ring system, which consists of three cyclohexane rings (A, B, and C) and one cyclopentane ring (D) (Figure 2). Their amphipathic nature, attributed to the presence of both hydrophilic and hydrophobic regions, allows them to integrate seamlessly into the phospholipid bilayer, where they regulate membrane properties. The hydroxyl (-OH) group in ring A is particularly significant, forming hydrogen bonds and other key molecular interactions and stabilizing membrane architecture. The B ring contributes to sterol planarity, while the C ring influences side-chain orientation at C20, which affects sterol function. The D ring and its bonded side chains further define sterol specificity and interactions, influencing their biochemical roles and structural adaptability (Fagundes et al., 2020). Due to their diverse biological functions, sterols have garnered significant interest in the pharmaceutical, nutraceutical, cosmetic, and food industries. They are extensively used as cholesterol-lowering agents in pharmaceuticals (Poli et al., 2021; Barkas et al., 2023), as functional supplements in nutraceuticals, as anti-aging compounds in cosmetics (Pérez-Sánchez et al., 2018; Bjørklund et al., 2022), and as stabilizers (Mohammadi et al., 2020) and bioactive ingredients in food formulations (T.B.D.A, 2024; Association, N.L, 2024). Given their versatility and industrial significance, ongoing research aims to explore alternative, sustainable sources of sterols to address growing market demand while ensuring environmental and economic feasibility.
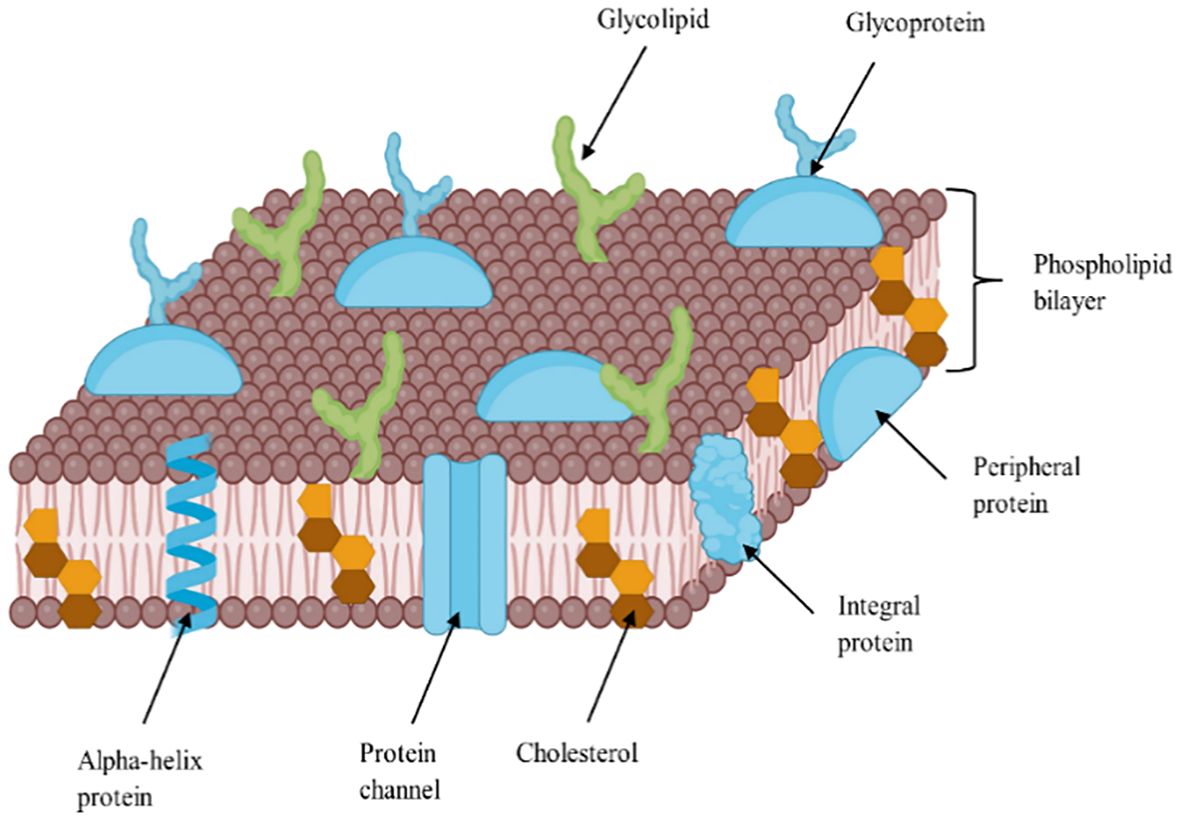
Figure 1. A detailed diagram showing sterol distribution in the lipid bilayer of cell membrane, adapted from (Bodenes, 2017).
Common sterols, commercial sources of sterols, and their applications
The production of sterols, vital for applications in food, healthcare, and pharmaceutical industries, relies on diverse feedstocks, including animal, fish, plant, microbial, and synthetic sources, producing distinct sterols such as cholesterol, squalene, phytosterols, and ergosterol (Zio et al., 2024).
Cholesterol, primarily sourced from animal fats and lanolin, is indispensable in the pharmaceutical industry for synthesizing steroid hormones such as corticosteroids, estrogens, and testosterone (Cerqueira et al., 2016). These are essential for regulating metabolism, immune and nervous responses, reproductive functions, and various therapies, including hormonal replacement and anti-inflammatory treatments. Cholesterol is also critical in manufacturing myelin sheaths and vitamin D (Cruz et al., 2013; Hofmaenner et al., 2022). Moreover, cholesterol also plays a role in bile acid synthesis, which is crucial for metabolic processes and utilized in the food industry to enhance the texture of dairy-based desserts and baking fats (Staels and Fonseca, 2009). Additionally, its emollient properties make it valuable in cosmetics for skin care products, and it is a fundamental component for lipid bilayer studies in research and diagnostics (Volkman, 2003).
Phytosterols (brassicasterol, stigmasterol, campesterol, and beta-sitosterol), similar in structure and function to cholesterol, are plant-derived and extracted from vegetable oils, tall oil, legumes, nuts, seeds, whole grains, and dried fruits (Fernandes and Cabral, 2007). and rice bran oil (Davis and Dean, 2016; Sujith Kumar et al., 2017). Known for reducing serum cholesterol levels by competing with dietary cholesterol for absorption in the gastrointestinal tract, phytosterols are widely used in cholesterol-lowering functional foods and nutritional supplements aimed at cardiovascular health (Poli et al., 2021). They are also valued in cosmetics for their antioxidative, anti-inflammatory, and anticancer properties and their benefits for skin barrier enhancement (Feng et al., 2020).
Squalene, a triterpene hydrocarbon primarily derived from shark liver oil or plants, is used across various industries due to its unique emollient properties. It enhances skin hydration and elasticity without leaving a greasy residue (Fiume et al., 2023). In the pharmaceutical industry, squalene is crucial as an adjuvant in flu and COVID-19 vaccines to enhance immunogenicity (Fox, 2009; Keech et al., 2020). It also finds applications in dietary supplements for its antioxidant benefits and is used in the food industry to inhibit oxidation and extend shelf life (Fiume et al., 2023).
Ergosterol is produced through microbial fermentation, offering a renewable alternative that sidesteps some ethical and sustainability issues associated with animal and plant sources (Papoutsis et al., 2020). It is used to produce vitamin D2 and is in demand primarily by the pharmaceutical and dietary supplement industries (Rangsinth et al., 2023).
Synthetic sterols are produced to meet the needs of various industries, which exceeds what can be feasibly extracted from natural sources (Kumari et al., 2013), and addresses several challenges associated with natural sterol extraction. Synthetic sterols offer several advantages over their natural counterparts. From an economic perspective, synthetic sterols can be produced more cost-effectively, particularly when scaled up to meet industrial demands. In terms of quality, synthetic sterols can be manufactured to have a consistent composition and high purity levels, which is critical for pharmaceutical applications (Feltrin et al., 2020). This consistency ensures that the sterols meet stringent regulatory standards and perform reliably in their intended applications. Moreover, synthetic production can mitigate the environmental impact associated with the large-scale extraction of natural sterols, offering a more sustainable solution (Tata et al., 2015).
Limitations of commercial sterol sources
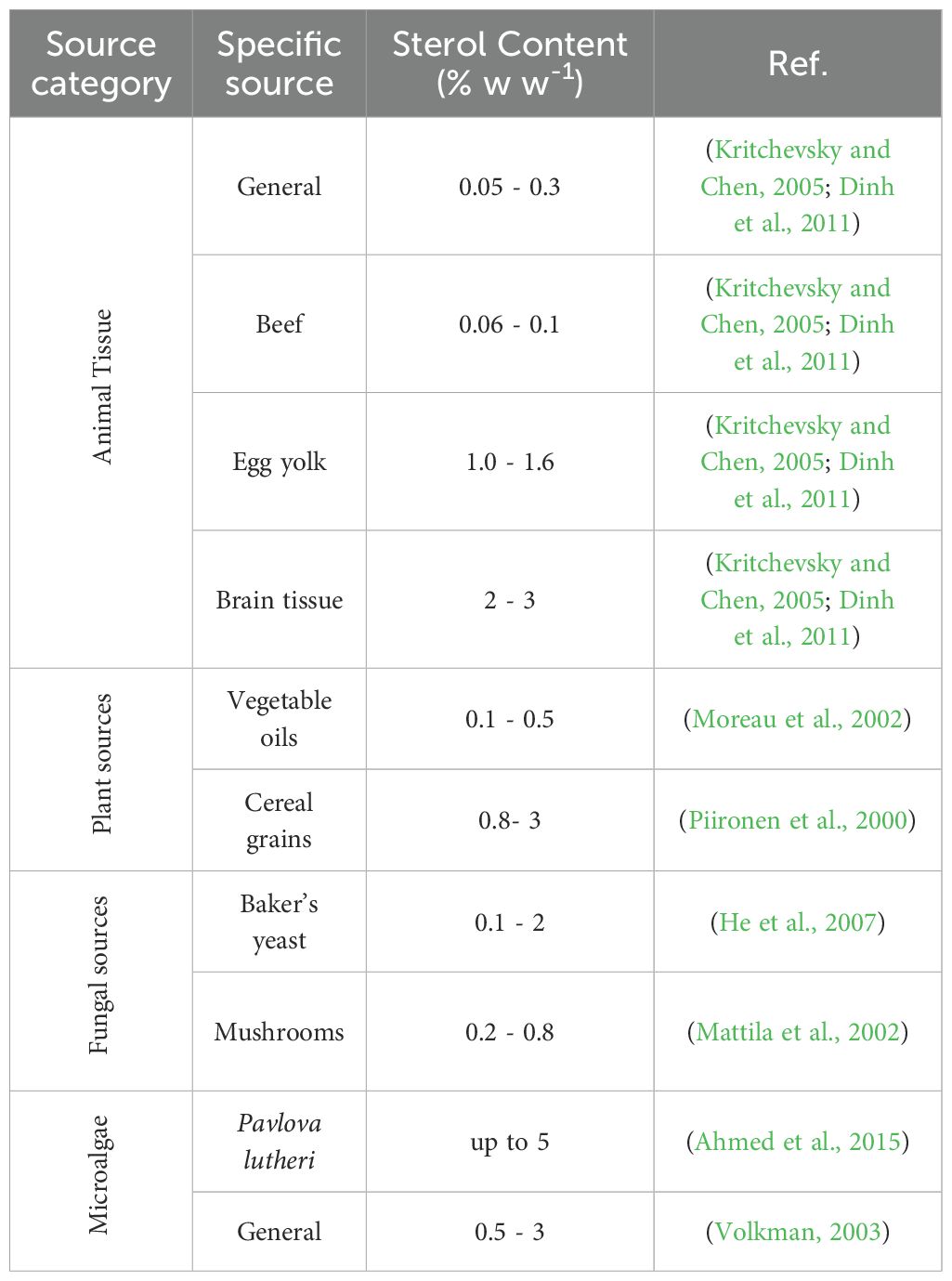
The current sources of sterols, whether derived from plants, animals, fungi, or synthesized chemically, face several limitations that can affect their sustainability, economic viability, and overall availability. For instance, the extraction process is often complex and costly due to the low concentrations of sterols in animal tissues (Table 1) (Kritchevsky and Chen, 2005; Dinh et al., 2011), which typically range from 0.05-0.3% (w/w) in most tissues, with variations depending on the source (e.g., 0.06-0.1% in beef, 1.0-1.6% in egg yolk, and 2-3% in brain tissue). Animal-derived sterols face health concerns, including the potential for contamination with pathogens or harmful substances. Moreover, ethical challenges related to animal welfare and the environmental implications of animal farming include significant greenhouse gas emissions and resource consumption (Randhir et al., 2020). These factors make animal-derived sterols less sustainable.
While phytosterols are generally more sustainable than animal sources, the scalability of phytosterols is dependent on agricultural factors such as crop yields and weather conditions. The main limitation of phytosterols is their low concentration in most plant sources, ranging from 0.8-3% (w/w) in cereal grains (Moreau et al., 2002) to 0.1-0.5% in vegetable oils (Piironen et al., 2000), which requires processing large amounts of raw material to obtain significant quantities of sterols (Fagundes et al., 2020). This process is resource-intensive and can be environmentally damaging, involving extensive land use, water consumption, and the application of pesticides and fertilizers. The processes required to extract and purify sterols to the high purity required for pharmaceutical and food-grade products are complex and expensive, posing economic challenges (Randhir et al., 2020). Furthermore, the variability in sterol content due to factors like seasonality and geographic location can lead to inconsistent quality and supply. Notably, plants do not produce cholesterol, a critical sterol for vitamin D3 synthesis, highlighting a gap in sterol source capabilities (Sijil et al., 2022).
Fungal sterols, particularly ergosterol, are derived from fungi like yeast and mushrooms. While fungi can be cultivated more sustainably than animals, developing fermentation-based sterols, the extraction and purification processes are still labor-intensive and costly. Additionally, the sterol content in fungi is relatively modest, with concentrations ranging from 0.1-2% (w/w) in baker’s yeast (He et al., 2007) and 0.2-0.8% in mushrooms (Mattila et al., 2002), which is comparable to microalgal sterol content. However, unlike microalgae, fungi cannot produce cholesterol, which limits their versatility as a sterol source. Additionally, there are also challenges related to maintaining sterile growth conditions and preventing contamination, which can complicate production and increase costs (Zhabinskii et al., 2022).
Synthetic sterols, while offering consistency and scalability, come with their own set of limitations. The production process is energy-intensive and relies heavily on petrochemical inputs, leading to a significant environmental footprint (Feng et al., 2020). Synthetic production can also be costly due to the complexity of chemical processes and the price of raw materials. Furthermore, consumer preference often leans towards natural products, posing a marketability challenge for synthetic sterols. Potential contaminants from chemical synthesis and stringent regulatory requirements further complicate the production and application of synthetic sterols (Tata et al., 2015).
Microalgae as an alternative sterol source
The limitations of commercial sterol sources have catalyzed research and development efforts to discover more sustainable, cost-effective, and socially acceptable alternatives. Microalgae stands out as a promising solution, offering numerous advantages regarding sustainability, efficiency, and biochemical diversity. While the sterol content in most microalgae species is also relatively low compared to some commercial sources, typically ranging from 0.5-3% (w w-1) of dry biomass (Volkman, 2003) (Table 1), particular species such as Pavlova lutheri achieving concentrations up to 5% (w w-1) (Ahmed et al., 2015) under optimized conditions, making them competitive with traditional sources. Additionally, microalgae produce a diverse range of sterols, including cholesterol and ergosterol, which plants cannot produce, providing a broad spectrum of bioactive molecules that can be tailored for specific applications (Sijil et al., 2022). Additionally, the ability to cultivate microalgae in controlled environments ensures consistent sterol production, mitigating the seasonal and environmental fluctuations observed in plant-derived sterols.
Beyond sterol content, microalgae present distinct advantages in scalability and environmental sustainability. Unlike plant sources, microalgae can be cultivated on non-arable land and in saline or brackish water, reducing competition with food crops (Abdelkarim et al., 2024). Additionally, microalgae can capture CO2 from industrial emissions, contributing to carbon reduction efforts. This starkly contrasts the environmental impact of animal farming, intensive agriculture, petrochemical-based synthetic production, and microbial fermentation, which often relies on agricultural feedstocks. Furthermore, microalgae exhibit rapid growth rates and high biomass productivity, translating to more efficient production processes (Abo-Shady et al., 2023). Under optimal conditions, some species can double their biomass within hours, leading to higher sterol yields in a shorter time frame than traditional sources, which require lengthy cultivation periods and extensive resource inputs (Gheda et al., 2021; Abo-Shady et al., 2023).
A significant advantage of microalgal cultivation is the ability to precisely control environmental parameters in closed systems, such as photobioreactors. These systems regulate light, temperature, pH, and nutrient supply, ensuring consistent sterol production with minimal contamination risks (Fagundes et al., 2020; Randhir et al., 2020). However, while laboratory-scale cultivation has demonstrated high sterol yield and quality, scaling up these controlled environments to industrial levels presents significant challenges. The high setup and operational costs of photobioreactors remain a barrier to commercialization. Nevertheless, advances in process optimization, photobioreactor engineering, and cost-effective nutrient sourcing steadily improve commercial feasibility (Wijffels et al., 2013).
Microalgae offer a sustainable and biochemically diverse alternative to traditional sterol sources, with advantages including rapid growth, environmental adaptability, and potential for controlled production. However, scaling up remains an ongoing challenge, requiring advancements in bioprocess engineering, metabolic optimization, and cost reduction strategies. If these barriers can be overcome, microalgae have the potential to become a high-yield, industrially viable source of sterols for nutraceutical, pharmaceutical, and green biotechnology applications.
The sterol composition across microalgae species displays remarkable diversity, reflecting complex biosynthetic pathways and environmental adaptation (Table 2). It is important to note that this review excludes dinoflagellates, a group known for producing highly unusual sterols such as dinosterol and its derivatives. Due to the complexity and variability of their metabolic networks, dinoflagellates warrant a separate and more detailed examination. Their metabolic plasticity and capacity for hybrid biosynthetic routes challenge current models of sterol evolution and remain an open area for future investigation.
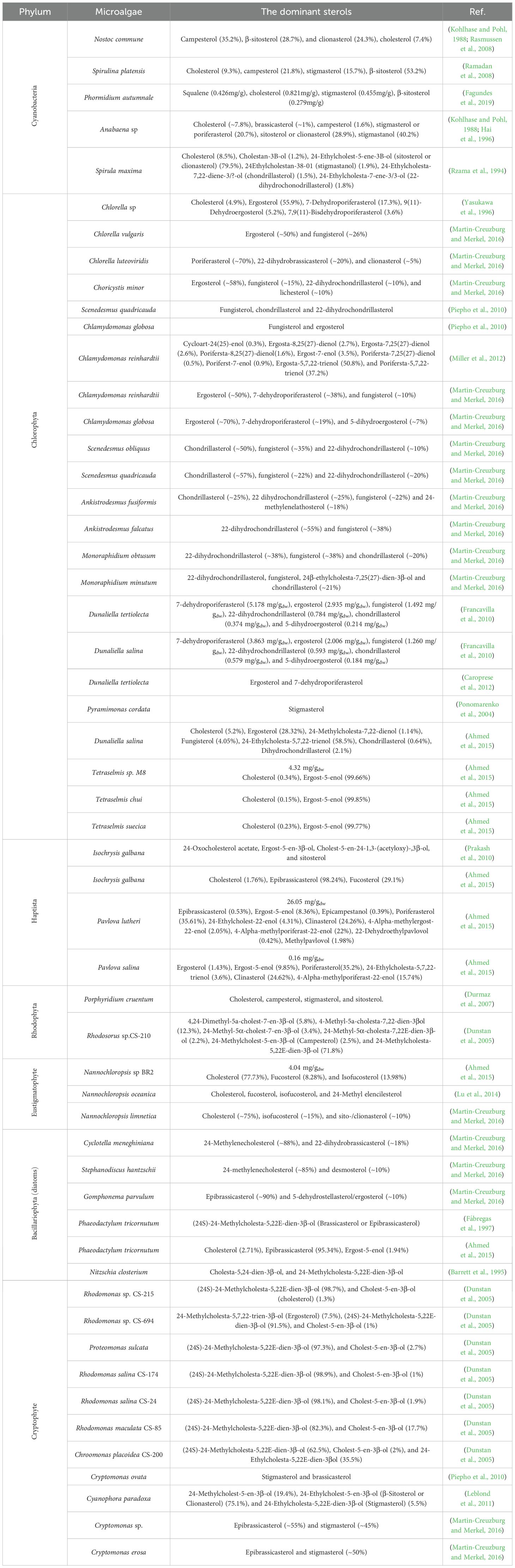
Table 2. Sterol profiles (% of total sterol) and total sterol (mg/gdw) in various microalgae species across different phyla.
The case of cyanobacteria also presents an ongoing scientific debate. Although numerous studies have reported sterol-like compounds in genera such as Nostoc, Anabaena, Spirulina, and Phormidium, compelling evidence suggests these may not be synthesized de novo. Volkman (2003; 2005) has argued that the sterols detected in cyanobacterial samples, typically in trace amounts below 0.03% of cell dry weight, could originate from external contamination, particularly by eukaryotic organisms such as fungi or yeasts (Hai et al., 1996). This interpretation is supported by studies demonstrating that cyanobacterial cultures become sterol-free when treated with cycloheximide, an inhibitor that selectively suppresses eukaryotic contaminants (Summons et al., 2001). Furthermore, genomic analyses reinforce the scepticism, while some cyanobacterial genomes contain genes with weak homology to sterol biosynthesis enzymes, they universally lack oxidosqualene cyclase (OSC), a critical enzyme that initiates the cyclization of 2,3-oxidosqualene into lanosterol or cycloartenol (Volkman, 2003; 2005). The absence of this key gene suggests that recognized sterol synthesis pathways are not native to cyanobacteria. This has significant implications for interpreting sterol biomarkers in ancient sedimentary rocks. If cyanobacteria are confirmed not to produce C-24 alkylated sterols, their presence in ancient geological records may serve as more robust evidence for early eukaryotic life, rather than ambiguous biosignatures subject to bacterial origin (Cavalier-Smith, 2002a; 2002b).
Among green algae (Chlorophyta), sterol profiles exhibit considerable biochemical diversity, underscoring this group’s evolutionary and ecological plasticity. Additionally, the lack of a universally dominant sterol across Chlorophyta orders and families and the range of compounds detected, such as ergosterol, fungisterol, and unique compounds like 7-dehydroporiferasterol, suggests distinctive cellular processes and adaptation mechanisms. The presence of ergosterol, commonly associated with fungi, in species like Chlorella and Scenedesmus, assumptions about the taxonomic exclusivity of specific sterols, underscores the conservation of certain biosynthetic traits across evolutionary boundaries. These findings raise questions about the possible horizontal gene transfer or ancient evolutionary retention of fungal-type sterol pathways in specific algal lineages. Notably, Dunaliella species within Chlorophyta further expand this diversity by synthesizing sterol derivatives like ergosterol peroxide, which has known antioxidant properties, highlighting the dual utility of microalgal sterols, not only as structural lipids but also as molecules with potential therapeutic and nutraceutical exploitation. Furthermore, sitosterol has been identified as the primary sterol in marine microalgae species like Isochrysis galbana, exemplifying the intra-group variability and ecological specialization of marine green algae (Yasukawa et al., 1996; Ponomarenko et al., 2004; Francavilla et al., 2010; Piepho et al., 2010; Prakash et al., 2010; Chen et al., 2014; Lv et al., 2015; Martin-Creuzburg and Merkel, 2016).
Red microalgae (Rhodophyta) predominantly synthesize C-27 sterols, with cholesterol frequently emerging as the most abundant. Porphyridium cruentum displays a sterol composition including cholesterol and stigmasterol, a sterol typically associated with animals and plants, respectively, which raises intriguing questions about evolutionary development. Such overlapping sterol signatures may reflect convergent evolution, where similar selective pressures in membrane structure or signaling led to independent development of comparable biosynthetic pathways, or gene transfer events that transmitted biosynthetic capabilities between distant lineages (Durmaz et al., 2007).
Among heterokontophytes, distinct patterns are evident. The Eustigmatophyceae (Gyrista) species Nannochloropsis exhibits a simpler profile with cholesterol and fucosterol (Lu et al., 2014; Ahmed et al., 2015; Martin-Creuzburg and Merkel, 2016). This simplicity may reflect genome streamlining or ecological specialization. In contrast, diatoms, another significant microalgal group, are characterized by a predominance of 24-methylenecholesterol, a compound that is thought to contribute to their membrane rigidity and is likely an adaptation to their siliceous frustules and aquatic habitats (Barrett et al., 1995; Fábregas et al., 1997; Martin-Creuzburg and Merkel, 2016). These differences reinforce the notion that sterol profiles are shaped not only by phylogeny but also by ecological and functional constraints. Cryptophyte species like Cryptomonas and Rhodomonas further enrich this spectrum with sterols such as epibrassicasterol and brassicasterol, adding to the biochemical repertoire available within microalgae. These compounds may confer selective advantages in specific niches, such as variable salinity or light environments (Piepho et al., 2010; Leblond et al., 2011; Martin-Creuzburg and Merkel, 2016). Their presence underscores the ecological versatility and adaptive potential of cryptophyte species.
The extensive variability in microalgal sterol profiles reveals a sophisticated interplay between genetic inheritance, ecological adaptation, and evolutionary innovation. These diverse biosynthetic capabilities not only provide fundamental insights into algal evolution and taxonomy but also hold immense promise for applied biotechnology. Unique sterols with antioxidant, antifungal, or membrane-modulating properties may be harnessed for use in functional foods, cosmetics, pharmaceuticals, or biomaterials.
Sterol biosynthesis in different organisms
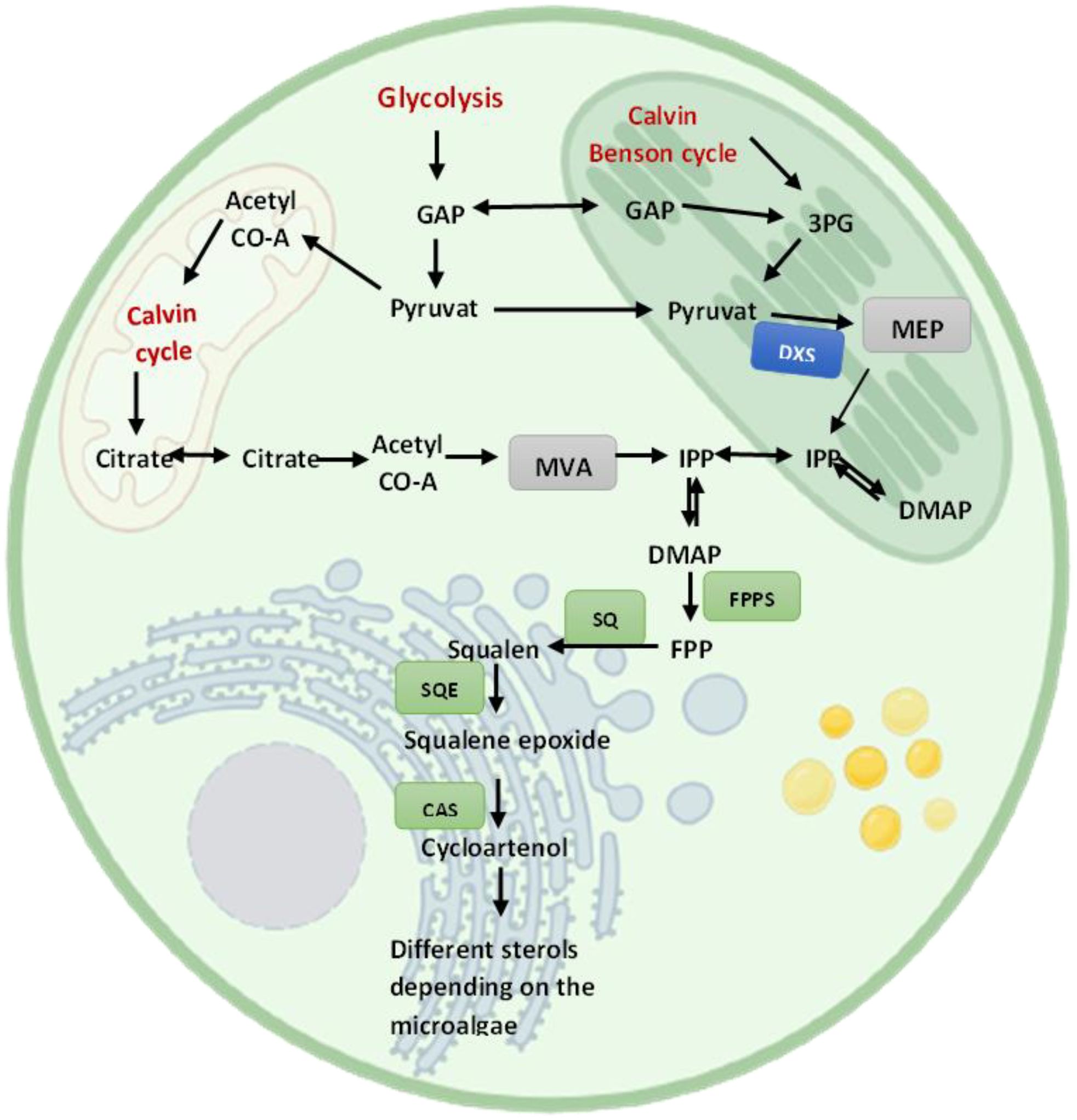
Sterol biosynthesis involves biochemical pathways that differ among various organisms, such as animals, plants, fungi, and microalgae (Figure 3).
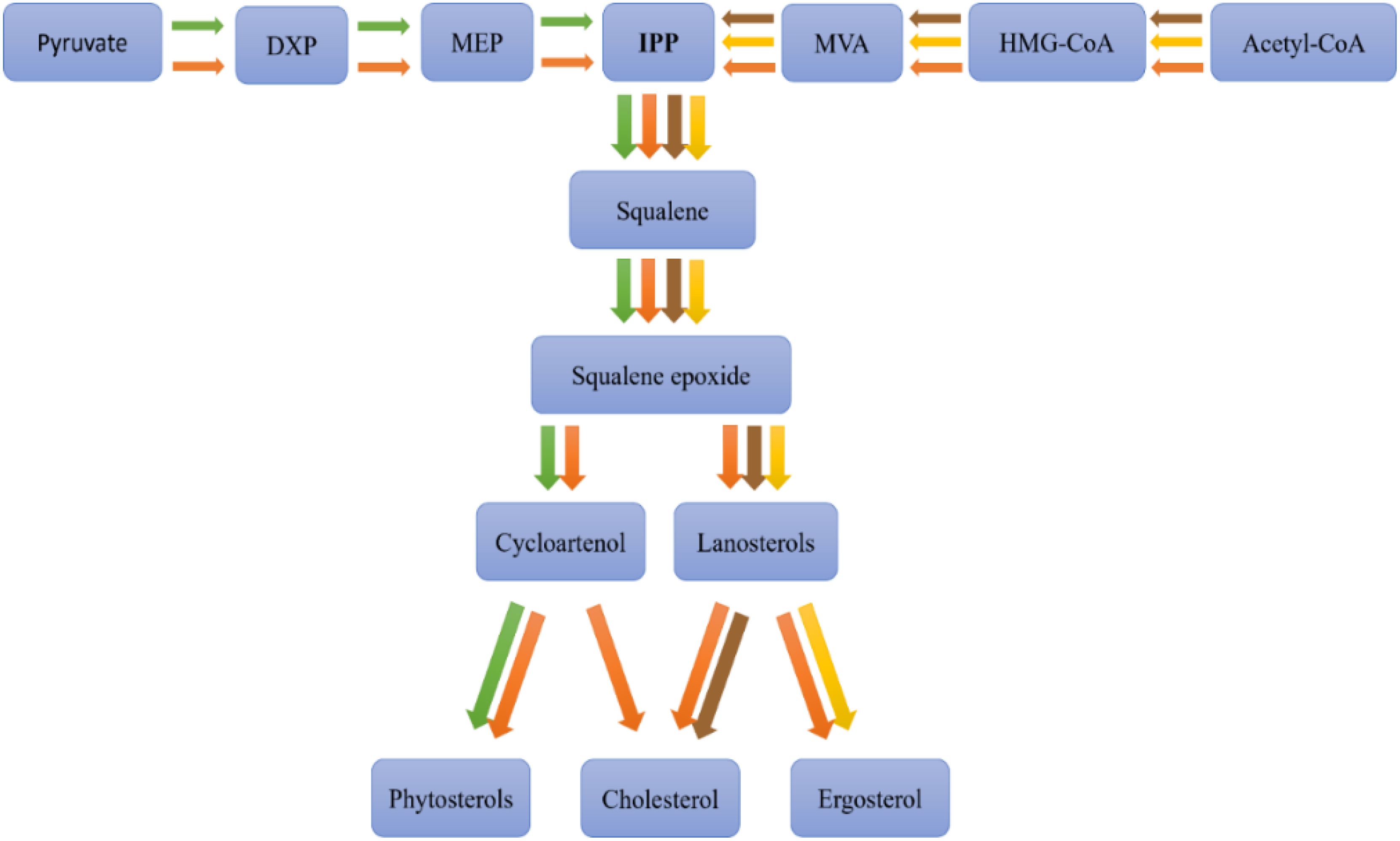
Figure 3. Sterols biosynthetic pathways among different organisms, animal (brown), plant (green), fungi (yellow), and microalgae (orange). Adapted from (Lu et al., 2014), DXP: 1-deoxy-D-xylulose-5-phosphate, MEP: 2-C-methyl-D-erythritol 4-phosphate, IPP: isopentenyl pyrophosphate, MVA: mevalonate, HMG-CoA: 3-hydroxy-3-methylglutaryl coenzyme A, Acetyl-CoA: acetyl coenzyme A.
In animals, cholesterol biosynthesis primarily occurs in the liver, following the mevalonic acid (MVA) pathway. This pathway initiates with the condensation of acetyl-CoA molecules to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), which is subsequently reduced to mevalonate by HMG-CoA reductase, a pivotal regulatory enzyme in cholesterol synthesis. The conversion of mevalonate through several phosphorylation and decarboxylation steps yields isopentenyl pyrophosphate (IPP), a foundational sterol building block. IPP is transformed into squalene via a sequence of reactions, which then cyclizes to produce lanosterol, the precursor to cholesterol, through multiple enzymatic modifications (Fagundes et al., 2020).
In plants, phytosterol biosynthesis commences similarly from acetyl-CoA, following either an analogous pathway to that in animals or diverging through the methylerythritol phosphate (MEP) pathway from pyruvate to form IPP and subsequently squalene. The plant pathway diverges post-squalene synthesis, with squalene converting to 2,3-oxidosqualene and then to cycloartenol, a critical intermediate. Cycloartenol undergoes various enzymatic transformations to yield phytosterols like sitosterol, stigmasterol, and campesterol (Du et al., 2022).
Fungi synthesize ergosterol, which serves a similar structural role to cholesterol in animals and phytosterols in plants, but follows a distinct biosynthetic pathway. Like other sterol-producing organisms, fungi begin with acetyl-CoA and progress through the mevalonate (MVA) pathway to generate isopentenyl pyrophosphate (IPP) and subsequently squalene. However, post-squalene synthesis, the pathway diverges, leading to lanosterol, which undergoes unique enzymatic modifications to form ergosterol rather than cholesterol (as in animals) or phytosterols (as in plants). These modifications involve specific enzymes that differentiate fungal sterol biosynthesis from other eukaryotic pathways (Singh et al., 2023).
Microalgae exhibit unique and variable sterol biosynthesis pathways (Fagundes and Wagner, 2021), reflecting their evolutionary lineage and the broad diversity of sterols they can produce (Figure 4). Sterol biosynthesis in microalgae typically begins with acetyl-CoA, converting to IPP via the mevalonate (MVA) pathway in the cytosol or, alternatively, some species utilize the MEP pathway in chloroplasts (Zhao et al., 2013). Following the synthesis of squalene, a series of enzymatic steps result in the formation of various key intermediates like lanosterol or cycloartenol, leading to a plethora of sterols, including cholesterol, ergosterol, fucosterol, sitosterol, and stigmasterol (Pollier et al., 2019) (Figure 5).
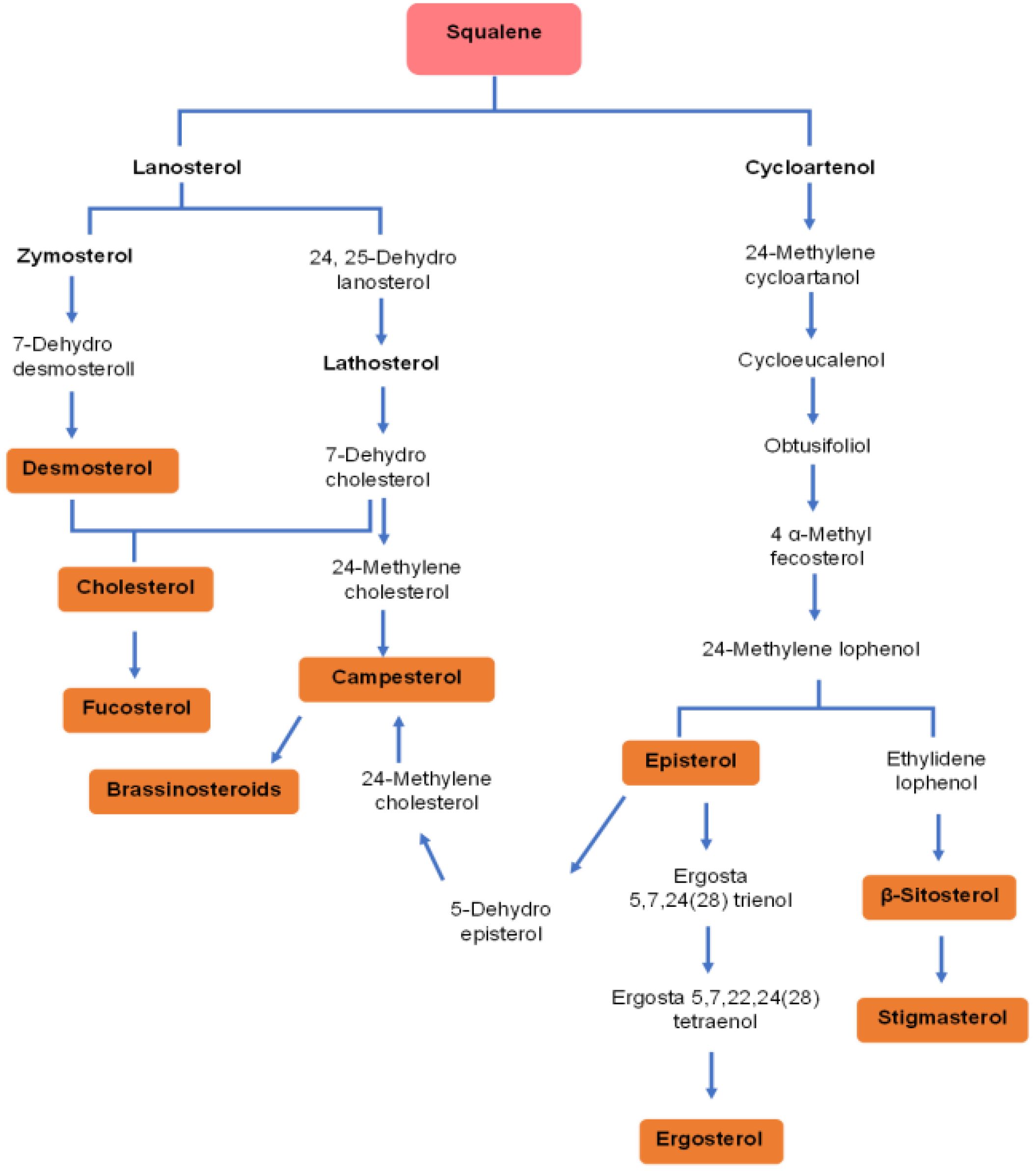
Figure 5. Diversity of sterols derived from squalene in microalgae. This pathway diagram illustrates the complex biosynthetic routes by which microalgae convert squalene into various bioactive sterols, via two primary branches: the lanosterol pathway (common in animals and some microalgae) or the cycloartenol (typical in plants and many microalgae). These pathways demonstrate the biochemical diversity of microalgal sterol synthesis, with end products including cholesterol, fucosterol, campesterol, ergosterol, β-sitosterol, and stigmasterol.
Specifically, the biosynthesis of stigmasterol and β-sitosterol involves the conversion of 2,3-oxidosqualene to cycloartenol by cycloartenol synthase (CAS), followed by enzymatic transformations, notably Δ24-sterol methyltransferase (SMT) converting cycloartenol to 24-methylenecycloartenol, leading sequentially to β-sitosterol. Stigmasterol synthesis from β-sitosterol involves desaturation by the cytochrome P450 enzyme CYP710A1 (Griebel and Zeier, 2010).
The cholesterol biosynthesis pathway in microalgae diverges after 2,3-oxidosqualene formation, with subsequent conversions leading to cholesterol (Randhir et al., 2020). For ergosterol, unique pathways, such as in Chlamydomonas reinhardtii, start with cycloartenol synthesis, indicating shared pathways with phytosterols (Brumfield et al., 2017).
Additionally, microalgae like Chlorella vulgaris can synthesize growth-promoting brassinosteroids from campesterol through complex transformations involving early or later C6-oxidation steps leading to brassinolide (Fagundes et al., 2020).
The regulation of sterol biosynthesis in microalgae is influenced by countless factors, including environmental conditions and developmental stages, underscoring the complexity of these pathways. A detailed understanding of these pathways enhances the biotechnological production of bioactive sterols for pharmaceutical and nutraceutical uses. Advances in genetic and metabolic engineering may further optimize these pathways, increasing sterols’ yield and diversity, thus boosting the commercial potential of microalgae as a source of valuable sterols.
Effect of culture conditions on the sterol content of microalgae
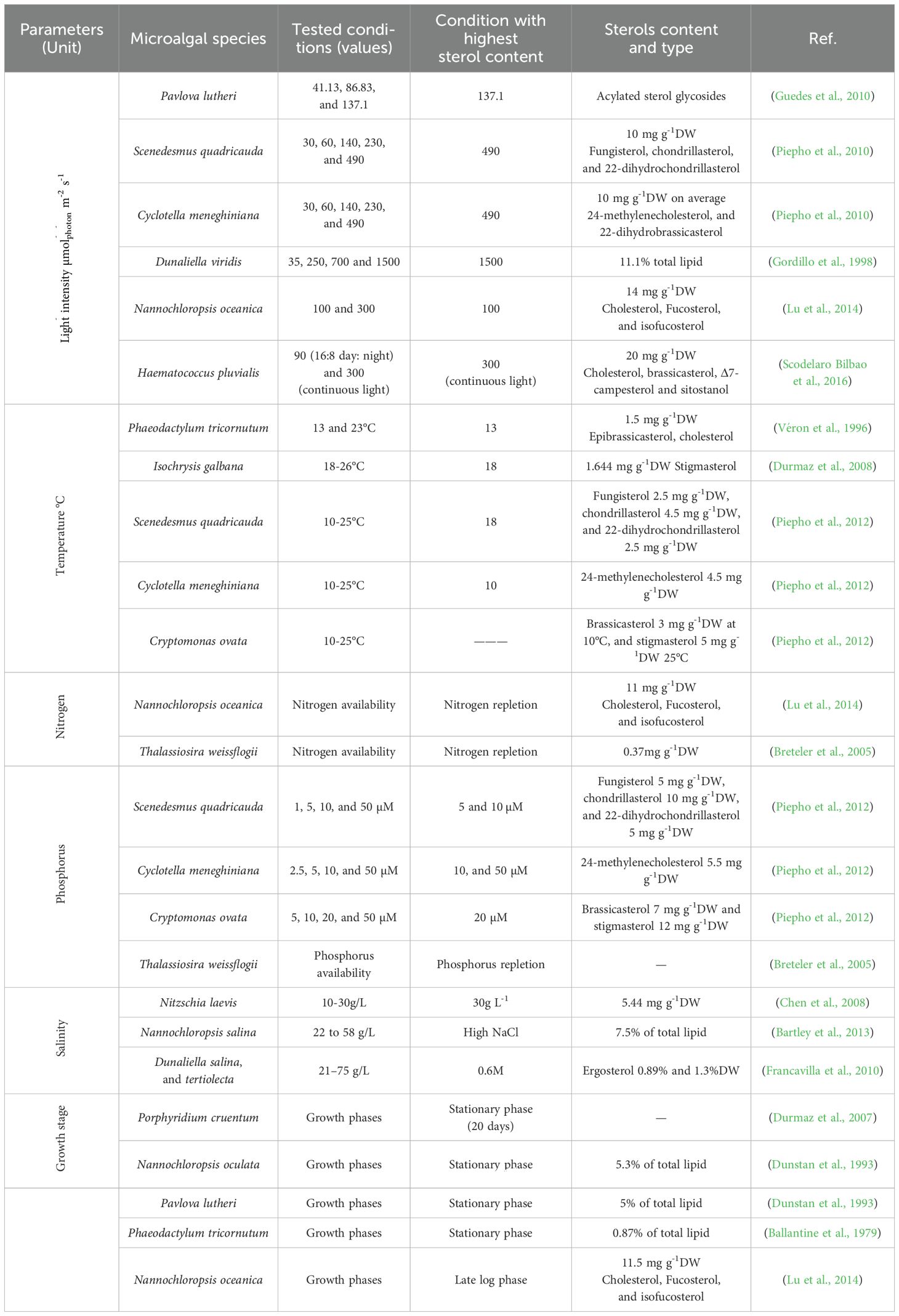
The sterol content of microalgae is reported to be influenced by several conditions (Figure 6), such as light intensity, temperature, nutrient availability, salinity, and growth phases (Lopes et al., 2013) (Table 3).
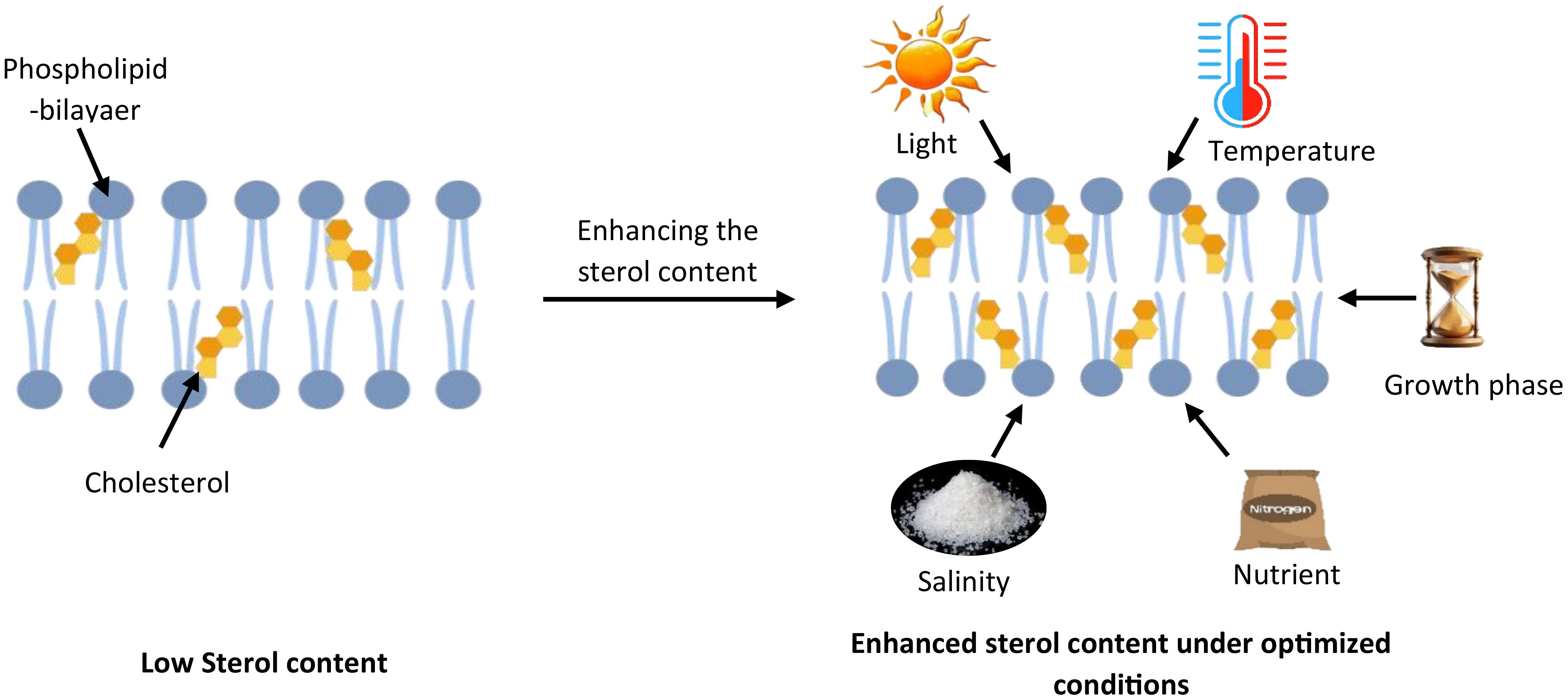
Figure 6. Environmental regulation of sterol composition in microalgal membranes. This schematic depicts how various environmental factors influence sterol content in microalgal cell membranes. The left side shows a phospholipid bilayer with low cholesterol content under standard conditions. The right side demonstrates enhanced cholesterol integration (shown as yellow molecules) within the membrane when environmental conditions are optimized. Key factors that regulate cholesterol biosynthesis and accumulation include light intensity, temperature, salinity, nutrient availability (particularly nitrogen and phosphorus), and growth phase timing.
Light intensity
Recent findings indicate that light intensity significantly affects sterol content in various microalgal species, though comparisons across studies should consider differences in experimental conditions such as biomass concentration, incident light intensity, and optical path length. Dunaliella viridis batch-cultured under varying photon flux rates (darkness, 35, 250, 700, and 1500 μmolphoton m−2 s−1 exhibited a marked increase in sterol content with increasing light intensity, particularly at the highest light intensities. The maximum sterol content, 11.1% of total lipid, was achieved at 1500 μmolphoton m−2 s−1, substantially higher than at lower photon fluence rates (1.6% of total lipid in darkness, 3.0% of total lipid at 35 μmolphoton m−2 s−1, 6.5% at 250 μmolphoton m−2 s−1,and 7.2% at 700 μmolphoton m−2 s−1) (Gordillo et al., 1998). Similarly, Pavlova lutheri showed increased acylated sterol glycosides with elevated light intensities (Guedes et al., 2010). This pattern was also observed in Scenedesmus quadricauda and Cyclotella meneghiniana, where higher light intensity increased sterol production (Piepho et al., 2010). The enhancement of sterol levels is hypothesized to be linked with increased photosynthesis activity under high light conditions. This relationship varies among species due to differences in their sterol biosynthetic pathways. Some algae utilize the mevalonate (MVA) pathway, while others rely on the methylerythritol phosphate (MEP) pathway, which is closely associated with chloroplast function. However, certain species have been found to utilize both pathways, integrating intermediates from both metabolic routes to regulate sterol biosynthesis in response to environmental conditions.
Species such as Haematococcus (H.) pluvialis and Nannochloropsis oceanica exhibit different responses to light intensity. H. pluvialis CCALA1081 was batch-cultured under two light regimes: 90 μmolphoton m−2 s−1 with a 16:8 h light/dark photoperiod and 300 μmolphoton m−2 s−1 continuous light. Under a light intensity of 300 μmolphoton m−2 s−1, total phytosterol content increased markedly, reaching approximately 20 mg gdw-1. However, this overall increase was accompanied by notable shifts in the composition of individual sterols, such as β-sitosterol and 24-methylene cholesterol, which decreased by 25% and 10%, respectively, compared to control conditions at 90 μmolphoton m−2 s−1. Conversely, other sterols such as cholesterol, brassicasterol, Δ7-campesterol, and sitostanol exhibited notable increases by 20%, 10%, 8%, and 5%, respectively, suggesting that elevated light triggers selective adjustment in sterol composition, potentially reflecting adaptive modifications in membrane structure and function (Scodelaro Bilbao et al., 2016). On the other hand, N. oceanica cultures were grown at standard light intensity 50 μmolphoton m−2 s−1 and then exposed to two specific light conditions, 100 and 300 μmolphoton m−2 s−1, which demonstrated a decrease in sterol content under high light (300 μmolphoton m−2 s−1) with the reduction primarily in cholesterol levels. However, some phytosterols, such as fucosterol and isofucosterol, increased in concentration under high light, indicating a complex response where overall sterol biosynthesis is repressed, yet specific sterols are selectively increased (Lu et al., 2014). These variations underscore the complexity of light’s impact on sterol metabolism in microalgae, which appears to be species-specific and possibly related to their distinct biosynthetic pathways. While these studies provide valuable insights, the variation in experimental conditions (culture conditions and mode, biomass concentrations, and optical path lengths) makes direct quantitative comparisons challenging. Consequently, further research involving diverse microalgal strains with standardized cultivation conditions is essential to fully understand sterol metabolism and its relationship with light intensity in these organisms.
Temperature
Temperature influences microalgae’s physiological and metabolic processes, impacting their growth rate, cell size, biochemical composition, and nutrient uptake capabilities. It plays a crucial role in regulating sterol metabolism, where the synthesis of ethyl groups branched at the C24 position serves as a cellular defense mechanism against thermal stress, as elucidated by Beck et al. (2007). This adaptation underscores the potential of manipulating temperature conditions to enhance the biotechnological production of valuable compounds such as squalene.
Further research into the temperature’s impact on sterol content across various microalgal species reveals nuanced responses. For instance, in Phaeodactylum tricornutum, sterol content decreased with increasing culture temperature from 13°C to 23°C, with the highest sterol content at 13°C, 1.5 mg gdw-1. Similarly, Isochrysis galbana demonstrated elevated sterol levels with 1.6 mg gdw-1 at lower temperatures (18°C compared to 26°C), indicating a possible thermal inhibition of sterol synthesis at higher temperatures (Durmaz et al., 2008).
Conversely, other studies have reported an opposite trend, where sterol production increases with rising temperatures. Investigations by Piepho et al. (2012) into Scenedesmus quadricauda, Cyclotella meneghiniana, and Cryptomonas ovata revealed that, while sterol content increased at 25°C compared to 10°C in S. quadricauda and C. meneghiniana, it remained unaffected in C. ovata’s. A similar trend was observed in Botryococcus braunii, where sterol levels were highest at 25°C but declined at both lower (18°C) and higher (32°C) temperatures (Kalacheva et al., 2002). These findings underscore the complexity of temperature effects on microalgal sterol biosynthesis, suggesting a species-specific and possibly strain-specific response that could be leveraged for targeted biotechnological applications.
Nutrient availability
Nutrient availability plays a critical role in modulating the biosynthesis and accumulation of sterols in microalgae, acting as a crucial environmental factor influencing lipid metabolism pathways. The relationship between nutrient supply, particularly nitrogen and phosphorus, and sterol content in microalgae is complex and varies across different species, reflecting their adaptive responses to nutrient stress conditions.
Nitrogen availability
Nitrogen starvation in microalgae leads to decreased sterol production primarily due to a reallocation of metabolic resources towards essential survival processes. For instance, several species like Ankistrodesmus falcatus (Kilham et al., 1997), Nannochloropsis oceanica (Lu et al., 2014), Eudorina unicocca, and Volvox aureus (Zhang et al., 2009) demonstrated a decrease in sterol content under nitrogen limitation. The reason is that under nitrogen deficiency, microalgae prioritize nitrogen conservation for critical functions such as protein synthesis and photosynthesis, diverting resources away from non-essential metabolite synthesis, including sterols. This metabolic shift also enhances the accumulation of energy-storage lipids, such as triacylglycerols (TAGs), competing with sterol biosynthesis for common precursors like acetyl-CoA. Moreover, nitrogen stress may affect the enzymatic activity within the sterol biosynthesis pathway and alter cell growth, reducing sterol production as the demand for membrane components decreases (Lu et al., 2014). This adaptation reflects the organism’s strategic response to nutrient limitation, emphasizing the importance of understanding metabolic regulation for optimizing microalgae-based production systems.
Contrarily, nitrogen limitation was found to elevate free sterol levels in Botryococcus braunii, while sterol ester content remained unchanged (Zhila et al., 2005). That is because, under conditions of nitrogen deficiency, microalgae undergo a metabolic shift from synthesizing membrane lipids to accumulating storage lipids, predominantly triacylglycerides (TAGs). This transition is a survival strategy that impacts cell growth and can increase cell volume, as observed in different microalgae species. The enlargement of cell volume during nitrogen starvation has been associated with an enhancement in sterol content attributed to the expansion of the cell membrane (Randhir et al., 2020). These observations underscore the nuanced relationship between nitrogen availability and sterol biosynthesis, indicating that nitrogen stress can either promote or inhibit sterol accumulation depending on the species and the specific metabolic adaptations invoked.
Phosphorus availability
The impact of phosphorus on sterol content in microalgae complements the intricate dynamics between nutrient availability and lipid metabolism. Phosphorus, a critical nutrient for algal growth, influences various metabolic processes, including nucleic acids and ATP synthesis. Its availability directly affects the production of sterols, components vital for maintaining cellular membrane integrity and fluidity. An adequate supply of phosphorus can enhance sterol biosynthesis, as evidenced by the increase in sterol content in Thalassiosira weissflogii (Breteler et al., 2005), Scenedesmus quadricauda and Cyclotella meneghiniana (Piepho et al., 2012) with elevated phosphorus levels. This enhancement is likely due to the role of phosphorus in supporting the overall metabolic activity, including the pathways involved in sterol synthesis. However, the response to phosphorus supplementation can vary among microalgae species, indicating species-specific metabolic strategies for managing nutrient stress. While some species exhibit increased sterol production with higher phosphorus availability, others show no significant change, such as Cryptomonas ovata, suggesting that the regulatory mechanisms governing sterol biosynthesis in response to phosphorus availability are complex and multifaceted (Piepho et al., 2012). This variability highlights the need for a deeper understanding of nutrient-sterol interactions to optimize microalgal cultivation for sterol production, underscoring the nuanced relationship between phosphorus supply and sterol content in microalgae (Piepho et al., 2012).
Findings on the impact of nutrient availability on sterol biosynthesis remain inconsistent and highlight species-dependent metabolic responses. While nitrogen and phosphorus levels influence sterol metabolism, their effects vary across different microalgae, necessitating further research to elucidate regulatory mechanisms and optimize cultivation strategies for enhanced sterol production in biotechnology applications.
Salinity
Salinity markedly influences the biochemical composition of algal cells, with a particular impact on their sterol content and membrane dynamics. Dunaliella species, renowned for their high salinity tolerance, have been extensively studied in this context. Research has shown that in Dunaliella tertiolecta and Dunaliella salina, sterol accumulation is highest at lower salinity levels, with maximum concentrations of 8.9 and 13 mg gdw-1, respectively, observed at 0.6 M NaCl. However, as salinity increases from approximately 21 to 75 g L-1, total sterol content progressively declines (Francavilla et al., 2010). This reduction can be linked to plasma membrane lipid composition adjustments, a response to salinity stress. In-depth studies, such as the analysis of the plasma membrane proteome of D. salina, reveal that these microalgae counteract salinity stress by up-regulating key metabolic and signal transduction pathways (Katz et al., 2007). This response is considered a strategic adaptation to modulate membrane fluidity and stability under saline conditions.
In contrast, other species like Nitzschia laevis exhibit a significant increase by 43.8% in sterol content when exposed to elevated sodium chloride concentrations, ranging from 10 to 30 g L−1 (Chen et al., 2008), reaching 5.44 mg gdw-1 at 30g L-1. A similar trend is observed in Nannochloropsis salina, where sterol content rises from ~4.5 to 7.5% of total lipid with salinity increments from 22 to 58 g L−1 (Bartley et al., 2013). However, in Pavlova lutheri, sterol levels remain unaffected across varying salinity ranges (15 to 45%), suggesting a more stable membrane composition under saline fluctuations (Ahmed et al., 2015). The enhanced salinity prompts a modification in algal cell membranes to reduce the influx of Na+ and Cl− ions by increasing membrane rigidity. This physiological adaptation is crucial for maintaining cellular integrity and function under high salt concentrations, showcasing the remarkable adaptability of microalgae in diverse environmental conditions.
Growth stages
In exploring microalgal species for sterol production, distinct growth phases significantly influence sterol biosynthesis, as evident from various species’ analyses. For most species, the stationary phase marks a critical period for sterol accumulation. Specifically, Phaeodactylum tricornutum (Ballantine et al., 1979), Nannochloropsis oculata and Pavlova lutheri (Dunstan et al., 1993) exhibit substantial sterol content at the stationary phase, accounting for 0.87%, 5.3% and 5% of total lipid, respectively. Contrastingly, Nannochloropsis oceanica diverges from this trend, with sterol levels showing modest increases during the early growth stages, reaching 0.75% of dry weight, followed by a rise to 1.15% of dry weight in the later stages of the culture cycle (Lu et al., 2014). This late-stage sterol increase is primarily attributed to a significant rise in cholesterol content. Concurrently, sterol biosynthetic genes displayed a coordinated upregulation as the culture progressed into the late log phase. All genes studied, except DXS and SMT1, showed elevated transcriptional activity, peaking in the late log phase. These observations suggest that sterol biosynthesis and accumulation are distinctive features of late cell growth as the culture nears the stationary phase (Lu et al., 2014). A different pattern was demonstrated in H. pluvialis CCALA 1081, where sterol accumulation is more closely linked to high light stress than to the growth phase (Scodelaro Bilbao et al., 2016). The highest sterol levels were observed within the first three days of exposure to high light, during which sterol content increased by approximately 1,200% relative to control conditions, reaching about 2.0% of dry weight. This elevated level of phytosterols (1.8% dry weight) was sustained after six days of high-light-induced stress, suggesting that light-induced oxidative stress, rather than culture age or biomass accumulation, played a dominant role in stimulating sterol biosynthesis in H. pluvialis.
The diversity in timing, conditions, and quantity of sterol production across these species underlines the importance of growth stage optimization in microalgal bioengineering for enhanced sterol yield.
Various studies on microalgal sterol content reveal that environmental conditions like light intensity, temperature, nutrient availability, salinity, and growth stages significantly affect sterol synthesis, with distinct responses among species. This variation underscores the importance of species-specific cultivation approaches and highlights a substantial gap in our understanding of the underlying metabolic and genetic mechanisms. The limited range of species studied and inadequate insights into how culture conditions influence cellular sterol biosynthesis are significant hurdles to optimizing sterol production. Addressing these challenges requires broader species research and deeper exploration into their sterol metabolic pathways. Such advancements are essential for developing effective cultivation strategies, enhancing sterol yields for nutraceuticals and other applications, and emphasizing the need for targeted efforts to tap into microalgae’s biotechnological potential for sustainable sterol production.
The potential health benefits of microalgal sterols
Sterols derived from microalgae have garnered significant interest due to their diverse biological effects and potential health benefits (Table 4), including anti-inflammatory, antioxidant, anti-cancer, acting in immunomodulation to reduce the impact of neurological diseases like Parkinson’s and Alzheimer’s, anti-hypercholesterolemic, and anti-diabetic (Khan et al., 2018). These compounds, integral components of cell membranes, play crucial roles in the microalgae, and when consumed by humans or animals (Lv et al., 2015).
The biological effects of microalgal sterols include cholesterol-lowering effects. Microalgal sterols are structurally similar to cholesterol and can inhibit its absorption in the human intestine. This leads to decreased blood cholesterol levels, which benefits cardiovascular health. Studies have shown that specific microalgal sterols can effectively reduce LDL (low-density lipoprotein) cholesterol, often called ‘bad’ cholesterol. The cyanobacterium Nostoc commune has emerged as a particularly promising source of cholesterol-reducing compounds, with its sterol profile dominated by campesterol, β-sitosterol, and clionasterol (Rasmussen et al., 2008). These sterols have been shown to inhibit the activation of sterol regulatory element binding proteins (SREBPs) in hepatic cells, a critical mechanism for regulating cholesterol homeostasis. Additionally, research by Rasmussen et al. (2008) demonstrated that lipid extracts from Nostoc commune significantly suppressed SREBP-regulated gene expression involved in cholesterol and fatty acid synthesis, suggesting multiple synergistic mechanisms contributing to its hypocholesterolemic effects.
In addition, some sterols from microalgae exhibit immunomodulatory and anti-inflammatory properties. These compounds can modulate the immune responses by interacting with specific cellular receptors and signaling cascades, leading to reduced expression of pro-inflammatory mediators and enhanced production of anti-inflammatory factors (Sanjeewa et al., 2016). This activity makes them potentially valuable therapeutic agents for treating chronic inflammatory disorders, autoimmune conditions, and inflammation-associated diseases.
Chlorella vulgaris contains several bioactive sterols, including ergosterol, 7-dehydroporiferasterol, and their oxidation products such as ergosterol peroxide, which demonstrate pronounced anti-inflammatory activity (Yasukawa et al., 1996). Mechanistic studies have revealed that these compounds suppress the production of pro-inflammatory cytokines, while inhibiting inflammatory enzymes (Yasukawa et al., 1996). Similarly, sterols isolated from Dunaliella tertiolecta, particularly ergosterol and 7-dehydroporiferasterol, significantly reduced inflammation markers in experimental models and modulated proliferation of peripheral blood mononuclear cells, suggesting potential applications in treating immune-mediated inflammatory conditions (Caroprese et al., 2012).
Recent research on the sterol-rich fraction extracted from the microalga Nannochloropsis oculata has demonstrated remarkable anti-inflammatory activity with therapeutic potential for treating inflammatory disorders and certain types of cancer, including promyelocytic leukemia (Sanjeewa et al., 2016). This fraction significantly inhibited the production of nitric oxide (NO), while downregulating the expression of pro-inflammatory genes, key molecular mechanisms implicated in chronic inflammation.
Microalgal sterols possess significant antioxidant properties that contribute to their overall therapeutic potential. These compounds effectively neutralize free radicals and reactive oxygen species (ROS), thereby protecting cellular components from oxidative damage implicated in various chronic diseases, including neurodegenerative disorders, cardiovascular diseases, and certain types of cancer. The sterol-rich fraction from Nannochloropsis oculata has demonstrated substantial radical scavenging activity, with the ability to neutralize DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) radicals at concentrations comparable to standard antioxidants (Sanjeewa et al., 2016). This antioxidant capacity was correlated with a significant reduction in intracellular ROS levels and enhanced expression of endogenous antioxidant enzymes, suggesting multifaceted mechanisms for oxidative stress mitigation. Particularly intriguing is the emerging evidence for neuroprotective effects of specific microalgal sterols. Dunaliella tertiolecta contains ergosterol and 7-dehydroporiferasterol, which have demonstrated neuromodulatory activities in experimental models (Francavilla et al., 2012). These compounds significantly improved behavioral parameters in animal models of neurological disorders while reducing levels of inflammatory mediators in brain tissue. The observed effects were attributed to multiple mechanisms, including inhibition of microglial activation, suppression of pro-inflammatory cytokine production, and modulation of neurotransmitter systems (Francavilla et al., 2012). Additionally, these sterols demonstrated antioxidant effects in neural tissue, protecting neurons from oxidative damage associated with neurodegenerative processes.
Research has also indicated that some sterols from microalgae may have anti-cancer properties. They can induce apoptosis (programmed cell death) in cancer cells, inhibit tumor growth, suppress angiogenesis, and modulate cell signaling pathways critical for cancer progression (Luo et al., 2015). The efficacy and specific mechanisms vary considerably depending on sterol structure and cancer type, highlighting the importance of structure-activity relationships in their therapeutic applications. The marine diatom Navicula incerta produces several bioactive sterols with promising anticancer activity, notably stigmasterol and 5β-hydroxysitostanol (Kim et al., 2014). Similarly, Chlorella vulgaris contains ergosterol peroxide, a sterol derivative with documented anticancer activity across multiple cancer cell lines (Yasukawa et al., 1996). These findings open avenues for exploring microalgae-derived sterols in cancer therapy.
Furthermore, there is growing evidence that microalgal sterols might have a role in regulating blood sugar levels, thereby offering potential benefits for managing diabetes. Their exact role and mechanism in glycemic control are still under investigation. Due to their antioxidant and anti-inflammatory properties, microalgal sterols may also benefit skin health. They can potentially aid in protecting the skin from UV radiation and reduce aging signs (Luo et al., 2015).
It is important to note that while the potential health benefits of microalgal sterols are promising, more research is needed to fully understand their biological effects, optimal dosages, and possible side effects, particularly in clinical settings.
Molecular advances for enhanced microalgal sterol production
Recent molecular advancements are transforming microalgal sterol production, bridging gaps in our understanding at the genetic level. The comprehensive sequencing of microalgal genomes has revealed the complex architecture of sterol biosynthetic pathways, enabling researchers to develop targeted molecular strategies for enhancing sterol output. These strategies encompass sophisticated genetic and metabolic engineering techniques, including precise gene editing through CRISPR/Cas9 systems (Summons et al., 2001; Naduthodi et al., 2019), strategic overexpression of rate-limiting enzymes, and selective gene silencing to redirect metabolic flux toward desired sterols (Bashir et al., 2016; Doron et al., 2016).
Foundational studies have demonstrated the efficacy of these approaches in model systems. The overexpression of squalene synthase, a key enzyme in the mevalonate pathway, in Chlamydomonas reinhardtii, has shown promising results in redirecting metabolic flows toward increased sterol production (Kajikawa et al., 2015). Similarly, gene silencing through RNA interference (RNAi) offers a powerful tool for downregulating competing pathways, further enhancing sterol synthesis (Kajikawa et al., 2015). D’adamo et al. (2019) pioneered this strategy by integrating three enzymes from the plant Lotus japonicus into Phaeodactylum tricornutum. This genetic intervention led to a significant upregulation in mRNA levels and amplified the activity within the native mevalonate pathway, a crucial precursor to sterol biosynthesis. As a result, this modification not only bolstered the production of essential sterols but also facilitated the synthesis of significant triterpenoids, demonstrating the potential for engineering microalgae as versatile biofactories for diverse high-value compounds.
Despite the promising outlook, the practical application of these molecular techniques in microalgae for sterol enhancement is still emerging and faces substantial real-world bottlenecks. A critical challenge is establishing stable transformation systems across diverse microalgal strains. Unlike model organisms such as Chlamydomonas reinhardtii and Phaeodactylum tricornutum, many commercially promising species remain recalcitrant to genetic manipulation due to robust cell walls, complex ploidy, or inefficient homologous recombination mechanisms (Hlavova et al., 2015; Muñoz et al., 2019). Moreover, the stability of transgene expression often diminishes over multiple generations, undermining the long-term viability of engineered strains in industrial settings (D’adamo et al., 2019). The regulatory landscape for genetically modified microalgae further complicates commercial deployment, with uncertain approval pathways and significant regional variations in permitted applications for biotechnology products (Beacham et al., 2017).
Challenges and future directions for microalgal sterols production
Despite their potential, several challenges hinder the commercial-scale production of microalgae-derived sterols. While certain species, such as Pavlova lutheri, exhibit impressive sterol concentrations under optimized conditions, most microalgal species have inherently lower sterol content compared to traditional sources (Ahmed et al., 2015), requiring careful strain selection and metabolic optimization to enhance sterol production at a commercially viable scale. Additionally, sterol biosynthesis pathways in diverse microalgal lineages are not yet fully understood. Unlike the well-characterized pathways in model organisms, the regulatory mechanisms and rate-limiting steps governing sterol production in commercially relevant species remain largely uncharacterized (Fagundes et al., 2020), making maximizing sterol production through genetic or metabolic engineering challenging.
Beyond sterol yield limitations, the diversity of sterols within microalgae species presents both an opportunity and a challenge for commercial development. This biochemical diversity could lead to the discovery of novel bioactive sterols with unique pharmaceutical properties, yet simultaneously complicates standardization and commercialization efforts since cultivation conditions can significantly alter sterol profiles (Randhir et al., 2020). Furthermore, while considerable research has characterized sterol compositions across taxonomic groups, the bioactivity and health benefits of specific microalgal sterols remain underexplored. Unlike macroalgae-derived sterols, which have been extensively studied for their antioxidant, cholesterol-lowering, and anti-inflammatory properties, microalgal sterols require further investigation to determine their functional applications and potential synergistic bioactivities in commercial formulations (Luo et al., 2015; Sanjeewa et al., 2016).
While sterol yield and bioactivity represent key biological challenges, the most significant barrier to commercializing microalgal sterols is translating laboratory success to industrial-scale production. Large-scale cultivation presents substantial logistical challenges absent from controlled laboratory environments. Open pond systems are vulnerable to contamination by competing microorganisms, seasonal fluctuations in environmental conditions, and adverse weather events that can dramatically reduce productivity (Liang et al., 2004; Day et al., 2012). Even in closed photobioreactor systems, biofilm formation progressively reduces light penetration, while temperature regulation becomes increasingly energy-intensive with scale. The fundamental requirement for precise environmental control over light intensity, temperature, pH, and nutrient availability to maintain consistent sterol profiles necessitates sophisticated monitoring and automation systems that substantially increase capital and operational costs.
Current approaches to cultivation system design present an unresolved tension between cost and control. Modern photobioreactors offer precise control but entail prohibitively high setup and maintenance expenses for sterol production alone. Conversely, more economical outdoor cultivation methods introduce significant variability in sterol yields due to fluctuating environmental conditions, making production less predictable and standardization more challenging (Ruiz et al., 2016; Hoffman et al., 2017). Furthermore, current harvesting and extraction technologies are not optimized for sterol recovery, resulting in product losses and increased processing costs. Developing efficient, sterol-specific downstream processing methods remains a significant technical hurdle that must be addressed to improve overall process economics (Gilbert-López et al., 2015).
The economic viability of microalgal sterol production depends on resolving multiple interrelated challenges across the value chain. Beyond the direct production costs, regulatory uncertainties surrounding genetically modified microalgae create significant commercial barriers. Approval pathways remain unclear in many jurisdictions, with substantial regional variations in permitted applications for biotechnology products. Additionally, microalgal sterols must compete with established plant and synthetic sources in terms of cost, consistency, and quality, creating significant market entry barriers despite potential sustainability advantages.
Nevertheless, several promising innovation pathways could substantially improve the commercial outlook. Recent advances in photobioreactor design have improved light penetration, gas exchange, and overall biomass productivity (Carvalho et al., 2006; Abdur Razzak et al., 2024; Penloglou et al., 2024), though their specific impact on sterol production economics requires further evaluation. Integration of real-time monitoring systems now enables automated adjustment of cultivation parameters while minimizing energy and resource consumption (Lee et al., 2022; Porras Reyes et al., 2024).
To realize the full potential of microalgae as sterol producers, research must address several critical knowledge gaps through integrated approaches. A fundamental limitation is our poor understanding of the functional significance of individual sterols within microalgal cells. In many commercially relevant species, the roles of specific sterols in membrane integrity, stress resilience, signaling, or ecological interactions remain largely unexplored. This mechanistic understanding would enable more rational engineering of strains for targeted sterol production and better prediction of how environmental factors influence sterol profiles. Priority should be given to elucidating the complete biosynthetic pathways responsible for microalgal sterol diversity, with particular attention to the enzymes involved in key structural modifications that determine bioactivity and commercial relevance. Mapping these pathways will not only clarify evolutionary relationships across microalgal lineages but also provide a critical foundation for bioengineering efforts to produce specific, high-value sterols.
To further reduce production costs, future efforts should focus on developing more efficient harvesting techniques, such as low-energy centrifugation, membrane filtration, and bioflocculation, which streamline processing and improve extraction efficiency (Barros et al., 2015; Matter et al., 2019; Deepa et al., 2023). Moreover, utilizing alternative nutrient sources, such as wastewater-based cultivation, presents a promising strategy to lower operational expenses while improving the sustainability of microalgal biotechnology (Do et al., 2022; Kundu et al., 2024).
Conclusion
Microalgae represent a compelling alternative to traditional sterol sources, offering unique advantages in biochemical diversity, environmental adaptability, and sustainable cultivation. The taxonomic diversity of microalgae corresponds to remarkable variation in sterol profiles, with particular species synthesizing unique compounds, including cholesterol and ergosterol, unlike plants, positioning them as a valuable platform for pharmaceutical, nutraceutical, and functional food applications.
However, despite significant progress, critical challenges hinder the full realization of microalgae as a commercially viable sterol source. The inherently low sterol content in many species, substantial variation in composition under different cultivation conditions, and the lack of complete understanding of biosynthetic regulation present significant biological barriers. Furthermore, the functional roles of microalgal sterols in cellular physiology, stress response, bioavailability, and therapeutic efficacy are poorly understood, limiting rational design approaches for enhanced yield and compositional specificity. Scaling from laboratory conditions to industrial production also presents remarkable technical and economic barriers. Current cultivation technologies struggle to deliver consistent sterol yields while maintaining economic feasibility.
Future efforts must adopt an integrative approach that bridges systems biology, synthetic biology, and bioprocess engineering. Unraveling the full regulatory networks governing sterol metabolism through multi-omics and genome editing will be critical for mapping complete sterol biosynthetic pathways across diverse microalgae and will provide the foundation for strain improvement and metabolic optimization. Simultaneously, process engineering innovations must focus on low-cost, scalable cultivation systems, particularly those leveraging waste streams and integrating carbon capture capabilities, combined with targeted process optimization for high-yield sterol production with specified molecular profiles.
In conclusion, the potential of microalgae as a sterol production platform is confirmed, but its realization hinges on overcoming significant biological, technological, and regulatory bottlenecks through coordinated research efforts. With strategic investment in fundamental research, applied technology development, and regulatory framework establishment, microalgae can be transformed from a promising alternative into a robust, industrially scalable source for sustainable sterol production, supporting the growing demands of health, nutrition, and green chemistry sectors.
Author contributions
OA: Writing – review & editing, Writing – original draft. RW: Writing – review & editing, Writing – original draft. MB: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project has received funding by a full scholarship (2020/2021) from the Ministry of Higher Education & Scientific Research, Egypt.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. ChatGPT (OpenAI) was used to enhance the language and clarity of the manuscript. The authors independently conducted all literature reviews, interpretation, analysis, and conclusions.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelkarim, O. H., Wijffels, R. H., and Barbosa, M. J. (2024). Microalgal lipid production: A comparative analysis of Nannochloropsis and Microchloropsis strains. J. Appl. Phycology., 1-20. doi: 10.1007/s10811-024-03318-7
Abdur Razzak, S., Bahar, K., Islam, K. M. O., Haniffa, A. K., Faruque, M. O., Hossain, S. M. Z., et al. (2024). Microalgae cultivation in photobioreactors: sustainable solutions for a greener future. Green Chem. Eng. 5, 418–439. doi: 10.1016/j.gce.2023.10.004
Abo-Shady, A. M., Gheda, S. F., Ismail, G. A., Cotas, J., Pereira, L., and Abdel-Karim, O. H. (2023). Antioxidant and antidiabetic activity of algae. Life 13 (2), 460. doi: 10.3390/life13020460
Ahmed, F., Zhou, W., and Schenk, P. M. (2015). Pavlova lutheri is a high-level producer of phytosterols. Algal Res. 10, 210–217. doi: 10.1016/j.algal.2015.05.013
Ballantine, J. A., Lavis, A., and Morris, R. J. (1979). Sterols of the phytoplankton—effects of illumination and growth stage. Phytochemistry 18, 1459–1466. doi: 10.1016/S0031-9422(00)98475-9
Barkas, F., Bathrellou, E., Nomikos, T., Panagiotakos, D., Liberopoulos, E., and Kontogianni, M. D. (2023). Plant sterols and plant stanols in cholesterol management and cardiovascular prevention. Nutrients 15 (13), 2845. doi: 10.3390/nu15132845
Barrett, S. M., Volkman, J. K., Dunstan, G. A., and Leroi, J.-M. (1995). Sterols of 14 species of marine diatoms (Bacillariophyta)1. J. Phycology 31, 360–369. doi: 10.1111/j.0022-3646.1995.00360.x
Barros, A. I., Gonçalves, A. L., Simões, M., and Pires, J. C. M. (2015). Harvesting techniques applied to microalgae: A review. Renewable Sustain. Energy Rev. 41, 1489–1500. doi: 10.1016/j.rser.2014.09.037
Bartley, M. L., Boeing, W. J., Corcoran, A. A., Holguin, F. O., and Schaub, T. (2013). Effects of salinity on growth and lipid accumulation of biofuel microalga Nannochloropsis salina and invading organisms. Biomass Bioenergy 54, 83–88. doi: 10.1016/j.biombioe.2013.03.026
Bashir, K. M. I., Kim, M.-S., Stahl, U., and Cho, M.-G. (2016). Microalgae engineering toolbox: Selectable and screenable markers. Biotechnol. Bioprocess Eng. 21, 224–235. doi: 10.1007/s12257-015-0386-4
Beacham, T. A., Sweet, J. B., and Allen, M. J. (2017). Large scale cultivation of genetically modified microalgae: A new era for environmental risk assessment. Algal Res. 25, 90–100. doi: 10.1016/j.algal.2017.04.028
Beck, J. G., Mathieu, D., Loudet, C., Buchoux, S., and Dufourc, E. J. (2007). Plant sterols in “rafts”: a better way to regulate membrane thermal shocks. FASEB J. 21, 1714–1723. doi: 10.1096/fj.06-7809com
Bjørklund, G., Shanaida, M., Lysiuk, R., Butnariu, M., Peana, M., Sarac, I., et al. (2022). Natural compounds and products from an anti-aging perspective. Molecules 27 (20), 7084. doi: 10.3390/molecules27207084
Bodenes, P. (2017). Study of the application of pulsed electric fields (PEF) on microalgae for the extraction of neutral lipids Etude de l’application de champs électriques pulsés sur des microalgues en vue de l’extraction de lipides neutres (Université Paris Saclay (COmUE).
Breteler, W. K., Schogt, N., and Rampen, S. (2005). Effect of diatom nutrient limitation on copepod development: role of essential lipids. Mar. Ecol. Prog. Ser. 291, 125–133. doi: 10.3354/MEPS291125
Brumfield, K. M., Laborde, S. M., and Moroney, J. V. (2017). A model for the ergosterol biosynthetic pathway in Chlamydomonas reinhardtii. Eur. J. Phycology 52, 64–74. doi: 10.1080/09670262.2016.1225318
Caroprese, M., Albenzio, M., Ciliberti, M. G., Francavilla, M., and Sevi, A. (2012). A mixture of phytosterols from Dunaliella tertiolecta affects proliferation of peripheral blood mononuclear cells and cytokine production in sheep. Veterinary Immunol. Immunopathology 150, 27–35. doi: 10.1016/j.vetimm.2012.08.002
Carvalho, A. P., Meireles, L. A., and Malcata, F. X. (2006). Microalgal reactors: A review of enclosed system designs and performances. Biotechnol. Prog. 22, 1490–1506. doi: 10.1002/btpr.v22:6
Cavalier-Smith, T. (2002a). The neomuran origin of archaebacteria, the negibacterial root of the universal tree and bacterial megaclassification. Int. J. Syst. Evol. Microbiol. 52, 7–76. doi: 10.1099/00207713-52-1-7
Cavalier-Smith, T. (2002b). The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa. Int. J. Syst. Evol. Microbiol. 52, 297–354. doi: 10.1099/00207713-52-2-297
Cerqueira, N.M.F.S.A., Oliveira, E. F., Gesto, D. S., Santos-Martins, D., Moreira, C., Moorthy, H. N., et al. (2016). Cholesterol biosynthesis: A mechanistic overview. Biochemistry 55, 5483–5506. doi: 10.1021/acs.biochem.6b00342
Chen, G. Q., Jiang, Y., and Chen, F. (2008). Salt-induced alterations in lipid composition of diatom nitzschia laevis (Bacillariophyceae) under heterotrophic culture condition(1). J. Phycol 44, 1309–1314. doi: 10.1111/j.1529-8817.2008.00565.x
Chen, J., Jiao, R., Jiang, Y., Bi, Y., and Chen, Z. Y. (2014). Algal sterols are as effective as β-sitosterol in reducing plasma cholesterol concentration. J. Agric. Food Chem. 62, 675–681. doi: 10.1021/jf404955n
Cruz, P. M., Mo, H., Mcconathy, W., Sabnis, N. A., and Lacko, A. G. (2013). The role of cholesterol metabolism and cholesterol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics. Front. Pharmacol. 4. doi: 10.3389/fphar.2013.00119
D'adamo, S., Schiano Di Visconte, G., Lowe, G., Szaub-Newton, J., Beacham, T., Landels, A., et al. (2019). Engineering the unicellular alga Phaeodactylum tricornutum for high-value plant triterpenoid production. Plant Biotechnol. J. 17, 75–87. doi: 10.1111/pbi.2019.17.issue-1
Davis, J. P. and Dean, L. L. (2016). “Chapter 11 - Peanut Composition, Flavor and Nutrition,” in Peanuts. Eds. Stalker, H. T. and Wilson, R. F. (Peanuts Genetics, Processing, and Utilization, Elsevier: AOCS Press), 289–345.
Day, J. G., Slocombe, S. P., and Stanley, M. S. (2012). Overcoming biological constraints to enable the exploitation of microalgae for biofuels. Bioresource Technol. 109, 245–251. doi: 10.1016/j.biortech.2011.05.033
Deepa, P., Sowndhararajan, K., and Kim, S. (2023). A review of the harvesting techniques of microalgae. Water 15 (17), 3074. doi: 10.3390/w15173074
Dinh, T. T. N., Thompson, L. D., Galyean, M. L., Brooks, J. C., Patterson, K. Y., and Boylan, L. M. (2011). Cholesterol content and methods for cholesterol determination in meat and poultry. Compr. Rev. Food Sci. Food Saf. 10, 269–289. doi: 10.1111/j.1541-4337.2011.00158.x
Do, C. V. T., Pham, M. H. T., Pham, T. Y. T., Dinh, C. T., Bui, T. U. T., Tran, T. D., et al. (2022). Microalgae and bioremediation of domestic wastewater. Curr. Opin. Green Sustain. Chem. 34, 100595. doi: 10.1016/j.cogsc.2022.100595
Doron, L., Segal, N., and Shapira, M. (2016). Transgene expression in microalgae—from tools to applications. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00505
Du, Y., Fu, X., Chu, Y., Wu, P., Liu, Y., Ma, L., et al. (2022). Biosynthesis and the roles of plant sterols in development and stress responses. Int. J. Mol. Sci. 23 (4), 2332. doi: 10.3390/ijms23042332
Dunstan, G. A., Brown, M. R., and Volkman, J. K. (2005). Cryptophyceae and rhodophyceae; chemotaxonomy, phylogeny, and application. Phytochemistry 66, 2557–2570. doi: 10.1016/j.phytochem.2005.08.015
Dunstan, G. A., Volkman, J. K., Barrett, S. M., and Garland, C. D. (1993). Changes in the lipid composition and maximisation of the polyunsaturated fatty acid content of three microalgae grown in mass culture. J. Appl. Phycology 5, 71–83. doi: 10.1007/BF02182424
Durmaz, Y., Donato, M., Monterio, M., Gouveia, L., Nunes, M. L., Gama, P. T., et al. (2008). Effect of temperature on growth and biochemical composition (sterols, α-tocopherol, carotenoids, fatty acid profiles) of the microalga, Isochrysis galbana. The Israeli Journal of Aquaculture - Bamidgeh 60 (3), 190-197. doi: 10.46989/001c.20492
Durmaz, Y., Monteiro, M., Koru, E., and Bandarra, N. (2007). Concentration of sterols of Porphyridium cruentum biomass at stationary phase. Pakistan J. Biol. Sci. 10, 1144–1146. doi: 10.3923/pjbs.2007.1144.1146
Fábregas, J., Arán, J., Morales, E. D., Lamela, T., and Otero, A. (1997). Modification of sterol concentration in marine microalgae. Phytochemistry 46, 1189–1191. doi: 10.1016/S0031-9422(97)80009-X
Fagundes, M. B., Falk, R. B., Facchi, M. M. X., Vendruscolo, R. G., Maroneze, M. M., Zepka, L. Q., et al. (2019). Insights in cyanobacteria lipidomics: A sterols characterization from Phormidium autumnale biomass in heterotrophic cultivation. Food Res. Int. 119, 777–784. doi: 10.1016/j.foodres.2018.10.060
Fagundes, M. B., Vendruscolo, R. G., and Wagner, R. (2020). “Sterols from microalgae,” in Handbook of Microalgae-Based Processes and Products, 573–596.
Fagundes, M. B. and Wagner, R. (2021). “Sterols Biosynthesis in Algae,” in Bioactive Compounds. Eds. Leila Queiroz, Z., Tatiele Casagrande Do, N., and Eduardo, J.-L. (IntechOpen, Rijeka), 9.
Feltrin, S., Ravera, F., Traversone, N., Ferrando, L., Bedognetti, D., Ballestrero, A., et al. (2020). Sterol synthesis pathway inhibition as a target for cancer treatment. Cancer Lett. 493, 19–30. doi: 10.1016/j.canlet.2020.07.010
Feng, S., Belwal, T., Li, L., Limwachiranon, J., Liu, X., and Luo, Z. (2020). Phytosterols and their derivatives: Potential health-promoting uses against lipid metabolism and associated diseases, mechanism, and safety issues. Compr. Rev. Food Sci. Food Saf. 19, 1243–1267. doi: 10.1111/1541-4337.12560
Fernandes, P. and Cabral, J. M. (2007). Phytosterols: applications and recovery methods. Bioresour Technol. 98, 2335–2350. doi: 10.1016/j.biortech.2006.10.006
Fiume, M., Bergfeld, W. F., Belsito, D. V., Hill, R. A., Klaassen, C. D., Liebler, D. C., et al. (2023). Squalane and squalene. Int. J. Toxicol. 42, 107s–109s. doi: 10.1177/10915818231204276
Fox, C. B. (2009). Squalene emulsions for parenteral vaccine and drug delivery. Molecules 14, 3286–3312. doi: 10.3390/molecules14093286
Francavilla, M., Colaianna, M., Zotti, M., Morgese, M. G., Trotta, P., Tucci, P., et al. (2012). Extraction, characterization and in vivo neuromodulatory activity of phytosterols from microalga Dunaliella tertiolecta. Curr. Med. Chem. 19, 3058–3067. doi: 10.2174/092986712800672021
Francavilla, M., Trotta, P., and Luque, R. (2010). Phytosterols from Dunaliella tertiolecta and Dunaliella salina: A potentially novel industrial application. Bioresource Technol. 101, 4144–4150. doi: 10.1016/j.biortech.2009.12.139
Gheda, S. F., Abo-Shady, A. M., Abdel-Karim, O. H., and Ismail, G. A. (2021). Antioxidant and Antihyperglycemic Activity of Arthrospira platensis (Spirulina platensis) Methanolic Extract: In vitro and in vivo Study. Egyptian J. Bot. 61, 71–93. doi: 10.21608/ejbo.2020.27436.1482
Gilbert-López, B., Mendiola, J. A., Fontecha, J., Van Den Broek, L. A. M., Sijtsma, L., Cifuentes, A., et al. (2015). Downstream processing of Isochrysis galbana: a step towards microalgal biorefinery. Green Chem. 17, 4599–4609. doi: 10.1039/C5GC01256B
Gordillo, F. J. L., Goutx, M., Figueroa, F. L., and Niell, F. X. (1998). Effects of light intensity, CO2 and nitrogen supply on lipid class composition of Dunaliella viridis. J. Appl. Phycology 10, 135–144. doi: 10.1023/A:1008067022973
Griebel, T. and Zeier, J. (2010). A role for beta-sitosterol to stigmasterol conversion in plant-pathogen interactions. Plant J. 63, 254–268. doi: 10.1111/j.1365-313X.2010.04235.x
Guedes, A. C., Meireles, L. A., Amaro, H. M., and Malcata, F. X. (2010). Changes in lipid class and fatty acid composition of cultures of pavlova lutheri, in response to light intensity. J. Am. Oil Chemists' Soc. 87, 791–801. doi: 10.1007/s11746-010-1559-0
Hai, T., Schneider, B., Schmidt, J., and Adam, G. (1996). Sterols and triterpenoids from the cyanobacterium Anabaena hallensis. Phytochemistry 41, 1083–1084. doi: 10.1016/0031-9422(95)00778-4
He, X., Guo, X., Liu, N., and Zhang, B. (2007). Ergosterol production from molasses by genetically modified Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 75, 55–60. doi: 10.1007/s00253-006-0807-6
Hlavova, M., Turoczy, Z., and Bisova, K. (2015). Improving microalgae for biotechnology — From genetics to synthetic biology. Biotechnol. Adv. 33, 1194–1203. doi: 10.1016/j.biotechadv.2015.01.009
Hoffman, J., Pate, R. C., Drennen, T., and Quinn, J. C. (2017). Techno-economic assessment of open microalgae production systems. Algal Res. 23, 51–57. doi: 10.1016/j.algal.2017.01.005
Hofmaenner, D. A., Kleyman, A., Press, A., Bauer, M., and Singer, M. (2022). The many roles of cholesterol in sepsis: A review. Am. J. Respir. Crit. Care Med. 205, 388–396. doi: 10.1164/rccm.202105-1197TR
Kajikawa, M., Kinohira, S., Ando, A., Shimoyama, M., Kato, M., and Fukuzawa, H. (2015). Accumulation of squalene in a microalga chlamydomonas reinhardtii by genetic modification of squalene synthase and squalene epoxidase genes. PloS One 10, e0120446. doi: 10.1371/journal.pone.0120446
Kalacheva, G. S., Zhila, N. O., Volova, T. G., and Gladyshev, M. I. (2002). The effect of temperature on the lipid composition of the green alga Botryococcus. Microbiology 71, 286–293. doi: 10.1023/A:1015898426573
Katz, A., Waridel, P., Shevchenko, A., and Pick, U. (2007). Salt-induced changes in the plasma membrane proteome of the halotolerant alga dunaliella salina as revealed by blue native gel electrophoresis and nano-LC-MS/MS analysis*. Mol. Cell. Proteomics 6, 1459–1472. doi: 10.1074/mcp.M700002-MCP200
Keech, C., Albert, G., Cho, I., Robertson, A., Reed, P., Neal, S., et al. (2020). Phase 1–2 trial of a SARS-coV-2 recombinant spike protein nanoparticle vaccine. New Engl. J. Med. 383, 2320–2332. doi: 10.1056/NEJMoa2026920
Khan, M. I., Shin, J. H., and Kim, J. D. (2018). The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microbial Cell Factories 17, 36. doi: 10.1186/s12934-018-0879-x
Kilham, S., Kreeger, D., Goulden, C., and Lynn, S. (1997). Effects of nutrient limitation on biochemical constituents of Ankistrodesmus falcatus. Freshw. Biol. 38, 591–596. doi: 10.1046/j.1365-2427.1997.00231.x
Kim, Y. S., Li, X. F., Kang, K. H., Ryu, B., and Kim, S. K. (2014). Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep. 47, 433–438. doi: 10.5483/BMBRep.2014.47.8.153
Kohlhase, M. and Pohl, P. (1988). Saturated and unsaturated sterols of nitrogen-fixing blue-green algae (cyanobacteria). Phytochemistry 27, 1735–1740. doi: 10.1016/0031-9422(88)80434-5
Kritchevsky, D. and Chen, S. C. (2005). Phytosterols—health benefits and potential concerns: a review. Nutr. Res. 25, 413–428. doi: 10.1016/j.nutres.2005.02.003
Kumari, P., Kumar, M., Reddy, C. R. K., and Jha, B. (2013). “Algal lipids, fatty acids and sterols,” in Functional Ingredients from Algae for Foods and Nutraceuticals, Woodhead Publishing Series in Food Science, Technology and Nutrition. 87–134.
Kundu, P., Dutta, N., and Bhattacharya, S. (2024). Application of microalgae in wastewater treatment with special reference to emerging contaminants: a step towards sustainability. Front. Analytical Sci. 4, 2024. doi: 10.3389/frans.2024.1513153
Leblond, J. D., Timofte, H. I., Roche, S. A., and Porter, N. M. (2011). Sterols of glaucocystophytes. Phycological Res. 59, 129–134. doi: 10.1111/j.1440-1835.2011.00610.x
Lee, J. S., Sung, Y. J., and Sim, S. J. (2022). Kinetic analysis of microalgae cultivation utilizing 3D-printed real-time monitoring system reveals potential of biological CO2 conversion. Bioresource Technol. 364, 128014. doi: 10.1016/j.biortech.2022.128014
Liang, S., Liu, X., Chen, F., and Chen, Z. (2004). Current microalgal health food R & D activities in China. Hydrobiologia 512, 45–48. doi: 10.1023/B:HYDR.0000020366.65760.98
Lopes, G., Sousa, C., Valentão, P., and Andrade, P. B. (2013). “Sterols in Algae and Health,” in Bioactive Compounds from Marine Foods: Plant and Animal Sources, Wiley Online Library, 173–191.
Lu, Y., Zhou, W., Wei, L., Li, J., Jia, J., Li, F., et al. (2014). Regulation of the cholesterol biosynthetic pathway and its integration with fatty acid biosynthesis in the oleaginous microalga Nannochloropsis oceanica. Biotechnol. Biofuels 7, 81. doi: 10.1186/1754-6834-7-81
Luo, X., Su, P., and Zhang, W. (2015). Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar. Drugs 13, 4231–4254. doi: 10.3390/md13074231
Lv, J., Yang, X., Ma, H., Hu, X., Wei, Y., Zhou, W., et al. (2015). The oxidative stability of microalgae oil (Schizochytrium aggregatum) and its antioxidant activity after simulated gastrointestinal digestion: Relationship with constituents. Eur. J. Lipid Sci. Technol. 117, 1928–1939. doi: 10.1002/ejlt.201400588
Martin-Creuzburg, D. and Merkel, P. (2016). Sterols of freshwater microalgae: potential implications for zooplankton nutrition. J. Plankton Res. 38, 865–877. doi: 10.1093/plankt/fbw034
Matter, I. A., Bui, V. K., Jung, M., Seo, J. Y., Kim, Y.-E., Lee, Y.-C., et al. (2019). Flocculation harvesting techniques for microalgae: A review. Appl. Sci. 9 (15), 3069. doi: 10.3390/app9153069
Mattila, P., Lampi, A.-M., Ronkainen, R., Toivo, J., and Piironen, V. (2002). Sterol and vitamin D2 contents in some wild and cultivated mushrooms. Food Chem. 76, 293–298. doi: 10.1016/S0308-8146(01)00275-8
Miller, M. B., Haubrich, B. A., Wang, Q., Snell, W. J., and Nes, W. D. (2012). Evolutionarily conserved Delta(25(27))-olefin ergosterol biosynthesis pathway in the alga Chlamydomonas reinhardtii. J. Lipid Res. 53, 1636–1645. doi: 10.1194/jlr.M027482
Mohammadi, M., Jafari, S. M., Hamishehkar, H., and Ghanbarzadeh, B. (2020). Phytosterols as the core or stabilizing agent in different nanocarriers. Trends Food Sci. Technol. 101, 73–88. doi: 10.1016/j.tifs.2020.05.004
Moreau, R. A., Whitaker, B. D., and Hicks, K. B. (2002). Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog. Lipid Res. 41, 457–500. doi: 10.1016/S0163-7827(02)00006-1
Muñoz, C. F., Weusthuis, R. A., D’adamo, S., and Wijffels, R. H. (2019). Effect of single and combined expression of lysophosphatidic acid acyltransferase, glycerol-3-phosphate acyltransferase, and diacylglycerol acyltransferase on lipid accumulation and composition in neochloris oleoabundans. Front. Plant Sci. 10, 2019. doi: 10.3389/fpls.2019.01573
Naduthodi, M. I. S., Mohanraju, P., Südfeld, C., D’adamo, S., Barbosa, M. J., and van der Oost, J. (2019). CRISPR–Cas ribonucleoprotein mediated homology-directed repair for efficient targeted genome editing in microalgae Nannochloropsis oceanica IMET1. Biotechnol. Biofuels 12, 66. doi: 10.1186/s13068-019-1401-3
Papoutsis, K., Grasso, S., Menon, A., Brunton, N. P., Lyng, J. G., Jacquier, J.-C., et al. (2020). Recovery of ergosterol and vitamin D2 from mushroom waste - Potential valorization by food and pharmaceutical industries. Trends Food Sci. Technol. 99, 351–366. doi: 10.1016/j.tifs.2020.03.005
Penloglou, G., Pavlou, A., and Kiparissides, C. (2024). Recent advancements in photo-bioreactors for microalgae cultivation: A brief overview. Processes 12 (6), 1104. doi: 10.3390/pr12061104
Pérez-Sánchez, A., Barrajón-Catalán, E., Herranz-López, M., and Micol, V. (2018). Nutraceuticals for skin care: A comprehensive review of human clinical studies. Nutrients 10 (4), 403. doi: 10.3390/nu10040403
Piepho, M., Martin-Creuzburg, D., and Wacker, A. (2010). Simultaneous effects of light intensity and phosphorus supply on the sterol content of phytoplankton. PloS One 5, e15828. doi: 10.1371/journal.pone.0015828
Piepho, M., Martin-Creuzburg, D., and Wacker, A. (2012). Phytoplankton sterol contents vary with temperature, phosphorus and silicate supply: a study on three freshwater species. Eur. J. Phycology 47, 138–145. doi: 10.1080/09670262.2012.665484
Piironen, V., Lindsay, D. G., Miettinen, T. A., Toivo, J., and Lampi, A.-M. (2000). Plant sterols: biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 80, 939–966. doi: 10.1002/(SICI)1097-0010(20000515)80:7<939::AID-JSFA644>3.0.CO;2-C
Poli, A., Marangoni, F., Corsini, A., Manzato, E., Marrocco, W., Martini, D., et al. (2021). Phytosterols, cholesterol control, and cardiovascular disease. Nutrients 13 (8), 2810. doi: 10.3390/nu13082810
Pollier, J., Vancaester, E., Kuzhiumparambil, U., Vickers, C. E., Vandepoele, K., Goossens, A., et al. (2019). A widespread alternative squalene epoxidase participates in eukaryote steroid biosynthesis. Nat. Microbiol. 4, 226–233. doi: 10.1038/s41564-018-0305-5
Ponomarenko, L. P., Stonik, I. V., Aizdaicher, N. A., Orlova, T. Y., Popovskaya, G. I., Pomazkina, G. V., et al. (2004). Sterols of marine microalgae Pyramimonas cf. cordata (Prasinophyta), Attheya ussurensis sp. nov. (Bacillariophyta) and a spring diatom bloom from Lake Baikal. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 138, 65–70. doi: 10.1016/j.cbpc.2004.02.007
Porras Reyes, L., Havlik, I., and Beutel, S. (2024). Software sensors in the monitoring of microalgae cultivations. Rev. Environ. Sci. Bio/Technology 23, 67–92. doi: 10.1007/s11157-023-09679-8
Prakash, S., Sasikala, S. L., and Aldous, V. H. J. (2010). Isolation and identification of MDR–Mycobacterium tuberculosis and screening of partially characterised antimycobacterial compounds from chosen marine micro algae. Asian Pacific J. Trop. Med. 3, 655–661. doi: 10.1016/S1995-7645(10)60158-7
Ramadan, F., Asker, M., and Ibrahim, Z. (2008). Functional bioactive compounds and biological activities of Spirulina platensis lipids. Czech J. Food Sci. 26, 211–222. doi: 10.17221/2567-CJFS
Randhir, A., Laird, D. W., Maker, G., Trengove, R., and Moheimani, N. R. (2020). Microalgae: A potential sustainable commercial source of sterols. Algal Res. 46, 1-13. doi: 10.1016/j.algal.2019.101772
Rangsinth, P., Sharika, R., Pattarachotanant, N., Duangjan, C., Wongwan, C., Sillapachaiyaporn, C., et al. (2023). Potential beneficial effects and pharmacological properties of ergosterol, a common bioactive compound in edible mushrooms. Foods 12 (13), 2529. doi: 10.3390/foods12132529
Rasmussen, H. E., Blobaum, K. R., Park, Y. K., Ehlers, S. J., Lu, F., and Lee, J. Y. (2008). Lipid extract of Nostoc commune var. sphaeroides Kutzing, a blue-green alga, inhibits the activation of sterol regulatory element binding proteins in HepG2 cells. J. Nutr. 138, 476–481. doi: 10.1093/jn/138.3.476
Ruiz, J., Olivieri, G., De Vree, J., Bosma, R., Willems, P., Reith, J. H., et al. (2016). Towards industrial products from microalgae. Energy Environ. Sci. 9, 3036–3043. doi: 10.1039/C6EE01493C
Rzama, A., Dufourc, E. J., and Arreguy, B. (1994). Sterols from green and blue-green algae grown on reused waste water. Phytochemistry 37, 1625–1628. doi: 10.1016/S0031-9422(00)89579-5
Sanjeewa, K. K. A., Fernando, I. P. S., Samarakoon, K. W., Lakmal, H. H. C., Kim, E.-A., Kwon, O. N., et al. (2016). Anti-inflammatory and anti-cancer activities of sterol rich fraction of cultured marine microalga Nannochloropsis oculata. Algae 31, 277–287. doi: 10.4490/algae.2016.31.6.29
Scodelaro Bilbao, P. G., Damiani, C., Salvador, G. A., and Leonardi, P. (2016). Haematococcus pluvialis as a source of fatty acids and phytosterols: potential nutritional and biological implications. J. Appl. phycology 28, 3283–3294. doi: 10.1007/s10811-016-0899-z
Sijil, P. V., Cherita, C., Jethani, H., and Chauhan, V. S. (2022). “Microalgae as a Renewable and Sustainable Source of High Value Metabolites,” in Microalgae for Sustainable Products: The Green Synthetic Biology Platform. Eds. Shekh, A. and Dasgupta, S. (The Royal Society of Chemistry) 1, 1-26.
Singh, A., Singh, K., Sharma, A., Kaur, K., Chadha, R., and Bedi, P. M. S. (2023). Recent advances in antifungal drug development targeting lanosterol 14α-demethylase (CYP51): A comprehensive review with structural and molecular insights. Chem. Biol. Drug Design 102, 606–639. doi: 10.1111/cbdd.v102.3
Staels, B. and Fonseca, V. A. (2009). Bile Acids and Metabolic Regulation: Mechanisms and clinical responses to bile acid sequestration. Diabetes Care 32, S237–S245. doi: 10.2337/dc09-S355
Sujith Kumar, M. S., Mawlong, I., and Singh, D. (2017). Phytosterol recovery from oilseeds: Recent advances. J. Food Process Eng. 40, e12466. doi: 10.1111/jfpe.2017.40.issue-3
Summons, R., Jahnke, L., Cullings, K., and Logan, G. (2001). Cyanobacterial bioamarkers: triterpenoids plus steroids? AGU Fall Meeting Abstracts 1, 0184.
Tata, D., Wasan, E., Wasan, K., and Gershkovich, P. (2015). Lipid-lowering activity of natural and semi-synthetic sterols and stanols. J. Pharm. Pharm. Sci. 18, 344–367. doi: 10.18433/J3GC84
Véron, B., Billard, C., Dauguet, J. C., and Hartmann, M. A. (1996). Sterol composition of Phaeodactylum tricornutum as influenced by growth temperature and light spectral quality. Lipids 31, 989–994. doi: 10.1007/BF02522694
Volkman, J. K. (2003). Sterols in microorganisms. Appl. Microbiol. Biotechnol. 60, 495–506. doi: 10.1007/s00253-002-1172-8
Volkman, J. K. (2005). Sterols and other triterpenoids: source specificity and evolution of biosynthetic pathways. Organic Geochemistry 36, 139–159. doi: 10.1016/j.orggeochem.2004.06.013
Wijffels, R. H., Kruse, O., and Hellingwerf, K. J. (2013). Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 24, 405–413. doi: 10.1016/j.copbio.2013.04.004
Yasukawa, K., Akihisa, T., Kanno, H., Kaminaga, T., Izumida, M., Sakoh, T., et al. (1996). Inhibitory effects of sterols isolated from Chlorella vulgaris on 12-0-tetradecanoylphorbol-13-acetate-induced inflammation and tumor promotion in mouse skin. Biol. Pharm. Bull. 19, 573–576. doi: 10.1248/bpb.19.573
Zhabinskii, V. N., Drasar, P., and Khripach, V. A. (2022). Structure and biological activity of ergostane-type steroids from fungi. Molecules 27 (7), 2103. doi: 10.3390/molecules27072103
Zhang, Z., Sachs, J. P., and Marchetti, A. (2009). Hydrogen isotope fractionation in freshwater and marine algae: II. Temperature and nitrogen limited growth rate effects. Organic Geochemistry 40, 428–439. doi: 10.1016/j.orggeochem.2008.11.002
Zhao, L., Chang, W. C., Xiao, Y., Liu, H. W., and Liu, P. (2013). Methylerythritol phosphate pathway of isoprenoid biosynthesis. Annu. Rev. Biochem. 82, 497–530. doi: 10.1146/annurev-biochem-052010-100934
Zhila, N. O., Kalacheva, G. S., and Volova, T. G. (2005). Effect of nitrogen limitation on the growth and lipid composition of the green alga botryococcus braunii kutz IPPAS H-252. Russian J. Plant Physiol. 52, 311–319. doi: 10.1007/s11183-005-0047-0
Keywords: microalgae, sterols production, environmental conditions, genetic engineering, biotechnological application
Citation: Abdelkarim OH, Wijffels RH and Barbosa MJ (2025) Exploiting microalgal diversity for sterol production. Front. Plant Sci. 16:1616863. doi: 10.3389/fpls.2025.1616863
Received: 23 April 2025; Accepted: 22 May 2025;
Published: 30 June 2025.
Edited by:
Maurycy Daroch, Peking University, ChinaReviewed by:
Jiangxin Wang, Shenzhen University, ChinaSergio Balzano, Anton Dohrn Zoological Station Naples, Italy
Copyright © 2025 Abdelkarim, Wijffels and Barbosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Omnia H. Abdelkarim, b21uaWEuaGFtZHlAd3VyLm5s; b21uaWEuaGFtZHlAc2NpZW5jZS50YW50YS5lZHUuZWc=
†ORCID: Omnia H. Abdelkarim, orcid.org/0000-0002-9149-1209
Rene H. Wijffels, orcid.org/0000-0001-7630-4295
Maria J. Barbosa, orcid.org/0000-0002-8246-0777
 Omnia H. Abdelkarim
Omnia H. Abdelkarim Rene H. Wijffels
Rene H. Wijffels Maria J. Barbosa
Maria J. Barbosa