- Department of Horticulture and Food Science, VIT School of Agricultural Innovations and Advanced Learning (VAIAL), Vellore Institute of Technology, Vellore, Tamil Nadu, India
Marigold (Tagetes spp.) is economically important flower crop widely cultivated for its vibrant flowers, use in religious ceremonies, landscaping, and extraction of carotenoids for industrial and pharmaceutical applications. Breeding advancements in marigold have primarily focused on enhancing yield, flower quality, and resistance to biotic and abiotic stresses. This review presents a comprehensive overview of progress in marigold breeding, covering traditional approaches such as selection, hybridization, and mutation breeding, as well as modern biotechnological tools, including marker-assisted selection (MAS), genomic selection, and CRISPR-based genome editing. Conventional breeding has led to the development of several high-yielding hybrids, including ‘Arka Abhi’ and ‘Arka Shubha,’ which are widely cultivated across India. Modern molecular approaches have facilitated the identification of quantitative trait loci (QTLs) associated with essential traits, improving the efficiency of breeding programs. In recent years, tissue culture techniques have played a pivotal role in the rapid propagation of elite varieties and the generation of somaclonal variants with desirable traits. Major challenges like a limited genetic base, climate change, pests and diseases still make sustainable production difficult. The integration of wild germplasm and advanced genomic tools offers promising avenues for addressing these limitations. Participatory breeding and interdisciplinary research play a crucial role in addressing location-specific demands and improving the economic viability of marigold cultivation. This analysis indicates the importance for sustainable breeding practices that match with growing market requirements and environmental issues. Hence, by integrating traditional knowledge with cutting-edge technologies, marigold breeding programs can unlock the crop’s full potential, contributing to the growth of India’s ornamental and agricultural sectors.
1 Introduction
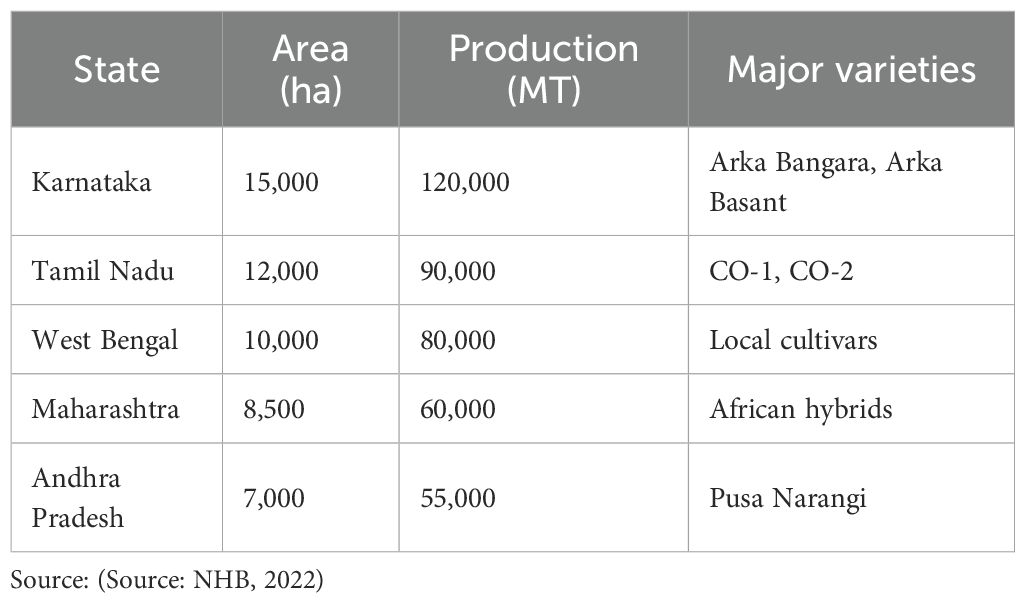
In India, marigold (Tagetes spp.) is an attractive crop with significant cultural, economic, and industrial value. Valued for their vibrant flowers, marigolds hold a major role in religious and social ceremonies, as well as in landscaping. Marigold is native to South and Central America especially Mexico and belong to the family Asteraceae (Compositae) (Shafiee et al., 2023). The genus Tagetes commonly cultivated species are Tagetes erecta (African Marigold), Tagetes patula (French Marigold) and Tagetes minuta. Marigold are long upright and quick growing habit (Udchachon et al., 2021). The height of plants ranges from 30 to 90 cm (Yadav and Dahiya, 2020). The flowers of these varieties are deep orange, light orange, golden yellow, bright yellow and lemon yellow in color. The size of flower may vary from 4 to 6cm in diameter. Marigold is good source of carotenoid pigment for poultry feed to intensify yellow color of egg yolks (Zhang et al., 2020). The crop is a critical raw material for the extraction of carotenoids, particularly lutein and zeaxanthin, which are widely used in the dye, food, and pharmaceutical industries (Shafique et al., 2021). India is the leading producers of marigold, with major cultivation areas in Karnataka, Tamil Nadu, and Maharashtra contributing significantly to domestic and export markets. Marigold cultivation is successful because it can adapt to a variety of agroclimatic conditions and yield large quantities of flowers quickly (Kumar et al., 2020). The crop is grown both as a field crop and in protected environments, supporting livelihoods across various regions. Improved varieties and hybrids such as ‘Arka Bangara,’ ‘Arka Agni,’ and ‘Pusa Narangi Gainda’ have enhanced marigold production, meeting the growing demands for high-quality flowers (Kaushal et al., 2024). Marigold also finds industrial application like preparation of natural dyes and essential oils. It is used as mosquito and nematode repellents. The marigold plants are highly useful for suppressing the population of nematodes in the field. The uses of marigold are extensive, often referred to as, “Versatile crop with golden harvest”. Marigolds produce thiopenes, which are toxic to nematodes and used as trap crop in tomato, brinjal, tobacco etc. Despite its adaptability, marigold cultivation faces challenges such as the limited availability of high-performing hybrids and susceptibility to pests like aphids and diseases such as alternaria blight and powdery mildew (Dedhia, 2024). Climate-induced stress factors, including drought and salinity, significantly impact flower yield and quality, further emphasizing the need for stress-resilient varieties (Akhtar et al., 2022). Efforts to enhance marigold cultivation have shifted from traditional breeding approaches to incorporating molecular tools, including marker-assisted selection (MAS) and genome editing, to develop superior varieties. This integration of traditional and modern techniques is essential to overcoming current challenges and unlocking the crop’s full potential for sustainable production (Susrama and Yuliadhi, 2020). The state-wise Marigold production in India for 2022–23 is presented in Table 1.
2 Methodology
A comprehensive review of the literature was carried out to collect scientific studies on the taxonomy, breeding objectives, conventional and modern methods of breeding in marigold. Research and review articles published up to December 2024 were sourced from databases like Scopus, Google Scholar, ScienceDirect, and others. Keywords like marigold breeding, pedigree selection, mutation breeding and micropropagation of marigold were used to guide the search. Studies that were out of scope, lacked sufficient details, or did not meet the relevance criteria were excluded. The selection process focused on relevance and relatedness to ensure only the most significant and appropriate information was included.
3 Taxonomy and genetic diversity
The genus Tagetes, belonging to the family Asteraceae, comprises approximately 50 species, with Tagetes erecta (African marigold) and Tagetes patula (French marigold) being the most widely cultivated species in India. These species differ in their morphology, flowering characteristics, and adaptability. Tagetes erecta is characterized by large flowers, upright growth habit, and suitability for cut flower production, while Tagetes patula produces small flowers and is favored for ornamental bedding purposes (Poulose et al., 2020). Taxonomic classification of Tagetes has traditionally relied on morphological traits, such as plant height, flower size, and leaf structure (Kaushal et al., 2024). However, recent advancements in molecular tools have provided a deeper understanding of genetic relationships within the genus. Highlighting the genetic proximity between species through phylogenetic analysis with molecular markers such as Random Amplified Polymorphic DNA (RAPD) and Simple Sequence Repeats (SSRs) enables breeders to take advantage of interspecific hybridization for transferring desired traits (Pangaribuan et al., 2022). Comparative genomic studies have also revealed that species within Tagetes share conserved genomic regions associated with traits like flower color and carotenoid biosynthesis, further aiding targeted breeding efforts (Cai et al., 2024).
Genetic diversity is a critical factor for the success of breeding programs, as it determines the extent of variation available for selection and hybridization. Assessments of genetic variability in marigold have been conducted using morphological, biochemical, and molecular markers (Namita et al., 2013). High levels of genetic diversity have been reported among marigold genotypes cultivated in different agro-climatic zones of India, reflecting the adaptability and evolutionary history. Molecular marker studies have been particularly effective in quantifying genetic diversity (Panwar et al., 2017). Efforts to conserve genetic resources of marigold are also gaining importance. Germplasm collections maintained by research institutes, such as the Indian Council of Agricultural Research (ICAR), play a vital role in preserving genetic diversity. These resources are crucial for the development of climate-resilient varieties and the incorporation of novel traits from wild relatives (Thirumalmurugan et al., 2020).
4 Breeding objectives
4.1 Yield improvement
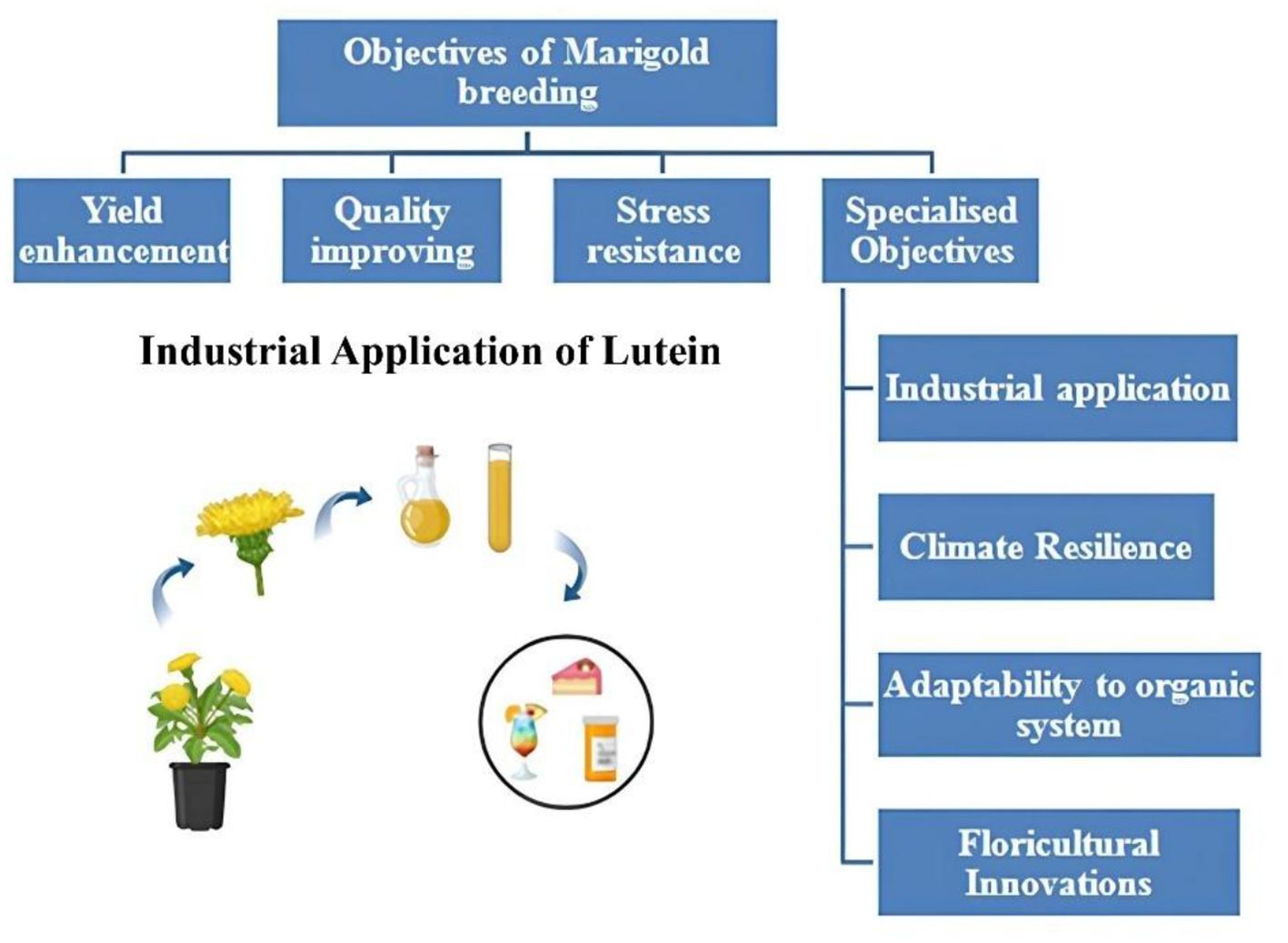
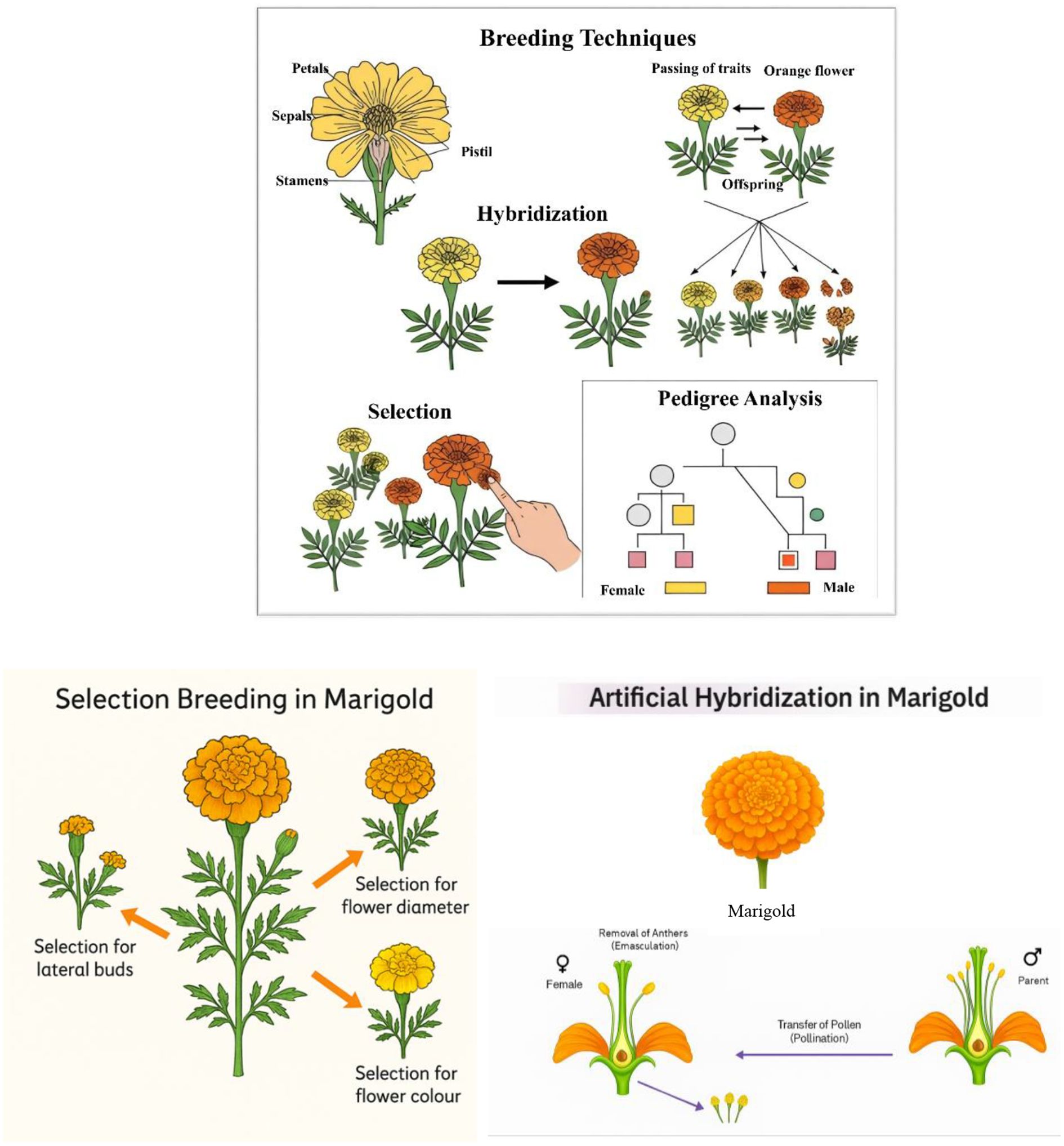
Yield improvement remains a top priority in marigold breeding due to its direct impact on profitability and market viability. High-yielding varieties are essential to meet the increasing demand for marigold in religious, ornamental, and industrial sectors. Recent research has introduced hybrids such as ‘Arka Abhi,’ ‘Arka Bhanu,’ and ‘Arka Vibha,’ which exhibit significantly improved flower yields, extended shelf life, and better transportability (Thirumalmurugan et al., 2020). These hybrids have shown potential for commercial cultivation in various agro-climatic zones, enhancing farmer incomes and market availability. Breeding programs have also focused on traits such as number of flowers per plant, flower diameter, and plant biomass, which contribute to overall yield. Studies have identified high heritability estimates for these traits, suggesting a strong genetic basis that facilitates effective selection (Adhikary et al., 2025). Marker-assisted selection (MAS) has emerged as a key tool in identifying and selecting genotypes with superior yield potential, enabling the development of hybrids with improved productivity under both irrigated and rainfed conditions (Singh et al., 2022; Joshi et al., 2024). The plant architecture, including growth habits and robust branching, has been optimized in new varieties to maximize flower production (Sumalatha et al., 2023). The overall objectives of marigold breeding are shown in Figure 1.
4.2 Quality improvement
Flower quality is a multifaceted objective in marigold breeding, encompassing attributes like size, shape, color, fragrance, and shelf life. Varieties with vibrant colors and uniform petal arrangements are highly desired in the ornamental and religious sectors. For instance, Arka Shubha, known for its high carotenoid content, has gained popularity for industrial uses such as pigment extraction for food and cosmetics (Sood et al., 2023). Marigolds (Tagetes erecta and Tagetes patula) are recognized for their medicinal and nutritional value, particularly as a rich source of lutein, a carotenoid beneficial for eye health (González-Morales et al., 2021). Recent advances in genetic transformation have further enhanced breeding efforts, improving germination rates and overall plant quality (Dedhia, 2024). Recent advances in breeding for quality traits have been supported by molecular studies identifying genes like PSY (Phytoene Synthase), PDS (Phytoene Desaturase), ZDS (ζ-Carotene Desaturase), LCY-B (Lycopene β-Cyclase), LCY-E (Lycopene ϵ-Cyclase), CCD (Carotenoid Cleavage Dioxygenase) involved in pigmentation pathways (Cooper and Messina, 2023; Yuan et al., 2023). Studies on the carotenoid biosynthesis pathway has led to the identification of key regulatory genes that enhance color intensity and stability (Toledo-Ortiz et al., 2010). The incorporation of such genes into breeding programs has resulted in varieties with deeper and more vibrant hues (Barbaś et al., 2025). Carotenoids are essential pigments responsible for the red, orange, and yellow colors in many plants (Zhang et al., 2020). The biosynthesis of carotenoids involves several key enzymes, including phytoene synthase (PSY), which catalyzes the first committed step in the pathway, converting geranylgeranyl pyrophosphate (GGPP) into phytoene (Yuan et al., 2015). Subsequent desaturation and isomerization steps are facilitated by enzymes such as phytoene desaturase (PDS) and ζ-carotene desaturase (ZDS), leading to the production of lycopene, a precursor for various carotenoids. The expression levels of these enzymes directly influence carotenoid accumulation and the pigmentation intensity in plant tissues (Toledo-Ortiz et al., 2010). Integrating these regulatory genes into breeding programs has been a successful strategy for developing plant varieties with enhanced pigmentation (Ingkasupart et al., 2015). The overexpression of the PSY gene in transgenic plants has resulted in increased carotenoid content, leading to fruits and flowers with intensified coloration (Yuan et al., 2015). Carotenoids are significant dietary antioxidants and precursors of vitamin A, playing a crucial role in human health by supporting vision, immune function, and overall cellular protection (Sultana et al., 2025). The use of gene editing tools can be made possible by an understanding of the molecular mechanisms supporting the production and control of carotenoid pigments., such as CRISPR/Cas9, to precisely manipulate these pathways (Zhu, 2022). This precision breeding approach enables the development of new varieties with desired pigmentation traits more efficiently and sustainably, meeting both consumer preferences and nutritional needs (Ahmar et al., 2020).
4.3 Stress resistance
Stress resistance is a crucial breeding objective for marigold. Biotic stresses, including pest infestations by aphids and thrips, as well as diseases such as Alternaria blight and powdery mildew, can cause significant yield losses. Commercial marigold varieties now have better tolerance to these risks due to breeding operations that have successfully included resistance genes from wild cultivars (Babaei et al., 2021; Oyebamiji et al., 2024). Abiotic stresses, such as drought, salinity, and heavy metal contamination, also adversely affect marigold growth and essential oil quality. Elevated levels of these stresses can result in the production of reactive oxygen species (ROS), which are harmful to plant cells (Riaz et al., 2013). To counteract this, wild marigold utilizes various tolerance mechanisms, such as boosting antioxidant activity to sustain cellular redox balance, increasing lipid peroxidation to protect cell wall integrity, synthesizing secondary metabolites, and accumulating osmolytes. Understanding these physiological and biochemical responses is vital for developing stress-tolerant marigold lines (Kaushal et al., 2024). By incorporating these resistance genes into breeding programs, marigold cultivars that are more tolerant to biotic and abiotic stress have been developed for guaranteed quality and sustainable production. High temperatures, salt, and drought are examples of abiotic stressors that are equally harmful to plant growth and productivity (Guo et al., 2022). Drought stress, affects water use efficiency, photosynthetic activity, and nutrient uptake, leading to reduced flower yield and quality (Ma et al., 2020). Studies have identified drought-responsive genes that regulate key physiological and biochemical pathways, enabling the development of drought-tolerant hybrids (Kumar T. et al., 2023). Similarly, salinity-tolerant varieties are being developed by targeting traits like osmotic adjustment and ion homeostasis, ensuring stable production in saline soils (Ma et al., 2020). Emerging technologies like CRISPR-Cas9 and transcriptomics are further aiding stress-resilience breeding by enabling precise gene editing and detailed analysis of stress-responsive pathways. These tools have facilitated the identification of genes responsible for abiotic stress tolerance, paving the way for the rapid development of resilient varieties (Xu et al., 2020). CRISPR-Cas9, in particular, has revolutionized the targeted modification of genes associated with drought, salinity, and temperature stress tolerance, while transcriptomics provides a comprehensive view of the gene expression changes under various stress conditions (Xu et al., 2020; Verma et al., 2023).
4.4 Specialized objectives
Breeding programs are targeting high carotenoid content for pigment extraction and medicinal properties. Varieties with enhanced lutein and zeaxanthin content are being developed to meet industrial demands (Buchori et al., 2024). As climate change introduces new challenges, breeding programs focus on developing varieties adapted to unpredictable weather conditions, such as unseasonal rainfall and extended dry periods (Cai et al., 2024). The demand for organic flowers has spurred breeding programs to develop varieties that thrive under organic cultivation with minimal chemical inputs (Zhang et al., 2022). Breeding for unique traits like double flowers, novel petal shapes, and bi-colored varieties is gaining traction to cater to high-value markets (Zhang et al., 2022).
5 Conventional breeding in marigold
5.1 Mass selection
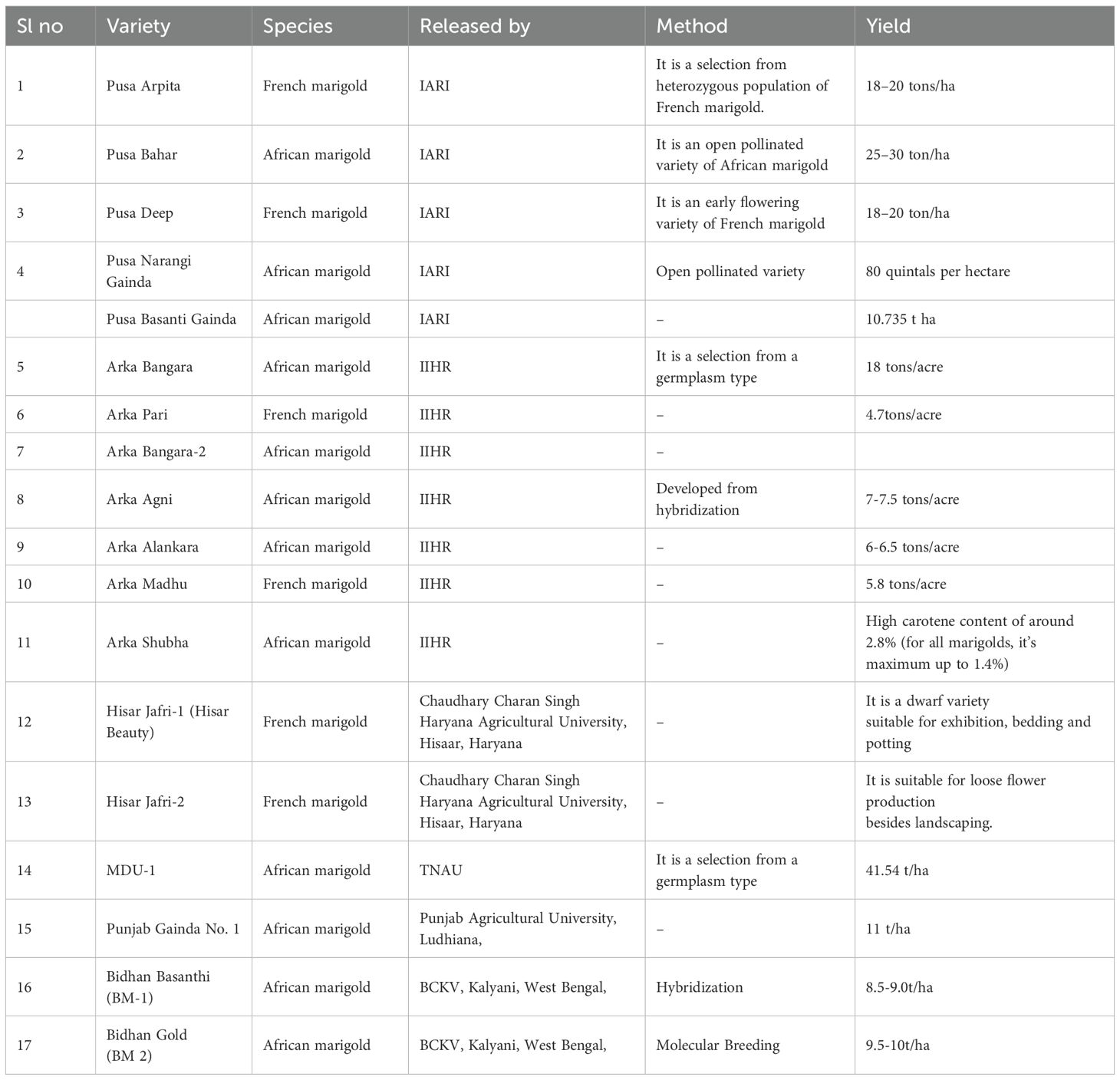
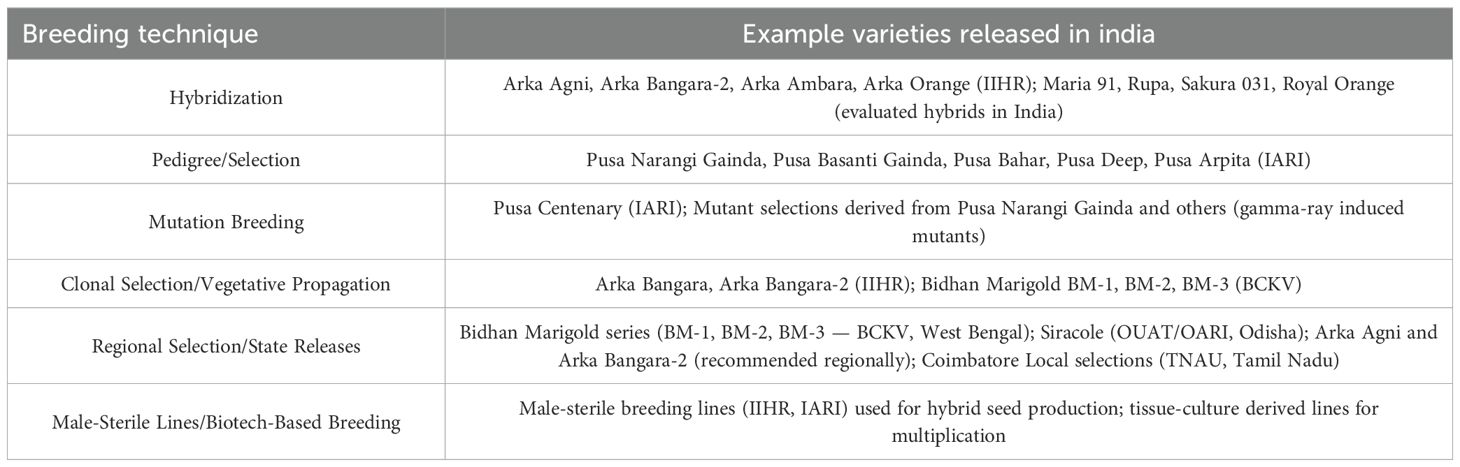
Mass selection is a traditional and straightforward breeding method used in marigold cultivation to enhance desirable traits such as flower size, color, and yield. In this approach, a large number of plants exhibiting these traits are selected, and their seeds are bulked together for the next generation (Barut et al., 2023). This method has been effective in developing open-pollinated varieties like ‘Calcutta Orange’ and ‘Calcutta Yellow,’ which are widely grown across India (Adhikari et al., 2020). The Indian Institute of Horticultural Research (IIHR) has developed marigold varieties using mass selection. One notable variety is ‘Arka Pari,’ a French marigold characterized by a dwarf growth habit and high flower production (Dulf et al., 2013). The flowers exhibit an orange hue, transitioning into various shades of orange depending on factors such as light, temperature, and the developmental stage. Blooming occurs year-round, beginning 30 days after planting and lasting for approximately nine weeks (Lenawaty et al., 2022). Furthermore, a study on the genetic diversity of African marigold genotypes assessed 33 genotypes based on 27 traits influencing yield, carotenoid levels, and lutein content. The study found that high heritability coupled with high genetic gain was observed for traits such as the number of secondary branches, fresh petal yield per flower, dry petal yield per flower, dry weight per flower, flower weight per plant, flower number per plant, zea-xanthin content, lutein content, total carotenoid content, seed number per flower, 100 seed weight, number of seeds per gram, and shelf life. This indicates that these traits are likely governed by additive gene effects, making selection effective for their improvement (Kumar A. et al., 2023). While mass selection is effective, modern breeding techniques such as marker-assisted selection (MAS) and in vitro regeneration are also being employed to enhance marigold breeding programs. For example, a study validated a SCAR marker linked to genic male sterility in marigold, facilitating MAS breeding programs (Asha et al., 2019). Furthermore, a protocol for in vitro regeneration and rapid mass multiplication of apetalous male sterile lines of marigold has been developed, enabling rapid propagation of desirable genotypes (Partap et al., 2023). Marigold varieties released through different breeding methods are listed in Table 2.
5.2 Pure-line selection
It is a crucial plant breeding method aimed at isolating and propagating genetically superior homozygous lines from a heterogeneous population. In marigold (Tagetes spp.), this technique is widely utilized to improve traits such as flower yield, carotenoid content, and plant architecture. Recent research highlights the significance of evaluating pre-breeding lines for desirable attributes (Ghosh et al., 2023; Ayiecho and Nyabundi, 2025). A study by (Singh et al., 2023) assessed various marigold lines for total carotenoid content and flower yield, identifying six pre-breeding lines with high carotenoid accumulation, which are valuable for hybrid development (da Silva and Viana, 2024; Maratovna et al., 2025). Selection techniques like Best Linear Unbiased Prediction (BLUP) and General Combining Ability (GCA) have been evaluated for their effectiveness in parental selection, with BLUP emerging as a dependable method for enhancing breeding success (McGowan et al., 2021; Anilkumar et al., 2022; Liu et al., 2024). Advancements in haploid induction methods, such as doubled haploid production through anther culture (Sharma et al., 2024), have also been investigated as a strategy to facilitate pure line development (Majumder et al., 2018b). Clonal fidelity assessment using microsatellite markers has further validated the uniformity and stability of these doubled haploid lines, reinforcing their role in ensuring genetic purity (Wang et al., 2020). In order to improve the effectiveness and precision of pure line selection in marigold breeding, these results highlight the significance of combining traditional selection techniques with molecular technologies (Majumder et al., 2018b; Biswas and Kumar, 2023; Sumalatha et al., 2023).
5.3 Pedigree selection
Pedigree selection is a breeding technique that selects superior individuals over several generations while keeping thorough records of their history in order to improve particular qualities in plants (Alemu et al., 2024; Hansen et al., 2025). In marigold (Tagetes spp.), this method is particularly useful for enhancing flower yield, color intensity, disease resistance, and growth habits (Merrick et al., 2022). The process involves selecting the best-performing plants from segregating populations and continuously evaluating their progeny over successive generations (Prasad et al., 2025). Recent studies have highlighted the efficiency of pedigree selection in marigold breeding (De, 2017; Begna, 2021). Pedigree selection has been successfully utilized to improve floral characteristics and resistance to Fusarium wilt, a major disease affecting marigold cultivation (Oyebamiji et al., 2024). It is found that focusing on high-heritability traits, such as flower size and petal count, led to substantial genetic improvements in later generations (Mekapogu et al., 2023). Molecular tools, such as SSR markers, have also been integrated into pedigree selection programs to ensure genetic purity and accelerate the breeding process (Dida, 2022). The success of pedigree selection in marigold depends on several factors, including the heritability of desired traits, the accuracy of selection, and environmental influences (Kumar T. et al., 2023). By maintaining detailed lineage records and continuously evaluating progeny, breeders can develop superior marigold varieties with enhanced ornamental and agronomic traits (Sinha et al., 2023). The integration of molecular markers further enhances selection efficiency, making pedigree selection a valuable strategy for marigold improvement (Ahmad and Anjum, 2018). Advancements in phenotyping tools have significantly enhanced the precision of selection methods in marigold (Tagetes erecta) breeding (Hansen et al., 2025). Automated imaging systems now enable breeders to quantify traits such as flower size, petal arrangement, and disease symptoms, thereby reducing subjectivity and increasing efficiency in trait selection (Gao et al., 2021; Jiang et al., 2024). For instance, hyper spectral imaging has been utilized to predict leaf nitrogen content and carbon-to-nitrogen ratios with high accuracy (Gill et al., 2022), underscoring the utility of automated imaging in assessing complex physiological traits in marigold (Ting et al., 2023).
5.4 Hybridization
Hybridization involves crossing two genetically distinct parent lines to produce offspring (F1 hybrids) that combine the best traits of both parents. Hybrid vigor, or heterosis, is commonly observed in marigold, resulting in higher yields and improved adaptability. The marigold hybrids ‘Arka Abhi,’ ‘Arka Bhanu,’ and ‘Arka Vibha’ have been developed by the ICAR-Indian Institute of Horticultural Research (IIHR) to enhance commercial cultivation (Sankar and Bharathi, 2021). These hybrids are noted for their high yield, larger flowers, and extended shelf life, making them particularly suitable for garland making, landscaping, and industrial applications. For instance, ‘Arka Vibha’ offers a shelf life of 8–9 days, which is advantageous for prolonged use in decorative purposes (Sankar and Bharathi, 2021). Hybrid seed production involves controlled pollination, including emasculation (removal of male parts) and hand pollination to prevent contamination. Male sterility systems have also been explored to simplify hybrid seed production, although they are not yet widely utilized in marigold breeding. Recent hybrids have shown resistance to pests and diseases, such as powdery mildew and nematodes, making them more sustainable for cultivation. Efforts to combine traits like drought tolerance and high carotenoid content have resulted in hybrids that are both resilient and commercially valuable.
5.5 Mutation breeding
Mutation breeding has traditionally been considered a secondary technique, it has gained prominence in recent years for creating novel genetic variability. This method uses mutagens like gamma rays and EMS (ethyl methanesulfonate) to induce changes in the DNA, leading to unique and desirable traits. Induced mutations have been used to develop marigold varieties with rare flower colors, including shades of pink and purple, expanding the ornamental appeal of marigold (Mir et al., 2023). Mutation breeding offers great potential but is a time-consuming process. The identification of useful mutations requires thorough screening and careful selection, which may involve backcrossing or repeated selection cycles.
5.6 Challenges in conventional breeding
Conventional breeding methods, though effective, face several challenges including the narrow genetic base of marigold populations in India limits the scope of selection and hybridization. Efforts to introduce genetic material from wild species like Tagetes minuta and Tagetes tenuifolia are underway but face technical challenges (Huang et al., 2024). Breeding cycles in marigold, particularly for developing hybrids or selecting pure lines, require several years to stabilize desirable traits. Conventional breeding struggles to match the rapid evolution of pests and pathogens, requiring complementary approaches such as molecular breeding.
6 Modern breeding approaches in marigold
6.1 Marker-assisted selection
Marker-Assisted Selection (MAS) is a powerful tool that leverages molecular markers to identify specific genes or regions of the genome associated with traits of interest. Breeders can efficiently select superior genotypes without relying on lengthy phenotypic evaluations by associating markers with traits like disease resistance, flower quality, and yield (Majumder et al., 2018a). Molecular markers have been used to identify Quantitative Trait Loci (QTLs) linked to key agronomic and ornamental traits in marigold, including flower size, color, and yield. This allows breeders to implement Marker-Assisted Selection (MAS) in early generations, expediting the development of high-quality varieties. For instance, the identification of a Sequence Characterized Amplified Region (SCAR) marker linked to genic male sterility in marigold has facilitated the efficient selection of parental lines in hybrid breeding program (Asha et al., 2019). In marigold, research has aimed at identifying QTLs associated with resistance to biotic stresses, including powdery mildew and nematodes, which pose significant challenges to cultivation in India. The combination of these tools in marigold breeding programs is accelerating the development of varieties with superior traits while reducing the trial and error associated with traditional breeding methods (Asha et al., 2019).
6.2 Genomic selection
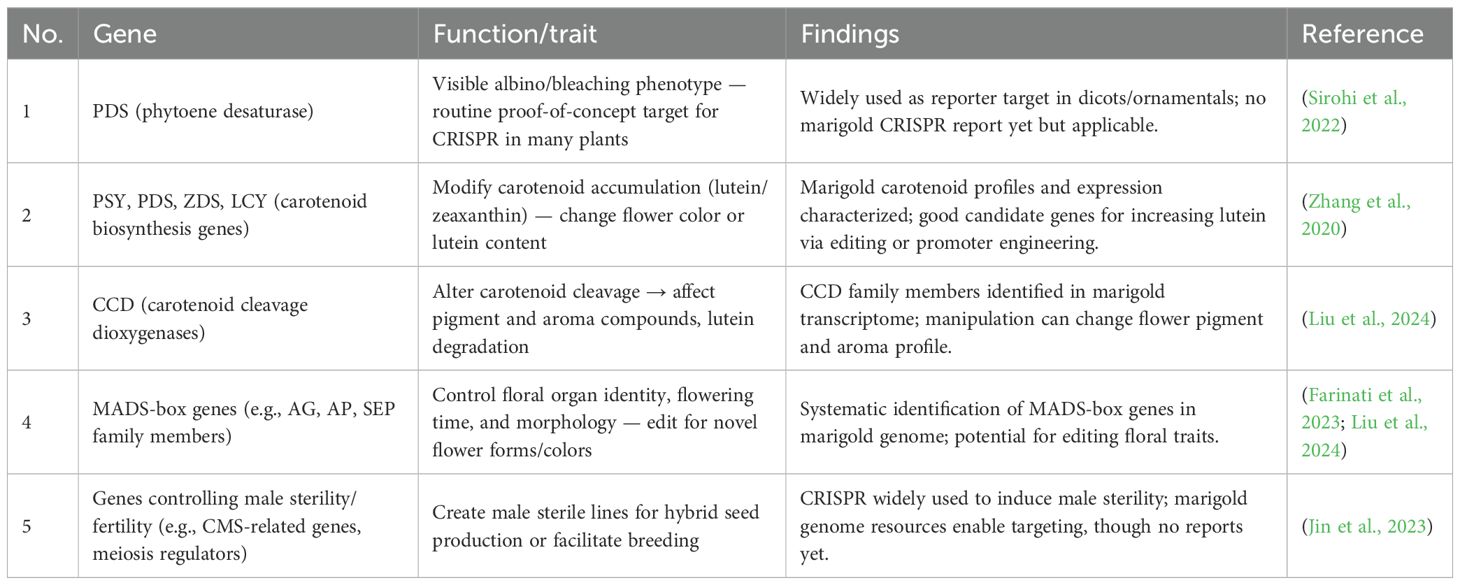
Genomic selection (GS) is an emerging tool in plant breeding that uses genomic data to predict the breeding value of individuals before they are evaluated for traits (Wang et al., 2020). This approach holds great promise for marigold, particularly in predicting traits like flower yield, carotenoid content, and abiotic stress tolerance (Lahkar et al., 2024). The integration of genomic data, such as single-nucleotide polymorphisms (SNPs), provides breeders with insights into the genetic makeup of marigold lines, enabling them to select individuals with desirable genetic profiles (Barbaś et al., 2025). Genomic selection can significantly reduce the time required to assess breeding lines by predicting their performance based on DNA markers rather than relying solely on phenotypic assessments (Tarakeshwari and Pavan, 2023; Majumder et al., 2018b). While genomic selection has been successfully applied to crops like rice and maize, its application in marigold (Tagetes erecta) breeding is still in the early stages (Sudhakaran et al., 2024). Ongoing research aims to identify effective markers for traits of interest in marigold and to develop strategies for incorporating genomic selection into existing breeding programs. In India, where marigold is a significant commercial crop, establishing a genomic selection framework could accelerate breeding progress, particularly in enhancing climate resilience and disease resistance (Mir et al., 2019). The key Genes and GM Technologies Used for Improvement of Flower Color in Marigold are tabulated in Table 3.
Table 3. Key genes and GM technologies used for improvement of flower color in marigold (Tagetes erecta).
6.3 CRISPR-Cas technology
The CRISPR-Cas9 system, a groundbreaking genome editing tool, has transformed plant breeding by enabling precise and targeted modifications of the plant genome. This approach allows for the incorporation of desirable traits without relying on traditional crossbreeding methods. The CRISPR-Cas technology presents significant potential for addressing challenges such as pest resistance, flower quality, and stress tolerance. Successful applications of CRISPR-Cas9 in ornamental plants, including modifications to flower color in Petunia hybrida, demonstrate its versatility (Nishihara et al., 2024). Recent studies have highlighted CRISPR-Cas9ability to enhance consumer-preferred traits in various crops (Verma et al., 2023), indicating that similar strategies could be explored in marigold to modify anthocyanin biosynthesis pathways and develop novel flower colors. Enhancing stress tolerance in marigold is another promising avenue for CRISPR-Cas9 technology. Introducing genes responsible for resistance to abiotic stresses such as drought and salinity could significantly improve the adaptability to harsh growing conditions. Research on other crops has demonstrated that CRISPR-mediated gene editing can strengthen responses to abiotic stress (Yadav et al., 2023), suggesting that similar methods could be applied to marigold for genetic improvement. Silencing genes linked to undesirable traits, such as short flower shelf life or reduced vase longevity, is another potential application of CRISPR. Targeting ethylene biosynthesis or signaling pathways, which are crucial to flower senescence, has been shown to extend vase life in ornamental plants (Xu et al., 2020). Implementing these techniques in marigold could enhance its commercial appeal by increasing flower longevity. Regulatory challenges currently limit the widespread adoption of CRISPR-Cas in India, but as the technology becomes more accessible and widely accepted, its potential for precision breeding in marigold will increase. Future research will aim to refine CRISPR-Cas9 protocols, minimize off-target effects, and navigate regulatory and ethical considerations (Dhanavel, 2022). The Potential Candidate Genes for CRISPR/Cas-Mediated Genome Editing in Marigold is tabulated in Table 4.
7 Tissue culture and micropropagation in marigold breeding
7.1 In vitro techniques for rapid multiplication
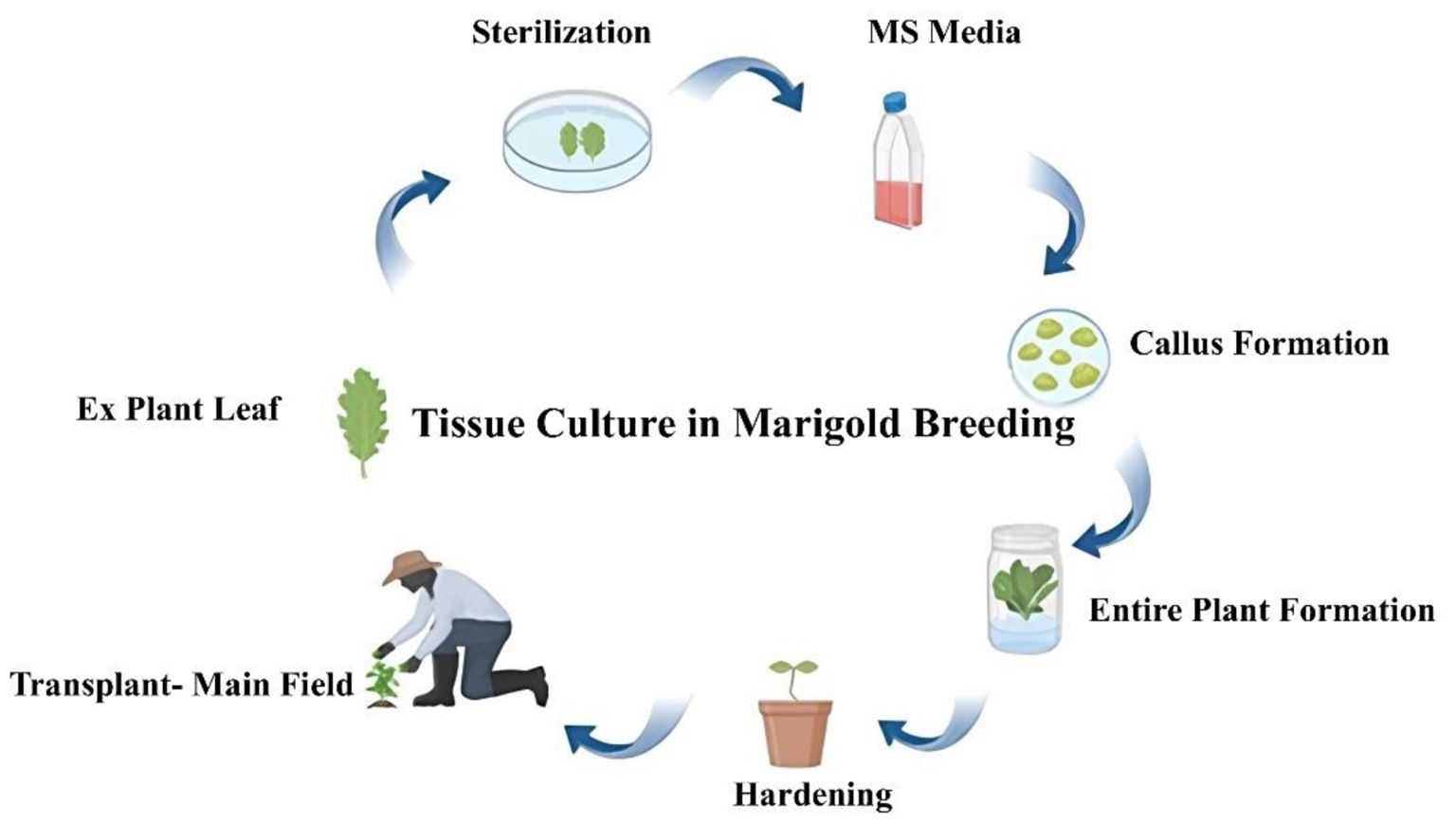
Tissue culture, specifically in vitro propagation, offers an efficient method for mass production of marigold plants. It bypasses the traditional seed propagation method, ensuring that desirable traits are consistently reproduced across generations. Recent advancements have allowed for the establishment of protocols for the regeneration of marigold from different explants such as shoot tips, leaf pieces, and flower tissues. Tissue culture and micropropagation techniques have important tools in modern marigold breeding programs. These methods offer rapid and efficient means for propagating elite genotypes, preserving genetic diversity, and enhancing breeding strategies (Kumar et al., 2018). Micropropagation has been employed extensively for the clonally multiplication of elite marigold genotypes with desired characteristics. This method allows breeders to obtain genetically uniform plants quickly and in large numbers. A key advantage of in vitro propagation is its ability to overcome the limitations of seed propagation, such as seed dormancy and low germination rates, which are sometimes seen in marigold cultivars. For instance, the use of cytokinin-based media has been shown to enhance shoot multiplication in marigold cultivars, leading to a rapid increase in plant numbers (Fibriani et al., 2023). The outline of tissue culture in marigold is shown in Figure 2.
7.2 Somaclonal variation in marigold
Somaclonal variation refers to the genetic variation observed in regenerated plants arising from changes in genetic material during in vitro culture. These variations can appear as changes in phenotypic traits, such as flower color, size, or disease resistance (Chen and Henny, 2003). The potential of somaclonal variation for developing new marigold cultivars with enhanced traits, such as improved yield, stress tolerance, and disease resistance, has been extensively studied. Somaclonal variation has been effectively utilized to develop drought-tolerant marigold lines (Mehraj et al., 2019). demonstrated that exposing marigold tissue cultures to osmotic stress using mannitol led to the selection of drought-tolerant genotypes. These selected clones exhibited higher proline and soluble sugar content, contributing to enhanced drought tolerance. Such drought-resistant lines are particularly valuable in regions facing water scarcity, ensuring sustainable marigold cultivation (Ferreira M dos, 2023). In addition to drought tolerance, somaclonal variation has also been successfully employed to develop marigold lines with enhanced pigment content, specifically higher levels of carotenoids, which are important for their ornamental and industrial value (Sarkar et al., 2016). It is also found that through somaclonal variation, marigold cultivars with significantly higher carotenoid content were developed, improving both the ornamental appeal and industrial value for uses in pharmaceuticals and natural dyes (Ferreira M dos, 2023). In chrysanthemums, has demonstrated that somaclonal variation can lead to desirable traits, including extended vase life and delayed senescence and a study on Chrysanthemum × morifolium protoplast regenerants observed significant variations in flower longevity and delayed senescence among the regenerants (Eeckhaut et al., 2020).
7.3 Applications of somaclonal variation in marigold
Somaclonal variation has played a crucial role in enhancing various traits in marigold breeding. In addition to improving stress tolerance and pigment content, it has been instrumental in increasing resistance to biotic stresses, including fungal infections, nematodes, and viral diseases. It is demonstrated that somaclonal variation in marigold resulted in greater resistance to fungal pathogens such as Fusarium oxysporum and Botrytis cinerea, both of which pose significant challenges to marigold cultivation (Dhanavel, 2021). This technique has also contributed to the economic growth of marigold farming by generating genetic diversity through tissue culture. As a result, breeders have successfully developed cultivars with novel flower colors and shapes, enhancing their market appeal. Reports indicate that somaclonal variation has led to the introduction of new marigold cultivars with distinctive flower traits, such as bi-colored petals, which cater to specialized ornamental markets (De, 2017). Somaclonal variation has also been utilized to enhance essential oil production in marigold. Studies indicate that inducing genetic variation in marigold lines has resulted in significant increases in essential oil yield, adding greater value to the flowers for the perfume and aromatherapy industries.
7.4 Challenges and limitations of somaclonal variation
While somaclonal variation presents a valuable opportunity for improving marigold traits, there are challenges associated with its application. One of the primary concerns is the stability of the traits induced through somaclonal variation. In some cases, the traits exhibited by somaclonal variants may not be stable across generations, resulting in a loss of desirable characteristics over time (Kumar, 2017). This poses a challenge for breeders who rely on stable traits for commercial production. Another limitation is the potential for undesirable traits to emerge as a result of somaclonal variation. While beneficial traits such as enhanced drought tolerance or improved pigment content may be induced, unwanted traits, such as reduced flower yield or poor plant architecture, can also emerge in the process (Zhang et al., 2020). Therefore, extensive evaluation and selection are required to ensure that somaclonal variants maintain the desired characteristics and remain stable over multiple generations. It is also emphasized the need for careful screening and long-term evaluation to ensure that somaclonal variants meet the rigorous standards required for commercial marigold production (Cicevan et al., 2022).
8 Achievements in marigold breeding
8.1 High-yielding varieties
Marigold breeding in India has achieved significant milestones over the years, especially in areas such as yield enhancement, quality improvement, and stress tolerance. The integration of both conventional and modern breeding techniques has led to the development of marigold cultivars that meet the increasing demands of the ornamental and pharmaceutical industries (Tang et al., 2020). One of the main goals of Indian breeding initiatives has been to create marigold cultivars with high yields. As the demand for marigold flowers continues to rise, particularly for use in religious ceremonies, decorations, and the extraction of carotenoids for industrial purposes, there is a growing need for varieties that offer high production efficiency and consistent yield (Youssef et al., 2020). One of the notable achievements in this area is the release of hybrid varieties such as ‘Arka Bangara,’ ‘Arka Bangara-2,’ and ‘Arka Agni,’ which have demonstrated significantly higher flower yields compared to traditional cultivars. It is reported that these hybrids have been specifically bred to enhance flower size, flower count per plant, and overall yield per hectare, contributing to greater productivity in marigold cultivation (Kumar et al., 2019; Panda et al., 2022). Furthermore, these varieties are known for their improved disease resistance, which has enhanced their adaptability in various climatic conditions across India. Breeding for high yield involves the selection of plants with desirable traits, such as larger flowers and increased branching (Kumar et al., 2019). Recent advancements in hybridization techniques have enabled the development of marigold cultivars that not only exhibit higher yields but also have enhanced shelf life, making them more commercially viable. It is highlighted the potential of hybrid vigor in marigold, where F1 hybrids display superior growth performance and resistance to biotic stresses (Modi et al., 2009). These hybrids have been widely adopted in marigold production, especially in states like Karnataka, Maharashtra, and Tamil Nadu, where marigold farming is economically significant. Different breeding techniques and Marigold varieties released in India are tabulated in Table 1 and Table 5. Different conventional breeding techniques followed in Marigold is shown in Figure 3.
8.2 Quality enhancement
Breeding programs in India have also focused on improving the quality of marigold flowers, especially their aesthetic and biochemical properties. Market demands for high-quality marigold flowers with improved color, size, and shelf life have led to the development of several superior cultivars (Lenawaty et al., 2022). Quality traits such as vibrant color, longer vase life, and higher carotenoid content have been prioritized in recent breeding efforts. Varieties like ‘Arka Shubha,’ known for its high carotenoid content, have been developed with industrial applications in mind. These varieties offer high yields of carotenoids, which are important for use in the pharmaceutical, food, and cosmetic industries (Ördögh, 2021). It is found that ‘Arka Shubha’ produced significantly higher levels of lutein and zeaxanthin, which are valuable carotenoids known for their antioxidant properties. These carotenoids have widespread applications in supplements, skincare products, and natural food colorants (Udchachon et al., 2021). In addition to carotenoid enhancement, flower characteristics such as color, size, and shelf life are crucial for meeting the demands of the cut flower industry. Research has led to the development of marigold cultivars with improved flower colors, ranging from deep orange to bi-colored variants, which cater to different market preferences. Ethylene, a plant hormone, plays a pivotal role in regulating processes such as flower wilting and petal drop. Inhibiting ethylene perception or action has been shown to extend the longevity of cut flowers and reduce post-harvest losses. The use of 1-methylcyclopropene (1-MCP), an ethylene action inhibitor, has successfully extended the vase life of several ornamental species by delaying ethylene-triggered aging (Baron et al., 2021). Recent studies have demonstrated that continuous application of 1-MCP during transport and storage can further enhance the post-harvest quality by blocking ethylene action and reducing botrytis incidence. Combining 1-MCP with antimicrobial agents, such as ajowan essential oil or silver nanoparticles, has been found to synergistically improve vase life and delay senescence in gerbera cut flowers (Baron et al., 2021).
8.3 Stress tolerance
Abiotic stress tolerance, particularly drought resistance, has been a major focus of marigold breeding programs due to the increasing unpredictability of weather patterns and water scarcity issues in many marigold-growing regions (Mohammed, 2025). Marigold, being a drought-sensitive plant, faces significant challenges in maintaining growth and flower production under water-limited conditions. However, recent research has led to the development of marigold lines exhibiting improved drought tolerance, a critical achievement in ensuring stable production (Hemmati et al., 2018). Research showed that drought stresses result in physiological and chemical changes in marigold plants, such as decreased production of essential oils and flower yield. In response to these challenges, breeders have worked to develop drought-tolerant varieties through conventional selection and genetic improvement (Riaz et al., 2013). The physiological responses of marigold to drought stress and identified key traits associated with drought tolerance, such as improved water use efficiency, osmotic adjustment, and increased root biomass (Eghlima et al., 2024). By selecting and propagating plants with these traits, they were able to develop marigold cultivars that are more resilient under drought conditions (Srinivasan and Jeyakumar, 2018). These cultivars not only exhibit better growth under limited water availability but also produce higher yields of essential oils, which is a major economic benefit. Moreover, the development of marigold cultivars with enhanced tolerance to salinity has also been an area of focus. It is found that marigold varieties like ‘Arka Agni’ exhibit superior tolerance to salinity stress, maintaining better physiological functioning and flower yield under saline conditions (Moradian et al., 2023). This achievement is important for regions where soil salinity is a limiting factor in agricultural productivity.
9 Challenges in marigold breeding
The narrow genetic diversity among cultivated marigold varieties remains a significant obstacle for breeders. Most commercial marigold varieties are derived from a limited pool of germplasm, which restricts the genetic variability available for breeding programs. This lack of diversity restricts the development of new traits, including disease resistance and tolerance to abiotic stresses (Bakshi and Ghosh, 2022). Efforts to incorporate wild germplasminto breeding programs are ongoing to broaden the genetic base. Wild relatives are valuable sources of traits such as pest resistance, higher carotenoid content, and adaptability to harsh environments (Patel et al., 2018). Moreover, preliminary breeding activities, including the development of intermediary populations, are essential for integrating wild germplasm into breeding programs. The production of marigolds is being challenged by climate change, which increases abiotic factors like heat, salinity, and drought. Marigold plants are sensitive to temperature fluctuations, which can affect flower size, color, and yield. Drought stress, in particular, has been shown to reduce essential oil content and overall plant vigor (Sachin and Homraj, 2021). To mitigate these effects, breeders are focusing on developing climate-resilient varieties. The primary challenge lies in simultaneously improving stress tolerance and maintaining high yield and flower quality. Marker-assisted selection (MAS) and genomic selection are being explored to accelerate the development of climate-resilient marigold varieties (Krzymińska et al., 2022). Marigold is susceptible to a range of pests and diseases, including Alternaria blight and aphids, which can cause significant yield losses. The limited genetic base has constrained the development of resistant varieties. Incorporating resistance genes from wild species and utilizing molecular tools like CRISPR-Cas for targeted genome editing are promising strategies (Shafiee et al., 2023).
10 Future prospectives
The future of marigold breeding holds exciting prospects, driven by advancements in genomics, biotechnology, and sustainable farming practices. One of the primary focuses will be the integration of genomic tools such as marker-assisted selection (MAS) and CRISPR-based genome editing, allowing breeders to develop marigold varieties with specific traits like enhanced carotenoid content and stress tolerance (Namita et al., 2013). The breeding of climate-resilient varieties will become increasingly important to address challenges like drought, salinity, and extreme temperature fluctuations. These varieties will be designed to thrive under changing environmental conditions, ensuring consistent production in diverse climates. Development of marigolds with pest and disease resistance will be a priority, especially in response to rising threats from pests like aphids and diseases such as powdery mildew (Burlec et al., 2021). Marigold breeding will also adapt to the growing demand for organic and sustainable practices, developing varieties suitable for low-input systems and resistant to organic pest control methods. Carotenoid production is also a key focus, as marigolds serve as a major source of natural pigments used in food, pharmaceuticals, and cosmetics (Aswath and Aswath, 2018). Breeders will focus on developing cultivars with higher carotenoid yields without compromising flower quality or stress resistance. Floricultural innovations will serve the ornamental market by producing marigolds with unique flower shapes, bi-colored petals, and improved post-harvest quality, such as longer shelf life and enhanced vase life. Advances in artificial intelligence (AI) and high-throughput phenotyping will streamline breeding efforts, enabling quicker identification of desirable traits and more precise breeding decisions. In the future, marigold breeding will integrate modern technology and environmentally friendly methods to satisfy the changing demands of the medicinal, agricultural, and ornamental sectors, providing a robust and superior crop for a variety of uses.
11 Conclusion
Marigold breeding in India has made significant progress over the years, contributing to the enhancement of yield, flower quality, and resistance to pests and diseases. As one of the most economically and culturally important ornamental crops, marigold holds immense potential for expanding its role in both domestic and international markets. However, challenges such as limited genetic diversity, the effects of climate change, and the need for improved pest and disease resistance continue to impede the full realization of its potential. The limited genetic diversity of cultivated marigold remains a major challenge, restricting opportunities for further enhancement. Modern breeding technologies, including marker-assisted selection (MAS) and genomic selection, provide valuable tools for accelerating genetic improvement in marigold. By identifying quantitative trait loci (QTLs) associated with desirable characteristics, these methods enhance selection efficiency. Genome editing techniques such as CRISPR-Cas offer the ability to introduce precise genetic modifications, strengthening marigold’s resistance to both biotic and abiotic stresses. Tissue culture and micro propagation have also proven beneficial in the rapid multiplication of elite genotypes, with the exploitation of somaclonal variation contributing to the development of marigold variants with enhanced stress tolerance and improved pigmentation. Developing climate-resilient varieties capable of withstanding these environmental challenges is a priority for future breeding programs. In conclusion, while considerable progress has been made in marigold breeding, ongoing research and the integration of modern breeding techniques are crucial to overcoming current challenges. Expanding genetic diversity, enhancing climate resilience, and improving pest and disease resistance, marigold breeding will play a pivotal role in ensuring the long-term sustainability and profitability of marigold cultivation in India.
Author contributions
VD: Writing – original draft, Methodology, Software, Data curation, Investigation, Writing – review & editing, Conceptualization. RS: Supervision, Writing – original draft, Formal Analysis, Resources, Funding acquisition, Validation, Project administration, Writing – review & editing, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors express their sincere gratitude to Vellore Institute of Technology, Vellore, Tamil Nadu, India for their valuable support and assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The authors utilized generative AI tools, such as Grammarly and ChatGPT, to perform basic grammar checks on their manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adhikari, P., Tripathi, K., Marasini, S., Neupane, R., Shrestha, A., Shrestha, J., et al. (2020). Effect of different doses of nitrogen on growth and yield of marigold (Tagetes erecta L.) in subtropical climate of Nepal. Fundam. Appl. Agric. 5 (3), 414–420. doi: 10.5455/faa.108327
Adhikary, K., Mondal, T., Majumder, J., Chowdhuri, T. K., Mukherjee, S., and Maherukh, K. (2025). Examine the impact of green-synthesized nanomaterials on the germination rates and seedling characteristics of African Marigold (Tagetes erecta L. var. Pusa Narangi Ganda and Pusa Basanti Ganda). Heliyon 11 (3). doi: 10.1016/j.heliyon.2025.e42319
Ahmad, R. and Anjum, M. A. (2018). Applications of molecular markers to assess genetic diversity in veget able and ornamental crops-A review. J. Hortic. Sci. Technol. 1 (1), 1–7. doi: 10.46653/jhst
Ahmar, S., Gill, R. A., Jung, K. H., Faheem, A., Qasim, M. U., Mubeen, M., et al. (2020). Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 21 (7), 2590. doi: 10.3390/ijms21072590
Akhtar, G., Faried, H. N., Razzaq, K., Ullah, S., Wattoo, F. M., Shehzad, M. A., et al. (2022). Chitosan-induced physiological and biochemical regulations confer drought tolerance in pot marigold (Calendula officinalis L.). Agronomy 12 (2), 474. doi: 10.3390/agronomy12020474
Alemu, A., Åstrand, J., Montesinos-Lopez, O. A., y Sanchez, J. I., Fernandez-Gonzalez, J., Tadesse, W., et al. (2024). Genomic selection in plant breeding: Key factors shaping two decades of progress. Mol. Plant 17, 552–578. doi: 10.1016/j.molp.2024.03.007
Anilkumar, C., Sunitha, N. C., Harikrishna, Devate, N. B., and Ramesh, S. (2022). Advances in integrated genomic selection for rapid genetic gain in crop improvement: a review. Planta 256, 87. doi: 10.1007/s00425-022-03996-y
Asha, K. M., Sane, A., Swini, T., Reddy, D. C. L., Patil, S. R., Cholin, S. S., et al. (2019). Validation of SCAR marker linked to genic male sterility in marigold: as a forward step towards marker assisted breeding programme. Int. J. Curr. Microbiol. Appl. Sci. 8, 3373–3383. doi: 10.20546/ijcmas.2019.802.393
Aswath, C. and Aswath, C. (2018). Effect of plant growth regulators and sucrose concentration on callus induction and shoot differentiation from ovary culture of marigold (Tagetes spp). Int. J. Chem. Stud. 6, 618–623.
Ayiecho, P. O. and Nyabundi, J. O. (2025). Development of pure line varieties. Conventional Contemp. Practices Plant Breeding Springer; p, 157–173. doi: 10.1007/978-3-031-74998-8
Babaei, K., Moghaddam, M., Farhadi, N., and Ghasemi Pirbalouti, A. (2021). Morphological, physiological and phytochemical responses of Mexican marigold (Tagetes minuta L.) to drought stress. Sci. Hortic. 284, 110116. doi: 10.1016/j.scienta.2021.110116
Bakshi, L. and Ghosh, R. (2022). Marigold biopesticide as an alternative to conventional chemical pesticides. J. Adv. Sci. Res. 13, 26–33. doi: 10.55218/jasr.202213503
Barbaś, P., Sawicka, B., Pszczółkowski, P., and Krochmal-Marczak, B. (2025). Application of biotechnological techniques in the breeding and sustainable production of marigold (Tagetes spp.). Breeding of Ornamental Crops. Annuals Cut Flowers 6, 297–330.
Baron, F., Mendoza, R., Melo, S. E., Clavijo, J., and Castellanos, D. A. (2021). Evaluation and representation of ethylene effect on vase life and quality of rose (Rosa hybrida) cv. Vendela. Acta Physiol. Plant. 43 (12), 161.
Barut, M., Ls, T., and Karaman, Ş. (2023). Unveiling the phytochemical variability of fatty acids in world marigold (Calendula officinalis L.) germplasm affected by genotype. Int. J. Agric. Environ. Food Sci. 7, 639–649. doi: 10.31015/jaefs.2023.3.18
Begna, T. (2021). Conventional breeding methods widely used to improve self-pollinated crops. Int. J. Res. 7, 1–16.
Biswas, P. and Kumar, N. (2023). “Application of molecular markers for the assessment of genetic fidelity of in vitro raised plants: Current status and future prospects,” in Molecular marker techniques. (Singapore: Springer Nature), 233–256.
Buchori, A., Sanjaya, I. P. W., Putra, R. P., Sukma, D., Suprapta, D. N., Syukur, M., et al. (2024). Assessment of tagetes patula mutants and its wild type for flower morphology, polyphenol contents, and antioxidant activity. SABRAO J. Breed Genet. 56, 1147–1158. doi: 10.54910/sabrao2024.56.3.21
Burlec, A. F., Pecio, Ł, Kozachok, S., Mircea, C., Corciovă, A., Vereştiuc, L., et al. (2021). Phytochemical profile, antioxidant activity, and cytotoxicity assessment of Tagetes erecta L. flowers. Molecules 26 (5), 1201. doi: 10.3390/molecules26051201
Cai, X., Wu, J., Lian, Y., Yang, S., Xue, Q., Li, D., et al. (2024). Characterization and discrimination of marigold oleoresin from different origins based on UPLC-QTOF-MS combined molecular networking and multivariate statistical analysis. Metabolites 14 (4), 225. doi: 10.3390/metabo14040225
Chen, J. and Henny, R. J. (2003). Somaclonal variation as a source for cultivar development of ornamental aroids.
Cicevan, R., Sestras, A. F., Plazas, M., Boscaiu, M., Vilanova, S., Gramazio, P., et al. (2022). Biological Traits and Genetic Relationships Amongst Cultivars of Three Species of Tagetes (Asteraceae). Plants 11 (6), 760. doi: 10.3390/plants11060760
Cooper, M. and Messina, C. D. (2023). Breeding crops for drought-Affected environments and improved climate resilience. Plant Cell 35, 162–186. doi: 10.1093/plcell/koac321
da Silva, J. P. A. and Viana, J. M. S. (2024). Efficiency of genomic selection for developing superior pure lines. doi: 10.21203/rs.3.rs-4889071/v1
De, L. (2017). Improvement of ornamental plants - A review. Int. J. Horticulture. doi: 10.5376/ijh.2017.07.0022
Dedhia, L. (2024). Performance evaluation of marigold pre-breeding lines for total carotenoids content, flower yield and associated traits. J. Ornamental Horticulture 27, 82–89. doi: 10.5958/2249-880X.2024.00012.X
Dhanavel, D. (2021). Induced physical and chemical mutagenesis on Marigold (Tagetes erecta L.) to determine the lethality, germination and seedling survivability. Int. J. Bot. Stud. 6 (3), 235–237.
Dhanavel, D. (2022). Germination studies on combined mutagenic treatment employing gamma rays and EMS in african marigold (Tagetes erecta L.). Indian J. Nat. Sci. 12 (70), 0976–0997.
Dida, G. (2022). Molecular markers in breeding of crops: recent progress and advancements. Int. J. Novel Res. Life Sci. 9, 10–21. doi: 10.5281/zenodo.7057206
Dulf, F. V., Pamfil, D., Baciu, A. D., and Pintea, A. (2013). Fatty acid composition of lipids in pot marigold (Calendula officinalis L.) seed genotypes. Chem. Cent J. 7 (1), 8. doi: 10.1186/1752-153X-7-8
Eeckhaut, T., Van Houtven, W., Bruznican, S., Leus, L., and Van Huylenbroeck, J. (2020). Somaclonal variation in chrysanthemum × morifolium protoplast regenerants. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.607171
Eghlima, G., Mohammadi, M., Ranjabr, M. E., Nezamdoost, D., and Mammadov, A. (2024). Foliar application of nano-silicon enhances drought tolerance rate of pot marigold (Calendula officinalis L.) by regulation of abscisic acid signaling. BMC Plant Biol. 24 (1), 1220. doi: 10.1186/s12870-024-05986-6
Farinati, S., Draga, S., Betto, A., Palumbo, F., Vannozzi, A., Lucchin, M., et al. (2023). Current insights and advances into plant male sterility: new precision breeding technology based on genome editing applications. Front. Plant Sci. 14, 1223861. doi: 10.3389/fpls.2023.1223861
Ferreira M dos, S. (2023). Rocha A de J, Nascimento F dos S, Oliveira WD dos S, Soares JM da S, Rebouças TA, et al. The Role of Somaclonal Variation in Plant Genetic Improvement: A Systematic Review. Agronomy 13 (3), 730. doi: 10.3390/agronomy13030730
Fibriani, S., Studi, P. D., Agribisnis, M., and Negeri Jember, P. (2023). Callus induction of marigold plants (Tagetes erecta L) with a combination of 2,4D and kinetin. Int. J. Economic Technol. Soc. Sciencesinjects 4, 79–85.
Gao, T., Zhu, F., Paul, P., Sandhu, J., Doku, H. A., Sun, J., et al. (2021). Novel 3D imaging systems for high-throughput phenotyping of plants. Remote Sens (Basel) 13, 2113. doi: 10.3390/rs13112113
Ghosh, S., Maherukh, K., and Ghosh, T. (2023). Breeding of annual flower crops: A potential area to explore. Agrobios Newsletter, Jodhpur. 53, 28.
Gill, T., Gill, S. K., Saini, D. K., Chopra, Y., de Koff, J. P., and Sandhu, K. S. (2022). A comprehensive review of high throughput phenotyping and machine learning for plant stress phenotyping. Phenomics 2, 156–183. doi: 10.1007/s43657-022-00048-z
Godoy-Hernández, G., Berzunza, E. A., Concha, L. C., and de Lourdes Miranda-Ham, M. (2006). Agrobacterium-mediated transient transformation of marigold (Tagetes erecta). Plant Cell Tissue Organ Cult 84, 365–368. doi: 10.1007/s11240-005-9031-9
González-Morales, S., Solís-Gaona, S., Valdés-Caballero, M. V., Juárez-Maldonado, A., Loredo-Treviño, A., and Benavides-Mendoza, A. (2021). Transcriptomics of biostimulation of plants under abiotic stress. Front. Genet. 12. doi: 10.3389/fgene.2021.583888
Guo, J., Shan, C., Zhang, Y., Wang, X., Tian, H., Han, G., et al. (2022). Mechanisms of salt tolerance and molecular breeding of salt-tolerant ornamental plants. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.854116
Gupta, V. and Ur Rahman, L. (2015). An efficient plant regeneration and Agrobacterium-mediated genetic transformation of Tagetes erecta. Protoplasma 252, 1061–1070. doi: 10.1007/s00709-014-0740-y
Hansen, L. S., Bouwman, A. C., Sahana, G., Slagboom, M., Nielsen, H. M., and Ellen, E. D. (2025). Comparative evaluation of phenotypic, pedigree, and family-based selection in insect breeding using stochastic simulation. Animal 19, 101475. doi: 10.1016/j.animal.2025.101475
Hemmati, K., Ebadi, A., Khomari, S., and Sedghi, M. (2018). Influence of ascorbic acid and 24-epibrassinolide on physiological characteristics of pot marigold under water-stress condition*. J. Plant Interact. 13, 364–372. doi: 10.1080/17429145.2018.1483033
Huang, W. Q., Meng, C., Zhang, L., Xu, F., Yang, X. M., Zhang, L. F., et al. (2024). A novel approach: enhancing marigold (Tagetes erecta L.) genetic transformation through seed priming technology. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1509720
Ingkasupart, P., Manochai, B., Song, W. T., and Hong, J. H. (2015). Antioxidant activities and lutein content of 11 marigold cultivars (Tagetes spp. ) grown Thailand. Food Sci Technol. (Brazil) 35, 380–385. doi: 10.1590/1678-457X.6663
Jiang, K., Chen, C., Jiang, G., Chi, Y., Xu, C., Kong, L., et al. (2024). Genetic improvement of oysters: current status, challenges, and prospects. Rev. Aquac 16, 796–817. doi: 10.1111/raq.12868
Jin, C., Dong, L., Wei, C., Wani, M. A., Yang, C., Li, S., et al. (2023). Creating novel ornamentals via new strategies in the era of genome editing. Front. Plant Sci. 14, 1142866. doi: 10.3389/fpls.2023.1142866
Joshi, A., Kumar, A., Adhikari, S., and Saroj, R. (2024). Marker-assisted selection: strategy for biotic and abiotic stress tolerance in plants. Climate-resilient Agric. p, 137–191.
Kaushal, N., Kashyap, B., and Dilta, B. S. (2024). Research on cuttings and flower yield improvement of marigold cv. Siracole for sustainable production through Jeevamrit application. Heliyon 10 (23), e40567. doi: 10.1016/j.heliyon.2024.e40567
Krzymińska, A., Frąszczak, B., Gąsecka, M., Magdziak, Z., and Kleiber, T. (2022). The content of phenolic compounds and organic acids in two tagetes patula cultivars flowers and its dependence on light colour and substrate. Molecules 27 (2), 527. doi: 10.3390/molecules27020527
Kumar, K. R. (2017). Studies on plant regeneration in marigold (Tagetes spp.) through in vitro culture of male and female gametophytes. Plant Cell Tissue Organ Cult. 143 (3), 549–564.
Kumar, A., Gautam, R. D., Kumar, A., Bisht, A., and Singh, S. (2020). Floral biology of wild marigold (Tagetes minuta L.) and its relation to essential oil composition. Ind. Crops Prod 145, 111996. doi: 10.1016/j.indcrop.2019.111996
Kumar, K. R., Pal Singh, K., Raju, D. V. S., Panwar, S., Bhatia, R., tia, B., et al. (2018). Standardization of in vitro culture establishment and proliferation of micro-shoots in african and french marigold genotypes. Int. J. Curr. Microbiol. Appl. Sci. 7, 2768–2781. doi: 10.20546/ijcmas.2018.701.332
Kumar, K. R., Singh, K. P., Raju, D. V. S., Bhatia, R., and Panwar, S. (2019). Influence of genotypes, growth regulators and basal media on direct differentiation of shoot buds from leaf segments of marigold (Tagetes spp.). Indian J. Exp. Biol. 57 (2), 30–39.
Kumar, A., Raza Jamali, A., Fatima Miano, T., Lal, R., Wahab Soomro, A., Suthar, M., et al. (2023). Response of farmyard manure (FYM) on growth and flowering of different marigold (Tagetes erecta) varieties. Am. J. Plant Biol. doi: 10.11648/j.ajpb.20230802.12
Kumar, T., Yadav, D., Kumar, N., and Dansena, V. (2023). A review of breeding for abiotic stress tolerance in ornamental crop. Int. J. Advanced Biochem. Res. 7 (2S), 194–199. doi: 10.33545/26174693.2023.v7.i2sc.209
Lahkar, C., Singh, S. K., Baruah, A. R., and Borkakati, R. P. (2024). Assessment of genetic purity in african marigold (Tagetes erecta) hybrids using microsatellite markers. Agric. Res. 13, 1–9. doi: 10.1007/s40003-023-00669-4
Lenawaty, D. Y., Sukma, D., Syukur, M., Suprapta, D. N., Nurcholis, W., and Aisyah, S. I. (2022). Increasing the diversity of marigold (Tagetes sp.) by acute and chronic chemical induced mutation of EMS (Ethyl Methane Sulfonate). Biodiversitas 23, 1399–1407. doi: 10.13057/biodiv/d230326
Liu, C., Wang, F., Li, R., Zhu, Y., Zhang, C., and He, Y. (2024). Marigold (Tagetes erecta) MADS-box genes: A systematic analysis and their implications for floral organ development. Agronomy 14, 1889. doi: 10.3390/agronomy14091889
Ma, Y., Dias, M. C., and Freitas, H. (2020). Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.591911
Majumder, J., Singh, S., Kumari, M., and Verma, M. (2018a). Variability and correlation studies on induced mutants of marigold (Tagetes erecta L.) for different traits and assessing them using molecular markers. Plant Tissue Cult Biotechnol. 28, 223–236. doi: 10.3329/ptcb.v28i2.39681
Majumder, J., Singh, S. K., and Verma, M. (2018b). Assessment of Mutation in Marigold (Tagetes erecta L.) using Morphological and Molecular Markers. Int. J. Curr. Microbiol. Appl. Sci. 7, 2588–2597. doi: 10.20546/ijcmas.2018.707.303
Maratovna, K. G., Qurbanalievich, K. A., and Aytmuratovna, M. T. (2025). Population and pure lines concept. Am. J. Of Multidiscip. Bull. 3, 90–94.
McGowan, M., Wang, J., Dong, H., Liu, X., Jia, Y., Wang, X., et al. (2021). Ideas in genomic selection with the potential to transform plant molecular breeding: a review. Plant Breed Rev. 45, 273–319.
Mehraj, U., Panwar, S., Kanwar, P. S., Namita, R. P., Amolkumar, U. S., Mallick, N. I. H. A. R. I. K. A., et al. (2019). Assessment of clonal fidelity of doubled haploid line of marigold (Tagetes erecta) using microsatellite markers. Indian J. Agric. Sci. 89, 1162–1166. doi: 10.56093/ijas.v89i7.91690
Mekapogu, M., Song, H. Y., Lim, S. H., and Jung, J. A. (2023). Genetic engineering and genome editing advances to enhance floral attributes in ornamental plants: an update. Plants 12 (23), 3983. doi: 10.3390/plants12233983
Merrick, L. F., Herr, A. W., Sandhu, K. S., Lozada, D. N., and Carter, A. H. (2022). Optimizing plant breeding programs for genomic selection. Agronomy 12, 714. doi: 10.3390/agronomy12030714
Mir, R. A., Ahanger, M. A., and Agarwal, R. M. (2019). Marigold: from mandap to medicine and from ornamentation to remediation. Am. J. Plant Sci. 10 (02), 309–338. doi: 10.4236/ajps.2019.102024
Mir, R. A., Irshad, S., Argal, S., Agarwal, R. M., and Khatoon, S. (2023). Quantitative analysis of polyphenolic compounds in two different cultivars of marigold (Tagetes erecta L.) using high-performance thin-layer chromatography. Front. Horticulture 2. doi: 10.3389/fhort.2023.1120267
Modi, P., Sinha, A., and Kothari, S. L. (2009). Floriculture and ornamental biotechnology reduction of hyperhydricity in micropropagated french marigold (Tagetes patula L.) plants by modified medium parameters. Floriculture and Ornamental Biotechnology 3 (1), 40–45.
Mohammed, L. S. (2025). Growth and flowering responses of two marigold species under water stress conditions with uses different mulches. Pakistan J. Life Soc. Sci. (PJLSS) 23 (1). doi: 10.57239/PJLSS-2025-23.1.00553
Moradian, T., Ghanbari, R., Seraj, M., and Asgharzade, A. (2023). Salicylic acid and boric acid improve flower growth, yield, and cold tolerance in French marigold (Tagetes patula). Iran. J. Plant Physiol. 13 (4), 4741–4751.
Namita, P. S., Sonah, H., Singh, S. P., and Sharma, T. R. (2013). Genetic diversity analysis of marigold (Tagetes sp) genotypes using RAPD and ISSR markers. Indian J. Agric. Sci. 83 (5), 484–490.
Narushima, M., Uesugi, M., Murai, Y., Katayama, Y., Iimura, Y., and Kajita, S. (2017). In vitro regeneration and Agrobacterium-mediated transformation of male-sterile marigold (Tagetes erecta L. ). Plant Biotechnol. 34, 125–129.
Nishihara, M., Hirabuchi, A., Teshima, T., Uesugi, S., and Takahashi, H. (2024). Flower color modification in Torenia fournieri by genetic engineering of betacyanin pigments. BMC Plant Biol. 24 (1), 614. doi: 10.1186/s12870-024-05284-1
Ördögh, M. (2021). The effect of different substrate on the morphological characteristics of hungarian tagetes patula cultivars. Acta Biol. Marisiensis 4, 73–82. doi: 10.2478/abmj-2021-0007
Oyebamiji, Y. O., Adigun, B. A., Shamsudin, N. A. A., Ikmal, A. M., Salisu, M. A., Malike, F. A., et al. (2024). Recent advancements in mitigating abiotic stresses in crops. Horticulturae 10 (2), 156. doi: 10.3390/horticulturae10020156
Panda, S., Grihalakshmi, K., Barman, S., and Pramita Mishra, P. (2022). Production and marketing of marigold in gajapati district, odisha, India: challenges and opportunities. Plant Arch. 22, 292–296. doi: 10.51470/plantarchives.2022.v22.no2.050
Pangaribuan, O., Hanafiah, D. S., Setiado, H., Simamora, J. R. M., and Sari, N. W. (2022). Quantity and quality test of DNA marigold plants (Tagetes erecta L.) for the suistainability of plant breeding. IOP Conf Ser. Earth Environ. Sci. 977 (1), 012047. doi: 10.1088/1755-1315/977/1/012047
Panwar, S., Singh, K. P., Janakiram, T., Bhardwaj, C., Banyal, N., Sonah, H., et al. (2017). Molecular characterization of African marigold (Tagetes erecta) genotypes using RAPD markers. Indian J. Agric. Sci. 87 (5), 663–668. doi: 10.56093/ijas.v87i5.70190
Partap, M., Verma, V., Thakur, M., and Bhargava, B. (2023). Designing of future ornamental crops: a biotechnological driven perspective. Hortic. Res. 10 (11), uhad192. doi: 10.1093/hr/uhad192
Patel, M. A., Chawla, S. L., Chauhan, D. A., Patil, S., and Chhatrola, H. N. (2018). Character association and path analysis studies in marigold (Tagetes spp.) under the South Gujarat region. ~ 3576 ~ J. ournal Pharmacognosy Phytochem. 7, 3576–3580.
Poulose, B., Paliwal, A., Bohra, M., Punetha, P., and Bahuguna, P. (2020). Namita. Character association and path analysis of quantitative traits among marigold (Tagetes sp.) genotypes. Indian J. Agric. Sci. 90, 2362–2368. doi: 10.56093/ijas.v90i12.110342
Prasad, S., Nath, P., Kumar, A., Choudhary, G., Kumari, A., Kumar, M., et al. (2025). Ecological association and diversity of self-recruiting wild fish species in aquacrop systems of northeastern bihar, India. J. Biol. Nat. 17, 214–221. doi: 10.56557/joban/2025/v17i19341
Riaz, A., Younis, A., Riaz Taj, A., Karim, A., Tariq, U., Munir, S., et al. (2013). Effect of drought stress on growth and flowering of marigold (Tagetes erecta L.). Pak. J. Bot. 45 (S1), 123–131.
Sachin, T. M. and Homraj, S. (2021). A review of marigold’s beneficial aspects. Pharma Innov. J. 10, 422–427.
Sankar, V. and Bharathi, U. (2021). Training e-manual on Advances in flower crops technologies including seed production.
Sarkar, J., Singh, S. K., Singh, K. P., and Guha, S. K. (2016). In-vivo and in-vitro mutagenesis in marigold (Tagetes erecta) using 60Co gamma rays. Indian J. Agric. Sci. 86, 870–875. doi: 10.56093/ijas.v86i7.59738
Shafiee, R., Abdollahi, M. R., Mirzaie-Asl, A., Moosavi, S. S., and Sarikhani, H. (2023). Effect of different factors on androgenesis induction in anther culture of African marigold (Tagetes erecta) and French marigold (Tagetes patula). J. Hortic. Sci 37, 787–799. doi: 10.22067/jhs.2023.79502.1207
Shafique, I., Andleeb, S., Aftab, M. S., Naeem, F., Ali, S., Yahya, S., et al. (2021). Efficiency of cow dung based vermi-compost on seed germination and plant growth parameters of Tagetes erectus (Marigold). Heliyon 7 (1), e05895. doi: 10.1016/j.heliyon.2020.e05895
Sharma, V., Kordrostami, M., Maan, S. S., Sarsu, F., and Penna, S. (2024). “Innovations in artificial induction of plant genetic diversity,” in Sustainable utilization and conservation of plant genetic diversity, eds. J. M. Al-Khayri, S. M. Jain. (Singapore: Springer), 259–287.
Singh, A., Kaur, L., and Singh, R. (2023). Management practices of diseases and insect pest in marigold and gladiolus flower in punjab state, India. Int. J. Environ. Climate Change 13, 2017–2022. doi: 10.9734/ijecc/2023/v13i102860
Singh, M., Nara, U., Kumar, A., Thapa, S., Jaswal, C., and Singh, H. (2022). Enhancing genetic gains through marker-assisted recurrent selection: from phenotyping to genotyping. Cereal Res. Commun. 50, 523–538. doi: 10.1007/s42976-021-00207-4
Sinha, S., Singh, S., Kumar, M., Singh, R. S., Satyendra, and Thakur, D. (2023). “Recent advancements in molecular marker technologies and their applications in crop improvement”. in Molecular marker techniques: a potential approach of crop improvement. (Singapore: Springer Nature Singapore), 319–337.
Sirohi, U., Kumar, M., Sharma, V. R., Teotia, S., Singh, D., Chaudhary, V., et al. (2022). CRISPR/Cas9 system: A potential tool for genetic improvement in floricultural crops. Mol. Biotechnol. 64, 1303–1318. doi: 10.1007/s12033-022-00523-y
Sood, V., Singh, A. K., Sinha, D., and Parwan, S. (2023). Screening of Marigold Germplasm against Leaf Spot and Flower Blight Disease under Field conditions in North Western Himalayas. Biol. Forum-An Int. J. 15, 430.
Srinivasan, A. and Jeyakumar, P. (2018). Estimation of genetic variability, heritability and genetic advance for high yield and tolerance to drought stress in marigold (Tagetes spp.) genotypes. J. Pharmacogn. Phytochem. 7 (6), 1847–1851.
Sudhakaran, S., Mandlik, R., Singh, P., Kumar, P., Meghwal, M., Mahakalkar, B., et al. (2024). “Transgenic approaches for accelerating breeding of ornamental crops,” in Ornamental horticulture: latest cultivation practices and breeding technologies (Singapore: Springer Nature Singapore), 151–174.
Sultana, H., Alakeel, K. A., Hassan, J., Mallick, S. R., Zakaria, M., Kayesh, E., et al. (2025). Nutrients, bioactive compounds and antinutritional properties of marigold genotypes as promising functional food. Sci. Rep. 15 (1), 4867. doi: 10.1038/s41598-025-88694-x
Sumalatha, A., Chandana, B. R., Dedhia, L., Reddy, L. D. C., Arivalagan, M., Bhaskar, V. V., et al. (2023). Comparative analysis of BLUP and GCA for parental selection in marigold (Tagetes erecta L.) for hybrid development. J. Hortic. Sci. 18, 307–314. doi: 10.24154/jhs.v18i2.2106
Susrama, I. G. K. and Yuliadhi, K. A. (2020). Induced mutagenesis in yellow flowering marigold with colchicine in hydrogen peroxide. Adv. Trop. Biodiversity Environ. Sci. 4, 44. doi: 10.24843/atbes.2020.v04.i02.p04
Tang, N., Liu, W., Zhang, W., and Tang, D. (2020). Integrative analysis of transcriptomic and proteomic changes related to male sterility in Tagetes erecta. Physiol. Mol. Biol. Plants 26, 2061–2074. doi: 10.1007/s12298-020-00886-z
Tarakeshwari, K. R. and Pavan, P. R.. (2023) “Advancements in breeding and genetics of ornamental plants” in Floriculture and landscaping chronicles: A collaborative insights N.D, (Kurukshetra, Haryana, India: Stella International Publication), 49.
Thirumalmurugan, V., Manivannan, K., and Nanthakumar, S. (2020). Genetic diversity in African marigold (Tagetes erecta L.) under Vellore conditions. Plant Arch. 20 (2), 3896–99.
Ting, T. C., Souza, A. C. M., Imel, R. K., Guadagno, C. R., Hoagland, C., Yang, Y., et al. (2023). Quantifying physiological trait variation with automated hyperspectral imaging in rice. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1229161
Toledo-Ortiz, G., Huq, E., and Rodríguez-Concepción, M. (2010). Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. U.S.A. 107, 11626–11631. doi: 10.1073/pnas.0914428107
Udchachon, S., Pongmanee, K., Boonruangrod, R., Attamangkune, S., and Ruangpanit, Y. (2021). Effect of marigold-derived products as pigment source on growth performance, antioxidant activity and liver enzymes of broiler chickens. Agric. Natural Resour. 55, 925–934. doi: 10.34044/J.ANRES.2021.55.6.03
Verma, V., Kumar, A., Partap, M., Thakur, M., and Bhargava, B. (2023). CRISPR-Cas: A robust technology for enhancing consumer-preferred commercial traits in crops. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1122940
Wang, X., Li, N., Li, W., Gao, X., Cha, M., Qin, L., et al. (2020). Advances in transcriptomics in the response to stress in plants. Glob Med. Genet. 07, 030–034. doi: 10.1055/s-0040-1714414
Xu, J., Kang, B. C., Naing, A. H., Bae, S. J., Kim, J. S., Kim, H., et al. (2020). CRISPR/Cas9-mediated editing of 1-aminocyclopropane-1-carboxylate oxidase1 enhances Petunia flower longevity. Plant Biotechnol. J. 18, 287–297. doi: 10.1111/pbi.13197
Yadav, P. and Dahiya, D. S. (2020). Knowledge and adoption of marigold cultivation practices of women farmers in Gurugram district of Haryana. Indian J. Extension Educ. 56, 99–102.
Yadav, R. K., Tripathi, M. K., Tiwari, S., Tripathi, N., Asati, R., Chauhan, S., et al. (2023). Genome editing and improvement of abiotic stress tolerance in crop plants. Life 13 (7), 1456. doi: 10.3390/life13071456
Youssef, H. A., Ali, S. M., Sanad, M. I., and Dawood, D. H. (2020). Chemical investigation of flavonoid, phenolic acids composition and antioxidant activity of mexican marigold (tagetes erecta l. ) flowers. Egypt J. Chem. 63, 2605–2615. doi: 10.21608/ejchem.2019.19839.2197
Yuan, Y., Ton, B. L., Thomas, W. J. W., Batley, J., and Edwards, D. (2023). Supporting crop plant resilience during climate change. Crop Sci. 63, 1816–1828. doi: 10.1002/csc2.21019
Yuan, H., Zhang, J., Nageswaran, D., and Li, L. (2015). Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2 (1). doi: 10.1038/hortres.2015.36
Zhang, H., Song, L., Li, L., Xin, H., Cui, R., Li, Z., et al. (2022). Interspecific hybridization with African marigold (Tagetes erecta) can improve flower-related performance in French marigold (T. patula). Not Bot. Horti Agrobot Cluj Napoca 50 (4), 12808–12808. doi: 10.15835/nbha50412808
Zhang, C., Wei, L., Wang, W., Qi, W., Cao, Z., Li, H., et al. (2020). Identification, characterization and functional analysis of AGAMOUS subfamily genes associated with floral organs and seed development in Marigold (Tagetes erecta). BMC Plant Biol. 20 (1), 439. doi: 10.1186/s12870-020-02644-5
Zhang, H., Zhang, S., Zhang, H., Chen, X., Liang, F., Qin, H., et al. (2020). Carotenoid metabolite and transcriptome dynamics underlying flower color in marigold (Tagetes erecta L.). Sci. Rep. 10 (1), 16835. doi: 10.1038/s41598-020-73859-7
Keywords: breeding, pedigree selection, landscape, mutation breeding, tissue culture
Citation: D. V and S. RK (2025) Marigold breeding in India: a comprehensive review of genetic advances, techniques and future prospects. Front. Plant Sci. 16:1619375. doi: 10.3389/fpls.2025.1619375
Received: 28 April 2025; Accepted: 08 September 2025;
Published: 26 September 2025.
Edited by:
Luisa M. Trindade, Wageningen University and Research, NetherlandsReviewed by:
Panagiotis Madesis, University of Thessaly, GreeceDionysia A. Fasoula, Agricultural Research Institute, Cyprus
Copyright © 2025 D. and S.. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramesh Kumar S., cmFtZXNoa3VtYXIuc0B2aXQuYWMuaW4=
 Venkatesan D.
Venkatesan D. Ramesh Kumar S.
Ramesh Kumar S.