- 1State Key Laboratory of North China Crop Improvement and Regulation, Hebei Agricultural University, Baoding, China
- 2Key Laboratory of North China Water-saving Agriculture, Ministry of Agriculture and Rural Affairs, Hebei Agricultural University, Baoding, China
- 3Key Laboratory of Crop Growth Regulation of Hebei Province, College of Agronomy, Hebei Agricultural University, Baoding, China
- 4Cotton Research Institute, Hebei Academy of Agriculture and Forestry Sciences, Shijiazhuang, China
- 5National Key Laboratory of Cotton Bio-breeding and Integrated Utilization, Institute of Cotton Research, Chinese Academy of Agricultural Sciences, Anyang, China
Drought stress detrimentally impacts leaf water transport, lowering transpiration and photosynthetic efficiency and ultimately reducing seed cotton yield. This study investigated the relationship between leaf hydraulic and photosynthetic traits in cotton under three moisture treatments: control (CK), moderate drought (MD), and severe drought (SD). By day 28 after drought stress, drought stress significantly impaired leaf hydraulics, as demonstrated by decreases in leaf hydraulic conductivity (Kleaf) (9.81% under MD, 12.93% under SD) and leaf water potential (5.79% under MD, 17.54% under SD). Key contributing factors included reduced xylem vessel diameter and number, diminished minor vein density, and decreased aquaporin gene expression. In addition, stomatal width and aperture were significantly reduced with increasing drought severity. Compared with CK, stomatal width and aperture decreased by 6.83% and 13.22% under MD, and by 20.59% and 19.92% under HD. These changes resulted in lower stomatal conductance, net photosynthetic rate, and biomass accumulation, inhibiting growth and reducing plant height, stem diameter, and leaf area. The results of this study provide insights into the anatomical and physiological mechanisms underlying leaf hydraulic conductivity under drought stress.
1 Introduction
Drought stress, a major constraint on crop production, impairs plant physiological activity and metabolism, ultimately leading to significant yield losses that threaten agricultural and economic sustainability (Cao et al., 2024). The severity of these impacts depends critically on the intensity, duration, and developmental stage of the stress event (Gray et al., 2016). Cotton is an important economic crop that is prone to drought stress during its growth process, which reduces its yield and quality (Zou et al., 2022). For instance, Bista et al. (2024) found that drought stress led to a reduction in lint and seed cotton yields by 61% and 62%, respectively. Statistically, yield loss caused by drought stress conditions exceeds the sum of losses due to other abiotic stressors (Abdelraheem et al., 2019). Drought stresses commonly result in negative impacts on growth parameters, such as reduced leaf area expansion, declines in the number of nodes and sympodial branches, reduces cotton the number of leaves, and biomass production, and stunted plant height thereby weakening plant growth and potentially leading to irreversible damage (Ahluwalia et al., 2021; Rehman et al., 2022; Zafar et al., 2023). These growth impairments are closely linked to the effects of drought on leaf physiology, which is a critical aspect of the plant’s response to water scarcity.
Drought stress has a profound impact on the physiological and biochemical processes, morphological structure, and overall function of leaves (Seleiman et al., 2021). As the “heart” of the plant, leaves are the primary site for photosynthesis and play a crucial role in the plant’s hydraulic system, serving as a safety valve to mitigate water imbalance (Sack and Scoffoni, 2013). Leaf hydraulic traits function as “regulators” of water transport within the leaf (Li et al., 2015) and are influenced by environmental factors. These traits mediate leaf gas exchange and overall water transport throughout the plant (Villagra et al., 2013). Leaf hydraulic conductivity (Kleaf), the water flow rate through a leaf at a given time and water potential gradient, reflects the water transport efficiency of the leaf and is a core indicator of leaf hydraulic traits (Sack and Frole, 2006). Studies have shown that Kleaf is affected by multiple water transport pathways, such as petioles, leaf vein xylem, vascular sheaths, and mesophyll cells, and its dynamics are not consistent among plant species, developmental periods, and environmental factors (Prado and Maurel, 2013). Under normal circumstances, drought stress decreases Kleaf, and the degree of its decline is positively correlated with stress severity (Lai et al., 2023), causing an imbalance in leaf hydraulic traits (Blackman et al., 2010). Previous studies have shown that the magnitude of Kleaf is directly related to xylem vessel diameter (Jafarikouhini and Sinclair, 2023), the degree of embolism (Dayer et al., 2020), tracheid size (Garcia-Forner et al., 2021), and cell wall thickness (Nardini et al., 2005). These leaf hydraulic traits play a pivotal role in a plant’s ability to adapt to drought conditions. Therefore, studying changes in leaf hydraulic characteristics under drought stress is of significant practical importance. Understanding these changes can reveal how plants adjust their leaf structure and function to cope with drought stress, thereby enhancing their drought resistance and improving growth performance.
Drought stress severely compromises the physiological and anatomical attributes of leaves, diminishing photosynthetic efficiency and water transport capabilities (Zafar et al., 2023). Characterizing these responses is vital for devising strategies to bolster drought resilience and sustain yield and quality in water-scarce environments. Kleaf and associated anatomical features, such as xylem vessel dimensions, are pivotal in determining water transport efficiency (Sack and Frole, 2006). Clarifying how drought stress impacts these traits can pinpoint genetic or agronomic interventions that enhance water use efficiency and preserve photosynthetic function. Moreover, stomatal traits, which are intricately connected to leaf hydraulics, regulate water loss and CO2 uptake, thereby influencing photosynthesis and plant growth (Ru et al., 2024). Thus, exploring the interplay between leaf hydraulic traits and stomatal characteristics under drought stress can uncover key mechanisms of drought adaptation in cotton. This understanding is crucial for breeding drought-resistant cotton varieties and optimizing irrigation practices to ensure sustainable production in drought-prone areas.
The integrity of the leaf hydraulic system is intrinsically linked to the functionality of leaves, exerting a profound influence on overall plant growth (Ziegler et al., 2023). Drought stress poses a significant challenge to plants by diminishing leaf photosynthetic capacity, which is a primary constraint on crop biomass accumulation and yield (Langhansová et al., 2024; Ru et al., 2024). Kleaf is a pivotal factor affecting the photosynthetic capacity of leaves, which are the principal sites of photosynthesis. Li et al. (2021a) found significant positive correlations among the net photosynthetic rate, stomatal conductance, transpiration rate, and leaf hydraulic conductivity in tomato, regardless of whether the plants were under normal water conditions or drought stress. Similarly, in cotton, leaf hydraulic traits are crucial in the plant’s drought response by modulating the efficiency of water transport and stomatal regulation, which are essential for sustaining photosynthesis and biomass production (Lai et al., 2024). When drought stress decreases leaf hydraulic conductivity, it triggers a cascade of physiological responses, including stomatal closure (Ru et al., 2024). This closure reduces stomatal conductance and the net photosynthetic rate, thereby affecting the plant’s ability to convert light energy into chemical energy, which is fundamental for growth and development (Wang et al., 2018; Ye et al., 2021). Building on these insights, our study aims to explore the relationship between leaf hydraulic traits and photosynthetic capacity under drought stress conditions, offering a deeper understanding of the mechanisms that cotton employs to cope with water scarcity.
Although the relationship between Kleaf and leaf photosynthetic capacity has been investigated, most related studies have focused on grasses. There is a gap in research on Malvaceae plants, especially cotton. Moreover, the relationship between leaf hydraulic traits and leaf photosynthetic traits remains poorly understood in cotton under drought stress. Therefore, the aims of the present study were (1) to explore the response pattern of leaf hydraulic conductivity to drought stress in cotton; (2) to clarify the anatomical mechanisms by which drought stress regulates leaf hydraulic conductivity; and (3) to determine the relationship between leaf hydraulic conductivity and leaf photosynthetic function under drought stress.
2 Materials and methods
2.1 Plant material and growth conditions
The experiment was carried out in the intelligent greenhouse of the College of Agronomy, Hebei Agricultural University, in 2023 (Hebei, China). Upland cotton (Gossypium hirsutum L.) variety ‘Guoxin Cotton No. 9’ was used as the experimental material, and the seeds were provided by the General Union of Rural Technical Services of Guoxin (Hebei, China). The seeds were soaked in an incubator at 25°C for 24 h. After the seeds showed white tips, they were sown in white PVC culture pots (with a diameter of 10 cm, a height of 20 cm, and a volume of 1.6 L) filled with 2.5 kg of culture medium (with a volume ratio of soil to sand of 3:1), containing 16.93 g kg−1 organic matter, 94.60 mg kg−1 alkaline dissolved nitrogen, 25.33 mg kg−1 effective phosphorus, and 202.07 mg kg−1 effective potassium. Three seeds were sown in each culture pot, and only one cotton seedling was retained after one true leaf emerged.
2.2 Experimental design
The experimental design employed a randomized complete block design. When the third true leaves of the cotton seedlings were fully expanded, water treatments were initiated: control (CK), moderate drought (MD), and severe drought (SD), with relative water contents of 70–75%, 55–60%, and 40–45%, respectively (Song et al., 2023). Each treatment was replicated in 70 pots. The soil moisture content was monitored by the weighing method every day, and each pot was supplemented with water to reach the set moisture content. The relative humidity in the culture room was constant at (70 ± 5) %. The light intensity was 600 μmol·m−2·s−1, and the photoperiod was 14/10 h, with day and night temperatures of 28°C/20°C.
2.3 Measurement of aboveground morphology
The aboveground morphological traits and biomass were measured at 0, 7, 14, 21, and 28 days after the initiation of the water treatments. Three plants were selected for each treatment to determine the following parameters:
Plant height: Measured from the cotyledon node to the apical growing point.
Stem diameter: Measured 1 cm above the cotyledon node using a vernier caliper.
Leaf area: Calculated using the length×width×0.75 method.
Aboveground dry matter mass: Determined after initial fresh weight recording, followed by kill-drying at 105°C for 30 minutes and drying at 80°C until constant weight.
2.4 Leaf hydraulic conductivity
After 0, 7, 14, 21, and 28 days of drought treatment, the third leaf from the top was sampled. There were three replicates for each treatment. The leaf hydraulic conductivity was measured using a plant high-pressure flowmeter (HPFM-Gen, Dynamax, Houston, TX, USA) in transient mode on the leaf, retaining a petiole length of 2 cm, with the applied pressure ranging from 0 to 5 kPa s−1 and the pressure and flow rate recorded every 2 seconds (Sack, 2002; Sack et al., 2005). The leaf hydraulic conductivity was calculated as follows:
The leaf area was measured using the leaf length and width method, which was calculated based on the leaf area correction factor method (Mao et al., 2014), as follows:
2.5 Leaf water potential
After 0, 7, 14, 21, and 28 days of drought treatment, the third leaf from the top was measured using a portable pressure chamber (PMS670, PMS Instrument Company, USA), with three replicates for each treatment. The leaves were first equilibrated in sealed black plastic bags for 20 min, then cut off at the base of the petiole and placed into a pressure chamber. Subsequently, pressure was slowly applied. The minimum pressure at which the first drop of water was observed exuding from the petiole under observation with a magnifying glass was regarded as the leaf water potential (Ψleaf) (Müllers et al., 2022).
2.6 Anatomical leaf and petiole structure
On day 28 post-treatment, the third leaves from the top were collected from each treatment for leaf and petiole anatomical analyses. For leaves, 0.5 cm × 0.5 cm sections were cut perpendicular to the veins, while petioles were cut into 0.5–1 cm segments, starting 1 cm from the leaf. Three replicates per treatment were prepared using the paraffin section method. Samples were fixed with formaldehyde–alcohol–acetic acid (FAA), treated with xylene and absolute ethanol, and then embedded in paraffin (Zhang et al., 2022). After saffron and solid green staining, the sections were sealed and stored at 4°C. Images were captured using a digital microscope (BX53, Olympus, Monolith, Japan) and analyzed using NIS-Elements software. Leaf parameters included thickness, cross-sectional area, and xylem vessel area, number, and diameter. Petiole analyses focused on the cross-sectional area, diameter, and the area and number of xylem vessels.
2.7 Leaf vein density
On day 28 post-treatment, the third leaves from the top were scanned (Epson Perfection V39; Epson, Suwa, Japan) with three replicates per treatment. The major (VLAmajor) and minor (VLAminor) vein densities were measured. Primary veins were assessed in intact leaves, and secondary veins were assessed in half of them for VLAmajor. Leaves were soaked in 95% ethanol for 1–2 days, stained with 1% saffron, and observed under a microscope (BX53, Olympus) to determine the vein length and field area (Lu et al., 2019). The vein density (VLAminor) was calculated using NIS-Elements software, reflecting the length of veins per unit leaf area.
2.8 Stomatal size and density
On day 28 post-treatment, the third leaf was selected from the main stems of cotton under each treatment (three replicates per treatment). The abaxial leaf surfaces were lightly coated with nail polish, allowed to dry for 3 min, and transferred onto clean slides using transparent tape. Observations and image acquisition were conducted using a digital microscope (BX53, Olympus, Monolith, Japan). The stomatal area and number were measured using NIS-Elements software, enabling the calculation of stomatal density (number of stomata per unit area).
2.9 Leaf gas exchange parameters
On days 0, 7, 14, 21, and 28 post-treatment, the environmental control mode of a portable photosynthesis system (LI-6400, Li-Cor, Lincoln, NE, USA) was adopted to measure the gas exchange parameters, including net photosynthetic rate (An), transpiration rate (E), stomatal conductance (gs), and intercellular CO2 concentration (Ci) in the third leaves from the top of cotton plants (Sáez et al., 2018). The light intensity of the red and blue light sources was 500 μmol·m−2·s−1, and the CO2 concentration was 400 μmol·mol−1. There were three replicates for each treatment. The instantaneous water use efficiency of the leaves was calculated as follows (Li et al., 2021b):
2.10 Chlorophyll fluorescence parameters of leaves
After 0, 7, 14, 21, and 28 days of drought treatment, a PAM-2500 portable chlorophyll fluorometer (PAM-2500, WALZ, Effeltrich, Germany) was used to measure the maximum photochemical efficiency of photosystem II (PSII; Fv/Fm) and the actual photochemical quantum yield of PSII (ΦPSII) for each treatment. The measurement sites were the same as those for the photosynthesis measurements, and there were three replicates for each treatment.
2.11 Aquaporin content and gene expression level
To determine the aquaporin content, the third leaves from the top of cotton seedlings were sampled on days 0, 7, 14, 21, and 28 post-drought treatment (three replicates per treatment). Samples were labeled, wrapped in aluminum foil, rapidly frozen in liquid nitrogen, and stored at –80°C. The aquaporin content was determined using an enzyme-linked immunosorbent assay (ELISA) kit (YJ191258) from Shanghai Yuanju Technology Center, following the manufacturer’s protocol.
To determine the expression of aquaporin genes, 50 mg of the same leaves was collected on day 28 post-treatment, frozen in liquid nitrogen, and stored at −80°C (three replicates per treatment). RNA was extracted, and its concentration and quality were assessed. RNA extraction and cDNA synthesis procedures are detailed in the appendix. All reagents were obtained from Sigma-Aldrich.
2.12 Statistical analysis
Data were recorded and organized using Microsoft Excel 2010. Statistical analyses were performed with IBM SPSS Statistics 25.0, employing one-way ANOVA followed by Duncan’s multiple comparison tests to assess significance. Graphs were plotted using GraphPad Prism 8.0, and correlation analyses were conducted with Origin 2023.
3 Results
3.1 Growth, photosynthetic, and fluorescence traits of cotton plants under drought stress
Drought stress exhibited progressive inhibitory effects on cotton seedling growth with increasing drought intensity and duration (Figure 1A). Quantitative analysis revealed significant reductions in key growth parameters, including plant height, stem diameter, leaf area, and aboveground dry matter mass. After 28 days of drought treatment, MD decreased these parameters by 28.52%, 15.32%, 43.51%, and 45.92%, respectively, compared with CK. SD induced more pronounced growth suppression, with corresponding reductions reaching 59.14% in plant height, 26.52% in stem diameter, 69.49% in leaf area, and 65.47% in aboveground dry matter mass (Figures 1B–E).
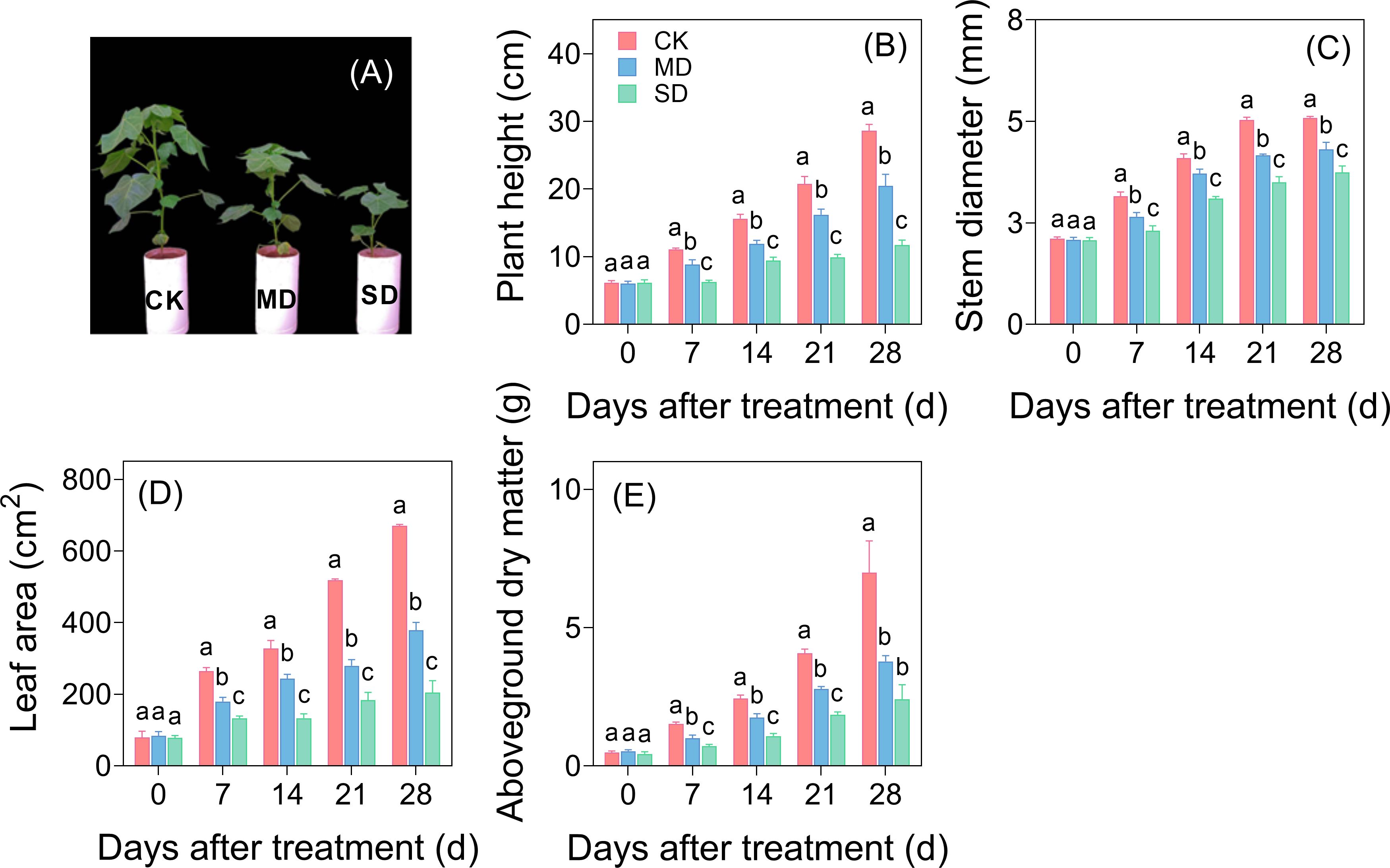
Figure 1. Effects of drought stress on aboveground morphology (A), plant height (B), stem diameter (C), leaf area (D), and aboveground dry matter (E) of cotton seedlings. Values are the mean ± SD (n = 3). Different lowercase letters indicate significant differences according to Duncan’s method (P< 0.05).
An and E of cotton seedlings that underwent drought treatment were substantially lower than those of the CK group starting after 7 days of treatment. This difference augmented progressively over time (Figures 2A, B). On day 28 of drought stress, gs, An, E, and Ci in the MD treatment had diminished by 39.07%, 14.68%, 10.88%, and 9.88%, respectively. In the SD treatment, these values decreased by 48.11%, 26.39%, 26.42%, and 22.69%, respectively (Figures 2A–D). The Fv/Fm and ΦPSII values of the leaves under drought stress conditions were lower than those in CK. On day 28 of drought stress, Fv/Fm of the leaves in the MD and SD treatments was significantly reduced by 11.95% and 15.94%, respectively, compared with CK (Figure 2E). Similarly, ΦPSII decreased significantly by 12.67% and 20.72% in the MD and SD treatments, respectively (Figure 2F).
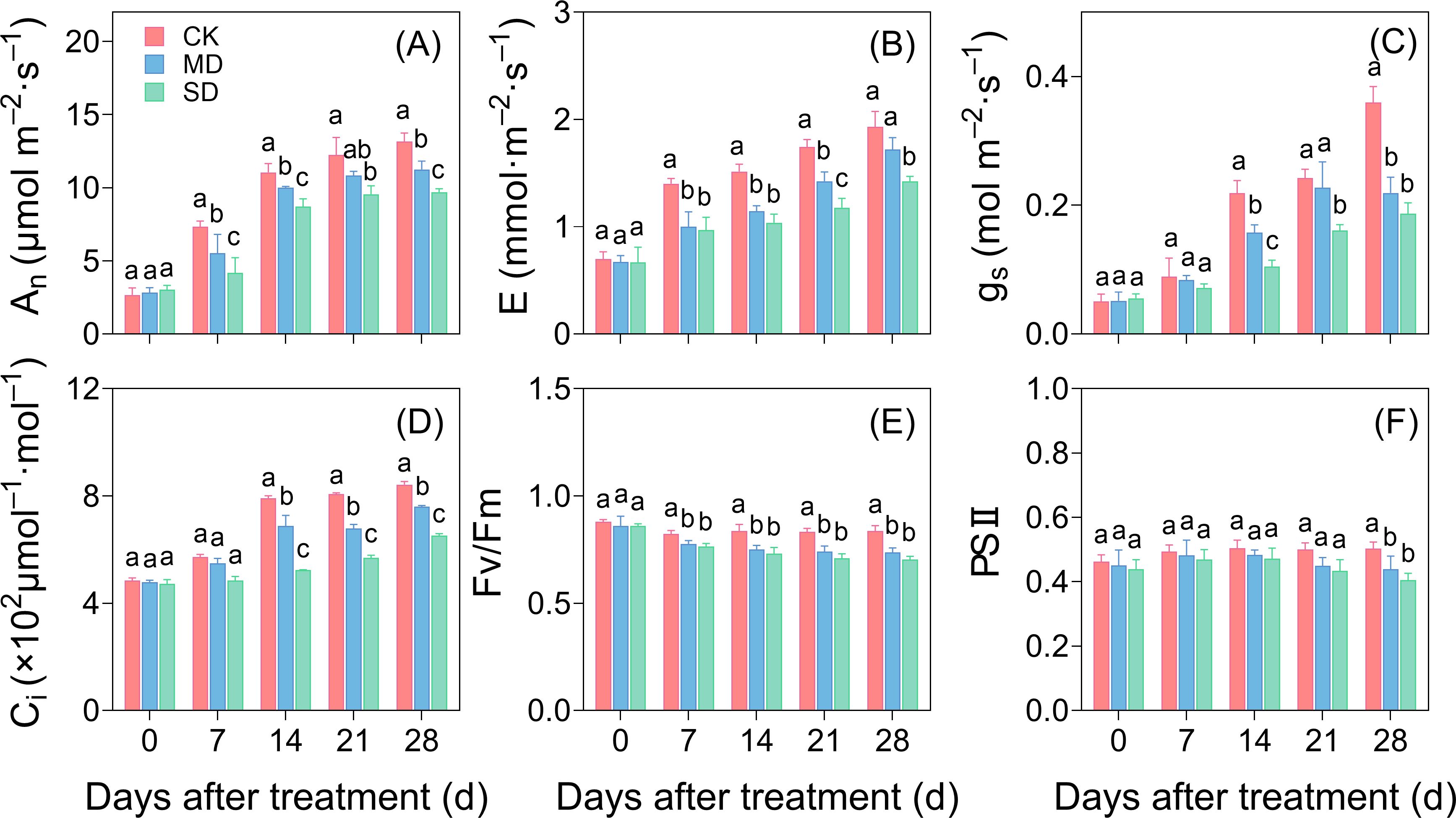
Figure 2. Effects of drought stress on photosynthetic fluorescence of cotton at different times. Effects of drought stress on the net photosynthetic rate (A), transpiration rate (B), stomatal conductance (C), intercellular carbon dioxide concentration (D), and chlorophyllin II fluorescence parameters in functional leaves from the main stem (E, F) of cotton seedlings. Values are the mean ± SD (n = 3). Different lowercase letters indicate significant differences according to Duncan’s method (P< 0.05).
3.2 Effect of drought stress on hydraulic traits of cotton plants
As the degree and duration of drought stress increased, Kleaf showed a decreasing trend compared with CK (Figure 3A), and by day 14 of drought treatment, a significant difference was observed, with Kleaf reduced by 7.17% and 18.77% in the MD and SD treatments, respectively, compared with CK. On days 21 and 28 of drought stress, Kleaf decreased by 6.54% and 9.18%, respectively, in the MD treatment and by 12.77% and 12.93%, respectively, in the SD treatment compared with CK (Figure 3A). Differences in Ψleaf were observed among treatments after 7 days of drought stress. Drought stress accelerated the decline in leaf water potential, and on day 28, leaf water potential in the SD treatment decreased significantly by 17.55% compared with CK (Figure 3B). On day 28 of drought stress, WUEi increased significantly by 47.42% and 42.28% in the MD and SD treatments, respectively, compared with CK (Figure 3C).
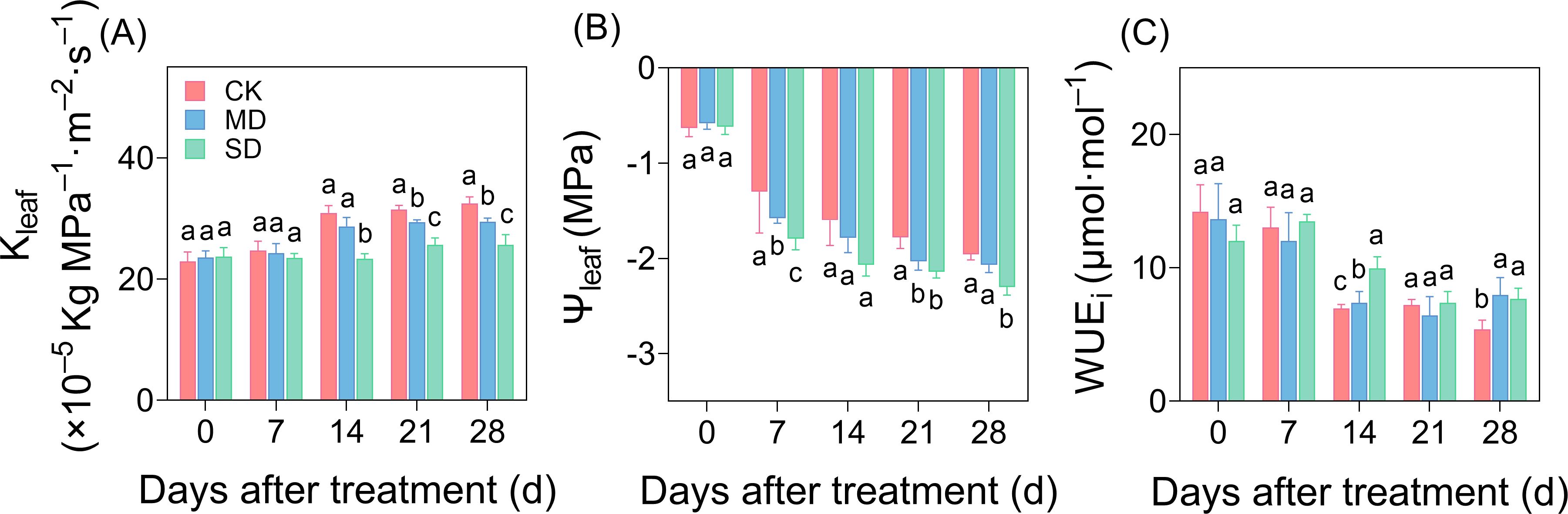
Figure 3. Effect of drought stress on leaf hydraulic traits over time in cotton. Effects of drought stress on hydraulic conductivity (A), water potential (B), and instantaneous water use efficiency (C) of cotton seedlings. Values are the mean ± SD (n = 3). Different lowercase letters indicate significant differences according to Duncan’s method (P< 0.05).
3.3 Effect of drought stress on aquaporins in cotton plants
After 7 days of drought stress, significant differences in aquaporin content were observed among the treatments (Figure 4A). By day 28 of drought stress, the aquaporin content in the MD and SD treatments had decreased by 31.07% and 36.73%, respectively, compared with the CK treatment (Figure 4A). Further analysis of the synthesized genes of water channel proteins showed that GhPIP2-1, GhPIP2-2, GhTIP1-2, and GhTIP1-3 were significantly downregulated under drought stress. The expression of GhPIP2-1, GhPIP2-2, and GhTIP1-2 was downregulated 2–2.5-fold (Figure 4B).
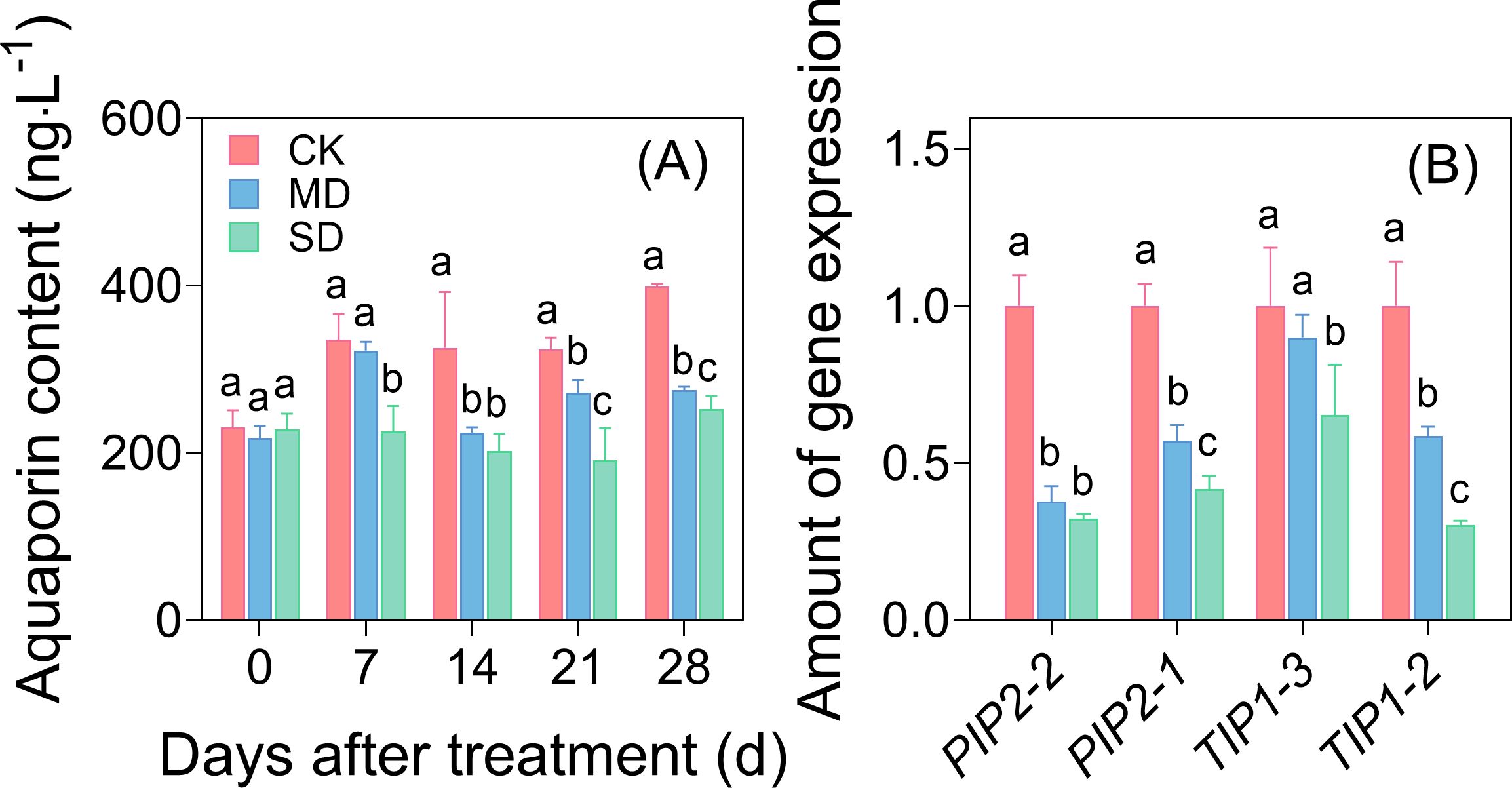
Figure 4. Effects of drought stress on aquaporin content (A) and the expression of aquaporin synthesis genes (B) in cotton seedling leaves. Values are the mean ± SD (n = 3). Different lowercase letters indicate significant differences according to Duncan’s method (P< 0.05).
3.4 Effect of drought stress on anatomical traits in cotton
3.4.1 Anatomical traits of leaves and petioles of cotton plants under drought stress
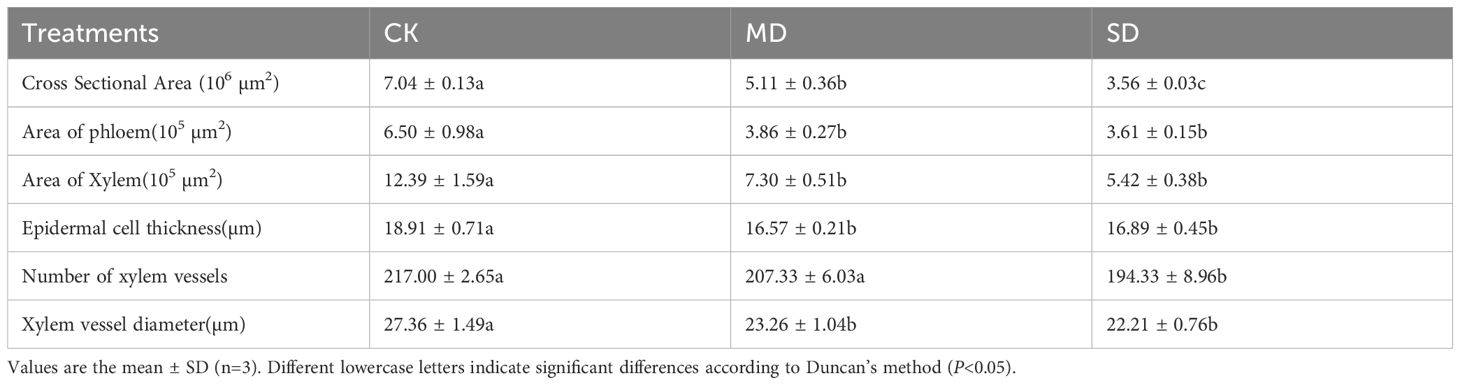
Figure 5 shows the anatomical structures of petioles (A) and leaves (B) on day 28 of drought treatment. Compared with CK, the cross-sectional area, phloem area, xylem area, and epidermal cell thickness of petioles were significantly reduced in the MD and SD treatments. Under MD treatment, these parameters decreased by 27.42%, 40.61%, 41.08%, and 10.68%, respectively, while under SD treatment, they decreased by 15.55%, 44.46%, 56.26%, and 12.37%, respectively. The diameter of the xylem vessels under MD treatment was significantly reduced by 17.61% compared with CK. Under SD treatment, the number and diameter of xylem vessels were significantly reduced by 10.45% and 18.83%, respectively, compared with CK (Table 1).
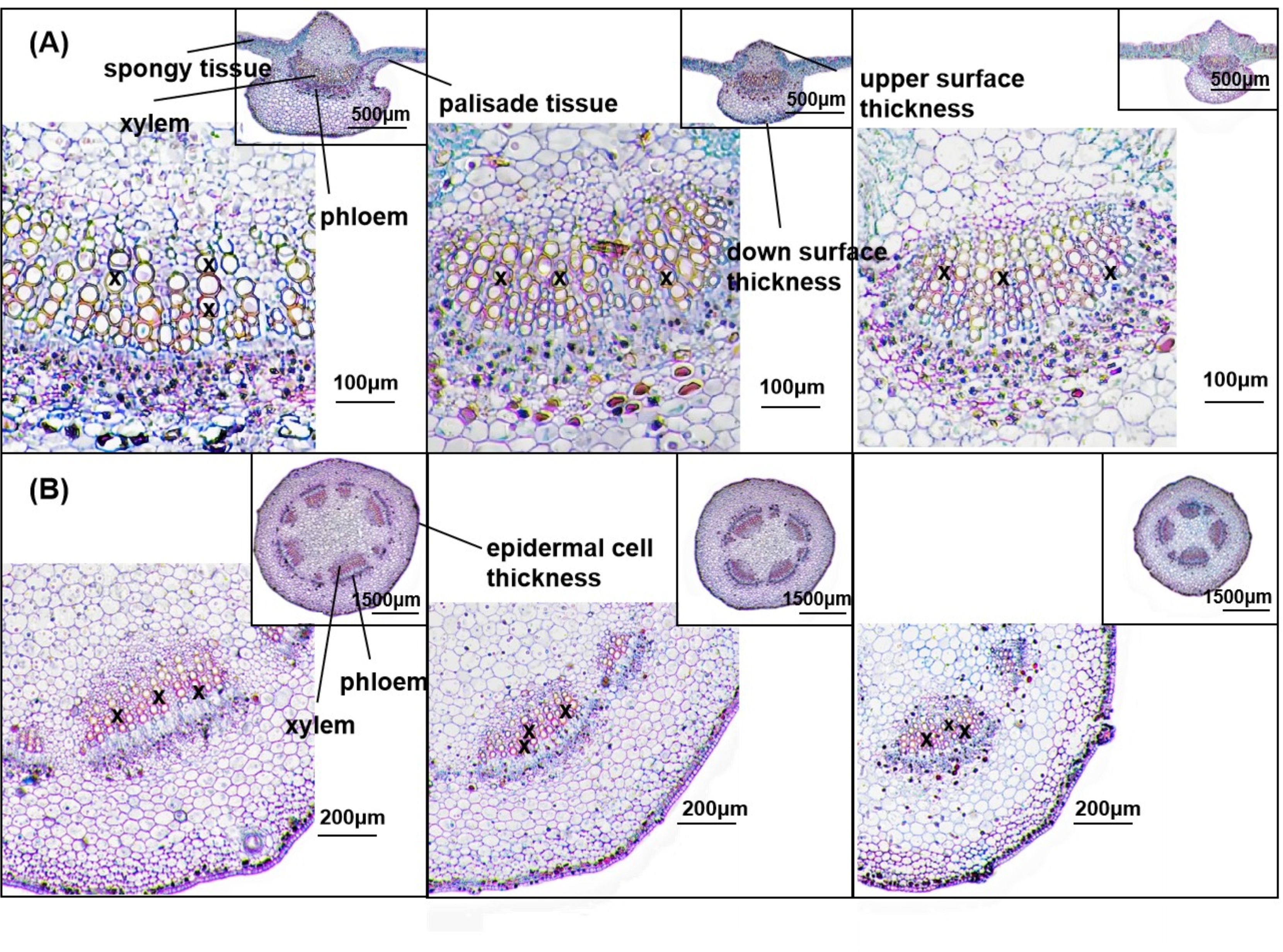
Figure 5. Effect of drought stress on plant anatomical traits. Paraffin sections of cotton leaves in CK, MD, and SD (A) and petioles in CK, MD, and SD (B) were evaluated after 28 days of drought stress. In (A), the image shows the petiole anatomy observed at 40× magnification, and the inset image in the upper right corner shows the complete leaf anatomy observed at 20× magnification. In (B), the image shows the petiole anatomy observed at 40× magnification, and the inset image in the upper right corner shows the complete petiole anatomy observed at 10× magnification. X marks the xylem vessels.
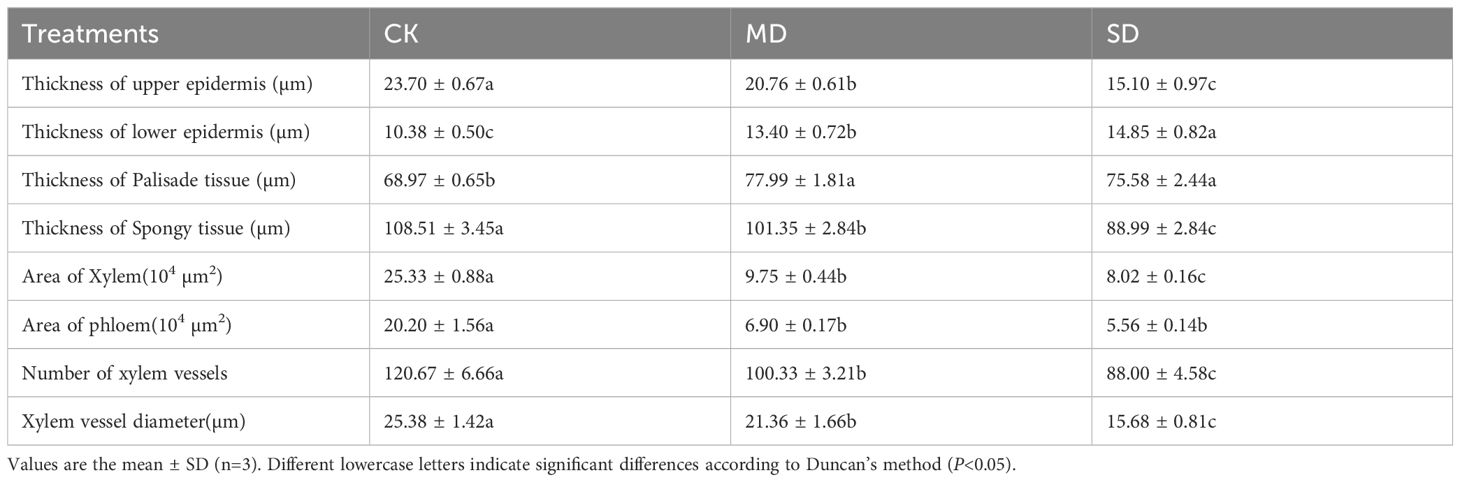
Table 2 shows that significant changes occurred in leaf anatomical parameters under drought treatments. Under MD treatment, the upper epidermal cell thickness, spongy tissue thickness, xylem area, phloem area, number of xylem vessels, and xylem vessel diameter decreased by 12.41%, 6.60%, 61.51%, 65.84%, 20.27%, and 18.84%, respectively. Under SD treatment, these parameters decreased more substantially, with reductions of 36.29%, 17.99%, 68.34%, 72.48%, 27.07%, and 38.21%, respectively. Compared with CK, the thickness of the lower epidermal cells and palisade tissue significantly increased by 29.09% and 13.08%, respectively, under MD treatment, and by 43.60% and 9.58%, respectively, under SD treatment.
3.4.2 Effect of drought stress on stomatal characteristics of cotton plants
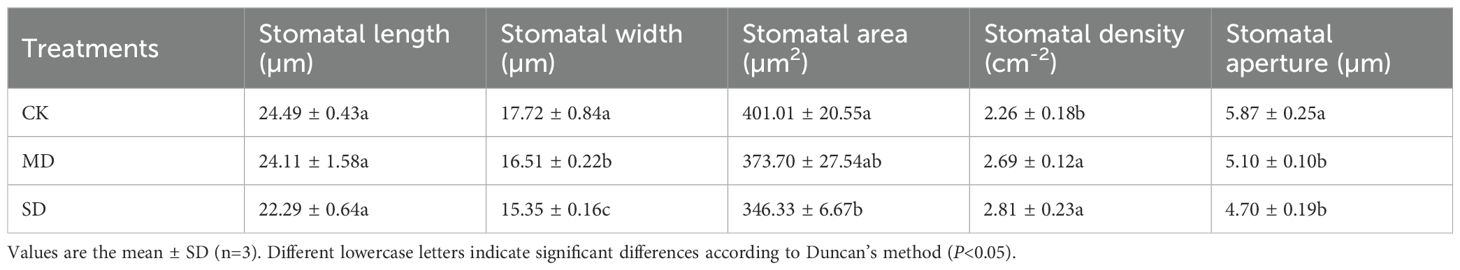
Figure 6 shows the stomatal images after 28 days of drought treatment. As shown in Table 3, stomatal width, stomatal area, and stomatal aperture decreased by 6.83%, 6.81%, and 13.22%, respectively, under MD treatment compared with CK. This was further aggravated in the SD treatment, with significant decreases of 13.37%, 13.64%, and 19.92%, respectively. Compared with the CK, the values in the MD and SD treatments increased by 18.96% and 24.14%, respectively.
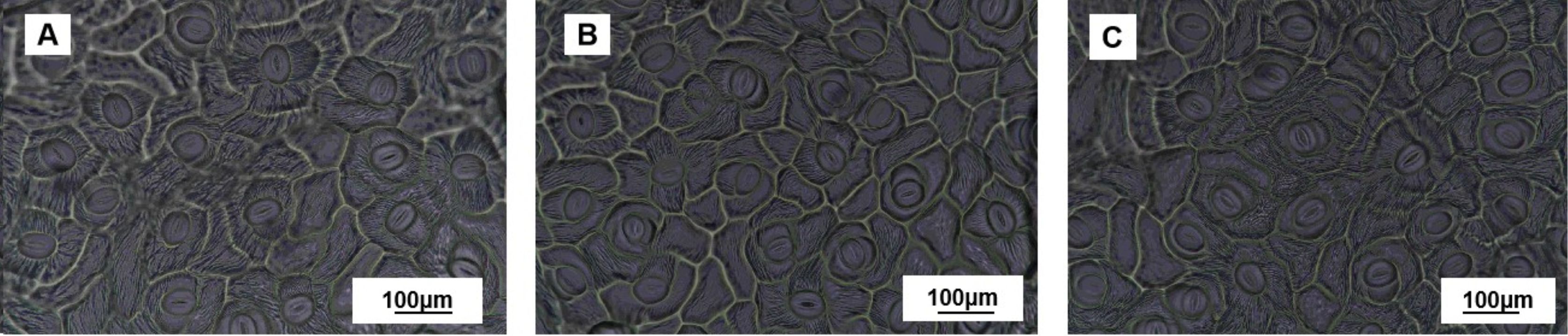
Figure 6. Measurement of stomata in the control (A), mild drought treatment (B), and severe drought treatment groups (C) after 28 days of drought treatment. Values are the mean ± SD (n = 3). Different lowercase letters indicate significant differences according to Duncan’s method (P< 0.05).
3.4.3 Effect of drought stress on leaf vein characteristics of cotton plants
Under SD treatment, VLAmajor was significantly higher than that under CK (Figure 7). Compared with CK, VLAmajor increased by 14.16% and 25.66% under the MD and SD treatments, respectively. The VLAminor density was significantly lower in the MD and SD treatments than in CK, with decreases of 40.31% and 41.86%, respectively.
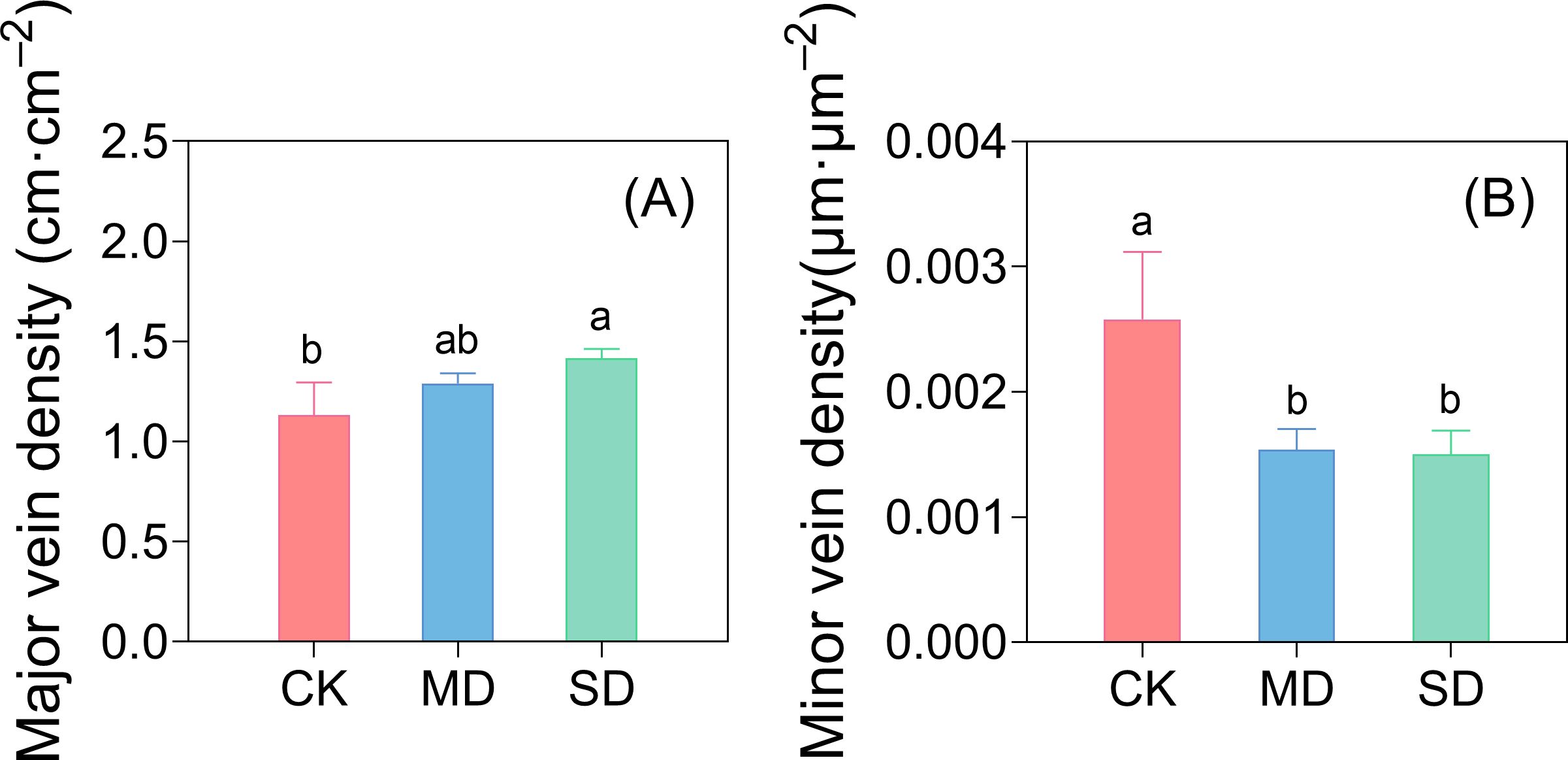
Figure 7. Effect of drought stress on major vein density (A) and minor vein density (B) in cotton seedlings. Values are means ± SD (n = 3). Different small letters mean significant differences according to the Duncan’s method (P< 0.05).
3.5 Xylem vessel diameter, number, and relationship between VLAminor and Kleaf
To identify the primary anatomical factors influencing Kleaf under drought stress, we conducted principal component analysis (PCA) on hydraulic conductivity and petiole anatomical traits (Figure 8A). The key factors influencing leaf hydraulic conductivity, including main vein density, secondary vein density, number of xylem vessels, xylem vessel diameter, xylem area, and epidermal cell thickness, accounted for 89.10% of the total variance. These factors were negatively correlated with primary vein density, and had extremely significant positive correlations with the number of xylem vessels, xylem vessel diameter, xylem area, and secondary vein density (Supplementary Table S4). The main factors affecting Kleaf were xylem vessel diameter and xylem area of the petiole.
PCA was conducted on Kleaf and leaf anatomical traits (Figure 8B). Leaf hydraulic conductivity and leaf anatomical factors accounted for 94.50% of the total variance. Kleaf was mainly influenced by the number and diameter of xylem vessels in the leaf, upper epidermal cell thickness in the leaf, spongy tissue thickness, and the number of xylem vessels in the petiole. Moreover, these indicators had extremely significant positive correlations with Kleaf (Supplementary Table S4). In conclusion, Kleaf was mainly affected by the diameter and number of xylem vessels in the leaf and petiole as well as secondary leaf vein density.
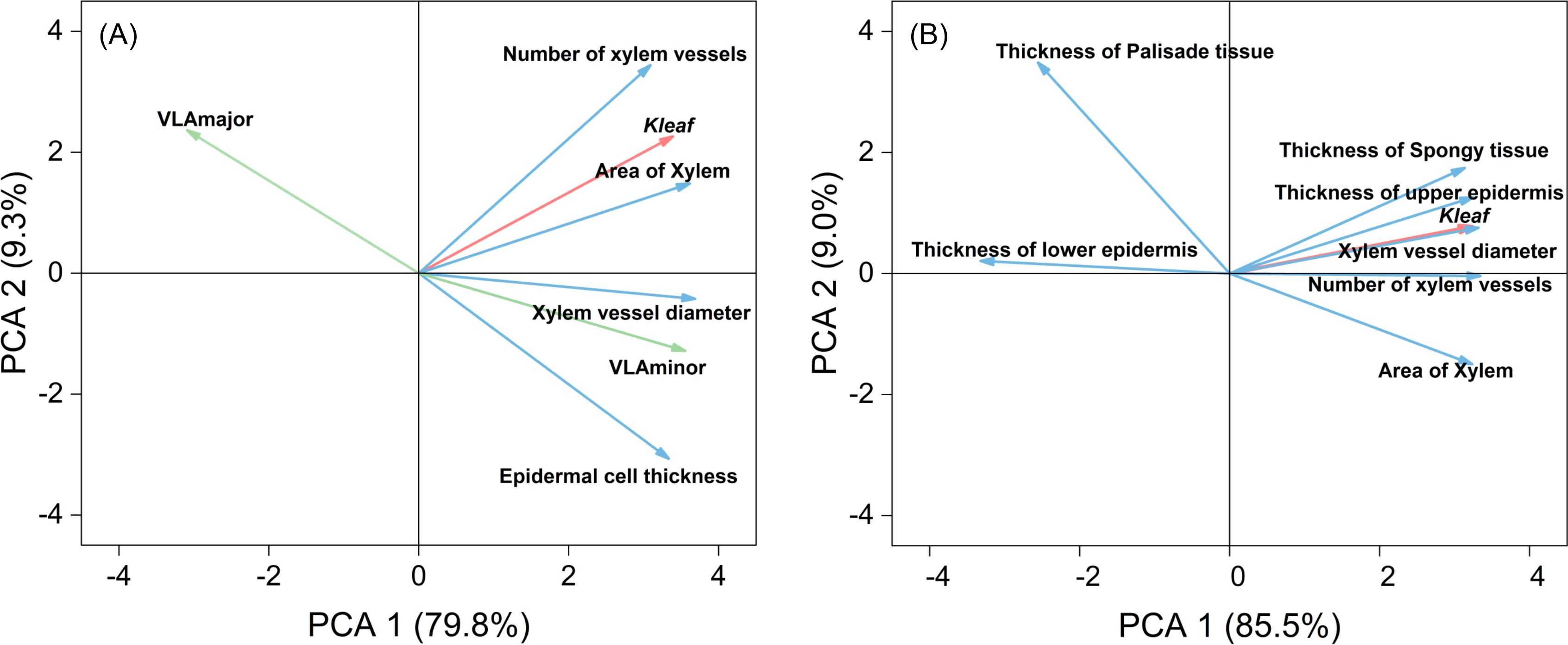
Figure 8. Principal component analysis (PCA) of hydraulic conductivity (red line), anatomical characteristics of the petiole (blue line), and vein density (green line) of leaves using original data (A), and PCA of hydraulic conductivity (red line) and anatomical characteristics of the leaf (blue line) using original data (B). Values in brackets are percentages explained by the first two components.
3.6 Kleaf in relation to the photosynthetic function and water status
To better understand the relationships between leaf hydraulic conductivity and stomatal traits, we conducted PCA on the relevant indicators under three water treatments (Figure 9). Kleaf was significantly negatively correlated with stomatal density and had extremely significant positive correlations with stomatal area, stomatal width, and stomatal aperture. Kleaf mainly influenced stomatal width and aperture.
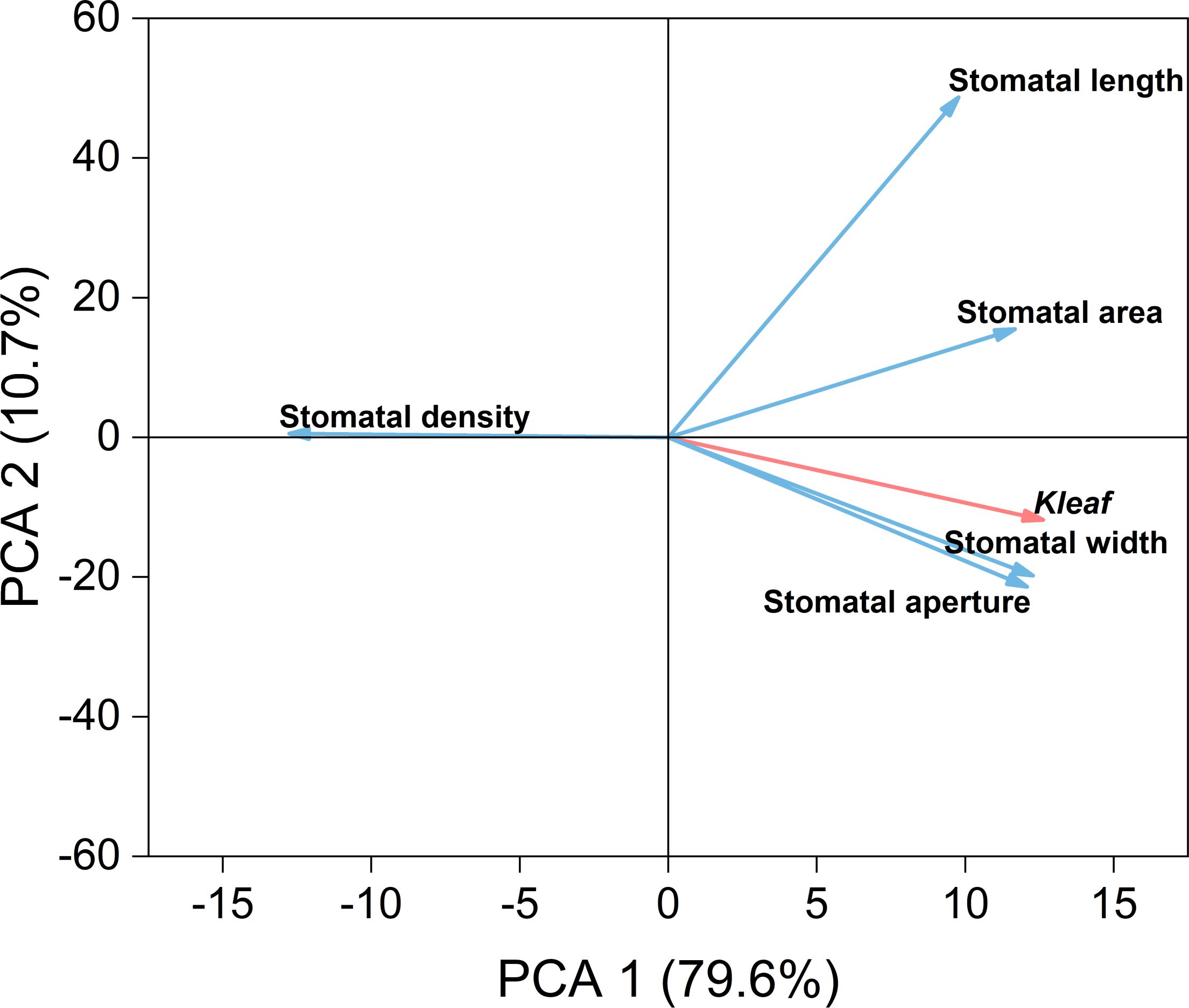
Figure 9. Principal component analysis (PCA) of hydraulic conductivity (red line) and stomatal characteristics (blue line) of leaves using original data. Values in brackets are percentages explained by the first two components.
Correlation analysis was carried out between leaf hydraulic conductivity and photosynthetic parameters. As shown in Figure 10, there were significant positive correlations between Kleaf and gs, An, E, and Ci. With the increase in drought stress severity, Kleaf decreased, and gs, An, E, and Ci decreased with the decline in Kleaf (Figures 10A–D). In conclusion, the decrease in Kleaf under drought stress led to the decline in stomatal aperture and width, decreasing gs, An, E, and Ci, attenuating photosynthetic function.
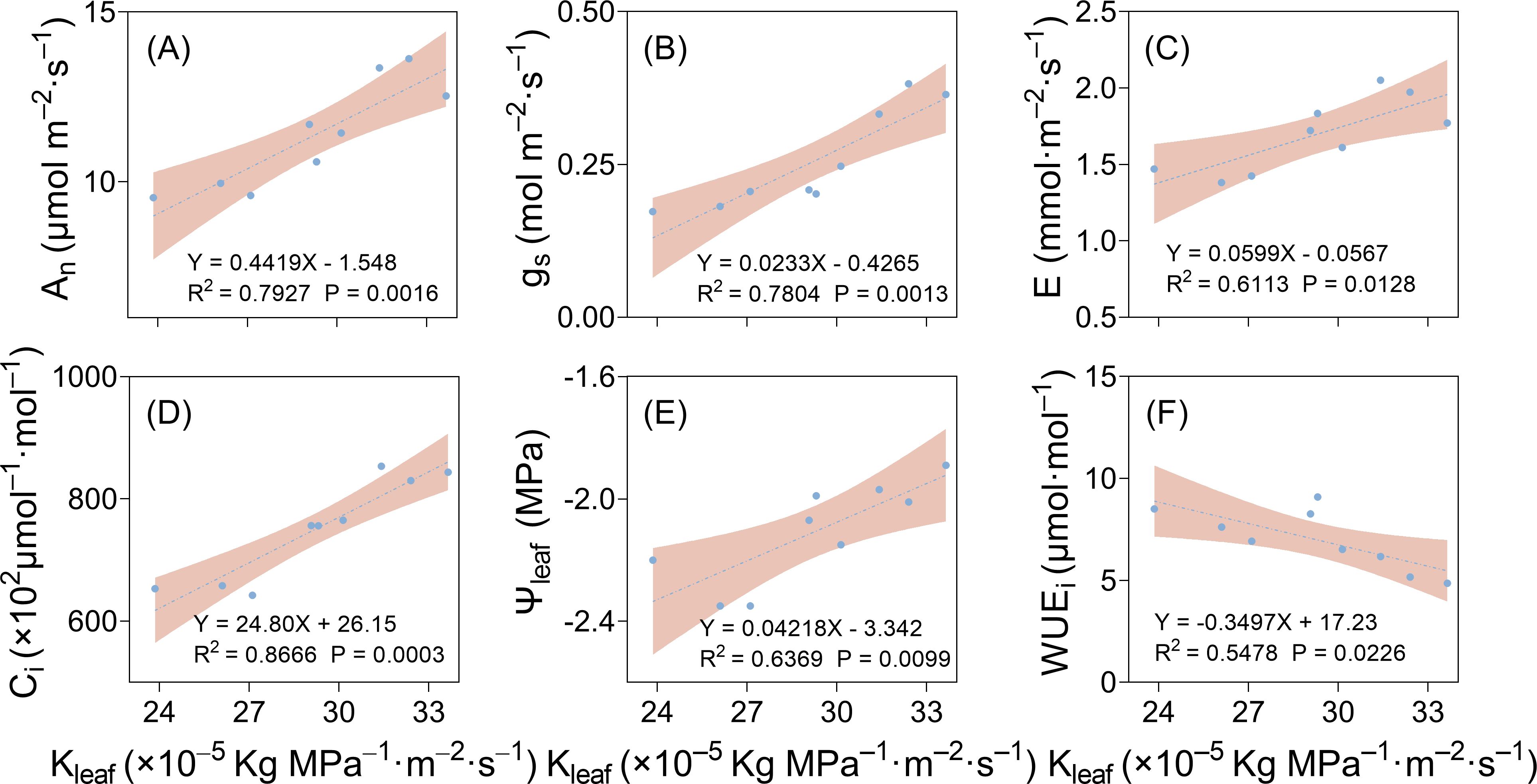
Figure 10. Linear regression results of cotton leaf hydraulic and stomatal conductance (A), net photosynthetic rate (B), transpiration rate (C), intercellular carbon dioxide concentration (D), leaf water potential (E), and instantaneous water use efficiency (F) of cotton plants.
There was a positive correlation between Kleaf and leaf water potential (Figure 10E) and a negative correlation between Kleaf and instantaneous leaf water use efficiency (Figure 10F). Therefore, drought stress decreased leaf xylem vessel diameter, number, and Kleaf, leading to a decrease in leaf water potential and an increase in instantaneous leaf water use efficiency.
4 Discussion
4.1 Effects of drought stress on hydraulic traits in cotton
Leaf hydraulic dysfunction is a pivotal adaptive response when plants face drought stress, critically influencing plant fitness under water stress conditions (Blackman et al., 2010). Hydraulic traits act as multifaceted functional indices, governing water transport efficiency and stomatal regulation (Creek et al., 2018), and shaping plant ecological strategies in growth dynamics and resource competition (Cosme et al., 2017; Poorter et al., 2017). Previous studies have revealed species-specific drought adaptation mechanisms: rice enhances water-use efficiency by reducing leaf water potential (Yang et al., 2024), while maize prioritizes drought tolerance by decreasing leaf hydraulic conductivity (Kleaf) (Qiao et al., 2020). These findings align with current experimental evidence suggesting that progressive drought intensification reduces vascular water supply capacity, with synchronously decreasing Kleaf (Figure 3A) and leaf water potential (Figure 3B). This indicates that cotton plants adapt to drought conditions by reducing Kleaf and leaf water potential, thereby maintaining normal growth.
4.2 Factors affecting Kleaf under drought stress
Kleaf is a critical hydraulic signal modulated by multifaceted anatomical and molecular factors (Sack and Scoffoni, 2013). Water transport in leaves operates through two sequential pathways: xylem vessels in petioles and vascular bundles, and the post-xylem pathway involving aquaporin-mediated membrane transport. These pathways contribute substantially to total leaf hydraulic resistance, with their coordinated regulation directly determining Kleaf (Prado and Maurel, 2013; Kaack et al., 2021). Our findings revealed that drought stress disrupted these pathways synergistically—reducing vein density, xylem vessel diameters and numbers, and aquaporin expression—collectively impairing hydraulic efficiency. This dual pathway suppression provides mechanistic insights into drought-induced Kleaf decline.
VLAminor plays a key role in leaf pulp hydraulic transport, especially under drought stress, and its regulatory role significantly affects leaf hydraulic efficiency and photosynthetic capacity. Unlike the VLAmajor, which mainly provides mechanical support and water redundancy, VLAminor is directly involved in water transport between leaf pulp cells, thereby affecting Kleaf and gas exchange efficiency (Baird et al., 2021). In this study, drought stress significantly reduced VLAminor (40.31% and 41.86% decrease in MD and SD treatments, respectively), which was significantly and positively correlated with the decrease in Kleaf (Figure 7B; Supplementary Figure S1B). This finding contrasts with the findings in rice, where there was no correlation between VLAminor and Kleaf under drought conditions (Xiong et al., 2015; Caringella et al., 2015; Scoffoni et al., 2011), whereas cotton exhibited a significant decrease in VLAminor, leading to a decrease in Kleaf. This difference may stem from the differences in leaf vein structure and water utilization strategies between cotton and rice. Rice, as a monocotyledon, has a parallel leaf vein structure with higher hydraulic redundancy under drought conditions, whereas the reticulate leaf vein structure of cotton is more susceptible to drought-induced embolism and cell wall thickening (Zou et al., 2022). In addition, the decrease in VLAminor may also be related to anatomical changes in cotton chloroplasts, such as a reduction in the thickness of spongy tissue (Table 2), which further limits the efficiency of water transport between chloroplasts (Sack and Frole, 2006). Thus, VLAminor is not only a key regulator of cotton leaf hydraulic efficiency, but also an important component of its drought adaptation strategy. Future studies should further explore the potential of increasing VLAminor through genetic improvement or agronomic measures to enhance the hydraulic efficiency and photosynthetic performance of cotton under drought conditions.
Plant water transport relies on the axial xylem vessel system (Cochard et al., 2004). This hydraulic pathway initiates soil water absorption via roots, progressing through root-to-stem xylem networks, petiolar vessels, and leaf vein vessels, ultimately delivering water to mesophyll cells for transpirational loss through stomata. Xylem vessel morphology directly regulates water transport efficiency from stems to foliar tissues (Kaack et al., 2021). Our investigation revealed marked anatomical alterations under drought conditions, including a diminished xylem vessel diameter, frequency, and cross-sectional area in both leaves and petioles (Figure 5; Tables 1, 2), which confirmed that stomatal change served as an adaptive strategy for mitigating water loss while maintaining plant viability. These structural modifications highlight the physiological acclimation mechanism in cotton under water deficit.
Previous studies have shown that the morphology of xylem vessels affects Kleaf. The larger the vessel diameters and the greater their number, the larger the Kleaf, resulting in enhanced water transport capacity. Conversely, smaller vessel diameters, reduced vessel areas, and fewer vessels increase the hydraulic resistance of the leaf, thereby reducing Kleaf (Aasamaa et al., 2005; Jafarikouhini and Sinclair, 2023). Our study identified four key anatomical determinants, namely, upper epidermal cell thickness, xylem area in leaf/petiole tissues, vessel diameter, and vascular bundle frequency, that exhibited significant positive correlations with Kleaf (Figures 8, 9; Supplementary Table S4). Notably, xylem vascular architecture emerges as the principal modulator of leaf hydraulic vulnerability under mild-to-moderate drought (Bouche et al., 2015; Trifiló et al., 2016). Under severe drought conditions, xylem embolism can lead to irreversible damage, significantly affecting water transport and leaf hydraulic conductivity (Knipfer et al., 2015). The phenomenon of embolism severely impedes water transport, leading to an increase in hydraulic resistance (Trifiló et al., 2016). Our study observed that under drought stress, both the number and diameter of xylem vessels in the leaves and petioles decreased under the MD and SD treatments (Tables 1, 2), indicating the occurrence of changes in the xylem vessels that resulted in a significant reduction in Kleaf (Figure 3A). Although embolism quantification remains technically challenging in herbaceous species, the observed structural degradation under SD conditions suggests its detrimental role in Kleaf reduction. Future studies should prioritize non-destructive embolism detection techniques to elucidate their long-term impacts on hydraulic performance and refine our understanding of drought adaptation strategies in crops.
Aquaporins, which are pivotal regulators of plant water homeostasis, mediate critical physiological processes, including transmembrane water transport and stress responses. Specifically, the decrease in aquaporins gene expression directly affected Kleaf, as aquaporins play a key role in water transport in chloroplasts and vascular sheath cells (Maurel et al., 2016). It is generally believed that under drought stress conditions, plants maintain their internal water by downregulating aquaporin synthesis gene expression levels and reducing aquaporin content (Afzal et al., 2016). In this study, the aquaporin content under the CK treatment was significantly higher than under the MD and SD treatments (Figure 4A). This aligns with the results of Šurbanovski et al. (2013), who demonstrated drought intensity-dependent suppression of PIP isoforms, and Xue et al. (2021), who reported coordinated PIP/TIP depression in drought-stressed strawberry. In this study, under the MD treatment, the expression of PIP-related synthesis genes in cotton leaves were down-regulated by 1.0–1.5-fold, while under the SD treatment, it decreased by 2.0–2.5-fold. Furthermore, under both drought treatments, the expression of TIP-related synthesis genes also decreased by 1.0–1.5-fold (Figure 4B). These results suggest that cotton adjusts aquaporin gene expression in its leaves to regulate the aquaporin content, thereby controlling the water transport efficiency. Thus, changes in aquaporins genes expression are not only an important molecular marker of cotton’s response to drought stress, but also a key driver of its reduced hydraulic efficiency and photosynthetic performance. Future studies could regulate the expression of aquaporins through gene editing or transgenic techniques to explore their potential in improving drought tolerance in cotton.
4.3 Decrease in leaf hydraulic conductivity under drought stress leads to decreases in photosynthetic functions
Green plants are confronted with a contradictory challenge—maximizing absorption of carbon dioxide for photosynthesis while minimizing water loss. Stomatal regulation plays a crucial role in this process (Harayama et al., 2019). Stomata not only regulate the entry of carbon dioxide but also control water evaporation. Therefore, stomatal behavior is vital for plant responses to water stress. Typically, plants regulate gs by changing stomatal size or stomatal density, which allows them to maintain growth and physiological activities under constantly changing environmental conditions (Fang et al., 2019). This experiment demonstrated that gs was significantly and positively correlated with stomatal width, stomatal area, and stomatal aperture, but it showed a highly significant negative correlation with stomatal density (Supplementary Figure S4A–D). These results suggest that changes in stomatal traits directly affect stomatal conductance, thereby influencing the plant’s photosynthetic capacity. Under drought conditions, leaves with smaller stomatal sizes and higher densities are more sensitive to changes in the external environment, with faster opening and closing speeds, which effectively reduce water loss and enhance water use efficiency (Raven, 2014; Kardiman and Ræbild, 2017). In this study, MD and SD treatments decreased stomatal width by 6.83% and 13.37%, respectively, and stomatal aperture by 20.59% and 27.58%, respectively, and Kleaf significantly decreased under both treatments (Figure 3A). These findings suggest that plants reduce water loss and maintain growth by decreasing stomatal size and increasing stomatal density.
Previous studies across various species have shown that under short-term environmental changes, gs and Kleaf exhibit a positive correlation, suggesting that Kleaf is a potential trigger for the decrease in gs (Theroux Rancourt et al., 2015; Xiong et al., 2018; Wang et al., 2018). Gleason et al. (2017) found that the plant’s water supply capacity weakened as drought stress intensified, decreasing Kleaf, which triggered stomatal closure, reduced the photosynthetic rate, and improved the water use efficiency in the leaves. This further confirms that Kleaf is significantly positively correlated with stomatal aperture and width (Figure 9; Supplementary Table S4), indicating that as Kleaf decreases, the stomatal width and aperture also decrease, directly affecting the plant’s photosynthetic function. In addition, Kleaf had an extremely significant positive correlation with gs and An under different drought treatments (Figures 10A, B). These findings suggested that drought stress affected hydraulic signaling by reducing Kleaf, which triggers rapid stomatal closure and a decrease in aperture, ultimately reducing water loss. However, this adaptive mechanism also decreases photosynthesis.
While our study provides valuable insights into the physiological and anatomical responses of cotton leaves to drought stress using potted plants in a greenhouse, it is important to acknowledge some limitations. The experimental setup, while allowing precise control of conditions, may not fully replicate field complexities. Consequently, yield and quality traits, which are critical for assessing the long-term impact of drought stress on cotton production, were not measured. Future research should extend these findings to field trials and explore the impacts of prolonged drought.
5 Conclusion
Our findings demonstrated that drought stress significantly reduced Kleaf in cotton by altering leaf anatomical traits, such as decreasing xylem vessel diameter, number, and area, as well as aquaporin content. The decline in Kleaf was closely associated with reductions in stomatal aperture, gs, and An, which impaired plant growth. Adaptive responses, such as increased VLAmajor and reduced leaf area, mitigated water loss under drought conditions. Notably, the strong correlation between Kleaf and VLAminor highlighted its critical role in maintaining hydraulic efficiency and photosynthetic function. Strategies aimed at improving Kleaf, such as optimizing xylem morphology and increasing VLAminor, could enhance drought tolerance in cotton.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
XLL: Conceptualization, Methodology, Investigation, Writing – review & editing, Software, Writing – original draft, Formal Analysis, Data curation. SW: Writing – review & editing, Writing – original draft, Formal Analysis, Methodology, Data curation, Conceptualization, Investigation. LZ: Writing – review & editing, Methodology, Software. PZ: Methodology, Supervision, Writing – review & editing. HQ: Methodology, Conceptualization, Writing – review & editing. KZ: Methodology, Supervision, Writing – review & editing. HS: Writing – review & editing, Supervision, Methodology, Software. YZ: Supervision, Software, Methodology, Writing – review & editing. XPL: Methodology, Conceptualization, Writing – review & editing. AL: Writing – review & editing, Methodology, Software. ZW: Writing – review & editing, Supervision, Methodology. CL: Methodology, Supervision, Writing – review & editing. LL: Conceptualization, Methodology, Supervision, Writing – review & editing, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (No.32272220), the Hebei Agriculture Research System (HBCT2024100207), S&T Program of Hebei (23567601H), and the Natural Science Foundation of Hebei Province (C2024204027).
Acknowledgments
We extend our sincere gratitude to LetPub (www.letpub.com.cn) and the other technicians for their assistance in conducting the field experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1622308/full#supplementary-material
References
Aasamaa, K., Niinemets, U., and Sober, A. (2005). Leaf hydraulic conductance in relation to anatomical and functional traits during Populus tremula leaf ontogeny. Tree Physiol. 25, 1409–1418. doi: 10.1093/treephys/25.11.1409
Abdelraheem, A., Esmaeili, N., O’Connell, M., and Zhang, J. (2019). Progress and perspective on drought and salt stress tolerance in cotton. Ind. Crops Prod. 130, 118–129. doi: 10.1016/j.indcrop.2018.12.070
Afzal, Z., Howton, T., Sun, Y., and Mukhtar, M. (2016). The roles of aquaporins in plant stress responses. JDB 4, 9. doi: 10.3390/jdb4010009
Ahluwalia, O., Singh, P. C., and Bhatia, R. (2021). A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 5, 100032. doi: 10.1016/j.resenv.2021.100032
Baird, A. S., Taylor, S. H., Pasquet-Kok, J., Vuong, C., Zhang, Y., Watcharamongkol, T., et al. (2021). Developmental and biophysical determinants of grass leaf size worldwide. Nature 592, 242–247. doi: 10.1038/s41586-021-03370-0
Bista, K. M., Adhikari, B., Sankarapillai, V. L., Pieralisi, B., Reddy, K. R., Jenkins, J., et al. (2024). Drought and heat stress induce differential physiological and agronomic trait responses in cotton. Ind. Crop Prod. 222, 11950. doi: 10.1016/j.indcrop.2024.119540
Blackman, C. J., Brodribb, T. J., and Jordan, G. J. (2010). Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytol. 188, 1113–1123. doi: 10.1111/j.1469-8137.2010.03439.x
Bouche, P. S., Delzon, S., Choat, B., Badel, E., Brodribb, T. J., Burlett, R., et al. (2015). Are needles of Pinus pinaster more vulnerable to xylem embolism than branches? New insights from X-ray computed tomography. Plant Cell Environ. 39, 860–870. doi: 10.1111/pce.12680
Cao, Y., Yang, W., Ma, J., Cheng, Z., Zhang, X., Liu, X., et al. (2024). An integrated framework for drought stress in plants. IJMS 25, 9347. doi: 10.3390/ijms25179347
Caringella, M. A., Bongers, F. J., and Sack, L. (2015). Leaf hydraulic conductance varies with vein anatomy across Arabidopsis thaliana wild-type and leaf vein mutants. Plant Cell Environ. 38, 2735–2746. doi: 10.1111/pce.12584
Cochard, H., Froux, F., Mayr, S., and Coutand, C. (2004). Xylem wall collapse in water-stressed pine needles. Plant Physiol. 134, 401–408. doi: 10.1104/pp.103.028357
Cosme, L. H. M., Schietti, J., Costa, F. R. C., and Oliveira, R. S. (2017). The importance of hydraulic architecture to the distribution patterns of trees in a central Amazonian forest. New Phytol. 215, 113–125. doi: 10.1111/nph.14508
Creek, D., Blackman, C. J., Brodribb, T. J., Choat, B., and Tissue, D. T. (2018). Coordination between leaf, stem, and root hydraulics and gas exchange in three arid-zone angiosperms during severe drought and recovery. Plant Cell Environ. 41, 2869–2881. doi: 10.1111/pce.13418
Dayer, S., Herrera, J. C., Dai, Z., Burlett, R., Lamarque, L. J., Delzon, S., et al. (2020). The sequence and thresholds of leaf hydraulic traits underlying grapevine varietal differences in drought tolerance. J. Exp. Bot. 71, 4333–4344. doi: 10.1093/jxb/eraa186
Fang, L., Abdelhakim, L. O. A., Hegelund, J. N., Li, S., Liu, J., Peng, X., et al. (2019). ABA-mediated regulation of leaf and root hydraulic conductance in tomato grown at elevated CO2 is associated with altered gene expression of aquaporins. Hortic. Res. 6, 104. doi: 10.1038/s41438-019-0187-6
Garcia-Forner, N., Carvalho, A., and Campelo, F. (2021). Water and stem girdling affect the tracheids’ number more than their shape in Pinus pinaster saplings. Trees 35, 1921–1931. doi: 10.1007/s00468-021-02160-5
Gleason, D. R. W., Bliss, C. A., Louise, H., and Comas (2017). Coordinated decline in photosynthesis and hydraulic conductance during drought stress in Zea mays. Flora 227, 1–9. doi: 10.1016/j.flora.2016.11.017
Gray, S. B., Dermody, O., Klein, S. P., Locke, A. M., McGrath, J. M., Paul, R. E., et al. (2016). Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nat. Plants. 2, 16132. doi: 10.1038/nplants.2016.132
Harayama, H., Kitao, M., Agathokleous, E., and Ishida, A. (2019). Effects of major vein blockage and aquaporin inhibition on leaf hydraulics and stomatal conductance. Proc. R. Soc B. 286, 20190799. doi: 10.1098/rspb.2019.0799
Jafarikouhini, N. and Sinclair, T. R. (2023). Hydraulic conductance and xylem vessel diameter of young maize roots subjected to sustained water-deficit. Crop Sci. 63, 2458–2464. doi: 10.1002/csc2.21023
Kaack, L., Weber, M., Isasa, E., Karimi, Z., Li, S., Pereira, L., et al. (2021). Pore constrictions in intervessel pit membranes provide a mechanistic explanation for xylem embolism resistance in angiosperms. New Phytol. 230, 1829–1843. doi: 10.1111/nph.17282
Kardiman, R. and Ræbild, A. (2017). Relationship between stomatal density, size and speed of opening in Sumatran rainforest species. Tree Physiol. 38, 696–705. doi: 10.1093/treephys/tpx149
Knipfer, T., Eustis, A., Brodersen, C., Walker, A. M., and Mcelrone, A. J. (2015). Grapevine species from varied native habitats exhibit differences in embolism formation/repair associated with leaf gas exchange and root pressure: Contrasting response of wild grapevines to drought stress. Plant Cell Environ. 38, 1503–1513. doi: 10.1111/pce.12497
Lai, Z., Zhang, K., Liao, Z., Kou, H., Pei, S., Dou, Z., et al. (2024). Stem hydraulic conductance, leaf photosynthesis, and carbon metabolism responses of cotton to short-term drought and rewatering. Agronomy 14, 71. doi: 10.3390/agronomy14010071
Langhansová, L., Dvořáková, M., Revutska, A., Petrová, Š., Hirnerová, A., Bouček, J., et al. (2024). The impact of the application of compochar on soil moisture, stress, yield and nutritional properties of legumes under drought stress. Sci. Total Environ. 914, 169914. doi: 10.1016/j.scitotenv.2024.169914
Li, S., Hamani, A. K. M., Zhang, Y., Liang, Y., Gao, Y., and Duan, A. (2021a). Coordination of leaf hydraulic, anatomical, and economical traits in tomato seedlings acclimation to long-term drought. BMC Plant Biol. 21, 536. doi: 10.1186/s12870-021-03304-y
Li, S., Liu, J., Liu, H., Qiu, R., Gao, Y., and Duan, A. (2021b). Corrigendum: Role of hydraulic signal and ABA in decrease of leaf Stomatal and mesophyll conductance in soil drought-stressed tomato. Front. Plant Sci. 12, 710792. doi: 10.3389/fpls.2021.710792
Li, L., McCormack, M. L., Ma, C., Kong, D., Zhang, Q., Chen, X., et al. (2015). Leaf economics and hydraulic traits are decoupled in five species-rich tropical-subtropical forests. Ecol. Lett. 18, 899–906. doi: 10.1111/ele.12466
Lu, Z., Xie, K., Pan, Y., Ren, T., Lu, J., Wang, M., et al. (2019). Potassium mediates coordination of leaf photosynthesis and hydraulic conductance by modifications of leaf anatomy. Plant Cell Environ. 42, 2231–2244. doi: 10.1111/pce.13553
Mao, L., Zhang, L., Zhao, X., Liu, S., Werf, W., Zhang, S., et al. (2014). Crop growth, light utilization and yield of relay intercropped cotton as affected by plant density and a plant growth regulator. Field Crops Res. 155, 67–76. doi: 10.1016/j.fcr.2013.09.021
Maurel, C., Verdoucq, L., and Rodrigues, O. (2016). Aquaporins and plant transpiration. Plant Cell Environ. 39, 2580–2587. doi: 10.1111/pce.12814
Müllers, Y., Postma, J. A., Poorter, H., and Van Dusschoten, D. (2022). Stomatal conductance tracks soil-to-leaf hydraulic conductance in faba bean and maize during soil drying. Plant Physiol. 190, 2279–2294. doi: 10.1093/plphys/kiac422
Nardini, A., Gortan, E., and Salleo, S. (2005). Hydraulic efficiency of the leaf venation system in sun- and shade-adapted species. Funct. Plant Biol. 32, 953–961. doi: 10.1071/FP05100
Poorter, L., van der Sande, M. T., Arets, E. J. M. M., Ascarrunz, N., Enquist, B. J., Finegan, B., et al. (2017). Biodiversity and climate determine the functioning of Neotropical forests. Global Ecol. Biogeogr. 26, 1423–1434. doi: 10.1111/geb.12668
Prado, K. and Maurel, C. (2013). Regulation of leaf hydraulics: from molecular to whole plant levels. Front. Plant Sci. 4. doi: 10.3389/fpls.2013.00255
Qiao, Y., Ren, J., Yin, L., Liu, Y., Deng, X., Liu, P., et al. (2020). Exogenous melatonin alleviates PEG-induced short-term water deficiency in maize by increasing hydraulic conductance. BMC Plant Biol. 20, 218. doi: 10.1186/s12870-020-02432-1
Rehman, T., Tabassum, B., Yousaf, S., Sarwar, G., and Qaisar, U. (2022). Consequences of drought stress encountered during seedling stage on physiology and yield of cultivated cotton. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.906444
Ru, C., Hu, X., Wang, W., and Yan, H. (2024). Impact of nitrogen on photosynthesis, remobilization, yield, and efficiency in winter wheat under heat and drought stress. Agric. Water Manage. 302, 109013. doi: 10.1016/j.agwat.2024.109013
Sack, L. (2002). The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods. J. Exp. Bot. 53, 2177–2184. doi: 10.1093/jxb/erf069
Sack, L. and Frole, K. (2006). Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology 87, 483–491. doi: 10.1890/05-0710
Sack, L. and Scoffoni, C. (2013). Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 198, 983–1000. doi: 10.1111/nph.12253
Sack, L., Tyree, M. T., and Holbrook, N. M. (2005). Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytol. 167, 403–413. doi: 10.1111/j.1469-8137.2005.01432.x
Sáez, P. L., Galmés, J., Ramírez, C. F., Poblete, L., Rivera, B. K., Cavieres, L. A., et al. (2018). Mesophyll conductance to CO2 is the most significant limitation to photosynthesis at different temperatures and water availabilities in Antarctic vascular species. Environ. Exp. Bot. 156, 279–287. doi: 10.1016/j.envexpbot.2018.09.008
Scoffoni, C., Rawls, M., McKown, A., Cochard, H., and Sack, L. (2011). Decline of leaf hydraulic conductance with dehydration: relationship to leaf size and venation architecture. Plant Physiol. 156, 832–843. doi: 10.1104/pp.111.173856
Seleiman, M. F., Al-Suhaibani, N., Ali, N., Akmal, M., Alotaibi, M., Refay, Y., et al. (2021). Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10, 259. doi: 10.3390/plants10020259
Song, W., Song, R., Zhao, Y., and Zhao, Y. (2023). Research on the characteristics of drought stress state based on plant stem water content. Sustain. Energy Technol. Assess. 56, 103080. doi: 10.1016/j.seta.2023.103080
Šurbanovski, N., Sargent, D. J., Else, M. A., Simpson, D. W., Zhang, H., and Grant, O. M. (2013). Expression of Fragaria vesca PIP aquaporins in response to drought stress: PIP down-regulation correlates with the decline in substrate moisture content. PloS One 8, e74945. doi: 10.1371/journal.pone.0074945
Theroux Rancourt, G., Ethier, G., and Pepin, S. (2015). Greater efficiency of water use in poplar clones having a delayed response of mesophyll conductance to drought. Tree Physiol. 35, 172–184. doi: 10.1093/treephys/tpv006
Trifiló, P., Raimondo, F., Savi, T., Gullo, M. A. L., and Nardini, A. (2016). The contribution of vascular and extra-vascular water pathways to drought-induced decline of leaf hydraulic conductance. J. Exp. Bot. 67, 5029–5039. doi: 10.1093/jxb/erw268
Villagra, M., Campanello, P. I., Bucci, S. J., and Goldstein, G. (2013). Functional relationships between leaf hydraulics and leaf economic traits in response to nutrient addition in subtropical tree species. Tree Physiol. 33, 1308–1318. doi: 10.1093/treephys/tpt098
Wang, X., Du, T., Huang, J., Peng, S., and Xiong, D. (2018). Leaf hydraulic vulnerability triggers the decline in stomatal and mesophyll conductance during drought in rice. J. Exp. Bot. 69, 4033–4045. doi: 10.1093/jxb/ery188
Xiong, D., Douthe, C., and Flexas, J. (2018). Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species: Coordination of CO 2 diffusion and H2O transport inside leaves. Plant Cell Environ. 41, 436–450. doi: 10.1111/pce.13111
Xiong, D., Yu, T., Zhang, T., Li, Y., Peng, S., and Huang, J. (2015). Leaf hydraulic conductance is coordinated with leaf morpho-anatomical traits and nitrogen status in the genus Oryza. J. Exp. Bot. 66, 741–748. doi: 10.1093/jxb/eru43
Xue, F., Liu, W., Cao, H., Song, L., Ji, S., Tong, L., et al. (2021). Stomatal conductance of tomato leaves is regulated by both abscisic acid and leaf water potential under combined water and salt stress. Physiol. Plant 172, 2070–2078. doi: 10.1111/ppl.13441
Yang, C., Lu, J., Xiong, Z., Wang, B., Ren, T., Cong, R., et al. (2024). Potassium deficiency enhances imbalances in rice water relations under water deficit by decreasing leaf hydraulic conductance. Physiol. Plant 176, e14360. doi: 10.1111/ppl.14360
Ye, M., Wu, M., Zhang, H., Zhang, Z., and Zhang, Z. (2021). High leaf vein density promotes leaf gas exchange by enhancing leaf hydraulic conductance in Oryza sativa L. Plants. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.693815
Zafar, M. M., Chattha, W. S., Khan, A. I., Zafar, S., Subhan, M., Saleem, H., et al. (2023). Drought and heat stress on cotton genotypes suggested agro-physiological and biochemical features for climate resilience. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1265700
Zhang, C., Li, W., Gao, Y., Xu, Z., and Tian, X. (2022). Artificial regulation effect of plant retardants on leaf anatomical characteristics of Elaeagnus angustifolia. Front. Environ. Sci. 10. doi: 10.3389/fenvs.2022.900960
Ziegler, C., Levionnois, S., Bonal, D., Heuret, P., Stahl, C., and Coste, S. (2023). Large leaf hydraulic safety margins limit the risk of drought-induced leaf hydraulic dysfunction in Neotropical rainforest canopy tree species. Funct. Ecol. 37, 1717–1731. doi: 10.1111/1365-2435.14325
Keywords: drought stress, leaf hydraulic conductivity, leaf anatomy, stomatal characteristics, photosynthetic traits
Citation: Li X, Wang S, Zhu L, Zhang P, Qi H, Zhang K, Sun H, Zhang Y, Lei X, Li A, Wang Z, Li C and Liu L (2025) Leaf hydraulic decline coordinates stomatal and photosynthetic limitations through anatomical adjustments under drought stress in cotton. Front. Plant Sci. 16:1622308. doi: 10.3389/fpls.2025.1622308
Received: 03 May 2025; Accepted: 16 June 2025;
Published: 10 July 2025.
Edited by:
Ahsan Ayyaz, Zhejiang University, ChinaReviewed by:
Muhammad Saeed, Government College University, Faisalabad, PakistanMohan K. Bista, Mississippi State University, United States
Copyright © 2025 Li, Wang, Zhu, Zhang, Qi, Zhang, Sun, Zhang, Lei, Li, Wang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liantao Liu, bGl1bHRkYXlAMTI2LmNvbQ==; Cundong Li, bnh5bGNkQGhlYmF1LmVkdS5jbg==; Zhanbiao Wang, d2FuZ196aGFuYmlhb0AxMjYuY29t
†These authors have contributed equally to this work
 Xiuli Li1,2,3†
Xiuli Li1,2,3† Lingxiao Zhu
Lingxiao Zhu Ke Zhang
Ke Zhang Hongchun Sun
Hongchun Sun Yongjiang Zhang
Yongjiang Zhang Zhanbiao Wang
Zhanbiao Wang Cundong Li
Cundong Li Liantao Liu
Liantao Liu