- 1National Key Laboratory of Ecological Environment of Arid Region Water Engineering, Inner Mongolia Agricultural University, Huhhot, China
- 2Collaborative Innovation Center for Integrated Management of Water Resources and Water Environment in the Inner Mongolia Reaches of the Yellow River, Hohhot, China
- 3Research and Development of Efficient Water-saving Technology and Equipment and Research Engineering Center of Soil and Water Environment Effect in Arid Area of Inner Mongolia Autonomous Region, Hohhot, China
Introduction: Nitrogen (N) can significantly affect the photosynthetic rate (Pn) of plants. Under traditional nitrogen fertilization (TNF) or inappropriate nitrogen application, leaf N is often redistributed to support the seed protein accumulation rather than the photosynthesis in the later stages of crop growth. Controlled-release fertilizers (CRF) have been reported to effectively reduce the nitrogen loss by matching the release pattern with crop N demand, thus increasing the yield. However, the changes in N allocation to enhance the photosynthesis under CRF have rarely been addressed.
Methods: A two-year field experiment was conducted in the Hetao Irrigation District, Inner Mongolia, China from 2019 to 2020 to evaluate the effects of different fertilization strategies on soil NO3-N concentration, leaf nitrogen content, photosynthetic characteristics, yield, and nitrogen use efficiency (NUE) in sunflowers. The treatments included the CRF application rates of 135, 225, and 315 kg/ha (CRF135, CRF225, and CRF315), and that of TNF at 225 kg/ha (TNF225).
Results: The results demonstrated that applying CRF at an appropriate rate maintained a high level of photosynthetic nitrogen content in the leaves during the later growth stages. This rate ensured a suitable soil NO3-N concentration (SNC), resulting in a 76.10% higher proportion of photosynthetic nitrogen (Npsn) than TNF at the same rate, significantly enhancing the photosynthetic nitrogen efficiency (PNUE) and highlighting the crucial role of nitrogen management in improving the crop productivity and NUE. Additionally, at CRF225, the net photosynthesis (Pn), stomatal conductance (Gs), and intercellular CO2 concentration (Ci) at maturity increased by 32.80%, 96.16%, and 13.56%, respectively, compared to TNF, leading to an 11.84% improvement in yield and a 9.70% increase in NUE.
Discussion: The correlation analysis confirmed a strong positive relationship between leaf N redistribution and photosynthetic efficiency, demonstrating the potential of CRF to improve the photosynthetic efficiency, optimize the N management, and promote the environmental sustainability in sunflower cultivation.
1 Introduction
The global demand for food has been expected to surge as the population will approach 9.7 billion by 2050 (ECONOMIC, U.N.D.F and AFFAIRS, S, 2023), presenting an urgent challenge to enhance the crop production efficiency and ensure the sustainable management (Li et al., 2016; Liu et al., 2020). The excessive and improperly managed nitrogen fertilizers not only elevate production costs but also lead to adverse environmental impacts, including water body eutrophication, soil degradation, and greenhouse gas emissions (Penuelas and Sardans, 2022; Zhao et al., 2024). Extensive field research has demonstrated that optimizing nitrogen fertilizer application patterns can produce more grains with less nitrogen. Specifically, the yields for wheat, corn, and rice have increased by 10% to 19%, while the nitrogen fertilizer applications have decreased by 15% to 19%, resulting in a 32–46% improvement in NUE and a 40% reduction in nitrogen surplus (Ren et al., 2022b). Furthermore, a comprehensive review of over 8,000 studies across multiple countries revealed that CRF significantly improved the crop yield by 5.1%, the farmer profitability by 8.2%, and the total nitrogen uptake by crops by 7.1%, while substantially reducing the environmental pollution, including the greenhouse gas emissions by 3.6% to 18.6% and the nitrogen losses by 32.6% to 49.1%, compared to the traditional fertilizers (Zhang et al., 2024). Thus, optimizing fertilization strategies and selecting appropriate fertilizer types are crucial for increasing NUE, enhancing crop yield, and mitigating environmental pollution risks.
The nitrogen fertilizer management is pivotal in modern agriculture as it directly influences the crop yield and ecosystem health (Huang et al., 2024; Seleiman et al., 2020; Wan et al., 2021). Represented by urea, traditional nitrogen fertilizers (TNF) can pose significant environmental and crop health risks when inappropriately applied (Carlson et al., 2016; Stolarski et al., 2017; Wang et al., 2022c). Although TNF can quickly enhance the crop nutrition, its high loss rates and low NUE contribute to increased production costs and environmental problems, such as soil acidification, groundwater pollution, and greenhouse gas emissions (Walling and Vaneeckhaute, 2020; Wang et al., 2022b). The nutrient losses from N fertilizers at approximately 50% highlight the inefficiencies in fertilizer use (Bindraban et al., 2020; Coskun et al., 2017). CRF designed with specific coating technologies to regulate nitrogen release offers a solution by aligning nutrient delivery with crop growth needs and absorption patterns (Hou et al., 2024; Salvagiotti et al., 2008; Sim et al., 2021). This approach not only improves the nitrogen utilization and crop nutrition (Cao et al., 2021; Vejan et al., 2021), but also reduces the environmental impact and supports healthy, stable crop growth, improving yield and quality (Hou et al., 2023; Li et al., 2020; Zhang et al., 2023). Despite evidence that CRF can enhance the nitrogen utilization efficiency and wheat yield by 32.49% and 18.20%, respectively, there is still potential for improvement (Ma et al., 2023). Sunflower is a major oilseed crop with high adaptability to arid and semi-arid climates (Ebrahimian et al., 2019; García-López et al., 2016). In northern China, particularly in regions like the Hetao Irrigation District, sunflower is widely cultivated due to its drought tolerance, relatively low water demand, and economic value (He and Liu, 2024; Ren et al., 2018). Compared to cereal crops, sunflower exhibits distinct nitrogen uptake dynamics and biomass partitioning patterns, making it a suitable model crop for studying NUE and the effectiveness of CRF under variable water-nitrogen conditions (Li et al., 2022; Ren et al., 2018). This is due to the need for a better understanding of CRF release dynamics and nitrogen application management. Therefore, further exploration of CRF’s role in the nitrogen release, crop responses, and environmental impacts is crucial for advancing agricultural practices towards greater efficiency and sustainability.
In exploring the sustainable optimization strategies for crop production, enhancing the photosynthetic efficiency is crucial, as it can directly affect the crop biomass accumulation and final yield (Mahmood et al., 2023; Wang et al., 2022a; Wu et al., 2019). The nitrogen supply can significantly influence photosynthesis by affecting the leaf structure and internal nitrogen distribution. The nitrogen deficiency can reduce photosynthesis, leaf area, and the lifespan of green leaves, thereby affecting the plant productivity (Liao et al., 2022; Nasar et al., 2022; Zhang et al., 2025). The nitrogen in crop leaves is categorized into four main types: photosynthetic nitrogen, respiratory nitrogen, storage nitrogen, and structural nitrogen (Ali et al., 2016; Dai et al., 2024; Liu et al., 2018b; Sun et al., 2020). The photosynthetic nitrogen can be further divided into three systems: the carboxylation system (Ncb), which includes proteins such as Rubisco involved in the Calvin cycle (Dai et al., 2024; Qiang et al., 2023); the electron transport system (Net) referring to the proteins involved in electron transfer (Nolfi-Donegan et al., 2020; Yoshida and Hisabori, 2024); and the light-harvesting system (Ncl) that consists of the proteins in photosystems I and II and other light-harvesting pigment-protein complexes (Grouneva et al., 2016; Liu et al., 2018b). The non-photosynthetic nitrogen is classified into respiratory nitrogen (Nresp) that includes the respiratory enzymes in the mitochondrial matrix (Hou et al., 2019); storage nitrogen (Nstore) stored in tissues and does not participate in metabolic processes; and structural nitrogen (Nstr) primarily adopted to build cell walls and nucleic acids (Hu et al., 2023a). The distribution patterns of nitrogen components among various crops lead to the differences in species-specific net photosynthetic rates and NUE (Gu, 2023; Li et al., 2019; Liu et al., 2018a; Tian et al., 2022). Proper nitrogen distribution among the different functions is essential for the crop growth and photosynthetic efficiency (Gao et al., 2022; Hu et al., 2023a; Jia et al., 2021). Research has indicated that CRF can optimize the nitrogen distribution in the soil with their slow-release properties aligning with the physiological nitrogen needs of crops to ensure an adequate supply (Trenkel, 2021; Vejan et al., 2021). However, there is limited research on how CRF affects nitrogen distribution in crop leaves to enhance photosynthesis-related parameters, such as photosynthetic rate (Pn), stomatal conductance (Gs), and intercellular CO2 concentration (Ci). An optimized nitrogen management strategy using CRF can create a healthier and more efficient photosynthetic environment, significantly improving the sunflower growth rate and yield. Additionally, the CRF application can contribute to the improved soil health and ecosystem services by reducing nitrogen loss (Ma et al., 2023; Xiao et al., 2019), protecting soil structure, and preserving microbial diversity (Gao et al., 2022; Trenkel, 2021), all of which support the sustained and efficient photosynthesis (Gao et al., 2024; Iriti et al., 2019; Radušienė et al., 2019). Therefore, it is crucial to further investigate the effect of varying soil NO3-N concentrations throughout the crop growth period under different CRF nitrogen application conditions. This approach seeks to enhance photosynthesis by altering the nitrogen distribution among different functions in crop leaves, ultimately improving NUE and increasing crop yield.
This study aimed to systematically assess the impact of CRF on sunflower growth and nitrogen management, addressing the gaps in existing research and offering actionable recommendations for agricultural practice. The specific objectives were: (1) to evaluate the physiological mechanisms of photosynthetic nitrogen use efficiency (PNUE) in the sunflowers influenced by CRF by analyzing the optimized distribution of nitrogen in sunflower leaves under suitable nitrogen application conditions; (2) to explore how CRF optimized the photosynthesis in sunflowers by measuring the key parameters such as Pn, Gs, and Ci, thereby enhancing the photosynthetic efficiency; (3) to analyze the impact of CRF on the nitrogen accumulation and distribution in the sunflower plants and assess how this mechanism affected the crop yield; and (4) to compare CRF with TNF to discuss the advantages of CRF in improving NUE, and to determine the optimal nitrogen application rate for CRF treatment to achieve the environmentally friendly and economically efficient nitrogen management strategies.
2 Materials and methods
2.1 Experimental materials and design
This study was conducted in the Ganzhaomiao Town experimental field (40°47’54”N, 107°16’42”E), Linhe District, Bayannaoer City, Inner Mongolia situated in a mid-temperate semi-arid continental climate zone. The field features the sandy loam soil ideal for sunflower growth (USDA) with the average bulk density of 1.40 g/cm3. The soil nutrient testing of the 0–100 cm layer before sowing in spring 2019 revealed the organic matter content of 6.19 g/kg, the available nitrogen of 34.43 mg/kg, the available phosphorus of 1.84 mg/kg, the available potassium of 113.04 mg/kg, and a soil pH of 8.5. The total rainfall during the growing seasons of 2019 and 2020 was 106.2 and 76.4 mm, respectively. All the climatic data were provided by an automatic weather station (Onset Computer Inc., U30, Hobo, USA) located in the experimental field (Figure 1).
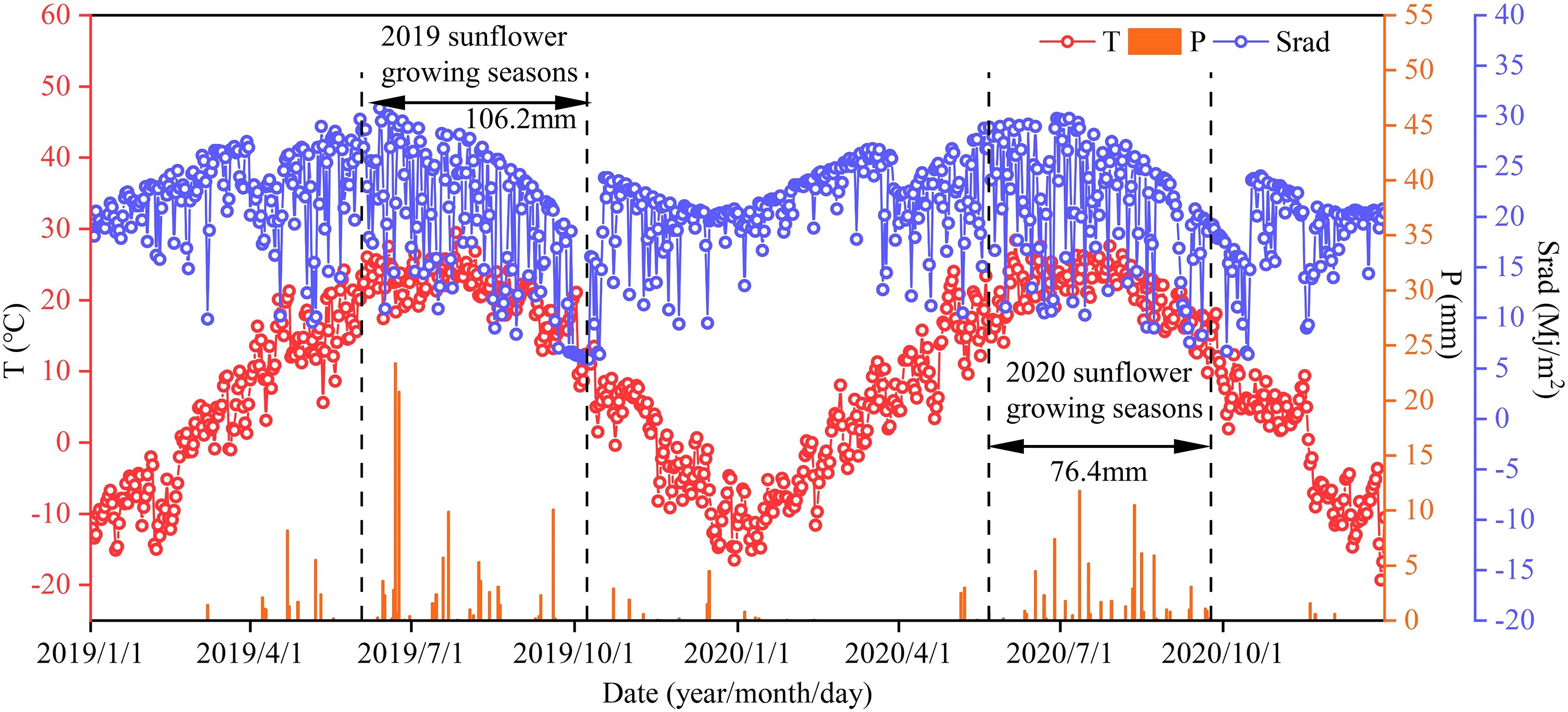
Figure 1. Average temperature (T), precipitation (P) and solar radiation (Srad) during the sunflower growing seasons in 2019 and 2020.
The sunflowers (Xinjiang Sanrui, SH361) were sown on June 3, 2019, and May 22, 2020, and harvested on October 8 and September 24, respectively. A conventional ridge–furrow planting system following a “one-film-two-rows” configuration was adopted. The study included four treatments: CRF at 135, 225, and 315 kg/ha, and TNF at 225 kg/ha. CRF was applied as a base fertilizer in a single application prior to sowing. TNF comprising the diammonium phosphate (18% N, 46% P2O5) as the base fertilizer and urea (46% N) as the top dressing was applied with the diammonium phosphate (1/3 N) before sowing, and the urea (2/3 N) was manually spread before irrigation at the budding stage. The furrow irrigation was performed on July 14 in both 2019 and 2020, with an irrigation amount of 120 mm each. Standard management measures were employed to control diseases, pests, and weeds.
The CRF used in this s experiment was the sixth-generation product developed by Tianjin Luyang Fertilizer Co., Ltd., with a nutrient composition of N:P:K = 28:12:10. It employs a bioactive double-membrane dual-control coating technology that enables precise regulation of nutrient release. The nutrient release rate in static water at 25°C is ≤5% within 24 hours, ≤15% within 7 days, and ≤65% within 28 days. The cumulative nutrient release over the release period is ≥80%. At an average soil temperature of 25°C, the nutrient release period is 70 d; at 15°C, it is 100 d; and at 10°C, it is 170 d.
2.2 Sampling and measurements
2.2.1 Soil NO3-N concentration
The soil NO3-N concentration (SNC) was measured using the semi-micro Kjeldahl method (Bremner, 1965). The soil samples were collected with a soil auger (Beijing New Landmark Soil Equipment Co., Ltd., 0301, XDB, CHN) from various depths (0–10 cm, 10–20 cm, 20–30 cm, 30–40 cm, 40–50 cm, 50–60 cm, 60–80 cm, and 80–100 cm) in both the mulched area (soil beneath the plastic film between the planted rows) and non-mulched area (soil between adjacent film strips without plastic cover) within 10- to 15-day intervals. To ensure the accuracy, three samples were collected for each measurement. The collected soil was air-dried, ground, thoroughly mixed to achieve uniformity, and passed through a 1 mm sieve. For the SNC determination, 5 g of soil was mixed with 25 mL of a 2 mol L-1 potassium chloride solution, shaken, and filtered, while the NO3-N concentration was quantified using an ultraviolet spectrophotometer (Beijing General Instrument Co., Ltd., TU-1901, CHN).
2.2.2 Photosynthetic characteristics
Three sunflower plants in each plot were selected and marked for the measurement of their photosynthetic characteristics at four stages: seedling (30 days after sowing (DAS), with 5–7 fully expanded leaves and 25–35 cm in height), budding (50 DAS, with visible apical flower buds and 12–16 leaves), flowering (70 DAS, with fully open capitulum and maximum leaf area development), and maturity (90 DAS, with top leaves still green and photosynthetically active). Measurements were performed nine times (three replicates, each repeated three times) using a portable photosynthesis system (Beijing, ECO Tech, Cpro T). The data collected included net photosynthetic rate (Pn, μmol CO2/m2/s), stomatal conductance (Gs, μmol H2O/m2/s), and intercellular CO2 concentration (Ci, μmol CO2/mol). Photosynthetically active radiation (PAR), CO2 concentration, flow rate, and leaf chamber temperature were set to 1700 μmol/m2/s, 380 μmol/mol, 500 μmol/s, and 30°C, respectively.
2.2.3 Leaf area
During the seedling, budding, flowering, and maturity stages of sunflowers, the length and width of the leaves from the bottom to the top of the plants were measured. The calculation formula (Equation 1) is as follows:
Where LA (cm2) is leaf area, Lenth (cm) is length of fully expanded leaves, width is length of fully expanded leaves, 0.75 is an empirical coefficient.
2.2.4 Dry matter accumulation and plant nitrogen uptake
Five sunflowers were randomly selected as the representative healthy specimens from both the diagonal and central areas of each plot using a five-point scale during their growth and development (Su et al., 2022). The specimens were divided into four parts: leaves, stems, seeds, and roots. The ground dry matter weight was determined by drying the samples in an oven at 105°C for 60 min, followed by drying at 80°C until a constant weight was achieved. The dried samples were subsequently ground into powder for the nitrogen content determination (Bremner, 1965). The samples were digested using H2SO4-H2O2, and the total nitrogen content was measured using a flow analyzer (China Ocean Energy Future Technology Group, K9860, China).
Sunflower four parts biomass (kg/ha) = plant four parts dry matter weight (kg) × the number of plants per hectare. The N concentration was expressed on a dry-weight basis, and total N uptake and accumulation were calculated as the product of concentration and dry weight.
2.2.5 Yield
At harvest time, ten mature sunflower plants were consecutively selected from each experimental plot, and all seeds from their flower heads were collected. The seeds were air-dried to a moisture content of approximately 8% before measuring the total yield. Additionally, the hundred-seed weight and number of seeds per head were determined annually to assess the yield composition.
2.3 Determining the distribution of nitrogen among functions
To determine the distribution of nitrogen among various functions, this study employed a portable photosynthesis system (Ecotech Ecology Technology, Beijing, model Cpro T) to measure CO2 response curves (Pn-Ci). The light intensity and leaf chamber temperature were maintained at 1000 μmol/m2 and 25°C, respectively. The CO2 concentrations were set in a specified sequence: 400, 300, 200, 150, 100, 80, 50, 400, 600, 800, 1000, and 1200 μmol/mol, and at each concentration, Pn (photosynthetic rate) and Ci (intercellular CO2 concentration) were measured and used to plot the CO2 response curves. After the CO2 concentration stabilized, the corresponding Pn and Ci values were recorded. Additionally, the maximum carboxylation rate (Vc,max) and maximum electron transport rate (Jmax) were calculated using the Farquhar, von Caemmerer, and Berry (FvCB) (Farquhar et al., 1980) model and the R package “plantcophys” (Duursma, 2015). The proportions of nitrogen distributed among the different functions were then calculated using formulas from previous studies (Jordan and Ogren, 1984; Makino and Osmond, 1991; Niinemets and Tenhunen, 1997).
Where Ncb represents the proportion of nitrogen allocated to the carboxylation system (Equation 2), Nr is the amount of nitrogen in Rubisco, assumed to be 0.16gN/(g Rubisco), and Vcr is the specific activity of Rubisco, assumed to be 20.5 μmol CO2/(g Rubisco)/s at 25°C.
Where Net represents the proportion of nitrogen in the electron transport components (Equation 3), Nb is the amount of nitrogen in cytochrome f, assumed to be 0.1240695 g N/(μmol cytochrome f), and Jmc is the capacity of electron transport per cytochrome f, set to 156 μmol electron/(μmol cytochrome f)/s.
where Ncl represents the nitrogen distribution in the light-harvesting system (Equation 4); and Cb is the chlorophyll binding of the thylakoid protein complexes, assumed to be 2.75 mmol chlorophyll (g chlorophyll N). To determine the chlorophyll content, 0.1 g of leaf tissue (excluding the main veins) was soaked in 25 mL of 95% ethanol for 48 h. The absorbance was then measured at the wavelengths of 665 and 649 nm, and the chlorophyll content was calculated using the specified formula (Equation 5):
Where, V is the volume of the extraction solution (25ml), and W is the weight of the leaf tissue being measured (0.1g).
Where, Nstore represents the proportion of nitrogen allocated to storage, Npsn represents the distribution of photosynthetic nitrogen, and Nnon-psn represents the non-photosynthetic components, which include Nstore, Nresp, and Nstr. Nresp represents the distribution of respiratory nitrogen, Nstr represents the distribution of structural nitrogen, and the photosynthetic components include Ncb, Net, and Ncl. Nstr, also known as SDS-insoluble N (Nin-SDS), was measured as described previously (Equations 6–9) (Takashima et al., 2004).
2.4 Photosynthetic nitrogen use efficiency and nitrogen utilization efficiency
PNUE (µmol CO2/g/s) reflects the rate of CO2 assimilation per unit leaf area (Equation 10):
where Pn is the net photosynthetic rate, μmol CO2/m2/s; LTNA is the amount of total leaf N accumulation, mg/plant; LA is the total leaf area, cm2/plant; and Narea denotes the leaf N content per unit area, mg/cm2.
In this study, three key nitrogen efficiency indices were adopted to comprehensively evaluate the sunflower’s efficiency in nitrogen uptake and utilization from the soil: Nitrogen Harvest Index (NHI), Partial Factor Productivity of Nitrogen (PFP), and NUE. NHI reflected the proportion of nitrogen in the harvested part of the crop and was applied as the important indicator of the crop’s ability to transfer and distribute nitrogen (Equation 11). PFP assessed the yield produced per unit of applied nitrogen and served as the indicator of nitrogen production efficiency (Equation 12). NUE provided the holistic view of the crop’s ability to utilize the available nitrogen resources (Equation 13).
Where GN is nitrogen content in grains; PNU is the uptake of N by the sunflower plant at maturity; Yield is the sunflower yield; F is the amount of applied N.
2.5 Statistical analysis
All data were processed and analyzed using Microsoft Excel 2021. Additionally, Origin software was employed to compare the mean values, calculate the least significant difference (LSD) at the 0.05 level, and create detailed graphical plots.
3 Results
3.1 Spatial and temporal distribution of soil NO3-N
Different fertilization strategies significantly influenced the distribution of NO3-N in the soil profile during the sunflower growth (Figure 2). In the CRF treatment, the soil NO3-N concentration (SNC) gradually increased with the nitrogen application, whereas in the TNF treatment, SNC was higher only in the 0–20 cm soil layer. During the seedling and budding stages, the CRF315 and CRF225 treatments increased the average 0–40 cm SNC by 63.94% and 130.68%, respectively, compared to CRF135, and by 44.85% and 101.97%, respectively, during the flowering and maturity stages. Under the same nitrogen application conditions, the CRF treatments raised the 0–40 cm SNC by 52.79% during the seedling and budding stages and by 14.82% during the flowering and maturity stages compared with the TNF treatments. In the 40–100 cm layer, CRF315 and CRF225 increased SNC by 1.47 and 2.11 times during the seedling and budding stages, and by 1.47 and 2.19 times during the flowering and maturity stages, respectively, compared to CRF135. Under the same conditions, the CRF treatments increased SNC by 2.93 times compared to the TNF treatments.
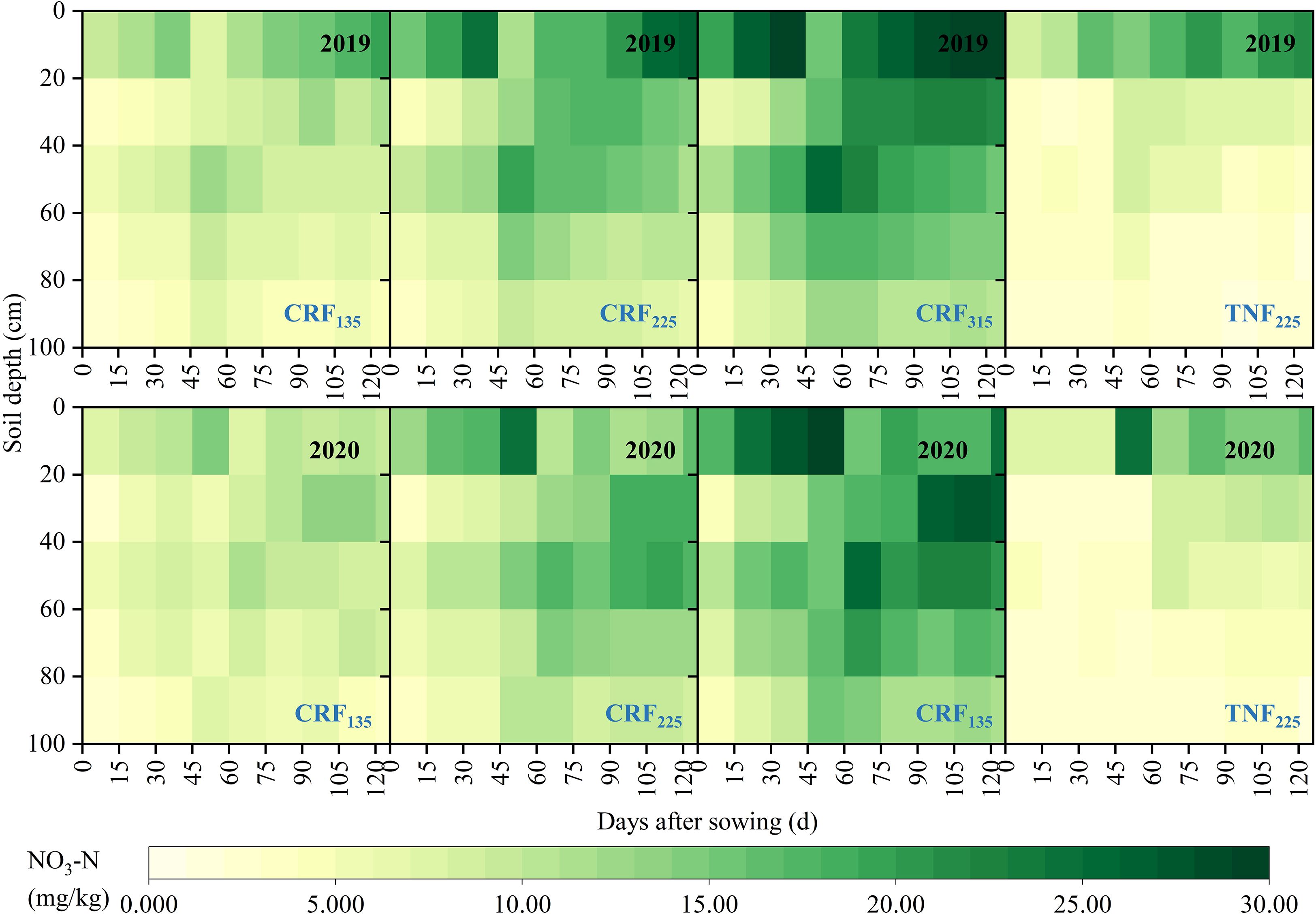
Figure 2. Soil NO3-N concentration (SNC) distribution during the growing season under the controlled-release fertilizer (CRF) three nitrogen application rates (subscripts 135, 225, and 315 kg/ha) and traditional nitrogen fertilizer (TNF) considering one nitrogen application rate (subscripts 225 kg/ha) from 2019 and 2020.
3.2 Sunflower Narea and PNUE under different nitrogen application conditions
During the study periods of 2019 and 2020, the dry matter and nitrogen contents of sunflower leaves were measured at the seedling, budding, flowering, and maturity stages to calculate Narea and PNUE. The fertilization treatments significantly affected both Narea and PNUE, with the correlation regression analysis demonstrating a positive relationship between SNC and Narea across all growth stages (Figure 3). Under CRF treatments, promoting the nitrogen application led to higher Narea and PNUE, and both metrics were consistently higher under CRF than under the TNF treatments at the equivalent nitrogen levels. On average, Narea in the CRF315 and CRF225 treatments was 21.27% and 10.08% higher, respectively, than in CRF135 over the two years. While Narea in TNF225 was 10.48% higher than that in CRF225 during the seedling and budding stages, it was 9.62% lower during the flowering and maturity stages. Narea increased initially but decreased later in the growth period, dropping by 60.81% (2.63 g/m2) at maturity compared to the flowering stage. The positive correlation between SNC and Narea suggested that Narea increased with the higher nitrate-nitrogen concentrations in the 0–40 cm soil layer throughout the sunflower growth cycle (Figure 4).
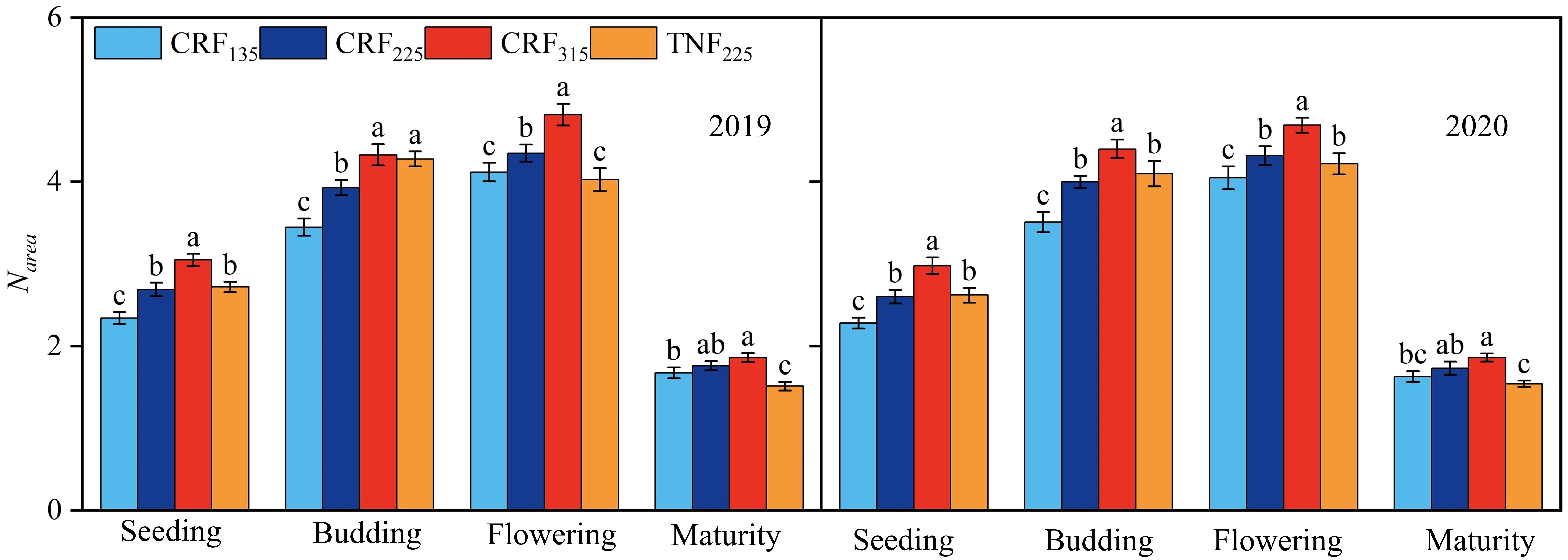
Figure 3. Nitrogen content per unit leaf area (Narea) of sunflower at seeding, budding, flowering, and maturity growth stages in 2019 and 2020 under the controlled-release fertilizer (CRF) three nitrogen application rates (subscripts 135, 225, and 315 kg/ha) and traditional nitrogen fertilizer (TNF) considering one nitrogen application rate (subscripts 225 kg/ha).
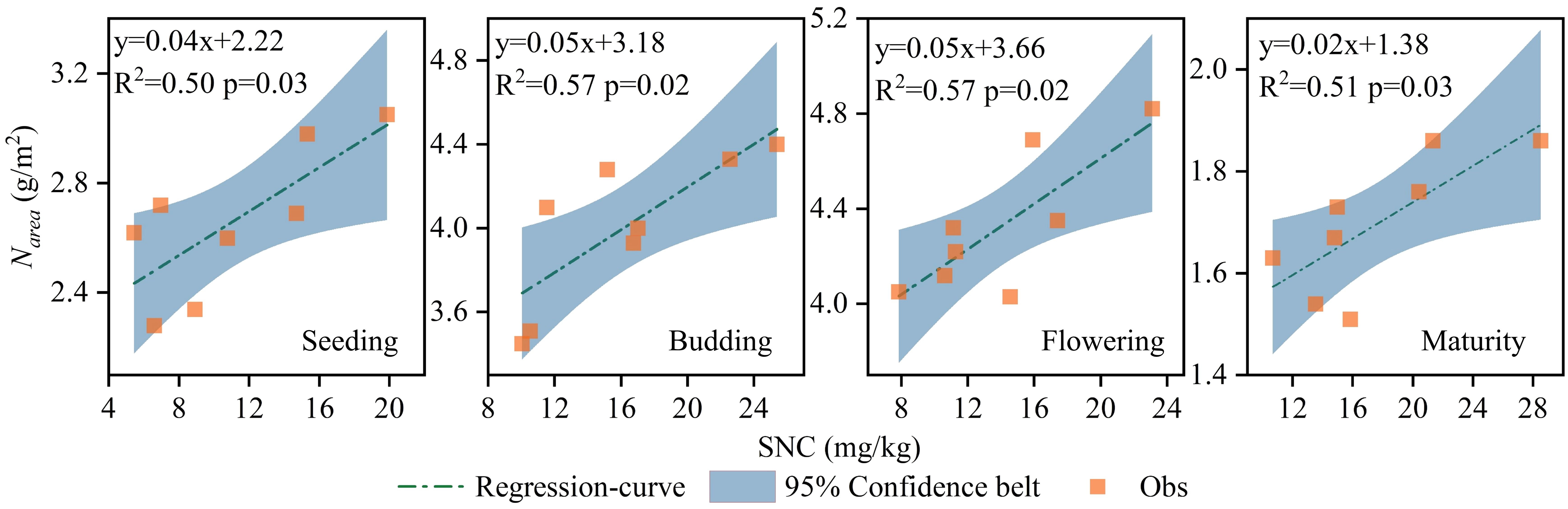
Figure 4. Correlation regression analysis between SNC and Narea of sunflower at seeding, budding, flowering and maturity growth stages in 2019 and 2020 under the controlled-release fertilizer (CRF) three nitrogen application rates (subscripts 135, 225, and 315 kg/ha) and traditional nitrogen fertilizer (TNF) considering one nitrogen application rate (subscripts 225 kg/ha).
Regarding PNUE (Table 1), CRF treatments consistently outperformed TNF225 across all growth stages. At the maturity stage, CRF315 exhibited the highest PNUE, followed by CRF225 and CRF135, indicating a stronger capacity to sustain photosynthetic efficiency relative to leaf nitrogen content under high nitrogen input. However, when considering all stages together, CRF225 showed more stable and balanced performance, especially at the budding and flowering stages where it maintained relatively high PNUE with lower nitrogen input compared to CRF315. These findings suggest that CRF treatments, particularly CRF225, can effectively enhance PNUE and nitrogen utilization efficiency across critical growth stages of sunflower.
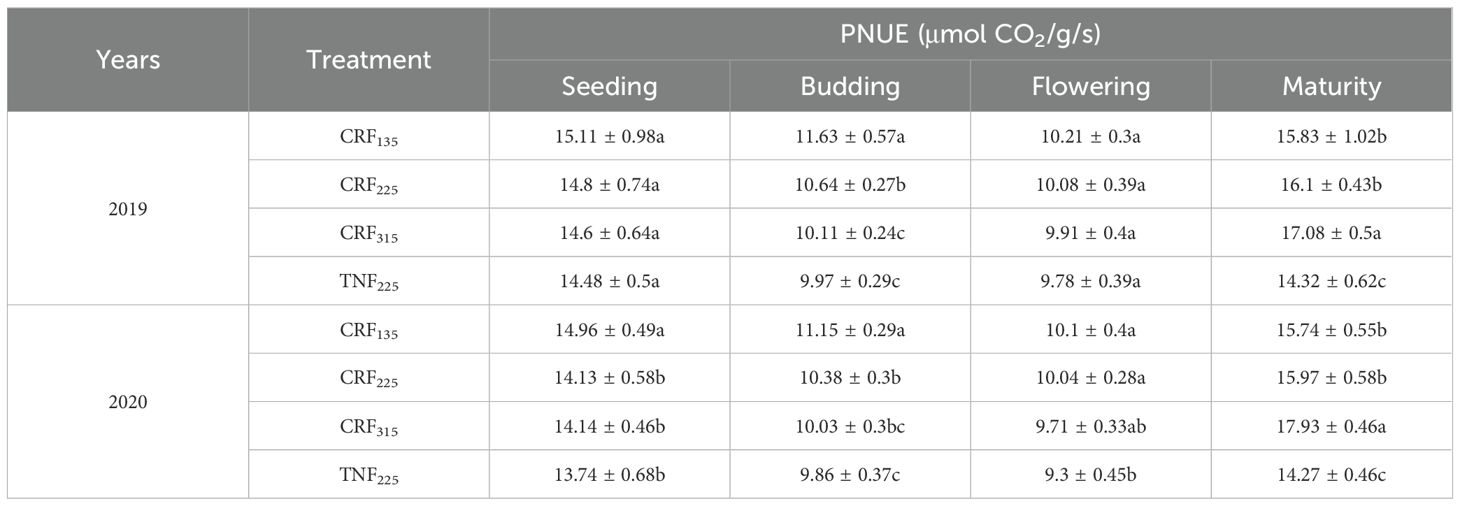
Table 1. Photosynthetic nitrogen use efficiency (PNUE) of sunflower at seeding, budding, flowering and maturity growth stages in 2019 and 2020 under the controlled-release fertilizer (CRF) three nitrogen application rates (subscripts 135, 225, and 315 kg/ha) and traditional nitrogen fertilizer (TNF) considering one nitrogen application rate (subscripts 225 kg/ha).
3.3 Proportion of N in leaves allocated to the photosynthetic system
The proportion of N allocated to the photosynthetic system in the leaves was higher under CRF225 conditions. During the two-year experiment under CRF135, the proportions of Nstore, Nresp, Nstr, and Npsn were 15.82%, 6.83%, 14.61%, and 62.75%, respectively (Figure 5). Compared to CRF225 and CRF315, Npsn under CRF225 increased by 2.43% and 35.84%, respectively, whereas Nnon-psn decreased by 18.12% and 42.24%, respectively. At the 225 kg/ha nitrogen application rate with TNF, the proportions of Nstore, Nresp, Nstr, and Npsn were 29.97%, 4.57%, 30.68%, and 34.79%, respectively, with Npsn under CRF225 being 76.10% higher than that under TNF225, and Nnon-psn decreased by 40.60%. The correlation analysis revealed that Npsn, Ncl, Net, Ncb, and Nresp were significantly positively correlated with PNUE, whereas Nstore and Nstr were significantly negatively correlated with PNUE (Figure 6).
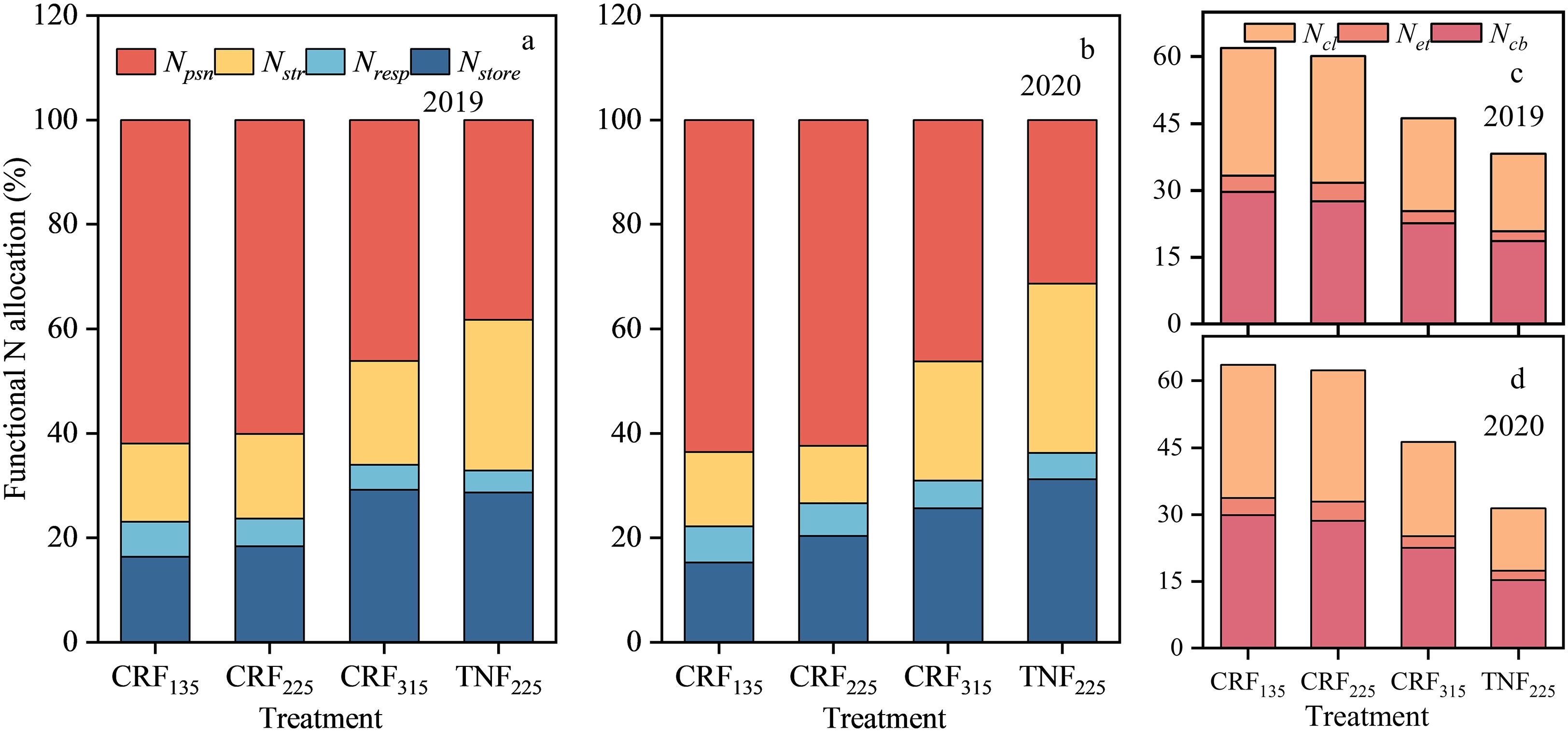
Figure 5. Distribution proportion of functional nitrogen (a and b), Nstore means the distribution proportion of storage nitrogen, Nresp means the distribution proportion of respiratory nitrogen, Nstr means the distribution proportion of structural nitrogen, Npsn means the distribution proportion of photosynthetic nitrogen; Distribution proportion of photosynthetic nitrogen (b and c), Ncb means the distribution proportion of carboxylation system, Net means the distribution proportion of electron transfer component, Ncl means the distribution proportion of light harvesting system in 2019 and 2020 under the controlled-release fertilizer (CRF) three nitrogen application rates (subscripts 135, 225, and 315 kg/ha) and traditional nitrogen fertilizer (TNF) considering one nitrogen application rate (subscripts 225 kg/ha).
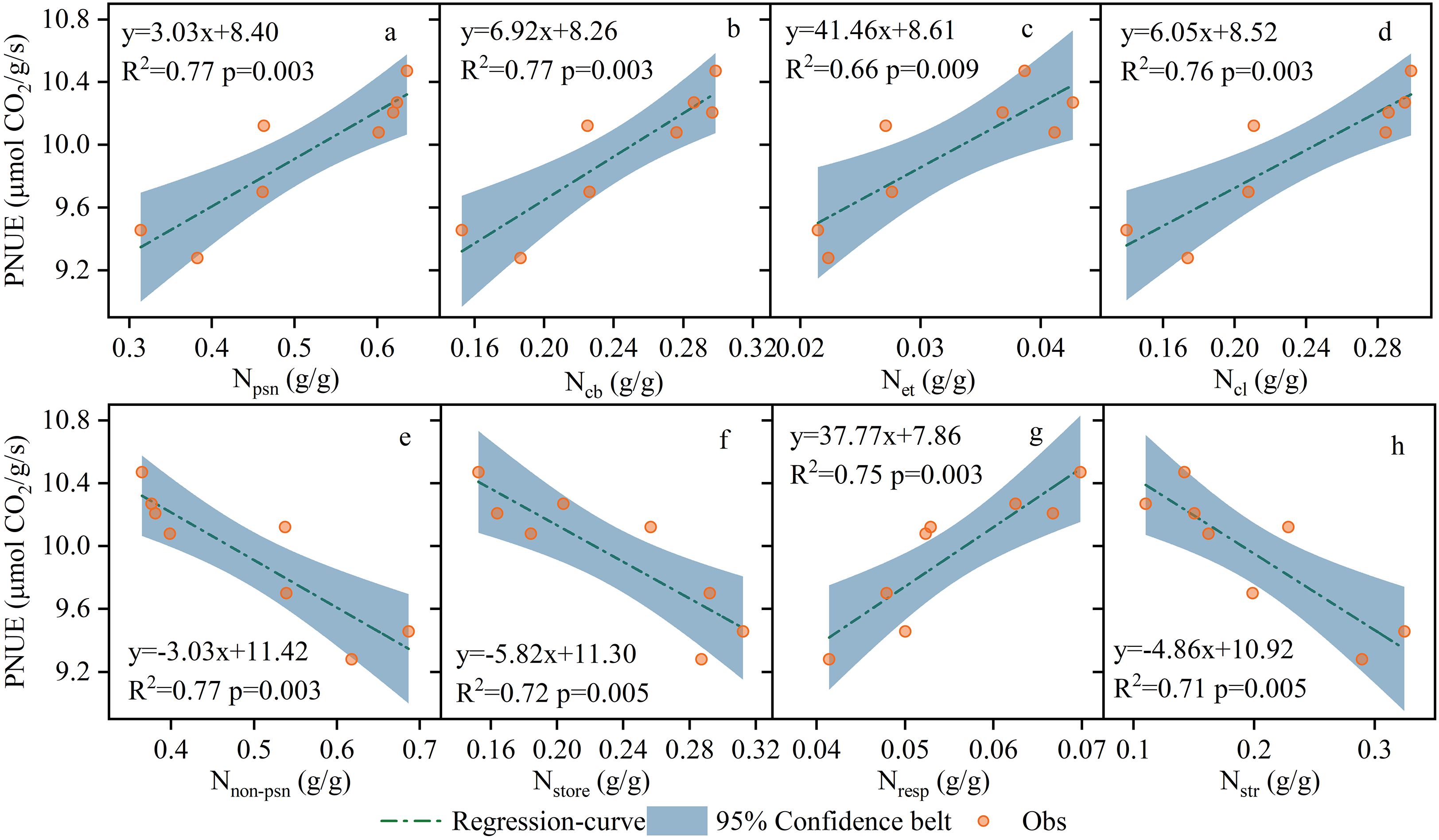
Figure 6. Correlation regression analysis between different N components and photosynthetic nitrogen use efficiency (PNUE), (a) Photosynthetic components (Npsn). (b) carboxylation systems (Ncb). (c) electron transfer components (Net). (d) light harvesting systems (Ncl). (e) non-photosynthetic components (Nnon-psn). (f) storage nitrogen (Nstore). (g) respiratory nitrogen (Nresp). (h) structural nitrogen (Nstr) in 2019 and 2020 under the controlled-release fertilizer (CRF) three nitrogen application rates (subscripts 135, 225, and 315 kg/ha) and traditional nitrogen fertilizer (TNF) considering one nitrogen application rate (subscripts 225 kg/ha).
Figure 5 Distribution proportion of functional nitrogen (a and b), Nstore means the distribution proportion of storage nitrogen, Nresp means the distribution proportion of respiratory nitrogen, Nstr means the distribution proportion of structural nitrogen, Npsn means the distribution proportion of photosynthetic nitrogen; Distribution proportion of photosynthetic nitrogen (b and c), Ncb means the distribution proportion of carboxylation system, Net means the distribution proportion of electron transfer component, Ncl means the distribution proportion of light harvesting system in 2019 and 2020 under the controlled-release fertilizer (CRF) three nitrogen application rates (subscripts 135, 225, and 315 kg/ha) and traditional nitrogen fertilizer (TNF) considering one nitrogen application rate (subscripts 225 kg/ha).
3.4 Photosynthetic characteristics of sunflowers under different CRF application conditions
The research data from 2019 and 2020 (Figure 7) exhibited the changes in the Pn, Gs, and Ci of sunflowers under different fertilization conditions. The results indicated that under the CRF treatment, increasing nitrogen application led to a gradual increase in all three indicators. At the seedling stage, compared with CRF135, the Pn for CRF225 and CRF315 increased by 10.25% and 21.27%, Gs by 4.48% and 7.13%, and Ci by 7.65% and 12.93%, respectively. By the budding stage, these increases were 4.15, 7.26, and 6.94% for CRF225 and 10.06%, 10.64%, and 10.24% for CRF315, respectively. The TNF225 treatment demonstrated the slightly higher Pn, Gs, and Ci than CRF with the same nitrogen amount, with Pn being 9.27% and 2.79% higher than CRF225 at the seedling and budding stages, respectively; Gs was 1.34% and 1.61% higher; and Ci was 3.27% and 1.29% higher. During the seedling and budding stages, Pn under TNF225 was 1.06% higher than that under CRF225.
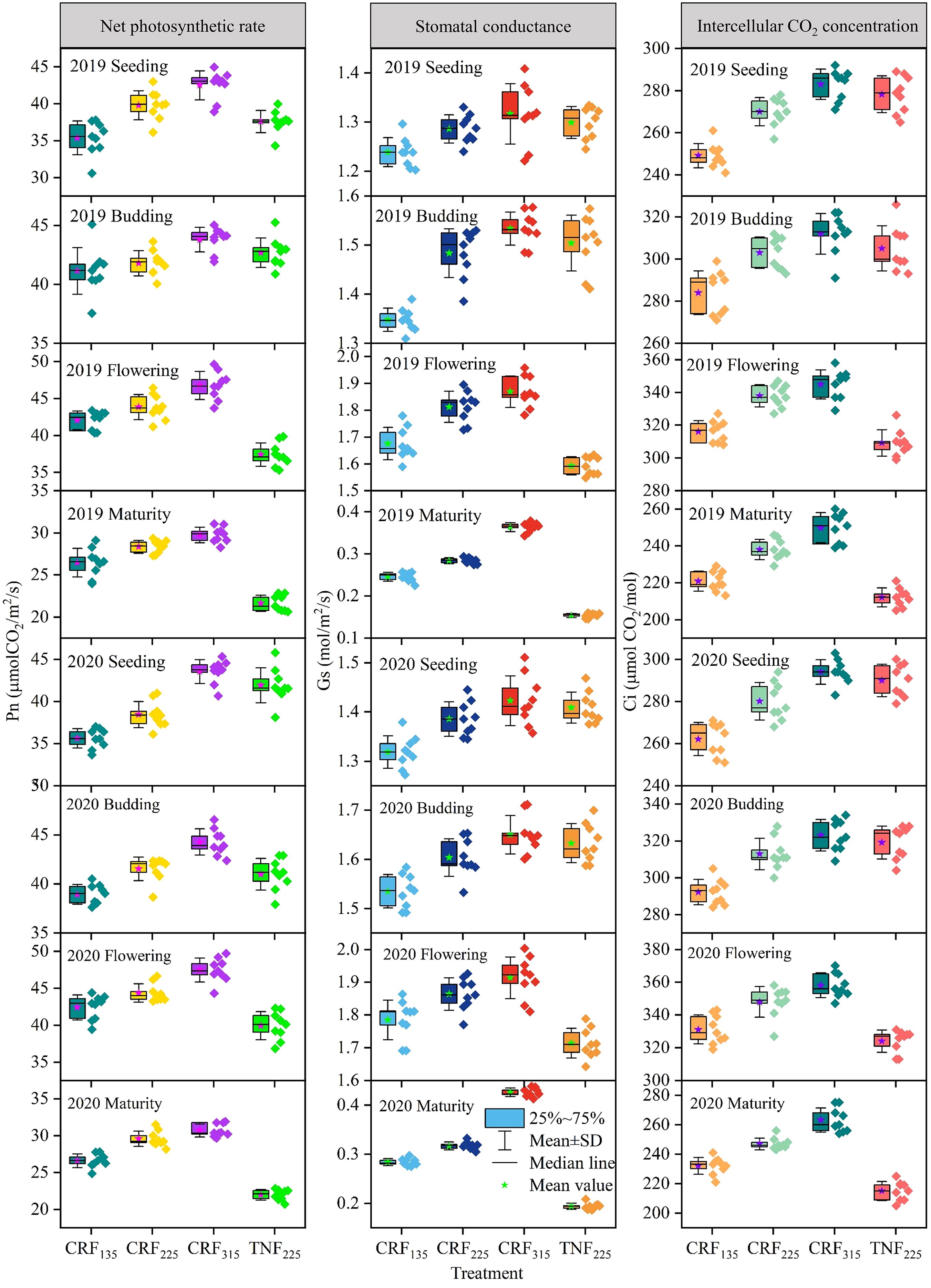
Figure 7. Variation trend of net photosynthetic rate (Pn, μmol CO2/m2/s), stomatal conductance (Gs, μmol H2O/m2/s) and intercellular CO2 concentration (Ci, μ mol CO2/mol) of sunflower under different treatments. CRF135, CRF225, CRF315 and TNF225 represent the controlled-release fertilizer (CRF) three nitrogen application rates (subscripts 135, 225, and 315 kg/ha) and traditional nitrogen fertilizer (TNF) considering one nitrogen application rate (subscripts 225 kg/ha).
By the flowering and maturity stages, the photosynthetic indicators under the TNF treatment had decreased to lower levels. At the maturity stage, except for the CRF315 treatment, all other treatments exhibited lower photosynthetic indicators. In particular, the Pn, Gs, and Ci of the CRF315 treatment were 15.02%, 31.29%, and 5.76% higher than those of the CRF225 treatment, respectively, whereas the CRF225 treatment showed decreases of 32.80%, 96.16%, and 13.56%, respectively, compared to TNF225.
3.5 Nitrogen accumulation and distribution
Throughout the sunflower growth period, significant differences in nitrogen accumulation in leaves, stems, seeds, roots, and total nitrogen at maturity were observed under different fertilization types and amounts (Figure 8). The total nitrogen accumulation increased by 10.17% and 39.33% for the CRF225 and CRF315 treatments, respectively, compared to CRF135, with CRF225 exhibiting a 4.17% increase over TNF225. As the nitrogen application rate increased under the CRF treatments, the nitrogen uptake by the leaves, stems, seeds, and roots also increased. When the application rate increased from 135 to 225 kg/ha, the nitrogen uptake by leaves, stems, seeds, and roots increased by 6.11%, 6.00%, 12.53%, and 15.06%, respectively. A further increase to 315 kg/ha resulted in significant increases in nitrogen uptake by leaves (61.75%), stems (59.91%), and roots (28.18%), whereas seed nitrogen uptake only rose by 6.10%. This suggested that higher nitrogen application primarily promoted the uptake in non-seed parts, with less impact on seed nitrogen uptake. The CRF treatments compared with TNF at the same nitrogen level increased the seed nitrogen uptake by 8.44%, indicating that CRF enhanced the nitrogen absorption by seeds more effectively. From the perspective of seed nitrogen uptake, the CRF application rate of 225 kg/ha was more suitable for the region.
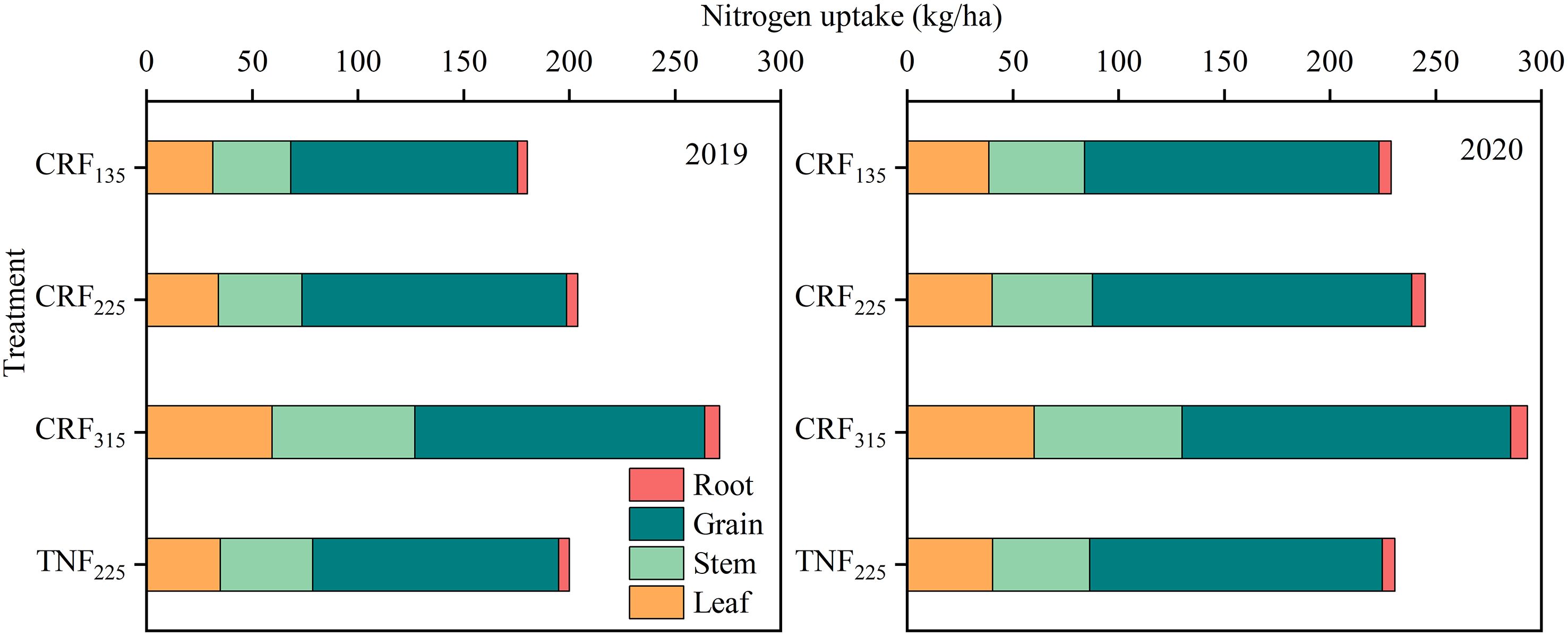
Figure 8. Nitrogen accumulation and distribution proportion in different organs at maturity stage of sunflower in 2019 and 2020 under the controlled-release fertilizer (CRF) three nitrogen application rates (subscripts 135, 225, and 315 kg/ha) and traditional nitrogen fertilizer (TNF) considering one nitrogen application rate (subscripts 225 kg/ha).
3.6 Yield and nitrogen utilization efficiency
Previous research has presented that as the nitrogen application increased, the nitrogen uptake by sunflowers also increased, with the crops treated with CRF consistently absorbing more nitrogen than those treated with TNF under the same conditions. Similarly, increasing the nitrogen application led to higher hundred-seed weight, seed number, and yield of sunflowers (Table 2). Compared with the 135 kg/ha CRF treatment, the 225 kg/ha and 315 kg/ha CRF treatments increased the hundred-seed weight, seed number, and yield by 5.18%, 6.79%, and 14.87%, and by 6.46%, 9.50%, and 21.16%, respectively. However, increasing the CRF treatment from to 315 kg/ha only resulted in the modest increases of 1.22%, 2.54%, and 5.50% in these metrics, respectively, indicating diminishing returns. Additionally, the CRF treatments outperformed the TNF treatments in the hundred-seed weight, seed number, and yield, with the 225 kg/ha CRF treatment demonstrating the increases of 4.99%, 6.74%, and 11.84%, respectively, over TNF at the same rate. Therefore, applying 225 kg/ha of controlled-release fertilizer was optimal for sunflower cultivation in the region, effectively increasing the yield while promoting the efficient resource use.
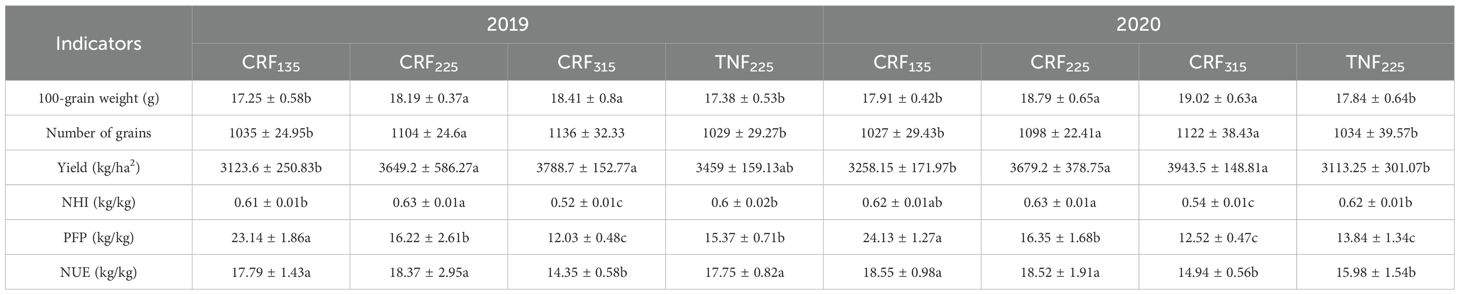
Table 2. Yield, yield components, nitrogen harvest index (NHI), partial factor productivity (PFP) and nitrogen utilization efficiency (NUE) of sunflower applied with controlled-release fertilizer (CRF) three nitrogen application rates (subscripts 135, 225, and 315 kg/ha) and traditional nitrogen fertilizer (TNF) considering one nitrogen application rate (subscripts 225 kg/ha) in the 2019 and 2020 growing seasons.
Under the CRF treatment, NHI and NUE initially increased and then decreased with the changes in nitrogen application, peaking at 225 kg/ha. The data from 2019 and 2020 indicated that compared to the CRF treatments of 135 and 315 kg/ha, the 225 kg/ha CRF treatment increased the average NHI by 2.24% and 18.90%, and NUE by 1.53% and 25.95%, respectively. However, PFP decreased as the nitrogen application increased, with the 135 kg/ha CRF treatment exhibiting the PFP 45.13% higher than the 225 kg/ha treatment, and the 225 kg/ha treatment presenting a PFP 32.73% higher than the 315 kg/ha treatment (Table 2).
Under the same nitrogen application conditions, the CRF treatments improved NHI, PFP, and NUE by 4.22%, 11.84%, and 9.70%, respectively, compared with the TNF treatments. These findings suggested that applying a moderate amount of nitrogen (225 kg/ha) with CRF optimized NHI and NUE in sunflowers while also enhancing PFP. Overall, CRF demonstrated a greater benefit than TNF in improving crop nitrogen utilization.
3.7 Correlation analysis among sunflower growth indicators
During the 2019–2020 research period, the comprehensive analysis of the correlations between sunflower growth indicators was conducted, revealing their interrelationships. Using the Pearson’s correlation coefficient, significant correlations were identified among Pn, Gs, Ci, NU, yield, hundred-weight (HW), grain number (GN), and Narea, whereas some relationships exhibited the variability (Figure 9).
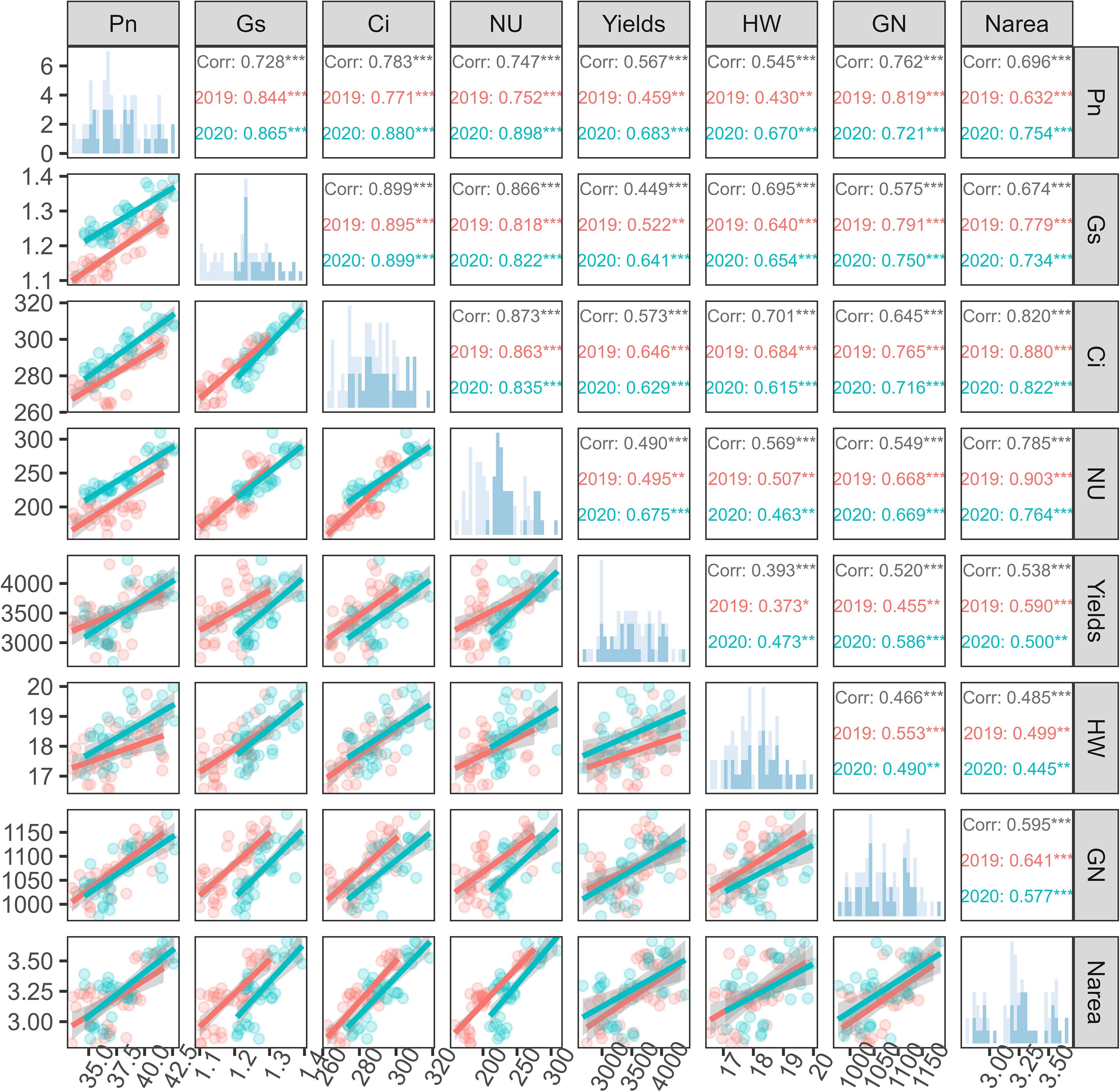
Figure 9. Pairwise correlations among net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), nitrogen uptake (NU), grain yield, hundred-weight per plant (HW), grain number per plant (GN), and leaf nitrogen content per unit area (Narea) under different nitrogen treatments in the 2019 and 2020 growing seasons. The upper triangle shows Pearson correlation coefficients (***P < 0.001, **P < 0.01), with values separated by year (red for 2019, blue for 2020). The lower triangle presents corresponding scatter plots with linear regression lines. Histogram distributions are shown on the diagonal. Red and blue colors indicate data from 2019 and 2020, respectively. CRF treatments include three nitrogen application rates (135, 225, and 315 kg N/ha), and TNF refers to traditional nitrogen fertilizer applied at 225 kg N/ha.
A strong positive correlation was observed between Pn and Gs (2019: r = 0.844***; 2020: r = 0.865***), emphasizing the close link between the photosynthesis and stomatal behavior. The correlations between Ci and both Pn and Gs were also significant over the two years, reflecting a synergistic effect in the gas exchange process. Pn and NU indicated a significant positive correlation (2019: r = 0.752***; 2020: r = 0.898***), revealing a strong link between the photosynthetic activity and the crop nitrogen uptake, which was also influenced by environmental factors. The correlation between crop nitrogen uptake and yield (2019: r = 0.495***, 2020: r = 0.675***) suggested that nitrogen fertilization strategies may affect the yield potential differently each year. Overall, the sunflower yield demonstrated the significant positive correlations with other growth indicators, while the coefficients were around 0.5, indicating that the yield was affected by a variety of complex factors. Notably, the correlations of Ci and Pn with yield were above 0.5, highlighting the importance of photosynthesis and stomatal behavior in affecting crop yield.
4 Discussion
4.1 Response of leaf nitrogen distribution and photosynthetic parameters to nitrogen fertilizer type and application rate
Nitrogen distribution is a crucial factor influencing PNUE (Onoda et al., 2017; Zhuo et al., 2024), with different plant species exhibiting varied nitrogen allocations. The plants with higher PNUE allocated more nitrogen to the photosynthetic system and demonstrated higher growth rates (Poorter et al., 1990), whereas those with lower PNUE allocated more nitrogen to the non-photosynthetic systems (Onoda et al., 2004; van Ommen Kloeke et al., 2011). In this study, CRF improved the nitrogen allocation to the photosynthetic system compared to traditional fertilizers by providing a more stable and sustained nitrogen supply, thus enhancing PNUE. Specifically, under the 225 kg/ha CRF treatment, the nitrogen allocation to the photosynthetic system was greater than at other application levels, which promoted higher PNUE (Figure 3). Pn mainly relied on the light capture, electron transfer, and carboxylation, and nitrogen allocation to these processes (Ncl, Net, and Ncb, respectively) was positively correlated with PNUE (Figure 4). Therefore, increasing the proportions of Ncl, Net, and Ncb was crucial for improving PNUE. Evolutionary algorithms have been used to simulate optimal nitrogen allocation in photosynthetic systems, suggesting that internal nitrogen redistribution may enhance photosynthetic capacity by up to 60% (Zhu et al., 2007). The nitrogen allocation to the photosynthetic system can be influenced by the plant growth environment. In cucumbers, as plants transition from high to low light intensity environments, the proportions of Ncb and Net can decrease, while Ncl can increase (Trouwborst et al., 2011). Generally, most Nnon-psn exist as Nstore, Nstr, and Nresp, serving as a buffering mechanism for environmental adaptation (Cao et al., 2021). The species with higher proportions of Nnon-psn exhibited greater tolerance to environmental stress (Onoda et al., 2004; van Ommen Kloeke et al., 2011). As the nitrogen application decreased, Nstore supported the plant growth and development, and could be converted into Npsn to sustain photosynthesis (Liu et al., 2018b). Reducing the nitrogen application from 315 to 225 kg/ha increased the proportion of Npsn, consistent with previous studies (Hou et al., 2019; Waring et al., 2023). This study also highlighted the significant impact of CRF on sunflower photosynthetic parameters (Pn, Gs, and Ci), indicating that these parameters could be optimized by adjusting the nitrogen fertilizer application rate (Figure 5). These results highlighted the critical role of nitrogen management strategies in promoting the crop photosynthetic efficiency. By providing a more stable and sustained nitrogen supply, CRF enhanced the leaf physiological states and the stomatal functions, thereby improving Pn and Gs. These findings aligned with the existing literature on the positive effects of nitrogen supply on crop growth and photosynthesis (Hong et al., 2022; Mu and Chen, 2021; Vos et al., 2005), demonstrating the complex interaction between nitrogen supply and plant photosynthetic mechanisms. Nitrogen is essential for synthesizing the chlorophyll and photosynthetic enzymes (Ali et al., 2019; Evans, 1989; Evans and Clarke, 2019), which are crucial for photosynthesis. Therefore, enhancing the nitrogen supply, particularly through the sustained and stable provision achieved with CRF, may boost chlorophyll synthesis and increase photosynthetic enzyme activity, thereby directly improving Pn. Additionally, an adequate nitrogen supply enhances plant stomatal regulation capabilities (Radin et al., 1982), as evidenced by increased stomatal conductance (Gs), which facilitated more efficient carbon dioxide absorption and further promoted the photosynthesis.
The study results demonstrated that the CRF treatment outperformed the TNF treatment in photosynthetic performance during key growth stages, indicating the importance of optimizing the nitrogen supply during critical crop growth periods. This advantage was attributed to the slow-release characteristic of CRF, which could provide an appropriate nitrogen supply when crop demand was the highest, thereby supporting the optimal growth and development (Shaviv, 2001; Zhang et al., 2024). This finding demonstrated the need to align the nitrogen management strategies with the temporal requirements of crop growth and development, which could have significant implications for improving the nitrogen fertilizer use efficiency and reducing the environmental impact. Selecting the appropriate type and amount of nitrogen fertilizer could significantly enhance the crop photosynthetic efficiency, increase yield, and mitigate the environmental impacts of nitrogen fertilizers.
4.2 Nitrogen accumulation, distribution, and utilization efficiency
The results of this study revealed the significant differences in the nitrogen accumulation and distribution at various sunflower growth stages under different CRF treatments, with the 225 kg/ha application rate achieving the optimal nitrogen accumulation and distribution in the leaves, stems, seeds, and roots (Figure 8). This finding demonstrated the complex interaction between the nitrogen supply and the crop physiological responses (Meng et al., 2021; Pan et al., 2019; Rurinda et al., 2020), highlighting the need for precise nitrogen application management to optimize the crop growth and enhance NUE (Oliveira et al., 2025; Raghuram et al., 2022; Yang et al., 2022). The continuous and stable nitrogen supply provided by CRF promoted the efficient nitrogen absorption and utilization, thereby affecting the nitrogen distribution pattern within the plant. Compared with TNF, CRF reduced the nitrogen accumulation in non-target parts, such as leaves and stems, and increased its transfer to seeds, which was crucial for improving the crop yield and quality. This optimized nitrogen distribution was linked to CRF’s enhancement of nitrogen assimilation and transport mechanisms by CRF, reflecting the adaptive response of the crop to the nitrogen fertilizer management strategies.
Improving NUE can be a key goal in the nitrogen fertilizer management for sustainable agricultural development (Ren et al., 2022a; Shi et al., 2024). This study discovered that under the CRF treatment, the sunflower NUE increased with the application rate to the peak of 225 kg/ha before declining, indicating that this rate provides the optimal NUE. This finding highlighted the importance of identifying an appropriate nitrogen application amount that met the crop growth needs without leading to excessive nitrogen accumulation or loss, which was essential for optimizing the nitrogen use and minimizing the environmental impact. Further analysis revealed that CRF, compared to TNF, more effectively enhanced the crop PNUE and NHI, demonstrating its superior ability to improve the direct nitrogen utilization efficiency and convert nitrogen into yield (Table 2). Although CRF315 resulted in higher biomass and grain yield, it also caused excessive nitrogen accumulation in vegetative tissues (i.e., leaves and stems), which led to a lower NHI and reduced NUE (Hu et al., 2023b; Li et al., 2022). In contrast, CRF225 achieved a more balanced nitrogen distribution between vegetative and reproductive organs, thereby contributing to improved NUE. This efficacy was attributed to the ability of CRF to reduce the nitrogen fluctuations and provide a more stable nitrogen source (Ali et al., 2024; Geng et al., 2015; Li et al., 2017; Trenkel, 2021), indicating the potential benefits of controlled-release technology in agricultural production.
The absorption, transformation, and distribution of nitrogen within plants involve complex biochemical reactions, including the regulation of nitrogen assimilation and transport protein expression (Tegeder and Masclaux-Daubresse, 2018; Xu et al., 2012). CRF may enhance the nitrogen utilization efficiency by influencing these biochemical pathways. For instance, a stable nitrogen supply could prompt plants to adjust their nitrogen assimilation enzyme activities (Xiong et al., 2021), improve the nitrogen fixation in organic forms, and increase the nitrogen availability during critical growth stages (Liu et al., 2022; Sharma et al., 2021). Future research should investigate how CRF specifically affects these biochemical processes and signaling pathways and how these effects interact with crop growth traits and yield performance. The practical optimization of nitrogen fertilizer management should not only aim to increase yield but also balance crop quality, resource use efficiency, and environmental impact. By precisely controlling the timing and amount of nitrogen supply, it is possible to maximize the crop nutritional value and economic return while minimizing the environmental burden. Additionally, considering the crop varieties, soil conditions, and climate change responses can further enhance the efficiency and sustainability of N management. Therefore, future efforts should integrate new nitrogen fertilizer technologies, such as CRF, with crop physiology research, precision agriculture, and environmental protection measures to promote comprehensive sustainable agricultural development.
5 Conclusion
This study comprehensively evaluated the effects of CRF application on the spatiotemporal distribution of soil NO3-N throughout the crop growth period and its subsequent effects on nitrogen distribution in crop leaves, photosynthetic characteristics, nitrogen accumulation and distribution, yield and its components, PNUE, and NUE. These findings demonstrated the significant benefits of CRF in optimizing the nitrogen management, enhancing the crop production efficiency, and promoting the environmental sustainability. Specifically, CRF maintained the stable concentration of NO3-N in the soil during crop growth, improved the proportion of Npsn in leaves, enhanced photosynthetic efficiency, and optimized the nitrogen accumulation and distribution within the plant, particularly at an application rate of 225 kg/ha. Moreover, CRF significantly improved NUE compared with TNF. These results proved the value of CRF and precise management strategies for enhancing the crop photosynthesis, yield, and NUE by altering the nitrogen distribution in crop leaves, thereby achieving the efficient and sustainable agricultural production. Future research should further investigate the CRF application effects on various crops and under different environmental conditions and evaluate the long-term impacts of nitrogen fertilizer management on agricultural ecosystem services.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WR: Validation, Methodology, Data curation, Visualization, Writing – review & editing, Formal analysis, Writing – original draft. XL: Project administration, Resources, Methodology, Writing – review & editing, Funding acquisition. TL: Writing – review & editing, Resources, Project administration. NC: Software, Writing – review & editing, Methodology. MX: Writing – review & editing, Software, Data curation. QQ: Writing – review & editing, Software, Supervision. BL: Software, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study received funding from multiple sources, including the National Natural Science Foundation of China (U24A20179), the Fundamental Research Funds for the Inner Mongolia Universities through the Science Foundation for Distinguished Young Scholars of Inner Mongolia Agricultural University (BR220302), the Inner Mongolia Science and Technology Program (2022YFHH0039), Inner Mongolia Water Conservancy Science and Technology Program (NSK202201), and First-Class Discipline in Inner Mongolia (Agricultural Engineering).
Acknowledgments
We are grateful to Maoxin Xin, Qian Qi and Bin Liu for their help with the experiments. We also would like to thank every reviewer for all of their careful, constructive, and insightful comments in relation to this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, A. A., Xu, C., Rogers, A., Fisher, R. A., Wullschleger, S. D., Massoud, E. C., et al. (2016). A global scale mechanistic model of photosynthetic capacity (LUNA V1.0). Geoscientific Model. Dev. 9, 587–606. doi: 10.5194/gmd-9-587-2016
Ali, M. F., Han, R., Lin, X., and Wang, D. (2024). Controlled-release nitrogen combined with ordinary nitrogen fertilizer improved nitrogen uptake and productivity of winter wheat. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1504083
Ali, S., Hafeez, A., Ma, X., Tung, S. A., Chattha, M. S., Shah, A. N., et al. (2019). Equal potassium-nitrogen ratio regulated the nitrogen metabolism and yield of high-density late-planted cotton (Gossypium hirsutum L.) in Yangtze River valley of China. Ind. Crops Products 129, 231–241. doi: 10.1016/j.indcrop.2018.12.009
Bindraban, P. S., Dimkpa, C. O., White, J. C., Franklin, F. A., Melse-Boonstra, A., Koele, N., et al. (2020). Safeguarding human and planetary health demands a fertilizer sector transformation. Plants People Planet 2, 302–309. doi: 10.1002/ppp3.10098
Bremner, J. (1965). “Total nitrogen,” in Methods of soil analysis: part 2 chemical and microbiological properties, Agronomy Monographs. vol. 9, 1149–1178. doi: 10.2134/agronmonogr9.2.c32
Cao, J.-B., He, L.-M., Nwafor, C. C., Qin, L.-H., Zhang, C.-Y., Song, Y.-T., et al. (2021). Ultrastructural studies of seed coat and cotyledon during rapeseed maturation. J. Integr. Agric. 20, 1239–1249. doi: 10.1016/s2095-3119(20)63189-6
Carlson, K. M., Gerber, J. S., Mueller, N. D., Herrero, M., MacDonald, G. K., Brauman, K. A., et al. (2016). Greenhouse gas emissions intensity of global croplands. Nat. Climate Change 7, 63–68. doi: 10.1038/nclimate3158
Coskun, D., Britto, D. T., Shi, W., and Kronzucker, H. J. (2017). Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 3, 17074. doi: 10.1038/nplants.2017.74
Dai, C., Lin, Y., Guan, J., Meng, T., Liu, Y., Cui, X., et al. (2024). Mechanism analysis: Nitrogen and potassium synergy regulate nitrogen distribution in photosynthetic system to enhance Panax notoginseng resistance to light stress. Ind. Crops Products 210, 118111. doi: 10.1016/j.indcrop.2024.118111
Duursma, R. A. (2015). Plantecophys–an R package for analysing and modelling leaf gas exchange data. PloS One 10, e0143346. doi: 10.1371/journal.pone.0143346
Ebrahimian, E., Seyyedi, S. M., Bybordi, A., and Damalas, C. A. (2019). Seed yield and oil quality of sunflower, safflower, and sesame under different levels of irrigation water availability. Agric. Water Manage. 218, 149–157. doi: 10.1016/j.agwat.2019.03.031
ECONOMIC, U.N.D.F and AFFAIRS., S (2023). World population prospects 2022: Summary of results (UN). Available at: https://www.un.org/development/desa/pd/content/World-Population-Prospects-2022
Evans, J. R. (1989). Photosynthesis and nitrogen relationships in leaves of C(3) plants. Oecologia 78, 9–19. doi: 10.1007/BF00377192
Evans, J. R. and Clarke, V. C. (2019). The nitrogen cost of photosynthesis. J. Exp. Bot. 70, 7–15. doi: 10.1093/jxb/ery366
Farquhar, G. D., von Caemmerer, S., and Berry, J. A. (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C 3 species. Planta 149, 78–90. doi: 10.1007/BF00386231
Gao, Y., Shao, Y., Wang, J., Hu, B., Feng, H., Qu, Z., et al. (2024). Effects of straw returning combined with blended controlled-release urea fertilizer on crop yields, greenhouse gas emissions, and net ecosystem economic benefits: A nine-year field trial. J. Environ. Manage 356, 120633. doi: 10.1016/j.jenvman.2024.120633
Gao, Y., Song, X., Zheng, W., Wu, L., Chen, Q., Yu, X., et al. (2022). The controlled-release nitrogen fertilizer driving the symbiosis of microbial communities to improve wheat productivity and soil fertility. Field Crops Res. 289, 108712. doi: 10.1016/j.fcr.2022.108712
García-López, J., Lorite, I. J., García-Ruiz, R., Ordoñez, R., and Dominguez, J. (2016). Yield response of sunflower to irrigation and fertilization under semi-arid conditions. Agric. Water Manage. 176, 151–162. doi: 10.1016/j.agwat.2016.05.020
Geng, J., Sun, Y., Zhang, M., Li, C., Yang, Y., Liu, Z., et al. (2015). Long-term effects of controlled release urea application on crop yields and soil fertility under rice-oilseed rape rotation system. Field Crops Res. 184, 65–73. doi: 10.1016/j.fcr.2015.09.003
Grouneva, I., Muth-Pawlak, D., Battchikova, N., and Aro, E. M. (2016). Changes in relative thylakoid protein abundance induced by fluctuating light in the diatom thalassiosira pseudonana. J. Proteome Res. 15, 1649–1658. doi: 10.1021/acs.jproteome.6b00124
Gu, L. (2023). Optimizing the electron transport chain to sustainably improve photosynthesis. Plant Physiol. 193, 2398–2412. doi: 10.1093/plphys/kiad490
He, H. and Liu, L. (2024). Study on irrigation scheme and nitrogen application to sunflower (Helianthus annuus L.) in saline farmland in the arid/semi-arid region of Hetao Irrigation District. Irrigation Sci. 43, 203–219. doi: 10.1007/s00271-024-00928-4
Hong, T., Cai, Z., Li, R., Liu, J., Li, J., Wang, Z., et al. (2022). Effects of water and nitrogen coupling on watermelon growth, photosynthesis and yield under CO2 enrichment. Agric. Water Manage. 259, 107229. doi: 10.1016/j.agwat.2021.107229
Hou, J., Tian, Y., Zhou, J., Liu, K., and Cao, B. (2023). An environmental and economic assessment of sustained cleaner production for double rice from the combination of controlled-released urea and water-saving irrigation. J. Cleaner Production 383, 135467. doi: 10.1016/j.jclepro.2022.135467
Hou, W., Trankner, M., Lu, J., Yan, J., Huang, S., Ren, T., et al. (2019). Interactive effects of nitrogen and potassium on photosynthesis and photosynthetic nitrogen allocation of rice leaves. BMC Plant Biol. 19, 302. doi: 10.1186/s12870-019-1894-8
Hou, Y., Xu, X., Kong, L., Zhang, Y., Zhang, L., and Wang, L. (2024). Combining time-variable controlled release urea formulations to improve spring maize yield and reduce nitrogen losses in northeastern China. Eur. J. Agron. 159, 127268. doi: 10.1016/j.eja.2024.127268
Hu, Y., Zeeshan, M., Wang, G., Pan, Y., Liu, Y., and Zhou, X. (2023b). Supplementary irrigation and varying nitrogen fertilizer rate mediate grain yield, soil-maize nitrogen accumulation and metabolism. Agric. Water Manage. 276, 108066. doi: 10.1016/j.agwat.2022.108066
Hu, K., Zhao, P., Wu, K., Yang, H., Yang, Q., Fan, M., et al. (2023a). Reduced and deep application of controlled-release urea maintained yield and improved nitrogen-use efficiency. Field Crops Res. 295, 108876. doi: 10.1016/j.fcr.2023.108876
Huang, X., Xu, X., Zhu, Q., and Zhang, Y. (2024). Optimizing water and nitrogen inputs for sustainable wheat yields and minimal environmental impacts. Agric. Syst. 220, 104061. doi: 10.1016/j.agsy.2024.104061
Iriti, M., Scarafoni, A., Pierce, S., Castorina, G., and Vitalini, S. (2019). Soil application of effective microorganisms (EM) maintains leaf photosynthetic efficiency, increases seed yield and quality traits of bean (Phaseolus vulgaris L.) plants grown on different substrates. Int. J. Mol. Sci. 20, 2327. doi: 10.3390/ijms20092327
Jia, M., Colombo, R., Rossini, M., Celesti, M., Zhu, J., Cogliati, S., et al. (2021). Estimation of leaf nitrogen content and photosynthetic nitrogen use efficiency in wheat using sun-induced chlorophyll fluorescence at the leaf and canopy scales. Eur. J. Agron. 122, 126192. doi: 10.1016/j.eja.2020.126192
Jordan, D. B. and Ogren, W. L. (1984). The CO2/O 2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase: Dependence on ribulosebisphosphate concentration, pH and temperature. Planta 161, 308–313. doi: 10.1007/BF00398720
Li, C., Feng, H., Luo, X., Li, Y., Wang, N., Wu, W., et al. (2022). Limited irrigation and fertilization in sand-layered soil increases nitrogen use efficiency and economic benefits under film mulched ridge-furrow irrigation in arid areas. Agric. Water Manage. 262, 107406. doi: 10.1016/j.agwat.2021.107406
Li, Y., Liu, N., Fan, H., Su, J., Fei, C., Wang, K., et al. (2019). Effects of deficit irrigation on photosynthesis, photosynthate allocation, and water use efficiency of sugar beet. Agric. Water Manage. 223, 105701. doi: 10.1016/j.agwat.2019.105701
Li, Y., Sun, Y., Liao, S., Zou, G., Zhao, T., Chen, Y., et al. (2017). Effects of two slow-release nitrogen fertilizers and irrigation on yield, quality, and water-fertilizer productivity of greenhouse tomato. Agric. Water Manage. 186, 139–146. doi: 10.1016/j.agwat.2017.02.006
Li, J., Wang, E., Wang, Y., Xing, H., Wang, D., Wang, L., et al. (2016). Reducing greenhouse gas emissions from a wheat–maize rotation system while still maintaining productivity. Agric. Syst. 145, 90–98. doi: 10.1016/j.agsy.2016.03.007
Li, G., Zhao, B., Dong, S., Zhang, J., Liu, P., and Lu, W. (2020). Controlled-release urea combining with optimal irrigation improved grain yield, nitrogen uptake, and growth of maize. Agric. Water Manage. 227, 105834. doi: 10.1016/j.agwat.2019.105834
Liao, Z., Zeng, H., Fan, J., Lai, Z., Zhang, C., Zhang, F., et al. (2022). Effects of plant density, nitrogen rate and supplemental irrigation on photosynthesis, root growth, seed yield and water-nitrogen use efficiency of soybean under ridge-furrow plastic mulching. Agric. Water Manage. 268, 107688. doi: 10.1016/j.agwat.2022.107688
Liu, X., Hu, B., and Chu, C. (2022). Nitrogen assimilation in plants: current status and future prospects. J. Genet. Genomics 49, 394–404. doi: 10.1016/j.jgg.2021.12.006
Liu, T., Ren, T., White, P. J., Cong, R., and Lu, J. (2018b). Storage nitrogen co-ordinates leaf expansion and photosynthetic capacity in winter oilseed rape. J. Exp. Bot. 69, 2995–3007. doi: 10.1093/jxb/ery134
Liu, N., Wu, S., Guo, Q., Wang, J., Cao, C., and Wang, J. (2018a). Leaf nitrogen assimilation and partitioning differ among subtropical forest plants in response to canopy addition of nitrogen treatments. Sci. Total Environ. 637-638, 1026–1034. doi: 10.1016/j.scitotenv.2018.05.060
Liu, L., Zhang, X., Xu, W., Liu, X., Li, Y., Wei, J., et al. (2020). Challenges for global sustainable nitrogen management in agricultural systems. J. Agric. Food Chem. 68, 3354–3361. doi: 10.1021/acs.jafc.0c00273
Ma, Q., Qian, Y., Yu, Q., Cao, Y., Tao, R., Zhu, M., et al. (2023). Controlled-release nitrogen fertilizer application mitigated N losses and modified microbial community while improving wheat yield and N use efficiency. Agriculture Ecosyst. Environ. 349, 108445. doi: 10.1016/j.agee.2023.108445
Mahmood, Y. A., DeSilva, J., King, I. P., King, J., and Foulkes, M. J. (2023). Leaf photosynthesis traits and associations with biomass and drought tolerance in amphidiploid and ancestral wheat genotypes. Eur. J. Agron. 147, 126846. doi: 10.1016/j.eja.2023.126846
Makino, A. and Osmond, B. (1991). Solubilization of ribulose-1,5-bisphosphate carboxylase from the membrane fraction of pea leaves. Photosynth Res. 29, 79–85. doi: 10.1007/BF00035378
Meng, X., Wang, X., Zhang, Z., Xiong, S., Wei, Y., Guo, J., et al. (2021). Transcriptomic, proteomic, and physiological studies reveal key players in wheat nitrogen use efficiency under both high and low nitrogen supply. J. Exp. Bot. 72, 4435–4456. doi: 10.1093/jxb/erab153
Mu, X. and Chen, Y. (2021). The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 158, 76–82. doi: 10.1016/j.plaphy.2020.11.019
Nasar, J., Wang, G. Y., Ahmad, S., Muhammad, I., Zeeshan, M., Gitari, H., et al. (2022). Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.988055
Niinemets, Ü. and Tenhunen, J. (1997). A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ. 20, 845–866. doi: 10.1046/j.1365-3040.1997.d01-133.x
Nolfi-Donegan, D., Braganza, A., and Shiva, S. (2020). Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 37, 101674. doi: 10.1016/j.redox.2020.101674
Oliveira, B. R. D., Ratke, R. F., Steiner, F., Al-Askar, A. A., Aguilera, J. G., Hashem, A. H., et al. (2025). Enhancing off-season maize production through tailored nitrogen management and advanced cultivar selection techniques. Agric. Syst. 224, 104239. doi: 10.1016/j.agsy.2024.104239
Onoda, Y., Hikosaka, K., and Hirose, T. (2004). Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct. Ecol. 18, 419–425. doi: 10.1111/j.0269-8463.2004.00847.x
Onoda, Y., Wright, I. J., Evans, J. R., Hikosaka, K., Kitajima, K., Niinemets, U., et al. (2017). Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 214, 1447–1463. doi: 10.1111/nph.14496
Pan, W. L., Kidwell, K. K., McCracken, V. A., Bolton, R. P., and Allen, M. (2019). Economically optimal wheat yield, protein and nitrogen use component responses to varying N supply and genotype. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01790
Penuelas, J. and Sardans, J. (2022). The global nitrogen-phosphorus imbalance. Science 375, 266–267. doi: 10.1126/science.abl4827
Poorter, H., Remkes, C., and Lambers, H. (1990). Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiol. 94, 621–627. doi: 10.1104/pp.94.2.621
Qiang, B., Zhou, W., Zhong, X., Fu, C., Cao, L., Zhang, Y., et al. (2023). Effect of nitrogen application levels on photosynthetic nitrogen distribution and use efficiency in soybean seedling leaves. J. Plant Physiol. 287, 154051. doi: 10.1016/j.jplph.2023.154051
Radin, J. W., Parker, L. L., and Guinn, G. (1982). Water relations of cotton plants under nitrogen deficiency: V. Environmental control of abscisic acid accumulation and stomatal sensitivity to abscisic acid. Plant Physiol. 70, 1066–1070. doi: 10.1104/pp.70.4.1066
Radušienė, J., Marksa, M., Ivanauskas, L., Jakštas, V., Çalişkan, Ö., Kurt, D., et al. (2019). Effect of nitrogen on herb production, secondary metabolites and antioxidant activities of Hypericum pruinatum under nitrogen application. Ind. Crops Products 139, 111519. doi: 10.1016/j.indcrop.2019.111519
Raghuram, N., Aziz, T., Kant, S., Zhou, J., and Schmidt, S. (2022). Nitrogen use efficiency and sustainable nitrogen management in crop plants. Front. Media SA 13, 862091. doi: 10.3389/978-2-88974-284-4
Ren, D., Xu, X., Engel, B., and Huang, G. (2018). Growth responses of crops and natural vegetation to irrigation and water table changes in an agro-ecosystem of Hetao, upper Yellow River basin: Scenario analysis on maize, sunflower, watermelon and tamarisk. Agric. Water Manage. 199, 93–104. doi: 10.1016/j.agwat.2017.12.021
Ren, K., Xu, M., Li, R., Zheng, L., Liu, S., Reis, S., et al. (2022b). Optimizing nitrogen fertilizer use for more grain and less pollution. J. Cleaner Production 360, 132180. doi: 10.1016/j.jclepro.2022.132180
Ren, C., Zhang, X., Reis, S., and Gu, B. (2022a). Socioeconomic barriers of nitrogen management for agricultural and environmental sustainability. Agriculture Ecosyst. Environ. 333, 107950. doi: 10.1016/j.agee.2022.107950
Rurinda, J., Zingore, S., Jibrin, J. M., Balemi, T., Masuki, K., Andersson, J. A., et al. (2020). Science-based decision support for formulating crop fertilizer recommendations in sub-Saharan Africa. Agric. Syst. 180, 102790. doi: 10.1016/j.agsy.2020.102790
Salvagiotti, F., Cassman, K. G., Specht, J. E., Walters, D. T., Weiss, A., and Dobermann, A. (2008). Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 108, 1–13. doi: 10.1016/j.fcr.2008.03.001
Seleiman, M. F., Santanen, A., and Mäkelä, P. S. A. (2020). Recycling sludge on cropland as fertilizer – Advantages and risks. Resources Conserv. Recycling 155, 104647. doi: 10.1016/j.resconrec.2019.104647
Sharma, S., Singh, P., Choudhary, O. P., and Neemisha (2021). Nitrogen and rice straw incorporation impact nitrogen use efficiency, soil nitrogen pools and enzyme activity in rice-wheat system in north-western India. Field Crops Res. 266, 108131. doi: 10.1016/j.fcr.2021.108131
Shaviv, A. (2001). Advances in controlled-release fertilizers. Advances in Agronomy. 71, 1–49. doi: 10.1016/S0065-2113(01)71011-5
Shi, X., Hao, X., Shi, F., Li, N., Tian, Y., Han, P., et al. (2024). Improving cotton productivity and nutrient use efficiency by partially replacing chemical fertilizers with organic liquid fertilizer under mulched drip irrigation. Ind. Crops Products 216, 118731. doi: 10.1016/j.indcrop.2024.118731
Sim, D. H. H., Tan, I. A. W., Lim, L. L. P., and Hameed, B. H. (2021). Encapsulated biochar-based sustained release fertilizer for precision agriculture: A review. J. Cleaner Production 303, 127018. doi: 10.1016/j.jclepro.2021.127018
Stolarski, M. J., Krzyżaniak, M., Warmiński, K., Tworkowski, J., and Szczukowski, S. (2017). Perennial herbaceous crops as a feedstock for energy and industrial purposes: Organic and mineral fertilizers versus biomass yield and efficient nitrogen utilization. Ind. Crops Products 107, 244–259. doi: 10.1016/j.indcrop.2017.05.059
Su, P., Wicaksono, W. A., Li, C., Michl, K., Berg, G., Wang, D., et al. (2022). Recovery of metagenome-assembled genomes from the phyllosphere of 110 rice genotypes. Sci. Data 9, 254. doi: 10.1038/s41597-022-01320-7
Sun, J., Li, W., Li, C., Chang, W., Zhang, S., Zeng, Y., et al. (2020). Effect of different rates of nitrogen fertilization on crop yield, soil properties and leaf physiological attributes in banana under subtropical regions of China. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.613760
Takashima, T., Hikosaka, K., and Hirose, T. (2004). Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ. 27, 1047–1054. doi: 10.1111/j.1365-3040.2004.01209.x
Tegeder, M. and Masclaux-Daubresse, C. (2018). Source and sink mechanisms of nitrogen transport and use. New Phytol. 217, 35–53. doi: 10.1111/nph.14876
Tian, P., Liu, J., Zhao, Y., Huang, Y., Lian, Y., Wang, Y., et al. (2022). Nitrogen rates and plant density interactions enhance radiation interception, yield, and nitrogen use efficiencies of maize. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.974714
Trenkel, M. E. (2021). Slow-and controlled-release and stabilized fertilizers: an option for enhancing Nutrient use effiiency in agriculture. International Fertilizer Industry Association (IFA).
Trouwborst, G., Hogewoning, S. W., Harbinson, J., and van Ieperen, W. (2011). Photosynthetic acclimation in relation to nitrogen allocation in cucumber leaves in response to changes in irradiance. Physiol. Plant 142, 157–169. doi: 10.1111/j.1399-3054.2011.01456.x
van Ommen Kloeke, A. E. E., Douma, J. C., Ordoñez, J. C., Reich, P. B., and van Bodegom, P. M. (2011). Global quantification of contrasting leaf life span strategies for deciduous and evergreen species in response to environmental conditions. Global Ecol. Biogeography 21, 224–235. doi: 10.1111/j.1466-8238.2011.00667.x
Vejan, P., Khadiran, T., Abdullah, R., and Ahmad, N. (2021). Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control Release 339, 321–334. doi: 10.1016/j.jconrel.2021.10.003
Vos, J., Putten, P., and Birch, C. J. (2005). Effect of nitrogen supply on leaf appearance, leaf growth, leaf nitrogen economy and photosynthetic capacity in maize (Zea mays L.). Field Crops Res. 93, 64–73. doi: 10.1016/j.fcr.2004.09.013
Walling, E. and Vaneeckhaute, C. (2020). Greenhouse gas emissions from inorganic and organic fertilizer production and use: A review of emission factors and their variability. J. Environ. Manage 276, 111211. doi: 10.1016/j.jenvman.2020.111211
Wan, X., Wu, W., and Liao, Y. (2021). Mitigating ammonia volatilization and increasing nitrogen use efficiency through appropriate nitrogen management under supplemental irrigation and rain–fed condition in winter wheat. Agric. Water Manage. 255, 107050. doi: 10.1016/j.agwat.2021.107050
Wang, J., Hussain, S., Sun, X., Zhang, P., Javed, T., Dessoky, E. S., et al. (2022a). Effects of nitrogen application rate under straw incorporation on photosynthesis, productivity and nitrogen use efficiency in winter wheat. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.862088
Wang, Y., Lu, Y., Yuan, J., and He, G. (2022c). Evaluating the risks of nitrogen fertilizer-related grain production processes to ecosystem health in China. Resources Conserv. Recycling 177, 105982. doi: 10.1016/j.resconrec.2021.105982
Wang, T., Wang, Z., Zhang, J., and Ma, K. (2022b). An optimum combination of irrigation amount, irrigation water salinity and nitrogen application rate can improve cotton (for fiber) nitrogen uptake and final yield. Ind. Crops Products 187, 115386. doi: 10.1016/j.indcrop.2022.115386
Waring, E. F., Perkowski, E. A., and Smith, N. G. (2023). Soil nitrogen fertilization reduces relative leaf nitrogen allocation to photosynthesis. J. Exp. Bot. 74, 5166–5180. doi: 10.1093/jxb/erad195
Wu, A., Hammer, G. L., Doherty, A., von Caemmerer, S., and Farquhar, G. D. (2019). Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 5, 380–388. doi: 10.1038/s41477-019-0398-8
Xiao, Y., Peng, F., Zhang, Y., Wang, J., Zhuge, Y., Zhang, S., et al. (2019). Effect of bag-controlled release fertilizer on nitrogen loss, greenhouse gas emissions, and nitrogen applied amount in peach production. J. Cleaner Production 234, 258–274. doi: 10.1016/j.jclepro.2019.06.219
Xiong, H., Ma, H., Hu, B., Zhao, H., Wang, J., Rennenberg, H., et al. (2021). Nitrogen fertilization stimulates nitrogen assimilation and modifies nitrogen partitioning in the spring shoot leaves of citrus (Citrus reticulata Blanco) trees. J. Plant Physiol. 267, 153556. doi: 10.1016/j.jplph.2021.153556
Xu, G., Fan, X., and Miller, A. J. (2012). Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 63, 153–182. doi: 10.1146/annurev-arplant-042811-105532
Yang, Y., Zou, J., Huang, W., Manevski, K., Olesen, J. E., Rees, R. M., et al. (2022). Farm-scale practical strategies to increase nitrogen use efficiency and reduce nitrogen footprint in crop production across the North China Plain. Field Crops Res. 283, 108526. doi: 10.1016/j.fcr.2022.108526
Yoshida, K. and Hisabori, T. (2024). Divergent protein redox dynamics and their relationship with electron transport efficiency during photosynthesis induction. Plant Cell Physiol. 65, 737–747. doi: 10.1093/pcp/pcae013
Zhang, G., Li, Z., Zhu, Q., Yang, C., Shu, H., Gao, Z., et al. (2025). Cropping patterns and plant population density alter nitrogen partitioning among photosynthetic components, leaf photosynthetic capacity and photosynthetic nitrogen use efficiency in field-grown soybean. Ind. Crops Products 226, 120680. doi: 10.1016/j.indcrop.2025.120680
Zhang, G., Liu, S., Wang, X., Wang, X., Zhang, Y., Zhao, D., et al. (2023). Mixed application of controlled-release urea and normal urea can improve crop productivity and reduce the carbon footprint under straw return in winter wheat-summer maize cropping system. Eur. J. Agron. 151, 127002. doi: 10.1016/j.eja.2023.127002
Zhang, Y., Ren, W., Zhu, K., Fu, J., Wang, W., Wang, Z., et al. (2024). Substituting readily available nitrogen fertilizer with controlled-release nitrogen fertilizer improves crop yield and nitrogen uptake while mitigating environmental risks: A global meta-analysis. Field Crops Res. 306, 109221. doi: 10.1016/j.fcr.2023.109221
Zhao, J., Yang, J., Xie, H., Qin, X., and Huang, R. (2024). Sustainable management strategies for balancing crop yield, water use efficiency and greenhouse gas emissions. Agric. Syst. 217, 103944. doi: 10.1016/j.agsy.2024.103944
Zhu, X. G., de Sturler, E., and Long, S. P. (2007). Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiol. 145, 513–526. doi: 10.1104/pp.107.103713
Keywords: sunflower, photosynthetic-nitrogen use efficiency, controlled-release fertilizer, leaf nitrogen allocation, sustainable agricultural development
Citation: Ren W, Li X, Liu T, Chen N, Xin M, Qi Q and Liu B (2025) Controlled-release fertilizer affects leaf nitrogen allocation and photosynthesis to improve nitrogen use efficiency and yield in the sunflower field. Front. Plant Sci. 16:1622766. doi: 10.3389/fpls.2025.1622766
Received: 04 May 2025; Accepted: 17 June 2025;
Published: 07 July 2025.
Edited by:
Abraham J. Escobar-Gutiérrez, l’alimentation et l’environnement (INRAE), FranceReviewed by:
Agnieszka Synowiec, University of Agriculture in Krakow, PolandMuhammad Talha Aslam, University of Agriculture, Faisalabad, Pakistan
Copyright © 2025 Ren, Li, Liu, Chen, Xin, Qi and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianyue Li, bGl4aWFueXVlODBAMTI2LmNvbQ==
 Wenhao Ren
Wenhao Ren Xianyue Li
Xianyue Li Tingxi Liu1,2
Tingxi Liu1,2 Ning Chen
Ning Chen