- Shandong Provincial Key Laboratory of Microbial Engineering, School of Biologic Engineering, Qilu University of Technology (Shandong Academy of Sciences), Jinan, Shandong, China
Soil salinization represents a significant global ecological challenge. Plants encounter salt stress in their growth environments. Suberin, a hydrophobic polymer, plays a critical role in plant salt tolerance. This review examines the mechanisms by which suberin contributes to salt tolerance. Suberin comprises polyaliphatic and polyphenolic domains. Its biosynthesis involves multiple enzymes, including fatty acid synthases, the fatty acid elongation complex, and various cytochrome P450 monooxygenases. ABCG transporters and lipid transfer proteins facilitate the transport of suberin monomers from the endoplasmic reticulum to the plasma membrane and cell wall. Plants utilize suberin lamellae to respond to salt stress through multiple mechanisms. Under salt stress, the structure and composition of suberin lamellae undergo modifications, including increased thickness and enhanced very-long-chain fatty acid components. In addition, salt stress elevates the expression of genes associated with suberin biosynthesis and transport. Mutations in these genes often result in salt-sensitive phenotypes. Fundamentally, suberin contributes to forming the hydrophobic component of the apoplastic barrier, thereby reducing passive Na+ influx and restricting sodium uptake to protect plants from ion toxicity. Understanding the mechanisms of suberin in salt tolerance offers potential strategies for enhancing crop salt tolerance through genetic engineering.
1 Introduction
Plants have developed regulatory processes to respond and adapt to the dynamic conditions of complex and variable terrestrial environments (Shams and Khadivi, 2023). Roots are particularly affected by soil abiotic stresses such as salt and drought. The regulation of root water absorption and ion selectivity constitutes the initial defense mechanism against plant stress. Root suberin lamellae have demonstrated significant importance in plant stress resistance by functioning as an apoplastic barrier that modulates the diffusion of aqueous solutes, gases, and water (Serra and Geldner, 2022).
2 The formation of suberin in plants
Suberin is a complex hydrophobic polymer consisting of polyaliphatic and polyphenolic domains. Two types of suberin lamellae have been identified based on electron density in electron microscopy, electron-dense lamellae (appearing dark) and electron-lucent lamellae (appearing semitransparent). This distinction stems from the chemical attributes, with the polyaliphatic domain primarily associated with the electron-lucent lamellae and the polyphenolic domain to be associated with the electron-dense lamellae (Graca and Santos, 2007). The polyaliphatic domain consists of very-long-chain fatty acids (VLCFAs), including ω-hydroxyacids, dicarboxylic acids and primary alcohols. These aliphatic components integrate into a polymeric matrix anchored to a glycerol backbone, establishing a hydrophobic and structurally diverse platform within the suberin macromolecule. The polyphenolic domain contains p-hydroxycinnamic acid derivatives, predominantly ferulic acid. These phenolic components contribute to the cross-linking and recalcitrance of the suberin polymer, providing enhanced resistance to chemical and biological degradation (Nomberg et al., 2022).
2.1 The biosynthesis of suberin monomers
Suberin biosynthesis is initiated with fatty acid synthesis. In plants, C16:0, C18:0 and C18:1 fatty acids are synthesized in plastids by the fatty acid synthase complex. These compounds enter the endoplasmic reticulum (ER) after the CoA group is added. C16:0-CoA, C18:0-CoA and C18:1-CoA form VLCFAs by fatty acid elongation complex (FAE) in the ER. The β-ketoacyl-CoA synthase (KCS) enzymes perform essential functions in this process, catalyzing the rate-limiting step and determining product length. KCS2/DAISY and KCS20 are essential enzymes for suberin polyaliphatic monomer biosynthesis in Arabidopsis thaliana (Lee et al., 2009; Franke et al., 2009). The atkcs2/daisy mutant displayed notable changes in suberin composition with decreased C22 and enriched C16, C18 and C20 derivatives. In addition, the atkcs20kcs2 double mutant exhibited abnormal endodermal suberin lamellae. Chemical analysis revealed significantly reduced C22 and C24 VLCFA levels, while C20 VLCFA derivatives accumulated excessively (Lee et al., 2009). Similar results have been reported in in potato (Solanum tuberosum). StKCS6 silencing in the tuber periderm reduced the levels of VLCFAs exceeding C28 in length (Serra et al., 2009a). All these indicated that the KCS family is closely related to the elongation of fatty acid which act as monomers of suberin lamella (Figure 1).
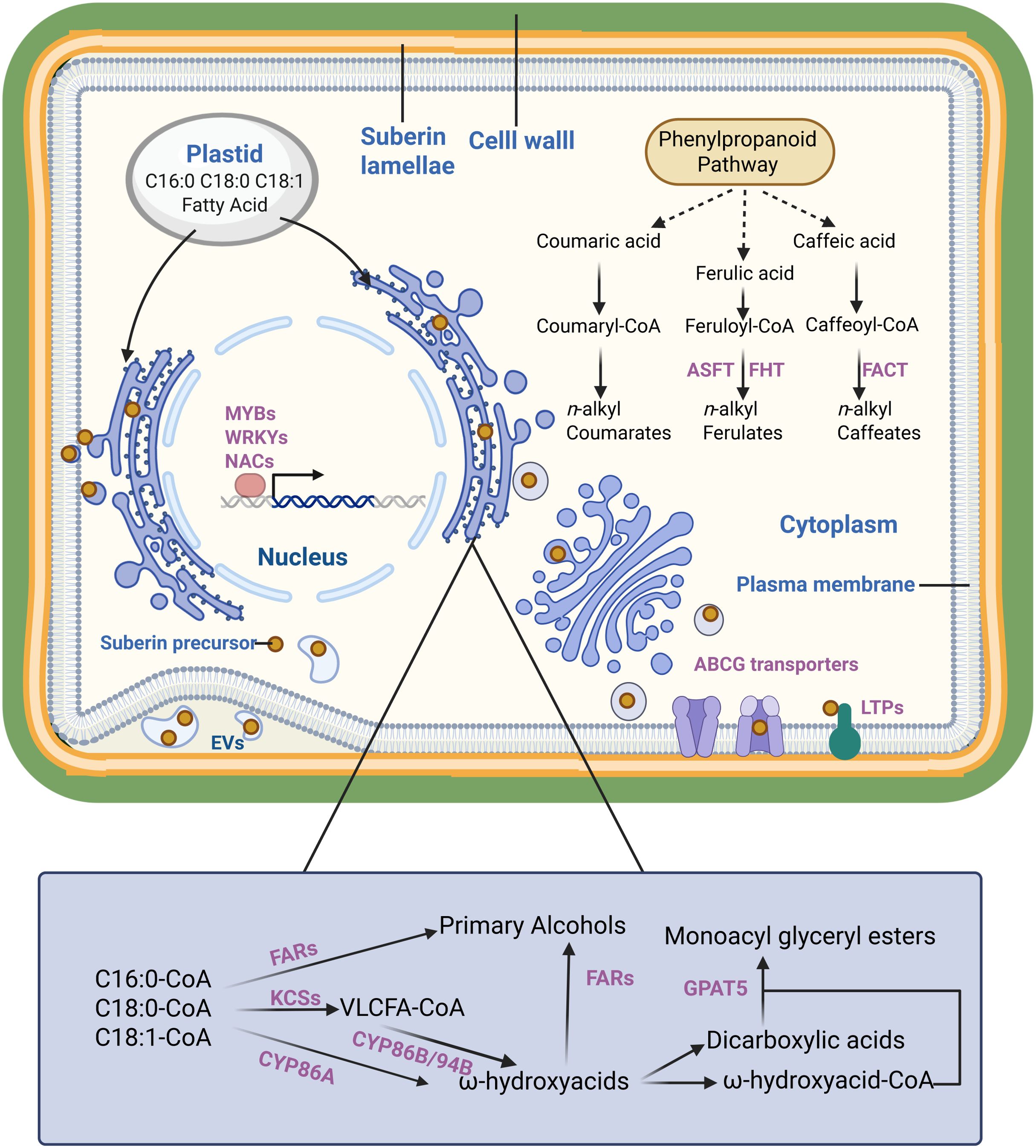
Figure 1. The formation of suberin lamellae in plants. The scheme is proposed based on previous studies. It presents suberin monomer (yellow dot) in plant cell, enzymes/transporters involved in suberin monomer biosynthesis, transportation and regulation (purple). Dashed lines represent more than one enzymatic step.
Compared to unmodified fatty acids, ω-hydroxy acids and α, ω-diacids are crucial for suberin polyester extension (Serra and Geldner, 2022). Cytochrome P450 monooxygenases are essential enzymes in ω-hydroxylate fatty acid biosynthesis in plants. The atcyp86a1/horst mutants exhibited significantly reduced levels of ω-hydroxy acids in roots compared with the wild type (Hofer et al., 2008). Similarly, CYP86B1/RALPH has been identified as a critical oxidase for suberin monomer ω-hydroxy acid and α,ω-dicarboxylic acid biosynthesis in Arabidopsis, with atcyp86b1/ralph mutants demonstrating a substantial reduction in C22 and C24 ω-hydroxy acid and α,ω-dicarboxylic acid contents (Molina et al., 2009; Compagnon et al., 2009). In potato, CYP86A33 silencing led to a 60% decrease in aliphatic suberin, particularly affecting C18:1 ω- hydroxyacid (70%) and α,ω-dicarboxylic acids (90%) levels (Serra et al., 2009b). In addition to CYP86s, CYP94s have also been reported to be closely related to fatty acid ω-hydroxylation process. The atcyp94b1 mutant exhibited marked reduction in ω-hydroxy acid and α,ω-dicarboxylic acid levels. Conversely, heterologous AoCYP94B1 overexpression in Arabidopsis resulted in enhanced accumulation of C18 octadecanol C16 ω-hydroxy acids, and C16 α,ω-dicarboxylic acids compared with the wild type (Krishnamurthy et al., 2020). Similar outcomes were observed with AoCYP94B3 (Krishnamurthy et al., 2021).
Primary alcohols and glycerol constitute essential components of suberin polyaliphatic monomers. The fatty acyl reductases facilitate the reduction in α-carboxylic groups of VLCFA-CoA to generate suberin monomer primary alcohols. Arabidopsis mutants atfar1, atfar4, and atfar5 showed modified suberin composition in the root and seed coat, with decreased levels of C22, C20 and C18 primary alcohols, respectively (Domergue et al., 2010).
The glycerol-3-phosphate acyltransferase (GPAT) transfers the acyl-CoA to glycerol-3 phosphate. GPAT5 demonstrates broad substrate specificity with the CoA-derivatives of C16–C22 fatty acids, ω-hydroxy acids, and α,ω-dicarboxylic acids. It is an essential role in suberin synthesis is evidenced by the atgpat5 mutant, which showed a 50% reduction in aliphatic suberin in roots and significantly decreased the suberin-related dicarboxylic acid and ω-hydroxy acid contents of seed coats (Yang et al., 2012; Beisson et al., 2007).
Ferulic acid, synthesized by phenylpropanoid pathway, is the primary aromatic monomer of suberin (Graça, 2015). Its significance in suberin formation was demonstrated through phenylpropanoid pathway inhibition studies, which showed blocked suberin deposition that can be restored by exogenous ferulic acid application (Andersen et al., 2021). Aliphatic suberin feruloyl transferase (ASFT) mediates the transfer of feruloyl-CoA acyl groups to ω-hydroxy acids and primary alcohols, a crucial step in suberin monomer biosynthesis. Arabidopsis atasft mutants demonstrated near-complete absence of ferulic acid in their polyphenolic domain affecting aliphatic suberin monomer levels (Molina et al., 2009; Gou et al., 2009). Furthermore, ENHANCED SUBERIN1 (ESB1) is involved in suberin synthesis, as evidenced by the Arabidopsis mutant atesb1 showing significantly increased root suberin content (Baxter et al., 2009).
2.2 The transport of suberin monomers
While numerous enzymes involved in suberin monomer synthesis or modification are located in the ER lumen or at the cytosolic face of the ER, suberin lamellae formation typically occurs on the plasma membrane surface or cell wall. The transport of suberin monomers from the ER to the plasma membrane and their transmembrane transport are essential steps in suberin lamellae formation (Serra and Geldner, 2022). The mechanisms underlying these processes remain incompletely understood, and several hypotheses exist regarding them. Lipid-like suberin monomers synthesized at the ER may transfer directly through the ER-plasma membrane contact site. Vesicle trafficking by the Golgi and trans-Golgi networks represents another potential mechanism, given its established role in cuticular wax delivery (Mcfarlane et al., 2014). Additionally, earlier studies documented plasma membrane invaginations containing extracellular tubules or vesicles. These structures were initially proposed as intermediates in lipid-like substance transport, including suberin monomers (Scott and Peterson, 1979; Peterson and Ma, 2001). This concept remained dormant until recent research identified similar structures called extracellular vesicular-tubular (EVs), which demonstrate a strong correlation with suberin lamellae formation. The accumulation of large numbers of EVs in suberizing cells were observed through electron-microscopy (De Bellis et al., 2022).
After suberin monomers reach the plasma membrane, they are transported across the membrane to the apoplast. Several ABC transporters of the G-clade (ABCG) have been implicated in this process. The Arabidopsis DSO/ABCG11 transporter influences cutin metabolism in reproductive organs and suberin content of roots (Panikashvili et al., 2010). In addition, three Arabidopsis transporters (ABCG2, ABCG6, and ABCG20) participate in suberin metabolism in roots and seed coats (Yadav et al., 2014; Fedi et al., 2017). The triple mutant of these genes exhibited changes in suberin lamellae structure, composition, and properties in the root and seed coat (Yadav et al., 2014). Further research confirmed AtABCG1’s role in suberin transport, as the atabcg1 mutant showed decreased root suberin content, particularly in VLCFAs, primary alcohols, and dicarboxylic acids (Shanmugarajah et al., 2019). Furthermore, homologs of these genes, such as StABCG1 and OsABCG5/RCN1, facilitate suberin precursor transport (Landgraf et al., 2014; Shiono et al., 2014). Beyond ABCG transporters, the LIPID TRANSFER PROTEIN (LTP) superfamily contributes to cuticular wax deposition and pollen wall formation (Chen et al., 2022; Gao et al., 2021; Edqvist et al., 2018). Evidence indicates that LTPs participate in suberin monomers transport. AtLTPI-4 contributes to suberin formation in Arabidopsis crown galls, as the atltpi4 mutant showed significantly reduced suberin accumulation. Protein expression in epidermal cells increased C24 and C26 VLCFA levels (Deeken et al., 2016). In addition, Arabidopsis LTPG15, expressed in the root endodermis and seed coat, facilitates suberin monomer transport (Lee and Suh, 2018). Collectively, both ABCG transporters and LTPs may transport suberin monomers across the membrane to the apoplast where suberin lamellae form.
2.3 Regulation of suberin biosynthesis
Suberin deposition exhibits cell and tissue specificity and responds to various environmental stresses, reflecting strict transcriptional regulation of suberin biosynthesis-related genes. The MYB family regulates suberin biosynthesis and deposition across species and tissues (Cesarino, 2022). AtMYB41 activates aliphatic suberin synthesis and deposition. AtMYB41 overexpression enhanced the transcription of suberin biosynthesis genes in leaves and induced suberin deposition on leaf cell walls, forming suberin-lamellae-like structures (Kosma et al., 2014). Subsequently, additional MYB transcription factors involved in suberin biosynthesis regulation were identified and functionally validated. MdMYB93 demonstrated a significant role in regulating suberin deposition in russeted apple (Malus domestica) skins (Legay et al., 2016). MYB9 and MYB107 regulate suberin deposition in seed coats and fruit skins (Lashbrooke et al., 2016). Shukla et al. identified four MYB transcription factors (MYB41, MYB53, MYB92, and MYB93) that individually respond to developmental and exogenous signals and promote endodermal suberin formation (Shukla et al., 2021). In potato wound-healing tissues, StMYB102 and StMYB74 function as regulators of wound suberin biosynthesis and deposition (Wahrenburg et al., 2021).
The NAC and WRKY families serve as transcriptional regulators of suberin biosynthesis. ANAC046 expression occurs primarily in the root endodermis and periderm, with wound-induced expression in leaves. ANAC046 overexpression enhanced the expression of suberin biosynthesis genes in the roots and leaves, increasing root wax and suberin accumulation. This indicated that ANAC046 functions as a key transcription factor promoting suberin biosynthesis in Arabidopsis roots (Mahmood et al., 2019). StNAC103 and StNAC101 act as suberin biosynthesis repressors in potato, evidenced by increased suberin and wax in RNAi-mediated mutants (Soler et al., 2020). WRKY33 functions as an upstream regulator of CYP94B1 in Arabidopsis, with atwrky33 mutants showing reduced suberin and salt-sensitive phenotypes (Krishnamurthy et al., 2020). Krishnamurthy et al. identified AtWRKY9 as a suberin biosynthesis regulator through its control of AtCYP94B31 and AtCYP86B1 expression in Arabidopsis (Krishnamurthy et al., 2021).
Various hormones, including abscisic acid (ABA) (Shiono et al., 2022; Shiono and Matsuura, 2024), ethylene (Liu et al., 2021), auxin (Ursache et al., 2021), and gibberellin (Binenbaum et al., 2023) participate in regulating suberin biosynthesis.
3 The role of suberin in salt stress resistance
Salt stress is a major environmental challenge affecting plant growth and production globally (Zhou et al., 2024; Shams et al., 2024, 2023). Plant survival under salt stress depends on maintaining low cytoplasmic Na+ concentration in shoots. Apoplastic transpiration bypass flow of water and solutes contributes substantially to Na+ entry into shoots (Ochiai and Matoh, 2002). However, suberin lamellae in the endodermis and exodermis can block this bypass flow of water and solutes (Hose et al., 2001; Steudle and Peterson, 1998). This characteristic establishes a strong connection between suberin lamellae and plant salt tolerance.
3.1 The structure and composition of suberin lamellae change in response to salt stress
Suberin lamellae serve as a natural barrier that restricts Na+ transport from roots to shoots through the bypass flow. Its thickness and location directly influence the effectiveness of salt exclusion in plants. Three rice (Oryza sativa) varieties cultivated under varying salt concentrations demonstrated increased root suberization and upregulation of suberin synthesis genes in response to salt stress. The formation of both the endodermal and exodermal suberin lamellae advanced toward the root tip, indicating accelerated root suberization under salt stress. The variety exhibiting the highest degree of root suberization displayed the lowest Na+ accumulation in shoots (Krishnamurthy et al., 2009, 2011). Similar observations were documented in Avicennia officinalis, where salt treatment increased root suberin lamellae thickness and significantly reduced Na+ transport to aboveground parts by the xylem (Krishnamurthy et al., 2014). Subsequent research using corn (Zea mays), olive (Olea europaea), and grape (Vitis vinifera) also showed salt stress induced thickening of the suberin lamellae (Rossi et al., 2015; Shen et al., 2015; Wang et al., 2023). These findings demonstrate that salt stress promotes suberin deposition and thickening, effectively inhibiting Na+ absorption and transportation, thereby establishing an inverse relationship between Na+ permeability and root suberization.
In addition to the change of the structure, suberin composition undergoes changes under salt stress. NaCl treatment induced a 22% increase in total suberin content in Arabidopsis after 100 mM NaCl exposure, with significant increases in dicarboxylic fatty acids and modest increases in 18:0 ferulate and 20:0 and 22:0 coumarates (De Silva et al., 2021). In Chenopodium album, salt stress significantly increased saturated and unsaturated VLCFAs with chain lengths of C20–C26 (Ivanova et al., 2016). Moreover, increasing saturation and length of fatty acids represents an adaptation strategy against salt stress in halophytes (Sui et al., 2018). These findings indicate that salt stress enhances VLCFA components in plant suberin, potentially strengthening the hydrophobic barrier, limiting Na+ flux, and maintaining membrane stability.
3.2 Expression of genes related to suberin biosynthesis were induced by salt stress
The structural changes in suberin lamellae structure under salt stress indicate the regulation of suberin synthesis and transport-related gene expression. Transcriptome analyses reveal the salt stress-induced transcription of biosynthesis genes (Wei et al., 2020; Yang et al., 2018). In rice, an increased transcript levels of suberin biosynthesis gene was detectable as early as 30 minutes after NaCl treatment (Krishnamurthy et al., 2009). Analysis of tissue-specific differential induction revealed that the expression of suberin-related genes in roots correlates most strongly with salt stress. In quinoa (Chenopodium quinoa), CqGPAT5a and CqGPAT5b demonstrated high expression in roots with rapid induction under high salt stress (Wang et al., 2025b). Similarly, VvKCS11 exhibits root-specific high expression and strong salt stress induction in grape (Yang et al., 2020). Salt treatment increases the expression of CYP94B3 and CYP86B1, key suberin precursor synthesis genes, in the roots of both Arabidopsis and medicinal plants (Krishnamurthy et al., 2021).
Studies using suberin-related gene mutants further demonstrate suberin’s significance in plant salt tolerance. In wheat (Triticum aestivum), TaGPAT6 overexpression enhanced suberin deposition in the seed coat and root tip outer layers, improving salt tolerance through reduced Na+ accumulation. Conversely, tagpat6 mutants exhibited decreased suberin deposition, enabling Na+ accumulation and resulting in salt sensitivity (Wang et al., 2025a). The Arabidopsis mutant cyp86a1 displayed salt sensitivity with increased Na+ and decreased K+ accumulation (Wang et al., 2020). Furthermore atmyb107 and atmyb9 mutants showed a significantly reduced seed suberin monomer content, leading to decreased germination rates under salt stress (Lashbrooke et al., 2016). Similarly, ABCG23 mutation reduced C24 ω-hydroxy fatty acids and 1, ω-dicarboxylic acids in the mutant seed coats, diminishing germination rates under salt stress (Kim et al., 2025). These mutant studies demonstrate that altered suberin composition or lamellae structure affects the ion and water absorption, often resulting in salt-sensitive phenotypes (De Silva et al., 2021).
4 Conclusions
Suberin functions as a natural hydrophobic barrier in plants. Salt stress induces the expression of genes related to suberin biosynthesis and transportation were induced by salt stress and transport, modifying suberin composition and lamellae structure. Increased suberin content, altered suberin monomer composition, and thickened lamellae enhance the hydrophobic barrier. This barrier can thus restrict sodium uptake, protecting plant photosynthetic organs from ion toxicity (Figure 2). The role of suberin in salt stress response suggests potential applications in genetic engineering to enhance suberin deposition for developing salt-tolerant crops.
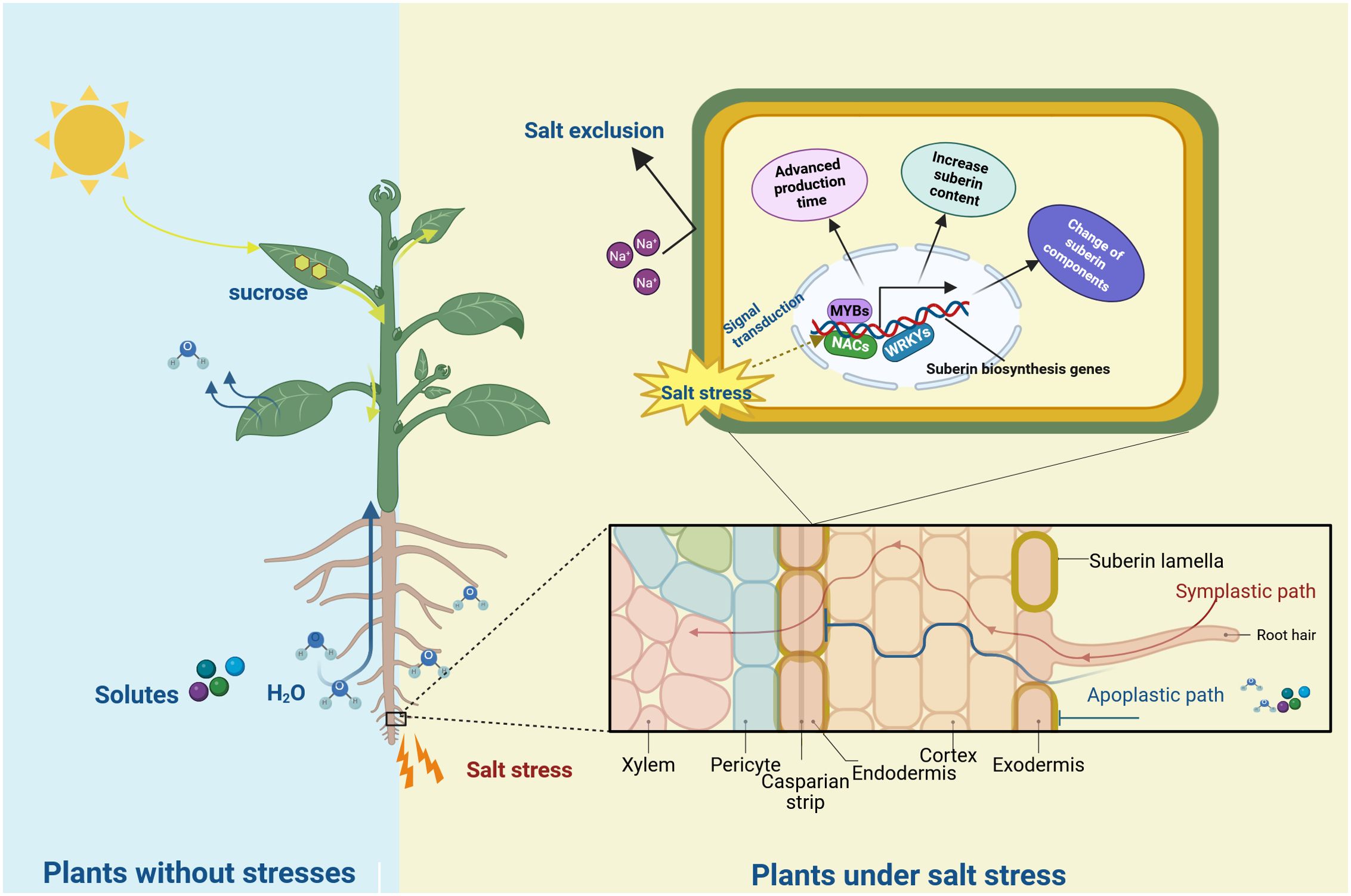
Figure 2. The role of suberin in plant in response to salt stress. The light blue background represents the non-stress environment, and the yellow background represents the salt-stress environment. Under salt stress, genes related to suberin lamellae foemation are induced by the regulation of transcription factors (such as MYBs, NACs and WRKYs), resulting in the advanced production time, the change of components and the increased content of suberin. The suberin lamellae (yellow circles) in roots was induced and block the bypass flow of Na+.
Author contributions
RC: Writing – original draft. PW: Writing – original draft. JL: Visualization, Writing – original draft. XY: Writing – review & editing, Software. XG: Writing – review & editing. HZ: Software, Writing – review & editing. NH: Funding acquisition, Writing – review & editing. ZY: Writing – original draft, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Fruit Innovation Team of Shandong Modern Agricultural Industry Technology System (SDAIT-06-14) and the Basic Research Projects of Integration of Science, Education and Industry of Qilu University of Technology (Shandong Academy of Sciences) (2022PX033).
Acknowledgments
We acknowledge TopEdit LLC for the linguistic editing and proofreading during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andersen, T. G., Molina, D., Kilian, J., Franke, R. B., Ragni, L., and Geldner, N. (2021). Tissue-autonomous phenylpropanoid production is essential for establishment of root barriers. Curr. Biol. 31, 965–977.e5. doi: 10.1016/j.cub.2020.11.070
Baxter, I., Hosmani, P. S., Rus, A., Lahner, B., Borevitz, J. O., Muthukumar, B., et al. (2009). Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PloS Genet. 5, e1000492. doi: 10.1371/journal.pgen.1000492
Beisson, F., Li, Y., Bonaventure, G., Pollard, M., and Ohlrogge, J. B. (2007). The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 19, 351–368. doi: 10.1105/tpc.106.048033
Binenbaum, J., Wulff, N., Camut, L., Kiradjiev, K., Anfang, M., Tal, I., et al. (2023). Gibberellin and abscisic acid transporters facilitate endodermal suberin formation in Arabidopsis. Nat. Plants 9, 785–802. doi: 10.1038/s41477-023-01391-3
Cesarino, I. (2022). With a little help from MYB friends: Transcriptional network controlling root suberization and lignification. Plant Physiol. 190, 1077–1079. doi: 10.1093/plphys/kiac318
Chen, L., Ji, C., Zhou, D., Gou, X., Tang, J., Jiang, Y., et al. (2022). OsLTP47 may function in a lipid transfer relay essential for pollen wall development in rice. J. Genet. Genomics 49, 481–491. doi: 10.1016/j.jgg.2022.03.003
Compagnon, V., Diehl, P., Benveniste, I., Meyer, D., Schaller, H., Schreiber, L., et al. (2009). CYP86B1 is required for very long chain omega-hydroxyacid and alpha, omega -dicarboxylic acid synthesis in root and seed suberin polyester. Plant Physiol. 150, 1831–1843. doi: 10.1104/pp.109.141408
De Bellis, D., Kalmbach, L., Marhavy, P., Daraspe, J., Geldner, N., and Barberon, M. (2022). Extracellular vesiculo-tubular structures associated with suberin deposition in plant cell walls. Nat. Commun. 13, 1489. doi: 10.1038/s41467-022-29110-0
Deeken, R., Saupe, S., Klinkenberg, J., Riedel, M., Leide, J., Hedrich, R., et al. (2016). The nonspecific lipid transfer protein Atltpi-4 is involved in suberin formation of Arabidopsis thaliana crown galls. Plant Physiol. 172, 1911–1927. doi: 10.1104/pp.16.01486
De Silva, N. D. G., Murmu, J., Chabot, D., Hubbard, K., Ryser, P., Molina, I., et al. (2021). Root suberin plays important roles in reducing water loss and sodium uptake in Arabidopsis thaliana. Metabolites 11(11):735. doi: 10.3390/metabo11110735
Domergue, F., Vishwanath, S. J., Joubes, J., Ono, J., Lee, J. A., Bourdon, M., et al. (2010). Three Arabidopsis fatty acyl-coenzyme A reductases, FAR1, FAR4, and FAR5, generate primary fatty alcohols associated with suberin deposition. Plant Physiol. 153, 1539–1554. doi: 10.1104/pp.110.158238
Edqvist, J., Blomqvist, K., Nieuwland, J., and Salminen, T. A. (2018). Plant lipid transfer proteins: are we finally closing in on the roles of these enigmatic proteins? J. Lipid Res. 59, 1374–1382. doi: 10.1194/jlr.R083139
Fedi, F., O’neill, C. M., Menard, G., Trick, M., Dechirico, S., Corbineau, F., et al. (2017). Awake1, an ABC-type transporter, reveals an essential role for suberin in the control of seed dormancy. Plant Physiol. 174, 276–283. doi: 10.1104/pp.16.01556
Franke, R., Hofer, R., Briesen, I., Emsermann, M., Efremova, N., Yephremov, A., et al. (2009). The DAISY gene from Arabidopsis encodes a fatty acid elongase condensing enzyme involved in the biosynthesis of aliphatic suberin in roots and the chalaza-micropyle region of seeds. Plant J. 57, 80–95. doi: 10.1111/j.1365-313X.2008.03674.x
Gao, H. N., Jiang, H., Lian, X. Y., Cui, J. Y., You, C. X., Hao, Y. J., et al. (2021). Identification And functional analysis of the MdLTPG gene family in apple. Plant Physiol. Biochem. 163, 338–347. doi: 10.1016/j.plaphy.2021.04.015
Gou, J. Y., Yu, X. H., and Liu, C. J. (2009). A hydroxycinnamoyltransferase responsible for synthesizing suberin aromatics in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 106, 18855–18860. doi: 10.1073/pnas.0905555106
Graça, J. (2015). Suberin: the biopolyester at the frontier of plants. Front. Chem. 3, 62. doi: 10.3389/fchem.2015.00062
Graca, J. and Santos, S. (2007). Suberin: a biopolyester of plants’ skin. Macromol. Biosci. 7, 128–135. doi: 10.1002/mabi.200600218
Hofer, R., Briesen, I., Beck, M., Pinot, F., Schreiber, L., and Franke, R. (2008). The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid omega-hydroxylase involved in suberin monomer biosynthesis. J. Exp. Bot. 59, 2347–2360. doi: 10.1093/jxb/ern101
Hose, E., Clarkson, D. T., Steudle, E., Schreiber, L., and Hartung, W. (2001). The exodermis: A variable apoplastic barrier. J. Exp. Bot. 52, 2245. doi: 10.1093/jexbot/52.365.2245
Ivanova, T. V., Maiorova, O. V., Orlova, Y. V., Kuznetsova, E. I., Khalilova, L. A., Myasoedov, N. A., et al. (2016). Cell ultrastructure and fatty acid composition of lipids in vegetative organs of Chenopodium album L. under salt stress conditions. Russian J. Plant Physiol. 63, 763–775. doi: 10.1134/S1021443716060054
Kim, R. J., Zhang, Y., and Suh, M. C. (2025). ATP-binding cassette G23 is required for Arabidopsis seed coat suberization. Plant Sci. 352, 112361. doi: 10.1016/j.plantsci.2024.112361
Kosma, D. K., Murmu, J., Razeq, F. M., Santos, P., Bourgault, R., Molina, I., et al. (2014). AtMYB41 activates ectopic suberin synthesis and assembly in multiple plant species and cell types. Plant J. 80, 216–229. doi: 10.1111/tpj.12624
Krishnamurthy, P., Jyothi-Prakash, P. A., Qin, L., He, J., Lin, Q., Loh, C. S., et al. (2014). Role of root hydrophobic barriers in salt exclusion of a mangrove plant Avicennia officinalis. Plant Cell Environ. 37, 1656–1671. doi: 10.1111/pce.12272
Krishnamurthy, P., Ranathunge, K., Franke, R., Prakash, H., Schreiber, L., and Mathew, M. (2009). The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.). Planta 230, 119–134. doi: 10.1007/s00425-009-0930-6
Krishnamurthy, P., Ranathunge, K., Nayak, S., Schreiber, L., and Mathew, M. (2011). Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). J. Exp. Bot. 62, 4215–4228. doi: 10.1093/jxb/err135
Krishnamurthy, P., Vishal, B., Bhal, A., and Kumar, P. P. (2021). WRKY9 transcription factor regulates cytochrome P450 genes CYP94B3 and CYP86B1, leading to increased root suberin and salt tolerance in Arabidopsis. Physiol. Plant. 172, 1673–1687. doi: 10.1111/ppl.13371
Krishnamurthy, P., Vishal, B., Ho, W. J., Lok, F. C. J., Lee, F. S. M., and Kumar, P. P. (2020). Regulation of a cytochrome P450 gene CYP94B1by WRKY33 transcription factor controls apoplastic barrier formation in roots to confer salt tolerance. Plant Physiol. 184, 2199–2215. doi: 10.1104/pp.20.01054
Landgraf, R., Smolka, U., Altmann, S., Eschen-Lippold, L., Senning, M., Sonnewald, S., et al. (2014). The ABC transporter ABCG1 is required for suberin formation in potato tuber periderm. Plant Cell 26, 3403–3415. doi: 10.1105/tpc.114.124776
Lashbrooke, J., Cohen, H., Levy-Samocha, D., Tzfadia, O., Panizel, I., Zeisler, V., et al. (2016). MYB107 and MYB9 homologs regulate suberin deposition in angiosperms. Plant Cell 28, 2097–2116. doi: 10.1105/tpc.16.00490
Lee, S. B., Jung, S. J., Go, Y. S., Kim, H. U., Kim, J. K., Cho, H. J., et al. (2009). Two Arabidopsis 3-ketoacyl CoA synthase genes, KCS20 and KCS2/DAISY, are functionally redundant in cuticular wax and root suberin biosynthesis, but differentially controlled by osmotic stress. Plant J. 60, 462–475. doi: 10.1111/j.1365-313X.2009.03973.x
Lee, S. B. and Suh, M. C. (2018). Disruption of glycosylphosphatidylinositol-anchored lipid transfer protein 15 affects seed coat permeability in Arabidopsis. Plant J. 96, 1206–1217. doi: 10.1111/tpj.14101
Legay, S., Guerriero, G., Andre, C., Guignard, C., Cocco, E., Charton, S., et al. (2016). MdMyb93 is a regulator of suberin deposition in russeted apple fruit skins. New Phytol. 212, 977–991. doi: 10.1111/nph.14170
Liu, Y., Tao, Q., Li, J., Guo, X., Luo, J., Jupa, R., et al. (2021). Ethylene-mediated apoplastic barriers development involved in cadmium accumulation in root of hyperaccumulator Sedum alfredii. J. Hazard Mater. 403, 123729. doi: 10.1016/j.jhazmat.2020.123729
Mahmood, K., Zeisler-Diehl, V. V., Schreiber, L., Bi, Y. M., Rothstein, S. J., and Ranathunge, K. (2019). Overexpression of ANAC046 promotes suberin biosynthesis in roots of Arabidopsis thaliana. Int. J. Mol. Sci. 20(24):6117. doi: 10.3390/ijms20246117
Mcfarlane, H. E., Watanabe, Y., Yang, W., Huang, Y., Ohlrogge, J., and Samuels, A. L. (2014). Golgi- and trans-Golgi network-mediated vesicle trafficking is required for wax secretion from epidermal cells. Plant Physiol. 164, 1250–1260. doi: 10.1104/pp.113.234583
Molina, I., Li-Beisson, Y., Beisson, F., Ohlrogge, J. B., and Pollard, M. (2009). Identification of an Arabidopsis feruloyl-coenzyme A transferase required for suberin synthesis. Plant Physiol. 151, 1317–1328. doi: 10.1104/pp.109.144907
Nomberg, G., Marinov, O., Arya, G. C., Manasherova, E., and Cohen, H. (2022). The key enzymes in the suberin biosynthetic pathway in plants: an update. Plants (Basel) 11(3):392. doi: 10.3390/plants11030392
Ochiai, K. and Matoh, T. (2002). Characterization of the Na+ delivery from roots to shoots in rice under saline stress: excessive salt enhances apoplastic transport in rice plants. Soil Sci. Plant Nutr. 48, 371–378. doi: 10.1080/00380768.2002.10409214
Panikashvili, D., Shi, J. X., Bocobza, S., Franke, R. B., Schreiber, L., and Aharoni, A. (2010). The Arabidopsis DSO/ABCG11 transporter affects cutin metabolism in reproductive organs and suberin in roots. Mol. Plant 3, 563–575. doi: 10.1093/mp/ssp103
Peterson, C. A. and Ma, F. J. C. J. O. B. (2001). Development of cell wall modifications in the endodermis and exodermis of Allium cepa roots. Canad. J. Bot. 79, 621–634. doi: 10.1139/b01-030
Rossi, L., Francini, A., Minnocci, A., and Sebastiani, L. (2015). Salt stress modifies apoplastic barriers in olive (Olea europaea L.): A comparison between a salt-tolerant and a salt-sensitive cultivar. Scientia Hortic. 192, 38–46. doi: 10.1016/j.scienta.2015.05.023
Scott, M. G. and Peterson, R. L. J. C. J. O. B. (1979). The root endodermis in Ranunculus acris. I. Struct. Ontogeny 57, 1040–1062. doi: 10.1139/b79-129
Serra, O. and Geldner, N. (2022). The making of suberin. New Phytol. 235, 848–866. doi: 10.1111/nph.18202
Serra, O., Soler, M., Hohn, C., Franke, R., Schreiber, L., Prat, S., et al. (2009a). Silencing of StKCS6 in potato periderm leads to reduced chain lengths of suberin and wax compounds and increased peridermal transpiration. J. Exp. Bot. 60, 697–707. doi: 10.1093/jxb/ern314
Serra, O., Soler, M., Hohn, C., Sauveplane, V., Pinot, F., Franke, R., et al. (2009b). CYP86A33-targeted gene silencing in potato tuber alters suberin composition, distorts suberin lamellae, and impairs the periderm’s water barrier function. Plant Physiol. 149, 1050–1060. doi: 10.1104/pp.108.127183
Shams, M. and Khadivi, A. (2023). Mechanisms of salinity tolerance and their possible application in the breeding of vegetables. BMC Plant Biol. 23, 139. doi: 10.1186/s12870-023-04152-8
Shams, M., Pokora, W., Khadivi, A., and Aksmann, A. (2024). Superoxide dismutase in Arabidopsis and Chlamydomonas: diversity, localization, regulation, and role. Plant Soil 503, 751–771. doi: 10.1007/s11104-024-06618-6
Shams, M., Yuksel, E. A., Agar, G., Ekinci, M., Kul, R., Turan, M., et al. (2023). Biosynthesis of capsaicinoids in pungent peppers under salinity stress. Physiol. Plant. 175, e13889. doi: 10.1111/ppl.13889
Shanmugarajah, K., Linka, N., Grafe, K., Smits, S. H. J., Weber, A. P. M., Zeier, J., et al. (2019). ABCG1 contributes to suberin formation in Arabidopsis thaliana roots. Sci. Rep. 9, 11381. doi: 10.1038/s41598-019-47916-9
Shen, J., Xu, G., and Zheng, H. Q. (2015). Apoplastic barrier development and water transport in Zea mays seedling roots under salt and osmotic stresses. Protoplasma 252, 173–180. doi: 10.1007/s00709-014-0669-1
Shiono, K., Ando, M., Nishiuchi, S., Takahashi, H., Watanabe, K., Nakamura, M., et al. (2014). RCN1/OsABCG5, an ATP-binding cassette (ABC) transporter, is required for hypodermal suberization of roots in rice (Oryza sativa). Plant J. 80, 40–51. doi: 10.1111/tpj.12614
Shiono, K. and Matsuura, H. (2024). Exogenous abscisic acid induces the formation of a suberized barrier to radial oxygen loss in adventitious roots of barley (Hordeum vulgare). Ann. Bot. 133, 931–940. doi: 10.1093/aob/mcae010
Shiono, K., Yoshikawa, M., Kreszies, T., Yamada, S., Hojo, Y., Matsuura, T., et al. (2022). Abscisic acid is required for exodermal suberization to form a barrier to radial oxygen loss in the adventitious roots of rice (Oryza sativa). New Phytol. 233, 655–669. doi: 10.1111/nph.17751
Shukla, V., Han, J. P., Cleard, F., Lefebvre-Legendre, L., Gully, K., Flis, P., et al. (2021). Suberin plasticity to developmental and exogenous cues is regulated by a set of MYB transcription factors. Proc. Natl. Acad. Sci. U.S.A. 118(39):e2101730118. doi: 10.1073/pnas.2101730118
Soler, M., Verdaguer, R., Fernandez-Pinan, S., Company-Arumi, D., Boher, P., Gongora-Castillo, E., et al. (2020). Silencing against the conserved NAC domain of the potato StNAC103 reveals new NAC candidates to repress the suberin associated waxes in phellem. Plant Sci. 291, 110360. doi: 10.1016/j.plantsci.2019.110360
Steudle, E. and Peterson, C. A. (1998). How does water get through roots? J. Exp. Bot. 49, 775–788. doi: 10.1093/jxb/49.322.775
Sui, N., Wang, Y., Liu, S., Yang, Z., Wang, F., and Wan, S. (2018). Transcriptomic and physiological evidence for the relationship between unsaturated fatty acid and salt stress in peanut. Front. Plant Sci. 9, 7. doi: 10.3389/fpls.2018.00007
Ursache, R., De Jesus Vieira Teixeira, C., Denervaud Tendon, V., Gully, K., De Bellis, D., Schmid-Siegert, E., et al. (2021). GDSL-domain proteins have key roles in suberin polymerization and degradation. Nat. Plants 7, 353–364. doi: 10.1038/s41477-021-00862-9
Wahrenburg, Z., Benesch, E., Lowe, C., Jimenez, J., Vulavala, V. K. R., Lu, S., et al. (2021). Transcriptional regulation of wound suberin deposition in potato cultivars with differential wound healing capacity. Plant J. 107, 77–99. doi: 10.1111/tpj.15275
Wang, W., Chi, M., Liu, S., Zhang, Y., Song, J., Xia, G., et al. (2025a). TaGPAT6 enhances salt tolerance in wheat by synthesizing cutin and suberin monomers to form a diffusion barrier. J. Integr. Plant Biol. 67, 208–225. doi: 10.1111/jipb.13808
Wang, Z., Liu, Y., Huang, H., Zheng, Z., Lu, S., Yang, X., et al. (2025b). Functional identification of two Glycerol-3-phosphate Acyltransferase5 homologs from Chenopodium quinoa. Plant Sci. 350, 112313. doi: 10.1016/j.plantsci.2024.112313
Wang, P., Wang, C.-M., Gao, L., Cui, Y.-N., Yang, H.-L., De Silva, N. D. G., et al. (2020). Aliphatic suberin confers salt tolerance to Arabidopsis by limiting Na+ influx, K+ efflux and water backflow. Plant Soil 448, 603–620. doi: 10.1007/s11104-020-04464-w
Wang, P., Wang, F., Li, L., Su, S., Han, N., and Yang, Z. (2023). Study on Effects of salt stress on the Suberin Lamella of grapevine roots. Bio Web Conf. 61, 01027. doi: 10.1051/bioconf/20236101027
Wei, X., Yang, Z., Han, G., Zhao, X., Yin, S., Yuan, F., et al. (2020). The developmental dynamics of the sweet sorghum root transcriptome elucidate the differentiation of apoplastic barriers. Plant Signal Behav. 15, 1724465. doi: 10.1080/15592324.2020.1724465
Yadav, V., Molina, I., Ranathunge, K., Castillo, I. Q., Rothstein, S. J., and Reed, J. W. (2014). ABCG transporters are required for suberin and pollen wall extracellular barriers in Arabidopsis. Plant Cell 26, 3569–3588. doi: 10.1105/tpc.114.129049
Yang, W., Simpson, J. P., Li-Beisson, Y., Beisson, F., Pollard, M., and Ohlrogge, J. B. (2012). A land-plant-specific glycerol-3-phosphate acyltransferase family in Arabidopsis: substrate specificity, sn-2 preference, and evolution. Plant Physiol. 160, 638–652. doi: 10.1104/pp.112.201996
Yang, Z., Yang, X., Dong, S., Ge, Y., Zhang, X., Zhao, X., et al. (2020). Overexpression of beta-Ketoacyl-CoA Synthase From Vitis vinifera L. Improves Salt Tolerance in Arabidopsis thaliana. Front. Plant Sci. 11, 564385. doi: 10.3389/fpls.2020.564385
Yang, Z., Zheng, H., Wei, X., Song, J., Wang, B., and Sui, N. (2018). Transcriptome analysis of sweet Sorghum inbred lines differing in salt tolerance provides novel insights into salt exclusion by roots. Plant Soil 430, 423–439. doi: 10.1007/s11104-018-3736-0
Keywords: suberin, salt stress, salt resistance, biosynthesis, transportation, salt exclusion
Citation: Chen R, Wang P, Liu J, Yang X, Gong X, Zhou H, Han N and Yang Z (2025) Suberin in plants: biosynthesis, regulation, and its role in salt stress resistance. Front. Plant Sci. 16:1624136. doi: 10.3389/fpls.2025.1624136
Received: 07 May 2025; Accepted: 16 June 2025;
Published: 30 June 2025.
Edited by:
Tabassum Hussain, University of Karachi, PakistanReviewed by:
Mostafakamal Shams, University of Gdansk, PolandCopyright © 2025 Chen, Wang, Liu, Yang, Gong, Zhou, Han and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Han, aG44MjY1QDE2My5jb20=; Zhen Yang, Z2luYTM1QDEyNi5jb20=
†These authors have contributed equally to this work
 Ruonan Chen†
Ruonan Chen† Ning Han
Ning Han Zhen Yang
Zhen Yang