- 1Inner Mongolia Key Laboratory of Desert Ecological System, Inner Mongolia Academy of Forestry Sciences, Hohhot, Inner Mongolia, China
- 2College of Desert Control Science and Engineering, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia, China
- 3Forest Resources Monitoring Office, Inner Mongolia Autonomous Region Forestry and Grassland Monitoring and Planning Institute, Hohhot, Inner Mongolia, China
- 4College of Computer Science, Inner Mongolia University, Hohhot, Inner Mongolia, China
- 5Northern Regional Office, Beijing Jianghe Huiyuan Technology Co., Ltd., Beijing, China
As one of the typical sand-fixation and soil and water conservation shrub species in the desert steppe, the effects of simulated nibbling intensity on the growth and physiological characteristics of Caragana tibetica are unclear. To solve the problem, in this paper, Caragana tibetica with complete configuration, good growth status, close location, the same terrain, and basically consistent morphological structure in the test area were selected as the research objects. The artificial pruning method was adopted to simulate different intensities of animal nibbling. The field in-situ sampling and indoor index determination methods were used to determine its growth and physiological characteristics, so as to clarify the growth and physiological response characteristics of Caragana tibetica to simulated nibbling. The results showed that: (1) The higher the nibbling intensity, the greater the number of new branches and the length of new branches. (2) Different simulated nibbling intensities had significant effects on the physiological characteristics of Caragana tibetica, whose effects are through antioxidant enzymes, osmoregulatory substances, and malondialdehyde. (3) During the whole growing season of Caragana tibetica, the value of physiological characteristics increased first and then decreased, reaching the maximum value in July. (4) The growth characteristics and physiological characteristics of Caragana tibetica have different degrees of correlation. This paper clarifies the impact of simulated nibbling intensity on the growth and physiological characteristics of Caragana tibetica, which can provide an important theoretical basis and practical significance for promoting the sustainable development and utilization of the desert steppe.
1 Introduction
As an important part of China’s ecosystem, the desert steppe plays a crucial role in the construction of a green ecological security barrier in the north of China, the maintenance of terrestrial ecosystem functions, and sustainable development. Various shrub, semi-shrub, and small shrub communities in the desert steppe are not only precious biological resources and natural feed bases for animal husbandry, but also the core function of the desert ecosystem, in which Caragana plants play an important role (Ma et al., 2008). Caragana tibetica, which has relatively strong drought resistance, is one of the typical sand-fixation and soil and water conservation shrub species in the desert steppe (Liu et al., 2024a). In vegetation zoning, the Caragana tibetica community is often regarded as an indicator species marking the transition from grassland to desert. However, it was found that, unlike the good condition of Caragana tibetica in the free range, the health status of the ones in the enclosed sample area decreased obviously, where the plants grew slowly and some died. Although enclosure is an effective measure to restore degraded grassland vegetation, the enclosure time should not be too long to avoid inhibiting plant regeneration and seedling formation, whose appropriate time and method should be determined according to the type of grassland and the degree of degradation. Therefore, it is necessary to find out how livestock nibbling or human disturbance affects the growth and physiology of Caragana tibetica in free grazing land, to determine the appropriate time and method for the enclosure of Caragana tibetica.
Only a few existing studies focus on Caragana tibetica. Liu et al. (2024a) examined eight Caragana tibetica communities using point pattern analysis to assess the spatial distribution pattern (SDP) and the influencing factors of Caragana tibetica scrubs. Han et al. (2024) investigated the relationship between soil physical & chemical properties and the diversity of bacteria and fungi throughout the evolutionary stages of the scrub root systems. Liu et al. (2024b) employed Illumina paired-end sequencing to investigate the genetic organization and structure of Caragana tibetica and Caragana turkestanica. He et al. (2024) revealed that the grazing process induces significant changes in the metabolic pathways of Caragana tibetica branches by analyzing 48 metabolomic profiles from Caragana tibetica. Song et al. (2015) performed HPLC-based activity profiling for targeted identification of anti-HCC activity from CT-EtOAc by MS-directed purification method. Zhang et al. (2011) studied whether fertile islands were present inside and underneath Caragana tibetica-formed nebkhas in the northwest portion of the Ordos on the Inner Mongolia Plateau, China, and if such a fertile island effect increased with the age of the nebkhas. Xiang et al. (2005) studied antioxidant constituents of Caragana tibetica and found that the 70% acetone extract of the stems of Caragana tibetica has a potent superoxide anion scavenging activity.
There are relatively many studies on Caragana species, where most scholars devote themselves to studying the physiological characteristics of Caragana species in terms of photosynthesis and water use efficiency. Yan et al. (2025) studied how high temperature, salinity, and CO2 affect endogenous phytohormones, photosynthesis, and redox homeostasis in Caragana korshinskii Kom leaves, as well as comprehensively evaluating the plant’s physiological response to multiple environmental stressors. Lu et al. (2024) calculated the instantaneous water use efficiency and short-term water use efficiency of these plantations to investigate the impact factors. Yan et al. (2023) evaluated the physiological responses of Korshinsk peashrub (Caragana korshinskii Kom.) to water deficit, photosynthetic gas exchange, chlorophyll fluorescence, and the levels of superoxide anion. Gao et al. (2023a) clarified the response of photosynthesis physiology of Caragana intermedia to soil water content and to determine the relevant threshold ranges in the sandy lands of Qinghai-Tibet Plateau. Zhao et al. (2021) studied water-use strategies and physiological characteristics of Caragana korshinskii and Salix psammophila in a semiarid revegetated ecosystem. Yang et al. (2020) studied photosynthesis and water potential of Caragana intermedia, and its adaptation to environmental changes while growing. Zheng et al. (2017) selected three Caragana species to investigate the cell water relations for these regional-level distribution characteristics. Bao et al. (2015) analyzed age-related changes in photosynthesis and water relations of revegetated Caragana korshinskii in the Tengger Desert, Northern China. Guo et al. (2014) compared the photosynthesis and antioxidative protection in Sophora moorcroftiana and Caragana maximovicziana under water stress. Yan et al. (2012) studied photosynthesis, PSII efficiency, contents of ions, and free amino acids in leaves of Caragana korshinskii Kom exposed to three levels of salinity. Ma et al, 2004, Ma et al, 2003) studied photosynthesis, transpiration, and water use efficiency of Caragana microphylla, Caragana intermedia, and Caragana korshinskii.
Some scholars also studied other physiological characteristics of Caragana species, including stress resistance, adaptability to water, nitrogen, and phosphorus, carbon sequestration ability, physiological adaptability, and the resulting species pattern distribution. Ma et al. (2023) evaluated the stress resistance ability by the roots exposure stress model test and compared the physiological growth indexes in Hedysarum scoparium and Caragana korshinskii. Roy et al. (2021) searched for suitable combinations of water, nitrogen, and phosphorus to improve physiological and biochemical adaptations of Caragana korshinskii to coal-mined spoils. Gong et al. (2016) used five experimental sites to evaluate the Caragana korshinskii’s functional and physiological features, particularly its carbon fixation capacity, as well as the relationships among these features. Ma et al. (2016) studied drought responses of three closely related Caragana species and the implications for their vicarious distribution. Xie et al. (2014) studied the relationships between the leaf ecological and physiological traits of Caragana species and the aridity index and solar radiation, to examine the mechanisms responsible for the Caragana species distribution pattern. Ma et al. (2012) proposed correlation analyses on the relationship between physiological and morphological traits and climatic factors (precipitation and solar radiation) of three Caragana species.
In summary, remarkable progress has been made in the study of the growth and physiological characteristics of Caragana species, which can provide important references for this study. However, the effects of different nibbling intensities on the growth and physiological characteristics of Caragana tibetica are still unclear. Therefore, in this study, Caragana tibetica, the main protective plant in the transition zone between steppe and desert, is taken as the research object. Comparative experiments are carried out to simulate different nibbling intensities, growth characteristics of new shoot number and new shoot length of Caragana tibetica after nibbling are analyzed, and the physiological response characteristics (including antioxidant enzyme activity, osmotic regulator, etc.) of Caragana tibetica are clarified. The relationship between physiological characteristics and growth indicators is also analyzed, which can provide an important theoretical basis and practical guiding significance for promoting the sustainable development and utilization of the desert steppe.
2 Materials and methods
2.1 Study area
The study area is located in the middle of the Darhan Muminggan United Banner, Baotou, Inner Mongolia, near the National Highway G210. The geographical location is 110°5’43” -110°6’ 42” east longitude and 42°1’41” -42°1’ 7” north latitude. The study area is deep in the hinterland, which is located in the middle temperate zone. It is a typical temperate semi-arid continental climate, whose daily temperature difference is large, and the average annual temperature is about 6.2°C. The maximum length from east to west is about 1.2 km, the maximum width from north to south is about 1.3 km, and the average altitude is 1376 m. The average annual precipitation in the study area is about 170 mm, and the average annual sunshine duration is 3105.1h. The main wind direction is southeast, and the average annual wind speed is 4.0m/s. Strong winds appear each year, while sandstorms, blowing sand, and hurricanes sometimes appear.
2.2 Experimental design and sample collection
The experimental samples were collected in mid-April 2023. Caragana tibetica with complete configuration, good growth conditions, same terrain, and consistent morphological structure was selected as the research object in the study area. Since the sites were all within a 200 m range, the geographical characteristics and meteorological factors were consistent. Based on previous investigations and research over the years, the study sites had similar vegetation and soil types and altitudes. The artificial pruning method was adopted to simulate animal nibbling of different intensities. According to the volume percentage, the aboveground parts of the Caragana tibetica were pruned by 25%, 50%, and 75%, respectively. The unpruned shrubbery was used as CK (i.e., the control group). 5 replicates were set in each group, with a total of 20 plants. The pruning intensity of each plant was marked and numbered with PVC (i.e., Poly Vinyl Chloride) pipe. The number and length of new branches after pruning were observed and recorded at the end of the growing season (September of the same year). Healthy Caragana tibetica leaves were collected in May, June, July, August, and September, respectively to determine plant physiological characteristics including POD, SOD, Catalase (CAT), PRO, SS, Soluble Protein (SP), and Malondialdehyde (MDA). The samples collected in each test were immediately stored in dry ice and transported to the laboratory.
2.3 Growth characteristics measurement
New branch number (NBN) and new branch length (NBL) are selected as growth characteristics of Caragana tibetica.
2.3.1 NBN
To measure NBN, at the end of the growing season (September), the number of new branches per plant of CK, 25% treatment group, 50% treatment group and 75% treatment group were recorded respectively, and the average number of new branches for 5 Caragana tibetica plants in each treatment group was treated as NBN in this group.
2.3.2 NBL
As for NBL, at the end of the growing season (September), the length of each new branch in the CK, the 25% treatment group, the 50% treatment group, and the 75% treatment group was measured by a vernier caliper (accuracy 0.01mm). The average length of all new branches in each plant was taken as the length of the new branch, and the average length of a new branch of 5 Caragana tibetica plants in each treatment group was taken as NBL in this group.
2.4 Physiological characteristics determination
2.4.1 POD
POD activity was determined using the Guaiacol colorimetry method (Yoshida et al., 2003). Extraction of plant samples was combined with H2O2 and PBA solutions, and absorbance at 470 nm was measured using a spectrophotometer to assess POD activity. Quantitative determination of POD is achieved by comparing the absorbance value to the standard curve of a standard sample.
2.4.2 SOD
SOD activity was determined by Nitrogen blue tetrazole photochemical reduction method (Gao et al., 2023b). The sample extract was reacted with NBT solution, xanthine oxidase solution, NaH2PO4 solution, and Na2AsO2 solution under light conditions. When the reaction stops, the absorbance at 560 nm was measured using a spectrophotometer and quantified by calculating the SOD activity from a standard curve.
2.4.3 CAT
CAT activity was determined by the Active ultraviolet absorption method (Mhamdi et al., 2010). The Caragana tibetica sample was diluted to a suspension, and the reaction mixture was prepared by mixing the diluted sample extract, phosphate buffer, KCoCl2, and H2O2 in a test tube. Start the CAT to catalyze H2O2, and observe the formation of bubbles after the reaction stops. A spectrophotometer was used to measure absorbance at wavelengths between 240 and 290 nm to assess CAT activity. The absorbance data is compared with the standard curve for quantitative determination.
2.4.4 PRO
PRO content was determined by the Sulfosalicylic acid extraction method (Ábrahám et al., 2010). After removing the precipitate by centrifugation, PRO was precipitated by trichloroacetic acid and redissolved. Using the reaction of diethylamine reagent at 60°C, the PRO content was calculated according to the standard curve.
2.4.5 SS
SS content was determined by the Anthrone colorimetric method (Somani et al., 1987). Caragana tibetica samples were ground into powder and placed in a 50 mL centrifuge tube first. Then, adding an appropriate amount of distilled water, a boiling water bath is used to extract SS. After the insoluble matter was removed by centrifugation, the supernatant was reacted with anthrone reagent, and the color was developed in a boiling water bath. After cooling, the absorbance was finally measured at 620 nm using a spectrophotometer, and the SS content in the test sample was calculated according to the standard curve.
2.4.6 SP
SP content was determined using the Coomassie brilliant blue method (Deans et al., 2018). First of all, Caragana tibetica samples were chopped and placed in a 50 mL centrifuge tube. Subsequently, 10 mL of Tris-HCl buffer was added, and the sample was dispersed using an ultrasonic instrument. After the cell fragments were removed by centrifugation, different concentrations of BSA standard and Bradford reagent were added to the standard and the test sample tubes, and the reaction time was 25 min. Finally, the absorbance was measured at 595 nm using a spectrophotometer, and the protein concentration in the test sample was calculated based on the standard curve.
2.4.7 MDA
MDA content was determined by the Thiobarbituric acid method (Davey et al., 2005). Caragana tibetica samples were heated in a phosphoric acid buffer, and the absorbance at 532 nm was measured using a spectrophotometer. MDA content is calculated according to standard curves.
2.5 Data processing and analysis
Differences between the groups for the given parameters were evaluated using Duncan’s significance difference test, with significance set at p<0.05. The correlation analysis between the growth characteristics and physiological characteristics of Caragana tibetica after simulated eating was carried out with the pheatmap package in R 4.3.2 software, to determine the key factors affecting the growth characteristics and physiological characteristics.
3 Results
3.1 Changes in the NBN and NBL of Caragana tibetica under different simulated nibbling intensities
As shown in Table 1, NBN and NBL under different simulated nibbling intensities show significant differences (p<0.001). With the increase of nibbling intensity, both NBN and NBL showed a significant increasing trend, and both reached the maximum value at 75% nibbling intensity. It can be calculated that NBN and NBL under three kinds of nibbling intensity were higher than those in the control group (i.e. CK), where the ones at 75% nibbling intensity were 11.00 times and 7.86 times of CK, the ones at 50% nibbling intensity were 5.00 times and 3.61 times of CK, the ones at 25% nibbling intensity were 2.00 times and 1.81 times of CK. There were no significant differences in the NBN and NBL at CK and 25% nibbling intensity (p>0.05), while there were significant differences at 50% and 75% nibbling intensity (p<0.05), which proves that nibbling can promote the growth of new branches of Caragana tibetica.
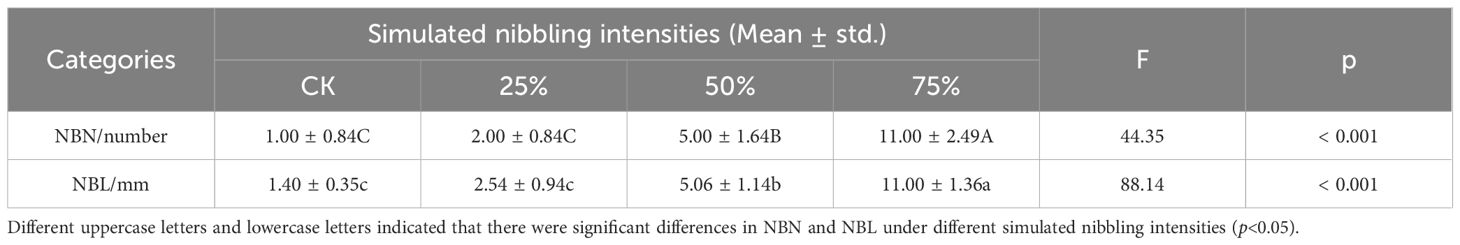
Table 1. Changes in the NBN and NBL of Caragana tibetica under different simulated nibbling intensities.
3.2 Changes of antioxidant enzyme activity in Caragana tibetica under different simulated nibbling intensities
The activity of the three antioxidant enzymes (i.e. POD, SOD, and CAT) was highest in July in the growing season (Table 2). POD content in Caragana tibetica leaves at CK, 25%, 50% and 75% nibbling intensity in July was 2.79 u•(g•min)-1, 2.91 u•(g•min)-1, 3.22 u•(g•min)-1 and 3.34 u•(g•min)-1, respectively. Compared with CK, 50% and 75% simulated nibbling intensity significantly increased by 15.41% and 19.71% (p<0.05). POD content was the lowest in May, when 75% nibbling intensity significantly increased by 32%, 8.55%, and 13.79% compared with CK, 25%, and 50% (p<0.05), respectively. It is clear that SOD content in July showed an increasing trend with the increase of nibbling intensity, when 25%, 50% and 75% nibbling intensity significantly increased by 14.39%, 21.43% and 35.27% compared with CK, respectively (p<0.05). However, there was no significant difference among the four treatment groups (CK, 25%, 50%, and 75%) in September (p>0.05). During the whole growing season, the CAT content of Caragana tibetica showed a trend of first increasing and then decreasing. There was no significant difference in CAT content between different treatment groups from May to August (p>0.05), while there was a significant difference between 50% and 75% nibbling intensity and CK in September (p<0.05).
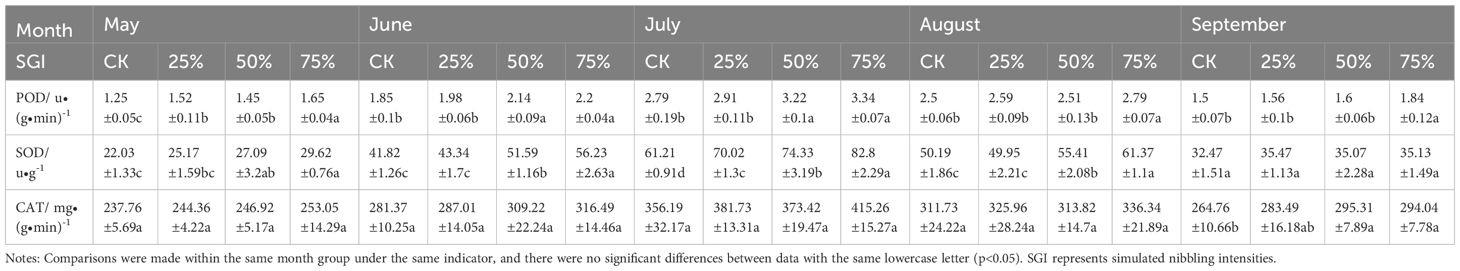
Table 2. Changes of three types of antioxidant enzymes content in Caragana tibetica leaves under different simulated nibbling intensities.
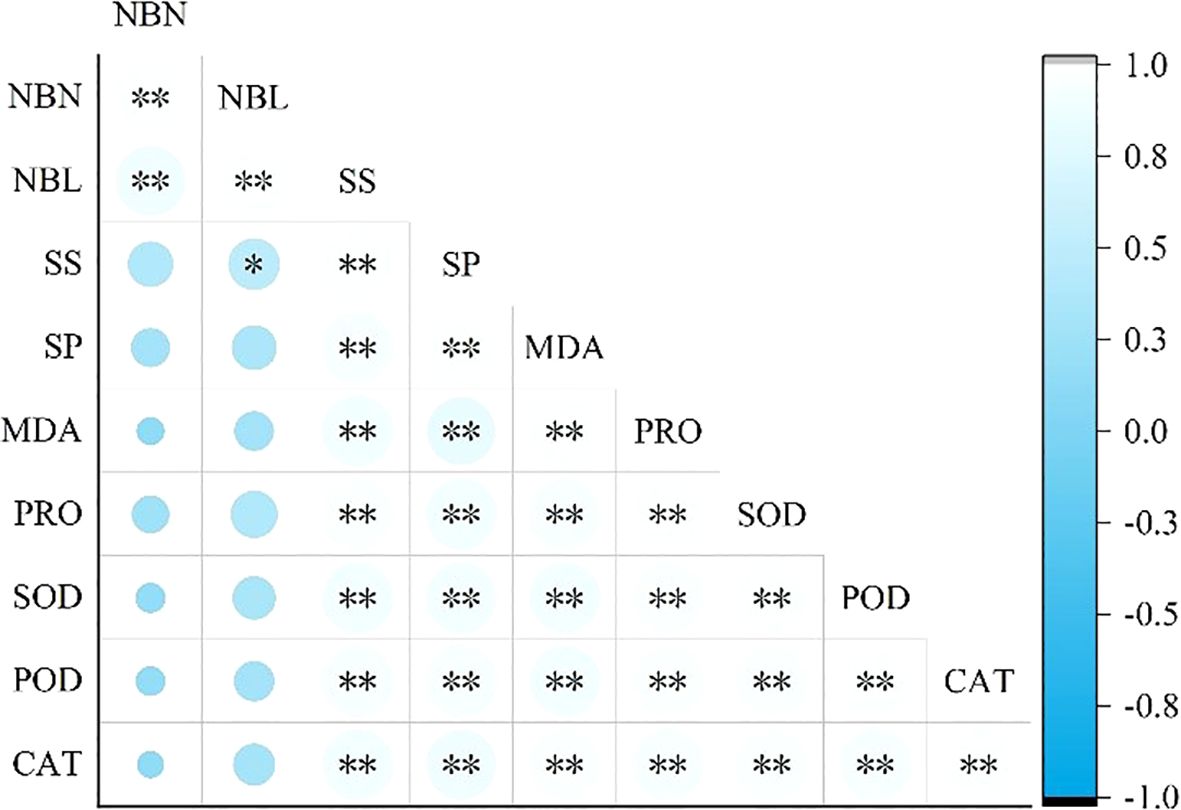
Figure 1. Correlation between growth and physiological characteristics of Caragana tibetica nibbling. * represents p<0.05; ** represents p<0.01.
3.3 Changes of osmoregulatory substances in Caragana tibetica under different simulated nibbling intensities
During the growing season from May to September, different simulated nibbling intensities had significant effects on the PRO content of Caragana tibetica. As shown in Table 3, the 75% nibbling intensity significantly increased by 22.93% compared with the 50% nibbling intensity in May (p<0.05). In June and August, PRO content showed a significant increasing trend with the increase of nibbling intensity, while in July, 50% and 75% nibbling intensity were significantly greater than CK and 25%. Among May to September, the PRO content of all treatment groups in July was higher than that of other groups, the highest value was 180.82 μg•g-1 at 75% nibbling intensity, and the lowest value was 119.72 μg•g-1 for CK. From the table, it can be seen that SS and SP content increased first and then decreased during the whole growing season, reaching the maximum value in July. SS content was between 3.34 mg•g-1 and 4.61 mg•g-1 in July, and was significantly increased by 17.37% and 27.55% compared with CK in 50% and 75% treatment groups, respectively (p<0.05). In May, 75% nibbling intensity was larger than in other groups, a significant increase of 16.2% compared with CK. SP content in July and August was higher than in other treatment groups. From June to August, SP content increased with the increase in nibbling intensity. In August, there was no significant difference in SP content among 25%, 50% and 75% treatment groups (p>0.05). In summary, PRO, SS, and SP contents reflect the physiological response and adaptability of Caragana tibetica in the growing season.
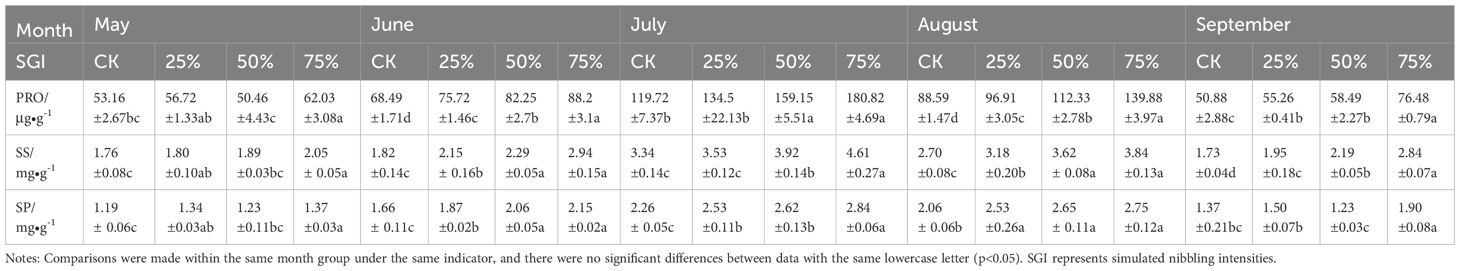
Table 3. Changes of three osmoregulatory substances content in Caragana tibetica leaves under different simulated nibbling intensities.
3.4 Changes of MDA in Caragana tibetica under different simulated nibbling intensities
MDA content reflects the physiological state and adaptive ability of Caragana tibetica. As shown in Table 4, under different nibbling intensities, the MDA content of Caragana tibetica increased first and then decreased from May to September, reaching the maximum value in July. The values were 3.34 μmol•g-1、3.53 μmol•g-1、3.92 μmol•g-1, and 4.61μmol•g-1 at CK, 25%, 50%, and 75% nibbling intensity, respectively, while MDA in 50% and 75% treatment groups was significantly higher than that in CK and 25% (p<0.05). MDA content was the lowest in May, at CK, 25%, 50%, and 75%, the values were 1.76 μmol•g-1, 1.80 μmol•g-1, 1.89 μmol•g-1, and 2.05 μmol•g-1, respectively, while 75% nibbling intensity was significantly higher than that of the other three groups (p<0.05). Besides, there was no significant difference between CK and 25% in May, June, August, and September, while the nibbling intensity of 25% in July was significantly higher than that of CK. In summary, the MDA content increased with the increase of nibbling intensity in each month of the whole growing season, indicating that the greater the nibbling intensity, the greater the effect on the MDA content of Caragana tibetica.

Table 4. Changes of MDA content in Caragana tibetica leaves under different simulated nibbling intensities.
3.5 Correlation analysis of growth and physiological characteristics of Caragana tibetica after simulated nibbling
As shown in Figure 1, NBN and NBL were positively correlated with SP, MDA, PRO, SOD, POD and CAT, but not significantly. There was a significant positive correlation between NBL and SS (p<0.05). SS, SP, MDA, PRO, SOD, POD and CAT were significantly positively correlated with each other (p<0.01).
4 Discussion
4.1 Growth characteristics of Caragana tibetica under different nibbling intensities
From the simulated nibbling experiment, it is clear that the nibbling treatment had an important effect on the growth performance of Caragana tibetica. After nibbling treatment, NBN and NBL of Caragana tibetica were significantly higher than those of the control group. Under different nibbling intensities, NBN and NBL showed the same trend, i.e., 75% nibbling>50% nibbling>25% nibbling>CK. This result may be due to compensatory growth mechanisms of Caragana tibetica. When the branches and leaves of Caragana tibetica are damaged, they may reduce the normal leaf fall or increase the photosynthetic rate of the Caragana tibetica to compensate for the nibbling loss. This compensatory growth mechanism helps Caragana tibetica recover quickly after injury and may even grow better than if it were undamaged. These results are consistent with the description of compensatory growth mechanisms and their ecological significance after nibbling or injury in Fornoni (2011).
4.2 Physiological characteristics of Caragana tibetica under different nibbling intensities
From the experiment, it can be found that the status of the active oxygen metabolism system in Caragana tibetica changed under different nibbling intensities. This change causes the protective enzyme system in the Caragana tibetica to be activated and participate in the clearance process of reactive oxygen species. The activity of antioxidant enzymes such as POD, SOD, and CAT in Caragana tibetica changes with the nibbling intensity. The results showed that during the whole growing season from May to September, the main protective enzymes such as POD, SOD, and CAT showed similar changes under different nibbling intensity. This tendency is usually manifested as an initial increase in growth (May to July) followed by a gradual decrease (July to September). This is consistent with the results of Zhu et al. (2011). Considering that large amounts of antioxidant enzymes are usually synthesized to protect cells from oxidative damage, maintaining the antioxidant enzyme activity in Caragana tibetica at a high level can help maintain its normal growth and development.
In addition, the results showed that osmoregulatory substances play an important role in the Caragana tibetica stress response. As an important amino acid, PRO can play functions such as anti-oxidation, anti-stress, and regulation of growth and development in Caragana tibetica. It was found that under different nibbling treatments, Caragana tibetica would synthesize more PRO to cope with external pressure with the increase of months. In the same month, the PRO content produced by Caragana tibetica under 75% nibbling treatment was significantly higher than CK, while there was no significant difference from 50% nibbling treatment in some months. Furthermore, this paper found that SS and SP show similar changes to PRO, which indicates that under nibbling treatment, SS and SP can also regulate osmotic regulation and cell protection to maintain cell stability. It was found that the contents of PRO, SS, and SP in Caragana tibetica were significantly affected by different nibbling intensities. Specifically, from May to September, the contents of three types of osmoregulatory substances increased first and then decreased, and reached their highest in July of the 75% nibbling treatment. The results indicate that 75% nibbling treatment may promote the ability of Caragana tibetica to adapt to external stress, while 50% and 25% nibbling treatment have a higher effect on membrane lipid permeability. The accumulation of PRO and stress response, the physiological function of SS and the role of SP in this paper are consistent with the conclusions of Lehmann et al. (2010).
As for MDA, its content level can reflect the degree of stress injury and the degree of membrane lipid peroxidation. In this paper, the results showed that the maximum MDA content under different nibbling intensity treatments was 1.19 times, 1.33 times, and 1.36 times that of the control treatment, respectively, which reflects that 75% nibbling intensity had the greatest effect on the biofilm lipid permeability of Caragana tibetica. With the progress of the growing season (from May to September), MDA content increased first and then decreased, which also reached its maximum value in July. In September, there were significant differences between 50% & 75% nibbling treatments and CK, while the difference between 75% nibbling treatments and other nibbling treatments is also remarkable. It showed that higher nibbling intensity may lead to greater stress in Caragana tibetica, thus intensifying oxidation and lipid peroxidation in leaves. In addition, nibbling pressure can lead to the intensification of membrane lipid peroxidation, which is manifested by the increase of MDA content. This reflects the degree of damage plant cell membrane, suggesting a change in cell membrane permeability and the impairment of physiological function in the face of nibbling pressure. The research results in this paper are consistent with the significance of MDA as a marker of membrane lipid peroxidation proposed by Davey et al (2005) and its change rule in plant stress response.
5 Conclusion
NBN and NBL under three different nibbling intensities were greater than those in CK. NBN and NBL increased with higher nibbling intensity, which indicates that nibbling can promote the growth of new branches of Caragana tibetica. Different simulated nibbling intensities had effects on the antioxidant enzyme activity of Caragana tibetica. The contents of POD, SOD and CAT reached highest in July during the growth season from May to September. Different simulated nibbling intensities had significant effects on the PRO content of Caragana tibetica. During the whole growing season, the contents of SS and SP increased first and then decreased, reaching the maximum value in July. MDA content increased with the nibbling intensity rise of the whole growing season, indicating that the greater the nibbling intensity, the greater the effect on the MDA content of Caragana tibetica. In future work, we need to further employ metabolomics techniques to reveal the effects of simulated nibbling on aspects of Caragana tibetica gene regulation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YL: Validation, Resources, Methodology, Writing – original draft. MH: Writing – original draft, Methodology. RJ: Resources, Writing – original draft, Investigation. QT: Validation, Writing – review & editing, Supervision, Writing – original draft, Software. JC: Writing – review & editing. ML: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Inner Mongolia Academy of Forestry Sciences Open Research Project, Hohhot 010010, China, Project NO. KF2024ZD05. National Natural Science Foundation of China (Grant NO. 52402383). The High-level/excellent Doctor Introduction Project of Inner Mongolia Agricultural University (Grant NO. NDYB2023-53).
Conflict of interest
Authors JC and ML were employed by the company Beijing Jianghe Huiyuan Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ábrahám, E., Hourton-Cabassa, C., Erdei, L., and Szabados, L. (2010). “Methods for determination of proline in plants,” in Plant stress tolerance: methods and protocols (Humana P, Totowa, NJ), (317–331).
Bao, J. T., Wang, J., Li, X. R., Zhang, Z. S., and Su, J. Q. (2015). Age-related changes in photosynthesis and water relations of revegetated Caragana korshinskii in the Tengger desert, Northern China. Trees. 29, 1749–1760. doi: 10.1007/s00468-015-1255-7
Davey, M. W., Stals, E., Panis, B., Keulemans, J., and Swennen, R. L. (2005). High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 347, 201–207. doi: 10.1016/j.ab.2005.09.041
Deans, C. A., Sword, G. A., Lenhart, P. A., Burkness, E., Hutchison, W. D., and Behmer, S. T. (2018). Quantifying plant soluble protein and digestible carbohydrate content, using corn (Zea mays) as an exemplar. J. Vis. Exp. 138, e58164. doi: 10.3791/58164
Fornoni, J. (2011). Ecological and evolutionary implications of plant tolerance to herbivory. Funct. Ecol. 25, 399–407. doi: 10.1111/j.1365-2435.2010.01805.x
Gao, Y., Jia, Z. Q., Wu, R. A., He, L. X. Z., Liu, T., Li, Q. X., et al. (2023a). Difference in response of Caragana intermedia photosynthesis to soil water content in different afforestation years and related threshold effects in alpine sandy lands. Forests. 14, 701. doi: 10.3390/f14040701
Gao, X. Q., Wang, X. Y., Jiao, W., Li, N., Wang, J., Zheng, L. Y., et al. (2023b). Physiological and transcriptomic analysis of Catalpa bungei seedlings in response to saline-alkali stress. For. Res. 36, 166–178. doi: 10.12403/j.1001-1498.20220180
Gong, C. M., Bai, J., Wang, J. H., Zhou, Y. L., Kang, T., Wang, J. J., et al. (2016). Carbon storage patterns of Caragana korshinskii in areas of reduced environmental moisture on the Loess Plateau, China. Sci. Rep. 6, 28883. doi: 10.1038/srep28883
Guo, Q. Q., Zhang, W. H., and Li, H. E. (2014). Comparison of photosynthesis and antioxidative protection in Sophora moorcroftiana and Caragana maximovicziana under water stress. J. Arid Land. 6, 637–645. doi: 10.1007/s40333-013-0212-9
Han, M., Liang, Y. M., Gao, Y., Yang, W. Y., and Guo, Y. F. (2024). Relationship between root system-soil C:N:P and soil microbial diversity at different evolutionary stages of Caragana tibetica scrub in arid desert grassland, Northern China. Front. Plant Sci. 15, 1423536. doi: 10.3389/fpls.2024.1423536
He, M. H., Han, Y. L., Gao, Y., Han, M., and Duan, L. Q. (2024). Decoding the metabolomic responses of Caragana tibetica to livestock grazing in fragile ecosystems. Front. Plant Sci. 15, 1339424. doi: 10.3389/fpls.2024.1339424
Lehmann, S., Funck, D., Szabados, L., and Rentsch, D. Proline metabolism and transport in plant development. Amino Acids (2010) 39, 949–962.
Liu, Y., Dong, L., Wang, J., Li, J. R., Yi, L. Q., Li, H. M., et al. (2024a). Spatial heterogeneity affects the spatial distribution patterns of Caragana tibetica scrubs. Forests. 15, 2072. doi: 10.3390/f15122072
Liu, L., Li, H. Y., Li, J. X., Li, X. J., Hu, N., Sun, J., et al. (2024b). Chloroplast genomes of Caragana tibetica and Caragana turkestanica: structures and comparative analysis. BMC Plant Biol. 24, 254. doi: 10.1186/s12870-024-04979-9
Lu, C., Cao, J. R., Guo, S. S., Yao, C., Wang, Z. X., Tong, X. G., et al. (2024). Water use efficiency and its influencing factors following plantations restoration with Caragana korshinskii and Robinia pseudoacacia in the loess hilly region. Ecol. Eng. 202, 107229. doi: 10.1016/j.ecoleng.2024.107229
Ma, C. C., Gao, Y. B., Guo, H. Y., and Wang, J. L. (2004). Photosynthesis, transpiration, and water use efficiency of Caragana microphylla, C. intermedia and C. korshinskii. Photosynthetica. 42, 65–70. doi: 10.1023/B:PHOT.0000040571.63254.c2
Ma, C. C., Gao, Y. B., Guo, H. F., Wang, J. L., and Guo, H. Y. (2003). Interspecific transition among Caragana microphylla, C. davazamcii and C. korshinskii along geographic gradient. II. Characteristics of photosynthesis and water metabolism. Acta Bot. Sin. 45, 1228–1237.
Ma, C. C., Gao, Y. B., Guo, H. Y., Wang, J. L., Wu, J. B., and Xu, J. S. (2008). Physiological adaptations of four dominant Caragana species in the desert region of the Inner Mongolia Plateau. J. Arid Environ. 72, 247–254. doi: 10.1016/j.jaridenv.2007.05.009
Ma, C. C., Guo, H. Y., Li, Q. F., Wang, J. L., Wu, J. B., and Gao, Y. ,. B. (2012). Geographical variation of physiological and morphological traits among populations of three Caragana species in Inner Mongolia Steppe. J. Food Agric. Environ. 10, 1233–1239.
Ma, F., Na, X. F., and Xu, T. T. (2016). Drought responses of three closely related Caragana species: Implication for their vicarious distribution. Ecol. Evol. 6, 2763–2773. doi: 10.1002/ece3.2044
Ma, J. L., Wang, H., Jin, L., and Zhang, P. (2023). Comparative analysis of physiological responses to environmental stress in Hedysarum scoparium and Caragana korshinskii seedlings due to roots exposure. PeerJ. 11, e14905. doi: 10.7717/peerj.14905
Mhamdi, A., Queval, G., Chaouch, S., Vanderauwera, S., Van Breusegem, F., and Noctor, G. (2010). Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 61, 4197–4220. doi: 10.1093/jxb/erq282
Roy, R., Mostofa, M. G., Wang, J. X., Fornara, D., Sarker, T., and Zhang, R. Q. (2021). Revegetation intervention of drought-prone coal-mined spoils using Caragana korshinskii under variable water and nitrogen-phosphorus resources. Agric. Water Manage. 246, 106712. doi: 10.1016/j.agwat.2020.106712
Somani, B. L., Khanade, J., and Sinha, R. (1987). A modified anthrone-sulfuric acid method for the determination of fructose in the presence of certain proteins. Anal. Biochem. 167, 327–330. doi: 10.1016/0003-2697(87)90172-2
Song, P., Wang, Q., Lv, J., Xu, C., Lin, Q. X., Ma, X. H., et al. (2015). HPLC-based activity profiling of anti-hepatocellular carcinoma constituents from the Tibetan medicine, Caragana tibetica. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 35, 450–455. doi: 10.1007/s11596-015-1452-z
Xiang, T., Uno, T., Ogino, F., Ai, C. Q., Duo, J., and Sankawa, U. (2005). Antioxidant constituents of Caragana tibetica. Chem. Pharm. Bull. 53, 1204–1206. doi: 10.1248/cpb.53.1204
Xie, L. N., Ma, C. C., Guo, H. Y., Li, Q. F., and Gao, Y. B. (2014). Distribution pattern of Caragana species under the influence of climate gradient in the Inner Mongolia region, China. J. Arid Land. 6, 311–323. doi: 10.1007/s40333-013-0227-2
Yan, H., Fu, K., Liu, X., Dai, Z. G., and Ru, C. (2025). Elevated CO2 alleviates negative impacts of high temperature and salinity on phytohormones, photosynthesis, and redox reactions in leaves of Caragana korshinskii Kom. Plant Physiol. Biochem. 220, 109475. doi: 10.1016/j.plaphy.2025.109475
Yan, H., Hu, X. T., and Li, F. S. (2012). Leaf photosynthesis, chlorophyll fluorescence, ion content and free amino acids in Caragana korshinskii Kom exposed to NaCl stress. Acta Physiol. Plant 34, 2285–2295. doi: 10.1007/s11738-012-1029-4
Yan, H., Liu, X. L., Ding, H., Dai, Z. G., Niu, X. L., and Zhao, L. (2023). Hormonal balance, photosynthesis, and redox reactions in the leaves of Caragana korshinskii Kom. under water deficit. Plants 12, 2076. doi: 10.3390/plants12112076
Yang, K. Y., Jia, Z. Q., Li, Q. X., Feng, L. L., He, L. X. Z., Dai, J., et al. (2020). Adaptation of Caragana intermedia to environmental changes while growing: photosynthesis and water potential. Agrociencia. 54, 977–994.
Yoshida, K., Kaothien, P., Matsui, T., Kawaoka, A., and Shinmyo, A. (2003). Molecular biology and application of plant peroxidase genes. Appl. Microbiol. Biotechnol. 60, 665–670. doi: 10.1007/s00253-002-1157-7
Zhang, P. J., Yang, J., Zhao, L. Q., Bao, S., and Song, B. Y. (2011). Effect of Caragana tibetica nebkhas on sand entrapment and fertile islands in steppe-desert ecotones on the Inner Mongolia Plateau, China. Plant Soil. 347, 79–90. doi: 10.1007/s11104-011-0813-z
Zhao, Y., Wang, L., Knighton, J., Evaristo, J., and Wassen, M. (2021). Contrasting adaptive strategies by Caragana korshinskii and Salix psammophila in a semiarid revegetated ecosystem. Agric. For. Meteorol. 300, 108323. doi: 10.1016/j.agrformet.2021.108323
Zheng, Z. R., Feng, C. Y., Zhao, N. X., Gao, Y. B., and Lv, S. H. (2017). Evidence of cell water relations for geographically substituted distribution of three Caragana species on the Mongolian plateau. J. Environ. Prot. Ecol. 18, 561–570.
Keywords: Caragana tibetica, simulated gnawing, physiological characteristics, windbreak and sand fixation, desert grassland
Citation: Liang Y, He M, Jia R, Tian Q, Chen J and Li M (2025) Effects of simulated nibbling intensity on growth and physiological characteristics of Caragana tibetica. Front. Plant Sci. 16:1625476. doi: 10.3389/fpls.2025.1625476
Received: 09 May 2025; Accepted: 22 July 2025;
Published: 23 September 2025.
Edited by:
Stephan Pollmann, National Institute of Agricultural and Food Research and Technology, SpainReviewed by:
Guo Dong Tang, China Institute of Water Resources and Hydropower Research, ChinaNebi Bilir, Isparta University of Applied Sciences, Türkiye
Copyright © 2025 Liang, He, Jia, Tian, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Tian, Y3N0aWFucWlAaW11LmVkdS5jbg==
 Yumei Liang
Yumei Liang Minghui He3
Minghui He3 Qi Tian
Qi Tian