- 1Tianjin Key Laboratory of Animal and Plant Resistance, College of Life Sciences, Tianjin Normal University, Tianjin, China
- 2Tsinghua-Peking Center for Life Sciences, College of Life Sciences, Tsinghua University, Beijing, China
Introduction: Plant growth regulation involves complex biochemical and signaling pathways. Tryptamine (Try), a polyamine derived from tryptophan, has been implicated in plant growth and stress responses, yet its specific regulatory mechanisms have not been fully understood.
Methods: This study investigates the physiological and molecular effects of Try on Lemna turionifera 5511, focusing on its role in growth regulation, photosynthesis, and hormonal balance. Our findings reveal that Try content increases in overgrown duckweed, suggesting its involvement in aging and stress responses. Exogenous Try application at concentrations ranging from 50 to 200 μM resulted in dose-dependent growth inhibition, with 150 μM Try significantly reducing growth rate, leaf area, and chlorophyll content.
Results: The Chlorophyll a (Chla) and Chlorophyll b (Chlb) levels were decreased by 37.5% and 40.43%, respectively. Try treatment also negatively impacted photosynthesis, as evidenced by reduced chlorophyll fluorescence parameters and downregulation of 16 photosynthesis-related genes. Additionally, Try induced oxidative stress, increasing reactive oxygen species (ROS) and peroxidase (POD) activity by 9.17% and 10.11%, respectively. While modulating endogenous hormone levels, particularly increasing abscisic acid (ABA) and decreasing cytokinin (CTK) content by 23.58% and 17.55%. Moreover, transcriptomic analysis revealed an upregulation of auxin (IAA) metabolism-related enzymes by Try addition. Meanwhile, changes in the expression of genes related to the tryptophan metabolism pathways indicate a metabolic change associated with aging.
Discussion: These results highlight the complex role of Try in regulating duckweed growth and stress responses, suggesting its potential as a regulatory molecule in plant development. Further research is needed to elucidate the molecular mechanisms underlying the influence of Try and its applications in agriculture and environmental management.
1 Introduction
Plants synthetize an extensive array of low molecular weight organic compounds, broadly classified as primary metabolites, secondary metabolites, or hormones (Erb and Kliebenstein, 2020), which play diverse roles in plant growth, development, and stress responses. Plant growth itself is a highly complex biological process governed by intricate networks of biochemical and signaling pathways (Olas et al., 2020). As plants reach certain developmental stages, their growth rates typically decrease, yet the inside regulatory mechanisms underlying this phenomenon, including the production and activity of these compounds that are tightly regulated through complex metabolic networks, remain elusive. Polyamines, a class of ubiquitous small aliphatic polycations in eukaryotic organisms (Seifi and Shelp, 2019), are characterized by their polymer-like carbon chain structures containing multiple amino groups (Ćavar Zeljković et al., 2024). Polyamines function as secondary metabolites in plants (Ćavar Zeljković et al., 2024) and play significant roles in various physiological processes, including seed germination, tuber dormancy break, root development, floral initiation and development, senescence delay, organogenesis, and stress responses (Yousefi et al., 2019). Among these polyamines, Try represents a particularly interesting yet understudied compound whose role in plant growth regulation remains poorly understood.
Tryptamines, a class of compounds derived from the essential amino acid tryptophan, are characterized by an indole ring structure consisting of a benzene ring fused to a pyrrole ring, with an amino group attached to a 2-carbon side chain (Zohairi et al., 2023). Try biosynthesis occurs through tryptophan metabolism via the Try biosynthetic pathway. These tryptophan-derived monoamines share structural similarities with 5-hydroxytryptamine (5-HT) (Bhattarai et al., 2018), and serve as crucial biosynthetic precursors for numerous natural alkaloids. Furthermore, tryptamines are frequently employed as fundamental chemical building blocks in the total synthesis of biologically active compounds and pharmaceutically important substances (Simonetti et al., 2021). The enzymatic conversion of Try to 5-hydroxytryptamine, mediated by tryptamine 5-hydroxylase (T5H), represents a critical biochemical pathway. 5-HT, a well-established neuromodulator, plays a pivotal role in the mechanism of various psychoactive drugs. Its complex interactions with other neuromodulators, including norepinephrine, dopamine, and oxytocin, significantly influence human subjective experiences and mental health (Ligneul and Mainen, 2023). Beyond its neurological significance, Try also serves as a key metabolite in tryptophan catabolism. Try plays an indispensable role in numerous physiological processes, particularly in IAA synthesize. However, the dynamics of Try levels during plant growth remain poorly characterized.
Auxin (IAA), a morphogen-like substance, is essential for plant growth and development, regulating processes such as branching, gravitropism, phototropism, and seed development (Benková et al., 2003; Perico et al., 2022). In higher plants, tryptophan-dependent IAA biosynthesis primarily proceeds through four well-characterized pathways: the indole-3-pyruvate (IPyA) pathway (Stepanova et al., 2008), the tryptamine (YUCCA) pathway (Zhao et al., 2001), the indole-3-acetaldoxime (IAOx) pathway (Bartel et al., 2001), and the indole acetamide pathway (Pollmann et al., 2002). While Try plays a crucial role in IAA synthesis under normal physiological conditions, both relevant literature (Wan et al., 2018) and our previous research revealed a paradoxical relationship between Try concentration and plant growth, with 150 μM of exogenous Try identified as the optimal concentration for inhibiting duckweed growth. This finding aligns with studies suggesting that elevated IAA levels may initiate senescence processes (Casanova-Sáez et al., 2021). Try, as one of these plant metabolites, has not been thoroughly investigated in terms of its endogenous levels and physiological effects. Our study focus on differential Try accumulation patterns between senescent and young duckweed, aiming to reveal that the change in Try content may affect the growth of plants.
Duckweeds, described as the “simplest and smallest of flowering plants” (Hillman, 1961), have small stature and morphological simplicity. As one of the fastest-growing higher plants, duckweeds can quickly cover water surfaces (Ziegler et al., 2015). This rapid growth is beneficial for fundamental research as it enables the quick accumulation of large sample sizes. Studies have shown that, depending on the species and culture medium, the population size of duckweed doubles within 1.43 to 4.54 days, thus achieving high biomass yield in a short period of time (Kang et al., 2025). The growth characteristics of duckweed make it an ideal model for studying the regulation of plant growth. Existing studies have shown that the exogenous addition of secondary metabolites such as ABA can have an impact on the growth of duckweed (Utami et al., 2018). However, the relationship between the growth of duckweed and the exogenous application of Try remains unclear. In this study, we employ Lemna turionifera 5511 as a model system to investigate the physiological role of Try. Our research aims to address three specific objectives: (i) to examine the relationship between endogenous Try levels and the reduced growth rate of duckweed; (ii) to evaluate the effects of exogenous Try application on duckweed growth; and (iii) to analyze the impact of exogenous Try on the IAA metabolic pathway. Through these investigations, we aim to elucidate the complex interplay between Try metabolism and plant growth regulation.
2 Materials and methods
2.1 Duckweed culture and try treatment settings
The duckweed used in this study (Lemna turionifera 5511) was originally obtained from the Fengchan river in Tianjin, China. The duckweed was cultivated according to our previous study (Wang et al., 2023). The culture condition was a temperature of 24°C/22°C at day/night, under 16 h light/8 h dark cycle, and with a light intensity of 95 µmol m-2·s-1. The duckweed was treated with 50, 100, 150 and 200 μM Try for 9 days. The treatment with or without 150 μM Try was used for the following experiments.
2.2 Enzyme linked immunosorbent assay
The determination of peroxidase (POD), reactive oxygen species (ROS), abscisic acid (ABA), auxin (IAA), cytokinin (CTK) and tryptamine (Try) activities was performed using double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) kits (Enzyme-linked Biotechnology Co., Shanghai, China). Plant samples from CK and Try groups were ground with 0.1 g of fresh weight in liquid nitrogen and then centrifuged with 1 mL of PBS to create a suspension. The supernatant was diluted 5 times before being used for sampling. By coating microplates with specific antibodies, followed by diluting supernatants, binding with HRP-conjugated secondary antibodies, developing the color reaction with TMB, and finally quantifying absorbance at 450 nm for results. The standard curve is:
POD: y=0.024x-0.0511 ROS: y=0.0125x+0.0835
ABA: y=0.0676x+0.1412 IAA: y=0.0742x+0.1168
CTK: y=0.0635x+0.0757 Try: y=0.343x+0.7281
2.3 Chlorophyll fluorescence parameter monitoring and photosynthetic pigment content determination
The Dual-PAM100 fluorometer from Waltz, Germany, was used to determine the duckweed chlorophyll fluorescence parameters including photosystem II complex (PSII), a photosynthetic unit in the thylakoid membrane, maximum quantum yield of PSII (Fv/Fm), quantum yield of PSII (Y (II)) and nonphotochemical quenching coefficient (qN). The samples were given a 30-min dark treatment prior to the measuring in order to reduce the amount of organic materials in the plant tissue before detection.
A total of 0.1 g of fresh duckweed from CK group and Try group were immersed in 10 mL of 95% alcohol within dark for 24 hours. The absorbance of the extract was measured at wavelengths of 645 nm and 663 nm using a multimode micro-plate reader (TECAN, Spark, Switzerland). Chlorophyll a (Chla), chlorophyll b (Chlb), and total chlorophyll (Chla+b) were calculated as follows:
Chla (mg/g)={(12.7A663-2.69A645)×10mL}/0.1g
Chlb (mg/g)={(22.9A645-4.68A663)×10mL}/0.1g
Chl(a+b) (mg/g)={(8.02A663 + 20.21A645)×10mL}/0.1g
2.4 RNA sequencing and analysis
Following 48 h of Try treatment, the RNA from both the CK and Try groups underwent sequencing and analysis at Novogene in Chaoyang, Beijing, China. Total RNA was collected using the QIAGEN Total RNA prep Pure Plant Kit (Beijing, China). The quality of the RNA was assessed using an Agilent 2100 Bioanalyzer (Santa Clara, CA, USA). Subsequently, the library was constructed and examined using the Illumina NovaSeq 6000 sequencing system (San Diego, CA, USA). Firstly, the integrity of the RNA extracted from the duckweed with two group was analyzed by agarose gel electrophoresis. A NanoPhotometer spectrophotometer was used to detect the purity of RNA. Secondly, total RNA (≥0.25 g) was used to prepare the library, and the effective concentration (≥2 nM) of the library was accurately quantified by qRT-PCR to ensure the quality of the library. Thirdly, clustering and sequencing index-coded samples clustering has been performed according to the manufacturer’s instructions. The library preparations were sequenced on an Illumina HiSeq platform (performed at Novogene Bioinformatics Technology Co., Ltd. Beijing, China). The gene functions were annotated according to Gene Ontology and KEGG databases. The DESeq R package (1.10.1) was used to analyze differential gene expression of “CK” and “Try_150 μM”. The criterion was that genes with adjusted p-value < 0.05 were designated as differently expressed. GO seq and KOBAS software were used to perform Gene Ontology functional enrichment and KEGG pathway enrichment analyzed on differential gene sets.
2.5 Statistical analysis
The data were presented as the mean ± SD triplicate. The area of frond was measured by imageJ. The graphs were prepared in Illustrator 2022 and Photoshop 2023. Microsoft Excel 2021 was used for data processing. GraphPad Prism 9 was used for Figure drawing. Statistical analyses were performed using Statistical Program for Social Science (SPSS). Statistical significance was assessed by independent samples t-test, with significant differences denoted by asterisks. (*: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001, ns: not significant).
3 Results
3.1 Influence of external application of Try on the growth of duckweed
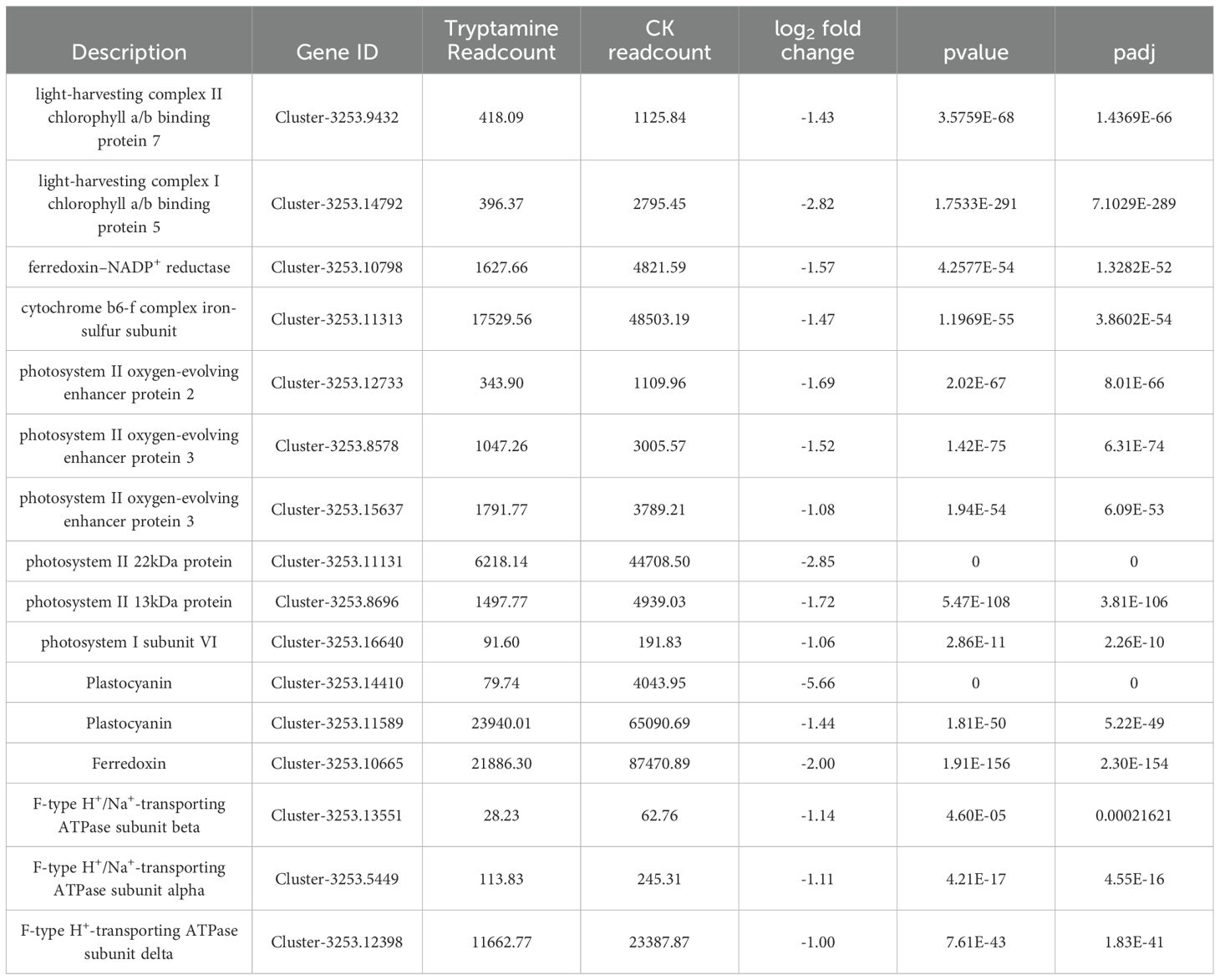
In order to study the effect of external application of Try on duckweed growth, we treated duckweed with different concentrations of Try, calculated its growth status, and then selected an optimal concentration for subsequent experiments. The results showed that the growth rate of duckweed decreased with the increase of exogenous Try (Figure 1A). On the fifth day of Try treatment, the 100 μM, 150 μM and 200 μM Try treatment groups showed significant changes compared with the blank control (CK) group (p < 0.05). The average growth rate of CK group was 6 plants per bottle, that of 50 μM group was 6 plants per bottle, that of 100 μM group was 4.25 plants per bottle, that of 150 μM group was 4.25 plants per bottle and that of 200 μM group was 3.5 plants per bottle. On the ninth day, this significant difference was even more pronounced (p < 0.001). At this time, the average growth of CK group was 16 plants per group, was 15 in 50 μM group (6.25% less than control), was 11.75 in 100 μM group (26.56% less than control), was 9 in 150 μM group (43.75% less than control) and was 8.75 in 200 μM group (45,31% less than control) (Figure 1B). The concentrations of 50 μM, 100 μM, 150 μM, and 200 μM Try were applied to the duckweed. We found that under 50 μM, 100 μM, the growth of duckweed showed no significant change compared to the CK group. And 150 μM Try addition lead to a decreased growth rate of duckweed. The treatment with or without 150 μM Try was used for the following experiments.
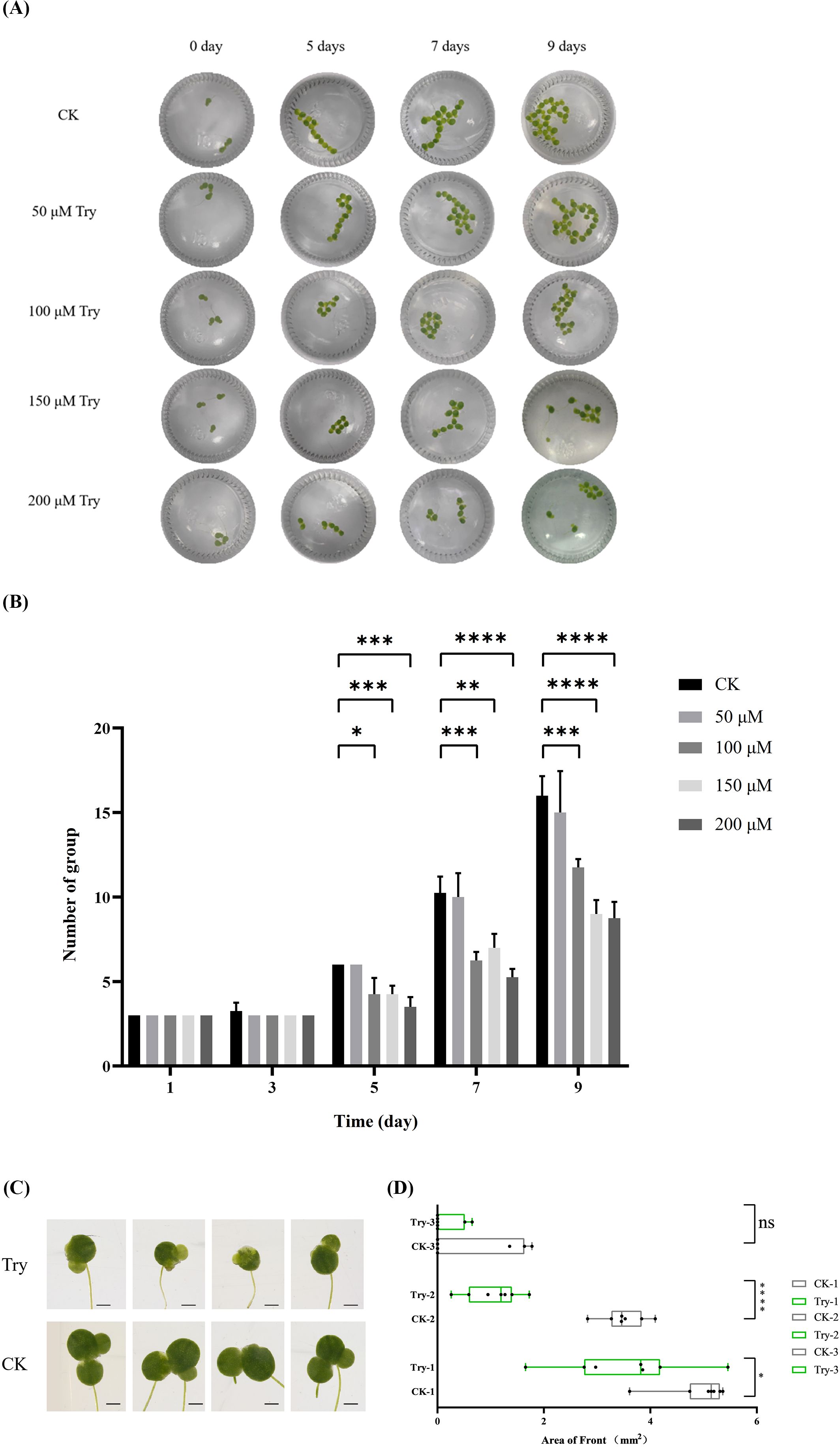
Figure 1. Influence of external application of Try on the growth of duckweed. (A) Leaf diagram; (B) Number of group; (C) Leaf morphology; (D) Leaf area. CK-1, Area of the largest leaf in the control group; CK-2, Area of the second largest leaf of the control group; CK-3, Area of the third largest leaf of the control group; Try-1, Area of the largest leaf in the Try treatment group; Try-2, Area of the second largest leaf of the control group; Try-3, Area of the third largest leaf of the control group. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns, not significant).
After treating duckweed with 150 μM Try for nine days, we observed the growth of duckweed leaves using a stereomicroscope. The pictures showed that the leaf edges of duckweed treated with Try showed yellowing and browning, and the hyalinization was more serious (Figure 1C). Based on this, we further measured the leaf area of duckweed in CK group and Try treated group. The leaf area of the first leaf in the Try treatment group decreased by 28.25% compared with the CK group, and the leaf area of the second leaf in the Try treatment group decreased by 69.91% compared with the CK group. The area of the first leaf and the second leaf in Try treatment were reduced significantly (p < 0.05, p < 0.0001) (Figure 1D). These results indicated that the phenotype of duckweed changed after external application of Try, indicating that Try could regulate the growth process of duckweed.
3.2 Influence of 150 μM concentration of Try on photosynthesis of duckweed
To investigate the changes in photosynthesis of duckweed after external treatment with Try, Fv/Fm value and chlorophyll content of duckweed leaves were determined. We also measured at the genetic level to reveal the effect of Try on photosynthesis of duckweed. The results indicated that the 150 μM Try treatment significantly reduced the value of Fv/Fm by 3.96%, compared to the CK (p < 0.05; Figure 2A).
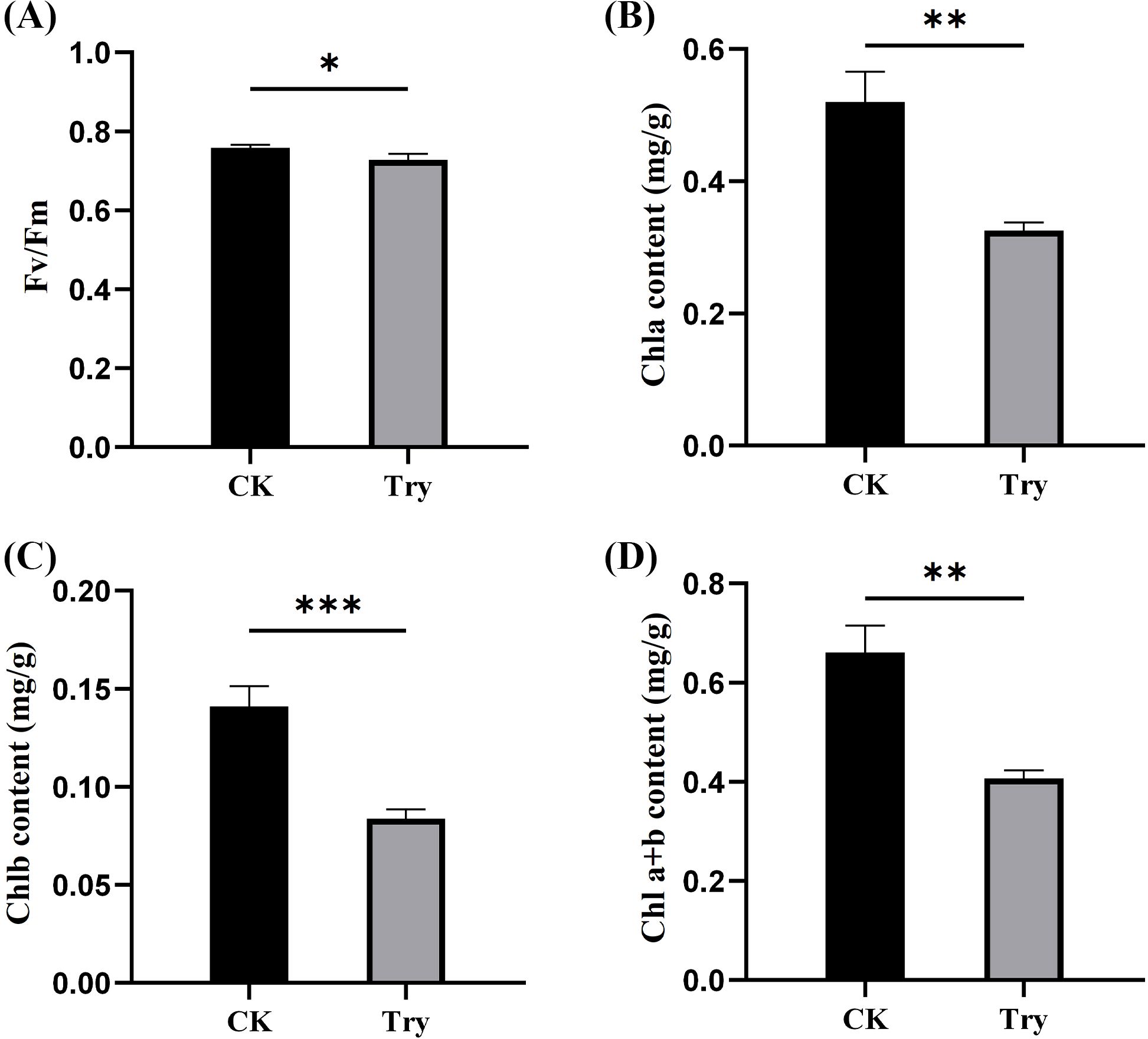
Figure 2. Influence of 150 μM concentration of Try on photosynthesis of duckweed. (A) maximum photochemical quantum efficiency of PSII (Fv/Fm); (B) chlorophyll a content; (C) chlorophyll b content; (D) total chlorophyll content. (*p < 0.05, **p < 0.01, ***p < 0.001).
After processing for 8 days, the chlorophyll contents of CK and Try samples were determined. It was found that compared with CK group, the content of chlorophyll a, chlorophyll b and total chlorophyll in Try treatment group reduced by 37.50%, 40.43% and 37.97%, respectively (p < 0.01; Figures 2B–D).
Moreover, the changes of gene expression levels showed that the Try treatment affected protein content by influencing gene expression levels. The levels of gene expression almost all have reduced (Table 1). These results indicated that exogenous Try could regulate the growth of duckweed by affecting photosynthesis.
3.3 Influence of endogenous Try content on Try metabolism and growth state of duckweed
To elucidate the role of Try in the development process of duckweed, we performed RNA-seq analysis to investigate the differential expression of genes involved in Try metabolism between young and senescent duckweed (Figure 3A). It was found that the three enzymes directly related to Try metabolism were: TDC, T5H, and SNAT. In senescent duckweed, the expressions of TDC and T5H did not change significantly, while the expression of SNAT was significantly downregulated. The expression of SNAT was downregulated by 0.46 log2 Fold Change. However, in the metabolic pathways related to Try, the expression of other enzymes had undergone significant changes. The expression of PHGDH was downregulated by 1.22 log2 Fold Change, that of PSPH was downregulated by 0.98 log2 Fold Change and that of trpA was downregulated by 0.05 log2 Fold Change. The expression of tnaA was upregulated by 0.05 log2 Fold Change, and the expression of PSAT1 was upregulated by 2.11 log2 Fold Change. In order to understand more clearly the content of Try in young and senescent duckweed, we examined the Try content in young and senescent duckweed respectively (Figure 3B). The Try content in the senescent duckweed was 14.76 µmol/L, nearly 7% higher than that in the control group (13.78 µmol/L). These results indicated that Try accumulates in senescent duckweed.
Figure 3. Influence of endogenous Try content on Try metabolism and growth state of duckweed. (A) Heatmap correlation between the Try relation metabolic pathway in young and senescent duckweed. Red arrows represent up-regulation, green arrows represent down-regulation. The legend colors in this figure were changed from red to blue, reflecting a decrease in log2 fold change values (red: high expression; blue: low expression). The column on the left of the heat map represents young duckweed and the column on the right represents senescent duckweed. The heatmap data is the log2 value of the readcount. PHGDH, phosphoglycerate dehydrogenase; trpA, tryptophan synthase alpha chain; PSAT1, Phosphoserine Aminotransferase 1; PSPH, phosphoserine phosphatase; TDC, tryptophan decarboxylase; tnaA, tryptophanase; SNAT, arylalkylamine N-acetyltransferase; COMT, Catechol-O-MethylTransferase; TPH, tryptophan 5-monooxygenase; T5H, tryptamine 5-hydroxylase; ASMT, acetylserotonin O-methyltransferase; SNAT, Serotonin N-Acetyltransferase; TPH, Tryptophan Hydroxylase; (B) The content of Try in senescent and young duckweed, asterisks indicate significant differences between senescent and young samples. (**p < 0.01).
3.4 Influence of 150 μM Try on endogenous hormones of duckweed
To reveal the effect of Try on the endogenous hormone content of duckweed and the expression of its related genes, we measured the content of ABA and CTK and the expression of their related genes. Our results indicated that the ABA content significantly increased by 23.58% (p < 0.05; Figure 4A) and the CTK content significantly decreased by 17.55% (p < 0.01; Figure 4B), compared to the CK.
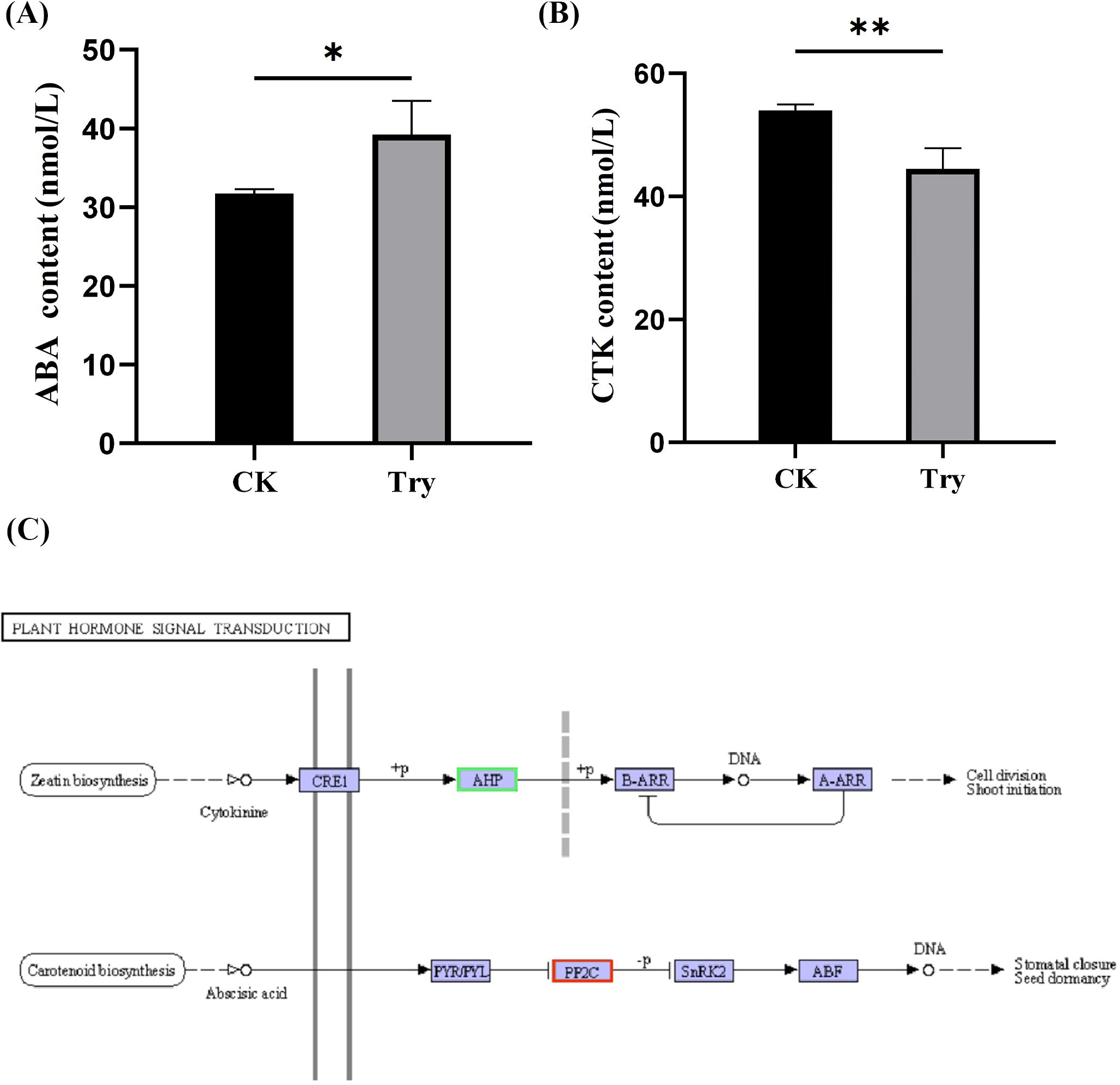
Figure 4. Influence of 150 μM Try on endogenous hormones of duckweed. (A) ABA content; (B) CTK content; (C) plant hormone signal transduction. CRE1, Cytokinin Response 1; AHP, Arabidopsis Histidine Phosphotransfer Protein; B-ARR, Type-B Arabidopsis Response Regulator; A-ARR, Type-A Arabidopsis Response Regulator; PYR/PYL, Pyrabactin Resistance/PYR1-Like; PP2C, Protein Phosphatase 2C; SnRK2, Sucrose Non-fermenting 1-Related Protein Kinase 2. (*p < 0.05, **p < 0.01).
Transcriptome sequencing results showed that Try treatment led to increased expression of genes related to ABA production and decreased expression of genes associated with CTK production. The impact of exogenous Try on the endogenous hormone content in duckweed has been uncovered at the level of gene expression (Figure 4C). The above results showed that Try regulated the endogenous hormone content of duckweed by regulating gene expression, and finally regulated the growth state of duckweed.
3.5 Influence of 150 μM Try on ROS and POD of duckweed
To investigate the oxidative stress response under 150 μM Try treatment, we measured reactive oxygen species (ROS) accumulation and peroxidase (POD) activity in both experimental and CK groups (Figure 5). The results showed that ROS levels in the Try group were 356.444 ng/g which was 9.17% more than those in CK (326.597 ng/g). Similarly, the Try treated duckweed exhibited a POD value of 170.140 ng/g, showing a 10.11% increase compared to the CK (154.513 ng/g). These findings suggested that the Try treatment induced oxidative stress, triggering ROS overproduction and simultaneously activating the antioxidant defense system, as evidenced by enhanced POD activity to scavenge excessive ROS and mitigate cellular damage.
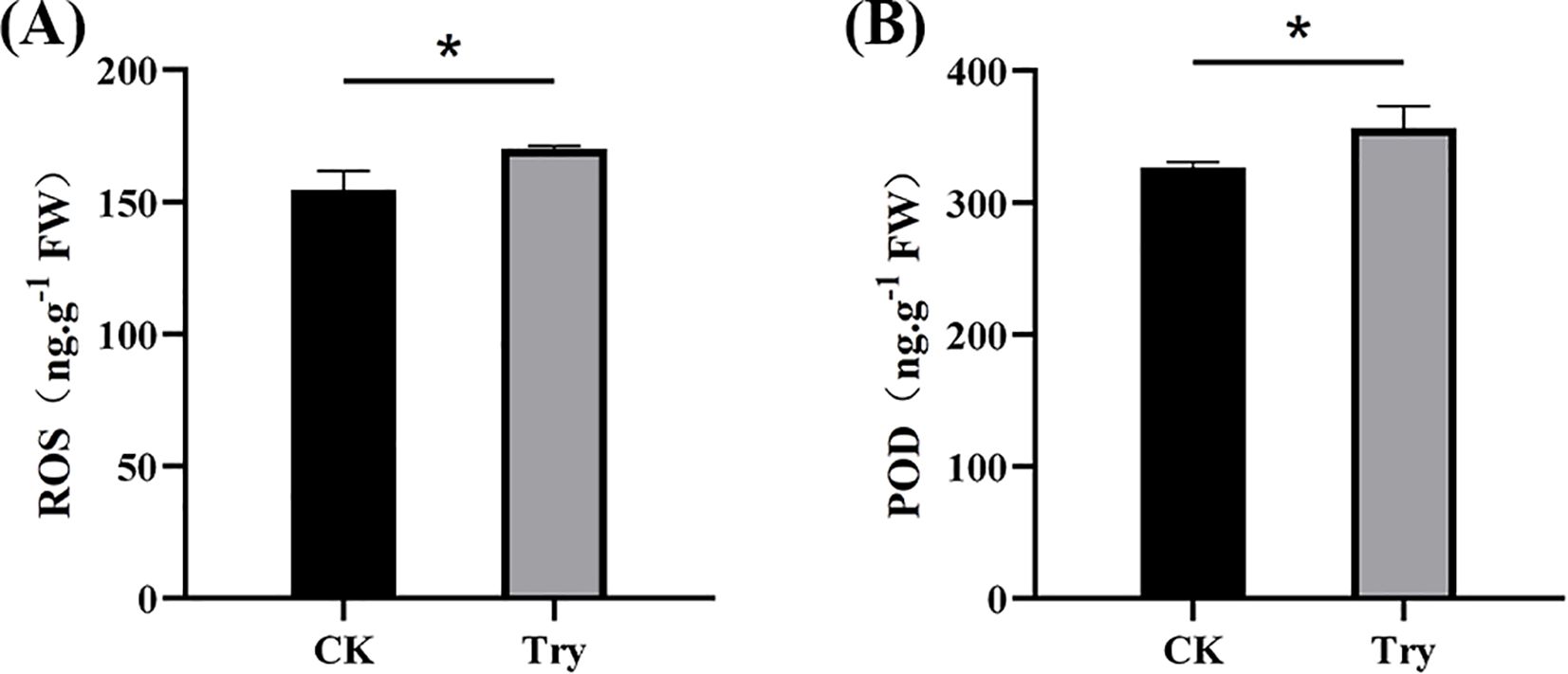
Figure 5. Influence of 150 μM Try on ROS and POD of duckweed. (A) ROS content; (B) POD content. (*p < 0.05).
3.6 Influence of exogenous Try on Try metabolism and endogenous auxin content of duckweed
To study the effect of exogenous application of Try on the Try metabolism-related genes of duckweed, we analyzed the expression profiles of the related genes using RNA-seq data (Figure 6A). In the Try metabolic pathway, there were four key enzymes that directly affected Try content, they were TDC, MAO, T5H and SNAT. The expression of MAO and T5H did not change much compared with CK. However, the expression of TDC was revised up by 2.25 log2 Fold Change and the expression of SNAT was revised down by 0.69 log2 Fold Change. Combining the expression of these four enzymes, we found that exogenous Try treatment of duckweed resulted in the increase of endogenous Try content. The content of Try in senescent duckweed also increased significantly. These results indicated that external application of Try could affect the growth of duckweed. From the expression of Try metabolism-related enzymes in duckweed after external application of Try, it could be seen that the expressions of YUCCA and ALDH, two enzymes directly related to IAA generation, were changed. The expression of YUCCA was revised up by 0.08 log2 Fold Change and the expression of ALDH was revised down by 0.04 log2 Fold Change. According to the expression of these two enzymes, exogenous application of Try can increase the content of endogenous IAA in duckweed. Furthermore, endogenous IAA levels were quantified (Figure 6B), demonstrating a 17.76% increase in IAA content in the experimental group (37.39 nmol/L) compared to the CK group (31.75 nmol/L). Collectively, these findings suggested that aging in duckweed was linked to dynamic reprogramming of Try metabolism and auxin biosynthesis, which might influence growth regulation and stress acclimation.
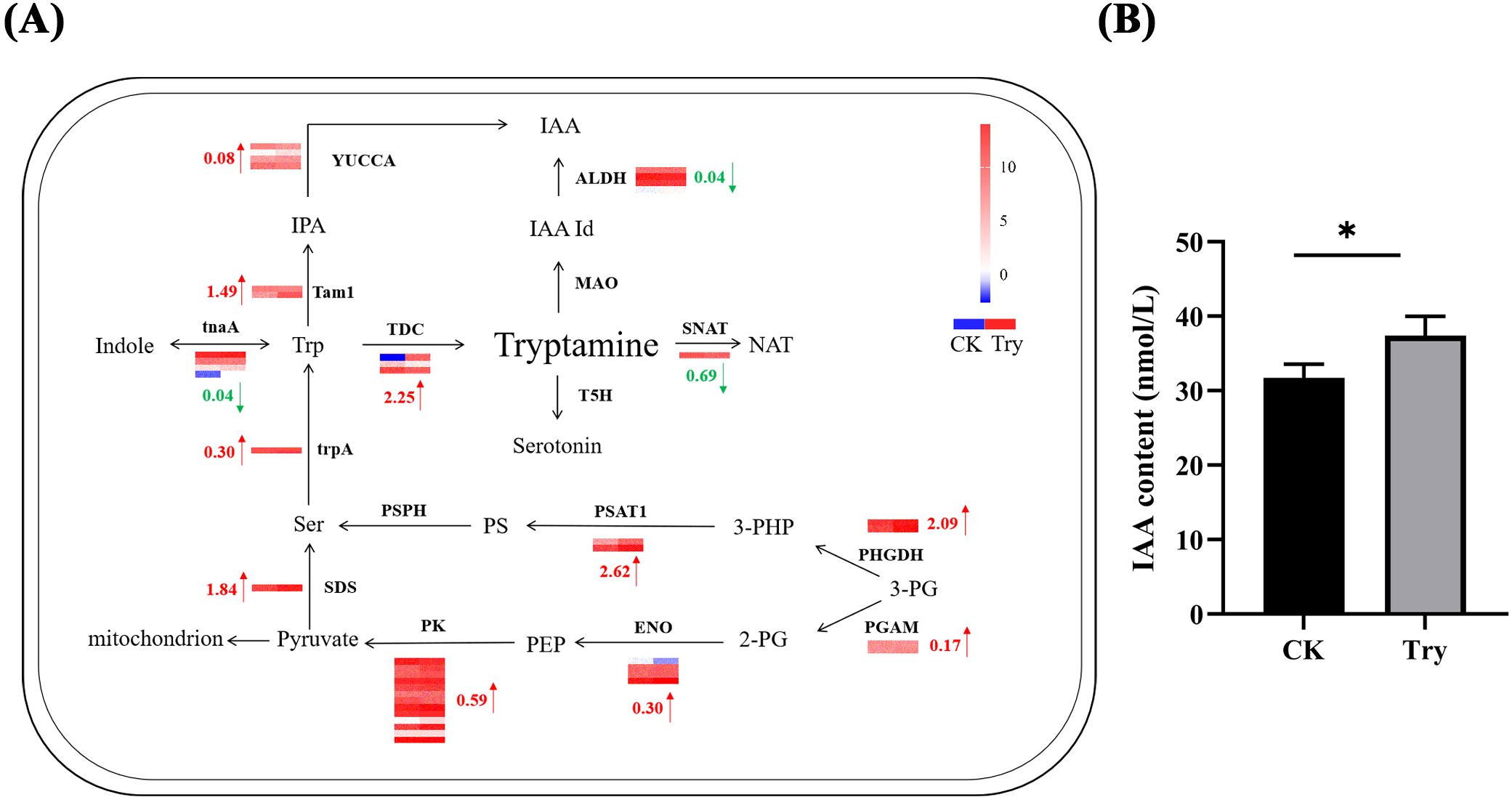
Figure 6. Influence of exogenous Try on Try metabolism and endogenous auxin content of duckweed (A) Showing the differential regulation of mRNA expression of Try pathway enzymes. Red arrows represent up-regulation, green arrows represent down-regulation. The legend colors in this figure were changed from red to blue, reflecting a decrease in log2 fold change values (red: high expression; blue: low expression). The left column of the heat map represents the CK group, and the right column represents the Try treatment group. The heatmap data is the log2 value of the readcount. PHGDH, phosphoglycerate dehydrogenase; trpA, tryptophan synthase alpha chain; PSAT1, Phosphoserine Aminotransferase 1; PSPH, phosphoserine phosphatase; TDC, tryptophan decarboxylase; tnaA, tryptophanase; SNAT, Catechol-O-MethylTransferase; TPH, tryptophan 5-monooxygenase; T5H, tryptamine 5-hydroxylase; MAO, monoamine oxidase; tam1, tryptophan aminotransferase; YUCCA, Indole-3-pyruvate monooxygenase; ALDH, aldehyde dehydrogenase; SDS, serine threonine ammonia-lyase; PGAM, 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase; ENO, enolase; PK, pyruvate kinase; (B) The content of IAA in CK and 150 μM of exogenous Try duckweed. (*p < 0.05).
4 Discussion
The present study provided comprehensive insights into the physiological and molecular effects of Try on Lemna turionifera 5511, particularly focusing on its role in growth regulation, photosynthesis and hormonal balance. Our findings revealed that Try, a polyamine derived from tryptophan, significantly influenced duckweed growth and metabolism, suggesting its potential as a regulatory molecule in plant development.
4.1 Try accumulation and growth regulation
Our results indicate that Try content increases in overgrown duckweed, suggesting a possible role in growth regulation. The accumulation of Try in senescent duckweed (14.76 µmol/L) compared to young duckweed (13.78 µmol/L) implies that Try may be involved in the aging process or stress responses in plants (Figure 3B). This is consistent with previous studies. It was shown that the levels of polyamines vary at various growth stages of plants. Try played crucial roles in plant growth, development, and stress responses (Kusano et al., 2008; Tiburcio et al., 2014). For instance, during the development processes such as flowering and cell division, there are complex interactions with plant hormones, which are crucial for the normal growth and development of plants (Kusano et al., 2008). In Arabidopsis, the SHT (spermidine hydroxycinnamoyltransferase) gene is expressed exclusively in tapetum cells of anthers (Tiburcio et al., 2014). Its product is necessary for proper pollen wall development. Under abiotic stresses such as drought and salinity, the levels of polyamines in plants increase to cope with this stress. Overexpression of ADC2 in Arabidopsis leads to putrescine accumulation, enhancing drought tolerance (Kusano et al., 2008). The downregulation of key Try-related metabolic enzymes such as PHGDH, PSPH, trpA, and SNAT in senescent duckweed further supports the idea that Try metabolism is closely linked to plant aging and growth regulation (Figure 3A).
The exogenous application of Try at concentrations ranging from 50 to 200 μM resulted in a dose-dependent inhibition of duckweed growth (Figure 1A). Notably, 150 μM Try significantly reduced the growth rate, leaf area, and chlorophyll content, indicating that Try can act as a growth inhibitor at higher concentrations (Figures 1B–D). This finding aligns with previous studies showing that excessive levels of certain polyamines can inhibit plant growth and development (Alcázar et al., 2010). The observed yellowing and browning of leaves in Try-treated duckweed further suggest that Try may induce oxidative stress, leading to cellular damage and growth inhibition (Figure 1C).
4.2 Try impact on photosynthesis and reactive oxygen species and peroxidase activity
The reduction in chlorophyll content and Fv/Fm in Try-treated duckweed indicates that Try negatively affects photosynthesis (Figure 2). The down-regulation of the genes of the proteins related to photosynthesis further supports this conclusion (Table 1). Although the application of polyamines can improve the photosynthesis of plants (Killiny and Nehela, 2020). Polyamines enhance the photosynthetic capacity of citrus plants by improving chlorophyll content, net photosynthetic rate and Fv/Fm. However, the high dose of PAs may destroy the structure of chloroplasts based on light conditions (Wu et al., 2012). This might be that polyamines have triggered the relevant stress response in chloroplasts, leading to excessive accumulation of ROS. When the production of ROS exceeds the clearance capacity of the chloroplast’s antioxidant system, photo-oxidative damage will be triggered.
The increase in reactive oxygen species (ROS) and peroxidase (POD) activity in Try-treated duckweed indicates that Try induces oxidative stress (Figure 5). ROS are known to cause cellular damage, including lipid peroxidation, protein oxidation, and DNA damage (Mittler, 2002). The increase in POD activity suggests that duckweed responds to Try-induced oxidative stress by enhancing its antioxidant defense mechanisms. Previous studies have shown that polyamines can modulate ROS levels and antioxidant enzyme activities in plants (Groppa et al., 2003). These observations indicates that Try triggers the stress response of plants, leading to a decline in photosynthesis and a reduction in chlorophyll content in duckweed. Duckweed eliminates the negative impact of ROS by increasing the content of POD.
4.3 Hormonal regulation and stress responses
Our study also highlights the role of Try in modulating endogenous hormone levels, particularly abscisic acid (ABA) and cytokinin (CTK) (Figures 4A, B). The significant increase in ABA content and decrease in CTK content in Try-treated duckweed suggests that Try may induce stress responses in plants. ABA is known to play a crucial role in plant stress responses, including stomatal closure and seed dormancy (Finkelstein, 2013), while CTK is involved in delaying senescence and maintaining chlorophyll content (Zwack and Rashotte, 2015). It has also been proposed that polyamines are involved in plant tolerance and acclimation to drought by modulating ABA and CTK biosynthesis (Asija et al., 2023; Napieraj et al., 2023). Under drought conditions, polyamines increase the ABA content in plants by inducing the expression of key genes for ABA biosynthesis (such as NCED), promote stomatal closure to reduce water loss, and enhance the plants’ response to drought stress by regulating the expression of related genes in the ABA signal transduction pathway. Meanwhile, polyamines can also cause changes in CTK content, regulate physiological processes such as cell division, chlorophyll biosynthesis and nutrient absorption, and control the growth and photosynthetic efficiency of plants (Napieraj et al., 2023). In our studies, ABA-related genes were upregulated and CTK-related genes were down-regulated in duckweed treated with Try, which supported the view that Try induced a stress response, triggers changes in endogenous related hormones in duckweed, and leads to growth inhibition and aging (Figure 4C).
5 Conclusion
In conclusion, our study demonstrates that Try plays a complex role in regulating duckweed growth, photosynthesis, and stress responses. The accumulation of Try in older duckweed, its growth-inhibiting effects at higher concentrations, and its impact on hormonal balance and oxidative stress responses highlight its potential as a regulatory molecule in plant development. Further research is needed to explore the molecular mechanisms underlying the effect of Try and its potential applications in agriculture and environmental management.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
QD: Writing – review & editing, Writing – original draft. WH: Writing – review & editing, Writing – original draft. YJH: Resources, Project administration, Formal Analysis, Writing – review & editing, Methodology, Supervision. SL: Methodology, Software, Formal Analysis, Writing – original draft. YH: Formal Analysis, Methodology, Writing – original draft. ZQ: Writing – review & editing, Formal Analysis, Methodology. YJ: Methodology, Writing – review & editing, Data curation, Formal Analysis. WS: Writing – original draft, Data curation. TQ: Data curation, Writing – original draft. LY: Validation, Investigation, Writing – review & editing, Conceptualization, Funding acquisition, Software, Methodology, Supervision, Resources, Formal Analysis, Project administration, Data curation, Writing – original draft, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Present research has been supported by National Natural Science Foundation of China (No.32471699, No. 32071620), Tianjin Natural Science Foundation of Tianjin (23JCYBJC00540), Tianjin Education Reform Project (B231006511) and undergraduate innovation training program (202410065040).
Acknowledgments
We greatly appreciate the help form Xuanbin Li, Ruishan Lu, Ying Chu, Yuan Wang and Hanfei Luo for providing valuable help in preparing experimental materials and data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fpls.2025.1663336.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alcázar, R., Altabella, T., Marco, F., Bortolotti, C., Reymond, M., Koncz, C., et al. (2010). Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231, 1237–1249. doi: 10.1007/s00425-010-1130-0, PMID: 20221631
Asija, S., Seth, T., Umar, S., and Gupta, R. (2023). Polyamines and their crosstalk with phytohormones in the regulation of plant defense responses. J. Plant Growth Regul. 42, 5224–5246. doi: 10.1007/s00344-022-10837-5
Bartel, B., LeClere, S., Magidin, M., and Zolman, B. K. (2001). Inputs to the active indole-3-acetic acid pool: de novo synthesis, conjugate hydrolysis, and indole-3-butyric acid β-oxidation. J. Plant Growth Regul. 20, 198–216. doi: 10.1007/s003440010025
Benková, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertová, D., Jürgens, G., et al. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602. doi: 10.1016/s0092-8674(03)00924-3, PMID: 14651850
Bhattarai, Y., Williams, B. B., Battaglioli, E. J., Whitaker, W. R., Till, L., Grover, M., et al. (2018). Gut microbiota-produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host Microbe 23, 775–785.e5. doi: 10.1016/j.chom.2018.05.004, PMID: 29902441
Casanova-Sáez, R., Mateo-Bonmatí, E., and Ljung, K. (2021). Auxin metabolism in plants. Cold Spring Harbor Perspect. Biol. 13, a039867. doi: 10.1101/cshperspect.a039867, PMID: 33431579
Ćavar Zeljković, S., De Diego, N., Drašar, L., Nisler, J., Havlíček, L., Spíchal, L., et al. (2024). Comprehensive LC-MS/MS analysis of nitrogen-related plant metabolites. J. Exp. Bot. 75, 5390–5411. doi: 10.1093/jxb/erae129, PMID: 38526483
Erb, M. and Kliebenstein, D. J. (2020). Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol. 184, 39–52. doi: 10.1104/pp.20.00433, PMID: 32636341
Finkelstein, R. (2013). Abscisic acid synthesis and response. Arabidopsis Book 11, e0166. doi: 10.1199/tab.0166, PMID: 24273463
Groppa, M. D., Benavides, M. P., and Tomaro, M. L. (2003). Polyamine metabolism in sunflower and wheat leaf discs under cadmium or copper stress. Plant Sci. 164, 293–299. doi: 10.1016/S0168-9452(02)00412-0
Hillman, W. S. (1961). The Lemnaceae, or duckweeds: a review of the descriptive and experimental literature. Botanical Rev. 27, 221–287. doi: 10.1007/BF02860083
Kang, J., Kim, K., Do, T. H. T., Han, M., and Lee, Y. (2025). Duckweeds for plant molecular farming: advances, challenges, and future directions. J. Plant Biol. 68, 77–91. doi: 10.1007/s12374-025-09458-8
Killiny, N. and Nehela, Y. (2020). Citrus polyamines: structure, biosynthesis, and physiological functions. Plants 9, 426. doi: 10.3390/plants9040426, PMID: 32244406
Kusano, T., Berberich, T., Tateda, C., and Takahashi, Y. (2008). Polyamines: essential factors for growth and survival. Planta 228, 367–381. doi: 10.1007/s00425-008-0772-7, PMID: 18594857
Ligneul, R. and Mainen, Z. F. (2023). Serotonin. Curr. Biol. 33, R1216–R1221. doi: 10.1016/j.cub.2023.09.068, PMID: 38052167
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. doi: 10.1016/s1360-1385(02)02312-9, PMID: 12234732
Napieraj, N., Janicka, M., and Reda, M. (2023). Interactions of polyamines and phytohormones in plant response to abiotic stress. Plants 12, 1159. doi: 10.3390/plants12051159, PMID: 36904019
Olas, J. J., Fichtner, F., and Apelt, F. (2020). All roads lead to growth: imaging-based and biochemical methods to measure plant growth. J. Exp. Bot. 71, 11–21. doi: 10.1093/jxb/erz406, PMID: 31613967
Perico, C., Tan, S., and Langdale, J. A. (2022). Developmental regulation of leaf venation patterns: monocot versus eudicots and the role of auxin. New Phytol. 234, 783–803. doi: 10.1111/nph.17955, PMID: 35020214
Pollmann, S., Müller, A., Piotrowski, M., and Weiler, E. W. (2002). Occurrence and formation of indole-3-acetamide in Arabidopsis thaliana. Planta 216, 155–161. doi: 10.1007/s00425-002-0868-4, PMID: 12430025
Seifi, H. S. and Shelp, B. J. (2019). Spermine differentially refines plant defense responses against biotic and abiotic stresses. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00117, PMID: 30800140
Simonetti, G., Boga, C., Durante, J., Micheletti, G., Telese, D., Caruana, P., et al. (2021). Synthesis of novel tryptamine derivatives and their biological activity as antitumor agents. Molecules 26. doi: 10.3390/molecules26030683, PMID: 33525621
Stepanova, A. N., Robertson-hoyt, J., Yun, J., Benavente, L. M., Xie, D.-Y., Doležal, K., et al. (2008). TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191. doi: 10.1016/j.cell.2008.01.047, PMID: 18394997
Tiburcio, A. F., Altabella, T., Bitrian, M., and Alcazar, R. (2014). The roles of polyamines during the lifespan of plants: from development to stress. Planta 240, 1–18. doi: 10.1007/s00425-014-2055-9, PMID: 24659098
Utami, D., Kawahata, A., Sugawara, M., Jog, R. N., Miwa, K., and Morikawa, M. (2018). Effect of exogenous general plant growth regulators on the growth of the duckweed lemna minor. Front. Chem. 6. doi: 10.3389/fchem.2018.00251, PMID: 30038905
Wan, J., Zhang, P., Wang, R., Sun, L., Ju, Q., and Xu, J. (2018). Comparative physiological responses and transcriptome analysis reveal the roles of melatonin and serotonin in regulating growth and metabolism in Arabidopsis. BMC Plant Biol. 18, 362. doi: 10.1186/s12870-018-1548-2, PMID: 30563469
Wang, W., Yang, Y., Ma, X., He, Y., Ren, Q., Huang, Y., et al. (2023). New Insight into the Function of Dopamine (DA) during Cd Stress in Duckweed (Lemna turionifera 5511). Plants (Basel) 12. doi: 10.3390/plants12101996, PMID: 37653913
Wu, Q., Zou, Y., Liu, M., and Cheng, K. (2012). Effects of exogenous putrescine on mycorrhiza, root system architecture, and physiological traits of glomus mosseae-colonized trifoliate orange seedlings. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 40, 80–85. doi: 10.15835/nbha4027926
Yousefi, F., Jabbarzadeh, Z., Amiri, J., and Rasouli-Sadaghiani, M. H. (2019). Response of roses (Rosa hybrida L. ‘Herbert stevens’) to foliar application of polyamines on root development, flowering, photosynthetic pigments, antioxidant enzymes activity and NPK. Sci. Rep. 9, 16025. doi: 10.1038/s41598-019-52547-1, PMID: 31690765
Zhao, Y., Christensen, S. K., Fankhauser, C., Cashman, J. R., Cohen, J. D., Weigel, D., et al. (2001). A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291, 306–309. doi: 10.1126/science.291.5502.306, PMID: 11209081
Ziegler, P., Adelmann, K., Zimmer, S., Schmidt, C., and Appenroth, K. J. (2015). Relative in vitro growth rates of duckweeds (Lemnaceae) - the most rapidly growing higher plants. Plant Biol. (Stuttgart Germany) 17 Suppl 1, 33–41. doi: 10.1111/plb.12184, PMID: 24803032
Zohairi, F., Khandelia, H., and Hakami Zanjani, A. A. (2023). Interaction of psychedelic tryptamine derivatives with a lipid bilayer. Chem. Phys. Lipids 251, 105279. doi: 10.1016/j.chemphyslip.2023.105279, PMID: 36627076
Keywords: tryptamine, Lemna turionifera 5511, growth regulation, photosynthesis, oxidative stress, hormonal balance
Citation: Di Q, Han W, Han Y, Liu S, Hu Y, Qu Z, Jiang Y, Sun W, Qiu T and Yang L (2025) Effects of tryptamine on duckweed growth. Front. Plant Sci. 16:1625939. doi: 10.3389/fpls.2025.1625939
Received: 09 May 2025; Accepted: 17 June 2025;
Published: 08 July 2025; Corrected: 01 August 2025.
Edited by:
João Serôdio, University of Aveiro, PortugalReviewed by:
Johann Hornbacher, Leibniz University Hannover, GermanyElžbieta Jankovska-Bortkevič, Nature Research Centre, Lithuania
Copyright © 2025 Di, Han, Han, Liu, Hu, Qu, Jiang, Sun, Qiu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Yang, c2t5eWxAdGpudS5lZHUuY24=
†These authors share first authorship
 Qiqi Di
Qiqi Di Wenqian Han
Wenqian Han Yujie Han2
Yujie Han2 Sizheng Liu
Sizheng Liu Ziyang Qu
Ziyang Qu Lin Yang
Lin Yang