- 1Centre for Advanced Material Application, Slovak Academy of Sciences, Bratislava, Slovakia
- 2Department of Nanobiology, Cancer Research Institute, Biomedical Research Center, Slovak Academy of Sciences, Bratislava, Slovakia
- 3Department of Genetics, Faculty of Natural Sciences, Comenius University Bratislava, Bratislava, Slovakia
Introduction
Nanoparticle-based elicitation represents an emerging technical advance in plant biotechnology that enables selective modulation of secondary metabolite pathways and induces mild stress, mainly due to its ability to influence plant growth, increase nutrient content, and improve both photosynthetic activity and metabolic processes (Wang et al., 2023b). The application of nanoparticles, such as TiO2, Fe3O4, or Ag-NPs, has been shown to be an effective way to stimulate the biosynthetic pathways of flavonoids, alkaloids, or phenolic acids in various medicinal plants (Gohari et al., 2020; Salih et al., 2022; Ahmed et al., 2023; Muhammad et al., 2025). As a result, they represent a promising tool for sustainable and economically advantageous production of plant extracts, which is important for phytotherapeutics with high demands on quality and consistency of composition.
This opinion highlights the need for a broader evaluation of nanoparticle-mediated enhancements in Silybum marianum beyond silybin, particularly focusing on the overlooked compound taxifolin and other flavonolignans. We seek to stimulate discussion on whether the observed therapeutic effects may result from a more complex phytochemical shift than currently appreciated, given that the biological activity of silymarin is influenced not only by silybin, but also by other constituents such as silychristin, isosilybin, silydianin, and taxifolin, some of which have demonstrated even stronger pharmacological effects in specific contexts.
Changes in silymarin composition after nanoparticle treatment
Zinc oxide nanoparticles (ZnO-NPs) are attracting increasing attention for their potential to enhance the production of specialized plant metabolites (García-López et al., 2018; Wang et al., 2023a). In a recent study, Fahad Almulhim et al. (2025) investigated the use of ZnO-NPs to enhance silybin accumulation in Silybum marianum fruits and evaluated the osteoprotective potential of the resulting extracts (Fahad Almulhim et al., 2025). The foliar application of ZnO-NPs led to an almost eightfold rise in silybin (A+B) content compared to untreated controls, as determined by HPLC. The results are consistent with the previous work Jafari et al. (2023), who monitored the increase in silybins after treatment with titanium dioxide nanoparticles (TiO2-NPs). Foliar application of TiO2-NPs and chitosan significantly increased the accumulation of silybin A and B in milk thistle seeds. This effect was associated with the downregulation of specific microRNAs, leading to increased expression of key biosynthetic genes (Jafari et al., 2023). This highlights that nanoparticle treatment actively modulates regulatory pathways involved in secondary metabolism. This outcome points to a practical way of enhancing phytochemical yields without the need for major genetic engineering or intensive breeding efforts.
In both of the studies mentioned above, the presence of silybins was monitored. Silybin is considered as the key active compound of silymarin, an extract from the seeds of milk thistle, valued for its liver-protective, anticancer, antioxidant, and bone-preserving properties (Ray et al., 2024). Approximately 40-60% of silymarin is silybin, consisting of two isomers (silybin A and silybin B in a 1:1 ratio). Furthermore, silymarin contains other major flavonolignans such as silychristin, isosilybin A, isosilybin B, silydianin, 2,3-dehydrosilybin, isosilychristin, and the flavonoid taxifolin (Figure 1A). Understanding the broader shifts in silymarin composition after nanoparticle exposure is crucial not only for optimizing extract yield but also for ensuring reproducible and effective therapeutic outcomes.
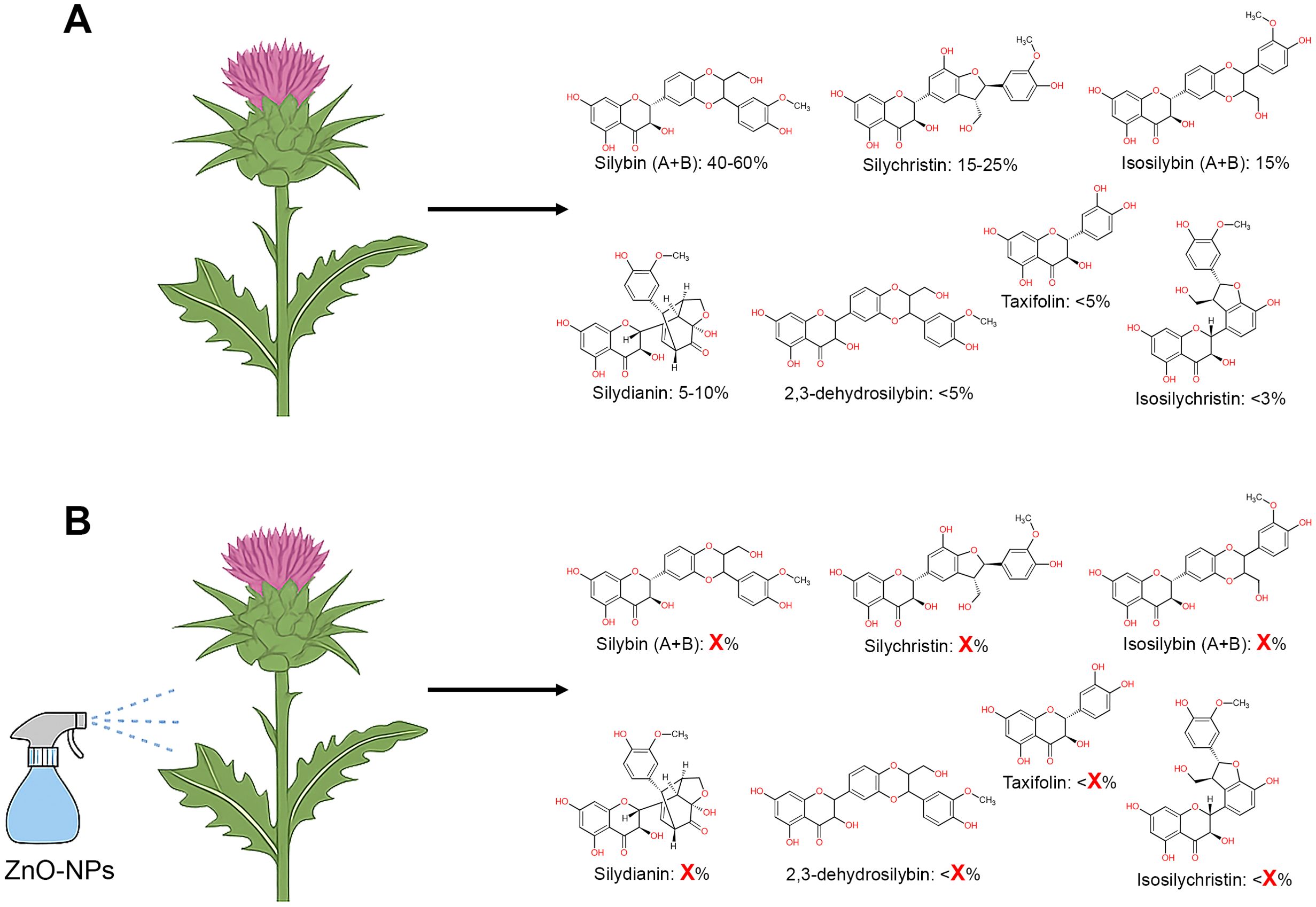
Figure 1. (A) Major components of silymarin, including their quantitative abundance. Silymarin consists of approximately 40-60% silybins, 15-25% silychristin, 15% isosilybins, 5-10% silydianin, less than 5% 2,3-dehydrosilybin or taxifolin, and less than 3% isosilychristin (Fenclova et al., 2019). (B) Hypothetical representation showing the unknown effect of nanoparticle treatment on individual flavonolignan and flavonoid content, emphasizing the need for detailed phytochemical profiling.
Discussion
Although the observed increase in silybin is promising, it raises some questions about the broader metabolic impacts of ZnO-NPs or TiO2-NPs treatment. Specifically, what exactly increased in the phytochemical profile after nanoparticle stimulation? (Figure 1B).
There are two main possibilities to consider:
1. Uniform enhancement of all flavonoids/flavonolignans. Nanoparticles treatment boosted the production of all flavonoids and flavonolignans across the board, representing a significant advantage. More bioactive compounds per biomass unit would mean smaller cultivation areas, reduced production costs, and more sustainable extraction process.
2. Selective enhancement or alteration of flavonoid/flavonolignan profiles. Nanoparticles may have selectively stimulated particular branches of the flavonoid biosynthetic pathway. This could have shifted the balance between different flavonoids and flavonolignans, creating an extract with evermore potent properties than the natural extract and potentially changing the biological properties of the extract.
Unfortunately, without a detailed comparison to extracts from untreated plants, it remains uncertain whether the profile of flavonoids/flavonolignans remained consistent.
Recent studies have shown that ZnO-NPs treatment leads to non-uniform changes in flavonoid synthesis. Wang et al. (2023a) reported that ZnO-NPs treatment in Ginkgo biloba caused an increase in the levels of kaempferol and isorhamnetin, while the content of quercetin remained largely unchanged (Wang et al., 2023a). This non-uniform shift suggests that nanoparticle treatment may not simply boost all flavonoid production equally. Similarly, other types of foliar spraying, including salicylic acid, spermine, and brassinosteroid, have revealed that the percentage changes across individual flavonolignans and flavonoid in silymarin are highly non-uniform. Some treatments led to a several-fold increase in one compound while simultaneously decreasing others, and vice versa in other cases, suggesting a highly selective and potentially competitive regulation within the silymarin biosynthetic pathway (Fanai et al., 2024). This supports the idea that nanoparticle treatment can induce localized or selective metabolic responses rather than a uniform boost in secondary metabolite content throughout the plant. These differences in flavonoid content are critical, as the biological activity of silymarin extracts depends not only on the amount of silybin but also on the presence and ratios of other flavonolignans (e.g., silychristin, isosilybin, silydianin) and flavonoid like taxifolin, which possess better medical properties than silybin. For example, isosilybin A and isosilybin B have shown superior and selective anticancer properties compared to silybin, particularly in hepatic and prostate cancer cell models (Deep et al., 2007; Polyak et al., 2010). Similarly, 2,3-dehydrosilybin and silychristin have demonstrated stronger antioxidant effects than silybin, especially in reducing intracellular ROS levels under oxidative stress (Jurčacková et al., 2025). Taxifolin and silydianin have also demonstrated more potent antioxidant and hepatoprotective activities than silybin, further supporting the idea that multiple constituents of silymarin may contribute significantly to its therapeutic profile (Anthony and Saleh, 2013; Kim et al., 2024).
Importantly, after the treatment with ZnO-NPs, or TiO2-NPs, increased expression of chalcone synthase (CHS), a key enzyme responsible for flavonoid biosynthesis pathway, was observed in milk thistle (Jafari et al., 2023; Fahad Almulhim et al., 2025). This upregulation is consistent with the general pattern of stress-related gene expression observed under nanoparticle exposure, likely mediated by ROS signaling and hormonal modulation. However, increased CHS expression alone does not indicate which downstream flavonoids or flavonolignans are preferentially synthetized, likely mediated by ROS signaling and hormonal modulation (Tripathi et al., 2022). In Silybum marianum, three CHS genes (SmCHS1, SmCHS2, and SmCHS3) with different expression patterns have been identified. SmCHS1 and SmCHS3 are highly expressed in petals during the early flowering stage and in stems and upper leaves at mid-flowering, and are most likely responsible for silymarin biosynthesis (Sanjari et al., 2015). SmCHS2 is weakly expressed across plant tissues. This is consistent with the observation that ZnO-NPs treatment the most significantly increased SmCHS3 expression, followed by SmCHS1 and lastly SmCHS2 (Fahad Almulhim et al., 2025).
Nevertheless, whether higher expression of CHS led predominantly to increased silybin, taxifolin, or a general rise in flavonoid flux remains unknown. The potential increase in taxifolin or other flavonoids would be noteworthy. Taxifolin is a flavonoid precursor in silymarin biosynthesis (AbouZid et al., 2017) with well-documented anti-osteoporotic effects. Satué et al. (2013) show that taxifolin promotes osteoblast differentiation and inhibits osteoclastogenesis, contributing to bone formation and preservation (Satué et al., 2013). These findings have been further confirmed by later studies, demonstrating that taxifolin promotes osteogenic differentiation of human bone marrow mesenchymal stem cells by enhancing the expression of osteogenic markers and inhibiting TNF-α-induced NF-κB signaling (Wang et al., 2017), and inhibits RANKL-induced osteoclastogenesis by modulating NF-κB signaling pathways (Zhang et al., 2019). Therefore, if ZnO-NPs treatment disproportionately upregulated taxifolin levels, the observed osteoprotective effects in the rat model might be partly or predominantly due to taxifolin rather than silybin (Fahad Almulhim et al., 2025).
Without a comprehensive phytochemical profile comparing nanoparticles-treated and untreated plant extracts, it is challenging to conclude which compounds are responsible for enhanced biological activity. However, what is certain is that monitoring only silybin levels is insufficient. Future studies should thus profile the full range of major flavonoids and flavonolignans and assess their relative abundances before and after nanoparticle treatment. In conclusion, published studies have made a valuable contribution to the field by demonstrating the potential of nanoparticles to enhance secondary metabolite accumulation in plants with therapeutical potential. Nevertheless, further investigations including more complex view are needed. Future research should focus on the follow:
1. Comparison of full flavonoid and flavonolignan profiles between treated and untreated plants.
2. Determination of disproportionality of taxifolin and/or other flavonoids increment.
3. Clarification of the observed therapeutical effects origin – its derivation from silybin or other components.
These findings represent an important step forward, and we hope that the insights presented here will guide future research. Our comments are intended to complement important findings and to support further advances in nanoparticle-assisted phytochemical enhancement.
Author contributions
MS: Writing – original draft, Conceptualization, Investigation, Writing – review & editing. RM: Formal analysis, Writing – original draft. AB: Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The fee for publishing this manuscript was supported by VEGA grant (No. 2/0116/22, Slovakia).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The authors declare that no generative AI tools were used in the writing of the manuscript. However, generative AI (OpenAI/DALL·E) was used to create the milk thistle plant and spray presented in Figure 1.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
AbouZid, S. F., Ahmed, H. S., Moawad, A. S., Owis, A. I., Chen, S.-N., Nachtergael, A., et al. (2017). Chemotaxonomic and biosynthetic relationships between flavonolignans produced by Silybum marianum populations. Fitoterapia 119, 175–184. doi: 10.1016/j.fitote.2017.04.002
Ahmed, M. A., Shafiei-Masouleh, S.-S., Mohsin, R. M., and Salih, Z. K. (2023). Foliar application of iron oxide nanoparticles promotes growth, mineral contents, and medicinal qualities of solidago virgaurea L. J. Soil Sci. Plant Nutr. 23, 2610–2624. doi: 10.1007/s42729-023-01218-2
Anthony, K. and Saleh, M. (2013). Free radical scavenging and antioxidant activities of silymarin components. Antioxidants 2, 398–407. doi: 10.3390/antiox2040398
Deep, G., Oberlies, N. H., Kroll, D. J., and Agarwal, R. (2007). Isosilybin B and isosilybin A inhibit growth, induce G1 arrest and cause apoptosis in human prostate cancer LNCaP and 22Rv1 cells. Carcinogenesis 28, 1533–1542. doi: 10.1093/carcin/bgm069
Fahad Almulhim, B., Sherif, F., Younis, N. S., Safwat, Y., and Khattab, S. (2025). Foliar spraying with zinc oxide nanoparticles enhances the anti-osteoporotic efficacy of the fruit extracts of Silybum marianum L. by stimulating silybin production. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1421485
Fanai, S., Bakhshi, D., and Abbaszadeh, B. (2024). Physiological and biochemical characteristics of milk thistle (Silybum marianum (L.) Gaertn) as affected by some plant growth regulators. Food Sci. Nutr. 12, 6022–6033. doi: 10.1002/fsn3.4233
Fenclova, M., Novakova, A., Viktorova, J., Jonatova, P., Dzuman, Z., Ruml, T., et al. (2019). Poor chemical and microbiological quality of the commercial milk thistle-based dietary supplements may account for their reported unsatisfactory and non-reproducible clinical outcomes. Sci. Rep. 9, 11118. doi: 10.1038/s41598-019-47250-0
García-López, J., Zavala-García, F., Olivares-Sáenz, E., Lira-Saldívar, R., Díaz Barriga-Castro, E., Ruiz-Torres, N., et al. (2018). Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination. Agronomy 8, 215. doi: 10.3390/agronomy8100215
Gohari, G., Mohammadi, A., Akbari, A., Panahirad, S., Dadpour, M. R., Fotopoulos, V., et al. (2020). Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci. Rep. 10, 912. doi: 10.1038/s41598-020-57794-1
Jafari, S., Mousavi-Fard, S., Rezaei Nejad, A., Mumivand, H., Sorkheh, K., and Nikoloudakis, N. (2023). Foliar application of chitosan and titanium dioxide enhances silybin content by orchestrating miRNA and gene targets transcription in Milk thistle (Silybum marianum L.). Curr. Plant Biol. 35–36, 100302. doi: 10.1016/j.cpb.2023.100302
Jurčacková, Z., Hrčková, G., Mudroňová, D., Matiašová, A. A., and Biedermann, D. (2025). Flavonolignans silybin, silychristin and 2,3-dehydrosilybin showed differential cytoprotective, antioxidant and anti-apoptotic effects on splenocytes from Balb/c mice. Sci. Rep. 15, 5631. doi: 10.1038/s41598-025-89824-1
Kim, J., Han, S. H., Kim, N. K., Tran, G. H., Shim, J., Chin, J. H., et al. (2024). Antioxidant activities and silymarin content of Silybum marianum using different extraction methods. J. Appl. Biol. Chem. 67, 55. doi: 10.3839/jabc.2024.055
Muhammad, S., Khan, A. A., Khan, M. R., Mukhtar, S., Kazmi, A., Ali, A., et al. (2025). Effective substitution of ferrous sulfate with iron oxide nanoparticles enhances growth, antioxidant activity, and stevioside accumulation in micro-propagated Stevia rebaudiana. Front. Plant Sci. 16. doi: 10.3389/fpls.2025.1569613
Polyak, S. J., Morishima, C., Lohmann, V., Pal, S., Lee, D. Y. W., Liu, Y., et al. (2010). Identification of hepatoprotective flavonolignans from silymarin. Proc. Natl. Acad. Sci. U.S.A. 107, 5995–5999. doi: 10.1073/pnas.0914009107
Ray, P. P., Islam, M. A., Islam, M. S., Han, A., Geng, P., Aziz, A., et al. (2024). A comprehensive evaluation of the therapeutic potential of silibinin: a ray of hope in cancer treatment. Front. Pharmacol. 15. doi: 10.3389/fphar.2024.1349745
Salih, A. M., Al-Qurainy, F., Khan, S., Nadeem, M., Tarroum, M., and Shaikhaldein, H. O. (2022). Biogenic silver nanoparticles improve bioactive compounds in medicinal plant Juniperus procera in vitro. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.962112
Sanjari, S., Shobbar, Z. S., Ebrahimi, M., Hasanloo, T., Sadat-Noori, S.-A., and Tirnaz, S. (2015). Chalcone synthase genes from milk thistle (Silybum marianum): isolation and expression analysis. J. Genet. 94, 611–617. doi: 10.1007/s12041-015-0560-7
Satué, M., Arriero, M., del, M., Monjo, M., and Ramis, J. M. (2013). Quercitrin and Taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells. Biochem. Pharmacol. 86, 1476–1486. doi: 10.1016/j.bcp.2013.09.009
Tripathi, D., Singh, M., and Pandey-Rai, S. (2022). Crosstalk of nanoparticles and phytohormones regulate plant growth and metabolism under abiotic and biotic stress. Plant Stress 6, 100107. doi: 10.1016/j.stress.2022.100107
Wang, Q., Xu, S., Zhong, L., Zhao, X., and Wang, L. (2023a). Effects of zinc oxide nanoparticles on growth, development, and flavonoid synthesis in ginkgo biloba. Int. J. Mol. Sci. 24, 15775. doi: 10.3390/ijms242115775
Wang, X., Xie, H., Wang, P., and Yin, H. (2023b). Nanoparticles in plants: uptake, transport and physiological activity in leaf and root. Materials 16, 3097. doi: 10.3390/ma16083097
Wang, Y.-J., Zhang, H.-Q., Han, H.-L., Zou, Y.-Y., Gao, Q.-L., and Yang, G.-T. (2017). Taxifolin enhances osteogenic differentiation of human bone marrow mesenchymal stem cells partially via NF-κB pathway. Biochem. Biophys. Res. Commun. 490, 36–43. doi: 10.1016/j.bbrc.2017.06.002
Zhang, H.-Q., Wang, Y.-J., Yang, G.-T., Gao, Q.-L., and Tang, M.-X. (2019). Taxifolin Inhibits Receptor Activator of NF-κB Ligand-Induced Osteoclastogenesis of Human Bone Marrow-Derived Macrophages in vitro and Prevents Lipopolysaccharide-Induced Bone Loss in vivo. Pharmacology 103, 101–109. doi: 10.1159/000495254
Keywords: nanoparticles, silymarin, silybin, flavonoid, taxifolin, elicitation
Citation: Selc M, Macova R and Babelova A (2025) Nanoparticle-boosted silymarin: Are we overlooking taxifolin and other key components? Front. Plant Sci. 16:1628672. doi: 10.3389/fpls.2025.1628672
Received: 15 May 2025; Accepted: 30 July 2025;
Published: 15 August 2025.
Edited by:
Vladimir Orbovic, University of Florida, United StatesReviewed by:
Ahmad Faraz, Glocal University, IndiaCopyright © 2025 Selc, Macova and Babelova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michal Selc, bWljaGFsLnNlbGNAc2F2YmEuc2s=
 Michal Selc
Michal Selc Radka Macova
Radka Macova Andrea Babelova
Andrea Babelova