- 1Department of Agriculture and Environmental Sciences, Lincoln University of Missouri, Jefferson City, MO, United States
- 2Department of Biochemistry, University of Missouri, Columbia, MO, United States
- 3IR-4 Project Headquarters, North Carolina State University, Raleigh, NC, United States
- 4Alabama Cooperative Extension System, Alabama A&M and Auburn Universities, Normal, AL, United States
Industrial hemp (Cannabis sativa L.) is a multipurpose crop primarily grown for fiber, grain, and cannabinoids. Due to its high-quality protein and oil composition, industrial hemp grain is increasingly an important crop for a nutritional source. Although the global demand for hemp grain is increasing, research exploring the genetic and climatic effects on agronomic and seed composition traits is limited. Furthermore, there has been very little research conducted on seed development and shattering. Therefore, to study this biological phenomenon and for optimal hemp grain production in Missouri, suitable cultivars for this production region were compared to identify the best-suited ones. Key physiological and seed compositional traits were studied. We found significant variation in plant height, diameter, biomass, grain yield, crude protein, and crude fat among the evaluated varieties. The dual type variety, Futura 83, showed superior performance and yield and has many suitable traits for the Missouri production region, with the grain yield ranging from 2434–2793 kg/ha. In addition, we studied the expression of two candidate genes associated with seed shattering resistance and flowering. Both genes were expressed differentially among various hemp tissues. The expression of the GmPdh1 homolog gene was higher in mature seeds, and the GmDt1 homolog was higher in flower tissues, suggesting their potential role in seed dispersal and flowering. However, further research is required for functional validation and increasing the crop yield and seed composition.
1 Introduction
Industrial hemp (Cannabis sativa L.) is a versatile crop grown for fiber, seeds, and flowers (Deguchi et al., 2020; Vasantha Rupasinghe et al., 2020; Muedi et al., 2024). Legally, they are defined as the cannabis plant that has less than 0.3 percent Δ-9 tetrahydrocannabinol (THC) (Agriculture Improvement Act, 2018). Use of this plant is significant across multiple sectors, including agriculture, manufacturing, healthcare, and biofuel (Gülizar et al., 2025).
Further, they are categorized based on their primary use (Deguchi et al., 2020; Vasantha Rupasinghe et al., 2020). The four main types are fiber-type, grain-type, dual-type, and cannabinoid-type, which are characterized by their distinct plant phenotypes and uses. Fiber-type hemp is primarily grown for its fibers (Islam and Hasan, 2025). Similarly, grain-type hemp is cultivated for its grain, dual-type hemp, which is used for both grain and fiber production, while cannabinoid hemp is grown for its cannabinoid and other secondary metabolites (ElSohly et al., 2017; Das et al., 2020; Muedi et al., 2024).
Industrial hemp has nine primary growth phases: seed germination and emergence, leaf growth, lateral shoot formation, stem elongation, first flowering, full flowering, fruit development, fruit ripening, and senescence (Mishchenko et al., 2017; Petit et al., 2020a; Rahemi et al., 2021).
Grain yield is the key trait for grain and dual-type hemp varieties. The seeds are a nutrient-packed source of excellent protein that ranges from 24.20 to 30%. The protein content in hemp is higher than that of chickpea and on par with that of soybeans, which are commonly used plant-based proteins (Liu et al., 2023). In addition to proteins, they are a good source of saturated and polyunsaturated fatty acids. Likewise, they are also widely used in the cosmetic industry (Callaway, 2004; Cattaneo et al., 2021).
Although some industrial hemp varieties are monoecious, most of the hemp varieties are dioecious, having both separate male and female flowers (Hall et al., 2012; Petit et al., 2020a; Toth et al., 2020; Cattaneo et al., 2021). Flowering is a complex trait in hemp that is influenced by numerous genes and their interactions (Ma, 2005; Liu et al., 2010; Petit et al., 2020a). The candidate genes that sense and transmit light signals in the flowering pathway include cry1 (cryptochrome), phyA and phyE (phytochromes), spa1 (a regulator of phyA), uvr8 (a UV-B receptor), and xap5 (a circadian regulator of light responses). Similarly, several transcription factors such as bed, bZIP, constans, floricaula, leafy, flowering locus D, flowering locus T, MADS-box genes, squamosa, and vrn1 are also related to flowering pathways (Petit et al., 2020). The circadian clock gene CsPRR37 in auto-flowering cannabis. Likewise, CsFT1 (LOC115700781), an ortholog of the Arabidopsis FLOWERING LOCUS T gene, was identified as a candidate gene controlling photoperiod-insensitive flowering in Cannabis sativa (Dowling et al., 2024). Similarly, circadian clock gene CsPRR37 is also related to flowering time in Cannabis sativa particularly as its role in repressing the FT gene (Leckie et al., 2024). In addition to these genes, one of the essential flowering genes is Centroradialis-like protein (CENLP) that causes shoot growth and flowering (Amaya et al., 1999). The Cannabis sativa CEN-like protein 2 (LOC115720824) is homologous to Glycine max Terminal Flower 1b (GmTFL1 b). The function of GmTFL1 b varies in different plant species. The function of the TFL1 gene is the suppression of flowering by repressing the role of florigen FT, which is responsible for the transition to the reproductive phase from the vegetative phase (Wickland and Hanzawa, 2015). In soybeans, the gene GmTFL1 b is a key regulator of stem termination, influencing whether the plant exhibits a determinate or indeterminate growth habit (Quach and Nguyen, 2015; Mohamedikbal et al, 2024). Additionally, knockout mutants tfl1c/tfl1d flowered earlier than the wild-type plants (Wang and Li, 2008). Similarly, in Arabidopsis, the TFL1 gene is the homolog of the GmTFL1b gene. In the tfl1 mutant, genes promoting floral identity are expressed prematurely, leading to early flowering and showing conversion of the shoot apical meristem into a terminal flower (Hanano and Goto, 2011). While several photoperiodism and flowering genes have been previously characterized in hemp, such as CsFT1, CsPRR37, and Autoflower1, our study focused on CsCENLP to explore an additional gene. Investigating this gene allows us to complement existing knowledge and uncover potentially new genes that regulate flowering time. Furthermore, qPCR enables us to directly assess whether its expression patterns correlate with flowering phenotypes.
While seed shattering is an essential trait for natural seed dispersion, it is one of the major issues in industrial hemp, where it reduces the total grain yield by 40-80% (Rahemi et al., 2021). A complex network of interacting genes governs seed dispersal. Generally, this phenomenon is a dominant trait in plants such as rice, wheat, cowpea, and turnip rape. In sorghum, the key shattering gene encodes a YABBY transcription factor. Its homologous genes in rice cause seed shattering by forming an abscission layer between the pedicel and spikelet (monocots) (Maity et al., 2021). In soybeans, seed shattering is closely linked to the dehiscence zone (DZ) characteristics between cells in the seed pod valve. Seed shattering is primarily driven by the loss of adhesion between cells in DZ. This loss of adhesion is influenced by two key enzymes: polygalacturonase (PG) and cellulase (CE). PG breaks down the galacturonan chains of pectin, while CE targets cytoskeletal elements in the cell wall, facilitating the breakdown of the middle lamella (Marsh et al., 2023). GmPdh1 is one of the key genes responsible for seed shattering because the pod abscission layer of homozygous loss-of-function pdh1 mutants remains intact longer, significantly reducing seed shattering (Funatsuki et al., 2014). We selected GmPdh1 because of its well-characterized, tissue-specific role in regulating lignin deposition and its direct effect on pod shattering in soybean. Given the similarity of the shattering process in hemp, we hypothesize that Pdh1 may play a comparable role in seed shattering.
The global industrial hemp market value is anticipated to increase and is expected to achieve a market valuation of $36 billion by 2026 (Dhoubhadel, 2021). Although grain and dual-type hemp have tremendous potential across multiple sectors, one of the major bottlenecks is the lack of reliable varieties for regional cultivation. Furthermore, most hemp varieties are imported from Europe, Canada, and China. And the lack of regional testing of the U.S.-based varieties presents another major challenge since there is climatic dissimilarity between these agroclimatic regions. Thus, there is a dire need for thorough varietal screening for selection (Wimalasiri et al., 2021). Hence, our primary goal is to identify suitable hemp varieties for Missouri and the Midwest production region.
2 Materials and methods
We compared the morphological traits of grain-type and dual-purpose hemp varieties, using a fiber-type variety as a positive control, which has been named the check variety in the results section. The check fiber variety was based on the previous studies conducted in the other part of the United States and the own Field Trial at Lincoln University of Missouri (Cornell Hemp Report, 2020). Since this is an early-stage experiment for the Missouri production region, we used two replications in this initial study and continued the research over a period of three years to assess consistency and performance.
2.1 Plant materials
We selected nine varieties in 2022 based on their suitability in other parts of the United States and their global performance (Rahemi et al., 2021; Cornell Hemp report, 2020; Ferfuia et al., 2021). The varieties selected can be found in Table 1. In 2023, a total of 16 varieties were screened, and the details of origin and use can be found in Table 2. Likewise, their flowering and maturation times can be found in Table 3. Likewise, for the planting season 2024, 10 varieties were selected for the varietal screening. The selection of the varieties was based on the previous year’s field performance and the availability of seeds for the planting season. Similarly, naturally growing (wild-type) American ferals were collected from different parts of Missouri. Their seeds were sorted and grown in the field. Among these, LHV-17, one of the best-adapted wild types, was selected for comparative studies. LHV-17 was then compared with domesticated varieties.
2.2 Land preparation and planting
The field experiments were conducted at the George Washington Carver Farm, Lincoln University, Jefferson City, Missouri, in 2022, 2023, and 2024. The site is located at an elevation of 213 meters above sea level. The experiment was conducted using a Randomized Complete Block Design (RCBD) experimental layout in two replications. Because this was an early-stage, multi-year study to screen hemp varieties, using two replications was considered sufficient (Kempton and Gleeson, 1997). Planting was carried out using a Great Plains 1006 NT No-Till Compact Drill, with a plot size of 185.89 m² and row spacing set at 19.15 cm. Fertilizer was applied at a ratio of 75:20:30 nitrogen: phosphorus: potassium (N: P: K). Seeds were planted at a depth of 0.63 cm.
2.3 Field data collection
Weekly field data were collected for each season as indicated in Table 4.
2.4 Seed compositional analysis
2.4.1 Proximate analysis
A total of 25 g of seeds were harvested from each plot, with three technical replicates per treatment. Seeds were cleaned to remove debris and sent to the University of Missouri Agricultural Experiment Station Chemical Laboratories (ESCL) for proximate composition analysis. Crude protein was determined using AOAC Method 984.13 (A–D, 2006), crude fat using AOAC Method 920.39 (A), crude fiber using AOAC Method 978.10 (2006), and ash content using AOAC Method 942.05.
2.4.2 Fractionation and quantification of seed storage proteins
200 mg of seeds were ground using liquid nitrogen in a pre-chilled mortar and pestle. Total and soluble proteins were extracted following the Osborne fractionation method (Osborne, 1924), separating proteins into water-soluble (albumins), salt-soluble (globulins), and alcohol-soluble (prolamins) fractions. To extract water-soluble protein, 1 ml of 25 mM Tris was added to the ground sample, which was then vortexed and centrifuged for 15 minutes at 13,000 rpm. The supernatant was collected and quantified after centrifugation using the Bradford Assay (Thermo Scientific, California). Subsequently, a protein extraction solution containing 10 mM Tris-HCl (pH 7.5-8), 1 M NaCl, 0.1% (w/v) DTT, and 10 mM EDTA was used to extract salt-soluble protein. 1 ml of the extraction solution was added to the remaining solid. The sample was placed in an orbital shaker for 2 hours. Afterward, it was centrifuged for 15 minutes at 15,000 g at 4°C, and the supernatant was again quantified using the Bradford Assay. Finally, the remaining pellet was treated with 1 ml of 70% ethanol to extract alcohol-soluble proteins (prolamins), incubated for 1 hour with shaking, and centrifuged. All protein fractions were quantified separately using the Bradford Assay (Thermo Scientific, California) and combined to estimate total storage proteins.
2.5 Candidate gene analysis for flowering time and seed shattering
Variation in flowering time and seed shattering are some of the major bottlenecks for hemp cultivation, with economic challenges. To address these issues, we investigated candidate genes such as CsCENLP and CsDrrp2, which may be involved in developmental processes related to flowering and cell wall composition, factors that contribute to seed pod dehiscence and dispersal. Understanding the molecular basis of these traits is crucial for developing improved cultivars with uniform agronomic performance and reduced seed shattering.
2.5.1 Selection of candidate genes
Two candidate genes were selected in Cannabis sativa based on homology to the Glycine max genome from the Pink Pepper Cannabis genome. A predicted gene, annotated as disease resistance response protein 206 (CsDrrp2), showed the highest sequence identity to the soybean GmPdh1 gene involved in pod shattering. Similarly, CEN-like protein 2 (CsCENLP), located on Chromosome 2 (LOC115720824), was identified as a homolog of the soybean terminal flower 1b (GmTFL1b or Dt1) gene, which regulates flowering.
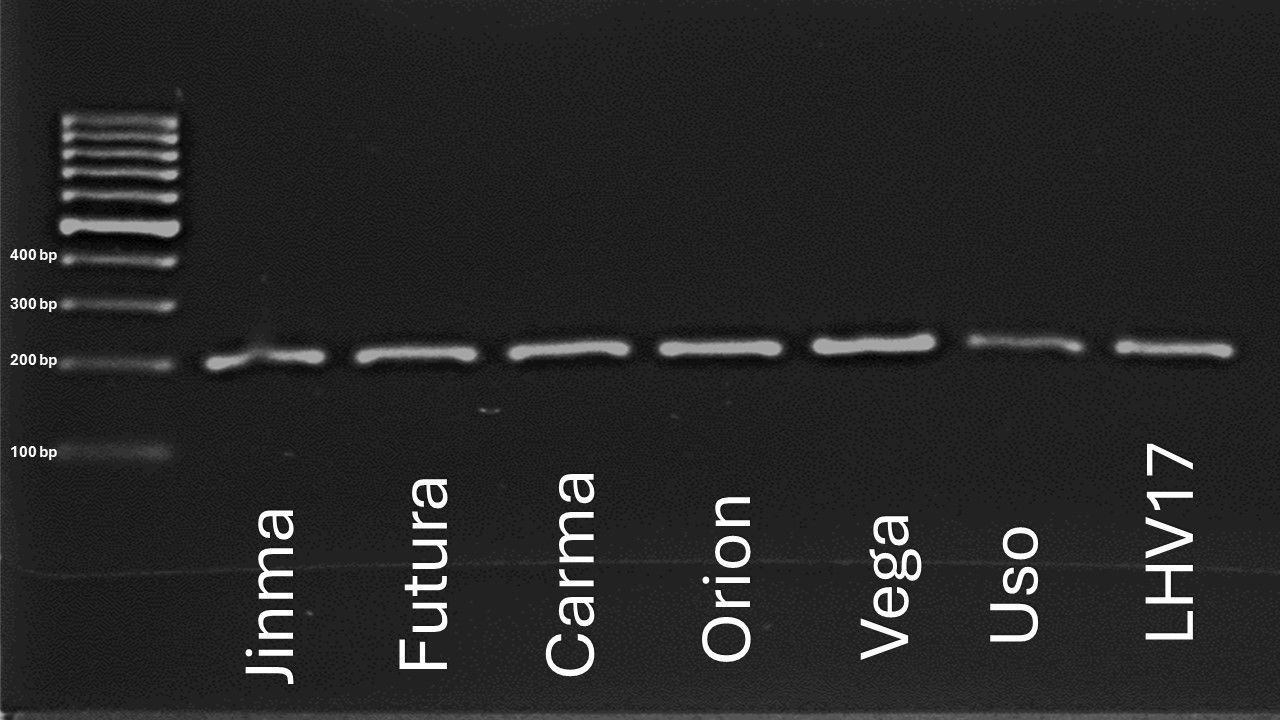
Gene-specific primers were designed for both candidate genes. The forward primer sequence for CsDrrp2 was CCCATTACACTCGACAACAATC, and the reverse primer was CCACCAACCGAAACATC. Similarly, the forward primer sequence for CsCENLP was GTGATAGGAGATGTGGTTGATGT, and the reverse primer was TGCTCCCTCAAATAAGGATCAC. The amplicon size for CsDrrp2 is 200 bp, while the male flower for CsCENLP is a 334 bp band, and the female flower is two bands of 334 and 380 bp.
2.5.2 Nucleic acid extraction
Plant tissues were collected at different developmental stages from the Carver field experiment and flash frozen. Total RNA was extracted using the TRIzol method according to the manufacturer’s instructions (Thermo Fisher Scientific, California). Genomic DNA was extracted using the modified CTAB method (Aboul-Maaty and Oraby, 2019). RNA and DNA concentrations and quality were quantified using a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific, California).
2.5.3 PCR
Polymerase chain reaction (PCR) amplification was performed using genomic DNA templates. The thermal profile was as follows: initial denaturation at 94°C for 5 minutes, followed by 35 cycles of denaturation at 94 °C for 30 seconds, annealing at 52 °C (CsDrrp2) and 47 °C (CsCENLP) for 30 seconds, and extension at 72 °C for 1 minute, with a final extension at 72 °C for 7 minutes. The amplicons were visualized on 1.5% agarose gels.
2.5.4 qRT-PCR analysis
First-strand cDNA synthesis was performed using the Thermo Fisher manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was conducted in a 15 µL reaction containing 1.5 µL of synthesized cDNA, 7.5 µL of SYBR Green Master Mix, 1.5 µL of a mixed 2 µM primer solution (forward and reverse primers for CsDrrp2 and CsCENLP), and 4.5 µL of nuclease-free water (Biorad MJ mini, California). The thermal cycling conditions were as follows: initial denaturation at 95 °C for 3 minutes, followed by 34 cycles of denaturation at 95 °C for 30 seconds, annealing at 52 °C (CsDrrp2) and 47 °C (CsCENLP) for 30 seconds, and extension at 72 °C for 30 seconds, with a final extension at 72 °C for 7 minutes.
Relative expression levels were calculated using the 2^−ΔΔCt method. The α-tubulin was used as the internal reference for normalization (Deguchi et al., 2021). Each reaction was performed in three biological replicates.
2.6 Data analysis
Statistical analysis was performed using RStudio version 4.3.2. Data from the 2022, 2023, and 2024 seasons were examined separately using one-way analysis of variance (ANOVA). Differences between means for each year were separated using Tukey’s Honest Significance Difference (HSD) test. A probability level of P< 0.05 was considered significant for F-statistic values and mean comparisons. Graphing was done using the ggplot command from the ggplot2 package in R (R Development Core Team, 2020). Only for the varieties that were grown in all three years, treatment x season interaction was performed using two-way ANOVA in R Studio.
3 Results
3.1 Germination and emergence percentage
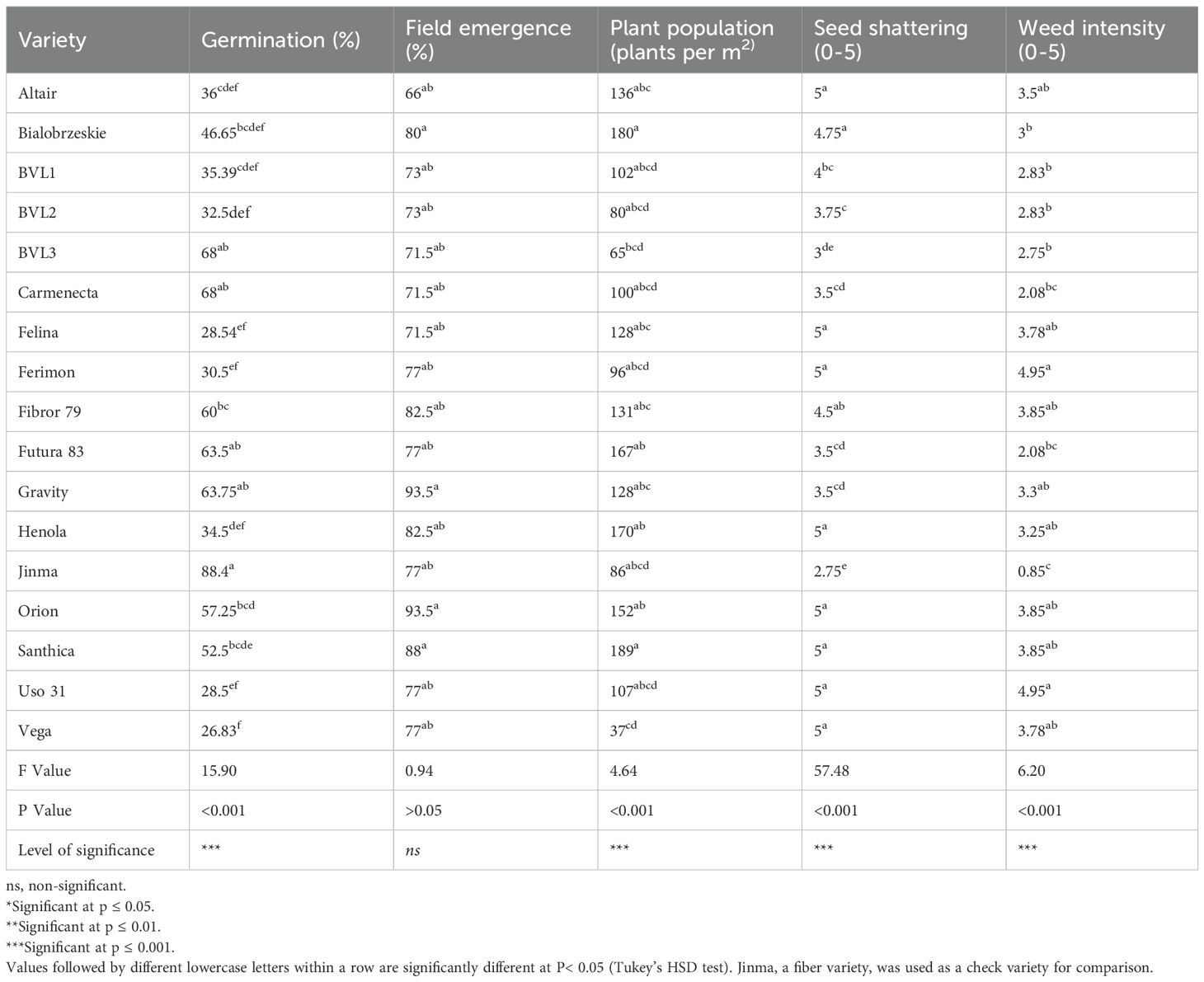
There was significant variation in germination percentage among the varieties in the greenhouse conditions (P< 0.001). Carmenecta had the highest germination percentage at 68%, followed by BVL3 and Gravity. On the other hand, Vega had the lowest germination percentage at 26.83% (Table 5).
However, under field conditions, all the varieties displayed emergence percentages ranging from 66% to 93.5% (Table 5). The variation among the genotypes at the field level was not statistically significant (P > 0.05). Gravity and Orion demonstrated the highest emergence percentage of 93.6%. In contrast, Altair had the lowest emergence percentage (66%), followed by Felina (70%) (Table 5).
3.2 Growth pattern and final plant height
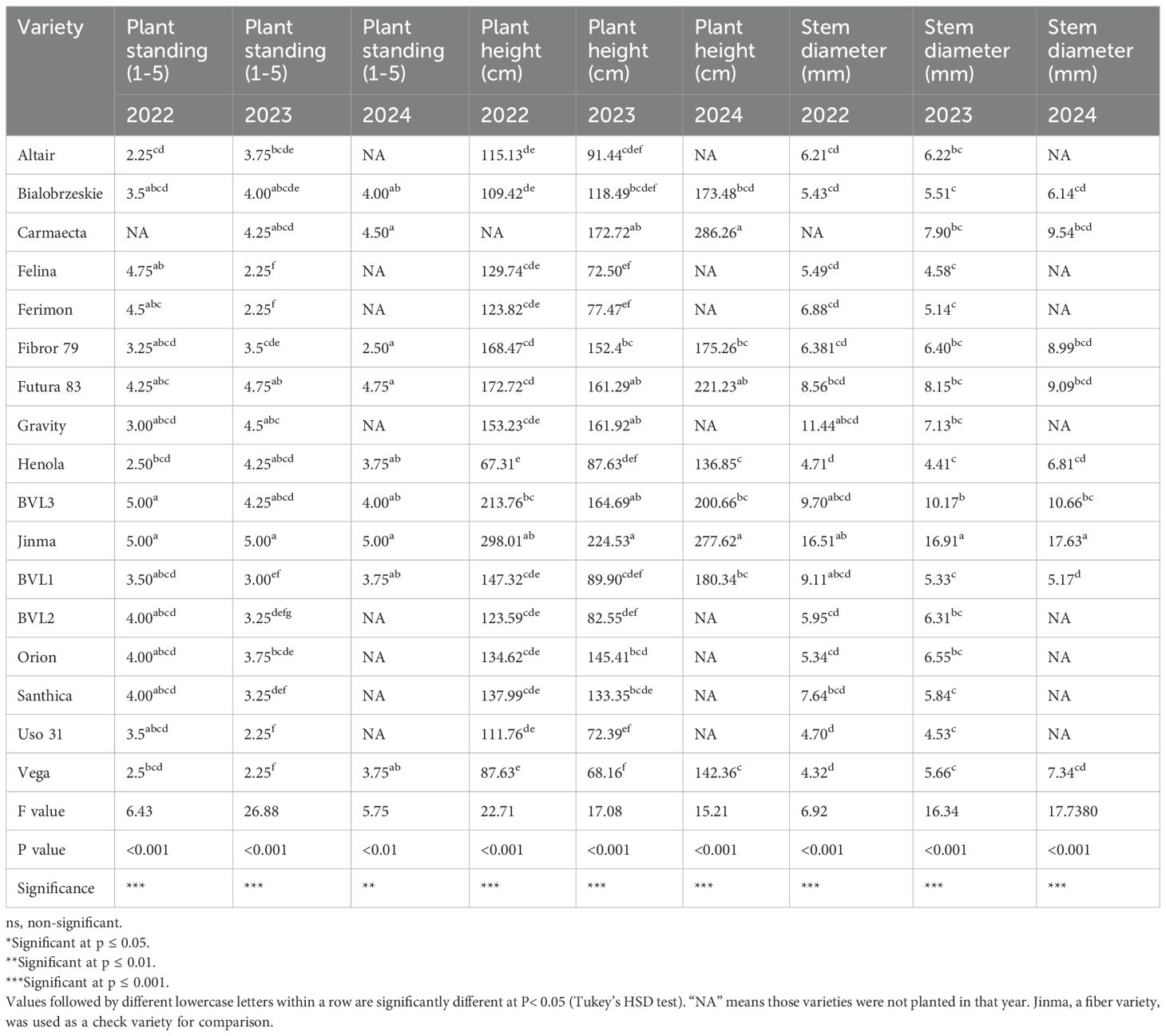
The growth pattern from the emergence until harvesting among the different hemp varieties is illustrated in Figure 1. Grain type varieties continued growing till 5th week of sowing, after which they transitioned to the flowering stage from the vegetative stage. After the flowering stage, grain-type cultivars Vega, Felina, BVL1, BVL2, Ferimon, Uso-31, and Altair showed minimal growth. Unlike grain-type varieties, dual-type and fiber-type varieties continued growing until the 10th week, providing evidence of longer maturation time (Figure 1).
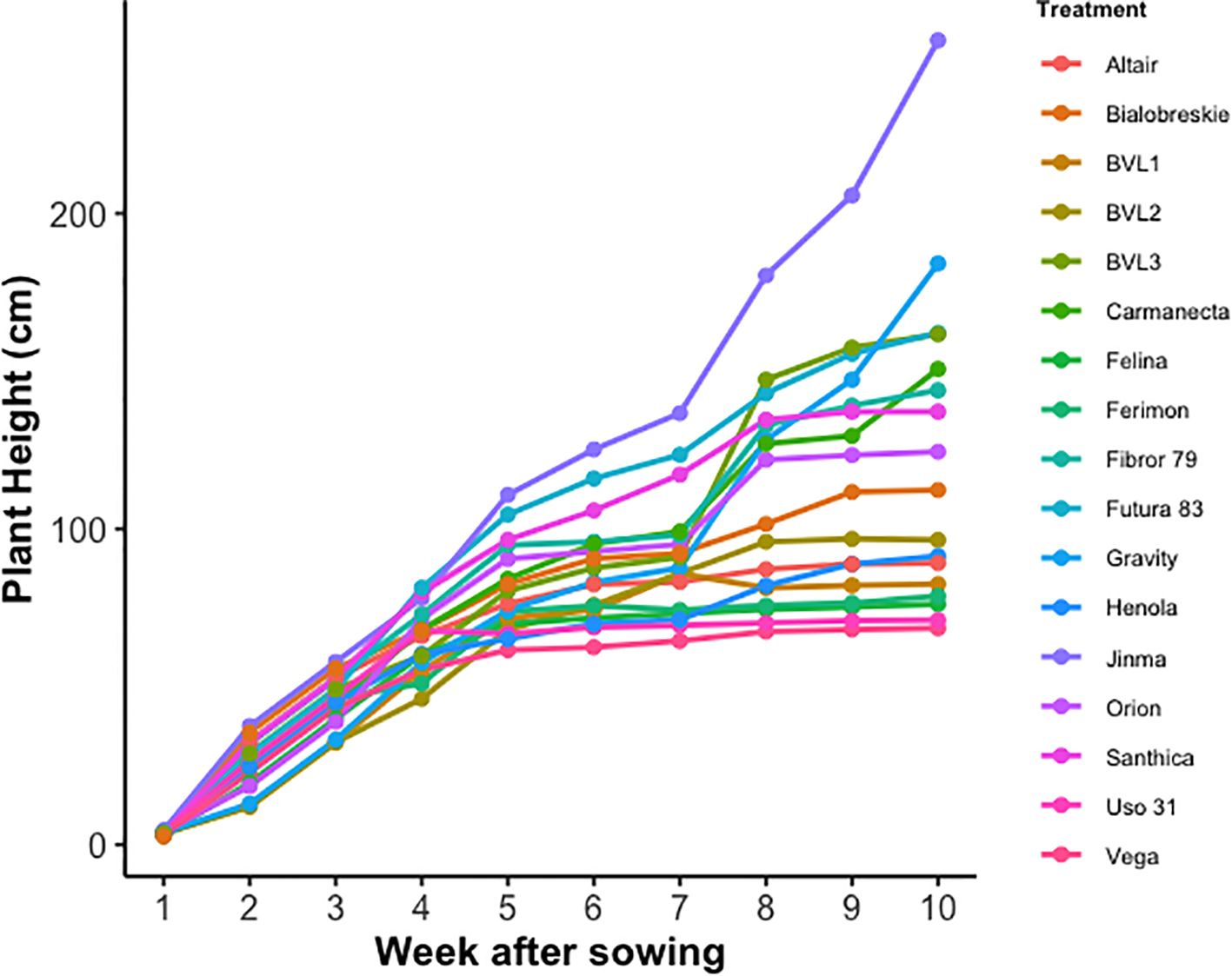
Figure 1. Time series measurement of plant height among fiber, grain, and dual-type industrial hemp varieties. The plant height within each week is the mean of the two replications studied.
Plants reach the final plant height (FPH) when they cease to grow vertically. FPH is a key plant performance trait that is correlated with plant biomass, lodging, and yield. Furthermore, it is essential for compatibility with mechanized harvesting. The study demonstrated the varietal effect on plant height among different hemp varieties (P value<0.001). The detailed plant height can be found in Table 1. BVL3 had the highest plant height, 164.59 cm, among the dual-type varieties tested for the year 2023. Similarly, Altair had the highest plant height of 91.44 cm among the grain-type varieties for the year 2023. On the other hand, Vega (68.07 cm) had the shortest plant height for the same year (Table 1).
3.3 Plant standing and plant population
For the planting seasons 2022/2023/2024, Futura 83 displayed the highest plant standing, indicating its robust field performance (Table 1). On the contrary, grain-type varieties, namely Ferimon, Uso-31, and Vega, displayed the lowest plant standing scores in all these years. While the plant standing provides some insight into the overall qualitative traits of the plants, future studies should incorporate more physiological measurements, such as chlorophyll content and photosynthetic rate, for improved precision. Based on the number of plants per square meter, Santhica (190 plants/m2) and Bialobrzeskie (180 plants/m2) had the highest plant population (P< 0.01) (Table 5).
3.4 Weed intensity
All the tested varieties displayed some degree of weed infestation. The dual-type varieties, such as Futura 83 and Carmenecta, had lower weed infestations per plot (weed infestation scores of 2.08 and 2.5) (Table 5). This still means weeds covered more than 45% of the plot, a considerable weed pressure. Conversely, the grain varieties were highly susceptible to weed infestation, with Ferimon, Uso-31, and Vega displaying the highest weed infestation. Weeds covered more than 80% of the plots grown with grain-type varieties. The major weed we found was cocklebur (Xanthium strumarium) and other weeds we found were Goose grass (Eleusine indica (L.) crab grass (Digitaria sanguinalis (L.) Scop and other monocots.
3.5 Stem diameter
We found significant stem diameter variation across industrial hemp varieties in three years (P value<0.001). Jinma (17.63 mm) had the largest stem diameter among the tested varieties in all three years. Among dual-type and grain-type, BVL3 had the largest stem diameter of 10.66 mm for the planting season 2023. In contrast, Henola, a grain-type variety, had the smallest stem diameter, 4.42 mm (Table 1). Like plant height, a discernible pattern was observed in the stem diameter across different hemp types. Fiber variety exhibited the largest diameters, followed by dual-type and grain-type varieties.
3.6 Stomatal conductance
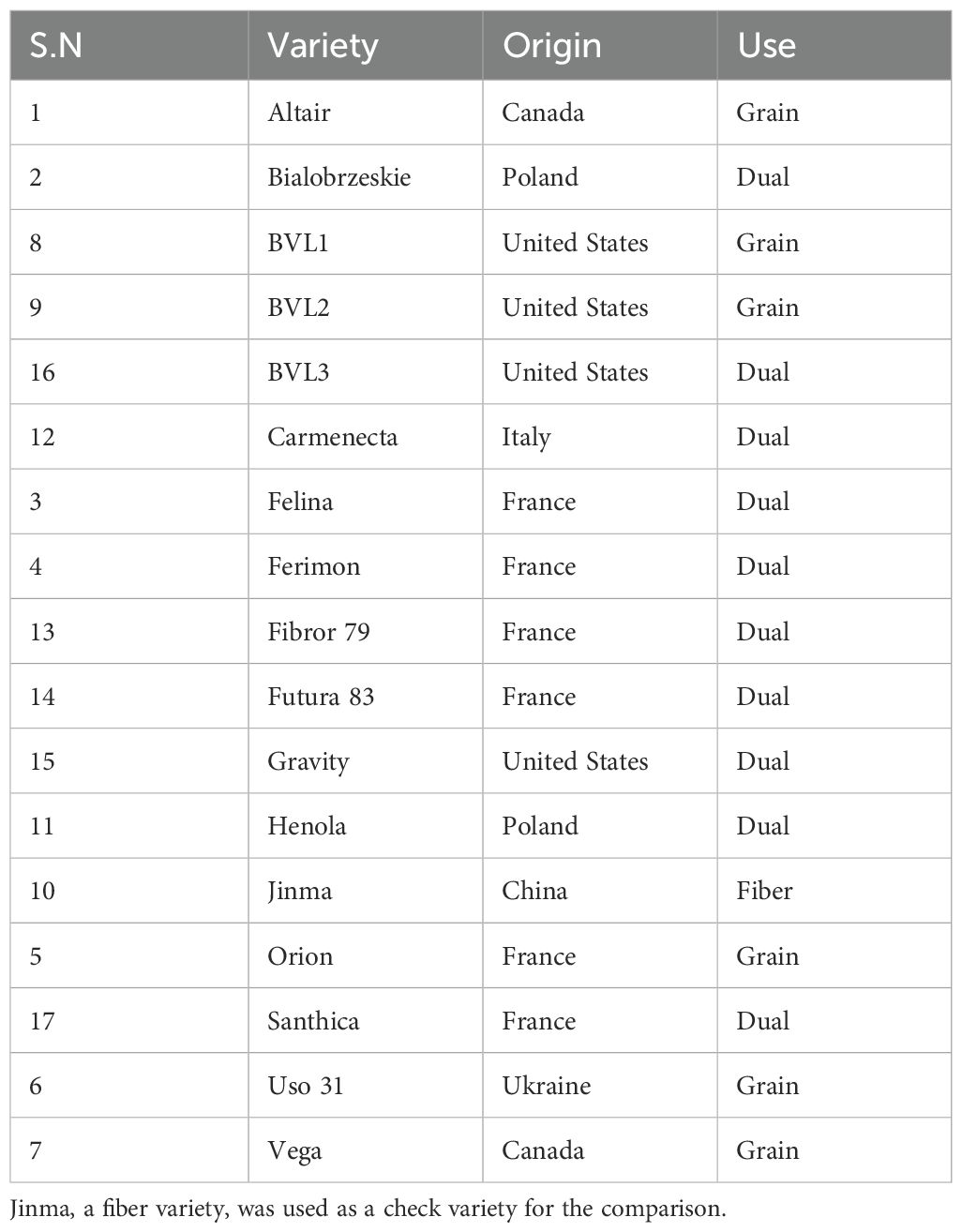
We observed that stomatal conductance (gs) increased with increasing apparent transpiration (Figure 2A). A positive linear relationship was observed between stomatal conductance and apparent transpiration rate, with an R² value of 0.57 (P< 0.001), indicating 57% of the variation in transpiration is explained by changes in stomatal conductance. Our result aligns with physiological expectations: higher stomatal conductance leads to more water loss through transpiration. Additionally, we found that the stomatal conductance decreased with increasing vapor pressure deficit (Figure 2B). These two variables had a weak relationship (R² = 0.28), meaning only 28 percent of the variation in the stomatal movement in the plants is due to Vapor Pressure deficit (VPD) (P value<0.05).
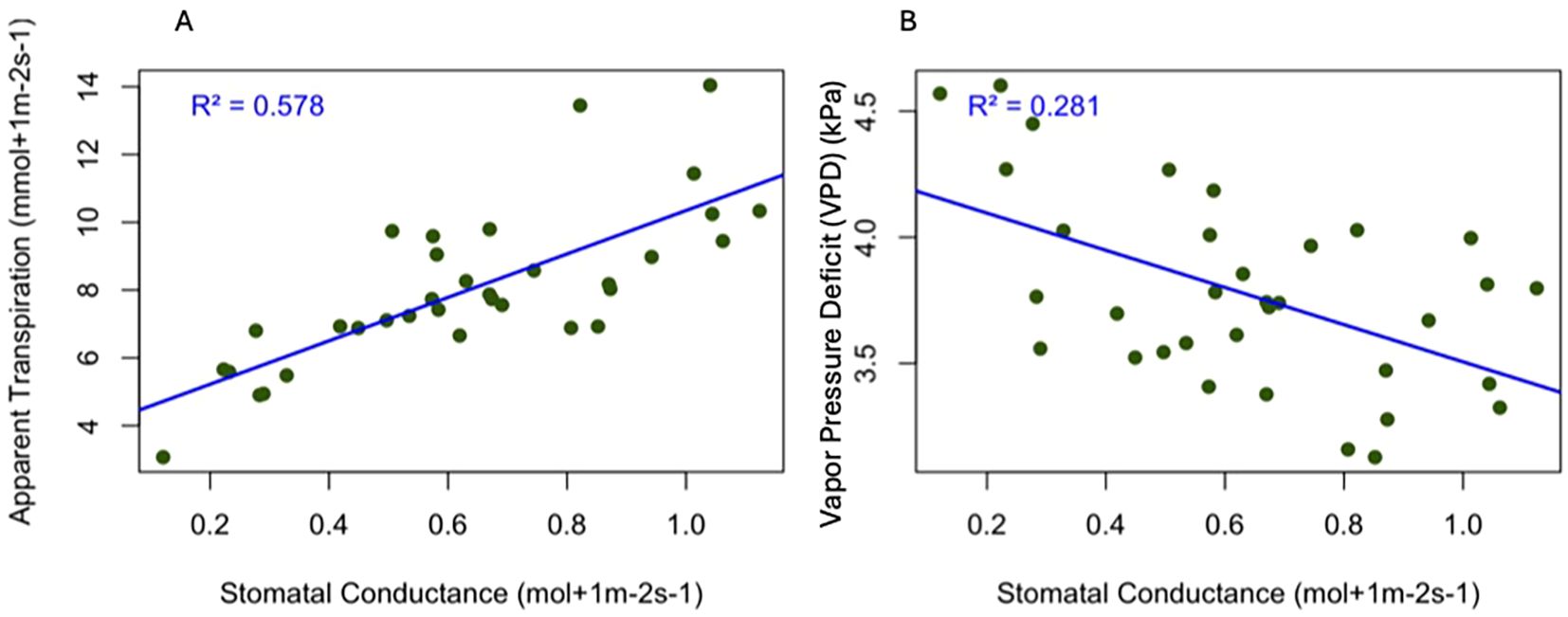
Figure 2. Relationship between stomatal conductance and physiological traits in the evaluated varieties in the hemp plants during the maturation stage. The blue line represents the regression line with data points in green (A) gs vs apparent transpiration (R² = 0.578, p< 0.001) (B) gs vs vapor pressure deficit (R2 = 0.281, p<0.05).
3.7 Flowering and maturity traits
Seeds are considered mature when their moisture content reaches between 18% and 20%. We found that most of the grain varieties flower and mature earlier than dual-type and fiber-type varieties under the photoperiodic conditions (Table 3). Additionally, male plants flowered 15 to 20 days earlier than female plants (Table 3). Ideally, industrial hemp should be harvested when the seed moisture content is 20-30% for optimal yield. Most of the grain-type varieties matured in 60 to 75 days (Table 3). However, two grain type varieties originated from the USA had an intermediate flowering (60–65 days) and maturation time (115–120 days). Likewise, dual-type varieties took 115 to 120 days to mature (Table 3). Varieties such as Carmenecta, Gravity, BVL1, and BVL2 had a higher ratio of male plants; nevertheless, they had consistent good yield (Table 3 and Table 6).
3.8 Biomass
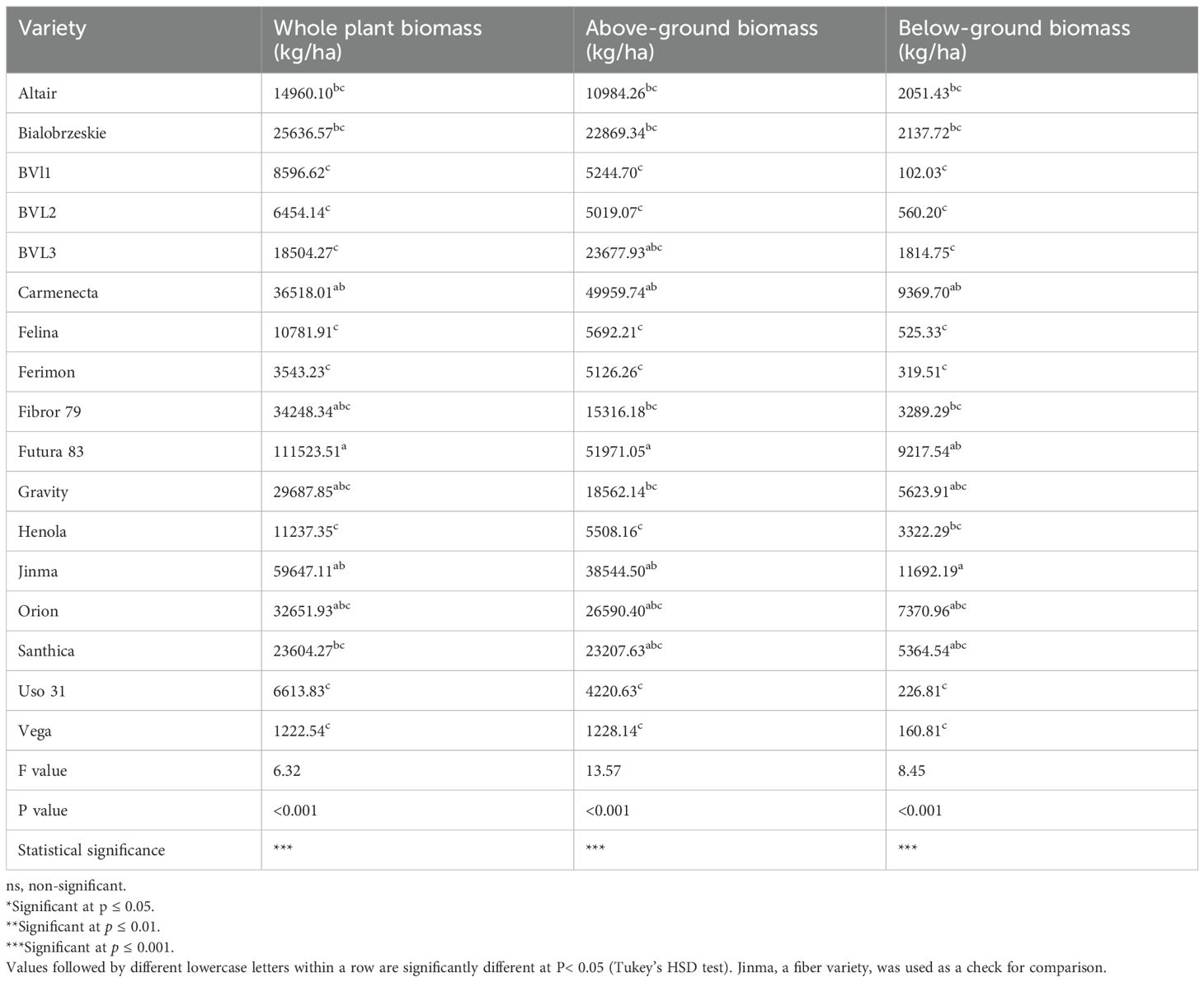
There was a statistically significant difference in the whole plant, above-ground, and below-ground biomass among the varieties studied (P value< 0.001). Based on this study, Futura 83 had the highest biomass, 111523.51 kg/ha, followed by Carmenecta. On the other hand, Vega had the lowest biomass (Table 7). Likewise, aboveground biomass, which provides the biomass for actual use, also differed significantly among the evaluated varieties (P value<0.001), with the variety Futura 83 posing the highest above-ground biomass. Likewise, below-ground biomass was highest on the dual type variety Carmenecta, comparable statistically with the check fiber type variety.
We performed linear regression analyses on whole plant biomass against other key traits influencing this characteristic. We found that stem diameter, final plant height, and below-ground biomass strongly correlated with the whole plant biomass, with R-squared values greater than 70% (Supplementary Document 1-File 9).
3.9 Grain yield
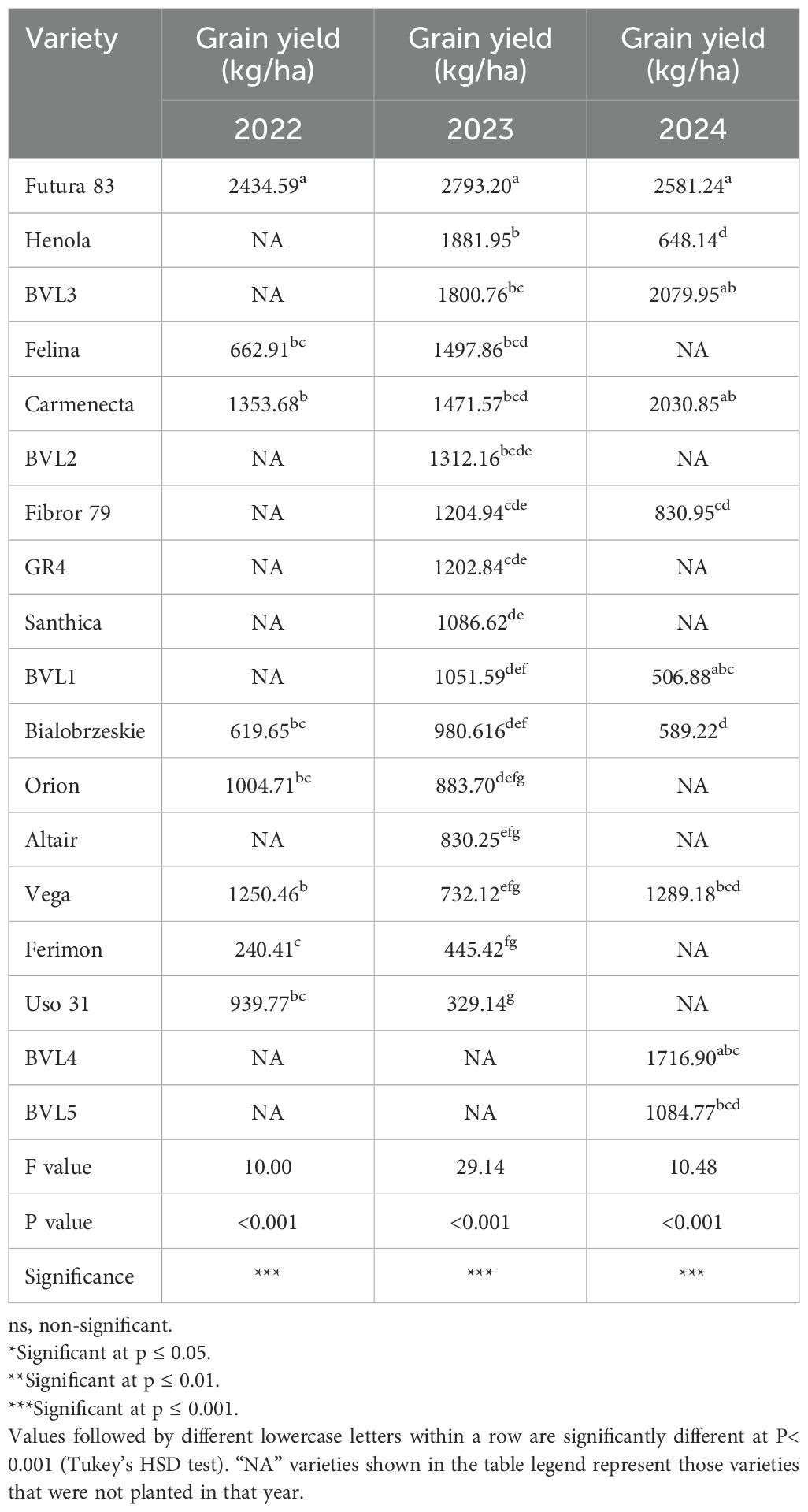
We conducted a multi-year varietal trial to screen suitable varieties for the Midwest production regions and to identify parental lines for breeding purposes. 2022 was the starting year for our research, and given the limited seeds available, we screened nine varieties. During the 2022 planting season, the highest grain yield among eight evaluated varieties was from Futura 83, a French-origin variety, with a grain yield of 2434 kg/ha. The field trial continued in 2023 with a total of 16 varieties, including Futura 83 and other good-performing varieties. During the 2023 planting season, the Futura 83 again achieved the highest grain yield of 2,793 kg/ha. There was an increase in the grain yield compared to 2023. Similarly, in 2024, among the 10 varieties, Futura 83 consistently had a higher grain yield compared to other varieties, but it was lower than in 2023, with a grain yield of 2581 kg/ha (Table 6). Despite different climatic conditions in multiple years (Supplementary Materials Document 1-file 2), Futura 83 had a consistent grain yield on average of 2602 kg/ha for three consecutive years, suggesting stable grain production. In contrast, grain varieties such as USO-31, Ferimon, and Bialobrzeskie had lower grain yield in all the planting years (Table 6).
In addition to Futura 83, BVL3, a USA-origin variety, showed good grain yield in 2023 and 2024. The grain yield for 2023 and 2024 was 1800 kg/ha and 2079 kg/ha, respectively. Additionally, it has a high plant biomass (22.26 g/plant). The percentage of male flowers in Futura 83 is very low, the male percentage in BVL3 is 45%.
3.10 Seed compositional trait
3.10.1 Crude protein and total seed storage protein
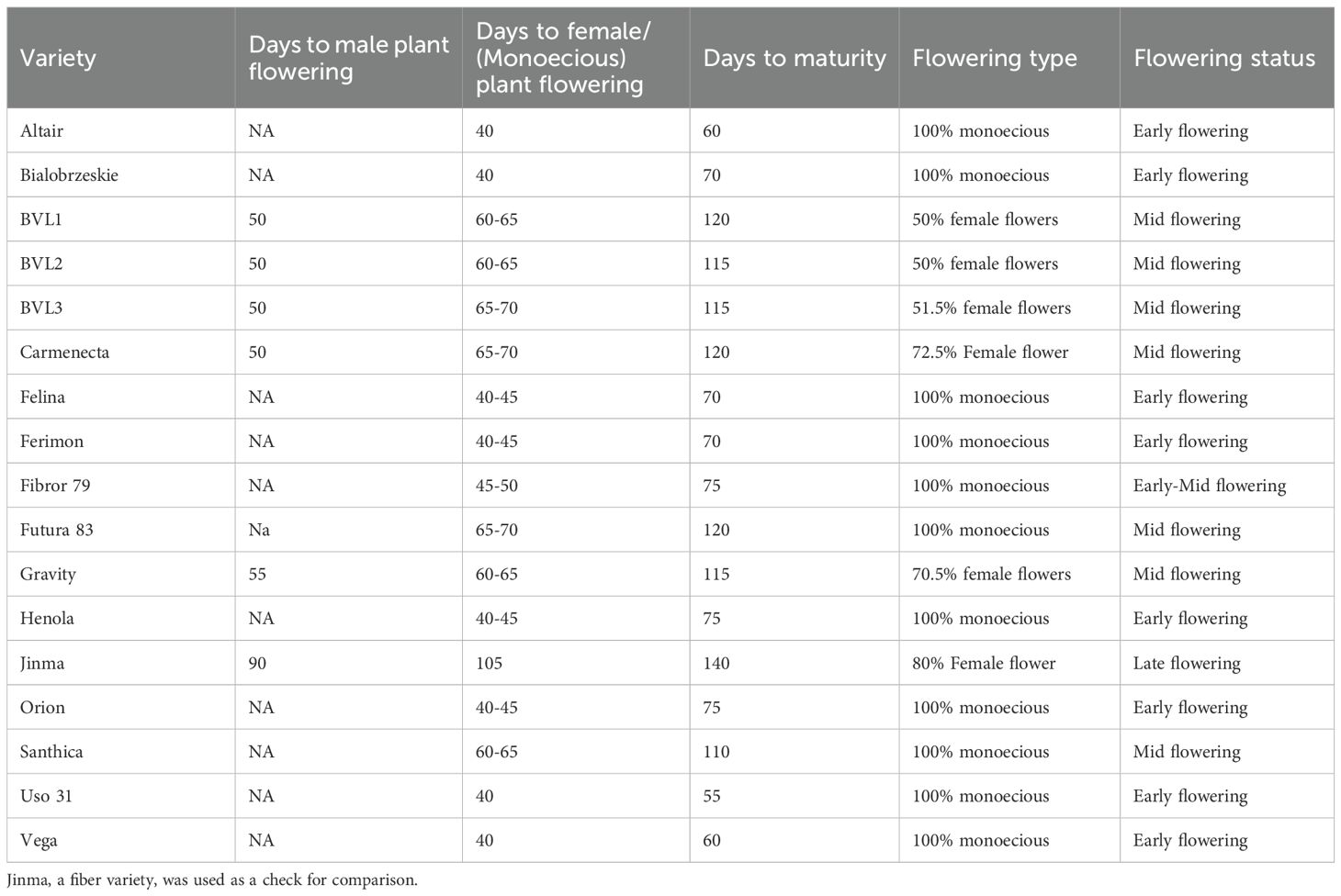
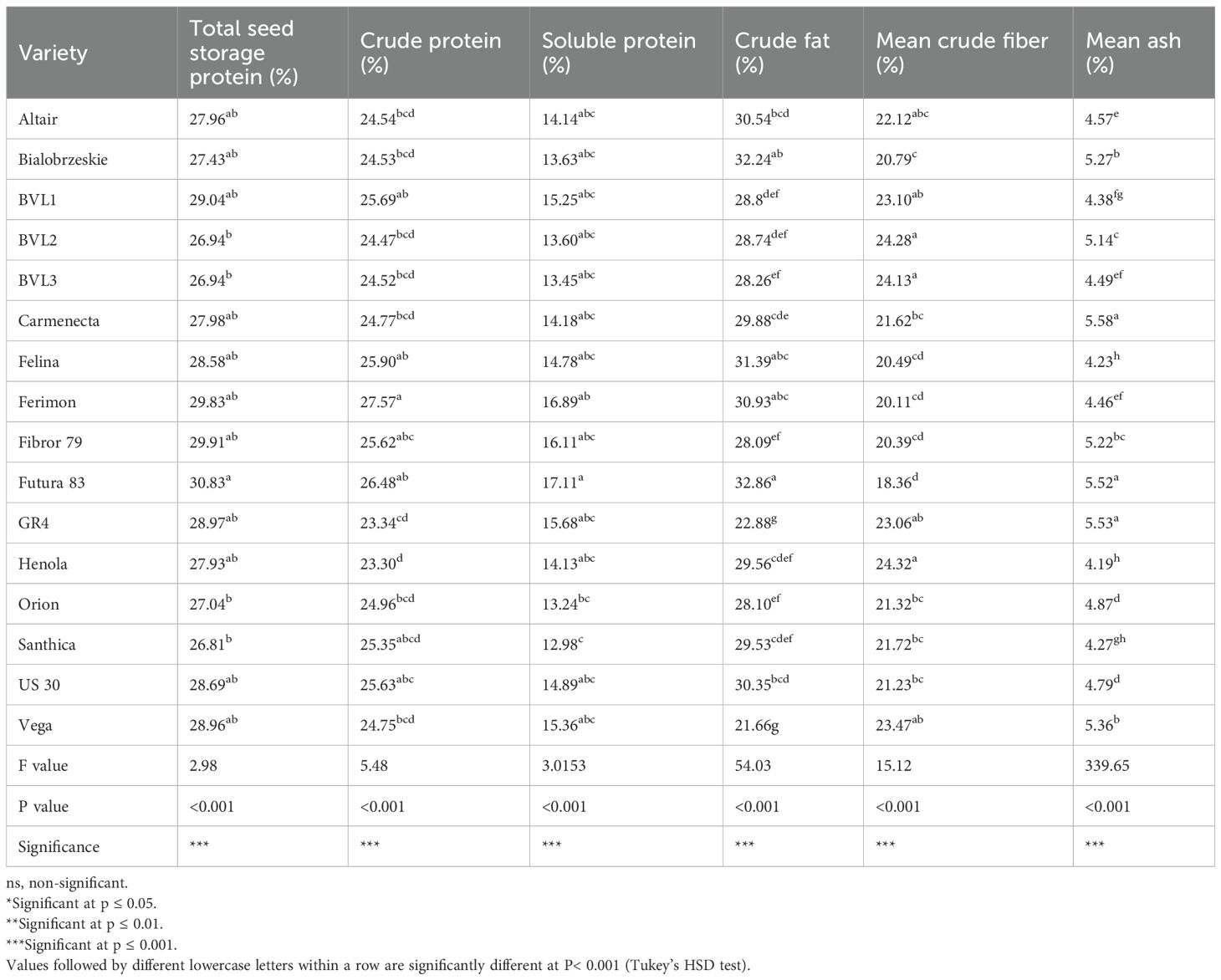
The statistical test revealed a significant difference in seed proteins among the evaluated varieties (P-value<0.001). We found that the crude protein ranged from 23% to 27% on a dry seed basis. Ferimon (27.57%) and Futura 83 (26.48%) had the highest crude protein content. Similarly, the total seed storage protein ranged from 26.81% to 30.35% per gram of dry seeds. The water-soluble protein concentration ranged from 12.98% to 17.11% per gram of dry seed. Among the varieties studied, Futura 83 had the highest soluble protein content of 17.12%, followed by Ferimon, 16.89% (Table 8). Among the seed compositional traits, crude protein had relatively less variability among the tested varieties compared to crude fat and ash content (Table 8 and Figure 3).
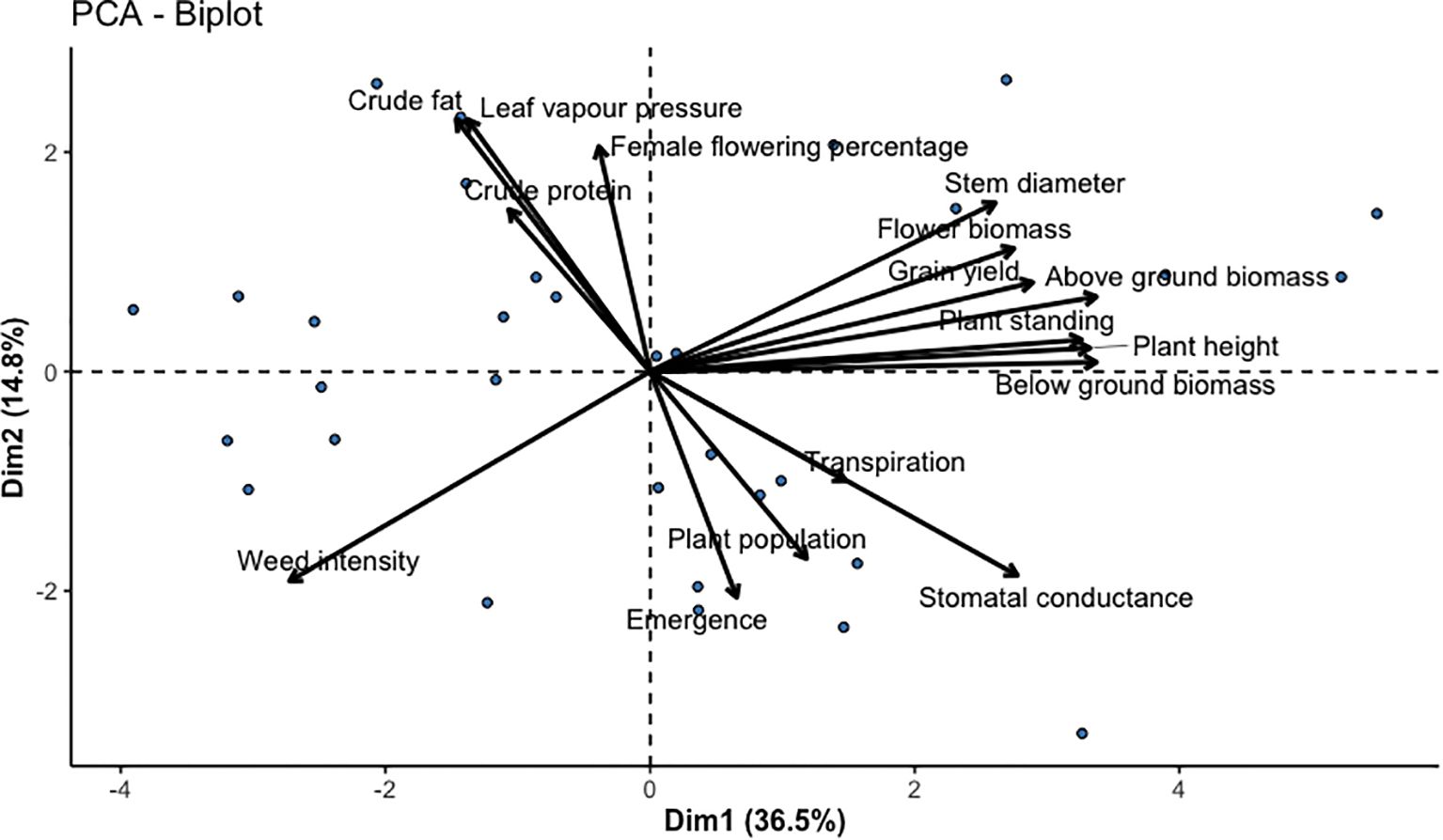
Figure 3. PCA biplot of 16 grain and dual-type industrial hemp varieties. The principal component analysis (PCA) of yield and plant performance traits.
3.10.2 Crude fat
Likewise, crude fat content significantly varied among the varieties (P<0.001). Crude fat in the evaluated varieties ranged from 21.66% to 32.86%. The highest crude fat content was found in Futura 83 (32.86%) and Bialobrzeskie (32.24%), which are primarily grown for grain yield, and the lowest crude fat content was seen in Vega (21.66%) (Table 8). Compared to protein, which exhibited moderate variability, crude fat showed greater phenotypic variability (Table 8 and Figure 3).
3.10.3 Crude fiber and ash
Similarly, there was also a significant variation in crude fiber and ash content across varieties (P< 0.01). The highest crude fiber content was found in BVL2 (24.28%), Henola (24.32%), and BVL3 (24.13%) (Table 8). Ash is made up of inorganic compounds and determines the total mineral content of a sample. Our result suggests the ash content to be highest in Carmenecta (5.58%) and GR4 (5.53%) (Table 8), which are both dual-type varieties.
3.10.4 Principal component analysis of the evaluated agronomic traits
Principal component analysis (PCA) was employed to evaluate the multivariate structure of 4 yield-related and 11 plant performance traits across diverse hemp varieties. PC1, explaining 38.8% of the variation, was predominantly influenced by above-ground biomass, whole plant biomass, below-ground biomass, and harvestable plant height, indicating that biomass accumulation and plant height are key drivers of inter-varietal differences (Figure 3). PC2, accounting for 14.6%, was shaped by plant population, emergence percentage, and stomatal conductance, suggesting that early vigor and physiological efficiency also play essential roles in varietal performance. The PCA biplot (Figure 3) illustrated the strength and direction of trait contributions, with closely grouped vectors indicating positive correlations, particularly among biomass-related traits. In contrast, opposing vectors highlighted negative trait associations, such as between emergence and weed percentage. PCA-reduced data were subjected to cluster analysis to explore phenotypic variation further, resulting in three distinct varietal groups visualized with convex hulls (Supplementary Document 1-File 18). Overall, this combined PCA provided a robust framework for selecting hemp genotypes with desirable agronomic traits and informs targeted breeding strategies for biomass, plant vigor, or physiological resilience.
3.10.5 Pearson’s correlation among agronomic traits
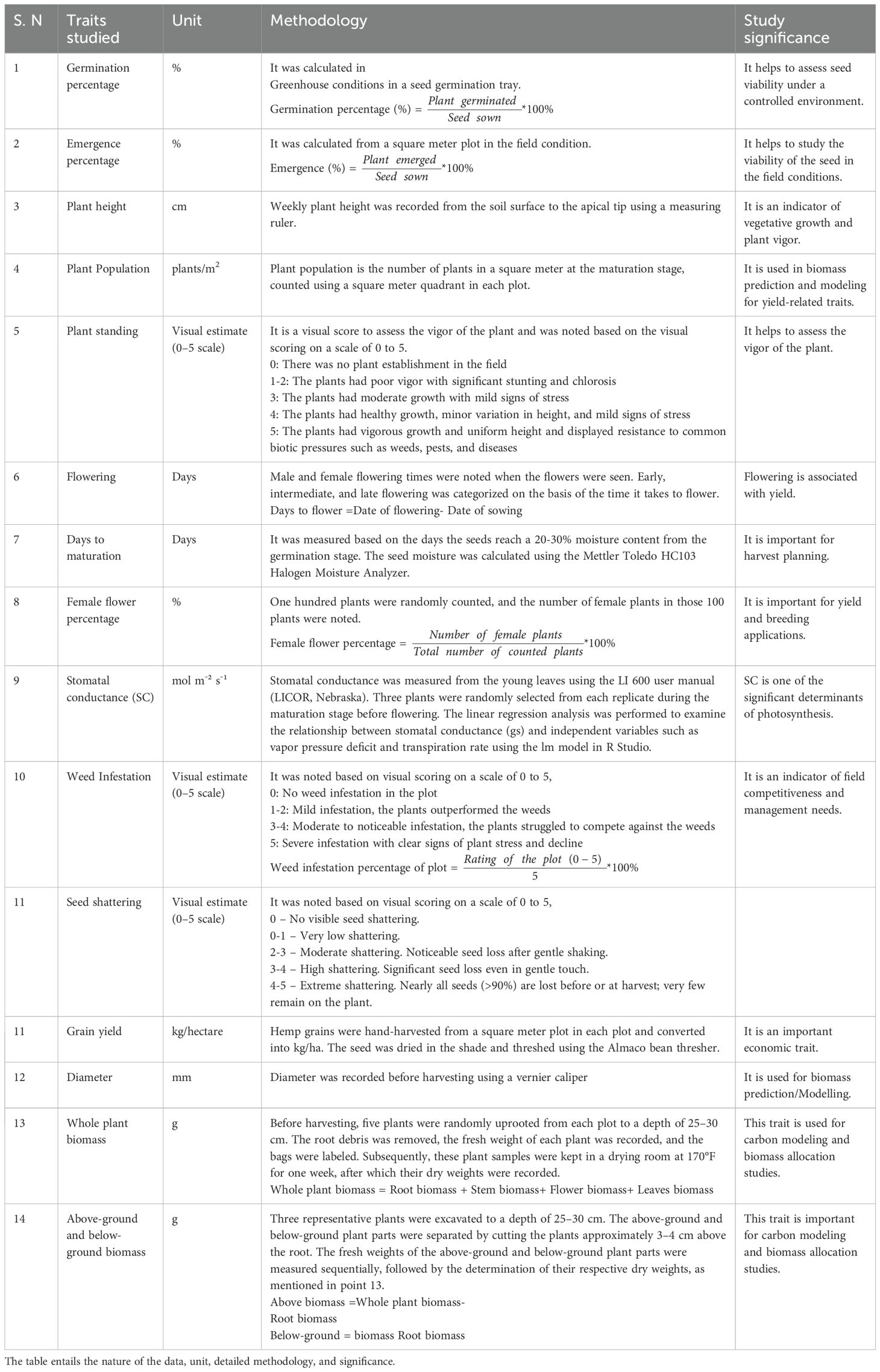
Grain yield, aboveground biomass, crude protein, and crude fat are economically essential traits in industrial hemp. In our study, grain yield was highly correlated with aboveground biomass (r = 0.72) and plant standing ability (r = 0.63). It also showed a moderate but significant positive correlation with plant height (r = 0.47) and below-ground biomass (r = 0.45). These findings suggest vigorous plants with greater aboveground biomass produce higher grain yields (Figure 4).
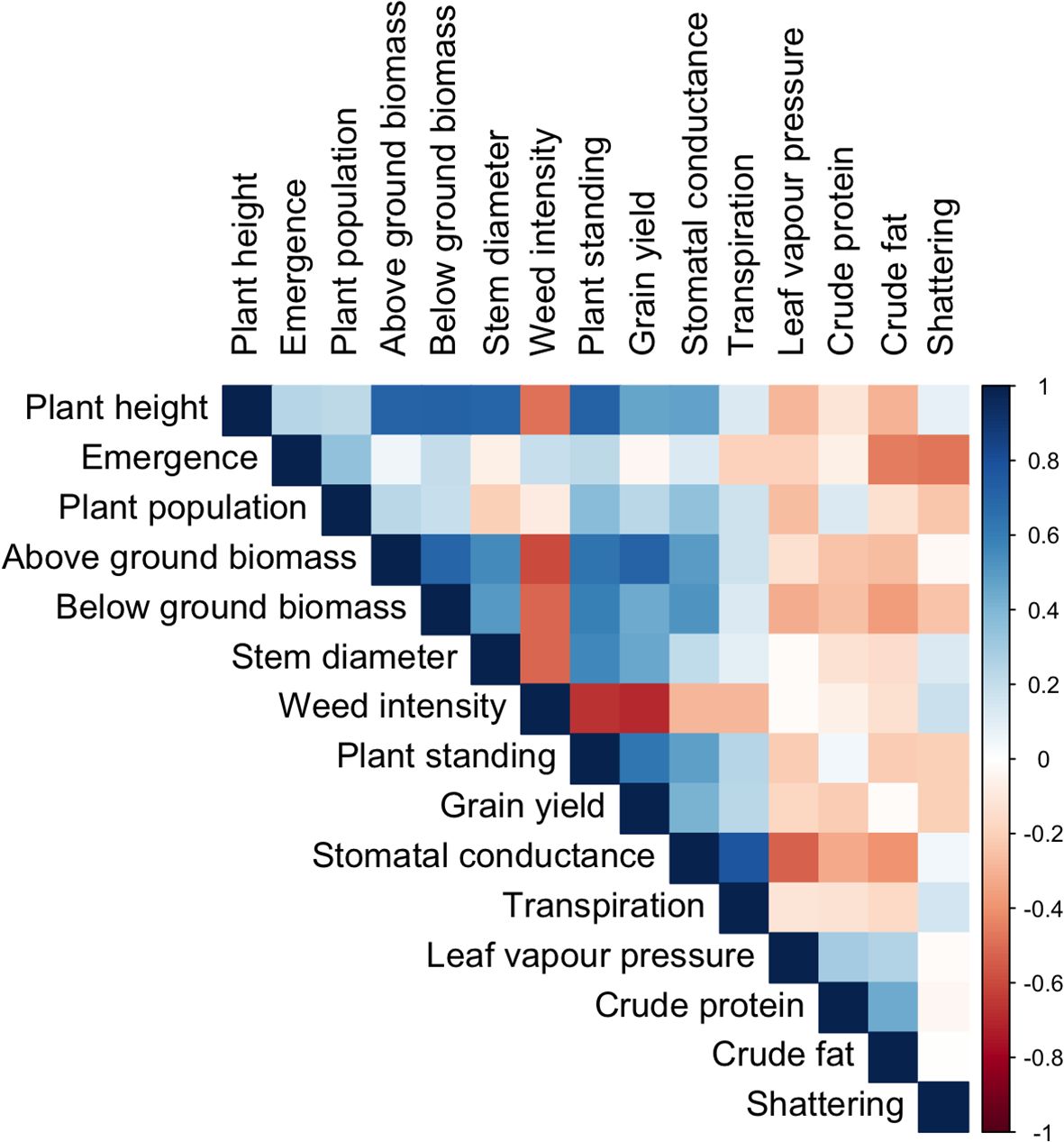
Figure 4. Pearson’s correlation matrix (r) shows an association between key physiological, agronomic, and seed compositional traits. ns p>0.05; *<0.05,**<0.01; and ***<0.001.
Aboveground biomass was also strongly correlated with plant height (r = 0.72), below-ground biomass (r = 0.71), and stem diameter (r = 0.56). In addition, stomatal conductance was moderately correlated with plant height, aboveground biomass, below-ground biomass, and plant standing. It also exhibited a moderate positive correlation with grain yield, indicating that stomatal function may regulate plant productivity and structural integrity under field conditions (Figure 4).
In contrast, crude protein and fat content did not correlate significantly with the measured agronomic traits, suggesting that distinct physiological or genetic mechanisms may govern these seed quality attributes (Figure 4). All the detailed correlation values can be found in the Figure 4 Correlation analysis result.
3.11 Linking agronomic traits to genetic regulation: Expression of CsDrrp2 and CsCENLP
We found that the homolog of the GmPdh1 gene (CsDrrp2) is present in the selected industrial hemp varieties, including the wild-type hemp accession (LHV17) (Figure 5). To further understand its gene expression pattern, RT-PCR was conducted to examine the GmPdh1 homolog gene (CsDrrp2) gene expression among various hemp tissues in the early and late reproductive stages, Futura 83.
The seed development in hemp undergoes the following stages (The figure is demonstrated in Supplementary Document 1 File 8).
Flowering stage (R1): The stage when the first female flower is seen.
Early seed development stage (R2): The stage when the seed begins developing from the female flower. No seed shattering was seen in this stage.
Early seed filling stage (R3): The stage when the seeds are developed but still soft and milky when crushed with a finger. There was no shattering in this stage.
Later seed filling stage (R4): The stage when the seeds become hard and begin shattering, detaching from the mother plant, and spreading away from the middle lamella. The shattering was mild in the beginning and severe in the later stage.
Mature seeds (R5): The stage when the seed has entirely shattered and lies on the ground.
We observed two dehiscence zones that induced shattering. One was in the bracts that contain each seed (Supplementary Document 1 File 8). Another zone was in the chalaza placental region, where the seed is physically attached to the mother plant. In most cases, shattering is because of the dehiscence zone I in the bracts (Supplementary Document 1 File 8).
Phenotypically, the early flowering variety, USO-31, shatters more than the later-flowering fiber variety Jinma and Futura 83 (Table 5). Our field study suggests that the varieties should have 65–70 days for maximum grain yield and less shattering (Table 5). We studied the expression levels of CsDrrp2 at different stages of seed development in Futura 83 and LHV 17. There was a higher expression of this gene in the reproductive tissues than vegetative tissues in both Futura 83 and LHV 17. However, there was not statistical significance of differential expression in LHV-17 (Figures 6A, B). Similarly, we found CsCENLP to be expressed in all the tissues, particularly more in flowers and seeds than root (Figure 7B).
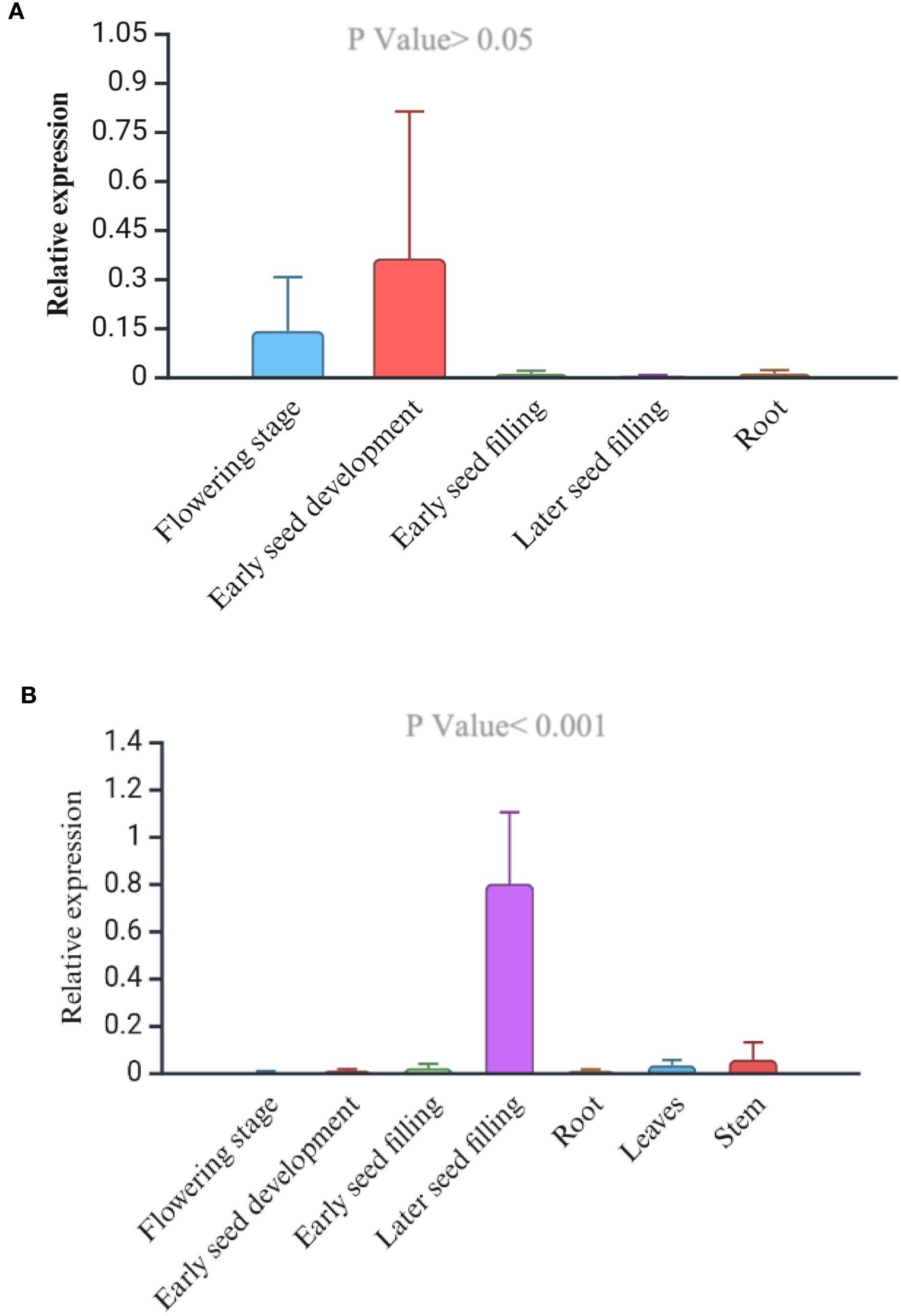
Figure 6. Relative expression of the CsDrrp2 gene (GmPdh1 homolog) in the successive plant development (A), Wild-type industrial hemp variety. The tissue-specific expression is not significant (P value>0.05, F value 0.51) (B). Futura 83, a cultivated dual-type hemp variety (P value<0.001, F value 19.226).
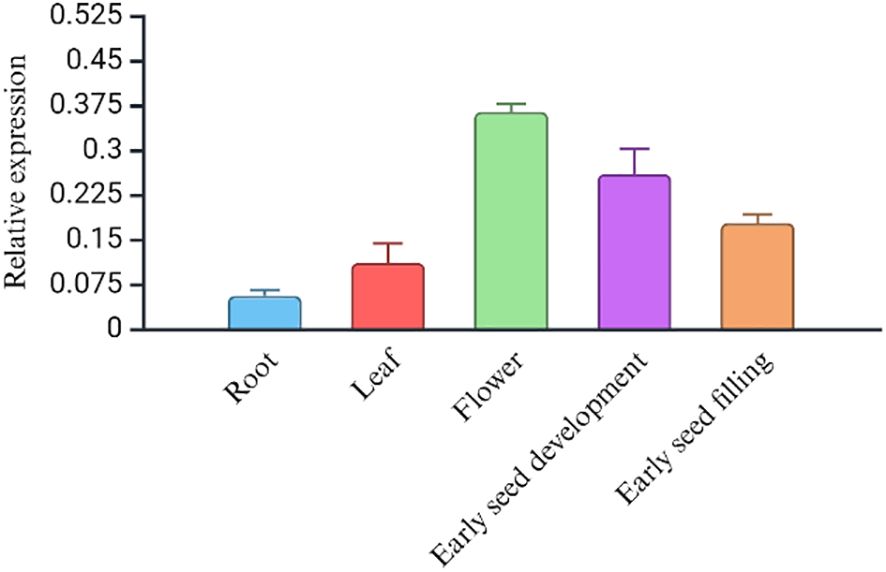
Figure 7. The gene expression profile (RT-PCR) of the CsCENLP in different tissues in Futura 83. (P value<0.001, F value 181.89).
4 Discussion
Most of the varieties we screened originated from Europe and Canada (Table 2). Missouri has distinct climatic differences compared to these regions. For instance, Missouri experiences hot and humid summers, whereas countries such as France, where varieties like Futura 83, Orion, and Fibror 79 were bred have milder summer conditions (NOAA, 2023). Missouri’s summer temperatures are also generally higher than those in Italy, Ukraine, Poland, and Canada (Ferfuia et al., 2021).
Additionally, Missouri’s lower latitude results in shorter summer photoperiods, which can trigger premature flowering in photoperiod-sensitive crops like hemp. European cultivars bred in France, Italy, and Poland typically come from more stable climates, and the uneven rainfall patterns in Missouri may negatively impact their performance (Campiglia et al., 2017). Similarly, Canadian varieties are bred for shorter, drier growing seasons and may not be well-suited to Missouri’s hot and humid climate. Although the use of two replications was appropriate for an early-stage screening trial, we acknowledge that it limits the statistical power and robust conclusions, particularly for traits such as yield and biomass influenced by environmental variability. Future experiments will include additional replications and site locations to bolster the varietal performance assessments and to delve deeper into genotype × environment interactions. The subsequent paragraphs describe the plant phenology and yield of the tested varieties in Missouri’s production environment.
4.1 Germination and plant growth
We observed all the varieties to have better germination in the field conditions than in the greenhouse conditions. Light irrigation in the field followed by seed sowing might have contributed to better germination in the field. In addition, fertilized soil, on the contrary to just the cocopeat used in the greenhouse, might have favored the germination in the field. However, further study should be conducted for further verification. We found that the different varieties had distinct growth patterns and time frames to transition from one growth phase to another. Grain-type varieties have earlier flowering and maturation times than fiber-type varieties. Like our study, Rahemi et al. (2021) and Ferfuia et al. (2021) also found that grain-type varieties, such as USO 31, had earlier flowering and maturation than fiber-type varieties. These phenological differences among the varieties might be because of the distinctive degree day requirement for each variety and the climatic differences between Missouri and the country where it was bred (Ferfuia et al., 2021). These findings would be critical for regional cultivar selection; short-maturing types could be selected in the Northern part of the United States, and later-maturing types could be selected for the Southern Region.
4.2 Planting population
We observed differences in plant population among the tested varieties. This might be due to a combination of factors, such as poor germination, adverse soil conditions, and biological threats like insects and pathogens (Van Roekel and Coulter, 2011). Like our experiment, Rahemi et al. (2021) also reported the grain varieties to have higher plant populations. The higher plant population found in Santhica and Bialobrzeskie v might be because of its smaller size and the intrinsic seed vigor traits that facilitate more germination and seedling emergence (Huang et al., 2019).
4.3 Weed suppression potential among varieties
Weed infestation is one of the significant challenges in industrial hemp cultivation, as there are limited herbicides available for industrial use. Our study did not use herbicides, thereby looking for inter-varietal weed suppression. We found that fiber variety, Jinma, contrary to grain and dual types, could suppress weeds efficiently. The fiber variety grew vigorously and formed a faster, denser leaf canopy, depriving weeds of sunlight and nutrients, thereby suppressing their growth. Furthermore, planting time also affected weed suppression. Notably, the earlier planting facilitated enhanced plant growth, outperforming weeds in fiber and dual-type varieties. Our findings were consistent with the similar findings in other cropping systems, which showed that early planting improves crop dominance over the weeds through faster establishment and resource capture (Williams, 2006). Based on our findings, fiber-type hemp varieties are recommended for fields with persistent weed pressure, primarily when herbicide use is restricted. Furthermore, we found an inverse relation of weed percentage with regard to the plant population (Figures 3, 4) and increasing seeding rate might potentially reduce the weeds; however, this requires further investigation for confirmation. Our weed intensity is based on a visual estimate and future research should include quantitative parameters such as weed biomass, nutrient competition, and additive findings.
4.4 Phenotypic variation in plant height and stem diameter:
Our findings on plant height and stem diameter were similar to those in previous research conducted in other parts of the United States. Notably, all the researchers found the fiber varieties to be the tallest, followed by the dual and grain types (Cornell Hemp Trial Report, 2020; Darby, 2021; Rahemi et al., 2021). Plant height (PH) is primarily influenced by stem elongation because of continuous cell division and the expansion of cells generated by the apical and intercalary meristems. In most plants, the plant height and diameter are polygenic traits (Wang and Li, 2008). In addition to genes, plant height is also regulated by plant hormones, including gibberellin (GA), brassinosteroid (BR), auxin (IAA), cytokinin (CK), and strigolactone (SL) (Tong et al., 2009). However, there has been a limited study conducted on the genetic architecture that determines the plant traits, such as plant height and stem diameter, in cannabis. While our study found the plant height to be associated as a varietal trait, further research should study what particular genetic mechanism and hormonal regulation contribute to the distinctive phenotype observed among the varieties (Petit et al., 2020b).
4.5 Grain yield
Grain yield is an important trait for the dual-type industrial hemp varieties (Alonso-Esteban et al., 2022). The grain yield among the tested varieties ranged from 328 kg/ha to 2,796 kg/ha. The average grain yield for the varietal trial was 1,283 kg/ha, which is higher than the national average of 896 kg/ha National Agricultural Statistics Service (2022). In a varietal screening study by Das et al. (2020), grain yields ranged from 27 kg/ha to 2,362 kg/ha, aligning with the range observed in this study. Similarly, Rahemi et al. (2021) reported an average grain yield of 771 kg/ha, which also falls within the range found in this study. As in the present research, Futura 83 demonstrated superior yield and performance traits in both New York and Vermont, confirming its suitability for cultivation across a wider geographic area (Cornell Hemp Report, 2020; Darby, 2021).
Grain yield is a complex trait. Hemp grain production varies among varieties and is influenced by environmental conditions such as soil type, moisture levels, and agronomic practices (Campbell et al., 2019; Campiglia et al., 2017; Poudel et al., 2020; Rahemi et al., 2021; Stack et al., 2021; Toth et al., 2020). In a study conducted across 14 cultivars in Latvia, the Czech Republic, France, and Italy, the grain yields were primarily determined by variety type and then by flowering time, which is consistent with our findings (Campiglia et al., 2017). We also found that grain yield was strongly influenced by variety and flowering time, as observed by Visković et al. (2024).
The climate study by Ferfuia et al. (2021) found that high temperatures during early grain filling negatively impact seed yield. While we did not study the impact of temperature on the yield, we found that early maturing varieties that had a maturation time of 55–60 days had lower yield. Dual-purpose varieties such as Futura 83 produced high grain and biomass yields, which has intermediate maturation times of 120 days. We observed higher biomass to be related to more grain yield, but this trait also additionally implies to higher fiber strength, assimilable sugars, making it more readily available for biofuel and fiber production (Williams et al., 2025). In addition to varietal effects and flowering time, grain yield is highly influenced by the Nitrogen dosage (Tang et al., 2022; Podder et al., 2024). The national average for grain yield is rising, probably because of the discovery of better agronomic practices, genetics, and screening stable varieties in the USA. However, no single variety has been identified that performs well in all the geographic regions of the United States, suggesting the paramount importance of this study (Williams et al., 2025). However, this study used only two replications per site-year, which may reduce the precision of traits such as flowering time and grain yield, especially given their strong environmental interactions. Despite this limitation, the consistent yield observed across multiple years suggests stable genetics in the Futura 83 variety. Nevertheless, future studies should incorporate more replications and multi-environment trials to allow for more robust statistical inference and to better support breeding decisions. Although our study did not delve deeper into the sexual dimorphism and grain yield, this factor might play an important role in this trait. While excessive male flower production can reduce seed yield in dioecious varieties, monoecious plants may produce inadequate pollination. Additionally, in hemp, monoecious might require allocating much resources to pollen production, reducing the resources available for seed development. On the contrary, in a variety of dioecious plants. In some cases, early male plants may senesce before females reach full reproductive maturity, enabling more effective seed development by females. While our study just explored the varietal effect on the yield, future research should explore how distribution of male and female flowers may impact yield parameters.
4.6 Seed protein and oil composition
We studied the phenotypic variation of key seed compositional traits such as total protein, crude protein, crude fat, and crude fiber, which are key components for human food and animal feed. The total protein observed in the hemp was 26-31% per gram of seed, similar to the previously identified range of 25%-30% (Aluko, 2017; Sun et al., 2021; M. Liu et al., 2023). Likewise, the crude protein in industrial hemp was 23-27% per gram of seed on a dry weight basis. Our study was consistent with the research findings by Alonso-Esteban et al. (2022), which found hemp seeds to contain between 25% and 35% crude protein and between 20% and 25% crude oil. We found Ferimon and Futura 83 to have the highest crude protein, with 27% and 26%, respectively. These studies further provide evidence of hemp seeds as a rich source of digestible proteins for humans and animal feed (House et al., 2010; Alonso-Esteban et al., 2022). Furthermore, Edestin could be used as a substitute for blood plasma when plasma is lost (such as in trauma and surgery) (Horska, 2005).
A complex genetic network controls grain filling. Edestin 1 and Edestin 2 encode for edestin protein, the predominant protein in industrial hemp grain (Deguchi et al., 2020; Cattaneo et al., 2021; Sun et al., 2021). Seed storage protein (SSP) encoding genes are primarily activated during the grain-filling and maturation stages. SSPs accumulate in the embryo or endosperm of the seed, depending on the plant species. The endosperm remains a single-cell layer in dicotyledonous plants such as industrial hemp. At the same time, the embryo cells develop protein storage vacuole (PSV) structures to store SSP (Yang et al., 2022). Although significant development has been made in understanding seed protein and storage reserve accumulation in model crops, little work has been conducted on this topic in industrial hemp (Yang et al., 2022). The cause of the variation in seed proteins among the tested genotypes is unknown. The higher total and crude protein observed in Futura 83 and Ferimon 79 varieties may be attributed to their distinct genetic makeup, the environment favoring this cultivar, or the interaction of both genes and the environment. These high-protein-containing cultivars, such as Futura 83 and Ferimon 79, likely possess traits that enhance the expression of these seed storage encoding genes. Increased expression of these genes could result in higher seed protein content in these varieties. However, further investigation is needed to understand the mechanisms governing protein synthesis in hemp comprehensively.
4.7 Genetic and environmental roles in flowering
One major challenge in hemp cultivation is the lack of uniform flowering and seed maturation. Some plants may remain vegetative within a single field while others reach full maturity. To exacerbate shattering, some flower clusters fully mature to seed within a single plant, while others are still in intermediate maturity (Supplementary Material Document 1 File 8). One way to fix the seed shattering in hemp is to restore the flowering time and engineer it to flower uniformly. The hemp breeding goal should target an intermediate flowering time to reduce seed shattering. Flowering in hemp is often triggered by signals received by the meristematic tissue related to alterations in environmental factors such as light intensity, daylength, influencing flowering time and intensity in industrial hemp (Downs and Borthwick, 1956; Petit et al., 2020b). The plants flower prematurely if they experience daylight periods shorter than 14 hours (Hall et al., 2012; Cheng et al., 2022).
Thus, understanding the mechanisms governing flowering, sexual orientation, and their relation to seed development and shattering is necessary for hemp breeding and improvement (Toth et al., 2020; Asiamah et al., 2025). Our study found the flowering time was different among the tested varieties. However this trait in hemp is very photoperiodic and future research should include how the light intensity, day length and other environmental factors and study whether thresholds vary among varieties to better determine which traits are most strongly conserved (Hall et al., 2012; Zhang et al., 2025). In the meantime, our study remains valuable for preparedness in cultivation and harvesting. Further study is required to characterize the gene expression among different early, late, and intermediate flowering varieties to better understand the flowering patterns and maturity in hemp varieties, and this will help design molecular breeding strategies for flowering time and maturity traits. Additionally, future research should sequence the sex specific gel bands and compare with the available pangenome for the validation (Lynch et al., 2025). While CsCENLP has not been directly linked to flowering and shattering, this gene might regulate the timing and the place in the tissues of lignin deposition, which is a very critical physical phenomenon for shattering. Future transcriptomics research and histological research on CsCENLP mutants or overexpression lines could give a better overview of the relationship between CsCENLP and seed shattering.
4.8 Molecular basis of seed shattering
In dicot plants, such as industrial hemp, seed shattering is caused by the development of a specialized dehiscence or abscission zone along the seed cover. During the seed development stage, cells in this zone differentiate into lignified and separation layers, which later undergo self-degradation, after which the seed cover splits open (Maity et al., 2021). Lignification is a necessary process involved in seed shattering. Alongside lignin, other key components of the plant’s secondary cell wall, such as cellulose and hemicellulose are related to cell wall strength and integrity, directly affecting pod dehiscence. Higher cellulose, hemicellulose, and lignin contents in pods are strongly associated with dehiscence (Hofhuis et al., 2016).
In soybean, GmPdh1 encodes a dirigent (DIR)-like protein. It enhances pod dehiscence by increasing the torsion of dried pod walls, generating a driving force for pod splitting under low humidity conditions (Funatsuki et al., 2014). The homolog of the GmPdh1 gene is the CsDrrp2, which we hypothesized to be associated with shattering. While the expression of the CsDrrp2 in the later seed development stage might associate with seed shattering, however, the exact role of this gene is unknown and we cannot provide its function based on the current result. It is unknown whether it causes lignin deposition in the dehiscence layer or cellulose deposition. The homolog of CsDrrp2 promotes lignification in the inner sclerenchyma of pod walls, the site of thick secondary cell wall formation (Funatsuki et al., 2014; Marsh et al., 2023). This gene may play a similar role in Cannabis sativa by promoting lignification in the inner sclerenchyma of the seed coat, as observed in legumes. Such activity could enhance the differential lignin deposition between the inner and outer layers of the seed coat. During consecutive drying cycles, these differences may generate dehiscence forces that lead to seed shattering. The future direction should be RNA sequencing of the highly shattering grain type varieties and the less shattering ones based on our screened varieties for seed development and shattering genes. Furthermore, additional seed shattering genes such as SHAT-5, SHP1 and SHP2 that are found to be associated with shattering should also be studied for further exploring the mechanism of shattering (Maity et al., 2021). Shattering occurs as a torsion in the bracts surrounding the seeds, pulling both the seed covers and exposing the seeds. Although our experiment included the bracts surrounding the seeds, further research should focus on identifying the specific tissue and the region where this gene is expressed. Furthermore, RNA in situ hybridization should be performed to determine the exact location of the expression of this gene and its role. Future work would be knocking out this gene and creating mutants to see if there are any quantifiable differences in the seed shattering.
5 Conclusion
We observed significant variation across major traits such as plant height, stem diameter, biomass, grain yield, and seed composition. Over three consecutive years, the variety Future 83 exhibited high biomass and grain yield, suggesting stable performance. Furthermore we observed seed shattering as a one of a major factor negatively impacting grain yield. Our study provided preliminary insight into the molecular basis of flowering and seed development. By comparing homologs of the soybean Pod dehiscence 1 (GmPdh1) and Terminal flower 1b (GmTFL1b or Dt1) genes in industrial hemp, we explored a novel aspect of hemp genetics. Further research should explore the molecular functions of these genes for validation. Additionally, future efforts should focus on producing hemp varieties with uniform growth and maturation, higher grain yield, and improved seed composition. This could be achieved by investigating the gene-environment interactions (G×E) to assess their adaptive suitability across the diverse agroecological zones of the Midwest. Overall, our findings will contribute to hemp breeding and crop improvement and support hemp farmers by enhancing cultivation practices.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://doi.org/10.6084/m9.figshare.30307579.v1.
Author contributions
KT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. TM: Conceptualization, Data curation, Formal analysis, Validation, Visualization, Writing – review & editing. JA: Data curation, Formal analysis, Writing – review & editing. CC: Supervision, Validation, Writing – review & editing. SM: Data curation, Writing – review & editing, Methodology. SS: Writing – review & editing. PK: Writing – review & editing. ER: Writing – review & editing. CM: Conceptualization, Supervision, Writing – review & editing. JP: Writing – review & editing, Supervision. BV: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Funding support to the corresponding author B. Valliyodan, USDA-NIFA project award number is 2021-38821-34726.
Acknowledgments
We want to acknowledge the USDA-NIFA Capacity Building Program for funding support to BV. Furthermore, we thank AESCL Analytical Services at the University of Missouri, Columbia, for conducting the seed compositional analysis. Similarly, we would also like to thank Taylor Geospatial Institute, Saint Louis University, for their assistance. Moreover, a special acknowledgment goes to the Valliyodan Laboratory at Lincoln University of Missouri for data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1632346/full#supplementary-material
References
Aboul-Maaty, N. A. F. and Oraby, H. A. S. (2019). Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method. Bull. Natl. Res. Cent 43, 25. doi: 10.1186/s42269-019-0066-1
Alonso-Esteban, Torija-Isasa, M.E., and de Cortes Sánchez-Mata, M. (2022). Mineral elements and related antinutrients, in whole and hulled hemp (Cannabis sativa L.) seeds. J. Food Composition Anal. 109, 104516. doi: 10.1016/j.jfca.2022.104516
Aluko, R. E. (2017). “Hemp seed (Cannabis sativa L.) proteins: Composition, structure, enzymatic modification, and functional or bioactive properties,” in Sustainable protein sources. Eds. Nadathur, S. R., Wanasundara, J. P. D., and Scanlin, &L. (Academic Press, New York, USA ), 121–132. doi: 10.1016/B978-0-12-802778-3.00007-X
Amaya, I., Ratcliffe, O. J., and Bradley, D. J. (1999). Expression of CENTRORADIALIS (CEN) and CEN-like genes in tobacco reveals a conserved mechanism controlling phase change in diverse species. Plant Cell 11, 1405–1417. doi: 10.1105/tpc.11.8.1405
Asiamah, J. Y., Haruna, M. S., Tamang, K. R., Carson, C. B., Koirala, P., Reed, E. A., et al. (2025). Genome editing in grain legumes for food security. Front. Genome Editing 7. doi: 10.3389/fgeed.2025.1572292
Callaway, J. C. (2004). Hempseed as a nutritional resource: An overview. Euphytica 140, 65–72. doi: 10.1007/s10681-004-4811-6
Campbell, B. J., Berrada, A. F., Hudalla, C., Amaducci, S., and McKay, J. K. (2019). Genotype × Environment interactions of industrial hemp cultivars highlight diverse responses to environmental factors. Agrosystems Geosciences Environ. 2, 1–11. doi: 10.2134/age2018.11.0057
Campiglia, E., Radicetti, E., and Mancinelli, R. (2017). Plant density and nitrogen fertilization affect the agronomic performance of industrial hemp (Cannabis sativa L.) in Mediterranean environment. Ind. Crops Products 100, 246–254. doi: 10.1016/J.INDCROP.2017.02.022
Cattaneo, C., Givonetti, A., Leoni, V., Guerrieri, N., Manfredi, M., Giorgi, A., et al. (2021). Biochemical aspects of seeds from Cannabis sativa L. plants grown in a mountain environment. Sci. Rep. 11, 3927. doi: 10.1038/s41598-021-83290-1
Cheng, X., Wang, R., Liu, X., Zhou, L., Dong, M., Rehman, M., et al. (2022). Effects of light spectra on morphology, gaseous exchange, and antioxidant capacity of industrial hemp. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.937436
Cornell Hemp (2020). 2020 Cornell Hemp trials for New York State: grain, dual purpose, and fiber production (Geneva, New York: Cornell University Extension). Available online at: https://bpb-us-e1.wpmucdn.com/blogs.cornell.edu/dist/a/7491/files/2021/05/2020HempGrain-FiberTrialReport_2021.03.17.pdf.
Das, L., Li, W., Dodge, L. A., Stevens, J. C., Williams, D. W., Hu, H., et al. (2020). Comparative evaluation of industrial hemp cultivars: agronomical practices, feedstock characterization, and potential for biofuels and bioproducts. ACS Sustain. Chem. Eng. 8, 6200–6210. doi: 10.1021/acssuschemeng.9b06145
Deguchi, M., Kane, S., Potlakayala, S., George, H., Proano, R., Sheri, V., et al. (2020). Metabolic engineering strategies of industrial hemp (Cannabis sativa L.): A brief review of the advances and challenges. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.580621
Deguchi, M., Potlakayala, S., Spuhler, Z., George, H., Sheri, V., Agili, R., et al. (2021). Selection and validation of reference genes for normalization of qRT-PCR data to study the cannabinoid pathway genes in industrial hemp. PloS One 16, e0260660. doi: 10.1371/journal.pone.0260660
Dhoubhadel, S. P. (2021). Challenges, opportunities, and the way forward for the U.S. hemp industry Vol. 19 (Western Economics Forum, Western Agricultural Economics Association, California). Available online at: https://ideas.repec.org/a/ags/weecfo/315938.html (Accessed 2021).
Dowling, C. A., Shi, J., Toth, J. A., Quade, M. A., Smart, L. B., McCabe, P. F., et al. (2024). A FLOWERING LOCUS T ortholog is associated with photoperiod-insensitive flowering in hemp (Cannabis sativa L.). Plant J. 119, 383–403. doi: 10.1111/tpj.16769
Downs, R. J. and Borthwick, H. A. (1956). Effects of photoperiod on the growth of trees. Botanical Gazette 117, 310–326. doi: 10.1086/335918
ElSohly, M. A., Radwan, M. M., Gul, W., Chandra, S., and Galal, A. (2017). “Phytochemistry of Cannabis sativa L,” in Phytocannabinoids. Progress in the Chemistry of Organic Natural Products, vol. 103 . Eds. Kinghorn, A., Falk, H., Gibbons, S., and Kobayashi, J. (Springer, Cham). doi: 10.1007/978-3-319-45541-9_1
Ferfuia, C., Zuliani, F., Danuso, F., Piani, B., Cattivello, C., Dorigo, G., et al. (2021). Performance and stability of different monoecious hemp cultivars in a multi-environments trial in North-Eastern Italy. Agronomy 11, 1424. doi: 10.3390/agronomy11071424
Funatsuki, H., Suzuki, M., Hirose, A., Inaba, H., Yamada, T., Hajika, M., et al. (2014). Molecular basis of a shattering resistance boosting the global dissemination of soybeans. Proc. Natl. Acad. Sci. 111, 17797–17802. doi: 10.1073/pnas.1417282111
Gülizar, KurtoğluA., Erdal, İbrahim, and Tosun, İsmail (2025). Phytoremediation potential of industrial hemp grown in sewage sludge: Growth performance and environmental impact. J. Environ. Chem. Eng. 13, 2213–3437. doi: 10.1016/j.jece.2025.117173
Hall, J., Bhattarai, S. P., and Midmore, D. J. (2012). Review of flowering control in industrial hemp. J. Natural Fibers 9, 23–36. doi: 10.1080/15440478.2012.651848
Hanano, S. and Goto, K. (2011). Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell 23, 3172–3184. doi: 10.1105/tpc.111.088641
Hofhuis, H., Moulton, D., Lessinnes, T., Routier-Kierzkowska, A. L., Bomphrey, R. J. J., Mosca, G., et al. (2016). Morphomechanical innovation drives explosive seed dispersal. Cell 166, 222–233. doi: 10.1016/J.CELL.2016.05.002
Horska, I. (2005). Edestin-comprising agent for substitution of blood plasma and a method of its production (WO2005058332A3) (World Intellectual Property Organization, Czech Republic). Available online at: https://patents.google.com/patent/WO2005058332A3/en (Accessed June 30, 2005).
House, J. D., Neufeld, J., and Leson, G. (2010). Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility-corrected amino acid score method. J. Agric. Food Chem. 58, 11801–11807. doi: 10.1021/JF102636B
Huang, J., Li, Y., Shi, Y., Wang, L., Zhou, Q., and Huang, X. (2019). Effects of nutrient level and planting density on population relationship in soybean and wheat intercropping populations. PloS One 14, e0225810. doi: 10.1371/journal.pone.0225810
Islam, S. and Hasan, B. (2025). An overview of the effects of water and moisture absorption on the performance of hemp fiber and its composites. SPE Polym 6. doi: 10.1002/pls2.10167
Kempton, R. A. and Gleeson, A. C. (1997). “Unreplicated trials,” in Statistical methods for plant variety evaluation. Plant breeding series 3 statistical methods for plant variety evaluation. Eds. Kempton, R. A., Fox, P. N., and Cerezo, M. (Springer, Dordrecht). doi: 10.1007/978-94-009-1503-9_6
Leckie, K. M., Sawler, J., Kapos, P., MacKenzie, J. O., Giles, I., Baynes, K., et al. (2024). Loss of daylength sensitivity by splice site mutation in Cannabis pseudo-response regulator. Plant J. 118, 2020–2036. doi: 10.1111/tpj.16726
Liu, M., Toth, J. A., Childs, M., Smart, L. B., and Abbaspourrad, A. (2023). composition and functional properties of hemp seed protein isolates from various hemp cultivars. J. Food Sci. 88. doi: 10.1101/2022.06.01.494437
Liu, B., Watanabe, S., Uchiyama, T., Kong, F., Kanazawa, A., Xia, Z., et al. (2010). The soybean stem growth Habit Gene Dt1 Is an ortholog of Arabidopsis TERMINAL FLOWER1. Plant Physiol. 153, 198–210. doi: 10.1104/pp.109.150607
Lynch, R. C., Padgitt-Cobb, L. K., Garfinkel, A. R., Knaus, A. R., Hartwick, B. J., Allsing, N. T, et al. (2025). Domesticated cannabinoid synthases amid a wild mosaic cannabis pangenome. Nature 643, 1001–1010. doi: 10.1038/s41586-025-09065-0
Ma, H. (2005). Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu. Rev. Plant Biol. 56, 393–434. doi: 10.1146/annurev.arplant.55.031903.141717
Maity, A., Lamichaney, A., Joshi, D. C., Bajwa, A., Subramanian, N., Walsh, M., et al. (2021). Seed shattering: A trait of evolutionary importance in plants. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.657773
Marsh, J. I., Nestor, B. J., Petereit, J., Tay Fernandez, C. G., Bayer, P. E., Batley, J., et al. (2023). Legume-wide comparative analysis of pod shatter locus PDH1 reveals phaseoloid specificity, high cowpea expression, and stress responsive genomic context. Plant J. 115, 68–80. doi: 10.1111/tpj.16209
Mishchenko, S., Mokher, J., Laiko, I., Burbulis, N., Kyrychenko, H., and Dudukova, S. (2017). Phenological growth stages of hemp (Cannabis sativa L.): codification and description according to the BBCH scale. Agricultural Sciences, Lithuanian Academy of Sciences, 24. doi: 10.6001/zemesukiomokslai.v24i2.3496
Mohamedikbal, S., Al-Mamun, H. A., Marsh, J. I., Upadhyaya, S., Danilevicz, M. F., Nguyen, H. T., et al. (2024). Local haplotyping reveals insights into the genetic control of flowering time variation in wild and domesticated soybean. Plant Genome 17, e20528. doi: 10.1002/tpg2.20528
Muedi, H. T. H., Kujoana, T. C., Shai, K., Mabelebele, M., and Sebola, N. A. (2024). The use of industrial hemp (Cannabis sativa) on farm animal’s productivity, health, and reproductive performance: a review. Anim. Production Sci. 64, AN23268. doi: 10.1071/AN23268
National Agricultural Statistics Service (2022). National hemp report 2022. Available online at: https://www.nass.usda.gov/Publications/Todays_Reports/reports/hempan22.pdf.
National Oceanic and Atmospheric Administration (2023). (NOAA: National Oceanic and Atmospheric Administration. U.S. Department of Commerce). Available online at: https://www.noaa.gov/ (Accessed July 13, 2025).
Petit, J., Salentijn, E. M. J., Paulo, M. J., Denneboom, C., and Trindade, L. M. (2020a). Genetic architecture of flowering time and sex determination in hemp (Cannabis sativa L.): A genome-wide association study. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.569958
Petit, J., Salentijn, E. M. J., Paulo, M.-J., Thouminot, C., van Dinter, B. J., Magagnini, G., et al. (2020b). Genetic variability of morphological, flowering, and biomass quality traits in hemp (Cannabis sativa L.). Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00102
Podder, S., Shafian, S., Thomason, W. E., Wilson, T. B., and Fike, J. H. (2024). Hemp seed yield responses to nitrogen fertility rates. Crops 4, 145–155. doi: 10.3390/crops4020011
Poudel, S., Poudel, B., Acharya, B., Kathayat, D., Pant, K., Tamang, K. R., et al. (2020). Effect of crop establishment methods on performance of rice in Rupandehi, Nepal. J. Institute Agric. Anim. Sci. 36. doi: 10.3126/jiaas.v36i1.48391
Quach, T. N. and Nguyen, H. T. M. (2015). Valliyodan, B. et al. Genome-wide expression analysis of soybean NF-Y genes reveals potential function in development and drought response. Mol. Genet. Genomics 290, 1095–1115. doi: 10.1007/s00438-014-0978-2
Rahemi, A., Dhakal, R., Temu, V. W., Rutto, L., and Kering, M. K. (2021). Performance of different-use type industrial hemp cultivars under mid-atlantic region conditions. Agronomy 11, 2321. doi: 10.3390/agronomy11112321
Stack, G. M., Toth, J. A., Carlson, C. H., Cala, A. R., Marrero-González, M. I., Wilk, R. L., et al. (2021). Season-long characterization of high-cannabinoid hemp (Cannabis sativa L.) reveals variation in cannabinoid accumulation, flowering time, and disease resistance. GCB Bioenergy 13, 546–561. doi: 10.1111/gcbb.12793
Sun, X., Sun, Y., Li, Y., Wu, Q., and Wang, L. (2021). Identification and characterization of the seed storage proteins and related genes of cannabis sativa L. Front. Nutr. 8. doi: 10.3389/fnut.2021.678421
Tang, K., Wang, J., Yang, Y., Deng, G., Yu, J., Hu, W., et al. (2022). Fiber hemp (Cannabis sativa L.) yield and its response to fertilization and planting density in China. Ind. Crops Products 177, 114542. doi: 10.1016/J.INDCROP.2022.114542
Tong, H., Jin, Y., Liu, W., Li, F., Fang, J., Yin, Y., et al. (2009). DWARF AND LOW-TILLERING, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J. 58, 803–816. doi: 10.1111/j.1365-313X.2009.03826.x
Toth, J. A., Stack, G. M., Cala, A. R., Carlson, C. H., Wilk, R. L., Crawford, J. L., et al. (2020). Development and validation of genetic markers for sex and cannabinoid chemotype in Cannabis sativa L. GCB Bioenergy 12, 213–222. doi: 10.1111/gcbb.12667
Van Roekel, R. J. and Coulter, J. A. (2011). Agronomic responses of corn to planting date and plant density. Agron. J. 103, 1414–1422. doi: 10.2134/agronj2011.0071
Vasantha Rupasinghe, H. P., Davis, A., Kumar, S. K., Murray, B., and Zheljazkov, V. D. (2020). Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging source for value-added functional food ingredients and nutraceuticals. Molecules 25. doi: 10.3390/molecules25184078
Visković, J., Sikora, V., Latković, D., Zeremski, T., Dunđerski, Dušan, Astatkie, T., et al. (2024). Optimization of hemp production technology for fiber and seed. Ind. Crops Products 219, 0926–6690. doi: 10.1016/j.indcrop.2024.119127
Wang, Y. and Li, J. (2008). Molecular basis of plant architecture. Annu. Rev. Plant Biol. 59, 253–279. doi: 10.1146/annurev.arplant.59.032607.092902
Wickland, D. P. and Hanzawa, Y. (2015). The FLOWERING LOCUS T/TERMINAL FLOWER 1 gene family: Functional evolution and molecular mechanisms. Mol. Plant 8, 983–997. doi: 10.1016/j.molp.2015.01.007
Williams, M. M. (2006). Planting date influences critical period of weed control in sweet corn. Weed Sci. 54, 928–933. doi: 10.1614/WS-06-005R.1
Williams, A., Brym, Z., Chen, C., Collins, A., Crawford, J., Darby, H., et al. (2025). Comparing agronomic performance of industrial hemp varieties for suitable production in the United States. Agron. J. 117. doi: 10.1002/agj2.70006
Wimalasiri, E. M., Jahanshiri, E., Chimonyo, V. G. P., Kuruppuarachchi, N., Suhairi, T. A. S. T. M., Azam-Ali, S. N., et al. (2021). A framework for the development of hemp (Cannabis sativa L.) as a crop for the future in tropical environments. Ind. Crops Products 172, 113999. doi: 10.1016/j.indcrop.2021.113999
Yang, J., Kornet, R., Diedericks, C. F., Yang, Q., Berton-Carabin, C. C., Nikiforidis, C. V., et al. (2022). Rethinking plant protein extraction: Albumin—From side stream to an excellent foaming ingredient. Food Structure 31, 100254. doi: 10.1016/J.FOOSTR.2022.100254
Keywords: industrial hemp, grain hemp, dual-type hemp, grain yield, flowering trait, seed shattering resistance, seed composition
Citation: Tamang KR, Mawhinney TP, Carson CB, Asiamah JY, Mahdi SH, Reed E, Sharma S, Koirala P, Patel J, Mensah CA and Valliyodan B (2025) Phenotypic and genetic characterization of sixteen grain and dual-type industrial hemp varieties (Cannabis sativa L.) for agronomic and yield component traits. Front. Plant Sci. 16:1632346. doi: 10.3389/fpls.2025.1632346
Received: 21 May 2025; Accepted: 17 September 2025;
Published: 20 October 2025.
Edited by:
Muhammad Qasim Shahid, South China Agricultural University, ChinaReviewed by:
Intikhab Alam, Fujian Agriculture and Forestry University, ChinaNikhil Malhotra, Indian Council of Agricultural Research (ICAR), India
Jacob Toth, Cornell University, United States
Francesco Tonolo, Leiden University, Netherlands
Copyright © 2025 Tamang, Mawhinney, Carson, Asiamah, Mahdi, Reed, Sharma, Koirala, Patel, Mensah and Valliyodan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Babu Valliyodan, VmFsbGl5b2RhbkJAbGluY29sbnUuZWR1
 Kusum Raj Tamang
Kusum Raj Tamang Thomas P. Mawhinney
Thomas P. Mawhinney Christian B. Carson1
Christian B. Carson1 Joshua Yeboah Asiamah
Joshua Yeboah Asiamah Sakina Haruna Mahdi
Sakina Haruna Mahdi Emily Reed
Emily Reed Prabesh Koirala
Prabesh Koirala Babu Valliyodan
Babu Valliyodan