- lnstitute for Multidisciplinary Research in Applied Biology (IMAB), Sciences Department, Public University of Navarre (UPNA), Pamplona, Spain
In quantitative terms, nitrogen (N) is the most important essential mineral element for plants, acquired mainly in the form of ammonium (NH4+) and nitrate (NO3-). Despite fluctuations in soil NH4+and NO3-availability, plants seek to balance their NH4+-to- NO3-uptake ratio to avoid the metabolic burden associated with the compensation of an intracellular proton excess or deficit. However, while the molecular mechanisms by which plants mediate and modulate the activity of their uptake systems for NO3-and NH4+have been well characterized, it has remained unclear to what extent these transport systems could interact. In this review, the potential contributions of AMTs and NRT1.1 to the overall acquisition of N are highlighted. Both NO3–independent and -dependent signaling of NRT1.1 in modulating NH4+tolerance, as well as the underestimated role of AMTs in nutrient and cellular pH homeostasis, are discussed. The interdependency between AMTs and NRT1.1 is considered highly relevant for optimized N uptake in field conditions, where both N forms typically coexist and act complementarily to maintain balanced pH and nutrient homeostasis for optimal plant growth
Introduction
Among all nutrients, nitrogen (N) is quantitatively the most important essential mineral element for plants and thus its availability a critical determinant in agricultural plant production. The accessibility of N for plant roots varies considerably through space and time, due to soil heterogeneity, anthropogenic N inputs, water flows, microbial activity, and other factors that dislocate or convert N forms (Bloom, 2015). Plants acquire N mostly in the form of ammonium (NH4+) and nitrate (NO3-), with the latter being the major form in most plants grown on crop soils (Miller et al., 2007). As plants have adapted to fluctuating NO3- concentrations and external pH levels, they evolved sophisticated transport systems that enable efficient NO3- uptake coupled with protons (H+), ensuring N acquisition even when NO3- availability is limited in acidic conditions (Tsai and Schmidt, 2021). At micromolar substrate concentrations, root uptake is mainly mediated by high-affinity NRT2-type transporters (Bouguyon et al., 2012), whereas at millimolar concentrations the high-affinity transporters become repressed and low-affinity transporters take over. Here, the transceptor NRT1.1/NPF6.3/CHL1 (hereby NRT1.1) plays a central role as it confers most of the low-affinity NO3- transport capacity (Krouk et al., 2010).
NRT1.1 also mediates NO3- signaling and triggers the downstream transcriptional upregulation of a plethora of NO3–inducible genes (Wang et al., 2009; Bouguyon et al., 2015). Moreover, NRT1.1 is extensively involved in cellular and physiological processes of plant growth and development. These include NO3- root-to-shoot translocation, shoot transpiration, auxin transport, seed dormancy relief, and enhanced tolerance to adverse environmental conditions such as H+ excess, sodium, cadmium, zinc, lead, or NH4+ toxicity, as well as iron or phosphorus (Pi) deficiency (Fang et al., 2021). Although specific topics associated with the NO3- transport and sensing functions of NRT1.1 have been discussed in multiple reviews, only a few studies have revealed its signaling function in the absence of NO3- (Wang et al., 2009; Jian et al., 2018; Hachiya et al., 2011, 2024).
With regard to NH4+ uptake systems, high-affinity transporters of the AMT family appear being of major importance in plant roots, because NH4+ concentrations in the majority of crop soils usually range between 20 and 200 μM (Lark et al., 2004). Out of the five AMT proteins expressed in Arabidopsis roots, AMT1.1, AMT1.2, AMT1.3, and AMT1.5, are responsible for high-affinity NH4+ uptake in N-deficient plants (Loqué et al., 2006; Yuan et al., 2007). Between 60 and 70% of this transport capacity is mediated by AMT1.1 and AMT1.3, while AMT1.2 with its lower affinity confers another approx. 20% and mainly retrieves NH4+ from the apoplastic transport route (Duan et al., 2018). As concluded from the remaining uptake capacity in the quadruple knock-out line qko (amt1.1, 1.2, 1.3, 2.1), AMT1.5 confers less than 10% of the overall high-affinity NH4+ uptake capacity, probably at fairly high affinity (estimated Km ~5 µM (Yuan et al., 2007)). On the other hand, under certain conditions, NH4+ concentrations can exceed those of NO3- and be the main source of N, such as in acidic or paddy soils, which can cause toxicity and arrested plant growth (Xu et al., 2012). Unlike H+ cotransport during NO3- uptake, only transport activities of AMT1.1 in wheat and common bean are pH-dependent, exhibiting an acid-stimulated regulatory mode (Søegaard, 2009; Ortiz-Ramirez et al., 2011), whereas the transport activities in Arabidopsis, tomato, and rice are pH-independent when expressed in oocytes.
Although the environmental challenge urges to reduce the input of NO3–based fertilizers (Howarth, 2008), NH4+ as the main source of N is problematic, and is partly overcome in agricultural practice by the coating of urea fertilizers with urease inhibitors to generate a slow and continuous release of NH4+. For this reason, the application of both N form in plant species-specific ratios has proven beneficial for crops (Britto and Kronzucker, 2013). However, the reason for NO3- and NH4+ positive synergy in plant growth and development is not fully understood. Furthermore, while the molecular mechanisms by which plants mediate and modulate the activity of their uptake systems for NO3- and NH4+ have been well characterized, it has remained unclear to what extent these transport systems interact to ensure optimal N uptake and growth. In this review, the interdependency of the AMT1s and NRT1.1 during N uptake balance is discussed. Emphasis is placed on how NRT1.1 signaling, both NO3–dependent and independent, affects NH4+ tolerance, alongside the underestimated role of AMT-dependent NH4+ uptake in nutrient homeostasis and cellular pH regulation.
The regulatory role of NRT1.1 in NH4+ nutrition
Hachiya and Noguchi (2011), and later Jian et al. (2018), pointed out that a NO3–independent function of NRT1.1 might exist in Arabidopsis, as functional disruption of NRT1.1 in the so-called chl1–5 mutant confers increased tolerance to sole NH4+ nutrition. The main reason for this phenomenon remains unclear. The reduction of shoot transpiration in the chl1–5 mutant (Guo et al., 2003) was excluded as the cause of the observed NH4+ tolerance, since no significant differences in the stomatal conductance were found with respect to the wild-type under sole NH4+ nutrition (Jian et al., 2018).
There is also some divergent findings regarding the possible transcriptional and post-transcriptional repression of AMTs in the chl1–5 as for its NH4+ tolerance. On one hand, Hachiya et al. (2011, 2024) did not observe differences in AMT transcript levels between wild-type Col-0 and chl1–5 under sole NH4+ nutrition. Similarly, Muños et al. (2004) did not find differences in the 15NH4+ uptake capacity under same conditions, leading to the conclusion that NH4+ tolerance is unlikely to result from AMT repression or lower NH4+ uptake. On the contrary, Jian et al. (2018) observed a significant downregulation of AMT genes in the mutant (specifically of AMT1.1, 1.3 and 1.5), together with a decline in the 15NH4+ uptake under sole NH4+ nutrition. Likewise, they observed a lower accumulation of free NH4+, which the authors attributed to decreased NH4+ uptake combined with the induction of glutamine synthetase activity in the roots. They suggested that the enhanced primary assimilation of NH4+ in chl1–5 plays a crucial role in its NH4+ tolerance. The later study by Hachiya et al. (2021) demonstrated that glutamine synthetase activity in roots attenuates NH4+ toxicity, whereas in shoot causes acidic stress that arrests cell growth in Arabidopsis.
Another process that appears to contribute to mitigating the NH4+ toxicity in chl1–5 is the promotion of glucosinolate (GSL) biosynthesis. According to the microarray analysis of Wang et al. (2009), several genes relevant to the aliphatic GSL biosynthetic pathway were induced in chl1–5 mutant in the absence of NO3-. Recently, Hachiya et al. (2024) confirmed that the biosynthesis of GSLs and their positive regulator genes MYB28 and MYB29 were indeed significantly induced in chl1–5 in the absence of NO3-. Since GSLs are N-rich secondary metabolites whose synthesis increases under NH4+ nutrition (Marino et al., 2016; Coleto et al., 2017), it is plausible that NH4+ tolerance of chl1–5 could be due in part to the promotion of NH4+ assimilation into these compounds rather than into Gln, a H+ generating process.
Taken together, despite some discrepancies in AMT expression and/or activity, the results suggest that the NH4+ tolerance observed in chl1–5 mutants is more closely linked to reduced NH4+ accumulation in the shoot. However, more studies are needed to elucidate how GSLs, other genes or hormones are involved in the NO3–independent signaling of NRT1.1 during NH4+ nutrition.
An additional detail is that the NH4+-tolerance of chl1–5 in the absence of NO3- was more pronounced at lower pH (Hachiya et al., 2011), so there might be a pH-dependent effect that could ‘amplify’ NRT1.1-dependent signaling. Indeed, excess NH4+ uptake mediated by AMTs leads to considerable acidification of the apoplastic pH, which causes ionic imbalances during nutrient uptake and an array of constraints to plant fitness in the long-term. Decreasing the medium pH from 6.5 to 5.5 increased the expression and activity of NRT1.1 irrespective of the presence of NO3-, indicating that pH alone can trigger NRT1.1 readiness in anticipation of reduced levels of available NO3- (Tsay et al., 1993; Ye et al., 2021). Although it has not yet been demonstrated which form of NRT1.1 exists predominantly under low pH/high NH4+ conditions, it is well known that CBL-INTERACTING PROTEIN KINASE 23 (CIPK23) stabilizes the NRT1.1 monomeric state by phosphorylation, enabling NRT1.1 to act as a high-affinity NO3- transceptor (Ho et al., 2009; Rashid et al., 2020). Since CIPK23 is also induced by low pH and excess NH4+ (see next section) (Straub et al., 2017; Ye et al., 2021), and post-translationally de-repressed by the protein phosphatase ABA-INSENSITIVE 1 and 2 (Ganz et al., 2022; Léran et al., 2015), CIPK23 likely enhances phosphorylation of AMT1 to prevent NH4+ uptake dependent toxicity, while NRT1.1 to stimulate NO3- once available. In addition to CIPK23, the kinase CIPK15 also represses the activity of AMT1.1 in Arabidopsis through phosphorylation under high ammonium conditions (Chen et al., 2020). Also, the phosphatases ABI1 and ABI2 inactivate CIPK15 via dephosphorylation, although there is currently no evidence that they also target NRTs. So far, one could speculate whether might be a ‘competition’ between NRT1.1 and AMTs as common targets for post-translational modifiers (e.g., CIPK23, ABI1, ABI2). This competition could potentially reduce the capacity of those kinases and phosphatases to control their activity, thereby influencing N uptake.
The other side of the coin: the contribution of AMTs to nutrient and pH homeostasis
In the field, NH4+ accumulation and low pH often arise simultaneously, because nitrification is generally inhibited under low pH condition. However, strict NH4+ conditions are unlikely in most crop soils, and even the presence of a low concentration of NO3- improves NH4+ tolerance (Garnica et al., 2010; Hachiya et al., 2012; Zheng et al., 2015; Fang et al., 2016; Du et al., 2021). Thus, under most soil conditions, the growth and tolerance of defected NRT1.1 mutants to NH4+/low pH are reduced due to their restricted NO3-/H+ uptake and primary NO3- response (Fang et al., 2016). At the gene expression level, NRT1.1 is also induced by low external pH that achieve a maximum at pH 5.0 in Arabidopsis (Ye et al., 2021). A low pH is compensated by more efficient NO3- uptake by NRT1.1, whose protonated H356 residue is essential for increasing the NO3- and H+ cotransport (Sun et al., 2014). Therefore, NRT1.1 is essential to avoid further H+ rhizotoxicity, and in turn the associated ionic imbalances such as excessive root accumulation of Fe2+ and Mn2+, or lower phosphorous (Pi) availability (Kochian et al., 2004).
In the context of NH4+ nutrition, apoplastic acidification is promoted under excessive NH4+ nutrition. The AMT-dependent NH4+ uptake contributes to depolarizing the electrical membrane potential, which in turn enhances net H+ efflux, primarily mediated by AHA2 (Meier et al., 2020). Therefore, the induction of NRT1.1 during NH4+-promoted apoplastic acidification is plausible for plants to ensure a correct charge balance, which this is in what lies partially the NO3–dependent alleviation of NH4+ toxicity when sufficient external NO3- is present. Under low external NO3- conditions, NRT1.1 is also involved in regulating the apoplastic pH through modulation of AHA2 via the co-receptor kinase QSK1 (Zhu et al., 2024). NRT1.1 interacts with QSK1, prompting QSK1 to phosphorylate AHA2 at the S899 site, which inhibits AHA2 activity and reduces the H+ efflux into the apoplast. This mechanism decreases external acidification and is associated with the repression of lateral root (LR) growth under unfavorable low NO3- conditions. This functional connection between NRT1.1 and AHA2 extends to the antagonistic role of AMTs, as NH4+ activates AMT1.3 signaling, promoting LR emergence and higher-order LR branching (Meier et al., 2020). While the role of auxin in NO3–mediated LR elongation is well established, further research is needed to determine whether AMTs and auxin also contribute to regulating the NRT1.1-QSK1-AHA2 module, particularly under varying N ratios and concentrations.
On top of NRT1.1 function, additional players have been added during adaptation of Arabidopsis to NH4+ nutrition and low pH, such as the S-type channel SLAH3 (Zheng et al., 2015; Xiao et al., 2022), and the C2H2-type zinc finger transcription factor SENSITIVE TO PROTON RHIZOTOXICITY 1 (STOP1) (Ye et al., 2021). SLAH3 form a functional unit with NRT1.1 and exports chloride and NO3- from the cytosol, whereas STOP1 plays a central role in the induction of NRT1.1 and CIPK23 as well as in the excretion of organic acids to solubilize external Pi. Hence, SLAH3 and STOP1 link external NH4+ with the reported efficient of NO3- and Pi acquisition, respectively (Tian et al., 2021; Ye et al., 2021). Considering that CIPK23 also phosphorylates NRT1.1, the potassium transporter AKT1, and the metal transporter IRT1, STOP1 appears to be behind in ensuring the nutrient balance and ion homeostasis during excessive NH4+ nutrition.
Lastly, there are regulatory elements influenced under co-provision of NH4+, altering the NRT1.1-dependent NO3- response, such as NIN LIKE PROTEIN 7 (NLP7) or the POLYADENYLATION FACTOR SUBUNIT 3 (CPSF30). Zhao et al. (2018) revealed that NLP7 acts as a positive regulatory factor upstream of NRT1.1 when NH4+ is present, modulating the NO3- signaling function of NRT1.1. Similarly, the presence of NH4+ prompts CPSF30 to act upstream of NRT1.1 in NO3- signaling without affecting the CPSF30 expression (Li et al., 2017).
Although the mechanisms underlying the NO3– and NH4+-dependent sensing function of NRT1.1 remain unclear, it could all respond to a constant fine adjustment in the intracellular pH. In fact, NH4+ and NO3- account for about 70% of the cations and anions taken up by plants, so they can exert a strong influence on intracellular pH despite the strong buffering capacity of the cytosol. The Figure 1 reveals a complex picture, involving for the first time an interconnection between NRTs and AMTs, whose contribution to regulating pH-dependent mechanisms of N uptake will depend on the balance between nitrate- and ammonium-based nutrition, and vice versa. In the case of NO3- nutrition, H+ consumption during NO3- reduction to nitrite and NH4+ increases cytosolic pH, and this declines plasma membrane (PM) H+-ATPase activity and the consequent respiratory expense. Therefore, cotransport of H+ together with a cytosolic pH increase during NO3- uptake and reduction contributes to overall plant cell alkalinization. On the other hand, the decrease in pH driven in part by the AMTs-mediated NH4+ uptake enrich the transcription of key players that activates not only the expression of NRT1.1, but also earlier genes involved in the acquisition of other nutrients whose availability or acquisition is particularly sensitive to external pH changes. For instance, Pi uptake is also an anion/H+ cotransport process and the second most important macronutrient for plants, aside from its close relationship with Fe availability and homeostasis. While, under low Pi conditions, the repression of NRT2s by NIGTs might be part of an adaptive response to reduce energy consumption and maintain the ionic balance, AMTs are upregulated to promote the NH4+ uptake and subsequent H+ release. For this reason, the AMT1 family is considered to play a role in local Pi signaling, and potentially increases Pi solubility in the medium (Paz-Ares et al., 2022). The discovery of NRT1.1 and AMT-dependent regulation of N and P-related genes may lead to a deeper understanding of the strategies employed by plants to adapt to changing nutrient environments.
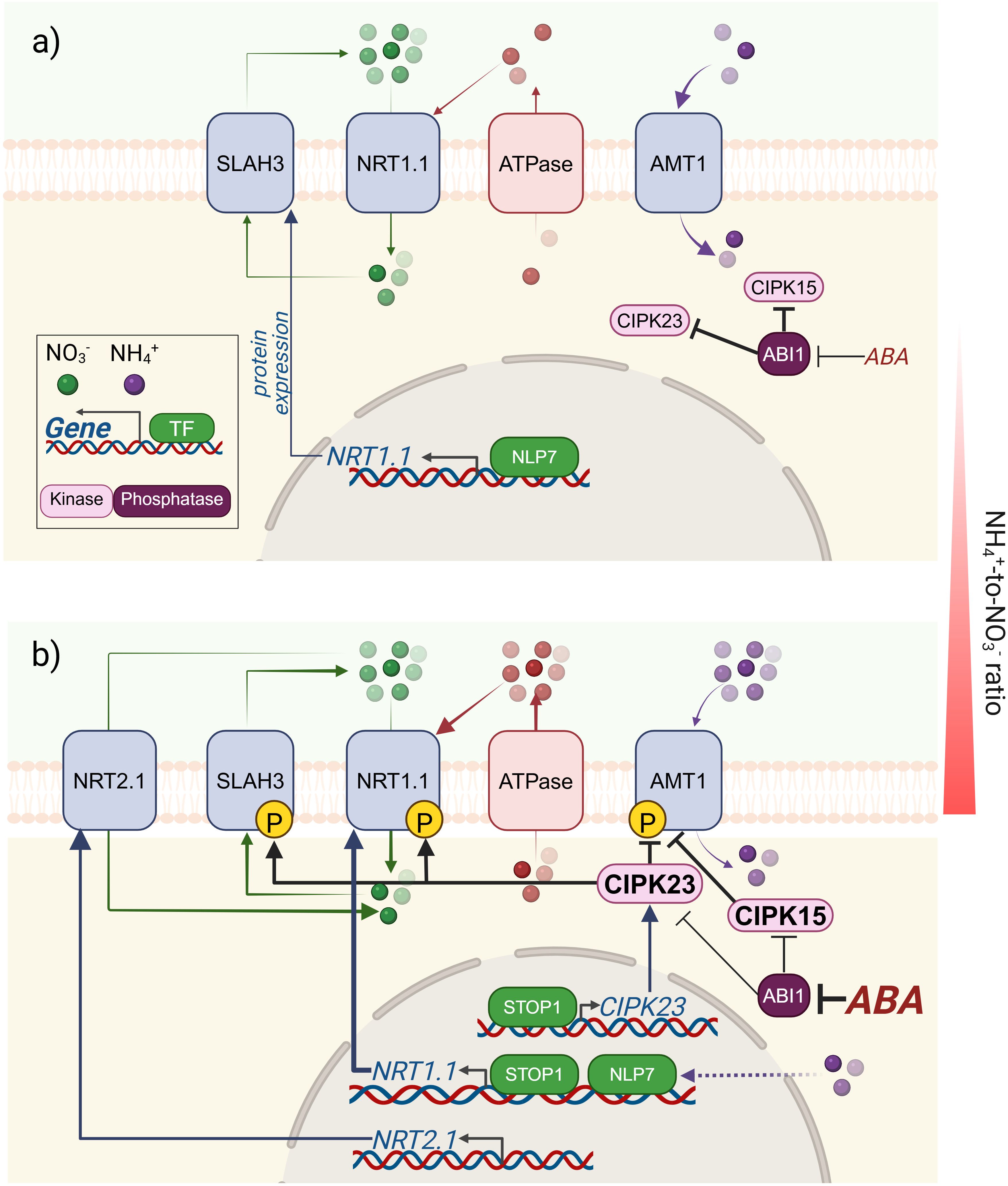
Figure 1. Schematic summary of the common factors that mediate the pH-dependent control of N uptake through NRTs and AMTs. (a) Under a balanced NH4+-to-NO3- ratio for proper plant growth, NO3- uptake is mainly sustained by NRT1.1, which imposes an as-yet-unclear repression on NRT2.1. In addition, in the presence of NH4+, NRT1.1 is enhanced by NLP7. In the case of NH4+, specific uptake occurs through AMTs, and the deactivation of AMTs by phosphorylation is repressed by the phosphatase ABI1. (b) As the external NH4+-NO3- ratio increases, it also increases the stress associated with the higher NH4+-dependent extrusion of H+ to the apoplast. Intracellular acidification promotes an enrichment of STOP1 in the nucleus, which induces the transcription of CIPK23 (the kinase that represses AMT1 activity by phosphorylation), but also induces SLAH3 and NRT1.1 to increase NO3- efflux/influx as a buffering cycle to alleviate NH4+-dependent H+ stress. ABA accumulation under this unfavorable situation inactivates the phosphatases ABI1 and 2, keeping active the phosphorylating activity of the kinases CIPK15 and CIPK23 towards their common N transporter targets. The figure was created using BioRender.
New perspectives
To date, attempts to enhance N use efficiency in crops through modulation of AMT gene expression have achieved limited success, likely due to NH4+ sensitivity. This phenomenon represents a bottleneck in improving NH4+-based nutrition, as NH4+ when supplied as the predominant N form proves highly deleterious for most crops (Britto and Kronzucker, 2002). Notably, NH4+ sensitivity exhibits substantial interspecies and intraspecies variability among cultivars, reflecting an intrinsic ecophysiological adaptation (Rivero-Marcos et al., 2024), where the role of AMTs in NH4+ acquisition and signaling/adaptation may also vary substantially across plant systems.
In contrast, NO3- transporters have been more thoroughly characterized, owing partly to their broader functional diversity across plant species (Ruffel et al., 2025). This disparity is not surprising given the predominance of NO3- as the primary inorganic N form in aerobic soils - a consequence of microbial competition for reduced N compounds, agricultural tillage practices, and other edaphic and climate factors (Mao et al., 2025). The nitrate transporter NRT1.1 has evolved dual functionality as a NO3- sensor, like the TF NLP7, and the overexpression of these components enhances growth and N use efficiency in several species (e.g., AtNRT1.1, Sakuraba et al., 2021; AtNLP7, Yu et al., 2016; OsNRT1.1A, Hu et al, 2015; OsNRT1.1B, Wang et al., 2018).
A key unresolved question concerns the synergistic growth response observed when NO3- and NH4+ are co-supplied. Remarkably, even micromolar NO3- concentrations (≤100 μM) - considered non-nutritional – to NH4+-fed plants can significantly alleviate NH4+ toxicity and stimulate growth in some NH4+-preferring species (Garnica et al., 2009; Yan et al., 2023). This strongly implicates the primary NO3- response (PNR) pathway in growth optimization, and likely includes downstream AMT-related components. Consequently, classical PNR components like NRT1.1, but also potentially AMTs - which may serve dual roles as NH4+ transporters and sensors (Pflüger et al., 2024) - represent promising targets for crop improvement strategies. Future research should investigate kinases and phosphatases regulated by NO3- signaling that may modulate AMT activity, and potential physical interactions between NRT1.1 and AMTs, which is plausible given the known ‘promiscuity’ among N transporters and key proteins regulating N uptake (Zhu et al., 2024).
Here, it has been shown how AMTs and NRTs sustain the vital uptake of N, thus affecting the N use efficiency of the plant and cellular pH homeostasis. While AMT-dependent NH4+ uptake largely contributes to apoplast and rhizosphere acidification, NO3- and other nutrient uptake such as Pi becomes more efficient through pH-dependent induction of TFs like STOP1. NRT1.1 has the most essential contribution not only in NO3- acquisition and signaling, but also in modulating NH4+ response in the absence of NO3-. Therefore, understanding the mechanisms by which plants regulate N acquisition in dependence or not of NRT1.1, and evaluating the contribution of AMTs to changes in internal and external pH, is critical for improving N use efficiency and nutrient uptake across diverse species and crop systems. These insights indicate that conventional approaches examining the responses to NO3- and NH4+ separately, or after N deprivation, are no longer sufficient. Field-relevant advances will require integrated approaches using both N forms and corresponding mutants, capitalizing on their synergistic potential to achieve growth optimization and yield improvement under reduced N inputs.
Author contributions
MR-M: Writing – original draft, Methodology, Formal Analysis, Visualization, Validation, Resources, Data curation, Supervision, Software, Investigation, Project administration, Conceptualization, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bloom, A. J. (2015). The increasing importance of distinguishing among plant nitrogen sources. Curr. Opin. Plant Biol. 25, 10–16. doi: 10.1016/j.pbi.2015.03.002
Bouguyon, E., Brun, F., Meynard, D., Kubeš, M., Pervent, M., Leran, S., et al. (2015). Multiple mechanisms of nitrate sensing by Arabidopsis nitrate transceptor NRT1.1. Nat. Plants 1, 1–8. doi: 10.1038/nplants.2015.15
Bouguyon, E., Gojon, A., and Nacry, P. (2012). Nitrate sensing and signaling in plants. Semin. Cell Dev. Biol. 23, 648–654. doi: 10.1016/j.semcdb.2012.01.004
Britto, D. T. and Kronzucker, H. J. (2002). NH4+ toxicity in higher plants: a critical review. J. Plant Physiol. 159, 567–584. doi: 10.1078/0176-1617-0774
Britto, D. T. and Kronzucker, H. J. (2013). Ecological significance and complexity of N-source preference in plants. Ann. Bot. 112, 957–963. doi: 10.1093/aob/mct157
Chen, H. Y., Chen, Y. N., Wang, H. Y., Liu, Z. T., Frommer, W. B., and Ho, C. H. (2020). Feedback inhibition of AMT1 NH4+-transporters mediated by CIPK15 kinase. BMC Biol. 18, 196. doi: 10.1186/s12915-020-00934-w
Coleto, I., de la Peña, M., Rodríguez-Escalante, J., Bejarano, I., Glauser, G., Aparicio-Tejo, P. M., et al. (2017). Leaves play a central role in the adaptation of nitrogen and sulfur metabolism to ammonium nutrition in oilseed rape (Brassica napus). BMC Plant Biol. 17, 157. doi: 10.1186/s12870-017-1100-9
Du, W., Zhang, Y., Si, J., Zhang, Y., Fan, S., Xia, H., et al. (2021). Nitrate alleviates ammonium toxicity in wheat (Triticum aestivum L.) by regulating tricarboxylic acid cycle and reducing rhizospheric acidification and oxidative damage. Plant Signal. Behav. 16, 12–12. doi: 10.1080/15592324.2021.1991687
Duan, F., Giehl, R. F. H., Geldner, N., Salt, D. E., and von Wirén, N. (2018). Root zone–specific localization of AMTs determines ammonium transport pathways and nitrogen allocation to shoots. PloS Biol. 16, e2006024. doi: 10.1371/journal.pbio.2006024
Fang, X. Z., Fang, S. Q., Ye, Z. Q., Liu, D., Zhao, K. L., and Jin, C. W. (2021). NRT1.1 dual-affinity nitrate transport/signalling and its roles in plant abiotic stress resistance. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.715694
Fang, X. Z., Tian, W. H., Liu, X. X., Lin, X. Y., Jin, C. W., and Zheng, S. J. (2016). Alleviation of proton toxicity by nitrate uptake specifically depends on nitrate transporter 1.1 in Arabidopsis. New Phytol. 211, 149–158. doi: 10.1111/nph.2016.211.issue-1
Ganz, P., Porras-Murillo, R., Ijato, T., Menz, J., Straub, T., Stührwohldt, N., et al. (2022). Abscisic acid influences ammonium transport via regulation of kinase CIPK23 and ammonium transporters. Plant Physiol. 190, 1275–1288. doi: 10.1093/plphys/kiac315
Garnica, M., Houdusse, F., Yvin, J. C., and Garcia-Mina, J. M. (2009). Nitrate modifies urea root uptake and assimilation in wheat seedlings. J. Sci. Food Agric. 89, 55–62. doi: 10.1002/jsfa.v89:1
Garnica, M., Houdusse, F., Zamarreño, A. M., and Garcia-Mina, J. M. (2010). The signal effect of nitrate supply enhances active forms of cytokinins and indole acetic content and reduces abscisic acid in wheat plants grown with ammonium. J. Plant Physiol. 167, 1264–1272. doi: 10.1016/j.jplph.2010.04.013
Guo, F.-Q., Young, J., and Crawford, N. M. (2003). The nitrate transporter atNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in arabidopsis. Plant Cell 15, 107–117. doi: 10.1105/tpc.006312
Hachiya, T., Inaba, J., Wakazaki, M., Sato, M., Toyooka, K., Miyagi, A., et al. (2021). Excessive ammonium assimilation by plastidic glutamine synthetase causes ammonium toxicity in Arabidopsis thaliana. Nat. Commun. 12, 1–10. doi: 10.1038/s41467-021-25238-7
Hachiya, T., Makita, N., Bach, L., Gojon, A., Nakagawa, T., and Sakakibara, H. (2024). Genetic and transcriptomic dissection of nitrate-independent function of Arabidopsis NRT1.1/NPF6.3/CHL1 under high ammonium condition. J. Soil Sci. Plant Nutr. 70, 321–325. doi: 10.1080/00380768.2024.2408295
Hachiya, T., Mizokami, Y., Miyata, K., Tholen, D., Watanabe, C. K., and Noguchi, K. (2011). Evidence for a nitrate-independent function of the nitrate sensor NRT1.1 in Arabidopsis thaliana. J. Plant Res. 124, 425–430. doi: 10.1007/s10265-010-0385-7
Hachiya, T. and Noguchi, K. (2011). Mutation of NRT1.1 enhances ammonium/low pH-tolerance in Arabiopsis thaliana. Plant Signaling Behav. 6, 706–708. doi: 10.4161/psb.6.5.15068
Hachiya, T., Watanabe, C. K., Fujimoto, M., Ishikawa, T., Takahara, K., Kawai-Yamada, M., et al. (2012). Nitrate addition alleviates ammonium toxicity without lessening ammonium accumulation, organic acid depletion and inorganic cation depletion in Arabidopsis thaliana shoots. Plant Cell Physiol. 53, 577–591. doi: 10.1093/pcp/pcs012
Ho, C.-H., Lin, S.-H., Hu, H.-C., and Tsay, Y.-F. (2009). CHL1 functions as a nitrate sensor in plants. Cell 138, 1184–1194. doi: 10.1016/j.cell.2009.07.004
Howarth, R. W. (2008). Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 8, 14–20. doi: 10.1016/j.hal.2008.08.015
Hu, B., Wang, W., Ou, S., Tang, J., Li, H., Che, R., et al. (2015). Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 47 (7), 834–838. doi: 10.1038/ng.3337
Jian, S., Liao, Q., Song, H., Liu, Q., Lepo, J. E., Guan, C., et al. (2018). NRT1.1-related NH4+ toxicity is associated with a disturbed balance between NH4+ uptake and assimilation. Plant Physiol. 178, 1473–1488. doi: 10.1104/pp.18.00410
Kochian, L. V., Hoekenga, O. A., and Piñeros, M. A. (2004). How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 55, 459–493. doi: 10.1146/annurev.arplant.55.031903.141655
Krouk, G., Lacombe, B., Bielach, A., Perrine-Walker, F., Malinska, K., Mounier, E., et al. (2010). Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 18, 927–937. doi: 10.1016/j.devcel.2010.05.008
Lark, R. M., Milne, A. E., Addiscott, T. M., Goulding, K. W. T., Webster, C. P., and O’Flaherty, S. (2004). Scale- and location-dependent correlation of nitrous oxide emissions with soil properties: an analysis using wavelets. Eur. J. Soil Sci. 55, 611–627. doi: 10.1111/j.1365-2389.2004.00620.x
Léran, S., Edel, K. H., Pervent, M., Hashimoto, K., Corratgé-Faillie, C., Offenborn, J. N., et al. (2015). Nitrate sensing and uptake in Arabidopsis are enhanced by ABI2, a phosphatase inactivated by the stress hormone abscisic acid. Sci. Signaling 8, ra43–ra43. doi: 10.1126/scisignal.aaa4829
Li, H., Hu, B., and Chu, C. (2017). Nitrogen use efficiency in crops: lessons from Arabidopsis and rice. J. Exp. Bot. 68, 2477–2488. doi: 10.1093/jxb/erx101
Loqué, D., Yuan, L., Kojima, S., Gojon, A., Wirth, J., Gazzarrini, S., et al. (2006). Additive contribution of AMT1;1 and AMT1;3 to high-affinity ammonium uptake across the plasma membrane of nitrogen-deficient Arabidopsis roots. Plant J. 48, 522–534. doi: 10.1111/j.1365-313X.2006.02887.x
Mao, J., Wang, J., Liao, J., Xu, X., Tian, D., Zhang, R., et al. (2025). Plant nitrogen uptake preference and drivers in natural ecosystems at the global scale. Plant Phytol. 246, 972–983. doi: 10.1111/nph.70030
Marino, D., Ariz, I., Lasa, B., Santamaría, E., Fernández-Irigoyen, J., González-Murua, C., et al. (2016). Quantitative proteomics reveals the importance of nitrogen source to control glucosinolate metabolism in Arabidopsis thaliana and Brassica oleracea. J. Exp. Bot. 67, 3313–3323. doi: 10.1093/jxb/erw147
Meier, M., Liu, Y., Lay-Pruitt, K. S., Takahashi, H., and von Wirén, N. (2020). Auxin-mediated root branching is determined by the form of available nitrogen. Nat. Plants 6, 1136–1145. doi: 10.1038/s41477-020-00756-2
Miller, A. J., Fan, X., Orsel, M., Smith, S. J., and Wells, D. M. (2007). Nitrate transport and signaling. J. Exp. Bot. 58, 2297–2306. doi: 10.1093/jxb/erm066
Muños, S., Cazettes, C., Fizames, C., Gaymard, F., Tillard, P., Lepetit, M., et al. (2004). Transcript profiling in the chl1–5 mutant of arabidopsis reveals a role of the nitrate transporter NRT1.1 in the regulation of another nitrate transporter, NRT2.1. Plant Cell 16, 2433–2447. doi: 10.1105/tpc.104.024380
Ortiz-Ramirez, C., Mora, S. I., Trejo, J., and Pantoja, O. (2011). PvAMT1;1, a highly selective ammonium transporter that functions as H+/NH4+ symporter. J. Biol. Chem. 286 (36), 31113–31122. doi: 10.1074/jbc.M111.261693
Paz-Ares, J., Puga, M. I., Rojas-Triana, M., Martínez-Hevia, I., Díaz, S., Poza-Carrión, C., et al. (2022). Plant adaptation to low phosphorus availability: Core signaling, crosstalks, and applied implications. Mol. Plant 15, 104–124. doi: 10.1016/j.molp.2021.12.005
Pflüger, T., Gschell, M., Zhang, L., Shnitsar, V., Zabadné, A. J., Zierep, P., et al. (2024). How sensor Amt-like proteins integrate ammonium signals. Sci. Adv. 10, eadm944. doi: 10.1126/sciadv.adm9441
Rashid, M., Bera, S., Banerjee, M., Medvinsky, A. B., Sun, G. Q., Li, B. L., et al. (2020). Feedforward control of plant nitrate transporter NRT1.1 biphasic adaptive activity. Biophys. J. 118, 898–908. doi: 10.1016/j.bpj.2019.10.018
Rivero-Marcos, M., Lasa, B., Neves, T., Zamarreño, A. M., García-Mina, J. M., García-Olaverri, C., et al. (2024). Plant ammonium sensitivity is associated with the external pH adaptation, repertoire of nitrogen transporters, and nitrogen requirement. J. Exp. Bot. 75, 3557–3578. doi: 10.1093/jxb/erae106
Ruffel, S., Del Rosario, J., Lacombe, B., Rouached, H., Gutiérrez, R. A., Coruzzi, G. M., et al. (2025). Nitrate sensing and signaling in plants: comparative insights and nutritional interactions. Annu. Rev. Plant Biology; 76, 25–52. doi: 10.1146/annurev-arplant-083123-053039
Sakuraba, Y., Chaganzhana, Mabuchi, A., Iba, K., and Yanagisawa, S. (2021). Enhanced NRT1.1/NPF6.3 expression in shoots improves growth under nitrogen deficiency stress in Arabidopsis. Commun. Biol. 4, 256. doi: 10.1038/s42003-021-01775-1
Søegaard, K. (2009). Nitrogen fertilization of grass/clover under cutting and grazing by dairy cows. Acta Agric. Scand. B Soil Plant Sci. 59, 139–150. doi: 10.1080/09064710802022911
Straub, T., Ludewig, U., and Neuhäuser, B. (2017). The kinase CIPK23 inhibits ammonium transport in arabidopsis thaliana. Plant Cell 29, 409–422. doi: 10.1105/tpc.16.00806
Sun, J., Bankston, J. R., Payandeh, J., Hinds, T. R., Zagotta, W. N., and Zheng, N. (2014). Crystal structure of the plant dual-affinity nitrate transporter NRT1.1. Nature 507, 73–77. doi: 10.1038/nature13074
Tian, W. H., Ye, J. Y., Cui, M. Q., Chang, J. B., Liu, Y., Li, G. X., et al. (2021). A transcription factor STOP1-centered pathway coordinates ammonium and phosphate acquisition in Arabidopsis. Mol. Plant 14, 1554–1568. doi: 10.1016/j.molp.2021.06.024
Tsai, H. H. and Schmidt, W. (2021). The enigma of environmental pH sensing in plants. Nat. Plants 7, 106–115. doi: 10.1038/s41477-020-00831-8
Tsay, Y. F., Schroeder, J. I., Feldmann, K. A., and Crawford, N. M. (1993). The herbicide sensitivity gene CHL1 of arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72, 705–713. doi: 10.1016/0092-8674(93)90399-B
Wang, W., Hu, B., Yuan, D., Liu, Y., Che, R., Hu, Y., et al. (2018). Expression of the nitrate transporter gene OsNRT1.1A/OsNPF6.3 confers high yield and early maturation in rice. Plant Cell 30, 638–651. doi: 10.1105/tpc.17.00809
Wang, R., Xing, X., Wang, Y., Tran, A., and Crawford, N. M. (2009). A genetic screen for nitrate regulatory mutants captures the nitrate transporter gene NRT1.1. Plant Physiol. 151, 472–478. doi: 10.1104/pp.109.140434
Xiao, C., Sun, D., Liu, B., Fang, X., Li, P., Jiang, Y., et al. (2022). Nitrate transporter NRT1.1 and anion channel SLAH3 form a functional unit to regulate nitrate-dependent alleviation of ammonium toxicity. J. Integr. Plant Biol. 64, 942–957. doi: 10.1111/jipb.13239
Xu, G., Fan, X., and Miller, A. J. (2012). Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 63, 153–182. doi: 10.1146/annurev-arplant-042811-105532
Yan, Y., Zhang, Z., Sun, H., Liu, X., Xie, J., Qiu, Y., et al. (2023). Nitrate confers rice adaptation to high ammonium by suppressing its uptake but promoting its assimilation. Mol. Plant; 16, 1871–1874. doi: 10.1016/j.molp.2023.11.008
Ye, J. Y., Tian, W. H., Zhou, M., Zhu, Q. Y., Du, W. X., Zhu, Y. X., et al. (2021). STOP1 activates NRT1.1-mediated nitrate uptake to create a favorable rhizospheric pH for plant adaptation to acidity. Plant Cell 33 (12), 3658–3674. doi: 10.1093/plcell/koab226
Yu, L. H., Wu, J., Tang, H., Yuan, Y., Wang, S. M., Wang, Y. P., et al. (2016). Overexpression of Arabidopsis NLP7 improves plant growth under both nitrogen-limiting and -sufficient conditions by enhancing nitrogen and carbon assimilation. Sci. Rep. 6, 27795. doi: 10.1038/srep27795
Yuan, L., Loqué, D., Kojima, S., Rauch, S., Ishiyama, K., Inoue, E., et al. (2007). The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell 19, 2636–2652. doi: 10.1105/tpc.107.052134
Zhao, L., Zhang, W., Yang, Y., Li, Z., Li, N., Qi, S., et al. (2018). The Arabidopsis NLP7 gene regulates nitrate signaling via NRT1.1-dependent pathway in the presence of ammonium. Sci. Rep. 8, 1–13. doi: 10.1038/s41598-018-20038-4
Zheng, X., He, K., Kleist, T., Chen, F., and Luan, S. (2015). Anion channel SLAH3 functions in nitrate-dependent alleviation of ammonium toxicity in Arabidopsis. Plant Cell Environ. 38, 474–486. doi: 10.1111/pce.2015.38.issue-3
Keywords: ammonium, AMTs, CIPK23, low pH, nitrate signaling, NRT1.1, SLAH3, STOP1
Citation: Rivero-Marcos M (2025) Functional crosstalk between nitrate and ammonium transporters in N acquisition and pH homeostasis. Front. Plant Sci. 16:1634119. doi: 10.3389/fpls.2025.1634119
Received: 23 May 2025; Accepted: 09 June 2025;
Published: 27 June 2025.
Edited by:
Ali Raza, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Angel Llamas, University of Cordoba, SpainKulasekaran Ramesh, Indian Institute of Oilseeds Research (ICAR), India
Copyright © 2025 Rivero-Marcos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mikel Rivero-Marcos, bWlrZWwucml2ZXJvQHVuYXZhcnJhLmVz
 Mikel Rivero-Marcos
Mikel Rivero-Marcos