- 1College of Life Science, Langfang Normal University, Langfang, Hebei, China
- 2State Key Laboratory of North China Crop Improvement and Regulation, Hebei Agricultural University, Baoding, China
- 3Deparment of Crop Science, Faculty of Plant and Animal Sciences and Technology, Marondera University of Agricultural Sciences and Technology, Marondera, Zimbabwe
Drought stress causes peculiar challenges to plant cells reliant on turgor pressure and a polysaccharides-enriched cell wall for growth and development. Appropriate cell wall changes in mechanical properties and biochemical composition under stress conditions constitute an indispensable stress adaptation strategy. A better understanding of stress-induced cell wall modifications is not only crucial for accruing fundamental scientific knowledge in plant biology, but will help us design novel strategies for enhancing crop drought tolerance. Here, we extensively reviewed how selected cell wall remodeling mechanisms, including cell wall demethylesterification, cell wall loosening and stiffening, stomata guard cell wall adjustment, cell wall lignification and root cell wall suberization orchestrate plant drought tolerance, revealing a potential target area for drought tolerance improvement in crops. Stress-induced demethylesterification of pectins, mediated by pectin methylesterases, permits calcium crosslinking of polyphenolics, which enhances cell wall rigidity and may help in intra-cell water preservation. Cell wall proteins such as xyloglucan endotransglucosylases/hydrolase, β-glucanases and expansins are regulated by drought stress, and orchestrate cell turgor-driven cell expansion, through modulating the loosening of cell wall polysaccharides, enabling cell and organ growth under those conditions. Meanwhile, overexpression of certain cell wall proteins/genes such as expansins may promote drought tolerance by improving cell water retention, antioxidant capacity, water use efficiency, and osmotic adjustment. We also discuss the genetic, transcriptional, and phytohormonal regulations of cell wall remodeling. Further, we highlight the recent advancements in elucidation of plant cell wall biosynthesis as aided by cutting-edge high-resolution imaging techniques that now facilitate direct visualization and quantitative in-situ (real-time) microanalysis of cell wall chemical composition and dynamics. Integrating latest cell wall imaging techniques to innovative single-cell omics, genome editing, and advanced data analysis approaches could facilitate appropriate cell wall modifications necessary for drought tolerance engineering in crop plants.
1 Introduction
Plants constantly encounter harsh environmental conditions throughout their lifespan. Among these conditions, drought stress is the primary abiotic factor hindering plant growth, development, and productivity, threatening crop production (Martignago et al., 2020; Bashir et al., 2021). Drought is often defined in a meteorological sense to mean a period of below normal precipitation that restricts plant growth and productivity in a natural or agricultural system (Boyer, 1982). However, drought stress (more relevant to plant physiology and referred to herein) is a different concept which refers to a plant water status when there is reduced water available for the plant, due to a decrease in water potential (ψw, or the free energy of water) and turgor, that is enough to disrupt the normal plant physiological functioning (Kramer and Boyer, 1995; Osmolovskaya et al., 2018; Fradera-Soler et al., 2022). Therefore, drought stress responses often mean responses to an altered plant water status due to reduced plant available water (as water acquires a lower free energy state in relation to unstressed conditions) (Verslues et al., 2006; Juenger and Verslues, 2023). Similar to plant cell turgor, drought stress is often quantified in pressure terms (as a decrease in ψw) (Kramer and Boyer, 1995; Verslues et al., 2006), permitting an assessment of the two to be made to establish the plant-water relationship and the direction of water movement along the soil-plant-continuum (Juenger and Verslues, 2023). Meanwhile, decreased ψw complicates the ability of the plant to take up water, which in turn prompts a repertoire of responses that enable the plant to avoid water loss, permit continual water uptake at decreased ψw, or help the plant to tolerate a state of reduced tissue water content (Verslues et al., 2006; Osmolovskaya et al., 2018). The severity of drought stress on a crop depends on its intensity and duration, genotypic capacity of species to resist, the plant developmental stage at occurrence, and the plant tissue affected (Zia et al., 2021). Whilst “drought” naturally causes drought stress over time, a short period (of just few days or even one day) without water may be sufficient enough to cause water deficit (drought stress) that negatively impact yield (Shao et al., 2009; Cluzel, 2024; Vadez et al., 2024), especially if it occurs at the critical stage of plant development such as anthesis or grain filling stages in maize (Vennam et al., 2023). The physiological effects of drought stress on plants include decreased leaf water potential, loss of cell turgor, disrupted plant water relations, reactive oxygen species (ROS) over-accumulation, impaired photosynthesis, inhibited cell growth, impaired metabolism of cell wall components, compromised stomatal functioning, etc. (detailed in (Le Gall et al., 2015; Osmolovskaya et al., 2018; Gupta et al., 2020)). In concert, all these factors retard plant growth and development, and decrease crop yields. Therefore, the increased drought incidences and severity associated with climate change (Osmolovskaya et al., 2018; Means, 2023), often causing moderate to severe drought stress that limit crop productivity and threatens global food security (Vadez et al., 2024), motivate the need to develop drought stress-tolerant crops for sustainable food production. This depends on first gaining a mechanistic understanding of how plants respond and adapt to such stress (Zhang et al., 2022).
Plants respond to drought stress by evoking elaborate cellular, physiological, biochemical, and anatomical changes, including cell wall modifications, root architectural and biochemical adjustments, phytohormonal elicitation, etc (Gupta et al., 2020; Seleiman et al., 2021). These morpho-physiological responses are tightly regulated by genetic and molecular mechanisms (Bashir et al., 2021; Liu and Qin, 2021). In particular, the cell wall is a complex and dynamic entity whose properties are tightly regulated via cell wall remodeling (see Table 1 for definitions), which refers to controlled modification, rearrangement, degradation or/and reconstruction of the cell wall in both growing and mature cells in response to various cues (Barnes and Anderson, 2018; Fradera-Soler et al., 2022). These modifications include cell wall loosening, cell wall stiffening, etc. and can be in response to biotic (Bacete et al., 2018; Swaminathan et al., 2022; Molina et al., 2024a) or abiotic stresses (Moore et al., 2008; Tenhaken, 2014; Oliveira et al., 2020; Jardine et al., 2022; Li et al., 2024) and/or developmental cues. For instance, cell wall components such as cellulose, hemicellulose and pectin are dynamically remodeled as an immune response against pathogen infection (Wan et al., 2021). Upon infection, cell wall hydrolysis induced by cell wall degrading enzymes releases carbohydrates (gylcans) that are sensed by plant receptors as alert signals to trigger plant immune response (Molina et al., 2024a; Molina et al., 2024b). Thus, infection-induced cell wall modification crucially mediates plant defense signaling and pathogen resistance (Wan et al., 2021; Molina et al., 2024a). Besides modulating biotic stress response, cell wall remodeling essentially mediates abiotic stress resistance in plants (Tenhaken, 2014; Houston et al., 2016; Jardine et al., 2022). In some instances, pathogen-triggered cell wall-related immune responses may partially overlap with abiotic (eg. salinity) stress-induced adaptive mechanisms, offering an exquisite machinery for combined stress resistance (Gigli-Bisceglia and Testerink, 2021), Thus, cell wall remodelng is an indispensable plant stress adaptation strategy.
Essentially, drought stress results in loss of cell turgor, decreased leaf and root water potentials, osmotic adjustment, decreased stomatal conductance, etc (Osmolovskaya et al., 2018; Hemati et al., 2022). Since turgor pressure drives cell expansion and growth (dependent upon cell wall extensibility), drought stress-triggered reduction in cell turgor pressure leads to reduced or ceased growth, due to reduced cell wall extensibility and cell expansion (Le Gall et al., 2015). In response, plant cell walls undergo dynamic mechanical and chemical composition modifications to cope with these drought stress effects (Tenhaken, 2014; Le Gall et al., 2015). Besides, drought stress, just like other abiotic or biotic stresses, induces cell wall damages, such as cellulose damage or reduction, or pectin breakdown, etc., which compromise the cell wall integrity (CWI) and proper functioning of the cell. These changes prompt appropriate cell wall damage responses, including cell wall stress sensing and signaling, cell wall remodeling mechanisms, and activation of downstream gene expressions and physiological responses, to ensure CWI maintenance and plant adaptation to drought stress (Le Gall et al., 2015; Vaahtera et al., 2019). In some instances, the plant cell wall elasticity is critical in facilitating differential root cell wall responses, such as loosening and stiffening within different root zones, necessary for continued root growth under drought stress conditions (Wu and Cosgrove, 2000; Cosgrove, 2016a). Furthermore, the guard cell wall is dynamically remodeled, for instance, through differential thickening and orientation of cellulose microfibrils, to permit continual stomatal opening and closing necessary for adaptation to different drought stress episodes (Amsbury et al., 2016; Jaafar and Anderson, 2024).
Cell wall remodeling processes are regulated by several loosening and stiffening enzymes/proteins, including pectin methylesterases (PMEs) which catalyze cell-wall esters hydrolysis, α-expansins (EXPAs), β-glucanases, peroxidases (PODs), etc (Tenhaken, 2014; Chebli and Geitmann, 2017; Wu et al., 2018; Perrot et al., 2022), with the resultant physicochemical shifts being critical for proper cell and tissue morphogenesis and stress adaptation (Chebli and Geitmann, 2017; Jardine et al., 2022). The activities of these cell wall remodeling-involved enzymes are tightly controlled, spatio-temporally (Barnes and Anderson, 2018). Besides, different phytohormones such as auxins (Jobert et al., 2023) and brassinosteroids (BRs) (Rao and Dixon, 2017) precisely regulate and potentiate the transcriptional output, actions and modulation of genes encoding cell wall remodeling-involved enzymes (Jobert et al., 2023). Meanwhile, several papers have uncovered the role of cell wall remodeling in enhancing plant abiotic stress tolerance, including salinity (Gigli-Bisceglia and Testerink, 2021; Dabravolski and Isayenkov, 2023), cold (Bilska-Kos et al., 2022; Kutsuno et al., 2023), cadmium (Loix et al., 2017), and heat (Wu H-C. et al., 2017; Wu et al., 2018), among others (Le Gall et al., 2015; Ezquer et al., 2020; García-Angulo and Largo-Gosens, 2022). For example, negatively charged pectin effectively sequester cadmium whereas lignification immobilizes cadmium to enhance cadmium tolerance in plants (Loix et al., 2017). Cell wall integrity (CWI) maintenance, lignin accumulation and amplified ascorbate-mediated antioxidant defense help plants adapt to salinity (Rui and Dinneny, 2020; Liu J. et al., 2021; Dabravolski and Isayenkov, 2023). Increased expression of lignin biosynthesis-related enzymes (such as peroxidases), and other cell-wall related proteins (including expansins) enhances thermotolerance acquisition (Wu and Jinn, 2010; Tenhaken, 2014; Wu et al., 2018). Thus, apt stress-induced cell wall compositional shifts constitute an effective strategy regulating plant abiotic stress adaptation (Oliveira et al., 2020). Despite all these examples revealing the role of cell wall remodeling in plant abiotic stress resistance, relatively less focus has been placed on its role in drought tolerance. Thus, our knowledge on how stress-induced cell wall modifications mediate drought tolerance remains fragmented.
More recently, an increased number of researches have revealed the role of cell wall remodeling as an important strategy for drought stress response and adaptation in several plant species. For example, cell wall O-acetylation (through enhanced O-acetyl esters, but down-regulated methyl ester hydrolysis) under drought stress in poplar (Populus trichocarpa) fine-tunes cell wall elasticity, regulates proper vascular tissue functioning, and influences growth-stress response trade-offs (Jardine et al., 2022). In soybean (Glycine max), increased cell wall plasticity and crosslinking under drought contribute to improved hydraulic conductance, water use efficiency, photosynthesis performance, and sustained higher plant growth under stress (Coutinho et al., 2021). Meanwhile, environmental-acclimation-triggered leaf cell wall modifications in Vitis vinifera have been suggested to crucially regulate leaf physiology by markedly affecting photosynthesis and water relations in an environmental condition-dependent manner (Roig-Oliver et al., 2020). These examples buttress the significant role of cell wall remodeling as a plant drought response strategy; hence, a budding area for research, and a promising trait for enhancing crop drought tolerance and yield (Ganie and Ahammed, 2021; García-Angulo and Largo-Gosens, 2022). Therefore, a better understanding of the stress-induced plant cell wall modifications may help us create novel strategies for enhancing crop drought tolerance (Piccinini et al., 2024).
In this review, we parse together the recent insights on how selected cell wall remodeling mechanisms, including cell wall demethylesterification, cell wall loosening and stiffening, stomata guard cell adjustment, cell wall lignification and root cell wall suberization orchestrate plant drought tolerance, revealing a potential target area for drought tolerance improvement in crops. First, we describe the primary structural composition of cell wall, cell wall-related sensing and signaling systems governing abiotic stress response, and forms of stress-induced cell wall modifications. We also review phytohormonal regulation of cell wall modifications that orchestrate drought tolerance in plants. Further, we highlight the recent advances in cell wall imaging techniques that now facilitate direct visualization and real-time quantification of native cell wall chemical composition and dynamics. We sum up by proffering future prospects related to the topic.
2 Cell wall composition and architecture
Plant cell wall is a complex assembly, primarily constituted by polysaccharide polymers (cellulose, hemicelluloses, and pectins), comprising distinct monosaccharides moieties joined by different type of bonds. The polymers may possess different biochemical decorations/modifications (such as methylations, acetylation, calcium bound ions) and may form connections with other cell wall components such as structural proteins (glycoproteins) and phenolic compounds (eg. polyphenolic lignin) (Cosgrove, 2005; Loqué et al., 2015; Höfte and Voxeur, 2017; Anderson and Kieber, 2020; Delmer et al., 2024). These different complex materials are woven into a strong, dynamically organized, and adaptable polymer structure primed for different functions (growth, pathogen defense, stress response, etc.) depending with the situation (Miedes et al., 2014; Wu et al., 2018; Zhang et al., 2021b). Although specific plant cell wall constituents and overall composition vary with plant species, tissue type, and tissue developmental state (Braidwood et al., 2014; Loqué et al., 2015), generally, polysaccharides constitute more than 80% of the total composite volume, with structural proteins and other minor components (eg., minerals) filling up the balance (Höfte and Voxeur, 2017). The plant cell wall is multi-layered or organized from the outside towards the plasma membrane (PM) as the middle lamella and primary cell wall (PCW), or/and secondary cell wall (SCW) in some species (Loqué et al., 2015; Höfte and Voxeur, 2017; Wu et al., 2018) (Figure 1). The dynamic PCW is formed in young dividing cells and functions to provide flexibility and basic structural support, protecting the cell and facilitating cell to cell interactions, whereas the thicker and more durable SCW is located between the PCW and the PM and is deposited beneath the primary wall of some specific cell types at a later stage after the cell has ceased growing and dividing (Hamann, 2012; Houston et al., 2016; Li Z. et al., 2016). The SCW is considered a vital adaptation characteristic that enables upright growth and environmental stress endurance in land plants (Houston et al., 2016).
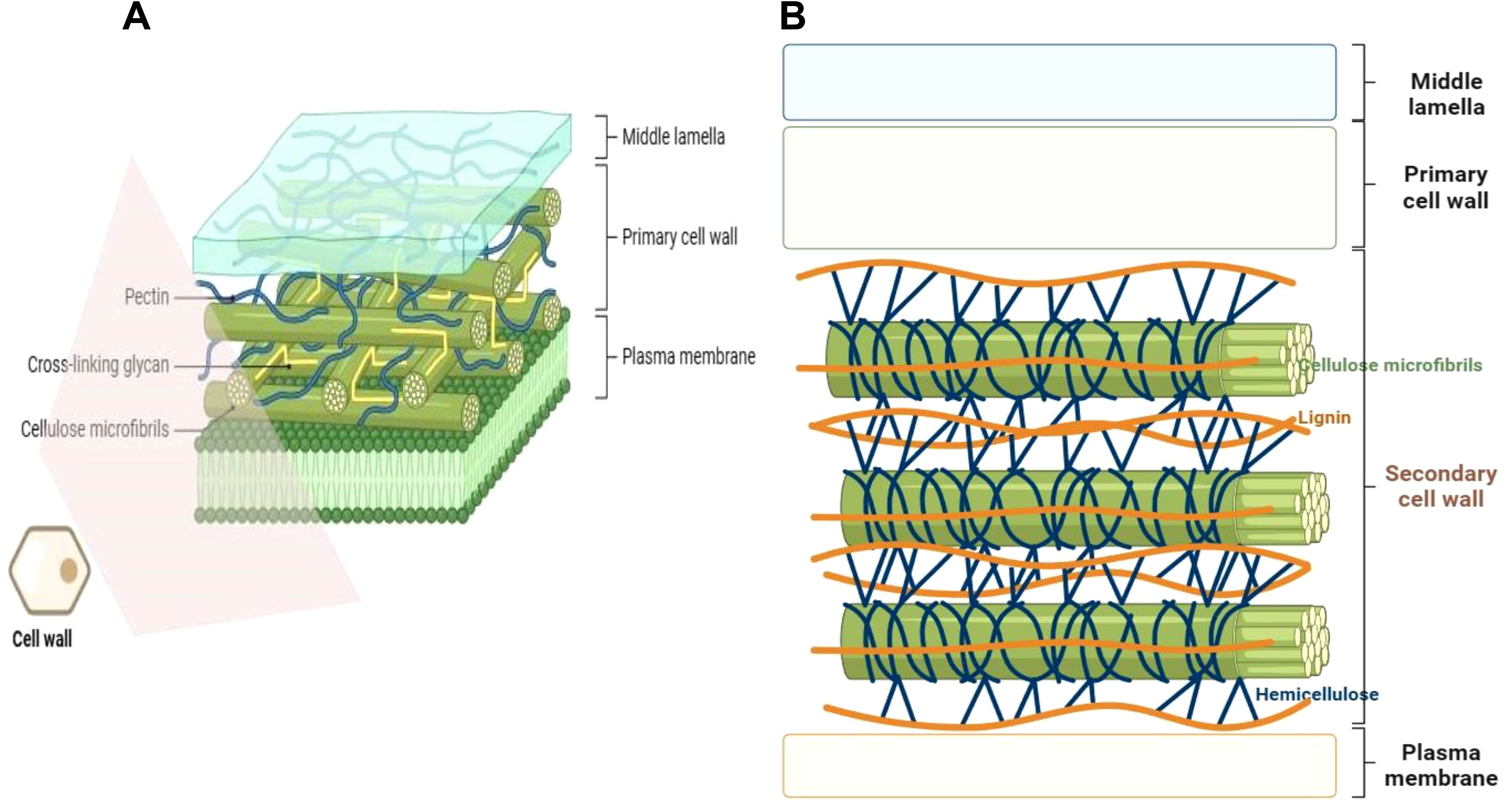
Figure 1. Plant cell wall position and structural configuration. (A) The primary cell wall is composed of cellulose microfibrils, pectin matrix, cross-linking xyloglucan hemicelluloses, and glycoproteins. It lacks lignin. (B) The secondary cell wall comprises of cellulose microfibrils and xylan hemicelluloses embedded with the hydrophobic polyphenol lignin and gylcoproteins. Some components like glycoproteins are not shown for simplicity purposes. The diagrams were created in Biorender.com (https://app.biorender.com/).
Sandwiched between the middle lamella and the plasma membrane, the thin layered primary cell wall is built up of polysaccharides cellulose, hemicelluloses and pectin (Figure 1A). The PCW lacks lignification, and is pervious to small molecules. At the same time, it is elastic and extensible to enable the growth of the cell via the acid growth mechanism (Cosgrove, 2005; Braidwood et al., 2014; Dabravolski and Isayenkov, 2023). Depending with the relative amounts (composition) of different polysaccharides, the PCW can be categorized into type I and type II cell walls. Type I PCWs are found in dicots, non-commelinoid monocots and gymnosperms (such as conifers), whilst type II PCWs are limited to commelinoid monocots, including grasses (Carpita, 1996; Vogel, 2008; Sarkar et al., 2009; Barnes and Anderson, 2018). Grass cell wall type and dicots cell wall type also exhibit further differences in types of lignin and ferulic acid esterification they possess (Sarkar et al., 2009). Both type I and type II PCWs possess same amounts of cellulose, but differ in proportion of hemicelluloses (Carpita and McCann, 2020). Whereas type I PCWs contain a lesser proportion of hemicelluloses and a greater pectin content, there is predomination of hemicellulose but less pectin content in type II PCWs (Vogel, 2008; Barnes and Anderson, 2018). Additionally, type I PCWs have a greater proportion of structural proteins as compared to type II PCWs. Furthermore, the two PCW types have different hemicellulose composition, whereby type I PCW hemicellulose is mainly comprised of xyloglucan (XyG) and minor amounts of glucomannans, arabinoxylans and glucuronoxylans. Contrariwise, type II PCW hemicellulose largely contains glucuroarabinoxylans (GAX) and less of mixed-linkage β-(1,3)-(1,4)-D-glucan (MLG) and XyG. Besides, type II PCWs possess hydroxycinnamic acids that crosslink with GAX (Carpita, 1996; Vogel, 2008; Sarkar et al., 2009; Le Gall et al., 2015; Barnes and Anderson, 2018; Carpita and McCann, 2020).
The thick layered, stiff, and often waterproof, secondary cell wall forms beneath the PCW in some specific cell types, and is constituted by cellulose, xylan hemicelluloses and lignin (Kang et al., 2019; Dabravolski and Isayenkov, 2023) (Figure 1B). Lignin is a major structural component of plant secondary cell walls. It is a complex polymer composed of covalently joined monolignol subunits (coniferyl, sinapyl, and hydroxyphenyl alcohols) derived from amino acid L-phenylalanine linked via free radical coupling. The monolignol subunits are subjected to redox-mediated polymerization to produce guaiacyl (G), syringyl (S), hydroxyphenyl (H) units of lignin, respectively (Vanholme et al., 2010). Gymnosperm lignins are almost entirely made of G units, whilst angiosperm lignins are composed of mainly G and S units, with much reduced percentage of H units (Vanholme et al., 2010). Due to their assemblage by non-enzymatic polymerization, lignin chains lack a definite structure (Delmer et al., 2024). Lignin is sometimes covalently connected to ferulated xylan side chains, and renders apoplastic cell wall domains stiff/firm (Swaminathan et al., 2022). This increases the plant tissue`s structural robustness or mechanical strength (Polo et al., 2020), and facilitates its better resistance to pathogen attack and acclimatization to environmental changes (Khasin et al., 2021; Yadav and Chattopadhyay, 2023). SCWs of certain boundary tissue layers of plants such as root endodermis may be enriched with suberin, a lipophilic polymer composed of phenolic-derived glycerol, fatty acids, and aromatics, that provide structural support to these SCWs (Woolfson et al., 2022). Besides, suberin may act as a protective barrier, controlling water and ion transport (Vishwanath et al., 2015) (discussed in detail later under section 4.5).
Cellulose constitutes the major polymer in most plant cell walls, and is comprised of unbranched β -(1,4)-linked glucan chains (Zhang et al., 2021b). Despite the simplistic structural nature of cellulose, its bundling into multiscale cellulosic fibrils creates a complex nanostructure with high tensile strength and an important load-bearing function (Zhang et al., 2021b). Each cellulose fibril is composed of fundamental units called microfibrils, of approximately 35 Å width, corresponding roughly to a 6×6 array of chains. These native cellulose chains (cellulose I) are in a parallel arrangement as has been revealed by diffraction patterns analyses, high-resolution electron microscopy, and atomic force microscopy (AFM) (see (Delmer et al., 2024) and references therein). The microfibrils are modelled in a crystalline form, with the parallel chains oriented in cellulose Iα or Iβ lattices, with hydrogen-bonded sheets of chains running diagonally across the rectangular cross section (see (Jarvis, 2017)). These microfibrils are synthesized by the cellulose synthase (CeSA) enzyme complexes (Turner and Kumar, 2017; Wilson et al., 2021). The cellulose synthase complexes are thought to be assembled in the Golgi apparatus and transported to the PM via vesicle trafficking (Zhang et al., 2021b).
Hemicelluloses comprise of xylans, xyloglucans, β-(1,3;1,4)-glucan, mannans, and glucomannans. They all contain β-(1,4)-glycosyl connected backbones with the same equatorial arrangements (Zhang et al., 2021b). Except for xylans (whose backbone is synthesized by the GT47 and GT43 family Type II membrane proteins (Wu et al., 2010)), backbones of most hemicelluloses are synthesized by the GT2 family cellulose synthase-like proteins (Scheller and Ulvskov, 2010). However, their glycosyl residues vary. The backbones are frequently replaced by different glycosyl residues with unique patterns, which explains their architectural and physiochemical differences (reviewed in (Zhang et al., 2021b)). Xylan is the most abundant hemicellulosic polysaccharide in vascular plants (most common in type II primary cell walls and eudicot secondary walls), with a β-(1,4)-linked xylosyl sugar backbone decked with non-/methylated or non-/feruloylated arabinose and glucuronic acid residues as side chains. Xyloglucan hemicellulose is the principal polysaccharide in type I (dicotyledonous) primary walls, possessing a β-(1,4)-linked glucan sugar backbone decked with side chains of xylose, fucose, and galactose residues (Zabotina, 2012). Other hemicelluloses include mannans and glucomannans. Mannans are commonly found in gymnosperms, and possess linear glycan chains with a β-(1,4)-linked mannose backbone. Glucomannan is just mannan with intercalary β-(1,4)-linked glucose in its backbone (Carpita and Gibeaut, 1993; Ebringerová, 2005; Anderson and Kieber, 2020).
Pectin is a highly complex and heterogeneous polysaccharide, with d-galacturonic acid (GalA) residues linked via α-1,4-glycosidic bonds (Mohnen, 2008). Pectin is synthesized in the Golgi apparatus in methylesterifed form (with homologalacturonan methylesterified at the C-6 position) and exported into the wall where it is demethylesterified by the action of PMEs (Pelloux et al., 2007; Mohnen, 2008; Harholt et al., 2010; Wu et al., 2022). Pectin is composed of four main types of domains, viz., homogalacturonan (HG), rhamnogalacturonan I (RGI), RGII, and xylogalacturonan (XGA), covalently linked to create a pectin matrix (Willats et al., 2001; Mohnen, 2008; Ropartz and Ralet, 2020). HG, or the pectin smooth region, is the dominant component of pectin, and comprises α-1,4-linked d-GalA chains, and contributes to structural elasticity (Ridley et al., 2001; Willats et al., 2001; Ropartz and Ralet, 2020). HG is synthesized by galacturonosyl transferases (GAUTs) of the GT8 family (Atmodjo et al., 2013). PME can act upon HG, in both random and blockwise demethylesterification, thus playing dual roles in both cell wall loosening and stiffening (Du et al., 2022). In the former, HG can become susceptible to the actions of other cell wall degrading enzymes such as polygalacturonases and pectate lyases, resulting in cell wall loosening. In the later, HG demethylesterified in a blockwise fashion exposes carboxyl groups which can cross-link with Ca2+ ions to form a pectate gel network, known as an “egg box”, which contributes to cell wall stiffening (Pelloux et al., 2007). RGI backbone is built upon galacturonic acid and rhamnose residues, laced with arabinan, arabinogalactan, and galactan side chains; whereas RGII comprises of HG backbone decked with side chains of several sugar units and diverse glycosyl linkages (Anderson and Kieber, 2020; Malacarne et al., 2024). Pectin makes up the main constituent of primary cell walls, in both dicotyledonous (~ 35%) and monocotyledonous (2-10% in Gramineae) species, playing essential roles in cell adhesion and cell wall plasticity (O’neill et al., 1990; Willats et al., 2001; Mohnen, 2008; Wu et al., 2018). Thus, pectins control cell and organ growth, development, cell-wall porosity, and response to environmental cues (Willats et al., 2001; Caffall and Mohnen, 2009; Wolf and Greiner, 2012; Wu et al., 2022). Despite also being localized in the PCW and SCW, pectins are more enriched in the middle lamella, and their biosynthesis requires different kinds of transferases, such as glycosyl-, methyl-, and acetyltransferases (Ridley et al., 2001; Mohnen, 2008; Caffall and Mohnen, 2009; Yang and Anderson, 2020).
Besides, cell wall-related proteins and enzymes, including expansins (α-expansins, β-expansins, EXPLA, EXPLB), xyloglucan endo-β-transglucosylases/hydrolases (XET/XTHs), endo-1,4-β-D-glucanase (EGase), extensins, proline rich proteins and glycine-rich proteins, as well as minor components (eg., minerals, small metabolites, etc.) are also important constituents of cell walls (Jamet et al., 2006; Qiu et al., 2021). Cell wall-related proteins regulate cell wall extensibility, which modulates cell enlargement and expansion (Le Gall et al., 2015). Expansins induce wall creep and wall relaxation, via loosening of the linkages between cellulose microfibrils (Cosgrove, 2000; Sampedro and Cosgrove, 2005; Cosgrove, 2016b) (discussed in detail hereafter in section 4.2.1). Extensins (eg. leucine-rich repeat extensins) and hydroxyproline-rich proteins (HRGPs) are involved in regulating cell wall expansion, cell growth, and cell wall integrity sensing (Herger et al., 2019). Other cell wall-modifying enzymes crucially regulate cell wall plasticity/rheology (Cosgrove, 1999; Le Gall et al., 2015). These cell wall-modifying proteins actively participate in cell wall remodeling processes in response to different growth and stress stimuli (Höfte and Voxeur, 2017; Anderson and Kieber, 2020). In summary, plant cell wall is a complex and highly dynamic entity whose components and structure are constantly and appropriately modified during growth and development and in response to environmental cues.
3 Molecular and genetic regulation of cell wall related sensing and signaling systems involved in abiotic stress response
Plant cells possess an effective CWI sensing mechanism that monitors functional and structural changes to the cell wall to ensure a balance between cell wall biosynthesis and turgor-driven cell wall extension/growth without compromising the CWI (Vaahtera et al., 2019; Rui and Dinneny, 2020). Most of the work on CWI sensing in plants is based on Arabidopsis thaliana (Arabidopsis), but its elucidation in other species is gaining traction. CWI signaling pathway is initiated by PM-localized cell surface sensors, including several members of the receptor-like Ser/Thr protein-kinase (RLK) and receptor-like protein (RLP) families, and relayed downstream via the mitogen-activated protein kinase (MAPK) cascades among other signaling modules (Bashline et al., 2014; He et al., 2018). The molecular mechanisms of CWI-modulated stress signaling share commonalities with the osmotic signaling cascade, that may be drought- or salinity-induced. For instance, alterations in cell water potential and turgor pressure are common among these stresses (Verslues et al., 2006; Novaković et al., 2018). In fully hydrated (> 60%) primary cell walls, reduced cell water status alters the gel-like matrix, which affects the organization and interaction of cell wall polymers and the wall-plasma membrane physical connection (Thompson and Islam, 2021). In ion toxicities, for instance high salinity-induced, monovalent ions (eg., Na+ and K+) may displace Ca2+ ions and disrupt the pectin “egg box” matrix structure (Peaucelle et al., 2012; Engelsdorf et al., 2018). These stress-induced alterations in cell wall mechanical properties yield CWI changes that are perceived by the CWI sensors such as Wall Associated Kinases (WAKs), Feronia (FER), and Receptor-Like Protein Kinases (RPKs) (Ringli, 2010; Hamann, 2012; Novaković et al., 2018; Vaahtera et al., 2019; Liu J. et al., 2021; Baez et al., 2022). Meanwhile, cold stress may cause the ice crystallization of the apoplastic space, which may result in cell wall deformation (Rajashekar and Lafta, 1996). Cold and drought stresses may similarly affect cellular exchanges through a decrease in membrane permeability or a decrease in water content and plasmolysis. Osmotic adjustment becomes critical in response to both stresses to ensure osmotic potential and a protective layer around cell structures and macromolecules (Charrier, 2021). Thus, plants have developed similar molecular response pathways for these stresses, mediated, in part, by abscisic acid (ABA) and Dehydration-Responsive Element (DRE) cis-acting element or C-Repeat (CRT) (Yamaguchi-Shinozaki and Shinozaki, 1994; Stockinger et al., 1997; Nakashima et al., 2014; Charrier, 2021; Kim et al., 2024); these pathways regulate responses to osmotic stress (Charrier, 2021). Besides, both drought and cold stresses induce stomatal closure, although, in cold stress, this mechanism seems to be ABA-independent (Wilkinson et al., 2001; Kim et al., 2024). It will be crucial to understand how these combined stresses are perceived by the cell wall and get integrated to the CWI maintenance pathway to produce a robust stress response. This will create a possibility to enhance plant drought tolerance via cell wall modification (Barbut et al., 2024).
The cell wall-perceived stress signal is transduced into the cell, prompting an eventual repertoire of responses to be coordinated (Ringli, 2010; Smokvarska et al., 2020). Cell wall-mediated stress responses encompass CWI sensing, ROS generation, and phytohormonal signaling pathways converging (Rao and Dixon, 2017; Novaković et al., 2018; Vaahtera et al., 2019) (Figure 2). Hyperosmotic stress triggers ROS accumulation, which act as secondary messengers for inducting several plant responses (Martinière et al., 2019). Ca2+ influx evokes Respiratory Burst Oxidase Homologues (RBOHs), triggering the production of ROS (H2O2 and OH•−) in the cell wall (Suzuki et al., 2011). The osmotically triggered ROS buildup requires Rho-of-plants 6 (ROP6), an upstream activator of RBOH D or/and F (Martinière et al., 2019). The ROP6 creates osmotic stimuli-dependent nanodomains within the plasma membrane, ensuring signal specificity (Smokvarska et al., 2020). When combined with POD activity, ROS production fuels radical coupling of extensins and signaling, resulting in pectate buildup and wall stiffening (Francoz et al., 2015) (Figure 2). However, limited POD activity or H2O2 induces OH•− radicals’ formation, triggering the severing of polycarbohydrate sugar bonds, and consequent cell wall loosening (Kidwai et al., 2020).
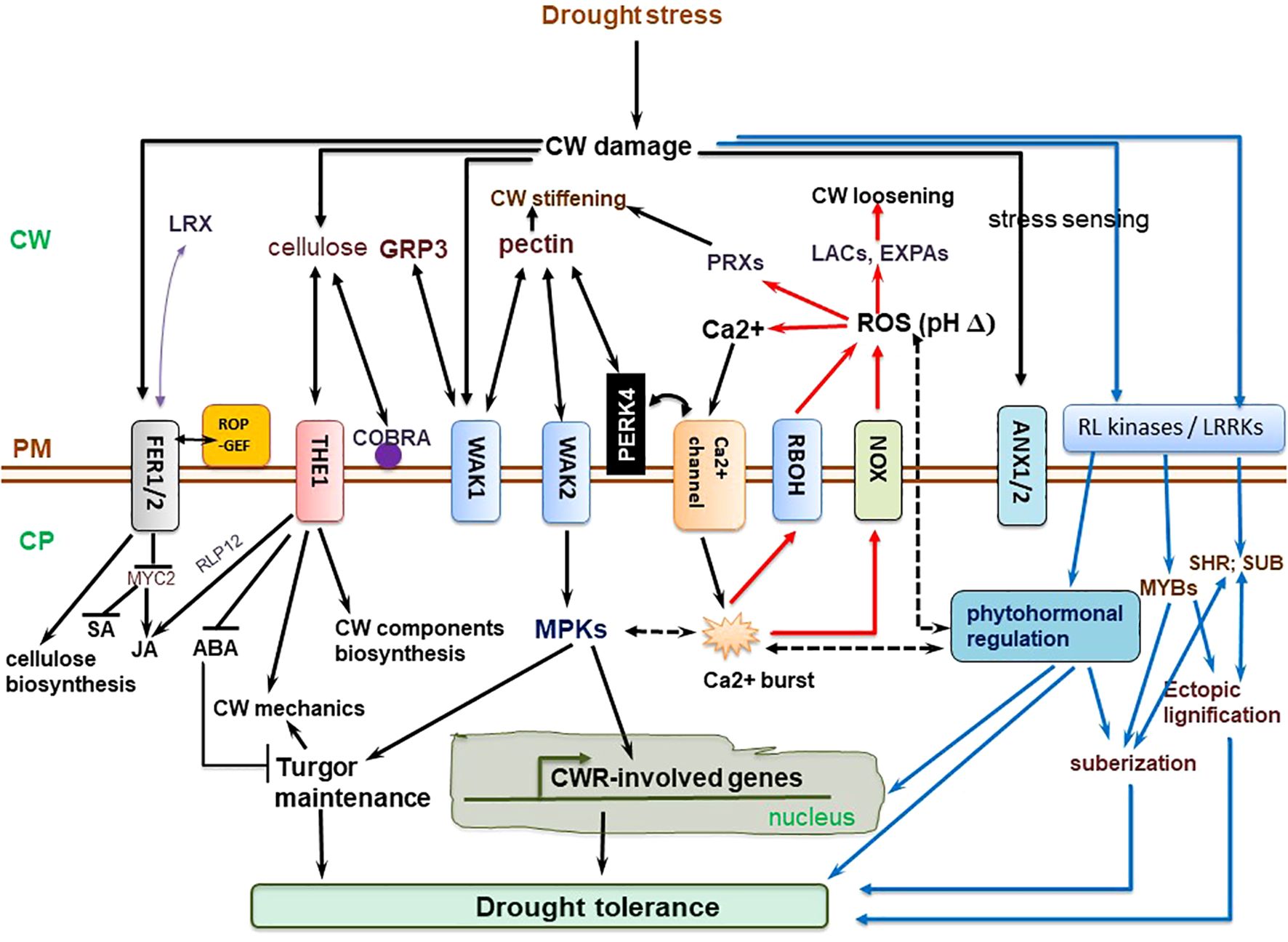
Figure 2. Molecular regulation mechanisms of cell wall (CW) remodeling related to plant drought tolerance. Receptor-like kinases (RLKs) such as leucine-rich repeat receptor-like kinases (LRRKs), wall-associated kinases (WAKs), and extracellular proteins, including extracellular leucine-rich repeat-containing extension 1(LRX1) perceive drought-triggered CW damage or composition and/or structural alterations to orchestrate CW stress signaling, which is mediated via mitogen-activated protein kinases (MAPKs). The MAPK cascade regulate turgor maintenance through vacuolar invertase, and together with NACs and MYB transcription factors, regulate the activation of downstream CW remodeling-involved genes. The CWI maintenance pathway crosstalk with ROS and phytohormonal signaling pathways to actuate plant drought tolerance. Plasma membrane (PM)-localized proline-rich extension-like receptor kinase 4 (PERK4) interacts with pectin and stimulates Ca2+ channels, resulting in cytosolic Ca2+ burst, which alters intracellular and extracellular pH, and trigger NADPH oxidase (NOX)-reliant reactive oxygen species (ROS) accumulation. The Ca2+ influx evokes respiratory burst oxidase homologues, causing ROS accumulation in the cell wall. This, together with peroxidase (PRX) activity, facilitates oxidative crosslinking of extensins and pectin accumulation, stiffening the cell wall. On the other hand, limited PRX activity or H2O2 generation trigger OH•− radicals accumulation, increased severing of sugar bonds in polysaccharides, and eventual CW loosening. THESEUS1 (THE1) perceives cellulose reduction-induced structural defects in CW, whereas COBRA is a PM-localized and GPI-anchored protein crucial for cellulose microfibrils alignment. ANX1/2, ANXUR1/2 (closest homologs of FER in Arabidopsis thaliana) modulate pollen tube rupture which encompass rapid alterations in CW composition and architecture (Ringli, 2010). Complete black arrows denote cell wall integrity (CWI) maintenance signaling/mechanisms, blue lines relate to phytohormonal regulation and lignification mechanisms already discussed in detail in other sections, red lines relate to reactive oxygen species (ROS) signaling, whereas black dotted lines imply crosstalk among these pathways. ABA, abscisic acid; CP, cytoplasm; EXPAs, expansins; FER1/2, FERONIA 1/2; GRP3, Glycine-rich protein 3; LACs, laccases; JA, jasmonic acid; ROP-GEF, Rho-of-plants-guanosine nucleotide exchange factors; SA, salicylic acid; SHR, SHORT-ROOT transcription factor; SUB, SUBERMAN transcription factor. The illustration is based on (Ringli, 2010; Novaković et al., 2018; Gigli-Bisceglia et al., 2020; Bacete et al., 2022) and others discussed in text.
Several receptor-like kinases, including WAKs family, Catharanthus roseus Receptor-Like Kinase1-Like (CrRLK1L), FER, Leucine-Rich Repeat Receptor-Like Kinases/Protein Kinases, etc. have been known to mediate cell wall stress sensing and signaling (Ringli, 2010; Steinwand and Kieber, 2010; Wolf and Greiner, 2012; Osakabe et al., 2013; Galindo-Trigo et al., 2016; Wolf, 2022). WAKs are the most studied RLKs and are highly conserved in Arabidopsis in five transmembrane protein families (Steinwand and Kieber, 2010). They harbor a cytoplasmic serine threonine kinase, a transmembrane domain, and an extracellular domain (Kohorn and Kohorn, 2012). WAKs, such as WAK1, seem to directly bind to polysaccharides in the wall, through their extracellular N terminus firmly attaching to Ca2+ cross-linked pectin-derived oligogalacturonides (He et al., 1996; Decreux and Messiaen, 2005), to initiate cell wall perception and signaling through MAPKs (such as MAPK3, MAPK6, etc.) and modulate vacuolar invertases and turgor maintenance (Kohorn et al., 2006; Kohorn and Kohorn, 2012). Meanwhile, WAK1 has also been shown to interact with the glycine-rich protein AtGRP-3, which is a cell wall-localized structural protein (Park et al., 2001). WAKs are involved in cell expansion (Wagner and Kohorn, 2001), and are also induced by, and participate in the response to, pathogen attack and several stresses such as wounding, heavy metals, etc. (reviewed in (Ringli, 2010; Kohorn and Kohorn, 2012)). Disruption of WAK expression using WAK2 antisense RNA led to reduced leaf cell expansion (Wagner and Kohorn, 2001), whereas wak2 loss-of-function mutants and WAK4 antisense-RNA-expressing seedlings showed impaired root cell elongation (Kohorn et al., 2006). The growth performance of wak2 loss-of-function mutants exhibited a dependence on extrinsic sugars, signifying that possibly WAKs provide a cell-wall-sensing function, mediated by pectins and sugar metabolism (Kohorn et al., 2006; Kohorn and Kohorn, 2012).
Other members of the RLK family also participate in CWI sensing. Theseus1 (THE1), a member of the CrRLK1L family, was identified as a suppressor of the short-hypocotyl phenotype in the cellulose-deficient procuste1-1 (pcr1-1) mutant (Hématy et al., 2007).Under control (non-stress) conditions, knockout mutants (the1-1, the1-2, the1-3 and the1-6) did not exhibit any phenotypic defects, signifying that THE1 is only activated upon CWI being compromised, reinforcing the idea that THEI functions as a CWI sensor (Hématy et al., 2007; Baez et al., 2022). Additionally, the1 attenuated hypocotyl growth inhibition in other cellulose-deficient mutants, such as cesa3eli1, cesa1rsw1, etc (Hématy et al., 2007; Hématy and Höfte, 2008), suggesting that THE1 is activated by cellulose synthesis perturbation, and may act as a CWI sensor, which in turn evoke the expression of downstream candidate genes that regulate cell elongation (Bashline et al., 2014; Novaković et al., 2018). Meanwhile, Feronia (FER) is a PM-localized receptor kinase and most characterized CWI sensor from the CrRLK1 family (Galindo-Trigo et al., 2016). FER synergizes with different Rapid Alkalinization Factor (RALF) peptide ligands to function in several growth, development, and stress response processes, including CWI maintenance in growing-tip or elongating root-tip cells (Duan et al., 2010; Stegmann et al., 2017; Feng et al., 2018; Cheung, 2024). FER acts as a receptor for numerous RALFs, including RALF1 and RALF34 (Li C. et al., 2016; Gonneau et al., 2018). FER-RALF1 interaction enhances FER phosphorylation ability but inhibits across PM proton transport directed by H+-ATPase (Li C. et al., 2016), which possibly influences cell wall remodeling according to the acid growth theory (Haruta et al., 2014; Baez et al., 2022). FER and its RLK relatives possess extracellular domains that interact with cell wall carbohydrate moieties to sense cell wall perturbations and initiate appropriate cellular responses (Li C. et al., 2016; Moussu et al., 2023). For example, the FER extracellular domain directly interacts with pectin to sense salinity-induced wall defects, and trigger corresponding stress responses (Feng et al., 2018). In addition to the PM and cell wall, the RALF-FER signaling cascades interact with molecules in the cytoplasm and nucleus to modulate a complex intertwined signaling network (Cheung, 2024). FER also interacts with ROP-GEF [Rho-of-plants (ROP)-guanosine nucleotide exchange factors (GEF)] to facilitate the ROP2 exchange of GDP to GTP (Igisch et al., 2022), RBOH activation, and ROS production (Galindo-Trigo et al., 2016; Novaković et al., 2018). Disruption of FER function decreases ROPs levels, hinders ROP-mediated and RBOH-reliant ROS synthesis (Duan et al., 2010). More recently, it has been shown that FER controls the accrual and nano-scale compartmentalization of phosphatidylserine in the PM, to regulate Rho GTPase signaling in Arabidopsis (Smokvarska et al., 2023). Overall, considering the functional diversity of FER and the mechanistic complexity of the FER-anchored signaling modules, FER provides a rich ground for research that could help uncover new insights on plant abiotic stress response and signaling (Li C. et al., 2016; Cheung, 2024).
FEI1 and FEI2, leucine-rich repeat RLKs, belong to RLK subfamily XIII, which is different from the WAK and THE1 subfamilies (Xu et al., 2008; Bashline et al., 2014). They are important for the non-uniform expansion of different root cells in Arabidopsis, as well as cell extension in Arabidopsis stamen (filaments) and etiolated seedling hypocotyls (Xu et al., 2008). FEI1 and FEI2 gene mutations interrupt the non-uniform (anisotropic) cell expansion, hinders biosynthesis of wall polymers, and fortify cellulose biosynthesis repressors (Xu et al., 2008). The fei1 fei2 roots with expansion defects were rescued by the disruption of only 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (an ethylene biosynthesis-related enzyme involved in conversion of Ado-Met to ACC), and not the entire ethylene (Et) response pathway, suggesting that FEI kinases crucially mediate a signaling pathway that integrates cell wall biosynthesis and ACC synthase in Arabidopsis (Xu et al., 2008). More recently, FEI1, FEI2, and Altered Root Hydrotropic Response 1 (ARH1), the three closely linked RLKs, have been shown to exhibit polar localization at the PM regions of Arabidopsis root tips (Chang et al., 2024). Overexpression of these three genes greatly reduced root hydrotropism, but their corresponding loss-of function mutants showed an increased root hydrotropic response tips (Chang et al., 2024). Additionally, the triple mutant arh1-2 fei1-C fei2-C showed cell wall, cutin, and wax (CCW) biosynthesis impairments in its root tips, suggesting that the root tip cell wall integrity, cutin and wax status mediate a balance between root hydrotropism and osmotic tolerance (Chang et al., 2024); this will need further exploration as it may also crucially regulate root responses to drought stress.
A PM-localized receptor-like protein, RLP44, mediates the response to pectin modification through activation of brassinosteroid (BR) signaling pathway (Wolf et al., 2014). RLP44 mediates this activation via direct connection with the BR co-receptor BAK1 (Brassinosteroid-Insensitive 1(BRI1)-Associated Receptor Kinase 1), to integrate cell wall surveillance with hormone signaling, and regulate CWI sensing and growth in Arabidopsis (Wolf et al., 2014). BAK1 can be activated upon both abiotic and biotic stresses, and can also act as a co-receptor for several RLPs mediating DAMP (Damage-Associated Molecular Patterns) and PAMP (Pathogen-Associated Molecular Patterns) recognition (Yasuda et al., 2017; Novaković et al., 2018; Molina et al., 2024a). DAMPs and PAMPs are unique CWI sensors that detect plant cell wall damage (eg. to cellulose and other polysaccharide components such as pectins) caused by pathogen infection, wounding, or other stresses, to activate RLKs or receptor kinases (RKs) that initiate stress signaling cascades (for reviews, see (Molina et al., 2024a; Molina et al., 2024b)). At the same time, these stress-induced cell wall damages prompt cell wall remodeling to ensure CWI maintenance (Hématy et al., 2007; Novaković et al., 2018). In case of DAMPs, the caused by these stresses leads to the release of carbohydrate-based wall molecules (glycans) that are recognized/perceived by the extracellular ectodomains (ECDs) of pattern recognition receptors (PRRs) as DAMPs to actuate pattern-triggered immunity (PTI) response and disease resistance (Molina et al., 2024b). For PAMPs, specific ECD-PRRs of RKs (eg., RKs with leucine-rich repeat and Malectin domains within their ECDs, LRR-MAL RKs (Martín-Dacal et al., 2023)) or RLPs (eg., lysine motif, Lys-M OsCERK1 (Shimizu et al., 2010)) can recognize oligosaccharide/polysaccharide molecules emanating from pathogens in the apoplast as PAMPs (Novaković et al., 2018; Molina et al., 2024b). Examples of PAMPs include chitooligosaccharides from fungi, flagellin from bacterial flagellae, etc (Boller and Felix, 2009; Novaković et al., 2018; Molina et al., 2024b). Likewise, specific ECD-PRRs recognize glycans emanating from the walls and extracellular layers of microbes interacting with plants as microbe-associated molecular patterns (MAMPs), evoking corresponding PTI responses (Boller and Felix, 2009; Molina et al., 2024a). Despite not directly linked to abiotic stress response, further elucidation of these unique glycan-based recognition mechanisms may reveal any possible similarities with abiotic-stress-specific cell wall sensors, which could help in identifying novel abiotic stress cell wall sensors in plants.
Meanwhile, another key receptor, Arabidopsis Receptor Like Protein Kinase 1 (RPK1), is only responsive to abiotic stress, and its overexpression enhances tolerance to drought, heat, salinity, and cold stresses (Osakabe et al., 2010; Novaković et al., 2018). Other RLKs involved in cell wall sensing, including PERK4 (Proline-rich Extension-like Receptor Kinase 4), are discussed in recent reviews ( (Baez et al., 2022; Wolf, 2022). The exact functions of these several RLKs and RLPs are yet to be elucidated. Nonetheless, we expect that as research on CWI sensing and stress signaling gathers much pace, new details will emerge that will help us assign receptor/gene functions and clarify the complex signaling crosstalk involved in abiotic stress response (Novaković et al., 2018). Downstream of these receptor complexes, MAPK cascades are among the key signaling modules which integrate signals and regulate diverse cellular and physiological responses via phosphorylation of several downstream targets (reviewed in (He et al., 2018)).
NAC [no apical meristem (NAM), Arabidopsis ATAF1/2, and CUC2 (cup-shaped cotyledon)] and MYB (myeloblastosis) TFs crucially modulate the induction of downstream SCW-biosynthetic or stress-responsive genes (Wang and Dixon, 2012; Cao Y. et al., 2020), with the transcriptional regulation exhibiting a high plasticity to abiotic stress, which helps plants to appropriately adapt to the stress (Nakano et al., 2015; Yoon et al., 2015; Houston et al., 2016; Choi et al., 2023). For instance, transcriptional actuation of SCW-biosynthesis-related genes by rice and maize secondary wall NACs (SWNs, that is, OsSWNs and ZmSWNs) rescued an Arabidopsis snd1 nst1 double mutant that had secondary wall thickening defects (Zhong et al., 2011). Overexpressed OsSWNs and ZmSWNs significantly activated several SCW-related TFs and biosynthetic genes in Arabidopsis, simultaneously increasing cellulose, xylan, and lignin accrual (Zhong et al., 2011). Additionally, OsMYB46 and ZmMYB46, the functional orthologs of AtMYB46/AtMYB83, effectively activated the whole SCW biosynthesis system after overexpression in Arabidopsis. OsSWNs and ZmSWNs activated OsMYB46 and ZmMYB46 by directly binding to the SCW NAC-binding elements (SNBEs) at their (OsMYB46 and ZmMYB46) promoters (Zhong et al., 2011). Reasonably, these NAC and MYB TFs could be candidates for manipulation to influence lignin biosynthetic genes expression changes that possibly modify plant cell walls, via increased lignin accumulation, to enhance plant drought tolerance (Miyamoto et al., 2020; Han et al., 2022).
Recently, SHORT-ROOT (SHR) transcription factor, a master regulator of endodermal development, has been observed to mediate a transcriptional interplay between lignification and suberization, integrated to stress signaling (Xu H. et al., 2022). Additionally, 13 key MYB TFs (including MYB74, MYB68, MYB36, MYB122, MYB41, MYB39, MYB52, MYB53, etc.) that form multiple sub-networks mediating feedback or feed-forward loops to balance this interplay were uncovered (Xu H. et al., 2022). Among them, sub-networks involving nine MYB TFs were shown to interact with ABA signaling to integrate stress response and root development, suggesting that SHR and these key MYB TFs crucially modulate and integrate multiple developmental and stress signals, and are key targets for genetic engineering for enhancing plant stress adaptation (Xu H. et al., 2022). Equally, the function of SUB TF (partly discussed in section 3 above) crucially modulates transcriptional networks related to suberin, lignin, and phenylpropanoid biosynthesis, as well as phytohormonal signaling (Cohen et al., 2020); hence, its characterization and potential targeting for genetic engineering may be a key step towards enhancing cell wall remodeling-mediated drought tolerance in crop plants (Cohen et al., 2020).
Photo-sensitive Leaf Rolling 1 (PSL1) gene encrypts a cell wall-localized polygalacturonase (PG) that alters cell wall structure and enhances rice drought tolerance (Zhang G. et al., 2021). A psl1 mutant, exhibiting ‘napping’ phenotype and reduced growth, displayed significant cell wall composition modifications as compared Wt plants (Zhang G. et al., 2021). Such cell wall composition alterations improved the mutant`s drought tolerance, through decreasing osmotic and drought stress-induced water loss. Collectively, these results suggested that PSL1, acting as PG, modifies cell wall biosynthesis, plant development, and enhances rice drought stress tolerance (Zhang G. et al., 2021). Equally, Curled Leaf and Dwarf 1/Semi-Rolled Leaf 1 (CLD1/SRL1) gene, that encrypts a glycophosphatidylinositol (GPI)-anchored protein (GAP) involved in controlling other growth and development facets in rice, functionally regulates rice leaf rolling by influencing cell wall synthesis, epidermis integrity, and water homeostasis (Li et al., 2017). A cld1 mutant shows substantially decreased cellulose and lignin contents in leaf SCWs, signifying that deterred CLD1/SRL1 function impacts cell wall development (Li et al., 2017). Additionally, CLD1/SRL1 function deficiency results in leaf epidermis defects (eg., formation of bulliform-like epidermal cells), which reduce the water retention capacity and causes water deficit in cld1 mutant leaves – the main contributor to leaf rolling. Due to the accelerated leaf water loss and reduced leaf water content, cld1 mutant shows decreased water deficit stress tolerance (Li et al., 2017). Overall, CLD1/SRL1 may play an essential role in improving plant drought tolerance, by functionally regulating leaf-rolling and minimizing leaf transpiration (Li et al., 2017), and thus, can be targeted for genetic engineering to enhance crop drought tolerance.
Cellulose synthase gene AtCesA8/IRX1 participates in SCW synthesis, and influences plant drought and osmotic stress tolerance (Chen et al., 2005). Disruption of AtCesA8/IRX1, combined with other two allelic mutants, leaf wilting 2-1 (lew2-1) and lew2-2, improved drought and osmotic stress tolerances in mutant plants as compared to Wt Arabidopsis (Chen et al., 2005). The lew2 mutant accrued greater ABA, proline, and soluble sugar contents in comparison to Wt plants, revealing that knocking down of LEW2 enhances drought tolerance, whereas cellulose synthesis is essential for plant response to osmotic and drought stresses (Chen et al., 2005). Other cell wall remodeling-involved genes important for improving plant drought tolerance are provided in Table 2. Leveraging on modern genomics tools such as genome-wide association studies, (GWAS), which now permit high-resolution mapping of QTLs, we can exploit the abundant natural genetic variation present in progenitor species (Bahri et al., 2020) and cultivars (Martínez et al., 2007), to identify and harness desirable cell wall properties for the genetic improvement of drought tolerance in crop plants (Yoshida et al., 2021).
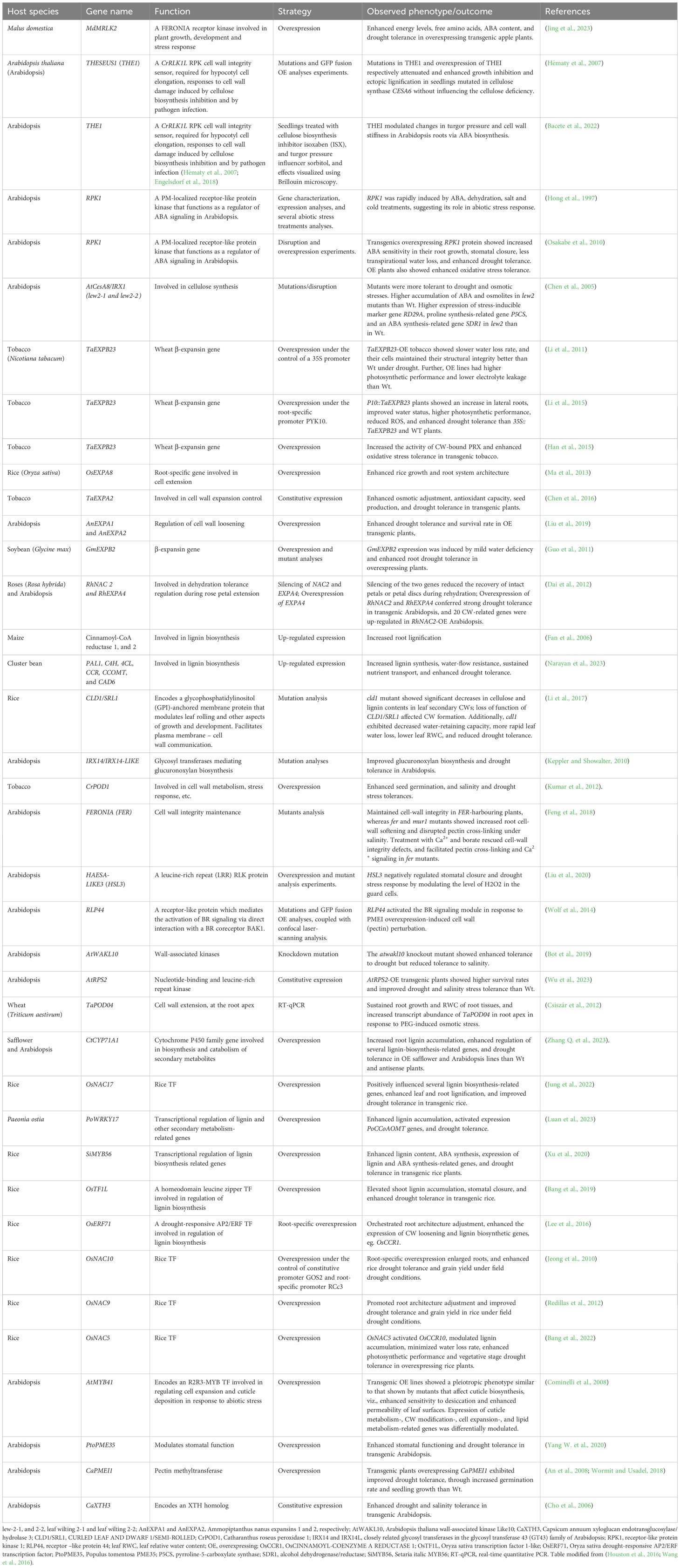
Table 2. Selected cell-wall-modification-related gene candidates for enhancing drought tolerance in plants.
4 Cell wall modifications necessary for plant drought tolerance
Drought stress response-associated cell wall plasticity conceivably contributes to cell turgor maintenance (Martínez et al., 2007), and as such, can be connected with plant drought tolerance (Le Gall et al., 2015). In common beans (Phaseolus vulgaris L.), for example, a comparative analysis of six genotypes showed that more drought tolerant genotypes had a huge drop in elasticity modulus (ϵ), coupled with greater cell wall elasticity, which enabled them to maintain better their turgescence (Martínez et al., 2007). In comparison, drought susceptible genotypes did not display any significant decrease in ϵ or cell wall elasticity, suggesting that cell wall elasticity may be critical for cell integrity maintenance and drought stress tolerance (Martínez et al., 2007; Hessini et al., 2009; Le Gall et al., 2015). Meanwhile, several enzymes, proteins and ions such as PMEs, expansins, β-glucanases, PODs, Ca2+ ions, etc. regulate cell wall remodeling-related loosening and stiffening processes that are critical for inducing growth changes, and drought tolerance (Tenhaken, 2014; Wu et al., 2018; Ganie and Ahammed, 2021; Qiu et al., 2021). Here, various forms of these cell wall modifications are discussed.
4.1 Cell wall pectin ester modifications
Cell wall pectin ester modifications are mainly achieved through the processes of demethylesterification and O-acetylation, discussed hereunder this section.
4.1.1 Demethylesterification by pectin methylesterases
A stress-challenged cell institutes specific cell-wall-protein-biosynthesis-related transcriptional responses, which modify cell wall components, including pectin, and significantly alter the cell wall architecture (Le Gall et al., 2015; Novaković et al., 2018; Wu et al., 2018; Baez et al., 2022; Wu et al., 2022). Pectin modifications are mediated by a large family of cell-wall-localized enzymes, the PMEs, which catalyze the removal of methyl esters from the d-GalA backbone of HG (Braidwood et al., 2014), with their activity being controlled by pectin methylesterase inhibitors (PMEIs) (Sénéchal et al., 2015; Wormit and Usadel, 2018). PMEs regulate apoplastic Ca2+ levels in response to stress (Wu and Jinn, 2010; Wu et al., 2018). The action of PMEs yields carbanions on the HG, permitting the creation of Ca2+ cross-linking networks of unmethylesterified GalA units (Braidwood et al., 2014; Wormit and Usadel, 2018; Cao L. et al., 2020; Kumar et al., 2023), which increases cell wall stiffness (Braybrook and Peaucelle, 2013). This possibly increases cell wall water preservation and limits dehydration. On the other hand, pectin breakdown, due to PME hydrolysis of pectic HG, leads to cell wall loosening and extensibility (Ezaki et al., 2005; Pelloux et al., 2007; Peaucelle et al., 2012). This pectin-induced alteration in cell wall mechanics essentially modulate cell wall growth and response to desiccation (Wu et al., 2018; Liu X. et al., 2021). However, to what extent this pectin-modification-induced cell wall flexibility under water deficit conditions effect plant drought tolerance is yet to be clarified.
Higher levels of demethylesterified HG were observed upon desiccation in desiccation-tolerant plant Craterostigma plantagineum, but this was reversed after rehydration (Jung et al., 2019). A greater amount of de-methylesterified HG, upon desiccation, and when combined with Ca2+ ions, results in the creation of pectate gel-like structures known as “egg-boxes”, which essentially regulate wall biomechanics and cell-cell bonding to enhance cell wall stiffness (Moore et al., 2008; Jung et al., 2019; Chen et al., 2020; Du et al., 2020), which may be critical in preserving cell water, minimizing dehydration and enhancing drought tolerance. Highly de-methylesterified HG also offers extra binding sites for pectin binding proteins (Pelloux et al., 2007), which may be crucial in perceiving cell wall hydration status (Chen et al., 2020). PME-mediated cell-wall demethylesterification has been shown to crucially regulate abiotic stress tolerance, including heat (Huang et al., 2017; Wu et al., 2018), stem lodging (Hongo et al., 2012), salinity (Yan et al., 2018b), etc. Besides, PMEs are also frequently modified in response to drought stress (Tenhaken, 2014; Ezquer et al., 2020). For instance, Capsicum annuum (pepper) gene CaPMEI1 is transcriptionally induced by drought stress, and may be involved in plant drought tolerance (An et al., 2008). Compared to Wt control, CaPMEI1-OE Arabidopsis lines displayed improved drought tolerance, evidenced by increased germination rate and seedling root growth (An et al., 2008). In poplar (Populus tomentosa) and Arabidopsis, PtoPME35 modulates stomatal functioning and drought response (Yang W. et al., 2020). Pectin methylesterification degree is reduced in PtoPME35-OE transgenic poplar plants, whereas overexpressing PtoPME35 in Arabidopsis inhibits stomatal opening, resulting in reduced leaf transpiration under drought stress conditions (Yang W. et al., 2020). In tomato (Solanum lycopersicum L.), transient silencing of the PMEI gene Slpmei27 by virus-induced gene silencing significantly improves drought resistance, through altering cell wall structure, stomatal permeability, and ROS balance, as well as reducing water loss rate (Cheng et al., 2022).
PME35-mediated PCW demethylesterification also regulates mechanical ability of Arabidopsis stems to resist lodging (Hongo et al., 2012), whereas PMEI5-mediated pectin modification enhances dehydration tolerance in onion (Forand et al., 2022), suggesting that it could also play a role in improving drought tolerance. It is known that increased aggregates of water bound within the cell wall preserves tissue hydration and turgor pressure, thereby enhancing cell wall rigidity (Ortega, 2010; Thompson and Islam, 2021). It has been shown that reduced cell-wall pectin content inhibits stress-induced root cell growth (Liu X. et al., 2021). Compared to Wt or mutants with decreased cellulose content, Arabidopsis mutants with decreased pectin or hemicellulose content exhibited no root cell growth under drought conditions suggesting that an appreciable quantity of pectin is needed for root cell growth under drought stress conditions (Liu X. et al., 2021). Although no relationship evidently linked the levels of pectin methylesterification to cell growth, analysis of cell wall composition, coupled with 2β-deoxy-Kdo experiments, suggested that RGII could play a crucial role in these processes (Liu X. et al., 2021). Remarkably, a comparative study in wheat revealed that, compared to drought-sensitive variety Creso, the percentages of RGI and RGII side chains were considerably increased in the drought-resistant variety Capeiti in response to water stress, supporting the role of pectin side chains in drought stress response (Leucci et al., 2008), which is possibly creation of hydrated gels that minimize cell damage (Leucci et al., 2008; Caffall and Mohnen, 2009; Tenhaken, 2014).
4.1.2 Pectin O-acetylation by pectin acetylesterases
Besides the methylesterification and demethylesterification of the α-(1,4)-linked GalA at the O-6 sites of the backbone, which influence the formation of Ca2+ bridges between HGs (Miao et al., 2011), occasional acetylation of pectin at O-2 and O-3 sites also occur as an essential architectural and functional feature of pectin (Zhang et al., 2021b; Shahin et al., 2023). O-acetylation of pectin also leads to the formation of pectic gel, and is mediated by the pectin acetylesterases (PAEs), as well as the Trichome-Birefringence Like (TBL) and Reduced Wall Acetylation (RWA) family proteins (Bischoff et al., 2010; Stranne et al., 2018). For instance, investigations of tbl mutants in Arabidopsis have shown that plant dwarfism, weak stems, and stunted growth are linked to the deficiency of TBL genes (Bischoff et al., 2010). This implies that pectin O-acetylation considerably impacts plant morphogenesis, development, and responses to abiotic and biotic stresses (Gille and Pauly, 2012; Shahin et al., 2023). In Arabidopsis, TBL10 (AT3G06080) has been shown to orchestrate O-acetylation of RGI and abiotic stress responses (Stranne et al., 2018). Compared to Wt plants, tbl10 mutants, displaying reduced RGI O-acetylation, had increased drought tolerance levels, suggesting that this alteration (O-acetylated RGI) may impact water uptake and transport (Stranne et al., 2018).
Plant cell wall O‐acetylation is also adjusted in response to drought stress. For instance, O‐acetylation level in Populus trichocarpa leaf cell walls was significantly elevated in response to drought stress (Jardine et al., 2022). This rapid response to stress, similar to other mechanisms of cell wall methylation of polysaccharides, could provide the plasticity necessary for plant growth changes and stress adaptation, including stomatal closure (Jardine et al., 2022), and influencing photosynthesis and water relations (Roig-Oliver et al., 2020; Ganie and Ahammed, 2021). Besides, plant cell wall O-acetylation is known to crucially regulate physicochemical, mechanical and architectural processes essential for curtailing degradation, simultaneously promoting intermolecular crosstalk among cell-wall polymers (Biely, 2012; Peaucelle et al., 2012; Shahin et al., 2023). Meanwhile, Arabidopsis PAE2, PAE4, and PAE8 have been shown to be induced, and highly expressed, by osmotic stress (Philippe et al., 2017). Considering that these PAEs may also have important roles in photosynthesis (Roig-Oliver et al., 2020), it will be plausible and interesting to functionally characterize these or other phylogenetically-related PAEs under drought stress conditions.
4.2 Cell wall loosening and stiffening
4.2.1 Xyloglucan endotransglucosylases and expansins underpin cell wall loosening-mediated morphogenesis and stress response
Plant cell and organ morphogenesis, under both benign and stress conditions, requires a specialized cell wall remodeling mechanism that relaxes cell wall tensions created by turgor pressure, permitting water influx to reestablish cell wall tension and cell expansion (Cosgrove, 2016a; Chebli and Geitmann, 2017). Cell wall loosening is a form of wall modification that achieves this purpose and underpin creep, ie., a protein-mediated process and irrevocable time-dependent cell extension (Cosgrove, 2018; Zhang et al., 2019). The loosening of cell wall polysaccharides is suggested to play a crucial role under osmotic, drought, or salinity stresses, that is, to sustain the possibility for cells and organs to expand under those conditions (Tenhaken, 2014). This cell wall loosening is mediated by cell wall proteins XTHs, β-glucanases, xyloglucan endo-transglycosylases (XETs), EXPAs, etc., which orchestrate cell turgor-driven cell enlargement (Cosgrove, 2000; Ezquer et al., 2020; Stratilová et al., 2020), and appear to be regulated by drought stress as observed in soybean (Coutinho et al., 2021). The XTHs, β-glucanases, and XETs regulate the remodeling of primary load-bearing components pectin matrix and cellulose/xylogucan network, to generate the morphological alterations necessary for plant development and stress defence (Chebli and Geitmann, 2017). By cleaving and ligating non-load-bearing xyloglucans, these proteins characteristically reduce the number of linkages between cellulose and the load bearing components, resulting in a more easily breakable wall. On the other hand, EXPAs induce creep (Cosgrove, 1999; Cosgrove, 2016b; Samalova et al., 2022).
Expansins (EXPs) modulate cell wall loosening by inducing cell wall stress relaxation and extension in a pH-dependent manner (Rayle and Cleland, 1992; McQueen-Mason and Cosgrove, 1995; Cosgrove, 2000; Cosgrove, 2015; Cosgrove, 2016a). Particularly, among the four different subgroups of plant expansins, viz., EXPA (α-expansin), EXPB (β-expansin), EXLA (expansin-like A), and EXLB (expansin-like B) (Kende et al., 2004; Sampedro and Cosgrove, 2005), EXPAs and EXLBs have been central in inducing cell wall loosening, via acid growth response or auxin-induced acidification of the cell wall space (McQueen-Mason et al., 1992; Rayle and Cleland, 1992; Cosgrove, 2016a; Chebli and Geitmann, 2017). EXPAs are the most abundant, and have been characterized in different crop species such as Arabidopsis, rice, wheat, poplar, etc. (see (Marowa et al., 2016; Samalova et al., 2022)). Meanwhile, the cell-wall loosening model (Cosgrove, 2015) enunciates that non-water-stressed quiescent cells are at osmotic balance, as wall stresses offset the externally exerted turgor pressure on the wall. However, in growing cells, the cells are loosened through the EXPs-modulated pH-dependent manner, which involves relaxation of the load-bearing cell wall components and release of turgor-generated wall stresses, allowing water flow into the cell and restoration of wall tension and cell expansion (Cosgrove, 2015; Cosgrove, 2018; Samalova et al., 2022). Although EXPs lack the capacity to hydrolize the polysaccharide substrates by themselves (McQueen-Mason et al., 1992; McQueen-Mason and Cosgrove, 1995), pH shifts facilitate EXP-mediated cell wall loosening, via cell wall components relaxation, which enables access to polysaccharide substrates by different hydrolases (Cosgrove, 2000; Cosgrove, 2005; Samalova et al., 2022).
Several EXPAs transcripts are up-regulated under abiotic stress (Tenhaken, 2014; Marowa et al., 2016), with enhanced EXPAs expression contributing to drought tolerance ( (Samalova et al., 2022) and references therein). For instance, overexpression of TaEXPA2 promotes drought tolerance in TaEXPA2-OE wheat plants, by improving cell water retention, antioxidant capacity, and lateral root proliferation under drought stress (Yang J. et al., 2020). When overexpressed in tobacco, TaEXPA2 enhances water deficit tolerance and seed production, by promoting osmotic adjustment, antioxidant capacity, and expression of numerous antioxidant-enzymes-encoding genes (Chen et al., 2016). TaEXPB23 overexpressed in tobacco enhanced water deficit tolerance, by reducing the rate of water loss (Li et al., 2011). Overexpression of GhEXLB2 improved drought tolerance in cotton (Gossypium hirsitum L.), by enhancing WUE, soluble sugar and chlorophyll contents (Zhang et al., 2021a). An Erianthus arundinaceus EXPA gene (EaEXPA1) overexpressed in sugarcane (Saccharum spp. cv. Co 86032) enhanced drought tolerance in transgenic sugarcane lines, via improved leaf relative water content and photosynthetic parameters (Ashwin Narayan et al., 2021). Ammopiptanthus nanus EXPA genes AnEXPA1 and AnEXPA2 overexpressed in Arabidopsis enhanced cold and drought tolerance in transgenic Arabidopsis plants, with AnEXPA2 being induced by both cold and drought, and responding to hormone induction (Liu et al., 2019); AnEXPA2 was suggested to enhance drought tolerance by improving ROS scavenging ability and EXP activity in overexpressing transgenic plants (Liu et al., 2019). Equally, an EXP gene AstEXPA1 from creeping bentgrass (Agrostis stolonifera), overexpressed in tobacco, improved tolerance to drought (and other stresses) by increasing soluble sugar content and osmoprotection in transgenic plants (Hao et al., 2017). What is revealing from these few examples is that progenitors may harbor important cell-wall loosening-related genes that can be harnessed for improving drought tolerance in elite crop species (Tucker et al., 2018).
Meanwhile, extensins (eg. leucine-rich repeat extensins, LRXs) are crucial hydroxyproline-rich glycoproteins that are known to reinforce plant cell walls, by forming intra and intermolecular crosslinking of tyrosine residues (Lamport et al., 2011; Castilleux et al., 2021), thereby improving mechanical protection against pathogen attack (Castilleux et al., 2021). Besides, LRXs interact with RALF peptide ligands that modify cell wall expansion (Moussu et al., 2023), and synergistically link with transmembrane receptor FER in cell growth regulation (Moussu et al., 2023). This may suggest that LRXs coordinate the cell-wall-PM connection that underpins extracellular signal perception or information transfer necessary to steer cell expansion or/and cell wall formation (Draeger et al., 2015; Herger et al., 2019). However, it remains to be examined whether extensins can steer cell growth under drought stress conditions. Nonetheless, the most plausible role of extensins in drought tolerance, due to their capacity to form cross-linked dendritic assemblies with peroxidases (Mishler-Elmore et al., 2021), may be cell wall stiffening, which enhances cell water preservation. Overall, several cell-wall-related proteins crucially regulate cell wall extensibility, by mediating cell turgor maintenance, enlargement and expansion, as well as morphological and physiological alterations necessary for both growth and stress response (Martínez et al., 2007; Le Gall et al., 2015; Chebli and Geitmann, 2017; Ezquer et al., 2020), and these could be targeted for genetic or metabolic modulation to improve plant drought stress tolerance.
4.2.2 The role of peroxidases and laccases
Cell wall PODs, ROS, and laccases (LACs) are also involved in cell wall loosening and stiffening (Tenhaken, 2014; Francoz et al., 2015; Xie et al., 2018). Due to their twofold (hydroxylic and peroxidative) catalytic cycles (Passardi et al., 2004), PODs may generate oxidative radicals (OH•, ·O2–, etc.), and at the same time oxidize glycoproteins or phenolics esterified with cell wall aromatic compounds (monolignols, cinnamic acids, aromatic amino acids, etc.) that are free or polysaccharides-linked (Tenhaken, 2014; Francoz et al., 2015). Thus, conceptually, it has been enunciated that under stress conditions, plant cellular growth is tightly coordinated by the antagonism or delicate balance between these ROS or POD-mediated cell wall stiffening and weakening processes (Tenhaken, 2014). On one hand, crosslinking of glycoproteins to polysaccharides-esterified phenolic compounds relies on LACs- or POD-generated ROS (oxygen radicals, ·OH) (Vanholme et al., 2010), yielding to cell wall rigidification, whereupon expansins or XTHs access to xyloglucan substrate is hindered, and cell growth is arrested (Tenhaken, 2014; Francoz et al., 2015; Alavarse et al., 2022). On the other hand, prolonged stress and ROS synthesis cause POD substrates depletion, which favor hydroxyl radicals formation (H2O2-driven); and the formed hydroxyl radicals induce direct cleavage of cell wall polysaccharides, through covalent bonds breakage, resulting in cell wall loosening, and consequent cell expansion almost similar to non-stress conditions (Schopfer, 2001; Tenhaken, 2014; Samalova et al., 2022). Thus, PODs crucially modulate cellular H2O2 and ROS homeostasis under stress conditions (Kidwai et al., 2020).
LACs, in concert with PODs, crucially catalyze monolignol polymerization in the apoplastic cell wall domains, to facilitate cell wall lignification (Wang et al., 2013; Zhao et al., 2013; Xie et al., 2018). In this process, monolignols are actuated by LAC or POD oxidation systems to produce the final lignin polymers (Tobimatsu and Schuetz, 2019). The ensuing cell wall stiffening or rigidification helps plants to resist drought stress, possibly by waterproofing tissues (Le Gall et al., 2015). Evidence from multiple LACs or PODs mutants-based genetic studies show that LACs and PODs cooperate in cell wall lignification. For instance, LAC4 and LAC17 crucially regulate tissue-specific lignin accumulation in Arabidopsis (Berthet et al., 2011). Double knockout (LAC4 and LAC17) mutants had ~20-40% reduction in stem lignin content when compared to Wt (Berthet et al., 2011). In concert with LAC11, they exhibit high expression in lignifying tissues, and, simultaneous disruption of LAC11, LAC4 and LAC17 severely arrests plant growth and vascular development, and considerably diminish root lignin accumulation (Zhao et al., 2013). Intriguingly, putative lignin POD genes were expressed at normal or higher levels in the LAC triple mutant, signifying that lignin LAC activity is essential and non-redundant with POD activity for stem and root vascular tissue lignification in Arabidopsis (Zhao et al., 2013). Similarly, quadruple and quintuple loss-of-function Arabidopsis mutants revealed that LAC5, LAC10, and LAC12 non-redundantly modify lignin accumulation in distinct lignified cell types (Blaschek et al., 2023). Meanwhile, Arabidopsis POD genes AtPOD2, AtPOD25 and AtPOD71 cooperatively modulate stem lignification (Shigeto et al., 2015), with three double mutants (atPOD2/atPOD25, atPOD2/atPOD71, and atPOD25/atPOD71) resulting in ~ 11-25% decrease in stem lignin content (Shigeto et al., 2015). Besides, AtPOD17 essentially mediates leaf, stem, flower, and silique lignification (Cosio et al., 2017), whereas AtPOD64 regulates Casparian strips lignification (Lee et al., 2013). All these observations suggest that different LAC and POD genes play prominent lignification-related roles in distinct cell- or tissue-types (Xie et al., 2018; Blaschek et al., 2023; Choi et al., 2023).
Meanwhile, overexpression of POD genes has been shown to enhance crop drought tolerance. For instance, several POD genes were up-regulated under heat, salinity and drought stresses in potato (Solanum tuberosum); specifically, five genes (StPRX19, StPRX28, StPRX40, StPRX41, and StPRX57) were upregulated under drought conditions (Yang X. et al., 2020), suggesting they could play a role in drought tolerance. However, their exact regulatory functions will need to be investigated. Microarray investigation revealed five candidate genes (ZmPRX26, ZmPRX42, ZmPRX71, ZmPRX75, and ZmPRX78) whose expression is considerably altered in response to both 20 mM NaCl and 20% PEG treatments in maize, particularly being highly expressed in the roots, suggesting their involvement in root-related salinity and drought stress responses (Wang et al., 2015). It may be that these ZmPRX genes regulate maize drought tolerance by enhancing root lignin accumulation, similar to AtPOD64 (Wu Y. et al., 2017), which promotes root system development and stress tolerance (Wu Y. et al., 2017). Arabidopsis seedlings with knocked out AtPOD33 exhibited shorter roots than WT controls, whilst seedlings overexpressing AtPOD34 exhibited significantly longer roots (Passardi et al., 2006), with the root length modifications linked to corresponding cell length changes (Passardi et al., 2006). Overexpression of the AtPOD64 gene also increased root growth in Arabidopsis (Wu Y. et al., 2017). It is well known that a well-developed root system can improve plant drought tolerance (Wasaya et al., 2018). Conceivably, overexpressed PODs enhance root elongation, which improves deeper water extraction, whereas root lignification helps preserve axial water transport, thereby improving crop drought tolerance (Zhao, 2016).
4.3 Stomata guard cell wall remodeling
Plant cell walls play a very important role in stomatal opening, a key process that determines drought resistance (Alonso Baez and Bacete, 2023). Stomata, which are small orifices localized on the leaf epidermis of plants, and bounded by two guard cells, facilitate an interface for plant-environmental gas exchange, thereby governing plant water balance (Auler et al., 2022; Liu C. et al., 2023). Dynamic alteration of guard cell wall (GCW) structure controls stomatal conductance, photosynthesis, and water loss frequencies, as well as pathogen entry (see details in (Jaafar and Anderson, 2024)). The opening and closing of stomata orifices is mediated by changes in turgor pressure, structure and composition of the two guard cells, as affected by various signals such as ABA, ROS, Ca2+, blue light and extracellular calmodulin (Kollist et al., 2014; Liu et al., 2022). Since there is generally reduced turgor pressure under drought stress conditions, stomatal closure is amplified (as the first reaction to drought stress) to minimize water loss from transpirational pathways and maintain turgor (Pirasteh-Anosheh et al., 2016). This improves water use efficiency and drought tolerance (Auler et al., 2022). GCWs are dynamically remodeled to facilitate this process. For instance, the differential thickening and orientation of cellulose microfibrils gets ramped up, permitting guard cells to act reversibly during repeated stomatal opening and closing (Jaafar and Anderson, 2024). Especially, the anisotropic behavior of GCWs (being elastic to structural and orientational modulation of cellulose microfibrils (Baskin, 2005)) allows for this stomatal functioning, through continuous swelling and deflation during drought stress episodes (Lawson and Matthews, 2020).
Meanwhile, Arabidopsis GCWs are endowed with unesterified HGs and arabinans, whilst methyl-esterified HGs and Ca2+ cross-linked blockwise de-esterified HGs are restricted to the exterior of the wall (Amsbury et al., 2016; Merced and Renzaglia, 2019). Under stress conditions, guard cells undergo recurring inflation-deflation cycles, with this process and the formation of guard cell orifice being necessitated by the modification of pectic HG. Augmenting pectic HG modification, via demethyleesterification, promotes pore formation, whereas hindering HG demethylesterification defers pore initiation and impedes pore enlargement (Rui et al., 2019). This may suggest that stomatal function is governed by GCW characteristics, and involves GCW demethylesterification (Amsbury et al., 2016; Lawson and Matthews, 2020) (Figure 3). GCW-expressed AtPME6 is necessary for stomata opening and closing. GCWs of pme6-1 mutant are enriched with methylesterified pectin and exhibit a decreased dynamic range when exposed to stomata closing/opening elicitors, suggesting the nullification of stomatal function to be a result of a GCW mechanical alteration (Amsbury et al., 2016). Similarly, a GCW-specific PME, AtPME53, crucially drives ABA-dependent stomatal function and heat stress response in Arabidopsis (Wu et al., 2022). This reveals a link between PME-mediated GCW pectin demethylesterification, stomatal functioning, and abiotic stress response (Amsbury et al., 2016; Wu et al., 2022). Besides, PME34 crucially regulates GCW plasticity modulating stomatal conductance in response to abiotic stress such as heat in Arabidopsis (Huang et al., 2017; Wu H-C. et al., 2017). Equally, a GCW-expressed AtEXPA1 controls stomatal functioning in Arabidopsis through modifying GCW structure (Wei et al., 2011). These studies show that PME and EXPAs activities regulating GCW remodeling are essential stress response strategies (Wu et al., 2022), and hence, could be possible targets for deliberate genetic manipulation to engineer drought tolerance in crops.
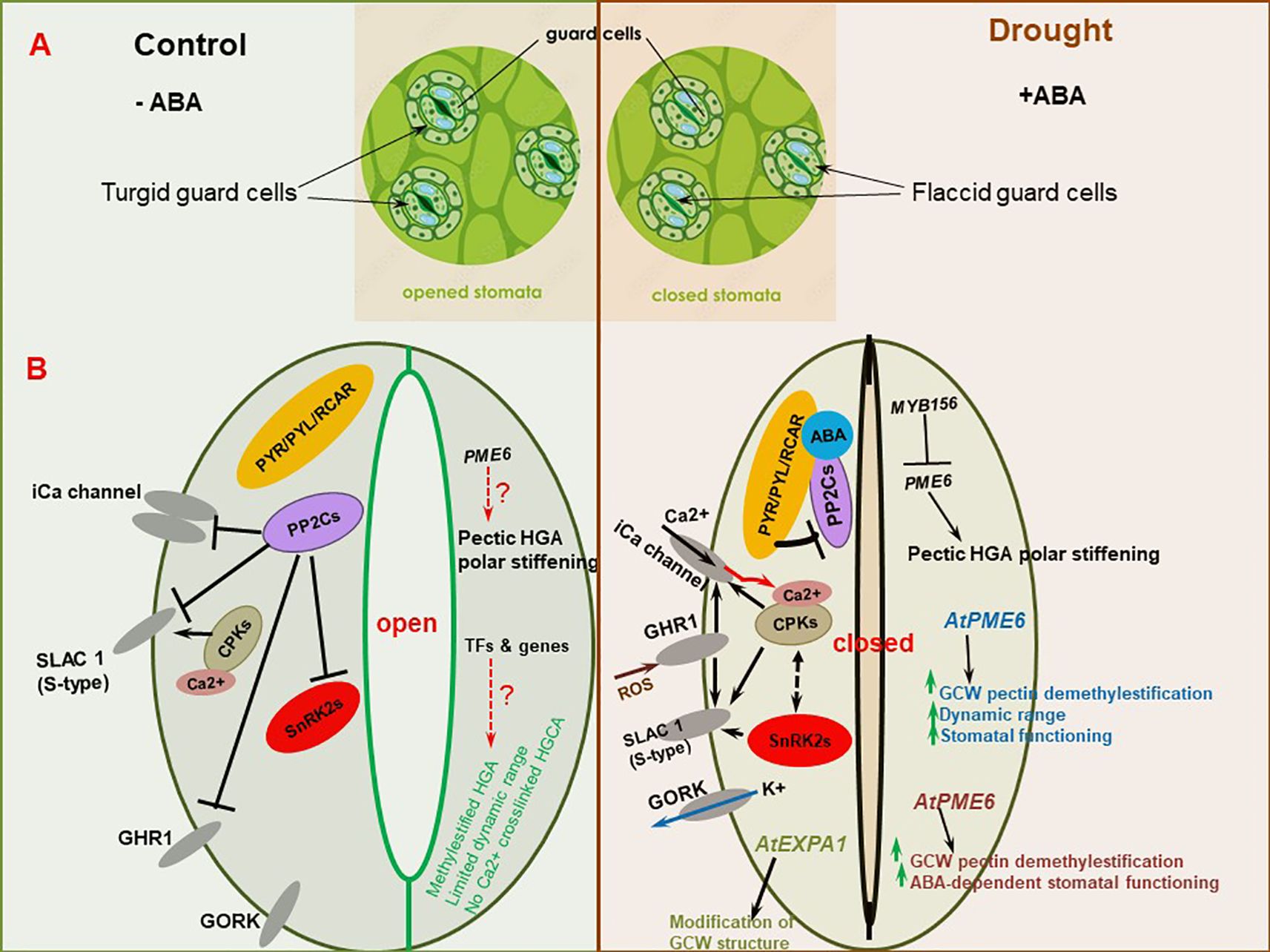
Figure 3. Guard cell wall (GCW) remodeling-mediated stomatal regulation. (A) Illustration of guard cell structure under control and drought stress conditions. (B) Abscisic acid (ABA) and GCW remodeling-mediated mechanisms regulating stomatal functioning under stress conditions. The canonical PYR/PYL/RCAR-mediated ABA signaling module regulates stomatal regulation in alliance with reactive oxygen species (ROS), calcium (Ca2+) signaling and GCW remodeling processes. Modulation of guard cell pectic matrix results in altered GCW properties, which consequently influence mechanical stomatal responses to exogenous cues, thereby regulating stomatal functioning. For simplicity/clarity purposes, the ABA-mediated signaling pathway is shown on the left side whereas the CW remodeling mechanisms are shown on the right side of each guard cell, respectively; however, in reality, these pathways are crosslinked and are distributed across entire guard cell. Dashed red arrow and question mark imply that the signaling pathway needs further clarification. Green upward-pointing arrows denote increased or improved trait/physiological process. The red arrow in Figure B (bottom right) represent cytosolic free calcium [Ca2+]cyt outburst. PYR/PYL/RCAR, pyrabactin resistance/pyrabactin resistance-like/regulatory component of ABA receptors; PP2Cs, type 2C protein phosphatases; SnRK2s; sucrose non-fermenting 1 (SNF1)-related protein kinases 2; CPKs, calcium protein kinases; GHR1, GUARD CELL HYDROGEN PEROXIDE-RESISTANT 1 channel; GORK, GUARD CELL OUTWARD RECTIFYING K+ CHANNEL; SLAC1 (S-type), slow anion channel 1 (slow type); iCa channel, Ca2+-permeable cation (iCa) channel; AtEXPA1, Arabidopsis thaliana expansin A1; AtPME6, Arabidopsis thaliana pectin methyltransferase 6; HG, homogalacturonan. Illustrations are based on (Cotelle and Leonhardt, 2019) (for ABA signaling), and (Amsbury et al., 2016; Carter et al., 2017; Zheng et al., 2023) (for pectin modifications).
More recently, it has been shown that MYB156 TF controls pectic HG-based polar stiffening in poplar (Poplar species), via downregulation of PME6 (Zheng et al., 2023). Polar stiffening is necessary for guard cell dynamics critical for normal stomatal morphology maintenance during stomatal movement in response to alterations in environmental conditions (Carter et al., 2017). MYB156-deficient plants had increased stomata polar stiffness and improved stomatal dynamics and response rate to environmental cues, whereas MYB156-overexpressed plants had decreased stomata polar stiffness and lessened stomatal dynamics, revealing the connection between the GCW structure and function in stomatal dynamics (Zheng et al., 2023) (Figure 3). This study provides a potential way for enhancing plant stomatal functioning and water deficit tolerance by engineering this specific property (Zheng et al., 2023). Taken together, the GCW properties underpin the dynamic stomata guard cell deformations that occur during guard cell expansion and contraction to drive stomatal opening and closing, whose precise regulation is critical for water transport, photosynthesis and growth, especially under drought conditions (Siqueira et al., 2021; Jaafar and Anderson, 2024), and could be appropriate targets for manipulation through genetic engineering approaches. It will be crucial to establish the crosstalk existing between GCW remodeling mechanisms, ABA signaling and CWI sensing system as it sheds more light on stomatal functioning dynamics and drought response.
4.4 Lignification
Lignin is biosynthesized through the general phenylpropanoid pathway (Vanholme et al., 2010) (Figure 4A). This pathway is mediated by several key enzymes, including phenylalanine ammonia lyase (PAL), ferulate 5-hydroxylase (F5H), p-coumarate 3-hydroxylase (C3H), 4-coumarate coenzyme A ligase (4CL), p-hydroxycinnamoyl-CoA:quinate/shikimate hydroxycinnamoyl transferase (HCT), cinnamate 4-hydroxylase (C4H), caffeoyl-CoA O-methyltransferase (CCoAOMT), caffeic acid O-methyltransferase (COMT), cinnamoyl-CoA reductase (CCR), cinnamyl alcohol dehydrogenase (CAD), etc (Dixon et al., 2001; Vogt, 2010; Wang et al., 2013; Dong and Lin, 2021; Han et al., 2022). Consequently, genetic modification of these enzymes has been shown to significantly alter lignin accumulation and/or composition, as well as stress defense (Xie et al., 2018). Monolignols secreted by lignifying cells are polymerized by cell-wall-localized O2-reliant laccases (LACs) and H2O2-dependent peroxidases (PODs) into lignin polymers (Tobimatsu and Schuetz, 2019; Han et al., 2022) (Figure 4A). Lignification involves the deposition of lignin into apoplastic cell wall domains (Vanholme et al., 2010), rendering them mechanically strong, firm, and hydrophobic (Zhao, 2016); this stiffens cell wall extensibility, and controls cell membrane permeability and water passage to maintain cell osmotic balance (Moura et al., 2010; Bang et al., 2022). Increased lignin accumulation helps plants adapt to drought stress (Xu et al., 2017). For instance, overexpression of poplar (Populus L.) transcription factor PdNF-YB21 promoted root lignification, enlarged xylem vessels and root growth, which improved drought tolerance (Zhou et al., 2020). Additionally, PdNF-YB21-PdFUS3-PdNCED3 module promoted root ABA content, auxin regulation of root growth and drought tolerance in poplar (Zhou et al., 2020). A Setaria italic transcription factor, SiMYB56, when overexpressed in rice, confers drought tolerance, by lowering MDA content, increasing lignin content, and modulating lignin biosynthesis and ABA signaling pathway under drought conditions (Xu et al., 2020).
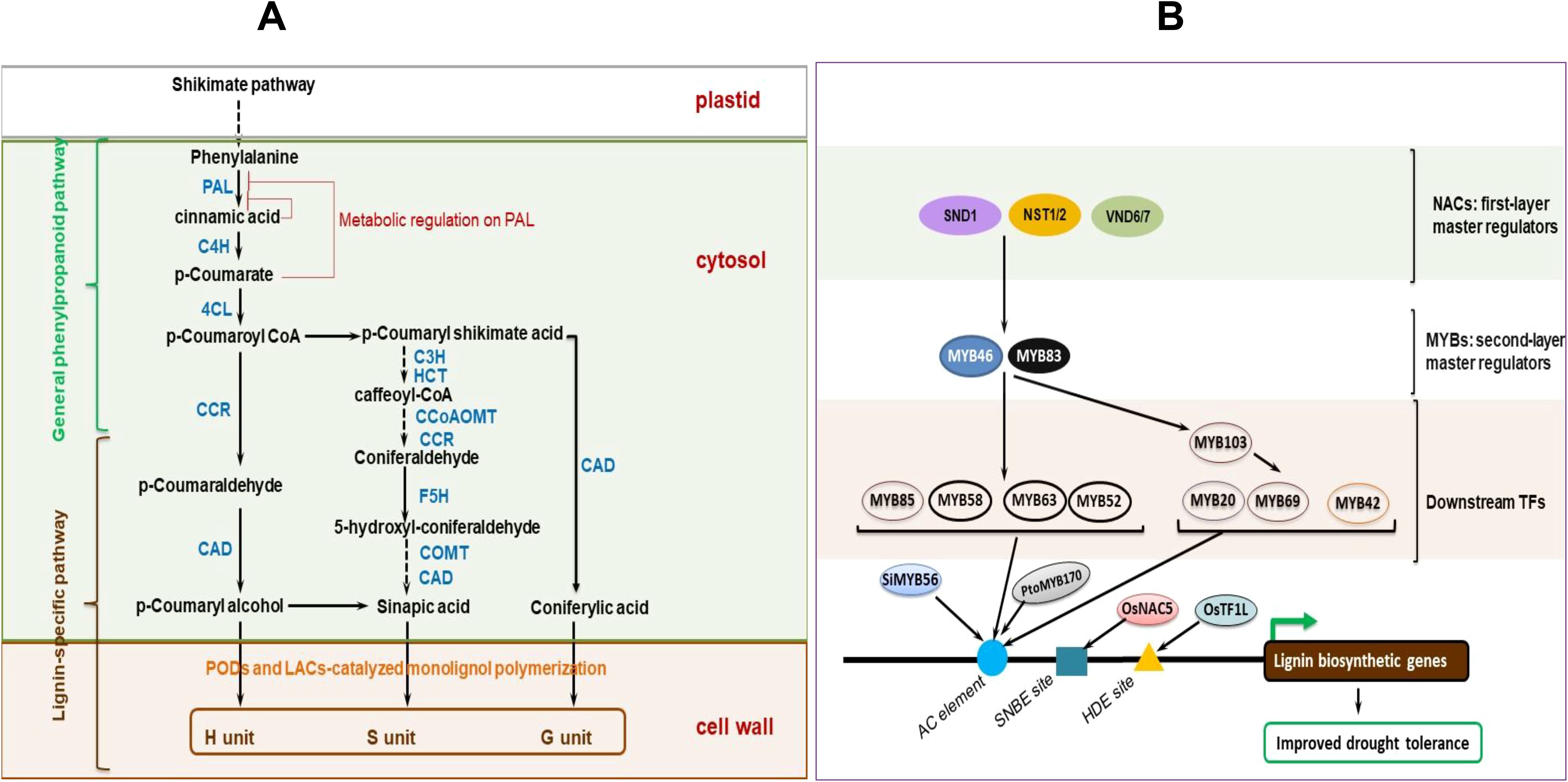
Figure 4. Lignin biosynthesis and modulation-mediated drought tolerance in plants. (A). Simplified pathway for lignin biosynthesis in plants. Phenylalanine ammonia lyase (PAL) act on the phenylalanine generated from the shikimate pathway to produce cinnamic acid. Downstream, diverse enzyme superfamilies catalyze series of steps to convert cinnamic acid into monolignols (p-Coumaryl alcohol, sinapic acid, and coniferylic acid), which are subsequently polymerized (through class III peroxidases- and laccases-mediated oxidative radical coupling) into lignin units (p-hydroxyphenyl, H; guaiacyl, G; and syringyl, S). The resultant lignification stiffens the cell wall. Note: Key enzymes (not exhaustive) are displayed in blue text, as follows: PAL, phenylalanine ammonia lyase; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumaroyl CoA-Ligase; CCR, cinnamoyl CoA reductase; CAD, (hydroxy)cinnamyl alcohol dehydrogenase; C3H, p-coumaroyl shikimate/quinate 3-hydroxylase; HCT, hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase; CCoAOMT, caffeoyl CoA O-methyltransferase; CCR, cinnamoyl-CoA reductase; F5H, ferulate 5-hydroxylase; COMT, caffeic acid/5-hydroxyferulic acid O-methyltransferase. Complete arrows signify single-step enzymatic reactions, whereas dashed arrows imply series of enzymatic steps (not fully shown) for clarity purposes. Figure adopted from (Nguyen et al., 2016; El-Azaz et al., 2020) and others discussed in text. (B) NAC-MYB-mediated transcriptional regulation of lignin biosynthetic genes involved in plant drought tolerance. Transcription factors bind to the cis-acting elements (TF binding sites), viz., AC element, secondary wall NAC binding element (SNBE), and HD-binding cis-elements (HDE) on the promoter of lignin biosynthetic genes to actuate their expression. TFs without prefixes relate to Arabidopsis (Arabidopsis thaliana) whereas those with prefixes are for other species. SiMYB56, Setaria italic MYB56; OsNAC5, Oryza sativa NAC5; PtoMYB170, Populus tomentosa MYB170; OsTF1L, Oryza sativa transcription factor 1-like; SND1, SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN 1; NST1/2, NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1/2; VND6/7, VASCULAR-RELATED NAC DOMAIN 6/7. Illustrations are based on (Nakano et al., 2015; Yoon et al., 2015; Han et al., 2022; Choi et al., 2023) and others discussed in text.
NAC and MYB TFs crucially modulate lignin biosynthesis, essential for drought stress tolerance in plants (Figure 4B). For instance, OsNAC5 TF actuates a rice gene OsCCR10 (Cinnamoyl-CoA Reductase 10) and enhances vegetative stage drought tolerance in overexpressing rice plants, by modulating root lignin accumulation (via increased H- and G-lignin biosynthesis), greater photosynthetic performance, and minimized leaf water loss rate than in non-transgenic controls (Bang et al., 2022). Overexpression of OsNAC17 positively influences numerous lignin biosynthesis-related genes, enhances leaf and root lignification, and improves drought tolerance in rice (Jung et al., 2022). Besides, ectopic expression of an HD-Zip TF OsTF1L elevates lignin biosynthesis, lignin accumulation in shoots, and stomatal closure, which enhances rice reproductive-stage drought tolerance (Bang et al., 2019). Equally, an overexpressed cytochrome P450 family gene CtCYP71A1 results in increased root lignification, positive regulation of several other lignin biosynthetic genes, and improved drought tolerance in overexpressing safflower (Carthamus tinctorius) and Arabidopsis lines than in wild-type and antisense plants (Zhang Q. et al., 2023). Overexpressing Paeonia ostia (an oil-producing woody crop) gene PoWRKY17 activates PoCCoAOMT expression, enhances lignin accumulation, increases leaf RWC, decreases relative electrical conductivity, minimizes MDA content and ROS accumulation, but enhances protective enzyme activities. All these positive physiological changes endow the crop plants with improved water deficit tolerance (Luan et al., 2023). Besides, a glycine-rich RNA-binding protein, OsGRP3, promotes rice drought tolerance by modulating phenylpropanoid biosynthesis and enhancing lignin accumulation (Xu W. et al., 2022). Upregulated expression of phenylpropanoid biosynthesis-related genes (PAL1, 4CL, CCR, C4H, CCOMT, and CAD6) improved lignin biosynthesis and confered cluster bean [Cyamopsis tetragonoloba (L.) Taub.] drought tolerance, by increasing water-flow resistance and maintaining steady nutrient transport under drought stress conditions (Narayan et al., 2023). In apple (Malus × domestica Borkh.), MdMYB88 and MdMYB124 improve water deficit tolerance by regulating root xylem development and root cell wall cellulose and lignin depositions (Geng et al., 2018). Overexpressing a CCCH-type TF, PuC3H35, promotes root proanthocyanidin (an effective non-enzymatic antioxidant) and lignin biosynthesis, as well as vascular tissue development, which confers improved drought stress tolerance in Populus ussuriensis, by actuating anti-oxidation and mechanical support (Li et al., 2022). Given than lignin biosynthesis pathway crosstalk with other signaling pathways, including phytohormonal, post-transcriptional, post-translational, and epigenetic regulations (Novaković et al., 2018; Rao and Dixon, 2018; Dong and Lin, 2021), untangling this crosstalk will aid in identifying key hub targets for genetic or metabolic pathways manipulation for improved plant drought tolerance (Xie et al., 2018; Liu S. et al., 2023).
Besides lignin, other components such as glucuronoxylan have been implicated in plant drought stress response. More recently (Barbut et al., 2024), used RGB (red, green, and blue light) monitoring and hyperspectral imaging (HSI) techniques to reveal that the integrity of xylan backbone in SCW affects Arabidopsis response to drought stress. Compared to the wild-type, all Arabidopsis lines with impaired xylan integrity exhibited better survival, increased stomatal density and delayed growth inhibition under moderate drought stress conditions, although the magnitude of response was genotype-dependent (Barbut et al., 2024). Amongst the three xylan biosynthesis mutants irx9, irx10 and irx14 (irx, irregular xylem) and xylanase-expressing lines, irx14 was the most drought-resistant, and the only genotype with increased lignin content and unchanged xylem conductivity (Barbut et al., 2024). These results suggest that modifying SCW integrity could be a potential strategy for developing drought-tolerant crop cultivars, although more studies are required to first understand the underlying molecular causes of SCW variation amongst genotypes. In support of these findings, engineered Arabidopsis plants with low xylan acetylation, and low lignin and xylan contents, have shown improved tolerance to severe drought stress than their wild-type counterparts (Yan et al., 2018a). Drought-tolerant plants displayed low leaf water loss rate and up-regulated expression of drought-responsive genes (RD29A, RD29B, DREB2A) under drought stress conditions, which did not occur under control conditions. Additionally, plants with low lignin content (as a result of expression of a 3-dehydroshikimate dehydratase, QsuB) exhibited a stronger response to ABA treatments, and accumulated more ABA in response to drought than the wild-type (Yan et al., 2018a). However, in plants with low xylan content or low xylan acetylation, drought tolerance was not related to ABA content or response differences, but, possibly, to increased galactose levels and increased sugar released under drought stress conditions (Yan et al., 2018a). Overall, these findings demonstrate the utility of enhancing plant drought tolerance through modification of lignin, xylans, and other SCW components.
4.5 Root suberization
The plant root endodermal cell layer essentially controls water and mineral nutrients uptake (Franke and Schreiber, 2007; Wang et al., 2019). Besides that, altering the extent of root cell wall suberization helps plants respond to abiotic and biotic stress (Chen et al., 2022; Kim and Sung, 2023). Suberized cells are generally waterproof, and act as barriers against pathogen invasion (Franke and Schreiber, 2007; Chen et al., 2022; Woolfson et al., 2022). Root suberin accumulation modulates salt and water transport, hence, is induced by salinity, cadmium (Cd), and ammonium stresses (Ranathunge et al., 2016; Wang et al., 2019) Suberization is also actuated under drought conditions (Henry et al., 2012), possibly via ABA (Kim and Sung, 2023), implying an essential role for suberin in preventing water loss (Chen et al., 2022). For instance, Arabidopsis root suberization has been shown to play crucial roles in minimizing water loss and NaCl uptake (de Silva et al., 2021). Prolonged water deficit stress increased the contents of suberin and suberin-related waxes in Wt Arabidopsis. Analysis of an Arabidopsis mutant collection showed that double mutant (cyp86a1-1 cyp86b1-1), with a significantly changed suberin composition and lamellae architecture, had elevated root peridermal cell water loss (de Silva et al., 2021). Equally, the triple mutant (abcg2-1 abcg6-1 abcg20-1), with changed suberin composition and lamellae structure, showed increased sensitivity to sodium, suggesting the essential role of suberin composition and lamellae architecture in minimizing peridermal cell water escape, and for limiting unrestrained sodium uptake, which assist plants to better tolerate drought and high-salinity stress conditions (de Silva et al., 2021).
During normal development, Arabidopsis Suberman (SUB) TF regulates root endodermal layer suberization. When transiently expressed in tobacco, SUB inducts several suberin-related genes, enhances suberin buildup, and lamellae deposition (Cohen et al., 2020). In drought-stressed rice roots, suberization of the endodermis has been shown to be increased, particularly in drought-tolerant genotypes, suggesting a critical water retention and drought tolerance strategy (Henry et al., 2012). Similarly, upregulated expression of suberin biosynthesis-related genes, and suberin lamellae accumulation is enriched in drought stressed barley roots (Kreszies et al., 2019), pointing to an essential plant drought stress tolerance mechanism (Kreszies et al., 2019). Meanwhile, overexpression of MYB39 in Arabidopsis roots dramatically enhanced root suberization (Wang et al., 2020). ABA has been shown to elicit the activation of root suberization under osmotic stress (Wang et al., 2020). Besides, water deficit conditions significantly increased the induction of suberin biosynthesis-, ABA biosynthesis-, and aquaporins (AQPs)-related genes, and heightened lamellae suberization of the root endodermis in rice, suggesting that suberization, ABA metabolism and AQPs activity could dependently or independently regulate root drought tolerance in rice (Kim and Sung, 2023).
In tomato, which lacks endodermal suberin during normal development, it has been shown that a suberized exodermis is necessary for drought tolerance (Cantó-Pastor et al., 2024). Modulation of the tomato MYB92 TF and Aliphatic Suberin Feruloyl Transferase (ASFT) enzyme could control exodermal suberin-related root responses to water deficit stress, and regulating the degree of exodermal suberization could be a new strategy for engineering drought tolerance in plants, whereas constitutive biosynthesis of endodermal suberin could aid in creating drought-tolerant plants, with enhanced CO2 sequestration capacity (Thompson, 2017). Several suberin metabolism–related genes (KCS2, KCS20, β-ketoacyl-CoA synthases, fatty acyl reductases, MYBs, etc.) and those of the phenylpropanoid biosynthesis pathway (compiled in (Vishwanath et al., 2015; Shukla et al., 2021; Chen et al., 2022; Woolfson et al., 2022; Cantó-Pastor et al., 2024)) can be targeted for modification, via overexpression, silencing, etc., to improve plant drought tolerance. Besides, agronomic interventions such as silicon-meditated suberization of roots can also aid in developing drought tolerance in plants such as rice and barley (Kreszies et al., 2020). However, tradeoffs or feedback regulations related to the modulation of suberin metabolism should be taken into account, since the outcomes of such modulation on plant growth, water and nutrient relations, plant-microbe interactions, and overall plant fitness are hardly predictable (Cohen et al., 2020; Woolfson et al., 2022; Cantó-Pastor et al., 2024).
4.6 Root cell wall remodeling related to root expansion and response to water extraction under drought
Drought stress or water deficit is accompanied with decreased soil water potential. Under these conditions, plants tend to extend their roots into deeper soil layers in search for water (Takahashi et al., 2020). Thus, root system architecture adjustment represents a highly dynamic physical network that orchestrates plant access to a heterogeneous distribution of soil water (Feng et al., 2016). This process involves growth of the root (tips) and simultaneous preservation of extracted water within the root itself. Drought tolerance encompasses adaptations to growth under reduced water potential and the associated remodeling of the cell wall that permit growth occurrence under lower water contents. As such, drought tolerance results in both loosening and tightening of the cell wall, in different root zones (Moore et al., 2008). Tissues that are necessary to maintain in a growth-ready state are loosened, whilst non-essential tissues are tightened to prioritize continual growth of vital growing points such as the root apex even under lower turgor pressure (Wu and Cosgrove, 2000; Moore et al., 2008) (Figure 5). To permit cell wall growth, the threshold turgor pressure in these growing points is altered.
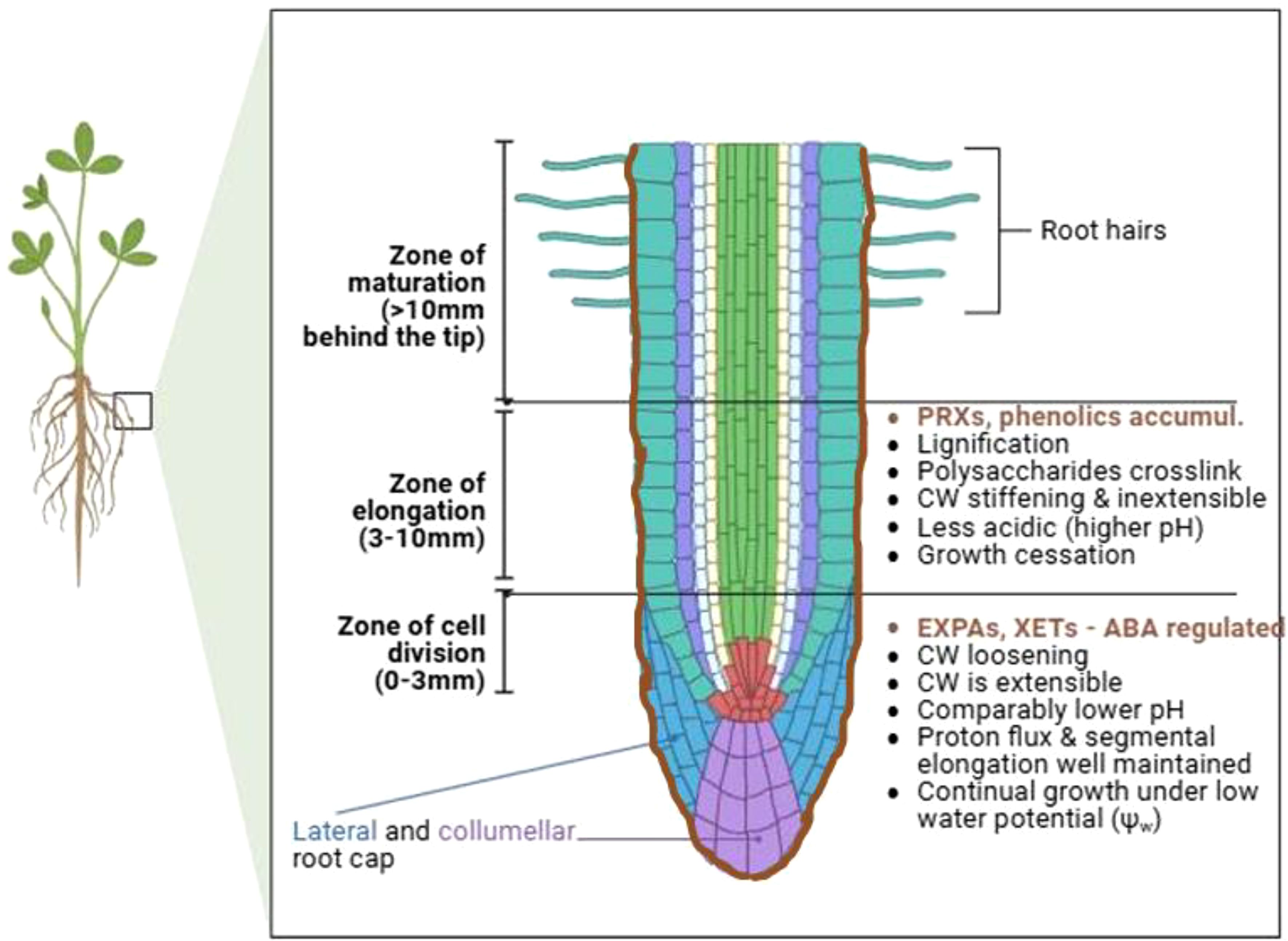
Figure 5. Differential cell wall (CW) responses occurring within different longitudinal root zones facilitate root adaptation to drought stress. Cell walls within the zone of cell division are loosened to allow continual growth even under conditions of low water potential, whereas those within the elongation zone are tightened (for instance, via lignification) to prioritize the growth of the apical segment and conservation of the extracted water within the root. Stiffening of the elongation zone also strengthens the root to facilitate root tip scavenging of deeper soil layers for water. For other parts labeling (from center to the outside), green, cream, sky-blue, purple, magenta, and brown represent the vascular cylinder, pericyte, endodermis, cortex, epidermis, and exodermis, respectively. EXPAs, expansins; XETs, xyloglucan endotransglucosylases; PRXs, peroxidases; ABA, abscisic acid; phenolics accum., phenolics accumulation. This illustration is based on (Wu and Cosgrove, 2000; Fan and Neumann, 2004; Fan et al., 2006) and others discussed in text. The figure was created using Biorender.com (https://app.biorender.com/).
Studies on maize root responses to drought stress treatment (Wu et al., 1994; Wu et al., 1996; Wu and Cosgrove, 2000) have been the most revealing about drought effects on cell wall structure and properties. Differential cell wall responses to drought stress treatment have been observed in different root zones (Voothuluru et al., 2016). On one hand, the cell walls within the root apical zone (0-3 mm behind the tip) are kept in a flexible/extensible state to permit slow tissue growth within this region even under low water potentials. On the other hand, cell walls within the elongation zone (3-9 mm behind the tip) are stiffened or made inextensible to halt further growth within that region (Fan and Neumann, 2004; Fan et al., 2006) (Figure 5). Different mechanisms have been shown to underpin these differential cell wall responses (loosening and stiffening) within these two different root zones. First, drought stress results in spatial difference in cell wall-associated pH between the two root zones, with the growing tip having a comparably lower pH to the elongation zone (Fan and Neumann, 2004). This observation resonates well with the canonical acid growth expansion theory, whereby auxin phosphorylation and activation of PM proton pumps (H+-ATPase) induces cell wall (apoplastic) acidification, triggers expansins and cell wall relaxation (McQueen-Mason et al., 1992; Wu et al., 1996; Fan and Neumann, 2004), which may promote organ expansion (Lin et al., 2021). Secondly, water channels are also believed to allow preferential water conveyance to the root tip cell walls, aiding cell wall loosening in that region (Moore et al., 2008). Thirdly, expansins and XETs may also be involved in cell wall loosening within the root growing tip, possibly regulated by ABA (Wu et al., 1994; Wu et al., 1996). Expansins modulate cell wall loosening via disruption of hydrogen bonding in the wall without compromising polysaccharide backbones (Cosgrove, 2024). XETs tend to increase their activity within the cell wall loosening (apical 6mm) region under low water potentials, as regulated by ABA (Wu et al., 1994). Meanwhile, Arabidopsis root responses to salinity have been shown to rely on pectin modification (mediated by PMEs) and cell wall sensing, whereby these responses partially require the functionality of FERONIA alone or HERKULES 1/THESEUS1 (HERK1/THE1) to diminish salt effects (Gigli-Bisceglia et al., 2022). Considering that salinity and drought are both osmotic stresses, these results may point to possible involvement of pectin remodeling in root responses to drought stress (Piro et al., 2003; Leucci et al., 2008); this will need further experimental investigation.
The accumulation of phenolics and lignin within the root elongation region, and their subsequent crosslinking to the cell wall polysaccharides, as mediated by peroxidases, stiffens the cell wall (Fan et al., 2006), whereas lignin accumulation induces removal of water from the wall, thus causing the cell wall to be inextensible and growth within the root elongation zone to cease (Fan and Neumann, 2004; Moore et al., 2008). This has been supported by the observation that lignin biosynthesis-related enzymes increase their activities with drought stress in the root elongation zone (Fan and Neumann, 2004). Taken together, differential cell wall responses (loosening and tightening) within the two different root zones facilitate for root adaptation to drought stress, by orchestrating continued growth of the root tip under water deficit conditions, and simultaneous preservation of the extracted water within the root itself. Besides, stiffening of the root elongation zone strengthens the growing tip to bore deeper through the “drier” soil layers in search of water.
5 Phytohormonal regulation of cell wall remodeling-mediated drought tolerance
Phytohormones regulate cell wall properties, growth, development, and cell wall stress signaling, as well as transcriptional output of some genes encoding cell-wall remodeling enzymes. In turn, the cell wall modulates the homeostasis of these phytohormones (Wolf et al., 2012), creating an interplay essential for stress response (Jobert et al., 2023). For instance, ABA, as already briefly highlighted above, ABA regulates suberization, AQP activity and drought tolerance in rice roots (Kim and Sung, 2023). Besides, endodermal ABA signaling mediates suberization responses to nutrient stress in Arabidopsis roots (Barberon et al., 2016). ABA signaling transduction mediates suberin lamellae formation (Wang et al., 2020), and cell-wall biosynthesis (Dalal et al., 2018) in drought-exposed roots, as has been observed in rice (Henry et al., 2012), wheat (Dalal et al., 2018), Arabidopsis (Wang et al., 2020), and barley (Kreszies et al., 2019). Similarly, ABA-treated wheat exhibited increased root suberin lamellae, whereas Arabidopsis showed accelerated suberin deposition under drought conditions (Grünhofer et al., 2021), suggesting that drought induces suberin biosynthesis, and ABA-signaling may coordinate root suberization and drought tolerance in such species (Wang et al., 2020; Kim et al., 2022) (Figure 6). However, the exact mechanism underlying ABA-mediated root suberization under varying water potentials remains unexplored (Kim and Sung, 2023).
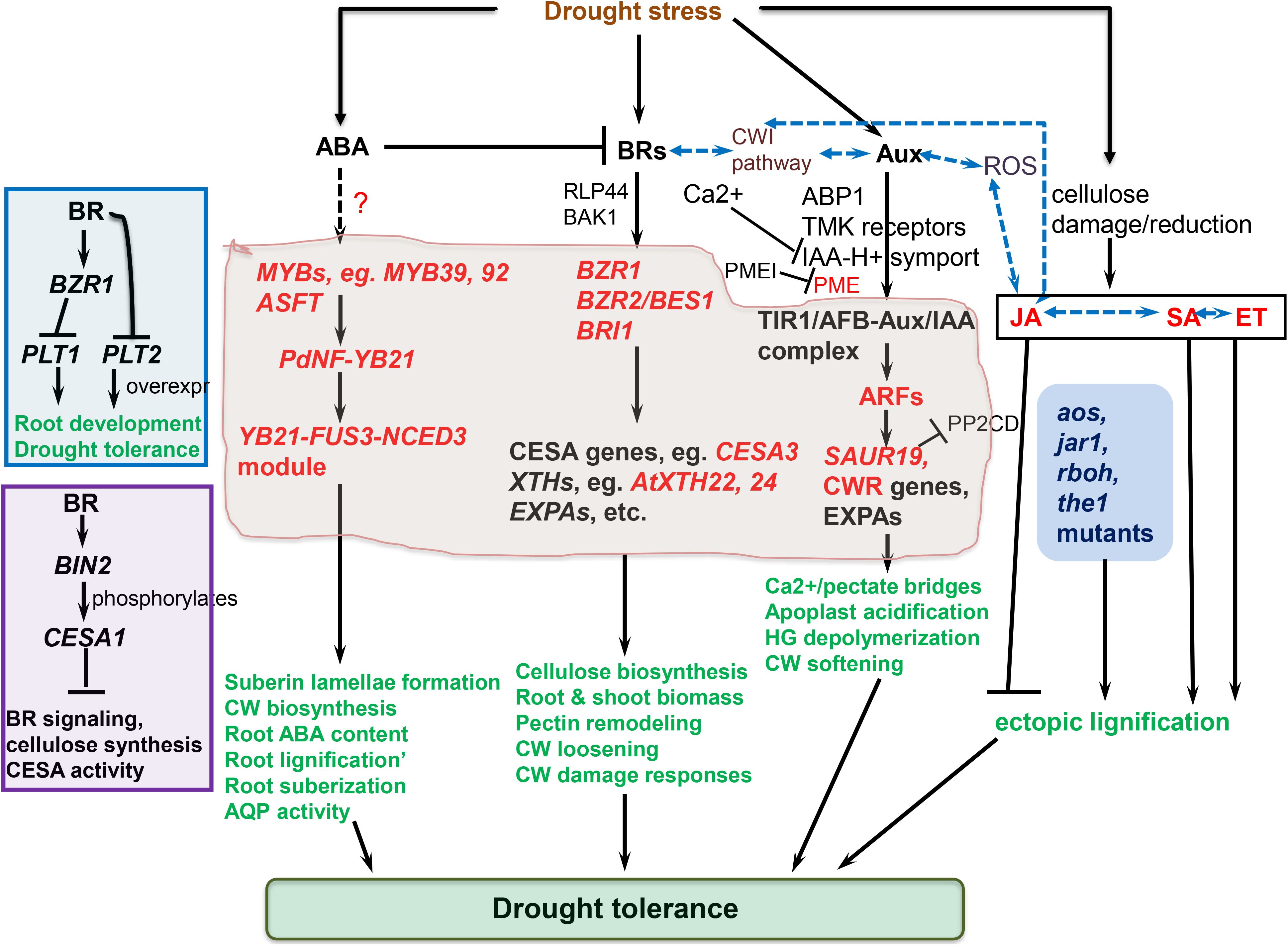
Figure 6. Phytohormonal regulation of cell wall remodeling (CWR) mechanisms related to drought stress tolerance discussed in this review. BRASSINAZOLE-RESISTANT 1/2 (BZR1/2) or BRASSINOSTEROID-INSENSITIVE (BRI)-mediated brassinosteroid (BR) signaling regulates the actuation of several CWR-related genes and processes. Abscisic acid (ABA) signaling and MYB transcription factors modulate auxin-regulated root growth, root suberization and/or lignification, and drought tolerance. Auxin signaling modulates expression of CWR enzymes regulating pectin properties. Stress-triggered cellulose damage or reduction elevates jasmonic acid, salicylic acid and ethylene levels, alters cell wall composition and architecture, and promotes ectopic lignin deposition (ELD). Dashed black arrow and question mark imply that the signaling pathway needs further clarification. Encasing in the center represent nuclear processes. Blue dashed arrows (not exhaustive) denote crosstalks, red font implies upregulated expression or elevated level, whilst green font implies improved physiological response/trait. NF-YB21, nuclear factor YB21; PdFUS3, a B3 domain transcription factor; ASFT, ALIPHATIC SUBERIN FERULOYL TRANSFERASE; NCED3, NINE-CIS-EPOXYCAROTENOID DIOXYGENASE 3; CWI pathway, cell wall integrity pathway; RLP44, receptor like protein 44; ROS, reactive oxygen species; BRI1, BRASSINOSTEROID-INSENSITIVE 1; BIN2, BRASSINOSTEROID INSENSITIVE 2; BES1, BRI1-EMS-Suppressor 1; BAK1, BRI1-associated receptor kinase 1; PLT1/PLT2, PLETHORA 1/2; CESA1, cellulose synthase A1; EXPAs, expansin As; XTHs, xyloglucan endotransglucosylases/hydrolases; PME, pectin methyltransferase; PMEI, PME inhibitor; SAUR19, SMALL AUXIN UP RNA 19; ARFs, auxin-responsive factors; AQP activity, aquaporin activity; aos, allene oxide synthase; jar1, jasmonic acid resistant 1; rboh, respiratory burst oxidase homologues; the1, theseus1 receptor-like kinase; Main figure is based on (Zhou et al., 2020) (for ABA), (Rao and Dixon, 2017; Novaković et al., 2018; Tiika et al., 2021) (for BRs), (Majda and Robert, 2018; Jobert et al., 2023) (for auxins), (Denness et al., 2011) (for jasmonic acid), and others discussed in text. Top left insert is based on (Zhao et al., 2023), whilst bottom left insert is based on (Sánchez-Rodríguez et al., 2017).
BR-mediated modulation of stress-triggered cellulose synthesis seems to be evolutionarily conserved in certain species (Kesten et al., 2017). For instance, increased levels of BRI1, CESA3, and other BR signaling-associated genes were observed in progenitor species Agropyron elongatum than in common/cultivated wheat genotypes in response to water stress (Placido et al., 2013). When introgressed into cultivated genotype, the wheat translocation line exhibited enhanced drought tolerance and greater root and shoot biomass in comparison with the control under stress (Placido et al., 2013), suggesting that the enhanced BR-signaling pathway of Agropyron elongatum could contribute to its higher drought stress adaptation, via improved root and shoot biomass, that possibly facilitate enhanced water extraction under stress (Placido et al., 2013; Rao and Dixon, 2017). Additionally, BRs mediate several cell wall remodeling processes, including cell wall damage responses, cell wall signaling, CWI maintenance, cell wall loosening, cellulose deposition, lignin accumulation, pectin modification, etc. (Zurek and Clouse, 1994; Rao and Dixon, 2017; Novaković et al., 2018; Rao and Dixon, 2018) (Figure 6). BR hormone signaling pathway also modulates the induction of several cell wall-associated genes (Wolf et al., 2012). In Arabidopsis, BR-activated TF BZR1 and its homology BZR2/BES1 directly bind to the promoter regions (such as the CANNTG-E motifs) of numerous cell wall-associated and BR-responsive genes such as the cellulose synthase A (CeSA) genes (Xie et al., 2011; Li et al., 2018), and NAC and MYB TFs linked to lignin biosynthesis pathways (Zhao and Dixon, 2011; Rao and Dixon, 2017; Rao and Dixon, 2018). However, Brassinosteroid Insensitive 2 (BIN2), a protein kinase, directly phosphorylates Arabidopsis CESA1, negatively regulating BR signaling, CESA activity and cellulose biosynthesis (Sánchez-Rodríguez et al., 2017) (Bottom left insert of Figure 6). CESA genes, such as CESA1, are transcriptionally and post-transcriptionally modulated via the BR-mediated signaling to regulate cellulose synthesis in Arabidopsis (Novaković et al., 2018). Besides, BRs regulate the expression of genes encoding cell wall loosening EXPAs and XTHs (Zurek and Clouse, 1994; Novaković et al., 2018). XTHs (eg., AtXTH22, AtXTH24, etc.) and EXPA genes have been shown to be considerably up-regulated by BR treatment in Arabidopsis and soybean (Zurek and Clouse, 1994; He et al., 2003), and in response to salinity and drought stress in halophytic plant Salicornia europaea (Tiika et al., 2021).
BRs also modulate stress-induced pectin remodeling. In particular, BR signaling crucially modulate cell wall modifying proteins, including PMEs (Wolf et al., 2012), whereupon stress-induced feedback mechanism from the cell wall potentiates the output of the BR pathway to ensure cell wall homeostasis and CWI in Arabidopsis (Wolf et al., 2012; Novaković et al., 2018). More recently, BR signaling has been shown to modulate root growth and drought tolerance in Arabidopsis, by repressing the expression of transcription factors PLETHORA 1 and 2 (PLT1 and PLT2, involved in root development, including apical meristem embryonic pattern formation (Aida et al., 2004)) (Zhao et al., 2023) (Top left insert of Figure 6). PLT1-OE and PLT2-OE transgenic Arabidopsis exhibited higher water deficit tolerance as compared to their Wt counterparts. Additionally, both in vivo and in vitro experiments have shown BZR1 to bind to PLT1 promoter region and hinder its transcription activation (Zhao et al., 2023). Further, BR signaling regulated PLT1 and PLT2 expression in root development, and PLT2 partly rescued the drought sensitivity of bes1-D mutant (Zhao et al., 2023). It might be probable that these genes could play other essential roles in cell wall remodeling processes linked to drought tolerance, and will need further exploration.
Auxin (Aux) is another phytohormone that regulate the expression and activities of cell wall remodeling-involved enzymes, including EXPAs, PMEs, PODs, etc (Cosgrove, 2015; Nafisi et al., 2015; Cosgrove, 2016b). These enzymes crucially mediate cross-linking of lignins, pectins, and proteins, resultantly influencing stress-induced cell elongation (Passardi et al., 2004; Passardi et al., 2006; Liu X. et al., 2021). Essentially, Aux signaling potentiates the transcriptional output of several pectin remodeling related enzymes-encoding genes, their local activity, and spatio-temporal distribution or modulation of pectin (Jobert et al., 2023). Using atomic force microscopy (AFM) (Braybrook and Peaucelle, 2013), established a link between Aux signaling and PME activity that modulates cell wall biochemical and mechanical changes necessary for organ formation in the Arabidopsis shoot apical meristematic region. Auxin also triggers low pH, which activates PME and hinders PMEI (see (Majda and Robert, 2018)). Besides, indole-3-acetic acid (IAA) orchestrate several plant growth and development processes (Sánchez-Rodríguez et al., 2010; Zhao, 2010), including the canonical acid growth cell expansion theory (McQueen-Mason et al., 1992). Auxin phosphorylates and activates PM proton pumps (H+-ATPase) to control apoplastic pH, which triggers expansins and relaxes the cell wall (McQueen-Mason et al., 1992). The transmembrane kinases also directly interact/phosphorylate PM H+-ATPases, evoking their activation, and inducing cell-wall (apoplastic) acidification, and hypocotyl cell elongation in Arabidopsis (Lin et al., 2021). Auxin acidification modulates the relaxation of cell wall matrix due to loosening of the binding between cell wall polysaccharides, as controlled by XTHs and cellulases. The expression of these XTHs and cellulases is upregulated by auxin. When cellulase activity is increased by auxin, the load-bearing hemicellulose chains are cleaved, causing modification of interactions between polysaccharides and other cell wall components. This leads to eventual cell wall loosening and extensibility (nicely reviewed in (Majda and Robert, 2018)). However, how these hormonal crosstalks regulate drought stress response and adaptation in crops is yet to be fully understood. It will be interesting to understand, and/or quantify, how cells balance growth/development- and stress-response-related portions of cell wall modifications.
Other phytohormones, including jasmonic acid (JA), salicylic acid (SA), ethylene, etc., also participate in cell wall remodeling-mediated abiotic stress response (Ellis et al., 2002; Novaković et al., 2018), and defense ( (Nafisi et al., 2015). The levels of these hormones are elevated in response to stress-induced cellulose damage or reduction, leading to cell wall compositional and mechanical shifts, including ectopic lignification (Kesten et al., 2017) (Figure 6). In certain situations, these phytohormone (eg., BR and JA) signaling pathways intricately interact with ROS and CWI pathways to regulate stress response and adaptation (Nafisi et al., 2015; Novaković et al., 2018). For instance, on one hand, JA inhibits stress-induced cell-wall-remodeling-related ectopic lignin accumulation in Arabidopsis (Denness et al., 2011). Mutants (eg., allene oxide synthase, aos; and jasmonic acid resistant 1, jar1) with stifled JA production exhibit elevated lignin accumulation prompted by the damage to the cell wall (Denness et al., 2011). On the other hand, mutants with repressed ROS generation and CWI detection (eg., rboh and the1), exhibit elevated contents of ectopic lignin caused by the damage to the cell wall (Denness et al., 2011), revealing the important crosstalk coupling JA, ROS and CWI pathways as necessary for cell wall damage-triggered responses. Decoding such crosstalk helps in clarifying how drought tolerance is regulated by each or combination of the cell-wall remodeling-involved components or pathways, and could facilitate for appropriate cell wall adjustments, or metabolic engineering for drought tolerance in crop plants (Neumann, 1995; Liu S. et al., 2023).
6 Advances in plant cell wall imaging are driving cell wall remodeling investigations
Over the past two decades, a repertoire of plant cell imaging techniques has been developed to aid the functional visualization of plant cell structural architecture and biosynthetic processes at different spatiotemporal scales, offering a better understanding of how the plant cell wall functions under different conditions (Higashiyama et al., 2021; Xu et al., 2021) (Figure 7). Particularly, the deployment of different microscopy models, including polarized light microscopy (Radosavljević et al., 2021), scanning electron microscopy (SEM) (Fromm et al., 2003; Wightman, 2022), field emission scanning electron microscopy (FESEM) (Zheng et al., 2017), etc., revolutionized plant cell/tissue/organ imaging, and significantly enhanced visualization and quantification of subtle cell wall compositional and morphological changes, which helped in resolving some complex biological questions that could not be answered by conventional biochemical assays (Komis et al., 2018; Cui et al., 2023).
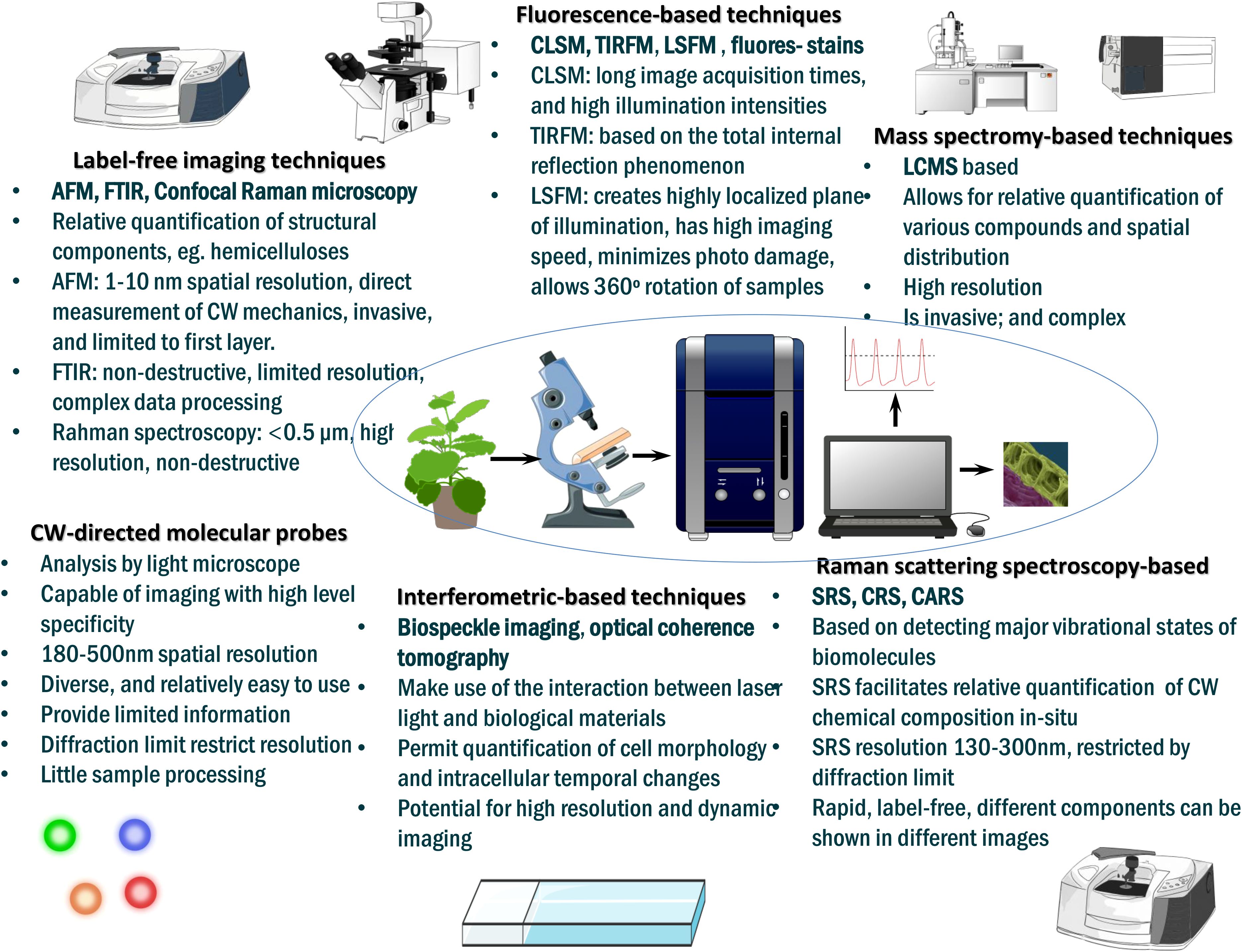
Figure 7. Summary of advanced plant cell wall imaging techniques (not exhaustive). CW, cell wall; AFM, atomic force microscopy; CLSM, confocal laser-scanning microscopy; CRS, coherent Raman scattering microscopy; CARS, coherent anti-Stokes Raman scattering (CARS) microscopy; FTIR, Fourier-transform infrared spectroscopy; LCMS, liquid chromatography-mass spectrometry; LSFM, light sheet fluorescent microscopy; SRS, stimulated Raman scattering microscopy; TIRFM, total internal reflection fluorescence microscopy. For detailed reviews, visit (Zhao et al., 2019; Mateu et al., 2020; DeVree et al., 2021; Xu et al., 2021; Alonso Baez and Bacete, 2023; Piccinini et al., 2024).
Fluorescence-based techniques, including confocal laser-scanning microscopy, total internal reflection fluorescence microscopy, light sheet fluorescent microscopy, etc., have provided insights into plant cell wall composition, structure, and biosynthetic machineries such as lignin and pectin cross-linking (reviewed in (Tobimatsu et al., 2013; Zeng et al., 2017; Kitin et al., 2020; DeVree et al., 2021)). Additionally, several histochemical and immunolabeling tools can be used for direct labeling, targeted analyses, and/or real-time visualization of dynamics of cell wall polysaccharides or other non-polysaccharide components, thereby offering new ways to explore the physiological mechanisms of cell-wall remodeling (Barnes and Anderson, 2018; Rydahl et al., 2018; Voiniciuc et al., 2018; DeVree et al., 2021). These histochemical and immunolabeling techniques include the monoclonal antibodies, small-molecule probes and fluorescently tagged monolignols (detailed in (Rydahl et al., 2018; Ursache et al., 2018)). For instance, 2F4 monoclonal antibody has been successfully used to detect how pectin HGs, one of the major plant cell wall components, can form pectate complexes with divalent Ca2+ ions (egg boxes) when GalA residues are blockwise de-esterified (Liners et al., 1989), since this mechanism crucially regulate wall biomechanics and mediates cell-cell bonding (Du et al., 2022). Meanwhile, turgor pressure changes within guard cells during drought stress regulate stomatal opening and closing (Auler et al., 2022), and these are governed by biochemical and mechanical shifts of the GCWs (Amsbury et al., 2016). A histological technique, monoclonal antibody LM20 – with binding affinity to highly methylesterified HGs, has been used to differentiate guard cell wall components from adjacent epidermal cells. Based on the variations in immunofluorescence, Arabidopsis GCWs are distinguished by low level of methylated pectins (Amsbury et al., 2016), thereby providing an effective way for identifying GCW components and understanding GCW modifications during drought stress (Alonso Baez and Bacete, 2023). Recently, an inventive oligogalacturonide-derived molecular probe (modeled on Ca2+-dependent attachment of fluorescently-labeled oligogalacturonides to endogenous de-esterified HGs) was developed to competently monitor HG crosslinking dynamics in elongating pollen tube- and root hair cell walls of Arabidopsis in real-time (Mravec et al., 2017), thereby further clarifying the mechanisms by which cell walls are remodeled. Equally (Besten et al., 2025), developed CarboTag, a modular approach for live and functional fluorescence imaging of plant cell walls. It is based on a small molecular motif, a pyridine boronic acid, that undergoes high-affinity binding with diols in the plant cell wall (Besten et al., 2025). CarboTag enabled the authors to develop several cell wall imaging probes (in different colors) for multiplexing, and novel functional reporters for live and quantitative imaging of key cell wall properties such as network porosity, cell wall pH, and the presence of ROS (Besten et al., 2025). This technique opens the way for dynamic and quantitative mapping of cell wall responses to various perturbations, including abiotic stress, at subcellular resolution. Moreover, it is applicable to diverse plant and brown algae species without the need for genetic manipulation of the organism of interest (Besten et al., 2025). Besides, fluorescent proteins (FP) can be conveniently tagged to cell wall-specific fluorophores, permitting simultaneous visualization of FP-labelled proteins and cell wall components (Ursache et al., 2018; Voiniciuc et al., 2018; DeVree et al., 2021). Further, coupling cell wall-directed molecular probes to light microscope-based analysis facilitates high-level-specificity imaging, minimal sample processing, and real time parsing of cell wall assemblies, thereby “simplifying” our understanding of plant cell wall architecture and stress-related response mechanisms (DeVree et al., 2021).
Meanwhile, high-resolution label-free imaging techniques, including AFM (Zhang et al., 2016; Song et al., 2020; Pu et al., 2022; Pu et al., 2025), Fourier-transformed infrared (FTIR) microspectroscopy (Smith-Moritz et al., 2015), hyperspectral imaging (HSI) (Sarić et al., 2022), transmission electron microscopy (TEM) (Fromm et al., 2003), confocal Raman microscropy (µ-Raman) (Gierlinger et al., 2012), etc., now enable direct and powerful visualization, and revelation of molecular and native chemical composition, as well as structural architecture of cell walls (Barnes and Anderson, 2018; Zhao et al., 2019; Alonso Baez and Bacete, 2023). For instance, AFM technique has been used to directly image PCWs and SCWs from fresh maize tissues under near-native conditions (Song et al., 2020). By analyzing cellulose structure in these different cell wall types, individual microfibrils and bundles in these cell walls were measured at nanometer scale, which helped to parse the different mechanisms of cellulose biosynthesis in PCWs and SCWs (Song et al., 2020). Similarly (Braybrook and Peaucelle, 2013), used AFM to reveal a feedback loop between Aux signaling and pectin-mediated cell wall remodeling that underpins organ formation and development in Arabidopsis (Braybrook and Peaucelle, 2013). The latest AFM-based infrared/Raman spectroscopy allows for in situ imaging of the multidimensional structure of the cell wall, revealing the mechanical characteristics of plant tissues or single cells, and specific single-molecule recognition of cell wall-related enzymes (Pu et al., 2025). As already discussed above, stomatal opening determines drought resistance, and guard cell walls have a crucial role in this process (Pirasteh-Anosheh et al., 2016). Since FTIR microspectroscopic imaging technique allows for a detailed analysis of the biochemical composition of specific cell walls in situ, and has already been used to classify cell wall mutants (Mouille et al., 2003), it could be a suitable fit for in-depth analysis of the composition and orientation of guard cell wall (GCW) polymers during drought stress (Alonso Baez and Bacete, 2023).
Hyperspectral imaging (HSI) is a noninvasive, label-free, rapid, and high-throughput plant phenotyping (HTPP) technique that acquires and processes both spectral (λ) and spatial (x, y) information and merges these into a 3D data matrix, referred to as ‘hyperspectral data cube’, with HSI sensors capturing information from the entire wavelength spectrum (UV, near-infrared, visible, and short-wave infrared in the 250–2500 nm regions) (Sarić et al., 2022). Moreover, HSI systems have a finer resolution (<5 nm) with tens to hundreds of spectral bands in a continuous range (Mehta et al., 2018). In comparison, RGB imaging collects light interacting with a sample at only three distinct wavelengths (red – 630 nm, green – 545 nm, and blue 435 nm) and the information on the location (spatial information) from which the light is being collected (Mehta et al., 2018). The additional spectral information provided by HSI facilitates for more accurate analysis and understanding of micro and nanoscale properties of the plant cell walls that are not detectable using RGB imaging (Mehta et al., 2018). HSI is a powerful and important tool for plant cell wall visualization and analysis, and has been applied in determining plant traits (eg., root traits), and detecting plant abiotic and biotic stress (eg., early phases of plant disease) responses (Sarić et al., 2022). For instance (Charrier et al., 2021), used a multimodal scattering near-field optical microscopy (SNOM) technique for in-situ HSI of poplar wood material. This technique facilitated determination of nanoscale properties of PCWs by correlating the local optical, chemical, and mechanical properties at a spatial resolution of 20 nm, which enabled monitoring of the delignification process and different lignin acetylation yields in relation to their structure and location in the PCW of poplar wood (Charrier et al., 2021). It is possible that this same technique can equally be applied to monitoring drought stress-induced cell wall modifications such as acetylation or demethylesterification of pectin. Meanwhile (Barbut et al., 2024), combined RGB and HSI techniques to reveal that the integrity of xylan backbone in SCW affects Arabidopsis response to drought stress. Coupling such advanced techniques to single cell analysis and machine learning approaches now permits for real-time in vivo monitoring of plant stress response, such as stress-related H2O2 signaling, and accurate differentiation of different types of stress, ultimately enhancing our understanding of the mechanisms of plant stress signaling (Hu et al., 2025).
TEM is a high-resolution microscopic technique that employs highly energetic electrons for the in situ analysis of the ultrastructure (at atomic scale) and direct visualization of the nanoscale morphologies of cellulosic materials (especially the “nanocelluloses” – nanofibers and nanocrystals) through a highly magnified image (Ogawa and Putaux, 2019). Now, time-resolved in situ TEM enables unprecedented understanding of the ultrastructure of materials and how structure is related to properties and function (Alcorn et al., 2023). Already, TEM technique has been applied in ultrastructural and immunohistological investigations of plant viral pathogens (Zechmann and Zellnig, 2009), and can be deployed for real-time assessment of cell wall remodeling dynamics during drought stress. When coupled to molecular probes, ie., colloidal gold-conjugated antibodies (to form immunogold TEM), TEM immunolocalizes polysaccharides across the cell wall thickness at much higher resolution (Sun et al., 2017). Additionally, immunogold TEM offers information on the relative abundances of target polysaccharides in a cell wall (Liners and Van Cutsem, 1992), thereby providing utility for analyzing the composition and distribution of cell wall polysaccharides at an ultrastructural level (Sun et al., 2017; Majda, 2019).
The latest cutting-edge Raman microscopy (µ-Raman), which integrates the chemical analysis technique, Raman spectroscopy (dependent on inelastic or Raman scattering of monochromatic laser light interacting with biomolecules), with a traditional light microscope (Gierlinger et al., 2012), is a non-destructive label-free technique that facilitates in-situ analysis of chemical composition and direct visualization of the structures of cell wall components such as pectins at micrometer (<0.5 μm) and nano-scale levels (Mateu et al., 2020). µ-Raman based approaches, including the coherent Raman scattering (CRS) microscopy, coherent anti-Stokes Raman scattering (CARS) microscopy, and stimulated Raman scattering (SRS) microscopy (Mateu et al., 2020; Xu et al., 2021), are modelled on detecting biomolecules` major vibrational states (Kumamoto et al., 2018), thereby offering label-free dynamics, rapid, high-specificity, and quantitative microanalysis of cell walls chemical compositions in their native states (Cui et al., 2023). µ-Raman is used in live imaging of cell wall biosynthetic processes (Xu et al., 2021) and cell wall lignification (Schmidt et al., 2010). Thus, µ-Raman spectroscopy is important for elucidating plant cell wall cross-linking chemistry and polymeric architecture (Zeng et al., 2017), improving our fundamental understanding of the dynamic processes involved in stress-induced cell wall remodeling (Zhao et al., 2019). When integrated to super-resolution microscopy, such as structured illumination microscopy, photoactivation localization microscopy, etc., and real-time analyses of dynamic cell wall structural changes (through use of light-sheet fluorescence microscopy), these label-free imaging technologies fine-tune the molecular characterization, and enhance our knowledge of temporal cell wall structural and compositional shifts under stress conditions (Komis et al., 2018; Zhao et al., 2019). This may help us to deconstruct individual constituents of CWI, phytohormonal, and ROS signaling networks, and to decipher cell wall regulation by their crosstalk (Novaković et al., 2018). Meanwhile, the surface-enhanced Raman scattering (SERS)-based nanoprobe is used for the real-time in vivo monitoring of multiple endogenous stress signaling molecules in plants (Son et al., 2023). The nanosensor is placed in the intercellular space, and is optically active in the near-infrared region (785 nm), enabling it to evade interferences from plant autofluorescence (Son et al., 2023). The method has been successfully used to detect abiotic and biotic stress-related molecules, including phytohormone salicylic acid, extracellular adenosine triphosphate (ATP), cruciferous phytoalexin, and glutathione in Nasturtium officinale, bread wheat, and barley species, signposting the possible onset of plant stress, including disease (Son et al., 2023). This paves the way for the possibility of monitoring the commencement or early processes of stress in plants, including the key molecules involved.
Furthermore, the latest label-free interferometric-based technologies, such as biospeckle imaging, optical coherence tomography, etc., can harness the interaction of laser light with biological materials to deduce (from interference patterns) important structural and activity information, permitting measurement of cell structure and temporal intracellular changes (Ebrahimi et al., 2023). For instance, laser speckle contrast imaging has been used to monitor the dynamic changes occurring during the growth of the apical root region of beetroot (Beta vulgaris) (Schott et al., 2022). It is possible that this technique can be harnessed for in situ monitoring of the physiological processes, and in particular the cell wall remodeling mechanisms occurring within the root apical region under drought stress. Besides, interferometric-based technologies have shown high utility in determining seed germination capacity, plant growth, plant disease, etc., and are envisaged to facilitate high-resolution and dynamic imaging of plant cell walls at various spatiotemporal scales (Ebrahimi et al., 2023). This will enhance our knowledge of abiotic stress-triggered cell wall modifications and crosstalk among multiple interconnected pathways.
Despite these recent advances, however, understanding drought stress-induced cell wall remodeling still remain conceptually and technically challenging. This is because of several reasons. First, disentangling the complex crosstalk among CWI maintenance, cell wall stress sensing and signaling, phytohormonal signaling, and ROS production mechanisms remains cumbersome, especially deconstruction of the individual components constituting each of those networks, and grasping how cell wall stress sensors decode or differentiate specific abiotic stress-induced cell wall modifications to institute narrowly targeted responses (Kumar et al., 2016; Novaković et al., 2018). Secondly, the non-quantitative nature of the data output of some of the low-end biochemical and cytological exploration techniques still in use, and their inability to monitor dynamic changes in plant development or biomass transformation limit their overall effectiveness (Zhao et al., 2019; Xu et al., 2021). Fortunately, the recent cutting-edge imaging techniques highlighted above are countering some of these shortcomings. Furthermore, despite the central role of cell wall-localized proteins like EXPAs, XTHs, or PMEs in assembly and spatiotemporal cell wall control, their precise localization or movement within the cell wall, remains largely unresolved (DeVree et al., 2021). Designing specific probes targeting these cell wall proteins will be crucial in understanding the connection between the cell wall protein activity, localization, and formation of cell wall microdomains, and whether engineering of these proteins could be useful in designing cell walls with desired characteristics to enhance plant stress tolerance (DeVree et al., 2021). Going forward, coupling these latest high-resolution imaging techniques to high-sensitivity mass spectromy-based analytical and spatially resolved singe-cell omics approaches (Zhang H. et al., 2023), and advanced data analysis (eg., multivariate) methods (Moore et al., 2020) will immensely improve our understanding of the cell wall composition, structure, biosynthetic machineries, function, and regulation at spatial and time-gated scales (DeVree et al., 2021). Besides, synthetic biology (Shelake et al., 2022) and genome-editing approaches (Zafar et al., 2020) are rapidly “simplifying” deconstruction of complex networks, and facilitating metabolic pathway engineering for enhanced stress tolerance (Liu S. et al., 2023). We anticipate progressive improvement in these technologies to facilitate appropriate cell wall modifications (for instance, via genetic, or in-planta biosynthetic pathways engineering (Yoshida et al., 2021)) necessary for developing more drought-tolerant crop plants tailor-fit for the new climatic environments.
7 Conclusion
Cell wall interfaces the cell interior with its surrounding environment, regulating stress sensing, signaling and response; therefore, cell wall plasticity under drought stress crucially helps in regulating plant stress acclimation and adaptation. Here, we have synthesized how several cell wall remodeling mechanisms orchestrate drought tolerance in plants (Figure 8). Stress-induced demethylesterification of pectins, mediated by PMEs, permits calcium crosslinking of polyphenolics, which enhances cell wall rigidity and may help in intra-cell water preservation. Lignin accumulation-mediated cell wall thickening increases the plant tissue`s structural robustness, and regulates cell water movement across the cell membrane, which potentiates better plant tolerance to drought. Root suberization creates a hydrophobic and protective secondary cell wall layer that minimizes water loss and enhance drought tolerance. Similarly, PMEs and expansin activities that regulate guard cell wall plasticity are essential in controlling stomatal aperture in response to drought stress, and are excellent targets for genetic engineering of drought tolerance in crops. At the same time, highly plastic transcriptional regulation of secondary cell wall biosynthetic or stress-responsive genes, modulated by several TFs such as MYBs, NACs, WRKYs, etc., orchestrates plant drought tolerance. Meanwhile, phytohormones such as BRs, auxins, etc., crosstalk with ROS and cell wall integrity pathways to modulate stress response and transcriptional output of some cell-wall remodeling-associated genes. However, fine-tuning plant cell wall properties to engineer stress tolerance requires a deep understanding of these cell wall biosynthesis and regulation mechanisms, and then deploy genome engineering techniques to tailor the desired modifications. Harnessing modern high-resolution plant cell wall imaging techniques, single cell omics, genome editing and synthetic biology approaches could help us achieve this feat.
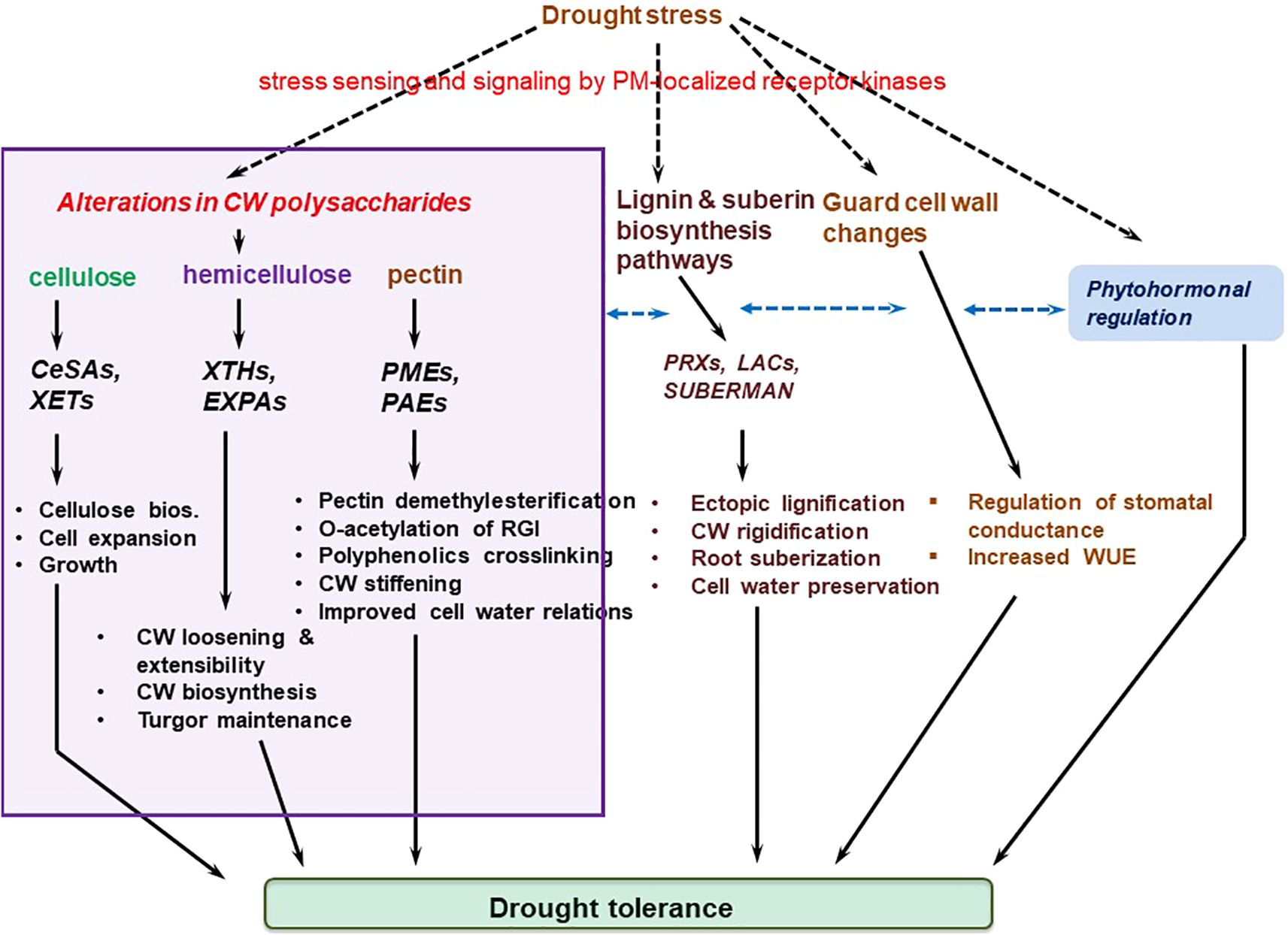
Figure 8. Summary of cell wall remodeling mechanisms orchestrating plant drought tolerance discussed in this review. Purple enclosure signifies cell wall integrity maintenance pathway, black dashed arrows represent stress sensing and signaling mediated by plasma membrane-localized receptor kinases/proteins, whereas blue dashed connectors represent crosstalk among the different signaling pathways. CeSA, cellulose synthase A; cellulose bios., cellulose biosynthesis; EXPAs, expansins; LACs, laccases; PAEs, pectin acetylesterases; PMEs, pectin methylesterases; PRXs, peroxidases; RGI, rhamnogalacturonan I; SUBERMAN, SUBERMAN transcription factor; WUE, water use efficiency; XETs, xyloglucan endotransglucosylases; XTHs, xyloglucan endotransglucosylases/hydrolases.
Author contributions
NZ: Formal Analysis, Writing – original draft, Visualization, Data curation, Writing – review & editing, Validation, Investigation, Conceptualization, Methodology. ZZ: Investigation, Formal Analysis, Writing – review & editing, Validation. SC: Investigation, Formal Analysis, Validation, Writing – review & editing. XZ: Writing – review & editing, Validation, Data curation, Investigation. SZ: Investigation, Writing – review & editing, Validation, Formal Analysis. YW: Investigation, Writing – review & editing, Formal Analysis, Data curation. TZ: Project administration, Formal Analysis, Visualization, Methodology, Conceptualization, Software, Writing – original draft, Validation, Investigation. LW: Formal Analysis, Project administration, Validation, Methodology, Writing – review & editing, Conceptualization, Supervision, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Science and Technology Research Project for Colleges and Universities in Hebei Province (No. QN2023004); Langfang Normal University Doctoral Research Initiation Fund (No. XBQ202307); Hebei Province Modern Agricultural Industrial Technology System Innovation Project (HBC2023090208).
Acknowledgments
The authors sincerely thank Dr. Songtao Liu (Hebei North University, China) for helping with valuable suggestions during draft manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aida, M., Beis, D., Heidstra, R., Willemsen, V., Blilou, I., Galinha, C., et al. (2004). The PLETHORA genes mediate patterning of the arabidopsis root stem cell niche. Cell 119, 109–120. doi: 10.1016/j.cell.2004.09.018
Alavarse, A. C., Frachini, E. C. G., da Silva, R. L. C. G., Lima, V. H., Shavandi, A., and Petri, D. F. S. (2022). Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 202, 558–596. doi: 10.1016/j.ijbiomac.2022.01.029
Alcorn, F. M., Jain, P. K., and van der Veen, R. M. (2023). Time-resolved transmission electron microscopy for nanoscale chemical dynamics. Nat. Rev. Chem. 7, 256–272. doi: 10.1038/s41570-023-00469-y
Alonso Baez, L. and Bacete, L. (2023). Cell wall dynamics: novel tools and research questions. J. Exp. Bot. 74 (21), 6448–6467. doi: 10.1093/jxb/erad310
Amsbury, S., Hunt, L., Elhaddad, N., Baillie, A., Lundgren, M., Verhertbruggen, Y., et al. (2016). Stomatal function requires pectin de-methyl-esterification of the guard cell wall. Curr. Biol. 26, 2899–2906. doi: 10.1016/j.cub.2016.08.021
An, S. H., Sohn, K. H., Choi, H. W., Hwang, I. S., Lee, S. C., and Hwang, B. K. (2008). Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta 228, 61–78. doi: 10.1007/s00425-008-0719-z
Anderson, C. T. and Kieber, J. J. (2020). Dynamic construction, perception, and remodeling of plant cell walls. Annu. Rev. Plant Biol. 71, 39–69. doi: 10.1146/annurev-arplant-081519-035846
Ashwin Narayan, J., Chakravarthi, M., Nerkar, G., Manoj, V. M., Dharshini, S., Subramonian, N., et al. (2021). Overexpression of expansin EaEXPA1, a cell wall loosening protein enhances drought tolerance in sugarcane. Ind. Crops Prod. 159, 113035. doi: 10.1016/j.indcrop.2020.113035
Atmodjo, M. A., Hao, Z., and Mohnen, D. (2013). Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 64, 747–779. doi: 10.1146/annurev-arplant-042811-105534
Auler, P. A., Freire, F. B. S., Lima, V. F., and Daloso, D. M. (2022). On the role of guard cells in sensing environmental signals and memorising stress periods. Theor. Exp. Plant Physiol. 34, 277–299. doi: 10.1007/s40626-022-00250-4
Bacete, L., Mélida, H., Miedes, E., and Molina, A. (2018). Plant cell wall-mediated immunity: cell wall changes trigger disease resistance responses. Plant J. Cell Mol. Biol. 93, 614–636. doi: 10.1111/tpj.13807
Bacete, L., Schulz, J., Engelsdorf, T., Bartosova, Z., Vaahtera, L., Yan, G., et al. (2022). THESEUS1 modulates cell wall stiffness and abscisic acid production in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 119, e2119258119. doi: 10.1073/pnas.2119258119
Baez, L. A., Tichá, T., and Hamann, T. (2022). Cell wall integrity regulation across plant species. Plant Mol. Biol. 109, 483–504. doi: 10.1007/s11103-022-01284-7
Bahri, B. A., Daverdin, G., Xu, X., Cheng, J.-F., Barry, K. W., Brummer, E. C., et al. (2020). Natural variation in lignin and pectin biosynthesis-related genes in switchgrass (Panicum virgatum L.) and association of SNP variants with dry matter traits. Bioenergy Res. 13, 79–99. doi: 10.1007/s12155-020-10090-2
Bang, S. W., Choi, S., Jin, X., Jung, S. E., Choi, J. W., Seo, J. S., et al. (2022). Transcriptional activation of rice CINNAMOYL-CoA REDUCTASE 10 by OsNAC5, contributes to drought tolerance by modulating lignin accumulation in roots. Plant Biotechnol. J. 20, 736–747. doi: 10.1111/pbi.13752
Bang, S. W., Lee, D.-K., Jung, H., Chung, P. J., Kim, Y. S., Choi, Y. D., et al. (2019). Overexpression of OsTF1L, a rice HD-Zip transcription factor, promotes lignin biosynthesis and stomatal closure that improves drought tolerance. Plant Biotechnol. J. 17, 118–131. doi: 10.1111/pbi.12951
Barberon, M., Vermeer, J. E. M., De Bellis, D., Wang, P., Naseer, S., Andersen, T. G., et al. (2016). Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164, 447–459. doi: 10.1016/j.cell.2015.12.021
Barbut, F. R., Cavel, E., Donev, E. N., Gaboreanu, I., Urbancsok, J., Pandey, G., et al. (2024). Integrity of xylan backbone affects plant responses to drought. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1422701
Barnes, W. J. and Anderson, C. T. (2018). Release, recycle, rebuild: cell-wall remodeling, autodegradation, and sugar salvage for new wall biosynthesis during plant development. Mol. Plant 11, 31–46. doi: 10.1016/j.molp.2017.08.011
Bashir, S. S., Hussain, A., Hussain, S. J., Wani, O. A., Zahid Nabi, S., Dar, N. A., et al. (2021). Plant drought stress tolerance: understanding its physiological, biochemical and molecular mechanisms. Biotechnol. Biotechnol. Equip. 35, 1912–1925. doi: 10.1080/13102818.2021.2020161
Bashline, L., Lei, L., Li, S., and Gu, Y. (2014). Cell wall, cytoskeleton, and cell expansion in higher plants. Mol. Plant 7, 586–600. doi: 10.1093/mp/ssu018
Baskin, T. I. (2005). Anisotropic expansion of the plant cell wall. Annu. Rev. Cell Dev. Biol. 21, 203–222. doi: 10.1146/annurev.cellbio.20.082503.103053
Berthet, S., Demont-Caulet, N., Pollet, B., Bidzinski, P., Cézard, L., Le Bris, P., et al. (2011). Disruption of LACCASE4 and 17 results in tissue-specific alterations to lignification of arabidopsis thaliana stems. Plant Cell. 23, 1124–1137. doi: 10.1105/tpc.110.082792
Besten, M., Hendriksz, M., Michels, L., Charrier, B., Smakowska-Luzan, E., Weijers, D., et al. (2025). CarboTag: a modular approach for live and functional imaging of plant cell walls. Nat. Methods 22, 1081–1090. doi: 10.1038/s41592-025-02677-4
Biely, P. (2012). Microbial carbohydrate esterases deacetylating plant polysaccharides. Biotechnol. Adv. 30, 1575–1588. doi: 10.1016/j.biotechadv.2012.04.010
Bilska-Kos, A., Pietrusińska, A., Suski, S., Niedziela, A., Linkiewicz, A. M., Majtkowski, W., et al. (2022). Cell wall properties determine genotype-specific response to cold in miscanthus × giganteus plants. Cells 11, 547. doi: 10.3390/cells11030547
Bischoff, V., Nita, S., Neumetzler, L., Schindelasch, D., Urbain, A., Eshed, R., et al. (2010). TRICHOME BIREFRINGENCE and its homolog AT5G01360 encode plant-specific DUF231 proteins required for cellulose biosynthesis in Arabidopsis. Plant Physiol. 153, 590–602. doi: 10.1104/pp.110.153320
Blaschek, L., Murozuka, E., Serk, H., Ménard, D., and Pesquet, E. (2023). Different combinations of laccase paralogs nonredundantly control the amount and composition of lignin in specific cell types and cell wall layers in Arabidopsis. Plant Cell. 35, 889–909. doi: 10.1093/plcell/koac344
Boller, T. and Felix, G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. doi: 10.1146/annurev.arplant.57.032905.105346
Bot, P., Mun, B.-G., Imran, Q. M., Hussain, A., Lee, S.-U., Loake, G., et al. (2019). Differential expression of AtWAKL10 in response to nitric oxide suggests a putative role in biotic and abiotic stress responses. PeerJ 7, e7383. doi: 10.7717/peerj.7383
Boyer, J. S. (1982). Plant productivity and environment. Science 218, 443–448. doi: 10.1126/science.218.4571.443
Braidwood, L., Breuer, C., and Sugimoto, K. (2014). My body is a cage: mechanisms and modulation of plant cell growth. New Phytol. 201, 388–402. doi: 10.1111/nph.12473
Braybrook, S. A. and Peaucelle, A. (2013). Mechano-chemical aspects of organ formation in arabidopsis thaliana: the relationship between auxin and pectin. PloS One 8, e57813. doi: 10.1371/journal.pone.0057813
Caffall, K. H. and Mohnen, D. (2009). The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 344, 1879–1900. doi: 10.1016/j.carres.2009.05.021
Cantó-Pastor, A., Kajala, K., Shaar-Moshe, L., Manzano, C., Timilsena, P., De Bellis, D., et al. (2024). A suberized exodermis is required for tomato drought tolerance. Nat. Plants 10, 118–130. doi: 10.1038/s41477-023-01567-x
Cao, Y., Li, K., Li, Y., Zhao, X., and Wang, L. (2020). MYB transcription factors as regulators of secondary metabolism in plants. Biology 9, 61. doi: 10.3390/biology9030061
Cao, L., Lu, W., Mata, A., Nishinari, K., and Fang, Y. (2020). Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 242, 116389. doi: 10.1016/j.carbpol.2020.116389
Carpita, N. C. (1996). Structure and biogenesis of the cell walls of grasses. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 445–476. doi: 10.1146/annurev.arplant.47.1.445
Carpita, N. C. and Gibeaut, D. M. (1993). Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. Cell Mol. Biol. 3, 1–30. doi: 10.1111/j.1365-313X.1993.tb00007.x
Carpita, N. C. and McCann, M. C. (2020). Redesigning plant cell walls for the biomass-based bioeconomy. J. Biol. Chem. 295, 15144–15157. doi: 10.1074/jbc.REV120.014561
Carter, R., Woolfenden, H., Baillie, A., Amsbury, S., Carroll, S., Healicon, E., et al. (2017). Stomatal opening involves polar, not radial, stiffening of guard cells. Curr. Biol. 27, 2974–2983.e2. doi: 10.1016/j.cub.2017.08.006
Castilleux, R., Plancot, B., Vicré, M., Nguema-Ona, E., and Driouich, A. (2021). Extensin, an underestimated key component of cell wall defence? Ann. Bot. 127 (6), 709–713. doi: 10.1093/aob/mcab001
Chang, J., Li, X., Shen, J., Hu, J., Wu, L., Zhang, X., et al. (2024). Defects in the cell wall and its deposition caused by loss-of-function of three RLKs alter root hydrotropism in Arabidopsis thaliana. Nat. Commun. 15, 2648. doi: 10.1038/s41467-024-46889-2
Charrier, G. (2021). Suffer from drought to withstand the cold. Plant Physiol. 186, 208–209. doi: 10.1093/plphys/kiab094
Charrier, A. M., Normand, A. C., Passian, A., Schaefer, P., and Lereu, A. L. (2021). In situ plant materials hyperspectral imaging by multimodal scattering near-field optical microscopy. Commun. Mater. 2, 1–12. doi: 10.1038/s43246-021-00166-7
Chebli, Y. and Geitmann, A. (2017). Cellular growth in plants requires regulation of cell wall biochemistry. Curr. Opin. Cell Biol. 44, 28–35. doi: 10.1016/j.ceb.2017.01.002
Chen, Y., Han, Y., Zhang, M., Zhou, S., Kong, X., and Wang, W. (2016). Overexpression of the wheat expansin gene taEXPA2 improved seed production and drought tolerance in transgenic tobacco plants. PloS One 11, e0153494. doi: 10.1371/journal.pone.0153494
Chen, Z., Hong, X., Zhang, H., Wang, Y., Li, X., Zhu, J.-K., et al. (2005). Disruption of the cellulose synthase gene, AtCesA8/IRX1, enhances drought and osmotic stress tolerance in Arabidopsis. Plant J. Cell Mol. Biol. 43, 273–283. doi: 10.1111/j.1365-313X.2005.02452.x
Chen, P., Jung, N. U., Giarola, V., and Bartels, D. (2020). The dynamic responses of cell walls in resurrection plants during dehydration and rehydration. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01698
Chen, A., Liu, T., Wang, Z., and Chen, X. (2022). Plant root suberin: A layer of defence against biotic and abiotic stresses. Front. Plant Sci. 13, 1056008. doi: 10.3389/fpls.2022.1056008
Cheng, M., Meng, F., Qi, H., Mo, F., Wang, P., Chen, X., et al. (2022). Escaping drought: The pectin methylesterase inhibitor gene Slpmei27 can significantly change drought resistance in tomato. Plant Physiol. Biochem. 192, 207–217. doi: 10.1016/j.plaphy.2022.10.008
Cheung, A. Y. (2024). FERONIA: A receptor kinase at the core of a global signaling network. Annu. Rev. Plant Biol. 75, 345–375. doi: 10.1146/annurev-arplant-102820-103424
Cho, S. K., Kim, J. E., Park, J.-A., Eom, T. J., and Kim, W. T. (2006). Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett. 580, 3136–3144. doi: 10.1016/j.febslet.2006.04.062
Choi, S. J., Lee, Z., Kim, S., Jeong, E., and Shim, J. S. (2023). Modulation of lignin biosynthesis for drought tolerance in plants. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1116426
Cluzel, X. (2024). What are the differences between drought and drought stress for crops? Elicit Plant. Available online at: https://www.elicit-plant.com/blog/drought-stress/what-are-the-differences-between-drought-and-drought-stress-for-crops/ (Accessed 29 Jun 2025).
Cohen, H., Fedyuk, V., Wang, C., Wu, S., and Aharoni, A. (2020). SUBERMAN regulates developmental suberization of the Arabidopsis root endodermis. Plant J. 102, 431–447. doi: 10.1111/tpj.14711
Cominelli, E., Sala, T., Calvi, D., Gusmaroli, G., and Tonelli, C. (2008). Over-expression of the Arabidopsis AtMYB41 gene alters cell expansion and leaf surface permeability. Plant J. 53, 53–64. doi: 10.1111/j.1365-313X.2007.03310.x
Cosgrove, D. J. (1999). Enzymes and other agents that enhance cell wall extensibility. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 391–417. doi: 10.1146/annurev.arplant.50.1.391
Cosgrove, D. J. (2000). Loosening of plant cell walls by expansins. Nature 407, 321–326. doi: 10.1038/35030000
Cosgrove, D. J. (2005). Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 6, 850–861. doi: 10.1038/nrm1746
Cosgrove, D. J. (2015). Plant expansins: diversity and interactions with plant cell walls. Curr. Opin. Plant Biol. 25, 162–172. doi: 10.1016/j.pbi.2015.05.014
Cosgrove, D. J. (2016a). Catalysts of plant cell wall loosening. F1000Res. 5, F1000 Faculty Rev-119. doi: 10.12688/f1000research
Cosgrove, D. J. (2016b). Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 67, 463–476. doi: 10.1093/jxb/erv511
Cosgrove, D. J. (2018). Diffuse growth of plant cell walls. Plant Physiol. 176, 16–27. doi: 10.1104/pp.17.01541
Cosgrove, D. J. (2024). Plant cell wall loosening by expansins. Annu. Rev. Cell. Dev. Biol. 40 (1), 329–352. doi: 10.1146/annurev-cellbio-111822-115334
Cosio, C., Ranocha, P., Francoz, E., Burlat, V., Zheng, Y., Perry, S. E., et al. (2017). The class III peroxidase PRX17 is a direct target of the MADS-box transcription factor AGAMOUS-LIKE15 (AGL15) and participates in lignified tissue formation. New Phytol. 213, 250–263. doi: 10.1111/nph.14127
Cotelle, V. and Leonhardt, N. (2019). “ABA signaling in guard cells1,” in Advances in botanical research. Eds. Seo, M. and Marion-Poll, A. (Academic Press), 115–170.
Coutinho, F. S., Rodrigues, J. M., Lima, L. L., Mesquita, R. O., Carpinetti, P. A., MaChado, J. P. B., et al. (2021). Remodeling of the cell wall as a drought-tolerance mechanism of a soybean genotype revealed by global gene expression analysis. aBIOTECH 2, 14–31. doi: 10.1007/s42994-021-00043-4
Csiszár, J., Gallé, Á., Horváth, E., Dancsó, P., Gombos, M., Váry, Z., et al. (2012). Different peroxidase activities and expression of abiotic stress-related peroxidases in apical root segments of wheat genotypes with different drought stress tolerance under osmotic stress. Plant Physiol. Biochem. 52, 119–129. doi: 10.1016/j.plaphy.2011.12.006
Cui, Y., Zhang, X., Li, X., and Lin, J. (2023). Multiscale microscopy to decipher plant cell structure and dynamics. New Phytol. 237, 1980–1997. doi: 10.1111/nph.18641
Dabravolski, S. A. and Isayenkov, S. V. (2023). The regulation of plant cell wall organisation under salt stress. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1118313
Dai, F., Zhang, C., Jiang, X., Kang, M., Yin, X., Lü, P., et al. (2012). RhNAC2 and RhEXPA4 are involved in the regulation of dehydration tolerance during the expansion of rose petals. Plant Physiol. 160, 2064–2082. doi: 10.1104/pp.112.207720
Dalal, M., Sahu, S., Tiwari, S., Rao, A. R., and Gaikwad, K. (2018). Transcriptome analysis reveals interplay between hormones, ROS metabolism and cell wall biosynthesis for drought-induced root growth in wheat. Plant Physiol. Biochem. 130, 482–492. doi: 10.1016/j.plaphy.2018.07.035
Decreux, A. and Messiaen, J. (2005). Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol. 46, 268–278. doi: 10.1093/pcp/pci026
Delmer, D., Dixon, R. A., Keegstra, K., and Mohnen, D. (2024). The plant cell wall—dynamic, strong, and adaptable—is a natural shapeshifter. Plant Cell. 36, 1257–1311. doi: 10.1093/plcell/koad325
Denness, L., McKenna, J. F., Segonzac, C., Wormit, A., Madhou, P., Bennett, M., et al. (2011). Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 156, 1364–1374. doi: 10.1104/pp.111.175737
de Silva, N. D. G., Murmu, J., Chabot, D., Hubbard, K., Ryser, P., Molina, I., et al. (2021). Root suberin plays important roles in reducing water loss and sodium uptake in arabidopsis thaliana. Metabolites 11, 735. doi: 10.3390/metabo11110735
DeVree, B. T., Steiner, L. M., Głazowska, S., Ruhnow, F., Herburger, K., Persson, S., et al. (2021). Current and future advances in fluorescence-based visualization of plant cell wall components and cell wall biosynthetic machineries. Biotechnol. Biofuels. 14, 78. doi: 10.1186/s13068-021-01922-0
Dixon, R. A., Chen, F., Guo, D., and Parvathi, K. (2001). The biosynthesis of monolignols: a “metabolic grid”, or independent pathways to guaiacyl and syringyl units? Phytochemistry 57, 1069–1084. doi: 10.1016/S0031-9422(01)00092-9
Dong, N.-Q. and Lin, H.-X. (2021). Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 63, 180–209. doi: 10.1111/jipb.13054
Draeger, C., Ndinyanka Fabrice, T., Gineau, E., Mouille, G., Kuhn, B. M., Moller, I., et al. (2015). Arabidopsis leucine-rich repeat extensin (LRX) proteins modify cell wall composition and influence plant growth. BMC Plant Biol. 15, 155. doi: 10.1186/s12870-015-0548-8
Du, J., Anderson, C. T., and Xiao, C. (2022). Dynamics of pectic homogalacturonan in cellular morphogenesis and adhesion, wall integrity sensing and plant development. Nat. Plants. 8, 332–340. doi: 10.1038/s41477-022-01120-2
Du, J., Kirui, A., Huang, S., Wang, L., Barnes, W. J., Kiemle, S. N., et al. (2020). Mutations in the pectin methyltransferase QUASIMODO2 influence cellulose biosynthesis and wall integrity in arabidopsis. Plant Cell. 32, 3576–3597. doi: 10.1105/tpc.20.00252
Duan, Q., Kita, D., Li, C., Cheung, A. Y., and Wu, H.-M. (2010). FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. 107, 17821–17826. doi: 10.1073/pnas.1005366107
Ebrahimi, S., Moreno-Pescador, G., Persson, S., Jauffred, L., and Bendix, P. M. (2023). Label-free optical interferometric microscopy to characterize morphodynamics in living plants. Front. Plant Sci. 14, 1156478. doi: 10.3389/fpls.2023.1156478
Ebringerová, A. (2005). Structural diversity and application potential of hemicelluloses. Macromol. Symp. 232, 1–12. doi: 10.1002/masy.200551401
El-Azaz, J., de la Torre, F., Pascual, M. B., Debille, S., Canlet, F., Harvengt, L., et al. (2020). Transcriptional analysis of arogenate dehydratase genes identifies a link between phenylalanine biosynthesis and lignin biosynthesis. J. Exp. Bot. 71, 3080–3093. doi: 10.1093/jxb/eraa099
Ellis, C., Karafyllidis, I., Wasternack, C., and Turner, J. G. (2002). The arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell. 14, 1557–1566. doi: 10.1105/tpc.002022
Engelsdorf, T., Gigli-Bisceglia, N., Veerabagu, M., McKenna, J. F., Vaahtera, L., Augstein, F., et al. (2018). The plant cell wall integrity maintenance and immune signaling systems cooperate to control stress responses in Arabidopsis thaliana. Sci. Signal 11, eaao3070. doi: 10.1126/scisignal.aao3070
Ezaki, N., Kido, N., Takahashi, K., and Katou, K. (2005). The role of wall ca2+ in the regulation of wall extensibility during the acid-induced extension of soybean hypocotyl cell walls. Plant Cell Physiol. 46, 1831–1838. doi: 10.1093/pcp/pci199
Ezquer, I., Salameh, I., Colombo, L., and Kalaitzis, P. (2020). Plant cell walls tackling climate change: insights into plant cell wall remodeling, its regulation, and biotechnological strategies to improve crop adaptations and photosynthesis in response to global warming. Plants Basel. Switz. 9, 212. doi: 10.3390/plants9020212
Fan, L., Linker, R., Gepstein, S., Tanimoto, E., Yamamoto, R., and Neumann, P. M. (2006). Progressive inhibition by water deficit of cell wall extensibility and growth along the elongation zone of maize roots is related to increased lignin metabolism and progressive stelar accumulation of wall phenolics. Plant Physiol. 140, 603–612. doi: 10.1104/pp.105.073130
Fan, L. and Neumann, P. M. (2004). The spatially variable inhibition by water deficit of maize root growth correlates with altered profiles of proton flux and cell wall pH. Plant Physiol. 135, 2291–2300. doi: 10.1104/pp.104.041426
Feng, W., Kita, D., Peaucelle, A., Cartwright, H. N., Doan, V., Duan, Q., et al. (2018). The FERONIA Receptor Kinase Maintains Cell-Wall Integrity during Salt Stress through Ca2+ Signaling. Curr. Biol. 28, 666–675.e5. doi: 10.1016/j.cub.2018.01.023
Feng, W., Lindner, H., Robbins NE, I. I., and Dinneny, J. R. (2016). Growing out of stress: the role of cell- and organ-scale growth control in plant water-stress responses. Plant Cell. 28, 1769–1782. doi: 10.1105/tpc.16.00182
Forand, A. D., Finfrock, Y. Z., Lavier, M., Stobbs, J., Qin, L., Wang, S., et al. (2022). With a little help from my cell wall: structural modifications in pectin may play a role to overcome both dehydration stress and fungal pathogens. Plants 11, 385. doi: 10.3390/plants11030385
Fradera-Soler, M., Grace, O. M., Jørgensen, B., and Mravec, J. (2022). Elastic and collapsible: current understanding of cell walls in succulent plants. J. Exp. Bot. 73, 2290–2307. doi: 10.1093/jxb/erac054
Francoz, E., Ranocha, P., Nguyen-Kim, H., Jamet, E., Burlat, V., and Dunand, C. (2015). Roles of cell wall peroxidases in plant development. Phytochemistry 112, 15–21. doi: 10.1016/j.phytochem.2014.07.020
Franke, R. and Schreiber, L. (2007). Suberin — a biopolyester forming apoplastic plant interfaces. Curr. Opin. Plant Biol. 10, 252–259. doi: 10.1016/j.pbi.2007.04.004
Fromm, J., Rockel, B., Lautner, S., Windeisen, E., and Wanner, G. (2003). Lignin distribution in wood cell walls determined by TEM and backscattered SEM techniques. J. Struct. Biol. 143, 77–84. doi: 10.1016/S1047-8477(03)00119-9
Galindo-Trigo, S., Gray, J. E., and Smith, L. M. (2016). Conserved roles of crRLK1L receptor-like kinases in cell expansion and reproduction from algae to angiosperms. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01269
Ganie, S. A. and Ahammed, G. J. (2021). Dynamics of cell wall structure and related genomic resources for drought tolerance in rice. Plant Cell Rep. 40, 437–459. doi: 10.1007/s00299-020-02649-2
García-Angulo, P. and Largo-Gosens, A. (2022). Plant cell wall plasticity under stress situations. Plants 11, 2752. doi: 10.3390/plants11202752
Geng, D., Chen, P., Shen, X., Zhang, Y., Li, X., Jiang, L., et al. (2018). MdMYB88 and mdMYB124 enhance drought tolerance by modulating root vessels and cell walls in apple. Plant Physiol. 178, 1296–1309. doi: 10.1104/pp.18.00502
Gierlinger, N., Keplinger, T., and Harrington, M. (2012). Imaging of plant cell walls by confocal Raman microscopy. Nat. Protoc. 7, 1694–1708. doi: 10.1038/nprot.2012.092
Gigli-Bisceglia, N., Engelsdorf, T., and Hamann, T. (2020). Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell Mol. Life Sci. CMLS. 77, 2049. doi: 10.1007/s00018-019-03388-8
Gigli-Bisceglia, N. and Testerink, C. (2021). Fighting salt or enemies: shared perception and signaling strategies. Curr. Opin. Plant Biol. 64, 102120. doi: 10.1016/j.pbi.2021.102120
Gigli-Bisceglia, N., van Zelm, E., Huo, W., Lamers, J., and Testerink, C. (2022). Arabidopsis root responses to salinity depend on pectin modification and cell wall sensing. Dev. Camb. Engl. 149, dev200363. doi: 10.1242/dev.200363
Gille, S. and Pauly, M. (2012). O-acetylation of plant cell wall polysaccharides. Front. Plant Sci. 3. doi: 10.3389/fpls.2012.00012
Gonneau, M., Desprez, T., Martin, M., Doblas, V. G., Bacete, L., Miart, F., et al. (2018). Receptor kinase THESEUS1 is a rapid alkalinization factor 34 receptor in arabidopsis. Curr. Biol. CB. 28, 2452–2458.e4. doi: 10.1016/j.cub.2018.05.075
Grünhofer, P., Schreiber, L., and Kreszies, T. (2021). “Suberin in monocotyledonous crop plants: structure and function in response to abiotic stresses,” in Rhizobiology: Molecular Physiology of Plant Roots. Eds. Mukherjee, S. and Baluška, F. (Springer International Publishing, Cham), 333–378.
Guo, W., Zhao, J., Li, X., Qin, L., Yan, X., and Liao, H. (2011). A soybean β-expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. Plant J. Cell Mol. Biol. 66, 541–552. doi: 10.1111/j.1365-313X.2011.04511.x
Gupta, A., Rico-Medina, A., and Caño-Delgado, A. I. (2020). The physiology of plant responses to drought. Science 368, 266–269. doi: 10.1126/science.aaz7614
Hamann, T. (2012). Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front. Plant Sci. 3. doi: 10.3389/fpls.2012.00077
Han, Y., Chen, Y., Yin, S., Zhang, M., and Wang, W. (2015). Over-expression of TaEXPB23, a wheat expansin gene, improves oxidative stress tolerance in transgenic tobacco plants. J. Plant Physiol. 173, 62–71. doi: 10.1016/j.jplph.2014.09.007
Han, X., Zhao, Y., Chen, Y., Xu, J., Jiang, C., Wang, X., et al. (2022). Lignin biosynthesis and accumulation in response to abiotic stresses in woody plants. For. Res. 2, 9. doi: 10.48130/FR-2022-0009
Hao, Z., Qian, X., Xiao, X., Huabo, L., Junkai, Z., and Jichen, X. (2017). Transgenic tobacco plants expressing grass AstEXPA1 gene show improved performance to several stresses. Plant Biotechnol. Rep. 11, 331–337. doi: 10.1007/s11816-017-0454-7
Harholt, J., Suttangkakul, A., and Vibe Scheller, H. (2010). Biosynthesis of pectin. Plant Physiol. 153, 384–395. doi: 10.1104/pp.110.156588
Haruta, M., Sabat, G., Stecker, K., Minkoff, B. B., and Sussman, M. R. (2014). A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343, 408–411. doi: 10.1126/science.1244454
He, J.-X., Fujioka, S., Li, T.-C., Kang, S. G., Seto, H., Takatsuto, S., et al. (2003). Sterols regulate development and gene expression in arabidopsis. Plant Physiol. 131, 1258–1269. doi: 10.1104/pp.014605
He, Y., Zhou, J., Shan, L., and Meng, X. (2018). Plant cell surface receptor-mediated signaling - a common theme amid diversity. J. Cell Sci. 131 (2), jcs209353. doi: 10.1242/jcs.209353
He, Z. H., Fujiki, M., and Kohorn, B. D. (1996). A cell wall-receptor-like associated protein kinase. J. Biol. Chem. 271 (33), 19789–19793. doi: 10.1074/jbc.271.33.19789
Hématy, K. and Höfte, H. (2008). Novel receptor kinases involved in growth regulation. Curr. Opin. Plant Biol. 11 (3), 321–328. doi: 10.1016/j.pbi.2008.02.008
Hemati, A., Moghiseh, E., Amirifar, A., Mofidi-Chelan, M., and Asgari Lajayer, B. (2022). “Physiological effects of drought stress in plants,” in Plant Stress Mitigators (Springer, Singapore), 113–124.
Hématy, K., Sado, P.-E., Tuinen, A. V., Rochange, S., Desnos, T., Balzergue, S., et al. (2007). A receptor-like kinase mediates the response of arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 17, 922–931. doi: 10.1016/j.cub.2007.05.018
Henry, A., Cal, A. J., Batoto, T. C., Torres, R. O., and Serraj, R. (2012). Root attributes affecting water uptake of rice (Oryza sativa) under drought. J. Exp. Bot. 63, 4751–4763. doi: 10.1093/jxb/ers150
Herger, A., Dünser, K., Kleine-Vehn, J., and Ringli, C. (2019). Leucine-rich repeat extensin proteins and their role in cell wall sensing. Curr. Biol. CB. 29, R851–R858. doi: 10.1016/j.cub.2019.07.039
Hessini, K., Martínez, J. P., Gandour, M., Albouchi, A., Soltani, A., and Abdelly, C. (2009). Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environ. Exp. Bot. 67, 312–319. doi: 10.1016/j.envexpbot.2009.06.010
Higashiyama, T., Maizel, A., and Simon, R. (2021). Seeing is believing: advances in plant imaging technologies. Plant Cell Physiol. 62, 1217–1220. doi: 10.1093/pcp/pcab133
Hoffmann, N., Benske, A., Betz, H., Schuetz, M., and Samuels, A. L. (2020). Laccases and peroxidases co-localize in lignified secondary cell walls throughout stem development. Plant Physiol. 184, 806–822. doi: 10.1104/pp.20.00473
Höfte, H. and Voxeur, A. (2017). Plant cell walls. Curr. Biol. CB. 27, R865–R870. doi: 10.1016/j.cub.2017.05.025
Hong, S. W., Jon, J. H., Kwak, J. M., and Nam, H. G. (1997). Identification of a receptor-like protein kinase gene rapidly induced by abscisic acid, dehydration, high salt, and cold treatments in arabidopsis thaliana. Plant Physiol. 113, 1203–1212. doi: 10.1104/pp.113.4.1203
Hongo, S., Sato, K., Yokoyama, R., and Nishitani, K. (2012). Demethylesterification of the primary wall by PECTIN METHYLESTERASE35 provides mechanical support to the arabidopsis stem. Plant Cell. 24, 2624–2634. doi: 10.1105/tpc.112.099325
Houston, K., Tucker, M. R., Chowdhury, J., Shirley, N., and Little, A. (2016). The plant cell wall: A complex and dynamic structure as revealed by the responses of genes under stress conditions. Front. Plant Sci. 7, 984. doi: 10.3389/fpls.2016.00984
Hu, H., Yuan, H., Sun, S., Feng, J., Shi, N., Wang, Z., et al. (2025). Machine learning-powered activatable NIR-II fluorescent nanosensor for in vivo monitoring of plant stress responses. Nat. Commun. 16, 5114. doi: 10.1038/s41467-025-60182-w
Huang, Y.-C., Wu, H.-C., Wang, Y.-D., Liu, C.-H., Lin, C.-C., Luo, D.-L., et al. (2017). PECTIN METHYLESTERASE34 contributes to heat tolerance through its role in promoting stomatal movement. Plant Physiol. 174, 748–763. doi: 10.1104/pp.17.00335
Igisch, C. P., Miège, C., and Jaillais, Y. (2022). Cell shape: A ROP regulatory tug-of-war in pavement cell morphogenesis. Curr. Biol. 32, R116–R118. doi: 10.1016/j.cub.2021.12.028
Jaafar, L. and Anderson, C. T. (2024). Architecture and functions of stomatal cell walls in eudicots and grasses. Ann. Bot. 134, 195–204. doi: 10.1093/aob/mcae078
Jamet, E., Canut, H., Boudart, G., and Pont-Lezica, R. F. (2006). Cell wall proteins: a new insight through proteomics. Trends Plant Sci. 11, 33–39. doi: 10.1016/j.tplants.2005.11.006
Jardine, K. J., Dewhirst, R. A., Som, S., Lei, J., Tucker, E., Young, R. P., et al. (2022). Cell wall ester modifications and volatile emission signatures of plant response to abiotic stress. Plant Cell Environ. 45, 3429–3444. doi: 10.1111/pce.14464
Jarvis, M. C. (2017). Structure of native cellulose microfibrils, the starting point for nanocellulose manufacture. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 376, 20170045. doi: 10.1098/rsta.2017.0045
Jeong, J. S., Kim, Y. S., Baek, K. H., Jung, H., Ha, S.-H., Do Choi, Y., et al. (2010). Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 153, 185–197. doi: 10.1104/pp.110.154773
Jing, Y., Liu, C., Liu, B., Pei, T., Zhan, M., Li, C., et al. (2023). Overexpression of the FERONIA receptor kinase MdMRLK2 confers apple drought tolerance by regulating energy metabolism and free amino acids production. Tree Physiol. 43, 154–168. doi: 10.1093/treephys/tpac100
Jobert, F., Yadav, S., and Robert, S. (2023). Auxin as an architect of the pectin matrix. J. Exp. Bot. 74 (22), 6933–6949. doi: 10.1093/jxb/erad174
Juenger, T. E. and Verslues, P. E. (2023). Time for a drought experiment: Do you know your plants’ water status? Plant Cell 35, 10–23. doi: 10.1093/plcell/koac324
Jung, N. U., Giarola, V., Chen, P., Knox, J. P., and Bartels, D. (2019). Craterostigma plantagineum cell wall composition is remodelled during desiccation and the glycine-rich protein CpGRP1 interacts with pectins through clustered arginines. Plant J. 100, 661–676. doi: 10.1111/tpj.14479
Jung, S. E., Kim, T. H., Shim, J. S., Bang, S. W., Bin Yoon, H., Oh, S. H., et al. (2022). Rice NAC17 transcription factor enhances drought tolerance by modulating lignin accumulation. Plant Sci. 323, 111404. doi: 10.1016/j.plantsci.2022.111404
Kang, X., Kirui, A., Dickwella Widanage, M. C., Mentink-Vigier, F., Cosgrove, D. J., and Wang, T. (2019). Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nat. Commun. 10, 347. doi: 10.1038/s41467-018-08252-0
Kende, H., Bradford, K., Brummell, D., Cho, H., Cosgrove, D., Fleming, A., et al. (2004). Nomenclature for members of the expansin superfamily of genes and proteins. Plant Mol. Biol. 55, 311–314. doi: 10.1007/s11103-004-0158-6
Keppler, B. D. and Showalter, A. M. (2010). IRX14 and IRX14-LIKE, two glycosyl transferases involved in glucuronoxylan biosynthesis and drought tolerance in arabidopsis. Mol. Plant 3, 834–841. doi: 10.1093/mp/ssq028
Kesten, C., Menna, A., and Sánchez-Rodríguez, C. (2017). Regulation of cellulose synthesis in response to stress. Curr. Opin. Plant Biol. 40, 106–113. doi: 10.1016/j.pbi.2017.08.010
Khasin, M., Bernhardson, L. F., O’Neill, P. M., Palmer, N. A., Scully, E. D., Sattler, S. E., et al. (2021). Pathogen and drought stress affect cell wall and phytohormone signaling to shape host responses in a sorghum COMT bmr12 mutant. BMC Plant Biol. 21, 391. doi: 10.1186/s12870-021-03149-5
Kidwai, M., Ahmad, I. Z., and Chakrabarty, D. (2020). Class III peroxidase: an indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 39, 1381–1393. doi: 10.1007/s00299-020-02588-y
Kim, J.-S., Kidokoro, S., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2024). Regulatory networks in plant responses to drought and cold stress. Plant Physiol. 195, 170–189. doi: 10.1093/plphys/kiae105
Kim, G., Ryu, H., and Sung, J. (2022). Hormonal crosstalk and root suberization for drought stress tolerance in plants. Biomolecules 12, 811. doi: 10.3390/biom12060811
Kim, G.-E. and Sung, J. (2023). ABA-dependent suberization and aquaporin activity in rice (Oryza sativa L.) root under different water potentials. Front. Plant Sci. 14, 1219610. doi: 10.3389/fpls.2023.1219610
Kitin, P., Nakaba, S., Hunt, C. G., Lim, S., and Funada, R. (2020). Direct fluorescence imaging of lignocellulosic and suberized cell walls in roots and stems. AoB. Plants 12, plaa032. doi: 10.1093/aobpla/plaa032
Kohorn, B. D., Kobayashi, M., Johansen, S., Riese, J., Huang, L.-F., Koch, K., et al. (2006). An Arabidopsis cell wall-associated kinase required for invertase activity and cell growth. Plant J. 46, 307–316. doi: 10.1111/j.1365-313X.2006.02695.x
Kohorn, B. and Kohorn, S. (2012). The cell wall-associated kinases, WAKs, as pectin receptors. Front. Plant Sci. 3. doi: 10.3389/fpls.2012.00088
Kollist, H., Nuhkat, M., and Roelfsema, M. R. G. (2014). Closing gaps: linking elements that control stomatal movement. New Phytol. 203, 44–62. doi: 10.1111/nph.12832
Komis, G., Novák, D., Ovečka, M., Šamajová, O., and Šamaj, J. (2018). Advances in imaging plant cell dynamics. Plant Physiol. 176, 80–93. doi: 10.1104/pp.17.00962
Kramer, P. J. and Boyer, J. S. (1995). Water relations of plants and soils (San Diego, California: Academic press).
Kreszies, T., Kreszies, V., Ly, F., Thangamani, P. D., Shellakkutti, N., and Schreiber, L. (2020). Suberized transport barriers in plant roots: the effect of silicon. J. Exp. Bot. 71, 6799–6806. doi: 10.1093/jxb/eraa203
Kreszies, T., Shellakkutti, N., Osthoff, A., Yu, P., Baldauf, J. A., Zeisler-Diehl, V. V., et al. (2019). Osmotic stress enhances suberization of apoplastic barriers in barley seminal roots: analysis of chemical, transcriptomic and physiological responses. New Phytol. 221, 180–194. doi: 10.1111/nph.15351
Kumamoto, Y., Harada, Y., Takamatsu, T., and Tanaka, H. (2018). Label-free molecular imaging and analysis by raman spectroscopy. Acta Histochem. Cytochem. 51, 101–110. doi: 10.1267/ahc.18019
Kumar, M., Campbell, L., and Turner, S. (2016). Secondary cell walls: biosynthesis and manipulation. J. Exp. Bot. 67, 515–531. doi: 10.1093/jxb/erv533
Kumar, S., Jaggi, M., and Sinha, A. K. (2012). Ectopic overexpression of vacuolar and apoplastic Catharanthus roseus peroxidases confers differential tolerance to salt and dehydration stress in transgenic tobacco. Protoplasma 249, 423–432. doi: 10.1007/s00709-011-0294-1
Kumar, R., Meghwanshi, G. K., Marcianò, D., Ullah, S. F., Bulone, V., Toffolatti, S. L., et al. (2023). Sequence, structure and functionality of pectin methylesterases and their use in sustainable carbohydrate bioproducts: A review. Int. J. Biol. Macromol. 244, 125385. doi: 10.1016/j.ijbiomac.2023.125385
Kutsuno, T., Chowhan, S., Kotake, T., and Takahashi, D. (2023). Temporal cell wall changes during cold acclimation and deacclimation and their potential involvement in freezing tolerance and growth. Physiol. Plant 175, e13837. doi: 10.1111/ppl.13837
Lamport, D. T. A., Kieliszewski, M. J., Chen, Y., and Cannon, M. C. (2011). Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 156, 11–19. doi: 10.1104/pp.110.169011
Lawson, T. and Matthews, J. (2020). Guard cell metabolism and stomatal function. Annu. Rev. Plant Biol. 71, 273–302. doi: 10.1146/annurev-arplant-050718-100251
Lee, D.-K., Jung, H., Jang, G., Jeong, J. S., Kim, Y. S., Ha, S.-H., et al. (2016). Overexpression of the osERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol. 172, 575–588. doi: 10.1104/pp.16.00379
Lee, Y., Rubio, M. C., Alassimone, J., and Geldner, N. (2013). A mechanism for localized lignin deposition in the endodermis. Cell 153, 402–412. doi: 10.1016/j.cell.2013.02.045
Le Gall, H., Philippe, F., Domon, J.-M., Gillet, F., Pelloux, J., and Rayon, C. (2015). Cell wall metabolism in response to abiotic stress. Plants 4, 112–166. doi: 10.3390/plants4010112
Leucci, M. R., Lenucci, M. S., Piro, G., and Dalessandro, G. (2008). Water stress and cell wall polysaccharides in the apical root zone of wheat cultivars varying in drought tolerance. J. Plant Physiol. 165, 1168–1180. doi: 10.1016/j.jplph.2007.09.006
Li, Z., Fernie, A. R., and Persson, S. (2016). Transition of primary to secondary cell wall synthesis. Sci. Bull. 61, 838–846. doi: 10.1007/s11434-016-1061-7
Li, A. X., Han, Y. Y., Wang, X., Chen, Y. H., Zhao, M. R., Zhou, S.-M., et al. (2015). Root-specific expression of wheat expansin gene TaEXPB23 enhances root growth and water stress tolerance in tobacco. Environ. Exp. Bot. 110, 73–84. doi: 10.1016/j.envexpbot.2014.10.002
Li, Q.-F., Lu, J., Yu, J.-W., Zhang, C.-Q., He, J.-X., and Liu, Q.-Q. (2018). The brassinosteroid-regulated transcription factors BZR1/BES1 function as a coordinator in multisignal-regulated plant growth. Biochim. Biophys. Acta BBA. - Gene Regul. Mech. 1861, 561–571. doi: 10.1016/j.bbagrm.2018.04.003
Li, C., Wu, H.-M., and Cheung, A. Y. (2016). FERONIA and her pals: functions and mechanisms1[OPEN. Plant Physiol. 171, 2379–2392. doi: 10.1104/pp.16.00667
Li, F., Xing, S., Guo, Q., Zhao, M., Zhang, J., Gao, Q., et al. (2011). Drought tolerance through over-expression of the expansin gene TaEXPB23 in transgenic tobacco. J. Plant Physiol. 168, 960–966. doi: 10.1016/j.jplph.2010.11.023
Li, D., Yang, J., Pak, S., Zeng, M., Sun, J., Yu, S., et al. (2022). PuC3H35 confers drought tolerance by enhancing lignin and proanthocyanidin biosynthesis in the roots of Populus ussuriensis. New Phytol. 233, 390–408. doi: 10.1111/nph.17799
Li, J., Zhang, Y., Chen, Y., Wang, Y., Zhou, Z., Tu, J., et al. (2024). The roles of cell wall polysaccharides in response to waterlogging stress in Brassica napus L. root. BMC Biol. 22, 191. doi: 10.1186/s12915-024-01972-4
Li, W.-Q., Zhang, M.-J., Gan, P.-F., Qiao, L., Yang, S.-Q., Miao, H., et al. (2017). CLD1/SRL1 modulates leaf rolling by affecting cell wall formation, epidermis integrity and water homeostasis in rice. Plant J. Cell Mol. Biol. 92, 904–923. doi: 10.1111/tpj.13728
Lin, W., Zhou, X., Tang, W., Takahashi, K., Pan, X., Dai, J., et al. (2021). TMK-based cell-surface auxin signalling activates cell-wall acidification. Nature 599, 278–282. doi: 10.1038/s41586-021-03976-4
Liners, F., Letesson, J.-J., Didembourg, C., and Van Cutsem, P. (1989). Monoclonal antibodies against pectin: recognition of a conformation induced by calcium. Plant Physiol. 91, 1419–1424. doi: 10.1104/pp.91.4.1419
Liners, F. and Van Cutsem, P. (1992). Distribution of pectic polysaccharides throughout walls of suspension-cultured carrot cells. Protoplasma 170, 10–21. doi: 10.1007/BF01384453
Liu, X., Cui, H., Zhang, B., Song, M., Chen, S., Xiao, C., et al. (2021). Reduced pectin content of cell walls prevents stress-induced root cell elongation in Arabidopsis. J. Exp. Bot. 72, 1073–1084. doi: 10.1093/jxb/eraa533
Liu, X., Liang, C., Hou, S., Wang, X., Chen, D., Shen, J., et al. (2020). The LRR-RLK protein HSL3 regulates stomatal closure and the drought stress response by modulating hydrogen peroxide homeostasis. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.548034
Liu, S. and Qin, F. (2021). Genetic dissection of maize drought tolerance for trait improvement. Mol. Breed. 41, 8. doi: 10.1007/s11032-020-01194-w
Liu, C., Sack, L., Li, Y., Zhang, J., Yu, K., Zhang, Q., et al. (2023). Relationships of stomatal morphology to the environment across plant communities. Nat. Commun. 14, 6629. doi: 10.1038/s41467-023-42136-2
Liu, H., Song, S., Zhang, H., Li, Y., Niu, L., Zhang, J., et al. (2022). Signaling transduction of ABA, ROS, and ca2+ in plant stomatal closure in response to drought. Int. J. Mol. Sci. 23, 14824. doi: 10.3390/ijms232314824
Liu, S., Zenda, T., Tian, Z., and Huang, Z. (2023). Metabolic pathways engineering for drought or/and heat tolerance in cereals. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1111875
Liu, Y., Zhang, L., Hao, W., Zhang, L., Liu, Y., and Chen, L. (2019). Expression of Two α-Type Expansins from Ammopiptanthus nanus in Arabidopsis thaliana Enhance Tolerance to Cold and Drought Stresses. Int. J. Mol. Sci. 20, 5255. doi: 10.3390/ijms20215255
Liu, J., Zhang, W., Long, S., and Zhao, C. (2021). Maintenance of cell wall integrity under high salinity. Int. J. Mol. Sci. 22, 3260. doi: 10.3390/ijms22063260
Loix, C., Huybrechts, M., Vangronsveld, J., Gielen, M., Keunen, E., and Cuypers, A. (2017). Reciprocal interactions between cadmium-induced cell wall responses and oxidative stress in plants. Front. Plant Sci. 8, 1867. doi: 10.3389/fpls.2017.01867
Loqué, D., Scheller, H. V., and Pauly, M. (2015). Engineering of plant cell walls for enhanced biofuel production. Curr. Opin. Plant Biol. 25, 151–161. doi: 10.1016/j.pbi.2015.05.018
Luan, Y., Chen, Z., Meng, J., Tao, J., and Zhao, D. (2023). PoWRKY17 promotes drought tolerance in Paeonia ostii by modulating lignin accumulation. Ind. Crops Prod. 204, 117228. doi: 10.1016/j.indcrop.2023.117228
Ma, N., Wang, Y., Qiu, S., Kang, Z., Che, S., Wang, G., et al. (2013). Overexpression of OsEXPA8, a root-specific gene, improves rice growth and root system architecture by facilitating cell extension. PloS One 8, e75997. doi: 10.1371/journal.pone.0075997
Majda, M. (2019). Visualization of plant cell wall epitopes using immunogold labeling for electron microscopy. Bio Protoc. 9 (7), e3205. doi: 10.21769/bioprotoc.3205
Majda, M. and Robert, S. (2018). The role of auxin in cell wall expansion. Int. J. Mol. Sci. 19, 951. doi: 10.3390/ijms19040951
Malacarne, G., Lagreze, J., Rojas San Martin, B., Malnoy, M., Moretto, M., Moser, C., et al. (2024). Insights into the cell-wall dynamics in grapevine berries during ripening and in response to biotic and abiotic stresses. Plant Mol. Biol. 114, 38. doi: 10.1007/s11103-024-01437-w
Marowa, P., Ding, A., and Kong, Y. (2016). Expansins: roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 35, 949–965. doi: 10.1007/s00299-016-1948-4
Martignago, D., Rico-Medina, A., Blasco-Escámez, D., Fontanet-Manzaneque, J. B., and Caño-Delgado, A. I. (2020). Drought resistance by engineering plant tissue-specific responses. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01676
Martín-Dacal, M., Fernández-Calvo, P., Jiménez-Sandoval, P., López, G., Garrido-Arandía, M., Rebaque, D., et al. (2023). Arabidopsis immune responses triggered by cellulose- and mixed-linked glucan-derived oligosaccharides require a group of leucine-rich repeat malectin receptor kinases. Plant J. 113, 833–850. doi: 10.1111/tpj.16088
Martínez, J. P., Silva, H., Ledent, J. F., and Pinto, M. (2007). Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur. J. Agron. 26, 30–38. doi: 10.1016/j.eja.2006.08.003
Martinière, A., Fiche, J. B., Smokvarska, M., Mari, S., Alcon, C., Dumont, X., et al. (2019). Osmotic stress activates two reactive oxygen species pathways with distinct effects on protein nanodomains and diffusion. Plant Physiol. 179, 1581–1593. doi: 10.1104/pp.18.01065
Mateu, B. P., Bock, P., and Gierlinger, N. (2020). Raman imaging of plant cell walls. Methods Mol. Biol. Clifton. NJ. 2149, 251–295. doi: 10.1007/978-1-0716-0621-6_15
McQueen-Mason, S. J. and Cosgrove, D. J. (1995). Expansin mode of action on cell walls. Analysis of wall hydrolysis, stress relaxation, and binding. Plant Physiol. 107, 87–100. doi: 10.1104/pp.107.1.87
McQueen-Mason, S., Durachko, D. M., and Cosgrove, D. J. (1992). Two endogenous proteins that induce cell wall extension in plants. Plant Cell. 4, 1425–1433. doi: 10.1105/tpc.4.11.1425
Means, T. (2023). Climate change and droughts: What’s the connection? » Yale Climate Connections (Yale School of the Environment, New Haven, Connecticut: Yale Climate Connections). Available online at: http://yaleclimateconnections.org/2023/05/climate-change-and-droughts-whats-the-connection/.
Mehta, N., Shaik, S., Devireddy, R., and Gartia, M. R. (2018). Single-cell analysis using hyperspectral imaging modalities. J. Biomech. Eng. 140, 0208021–02080216. doi: 10.1115/1.4038638
Merced, A. and Renzaglia, K. S. (2019). Contrasting pectin polymers in guard cell walls of Arabidopsis and the hornwort Phaeoceros reflect physiological differences. Ann. Bot. 123, 579–585. doi: 10.1093/aob/mcy168
Miao, Y., Li, H.-Y., Shen, J., Wang, J., and Jiang, L. (2011). QUASIMODO 3 (QUA3) is a putative homogalacturonan methyltransferase regulating cell wall biosynthesis in Arabidopsis suspension-cultured cells. J. Exp. Bot. 62, 5063–5078. doi: 10.1093/jxb/err211
Miedes, E., Vanholme, R., Boerjan, W., and Molina, A. (2014). The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00358
Mishler-Elmore, J. W., Zhou, Y., Sukul, A., Oblak, M., Tan, L., Faik, A., et al. (2021). Extensins: self-assembly, crosslinking, and the role of peroxidases. Front. Plant Sci. 12, 664738. doi: 10.3389/fpls.2021.664738
Miyamoto, T., Tobimatsu, Y., and Umezawa, T. (2020). MYB-mediated regulation of lignin biosynthesis in grasses. Curr. Plant Biol. 24, 100174. doi: 10.1016/j.cpb.2020.100174
Mohnen, D. (2008). Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11, 266–277. doi: 10.1016/j.pbi.2008.03.006
Molina, A., Jordá, L., Torres, M. Á., Martín-Dacal, M., Berlanga, D. J., Fernández-Calvo, P., et al. (2024a). Plant cell wall-mediated disease resistance: Current understanding and future perspectives. Mol. Plant 17, 699–724. doi: 10.1016/j.molp.2024.04.003
Molina, A., Sánchez-Vallet, A., Jordá, L., Carrasco-López, C., Rodríguez-Herva, J. J., and López-Solanilla, E. (2024b). Plant cell walls: source of carbohydrate-based signals in plant-pathogen interactions. Curr. Opin. Plant Biol. 82, 102630. doi: 10.1016/j.pbi.2024.102630
Moore, J. P., Gao, Y., Zietsman, A. J. J., Fangel, J. U., Trygg, J., Willats, W. G. T., et al. (2020). Analysis of plant cell walls using high-throughput profiling techniques with multivariate methods. Methods Mol. Biol. Clifton. NJ. 2149, 327–337. doi: 10.1007/978-1-0716-0621-6_18
Moore, J. P., Vicré-Gibouin, M., Farrant, J. M., and Driouich, A. (2008). Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiol. Plant 134, 237–245. doi: 10.1111/j.1399-3054.2008.01134.x
Mouille, G., Robin, S., Lecomte, M., Pagant, S., and Höfte, H. (2003). Classification and identification of Arabidopsis cell wall mutants using Fourier-Transform InfraRed (FT-IR) microspectroscopy. Plant J. 35, 393–404. doi: 10.1046/j.1365-313X.2003.01807.x
Moura, J. C. M. S., Bonine, C. A. V., de Oliveira Fernandes Viana, J., Dornelas, M. C., and Mazzafera, P. (2010). Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 52, 360–376. doi: 10.1111/j.1744-7909.2010.00892.x
Moussu, S., Lee, H. K., Haas, K. T., Broyart, C., Rathgeb, U., De Bellis, D., et al. (2023). Plant cell wall patterning and expansion mediated by protein-peptide-polysaccharide interaction. Science 382, 719–725. doi: 10.1126/science.adi4720
Mravec, J., Kračun, S. K., Rydahl, M. G., Westereng, B., Pontiggia, D., De Lorenzo, G., et al. (2017). An oligogalacturonide-derived molecular probe demonstrates the dynamics of calcium-mediated pectin complexation in cell walls of tip-growing structures. Plant J. 91, 534–546. doi: 10.1111/tpj.13574
Nafisi, M., Fimognari, L., and Sakuragi, Y. (2015). Interplays between the cell wall and phytohormones in interaction between plants and necrotrophic pathogens. Phytochemistry 112, 63–71. doi: 10.1016/j.phytochem.2014.11.008
Nakano, Y., Yamaguchi, M., Endo, H., Rejab, N., and Ohtani, M. (2015). NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00288
Nakashima, K., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2014). The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 5, 170. doi: 10.3389/fpls.2014.00170
Narayan, S., Sharma, R. K., Kumar, V., Sanyal, I., and Shirke, P. A. (2023). Alterations in plant anatomy and higher lignin synthesis provides drought tolerance in cluster bean [Cyamopsis tetragonoloba (L.) Taub. Plant Physiol. Biochem. PPB 201, 107905. doi: 10.1016/j.plaphy.2023.107905
Neumann, P. M. (1995). The role of cell wall adjustments in plant resistance to water deficits. Crop Sci. 35 (5), 1258–1266. cropsci1995.0011183X003500050002x. doi: 10.2135/cropsci1995.0011183X003500050002x
Nguyen, T.-N., Son, S., Jordan, M. C., Levin, D. B., and Ayele, B. T. (2016). Lignin biosynthesis in wheat (Triticum aestivum L.): its response to waterlogging and association with hormonal levels. BMC Plant Biol. 16, 28. doi: 10.1186/s12870-016-0717-4
Novaković, L., Guo, T., Bacic, A., Sampathkumar, A., and Johnson, K. L. (2018). Hitting the wall-sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants Basel. Switz. 7, 89. doi: 10.3390/plants7040089
O’neill, M., Albersheim, P., and Darvill, A. (1990). “12 - the pectic polysaccharides of primary cell walls,” in Methods in Plant Biochemistry. Ed. Dey, P. M. (San Diego, California: Academic Press), 415–441.
Ogawa, Y. and Putaux, J.-L. (2019). Transmission electron microscopy of cellulose. Part 2: technical and practical aspects. Cellulose 26, 17–34. doi: 10.1007/s10570-018-2075-x
Oliveira, D. M., Mota, T. R., Salatta, F. V., Sinzker, R. C., Končitíková, R., Kopečný, D., et al. (2020). Cell wall remodeling under salt stress: Insights into changes in polysaccharides, feruloylation, lignification, and phenolic metabolism in maize. Plant Cell Environ. 43, 2172–2191. doi: 10.1111/pce.13805
Ortega, J. K. E. (2010). Plant cell growth in tissue. Plant Physiol. 154, 1244–1253. doi: 10.1104/pp.110.162644
Osakabe, Y., Mizuno, S., Tanaka, H., Maruyama, K., Osakabe, K., Todaka, D., et al. (2010). Overproduction of the membrane-bound receptor-like protein kinase 1, RPK1, enhances abiotic stress tolerance in Arabidopsis. J. Biol. Chem. 285, 9190–9201. doi: 10.1074/jbc.M109.051938
Osakabe, Y., Yamaguchi-Shinozaki, K., Shinozaki, K., and Tran, L.-S. P. (2013). Sensing the environment: key roles of membrane-localized kinases in plant perception and response to abiotic stress. J. Exp. Bot. 64, 445–458. doi: 10.1093/jxb/ers354
Osmolovskaya, N., Shumilina, J., Kim, A., Didio, A., Grishina, T., Bilova, T., et al. (2018). Methodology of drought stress research: experimental setup and physiological characterization. Int. J. Mol. Sci. 19, 4089. doi: 10.3390/ijms19124089
Park, A. R., Cho, S. K., Yun, U. J., Jin, M. Y., Lee, S. H., Sachetto-Martins, G., et al. (2001). Interaction of the arabidopsis receptor protein kinase wak1 with a glycine-rich protein, atGRP-3*. J. Biol. Chem. 276, 26688–26693. doi: 10.1074/jbc.M101283200
Passardi, F., Penel, C., and Dunand, C. (2004). Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci. 9, 534–540. doi: 10.1016/j.tplants.2004.09.002
Passardi, F., Tognolli, M., De Meyer, M., Penel, C., and Dunand, C. (2006). Two cell wall associated peroxidases from Arabidopsis influence root elongation. Planta 223, 965–974. doi: 10.1007/s00425-005-0153-4
Peaucelle, A., Braybrook, S., and Höfte, H. (2012). Cell wall mechanics and growth control in plants: the role of pectins revisited. Front Plant Sci. 3, 121. doi: 10.3389/fpls.2012.00121
Peaucelle, A., Braybrook, S. A., Le Guillou, L., Bron, E., Kuhlemeier, C., and Höfte, H. (2011). Pectin-induced changes in cell wall mechanics underlie organ initiation in arabidopsis. Curr. Biol. 21, 1720–1726. doi: 10.1016/j.cub.2011.08.057
Pelloux, J., Rustérucci, C., and Mellerowicz, E. J. (2007). New insights into pectin methylesterase structure and function. Trends Plant Sci. 12, 267–277. doi: 10.1016/j.tplants.2007.04.001
Perrot, T., Pauly, M., and Ramírez, V. (2022). Emerging roles of β-glucanases in plant development and adaptative responses. Plants 11, 1119. doi: 10.3390/plants11091119
Philippe, F., Pelloux, J., and Rayon, C. (2017). Plant pectin acetylesterase structure and function: new insights from bioinformatic analysis. BMC Genomics 18, 456. doi: 10.1186/s12864-017-3833-0
Piccinini, L., Nirina Ramamonjy, F., and Ursache, R. (2024). Imaging plant cell walls using fluorescent stains: The beauty is in the details. J. Microsc. 295, 102–120. doi: 10.1111/jmi.13289
Pirasteh-Anosheh, H., Saed-Moucheshi, A., Pakniyat, H., and Pessarakli, M. (2016). “Stomatal responses to drought stress,” in Water Stress and Crop Plants (Chichester, West Sussex: John Wiley & Sons, Ltd), 24–40.
Piro, G., Leucci, M. R., Waldron, K., and Dalessandro, G. (2003). Exposure to water stress causes changes in the biosynthesis of cell wall polysaccharides in roots of wheat cultivars varying in drought tolerance. Plant Sci. 165, 559–569. doi: 10.1016/S0168-9452(03)00215-2
Placido, D. F., Campbell, M. T., Folsom, J. J., Cui, X., Kruger, G. R., Baenziger, P. S., et al. (2013). Introgression of novel traits from a wild wheat relative improves drought adaptation in wheat. Plant Physiol. 161, 1806–1819. doi: 10.1104/pp.113.214262
Polo, C. C., Pereira, L., Mazzafera, P., Flores-Borges, D. N. A., Mayer, J. L. S., Guizar-Sicairos, M., et al. (2020). Correlations between lignin content and structural robustness in plants revealed by X-ray ptychography. Sci. Rep. 10, 6023. doi: 10.1038/s41598-020-63093-6
Pu, J., Ma, J., Zhai, H., Wu, S., Wang, Y., Putnis, C. V., et al. (2025). Atomic force microscopy imaging of plant cell walls. Plant Physiol. 197, kiae655. doi: 10.1093/plphys/kiae655
Pu, J., Putnis, C. V., and Wang, L. (2022). AFM imaging and single-molecule recognition of plant cell walls. Trends Plant Sci. 27, 412–413. doi: 10.1016/j.tplants.2021.11.010
Qiu, D., Xu, S., Wang, Y., Zhou, M., and Hong, L. (2021). Primary cell wall modifying proteins regulate wall mechanics to steer plant morphogenesis. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.751372
Radosavljević, J. S., Mitrović, A. L., Radotić, K., Zimányi, L., Garab, G., and Steinbach, G. (2021). Differential polarization imaging of plant cells. Mapping the anisotropy of cell walls and chloroplasts. Int. J. Mol. Sci. 22, 7661. doi: 10.3390/ijms22147661
Rajashekar, C. B. and Lafta, A. (1996). Cell-wall changes and cell tension in response to cold acclimation and exogenous abscisic acid in leaves and cell cultures. Plant Physiol. 111, 605–612. doi: 10.1104/pp.111.2.605
Ranathunge, K., Schreiber, L., Bi, Y.-M., and Rothstein, S. J. (2016). Ammonium-induced architectural and anatomical changes with altered suberin and lignin levels significantly change water and solute permeabilities of rice (Oryza sativa L.) roots. Planta 243, 231–249. doi: 10.1007/s00425-015-2406-1
Rao, X. and Dixon, R. A. (2017). Brassinosteroid mediated cell wall remodeling in grasses under abiotic stress. Front. Plant Sci. 8, 806. doi: 10.3389/fpls.2017.00806
Rao, X. and Dixon, R. A. (2018). Current models for transcriptional regulation of secondary cell wall biosynthesis in grasses. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00399
Rayle, D. L. and Cleland, R. E. (1992). The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiol. 99, 1271–1274. doi: 10.1104/pp.99.4.1271
Redillas, M. C., Jeong, J. S., Kim, Y. S., Jung, H., Bang, S. W., Choi, Y. D., et al. (2012). The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol. J. 10 (7), 792–805. doi: 10.1111/j.1467-7652.2012.00697.x
Ridley, B. L., O’Neill, M. A., and Mohnen, D. (2001). Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57, 929–967. doi: 10.1016/S0031-9422(01)00113-3
Ringli, C. (2010). Monitoring the outside: cell wall-sensing mechanisms. Plant Physiol. 153, 1445–1452. doi: 10.1104/pp.110.154518
Roig-Oliver, M., Nadal, M., Clemente-Moreno, M. J., Bota, J., and Flexas, J. (2020). Cell wall components regulate photosynthesis and leaf water relations of Vitis vinifera cv. Grenache acclimated to contrasting environmental conditions. J. Plant Physiol. 244, 153084. doi: 10.1016/j.jplph.2019.153084
Ropartz, D. and Ralet, M.-C. (2020). “Pectin structure,” in Pectin: Technological and Physiological Properties. Ed. Kontogiorgos, V. (Springer International Publishing, Cham), 17–36.
Rui, Y., Chen, Y., Yi, H., Purzycki, T., Puri, V. M., and Anderson, C. T. (2019). Synergistic pectin degradation and guard cell pressurization underlie stomatal pore formation. Plant Physiol. 180, 66–77. doi: 10.1104/pp.19.00135
Rui, Y. and Dinneny, J. R. (2020). A wall with integrity: surveillance and maintenance of the plant cell wall under stress. New Phytol. 225, 1428–1439. doi: 10.1111/nph.16166
Rydahl, M. G., Hansen, A. R., Kračun, S. K., and Mravec, J. (2018). Report on the current inventory of the toolbox for plant cell wall analysis: proteinaceous and small molecular probes. Front. Plant Sci. 9, 581. doi: 10.3389/fpls.2018.00581
Samalova, M., Gahurova, E., and Hejatko, J. (2022). Expansin-mediated developmental and adaptive responses: A matter of cell wall biomechanics? Quant. Plant Biol. 3, e11. doi: 10.1017/qpb.2022.6
Sampedro, J. and Cosgrove, D. J. (2005). The expansin superfamily. Genome Biol. 6, 242. doi: 10.1186/gb-2005-6-12-242
Sánchez-Rodríguez, C., Ketelaar, K., Schneider, R., Villalobos, J. A., Somerville, C. R., Persson, S., et al. (2017). BRASSINOSTEROID INSENSITIVE2 negatively regulates cellulose synthesis in Arabidopsis by phosphorylating cellulose synthase 1. Proc. Natl. Acad. Sci. 114, 3533–3538. doi: 10.1073/pnas.1615005114
Sánchez-Rodríguez, C., Rubio-Somoza, I., Sibout, R., and Persson, S. (2010). Phytohormones and the cell wall in Arabidopsis during seedling growth. Trends Plant Sci. 15, 291–301. doi: 10.1016/j.tplants.2010.03.002
Sarić, R., Nguyen, V. D., Burge, T., Berkowitz, O., Trtílek, M., Whelan, J., et al. (2022). Applications of hyperspectral imaging in plant phenotyping. Trends Plant Sci. 27, 301–315. doi: 10.1016/j.tplants.2021.12.003
Sarkar, P., Bosneaga, E., and Auer, M. (2009). Plant cell walls throughout evolution: towards a molecular understanding of their design principles. J. Exp. Bot. 60, 3615–3635. doi: 10.1093/jxb/erp245
Scheller, H. V. and Ulvskov, P. (2010). Hemicelluloses. Annu. Rev. Plant Biol. 61, 263–289. doi: 10.1146/annurev-arplant-042809-112315
Schmidt, M., Perera, P., Schwartzberg, A. M., Adams, P. D., and Schuck, P. J. (2010). Label-free in situ imaging of lignification in plant cell walls. J. Vis. Exp. JoVE. 45, 2064. doi: 10.3791/2064
Schopfer, P. (2001). Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J. 28, 679–688. doi: 10.1046/j.1365-313x.2001.01187.x
Schott, C., Bley, T., Walter, T., Brusius, J., and Steingroewer, J. (2022). Monitoring the apical growth characteristics of hairy roots using non-invasive laser speckle contrast imaging. Eng. Life Sci. 22, 288–298. doi: 10.1002/elsc.202100086
Seleiman, M. F., Al-Suhaibani, N., Ali, N., Akmal, M., Alotaibi, M., Refay, Y., et al. (2021). Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10, 259. doi: 10.3390/plants10020259
Sénéchal, F., Mareck, A., Marcelo, P., Lerouge, P., and Pelloux, J. (2015). Arabidopsis PME17 Activity can be Controlled by Pectin Methylesterase Inhibitor4. Plant Signal Behav. 10, e983351. doi: 10.4161/15592324.2014.983351
Shahin, L., Zhang, L., Mohnen, D., and Urbanowicz, B. R. (2023). Insights into pectin O-acetylation in the plant cell wall: structure, synthesis, and modification. Cell Surf. Amst. Neth. 9, 100099. doi: 10.1016/j.tcsw.2023.100099
Shao, H.-B., Chu, L.-Y., Jaleel, C. A., Manivannan, P., Panneerselvam, R., and Shao, M.-A. (2009). Understanding water deficit stress-induced changes in the basic metabolism of higher plants - biotechnologically and sustainably improving agriculture and the ecoenvironment in arid regions of the globe. Crit. Rev. Biotechnol. 29, 131–151. doi: 10.1080/07388550902869792
Shelake, R. M., Kadam, U. S., Kumar, R., Pramanik, D., Singh, A. K., and Kim, J.-Y. (2022). Engineering drought and salinity tolerance traits in crops through CRISPR-mediated genome editing: Targets, tools, challenges, and perspectives. Plant Commun. 3, 100417. doi: 10.1016/j.xplc.2022.100417
Shigeto, J., Itoh, Y., Hirao, S., Ohira, K., Fujita, K., and Tsutsumi, Y. (2015). Simultaneously disrupting AtPrx2, AtPrx25 and AtPrx71 alters lignin content and structure in Arabidopsis stem. J. Integr. Plant Biol. 57, 349–356. doi: 10.1111/jipb.12334
Shimizu, T., Nakano, T., Takamizawa, D., Desaki, Y., Ishii-Minami, N., Nishizawa, Y., et al. (2010). Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J. Cell Mol. Biol. 64, 204–214. doi: 10.1111/j.1365-313X.2010.04324.x
Shukla, V., Han, J.-P., Cléard, F., Lefebvre-Legendre, L., Gully, K., Flis, P., et al. (2021). Suberin plasticity to developmental and exogenous cues is regulated by a set of MYB transcription factors. Proc. Natl. Acad. Sci. 118, e2101730118. doi: 10.1073/pnas.2101730118
Siqueira, J. A., de Oliveira, H. O., Nunes-Nesi, A., and Araújo, W. L. (2021). Guard cell regulation: pulling the strings behind the scenes. Trends Plant Sci. 26, 1093–1095. doi: 10.1016/j.tplants.2021.07.005
Smith-Moritz, A. M., Hao, Z., Fernández-Niño, S. G., Fangel, J. U., Verhertbruggen, Y., Holman, H.-Y. N., et al. (2015). Structural characterization of a mixed-linkage glucan deficient mutant reveals alteration in cellulose microfibril orientation in rice coleoptile mesophyll cell walls. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00628
Smokvarska, M., Bayle, V., Maneta-Peyret, L., Fouillen, L., Poitout, A., Dongois, A., et al. (2023). The receptor kinase FERONIA regulates phosphatidylserine localization at the cell surface to modulate ROP signaling. Sci. Adv. 9, eadd4791. doi: 10.1126/sciadv.add4791
Smokvarska, M., Francis, C., Platre, M. P., Fiche, J.-B., Alcon, C., Dumont, X., et al. (2020). A plasma membrane nanodomain ensures signal specificity during osmotic signaling in plants. Curr. Biol. 30, 4654–4664.e4. doi: 10.1016/j.cub.2020.09.013
Son, W. K., Choi, Y. S., Han, Y. W., Shin, D. W., Min, K., Shin, J., et al. (2023). In vivo surface-enhanced Raman scattering nanosensor for the real-time monitoring of multiple stress signalling molecules in plants. Nat. Nanotechnol. 18, 205–216. doi: 10.1038/s41565-022-01274-2
Song, B., Zhao, S., Shen, W., Collings, C., and Ding, S.-Y. (2020). Direct measurement of plant cellulose microfibril and bundles in native cell walls. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00479
Stegmann, M., Monaghan, J., Smakowska-Luzan, E., Rovenich, H., Lehner, A., Holton, N., et al. (2017). The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355, 287–289. doi: 10.1126/science.aal2541
Steinwand, B. J. and Kieber, J. J. (2010). The role of receptor-like kinases in regulating cell wall function. Plant Physiol. 153, 479–484. doi: 10.1104/pp.110.155887
Stockinger, E. J., Gilmour, S. J., and Thomashow, M. F. (1997). Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. U. S. A. 94, 1035–1040. doi: 10.1073/pnas.94.3.1035
Stranne, M., Ren, Y., Fimognari, L., Birdseye, D., Yan, J., Bardor, M., et al. (2018). TBL10 is required for O-acetylation of pectic rhamnogalacturonan-I in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 96, 772–785. doi: 10.1111/tpj.14067
Stratilová, B., Kozmon, S., Stratilová, E., and Hrmova, M. (2020). Plant xyloglucan xyloglucosyl transferases and the cell wall structure: subtle but significant. Mol. Basel. Switz. 25, 5619. doi: 10.3390/molecules25235619
Sun, Q., Sun, Y., and Juzenas, K. (2017). Immunogold scanning electron microscopy can reveal the polysaccharide architecture of xylem cell walls. J. Exp. Bot. 68, 2231–2244. doi: 10.1093/jxb/erx103
Suzuki, N., Miller, G., Morales, J., Shulaev, V., Torres, M. A., and Mittler, R. (2011). Respiratory burst oxidases: the engines of ROS signaling. Curr. Opin. Plant Biol. 14, 691–699. doi: 10.1016/j.pbi.2011.07.014
Swaminathan, S., Lionetti, V., and Zabotina, O. A. (2022). Plant cell wall integrity perturbations and priming for defense. Plants 11, 3539. doi: 10.3390/plants11243539
Takahashi, F., Kuromori, T., Urano, K., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2020). Drought stress responses and resistance in plants: from cellular responses to long-distance intercellular communication. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.556972
Tenhaken, R. (2014). Cell wall remodeling under abiotic stress. Front. Plant Sci. 5, 771. doi: 10.3389/fpls.2014.00771
Thompson, A. (2017). The New Plants That Could Save Us from Climate Change (New York: Popular Mechanics). Available online at: https://www.popularmechanics.com/science/green-tech/a14000753/the-plants-that-could-save-us-from-climate-change/.
Thompson, D. S. and Islam, A. (2021). Plant cell wall hydration and plant physiology: an exploration of the consequences of direct effects of water deficit on the plant cell wall. Plants Basel. Switz. 10, 1263. doi: 10.3390/plants10071263
Tiika, R. J., Wei, J., Cui, G., Ma, Y., Yang, H., and Duan, H. (2021). Transcriptome-wide characterization and functional analysis of Xyloglucan endo-transglycosylase/hydrolase (XTH) gene family of Salicornia europaea L. under salinity and drought stress. BMC Plant Biol. 21, 491. doi: 10.1186/s12870-021-03269-y
Tobimatsu, Y. and Schuetz, M. (2019). Lignin polymerization: how do plants manage the chemistry so well? Curr. Opin. Biotechnol. 56, 75–81. doi: 10.1016/j.copbio.2018.10.001
Tobimatsu, Y., Wagner, A., Donaldson, L., Mitra, P., Niculaes, C., Dima, O., et al. (2013). Visualization of plant cell wall lignification using fluorescence-tagged monolignols. Plant J. Cell Mol. Biol. 76, 357–366. doi: 10.1111/tpj.12299
Tucker, M. R., Lou, H., Aubert, M. K., Wilkinson, L. G., Little, A., Houston, K., et al. (2018). Exploring the role of cell wall-related genes and polysaccharides during plant development. Plants Basel. Switz. 7, 42. doi: 10.3390/plants7020042
Turner, S. and Kumar, M. (2017). Cellulose synthase complex organization and cellulose microfibril structure. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 376, 20170048. doi: 10.1098/rsta.2017.0048
Ursache, R., Andersen, T. G., Marhavý, P., and Geldner, N. (2018). A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J. Cell Mol. Biol. 93, 399–412. doi: 10.1111/tpj.13784
Vaahtera, L., Schulz, J., and Hamann, T. (2019). Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants. 5, 924–932. doi: 10.1038/s41477-019-0502-0
Vadez, V., Grondin, A., Chenu, K., Henry, A., Laplaze, L., Millet, E. J., et al. (2024). Crop traits and production under drought. Nat. Rev. Earth Environ. 5, 211–225. doi: 10.1038/s43017-023-00514-w
Vanholme, R., Demedts, B., Morreel, K., Ralph, J., and Boerjan, W. (2010). Lignin biosynthesis and structure. Plant Physiol. 153, 895–905. doi: 10.1104/pp.110.155119
Vennam, R. R., Poudel, S., Ramamoorthy, P., Samiappan, S., Reddy, K. R., and Bheemanahalli, R. (2023). Impact of soil moisture stress during the silk emergence and grain-filling in maize. Physiol. Plant 175, e14029. doi: 10.1111/ppl.14029
Verslues, P. E., Agarwal, M., Katiyar-Agarwal, S., Zhu, J., and Zhu, J.-K. (2006). Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 45, 523–539. doi: 10.1111/j.1365-313X.2005.02593.x
Vishwanath, S. J., Delude, C., Domergue, F., and Rowland, O. (2015). Suberin: biosynthesis, regulation, and polymer assembly of a protective extracellular barrier. Plant Cell Rep. 34, 573–586. doi: 10.1007/s00299-014-1727-z
Vogel, J. (2008). Unique aspects of the grass cell wall. Curr. Opin. Plant Biol. 11, 301–307. doi: 10.1016/j.pbi.2008.03.002
Voiniciuc, C., Pauly, M., and Usadel, B. (2018). Monitoring polysaccharide dynamics in the plant cell wall. Plant Physiol. 176, 2590–2600. doi: 10.1104/pp.17.01776
Voothuluru, P., Anderson, J. C., Sharp, R. E., and Peck, S. C. (2016). Plasma membrane proteomics in the maize primary root growth zone: novel insights into root growth adaptation to water stress. Plant Cell Environ. 39, 2043–2054. doi: 10.1111/pce.12778
Wan, J., He, M., Hou, Q., Zou, L., Yang, Y., Wei, Y., et al. (2021). Cell wall associated immunity in plants. Stress Biol. 1, 3. doi: 10.1007/s44154-021-00003-4
Wagner, T. A. and Kohorn, B. D. (2001). Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell 13 (2), 303–318. doi: 10.1105/tpc.13.2.303
Wang, P., Calvo-Polanco, M., Reyt, G., Barberon, M., Champeyroux, C., Santoni, V., et al. (2019). Surveillance of cell wall diffusion barrier integrity modulates water and solute transport in plants. Sci. Rep. 9, 4227. doi: 10.1038/s41598-019-40588-5
Wang, Y., Chantreau, M., Sibout, R., and Hawkins, S. (2013). Plant cell wall lignification and monolignol metabolism. Front. Plant Sci. 4. doi: 10.3389/fpls.2013.00220
Wang, H.-Z. and Dixon, R. A. (2012). On–off switches for secondary cell wall biosynthesis. Mol. Plant 5, 297–303. doi: 10.1093/mp/ssr098
Wang, Y., Fan, C., Hu, H., Li, Y., Sun, D., Wang, Y., et al. (2016). Genetic modification of plant cell walls to enhance biomass yield and biofuel production in bioenergy crops. Biotechnol. Adv. 34, 997–1017. doi: 10.1016/j.biotechadv.2016.06.001
Wang, C., Wang, H., Li, P., Li, H., Xu, C., Cohen, H., et al. (2020). Developmental programs interact with abscisic acid to coordinate root suberization in Arabidopsis. Plant J. 104, 241–251. doi: 10.1111/tpj.14920
Wang, Y., Wang, Q., Zhao, Y., Han, G., and Zhu, S. (2015). Systematic analysis of maize class III peroxidase gene family reveals a conserved subfamily involved in abiotic stress response. Gene 566, 95–108. doi: 10.1016/j.gene.2015.04.041
Wasaya, A., Zhang, X., Fang, Q., and Yan, Z. (2018). Root phenotyping for drought tolerance: A review. Agronomy 8, 241. doi: 10.3390/agronomy8110241
Wei, P.-C., Zhang, X.-Q., Zhao, P., and Wang, X.-C. (2011). Regulation of stomatal opening by the guard cell expansin AtEXPA1. Plant Signal Behav. 6, 740–742. doi: 10.4161/psb.6.5.15144
Wightman, R. (2022). An overview of cryo-scanning electron microscopy techniques for plant imaging. Plants 11, 1113. doi: 10.3390/plants11091113
Wilkinson, S., Clephan, A. L., and Davies, W. J. (2001). Rapid low temperature-induced stomatal closure occurs in cold-tolerant Commelina communis leaves but not in cold-sensitive tobacco leaves, via a mechanism that involves apoplastic calcium but not abscisic acid. Plant Physiol. 126, 1566–1578. doi: 10.1104/pp.126.4.1566
Willats, W. G. T., McCartney, L., Mackie, W., and Knox, J. P. (2001). Pectin: cell biology and prospects for functional analysis. Plant Mol. Biol. 47, 9–27. doi: 10.1023/A:1010662911148
Wilson, T. H., Kumar, M., and Turner, S. R. (2021). The molecular basis of plant cellulose synthase complex organisation and assembly. Biochem. Soc. Trans. 49, 379–391. doi: 10.1042/BST20200697
Wolf, S. (2022). Cell wall signaling in plant development and defense. Annu. Rev. Plant Biol. 73, 323–353. doi: 10.1146/annurev-arplant-102820-095312
Wolf, S. and Greiner, S. (2012). Growth control by cell wall pectins. Protoplasma 249 Suppl 2, S169–S175. doi: 10.1007/s00709-011-0371-5
Wolf, S., Mravec, J., Greiner, S., Mouille, G., and Höfte, H. (2012). Plant cell wall homeostasis is mediated by brassinosteroid feedback signaling. Curr. Biol. 22, 1732–1737. doi: 10.1016/j.cub.2012.07.036
Wolf, S., van der Does, D., Ladwig, F., Sticht, C., Kolbeck, A., Schürholz, A.-K., et al. (2014). A receptor-like protein mediates the response to pectin modification by activating brassinosteroid signaling. Proc. Natl. Acad. Sci. 111, 15261–15266. doi: 10.1073/pnas.1322979111
Woolfson, K. N., Esfandiari, M., and Bernards, M. A. (2022). Suberin biosynthesis, assembly, and regulation. Plants 11, 555. doi: 10.3390/plants11040555
Wormit, A. and Usadel, B. (2018). The multifaceted role of pectin methylesterase inhibitors (PMEIs). Int. J. Mol. Sci. 19, 2878. doi: 10.3390/ijms19102878
Wu, H.-C., Bulgakov, V. P., and Jinn, T.-L. (2018). Pectin methylesterases: cell wall remodeling proteins are required for plant response to heat stress. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01612
Wu, Y. and Cosgrove, D. J. (2000). Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J. Exp. Bot. 51, 1543–1553. doi: 10.1093/jexbot/51.350.1543
Wu, A.-M., Hörnblad, E., Voxeur, A., Gerber, L., Rihouey, C., Lerouge, P., et al. (2010). Analysis of the Arabidopsis IRX9/IRX9-L and IRX14/IRX14-L pairs of glycosyltransferase genes reveals critical contributions to biosynthesis of the hemicellulose glucuronoxylan. Plant Physiol. 153, 542–554. doi: 10.1104/pp.110.154971
Wu, H.-C., Huang, Y.-C., Stracovsky, L., and Jinn, T.-L. (2017). Pectin methylesterase is required for guard cell function in response to heat. Plant Signal Behav. 12, e1338227. doi: 10.1080/15592324.2017.1338227
Wu, H.-C. and Jinn, T.-L. (2010). Heat shock-triggered Ca2+ mobilization accompanied by pectin methylesterase activity and cytosolic Ca2+ oscillation are crucial for plant thermotolerance. Plant Signal Behav. 5, 1252–1256. doi: 10.4161/psb.5.10.12607
Wu, Y., Sharp, R. E., Durachko, D. M., and Cosgrove, D. J. (1996). Growth maintenance of the maize primary root at low water potentials involves increases in cell-wall extension properties, expansin activity, and wall susceptibility to expansins. Plant Physiol. 111, 765–772. doi: 10.1104/pp.111.3.765
Wu, Y., Spollen, W. G., Sharp, R. E., Hetherington, P. R., and Fry, S. C. (1994). Root growth maintenance at low water potentials (Increased activity of xyloglucan endotransglycosylase and its possible regulation by abscisic acid). Plant Physiol. 106, 607–615. doi: 10.1104/pp.106.2.607
Wu, X., Wang, Z., Liu, X., Gao, Z., and Li, Z. (2023). Constitutive expression of nucleotide-binding and leucine-rich repeat gene AtRPS2 enhanced drought and salt tolerance in rice. J. Plant Physiol. 287, 154048. doi: 10.1016/j.jplph.2023.154048
Wu, Y., Yang, Z., How, J., Xu, H., Chen, L., and Li, K. (2017). Overexpression of a peroxidase gene (AtPrx64) of Arabidopsis thaliana in tobacco improves plant’s tolerance to aluminum stress. Plant Mol. Biol. 95, 157–168. doi: 10.1007/s11103-017-0644-2
Wu, H.-C., Yu, S.-Y., Wang, Y.-D., and Jinn, T.-L. (2022). Guard cell-specific pectin METHYLESTERASE53 is required for abscisic acid-mediated stomatal function and heat response in arabidopsis. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.836151
Xie, L., Yang, C., and Wang, X. (2011). Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J. Exp. Bot. 62, 4495–4506. doi: 10.1093/jxb/err164
Xie, M., Zhang, J., Tschaplinski, T. J., Tuskan, G. A., Chen, J.-G., and Muchero, W. (2018). Regulation of lignin biosynthesis and its role in growth-defense tradeoffs. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01427
Xu, W., Dou, Y., Geng, H., Fu, J., Dan, Z., Liang, T., et al. (2022). OsGRP3 enhances drought resistance by altering phenylpropanoid biosynthesis pathway in rice (Oryza sativa L.). Int. J. Mol. Sci. 23, 7045. doi: 10.3390/ijms23137045
Xu, C., Fu, X., Liu, R., Guo, L., Ran, L., Li, C., et al. (2017). PtoMYB170 positively regulates lignin deposition during wood formation in poplar and confers drought tolerance in transgenic Arabidopsis. Tree Physiol. 37, 1713–1726. doi: 10.1093/treephys/tpx093
Xu, H., Liu, P., Wang, C., Wu, S., Dong, C., Lin, Q., et al. (2022). Transcriptional networks regulating suberin and lignin in endodermis link development and ABA response. Plant Physiol. 190, 1165–1181. doi: 10.1093/plphys/kiac298
Xu, S.-L., Rahman, A., Baskin, T. I., and Kieber, J. J. (2008). Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in arabidopsis. Plant Cell. 20, 3065–3079. doi: 10.1105/tpc.108.063354
Xu, W., Tang, W., Wang, C., Ge, L., Sun, J., Qi, X., et al. (2020). SiMYB56 confers drought stress tolerance in transgenic rice by regulating lignin biosynthesis and ABA signaling pathway. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00785
Xu, H., Zhao, Y., Suo, Y., Guo, Y., Man, Y., Jing, Y., et al. (2021). A label-free, fast and high-specificity technique for plant cell wall imaging and composition analysis. Plant Methods 17, 29. doi: 10.1186/s13007-021-00730-9
Yadav, S. and Chattopadhyay, D. (2023). Lignin: the building block of defense responses to stress in plants. J. Plant Growth Regul. 42, 6652–6666. doi: 10.1007/s00344-023-10926-z
Yamaguchi-Shinozaki, K. and Shinozaki, K. (1994). A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell. 6, 251–264. doi: 10.1105/tpc.6.2.251
Yan, J., Aznar, A., Chalvin, C., Birdseye, D. S., Baidoo, E. E. K., Eudes, A., et al. (2018a). Increased drought tolerance in plants engineered for low lignin and low xylan content. Biotechnol. Biofuels. 11, 195. doi: 10.1186/s13068-018-1196-7
Yan, J., He, H., Fang, L., and Zhang, A. (2018b). Pectin methylesterase31 positively regulates salt stress tolerance in Arabidopsis. Biochem. Biophys. Res. Commun. 496, 497–501. doi: 10.1016/j.bbrc.2018.01.025
Yang, Y. and Anderson, C. T. (2020). “Biosynthesis, localisation, and function of pectins in plants,” in Pectin: Technological and Physiological Properties, vol. p . Ed. Kontogiorgos, V. (Springer International Publishing, Cham), 1–15.
Yang, W., Ruan, M., Xiang, M., Deng, A., Du, J., and Xiao, C. (2020). Overexpression of a pectin methylesterase gene PtoPME35 from Populus tomentosa influences stomatal function and drought tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 523, 416–422. doi: 10.1016/j.bbrc.2019.12.073
Yang, X., Yuan, J., Luo, W., Qin, M., Yang, J., Wu, W., et al. (2020). Genome-wide identification and expression analysis of the class III peroxidase gene family in potato (Solanum tuberosum L.). Front. Genet. 11, 593577. doi: 10.3389/fgene.2020.593577
Yang, J., Zhang, G., An, J., Li, Q., Chen, Y., Zhao, X., et al. (2020). Expansin gene TaEXPA2 positively regulates drought tolerance in transgenic wheat (Triticum aestivum L.). Plant Sci. 298, 110596. doi: 10.1016/j.plantsci.2020.110596
Yasuda, S., Okada, K., and Saijo, Y. (2017). A look at plant immunity through the window of the multitasking coreceptor BAK1. Curr. Opin. Plant Biol. 38, 10–18. doi: 10.1016/j.pbi.2017.04.007
Yoon, J., Choi, H., and An, G. (2015). Roles of lignin biosynthesis and regulatory genes in plant development. J. Integr. Plant Biol. 57, 902–912. doi: 10.1111/jipb.12422
Yoshida, K., Sakamoto, S., and Mitsuda, N. (2021). In planta cell wall engineering: from mutants to artificial cell walls. Plant Cell Physiol. 62, 1813–1827. doi: 10.1093/pcp/pcab157
Zabotina, O. A. (2012). Xyloglucan and its biosynthesis. Front. Plant Sci. 3, 134. doi: 10.3389/fpls.2012.00134
Zafar, S. A., Zaidi, SS-E-A, Gaba, Y., Singla-Pareek, S. L., Dhankher, O. P., Li, X., et al. (2020). Engineering abiotic stress tolerance via CRISPR/Cas-mediated genome editing. J. Exp. Bot. 71, 470–479. doi: 10.1093/jxb/erz476
Zechmann, B. and Zellnig, G. (2009). Rapid diagnosis of plant virus diseases by transmission electron microscopy. J. Virol. Methods 162, 163–169. doi: 10.1016/j.jviromet.2009.07.032
Zeng, Y., Himmel, M. E., and Ding, S.-Y. (2017). Visualizing chemical functionality in plant cell walls. Biotechnol. Biofuels. 10, 263. doi: 10.1186/s13068-017-0953-3
Zhang, Q., Ahmad, N., Li, Z., He, J., Wang, N., Naeem, M., et al. (2023). CtCYP71A1 promotes drought stress tolerance and lignin accumulation in safflower and Arabidopsis. Environ. Exp. Bot. 213, 105430. doi: 10.1016/j.envexpbot.2023.105430
Zhang, B., Chang, L., Sun, W., Ullah, A., and Yang, X. (2021a). Overexpression of an expansin-like gene, GhEXLB2 enhanced drought tolerance in cotton. Plant Physiol. Biochem. 162, 468–475. doi: 10.1016/j.plaphy.2021.03.018
Zhang, H., Delafield, D. G., and Li, L. (2023). Mass spectrometry imaging: the rise of spatially resolved single-cell omics. Nat. Methods 20, 327–330. doi: 10.1038/s41592-023-01774-6
Zhang, B., Gao, Y., Zhang, L., and Zhou, Y. (2021b). The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 63, 251–272. doi: 10.1111/jipb.13055
Zhang, G., Hou, X., Wang, L., Xu, J., Chen, J., Fu, X., et al. (2021). PHOTO-SENSITIVE LEAF ROLLING 1 encodes a polygalacturonase that modifies cell wall structure and drought tolerance in rice. New Phytol. 229, 890–901. doi: 10.1111/nph.16899
Zhang, T., Tang, H., Vavylonis, D., and Cosgrove, D. J. (2019). Disentangling loosening from softening: insights into primary cell wall structure. Plant J. Cell Mol. Biol. 100, 1101–1117. doi: 10.1111/tpj.14519
Zhang, T., Zheng, Y., and Cosgrove, D. J. (2016). Spatial organization of cellulose microfibrils and matrix polysaccharides in primary plant cell walls as imaged by multichannel atomic force microscopy. Plant J. Cell Mol. Biol. 85, 179–192. doi: 10.1111/tpj.13102
Zhang, H., Zhu, J., Gong, Z., and Zhu, J.-K. (2022). Abiotic stress responses in plants. Nat. Rev. Genet. 23, 104–119. doi: 10.1038/s41576-021-00413-0
Zhao, Y. (2010). Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 61, 49–64. doi: 10.1146/annurev-arplant-042809-112308
Zhao, Q. (2016). Lignification: flexibility, biosynthesis and regulation. Trends Plant Sci. 21, 713–721. doi: 10.1016/j.tplants.2016.04.006
Zhao, Q. and Dixon, R. A. (2011). Transcriptional networks for lignin biosynthesis: more complex than we thought? Trends Plant Sci. 16, 227–233. doi: 10.1016/j.tplants.2010.12.005
Zhao, Y., Man, Y., Wen, J., Guo, Y., and Lin, J. (2019). Advances in imaging plant cell walls. Trends Plant Sci. 24, 867–878. doi: 10.1016/j.tplants.2019.05.009
Zhao, Q., Nakashima, J., Chen, F., Yin, Y., Fu, C., Yun, J., et al. (2013). LACCASE is necessary and nonredundant with PEROXIDASE for lignin polymerization during vascular development in arabidopsis[C][W. Plant Cell. 25, 3976–3987. doi: 10.1105/tpc.113.117770
Zhao, Z., Wu, S., Gao, H., Tang, W., Wu, X., and Zhang, B. (2023). The BR signaling pathway regulates primary root development and drought stress response by suppressing the expression of PLT1 and PLT2 in Arabidopsis thaliana. Front. Plant Sci. 14, 1187605. doi: 10.3389/fpls.2023.1187605
Zheng, L., Chen, Y., Ding, L., Zhou, Y., Xue, S., Li, B., et al. (2023). The transcription factor MYB156 controls the polar stiffening of guard cell walls in poplar. Plant Cell. 35, 3757–3781. doi: 10.1093/plcell/koad198
Zheng, Y., Cosgrove, D. J., and Ning, G. (2017). High-resolution field emission scanning electron microscopy (FESEM) imaging of cellulose microfibril organization in plant primary cell walls. Microsc. Microanal. 23, 1048–1054. doi: 10.1017/S143192761701251X
Zhong, R., Lee, C., McCarthy, R. L., Reeves, C. K., Jones, E. G., and Ye, Z.-H. (2011). Transcriptional activation of secondary wall biosynthesis by rice and maize NAC and MYB transcription factors. Plant Cell Physiol. 52, 1856–1871. doi: 10.1093/pcp/pcr123
Zhou, Y., Zhang, Y., Wang, X., Han, X., An, Y., Lin, S., et al. (2020). Root-specific NF-Y family transcription factor, PdNF-YB21, positively regulates root growth and drought resistance by abscisic acid-mediated indoylacetic acid transport in Populus. New Phytol. 227, 407–426. doi: 10.1111/nph.16524
Zia, R., Nawaz, M. S., Siddique, M. J., Hakim, S., and Imran, A. (2021). Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 242, 126626. doi: 10.1016/j.micres.2020.126626
Keywords: cell wall modifications, drought tolerance, pectin methyltransferases, stomata guard cell wall, lignification, cell wall imaging
Citation: Zhao N, Zhou Z, Cui S, Zhang X, Zhu S, Wang Y, Zenda T and Wenjing L (2025) Advanced imaging-enabled understanding of cell wall remodeling mechanisms mediating plant drought stress tolerance. Front. Plant Sci. 16:1635078. doi: 10.3389/fpls.2025.1635078
Received: 25 May 2025; Accepted: 21 July 2025;
Published: 08 August 2025.
Edited by:
Wajid Zaman, Yeungnam University, Republic of KoreaReviewed by:
Luis Morales-Quintana, Autonomous University of Chile, ChileFélix Barbut, University of Georgia, United States
Copyright © 2025 Zhao, Zhou, Cui, Zhang, Zhu, Wang, Zenda and Wenjing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tinashe Zenda, dHplbmRhQG11YXN0LmFjLnp3; Li Wenjing, bGl3ZW5qaW5nQGxmbnUuZWR1LmNu
 Nannan Zhao
Nannan Zhao Zhiguo Zhou1
Zhiguo Zhou1 Tinashe Zenda
Tinashe Zenda Li Wenjing
Li Wenjing