- College of Landscape Architecture, Beijing University of Agriculture, Beijing, China
Glutathione S-transferases (GSTs), a superfamily of multifunctional enzymes, are involved in plant growth, development, and response to biotic and abiotic stresses. In this study, 86 members of the GST family, denoted QdGST, were identified in the Quercus dentata genome and found to be distributed among six of the GST classes, with the majority in the tau class, followed by the lambda and phi classes. This uneven distribution of QdGST genes was observed across 11 chromosomes. Thirty-one tandem and seven segmental duplication events were found to have contributed to the expansion of the QdGST family. Moreover, a total of 29 categories of cis-acting elements were identified in the promoters of the QdGST genes, most of which were involved in defense and stress responses. RNA sequencing analysis revealed that most QdGST genes displayed tissue-specific expression patterns, and that cadmium or lead treatment induced the expression of 31 of them, most of which belonged to the tau class. Quantitative real-time PCR analysis confirmed the expression of cadmium- and lead-induced QdGST genes, with QdGSTU20 and QdGSTU36 in particular showing strong upregulation. QdGSTU36 also enhanced yeast growth under cadmium and lead stresses when expressed in yeast. These findings lay a crucial foundation for further work to clarify the biological functions of QdGST genes associated with heavy metal tolerance in Q. dentata.
1 Introduction
The glutathione S-transferase (GST) superfamily comprises a group of multifunctional enzymes (EC 2.5.1.18) that are widely distributed across diverse living organisms, ranging from bacteria and fungi to plants and animals (Wang et al., 2023). The amino acid sequences of GSTs differ greatly among different subfamilies, but their overall structures remain remarkably similar (Duan et al., 2022). Typically, a GST protein contains two distinct functional regions: an N-terminal glutathione-binding site (G-site) and a C-terminal substrate binding site (H-site) (Edwards and Dixon, 2005). The G-site is highly conserved, whereas the H-site shows considerable variation, enabling binding of a variety of different substrates (Zhao et al., 2021). These two domains are in close proximity to each other in the three-dimensional structure and form catalytic sites with specific functions in different subcellular locations (Zhao et al., 2021). In general, GSTs facilitate the conjugation of reduced glutathione to diverse hydrophobic and electrophilic substrates (Mo et al., 2023).
GSTs have vital role in plant growth and development (Gong et al., 2005), as well as transport and metabolism of secondary compounds (Dixon et al., 2010) and response to various stresses including exposure to bacterial and fungal pathogens (Frova, 2006; Perperopoulou et al., 2018), cold (Kayum et al., 2018; Song et al., 2021), chemical toxicity (Xu et al., 2016), salinity (Xu et al., 2016), drought (Chen et al., 2012; Xu et al., 2016), ultraviolet radiation (Liu and Li, 2002), and heavy metals (Gao et al., 2020; Jing et al., 2020). Plant GSTs can be divided into 14 classes on the basis of protein sequence, gene structure, gene function, and immunological characteristics (Wang et al., 2020; Zhao et al., 2021; Liu et al., 2023). The phi (GSTF), tau (GSTU), lambda (GSTL), and dehydroascorbate reductase (DHAR) classes are exclusive to plants, with phi and tau being the most prevalent (Wang et al., 2020).
Developments in sequencing technology and the associated reductions in the costs of sequencing have led to the discovery of increasing numbers of GSTs in both model and non-model plants, including Arabidopsis (Sappl et al., 2009), rice (Jain et al., 2010), poplar (Lan et al., 2009), tomato (Islam et al., 2017), sweet potato (Ding et al., 2017), pumpkin (Kayum et al., 2018), Brassica rapa (Khan et al., 2018), Physcomitrella patens (Liu et al., 2013), Gossypium hirsutum (Xu et al., 2017), Cucumis melo (Wang et al., 2020), Malus (Fang et al., 2020), Capsicum annuum (Islam et al., 2019), maize, and soybean (McGonigle et al., 2000). However, no such research has yet focused on GSTs in plants of the Fagaceae family, despite their ecological and economic significance of these species.
GSTs participate in resistance to heavy metal stresses in various organisms, including plants (Kumar et al., 2013; Liu et al., 2013; Lim et al., 2005; Ezaki et al., 2000), animals (Xu et al., 2016; Park et al., 2019; Takenaka et al., 2014; Nair and Choi, 2011), and fungi (Shen et al., 2015; Dixit et al., 2011). For instance, the fungal TvGST in Trichoderma virens has a role in tolerance to cadmium (Cd) stress (Dixit et al., 2011), whereas tobacco GST gene parB confers resistance to copper (Cu) and aluminum (Al) in Arabidopsis (Ezaki et al., 2000). Overexpression of the Nt107 gene from the tobacco tau subfamily has been reported to result in accumulation of Cu in Dianthus superbus (Lim et al., 2005); similarly, overexpression of PpGST, a zeta GST gene from Pyrus pyrifolia, enhanced the tolerance of transgenic tobacco lines to Cd stresses (Liu et al., 2013), and Arabidopsis with heterologous expression of rice lambda-class OsGSTL2 exhibited tolerance to arsenic (As), Cd, and chromium (Cr) treatments (Kumar et al., 2013). Overexpression of OsGSTU6 in rice reduces accumulation of Cd in leaves and enhances the tolerance of the plant to Cd stress; conversely, reduced OsGSTU6 expression levels are associated with Cd accumulation and diminished tolerance to heavy metal stress (Jing et al., 2020). The expression of tau- and theta-class GSTs can be induced by Cd, Cr, and lead (Pb) stresses in radish (Gao et al., 2020); some tau GST genes can be induced by Cd and As stresses in rice (Moons, 2003; Ahsan et al., 2008; Norton et al., 2008; Lin et al., 2013); and Cd treatment can trigger GST gene expression in wheat and maize (Mauch and Dudler, 1993; Marrs and Walbot, 1997). Moreover, accumulation of GST proteins has been observed in poplar and soybean under Cd stress (Sobkowiak and Deckert, 2006; Kieffer et al., 2009).
Quercus is the largest genus in the Fagaceae family and contains the most abundant and economically important woody plants (Wang et al., 2023). Quercus species can tolerate multiple heavy metal stresses, such as cobalt (Co), Pb, Cu, zinc (Zn), Cd, antimony (Sb), and nickel (Ni), and thus contribute significantly to the restoration of environments affected by heavy metal pollution (Gogorcena et al., 2011; Shi et al., 2017, Shi et al., 2019). However, the molecular mechanism underlying this heavy metal tolerance in Quercus plants remains unclear, and no research on GST genes in this genus has previously been published. Quercus dentata is a key species in northern China. Here, we systematically evaluated the potential roles of Quercus GSTs in heavy metal tolerance using high-quality whole-genome and transcriptome data for Q. dentata (Wang et al., 2023). We identified 86 QdGST genes and characterized their protein products using bioinformatics techniques; then, we analyzed their expression patterns under exposure to different heavy metals (Cd and Pb) using quantitative real-time PCR (qRT-PCR). Candidate QdGST genes with potential roles in heavy metal tolerance were expressed in yeast to confirm their effects. The results demonstrated that several QdGST genes of the tau subfamily contributed to the heavy metal tolerance of Q. dentata. These findings provide new insight into the evolutionary history and functional roles of QdGST genes and will provide a valuable reference for breeding efforts to develop plants with heavy metal resistance.
2 Materials and methods
2.1 Plant materials, growth condition and treatment
A mixture of Q. dentata seeds and sand with a humidity of 60% was stored in a refrigerator at 4°C for 3 weeks and then soaked in water at 40°C three times at room temperature (Zhang et al., 2023). After treatment, the Q. dentata seeds were sown in a 1:1 vermiculite-peat substrate and cultivated at 25°C/20°C with a 16 h/8 h light/dark cycle at 60% humidity. The 1/2 Hoagland nutrient solution was prepared without calcium nitrate according to the manufacturer’s protocol (Hope Bio-Technology Corporation Ltd., Qingdao, China). Seedlings of the same size and state were transplanted into 1/2 Hoagland nutrient solution and grown under the same conditions as used for sowing. Cd-grown and Pb-grown seedlings were treated with 200 mg/L CdSO4·8/3H2O and 1000 mg/L PbCl2, respectively (Gao et al., 2020). Seedlings cultivated in standard 1/2 Hoagland solution were used as controls. Roots, stems, and leaves of Cd-grown, Pb-grown and control seedlings were separately harvested for RNA extraction at 0 h and 24 h after transplantation. For each treatment, three culture bottles were used, with one seedling per bottle.
2.2 Characterization and identification of GST genes in Q. dentata
Genomic data of Q. dentata were obtained from the National Genomics Data Center (https://ngdc.cncb.ac.cn/) under BioProject PRJCA013491 with accession number GWHBRAD00000000) (Wang et al., 2023). To locate QdGST genes within the genome, a BLAST search was conducted with GST genes from Arabidopsis thaliana as the query sequences. In addition, Arabidopsis GST protein sequences were retrieved from the TAIR database (https://www.arabidopsis.org/) and used to screen for Q. dentata GSTs on the basis of sequence similarity (BLASTP; e-value ≤ 10–10). Hidden Markov model profiles for the GST_N (PF02798) and GST_C (PF00043) domains were obtained from the Pfam database and used to identify GST domains. Q. dentata protein sequences identified in homology searches were subsequently analyzed using the NCBI conserved domain database, HMMER, Pfam, and SMART (Letunic et al., 2004). The resulting QdGSTs were classified on the basis of their homology to Arabidopsis GSTs using a previously described standard method (Ghangal et al., 2020).
2.3 Phylogenetic analysis
Sequence alignment of GST proteins from Arabidopsis and Q. dentata was performed using the MUSCLE wrapper in TBtools with default parameters to examine evolutionary relationships among the proteins (Chen et al., 2020). A maximum likelihood phylogenetic tree was built using the ‘One Step Build a ML Tree’ feature in TBtools, applying the JTT substitution model with a 95% site coverage cut-off. Node confidence was evaluated using 1000 bootstrap replicates. GST classes were visualized using with distinct colors for clarity.
2.4 Amino acid characteristic analysis of QdGSTs
The characteristics of QdGST proteins, including molecular weight, isoelectric point, and grand average of hydropathicity, were analyzed using the ExPasy tool (http://web.expasy.org/). Their subcellular localizations were predicted with Cell-PLoc 2.0 (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2/).
2.5 Genome structure, chromosomal localization, gene duplication, and collinearity analysis
The chromosomal locations of 86 QdGST genes were obtained from a genome annotation file and mapped to chromosomes using TBtools (Chen et al., 2020). Genomic data for A. thaliana, C. annuum, Vitis vinifera, Triticum aestivum, and Oryza sativa were obtained from EnsemblPlants (http://plants.ensembl.org); those for Quercus mongolica were obtained from the NCBI Sequence Read Archive under accession codes PRJNA609556 and PRJNA607679. All the genomic data were analyzed for collinear relationships using the ‘One Step MCScanX’ feature in TBtools with default settings (Chen et al., 2020).
2.6 Analysis of QdGST gene structure, conserved motifs, and domains
Structural information regarding the QdGST genes was obtained from a GFF file and used to visualize the conserved domains and exon–intron organization with TBtools. Conserved motif sequences and types of QdGST genes were analyzed using MEME Suite 5.5.3 (http://meme-suite.org/tools/meme) with the following parameters: motif site distribution = any number of repetitions; and maximum motif number = 10. The structures and motif distributions of QdGST genes were grouped according to a phylogenetic tree and visualized using TBtools (Chen et al., 2020).
2.7 Analysis of cis-acting elements of QdGST gene promoters
Promoter regions (2,000 bp upstream of the translation initiation site) were extracted from all QdGST genomic sequences using TBtools (Chen et al., 2020). Potential cis-regulatory elements within these regions were identified by searching the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) with default settings.
2.8 Expression analysis of QdGST genes using RNA sequencing
To investigate the tissue-specific expression of the QdGST genes, we obtained RNA expression data for Q. dentata from the National Genomics Data Center (accession code GWHBRAD00000000). For analysis of the expression of QdGST genes under Pb and Cd stresses, samples were collected using the method described above. Total RNA extraction and mRNA library construction and sequencing were performed according to the methods described by Chen et al. (2022). All RNA-seq data have been submitted to the Genome Sequence Archive (accession CRA013085) of the National Genomics Data Center (CNCB-NGDC Members and Partners, 2022). Expression levels were normalized to FPKM (fragments per kilobase million), and a heatmap was generated by applying the logarithmic transformation log10(FPKM+1) using TBtools.
2.9 qRT-PCR analysis
Total RNA was extracted from the samples using a SteadyPure Plant RNA Extraction Kit (Accurate Biotechnology, Hunan, China) according to the protocol from manufacturer. Complementary DNA (cDNA) was synthesized using Evo M-MLV RT Premix for qPCR (Accurate Biotechnology). The primers for the qRT-PCR experiments were designed using Primer3plus (https://www.primer3plus.com/) and are listed in Supplementary Table S3. The CaCs gene was selected as the internal reference gene for roots, whereas the EF1-α gene was used for stems and leaves (Supplementary Table S3). Subsequently, the qPCRs were performed on a Bio-Rad CFX96 Touch Real-Time PCR Detection System (USA) using an SYBR Green Premix ProTaq HS qPCR Kit (Accurate Biotechnology) under the following conditions: 95°C for 30 s, followed by 40 cycles of 95°C for 15 s and 60°C for 30 s. The 2−ΔΔCt method with three technical replicates per sample was used to calculate relative gene expression (Livak and Schmitten, 2001).
2.10 Heterologous expression of QdGST in yeast
Gene-specific primers (Supplementary Table S3) for QdGSTU20 and QdGSTU36 were used to amplify their coding DNA sequence regions. To enable expression in yeast (Saccharomyces cerevisiae), cDNAs for QdGSTU20 and QdGSTU36, obtained through PCR amplification, were cloned into the KpnI-XbaI restriction sites of the pYES2.0 plasmid. A transformation kit (Huayueyang Biotechnology, China) was used to introduce the empty plasmid and recombinant vector into S. cerevisiae INVSc1. Jiang’s method with minor modifications was used to evaluate the metal resistance of the transgenic yeast (Jiang et al., 2024). Yeast harboring either the recombinant plasmid or the empty plasmid was cultured in liquid medium (SC-U/Glu) and incubated at 28°C and 200 rpm until the optical density at 600 nm reached 1.0. Subsequently, serial ten-fold dilutions were performed using sterile water, and 2-μL aliquots from each dilution were dropped onto solid SC-U/Gal induction medium supplemented with various heavy metals (10 μM CdSO4·8/3H2O, 3 mM PbCl2, or 15 mM MnSO4·H2O) or control medium (no additional metal ions). Three independent biological replicates were included in each treatment. Growth phenotypes were recorded after 3 days of incubation at 28°C.
2.11 Statistical analysis
GraphPad Prism 9.4.1 was selected for statistical analysis and graph generation. In figures showing the results of qPCR analyses, error bars represent the standard deviation from three independent biological replicates. Statistical analysis was performed using one-way analysis of variance and post hoc least significant difference tests to identify differences among group means, at a significance level of 0.05.
3 Results
3.1 Identification and phylogenetic and characteristic analysis of the GSTs in Q. dentata
Eighty-six GSTs in Q. dentata were identified and systematically classified on the basis of their chromosomal locations and the homology of their encoded proteins with that of Arabidopsis GSTs (Supplementary Table S1) (Zhao et al., 2021; Mo et al., 2023). GST proteins from Q. dentata (86) and Arabidopsis thaliana (56) were used to construct a phylogenetic tree for investigation of the phylogenetic relationships among the QdGSTs. QdGSTs belonging to six classes (tau, lambda, phi, TCHQD, theta, and DHAR) were identified (Figure 1), with the majority belonging to the tau class (56 members, 65.1%), followed by the lambda (13 members, 15.1%), phi (eight members, 9.3%), and theta (five members, 5.8%) classes. Only two members each belonged to the TCHQD and DHAR classes, and no QdGST belonged to the zeta class (Figure 1).

Figure 1. Phylogenetic analysis of GSTs from Q. dentata and A. thaliana. Different colors represent different classes of GST genes. Species names are abbreviated as follows: At, A. thaliana; Qd, Q. dentata. Different shapes indicate different species (At, black background star; Qd, blue background star). The tree was constructed using TBtools and the maximum likelihood method with 1,000 bootstrap replications.
The coding DNA sequences of QdGSTs varied in length from 426 bp (QdGSTU39) to 1467 bp (QdGSTT1), encoding proteins that ranged from 141 to 488 amino acids. The molecular weights (MWs) of the QdGSTs ranged from 15.9 kDa (QdGSTU39) to 55.1 kDa (QdGSTT1), and their isoelectric point values ranged from 4.48 (QdGSTL1) to 9.38 (QdTCHQD1 and QdTCHQD2). The grand average of hydropathicity values of all QdGSTs, except for QdGSTU3 (0) and QdGSTU54 (0.133), were negative and ranged from −0.02 (QdGSTU24) to −0.57 (QdMTP8.3). Most of the QdMTPs had weak hydrophilicity, and the majority were predicted to have cytoplasmic localization, with limited numbers in the chloroplast and the nucleus (Supplementary Table S1).
3.2 Chromosomal distribution and duplication of QdGST family members
The 86 QdGST genes were unevenly dispersed across 11 of the 12 chromosomes. Twenty-one (24.4%) and 20 (23.3%) genes, belonging to the tau and lambda members, were located on chromosomes 4 and 7, respectively; chromosome 9 contained 13 QdGST genes; and chromosomes 6, 2, 3, 12, 10, and 8 had 8, 7, 6, 4, 3, and 2 QdGST genes, respectively. Chromosomes 5 and 11 contained only one QdGST gene each (Figure 2). No significant association was observed between chromosomal length and number of QdGST genes (Figure 2). Segmental and tandem duplication have important roles in driving the expansion of gene families (Wang et al., 2019). A total of 31 gene pairs distributed on seven chromosomes were identified as tandem duplication types; 22 of these (71.0%) involved genes from the tau class, and seven (22.6%) involved genes from the lambda class. There was one tandem duplication (0.03%) corresponding to each of the theta and phi classes (Figure 2). In addition, seven QdGST gene pairs (QdGSTU6/QdGSTU54, QdGSTU31/QdGSTU48, QdGSTU34/QdGSTU49, QdGSTF3/QdGSTF5, QdGSTL3/QdGSTL13, QdGSTL7/QdDHAR2, and QdGSTL7/QdGSTL13), located on chromosomes 3, 6, 7, 8, 9, 10, and 12, respectively, were identified as segmental duplications (Figure 3). Three of these gene pairs were from the tau class, two from the lambda class, and one from the phi class. Notably, one segmental duplication pair (QdGSTL7/QdDHAR2) contained genes from both the lambda and DHAR subfamilies. QdGSTU31, QdGSTU34, and QdGSTL7 underwent both segmental and tandem duplication. Overall, these results suggest that the duplication events identified here contributed primarily to the expansion of the tau and lambda classes of GST genes in Q. dentata.

Figure 2. Chromosomal localization of QdGST genes in Q. dentata. Sky-blue vertical bars represent the Q. dentata chromosomes. The chromosome number is shown on the left side of each chromosome. Tandem duplication gene pairs are marked by red curves. The size of the chromosome is indicated by a vertical scale bar.
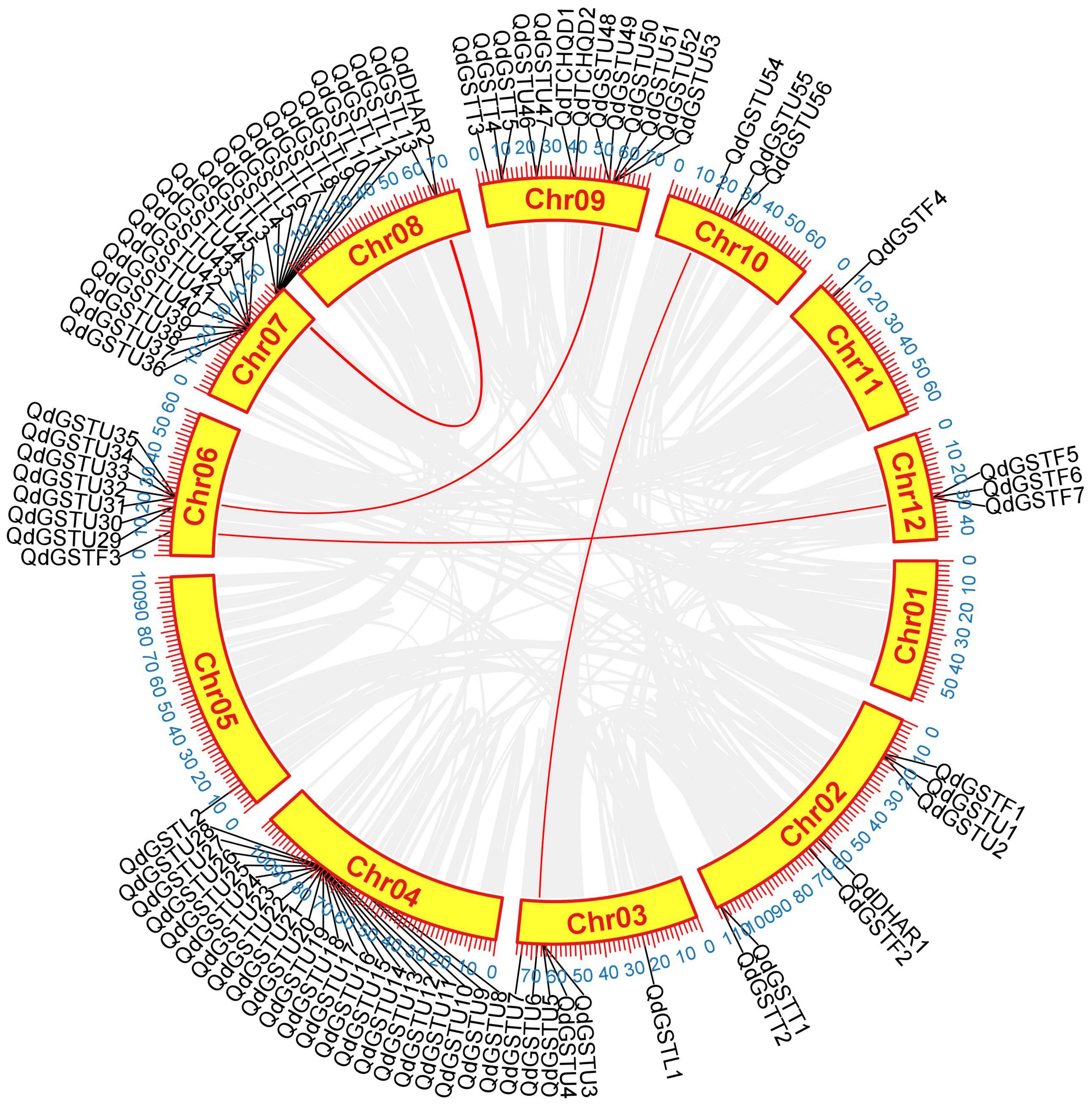
Figure 3. Segmental duplication analysis of QdGST genes. Segmental duplication of QdGST is shown using a Circos plot. Red curves represent segmental duplications of the GSTs. The gray background shows the genomic positions of all the collinear gene pairs in Q. dentata.
3.3 Analysis of GST gene collinearity
To explore the evolutionary relationships of GST genes in Q. dentata and other species, we constructed a synteny map based on six species (A. thaliana, O. sativa, C. annuum, T. aestivum, V. vinifera, and Q. mongolica) and previously published genome-wide GST data (Wang et al., 2019; Islam et al., 2019; Li et al., 2022). We identified 135 orthologous GST gene pairs between Q. dentata and the six angiosperm species (Figure 4). Of these, 42 collinear blocks of QdGST genes were found between Q. dentata and Q. mongolica, followed by 38 between Q. dentata and V. vinifera, 23 between Q. dentata and C. annuum, and 20 between Q. dentata and A. thaliana. In addition, eight and four orthologous GST gene pairs were detected for T. aestivum and O. sativa, respectively (Figure 4). No QdGST gene syntenic regions were found on chromosome 5 or 11 of the Q. dentata genome. Three QdGST (QdGSTL13, QdGSTL7, and QdGSTU46) gene pairs were detected between Q. dentata and two monocot plants (T. aestivum and O. sativa); this indicates that the majority of QdGST genes were formed after the divergence of their common ancestor. Notably, 36 QdGST genes lacked detectable orthologs across other species.
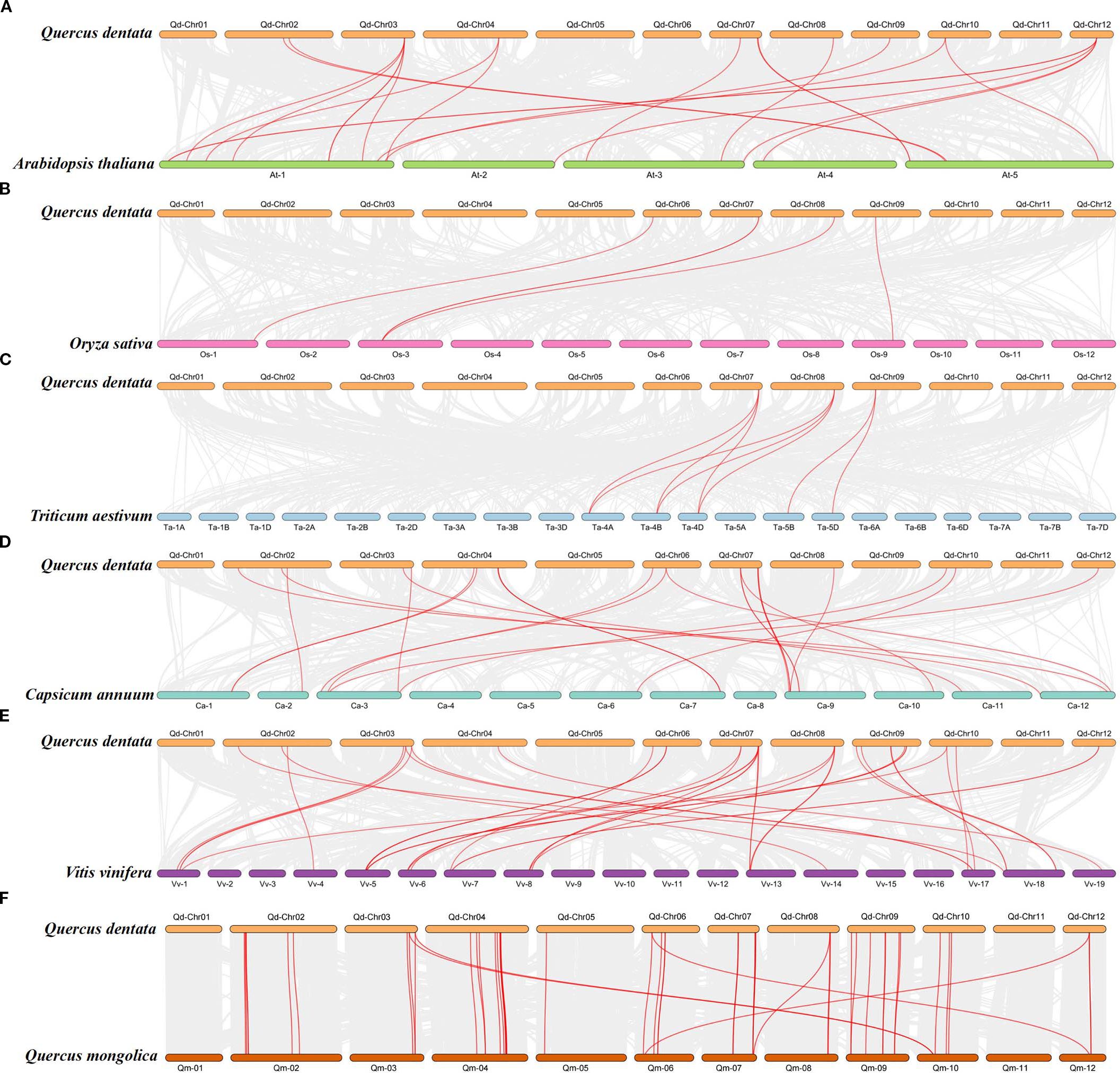
Figure 4. Collinearity analysis of GST genes in Q. dentata and six other species: (A)A) thaliana, (B) O. sativa, (C) T. aestivum, (D) C annuum, (E) V. vinifera, (F) Q. mongolica. Representative homologous GST gene pairs (red) are overlaid on the collinear gene pairs (gray).
3.4 Examination of QdGST gene structures and conserved protein motifs
We analyzed the composition of QdGST proteins and found ten conserved motifs among the 86 QdGSTs. The presence of these motifs varied among QdGST from different classes, with motif 9 found only in the lambda class, whereas motifs 5 and 10 were specific to the tau class. Motifs 4, 1, and 3 were consistently present in all proteins of the DHAR and TCHQD classes; all members of the theta class had motifs 1 and 3; and all members of the phi class had motifs 4, 1, 2, and 3. All members of the lambda class except for QdGSTL1 harbored motifs 9, 4, 1, 2, and 7; and most members of the tau class (50/56, 89.3%) contained motifs 4, 6, 1, 2, 8, and 3 (Figure 5A). To investigate the evolution of the QdGST genes, we analyzed their structures using the Q. dentata genome annotation file. We found that the number of introns in QdGST genes varied from 0 to 12, with genes within the same class exhibiting similar structural patterns. Most QdGST genes (49/56) in the tau subfamily had one intron, and five genes had two introns, and the QdGSTU2 gene had 11 introns. Most members (11/13) of the lambda class had 9–10 introns. Seven of eight QdGST genes in the phi class had two introns; the exception was QdGSTF8, which had three. TCHQD- and DHAR-class genes contained three and five introns, respectively, and intron numbers in the theta class ranged from four (QdGSTT1) to 12 (QdGSTT5) (Figure 5B).
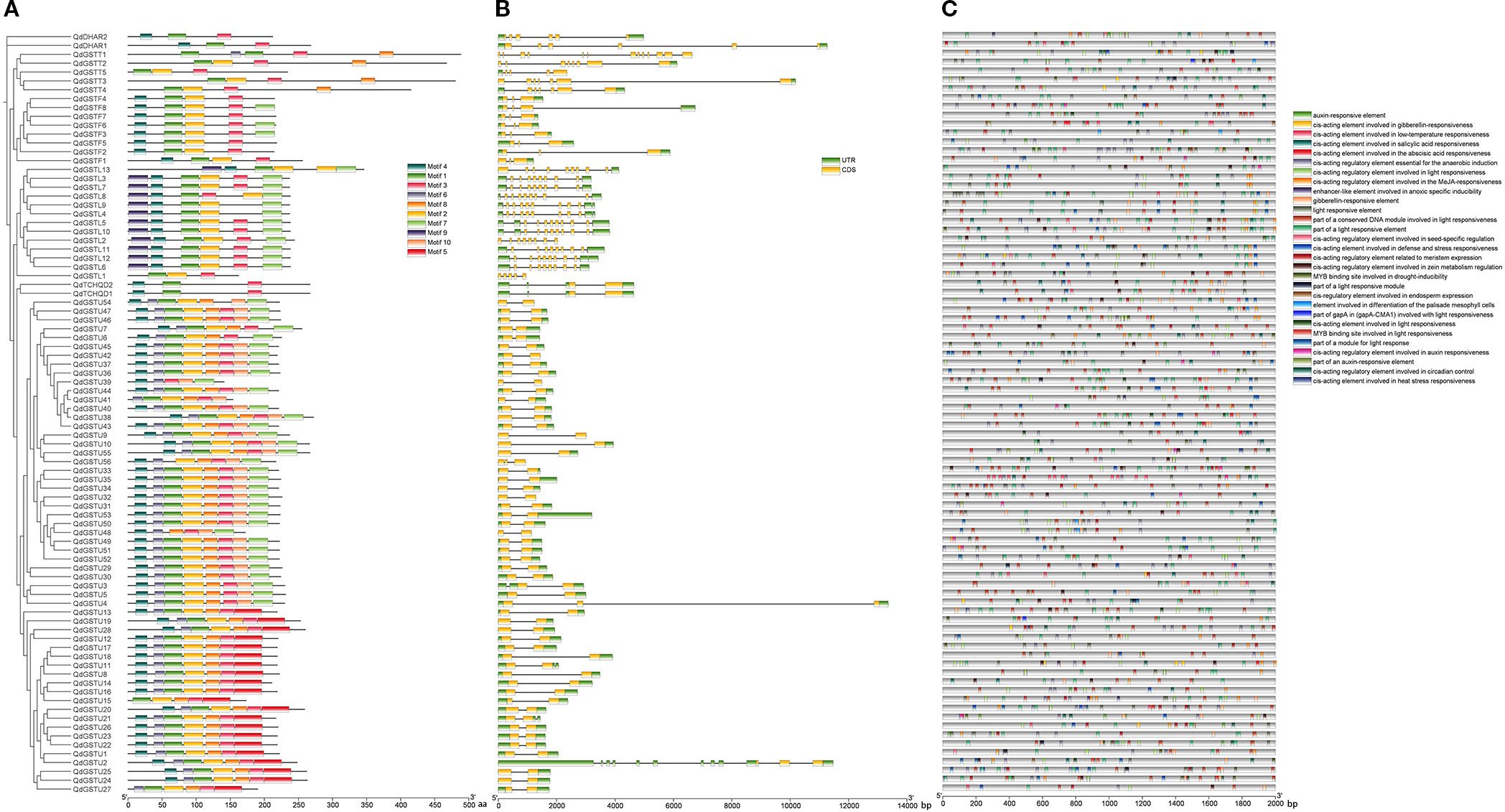
Figure 5. Conserved protein motifs, gene structures, and cis-acting elements of QdGST genes. (A) Composition and distributions of conserved motifs in the QdGST genes. Different motifs are shown in boxes of different colors. (B) Exon–intron organization of QdGST genes. (C) Prediction of cis-acting elements in the promoters of QdGST genes. Different colored boxes indicate different regulatory elements.
3.5 Identification of cis-regulatory elements of QdGST genes
We screened the 2,000-bp promoter regions upstream of the translation initiation sites of the 86 QdGST genes using PlantCARE to detect cis-acting elements. The results revealed 1,924 cis-regulatory elements that could be classified into 29 categories (Figure 5C) on the basis of their functions. These functions included response to light, plant hormones, stresses, and plant development. More than 50% of the identified elements were related to light response; these included ACE, G-box, 4cl-CMA2b, MRE, Box 4, ATCT-motif, ATC-motif, chs-CMA2a, AT1-motif, AE-box, and ACA-motif, which were ubiquitously present in the promoter regions of all QdGSTs (Figure 5C, Supplementary Table S2).
A total of 527 cis-acting elements were found to be related to signaling by plant hormones, including gibberellin (TATC-box, P-box, GARE-motif), salicylic acid (TCA-element), abscisic acid (ABRE), auxin (TGA-element, AuxRR-core, AuxRE, and TGA-box), and methyl jasmonate (TGACG-motif and CGTCA-motif), as well as zein metabolism regulation (O2-site). A total of 326 cis-regulatory elements were implicated in stress responses such as anaerobic induction (ARE, GC-motif), drought response (MBS), low-temperature response (LTR), defense and stress response (TC-rich repeats), and heat response (HSE). Twenty-four and 83 cis-acting elements were related to circadian control and plant development (CAT-box, GCN4_motif, RY-element, and HD-Zip 1), respectively (Figure 5C, Supplementary Table S2).
3.6 Expression profiles of QdGST genes based on RNA-seq data
The expression patterns of QdGST genes were characterized using transcriptome sequencing data from diverse tissues and developmental stages. We detected expression (log10(FPKM+1) > 0) of all QdGST genes except QdGSTU41 in at least one tissue at each development stage (Figure 6A). Nine QdGST genes (QdDHAR2, QdGSTL3, QdGSTL7, QdGSTL12, QdGSTL6, QdGSTU5, QdGSTU11, QdGSTT2, and QdGSTT3) showed high expression in all samples (log10(FPKM+1) > 1). The eighty-six QdGST genes could be divided into three classes according to their tissue-specific expression profiles: (a) QdGST genes with extremely low expression in almost all tissues; (b) QdGST genes exhibiting low-to-medium expression across different tissues; and (c) QdGST genes with high expression in some tissues. These findings were consistent with previous reports (Islam et al., 2019; Hasan et al., 2021).
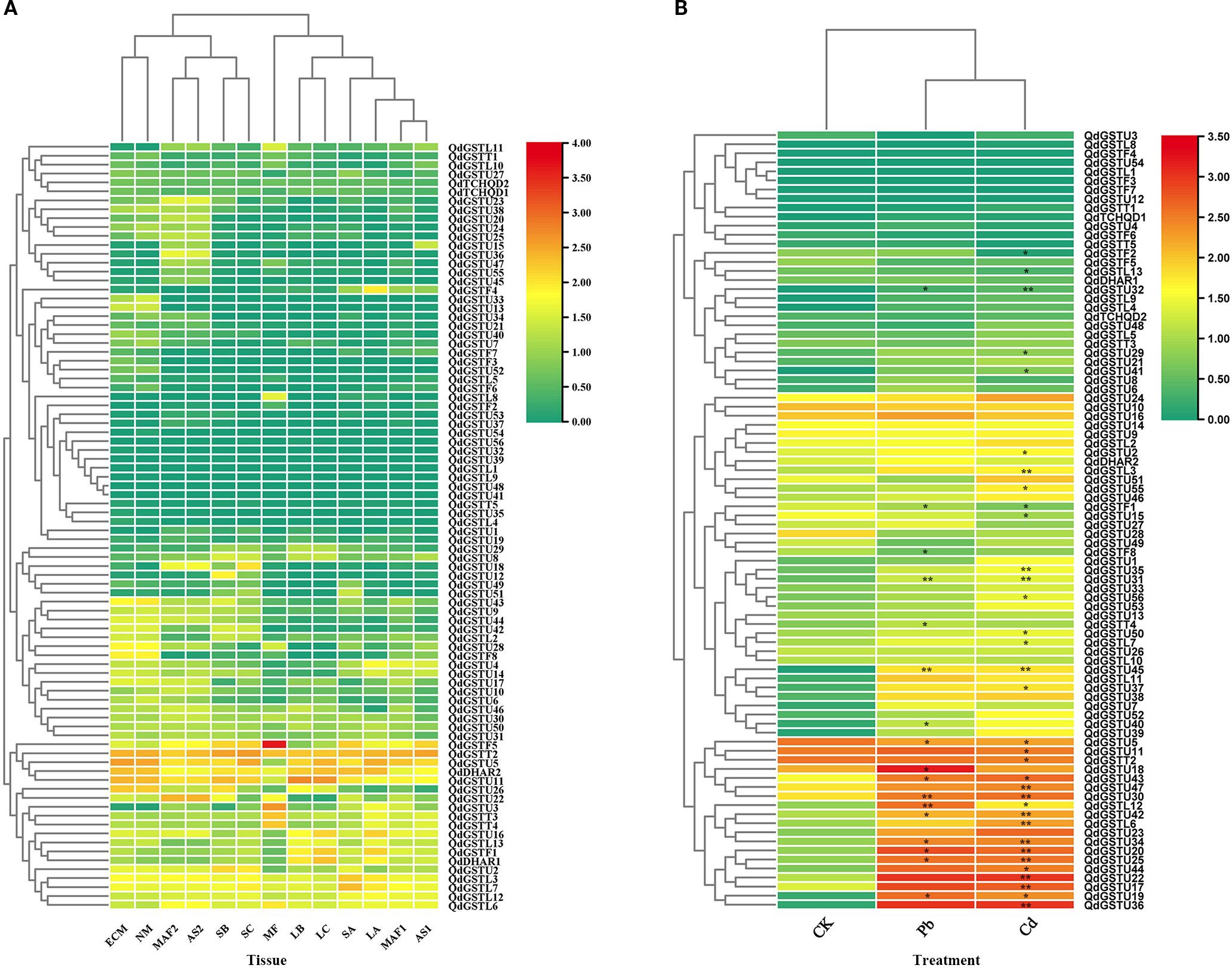
Figure 6. Analysis of expression profiles of QdGST genes. (A) Heatmaps of the expression profiles of QdGST genes in various tissues of Q. dentata, including ectomycorrhiza (ECM), root (NM), fruit (MF, MAF1 and MAF2), shell (AS1 and AS2), leaves (LA, LB, and LC), and stems (SA, SB, and SC). (B) Expression patterns of QdGST genes in roots under Cd and Pb treatments. Black asterisks denote statistically significant differences between heavy metal treatment and control groups (*p < 0.05; **p < 0.01). The color bar represents log10(FPKM+1).
To investigate the responses of QdGST genes to heavy metal stresses, we analyzed their expression profiles under Pb and Cd treatment using data from transcriptome sequencing. Nine QdGST genes of the tau class (QdGSTU19, QdGSTU20, QdGSTU25, QdGSTU30, QdGSTU31, QdGSTU32, QdGSTU34, QdGSTU42, and QdGSTU43) and one member of the lambda class (QdGSTL12) were upregulated in response to both Pb and Cd treatment. Two members of the tau class (QdGSTU18 and QdGSTU40), one of the theta class (QdGSTT4), and one of the phi class (QdGSTF8) were upregulated only in response to Pb treatments, whereas 13 tau-class (QdGSTU2, QdGSTU17, QdGSTU22, QdGSTU29, QdGSTU35, QdGSTU36, QdGSTU37, QdGSTU41, QdGSTU44, QdGSTU47, QdGSTU50, QdGSTU55, and QdGSTU56) and four lambda-class (QdGSTL3, QdGSTL6, QdGSTL7, and QdGSTL11) genes were upregulated only by Cd treatment. QdGSTU19, QdGSTU20, QdGSTU36, QdGSTU39, QdGSTU44, QdGSTU45, and QdGSTL11 showed particularly marked upregulation (>100-fold increase in expression) under Pb and/or Cd treatment (Figure 6B).
3.7 Expression of QdGST genes under Pb and Cd stresses according to qRT-PCR
The results of the RNA-seq analysis for eight QdGST genes with high expression levels under Pb and Cd treatments were validated using qRT-PCR. Under normal growth conditions, the expression patterns of these genes showed tissue specificity, with QdGSTL6, QdGSTL12, QdGSTU17, and QdGSTU36 having higher levels in stems and leaves than in roots (Figure 7), whereas QdGSTU20 and QdGSTU44 had higher expression levels in roots and stems compared to leaves, and QdGSTU19 and QdGSTU34 were highly expressed in stems and roots, respectively. All the tested QdGST genes were upregulated under both Pb and Cd treatments in stems, and all except QdGSTL6 were upregulated under Cd treatment in roots. Expression of QdGSTL12, QdGSTU19, QdGSTU20, QdGSTU34, QdGSTU36 and QdGSTU44 was induced by Pb treatment. In leaves, the expression of QdGSTU20, QdGSTU34, QdGSTU36, and QdGSTU44 was induced by Cd treatment, whereas that of QdGSTL6 was upregulated by Pb treatment. However, four QdGST genes (QdGSTL6, QdGSTL12, QdGSTU17, and QdGSTU19) were inhibited by Cd and three (QdGSTL12, QdGSTU20, and QdGSTU34) by Pb in leaves.
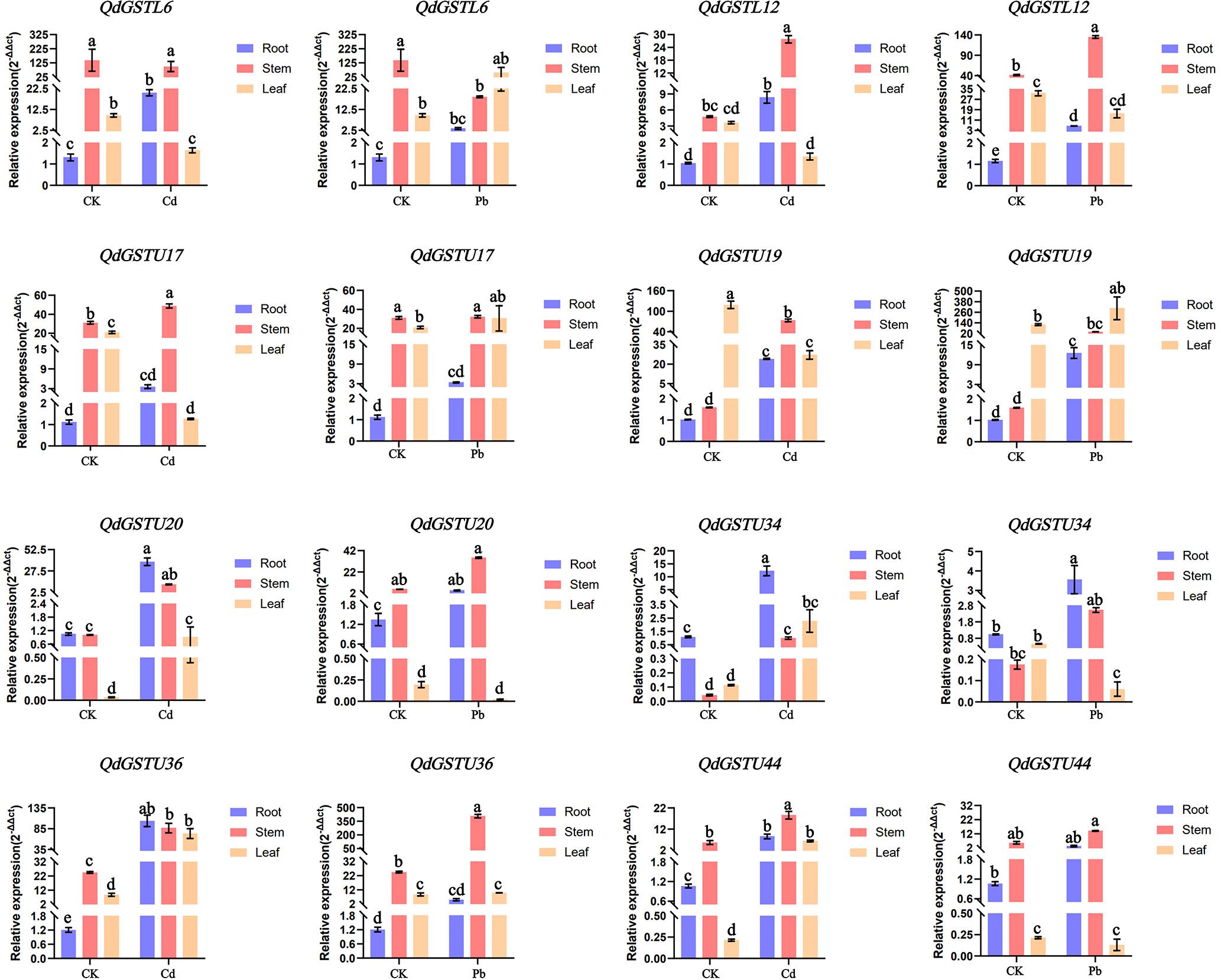
Figure 7. Relative expression patterns of eight QdGST genes in Q. dentata seedlings under Pb and Cd stresses. Expression was analyzed by qRT-PCR with three biological replicates; the results are presented as the mean ± standard deviation. Untreated controls are denoted CK. Lowercase letters indicate significant differences between control and treatment groups (p < 0.05).
3.8 Growth of recombinant yeast under heavy metal stresses
To further characterize the involvement of QdGST genes in heavy metal resistance, QdGSTU20 and QdGSTU36, which were highly expressed under both Pb and Cd treatments, were introduced into wild-type yeast for metal sensitivity analysis. Under normal conditions, yeast strains with empty vectors and those expressing QdGSTU20 or QdGSTU36 showed no significant difference in growth. However, when expressed in yeast, QdGSTU36 conferred Pb and Cd tolerance. Expression of QdGSTU20 did not alter the sensitivity of yeast to Pb or Cd; however, it did confer Mn tolerance (Figure 8).

Figure 8. Tolerance of yeast expressing heterologous QdGST to heavy metal stresses. S. cerevisiae strain INVSc1 was genetically modified to harbor either the empty pYES2 plasmid or a recombinant vector containing the QdGSTU20 (A) or QdGSTU36 (B) gene. Two-microliter serial dilutions of yeast cultures were inoculated onto SD Ura/Gal medium treated with heavy metals.
4 Discussion
The GST superfamily represents an evolutionarily conserved group of proteins found across diverse living organisms. Plant GSTs have important roles in regulation of growth and development and improve plant resistance to various stresses (Pinkus et al., 1996; Gullner et al., 2018; Kayum et al., 2018; Shokat et al., 2020). Many GST genes have been detected in various plants, with their numbers varying greatly among species. Until now, the GST family in Quercus species had not been analyzed. In the present study, we identified 86 GST genes in Q. dentata, a number larger than that in rice (Jain et al., 2010), Arabidopsis (Sappl et al., 2009), barley (Rezaei et al., 2013), maize (McGonigle et al., 2000), Capsella rubella (He et al., 2016), radish (Gao et al., 2020), or pepper (Islam et al., 2019), but smaller than that in wheat (Wang et al., 2019), soybean (Hasan et al., 2020), potato (Islam et al., 2018), litchi (Hu et al., 2016), or tomato (Lan et al., 2009). These QdGST genes were found to be distributed among six of the GST classes, with the greatest abundance in the tau class (56 members), followed by the lambda (13) and phi (8) classes. These results are in contrast to those of previous research in other species, which found that the phi- and tau-class GSTs were most abundant, with numbers of lambda-class members ranging from only two to five in many plant species (He et al., 2016; Islam et al., 2019; Wang et al., 2019; Liu et al., 2023). Members of the zeta class have been identified in various monocotyledonous and dicotyledonous plants (Wang et al., 2019). However, we found no QdGST belonging to this class, similar to findings in apple (Fang et al., 2020). Therefore, we speculate that the zeta class of GSTs may have been lost during the evolution of Q. dentata.
Gene family expansion depends on various duplication mechanisms, including tandem, segmental, and whole-genome duplications, as well as transpositions (Cannon et al., 2004; Flagel and Jonathan, 2009). Previous studies have shown that tandem and segmental duplications, which are distinctive features of plant genomes, resulted in most of the members of the tau and phi classes of GST (Islam et al., 2019; Hao et al., 2021; Wang et al., 2023). In the present study, 48 QdGST genes were found to be associated with tandem duplication, whereas 12 were associated with segmental duplication. These results show that tandem duplication had a significant role in the evolution of QdGST genes, consistent with findings in other plant species (Jain et al., 2010; He et al., 2016; Fang et al., 2020). Duplication events can drive the expansion of gene families and generate novel gene functions, which can enable plants to quickly adapt to adverse environmental conditions (Freeling, 2009). In Q. dentata, most tandem duplication events occurred between gene pairs in the tau class, resulting in tau QdGSTs being the most common. The tau GSTs also seemed to have essential roles in the tolerance of Q. dentata to different stresses. The lambda class was the second largest, owing to both tandem and segmental duplication events. This phenomenon has not been observed in previous studies. Therefore, further research is needed to understand the roles of the expanded lambda class of GSTs in Q. dentata.
GST genes of the tau class in plants have been reported to have important roles in adaptation to diverse environmental stresses. For instance, DsGSTU1 in Digitaria sanguinalis is associated with resistance to haloxyfop-P-methyl, an acetyl-CoA-carboxylase-inhibiting herbicide (Liu et al., 2023), and ectopic overexpression of a BcGSTU gene from Brassica campetris subsp. Chinensis in Arabidopsis results in better performance of plants under abiotic (NaCl and PEG) and biotic (Alternaria brassicae infection) stresses (Kao et al., 2016). Moreover, OsGSTU17 in O. sativa and MruGSTU39 in Medicago ruthenica improve drought stress tolerance (Wang et al., 2022; Li et al., 2023), overexpression of SbGST from Salicornia brachiata in transgenic tobacco has been found to enhance seed germination and growth under salt stress (Jha et al., 2011), GmGSTU23 increases salt tolerance in soybean (Li et al., 2023), PeGSTU58 from Populus euphratica enhances salt and drought stress tolerance (Meng et al., 2023), a rice OsGSTU4 improves tolerance to salinity and oxidative stresses in Arabidopsis (Sharma et al., 2014), and JrGSTTau1 improves plant tolerance to chilling (Yang et al., 2016). Tau-class GST genes are predominant in woody plants, including V. vinifera (88 tau GSTs of a total of 132), Populus trichocarpa (66/79), Coffee canephora (34/54), Citrus sinensis (12/25), Prunus avium (52/67), Amborella trichopoda (36/52), and Picea abies (73/104) (Monticolo et al., 2017; Sabir et al., 2022). Woody plants are constantly exposed to various environmental stresses such as drought, extreme temperatures, pathogens, and herbivores. Thus, the expansion of the tau class of GST genes in these plants may represent a key evolutionary innovation underpinning their adaptation to their environments. As in other woody species, the tau class (56 of 86) represents the majority of GSTs in Q. dentata. The heavy-metal-inducible expression of QdGSTU genes demonstrated here and its functional validation in yeast indicate a critical role of the tau class in adaptation to environmental stress.
With increasing industrialization, contamination of soil by heavy metals is a growing environmental concern (Li et al., 2022). Research has demonstrated that Quercus species exhibit significant tolerance to multiple heavy metals (e.g., Sb, Cd, Cu, Pb, Zn, Co, and Ni) and serve as effective agents in phytoremediation of contaminated soils (Gogorcena et al., 2011; Shi et al., 2017, Shi et al., 2019). Although various plant GSTs have been reported to respond to exposure to different heavy metals (Sappl et al., 2009; Lin et al., 2013; Kumar et al., 2013), the role of the Quercus GST family remained unclear. Here, we found that 31 GST genes in Q. dentata were upregulated under Cd or Pb treatment, most of which were members of the tau class. In particular, expression levels of tau-class QdGSTU19, QdGSTU20, QdGSTU36, and QdGSTU44 were strongly upregulated under Pb and/or Cd treatment according to our RNA-seq analysis; these results were validated by qRT-PCR. Our findings are consistent with those previously reported in other plant species (Lin et al., 2013; Gao et al., 2020; Jing et al., 2020), confirming that GST genes of the tau class have important roles in plant tolerance to heavy metal stress. Heterologous expression of QdGSTU36 in yeast conferred the ability to tolerate Cd and Pb stresses, consistent with the expression profiles of this gene obtained in the qRT-PCR analysis. However, heterologous expression of QdGSTU20 provided tolerance only to Mn. We hypothesize that expansion of the tau class has enabled Q. dentata to develop more precise adaptation mechanisms under complex environmental conditions. Our results provide candidate genes for future development of heavy-metal-tolerant plants, with QdGSTU36 and QdGSTU20 showing particular importance in this regard. However, the lack of in-plant validation (e.g., overexpression/knock-out studies of QdGST genes in Arabidopsis or Q. dentata) limits the biological relevance of these findings to actual plant stress responses. Therefore, future work should include generation of stable transgenic Arabidopsis or Q. dentata plants via over-expression, CRISPR–Cas, or RNA interference; these could be used to (a) evaluate growth and physiological parameters under heavy metal stresses; and (b) quantify metal accumulation and localization. Integrating the results of these plant assays with our yeast data will provide a more complete understanding of how tau-class GSTs mediate heavy-metal detoxification in Quercus plants.
Previous research has shown that GST gene expression is regulated by multiple signaling molecules, including reactive oxygen intermediates (hydrogen peroxide) and plant growth regulators such as auxins, salicylic acid, ethylene, jasmonic acid, and nitric oxide (Moons, 2005). Several upstream regulators of GST genes have been identified through yeast one-hybrid and dual luciferase reporter assays. For instance, JrDREB2A, JrMYC2, JrMYB44, JrDof1, and JrWRKY7 were shown to directly activate JrGSTTau1 expression to regulate osmotic stress response in Juglans regia (Yang et al., 2019); LhGST, which belongs to the phi class, was found to be crucial for anthocyanin transport and accumulation in lily tepals, and its promoter could be activated by LhMYB12-lat (Cao et al., 2021); similarly, MrGST1, another phi-class GST, was activated by MrMYB1.1 and regulated anthocyanin accumulation in Chinese bayberry (Morella rubra) fruit (Xue et al., 2022). In the present study, our analysis of the promoter sequences of QdGSTU36 and QdGSTU20 showed the presence of salicylic acid-, abscisic acid-, and methyl jasmonate-responsive elements, as well as an MYB recognition site. These results indicate potential roles of plant hormone signaling in the heavy metal tolerance of Q. dentata, including interactions with GST genes. However, further experiments are need to clarify the pathways contributing to heavy metal stress tolerance in Q. dentata.
5 Conclusions
A total of 86 GST genes were identified in the Q. dentata genome and found to comprise members of six classes, with an uneven distribution across 11 chromosomes. Promoter analysis revealed the presence of 29 categories of cis-acting elements, most of which were involved in defense and stress responses. RNA-seq and qRT-PCR analyses demonstrated that the QdGST genes of the tau class are crucial for heavy metal tolerance; when expressed in yeast, QdGSTU36 conferred Cd and Pb tolerance, whereas QdGSTU20 conferred Mn tolerance. These findings lay a foundation for further functional verification of QdGST genes. Moreover, they provide a basis for genetic engineering to develop heavy-metal-tolerant crops via overexpression of QdGST genes or CRISPR-based genome editing.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
JS: Conceptualization, Data curation, Investigation, Software, Validation, Writing – original draft. XW: Conceptualization, Data curation, Investigation, Software, Validation, Writing – original draft. AS: Data curation, Investigation, Validation, Writing – original draft. XP: Data curation, Validation, Writing – original draft. WW: Methodology, Writing – original draft. PL: Methodology, Writing – original draft. ZH: Methodology, Writing – original draft. YZ: Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. XH: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the 2023 Student Internship and Farming Education Project of Landscape Architecture College and the Building Project of Beijing Laboratory of Urban and Rural Ecological Environment (grant number PXM2015-014207-000014).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1641553/full#supplementary-material
References
Ahsan, N., Lee, D. G., Alam, I., Kim, P. J., Lee, J. J., Ahn, Y. O., et al. (2008). Comparative proteomic study of arsenic-induced differentially expressed proteins in rice roots reveals glutathione plays a central role during As stress. Proteomics 8, 3561–3576. doi: 10.1002/pmic.200701189
Cannon, S. B., Mitra, A., Baumgarten, A., Young, N. D., and May, G. (2004). The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 4, 10. doi: 10.1186/1471-2229-4-10
Cao, Y. W., Xu, L. F., Xu, H., Yang, P. P., He, G. R., Tang, Y. C., et al. (2021). LhGST is an anthocyanin-related glutathione S-transferase gene in Asiati hybrid lilies (Lilium spp.). Plant Cell Rep. 40, 85–95. doi: 10.1007/s00299-020-02615-y
Chen, C. J., Chen, H., Zhang, Y., Thomas, H. R., Frank, M. H., He, Y. H., et al. (2020). TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202. doi: 10.1016/j.molp.2020.06.009
Chen, T. T., Chen, X., Zhang, S. S., Zhu, J. W., Tang, B. X., Wang, A., et al. (2021). The genome sequence archive family: toward explosive data growth and diverse data types. Genom. Proteom. Bioinf. 19, 578–583. doi: 10.1016/j.gpb.2021.08.001
Chen, M., Guo, L., Ramakrishnan, M., Fei, Z. J., Vinod, K. K., Ding, Y. L., et al. (2022). Rapid growth of Moso bamboo (Phyllostachys edulis): cellular roadmaps transcriptome dynamics and environmental factors. Plant Cell 34, 3577–3610. doi: 10.1093/plcell/koac193
Chen, J. H., Jiang, H. W., Hsieh, E. J., Chen, H. Y., Chien, C. T., Hsieh, H. L., et al. (2012). Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol. 158, 340–351. doi: 10.1104/pp.111.181875
CNCB-NGDC Members and Partners (2022). Database resources of the national genomics data center China national center for bioinformation in 2022. Nucleic Acids Res. 50, D27–D38. doi: 10.1006/meth.2001.1262
Ding, N., Wang, A., Zhang, X. J., Wu, Y. X., Wang, R. Y., Cui, H. H., et al. (2017). Identification and analysis of glutathione S-transferase gene family in sweet potato reveal divergent GST-mediated networks in aboveground and underground tissues in response to abiotic stresses. BMC Plant Biol. 17, 225. doi: 10.1186/s12870-017-1179-z
Dixit, P., Mukherjee, P. K., Ramachandran, V., and Eapen, S. (2011). Glutathione transferase from Trichoderma virens enhances cadmium tolerance without enhancing its accumulation in transgenic Nicotiana tabacum. PloS One 6, e16360. doi: 10.1371/journal.pone.0016360
Dixon, D. P., Skipsey, M., and Edwards, R. (2010). Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 71, 338–350. doi: 10.1016/j.phytochem.2009.12.012
Duan, Q., Li, G. R., Qu, Y. P., Yin, D. X., Zhang, C. L., and Chen, Y. S. (2022). Genome-wide identification evolution and expression analysis of the glutathione S-transferase supergene family in Euphorbiaceae. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.808279
Edwards, R. and Dixon, D. P. (2005). Plant glutathione transferases. Methods Enzymol. 401, 169–186. doi: 10.1016/S0076-6879(05)01011-6
Ezaki, B., Gardner, R. C., Ezaki, Y., and Matsumoto, H. (2000). Expression of aluminum-induced genes in transgenic arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol. 122, 657–665. doi: 10.1104/pp.122.3.657
Fang, X., An, Y. Y., Zheng, J., Shangguan, L. F., and Wang, L. J. (2020). Genome-wide identification and comparative analysis of GST gene family in apple (Malus domestica) and their expressions under ALA treatment. 3 Biotech. 10, 307. doi: 10.1007/s13205-020-02299-x
Flagel, L. E. and Jonathan, F. W. (2009). Gene duplication and evolutionary novelty in plants. New Phytol. 183, 557–564. doi: 10.1111/j.1469-8137.2009.02923.x
Freeling, M. (2009). Bias in plant gene content following different sorts of duplication: Tandem whole-genome segmental or by transposition. Annu. Rev. Plant Biol. 60, 433–453. doi: 10.1146/annurev.arplant.043008.092122
Frova, C. (2006). Glutathione transferases in the genomics era: new insights and perspectives. Biomol. Eng. 23, 149–169. doi: 10.1016/j.bioeng.2006.05.020
Gao, J., Chen, B. W., Lin, H. J., Liu, Y., Wei, Y., Chen, F. B., et al. (2020). Identification and characterization of the glutathione S-transferase (GST) family in radish reveals a likely role in anthocyanin biosynthesis and heavy metal stress tolerance. Gene 743, 144484. doi: 10.1016/j.gene.2020.144484
Ghangal, R., Rajkumar, M. S., Garg, R., and Jain, M. (2020). Genome-wide analysis of glutathione S-transferase gene family in chickpea suggests its role during seed development and abiotic stress. Mol. Biol. Rep. 47, 2749–2761. doi: 10.1007/s11033-020-05377-8
Gogorcena, Y., Larbi, A., Andaluz, S., Carpena, R. O., and Abadía, A. (2011). Effects of cadmium on corkoak (Quercus suber L.) plants grown in hydroponics. Tree Physiol. 31, 1401–1412. doi: 10.1093/treephys/tpr114
Gong, H. B., Jiao, Y. X., Hu, W. W., and Pua, E. C. (2005). Expression of glutathione-S-transferase and its role in plant growth and development in vivo and shoot morphogenesis in vitro. Plant Mol. Biol. 57, 53–66. doi: 10.1007/s11103-004-4516-1
Gullner, G., Komives, T., Király, L., and Schröder, P. (2018). Glutathione S-transferase enzymes in plant-pathogen interactions. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01836
Hao, Y. C., Xu, S. S., Lyu, Z. F., Wang, H. W., Kong, L. R., and Sun, S. L. (2021). Comparative analysis of the glutathione S-transferase gene family of four Triticeae species and transcriptome analysis of GST genes in common wheat responding to salt stress. Int. J. Genomics 2021, 6289174. doi: 10.1155/2021/6289174
Hasan, M. S., Islam, S., Hasan, M. N., Sajib, S. D., Ahmed, S., Islam, T., et al. (2020). Genome-wide analysis and transcript profiling identify several abiotic and biotic stress-responsive Glutathione S-transferase genes in soybean. Plant Gene 23, 100239. doi: 10.1016/j.plgene.2020.100239
Hasan, M. S., Singh, V., Islam, S., Islam, M. S., Ahsan, R., Kaundal, A., et al. (2021). Genome-wide id-entification and expression profiling of glutathione S-transferase family under multiple abiotic and biotic stresses in Medicago truncatula L. PloS One 16, e0247170. doi: 10.1371/journal.pone.0247170
He, G., Guan, C. N., Chen, Q. X., Gou, X. J., Liu, W., Zeng, Q. Y., et al. (2016). Genome-wide analysis of the glutathione S-transferase gene family in Capsella rubella: identification expression and biochemical functions. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01325
Hu, B., Zhao, J. T., Lai, B., Qin, Y. H., Wang, H. C., and Hu, G. B. (2016). LcGST4 is an anthocyanin-related glutathione S-transferase gene in Litchi chinensis Sonn. Plant Cell Rep. 35, 831–843. doi: 10.1007/s00299-015-1924-4
Islam, M. S., Choudhury, M., Majlish, A. K., Islam, T., and Ghosh, A. (2018). Comprehensive genome-wide analysis of glutathione S-transferase gene family in potato (Solanum tuberosum L.) and their expression profiling in various anatomical tissues and perturbation conditions. Gene 639, 149–162. doi: 10.1016/j.gene.2017.10.007
Islam, S., Rahman, I. A., Islam, T., and Ghosh, A. (2017). Genome-wide identification and expression analysis of glutathione S-transferase gene family in tomato: Gaining an insight to their physiological and stress-specific roles. PloS One 12, e0187504. doi: 10.1371/journal.pone.0187504
Islam, S., Sajib, S. D., Jui, Z. S., Arabia, S., Islam, T., and Ghosh, A. (2019). Genome-wide identification of glutathione S-transferase gene family in pepper its classification and expression profiling under different anatomical and environmental conditions. Sci. Rep. 9, 9101. doi: 10.1038/s41598-019-45320-x
Jain, M., Ghanashyam, C., and Bhattacharjee, A. (2010). Comprehensive expression analysis suggests overlapping and specific roles of rice glutathione S-transferase genes during development and stress responses. BMC Genomics 11, 73. doi: 10.1186/1471-2164-11-73
Jha, B., Sharma, A., and Mishra, A. (2011). Expression of SbGSTU (tau class glutathione S-transferase) gene isolated from Salicornia brachiata in tobacco for salt tolerance. Mol. Biol. Rep. 38, 4823–4832. doi: 10.1007/s11033-010-0625-x
Jing, X. Q., Zhou, M. R., Nie, X. M., Zhang, L., Shi, P., Shalmani, A., et al. (2020). OsGSTU6 contributes to Cadmium stress tolerance in rice by involving in intracellular ROS homeostasis. J. Plant Growth Regul. 40, 945–961. doi: 10.1007/s00344-020-10148-7
Kao, C. W., Bakshi, M., Sherameti, I., Dong, S., Reichelt, M., Oelmüller, R., et al. (2016). A Chinese cabbage (Brassica campetris subsp. Chinensis) τ-type glutathione-S-transferase stimulates Arabidopsis development and primes against abiotic and biotic stress. Plant Mol. Biol. 92, 643–659. doi: 10.1007/s11103-016-0531-2
Kayum, M. A., Nath, U. K., Park, J. I., Biswas, M. K., Choi, E. K., Song, J. Y., et al. (2018). Genome-wide identification characterization and expression profiling of glutathione S-transferase (GST) family in Pumpkin reveals likely role in cold-stress tolerance. Genes (Basel) 9, 84. doi: 10.3390/genes9020084
Khan, N., Hu, C. M., Amjad, K. W., and Hou, X. L. (2018). Genome-wide identification classification and expression divergence of glutathione-transferase family in Brassica rapa under multiple hormone treatments. BioMed. Res. Int. 2018, 6023457. doi: 10.1155/2018/6023457
Kieffer, P., Schröder, P., Dommes, J., Hoffmann, L., Renaut, J., and Hausman, J. F. (2009). Proteomic and enzymatic response of poplar to cadmium stress. J. Proteomics 72, 379–396. doi: 10.1016/j.jprot.2009.01.014
Kumar, S., Asif, M. H., Chakrabarty, D., Tripathi, R. D., Dubey, R. S., Trivedi, P. K., et al. (2013). Expression of a rice lambda class of glutathione S-transferase OsGSTL2 in Arabidopsis provides tolerance to heavy metal and other abiotic stresses. J. Hazard. Mater., 248–249. doi: 10.1016/j.jhazmat.2013.01.004
Lan, T., Yang, Z. L., Yang, X., Liu, Y. J., Wang, X. R., and Zeng, Q. Y. (2009). Extensive functional diversification of the Populus glutathione S-transferase supergene family. Plant Cell 21, 3749–3766. doi: 10.1105/tpc.109.070219
Letunic, I., Copley, R. R., Schmidt, S., Ciccarelli, F. D., Doerks, T., Schultz, J., et al. (2004). SMART 4.0: towards genomic data integration. Nucleic Acids Res. 32, D142–D144. doi: 10.1093/nar/gkh088
Li, J. Y., Meng, L. J., Ren, S. H., Jia, C. Y., Liu, R., Jiang, H. Z., et al. (2023). OsGSTU17, a Tau class glutathione S-transferase gene, positively regulates drought stress tolerance in Oryza sativa. Plants (Basel) 12, 3166. doi: 10.3390/plants12173166
Li, X. G., Pang, Y. T., Zhong, Y. W., Cai, Z. D., Ma, Q. B., Wen, K., et al. (2023). GmGSTU23 encoding a Tau class glutathione S-transferase protein enhances the salt tolerance of Soybean (Glycine max L.). Int. J. Mol. Sci. 23, 5547. doi: 10.3390/ijms24065547
Li, J. H., Xia, C. G., Cheng, R., Lan, J. R., Chen, F. Y., Li, X. L., et al. (2022). Passivation of multiple heavy metals in lead-zinc tailings facilitated by straw biochar-loaded N-doped carbon aerogel nanoparticles: mechanisms and microbial community evolution. Sci. Total. Environ. 803, 149866. doi: 10.1016/j.scitotenv.2021.149866
Li, H., Yang, Y. X., Li, H., Wang, W., Zheng, H., and Tao, J. M. (2022). Genome-wide identification of glutathione S-transferase and expression analysis in response to anthocyanin transport in the flesh of the new teinturier grape germplasm ‘Zhongshan-HongYu’. Int. J. Mol. Sci. 23, 7717. doi: 10.3390/ijms23147717
Lim, J. D., Hahn, S. J., Yu, C. Y., and Chung, I. M. (2005). Expression of the glutathione S-transferase gene (NT107) in transgenic Dianthus superbus. Plant Cell Tiss. Org. 80, 277–286. doi: 10.1007/s11240-004-1032-6
Lin, C. Y., Trinh, N. N., Fu, S. F., Hsiung, Y. C., Chia, L. C., Lin, C. W., et al. (2013). Comparison of early transcriptome responses to copper and cadmium in rice roots. Plant Mol. Biol. 81, 507–522. doi: 10.1007/s11103-013-0020-9
Liu, Y. J., Han, X. M., Ren, L. L., Yang, H. L., and Zeng, Q. Y. (2013). Functional divergence of the glutathione S-transferase supergene family in Physcomitrella patens reveals complex patterns of large gene family evolution in land plants. Plant Physiol. 161, 773–786. doi: 10.1104/pp.112.205815
Liu, X. Y., Hou, Z. L., Zhang, Y. Y., Merchant, A., Zhong, M. E., Ma, G. L., et al. (2023). Cloning and functional characterization of a tau class glutathione transferase associated with haloxyfop-P-methyl resistance in Digitaria sanguinalis. Pest Manag Sci. 79, 3950–3958. doi: 10.1002/ps.7588
Liu, X. F. and Li, J. Y. (2002). Characterization of an ultra-violet inducible gene that encodes glutathione S-transferase in Arabidopsis thaliana. Yi Chuan Xue Bao 29, 458–460. doi: 10.1007/s11769-002-0028-6
Livak, K. J. and Schmitten, T. D. (2001). Analysis of relative gene expression data using real-time quantit-ative PCR and the 2–ΔΔCt method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Marrs, K. A. and Walbot, V. (1997). Expression and RNA splicing of the maize glutathione S-transferase Bronze2 gene is regulated by cadmium and other stresses. Plant Physiol. 113, 93–102. doi: 10.1104/pp.113.1.93
Mauch, F. and Dudler, R. (1993). Differential induction of distinct glutathione-S-transferases of wheat by xenobiotics and by pathogen attack. Plant Physiol. 102, 1193–1201. doi: 10.1104/pp.102.4.1193
McGonigle, B., Keeler, S. J., Lau, S. M., Koeppe, M. K., and O’Keefe, D. P. (2000). A genomics approach to the comprehensive analysis of the glutathione S-transferase gene family in soybean and maize. Plant Physiol. 124, 1105–1120. doi: 10.1104/pp.124.3.1105
Meng, H. J., Zhao, J. N., Yang, Y. F., Diao, K. H., Zheng, G. S., Li, T., et al. (2023). PeGSTU58, a glutathione S-transferase from Populus euphratica, enhances salt and drought stress tolerance in transgenic arabidopsis. Int. J. Mol. Sci. 24, 9354. doi: 10.3390/ijms24119354
Mo, Z. J., Huang, Y., Pu, T. X., Duan, L. L., Pi, K., Luo, J. J., et al. (2023). Genome-wide identification and characterization of glutathione S-transferases (GSTs) and their expression profile under abiotic stresses in tobacco (Nicotiana tabacum L.). BMC Genomics 24, 341. doi: 10.1186/s12864-023-09450-x
Moons, A. (2005). Regulatory and functional interactions of plant growth regulators and plant glutathione S-transferases (GSTs). Vitam. Horm. 72, 155–202. doi: 10.1016/S0083-6729(05)72005-7
Monticolo, F., Colantuono, C., and Chiusano, M. L. (2017). Shaping the evolutionary tree of green plants: evidence from the GST family. Sci. Rep. 7, 14363. doi: 10.1038/s41598-017-14316-w
Moons, A. (2003). Osgstu3 and osgtu4 encoding tau class glutathione S-transferases are heavy metal and hypoxic stress-induced and differentially salt stress-responsive in rice roots. FEBS Lett. 553, 427–432. doi: 10.1016/s0014-5793(03)01077-9
Nair, P. M. and Choi, J. (2011). Identification characterization and expression profiles of Chironomus riparius glutathione S-transferase (GST) genes in response to cadmium and silver nanoparticles exposure. Aquat Toxicol. 101, 550–560. doi: 10.1016/j.aquatox.2010.12.006
Norton, G. J., Lou-Hing, D. E., Meharg, A. A., and Price, A. H. (2008). Rice-arsenate interactions in hydroponics: whole genome transcriptional analysis. J. Exp. Bot. 59, 2267–2276. doi: 10.1093/jxb/ern097
Park, J. C., Lee, M. C., Yoon, D. S., Han, J., Park, H. G., Hwang, U. K., et al. (2019). Genome-wide identification and expression of the entire 52 glutathione S-transferase (GST) subfamily genes in the Cu2+-exposed marine copepods Tigriopus japonicus and Paracyclopina nana. Aquat Toxicol. 209, 56–69. doi: 10.1016/j.aquatox.2019.01.020
Perperopoulou, F., Pouliou, F., and Labrou, N. E. (2018). Recent advances in protein engineering and biotechnological applications of glutathione transferases. Crit. Rev. Biotechnol. 38, 511–528. doi: 10.1080/07388551.2017.1375890
Pinkus, R., Weiner, L. M., and Daniel, V. (1996). Role of oxidants and antioxidants in the induction of AP-1 NF-kappaB and glutathione S-transferase gene expression. J. Biol. Chem. 271, 13422–13429. doi: 10.1074/jbc.271.23.13422
Rezaei, M. K., Shobbar, Z. S., Shahbazi, M., Abedini, R., and Zare, S. (2013). Glutathione S-transferase (GST) family in barley: identification of members enzyme activity and gene expression pattern. J. Plant Physiol. 170, 1277–1284. doi: 10.1016/j.jplph.2013.04.005
Sabir, I. A., Manzoor, M. A., Shah, I. H., Liu, X., Jiu, S., Wang, J., et al. (2022). Identification and comprehensive genome-wide analysis of glutathione S-transferase gene family in sweet cherry (Prunus avium) and their expression profiling reveals a likely role in anthocyanin accumulation. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.938800
Sappl, P. G., Carroll, A. J., Clifton, R., Lister, R., Whelan, J., Millar, A. H., et al. (2009). The Arabidopsis glutathione transferase gene family displays complex stress regulation and co-silencing multiple genes results in altered metabolic sensitivity to oxidative stress. Plant J. 58, 53–68. doi: 10.1111/j.1365-313X.2008.03761.x
Sharma, R., Sahoo, A., Devendran, R., and Jain, M. (2014). Over-expression of a rice tau class glutathione s-transferase gene improves tolerance to salinity and oxidative stresses in Arabidopsis. PloS One 9, e92900. doi: 10.1371/journal.pone.0092900
Shen, M., Zhao, D. K., Qiao, Q., Liu, L., Wang, J. L., Cao, G. H., et al. (2015). Identification of glutathione S-transferase (GST) genes from a dark septate endophytic fungus (Exophiala pisciphila) and their expression patterns under varied metals stress. PloS One 10, e0123418. doi: 10.1371/journal.pone.0123418
Shi, X., Wang, S. F., Chen, Y. T., Xu, Q. D., Sun, H. J., An, R., et al. (2019). Tolerance and vegetation restoration prospect of seedlings of five oak species for Pb/Zn mine tailing. Ying Yong Sheng Tai Xue Bao 30, 4091–4098. doi: 10.13287/j.1001-9332.201912.039
Shi, X., Wang, S. F., Sun, H. J., Chen, Y. T., Wang, D. X., Pan, H. W., et al. (2017). Comparative of Quercus spp. and Salix spp. for phytoremediation of Pb/Zn mine tailings. Environ. Sci. pollut. Res. Int. 24, 3400–3411. doi: 10.1007/s11356-016-7979-0
Shokat, S., Großkinsky, D. K., Roitsch, T., and Liu, F. (2020). Activities of leaf and spike carbohydrate-metabolic and antioxidant enzymes are linked with yield performance in three spring wheat genotypes grown under well-watered and drought conditions. BMC Plant Biol. 20, 400. doi: 10.1186/s12870-020-02581-3
Sobkowiak, R. and Deckert, J. (2006). Proteins induced by cadmium in soybean cells. J. Plant Physiol. 163, 1203–1206. doi: 10.1016/j.jplph.2005.08.017
Song, W., Zhou, F., Shan, C. H., Zhang, Q., Ning, M., Liu, X. M., et al. (2021). Identification of glutathione S-transferase genes in Hami melon (Cucumis melo var. saccharinus) and their expression analysis under cold stress. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.672017
Takenaka, Y., Haga, N., Inoue, I., Nakano, T., Ikeda, M., Katayama, S., et al. (2014). Identification of two nickel ion-induced genes NCI16 and PcGST1 in Paramecium caudatum. Eukaryot Cell 13, 1181–1190. doi: 10.1128/EC.00112-14
Wang, L. L., Fu, H. B., Zhao, J., Wang, J. G., Dong, S. Q., Yuan, X. Y., et al. (2023). Genome-wide identification and expression profiling of glutathione S-transferase gene family in Foxtail Millet (Setaria italica L.). Plants 12, 1138. doi: 10.3390/plants12051138
Wang, W. B., He, X. F., Yan, X. M., Ma, B., Lu, C. F., Wu, J., et al. (2023). Chromosome-scale genome assembly and insights into the metabolome and gene regulation of leaf color transition in an important oak species, Quercus dentata. New Phytol. 238, 2016–2032. doi: 10.1111/nph.18814
Wang, R. B., Ma, J. F., Zhang, Q., Wu, C. L., Zhao, H. Y., Wu, Y. N., et al. (2019). Genome-wide identification and expression profiling of glutathione transferase gene family under multiple stresses and hormone treatments in wheat (Triticum aestivum L.). BMC Genomics 20, 986. doi: 10.1186/s12864-019-6374-x
Wang, T. Z., Zhang, D., Chen, L., and Wang, J. (2022). and Zhang, W.H. Genome-wide analysis of the Glutathione S-Transferase family in wild Medicago ruthenica and drought-tolerant breeding application of MruGSTU39 gene in cultivated alfalfa. Theor. Appl. Genet. 135, 853–864. doi: 10.1007/s00122-021-04002-x
Wang, J. R., Zhang, Z. D., Wu, J. H., Huang, X. Y., Wang-Pruski, G. F., and Zhang, Z. Z. (2020). Genome-wide identification characterization and expression analysis related to autotoxicity of the GST gene family in Cucumis melo L. Plant Physiol. Biochem. 155, 59–69. doi: 10.1016/j.plaphy.2020.06.046
Xu, L., Chen, W., Si, G. Y., Huang, Y. Y., Lin, Y., Cai, Y. P., et al. (2017). Genome wide analysis of the GST gene family in Gossypium hirsutum L. Yi Chuan 39, 737–752. doi: 10.16288/j.yczz.16-435
Xu, P. F., Han, N. N., Kang, T. H., Zhan, S., Lee, K. S., Jin, B. R., et al. (2016). SeGSTo a novel glutathione S-transferase from the beet armyworm (Spodoptera exigua) involved in detoxification and oxidative stress. Cell Stress Chaperones 21, 805–816. doi: 10.1007/s12192-016-0705-5
Xu, J., Tian, Y. S., Xing, X. J., Peng, R. H., Zhu, B., Gao, J. J., et al. (2016). Over-expression of AtGSTU19 provides tolerance to salt drought and methyl viologen stresses in Arabidopsis. Physiol. Plant 156, 164–175. doi: 10.1111/ppl.12347
Xue, L., Huang, X. R., Zhang, Z. H., Lin, Q. H., Zhong, Q. Z., Zhao, Y., et al. (2022). An anthocyanin-related glutathione S-transferase, MrGST1, plays an essential role in fruit coloration in Chinese bayberry (Morella rubra). Front. Plant Sci. 13, 903333. doi: 10.3389/fpls.2022.903333
Yang, G. Y., Xu, Z. G., Peng, S. B., Sun, Y. D., Jia, C. X., and Zhai, M. Z. (2016). In planta characterization of a tau class glutathione S-transferase gene from Juglans regia (JrGSTTau1) involved in chilling tolerance. Plant Cell Rep. 35, 681–692. doi: 10.1007/s00299-015-1912-8
Yang, G. Y., Chen, S. W., Li, D. P., Gao, X. Q., Su, L. Y., Peng, S. B., et al. (2019). Multiple transcriptional regulation of walnut JrGSTTau1 gene in response to osmotic stress. Physiol. Plant. 166, 748–761. doi: 10.1111/ppl.12833
Zhang, M. J., Zhang, Y. Z., Wang, Y., Xian, C. C., Cui, X. M., Xie, B. Q., et al. (2023). Desiccation sensitivity characteristics and low-temperature storage of recalcitrant Quercus variabilis seed. Forests 14, 1837. doi: 10.3390/f14091837
Zhao, Y. W., Wang, C. K., Huang, X. Y., and Hu, D. G. (2021). Genome-wide analysis of the glutathione S-transferase (GST) genes and functional identification of MdGSTU12 reveals the involvement in the regulation of anthocyanin accumulation in apple. Genes (Basel) 12, 1733. doi: 10.1016/j.ijbiomac.2021.11.038
Keywords: glutathione S-transferases, Quercus dentata, heavy metal, cadmium, lead, gene family
Citation: Sha J, Wu X, Shen A, Pang X, Wang W, Leng P, Hu Z, Zhao Y and He X (2025) Genome-wide identification, expression and functional analysis of glutathione s-transferase family members in Quercus dentata under heavy metal stresses. Front. Plant Sci. 16:1641553. doi: 10.3389/fpls.2025.1641553
Received: 05 June 2025; Accepted: 12 August 2025;
Published: 09 September 2025.
Edited by:
Fei Shen, Beijing Academy of Agricultural and Forestry Sciences, ChinaReviewed by:
Ning Xu, China Agricultural University, ChinaRuchika Rajput, Oak Ridge National Laboratory (DOE), United States
Copyright © 2025 Sha, Wu, Shen, Pang, Wang, Leng, Hu, Zhao and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangfeng He, aHhmNzkxMjMwQDE2My5jb20=; Yazhou Zhao, eWF6aG91MTY2N0BzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Jingjing Sha†
Jingjing Sha† Xiangyue Wu
Xiangyue Wu Xin Pang
Xin Pang Pingsheng Leng
Pingsheng Leng Zenghui Hu
Zenghui Hu Xiangfeng He
Xiangfeng He