- Xinjiang Production and Construction Corps Key Laboratory of Protection and Utilization of Biological Resources in Tarim Basin, College of Life Science and Technology, Tarim University, Alar, Xinjiang, China
Introduction: The REGULATOR OF CHLOROPLAST BIOGENESIS (RCB) is a novel protein component in plant temperature signaling that functions by synergizing with HEMERA (HMR) to initiate thermomorphogenesis by stabilizing PHYTOCHROME INTERACTING TRANSCRIPTION FACTOR 4 (PIF4) during the day.
Methods: In this study, we successfully cloned the heat-responsive gene KcRCB from Karelinia caspia, a desert-adapted plant species. KcRCB transcript levels were significantly elevated when the plants were exposed to high temperatures. Furthermore, KcRCB demonstrated differential expression in the Karelinia caspia roots, stems, and leaves, with optimal expression in the leaves. Subsequently, KcRCB transgene was overexpressed in Arabidopsis thaliana and cotton plants to characterize its thermomorphogenesis effects. In comparison with the wild-type Arabidopsis thaliana plants, KcRCB-overexpressing Arabidopsis thaliana plants exhibited reduced incidence of leaf damage and enhanced capacity to withstand elevated temperatures. KcRCB-overexpressing cotton plants subjected to elevated temperatures also exhibited reduced leaf damage.
Results and Discussion: Physiological assays demonstrated that KcRCB expression enhances plant resilience to high-temperature stress by maintaining cell membrane stability and reducing the accumulation of reactive oxygen species (ROS). Moreover, we observed increased stomatal density and opening in the leaves of the KcRCB overexpressing lines compared to the control group when exposed to high temperatures. Subcellular localization experiments showed that KcRCB was localized to the stomatal guard cell membranes. This suggested that KcRCB protects plant cells from high-temperature-related damage by regulating stomatal openness, increasing the transpiration rate, and improving the efficiency of heat dissipation, thereby. These findings enhance the understanding of the mechanisms underlying high-temperature tolerance in the desert plant species. Specifically, this study expands our understanding regarding the biological roles of KcRCB and the molecular regulatory networks underlying heat stress responses in Karelinia caspia.
1 Introduction
In recent years, global warming has become a serious problem and is expected to significantly affect crop yields by 2040 (Challinor et al., 2014). Higher temperatures can significantly impact crop growth (Zhao et al., 2017), as well as plant phenology, distribution, and diversity, thereby significantly reducing crop productivity (Arnold et al., 2019; Rathore et al., 2020; Sadok et al., 2020). Plant photosynthesis is irreversibly damaged at temperatures beyond the critical threshold for plant growth (Bernacchi et al., 2025). Plants have an inherent ability to perceive and respond to environmental fluctuations and stresses (Lamers et al., 2020). Plants respond and adapt to rising temperatures by undergoing morphological changes through a process known as thermomorphogenesis (Franklin et al., 2011; Quint et al., 2016). The mechanisms underlying thermomorphogenesis and identification of heat responsive genes are current hotspot areas in plant research. Arabidopsis thaliana is the commonly used plant for studying thermal morphogenesis. It exhibits optimal growth at 22°C, but temperatures above 29°C significantly affect its growth, distort its structure and morphology, reduce seed yield, and cause significant temperature-related morphological adjustments (Gil and Park, 2018). The specific high temperature-related characteristics include elongation of hypocotyls, petioles, and stem, and leaf drooping (Casal and Balasubramanian, 2019). In terrestrial eudicots, elongation of hypocotyls and petioles represent significant thermal morphological responses of seedlings. For example, sustained warm temperatures cause elongation of hypocotyls and petioles in Arabidopsis thaliana (Blázquez et al., 2003; Crawford et al., 2012). Enhanced blade evaporative cooling is an adaptation to alleviate high temperatures (Blázquez et al., 2003). Furthermore, higher temperatures also lead to an increased ratio between stomata and epidermal cells, as well as narrower and longer taproots. Moreover, continuous higher temperatures will reduce the stomatal index (Legris et al., 2017; Pérez-Bueno et al., 2022).
PIF4 plays a key regulatory role in thermomorphogenesis when Arabidopsis thaliana plants are exposed to elevated temperatures. PIF4 directly regulates the expression of auxin biosynthesis genes and members of the SAUR (SMALL AUXIN UP RNA) gene family, leading to cell elongation and phenotypes such as elongated hypocotyls (Sun et al., 2016). PIF4 also interacts with MRG1/2 (MORF-RELATED GENE 1/2) to facilitate transcription of heat-responsive genes through histone modifications; concurrently, MRG2 enhances its regulatory function by stabilizing the PIF4 protein (Zhou et al., 2024). Furthermore, HSFA1s (HEAT SHOCK TRANSCRIPTION FACTOR A1s) promote thermomorphogenesis during high daytime temperatures by stabilizing PIF4 (Tan et al., 2023). HSFB2a/B2b (HEAT SHOCK TRANSCRIPTION FACTOR B2a/B2b) are negative regulators of heat stress responses. They undergo ubiquitin-mediated degradation by interacting with XBAT31 (XB3 ORTHOLOG 1 IN ARABIDOPSIS THALIANA), an E3 ligase. This enhances the expression of heat stress-responsive genes and improves plant thermotolerance (Zhang et al., 2024). MicroRNAs (miRNAs) also play a critical role in the regulation of plant growth and various stress response processes (Huang et al., 2025). During heat stress, most miRNAs enhance nuclear-localization of HYL1 (HYPONASTIC LEAVES 1) to induce plant tolerance (Cao et al., 2025). The SUMO E3 ligase SIZ1 participates in the post-transcriptional regulation of thermomorphogenesis by mediating SUMOylation of CPSF100 (CLEAVAGE AND POLYADENYLATION SPECIFICITY FACTOR 100), which in turn modulates the poly(A) site usage of key thermomorphogenesis-related genes (Yu et al., 2024). When exposed to higher temperatures, PIF4 accumulates in the stomatal precursors and binds to the promoter of SPCH (SPEECHLESS), a master regulator of stomatal lineage initiation, thereby repressing SPCH expression and reducing the formation of stomata (Lau et al., 2018). The transcription factor HSFA1b functions as a heat sensor by modulating stomatal movement under high-temperature stress through inhibition of the kinase activity of OST1 (OPEN STOMATA 1) via its intrinsic adenylate cyclase activity (Zhang et al., 2025). These genes and their interactions collectively form a complex regulatory network that precisely modulates thermomorphogenesis in Arabidopsis thaliana.
Thermomorphogenesis in Arabidopsis thaliana during both day and night is triggered by the accumulation of PIF4, a central temperature regulator induced by warm temperatures. During the day, PIF4 requires specific stabilization to avoid degradation promoted by active PHYB (Foreman et al., 2010). This stabilization of PIF4 during the day depends on its interaction with HMR, a transcription activator (Qiu et al., 2019). A forward genetic screen using suppressors of the hmr-22 allele resulted in the identification of a novel daytime temperature signaling component called as RCB, which is involved in the modulation of chloroplast biogenesis (Qiu et al., 2021).
In Arabidopsis thaliana, non-catalytic thioredoxins are by-products of NUCLEAR CONTROL OF PEP (NCP) activity. They perform distinct and crucial roles in phytochrome signaling across seed plants (Yang et al., 2019). RCB, also known as MRL7, is a thioredoxin, which acts in concert with HMR to initiate thermomorphogenesis by selectively stabilizing PIF4 during the daytime (Qiu et al., 2019, 2021). Plants contain six primary types of thioredoxin proteins (Trx-f, Trx-m, Trx-x, Trx-y, Trx-h, and Trx-o). Different plant cell compartments contain distinct types of thioredoxins. For example, chloroplasts in plants contain Trx-f, Trx-m, Trx-x, and Trx-y (Yua et al., 2014).
Chloroplasts play a critical role in plant responses to environmental stresses (Gan et al., 2019). Under stressful conditions, chloroplasts mediate retrograde signaling and transmit information from the plastids to the nucleus (Song et al., 2021), thereby optimizing nuclear gene expression according to the environmental conditions (Leister et al., 2017). Therefore, chloroplasts regulate transcription and translation of several nuclear genes and modulate specific adaptive responses to environmental stresses. These regulatory mechanisms help plants to survive and grow under stressful conditions. Chloroplast development is a complex process involving multiple proteins. MRL7 is a chloroplast-associated protein in Arabidopsis thaliana. MRL7 performs essential functions in chloroplast development and in plastid gene expression (Qiao et al., 2011).
Karelinia caspica is a perennial herb belonging to the genus Karelinia of the Asteraceae family. It thrives at the edge of deserts and is characterized by flat and distinctly succulent leaves and well-developed water-storage tissues. Therefore, it demonstrates high tolerance to elevated temperatures and drought (Zhang et al., 2014). Karelinia caspica can withstand temperatures exceeding 40°C. The inflection point in its tolerance duration at 45°C is approximately 8 hours, whereas its maximum tolerance duration at 50°C is about 1 hour. However, the molecular mechanisms underlying its adaptation to extreme environments remain unclear (Wang et al., 2021). RCB gene, a key player in the thermomorphogenesis mechanisms in Arabidopsis thaliana, is conserved across plant species and plays a critical role in chloroplast development and temperature signal transduction (Qiu et al., 2021). In Arabidopsis thaliana, RCB modulates thermomorphogenesis by synergizing with HEMERA to stabilize PIF4. As a desert plant, Karelinia caspica persists in an environment characterized by intense light and extreme temperatures year-round. Therefore, it requires robust chloroplast biogenesis regulatory machinery to maintain photosynthetic efficiency (Zeng et al., 2021). Our transcriptome analysis of Karelinia caspica under high-temperature stress demonstrates upregulation of KcRCB. Therefore, we hypothesized that KcRCB regulates thermotolerance in Karelinia caspica.
There have been significant advances in plant thermotolerance research across diverse plant taxa (Kan et al., 2023). However, mechanistic insights into heat adaptation in the desert plant Karelinia caspica are unclear. Discovery of the RCB gene has facilitated further investigations into the regulatory networks underlying thermotolerance in Karelinia caspica. As a keystone species at desert margins, K. caspica excels in desert edge stabilization and plays a pivotal role in mitigating land desertification through its ecological functions. In this study, we cloned the KcRCB gene and analyzed its expression using the high-temperature transcriptome dataset of Karelinia caspica. To evaluate the high-temperature tolerance of KcRCB from Karelinia caspica, we expressed KcRCB in Arabidopsis thaliana and cotton and performed physiological experiments.
2 Results
2.1 Cloning and analysis of the KcRCB gene based on transcriptome data of high-temperature-treated Karelinia caspica
The transcriptome of high temperature-treated Karelinia caspica was compared with the RCB gene sequence from Arabidopsis thaliana to identify the homologous gene. The full-length KcRCB coding sequence (CDS) was amplified by PCR. The KcRCB CDS was 1005 bp in length and encoded 334 amino acids (Supplementary Figure 1). Based on the amino acid composition, molecular formula of KcRCB protein was predicted to be C1727H2703N487O526 S9 by ProtParam. Its molecular mass was 38,992.95 and the theoretical isoelectric point (IEP) was 5.57. It consisted of 65 positively charged residues and 5.57 negatively charged residues. ProtScale analysis showed that the KcRCB protein had a maximum hydrophobicity value of 2.144 and a maximum hydrophilicity value of -3.678, indicating that most of its amino acids were hydrophilic.
Phylogenetic tree construction and conserved domain analysis demonstrated high sequence identify of the amino acid sequence of KcRCB with the RCB proteins of various plant species, including Lactuca sativa (82.49% identity), Cichorium endivia (81.90% identity), Cynara cardunculus (79.06% identity), Mikania micrantha (78.81% identity), Arctium lappa (78.44% identity), Tagetes erecta (77.19%), Nicotiana tomentosiformis (70.53%), and Arabidopsis thaliana (67.05% identity). Phylogenetic analysis using MEGA 11.0 for sequence alignment combined with MEME-based motif prediction demonstrated that KcRCB was most closely related with the RCB proteins from Lactuca sativa and Cichorium endivia (Asteraceae). KcRCB contains 9 conserved motifs. Among these, motifs 8 and 10 are additional to those found in the RCB protein from Arabidopsis thaliana. Compared with the RCB protein from cotton, KcRCB possesses an extra motif 8 but lacks motif 7; compared to the soybean RCB, it contains additional motifs 7 and 8; compared to maize RCB, it has extra motifs 3, 7, and 8. These differences in motif composition suggest potential functional divergence of RCB proteins across different species (Figure 1).

Figure 1. Phylogenetic analysis of RCB proteins in different plant species and identification of protein structure motifs.
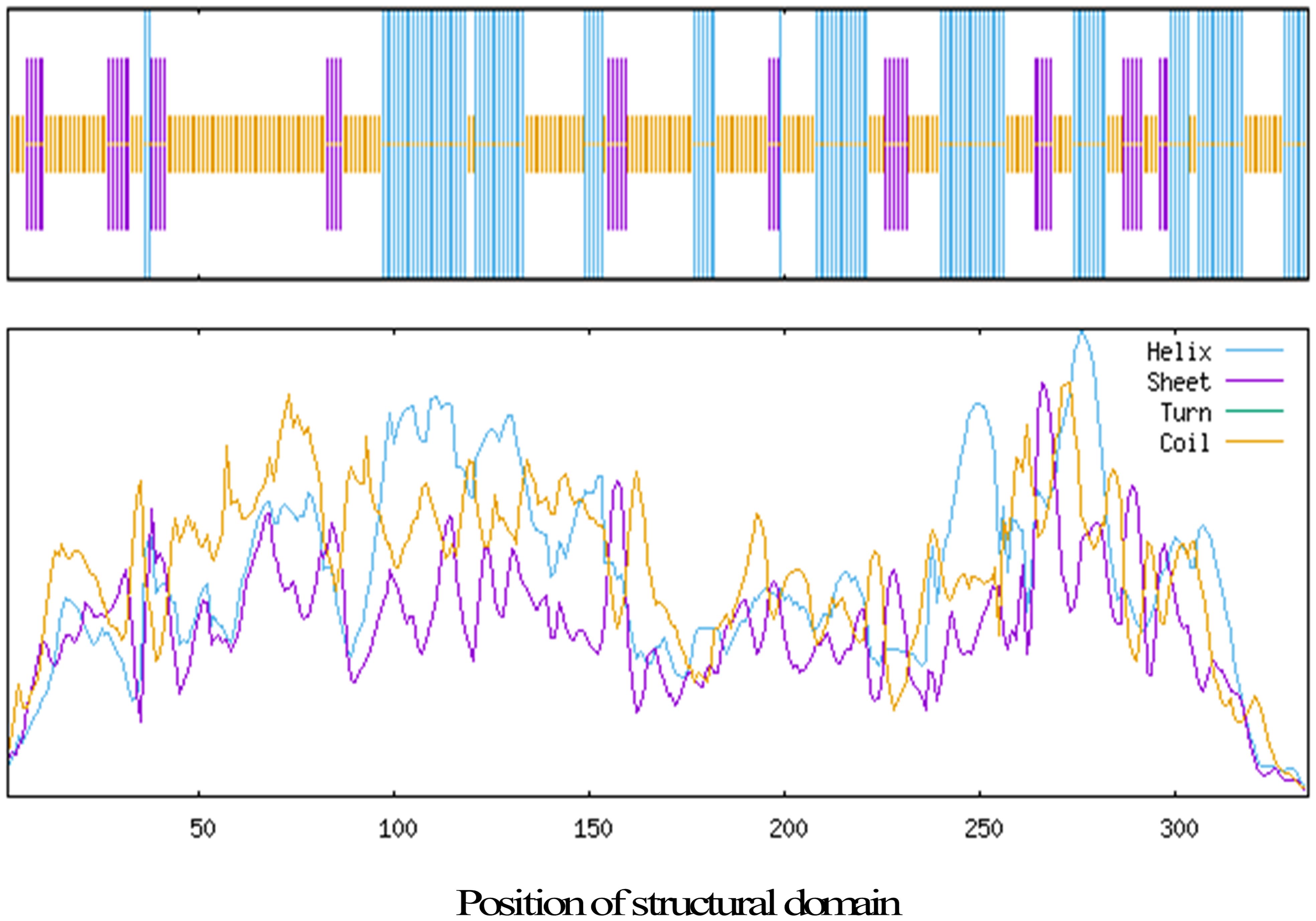
We then analyzed the secondary structure of the KcRCB protein using SignalP 5.0 and TMHMM online tools and found that both signal peptides and transmembrane domains were absent. SOPMA-based secondary structure prediction showed that KcRCB protein primarily consists of 49.10% random coils, followed by α-helices at 32.04%, extended strands at 14.37%, and β-turns at 4.49% (Figure 2).
Domain architecture analysis of KcRCB using InterPro and Prosite identified a thioredoxin-like domain, which shared homology with RCB orthologs from other plant species (Supplementary Figure 2). Sequence alignment of KcRCB with RCB proteins from ten additional plant species (Supplementary File) confirmed high conservation of this thioredoxin-like domain, despite several species-specific amino acid variations as follows within the domain: (1) KcRCB at position 236 contains aspartic acid (an acidic residue), whereas all other analyzed species possess a basic amino acid at this site; (2) amino acid at position 257 in KcRCB and tobacco homologs is occupied by serine (a hydroxyl-containing residue) compared to asparagine (an amide) in Arabidopsis thaliana; (3) glutamine (an amide) at position 302 in KcRCB is substituted by glutamate, serine, threonine, proline, leucine, or lysine in other homologs; (4) lysine (a basic residue) at position 306 in KcRCB is replaced by threonine (hydroxyl-containing) in Arabidopsis thaliana and tobacco RCBs, and by serine (hydroxyl-containing) or methionine (sulfur-containing) in other species. These conserved domain variations may contribute to functional divergence of RCB proteins across plant species.
2.2 Analysis of the expression pattern of KcRCB in Karelinia caspia (Pall.) Less.
Phenotypic changes in the Karelinia caspica seedlings under a high temperature stress of 45°C are shown in Figure 3A. After 5 min of high temperature treatment, Karelinia caspica leaves began to exhibit thermomorphic changes and demonstrated upturning. After 30 min of high temperature treatment, Karelinia caspica exhibited more obvious thermomorphic changes with most of the leaves gathering at the center. At 120 min of high temperature treatment, the leaves began to spread slowly. By 240 min of high temperature treatment, the leaves spread to a degree close to the untreated state.
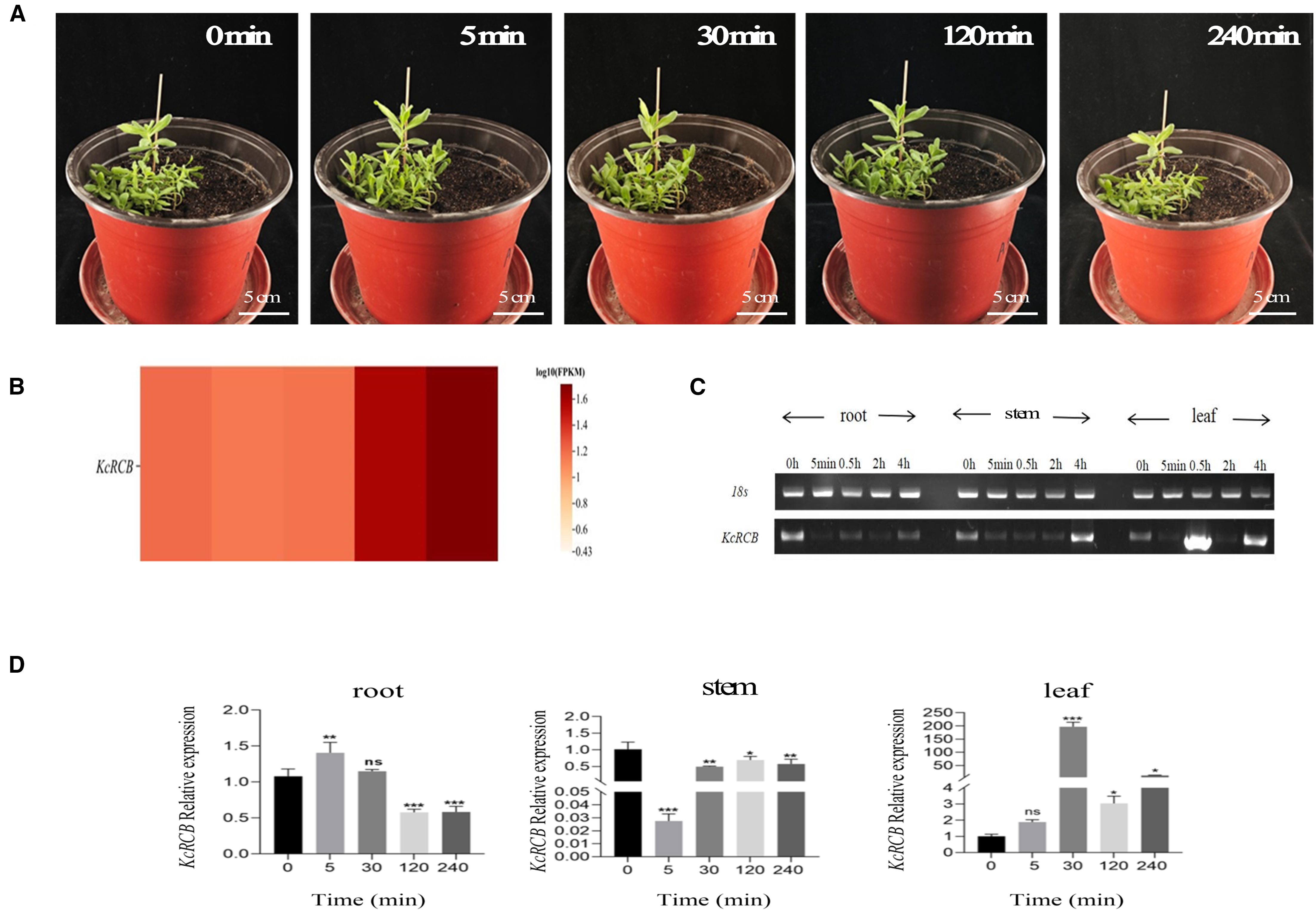
Figure 3. Phenotypic analysis of Karelinia caspia during 45°C heat treatment and the expression profile of KcRCB. (A) Representative images of Karelinia caspica phenotype at 0, 5, 30, 120, and 240 min of heat treatment at 45°C. (B) Heatmap of KcRCB expression in Karelinia caspica at 0, 5, 30, 120, and 240 min of heat treatment at 45°C. (C) RT-PCR analysis of KcRCB gene expression in the root, stem, and leaf tissues of Karelinia caspica at different time points during heat treatment at 45°C. (D) qRT-PCR analysis of KcRCB gene expression in the root, stem, and leaf tissues of Karelinia caspica at different time points during heat treatment at 45°C. Data are presented as the mean ± standard deviation (SD) of three biological replicates. ns, no significant difference (P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001.
Subsequently, we performed transcriptome sequencing of the high temperature-treated Karelinia caspica leaves and used the heatmap to evaluate KcRCB gene expression. The calculated FPKM value of KcRCB showed dynamic changes over time in the leaves of plants subjected to heat stress 45°C. As shown in Figure 3B, FPKM of KcRCB initially decreased from 1.21 to 1.16 at 5 min post-treatment, followed by a gradual increase to 1.18 at 30 min, 1.58 at 120 min, and 1.76 at 240 min.
We then performed RT-PCR expression analysis of Karelinia caspica plants subjected to high-temperature stress. KcRCB exhibited enhanced heat tolerance at 45°C, especially in the leaf tissues. Overall, KcRCB expression was lower in the roots compared to the control, thereby indicating that KcRCB did not function significantly in the roots. In the stem, KcRCB expression decreased initially, followed by a gradual increase with the time of higher temperature treatment. In the leaves, KcRCB gene expression showed a fluctuating trend with increasing treatment time and reached maximum expression at 30 min (Figure 3C).
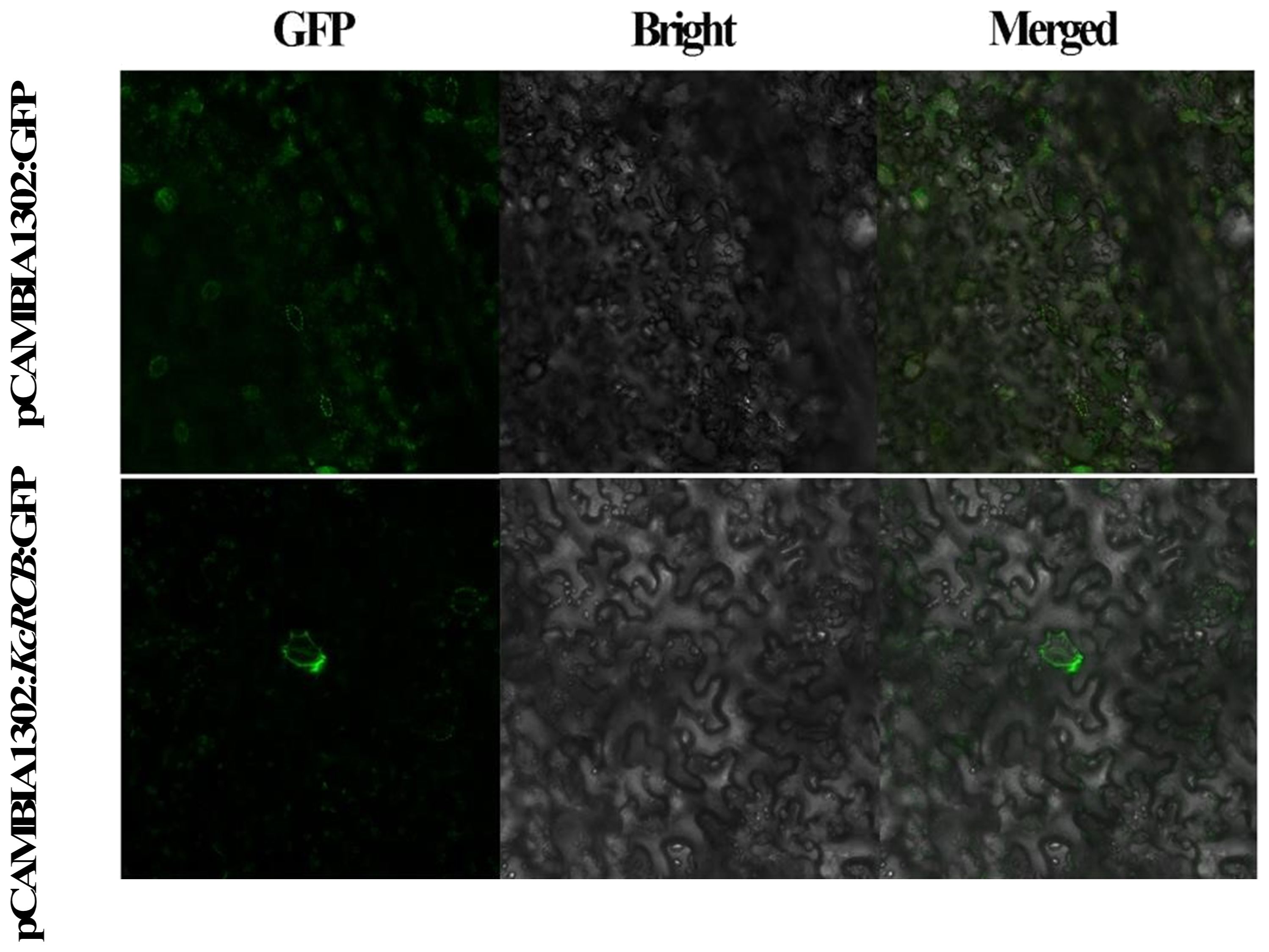
We then performed real-time quantitative PCR (RT-qPCR) analysis and found that the relative expression of KcRCB was low in the roots and decreased with increasing high temperature treatment time. In the stem, relative expression of KcRCB decreased initially, but then increased as the time of higher temperature treatment progressed. The relative expression levels of KcRCB transcripts in the stem were significantly lower than those before treatment. In the leaves, relative expression levels of KcRCB transcripts increased at 30 min of treatment, then decreased briefly at 120 min, and increased again at 240 min to reach a maximum. Relative expression of KcRCB in the leaves reached maximum level at 30 min of treatment, then decreased at 120 min, and subsequently increased again to nearly the same maximal level at 240 min (Figure 3D). This demonstrated that KcRCB responded significantly to high temperatures in the leaves. The subcellular localization analysis in tobacco demonstrated that KcRCB was localized in the leaf stomatal guard cell membrane (Figure 4).
2.3 Overexpression of KcRCB enhances the heat tolerance of Arabidopsis thaliana
Wild-type and KcRCB-overexpressing (KcRCB-OE) transgenic Arabidopsis thaliana plants were cultivated until they reached the seedling stage. Then, they were placed in a 45°C artificial climate chamber to undergo high-temperature stress treatment. As shown in Figure 5A, there were no differences in the growth patterns between the wild-type and KcRCB OE2 Arabidopsis thaliana plants cultured at 21°C. However, KcRCB OE1 Arabidopsis thaliana plants grew relatively luxuriantly. After 6 h of high-temperature stress, the leaves of wild-type plants showed severe wilting, but the two KcRCB-overexpression strains (OE1 and OE2) showed less wilting than the wild-type. After high-temperature treatment for 6 h, the wild-type and KcRCB-OE Arabidopsis thaliana plants were transferred to a 21°C incubator and cultivated normally for three days. We observed that all the KcRCB-overexpressing plants grew new leaves, but the wild-type Arabidopsis thaliana plants did not show recovery.
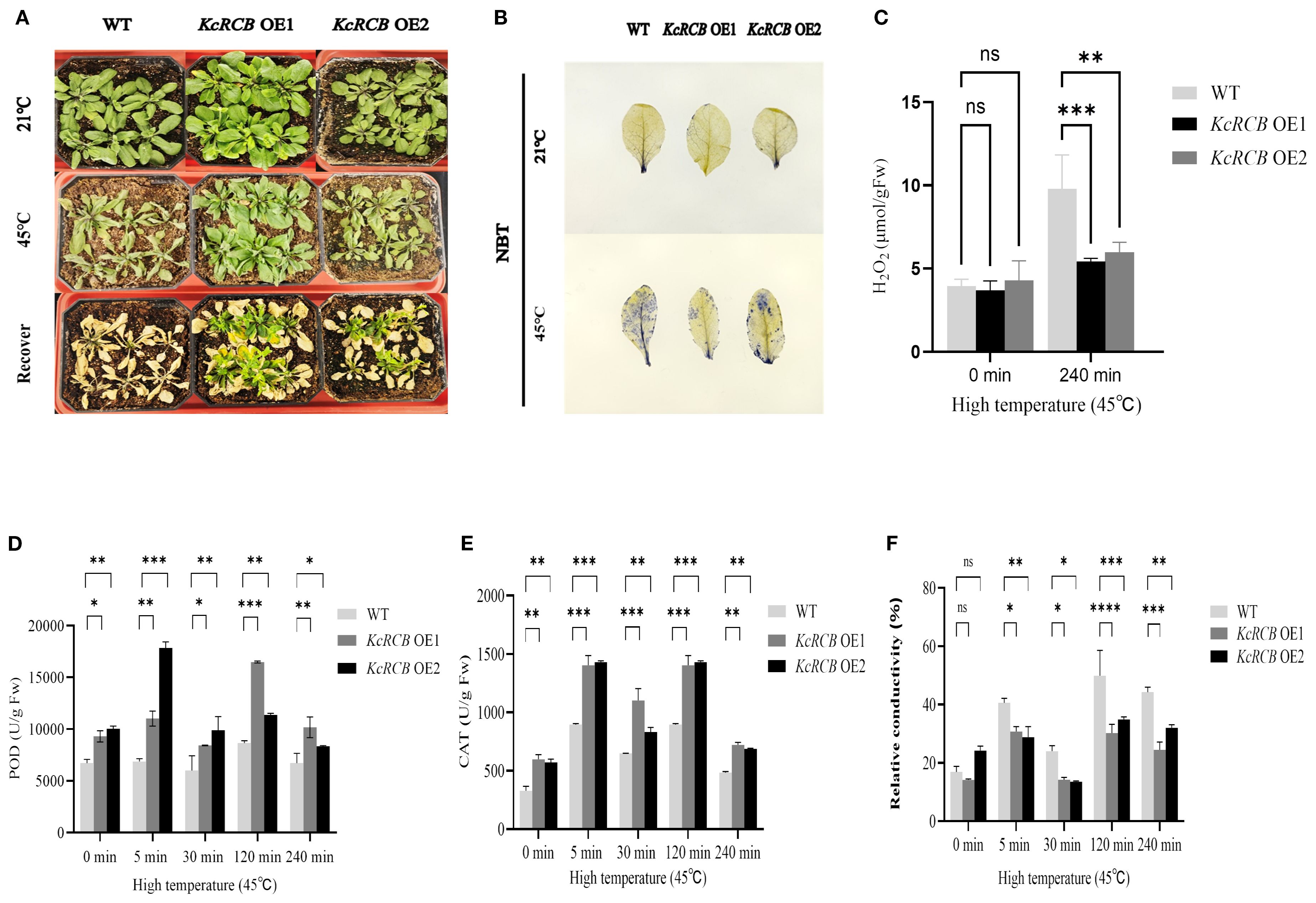
Figure 5. KcRCB overexpression enhances heat tolerance in Arabidopsis thaliana. (A) Comparison of phenotypes in the wild-type Arabidopsis thaliana and KcRCB-overexpressing lines (OE1, OE2) under normal cultivation, heat treatment at 45°C for 6 h, and 3 days of recovery after heat treatment. (B) NBT staining of wild-type Arabidopsis thaliana and KcRCB overexpression lines (OE1, OE2) leaves under normal culture conditions and after heat treatment at 45°C for 240 min. (C) Estimation of H2O2 content in the wild-type and KcRCB-transgenic Arabidopsis thaliana under normal conditions and heat treatment at 45°C for 240 min. (D-E) Estimation of (D) peroxidase (POD) and (E) Catalase (CAT) activity activities in the wild-type and KcRCB-transgenic Arabidopsis thaliana under normal conditions and heat treatment at 45°C. (F) Relative conductivity measurements in the wild-type and KcRCB-transgenic Arabidopsis thaliana under normal conditions and heat treatment at 45°C. Data are presented as the mean ± standard deviation (SD) of three biological replicates. “ns”, no significant difference (P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The KcRCB-OE transgenic plants (OE1 and OE2) and wild-type Arabidopsis thaliana plants were transplanted into soil and cultivated until the seedling stage. They were subjected to heat stress treatment at 45°C. Then, we performed NBT staining and H2O2 estimation in the leaves of plants cultivated at 21°C (control) and those subjected to 240 min heat treatment at 45°C. Furthermore, we collected leaf tissues from each group at 0, 5, 30, 120, and 240 min after the onset of heat treatment and measured catalase (CAT) and peroxidase (POD) activities, as well as relative electrical conductivity.
NBT staining did not show significant differences between wild-type and KcRCB-OE plants under normal cultivation conditions (Figure 5B). However, after 240 min of heat stress treatment, we observed significant concentrations of superoxide anions in the entire leaf of the wild-type plants, but significantly lower levels of superoxide anions in the leaves of KcRCB OE1 and KcRCB OE2 Arabidopsis thaliana plants. Moreover, ROS levels were slightly higher in the leaves of KcRCB OE2 plants than those of the KcRCB OE1 plants.
We then estimated H2O2 levels in the wild-type and KcRCB-OE Arabidopsis thaliana leaves before and after high-temperature treatment (Figure 5C). We did not observe any significant differences in the H2O2 levels content between the leaves of the wild-type and KcRCB-OE lines before high-temperature treatment. However, after 240 min of high-temperature stress, the H2O2 content in the leaves of the wild-type Arabidopsis thaliana plants was significantly higher than that of the overexpression lines. Moreover, H2O2 content in the leaves of the KcRCB OE2 plants was slightly higher than those of the KcRCB OE1 plants.
POD activity reflects plant tolerance to various stresses. POD activity significantly increased at 5 and 120 min and decreased at 240 min in both the wild-type and KcRCB-OE lines (Figure 5D). Catalase activity of the transgenic plants overexpressing KcRCB was consistently higher than those of the wild type, thereby indicating enhanced antioxidant capacity (Figure 5E). Before undergoing high-temperature treatment, there were no significant differences in the relative conductivity between the overexpression lines and the wild type. However, relative conductivity in both the wild-type and KcRCB-OE lines increased after 5 min of heat treatment, then showed a decline at 30 min, and subsequently increased again at 120 min and 240 min of heat treatment (Figure 5F). The relative electrical conductivity of the two overexpression plants was significantly lower than that of the wild type. This suggested that the electrolyte leakage in the overexpression plants was lower than in the wild type plants.
2.4 Overexpression of KcRCB reduces cotton leaf damage
In this study, the cotton cultivar “Tahe 2” employed was developed by Xinjiang Tarim River Seed Industry Co., Ltd. This cultivar is an medium-maturity, non-transgenic conventional upland cotton with medium fiber, exhibiting resistance to Fusarium wilt and tolerance to Verticillium wilt. We obtained transgenic lines overexpressing the KcRCB gene by tissue culturing and verified their high-temperature tolerance. The KcRCB-overexpressing plants and control plants were subjected to heat stress treatment at 45°C in an artificial climate chamber. As shown in Figure 6A, we did not observe phenotypic differences between the KcRCB-overexpressing and control plants at room temperature. After 30 min of cultivation in a 45°C incubator, the control plants began exhibited leaf drooping and mild leaf wilting, whereas KcRCB-overexpressing plants showed slightly drooping leaves. After 120 min of heat stress, the leaf drooping angle of the control group increased significantly with curling, whereas the leaf drooping angle of the KcRCB-overexpressing plants increased slightly. After 240 min of heat stress, the leaf drooping angle of the control plants approached 90°, whereas leaf drooping angle of the KcRCB-overexpressing plants showed a slight increase in drooping angle.
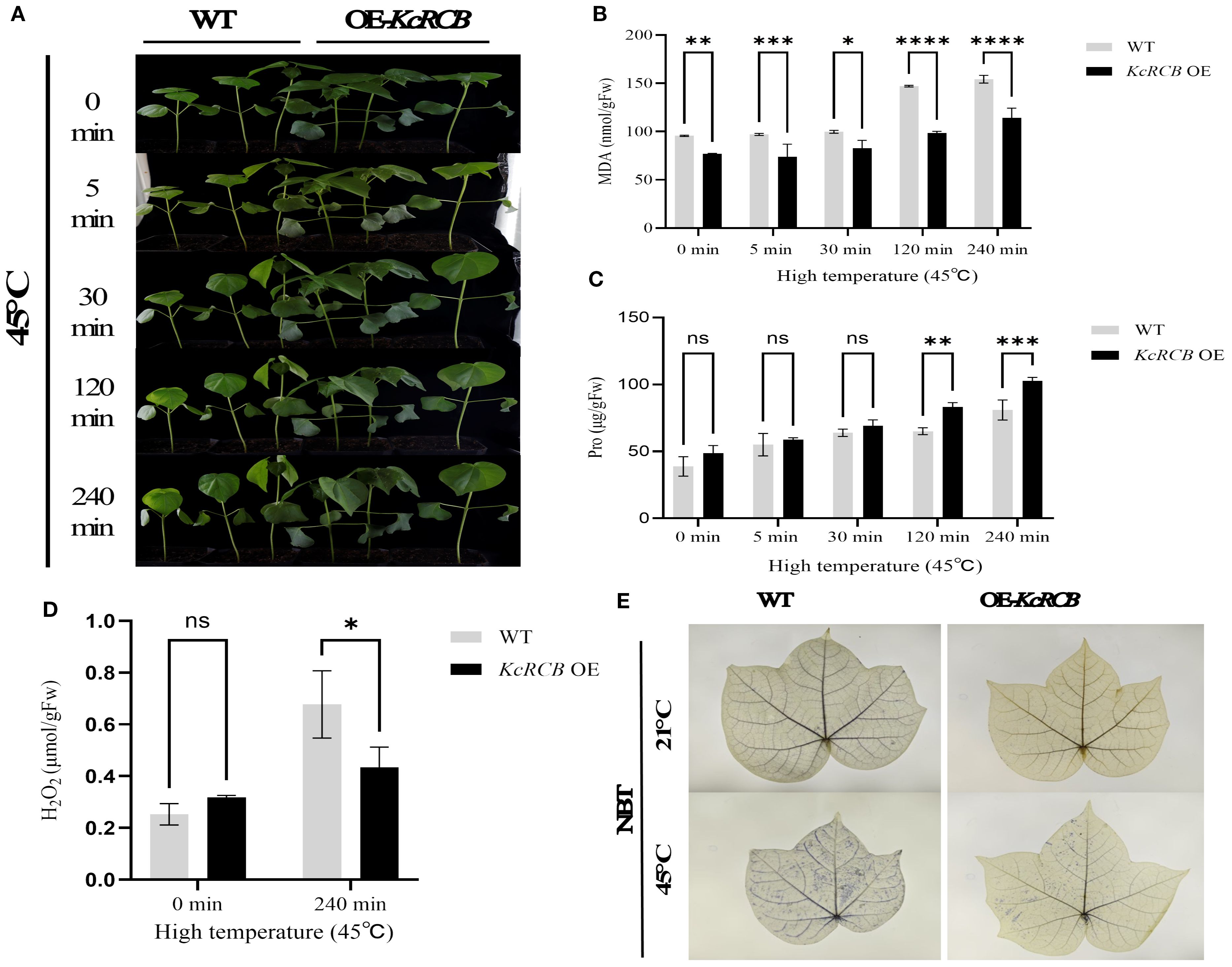
Figure 6. KcRCB overexpression alleviates heat-induced damage in the cotton leaves. (A) Phenotypic changes in the KcRCB-overexpressing and non-transgenic cotton plants subjected to heat treatment at 45°C for 0, 5, 30, 120, and 240 min. (B) Estimation of malondialdehyde (MDA) content in the leaves of KcRCB-overexpressing and non-transgenic cotton plants subjected to heat treatment at 45°C. (C) Estimation of hydrogen peroxide (H2O2) content in the leaves of KcRCB-overexpressing and non-transgenic cotton plants subjected to heat treatment at 45°C. (D) Nitroblue tetrazolium (NBT) staining of leaves from the KcRCB-overexpressing and non-transgenic cotton plants subjected to heat treatment at 45°C. Data are presented as the mean ± standard deviation (SD) of three biological replicates. “ns”, no significant difference (P > 0.05), *P< 0.05, **P< 0.01, ***P< 0.001, ****P< 0.0001. Fw: fresh weight.
Subsequently, we measured MDA activity and proline (Pro) content in the control and KcRCB-overexpressing plants under heat stress at different treatment times. MDA activity in both the control and KcRCB-overexpressing plants exhibited an upward trend with increasing treatment time. However, the control group showed a significant increase in MDA activity after 120 min of treatment, whereas KcRCB-overexpressing plants showed only a mild increase in MDA activity. Throughout the entire heat stress period, MDA activity in the KcRCB-overexpressing plants was lower than in the control plants and reached significant levels at 120 min and 240 min (Figure 6B). MDA activity results suggested that plasma membrane damage during heat stress was significantly lower in the KcRCB-overexpressing plants.
During the entire treatment period, proline (Pro) content showed an upward trend in both the control and KcRCB-overexpressing plants. In the early stage of heat treatment, Pro content was similar in both between the control and KcRCB-overexpressing plants, but increased significantly after 120 min of treatment. Throughout the heat stress period, Pro content in the KcRCB-overexpressing plants was consistently higher than in the control group and showed significant differences from the control group after 120 min (Figure 6C). The Pro content data demonstrated that stress tolerance was significantly higher in the KcRCB-overexpressing plants than in the control group.
After 240 min of heat stress, we performed NBT staining and estimated H2O2 content in the leaves of the KcRCB-overexpressing and control plants. NBT staining results showed that both the control and the KcRCB-overexpressing plants did not show any differences in ROS accumulation or phenotype before heat treatment. However, after heat treatment, the leaves of control plants exhibited darker staining than the KcRCB-overexpressing plants, thereby indicating higher ROS accumulation in the control plants (Figure 6E).
Estimation of H2O2 content in the cotton leaves before and after heat treatment (Figure 6D) showed that there were no significant differences between the control and KcRCB-overexpressing plants prior to heat treatment. However, after 240 min of heat stress, leaves of the control plants exhibited significantly higher H2O2 levels than those from the KcRCB-overexpressing plants. This further validated the NBT staining results and showed significant accumulation of ROS in the control plant leaves after heat stress compared with the KcRCB-overexpressing plants.
We analyzed the stomatal aperture of the cotton leaves in the KcRCB-overexpressing and control plants before and after heat treatment. There were no significant differences in the stomatal aperture of the cotton leaves of the control and KcRCB-overexpressing plants cultivated at room temperature. However, after heat stress, the KcRCB-overexpressing plants exhibited a higher degree of stomatal opening (Figure 7A). Stomatal aperture was quantified by calculating the stomatal opening rate in three biological replicates per group. As shown in Figure 7B, there were no significant differences in the stomatal opening rate between the KcRCB-overexpressing and the control plants before heat treatment. However, stomatal opening rate in the KcRCB-overexpressing plants after 240 min of heat stress was approximately 2-fold higher than that of the control group. These results suggest that overexpression of KcRCB enhances stomatal opening under heat stress, thereby promoting transpiration to reduce leaf temperature and mitigate leaf damage.
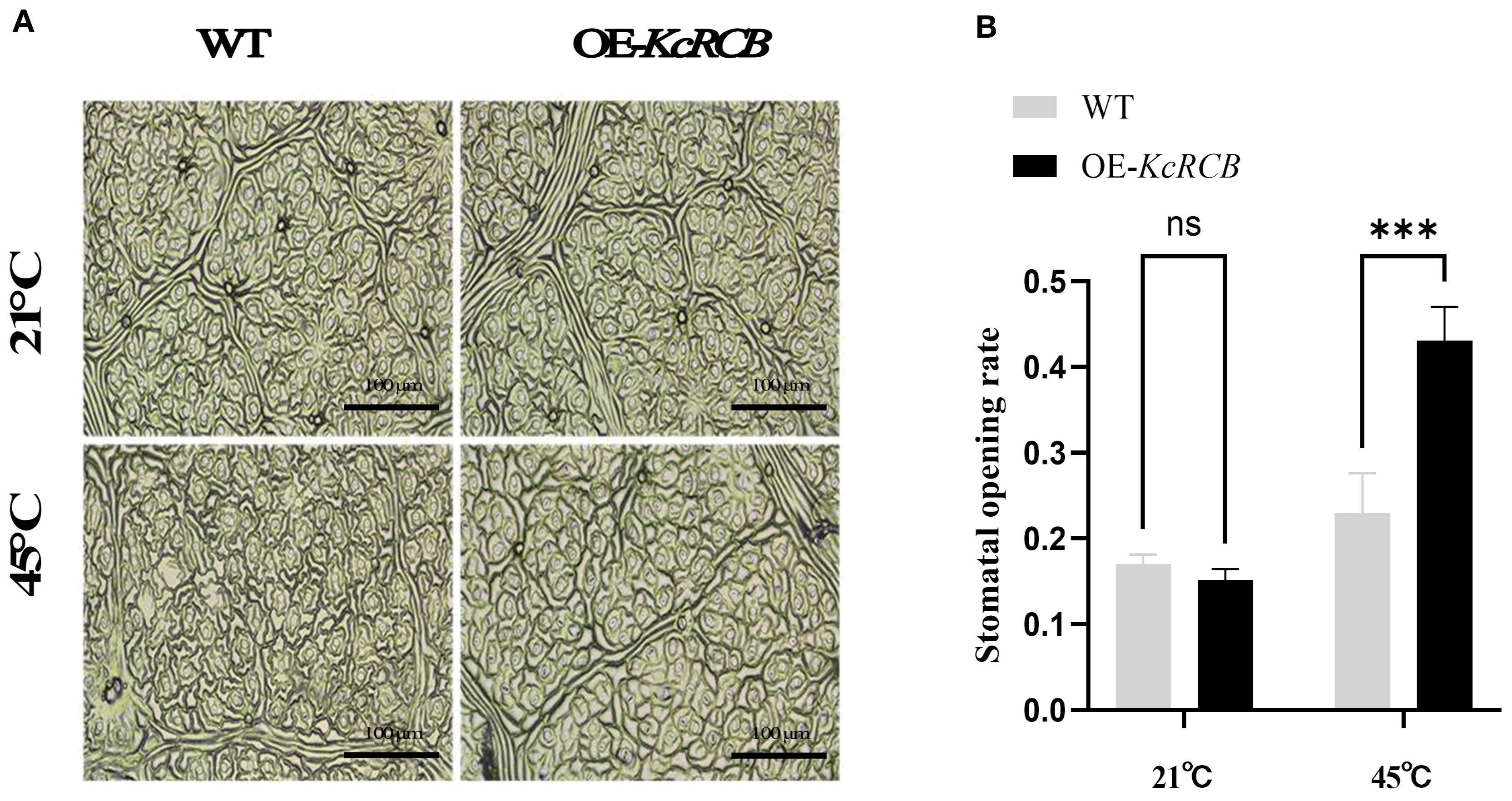
Figure 7. KcRCB overexpression promotes stomatal opening in cotton under heat stress. (A) Stomatal changes in the leaves of KcRCB-overexpressing and non-transgenic cotton plants subjected to heat treatment at 45°C for 240 min. (B) Stomatal opening/closing rates in the leaves of KcRCB-overexpressing and non-transgenic cotton plants before and after heat treatment at 45°C. Data are presented as the mean ± standard deviation (SD) of three biological replicates. “ns”, no significant difference (P > 0.05), ***P < 0.001.
3 Discussion
Our study shows that the KcRCB protein enhances plant tolerance to high temperatures by regulating stomatal opening. Validation experiments in Arabidopsis thaliana (model plant) and cotton (cash crop) plants demonstrate that overexpression of the Karelinia caspica RCB gene mitigates heat-induced leaf damage, delays the onset of heat stress morphology, and improves plant thermotolerance.
Karelinia caspica is a perennial herbaceous plant belonging to the genus Karelinia in the Asteraceae family. It primarily inhabits sand dunes, Gobi deserts, saline-alkaline lands, and the vicinity of reed marshes and paddy fields, often forming large-scale populations. This species exhibits strong stress resistance traits, including high tolerance to salinity, drought, and extreme temperatures. In China, Karelinia caspica is mainly distributed in the desert regions. Its flat and fleshy leaves are well-adapted for water storage and retention to thrive in arid conditions. Karelinia caspica can flower normally in the Taklamakan Desert when the temperatures exceeds 45°C in the summer, thereby signifying its exceptional heat tolerance (Wang et al., 2021). Our research findings show that the KcRCB gene expression levels in heat-stressed Karelinia caspica show a decreasing trend first but increase significantly with prolonged exposure to high temperatures. The high expression of KcRCB is synchronized with the heat-tolerant phenotype. This suggests that KcRCB is involved in the response of Karelinia caspica to high-temperature stress.
3.1 Identification and analysis of KcRCB
Current research on RCB genes is limited to the model plant Arabidopsis thaliana (Yoo et al., 2019; Qiu et al., 2021). In this study, we cloned the KcRCB gene using transcriptomic data from Karelinia caspica, based on the sequence of Arabidopsis thaliana RCB (AT4G28590). Domain analysis, phylogenetic tree construction, and motif prediction analysis showed that the sequence of the RCB protein was conserved and belonged to the MRL7 thioredoxin-like superfamily, consistent with findings in Arabidopsis thaliana (Yang et al., 2019). Thioredoxins are ubiquitous and thermostable proteins that function as hydrogen carriers. KcRCB exhibits amino acid variations compared to other plant species in the conserved region. These variations may contribute to differences in thermostability. These motif variations across species may also be responsible for the functional divergence of KcRCB compared to other plant RCB proteins.
In Arabidopsis thaliana, MRL7 was initially identified as a regulator of chloroplast growth and development, influencing plastid gene expression (Qiao et al., 2011). Subsequent studies demonstrated that Arabidopsis thaliana Early Chloroplast Biogenesis 1 (AtECB1), an allelic variant of MRL7 encoded a thioredoxin-like fold protein, and modulated the activity of the plastid-encoded RNA polymerase (PEP) and chloroplast biogenesis (Yua et al., 2014). Furthermore, immunological analyses showed that Suppressor of Variegation 4 (SVR4, also known as MRL7) was a component of the Transcriptionally Active Chromosome (TAC) complex in the barley chloroplasts. Gene expression studies further demonstrated that SVR4 and SVR4-like proteins are essential for the normal functioning of the plastid transcriptional machinery (Powikrowska et al., 2013).
Recent studies have shown that the RCB protein is a dual-targeted nucleus/plastid component required for PEP assembly. RCB interacts with phytochromes in the nucleus and promotes degradation of transcription factors PIF1 and PIF3, which localize to the photobodies and regulate PhAPG (plastid-encoded photosynthetic genes) expression levels (Yoo et al., 2019). RCB-dependent PIF degradation in the nucleus signals PEP assembly and PhAPG expression in the plastids. Emerging evidence suggests that RCB acts as a novel temperature signaling component and interacts with HMR to selectively stabilize PIF4 during the day, thereby initiating thermomorphogenesis.
We then performed RT-PCR and qRT-PCR analyses to investigate the expression patterns of KcRCB in various tissues of Karelinia caspica under heat stress. KcRCB is predominantly expressed in the leaves and shows time-dependent increase in the KcRCB transcript levels during heat treatment. Furthermore, subcellular localization data demonstrated KcRCB accumulation in the guard cells surrounding the stomatal pores. Based on these data, we hypothesize that KcRCB contributes to temperature regulation by enhancing stomatal conductance and transpiration. This mechanism facilitates leaf cooling and alleviates heat-induced damage (Zhou et al., 2023). Collectively, these findings indicate that KcRCB plays a positive regulatory role in mediating thermotolerance in Karelinia caspica.
3.2 KcRCB enhances heat tolerance in Arabidopsis thaliana
To further investigate the function of KcRCB, we conducted preliminary functional validation in the model plant Arabidopsis thaliana. We did not observe significant phenotypic differences between KcRCB-overexpressing transgenic plants and non-transgenic controls under normal conditions. However, the KcRCB-overexpressing transgenic plants exhibited significantly enhanced thermotolerance during heat stress.
Heat stress commonly affects multiple physiological and biochemical processes in plants, including membrane thermostability, antioxidant enzyme activity, osmotic regulators, and photosynthetic characteristics. During heat stress, excessive accumulation of reactive oxygen species (ROS) such as H2O2, O2-, and ·OH, causes significant lipid peroxidation and electrolyte leakage, thereby damaging the plants significantly (Dvořák et al., 2021). In our study, transgenic Arabidopsis thaliana showed lower malondialdehyde (MDA) concentration and relatively conductivity than the wild-type plants under heat stress, thereby indicating improved protection of the leaf cell membranes. Furthermore, activities of antioxidant enzymes catalase (CAT) and peroxidase (POD) were significantly higher in the KcRCB-overexpressing transgenic plants than in wild-type controls. NBT staining and H2O2 content assays also demonstrated excessive ROS accumulation in the wild-type plants but lower ROS levels in the KcRCB-overexpressing transgenic lines after heat stress. These results suggest that KcRCB mitigates heat-induced damage by enhancing ROS scavenging.
3.3 Overexpression of KcRCB reduces cotton leaf damage
Our study showed that KcRCB overexpression enhanced heat tolerance by decreasing membrane damage and increasing ROS scavenging in Arabidopsis thaliana. To verify whether KcRCB is stably expressed in other plant species, we further verified the effects of KcRCB expression in cotton. Transgenic cotton was obtained by Agrobacterium-mediated transformation via tissue culturing. We selected KcRCB overexpressing cotton as the experimental group for analyzing high-temperature stress at 45°C. Phenotypic observations demonstrated that the wilting and recurved angle of cotton leaves in the high-temperature treated control group were significantly higher than in the corresponding experimental group. Furthermore, NBT staining and hydrogen peroxide estimations showed that the ROS levels were significantly elevated in the control group than in the KcRCB overexpressed plants. MDA analysis demonstrated that KcRCB overexpression reduced membrane damage in the leaf cells.
3.4 KcRCB is localized to the leaf stomatal guard cell membrane
Heat-tolerant plants mitigate the effects of high-temperature stress by increasing transpiration rates to facilitate efficient leaf cooling. This adaptive strategy is attributed to increased stomatal density and total stomatal pore area per leaf, which enables optimal gas exchange and thermoregulation. By modulating stomatal density, aperture size, and morphology, heat-tolerant plants protect chloroplast structure and function while maintaining membrane thermostability during heat stress (Hao et al., 2019).
In our study, statistical analysis of stomatal aperture and density in the transgenic cotton plants overexpressing KcRCB demonstrated a significant increase in the stomatal opening during heat stress compared to the wild-type control plants, which exhibited widespread stomatal closure. These findings suggest that KcRCB enhances thermotolerance by promoting stomatal conductance, thereby increasing transpiration and reducing leaf temperature. This mechanism effectively safeguards the leaves from heat-induced damage and underscores the critical role of KcRCB in regulating stomatal dynamics and improving heat resilience in the transgenic cotton plants.
Current knowledge of the molecular mechanisms underlying thermomorphogenesis primarily focuses on the following three aspects: protein-level regulation; expression of PIF4; and chromatin state modifications via promoter binding (Quint et al., 2016). The core pathway of thermomorphogenesis is governed by PIF4 and other regulatory factors, which coordinate plant growth and development in response to temperature and light cues (Proveniers and van Zanten, 2013). High temperatures inhibit stomatal production and alter stomatal morphology. KcRCB is localized in the plasma membrane of the leaf guard cells. Previous studies have shown that RCB plays a key role in the PIF4 regulatory pathway (Qiu et al., 2021). As a central component of high-temperature signaling, PIF4 accumulates in the stomatal precursors upon heat exposure and binds to the promoter of SPCH. Temperature-activated PIF4 represses SPCH expression to limit stomatal production under heat stress (Lau et al., 2018). Future investigations are necessary to determine whether KcRCB participates in this regulatory pathway and its potential interactions with the PIF4 network.
Our study has a few limitations. Although our experiments showed that stomatal opening of the leaves in the KcRCB-overexpressing plants was enhanced under high-temperature stress, the molecular mechanisms underlying these effects are not clear. The genomic data of Karelinia caspica is not available. Moreover, transcriptomic data is incomplete and chromosomal localization results are unclear. Therefore, it is difficult to perform in-depth investigations of the regulatory network underlying the functions of KcRCB. In the future, mining of the complete Karelinia caspica transcriptome database is necessary to identify the upstream and downstream genes of the KcRCB pathway. The results of our study provide a molecular basis for investigating the regulatory mechanisms of KcRCB in adverse conditions.
4 Conclusions
In this study, we cloned the chloroplast development-related gene KcRCB from Karelinia caspica, a plant adapted to extreme environments. The full-length cDNA of KcRCB is 1005 bp and encodes 335 amino acids. The amino acid sequence analysis demonstrates a high degree of evolutionary conservation between KcRCB and its homologous genes in Asteraceae plants. Subcellular localization experiments suggest that KcRCB is localized in the leaf stomata, but further co-localization assays are necessary to determine its precise localization. Functional analysis demonstrated that overexpression of KcRCB significantly improves the heat tolerance of transgenic Arabidopsis thaliana and cotton plants by enhancing the stomatal opening of the leaves, thereby, highlighting its important role in plant thermomorphogenesis.
5 Materials and methods
5.1 Cloning and analysis of the KcRCB gene
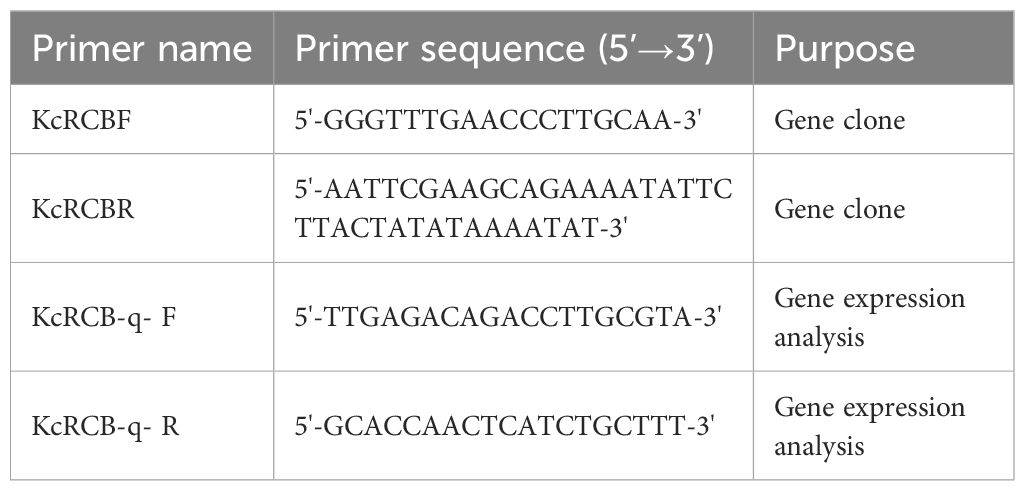
KcRCB was identified through a two-step selection process from the Karelinia caspica transcriptome datasets. Initially, genes exhibiting constitutively high expression (top 5% FPKM values in both control and heat-stressed Karelinia caspica leaf samples) were prioritized because highly abundant transcripts are typically indicative of core components in essential biological pathways (e.g., chloroplast maintenance). In the next step, we selected differential expressing genes (p < 0.05 under high-temperature stress) among these high-abundance candidates that also display stress-responsive expression patterns. Therefore, we selected genes with putative dual functions in both basal physiological processes as well as in adaptive stress responses. The homologous KcRCB gene was cloned by comparing the third-generation full-length transcriptome data of high-temperature-treated Karelinia caspica with the AtRCB gene, and cloned using the gene-specific primers listed in Table 1. The amino acid sequence of KcRCB was compared with the RCB protein sequences of other plants using the NCBI BLAST website (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Hydrophobicity of the KcRCB protein was analyzed using the Protscale online tool on the EXPASY website (https://www.expasy.org/). The conserved structural domains of the KcRCB protein were predicted using the Prosite and Interpro online databases (https://www.ebi.ac.uk/interpro/). The physicochemical properties of the KcRCB protein, including isoelectric point (PI) and amino acid composition were predicted using ProtParam tool on the EXPASY website. The secondary structure of KcRCB protein was analyzed using the SOPMA online tool (https://npsa.lyon.inserm.fr/). The motif analysis of the KcRCB protein was performed using MEME (https://meme-suite.org/meme/) to predict the signal peptide. The transmembrane structural domain of the KcRCB protein was predicted by using TMHMM tool (https://services.healthtech.dtu.dk/services/TMHMM-2.0/). A phylogenetic tree was constructed via sequence alignment using MEGA11.0, and the evolutionary relationships, protein phylogenetic analysis, and conserved protein motifs among several plant species with protein sequences homologous to KcRCB were analyzed.
5.2 Expression pattern of KcRCB in Karelinia caspia (Pall.) Less.
Seeds of Karelinia caspia were sown in a growth substrate composed of nutrient soil and vermiculite at a volume ratio of 3:1. The seeds were then covered with a membrane. The seeds were gently prodded to encourage seedling development. The membrane was removed after two days and the seedlings were cultivated for two months at 28°C, with 16 h of light and 8 h of darkness. Karelinia caspica seedlings exhibiting similar growth patterns were then exposed to a temperature of 45°C for 5, 30, 120, and 240 min. Each treatment was repeated thrice. The entire process was photographed. After the treatment, we collected the roots, stems, and leaves for RNA extraction using the RNAprep Pure Plant Kit (Beijing Tiangen Biochemical Technology). Then, reverse transcription was performed to synthesize cDNA using a Easy Script® One-Step gDNA Removal and cDNA Synthesis Kit (Beijing Quan’s Jin Biotechnology). We then estimated the concentration of cDNA samples. RT-PCR analysis was performed to determine the relative expression levels of KcRCB in plants treated at 45°C. The PCR products were analyzed by electropherogram and the expression pattern was evaluated based on the brightness of specific bands. The relative expression of KcRCB during 45°C treatment was analyzed by real-time fluorescence quantitative PCR (RTqPCR) using Beijing Quan’s Gold Biotechnology PerfectStart® Fast Green qPCR SuperMix. The q-PCR reaction was performed using the KcRCB-q- F and KcRCB-q- R primers (Table 1).Data analysis and graphical representation was performed using the GraphPad Prism software.
5.3 Subcellular localization of KcRCB in Nicotiana tabacum L.
To determine subcellular localization of KcRCB, the target gene was cloned into the pCAMBIA-1302 vector using the 2×Seamless Cloning Mix kit (Beijing Bomade Biologicals) and KcRCB-1302 F and KcRCB-1302 R primers shown in Table 1. The T vector plasmid with KcRCB was used as the template for PCR amplification of the target gene. The pCAMBIA1302 vector was double digested with the Bgl II and Spe I enzymes. The digested product was isolated from the agarose gel and mixed with the PCR amplification product of KcRCB according to the manufacturer’s instructions. The ligated product was then incubated at 50°C for 30 min. The ligated product was subsequently transferred into the E. coli DH5α cells and the positive clones were selected. The recombinant plasmid was then electroporated into the GV3101 electrocompetent cells at appropriate conditions. The capacitance of the electroporation cup was set at 25 μF. The resistance of the power supply was 200 ohms, and the voltage was 2400 V. The electroporation cup was quickly inserted into the electro transferring tank and ‘single colony activation’ option was used to identify the positive clones.
To determine the subcellular localization of KcRCB, the transformed Agrobacterium pCAMBIA1302-KcRCB was cultured until an OD600 of 1.2. Then, the bacterial solution was resuspended with 5 mL of MMA (10 mM MgCl2, 150 μM AS, 10 mM MES, adjusted to pH 5.6), and incubated at room temperature for 2–6 h until an OD600 of 0.6–0.8 was obtained. Subsequently, we selected 4-week-old Benjamin’s tobacco and injected the bacterial solution with the target genes and the nuclear marker solution with a 1 mL syringe at a ratio of 1:1 into the three upper full-spreading leaves, which were then marked for identification. The darkening of leaves were observed after 2 days, followed by a 2-day period of normal incubation. The leaves were then labeled, harvested, inverted on a slide, and observed under a laser confocal microscope.
5.4 Analysis of the high-temperature tolerance in KcRCB-overexpressing Arabidopsis thaliana (L.) Heynh.
The KcRCB gene was ligated into pONDR221 using the Gateway™ BP Clonase™ Enzyme Mix (Thermo Fisher Scientific). Then, the target gene was cloned into the pK2GW7 overexpression vector and transferred into the GV3101 Electroporation-Competent cells (Shanghai Vidi Biotech) using the Gateway™ LR Clonase™ enzyme mixture.
Arabidopsis thaliana was infected using the flower-dipping method (Zhang et al., 2006). At the time of full bloom, the flowers and pods were pollinated and then removed. The flower buds were then infected. The plants were watered for 24 h before infection. The transformed Agrobacterium spp. with the target gene were activated until they reached an OD600 of approximately 1.2. The sample was resuspended in a 5% sucrose solution to adjust the OD600 value to 0.6–0.8. Subsequently, 100 μL of Silwet L-77 was added to 1 L of the 5% sucrose solution (this was done at the point of infection). All flower buds were immersed in the infection solution for 1–2 min. Subsequently, the immersed plants were kept in the dark for 1 day. Then, the plants were returned to normal growing conditions until seed maturity.
T1 (These represent the T0 generation transgenic seeds directly obtained following Agrobacterium-mediated transformation.) seeds were planted on 1/2 MS medium with kanamycin (50 µg/ml) to select transgenic plants. The stable transformation was confirmed in the T2 (These seeds are produced by self-fertilization of T1 plants.) and T3 (These seeds are derived from self-fertilization of confirmed homozygous T2 plants.) generations.
Subsequently, two independent homozygous lines (KcRCB-OE1 or OE1 and KcRCB-OE2 or OE2) with robust growth were selected for the study. The seedlings were cultivated in a controlled growth chamber at 21°C under a 16-h light/8-h dark photoperiod (light intensity: 150–200 μmol·m-²·s-¹) for four weeks before heat stress treatments. Four-week-old transgenic lines (OE1, OE2) and wild-type Arabidopsis thaliana (Col-0) were exposed to 45°C for heat stress in a constant-temperature incubator under dark conditions. The samples were harvested at 0, 5, 30, 120, and 240 min post-stress for quantifying the catalase (CAT) and peroxidase (POD) activities, as well as the relative electrical conductivity. We also estimated H2O2 levels and performed NBT staining of samples at 0 and 240 min to visualize accumulation of hydrogen peroxide and superoxide anions, respectively. Following sampling, heat treatment persisted until distinct phenotypic alterations (e.g., leaf wilting, chlorosis) were observed. Stressed plants were then transferred to a 21°C light incubator (16 h light/8 h dark cycle, 150 µmol m-² s-¹) for 3 days of recovery. Subsequent phenotypic assessments were performed after recovery using a kit manufactured by the Beijing Solepol Technology Company. We accurately measured 1 g of young green leaves and added 10 mL of distilled water. The mixture was then left to stand for 12 h and the conductivity was estimated as R1. The mixture was subsequently boiled for 30 min and then allowed to cool to room temperature and the conductivity was estimated as R2. The relative conductivity was then calculated according to the following formula: Relative conductivity = R1/R2. The data were analyzed using the Excel and Graphpad 9.0 software packages (Jambunathan, 2010). All kits for the determination of physiological and biochemical indicators were purchased from Solarbio Science & Technology Co., Ltd.
5.5 Analysis of high temperature tolerance in the KcRCB-overexpressing cotton plants
Cotton seeds were sterilized in histoculture flasks containing MS medium, followed by culturing in a constant temperature incubator set at 28°C in dark. This procedure was undertaken to obtain cotton stem segments. The Agrobacterium strain was used to infect the cotton stem segments. The segments were then cultured in the dark for three days. Thereafter, they were cultured under standard conditions to allow healing of wounds, root growth, and transplantation into soil after the growth of cotyledons for positive validation (Srivastava et al., 2023). The KcRCB-overexpressing transgenic and non-transgenic plants were cultivated at 28°C and then grown to 2–3 true leaves for high temperature tolerance analysis. The plants were treated at 45°C for 0, 5, 30, 120, and 240 min, respectively. Three biological replicates were set up for each treatment. Malondialdehyde (MDA) and proline (Pro) levels were evaluated at all time points using kits from the Beijing Solepol Science and Technology Company. The H2O2 and superoxide (NBT staining) levels were measured in the control and KcRCB-overexpressing plant leaves after 240 min of treatment. The data was analyzed and processed using the Excel and GraphPad 9.0 software.
5.6 Analysis of stomata in cotton leaves by blotting method
Stomata in the cotton leaves were observed using the nail polish and transparent tape blotting method (Yuan et al., 2020). A uniform layer of nail polish was applied to the vein-free area on the abaxial surface of cotton leaves and air-dried at room temperature for approximately 5–10 min. Then, a transparent tape with the adhesive side was pressed gently by fingers to fully contact the dried nail polish and peeled off to obtain the imprint, pasted onto a glass slide with the adhesive side, and observed under a light microscope at 40× magnification.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
WL: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. WH: Investigation, Methodology, Visualization, Writing – original draft. YZ: Methodology, Software, Supervision, Writing – original draft. GL: Formal Analysis, Methodology, Validation, Writing – original draft. SL: Investigation, Validation, Writing – original draft. YW: Conceptualization, Funding acquisition, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by funding from the National Science and Technology Support Plan Project (Grant No. 2023ZD04040-4-2), Science and Technology Plan Projects of Autonomous Region (Grant No. XL202403-09), and Postgraduate Scientific Research and Innovation Project of Tarim University (Grant No. TDGRI2024002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1641916/full#supplementary-material
Supplementary Figure 1 | The amino acid sequence of KcRCB.
Supplementary Figure 2 | The conserved domain of RCB protein.
Supplementary File | Amino acid information for RCB proteins from different plant species used for phylogenetic tree construction.
References
Arnold, P. A., Kruuk, L. E. B., and Nicotra, A. B. (2019). How to analyse plant phenotypic plasticity in response to a changing climate. New Phytol. 222, 1235–1241. doi: 10.1111/nph.15656
Bernacchi, C. J., Long, S. P., and Ort, D. R. (2025). Safeguarding crop photosynthesis in a rapidly warming world. Science 388, 1153–1160. doi: 10.1126/science.adv5413
Blázquez, M. A., Ahn, J. H., and Weigel, D. (2003). A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat. Genet. 33, 168–171. doi: 10.1038/ng1085
Cao, Y., Zhang, J., Zhao, Z., Tang, G., and Yan, J. (2025). Heat stress triggers enhanced nuclear localization of HYPONASTIC LEAVES 1 to regulate microRNA biogenesis and thermotolerance in plants. Plant Cell 37, koaf092. doi: 10.1093/plcell/koaf092
Casal, J. J. and Balasubramanian, S. (2019). Thermomorphogenesis. Annu. Rev. Plant Biol. 70, 321–346. doi: 10.1146/annurev-arplant-050718-095919
Challinor, A. J., Watson, J., Lobell, D. B., Howden, S. M., Smith, D. R., and Chhetri, N. (2014). A meta-analysis of crop yield under climate change and adaptation. Nat. Climate Change 4, 287–291. doi: 10.1038/nclimate2153
Crawford, A. J., Mclachlan, D. H., Hetherington, A. M., and Franklin, K. A. (2012). High temperature exposure increases plant cooling capacity. Curr. Biol. 22, R396–R397. doi: 10.1016/j.cub.2012.03.044
Dvořák, P., Krasylenko, Y., Zeiner, A., Šamaj, J., and Takáč, T. (2021). Signaling toward reactive oxygen species-scavenging enzymes in plants. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.618835
Foreman, J., Johansson, H., Hornitschek, P., Josse, E. M., Fankhauser, C., and Halliday, K. J. (2010). Light receptor action is critical for maintaining plant biomass at warm ambient temperatures. Plant J. 65, 441–452. doi: 10.1111/j.1365-313X.2010.04434.x
Franklin, K. A., Lee, S. H., Patel, D., Kumar, S. V., Spartz, A. K., Gu, C., et al. (2011). Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc. Natl. Acad. Sci. United States America 108, 20231–20235. doi: 10.1073/pnas.1110682108
Gan, P., Liu, F., Li, R., Wang, S., and Luo, J. (2019). Chloroplasts- beyond energy capture and carbon fixation: tuning of photosynthesis in response to chilling stress. Int. J. Mol. Sci. 20, 5046. doi: 10.3390/ijms20205046
Gil, K. E. and Park, C. M. (2018). Thermal adaptation and plasticity of the plant circadian clock. New Phytol. 221, 1215–1229. doi: 10.1111/nph.15518
Hao, L., Guo, L., Li, R., Cheng, Y., Huang, L., Zhou, H., et al. (2019). Responses of photosynthesis to high temperature stress associated with changes in leaf structure and biochemistry of blueberry (Vaccinium corymbosum L.). Scientia Hortic. 246, 251–264. doi: 10.1016/j.scienta.2018.11.007
Huang, Z., Lin, R., Dong, Y., Tang, M., Xia, X., Fang, L., et al. (2025). MiR164a-targeted NAM3 inhibits thermotolerance in tomato by regulating HSFA4b-mediated redox homeostasis. Plant Physiol. 197, kiaf113. doi: 10.1093/plphys/kiaf113
Jambunathan, N. (2010). Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods Mol. Biol. (Clifton N.J.) 639, 292–298. doi: 10.1007/978-1-60761-702-0_18
Kan, Y., Mu, X. R., Gao, J., Lin, H. X., and Lin, Y. (2023). The molecular basis of heat stress responses in plants. Mol. Plant 16, 1612–1634. doi: 10.1016/j.molp.2023.09.013
Lamers, J., van der Meer, T., and Testerink, C. (2020). How plants sense and respond to stressful environments. Plant Physiol. 182, 1624–1635. doi: 10.1104/pp.19.01464
Lau, O. S., Song, Z., Zhou, Z., Davies, K. A., Chang, J., Yang, X., et al. (2018). Direct control of SPEECHLESS by PIF4 in the high-temperature response of stomatal development. Curr. Biology: CB 28, 1273–1280.e3. doi: 10.1016/j.cub.2018.02.054
Legris, M., Nieto, C., Sellaro, R., Prat, S., and Casal, J. J. (2017). Perception and signalling of light and temperature cues in plants. Plant J. 90, 683–697. doi: 10.1111/tpj.13467
Leister, D., Wang, L., and Kleine, T. (2017). Organellar gene expression and acclimation of plants to environmental stress. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00387
Pérez-Bueno, M. L., Illescas-Miranda, J., Martín-Forero, A. F., de Marcos, A., Barón, M., Fenoll, C., et al. (2022). An extremely low stomatal density mutant overcomes cooling limitations at supra-optimal temperature by adjusting stomatal size and leaf thickness. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.919299
Powikrowska, M., Khrouchtchova, A., Martens, H. J., Zygadlo-Nielsen, A., Melonek, J., Schulz, A., et al. (2013). SVR4 (suppressor of variegation 4) and SVR4-like: two proteins with a role in proper organization of the chloroplast genetic machinery. Physiologia Plantarum 150, 477–492. doi: 10.1111/ppl.12108
Proveniers, M. C. G. and Van Zanten, M. (2013). High temperature acclimation through PIF4 signaling. Trends Plant Sci. 18, 59–64. doi: 10.1016/j.tplants.2012.09.002
Qiao, J., Ma, C., Wimmelbacher, M., Börnke, F., and Luo, M. (2011). Two novel proteins, mrl7 and its paralog mrl7-l, have essential but functionally distinct roles in chloroplast development and are involved in plastid gene expression regulation in Arabidopsis. Plant Cell Physiol. 52, 1017–1030. doi: 10.1093/pcp/pcr054
Qiu, Y., Li, M., Kim, R. J., Moore, C. M., and Chen, M. (2019). Daytime temperature is sensed by phytochrome B in Arabidopsis through a transcriptional activator HEMERA. Nat. Commun. 10, 140. doi: 10.1038/s41467-018-08059-z
Qiu, Y., Pasoreck, E. K., Yoo, C. Y., He, J., Wang, H., Bajracharya, A., et al. (2021). RCB initiates Arabidopsis thermomorphogenesis by stabilizing the thermoregulator PIF4 in the daytime. Nat. Commun. 12, 2042. doi: 10.1038/s41467-021-22313-x
Quint, M., Delker, C., Franklin, K. A., Wigge, P. A., Halliday, K. J., and van Zanten, M. (2016). Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2, 15190. doi: 10.1038/nplants.2015.190
Rathore, S., Bindoff, N. L., Phillips, H. E., and Feng, M. (2020). Recent hemispheric asymmetry in global ocean warming induced by climate change and internal variability. Nat. Commun. 11, 2008. doi: 10.1038/s41467-020-15754-3
Sadok, W., Lopez, J. R., and Smith, K. P. (2020). Transpiration increases under high-temperature stress: potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 44, 2102–2116. doi: 10.1111/pce.13970
Song, Y., Feng, L., Alyafei, M. A. M., Jaleel, A., and Ren, M. (2021). Function of chloroplasts in plant stress responses. Int. J. Mol. Sci. 22, 13464. doi: 10.3390/ijms222413464
Srivastava, A., Shukla, A. K., Srivastava, S., Dubey, R. S., Singh, P. K., and Verma, P. C. (2023). Agrobacterium-mediated genetic transformation of cotton and regeneration via somatic embryogenesis. Bio Protocol 13, e4677. doi: 10.21769/BioProtoc.4677
Sun, N., Wang, J., Gao, Z., Dong, J., He, H., Terzaghi, W., et al. (2016). Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc. Natl. Acad. Sci. 113, 6071–6076. doi: 10.1073/pnas.1604782113
Tan, W., Chen, J., Yue, X., Chai, S., Liu, W., Li, C., et al. (2023). The heat response regulators HSFA1s promote Arabidopsis thermomorphogenesis via stabilizing PIF4 during the day. Sci. Adv. 9, eadh1738. doi: 10.1126/sciadv.adh1738
Wang, Y., Guo, Y., Li, F., Liu, Y., and Jin, S. (2021). Overexpression of KcNHX1 gene confers tolerance to multiple abiotic stresses in Arabidopsis thaliana. J. Plant Res. 134, 613–623. doi: 10.1007/s10265-021-01280-w
Yang, E. J., Yoo, C. Y., Liu, J., Wang, H., Cao, J., Li, F. W., et al. (2019). NCP activates chloroplast transcription by controlling phytochrome-dependent dual nuclear and plastidial switches. Nat. Commun. 10, 2630. doi: 10.1038/s41467-019-10517-1
Yoo, C. Y., Pasoreck, E. K., Wang, H., Cao, J., Blaha, G. M., Weigel, D., et al. (2019). Phytochrome activates the plastid-encoded RNA polymerase for chloroplast biogenesis via nucleus-to-plastid signaling. Nat. Commun. 10, 2629. doi: 10.1038/s41467-019-10518-0
Yu, Z., Wang, J., Zhang, C., Zhan, Q., Shi, L., Song, B., et al. (2024). SIZ1-mediated SUMOylation of CPSF100 promotes plant thermomorphogenesis by controlling alternative polyadenylation. Mol. Plant 17, 1392–1406. doi: 10.1016/j.molp.2024.07.011
Yua, Q. B., Ma, Q., Kong, M. M., Zhao, T. T., Zhang, X. L., Zhou, Q., et al. (2014). AtECB1/MRL7, a thioredoxin-like fold protein with disulfide reductase activity, regulates chloroplast gene expression and chloroplast biogenesis in Arabidopsis thaliana. Mol. Plant 7, 206–217. doi: 10.1093/mp/sst092
Yuan, J., Wang, X., Zhou, H., Li, Y., Zhang, J., Yu, S., et al. (2020). Comparison of sample preparation techniques for inspection of leaf epidermises using light microscopy and scanning electronic microscopy. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00133
Zeng, C., Jia, T., Gu, T., Su, J., and Hu, X. (2021). Progress in research on the mechanisms underlying chloroplast-involved heat tolerance in plants. Genes 12, 1343. doi: 10.3390/genes12091343
Zhang, X., Henriques, R., Lin, S. S., Niu, Q. W., and Chua, N. H. (2006). Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1, 641–646. doi: 10.1007/s10265-017-0982-9
Zhang, X., Liao, M., Chang, D., and Zhang, F. (2014). Comparative transcriptome analysis of the Asteraceae halophyte Karelinia caspica under salt stress. BMC Res. Notes 7, 927. doi: 10.1186/1756-0500-7-927
Zhang, Y., Song, R. F., Hu, X. Y., Zhou, H., Wang, W., Zhang, J., et al. (2025). Arabidopsis HSFA1b functions as a heat sensor inhibiting OST1-mediated stomatal closure through its adenylate-cyclase activity. Mol. Plant 18 (9), 1549–1566. doi: 10.1016/j.molp.2025.07.018
Zhang, L. L., Zhu, Q. Y., Sun, J. L., Yao, Z. W., Qing, T., Ma, H., et al. (2024). XBAT31 regulates reproductive thermotolerance through controlling the accumulation of HSFB2a/B2b under heat stress conditions. Cell Rep. 43, 114349. doi: 10.1016/j.celrep.2024.114349
Zhao, C., Liu, B., Piao, S., Wang, X., Lobell, D. B., Huang, Y., et al. (2017). Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. United States America 114, 9326–9331. doi: 10.1073/pnas.1701762114
Zhou, N., Li, C., Xie, W., Liang, N., Wang, J., Wang, B., et al. (2024). Histone methylation readers MRG1/2 interact with PIF4 to promote thermomorphogenesis in Arabidopsis. Cell Rep. 43, 113726. doi: 10.1016/j.celrep.2024.113726
Keywords: Karelinia caspia, KcRCB, high temperature, expression analysis, functional exploration
Citation: Li W, Huang W, Zhao Y, Li G, Li S and Wang Y (2025) Cloning and high temperature tolerance analysis of the thermal response related gene KcRCB in Karelinia caspia (Pall.) Less. Front. Plant Sci. 16:1641916. doi: 10.3389/fpls.2025.1641916
Received: 05 June 2025; Accepted: 01 September 2025;
Published: 17 September 2025.
Edited by:
Muthusamy Ramakrishnan, Nanjing Forestry University, ChinaReviewed by:
Xin Zhang, Henan Institute of Science and Technology, ChinaJialu Wang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Tingkai Zhai, Fujian Agriculture and Forestry University, China
Copyright © 2025 Li, Huang, Zhao, Li, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanqin Wang, d3lxd3hmQDEyNi5jb20=
 Wenlong Li
Wenlong Li Wanyi Huang
Wanyi Huang