- 1Departamento de Recursos Ambientales, Facultad de Ciencias Agronómicas, Universidad de Tarapacá, Arica, Chile
- 2Química y Farmacia, Facultad de Ciencias de la Salud, Universidad Arturo Prat, Iquique, Chile
- 3Chemical Ecology Laboratory, Instituto de Investigaciones Agropecuarias, Instituto de Investigaciones Agropecuarias (INIA) Quilamapu, Chillán, Chile
Citriculture faces significant constraints in expanding into new environments and agroecological zones. Grafting onto tolerant rootstocks has helped overcome some of these limitations, enabling cultivation under diverse conditions. Nevertheless, citrus production remains vulnerable to multiple abiotic and biotic stressors, among which red mite (Panonychus citri) herbivory can markedly reduce yield and fruit quality. While rootstocks are known to influence scion physiology and defence capacity, their specific role in modulating responses to pest attack is still poorly understood. To address this, we evaluated 18-month-old ‘W. Murcott’ mandarin grafted onto four citrus rootstocks (‘Macrophylla’, ‘C35’, ‘Citrumelo’, ‘Carrizo citrange’) under semi-field conditions, infested or not with P. citri. After seven days of infestation (100–160 eggs/leaf), we quantified stress markers (malondialdehyde, proline, salicylic acid), physiological parameters, primary and secondary metabolites, volatile organic compounds (VOCs), and defence-related gene expression. Rootstocks significantly modulated constitutive and inducible responses. ‘Citrumelo’ and ‘Carrizo’ showed the lowest MDA accumulation and strongest induction of SA, PR5, and GLR transcripts, coupled with increased emission of herbivory-induced plant volatiles (HIPVs, e.g., β-pinene, methyl salicylate, β-ocimene). ‘Macrophylla’ exhibited limited changes, whereas ‘C35’ displayed high MDA content and PITY1 induction, suggesting greater oxidative stress. Photosynthetic pigments declined across all combinations after infestation, while soluble sugars and flavonoids decreased in susceptible rootstocks. VOC profiles shifted both qualitatively and quantitatively in a rootstock-dependent manner. These results show that P. citri herbivory can amplify rootstock-driven differences in physiological, biochemical, and molecular traits, providing a basis for further studies on the role of rootstock–scion interactions in citrus resistance to mite attack.
1 Introduction
Citrus is one of the most important fruit trees worldwide, covering 10.55 million hectares and yielding over 169.38 million tons during 2023 (FAOSTAT, 2025). Among citrus species, mandarins, clementines and tangerines rank second in terms of productive importance, with a combined production of 52,556,927 tons (FAOSTAT, 2025). In Chile, mandarins are primarily cultivated in the north-central regions, spanning from the extremely arid climate of Arica y Parinacota (Azapa Valley, 18°31’ S, 70°10’ W) and Atacama (27°22’ S, 70°19’ W) to the Mediterranean conditions of central Chile (34°22’ S, 71°07’ W), with a total of 12,405.3 hectares (ODEPA, 2025). However, its productivity and that of other Citrus species can be significantly affected by both abiotic and biotic factors, which have been further exacerbated by climate change (Syvertsen and García-Sanchez, 2014; Agut et al., 2016; Nawaz et al., 2021).
Grafting techniques have enabled cultivation of fruit trees in soil-limiting conditions through the use of tolerant and resistant rootstocks (Rasool et al., 2020). These rootstocks can positively influence various characteristics of the scions at the molecular and physiological levels, including vigour, organoleptic fruit quality, yield, and nutrient uptake, among other agronomic traits (Agusti et al., 2020; He et al., 2020; Zhou et al., 2022). It has been reported that rootstocks can confer tolerance to diseases and pests (Agut et al., 2015; Jones and Killiny, 2021; Guarino et al., 2022; Alfaro-Quezada et al., 2023). Hence, rootstocks may significantly impact on features and products of the scion’s primary and secondary metabolism.
It is worth noting that several published studies have investigated the physiological and biochemical parameters of citrus cultivars (Simpson et al., 2014; Arjona-López et al., 2023). However, these studies typically involved non-grafted plants or different cultivars grafted onto the same rootstock under abiotic stress conditions (Long et al., 2017; Huang et al., 2020). Less is known, however, about the effect of citrus rootstocks on scions attacked by pests.
Plants detect herbivores through elicitors/effectors known as damage-associated molecular patterns (DAMPs) and herbivore-associated molecular patterns (HAMPs) (Erb et al., 2012), which activate the production of oxidative molecules, defence-related phytohormones, and expression of genes (Erb et al., 2012; Mishra et al., 2024; Sheri et al., 2023). In addition, plants emit volatile organic compounds (VOCs), specifically herbivory-induced plant volatiles (HIPVs; Erb et al., 2015; Ali et al., 2023), which act as indirect defences agents (Alsabte et al., 2022; Zhou and Jander, 2022) by attracting predators and parasitoids, establishing tri-trophic interactions (Erb et al., 2010; Abdala-Roberts et al., 2019). HIPV emissions can vary depending on Citrus rootstock, as observed in ‘Sugar Belle’ hybrid mandarin scions infested with Diaphorina citri (Hemiptera: Liviidae) (Jones and Killiny, 2021).
Biochemical and physiological traits, and several growth attributes have been helpful in identifying plant tolerance against pests (Mitchell et al., 2016). Proline and other amino acids increased in vine (Vinis vinifera L.), wheat (Triticum aestivum L.), and potato (Solanum tuberosum L.) plants infected by pathogens (Anzano et al., 2022); likewise, malondialdehyde (MDA) content, a key bioindicator of plant cell membrane lipid peroxidation, plays a role in plant response to herbivory (Morales and Munné-Bosch, 2019). Similarly, soluble sugars, proteins, and antioxidant molecules are vital for plant development, with critical functions in their defence mechanisms against biotic stresses (Anzano et al., 2022; Hayat et al., 2022; Sheri et al., 2023). Also, polyphenols are pivotal in plant defence mechanisms, acting as crucial deterrents against biotic threats, including herbivores, and serving as protectors against abiotic stresses (Singh et al., 2021).
The main player in plants infested with mites, aphids, and whiteflies is salicylic acid (SA; Mishra et al., 2024), but ethylene (ET), abscisic acid (ABA) and jasmonate (JA) also modulate the expression of defence-related genes (Erb et al., 2010; Li et al., 2019; Yu et al., 2021). In sour orange plants infested with T. urticae, EIN3, an ET- related transcription factor (TF), and ABA4, an ABA biosynthesis-related gene (North et al., 2007), were involved in defence pathways (Agut et al., 2014). SA marker genePR-5 (Pathogenesis Related-5) is induced early, followed by JA-related PR-3 (Pathogenesis Related-3). PI Citrus TYPE 1 (PITY1) is a proposed infestation marker glutamate receptor-like genes (GLR) that act as non-specific amino acid sensors in plant defence signalling pathways (Yan et al., 2024).
Panonychus citri (McGregor) (citrus red mite; Acari: Tetranychidae), is a major foliar pest of Citrus (Zanardi et al., 2015), feeding on the adaxial leaf surface by extracting cell contents, such as chloroplasts (Hoy, 2011) depositing its eggs there. This behavior affects citrus varieties at morphological, physiological, and molecular levels (Agut et al., 2014, 2016), reducing photosynthesis, stomatal conductance, and transpiration, as shown in Jatropha curcas plants (Hsu et al., 2015), unlike mite-tolerant varieties. Citrus rootstocks may mitigate infestation effects, making it essential to explore their role in enhancing scion resistance to phytophagous mites (Agut et al., 2014).
This research evaluates four different citrus rootstocks on the commercial mandarin ‘W. Murcott’ when scions were infected by P. citri under semi-field conditions. To this purpose, stress markers together with several physiological traits, VOCs, and expression of defence-related genes were analyzed. Our results contribute to refining nursery protocols and identifying optimal scion/rootstock interactions in young citrus plants.
2 Materials and methods
2.1 Plant material and growth conditions
The study was conducted during the summer of 2022, in 18-month-old mandarin ‘W. Murcott’ (Citrus reticulata Blanco) plants at Huayquique, Chile (20° 16’ S; 70° 07’ W; 28 m. a.s.l.). Each scion was grafted onto one of four different rootstocks: ‘Macrophylla’ (MA), ‘C35’ (C35), ‘Citrumelo’ (CI), and ‘Carrizo’ (CA) (Table 1). The selected mandarin ‘W. Murcott’ is always propagated by grafting in commercial production. Therefore, the use of grafted plants in this study reflects standard commercial practices and ensures relevance to filed conditions. The individuals were divided into 1) non-infested and 2) infested plants and cultivated separately, under semi-field conditions, in two anti-aphid screened greenhouses (4 m × 8 m × 3.5 m) to avoid plant-to-plant communication. The plants were grown in pots (20 L) filled with a substrate mix consisting of peat: organic soil: perlite (2: 2: 1) and watered three times per week. The soil was provided with N:P:K in solution [Ultrasol® Multipurpose 18-18-18, Soquimich, Chile] once a week. Up to the beginning of experiments, macro- and micronutrients were also supplied by foliar applications of Basfoliar SP 25-10-17 [COMPO EXPERT, Chile]. The meteorological data were obtained from a local weather station (Supplementary Figure S1).

Table 1. Main traits of ‘Macrophylla’ (Citrus macrophylla Wester); ‘C35’ [C. sinensis × P. trifoliata (South African)]; ‘Citrumelo’ (Citrus paradisi Macf. ‘Duncan’ grapefruit × P. trifoliata), and ‘Carrizo citrange’ (Citrus sinensis (L.) Osbeck × Poncirus trifoliata (L.) Raf.) rootstocks (Agusti et al., 2020).
2.2 Stock of Panonychus citri colonies
The citrus red mite Panonychus citri colonies were collected in citrus orchards at Pica Oasis, Chile (20°29’ S; 69°19’ W; 1,346 m.a.s.l.). It was reared on grapefruit (Citrus × paradisi Macfad.) fruits, placed on discs of PVC (diam. = 13 cm, H = 8 cm). To prevent the escape of mites, a small layer of vaseline was applied to contact surface between the fruit surface and the PVC disc. All inoculated fruits were put in a growth chamber at 25°C, 50% RH, and 16/8 h light/dark photoperiod.
2.3 Mite treatment and sampling
Infestation with mites was carried out in a greenhouse following a 30-day acclimation period. One shoot from the central part of the plant with 10 - 14 fully expanded leaves of each W. Murcott scion/rootstock combination was selected for treatment: a) WM/MA; b) WM/C35; c) WM/CI; d) WM/CA. The plants were inoculated with 20 gravid females of P. citri using a plastic micropipette tip. The tip was carefully attached by a clip to the abaxial side of each leaf, allowing P. citri to establish itself over the leaves. After 24 h, the micropipette tips and the clips were removed. The number of P. citri females was verified daily using a 10× handheld magnifying glass. Non-infested shoots with similar features as described above were chosen as controls. A seven-day infestation period was selected to ensure a robust and consistent physiological and molecular response to the imposed mite density of 20 adult females per leaf. This timeframe was chosen to precede the hatching of eggs, which typically occurs after approximately seven days and leads to a rapid and uneven increase in the Panonychus citri population. Extending the infestation period beyond this point could introduce uncontrolled variability due to asynchronous population growth and differing plant stress levels, thereby compromising the reproducibility of the observed responses.
At the end of the seventh day, the number of eggs per leaf from each scion/rootstock combination was assessed by counting under a stereoscope. Net assimilation and other photosynthesis-related parameters were evaluated using an infra-red gas analyzer (IRGA). Several leaves from control and treated plants were pooled in three different biological replicates. They were frozen in liquid nitrogen, freeze-dried and then stored until use. For RNA extraction, fresh leaf samples were collected in liquid nitrogen, and then stored at -80°C until analysis.
2.4 Stress-related biological markers
2.4.1 Malondialdehyde content (MDA)
MDA content was determined according to Heath and Packer (1968) and Alfaro-Quezada et al. (2023).
Approximately 0.25 g dry weight (DW) of leaf tissue was homogenized with 5 mL of a 5% trichloroacetic acid (TCA) solution and 1.25% glycerol. After centrifugation at 6,700 g for 10 min at 4°C and filtration through Whatman N° 1 filter paper, the supernatant was mixed with 2 mL of 0.67% thiobarbituric acid (TBA). The mixture was incubated for 30 min at 100°C, ice-cooled for 5 min, and centrifuged at 6,700 g for 1 min at 4°C. The absorbance was measured at 532 nm by UV-Vis spectrophotometry (BioTeK Instruments).
2.4.2 Proline content
Proline content was determined in 0.5 g of leaf tissue by ninhydrin reaction (Bates et al., 1973).
A standard curve using L-proline was made and absorbance was read at 520 nm by UV-Vis spectrophotometry.
2.4.3 Salicilyc acid (SA) content
SA content was determined by a colorimetric reaction according to Warrier et al. (2013) with some modifications.
About 0.05 g DW of leaf tissue was powdered and added with 1 mL of double distilled water, vortexed and placed in a dry bath at 60°C for 10 min. After centrifuging at 10,000 g for 10 min, an aliquot of 6.6 μL of supernatant was combined with 193.4 μL of fresh ferric chloride (FeCl). A SA (M.W. 138.12 g mol-1) standard was used for the calibration curve. The absorbance was read at 540 nm by UV-Vis spectrophotometry.
2.5 Gas exchange rates
The net assimilation rate of CO2 (A), stomatal conductance (gs), and transpiration (E) were measured using a portable apparatus (IRGA, LI-6800®, LI-COR Inc, Lincoln, Nebraska, USA). Fully expanded leaves from the central part of young trees were carefully extended and placed in the gas exchange chamber. The chamber was set to maintain a constant photosynthetically active radiation (PAR) of 1,200 µmol m-2s-1, and the carbon dioxide concentration (CO2) was held at 420 µmol mol-1 using the instrument’s internal CO2 injection system. The measurements were conducted at midday, between 11:30 a.m. and 12:30 p.m., on sunny days in February 2022, in three replicates per scion/rootstock combination and treatment.
2.6 Photosynthetic pigment, total sugar, and protein contents
2.6.1 Photosynthetic pigment
About 0.5 g DW of leaf tissue were used for measuring chlorophylls and carotenoids (Lichtenthaler, 1987).
Pigments were extracted in 10 mL of 80% (v/v) acetone and centrifuged for 10 min at 4,500 g. The absorbance was measured in the supernatant at 663, 646, and 470 nm by UV-Vis spectrophotometry.
2.6.2 Total sugar content
Total sugar content was determined according to Dubois et al. (1956).
Total sugars were extracted from 0.1 g DW using 5 mL of distilled water and shaken for 60 min. Then, they were centrifuged at 4,500 g for 30 min at 12 ± 2°C. An aliquot of 30 µL of supernatant was added with 180 µL of distilled water, 200 µL of phenol (80%), 1 mL of concentrated H2SO4 and cooled at RT in darkness. A standard curve with D-glucose was used, and the absorbance was read at 490 nm by UV-Vis spectrophotometry.
2.6.3 Total protein content
Total proteins were extracted according to Nunes et al. (2015) with some modifications.
About 1 g DW of leaf tissue was ground in buffer containing KH2PO4 50 mM, pH 7.0, 2 mM EDTA, and 1% (w/v) PVP. The homogenate was then centrifuged at 10,000 g for 10 min at 4°C. The assay was performed using the Protein Assay Kit Pierce™ BCA (Thermo Scientific, USA) following the manufacturer’s instructions, with bovine serum albumin (BSA) as standard. The absorbance was read at 562 nm by UV-Vis spectrophotometry.
2.7 Total phenolic and flavonoid contents
Total phenolic and flavonoid contents were determined as described by Rao et al. (2019) with some modifications.
About 0.1 g of leaf tissue was homogenised with 5 mL of cooled 80% (v/v) methanol and shaken on an orbital shaker at 200 rpm for 2 h at RT. The homogenates were centrifuged at 2,500 g for 15 min.
2.7.1 Total phenolic content (TPC)
The TPC was determined in a 300-μl aliquot of the supernatant added with Folin reagent (Folin: distilled water 1: 10) and was incubated for 5 min at RT. Then, 2.25 mL of Na2CO3 solution (60 g L-1) was added and allowed to react in darkness for 2h at RT. The absorbance was measured at 725 nm using a UV-Vis spectrophotometer and the results are expressed in mg gallic acid equivalents (GAEs) per gram dry weight (mg GAEs g-1 DW).
2.7.2 Total flavonoids content (TFC)
The TFC was determined in a 500 µL of methanolic extract combined with 2.25 mL of distilled water.
An aliquot of 150 µL of 5% (w/v) NaNO2 in water solution was added and incubated for 6 min at RT. Then, 300 µL of 10% (w/v) of AlCl3 solution were added. After incubation at RT for 5 min, 1 mL of 1 M NaOH was added and vortexed for 30 s. The absorbance was measured by a UV-Vis spectrophotometer at 510 nm. The results are expressed as mg rutin equivalents (REs) per gram dry weight (mg REs g-1 DW).
2.8 VOCs collection and chemical analysis
Volatile organic compounds (VOCs) were collected during the summer of 2022 using a dynamic headspace technique, as described by Rioja et al. (2016). Briefly, a shoot with 10–14 leaves was selected and enclosed in a 1-L oven bag (food-grade) while still attached to the plant. Filtered air (charcoal, 8–20 mesh, Sigma-Aldrich, St. Louis, Missouri, USA) was delivered into the bag at 1000 mL min⁻¹, and pulled it out at 900 mL min⁻¹ using a vacuum pump (BOECO, Hamburg, Germany) through a glass column containing 100 mg of Porapak Q adsorbent (80–100 mesh, Waters Associates, Milford, Massachusetts, USA) for 24 h (Figure 1). After sampling, each column was eluted with 1 mL of chromatographic-grade hexane (≥99%, Sigma-Aldrich) into a glass vial with PTFE-lined caps, and stored in amber vials at -80°C until chemical analysis. Porapak Q columns were cleaned and conditioned with 1 mL of redistilled diethyl ether (Merck, Darmstadt, Germany) under a nitrogen stream (70 mL min-1) at 150°C for 2 hours.

Figure 1. Dynamic headspace collection from citrus shoots under semi-field conditions. An air flow is passed through an activated charcoal filter and pumped into the bag. Simultaneously, the volatile organic compounds (VOCs) released from citrus shoots were extracted by a vacuum pump (BOECO, Hamburg, Germany), passing through glass traps filled with 100 mg of Porapak Q(™) (80-100 mesh, Waters Associates, Milford, Massachusetts, USA). The VOCs were eluted and stored in a laboratory ultrafreezer for chemical analyses and identification.
A 1-µL aliquot of the eluted VOCs was injected in splitless mode into a gas chromatograph coupled to a mass spectrometer (GC-MS; QP2010 Ultra, Shimadzu, Kyoto, Japan) equipped with an RTx5 capillary column (30 m, 0.25 mm internal diameter, 0.25 µm film thickness; Restek, Bellefonte, Pennsylvania, USA). The oven temperature program began at 40°C (held for 1 min), increased at 5°C min⁻¹ to 280°C, and was held for 5 min. Helium was used as carrier gas at a constant flow of 1 mL min⁻¹. Electron impact ionization was set at 70 eV, with a source temperature of 230°C, and mass spectra were acquired in the range of 50 to 500 m/z. VOC identification was carried out using LabSolutions GCMS software (v4.30, Shimadzu) and the NIST library (version 2.0). Although no retention indices or co-injection with authentic standards were conducted, compound identifications were based on high-quality spectral matches (≥90%) and are thus considered tentative unless otherwise specified. Quantification was conducted using the internal standard method, with tridecane (Sigma-Aldrich) as the analytical standard. Compound concentrations are expressed in µg mL-1.
2.9 Gene expression analysis by qRT-PCR
Total RNA was extracted from 100 mg of fresh leaf samples (Chang et al., 1993) collected three days after infestation. RNA yield and purity were checked by UV spectrophotometry, and RNA integrity was determined by electrophoresis. DNA was removed from 15 μg aliquots of total RNA using the TURBO DNA-free kit (Thermo, Applied Biosystems). The cDNA was synthesized from 6 μg of the DNaseI-treated RNA by means of the HighCapacity cDNA Kit (Thermo, Applied Biosystems), using random primers. Real-time qPCR was performed in a reaction mixture, final volume 25 µL, containing 100 ng of cDNA, 5 pmol of each primer, and 12.5 µL of the PowerUp SYBR Green PCR master mix (Thermo, Applied Biosystems), according to the manufacturer’s instructions. The oligonucleotides CrEF1a and CrGAPDH, annealing to the internal transcribed spacer of rRNA and encoding a member of the glyceraldehyde-3-phosphate dehydrogenase protein family, respectively, were used to amplify the internal standard with Citrus samples. The primer sequences used for the real-time qRT-PCR analysis are listed in Annexes (Supplementary Table S1; Agut et al., 2014; 2016). qPCRs were carried out using the QuantStudio(™) 3 Real-Time PCR System (ThermoFisher) following the kit instructions as follows: for 2 min at 50°C, 2 min at 95°C and then for 40 cycles of 95°C for 15 s and 60°C for 15 s, including the melt curve. The obtained Ct values were analyzed using the comparative threshold cycle or 2-ΔΔCt method (Livak and Schmittgen, 2001). Transcript levels were normalized against Elongation Factor 1-alpha (EF1a), used as the internal reference gene due to its stable expression across all scion/rootstock combinations and under Panonychus citri infestation.
2.10 Experimental design and statistical analysis
A factorial design to determine the rootstock influence on physiological and biochemical traits in the commercial mandarin scions after seven days of continuous herbivory by P. citri was applied as follows: factor 1) four rootstock levels [(WM/MA), (WM/C35), (WM/CI), and (WM/CA)], and factor 2) infestation levels (non-infested or ‘control’, and infested plants). The physiological and biochemical parameters were evaluated using three biological replicates for each scion/rootstock combination with three technical replicates each. At least five biological replicates were collected in vivo to characterise the VOCs emitted by mandarin shoots. All data were transformed using natural logarithm (Ln) transformation [ln(x+1)] to meet normality requirements. To verify the effect of four levels of rootstocks on the physio- and biochemical traits of mandarin scion in two different infestation levels, a General Linear Model (GLM) was applied. This model is statistically equivalent to a two-way ANOVA. Where significant interaction effects were found, it was conducted an post hoc analysis using a one-way ANOVA followed by Tukey’s test (P < 0.05) to compare scion/rootstock combinations within infestation levels. Additionally, to compare non-infested vs. infested plants within each scion/rootstock combination were performed Student’s t-tests (P < 0.05). gene expression values are given as the mean of the normalized expression values of five technical replicates. Genes were considered up- or down- regulated when the fold change (FC) was ≥2 relative to the non-infested control. All statistical analyses were performed using software JASP (Version 0.19.3) (JASP Team, 2024).
3 Results
3.1 Physiological parameters linked to plant defence against mite attack
The oviposition preference by P. citri, calculated as the number of eggs per leaf, showed that all scion/rootstock combinations were significantly affected by the red mite attack (F = 5.13; P = 0.0287) (Figure 2A). After seven days of infestation, the females deposited 100 to 160 eggs per leaf. The scion/rootstock combination with lowest preference was CA, followed by MA and C35, while CI was the most affected by eggs deposition.
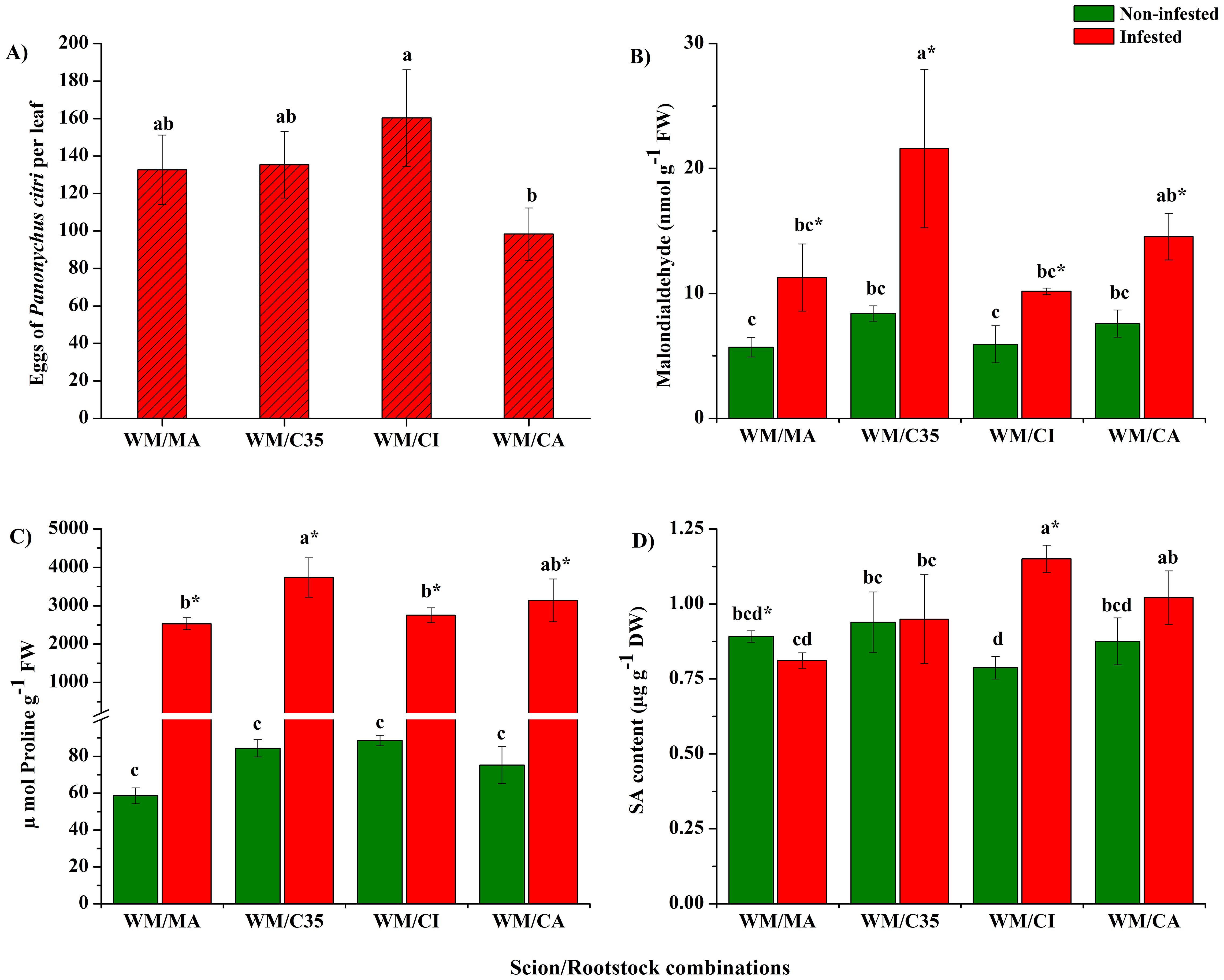
Figure 2. Oviposition preference expressed as number of eggs per leaf (A), content of malondialdehyde (MDA; B), proline (C) and salicylic acid (SA; D) in citrus leaves infested with mites (Panonychus citri) on ‘W. Murcott’s mandarin grafted on different rootstocks: Macrophylla (MA), C35, Citrumelo (CI), and Carrizo (CA). Each bar indicates the mean of three biological replicates ± SD. Significant differences between scion/rootstock combinations in non-infested and infested plants were analyzed by ANOVA followed by Tukey’s post-hoc test (different letters = all against all; P < 0.05). The asterisk indicates statistically significant differences between control and infested leaves in the same scion/rootstock combination according to Student’s t-test (P < 0.05).
Lower MDA contents were recorded in non-infested mandarin leaves from all scion/rootstock combinations than infested ones (F = 2.60; P = 0.2269). However, after infestation, the MDA levels were significantly higher in the mandarin grafted on ‘C35’ (F = 14.35; P = 0.0323; Figure 2B); therefore, P. citri injury exacerbated rootstock influence on foliar MDA content in ‘W. Murcott’ scions (F = 3.40; P = 0.0434).
Proline contents of mandarin leaves were significantly enhanced by P. citri feeding in all scion/rootstock combinations (F = 574.27; P = 0.0001; Figure 2C). Furthermore, both factors. i.e., rootstock and herbivory, affected the levels of this stress-protective metabolite (F = 8.39; P = 0.0014), with the ‘WM/C35’ combination yielding higher values than the others (Figure 2C).
As regards SA levels in mandarin leaves, results showed significant variations induced by rootstocks (F = 5.16; P = 0.0041; Figure 2D). Different from non-infested plants, SA contents increased significantly in infested mandarin grafted on ‘Citrumelo’ rootstock (F = 22.84; P = 0.0001). Thus, herbivory and rootstock interactions influenced foliar contents of SA (F = 17.58; P = 0.0001).
3.2 Rootstock influence on photosynthetic traits and pigments from ‘W. Murcott’ mandarin under mite attack
The net assimilation rate of CO2 (A) ranged from 9.356 to 11.518 µmol CO2 m-2 s-1 in control plants with the ‘WM/CI’ combination displaying the highest values. Seven days after infestation by the citrus red mite, A varied from 7.960 to 9.367 CO2 m-2 s-1 (F = 2.43; P = 0.0667; Table 2). The transpiration rate (E) of ‘W. Murcott’ mandarin leaves did not exhibit significant differences among the four scion/rootstock combinations and two P. citri infestation levels (F = 2.22; P = 0.0888; Table 2). By contrast, stomatal conductance (gs) varied depending on the scion/rootstock combination (F = 13.50; P = 0.0001), whereas herbivory had no effect on this parameter (F = 7.39; P = 0.0727).
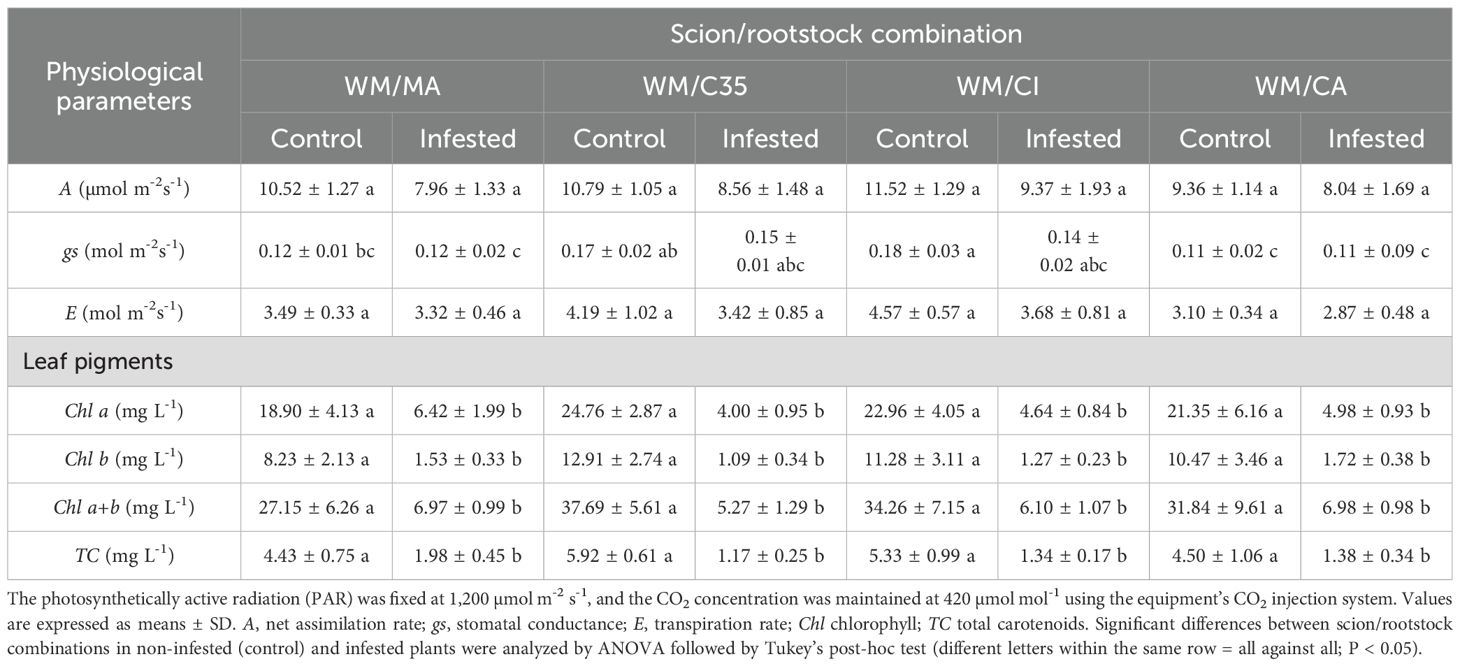
Table 2. Physiological parameters and photosynthetic pigments of ‘W. Murcott’ mandarin leaves grafted onto ‘Macrophylla’ (Citrus macrophylla Wester), ‘C35’ [C. sinensis × P. trifoliata (South African)] ‘Citrumelo’ (Citrus paradisi Macf. ‘Duncan’ grapefruit × P. trifoliata), and ‘Carrizo citrange’ (Citrus sinensis (L.) Osbeck × Poncirus trifoliata (L.) Raf.) rootstocks under Panonychus citri attack under semi-field conditions.
Rootstocks did not exert a significant influence on the concentrations of chlorophyll a and b, as well as carotenoids (chlorophyll a, F = 0.31; P = 0.8149; chlorophyll b, F = 1.09, P = 0.3828; chlorophyll a+b, F = 0.76, P = 0.5343; carotenoids, F = 0.89, P = 0.4658; Table 2). After seven days of herbivory, however, pigment concentrations for ‘W. Murcott’ tended to decrease significantly on all rootstocks (chlorophyll a, F = 160.99, P = 0.0001; chlorophyll b, F = 122.27, P = 0.0001; chlorophyll a+b, F = 152.96, P = 0.0001; carotenoids, F = 178.26, P = 0.0001).
3.3 Rootstock influence on biochemical and metabolic traits from ‘W. Murcott’ under herbivory
Citrus rootstocks did not affect soluble sugar levels (F = 1.09; P = 0.4727) in mandarin scions. However, these decreased significantly after P. citri injury when grafted on ‘MA’ and ‘CI’, going below constitutive levels of leaf sugar content (F = 20.91; P = 0.0196) (Figure 3A).

Figure 3. Total sugar (A) and soluble protein (B) contents in leaves of ‘W. Murcott’ mandarin scions grafted on Macrophylla (MA), C35, Citrumelo (CI), or Carrizo (CA) rootstocks and infested or not with mites (Panonychus citri) on Values are the means of five biological replicates ± SD. Significant differences between scion/rootstock combinations in control and non-infested plants were analyzed by ANOVA followed by Tukey’s post-hoc test (different letters = all against all; P < 0.05). Asterisks indicate statistically significant differences between control and infested leaves in the same scion/rootstock combination according to Student’s t-test (P < 0.05).
Soluble protein levels were higher in non-infested mandarin plants grafted on ‘Citrumelo’ than on other rootstocks (F = 9.09; P = 0.0001) (Figure 3B). Thus, results indicate that rootstocks influence leaf protein contents (F = 5.85; P = 0.0068). Moreover, after P. citri infestation, soluble proteins diminished in mandarin leaves in three of the four combinations compared to non-infested plants, except on the ‘CA’ rootstock (F = 27.17; P = 0.0001; Figure 3B), when it increased slightly.
Rootstocks did not significantly affect TPC (F = 4.14; P = 0.2037; Figure 4A), which ranged between 0.46 ± 0.11 and 0.67 ± 0.12 mg GAEs g-1 DW, nor TFC (F = 0.77; P = 0.5268; Figure 4B) in mandarin leaves. On all rootstocks, TFC, that ranged from 0.035 ± 0.007 to 0.042 ± 0.011 mg REs g-1 DW, diminished significantly (at least three-fold) after P. citri infestation (F = 155.82; P = 0.0001; Figure 4B) compared to non-infested plants.
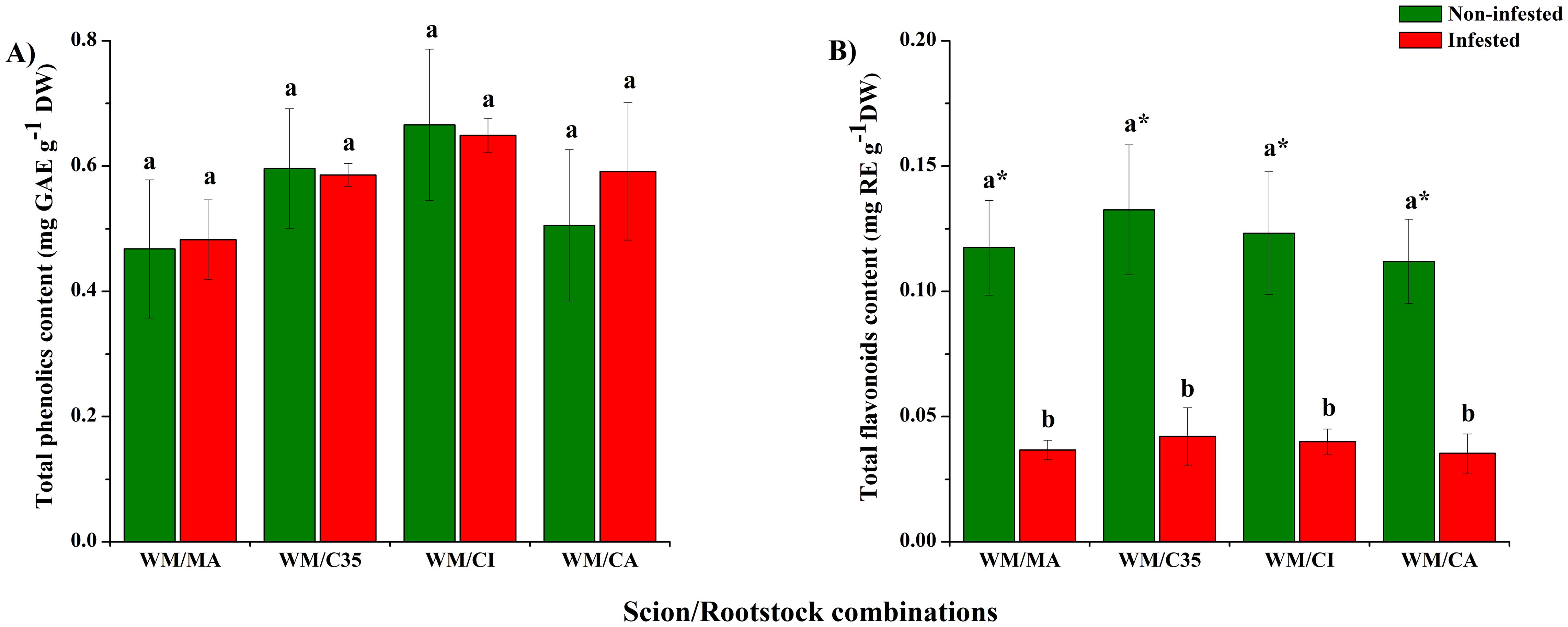
Figure 4. Total content of phenolic compounds (A) and flavonoids (B) in leaves of ‘W. Murcott’s mandarin grafted on Macrophylla (MA), C35, Citrumelo (CI), or Carrizo (CA). Rootstocks and infested or not with mites (Panonychus citri).: Values are the means of three biological replicates ± SD. Significant differences between scion/rootstock combinations in control and non-infested plants were analyzed by ANOVA followed by Tukey’s post-hoc (different letters = all against all; P < 0.05). Asterisks indicate statistically significant differences between control and infested leaves in the same scion/rootstock combination according to Student’s t-test (P < 0.05).
3.4 Scion/rootstock interaction on VOCs emitted by ‘W. Murcott’ leaves infested with P. citri
As shown in Table 3, chemical profiles showed slight differences between ‘W. Murcott’/rootstock combinations, mainly with respect to undetected compounds, whereas 2,3,3-trimethylhexane, and 2,4-dimethylhept-1-ene were only registered from scions grafted on ‘MA’. hexan-3-ol (3-hexanol), 2,2,4-trimethyldecane and 3-ethylbenzaldehyde were identified only in two or three scion/rootstock combinations. After seven days of infestation, (1R)-2-methyl-5-propan-2- ylbicyclo [3.1.0] hex-2-ene (α-thujene), [(Z)-hex-3-enyl] acetate (cis-3-Hexenyl acetate), methyl 2-hydroxybenzoate (methyl salicylate, MeSA), (3E, 6E) - 3, 7, 11 -trimethyldodeca-1,3,6,10-tetraene (α-farnesene),(3E)-3,7-dimethylocta-1,3,6-triene(β- ocimene), 3,7-dimethylocta-1,6-dien-3-ol (linalool), and (6R)-3-methylidene-6-propan-2-ylcyclohexene (β-phellandrene) were detected only from infested scions (Table 3). Moreover, significant variations were caused by rootstocks after herbivory (Supplementary Figure S2); thus, HIPVs released from ‘W. Murcott’ scions, such as (1S,5S)-6,6-dimethyl-2-methylidenebicyclo[3.1.1]heptane (β-pinene), cis-3-Hexenyl acetate, and linalool, varied significantly between scion/rootstock combinations (Table 3). The amounts of MeSA and (4R)-1-methyl-4-prop-1-en-2-ylcyclohexene (D-limonene) released from infested shoots did not show significant differences in any of the scion/rootstock combinations (Table 3). Although identification was not confirmed via retention indices or co-injection with authentic standards, the observed differences in compound abundance support biologically meaningful interpretations and provide a basis for selecting candidate VOCs for functional assays.

Table 3. Concentrations of volatile organic compounds emitted from (Citrus reticulata Blanco) ‘W. Murcott’ scion grafted onto ‘Macrophylla’ (Citrus macrophylla Wester), ‘C35’ (C. sinensis × P. trifoliata (South African)) ‘Citrumelo’ (Citrus paradisi Macf. ‘Duncan’ grapefruit × P. trifoliata), and ‘Carrizo citrange’ (Citrus sinensis (L.) Osbeck × Poncirus trifoliata (L.) Raf.) rootstocks. Values represent mean concentration (n = 6) ± standard error (µg mL-1) of volatile organic compounds (VOCs) identified based on ≥90% spectral match with the NIST library (v2.0).
3.5 Gene expression of infested scions during P. citri attack
This section presents gene expression data from infested scions. The control (fold change = 1) corresponds to a pooled baseline composed of all non-infested scion/rootstock combinations, calculated separately for each transcript. Specifically, CNT represents the average Ct value of non-infested controls across all rootstocks (Supplementary Figure S3) shows the Ct values of non-infested controls for each rootstock). This pooled control was used to normalize gene expression levels, allowing consistent comparison of infestation-induced responses among genotypes. Of all the genes explored, EIN3, PR3, and GLR did not show detectable and stable levels of transcripts through replicates in non-infested control ‘W. Murcott’ scions. Transcript accumulation of ABA4 (Figure 5A) was significantly down-regulated in scions grafted onto ‘CI’, ‘CA’, and ‘MA’ rootstocks, up to 12-fold in the case of ‘Carrizo citrange’. Conversely, ‘C35’ rootstock significantly increased ABA4 transcript levels in response to insect attack.
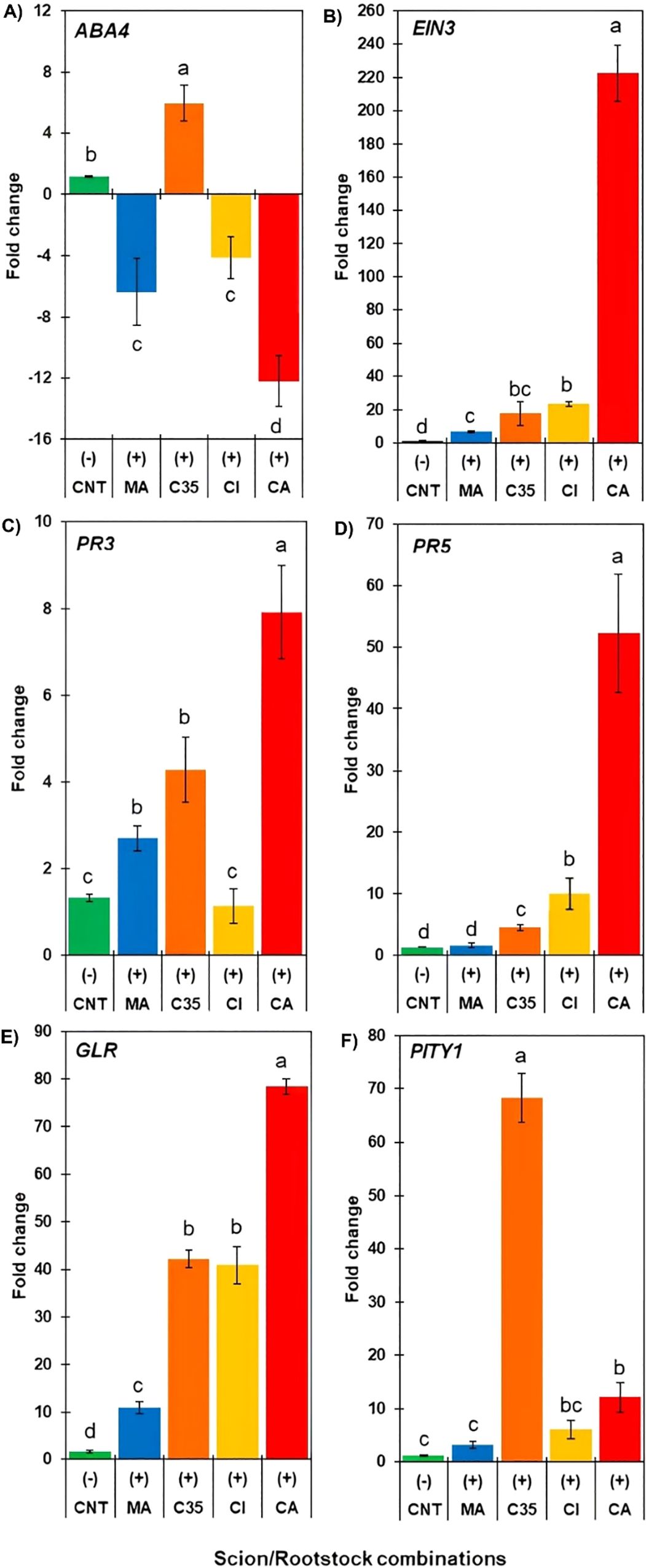
Figure 5. qRT-PCR analysis (FC, fold change) of genes related to phytohormone biosynthesis [(A) abscisic acid (B)] and ethylene) and defence (C–F) in mandarin scions grafted on different Citrus rootstocks (MA, C35, CI, and CA) seven days after being infested with red mite. Expression values are means of five technical replicates, normalized against EF1a as a reference gene. Gene expression was analyzed relative to a pooled non-infested control (CNT), composed of all non-infested scion/rootstock combinations, and calculated separately for each gene. Genes were considered up- or down-regulated when fold change (FC) relative to the non-infested control on the same rootstock (CNT) was ≥ 2. Different letters indicate statistically significant differences among combinations according to Tukey’s test (P < 0.05). Error bars represent standard error of the mean.
EIN3 was significantly up-regulated in mandarin scions grafted onto ‘CI’, ‘C35’, and ‘CA’ rootstocks, with the latter being upregulated over 200-fold compared to the control. It exhibits one of the highest expression levels among the genes evaluated in this study (Figure 5B).
The expression of PR3 was significantly higher in the ‘W. M./CA’ combination, reaching up to 8-fold control levels; ‘WM/MA’ and ‘WM/C35’ combinations also showed a lower but significant increase, while on ‘Citrumelo’ rootstock, scions maintained similar transcript levels to the controls (Figure 5C). As for PR5, ‘WM/CA’, ‘WM/CI’, and ‘WM/C35’ showed a significant increase compared to the control, while ‘WM/MA’ remained unchanged in response to red mite herbivory. PR3 was generally less expressed than PR5, the latter being up-regulated up to 50-fold control level ‘WM/CA’ (Figure 5D). The expression of GLR significantly increased for all rootstocks in response to red mite attacks, with scions grafted on ‘CA’ showing the most significant increase (80-fold over the control), followed by ‘CI’, ‘C35’, and ‘Macrophylla’ (10-fold control levels; Figure 5E). PITY1 transcript accumulation significantly increased in mandarin grafted on ‘C35’ (up to 70-fold over the control), followed by ‘CA’ (12-fold), while ‘CI’ and ‘MA’ did not induce significant differences compared to the control (Figure 5F).
4 Discussion
4.1 Red mite attack activates stress responsive biological markers
The number of eggs of P. citri after seven days of infestation ranged from approximately 100 to 160 eggs per leaf and can be considered detrimental to the physio-biochemical functioning of W. Murcott, as in other mandarin cultivars (Agut et al., 2016).
MDA is widely used as a marker of membrane integrity and stress tolerance in plants (Morales and Munné-Bosch, 2019; Sheri et al., 2023), as it activates regulatory genes related to plant defence and development. After seven days of continuous P. citri infestation, all scion/rootstock combinations showed elevated MDA levels, with ‘WM/C35’ exhibiting the highest. Similar MDA increases have been reported in T. urticae - infested bean plants (Farouk and Osman, 2012), whereas in cucumber, MDA initially rose but later declined under sustained mite feeding (Shahtousi and Talaee, 2023), suggesting that lower levels of MDA may reflect reduced damage, greater antioxidant capacity, and increased tolerance to herbivores. Our findings support the idea that both ‘C35’ and ‘CA’ rootstock are less effective in limiting membrane damage, as reflected by higher MDA accumulation. This agreed with increased proline and ABA4 expression in the ‘C35’ rootstock/scion combination, pointing to greater susceptibility to red mite attack.
The osmolyte proline has been extensively studied in grafted citrus under abiotic stressors such as salinity, drought, and heat (Shahid et al., 2019; Balfagón et al., 2022). Our results show that the different rootstocks did not significantly influence constitutive levels of this stress-protective compound. By contrast, insect attacks are known to induce osmolyte accumulation, including proline, as reported in plants infected by fungi, viruses, or infested by T. urticae (Qamar et al., 2015; Anzano et al., 2022; Farouk and Osman, 2012). Notably, proline levels decrease in mite-susceptible wild rice (Oryza barthii) leaves but increase in tolerant cultivars following Schizotetranychus oryzae attack (Acari: Tetranychidae; Buffon et al. (2021). Similarly, we observed a significant post-infestation increase in proline across all scion/rootstock combinations, with the highest levels in ‘C35’ rootstock. This suggest that while constitutive proline remained stable, its inductibility under mite stress may reflect an active, though not necessarily protective, response.
Our results show that basal SA levels in mandarin leaves were rootstock-dependent, with the lowest levels observed in the ‘CI’ combination. This agrees with Agut et al. (2016), who found significant differences in constitutive SA content in ‘Clemenules’ scions grafted onto different rootstocks. Beyond its developmental roles, SA is a key phytohormone in plant defence (Mishra et al., 2024). Upon T. urticae infestation, SA levels increased in ‘Clemenules’/’Cleopatra’ after three days, while ungrafted ‘Sour orange’ exhibited higher SA than ‘Cleopatra’ after seven days (Agut et al., 2014, 2016). Similarly, Leus et al. (2022) reported elevated SA in R. simsii cultivars infested by P. latus. Consistent with these findings, we observed significant SA accumulation in ‘W. Murcott’ grafted onto ‘CI’ and ‘CA’ rootstocks following seven days of P. citri infestation.
4.2 Scion/rootstock combinations modulates photo-assimilation and photosynthetic pigments under red mite attack
Yulianti and Agisimanto (2023) reported that grafting ‘Pontianak’ tangerine onto ‘Japansche citroen’ and ‘Citrumelo’ rootstocks significantly affected photosynthetic rate, whereas no differences were observed with ‘Montaji’ lemon. In our study, although P. citri infestation led to reductions in A and E in ‘W. Murcott’, the differences were not significant; however, gs was significantly influenced by rootstock. Similarly, Yulianti and Agisimanto (2023) observed no gs variation in their grafting experiments. Earlier, Hare and Youngman (1987) found no significant physiological changes in ‘Washington Navel’ oranges infested by P. citri, suggesting leaf tolerance. In contrast, T. urticae infestation significantly reduced photosynthesis in cotton (Reddall et al., 2007) and lowered A, gs, and E in J. curcas (Hsu et al., 2015). Overall, our data suggest that ‘W. Murcott’ grafted onto the tested rootstocks tolerates P. citri infestation for at least seven days without marked impairment of photosynthetic performance.
Photosynthetic pigments are known to be affected by biotic stresses (Li et al., 2024). In our study, rootstocks did not alter chlorophyll or carotenoid levels in mandarin leaves, but P. citri feeding significantly reduced both pigments, potentially contributing to the observed, albeit non-significant, decline in photosynthetic parameters. Similarly, Blasi et al. (2017) reported chlorophyll loss in S. oryzae-infested rice leaves. In contrast, T. urticae infestation increased carotenoid content in beans (Farouk and Osman, 2012), while J. curcas showed no pigment changes under similar infestation (Hsu et al., 2015).
4.3 Rootstocks influence primary and secondary metabolites in ‘W. Murcott’ leaves under mite attack
Our results show that citrus rootstocks did not significantly affect constitutive soluble sugar levels in ‘W. Murcott’ mandarin. However, following P. citri infestation, sugar content declined significantly, particularly in plants grafted onto ‘CI’ and ‘MA’. This aligns with studies reporting sugar level changes under herbivory: increases in T. urticae-infested beans (Farouk and Osman, 2012) and T. evansi-infested tomato (Ximénez-Embún et al., 2018), but decreases in J. curcas (Hsu et al., 2015). Hayat et al. (2022) also noted rootstock-driven sugar variability in mandarin leaves, with ‘Trifoliate Orange’ inducing the highest content. The observed sugar depletion in our study may reflect a resource reallocation strategy under biotic stress, where breakdown of reserves contributes to defence signaling (Van den Ende and El-Esawe, 2014). In particular, the ‘WM/CI’ combination appears especially reactive to mite attack, mirroring findings in sugarcane under aphid pressure (Koch et al., 2020).
Soluble proteins are crucial for plant growth and defence against biotic stress (Han et al., 2023). In our study, ‘W. Murcott’ grafted onto ‘CI’ exhibited significantly higher basal protein levels, indicating a rootstock effect. Similar influences have been reported in ‘Shatangju’ and ‘March Seedless’ grafted onto protein-promoting rootstocks like ‘Citrange’, ‘Flying Dragon’, and ‘Troyer citrange’ (Hayat et al., 2022; Sharma et al., 2015). Upon P. citri infestation, protein levels declined significantly across most combinations, except for ‘CA’, suggesting a rootstock-dependent response. Such reductions mirror those observed in T. urticae-infested J. curcas and T. evansi-infested tomato (Hsu et al., 2015; Ximénez-Embún et al., 2018). Mite-secreted effectors are known to manipulate plant proteomes, including components of the ubiquitin-proteasome system, autophagy, phytohormone signaling, and transcription regulation (Blaazer et al., 2018; Zhao and Wang, 2024). Likewise, S. oryzae infestation down-regulated defence- and metabolism-related proteins in rice (Blasi et al., 2017). These findings suggest that P. citri may similarly suppress host protein-based defences to enhance its fitness.
Rootstocks did not significantly affect constitutive total phenolic content (TPC) in ‘W. Murcott’, although higher levels were observed in scions grafted onto ‘C35’ and ‘CI’. Similarly, Legua et al. (2014) found increased TPC in ‘Clemenules’ grafted onto ‘Volkameriana’. After seven days of P. citri infestation, TPC remained unchanged in most combinations but showed an increasing trend in ‘WM/CA’. In contrast, T. urticae infestation led to significantly higher TPC in beans (Farouk and Osman, 2012), suggesting species-specific or stress duration-dependent phenolic responses.
As with TPC, rootstocks did not influence constitutive flavonoid content in ‘W. Murcott’ leaves. However, other studies have shown rootstock effects: higher flavonoid levels were reported in ‘Maltese half-blood’ orange grafted onto ‘Volkameriana’ (Zouaghi et al., 2018) and in ‘Newhall’/P. trifoliata compared to ‘Newhall’/C. junos (Li et al., 2023). Flavonoids contribute to plant defence through deterrent and antifungal properties and accumulate in response to bacterial infections (Anzano et al., 2022). In our study, total flavonoid content (TFC) significantly declined after seven days of P. citri infestation, suggesting mite-mediated suppression of defence pathways. This is consistent with findings in tomato, where T. evansi and T. urticae reduced flavonoid levels and suppressed associated signalling pathways (Knegt et al., 2020; Su et al., 2020). P. citri may act similarly, downregulating flavonoid-dependent defences in mandarin.
Citrus VOC emissions are influenced by rootstocks (Jones and Killiny, 2021; Guarino et al., 2022). For example, ‘Minneola’ grafted onto ‘MA’ releases β-phellandrene, caryophyllene, citronellol, and cis-p-mentha-2,8-dien-1-ol—compounds absent in lime leaf emissions (Rioja and Ceballos, 2024). Rootstock-dependent changes in VOC profiles have also been observed under Citrus tristeza virus infection (Guarino et al., 2022). In our study, ‘W. Murcott’ VOC profiles were only slightly affected by rootstock, with minor variations in alcohols, alkanes, and aromatic aldehydes across combinations, similar to findings by Jones and Killiny (2021). Herbivory can trigger the release of HIPVs as indirect defences. For instance, increased emissions of D-limonene, ocimene, and MeSA have been documented in citrus infested by Aonidiella aurantii (Alsabte et al., 2022), and higher levels of MeSA, azulene, and 2-ethylhexan-1-ol were detected in mite-infested ‘Minneola’ (Rioja and Ceballos, 2024). In avocado, O. yothersi induced exclusive emissions of β-ocimene, linalool, α-farnesene, and MeSA, which also act as repellents (Rioja et al., 2016, 2018). In our study, MeSA was consistently detected in all P. citri-infested scion/rootstock combinations, reinforcing its role as a key HIPV mediating tri-trophic interactions (Abdala-Roberts et al., 2019). Seemingly, the citrus rootstocks do not appear to affect the tritrophic interactions; therefore, studies on the behavioural responses in predators of P. citri are required. Present results show that the chemical profiles changed both quantitatively and qualitatively after P. citri infestation. High emissions of α-thujene, β-pinene, and β-phellandrene were registered only in the ‘WM/CI’ combination, indicating that citrus rootstocks markedly affect the indirect induced defences in mandarin scions.
4.4 Scion/rootstock combinations differentially affect phytohormone- and defence- related genes under mite attack
Gene expression is a sensitive indicator of plant responses of early molecular responses to stress (Wang et al., 2023). Phytohormones like ethylene (ET) and abscisic acid (ABA) modulate defence gene expression, often via antagonistic pathways (Li et al., 2019; Yu et al., 2021; Müller, 2021). ABA4, which encodes a membrane protein involved in neoxanthin synthesis and stress-induced ABA accumulation (North et al., 2007), was generally downregulated in our study, except in the ‘C35’ combination, where it was significantly upregulated. Elevated ABA4 expression, along with high MDA levels, suggests greater membrane damage and stress in this rootstock under P. citri infestation, consistent with responses observed in A. thaliana (Barczak-Brzyżek et al., 2017; Rosa-Diaz et al., 2024), Tamarix nilotica (Younis, 2021), and ‘Cleopatra’ mandarin (Agut et al., 2014). Conversely, EIN3, an ET-responsive transcription factor, was strongly upregulated across all combinations, reaching a 220-fold increase in ‘WM/CA’. EIN3 is a central regulator of ET signaling and downstream defence responses (Solano et al., 1998; Binder, 2020; Pérez-Hedo et al., 2024) and has been similarly induced in cassava infested by T. urticae (Yang et al., 2019). These findings highlight distinct hormonal response strategies to mite attack among rootstock combinations.
Pathogenesis-related (PR) proteins play key roles in plant defence by reinforcing cell structures and exerting enzymatic activity against pathogens (Van Loon et al., 2006; Han et al., 2023). Members of the PR family have specific functions: chitinases (PR-3) act via the JA pathway, while thaumatin/osmotin-like proteins (TLPs; PR-5) are SA-responsive. In our study, both PR-3 and PR-5 were strongly upregulated by P. citri infestation in ‘WM/CA’ and ‘WM/C35’ combinations, with PR-5 reaching transcript levels ten times higher than PR-3. PR-5 is known to be recruited by PR-1 to enhance resistance through ROS-dependent amplification of immune responses (Han et al., 2023). The accumulation of PR gene transcripts is a hallmark of SA- and JA-mediated defence and is associated with the production of antimicrobial proteins such as glucanases (PR-2), chitinases (PR-3, PR-4), and TLPs (PR-5; Gkizi et al., 2016). In citrus infested by Tetranychus spp., PR-5 expression increases early, while PR-3 induction is delayed but sustained (Agut et al., 2014, 2015), consistent with our findings.
Some mite species can suppress defence-related gene expression. In tomato (S. lycopersicum var. Santa Clara I-5300), T. evansi suppressed WIPI-II and PR-P6, genes linked to JA and SA pathways, respectively, whereas T. urticae upregulated both (Sarmento et al., 2011). Similarly, in azalea, P. latus initially induced JA accumulation, but later significantly increased SA levels, suggesting suppression of JA-mediated defences to enable sustained infestation without compromising mite fitness (Leus et al., 2022).
The citrus Protein Inhibitor Type 1 (PI TYPE1) gene is a known marker of arthropod-induced defence (Agut et al., 2014). In our study, PITY1 was significantly upregulated across all scion/rootstock combinations, with the highest expression observed in ‘WM/C35’ (65-fold), followed by ‘CA’ (12-fold), and lower increases in ‘CI’ and ‘MA’ (7- and 3-fold, respectively). Similar strong induction of PI genes has been reported in tomato under T. urticae attack, where they emerged as prominent defence-related transcripts in microarray analyses (Martel et al., 2015), reinforcing their role as key molecular markers in plant responses to mite herbivory.
The putative glutamate receptor-like (GLR) gene was strongly upregulated in all scion/rootstock combinations, with expression increasing 10-fold in ‘MA’, 40-fold in ‘C35’ and ‘CI’, and up to 80-fold in ‘CA’. GLR proteins play key roles in sensing leaf damage and regulating defence signalling pathways, as well as in wound and pathogen responses (Mousavi et al., 2013; Yan et al., 2024). In mite-infested sour orange, GLR overexpression and glutamate accumulation were linked to systemic resistance (Agut et al., 2016). Exogenous glutamate application also primed plants for stronger, faster responses to pests and pathogens, highlighting the role of GLRs in early defence signalling.
In conclusion, Panonychus citri herbivory amplifies rootstock-driven differences in the physiological, biochemical, and molecular responses of ‘W. Murcott’ mandarin scions under semi-field conditions. Rootstocks significantly influenced stress markers—including MDA, proline, SA, soluble sugars, and proteins—as well as VOC emission profiles, indicating modulation of both primary and secondary metabolism. Among the combinations tested, ‘WM/CI’ and ‘WM/CA’ emerged as promising rootstocks for enhancing scion performance and red mite tolerance. ‘WM/CI’ showed the lowest MDA levels and highest accumulation of defence-related metabolites, while ‘WM/CA’ promoted reprogramming of defence genes, including ABA4 suppression. The consistently higher expression of PR5 over PR3 across combinations support a predominantly SA-mediated defence response. These integrated responses are visually summarized in the heatmap (Figure 6), which highlights distinct biochemical and transcriptional patterns across scion/rootstock combinations induced by infestation. Both ‘WM/CI’ and ‘WM/CA’ showed increased VOC emission (e.g., β-pinene, MeSA, β-ocimene) and upregulated PR5 and GLR expression, suggesting strong inducible defenses. In contrast, ‘WM/MA’ displayed limited changes in stress markers and gene expression, indicating weaker inducible responses due to red mite attack. Meanwhile, ‘WM/C35’ was distinguished by its high MDA accumulation and strong induction of PITY1, pointing to more pronounced oxidative stress and activation of damage-related pathways.
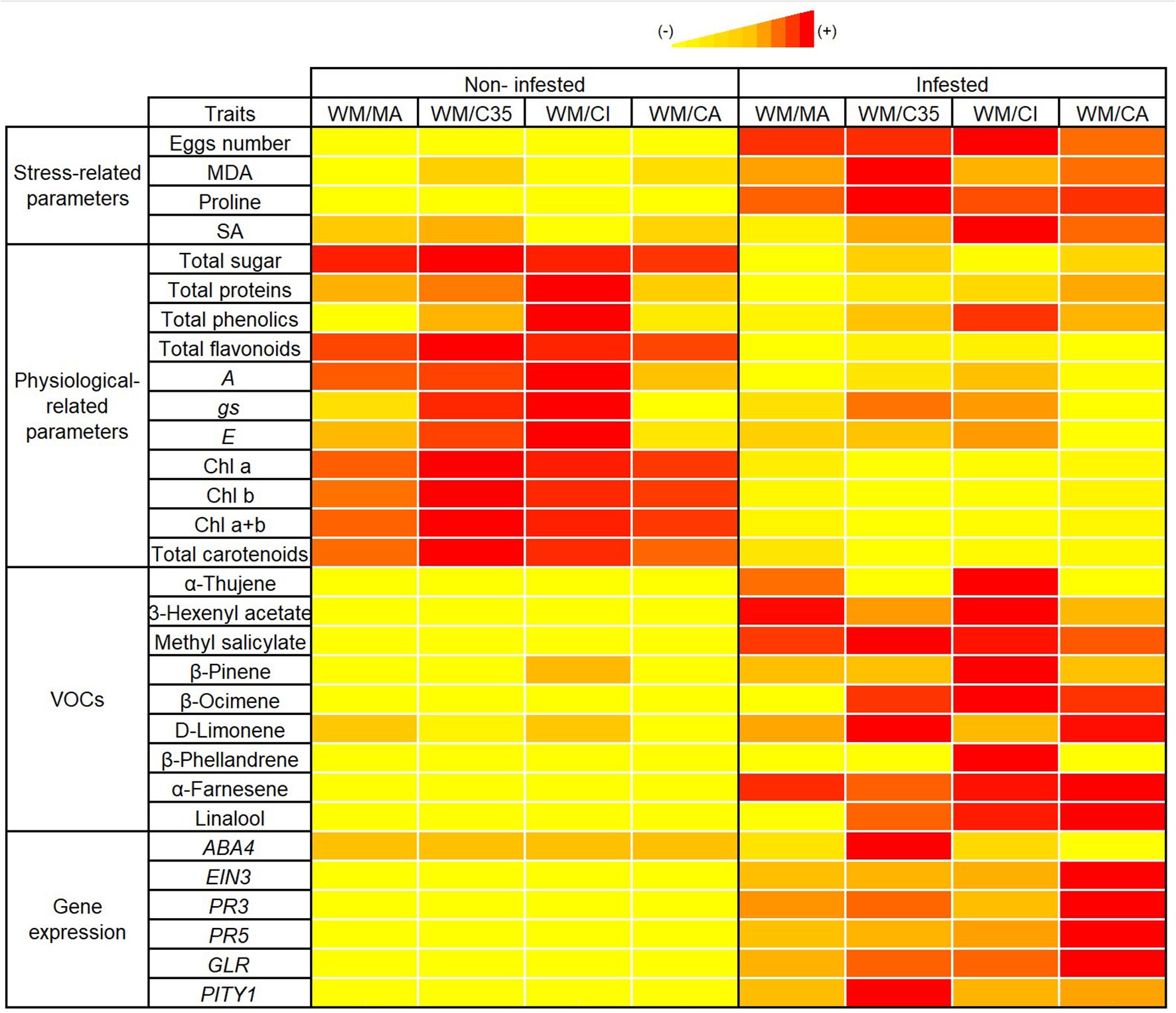
Figure 6. Heatmap showing changes in physiological, biochemical, molecular, and volatile organic compound (VOC) traits in four Citrus scion–rootstock combinations, comparing non-infested and Panonychus citri-infested plants. Combinations include ‘WM/MA’ ('Macrophylla', Citrus macrophylla Wester), ‘WM/C35’ [C. sinensis × P. trifoliata (South African)], ‘WM/CI’ [‘Citrumelo’ (Citrus paradisi Macf. ‘Duncan’ grapefruit × P. trifoliata)], and ‘WM/CA’ ['Carrizo citrange' (Citrus sinensis (L.) Osbeck × Poncirus trifoliata (L.) Raf.)], each grafted with ‘W. Murcott’ (WM) as the scion. Traits are grouped into stress-related parameters, physiological parameters, VOCs, and defense-related gene expression. Color intensity represents the relative value of each trait within the non-infested vs. infested comparison for each combination, with red indicating the highest value, followed by orange and yellow, reflecting decreasing relative levels. Parameters include malondialdehyde (MDA), proline, salicylic acid (SA), photosynthesis (A), stomatal conductance (gs), transpiration (E), chlorophyll (Chl a, Chl b, Chl a+b), carotenoids, selected VOCs (e.g., α-thujene, β-pinene, methyl salicylate), and defense-related gene expression (ABA4, EIN3, PR3, PR5, GLR, PITY1). Each trait was independently normalized using min–max scaling across both infestation conditions for a given combination, allowing comparison of within-combination variation. This multivariate representation provides an integrated overview of rootstock-modulated responses to red mite infestation.
Despite extensive research on ungrafted citrus rootstocks and abiotic stress (Simpson et al., 2014; Long et al., 2017; Huang et al., 2020; Arjona-López et al., 2023), few studies have addressed how rootstocks modulate scion responses to herbivory (Agut et al., 2016; Shaltiel-Harpaz et al., 2018). Our findings underscore the pivotal role of rootstock selection in shaping scion resilience under biotic stress, through coordinated changes in metabolite profiles and gene expression. Future research should explore the functional roles of key metabolites and regulatory genes, and evaluate resistance priming through exogenous hormone applications. Ultimately, integrating multi-level markers - from metabolic to transcriptional -offers a robust framework for rootstock selection in breeding and nursery programmes aimed at developing citrus cultivars resilient to evolving agroecological challenges.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://github.com/TOMMYRIOJA/data-repository-publication-citrus-Panonychus-citri.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
TR: Investigation, Supervision, Conceptualization, Writing – review & editing, Funding acquisition, Writing – original draft, Methodology, Formal Analysis, Resources, Visualization. KR: Writing – review & editing, Formal Analysis, Writing – original draft, Methodology, Conceptualization, Supervision, Visualization. RC: Formal Analysis, Writing – review & editing, Methodology.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the ‘Agencia Nacional de Investigación y Desarrollo de Chile’ (ANID), FONDECYT Iniciación project INI 11200852 and project UTA-MAYOR 9734-23. We would like to express our gratitude to the ‘Agencia Nacional de Investigación y Desarrollo de Chile’ (ANID) for their support through the FONDECYT Iniciación project INI 11200852, and project UTA-MAYOR 9734-23.
Acknowledgments
We thank FONDEQUIP Project EQM190088 for making available the IRGA6800 to acquire photosynthetic data, and to Ms. Carolina Navea, biotechnologist (bmF2ZWEuY2Fyb2xpbmFAZ21haWwuY29t) for the figures design presented. The authors would like to thank Stefania Biondi and Francesca Rapparini, senior plant biologists, for their valuable contributions to improving the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1645535/full#supplementary-material
References
Abdala-Roberts, L., Puentes, A., Finke, D. L., Marquis, R. J., Montserrat, M., Poelman, E. H., et al. (2019). Tri-trophic interactions: bridging species, communities and ecosystems. Ecol. Lett. 22, 2151–2167. doi: 10.1111/ele.13392
Agusti, M., Mesejo, C., and Reig, C. (2020). Citricultura. Tercera edición (Madrid, España: Ediciones Mundi-Prensa).
Agut, B., Gamir, J., Jacas, J. A., Hurtado, M., and Flors, V. (2014). Different metabolic and genetic responses in citrus may explain relative susceptibility to Tetranychus urticae. Pest Manag Sci. 70, 1728–1741. doi: 10.1002/ps.3718
Agut, B., Gamir, J., Jaques, J. A., and Flors, V. (2015). Tetranychus urticae - triggered responses promote genotype-dependent conspecific repellence or attractiveness in citrus. New Phytol. 207, 790–804. doi: 10.1111/nph.13357
Agut, B., Gamir, J., Jaques, J. A., and Flors, V. (2016). Systemic resistance in citrus to Tetranychus urticae induced by conspecifics is transmitted by grafting and mediated by mobile amino acids. J. Exp. Bot. 67, 5711–5723. doi: 10.1093/jxb/erw335
Alfaro-Quezada, J. F., Martínez, J. P., Molinett, S., Valenzuela, M., Montenegro, I., Ramírez, I., et al. (2023). Rootstock increases the physiological defence of tomato plants against Pseudomonas syringae pv. tomato infection. J. Exp. Bot. 74, 2891–2911. doi: 10.1093/jxb/erad040
Ali, M. Y., Naseem, T., Holopainen, J. K., Liu, T., Zhang, J., and Zhang, F. (2023). Tritrophic Interactions among Arthropod Natural Enemies, Herbivores and Plants Considering Volatile Blends at Different Scale Levels. Cells 12, 251. doi: 10.3390/cells12020251
Alsabte, A., Ahmed, Q. H., Kayahan, A., and Karaca, I. (2022). Effects of Volatile Organic Compounds (VOCs) emitted by citrus infested with Aonidiella aurantii on the predator Rhyzobius lophanthae attraction. Phytoparasitica 50, 645–653. doi: 10.1007/s12600-022-00978-4
Anzano, A., Bonanomi, G., Mazzoleni, S., and Lanzotti, V. (2022). Plant metabolomics in biotic and abiotic stress: a critical overview. Phytochem. Rev. 21, 503–524. doi: 10.1007/s11101-021-09786-w
Arjona-López, J. M., Aparicio-Durán, L., Gmitter, F. G., Jr., Romero-Rodríguez, E., Grosser, J. W., Hervalejo, A., et al. (2023). Physiological influence of water stress conditions on novel HLB-tolerant citrus rootstocks. Agronomy 13, 63. doi: 10.3390/agronomy13010063
Balfagón, D., Rambla, J. L., Granell, A., Arbona, V., and Gómez-Cadenas, A. (2022). Grafting improves tolerance to combined drought and heat stresses by modifying metabolism in citrus scion. Environ. Exp. Bot. 195, 104793. doi: 10.1016/j.envexpbot.2022.104793
Barczak-Brzyżek, A., Kiełkiewicz, M., Górecka, M., Kot, K., Karpińska, B., and Filipecki, M. (2017). Abscisic Acid Insensitive 4 transcription factor is an important player in the response of Arabidopsis thaliana to two-spotted spider mite (Tetranychus urticae) feeding. Exp. Appl. Acarol. 73, 317–326. doi: 10.1007/s10493-017-0203-1
Bates, L. S., Waldren, R. P., and Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. doi: 10.1007/BF00018060
Binder, B. M. (2020). Ethylene signaling in plants. J. Biol. Chem. 295, 7710–7725. doi: 10.1074/jbc.REV120.010854
Blaazer, C. J. H., Villacis-Perez, E. A., Chafi, R., Van Leeuwen, T., Kant, M. R., and Schimmel, B. C. J. (2018). Why do herbivorous mites suppress plant defenses? Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01057
Blasi, É.A.R., Buffon, G., Rativa, A. G. S., Lopes, M. C. B., Berger, M., Santi, L., et al. (2017). High infestation levels of Schizotetranychus oryzae severely affects rice metabolism. J. Plant Physiol. 219, 100–111. doi: 10.1016/j.jplph.2017.10.005
Buffon, G., Blasi, É.A.R., Lamb, T. I., Adamski, J. M., Schwambach, J., Ricachenevsky, F. K., et al. (2021). Oryza sativa cv. Nipponbare and Oryza barthii as Unexpected Tolerance and Susceptibility Sources Against Schizotetranychus oryzae (Acari: Tetranychidae) Mite Infestation. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.613568
Chang, S., Puryear, J., and Cairney, J. A. (1993). simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 11, 113–116. doi: 10.1007/BF02670468
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
Erb, M., Foresti, N., and Turlings, T. (2010). A tritrophic signal that attracts parasitoids to host-damaged plants withstands disruption by non-host herbivores. BMC Plant Biol. 10, 247. doi: 10.1186/1471-2229-10-247
Erb, M., Meldau, S., and Howe, G. A. (2012). Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 17, 250–259. doi: 10.1016/j.tplants.2012.01.003
Erb, M., Veyrat, N., Robert, C. A., Xu, H., Frey, M., Ton, J., et al. (2015). Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 6, 6273. doi: 10.1038/ncomms7273
FAOSTAT (2025). Statistical database from the Food and Agriculture (Rome, Italy: Organization (FAO) of the United Nations). Available online at: https://www.fao.org/faostat/en/data/QCL.
Farouk, S. and Osman, M. A. (2012). Alleviation of oxidative stress induced by spider mite invasion through application of elicitors in bean plants. Egypt J. Biol. 14, 1–13. doi: 10.4314/ejb.v14i1.1
Gkizi, D., Lehmann, S., L’Haridon, F., Serrano, M., Paplomatas, E. J., Métraux, J. P., et al. (2016). The innate immune signaling system as a regulator of disease resistance and induced systemic resistance activity against Verticillium dahliae. Mol. Plant Microbe Interact. 29, 313–323. doi: 10.1094/MPMI-11-15-0261-R
Guarino, S., Mercati, F., Fatta Del Bosco, S., Motisi, A., and Abbate, L. (2022). Rootstocks with different tolerance grade to citrus tristeza virus induce dissimilar volatile profile in Citrus sinensis and avoidance response in the vector Aphis gossypii Glover. Plants 11, 3426. doi: 10.3390/plants11243426
Han, Z., Xiong, D., Schneiter, R., and Tian, C. (2023). The function of plant PR1 and other members of the CAP protein superfamily in plant–pathogen interactions. Mol. Plant Pathol. 24, 651–668. doi: 10.1111/mpp.13320
Hare, D. J. and Youngman, R. R. (1987). Gas exchange of orange (Citrus sinensis) leaves in response to feeding injury by the citrus red mite (Acari: tetranychidae). J. Econ Entomol. 80, 1249–1253. doi: 10.1093/jee/80.6.1249
Hayat, F., Li, J., Liu, W., Li, C., Song, W., Iqbal, S., et al. (2022). Influence of citrus rootstocks on scion growth, hormone levels, and metabolites profile of ‘Shatangju’ Mandarin (Citrus reticulata blanco). Hortic. 8, 608. doi: 10.3390/horticulturae8070608
He, J., Zhou, J., Wan, H., Zhuang, X., Li, H., Qin, S., et al. (2020). Rootstock–scion interaction affects cadmium accumulation and tolerance of malus. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.01264
Heath, R. L. and Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198. doi: 10.1016/0003-9861(68)90654-1
Hoy, M. A. (2011). Agricultural Acarology: Introduction to Integrated Mite Management. 1st ed (CRC Press). doi: 10.1201/b10909
Hsu, M. H., Chen, C. C., Lin, K. H., Huang, M. Y., Yang, C. M., and Huang, W. D. (2015). Photosynthetic responses of Jatropha curcas to spider mite injury. Photosynthetica 53, 349–355. doi: 10.1007/s11099-015-0132-3
Huang, W. L., Wu, F. L., Huang, H. Y., Huang, W. T., Deng, C. L., Yang, L. T., et al. (2020). Excess copper-induced alterations of protein profiles and related physiological parameters in citrus leaves. Plants 9, 291. doi: 10.3390/plants9030291
JASP Team (2024). JASP (Version 0.19.3) (Computer software). Available online at: https://jasp-stats.org/download/.
Jones, S. E. and Killiny, N. (2021). Influence of rootstock on the leaf volatile organic compounds of citrus scion is more pronounced after the infestation with Diaphorina citri. Plants 10, 2422. doi: 10.3390/plants10112422
Knegt, B., Meijer, T. T., Kant, M. R., Kiers, E. T., and Egas, M. (2020). Tetranychus evansi spider mite populations suppress tomato defenses to varying degrees. Ecol. Evol. 10, 4375–4390. doi: 10.1002/ece3.6204
Koch, K. G., Palmer, N. A., Donze-Reiner, T., Scully, E. D., Seravalli, J., Amundsen, K., et al. (2020). Aphid-responsive defense networks in hybrid switchgrass. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.01145
Legua, P., Forner, J. B., Hernández, F., and Forner-Giner, M. A. (2014). Total phenolics, organic acids, sugars and antioxidant activity of mandarin (Citrus clementina Hort. Ex Tan.): Variation from rootstock. Sci. Hortic. 174, 60–64. doi: 10.1016/j.scienta.2014.05.004
Leus, L., Luypaert, G., Dhooghe, E., Witters, J., Pauwels, E., Van Poucke, C., et al. (2022). Jasmonic Acid and Salicylic Acid Levels in Defense Response of Azalea (Rhododendron simsii Hybrid) to Broad Mite (Polyphagotarsonemus latus). Hortic. 8, 840. doi: 10.3390/horticulturae8090840
Li, N., Han, X., Feng, D., Yuan, D., and Huang, L. J. (2019). Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: do we understand what they are whispering? Int. J. Mol. Sci. 20, 671. doi: 10.3390/ijms20030671
Li, Q., Yao, J., Zheng, W., Wang, J., Liao, L., Sun, G., et al. (2023). Hetero-grafting affects flavonoid biosynthesis in sweet orange ‘Newhall’ (Citrus sinensis) peels: a metabolomics and transcriptomics analysis. Front. Plant Sci. 14. doi: 10.3389/fpls.2023
Li, X., Zhang, W., Niu, D., and Liu, X. (2024). Effects of abiotic stress on chlorophyll metabolism. Plant Sci. 342, 112030. doi: 10.1016/j.plantsci.2024.112030
Lichtenthaler, H. K. (1987). “Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes,” in Methods in enzymology. Eds. Jura, N. and Murphy, J. M. (Academic Press), 350–382. doi: 10.1016/0076-6879(87)48036-1
Livak, K. J. and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) methods. Methods 25, 402–408. doi: 10.1006/meth.2001
Long, A., Zhang, J., Yang, L. T., Ye, X., Lai, N. W., Tan, L. L., et al. (2017). Effects of low pH on photosynthesis, related physiological parameters, and nutrient profiles of citrus. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00185
Martel, C., Zhurov, V., Navarro, M., Martinez, M., Cazaux, M., Auger, P., et al. (2015). Tomato whole genome transcriptional response to Tetranychus urticae identifies divergence of spider mite-induced responses between tomato and Arabidopsis. Mol. Plant Microbe Interact. 3, 343–361. doi: 10.1094/MPMI-09-14-0291-FI
Mishra, S., Roychowdhury, R., Ray, S., Hada, A., Kumar, A., Sarker, U., et al (2024). Salicylic acid (SA)-mediated plant immunity against biotic stresses: An insight on molecular components and signaling mechanism. Plant Stress 11, 100427. doi: 10.1016/j.stress.2024.100427
Mitchell, C., Brennan, R. M., Graham, J., and Karley, A. (2016). Plant defense against herbivorous pests: Exploiting resistance and tolerance traits for sustainable crop protection. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01132
Morales, M. and Munné-Bosch, S. (2019). Malondialdehyde: facts and artifacts. Plant Physiol. 180, 1246–1250. doi: 10.1104/pp.19.00405
Mousavi, S. A., Chauvin, A., Pascaud, F., Kellenberger, S., and Farmer, E. E. (2013). GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500, 422–426. doi: 10.1038/nature12478
Müller, M. (2021). Foes or friends: ABA and ethylene interaction under abiotic stress. Plants 10, 448. doi: 10.3390/plants10030448
Nawaz, R., Abbasi, N. K., Hafiz, I. A., Khan, M. F., and Khalid, A. (2021). Environmental variables influence the developmental stages of the citrus leafminer, infestation level and mined leaves physiological response of Kinnow mandarin. Sci. Rep. 11, 7720. doi: 10.1038/s41598-021-87160-8
North, H. M., De Almeida, A., Boutin, J. P., Frey, A., To, A., Botran, L., et al. (2007). The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J. 50, 810–824. doi: 10.1111/j.1365-313X.2007.03094.x
Nunes, P. M., da Silva, C., Paula, C., Smolarek, F., Zeviani, W., Chaves, S., et al. (2015). Residues of Citrus sinensis (L.) Osbeck as agents that cause a change in antioxidant defense in plants. Braz. J. Pharm. Sci. 51, 479–493. doi: 10.1590/S1984-82502015000200025
ODEPA (2025). Oficina de Estudios y Políticas Agrarias (ODEPA). Available online at: https://apps.odepa.gob.cl/powerBI/boletin_fruta.html.
Pérez-Hedo, M., Gallego-Giraldo, C., Forner-Giner, M.Á., Ortells-Fabra, R., and Urbaneja, A. (2024). Plant volatile-triggered defense in citrus against biotic stressors. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1425364
Qamar, A., Mysore, K. S., and Senthil-Kumar, M. (2015). Role of proline and pyrroline-5-carboxylate metabolism in plant defense against invading pathogens. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.00503
Rao, M. J., Xu, I., Huang, Y., Tang, X., Deng, X., and Xu, Q. (2019). Ectopic expression of citrus UDP-GLUCOSYL TRANSFERASE gene enhances anthocyanin and proanthocyanidins contents and confers high light tolerance in Arabidopsis. BMC Plant Biol. 19, 603. doi: 10.1186/s12870-019-2212-1
Rasool, A., Mansoor, S., Bhat, K. M., Hassan, G. I., Baba, T. R., AlYemeni, M. N., et al. (2020). Mechanisms underlying graft union formation and rootstock scion interaction in horticultural plants. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.590847
Reddall, A. A., Wilson, L. J., Gregg, P. C., and Sadras, V. O. (2007). Photosynthetic response of cotton to spider mite damage: interaction with light and compensatory mechanisms. Crop Sci. 47, 2047–2057. doi: 10.2135/cropsci2006.11.0707
Rioja, T. and Ceballos, R. (2024). Citrus volatiles induced by herbivory of Aleurothrixus floccosus (Hemiptera: Aleyrodidae) elicit attraction to the exotic ladybird Clitostethus arcuatus (Coleoptera: Coccinellidae). Chil J. Agric. Res. 84, 181–194. doi: 10.4067/s0718-58392024000200181
Rioja, T., Ceballos, R., and Holuigue, L. (2018). Herbivore-induced plant volatiles emitted from avocado shoots infested by Oligonychus yothersi (Acari: Tetranychidae) increases the attraction of micro-coleopterans. Chil J. Agric. Res. 78, 447–458. doi: 10.4067/S0718-58392018000300447
Rioja, T., Ceballos, R., Holuigue, L., and Vargas, R. (2016). Different population densities and continuous feeding by Oligonychus yothersi (McGregor) (Acari: Tetranychidae) affect the emissions of herbivore-induced plant volatiles on avocado (Persea americana Mill. cv. Hass) Shoots under Semi-field Conditions. Int. J. Acarol. 42, 310–318. doi: 10.1080/01647954.2016.1191539
Rosa-Diaz, I., Rowe, J., Cayuela-Lopez, A., Arbona, V., Díaz, I., and Jones, A. M. (2024). Spider mite herbivory induces an ABA-driven stomatal defense. Plant Physiol. 195, 2970–2984. doi: 10.1093/plphys/kiae215
Sarmento, R. A., Lemos, F., Bleeker, P. M., Schuurink, R. C., Pallini, A., Oliveira, M. G., et al. (2011). A herbivore that manipulates plant defence. Ecol. Lett. 14, 229–236. doi: 10.1111/j.1461-0248.2010.01575.x
Shahid, M. A., Balal, R. M., Khan, N., Simón-Grao, S., Alfosea-Simón, M., Cámara-Zapata, J. M., et al. (2019). Rootstocks influence the salt tolerance of Kinnow mandarin trees by altering the antioxidant defense system, osmolyte concentration, and toxic ion accumulation. Sci. Hortic. 250, 1–11. doi: 10.1016/j.scienta.2019.02.028
Shahtousi, S. and Talaee, L. (2023). The effect of spermine on Tetranychus urticae-Cucumis sativus interaction. BMC Plant Biol. 23, 575. doi: 10.1186/s12870-023-04573-5
Shaltiel-Harpaz, L., Gerchman, Y., Ibdah, M., Kedoshim, R., Rachmany, D., Hatib, K., et al. (2018). Grafting on resistant interstocks reduces scion susceptibility to pear psylla, Cacopsylla bidens. Pest Manag Sci. 74, 617–626. doi: 10.1002/ps.4745
Sharma, R. M., Dubey, A. K., and Awasthi, O. P. (2015). Physiology of grapefruit (Citrus paradisi Macf.) cultivars as affected by rootstock. J. Hortic. Sci. Biotech. 90, 325–331. doi: 10.1080/14620316.2015.11513190
Sheri, V., Kumar, M., Jaconis, S., and Zhang, B. (2023). Antioxidant defense in cotton under environmental stresses: Unraveling the crucial role of a universal defense regulator for enhanced cotton sustainability. Plant Physiol. Biochem. 204, 108141. doi: 10.1016/j.plaphy.2023.108141
Simpson, C. R., Nelson, S. D., Melgar, J. C., Jifon, J., King, S. R., Schuster, G., et al. (2014). Growth response of grafted and ungrafted citrus trees to saline irrigation. Sci. Hortic. 169, 199–205. doi: 10.1016/j.scienta.2014.02.020
Singh, S., Kaur, I., and Kariyat, R. (2021). The multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 22, 1442. doi: 10.3390/ijms22031442
Solano, R., Stepanova, A., Chao, Q., and Ecker, J. R. (1998). Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 12, 3703–3714. doi: 10.1101/gad.12.23.3703
Su, Q., Yang, F., Yao, Q., Peng, Z., Tong, H., Wang, S., et al. (2020). A non-vector herbivore indirectly increases the transmission of a vector-borne virus by reducing plant chemical defences. Funct. Ecol. 34, 1091–1101. doi: 10.1111/1365-2435.13535
Syvertsen, J. P. and García-Sanchez, F. (2014). Multiple abiotic stresses occurring with salinity stress in citrus. Environ. Exp. Bot. 103, 128–137. doi: 10.1016/j.envexpbot.2013.09.015
Van den Ende, W. and El-Esawe, S. K. (2014). Sucrose signalling pathways leading to fructan and anthocyanin accumulation: A dual function in abiotic and biotic stress responses? Environ. Exp. Bot. 108, 4–13. doi: 10.1016/j.envexpbot.2013.09.017
Van Loon, L. C., Rep, M., and Pieterse, C. M. (2006). Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 44, 135–162. doi: 10.1146/annurev.phyto.44.070505.143425
Wang, T., Zheng, Z., Deng, L., Li, W., Yuan, Y., Zhang, M., et al. (2023). Effect of natural variation and rootstock on fruit quality and volatile organic compounds of ‘Kiyomi tangor’ (Citrus reticulata Blanco) Citrus. Int. J. Mol. Sci. 24, 16810. doi: 10.3390/ijms242316810
Warrier, R. R., Paul, M., and Vineetha, M. V. (2013). Estimation of salicylic acid in Eucalyptus leaves using spectrophotometric methods. Genet. Plant Physiol. 3, 90–97.
Ximénez-Embún, M. G., González-Guzmán, M., Arbona, V., Gómez-Cadenas, A., Ortego, F., and Castañera, P. (2018). Plant-mediated effects of water deficit on the performance of tetranychus evansi on tomato drought-adapted accessions. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01490
Yan, C., Gao, Q., Yang, M., Shao, Q., Xu, X., Zhang, Y., et al. (2024). Ca2+/calmodulin-mediated desensitization of glutamate receptors shapes plant systemic wound signalling and anti-herbivore defence. Nat. Plants. 10, 145–160. doi: 10.1038/s41477-023-01578-8
Yang, J., Wang, G. Q., Zhou, Q., Lu, W., Ma, J. Q., and Huang, J. H. (2019). Transcriptomic and proteomic response of Manihot esculenta to Tetranychus urticae infestation at different densities. Exp. Appl. Acarol. 78, 273–293. doi: 10.1007/s10493-019-00387-z
Younis, A. A. (2021). Structural, physiological, and biochemical alterations of the galled stems of Tamarix nilotica. Egypt J. Bot. 61, 747–758. doi: 10.21608/ejbo.2021.50503.1585
Yu, Q., Hua, X., Yao, H., Zhang, Q., He, J., Peng, L., et al. (2021). Abscisic acid receptors are involves in the Jasmonate signaling in Arabidopsis. Plant Signal Behav. 16, 1948243. doi: 10.1080/15592324.2021.1948243
Yulianti, F. and Agisimanto, D. (2023). “Leaf physiological responses of the citrus commercial varieties grafted onto rootstocks,” in IOP Conf. Series: Earth and Environmental Science 1172, 012017. 5th International Conference on Sustainable Agriculture. (Gothenburg, Sweden: Department of Agrotechnology, Faculty of Agriculture, Universitas Muhammadiyah Yogyakarta (UMY)). doi: 10.1088/1755-1315/1172/1/012017
Zanardi, O. Z., Bordini, G. P., Franco, A. A., de Morais, M. R., and Yamamoto, P. T. (2015). Development and reproduction of Panonychus citri (Prostigmata: Tetranychidae) on different species and varieties of citrus plants. Exp. Appl. Acarol. 67, 565–581. doi: 10.1007/s10493-015-9968-2
Zhao, Y. and Wang, Y. (2024). Protein dynamics in plant immunity: insights into plant–pest interactions. Int. J. Mol. Sci. 25, 12951. doi: 10.3390/ijms252312951
Zhou, S. and Jander, G. (2022). Molecular ecology of plant volatiles in interactions with insect herbivores. J. Exp. Bot. 73, 449–462. doi: 10.1093/jxb/erab413
Zhou, Z., Yuan, Y., Wang, K., Wang, H., Huang, J., Yu, H., et al. (2022). Rootstock-scion interactions affect fruit flavor in grafted tomato. Hortic. Plant J. 8, 499–510. doi: 10.1016/j.hpj.2022.01.001
Keywords: biotic stress marker genes, fruit trees, plant-insect interaction, scion/rootstock interaction, volatile organic compounds, salicylic acid
Citation: Rioja T, Ruiz KB and Ceballos R (2025) Red mite (Panonychus citri) attack amplifies citrus rootstock-driven responses in physiological and biochemical traits, VOC emission, and expression of defence-related genes in mandarin scions. Front. Plant Sci. 16:1645535. doi: 10.3389/fpls.2025.1645535
Received: 11 June 2025; Accepted: 01 August 2025;
Published: 04 September 2025.
Edited by:
Paloma Sanchez-Bel, University of Jaume I, SpainReviewed by:
John Caulfield, Rothamsted Research, United KingdomSiquan Ling, Guangdong Academy of Forestry, China
Copyright © 2025 Rioja, Ruiz and Ceballos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tommy Rioja, dHJpb2phQGFjYWRlbWljb3MudXRhLmNs
†ORCID: Ricardo Ceballos, orcid.org.0000-0003-1321-3454
 Tommy Rioja
Tommy Rioja Karina B. Ruiz
Karina B. Ruiz Ricardo Ceballos
Ricardo Ceballos