- 1College of Resources and Environment, Henan Agricultural University, Zhengzhou, China
- 2College of Geography and Planning, Chizhou University, Chizhou, China
- 3Key Laboratory of Arable Land Quality Conservation in the Huanghuaihai Plain, Ministry of Agriculture and Rural Affairs, Zhengzhou, China
Delayed nitrogen (N) application increases N use efficiency in a broadacre cropping system. However, its effect on N2O emissions and the underlying microbial mechanisms remains poorly understood. A field-plot experiment was carried out to examine the effects of biochar and a nitrification inhibitor (DMPP) on soil N2O emissions with six treatments: without N application (control), optimal N application (ON), farmer conventional N application (FN), biochar + ON (ONB), DMPP + ON (OND), and biochar + OND (ONDB). In comparison to the ON treatments, cumulative N2O emissions from the OND and ONDB treatments were significantly reduced by 32% and 38%, respectively, whereas emissions from the FN and ONB treatments exhibited increases of 38% and 4%, respectively. N application or biochar amendment increased the abundance of AOA and AOB, whereas DMPP amendment led to a reduction in AOB abundance. The OND and ONDB treatments enhanced the relative proportion of Nitrospira in the AOB community. The ONB treatment altered the most dominant genus of nirS and nosZ communities. Correlation analysis revealed that AOB, nirK, and nirK/nosZ were the predominant microorganism communities influencing soil N2O emissions. Random forest analysis identified Nitrospira in AOB communities, Cronobacter in nirK-containing communities, and Ramlibacter and Methylobacillus in the nosZ-containing community as key microbial taxa contributing to N2O emissions. We propose that the ONBD treatment provides dual advantages by reducing N2O emissions and enhancing N use efficiency under the delayed N application regime.
1 Introduction
Nitrous oxide (N2O) contributes approximately 7% to the overall global warming phenomenon (Li et al., 2020). Since 1975, the atmospheric concentration of N2O has risen by 23%, reaching to the current level of 332 ppb, the highest concentration documented in more than 800,000 years (IPCC, 2023). Emissions of N2O from agricultural systems are largely attributed to the application of nitrogen (N) fertilizers, resulting in the annual release of more than 4 Tg N2O-N (Yu et al., 2023). To address the escalating food demands of the world population, the quantity of synthetic N fertilizer applied in crop production continues to rise (Aryal et al., 2022). Urea, a globally prevalent synthetic N fertilizer, exhibits suboptimal utilization efficiency, resulting in significant N loss (approximately 40%) through various pathways (Liu et al., 2010), such as gaseous N emissions (e.g., N2O, NO) and nitrate-nitrogen (NO3−-N) (Klimczyk et al., 2021). From a sustainable development perspective, agricultural modernization must achieve precise N management to ensure food security and mitigate climate change.
The conventional approach to minimizing N2O emissions in agricultural production involves optimizing N application regimes and reducing the overall amount of N applied (Hartmann et al., 2015). Several studies have assessed the effects of various N application management strategies on mitigating N2O emissions, including deep application of N fertilizer (Wu et al., 2021), integration of urea and organic fertilizers (Wei et al., 2024), optimization of agricultural practices (Ashiq et al., 2021), and advances in irrigation techniques (Zhong et al., 2021). However, the impact of the timing of crop N application on N2O emissions has been largely overlooked in recent decades. Improvement of N use efficiency (NUE) cannot be accomplished instantly owing to the complexity of N uptake and utilization by crops (Qiao et al., 2015). Premature application of N fails to consider appropriate matching of N supply and N demand of winter wheat (Cui et al., 2010), resulting in significant N loss (Ding et al., 2010). Indeed, the N requirements of winter wheat differ among developmental stages, and soil N mineralization can effectively meet the early N demands of wheat (Sylvester-Bradley et al., 2001). Engel et al. (2017) considered that deferral of N application until spring was more appropriate to fulfill the N requirements of winter wheat. Application of a basic N fertilizer during the tillering stage of winter wheat has been shown to significantly enhance NUE (Wallace et al., 2020). Similarly, Yao et al. (2024) reported that delayed application of fertilizers is beneficial for increasing wheat yield. Thus, delaying N application until spring and applying N fertilizer as a topdressing during the critical phase for N demand by winter wheat may represent a viable approach to mitigate N2O emissions. The combined application of N fertilizers and synergistic agents represents a robust strategy for mitigating yield losses in crops caused by diminished N application (Huang et al., 2019). Nitrification inhibitors (NIs), serving as soil synergists, exhibit remarkable advantages in mitigating N2O emissions and reducing N losses (Liu C. et al., 2021; Dawar et al., 2021). Notably, 3,4-dimethylpyrazole phosphate (DMPP) has been shown to effectively reduce N2O emissions and NO3−-N leaching in agricultural systems. As a sustainable material for soil improvement, the potential environmental benefits of biochar are being increasingly validated. In the North China Plain, biochar amendment at a rate of 15 t ha−1 represents an optimal strategy for achieving high grain yields while substantially reducing N fertilizer inputs (Huang et al., 2022). He et al. (2018) reported that the application of biochar at 15 t ha−1 markedly reduced N2O emissions in wheat fields by 49.69%. Both positive and negative impacts on N2O emissions of N fertilizer applied in conjunction with synergistic agents have been reported (An et al., 2022; Duan et al., 2019; Verhoeven and Six, 2014). The inconsistent findings present a significant challenge to the predictive analysis of the impact of synergistic agents on N2O emissions. To date, it remains unclear how do DMPP and biochar interact under delayed N regimes to shape microbial N2O pathways.
Production of N2O from agricultural soils is primarily driven by microbial involvement in the nitrification and denitrification processes (Pihlatie et al., 2004). The investigation of nitrifying and denitrifying microorganisms provides vital insights into the mechanisms that govern N2O emission (Wang et al., 2024). The nitrification pathway contributes to N2O emissions from dryland soils (Shaaban, 2024). Nitrification plays a crucial role in mediating N2O emissions, particularly through ammonia oxidation and nitrifier denitrification, which are predominantly regulated by ammonia-oxidizing microorganisms (Martens-Habbena et al., 2015). An increasing body of evidence indicates that DMPP mitigates N2O emissions primarily by inhibiting nitrification, particularly through suppression of AOA and AOB activities. Biochar application significantly enhances N2O emission, which is attributable to biochar-stimulated increase in the activity of AOB and AOA (Lin et al., 2017). Research on denitrifying bacteria is crucial to elucidate the mechanisms of N2O emission under various fertilization practices (Huang et al., 2021). The microbial genes nirS, nirK, and nosZ play pivotal roles in denitrification (Shen et al., 2021; Li et al., 2020). The reduction of nitrite to NO, primarily mediated by nirS and nirK, is a rate-limiting step in the denitrification pathway (Liang et al., 2021). The transformation of N2O to dinitrogen is predominantly catalyzed by a N2O reductase encoded by nosZ (Shaaban et al., 2023). However, the impacts of DMPP and biochar application on N2O generation mediated by denitrifying bacteria in dryland soils remain unclear. In addition, the response of soil N2O emissions to DMPP and biochar application, together with microbe-mediated mechanisms of N2O production in dryland soils, under delayed N application is poorly understood.
The North China Plain is an important dryland agricultural region in China and accounts for 66% of the total wheat production area in the country. More than 70% of the farmland is subjected to excessive N application, with the annual input of synthetic N at 550–600 kg N ha−1 (Song et al., 2018). The region has emerged as a ‘hot spot’ for N2O emissions in China. Given this context, we investigated the effects of combined application of synergists on N2O emission and its underlying microbial mechanisms under a delayed N application regime. We hypothesized that: 1) delayed N application may effectively reduce soil N2O emissions; 2) the combined application of biochar and DMPP represents the most effective strategy for mitigating N2O emissions; and 3) the inhibitory mechanism of the combination of biochar and DMPP on N2O emissions via microbial community modulation. The aims of the field-plot experiment were 1) to elucidate the impact of N fertilizer application in combination with synergists on N2O emission under delayed N application, and 2) to investigate the influence of DMPP and biochar on the abundance and diversity of microbial functional genes associated with N2O emission.
2 Materials and methods
2.1 Field site
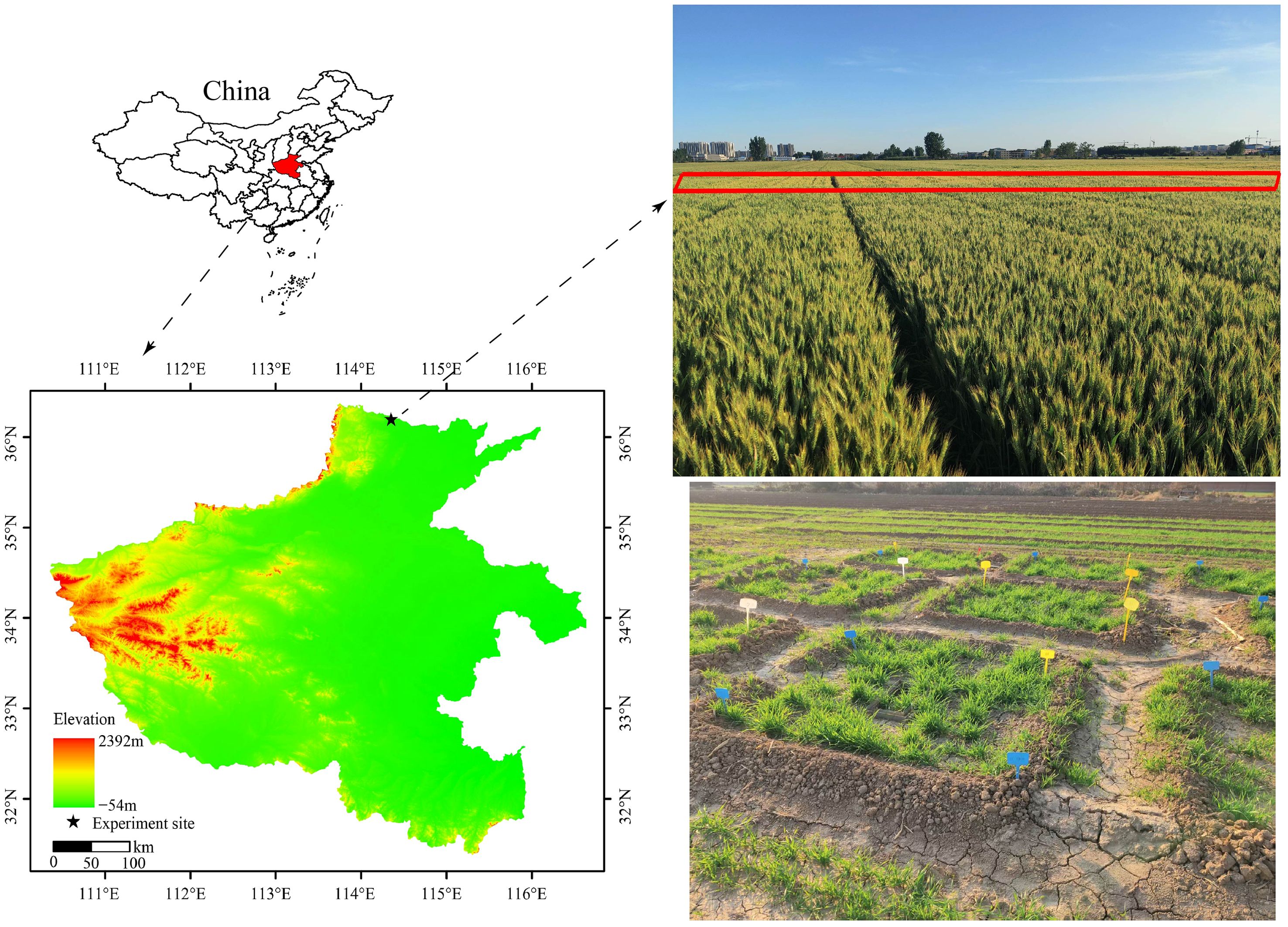
The field-plot experiment was carried out in 2022–2024 at Anyang (36°11’51’’N, 114°20’56’’E), Henan Province, China (Figure 1). The soil type is classified as a Fluvisols. The study site has an average elevation of 84.3 m above sea level, and the mean annual temperature and rainfall is 14°C and 557 mm, respectively. The soil pH was 7.57, and the contents of soil organic carbon (SOC), total N (TN), available phosphorus, and available potassium were 11.22 g kg−1, 1.09 g kg−1, 12.9 mg kg−1, and 89.2 mg kg−1, respectively.
2.2 Experimental design
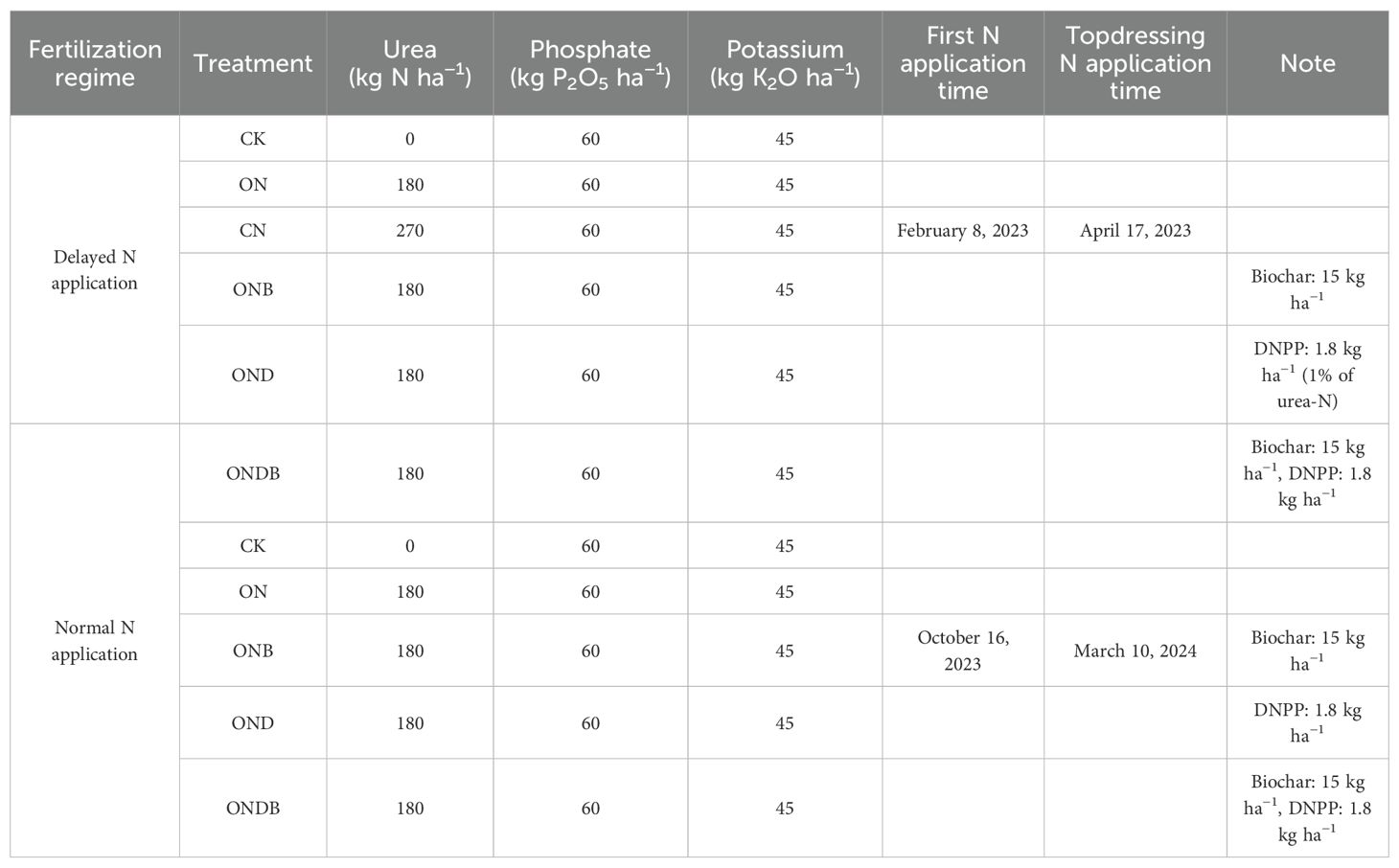
Six treatments were applied in the delayed N application experiment during the 2022-2023 winter wheat growing season: a control group without N fertilizer application (CK); two rates of N fertilizer application, namely, 180 kg N ha−1 (optimal N application; ON) and 270 kg N ha−1 (farmer conventional N application; FN); ON + biochar at the rate of 15 t ha−1 (ONB); ON + DMPP (OND); and ON + biochar + DMPP (ONDB). Five treatments were applied in the normal N application experiment during the 2023-2024 winter wheat growing season. The experimental treatments included CK, ON, ONB, OND, and ONDB. It is well established that elevated N application rates lead to increased N2O emissions and higher emission factors, the experimental results from the first wheat-growing season fully support this conclusion. This study focuses on the environmental effects resulting from the integration of optimal N application (ON) with biochar or DMPP. Therefore, the FN treatment was excluded from the normal N application regime.
Urea was utilized as the N fertilizer applied in two distinct phases: 60% of the N fertilizer was applied during the first fertilization, and the remaining 40% was applied as topdressing (Table 1). In addition, phosphate and potassium fertilizers, together with biochar, were applied on October 20, 2022 and October 16, 2023. Biochar was prepared from corn stalks at 450°C. The biochar C and N contents were 507 g kg−1 and 2.1 g kg−1, respectively, and the pH was 9.7. Each experimental plot has an area of 2 m × 2 m, with three replicates per treatment. Wheat seeds were sown on October 25, 2022, and October 16, 2023, while the mature grains were harvested on June 10, 2023 and June 4, 2024, respectively.
2.3 N2O gas sampling and measurements
Nitrous oxide gas was collected in a sealed chamber, following the methodology described by Wu et al. (2024). Eighteen static opaque chamber bottoms were inserted into the soil within the study plot at 8 cm depth until the harvest of winter wheat. The static chambers were equipped with an electric fan and a thermometer on top. Gas samples were extracted from the chamber at four time points (0, 15, 30, and 45 min) using a 50 ml plastic syringe following its closure. Simultaneously, the temperature inside the static chambers was recorded. The electric fan operated continuously throughout the sampling process to maintain air homogeneity within the enclosed space. A total of 72 gas samples were collected each sampling day over a continuous 7-day period following N application; thereafter, the sampling was conducted at 7- to 10-day intervals.
N2O flux was determined using a GC-2010 Plus gas chromatograph. The emission flux of N2O (f) was calculated with the following formula:
where ρ (kg m−3) is the N2O density, V is the volume of the sealing chamber (m3), A is the bottom area of the chamber (m2), ΔC/ΔT (μL L−1 h−1) is the temporal variation in N2O concentration in the sealed chamber, and T (C) is the mean temperature inside the chamber. The cumulative emission of nitrous oxide (CE-N2O) was estimated by employing linear interpolation of N2O flux and time (Allen et al., 2010).
The N2O emission factor was calculated as follows:
where CEN fertilizer and CEno N fertilizer represents CE-N2O from treatments with N application and without, respectively. Ninput represents the amount of N fertilizer applied.
2.4 Analysis of soil physicochemical parameters
Fresh soil samples were collected subsequent to gas sampling for determination of the ammonia-N (NH4+-N) and Nitrate-N (NO3−-N) contents, which were extracted using 2 M L−1 KCl solution and subsequently determined with a flow analyzer. Soil samples collected at harvest were used to determine physicochemical parameters. Dissolved organic N (DON) was measured by subtracting NH4+-N and NO3−-N from the total soluble N (TSN) content. Soil bulk density, pH, TSN, SOC), and TN were determined following soil agricultural chemistry analysis (Lu, 2000). Each sample is analyzed in duplicate, and the relative deviation between the duplicate samples must not exceed 5%. The soil water-filled pore space (WFPS) was determined as follows.
Where, θv represents the volumetric water content, ρ represents for soil bulk density.
2.5 DNA extraction and qPCR
Total DNA from 0.5 g soil samples was extract by using the E.Z.N.A.® Soil DNA Kit (Omega Bio-Tek, USA). The purified DNA was stored at −80°C until analysis. Gene copy numbers were determined using fluorescent qPCR (ABI 7500, USA) following protocols described by Huang. Information on the primers used and the parameters for the qPCR reactions are presented in Supplementary Table S1.
2.6 Microbial diversity detection and taxonomic analysis
The qPCR products were identified, purified, and quantified using 2% agarose gel electrophoresis, the AxyPrep DNA Gel Extraction Kit, and a Quantus™ Fluorometer, respectively. The NEXTFLEX Rapid DNA-Seq Kit was used to construct a DNA library, which was sequenced using an Illumina platform (NovaSeq PE250). The initial sequences were subsequently refined and concatenated to yield high-quality sequences. The UPARSE software was employed for clustering of operational taxonomic units (OTUs), following the clustering protocols and methodologies outlined by Bi et al. (2023). The RDP Classifier was used to annotate the species classification of the sequences, and the classification information for each OTU was derived by comparison with the Silva 16S rRNA database. The UCLUST algorithm was used for further taxonomic analysis of the representative OTU sequences.
2.7 Statistical analysis
The data were analyzed statistically with SPSS version 25.0. ANOVA was employed to assess the significance of differences among the indexes, post hoc tests were executed using the LSD, with a significance threshold of P < 0.05. Redundancy analysis was conducted using canoco 5.0 to examine the relationships between soil indicators and microbial communities. Correlation analysis was conducted using Origin 2021 software. A random forest analysis was conducted to identify microbial genera that significantly influence N2O emission using the ‘rfPermute’ package for R.
3 Results
3.1 N2O flux
The temporal dynamics of N2O emission showed discernible fluctuations. N fertilizer application greatly stimulated soil N2O emission. A distinct difference in soil N2O emissions between the first and second N applications was observed (Figure 2). The N2O flux increased markedly following the second N application. The N application treatments exhibited significantly higher N2O emissions compared with those of the CK. Under the delayed N application regime, the highest CE-N2O (1.96 kg ha−1) was observed under the FN treatment. The ONB treatment (1.47 kg ha−1) led to a slightly higher CE-N2O than in the ON treatment (1.42 kg ha−1) (Figure 2A). Addition of DMPP resulted in significant reduction of N2O emissions; the OND and ONDB treatments exhibited reductions in CE-N2O of 32% and 38%, respectively, compared with the CE-N2O of the ON treatment. The N2O emission factors for different treatments under the delayed N application regime ranged from 0.23% to 0.56%, which were substantially lower than the factors ranging from 0.60% to 1.03% under the normal N application regime (Figure 2B). These results indicate that implementing a delayed N application strategy can effectively mitigate N2O emissions.
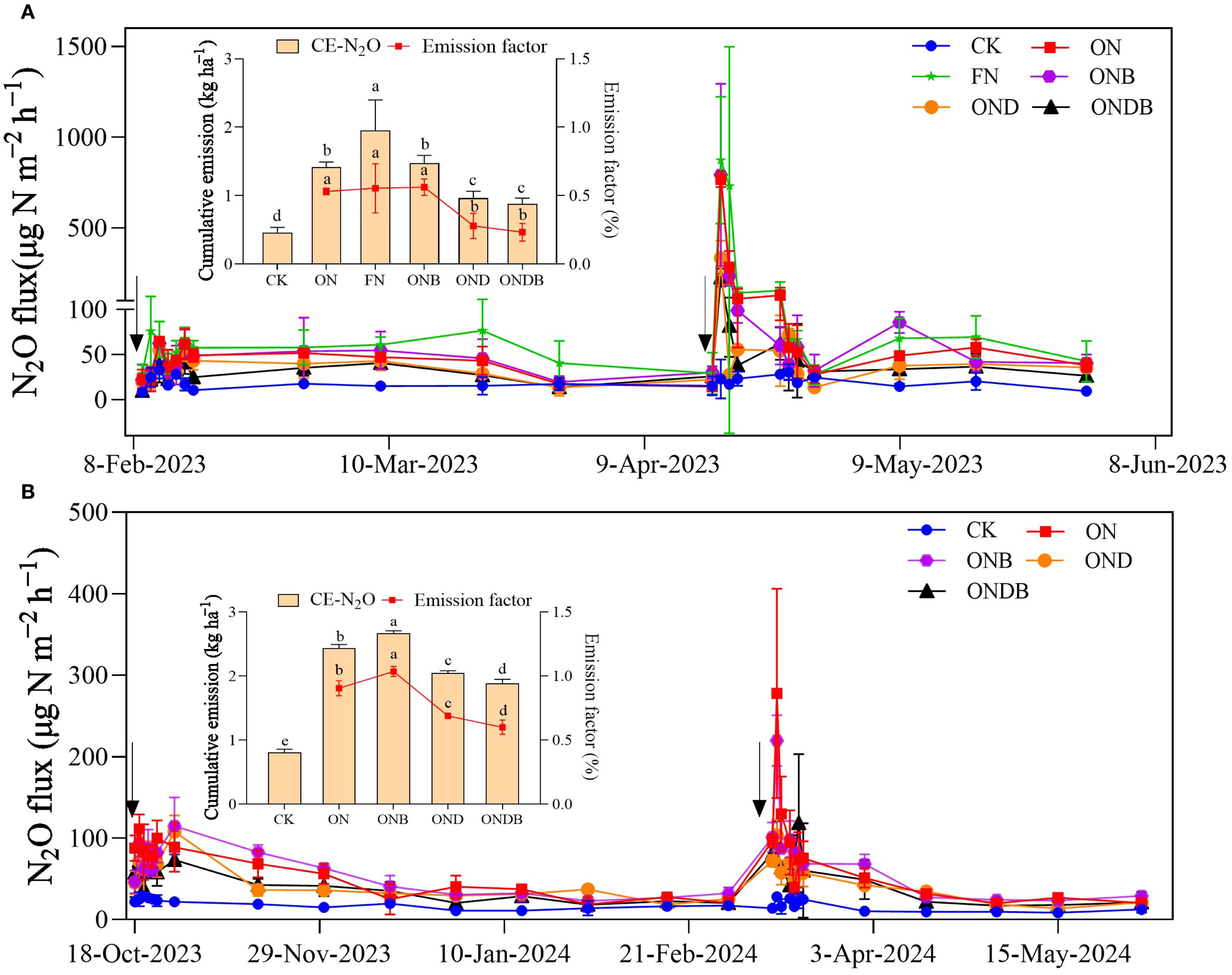
Figure 2. N2O emissions under delayed N application (2022–2023 wheat growing season) (A) and Normal N application (2023–2024 wheat growing season) (B).The black arrows indicate the time points at which N fertilizer was applied. CK, control; ON, 180 kg N ha−1; FN, 270 kg N ha−1; ONB, ON + biochar;OND, ON + DMPP; ONDB, ONB + biochar. Different letters indicate statistically significant differences among treatments (P < 0.05).
3.2 Variation in soil characteristics
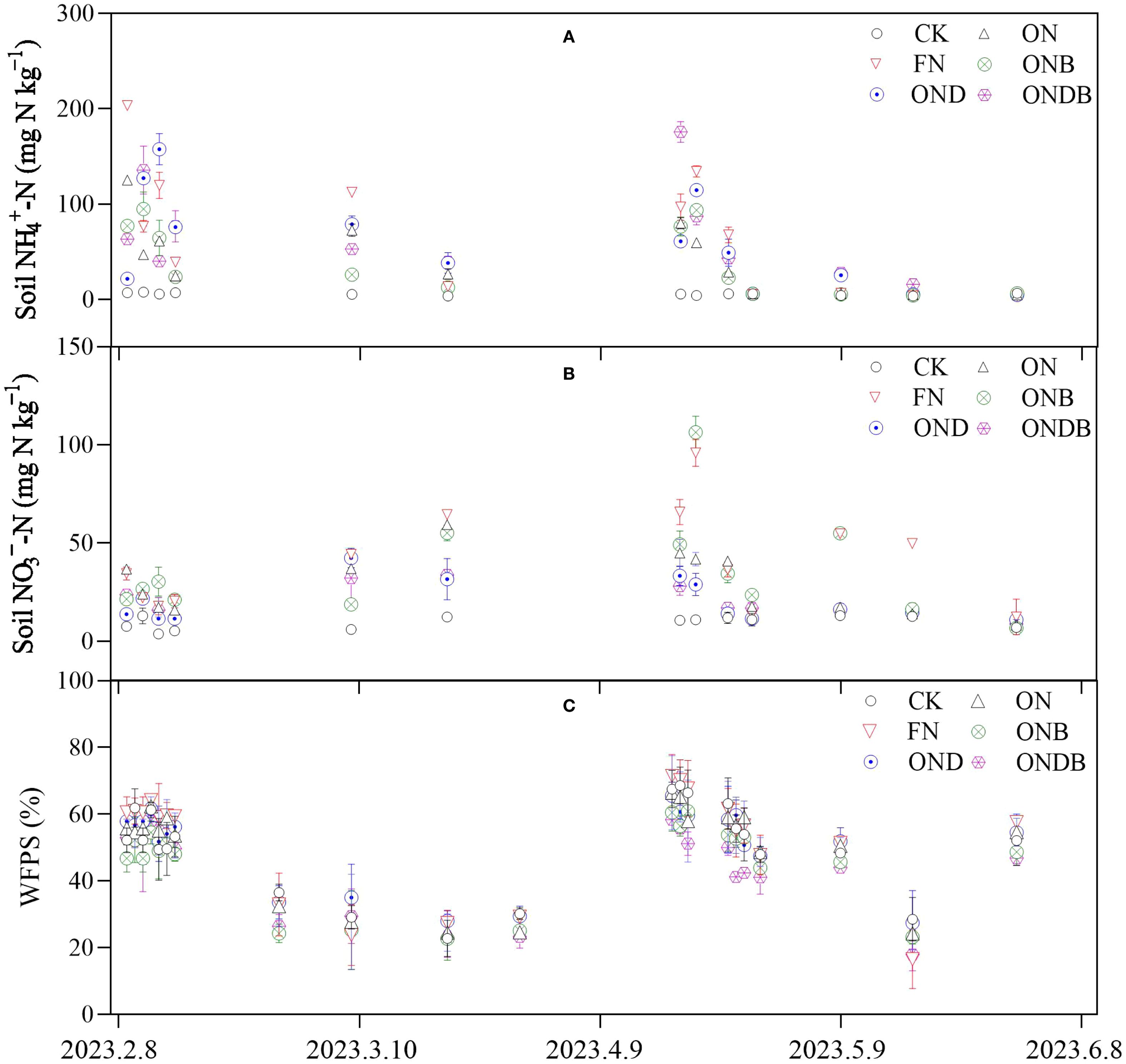
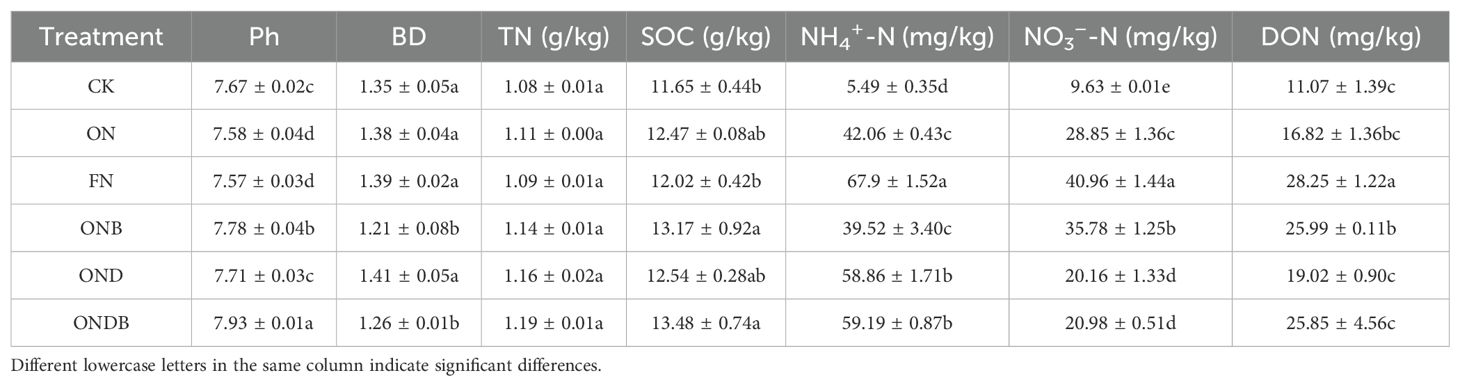
Soil NO3−-N and NH4+-N contents were significantly increased following application of N. The NH4+-N content under the ONB treatment was lower than the ON treatment following N fertilization. The NO3−-N content in response to DMPP application (the OND and ONDB treatments) was comparatively low (Figures 3A, B). The WFPS ranged between 16.58% and 71.46%, exhibiting similar tendencies under the different treatments (Figure 3C). According to the average inorganic-N content of the soil from the initial N application until the wheat harvesting period, the FN treatment resulted in the highest NH4+-N and NO3−-N contents (Table 2). The ONB treatment decreased NH4+-N content and significantly increased NO3−-N content, whereas treatment with DMPP (OND and ONDB) led to a significant increase in NH4+-N content and a significant decrease in NO3−-N content. The NO3−-N content differed significantly among the treatments, except for OND and ONDB. Biochar amendment significantly enhanced the soil pH and SOC content, compared with the ON treatment; the ONB and ONDB treatments increased pH by 3% and 5%, respectively, and SOC by 7% and 8%, respectively. Application of DMPP led to slight, but non-significant, increases in soil SOC and pH. The soil DON concentration increased markedly with increase in the N application rate. The ONB and ONDB treatments slightly enhanced the soil DON concentration, whereas the OND treatment had the opposite effect.
3.3 Abundance of N functional genes
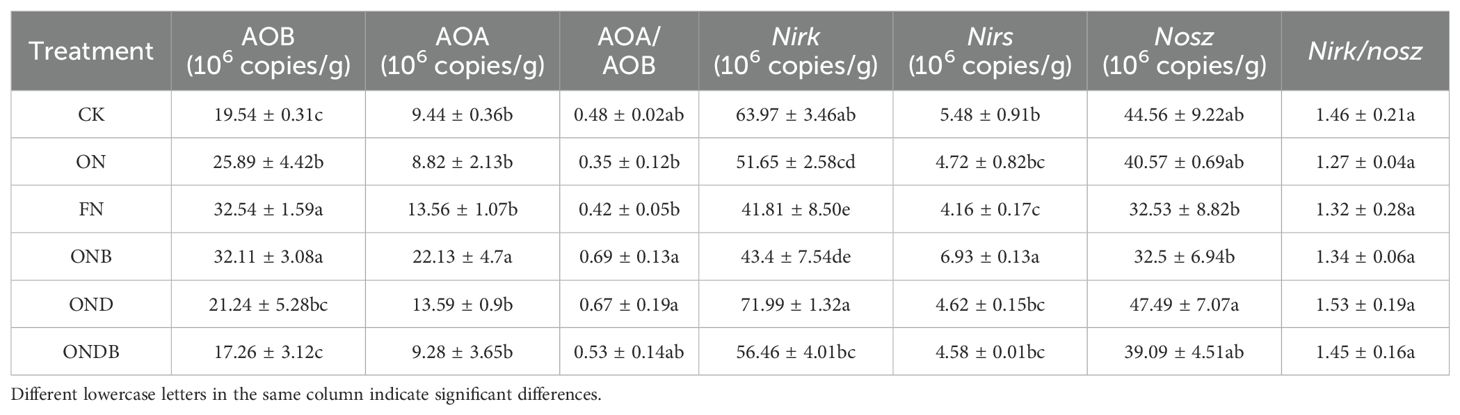
Ammonia-oxidizing and denitrifying bacteria exhibited distinct variation in abundance among the treatments (Table 3), as indicated by the copy numbers of microbial functional genes. The CK, ON, and FN treatments exhibited significant elevation in AOB gene copy numbers with increasing N input, whereas no notable differences in AOA were observed. In comparison with the ON treatment, the ONB treatment markedly enhanced the abundance of AOB gene copies, whereas ONDB treatment had the most pronounced effect in reducing AOB gene copy numbers. The ONB treatment significantly enhanced the AOA abundance, whereas the OND and ONDB treatments had no significant effect. The ratio of AOA to AOB gene copy numbers was smallest under the ON treatment (0.35) and largest under the ONB treatment (0.69). The quantity of nirS, nirK, and nosZ gene copies declined with increase in the N application rate. Relative to the ON treatment, the ONB treatment substantially increased the nirS abundance, whereas the OND treatment significantly increased the abundance of nirK (Table 3). The highest number of gene copies was observed for nirK, whereas the lowest number of copies detected was for nirZ. The nirK/nosZ ratio varied between 1.27 and 1.53, with no statistically significant differences among treatments detected.
3.4 Diversity and composition of N functional genes
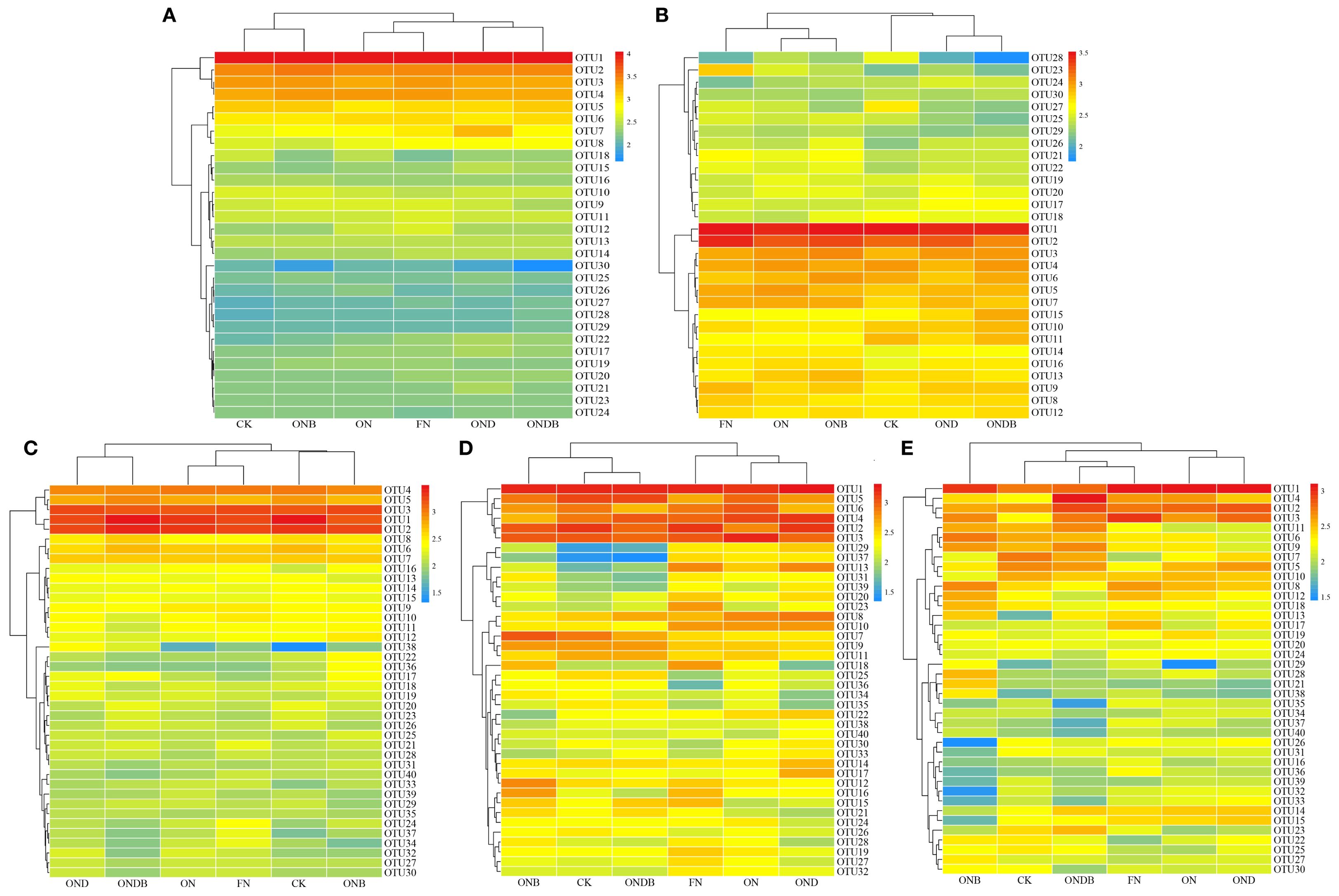
To graphically illustrate the effects of different treatments on the N cycling microbial community, hierarchical clustering analysis of OTUs was conducted across treatments (Figure 4). Based on the OTU clustering results for AOB, OND and ONDB were initially grouped before being clustered with CK, whereas ON and ONB were preferentially grouped and then clustered with FN. The CK, ON, and FN treatments were grouped into distinct clusters, indicating that the different N fertilizer rates significantly influenced the soil AOB community. It is noteworthy that the cluster heatmaps for the AOA, AOB, and nirK-containing communities revealed preferential combination of the OND and ONDB treatments (Figures 4A–C), indicating that DMPP application altered the composition of the amoA- and nirK-containing communities. The ON and OND treatments were initially grouped, suggesting that DMPP application alone had a minimal impact on the nirS- and nosZ-containing communities (Figures 4D, E). In contrast, the different N application treatments (CK, ON, FN) were grouped into separate clusters, suggesting that N application significantly influenced the nirS- and nosZ-containing communities.
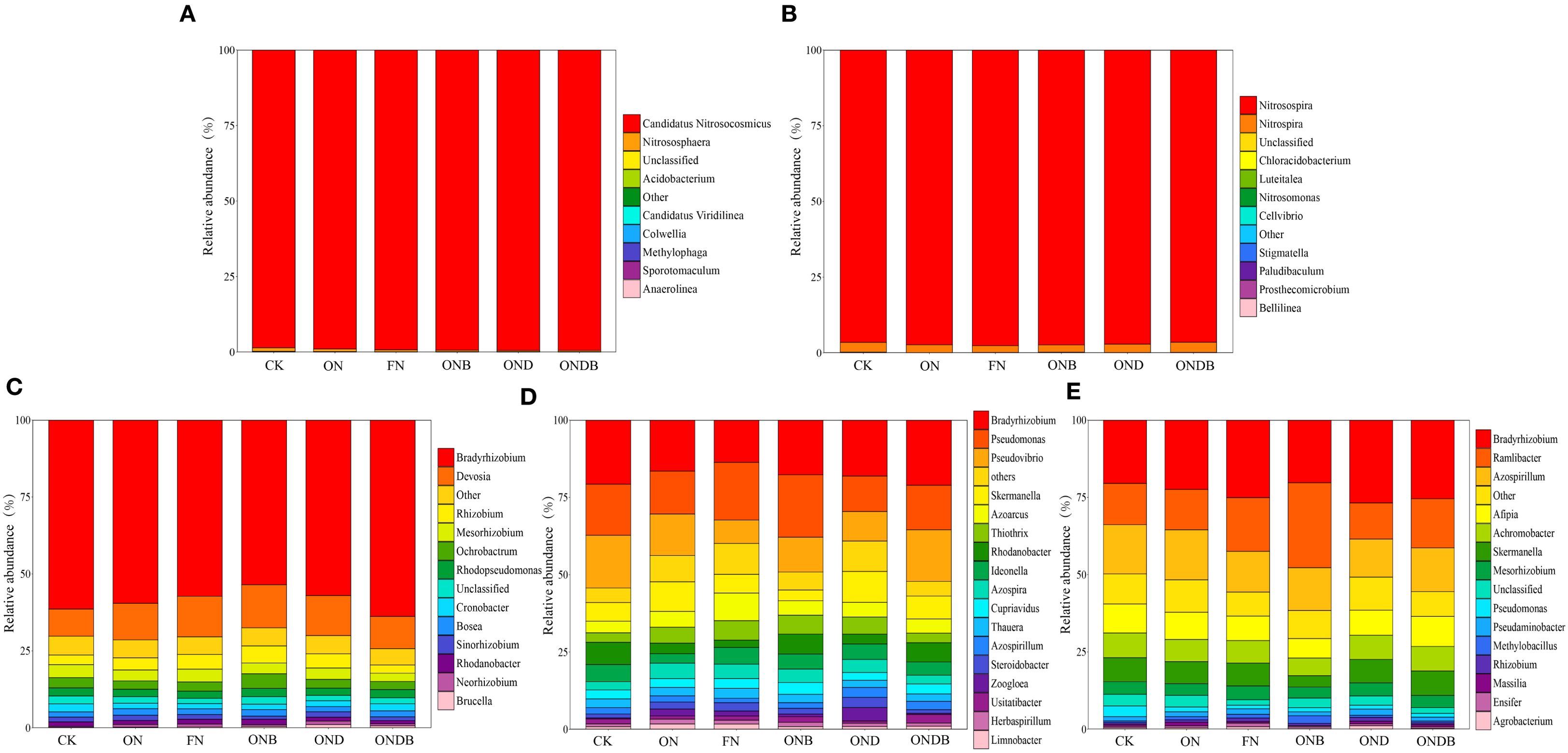
Based on the microbial communities at the genus level (Figure 5), the AOA community structure was relatively simple, with Candidatus Nitrosocosmicus identified as the dominant genus (Figure 5A). The AOB community was primarily composed of the genera Nitrosospira and Nitrospira at the genus level (Figure 5B), with Nitrosospira exhibiting the highest relative abundance. Application of N or biochar decreased the relative abundance of Nitrospira, whereas DMPP had a stimulatory effect on Nitrospira abundance. Notably, combined application of DMPP and biochar led to a more pronounced stimulation of Nitrospira abundance. The composition of the nirK-, nirS-, and nosZ-containing communities exhibited greater diversity at the genus level (Figures 5C–E). A high rate of N input or biochar application resulted in a shift of the most dominant nirS-containing genus from Bradyrhizobium to Pseudomonas. The most dominant genus for the nirK- and nosZ-containing communities was Bradyrhizobium. Biochar amendment markedly enhanced the relative abundance of Ramlibacter in the nosZ-containing community, resulting in a shift of the most dominant genus to Ramlibacter under the ONB treatment.
3.5 Relationships among N2O emission, soil properties, and microbial communities
Redundancy analysis was conducted to examine the inter-relationships among N2O emission, soil physicochemical properties, and microbial gene abundance. Axes 1 and 2 accounted for 67.99% of the total variance (Figure 6). The ON, ONB, and DMPP addition treatments were resolved as distinct on axis 1 (49.80%). The contents of NO3−-N and inorganic N, DOC, pH, and TN were critical indicators that influenced the experimental system. Correlation analysis indicated that NO3−-N, inorganic N, AOB, nirK, DON, and NH4+-N were key indicators that influenced soil N2O emission (Figure 7). Random forest analysis further revealed that Nitrospira was the genus within the AOB community that most significantly affected N2O emission, Cronobacter was the dominant genus responsible for N2O emission in the nirK-containing community, whereas Ramlibater and Methylobacillus in the nosZ-containing community were the significantly predictors of N2O emission (Figure 8).
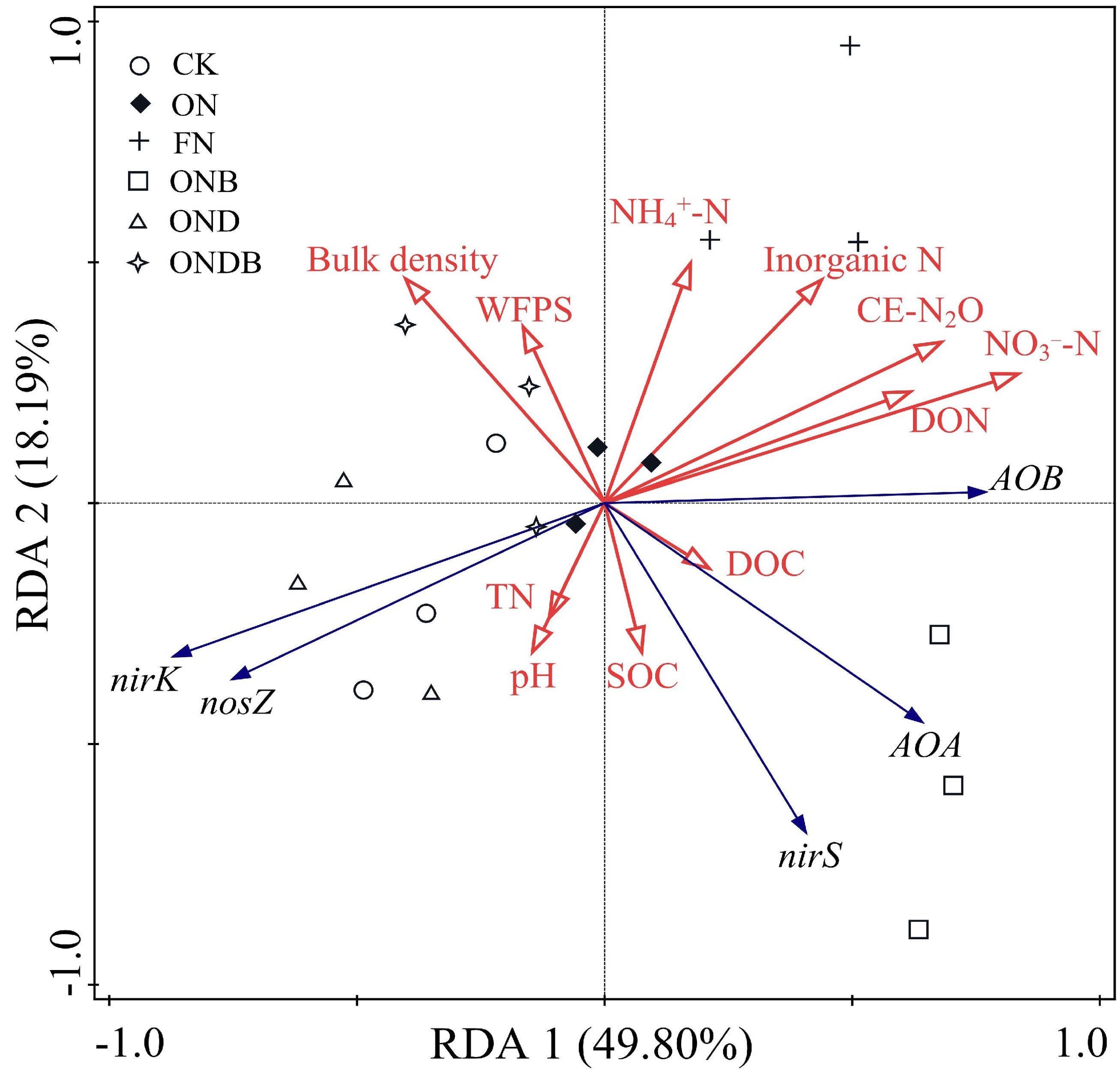
Figure 6. Redundancy analysis of copy number of N2O related functional genes and soil physicochemical properties.
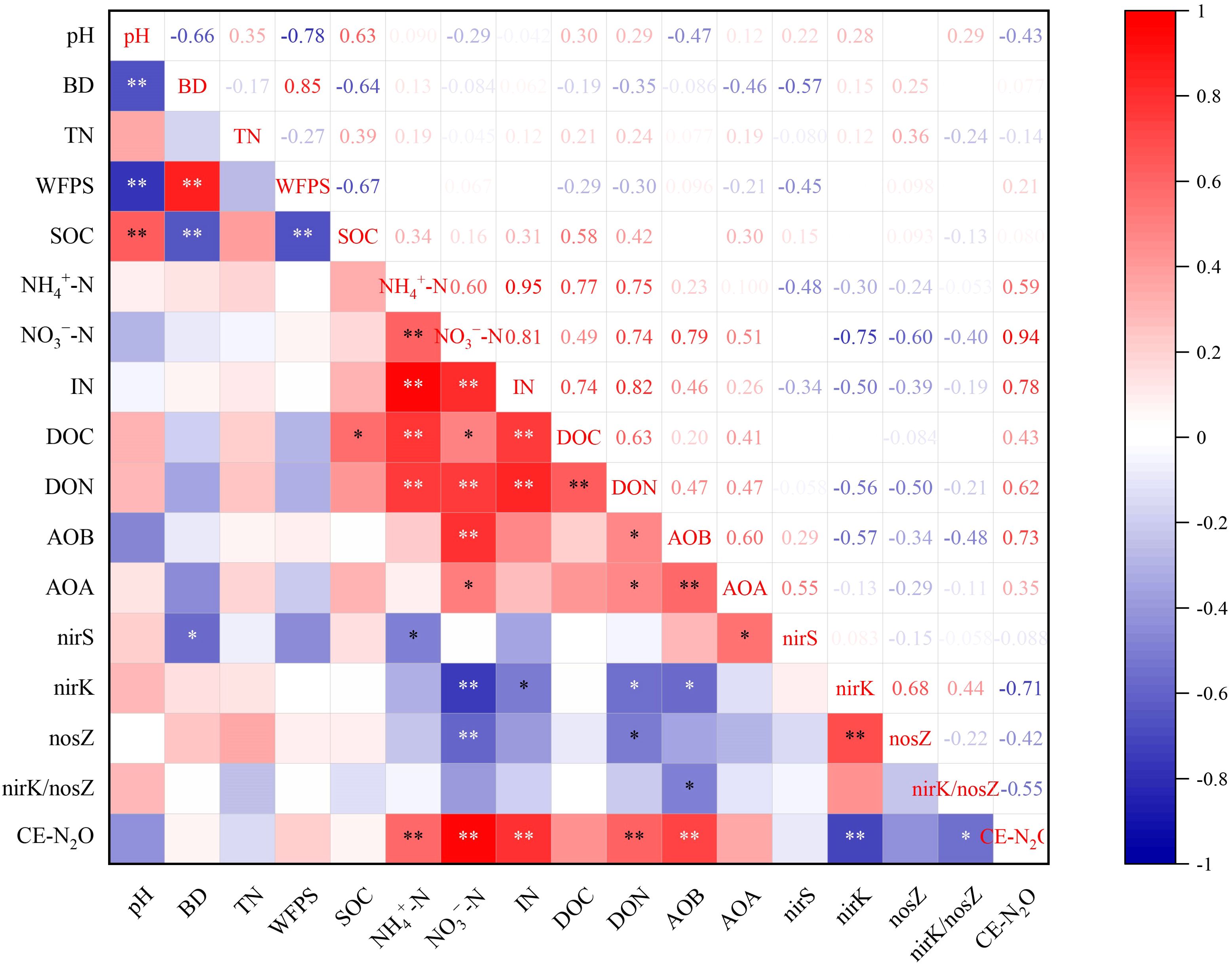
Figure 7. Correlation analysis of N2O emissions with soil properties and the abundance of N-related functional gene. * P < 0.05; **P < 0.01
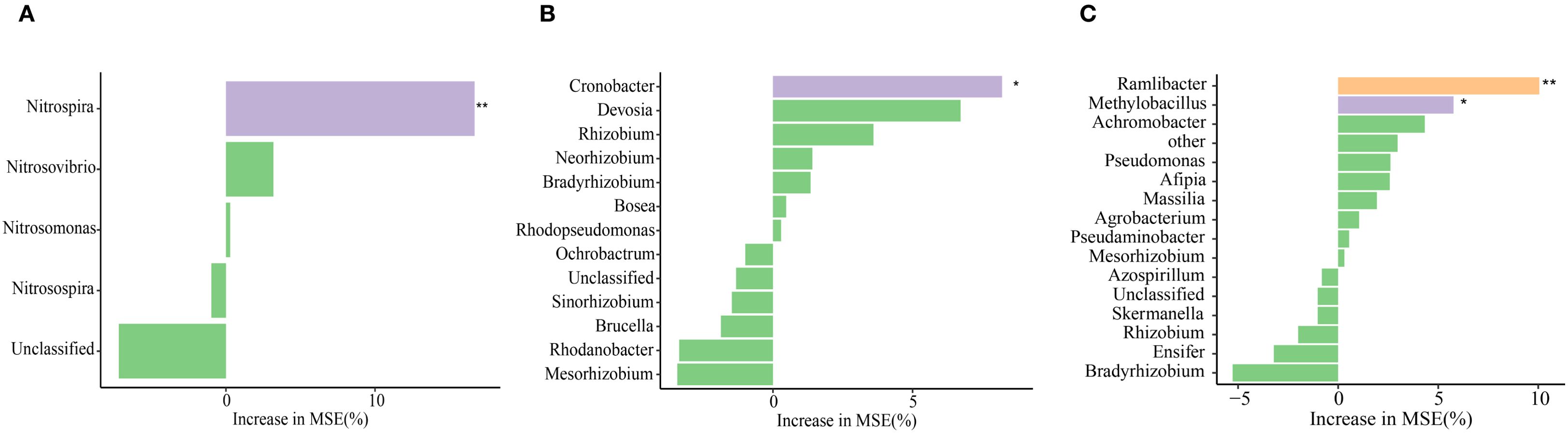
Figure 8. The effects of various genera of AOB (A), nirK- (B) and nosZ- communities (C) on N2O emissions was elucidated base on random forest analysis. * P < 0.05; **P < 0.01.
4 Discussion
4.1 Impacts of biochar and DMPP on N2O emission
The N2O released from agricultural soils is a byproduct of nitrification and denitrification. Carbon and N play crucial roles influencing the emission of N2O (Cayuela et al., 2014; Li et al., 2022). Our findings indicate that the co-application of biochar and DMPP caused the most effective inhibition of N2O emission. Compared with the ON treatment, the ONDB treatment led to a 38% reduction in CE-N2O. This effect is attributed to the substantial decrease in content of the denitrification substrate (NO3−-N) under the ONDB treatment, which consequently inhibited the activity of denitrifying bacteria. The regression analysis revealed a significant negative correlation between pH and CE-N2O (P < 0.01, Supplementary Figure S1). The ONDB treatment significantly increased the soil pH, which may be an additional factor that contributes to the synergistic effects of biochar and DMPP in mitigating N2O emission. Therefore, we proposed that DMPP’s inhibition of AOB combined with biochar’s pH modulation jointly reduced N2O. Recent studies have demonstrated that biochar has a markedly superior capacity for N2O adsorption compared with soil and its mineral constituents (Xiao et al., 2018). Consequently, the ONDB treatment may enhance the adsorption and stabilization of specific N2O molecules within the biochar matrix. However, Li et al. (2023) reported that combination of biochar and DMPP did not lead to a significant reduction in N2O emissions in agricultural systems, which may be attributable to regional soil characteristics and the intrinsic properties of biochar.
Biochar application alone resulted in an increase in soil CE-N2O compared with that of the ON treatment. The ONB treatment significantly enhanced the soil NO3−-N content (Table 2), indicating that biochar incorporation significantly enhanced soil nitrification, consistent with the findings of Chen et al. (2019). Previous research has demonstrated that biochar is abundant in various volatile compounds and serves as an organic C source for denitrifying bacteria (Fu et al., 2022), thereby stimulating N2O emission. The present study revealed that the ONB treatment significantly increased the DOC compared with the ON treatment, thereby confirming that the incorporation of biochar (an exogenous source of organic C) enhanced soil denitrification (Weldon et al., 2019). Furthermore, biochar application significantly enhanced the SOM, thereby increasing the availability of C and N within the soil (Chagas et al., 2022). This enhancement fosters elevated diversity and activity of soil microorganisms, leading to increased oxygen consumption (Xu W. et al., 2023), and as a result, localized anoxic conditions are more conducive to denitrification.
Li et al. (2023) reported DMPP decreases N2O emissions by disrupting the N conversion processes within the soil, ultimately causing reduced availability of N for nitrification and denitrification. A experiment conducted by Zhao on a wheat–maize rotation system demonstrated that DMPP significantly mitigated soil N2O emissions, consistent with the present findings. The current study demonstrated that DMPP application resulted in a significant 32% reduction in CE-N2O compared with that of the ON treatment, which was largely consistent with the results of a meta-analysis of agricultural systems conducted by Ekwunife et al. (2022). Nevertheless, this reduction was markedly less than in lab experiments (Fan et al., 2019).
Fluctuations in air temperature and precipitation affect soil aeration and oxygen concentrations, which subsequently impact on N2O production (Yang Y. et al., 2021); in addition, the redox environment of the soil plays a critical role (Xu P. et al., 2023). In the present study, a notable increase in soil N2O flux emissions was observed following the second N application. Fluctuations in soil moisture, in combination with optimal surface-soil temperatures ranging from 19 to 27°C, resulted in frequent cycles of drying and wetting within the soil environment (Supplementary Figure S2). This dynamic created alternating conditions of oxidation and reduction, which further enhanced the denitrification process facilitated by both nitrifying and denitrifying bacteria, thereby increasing N2O emissions. Theodorakopoulos et al. (2017) reported that N2O emissions were predominantly attributable to nitrification at WFPS < 60%, and by denitrification at WFPS > 60%. The soil WFPS ranged between 17% and 71% in the present study. Consequently, it is probable that N2O emissions from the soil primarily originated from the nitrification pathway. Significant correlations between NO3−-N, inorganic N, NH4+-N, and CE-N2O were observed, which is inconsistent with the findings of Huang et al. (2019). We propose that the N2O emissions observed in the present study primarily originated from oxidation of soil ammonia, particularly through hydroxylamine decomposition. Furthermore, the notable positive correlation between soil DON and CE-N2O reinforces that DON-mediated heterotrophic ammoxidation may serve as a pivotal contributor to N2O production. However, the findings of this study were derived from two wheat growing seasons, and the long-term efficacy of combined biochar and DMPP application in mitigating N2O emissions remains to be confirmed.
4.2 Response of ammonia oxidizing microbial communities to N and synergist
The activity of the nitrifying bacterial community is significantly influenced by the soil environment (Zheng et al., 2019). AOA and AOB display distinct adaptations to soil NH4+ environments, with AOB predominant under elevated NH4+ conditions, whereas AOA exhibits the opposite trend (Fang et al., 2023). Previous investigations have revealed that fertilizer application markedly increases nitrifying microbial activity in the soil, leading to elevated N2O emissions (Li et al., 2020). The microcosmic examination of ammonia-oxidizing processes under different N fertilization regimes is rather complex, owing to the participation of a diverse array of ammonia-oxidizing microorganisms (Yang L. et al., 2021).
The present study detected a positive correlation between N2O emission and the abundance of AOB, which in turn increases with elevation of the N application rate. This result accords with a meta-analysis of 157 field observation datasets conducted by Ouyang et al. (2018). However, their study indicated that the abundance of AOA increases in response to N application. The present findings showed that biochar application significantly enhanced the abundance of both AOA and AOB, consistent with previous research demonstrating that biochar stimulates nitrification activity and fosters the proliferation of ammonia-oxidizing microorganisms (Xu W. et al., 2023). Application of DMPP mitigated the impact of N fertilization on the abundance of AOB. Furthermore, the synergistic effect of biochar and DMPP significantly decreased the abundance of AOA and AOB. The copy number of AOA genes was lower than that for AOB genes (AOA/AOB=0.52) at wheat harvesting (Table 3). These findings indicate that AOB may exhibit greater abundance and demonstrate enhanced ammoxidation activity in agricultural soils with elevated N contents (Yan et al., 2018). With regard to OTU clustering within AOB, the OND and ONDB treatments were clustered and subsequently linked with CK to form a single cluster. The ONB and ON treatments were preferentially linked before being grouped with FN to establish a distinct cluster. This finding elucidates the variation in CE-N2O under the different treatments. Redundancy analysis indicated that AOB was the most significant positive factor that influenced N2O emissions, while correlation analysis revealed that AOB contributes substantially more to N2O emissions than AOA.
The impact of AOB on N2O emissions is significantly greater than that of AOA. Further identification of specific microorganisms within AOB that modulate N2O emissions is warranted. Cytryn et al. (2012) amplified amoA gene fragment and revealed that the AOB community in paddy soil is predominantly composed of Nitrosomonas. The relative abundance of Nitrosospira decreased compared with Nitrosomonas as N application increased., as the addition of N promotes a shift from a less nutrient-rich bacterial community to a more symbiotic community. However, high-throughput sequencing revealed that the predominant genus of AOB was Nitrosospira in this study and that the proportion of Nitrosospira rose with increase in the N application rate, whereas Nitrospira showed an inverse relationship. Similarity, Bi et al. (2023) reported that Nitrosospira is the predominant ammonia-oxidizing genus in agricultural soils. Liu et al. (2021) identified Nitrosospira as the dominant genus in environments with high NH4+ concentrations, demonstrating an enhanced capacity for ammonia oxidation. This genus plays a crucial role in N2O emissions from soils characterized by high concentrations of NH4+. In the current study, the application of biochar alone (ONB) significantly enhanced the proportion of Nitrosospira compared with the ON treatment, while concurrently reducing Nitrospira abundance. However, Lin et al. (2017) demonstrated that exogenous organic C altered the AOB community, shifting from Nitrosospira to Nitrosomonas.
We propose that the primary factor contributing to this discrepancy is soil pH. In acidic conditions, the nitrification activity of Nitrosomonas surpasses that of Spirulina Nitrosomonas; however, the adaptability of Nitrosomonas to alkaline environmental stress is significantly lower than that of Spirulina Nitrosomonas. Nitrospira can oxidize nitrite and convert urea into ammonia, promoting the growth of nitrifying bacteria. Although its abundance remains relatively stable with increasing N application rates, it exhibits a significant correlation with N2O emissions (Liu et al., 2024). Likewise, in the present study, treatments that included DMPP were observed to enhance the relative abundance of Nitrospira, whereas the ONB and FN treatments led to a decrease in its relative abundance. Random forest analysis further demonstrated that Nitrospira exhibit a significant predictive capacity for N2O emissions. Additionally, in comparison with the ON treatment, the combination of biochar and DMPP significantly reduced the α-diversity indices (Supplementary Table S2), indicating that ONDB treatment significantly reduced AOB richness and diversity. Consequently, we propose that DMPP exerts an inhibitory effect on N2O emissions by diminishing the abundance and α-diversity of AOB, as well as by increasing the relative proportion of Nitrospira within the AOB community.
4.3 Response of denitrifying microbial communities to N and synergist
Denitrification, mediated by heterotrophic bacteria and fungi, primarily occurs in anaerobic environments, where nitrate undergoes a series of transformations (e. g. NO2−, NO, N2O) that ultimately yield N2. Previous studies have established that denitrifying bacteria harboring nirS and nirK are the primary contributors to denitrification-mediated N2O production, primarily because of inadequate genetic capacity for the reduction of N2O (Huang et al., 2019). The nirK and nosZ copy numbers under the ONB treatment were lower than those detected under the ON treatment (Table 3). Biochar application can foster a soil environment with an elevated C/N ratio, thereby promoting N assimilation within the soil and diminishing the availability of N substrates for denitrification (Liu Z. et al., 2021). The OTU cluster heatmaps revealed that the nirS-, nirK-, and nosZ-containing communities exhibited distinct responses to the various treatments. Our findings indicated that DMPP treatment (OND and ONDB) significantly altered the composition of nirK-containing communities (Figure 4C); however, DMPP application alone exhibited limited effects on the nirS- and nosZ-containing communities (Figures 4D, E). Conversely, biochar application alone had a pronounced impact on the nirS- and nosZ-containing communities (Figures 4D, E). Xiao et al. (2021) reported that application of exogenous N or C influences the soil microbial community, while the simultaneous addition may alter the dominant genera within the denitrification gene community. Similarly, in the present study, we observed a shift in the most dominant genus of nirS-containing community from Bradyrhizobium under the ON treatment to Pseudomonas under the FN and ONB treatments. The most dominant genus of nosZ-containing community transitioned from Bradyrhizobium to Ramlibacter under the ONB treatment. Bradyrhizobium is an aerobic azotobacter within the rhizobia order (Geddes et al., 2020), and was the most dominant genus in the nosZ-containing community in all treatments except for the ONB treatment. This finding emphasizes the possibility for denitrification within an aerobic environment. Both nirK and nosZ were significantly correlated with DON. The correlation coefficient between SOC and nirS exceeded that of SOC with the other denitrification genes (nirK, nosZ) (Figure 7), indicating that nirK- or nosZ-containing bacteria showed heightened sensitivity to exogenous N, whereas nirS-containing bacteria exhibited greater sensitivity to exogenous C compared with that of nirK- or nosZ-containing bacteria.
In this study, CE-N2O was significantly negatively correlated with the nirK gene copy number (P < 0.01). This finding is consistent with the conclusions by Wu et al. (2023), who established that the presence of nirK-containing denitrifying bacteria are critical determinants in both N2O consumption and production. Random forest analysis identified Cronobacter as a critical genus driving N2O emissions in nirK-containing communities. Similarly, Xie et al. (2024) reported that substitution with organic fertilizers affected the relative abundance of Cronobacter in the nirK-containing community, thereby mitigating N2O emissions. Interestingly, our findings demonstrate that despite the lack of a markedly correlation between CE-N2O emissions and nosZ gene copy numbers, while a significant negative correlation was observed with the nirK/nosZ ratio (Shi et al., 2019). Furthermore, the present findings revealed that Ramlibater and Methylobacillus in the nosZ-containing community exhibited a significant predictive capacity for N2O emissions. Consequently, the nosZ gene may still be among the key contributors to N2O emissions mediated by denitrification. Tang et al. (2024) reported that niche variations in the denitrification genes nirK and nirS resulted in differential N2O emissions. However, a weak correlation was observed between CE-N2O and nirS gene copy number in the present study, indicating that nirS was not the most critical factor influencing N2O emissions. Nevertheless, correlation analysis revealed a significant correlation between AOB and nirK copy number with N2O emissions; however, this does not provide direct evidence for microbiome-mediated N2O emission. Therefore, it is essential to conduct a more in-depth analysis in future to elucidate the contributions of various microorganisms to N2O emissions using microbial molecular ecology and isotope tracer methodologies.
5 Conclusion
Compared to the normal N application regime, delayed N application significantly reduced both CE-N2O and EF-N2O. Under the delayed N application regime, the ONB treatment increases N2O emissions, whereas treatment with DMPP (OND and ONDB) significantly mitigates N2O emissions by 32% - 38%. The CE-N2O exhibited a positive correlation with the copy number of AOB genes, and a negative correlation with nirK gene copy number and nirK/nosZ ratio. Random forest analysis identified that the community species of the AOB, nirK, and nosZ-containing communities are sensitive biomarkers for evaluating of N2O emissions in agricultural ecosystems. Consequently, the ONDB treatment is a promising strategy for mitigation of N2O emissions under the delayed N application regime. This approach is feasible for regions with high N inputs but requires cost-benefit analysis for farmer adoption. Future studies are encouraged to employ isotopic tracing techniques to confirm microbial pathways and conduct field trials across diverse soil types.
Data availability statement
The raw sequencing data generated from microbial diversity analyses in this study have been deposited in the Sequence Read Archive (SRA) database at NCBI under the following BioProject accession numbers: AOA (PRJNA1334209), AOB (PRJNA1334285), nirK (PRJNA1334144), nirS (PRJNA1334174), and nosZ (PRJNA1334121).
Author contributions
HW: Data curation, Formal Analysis, Funding acquisition, Investigation, Writing – review & editing. DZ: Investigation, Visualization, Writing – original draft. XS: Visualization, Writing – original draft. GM: Visualization, Writing – original draft. QY: Visualization, Writing – original draft. HZ: Visualization, Writing – original draft. SL: Project administration, Visualization, Writing – original draft. XJ: Supervision, Visualization, Writing – original draft. DW: Data curation, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by National Key Research and Development Program of China (grant number 2021YFD1700900; grant number 2023YFD1900203).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1647453/full#supplementary-material
References
Allen, D. E., Kingston, G., Rennenberg, H., Dalal, R. C., and Schmidt, S. (2010). Effect of nitrogen fertilizer management and waterlogging on nitrous oxide emission from subtropical sugarcane soils. Agric. Ecosyst. Environ. 136, 209–217. doi: 10.1016/j.agee.2009.11.002
An, N., Zhang, L., Liu, Y., Shen, S., Li, N., Wu, Z., et al. (2022). Biochar application with reduced chemical fertilizers improves soil pore structure and rice productivity. Biochar application with reduced chemical fertilizers improves soil pore structure and rice productivity. Chemosphere 298, 134304. doi: 10.1016/j.chemosphere.2022.134304
Aryal, B., Gurung, R., Camargo, A. F., Fongaro, G., Treichel, H., Mainali, B., et al. (2022). Nitrous oxide emission in altered nitrogen cycle and implications for climate change. Environ. Pollut. 314, 120272. doi: 10.1016/j.envpol.2022.120272
Ashiq, W., Vasava, H., Cheema, M., Dunfield, K., Daggupati, P., and Biswas, A. (2021). Interactive role of topography and best management practices on N2O emissions from agricultural landscape. Soil Till Res. 212, 105063. doi: 10.1016/j.still.2021.105063
Bi, R., Xu, X., Zhan, L., Chen, A., Zhang, Q., and Xiong, Z. (2023). Proper organic substitution attenuated both N2O and NO emissions derived from AOB in vegetable soils by enhancing the proportion of Nitrosomonas. Sci. Total Environ. 866, 161231. doi: 10.1016/j.scitotenv.2022.161231
Cayuela, M. L., van Zwieten, L., Singh, B. P., Jeffery, S., Roig, A., and Sanchez-Monedero, M. A. (2014). Biochar's role in mitigating soil nitrous oxide emissions: a review and meta-analysis. Agric. Ecosyst. Environ. 191, 5–16.
Chagas, J. K. M., de Figueiredo, C. C., and Ramos, M. L. G. (2022). Biochar increases soil carbon pools: Evidence from a global meta-analysis. J. Environ. Manage 305, 114403. doi: 10.1016/j.jenvman.2021.114403
Chen, H., Yin, C., Fan, X., Ye, M., Peng, H., Li, T., et al. (2019). Reduction of N2O emission by biochar and/or 3, 4-dimethylpyrazole phosphate (DMPP) is closely linked to soil ammonia oxidizing bacteria and nosZI-N2O reducer populations. Sci. Total Environ. 694, 133658. doi: 10.1016/j.scitotenv.2019.133658
Cui, Z., Zhang, F., Chen, X., Dou, Z., and Li, J. (2010). In-season nitrogen management strategy for winter wheat: maximizing yields, minimizing environmental impact in an over-fertilization context. Field Crops Res. 116, 140–146. doi: 10.1016/j.fcr.2009.12.004
Cytryn, E., Levkovitch, I., Negreanu, Y., Dowd, S., Frenk, S., and Silber, A. (2012). Impact of short-term acidification on nitrification and nitrifying bacterial community dynamics in soilless cultivation media. Appl. Environ. Microb. 78, 6576–6582. doi: 10.1128/AEM.01545-12
Dawar, K., Khan, A., Sardar, K., Fahad, S., Saud, S., Datta, R., et al. (2021). Effects of the nitrification inhibitor nitrapyrin and mulch on N2O emission and fertilizer use efficiency using 15N tracing techniques. Sci. Total Environ. 757, 143739. doi: 10.1016/j.scitotenv.2020.143739
Ding, Y., Liu, Y., Wu, W., Shi, D., Yang, M., and Zhong, Z. (2010). Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil pollut. 213, 47–55. doi: 10.1007/s11270-010-0366-4
Duan, P., Zhang, Q., Zhang, X., and Xiong, Z. (2019). Mechanisms of mitigating nitrous oxide emissions from vegetable soil varied with manure, biochar and nitrification inhibitors. Agr For. Meteorol. 278, 107672. doi: 10.1016/j.agrformet.2019.107672
Ekwunife, K. C., Madramootoo, C. A., and Abbasi, N. A. (2022). Assessing the impacts of tillage, cover crops, nitrification, and urease inhibitors on nitrous oxide emissions over winter and early spring. Biol. Fert Soils (2022) 58.3, 195–206. doi: 10.1007/s00374-021-01605-w
Engel, R., Jones, C., Romero, C., and Wallander, R. (2017). Late-fall, winter and spring broadcast applications of urea to no-till winter wheat I. Ammonia loss and mitigation by NBPT. Soil Sci. Soc. Am. J. 81, 322–330. doi: 10.2136/sssaj2016.10.0332
Fan, X., Yin, C., Chen, H., Ye, M., Zhao, Y., Li, T., et al. (2019). The efficacy of 3, 4-dimethylpyrazole phosphate on N2O emissions is linked to niche differentiation of ammonia oxidizing archaea and bacteria across four arable soils. Soil Biol. Biochem. 130, 82–93. doi: 10.1016/j.soilbio.2018.11.027
Fang, J., Lyu, T., Liu, J., He, S., Yang, X., Dou, H., et al. (2023). Response of nitrogen cycling and related microorganisms to brackish wetlands formed by evapotranspiration. Pedosphere 33, 252–266. doi: 10.1016/j.pedsph.2023.07.007
Fu, X., Hou, R., Yang, P., Qian, S., Feng, Z., Chen, Z., et al. (2022). Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total Environ. 817, 153061. doi: 10.1016/j.scitotenv.2022.153061
Geddes, B. A., Kearsley, J., Morton, R., and Finan, T. M. (2020). The genomes of rhizobia. Advances in Botanical Research Vol. 94 (The Netherlands: Academic Press), 213–249.
Hartmann, T. E., Yue, S., Schulz, R., He, X., Chen, X., Zhang, F., et al. (2015). Yield and N use efficiency of a maize–wheat croping system as affected by different fertilizer management strategies in a farmer's field of the North China Plain. Field Crops Res. 174, 30–39. doi: 10.1016/j.fcr.2015.01.006
He, T., Liu, D., Yuan, J., Luo, J., Lindsey, S., Bolan, N., et al. (2018). Effects of application of inhibitors and biochar to fertilizer on gaseous nitrogen emissions from an intensively managed wheat field. Sci. Total Environ. 628, 121–130. doi: 10.1016/j.scitotenv.2018.02.048
Huang, C., Liu, Q., Li, Z. L., Ma, X. D., Hou, Y. N., Ren, N. Q., et al. (2021). Relationship between functional bacteria in a denitrification desulfurization system under autotrophic, heterotrophic, and mixotrophic conditions. Water Res. 188, 116526. doi: 10.1016/j.watres.2020.116526
Huang, R., Wang, Y., Liu, J., Li, J., Xu, G., Luo, M., et al. (2019). Variation in N2O emission and N2O related microbial functional genes in straw-and biochar-amended and non-amended soils. Appl. Soil Ecol. 137, 57–68. doi: 10.1016/j.apsoil.2019.01.010
Huang, M., Wang, C., Qi, W., Zhang, Z., and Xu, H. (2022). Modelling the integrated strategies of deficit irrigation, nitrogen fertilization, and biochar addition for winter wheat by AquaCrop based on a two-year field study. Field Crops Res. 282, 108510. doi: 10.1016/j.fcr.2022.108510
IPCC. (2023). IPCC, (2023): summary for policymakers. In: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland, pp. 1–34
Klimczyk, M., Siczek, A., and Schimmelpfennig, L. (2021). Improving the efficiency of urea-based fertilization leading to reduction in ammonia emission. Sci. Total Environ. 771, 145483. doi: 10.1016/j.scitotenv.2021.145483
Li, C., Hu, H. W., Chen, Q. L., Chen, D., and He, J. Z. (2020). Growth of comammox Nitrospira is inhibited by nitrification inhibitors in agricultural soils. J. Soil Sed. 20, 621–628. doi: 10.1007/s11368-019-02442-z
Li, Z., Xu, P., Han, Z., Wu, J., Bo, X., Wang, J., et al. (2023). Effect of biochar and DMPP application alone or in combination on nitrous oxide emissions differed by soil types. Biol. Fert Soils 59, 123–138. doi: 10.1007/s00374-022-01688-z
Li, Z., Zeng, Z., Song, Z., Tian, D., Huang, X., Nie, S., et al. (2022). Variance and main drivers of field nitrous oxide emissions: a global synthesis. J. Clean Prod. 353, 131686. doi: 10.1016/j.jclepro.2022.131686
Li, L., Zheng, Z., Wang, W., Biederman, J. A., Xu, X., Ran, Q., et al. (2020). Terrestrial N2O emissions and related functional genes under climate change: A global meta-analysis. Global Change Biol. 26, 931–943. doi: 10.1111/gcb.14847
Liang, Y., Wu, C., Wei, X., Liu, Y., Chen, X., Qin, H., et al. (2021). Characterization of nirS-and nirK-containing communities and potential denitrification activity in paddy soil from eastern China. Agric. Ecosyst. Environ. 319, 107561. doi: 10.1016/j.agee.2021.107561
Lin, Y. X., Ding, W. X., Liu, D. Y., He, T. H., Yoo, G. Y., Yuan, J. J., et al. (2017). Wheat straw-derived biochar amendment stimulated N2O emissions from rice paddy soils by regulating the amoA genes of ammonia-oxidizing bacteria. Soil Biol. Biochem. 113, 89–98. doi: 10.1016/j.soilbio.2017.06.001
Liu, Y., Chen, Y., Duan, P., Lu, H., Gao, Y., and Xu, K. (2024). Microbially mediated mechanisms underlie the increased soil N2O emissions under nitrogen fertilization in purple soil. Appl. Soil Ecol. 204, 105725. doi: 10.1016/j.apsoil.2024.105725
Liu, C., Liu, H., Liu, X., Zhang, Y., Wang, L., Guan, D., et al. (2021). Nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP) reduces N2O emissions by altering the soil microbial community in a wheat–maize rotation on the North China Plain. Eur. J. Soil Sci. 72, 1270–1291. doi: 10.1111/ejss.13017
Liu, Z., Wu, X., Li, S., Liu, W., Bian, R., Zhang, X., et al. (2021). Quantitative assessment of the effects of biochar amendment on photosynthetic carbon assimilation and dynamics in a rice–soil system. N. Phytol. 232, 1250–1258. doi: 10.1111/nph.17651
Liu, J., You, L., Amini, M., Obersteiner, M., Herrero, M., Zehnder, A. J. B., et al. (2010). A high-resolution assessment on global nitrogen flows in cropland. Proc. Natl. Acad. Sci. U. S. A. 107, 8035–8040. doi: 10.1073/pnas.0913658107
Lu, R. (2000). Soil Agricultural Chemistry Analysis (Beijing, China: Chinese Agriculture and Technology Press).
Martens-Habbena, W., Qin, W., Horak, R. E., Urakawa, H., Schauer, A. J., Moffett, J. W., et al. (2015). The production of nitric oxide by marine ammonia-oxidizing archaea and inhibition of archaeal ammonia oxidation by a nitric oxide scavenger. Environ. Microbiol. 17, 2261–2274. doi: 10.1111/1462-2920.12677
Ouyang, Y., Evans, S. E., Friesen, M. L., and Tiemann, L. K. (2018). Effect of nitrogen fertilization on the abundance of nitrogen cycling genes in agricultural soils: a meta-analysis of field studies. Soil Biol. Biochem. 127, 71–78. doi: 10.1016/j.soilbio.2018.08.024
Pihlatie, M., Syväsalo, E., Simojoki, A., Esala, M., and Regina, K. (2004). Contribution of nitrification and denitrification to N2O production in peat, clay and loamy sand soils under different soil moisture conditions. Nutr. Cycl. Agroecosys. 70, 135–141. doi: 10.1023/B:FRES.0000048475.81211.3c
Qiao, C., Liu, L., Hu, S., Compton, J. E., Greaver, T. L., and Li, Q. (2015). How inhibiting nitrification affects nitrogen cycle and reduces environmental impacts of anthropogenic nitrogen input. Global Change Biol. 21, 1249–1257. doi: 10.1111/gcb.12802
Shaaban, M. (2024). Microbial pathways of nitrous oxide emissions and mitigation approaches in drylands. J. Environ. Manage 354, 120393. doi: 10.1016/j.jenvman.2024.120393
Shaaban, M., Wang, X. L., Song, P., Hou, X., Wu, Y., and Hu, R. (2023). Ascription of nosZ gene, pH and copper for mitigating N2O emissions in acidic soils. Environ. Res. 237, 117059. doi: 10.1016/j.envres.2023.117059
Shen, Q., Redmile-Gordon, M., Song, J., Li, J., Zhang, K., Voroney, P., et al. (2021). Amendment with biodiesel co-product modifies genes for N cycling (nirK, nirS, nosZ) and greenhouse gas emissions (N 2 O, CH 4, CO 2) from an acid soil. Biol. Fert Soils 57, 629–642. doi: 10.1007/s00374-021-01546-4
Shi, Y., Liu, X., and Zhang, Q. (2019). Effects of combined biochar and organic fertilizer on nitrous oxide fluxes and the related nitrifier and denitrifier communities in a saline-alkali soil. Sci. Total Environ. 686, 199–211. doi: 10.1016/j.scitotenv.2019.05.394
Song, X., Liu, M., Ju, X., Gao, B., Su, F., Chen, X., et al. (2018). Nitrous oxide emissions increase exponentially when optimum nitrogen fertilizer rates are exceeded in the North China Plain. Environ. Sci. Technol. 52, 12504–12513. doi: 10.1021/acs.est.8b03931
Sylvester-Bradley, R., Stokes, D. T., and Scott, R. K. (2001). Dynamics of nitrogen capture without fertilizer: the baseline for fertilizing winter wheat in the UK. J. Agric. Sci. 136, 15–33. doi: 10.1017/S0021859600008479
Tang, Q., Moeskjær, S., Cotton, A., Dai, W., Wang, X., Yan, X., et al. (2024). Organic fertilization reduces nitrous oxide emission by altering nitrogen cycling microbial guilds favouring complete denitrification at soil aggregate scale. Sci. Total Environ. 946, 174178. doi: 10.1016/j.scitotenv.2024.174178
Theodorakopoulos, N., Lognoul, M., Degrune, F., Broux, F., Regaert, D., Muys, C., et al. (2017). Increased expression of bacterial amoA during an N2O emission peak in an agricultural field. Agric. Ecosyst. Environ. 236, 212–220. doi: 10.1016/j.agee.2016.12.002
Verhoeven, E. and Six, J. (2014). Biochar does not mitigate field-scale N2O emissions in a Northern California vineyard: an assessment across two years. Agric. Ecosyst. Environ. 191, 27–38. doi: 10.1016/j.agee.2014.03.008
Wallace, A. J., Armstrong, R. D., Grace, P. R., Scheer, C., and Partington, D. L. (2020). Nitrogen use efficiency of 15N urea applied to wheat based on fertiliser timing and use of inhibitors. Nutr. Cycl Agroecosys. 116, 41–56. doi: 10.1007/s10705-019-10028-x
Wang, C., Xiao, G., Guan, Y., Li, Y., Chen, D., and Shen, W. (2024). Contrasting effects of intensified dry-season drought and extended dry-season length on soil greenhouse gas emissions in a subtropical forest. Sci. Total Environ. 906, 167419. doi: 10.1016/j.scitotenv.2023.167419
Wei, Z., Well, R., Ma, X., Lewicka-Szczebak, D., Rohe, L., Zhang, G., et al. (2024). Organic fertilizer amendment decreased N2O/(N2O+ N2) ratio by enhancing the mutualism between bacterial and fungal denitrifiers in high nitrogen loading arable soils. Soil Biol. Biochem. 198, 109550. doi: 10.1016/j.soilbio.2024.109550
Weldon, S., Rasse, D. P., Budai, A., Tomic, O., and Dörsch, P. (2019). The effect of a biochar temperature series on denitrification: which biochar properties matter? Soil Biol. Biochem. 135, 173–183. doi: 10.1016/j.soilbio.2019.04.018
Wu, P., Chen, G., Liu, F., Cai, T., Zhang, P., and Jia, Z. (2021). How does deep-band fertilizer placement reduce N2O emissions and increase maize yields? Agr Ecosyst. Environ. 322, 107672. doi: 10.1016/j.agee.2021.107672
Wu, Z., Wang, Y., Liu, C., Yin, N., Hu, Z., Shen, L., et al. (2023). Characteristics of soil N2O emission and N2O-producing microbial communities in paddy fields under elevated CO2 concentrations. Environ. pollut. 318, 120872. doi: 10.1016/j.envpol.2022.120872
Wu, H., Wang, D., Zhang, D., Rao, W., Yuan, Q., Shen, X., et al. (2024). Responses of N2O, CO2, and NH3 emissions to biochar and nitrification inhibitors under a delayed nitrogen application regime. Agriculture 14, 1986. doi: 10.3390/agriculture14111986
Xiao, F., Gámiz, B., and Pignatello, J. J. (2018). Adsorption and desorption of nitrous oxide by raw and thermally air-oxidized chars. Sci. Total Environ. 643, 1436–1445. doi: 10.1016/j.scitotenv.2018.06.280
Xiao, X., Xie, G., Yang, Z., He, N., Yang, D., and Liu, M. (2021). Variation in abundance, diversity, and composition of nirK and nirS containing denitrifying bacterial communities in a red paddy soil as affected by combined organic-chemical fertilization. Appl. Soil Ecol. 166, 104001. doi: 10.1016/j.apsoil.2021.104001
Xie, L., Li, L., Xie, J., Wang, J., Mumtaz, M. Z., Effah, Z., et al. (2024). Optimal substitution of inorganic fertilizer with organic amendment sustains rainfed maize production and decreases soil N2O emissions by modifying denitrifying bacterial communities in Northern China. Eur. J. Agron. 160, 127287. doi: 10.1016/j.eja.2024.127287
Xu, P., Jiang, M., Khan, I., Shaaban, M., Zhao, J., Yang, T., et al. (2023). The effect of upland crop planting on field N2O emission from rice-growing seasons: A case study comparing rice-wheat and rice-rapeseed rotations. Agr Ecosyst. Environ. 347, 108365. doi: 10.1016/j.agee.2023.108365
Xu, W., Xu, H., Delgado-Baquerizo, M., Gundale, M. J., Zou, X., and Ruan, H. (2023). Global meta-analysis reveals positive effects of biochar on soil microbial diversity. Geoderma 436, 116528. doi: 10.1016/j.geoderma.2023.116528
Yan, L., Wang, G., Ai, S., Huo, Z., Wang, Y., Gu, J. D., et al. (2018). Abundance of ammonia-oxidizing bacteria and archaea under different ventilation strategies during cattle manure composting. J. Environ. Manage 212, 375–383. doi: 10.1016/j.jenvman.2018.02.032
Yang, Y., Xiao, Y., Li, C., Wang, B., Gao, Y., and Zhou, G. (2021). Nitrogen addition, rather than altered precipitation, stimulates nitrous oxide emissions in an alpine steppe. Ecol. Evol. 11, 15153–15163. doi: 10.1002/ece3.8196
Yang, L., Zhu, G., Ju, X., and Liu, R. (2021). How nitrification-related N2O is associated with soil ammonia oxidizers in two contrasting soils in China? Sci. Total Environ. 770, 143212. doi: 10.1016/j.scitotenv.2020.143212
Yao, C., Li, J., Gao, Y., Zhang, Z., Liu, Y., Sun, Z., et al. (2024). Delayed application of water and fertilizer increased wheat yield but did not improve quality parameters. Field Crop Res. 319, 109649. doi: 10.1016/j.fcr.2024.109649
Yu, H., Han, X., Zhang, X., Meng, X., Yue, Z., Liu, X., et al. (2023). Fertilizer-induced N2O and NO emissions in tea gardens and the main controlling factors: A recent three-decade data synthesis. Sci. Total Environ. 871, 162054. doi: 10.1016/j.scitotenv.2023.162054
Zheng, Q., Hu, Y., Zhang, S., Noll, L., Böckle, T., Dietrich, M., et al. (2019). Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 136, 107521. doi: 10.1016/j.soilbio.2019.107521
Keywords: N2O, biochar, DMPP, AOB, nirK, nosZ
Citation: Wu H, Zhang D, Shen X, Ma G, Yuan Q, Zhao H, Liu S, Jie X and Wang D (2025) Combined biochar and DMPP reduce N2O emissions in wheat crops via microbial community modulation. Front. Plant Sci. 16:1647453. doi: 10.3389/fpls.2025.1647453
Received: 15 June 2025; Accepted: 05 September 2025;
Published: 01 October 2025.
Edited by:
Jie Zhou, Nanjing Agricultural University, ChinaReviewed by:
Hanuman Singh Jatav, Sri Karan Narendra Agriculture University, IndiaMohamed T. El-Saadony, Zagazig University, Egypt
Copyright © 2025 Wu, Zhang, Shen, Ma, Yuan, Zhao, Liu, Jie and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daichang Wang, ZHp3YW5nQGhlbmF1LmVkdS5jbg==
 Haizhong Wu
Haizhong Wu Dengxiao Zhang1,3
Dengxiao Zhang1,3