- 1Laboratory of Ecology and Environmental Management, Science and Technology Advanced Institute, Van Lang University, Ho Chi Minh City, Vietnam
- 2Faculty of Applied Technology, Van Lang School of Technology, Van Lang University, Ho Chi Minh City, Vietnam
- 3Department of Life Sciences, National Chung Hsing University, Taichung, Taiwan
- 4Advanced Plant and Food Crop Biotechnology Center, National Chung Hsing University, Taichung, Taiwan
- 5Innovation and Development Center of Sustainable Agriculture, National Chung Hsing University, Taichung, Taiwan
Antimicrobial peptides (AMPs) from plants and microorganisms have emerged as promising tools due to their multifunctional roles in plant defense. These small, bioactive molecules, such as thionins, systemins, defensins, cyclotides, hevein-like peptides, and cyclic dipeptides, exhibit broad-spectrum activity against fungal pathogens, bacteria, and insect pests. Recent studies have further elucidated their supportive roles in conferring tolerance to abiotic stresses, including salinity, drought, and heavy metals exposure, thus expanding their potential applications. Previous studies demonstrated that the integration of AMPs genes into transgenic crops has shown significant potential in improving plant resistance to both biotic and abiotic stresses. Importantly, in our recent study, a cyclic dipeptide cyclo(L-Ala-Gly) from Priestia megaterium BP01R2 enables salinity stress alleviation in plants. The latest finding revealed that cyclo(His-Pro) in Arabidopsis navigated carbon flux from glycolysis to the pentose phosphate pathway and its supplementation increased NADPH levels and the NADPH/NADP+ ratio in plants. This review explores the latest advances in the application of plant- and microorganisms-derived AMPs, with a focus on their functional mechanisms and their roles in the development of stress-resilient crops. It also provides an overview of ongoing efforts to harness peptides in sustainable agricultural practices.
1 Introduction
The remarkable diversity, ubiquity, and versatility of antimicrobial peptides (AMPs) make them an abundant source of novel metabolites with potential applications in medicine, agriculture, and the food industry (Campos et al., 2018; Bakare et al., 2022; Simonsen et al., 2005). Various strategies have been implemented to alleviate the adverse effects of biotic and abiotic stresses on plants, including plant breeding (Hill et al., 1998), acclimation (Pandolfi et al., 2016), seed biopriming (Corbineau et al., 2023; Aizaz et al., 2023), plant growth-promoting rhizobacteria (PGPR) (Vaishnav et al., 2019), and the use of non-protein amino acids (NPAAs) (e.g., 5-hydroxynorvaline, meta-tyrosine, GABA, BABA, Canavanine, L-DOPA, Mimosine, etc.) (Silva Rodrigues-Corrêa and Fett-Neto, 2019). Research has also investigated the roles of various AMPs in plant defense systems to enhance resistance to phytopathogens (Maximiano et al., 2022) and to bolster adaptive mechanisms against abiotic stresses (Kulaeva et al., 2020). These studies show that AMPs can act directly as insecticidal molecules, inhibiting the growth of insect larvae (Mulla and Tamhane, 2023), or as inhibitors that suppress the growth of pathogenic bacteria or fungi (Huang et al., 2021).
Despite extensive investigation into their roles in suppressing bacteria and fungi (Leannec-Rialland et al., 2022; McLaughlin et al., 2021; Nawrot et al., 2014), the contributions of AMPs to mitigating the adverse effects of abiotic stresses remain less explored. Specifically, relatively few studies have examined the functions of AMPs in improving plant tolerance to salinity and drought stress (Li et al., 2021; Kumar et al., 2019; Hung et al., 2024), heavy metal toxicity (Mirakhorli et al., 2019), or wound stress (Rawat et al., 2017). This disparity in research emphasis can be attributed to several factors. Historically, AMPs were primarily identified and characterized for their antimicrobial properties, leading to a predominant focus on biotic stress responses, where their inhibitory effects on pathogens are straightforward to assay and quantify (Tang et al., 2023). In contrast, abiotic stresses, such as salinity and drought, involve complex physiological and molecular pathways (e.g., osmotic regulation or ion homeostasis), making it less intuitive and more challenging to evaluate the indirect effects or multifunctional roles of AMPs (Kulaeva et al., 2020). For instance, elucidating mechanisms of membrane permeability modulation and hormone signaling under abiotic conditions requires interdisciplinary approaches using transcriptomics, proteomics, and functional genomics, which demand specialized expertise and significant facilities (Deshmukh et al., 2025). Furthermore, abiotic stress experiments often necessitate controlled environments, which are more complicated than in vitro antimicrobial assays. While funding priorities have shifted toward abiotic stress research in recent years, driven by climate change concerns and the need for resilient crops, the focus remains on broader molecular mechanisms, leaving AMP-specific studies underexplored (Oyebamiji et al., 2024). This knowledge gap is critical, as emerging evidence suggests AMPs could enhance plant resilience through mechanisms like reactive oxygen species scavenging and hormone crosstalk, yet comprehensive studies remain sparse (Kaya and Adamakis 2025; González Ortega-Villaizán et al., 2024). Addressing this gap through targeted research could unlock novel applications for AMPs in sustainable agriculture. Our recent research, which demonstrated that the exogenous application of the cyclic dipeptide cyclo(L-Ala-Gly) contributes to salinity stress tolerance in plants, further highlights the potential of small bioactive molecules to enhance crop resilience against escalating global abiotic stresses (Hung et al., 2024). Beyond their direct application, AMP-encoding genes have also been widely incorporated into transgenic crops to confer new agronomic traits, including resistance to both biotic and abiotic stresses (Su et al., 2020; Parisi et al., 2020). To address this critical gap, this review synthesizes recent advances in the development of transgenic plants engineered with AMP-encoding genes from diverse plant and microbial sources. Furthermore, we explore the emerging potential of cyclic dipeptides to enhance the resistance of major crops to both biotic and abiotic stresses. By consolidating these findings, this manuscript offers valuable insights for researchers and agricultural scientists, guiding the development of novel, sustainable strategies to improve crop resilience and productivity in the face of diverse environmental challenges.
2 Classification and mechanisms of AMPs
2.1 Thionins
2.1.1 Family and structure
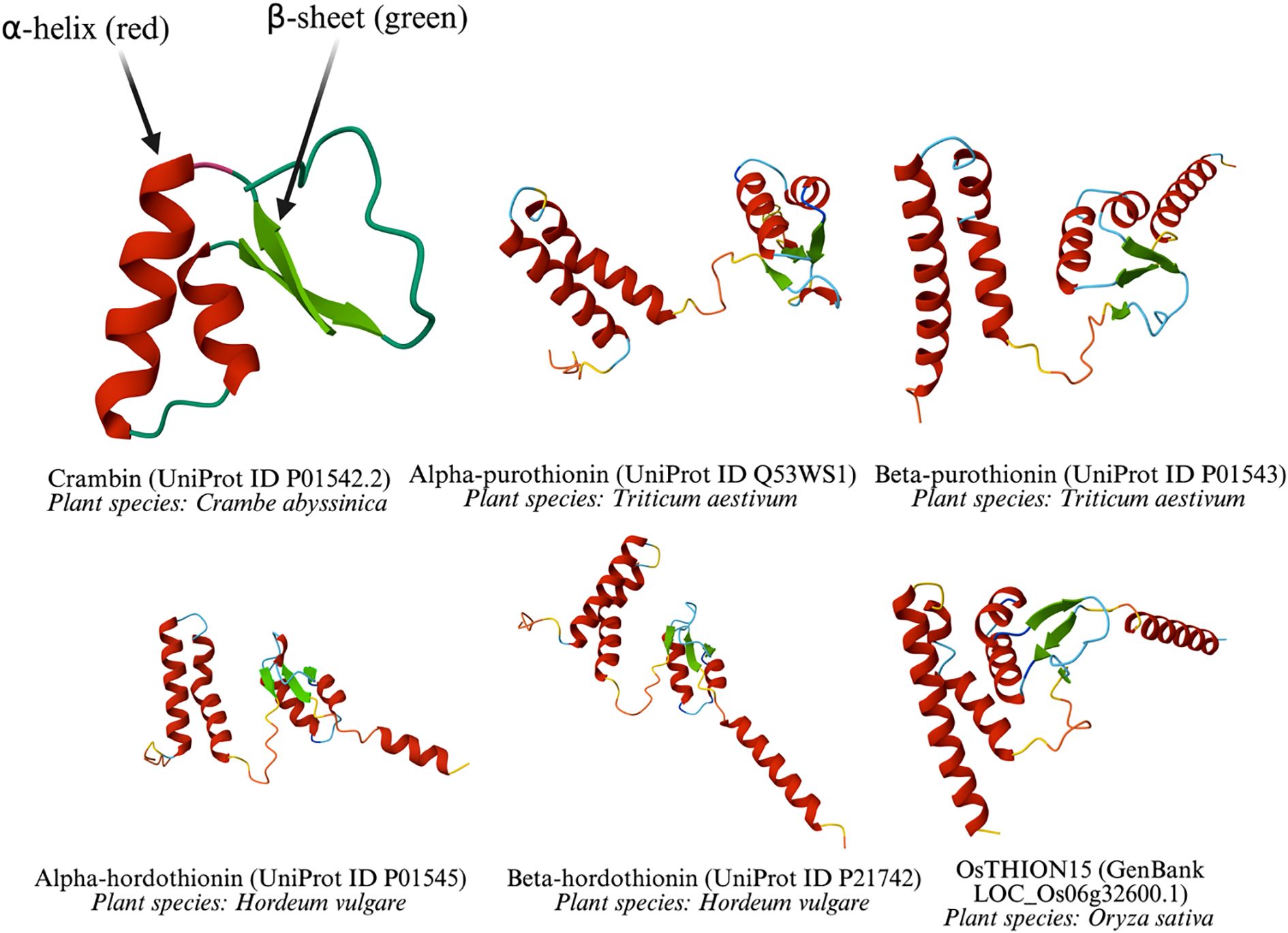
Thionins are a major class of plant-derived antimicrobial peptides (AMPs), alongside defensins, non-specific lipid transfer proteins, hevein-like peptides, knottin-like peptides, and cyclotides, distinguished by their cysteine spacing motifs and three-dimensional (3D) structures (Odintsova et al., 2018). As the first plant AMPs identified with in vitro antipathogenic activity, thionins are small peptides of approximately 5 kDa and 45–47 amino acids (aa), found predominantly in certain angiosperm families, including Poaceae (e.g., barley, wheat), Brassicaceae (e.g., Arabidopsis), and Ranunculaceae. Their compact structure, characterized by 6 or 8 cysteine residues forming 3 or 4 disulfide bridges, confers exceptional stability under diverse environmental conditions (Odintsova et al., 2018). Some common thionin structures were predicted using AlphaFold3 (Abramson et al., 2024) and are shown in Figure 1.
All depicted thionins share a common structural motif, including at least one α-helix and one β-sheet, which are critical for their antimicrobial function. These elements are stabilized by disulfide bonds, a hallmark of thionins, enabling them to disrupt microbial membranes. Differences in loop lengths and helix-sheet arrangements reflects species-specific variations, yet the conserved core structure underscores their shared evolutionary origin and functional role in plant defense. The diversity of plant species (Crambe abyssinica, Triticum aestivum, Hordeum vulgare, and Oryza sativa) highlights the widespread occurrence and adaptation of thionins across monocot and dicot plants.
2.1.2 Mechanisms of antimicrobial action
Thionins primarily target pathogen cell membranes by interacting with negatively charged phospholipids, such as phosphatidic acid or phosphatidylserine, forming proteolipid complexes that disrupt membrane integrity through solubilization and lysis (Ji et al., 2015). This interaction also involves the extraction of phospholipids, reducing membrane fluidity and causing irreparable damage (Stec et al., 2004). Beyond their direct antimicrobial activity, thionins contribute to plant defense by activating signal transduction pathways mediated by phytohormones, including salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) (Künstler et al., 2020). Previous studies further suggest that thionins enhance plant immunity by modulating reactive oxygen species (ROS) production and synergizing with other AMPs to bolster resistance against fungal and bacterial pathogens (Chan et al., 2005; Hao et al., 2020).
2.1.3 Roles of thionins in biotic stress mitigation
2.1.3.1 Antimicrobial activities
Thionins exhibit broad-spectrum activity against diverse biotic stressors, including bacteria, fungi, insects, and nematodes. A thionin-like peptide, CaThi, from Capsicum annuum, inhibited Candida spp. with half-maximal inhibitory concentrations (IC50) ranging from 10 to 40 µg/mL (Taveira et al., 2016). CaThi was shown to permeabilize the plasma membrane of all tested Candida spp. and induce oxidative stress in Candida tropicalis, with synergistic interactions with fluconazole significantly enhancing candidacidal activity (Taveira et al., 2016). Similarly, a thionin (Thi2.1) from Arabidopsis thaliana, when expressed in the BVE-E6E7 bovine endothelial cell line, reduced the viability of Escherichia coli, Staphylococcus aureus, and Candida albicans (Loeza-Ángeles et al., 2008). A cowpea thionin II expressed antifungal activity against Fusarium culmorum at minimum inhibitory concentration (MIC) ~ 50 µg mL−1, but Aspergillus niger and Penecillium expansum at MIC greater than 500 µg mL−1 (Schmidt et al., 2019). The thionin II activity remained at elevated temperature (100°C, 15 min) but lost its antifungal property in the presence of cations such as Na+, K+, Ca2+, and Mg2+ (Schmidt et al., 2019). A study by Moghadam et al. (2023) evaluated the antifungal efficacy of aqueous and alcoholic extracts from three varieties of C. annuum against various Candida species (Moghadam et al., 2023). The results, however, indicated that these extracts exhibited minimal inhibitory effects, with MIC exceeding 512 µg mL−1, suggesting limited antifungal activity in their crude form (Moghadam et al., 2023).
2.1.3.2 Antifungal defense
A 2004 study by Oard et al. showed that, of 12 natural and synthetic AMPs, purothionin, a type I thionins from wheat seed, exhibited the strongest inhibitory activity against Rhizoctonia solani LR172, causal agent of rice sheath blight and aerial blight of soyabeans in the USA (Oard et al., 2004). Also in Oard et al. study, a strong correlation between membrane permeabilization and antifungal activity of the tested peptides was found with the most significant changes in membrane integrity at ≥ 0.5 µmol L−1 of purothionin, followed by 2 µmol L−1 of cecropin B, a natural peptide from cecropia moth (Oard et al., 2004). A 15 kDa thionin protein, Thi2.4, from A. thaliana was shown to interact with the fungal virulence factor fruit body lectin (FFBL), thereby reducing the toxicity of Fusarium graminearum. In addition to this protein–protein interaction, Thi2.4 exhibits direct antifungal activity by disrupting fungal cell membranes (Asano et al., 2013).
2.1.3.3 Insect and nematode resistance
In barley (Hordeum vulgare), the thionin genes THIO1567 and THIO1570 exhibited significantly higher transcript levels in the bird cherry–oat aphid (Rhopalosiphum padi L.)-resistant genotype Hsp5 compared to the susceptible genotype Lina, suggesting a role in aphid resistance (Mehrabi et al., 2014). Similarly, in rice, thionin gene expression was more strongly suppressed in the susceptible genotype Nipponbare (Oryza sativa) than in the resistant accession TOG5681U (Oryza glaberrima) following nematode infection, indicating a potential contribution of thionins to nematode resistance (Petitot et al., 2017).
2.1.4 Roles of thionins in abiotic stress mitigation
Under drought stress conditions, the susceptible barley cultivar Concerto showed increased expression of the HvTHIO1 gene, suggesting a potential protective role (Leybourne et al., 2022). In rice, the defensin-dissimilar thionin gene OsThi9 was strongly expressed in roots and stems under cadmium (Cd) exposure (0.1 µM CdCl2). Overexpression of OsThi9 reduced Cd translocation to shoots, lowering Cd levels in leaves and grains (Liu et al., 2023). A combination of osmotic and heat stress caused higher susceptibility of Arabidopsis plants to Botrytis cinerea, possibly due to the reduced expression of defense genes, including PLANT DEFENSIN 1.3 (PDF1.3), BOTRYTIS SUSCEPTIBLE 1 (BOS1), THIONIN2.2 (THI2.2), and cell wall-related genes (Sewelam et al., 2021). Recently, Yan et al. (2025) found that OsTHION15 was significantly upregulated under drought stress, while the Osthion15 mutant led to higher sensitivity to drought and abscisic acid (ABA) stress, indicating its key roles in abiotic stress resistance (Yan et al., 2025). While these studies indicate altered expression of thionin genes under abiotic stress conditions, such changes do not directly confirm the functional roles of thionins in stress tolerance. Therefore, further research is needed to elucidate the direct involvement of the thionins themselves in abiotic stress tolerance.
2.1.5 Applications of thionin genes in transgenic plant development
Thi2.1, encoding 5 kDa cysteine-rich antimicrobial peptides, was upregulated in the resistant Arabidopsis ecotype UK-4 upon Fusarium oxysporum f. sp. matthiolae infection. Its overexpression in susceptible Col-2 seedlings delayed chlorophyll loss, inhibited fungal growth, and triggered severe fungal phenotype abnormalities (Epple et al., 1997). When Thi2.1 was introduced into the tomato genome, the resulting transgenic plants showed enhanced resistance to Fusarium wilt (FW) (F. oxysporum f. sp. lycopersici) and bacterial wilt (BW) (Ralstonia solanacearum) (Chan et al., 2005). These Thi2.1 transgenic lines matched the BW-resistant variety H7996 in disease incidence. Similarly, Thi2.1 transgenic lines R7 and R11 showed a disease resistance comparable to the FW resistant variety MH1 and significantly lower disease incidence than WT plants (Chan et al., 2005). Meanwhile, β-purothionin, a 45-aa wheat endosperm thionin with 4 disulfide bonds and a strong cationic charge, offers environmental stability (Oard and Enright, 2006; Kaas et al., 2010; Stec, 2006). Transgenic Arabidopsis expressing β-purothionin gene driven by a leaf-specific chloroplast carbonic anhydrase promoter showed top-tier resistance to Pseudomonas syringae strain DC3000, with no leaf infection symptoms. In vitro tests against F. oxysporum revealed transgenic seedlings surviving 12–15 days with minimal necrosis and discoloration, while control died within 6–8 days (Oard and Enright, 2006). Thio-60 extracted from transgenic onion (Allium cepa L.) outperformed its non-transgenic counterpart, inhibiting A. niger spore germination by 52% versus 37% (Tawfik et al., 2022). A barley α-hordothionin (α-HT) gene with 384 bp in length, driven by the constitutive El2Ω or β-amylase (β-Amy) promoter (the 5’-UTR region), was transformed into sweet potato cultivar Kokei No. 14, to tackle Ceratocystis fimbriata, the top postharvest disease of sweet potato (Muramoto et al., 2012). C. fimbriata causes the most damaging postharvest disease of sweet potato (Ipomoea batatas (L.) Lam.) worldwide (Zhang et al., 2018). Wounds on storage roots were inoculated with a suspension of C. fimbriata spores to examine the resistance. Transgenic lines El2Ω: α-HT No. 1 and β-Amy: α-HT No. 060201 had much smaller black lesions than non-transgenic Kokei No. 14, with lesion areas of 119 mm2 and 111 mm2 compared to 283 mm2, respectively, indicating that the transgenic lines acquired solid resistance (Muramoto et al., 2012). In non-transgenic rice, OsTHI7 normally exhibited root tip-specific expression, but its promoter activity was not detectable inside Meloidogyne graminicola-induced galls (Ji et al., 2015). This down-regulation might be a strategy by M. graminicola to suppress plant defense, thereby preventing the thionins from reaching toxic levels against the nematode. In contrast, transgenic rice lines that overexpressed OsTHI7 showed decreased susceptibility to M. graminicola infection. These lines exhibited a significant reduction, approximately 39.2%, in the number of females and total nematodes per plant compared to control plants (Ji et al., 2015). This suggests a protective effect of OsTHI7 against the nematode, likely due to its direct toxic effect, as it possesses characteristics to alter cell membrane permeability and contribute to toxicity. Similarly, overexpression of OsTHI7 in rice decreased susceptibility to Pythium graminicola colonization (Ji et al., 2015). Transgenic line OX22 displayed significantly healthier shoots with a lower disease rate and greater shoot length compared to control plants. Quantitative PCR analysis revealed that the quantity of P. graminicola DNA was significantly lower in the roots of OsTHI7-overexpressing lines (70% of total DNA) compared to wild-type and empty vector controls (97% and 98% respectively) (Ji et al., 2015).
Huanglongbing (HLB), the deadliest threat to global citrus production, has spurred extensive resistance engineering (Hao et al., 2017). A modified citrus thionin gene (Mthionin) gene, derived from an endogenous citrus thionin, was inserted into the Carrizo citrus genome. The transgenic Carrizo plants resisted HLB disease (Candidatus Liberibacter asiaticus) (Las) and citrus canker disease (Xanthomonas citri) (Hao et al., 2016). Leaf infiltration assays showed Mthionin transgenic leaves with little to no canker at X. citri concentration of 104–107 CFU mL−1. Grafted plants with transgenic Carrizo rootstock had significantly lower Las titers in young leaves and roots than controls, indicating that Mthionin is a promising HLB fighter (Hao et al., 2016). In another study, Mthionin gene, driven by a double 35S promoter, was inserted into the A. thaliana genome (Hao et al., 2020). Lines A24 and A52, with peak Mthionin expression, showed reduced water soaking, lesions, and fungal biomass in detached leaf assays. Sprayed with F. graminearum (5 x 105 conidia mL−1), GUS transgenic plants suffered severe symptoms, such as dry flowers, dry siliques, and dead branches, while Mthionin plants remained symptom-free. Furthermore, F. graminearum conidia barely germinated on Mthionin leaves. At 48 h post-inoculation, the DEFENSIN1.2 expression in Mthionin plants was significantly higher than in GUS plants, hinting that Mthionin bolsters resistance to F. graminearum via defense genes and phytohormone signaling. Ectopic expression of two barley thionin genes AK252675.1 and AK359149 in Nicotiana benthamiana reduced host susceptibility to Myzus persicae, underscoring the important role of thionin genes against aphids (Escudero-Martinez et al., 2017). The Thio-60 gene from A. thaliana was transformed into date palm (Phoenix dactylifera L.) cultivars Barhy, Sakkoti, and Shamia, yielding transgenic plants with higher resistance to F. oxysporum, confirmed by detached leaf pathogenicity test (Allah et al., 2023). In a more recent advancement, thionin genes (e.g., thio-60 and thio-63) were introduced into Paulownia trees using chitosan nanoparticles as a delivery system for genetic transformation. The resulting transgenic lines exhibited enhanced resistance to fungal pathogens. Specifically, thio-60 transgenic lines increased resistance to Fusarium equiseti, while thio-63 transgenic lines enhanced resistance to A. niger (Bouqellah et al., 2024).
The integration of thionin genes into transgenic plant systems reveals consistent patterns of enhanced resistance across diverse biotic stressors and crop species. In response to fungal pathogens such as Fusarium spp., A. niger, C. fimbriata, and F. graminearum, overexpression of thionin variants (e.g., Thi2.1, β-purothionin, α-HT, Mthionin, Thio-60) has been shown to significantly reduce lesion sizes in sweet potato, inhibit spore germination in onion, delay seedling mortality A. thaliana, and produce symptom-free phenotypes. These effects are primarily attributed to direct antimicrobial activity and the upregulation of defense-related genes.
Comparable efficacy is observed under bacterial stress conditions, including infections by R. solanacearum, P. syringae, X. citri, and Ca. L. asiaticus. Transgenic lines exhibit reduced pathogen titers, absence of visible symptoms, and resistance levels on par with commercial cultivars. Although less frequently reported, insect resistance has also been demonstrated, notably against M. persicae in N. benthamiana, where ectopic thionin expression decreases host plant susceptibility. While model species such as A. thaliana and N. benthamiana continue to serve as valuable platforms for mechanistic studies, economically important crops—tomato, sweet potato, citrus, onion, date palm, and Paulownia spp.—exhibit promising translational outcomes, including postharvest protection and reduced disease incidence under laboratory- and field-relevant conditions. Collectively, these findings underscore the potential of thionin genes to confer broad-spectrum biotic resistance through membrane disruption and activation of defense pathways, supporting their utility in multi-pathogen engineering strategies across diverse plant systems.
2.2 Defensins
2.2.1 Family and structure
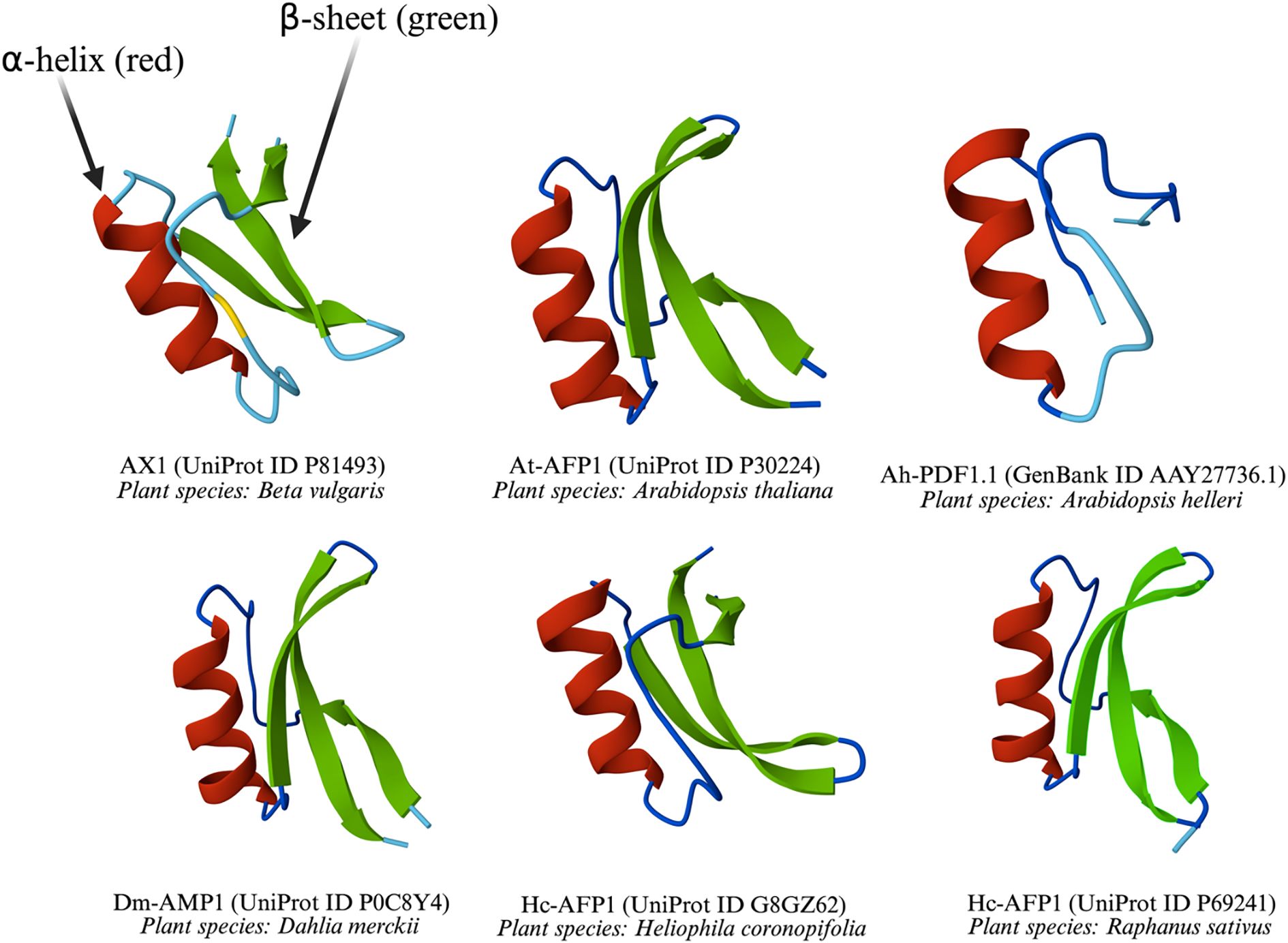
Like thionins, defensins are a key family of small, cysteine-rich, cationic peptides, but they are distinguished by a different structural architecture. They typically range from 45 to 54 aa, and feature eight conserved cysteine residues forming four disulfide bridges, which confer stability against protease degradation, extreme pH, and high temperatures (Azmi and Hussain, 2021; Kovaleva et al., 2020). The disulfide bonds cysteine residues in plant defensins possess the same pattern: Cys1-Cys8, Cys2-Cys5, Cys3-Cys6, and Cys4-Cys7 (Leannec-Rialland et al., 2022) with few exceptions (Janssen et al., 2003; Lay et al., 2003). Found across plant families such as Brassicaceae, Fabaceae, and Solanaceae families, plant defensins belong to the cis-defensin superfamily, distinct from the trans-defensin superfamily of mammals (Parisi et al., 2019). GMA4CG_V6, derived from MtDef4—a 47-aa defensin from Medicago truncatula—exhibited a multifaceted mode of action and antifungal activity against B. cinerea. Its 17-aa frame, containing the gamma-core motif of MtDef4, retains potent antifungal activity even at elevated salt concentrations (Tetorya et al., 2023). A characteristic γ-core motif (GXCX3–9C) is critical for their antimicrobial activity (Slezina et al., 2022; 2023; Sonderegger et al., 2018). The γ-core motif also plays role in mediating the entry of defensins into fungal cells (Sagaram et al., 2013; Tetorya et al., 2023). The Scots pine (Pinus sylvestris L.) defensin PsDef5.1 features a cysteine-rich α-motif (CX5CX3CX7CX9CXC) and a γ-core motif (GXCX9C), underscoring its structural complexity (Shalovylo et al., 2021). PsDef2 (RMCKTPSAKFKGYCVSSTNCKNVCRTEGFPTGSCDFHITSRKCYCYKPCP) and PsDef1 (RMCKTPSGKFKGYCVNNTNCKNVCRTEGFPTGSCDFHVAGRKCYCYKPCP), both defensins derived from P. sylvestris, differ by six aa in their primary sequences (Bukhteeva et al., 2022). Although this significant divergence is notable, with approximately 10–13% of the sequence, did not strongly affect their biological activities, including antimicrobial, antibacterial, and insect α-amylase inhibitory effects, implying the structural core, likely the cysteine-stabilized α-β (CSαβ) fold typical of plant defensins, is preserved (Bukhteeva et al., 2022). The CSαβ fold is likely critical for structural stability, functional scaffold, and dynamic flexibility of plant defensins. Some common defensin structures were predicted using AlphaFold3 (Abramson et al., 2024) and are shown in Figure 2.
All the defensins share a common structural motif, including at least one α-helix and one β-sheet, which are stabilized by disulfide bonds typical of defensins. These elements are critical for their ability to disrupt microbial membranes. The variation in loop lengths and helix-sheet arrangements reflects species-specific adaptations, while the conserved core structure underscores their shared evolutionary role in plant defense. The diversity of plant species such as Beta vulgaris, A. thaliana, Arabidopsis helleri, Dahlia merckii, Heliophila coronopifolia, and Raphanus sativus highlights the widespread occurrence of these defensins across different plant families.
2.2.2 Mechanism of action
Similar to thionins, defensins and other AMPs deploy one or both primary antimicrobial strategies: membrane disruption and inhibition of cellular machinery (Figure 3). Their amphipathic nature enables them to target pathogen membranes, while specific residues enhance their efficacy. DefSm2-D (KLCEKPSKTWFGNCGNPRHCGDQCK-SWEGVHGACHVRNGKHMCFCYFNCPQAE), an antifungal defensin from wild thistle (Silybum marianum): truncated versions like SmAPγ27-44 (WEGAVHGACHVRNGKHMC), with its Arg38 residue in the γ-core domain, exhibited potent antagonism against F. graminearum (MIC50 of 20 μM), outperforms SmAPα1-21 (KLCEKPSKTWFGNCGNPRHCG) (MIC50 of 32 μM) and SmAPα10-21 (WFGNCGNPRHCG) (MIC50 of 70 μM) (Sagaram et al., 2013). The presence of three cationic lysine (Lys) residues, one anionic glutamic acid (Glu), and a tryptophan (Trp) in SmAPα1–21 likely enhances its membrane-binding capacity. Moreover, its selective action—targeting fungal cell walls over host cells—hinges on compositional differences between pathogen and plant membranes (Fernández et al., 2021).
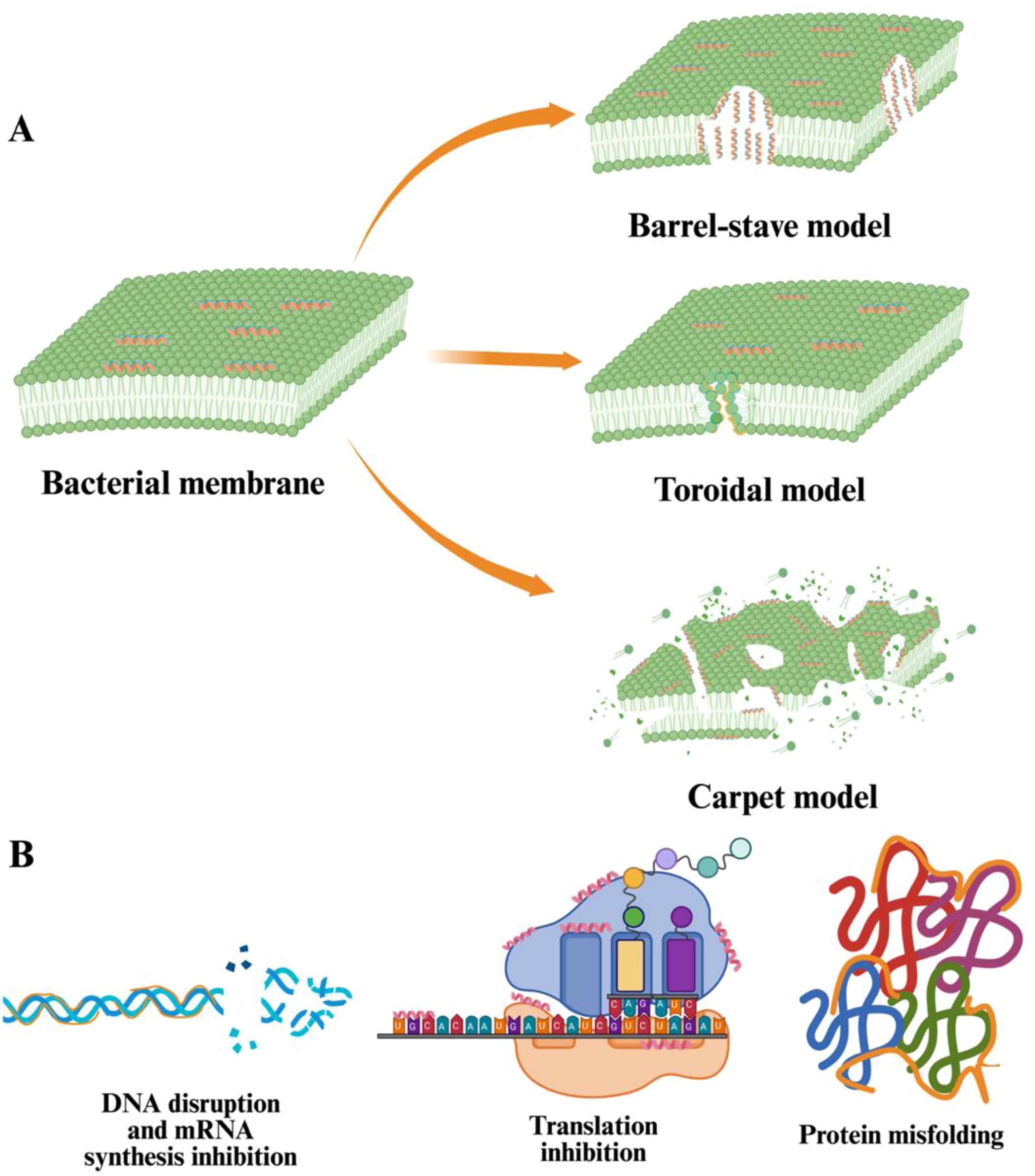
Figure 3. Two modes of action of AMPs. (A) In the barrel-stave model, the attached AMPs insert perpendicularly into the bacterial membrane. Their hydrophobic surfaces (shown in orange) bind to the lipid core of the bilayer, and their hydrophilic surfaces (shown in blue) form cylindrical pores, disrupting the membrane integrity. In the toroidal model, AMPs insert themselves into the lipid bilayer and induced the membrane to bend inward, creating a pore lined by both AMPs and the lipid headgroups. This results in a toroidal structure, where the lipids are significantly distorted. In the barrel-stave model, AMPs alone form a rigid pore structure, while the toroidal model triggers the interaction between the peptides and the lipids. In the carpet model, AMPs cover the bacterial membrane surface in a manner similar to a carpet. Initially, the AMPs do not form distinct pores. As the concentration of AMPs increases, they align parallel to the membrane surface and interact with the lipid headgroups through electrostatic and hydrophobic forces. This accumulation disrupts the membrane through a detergent-like mechanism involving partial micellization. Finally, the membrane undergoes disintegration and fragmentation, causing the release of cellular components and subsequent bacterial cell death. (B) AMPs can pass through cellular barriers to attach to nucleic acids (DNA, mRNA). AMPs exhibit high binding affinity to single- or double-stranded nucleic acids, allowing them to break down DNA molecules and inhibit mRNA synthesis. The attachment of AMPs on the double-helix DNA structure inhibits mRNA synthesis and disrupts DNA integrity. Also, AMPs can cause the blockage of protein translation by targeting ribosomes and protein misfolding by forming a complex with DnaK, a critical protein functioning in stabilizing de novo proteins. Figure 3 is reproduced and adapted from earlier studies (Le et al. 2017; Matsuzaki 2019).
[A] In the barrel-stave model, the attached AMPs insert perpendicularly into the bacterial membrane. Their hydrophobic surfaces (shown in orange) bind to the lipid core of the bilayer, and their hydrophilic surfaces (shown in blue) form cylindrical pores, disrupting the membrane integrity. In the toroidal model, AMPs insert themselves into the lipid bilayer and induced the membrane to bend inward, creating a pore lined by both AMPs and the lipid headgroups. This results in a toroidal structure, where the lipids are significantly distorted. In the barrel-stave model, AMPs alone form a rigid pore structure, while the toroidal model triggers the interaction between the peptides and the lipids. In the carpet model, AMPs cover the bacterial membrane surface in a manner similar to a carpet. Initially, the AMPs do not form distinct pores. As the concentration of AMPs increases, they align parallel to the membrane surface and interact with the lipid headgroups through electrostatic and hydrophobic forces. This accumulation disrupts the membrane through a detergent-like mechanism involving partial micellization. Finally, the membrane undergoes disintegration and fragmentation, causing the release of cellular components and subsequent bacterial cell death. [B] AMPs can pass through cellular barriers to attach to nucleic acids (DNA, mRNA). AMPs exhibit high binding affinity to single- or double-stranded nucleic acids, allowing them to break down DNA molecules and inhibit mRNA synthesis. The attachment of AMPs on the double-helix DNA structure inhibits mRNA synthesis and disrupts DNA integrity. Also, AMPs can cause the blockage of protein translation by targeting ribosomes and protein misfolding by forming a complex with DnaK, a critical protein functioning in stabilizing de novo proteins. Figure 3 is reproduced and adapted from earlier studies (Le et al., 2017; Matsuzaki, 2019).
2.2.3 Roles of defensins in biotic stress
Defensins play important roles in plant immunity, with expression often triggered by the mitogen-activated protein kinase (MAPK) cascade via effector-triggered immunity (ETI) and MAMP-triggered immunity (MTI) in response to fungal and bacterial assaults (Contreras et al., 2020). In pepper, the defensin gene CADEF1 remained silent in healthy leaves but surged under infection by Xanthomonas campestris pv. vesicatoria (Mee Do et al., 2004). In rice, OsDEF7 and OsDEF8 ramped up expression following Xanthomonas oryzae pv. oryzae infection (Weerawanich et al., 2018). Arabidopsis defensin-like (DEFL) genes spiked in leaves challenged by Alternaria brassicicola or P. syringae, likely via JA signaling (Tesfaye et al., 2013). The transcription factor WRKY75 further amplifies this response, upregulating JA pathway genes like ORA59 and PDF1.2 in overexpressing plants, while their expression drops in wrky75 mutants (Chen et al., 2021). Defensins also exhibit direct antimicrobial activity, for example, cowpea Cp-thionin II inhibited S. aureus (MIC ~ 128 μg mL−1), E. coli (MIC ~ 64 μg mL−1), and P. syringae (MIC ~ 42 μg mL−1) (Franco et al., 2006), while Scots pine PsDef5.1, fused with thioredoxin, suppressed pathogens like Fusarium sporotrichiella and Bacillus pumilus (Shalovylo et al., 2021). In resistant wild chickpea (ICC17160), six defensin genes (CaDEF1.1B, CaDEF2.4, CaDEF2.5a, CaDEF3, CaDEF5, and CaDEFL2) showed heightened expression against F. oxysporum and Rhizoctonia bataticola compared to the susceptible JG62 variety (Nitnavare et al., 2023). In a recent study by Kalunke et al. (2025), the bi-domain defensin MtDef5 from Medicago truncatula demonstrated antifungal properties at very low concentrations (Kalunke et al., 2025). It consists of two single-domains, MtDef5A and MtDef5B, linked by a short peptide APKKVEP. Between these two domains, MtDef5B showed greater antifungal potency compared with MtDef5A, exhibiting potent broad-spectrum inhibitory activity against Botrytis cinerea (Bc), Fusarium graminearum (Fg), Fusarium virguliforme (Fv), and the oomycete Phytophthora capsici (Pc), with MICs ranging from 0.75 µM to 1.5 µM for these pathogens. In contrast, a variant called GMA5AC_V2, derived from carboxy-terminal γ-core motif of MtDef5A (GMA5AC), exhibited two-fold higher activity (MIC ~ 1.5 µM) than GMA5AC (MIC ~ 3 µM) against Bc. It was at least four-fold more potent than GMA5AC against Colletotrichum gloeosporioides (Cg), Fg, and Fv. Notably, while GMA5AC had a MIC value of >12 µM against Cg, GMA5AC_V2 had a MIC value of 3 µM against this pathogen (Kalunke et al., 2025). The GMA5AC_V2 potent antifungal activity could be attributed to the replacement of two cysteine residues (C12 and C14) with phenylalanine in its structure (Kalunke et al., 2025). Furthermore, GMA5AC_V2 also displayed a multi-faceted mode of action similar to MtDef5B as it disrupted the fungal plasma membrane, induced the production of ROS in both Bc and Cg germlings, and inhibited protein translation in vitro (Kalunke et al., 2025). In summary, plant defensins contribute to biotic stress mitigation by acting as both immune response regulators and direct antimicrobial agents, enhancing resistance against a broad spectrum of fungal and bacterial pathogens.
2.2.4 Roles of defensins in abiotic stresses
2.2.4.1 Defensin-mediated mechanisms for heavy metal tolerance
A primary mechanism for heavy metal tolerance is the chelation of metal ions by the cysteine-rich domains of defensins, followed by the efflux of the metal-defensin complex from the cytoplasm to less sensitive extracellular compartments like cell walls or the apoplast, or into vascular tissues for transport (Luo et al., 2018; Luo et al., 2019; Li et al., 2025; Luo et al., 2019). Defensins mitigate abiotic stresses through these mechanisms, which include metal chelation, regulation of metal transport, and modulation of physiological responses. Many defensins (e.g., CAL1, DEF8, AtPDF1.5, AtPDF2.5, NtCAL1) are secreted to the cell wall or extracellular spaces (Luo et al., 2018; Gu et al., 2023; Liu et al., 2021; Luo et al., 2020, 2019; Gu et al., 2023; Jin et al., 2024), while others, like AtPDF2.6 and SpPDF, are localized in the cytoplasm, where they directly chelate metals to mitigate toxicity (Luo et al., 2019; Li et al., 2025).
2.2.4.2 Specific roles of defensins in Cd stress mitigation
Cd is a major heavy metal pollutant posing significant threats to food security and human health, making Cd detoxification and accumulation a critical area of study (Luo et al., 2018; Luo et al., 2019; Li et al., 2024; Luo et al., 2020). Several defensins play key roles in managing Cd stress by regulating its chelation, transport, and accumulation in different plant tissues. Some defensins are regulators of root-to-shoot translocation, acting as secreted proteins that primarily control the movement of Cd from roots to shoots. For example, CAL1 (Cd Accumulation in Leaf 1) in rice is a cell wall-localized defensin-like protein (Luo et al., 2018) that positively regulates Cd accumulation in leaves (Luo et al., 2018; 2020). It chelated Cd in the cytosol and drove its secretion to extracellular spaces, promoting its loading into xylem vessels for root-to-shoot translocation and accumulation in leaves and straws, but not in rice grains (Jin et al., 2024). Similarly, NtCAL1 in tobacco functions as a positive factor in plant Cd accumulation and resistance (Jin et al., 2024), with its overexpression enhancing Cd translocation to the shoot. Overexpression of NtCAL1 enhanced the content of ascorbic acid and the activities of antioxidant enzymes such as catalase (CAT) and ascorbate peroxidase (APX), helping to scavenge oxidative stress induced by Cd (Jin et al., 2024). It also positively regulated the expression of metal transport-related genes such as NtRAMP3, NtHMAα, NtHMAβ (Jin et al., 2024). AtPDF2.5 in Arabidopsis mediated Cd tolerance and accumulation (Luo et al., 2019), and its expression, which is induced by Cd, was primarily expressed in root xylem vascular bundles (Luo et al., 2020). This defensin has Cd-chelating activity (Luo et al., 2019) and promotes cytoplasmic Cd efflux and subsequent accumulation in the apoplast of cell walls, thereby enhancing detoxification and apoplastic accumulation (Luo et al., 2020). Additionally, OsThi9, a defensin-dissimilar thionin, alleviates Cd toxicity by sequestering Cd in cell walls and reducing its translocation to upper parts, which diminishes Cd accumulation in stems and brown rice (Liu et al., 2023). Other defensins act as modulators of shoot and grain accumulation, controlling Cd allocation within the plant. CAL2, a close homolog of CAL1, is a cell wall-localized protein with Cd chelation activity. Overexpression of CAL2 has been shown to increase Cd accumulation in both rice shoots and grains (Luo et al., 2020). Its expression is unaffected by Cd stress but is positively regulated by the endoplasmic reticulum stress response regulator OsbZIP39 (Li et al., 2024). DEFENSIN 8 (DEF8) in rice is a dual-function protein that mediates both xylem Cd loading and phloem Cd unloading (Gu et al., 2023). It was highly expressed in rice grains and was induced by Cd exposure (Gu et al., 2023). While DEF8 facilitates Cd transport from roots to shoots, it prevents Cd loading from the phloem into the grains during filling, making it an ideal target for breeding low-Cd rice varieties (Kazachkova 2023). AtPDF1.5 enhanced adaptation to low nitrogen levels and Cd stress in Arabidopsis (Wu et al., 2021). Its mechanism for Cd tolerance may be attributed to chelation and signal transmission, as it regulates the expression of metal transporter genes such as AtHMP07, AtNRAMP4, AtNRAMP1, AtHIPP3, resulting in higher Cd accumulation in shoots and promoting Cd transport (Wu et al., 2021). Some defensins are cytoplasmic chelators that act within the cytoplasm to directly sequester metals. AtPDF2.6 in A. thaliana is a non-secreted, cytoplasm-localized protein that actively chelates cytoplasmic Cd to mitigate its toxicity (Luo et al., 2019). Similarly, SpPDF in Sedum plumbizincicola, a hyperaccumulator plant, is another cytoplasm-localized defensin that confers Cd accumulation via its chelation (Li et al., 2025). Its chelation activity leads to increased Cd accumulation in the roots and reduced translocation to the shoots, helping the plant compartmentalize metals away from sensitive aerial parts (Li et al., 2025).
2.2.4.3 Roles of defensins in other abiotic stress mitigation
Apart from heavy metal responses, defensins actively contribute to abiotic stress responses, including drought, salinity, and heavy metal tolerance. The pepper CADEF1 gene, for example, was activated by abiotic elicitors (e.g., H2O2, wounding, salinity, drought) and stress hormones like SA, Methyl Jasmonate (MeJA), JA, abscisic acid (ABA), and ET (Contreras et al., 2020). Similarly, rice OsDEF7 and OsDEF8 responded to imbibition, anoxia, drought, and cold (Weerawanich et al., 2018). In Arabidopsis halleri ssp. halleri, a zinc (Zn)-tolerant species, defensin proteins accumulated in shoots and gene expression increased under Zn exposure (Mirouze et al., 2006). Arabidopsis AtPDF1.1 is upregulated by iron overload and Pectobacterium carotovorum infection, enhancing tolerance by chelating iron and disrupting pathogen homeostasis (Hsiao et al., 2017). These previous studies, however, did not directly confirm the functional roles of defensin genes in plant stress tolerance under various abiotic stresses, underscoring the need for further mechanistic studies to validate their contributions.
2.2.5 Applications of defensin genes in transgenic plant development
Defensin genes have been harnessed to engineer stress-resistant crops, demonstrating their versatile applications against both biotic and abiotic challenges. The first attempt to transform defensin genes into plant genomes to increase resistance towards phytopathogenic fungi was made by Bondt et al. (1998) (Bondt et al., 1998). Subsequently, the expression of alfalfa antifungal peptide (alfAFP) gene, a defensin isolated from Medicago sativa seeds, in transgenic potato plants, rendered high levels of field resistance against Verticillium dahliae, the causative agent of “early dying” disease of potato (Gao et al., 2000). In vitro assays showed that alfAFP inhibited hyphal elongation of V. dahliae, Alternaria solani and Fusarium culmorum, but not Phytophthora infestans. The secretion of alfAFP in the intercellular spaces of stem tissues protected transgenic potato in both greenhouse and multi-year trials (Gao et al., 2000). Transgenic wheat (Bobwhite and Xin Chun 9 genotypes) expressing an apoplast-targeted antifungal plant defensin, MtDef4.2, from Medicago truncatula displayed significant resistance to the leaf rust pathogen Puccinia triticina (Pt) (Kaur et al., 2017). This resistance was observed in growth chamber bioassays, where transgenic lines showed highly resistant infection types compared to non-transgenic controls. Interestingly, while MtDef4.2 reduced the proliferation of infection hyphae and minimized lesion sizes on infected wheat leaves, it did not negatively affect symbiotic relationship between the host plant and the beneficial arbuscular mycorrhizal fungus Rhizophagus irregularis (Kaur et al., 2017). This is a significant advantage for its agricultural application.
While early efforts focused on introducing defensins to combat phytopathogenic fungi (Bondt et al., 1998; Wang et al., 1999; Lebedev et al., 2002; Cary et al., 2000; Park et al., 2002; Swathi Anuradha et al., 2008; Abdallah et al., 2010), recent applications have expanded to include environmental stressors. Overexpression of the NaD1 gene from Nicotiana alata in tobacco (Nicotiana tabacum) enhanced drought tolerance by maintaining photosynthetic pigments and boosting antioxidant enzyme activity, which reduces oxidative damage (Royan et al., 2023). In the context of heavy metal tolerance, the AhPDF1.1 defensin from a Zn-tolerant species, when transformed into Arabidopsis, conferred increased Zn tolerance (Mirouze et al., 2006). Further findings highlight the multifaceted roles of defensins in heavy metal mitigation. Overexpression of AtPDF2.5 significantly enhanced tolerance and accumulation of Cd in both the host Arabidopsis and in rice through heterologous expression. This suggests that AtPDF2.5 promotes cytoplasmic Cd efflux via chelation, thereby enhancing detoxification and apoplastic accumulation (Luo et al., 2019). Similarly, AtPDF2.6 overexpression also enhanced Cd tolerance, while its knockout increased Cd sensitivity, emphasizing its role in detoxification via chelation and efflux (Luo et al., 2019). Beyond direct chelation, defensins can be regulated by stress-signaling pathways. The OsbZIP39 transcription factor, a key regulator of the unfolded protein response in the endoplasmic reticulum, positively regulates Cd accumulation in rice. Upon Cd exposure, OsbZIP39 is activated and enhances the expression of the defensin-like protein OsCAL2, which in turn facilitates Cd accumulation in the plant (Li et al., 2024). These findings show that defensin-based strategies can be leveraged to develop crops with resilience to various environmental stresses through distinct mechanisms, including antioxidant activity, metal chelation, and ion efflux.
2.3 Cyclotides
2.3.1 Family and structure
Cyclotides represent another fascinating class of AMPs with a unique, highly stable structure, which enables them to resist harsh environmental conditions. This is a salient group of plant-derived macrocyclic peptides, typically containing 28–37 aa and featuring an embedded cystine knot. These peptides have been identified in five major plant families, including Rubiaceae, Violaceae, Solanaceae, Cucurbitaceae, and Fabaceae (Slazak et al., 2020). Cyclotide-like genes have also been identified in members of the Poaceae family, including O. sativa, Zea mays, T. aestivum, Agrostis stolonifera, Schedonorus arundinaceus, Pennisetum glaucum, Sorghum bicolor, Hordeum vulgare, Saccharum officinarum, and Setaria italica. Cyclotides are distinguished by their unique structural characteristics, which include a head-to-tail cyclic peptide backbone and a cystine knot motif, where two disulfide bonds are interlinked by a third. This structural conformation confers exceptional stability, enabling cyclotides to remain functional under extreme temperatures, pH variations, and enzymatic degradation while maintaining solubility in both organic and aqueous environments (Veer et al., 2019).
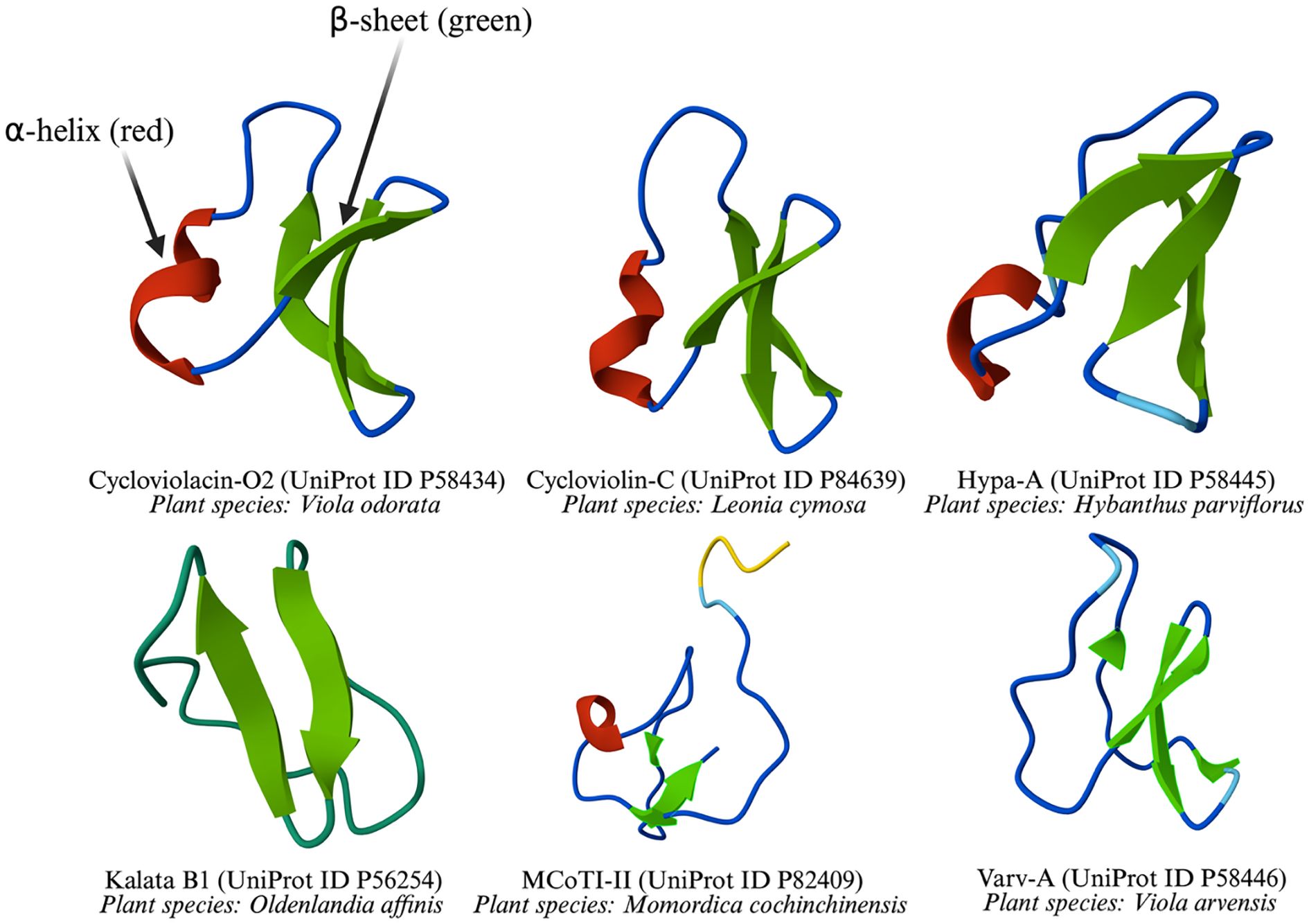
One of the most well-characterized cyclotides, kalata B1, was initially isolated from Oldenlandia affinis and has since been identified in several Viola species, including Viola tricolor, Viola yedoensis, Viola philippica, Viola baoshanensis, and Viola odorata. It was the first cyclotide to have its structure elucidated (Saether et al., 1995; Veer et al., 2019). The exceptional stability of cyclotides is attributed to their cyclic peptide backbone and cystine knot motif, which enable them to withstand extreme conditions such as high temperatures and enzymatic degradation within the human digestive system. The stability of kalata B1 has been linked to these structural features, which historically contributed to the use of O. affinis as a medicinal tea in Congolese tribes, where it was known as “kalata-kalata” in the Tsjiluba language and used to facilitate childbirth (Gran et al., 2000). Some cyclotides structures were predicted using AlphaFold3 (Abramson et al., 2024) and shown in Figure 4.
The figure illustrates the 3D-structures of six common cyclotides, which are characterized by one or more β-sheets, with and without α-helix. These representations highlight structural diversity across different plant species.
2.3.2 Mechanism of action
As with other AMP classes, cyclotides exert their bioactivity through multiple mechanisms, including membrane disruption and targeting of insect physiology. Cycloviolacin-O2 (CyO2) from V. odorata exhibits antimicrobial properties by disrupting cellular membranes and interfering with intracellular targets, with MICs ranging from 0.8 to 100 µM against fungal and bacterial pathogens such as F. oxysporum, Botrytis cinerea, and P. syringae (Oguis et al., 2019). Additionally, insecticidal cyclotides such as kalata B1 and kalata B2 target the physiological integrity of insect midgut cells. Kalata B1, for example, induces rupture of epithelial cells in the midgut of Helicoverpa armigera larvae, a mode of action similar to that of Bacillus thuringiensis δ-endotoxins (English and Slatin, 1992; Barbeta et al., 2008). Furthermore, the high metal-binding affinity of cyclotides suggests their potential involvement in detoxification processes, though the underlying mechanisms require further investigation (Zhang et al., 2015). The paper by Miszczak et al. (2025) tested whether differences in cyclotide production between metallicolous (M) and non-metallicolous (NM) populations under Zn and lead (Pb) stress and their functions in alleviating metal stress (Miszczak et al., 2025). In M population, the decrease in cyclotide production under heavy metal stress may be explained the reallocation of resources towards other tolerance mechanisms (e.g., metal sequestration or callose production), while in NM population, the increase in cyclotide production suggests an inducible defense response to mitigate oxidative stress or cellular damage caused by heavy metals. In these conditions, cyclotides may act as metal chelators or membrane stabilizers to protect metal-sensible plants (Miszczak et al., 2025). However, the paper did not provide direct evidence of cyclotide function, such as metal binding, detoxification, or cellular protection, leaving their mechanistic role unclear.
2.3.3 Roles of cyclotides in biotic stress
Cyclotides, like thionins and defensins, serve as potent natural defenses against biotic stressors. Kalata B1 and B2, derived from O. affinis, exhibited significant insecticidal activity. Kalata B1 effectively inhibited the growth of Helicoverpa punctigera and H. armigera larvae at a concentration of 0.13% (w/v), while reducing nutrient uptake at 0.24% (w/v) (Jennings et al., 2001; 2005). Kalata B2 demonstrated superior molluscicidal efficacy compared to metaldehyde in controlling the golden apple snail (Pomacea canaliculata), a major pest in rice cultivation, with an LC50 of 53 μM, whereas metaldehyde exhibited an LC50 of 133 μM (Plan et al., 2008). Unlike metaldehyde, which poses toxicity risks to non-target mammals, kalata B2 presents an environmentally safer alternative (Tan et al., 2022). Furthermore, the cyclotide Cter M, isolated from Clitoria ternatea, demonstrated efficacy against H. armigera, contributing to the development of Sero-X®, a bioinsecticide approved for commercial use in Australia in 2017 (Oguis et al., 2019). In terms of antimicrobial properties, cycloviolacin-O2 (CyO2) from V. odorata exhibits antifungal and antibacterial activity against pathogens such as Colletotrichum utrechtense, Alternaria alternata, and Dickeya dadantii (Oguis et al., 2019). Additionally, cyclotide-like genes in Z. mays (Zmcyc1 and Zmcyc5) were reported to contribute to plant defense against fungal pathogens such as F. graminearum and insect pests like Rhopalosiphum maydis, suggesting a broader protective function in plant immunity (Salehi et al., 2017).
2.3.4 Roles of cyclotides in abiotic stress
Emerging evidence suggests that cyclotides also contribute to abiotic stress tolerance, although their exact mechanisms remain underexplored. V. baoshanensis, a known Cd hyperaccumulator, accumulates Cd concentrations of 1168 mg kg−1 in shoots and 981 mg/kg in roots when grown in Pb-Zn mining areas (Liu et al., 2004). In response to Cd exposure, cyclotide precursor mRNAs VbCP7S and its spliced variant VbCP6S were upregulated in the leaves, indicating a potential role in metal binding and detoxification (Zhang et al., 2009). In Z. mays, cyclotide-like genes Zmcyc1 and Zmcyc5 are induced under abiotic stress conditions, including drought, salinity, and mechanical wounding. Their expression is also modulated by phytohormones such as SA and MeJA, suggesting involvement in stress response pathways linked to plant defense (Salehi et al., 2017). Additionally, studies have highlighted the strong metal-binding affinity of cyclotides, raising the possibility that they contribute to heavy metal detoxification, though further research is required to elucidate their precise function in abiotic stress adaptation (Zhang et al., 2015).
2.3.5 Applications of cyclotide genes in transgenic plant development
Cyclotides present promising applications in transgenic plant development, demonstrating a dual role in conferring resistance to both biotic and abiotic stresses. In the context of abiotic stress, cyclotides show potential for heavy metal detoxification. For example, five cyclotide precursor genes (VbCP1–VbCP5) from V. baoshanensis enhanced copper (Cu) tolerance when expressed in Saccharomyces cerevisiae (Zhang et al., 2009), demonstrating their metal-binding capabilities. This approach is similar to the use of a chicken-derived metallothionein gene, which significantly improved Cd sequestration in Chlamydomonas reinhardtii (Cai et al., 1999). This highlights that cyclotides, like other metal-binding proteins, can be leveraged to increase tolerance to heavy metal contamination. In parallel, cyclotides have been successfully engineered for biotic stress resistance. The kalata B1 (Oak1) gene from O. affinis was introduced into rice, resulting in transgenic plants that provided protection against a specific agricultural pest, the golden apple snail (Lim and Lai, 2017). These findings collectively underscore the exceptional versatility of cyclotides as valuable molecular tools for developing crops with improved resilience to both herbivorous pests and heavy metal contamination, using distinct mechanisms for each stress type. More recently, a study by Qu et al. (2020) successfully produced a structurally validated cyclotide in rice suspension cells in the presence of a supporting biosynthetic enzyme asparaginyl endopeptidase (AEP), which is derived from the O. affinis plant (Qu et al., 2020). This enzyme is crucial in performing the final, essential step in cyclotide biosynthesis—the head-to-tail cyclization of the linear precursor peptide (Tang and Luk 2021). Without it, the plant would only produce a linear peptide, not the highly stable, circular cyclotide. The study’s main finding was that co-expressing this enzyme with the cyclotide precursor gene was necessary to achieve the correct, functional structure of the kalata B1 cyclotide in the rice suspension cell (Qu et al., 2020). This research confirms that the biological machinery of a plant can be harnessed to correctly produce complex cyclotides, validating their potential for crop engineering.
2.4 Hevein-like antimicrobial peptides
2.4.1 Family and structure
Hevein-like AMPs are a family of structurally related peptides characterized by their similarity to hevein, a peptide originally identified in the latex of the rubber tree (Hevea brasiliensis) (Van Parijs et al., 1991). These peptides are cysteine- and glycine-rich, typically containing 6–8 cysteine residues that form disulfide bonds, contributing to their stable, compact structures. They are found across various plant families, including both monocots and dicots, indicating their widespread distribution in the plant kingdom (Slavokhotova et al., 2017). A key structural feature of hevein-like AMPs is the chitin-binding domain, which facilitates interaction with chitin and related oligosaccharides, a critical component of fungal cell walls and insect exoskeletons. For instance, the 40-amino-acid Pn-AMP2 and the 8C-hevein-like ginkgotide gB5 from Ginkgo biloba exemplify this structural motif, which underpins their biological activities (Koo et al., 1998; Wong et al., 2016).
2.4.2 Mechanisms of antimicrobial action
Hevein-like AMPs exhibit broad-spectrum antimicrobial activity through mechanisms primarily based on their chitin-binding capability. The chitin-binding domain allows these peptides to target chitin-containing structures in fungal cell walls, disrupting membrane integrity and inhibiting fungal growth. Pn-AMP2 demonstrated antifungal activity against both chitin-containing and non-chitin-containing fungi, suggesting additional mechanisms such as membrane permeabilization or interference with cellular processes (Koo et al., 1998). Similarly, the PMAPI protein from paper mulberry (Broussonetia papyrifera) inhibited Trichoderma viride with an IC50 of 0.1 μg/μL (Zhao et al., 2011). Additionally, some hevein-like AMPs, such as WAMP-1a from Triticum kiharae, exhibited antibacterial activity against Gram-positive and Gram-negative bacteria by disrupting bacterial cell membranes (Odintsova et al., 2009). These peptides also counteract fungal proteases, such as fungalysin from Fusarium verticillioides, which degrades plant chitinases, thereby preserving plant defense mechanisms (Slavokhotova et al., 2014).
2.4.3 Roles in biotic stress mitigation
Hevein-like AMPs play a critical role in plant defense against biotic stresses, including fungal, bacterial, and insect challenges. Their antifungal properties are well-documented, with peptides like WAMP1 and WAMP2 from Triticum kiharae showing upregulated expression in response to Fusarium oxysporum infection and inhibiting fungal growth (Andreev et al., 2012). Ginkgotide gB5 from Ginkgo biloba inhibited fungi such as A. niger (IC50 ~ 6.8 μg mL−1) and R. solani (IC50 ~ 20 μg mL−1) (Wong et al., 2016). Beyond fungi, WjAMP1 from wasabi (Wasabia japonica) suppressed bacterial growth, including E. coli (IC50 ~ 27.5 μg mL−1) and Pseudomonas cichorii (IC50 ~ 13.8 μg mL−1) (Kiba et al., 2003). Hevein-like AMPs also contributed to insect resistance, as seen with the MLX56 protein from mulberry, which exhibited antagonistic activity against herbivores like Spodoptera litura larvae and Henosepilachna vigintioctopunctata (Murata et al., 2021). Additionally, WAMP2-derived peptides enhanced the efficacy of commercial fungicides like Folicur® EC 250, achieving synergistic suppression of fungal spore germination (≥ 90%) (Odintsova et al., 2020).
2.4.4 Roles of hevein-like AMPs in abiotic stress alleviation
In addition to biotic stress, hevein-like AMPs contribute to abiotic stress responses, particularly salinity stress. The expression of wamp genes encoding WAMP1 and WAMP2 in T. kiharae is significantly induced by elevated NaCl levels (100–200 mM), suggesting a role in mitigating salinity stress (Andreev et al., 2012). Furthermore, transgenic asparagus plants expressing a hevein-like gene show enhanced resistance to stem wilt, accompanied by increased activities of antioxidant enzymes such as CAT, superoxide dismutase (SOD), and phenylalanine ammonia lyase (PAL), and reduced malondialdehyde levels, indicating a broader role in oxidative stress alleviation (Chen et al., 2019).
2.4.5 Applications of hevein-like AMPs genes in transgenic plant development
Hevein-like AMP genes have been extensively utilized in transgenic plant development to enhance resistance to a wide range of biotic stresses, particularly against fungal pathogens and herbivorous insects. As the primary mechanism of hevein-like AMPs is their ability to bind to chitin, a major component of fungal cell walls and insect skeletons, the core function of these peptides does not directly contribute to abiotic stress tolerance. Their application across different crop species demonstrates their broad potential. Several hevein-like genes have been successfully used to combat fungal diseases. The pnAMP-h2 gene from Pharbitis nil conferred improved resistance to Phytophthora parasitica in tobacco (Koo et al., 2002), while the amp1 and amp2 genes from T. kiharae significantly reduced lesion areas caused by Phytophthora infestans in tomatoes (Khaliluev et al., 2011). Similarly, the SmAMP2 gene from Stellaria media and a hevein-like gene in asparagus both enhanced resistance to fungal pathogens, including Phoma asponaqi and the agent of stem wilt, respectively (Odintsova et al., 2009; Chen et al., 2019). Beyond fungi, hevein-like AMPs also offer protection against insect pests. The MLX56 gene in transgenic tomatoes provided resistance to herbivorous insects, highlighting its potential as a biological alternative to traditional crop protection methods (Murata et al., 2021). These applications collectively underscore the critical role of hevein-like AMPs in developing resilient, disease-resistant crops by targeting distinct biotic threats.
From the structural stability and direct antimicrobial activity of hevein-like AMPs—which utilize disulfide bonds and chitin-binding domains for pathogen recognition—attention shifts to systemins, a class of peptide hormones that amplify plant defense responses through signaling cascades, notably via the JA pathway. This progression from direct antimicrobial effectors to systemic signaling molecules underscores the functional diversification of AMPs in plant immunity, contributing not only to resistance against biotic stressors but also to enhanced tolerance of abiotic challenges.
2.5 Systemins
2.5.1 Family and structure
Systemin (SYS) is an 18-amino-acid peptide hormone derived from a 200-amino-acid precursor, prosystemin (PS), in plants, notably within the Solanaceae family (Zhang et al., 2020). SYS is processed from PS and functions as a long-distance signaling molecule, capable of traveling 40 cm from the injection site to the plant apex at a rate of 2.5 cm h−1 in tomato plants (Mucha et al., 2019). Hydroxyproline-rich systemins (HypSYS) are related glycopeptides, first identified in Solanaceae species such as tobacco (N. tabacum) and tomato (Solanum lycopersicum) (Pearce et al., 1991). HypSYS peptides are small, defense-signaling molecules characterized by hydroxyproline modifications, which enhance their stability and activity. In petunia (Petunia hybrida), HypSYS peptides, termed PhHypSYS, exhibited distinct functional roles compared to their tobacco and tomato counterparts (Pearce et al., 2007).
2.5.2 Mechanisms of antimicrobial and defense action of systemin and prosystemin
SYS and HypSYS peptides activate plant defense responses primarily through the JA signaling pathway. In tomato, SYS enhances resistance to necrotrophic fungi like Plectosphaerella cucumerina by upregulating JA-biosynthesis gene LOX2 and JA-responsive genes such as JASMONATE ZIM-DOMAIN PROTEIN 10 (JAZ10), DEFENSIN1.2 (PDF1.2), and DEFENSIN1.3 (PDF1.3) (Zhang et al., 2018; Pastor-Fernández et al., 2022). SYS also interacts with membrane receptors BAK1 and BIK1, which are markers of pattern-triggered immunity (PTI), showing elevated expression only under pathogen challenge, suggesting a unique perception mechanism distinct from classical damage-associated molecular patterns (Lin et al., 2014). Additionally, SYS-treated plants exhibited increased hydrogen peroxide (H2O2) production following flagellin 22 (flg22) treatment, amplifying defense responses (Zhang et al., 2018). HypSYS peptides from tobacco and tomato, induced defense genes against herbivores, while PhHypSys in petunia upregulated defensin I, enhancing pathogen resistance (Pearce and Ryan, 2003; Pearce et al., 2007).
2.5.3 Roles in biotic stress mitigation
SYS and HypSYS peptides are pivotal in mitigating biotic stresses, including fungal, bacterial, and insect challenges. SYS enhanced resistance to necrotrophic fungi such as B. cinerea in Arabidopsis and tomato by activating JA-responsive defenses, reducing fungal colonization (Coppola et al., 2019). Exogenous SYS application to tomato plants restricts Spodoptera littoralis larval growth across generations and attracts natural enemies through volatile compound emission. Similarly, PS application increases S. littoralis mortality and suppresses B. cinerea colonization (Molisso et al., 2022). HypSYS peptides from tobacco and tomato induce anti-herbivore defenses, such as protease inhibitors, while PhHypSYS in petunia targets pathogen defenses via defensin I upregulation, demonstrating functional diversity within the HypSYS family (Pearce and Ryan, 2003; Pearce et al., 2007). SYS treatment increased the expression of pathogenesis-related (PR) gene PR1 and PR10, as well as the PTI market genes Pti5 and GRAS2. It also activated the MAPK signaling pathway, with peak MAPK observed at 3 hours after treatment. In addition, the H2O2 content in SYS-treated tomato fruits reached the highest level at 12 hours (Wang et al., 2024).
2.5.4 Roles of SYS and PS in abiotic stress alleviation
While the primary role of SYS and HypSys is in biotic stress response, their involvement in abiotic stress alleviation is less direct but notable. The activation of JA-signaling pathways by SYS and HypSys can indirectly enhance resilience to oxidative stress, as JA-responsive genes like glutathione S-transferase (GST) contribute to detoxification and attenuation of oxidative damage (Gullner et al., 2018). Additionally, the upregulation of heat shock proteins (HSPs) in SYS- or PS-treated plants under biotic stress conditions suggests a potential overlap with abiotic stress responses, as HSPs stabilize proteins under various stresses (Corrado et al., 2016; ul Haq et al., 2019). However, specific roles in abiotic stresses like salinity or drought require further investigation.
2.5.5 Applications of SYS and PS genes in transgenic plant development
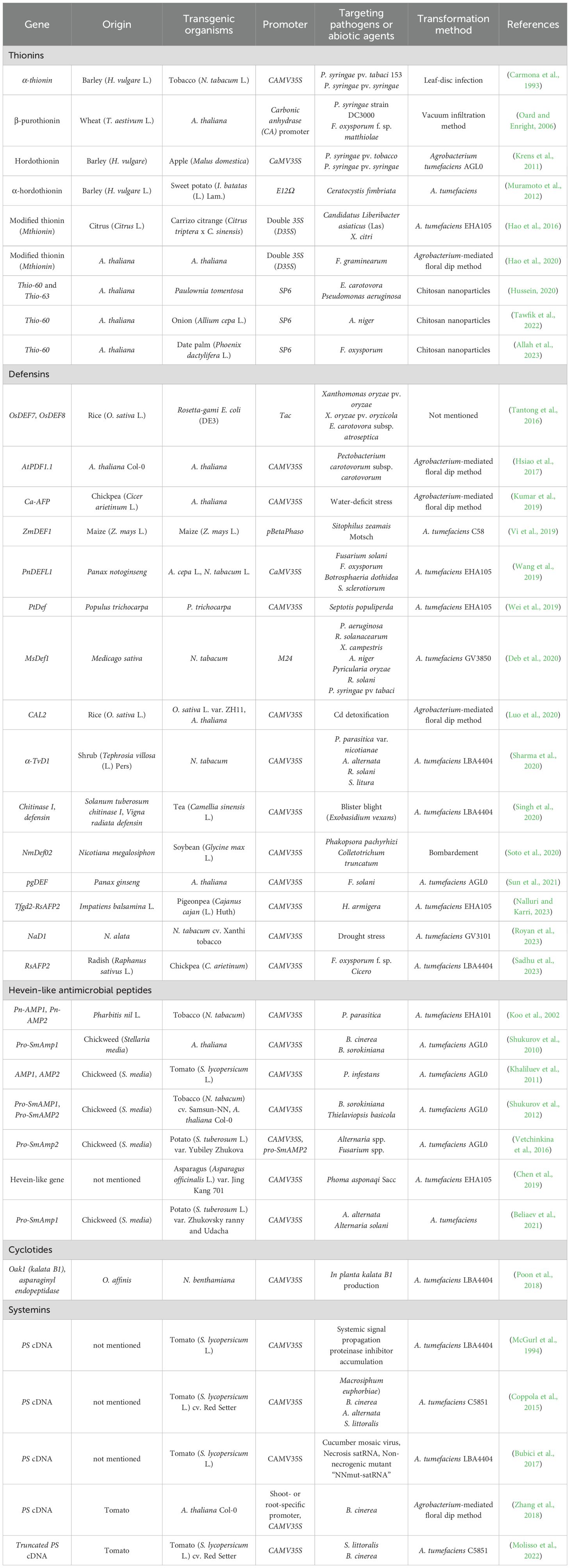
The genes encoding SYS and PS have been leveraged in transgenic plant development to enhance resistance to a range of biotic stresses, particularly against insects and fungal pathogens. Overexpression of the tomato PS gene in Arabidopsis conferred higher resistance to the necrotrophic fungus B. cinerea by modulating JA-responsive genes (Zhang et al., 2018). Further research on a truncated PS (tPS) cDNA, which lacks the SYS sequence, showed its remarkable effectiveness in two different host plants. In tomato, it resulted in reduced larval growth and higher mortality of the herbivorous insect S. littoralis and suppressed necrosis from B. cinerea (Molisso et al., 2022). A similar transformation in tobacco demonstrated that tPS enhances resistance to B. cinerea by upregulating a suite of defense genes, including heat shock proteins (HSP), glutathione S-transferases (GST), and proteinase inhibitor II (PI II) (Corrado et al., 2016). These findings show that both the full PS protein and its truncated version act as potent signaling molecules that fortify plant defenses through different pathways. Extending the theme of peptide-mediated signaling seen in SYSs, which amplify defense through JA pathways, cyclic dipeptides (CDPs) represent an even more compact and stable class of AMPs. These small molecules provide direct antimicrobial effects while also inducing resistance mechanisms, akin to those seen in hevein-like AMPs and SYSs. This dual functionality reinforces the interconnected roles of diverse AMPs in holistic plant stress management and transgenic innovation. Table 1 summarizes the application of SYS and PS genes, along with other AMP genes, in transgenic engineering strategies aimed at improving plant tolerance to both biotic and abiotic stresses.
2.6 Cyclic dipeptides in plant defense and stress mitigation
2.6.1 Family and structure
CDPs, also referred to as 2,5-diketopiperazines (DKPs), are the smallest cyclic peptides, characterized by a stable 2,5-DKP ring structure (Mishra et al., 2017). These naturally occurring compounds are found in bacteria, yeast, fungi, plants, and animals. Synthesized as secondary metabolites or protein metabolism byproducts, CDPs are produced via CDP synthases and non-ribosomal peptide synthetases (NRPSs), utilizing aminoacyl-tRNAs as substrates, with additional modifications by tailoring enzymes (Fizza et al., 2025). Approximately 100 CDP synthases have been identified, generating 75 of the 210 possible natural CDPs. The cyclization process, involving macrolactomization and macrolactamization, enhances their resistance to peptidase degradation, rendering CDPs highly stable and promising for applications in medicine, industry, and agriculture (Bechtler and Lamers, 2021).
2.6.2 Mechanisms of CDPs in biotic stress mitigation
CDPs enhance plant resistance to biotic stresses through direct pathogen inhibition, insect and nematode deterrence, and induction of systemic resistance. CDPs such as cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) from Lactobacillus plantarum MiLAB 393 inhibited fungi including Fusarium sporotrichioides, Aspergillus fumigatus, and Kluyveromyces marxianus (Ström et al., 2002). Similarly, Bacillus sp. strain N produced CDPs like cyclo(L-Pro-L-Leu), cyclo(D-Pro-L-Leu), and cyclo(D-Pro-L-Tyr), which suppressed Penicillium expansum (MIC ~ 4 µg mL−1), outperforming the fungicide Bavistin, and bacteria such as Bacillus subtilis and Escherichia coli (Kumar et al., 2012). Cyclo(L-Pro-L-Asp) exhibited potent antifungal activity against Aspergillus flavus (MIC ~ 16 µg mL−1) and Trichophyton rubrum (MIC ~ 2 µg mL−1), surpassing standard fungicides (Challa et al., 2014). Cyclo(L-Val-L-Pro) and cyclo(L-Phe-L-Pro) from Lactobacillus plantarum LBP-K10 inhibited Ganoderma boninense, a major oil palm pathogen (Kwak et al., 2014). Additionally, cyclo(L-Leu-L-Pro) from Achromobacter xylosoxidans suppressed aflatoxin production in Aspergillus species by targeting glutathione S-transferase (Yan et al., 2004).
CDPs also confer resistance to insects and nematodes (Sun et al., 2021; Sathya et al., 2016). Cyclo(L-Leu-L-Pro) from Bacillus gaemokensis PB69 enhanced cucumber resistance to Pseudomonas syringae pv. lachrymans and increased Spodoptera litura larval mortality (Song et al., 2017). Cyclo(L-Pro-L-Ile) from Bacillus thuringiensis JCK-1233 suppressed pine wilt disease caused by Bursaphelenchus xylophilus in pine seedlings by modulating defense gene expression and preventing hypersensitive reactions (Park et al., 2020). Furthermore, CDPs act as elicitors of induced systemic resistance (ISR). Cyclo(L-Pro-L-Pro) and cyclo(D-Pro-D-Pro) induced resistance in Nicotiana benthamiana against Phytophthora nicotianae and Tobacco mosaic virus via SA-mediated pathways, triggering stomatal closure, H2O2 production, and nitric oxide accumulation (Wu et al., 2017). Cyclo(L-Leu-L-Pro) from Bacillus vallismortis BS07 activated SA-dependent defenses in chili peppers, reducing disease severity from Pectobacterium carotovorum and Phytophthora capsici (Noh et al., 2017).
The stability, bioactivity, and simple chemical structures of CDPs make them promising for transgenic plant development. Genes encoding CDP synthases could be integrated into crop genomes to produce antifungal CDPs like cyclo(L-Pro-L-Leu), enhancing resistance to Fusarium or Aspergillus spp (Boecker et al., 2018). The heat stability and resistance to enzymatic degradation of CDPs ensure their efficacy in field conditions, supporting their use in engineering crops with enhanced biotic stress tolerance (Zhao et al., 2021). Additionally, CDPs’ role in inducing ISR via SA or JA pathways could be leveraged to develop transgenic plants with enhanced systemic immunity (Solis-Ortiz et al., 2022). In summary, CDPs mitigate biotic stresses in crops such as cucumber, chili pepper, pine, and oil palm through direct antimicrobial action, toxin suppression, and SA-mediated ISR, offering versatile mechanisms for enhancing plant immunity against diverse pathogens and pests.
2.6.3 Roles of antimicrobial peptides in abiotic stress alleviation
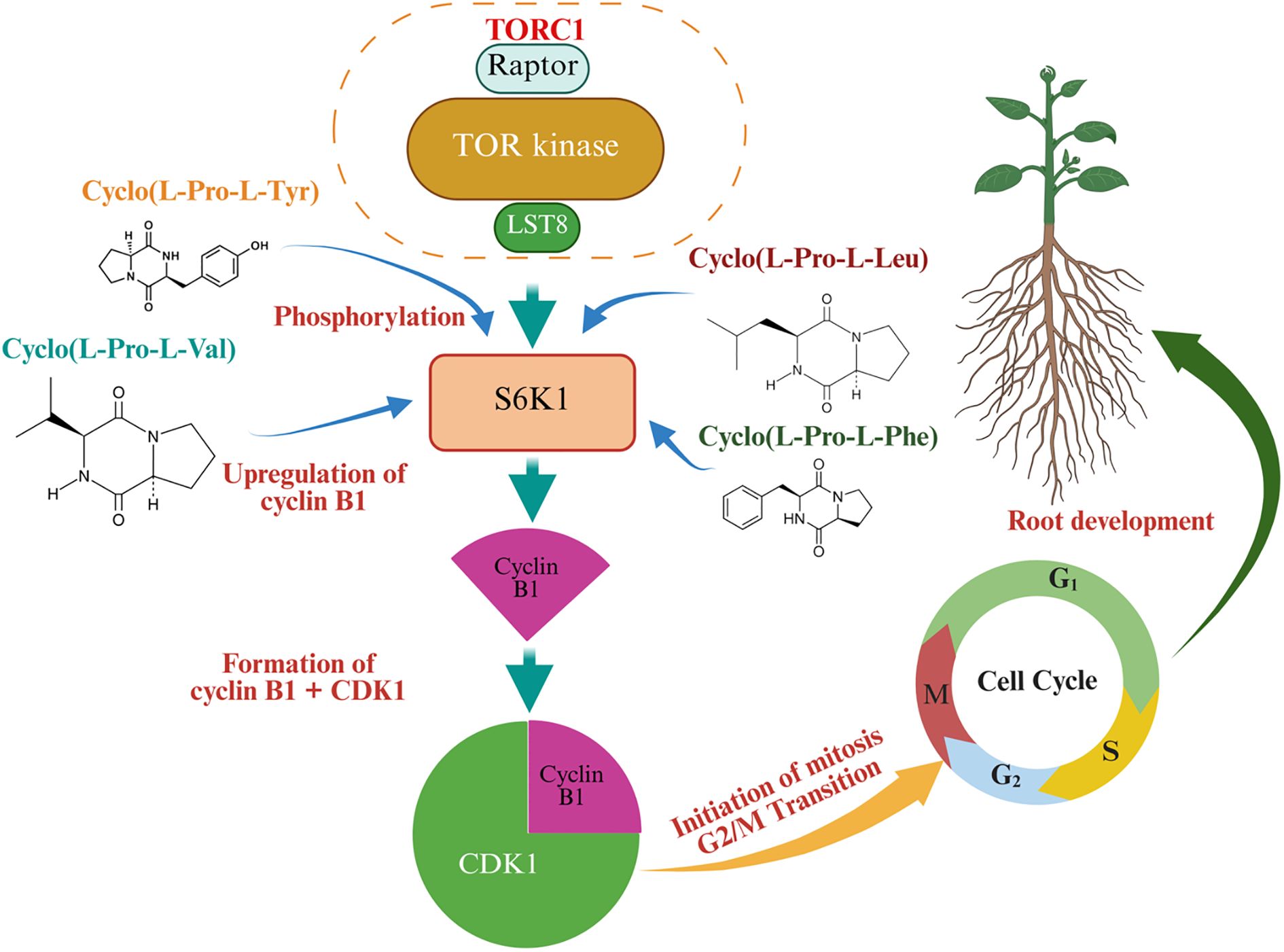
While research on CDPs’ roles in abiotic stress alleviation is limited, emerging evidence suggests their involvement. A mixture of CDPs (cyclo(L-Pro-L-Tyr), cyclo(L-Pro-L-Val), cyclo(L-Pro-L-Leu), cyclo(L-Pro-L-Phe)) produced by P. aeruginosa has been shown to enhance Arabidopsis growth and root system architecture (González-López et al., 2021). This is achieved by increasing the phosphorylation of the S6 ribosomal protein kinase (S6K), a downstream substrate of the target of rapamycin (TOR) kinase (Figure 5). The TOR pathway is a central hub regulating plant growth, cell proliferation, and metabolism, and its modulation by CDPs suggests a mechanism for improving stress resilience through post-translational modifications (Shi et al., 2018; Caldana et al., 2019). Furthermore, recent studies shed light on the intricate interplay between CDPs and metabolic pathways involved in abiotic stress tolerance. For example, the apparent controversy regarding the role of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in plant salinity stress tolerance—where some studies report that its upregulation enhanced tolerance (Qiu et al., 2025; Zhang et al., 2024), while others suggest its inhibition by cyclo(His-Pro) improved tolerance (Minen et al., 2025)—can be reconciled by considering the distinct roles, isoforms, and regulatory mechanisms of GAPDH in plant metabolism, as well as the context-specific effects of salinity stress. Upregulation of plastidial GAPDH (pGAPDH) supports photosynthesis and energy production, critical for maintaining growth and osmolyte synthesis under salinity (Li et al., 2019). Conversely, inhibition of cytosolic GAPDH (cGAPDH) by cyclo(His-Pro) redirects carbon flux from glycolysis to the pentose phosphate pathway (PPP), enhancing nicotinamide adenine dinucleotide phosphate (NADPH) production and antioxidant defense, which is vital for mitigating oxidative stress (Minen et al., 2025) (Figure 6). These mechanisms are not mutually exclusive but reflect a dynamic metabolic balance tailored to specific stress conditions, tissues, and plant species. For instance, under normal conditions, the microbial inoculant Priestia megaterium BP01R2, which likely produces CDPs, induced lateral root formation, created more root hairs, increased fresh weight, and promoted main root length (Hung et al., 2024). Under salinity stress, P. megaterium BP01R2 mitigated adverse effects by reducing chlorotic and curled leaves and minimizing the decline in root development. The expression pattern of P. megaterium BP01R2 -inoculated Arabidopsis plants under salt stress resembled that of control plants rather than NaCl-treated plants, suggesting a significant stress-mitigating effect. Under stress-free conditions, genes related to root cell differentiation, root morphology, cytoskeleton, and various enzyme activities (ATPase, helicase, ligase) were upregulated, aligning with enhanced plant growth. Under stress conditions, genes involved in cell death, hypersensitive response, JA biosynthesis and response, ET response, MAPK signaling, and glutathione metabolism were also upregulated, indicating a broader activation of stress response pathways (Hung et al., 2024). Intriguingly, the exogenous application of cyclo(L-Ala-Gly), which was identified in the metabolomic data of P. megaterium BP01R2, mirrored the performance of its host bacterium BP01R2 in alleviating salinity stress in Arabidopsis (Hung et al., 2024), suggesting potential applications of these small molecules as biostimulants in agriculture. These initial studies paved the way for a partial understanding of the underlying mechanisms behind CDPs’ properties in abiotic stress mitigation. However, further investigations integrating transcriptomic, proteomic, and metabolomic approaches are needed to fully elucidate the complex mechanisms underlying CDPs’ contributions to abiotic stress tolerance, such as drought, salinity, or temperature extremes.
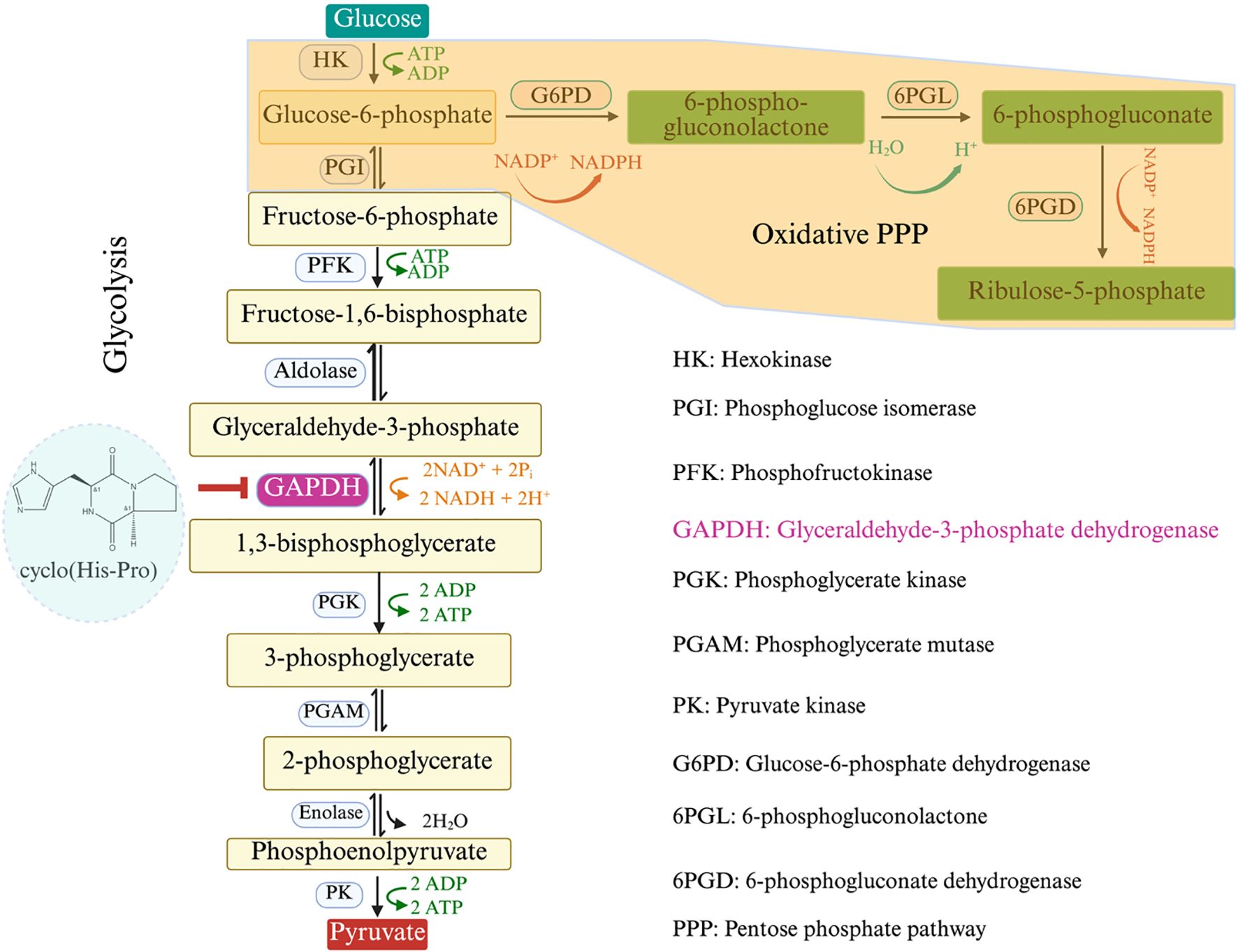
Figure 6. Metabolic pathways of glycolysis and the oxidative pentose phosphate pathway in plants. Figure 5 illustrates the metabolic pathways of glycolysis and the oxidative pentose phosphate pathway (PPP) in plants, highlighting the interconversion of key intermediates and the enzymes involved. The inhibition of key enzyme glyceraldehyde-3-phosphate dehydrogenase in the glycolysis (on the left) navigates the carbon flux towards the oxidative PPP (on the right), boosting NADPH production for redox balance. Figure 6 was conceptualized based on the mechanisms described in (Minen et al., 2025).
Figure 5 illustrates the molecular mechanisms involved in root development, with a focus on the Target of Rapamycin (TOR) signaling pathway. TOR kinase complex, consisting of TOR kinase, Raptor, and LST8, functions as the central regulator. The complex is induced by cyclic dipeptides [e.g., Cyclo(L-Pro-L-Tyr), Cyclo(L-Pro-L-Val), Cyclo(L-Pro-L-Leu), and Cyclo(L-Pro-L-Phe)], and subsequently facilitates the phosphorylation process to activate S6K1. S6K1 upregulates the expression of Cyclin B1, which leads to the formation of cyclin B1 and cyclin-dependent kinase 1 (CDK1). The Cyclin B1–CDK1 complex drives the transition from the G2 phase to the M phase (mitosis) in the cell cycle, thereby promoting root development in plants. Figure 5 was conceptualized based on the mechanisms described in (Caldana et al., 2019; González-López et al., 2021).
3 Future perspectives
From an agricultural perspective, AMPs have been extensively studied for their antifungal and antibacterial properties, with significant potential for enhancing crop resistance to biotic stresses. Recent studies also highlight salient roles of AMPs in abiotic stress responses. However, the mechanisms underlying AMPs’ involvement in abiotic stress responses remain underexplored, which necessitate comprehensive evidence on molecular features, signaling pathways, phytohormone interactions, and field-based assessments to validate their efficacy in crops. The potential of AMPs as biocontrol agents, either through direct application or transgenic approaches, is promising, given their stability and broad-spectrum activity. Nevertheless, utilizing AMPs as elicitors to induce abiotic stress tolerance requires further investigation to elucidate their modes of action and optimize their application. Bridging this knowledge gap is critical for developing resilient crop varieties to address rising environmental challenges. Additionally, the release of bioactive AMPs from genetically modified crops raises concerns for human health and ecosystems. The high stability of AMPs suggests persistence in soil and water, therefore, thorough assessments of their environmental fate, interactions with soil microbiota, and degradation dynamics, are highly recommended, to ensure safe deployment.
To advance the application of AMPs in agriculture and address current knowledge gaps, the following research directions and recommendations are proposed:
● Elucidate Molecular Mechanisms: Investigate the signaling pathways and molecular interactions of AMPs in abiotic stress responses, focusing on their crosstalk with phytohormones like JA, ABA, SA to uncover synergistic effects.
● Expand Field-Based Studies: Conduct greenhouse and in-field trials to evaluate AMP efficacy across diverse crop species under combined biotic (pathogens, insects) and abiotic (salinity, drought, heavy metals) stresses, ensuring translational relevance.
● Assess Environmental Impact: Perform comprehensive studies on the persistence, degradation, and ecological interactions of AMPs in soil and water, including their effects on non-target organisms and microbial communities, to ensure biosafety.
● Integrate Multi-Stress Resistance: Explore gene stacking strategies combining AMPs (e.g., SYS with SOS1, NaD1 with HSP90) to engineer crops with simultaneous resistance to biotic and abiotic stresses, balancing efficacy with yield stability.
These directions aim to harness AMPs potential for sustainable agriculture while addressing ecological and safety concerns, paving the way for resilient crop systems amidst global environmental challenges.
Author contributions
DH-T: Writing – original draft, Writing – review & editing, Visualization, Conceptualization, Software. C-CH: Supervision, Writing – original draft, Writing – review & editing, Data curation, Project administration, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research is funded by National Science and Technology Council of Taiwan under grant number NSTC 113-2321-B-005-011, and the Ministry of Education of Taiwan (the Higher Education Sprout Project).
Acknowledgments
We would like to express our gratitude to Dr. Tan Phong Nguyen, Faculty of Environment and Labour Safety, Ton Duc Thang University, Vietnam, and Dr. Nhuan Nghiem, Department of Environmental Engineering and Earth Sciences, Clemson University, Clemson, South Carolina, USA for helping us find the latest papers used in this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
aa, amino acid; ABA, abscisic acid; A. alternata, Alternaria alternata; AMPs, antimicrobial peptides; APX, Ascorbate peroxidase; A. thaliana, Arabidopsis thaliana; B. thuringiensis, Bacillus thuringiensis; B. cinerea, Botrytis cinerea; CAT, catalase; Cd, cadmium; CDP, cyclic dipeptide; DKP, 2,5-kiketopiperazines; E. carotovora, Erwinia carotovora; ET, Ethylene; ETI, effector-triggered immunity; F. oxysporum, Fusarium oxysporum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HypSYS, Hydroxyproline-rich systemins; ISR, induced systemic resistance; JA, Jasmonic acid; MAMP, microbe-associated molecular pattern; MAPK, mitogen-activated protein kinase; MIC, Minimum inhibitory concentration; NADPH, nicotinamide adenine dinucleotide; N. tabacum, Nicotiana tabacum; O. sativa, Oryza sativa; PAL, Phenylalanine ammonia lyase; P. infestans, Phytophthora infestans; PR, Pathogenesis-related; PS, prosystemin; P. syringae, Pseudomonas syringae; R. solanacearum, Ralstonia solanacearum; R. solani, Rhizoctonia solani; S. aureus, Staphylococcus aureus; S. lycopersicum, Solanum lycopersicum; S. littoralis, Spodoptera littoralis; S. litura, Spodoptera litura; S. tuberosum, Solanum tuberosum; SOD, Superoxide dismutase; S. sclerotiorum, Sclerotinia sclerotiorum; SYS, systemin; T. aestivum, Triticum aestivum; T. turgidum, Triticum turgidum; X. campestris, Xanthomonas campestris; Zn, zinc.
References
Abdallah, N. A., Shah, D., Abbas, D., and Madkour, M. (2010). Stable integration and expression of a plant defensin in tomato confers resistance to fusarium wilt. GM Crops 1, 344–505. doi: 10.4161/gmcr.1.5.15091
Abramson, J., Adler, J., Dunger, J., Evans, R., Green, T., Pritzel, A., et al. (2024). Accurate structure prediction of biomolecular interactions with alphaFold 3. Nature 630, 493–500. doi: 10.1038/s41586-024-07487-w
Aizaz, M., Ahmad, W., Asaf, S., Khan, I., Jan, S. S., Alamri, S. S., et al. (2023). Characterization of the seed biopriming, plant growth-promoting and salinity-ameliorating potential of halophilic fungi isolated from hypersaline habitats. Int. J. Mol. Sci. 24, 49045. doi: 10.3390/ijms24054904
Allah, K. W. A., Alabasey, E. E. D. G., Ahmed, K. Z., Hussien, E. T., and Razik, A. B. A. (2023). Phoenix dactylifera in vitro culture and transformation of thio-60 antifungal gene via chitosan nanoparticle. Plant Cell Tissue Organ Culture (PCTOC) 155, 603–612. doi: 10.1007/s11240-023-02505-7
Andreev, Y. A., Korostyleva, T. V., Slavokhotova, A. A., Rogozhin, E. A., Utkina, L. L., Vassilevski, A. A., et al. (2012). Genes encoding hevein-like defense peptides in wheat: distribution, evolution, and role in stress response. Biochimie 94, 1009–1165. doi: 10.1016/j.biochi.2011.12.023
Asano, T., Miwa, A., Maeda, K., Kimura, M., and Nishiuchi, T. (2013). The secreted antifungal protein thionin 2.4 in arabidopsis thaliana suppresses the toxicity of a fungal fruit body lectin from fusarium graminearum. Edited by brett tyler. PloS Pathog. 9, e10035815. doi: 10.1371/journal.ppat.1003581
Azmi, S. and Hussain, M. K. (2021). Analysis of structures, functions, and transgenicity of phytopeptides defensin and thionin: A review. Beni-Suef Univ. J. Basic Appl. Sci. 10, 55. doi: 10.1186/s43088-020-00093-5
Bakare, O. O., Gokul, A., Fadaka, A. O., Wu, R., Niekerk, L.-A., Barker, A. M., et al. (2022). Plant antimicrobial peptides (PAMPs): features, applications, production, expression, and challenges. Molecules 27, 3703. doi: 10.3390/molecules27123703
Barbeta, B. L., Marshall, A. T., Gillon, A. D., Craik, D. J., and Anderson, M. A. (2008). Plant cyclotides disrupt epithelial cells in the midgut of lepidopteran larvae. Proc. Natl. Acad. Sci. 105, 1221–1255. doi: 10.1073/pnas.0710338104
Bechtler, C. and Lamers, C. (2021). Macrocyclization strategies for cyclic peptides and peptidomimetics. RSC Medicinal Chem. 12, 1325–1515. doi: 10.1039/D1MD00083G
Beliaev, D. V., Yuorieva, N. O., Tereshonok, D. V., Tashlieva, I. I., Derevyagina, M. K., Meleshin, A. A., et al. (2021). High resistance of potato to early blight is achieved by expression of the pro-SmAMP1 gene for hevein-Like antimicrobial peptides from common chickweed (Stellaria media). Plants 10, 13955. doi: 10.3390/plants10071395
Boecker, S., Grätz, S., Kerwat, D., Adam, L., Schirmer, D., Richter, L., et al. (2018). Aspergillus Niger is a superior expression host for the production of bioactive fungal cyclodepsipeptides. Fungal Biol. Biotechnol. 5, 45. doi: 10.1186/s40694-018-0048-3
Bouqellah, N. A., Hussein, E. T., Abdel Razik, A. B., Ahmed, M. F., and Faraag, A. H. I. (2024). Development of transgenic Paulownia trees expressing antimicrobial thionin genes for enhanced resistance to fungal infections using chitosan nanoparticles. Microb. pathog. 191, 106659. doi: 10.1016/j.micpath.2024.106659
Bondt, A., Zaman, S., Broekaert, W., Cammue, B., and Keulemans, J. (1998). Genetic transformation of apple (Malus pumila mill.) for increased fungal resistance: in vitro antifungal activity in protein extracts of transgenic apple expressing rs-afp2 or ace-amp1. Acta Hortic. 484, 565–570. doi: 10.17660/ActaHortic.1998.484.96
Bubici, G., Carluccio, A. V., Stavolone, L., and Cillo, F. (2017). Prosystemin overexpression induces transcriptional modifications of defense-Related and receptor-like kinase genes and reduces the susceptibility to cucumber mosaic virus and its satellite RNAs in transgenic tomato plants. Edited by timothy P devarenne. PloS One 12, e01719025. doi: 10.1371/journal.pone.0171902
Bukhteeva, I., Hrunyk, N. I., Yusypovych, Y. M., Shalovylo, Y. I., Kovaleva, V., and Nesmelova, I. V. (2022). Structure, dynamics, and function of psDef2 defensin from pinus sylvestris. Structure 30, 753–7625.e5. doi: 10.1016/j.str.2022.03.001
Cai, X.-H., Brown, C., Adhiya, J., Traina, S. J., and Sayre, R. T. (1999). Growth and heavy metal binding properties of transgenic chlamydomonas expressing a foreign metallothionein gene. Int. J. Phytoremediation 1, 53–655. doi: 10.1080/15226519908500004
Caldana, C., Martins, M. C.M., Mubeen, U., and Urrea-Castellanos, R. (2019). The magic ‘Hammer’ of TOR: the multiple faces of a single pathway in the metabolic regulation of plant growth and development. J. Exp. Bot. 70, 2217–2255. doi: 10.1093/jxb/ery459
Campos, M. L., Liao, L. M., Alves, E. S., Migliolo, L., Dias, S. C., and Franco, O. L. (2018). A structural perspective of plant antimicrobial peptides. Biochem. J. 475, 3359–3375. doi: 10.1042/BCJ20180213
Carmona, M. J., Molina, A., Fernández, J. A., López-Fando, J. J., and García-Olmedo, F. (1993). Expression of the A-thionin gene from barley in tobacco confers enhanced resistance to bacterial pathogens. Plant J. 3, 457–625. doi: 10.1111/j.1365-313X.1993.tb00165.x
Cary, J. W., Rajasekaran, K., Jaynes, J. M., and Cleveland, T. E. (2000). Transgenic expression of a gene encoding a synthetic antimicrobial peptide results in inhibition of fungal growth in vitro and in planta. Plant Sci 154, 171–815. doi: 10.1016/S0168-9452(00)00189-8
Challa, C., Kumar, N., John, M., and Lankalapalli, R. S. (2014). A comparative study of antimicrobial properties of cyclo(L-pro- L-asp) with its 2-ketopiperazine analog. Medicinal Chem. Res. 23, 2377–2385. doi: 10.1007/s00044-013-0836-5
Chan, Y.-L., Prasad, V., Sanjaya, Chen, K. H., Liu, P. C., Chan, M.-T., et al. (2005). Transgenic tomato plants expressing an arabidopsis thionin (Thi2.1) driven by fruit-Inactive promoter battle against phytopathogenic attack. Planta 221, 386–935. doi: 10.1007/s00425-004-1459-3
Chen, H., Guo, A., Lu, Z., Tan, S., Wang, J., Gao, J., et al. (2019). Agrobacterium tumefaciens-mediated transformation of a hevein-like gene into asparagus leads to stem wilt resistance. Edited by jen-tsung chen. PloS One 14, e0223331. doi: 10.1371/journal.pone.0223331
Chen, L., Zhang, L., Xiang, S., Chen, Y., Zhang, H., and Yu, D. (2021). The transcription factor WRKY75 positively regulates jasmonate-Mediated plant defense to necrotrophic fungal pathogens. Edited by karl-Josef dietz. J. Exp. Bot. 72, 1473–1895. doi: 10.1093/jxb/eraa529
Contreras, G., Shirdel, I., Braun, M. S., and Wink, M. (2020). Defensins: transcriptional regulation and function beyond antimicrobial activity. Dev. Comp. Immunol. 104, 103556. doi: 10.1016/j.dci.2019.103556
Coppola, M., Corrado, G., Coppola, V., Cascone, P., Martinelli, R., Digilio, M. C., et al. (2015). Prosystemin overexpression in tomato enhances resistance to different biotic stresses by activating genes of multiple signaling pathways. Plant Mol. Biol. Rep. 33, 1270–1855. doi: 10.1007/s11105-014-0834-x
Coppola, L., Romanelli, Gualtieri, Molisso, Ruocco, Avitabile, et al. (2019). Tomato plants treated with systemin peptide show enhanced levels of direct and indirect defense associated with increased expression of defense-related genes. Plants 8, 395. doi: 10.3390/plants8100395
Corbineau, F., Taskiran-Özbingöl, N., and El-Maarouf-Bouteau, a. H. (2023). Improvement of seed quality by priming: concept and biological basis. Seeds 2, 101–155. doi: 10.3390/seeds2010008
Corrado, G., Arena, S., Araujo-Burgos, T., Coppola, M., Rocco, M., Scaloni, A., et al. (2016). The expression of the tomato prosystemin in tobacco induces alterations irrespective of its functional domain. Plant Cell Tissue Organ Culture (PCTOC) 125, 509–195. doi: 10.1007/s11240-016-0967-8
Deb, D., Shrestha, A., Sethi, L., Das, N. C., Rai, V., Das, A. B., et al. (2020). Transgenic tobacco expressing medicago sativa defensin (Msdef1) confers resistance to various phyto-Pathogens. Nucleus 63, 179–905. doi: 10.1007/s13237-020-00307-2
Deshmukh, R., Gupta, S. K., and Patil, G. B. (2025). Integrated omics approaches for biotic and abiotic stress tolerance in plants. J. Plant Biochem. Biotechnol. 34, 1–5.
English, L. and Slatin, S. L. (1992). Mode of action of delta-Endotoxins from bacillus thuringiensis: A comparison with other bacterial toxins. Insect Biochem. Mol. Biol. 22, 1–75. doi: 10.1016/0965-1748(92)90093-T
Epple, P., Apel, K., and Bohlmann, H. (1997). Overexpression of an endogenous thionin enhances resistance of arabidopsis against fusarium oxysporum. Plant Cell 9, 509–520. doi: 10.1105/tpc.9.4.509
Escudero-Martinez, C. M., Morris, J. A., Hedley, P. E., and Bos, J. I. B. (2017). Barley transcriptome analyses upon interaction with different aphid species identify thionins contributing to resistance. Plant Cell Environ. 40, 2628–2643. doi: 10.1111/pce.12979
Fernández, A., Colombo, M. L., Curto, L. M., Gómez, G. E., Delfino, J. M., Guzmán, F., et al. (2021). Peptides derived from the α-core and γ-core regions of a putative silybum marianum flower defensin show antifungal activity against fusarium graminearum. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.632008
Fizza, C., Israr, M., Khan, M. J., Sarwar, A., Aziz, T., Chun, C., et al. (2025). Cyclodipeptides (CDPs), the enzymatic synthesis, and the potential functional properties to exhibit broad varieties of pharmacological properties—A comprehensive review. Ital. J. Food Sci 37, 106–127. doi: 10.15586/ijfs.v37i1.2829
Franco, O. L., Murad, A. M., Leite, J. R., Mendes, P. A. M., Prates, M. V., and Bloch, C., Jr. (2006). Identification of a cowpea γ-thionin with bactericidal activity. FEBS J. 273, 3489–3975. doi: 10.1111/j.1742-4658.2006.05349.x
González-López, O., Palacios-Nava, B. B., Peña-Uribe, C. A., Campos-García, J., López-Bucio, J., García-Pineda, E., et al. (2021). Growth promotion in arabidopsis thaliana by bacterial cyclodipeptides involves the TOR/S6K pathway activation. J. Plant Physiol. 257, 153343. doi: 10.1016/j.jplph.2020.153343
González Ortega-Villaizán, A., King, E., Patel, M. K., and Pollmann, S. (2024). Plant hormone crosstalk under abiotic stress conditions. In: Lüttge U, Cánovas FM, Risueño Almeida MC, Leuschner C, Pretzsch H (eds) Progress in Botany, 85. Switzerland: Springer Nature. doi: 10.1007/124_2024_80
Gran, L., Sandberg, F., and Sletten, K. (2000). Oldenlandia affinis (R&S) DC: A plant containing uteroactive peptides used in african traditional medicine. J. Ethnopharmacology 70, 197–2035. doi: 10.1016/S0378-8741(99)00175-0
Gao, A. G., Hakimi, S. M., and Mittanck, C. A. (2000). Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat. Biotechnol. 18, 1307–1310. doi: 10.1038/82436
Gu, T. Y., Qi, Z. A., and Chen, S. Y. (2023). Dual-function DEFENSIN 8 mediates phloem cadmium unloading and accumulation in rice grains. Plant Physiol. 191, 515–527. doi: 10.1093/plphys/kiac423
Gullner, G., Komives, T., Király, L., and Schröder, P. (2018). Glutathione S-transferase enzymes in plant-pathogen interactions. Front. Plant Sci 9. doi: 10.3389/fpls.2018.01836
Hao, G., Bakker, M. G., and Kim, H.-S. (2020). Enhanced resistance to fusarium graminearum in transgenic arabidopsis plants expressing a modified plant thionin. Phytopathology® 110, 1056–1665. doi: 10.1094/PHYTO-12-19-0447-R
Hao, G., Stover, E., and Gupta, G. (2016). Overexpression of a modified plant thionin enhances disease resistance to citrus canker and huanglongbing (HLB). Front. Plant Sci 7. doi: 10.3389/fpls.2016.01078
Hao, G., Zhang, S., and Stover, E. (2017). Transgenic expression of antimicrobial peptide D2A21 confers resistance to diseases incited by pseudomonas syringae pv. Tabaci and xanthomonas citri, but not candidatus liberibacter asiaticus. PloS One 12, e01868105. doi: 10.1371/journal.pone.0186810
Hill, J., Becker, H. C., and Tigerstedt, P. M. A. (1998). “Breeding for biotic and abiotic stress,” in Quantitative and ecological aspects of plant breeding (Springer Netherlands, Dordrecht), 212–234. doi: 10.1007/978-94-011-5830-5_8
Hsiao, P.-Y., Cheng, C.-P., Koh, K. W., and Chan, M.-T. (2017). The Arabidopsis Defensin Gene, AtPDF1.1, Mediates Defence against Pectobacterium Carotovorum Subsp. Carotovorum via an Iron-Withholding Defence System. Sci. Rep. 7, 91755. doi: 10.1038/s41598-017-08497-7
Huang, C.-Y., Araujo, K., Sánchez, J. N., Kund, G., Trumble, J., Roper, C., et al. (2021). A stable antimicrobial peptide with dual functions of treating and preventing citrus huanglongbing. Proc. Natl. Acad. Sci. 118, e2019628118. doi: 10.1073/pnas.2019628118
Hung, S.-H. W., Yeh, P.-H., Huang, T.-C., Huang, S.-Y., Wu, I.-C., Liu, C.-H., et al. (2024). A cyclic dipeptide for salinity stress alleviation and the trophic flexibility of endophyte provide insights into saltmarsh plant–microbe interactions. ISME Commun. 4, ycae041. doi: 10.1093/ismeco/ycae041
Hussein, E. (2020). Production of transgenic paulownia tomentosa (Thunb.) steud. Using chitosan nanoparticles to express antimicrobial genes resistant to bacterial infection. Mol. Biol. Res. Commun. 9, 55–62. doi: 10.22099/mbrc.2019.35331.1454
Janssen, B. J.C., Schirra, H. J., Lay, F. T., Anderson, M. A., and Craik, D. J. (2003). Structure of petunia hybrida defensin 1, a novel plant defensin with five disulfide bonds. Biochemistry 42, 8214–8225. doi: 10.1021/bi034379o
Jennings, C. V., Rosengren, K.J., Daly, N. L., Plan, M., Stevens, J., Scanlon, M. J., et al. (2005). Isolation, solution structure, and insecticidal activity of kalata B2, a circular protein with a twist: do möbius strips exist in nature? Biochemistry 44, 851–605. doi: 10.1021/bi047837h
Jennings, C., West, J., Waine, C., Craik, D., and Anderson, M. (2001). Biosynthesis and insecticidal properties of plant cyclotides: the cyclic knotted proteins from oldenlandia affinis. Proc. Natl. Acad. Sci. 98, 10614–10195. doi: 10.1073/pnas.191366898
Ji, H., Gheysen, G., Ullah, C., Verbeek, R., Shang, C., Vleesschauwer, D. D., et al. (2015). The role of thionins in rice defence against root pathogens. Mol. Plant Pathol 16, 870–815. doi: 10.1111/mpp.12246
Jin, W., Wang, H., and Liu, Q. (2024). The defensin protein NtCAL1 functions as a positive factor in plant cadmium accumulation and resistance in tobacco. Environ. Exp. Bot., 225:105866. doi: 10.1016/j.envexpbot.2024.105866
Kaas, Q., Westermann, J. C., Henriques, S. T., and Craik, D. J. (2010). Antimicrobial peptides in plants. In: Wang G (ed) Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies. CABI. doi: 10.1079/9781845936570.0040
Kazachkova, Y. (2023). Seeds and heavy metal: defensin-like protein DEF8 mediates cadmium accumulation in rice and phloem unloading. Plant Physiol. 191, 12–14. doi: 10.1093/plphys/kiac475
Kaya, C. and Adamakis, I. D. S. (2025). Redox-epigenetic crosstalk in plant stress responses: the roles of reactive oxygen and nitrogen species in modulating chromatin dynamics. Int. J. Mol. Sci. 26, 7167. doi: 10.3390/ijms26157167
Kaur, J., Fellers, J., and Adholeya, A. (2017). Expression of apoplast-targeted plant defensin MtDef4.2 confers resistance to leaf rust pathogen Puccinia triticina but does not affect mycorrhizal symbiosis in transgenic wheat. Transgenic Res. 26, 37–49. doi: 10.1007/s11248-016-9978-9
Khaliluev, M. R., Mamonov, A. G., Smirnov, A. N., Kharchenko, P. N., and Dolgov, S. V. (2011). Expression of genes encoding chitin-binding proteins (PR-4) and hevein-like antimicrobial peptides in transgenic tomato plants enhanced resistanse to phytophthora infestance. Russian Agric. Sci. 37, 297–302. doi: 10.3103/S1068367411040082
Kalunke, R. M., Pokhrel, A., Tetorya, M., Godwin, J., Nath, V. S., Czymmek, K. J., et al (2025). Modes of Action and Bio-Fungicide Potential of Peptides Derived from the Medicago truncatula Bi-Domain Defensin MtDef5. J. Exp. Bot. 22, eraf166. doi: 10.1093/jxb/eraf166
Kiba, A., Saitoh, H., Nishihara, M., Omiya, K., and Yamamura, S. (2003). C-terminal domain of a hevein-like protein from wasabia japonica has potent antimicrobial activity. Plant Cell Physiol. 44, 296–3035. doi: 10.1093/pcp/pcg035
Koo, J. C., Lee, S. Y., Chun, H. J., Cheong, Y. H., Choi, J. S., Kawabata, S.-I., et al. (1998). Two hevein homologs isolated from the seed of pharbitis nil L. Exhibit potent antifungal activity. Biochim. Biophys. Acta 1382, 80–90. doi: 10.1016/s0167-4838(97)00148-9
Koo, J. C., Chun, H. J., and Park, H. C. (2002). Over-expression of a seed-specific hevein-like antimicrobial peptide from Pharbitis nil enhances resistance to a fungal pathogen in transgenic tobacco plants. Plant Mol. Biol. 50:441–452. doi: 10.1023/A:1019864222515
Kovaleva, V., Bukhteeva, I., Kit, O. Y., and Nesmelova, I. V. (2020). Plant defensins from a structural perspective. Int. J. Mol. Sci. 21, 5307. doi: 10.3390/ijms21155307
Krens, F. A., Schaart, J. G., Groenwold, R., Walraven, A. E., Hesselink, T., and Thissen, J. T. (2011). Performance and long-term stability of the barley hordothionin gene in multiple transgenic apple lines. Transgenic Res. 20, 11123–11133. doi: 10.1007/s11248-011-9484-z
Kulaeva, O., Kliukova, M., Afonin, A., Sulima, A., Zhukov, V., and Tikhonovich, I. (2020). The role of plant antimicrobial peptides (AMPs) in response to biotic and abiotic environmental factors. Biol. Commun. 65, 187–199. doi: 10.21638/spbu03.2020.205
Kumar, S. N., Mohandas, C., Siji, J. V., Rajasekharan, K. N., and Nambisan, B. (2012). Identification of antimicrobial compound, diketopiperazines, from a bacillus sp. N strain associated with a rhabditid entomopathogenic nematode against major plant pathogenic fungi. J. Appl. Microbiol. 113, 914–924. doi: 10.1111/j.1365-2672.2012.05385.x
Kumar, M., Yusuf, M. A., Yadav, P., Narayan, S., and Kumar, M. (2019). Overexpression of chickpea defensin gene confers tolerance to water-deficit stress in arabidopsis thaliana. Front. Plant Sci 10. doi: 10.3389/fpls.2019.00290
Künstler, A., Gullner, G., Ádám, A. L., Nagy, J. K. K., and Király, L. (2020). The versatile roles of sulfur-Containing biomolecules in plant defense—A road to disease resistance. Plants 9, 17055. doi: 10.3390/plants9121705
Kwak, M.-K., Liu, R., Kim, M.-K., Moon, D., Kim, A. H., Song, S.-H., et al. (2014). Cyclic dipeptides from lactic acid bacteria inhibit the proliferation of pathogenic fungi. J. Microbiol. 52, 64–705. doi: 10.1007/s12275-014-3520-7
Lay, F. T., Brugliera, F., and Anderson, M. A. (2003). Isolation and properties of floral defensins from ornamental tobacco and petunia. Plant Physiol. 131, 1283–1935. doi: 10.1104/pp.102.016626
Le, C.-F., Fang, C.-M., and Sekaran, S. D. (2017). Intracellular targeting mechanisms by antimicrobial peptides. Antimicrobial Agents and Chemotherapy, 61, e02340-16. doi: 10.1128/AAC.02340-16
Leannec-Rialland, V., Atanasova, V., Chereau, S., Tonk-Rügen, M., Cabezas-Cruz, A., and Richard-Forget, F. (2022). Use of defensins to develop eco-Friendly alternatives to synthetic fungicides to control phytopathogenic fungi and their mycotoxins. J. Fungi 8, 2295. doi: 10.3390/jof8030229
Lebedev, V. G., Dolgov, S. V., Lavrova, N., Lunin, V. G., and Naroditski, B. S. (2002). Plant-defensin genes introduction for improvement of pear phytopathogen resistance. Acta Hortic. 596, 167–172. doi: 10.17660/ActaHortic.2002.596.21
Leybourne, D. J., Valentine, T. A., Binnie, K., Taylor, A., Karley, A. J., and Bos, J. I.B. (2022). Drought stress increases the expression of barley defence genes with negative consequences for infesting cereal aphids. Edited by christine foyer. J. Exp. Bot. 73, 2238–2505. doi: 10.1093/jxb/erac010
Li, J., Hu, S., Jian, W., Xie, C., and Yang, X. (2021). Plant antimicrobial peptides: structures, functions, and applications. Botanical Stud. 62. doi: 10.1186/s40529-021-00312-x
Li, J., Wang, L.-Y., and Huang, H.-C. (2024). Endoplasmic reticulum stress response modulator OsbZIP39 regulates cadmium accumulation via activating the expression of defensin-like gene OsCAL2 in rice. J. Hazard. Mater. 476:135007. doi: 10.1016/j.jhazmat.2024.135007
Li, X., He, Z., and Li, Y. (2025). The Sedum plumbizincicola defensin gene, SpPDF, conferred cadmium accumulation via its chelation. Ecotoxicol. environ. saf. 294:118095. doi: 10.1016/j.ecoenv.2025.118095
Li, X., Wei, W., Li, F., Zhang, L., Deng, X., Liu, Y., et al. (2019). The plastidial glyceraldehyde-3-Phosphate dehydrogenase is critical for abiotic stress response in wheat. Int. J. Mol. Sci. 20, 11045. doi: 10.3390/ijms20051104
Lim, Y. Y. and Lai, K. S. (2017). Generation of transgenic rice expressing cyclotide precursor oldenlandia affinis kalata B1 protein. J. Anim. Plant Sci. 27, 667–671.
Lin, W., Li, B., Lu, D., Chen, S., Zhu, N., He, P., et al. (2014). Tyrosine phosphorylation of protein kinase complex BAK1/BIK1 mediates arabidopsis innate immunity. Proc. Natl. Acad. Sci. 111, 3632–3375. doi: 10.1073/pnas.1318817111
Liu, Y., Hua, Y. P., Chen, H., Zhou, T., Yue, C. P., Huang, J. Y., et al. (2021). Genome-scale identification of plant defensin PDF family genes and molecular characterization of their responses to diverse nutrient stresses in allotetraploid rapeseed. Peer J 9:e12007. doi: 10.7717/peerj.12007
Liu, X., Gong, X., Zhou, D., Jiang, Q., Liang, Y., Ye, R., et al. (2023). Plant defensin-dissimilar thionin osThi9 alleviates cadmium toxicity in rice plants and reduces cadmium accumulation in rice grains. J. Agric. Food Chem. 71, 8367–8380. doi: 10.1021/acs.jafc.3c01032
Liu, W., Shu, W., and Lan, C. (2004). Viola baoshanensis, a plant that hyperaccumulates cadmium. Chin. Sci Bull. 49, 29–325. doi: 10.1007/BF02901739
Loeza-Ángeles, H., Sagrero-Cisneros, E., Lara-Zárate, L., Villagómez-Gómez, E., López-Meza, J. E., and Ochoa-Zarzosa, A. (2008). Thionin thi2.1 from arabidopsis thaliana expressed in endothelial cells shows antibacterial, antifungal and cytotoxic activity. Biotechnol. Lett. 30, 1713–1195. doi: 10.1007/s10529-008-9756-8
Luo, J.-S., Huang, J., and Zeng, D.-L. (2018). A defensin-like protein drives cadmium efflux and allocation in rice. Nat. Commun., 9, 645. doi: 10.1038/s41467-018-03088-0
Luo, J.-S., Gu, T., Yang, Y., and Zhang, Z. (2019). A non-secreted plant defensin atPDF2.6 conferred cadmium tolerance via its chelation in arabidopsis. Plant Mol. Biol. 100, 561–569. doi: 10.1007/s11103-019-00878-y
Luo, J.-S., Xiao, Y., Yao, J., Wu, Z., Yang, Y., Ismail, A. M., et al. (2020). Overexpression of a defensin-like gene CAL2 enhances cadmium accumulation in plants. Front. Plant Sci 11. doi: 10.3389/fpls.2020.00217
Matsuzaki, K. (2019). Membrane permeabilization mechanisms. In: Matsuzaki K (ed) Antimicrobial Peptides. Adv. Exp. Med. Biol., 1117. Springer Singapore. doi: 10.1007/978-981-13-3588-4_2
Maximiano, M. R., Rios, T. B., Campos, M. L., Prado, G. S., Dias, S. C., and Franco, O. L. (2022). Nanoparticles in association with antimicrobial peptides (NanoAMPs) as a promising combination for agriculture development. Front. Mol. Biosci. 9. doi: 10.3389/fmolb.2022.890654
McGurl, B., Orozco-Cardenas, M., Pearce, G., and Ryan, C. A. (1994). Overexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase inhibitor synthesis. Proc. Natl. Acad. Sci. 91, 9799–9802. doi: 10.1073/pnas.91.21.9799
McLaughlin, J. E., Darwish, N. I., Garcia-Sanchez, J., Tyagi, N., Trick, H. N., McCormick, S., et al. (2021). A lipid transfer protein has antifungal and antioxidant activity and suppresses fusarium head blight disease and DON accumulation in transgenic wheat. Phytopathology 111, 671–835. doi: 10.1094/PHYTO-04-20-0153-R
Mee Do, H., Lee, S. C., Jung, H. W., Sohn, K. H., and Hwang, B. K. (2004). Differential expression and in situ localization of a pepper defensin (CADEF1) gene in response to pathogen infection, abiotic elicitors and environmental stresses in capsicum annuum. Plant Sci 166, 1297–13055. doi: 10.1016/j.plantsci.2004.01.008
Mehrabi, S., Åhman, I., and Jonsson, L. M.V. (2014). Transcript abundance of resistance- and susceptibility-Related genes in a barley breeding pedigree with partial resistance to the bird cherry-Oat aphid (Rhopalosiphum padi L.). Euphytica 198, 211–225. doi: 10.1007/s10681-014-1093-5
Minen, R. I., Camalle, M. D., Schwertfeger, T. J., Abdulhakim, F., Reish, H., Souza, L. P. D., et al. (2025). Characterization of the cyclic dipeptide cyclo(His-pro) in arabidopsis. Plant Physiol. 198, kiaf174. doi: 10.1093/plphys/kiaf174
Mirakhorli, N., Norolah, Z., Foruzandeh, S., Shafizade, F., Nikookhah, F., Saffar, B., et al. (2019). Multi-Function plant defensin, antimicrobial and heavy metal adsorbent peptide. Iranian J. Biotechnol. 17, 43–495. doi: 10.29252/ijb.1562
Mirouze, M., Sels, J., Richard, O., Czernic, P., Loubet, S., Jacquier, A., et al. (2006). A putative novel role for plant defensins: A defensin from the zinc hyper-accumulating plant, arabidopsis halleri, confers zinc tolerance. Plant J. 47, 329–342. doi: 10.1111/j.1365-313X.2006.02788.x
Mishra, A. K., Choi, J., Choi, S.-J., and Baek, K.-H. (2017). Cyclodipeptides: an overview of their biosynthesis and biological activity. Molecules 22, 1–13. doi: 10.3390/molecules22101796
Miszczak, R., Slazak, B., Sychta, K., Göransson, U., Nilsson, A., and Słomka, A. (2025). Interpopulational variation in cyclotide production in heavy-Metal-Treated pseudometallophyte (Viola tricolor L.). Plants 14, 4715. doi: 10.3390/plants14030471
Moghadam, S., Azari, B., Rashidi, R., Bafghi, M. H., Rakhshandeh, H., Selman, S. M., et al. (2023). Antifungal activity of three different varieties of capsicum annuum against clinical isolates of candida species. Trop. Diseases Travel Med. Vaccines 9, 95. doi: 10.1186/s40794-023-00194-w
Molisso, D., Coppola, M., Buonanno, M., Di Lelio, I., Aprile, A. M., Langella, E., et al. (2022). Not only systemin: prosystemin harbors other active regions able to protect tomato plants. Front. Plant Sci 13. doi: 10.3389/fpls.2022.887674
Molisso, D., Coppola, M., Buonanno, M., Lelio, I. D., Monti, S. M., Melchiorre, C., et al. (2022). Tomato prosystemin is much more than a simple systemin precursor. Biology 11, 124. doi: 10.3390/biology11010124
Mucha, P., Ruczynski, J., Dobkowski, M., Backtrog, E., and Rekowski, P. (2019). Capillary electrophoresis study of systemin peptides spreading in tomato plant. Electrophoresis 40, 336–342. doi: 10.1002/elps.201800206
Mulla, J. A. and Tamhane, V. A. (2023). Novel insights into plant defensin ingestion induced metabolic responses in the polyphagous insect pest helicoverpa armigera. Sci. Rep. 13, 31515. doi: 10.1038/s41598-023-29250-3
Muramoto, N., Tanaka, T., Shimamura, T., Mitsukawa, N., Hori, E., Koda, K., et al. (2012). Transgenic sweet potato expressing thionin from barley gives resistance to black rot disease caused by ceratocystis fimbriata in leaves and storage roots. Plant Cell Rep. 31, 987–975. doi: 10.1007/s00299-011-1217-5
Murata, M., Konno, K., Wasano, N., Mochizuki, A., and Mitsuhara, I. (2021). Expression of a gene for an MLX56 defense protein derived from mulberry latex confers strong resistance against a broad range of insect pests on transgenic tomato lines. Edited by yonggen lou. PloS One 16, e02399585. doi: 10.1371/journal.pone.0239958
Nalluri, N. and Karri, V. (2023). Over-Expression of trigonella foenum-Graecum defensin (Tfgd2) and raphanus sativus antifungal protein (RsAFP2) in transgenic pigeonpea confers resistance to the helicoverpa armigera. Plant Cell Tissue Organ Culture (PCTOC) 152, 569–825. doi: 10.1007/s11240-022-02431-0
Nawrot, R., Barylski, J., Nowicki, G., Broniarczyk, J., Buchwald, W., and Goździcka-Józefiak, A. (2014). Plant antimicrobial peptides. Folia Microbiologica 59, 181–965. doi: 10.1007/s12223-013-0280-4
Nitnavare, R. B., Pothana, A., Richa, K., Bhattacharya, J., Sapara, V., et al. (2023). Chickpea defensin gene family: promising candidates for resistance against soil-Borne chickpea fungal pathogens. J. Plant Growth Regul. 42, 6244–6605. doi: 10.1007/s00344-022-10811-1
Noh, S. W., Seo, R., Park, J. K., Manir, M. M., Park, K., Sang, M. K., et al. (2017). Cyclic dipeptides from bacillus vallismortis BS07 require key components of plant immunity to induce disease resistance in arabidopsis against pseudomonas infection. Plant Pathol J. 33, 402–495. doi: 10.5423/PPJ.OA.11.2016.0255
Oard, S. V. and Enright, F. M. (2006). Expression of the antimicrobial peptides in plants to control phytopathogenic bacteria and fungi. Plant Cell Rep. 25, 561–572. doi: 10.1007/s00299-005-0102-5
Oard, S., Rush, M. C., and Oard, J. H. (2004). Characterization of antimicrobial peptides against a US strain of the rice pathogen rhizoctonia solani. J. Appl. Microbiol. 97, 169–180. doi: 10.1111/j.1365-2672.2004.02291.x
Odintsova, T., Shcherbakova, L., Slezina, M., Pasechnik, T., Kartabaeva, B., Istomina, E., et al. (2020). Hevein-Like antimicrobial peptides wamps: structure–Function relationship in antifungal activity and sensitization of plant pathogenic fungi to tebuconazole by WAMP-2-Derived peptides. Int. J. Mol. Sci. 21, 79125. doi: 10.3390/ijms21217912
Odintsova, T. I., Slezina, M. P., and Istomina, E. A. (2018). Plant thionins: structure, biological functions and potential use in biotechnology. Vavilov J. Genet. Breed. 22, 667–675. doi: 10.18699/VJ18.409
Odintsova, T. I., Vassilevski, A. A., Slavokhotova, A. A., Musolyamov, A. K., Finkina, E. I., Khadeeva, N. V., et al. (2009). A novel antifungal hevein-type peptide from triticum kiharae seeds with a unique 10-cysteine motif. FEBS J. 276, 4266–4275. doi: 10.1111/j.1742-4658.2009.07135.x
Oguis, G. K., Gilding, E. K., Jackson, M. A., and Craik, D. J. (2019). Butterfly pea (Clitoria ternatea), a cyclotide-bearing plant with applications in agriculture and medicine. Front. Plant Sci 10. doi: 10.3389/fpls.2019.00645
Oyebamiji, Y. O., Adigun, B. A., and Shamsudin, N. A. A. (2024). Recent advancements in mitigating abiotic stresses in crops. Hortic. 10, 156. doi: 10.3390/horticulturae10020156
Pandolfi, C., Azzarello, E., Mancuso, S., and Shabala, S. (2016). Acclimation improves salt stress tolerance in zea mays plants. J. Plant Physiol. 201, 1–8. doi: 10.1016/j.jplph.2016.06.010
Parijs, J. V., Broekaert, W. F., Goldstein, I. J., and Peumans, W. J. (1991). Hevein: an antifungal protein from rubber-Tree (Hevea brasiliensis) latex. Planta 183, 258–645. doi: 10.1007/BF00197797
Parisi, K., Poon, S., Renda, R. F., Sahota, G., English, J., Yalpani, N., et al. (2020). Improving the digestibility of plant defensins to meet regulatory requirements for transgene products in crop protection. Front. Plant Sci 11. doi: 10.3389/fpls.2020.01227
Parisi, K., Shafee, T. M. A., Quimbar, P., Weerden, N. L.v. d., Bleackley, M. R., and Anderson, M. A. (2019). The evolution, function and mechanisms of action for plant defensins. Semin. Cell Dev. Biol. 88, 107–118. doi: 10.1016/j.semcdb.2018.02.004
Park, A. R., Jeong, S.-I., Jeon, H. W., Kim, J., Kim, N., Ha, M. T., et al. (2020). Diketopiperazine, cyclo-(L-pro-L-ile), derived from bacillus thuringiensis JCK-1233 controls pine wilt disease by elicitation of moderate hypersensitive reaction. Front. Plant Sci 11. doi: 10.3389/fpls.2020.01023
Park, H. C., Kang, Y. H., Chun, H. J., Koo, J. C., Cheong, Y. H., Kim, C. Y., et al. (2002). Characterization of a stamen-specific CDNA encoding a novel plant defensin in chinese cabbage. Plant Mol. Biol. 50, 57–68. doi: 10.1023/A:1016005231852
Pastor-Fernández, J., Sánchez-Bel, P., and Gamir, J. (2022). Tomato systemin induces resistance against Plectosphaerella cucumerina in Arabidopsis through the induction of phenolic compounds and priming of tryptophan derivatives. Plant Sci. 321:111321. doi: 10.1016/j.plantsci.2022.111321
Pearce, G. and Ryan, C. A. (2003). Systemic signaling in tomato plants for defense against herbivores. J. Biol. Chem. 278, 30044–30505. doi: 10.1074/jbc.M304159200
Pearce, G., Siems, W. F., Bhattacharya, R., Chen, Y.-C., and Ryan, C. A. (2007). Three hydroxyproline-rich glycopeptides derived from a single petunia polyprotein precursor activate defensin I, a pathogen defense response gene. J. Biol. Chem. 282, 17777–17784. doi: 10.1074/jbc.M701543200
Pearce, G., Strydom, D., Johnson, S., and Ryan, C. A. (1991). A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253, 895–897. doi: 10.1126/science.253.5022.895
Petitot, A.-S., Kyndt, T., Haidar, R., Dereeper, A., Collin, M., Engler, J. D. A., et al. (2017). Transcriptomic and histological responses of african rice (Oryza glaberrima) to meloidogyne graminicola provide new insights into root-Knot nematode resistance in monocots. Ann. Bot. 119, 885–995. doi: 10.1093/aob/mcw256
Plan, M. R. R., Saska, I., Cagauan, A. G., and Craik, D. J. (2008). Backbone cyclised peptides from plants show molluscicidal activity against the rice pest pomacea canaliculata (Golden apple snail). J. Agric. Food Chem. 56, 5237–5415. doi: 10.1021/jf800302f
Poon, S., Harris, K. S., Jackson, M. A., McCorkelle, O. C., Gilding, E. K., Durek, T., et al. (2018). Co-Expression of a cyclizing asparaginyl endopeptidase enables efficient production of cyclic peptides in planta. J. Exp. Bot. 69, 633–415. doi: 10.1093/jxb/erx422
Qiu, J., Chen, M., Cai, Z., Chen, X., Pang, Z., Chen, H., et al. (2025). Cytosolic glyceraldehyde-3-Phosphate dehydrogenase regulates plant stem cell maintenance under oxidative stress. Plant Cell Rep. 44, 1215. doi: 10.1007/s00299-025-03507-9
Qu, H., Jackson, M. A., Yap, K., Harvey, P. J., Gilding, E. K., Craik, D. J., et al. (2020). Production of a structurally validated cyclotide in rice suspension cells is enabled by a supporting biosynthetic enzyme. Planta 252, 97. doi: 10.1007/s00425-020-03505-z
Rawat, S., Ali, S., Chamil Nayankantha, N. N., Chandrashekar, N., Mittra, B., and Grover, A. (2017). Isolation and expression analysis of defensin gene and its promoter from brassica juncea. J. Plant Dis. Prot. 124, 591–6005. doi: 10.1007/s41348-017-0103-y
Royan, S., Shirzadian-Khorramabad, R., Zibaee, A., Bagherieh-Najjar, M. B., and Firouzabadi, F. N. (2023). Tobacco plants expressing the defensin naD1 enhance drought tolerance characteristics in transgenic lines. Plant Growth Regul. 101, 503–185. doi: 10.1007/s10725-023-01037-6
Sadhu, S., Jogam, P., Gande, K., Marapaka, V., Penna, S., and Peddaboina, V. (2023). Expression of radish defensin (RsAFP2) gene in chickpea (Cicer arietinum L.) confers resistance to fusarium wilt disease. Mol. Biol. Rep. 50, 11–185. doi: 10.1007/s11033-022-08021-9
Saether, O., Craik, D. J., Campbell, I. D., Sletten, K., Juul, J., and Norman, D. G. (1995). Elucidation of the primary and three-Dimensional structure of the uterotonic polypeptide kalata B1. Biochemistry 34, 4147–4585. doi: 10.1021/bi00013a002
Sagaram, U. S., El-Mounadi, K., Garry, W., Berg, H. R., Kaur, J., et al. (2013). Structural and functional studies of a phosphatidic acid-Binding antifungal plant defensin mtDef4: identification of an RGFRRR motif governing fungal cell entry. PloS One 8, e824855. doi: 10.1371/journal.pone.0082485
Salehi, H., Bahramnejad, B., and Majdi, M. (2017). Induction of two cyclotide-like genes zmcyc1 and zmcyc5 by abiotic and biotic stresses in zea mays. Acta Physiologiae Plantarum 39, 1315. doi: 10.1007/s11738-017-2425-6
Sathya, A., Vijayabharathi, R., Kumari, B. R., Srinivas, V., Sharma, H. C., Sathyadevi, P., et al. (2016). Assessment of a diketopiperazine, cyclo(Trp-phe) from streptomyces griseoplanus SAI-25 against cotton bollworm, helicoverpa armigera (Lepidoptera: noctuidae). Appl. Entomology Zoology 51, 11–22. doi: 10.1007/s13355-015-0366-3
Schmidt, M., Arendt, E. K., and Thery, T. L. C. (2019). Isolation and characterisation of the antifungal activity of the cowpea defensin cp-thionin II. Food Microbiol. 82, 504–514. doi: 10.1016/j.fm.2019.03.021
Sewelam, N., El-Shetehy, M., Mauch, F., and Maurino, V. G. (2021). Combined abiotic stresses repress defense and cell wall metabolic genes and render plants more susceptible to pathogen infection. Plants 10, 19465. doi: 10.3390/plants10091946
Shalovylo, Y. I., Yusypovych, Y. M., Hrunyk, N. I., Roman, I. I., Zaika, V. K., Krynytskyy, H. T., et al. (2021). Seed-derived defensins from scots pine: structural and functional features. Planta 254, 1295. doi: 10.1007/s00425-021-03788-w
Sharma, A., Sambasivam, V., Shukla, P., Rampuria, S., and Kirti, P. B. (2020). An in vitro generated variant of tephrosia villosa defensin (α-TvD1) enhances biotic stress tolerance in transgenic tobacco. J. Plant Pathol 102, 1133–1435. doi: 10.1007/s42161-020-00591-6
Shi, L., Wu, Y., and Sheen, J. (2018). TOR signaling in plants: conservation and innovation. Development 145, dev160887. doi: 10.1242/dev.160887
Shukurov, R. R., Voblikova, V. D., Nikonorova, A. K., Egorov, T. S. A., Grishin, E. V., and Babakov, A. V. (2010). Increase of resistance of arabidopsis thaliana plants to phytopathogenic fungi expressing hevein-like peptides from weed plant stellaria media. Russian Agric. Sci. 36, 265–675. doi: 10.3103/S1068367410040117
Shukurov, R. R., Voblikova, V. D., Nikonorova, A. K., Komakhin, R. A., Komakhina, V. V., Egorov, T. A., et al. (2012). Transformation of tobacco and arabidopsis plants with stellaria media genes encoding novel hevein-like peptides increases their resistance to fungal pathogens. Transgenic Res. 21, 313–255. doi: 10.1007/s11248-011-9534-6
Silva Rodrigues-Corrêa, K.C.D. and Fett-Neto, A. G. (2019). Abiotic stresses and non-protein amino acids in plants. Crit. Rev. Plant Sci. 38, 411–430. doi: 10.1080/07352689.2019.1707944
Simonsen, S. M., Sando, L., Ireland, D. C., Colgrave, M. L., Bharathi, R., Göransson, U., et al. (2005). A continent of plant defense peptide diversity: cyclotides in Australian hybanthus (Violaceae). Plant Cell 17, 3176–3895. doi: 10.1105/tpc.105.034678
Singh, H.R., Hazarika, P., Deka, M., and Das, S. (2020). Study of agrobacterium-Mediated co-Transformation of tea for blister blight disease resistance. J. Plant Biochem. Biotechnol. 29, 24–355. doi: 10.1007/s13562-019-00508-0
Slavokhotova, A. A., Naumann, T. A., Price, N. P., Rogozhin, E. A., Andreev, Y. A., Vassilevski, A. A., et al. (2014). Novel mode of action of plant defense peptides – hevein-like antimicrobial peptides from wheat inhibit fungal metalloproteases. FEBS J. 281, 4754–4764. doi: 10.1111/febs.13015
Slavokhotova, A. A., Shelenkov, A. A., Andreev, Y. A., and Odintsova, T. I. (2017). Hevein-like Antimicrobial Peptides of Plants. Biochem Mosc. 82 (13), 1659–1674.
Slazak, B., Haugmo, T., Badyra, B., and Goransson, U. (2020). The life cycle of cyclotides: biosynthesis and turnover in plant cells. Plant Cell Rep. 39, 1359–1367. doi: 10.1007/s00299-020-02569-1
Slezina, M. P., Istomina, E. A., Korostyleva, T. V., and Odintsova, T. I. (2023). The γ-core motif peptides of plant AMPs as novel antimicrobials for medicine and agriculture. Int. J. Mol. Sci. 24, 483. doi: 10.3390/ijms24010483
Slezina, M. P., Istomina, E. A., Kulakovskaya, E. V., Korostyleva, T. V., and Odintsova, T. I. (2022). The γ-core motif peptides of AMPs from grasses display inhibitory activity against human and plant pathogens. Int. J. Mol. Sci. 23, 8383. doi: 10.3390/ijms23158383
Solis-Ortiz, C. S., Gonzalez-Bernal, J., Kido-Díaz, H. A., Peña-Uribe, C. A., López-Bucio, J. S., López-Bucio, J., et al. (2022). Bacterial cyclodipeptides elicit arabidopsis thaliana immune responses reducing the pathogenic effects of pseudomonas aeruginosa PAO1 strains on plant development. J. Plant Physiol. 275, 153738. doi: 10.1016/j.jplph.2022.153738
Sonderegger, C., Váradi, G., Galgóczy, L., Kocsubé, S., Posch, W., Borics, A., et al. (2018). The evolutionary conserved γ-core motif influences the anti-candida activity of the penicillium chrysogenum antifungal protein PAF. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.01655
Song, G. C., Choi, H. K., Kim, Y. S., Choi, J. S., and Ryu, C. M. (2017). Seed defense biopriming with bacterial cyclodipeptides triggers immunity in cucumber and pepper. Sci. Rep. 7. doi: 10.1038/s41598-017-14155-9
Soto, N., Hernández, Y., Delgado, C., Rosabal, Y., Ortiz, R., Valencia, L., et al. (2020). Field resistance to phakopsora pachyrhizi and colletotrichum truncatum of transgenic soybean expressing the nmDef02 plant defensin gene. Front. Plant Sci 11. doi: 10.3389/fpls.2020.00562
Stec, B., Markman, O., Rao, U., Heffron, G., Henderson, S., Vernon, L. P., et al. (2004). Proposal for molecular mechanism of thionins deduced from physico-chemical studies of plant toxins. J. Pept. Res. 64, 210–224. doi: 10.1111/j.1399-3011.2004.00187.x
Stec, B. (2006). “Plant Thionins – the Structural Perspective.” Cell. Mol. Life Sci. 63, 1370–85. doi: 10.1007/s00018-005-5574-5
Ström, K., Sjögren, J., Broberg, A., and Schnürer, J. (2002). Lactobacillus plantarum miLAB 393 produces the antifungal cyclic dipeptides cyclo(l-phe-l-pro) and cyclo(l-phe-trans-4-OH-l-pro) and 3-phenyllactic acid. Appl. Environ. Microbiol. 68, 4322–4327. doi: 10.1128/AEM.68.9.4322-4327.2002
Su, Q., Wang, K., and Zhang, Z. (2020). Ecotopic expression of the antimicrobial peptide dmAMP1W improves resistance of transgenic wheat to two diseases: sharp eyespot and common root rot. Int. J. Mol. Sci. 21, 6475. doi: 10.3390/ijms21020647
Sun, X., Zhang, R., Ding, M., Liu, Y., and Li, L. (2021). Biocontrol of the root-knot nematode meloidogyne incognita by a nematicidal bacterium pseudomonas simiae MB751 with cyclic dipeptide. Pest Manage. Sci 77, 4365–4374. doi: 10.1002/ps.6470
Sun, T., Zhang, Y., Wang, Q., Jiang, Y., Li, H., Ma, R., et al. (2021). Overexpression of panax ginseng defensin enhances resistance to fusarium solani in transgenic arabidopsis thaliana. Australas. Plant Pathol 50, 705–714. doi: 10.1007/s13313-021-00821-0
Swathi Anuradha, T., Divya, K., Jami, S. K., and Kirti, P. B. (2008). Transgenic tobacco and peanut plants expressing a mustard defensin show resistance to fungal pathogens. Plant Cell Rep. 27, 1777–1786. doi: 10.1007/s00299-008-0596-8
Tan, Y.-J., Khoo, S. Q.-X., and Tan, S.-T. (2022). Acute metaldehyde poisoning from ingestion: clinical features and implications for early treatment. Acute Med. Surg 9, e7665. doi: 10.1002/ams2.766
Tang, R., Tan, H., and Dai, Y. (2023). Application of antimicrobial peptides in plant protection: making use of the overlooked merits. Front. Plant Sci. 14:1139539. doi: 10.3389/fpls.2023.1139539
Tang, T. M. S. and Luk, L. Y. P. (2021). Asparaginyl endopeptidases: enzymology, applications and limitations. Org. Biomol. Chem. 19, 5048–5062. doi: 10.1039/D1OB00608H
Tantong, S., Pringsulaka, O., Weerawanich, K., Meeprasert, A., Rungrotmongkol, T., Sarnthima, R., et al. (2016). Two novel antimicrobial defensins from rice identified by gene coexpression network analyses. Peptides 84, 7–16. doi: 10.1016/j.peptides.2016.07.005
Taveira, G. B., Carvalho, A. O., Rodrigues, R., Trindade, F. G., cunha, M. D., and Gomes, v. M. (2016). Thionin-like peptide from capsicum annuum fruits: mechanism of action and synergism with fluconazole against candida species. BMC Microbiol. 16, 125. doi: 10.1186/s12866-016-0626-6
Tawfik, E., Hammad, I., and Bakry, A. (2022). Production of transgenic allium cepa by nanoparticles to resist aspergillus Niger infection. Mol. Biol. Rep. 49, 1783–1905. doi: 10.1007/s11033-021-06988-5
Tesfaye, M., Silverstein, K. A. T., Nallu, S., Wang, L., Botanga, C. J., Gomez, S.K., et al. (2013). Spatio-temporal expression patterns of arabidopsis thaliana and medicago truncatula defensin-like genes. Edited by cynthia gibas. PloS One 8, e58992. doi: 10.1371/journal.pone.0058992
Tetorya, M., Li, H., Djami-Tchatchou, A. T., Buchko, G. W., Czymmek, K. J., and Shah, D. M. (2023). Plant defensin mtDef4-derived antifungal peptide with multiple modes of action and potential as a bio-inspired fungicide. Mol. Plant Pathol 24, 896–9135. doi: 10.1111/mpp.13336
ul Haq, S., Khan, A., Ali, M., Khattak, A. M., Gai, W.-X., Zhang, H.-X., et al. (2019). Heat shock proteins: dynamic biomolecules to counter plant biotic and abiotic stresses. Int. J. Mol. Sci. 20, 5321. doi: 10.3390/ijms20215321
Vaishnav, A., Shukla, A. K., Sharma, A., Kumar, R., and Choudhary, D. K. (2019). Endophytic bacteria in plant salt stress tolerance: current and future prospects. J. Plant Growth Regul. 38, 650–668. doi: 10.1007/s00344-018-9880-1
Veer, S. J. D., Kan, M.-W., and Craik, D. J. (2019). Cyclotides: from structure to function. Chem. Rev. 119, 12375–14215. doi: 10.1021/acs.chemrev.9b00402
Vetchinkina, E. M., Komakhina, V. V., Vysotskii, D. A., Zaitsev, D. V., Smirnov, A. N., Babakov, A. V., et al. (2016). Expression of plant antimicrobial peptide pro-smAMP2 gene increases resistance of transgenic potato plants to alternaria and fusarium pathogens. Russian J. Genet. 52, 939–951. doi: 10.1134/S1022795416080147
Vi, T. X. T., Nguyen, T. N. L., Pham, T. T. N., Nguyen, H. Q., Nguyen, T. H. Y., Tu, Q. T., et al. (2019). Overexpression of the zmDEF1 gene increases the resistance to weevil larvae in transgenic maize seeds. Mol. Biol. Rep. 46, 2177–2855. doi: 10.1007/s11033-019-04670-5
Wang, Y., Nowak, G., Culley, D., Hadwiger, L. A., and Fristensky, B. (1999). Constitutive expression of pea defense gene DRR206 confers resistance to blackleg (Leptosphaeria maculans) disease in transgenic canola (Brassica napus). Mol. Plant-Microbe Interactions® 12, 410–185. doi: 10.1094/MPMI.1999.12.5.410
Wang, Q., Qiu, B. L., Li, S., Zhang, Y. P., Cui, X. M., Ge, F., et al. (2019). A Methyl Jasmonate Induced Defensin like Protein from Panax Notoginseng Confers Resistance against Fusarium Solani in Transgenic Tobacco. Biol. Plantarum 63, 797–807. doi: 10.32615/bp.2019.123
Wang, P., Wu, T., Cheng, Y., Gao, Y., Huang, B., Li, Z., et al. (2024). The phytocytokine systemin enhances postharvest tomato fruit resistance to Botrytis cinerea. Postharvest Biol. Technol. 210:112738. doi: 10.1016/j.postharvbio.2023.112738
Weerawanich, K., Webster, G., Ma, J. K.-C., Phoolcharoen, W., and Sirikantaramas, S. (2018). Gene expression analysis, subcellular localization, and in planta antimicrobial activity of rice (Oryza sativa L.) defensin 7 and 8. Plant Physiol. Biochem. 124, 160–166. doi: 10.1016/j.plaphy.2018.01.011
Wei, H., Movahedi, A., Xu, C., Sun, W., Li, L., Li, D., et al. (2019). Characterization, expression profiling, and functional analysis of a populus trichocarpa defensin gene and its potential as an anti-Agrobacterium rooting medium additive. Sci. Rep. 9, 153595. doi: 10.1038/s41598-019-51762-0
Wong, K., Tan, W. L., Serra, A., Xiao, T., Sze, S. K., Yang, D., et al. (2016). Ginkgotides: proline-rich hevein-like peptides from gymnosperm ginkgo biloba. Front. Plant Sci 7. doi: 10.3389/fpls.2016.01639
Wu, L., Wu, H., Chen, L., Zhang, H., and Gao, X. (2017). Induction of systemic disease resistance in nicotiana benthamiana by the cyclodipeptides cyclo (L-pro-l-pro) and cyclo (D-pro-d-pro). Mol. Plant Pathol 18, 67–74. doi: 10.1111/mpp.12381
Wu, Z., Liu, D., Yue, N., Song, H., Luo, J., Zhang, Z., et al. (2021). PDF1.5 enhances adaptation to low nitrogen levels and cadmium stress. Int. J. Mol. Sci. 22, 10455. doi: 10.3390/ijms221910455
Yan, M., Chai, M., An, C., Jiang, X., Yang, F., Fang, X., et al. (2025). Genome-wide identification and expression analysis of thionin family in rice (Oryza sativa) and functional characterization of osTHION15 in drought stress and ABA stress. Int. J. Mol. Sci. 26, 3447. doi: 10.3390/ijms26073447
Yan, P.-S., Song, Y., Sakuno, E., Nakajima, H., Nakagawa, H., and Yabe, K. (2004). Cyclo(L-leucyl-L-prolyl) produced by achromobacter xylosoxidans inhibits aflatoxin production by aspergillus parasiticus. Appl. Environ. Microbiol. 70, 7466–7473. doi: 10.1128/AEM.70.12.7466–7473.2004
Zhang, J., Li, J., Huang, Z., Yang, B., Zhang, X., Li, D., et al. (2015). Transcriptomic screening for cyclotides and other cysteine-rich proteins in the metallophyte viola baoshanensis. J. Plant Physiol. 178, 17–26. doi: 10.1016/j.jplph.2015.01.017
Zhang, J., Liao, B., Craik, D. J., Li, J.-T., Hu, M., and Shu, W.-S. (2009). Identification of two suites of cyclotide precursor genes from metallophyte viola baoshanensis: CDNA sequence variation, alternative RNA splicing and potential cyclotide diversity. Gene 431, 23–32. doi: 10.1016/j.gene.2008.11.005
Zhang, M., Liu, M., Pan, S., Pan, C., Li, Y., and Tian, J. (2018). Perillaldehyde controls postharvest black rot caused by ceratocystis fimbriata in sweet potatoes. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.01102
Zhang, H., Yu, P., Zhao, J., Jiang, H., Wang, H., Zhu, Y., et al. (2018). Expression of tomato prosystemin gene in arabidopsis reveals systemic translocation of its MRNA and confers necrotrophic fungal resistance. New Phytol. 217, 799–812. doi: 10.1111/nph.14858
Zhang, H., Zhang, H., and Lin, J. (2020). Systemin-mediated long-distance systemic defense responses. New Phytol. 226, 1573–1825. doi: 10.1111/nph.16495
Zhang, X., Zhang, J., Tuluhong, G., and Zhang, F. (2024). A nuclear-located glyceraldehyde-3-phosphate dehydrogenase affects salt stress response processes in arabidopsis thaliana as a senescence component. J. Plant Biochem. Biotechnol. 33, 24–335. doi: 10.1007/s13562-022-00815-z
Zhao, M., Ma, Y., Pan, Y.-H., Zhang, C.-H., and Yuan, W.-X. (2011). A hevein-like protein and a class I chitinase with antifungal activity from leaves of the paper mulberry. Biomed. Chromatogr. 25, 908–125. doi: 10.1002/bmc.1543
Keywords: antimicrobial peptides, biotic and abiotic stresses, salinity, drought, pathogenic fungi, plant diseases, insect pests, transgenic plants
Citation: Ha-Tran DM and Huang C-C (2025) Peptides from plants and microorganisms: a defense strategy for biotic and abiotic stresses. Front. Plant Sci. 16:1649512. doi: 10.3389/fpls.2025.1649512
Received: 18 June 2025; Accepted: 29 September 2025;
Published: 21 October 2025.
Edited by:
Ana Elisa Rato, University of Evora, PortugalReviewed by:
James Godwin, Donald Danforth Plant Science Center, United StatesNoor Alam Chowdhary, Banaras Hindu University, India
Copyright © 2025 Ha-Tran and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chieh-Chen Huang, Y2NodWFuZ0BkcmFnb24ubmNodS5lZHUudHc=
 Dung Minh Ha-Tran
Dung Minh Ha-Tran Chieh-Chen Huang3,4,5*
Chieh-Chen Huang3,4,5*