- 1Engineering Technology Institute of Maize Breeding in Anhui Province, Chuzhou, China
- 2College of Agriculture, Anhui Science and Technology University, Chuzhou, China
- 3College of Resources and Environment, Anhui Science and Technology University, Chuzhou, China
- 4Anhui Province International Joint Research Center of Forage Bio-breeding, Chuzhou, China
Introduction: Phenylalanine ammonia-lyase (PAL), as the rate-limiting enzyme in plant phenylpropanoid metabolism, catalyzes the conversion of L-phenylalanine to trans-cinnamic acid and plays a pivotal role in plant-insect resistance mechanisms.
Methods: Utilizing a maize pangenome constructed from 26 high-quality genomes, we systematically identified the ZmPAL gene family members. Evolutionary pressure and structural variation (SV) analyses were conducted, alongside reanalysis of publicly available RNA-seq datasets under lepidopteran stress conditions. Temporal expression patterns were further validated via qRT-PCR.
Results: This investigation identified 29 ZmPAL genes, comprising 7 core, 2 near-core, 12 dispensable, and 8 private genes, revealing substantial limitations of single-reference genome-based studies. Evolutionary analysis indicated positive selection of ZmPAL8 in specific germplasms, while SV-affected ZmPAL5 exhibited significantly divergent expression patterns. Conserved expression profiles were observed among ZmPAL members under diverse lepidopteran stresses. Temporal-specific regulation was established: ZmPAL7, ZmPAL10, and ZmPAL23 dominated early defense responses, whereas ZmPAL10 and ZmPAL23 maintained predominance during mid-late phases.
Discussion: This pangenome-based study provides novel insights into PAL-mediated phytoprotective mechanisms against lepidopteran pests and establishes a theoretical framework for understanding maize's molecular adaptation to biotic stressors.
1 Introduction
Plant metabolic systems are categorized into primary and secondary pathways, with the phenylpropanoid pathway constituting one of three principal secondary metabolic routes (Crozier et al., 2006; Kumar et al., 2023; Salam et al., 2023; Han et al., 2025). Initiated by PAL, this pathway catalyzes the first enzymatic conversion of phenylpropanoid compounds to generate intermediates including coumaric acid, ferulic acid, and sinapic acid (MacDonald and D’Cunha, 2007; Levy et al., 2018; Zheng et al., 2024). Through sequential enzymatic conversions, these precursors form coumarins, chlorogenic acids, and phenylpropanoyl-CoA esters, ultimately yielding diverse phenylpropanoids such as flavonoids, lignin, cinnamates, and alkaloids (Rhodes and Wooltorton, 1976; Lavhale et al., 2018; Zhou et al., 2025). The phenylpropanoid pathway produces abundant phenolic derivatives that serve as precursors for phytohormones, anthocyanins, phytoalexins, and structural polymers (Dong and Lin, 2021). Particularly, phenylpropanoid derivatives play critical functions in plant defense mechanisms against phytophagous insects and microbial pathogens through their roles in physical barrier formation and antimicrobial compound biosynthesis (Dixon et al., 2002; Dong and Lin, 2021).
Phenylalanine ammonia-lyase (PAL; EC 4.3.1.5), functioning as the pivotal rate-limiting enzyme in phenylpropanoid metabolism (Vogt, 2010), catalyzes the deamination of L-phenylalanine to trans-cinnamic acid, thereby initiating biosynthetic cascades that yield critical secondary metabolites including flavonoids, anthocyanins, and lignin (Liu et al., 2023b; Qiu et al., 2024). This enzyme exhibits ubiquitous presence across photosynthetic organisms, being phylogenetically conserved in plants, fungi, yeasts, and algae, though notably absent in metazoans (Emiliani et al., 2009; Kawatra et al., 2020). Molecular characterization of PAL isoforms has been documented in numerous taxonomically diverse species, including but not limited to Arabidopsis thaliana (Kawatra et al., 2020), Brassica napus (Zhang et al., 2023), Populus tremula (Subramaniam et al., 1993), Sorghum bicolor (Pant and Huang, 2022), Musa acuminata (Wuyts et al., 2006), Solenostemon scutellarioides (Zhu et al., 2015), Oryza sativa (Yu et al., 2018), Triticum aestivum (Feduraev et al., 2020), and Juglans regia (Xu et al., 2012; Yan et al., 2019), demonstrating its evolutionary significance in secondary metabolic processes.
The PAL gene family members function as key regulators in plant developmental processes (Elkind et al., 1990; Ramzan et al., 2023). In Arabidopsis thaliana, AtPAL1, AtPAL2, and AtPAL4 demonstrate tissue-specific expression patterns, with preferential accumulation in stems and seeds (Cochrane et al., 2004). Similarly, 11 out of 12 identified ClPAL genes in watermelon (Citrullus lanatus) exhibit substantial transcriptional activity in stems and floral organs (Dong and Shang, 2013). Beyond developmental regulation, PAL isoforms mediate plant responses to diverse biotic and abiotic stresses (Wang et al., 2025). For instance, AtPAL1 and AtPAL2 upregulation under nitrogen deficiency and thermal fluctuations drives flavonoid accumulation in Arabidopsis (Ni et al., 2008c). Maize cultivars exposed to heat stress show elevated ZmPAL2 and ZmPAL11 expression, correlating with enhanced PAL enzymatic activity and concurrent increases in total phenolics/flavonoids (Wu et al., 2020). Conversely, AevPAL1 overexpression in bread wheat (Aegilops variabilis) confers resistance against cereal cyst nematode (Heterodera avenae) (Zhang et al., 2021), while Puccinia striiformis infection differentially modulates 25 wheat PAL homologs (11 upregulated, 14 downregulated) (Sørensen et al., 2016). Functional studies in rice reveal that OsPAL6 and OsPAL8 overexpression elevates lignin and salicylic acid biosynthesis, enhancing resistance to brown planthopper (Nilaparvata lugens) (He et al., 2019; Yang et al., 2024). Notably, PAL induction exhibits systemic signaling properties - both herbivore-damaged and adjacent undamaged cotton (Gossypium hirsutum) and maize seedlings display synchronized PAL activation, suggesting interspecies communication through phenylpropanoid-mediated defense priming (Lv et al., 2016; Hu et al., 2022).
Maize (Zea mays L.), a cornerstone crop in global food security, plays pivotal roles in human nutrition, livestock feed production, industrial applications, and bioenergy development (Lu et al., 2018; Yan and Tan, 2019; Yin et al., 2024). Escalating challenges from climate change and ecological shifts have intensified crop vulnerability to phytophagous pests, particularly lepidopteran species that severely compromise maize productivity (Zeng et al., 2020; Bebber, 2021). Key defoliators including fall armyworm (Spodoptera frugiperda, FAW), Asian corn borer (Ostrinia furnacalis, ACB), and beet armyworm (Spodoptera exigua, BAW) inflict systemic damage through folivory, stem boring, and ear feeding during critical growth stages (Tzin et al., 2017; Lu et al., 2024; Zhao et al., 2024). This biotic stress necessitates urgent identification of stress-resistance genes and development of resilient cultivars - strategies crucial for yield optimization, quality enhancement, and climate-smart agricultural adaptation.
Investigation of ZmPAL genes holds significant agricultural importance given phenylpropanoids’ critical roles in phytophagous insect resistance (Li et al., 2021; Ramaroson et al., 2022). While previous studies have characterized maize PAL family members employing conventional single-reference genome approaches, systematic analyses under lepidopteran herbivory remain unexplored (Wu et al., 2020). Traditional gene family identification methods, constrained by single-genome frameworks, inherently fail to detect non-reference members existing in other germplasm (Bi et al., 2025; Tong et al., 2025). The maize pangenome resource established by Hufford et al., comprising 26 high-quality genomes with extensive presence-absence variations (PAVs) and structural variations (SVs), provides unprecedented resolution for pan-genomic studies (Funk and Zahn, 2021; Hufford et al., 2021). Leveraging this pangenomic architecture, researchers have successfully delineated multiple pan-gene families including TPS (Sun et al., 2023), ARF (Man et al., 2025), and Ann (Liu et al., 2025), demonstrating the framework’s capacity to reveal previously undetected genetic diversity. This approach enables comprehensive investigation of gene family evolution and functional diversification across maize germplasm.
This study systematically characterized the ZmPALs pangenome family across 26 maize genomes, profiling presence-absence variations, selection pressures, cis-regulatory elements, and structural motifs. By integrating publicly available RNA-seq datasets with qRT-PCR validation, the expression dynamics of ZmPAL members under lepidopteran infestation were comprehensively deciphered. The findings yield foundational insights into ZmPAL-mediated molecular responses to lepidopteran stressors while establishing a molecular framework for understanding regulatory mechanisms during herbivore challenges. These results provide actionable genetic targets for developing insect-resistant maize cultivars through molecular breeding strategies, addressing critical agricultural demands for sustainable pest management solutions.
2 Materials and methods
2.1 Plant material preparation and insect rearing
All experiments were conducted at the Plant Growth Facility of Anhui Science and Technology University (32°55’N, 117°23’E), located in Chuzhou, Anhui Province, China. The facility maintains controlled-environment chambers with standardized conditions for photoperiod, temperature, and humidity control. The maize inbred line B73 was cultivated in a controlled-environment growth chamber using a specific substrate composed of a sterilized potting mixture with a ratio of peat:vermiculite:perlite of 3:1:1. Regular irrigation and fertilization were conducted to maintain optimal growth conditions. Fertilization was performed with 5 grams per pot of NPK 15-15–15 compound fertilizer, applied once every 8 days after seedling emergence. Plants were grown in plastic pots with a diameter of 15 centimeters, filled with the sterilized potting mixture. The cultivation was carried out under controlled conditions: 28°C, a photoperiod of 16 hours of light/8 hours of darkness, and relative humidity of 50-60%. The substrate was sterilized prior to use to prevent microbial contamination.
Experimental treatments commenced at the three-leaf developmental stage, with triplicate biological replicates implemented throughout. Lepidopteran test species (Spodoptera frugiperda, Ostrinia furnacalis, and Spodoptera exigua) were obtained as laboratory-adapted colonies from the Engineering Technology Institute of Maize Breeding in Anhui Province. Larvae were maintained in climate-controlled chambers under standardized rearing conditions (25 ± 1°C, 60 ± 5% RH, 16h light cycle), fed artificial diet until reaching uniform 2nd-3rd instar developmental stages. Adult specimens received nutritional supplementation via 10% honey solution. All insect bioassays included triplicate biological replicates to ensure experimental robustness. The study employed a completely randomized design with three biological replicates per treatment. For lepidopteran infestation experiments, three-leaf stage maize plants were randomly assigned to four treatment groups: (1) control (no infestation), (2) Spodoptera frugiperda infestation, (3) Ostrinia furnacalis infestation, and (4) Spodoptera exigua infestation. Each treatment group contained 15 plants (3 plants per replicate). Infestation was performed using 2nd-instar larvae at a density of 5 larvae per plant.
2.2 Identification of the ZmPALs pan gene family
The 26 maize pangenome datasets were retrieved from MaizeGDB following Hufford’s genomic framework (Hufford et al., 2021). The PAL-specific HMM profile (PF00320) was acquired from the InterPro database (Paysan-Lafosse et al., 2023). A dual-algorithm approach employing HMMER v3.3.2 and BLASTP identified candidate sequences containing PAL domains, with candidates selected using an E-value threshold <1e-10. Following sequence extraction and redundancy elimination, structural validation through SMART and functional annotation via UniProt confirmed definitive PAL family members across all pangenome assemblies (Bateman et al., 2021; Letunic et al., 2021) (Please refer to Supplementary Table S1 for the URLs and access times of all the websites in Section 2).
2.3 Phylogenetic analysis of the ZmPALs pan gene family
Phylogenetic relationships among ZmPALs proteins were reconstructed through comparative analysis with Arabidopsis and rice homologs. Reference PAL protein sequences from Arabidopsis thaliana and Oryza sativa were retrieved from NCBI and Phytozome13 databases (Pruitt et al., 2011; Goodstein et al., 2012). Multiple sequence alignment of maize, rice, and Arabidopsis PAL homologs was performed using the ClustalW algorithm, followed by phylogenetic tree construction via the neighbor-joining method in MEGA11 with 1000 bootstrap replicates. The resultant tree topology was visualized and annotated using Evolview to enhance interpretative resolution of evolutionary relationships (Subramanian et al., 2019).
2.4 Presence-absence variation in the ZmPALs pan gene family
Presence-absence variation (PAV) profiling of ZmPALs was conducted using genomic data from Hufford’s pangenome study. A custom script generated binary PAV matrices, which were subsequently processed for phylogenomic representations of 26 genotypes through the ggplot2 package in R. Presence/absence patterns across genotypes were visualized as heatmaps via the ComplexHeatmap R package, enabling systematic evaluation of PAL family genomic architecture heterogeneity.
2.5 Ka/Ks calculations for the ZmPALs pan gene family
Coding sequences (CDS) and corresponding protein sequences of PAL family members were extracted from 26 maize genomes. Evolutionary selection pressures were quantified using KaKs_Calculator v2.0 to compute Ka/Ks ratios for each PAL homolog. A visualization pipeline integrating three R packages (ggridges, ggplot2, and pheatmap) generated comparative evolutionary profiles - ridgeline plots for full Ka/Ks distribution analysis and heatmaps specifically highlighting positive selection candidates (Ka/Ks>1). The evolutionary framework defined neutral mutations at Ka/Ks=1, positive selection at ratios >1, and purifying selection at ratios <1, enabling systematic detection of selection signatures across phylogenetic contexts (Li et al., 2023b).
2.6 Analysis of overlapping expression of structural variants of ZmPALs
SV data and corresponding gene expression profiles across cultivars were retrieved from Hufford’s genomic resource, with B73 serving as the reference genome for structural variant calling. SV annotations were performed using ANNOVAR, followed by targeted extraction of PAL-associated structural variants. Correlation analyses between SV status and transcriptional activity of PAL members were conducted, with differentially expressed candidates visualized through histogram representations. Genome annotation files (GFF3 format) for all 26 maize lines were acquired from MaizeGDB to resolve structural configurations of significant PAL variants. Motif discovery in ZmPAL protein sequences was executed via the MEME Suite (v5.5.5) using default parameters except for specifying 10 motifs (Bailey et al., 2015). Final gene structure diagrams integrating motif patterns and genomic architectures were generated using TBtools, leveraging GFF annotations and motif prediction outputs for comparative visualization (Chen et al., 2023).
2.7 Analysis of cis-elements and structures of ZmPALs from different maize
ZmPAL protein sequences from insect-resistant accession CML333 and reference genome B73 were submitted to the MEME Suite for comparative motif analysis, specifying 10 conserved motifs and generating WebLogo outputs to visualize sequence conservation patterns. Promoter regions (-2000 bp upstream) of ZmPALs were extracted from both genotypes for cis-element identification using PlantCARE database, prioritizing elements associated with stress responsiveness, developmental regulation, and hormonal signaling pathways (Rombauts et al., 1999).
2.8 Analyzing the ZmPALs pan gene family using published RNA-Seq data
Publicly available RNA-seq datasets documenting maize responses to lepidopteran infestations (Spodoptera frugiperda: PRJNA675077, Ostrinia furnacalis: PRJNA772910, Spodoptera exigua: PRJNA625224) were retrieved from NCBI for expression profiling of ZmPALs pan-genes (Tzin et al., 2017; Tang et al., 2021; Ye et al., 2022). Raw sequence quality was assessed using FastQC v0.12.1, followed by adapter removal and quality filtering through Trimmomatic v0.40. Processed reads were aligned to the B73_V5 reference genome using TBtools’ One-Step RNAseq plugin, implementing HISAT2 for alignment and StringTie for transcript quantification to generate FPKM expression matrices. Differential expression patterns were visualized as log2(RPKM+1)-transformed heatmaps using the ComplexHeatmap package (v2.6.2) in R v4.0.3, enabling cross-infestation comparative analysis of PAL transcriptional dynamics.
2.9 Expression properties of ZmPALs genes under lepidopteran infestation
Lepidopteran infestation was performed by placing five 2nd-instar larvae (starved for 4 hr) on the abaxial surface of the third fully expanded leaf using a fine brush. Larvae were confined to individual plants using mesh bags (30×40 cm, 120-mesh) to prevent escape and ensure controlled feeding. Infestation duration was standardized to 24 hours for all experiments, after which larvae were removed and leaf damage was quantified. Leaf samples were collected from maize plants at 0h (uninfested control), 4h, 12h, and 24h post-infestation by lepidopteran larvae (Spodoptera frugiperda, Ostrinia furnacalis, Spodoptera exigua). Total RNA was isolated using the RNAprep Pure Plant Kit (TIANGEN, Beijing, China) following manufacturer protocols, with subsequent cDNA synthesis performed via HiscriptII Reverse Transcriptase (Vazyme, Nanjing, China). qRT-PCR primers targeting ZmPALs were designed using Primer 6 software, with maize elongation factor 1-alpha (EF-1α) serving as the endogenous control (Ye et al., 2012) (Supplementary Table S2). Amplification reactions (20μL) containing 5μL cDNA template, 0.1μM primers, and SYBR Green Master Mix were conducted in triplicate on an ABI ViiA 7 system under standardized thermal cycling conditions: 95°C for 30 sec, 40 cycles of 95°C/10 sec and 60°C/30 sec, followed by melt curve analysis (95°C/15 sec, 60°C/60 sec, 95°C/15 sec). Melting curve profiles and cycle threshold (Ct) values were acquired using QuantStudio™ Software v1.6.1, with relative expression quantified through the 2−ΔΔCt method (Livak and Schmittgen, 2001). All data were analyzed using SPSS 26.0 (IBM Corp.). One-way ANOVA followed by Tukey’s HSD test was employed for multiple comparisons between treatment groups. For temporal expression patterns, two-way ANOVA was used to examine the effects of time and treatment. Data are presented as mean ± standard error (SE) of three biological replicates, with statistical significance set at p < 0.05. Data visualization was performed using GraphPad Prism 8 software (Berkman et al., 2018). All qRT-PCR reactions were performed in triplicate for each sample.
3 Results
3.1 Identification and phylogenetic analysis of the ZmPALs pan-genome
A total of 29 ZmPALs family members were identified across 26 maize pan-genome assemblies. Comparative analysis using B73 as the reference genome revealed marked disparities in both gene count and protein sequence length among different maize lines (Figure 1a). The Ki3 and MS71 lines exhibited the highest PALs content with 16 members each, while M162W showed the minimal count of 11 members. Structural classification of ZmPALs demonstrated a composition of 7 core genes, 2 near-core genes, 12 non-core genes, and 8 line-specific private genes (Figure 1b). The identification of 29 ZmPALs across 26 maize genomes revealed four distinct categories: (1) 7 core genes present in all accessions, likely maintaining essential functions in phenylpropanoid metabolism; (2) 2 near-core genes absent in only 1–2 lines, suggesting conserved but potentially specialized roles; (3) 12 non-core genes showing intermediate frequencies (present in 3–24 genomes), which may contribute to lineage-specific adaptations; and (4) 8 private genes unique to single accessions, representing recent evolutionary innovations or deletions in other lines. This distribution pattern reflects the dynamic evolutionary history of the ZmPAL family, where core genes preserve fundamental functions while non-core and private genes may enable metabolic diversification and environmental adaptation. Particularly, the substantial proportion of non-core and private genes (20/29, ~69%) highlights the remarkable genetic plasticity of phenylpropanoid pathways in maize, potentially facilitating rapid responses to biotic stresses like lepidopteran herbivory across different environments.
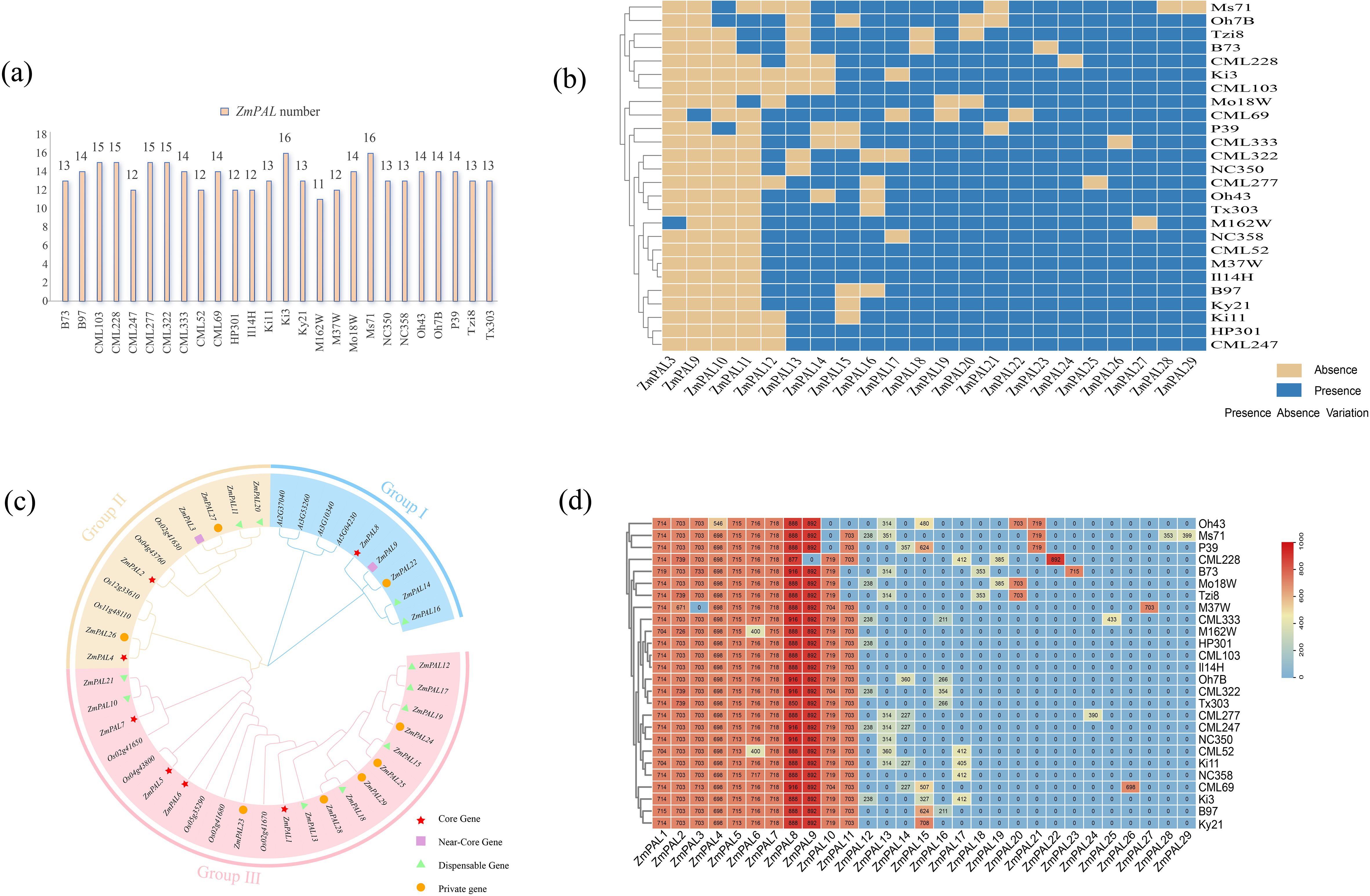
Figure 1. Identification and phylogenetic analysis of ZmPALs in pan-genome. (a) Number of ZmPALs. (b) Heatmap of the presence and absence of 21 ZmPALs in 26 maize varieties except for the core genes. (c) Phylogenetic tree of PALs from Arabidopsis and Maize. (d) Heatmap of ZmPALs protein length.
Protein length comparisons revealed that ZmPAL8 (core) displayed the longest sequence, followed by ZmPAL7. The near-core ZmPAL9 maintained consistent protein length in 25 lines despite its absence in CML228. Among non-core genes, ZmPAL21 exhibited the longest average protein length compared to the shortest ZmPAL12. Substantial variation in protein lengths was observed across other family members (Figure 1d). Comprehensive data for all PAL family members are provided in Supplementary Table S3.
Phylogenetic analysis utilizing protein sequences from maize, rice, and Arabidopsis established three distinct subfamilies following the classification by Wu et al. (Figure 1c). Subfamily III contained the highest number of ZmPALs (17 members), followed by Subfamily II with 7 members, while Subfamily I exhibited the smallest contingent (5 members). Core, non-core, and private genes predominantly accumulated in Subfamily III, though near-core genes were notably absent from this subfamily. Arabidopsis PALs were exclusively clustered in Subfamily I, whereas rice PALs distributed across both Subfamilies II and III. This evolutionary pattern demonstrates closer phylogenetic relationship between maize and rice compared to their divergence from Arabidopsis.
3.2 ZmPALs are subject to different selection pressures among maize varieties
The Ka/Ks ratio served as a critical evolutionary indicator to investigate selection pressures on PAL family members across 26 maize genomes. Comprehensive calculation of Ka/Ks values revealed that except for private genes (uncalculable ratios), only ZmPAL8 exhibited a ratio exceeding 1, while most members maintained ratios below this threshold (Figure 2a). Distinct positive selection signals were detected for ZmPAL8 in Ki3 and Tx303 lines, contrasting with purifying selection patterns in other genes. Heatmap analysis of ratios >1 confirmed ZmPAL8 as the sole gene demonstrating elevated Ka/Ks values, indicative of sustained selective pressure during maize evolution (Figure 2b).
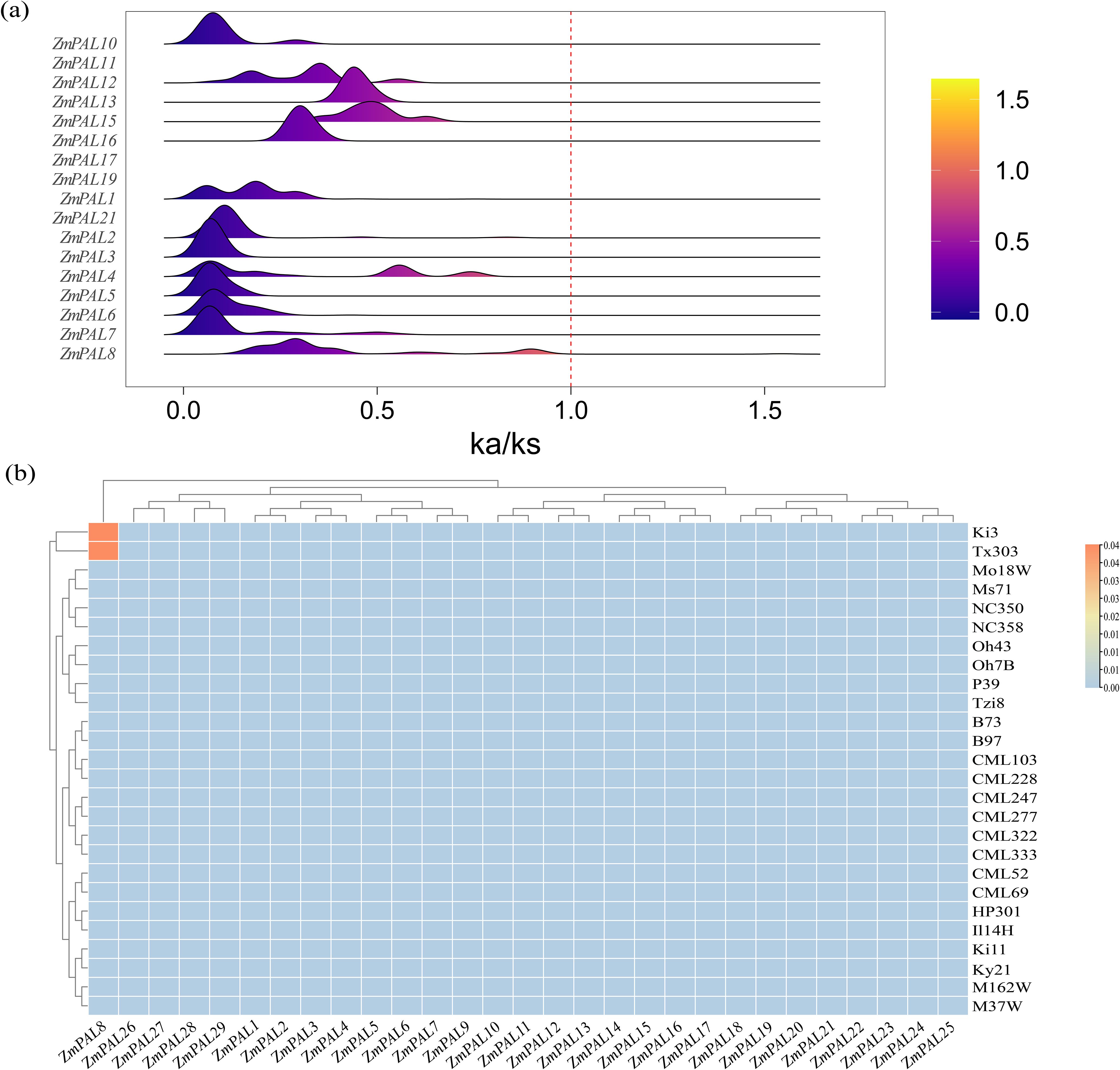
Figure 2. Ka/Ks values of ZmPALs. (a) Distribution of Ka/Ks values of ZmPAL for 26 maize varieties; (b) heat map of the frequency of occurrence of different maize varieties with Ka/Ks ratios > 1 in each PAL.
3.3 Effect of SV on gene expression, structure and motifs of ZmPALs
Structural variation (SV) analysis of PAL family members revealed distinct expression patterns through Pearson correlation coefficients comparing SV-overlapping and non-overlapping genes. Expression profiling demonstrated elevated transcript levels for ZmPAL3, ZmPAL7, and ZmPAL10, while ZmPAL1 and ZmPAL13 showed complete transcriptional silence (Figure 3a). Notably, ZmPAL5 exhibited significant differential expression between SV-present and SV-absent conditions (p<0.05), demonstrating substantial SV-mediated regulation of its transcriptional activity (Figure 3b).
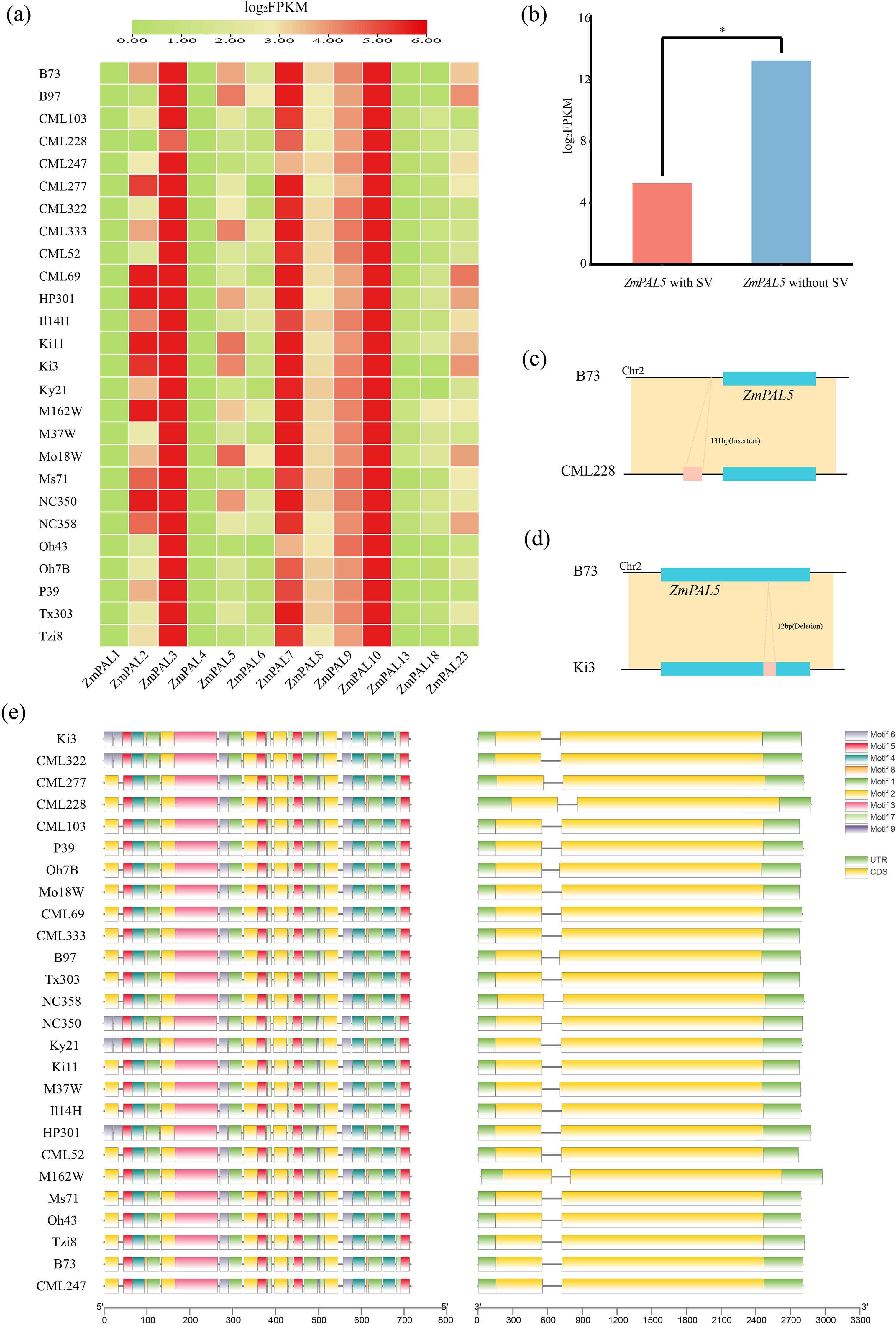
Figure 3. SV affects the expression, structure and motifs of 26 maize genomes. (a) SV on the expression of 26 maize pan-genomes; (b) SV significantly affects the expression of ZmPAL5 (*: P<0.05); (c) effect of SV insertion on ZmPAL5; (d) effect of SV deletion on ZmPAL5; (e) structure and motifs of ZmPAL5 in the maize pan-genome.
Genomic analysis identified 196 structural variations (SVs) overlapping with 29 ZmPALs loci and their flanking 2-kb regulatory regions. Comparative profiling against the B73 reference genome revealed SVs predominantly manifested as insertions (Figure 3c) and deletions (Figure 3d). Notably, line CML228 exhibited a 131-bp upstream insertion, while Ki3 displayed a 12-bp intragenic deletion. Comparative structural mapping of ZmPAL5 across 26 genomes demonstrated conserved gene architecture and conserved domains identical to the B73 reference, with no substantial structural divergences observed (Figure 3e).
3.4 Cis-elements and structural analysis of ZmPALs
Comparative analysis of cis-acting elements in ZmPALs promoters was conducted between CML333 and B73, focusing on hormone responsiveness, plant growth/development, and stress-related regulatory motifs (Figure 4a). The B73 genome exhibited greater cis-element diversity compared to CML333. Hormonal response elements for abscisic acid, gibberellin, and salicylic acid were prevalent across most ZmPAL promoters. Light-responsive elements were ubiquitously present except in ZmPAL9_CML333. Stress-inducible motifs including low-temperature responsiveness, anaerobic induction, and drought-inducibility were systematically identified. These findings suggest that ZmPALs may orchestrate maize growth, development, and stress adaptation through combinatorial regulation by diverse promoter cis-elements.
Based on the conserved motifs of ZmPALs in the reference genomes B73 and CML333, a conserved domain alignment diagram was generated (Figure 4b). The results indicated that all ten conserved domains identified in the CML333 genome corresponded to those in the B73 reference genome. However, the amino acids within the corresponding conserved domains of each group did not exhibit complete alignment, a phenomenon potentially attributable to selective pressures affecting the conserved domains of ZmPALs.
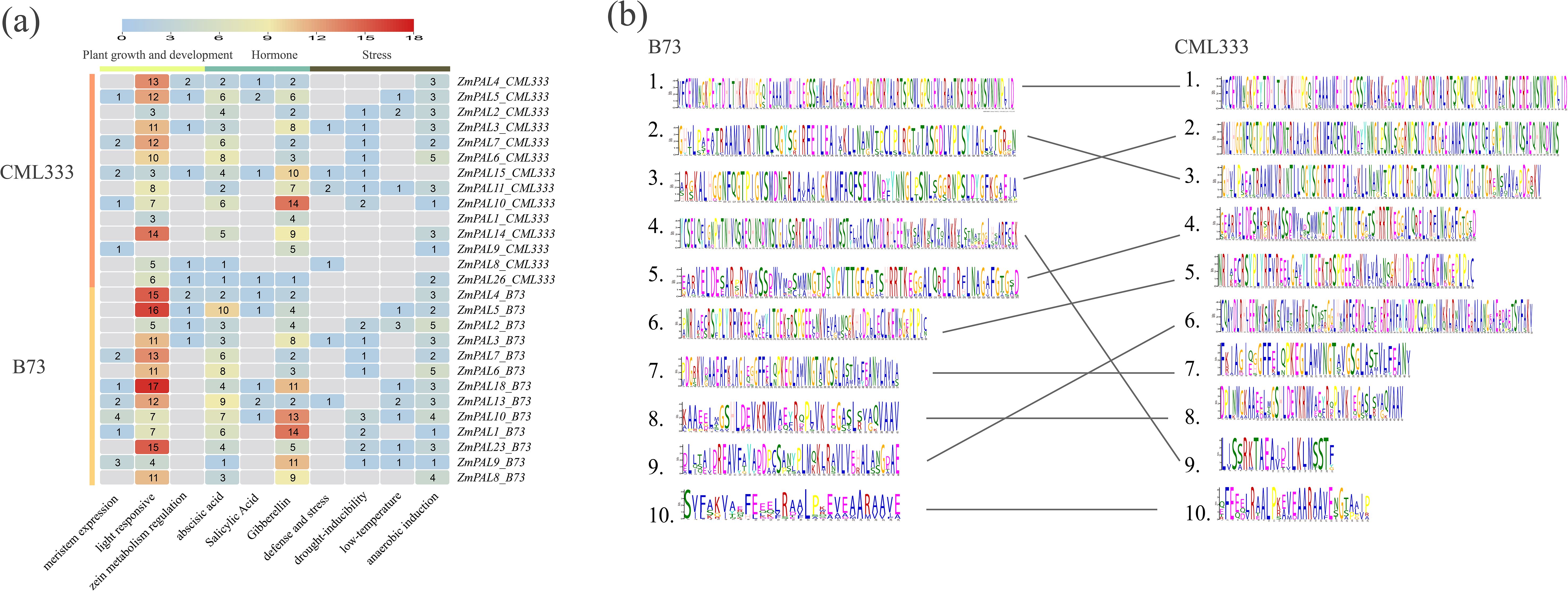
Figure 4. Cis-elements and structural analysis of ZmPALs. (a) Number and functional classification of cis-acting elements in the B73 and CML333 genomes. (b) Web logos of ZmPALs in CML333 and B73 are shown on the left and right, respectively. Web logos connected by lines indicate that they correspond. webLogos are arranged in the order of E-values.
3.5 Transcriptome analysis of the ZmPALs gene family under stress in different lepidopteran insects
Transcriptomic re-analysis of published datasets (PRJNA675077: Spodoptera frugiperda; PRJNA772910: Ostrinia furnacalis; PRJNA625224: Spodoptera exigua) using the B73_V5 reference genome revealed conserved expression patterns among ZmPALs pan-family members under Lepidopteran herbivory (Figure 5). Two members (ZmPAL4 and ZmPAL13) exhibited transcriptional silence across all stress conditions, while five genes (ZmPAL2, ZmPAL3, ZmPAL7, ZmPAL10 and ZmPAL23) demonstrated elevated expression profiles. The pan-family displayed coordinated transcriptional responses to these biotic stressors, suggesting functional conservation in insect defense mechanisms.
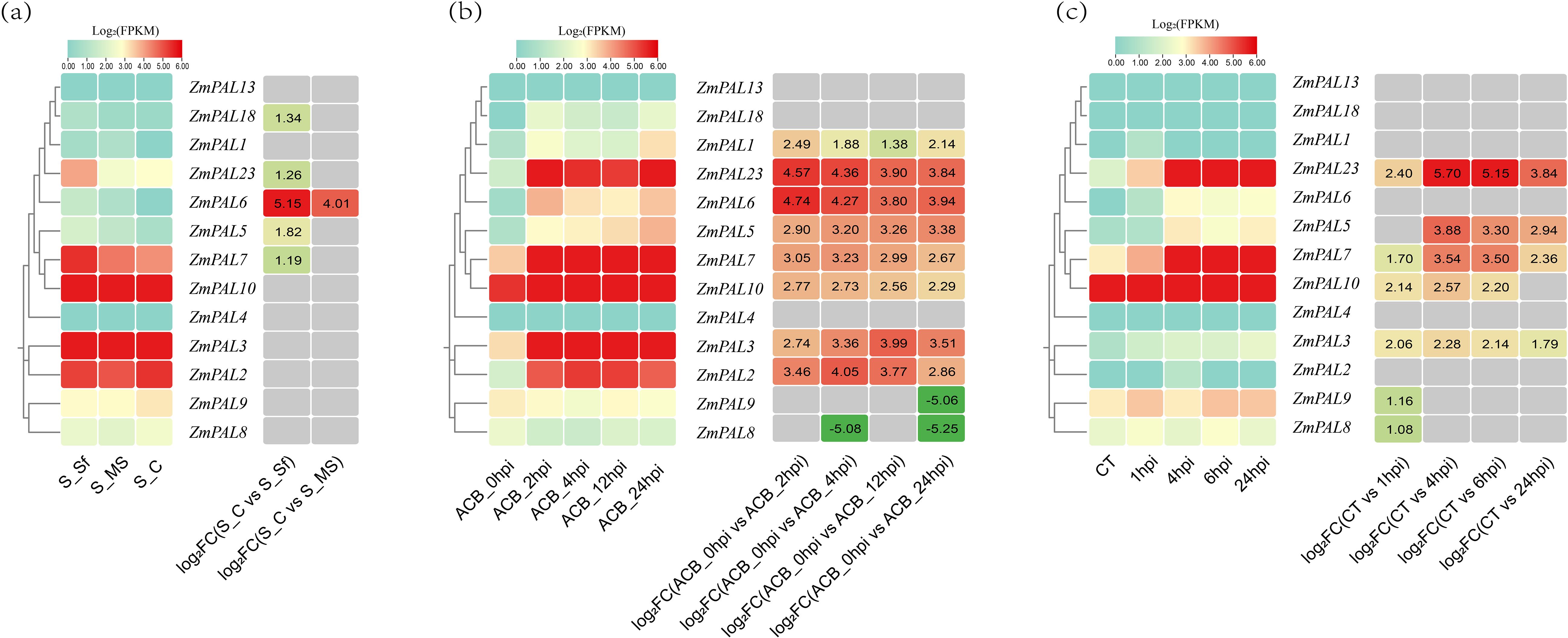
Figure 5. Expression profiles of ZmPALs under Lepidopteran herbivory. (a) Spodoptera frugiperda challenge: S_C (Control), S_Sf (Third-day larval feeding), S_MS (Mechanical wounding control). (b) Dynamic response to Ostrinia furnacalis infestation: Temporal expression patterns at 0, 4, 12, and 24 hours post-infestation (hpi). (c) Spodoptera exigua exposure: CT (Untreated control) and stress responses at 1, 4, 6, 24 hpi. Note: left matrix shows normalized FPKM values, while the right matrix illustrates differential expression through log2(fold change) values (|FC|≥1 threshold), with red and green color gradients denoting upregulation and downregulation respectively.
Lepidopteran-specific induction patterns revealed distinct regulatory responses among ZmPALs members. Spodoptera frugiperda infestation (Figure 5a) triggered significant upregulation of five genes (ZmPAL5, ZmPAL6, ZmPAL7, ZmPAL18 and ZmPAL23), with ZmPAL6 showing the most pronounced induction. Ostrinia furnacalis challenge (Figure 5b) upregulated eight ZmPALs (ZmPAL1, ZmPAL2, ZmPAL3, ZmPAL5, ZmPAL6, ZmPAL7, ZmPAL10 and ZmPAL23), particularly ZmPAL6 and ZmPAL23, while downregulating ZmPAL8 and ZmPAL9. Spodoptera exigua exposure (Figure 5c) elevated seven transcripts (ZmPAL3, ZmPAL5, ZmPAL7, ZmPAL8, ZmPAL9, ZmPAL10 and ZmPAL23), with ZmPAL23 demonstrating the highest induction magnitude.
3.6 Analysis of the expression pattern of the ZmPALs gene family after FAW infestation
To elucidate ZmPALs pan-family functions in maize defense against fall armyworm (FAW), a time-course induction experiment was conducted in B73 plants. Quantitative PCR analysis revealed dynamic temporal expression patterns of six ZmPALs (ZmPAL3, ZmPAL5, ZmPAL6, ZmPAL7, ZmPAL10 and ZmPAL23) at 0, 4, 12, and 24 hours post-infection (hpi) (Figure 6). All examined genes exhibited FAW-responsive regulation, with significant upregulation peaking during early to mid-stages (4–12 hpi). Transcript levels subsequently declined to basal levels by 24 hpi, indicating transient induction kinetics characteristic of biotic stress responses.
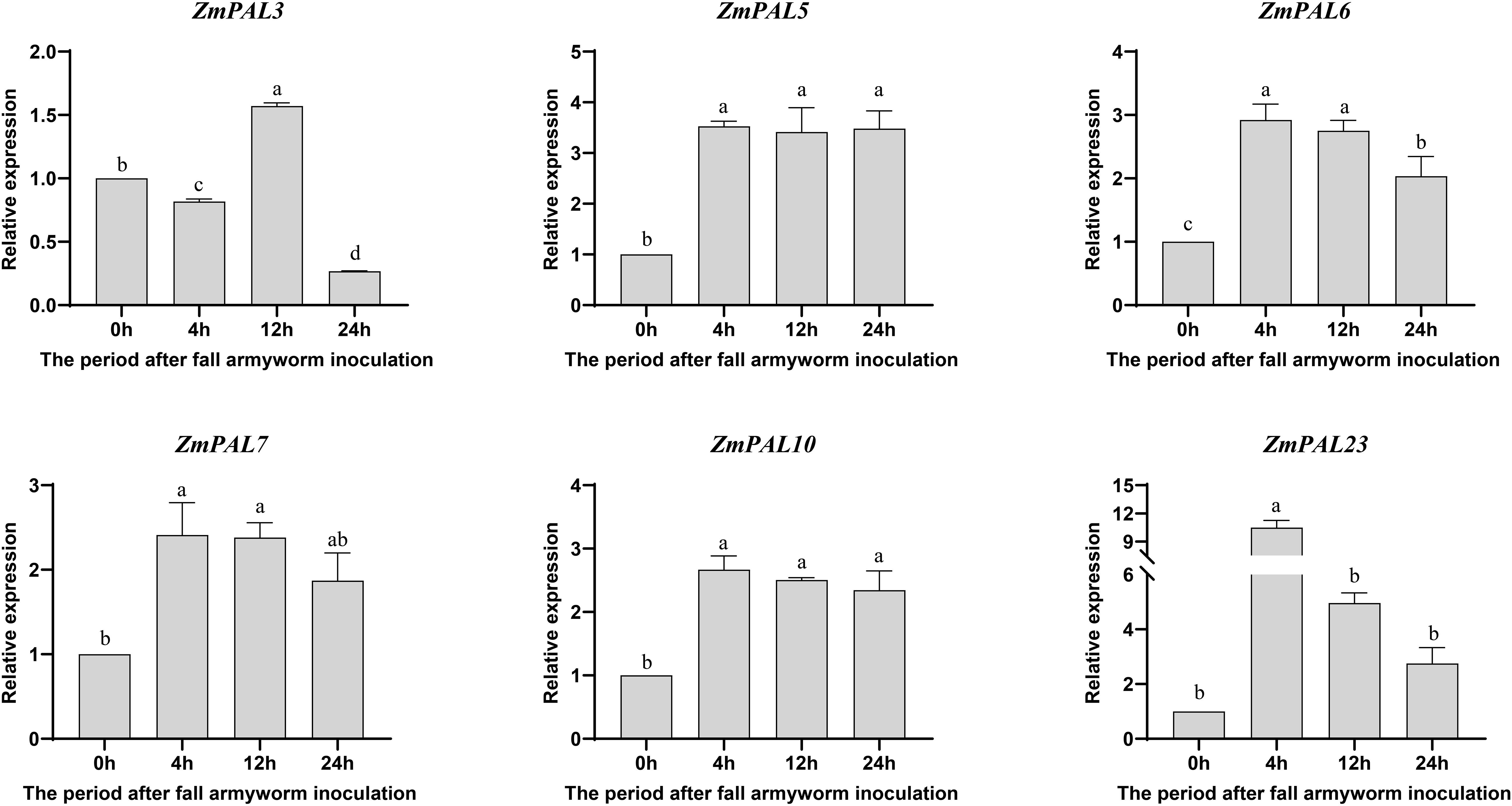
Figure 6. Expression pattern of ZmPALs pan gene family after FWA infestation. The time of infestation was set to four time points (0, 4, 12 and 24 hpi after infestation), with 0h as a control. Significant differences are indicated by lower case letters (p < 0.05).
Five ZmPALs members (excluding ZmPAL3) exhibited significant induction across FAW infestation stages. ZmPAL5 and ZmPAL6 peaked at 12 hpi, whereas ZmPAL23 showed an early expression surge at 4 hpi (Figure 6). The transient ZmPAL3 upregulation at 12 hpi coincided with defense signaling activation, while its downregulation at 4 hpi and 24 hpi suggests dynamic transcriptional reprogramming coordinating anti-herbivore responses. These differential patterns implicate ZmPALs in FAW defense through temporal-specific regulation, potentially mediated by phased induction of defense-related signaling cascades. The observed temporal divergence in gene activation (early vs mid-phase peaks) reflects functional specialization within the PAL regulatory network during biotic stress adaptation.
3.7 Expression pattern analysis of ZmPALs gene family after ACB infestation
Functional investigation of ZmPALs pan-family members under Asian corn borer (ACB) infestation revealed temporally differentiated expression dynamics in B73 (Figure 7). ZmPAL5 and ZmPAL10 showed no significant expression alterations, suggesting limited involvement in ACB response. Notably, ZmPAL7 exhibited significant upregulation at 4 hpi, indicating potential early defense activation, while ZmPAL3 demonstrated delayed induction peaking at 24 hpi, possibly mediating late-phase resistance mechanisms. ZmPAL6 displayed consistent downregulation across all timepoints, suggesting suppression during ACB challenge. In contrast, ZmPAL23 demonstrated sustained induction throughout infestation stages, highlighting its central regulatory role. These divergent expression profiles reflect functional specialization within the ZmPALs network during ACB defense, with specific members orchestrating phase-specific resistance strategies. The identification of temporally regulated PAL isoforms provides both mechanistic insights into maize-insect interactions and potential genetic targets for breeding ACB-resistant varieties.
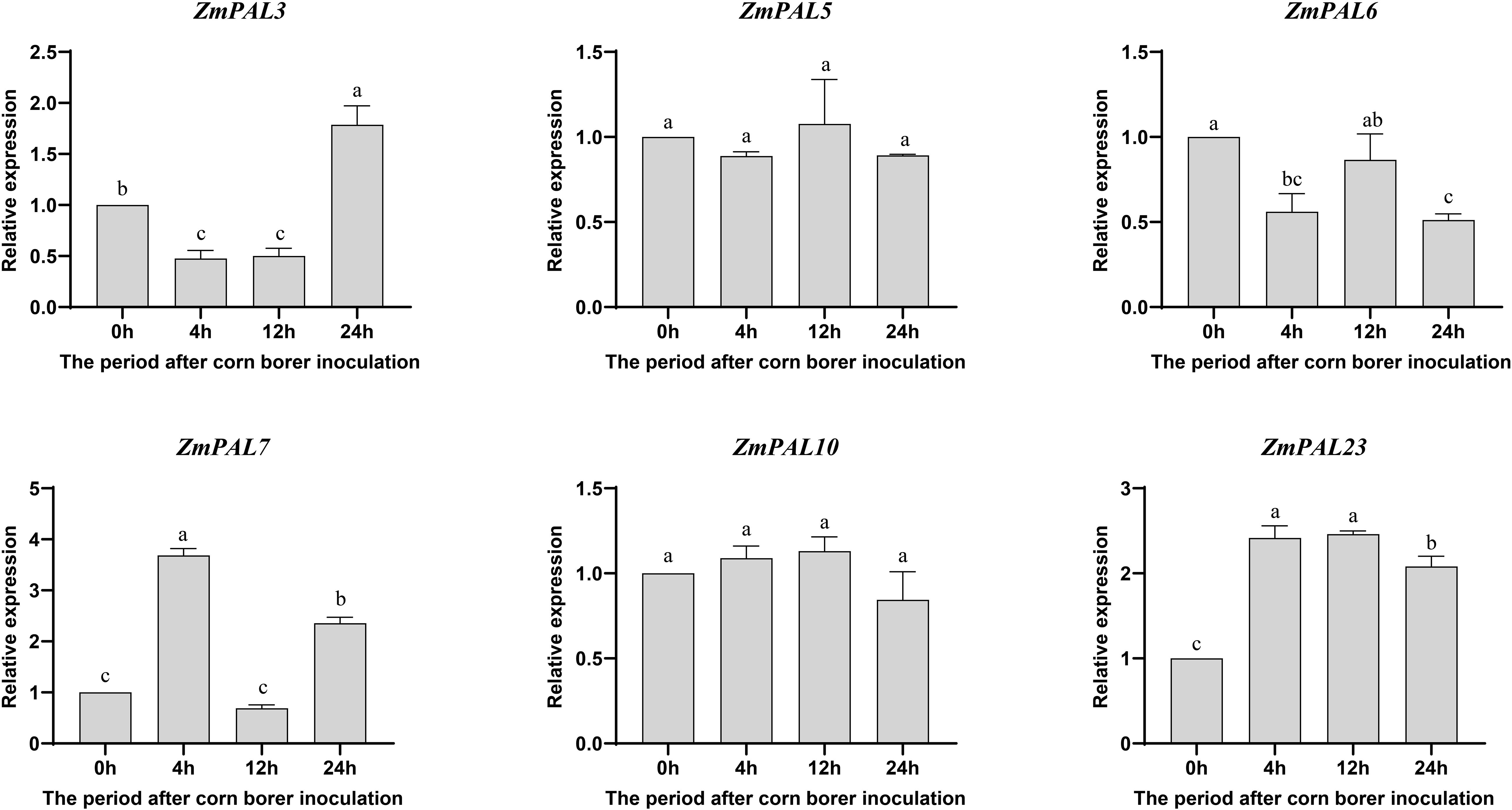
Figure 7. Expression pattern of ZmPALs pan gene family after ACB infestation. The time of infestation was set to four time points (0, 4, 12 and 24 hpi after infestation), with 0h as a control. Significant differences are indicated by lower case letters (p < 0.05).
3.8 Expression pattern analysis of the ZmPALs gene family after BAW infestation
qRT-PCR analysis of ZmPALs expression in B73 following beet armyworm (BAW) infestation revealed temporally stratified transcriptional dynamics across four timepoints (0, 2, 12, 24 hpi) (Figure 8). All responsive ZmPALs exhibited phased induction patterns: ZmPAL23 showed marked upregulation at 4 hpi, while ZmPAL7 peaked at 24 hpi. Early response genes (ZmPAL10 and ZmPAL23) demonstrated significant induction by 4 hpi, suggesting initiation of primary defense mechanisms. Mid-phase induction (12 hpi) involved ZmPAL5, ZmPAL10, and ZmPAL23, potentially coordinating intermediate defense signaling. Late-stage upregulation (24 hpi) characterized ZmPAL3 and ZmPAL7, with ZmPAL10 maintaining sustained 2–3 fold induction post-4 hpi. Contrastingly, ZmPAL6 displayed consistent downregulation, indicating negative regulatory functions. The observed temporal divergence in PAL activation suggests stage-specific defensive roles, particularly mid-phase induction potentially mediating defense pathway modulation. This dynamic transcriptional reprogramming reflects ongoing plant-insect interplay through successive defense phases.
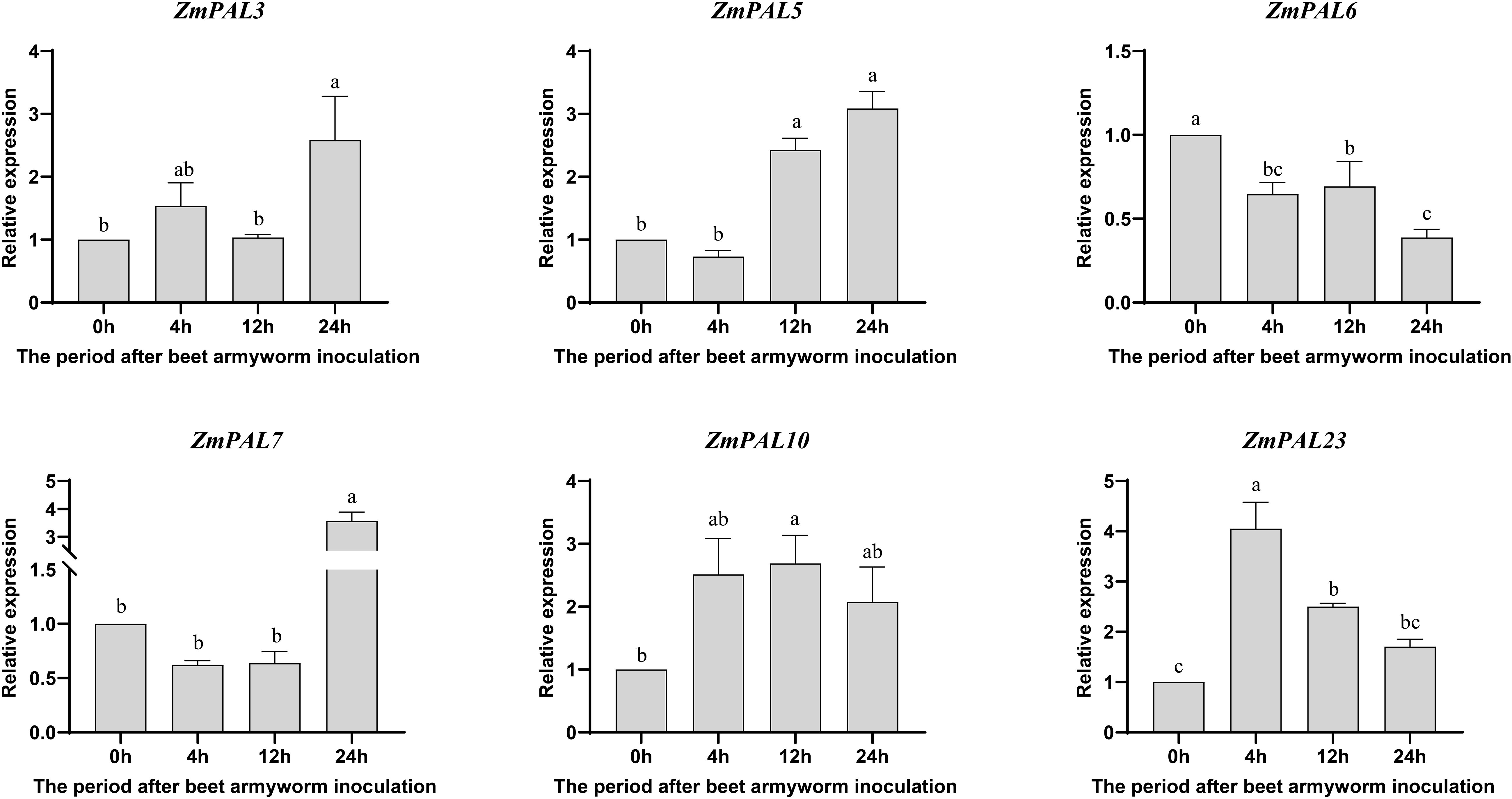
Figure 8. Expression pattern of ZmPALs pan gene family after BAW infestation. The time of infestation was set to four time points (0, 4, 12 and 24 hpi after infestation), with 0h as a control. Significant differences are indicated by lower case letters (p < 0.05).
4 Discussion
The PAL gene family in maize was first identified by the B73 reference genome (B73 RefGen_v3) (Lv et al., 2016). Traditional gene family analyses are usually based on a single reference genome, and given that a single reference genome is insufficient to capture the full extent of genetic diversity within a species, determining the presence or absence of genes in multiple reference genomes becomes challenging (Golicz et al., 2016; Silva-Arias et al., 2024). Recently, a pan-genome of 26 high-quality maize genomes containing genes missing from the reference genome has been published, which provides a more precise assembly and annotation than the B73 reference genome when compared to reference genome-based gene family analyses (Schnable et al., 2009; Hirsch et al., 2014). In this study, 29 ZmPALs were identified using the maize pan-genome, including 7 core genes, 2 near-core genes, 12 non-core genes and 8 private genes. This is an increase of 16 genes compared to the 13 genes identified in the reference genome (Wu et al., 2020). PAV analysis showed that only 7 of the 29 gene family members were consistently present in all maize varieties and the core genes were distributed in all three subgroups, and these core genes may play important regulatory roles in maize growth and development. In addition, the remaining genes are not universally absent in all varieties, thus ensuring genomic complementarity between varieties (Lin et al., 2024). ZmPAL28 and ZmPAL29 are present only in Ms71 and regulate traits that are unique to this line. Despite the variation in the number of ZmPALs, total gene expression showed no correlation. This may be due to the fact that some members of the gene family have similar functions and can compensate each other to maintain normal physiological processes (Iohannes and Jackson, 2023).
Structural variations (SVs), encompassing deletions, insertions, inversions, and translocations, represent critical genetic modifications that can alter gene architecture, conserved domains, and transcriptional regulation (Tang, 2020; Kalakoti et al., 2025). SVs substantially influence crop resilience to biotic and abiotic stressors, as exemplified by the SNP-465 variant in ZmICE1’s promoter region modulating cold tolerance through transcriptional reprogramming (Jiang et al., 2022), and ZmBGLU17’s promoter SV enhancing resistance against Phytophthora pathogens and Asian corn borer (Liu et al., 2023a). This study identified SV-mediated regulation of ZmPAL5 expression warranting mechanistic investigation. Comparative analysis revealed a 131-bp insertion in CML333’s upstream region disrupted coding sequence alignment, resulting in complete ZmPAL5 transcriptional silencing. Conversely, the 12-bp intragenic deletion in Ki3’s non-coding region exhibited minimal impact on ZmPAL5 functionality, highlighting the positional sensitivity of structural variations in gene regulation. The Ka/Ks analysis in this study revealed that ZmPAL8 underwent positive selection in both Ki3 and Tx303, whereas the other ZmPALs experienced purifying selection (Figure 2). This suggests that ZmPAL8 may contribute to local adaptation by enhancing phenylpropanoid biosynthesis related to defense mechanisms. In contrast, ZmPAL7 and ZmPAL10 exhibited conservative Ka/Ks ratios across different varieties, reflecting their non-redundant roles in fundamental metabolism. This differential selective pressure is consistent with the observed PAVs and SVs, highlighting how genetic diversity shapes the functional specialization of the ZmPAL family.
The enhanced insect resistance in CML333 correlates with its specialized chemical defense system, particularly benzoxazinoid (Bx) biosynthesis known for insect deterrence and toxicity (Kumar, 2002; Ni et al., 2008b). Comparative cis-regulatory element analysis of ZmPALs promoters revealed greater element diversity in B73 versus CML333. However, evolutionary optimization in CML333 under prolonged natural and artificial selection pressures appears to have enhanced cis-element functional efficiency, potentially enabling rapid anti-herbivore gene activation. This regulatory refinement complements CML333’s predominant Bx-mediated defense strategy, contrasting with B73’s reliance on Bt protein expression and volatile organic compound release (Ni et al., 2008a; Matova et al., 2020). The observed inverse relationship between cis-element quantity and functional efficacy suggests evolutionary trajectory differences in pest resistance mechanisms between these genotypes. Such specialization aligns with ecological adaptation theory, where sustained pest pressure drives optimization of specific defense pathways rather than generalized regulatory complexity.
Plant insect resistance represents a pivotal adaptive trait in maize defense against Lepidopteran pests, mediated through intricate metabolic network regulation (Peterson et al., 2016; War et al., 2018; Prasanna et al., 2022). Studies demonstrate herbivory-induced activation of secondary metabolic pathways, with the phenylpropanoid pathway serving as a central hub for biosynthesis of lignin, phenolic compounds, and flavonoids (Singer et al., 2003; Singh et al., 2021; Dong et al., 2024). These metabolites function synergistically through physical barrier formation and phytochemical accumulation to disrupt insect feeding and development (Koul, 2008; Gupta and Roy, 2021). In Spodoptera frugiperda-infested maize, the gl8 cuticular wax-deficient mutant exemplifies metabolic compensation mechanisms, exhibiting significant upregulation of phenylpropanoid-related genes concurrent with enhanced jasmonic acid (JA) signaling and benzoxazinoid accumulation (Liu et al., 2022). This metabolic reprogramming reveals plants’ adaptive strategies in balancing physical defenses (cuticular layers) with chemical defenses (specialized metabolites) (Ahmad et al., 2024). The preferential activation of phenylpropanoid metabolism under compromised physical barriers suggests its critical role as a compensatory defense mechanism against herbivory pressure, potentially through toxin-mediated larval growth inhibition.
Phenylalanine ammonia-lyase the rate-limiting enzyme in phenylpropanoid metabolism (Kong, 2015), serves as a central regulator in plant anti-herbivore responses (Li et al., 2023a; Zhu et al., 2024). Studies demonstrate that maize ZmPALs members (e.g., ZmPAL5) mediate drought-induced resistance through catalytic production of trans-cinnamic acid from phenylalanine (Amorim-Silva and Botella, 2025), stimulating lignin biosynthesis to fortify cell wall rigidity against insect mandible penetration (Al-Khayri et al., 2023). This mechanism aligns with findings by Rojanaridpiched et al. linking lignification and silicon deposition to reduced leaf herbivory rates (Rojanaridpiched et al., 1984; Jiménez-Galindo et al., 2019), while Williams’ research implicates hemicellulose content in Spodoptera frugiperda resistance (Williams et al., 1998). PAL activity positively correlates with flavonoid phytoalexin accumulation, exemplified by PAL-mediated flavonoid biosynthesis disrupting lepidopteran gut microbiota homeostasis, thereby inducing larval developmental arrest and mortality (Sun et al., 2022). Notably, PAL functionality integrates with defense signaling networks through cross-talk with ethylene and jasmonic acid (JA) pathways (Yang et al., 2015; Li et al., 2019). For instance, the maize expansin EXP-A20 enhances PAL-dependent lignification via ethylene biosynthesis gene ACO31 activation, establishing multi-layered defense architecture against herbivores (Batool et al., 2024).
Reprocessed transcriptomic datasets from maize under Lepidopteran herbivory (Spodoptera frugiperda, Ostrinia furnacalis, Spodoptera exigua) using the B73_V5 reference genome revealed conserved transcriptional responses across ZmPALs family members. Two genes (ZmPAL4 and ZmPAL13) exhibited complete transcriptional silence, while five members (ZmPAL2, ZmPAL3, ZmPAL7, ZmPAL10 and ZmPAL23) demonstrated constitutively elevated expression profiles. Comparative analysis identified six candidate genes (ZmPAL3, ZmPAL5, ZmPAL6, ZmPAL7, ZmPAL10 and ZmPAL23) with significant upregulation patterns relative to control conditions, suggesting their prioritized roles in insect defense signaling cascades. The conserved stress-responsive expression architecture across phylogenetically distinct Lepidopteran species implies evolutionary conservation of PAL-mediated defense mechanisms in maize.
qRT-PCR validation in B73 under Lepidopteran herbivory (Spodoptera frugiperda, Ostrinia furnacalis, Spodoptera exigua) revealed temporally stratified expression dynamics of ZmPALs members. ZmPAL7, ZmPAL10, and ZmPAL23 demonstrated marked induction during initial defense phases, while ZmPAL10 and ZmPAL23 maintained elevated expression through mid-late infestation stages. Intriguingly, ZmPAL6 exhibited contrasting regulation - significant upregulation during FAW challenge versus downregulation under BAW stress, indicating insect-specific transcriptional reprogramming. ZmPAL23 emerged as a central regulator, showing sustained upregulation across all Lepidopteran stressors and timepoints. These findings provide novel insights into PAL-mediated defense mechanisms against herbivores, revealing both conserved and species-specific regulatory strategies. The temporally coordinated activation patterns suggest hierarchical gene network functionality, where early-responsive genes initiate defense signaling while persistent effectors maintain resistance throughout infestation phases. This temporal specialization in PAL isoform activation advances understanding of maize’s molecular adaptation to Lepidopteran pressures.
While this study provides valuable insights into expression patterns and potential functions of ZmPALs pan-family members in maize, several constraints warrant consideration. The analysis primarily relies on transcriptomic data, necessitating integrated multi-omics analyses encompassing proteomic and metabolomic dimensions to fully characterize PAL-mediated defense mechanisms. Although qRT-PCR validated expression profiles of selected genes, empirical validation through transgenic approaches or CRISPR-based functional genomics remains essential to establish causal relationships. These findings establish a foundational framework for understanding PAL-mediated Lepidopteran resistance while highlighting key knowledge gaps in isoform-specific regulatory networks. Subsequent investigations should prioritize mechanistic studies elucidating temporal coordination between PAL isoforms and downstream defense pathways. Such efforts will advance molecular breeding strategies aimed at enhancing maize resilience through optimized PAL-mediated defense architectures.
5 Conclusions
Pan-genomic comparative analysis identified 29 ZmPALs family members across 26 maize genomes, comprising 7 core, 2 near-core, 12 non-core, and 8 private genes. Phylogenetic classification revealed three evolutionary subgroups, with Group III containing the largest membership (17 genes). Evolutionary pressure analysis demonstrated positive selection acting on ZmPAL8 in specific accessions, while purifying selection dominated other members. SV analysis revealed SV-mediated regulation of ZmPAL5 expression, where a 131-bp upstream insertion in CML333 resulted in transcriptional silencing.
Transcriptomic profiling under Lepidopteran herbivory (Spodoptera frugiperda, Ostrinia furnacalis, Spodoptera exigua) revealed temporally dynamic expression patterns. qRT-PCR validation confirmed stage-specific regulation: ZmPAL7, ZmPAL10, and ZmPAL23 showed early-phase induction, while ZmPAL10 and ZmPAL23 maintained elevated expression through mid-late infestation stages. Notably, ZmPAL6 exhibited contrasting regulation - upregulation during Spodoptera frugiperda challenge versus downregulation under Spodoptera exigua stress, indicating insect-specific transcriptional responses. ZmPAL23 demonstrated sustained upregulation across all temporal phases, suggesting its central regulatory role in coordinated defense mechanisms. These findings delineate temporal specialization and functional divergence within ZmPALs, providing mechanistic insights into phenylpropanoid pathway regulation during insect resistance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
TW: Formal analysis, Writing – original draft, Writing – review & editing. YZ: Formal analysis, Writing – original draft, Writing – review & editing. LS: Writing – review & editing. MG: Writing – review & editing. YH: Writing – review & editing. HY: Conceptualization, Writing – review & editing. DW: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – review & editing. JD: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the Key Discipline Construction Funds for Crop Science of Anhui Sciences and Technology University (No.XKXJGF001), the Natural Science Foundation of Education Department of Anhui Province (2023AH051852), the Anhui Province International Joint Research Center of Forage Biobreeding (No.ANJIRCFB202303), and the Transformation of high-yielding and stress-resistant corn varieties and precision cultivation technology for high planting density (2024ZH017). The authors declare that this study received funding from the Collaborative Breeding and Development of New Ordinary Corn Varieties (Horizontal Project of Zhejiang Wuwangnong Seed Co., Ltd.). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1651563/full#supplementary-material
References
Ahmad, N., Xu, Y., Zang, F., Li, D., and Liu, Z. (2024). The evolutionary trajectories of specialized metabolites towards antiviral defense system in plants. Mol. Horticulture 4, 2. doi: 10.1186/s43897-023-00078-9
Al-Khayri, J. M., Rashmi, R., Toppo, V., Chole, P. B., Banadka, A., Sudheer, W. N., et al. (2023). Plant secondary metabolites: The weapons for biotic stress management. Metabolites 13, 716. doi: 10.3390/metabo13060716
Amorim-Silva, V. and Botella, M. A. (2025). The whole is not always the sum of the parts: Synergistic plant responses to combined environmental stresses. Plant Cell Environ. 48, 6336. doi: 10.1111/pce.15616
Bailey, T. L., Johnson, J., Grant, C. E., and Noble, W. S. (2015). The MEME suite. Nucleic Acids Res. 43, W39–W49. doi: 10.1093/nar/gkv416
Bateman, A., Martin, M.-J., Orchard, S., Magrane, M., Agivetova, R., Ahmad, S., et al. (2021). UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 49, D480–D489. doi: 10.1093/nar/gkaa1100
Batool, R., Umer, M. J., Zhang, Y., Guo, J., and Wang, Z. (2024). Phytol-induced interplant signaling in maize facilitates EXP-A20-driven resistance through ACO31-dependent ethylene accumulation against Ostrinia furnacalis. Plant J. 121, e17186. doi: 10.1111/tpj.17186
Bebber, D. P. (2021). Global warming and China’s crop pests. Nat. Food 3, 6–7. doi: 10.1038/s43016-021-00427-1
Berkman, S. J., Roscoe, E. M., and Bourret, J. C. (2018). Comparing self-directed methods for training staff to create graphs using Graphpad Prism. J. Appl. Behav. Anal. 52, 188–204. doi: 10.1002/jaba.522
Bi, Z., Li, H., Liang, Y., Sun, D., Liu, S., Chen, W., et al. (2025). Emerging paradigms for target discovery of traditional medicines: A genome-wide pan-GPCR perspective. Innovation 6, 100774. doi: 10.1016/j.xinn.2024.100774
Chen, C., Wu, Y., Li, J., Wang, X., Zeng, Z., Xu, J., et al. (2023). TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 16, 1733–1742. doi: 10.1016/j.molp.2023.09.010
Cochrane, F. C., Davin, L. B., and Lewis, N. G. (2004). The Arabidopsis phenylalanine ammonia lyase gene family: kinetic characterization of the four PAL isoforms. Phytochemistry 65, 1557–1564. doi: 10.1016/j.phytochem.2004.05.006
Crozier, A., Clifford, M. N., and Ashihara, H. (2006). “Plant secondary metabolites,” in Occurrence, Structure Role in the Human Diet (Oxford, UK: Blackwell Publishers).
Dixon, R. A., Achnine, L., Kota, P., Liu, C. J., Reddy, M. S., and Wang, L. (2002). The phenylpropanoid pathway and plant defence—a genomics perspective. Mol. Plant Pathol. 3, 371–390. doi: 10.1046/j.1364-3703.2002.00131.x
Dong, X., Li, W., Li, C., Akan, O. D., Liao, C., Cao, J., et al. (2024). Integrated transcriptomics and metabolomics revealed the mechanism of catechin biosynthesis in response to lead stress in tung tree (Vernicia fordii). Sci. Total Environ. 930, 172796. doi: 10.1016/j.scitotenv.2024.172796
Dong, N. Q. and Lin, H. X. (2021). Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 63, 180–209. doi: 10.1111/jipb.13054
Dong, C.-J. and Shang, Q.-M. (2013). Genome-wide characterization of phenylalanine ammonia-lyase gene family in watermelon (Citrullus lanatus). Planta 238, 35–49. doi: 10.1007/s00425-013-1869-1
Elkind, Y., Edwards, R., Mavandad, M., Hedrick, S. A., Ribak, O., Dixon, R. A., et al. (1990). Abnormal plant development and down-regulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene. Proc. Natl. Acad. Sci. 87, 9057–9061. doi: 10.1073/pnas.87.22.9057
Emiliani, G., Fondi, M., Fani, R., and Gribaldo, S. (2009). A horizontal gene transfer at the origin of phenylpropanoid metabolism: a key adaptation of plants to land. Biol. Direct 4, 1–12. doi: 10.1186/1745-6150-4-7
Feduraev, P., Skrypnik, L., Riabova, A., Pungin, A., Tokupova, E., Maslennikov, P., et al. (2020). Phenylalanine and tyrosine as exogenous precursors of wheat (Triticum aestivum L.) secondary metabolism through PAL-associated pathways. Plants 9, 476. doi: 10.3390/plants9040476
Funk, M. and Zahn, L. M. (2021). An a-maize-ing set of genomes. Science 373, 637.1–63637. doi: 10.1126/science.373.6555.637-a
Golicz, A. A., Bayer, P. E., Barker, G. C., Edger, P. P., Kim, H., Martinez, P. A., et al. (2016). The pangenome of an agronomically important crop plant Brassica oleracea. Nat. Commun. 7, 13390. doi: 10.1038/ncomms13390
Goodstein, D. M., Shu, S., Howson, R., Neupane, R., Hayes, R. D., Fazo, J., et al. (2012). Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40, D1178–D1186. doi: 10.1093/nar/gkr944
Gupta, S. and Roy, A. (2021). “Deciphering the role of phytoanticipins, phytoalexins, and polyphenols in plant-insect defense,” in Plant-Pest Interactions: From Molecular Mechanisms to Chemical Ecology: Chemical Ecology. Singapore: Springer Singapore, 305–335.
Han, H., Zhang, L., Li, S., Zhao, R., Wang, F., Zhang, N., et al. (2025). Transcriptome analysis reveals the involvement of phenylpropane metabolic pathway in cold tolerance of cinnamomun bodinieri. Russian J. Plant Physiol. 72, 24. doi: 10.1134/S1021443724609157
He, J., Liu, Y., Yuan, D., Duan, M., Liu, Y., Shen, Z., et al. (2019). An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. 117, 271–277. doi: 10.1073/pnas.1902771116
Hirsch, C. N., Foerster, J. M., Johnson, J. M., Sekhon, R. S., Muttoni, G., Vaillancourt, B., et al. (2014). Insights into the maize pan-genome and pan-transcriptome. Plant Cell 26, 121–135. doi: 10.1105/tpc.113.119982
Hu, Z., Zhong, X., Zhang, H., Luo, X., Wang, Y., Wang, Y., et al. (2022). GhMYB18 confers Aphis gossypii Glover resistance through regulating the synthesis of salicylic acid and flavonoids in cotton plants. Plant Cell Rep. 42, 355–369. doi: 10.1007/s00299-022-02961-z
Hufford, M. B., Seetharam, A. S., Woodhouse, M. R., Chougule, K. M., Ou, S., Liu, J., et al. (2021). De novo assembly, annotation, and comparative analysis of 26 diverse maize genomes. Science 373, 655–662. doi: 10.1126/science.abg5289
Iohannes, S. D. and Jackson, D. (2023). Tackling redundancy: genetic mechanisms underlying paralog compensation in plants. New Phytol. 240, 1381–1389. doi: 10.1111/nph.19267
Jiang, H., Shi, Y., Liu, J., Li, Z., Fu, D., Wu, S., et al. (2022). Natural polymorphism of ZmICE1 contributes to amino acid metabolism that impacts cold tolerance in maize. Nat. Plants 8, 1176–1190. doi: 10.1038/s41477-022-01254-3
Jiménez-Galindo, J. C., Malvar, R. A., Butrón, A., Santiago, R., Samayoa, L. F., Caicedo, M., et al. (2019). Mapping of resistance to corn borers in a MAGIC population of maize. BMC Plant Biol. 19, 431. doi: 10.1186/s12870-019-2052-z
Kalakoti, Y., Sanjeev, A., and Wallner, B. (2025). Prediction of structural variation. Curr. Opin. Struct. Biol. 91, 103003. doi: 10.1016/j.sbi.2025.103003
Kawatra, A., Dhankhar, R., Mohanty, A., and Gulati, P. (2020). Biomedical applications of microbial phenylalanine ammonia lyase: Current status and future prospects. Biochimie 177, 142–152. doi: 10.1016/j.biochi.2020.08.009
Kong, J.-Q. (2015). Phenylalanine ammonia-lyase, a key component used for phenylpropanoids production by metabolic engineering. RSC Adv. 5, 62587–62603. doi: 10.1039/C5RA08196C
Koul, O. (2008). Phytochemicals and insect control: an antifeedant approach. Crit. Rev. Plant Sci. 27, 1–24. doi: 10.1080/07352680802053908
Kumar, H. (2002). Plant damage and grain yield reduction by fall armyworm and stem borers on certain maize hybrids containing resistance genes from varying sources under experimental and farmers field conditions. Crop Prot. 21, 563–573. doi: 10.1016/S0261-2194(01)00146-6
Kumar, S., Korra, T., Thakur, R., Arutselvan, R., Kashyap, A. S., Nehela, Y., et al. (2023). Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 8, 100154. doi: 10.1016/j.stress.2023.100154
Lavhale, S. G., Kalunke, R. M., and Giri, A. P. (2018). Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants. Planta 248, 1063–1078. doi: 10.1007/s00425-018-2965-z
Letunic, I., Khedkar, S., and Bork, P. (2021). SMART: recent updates, new developments and status in 2020. Nucleic Acids Res. 49, D458–D460. doi: 10.1093/nar/gkaa937
Levy, H. L., Sarkissian, C. N., and Scriver, C. R. (2018). Phenylalanine ammonia lyase (PAL): From discovery to enzyme substitution therapy for phenylketonuria. Mol. Genet. Metab. 124, 223–229. doi: 10.1016/j.ymgme.2018.06.002
Li, Y., Cheah, B. H., Fang, Y.-F., Kuang, Y.-H., Lin, S.-C., Liao, C.-T., et al. (2021). Transcriptomics identifies key defense mechanisms in rice resistant to both leaf-feeding and phloem feeding herbivores. BMC Plant Biol. 21, 1–18. doi: 10.1186/s12870-021-03068-5
Li, N., Han, X., Feng, D., Yuan, D., and Huang, L.-J. (2019). Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: do we understand what they are whispering? Int. J. Mol. Sci. 20, 671. doi: 10.3390/ijms20030671
Li, G., Song, C., Manzoor, M. A., Li, D., Cao, Y., and Cai, Y. (2023a). Functional and kinetics of two efficient phenylalanine ammonia lyase from Pyrus bretschneideri. BMC Plant Biol. 23, 612. doi: 10.1186/s12870-023-04586-0
Li, L.-L., Xiao, Y., Wang, X., He, Z.-H., Lv, Y.-W., Hu, X.-S., et al. (2023b). The Ka/Ks and πa/πs ratios under different models of gametophytic and sporophytic selection. Genome Biol. Evol. 15, evad151. doi: 10.1093/gbe/evad151
Lin, Y.-C., Mayer, M., Valle Torres, D., Pook, T., Hölker, A. C., Presterl, T., et al. (2024). Genomic prediction within and across maize landrace derived populations using haplotypes. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1351466
Liu, C., He, S., Chen, J., Wang, M., Li, Z., Wei, L., et al. (2023a). A dual-subcellular localized β-glucosidase confers pathogen and insect resistance without a yield penalty in maize. Plant Biotechnol. J. 22, 1017–1032. doi: 10.1111/pbi.14242
Liu, J., Li, L., Robert, C. A. M., Li, B., He, S., Xiong, Z., et al. (2022). A reductase in the lipid metabolism at cross roads between cuticular wax production and jasmonic acid-mediated defenses in maize. bioRxiv - Plant Biol. 2022-08. doi: 10.1101/2022.08.10.503514
Liu, S., Zenda, T., Tian, Z., and Huang, Z. (2023b). Metabolic pathways engineering for drought or/and heat tolerance in cereals. Front. Plant Sci. 14, 1111875. doi: 10.3389/fpls.2023.1111875
Liu, X., Zhang, M., Zhao, X., Shen, M., Feng, R., and Wei, Q. (2025). The evolution, variation and expression patterns of the annexin gene family in the maize pan-genome. Sci. Rep. 15, 5711. doi: 10.1038/s41598-025-89119-5
Livak, K. J. and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lu, C., Yu, Z., Tian, H., Hennessy, D. A., Feng, H., Al-Kaisi, M., et al. (2018). Increasing carbon footprint of grain crop production in the US western corn belt. Environ. Res. Lett. 13, 124007. doi: 10.1088/1748-9326/aae9fe
Lu, J., Zhuang, M., Long, J., Jiang, X., Zhang, Y., Ren, M., et al. (2024). Linking life table and consumption rate of the fall armyworm, Spodoptera frugiperda reared on different maize cultivars. Entomologia Generalis 44, 961–969. doi: 10.1127/entomologia/2024/2492
Lv, M., Kong, H., Liu, H., Lu, Y., Zhang, C., Liu, J., et al. (2016). Induction of phenylalanine ammonia-lyase (PAL) in insect damaged and neighboring undamaged cotton and maize seedlings. Int. J. Pest Manage. 63, 166–171. doi: 10.1080/09670874.2016.1255804
MacDonald, M. J. and D’Cunha, G. B. (2007). A modern view of phenylalanine ammonia lyase. Biochem. Cell Biol. 85, 273–282. doi: 10.1139/O07-018
Man, Q.-c., Wang, Y.-q., Gao, S.-j., Gao, Z.-c., Peng, Z.-p., and Cui, J.-h. (2025). Pan-genome analysis and expression verification of the maize ARF gene family. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1506853
Matova, P. M., Kamutando, C. N., Magorokosho, C., Kutywayo, D., Gutsa, F., and Labuschagne, M. (2020). Fall-armyworm invasion, control practices and resistance breeding in Sub-Saharan Africa. Crop Sci. 60, 2951–2970. doi: 10.1002/csc2.20317
Ni, X., Da, K., Buntin, G. D., and Brown, S. L. (2008a). Physiological basis of fall armyworm (Lepidoptera: Noctuidae) resistance in seedlings of maize inbred lines with varying levels of silk maysin. Florida Entomologist 91, 537–545. doi: 10.1603/0022-0493(2008)101[1455:iomeia]2.0.co;2
Ni, Y., Jiang, H.-L., Lei, B., Li, J.-N., and Chai, Y.-R. (2008c). Molecular cloning, characterization and expression of two rapeseed (Brassica napus L.) cDNAs orthologous to Arabidopsis thaliana phenylalanine ammonia-lyase 1. Euphytica 159, 1–16. doi: 10.1007/s10681-007-9448-9
Ni, X., Krakowsky, M. D., Buntin, G. D., Rector, B. G., Guo, B., and Snook, M. E. (2008b). Identification of multiple ear-colonizing insect and disease resistance in CIMMYT maize inbred lines with varying levels of silk maysin. J. Economic Entomology 101, 1455–1465. doi: 10.1093/jee/101.4.1455
Pant, S. and Huang, Y. (2022). Genome-wide studies of PAL genes in sorghum and their responses to aphid infestation. Sci. Rep. 12, 22537. doi: 10.1038/s41598-022-25214-1
Paysan-Lafosse, T., Blum, M., Chuguransky, S., Grego, T., Pinto, B. L., Salazar, et al. (2023). InterPro in 2022. Nucleic Acids Res. 51, D418–D427. doi: 10.1093/nar/gkac993
Peterson, J. A., Ode, P. J., Oliveira-Hofman, C., and Harwood, J. D. (2016). Integration of plant defense traits with biological control of arthropod pests: challenges and opportunities. Front. Plant Sci. 7, 1794. doi: 10.3389/fpls.2016.01794
Prasanna, B. M., Bruce, A., Beyene, Y., Makumbi, D., Gowda, M., Asim, M., et al. (2022). Host plant resistance for fall armyworm management in maize: relevance, status and prospects in Africa and Asia. Theor. Appl. Genet. 135, 3897–3916. doi: 10.1007/s00122-022-04073-4
Pruitt, K. D., Tatusova, T., Brown, G. R., and Maglott, D. R. (2011). NCBI Reference Sequences (RefSeq): current status, new features and genome annotation policy. Nucleic Acids Res. 40, D130–D135. doi: 10.1093/nar/gkr1079
Qiu, M., Jiang, J., Jiang, W., Zhang, W., Jiang, Y., Xin, F., et al. (2024). The biosynthesis of L-phenylalanine-derived compounds by engineered microbes. Biotechnol. Adv. 77, 108448. doi: 10.1016/j.biotechadv.2024.108448
Ramaroson, M.-L., Koutouan, C., Helesbeux, J.-J., Le Clerc, V., Hamama, L., Geoffriau, E., et al. (2022). Role of phenylpropanoids and flavonoids in plant resistance to pests and diseases. Molecules 27, 8371. doi: 10.3390/molecules27238371
Ramzan, T., Shahbaz, M., Maqsood, M. F., Zulfiqar, U., Saman, R. U., Lili, N., et al. (2023). Phenylalanine supply alleviates the drought stress in mustard (Brassica campestris) by modulating plant growth, photosynthesis, and antioxidant defense system. Plant Physiol. Biochem. 201, 107828. doi: 10.1016/j.plaphy.2023.107828
Rhodes, M. J. and Wooltorton, L. S. (1976). The enzymic conversion of hydroxycinnamic acids to p-coumarylquinic and chlorogenic acids in tomato fruits. Phytochemistry 15, 947–951. doi: 10.1016/S0031-9422(00)84376-9
Rojanaridpiched, C., Gracen, V., Everett, H., Coors, J., Pugh, B., and Bouthyette, P. (1984). Multiple factor resistance in maize to European corn borer. Maydica 29, 305–315.
Rombauts, S., Dehais, P., Van Montagu, M., and Rouze, P. (1999). PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 27, 295–296. doi: 10.1093/nar/27.1.295
Salam, U., Ullah, S., Tang, Z.-H., Elateeq, A. A., Khan, Y., Khan, J., et al. (2023). Plant metabolomics: an overview of the role of primary and secondary metabolites against different environmental stress factors. Life 13, 706. doi: 10.3390/life13030706
Schnable, P. S., Ware, D., Fulton, R. S., Stein, J. C., Wei, F., Pasternak, S., et al. (2009). The B73 maize genome: complexity, diversity, and dynamics. Science 326, 1112–1115. doi: 10.1126/science.1178534
Silva-Arias, G. A., Gagnon, E., Hembrom, S., Fastner, A., Khan, M. R., Stam, R., et al. (2024). Patterns of presence–absence variation of NLRs across populations of Solanum Chilense are clade-dependent and mainly shaped by past demographic history. New Phytol. 245, 1718–1732. doi: 10.1111/nph.20293
Singer, A. C., Crowley, D. E., and Thompson, I. P. (2003). Secondary plant metabolites in phytoremediation and biotransformation. Trends Biotechnol. 21, 123–130. doi: 10.1016/S0167-7799(02)00041-0
Singh, S., Kaur, I., and Kariyat, R. (2021). The multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 22, 1442. doi: 10.3390/ijms22031442
Sørensen, C. K., Thach, T., and Hovmøller, M. S. (2016). Evaluation of spray and point inoculation methods for the phenotyping of Puccinia striiformis on wheat. Plant Dis. 100, 1064–1070. doi: 10.1094/PDIS-12-15-1477-RE
Subramaniam, R., Reinold, S., Molitor, E. K., and Douglas, C. (1993). Structure, inheritance, and expression of hybrid poplar (Populus trichocarpaxPopulus deltoides) phenylalanine ammonia-lyase genes. Plant Physiol. 102, 71–83. doi: 10.1104/pp.102.1.71
Subramanian, B., Gao, S., Lercher, M. J., Hu, S., and Chen, W.-H. (2019). Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 47, W270–W275. doi: 10.1093/nar/gkz357
Sun, Y., Luo, M., Ge, W., Zhou, X., Zhou, Q., Wei, B., et al. (2022). Phenylpropanoid metabolism in relation to peel browning development of cold-stored ‘Nanguo’ pears. Plant Sci. 322, 111363. doi: 10.1016/j.plantsci.2022.111363
Sun, Y., Xiao, W., Wang, Q.-n., Wang, J., Kong, X.-d., Ma, W.-h., et al. (2023). Multiple variation patterns of terpene synthases in 26 maize genomes. BMC Genomics 24, 46. doi: 10.1186/s12864-023-09137-3
Tang, L. (2020). Single-cell structural variations. Nat. Methods 17, 252–252. doi: 10.1038/s41592-020-0787-y
Tang, Y., Guo, J., Zhang, T., Bai, S., He, K., and Wang, Z. (2021). Genome-wide analysis of WRKY gene family and the dynamic responses of key WRKY genes involved in Ostrinia furnacalis attack in Zea mays. Int. J. Mol. Sci. 22, 13045. doi: 10.3390/ijms222313045
Tong, C., Jia, Y., Hu, H., Zeng, Z., Chapman, B., and Li, C. (2025). Pangenome and pantranscriptome as the new reference for gene-family characterization: A case study of basic helix-loop-helix (bHLH) genes in barley. Plant Commun. 6, 101190. doi: 10.1016/j.xplc.2024.101190
Tzin, V., Hojo, Y., Strickler, S. R., Bartsch, L. J., Archer, C. M., Ahern, K. R., et al. (2017). Rapid defense responses in maize leaves induced by Spodoptera exigua caterpillar feeding. J. Exp. Bot. 68, 4709–4723. doi: 10.1093/jxb/erx274
Wang, L., Li, J., Liu, L., Dong, R., Liu, G., Rao, I. M., et al. (2025). Phenylalanine ammonia-lyase 2 regulates secondary metabolism and confers manganese tolerance in Stylosanthes guianensis. Plant Physiol. 197, kiaf005. doi: 10.1093/plphys/kiaf005
War, A. R., Taggar, G. K., Hussain, B., Taggar, M. S., Nair, R. M., and Sharma, H. C. (2018). Plant defence against herbivory and insect adaptations. AoB Plants 10, ply037. doi: 10.1093/aobpla/ply037
Williams, P. W., Davis, F. M., Buckley, P. M., Hedin, P. A., Baker, G. T., and Luthe, D. S. (1998). Factors associated with resistance to fall armyworm (Lepidoptera: Noctuidae) and southwestern corn borer (Lepidoptera: Crambidae) in corn at different vegetative stages. J. Economic Entomology 91, 1471–1480. doi: 10.1093/jee/91.6.1471
Wu, D.-G., Zhan, Q.-W., Yu, H.-B., Huang, B.-H., Cheng, X.-X., Li, W.-Y., et al. (2020). Genome-wide identification and analysis of maize pal gene family and its expression profile in response to high-temperature stress. Pak. J. Bot. 52, 1577–1587. doi: 10.30848/PJB2020-5(28)
Wuyts, N., De Waele, D., and Swennen, R. (2006). Activity of phenylalanine ammonia-lyase, peroxidase and polyphenol oxidase in roots of banana (Musa acuminata AAA, cvs Grande Naine and Yangambi km5) before and after infection with Radopholus similis. Nematology 8, 201–209. doi: 10.1163/156854106777998674
Xu, F., Deng, G., Cheng, S., Zhang, W., Huang, X., Li, L., et al. (2012). Molecular cloning, characterization and expression of the phenylalanine ammonia-lyase gene from Juglans regia. Molecules 17, 7810–7823. doi: 10.3390/molecules17077810
Yan, F., Li, H., and Zhao, P. (2019). Genome-wide identification and transcriptional expression of the PAL gene family in common walnut (Juglans regia L.). Genes 10, 46. doi: 10.3390/genes10010046
Yan, J. and Tan, B. C. (2019). Maize biology: From functional genomics to breeding application. J. Integr. Plant Biol. 61, 654–657. doi: 10.1111/jipb.12819
Yang, Y.-X., J Ahammed, G., Wu, C., Fan, S.-y., and Zhou, Y.-H. (2015). Crosstalk among jasmonate, salicylate and ethylene signaling pathways in plant disease and immune responses. Curr. Protein Pept. Sci. 16, 450–461. doi: 10.2174/1389203716666150330141638
Yang, H., Yu, H., Wang, S., Huang, H., Ye, D., Zhang, X., et al. (2024). Comparative transcriptomics reveals the key pathways and genes of cadmium accumulation in the high cadmium-accumulating rice (Oryza Sativa L.) line. Environ. Int. 193, 109113. doi: 10.1016/j.envint.2024.109113
Ye, W., Bustos-Segura, C., Degen, T., Erb, M., and Turlings, T. C. J. (2022). Belowground and aboveground herbivory differentially affect the transcriptome in roots and shoots of maize. Plant Direct 6, e426. doi: 10.1002/pld3.426
Ye, J., Coulouris, G., Zaretskaya, I., Cutcutache, I., Rozen, S., and Madden, T. L. (2012). Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinf. 13, 134. doi: 10.1186/1471-2105-13-134
Yin, Z., Wei, X., Cao, Y., Dong, Z., Long, Y., and Wan, X. (2024). Regulatory balance between ear rot resistance and grain yield and their breeding applications in maize and other crops. J. Advanced Res. doi: 10.1016/j.jare.2024.10.024
Yu, X.-Z., Fan, W.-J., Lin, Y.-J., Zhang, F.-F., and Gupta, D. K. (2018). Differential expression of the PAL gene family in rice seedlings exposed to chromium by microarray analysis. Ecotoxicology 27, 325–335. doi: 10.1007/s10646-018-1897-5
Zeng, J., Liu, Y., Zhang, H., Liu, J., Jiang, Y., Wyckhuys, K. A. G., et al. (2020). Global warming modifies long-distance migration of an agricultural insect pest. J. Pest Sci. 93, 569–581. doi: 10.1007/s10340-019-01187-5
Zhang, H., Huang, Q., Yi, L., Song, X., Li, L., Deng, G., et al. (2021). PAL-mediated SA biosynthesis pathway contributes to nematode resistance in wheat. Plant J. 107, 698–712. doi: 10.1111/tpj.15316
Zhang, H., Zhang, X., Zhao, H., Hu, J., Wang, Z., Yang, G., et al. (2023). Genome-wide identification and expression analysis of phenylalanine ammonia-lyase (PAL) family in rapeseed (Brassica napus L.). BMC Plant Biol. 23, 481. doi: 10.1186/s12870-023-04472-9
Zhao, M., Huang, S., Zhang, Q., Wei, Y., Tao, Z., Wang, C., et al. (2024). The plant terpenes DMNT and TMTT function as signaling compounds that attract Asian corn borer (Ostrinia furnacalis) to maize plants. J. Integr. Plant Biol. 66, 2528–2542. doi: 10.1111/jipb.13763
Zheng, J., Sun, R., Wu, D., Chen, P., and Zheng, P. (2024). Engineered Zea mays phenylalanine ammonia-lyase for improve the catalytic efficiency of biosynthesis trans-cinnamic acid and pcoumaric acid. Enzyme Microbial Technol. 176, 110423. doi: 10.1016/j.enzmictec.2024.110423
Zhou, Z., Duan, Y., Li, Y., Zhang, P., Li, Q., Yu, L., et al. (2025). CYP98A monooxygenases: a key enzyme family in plant phenolic compound biosynthesis. Horticulture Res. 12, uhaf074. doi: 10.1093/hr/uhaf074
Zhu, Z., Chen, R., and Zhang, L. (2024). Simple phenylpropanoids: recent advances in biological activities, biosynthetic pathways, and microbial production. Natural Product Rep. 41, 6–24. doi: 10.1039/D3NP00012E
Keywords: maize pan-genome, phenylalanine ammonia-lyase, structural variation, selection pressure, insect stress
Citation: Wang T, Zheng Y, Sun L, Guan M, Hu Y, Yu H, Wu D and Du J (2025) Analysis of maize PAL pan gene family and expression pattern under lepidopteran insect stress. Front. Plant Sci. 16:1651563. doi: 10.3389/fpls.2025.1651563
Received: 22 June 2025; Accepted: 18 August 2025;
Published: 01 September 2025.
Edited by:
Akshaya Kumar Biswal, The University of Georgia, United StatesReviewed by:
Dr. Richard Dormatey, CSIR Crops Research Istitute, GhanaShengli Jing, Xinyang Normal University, China
Copyright © 2025 Wang, Zheng, Sun, Guan, Hu, Yu, Wu and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tonghan Wang, MTg3Njc0MDE1MjFAMTYzLmNvbQ==; Degong Wu, d3VkZ0BhaHN0dS5lZHUuY24=; Junli Du, YWR1ODM0MTlAMTYzLmNvbQ==
†ORCID: Tonghan Wang, orcid.org/0009-0001-7927-6127
Degong Wu, orcid.org/0000-0002-8288-1184
 Tonghan Wang
Tonghan Wang Yaohui Zheng1,2
Yaohui Zheng1,2 Minghui Guan
Minghui Guan