- 1College of Agriculture and Biology, Liaocheng University, Liaocheng, China
- 2Mudanjiang Branch of Heilongjiang Academy of Agricultural Sciences, Mudanjiang, China
Introduction: Wall-associated receptor kinases (WAKs) are a family of receptor-like kinases (RLKs) that play important roles in the communication between the plant cell wall and the cytoplasm. WAKs have been identified in several plants. However, a comprehensive investigation of maize WAKs has not been performed yet.
Methods: In this study, the maize WAK gene family was identified through whole-genome scanning. y -30The physicochemical characteristics, chromosomal locations, phylogenetic tree, gene structures, conserved motifs, gene duplication, collinearity, and cis-acting elements of maize WAKs were analyzed.
Results: A total of 56 ZmWAKs were identified in the maize genome and divided into seven subgroups. Among these, 54 genes were successfully mapped to maize chromosomes. Gene duplication events were detected in 13 ZmWAKs, with nine segmental (SD) and two tandem duplication (TD) events. Maize WAKs exhibited zero, eight, 27, and 41 collinear links with the WAKs from Arabidopsis, soybean, rice, and sorghum, respectively. In the promoter regions of ZmWAKs, a total of 107 types of cis-acting elements were predicted. Among them, the functions of 82 elements are known. These elements are associated with plant growth and development and light, hormones, stress, and defense responses. The transcriptome data analysis showed that ZmWAKs displayed tissue-specific expression and are involved in the responses to various abiotic and biotic stresses, including cold, salt, drought, waterlogging, pathogens, and pests. ZmWAK9, ZmWAK15, ZmWAK27, ZmWAK41, and ZmWAK49 are significantly induced by multiple stress conditions, indicating their crucial roles in stress responses and potential value for further research.
Discussion: Our results provide insights into the function of maize WAKs in response to abiotic and biotic stresses and offer a theoretical foundation for understanding their mechanisms of action.
1 Introduction
Plant receptor-like kinases (RLKs) are a subgroup of serine/threonine (Ser/Thr) kinases and play a crucial role in plant growth, development, and stress responses (Shiu and Bleecker, 2001). The RLK family is a large protein family in plants. It is divided into different subfamilies based on the extracellular domains and the kinase domains, including cysteine-rich receptor-like kinases (CRKs), lysin motif receptor-like kinases (LysM-RLKs), cell wall-associated receptor kinases (WAKs), and leucine-rich receptor-like kinases (LRR-RLKs), etc. (Yu et al., 2025; Abedi et al., 2021; Zhang et al., 2025; Yang et al., 2017). WAKs are a unique group of RLKs, which are localized in the cell wall and identified as key cell wall integrity sensors (Yao et al., 2025). WAKs are involved in signal transduction between the extracellular matrix and the intracellular compartments (Kanneganti and Gupta, 2008; Decreux and Messiaen, 2005). These kinases have been demonstrated to play a crucial role in plant cell expansion and elongation as well as in mediating plant defense responses to both abiotic stresses and fungal diseases (Lally et al., 2001; Wang et al., 2019b; Zuo et al., 2015).
In addition, WAKs possess Ser/Thr kinase activity and typically contain the extracellular WAK galacturonan-binding (GUB_WAK_bind), epidermal growth factor-like (EGF-like), transmembrane, and Pkinase domains (He et al., 1999). WAKs span the plasma membrane and extend into the extracellular region to bind tightly to the cell wall, providing a physical and signaling continuum between the cell wall and the cytoplasm (He et al., 1999). In Arabidopsis, AtWAKs were expressed at organ junctions, in shoot and root apical meristems, and in developing leaves. Additionally, their expression was induced in response to perturbations in the cell wall. The antisense of AtWAKs resulted in reduced WAK levels and lead to a loss of cell expansion (Wagner and Kohorn, 2001). In rice, OsWAK12 was predominantly expressed in the roots. It positively regulated the growth and development of the rice root system by participating in auxin (IAA) signaling and modulating the expression of plasma membrane H+-ATPase-encoding genes, thereby influencing agronomic traits, including plant height and effective tillering (Hu, 2023). In cotton, 97% of GhWAKs were highly expressed in cotton fibers and ovules. Among them, 14 and 10 GhWAKs were found to possess putative gibberellin (GA) and IAA response elements in their promoter regions, respectively, and 13 and nine of these were significantly induced by GA and IAA treatment, respectively (Dou et al., 2021).
The WAK gene family plays a critical role in abiotic stress response. Previously, OsWAK112d had been identified as the candidate gene contributing to cold stress tolerance at the seedling stage of rice. Its expression is 200-fold higher in cold-tolerant plants than in cold-sensitive plants (Zhang et al., 2012). Furthermore, Arabidopsis thaliana WALL-ASSOCIATED KINASE LIKE10 (AtWAKL10) has been shown to positively regulate salt stress tolerance in Arabidopsis, as evidenced by a reduced germination rate in the loss-of-function mutant atwakl10 compared to the wild type under salt stress (Bot et al., 2019). In cotton, GhWAKL26 was significantly activated by salt stress, with the transgenic plants displaying significantly increased dry weight, fresh weight, and root length. Further analysis showed that GhWAKL26 primarily improves salt stress tolerance in cotton seedlings by regulating the Na+/K+ balance (Gao et al., 2024). Another study reported that GhWAK9, GhWAK12, GhWAKL46, and GhWAKL47 were markedly upregulated under drought stress and that the corresponding proteins primarily localized to the plasma membrane (Zhang et al., 2021). Under heavy metal stress, the Arabidopsis protein AtWAK1 was found to accumulate in the plasma membrane region, and its overexpression restored aluminum (Al)-stress-mediated root growth inhibition (Sivaguru et al., 2003). Rice OsWAK124 had been shown to localize to the cell wall, with overexpression lines exhibiting enhanced tolerance to heavy metals Al3+, Cu2+, and Cd2+ (Yin and Hou, 2017).
The WAK gene family also plays essential roles in biotic stress responses. In rice, OsWAK91 is located within a major sheath blight disease (SB)-resistant quantitative trait locus (QTL) region on chromosome 9. Its resistant allele resulted in the loss of a stop codon, conferring resistance to SB in rice (Al-Bader et al., 2019). In maize, ZmWAK has been reported to activate ZmSnRK1 after infection by the pathogen Sporisorium reilianum (Zhang et al., 2024). ZmSnRK1 participates in gene transcription, resource allocation, energy metabolism, and programmed cell death (PCD), preventing pathogens from penetrating into the shoot apical meristem (SAM), thereby triggering smut disease resistance in plants (Zhang, 2016). In wheat, at least 30 TaWAKs were involved in the responses to Fusarium infection, with most of these genes contributing to pectin- and chitin-induced defense pathways. Furthermore, 45 TaWAKs has been detected within the resistance QTL regions of Fusarium head blight (FHB) disease (Xia et al., 2022).
With the advancement and extensive application of genome sequencing technologies, a substantial volume of plant genomic sequencing data has been released. Accordingly, genome-wide identification of gene families has become a crucial approach for functional gene discovery in plants (Bai et al., 2024; Wang et al., 2024a; Sun et al., 2023; Wang et al., 2024b). In previous studies, the functions of some maize WAK/WAKL genes have been investigated. For example, ZmWAKL38, ZmWAKL42, and ZmWAKL52 were highly expressed in maize kernels (Hu et al., 2023). ZmWAK02, ZMWAK17, and ZmWAK-RLK1 mediated resistance to maize gray leaf spot, stalk rot, and northern corn leaf blight, respectively (Dai et al., 2024; Zuo et al., 2022; Hurni et al., 2015). However, there is still a lack of a thorough examination of the physicochemical characteristics, evolutionary relationships, and transcriptome landscapes of the maize WAK gene family. In this study, the maize WAK gene family was systematically identified based on the maize B73_V5 genome, and extensive transcriptome data were utilized to explore the expression patterns of these genes in various tissues and under different stress conditions. Our findings might prove valuable for further elucidating the functional roles of maize WAKs.
2 Materials and methods
2.1 Identification of the WAK gene family in maize
The maize genome sequence (Zm-B73-REFERENCE-NAM-5.0) was downloaded from Ensembl Plants (https://plants.ensembl.org/index.html). The hidden Markov model (HMM) files of the WAK gene family were obtained from the InterPro database (https://www.ebi.ac.uk/interpro/). They included the typical domains GUB_WAK_bind (PF13947), EGF (PF07645), and Pkinase (PF00069). The typical domains were used as query files to identify the WAKs in the maize genome using the Advanced HMMER search tool in the TBtools-II software. The screening threshold value (E) was set to e−3. Subsequently, secondary identification of the WAK gene family was conducted using the InterPro (https://www.ebi.ac.uk/interpro/), SMART (http://smart.embl-heidelberg.de/), and NCBI CDD (https://www.ncbi.nlm.nih.gov/cdd) databases. Finally, 56 WAK genes were confirmed in the maize genome. The lengths and locations of these genes were obtained from the genome annotation files, and gene distribution on chromosomes was visualized using the MapChart2.32 software.
2.2 Physicochemical characteristics and phylogenetic analysis of the WAK gene family in maize
The physicochemical characteristics of the maize WAK gene family were assessed using the protein parameter Calc tool in the TBtools-II software. These characteristics included molecular weight (MW), isoelectric point (pI), instability coefficient, aliphatic index, and grand average of hydropathy (GRAVY). The subcellular localization of maize WAKs was predicted via the DeepLoc 2.1 website (https://services.healthtech.dtu.dk/services/DeepLoc-2.1/) and was visualized by the HeatMap tool in the TBtools-II software (Chen et al., 2023).
The Arabidopsis WAK protein sequence was obtained from the TAIR database (https://www.arabidopsis.org/). A multiple sequence alignment of the WAKs for maize and Arabidopsis was performed using the MUSCLE software. A phylogenetic tree was developed via the MEGA 11 software using the following parameters: Neighbor-joining (NJ) method, Poisson model, complete deletion, and 1000 bootstrap replications. The phylogenetic tree was constructed in the FigTree v1.4.4 software and optimized in EvolView v2 (He et al., 2016).
2.3 Gene structure and conserved motif analysis of the WAK gene family in maize
The structural analysis of the maize WAK gene family members was conducted using the GXF Stat tool in TBtools-II. The conserved motif analysis was performed using the Simple MEME Wrapper tool in TBtools-II. The parameters were set as follows: The maximum number of motifs was 10, the motif length was 6–50 amino acids, and the maximum value of E was 1×e−10. Finally, the phylogenetic tree, gene structure, and conserved motifs for the maize WAK gene family were visualized in the Gene Structure View tool of TBtools-II (Chen et al., 2023).
2.4 Gene duplication and collinearity analysis of the WAK gene family in maize
With the One Step MCScanX tool of TBtools-II (Chen et al., 2023), the gene duplication events and collinearity of the WAK gene family were analyzed across maize and other species, including dicotyledonous plants (Arabidopsis thaliana and Glycine max), and monocotyledonous plants (Sorghum bicolor and Oryza sativa). The duplication and collinear gene pairs of the WAK gene family were identified in the genomes of maize and other species, and the nonsynonymous (Ka) and synonymous substitution rates (Ks) of the collinear gene pairs were calculated using the Simple Ka/Ks Calculator tool in TBtools-II. The occurrence time of the gene duplication events was estimated by the equation T = (Ks/2λ) × 10−6, where λ = 6.161029 × 10−9 (Lynch and Conery, 2000). The visualization was performed using the Advanced Circos and Multiple Synteny Plot tools in TBtools-II.
2.5 Functional analysis of the WAK gene family in maize
The background file was downloaded from TBtools-II to analyze the function of the maize WAK gene family members. The maize protein sequences were uploaded to the egg-NOG-mapper website (http://eggnog6.embl.de/#/app/emapper) to obtain the annotation information. By inputting the above files into the GO Enrichment tool of Tbtools-II, the Gene Ontology (GO) enrichment analysis of maize WAKs was conducted. The enrichment result was displayed in a bubble plot format, which was drawn on the Weishengxin website (https://www.bioinformatics.com.cn/).
2.6 Expression analysis of maize WAKs in various tissues and under abiotic and biotic stresses
The maize transcriptome data were downloaded from the NCBI SRA database (https://www.ncbi.nlm.nih.gov/sra/), encompassing the various tissues (root, leaf, and seed), abiotic stresses (temperature, salt, drought, and waterlogging), and biotic stresses (smut, gray leaf spot, beet armyworm, and aphid) (Stelpflug et al., 2016; Li et al., 2020; Yu et al., 2020; Schurack et al., 2021; Yu et al., 2018; Tzin et al., 2015). With the Convert SRA to Fastq Files tool in TBtools-II, the transcriptome sequencing files were converted from the SRA format to the FASTQ format. The gene expression levels were calculated by aligning the FASTQ sequences to the maize B73_V5 genome in the Kallisto Super GUI Wrapper tool of TBtools-II. The calculation parameters were set as follows: Bias correction, kmer size was 31, BootStrap was 0, threadNum was 2, FragLen was 200, and LengthSD was 30. Finally, the gene expression patterns were visualized using the HeatMap tool in TBtools-II. The heatmaps of gene expression and expression changes under stress treatment were drawn based on normalized expression values, which were respectively calculated using the formulas log2(TPM + 1) and log2(Fold Change).
2.7 Expression analysis of maize WAKs under various hormonal treatments
In order to investigate the effects of hormones on the maize WAK gene family, the maize seedlings were treated with either indole-3-acetic acid (IAA), abscisic acid (ABA), or gibberellic acid (GA3). Briefly, the seeds of Zea mays cv. B73 were sown in commercial soil in the greenhouse, with a temperature of 28°C and a photoperiod of 16-h light/8-h dark. After 14 days of cultivation, the maize seedlings entered the three-leaf stage. The IAA, ABA, and GA3 were dissolved in 0.1% Tween-20 to prepare 100 µmol/L solutions of each. The three-leaf seedlings were treated with either of these three solutions. The controls were treated with 0.1% Tween-20. The leaves of seedlings were collected at 0, 3, 6, and 12 hours post-treatment. Each treatment was performed in three replicates, with three plants per replicate. All samples were flash-frozen in liquid nitrogen and subsequently stored at −80°C for further analysis.
RNA sequencing (RNA-seq) analysis was performed by the Gene Denovo company in Guangzhou. The total RNA of samples was extracted using the Trizol RNA extraction kit (Invitrogen, Carlsbad, CA, USA). The RNA quality was evaluated with the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The transcripts were randomly fragmented, generating a large number of fragments, approximately 200 nucleotides in length. The fragments were converted to cDNA, and the cDNA library was constructed using the NEBNext Ultra RNA Library Prep kit for Illumina (NEB#7530, New England Biolabs, Ipswich, MA, USA). RNA-seq was performed using the Illumina Novaseq 6000 system. Quality control of the RNA-seq data was performed using the fastp software (Chen et al., 2018). The clean reads were rapidly aligned to the reference genome with HISAT (Kin et al., 2015). Finally, the RNA-Seq data was quantified in the RSEM software, and the gene expression levels were expressed as fragments per kilobase of transcript per million mapped reads (FPKM) (Li and Dewey, 2011). The expression patterns of WAK gene family members were visualized in Graphpad Prism 9.5, and the significance analysis of these genes was performed using SPSS 12.
2.8 Roles of maize WAKs under abiotic and biotic stresses, and hormonal treatments
We selected genes with high expression levels (TPM >10) and the most significant variation under different stresses for further analysis (Supplementary Tables S11-S18). A total of 34 genes were selected, including 6 genes under temperature stress, 9 genes under salt stress, 6 genes under drought stress, 11 genes under waterlogging stress, 16 genes under smut stress, 18 genes under gray leaf spot stress, 9 genes under beet armyworm stress, 12 genes under aphid stress, and 6 genes under hormonal treatments (IAA, ABA, GA). Except for ZmWAK15, 22, 25, 34, and 48, the remaining genes were significantly induced by multiple stresses.
To elucidate the functions of maize WAK genes in abiotic and biotic stresses as well as hormonal treatments, we generated a heatmap depicting the expression patterns of 34 genes. For each gene, the expression value used in the heatmap was determined by the most significant change [Log2(Fold Change)] observed under the respective stresses. For instance, under temperature stress [encompassing the normal temperature (25°C), the low temperature (16°C), the extremely low temperature (4°C), the moderately low temperature (10°C), the high temperature (37°C), the moderately high temperature (42°C), the extremely high temperature (48°C)], the heatmap value for each gene was derived from the condition exhibiting the maximum expression variation. Finally, the gene expression patterns were visualized using the HeatMap tool in TBtools-II.
2.9 Construction of the maize WAK protein interaction network
To investigate the interaction among the maize WAK gene family members, the corresponding protein sequences were uploaded to the Orthovenn2 database (https://orthovenn2.bioinfotoolkits.net/home), and were aligned with the protein sequences of A. thaliana and rice for homology analysis (Wang et al., 2019a).
The maize WAK protein interaction analysis was conducted in the STRING website (https://cn.string-db.org/), with a credibility value of 0.4, and the number of interacting proteins was limited to within 20. The maize WAK protein interaction network was constructed using the Cytoscape3.8.2 software (Shannon et al., 2003).
3 Results
3.1 Identification of the WAK gene family in the maize genome
A total of 56 WAK genes were identified at the whole-genome level in maize. According to their locations on the chromosome, the genes were named ZmWAK1–ZmWAK56 (Supplementary Table S1). The polypeptides encoded by ZmWAKs contained 319–1016 amino acids, with the longest being ZmWAK29. The MW of the encoded proteins ranged from 35.20 kDa to 110.83 kDa. Furthermore, their theoretical pI ranged from 4.87 to 9.70, indicating a predominantly acidic nature. The instability index varied from 32.11 to 60.50, with 46.43% for unstable protein. The aliphatic index ranged between 69.85 and 94.22. Finally, the GRAVY values ranged from -0.504 to 0.061, with negative values for 91.07% WAKs, reflecting their hydrophilic nature. The subcellular localization predictions indicated that maize WAK proteins predominantly localized to the plasma membrane, chloroplast, and vacuole, with lesser function in the endoplasmic reticulum, cytosol, extracellular matrix, and nucleus (Figure 1).
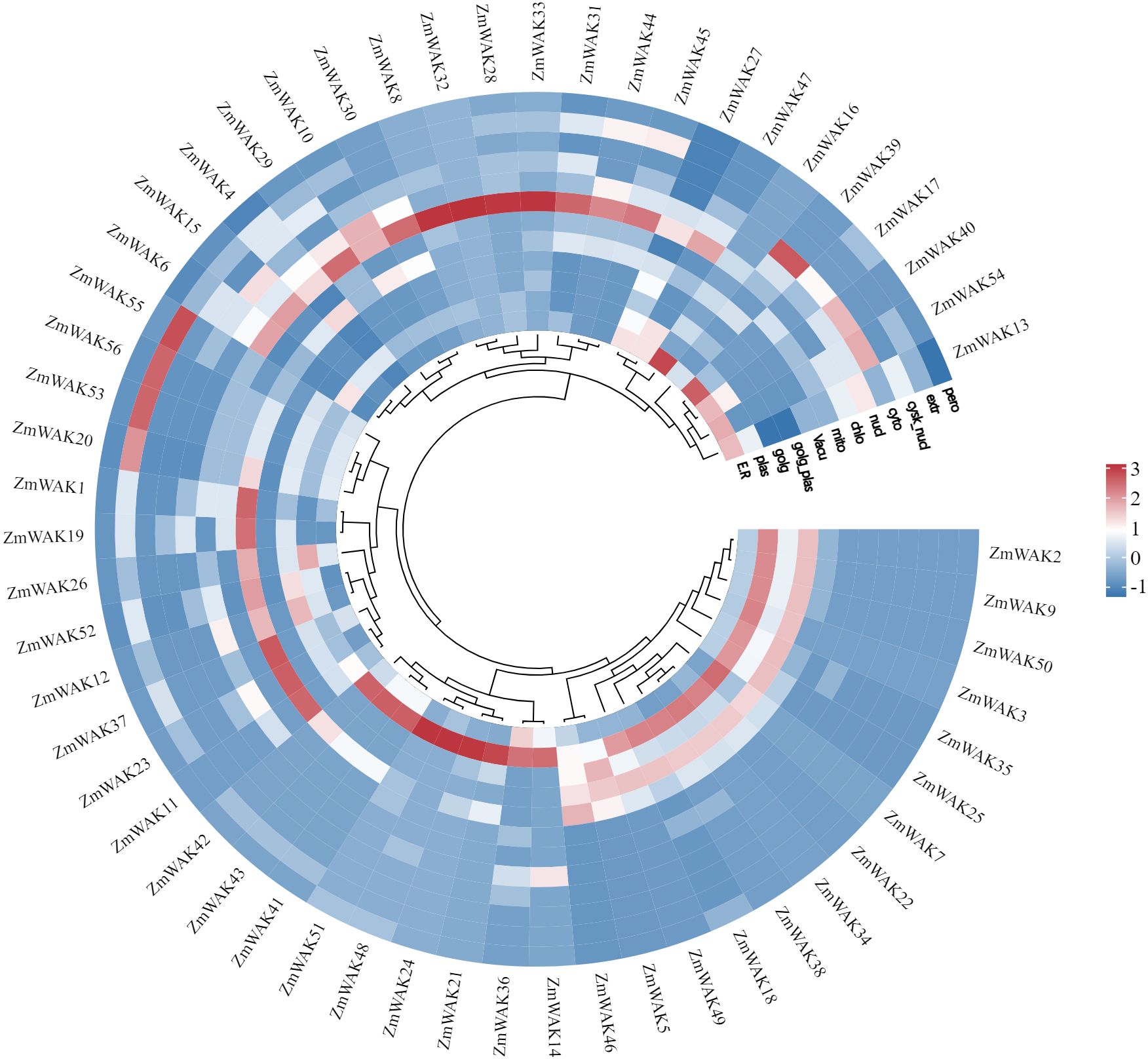
Figure 1. Heat map of the subcellular localization of ZmWAK genes in plants. E.R, Endoplasmic Reticulum; plas, plasma membrane; Golg, Golgi apparatus; Golg_plas, Golgi membrane; Vacu, vacuole; mito, mitochondria; chlo, chloroplast; nucl, nucleus; cyto, cytosol; cysk_nucl, nuclear cytoskeleton; extr, extracellular matrix; pero, peroxisome. The minimal functional presence of the gene is indicated by blue, and the maximum functional significance of the gene is represented by red.
3.2 Chromosomal location of ZmWAKs
The chromosomal location of maize WAK genes (Zm-B73-REFERENCE-NAM-5.0) was analyzed to understand the distribution of the maize WAK gene family across chromosomes (Supplementary Table S1, Figure 2). Among the 56 identified genes, 54 ZmWAKs were unevenly distributed on all maize chromosomes. Chromosome 8 contained the highest number of ZmWAKs (n = 12), followed by chromosomes 3, 2, 1, 4, 6, and 5 (n = 9, 8, 7, 6, 4, and 3). Chromosomes 7, 9, and 10 possessed the fewest ZmWAKs (n = 1 or 2). The WAKL proteins share similar structural features with the WAK proteins. In a previous study, a total of 58 ZmWAKL genes were identified in the maize genome of the Zm-B73-REFERENCE-GRAMENE-4.0 version, and were designated as ZmWAKL1 to ZmWAKL58 (Hu et al., 2023). The 58 ZmWAKL genes are unevenly distributed across the 10 chromosomes, with the highest number (15 genes) located on chromosome 8 and the lowest number (1 gene) on chromosome 7. The variation in the number of ZmWAK/WAKL genes may be due to the use of different genome versions during the identification process of the gene family.
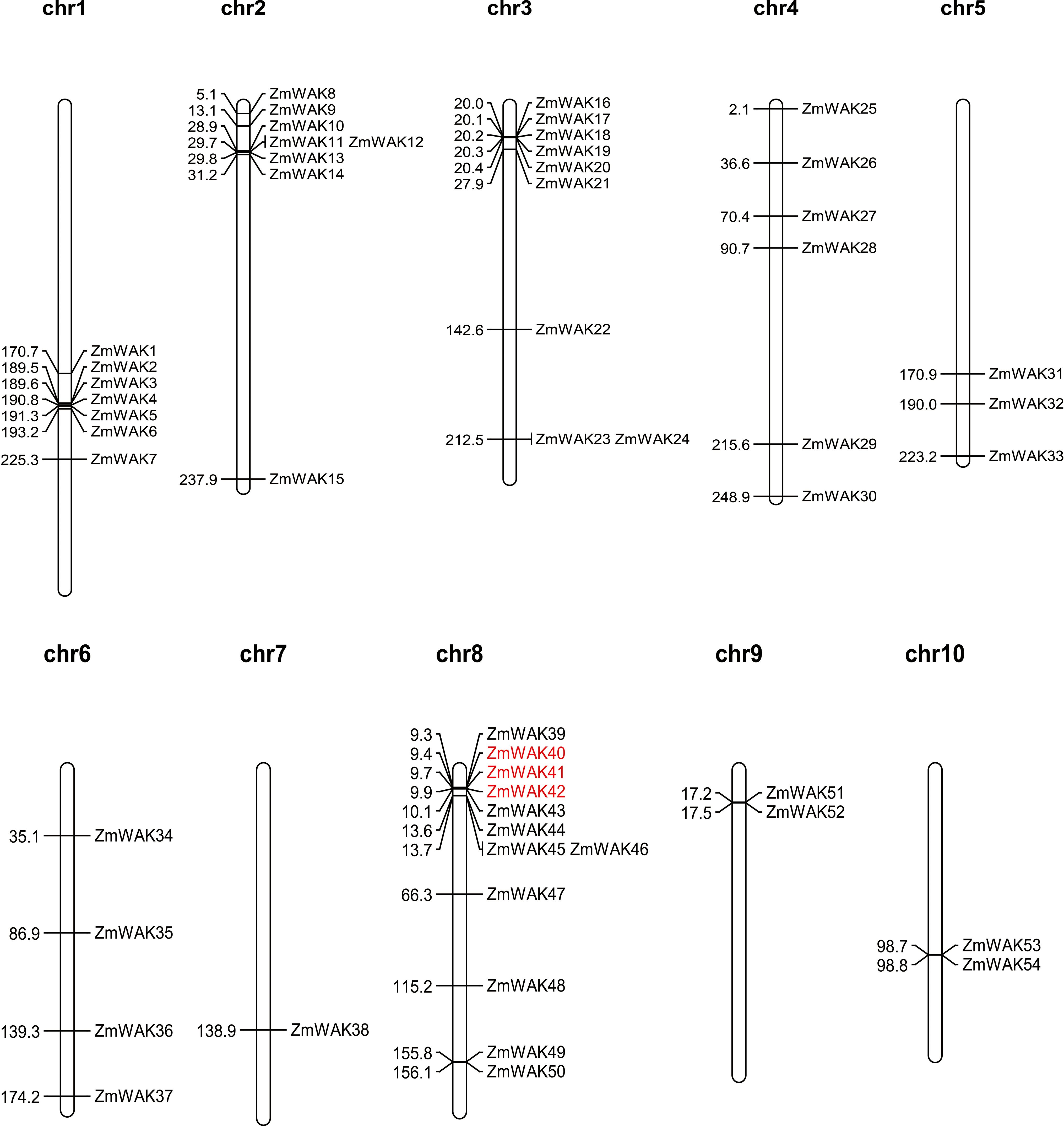
Figure 2. Distribution of ZmWAKs in maize genome. Red color represents these genes underwent tandem duplication.
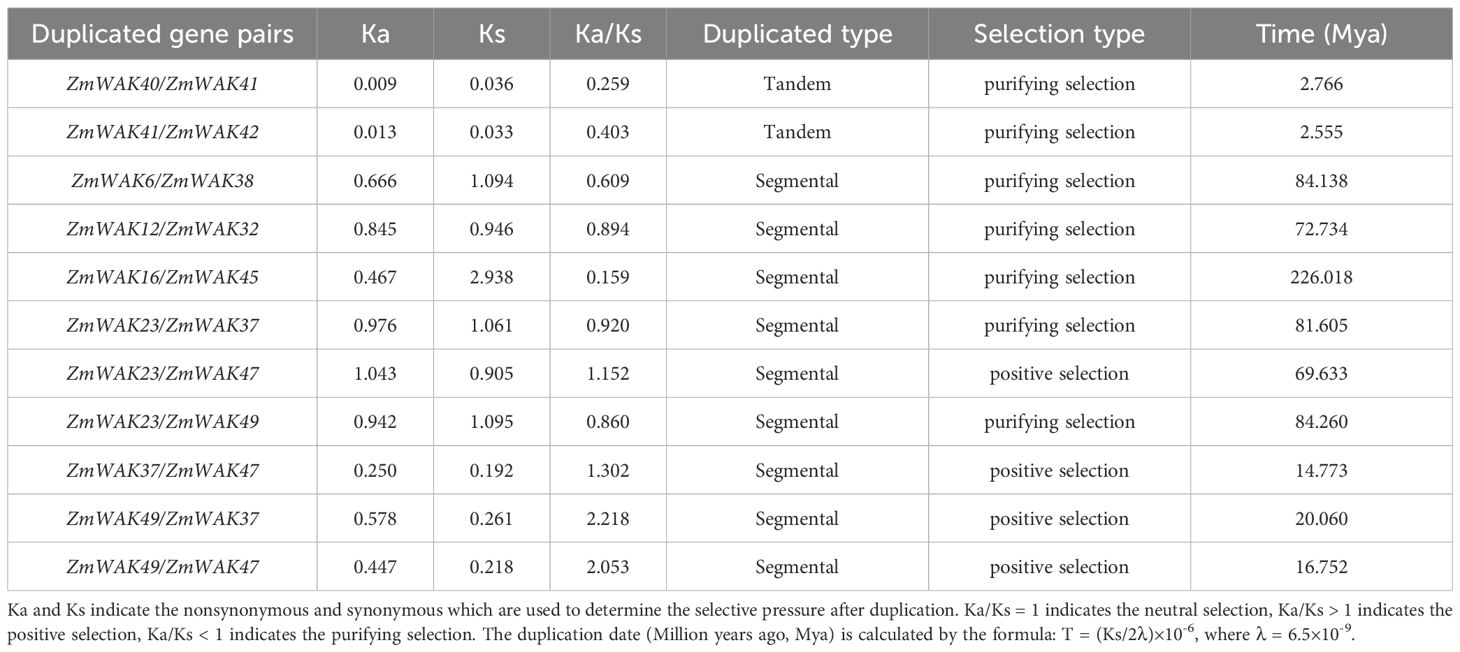
To obtain the evolutionary relationships among the ZmWAKs, we detected gene duplication events, including both segmental (SD) and tandem duplication (Table 1). Nine pairs of SD genes, including ZmWAK6/38, ZmWAK12/32, ZmWAK16/45, ZmWAK23/37, ZmWAK23/47, ZmWAK23/49, ZmWAK37/47, ZmWAK49/37, and ZmWAK49/47, and two pairs of TD genes, including ZmWAK40/41 and ZmWAK41/42 were identified in maize. ZmWAK40/41 and ZmWAK41/42 are two connected tandem duplication events, which together make these genes tandem triplicates. ZmWAK23, ZmWAK37, ZmWAK47, and ZmWAK49 exhibited 2–3 SD events, indicating these genes were the most active in the expansion of the maize WAK gene family. The SD and TD events occurred over 14.77–226.02 million and 2.55–2.76 million years ago, respectively. The Ka and Ks values of the 11 duplication gene pairs were calculated to estimate the selection pressure in gene differentiation. Four gene pairs exhibited Ka/Ks values greater than 1, suggesting that these gene pairs may have undergone positive selection. This indicates that the amino acid substitutions in these genes could potentially give rise to novel protein functions, thereby facilitating the adaptation of the organisms to environmental changes.
3.3 Phylogenetic analysis of the maize WAK gene family
In order to elucidate the phylogenetic relationships of ZmWAKs, a phylogenetic analysis was conducted with the WAK gene family members from Arabidopsis and maize. A total of nine subgroups were identified: I, II, III, IV, V, VI, VII, VIII, and IX (Figure 3). Among them, subgroup IX was the largest, comprising 28 ZmWAKs, followed by subgroup V, containing 13 ZmWAKs and four AtWAKs/WAKLs; subgroup VII, containing two ZmWAKs and seven AtWAKs/WAKLs; subgroup VI, containing nine AtWAKs/WAKLs; subgroup VIII, containing eight ZmWAKs; subgroup II, containing two ZmWAKs and two AtWAKs/WAKLs; subgroup IV, containing two ZmWAKs; subgroup III, containing one ZmWAK and one AtWAK/WAKL; and subgroup I, containing one AtWAK/WAKL. These data indicate that the expansion of the plant WAK gene family occurred unevenly across different species during evolution. In addition, subcellular localization reveals that 22 ZmWAK proteins are localized to the plasma membrane, predominantly in subgroups IV, V, VIII, and IX, which account for 50.00%, 53.84%, 37.50%, and 39.28% of the genes in these subgroups, respectively (Supplementary Table S1). In a previous study, 58 ZmWAKL genes in maize were identified three main clusters: Group I, Group II, and Group III, with 29, 19, and 10 genes, respectively (Hu et al., 2023). In this study, 56 ZmWAK genes were identified, of which 8, 13, and 28 were respectively assigned to subgroups V, VIII, and IX, with the remaining 7 genes allocated to other subgroups. This difference in grouping may result from the use of both Arabidopsis and maize WAK/WAKL genes in this study.
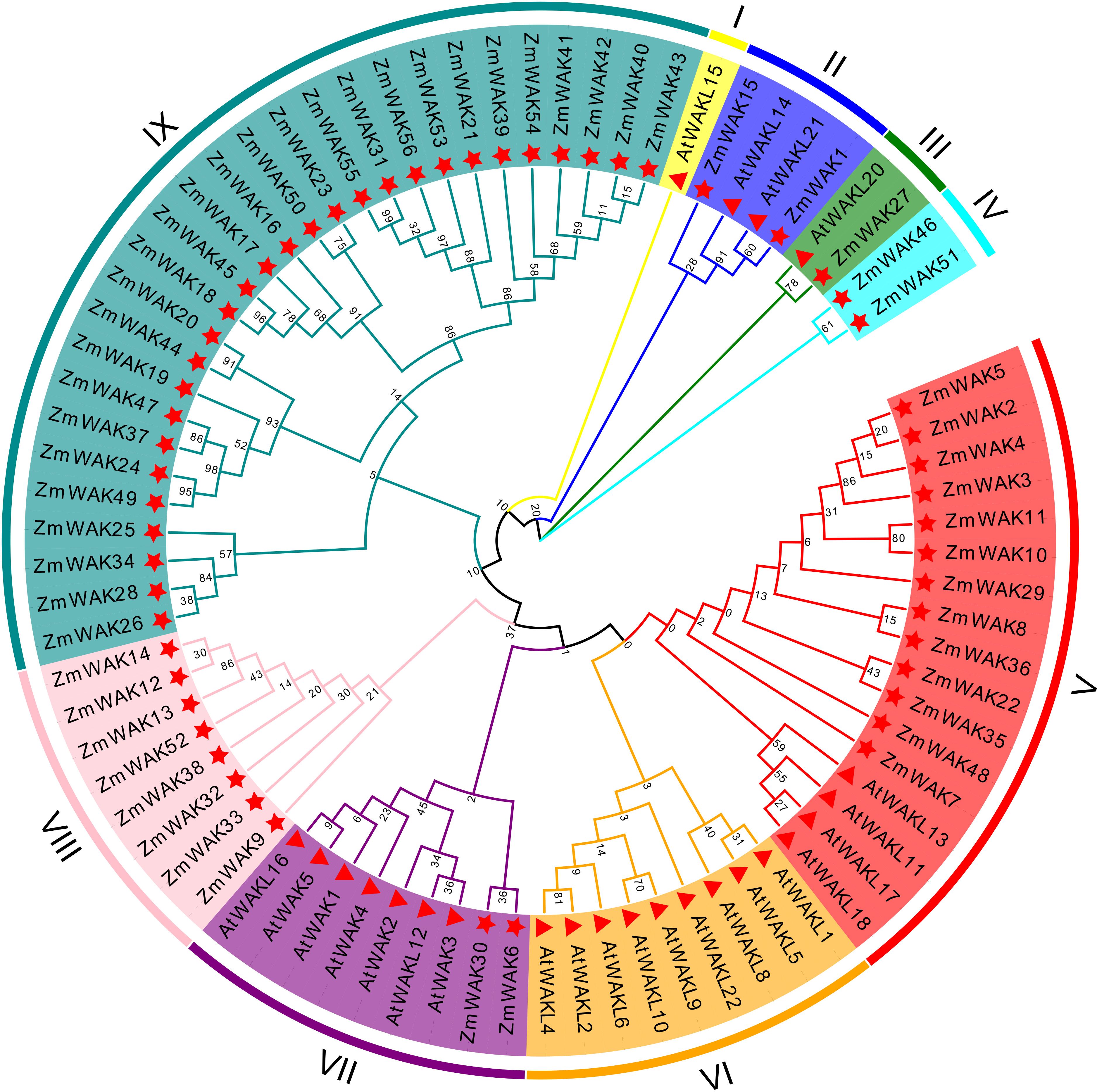
Figure 3. Phylogenetic relationships of the WAK proteins from Arabidopsis (At) and maize (Zm). Distinct color blocks represent different subgroups. The Star shape represents maize WAK proteins, and the triangle represents Arabidopsis WAK proteins.
3.4 Gene structure and conserved motif analysis of the maize WAK gene family
To further explore the evolution of the ZmWAK gene family, the gene structure and conserved motifs of ZmWAKs were analyzed. As shown in Supplementary Table S1, a total of five typical domains were detected in ZmWAKs, including signal peptide, GUB_WAK_bind domain, EGF domain, Pkinase_Tyr domain, and transmembrane domain. We observe that 28.57%, 21.42%, 17.85%, and 32.14% of ZmWAKs contain all five, four, three, and two typical domains, respectively. No ZmWAKL proteins were identified, because all identified ZmWAK proteins contain at least one extracellular domain (GUB_WAK_bind, EGF) and an intracellular domain (Pkinase_Tyr), based on the identification method used by previous researchers (Yang et al., 2021; Li et al., 2025). However, in a previous study, 58 ZmWAKL proteins were identified, which contained EGFs and a protein kinase domain (Hu et al., 2023). In this study, all 56 ZmWAK proteins contain an extracellular domain (GUB_WAK_bind,EGF) and an intracellular domain (Pkinase_Tyr). This suggests that the differences in gene number between the two studies may be attributed to variations in identification methods and screening criteria.
The ZmWAK proteins in the same group displayed similar conserved domains and structures. For example, groups V and IX are the two largest subgroups within the maize WAK gene family, comprising 13 and 28 ZmWAK proteins, respectively. In subgroup V, 76.92% of the ZmWAK proteins harbor the signal peptide, GUB_WAK_bind, EGF, transmembrane, and Pkinase_Tyr domains, with either two GUB_WAK_bind domains or two EGF domains present. In subgroup IX, 89.28% of the ZmWAK proteins are devoid of the EGF domain, while 42.8% of the ZmWAK proteins contain the WAK_assoc domain. Furthermore, we find that AtWAKL20 and ZmWAK27 (in subgroup III) and AtWAKL21 and ZmWAK1 (in subgroup II) are sub-clustered despite having different domain compositions. We speculate that these genes may have retained conserved domains or amino acid sequences during evolution. Conserved motif analysis reveals that around 89.29% of ZmWAKs contain three or four exons, whereas 10.71% of ZmWAKs contain two or five exons (Supplementary Table S1, Figure 4). In a previous study, the number of exons in ZmWAKL genes varied from 2 to 5 (Hu et al., 2023). This is similar to the results of this study. Additionally, Similar intron-exon distribution has been reported in other plants, implying that this feature of the WAK gene family might be conserved across different plant species.
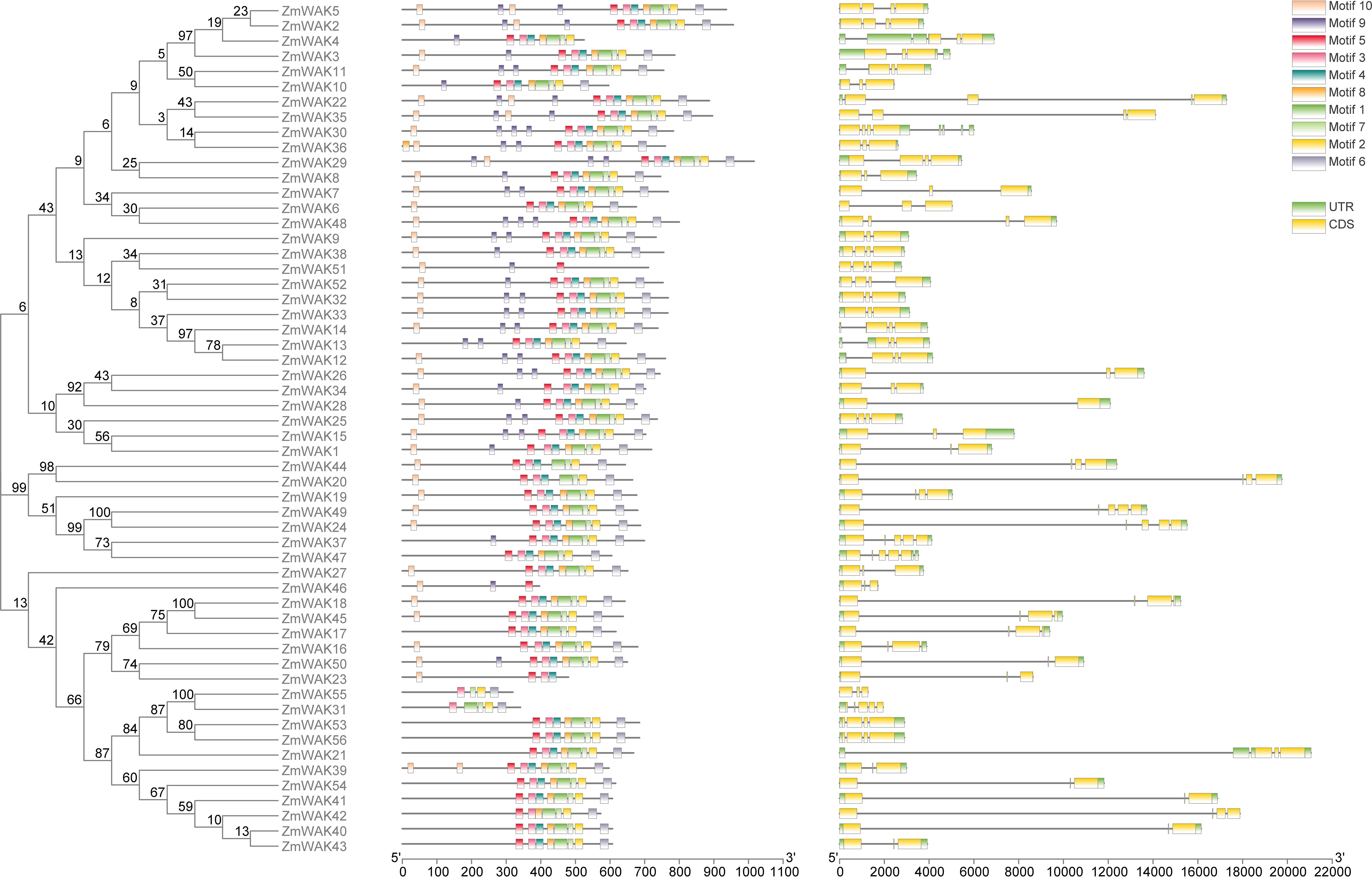
Figure 4. The gene structure and conserved motifs of ZmWAK gene family. From left to right, they are as follows: the phylogenetic tree of the 56 ZmWAK proteins, the motif distribution of the 56 ZmWAK proteins, The exon-intron structure of the ZmWAK genes.
A total of ten conserved motifs were predicted via the MEME analysis (Supplementary Table S2). Most of the ZmWAKs from subgroups II, V, and VIII contained all ten motifs. Around 78.57% and 46.42% of ZmWAKs from subgroup IX lacked motifs 9 and 10, respectively. ZmWAKs from subgroup IV contained the least number of motifs, harboring only motifs 5, 9, and 10. ZmWAKs from subgroups III and VII displayed a trend of losing motifs or having multiple copies of motif 9 (Supplementary Tables S2, S3, Figure 4). The gene structure and motif composition of ZmWAKs were similar within the same subgroup, indicating functional redundancy in plant growth and development. In a previous study, there were ten motifs identified in ZmWAKL proteins that are associated with the wall-associated kinase or protein kinase domain (Hu et al., 2023). A similar distribution of conserved motifs was observed between the ZmWAKL and ZmWAK gene families.
3.5 Analysis of the cis-acting elements of the maize WAK gene family
A total of 107 distinct types of cis-acting elements were identified in the promoter regions of maize WAK genes, including 82 elements with known functions and 25 elements with unknown functions (Supplementary Tables S4, S5, Figure 5A). A significant proportion (41.92%) of cis-acting elements were core promoter elements, followed by light-responsive elements (7.34%). Furthermore, specific cis-acting elements were predicted to participate in the responses to various hormones (methyl jasmonate/jasmonic acid (MeJA/JA), ABA, ethylene (ETH), salicylic acid (SA), IAA, and GA) and stress factors (drought, anaerobic, injury, low temperature (LT), salt, and fungus), defense responses, and plant development processes. Among them, the defense-responsive elements, elements related to plant development, and abiotic stress-responsive elements (MeJA/JA, ABA, ETH, and drought) were widely distributed in the promoter sequences of ZmWAKs (found in 2.54%, 3.46%, 6.93%, 3.86%, 3.16%, and 4.94% of the sequences, respectively; Figure 5B). This result suggests that ZmWAKs might be involved in stress responses and might regulate plant growth and development via hormonal pathways.
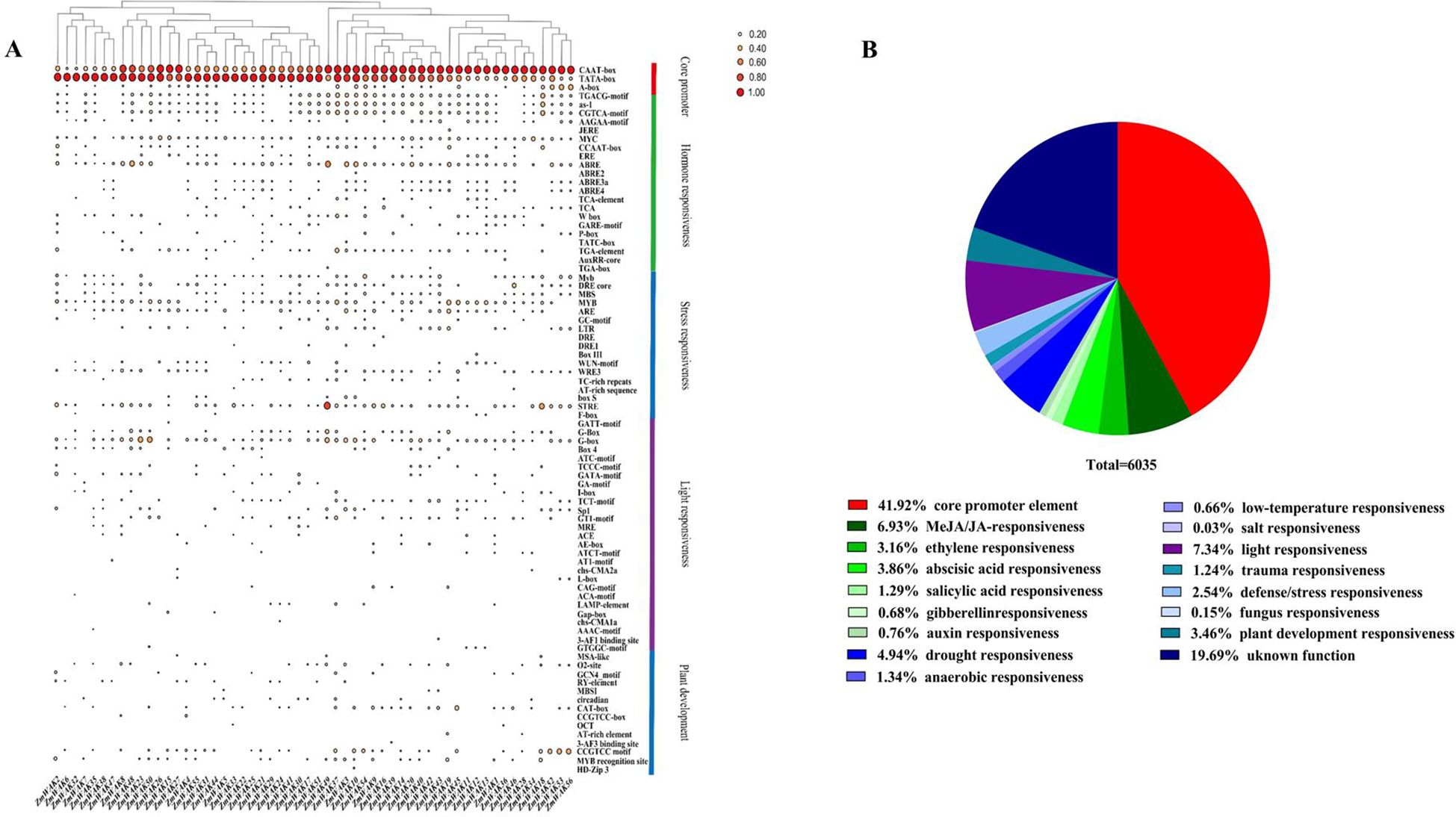
Figure 5. Predicted cis-elements in promoter regions of ZmWAK genes. (A) Distribution of different cis-regulatory elements. (B) The relative proportion of various cis-elements within promoter regions.
3.6 GO annotation analysis of the maize WAK gene family
Upon GO enrichment analysis, a total of eight ZmWAK genes were successfully annotated (Supplementary Figure S1). The molecular function enrichment analysis revealed that the genes were primarily associated with guanylate cyclase, phosphorus-oxygen lyase, and cyclase activities. The cellular component enrichment analysis showed that they were mainly distributed in the plant cell wall, plasma membrane, and cell periphery. The biological process enrichment analysis highlighted a significant enrichment of the genes in cGMP biosynthesis, cyclic purine nucleotide metabolism, and cyclic nucleotide metabolism/biosynthesis.
3.7 Collinearity analysis of the maize WAK gene family
To understand the collinearity of ZmWAKs in different species, a collinearity analysis was performed between maize WAKs and corresponding genes of monocotyledonous and dicotyledonous plants. As shown in Figure 6, a total of 76 interspecific collinear relationships were identified with maize WAKs, including eight, 27, and 41 collinear links with soybean, rice, and sorghum, respectively (Supplementary Table S6). However, no collinear links were detected between maize and Arabidopsis. Further analysis found that ZmWAK37 exhibited the highest collinearity, with six collinear links, followed by ZmWAK11, ZmWAK14, ZmWAK44, ZmWAK47, and ZmWAK49 (Supplementary Table S6). The Ka/Ks analysis revealed higher Ka, Ks, and Ka/Ks values for SDs than TDs for maize WAKs. The average Ks value of the maize gene pairs with rice homologs was markedly greater than 1. However, the Ka, Ks, and Ka/Ks values of the maize gene pairs with soybean and sorghum homologs were <1. In addition, based on these data, it was calculated that maize diverged from rice and sorghum around 227.56 and 211.68 million years ago, respectively.
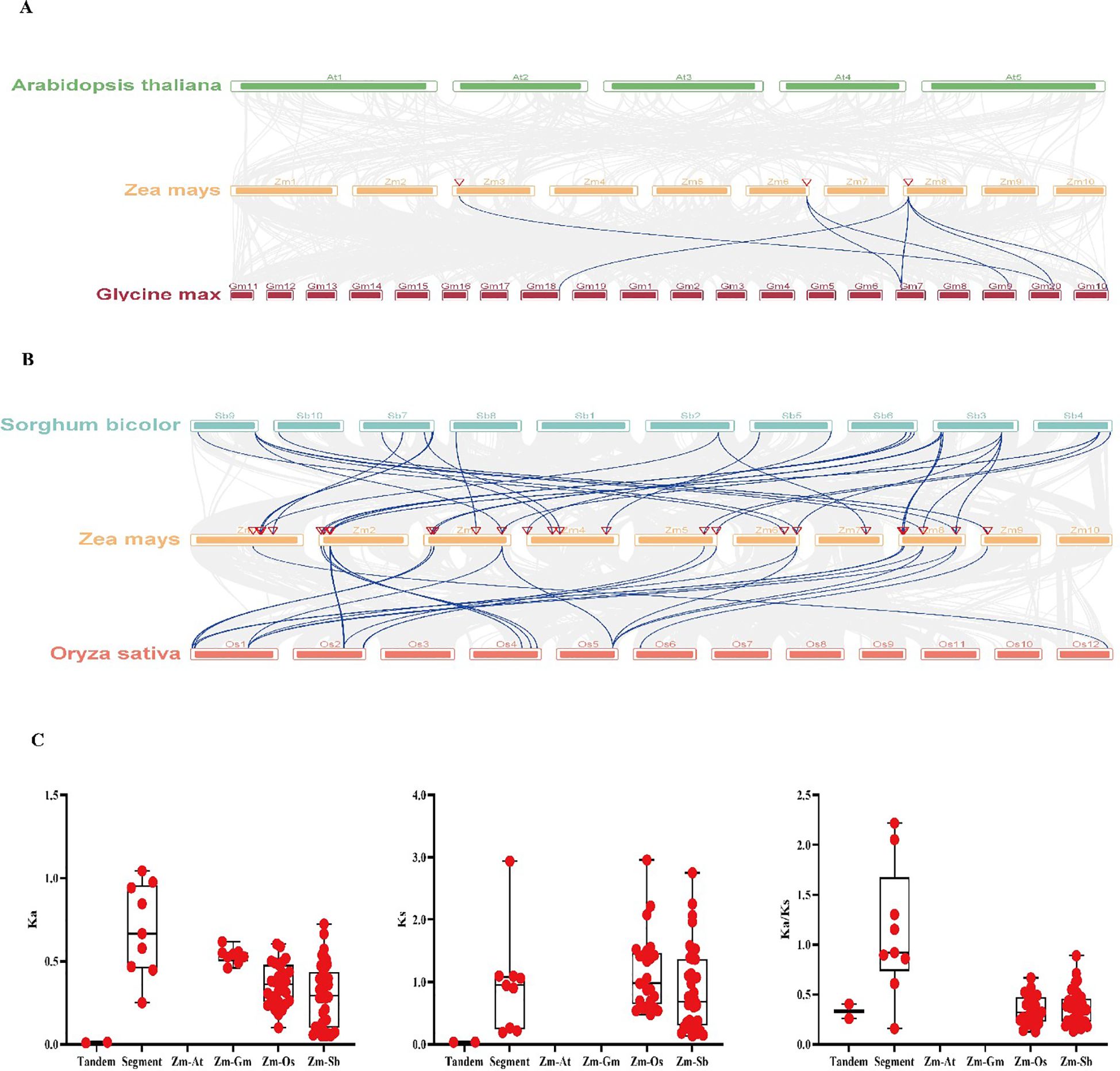
Figure 6. Gene duplication and collinearity of the WAK genes in maize. (A) The collinearity analysis of WAK genes among maize, Arabidopsis and soybean. (B) The collinearity analysis of WAK genes among maize, rice and sorghum. (C) The tandem repeats, segment repeats, and repeats among maize, Arabidopsis (Zm-At), soybean (Zm-Gm), rice (Zm-Os) and sorghum (Zm-Sb), respectively.
3.8 Tissue-specific expression analysis of ZmWAKs
3.8.1 Expression of ZmWAKs in the root
The expression of ZmWAKs in the root was analyzed using the transcriptome data (PRJNA171684) derived from a previous study (Stelpflug et al., 2016). The expression levels of 34 ZmWAKs identified in the root exceeded 1 transcript per million reads (TPM > 1) (Supplementary Table S7; Figure 7A). Among them, the highest expression levels were observed for ZmWAK15 (TPM > 314) and several other genes: ZmWAK13, ZmWAK22, ZmWAK25, ZmWAK43, ZmWAK49 (TPM > 10). At 5 days post-sowing, ZmWAK15 was highly expressed in various parts of the root, except the root elongation and meristem zone. At 7 days post-sowing, ZmWAK13, ZmWAK22, ZmWAK25, ZmWAK43, and ZmWAK49 displayed peak expression levels at the Z4 region of taproot. However, these five genes were hardly expressed in the root elongation and meristem zone at 5 days post-sowing.
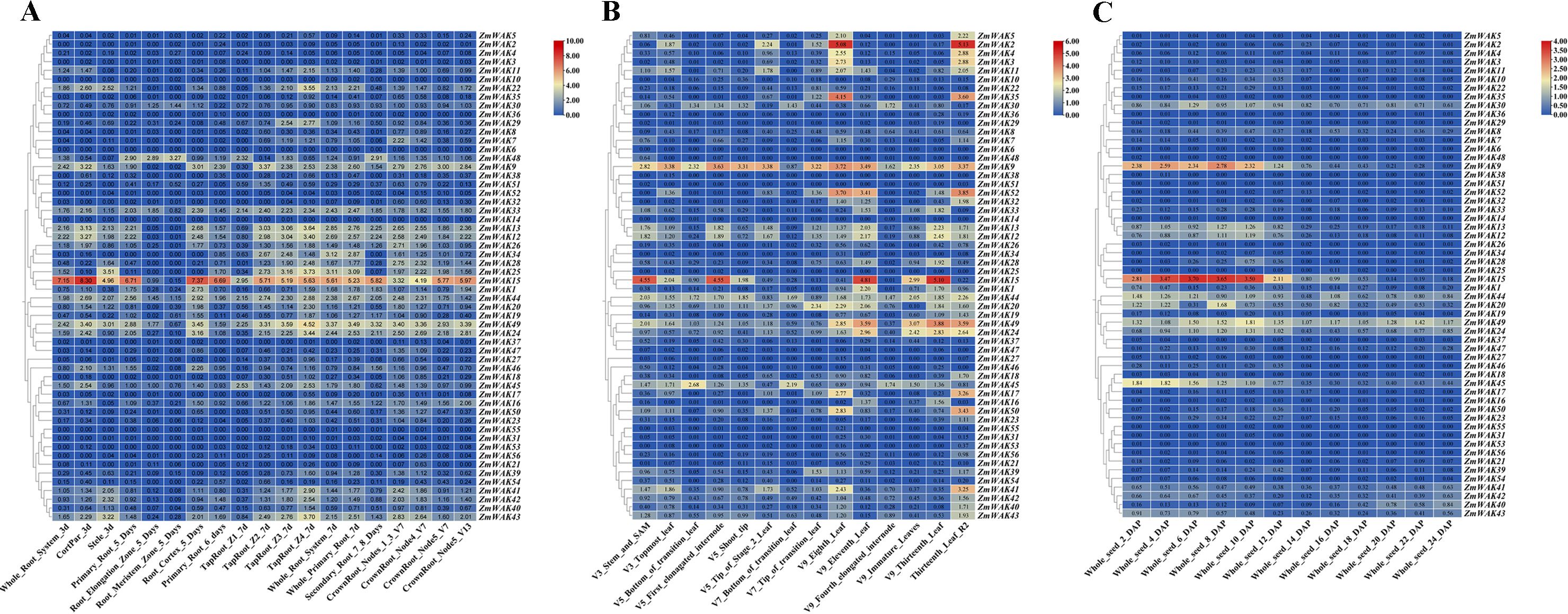
Figure 7. Maize WAK gene family expression heat map in maize development. (A)Maize WAK gene family expression heat map in root development. (B) Maize WAK gene family expression heat map in leaf development. (C) Maize WAK gene family expression heat map in seed development. The numbers in the colored boxes are calculated based on the transcript per million reads (TPM) values. The calculation formula is log2 (TPM + 1).
3.8.2 Expression of ZmWAKs in the leaf
The expression of ZmWAKs in the leaf was analyzed using previously published transcriptome data (Stelpflug et al., 2016). The expression levels of 32 ZmWAKs identified in the leaf were >1 (Supplementary Table S8; Figure 7B). Among them, the highest expressions were observed for ZmWAK2, ZmWAK9, ZmWAK15, ZmWAK35, ZmWAK49, and ZmWAK52 (TPM > 10). ZmWAK15 expression peaked in V3 stem and SAM, V5 first elongated internode, and V9 eleventh and thirteenth leaves. ZmWAK2, ZmWAK35, and ZmWAK52 were highly expressed in V9 eighth leaf and R2 thirteenth leaf. ZmWAK9 and ZmWAK49 displayed peak expression levels in V9 eighth leaf and thirteenth leaf, respectively.
3.8.3 Expression of ZmWAKs in the seed
Using previously published transcriptome data, ten ZmWAKs were found to be highly expressed in the seed (TPM > 1) (Stelpflug et al., 2016). Among them, ZmWAK9 and ZmWAK15 exhibited the highest expression levels in the early stages of seed growth, such as 2-, 4-, 6-, 8-, and 10 days post-pollination (Supplementary Table S9; Figure 7C).
3.9 Expression patterns of ZmWAKs under abiotic stresses
3.9.1 Expression patterns of ZmWAKs under temperature stress
The changes in the expression of ZmWAKs in response to temperature stress were assessed using the transcriptome data (PRJNA645274) derived from a previous study (Li et al., 2020). A total of 38 ZmWAKs genes were expressed in maize seedling leaves under temperature stress (TPM > 1) (Supplementary Table S10; Figures 8A, B). Of them, 23 ZmWAKs displayed highly expression levels (TPM > 10). Among these 23 genes, compared to normal temperature (NT) condition, 16 genes were significantly downregulated, and one gene (ZmWAK30) was significantly upregulated under both low-and high-temperature conditions. The expression level of ZmWAK40 was upregulated under high temperature (HT) and moderately high temperature (MHT) conditions compared to NT. ZmWAK41, ZmWAK42 and ZmWAK43 were upregulated under HT condition. ZmWAK52 was upregulated under HT and extremely low temperature (ELT) conditions. ZmWAK44 exhibited contrasting expression patterns under low-and high-temperature conditions: its expression level rose with decreasing temperature and fell with increasing temperature. Under temperature stress, eight ZmWAKs displayed the most significant changes. Among them, ZmWAK1, ZmWAK2, ZmWAK3, ZmWAK4, ZmWAK24, ZmWAK27, and ZmWAK35 exhibited 51.47–99.62% downregulation compared to NT condition, while ZmWAK30 exhibited 0.58–35.37-fold upregulation.
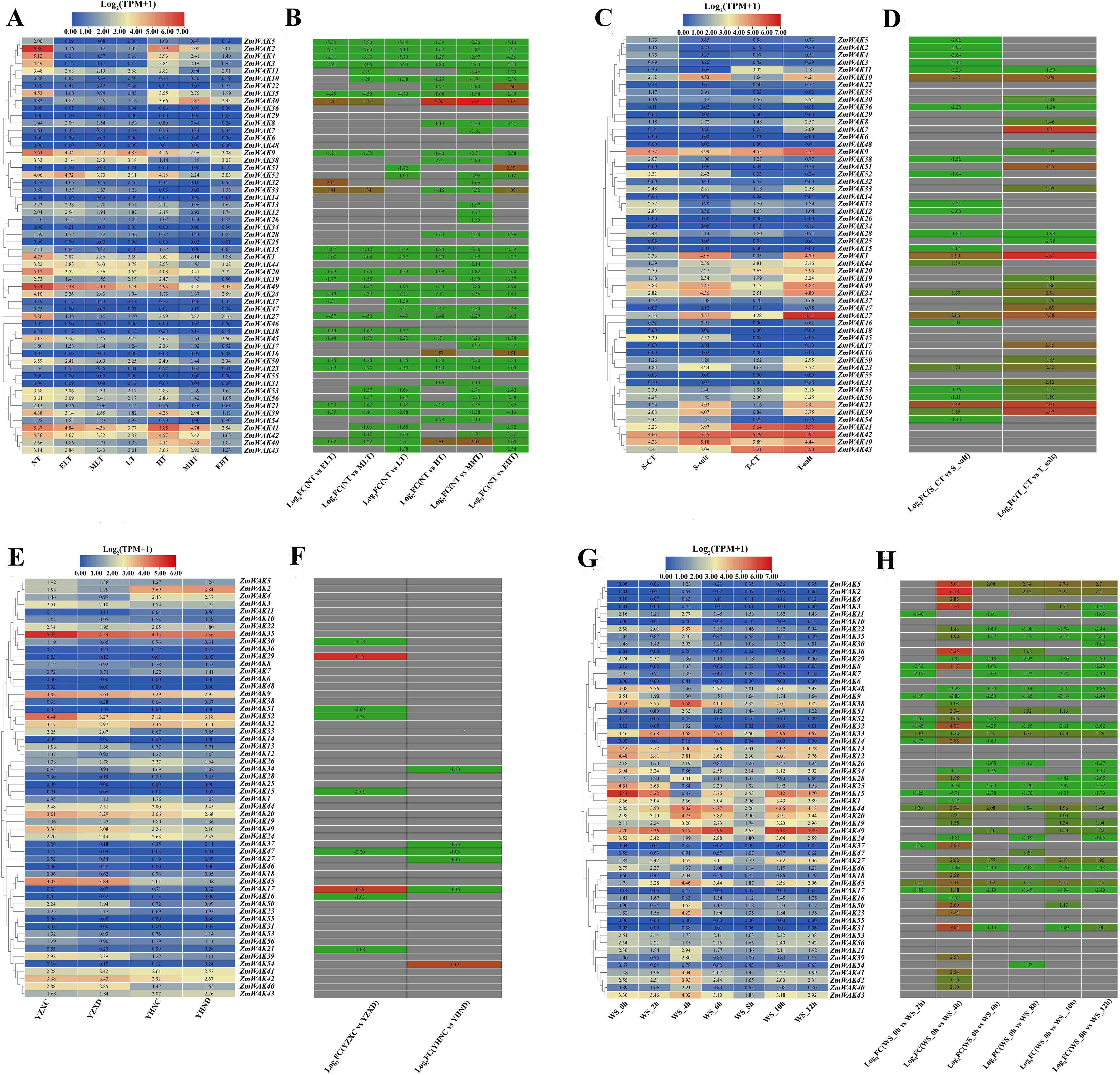
Figure 8. Maize WAK gene family expression heat map in abiotic stress. (A) Temperature stress expression pattern of WAK genes in maize. NT represents the normal temperature (25 °C), LT represents the low temperature (16 °C), ELT represents the extremely low temperature (4 °C), MLT represents the moderately low temperature (10 °C), HT represents the high temperature (37 °C), MHT represents the moderately high temperature (42 °C), EHT represents the extremely high temperature (48 °C). (B) Expression fold changes of the maize WAK gene family in temperature stress. (C) Salt stress expression pattern of WAK genes in maize. T-salt represents the inbred salt-tolerant maize line L87 treated with salt (220 mM NaCl); S-salt represents the salt sensitivity inbred line L29 treated with salt (220 mM NaCl), T−CT represents the inbred salt-tolerant maize line (L87) treated with water, S−CT represents the inbred salt-sensitive maize line (L29) treated with water. (D) Expression fold changes of the maize WAK gene family in salt stress. (E) Drought stress expression pattern of WAK genes in maize. YHND represents the drought-tolerant hybrid (ND476) treated with drought, YHNC represents the drought-tolerant hybrid (ND476) treated with water, YZXD represents the drought-intolerant hybrid (ZX978) treated with drought, YZXC represents the drought-intolerant hybrid (ZX978) treated with water. (F) Expression fold changes of the maize WAK gene family in drought stress. (G) Waterlogging stress expression pattern of WAK genes in maize. WS_0h represents waterlogging 0 hour, WS_2h represents waterlogging 2 hours, WS_4h represents waterlogging 4 hours, WS_6h represents waterlogging 6 hours, WS_8h represents waterlogging 8 hours, WS_10h represents waterlogging 10 hours, WS_12h represents waterlogging 12 hours. (H) Expression fold changes of the maize WAK gene family in Waterlogging stress. There are two figures in each stress treatment—the left figure represents the log2 (TPM + 1) and the right figure represents the log2 (Fold Change), indicated in red for up-regulation and green for down-regulation. When the absolute value of log2 (Fold Change) is greater than 1, it will be shown in the figure.
3.9.2 Expression patterns of ZmWAKs under salt stress
The changes in the expression of ZmWAKs in response to salt stress were assessed using the transcriptome data (PRJNA414300) downloaded from the NCBI website (http://www.ncbi.nlm.nih.gov). A total of 36 ZmWAKs genes were expressed under salt stress (TPM > 1) (Supplementary Table S11; Figures 8C, D). Among them, 14 ZmWAKs demonstrated high expression levels, with TPM values exceeding 10. Nine ZmWAKs exhibited significant differential expression in maize inbred lines after salt treatment (|Log2 (Fold Change)| > 1). Among the differentially expressed genes, eight genes (ZmWAK1, ZmWAK10, ZmWAK21, ZmWAK23, ZmWAK24, ZmWAK27, ZmWAK39, and ZmWAK49) were significantly upregulated under salt stress. The levels of these genes increased by 0.59–10.23-fold in the salt-sensitive (S-salt) maize line and 2.63–27.40-fold in the salt-tolerant (T-salt) maize line. The remaining gene (ZmWAK9) exhibited opposite expression patterns under salt stress, it was downregulated by 43.51% in the salt-sensitive maize inbred line, but upregulated 1.03-fold in the salt-tolerant maize inbred line.
3.9.3 Expression patterns of ZmWAKs under drought stress
The changes in the expression of ZmWAKs in response to drought stress were assessed using the transcriptome data (PRJNA576545) downloaded from the NCBI website (http://www.ncbi.nlm.nih.gov). A total of 34 ZmWAKs were found to be expressed in drought-tolerant and drought-intolerant hybrids under drought stress, among which six genes exhibited significantly high expression levels (TPM > 10) (Supplementary Table S12; Figures 8E, F). Among the highly expressed genes, three genes (ZmWAK9, ZmWAK20, and ZmWAK45) displayed similar expression patterns under drought stress, with downregulation by 13.40–21.5% in the drought-intolerant hybrid (ZX978) and by 23.40-41.05% in the drought-tolerant hybrid (ND476). The remaining three ZmWAKs (ZmWAK2, ZmWAK35, and ZmWAK52) displayed opposite expression patterns under drought stress, their expression levels were downregulated by 41.06–57.94% in the drought-intolerant hybrid and upregulated by 0.79–11.80% in the drought-tolerant hybrid.
3.9.4 Expression patterns of ZmWAKs under waterlogging stress
The changes in the expression of ZmWAKs in response to waterlogging stress were assessed using previously published transcriptome data (PRJNA606824) (Yu et al., 2020). We observed that 43 ZmWAKs were expressed under waterlogging stress, among which 21 ZmWAKs displayed high expression levels (TPM > 10) (Supplementary Table S13; Figures 8G, H). Among the 21 genes, the expression levels of nine genes were consistently downregulated under waterlogging stress, while those of seven genes were consistently upregulated, compared to the waterlogging 0 hour (WS_0h). The expression levels of three genes (ZmWAK22, ZmWAK38, ZmWAK43) were upregulated at 2 or 4 hours under waterlogging stress (WS_2h, WS_4h), but subsequently downregulated compared to WS_0h. One gene (ZmWAK42) exhibited elevated expression levels at 4 and 10 hours under waterlogging stress (WS_4h, WS_10h), but lower expression levels at other time points. The remaining gene ZmWAK41 was upregulated at all time points under waterlogging stress except at 8 hours, with a peak increase observed at 4 hours post-treatment. Under waterlogging stress, 11 ZmWAKs displayed the most significant changes. Among them, five genes (ZmWAK9, ZmWAK15, ZmWAK25, ZmWAK34, and ZmWAK48) were significantly downregulated by 85.97–99.04%, and four genes (ZmWAK23, ZmWAK27, ZmWAK44, and ZmWAK45) were significantly upregulated by 3.05–8.45-fold at WS_4h compared to WS_0h. The remaining two genes, ZmWAK22 and ZmWAK41, showed peak expression levels at 4 hours under waterlogging stress, which were increased by 1.75-fold and 4.8-fold compared to WS_0h, respectively.
3.10 Expression patterns of ZmWAKs under biotic stresses
3.10.1 Expression patterns of ZmWAKs under smut stress
The expression patterns of ZmWAKs under smut (Sphacelotheca reiliana) stress were analyzed using the transcriptome data (PRJNA673988) obtained from six maize inbred lines with distinct resistance levels (CML322 > B73 > EGB > Ky21 > Oh43 > T×303) (Schurack et al., 2021). Under smut stress, a total of 48 ZmWAKs were expressed in maize inbred lines (TPM > 1), with 25 genes displaying high expression levels (TPM > 10) (Supplementary Table S14; Figures 9A, B). Among the highly expressed genes, 23 ZmWAKs exhibited upregulation across all six maize inbreds following inoculation, except for ZmWAK26 and ZmWAK52. The expression levels of 16 ZmWAKs (ZmWAK2, ZmWAK5, ZmWAK11, ZmWAK12, ZmWAK13, ZmWAK21, ZmWAK27, ZmWAK35, ZmWAK39, ZmWAK40, ZmWAK41, ZmWAK42, ZmWAK43, ZmWAK49, ZmWAK53, and ZmWAK56) exhibited the most significant changes under smut stress (TPM > 10, |Log2 (Fold Change)| > 1). Among them, 12, 10, 8, 2, 1, and 1 ZmWAKs were significantly upregulated under smut stress in the maize inbred lines B73, Ky21, T×303, oh43, EGB, and CML322, respectively. Further analysis revealed that ZmWAK2, ZmWAK5, and ZmWAK11 were highly expressed exclusively in the resistant line B73, exhibiting respective upregulation of 255.19-, 396.26-, and 7.23-fold at 3 days post-infection.
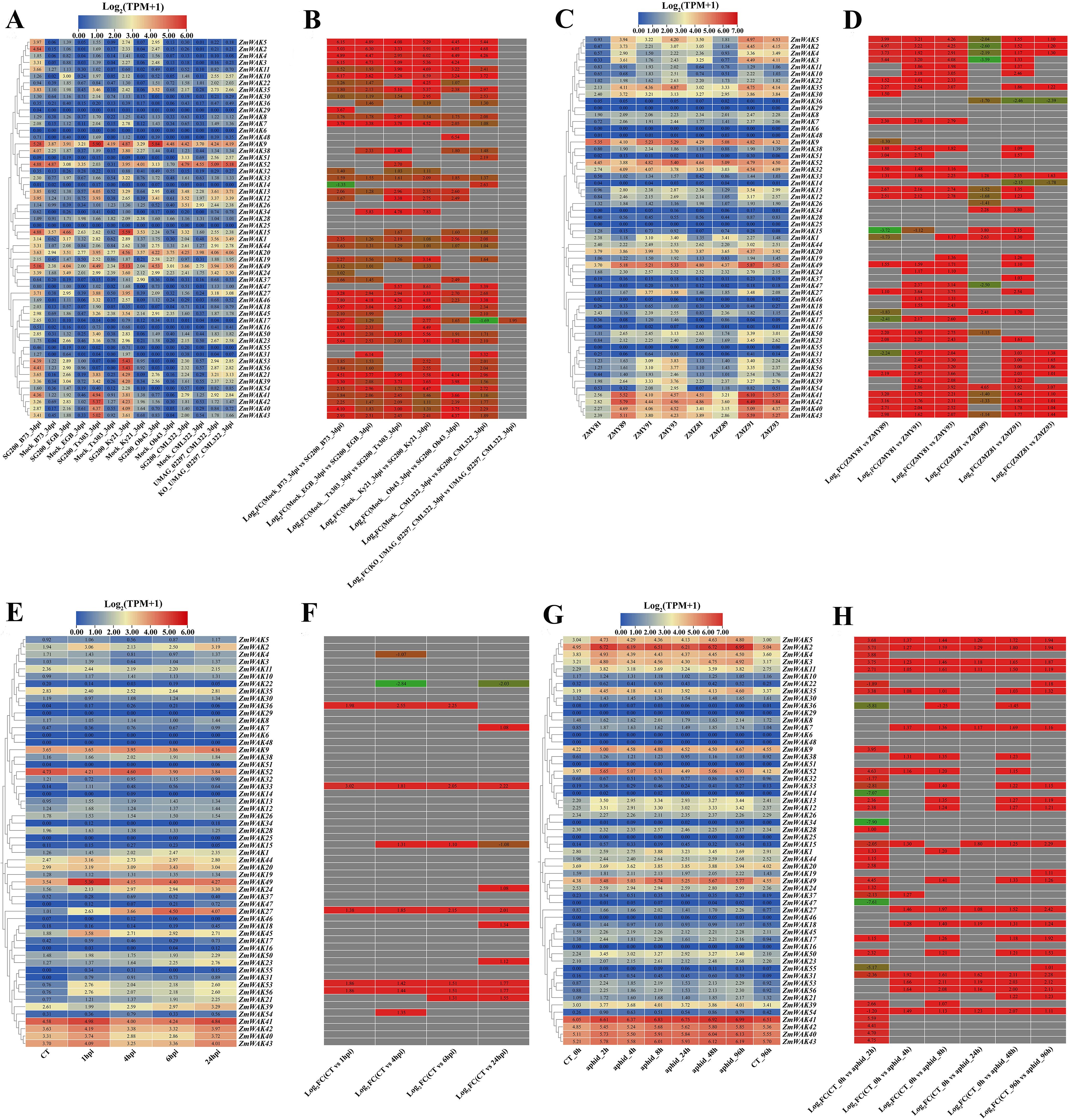
Figure 9. Maize WAK gene family expression heat map under biotic stress. (A) Expression pattern of maize ZmWAK genes under smut stress. SG 200 and UMAG_02297 are strains of the active vegetative fungus, which lead to maize smut. KO_UMAG_02297 is a mutant strain of UMAG_02297. Mock is the simulation control treatment involved uninfected plants. The term 3dpi refers to 3 days after infection. The six maize lines are B73, CML 322, EGB, Ky 21, Oh 43, and Tx 303. (B) Expression fold changes of the maize WAK gene family under smut stress. (C) Expression pattern of maize ZmWAK genes under gray leaf spot stress. ZMY represents the resistant inbreed line ‘Yayu 889’ and ZMZ represented the susceptible inbreed line ‘Zheng Hong 532’, they are sown in an field infected by Mycosphaerella maydis and the leaves of the infected plants are collected at 81, 89, 91, and 93 days after planting. (D) Expression fold changes of the maize WAK gene family under gray leaf spot stress. (E) Expression pattern of maize ZmWAK genes under beet armyworm stress. CT represents the uninfected plants, 1hpi represents 1 hour after infection, 4hpi represents 4 hours after infection, 6hpi represents 6 hours after infection, 24hpi represents 24 hours after infection. (F) Expression fold changes of the maize WAK gene family under beet armyworm stress. (G) Expression pattern of maize ZmWAK genes under aphid stress. CT_0h and CT_96h represent uninfected plants at 0 hour and 96 hours. aphid_2h, aphid_4h, aphid_8h, aphid_24h, aphid_48h, and aphid_96h represent infected plants at 2, 4, 8, 24, 48 and 96 hours, respectively. (H) Expression fold changes of the maize WAK gene family under aphid stress. There are two figures in each stress treatment—the left figure represents the log2(TPM + 1) and the right figure represents the log2 (Fold Change), indicated in red for up-regulation and green for down-regulation. When the absolute value of log2(Fold Change) is greater than 1, it will be shown in the figure.
3.10.2 Expression patterns of ZmWAKs under gray leaf spot stress
The expression patterns of ZmWAKs under gray leaf spot (Mycosphaerella maydis) stress were analyzed using transcriptome data (PRJNA436207) obtained from a previous study (Yu et al., 2018). A total of 41 ZmWAKs were expressed in resistant (ZMY) and susceptible (ZMZ) maize cultivars (Supplementary Table S15; Figures 9C, D). Among them, 18 genes exhibited high expression levels (TPM > 10) and displayed significant expression variation under leaf spot stress (|Log2 (Fold Change)| > 1). Further analysis showed that five genes (ZmWAK27, ZmWAK35, ZmWAK39, ZmWAK53, and ZmWAK56) were upregulated in both resistant and susceptible cultivars under gray leaf spot stress, compared to plants at 81 days post-infection. Twelve genes (ZmWAK2, ZmWAK3, ZmWAK4, ZmWAK5, ZmWAK13, ZmWAK30, ZmWAK32, ZmWAK40, ZmWAK41, ZmWAK42, ZmWAK43, and ZmWAK49) were upregulated in the resistant cultivar, while in the susceptible cultivar, these genes were initially downregulated at 89 days post-infection and subsequently upregulated. One gene (ZmWAK9) showed opposite expression patterns in resistant and susceptible cultivars, its expression level was downregulated by 4.59–59.42% in the resistant cultivar and upregulated by 1.74–76.31% in the susceptible cultivar.
3.10.3 Expression patterns of ZmWAKs under beet armyworm stress
The expression patterns of ZmWAKs under beet armyworm (Spodoptera exigua) stress were analyzed using maize leaf transcriptome data (PRJNA625224) downloaded from the NCBI website (http://www.ncbi.nlm.nih.gov). We detected a total of 36 ZmWAKs, with nine genes exhibiting relatively high expression levels (TPM > 10) (Supplementary Table S16; Figures 9E, F). Further analysis revealed that three ZmWAKs (ZmWAK27, ZmWAK45, and ZmWAK49) were consistently upregulated, and one gene (ZmWAK52) was consistently downregulated under beet armyworm stress. Four ZmWAKs (ZmWAK40, ZmWAK41, ZmWAK42, and ZmWAK43) exhibited biphasic expression patterns, being upregulated at 1 hour and 24 hours after infestation but downregulated at 4 hours and 6 hours after infestation. One gene (ZmWAK9) displayed a slight downregulation at 1 hour after infestation, followed by an increase in expression. Among these genes, ZmWAK27 displayed the most significant expression variation, exhibiting 4.09–20.32-fold upregulation after infestation.
3.10.4 Expression patterns of ZmWAKs under aphid stress
The expression pattern of ZmWAKs under aphid (Rhopalosiphum maidis) stress was analyzed using the transcriptome data (PRJNA295410) of maize foliar responses to aphid feeding obtained from a previous study (Tzin et al., 2015). A total of 38 ZmWAKs were expressed in maize foliar tissue, with 18 genes displaying high expression levels (Supplementary Table S17; Figures 9G, H). Among them, the expression levels of 16 genes were consistently upregulated under aphid stress. One gene (ZmWAK1) was downregulated at 2 and 4 hours after infestation, but was subsequently upregulated. The remaining gene, ZmWAK20, exhibited a dynamic expression pattern characterized by downregulation at 2, 4, and 96 hours after infestation and upregulation at 8, 24, and 48 hours after infestation. Further analysis revealed that 12 ZmWAKs showed the most significant expression variations (|Log2 (Fold Change)| >1). Among them, the expression of five genes (ZmWAK4, ZmWAK11, ZmWAK12, ZmWAK13, and ZmWAK52) peaked at 2 hours after infestation, with expression levels increasing by 1.22- to 2.37-fold. The expression of one gene (ZmWAK1) peaked at 8 hours after infestation, with expression level rising by 1.29-fold. The remaining six genes (ZmWAK2, ZmWAK3, ZmWAK5, ZmWAK35, ZmWAK39, and ZmWAK49) peaked at 96 hours after infestation, with expression levels increasing by 0.57- to 2.83-fold.
3.11 Expression patterns of ZmWAKs under hormonal treatments
RNA-seq was performed to explore the expression patterns of ZmWAKs under IAA, ABA, and GA treatments. A total of 29 ZmWAKs were detected to be expressed in treated maize leaf tissue (FPKM > 1; Supplementary Table S18; Figure 10). Under IAA treatment, the expression levels of 26 ZmWAKs significantly varied. Among them, the levels of three, two, and 14 genes peaked at 3, 6, and 12 hours post-treatment, respectively. Three genes showed the lowest expression levels at 3 or 6 hours post-treatment. The remaining four genes displayed a significant downregulation at 3 or 6 hours post-treatment, followed by a significant upregulation. Under ABA treatment, the expression levels of 26 ZmWAKs varied significantly. Among them, the levels of one, two, and 13 genes peaked at 3, 6, and 12 hours post-treatment. Three genes were significantly downregulated at 3 hours post-treatment. The levels of six genes significantly decreased, followed by a subsequent increase. In contrast, one gene showed an opposite expression pattern by initially upregulating and then downregulating. Under GA treatment, the levels of 26 genes varied significantly. Among them, the levels of three, one, and 19 genes peaked at 3, 6, and 12 hours post-treatment. Three genes significantly downregulated at 6 or 12 hours post-treatment. Overall, six genes (ZmWAK9, ZmWAK30, ZmWAK40, ZmWAK41, ZmWAK42, and ZmWAK49) displayed the highest expression and the most significant variations under IAA, ABA, and GA treatments, upregulating by 0.88–3.42-fold, 0.79–1.95-fold, and 0.30–4.26 fold, respectively.
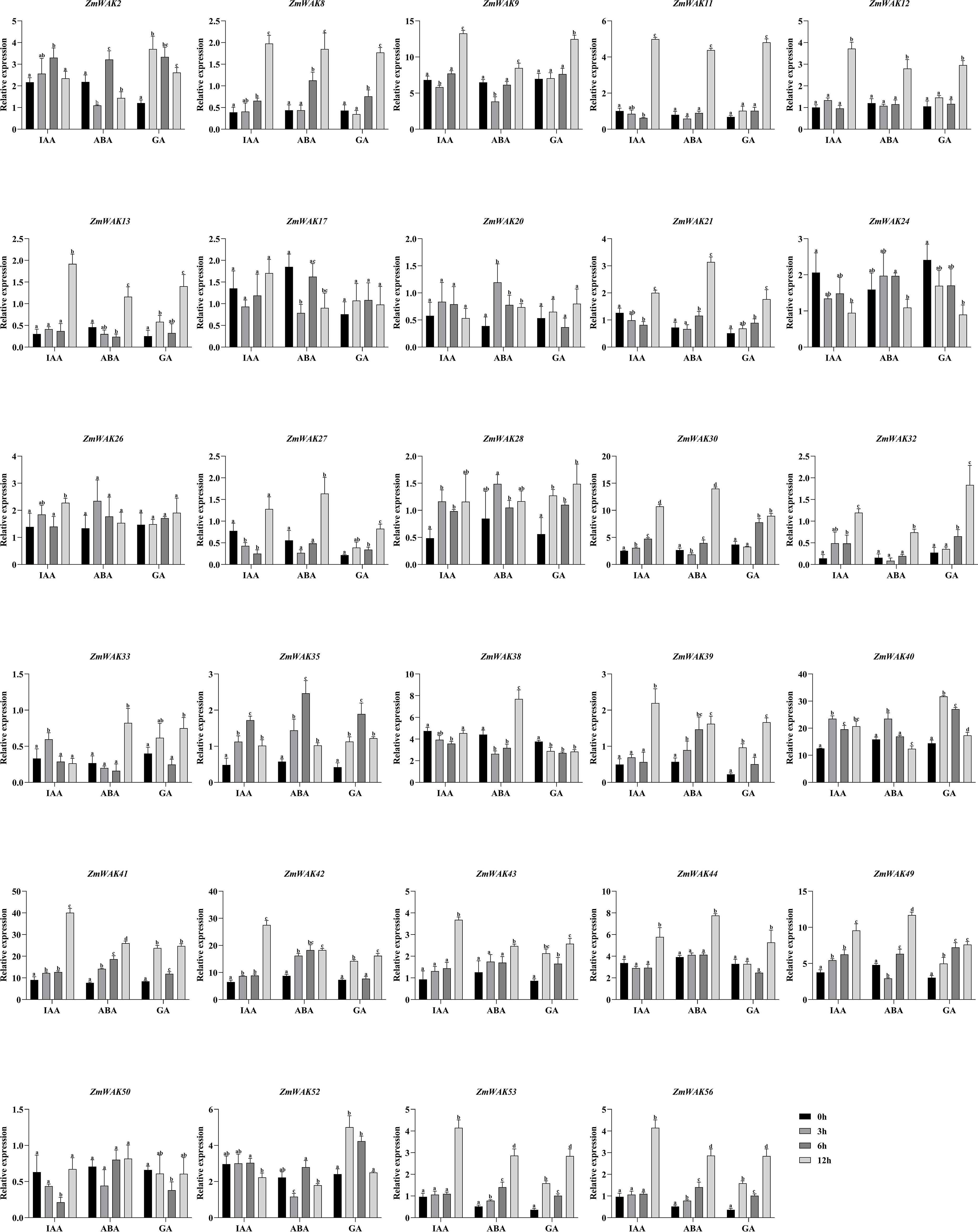
Figure 10. The expression pattern of WAK genes in maize under hormonal treatments. The black column represents 0 hour after hormonal treatments. The light gray column represents 3 hours after hormonal treatments. The dark gray column represents 6 hours after hormonal treatments. The gray column represents 12 hours after hormonal treatments. The level of significance (p < 0.05) among the different treatments is indicated by different letters.
3.12 Analysis of differentially expressed ZmWAKs under abiotic, biotic, and hormonal treatments
The expression profiles of maize WAK genes were analyzed under various abiotic, biotic, and hormonal treatments and heat maps were generated to visualize the differentially expressed genes (Supplementary Table S19, Supplementary Figure S2). Overall, the expression of 34 ZmWAKs significantly varied in response to abiotic, biotic, and hormonal treatments. Further analysis revealed that ZmWAK10, ZmWAK15, ZmWAK20, ZmWAK22, ZmWAK23, ZmWAK24, ZmWAK25, ZmWAK34, ZmWAK44, and ZmWAK48 were differentially expressed under abiotic stresses. Conversely, ZmWAK5, ZmWAK11, ZmWAK12, ZmWAK13, ZmWAK32, ZmWAK43, ZmWAK53, and ZmWAK56 were differentially expressed under biotic stresses. Additionally, ZmWAK1, ZmWAK2, ZmWAK3, ZmWAK4, ZmWAK9, ZmWAK21, ZmWAK27, ZmWAK30, ZmWAK35, ZmWAK39, ZmWAK40, ZmWAK41, ZmWAK42, ZmWAK45, ZmWAK49, and ZmWAK52 were differentially expressed under multiple stresses. Among these genes, the expression of ZmWAK9, ZmWAK27, ZmWAK41, and ZmWAK49 varied significantly under more than six types of stresses.
3.13 Analysis of the maize WAK protein interaction network
To further explore the function of the maize WAK gene family, an interaction network of maize WAK proteins was predicted using the STRING database. Our results showed that the maize WAK genes interacted with several other genes, including Cl3398_1, IDP4123, LOC100384169, K7U0Q4, LAZ1-8, ATL46-5, and YLS9 (Supplementary Figure S3). Cl3398_1 encodes a cAGP10-like protein, which displays homology to canonical arabinogalactan protein 10 (AGP10). AGP10 anchors in the plasma membrane and plays crucial roles in cell recognition, cell wall extension, and signal transduction. IDP4123 encodes a pollen Ole 1 allergen and extensin family protein (POE), which functions as developmental regulators in several plant tissues (Hu et al., 2014). LOC100384169 encodes a putative 3-deoxy-D-manno-octulosonic acid transferase (KDTA), which localizes in the mitochondria and is involved in the synthesis of a mitochondrial lipid A-like molecule (Lerouge, 2010). K7U0Q4 encodes germin-like protein subfamily 1 member 8, which is differentially expressed under salt stress (Soares et al., 2018). LAZ1–8 encodes a protein with a DUF300 domain, which positively regulates systemic acquired resistance (SAR) via the regulation of the expression of CBP60g, SARD1, and SA and BHP biosynthetic genes (Chen et al., 2024). ATL46–5 and YLS9 encode RING-type domain-containing protein and late embryogenesis abundant protein, respectively.
4 Discussion
In this study, a total of 56 ZmWAKs were identified in the maize genome (Supplementary Table S1). Phylogenetic analysis showed that the maize WAK gene family members can be classified into seven subgroups (Figure 3). In Arabidopsis, rice, soybean, and sorghum, the WAK gene families comprise 27, 125, 74, and 98 WAKs/WAKLs, respectively (He et al., 1999; Verica and He, 2002; Zhang et al., 2005; Li et al., 2025; Whyte and Sipahi, 2024). This finding revealed that the number of the WAK gene family members significantly varies among different plant species. Maize WAK genes in the same subgroups share similar gene structures and conserved motifs, implying that they might mediate similar functions in plants (Supplementary Table S1; Figure 4).
ZmWAK1 and ZmWAK15, classified in subgroup II, are closely related to AtWAKL14 and AtWAKL21. In Arabidopsis, AtWAKL14 interacts with pectin and oligogalacturonides (Ogs) and regulates vascular tissue development (Ma et al., 2024). ZmWAK27, classified in subgroup III, is closely related to AtWAKL20. AtWAKL20 is significantly downregulated under ABA and JA treatments (by 63.1% and 84.5%, respectively) (Chae et al., 2009). A T-DNA mutant of AtWAKL20 revealed that atwakl20 mutation did not affect root gravitropism (Han et al., 2024). In addition, a total of 13 ZmWAKs (ZmWAK2, ZmWAK3, ZmWAK4, ZmWAK5, ZmWAK7, ZmWAK8, ZmWAK10, ZmWAK11, ZmWAK22, ZmWAK29, ZmWAK35, ZmWAK36, and ZmWAK48) from subgroup V and four AtWAKLs (AtWAKL11, AtWAKL13, AtWAKL17, and AtWAKL18) are clustered together. AtWAKL11 interacts with NAC102, which is involved in modulating cell wall pectin metabolism and the binding of cadmium (Cd) to the cell wall, thereby enhancing Cd tolerance in Arabidopsis (Han et al., 2023). Two ZmWAKs (ZmWAK6 and ZmWAK30) from subgroup VII group together with seven AtWAKs/WAKLs (AtWAK1, AtWAK2, AtWAK3, AtWAK4, AtWAK5, AtWAKL12, and AtWAKL16). The overexpression of AtWAK1 had been shown to enhance resistance to Botrytis cinerea in transgenic plants (Brutus et al., 2010). In a previous study, expression of the dominant hyperactive allele for AtWAK2 was found to lead to a dwarf phenotype and activation of stress response and reactive oxygen species (ROS) production (Kohorn et al., 2021). The antisense expression of AtWAK4 had been found to inhibit cell elongation and alter cellular morphology (Lally et al., 2001). In the current study, the promoter regions of ZmWAK6 and ZmWAK30 are found to harbor several elements related to MeJA and JA stresses, defense responses, and meristem expression (Supplementary Table S4). MeJA and JA are important regulators of plant responses to biotic and abiotic stresses as well as plant development (Wasternack and Hause, 2013). Thus, ZmWAK6 and ZmWAK30 might be involved in plant growth, development, and stress responses via the MeJA and JA signaling pathways.
Gene duplication events, including SDs and TDs, have been recognized as predominant forces driving the expansion of gene families (Cannon et al., 2004). The analysis of gene duplication within the maize WAK gene family reveals that a total of nine SD and two TD events have contributed to the expansion of this gene family, collectively accounting for 23.2% of ZmWAKs (Table 1). In soybean, 37.83%, 20.27%, and 12.16% of GmWAKs are produced by SD, TD, and proximal duplication events, respectively (Zhang et al., 2005). In cotton, 62.07%, 17.24%, and 17.24% of GhWAKs are produced by SD, TD, and proximal duplication events, respectively (Dou et al., 2021). These results indicate that whole genome duplication (WGD) events, particularly SD events, play significant roles in the expansion of the WAK gene families of several plant species.
During whole genome duplication (WGD), the accumulated base mutations might facilitate the emergence of genes undergoing neofunctionalization or subfunctionalization (Roulin et al., 2013). Neofunctionalization gives rise to novel functional genes, which usually undergoes positive selection (Ka/Ks > 1) (Conant et al., 2014). Subfunctionalization leads to the function partitioning of the ancestral genes that experience purifying selection (Ka/Ks < 1) (Lynch and Conery, 2000). In the current study, the Ka/Ks analysis of 11 duplication gene pairs show that four gene pairs exhibit a Ka/Ks ratio of >1, suggesting that these genes have undergone positive selection and might have diversified to acquire new functions (Table 1). The remaining seven gene pairs have a Ka/Ks ratio of <1, indicating that they might have experienced purifying selection, leading to the subfunctionalization of the genes.
The interspecies collinearity analysis (Figure 6) of the WAK gene families from Arabidopsis, maize, soybean, rice, and sorghum demonstrates that 35 maize WAK genes exhibits collinearity with the WAK genes of soybean, rice, and sorghum, demonstrating a total of 76 collinear relationships. Maize WAK genes exhibits eight, 27, and 41 collinear relationships with soybean, rice, and sorghum WAKs, respectively, with ZmWAK37 showing the most collinear relationships (n=6), and ZmWAK11, ZmWAK14, ZmWAK44, ZmWAK47, and ZmWAK49 showing four collinear relationships each. However, none of the ZmWAKs exhibit collinear relationships with Arabidopsis WAKs (Supplementary Table S6, Figure 6). The SD and TD events of maize WAKs were predicted to have occurred around 2.55–226.02 million years ago, with maize diverging from rice and sorghum approximately 227.56 and 211.68 million years ago, respectively (Table 1). These results imply that maize exhibited higher homology with the monocotyledonous plants (rice and sorghum) than with the dicotyledonous plants (Arabidopsis and soybean).
Tissue-specific expression of maize WAK gene family members was analyzed using publicly available transcriptome data. Maize WAK gene family exhibits distinct expression patterns (Supplementary Tables S7–S9). ZmWAK13, ZmWAK15, ZmWAK22, ZmWAK25, ZmWAK43, and ZmWAK49 were highly expressed in the root. ZmWAK2, ZmWAK9, ZmWAK15, ZmWAK35, ZmWAK49, and ZmWAK52 were highly expressed in the leaf. ZmWAK9 and ZmWAK15 were highly expressed in the seed during the early growth stage. Among these genes, ZmWAK9, ZmWAK15, and ZmWAK49 were highly expressed in multiple tissues, including root, leaf, and seed. The expression of ZmWAK9 peaked in the V9 eighth leaf and the seed in its early growth stage. ZmWAK49 levels peaked in the Z4 region of the taproot at 7 days post-sowing and in the V9 thirteenth leaf. ZmWAK15 was highly expressed in the root, stem, and SAM at the V3 stage, in the first elongated internode at the V5 stage, in the V9 eleventh and thirteenth leaves, and in seeds during the early development stages.
Cis-acting element analysis demonstrated that the promoter regions of ZmWAK9, ZmWAK15, and ZmWAK49 contained MeJA- and JA-responsive elements (TGACG-motif, as-1, and CGTCA-motif), IAA-responsive elements (TGA-element and TGA-box), as well as meristem expression-related elements (CAT-box and CCGTCC motif; Supplementary Table S4). This result implies that these genes might participate in the regulation of plant growth and development by responding to MeJA, JA, and IAA.
The analysis of stress response patterns of maize WAK gene family showed that the expression of ZmWAK9, ZmWAK27, ZmWAK41, and ZmWAK49 significantly varied under different abiotic, biotic, or hormonal treatments (Figures 8-10). ZmWAK9 was downregulated under drought, waterlogging, and gray leaf spot stresses but upregulated under beet armyworm, IAA, ABA, and GA treatments. Under salt stress, ZmWAK9 displayed opposite expression patterns in the salt-tolerant (T-salt) and salt-sensitive (S-salt) maize inbred lines, with upregulation under T-salt and downregulation under S-salt. ZmWAK27 was significantly downregulated under temperature stress but upregulated under salt, waterlogging, smut, leaf spot, and beet armyworm stresses. ZmWAK41 was significantly induced by waterlogging, smut, gray leaf spot, beet armyworm, IAA, ABA, and GA treatments. In addition, ZmWAK49 was robustly induced by salt, smut, gray leaf spot, beet armyworm, aphid, IAA, ABA, and GA treatments.
In summary, the expression of ZmWAK9, ZmWAK15, ZmWAK27, ZmWAK41, and ZmWAK49 significantly varied during plant growth and development and under different stress conditions (Supplementary Tables S7-S18). ZmWAK9 encodes receptor-like protein kinase rlk13, which localizes on the plasma membrane and is involved in the cell surface receptor signaling pathway and protein phosphorylation. ZmWAK9 was found to be homologous to the rice OsWAK53 (Os04g0599000), with a homology of 52.91% (Figure 6). OsWAK53 had been identified as an upstream gene of OsPID, and they participated in the adaptation to low-phosphorus stress by maintaining chlorophyll content in rice leaves (Zhou, 2022). In the present study, ZmWAK9 was highly expressed in the V9 eighth leaf and during the early stages of seed development. It was significantly upregulated under armyworm, IAA, ABA, and GA treatments. Under salt stress, ZmWAK9 was upregulated by 103.1% in salt-tolerant material but downregulated by 43.5% in salt-sensitive material. This finding suggests that ZmWAK9 might be involved in the regulation of plant growth and development and responses to multiple stresses via hormone signaling pathways.
ZmWAK15 was significantly upregulated by 1.61–5.67-fold under smut stress compared to the control. A previous study reported that the pathogen Sporisorium reilianum activated ZmWAK15 (Zhang et al., 2024). This activation triggered the WAK-SnRK1a2-WRKY53 module, which led to the downregulation of genes associated with transmembrane transport and carbohydrate metabolism. Consequently, the fungus experienced nutrient starvation in the intercellular spaces, resulting in enhanced resistance to smut disease in maize (Zhang et al., 2024). In the present study, ZmWAK15 was highly expressed in multiple tissues of maize, including the root, stem, SAM, leaf, and seed, with the highest expression level observed in the roots. This finding shows that ZmWAK15 is involved not only in maize smut resistance but also in the regulation of maize growth and development. It suggests that ZmWAK15 is one of the most important members in maize WAK gene family and plays an important role in maize.
ZmWAK27 encodes a WAK-related receptor-like protein kinase. ZmWAK27 and AtWAKL20 fall within the same evolutionary clade, sharing a homology of 49.12%. Under ABA and JA treatments, The expression of AtWAKL20 was significantly suppressed (Chae et al., 2009). AtWAKL20 had been found to negatively regulate the immune response by phosphorylating the Ser470 residue in the NB-ARC domain of BSR1 (Zhong et al., 2025). In the current study, under temperature stress, ZmWAK27 was markedly downregulated by 78.3–95.78%. In contrast, under salt stress, ZmWAK27 was considerably upregulated by 5.31- and 9.39-fold in salt-sensitive and -tolerant inbred lines, respectively. Furthermore, under waterlogging, smut, gray leaf spot, and beet armyworm stresses, ZmWAK27 was upregulated by 50.67–3.52-fold, 12.08–45.48-fold, 0.72–12.55-fold, and 4.07–20.26-fold, respectively. Thus, ZmWAK27 can be induced by various stresses, suggesting its involvement in the responses to both biotic and abiotic stresses.
ZmWAK41 encodes a serine/threonine receptor-like kinase, which is homologous to rice RLG2 with a sequence similarity of 67.27%. Rice RLG2 encodes the rust resistance kinase Lr10, which was initially discovered in wheat and plays a crucial role in the plant’s defense mechanism against leaf rust pathogens (Loutre et al., 2009; Kurt and Yagdi, 2021). ZmWAK41 was substantially upregulated by 4.79-fold at 4 hours after waterlogging treatment. Under smut and gray leaf spot stresses, ZmWAK41 was considerably upregulated by 6.34–33.1-fold and 0.67–12.79-fold compared to the control, respectively. At 1 and 24 hours after beet armyworm treatment, ZmWAK41 was upregulated by 33.85% and 20.77%, respectively. Furthermore, under IAA, ABA, and GA treatments, ZmWAK41 was significantly upregulated by 1.94–3.42-fold compared to the control. The marked inductions of ZmWAK41 under waterlogging, smut, gray leaf spot, beet armyworm, IAA, ABA, and GA treatments indicate its potential role in mediating resistance to both biotic and abiotic stresses.
ZmWAK49 is homologous to Arabidopsis AtLRK10L1 (AT1G18390), with an amino acid sequence homology of 37.98%. AtLRK10L1 generates two different transcripts, AtLRK10L1.1 and AtLRK10L1.2, under the action of two different promoters (Shin et al., 2015). At-LRK10L1.2 is involved in ABA-mediated signaling. Its T-DNA insertion mutant exhibites reduced sensitivity to ABA but increased sensitivity to drought stress (Lim et al., 2015). We find that ZmWAK49 exhibites synteny with the rice gene LOC_Os05g47770 (Supplementary Table S6). In a previous study, LOC_Os05g47770 had been identified as the candidate gene associated with salt tolerance during the flowering stage of rice (Lekklar et al., 2019). At 24 and 72 hours post-inoculation of Magnaporthe oryzae, the expression of LOC_Os05g47770 in the near-isogenic lines, which carried resistance genes, were significantly upregulated by 4.5- and 10.49-fold, respectively (Jain et al., 2019). ZmWAK49 was highly expressed in the Z4 region of root tips at 7 days after sowing and in the V9 thirteenth leaf. Under salt stress, ZmWAK49 was upregulated by 0.59-fold in the salt-sensitive inbred line and by 2.63-fold in the salt-tolerant inbred line. Moreover, under smut, gray leaf spot, beet armyworm, and aphid stresses, ZmWAK49 was upregulated by 1.03–10.0-fold, 0.63–3.79-fold, 0.72–2.6-fold, and 0.6–1.7-fold, respectively. Furthermore, ZmWAK49 levels peaked at 24 hours post-treatment with IAA, ABA, and GA treatments, upregulating by 2.54-, 2.44-, and 2.51-fold compared to the control, respectively. Thus, ZmWAK49 was significantly induced by various hormones and stresses, suggesting that ZmWAK49 might contribute to plant development and stress responses via hormone signaling pathways.
5 Conclusion
In this study, a total of 56 ZmWAKs were identified in the maize genome. Based on chromosomal location, phylogenetic tree, gene structure, and conserved motif analyses, the identified genes were divided into seven subgroups, with 54 genes being mapped to maize chromosomes. Every subgroup displayed an analogous exon-intron structure and conserved motifs. Gene duplication and collinearity analyses revealed that the maize WAK gene family experienced SD and TD events. Among the duplication gene pairs, four pairs experienced positive selection, whereas seven pairs experienced purifying selection. A high degree of collinearity was observed between maize and monocotyledonous plants, such as rice and sorghum. Transcriptome data analysis showed that the expression patterns of ZmWAK in maize were distinct in various plant tissues and under different biotic and abiotic stresses. Notably, ZmWAK9, ZmWAK15, ZmWAK27, ZmWAK41, and ZmWAK49 were simultaneously and significantly induced by multiple stresses, implying that they might be crucial for maize growth and development as well as stress responses. Our results provided valuable insights into the function and evolution of maize WAKs, revealing potential candidate genes for stress tolerance research in maize.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. The transcriptome data from the hormonal treatment of maize reported in this study have been deposited in the National Center for Biotechnology Information (NCBI) under the BioProject accession number PRJNA1309972.
Author contributions
XL: Writing – original draft, Investigation, Methodology, Writing – review & editing. YB: Data curation, Writing – review & editing, Formal analysis. HF: Writing – review & editing, Formal analysis. YC: Writing – review & editing, Data curation. HC: Writing – review & editing, Formal analysis. PS: Validation, Writing – review & editing. YS: Investigation, Writing – review & editing. NL: Writing – review & editing, Formal analysis. YF: Project administration, Writing – review & editing. LL: Writing – review & editing, Project administration. LM: Writing – review & editing, Funding acquisition. CZ: Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the Doctoral Research Start-up Foundation of Liaocheng University (Project Number: 318052325), Shandong Provincial Natural Science Foundation (Project Number: K22LB56), Luxi Agricultural Standardization Internship Base Project (Project Number: K20LC0501), Investigation and Innovation of the Maize-Soybean Strip Intercropping System (Project Number: K24LD147).
Acknowledgments
We thank Chengjie Chen for providing technical assistance in bioinformatics, and we appreciate the linguistic assistance provided by Charlesworth during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1652811/full#supplementary-material
Supplementary Figure 1 | GO annotation analysis of ZmWAKs in maize genome. MF indicates molecular function. CC indicates cellular component. BP indicates biological process. Red dot plots indicate the most genes involved in that process, while small green plots indicate the least genes involved.
Supplementary Figure 2 | The expression pattern of WAK genes in maize under abiotic, biotic and hormonal treatments. Expression analysis was conducted on genes with high expression levels (TPM > 10) and the most significant variation under different stresses. For each gene, the expression value exhibiting the maximum variation (log2(Fold Change)) under each stress category was selected as the representative data for heatmap construction. Temperature represents the temperature stress. S_Salt and T_Salt represent the salt-stress sensitive and tolerant materials under salt stress, respectively. Drought_intolerant and Drought _tolerant represent the drought-stress intolerant and tolerant materials under drought stress, respectively. Waterlogging represents the waterlogging stress. Smut disease represents the smut stress. Leaf spot represents the gray leaf spot stress. Armyworm represents the beet armyworm stress. Aphid represents the aphid stress. IAA, ABA and GA represent the IAA, ABA, and GA treatments, respectively. The red color represents an escalation in expression, the green color represents a reduction in expression.
Supplementary Figure 3 | The function interaction network of maize WAK proteins. (A) The maize WAK protein interaction network constructed via homology modeling based on Arabidopsis thaliana proteins. (B) The maize WAK protein interaction network constructed via homology modeling based on rice proteins.
Supplementary Table 1 | Physicochemical parameters of 56 ZmWAK genes in maize genome.
Supplementary Table 2 | Conserved motif information in ZmWAK proteins. A total of 10 motifs are shown, and their E-values and sequence with are provided. The height of each letter represents the probability of amino acids at the respective position.
Supplementary Table 3 | Protein motif structure analysis of ZmWAK genes.
Supplementary Table 4 | Cis-acting elements in promoter regions of ZmWAK genes in maize.
Supplementary Table 5 | Function information of the cis-acting elements in the promoters of ZmWAK genes.
Supplementary Table 6 | Collinearity analysis of the WAK family genes among maize, Arabidopsis, soybean, rice and sorghum.
Supplementary Table 7 | Expression profiles of ZmWAK genes during root development.
Supplementary Table 8 | Expression profiles of ZmWAK genes during leaf development.
Supplementary Table 9 | Expression profiles of ZmWAK genes during seed development.
Supplementary Table 10 | Expression pattern of maize ZmWAK genes under temperature stress.
Supplementary Table 11 | Expression pattern of maize ZmWAK genes under salt stress.
Supplementary Table 12 | Expression pattern of maize ZmWAK genes under drought stress.
Supplementary Table 13 | Expression pattern of maize ZmWAK genes under waterlogging stress.
Supplementary Table 14 | Expression pattern of maize ZmWAK genes under smut stress.
Supplementary Table 15 | Expression pattern of maize ZmWAK genes under gray leaf spot stress.
Supplementary Table 16 | Expression pattern of maize ZmWAK genes under beet armyworm stress.
Supplementary Table 17 | Expression pattern of maize ZmWAK genes under aphid stress.
Supplementary Table 18 | Expression pattern of maize ZmWAK genes under the hormonal treatment.
Supplementary Table 19 | Expression patterns of maize ZmWAK genes under abiotic, biotic and hormonal treatments.
References
Abedi, A., Hajiahmadi, Z., Kordrostami, M., Esmaeel, Q., and Jacquard, C. (2021). Analyses of lysin-motif receptor-like kinase (LysM-RLK) gene family in allotetraploid Brassica napus L. and its progenitor species: an in silico study. Cells. 11 (1), 37. doi: 10.3390/cells11010037
Al-Bader, N., Meier, A., Geniza, M., Gongora, Y. S., and Jaiswal, P. (2019). Loss of premature stop codon in the wall-associated kinase 91 (oswak91) gene confers sheath blight disease resistance in rice. BioRxiv. 625509. doi: 10.1101/625509
Bai, W., Li, J., Zhang, D., Sun, F., Niu, Y., Wang, P., et al. (2024). A tyrosine kinase-like gene BdCTR1 negatively regulates flowering time in the model grass plant Brachypodium distachyon. J. Plant Growth Regul. 43, 4577–4587. doi: 10.1007/s00344-024-11418-4
Bot, P., Mun, B. G., Imran, Q. M., Hussain, A., Lee, S. U., Loake, G., et al. (2019). Differential expression of ATWAKL10 in response to nitric oxide suggests a putative role in biotic and abiotic stress responses. Peer J. 7, e7383. doi: 10.7717/peerj.7383
Brutus, A., Sicilia, F., Macone, A., Cervone, F. De, and Lorenzo., G. (2010). A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. U.S.A. 107, 9452–9457. doi: 10.1073/pnas.1000675107
Cannon, S. B., Mitra, A., Baumgarten, A., Young, N. D., and May, G. (2004). The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 4, 10. doi: 10.1186/1471-2229-4-10
Chae, L., Sudat, S., Dudoit, S., Zhu, T., and Luan, S. (2009). Diverse transcriptional programs associated with environmental stress and hormones in the Arabidopsis receptor-like kinase gene family. Mol. Plant. 2, 84–107. doi: 10.1093/mp/ssn083
Chen, Y., Han, Y., Huang, W., Zhang, Y., Chen, X., Li, D., et al. (2024). Lazarus 1 functions as a positive regulator of plant immunity and systemic acquired resistance. Front. Plant Sci. 20. doi: 10.3389/fpls.2024.1490466
Chen, C., Wu, Y., Li, J., Wang, X., Zeng, Z., Xu, J., et al. (2023). TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant. 16, 1733–1742. doi: 10.1016/j.molp.2023.09.010
Chen, S., Zhou, Y., Chen, Y., and Gu, J. (2018). Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 34, i884–i890. doi: 10.1093/bioinformatics/bty560
Conant, G. C., Bircher, J. A., and Pires, J. C. (2014). Dosage, duplication and diploidization: clarifying the interplay of multiple models for duplicate gene evolution over time. Curr. Plant Biol. 19, 91–98. doi: 10.1016/J.PBI.2014.05008
Dai, Z., Pi, Q., Liu, Y., Hu, L., Li, B., Zhang, B., et al. (2024). ZmWAK02 encoding an RD-WAK protein confers maize resistance against gray leaf spot. New Phytologist. 241, 1780–1793. doi: 10.1111/nph.19465
Decreux, A. and Messiaen, J. (2005). Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol. 46, 268–278. doi: 10.1093/pcp/pci026
Dou, L. L., Li, Z. F., Shen, Q., Shi, H. R., Li, H. Z., Wang, W. B., et al. (2021). Genome-wide characterization of the WAK gene family and expression analysis under plant hormone treatment in cotton. BMC Genomics. 22, 85. doi: 10.1186/s12864-021-07378-8
Gao, S., Zhang, Z., Zhao, Y., Li, X., Wu, Y., Huo, W., et al. (2024). Cell wall-associated receptor kinase GbWAKL26 positively regulate salt tolerance by maintaining Na+ and K+ homeostasis in cotton. Environ. Exp. Bot. 226, 105926. doi: 10.1016/j.envexpbot.2024.105926
Han, E., Geng, Z., Qin, Y., Wang, Y., and Ma, S. (2024). Single-cell network analysis reveals gene expression programs for Arabidopsis root development and metabolism. Plant Commun. 5, 100978. doi: 10.1016/j.sxplc.2024.100978
Han, G. H., Huang, R. N., Hong, L. H., Xu, J. X., Hong, Y. G., Wu, Y. H., et al. (2023). The transcription factor NAC102 confers cadmium tolerance by regulating WAKL11 expression and cell wall pectin metabolism in Arabidopsis. J. Integr. Plant Biol. 65, 2262–2278. doi: 10.1111/jipb.13557
He, Z. H., Cheeseman, I., He, D., and Kohorn, B. D. (1999). A cluster of five cell wall-associated receptor kinase genes, WAK1-5, are expressed in specific organs of Arabidopsis. Plant Mol. Biol. 39, 1189–1196. doi: 10.1023/A:1006197318246
He, Z., Zhang, H., Gao, S., Lercher, M. J., Chen, W. H., and Hu, S. (2016). Evolview v2: an online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res. 44, W236–W241. doi: 10.1093/nar/gkw370
Hu, Z. (2023). The functional study of wall-associated receptor kinase OsWAK12 in regulating rice root development. (Zhengzhou, China: Henan Agriculture University). doi: 10.27117/d.cnki.ghenu.2023.000383
Hu, K., Dai, Q., Ajayo, B. S., Wang, H., Hu, Y., Li, Y., et al. (2023). Insights into ZmWAKL in maize kernel development: genome-wide investigation and ga-mediated transcription. BMC Genomics. 24, 15. doi: 10.1186/s12864-023-09849-6
Hu, B., Liu, B., Liu, L., Liu, X., Xu, L., and Ruan, Y. (2014). Epigenetic control of Pollen Ole e1 allergen and extensin family gene expression in Arabidopsis thaliana. Acta Physiol. Plant. 36, 2203–2209. doi: 10.1007/s11738-014-1597-6
Hurni, S., Scheuermann, D., Krattinger, S. G., Kessel, B., Wicker, T., Herren, G., et al. (2015). The maize disease resistance gene Htn1 against northern corn leaf blight encodes a wall-associated receptor-like kinase. Proc. Natl. Acad. Sci. U. S. A. 112, 8780–8785. doi: 10.1073/pnas.1502522112
Jain, P., Dubey, H., Singh, P. K., Solanke, A. U., and Sharma, T. R. (2019). Deciphering signaling network in broad spectrum near isogenic lines of rice resistant to Magnaporthe oryzae. Sci. Rep. 9, 16939. doi: 10.1038/s41598-019-50990-8
Kanneganti, V. and Gupta, A. K. (2008). Wall associated kinases from plants-an overview. Physiol. Mol. Bio Plants. 14, 109–118. doi: 10.1007/s12298-008-0010-6
Kin, D., Langmead, B., and Salzberg, S. L. (2015). HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 12, 357. doi: 10.1038/nmeth.3317
Kohorn, B. D., Greed, B. E., Mouille, G., Verger, S., and Kohorn, S. L. (2021). Effects of Arabidopsis wall associated kinase mutations on ESMERALDA1 and elicitor induced ROS. PloS One. 16, e0251922. doi: 10.1371/journal.pone.0251922
Kurt, P. and Yagdi, K. (2021). Identifying leaf rust resistance gene Lr10 in some bread wheat using simple sequence repeat (SSR) marker. Kafkas University Journal of Faculty of Agriculture. 24, 850–858. doi: 10.5555/20210359599
Lally, D., Ingmire, P., Tong, H. Y., and He, Z. H. (2001). Antisense expression of a cell wall-associated protein kinase, WAK4, inhibits cell elongation and alters morphology. Plant Cell. 13, 1317–1331. doi: 10.2307/3871298
Lekklar, C., Pongpanich, M., Suriya-Arunroj, D., Chinpongpanich, A., Tsai, H., Comai, L., et al. (2019). Genome-wide association study for salinity tolerance at the flowering stage in panel of rice accessions from Thailand. BMC Genomics. 20, 76. doi: 10.1186/s12864-018-5317-2
Lerouge, P. (2010). Characterization of a putative 3-deoxy-D-mano-2-octulosonic acid (kdo) tranferase gene from Arabidopsis thaliana. Glycobiology. 20, 617–628. doi: 10.1093/glycob/cwq011
Li, B. and Dewey, C. N. (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 12, 323. doi: 10.1186/1471-2105-12-323
Li, X., Qi, S. F., Meng, L. Z., Su, P. S., Sun, Y. W., Li, N., et al. (2025). Genome-wide identification of the wall-associated kinase gene family and their expression patterns under various abiotic stresses in soybean (Glycine max (L.) Merr). Front. Plant Sci. 15, 1511681. doi: 10.3389/fpls.2024.1511681
Li, Y., Wang, X., Li, Y., Zhang, Y., Gou, Z., Qi, X., et al. (2020). Transcriptomic analysis revealed the common and divergent responses of maize seedling leaves to cold and heat stresses. Genes (Basel). 11, 881. doi: 10.3390/genes11080881
Lim, C. W., Yang, S. H., Shin, K. H., Lee, S. C., and Kim, S. H. (2015). The AtLRK10L1.2, Arabidopsis ortholog of wheat LRK10, is involved in ABA-mediated signaling and drought resistance. Plant Cell Rep. 34, 447–455. doi: 10.1007/s00299-014-1724-2
Loutre, C., Wicker, T., Travella, S., Galli, P., Scofield, S., Fahima, T., et al. (2009). Two different CC-NBS-LRR genes are required for Lr10-mediated leaf rust resistance in tetraploid and hexaploid wheat. Plant J. 60, 1043–1054. doi: 10.1111/j.1365-313x.2009.04024.x
Lynch, M. and Conery, J. S. (2000). The evolutionary fate and consequences of duplicate genes. Science. 290, 1151–1155. doi: 10.1126/science.290.5494.1151
Ma, Y., Wang, Z., Humphries, J., Ratcliffe, J., Bacic, A., Johnson, K. L., et al. (2024). Wall-associated kinase like 14 regulates vascular tissue development in Arabidopsis and tomato. Plant Sci. 341, 112013. doi: 10.1016/j.plantsci.2024.112013
Roulin, A., Auer, P. L., Libault, M., Schlueter, J., Farmer, A., May, G., et al. (2013). The fate of duplicated genes in a polyploid plant genome. Plant J. 73, 143–153. doi: 10.1111/tpj.12026
Schurack, S., Depotter, J., Gupta, D. K., Thines, M., and Doehlemann, G. (2021). Comparative transcriptome profiling identifies maize line specificity of fungal effectors in the maize: stilago maydis interaction. Plant J. 106, 733–752. doi: 10.1111/tpj.15195
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. doi: 10.1101/gr.1239303
Shin, K. H., Yang, S. H., Lee, J. Y., Lim, C. W., Brown, J. W. S., and Kim, H. H. (2015). Alternative splicing of mini-exons in the Arabidopsis leaf rust receptor-like kinase lrk10 genes affects subcellular localisation. Plant Cell Rep. 34, 495–505. doi: 10.1007/s00299-014-1729-x
Shiu, S. H. and Bleecker, A. B. (2001). Plant receptor-like kinase gene family: diversity, function, and signaling. Stke. 113, re22. doi: 10.1126/stke.2001.113.re22
Sivaguru, M., Ezaki, B., He, Z. H., Tong, H., Osawa, H., Baluska, F., et al. (2003). Aluminum-induced gene expression and protein localization of a cell wall-associated receptor kinase in Arabidopsis. Plant Physiol. 132, 2256–2266. doi: 10.1104/pp.103.022129
Soares, A. L. C., Geilfus, C. M., and Carpentier, S. C. (2018). Genotype-specific growth and proteomic responses of maize toward salt stress. Front. Plant Sci. 30. doi: 10.3389/fpls.2018.00661
Stelpflug, S. C., Sekhon, R. S., Vaillancourt, B., Hirsch, C. N., Buell, C. R., Leon, N. D., et al. (2016). An expanded maize gene expression atlas based on RNA sequencing and its use to explore root development. Plant Genome. 9 (1), 1–16. doi: 10.3835/plantgenome2015.04.0025
Sun, Y., Wang, D., Shi, M., Gong, Y., Yin, S., Jiao, Y., et al. (2023). Genome-wide identification of actin-depolymerzing fator gene family and their expression partterns under various abiotic stresses in soybean (Glycine max). Front. Plant Sci. 14, 1236175. doi: 10.3389/fpls.2023.1236175
Tzin, V., Fernande-Pozo, N., Richter, A., Schmelz, E. A., Schoettner, M., Schäfer, M., et al. (2015). Dynamic maize responses to aphid feeding are revealed by a time series of transcriptomic and metabolomic assays. Plant Physiol. 169, 1727–1743. doi: 10.1104/pp.15.01039
Verica, J. A. and He, Z. H. (2002). The cell wall-associated kinase (WAK) and WAK-like kinase gene family. Plant Physiol. 129, 455–459. doi: 10.1104/pp.011028
Wagner, T. A. and Kohorn, W. D. B. (2001). Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell. 13, 303–318. doi: 10.1105/tpc.13.2.303
Wang, Y., Derr, D. C., Chen, G. P., and Gu, Y. Q. (2019a). Orthovenn2: a web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nuclc Acids Res. 43, W52–W58. doi: 10.1093/nar/gkv487
Wang, D., Du, M., Lyu, P., Li, J., Meng, H., Liu, X., et al. (2024a). Functional characterization of the soybean Glycine max actin depolymenrization factor GmADF13 for plant resistance to drought stress. Plants. 13, 1651. doi: 10.3390/plants13121651
Wang, S., Meng, Y., Ding, F., Yang, K., Wang, C., Zhang, H., et al. (2024b). Comparative analysis of TPR gene family in Cuccurbitaceae and expression profiling under abiotic stress in Cucumis melo L. Horticulturae. 10, 83. doi: 10.3390/horticulturae10010083
Wang, H., Niu, H. H., Liang, M. M., Zhai, Y. F., Huang, W., Ding, Q., et al. (2019b). A wall-associated kinase gene CaWAKL20 from pepper negatively modulates plant thermotolerance by reducing the expression of ABA-responsive genes. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00591
Wasternack, C. and Hause, B. (2013). Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Botany. 111, 1021–1058. doi: 10.1093/aob/mct067
Whyte, T. D. and Sipahi, H. (2024). Bioinformatic and expression analyses of the wall-associated kinase genes under high-temperature stress in sorghum. Physiol. Plant. 176, e14125. doi: 10.1111/ppl.14125
Xia, X. B., Zhang, X., Zhang, Y. C., Wang, L. R., An, Q., Tu, Q., et al. (2022). Characterization of the WAK gene family reveals genes for FHB resistance in bread wheat (Triticum aestivum L.). Int. J. Mol. Sci. 23, 7157. doi: 10.3390/ijms23137157
Yang, M., Han, Y. Z., HABAIKE, A. Y. J., and Wang, C. L. (2017). Study of RLK6, one member of leucine-rich repeat receptor-like kinases (LRR-RLKs) subfamily genes, on process of flowering in Arabidopsis. J. Nucl. Agric. Sci. 31, 0654–0662. doi: 10.11869/j.issn.100-8551.2017.04.0654
Yang, J., Xie, M., Wang, X., Wang, G., Zhang, Y., Li, Z., et al. (2021). Identification of cell wall-associated kinases as important regulators involved in Gossypium hirsutum resistance to Verticillium dahliae. BMC Plant Biol. 21, 220. doi: 10.1186/s12870-021-02992-w
Yao, X. C., Humphries, J., Johnson, K. L., Chen, J. H., and Ma, Y. X. (2025). Function of WAKs in regulating cell wall development and responses to abiotic stress. Plants. 14, 343. doi: 10.3390/plants14030343
Yin, X. Y. and Hou, X. W. (2017). Role of OsWAK124, a rice wall-associated kinase, in response to environmental heavy metal stresses. Pak J. Bot. 49, 1255–1261.
Yu, Y., Shi, J., Li, X., Liu, J., Geng, Q., Shi, H., et al. (2018). Transcriptome analysis reveals the molecular mechanisms of the defense response to gray leaf spot disease in maize. BMC Genomics. 19, 1–17. doi: 10.1186/s12864-018-5072-4
Yu, F., Tan, Z., Fang, T., Tang, K., Liang, K., and Qiu, F. (2020). A comprehensive transcriptomics analysis reveals long non-coding RNA to be involved in the key metabolic pathway in response to waterlogging stress in maize. Genes (Basel). 11, 267. doi: 10.3390/genes11030267
Yu, Q., Zhang, Y. C., Liu, T. Y., Wang, L., Liu, Y., Yu, S. W., et al. (2025). Genome-wide characterization of cysteine-rich receptor-like kinase (CRK) gene family in rice and OsCRK26 functional analysis in response to drought stress. Plant Stress. 15, 100733. doi: 10.1016/j.stress.2024.100733
Zhang, N. (2016). ZmWAK-mediated head smut resistance. [PhD thesis]. Beijing, China: Chinese Agricultural University. doi: 10.27628/d.cnki.gzndu.2016.000080
Zhang, S., Chen, C., Li, L., Meng, L., Singh, J., Jiang, N., et al. (2005). Evolutionary expansion, gene structure, and expression of the rice wall-associated kinase gene family. Plant Physiol. 139, 1107–1124. doi: 10.1104/pp.105.069005
Zhang, F., Huang, L. Y., Wang, W. S., Zhao, X. Q., Zhu, L. H., Fu, B. Y., et al. (2012). Genome-wide gene expression profiling of introgressed indica rice alleles associated with seeding cold tolerance improvement in a japonica rice background. BMC Genomics. 13, 461. doi: 10.1186/1471-2164-13-461
Zhang, Z., Ma, W., Ren, Z., Wang, X., Zhao, J., Pei, X., et al. (2021). Characterization and expression analysis of wall-associated kinase (WAK) and WAK-like family in cotton. Int. J. Biol. Macromol. 187, 867–879. doi: 10.1016/j.ijbiomac.2021.07163
Zhang, Z. Q., Ma, W. Y., Wang, H. J., Ren, Z. Y., Liu, Y. G., He, K. L., et al. (2025). Characterization of the wall-associated kinase (WAK) gene family in Gossypium barbadense reveals the positive role of GbWAK5 in salt tolerance. Plant Cell Rep. 44, 1–18. doi: 10.1007/s00299-024-03407-4
Zhang, Q. Q., Xu, Q. Y., Zhang, N., Zhang, T., Xing, Y. X., Fan, Z., et al. (2024). A maize WAK-SnRK12-WRKY module regulates nutrient availability to defend against head smut disease. Mol. Plant. 17, 1654–1671. doi: 10.1016/j.molp.2024.09.013
Zhong, C., Li, W., Zhang, X., Zhang, D., Wen, Z., Song, W., et al. (2025). A cell wall-associated kinase phosphorylates NLR immune receptor to negatively regulate resistosome formation. Nat. Plants. 11, 561–579. doi: 10.1038/s41477-025-01949-3
Zhou, W. F. (2022). Effects of OsPID gene overexpression on rice morphology and physiology and its role in low phosphprus response. [Master’s thesis]. (Yangzhou, China: Yangzhou University). doi: 10.27441/d.cnki.gyzdu.2022.000646
Zuo, N., Bai, W., Wei, W. Q., Yuan, T. L., Zhang, D., Wang, Y. Z., et al. (2022). Fungal CFEM effectors negatively regulate a maize wall-associated kinase by interacting with its alternatively spliced variant to dampen resistance. Cell Rep. 41, 111877. doi: 10.1016/j.celrep.2022.111877
Keywords: maize (Zea mays L.), WAK gene family, abiotic and biotic stresses, hormonal treatments, expression pattern analysis
Citation: Li X, Bi Y, Feng H, Cai Y, Chen H, Su P, Song Y, Li N, Fan Y, Liu L, Meng L and Zong C (2025) Roles of maize WAK gene family in responses to abiotic and biotic stresses, and hormonal treatments. Front. Plant Sci. 16:1652811. doi: 10.3389/fpls.2025.1652811
Received: 27 June 2025; Accepted: 25 August 2025;
Published: 16 September 2025.
Edited by:
Martin Raspor, University of Belgrade, SerbiaReviewed by:
Wei-Hua Tang, Chinese Academy of Sciences (CAS), ChinaBin Zhang, Beijing Vegetable Research Center, China
Terik Djabeng Whyte, Eskişehir Osmangazi University, Türkiye
Jelena Rudic, Institute for Biological Research “Siniša Stanković” – National Institute of Republic of Serbia, Serbia
Copyright © 2025 Li, Bi, Feng, Cai, Chen, Su, Song, Li, Fan, Liu, Meng and Zong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingzhi Meng, bWVuZ2xpbmd6aGk2QDE2My5jb20=; Chunmei Zong, em9uZ2NtQGhhYXMuY24=
 Xiangnan Li
Xiangnan Li Yuwei Bi1
Yuwei Bi1 Peisen Su
Peisen Su Yong Song
Yong Song Nan Li
Nan Li Lingzhi Meng
Lingzhi Meng Chunmei Zong
Chunmei Zong