- College of Life Sciences, Zhejiang Normal University, Jinhua, China
Boron (B) is a vital micronutrient necessary for the proper development of plants. However, B exhibits a very narrow concentration range between deficiency and toxicity in plants, making precise regulatory control over its uptake, translocation, and cellular efflux critical for maintaining overall B homeostasis. Genetic analyses of Arabidopsis thaliana show that boron uptake and translocation are mediated by two families of transmembrane transporter proteins: NIPs (nodulin-26-like intrinsic proteins), which facilitate the permeation of boric acid, and BORs, responsible for exporting borate from cells. Importantly, the identification and characterization of NIPs and BORs have been essential for elucidating B homeostasis and its physiological roles not only in Arabidopsis but also in diverse plant species. Furthermore, the homeostasis of B is maintained by multi-level regulation of its transport proteins, including transcriptional modulation, mRNA stability, translational repression, and endocytic degradation. Moreover, modulating B transport gene expression to enhance tolerance to B deficiency or toxicity can improve plant growth under unfavorable B nutrient conditions. Therefore, generating B-efficient or B-tolerant plants is a cost-effective and sustainable agricultural strategy. In this review, we discuss the physiological roles of B transport proteins and their regulatory mechanisms, focusing on intracellular localization and abundance.
1 Introduction
Nutrients are categorized as either macronutrients or micronutrients based on the quantities required for growth. These nutrients play a crucial role in regulating cellular electrochemical balance, function as biochemical cofactors, and serve as structural components within biomolecules and complexes (Baxter, 2009). Boron (B) is an essential micronutrient for normal development of plants, naturally present in the soil as boric acid (H3BO3) or borate [B(OH4)-] depending on the pH of the soil solution (Warrington, 1923; Lilay et al., 2024). Under physiological conditions, B is present primarily as boric acid in solution; boric acid is a weak Lewis acid with a pKa of 9.24, [B(OH)3 +H2O ⇋ B(OH)4– +H+] (Power and Woods, 1997). Boron plays varied and complex roles in plant development, as shown by the diverse phenotypes of deficient plants. One of the primary functions of B is to facilitate the cross-linking of the pectic polysaccharide RG-II within cell walls (Kobayashi et al., 1996; Ishii and Matsunaga, 1996; Ishii et al., 1999; O’Neill et al., 2001), where over 90% of the RG-II in the plant cell wall is cross-linked by B (O’Neill et al., 2004; Matsunaga et al., 2004). In addition, it has been proposed that B serves as a component of both the plasma membrane (PM) and the cytoskeleton of the cell (Bassil et al., 2004; Voxeur and Fry, 2014).
There is a narrow range of B concentrations that supports plant growth, outside this range B can be toxic or cause deficiency symptoms. B deficiency symptoms primarily occur during plant growth, leading to inhibited expansion of young leaves, reduced root elongation, and loss of fertility (Dell and Huang, 1997; Shorrocks, 1997). On the other hand, B toxicity disrupts cellular metabolism, induces oxidative stress, promotes membrane lipid peroxidation, and triggers DNA damage, often leading to tissue necrosis (Reid et al., 2004; Sakamoto et al., 2011). Therefore, to prevent B deficiency or toxicity, plants require B transport systems in response to B levels. Since B cannot be readily re-translocated from mature to developing organs, B must be continuously absorbed from soil and transport to growing tissues in plants (Brown and Shelp, 1997). There were three distinct mechanisms reported for plants to acquire B from soil: (1) passive diffusion of uncharged boric acid under sufficient or high B availability; (2) active uptake, primarily under B-deficient conditions; and (3) facilitated diffusion mediated by channel proteins (Wimmer and Eichert, 2013). Recent findings have provided important insights into B transport in plants, along with advances in understanding its regulation. Here, we investigate B transport mechanisms, focusing on the key transporters involved, their physiological functions, and regulatory pathways.
2 Boron channels and transporters
2.1 Characterization of boron transporters
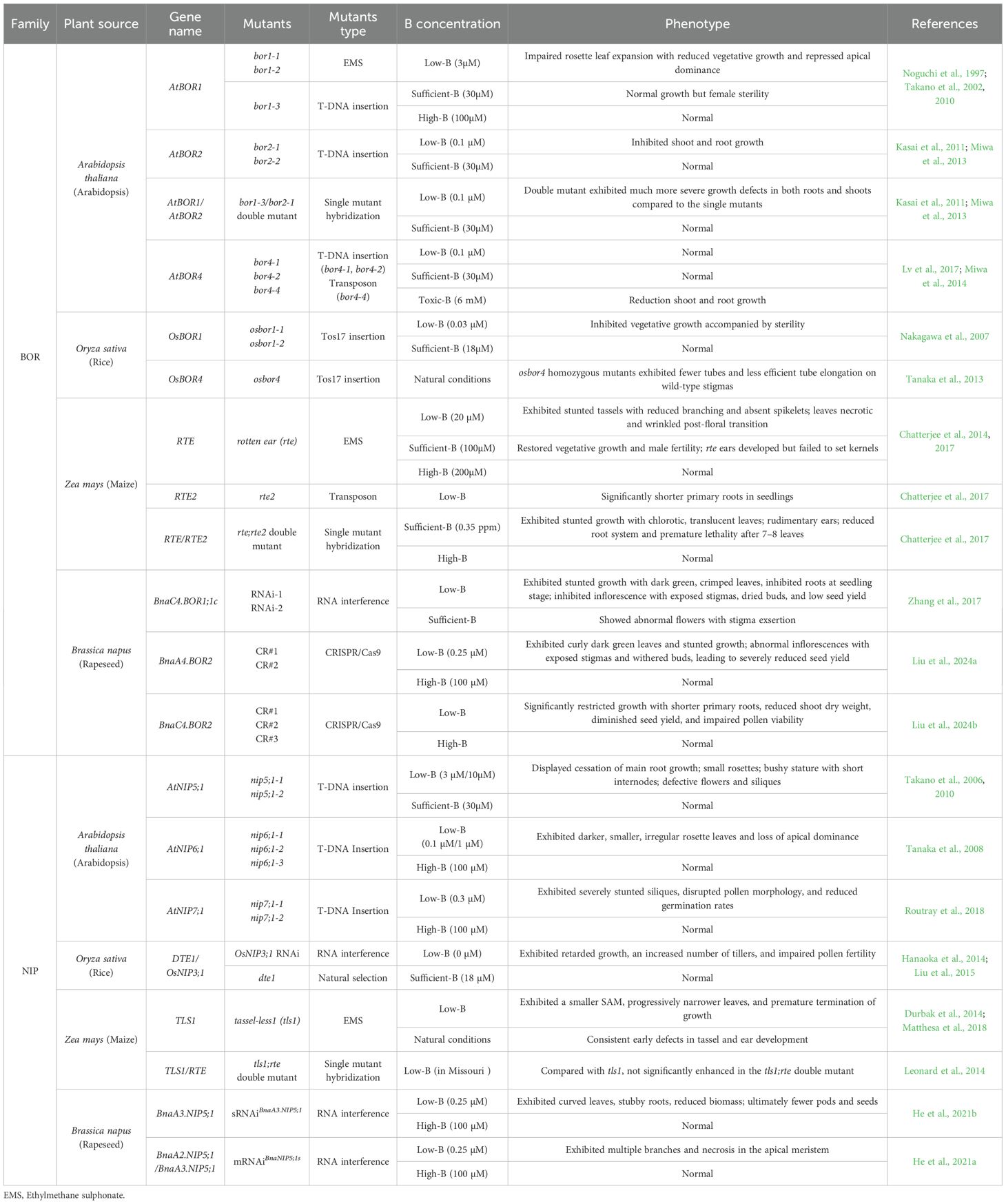
B transport processes have traditionally been regarded as predominantly passive (Marschner, 1995). This perspective is largely due to the fact that boric acid, which is a principal form of B under physiological conditions, exists as an uncharged molecule that readily diffuses across the plasma membrane (Takano et al., 2008). However, several physiological experiments have identified active mechanisms for B transport. Dannel et al. (2000) demonstrated that B transport in sunflower (Helianthus annuus) occurs via carrier or channel-mediated processes. Major breakthroughs in understanding B transport mechanisms began with the identification of Arabidopsis BOR1 (AtBOR1) as the first known biological B transporter (Takano et al., 2002). Regarding the uptake and translocation of B in plants, this process is ensured by two transmembrane transporter protein families (Figure 1): (1) channel proteins from the NIPs (nodulin-26 like intrinsic proteins) family, which are boric acid channels that enable the passive transmembrane flow of uncharged boric acid, driven by concentration gradients; and (2) efflux transporters belonging to the BOR family, which mediate the efflux of borate ions (Miwa and Fujiwara, 2010).
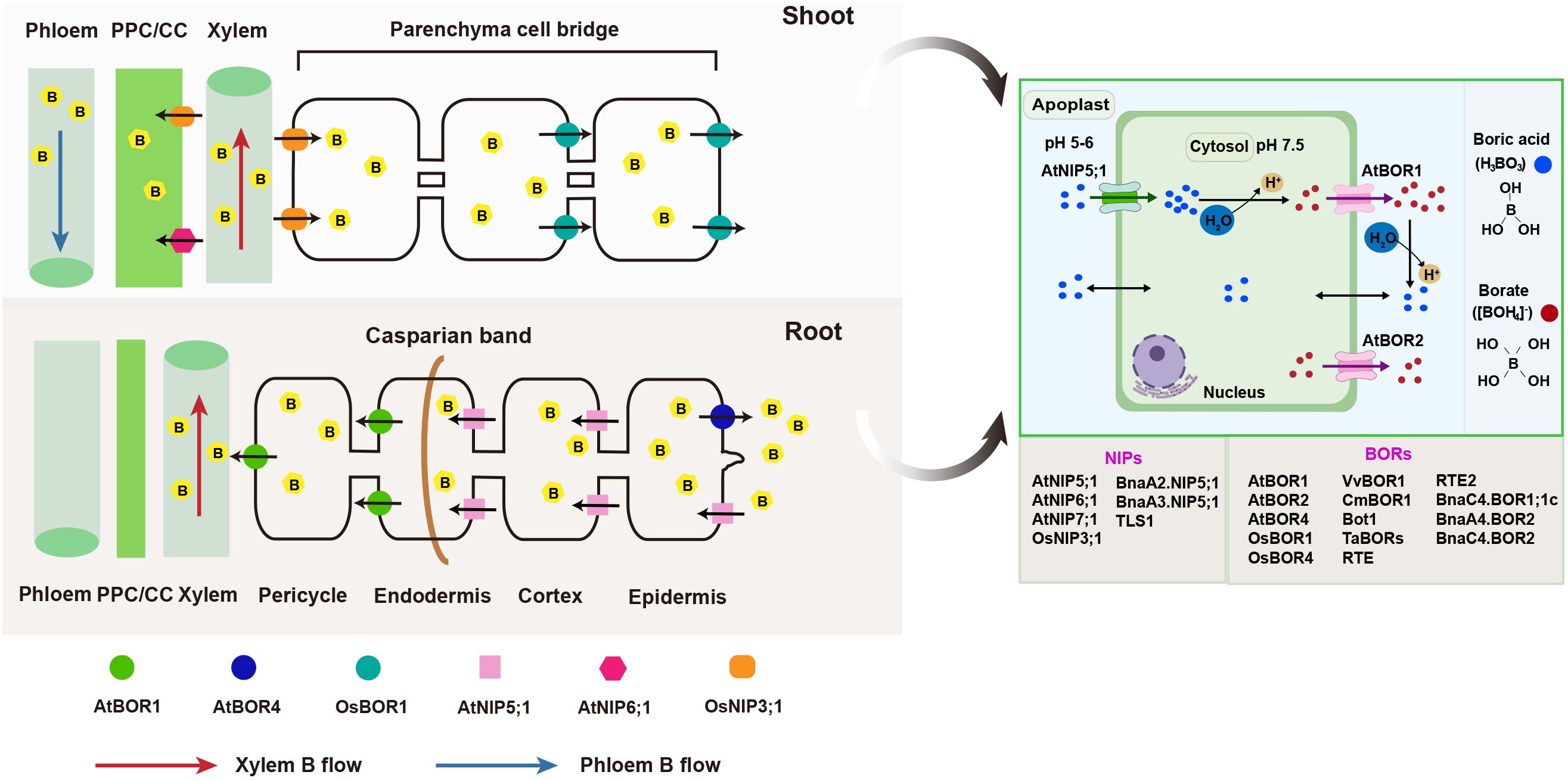
Figure 1. Overview of boron transporters in plants. Under low B conditions, AtNIP5;1 imports boric acid into epidermal, cortical, and endodermal cells, while AtBOR1 export boric acid/borate from stelar cells via xylem loading. Under excess B conditions, AtBOR4 enhances plant tolerance by mediating B export from roots to the soil. B is transported from roots to nodal regions via xylem, then unloaded and transferred across companion and phloem parenchyma cells to the phloem through AtNIP6;1. In rice, OsNIP3;1 is polarly localized at the xylem parenchyma cells and mediates the unloading of B from the xylem for intervascular transfer. The efflux of B for this intervascular transfer is then mediated by OsBOR1. Additionally, OsNIP3;1, located at the phloem cells, also facilitates B influx into the phloem for preferential distribution. Under physiological conditions, boric acid enters cells via specific channels. In the slightly alkaline cytosol (pH ~7.5), it is converted into borate anions and exported by borate uniporters. These anions are then reconverted to boric acid in the lower-pH (5-6) apoplast. NIPs and BORs from different plant species are listed in the colored boxes. At, Arabidopsis thaliana; Bna, Brassica napus; Cm, Citrus macrophylla; Os, Oryza sativa; PPC/CC, phloem parenchyma cells/companion cells; Ta, Triticum aestivum; Vv, Vitis vinifera.
2.2 Functions of NIPs in B transport
The molecular mechanisms of B uptake and transport in plants have been most extensively studied in Arabidopsis (Miwa and Fujiwara, 2010; Onuh and Miwa, 2021). Major intrinsic proteins (MIPs) family have been identified as boric acid channels (Wallace et al., 2006). Plant MIPs are classified into four distinct groups: the tonoplast intrinsic proteins (TIPs), the plasma membrane intrinsic proteins (PIPs), the nodulin 26 (NOD26)-like intrinsic proteins (NIPs) and the small basic intrinsic proteins (SIPs) (Wallace et al., 2006; Maurel et al., 2015). NIPs are further classified into three subclasses (I-III) based on their pore structures, and the physiological function of NIP I proteins remains unclear, while NIP II and III are known to transport boric acid and silicic acid, respectively (Wallace and Roberts, 2004; Danielson and Johanson, 2010; Roberts and Routray, 2017). In Arabidopsis, the NIP subfamily consists of nine genes (Johanson et al., 2001), including three members belonging to the NIP II subgroup: AtNIP5;1, AtNIP6;1 and AtNIP7;1 (Wallace and Roberts, 2005).
AtNIP5;1, a major boric acid channel played a crucial role in B uptake from soil under B-limited conditions (Takano et al., 2006, 2010). AtNIP5;1 has been shown to be localized on plasma membrane of lateral root cap (LRC) and epidermal cells (Takano et al., 2010). A ThrProGly (TPG) repeat in the N-terminus of AtNIP5;1 was crucial for its polar localization and effective B transport in roots (Wang et al., 2017). Expression of the AtNIP5;1 was transcriptionally enhanced 10-fold in response to B limitation in roots (Takano et al., 2006). AtNIP6;1 was the most similar gene to AtNIP5;1 among the nine NIP genes in Arabidopsis and played a key role in the preferential translocation of B into young growing leaves (Wallace and Roberts, 2005; Tanaka et al., 2008). Limitation treatment and tracer experiments showed that B concentration were significantly reduced in young rosette leaves and shoot apices (reduced by 20% to 27%) under the conditions of B limitation in atnip6;1 mutants, suggesting that AtNIP6;1 was required for preferential distribution of B to sink tissues (e.g., young rosette leaves, shoot apices; Tanaka et al., 2008). Both AtNIP5;1 and AtNIP6;1 were the boric acid channels on plasma membrane, and AtNIP6;1 was completely impermeable to water and involved in xylem-phloem B transfer (Takano et al., 2006; Tanaka et al., 2008). Unlike AtNIP5;1, B limitation resulted in a slight transcriptional upregulation (1.4-fold) of AtNIP6;1 in stems, but no significant difference was observed in shoots (Tanaka et al., 2008). AtNIP7;1 was also identified as a boric acid channel expressed in floral anthers, functions as a water-tight boric acid permease and also transports glycerol at a lower rate (Li et al., 2011; Routray et al., 2018).
To date, several AtNIP5;1 homologous genes have been identified in different crops species, such as rice (Oryza sativa), rapeseed (Brassica napus) and maize (Zea mays) (Wallace et al., 2006; Durbak et al., 2014; Hua et al., 2016; He et al., 2021a). Rice OsNIP3;1 exhibited the highest degree of similarity to AtNIP5;1 (Wallace et al., 2006), and was expressed in the vascular bundles of both leaf sheaths and blades, as well as in the root exodermis and stele (Figure 2) (Hanaoka et al., 2014). In the nodes, OsNIP3;1 was polarly localized at the xylem parenchyma cells of enlarged vascular bundles (EVBs), facing toward the xylem vessels (Shao et al., 2018). OsNIP3;1 RNAi plants showed disrupted B distribution between leaf blades and sheaths (Hanaoka et al., 2014). Subsequently, it was demonstrated that OsNIP3;1 mediated the unloading B from xylem of EVBs in the nodes, thus promoting its preferential distribution to developing tissues under B-limited conditions (Shao et al., 2018). TLS1/ZmNIP3;1 protein possessed the ability to transport both water and boric acid in Xenopus laevis oocytes, widely expressed across multiple tissue types, with highest levels in floral tissues and particularly in silks (Durbak et al., 2014; Leonard et al., 2014). Two orthologous AtNIP5;1 genes, BnaA2.NIP5;1 and BnaA3.NIP5;1, each with distinct functions, playing a crucial role in the growth of B. napus under B deficiency (He et al., 2021b). BnaA2.NIP5;1 and BnaA3.NIP5;1 functioned coordinately for efficient boron uptake. BnaA2.NIP5;1 was primarily expressed in root epidermal cells, mediated uptake, while BnaA3.NIP5;1 was polar-localized in the distal part of LRC cells and promoted root growth under deficiency to support translocation to the shoot (He et al., 2021a, 2021).
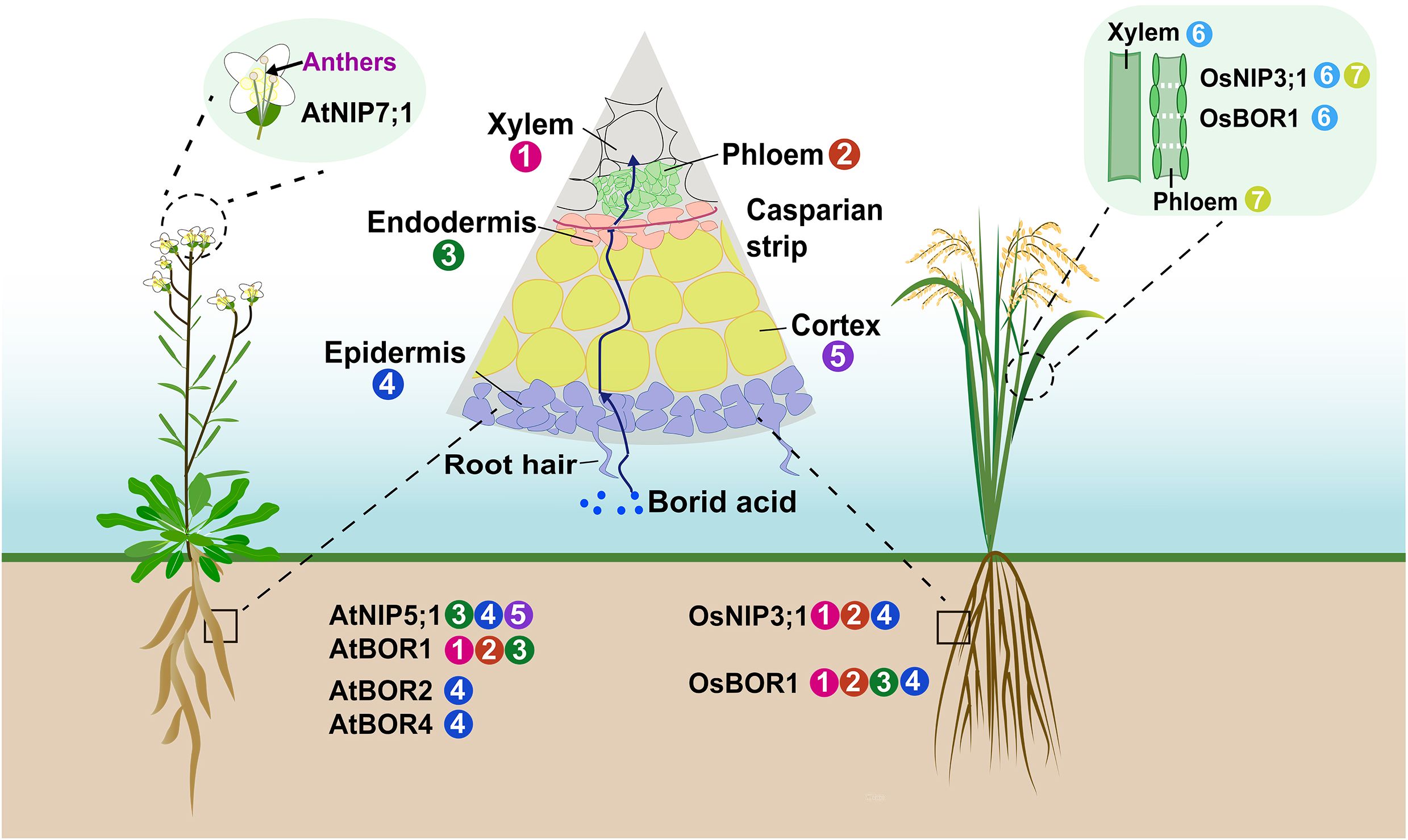
Figure 2. Distribution of boron transporters in various tissues. Illustration of Arabidopsis and rice plants, featuring magnifications that highlight various organs. The circled numbers adjacent to tissues indicated which transporters were predominantly expressed in each tissue. Note that not all tissues expressed these transporters.
2.3 Functions of BORs in B transport
AtBOR1 was an efflux-type B transporter that expressed in pericycle cells of the root stele (Figure 2), functioned in xylem loading and essential for preventing B deficiency in shoots (Noguchi et al., 2000; Takano et al., 2002). Subsequently, six AtBOR1-homology genes were identified in Arabidopsis (Frommer and Wiren, 2002). AtBOR2, the most similar paralog of AtBOR1, functioned in root cell elongation under conditions of B limitation (Miwa et al., 2013). The concentrations of shoot B were lower in atbor2 mutant than that in wild-type plants, but a more significant reduction was observed in atbor1–3 under low-B conditions (Miwa et al., 2013). Thus, the role of AtBOR2 contributed to the root-to-shoot translocation was minor than that of AtBOR1 (Miwa et al., 2013). Additionally, AtBOR4 encoded an efflux-type B transporter localized to the plasma membrane of the distal side of epidermal cells in roots and mitigated toxic levels of B in roots (Miwa et al., 2007; Miwa and Fujiwara, 2011; Miwa et al., 2014).
To date, functional BOR genes have been identified from different plants such as rice (Oryza sativa), wheat (Triticum aestivum), barley (Hordeum vulgare) and maize (Zea mays) (Nakagawa et al., 2007; Reid, 2007; Sutton et al., 2007; Chatterjee et al., 2014). In rice, OsBOR1, a close paralog of AtBOR1, functioned as an efflux transporter for B and played a crucial role in both the xylem loading of B and its uptake into roots (Nakagawa et al., 2007). This function, unlike AtBOR1’s exclusive role in xylem loading, resembled the combined roles of AtBOR1 and AtNIP5;1 in Arabidopsis. A recent study showed that OsBOR1 was highly expressed in the nodes, where it mediated B efflux from cells toward diffuse vascular bundles (DVBs) for delivering B to developing tissues (Shao et al., 2021). OsBOR1 cooperated with OsNIP3;1 to establish a coordinated system for the preferential distribution of B to developing tissues (Shao et al., 2021). In wheat, three functional BORs (TaBOR1.1, TaBOR1.2 and TaBOR1.3) were reported to localize on the plasma membrane in Arabidopsis leaf cells, and exhibit B efflux activity in BY-2 cells (Leaungthitikanchana et al., 2013). In maize, the B efflux transporter ROTTEN EAR (RTE) functioned as a co-ortholog of AtBOR1 and was predominantly expressed in the cells surrounding the xylem within both vegetative and reproductive tissues (Chatterjee et al., 2014); RTE2 encoded a protein similar to its paralog RTE and could completely recover the deficiency of atbor1 mutant in Arabidopsis (Chatterjee et al., 2017). RTE and RTE2 were all predominantly localized on the plasma membrane (Chatterjee et al., 2017).
AtBOR homologous genes have also been isolated from dicotyledonous species, such as grapevine (Vitis vinifera), citrus (Citrus macrophylla) and rapeseed (Brassica napus) (Pérez-Castro et al., 2012; Sun et al., 2012; Cañon et al., 2013). CmBOR1 from C. macrophylla and VvBOR1 from V. vinifera were both homologous to AtBOR1 (Cañon et al., 2013; Pérez-Castro et al., 2012). Functional assays in yeast showed that CmBOR1 mediated B efflux (Cañon et al., 2013), while VvBOR1 localized to the proximal plasma membrane of root pericycle cells and restored the wild-type phenotype in Arabidopsis atbor1–3 mutants under B deficiency (Pérez-Castro et al., 2012). In B. napus, BnaC4.BOR1;1c was widely expressed in shoot nodes and localized to the plasma membrane, displaying characteristics generally similar to AtBOR1 (Zhang et al., 2017); Nevertheless, BnaC4.BOR1;1c showed distinctive features compared with AtBOR1, it was also extensively expressed in immature floral buds, and preferential distribution of B to the reproductive organs (Zhang et al., 2017). Two homologous genes of AtBOR2 were identified in B. napus: BnaC4.BOR2 and BnaA4.BOR2, both of which were mainly localized to the plasma membrane and showed B transport activity in yeast (Liu et al., 2024a, 2024). BnaC4.BOR2, expressed in lateral root caps and steles, was involved in B absorption in roots and its translocation to shoots (Liu et al., 2024b). BnaA4.BOR2 was primarily expressed in the cortex and endodermis of the root tip meristem zone, as well as in the mature endodermis, it facilitated the transport of B from roots to shoots and its distribution within shoots (Liu et al., 2024a).
Conversely, while boric acid is an essential plant micronutrient, excess B inhibits plant growth, impairing various cellular functions and often causes necrosis of tissues (CamachoCristóbal et al., 2008; Landi et al., 2019; Wu et al., 2019). AtBOR4 mediated B efflux and was located on the distal surface of epidermal cells, where it reduced B concentrations in roots and shoots, thereby protecting plants from B accumulation and toxicity (Miwa et al., 2007; Takeda and Matsuoka, 2008). Unlike AtBOR4, OsBOR4 in rice exhibited distinct functional characteristics, showed an anther-specific expression pattern, and was involved in maintaining boron homeostasis during fertilization (Tanaka et al., 2013). Moreover, in barley, borate exporters Bot1/HvBOR2 was responsible for the high B tolerance and protected plants from B accumulation and subsequent toxicity (Miwa et al., 2007; Sutton et al., 2007).
3 Physiological functions of B transporters
The roles of B in plant development seem to be diverse and intricate, as demonstrated by the variety of phenotypes observed in plants exhibiting deficiency. Depending on the growth stage or period of plant undergoing the B deficiency, their vegetative and reproductive development might be significantly or completely suppressed. In higher plants, the symptoms of B deficiency vary widely, including stunted root and shoot growth, curled and reddish leaves, aborted floral buds, reduced pod formation, and poor seed yield (Yang et al., 2013; Durbak et al., 2014).
Mutations of B transporters in plant lead to significant developmental defects (Table 1). AtBOR1 was crucial for xylem loading, supporting normal shoot and reproductive development under low B conditions (Noguchi et al., 1997, 2000; Takano et al., 2002). The atbor1–1 mutant exhibited impaired rosette leaf expansion at 3 μM B and showed normal growth but female sterility at 30 μM B, while the wild-type plants thrived under the same conditions; both defects could be fully rescued by supplementation with 100 μM B (Noguchi et al., 1997). The atbor2–1 mutants under B deficiency exhibited impaired root cell elongation due to reduced RG-II-B dimer formation, indicating that a 50% RG-II cross-linking level was the minimum threshold for normal root elongation (Miwa et al., 2013). Moreover, the bor1-3/bor2–1 double mutant displayed significantly more pronounced growth defects in both roots and shoots under B-limited conditions compared to the bor1–3 or bor2–1 single mutant, indicating partially redundant roles for AtBOR1 and AtBOR2 in root and shoot development under B deficiency (Miwa et al., 2013; Chatterjee et al., 2017). Similarly, growth defects in both roots and shoots were significantly diminished in loss-of-function mutants of AtNIP5;1, a boric acid channel essential for B uptake that was necessary for growth under B-limited conditions (Takano et al., 2006). Under 0.1uM B conditions, the atnip6;1 mutant plants exhibited smaller, dark green color and irregular shape in young rosette leaves at vegetative stages, and loss of apical dominance at reproductive stages (Tanaka et al., 2008).
B deficiency not only impaired vegetative growth, including inhibited root elongation and leaf expansion, but also severely disrupted reproductive development, causing early defects in the inflorescence meristem (IM) (Durbak et al., 2014). However, most studies have primarily focused on roots, with limited analysis dedicated to how these genes affect reproductive development. A higher quantity of B is required during the reproductive development phase in cereals (Shorrocks, 1997; Blevins and Lukaszewski, 1998). This increased demand may be attributed to pectin in the primary cell wall of grasses, whose content is initially low but increases throughout reproductive development (Hu et al., 1996; Matoh et al., 1996). Such an increase in pectin content impacts the key processes, including flowering, fruit set, and seed formation (Dell and Huang, 1997; Huang et al., 2000). Since B is essential for cross-linking RG-II chains, its availability in developing tissues is critical for reproductive processes like pollen germination and pollen tube growth (Dell and Huang, 1997; Blevins and Lukaszewski, 1998). AtNIP7;1 was primarily expressed in the anthers of young flowers during a specific developmental phase, particularly at floral stages 9 and 10 (Routray et al., 2018). AtNIP7;1 loss-of-function disrupted pollen morphology and lowered germination rates under B deficiency, indicating that AtNIP7;1 was crucial for B transport during pollen development and fertilization under low-B conditions (Routray et al., 2018).
Mutations in borate/boric acid transporters disrupt B homeostasis globally, resulting in sterile phenotypes and reproductive growth deficiencies observed in crops, including rice, maize and rapeseed. Rice and other monocot cereals have a lower boron demand than dicots due to the reduced levels of pectic compounds in their cell walls (Matoh et al., 1996). In rice, B deficiency has a more pronounced effect on reproductive growth than on vegetative growth (Uraguchi and Fujiwara, 2011). Under B-deficient conditions, osbor1 mutants showed the sterile phenotype (Nakagawa et al., 2007). Furthermore, heterozygous osbor4 mutants exhibited abnormal segregation ratios in their progeny, and homozygous mutants displayed defects in pollen tube germination and/or elongation, suggesting that OsBOR4 plays a role in fertilization, a process known to require adequate boron nutrition, which is also consistent with its specific expression in anthers (Tanaka et al., 2013). The rice gene Dwarf and Tiller-Enhancing 1 (DTE1), an allele of OsNIP3;1, was identified as the ortholog of AtNIP5;1, and regulates the B-dependent growth and development (Liu et al., 2015). Loss of DTE1 function leads to vegetative and reproductive defects under low-B conditions, including growth retardation, excessive tillering and impaired pollen fertility (Liu et al., 2015). In maize, the early stages of tassel and ear development were especially sensitive to B deficiency (Durbak et al., 2014). Consistent with this notion, the maize RTE gene encoded a functional ortholog of the AtBOR1 (Chatterjee et al., 2014). The rte mutant exhibited developmental defects in both vegetative and reproductive tissues, which impact both male and female inflorescences due to an inability to maintain activity in the inflorescence and axillary meristems (Chatterjee et al., 2014). Exogenous B application restored reproductive growth phenotypes in a dose-dependent manner (Chatterjee et al., 2014). Transmission electron microscopy (TEM) analysis of rte mutant ears revealed developmental-stage-dependent defects in cell wall integrity, indicating that B deficiency disrupted cell wall structure, caused expansion defects and led to cell death in meristems and floral organs (Chatterjee et al., 2014). Different from RTE, the disruption of RTE2 did not affect vegetative or inflorescence development, rte2 mutant exhibited slightly shorter roots in B-deficient conditions during early seedling growth (Chatterjee et al., 2017). However, the rte/rte2 double mutant displayed more severe defects than its single mutants, showing complete growth arrest under B-deficient soils (Chatterjee et al., 2017). This B deficiency dependent phenotype was observer in poor soils but not nutrient-rich conditions, and could be fully rescued by boric acid supplementation (Chatterjee et al., 2017).
The maize TLS1 was an allele of ZmNIP3;1, which predominantly expressed in floral tissues, particularly within the silks, tassel-less1 (tls1) mutant displayed defects in vegetative and inflorescence development (Leonard et al., 2014). Under normal conditions, tls1 mutants exhibited early abnormalities in tassel and ear formation (Durbak et al., 2014; Leonard et al., 2014). However, under low B conditions, they additionally showed impaired vegetative growth, characterized by a smaller shoot apical meristem (SAM), progressively narrower leaves, and premature growth termination (Durbak et al., 2014). The developmental phenotypic defects of tls1 mutant could be rescued by application of sufficient B (Leonard et al., 2014; Durbak et al., 2014). The tls1 mutant displayed impaired vegetative-to-reproductive transition and floral meristem development, accompanied by reduced RG-II cross-linking in immature inflorescence cell walls (Leonard et al., 2014; Durbak et al., 2014). Moreover, light intensity affected the tls1 phenotypes: the combination of high-pressure sodium and metal halide (MH) lamps reduced the tassel phenotype severity in the tls1 mutant under low-boron conditions by significantly increasing both transpiration and boron content (Matthesa et al., 2018).
B. napus is a vital oil crop with high B demand and great sensitivity to B deficiency (Xu et al., 2002). Under B deficiency, B. napus exhibits severe growth defects in both vegetative (inhibited root growth, leaf curling and necrosis) and reproductive (branch proliferation and stigma protrusion) organs, ultimately leading to substantial yield loss (Wang et al., 2017). BnaC4.BOR1;1c RNAi plants caused severe inhibition of inflorescence growth, including exposed stigma, dried-up and dropped floral buds and significantly lower seed yield (Zhang et al., 2017). Mutations in either BnaC4.BOR2 or BnaA4.BOR2 increased B deficiency sensitivity in B. napus, inhibited root growth, reduced root and shoot biomass, and severely impaired inflorescence development under low B condition (Liu et al., 2024a, 2024). These defects caused substantial yield losses, highlighting the gene’s critical role in flower organ development and seed production under low-B conditions (Liu et al., 2024a, 2024). BnaA3.NIP5;1 RNAi plants exhibited severe developmental defects, including curved leaves and stubby roots, and caused a more than 85% decrease in seed yield per plant under low boron conditions, indicating that BnaA3.NIP5;1 was essential for seed production in B. napus under boron limitation (He et al., 2021a, 2021). Compared with the BnaA3.NIP5;1 single RNAi plants, the multiple-target knockdown lines of both BnaA2.NIP5;1 and BnaA3.NIP5;1 (mRNAiBnaNIP5;1s) exhibited more severe defects, such as multiple branches and apical meristem necrosis (He et al., 2021b).
In grapevine, VvBOR1 expression level was in a stage-dependent manner during grapevine reproductive growth, with a peak in flowers at anthesis (Pérez-Castro et al., 2012). B accumulation during grapevine fruit development exhibited a biphasic pattern, peaking during the rapid growth phases (pre-veraison and post-veraison) while declining during the growth-arrested stage (Pérez-Castro et al., 2012). VvBOR1 gene expression preceded B content increases, showing significant stage-to-stage correlation between transcriptional levels and subsequent B accumulation (Pérez-Castro et al., 2012).
4 Molecular mechanisms of plant responses to boron deficiency and toxicity stress
Due to the dual effects of B deficiency and toxicity on plant growth and development, it is important for plants to maintain B homeostasis for proper growth, and the regulation of the B transport process plays a crucial role in B homeostasis. The accumulation of B transporters is regulated by the availability of B through various regulatory mechanisms. Multiple transcriptional and post-transcriptional regulatory mechanisms have been identified to medicate acclimation to nutrient-rich (high-B) conditions (Figure 3). These mechanisms, regulated by B availability, ensure precise control of B uptake to prevent both toxicity and deficiency.
Figure 3. Intracellular and signaling mechanisms involved in cellular-level boron homeostasis within root cells. Under low-B conditions, AtBOR1 underwent continuous internalization from the plasma membrane into trans-Golgi network/early endosome (TGN/EE), where it was recycled back to the PM to sustain B uptake. Moreover, the transcription factor BnaA9.WRKY47 specifically activated the expression of BnaA3.NIP5;1 by binding to the W box elements. Under high-B conditions, AtBOR1 undergoes ubiquitination, the ubiquitinated BOR1 is transported from the TGN/EE into multi-vesicular bodies/late endosomes (MVB/LE) by TOLs and endosomal sorting complex required for transport (ESCRT) machinery for vacuolar degradation, preventing excessive B transport. The expression of BnaA3.NIP5;1 was repressed in response to boron deficiency. Additionally, ribosome stalling at AUG-stops in the 5’-UTR of AtNIP5;1 increased under high-B conditions and was coupled with mRNA degradation. AtNGAL1 positively regulated the expression of AtBOR1, AtNIP5;1, AtNIP6;1 and AtNIP7;1 in response to low B, and up-regulated AtBOR4 in response to high B.
4.1 Endocytic degradation of AtBORs regulates boron levels
The mRNA levels of AtBOR1 remained largely stable across the tested B conditions, and B translocation from roots to shoots increased under low B and decreased rapidly under high B treatment, suggesting there was post-transcriptional control of AtBOR1 (Takano et al., 2005). The trafficking of AtBOR1 shifted from PM-endosome recycling under B deficiency to endocytosis and vacuolar degradation under high B conditions, thereby regulating B homeostasis (Takano et al., 2010; Kasai et al., 2011). A series of forward studies demonstrated that DYNAMIN-RELATED PROTEIN 1A (DRP1A) and the clathrin adaptor protein ADAPTOR PROTEIN 2 (AP2)-mediated endocytosis maintained the polar localization of BOR1, thereby supporting plant growth under low-B conditions (Yoshinari et al., 2016, 2019). In contrast, boron-induced vacuolar sorting of BOR1 was DRP1-dependent but occurred through an AP2-independent endocytic pathway (Yoshinari et al., 2016, 2019). Additionally, K63-linked polyubiquitination of BOR1 at lysine 590 proved essential for its high B-induced endocytosis and degradation (Yoshinari et al., 2021a). GNOM, a guanine-nucleotide exchange factor (ARF-GEF), mediated endocytosis that contributed to maintaining BOR1 polar localization under boron-limited conditions (Yoshinari et al., 2021b). Similarly, AtBOR2, which was degraded under high B conditions, exhibited cycling behavior between the plasma membrane and endosomes under low B conditions, mirroring the dynamics of AtBOR1 (Miwa et al., 2013). In addition, OsBOR1 underwent gradual degradation in response to high B, however, its degradation pathway differs from that of AtBOR1 (Shao et al., 2021).
4.2 B-dependent regulation of mRNA levels
In eukaryotes, short open reading frames (ORFs) in the 5’-untranslated region (5’-UTR), known as upstream ORFs (uORFs), are often affected the translation of the downstream ORF (Jackson et al., 2010; Hellens et al., 2016). The 5’-UTR mediated B-dependent AtNIP5;1 mRNA degradation for plant acclimation to high-B conditions (Tanaka et al., 2011). AtNIP5;1 had two minimum ORFs (AUG-stops) in its 5’-UTR, and ribosome stalling at these AUG-stops, which was enhanced under high-B conditions and led to suppressed translation and mRNA degradation, depended on a well-conserved region 12 to 19 nucleotides upstream that acted in enhancing mRNA degradation but not in ribosome stalling (Tanaka et al., 2016). The 5’-UTRs was highly conserved between OsNIP3;1 and AtNIP5;1 (Tanaka et al., 2011). In rice protoplasts, the luciferase activity driven by the 5’UTR of DTE1/OsNIP3;1 exhibited a dual B-dependent response, increasing at 1 μM B but decreasing at 100 μM B, indicating the 5’UTR’s essential role in B-responsive regulation and suggesting an AtNIP5;1-like mRNA control mechanism to prevent excessive B accumulation under high-B conditions (Liu et al., 2015).
In contrast, AtBOR1 protein abundance was regulated through two distinct mechanisms: protein endocytic degradation and B-dependent mRNA level regulation. When the B supply was sufficient (100 μM), AtBOR1 level was down-regulated by endocytic protein degradation (Takano et al., 2005; Kasai et al., 2011). However, at higher B concentrations, AtBOR1 level was decreased further by both translational suppression and protein degradation to avoidance of B toxicity in plants (Aibara et al., 2018). Furthermore, a ribosome profiling analysis revealed that transcripts with reduced translation efficiency under high-B conditions were rich in uORFs, and B played a general role in termination of translation by high B induced global ribosome stalling at the stop codon of main open reading frame (mORFs) (Sotta et al., 2021).
The abundance of B transporters in diverse plant species is coordinately controlled through B-responsive mRNA regulation. The OsBOR1 promoter exhibited a progressive shift in its cell-specific activity between the stele and exodermis under varying B conditions, which reflected its functional adaptation to B availability (Nakagawa et al., 2007). The CTTTC tandem repeats in the BnaA3.NIP5;1 5’UTR negatively regulated its expression, and their deletion enhanced BnaA3.NIP5;1 expression, which promoted root growth and increased seed yield under B limitation (He et al., 2021a). In roots, CmBOR1 expression remained unchanged under both B deficiency and excess conditions, whereas in shoots, its expression was upregulated under B deficiency but unaffected by excess B (Cañon et al., 2013). RT-qPCR analysis of TaBOR1s revealed that the accumulation of TaBOR1.1 and TaBOR1.3 mRNA was up-regulated under B limitation, whereas TaBOR1.2 mRNA accumulation increased under excess B conditions compared with low or normal B conditions in roots (Leaungthitikanchana et al., 2013). In contrast, TaBORs and CmBOR1 exhibited distinct regulation, implying functional diversification among BOR1 genes (Cañon et al., 2013; Leaungthitikanchana et al., 2013). This divergence may reflect species-specific adaptations, particularly in plants with complex genomes, where different BOR1 paralogs could fulfill varied physiological roles.
Transcription factors play pivotal roles in multiple biological processes by activating or repressing the transcription of target genes (Levine and Davidson, 2005). Accumulating evidence has highlighted the importance of transcription factors in responding to nutrient conditions in plants. AtWRKY6 was the first transcription factor reported to involve in the response to B deficiency, with its promoter activity and transcription being induced by low B conditions (Kasajima et al., 2010). BnaA9.WRKY47 positively regulated low-B tolerance through up-regulating BnaA3.NIP5;1 expression to facilitate efficient B uptake (Feng et al., 2020). The Arabidopsis homolog AtWRKY47 acted as a negative regulator that involved in boron homeostasis (Feng et al., 2021). NGATHA-Like 1 (NGAL1, also known as ABNORMAL SHOOT 2, ABS2) was a B-responsive gene regulated in a B-dependent manner through AUG-Stop, similar to AtNIP5;1 (Tanaka et al., 2016). NGAL1 positively regulated the expression of AtBOR1, AtNIP5;1, AtNIP6;1 and AtNIP7;1 in response to low B, and up-regulated AtBOR4 in response to high B to enhance B transport and distribution in both conditions (Tsednee et al., 2022).
5 Transgenic plant development to address B deficiency and toxicity
The inadequate uptake of B due to poor soil quality has emerged as a significant agricultural challenge in various regions worldwide, and crops cultivated in B-deficient soils often experience reductions in both yield and fruit quality (Shorrocks, 1997; Dell and Huang, 1997). Although B fertilizer can alleviate plant B deficiency, borate rock is a non-renewable resource. To address this problem, molecular breeding to enhance B-transporter activity represents a promising strategy for combating B deficiency in crops. On the other hand, B exhibits toxic effects when present in excessive amounts. The generation of B-deficient or tolerant plants represents a cost-effective and environmentally sustainable strategy for agriculture. There were several reports on improvement of B deficiency tolerance or toxicity by modulating expression of B channel genes to improve plant growth under unfavorable B nutrient conditions.
5.1 Generation of transgenic plants to mitigate B deficiency
Overexpression of AtBOR1 enhanced root-to-shoot translocation of B, and improved shoot growth and fertility under B-deficient conditions but not root growth (Miwa et al., 2006). This was attributed to the degradation of AtBOR1 under high-B supply, and enhanced the translocation of B from root-to-shoot under low-B conditions (Takano et al., 2005; Miwa et al., 2006). Furthermore, tomato (Solanum lycopersicum) plants overexpressing AtBOR1 maintained normal leaf development under B deficiency, and elevated B accumulation in shoots and fruits (Uraguchi et al., 2014). In addition, overexpression of CmBOR1 in Arabidopsis resulted in enhanced shoot growth with limited B supply, as did overexpression of AtBOR1 (Cañon et al., 2013). Moreover, Overexpression of BnaC4.BOR1;1c in the B-inefficient B. napus cultivar W10 alleviated shoot B-deficiency symptoms by improving boron distribution from roots to shoots (Chen et al., 2018).
AtNIP5;1 was a major boric acid channel required for efficient import of B into roots (Takano et al., 2006). Arabidopsis plants with AtNIP5;1 activated by a T-DNA insertion with a enhancer improved root growth under B limitation, but did not improved shoot growth (Kato et al., 2009). Furthermore, introduction of Pro(35S+NIP5;1):NIP5;1 into the AtBOR1 over expressor improved root elongation, fertility and short-term B uptake under low-B supply (Kato et al., 2009). Elevated BnaA3.NIP5;1 expression improved low-B tolerance in transgenic lines at both seedling and mature stages, and field trials demonstrated that the BnaA3.NIP5;1Q allele significantly increased seed yield under B deficiency conditions (He et al., 2021a).
5.2 Generation of transgenic plants to combat B toxicity
Overexpression of AtBOR4 improved growth under conditions of B toxicity through AtBOR4-mediated B efflux that decreased B concentrations in roots and shoots (Miwa et al., 2007). AtBOR4-overexpressing transgenic plants were more capable of expanding leaves and accumulating chlorophyll in shoot tissues under high-B concentration, suggesting overexpressed AtBOR4 alters B distribution in leaves by exporting B from cytoplasm into apoplasm for enhancing high-B tolerance in shoots (Miwa and Fujiwara, 2011). Arabidopsis SHB1/HY1 gene, encoded HO1 (heme oxygenase 1), was up-regulated under excessive B stimulation, and the shb1 seedlings exhibited root inhibition under excessive B treatments (Lv et al., 2017). However, overexpressing SHB1/HY1 or applying the HO1 catalytic products could induced BOR4 transcription, reduced B accumulation in roots and restored primary root growth that confers high B tolerance (Lv et al., 2017).
Moreover, in a B-stress tolerant cultivar ‘Sahara’ of barley, unlike intolerant genotypes, which had four tandem copies of the Bot1 gene with higher transcript levels, and Bot1 expression levels directly correlating with tolerance across various landraces (Hayes and Reid, 2004; Reid, 2007; Sutton et al., 2007; Mickelbart et al., 2015). Similarly, TaBOR2 and HvBOR2 reduced root B concentrations in the tolerant cultivars, and their expression levels showed positive correlations with tolerance (Reid, 2007; Sutton et al., 2007).
6 Conclusion
Boron is an essential micronutrient for plant growth. Regulating the activity of transport proteins is essential for plants to adapt to changing nutrient availability. Plants use complex homeostasis networks to regulate boron uptake, mobilization, distribution, and storage to assure proper growth. While characterizing BOR and NIP II family members has greatly advanced our understanding of boron transport systems, further research on boron transport mechanisms in cereals remains essential to optimize boron nutrient use efficiency. The regulatory mechanisms of B transport proteins include B-induced ribosome stalling and AtNIP5;1 mRNA degradation mediated by its 5’UTR (Tanaka et al., 2011, 2016), as well as B-triggered endocytosis and degradation of AtBOR1 through its self-regulatory transceptor function (Takano et al., 2005, 2010; Yoshinari et al., 2021a). However, the involvement of additional regulatory elements or mechanisms in boron transport protein modulation remains unclear. Therefore, a systematic characterization of these proteins, including their regulatory components and interaction networks, is essential for future research. Current research on boron efficiency in plants has mainly focused on roots, leaving the mechanisms during reproductive growth poorly understood. In particular, floral organ responses to boron deficiency and their molecular regulation require urgent investigation. The development of B-deficient and B-tolerant transgenic plants by manipulating B transport proteins presents a promising strategy to reduce fertilizer use and mitigate toxicity risks. Current successes in creating plants that tolerate both low and high B levels should be optimized for crop species, promoting sustainable agriculture in areas affected by B deficiency or excess.
Author contributions
DZ: Writing – original draft, Writing – review & editing. RL: Writing – original draft, Writing – review & editing. BM: Writing – review & editing, Supervision. XC: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Zhejiang Provincial Natural Science Foundation of China (LQ23C020005), Zhejiang Province Higher Education Institution Laboratory Work Research Project (YB202356), Science and Technology Key Projects of Jinhua City (2024-2-017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aibara, I., Hirai, T., Kasai, K., Takano, J., Onouchi, H., Naito, S., et al. (2018). Boron-dependent translational suppression of the borate exporter BOR1 contributes to the avoidance of boron toxicity. Plant Physiol. 177, 759–774. doi: 10.1104/pp.18.00119
Bassil, E., Hu, H., and Brown, P. H. (2004). Use of phenylboronic acids to investigate boron function in plants. Possible role of boron in transvacuolar cytoplasmic strands and cell-to-wall adhesion. Plant Physiol. 136, 3383–3395. doi: 10.1104/pp.104.040527
Baxter, I. (2009). Ionomics: studying the social network of mineral nutrients. Curr. Opin. Plant Biol. 12, 381–386. doi: 10.1016/j.pbi.2009.05.002
Blevins, D. G. and Lukaszewski, K. M. (1998). Boron in plant structure and function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 481–500. doi: 10.1146/annurev.arplant.49.1.481
Brown, P. H. and Shelp, B. J. (1997). Boron mobility in plants. Plant Soil 193, 85–101. doi: 10.1023/A:1004211925160
CamachoCristóbal, J. J., Rexach, J., and GonzálezFontes, A. (2008). Boron in plants: deficiency and toxicity. J. Integr. Plant Biol. 50, 1247–1255. doi: 10.1111/j.1744-7909.2008.00742.x
Cañon, P., Aquea, F., Amparo Rodríguez-Hoces De La Guardia, A., and Arce-Johnson, P. (2013). Functional characterization of Citrus macrophylla BOR1 as a boron transporter. Physiol. Plant 149, 329–339. doi: 10.1111/ppl.12037
Chatterjee, M., Liu, Q., Menello, C., Galli, M., and Gallavotti, A. (2017). The combined action of duplicated boron transporters is required for maize growth in boron-deficient conditions. Genetics 206, 2041–2051. doi: 10.1534/genetics.116.198275
Chatterjee, M., Tabi, Z., Galli, M., Malcomber, S., Buck, A., Muszynski, M., et al. (2014). The boron efflux transporter ROTTEN EAR is required for maize inflorescence development and fertility. Plant Cell 26, 2962–2977. doi: 10.1105/tpc.114.125963
Chen, H. F., Zhang, Q., He, M. L., Wang, S. L., Shi, L., and Xu, F. S. (2018). Molecular characterization of the genome wide BOR transporter gene family and genetic analysis of BnaC04.BOR1;1c in Brassica napus. BMC Plant Biol. 18, 193. doi: 10.1186/s12870-018-1407-1
Danielson, J. A. and Johanson, U. (2010). Phylogeny of major intrinsic proteins. Adv. Exp. Med. Biol. 679, 19–31. doi: 10.1007/978-1-4419-6315-4_2
Dannel, F., Pfeffer, H., and Römheld, V. (2000). Characterization of root boron pools, boron uptake and boron translocation in sunflower using the stable isotopes 10 B and 11 B. Aust. J. Plant Physiol. 27, 397–405. doi: 10.1071/PP99086
Dell, B. and Huang, L. (1997). Physiological response of plants to low boron. Plant Soil 193, 103–120. doi: 10.1023/A:1004264009230
Durbak, A. R., Phillips, K. A., Pike, S., O’Neill, M. A., Mares, J., Gallavotti, A., et al. (2014). Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 26, 2978–2995. doi: 10.1105/tpc.114.125898
Feng, Y. N., Cui, R., Huang, Y. P., Shi, L., Wang, S. L., and Xu, F. S. (2021). Repression of transcription factor AtWRKY47 confers tolerance to boron toxicity in Arabidopsis thaliana. Ecotoxicol Environ. Saf. 220, 112406. doi: 10.1016/j.ecoenv.2021.112406
Feng, Y. N., Cui, R., Wang, S. L., He, M. L., Hua, Y. P., Shi, L., et al. (2020). Transcription factor BnaA9.WRKY47 contributes to the adaptation of Brassica napus to low boron stress by up-regulating the boric acid channel gene BnaA3.NIP5;1. Plant Biotechnol. J. 18, 1241–1254. doi: 10.1111/pbi.13288
Frommer, W. B. and Wiren, N. (2002). Plant biology: ping-pong with boron. Nature 420, 282–283. doi: 10.1038/420282a
Hanaoka, H., Uraguchi, S., Takano, J., Tanaka, M., and Fujiwara, T. (2014). OsNIP3;1, a rice boric acid channel, regulates boron distribution and is essential for growth under boron-deficient conditions. Plant J. 78, 890–902. doi: 10.1111/tpj.12511
Hayes, J. E. and Reid, R. J. (2004). Boron tolerance in barley is mediated by efflux of boron from the roots. Plant Physiol. 136, 3376–3382. doi: 10.1104/pp.103.037028
He, M. L., Wang, S. L., Zhang, C., Liu, L., Zhang, J. Y., Qiu, S., et al. (2021a). Genetic variation of BnaA3.NIP5;1 expressing in the lateral root cap contributes to boron deficiency tolerance in Brassica napus. PloS Genet. 17, e1009661. doi: 10.1371/journal.pgen.1009661
He, M. L., Zhang, C., Chu, L. Y., Wang, S. L., Shi, L., and Xu, F. S. (2021b). Specific and multiple-target gene silencing reveals function diversity of BnaA2.NIP5;1 and BnaA3.NIP5;1 in Brassica napus. Plant Cell Environ. 44, 3184–3194. doi: 10.1111/pce.14077
Hellens, R. P., Brown, C. M., Chisnall, M. A., Waterhouse, P. M., and Macknight, R. C. (2016). The emerging world of small ORFs. Trends Plant Sci. 21, 317–328. doi: 10.1016/j.tplants.2015.11.005
Hu, H., Brown, P. H., and Labavitch, J. M. (1996). Species variability in boron requirement is correlated with cell wall pectin. J. Exp. Bot. 47, 227–232. doi: 10.1093/jxb/47.2.227
Hua, Y. P., Zhang, D. D., Zhou, T., He, M. L., Ding, G. D., Shi, L., et al. (2016). Transcriptomics-assisted quantitative trait locus fine mapping for the rapid identification of a nodulin 26-like intrinsic protein gene regulating boron efficiency in allotetraploid rapeseed. Plant Cell Environ. 39, 1601–1618. doi: 10.1111/pce.12731
Huang, L., Pant, J., Dell, B., and Bell, R. W. (2000). Effects of boron deficiency on anther development and floret fertility in wheat (Triticum aestivum L. ‘Wilgoyne’). Ann. Bot. 85, 493–500. doi: 10.1006/anbo.1999.1095
Ishii, T. and Matsunaga, T. (1996). Isolation and characterization of a boron-rhamnogalacturonan-II complex from cell walls of sugar beet pulp. Carbohydr. Res. 284, 1–9. doi: 10.1016/0008-6215(96)00010-9
Ishii, T., Matsunaga, T., Pellerin, P., O’Neill, M. A., Darvill, A., and Albersheim, P. (1999). The plant cell wall polysaccharide rhamnogalacturonan II self-assembles into a covalently cross-linked dimer. J. Biol. Chem. 274, 13098–13104. doi: 10.1074/jbc.274.19.13098
Jackson, R. J., Hellen, C. U., and Pestova, T. V. (2010). The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127. doi: 10.1038/nrm2838
Johanson, U., Karlsson, M., Johansson, I., Gustavsson, S., Sjövall, and Fraysse, L. (2001). The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomen clature for major intrinsic proteins in plants. Plant Physiol. 126, 1358–1369. doi: 10.1104/pp.126.4.1358
Kasai, K., Takano, J., Miwa, K., Toyoda, A., and Fujiwara, T. (2011). High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana. J. Biol. Chem. 286, 6175–6183. doi: 10.1074/jbc.M110.184929
Kasajima, I., Ide, Y., Hirai, M. Y., and Fujiwara, T. (2010). WRKY6 is in volved in the response to boron deficiency in Arabidopsis thaliana. Physiol. Plant 139, 80–92. doi: 10.1111/j.1399-3054.2010.01349.x
Kato, Y., Miwa, K., Takano, J., Wada, M., and Fujiwara, T. (2009). Highly boron deficiency-tolerant plants generated by enhanced expression of NIP5;1, a boric acid channel. Plant Cell Physiol. 50, 58–66. doi: 10.1093/pcp/pcn168
Kobayashi, M., Matoh, T., and Azuma, J. (1996). Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol. 110, 1017–1020. doi: 10.1104/pp.110.3.1017
Landi, M., Margaritopoulou, T., Papadakis, I. E., and Araniti, F. (2019). Boron toxicity in higher plants: an update. Planta 250, 1011–1032. doi: 10.1007/s00425-019-03220-4
Leaungthitikanchana, S., Fujibe, T., Tanaka, M., Wang, S. L., Sotta, N., Takano, J., et al. (2013). Differential expression of three BOR1 genes corresponding to different genomes in response to boron conditions in Hexaploid Wheat (Triticum aestivum L.). Plant Cell Physiol. 54, 1056–1063. doi: 10.1093/pcp/pct059
Leonard, A., Holloway, B., Guo, M., Rupe, M., Yu, G. X., and Beatty, M. (2014). tassel-less1 encodes a boron channel protein required for inflorescence development in Maize. Plant Cell Physiol. 55, 1044–1054. doi: 10.1093/pcp/pcu036
Levine, M. and Davidson, E. H. (2005). Gene regulatory networks for development. Proc. Natl. Acad. Sci. 102, 4936–4942. doi: 10.1073/pnas.0408031102
Li, T., Choi, W. G., Wallace, I. S., Baudry, J., and Roberts, D. M. (2011). Arabidopsis thaliana NIP7;1: an anther-specific boric acid transporter of the aquaporin super family regulated by an unusual tyrosine in helix 2 of the transport pore. Biochemistry 50, 6633–6641. doi: 10.1021/bi2004476
Lilay, G. H., Thiébaut, N., Mee., D., Assunção, A. G. L., Schjoerring, J. K., Husted, S., et al. (2024). Linking the key physiological functions of essential micronutrients to their deficiency symptoms in plants. New Phytol. 242, 881–902. doi: 10.1111/nph.19645
Liu, K., Liu, L. L., Ren, Y. L., Wang, Z. Q., Zhou, K. N., Liu, X., et al. (2015). Dwarf and tiller-enhancing 1 regulates growth and development by influencing boron uptake in boron limited conditions in rice. Plant Sci. 236, 18–28. doi: 10.1016/j.plantsci.2015.03.015
Liu, W., Wang, S. L., Ye, X. S., and Xu, F. S. (2024a). BnaA4.BOR2 contributes the tolerance of rapeseed to boron deficiency by improving the transport of boron from root to shoot. Plant Physiol. Biochem. 208, 108508. doi: 10.1016/j.plaphy.2024.108508
Liu, W., Xu, F. S., Ye, X. S., Cai, H. M., Shi, L., and Wang, S. L. (2024b). BnaC4.BOR2 mediates boron uptake and translocation in Brassica napus under boron deficiency. Plant Cell Environ. 47, 3732–3748. doi: 10.1111/pce.14959
Lv, Q., Wang, L., Wang, J. Z., Li, P., Chen, Y. L., Du, J., et al. (2017). SHB1/HY1 alleviates excess boron stress by increasing BOR4 expression level and maintaining boron homeostasis in Arabidopsis roots. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00790
Matoh, T., Kawaguchi, S., and Kobayashi, M. (1996). Ubiquity of a borate rhamnogalacturonan II complex in the cell walls of higher plants. Plant Cell Physiol. 37, 636–640. doi: 10.1093/oxfordjournals.pcp.a028992
Matsunaga, T., Ishii, T., Matsumoto, S., Higuchi, M., Darvil, A., Albersheim, P., et al. (2004). Occurrence of the primary cell wall polysaccharide rhamnogalacturonan II inpteridophytes, lycophytes, and bryophytes. Implications for the evolution of vascular plants. Plant Physiol. 134, 339–351. doi: 10.1104/pp.103.030072
Matthesa, M. S., Robila, J. M., Tranb, T., Kimble, A., and McSteen, P. (2018). Increased transpiration is correlated with reduced boron deficiency symptoms in the maize tassel-less1 mutant. Physiol. Plant 163, 344–355. doi: 10.1111/ppl.12717
Maurel, C., Boursiac, Y., Luu, D. T., Santoni, V., Shahzad, Z., and Verdoucq, L. (2015). Aquaporins in plants. Physiol. Rev. 95, 1321–1358. doi: 10.1152/physrev.00008.2015
Mickelbart, M. V., Hasegawa, P. M., and Bailey-Serres, J. (2015). Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 16, 237–251. doi: 10.1038/nrg3901
Miwa, K., Aibara, I., and Fujiwara, T. (2014). Arabidopsis thaliana BOR4 is upregulated under high boron conditions and confers tolerance to high boron. Soil Sci. Plant Nutr. 60, 349–355. doi: 10.1080/00380768.2013.866524
Miwa, K. and Fujiwara, T. (2010). Boron transport in plants: co-ordinated regulation of transporters. Ann. Bot. 105, 1103–1108. doi: 10.1093/aob/mcq044
Miwa, K. and Fujiwara, T. (2011). Role of overexpressed BOR4, a boron exporter, in tolerance to high level of boron in shoots. Soil Sci. Plant Nutr. 57, 558–565. doi: 10.1080/00380768.2011.596473
Miwa, K., Takano, J., and Fujiwara, T. (2006). Improvement of seed yields under boron-limiting conditions through overexpression of BOR1, a boron transporter for xylem loading, in Arabidopsis thaliana. Plant J. 46, 1084–1091. doi: 10.1111/j.1365-313X.2006.02763.x
Miwa, K., Takano, J., Omori, H., Seki, M., Shinozaki, K., and Fujiwara, T. (2007). Plants tolerant of high boron levels. Science 318, 1417–1417. doi: 10.1126/science.1146634
Miwa, K., Wakuta, S., Takada, S., Ide, K., Takano, J., Naito, S., et al. (2013). Roles of BOR2, a boron exporter, in cross linking of rhamnogalacturonan II and root elongation under boron limitation in Arabidopsis. Plant Physiol. 163, 1699–1709. doi: 10.1104/pp.113.225995
Nakagawa, Y., Hanaoka, H., Kobayashi, M., Miyoshi, K., Miwa, K., and Fujiwara, T. (2007). Cell-Type specificity of the expression of OsBOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem Loading. Plant Cell 19, 2624–2635. doi: 10.1105/tpc.106.049015
Noguchi, K., Dannel, F., Pfeffer, H., Römheld, V., Hayashi, H., and Fujiwara, T. (2000). Defect in root-shoot translocation of boron in Arabidopsis thaliana mutant bor1-1. J. Plant Physiol. 156, 751–755. doi: 10.1016/S0176-1617(00)80242-4
Noguchi, K., Yasumori, M., Imai, T., Naito, S., Matsunaga, T., Oda, H., et al. (1997). bor1-1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiol. 115, 901–906. doi: 10.1104/pp.115.3.901
O’Neill, M. A., Eberhard, S., Albersheim, P., and Darvill, A. G. (2001). Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294, 846–849. doi: 10.1126/science.1062319
O’Neill, M. A., Ishii, T., Albersheim, P., and Darvill, A. G. (2004). Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 55, 109–139. doi: 10.1146/annurev.arplant.55.031903.141750
Onuh, A. F. and Miwa, K. (2021). Regulation, diversity and evolution of boron transporters in plants. Plant Cell Physiol. 62, 590–599. doi: 10.1093/pcp/pcab025
Pérez-Castro, R., Kasai, K., Gainza-Cortés, F., Ruiz-Lara, S., Casaretto, J. A., Peña-Cortés, H., et al. (2012). VvBOR1, the Grapevine ortholog of AtBOR1, encodes an efflux boron transporter that is differentially expressed throughout reproductive development of Vitis vinifera L. Plant Cell Physiol. 53, 485–494. doi: 10.1093/pcp/pcs001
Power, P. P. and Woods, W. G. (1997). The chemistry of boron and its speciation in plants. Plant Soil 193, 1–13. doi: 10.1023/A:1004231922434
Reid, R. (2007). Identification of boron transporter genes likely to be responsible for tolerance to boron toxicity in wheat and barley. Plant Cell Physiol. 48, 1673–1678. doi: 10.1093/pcp/pcm159
Reid, R. J., Hayes, J. E., Post, A., Stangoulis, J. C. R., and Graham, R. D. (2004). A critical analysis of the causes of boron toxicity in plants. Plant Cell Environ. 27, 1405–1414. doi: 10.1111/j.1365-3040.2004.01243.x
Roberts, D. M. and Routray, P. (2017). “The nodulin 26 intrinsic protein subfamily,” in Plant Aquaporins: From Transport to Signaling (Springer International Publishing AG, Cham, Switzerland), 267–296.
Routray, P., Li, T., Yamasaki, A., Yoshinari, A., Takano, J., Choi, W. G., et al. (2018). Nodulin intrinsic protein 7;1 is a tapetal boric acid channel involved in pollen cell wall formation. Plant Physiol. 178, 1269–1283. doi: 10.1104/pp.18.00604
Sakamoto, T., Inui, Y. T., Uraguchi, S., Yoshizumi, T., Matsunaga, S., Mastui, M., et al. (2011). Condensin II alleviates DNA damage and is essential for tolerance of boron overload stress in Arabidopsis. Plant Cell 23, 3533–3546. doi: 10.1105/tpc.111.086314
Shao, J. F., Yamaji, N., Huang, S., and Ma, J. F. (2021). Fine regulation system for distribution of boron to different tissues in rice. New Phytol. 230, 656–668. doi: 10.1111/nph.17169
Shao, J. F., Yamaji, N., Liu, X. W., Yokosho, K., Shen, R. F., Ma, J. F., et al. (2018). Preferential distribution of boron to developing tissues is mediated by the intrinsic protein OsNIP3. Plant Physiol. 176, 1739–1750. doi: 10.1104/pp.17.01641
Shorrocks, V. M. (1997). The occurrence and correction of boron deficiency. Plant Soil 193, 121–148. doi: 10.1023/A:1004216126069
Sotta, N., Chiba, Y., Miwa, K., Takamatsu, S., Tanaka, M., Yamashita, Y., et al. (2021). Global analysis of boron-induced ribosome stalling reveals its effects on translation termination and unique regulation by AUG-stops in Arabidopsis shoots. Plant J. 106, 1455–1467. doi: 10.1111/tpj.15248
Sun, J., Shi, L., Zhang, C. Y., and Xu, F. (2012). Cloning and characterization of boron transporters in Brassica napus. Mol. Biol. Rep. 39, 1963–1973. doi: 10.1007/s11033-011-0930-z
Sutton, T., Baumann, U., Hayes, J., Collins, N. C., Shi, B. J., Schnurbusch, T., et al. (2007). Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science 318, 1446–1449. doi: 10.1126/science.1146853
Takano, J., Miwa, K., and Fujiwara, T. (2008). Boron transport mechanisms: collaboration of channels and transporters. Trends Plant Sci. 13, 451–457. doi: 10.1016/j.tplants.2008.05.007
Takano, J., Miwa, K., Yuan, L., Von Wiren, N., and Fujiwara, T. (2005). Endocytosis and degradation of BOR1, a boron trans porter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl. Acad. Sci. U.S.A. 102, 12276–12281. doi: 10.1073/pnas.0502060102
Takano, J., Noguchi, K., Yasumori, M., Kobayashi, M., Gajdos, Z., Miwa, K., et al. (2002). Arabidopsis boron transporter for xylem loading. Nature 420, 337–340. doi: 10.1038/nature01139
Takano, J., Tanaka, M., Toyoda, A., Miwa, K., Kasai, K., Fuji, K., et al. (2010). Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc. Natl. Acad. Sci. U.S.A. 107, 5220–5225107. doi: 10.1073/pnas.0910744107
Takano, J., Wada, M., Ludewig, U., Schaaf, G., Von Wirén, N., and Fujiwara, T. (2006). The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18, 1498–1509. doi: 10.1105/tpc.106.041640
Takeda, S. and Matsuoka, M. (2008). Genetic approaches to crop improvement: responding to environmental and population changes. Nat. Rev. Genet. 9, 444–457. doi: 10.1038/nrg2342
Tanaka, M., Sotta, N., Yamazumi, Y., Yamashita, Y., Miwa, K., Murota, K., et al. (2016). The minimum open reading frame, AUG-stop, induces boron-dependent ribosome stalling and mRNA degradation. Plant Cell 28, 2830–2849. doi: 10.1105/tpc.16.00481
Tanaka, M., Takano., J., Chiba, Y., Lombardo, F., Ogasawara, Y., Onouchi, H., et al. (2011). Boron-dependent degradation of NIP5;1 mRNA for acclimation to excess boron conditions in Arabidopsis. Plant Cell 23, 3547–3559. doi: 10.1105/tpc.111.088351
Tanaka, N., Uraguchi, S., Saito, A., Kajikawa, M., Kasai, K., Sato, Y., et al. (2013). Roles of pollen-specific boron efflux transporter, OsBOR4, in the rice fertilization process. Plant Cell Physiol. 54, 2011–2019. doi: 10.1093/pcp/pct136
Tanaka, M., Wallace, I. S., Takano, J., Roberts, M. D., and Fujiwara, T. (2008). NIP6;1 Is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 20, 2860–2875. doi: 10.1105/tpc.108.058628
Tsednee, M., Tanaka, M., Giehl, R. F., Von Wiren, N., and Fujiwara, T. (2022). Involvement of NGATHA-Like 1 transcription factor in boron transport under low and high boron conditions. Plant Cell Physiol. 63, 1242–1252. doi: 10.1093/pcp/pcac099
Uraguchi, S. and Fujiwara, T. (2011). Significant contribution of boron stored in seeds to initial growth of rice seedlings. Plant Soil 340, 435–442. doi: 10.1007/s11104-010-0614-9
Uraguchi, S., Kato, Y., Hanaoka, H., Miwa, K., and Fujiwara, T. (2014). Generation of boron-deficiency-tolerant tomato by overexpressing an Arabidopsis thaliana borate transporter AtBOR1. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00125
Voxeur, A. and Fry, S. C. (2014). Glycosylinositol phosphorylceramides from Rosa cell cultures are boron-bridged in the plasma membrane and form complexes with rhamnogalacturonan II. Plant J. 79, 139–149. doi: 10.1111/tpj.12547
Wallace, I. S., Choi, W. G., and Roberts, D. M. (2006). The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim. Biophys. Acta 1758, 1165–1175. doi: 10.1016/j.bbamem.2006.03.024
Wallace, I. S. and Roberts, D. M. (2004). Homology modeling of representative sub families of Arabidopsis major intrinsic proteins: classification based on the aromatic/arginine selectivity filter. Plant Physiol. 135, 1059–1068. doi: 10.1104/pp.103.033415
Wallace, I. S. and Roberts, D. M. (2005). Distinct transport selectivity of two structural subclasses of the nodulin-like intrinsic protein family of plant aquaglyceroporin channels. Biochemistry 44, 16826–16834. doi: 10.1021/bi0511888
Wang, S., Yoshinari, A., Shimada, T., Hara-Nishimura, I., Mitani-Ueno, N., Ma, J., et al. (2017). Polar localization of the NIP5;1 boric acid channel is maintained by endocytosis and facilitates boron transport in Arabidopsis roots. Plant Cell 29, 824–842. doi: 10.1105/tpc.16.00825
Warrington, K. (1923). The effect of boric acid and borax on the broad bean and certain other plants. Ann. Bot. 37, 457–466. doi: 10.1093/oxfordjournals.aob.a089871
Wimmer, M. A. and Eichert, T. (2013). Review: mechanisms for boron deficiency-mediated changes in plant water relations. Plant Sci. 203-204, 25–32. doi: 10.1016/j.plantsci.2012.12.012
Wu, X., Lu, X., Riaz, M., Yan, L., and Jiang, C. (2019). Boron toxicity induced specific changes of cell ultrastructure and architecture of components in leaf center and tip of trifoliate orange [Poncirus trifoliate (L.) Raf. J. Environ. Manage 246, 426–433. doi: 10.1016/j.jenvman.2019.05.148
Xu, F. S., Wang, Y. H., Ying, W. H., and Meng, J. L. (2002). Inheritance of boron nutrition efficiency in Brassica napus. J. Plant Nutr. 25, 901–912. doi: 10.1081/PLN-120002968
Yang, L., Zhang, Q., Dou, J., Li, L., Guo, L., Shi, L., et al. (2013). Characteristics of root boron nutrition confer high boron efficiency in Brassica napus cultivars. Plant Soil. 371, 95–104. doi: 10.1007/s11104-013-1669-1
Yoshinari, A., Fujimoto, M., Ueda, T., Inada, N., Naito, S., and Takano, J. (2016). DRP1-dependent endocytosis is essential for polar localization and boron-induced degradation of the borate transporter BOR1 in Arabidopsis thaliana. Plant Cell Physiol. 57, 1985–2000. doi: 10.1093/pcp/pcw121
Yoshinari, A., Hosokawa, T., Amano, T., Beier, M. P., Kunied, T., Shimada, T., et al. (2019). Polar localization of the borate exporter BOR1 requires AP2 dependent endocytosis. Plant Physiol. 179, 1569–1580. doi: 10.1104/pp.18.01017
Yoshinari, A., Hosokawa, T., Beier, M. P., Oshima, K., Ogino, Y., Hori, C., et al. (2021a). Transport-coupled ubiquitination of the borate transporter BOR1 for its boron-dependent degradation. Plant Cell 33, 420–438. doi: 10.1093/plcell/koaa020
Yoshinari, A., Toda, Y., and Takano, J. (2021b). GNOM-dependent endocytosis maintains polar localisation of the borate exporter BOR1 in Arabidopsis. Biol. Cell 113, 264–269. doi: 10.1111/boc.202000106
Keywords: boron, transporters, NIP, BOR, regulation
Citation: Zhou D, Luo R, Ma B and Chen X (2025) Transport and regulatory mechanisms of boron in plants. Front. Plant Sci. 16:1653484. doi: 10.3389/fpls.2025.1653484
Received: 25 June 2025; Accepted: 29 October 2025;
Published: 17 November 2025.
Edited by:
Haijun Gong, Northwest A&F University, ChinaReviewed by:
Zhongxian Li, Henan Academy of Sciences, ChinaMunkhtsetseg Mugi Tsednee, Academia Sinica, Taiwan
Copyright © 2025 Zhou, Luo, Ma and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bojun Ma, bWJqQHpqbnUuY24=; Xifeng Chen, eGZjaGVuQHpqbnUuY24=
 Dan Zhou
Dan Zhou Rui Luo
Rui Luo Bojun Ma
Bojun Ma Xifeng Chen
Xifeng Chen