- 1College of Forestry, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China
- 2Fujian Forestry Survey and Design Institute, Fuzhou, Fujian, China
- 3College of Landscape Architecture and Art, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China
- 4Zhangpu Zhongxi State-owned Forest Farm in Fujian Province, Zhangzhou, Fujian, China
- 5Nan’an Luoshan State-owned Forest Farm in Fujian Province, Nan’an, Fujian, China
- 6Xiqin Teaching Forest Farm, Fujian Agriculture and Forestry University, Nanping, Fujian, China
Acacia melanoxylon is a high-quality timber species renowned for its superior heartwood and wide global use. To assess its stability, adaptability, and productivity in Fujian Province, China, a regional trial involving 47 A. melanoxylon families was conducted across four sites. The study utilized best linear unbiased prediction (BLUP) breeding values were estimated using 5-year-old diameter at breast height (DBH) and tree height data, and genotype plus genotype-by-environment (GGE) biplots were used to evaluate families and trial sites. The biplot generated based on BLUP values for DBH revealed that the four trial sites clustered into two groups. Group 1 comprised sites ZX (Zhangpu Zhongxi State-owned Forest Farm, Zhangpu County), LX (Quanzhou Luoxi State-owned Forest Farm, Quanzhou City), and XQ (Nanping Xiqin Teaching Forest Farm, Nanping City), while Group 2 included site LS (Nan’an Luoshan State-owned Forest Farm, Nan’an City). Sites LX and XQ showed strong positive correlations with each other and moderate correlations with ZX, indicating similar family rankings. In contrast, LX was negatively correlated with LS, reflecting opposing trends in genotype performance. Site ZX exhibited high discriminative power and representativeness, identifying it as the most effective location for evaluating families. Families 38, 35, 9, 17, and 24 demonstrated a combination of high yield and stability, underscoring their potential for extensive cultivation. The measurement of DBH is more cost-effective and accurate compared to tree height, and it aligns with our breeding objectives. Therefore, this study primarily focused on DBH for selection, and the results provide a theoretical foundation for promoting Acacia melanoxylon in Fujian Province.
1 Introduction
Acacia melanoxylon, an evergreen tree native to Australia, is a multipurpose timber species prized for its rapid growth, nitrogen-fixing ability, and high-quality wood (Hussain et al., 2020; Martins et al., 2020; Pereira and Ferreira, 2021). Since China's systematic introduction of Acacia species in 1985, A. melanoxylon has become an important component of plantations in southern Fujian Province, serving both ecological restoration and timber production needs (Fan et al., 2016). While the cultivation of A. melanoxylon in China has expanded, its genetic improvement program faces a significant challenge: a lack of attention of genotype-by-environment (G×E) interactions. Previous studies focused on selecting superior families have been confined to single-site trials. The absence of regional multi-environment trials (METs) makes it impossible to determine whether a superior genotype in one location will perform well in others, severely limiting the efficiency and effectiveness of breeding and deployment strategies.
Regionalized trials are essential in cultivar breeding and promotion. By analyzing the performance of various genotypes across diverse environmental conditions, METs elucidate genotype-by-environment (GE) interactions (G×E). They identify high-yielding, stable, and adaptable varieties, determining optimal planting zones (Li et al., 2023; Prášil et al., 2025). Statistical models for MET data have progressed from early multi-site joint ANOVA to combinations of ANOVA with regression or principal component analysis (PCA). More recently, the widely used Additive Main Effects and Multiplicative Interaction (AMMI) model and genotype plus GE (GGE) biplot have become prominent (Istanbuli et al., 2025; Vymyslický et al., 2025; Yousaf et al., 2025). Breeders consistently emphasize integrating G and GE effects to select superior genotypes in field trials (He et al., 2025; Skrøppa and Fundova, 2025). Yan et al (Yan et al., 2007). compared the AMMI model and the GGE biplot, concluding that the GGE biplot outperforms the AMMI1 model in mega-environment analysis and genotype evaluation. The GGE biplot excels in combining discriminative power with representativeness in environmental assessment, a feature the AMMI analysis lacks. Currently, mixed linear models with random effects are extensively used in trial data analysis (Khan et al., 2024). Best linear unbiased prediction (BLUP) values, more reliable than observed values and suitable for unbalanced data, offer higher prediction accuracy (Yuan et al., 2022; Amrate et al., 2024).
In recent years, the integrated BLUP-GGE approach has gained growing recognition in forest tree breeding owing to its excellent performance in evaluating genotype-by-environment interactions. For example, this approach has been successfully employed to assess growth stability and select superior genotypes in a variety of tree species, including Populus euramericana (Liu et al., 2021), Castanopsis hystrix (Liu et al., 2023), and Larix gmelinii var. principis-rupprechtii (Mayr.) Pilger (Zheng et al., 2023). These studies have demonstrated the scientific validity of the method. However, despite its demonstrated effectiveness in other species, the integrated BLUP-GGE biplot approach has not yet been systematically applied to the genetic evaluation and selection of Acacia melanoxylon families in Fujian Province. To bridge this critical gap, the present study was conducted with the following objectives: (1) to comprehensively evaluate the growth performance and stability of 47 A. melanoxylon families across four representative test sites in Fujian using the integrated BLUP-GGE biplot methodology; (2) to delineate mega-environments and assess the discriminative power and representativeness of test sites; and (3) to identify superior families with specific or broad adaptation for targeted breeding programs.
2 Materials and methods
2.1 Test site overview
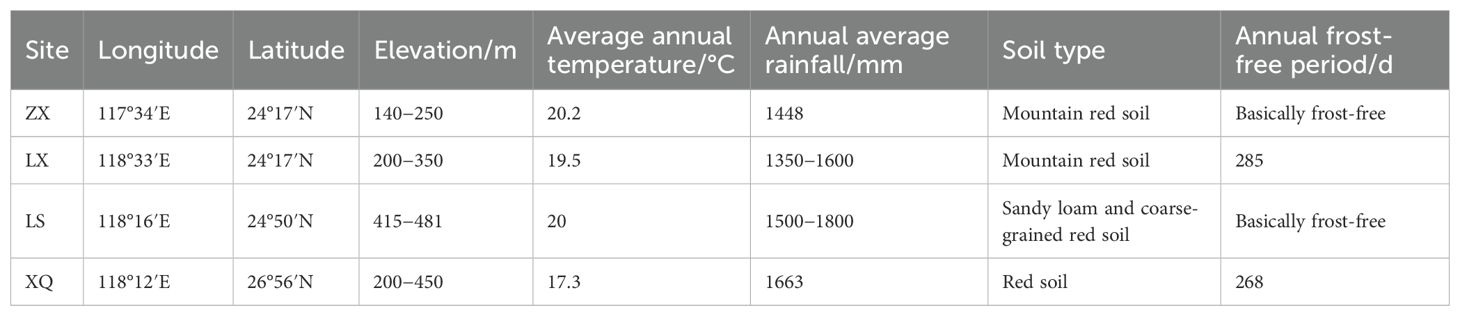
The study selected four trial sites located in the primary plantation regions of Fujian Province, China: Zhangpu Zhongxi State-owned Forest Farm (ZX), Quanzhou Luoxi State-owned Forest Farm (LX), Nan’an Luoshan State-owned Forest Farm (LS), and Nanping Xiqin Teaching Forest Farm (XQ). These sites were chosen to represent the diverse ecological conditions, including variations in topography and microclimate, typical for Acacia melanoxylon cultivation in southern China. Detailed site conditions, including geographic coordinates, elevation range, soil type, and key climate parameters, are provided in Table 1.
2.2 Test materials and design
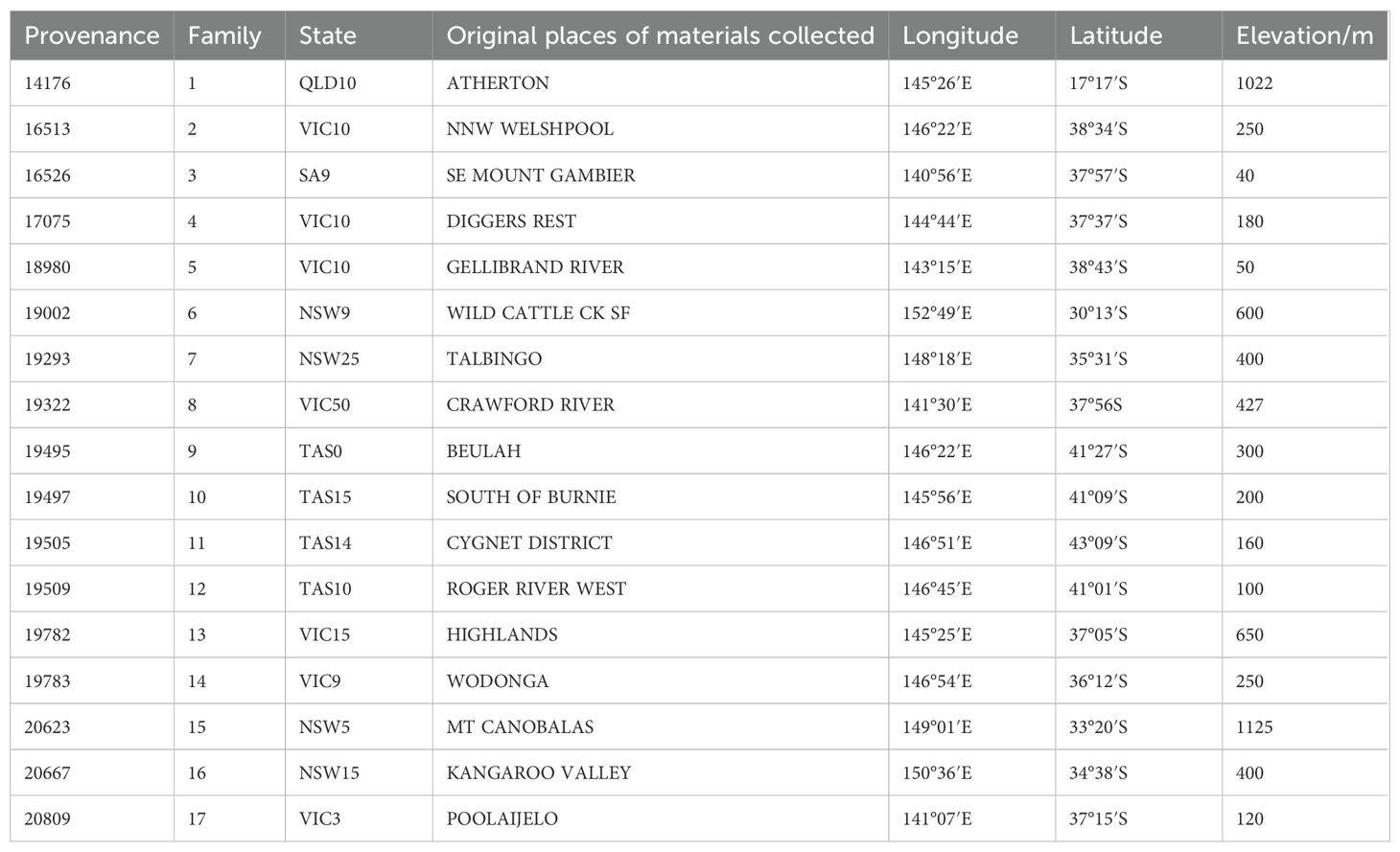
The regional trial involved 47 Acacia melanoxylon families. Families 1 through 17 were derived from seed material collected in 2018 from superior individual trees in Australia (Table 2). Families 18 to 47 originated from a primary clonal seed orchard of A. melanoxylon at Luoxi State-owned Forest Farm, located in Quanzhou City, Fujian Province. This orchard was established by grafting preselected elite trees. Seeds were collected in 2018, germinated at the Zhangpu Zhongxi Forest Farm nursery in the same year, and planted in 2019 across four trial sites in Fujian Province.
The experimental forest uniformly adopts a random complete block design (RCB), setting up 3, 4, 7, and 7 replicates (blocks) respectively in XQ, LS, ZX and LX. Each plot consisted of three trees arranged in a single column (1 row × 3 trees) with spacing of 2 × 3 m. Annual surveys were conducted in the experimental plantation. Diameter at breast height (DBH) was measured at 1.3 meters above ground level using a diameter tape, with an accuracy of 0.1 cm. Tree height was measured using an ultrasonic hypsometer, precise to 0.1 m. The growth data for DBH and tree height from 5-year-old Acacia melanoxylon used in this study consisted of individual tree measurements.
2.3 Statistical analysis method
The statistical analysis followed an integrated BLUP-GGE biplot framework to ensure accurate genetic parameter estimation and intuitive visualization. The process began with the estimation of breeding values using a mixed linear model fitted with the lmer() function from the R lme4 package (Gao et al., 2025). The model was specified as follows:
Where Xijkl represents the observed DBH or tree height of the k-th family in the j-th block at the i-th location (Yuan et al., 2022), μ is the overall mean, Li is the fixed effect of location, Bj(Li) is the random block effect nested within location, Fk is the random family effect, Li×Fk is the random family-by-location interaction effect, and eijkl is the residual error.
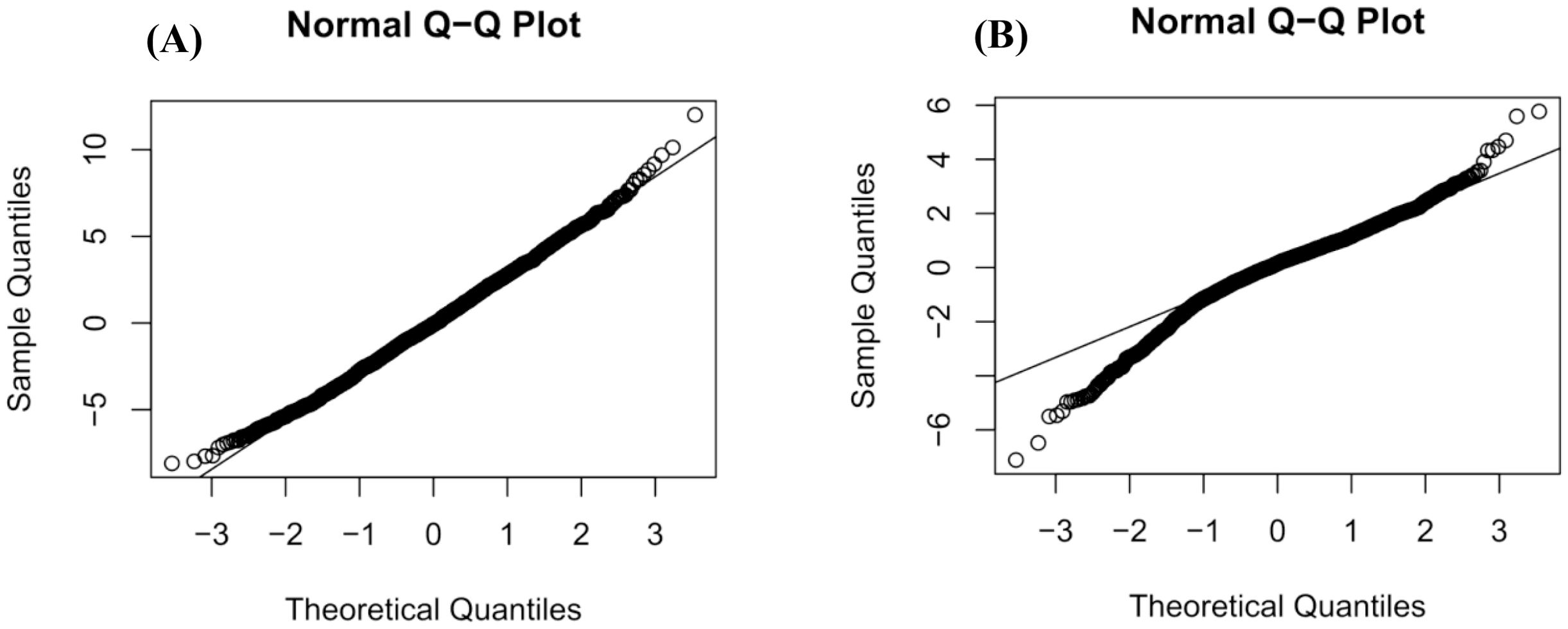
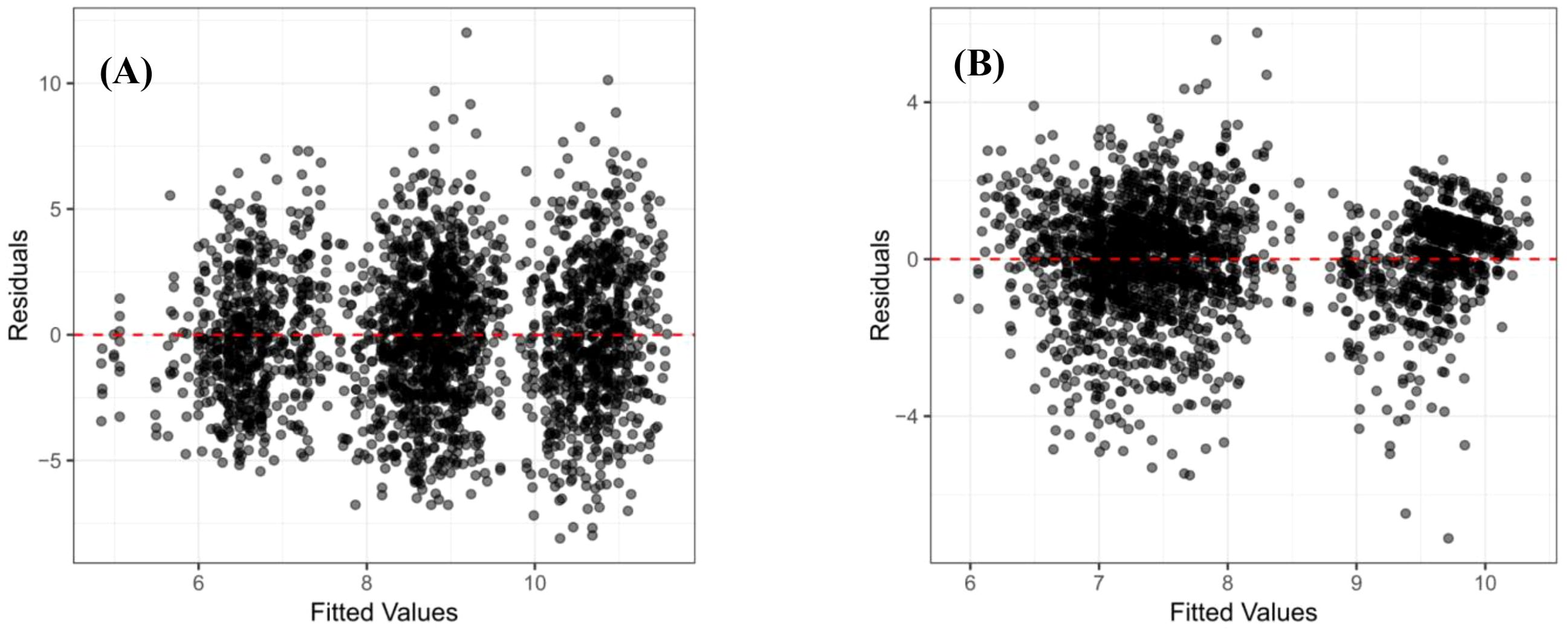
The model was fitted using the Restricted Maximum Likelihood (REML) algorithm, and the Best Linear Unbiased Predictions (BLUPs) for each family were extracted as the estimated breeding values. Prior to further analysis, the model’s adequacy was rigorously validated; the normality of residuals was assessed using a Q-Q plot, and the assumption of homoscedasticity was verified by plotting residuals against fitted values, with no substantial violations observed. In addition, significance testing for the fixed and random effects in the model was performed using the anova() and ranova() functions from the lmerTest package.
Finally, the validated BLUP values were used as inputs to generate GGE biplots using the GGEBiplotGUI package. The following parameter settings were selected based on the study’s objective to effectively visualize the genetic (G) and genotype-by-environment (GE) interaction patterns: Scaled = 0 (no standardization), Centering = ‘G+GE’ (focused on the GGE model), and singular value partitioning (SVP) = ‘JK’ (symmetric scaling focused on genotype discrimination) (Li et al., 2022).
3 Results
3.1 Estimation of breeding values using the BLUP method
The output of the mixed model operation displayed “REML criterion at convergence: []”, indicating successful model convergence. This was further verified by the message “Convergence achieved (0)”, confirming convergence. The mixed model residuals satisfied the assumptions of normality (Figure 1) and homoscedasticity (Figure 2). Furthermore, the DBH model demonstrated better performance compared to the tree height model.
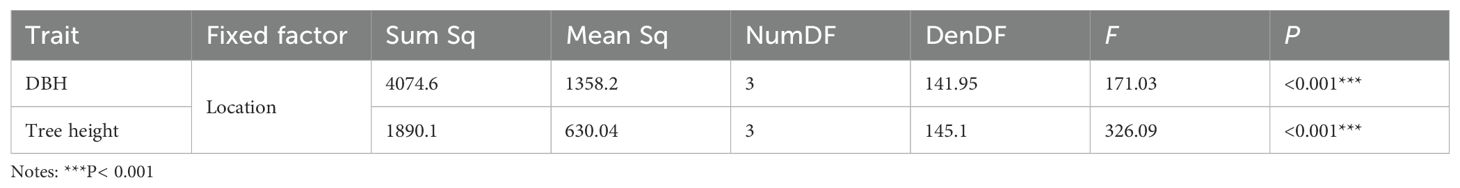
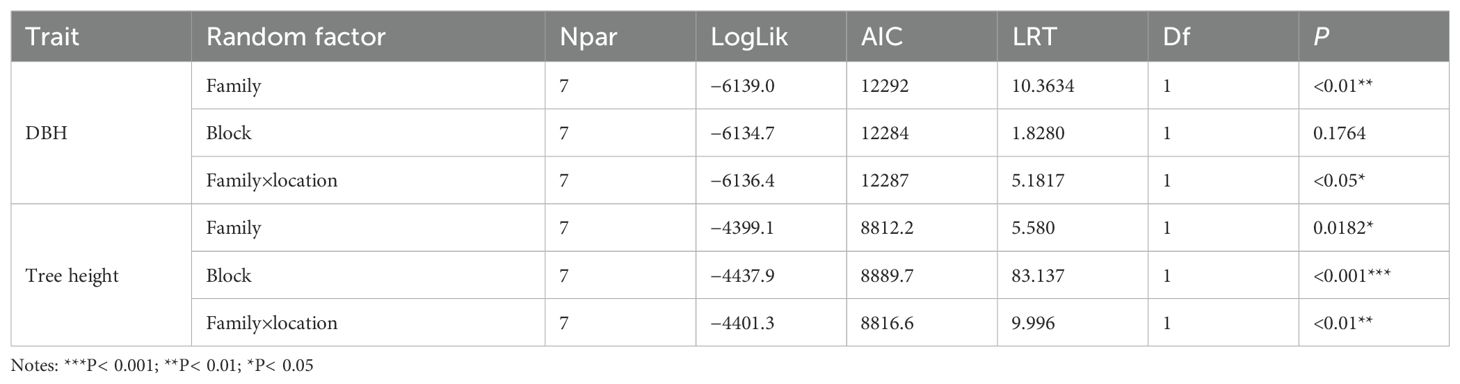
Significance testing for fixed effects in the mixed model (Table 3) revealed that location effects were highly significant (P< 0.001). This indicates strong environmental influences on the 5-year-old DBH and tree height. Regarding random effects (Table 4), block effects were non-significant for DBH but highly significant for tree height (P< 0.001). Family and family × location interaction effects were significant or highly significant for both traits (P< 0.05; P< 0.01). These findings demonstrate that DBH and tree height are significantly influenced by genotype and GE interactions.
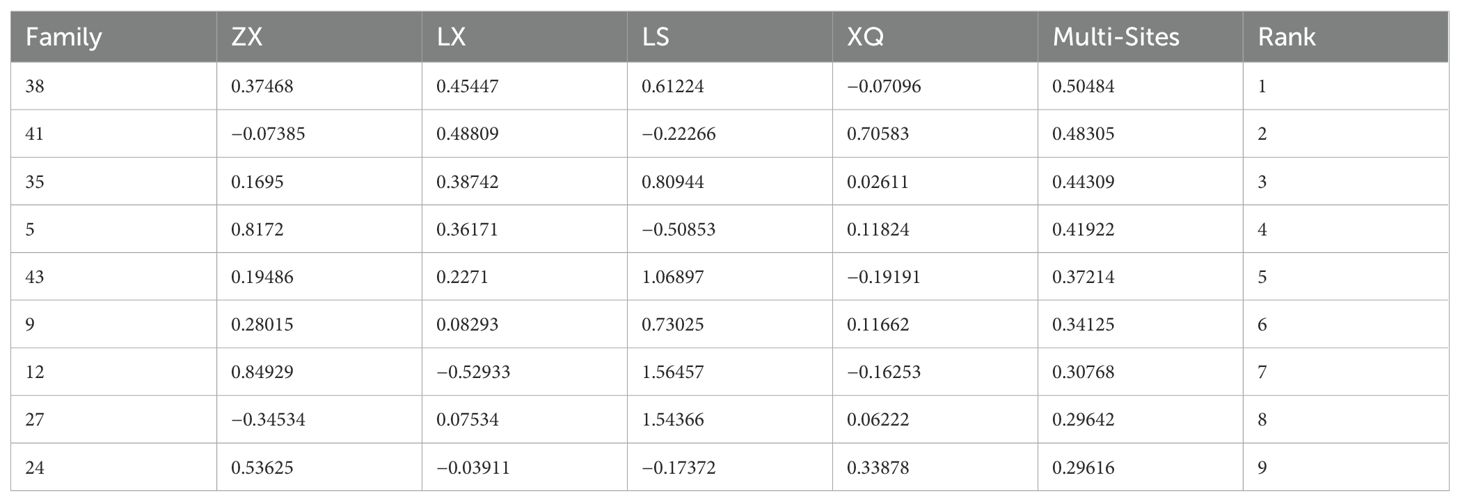
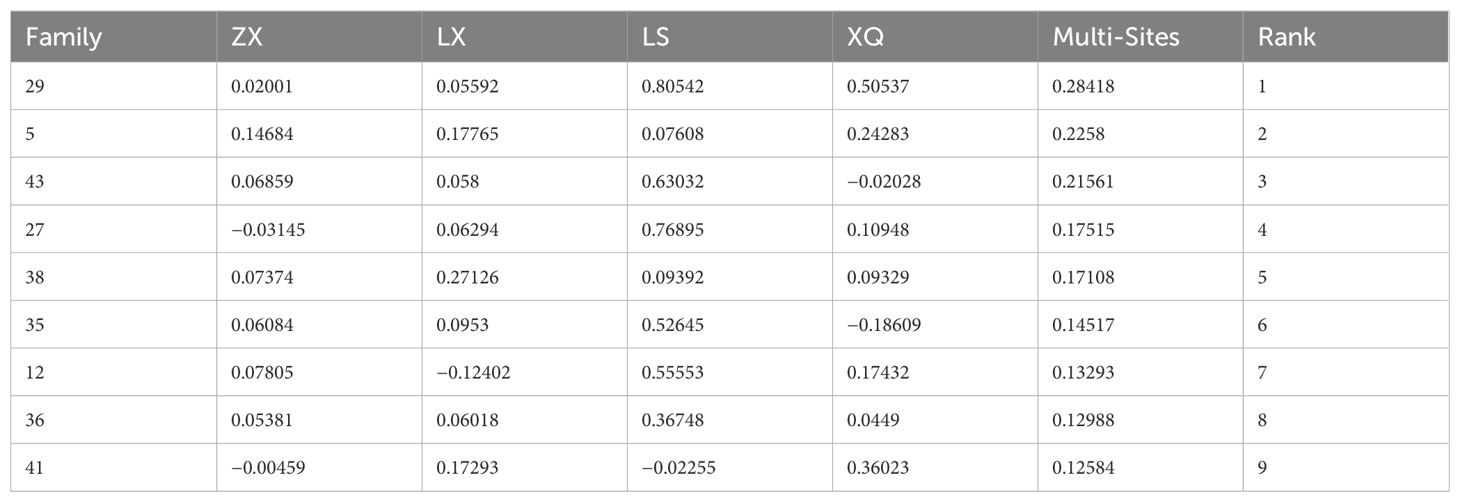
Using the R lme4 package, we fitted mixed linear models to assess DBH and tree height across four trial sites, as well as for the combined multi-site data, to estimate breeding values (Tables 5 and 6, The complete table can be found in the Supplementary Materials). For ranking purposes, multi-site breeding values were employed to evaluate family growth performance comprehensively. We applied a selection threshold of 20% to pinpoint elite families, ultimately identifying the top nine families with superior DBH and tree height.
The DBH breeding values ranged from −1.27462 to 0.50484, with 25 families registering values above 0. The top performing families were 38, 41, 35, 5, 43, 9, 12, 27, and 24. For tree height, breeding values ranged from −0.42664 to 0.28418, with 23 families showing values greater than 0. The highest-ranking families were 29, 5, 43, 27, 38, 35, 12, 36, and 41. These selected Acacia melanoxylon families demonstrate superior growth traits in both DBH and height, as well as greater genetic potential, making them ideal candidates for large-scale promotion.
3.2 Multi-location analysis based on GGE biplot
The GGE biplots for DBH and tree height (Figures 1-4), constructed from the first two principal components (AXIS1 for genetic and AXIS2 for environmental variation), demonstrated high explanatory power, accounting for 83.38% and 92.21% of the total variation, respectively. This indicates that the key patterns of genotype performance and environmental interaction were effectively captured by the model, ensuring the reliability of the subsequent conclusions regarding family selection and site evaluation (Zhao et al., 2022).
3.2.1 Results of site grouping
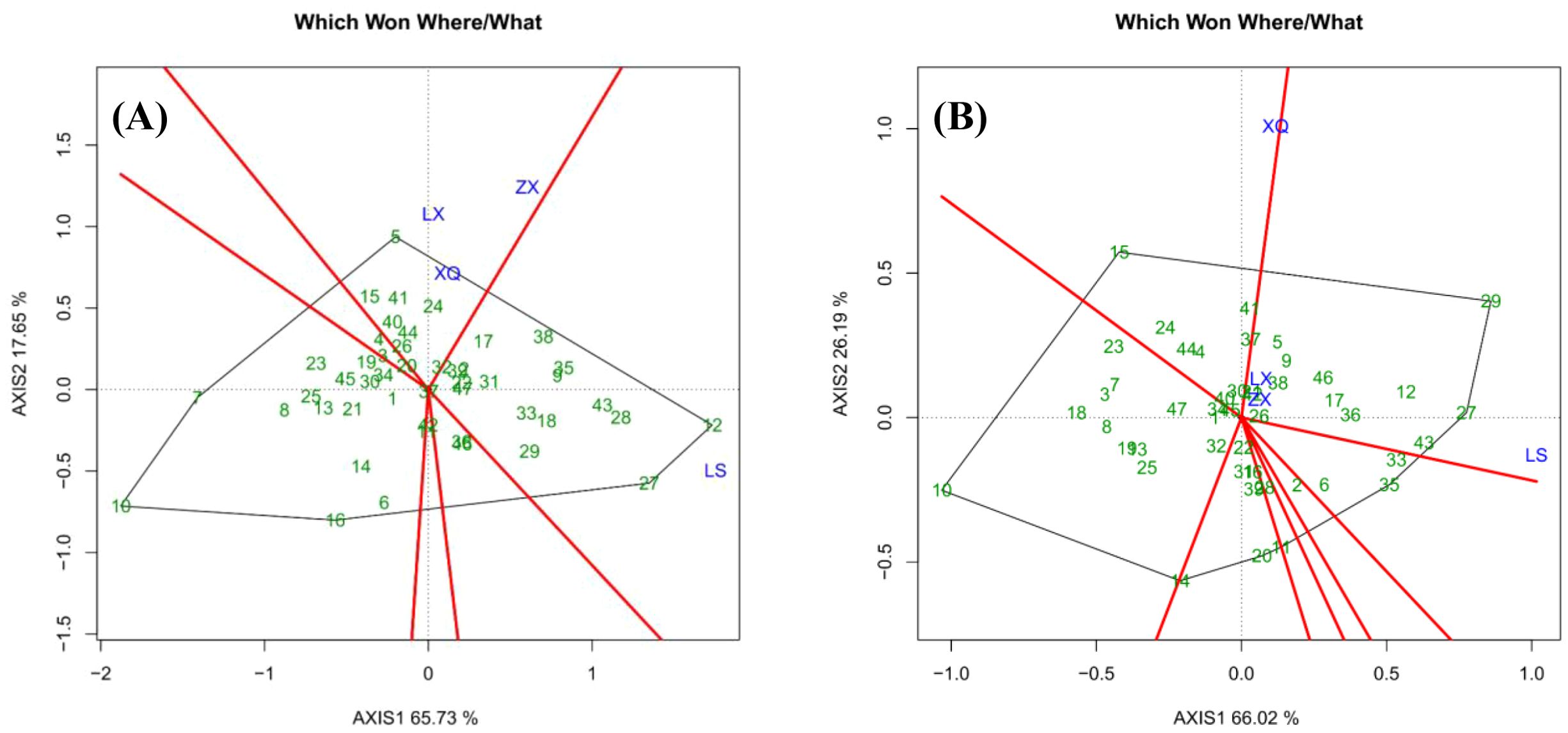
Figure 3 presents a “Which Won Where/What” biplot that categorizes trial sites and identifies the most advantageous genotypes within each group. The polygon is formed by the outermost families, with perpendicular lines extending from the origin to partition it into distinct sectors. Each sector’s vertex family represents the top performer for the sites within that sector. In the case of DBH (Figure 3A), sites ZX, XQ, and LX constitute Group 1, where Family 5 was the most successful. Site LS forms Group 2, with Families 27 and 12 at the sector vertices; however, Family 12 had the highest BLUP value. Similarly, regarding tree height (Figure 3B), sites LX, ZX, and LS make up Group 1, with Family 27 being the top-performing genotype. In Group 2, Site XQ demonstrated Family 15 as the top performer.
We observed a discrepancy between the BLUP rankings and the interpretation derived from the " Which Won Where/What " functional plot. For instance, in the case of DBH, Family 5 was identified as the top-performing genotype in Group 1 based on the vertex of the biplot, whereas Family 38 exhibited the highest multi-site BLUP value. This divergence can be well explained: the multi-site BLUP ranking reflects the comprehensive performance of each family across all four trial sites, while the biplot illustrates their performance within specific environmental groups. Thus, Family 5 demonstrated superior adaptation to the environments in Group 1, whereas Family 38 possessed the highest overall genetic potential.
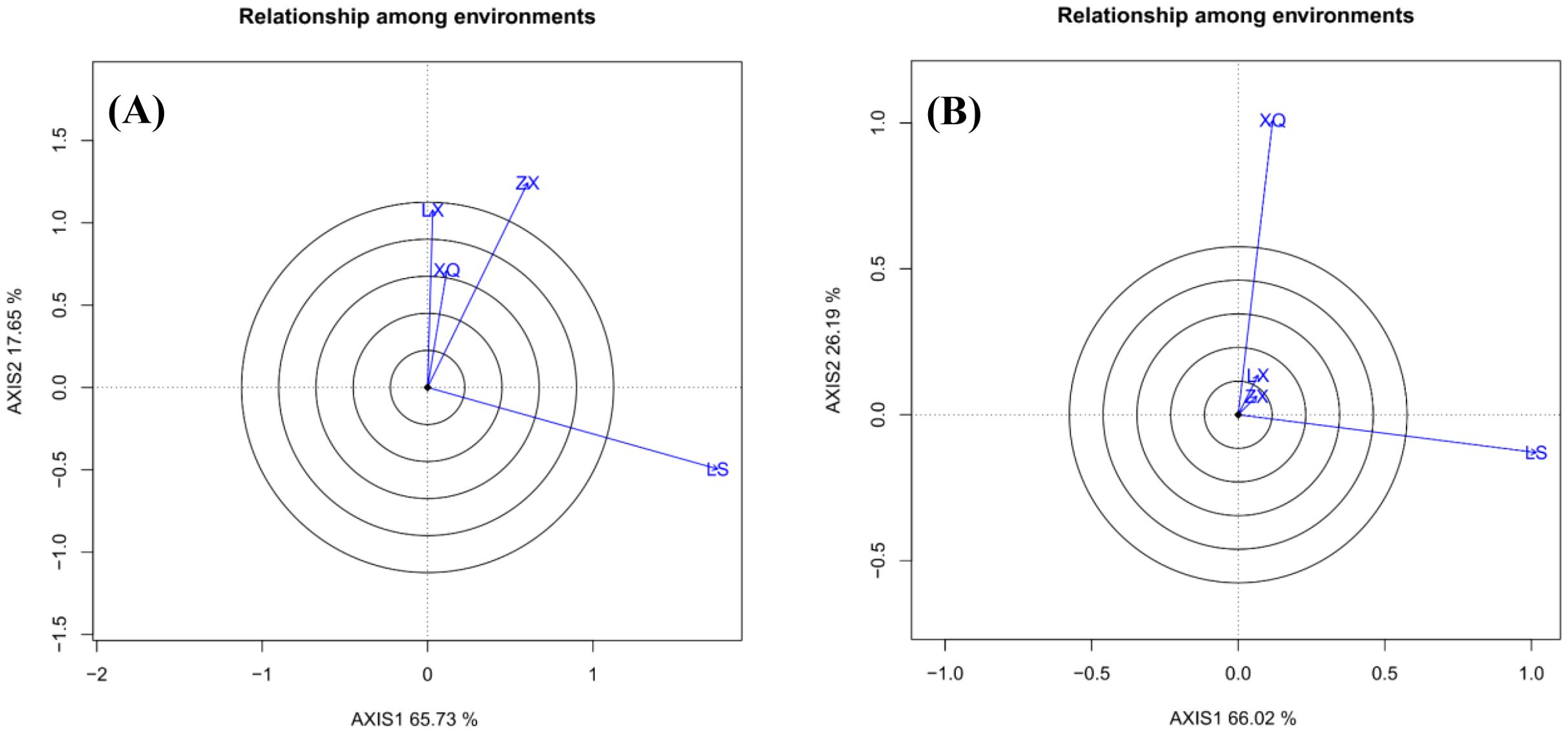
Figure 4 “Relationship Among Environments” biplot illustrates the similarity between trial sites during family evaluation. Sites with vectors forming angles smaller than 90° are positively correlated, with smaller angles indicating stronger correlations. Angles greater than 90° indicate negative correlations, while angles around 90° suggest no correlation. In the case of DBH (Figure 4A), sites LX and XQ are highly correlated and moderately correlated with ZX, suggesting consistent family rankings. Conversely, site LS is negatively correlated with LX, reflecting opposing rankings. Regarding tree height (Figure 4B), sites LX and ZX show a strong correlation, whereas XQ and LS are nearly uncorrelated, with an angle of approximately 90°.
3.2.2 Discrimination and representativeness of the test sites
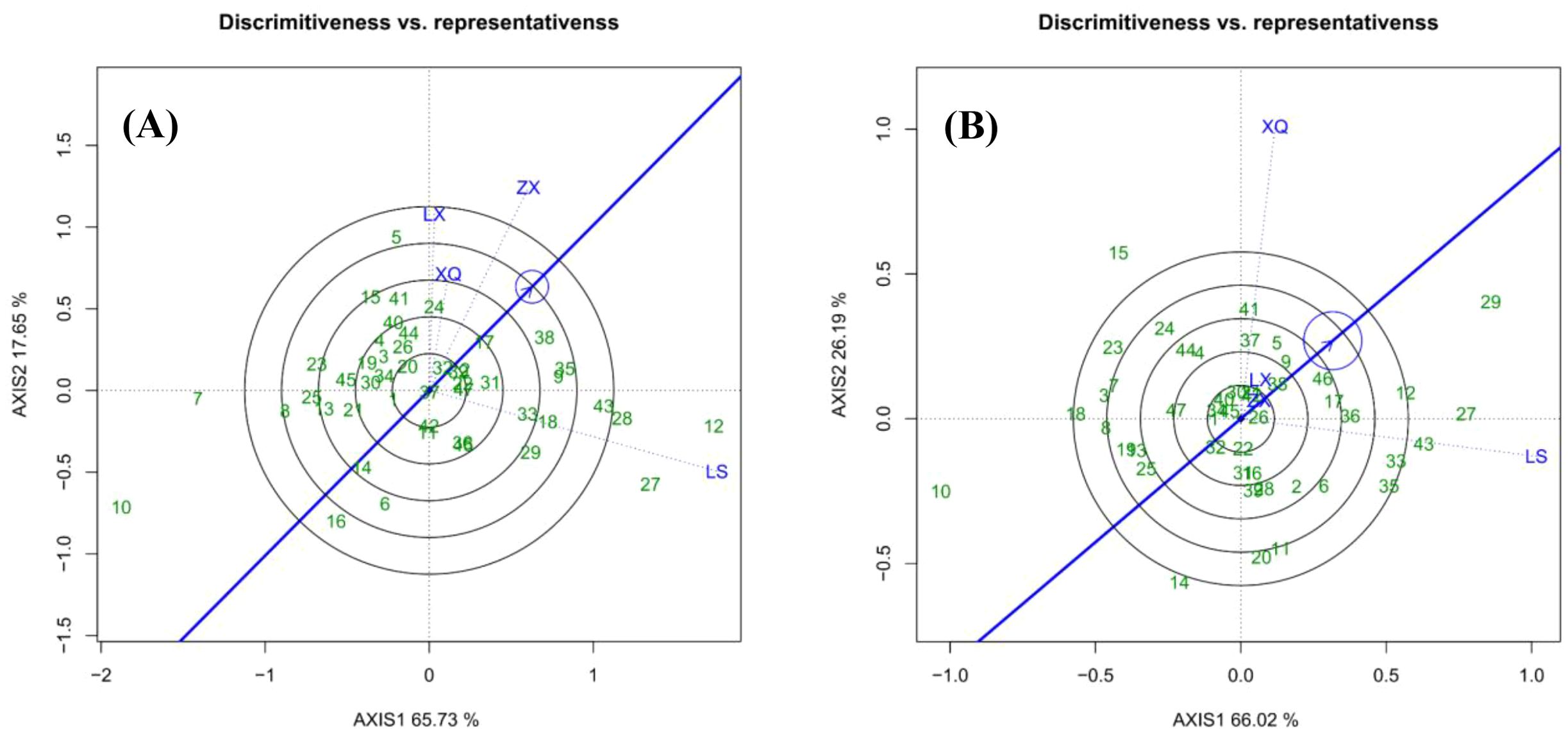
Figure 5 assesses trial sites in terms of their discriminative power (indicated by vector length) and representativeness (indicated by the angle from the average environment axis). Sites with longer vectors and smaller angles are more effective for selecting high-yield, stable families. In the case of DBH (Figure 5A), Site LS exhibited the highest discriminative power, with ZX, LX, and XQ following in sequence. ZX also demonstrated the highest representativeness, followed by XQ, with LS and LX showing similar levels. Consequently, ZX emerged as the most effective site for identifying elite families due to its high representativeness and second-highest discriminativity. For tree height (Figure 5B), no trial site could be identified that effectively selects families.
3.2.3 High yield and stability of families
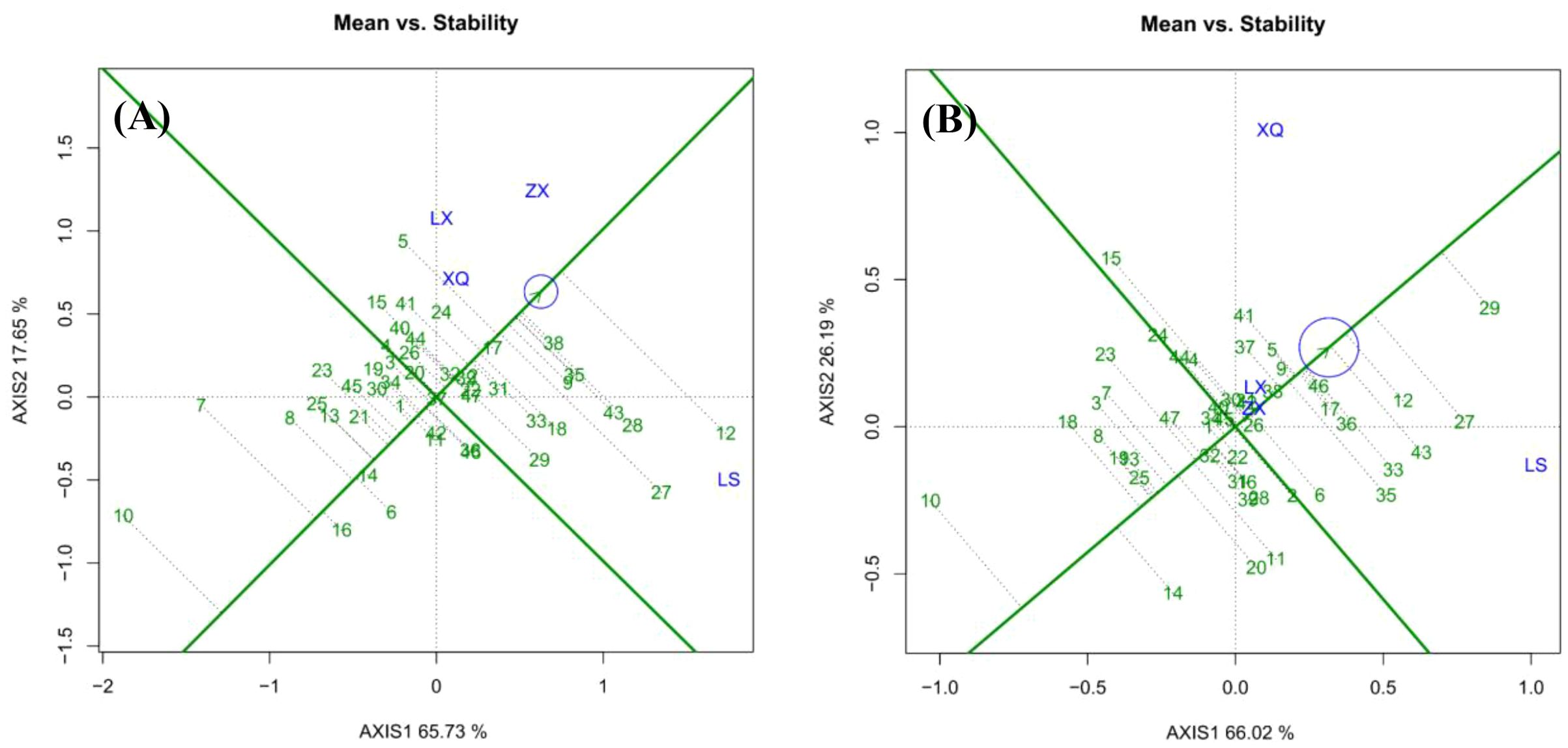
Within a specific family ecological zone, ideal families should achieve high yield and stability. The perpendicular line to the average environment axis, which passes through the origin, represents the overall mean growth value of the trait (Figure 6). Families positioned on the right side of this axis have growth values exceeding the mean, where a greater distance from the axis signifies a larger DBH or tree height. Conversely, families on the left side display below-average growth, with increased distance indicating smaller trait values.
For DBH (Figure 6A), Family 12 exhibited the highest growth, followed by Families 38, 28, and 43. Conversely, Family 10 demonstrated the lowest growth, with Families 7, 16, and 8 also performing poorly. Families 4, 20, and 37 displayed DBH values close to the overall mean. Regarding tree height (Figure 6B), Family 29 achieved the highest growth. Again, Family 10 ranked lowest. Families 24, 44, 40, and 2 exhibited tree heights near the overall mean.
The length of the vertical dashed line from each family to the average environment axis indicates stability, with shorter lines signifying greater stability. For DBH (Figure 6A), Family 12 exhibited the least stability, followed by Families 27 and 7. Conversely, Family 37 demonstrated the highest stability, followed by Families 17 and 14. Families 38, 35, 9, 33, 31, and 24 showed moderate stability. Concerning tree height (Figure 6B), Families 46, 17, 36, 29, 37, and 5 displayed moderate stability. The families within the top 25% growth rate were assessed and chosen for both high and stable yield, with Nos. 38, 35, 9, 17, and 24 demonstrating strong DBH rapidness and stability. Families 46, 36, 17, 5, and 9 were identified as having robust high-speed growth and stability.
4 Discussion
4.1 Data selection for GGE biplot analysis
Traditional biplots relying on fixed-effects models face limitations in forestry METs due to their assumptions of homogenous environmental variances and balanced data—conditions rarely met in practice given inherent spatial heterogeneity and occasional missing observations (Gauch et al., 2008). Our study substantiates these challenges, as evidenced by the unbalanced number of replicates across our four test sites. To overcome these constraints, we employed a mixed linear model, from which BLUP breeding values were extracted (Ling et al., 2021). The validity of our model was confirmed through convergence checks and residual diagnostics, ensuring that the BLUPs accurately reflected genetic effects (Piepho et al., 2008). The high discriminative power of sites like ZX and the clear differentiation of stable families (e.g., 38, 35) in our resulting biplots empirically demonstrate that BLUP-based data enhance the reliability of stability assessments and selection accuracy compared to analyses based on raw phenotypic data (Ferreira et al., 2012; Amrate et al., 2024).
The GGE biplots for DBH and tree height in this study explained 83.38% and 92.21% of the total variation, respectively, indicating reliable results, with tree height exhibiting higher reliability than DBH. However, considering the breeding objective for large-diameter timber and uncertainties in volume calculation, along with the inability of the "Discriminiveness vs. representativeness" plot for tree height to effectively identify test sites with both high discriminative power and strong representativeness, the GGE biplot analysis for DBH was ultimately adopted as the primary reference. Furthermore, compared to tree height measurement, DBH measurement offers higher accuracy. Genetic correlations among traits suggest that breeders can select high-productivity individuals based on larger DBH, which is easier to measure and more cost-effective (Adams et al., 2007; Kroon et al., 2008; Munhoz et al., 2021) . This further supports the feasibility of prioritizing the DBH-based GGE biplot analysis.
Acacia melanoxylon has been introduced to China as an important timber species for promotion. As a fast-growing tree species, its rotation period in China is approximately 8 years. Research on tree trials both domestically and internationally suggests that volume growth can be reliably evaluated at 1/3 to 1/2 of the rotation period. Thus, using DBH data from 5-year-old Acacia melanoxylon for evaluation and selection provides valuable reference. Additionally, while this study is based on predictive analysis using 5-year growth data, it should be noted that long-term growth patterns may evolve as trees develop. Continuous monitoring and integration with practical breeding efforts are essential for guiding future promotion and utilization.
4.2 Evaluation of tested families and sites
Regional METs are a primary and widely used method in forestry research. By analyzing data from multiple sites, METs facilitate the evaluation of genotype adaptability and stability, the identification of genotypes suited to diverse environments, and the delineation of optimal cultivation zones (Sixto et al., 2014; Nelson et al., 2018). On the average environment axis (Figure 4A), Family 12 demonstrated the largest DBH growth but exhibited low stability. In conjunction with breeding value analysis, Family 12 performed optimally at the Luoshan Forest Farm (LS) and moderately well at Zhongxi Forest Farm (ZX), while performing poorly at the other sites. It is recommended for planting in regions with ecological conditions akin to LS and ZX. Notably, stability is only practically significant when it coincides with high yield. For example, although Family 14 showed a “stable” performance, it was categorized as a “stable low-yield” family, making it economically unviable for DBH growth and unsuitable for promotion.
There is a strong correlation among the test sites ZX, LX, and XQ, with the ordering of family performances being similar across these locations, suggesting they may represent a similar mega-environment. This close relationship can be interpreted by examining the environmental data in Table 1.
While the soil types (mountain red soil) are similar between ZX, LX, and XQ, and differ from LS’s sandy loam, the climatic data reveal a more compelling pattern. Site XQ, situated in northern Fujian Province, has the lowest annual average temperature (17.3°C) among the four test sites. Notably, sites ZX and LX also experience relatively moderate average temperatures (20.2°C and 19.5°C, respectively) compared to the overall regional variation. Furthermore, the author's on-site investigations confirmed that Acacia melanoxylon at these sites grows primarily on shady slopes, which likely maintains a cooler microclimate than the broader forest farm averages might suggest. This consistent exposure to comparatively lower temperatures is the most parsimonious explanation for the strong genetic correlation among sites ZX, LX, and XQ.
In contrast, site LS appears independent and shows a weak correlation with the other three sites. Although LS has a reported average temperature of 20°C, it is located at a substantially higher elevation (415–481 m) compared to ZX (140–250 m) and LX (200–350 m). Higher elevation is often associated with greater temperature fluctuations and cooler conditions, which could differentiate its environmental profile. The combined factors of its distinct soil type and potentially unique microclimate at higher elevation may explain its divergence. However, to definitively pinpoint the causative environmental factors, further controlled experiments and more granular environmental monitoring are necessary.
According to the ranking of multi-site breeding values for DBH, a total of 25 families exhibited breeding values greater than 0. Among them, only 7 out of 17 families from the Australian introduced populations were selected, accounting for 41.18%, while 18 out of 30 families from the Luoxi Forest Farm seed orchard performed excellently, representing 60% of this group. This notable difference highlights the critical role of artificial selection in breeding: the proportion of superior genotypes in the locally pre-selected seed orchard population was significantly higher than that in the directly introduced populations. From the perspective of genetic gain, these results demonstrate that scientific selection can effectively enrich superior genotypes, enhance population growth and adaptive potential, and thereby offer more efficient breeding strategies for forest genetic improvement.
5 Conclusion
This study establishes the integrated BLUP-GGE biplot analysis as an effective framework for evaluating genotype-environment interactions and selecting superior genetic material in Acacia melanoxylon. The methodology's success in delineating mega-environments and identifying stable, high-performing genotypes demonstrates its substantial potential to streamline breeding programs for perennial trees characterized by long cycles and significant G×E effects.
Beyond its immediate application to Acacia melanoxylon, the research provides a validated, efficient strategy for the targeted deployment of genetic resources, which is crucial for enhancing forest productivity and adaptability under changing climatic conditions. Future work should focus on advancing the selected elite material into large-scale breeding programs and extending this analytical framework to assess other critical traits, such as wood properties and stress resilience, as well as to other economically important timber species.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
S-NR: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing, Resources, Funding acquisition. L-BC: Data curation, Visualization, Formal Analysis, Investigation, Writing – original draft. BZ: Data curation, Investigation, Writing – original draft. W-HZ: Writing – original draft, Data curation, Investigation. G-CD: Methodology, Supervision, Conceptualization, Writing – review & editing. G-QC: Methodology, Conceptualization, Supervision, Writing – review & editing. L-TY: Investigation, Resources, Writing – original draft. S-BH: Writing – original draft, Resources, Investigation. D-YS: Investigation, Writing – original draft, Resources. QY: Investigation, Resources, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Forestry Science and Technology Project of Fujian Province (2024FKJ29), the Fujian Province Forest Tree Seedling Science and Technology Research Project (ZMGG-0810), and the Science and Technology Innovation Project of Fujian Agriculture and Forestry University (KFB23060A).
Acknowledgments
The authors are grateful to Zhangpu Zhongxi State-owned Forest Farm(Zhangpu County, Zhangzhou City, Fujian Province, China), Quanzhou Luoxi State-owned Forest Farm(Quanzhou City, Fujian Province, China), Nanping Xiqin Teaching Forest Farm(Nanping City, Fujian Province, China), and Nan’an Luoshan State-owned Forest Farm(Nan’an City, Fujian Province, China) for providing the experimental site and technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1656136/full#supplementary-material
References
Adams, J. P., Rousseau, R. J., and Adams, J. C. (2007). Genetic performance and maximizing genetic gain through direct and indirect selection in cherrybark oak. Silvae Genet. 56, 80–87. doi: 10.1515/sg-2007-0012
Amrate, P. K., Nataraj, V., Shivakumar, M., Shrivastava, M. K., Rajput, L. S., Mohare, S., et al. (2025). Best linear unbiased prediction (BLUP)-based models aided in selection of high yielding charcoal rot and yellow mosaic resistant soybean genotypes. Genet. Resour. Crop Evol. 72, 5593–5611. doi: 10.1007/s10722-024-02289-5
Fan, C., Liu, Q., Zeng, B., Qiu, Z., Zhou, C., Chen, K., et al. (2016). Development of simple sequence repeat (SSR) markers and genetic diversity analysis in blackwood (acacia melanoxylon ) clones in China. Silvae Genet. 65, 49–54. doi: 10.1515/sg-2016-0006
Ferreira, R. T., Viana, A. P., Barroso, D. G., de Resende, M. D. V., and Júnior, A. T. D. A. (2012). Toona ciliata genotype selection with the use of individual BLUP with repeated measures. Sci. Agric. 69, 210–216. doi: 10.1590/S0103-90162012000300006
Gao, M. X., Ye, Y. Y., Zheng, Y., and Lai, J. S. (2025). A comprehensive analysis of r's application in ecological research from 2008 to 2023. J. Plant Ecol. 18 (1), rtaf010. doi: 10.1093/jpe/rtaf010
Gauch, H. G., Jr., Piepho, H., and Annicchiarico, P. (2008). Statistical analysis of yield trials by AMMI and GGE: further considerations. Crop Sci. 48, 866–889. doi: 10.2135/cropsci2007.09.0513
He, Y., Junker, R. R., Xiao, J., Lasky, J. R., Cao, M., Asefa, M., et al. (2025). Genetic and environmental drivers of intraspecific variation in foliar metabolites in a tropical tree community. New Phytologist. 246, 2551–2564. doi: 10.1111/nph.70146
Hussain, M. I., El-Sheikh, M. A., and Reigosa, M. J. (2020). Allelopathic potential of aqueous extract from acacia melanoxylon r. Br. On lactuca sativa. Plants 9, 1228. doi: 10.3390/plants9091228
Istanbuli, T., Alsamman, A. M., Al-Shamaa, K., Abu Assar, A., Adlan, M., Kumar, T., et al. (2025). Selection of high nitrogen fixation chickpea genotypes under drought stress conditions using multi-environment analysis. Front. Plant Sci. 16. doi: 10.3389/fpls.2025.1490080
Khan, A. M., Ali, Z., Mohsin, A. U., Soomro, A. A., Hussain, K., Rafiq, M., et al. (2024). Genotype × environment interactions analysis for chickpea grain yield and related traits by a mixed model approach. Discover Agric. 2, 90. doi: 10.1007/s44279-024-00110-6
Kroon, J., Andersson, B., and Mullin, T. J. (2008). Genetic variation in the diameter-height relationship in scots pine (pinus sylvestris). Can. J. For. Res. 38, 1493–1503. doi: 10.1139/X07-233
Li, L., Du, J., Ma, L., Ding, C., Zhang, P., and Kang, X. (2023). Regional testing of triploid hybrid clones of populus tomentosa. BMC Plant Biol. 23, 277. doi: 10.1186/s12870-023-04304-w
Li, Z., Liu, N., Zhang, W., Dong, Y., Ding, M., Huang, Q., et al. (2022). Application of BLUP-GGE in growth variation analysis in southern-type populus deltoides seedlings in different climatic regions. Forests 13, 2120. doi: 10.3390/f13122120
Ling, J., Xiao, Y., Hu, J., Wang, F., Ouyang, F., Wang, J., et al. (2021). Genotype by environment interaction analysis of growth of picea koraiensis families at different sites using BLUP-GGE. New For. 52, 113–127. doi: 10.1007/s11056-020-09785-3
Liu, G. J., Tian, Z. W., Jia, H. Y., Shen, W. H., Li, Z. H., Tang, L., et al. (2023). Genetic parameter estimates for the growth and morphological traits of castanopsis hystrix families and the genotype x environment interaction effects. Forests 14, 1619. doi: 10.3390/f14081619
Liu, N., Ding, C., Li, B., Ding, M., Su, X., and Huang, Q. (2021). Analysis of the genotype interaction of four-year-old populus euramericana using the BLUP-GGE technique. Forests 12, 1759. doi: 10.3390/f12121759
Martins, C., Monteiro, S., Knapic, S., and Dias, A. (2020). Assessment of bending properties of sawn and glulam blackwood in Portugal. Forests 11, 418. doi: 10.3390/F11040418
Munhoz, L. V., Santos, O. P. D., Valente, B. M. D. R., and Tambarussi, E. V. (2021). Genetic control of productivity and genotypes by environments interaction for eucalyptus dorrigoensis in southern Brazil. Cerne 27, e-102594. doi: 10.1590/01047760202127012594
Nelson, N. D., Berguson, W. E., McMahon, B. G., Cai, M., and Buchman, D. J. (2018). Growth performance and stability of hybrid poplar clones in simultaneous tests on six sites. Biomass Bioenergy 118, 115–125. doi: 10.1016/j.biombioe.2018.08.007
Pereira, A. and Ferreira, V. (2021). Invasion of native riparian forests by acacia species affects in-stream litter decomposition and associated microbial decomposers. Microb. Ecol. 81, 14–25. doi: 10.1007/s00248-020-01552-3
Piepho, H. P., Möhring, J., Melchinger, A. E., and Büchse, A. (2008). BLUP for phenotypic selection in plant breeding and variety testing. Euphytica 161, 209–228. doi: 10.1007/s10681-007-9449-8
Prášil, I. T., Musilová, J., Prášilová, P., Janáček, J., Coufová, M., Kosová, K., et al. (2025). Effect of geographical origin, regional adaptation, genotype, and release year on winter hardiness of wheat and triticale accessions evaluated for six decades in trials. Sci. Rep. 15, 5961. doi: 10.1038/s41598-025-89291-8
Sixto, H., Gil, P., Ciria, P., Camps, F., Sánchez, M., Cañellas, I., et al. (2014). Performance of hybrid poplar clones in short rotation coppice in mediterranean environments: analysis of genotypic stability. Glob. Change Biol. Bioenergy 6, 661–671. doi: 10.1111/gcbb.12079
Skrøppa, T. and Fundova, I. (2025). Lammas shoots in Norway spruce families in short-term trials; Genetic variation, inheritance patterns and implications of micro-environmental influence. Silvae Genet. 74, 1–10. doi: 10.2478/sg-2025-0001
Vymyslický, T., Trněný, O., Rietman, H., Balko, C., Đorđević, V., Ranđelović, P., et al. (2025). Phenotypic characterization of soybean genetic resources at multiple locations: breeding implications for enhancing environmental resilience, yield and protein content. Front. Plant Sci. 16. doi: 10.3389/fpls.2025.1422162
Yan, W., Kang, M. S., Ma, B., Woods, S., and Cornelius, P. L. (2007). GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci. 47, 643–653. doi: 10.2135/cropsci2006.06.0374
Yousaf, M. F., Tomar, V., Romé, H., Bagge, M., Timmermann, M., Chu, T. T., et al. (2025). Rate of double reduction and genetic variability in yield, quality, and senescence related traits in tetraploid potato (solanum tuberosum l.). Front. Plant Sci. 16. doi: 10.3389/fpls.2025.1560123
Yuan, Z., Dong, Y., Liu, N., Li, S., Zhang, W., Wang, Y., et al. (2022). G×e analysis of early growth traits of populus deltoides in east China by using BLUP-GGE. Forests 13, 1808. doi: 10.3390/f13111808
Zhao, Y., Feng, Y., Yang, C., Qiao, J., Zhou, H., Li, F., et al. (2022). Genetic parameters and genotype–environment interactions in paulownia clonal tests in temperate and subtropical regions of China. Forests 13, 2113. doi: 10.3390/f13122113
Keywords: Acacia melanoxylon, best linear unbiased prediction, GGE biplot, regional trial, family selection
Citation: Ruan S-N, Chen L-B, Zhang B, Zhang W-H, Ding G-C, Cao G-Q, Ye L-T, He S-B, Shen D-Y and Yan Q (2025) Genotype by environment interaction for growth traits of families of Acacia melanoxylon based on BLUP and GGE biplot. Front. Plant Sci. 16:1656136. doi: 10.3389/fpls.2025.1656136
Received: 29 June 2025; Accepted: 28 October 2025;
Published: 14 November 2025.
Edited by:
Mohan Lal, North East Institute of Science and Technology (CSIR), IndiaReviewed by:
Anupam Singh, Shree Guru Gobind Singh Tricentenary University, IndiaYe Zhao, Beijing Forestry University, China
Copyright © 2025 Ruan, Chen, Zhang, Zhang, Ding, Cao, Ye, He, Shen and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shao-Ning Ruan, Zmpyc25AMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Shao-Ning Ruan1*†
Shao-Ning Ruan1*† Liu-Ben Chen
Liu-Ben Chen Guang-Qiu Cao
Guang-Qiu Cao