- 1Department of Crop and Animal Production, Safiye Cikrikcioglu Vocational College, Kayseri University, Kayseri, Türkiye
- 2Genome and Stem Cell Research Center, Erciyes University, Kayseri, Türkiye
Introduction: This study is the first to investigate the genetic diversity of plant growth-promoting genes in rhizobacteria isolated from the wild ecology of Mount Erciyes, Türkiye. It has a flora rich in flowering plants, with 1170 plant taxa, 194 of which are endemic to the area.
Methods: A total of 165 bacterial isolates, including Azotobacter sp., Azospirillum sp., and Bacillus sp., were screened for genes associated with plant growth promotion: nitrogen fixation (nif), indole pyruvate decarboxylase (ipdC), 1-aminocyclopropane-1-carboxylate deaminase (accd), phosphate-solubilizing genes (Acpho, Alpho and phy), and siderophore biosynthesis (sd).
Results: The analysis revealed significant genetic variability across isolates, particularly for nif and sd genes, with distinct band patterns indicating genetic diversity among Azospirillum and Bacillus isolates.
Discussion: The findings emphasize the role of these rhizobacteria in nutrient cycling and stress resilience, potentially enhancing plant growth in nutrient-limited soils. In the current study, it contributes to understanding microbial biodiversity in Mount Erciyes and suggests a promising potential for sustainable agriculture through plant-microbe interactions.
1 Introduction
Recent advancements in agricultural research have increasingly focused on sustainable practices to enhance crop productivity while minimizing environmental impacts. In semi-arid regions like the southeastern part of the Central Anatolian Plateau, including Kayseri, Türkiye, cereal and legume cultivation faces challenges due to nutrient-poor aridisols and limited water availability. Plant growth-promoting rhizobacteria (PGPR) have emerged as a promising solution to these challenges, offering eco-friendly alternatives to chemical fertilizers. Studies have demonstrated that PGPR strains, such as Azospirillum and Bacillus, can significantly enhance crop yields. For instance, a study by Çakmakçı et al. (2017) reported that Azospirillum brasilense and Bacillus subtilis increased wheat grain yields by up to 30% in semi-arid conditions through mechanisms like nitrogen fixation and phosphate solubilization. Similarly, Erturk et al. (2012) found that co-inoculation of Bacillus and Rhizobium improved chickpea nodulation and yield by 25% in nutrient-deficient soils, highlighting the potential of PGPR for sustainable agriculture in regions like Kayseri.
The application of PGPR extends beyond yield enhancement to include stress tolerance and soil health improvement. Research by Swarnalakshmi et al. (2020) emphasized that PGPR, including Azospirillum and Bacillus, promote legume growth by producing phytohormones like indole-3-acetic acid (IAA) and solubilizing phosphorus, reducing reliance on synthetic fertilizers. Additionally, PGPR contribute to biocontrol by producing siderophores and antimicrobial compounds, as noted in a meta-analysis by Zeffa et al. (2020), which reported an 11.4% increase in soybean nodulation with Bradyrhizobium and Bacillus co-inoculation. These findings underscore the multifaceted roles of PGPR in enhancing nutrient cycling and plant resilience, critical for sustainable farming in semi-arid ecosystems.
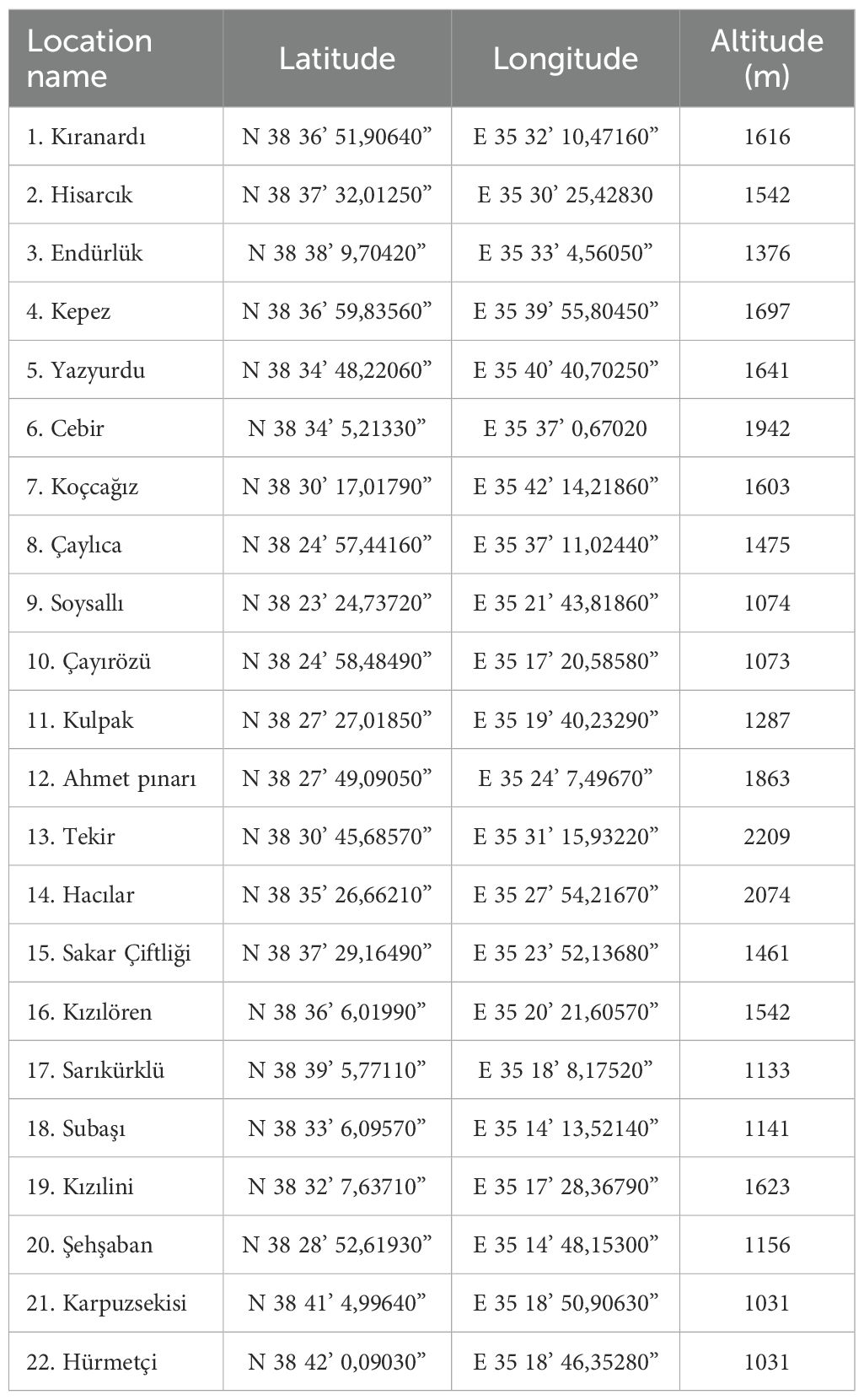
Türkiye’s diverse ecological landscape, particularly around Mount Erciyes in Kayseri, supports a rich biodiversity that remains underexplored for microbial resources. The southeastern part of the Central Anatolian Plateau, including the Kayseri region, is dominated by cereal and legume cultivation on semi-arid, nutrient-poor aridisols. Plant growth-promoting rhizobacteria (PGPR) strains such as Azospirillum and Bacillus have been shown to increase wheat and barley grain yields by up to 40%, providing a sustainable, low-input alternative to chemical fertilizers that could greatly benefit local farmers (Barış et al., 2014). Türkiye’s diverse ecological structure fosters high levels of endemism and genetic diversity. Mount Erciyes, a prominent stratovolcano situated approximately 25 km southwest of Kayseri, rises from the plains of Sultansazlığı and hosts a flora rich in flowering plant species. Of the 1170 plant taxa identified in this region, 194 are endemic (Vural and Aytaç, 2005). Although many studies have explored the biodiversity of plant and animal life on Mount Erciyes, a significant knowledge gap remains regarding the rhizobacteria inhabiting this unique environment. To date, there has been no research on PGPR or their associated functional genes in this area. PGPR are beneficial bacteria commonly isolated from soil and are known to promote plant growth through various mechanisms. These bacteria belong to diverse genera, including Alcaligenes, Agrobacterium, Azospirillum, Azotobacter, Arthrobacter, Bacillus, Bradyrhizobium, Burkholderia, Caulobacter, Chromobacterium, Enterobacter, Erwinia, Flavobacterium, Herbaspirillum, Klebsiella, Mesorhizobium, Micrococcus, Pseudomonas, Rhizobium, Rhodococcus, and Serratia (Egamberdiyeva, 2005). Among these genera, Azospirillum and Azotobacter are noteworthy for their nitrogen-fixing abilities, while Bacillus is notable for producing plant hormones, siderophores, and phosphate-solubilizing enzymes.
In this study, we focused on characterizing the genetic diversity of plant growth-promoting genes within rhizobacterial populations, specifically targeting Azospirillum sp., Azotobacter sp., and Bacillus sp., isolated from the unique flora of Mount Erciyes. To assess their plant growth-promoting potential, we screened for genes associated with key PGPR functions. These included nitrogen fixation genes (nif), indole pyruvate decarboxylase (ipdC) involved in auxin (IAA) biosynthesis, 1-aminocyclopropane-1-carboxylate deaminase (accd) associated with ethylene modulation, and genes involved in phosphate solubilization, such as acid phosphatase (Acpho), alkaline phosphatase (Alpho), and phytase (phy). Additionally, we screened for siderophore biosynthesis genes (sd) essential for iron acquisition and plant growth enhancement.
2 Materials and methods
2.1 Collection of soil samples
Soil samples were collected from various soil types (clayey, sandy, and loamy soils) within the vegetative regions of Mount Erciyes, with each sample consisting of a 1 kg mixture obtained by combining 100 g of soil from 10 different spots within an area. The sampling sites were strategically distributed to capture a range of ecological niches, including areas near endemic plant species and agricultural fields, to ensure a comprehensive representation of the rhizobacterial populations associated with the region’s unique flora. Samples were taken from the plant root-soil interface area (5-10 cm depth) in locations free from commercial microbial fertilizers. In total, 22 soil samples were collected, with GPS coordinates recorded for each sampling location (Table 1).
2.2 Physicochemical characterization of soil samples
A total of 22 different soil samples were analyzed for texture, pH (potential of hydrogen), lime (calcium carbonate equivalent, CaCO3), organic matter (OM), electrical conductivity (EC), phosphorus (P), total nitrogen (N), iron (Fe), copper (Cu), zinc (Zn), and manganese (Mn) properties. Soil texture was determined using the Bouyoucos hydrometer method (Bauder, 2000). Soil pH was measured potentiometrically with a glass electrode pH meter (Cole-Parmer Jenway 3510 Standard Digital pH Meter) using a 1:2.5 soil-to-water ratio (Mclean, 1982). Lime content was determined volumetrically with a Scheibler calcimeter. Organic matter content was measured using the Walkley–Black wet oxidation method (Walkley and Black, 1934). Exchangeable cations (Na+, Ca²+, Mg²+, and K+) were extracted by shaking the soils with 1 N ammonium acetate (pH 7.0) and quantified by inductively coupled plasma optical emission spectrometry (ICP-OES) (Rhoades, 1982). Available phosphorus was determined using the molybdenum blue colorimetric method by measuring the absorbance of the resulting blue solution at 660 nm with a spectrophotometer (Olsen and Sommers, 1982). Total nitrogen content was calculated using the micro-Kjeldahl method after wet digestion with a mixture of salicylic acid, sulfuric acid, and salt. Micronutrients, including Fe, Cu, Zn, and Mn, were extracted using the DTPA (diethylenetriaminepentaacetic acid) method and quantified by ICP-OES (Lindsay and Norvell, 1978).
2.3 Bacteria isolation and culture conditions
2.3.1 Isolation and identification of Azotobacter sp.
To isolate Azotobacter sp. from soil samples, 10 grams of each soil sample were mixed with 90 ml of dH2O. After thorough vortex mixing, the samples were left to settle at room temperature for approximately 20 min. Following this, 1 ml of the supernatant was transferred to 50 ml flasks containing Ashby liquid medium (pH 7.3) (per liter: 20 g mannitol, 0.2 g K2HPO4, 0.2 g MgSO4•7H2O, 0.2 g NaCl, 0.1 g K2SO4, 5 g CaCO3, and 1 ml of a microelement solution containing: 1 g H3BO3, 2 g FeSO4•7H2O, 1 g MnCl2•4H2O, 1 g Na2MoO4•2H2O, 0.5 g NaBr, and 0.2 g ZnSO4•7H2O) and incubated at 28°C for 5-7 days at 200 rpm in a shaking incubator (Bakulin et al., 2007). From the resulting culture, 1 ml was taken and serially diluted (10-4 to 10-7) before being plated on Ashby agar (Azotobacter Agar with Mannitol). Plates were incubated at 28°C for 5-7 days. Azotobacter colonies grown on the selective medium were stored at +4°C until DNA isolation (Bakulin et al., 2007).
2.3.2 Isolation and identification of Azospirillum sp.
To isolate Azospirillum sp., 10 grams of each soil sample were mixed with 90 ml of sterile distilled water. After thorough vortex mixing, the samples were left to settle at room temperature for approximately 20 minutes. Then, 1 ml of the liquid portion was serially diluted (10-² to 10-7) (Baldani and Reis, 2014), and added to 10 ml of N-free medium (liquid NFb medium, 1 L, pH 6.5: 5 g malic acid, 0.05 g yeast extract, 0.5 g K2HPO4, 0.2 g MgSO4•7H2O, 0.1 g NaCl, 0.02 g CaCl2•2H2O, 0.04 g CuSO4•5H2O, 0.12 g ZnSO4•7H2O, 1.4 g H3BO3, 1 g Na2MoO4•2H2O, 1.175 g MnSO4•H2O, 2 ml bromothymol blue [5 g/L in 0.2 N KOH], 2 ml Fe-EDTA solution [16.4 g/L], 4 ml vitamin solution [10 mg biotin; 20 mg pyridoxal-HCl, pH adjusted with KOH; for solid NFb medium, add 15 g agar]) (Baldani and Reis, 2014). Azospirillum sp. colonies grown on the selective medium were stored at +4°C until DNA isolation.
2.3.3 Isolation and identification of Bacillus sp.
To isolate Bacillus sp., 10 grams of each soil sample were mixed with 90 ml of sterile distilled water. After thorough vortexing, the samples were allowed to settle at room temperature for approximately 5 minutes. Then, 1 ml of the supernatant was transferred to 50 ml flasks containing 10 ml of Luria-Bertani (LB) Broth medium buffered with 0.25 M sodium acetate. The flasks were incubated in a shaking incubator at 30°C for 4 hours at 200 rpm. Following incubation, 1 ml of each sample was placed in sterile eppendorf tubes and heat-treated at 80°C for 5-10 minutes. Afterward, samples were plated on LB agar plates and incubated overnight at 30°C (Travers et al., 1987; Katı et al., 2016). Bacillus bacterial colonies grown on the selective medium were stored at +4°C until DNA isolation.
2.4 DNA isolation and amplification of plant-growth-promoting genes
DNA isolation was performed using the Bio Basic Bacterial Genomic DNA Isolation Kit (BS624), following the manufacturer’s recommended protocol. From each isolated DNA sample, 5 μl were loaded onto a 1% (w/v) agarose gel prepared in 1x TAE buffer (Tris-Acetic Acid-EDTA, pH 8.0) containing 10 μl ethidium bromide, and electrophoresed at 80 V for 1 hour. DNA presence was verified using a Biorad gel imaging system.
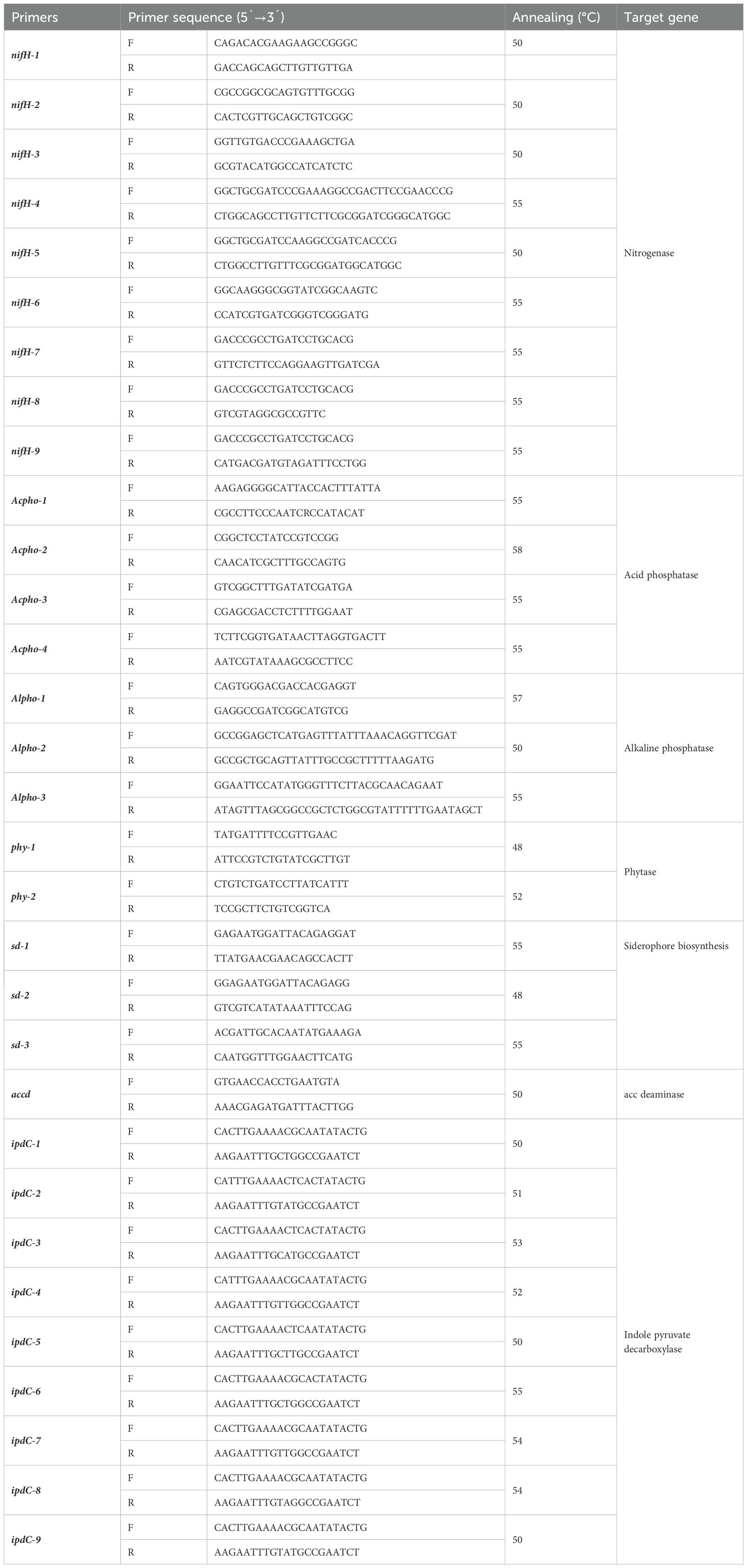
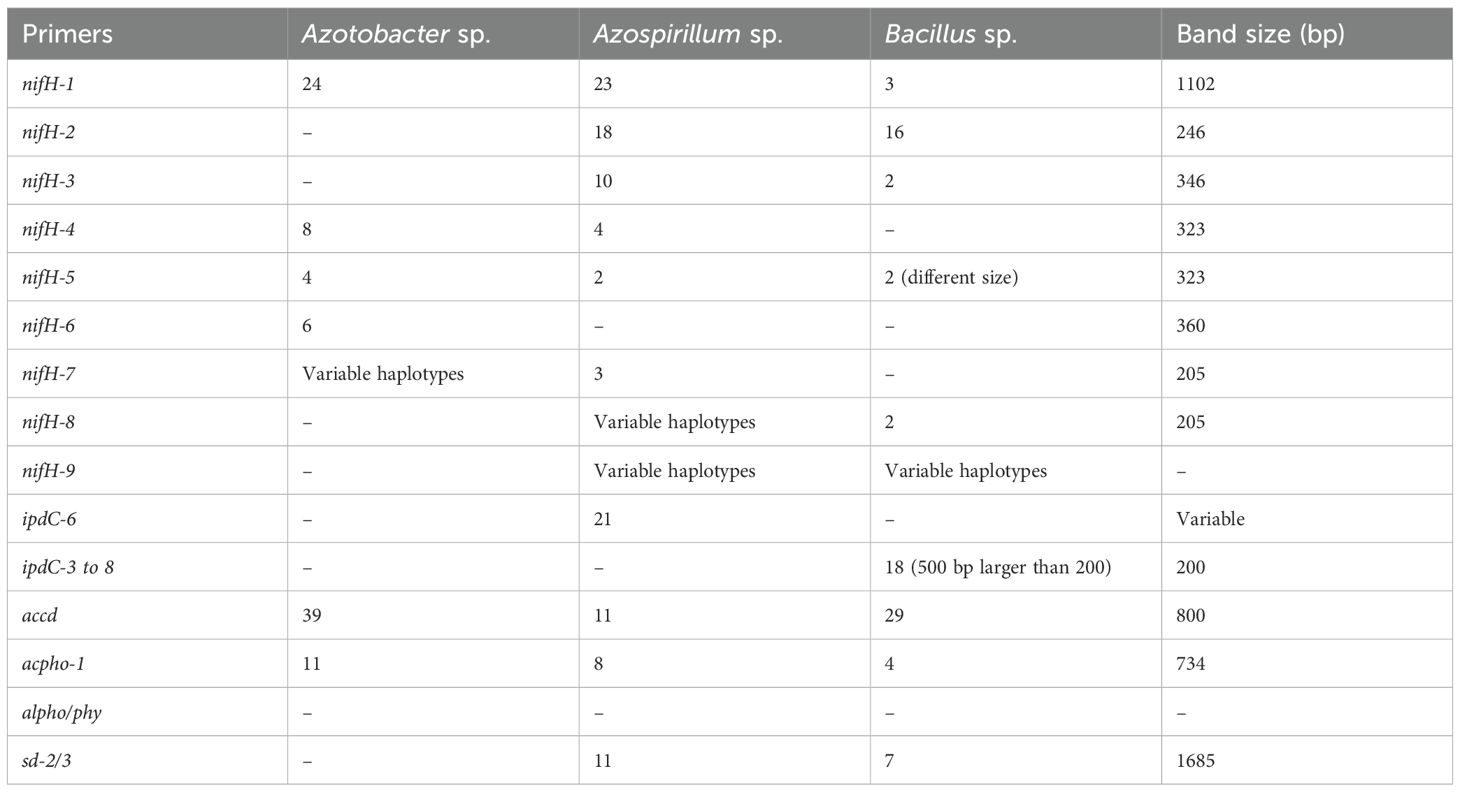
To determine whether the rhizobacteria identified as Azotobacter, Azospirillum, and Bacillus carried genes promoting plant growth, PCR analysis was conducted using primers (nifH-1, nifH-2, nifH-3, nifH-4, nifH-5, nifH-6, nifH-7, nifH-8, nifH-9, Acpho-1, Acpho-2, Acpho-3, Acpho-4, Alpho-1, Alpho-2, Alpho-3, phy-1, phy-2, sd-1, sd-2, sd-3, accd, ipdC-1, ipdC-2, ipdC-3, ipdC-4, ipdC-5, ipdC-6, ipdC-7, ipdC-8 and ipdC-9) for the nif, ipdC, accd, Acpho, Alpho, phy, and sd genes (Table 2) (Helmut et al., 2004; Ding et al., 2005; Wilson et al., 2006; Sakurai et al., 2008; Raddadi et al., 2008; Bawane et al., 2011; Shime-Hattori et al., 2011; Susilowati and Setyowati, 2016; Ahmed et al., 2017; Kirillov et al., 2017; Gaiero et al., 2018; Meng et al., 2019).
Each reaction contained the reagents at a final concentration as 2.4 mM MgCl2, 1× taq buffer, 0.25 mM dNTPmix, 0.3 pmol primers (each), 0.5 U taq DNA polymerase, and 30–100 ng of template DNA. The PCR amplification was performed under the following conditions: Initial denaturation at 95°C for 5 min, followed by 30 cycles at 94°C for 1 min, Tm C for 1 min, 72°C for 2 min, and a final extension step at 72°C for 10 min (Raddadi et al., 2008). Following PCR, 5 μl of each product was loaded onto a 1% (w/v) agarose gel prepared in 1x TAE buffer (Tris-Acetic Acid-EDTA, pH 8.0) containing 10 μl ethidium bromide, and electrophoresed at 80 V for 1 hour. DNA bands were visualized on a Biorad gel imaging system. GeneRuler 100 bp Plus DNA Ladder (Thermo Scientific), ranging from 100 to 3000 bp, was used as the molecular size marker in all gels to estimate the fragment sizes.
2.5 Statistical analysis
Statistical analyses were performed using the MSTAT-C statistical software (Michigan State University, USA). A one-way analysis of variance (ANOVA) was applied to assess differences in soil physicochemical properties among the 22 sampling sites on Mount Erciyes. Mean comparisons were conducted using the Tukey-Kramer Honestly Significant Difference (HSD) post hoc test at a 99% confidence level (p < 0.01). All parameters presented in Table 3, including pH, electrical conductivity (EC), organic matter (OM), nitrogen (N), lime content, phosphorus (P), calcium (Ca), potassium (K), magnesium (Mg), sodium (Na), copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn), were subjected to this analysis. However, due to the absence of replicate measurements per location (n=1), within-group variance could not be estimated, rendering traditional significance testing inconclusive. Consequently, the data were interpreted descriptively, focusing on ranges and patterns across locations.
3 Results
3.1 Physicochemical characterization of soil samples
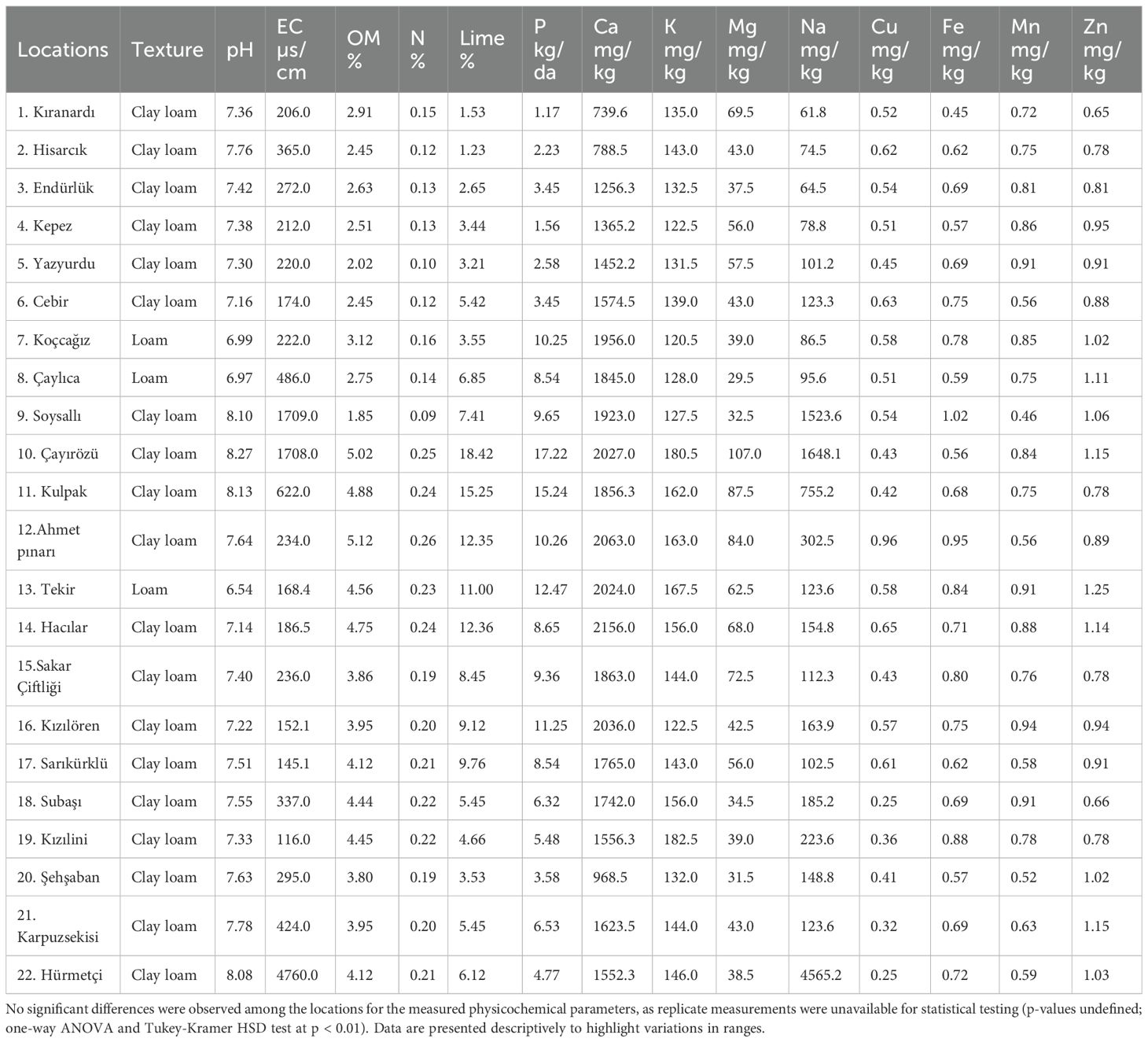
Table 3 summarizes the main physicochemical properties of the soil samples collected from the sampling sites. Soil samples collected from 22 distinct locations within the vegetative regions of Mount Erciyes were subjected to comprehensive physicochemical characterization to assess their properties and suitability for supporting rhizobacterial populations. These locations, spanning diverse microenvironments across the slopes of Mount Erciyes, were selected to capture variations in soil texture, nutrient content, and ecological niches. Table 3 summarizes the primary physicochemical properties of the soil samples, including texture, pH, electrical conductivity (EC), organic matter (OM) content, total nitrogen (N), lime content, and concentrations of key nutrients such as phosphorus (P), calcium (Ca), potassium (K), magnesium (Mg), sodium (Na), copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn).
Soil texture analysis revealed that the majority of the samples (19 out of 22) were classified as clay loam, while three locations (Koçcağız, Çaylıca, and Tekir) exhibited a loamy texture. This predominance of clay loam soils suggests a high capacity for nutrient and water retention, which is critical for supporting microbial activity and plant growth in the semi-arid conditions of the Central Anatolian Plateau. The pH values ranged from slightly acidic to moderately alkaline (6.54 to 8.27), with most samples falling within a neutral to slightly alkaline range (7.14–8.13). Notably, the Tekir location exhibited the most acidic pH (6.54), while Çayırözü had the highest pH (8.27), potentially influencing the solubility and availability of nutrients and the activity of rhizobacteria.
Electrical conductivity (EC), an indicator of soil salinity, varied significantly across the sampling sites, ranging from 116.0 µS/cm (Kızılini) to 4760.0 µS/cm (Hürmetçi). The exceptionally high EC at Hürmetçi suggests potential salinity stress, which could impact microbial communities and plant growth. Organic matter (OM) content ranged from 1.85% (Soysallı) to 5.12% (Ahmet Pınarı), indicating moderate to high organic content that supports microbial activity. Total nitrogen (N) content followed a similar trend, ranging from 0.09% (Soysallı) to 0.26% (Ahmet Pınarı), reflecting the variability in soil fertility across the sites.
Lime content, which affects soil structure and nutrient availability, was notably high at Çayırözü (18.42%) and Kulpak (15.25%), potentially influencing pH and microbial processes.
Nutrient analysis revealed variation in phosphorus (P), calcium (Ca), potassium (K), magnesium (Mg), and sodium (Na) concentrations. Phosphorus levels ranged from 1.17 kg/da (Kıranardı) to 17.22 kg/da (Çayırözü), indicating a wide range of phosphate availability that could influence the activity of phosphate-solubilizing rhizobacteria. Calcium concentrations were consistently high, ranging from 739.6 mg/kg (Kıranardı) to 2156.0 mg/kg (Hacılar), reflecting the calcareous nature of the region’s soils. Potassium and magnesium levels showed moderate variation, with K ranging from 120.5 mg/kg (Koçcağız) to 182.5 mg/kg (Kızılini) and Mg from 29.5 mg/kg (Çaylıca) to 107.0 mg/kg (Çayırözü). Sodium levels were notably elevated at Hürmetçi (4565.2 mg/kg) and Soysallı (1523.6 mg/kg), further indicating potential salinity challenges at these sites. Trace elements, including copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn), were present in low but variable concentrations, with Zn showing the highest values at Tekir (1.25 mg/kg) and Çayırözü (1.15 mg/kg), which may support specific microbial functions such as siderophore production. Due to the lack of replicate samples per location, one-way ANOVA and Tukey-Kramer HSD tests (p < 0.01) could not detect significant differences among locations for any parameter, as within-group variance was inestimable.
3.2 Bacterial diversity
A total of 165 microbial isolates were collected from 22 distinct soil samples taken from the wild rhizosphere region of Mount Erciyes. Based on colony morphology and Gram staining, the isolates were identified as follows: 50 Azotobacter sp. (30.3%), 42 Azospirillum sp. (25.5%), and 73 Bacillus sp. (44.2%). Genomic DNA was extracted from all bacterial isolates, and PCR analysis was performed to assess the presence and genetic diversity of several plant growth-promoting genes, including nif, ipdC, accd, Acpho, Alpho, phy, and sd. This approach enabled the identification of functional gene variants associated with plant growth promotion across the microbial community. Soil samples collected from Mount Erciyes displayed varying physicochemical characteristics based on location, which likely influenced microbial diversity and genetic functions observed. For example, the Kıranardı and Hisarcık soils, both with clay loam textures and slightly alkaline pH (7.3–7.7), had moderate organic matter (2.45–2.91%) and nitrogen content (0.12–0.15%). These conditions are suitable for nitrogen-fixing bacteria like Azotobacter and Azospirillum, which were prevalent in these regions due to the availability of nutrients and optimal pH levels.
Samples from Koçcağız and Çaylıca, featuring loamy soils with higher organic matter (3.12% and 2.75%, respectively), provided a nutrient-rich environment, which likely supports a diverse microbial community including Bacillus species. The variability in soil texture, organic matter, and pH across these sampling sites underscores how soil type influences the presence and diversity of plant growth-promoting genes, including those involved in nitrogen fixation and phosphate solubilization. Such diversity in microbial genes highlights the adaptive mechanisms and potential for these soils to support sustainable agriculture in response to nutrient demands.
3.3 Molecular screening of plant growth-promoting genes
3.3.1 Nitrogen fixation genes
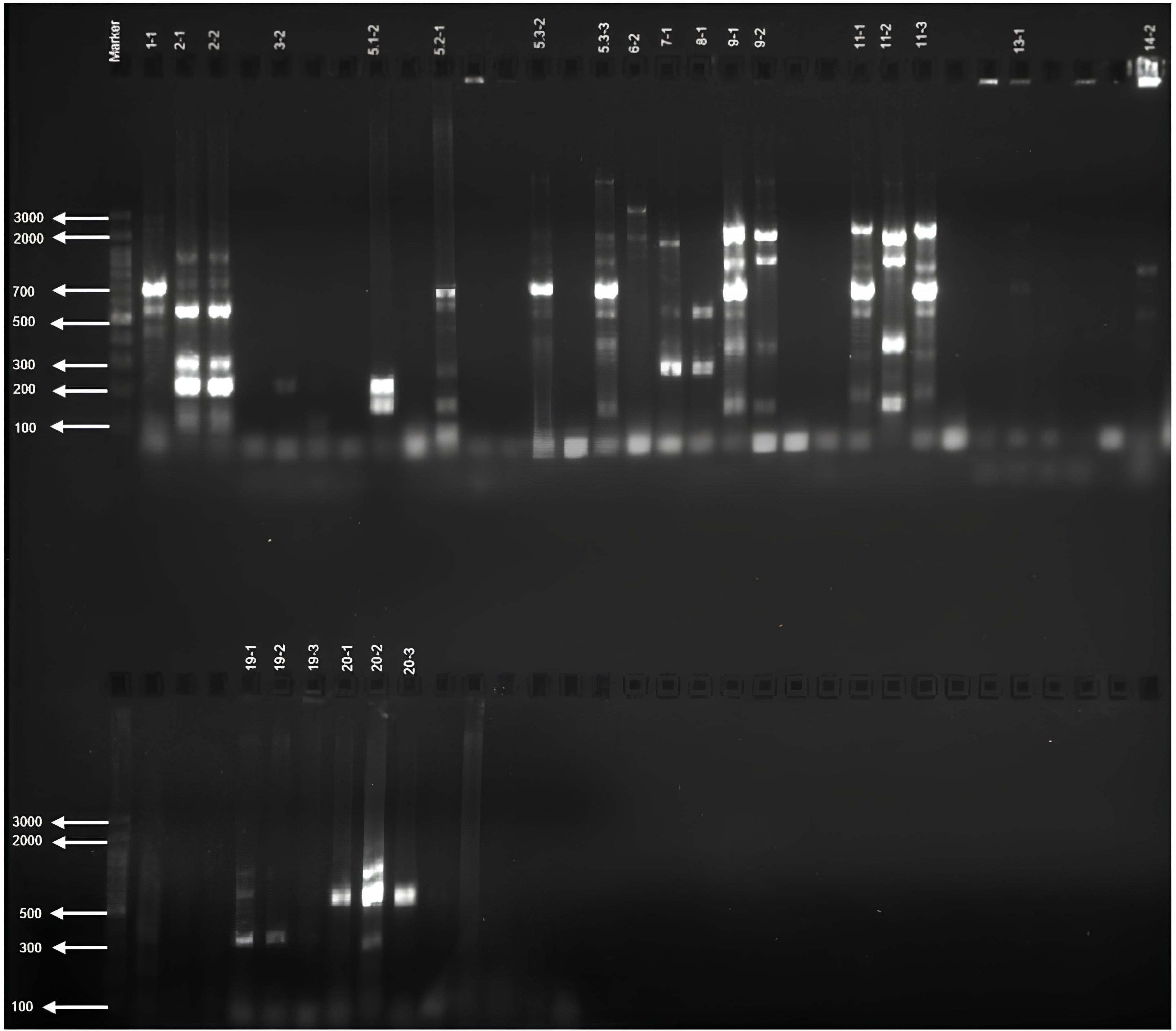
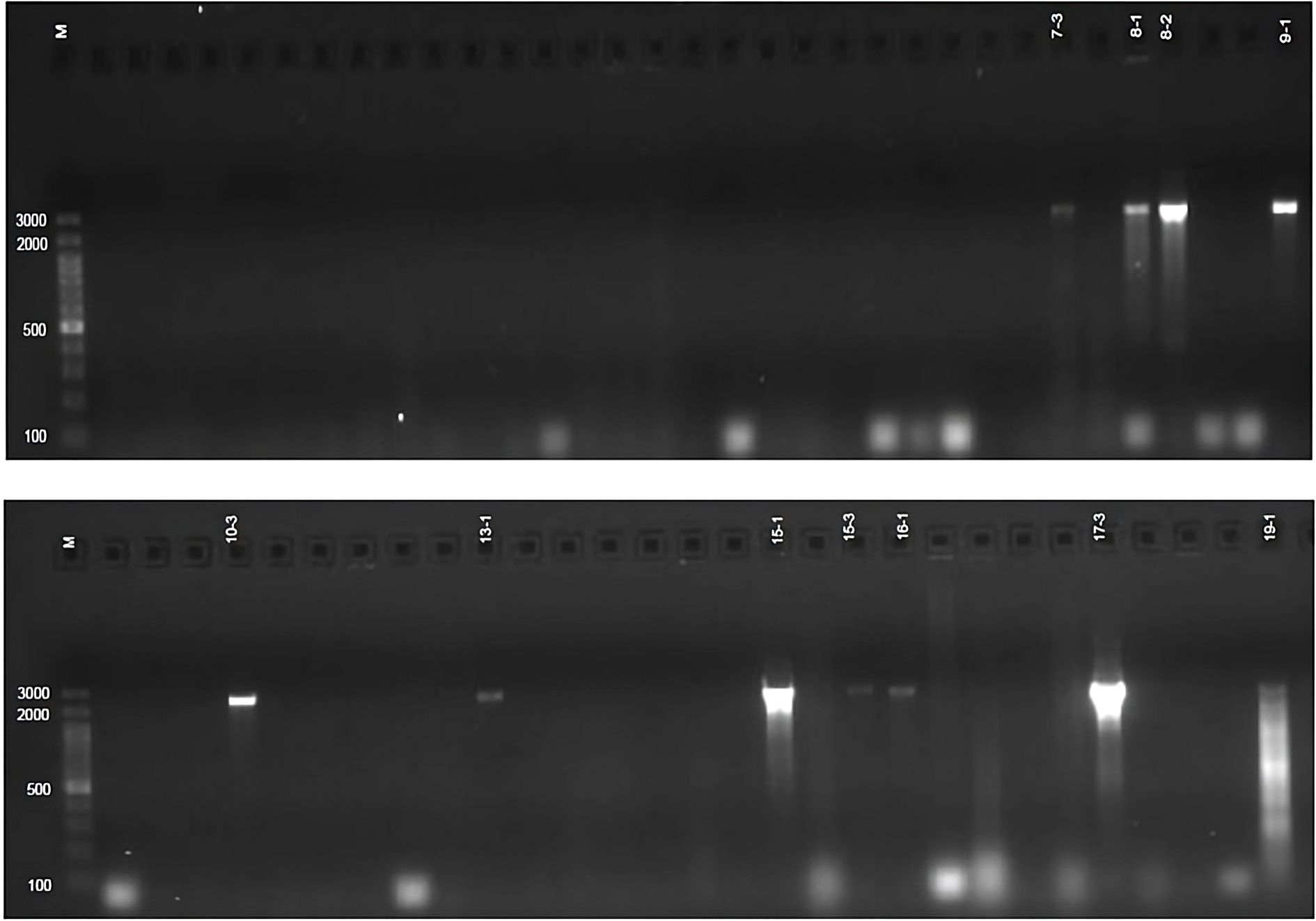
Screening of 50 Azotobacter sp. isolates for nifH genes using various primers revealed diverse amplification patterns. The nifH-1 primer was expected to produce a 1102 bp band (Figure 1); however, 24 isolates exhibited bands of varying haplotypes with sizes deviating from this expectation (Table 4).
Screening with the nifH-4 primer resulted in eight isolates producing the expected 323 bp band, while the nifH-5 primer yielded the anticipated 323 bp band in four isolates. The nifH-6 primer generated a 360 bp band in six isolates, consistent with expectations. In contrast, the nifH-7 primer produced gene patterns with a range of haplotypes significantly different from the expected 205 bp size. These findings indicate considerable genetic diversity in nifH genes among Azotobacter sp. isolates from different regions (Supplementary Figure 1).
Screening of 42 Azospirillum sp. isolates with nifH primers also showed varied results. The nifH-1 primer was expected to amplify a 1102 bp band, but 23 isolates displayed bands of different haplotypes with sizes deviating from this standard. The nifH-2 primer produced the expected 246 bp band in 18 isolates, while the nifH-3 primer yielded a 346 bp band in 10 isolates. For the nifH-4 primer, four isolates generated the expected 323 bp band, and the nifH-5 primer produced the anticipated 323 bp band in two isolates. The nifH-7 primer resulted in three isolates showing the expected 205 bp band. Additionally, screening with the nifH-8 primer revealed gene patterns with diverse haplotypes (Supplementary Figures 2A, B).
For 73 Bacillus sp. isolates, screening with nifH primers identified 37 isolates producing bands with various primers. The nifH-1 primer was expected to yield a 1102 bp band, and three isolates met this expectation. The nifH-2 primer produced the anticipated 246 bp band in 16 isolates, while the nifH-3 primer resulted in two isolates showing the expected 346 bp band. Screening with the nifH-5 primer revealed three isolates with bands deviating from the expected 323 bp size, though two isolates produced the anticipated 323 bp band. The nifH-8 primer identified two isolates with the expected 205 bp band. Finally, screening with the nifH-9 primer indicated gene patterns with diverse haplotypes (Supplementary Figure 3).
3.3.2 Indole pyruvate decarboxylase genes
Screening of 50 Azotobacter sp. isolates with nine ipdC primers yielded no amplification products, indicating the absence of detectable ipdC genes in these isolates. In contrast, screening of 42 Azospirillum sp. isolates revealed that 21 isolates produced bands of varying sizes with the ipdC-6 primer (Supplementary Figure 4), suggesting the presence of diverse ipdC gene haplotypes. Among 73 Bacillus sp. isolates, 18 were found to carry the ipdC gene. However, all positive isolates displayed band sizes approximately 500 bp larger than the expected 200 bp, indicating significant genetic variation from the anticipated profile (Table 4, Supplementary Figure 5).
3.3.3 1-aminocyclopropane-1-carboxylate deaminase genes
Screening of 50 Azotobacter sp. isolates for the accd gene revealed that 39 isolates produced bands of approximately 800 bp, consistent with the expected size (Supplementary Figure 6). Similarly, among 42 Azospirillum sp. isolates, 11 displayed bands of the anticipated size for the accd gene (Supplementary Figure 7). For Bacillus sp., 29 out of 73 isolates were found to carry the accd gene, all producing bands of the expected size (Table 4, Supplementary Figure 8).
3.3.4 Phosphate-solubilizing genes (acpho, alpho and phy)
Screening with the Acpho-1 primer identified 11 Azotobacter sp., 8 Azospirillum sp., and 4 Bacillus sp. isolates that produced a band of the expected 734 bp size, indicating the presence of the acid phosphatase gene (Supplementary Figures 9-11). In contrast, PCR amplification using Alpho and phy primers yielded no bands for any of the tested bacterial isolates. However, some Bacillus sp. isolates produced non-specific bands (Table 4), which do not confirm the presence of these genes due to their lack of specificity.
3.3.5 Siderophore biosynthesis genes
PCR screening for siderophore biosynthesis (sd) genes using sd-2 and sd-3 primers revealed that none of the 50 Azotobacter sp. isolates carried these genes. In contrast, 11 Azospirillum sp. isolates and 7 Bacillus sp. isolates produced the expected 1685 bp band, confirming the presence of sd genes (Figure 2). Additionally, some of these isolates exhibited extra bands of significantly larger sizes (Table 4, Supplementary Figures 12, 13), indicating potential genetic diversity in siderophore biosynthesis pathways.
4 Discussion
Nitrogen (N2), a critical component of nucleotides, proteins, and chlorophyll in plants, is one of the most essential elements for life. Despite constituting approximately 78% of the atmosphere, nitrogen is not readily available in a form usable by plants (Galloway et al., 2003). Soil is prone to nitrogen loss through processes such as denitrification, volatilization, and leaching due to nitrogen’s high reactivity and mobility. The leached reactive forms can cause environmental issues and adverse health effects (Williamson, 2011). Nitrogen fixation, the process of converting atmospheric N2 into ammonia (NH3), varies widely among bacteria and is catalyzed by the nitrogenase enzyme complex, which consists of two main components: an iron (Fe) protein encoded by the nifH gene and a molybdenum-iron (MoFe) protein encoded by the nifDK genes. The nifH gene is evolutionarily conserved and serves as a marker for nitrogen fixation, with primers designed from its sequence widely used to detect the genetic potential for nitrogen fixation in bacteria. While nif genes are sometimes located on chromosomes, they are often found on plasmids alongside other nitrogen-fixing genes, such as nod genes (Auman and Speake, 2001; Mehta et al., 2003; Rosch et al., 2002). In addition to encoding nitrogenase, nif genes regulate other enzymes involved in nitrogen fixation. Their expression is triggered by low nitrogen and oxygen levels, particularly around the root zone. Although the nif genes encoding nitrogenase are highly conserved across nitrogen-fixing bacteria, their regulation varies among diazotrophs depending on their evolutionary lineage. Approximately 20 nif genes encode the nitrogenase complex.
Transcription of nif genes is initiated under nitrogen stress, often activated by the nitrogen-sensitive nifA protein. When fixed nitrogen is scarce, nifC, an RNA polymerase, induces nifA expression, which in turn activates other transcription factors. In environments with sufficient reduced nitrogen or oxygen, the nifL protein suppresses nifA, halting nitrogenase production. Studies have identified nitrogenase activity in various Bacillus species isolated from rhizosphere soils, including B. megaterium, B. cereus, B. pumilus, B. circulans, B. licheniformis, B. subtilis, B. brevis, and B. firmus (Ahmad et al., 2008; Beneduzi and Passaglia, 2011; Ding et al., 2005; Sorokin et al., 2008; Xie et al., 1998). For instance, Xie et al. (1998) isolated 14 Bacillus strains capable of acetylene reduction from rice fields along the Yangtze River in China. Ahmad et al. (2008) reported high nitrogenase activity in B. fusiformis from Chungbuk Province, South Korea, while Sorokin et al. (2008) highlighted the nitrogenase activity of B. alkalidiazotrophicus, a low salt-tolerant alkaliphile from Mongolian soda soils, demonstrating the genetic diversity of nitrogenase activity in Bacillus species across different ecosystems. This nitrogen fixation capacity directly contributes to plant growth promotion by increasing the availability of nitrogen, a limiting nutrient for plant development. For example, nitrogen-fixing Bacillus species have been shown to enhance the growth of crops such as rice and wheat by improving nitrogen uptake, leading to increased biomass and grain yield (Kuan et al., 2016).
Phytohormones are vital for signaling and regulating plant growth and development. Auxins, particularly indole-3-acetic acid (IAA), are among the most studied plant growth regulators (Del Pozo et al., 2005; Figueredo et al., 2023). Bacteria associated with plants produce phytohormones that influence plant growth (Patten and Glick, 2002), plant pathology (Glickmann et al., 1998), and microbial interactions (Sergeeva et al., 2002). Tryptophan serves as a precursor for IAA biosynthesis (Kundan et al., 2015). Over 80% of rhizosphere bacteria can produce IAA, which enhances root branching, weight, size, and surface area, improving nutrient exchange in plants (Kashyap et al., 2019). Bacillus species possess the ipdC gene, which encodes indole pyruvate decarboxylase, a key enzyme in IAA production via the indole-3-pyruvate (IPyA) pathway (Raddadi et al., 2008). The production of IAA by Bacillus species promotes root system development, which enhances nutrient and water uptake, leading to improved plant vigor and yield. For instance, Bacillus subtilis inoculation has been shown to increase root biomass and grain yield in maize by up to 15% under field conditions, demonstrating its potential for agricultural applications (Kuan et al., 2016).
Azospirillum species, widely distributed in tropical, subtropical, and temperate soils, produce phytohormones such as IAA, gibberellins, cytokinins, and abscisic acid, promoting plant growth (Bashan et al., 2014; Creus et al., 2004). Of the 18 identified Azospirillum species (Baldani et al., 2014), few are known to enhance plant growth, with A. brasilense being the most commonly used to improve crop yields under field conditions. A. brasilense synthesizes IAA from tryptophan through three pathways: indole pyruvic acid (IPyA), tryptamine, and indole acetonitrile (Carreño-López et al., 2000; Spaepen et al., 2007a, 2007b). The IPyA pathway, involving transamination of tryptophan to IPyA by aromatic amino acid aminotransferases, followed by decarboxylation to indole acetaldehyde and oxidation to IAA, is the best-characterized. The ipdC gene, encoding phenylpyruvate decarboxylase, is critical in this pathway, and its presence has been confirmed in many Azospirillum strains (Jijón-Moreno et al., 2015). The application of A. brasilense has been associated with significant increases in crop productivity, including a 20–30% yield improvement in wheat and maize under field conditions, primarily due to enhanced root development and nutrient assimilation (Bashan et al., 2014).
1-Aminocyclopropane-1-carboxylic acid (ACC), a natural ethylene precursor, regulates seed germination, senescence, fruit ripening, wound healing, and plant development (Deikman, 1997). ACC deaminase, found in plant growth-promoting bacteria, converts ACC into α-ketobutyrate and ammonium, promoting root elongation during seed germination (Wenbo et al., 2003). Bacillus species with the accd gene enhance root-shoot development and increase dry and wet weights in plants (Ghosh et al., 2003; Raddadi et al., 2008). By lowering ethylene levels, ACC deaminase-producing bacteria mitigate stress responses in plants, leading to improved growth under adverse conditions such as drought or salinity. For example, Bacillus amyloliquefaciens has been shown to increase tomato plant biomass by 25% under salt stress, highlighting its role in enhancing plant resilience and productivity (Raddadi et al., 2008).
Although phosphorus (P) is abundant in soil, its insoluble form limits plant uptake, making it a critical constraint for growth. Soil phosphorus exists as mineral phosphates (e.g., calcium phosphates, hydroxyapatite, and rock phosphate) and organic phosphates (e.g., phosphoesters, phytates, or inositol phosphates), but plants can only absorb it as soluble monobasic or dibasic ions (Glass, 1989). Phosphate-solubilizing bacteria produce enzymes that convert insoluble phosphates into soluble forms (Nautiyal, 1999). The primary mechanism involves organic acid production, which acidifies the microbial environment, displacing Ca²+ ions to release ionic phosphate. Bacillus species are key phosphate-solubilizing bacteria, utilizing enzymes like acid phosphatases (Acpho), alkaline phosphatases (Alpho), and phytases (phy) for dephosphorylation (Abdallah et al., 2018; Fitriatin et al., 2014; Raddadi et al., 2008; Rodríguez and Fraga, 1999). Phosphatase genes in B. cereus and B. thuringiensis show 99% homology with those of B. cereus ATCC 14579T (Raddadi et al., 2008). Alkaline phosphatases, encoded by phoA, phoD, and phoX gene families, and phytases, with varying catalytic mechanisms, exhibit high microbial diversity (Lim et al., 2007; Mullaney and Ullah, 2003; Ragot et al., 2015; Zimmerman et al., 2013). In marine systems, phoD and phoX predominate over phoA, while phoD is the most abundant alkaline phosphatase gene in terrestrial soils, correlating with potential phosphatase activity (Fraser et al., 2015; Luo et al., 2009; Neal et al., 2018; Sebastian and Ammerman, 2009). Phosphate solubilization by Bacillus species enhances phosphorus availability, leading to improved plant growth and yield. For instance, B. megaterium has been reported to increase phosphorus uptake in soybean, resulting in a 10–15% increase in seed yield under greenhouse conditions (Fitriatin et al., 2014).
Iron (Fe) deficiency, an abiotic stress, can reduce crop yields (Kobayashi et al., 2005). Plants release chelators and phytosiderophores to bind Fe³+, enhancing its solubility and reducing it to Fe²+ for uptake. Rhizospheric bacteria, including Bacillus species like B. cereus, B. anthracis, and B. thuringiensis, produce siderophores via non-ribosomal peptide synthesis (Cendrowski et al., 2004; Chaabouni et al., 2012; Raddadi et al., 2008; Wilson et al., 2006). These bacteria carry siderophore biosynthesis genes (sd) and produce siderophores like bacillibactin (a catecholate-type siderophore) in Bacillus and azotobactin in A. vinelandii (Raddadi et al., 2008). Plants in non-sterile soils show no iron deficiency symptoms, indicating the role of microbial siderophores in enhancing iron uptake (Masalha et al., 2000). Siderophores also combat plant pathogens by competing for iron, thus supporting plant growth (Carrillo-Castaneda et al., 2005; Chaabouni et al., 2012; Vessey, 2003). The application of siderophore-producing Bacillus species has been shown to improve iron nutrition in crops like maize, leading to a 10% increase in chlorophyll content and a 12% increase in biomass under iron-deficient conditions (Chaabouni et al., 2012).
In summary, the multifaceted contributions of nitrogen-fixing, phytohormone-producing, ACC deaminase-active, phosphate-solubilizing, and siderophore-producing bacteria, particularly Bacillus and Azospirillum species, significantly enhance plant growth and productivity. These mechanisms collectively improve nutrient availability, stimulate root development, mitigate stress, and enhance resistance to pathogens, leading to increased crop yields and sustainable agricultural practices. For example, field studies have demonstrated that inoculation with Bacillus and Azospirillum strains can increase crop yields by 10–30% across various crops, including maize, wheat, and soybean, under diverse environmental conditions (Bashan et al., 2014; Kuan et al., 2016). These findings underscore the potential of these bacteria as biofertilizers to enhance plant production, offering a sustainable alternative to chemical fertilizers while addressing global food security challenges.
5 Conclusion
Plant growth-promoting rhizobacteria (PGPR) exhibit significant genetic diversity in key genes, including those for nitrogen fixation (nif), indole pyruvate decarboxylase (ipdC), 1-aminocyclopropane-1-carboxylate deaminase (accd), phosphate solubilization (Acpho, Alpho, phy), and siderophore biosynthesis (sd). These genes enable PGPR to fix atmospheric nitrogen, synthesize phytohormones, and enhance the availability of phosphate and iron, thereby promoting plant growth and resilience under diverse environmental conditions. This study investigates the genetic diversity of PGPR traits in bacterial isolates from Mount Erciyes, Türkiye, revealing the influence of local soil properties:
Hisarcık: Nutrient-rich, neutral to slightly alkaline soils harbor diverse nif genes, particularly in Azotobacter species, supporting robust nitrogen fixation in fertile environments.
Kıranardı: Soils with moderate pH and balanced micronutrients foster diversity in accd and phosphate-solubilizing genes, enhancing plant stress tolerance and nutrient uptake.
Kepez: Iron-deficient soils at higher altitudes show increased diversity in sd genes among Bacillus isolates, improving iron acquisition for both plants and microbes.
Endürlük: Acidic soils with variable mineral content promote diversity in phosphate-solubilizing genes, reflecting microbial adaptation to phosphorus scarcity.
The variability in PGPR traits closely corresponds to soil composition and altitude, highlighting the adaptability of rhizobacteria to specific ecological niches. These insights emphasize the potential of tailored microbial consortia to support sustainable agriculture.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
UA: Writing – original draft, Methodology, Formal Analysis, Funding acquisition, Supervision, Conceptualization, Writing – review & editing, Project administration, Data curation, Investigation, Validation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by Scientific and Technological Research Council of Türkiye (TÜBİTAK) under the Grant Number 123O376 and Kayseri University Scientific Project Unit the Grant Number FOA-2023-1115. The authors thank to TÜBİTAK and Kayseri University Scientific Project Unit for their supports.
Acknowledgments
The author thanks Dr. Adem Güneş for his valuable assistance about soil analysis and Dr. Salih Karabörklü for statistical data analysis.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1657785/full#supplementary-material
References
Abdallah, D. B., Frikha-Gargouri, O., and Tounsi, S. (2018). Rhizospheric competence, plant growth promotion and biocontrol efficacy of Bacillus amyloliquefaciens subsp. plantarum strain 32a. Biol. Control. 124. doi: 10.1016/j.biocontrol.2018.01.013
Ahmad, F., Ahmad, I., and Khan, M. S. (2008). Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 163, 173–181. doi: 10.1016/j.micres.2006.04.001
Ahmed, A. S., Khider, A. K., and Muniandy, S. (2017). Transfer of nif genes from nitrogen fixer Azotobacter chroococcum to phosphorus solubilizing Bacillus megaterium var. phosphaticum by conjugation. Zanco J. Pure Appl. Sci. 29, 97–111.
Auman, N. M. and Speake, C. C. (2001). nifH sequences and nitrogen fixation in type I and type II methanotrophs. Appl. Environ. Microbiol. 67, 4009–4016. doi: 10.1128/AEM.67.9.4009
Bakulin, M. K., Grudtsyna, A. S., and Pletneva, A. Y. (2007). Biological fixation of nitrogen and growth of bacteria of the genus Azotobacter in liquid media in the presence of perfluorocarbons. Appl. Biochem. Microbiol. 43, 399–402. doi: 10.1134/S0003683807040072
Baldani, J. I. and Reis, V. M. (2014). The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil 384, 413–431. doi: 10.1007/s11104-014-2186-6
Baldani, I., Sampaio, S., Massena, V., Andre, V. R., and Ka, V. (2014). “The family Rhodospirillaceae,” in The Prokaryotes: Alphaproteobacteria and Betaproteobacteria. Ed. Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., and Thompson, F. (Springer, Berlin), 533–618. doi: 10.1007/978-3-642-30197-1
Barış, Ö., Şahin, F., Turan, M., Orhan, F., and Gulluce, M. (2014). Use of plant−growth−promoting rhizobacteria (PGPR) seed inoculation as alternative fertilizer inputs in wheat and barley production. Commun. Soil Sci. Plant Anal. 45, 2457–2467. doi: 10.1080/00103624.2014.912296
Bashan, Y., de-Bashan, L., Prabhu, S. R., and Hernandez, J. P. (2014). Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives. Plant Soil 378, 1–33. doi: 10.1007/s11104-013-1956-x
Bauder, J. W. (2000). “Particle-size analysis,” in Methods of Soil Analysis: Part 3—Chemical Methods (Soil Science Society of America, Madison), 255–293.
Bawane, R., Tantwai, K., Rajput, L. P. S., Kadam-Bedekar, M., Kumar, S., Gontia, I., et al. (2011). Molecular analysis of phytase gene cloned from Bacillus subtilis. Adv. Stud. Biol. 3, 103–110.
Beneduzi, A. and Passaglia, L. M. P. (2011). “Genetic and phenotypic diversity of plant growth promoting bacilli,” in Bacteria in Agrobiology: Plant Growth Responses. Ed. Maheshwari, D. K. (Springer, Berlin), 1–27.
Çakmakçı, R., Dönmez, M. F., and Erdoğan, Ü. (2017). The effect of plant growth promoting rhizobacteria on barley seedling growth, nutrient uptake, some soil properties, and bacterial counts. Turk. J. Agric. For. 41, 189–199. doi: 10.3906/tar-1610-29
Carreño-López, R., Campos-Reales, N., Elmerich, C., and Baca, B. (2000). Physiological evidence for differently regulated tryptophan-dependent pathways for indole-3-acetic acid synthesis in Azospirillum brasilense Sp7. Mol. Gen. Genet. 264, 521–530. doi: 10.1007/s004380000340
Carrillo-Castaneda, G., Muñoz, J. J., and Peralta-Videa, J. R. (2005). A spectrophotometric method to determine the siderophore production by strains of fluorescent Pseudomonas in the presence of copper and iron. Microchem. J. 81, 35–40. doi: 10.1016/j.microc.2005.01.018
Cendrowski, S., MacArthur, W., and Hanna, P. (2004). Bacillus anthracis requires siderophore biosynthesis for growth in macrophages and mouse virulence. Mol. Microbiol. 51, 407–417. doi: 10.1046/j.1365-2958.2003.03861.x
Chaabouni, I., Guesmi, A., and Cherif, A. (2012). “Secondary metabolites of Bacillus: potentials in biotechnology,” in Bacteria in Agrobiology: Plant Growth Responses. Ed. Maheshwari, D. K. (Springer, Berlin), 347–366. doi: 10.1007/978-94-007-3021-2_16
Creus, C. M., Sueldo, R. J., and Barassi, C. A. (2004). Water relations and yield in Azospirillum-inoculated wheat exposed to drought in the field. Can. J. Microbiol. 50, 273–281. doi: 10.1139/B03-119
Deikman, J. (1997). Molecular mechanisms of ethylene regulation of gene transcription. Physiol. Plant 100, 561–566. doi: 10.1111/j.1399-3054.1997.tb03061.x
Del Pozo, J. C., Lopez-Matas, M. A., Ramirez-Parra, E., and Gutierrez, C. (2005). Hormonal control of the plant cell cycle. Physiol. Plant 123, 173–183. doi: 10.1111/j.1399-3054.2004.00420.x
Ding, Y., Wang, J., Liu, Y., and Chen, S. (2005). Isolation and identification of nitrogen-fixing bacilli from plant rhizospheres in Beijing region. J. Appl. Microbiol. 99, 1271–1281. doi: 10.1111/j.1365-2672.2005.02738.x
Egamberdiyeva, D. (2005). Plant-growth-promoting rhizobacteria isolated from a Calcisol in a semi-arid region of Uzbekistan: biochemical characterization and effectiveness. Z. Pflanzenernähr. Bodenk. 168, 94–99. doi: 10.1002/jpln.200321283
Erturk, Y., Ercisli, S., and Cakmakci, R. (2012). Yield and growth response of chickpea to co-inoculation with Rhizobium and plant growth promoting rhizobacteria under different irrigation levels. J. Anim. Plant Sci. 22, 822–828.
Figueredo, E. F., Cruz, T. A., Almeida, J. R., Batista, B. D., Marcon, J., Andrade, P. A., et al. (2023). The key role of indole-3-acetic acid biosynthesis by Bacillus thuringiensis RZ2MS9 in promoting maize growth revealed by the ipdC gene knockout mediated by the CRISPR-Cas9 system. Microbiol. Res. 266, 127218. doi: 10.1016/j.micres.2022.127218
Fitriatin, B. N., Yuniarti, A., Turmuktini, T., and Kennedy, I. R. (2014). The effect of phosphate solubilizing microbe producing growth regulators on soil phosphate, growth and yield of maize and fertilizer efficiency on Ultisol. Eurasian J. Soil Sci. 3, 101–107. doi: 10.18393/ejss.34313
Fraser, T. D., Lynch, D. H., Entz, M. H., and Dunfield, K. E. (2015). Linking alkaline phosphatase activity with bacterial phoD gene abundance in soil from a long-term management trial. Geoderma 257–258, 115–122. doi: 10.1016/j.geoderma.2014.10.016
Gaiero, J. R., Bent, E., and Fraser, T. D. (2018). Validating novel oligonucleotide primers targeting three classes of bacterial non-specific acid phosphatase genes in grassland soils. Plant Soil 427, 39–51. doi: 10.1007/s11104-018-3660-2
Galloway, J. N., Aber, J. D., Erisman, J. W., Seitzinger, S. P., Howarth, R. W., Cowling, E. B., et al. (2003). The nitrogen cascade. Bioscience 53, 341–356. doi: 10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2
Ghosh, S., Penterman, J. N., Little, R. D., Chavez, R., and Glick, B. R. (2003). Three newly isolated plant growth-promoting bacilli facilitate the seedling growth of canola, Brassica campestris. Plant Physiol. Biochem. 41, 277–281. doi: 10.1016/S0981-9428(03)00019-6
Glass, A. D. M. (1989). Plant nutrition: An introduction to current concepts (Boston: Jones and Bartlett Publishers).
Glickmann, E., Gardan, L., Jacquet, S., Hussain, S., Elasri, M., and Petit, A. (1998). Auxin production is a common feature of most pathovars of Pseudomonas syringae. Mol. Plant-Microbe Interact. 11, 156–162. doi: 10.1094/MPMI.1998.11.2.156
Helmut, B. F., Widmer, W., Sigler, V., and Zeyer, J. (2004). New molecular screening tools for analysis of free-living diazotrophs in soil. Appl. Environ. Microbiol. 70, 240–247. doi: 10.1128/AEM.70.1.240-247.2004
Jijón-Moreno, S., Marcos-Jiménez, C., and Pedraza, R. O. (2015). The ipdC, hisC1 and hisC2 genes involved in indole-3-acetic production used as alternative phylogenetic markers in Azospirillum brasilense. Antonie Van Leeuwenhoek 107, 1501–1517. doi: 10.1007/s10482-015-0444-0
Kashyap, B. K., Solanki, M. K., and Pandey, A. K. (2019). “Bacillus as plant growth promoting rhizobacteria (PGPR): A promising green agriculture technology,” in Plant health under biotic stress. Eds. Ansari, R. and Mahmood, I. (Springer, Singapore), 219–236. doi: 10.1007/978-981-13-6040-4_10
Katı, H., Karaca, B., and Gülşen, Ş.H. (2016). Identification of Bacillus species isolated from soil and investigation of their biological properties. Sakarya Univ. J. Sci. 20, 281–290.
Kirillov, S. O., Khassenov, B. B., and Silayev, D. V. (2017). Biochemical properties of recombinant alkaline phosphatase from Bacillus licheniformis T5. Eurasian J. Appl. Biotechnol. 4, 4. doi: 10.11134/btp.4.2017.4
Kobayashi, T., Suzuki, M., Inoue, H., Itai, R. N., and Takahashi, M. (2005). Expression of iron-acquisition-related genes in iron-deficient rice is co-ordinately induced by partially conserved iron-deficiency-responsive elements. J. Exp. Bot. 56, 1305–1316. doi: 10.1093/jxb/eri131
Kuan, K. B., Othman, R., Abdul Rahim, K., and Shamsuddin, Z. H. (2016). Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation, and nitrogen remobilization of maize under greenhouse conditions. PloS One 11, e0152478. doi: 10.1371/journal.pone.0152478
Kundan, R., Pant, G., Jadon, N., and Agrawal, P. K. (2015). Plant growth promoting rhizobacteria: Mechanism and current prospective. J. Fertil. Pestic. 6, 1–7. doi: 10.4172/2471-2728.1000155
Lim, B. L., Yeung, P., Cheng, C., and Hill, J. E. (2007). Distribution and diversity of phytate-mineralizing bacteria. ISME J. 1, 321–330. doi: 10.1038/ismej.2007.40
Lindsay, W. L. and Norvell, W. A. (1978). Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc Am. J. 42, 421–428. doi: 10.2136/sssaj1978.03615995004200030009x
Luo, H., Benner, R., Long, R. A., and Hu, J. (2009). Subcellular localization of marine bacterial alkaline phosphatases. Proc. Natl. Acad. Sci. U.S.A. 106, 21219–21223. doi: 10.1073/pnas.0907586106
Masalha, J., Kosegarten, H., Elmaci, Ö., and Mengel, K. (2000). The central role of microbial activity for iron acquisition in maize and sunflower. Biol. Fertil. Soils 30, 433–439. doi: 10.1007/s003740050021
Mclean, E. O. (1982). “Soil pH and lime requirement”, in Methods of Soil Analysis: Part 2—Chemical and Microbiological Properties. Ed. Page, A. L., Miller, R. H., and Keeney, D. R. (American Society of Agronomy, Madison), 199–224.
Mehta, M. P., Butterfield, D. A., and Baross, J. A. (2003). Phylogenetic diversity of nitrogenase (nifH) genes in deep-sea and hydrothermal vent environments of the Juan de Fuca Ridge. Appl. Environ. Microbiol. 69, 960–970. doi: 10.1128/AEM.69.2.960
Meng, D., Jiang, W., Li, J., Huang, L., Zhai, L., Zhang, L., et al. (2019). An alkaline phosphatase from Bacillus amyloliquefaciens YP6 of new application in biodegradation of five broad-spectrum organophosphorus pesticides. J. Environ. Sci. Health 54, 336–343. doi: 10.1080/03601234.2019.1571363
Mullaney, E. J. and Ullah, A. H. J. (2003). The term phytase comprises several different classes of enzymes. Biochem. Biophys. Res. Commun. 312, 179–184. doi: 10.1016/j.bbrc.2003.09.176
Nautiyal, C. S. (1999). An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 170, 265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x
Neal, A. L., Blackwell, M., Akkari, E., Guyomar, C., and Hirsch, P. R. (2018). Phylogenetic distribution, biogeography and the effects of land management upon bacterial non-specific acid phosphatase gene diversity and abundance. Plant Soil 427, 175–189. doi: 10.1007/s11104-017-3301-2
Olsen, S. R. and Sommers, L. E. (1982). “Phosphorus,” in Methods of Soil Analysis: Part 2—Chemical and Microbiological Properties. Ed. Page, A. L., Miller, R. H., and Keeney, D. R. (American Society of Agronomy, Madison), 403–430.
Patten, C. L. and Glick, B. R. (2002). Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 68, 3795–3801. doi: 10.1128/AEM.68.8.3795
Raddadi, N., Cherif, A., Boudabous, A., and Daffonchio, D. (2008). Screening of plant growth promoting traits of Bacillus thuringiensis. Ann. Microbiol. 58, 47–52. doi: 10.1007/BF03179444
Ragot, S. A., Kertesz, M. A., and Bünemann, E. K. (2015). phoD alkaline phosphatase gene diversity in soil. Appl. Environ. Microbiol. 81, 7281–7289. doi: 10.1128/AEM.01823-15
Rhoades, J. D. (1982). “Cation exchange capacity,” in Methods of Soil Analysis: Part 2—Chemical and Microbiological Properties. Ed. Page, A. L., Miller, R. H., and Keeney, D. R. (American Society of Agronomy, Madison), 149–157.
Rodríguez, H. and Fraga, R. (1999). Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 17, 319–339. doi: 10.1016/S0734-9750(99)00014-2
Rosch, C., Mergel, A., and Bothe, H. (2002). Biodiversity of denitrifying and dinitrogen-fixing bacteria in an acid forest soil. Appl. Environ. Microbiol. 68, 3818–3829. doi: 10.1128/AEM.68.8.3818
Sakurai, M., Wasaki, J., Tomizawa, Y., Shinano, T., and Osaki, M. (2008). Analysis of bacterial communities on alkaline phosphatase genes in soil supplied with organic matter. Soil Sci. Plant Nutr. 54, 62–71. doi: 10.1111/j.1747-0765.2007.00210.x
Sebastian, M. and Ammerman, J. W. (2009). The alkaline phosphatase PhoX is more widely distributed in marine bacteria than the classical PhoA. ISME J. 3, 563–572. doi: 10.1038/ismej.2009.10
Sergeeva, E., Liaimer, A., and Bergman, B. (2002). Evidence for production of the phytohormone indole-3-acetic acid by cyanobacteria. Planta 215, 229–238. doi: 10.1007/s00425-002-0749-x
Shime-Hattori, A., Kobayashi, S., Ikeda, S., Asano, R., Shime, H., and Shinano, T. (2011). A rapid and simple PCR method for identifying isolates of the genus Azospirillum within populations of rhizosphere bacteria. J. Appl. Microbiol. 111, 915–924. doi: 10.1111/j.1365-2672.2011.05115.x
Sorokin, I. D., Kravchenko, I. K., Tourova, T. P., Kolganova, T. V., Boulygina, E. S., and Sorokin, D. Y. (2008). Bacillus alkalidiazotrophicus sp. nov., a diazotrophic, low salt-tolerant alkaliphile isolated from Mongolian soda soil. Int. J. Syst. Evol. Microbiol. 58, 2459–2464. doi: 10.1099/ijs.0.65655-0
Spaepen, S., Vanderleyden, J., and Remans, R. (2007a). Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 31, 425–448. doi: 10.1111/j.1574-6976.2007.00072.x
Spaepen, S., Verse, W., Pohl, M., Steyaert, J., and Vanderleyden, J. (2007b). Characterization of phenylpyruvate decarboxylase, involved in auxin production of Azospirillum brasilense. J. Bacteriol. 189, 7626–7633. doi: 10.1128/JB.00830-07
Susilowati, D. N. and Setyowati, M. (2016). Analisis aktivitas nitrogenase dan gen nifH isolat bakteri rhizosfer tanaman padi dari lahan sawah pesisir Jawa Barat. Al-Kauniyah 9, 125–138.
Swarnalakshmi, K., Senthilkumar, M., and Ramakrishnan, B. (2020). Role of plant growth-promoting rhizobacteria in sustainable pulses production. Indian. J. Agron. 65, 4–12.
Travers, R. S., Martin, P. A. W., and Reichelderfer, C. F. (1987). Selective process for efficient isolation of soil Bacillus spp. Appl. Environ. Microbiol. 53, 1263–1266. doi: 10.1128/aem.53.6.1263-1266.1987
Vessey, J. K. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255, 571–586. doi: 10.1023/A:1026037216893
Vural, C. and Aytaç, Z. (2005). The flora of erciyes dağı (Kayseri, Turkey). Turk. J. Bot. 29, 185–236.
Walkley, A. and Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
Wenbo, M., Sebastianova, S. B., Sebastian, J., Burd, G. I., and Glick, B. R. (2003). Prevalence of 1-aminocyclopropane-1-carboxylate deaminase in Rhizobium spp. Antonie Van Leeuwenhoek 83, 285–291. doi: 10.1023/A:1023360919140
Williamson, J. M. (2011). The role of information and prices in the nitrogen fertilizer management decision: new evidence from the agricultural resource management survey. J. Agric. Resour. Econ. 36, 552–572.
Wilson, M. K., Abergel, R. J., Raymond, K. N., Arceneaux, J. E. L., and Byers, B. R. (2006). Siderophores of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Biochem. Biophys. Res. Commun. 348, 320–325. doi: 10.1016/j.bbrc.2006.07.055
Xie, G., Su, B., and Cui, Z. (1998). Isolation and identification of N2-fixing strains of Bacillus in rice rhizosphere of the Yangtze River valley. Acta Microbiol. Sin. 38, 125–129.
Zeffa, D. M., Peres, L. E. P., and dos Santos, O. J. A. P. (2020). Meta-analysis of plant growth-promoting rhizobacteria effects on soybean nodulation and yield. Rhizosphere 14, 100203. doi: 10.1016/j.rhisph.2020.100203
Keywords: plant hormones, gene analyses, biodiversity, rhizobacteria, PGPR
Citation: Azizoglu U (2025) Ecological and genetic diversity of plant growth-promoting genes in rhizobacteria isolated from the rhizosphere of wild flora on Mount Erciyes, Türkiye. Front. Plant Sci. 16:1657785. doi: 10.3389/fpls.2025.1657785
Received: 01 July 2025; Accepted: 18 August 2025;
Published: 03 September 2025.
Edited by:
Debasis Mitra, Graphic Era University, IndiaReviewed by:
Chitra Bhattacharya, Atmiya University, IndiaOlivia Landau, USDA-ARS Wheat Health Genetics and Quality Research, United States
Copyright © 2025 Azizoglu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ugur Azizoglu, YXppem9nbHVAa2F5c2VyaS5lZHUudHI=; YXppem9nbHVAZXJjaXllcy5lZHUudHI=; YXppem9nbHV1Z3VyQGhvdG1haWwuY29t
 Ugur Azizoglu
Ugur Azizoglu