- 1Guangxi Key Laboratory of Agro-environment and Agro-products Safety, Guangxi Colleges and Universities Key Laboratory of Crop Cultivation and Tillage, Agricultural College, Guangxi University, Nanning, China
- 2Guangxi Key Laboratory of Forest Ecology and Conservation, State Key Laboratory for Conservation and Utilization of Agro-bioresources, College of Forestry, Guangxi University, Nanning, Guangxi, China
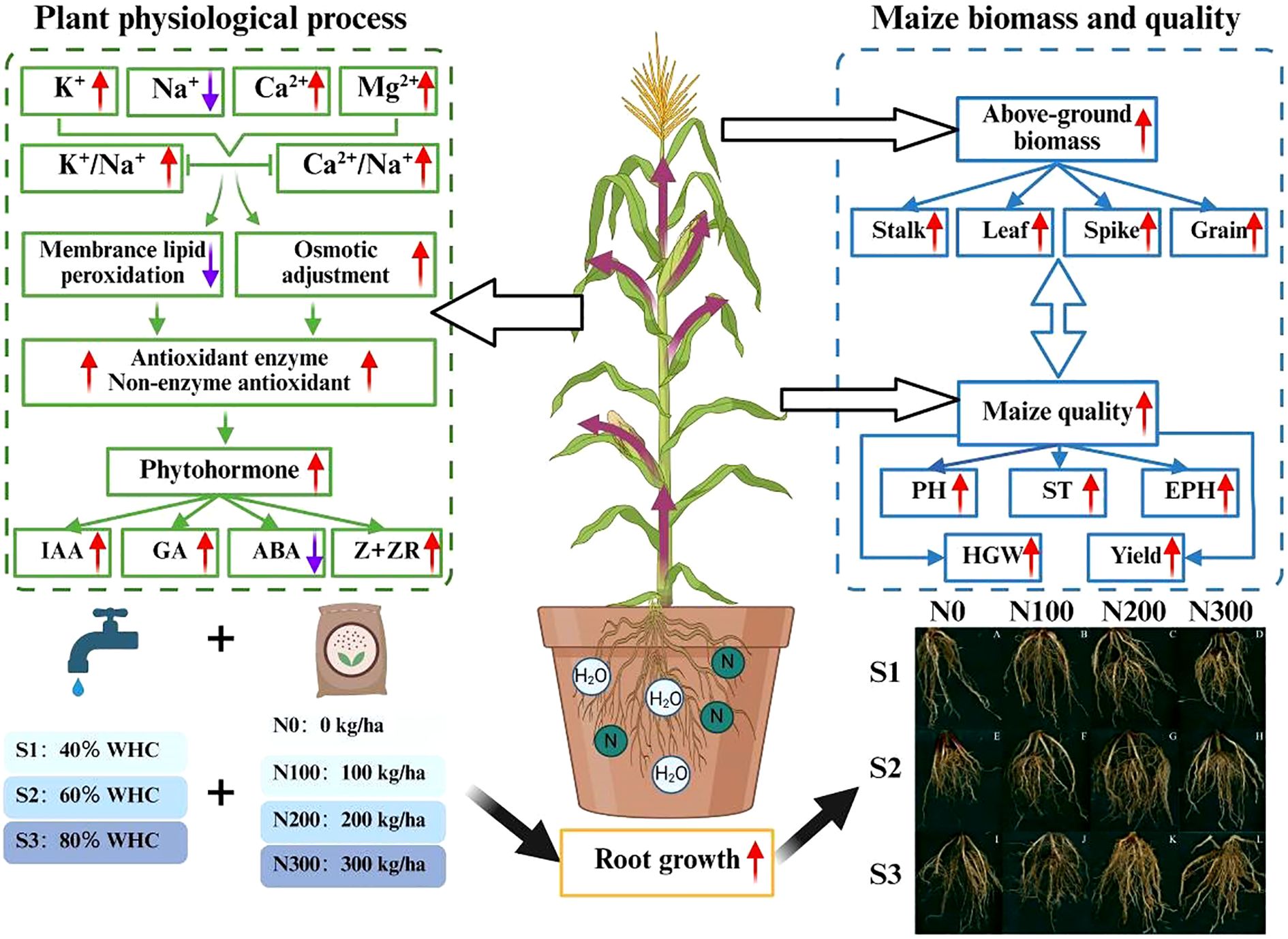
Introduction: Water constraints and excessive nitrogen (N) application hinder root development in spring maize in cold semi-arid regions (CSR), limiting growth and yield. In this study, we focused on the CSR where water scarcity and high fertilizer use are major challenges. Optimizing water-N interactions can enhance root distribution and nutrient uptake, offering a key strategy for improving crop productivity.
Materials and methods: To determine the optimal water-N management strategy under local climatic conditions and evaluate its effects on root physiology and yield performance of spring maize. A two-year field experiment (2020-2021) tested four N application rates (N0: 0 kg ha-1, N100: 100 kg ha-1, N200: 200kg ha-1, N300: 300 kg ha-1) and three soil moisture levels (S1: 40%, S2: 60%, S3: 80% field capacity). Water was managed by maintaining target soil moisture using TDR-based measurements and supplemental irrigation.
Results: Compared with S3-N300, S3-N200 increased plant height (7.89%), stalk thickness (10.48%), and spike position height (5.14%), while substantially boosting root antioxidant enzymes (7.72%), lowering reactive oxygen species (11.81%) and raising K+ (18.22%), Ca2+ (16.35%), Mg2+ (20.01%), and reduced Na+ (3.83%) levels. It also elevated Indole-3-acetic acid (IAA), Gibberellins (GAs), and Zeatin + Zeatin Riboside (Z+ZR) by 45%, 43%, and 30%, respectively. Biomass accumulation rose in spike (11.98%), leaf (23.21%), stalk (16.63%), and grain (6.95%), resulting in 8.01% yield improvement. Structural equation modeling (SEM) showed that water-N interactions explained 94% of the variation in yield, 89% in ion content, 94% in hormones, and 91% in ROS levels.
Conclusion: These findings confirm that S3-N200 (80% field capacity + 200 kg N ha-1) treatment improved root function, stress resilience, and nutrient uptake, thereby enhancing growth and yield compared to conventional local practice (>250-300 Kg N ha-1) without optimized water management. Optimizing water-N strategies in North China’s CSR supports sustainable maize production and strengthens agricultural resilience under water-limited conditions.
1 Introduction
Maize (Zea mays L.) is one of the primary food sources for a significant portion of the world’s population (FAO, 2020). Globally, maize is ranked as the third most important cereal crop after wheat and rice. Global maize consumption continues to rise, with enhancing pressure to maintain high yield under resource-limited and climate-stressed conditions (Erenstein et al., 2022). In cold semi-arid regions (CSR) of northern China, maize production is constrained by low precipitation, high evapotranspiration, and limited water availability, by imbalanced fertilization use. Water stress from both inadequate irrigation or drought is a dominant environmental factor that restricts crop growth, development, and reduces crop yield (Muhammad et al., 2025). Excessive N fertilization, mostly used to compensate for low soil fertility, which can further deteriorate soil health, increase N leaching, lodging risk, and contribute to environmental pollution (Muhammad et al., 2022a). Therefore, proper water-N management strategy is crucial for improving resource use efficiency and enhancing sustainable maize production under CSR environmental conditions. In this scenario, root development plays a crucial role in plant adaptation to water and nutrient availability during vegetative growth, especially in arid and semi-arid regions (Araus et al., 2008). The nutrients acquired by roots are essential for maize plant growth, and a restricted nutrient supply can hinder plant growth, leading to discernible phenotypical changes (Hu and Chu, 2020).
Water stress is one of the most critical environmental factors regulating plant growth and development and limiting crop production in CSR. Additionally, plants can respond and adapt to water stress by altering their cellular metabolism and activating various defense systems (Muhammad et al., 2022b). Plant survival under stress conditions relies on perceiving stimuli, generating and transmitting signals, and initiating various physiological and chemical changes (Wang et al., 2016, Wang et al., 2018). The mitotic activity of plant cells was always inhibited under drought stress. The drought-induced inhibition of maize leaf growth is associated with a significant reduction in the number of cells in the meristematic tissue (Sun et al., 2023). Meanwhile, drought stress also causes excessive accumulation of ROS in plants, which causes secondary oxidative stress, membrane damage, electrolyte leakage, lipid peroxidation, and consequently plant cell death (Voothuluru et al., 2020). Drought stress reduces N accumulation in roots, thereby restricting root growth and their distribution (Xia et al., 2021a). Although total root biomass reduces during drought stress, however, it increases the proportion of roots in deep soil layers, which favors water and nutrient absorption and stable grain yields (Xia et al., 2021b). Excessive irrigation adversely affects maize growth during waterlogging, where soil oxygen deficiency hampers root growth and premature plant aging, which ultimately reduces grain yield (Wang et al., 2019).
Nitrogen limitation hinders the growth and productivity of crops, resulting in the expansion of root architecture, an increase in biomass, and changes in root exudate composition (Zhang W, et al., 2021). Likewise, N deficiency triggers root branching and elongation to improve soil exploration, while high N fertilization alters root metabolic processes and morphology (Schlüter et al., 2012). These studies primarily focus on probing particular classes of metabolites, such as amino acids or fatty acids. However, capturing the aggregate effect of maize root physiology on biomass production under both N-deficient and sufficient conditions is essential for understanding metabolism throughout the plant. Plants have evolved inherent mechanisms to regulate growth and development in response to various environmental stresses. The control of cell expansion plays a crucial role in water stress responses and plant growth (Maggio et al., 2006). Additionally, cell growth caused by cell expansion is regulated by turgor pressure, which is the physical force against the cell wall and is maintained by osmotic regulation through osmotically active substances, including potassium ions (K+), magnesium ions (Mg2+), calcium ions (Ca2+), sugars, and amino acids (Xia et al., 2013). During water deficit stress, osmotic stress sensing and signaling are essential for maintaining plant water status, leading to rapid changes in gene expression (Yamaguchi-Shinozaki and Shinozaki, 2006; Osakabe et al., 2011) and turgor-dependent stomatal closure, which responds to hydraulic properties in the xylem (Aksu and Altay, 2020; Roelfsema et al., 2012).
To understand plant adaptation to N availability, systems biology methodologies are essential to comprehensively study the metabolic response of maize roots under N-deficient and N-sufficient conditions. Root systems have evolved structural and physiological strategies to forage resources in complex soil environments (Lynch, 2015; York et al., 2013). Efficient use of soil water and nutrients significantly influences crop growth and yield, which largely depends on root morphological traits and their temporal and spatial distributions (Ortega, 2010). Understanding root phenotypes under variable N regimes, contrasting water conditions, and their interactions, is crucial for integrating root phenotypes in crop breeding (Lynch, 2019). However, the impact of optimizing combined N and water management on maize yield through enhancing the spatial match between roots and soil water distribution remains unclear.
The purposes of this study were: (1) to examine the interactive effect water and N on root distribution, growth dynamics, morphology, osmotic adjustment, oxidative stress, and maize yield; (2) to identify the most effective water-N interaction for cold and semi-arid areas of north China; (3) to optimize N application levels to enhance water use efficiency and support sustainable maize production. We hypothesized that the interaction of optimized water and N levels would enhance root development and physiological function, thereby improving nutrient uptake, stress tolerance, and ultimately increasing maize yield in cold semi-arid regions.
2 Materials and methods
2.1 Experimental site, plant materials, and growing conditions
This study was conducted at the experimental station of the Qiqihar Branch, Heilongjiang Academy of Agricultural Sciences, China (47°16′26″N, 123°41′46″E), located in a semi-arid region of the western Songnen plain. The soil was classified as Aeolian sandy, with a depth of 0–20 cm, a pH of 7.82, a bulk density of 1.26 g cm2, and an organic matter content of 26.52 g kg-1. Available N, phosphorus (P), and potassium (K) were 100.05, 16.91, and 134.03 mg kg-1, respectively. Figure 1 shows the daily temperature, precipitation, and sunshine duration during the maize growing and fallow seasons of 2020 and 2021. The mean annual air highest temperature, lowest temperature, sunshine duration, and precipitation of the experimental site were 22.4°C, 12.7°C, 664.1 h, and 604 mm, respectively.

Figure 1. Temperature and precipitation of the upper, middle, and lower ten days per month and daily sunshine duration in 2020-2021.
Zea mays L., hybrid cultivar Nendan 19 (ND19) was used as a test crop. Plants were established at a density of 75,000 plants ha-1, with row to row spacing of 65 cm and plant to plant spacing of 25 cm, consistent with field conditions. To ensure experimental accuracy, soil samples were collected from conventional farms and subjected to a drought-resistant environment. Intact and uniform seeds (germination rate > 90%) were selected for planting. Each pot, measuring 29.5 cm in diameter and 38 cm in height, was filled with 30 kg Aeolian sandy soil collected from normal farmland, and the planting arrangement replicated field spacing.
2.2 Experimental design
The experiment consisted of four N rates: 0 kg N ha-1 (N0), 100 kg N ha-1 (N100), 200 kg N ha-1 (N200), and 300 kg N ha-1 (N300), and three water treatment levels: S1 (40% of field holding capacity (FHC), S2 (60% FHC), S3 (80% FHC). This factorial design resulted in twelve distinct treatments, each replicated thrice, comprising thirty-six experimental units. Fertilization included urea (46.4% N) applied at 0, 1.02, 2.02, and 3.02 g pot-1 corresponding to N0: 0 kg ha-1, N100: 100 kg ha-1, N200: 200kg ha-1, N300: 300 kg ha-1; P2O5 at100 kg ha-1 (18% P at 3.59 g pot-1); and KCl at100 kg ha-1 (60% K at 3.59 g pot-1). Two-thirds of the fertilizer was applied as base fertilizer at sowing, and the remaining one-third was applied at the 12th leaf stage. Soil moisture was measured using a TDR-100 meter (Spectrum Technologies Inc., California, USA), with readings taken daily at 06:00 and 18:00. If water conditions do not meet test requirements, artificial irrigation is required. Soil moisture was measured in the 0–20 cm layer. Before rainfall events, the experiment utilized automatically operated rain shelters with a triple-folding mechanism to shield the test area from natural precipitation. Insecticides were deemed unnecessary, but herbicides were applied before and after the planting. Seeds were manually sown on May 12, and harvesting occurred on September 25 during the 2020 and 2021 growing seasons.
3 Measurements and calculations
3.1 Plant sampling and data collection
The whole maize plant was harvested and washed with distilled water at the maturity stage. Plant height was recorded from the base of the maize stem to the highest point with a ruler, and stem diameter was measured with a Vernier Caliper. The plant were dried at 105°C for 30 min and then dried to a constant weight at 80°C for 72 h for dry weight calculation.
3.2 Root length, surface area, tips, diameter, and root-to-shoot ratio measurements
The roots were scanned using the LA-S plant root fine analyzer (MRS-9600TFU2L). LA-S Plant Root Analysis System was then used to analyze the data. The root length density (RLD) and root surface area density (RSD) were calculated using the following equations (Wu et al., 2022).
3.3 Determination of metal ions
To determine the Na+, K+, Ca2+,and Mg2+ concentrations in the roots, approximately 0.1 g of fresh root samples were ground in liquid N. Inductively coupled plasma mass spectrometry was used to determined Na+, K+, Ca2+,and Mg2+ concentrations in roots (mg kg-1) (ICP-MS, NexION 350x, PerkinElme Co., America).
3.4 Hormone content
Different treatments were applied to root samples for measuring hormone contents, including GA, IAA, ABA, and Z+ZR. Approximately 0.5 g of maize roots was ground in a mortar with 5 mL of methanol extraction buffer and 1 mmol L-1 dibutyl hydroxytoluene as an antioxidant. The extract was allowed to stand at 4°C for 4 h and then centrifuged at 10,000 × g for 15 minutes at 4°C. The supernatant was passed through a Chromosep C18 column, pre-washed with 10 mL of 100% methanol, and then washed with 5 mL of 80% methanol. The extracted hormones were dried in 1 mL of phosphate buffer and then dissolved in 0.1% (v/v) N2 between 20% (surfactant) and 0.1% (w/v) gelatin (pH 7.5) for enzyme analysis and immunoassay evaluation (ELISA).
3.5 Assay of osmoregulation substance, ROS, and antioxidant activity
Soluble sugar (SS), proline (pro), soluble protein (SP), superoxide anion (O2-), malondialdehyde (MDA), and H2O2 content were determined using the method of Muhammad et al. (2023).
The antioxidant enzyme activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), glutathione peroxidase (GPX), and glutathione reductase (GR) were detected according to the method by Ahmad et al. (2022).
To determine antioxidant enzyme activities, 0.2 g of fresh leaf sample was homogenized with 5 mL of phosphate buffer (0.1 M, pH 6.8). The mixed liquid sample was centrifuged at 12,000 × g for 20 min at 4 °C. The resultant supernatants were used for SOD, POD, CAT, APX, GPX, and GR activities, and the results were presented as U mg−1 min−1 FW.
3.6 Grain yield
At the physiological maturity stage (R6), all of the remaining ears were harvested to determine yield (length of the spike, spike diameter, rows per ear, and grain per row were counted manually from the selected cobs after the measurement of 100-grain weight, and moisture content was approximately 14%).
3.7 Statistical analysis
The experimental data were sorted and calculated using Excel 2019 (Microsoft Corp., Redmond, WA, USA). They were subjected to an analysis of variance (ANOVA) using SPSS 21.0 Statistics (Ver. 21.0, SPSS Inc., Chicago, IL, USA). Comparisons between means were conducted using the Least Significant Difference (LSD) test at p < 0.001 and p ≤ 0.05. OriginLab 2021 (Northampton, MA, USA) was used to illustrate the figures.
Spearman correlation analysis was used to determine the relationships among environmental variables, maize root growth indexes, and yields, using the R package “Mantel”, and the correlation results were visualized by the ggplot2, dplyr, corrplot, RColorBrewer, and ggsci mixed function of the R package “linKET”. AMOS 24.0 was used to construct structural equation models (SEMs) and test the relationship between different variables (including regression analysis, factor analysis, correlation analysis, and variance analysis). The optimal SEMs with statistical significance were selected for path construction.
4 Results
4.1 Interactive effects of nitrogen and water on plant growth performance
Significant effects of water treatments, N fertilization, and their interaction were observed for plant height (PH), stalk thickness (ST), and spike position height (SPH) (p < 0.05; Table 1). Similar trends were recorded in both years (2020 and 2021)., Water stress notably reduced PH, ST, and EPH under S1 and S2 conditions compared with S3 (Table 1). The N200 treatment increased PH, ST, and SPH compared with N100 by 7.08%, 3.69%, and 1.93% under S1; 11.79%, 9.62%, and 4.86% under S2; 15.25%, 25.39%, and 9.95% under S3, respectively. Conversely, N300 treatment decreased PH, ST, and EPH by 7.39%, 4.70%, and 1.57%, respectively (Table 1). While N variability and water treatments significantly affected PH (p < 0.05), no significant interaction was observed between year and N levels (p > 0.05). However, the water-N interaction was highly significant (p < 0.01), indicating that the availability of water influences the impact of N on growth metrics. The three-way interaction among (year × water × N) significantly affected PH but not ST or SPH (Table 1).
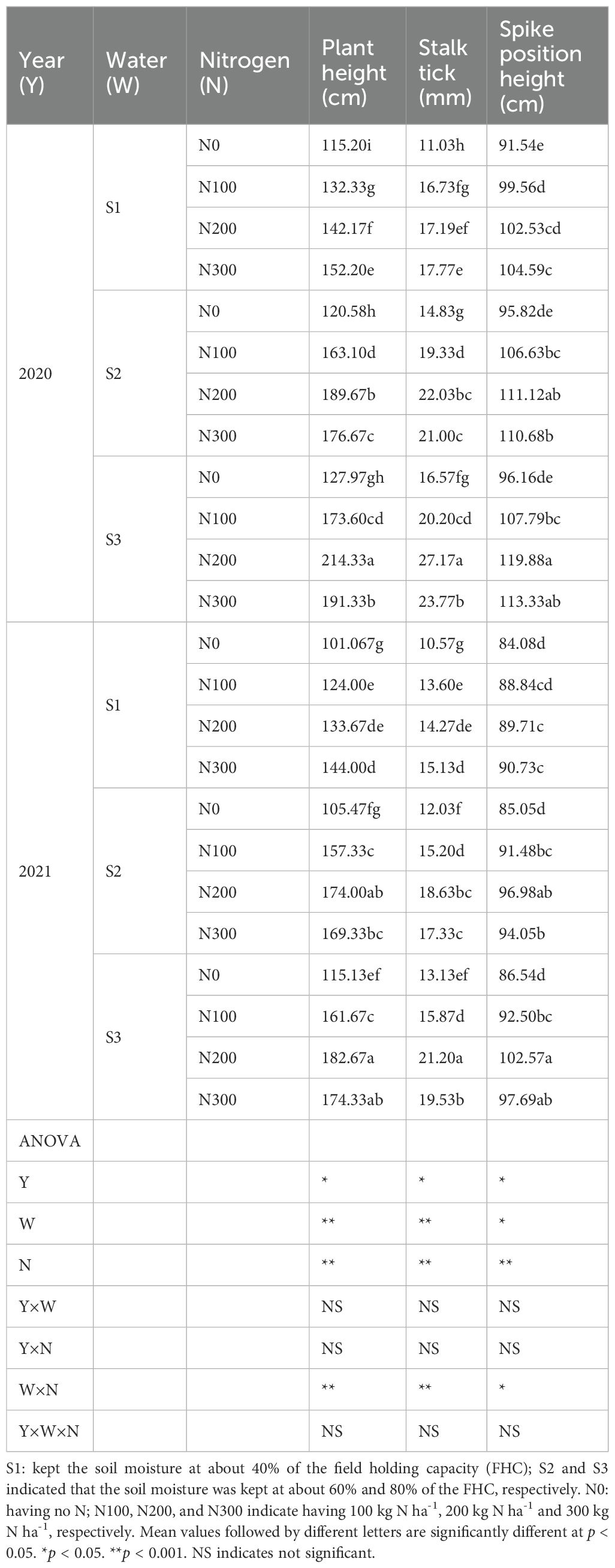
Table 1. Effects of water-nitrogen interaction on plant height, stalk tick, and spike position height of maize in 2020 and 2021.
4.2 Effects of water-N interaction on root growth
Key growth parameters, including root length (RL), stem diameter (SD), root specific surface area (RSSA), root/shoot ratio (R/S), water content, and total dry weight, were markedly influenced by N application and water availability, highlighting the complex interactions between nutritional and environmental stress factors (Supplementary Figure S1; Table S1). Across both 2020 and 2021, RL increased with higher N levels across all water conditions, with the greatest growth observed in the N200 treatment, especially under S3 (Figure 2). Suggesting that adequate N availability strongly promotes root development in well-watered conditions.
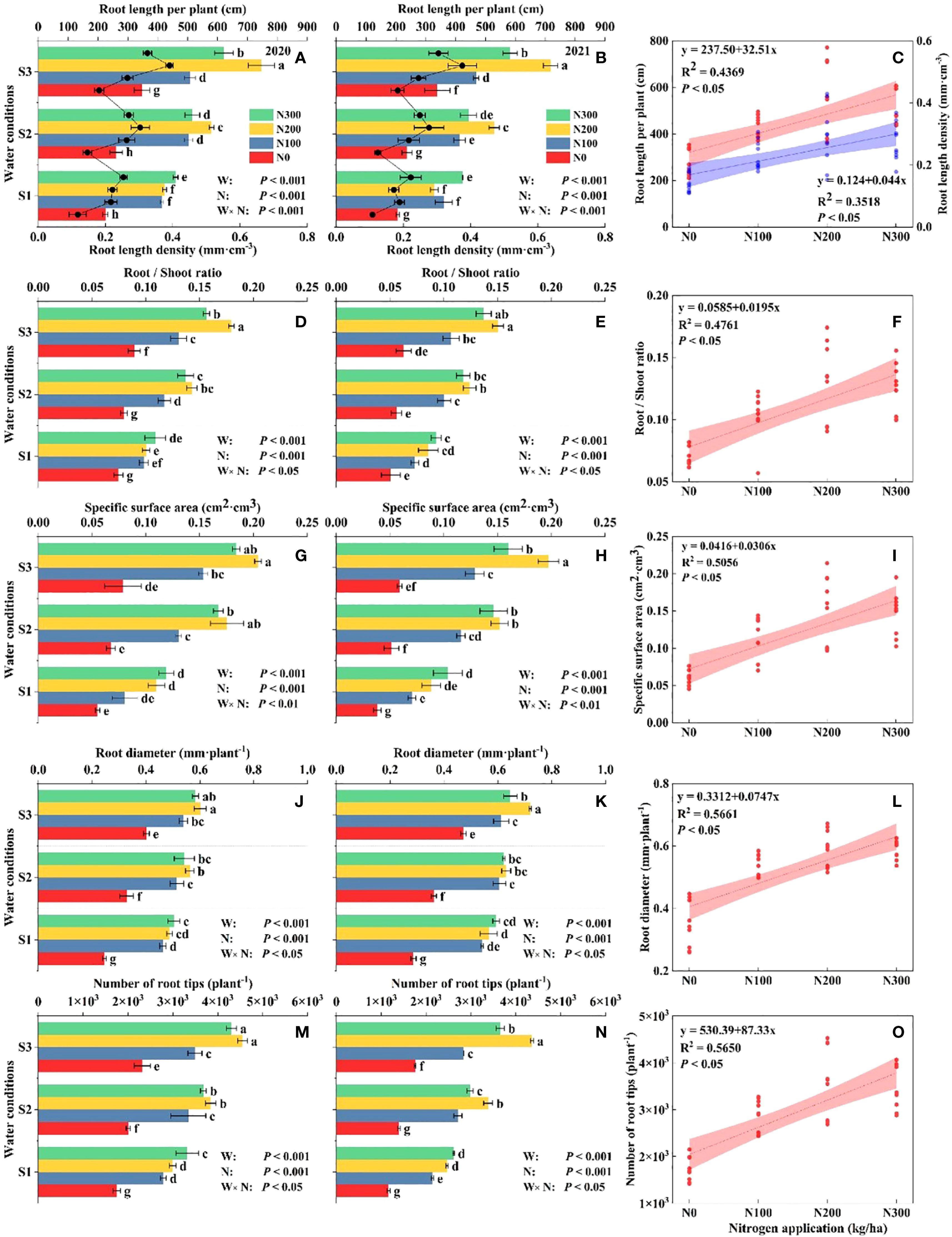
Figure 2. Different water conditions and N application affect the maize root. Root length (RL, A–C), root length density (RLD, A–C), root/shoot ratio (R/S, D–F), root-specific surface area (RSSA, G–I), root diameter (RD, J–L), and the number of root tips (RT, M–O) at different water-N interaction at maturity stage and relationship during 2020 and 2021.
The R/S ratio decreased as N increased, demonstrating a higher allocation of biomass to shoot growth at higher N levels, particularly under optimal water conditions. Similarly, N application had a positive influence on the specific area, with the highest values recorded for N200, suggesting improved root expansion and higher potential for water and nutrient absorption, which support overall plant growth. Root diameter (RD) consistently increased with higher N levels, particularly under N200, across both years, reflecting enhanced structural support and nutrient uptake capabilities. Leaf N content also increased with higher N applications, reaching peak levels under N200, a trend consistent across all water conditions, highlighting efficient N assimilation essential for photosynthesis and biomass production. Specifically, under S3-N200, RL increased by 48.75% and 24.31%, and RLD by 48.93% and 24.64% compared with S1-N200 and S2-N200, respectively (Figures 2A, B). Water-N interaction increased RSSA in the S3-N200 level by 44.54%, 21.95%, and 14.43% (Figures 2G, H), and RT by 33.46%, 25.26%, and 10.76% (Figures 2M, N) compared to S1-N300, S2-N300, and S3-N300 treatments, respectively.
Note: S1: kept the soil moisture at about 40% of the field holding capacity (FHC); S2 and S3 indicated that the soil moisture was kept at about 60% and 80% of the FHC, respectively. N0: having no N; N100, N200, and N300 indicate having 100 kg N ha-1, 200 kg N ha-1 and 300 kg N ha-1, respectively. Error bars represent the standard error, and different lowercase letters indicate significant differences at p < 0.05 (n = 3, Student’s t-test).
4.3 Effects of water-N interaction on root ions
The data revealed that ion concentrations were significantly elevated in 2020 compared to 2021, suggesting annual variations in ion uptake that are potentially influenced by external environmental factors beyond the experimental conditions. The increase in water conditions from S1 to S3 resulted in a significant decrease in Na+ levels and an increase in K+, Ca2+, and Mg2+ levels, emphasizing the crucial role of water availability in controlling nutrient absorption. The Na+ concentrations decreased by 34.12% and 15.02% in S3, respectively, compared to S1 (Supplementary Table S2). Compared to S3-N300, the K+, Ca2+, and Mg2+ content in S3-N200 were higher by 18.22%, 16.35%, and 20.02%, respectively, and by 50.49%, 53.48%, and 48.05% in S3-N100 (Figures 3D–L, p < 0.001). Nitrogen fertilization in S2 water condition increased K+, Ca2+, and Mg2+ by 40.08%, 38.47%, and 45.21% in N200 and by 25.66%, 25.41%, and 39.04% in N300 compared to N100 (Figures 3D–L, p < 0.001).
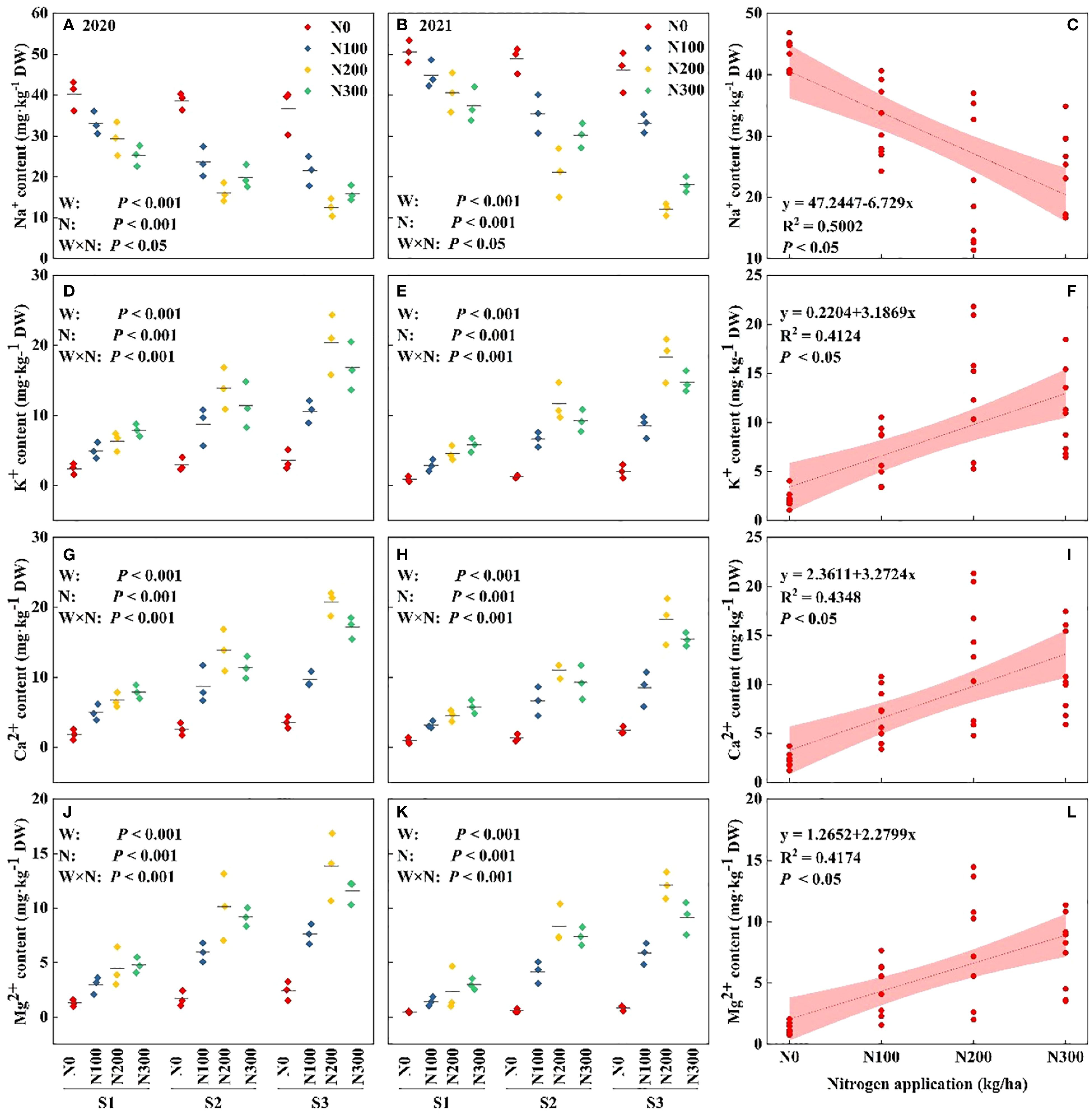
Figure 3. Interactive effects of water stress and N on nutrient contents in the maize root. Roots iron from (A, B) Na+, (D, E) K+, (G, H) Ca2+, and (J, K) Mg2+ in water-N interaction during 2020 and 2021. Relationship between different water conditions and Na+ (C), K+(F), Ca2+(I), and Mg2+(L) under different N levels during 2020-2021.
N200 significantly increased the concentrations of ions K+, Ca2+, and Mg2+ under different water conditions. In contrast, low and lack of N levels (N0 and N100) resulted in the lowest ion levels, highlighting the crucial role of N in enhancing nutrient absorption efficiency during stressful situations. The correlation between water and N was significant (p < 0.05), indicating the interconnected impact of these components on nutrient dynamics (Figures 3C, F, I, L). Nevertheless, the intricate interaction among year, water, and N indicates that external environmental conditions also have a substantial impact on nutrient absorption, extending beyond the controlled variables of water and N. These findings highlighting the complex relationships between environmental factors and agronomic techniques in determining plant mineral nutrition, particularly under variable water availability (Figures 3C, F, I, L, p < 0.05).
Note: S1: kept the soil moisture at about 40% of the field holding capacity (FHC); S2 and S3 indicated that the soil moisture was kept at about 60% and 80% of the FHC, respectively. N0: having no N; N100, N200, and N300 indicate having 100 kg N ha-1, 200 kg N ha-1 and 300 kg N ha-1, respectively. Error bars represent the standard error, and different lowercase letters indicate significant differences at p < 0.05 (n = 3, Student’s t-test).
4.4 Effects of water-N interaction on root hormones
The findings elucidate the intricate relationship between water conditions, N levels, and the hormonal equilibrium in maize roots (Figure 4). Across both years, increasing water levels from S1 to S3 consistently reduced ABA concentrations while increasing IAA, GA, and Z+ZR. The changes were more significant in 2021, suggesting a potential cumulative stress effect over successive years. Notably, under S3, ABA content decreased by 46.04% (9.69 ng g-1) and 19.01% (2.67 ng g-1) in 2020 and 48.17% (8.19 ng g-1), 18.23% (1.96 ng g-1) in 2021, compared with S1 and S2, respectively (Supplementary Table S3).
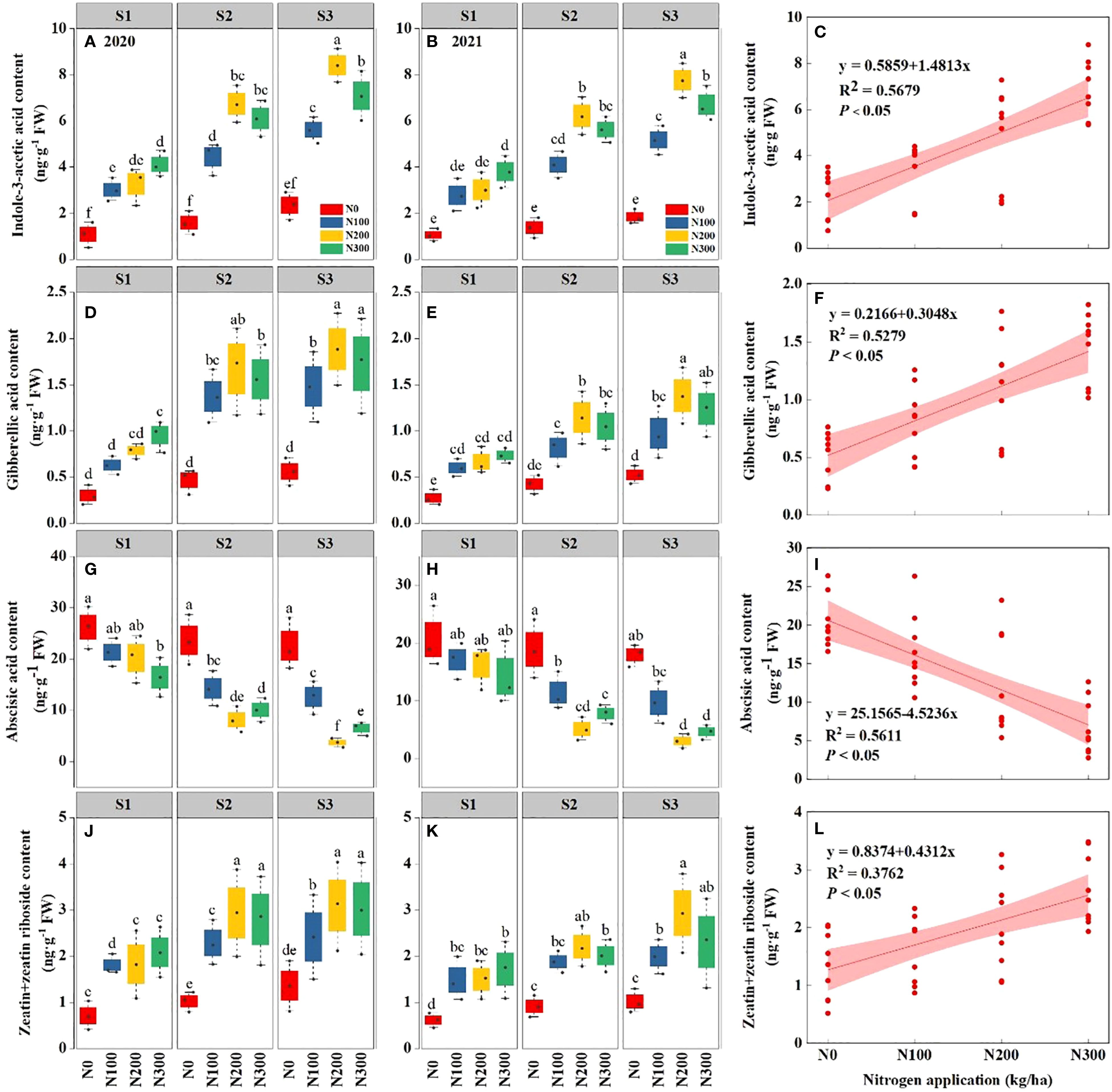
Figure 4. Interactive effects of water stress and nitrogen interaction on different hormones in maize roots (Maize L. cv. Nendan19) during 2020 and 2021. (A, B, D, E, G, H), and (J, K) indicate the Indole-3-acetic acid (IAA), gibberellic acid (GA), abscisic acid (ABA), and zeatin+zeatin riboside (Z + ZR), respectively. Relationship between different water conditions and IAA (C), GA (F), ABA (I), and Z + ZR (L) under different nitrogen levels during 2020-2021. S1: kept the soil moisture at about 40% of the field holding capacity (FHC); S2 and S3 indicated that the soil moisture was kept at about 60% and 80% of the FHC, respectively. N0: having no N; N100, N200, and N300 indicate having 100 kg N/ha, 200 kg N/ha and 300 kg N/ha, respectively. Error bars represent the standard error, and different lowercase letters indicate significant differences at p < 0.05 (n = 3, Student’s t-test).
Nitrogen application, particularly N200 (2.02 g pot-1) markedly increased all hormones concentrations compared to low (N0 and N100) and high N dosage (N300). This suggests that sufficient N availability can alleviate certain hormonal disturbances induced by water stress. A significant interaction between water and N, particularly for IAA and ABA, highlighting the complex hormonal response to environmental pressures (Figures 4A–C and Figures 4G–I). These findings underscore the importance of managing water and N synergistically to maintain hormonal equilibrium and enhance the ability of plants to withstand stressful conditions.
4.5 Effects of water-N interaction on root osmoregulation substance, ROS
The study highlights variations in reactive oxygen species (ROS), including O2- and H2O2, along with Pro, SS, MDA, and SP, in response to different environmental conditions (Figure 5). From 2020 to 2021, all markers declined, suggesting either adaptive plant responses or changes in experimental settings that affect plant stress reactivity. Notably, the S3 consistently showed elevated markers, suggesting a fundamental resistance to oxidative stress. Conversely, N200 noticeably increased stress indicators, indicating a heightened metabolic activity and probable relief from stress (Figure 5; Supplementary Table S4).
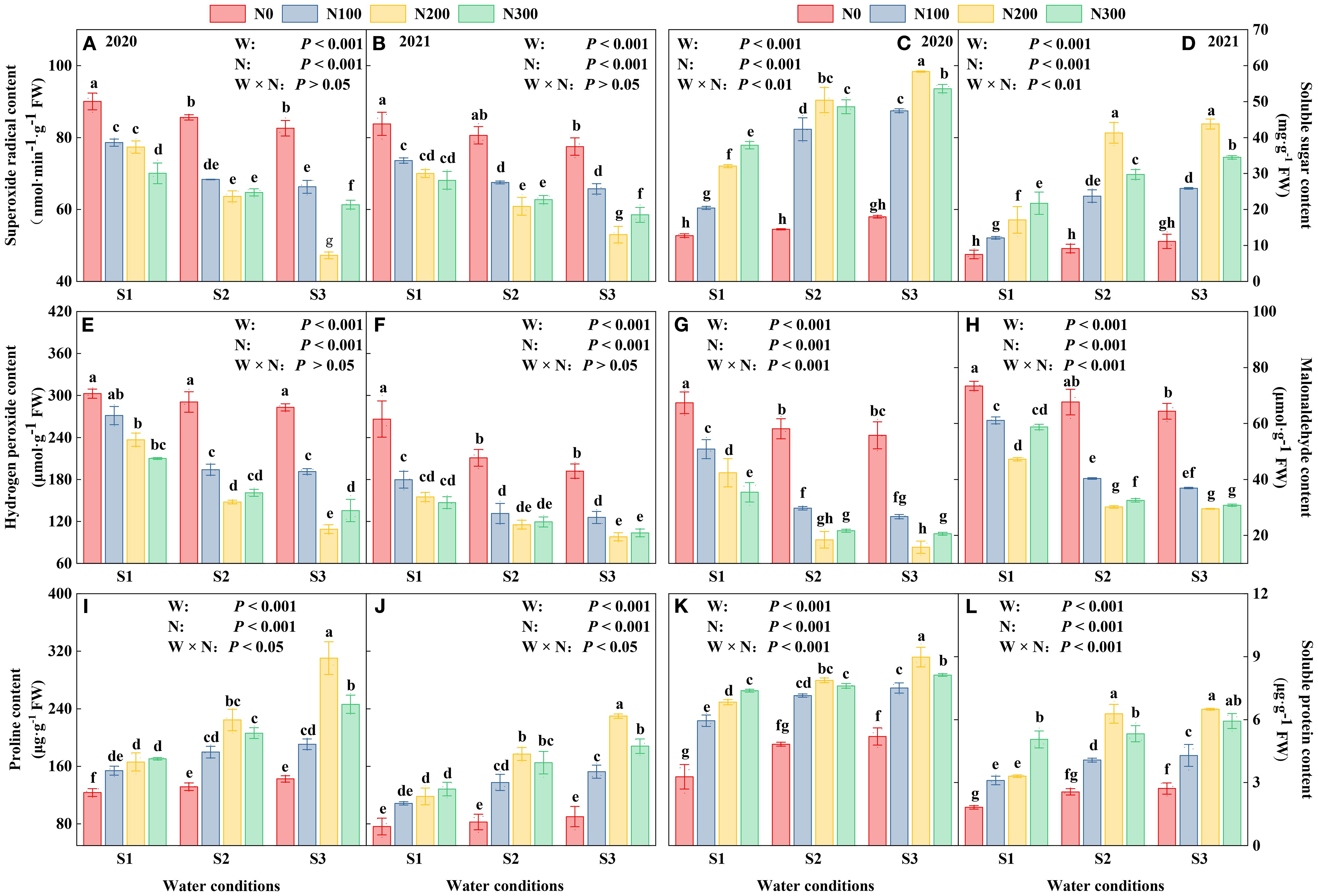
Figure 5. Interactive effects of water stress and nitrogen on the roots of maize (Maize L. cv. Nendan19) superoxide radical (A, B), soluble sugar (C, D), hydrogen peroxide (E, F), malonaldehyde (G, H), proline (I, J), soluble protein (K, L) during 2020 and 2021.
Water conditions from S1 to S3 gradually decreased stress indicators, with the lowest values observed in the S1 treatment. This suggests a possible slowdown or halt in metabolic activities under severe water deficit. The synergistic effects of N levels and water conditions were especially evident in the levels of O2-, H2O2, and MDA (Figures 5A, B, E–H). These results demonstrate the complex relationship between nutrient availability and water conditions in controlling plant physiological responses. Additionally, these results highlight the importance of accurate N management and a comprehensive understanding of water conditions in enhancing plant resilience against environmental pressures. On average, N200 treatment reduced Proline, SS, and SP contents by 24.39-44.63%, 15.94-41.59%, and 10.11-19.62% compared to N300 and by 36.46-51.41%, 28.22-68.10%, and 23.73-41.52% compared to N100 (Supplementary Table S4).
Note: S1 (with soil moisture maintained at about 40% of field holding capacity); S2 and S3 indicated that kept the soil moisture at about 60% and 80% of the soil’s FHC. Error bars represent the standard error, and different lowercase letters indicate significant differences at p < 0.05 (n = 3, Student’s t-test).
4.6 Effects of water-N interaction on root antioxidant
Oxidant enzyme activities, including SOD, POD, CAT, APX, GPX, and GR, were quantified in plant tissues exposed to water conditions and varying N levels (Figure 6). Enzyme activities in 2020 were greater than those in 2021, suggesting a difference in oxidative stress responses or environmental factors influencing enzyme expression (Supplementary Table S5). The highest activities were observed under S3, with a notable increase as water condition intensified from S1 to S3, emphasizing the critical role of water condition in enhancing antioxidant defense systems. Nitrogen had a dose-dependent impact, N200 consistently leading to the highest levels of enzyme activity (Supplementary Table S5).
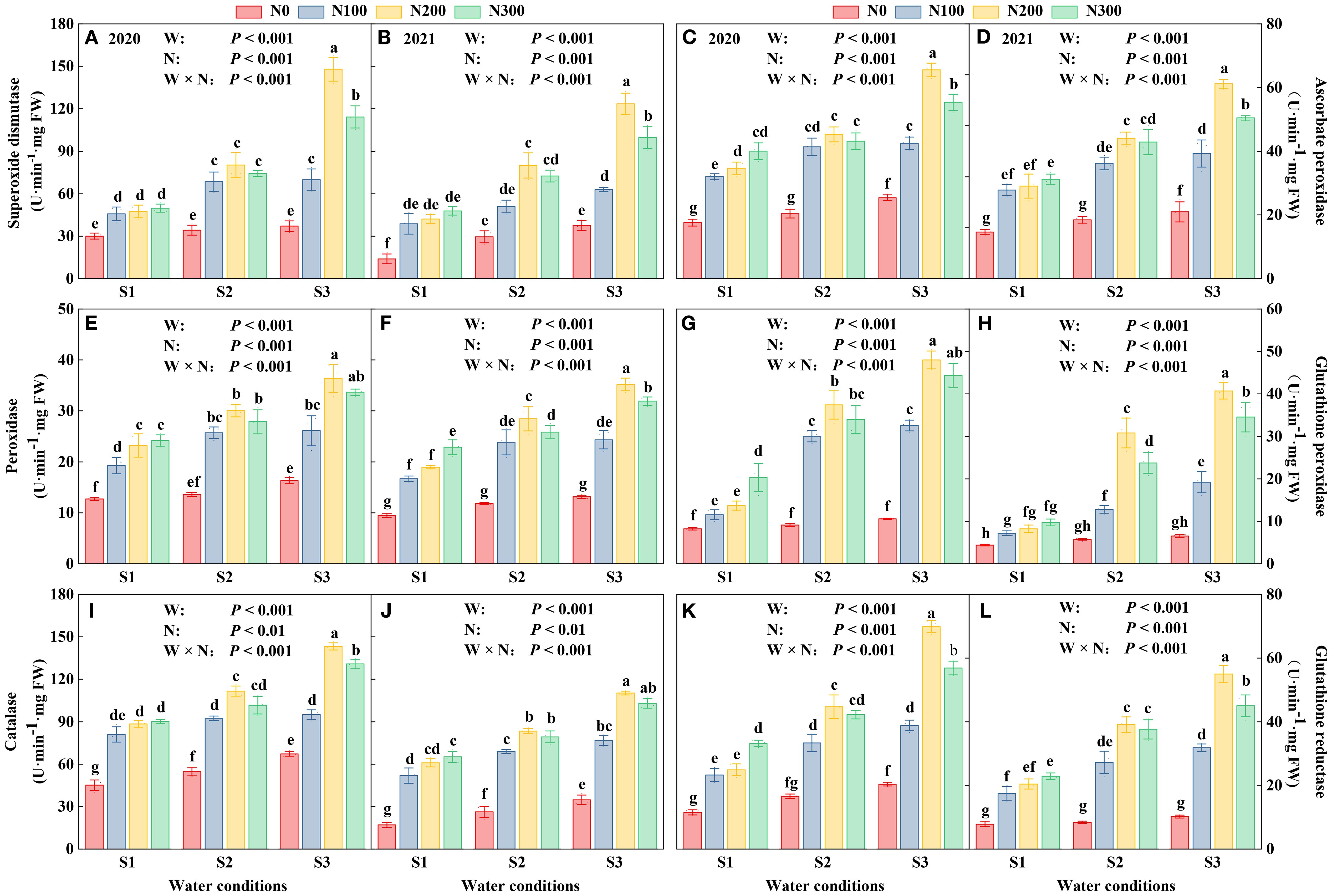
Figure 6. Interactive effects of water stress and N on the roots of maize (Maize L. cv. Nendan19) superoxide dismutase (A, B), ascorbate peroxidase (C, D), peroxidase (E, F), glutathione peroxidase (G, H), catalase (I, J), glutathione reductase (K, L) during 2020 and 2021. S1 (with soil moisture maintained at about 40% of field holding capacity); S2 and S3 indicated that they kept the soil moisture at about 60% and 80% of the soil’s FHC. Error bars represent the standard error, and different lowercase letters indicate significant differences at p < 0.05 (n = 3, Student’s t-test).
The activity of antioxidant enzymes showed significant variation due to the interplay between water and N, indicating intricate physiological responses to the environmental stressors. These findings underscore the need for tailored agronomic strategies to maximize plant health and enhance stress resistance in managing water and N levels. Under S3 conditions, the activities of antioxidant enzymes showed significant enhancements compared to S1 and S2 across all N levels. Specifically, SOD activity increased by 36.35%, 67.01%, and 54.37% at N100, N200, and N300, respectively, relative to S1, and by 10.05%, 41.01%, and 31.39% relative to S2 (Figures 6A, B; Supplementary Table S5). APX activity increased by 26.01%, 49.48%, and 32.12% over S1, and by 4.89%, 29.71%, and 19.10% over S2 at the respective N levels (Figures 6C, D; Supplementary Table S5). Similarly, CAT activity showed increases of 22.59%, 41.02%, and 33.52% over S1, and 6.05%, 23.04%, and 22.60% over S2 at N100, N200, and N300, respectively (Figures 6I, J; Supplementary Table S5).
4.7 Effects of water-N interaction on root non-enzyme antioxidants
The concentration of GSSG+GSH, GSH/GSSH, ASA+DSH, and ASA/DSH was significantly affected by N, water, and their interactions (Figures 7A–D). Over the two-year study, elevated N rates consistently enhanced GSSG+GSH and ASA+DS, with higher N rates yielding greater improvements. This highlights the crucial role of N in improving the plant’s antioxidant capacity. Notably, the N200 treatment demonstrated the highest glutathione levels among all water treatments. There was a marked increase in these levels as water conditions intensified from S1 to S3. Similarly, the ratio of GSH to GSSG was found to be highest in the N200 treatment. This indicates that plants receiving higher N levels can more efficiently control oxidative stress. The ascorbate levels exhibited a comparable trend, reaching their peak under conditions of S3-N200, suggesting that the accumulation of these crucial antioxidants is promoted by adequate N and water availability.
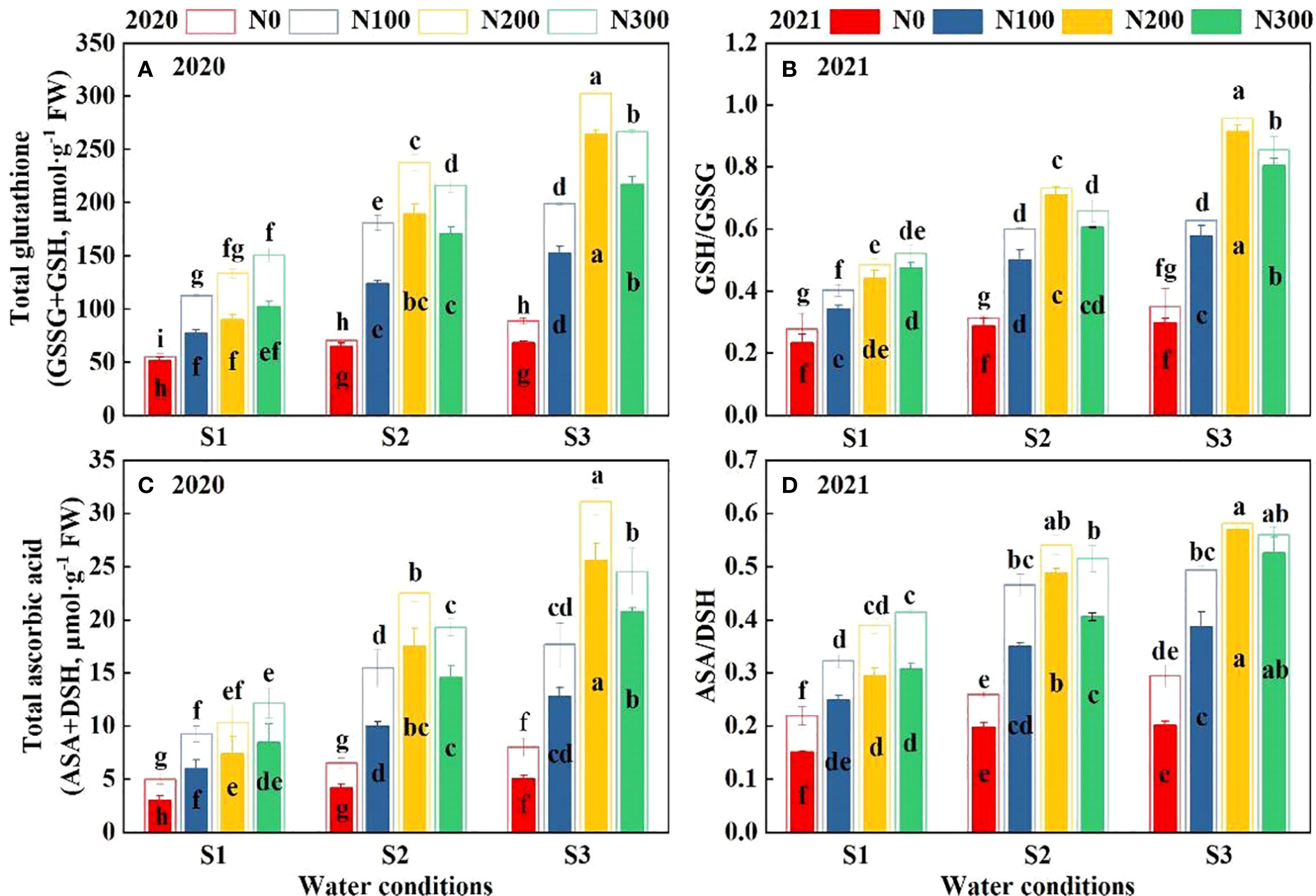
Figure 7. Interactive effects of water stress and N on total glutathione (A), GSH/GSSG ratio (B), total ascorbic acid (C), and ASA/DHA ratio (D) content of roots in 2020 and 2021. S1: kept the soil moisture at about 40% of the field holding capacity (FHC); S2 and S3 indicated that the soil moisture was kept at about 60% and 80% of the FHC, respectively. N0: having no N; N100, N200, and N300 indicate having 100 kg N ha-1, 200 kg N ha-1 and 300 kg N ha-1, respectively. Error bars represent the standard error, and different lowercase letters indicate significant differences at p < 0.05 (n = 3, Student’s t-test).
In 2021, although the levels of GSSG+GSH and ASA+DSH were reduced compared to 2020. Nitrogen application significantly influenced non-enzyme antioxidant levels (Supplementary Table S6, p < 0.01). Nitrogen, water, and their interactions significantly affect the GSSG+GSH, GSH/GSSH, ASA+DSH, and ASA/DSH (Supplementary Table S6; p < 0.01). The N200 treatment significantly increased antioxidant components compared with N100 and N300 across all water conditions in 2020-2021. Particularly, GSSG+GSH increased by 66.76% and 55.71% under S1, 46.60% and 31.91% under S2, and 38.31% and 14.83% under S3 (Figure 7A). Likewise, GSH/GSSH increased by 60.17% and 46.75% (S1), 41.23% and 29.69% (S2), 35.59% and 11.28% (S3) (Figure 7B). ASA+DSH increased by 73.34% and 63.83% (S1), 55.51% and 40.52% (S2), 46.48% and 19.91% (S3) (Figure 7C). ASA/DSH increased by 50.27% and 37.24% under S1, 29.08% and 19.98% under S2, 23.51% and 5.53% under S3, respectively (Figure 7D).
4.8 Effects of water-N interaction on biomass accumulation and partitioning and maize yield
The allocation of biomass across various plant components (spike, leaf, stalk, and grain) in 2020 and 2021 was significantly influenced by varying water conditions and N application rates (Figure 8). During both years, observable shift occurred in dry matter distribution from vegetative parts such as leaves and stalks, to reproductive parts, including spikes and grains, as the water condition increased from S1 to S3. This indicates that there were adaptive responses to the environmental stress.
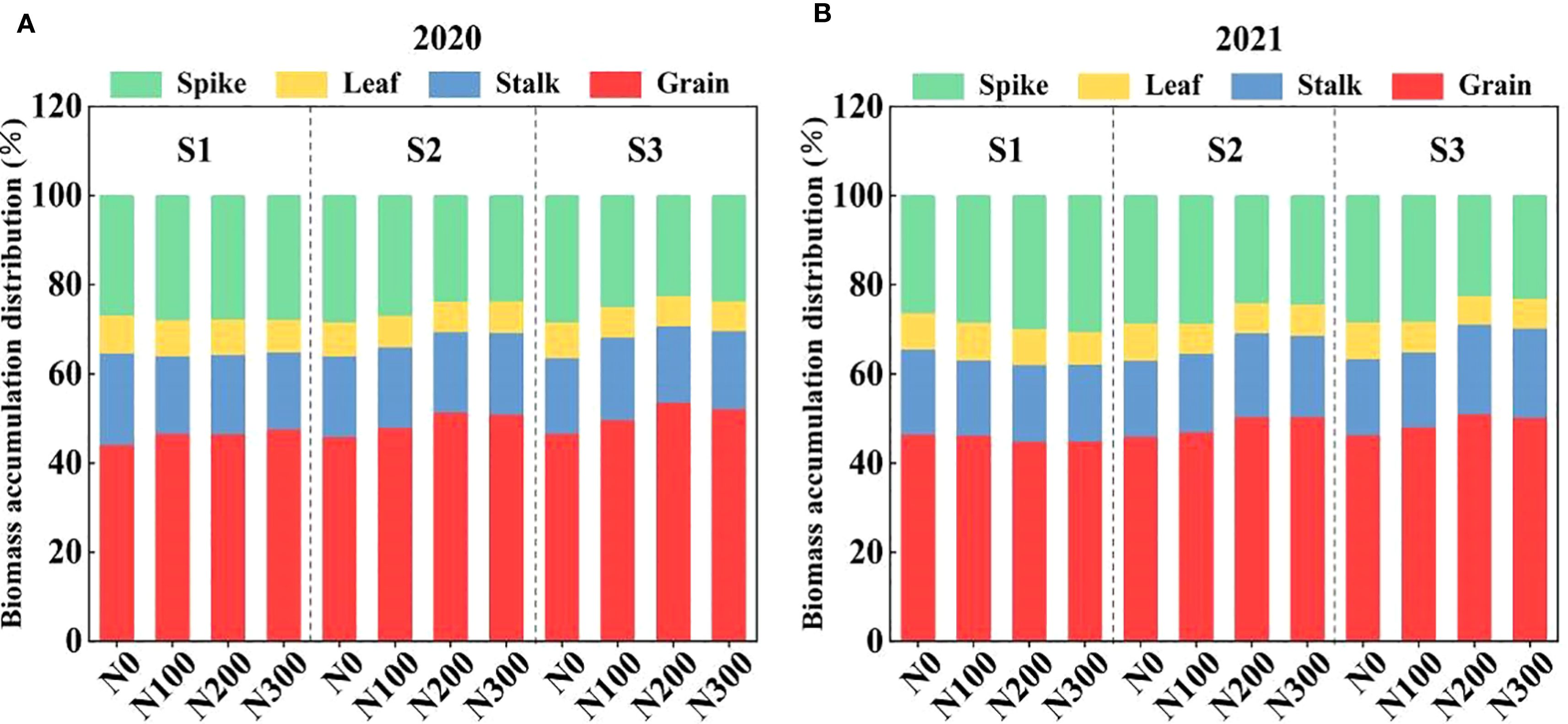
Figure 8. Interactive effects of water stress and N on biomass accumulation and distribution in spike, leaf, stalk, and grain of maize after maturity during 2020 (A) and 2021 (B). S1: kept the soil moisture at about 40% of the field holding capacity (FHC); S2 and S3 indicated that the soil moisture was kept at about 60% and 80% of the FHC, respectively. N0: having no N; N100, N200, and N300 indicate having 100 kg N ha-1, 200 kg N ha-1 and 300 kg N ha-1, respectively.
In 2020, N200 consistently resulted in more biomass being allocated to the grain, regardless of the water treatments. These results suggest that N plays a crucial role in enhancing the reproductive performance of plants, even under varying water conditions. Conversely, N0 and N300 treatments decreased dry matter allocation in grain, especially when subjected to lower water conditions (S1). Under S2 condition, N200 treatment resulted in higher biomass accumulation by 34.51% and 57.98% (spike), 41.97% and 56.30% (leaf), 31.59% and 48.01% (stalk), 30.66% and 45.01% (grain) compared to N100 and N300, respectively. Similarly, N200 treatment increased biomass accumulation by 42.23% and 61.29% (spike), 43.65% and 61.23% (leaf), 28.29% and 51.93% (stalk), and 29.78% and 48.03% (grain), respectively, compared to N100 and N300 under S3 (Figure 8A). The biomass accumulation was increased by 38.95% and 65.61% (spike), 48.82% and 66.26% (leaf), 38.93% and 50.25% (stalk), and 35.77% and 54.23% (grain) in S3-N200 compared to the S3-N100 and S3-N300 treatments (Figure 8B).
In 2020, the N200 treatment consistently yielded the highest values for each parameter, including a greater number of rows per ear, grain number per row, 100-grain weight, total grains per ear, and yield per plant (Table 2). Conversely, N0 resulted in the lowest values for the above parameters, emphasizing the crucial importance of optimizing water-N in promoting strong ear growth and grain filling. As the level of water increased, and there was a corresponding rise in all the measured metrics. S3 exhibited the most significant values, but S1 had the lowest values. The observed pattern was consistent across both N treatments and years, highlighting the cumulative impact of increased water availability on crop productivity. Under S3-N200 treatments, improvements were noted in 100-grain weight (2020: 17.76%, 8.64% and 4.07%), and (2021: 14.71%, 8.99%, and 3.96%), Grains per ear (2020: 17.94%, 10.07%, and 3.37%), and (2021: 17.32%, 7.09%, and 3.54%), yield (2020: 32.64%, 18.03%, and 7.39%), and (2021: 29.45%, 15.49%, and 7.42%) compared with S1-N300, S2-N300, and S3-N300 treatments (Table 2). These findings suggest that the management of water and N is crucial for optimizing crop outcomes in varying environmental conditions. Occasionally, the non-significant interactions among year, water, and N (Y × W × N) suggest that the separate impacts of year, water, and N may sometimes be more prominent than their combined contributions (p < 0.01).
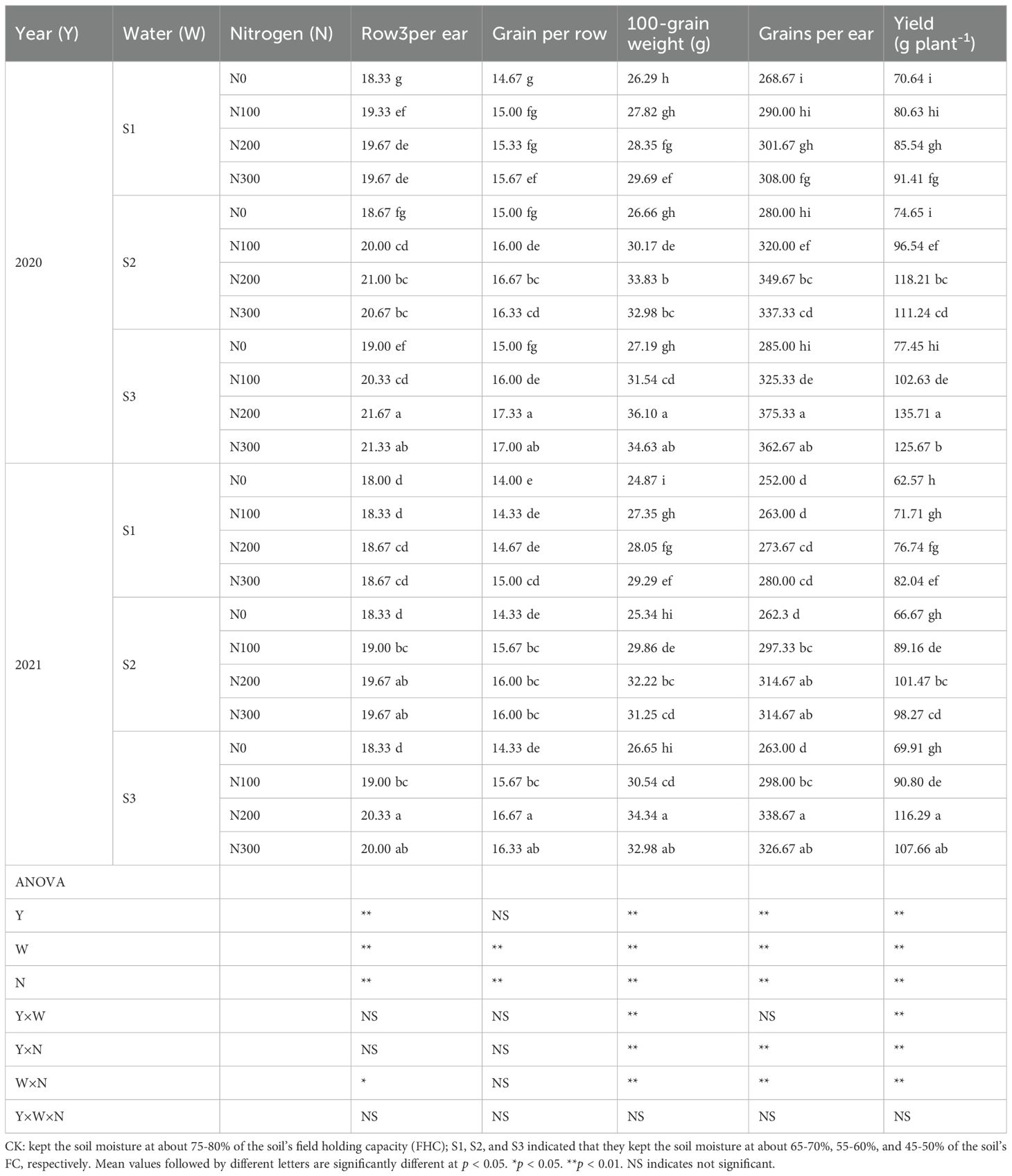
Table 2. Effects of water-N interaction on row per ear, grain per row, 100-grain weight, grain per ear, and yield of maize.
4.9 Correlations between variables
The positive correlations were observed between root traits, such as RL, root length density, R/S ratio, RSSA, root diameter (RD), number of root tips, and some biochemical and physiological parameters. These include IAA (R2 = 0.54-0.78, p < 0.01), GA (R2 = 0.67-0.90, p < 0.01), Z+ZR (R2 = 0.40-0.70, p < 0.05), and enzymes such as SOD (R2 = 0.48-0.89, p < 0.05), POD (R2 = 0.61-0.77, p < 0.01), CAT (R2 = 0.73-0.89, p < 0.01), APX (R2 = 0.64-0.82, p < 0.01), GPX (R2 = 0.70-0.84, p < 0.01), and GR (R2 = 0.64-0.86, p < 0.01). Positive correlations were found with K+ (R2 = 0.68-0.91, p < 0.01), Ca2+ (R2 = 0.69-0.85, p < 0.01), Mg2+ (R2 = 0.65-0.85, p < 0.01), Pro (R2 = 0.72-0.90, p < 0.01), sugar (R2 = 0.57-0.90, p < 0.01), SP (R2 = 0.40-0.81, p < 0.05) and yield (R2 = 0.65-0.82, p < 0.01; Figure 9A). Conversely, ABA, Na+, O2-, and H2O2 had a strong negative correlation with these parameters, whereas MDA content showed a lower negative correlation with root growth but was strongly negatively correlated with other parameters (Figure 9A).
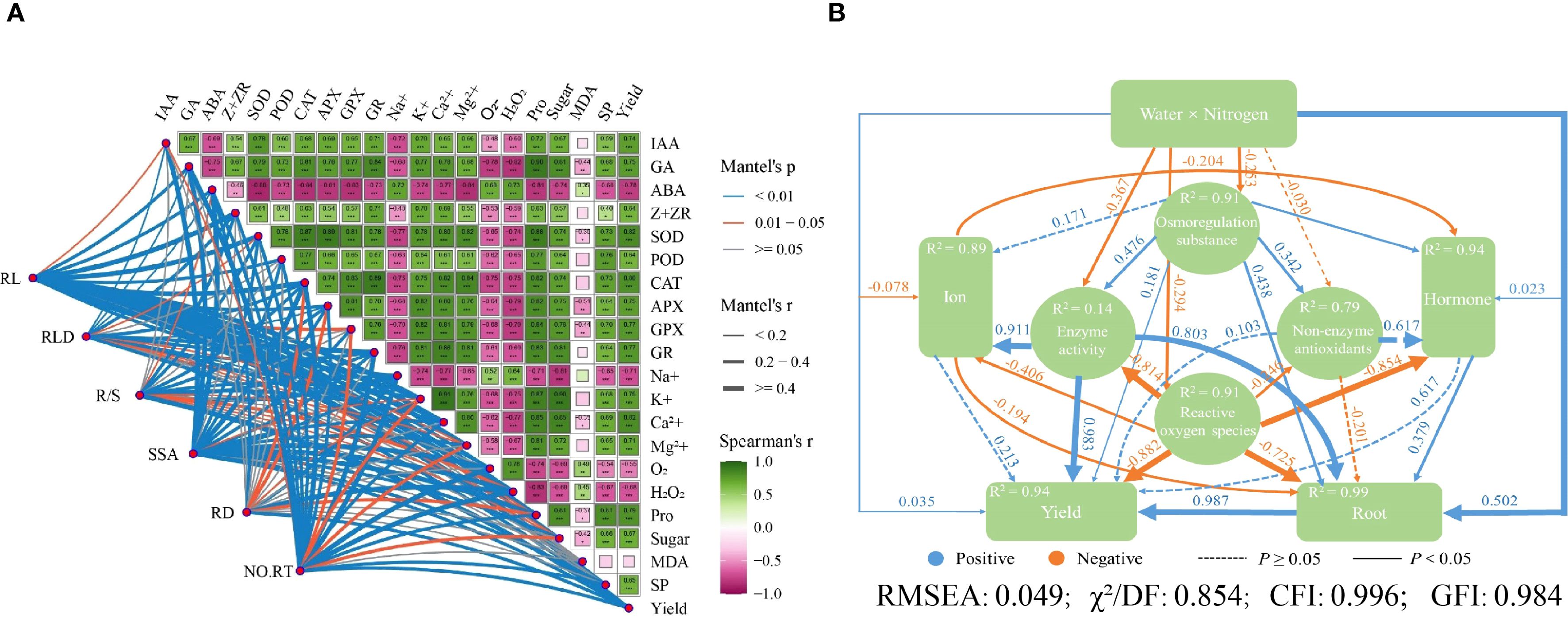
Figure 9. (A) Correlation analysis of enzyme activity, ion, hormone, root growth, and maize yield. Green represents a positive correlation, and pink represents a negative correlation. The darker the color, the stronger the correlation, and vice versa. The numbers in the boxes represent the correlation coefficients. RL, Root length; RLD, root length density; R/S, root/shoot ratio; RSSA, root specific surface area; RD, root diameter; NO.RT, number of root of tips; IAA, Indole-3-acetic acid; GA, gibberellic acid; ABA, abscisic acid; zeatin+zeatin riboside, Z+ZR; SOD, superoxide radical; POD, peroxidase; CAT, catalase; APX, ascorbate peroxidase; GPX, glutathione peroxidase; GR, glutathione reductase; O2-, superoxide anion; H2O2, hydrogen peroxide; Pro, proline; MDA, malonaldehyde; SP, soluble protein. (B) Structural equation modeling results based on the correlations among different water-N interactions (P = 0.426). The path coefficient represents the direction and intensity of the linear relationship between latent variables, and R2 represents the percentage of variation explained by other variables. Numbers near the boxes indicate the amount of variance explained by the model (R2).
Structural equation modeling (SEM) revealed the possible pathways by which root growth and morphology influence yield under water-N interaction, aiming to reduce the interactions among causal factors during 2020-2021 (Figure 9B). The model showed the best fit to the data at P = 0.426. In 2020-2021, water-N interaction influenced maize root growth, causing changes in osmoregulation substances, enzyme activity, reactive oxygen species, ion accumulation, hormones, and maize yield (Figure 9B). The model provided an excellent fit, explaining 94% variation in maize yield, 89% in ion accumulation, 94% in hormone regulation, and 91% in ROS modulation through root traits.
5 Discussion
Maize root distribution depends on the form of phenotypes, N concentration supplied, and water conditions. Water-N interaction increased the root parameters (RL, RLD, R/S, RSSA, RD, and RT) in 2020 and 2021 (Figure 2), as there were significant increases in the growth and development of roots (Zhang G, et al., 2021). The interaction between water and N increased RLD at the R6 growth stage, resulting in compensatory root growth in response to water stress (Qi et al., 2012). The root system searches for N to meet the required demand by increasing its length, number of root tips, and surface area in N-deficient conditions during the growth stage (Liu et al., 2017). Consequently, root formation also depends on soil-related properties such as soil texture, moisture content, and soil nutrients (Adu et al., 2017; De-la-Fuente et al., 2020).
5.1 Water-N interaction responsiveness is related to fast root growth
The root system comprised the primary, seminal, and shoot-borne nodal roots that form in whorls initiated below and above ground, as well as lateral roots in all axial root classes. Furthermore, nodal roots emerge acropetally in whorls through development either belowground or aboveground (Dowd et al., 2020). These nodal roots comprise the bulk of the axial root system, with their lateral roots playing a pivotal role in water and nutrient uptake (Schneider et al., 2020). These nodal root positions influence size-related traits, such as diameter and number (York et al., 2013). Previous research reported that RLD is essential for modeling water and nutrient movement in the vadose zone and root-shoot-atmosphere interactions (Shi et al., 2020). Similarly, RLD is the most suitable parameter to describe water uptake by plant roots compared with other root traits (Ng et al., 2019). The water and nutrient uptake increases with greater root length than root mass, and a higher specific root length tended to lead to greater plasticity in root growth and a greater physiological capacity, but shorter root longevity (Schneider et al., 2020).
The lower soil water conditions in the pots induced the roots to explore to find nutrient material, facilitating lateral and aerial root development, which explains why RL and RLD were significantly higher for roots between N concentrations with S1 than S2 (Fenn et al., 2013). We found that RT, RD, and RSSA under different water conditions were higher under N200 than N300, N100, and N0 due to soil water depletion varying rapidly in the higher N compared to the lower, and no N treatments, where the root system failed to fulfill the N requirements of the crop. Thus, suitable water conditions promote the strong growth of roots near the N supply, increase the R/S ratio (Figure 2), and even improve MGP, such as PH, ST, and EPH (Table 1). However, a lack of N increases the root length in the non-fertilizer strip to search for nutrient absorption and utilization (Su et al., 2015). Our results showed that S3-N200 significantly promoted the root length, root/shoot ratio, root-specific surface area, root diameter, and number of root tips. The maize growth performance in the roots developed under S3-N200 and S3-N300 showed no noticeable difference. In addition, the greater amounts of water and nutrients absorbed by the lateral and aerial roots were stored in the plants to enhance the growth of the above-ground parts (Ma et al., 2013).
5.2 Osmotic regulation and hormones in root growth ensure suitable water-N interaction responsiveness
The effect of the root system in improving nutrient absorption under water deficit conditions, ultimately leads to improving water-root-plant relations (Basirat et al., 2019). The application of N levels decreased IAA, GAs, and Z+ZR in 2020-2021, particularly under S1 water conditions (Figure 4). This implies that the root system was established to absorb a large amount of soil moisture and nutrients, which is consistent with the results that showed that water stress reduced root growth (Figure 2). The structural equation model revealed that the endogenous hormones enhanced the growth and distribution of roots (Figure 9), regulated root senescence and nutrient and water transport, which in turn delayed natural death and increased root vigor (Jia et al., 2018). Appropriate soil moisture enables roots to search for water in the soil to meet plant developmental needs (Elhani et al., 2019). In addition to keeping soil moist, mucilage can help to alter the redistribution of water within the soil pore space during the drying cycle. Thus, maintaining high fluid connectivity within the soil pore space reduces ABA in the root system and promotes growth (Benard et al., 2021). The ABA in S1, S2, and S3 water conditions was slightly lower under N200 than under other N levels, thereby indicating that the root system could increase ABA production despite reduced water conditions. Our result showed that the ABA content in S3 was lower than in S1 and S2 during 2020-2021. The IAA, GAs, and Z+ZR decreased progressively with decreasing water content, as described by Nelissen et al. (2018). On the other hand, the ABA content in N0 treatment remained highest in 2020 or 2021, likely due to a lack of nutrient supply (Figures 4G–I). In contrast, root development under sufficient water and N treatments resulted in a gradual decrease in ABA accumulation in roots, where the S3-N200 and S3-N300 interactions played a significant role in contributing to the decrease in ABA content.
The root sheath is a key factor in plant nutrient uptake and water absorption, as it plays an essential role in enhancing the efficiency of nutrient acquisition from the soil, reflected by the ions that promote root growth (Dong et al., 2023). Under different water conditions, N levels have various effects (promotion or inhibition), not only on K+, Ca2+, and Mg2+, but also on Na+. (Figure 3). From the 2020–2021 mean value, we found no significant difference in N levels between N200 and N300, probably because the water was fully absorbed and utilized. As a result of changing moisture conditions, which enhanced the lateral root and aerial root due to the compensating effect of root growth, S3 was significantly better than S1 and S2. The K+, Ca2+, and Mg2+ in roots were positively correlated with N level, water conditions, and water-N interaction, but there was no significant water-N interaction in Na+. This suggests that it impacts water availability and hydraulic contact (Benard et al., 2019). The root ions possess unique biological properties that may influence the growth of the root system (Wen et al., 2021). These results are consistent with a previous demonstration that osmotically active chemicals are necessary for maintaining the development and growth of crop root cells (Kim et al., 2010).
5.3 Suitable water-N interaction anti-aging senescence of the root system
The maize root accumulates large amount of ROS, inducing a variety of scavengers and non-enzymatic low-molecular-weight metabolites to counteract oxidative damage under different water conditions, and even under water deficit (Mittler et al., 2022). A higher N application contributes to the accumulation of ROS in roots, particularly under a gradual increase in moisture content. Meanwhile, the root contains various organic compounds, including sugars, organic acids, organic chelators, and enzymes, which are secreted by both roots and microorganisms (Chen et al., 2022; Opoku et al., 2022). These compounds play a crucial role in enhancing the mobility of nutrients in the soil and the exchange capacity (Sharma et al., 2021). We found that the O2-, H2O2, and MDA values under S3 in the N200 level were significantly lower than those under the other water conditions during 2020 and 2021, mainly because the root system absorbed large amounts of soil nutrients and moisture. In osmoregulation, substances such as proline, soluble sugar, and soluble protein showed an opposite trend to ROS, whether under the same water conditions or N levels. Therefore, the osmoregulation substance was increased at the S3-N200 treatments. In contrast, when the maize root was treated with S1 and S2 water conditions, the levels of proline, soluble sugar, and soluble protein in N200 and N300 consistently remained at a lower level, which may have been associated with less water, as it accumulates ROS in the root systems. The N may not be fully dissolved under S1 and S2 water conditions; therefore, the root system did not meet the corresponding water and nutrient requirements due to water stress treatment. Compared to other water conditions, the water and nutrients in the roots of S3 were rapidly depleted, forcing the roots to develop incompletely and affecting maize growth (Wang et al., 2018).
Maximum root enzymatic and nonenzymatic antioxidants occurred at S3-N200 treatment in both years. The SOD and CAT were highest in different water-N interaction, followed by APX and GR, and the lowest value was measured in GPX and POD. Previous studies showed that SOD and CAT with different metal cofactors reside in the apoplast, cytosol, chloroplasts, mitochondria, nuclei, and peroxisomes, in which they process O2- to H2O2 and also engage in H2O2 dismutation (Dvořák et al., 2020). These enzymes have been overexpressed individually or in combinations in various plant species, resulting in an enhanced performance under water stress conditions, such as preventing excessive levels of intracellular ROS, catalyzing the decomposition of H2O2 (Gomez et al., 2019). In the present study, the total glutathione during the S3-N200 treatment in 2020 and 2021 were 302.81μmol·g-1 FW and 263.88 μmol·g-1 FW, respectively, and was significantly higher than other water conditions (p < 0.01; Figures 7A–C). Under the same water condition, the N200 treatment resulted in higher GSSG/GSH and ASA/DSH ratios than the N0, N100, and N300 treatments. By contrast, if the same N application, the GSSG/GSH, and ASA/DSH gradually decreased with the decrease of moisture, and the lowest ratio was in S1 water conditions (Figure 7B–D). Thus, limited water availability is one of the most restrictive factors that affect the combined action of enzymatic and non-enzymatic antioxidants that regulating the growth of maize roots (Waltz, 2014). Very often, drought spells are accompanied by N deficiency, which critically exacerbates the adverse drought effects; thus, water was not the limiting factor for maize growth (Wu et al., 2022). S3-N200 and S3-N300 treatments can significantly alleviate root senescence; however, the difference was not significant.
5.4 Optimizing water and N management improves maize productivity
The premise of increasing grain yield is to enhance biomass quality, which is influenced by various factors, including nutrient availability, drought, flooding, and soil erosion. The dry matter accumulation and yield were closely related to the degree of an ideal root structure, to capture the water and N in the soil (Lynch, 2019). Correlation analysis showed that root morphology was significantly correlated with yield (Figure 9A), indicating that water-N interaction influenced maize agronomic traits (Table 2) and BAD (Figure 8). The AMOS structural equation modeling further showed that the water-N interaction can influence yield by affecting root morphology (Figure 9A). Under the S3 water conditions, N200 was most suitable because it promoted the growth and distribution of RL, RLD, root/shoot ratio, root specific surface area, RD, and the number of root tips. Moreover, increasing the root ions and hormones to promote biomass accumulation in the aboveground plant parts increases maize yield (Figure 9B). Therefore, adjusting the root development and distribution by regulating water-N interactions effectively improves water and fertilizer use efficiency, promoting biomass accumulation and increasing grain yield.
5.5 Limitations and implications
This study offers valuable insight into water–nitrogen interaction in maize, but it is limited to a single genotype and specific climatic conditions. Such specificity may restrict the generalizability of the findings to other environments. Additionally, soil biological factors, particularly microbial interactions, were not considered, although they may significantly influence root development and nutrient uptake. The precise water-nitrogen regulation used in this study also involves labor-intensive practices, which could hinder large-scale implementation. Nevertheless, the results highlight the potential of optimized irrigation and nitrogen management to improve productivity, conserve resources, and minimize environmental impacts on semi-arid regions facing water scarcity.
6 Conclusions
Optimizing water and nitrogen input significantly improves maize root architecture, nutrient uptake, and dry matter accumulation. The S3-N200 treatment achieved comparable yield and root performance to S3-N300 while reducing nitrogen use, offering a more resource-efficient and environmentally sustainable alternative. This strategy enhances maize productivity while conserving water and nitrogen, making it particularly suitable for cold semi-arid regions where water availability is declining.
Looking ahead, the future research should build on these findings by conducting multi-genotypic trials across diverse agro-climatic zones in China. Further investigation into soil microbial communities and their interaction with maize under varying water-nitrogen regimes will offer deeper insights into long-term soil health and nutrient cycling. Such integrative approaches will help develop a robust, climate–resilient crop management systems that promote sustainable agriculture and ensures food security in water-limited environments.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YC: Writing – original draft, Formal Analysis, Data curation. IM: Writing – review & editing, Data curation, Formal Analysis. SA: Writing – review & editing, Formal Analysis. MS-J: Writing – review & editing, Investigation. LY: Investigation, Writing – review & editing. XZ: Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was financially supported by the Natural Science Foundation of Guangxi Province (2024GXNSFBA010425 and 2024GXNSFBA010391) and the Science Foundation of China Postdoctoral (2023MD744185).
Acknowledgments
We are grateful to Xunbo Zhou, Li Yang, Mohammad Shah Jahan, and Muhammad Ihsan for their help with the experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1658353/full#supplementary-material
References
Adu, M. O., Asare, P. A., Yawson, D. O., Ackah, F. K., Amoah, K. K., Nyarko, M. A., et al. (2017). Quantifying variations in rhizosheath and root system phenotypes of landraces and improved varieties of juvenile maize. Rhizosphere-Neth. 3, 29–39. doi: 10.1016/j.rhisph.2016.12.004
Ahmad, S., Wang, G.-Y., Muhammad, I., Zeeshan, M., and Zhou, X.-B. (2022). Melatonin and KNO3 application improves growth, physiological and biochemical characteristics of maize seedlings under waterlogging stress conditions. Biology 11 (1), 99.
Aksu, G. and Altay, H. (2020). The effects of potassium applications on drought stress in sugar beet. Sugar Tech. 22, 1092–1102. doi: 10.1007/s12355-020-00851-w
Araus, J. L., Slafer, G. A., Royo, C., and Serret, M. D. (2008). Breeding for yield potential and stress adaptation in cereals. Crit. Rev. Plant Science. 27, 377–412. doi: 10.1080/07352680802467736
Basirat, M., Mousavi, S. M., Abbaszadeh, S., Ebrahimi, M., and Zare-banadkouki, M. (2019). The rhizosheath: a potential root trait helping plants to tolerate drought stress. Plant Soil. 445, 565–575. doi: 10.1007/s11104-019-04334-0
Benard, P., Schepers, J. R., Crosta, M., Zarebanadkouki, M., and Carminati, A. (2021). Physics of viscous bridges in soil biological hotspots. Water Resour. Res. 57, e2021WR030052. doi: 10.1029/2021WR030052
Benard, P., Zarebanadkouki, M., and Carminati, A. (2019). Physics and hydraulics of the rhizosphere network. J. Plant Nutr. Soil Sci. 182, 5–8. doi: 10.1002/jpln.201800042
Chen, Y., Yao, Z., Sun, Y., Wang, E., Tian, C., Sun, Y., et al. (2022). Current studies of the effects of drought stress on root exudates and rhizosphere microbiomes of crop plant species. Int. J. Mol. Sci. 23, 2374. doi: 10.3390/ijms23042374
De-la-Fuente, C. C., Simonin, M., King, E., Moulin, L., Bennett, M. J., Castrillo, G., et al. (2020). An extended root phenotype: the rhizosphere, its formation and impacts on plant fitness. Plant J. 103, 951–964. doi: 10.1111/tpj.14781
Dong, J., Norman, J. V., Žárský, V., and Zhang, Y. (2023). Plant cell polarity: The many facets of sidedness. Plant Physiol. 193, 1–5. doi: 10.1093/plphys/kiad436
Dowd, W. T., Braun, D. M., and Sharp, R. E. (2020). Maize lateral root developmental plasticity induced by mild water stress. II: Genotype-specific spatio-temporal effects on determinate development. Plant Cell Environ. 43, 2409–2427. doi: 10.1111/pce.13840
Dvořák, P., Krasylenko, Y., Ovečka, M., Basheer, J., Zaplová, V., Šamaj, J., et al. (2020). FSD1: a plastidial, nuclear and cytoplasmic enzyme relocalizing to the plasma membrane under salinity. bioRxiv 2020.2003. 2024.005363.
Elhani, S., Haddadi, M., Csákvári, E., Zantar, S., Hamim, A., Villányi, V., et al. (2019). Effects of partial root-zone drying and deficit irrigation on yield, irrigation water-use efficiency and some potato (Solanum tuberosum L.) quality traits under glasshouse conditions. Agric. Water Manage. 224, 105745. doi: 10.1007/s00271-009-0159-y
Erenstein, O., Jaleta, M., Sonder, K., Mottaleb, K., and Prasanna, B. M. (2022). Global maize production, consumption and trade: trends and R&D implications. Food Secur. 14 (5), 1295–1319.
Fenn, M. E., Ross, C. S., Schilling, S. L., Baccus, W. D., Larrabee, M. A., and Lofgren, R. A. (2013). Atmospheric deposition of nitrogen and sulfur and preferential canopy consumption of nitrate in forests of the Pacific Northwest, USA. For. Ecol. Manage. 302, 240–253. doi: 10.1016/j.foreco.2013.03.042
Gomez, R., Vicino, P., Carrillo, N., and Lodeyro, A. F. (2019). Manipulation of oxidative stress responses as a strategy to generate stress-tolerant crops. From damage to signaling to tolerance. Crit. Rev. Biotechnol. 39, 693–708. doi: 10.1080/07388551.2019.1597829
Hu, B. and Chu, C. (2020). Nitrogen-phosphorus interplay: old story with molecular tale. New Phytol. 225, 1455–1460. doi: 10.1111/nph.16102
Jia, Q., Xu, Y., Ali, S., Sun, L., Ding, R., Ren, X., et al. (2018). Strategies of supplemental irrigation and modified planting densities to improve the root growth and lodging resistance of maize (Zea mays L.) under the ridge-furrow rainfall harvesting system. Field Crop Res. 224, 48–59. doi: 10.1016/j.fcr.2018.04.011
Kim, T. H., Böhmer, M., Hu, H., Nishimura, N., and Schroeder, J. I. (2010). Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61, 561–591. doi: 10.1146/annurev-arplant-042809-112226
Liu, Z., Zhu, K., Dong, S., Liu, P., Zhao, B., and Zhang, J. (2017). Effects of integrated agronomic practices management on root growth and development of summer maize. Euro. J. Agron. 84, 140–151. doi: 10.1016/j.eja.2016.12.006
Lynch, J. P. (2015). Root phenes that reduce the metabolic costs of soil exploration: Opportunities for 21st century agriculture. Plant Cell Environ. 38, 1775–1784. doi: 10.1111/pce.12451
Lynch, J. P. (2019). Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytol. 223 (2), 548–564.
Ma, Q., Zhang, F., Rengel, Z., and Shen, J. (2013). Localized application of NH4+-N plus P at the seedling and later growth stages enhances nutrient uptake and maize yield by inducing lateral root proliferation. Plant Soil. 372 (1), 65–80
Maggio, A., Zhu, J. K., Hasegawa, P. M., and Bressan, R. A. (2006). Osmogenetics: aristotle to arabidopsis. Plant Cell. 18, 1542–1557. doi: 10.1105/tpc.105.040501
Mittler, R., Zandalinas, S. I., Fichman, Y., and Breusegem, F. V. (2022). Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Bio. 23, 663–679. doi: 10.1038/s41580-022-00499-2
Muhammad, I., Fahad, S., Khalofah, A., Zheng, B., and Shen, W. (2025). Melatonin enhances antioxidant defense systems and stress tolerance in plants under variable environmental conditions. Rice. 18, 70. doi: 10.1186/s12284-025-00825-0
Muhammad, I., Yang, L., Ahmad, S., Farooq, S., Khan, A., Zeeshan, M., et al. (2022a). Low irrigation water improves biomass saccharification, photosynthetic pigments of maize, and minimizes nitrate nitrogen leaching. J. Plant Nutr. Soil Sci. 22, 4897–4912. doi: 10.1007/s42729-022-00969-8
Muhammad, I., Yang, L., Ahmad, S., Mosaad, I. S. M., Al-Ghamdi, A. A., Abbasi, A. M., et al. (2022b). Melatonin application alleviates stress-induced photosynthetic inhibition and oxidative damage by regulating antioxidant defense system of maize: a meta-analysis. Antioxidants. 11, 512. doi: 10.3390/antiox11030512
Muhammad, I., Yang, L., Ahmad, S., Farooq, S., Khan, A., Muhammad, N., et al. (2023). Melatonin-priming enhances maize seedling drought tolerance by regulating the antioxidant defense system. Plant Physiol. 191 (4), 2301–2315.
Nelissen, H., Sun, X. H., Rymen, B., Jikumaru, Y., Kojima, M., Takebayashi, Y., et al. (2018). The reduction in maize leaf growth under mild drought affects the transition between cell division and cell expansion and cannot be restored by elevated gibberellic acid levels. Plant Biotechnol. J. 16, 615–627. doi: 10.1111/pbi.12801
Ng, C., Wang, Z., Leung, A., and Ni, J. (2019). A study on effects of leaf and root characteristics on plant root water uptake. Géotechnique. 69 (2), 151–157.
Opoku, V. A., Yawson, D. O., Asare, P. A., Afutu, E., Kotochi, M. C., Amoah, K. K., et al. (2022). Root hair and rhizosheath traits contribute to genetic variation and phosphorus use efficiency in cowpea (Vigna unguiculata L. Walp). Rhizosphere. 21, 100463. doi: 10.1016/j.rhisph.2021.100463
Ortega, J. K. E. (2010). Plant cell growth in tissue. Physiol. Plantarum. 154, 1244–1253. doi: 10.1104/pp.110.162644
Osakabe, Y., Kajita, S., and Osakabe, K. (2011). Genetic engineering of woody plants: Current and future targets in a stressful environment. Physiol. Plantarum. 142, 105–117. doi: 10.1111/j.1399-3054.2011.01451.x
Qi, W.-Z., Liu, H.-H., Liu, P., Dong, S.-T., Zhao, B.-Q., So, H. B., et al. (2012). Morphological and physiological characteristics of corn (Zea mays L.) roots from cultivars with different yield potentials. Eur. J. Agron. 38, 54–63.
Roelfsema, M. R., Hedrich, R., and Geiger, D. (2012). Anion channels: Master switches of stress responses. Trends Plant Sci. 17, 221–229. doi: 10.1016/j.tplants.2012.01.009
Schlüter, U., Mascher, M., Colmsee, C., Scholz, U., Bräutigam, A., Fahnenstich, H., et al. (2012). Maize source leaf adaptation to nitrogen deficiency affects not only nitrogen and carbon metabolism but also control of phosphate homeostasis. Plant Physiol. 160, 1384–1406. doi: 10.1104/pp.112.204420
Schneider, H. M., Postma, J. A., Kochs, J., Daniel, P., Lynch, J. P., and Van, D. D. (2020). Spatio-temporal variation in water uptake in seminal and nodal root systems of barley plants grown in soil. Front. Plant Sci. 11, 1–13. doi: 10.3389/fpls.2020.01247
Sharma, M., Pang, J., Wen, Z., De-Borda, A., Kim, H. S., Liu, Y., et al. (2021). A significant increase in rhizosheath carboxylates and greater specific root length in response to terminal drought is associated with greater relative phosphorus acquisition in chickpea. Plant Soil. 460, 51–68. doi: 10.1007/s11104-020-04776-x
Shi, J., Wu, X., Wang, X., Zhang, M., Han, L., Zhang, W., et al. (2020). Determining threshold values for root-soil water weighted plant water deficit index based smart irrigation. Agric. Water Manage. 230, 105979. doi: 10.1016/j.agwat.2019.105979
Su, W., Liu, B., Liu, X. W., Li, X. K., Ren, T., Cong, R. H., et al. (2015). Effect of depth of fertilizer banded-placement on growth, nutrient uptake and yield of oilseed rape (Brassica napus L.). Euro. J. Agron. 62, 38–45. doi: 10.1016/j.eja.2014.09.002
Sun, G., Dong, Z., Li, G., Yuan, H., Liu, J., Yao, X., et al. (2023). Mn3o4 nanoparticles alleviate ROS-inhibited root apex mitosis activities to improve maize drought tolerance. Adv. Biol. 7 (7), 2200317. doi: 10.1002/adbi.202200317
Voothuluru, P., Mäkelä, P., Zhu, J. M., Yamaguchi, M., Cho, I.-J., Oliver, M. J., et al. (2020). Apoplastic hydrogen peroxide in the growth zone of the maize primary root. increased levels differentially modulate root elongation under well-watered and water-stressed conditions. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00392
Wang, F. B., Liu, J. C., Zhou, L. J., Pan, G., Li, Z. W., Zaidi, S. H. R., et al. (2016). Senescence-specific change in ROS scavenging enzyme activities and regulation of various SOD isozymes to ROS levels in psf mutant rice leaves. Plant Physiol. Bioch. 109, 248–261. doi: 10.1016/j.plaphy.2016.10.005
Wang, J., Wen, X. F., Zhang, X. Y., Li, S. G., and Zhang, D. Y. (2018). Co-regulation of photosynthetic capacity by nitrogen, phosphorus and magnesium in a subtropical Karst Forest in China. Sci. Rer-UK. 8, 7406. doi: 10.1038/s41598-018-25839-1
Wang, Y., Zhang, X. Y., Chen, J., Chen, A. J., Wang, L. Y., Guo, X. Y., et al. (2019). Reducing basal nitrogen rate to improve maize seedling growth, water and nitrogen use efficiencies under drought stress by optimizing root morphology and distribution. Agric. Water Manage. 212, 328–337. doi: 10.1016/j.agwat.2018.09.010
Wen, Z., Pang, J., Ryan, M. H., Shen, J., Siddique, K. H., and Lambers, H. (2021). In addition to foliar manganese concentration, both iron and zinc provide proxies for rhizosheath carboxylates in chickpea under low phosphorus supply. Plant Soil. 465, 31–46. doi: 10.1007/s11104-021-04988-9
Wu, P., Liu, F., Li, H., Wang, J. Y., Liu, Y. H., Gao, Y., et al. (2022). Suitable fertilization depth can improve the water productivity and maize yield by regulating development of the root system. Agric. Water Manage. 271, 107784. doi: 10.1016/j.agwat.2022.107784
Xia, Z. Q., Si, L. Y., Jin, Y., Fu, Y. F., Wang, Q., and Lu, H. D. (2021a). Effects of root zone temperature increase on physiological indexes and photosynthesis of different genotype maize seedlings. Russ. J. Plant Physiol. 68, 169–178. doi: 10.1134/S1021443721010180
Xia, Z. Q., Zhang, G. X., Zhang, S. B., Wang, Q., Fu, Y. F., and Lu, H. D. (2021b). Efficacy of root zone temperature increase in root and shoot development and hormone changes in different maize genotypes. Agriculture-Basel. 11, 477. doi: 10.3390/agriculture11060477
Xia, H. Y., Zhao, J. H., Sun, J. H., Bao, G. X., Christie, P., Zhang, F. S., et al. (2013). Dynamics of root length and distribution and shoot biomass of maize as affected by intercropping with different companion crops and phosphorus application rates. Field Crops Res. 150, 52–62. doi: 10.1016/j.fcr.2013.05.027
Yamaguchi-Shinozaki, K. and Shinozaki, K. (2006). Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57(1), 781–803. doi: 10.1146/annurev.arplant.57.032905.105444
York, L. M., Nord, E. A., and Lynch, J. P. (2013). Integration of root phenes for soil resource acquisition Integration of root phenes for soil resource acquisition. Front. Plant Sci. 4, 355. doi: 10.3389/fpls.2013.00355
Zhang, W., Li, S. Q., Shen, Y. F., and Yue, S. C. (2021). Film mulching affects root growth and function in dryland maize-soybean intercropping. Field Crops Res. 271, 108240. doi: 10.1016/j.fcr.2021.108240
Keywords: maize, enzymes, crop yield, nitrogen, root development, water stress
Citation: Chi YX, Muhammad I, Ali S, Shah Jahan M, Yang L and Zhou XB (2025) Optimizing water and nitrogen management improves maize productivity by regulating root development in the cold semi-arid Songnen plains of Northeast China. Front. Plant Sci. 16:1658353. doi: 10.3389/fpls.2025.1658353
Received: 03 July 2025; Accepted: 29 August 2025;
Published: 19 September 2025.
Edited by:
Asif Ameen, Ayub Agriculture Research Institute, PakistanReviewed by:
Sanjeev Kumar, National Dairy Research Institute (ICAR), IndiaJihong Zhang, Shihezi University, China
Copyright © 2025 Chi, Muhammad, Ali, Shah Jahan, Yang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Yang, bTE4NzQ2NjEwNTcxQDE2My5jb20=
 Yu Xin Chi
Yu Xin Chi Ihsan Muhammad
Ihsan Muhammad Shahid Ali
Shahid Ali Mohammad Shah Jahan
Mohammad Shah Jahan Li Yang
Li Yang Xun Bo Zhou
Xun Bo Zhou