- Key Laboratory of Plant Secondary Metabolism and Regulation of Zhejiang Province, College of Life Sciences and Medicine, Zhejiang Sci-Tech University, Hangzhou, China
The capsid (Cap) protein encoded by the ORF2 gene of porcine circovirus type 2 is the major immunogen for the development of vaccines and can effectively reduce the incidence of porcine circovirus-associated diseases. In order to explore an efficient expression pathway of the recombinant Cap protein, using the Chlamydomonas reinhardtii chloroplast as the expression platform of the Cap protein, C. reinhardtii chloroplast expression vector pCR02 of the optimized ORF2 gene was constructed and transferred into the C. reinhardtii chloroplast using the biolistic bombardment method. After multiple rounds of resistance screening and culturing, PCR, RT-PCR, Western blotting, and ELISA were used to detect the ORF2 gene and the expression of the Cap protein in C. reinhardtii transformants. The results of the study showed that the ORF2 antigen gene had been correctly integrated into the specific site of the C. reinhardtii chloroplast genome, and the Cap protein was effectively expressed. These results suggested that the C. reinhardtii chloroplast is a potential platform for the production of recombinant antigen proteins and provided important support for exploring new production pathways of recombinant pharmaceutical proteins.
1 Introduction
Porcine circovirus type 2 (PCV2), a member of the Circoviridae family, is the main pathogen of post-weaning multisystemic wasting syndrome (PMWS). PCV2 is not only related to a series of diseases, such as porcine respiratory disease complex and porcine dermatitis, but also susceptible to co-infection with other pathogens (Segalés et al., 2004; Opriessnig et al., 2007; Ramamoorthy and Meng, 2009; Alarcon et al., 2014; Lv et al., 2014), which has caused great economic losses to the swine industry around the world (Gillespie et al., 2009; Chi et al., 2014; Zhai et al., 2014). Since management strategies and co-infection control show limited efficacy in reducing porcine circovirus-associated disease (PCVAD) incidence, vaccination remains the most effective approach for preventing PCV2 infection (Chae, 2012). The immunogenic capsid (Cap) protein encoded by the ORF2 gene is the target antigen of commercial vaccines due to its multiple antigenic epitopes (Cheung, 2003; Khayat et al., 2011; Zhang et al., 2015; Gava et al., 2018). Recombinant Cap proteins have been expressed in the Escherichia coli expression system (Wu et al., 2016), the yeast expression system (Bucarey et al., 2009; Tu et al., 2013), and the baculovirus/insect cell expression system (Liu et al., 2015; López-Vidal et al., 2015), and several baculovirus/insect cell-expressed proteins have successfully entered the marketing stage (Chae, 2012). However, the high production costs remain a significant bottleneck in expanding the application of the Cap antigen protein (Alarcon et al., 2014; Chi et al., 2014).
In recent years, the expression of pharmaceutical proteins in plant chloroplasts has become a research hotspot in plant biotechnology. Compared with the nucleus expression system, the chloroplast expression system has some unique advantages. For example, there is no gene silencing phenomenon and mechanism that inhibits the expression of recombinant proteins (Bock, 2007); the exogenous genes do not escape with the transmission of pollen, which contributes to improving bio-safety (Daniell, 2007). Chlamydomonas reinhardtii, also known as the “green yeast”, is the lower model plant in chloroplast genetic transformation research (Rochaix, 1995). Its growth cycle is short and culture is simple, which greatly reduces the production costs (Rochaix, 1995; Specht et al., 2010; Rasala and Mayfield, 2015). In addition, C. reinhardtii has a single large chloroplast, which contains many molecular chaperones and foldable enzymes (disulfide isomerase, proline isomerase, etc.) and provides a certain guarantee for the correct folding and assembly of recombinant proteins (Tran et al., 2013). At present, several pharmaceutical proteins have been successfully expressed in C. reinhardtii chloroplasts. In 2015, Barrera et al. (2015) expressed antitoxin drugs containing different domains of camel VHH antitoxin that can neutralize botulinum neurotoxin; in 2016, Ochoa-Méndez et al. (2016) constructed the expression vector of an anti-hypertensive drug (AHD) and successfully expressed it in the C. reinhardtii chloroplast; feeding results in mice with severe hypertension also showed that the drug can significantly decrease blood pressure. However, to date, there are a few reports on recombinant proteins expressed in the algal chloroplast (You-hong et al., 2017). It is unclear if this is due to a few attempts or to limitations of the system that preclude the expression of many proteins. In this study, we transformed the optimized ORF2 gene of PCV2 into the chloroplast genome of C. reinhardtii by biolistic bombardment and expressed it to assess the capacity of transgenic algae as a recombinant protein production platform.
2 Materials and methods
2.1 Algal strains and materials
The wild-type C. reinhardtii strain is CC-137. Wild-type strains were cultured in 50 mL liquid Tris–acetate–phosphate (TAP) medium at 160 rpm and 25 °C under a photoperiod of 12 h/12 h (Harris, 1989). The precipitates were collected from 1.5 mL algal fluid of the logarithmic growth stage (3,000 rpm, 5 min), then suspended and coated on solid medium, and used for biolistic bombardment transformation after being cultured for 3–4 days.
Plasmids patpX (approximately 3.95 kb) and p64D (approximately 9.8 kb) were preserved in our laboratory. patpX contains a group of polyclonal sites and carries the 5′ promoter (including 5′-UTR) of the atpA gene and the 3′ terminator of the rbcL gene, which were the C. reinhardtii chloroplast genomic endogenous genes. Plasmid p64D contains the aadA resistance gene cassette and the homologous recombinant fragment (clpP–trnL–petB–chlL–rpl23–rpl2).
2.2 Optimization and synthesis of ORF2 gene
In order to improve the expression efficiency of the major antigen gene-ORF2 (GenBank: AY035820.1) of PCV2, the ORF2 gene sequence was optimized and synthesized according to the preference of the chloroplast genome of C. reinhardtii by GenScript (Nanjing) Co., Ltd, Nanjing China. The NcoI and XbaI restriction sites were added at the 5′- and 3′-terminals of the codon-optimized ORF2 sequence, respectively, to facilitate the construction of a chloroplast expression vector.
2.3 Construction of C. reinhardtii chloroplast expression vector
Plasmid patpX and the optimized ORF2 gene were both excised by digestion with NcoI and XbaI, and the ORF2 gene was ligated to the large fragment of patpX. The ORF2 coding region was located between the chloroplast-specific promoter PatpA, 5’-UTR of atpA and the terminator of rbcL. ORF2 gene expression cassette was obtained from the chloroplast expression intermediate vector pAF25. Plasmid pAF25 was excised by digestion with EcoRV and NotI, and then the smaller fragment (approximately 1.8 kb) was blunted with the Klenow large fragment enzyme and subcloned into p64D plasmid, which was excised with EcoRV. The specific C. reinhardtii chloroplast expression vector pCR02 of the ORF2 gene was constructed.
2.4 C. reinhardtii chloroplast transformation and resistance screening
Gold particles coated with plasmid pCR02 were bombarded into the wild-type C. reinhardtii using the biolistic device PDS100/He (Bio-Rad, California, USA) (Kindle et al., 1991). After being cultured in darkness for 12 h, the bombarded C. reinhardtii cells were washed with TAP liquid medium and distributed on TAP solid medium containing 100 μg/mL spectinomycin. Under the conditions of a photoperiod of 12 h/12 h and 25 °C for approximately 10–14 days, the cells of C. reinhardtii without resistance gradually whitened and died, and the resistant cells grew into single green colonies on the solid selective medium. The single colonies of C. reinhardtii were selected and cultured in liquid selective medium (containing 100 μg/mL spectinomycin) for PCR analysis.
2.5 PCR detection of resistant algal cells
The total DNA of resistant algal cells and the wild-type strain was extracted using the cetyltrimethylammonium bromide (CTAB) method. Specific primers P1 (5′-CCATGGCTATGACTTATCCAC-3′) and P2 (5′-TCTAGATTATTTTGGATTTAATGGT-3′) were designed to detect the ORF2 gene in resistant algal cells. Plasmid pCR02 was used as the positive control and wild-type C. reinhardtii as the negative control. The PCR procedures were as follows: 98 °C 3 min, 98 °C 10 s, 50 °C 10 s, 72 °C 15 s, and 72 °C 2 min, 35 cycles.
In addition, the integration site of the ORF2 gene cassette in the C. reinhardtii chloroplast genome was detected using primers P3 (5′-CCGAACAATGTTTTTATTCCTGGAG-3′) and P4 (5′-TTCGAAAGCTGTACCTAAACCTACA-3′), which are complementary to the ORF2 gene and chlL gene of the chloroplast genome of C. reinhardtii, respectively. Plasmid pCR02 was used as the positive control and wild-type C. reinhardtii as the negative control. PCR was performed as follows: 94 °C 5 min, 94 °C 30 s, 55 °C 30 s, 72 °C 2 min, and 72 °C 5 min, 35 cycles.
2.6 RT-PCR analysis of the transgenic algal cells
The total RNA of transgenic algal cells and wild-type C. reinhardtii was prepared to detect the transcription level of the ORF2 gene using a total RNA extraction kit [Tiangen Biotech (Beijing) Co., Ltd., Beijing, China]. Total RNA (1 μg) was used to produce the cDNA of each line with the PrimeScript™ RT reagent kit with gDNA Eraser (Perfect Real Time, Takara, Dalian, China), and the RT-PCR detection of cDNA was performed using ORF2-specific primers P1 and P2 under the following conditions: 94 °C 5 min, 94 °C 30 s, 55 °C 30 s, 72 °C 1 min, and 72 °C 5 min, 35 cycles.
2.7 SDS–PAGE and Western blotting analysis
Crude proteins of C. reinhardtii transformants and the wild-type strain were extracted as described previously (Franklin et al., 2002). Liquid algal cells (15 mL) at the logarithmic growth phase were collected through centrifugation. The precipitate was suspended in 200 μL protein lysate (750 mM Tris–HCl, pH 8.0, 15% sucrose, 100 mM β-mercaptoethanol, and 1 mM Phenylmethanesulfonyl Fluoride (PMSF)) and centrifuged for 20 min at 12,000 r/min and 4 °C. The supernatants were composed of total soluble protein (TSP) and used for Cap protein analysis. Protein concentrations were determined using the Bradford protein assay. Approximately 10 μg total soluble protein from each sample was boiled for 5 min and loaded in the wells of a 12% polyacrylamide gel.
Protein samples were separated by 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS–PAGE) and directly transferred onto a polyvinylidene fluoride (PVDF) membrane (300 mA, 1 h). The membrane was then saturated in 5% skim milk powder for 2 h. After washing with tris-buffered saline with tween 20 (TBST), the membrane was successively incubated with rabbit anti-PCV2 Cap polyclonal antibody (1:10,000) (Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) at 4 °C overnight and then incubated with 1:5,000 diluted goat anti-rabbit IgG antibody for 1–2 h at room temperature. The target protein was visualized by adding Enhanced Chemiluminescence (ECL) chemiluminescence chromogenic solution (Yeasen Biotech Co., Ltd., Shanghai China).
2.8 ELISA analysis
The antigenicity of the Cap protein in the crude extract of C. reinhardtii transformants was determined using ELISA. The extraction of protein samples was the same as above. Crude protein samples were added to a 96-well microtiter plate that had been coated with PCV2 Cap antibody to react at 37 °C for 30 min, washed with PBST, and treated with Horseradish Peroxidase (HRP)–PCV2 Cap antibody at 37 °C for 30 min. Then, the color solution was added and incubated at 37 °C for 15 min, and the absorbance was monitored at A450 after termination of the color reaction.
3 Results
3.1 Construction of the C. reinhardtii chloroplast expression vector
The ORF2 gene sequence was optimized by GenScript (Nanjing) Co., Ltd., according to the preference of chloroplast gene expression in C. reinhardtii. A fragment of the ORF2 gene expression cassette was excised from pAF25 with EcoRV and NotI, blunted with the Klenow large fragment enzyme, and then inserted into the EcoRV site of vector p64D to construct C. reinhardtii chloroplast expression vector pCR02. The construction process is shown in Figure 1.
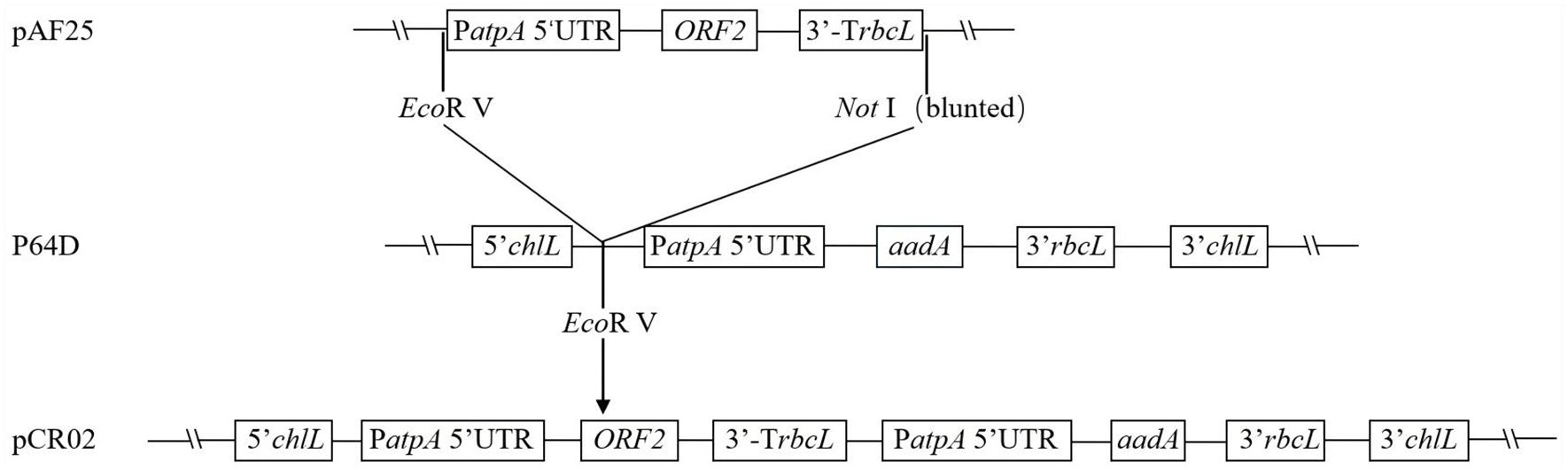
Figure 1. Construction of chloroplast expression vector pCR02. The pCR02 vector contains the optimized ORF2 antigen gene cassette, aadA resistance gene cassette, and the homologous fragment (clpP–trnL–petB–chlL–rpl23–rpl2). The ORF2 antigen gene is flanked by Chlamydomonas reinhardtii chloroplast-specific regulatory elements PatpA and TrbcL.
3.2 Acquisition of resistant algal cells
Wild-type C. reinhardtii cells were bombarded with gold particles coated with plasmid pCR02 using the biolistic PDS1000/He (Bio-Rad) system (1,100 psi) and cultured on the TAP solid medium containing 100 μg/mL spectinomycin. After 10–14 days, 13 resistant green algal colonies appeared on the plates, while most algal cells were albino and died (Figure 2). This indicated that the ORF2 gene and the aadA resistance gene may have been introduced into the chloroplast of C. reinhardtii, and the resistance gene has been expressed.
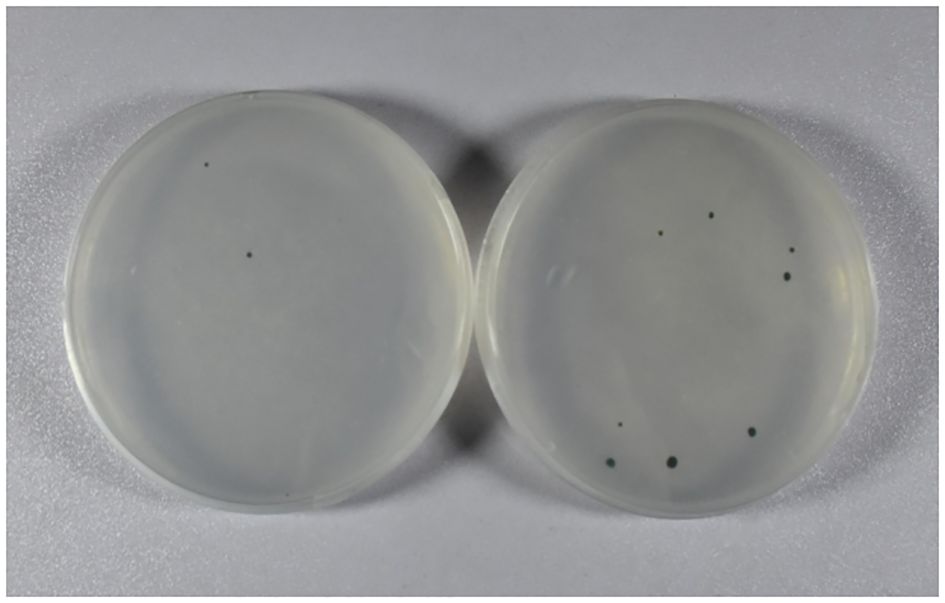
Figure 2. Acquisition of resistant algal cells. Resistant green algal colonies were obtained using the TAP solid medium containing 100 μg/mL spectinomycin. The other three resistant green algal colonies are not shown. TAP, Tris–acetate–phosphate.
3.3 PCR analysis of resistant algal cells
The total DNA of wild-type and resistant algal cells was extracted and detected using specific primers P1 and P2 of the ORF2 gene. As shown in Figure 3A, the expected ORF2 fragment, the 0.75-kb PCR product, was detected in four resistant algal cells and the positive control, but not in the wild-type algal strain. This indicated that the ORF2 gene may be integrated into the chloroplast genome of C. reinhardtii.
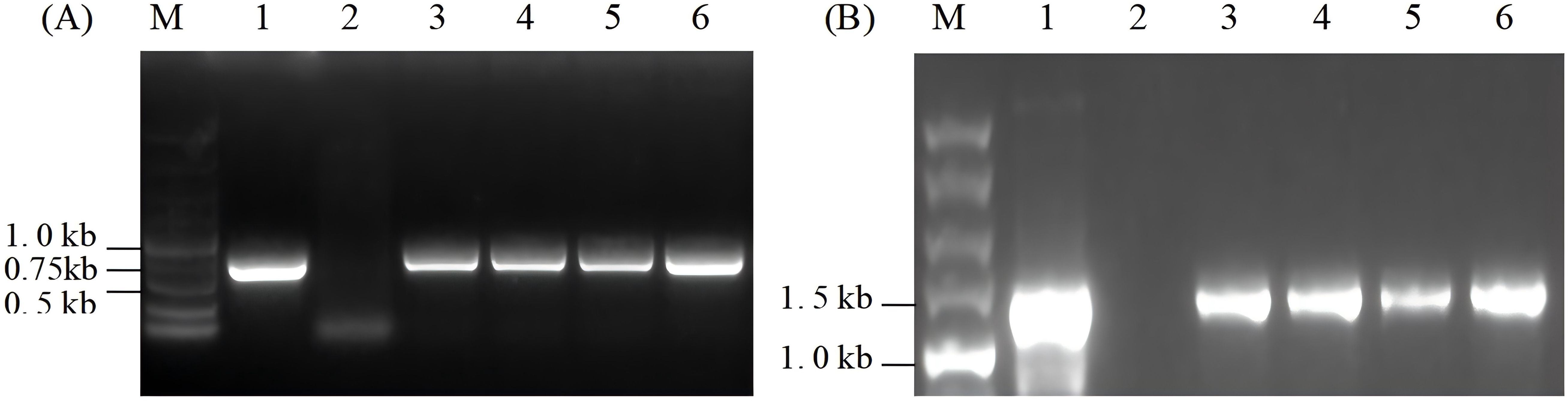
Figure 3. PCR analysis of resistant algal cells. (A) PCR detection of ORF2 gene in resistant algal transformants. (B) Integration site analysis. Lane M, 5.0-kb DNA marker; Lane 1, the positive control; Lane 2, wild-type algal cells; Lanes 3–6, transformants of Chlamydomonas reinhardtii.
To verify whether the ORF2 gene was correctly integrated into the specific site of the chloroplast genome, primers P3 and P4, which are complementary to the chloroplast chlL gene and ORF2 gene, respectively, were designed to detect the insertion site. Approximately 1.4-kb products were expected in algal transformants and the positive control, but no corresponding size product appeared in the wild-type. As shown in Figure 3B, 1.4-kb fragments were obtained in algal transformants that had undergone seven rounds of spectinomycin resistance screening, but did not appear in wild-type algal cells. The results suggested that the exogenous ORF2 gene had been site-specifically integrated into the chloroplast genome of C. reinhardtii.
3.4 Transcription level assay of the ORF2 gene in transgenic C. reinhardtii
The transcription level of the ORF2 gene was determined through RT-PCR of the cDNA using specific primers P1 and P2 of the ORF2 gene. As indicated in Figure 4A, the single 0.5-kb band in transgenic algal transformants displayed normal transcriptional status, whereas no band was amplified in wild-type algal cells. In addition, total RNA was directly used for PCR amplification to remove the samples that were possibly contaminated with DNA. The results showed that no bands were found in the same system and conditions, thus verifying the specificity of the RT-PCR (Figure 4B).
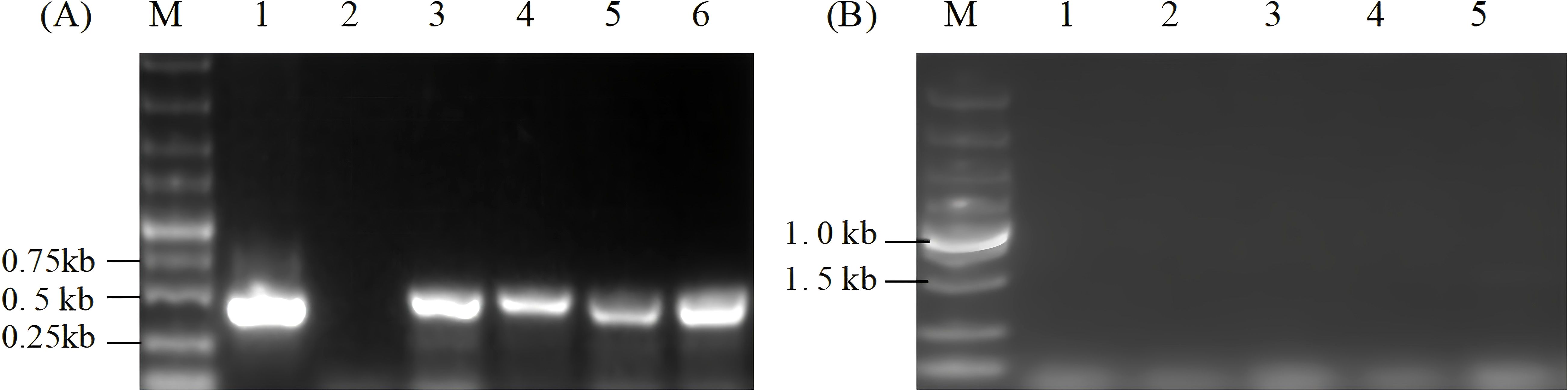
Figure 4. RT-PCR analysis of the ORF2 gene in transgenic Chlamydomonas reinhardtii of resistant algal cells. (A) RT-PCR was performed using ORF2-specific primers P1 and P2. Lane M, 5.0-kb DNA markers; Lane 1, plasmid pCR02; Lane 2, cDNA of wild-type C. reinhardtii; Lanes 3–6, cDNA of independent transgenic C. reinhardtii. (B) RT-PCR analysis using total RNA without reverse transcription as templates. Lane M, 5-kb DNA marker; Lane 1, total RNA of wild-type C. reinhardtii; Lanes 2–5, total RNA of algal transformants.
3.5 Western blotting analysis
To verify the expression of the Cap protein in transgenic algal transformants, total soluble protein of transformants and wild-type C. reinhardtii were extracted and analyzed using SDS–PAGE and Western blotting. Rabbit anti-PCV2 Cap polyclonal antibody and goat anti-rabbit IgG antibody were used for hybridization. As shown in Figure 5, the 28-kDa protein was only detected in four C. reinhardtii transformants, but no protein was detected in the wild-type strain, which indicates that the Cap protein had accumulated in the chloroplast of C. reinhardtii.
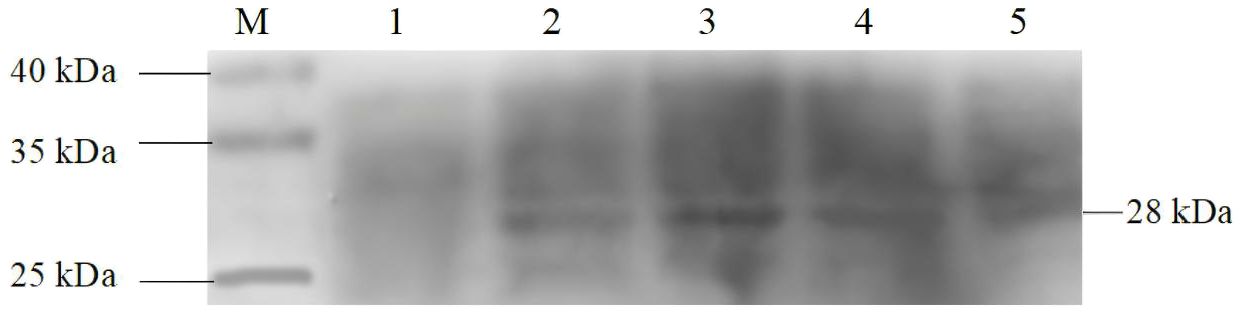
Figure 5. Western blotting analysis of the Cap protein in transgenic Chlamydomonas reinhardtii. Lane M, 10–250-kDa protein markers; Lane 1, wild-type C. reinhardtii; Lanes 2–5, C. reinhardtii transformants.
3.6 ELISA analysis
The antigenicity of the Cap protein expressed in C. reinhardtii chloroplasts was detected using ELISA. Total soluble protein samples extracted from wild-type and transgenic C. reinhardtii were reacted with PCV2 Cap antibody and HRP-conjugated PCV2 Cap antibody, and the absorbance was monitored at OD450. The results showed that significant color reactions were observed in four C. reinhardtii transformants (Figure 6A) and that absorption values were all detected (Figure 6B), indicating that the Cap protein expressed in C. reinhardtii chloroplasts had antigenicity.
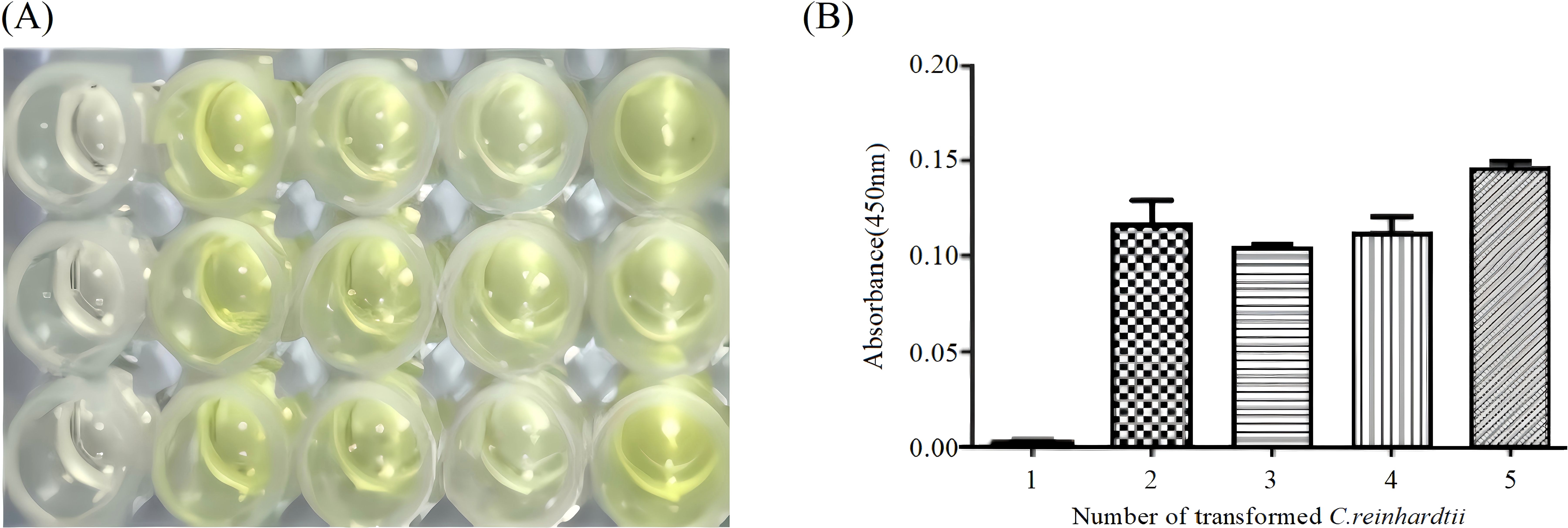
Figure 6. ELISA assay of Cap protein in Chlamydomonas reinhardtii transformants. (A) Color reaction of the Cap protein in four C. reinhardtii transformants and wild-type C. reinhardtii. Lane 1, wild-type C. reinhardtii; Lanes 2–5, C. reinhardtii transformants. (B) Absorbance of the Cap protein in four independent transgenic algal transformants and wild-type C. reinhardtii. Lane 1, wild-type C. reinhardtii; Lanes 2–5, C. reinhardtii transformants.
4 Discussion
Although the Cap antigen vaccine expressed in the insect/baculovirus system is available, the production of genetically engineered vaccines in C. reinhardtii has received much attention recently due to several advantages: low production cost, the product enables oral delivery without purification, and both eukaryotic and prokaryotic proteins can be expressed in C. reinhardtii chloroplasts (Rasala and Mayfield, 2015). In this study, the optimized ORF2 gene was recombined and expressed in the chloroplast of C. reinhardtii. The results showed that the Cap protein had been successfully expressed and had antigenicity, which proved that the C. reinhardtii chloroplast is a feasible expression platform for important recombinant proteins.
At present, the expression levels of exogenous proteins expressed in Chlamydomonas chloroplasts are generally low (Mayfield and Franklin, 2005; Liang et al., 2022; Jiang et al., 2025). The early-expressed β-glucuronidase (GUS) (Ishikura et al., 1999) and Renilla luciferase reporter proteins (Minko et al., 1999) in the chloroplasts of C. reinhardtii were almost undetectable in high endogenous protein backgrounds. There are several factors that may affect the expression of exogenous gene and protein accumulation in the C. reinhardtii chloroplast, including the promoter, 5′-UTR, and 3′-UTR, and optimization of the gene according to the preference of the chloroplast genome of C. reinhardtii. The transcription and expression of chloroplast genes are mainly determined using the promoter and 5′-UTR. Endogenous promoters from the C. reinhardtii chloroplast genome are required for the transcription and expression of exogenous genes (You-hong et al., 2017). The promoters of chloroplast endogenous genes, such as atpA, psbA, and psbD, were usually used to regulate the expression of foreign genes (Ishikura et al., 1999). Ishikura et al. (1999) expressed the unid gene in C. reinhardtii chloroplasts using the promoters of rbcL, psbA, and atpA genes and found that the atpA promoter had the best effect on the expression of the unid gene, while the promoter of psbA was almost ineffective. Michelet et al. (2011) found that the psaA-exon1 promoter was stronger than atpA and psbA in regulating the expression of vap A and acr V genes. The atpA promoter was examined and effectively regulated the transcription and expression of the ORF2 gene in C. reinhardtii chloroplasts in this study. Other regulatory factors, such as 5′-UTRs of endogenous genes from the C. reinhardtii chloroplast genome, also affect the expression of foreign genes (Kasai et al., 2003; Barnes et al., 2005). Barnes et al. (2005) studied the effects of 5′-UTR and 3′-UTR of different genes on gfp gene expression and found that the 5′-UTR has a greater impact on the accumulation of exogenous proteins and mRNA than the 3′-UTR, which is almost ineffective on mRNA and protein accumulation. The 5′-UTR, including the promoter, of the plastid atpA and psbD genes produced the highest levels of chimeric mRNA and protein accumulation, while the 5′-UTR of the rbcL and psbA genes produced less mRNA and protein. Here, we show that the 5′-UTR of the atpA gene fusing with the atpA promoter can effectively drive mRNA and protein accumulation in C. reinhardtii chloroplasts. The C. reinhardtii chloroplast genome shows a high adenine and thymidine content as much as 66.3% and 80% of the third nucleotide of the codon including adenine or thymidine. It was reported that the expression level of GUS structural gene (uidA) (Ishikura et al., 1999) and Renilla luciferase reporter genes (Minko et al., 1999) in Chlamydomonas chloroplasts is almost undetectable under a high endogenous protein background. Mayfield et al. (Franklin and Mayfield, 2004; Mayfield and Franklin, 2005) suggested that the preference for codons in the chloroplast genome of C. reinhardtii may be one of the reasons for the low expression of exogenous genes in C. reinhardtii chloroplasts. To achieve protein expression, the sequence of the ORF2 gene was optimized according to the preference of the C. reinhardtii chloroplast genome, and the Cap protein was expressed effectively.
Data availability statement
The original contributions presented in the study are publiclyavailable at the Jianguo cloud, which include the original images and date of ELISA. https://www.jianguoyun.com/p/DYT3digQ1NPfDRjfi4oGIAA.
Author contributions
YS: Writing – original draft, Validation, Formal analysis, Visualization. YLin: Validation, Writing – original draft, Visualization, Formal analysis. XC: Writing – review & editing. WL: Writing – review & editing. YLi: Writing – review & editing. ZY: Methodology, Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (No. 31570370) and the Natural Science Foundation of Zhejiang Province (No. LY15C020005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1661360/full#supplementary-material
References
Alarcon, P., Wieland, B., Mateus, A. L., and Dewberry, C. (2014). Pig farmers’ perceptions, attitudes, influences and management of information in the decision-making process for disease control. Prev. Vet. Med. 116, 223–242. doi: 10.1016/j.prevetmed.2013.08.004
Barnes, D., Franklin, S., Schultz, J., Henry, R., Brown, E., Coragliotti, A., et al. (2005). Contribution of 5’- and 3’-untranslated regions of plastid mRNAs to the expression of Chlamydomonas reinhardtii chloroplast genes. Mol. Genet. Genomics 274, 625–636. doi: 10.1007/s00438-005-0055-y
Barrera, D. J., Rosenberg, J. N., Chiu, J. G., Chang, Y. N., Debatis, M., Ngoi, S. M., et al. (2015). Algal chloroplast produced camelid VH H antitoxins are capable of neutralizing botulinum neurotoxin. Plant Biotechnol. J. 13, 117–124. doi: 10.1111/pbi.12244
Bock, R. (2007). Plastid biotechnology: prospects for herbicide and insect resistance, metabolic engineering and molecular farming. Curr. Opin. Biotechnol. 18, 100–106. doi: 10.1016/j.copbio.2006.12.001
Bucarey, S. A., Noriega, J., Reyes, P., Tapia, C., Sáenz, L., Zuñiga, A., et al. (2009). The optimized capsid gene of porcine circovirus type 2 expressed in yeast forms virus-like particles and elicits antibody responses in mice fed with recombinant yeast extracts. Vaccine 27, 5781–5790. doi: 10.1016/j.vaccine.2009.07.061
Chae, C. (2012). Commercial porcine circovirus type 2 vaccines: efficacy and clinical application. Vet. J. 194, 151–157. doi: 10.1016/j.tvjl.2012.06.031
Cheung, A. K. (2003). Transcriptional analysis of porcine circovirus type 2. Virology 305, 168–180. doi: 10.1006/viro.2002.1733
Chi, J. N., Wu, C. Y., Chien, M. S., Wu, P. C., Wu, C. M., and Huang, C. (2014). The preparation of porcine circovirus type 2 (PCV2) virus-like particles using a recombinant pseudorabies virus and its application to vaccine development. J. Biotechnol. 181, 12–19. doi: 10.1016/j.jbiotec.2014.04.006
Daniell, H. (2007). Transgene containment by maternal inheritance: effective or elusive? Proc. Natl. Acad. Sci. U.S.A. 104, 6879–6880. doi: 10.1073/pnas.0702219104
Franklin, S. E. and Mayfield, S. P. (2004). Prospects for molecular farming in the green alga Chlamydomonas. Curr. Opin. Plant Biol. 7, 159–165. doi: 10.1016/j.pbi.2004.01.012
Franklin, S., Ngo, B., Efuet, E., and Mayfield, S. P. (2002). Development of a GFP reporter gene for Chlamydomonas reinhardtii chloroplast. Plant J. 30, 733–744. doi: 10.1046/j.1365-313x.2002.01319.x
Gava, D., Serrão, V. H. B., Fernandes, L. T., Cantão, M. E., Ciacci-Zanella, J. R., Morés, N., et al. (2018). Structure analysis of capsid protein of Porcine circovirus type 2 from pigs with systemic disease. Braz. J. Microbiol. 49, 351–357. doi: 10.1016/j.bjm.2017.08.007
Gillespie, J., Opriessnig, T., Meng, X. J., Pelzer, K., and Buechner-Maxwell, V. (2009). Porcine circovirus type 2 and porcine circovirus-associated disease. J. Vet. Intern. Med. 23, 1151–1163. doi: 10.1111/j.1939-1676.2009.0389.x
Harris, E. H. (1989). “The chlamydomonas sourcebook,” in A Comprehensive Guide to Biology and Laboratory Use, vol. 246 . Ed. Harris, E. H. (Academic Press, San Diego, CA), 31–52. doi: 10.1126/science.246.4936.1503-a
Ishikura, K., Takaoka, Y., Kato, K., Sekine, M., Yoshida, K., and Shinmyo, A. (1999). Expression of a foreign gene in Chlamydomonas reinhardtii chloroplast. J. Biosci. Bioeng 87, 307–314. doi: 10.1016/s1389-1723(99)80037-1
Jiang, Y., Zhang, G., Wang, S., Raza, A., Liu, X., Guo, C., et al. (2025). Expressing exogenous gene in Chlamydomonas reinhardtii chloroplast with viral replication elements. Bioresour Technol. 434, 132784. doi: 10.1016/j.biortech.2025.132784
Kasai, S., Yoshimura, S., Ishikura, K., Takaoka, Y., Kobayashi, K., Kato, K., et al. (2003). Effect of coding regions on chloroplast gene expression in Chlamydomonas reinhardtii. J. Biosci. Bioeng 95, 276–282. doi: 10.1016/s1389-1723(03)80029-4
Khayat, R., Brunn, N., Speir, J. A., Hardham, J. M., Ankenbauer, R. G., Schneemann, A., et al. (2011). The 2.3-angstrom structure of porcine circovirus 2. J. Virol. 85, 7856–7862. doi: 10.1128/jvi.00737-11
Kindle, K. L., Richards, K. L., and Stern, D. B. (1991). Engineering the chloroplast genome: techniques and capabilities for chloroplast transformation in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. U.S.A. 88, 1721–1725. doi: 10.1073/pnas.88.5.1721
Liang, W., Qiu, J., Zhang, M., and Wang, C. (2022). Heterologous expression of human C-reactive protein in the green alga Chlamydomonas reinhardtii. J. Food Biochem. 46, e14067. doi: 10.1111/jfbc.14067
Liu, Y., Zhang, Y., Yao, L., Hao, H., Fu, X., Yang, Z., et al. (2015). Enhanced production of porcine circovirus type 2 (PCV2) virus-like particles in Sf9 cells by translational enhancers. Biotechnol. Lett. 37, 1765–1771. doi: 10.1007/s10529-015-1856-7
López-Vidal, J., Gómez-Sebastián, S., Bárcena, J., Nuñez Mdel, C., Martínez-Alonso, D., Dudognon, B., et al. (2015). Improved production efficiency of virus-like particles by the baculovirus expression vector system. PloS One 10, e0140039. doi: 10.1371/journal.pone.0140039
Lv, Q. Z., Guo, K. K., and Zhang, Y. M. (2014). Current understanding of genomic DNA of porcine circovirus type 2. Virus Genes 49, 1–10. doi: 10.1007/s11262-014-1099-z
Mayfield, S. P. and Franklin, S. E. (2005). Expression of human antibodies in eukaryotic micro-algae. Vaccine 23, 1828–1832. doi: 10.1016/j.vaccine.2004.11.013
Michelet, L., Lefebvre-Legendre, L., Burr, S. E., Rochaix, J. D., and Goldschmidt-Clermont, M. (2011). Enhanced chloroplast transgene expression in a nuclear mutant of Chlamydomonas. Plant Biotechnol. J. 9, 565–574. doi: 10.1111/j.1467-7652.2010.00564.x
Minko, I., Holloway, S. P., Nikaido, S., Carter, M., Odom, O. W., Johnson, C. H., et al. (1999). Renilla luciferase as a vital reporter for chloroplast gene expression in Chlamydomonas. Mol. Gen. Genet. 262, 421–425. doi: 10.1007/s004380051101
Ochoa-Méndez, C. E., Lara-Hernández, I., González, L. M., Aguirre-Bañuelos, P., Ibarra-Barajas, M., Castro-Moreno, P., et al. (2016). Bioactivity of an antihypertensive peptide expressed in Chlamydomonas reinhardtii. J. Biotechnol. 240, 76–84. doi: 10.1016/j.jbiotec.2016.11.001
Opriessnig, T., Meng, X. J., and Halbur, P. G. (2007). Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet. Diagn. Invest. 19, 591–615. doi: 10.1177/104063870701900601
Ramamoorthy, S. and Meng, X. J. (2009). Porcine circoviruses: a minuscule yet mammoth paradox. Anim. Health Res. Rev. 10, 1–20. doi: 10.1017/s1466252308001461
Rasala, B. A. and Mayfield, S. P. (2015). Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth Res. 123, 227–239. doi: 10.1007/s11120-014-9994-7
Rochaix, J. D. (1995). Chlamydomonas reinhardtii as the photosynthetic yeast. Annu. Rev. Genet. 29, 209–230. doi: 10.1146/annurev.ge.29.120195.001233
Segalés, J., Rosell, C., and Domingo, M. (2004). Pathological findings associated with naturally acquired porcine circovirus type 2 associated disease. Vet. Microbiol. 98, 137–149. doi: 10.1016/j.vetmic.2003.10.006
Specht, E., Miyake-Stoner, S., and Mayfield, S. (2010). Micro-algae come of age as a platform for recombinant protein production. Biotechnol. Lett. 32, 1373–1383. doi: 10.1007/s10529-010-0326-5
Tran, M., Van, C., Barrera, D. J., Pettersson, P. L., Peinado, C. D., Bui, J., et al. (2013). Production of unique immunotoxin cancer therapeutics in algal chloroplasts. Proc. Natl. Acad. Sci. U.S.A. 110, E15–E22. doi: 10.1073/pnas.1214638110
Tu, Y., Wang, Y., Wang, G., Wu, J., Liu, Y., Wang, S., et al. (2013). High-level expression and immunogenicity of a porcine circovirus type 2 capsid protein through codon optimization in Pichia pastoris. Appl. Microbiol. Biotechnol. 97, 2867–2875. doi: 10.1007/s00253-012-4540-z
Wu, P. C., Chen, T. Y., Chi, J. N., Chien, M. S., and Huang, C. (2016). Efficient expression and purification of porcine circovirus type 2 virus-like particles in Escherichia coli. J. Biotechnol. 220, 78–85. doi: 10.1016/j.jbiotec.2016.01.017
You-hong, L., Xia-ying, C., Yi-wen, Y., Zong-suo, L., and Zong-qi, Y. (2017). Expression and optimization strategy of RecombinantProteins in chlamydomonas chloroplast. China Biotechnol. 37, 118–125. doi: 10.13523/j.cb.20171016
Zhai, S. L., Chen, S. N., Xu, Z. H., Tang, M. H., Wang, F. G., Li, X. J., et al. (2014). Porcine circovirus type 2 in China: an update on and insights to its prevalence and control. Virol. J. 11, 88. doi: 10.1186/1743-422x-11-88
Keywords: porcine circovirus type 2, ORF2 antigen gene, capsid protein, Chlamydomonas reinhardtii, chloroplast transformation
Citation: Song Y, Lin Y, Cheng X, Li W, Li Y and Yang Z (2025) Expression of recombinant Cap antigen of porcine circovirus type 2 in the chloroplast of Chlamydomonas reinhardtii. Front. Plant Sci. 16:1661360. doi: 10.3389/fpls.2025.1661360
Received: 07 July 2025; Accepted: 12 September 2025;
Published: 09 October 2025.
Edited by:
Linchun Shi, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Bin Wu, Zhejiang University, ChinaYun Wei, Northeastern University, United States
Zaibiao Zhu, Nanjing Agricultural University, China
Copyright © 2025 Song, Lin, Cheng, Li, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zongqi Yang, eWFuZ3pvbmdxaUB6c3R1LmVkdS5jbg==
 Yiran Song
Yiran Song Youhong Lin
Youhong Lin Wei Li
Wei Li