- Institute of Resources, Environment and Soil Fertilizer, Fujian Academy of Agricultural Sciences, Fuzhou, China
Agriculture faces mounting challenges from climate change, soil degradation, and unsustainable agrochemical use, highlighting the need for eco-friendly solutions. Azolla, a fast-growing aquatic fern, has emerged as a multifunctional resource for sustainable farming and climate resilience. Through its symbiosis with Anabaena azollae, it fixes atmospheric nitrogen, reducing dependence on synthetic fertilizers and improving soil health. Azolla also serves as a protein-rich feed for livestock and aquaculture, suppresses weeds and pests in rice systems, and supports water conservation. Beyond agriculture, it contributes to carbon sequestration, mitigates methane emissions, and shows promise in wastewater treatment, bioremediation, and as a feedstock for biofuels and bioplastics. However, large-scale adoption is limited by challenges such as short shelf life, ecological risks, and preservation constraints. This review synthesizes current knowledge on Azolla, emphasizing its biological and ecological functions, highlights practical applications across agriculture, livestock, aquaculture, and environmental management, and outlines key research priorities needed to overcome limitations and enable its integration into climate-smart agricultural and environmental systems.
1 Introduction
Rapid population growth, climate change, and natural resource depletion create an urgent global challenge for agricultural sustainability (Maja and Ayano, 2021; Ramesh and Rajendran, 2022). Sustainable farming practices aim to balance food production with environmental conservation through strategies that include minimizing chemical use, managing water effectively, and restoring ecosystems while reducing greenhouse gas emissions (Muhie, 2022). Conventional farming practices heavily reliant on synthetic fertilizers and intensive irrigation have led to soil degradation, biodiversity loss, and increased greenhouse gas emissions. The goal is to meet current needs without jeopardizing future generations. Industrial development and agriculture are major contributors to environmental imbalance, necessitating eco-friendly strategies to mitigate climate change impacts. The existing agricultural challenges have escalated the urgency in finding sustainable and regenerative farming methods (Ramesh and Rajendran, 2023; Xu et al., 2024).
Among various alternatives, Azolla, a fast-growing aquatic fern, has gained significant attention for its unique biological properties and potential role in sustainable agriculture and climate resilience (Kour et al., 2024). Agricultural systems benefit from Azolla integration because it effectively lowers emissions while improving environmental sustainability (Kollah et al., 2016). This aquatic fern forms a symbiotic relationship with Anabaena azollae to fix atmospheric nitrogen, enabling it to function as a biofertilizer that reduces synthetic fertilizer use while preventing soil acidification and nitrous oxide emissions (Marzouk et al., 2023). Previous research demonstrates that Azolla performs better than inorganic fertilizers (Sood et al., 2012). In addition, Azolla contributes to rapid biomass generation, carbon sequestration, and methane reduction (Malyan et al., 2020; Korsa et al., 2024). Azolla also purifies water by absorbing heavy metals and pollutants, while serving as a high-protein livestock and aquaculture feedstock (25–33% crude protein), making it both a sustainable and economical supplement. Beyond agriculture, this nutrient-dense resource has uses in industry and healthcare and has even been featured in space diets (Ahluwalia et al., 2002; Prabakaran et al., 2022; Yohana et al., 2023).
The current review highlights Azolla’s role in sustainable agriculture and climate resilience by examining its biological properties, nitrogen fixation capacity, carbon sequestration potential, animal feed applications, phytoremediation functions, and industrial uses. The primary focus is on Azolla’s role in rice and crop-based systems, while livestock and aquaculture are discussed as complementary but integral components of agricultural systems. In addition, the review identifies key research gaps and proposes future directions to advance Azolla-based solutions for climate-smart agriculture.
2 Biological and ecological characteristics of Azolla
2.1 Taxonomy and species diversity
Though its precise classification is still under discussion, Jean-Baptiste Lamarck initially identified the genus Azolla in 1783 (Bujak and Bujak, 2024). Initially grouped with Salviniaceae, phylogenetic studies later confirmed its distinct evolutionary lineage (Saunders and Fowler, 1993).
The classification of Azolla proves difficult because the genus shows significant morphological variability, vegetative reproduction, and environmental adaptability, which make species identification challenging (Lydia et al., 2023). Azolla is divided into two subgenera: EuAzolla (A. filiculoides, A. rubra, A. microphylla, A. mexicana, A. caroliniana) and Rhizosperma (A. pinnata, A. nilotica), differentiated by morphology and reproduction. There are seven extinct and twenty-five fossil species of Azolla. The distribution, characteristic features, and uses of different Azolla species have been discussed in detail by Kour et al (Kour et al., 2024). Native to America, Africa, Asia, and Australia, Azolla has expanded globally due to its invasive nature, though no species are native to Europe. While fossil evidence shows that Azolla existed in Europe at one time, it was reintroduced to the continent in 1880 (Korsa et al., 2024; Kour et al., 2024).
Several species have become invasive outside their native ranges, forming dense mats that disrupt ecosystems and economic activities. Examples include Azolla cristata (syn. A. caroliniana) originated from North and Central America and is now growing in Africa, Asia, and Europe (Korsa et al., 2024; Kour et al., 2024). The native South and Central American A. microphylla has been introduced throughout the world (Kour et al., 2024). In contrast, A. mexicana remains primarily confined to North and Central America (Kour et al., 2024). Azolla pinnata, native to Asia, Africa, and Australia, has been introduced to the USA and South America. Azolla filiculoides, tolerant of cold climates, was introduced to China from East Germany in 1977 (Madeira et al., 2019; Kour et al., 2024). Through the introduction, Egypt received A. caroliniana, A. filiculoides, and A. pinnata (Serag et al., 2000b). Azolla caroliniana developed into an invasive species in the Danube Delta of Ukraine by 1978 (Prokopuk, 2016). Reflecting evolutionary adaptations, phylogenetic studies utilizing rbcL gene sequences confirm the split of Azolla into Euazolla and Rhizosperma (Mahmood et al., 2020). Species like A. pinnata and A. filiculoides are widely used in agriculture, while others remain underexplored for potential applications (Kour et al., 2024).
2.2 Growth and reproduction
The aquatic fern Azolla doubles its biomass roughly every 2 to 5 days, producing 3–9 tons of dry matter per hectare annually (Lumpkin and Plucknett, 1980; Wagner, 1997). Critical factors affecting Azolla growth and nutrient composition have been discussed in detail previously (Marzouk et al., 2023). Briefly, growth depends on temperature, light, nutrients, and water pH, with an optimum of 18–28°C; growth slows below 15°C and stops above 35°C (Sadeghi et al., 2013). Its symbiosis with Anabaena azollae enables survival in low-nitrogen conditions, though it thrives in nutrient-rich waters (Lechno-Yossef and Nierzwicki-Bauer, 2002).
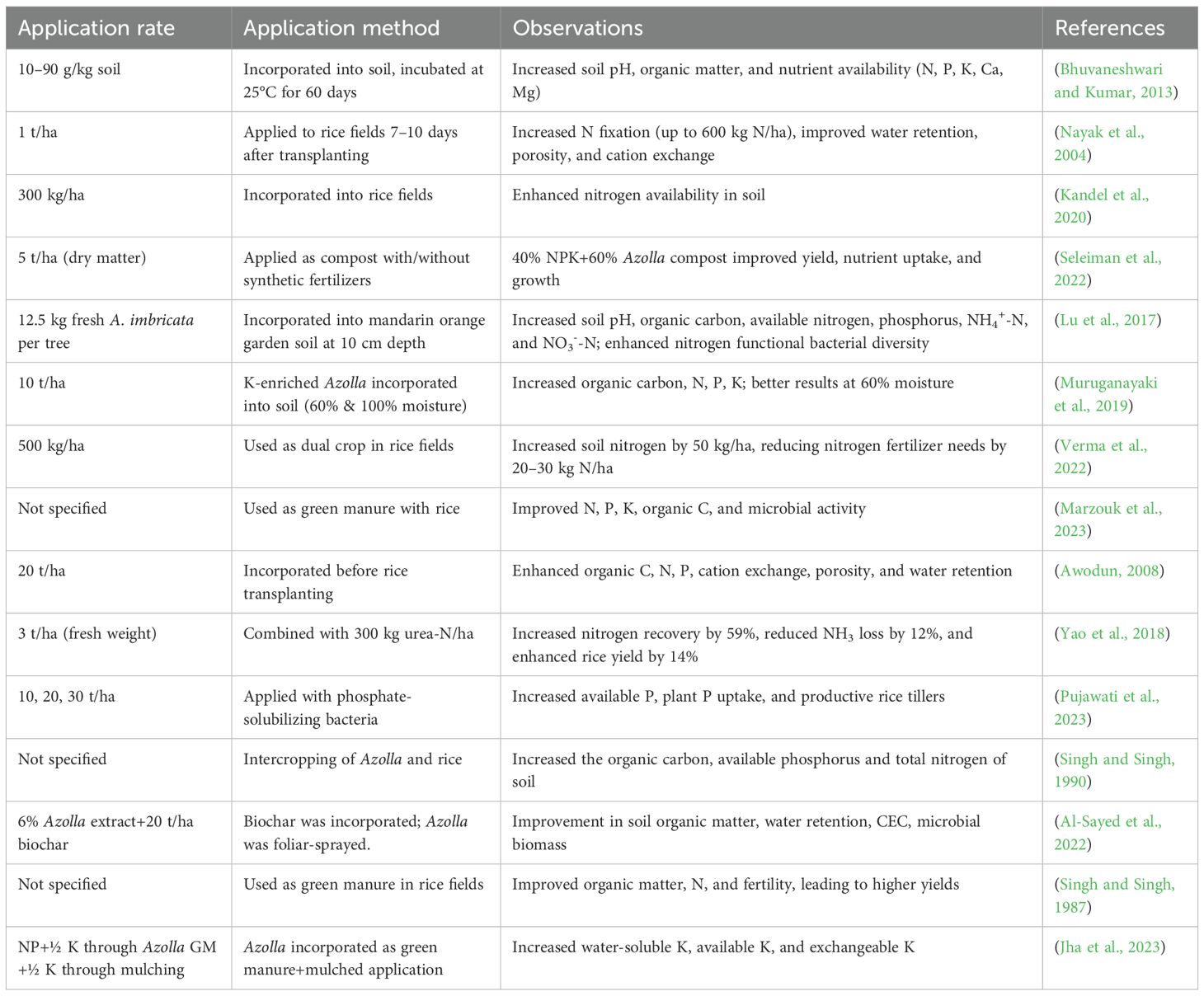
Reproduction occurs mainly through vegetative propagation, via detachment of rhizome branches, which allows rapid spread. Sexual reproduction is less common, involving heterosporous sporocarps containing microspores and megaspores (Sebastian et al., 2021; Schluepmann et al., 2022). The life cycle of Azolla varies by species. The process of sexual reproduction starts when paired sporocarps develop from shoot apical meristems, including both a megasporocarp with one megasporangium and a microsporocarp with several microsporangia (Dijkhuizen et al., 2021; Schluepmann et al., 2022). During sporocarp formation, A. azollae is recruited into the indusium cap near root-forming branches. While microsporocarps discharge massulae, including microspores, megasporocarps develop into megagametophytes, creating archegonia (Figure 1). Fertilization occurs when flagellate gametes reach the archegonia, leading to diploid growth, though the timing of microgametophyte and gamete development remains unclear (Schluepmann et al., 2022).
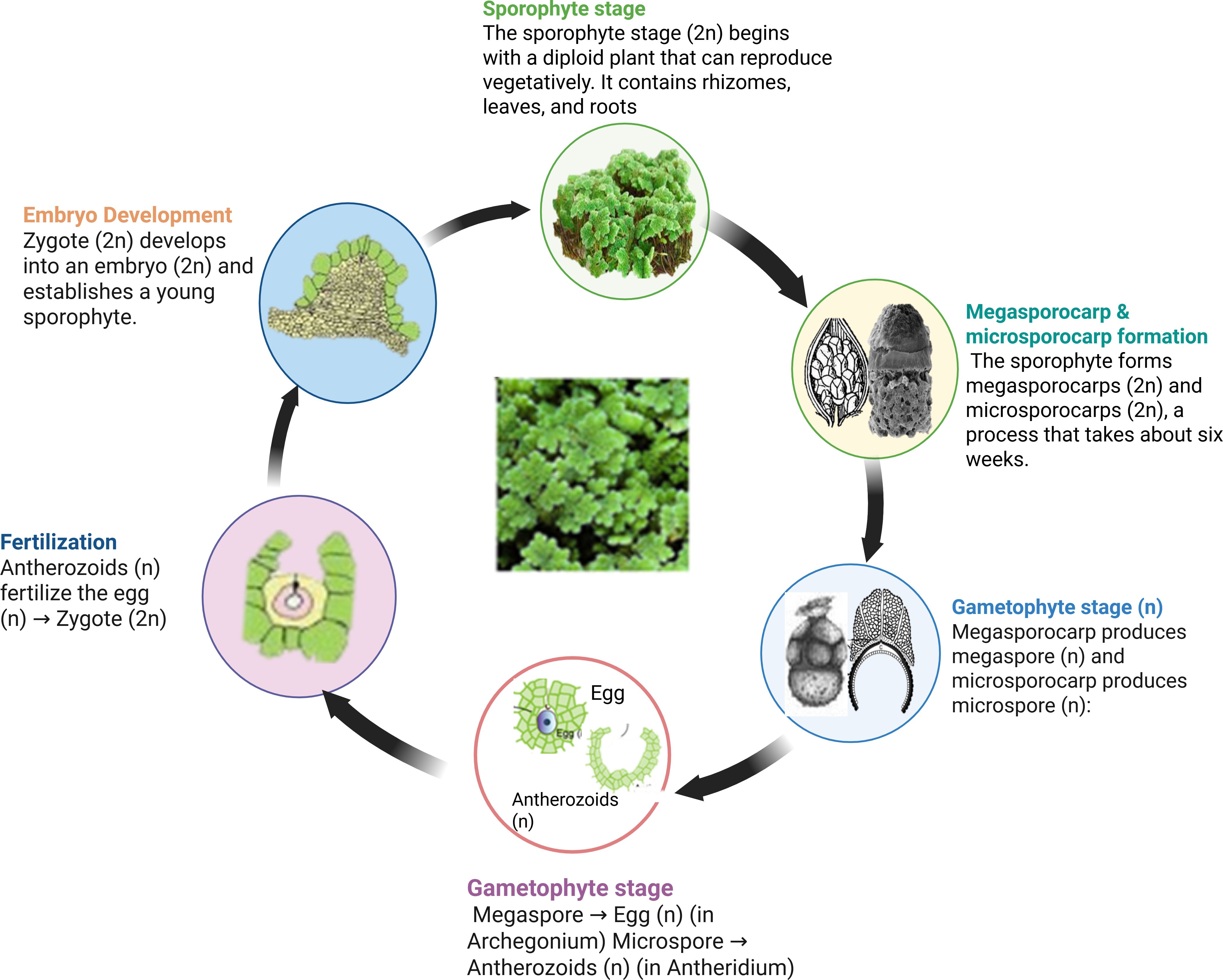
Figure 1. An example of the general life cycle of Azolla species showing various developmental stages. The sporophyte had a rhizome, leaves, and roots. Rhizome develops dense leaves containing cyanophycean algae on the upper surface. Adapted from (Sebastian et al., 2021), with permission from John Wiley & Sons.
Sporocarps in A. filiculoides can remain viable for up to four years at 4°C or indefinitely if dried and cryopreserved at -80°C, whereas fragile, water-rich sporophytes cannot be stored (Li et al., 2018). The shift to the haploid phase happens during the start of sporangial development, which depends on light conditions, temperature, and nutrient levels (White, 1971). Unlike seed plants, Azolla exhibits high plasticity in sporangial meristem formation, occurring in both sporophytes and gametophytes. Different Azolla species demonstrate variable sporangial responses when exposed to distinct environmental stimuli. For example, A. filiculoides produces sporocarps when exposed to far-red light, but this formation stops under open-field red light conditions (Dijkhuizen et al., 2021). Sporocarp formation is likely controlled by a conserved phase transition network involving regulatory elements known from seed plants, such as MIKCC, AP2, and GAMYB-microRNA319 interactions (Ambrose and Vasco, 2016). The processes controlling spore germination and gametophyte growth are probably controlled by the sporocarp itself, given the protected nature of Azolla gametophytes. During periods of environmental stress, sporocarps descend to the depths of aquatic environments and stay dormant until the conditions improve (Sood and Ahluwalia, 2009). Different Azolla species thrive in diverse habitats. Azolla pinnata, for instance, likes higher temperatures; A. filiculoides may survive in colder temperatures (Metzgar et al., 2007). However, other factors, such as high salinity, UV radiation, and heavy metals, can affect their growth (Korsa et al., 2024). Azolla plants in cold regions submerge during the winter and then emerge when the temperature increases. It can change their color from grey green to red-purple when exposed to intense sunlight. Azolla thrives in freshwater bodies like ditches, swamps, lakes, and rivers and is also called duckweed, mosquito, or water fern (Kour et al., 2024). While modern species are free-floating, fossils suggest that extinct species had suberect growth (Watanabe and Berja, 1983). Molecular research highlights genetic traits that enhance stress resistance, offering potential for selective breeding. Its sporophyte phase features a floating rhizome with leaf-like fronds and submerged roots.
2.3 Symbiotic nitrogen fixation
The nitrogen-fixing cyanobacterium A. azollae resides in specialized cavities of Azolla leaves, forming a mutualistic symbiosis first observed by Strasburger in 1873 and later described by De Bary (Carrapiço, 2010; Kour et al., 2024). This relationship enables Azolla to thrive in nitrogen-poor waters and function as an effective organic fertilizer (Figure 2) (Peters and Meeks, 1989).
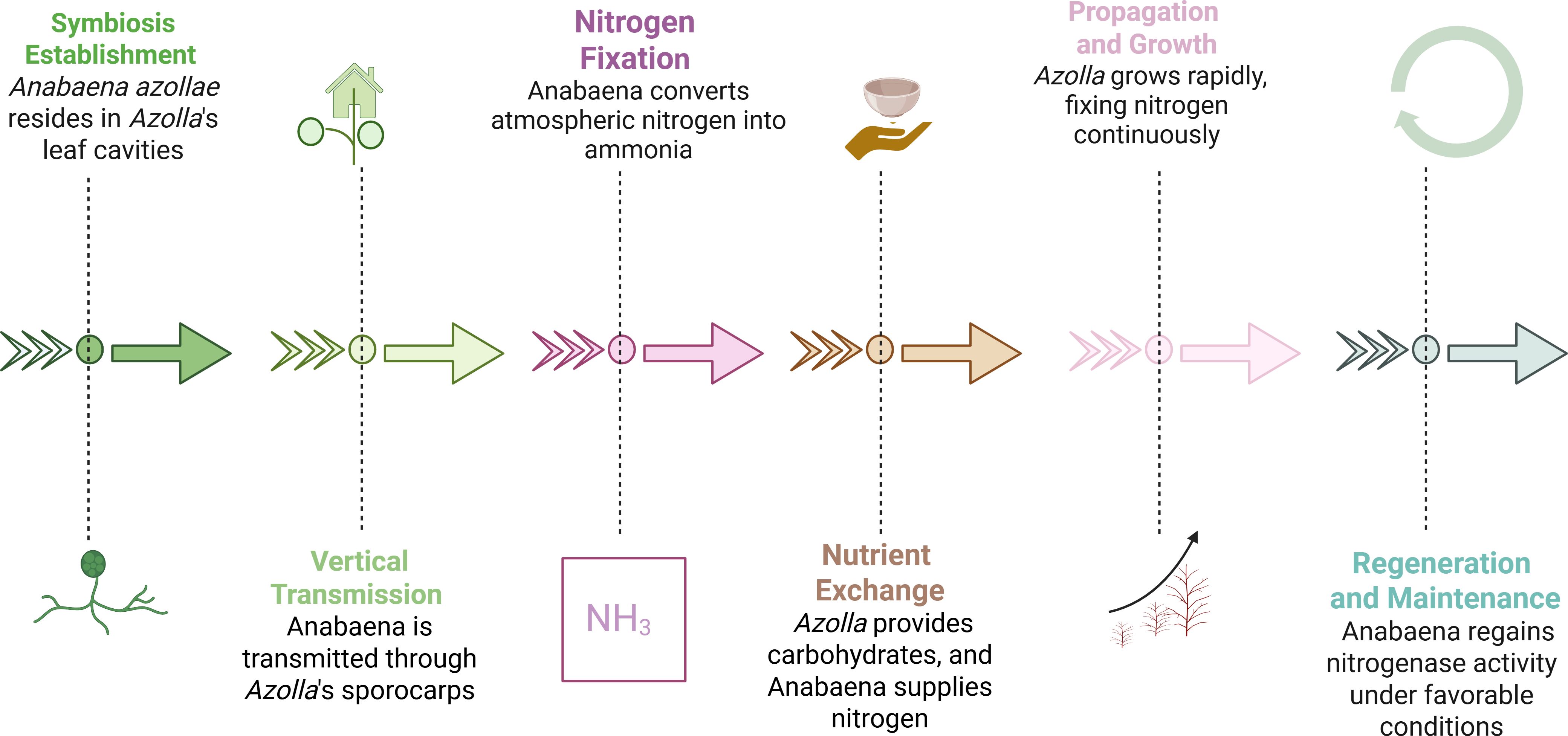
Figure 2. Example of Azolla-Anabaena symbiosis process (Peters and Meeks, 1989).
Molecular studies confirm the long-term coevolution of Azolla and its cyanobiont (Qiu and Yu, 2003; Papaefthimiou et al., 2008; Pereira and Vasconcelos, 2014). Phylogenetic analyses indicate a single evolutionary origin of the symbiosis, which has remained stable for over 100 million years (Bujak and Bujak, 2024). Vertical transmission through megasporocarps ensures that each new generation inherits its cyanobiont without external inoculation, maintaining high nitrogen-fixation efficiency (Ran et al., 2010).
The system functions without requiring external inoculation while preserving strong nitrogen-fixing efficiency (Carrapiço, 2010). The propagation of A. azollae within Azolla ferns depends on its apical colony in the shoot apex and the movement of its motile filaments (hormogonia) to organ initials like leaf cavities and sporocarps. The regulation of hormogonia movement and cell differentiation in Azolla is mostly unknown. Some evidence suggests that secretory trichomes and deoxyanthocyanins might affect this process (Cohen et al., 2002). The leaf cavity functions as a microhabitat that controls oxygen levels to protect nitrogenase from deactivation, thereby enabling nitrogen fixation. Azolla’s leaf cavities and sporocarps host a diverse microbial (Rai et al., 2002). Some studies suggested that some bacteria synthesize plant hormones like indole-3-acetic acid, which can improve the growth of Azolla (Kumar et al., 2022). Therefore, the Azolla-Anabaena relationship forms a complex microbial network that functions as a superorganism beyond its initial binary symbiosis (Carrapiço, 2017). Azolla maintains association with one cyanobacterial species, which contrasts with legumes hosting multiple symbiotic partners and prompts further investigation into its coevolution and metabolic interactions. Genetic research has identified regulatory differences in nitrogen fixation, which may lead to agricultural improvements (Pabby et al., 2003; Devaprakash et al., 2024).
3 Role of Azolla in sustainable agriculture
Azolla has long been used in agriculture mostly for water conservation, weed control, and soil fertility enhancement. Its use as a biofertilizer in rice systems dates back to China’s Tang Dynasty (618–907 AD), when farmers applied it as green manure to boost rice yields (Lumpkin and Plucknett, 1980). By the Ming Dynasty (17th century), its use had become widespread (Tarif, 2021; Kour et al., 2024). Cultivation began in Fujian and Guangdong, later spreading south of the Yangtze; after the establishment of the People’s Republic, its use expanded northward as both manure and animal feed. In central and southern China, it is still grown before early rice planting. In Vietnam, the use of A. pinnata as green manure dates back to the 11th century, predating its spread to China, India, and the Philippines (Tarif, 2021). Oral traditions suggest its domestication in La Van village, Thai Binh province, where villagers reared Azolla starter cultures from April to November and sold them to farmers at premium prices before the Vietnamese revolution (Watanabe, 1982; Tarif, 2021).
The symbiotic relationship between Azolla and A. azollae enables Azolla to function as a natural source of nitrogen through direct atmospheric nitrogen fixation into the plant. In flooded rice systems, fixation rates of 2–4 kg N per hectare per day have been reported, substantially reducing the need for synthetic fertilizers and positioning Azolla as an important component of sustainable agriculture (Pabby et al., 2003). This biologically sourced nitrogen not only lowers production costs but also minimizes environmental contamination compared to chemical fertilizers (Wagner, 1997). In rice paddies, the dense floating mat of Azolla suppresses weeds by blocking sunlight and reduces water loss through evaporation, thereby decreasing reliance on herbicides and manual weeding (Pabby et al., 2003). Its water-retention capacity also helps maintain soil moisture in drought-prone areas (Peters and Meeks, 1989). Beyond soil fertility, Azolla has long been used as livestock and aquaculture feed due to its protein-rich composition and balanced amino acid profile. More recently, it has been adopted in Iran, Africa, and parts of Europe for rice cultivation and aquatic farming (Madeira et al., 2016). Research in the 20th century further revealed its potential in carbon sequestration, organic farming, and phytoremediation. Its ability to absorb heavy metals and pollutants highlights its value in environmental remediation (Figure 3), reinforcing its role in modern sustainable agricultural systems (Yao et al., 2018).
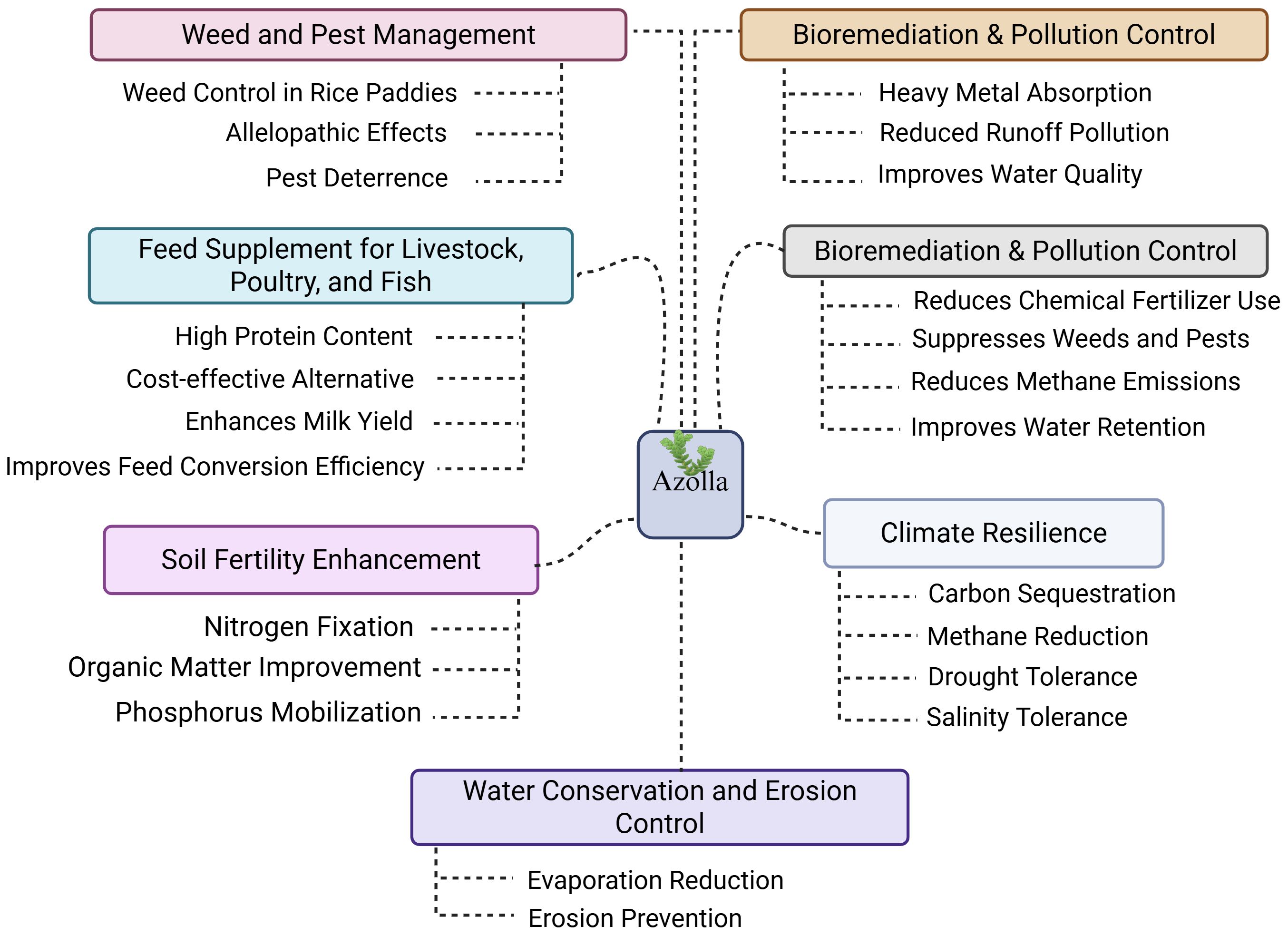
Figure 3. Examples of Azolla’s multifunctional benefits in agriculture and environmental sustainability.
3.1 Sustainable biofertilizer for soil health
Through its symbiosis with A. azollae, Azolla contributes 30–60 kg N ha-¹ per season via biological nitrogen fixation, improving soil fertility and raising nitrogen-use efficiency by up to 70% compared with synthetic fertilizers (Kollah et al., 2016; Kour et al., 2024) (Table 1). With a rapid growth rate that doubles biomass in 3–5 days, Azolla fixes 1.1–3.5 kg N ha-¹ day-¹, exceeding many legumes (Pillai, 2001; Vijayan et al., 2024). When used as a dual crop in rice paddies, it supplies 40–60 kg N ha-¹ per cycle (Adhikari et al., 2020). Azolla inoculation with 16.5–17.5 t fresh weight ha-¹ fixes 52.5–55.1 kg N ha-¹, while 12.2 t dry matter ha-¹ provides 33.8 kg N ha-¹ (Raja et al., 2012). The efficiency of nitrogen fixation varies depending on climatic conditions, floodwater nutrient levels, Azolla species, and rice growth stages (Kour et al., 2024).
Beyond nitrogen, after incorporation, Azolla enhances soil organic matter, microbial activity, and physical structure. Its decomposition increases aggregate stability, porosity, water retention, and permeability while reducing bulk density (Marzouk et al., 2023; Sun et al., 2024; Ansabayeva et al., 2025). These changes support higher crop yields. Humus derived from Azolla improves cation exchange capacity and nutrient availability (Ca²+, Mg²+, K+, P) (Kour et al., 2024). The breakdown of Azolla in soil helps various nitrogen-fixing bacteria and fungi to flourish, which in turn enhances nutrient cycling and crop nutrition (Samarajeewa et al., 2005; Adhikari et al., 2020).
Compared to synthetic nitrogen sources, Azolla-derived nitrogen is more efficient in terms of plant uptake and fertilizer use efficiency (Seleiman et al., 2022; Marzouk et al., 2023). Integrating organic and inorganic fertilizers sustains crop productivity and enhances soil health (Pushpanathan et al., 2004). Several studies demonstrate that mixing Azolla into soil helps improve nitrogen mineralization and its usage. The efficiency of fertilizers is enhanced when Azolla is added to the soil (Bhuvaneshwari and Singh, 2015; Adhikari et al., 2020). A previous study reported that the application of 86 kg N ha-¹+1000 kg Azolla ha-¹ increased rice growth by 15.54%, yield by 25.49%, and nitrogen-use efficiency (Safriyani et al., 2020).
Beyond nitrogen, Azolla increases phosphorous availability by 20–30%, hence very helpful for soils lacking phosphorus (Raja et al., 2012). With 3–5% nitrogen and 3–6% potassium in its biomass, it exceeds traditional green manures in nutrient value (Kour et al., 2024). Azolla breakdown increases urease and phosphatase activity, encouraging mineralization of nutrients (Herath et al., 2023). Moreover, Azolla is essential for the control of soil pH since it reduces acidification in acidic soils and increases phosphorus solubility in alkaline soils, thus boosting the availability of nutrients in several agroecosystems (Herath et al., 2023; Marzouk et al., 2024).
Azolla reduces runoff, prevents erosion, and improves aggregation, particularly when cultivated along contour lines or irrigation channels (Adhikari et al., 2020; Herath et al., 2023). Floating mats in rice paddies protect against sediment loss, while fine rootlets deposit silt in wetlands and channels, limiting nutrient depletion (Raja et al., 2012; Kollah et al., 2016). These processes enhance root development and water-use efficiency in rice fields (Razavipour et al., 2018).
Azolla contributes to abiotic stress management. It tolerates moderate salinity, removing excess salts from soil and water (Sadeghi et al., 2014). Its mats limit evaporation and salt buildup, reducing crop salinity stress (Serag et al., 2000a). Compost from Azolla enhances rice growth on saline soils by releasing organic acids that improve nutrient availability while aiding salt removal (Razavipour et al., 2018). Collectively, these properties establish Azolla as a cost-effective alternative to conventional soil amendments.
3.1.1 Azolla application in rice cultivation
Azolla significantly enhances rice grain yield, straw yield, caryopsis formation, and dry matter production when incorporated into paddy fields (Pabby et al., 2003). It is applied either as green manure before transplanting or as a dual crop after transplanting, with the latter being more widely adopted due to its greater agronomic benefits (Kimani et al., 2022). In the green manure system, Azolla is collected from nurseries, ponds, or ditches and applied 2–3 weeks before rice transplanting. Healthy, fresh Azolla inoculum is essential for efficient production, with inoculum density playing a crucial role (Adhikari et al., 2020). Singh recommends 2 t ha-¹, while in Vietnam, 5 t ha-¹ or more is preferred (Singh, 1981; Marzouk et al., 2023). Insufficient density can lead to overgrowth by algae and weeds. Various Azolla cultivation methods are used globally, with Vietnam favoring the half-saturation method. Azolla pinnata reaches a saturated density of 10–20 t ha-¹. The process begins by spreading inoculum at 0.5 kg m-². After one week, when the surface is fully covered, half of the Azolla is transferred to a new area of equal size. Within another week, both areas will reach full coverage. This cycle is repeated, doubling the covered area each time, leading to exponential expansion (Watanabe, 1982).
Azolla forms a thick mat that decomposes into the soil, supplying 20–40 kg N/ha and enhancing soil fertility and crop yields (Kulasooriya and De Silva, 1977; Watanabe et al., 1977). In dual cropping systems, introducing 0.5–1 t/ha of fresh Azolla after transplanting allows a dense mat to form within 15–20 days. Decomposing in 8–10 days, it releases nitrogen to support rice growth throughout the crop cycle, providing approximately 30 kg N/ha per cycle. To optimize nitrogen fixation, superphosphate (20 kg/ha) is applied in split doses (Watanabe et al., 1977; Yadav et al., 2014).
Yield impacts are well-documented. Azolla compost at 5% soil weight raised grain yield by 13.8% (Razavipour et al., 2018). A 1975 review of 1,500 trials in southern China reported yield increases of 600–750 kg ha-¹ (FAO-Rome, 1979; Liu, 1979). In Chekiang Province, 90% of 422 trials reported an average yield gain of 700 kg ha-¹ (18.6%) (Liu, 1979). Vietnamese studies found 1 t fresh Azolla increased yield by 28 kg, with 20 t ha-¹ raising yields by 0.5 t ha-¹ (Ventura et al., 1992; Nyoni, 2011). Dual cropping improved yields by 36–38% (Barthakur and Talukdar, 1983), while A. pinnata specifically increased grain yield by 6–29% (Moore, 1969). Integrating Azolla with neem cake-coated urea further maximized yield (Sukumar et al., 1988). Several other studies have demonstrated substantial yield improvements associated with Azolla application. Peters found that using Azolla as a monocrop biofertilizer increased rice yield by 112% compared to unfertilized controls, while intercropping with rice resulted in a 23% yield increase (Peters, 1978). When applied as both a monocrop and an intercrop, the yield increase reached 216%. Singh observed that the application of 30–40 kg N/ha from ammonium sulphate or 8–10 t/ha of fresh Azolla led to a 47% increase in grain yield (Singh, 1977). A review of multiple studies indicated that Azolla-based cropping systems increased grain yields by 14–40%, while monocropping during the fallow season resulted in a 15–20% yield increase (Samal et al., 2020).
Studies also indicate that incorporating Azolla enhances nitrogen recovery by 49–64% while reducing nitrogen loss by 26–48% (Yao et al., 2018). The nitrogen fixation capacity of Azolla varies across species, with A. filiculoides fixing 128 kg N/ha in 50 days, A. pinnata fixing 0.3–0.6 kg N/ha/day, and A. africana fixing 0.6–1.8 kg N/ha/day (Kumarasinghe and Eskew, 1993). Basal applications of 10–12 t ha-¹ increased soil N by 50–60 kg ha-¹, reducing fertilizer needs by 30–35 kg ha-¹ (Roy et al., 2016). Similarly, adding 500 kg/ha of green Azolla has been reported to raise soil nitrogen by 50 kg/ha, further reducing the need for nitrogenous fertilizers by 20–30 kg/ha (Roy et al., 2016). Additionally, Azolla application reduces NH3 volatilization by 12–42%, minimizing nitrogen loss in flooded rice systems (Yao et al., 2018).
Azolla’s effectiveness in rice production extends to its role in nitrogen management strategies. Studies indicate that applying Azolla with reduced nitrogen levels achieves yields comparable to full nitrogen applications, making it a viable alternative to synthetic fertilizers. For instance, applying 60 kg N/ha from Azolla along with 30 kg N/ha from urea resulted in yields equivalent to those obtained with a full 60 kg N/ha urea application (Setiawati et al., 2020). Additionally, Azolla lowers flooded water pH and temperature, contributing to reduced NH3 volatilization and improved nitrogen use efficiency (Yao et al., 2018). Beyond its contribution to nitrogen supply, as discussed in section 3.1, Azolla improves soil structure, enhances organic matter accumulation, and increases the availability of essential micronutrients such as Zn, Fe, and Mn (Subedi and Shrestha, 2015). Moreover, it releases plant growth regulators and vitamins that further promote rice growth and yield (Thapa and Poudel, 2021).
Integrated systems further boost sustainability. Azolla, integrated with rice, fish, and ducks, enhances nutrient cycling, soil fertility, and pest control while reducing chemical inputs. This sustainable system improves productivity and biodiversity while minimizing environmental impact (Sanginga and Van Hove, 1989; Van Hove, 1989). In the rice-fish-Azolla system, Azolla acts as a biofertilizer and fish feed, improving rice and fish production (Shanmugasundaram and Ravi, 1992). Azolla application at 2 t/ha increased yields and the benefit-cost ratio (1.88) (Sivakumar and Solaimalai, 2003). Fish stocked at 6,000/ha with Azolla feed generated a net income of $258/ha, surpassing rice monoculture by $51 (Van Hove, 1989; Cagauan and Pullin, 1994).
The rice-fish-Azolla-duck system (Figure 4) builds upon this approach by introducing ducks, which help control weeds and pests while enriching soil with their droppings (Sow and Ranjan, 2020). Ducks introduced 15–20 days after rice transplantation reduce reliance on pesticides, while Azolla supports soil health and serves as feed for both fish and ducks (Lumpkin and Plucknett, 1980). Fish benefit from organic matter derived from duck manure and decomposed Azolla, improving growth and productivity (Sow and Ranjan, 2020). Studies report up to a 58% increase in rice yield compared to monoculture due to improved nutrient cycling and pest control (Cagauan et al., 2000).
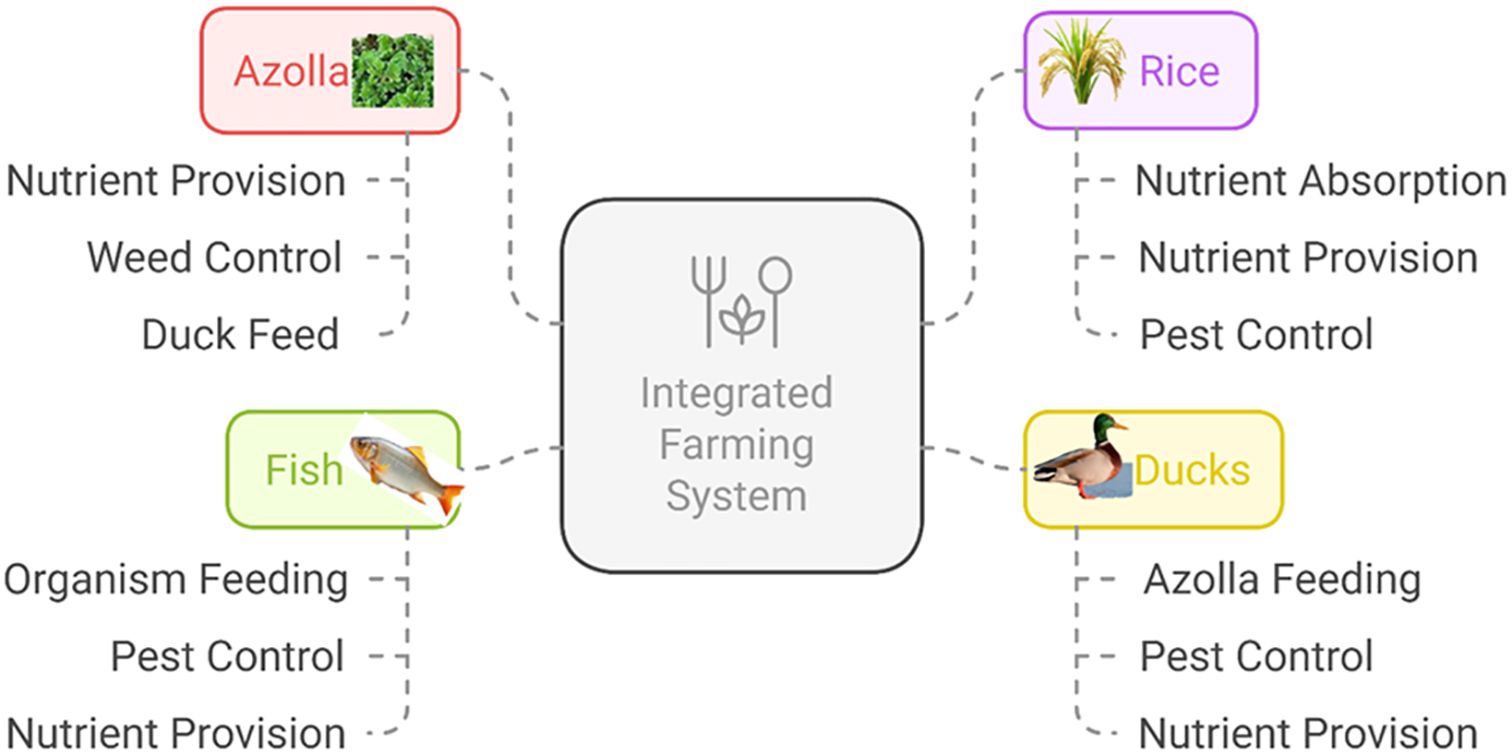
Figure 4. Schematic representation of rice–Azolla–duck–fish Interrelationships in an integrated farming system.
These systems significantly enhance pest control efficiency, reducing populations of rice pests such as green leafhoppers, brown planthoppers, stem borers, leaf folders, whorl maggots, and gall midges (Cagauan et al., 2000; Sapcota and Begum, 2022). Combined use of Azolla, fish, ducks, liquid biofertilizer, and nano-urea extends nutrient availability and boosts physiological traits, delivering high productivity with reduced chemical inputs (Sow and Ranjan, 2020).
Finally, Azolla benefits extend to non-rice crops. In taro (Colocasia esculenta), its use as green manure significantly raised yields (Tekle-Haimanot and Doku, 1995). In rice–wheat systems, it enhanced wheat yields, particularly when combined with Sesbania (Mahapatra and Sharma, 1989). It is also harvested from water bodies for use in wheat and vegetables (Pabby et al., 2003). In banana plantations, it serves as nutrient rich-mulch (Van Hove, 1989; Wijeysingha and Amarasinghe, 2023).
3.2 Azolla in water conservation, weed, and pest control
Azolla forms a dense floating mat on the water surface, reducing evaporation by up to 60% and conserving soil moisture (Kour et al., 2024). By covering the water surface, Azolla limits sunlight penetration, thereby lowering water temperature and evaporation rates, which is particularly beneficial in regions with water scarcity or irregular rainfall (Marzouk et al., 2023). Additionally, In non-flooded cropping systems, Azolla can also be applied as a living mulch, improving soil water retention and reducing moisture loss (Raja et al., 2012).
The thick Azolla mat prevents sunlight from reaching submerged weed seeds, inhibiting germination and growth. This eco-friendly method provides an alternative to herbicides (Adhikari et al., 2020). Previous studies show that Azolla can reduce weed biomass by up to 50% (Herath et al., 2023). Since weeds compete with rice for nutrients, light, and water, infestations can cause yield losses of 16–100% depending on severity (Geetha et al., 2022). Unlike herbicides such as 2,4-D, glyphosate, and propanil, which harm non-target organisms and degrade soil and water quality (Shekhawat et al., 2022), Azolla offers a safer alternative. Its potential in smallholder systems, first recognized in 1927, remains underexploited (Herath et al., 2023).
Azolla effectively suppresses multiple weeds, including Echinochloa crus-galli, Cyperus serotinus, Monochoria vaginalis, Eclipta prostrata, Fimbristylis miliacea, and Cyperus rotundus (Herath et al., 2023). By blocking light, altering microclimate (reducing evaporation and soil temperature), and improving soil structure when incorporated as green manure, Azolla reduces weed competition and enhances rice growth (Marzouk et al., 2023). Azolla also exhibits allelopathic properties that inhibit weed germination and growth. It releases secondary metabolites such as phenolic compounds, flavonoids, and tannins, which suppress invasive weed species (Bahadur et al., 2015; Ameena et al., 2024). Some studies have demonstrated that Azolla extracts negatively affect root elongation in weeds, further reinforcing its potential as a natural weed management tool (Herath et al., 2023).
The floating Azolla mat also disrupts pest life cycles. It prevents mosquitoes from laying eggs on water surfaces, lowering mosquito populations and reducing vector-borne disease risk (Kour et al., 2024). It interferes with the breeding of rice stem borers and leafhoppers, which require open water for egg-laying (Marzouk et al., 2023). Furthermore, Azolla supports beneficial organisms such as predatory insects, frogs, and fish, which feed on pest larvae, thereby enhancing natural pest control mechanisms and reducing the need for chemical pesticides (Raja et al., 2012). In integrated rice–fish systems, Azolla not only acts as biofertilizer and fish feed but also suppresses weeds and pests, reducing chemical inputs and maintaining ecological balance (Pabby et al., 2003). Azolla extracts possess antifungal and antibacterial properties, helping to mitigate plant diseases such as rice blast and sheath blight (Phukon et al., 2017). Azolla bioactive compounds have also demonstrated effectiveness against fungal infections in other crops, highlighting its potential in sustainable disease management (Pereira et al., 2015).
3.3 Azolla as animal feed
Azolla is a nutrient-rich, high-protein feed supplement for livestock, poultry, and fish. It provides an excellent amino acid profile, high digestibility, and essential micronutrients, serving as a sustainable alternative to conventional protein feeds such as soybean meal and fish meal, which are costly and environmentally intensive (Kour et al., 2024).
The nutrient composition of Azolla varies by species, geography, production methods, and soil conditions (Shaltout et al., 2012; Bhavyasree, 2015). Several studies have reported different compositions of different species of Azolla (Katole et al., 2017; Khursheed et al., 2019). Azolla microphylla and A. filiculoides, which are most commonly used, contain 91.77%–92.25% moisture, 3.9%–5.2% crude protein, 0.6%–1.8% crude fat, and 2% ash (Bhaskaran and Kannapan, 2015). On a dry matter basis, Azolla has 25–35% crude protein, 10–15% minerals, and up to 10% amino acids, making it comparable to commercial protein feeds (Marzouk et al., 2023). Despite its high nutritional value, inclusion in animal diets is generally limited to 25% due to anti-nutritional compounds.
Among species, A. pinnata, native to warm regions, has higher polyphenolic tannins, reducing digestibility, while A. filiculoides, found in the Americas and Europe, contains lower polyphenols and higher protein, making it a better protein source (Brouwer et al., 2018)). Azolla is also rich in lysine, methionine, and arginine, essential for muscle growth, as well as key minerals like calcium, phosphorus, magnesium, iron, and potassium, which support bone health and metabolism (Herath et al., 2023; Yohana et al., 2023). High beta-carotene and vitamin A levels enhance vision, immunity, and reproduction (Adhikari et al., 2020). Its low lignin content (<5%) ensures high digestibility for both ruminants and non-ruminants (Raja et al., 2012), while bioactive compounds such as flavonoids and phenolics improve gut health, feed efficiency, and disease resistance (Kour et al., 2024).
3.3.1 Azolla in dairy and meat production
Azolla supplementation in dairy cattle diets improves milk yield, enhances milk quality, and reduces feed costs. Replacing 15–25% of commercial feed with Azolla in crossbred cows increased milk and fat percentage and yield by 7–13%, while reducing feed costs by 20–25% (Katole et al., 2017; Bujak and Bujak, 2022; Nasir et al., 2022; Alebachew Chekol et al., 2024). A 10–15% replacement of conventional cattle feed increased milk yield by 15–20%, with improved fat and protein content (Herath et al., 2023). Fresh supplementation of up to 1 kg/day increased yield by 7–13%, with extended feeding (28–63 days) further enhancing production (Roy et al., 2018). In buffaloes, daily feeding of 1.5 kg Azolla increased milk yield by 15–20% (Meena et al., 2017), while supplementation with cottonseed cake raised output from 8.0 to 9.3 L/day (Chatterjee et al., 2013).
In beef cattle and meat production, feeding Azolla to cattle and goats for two months increased milk production by 10–15% and meat yield by 8–10% (Alebachew Chekol et al., 2024). Feeding 5% dried Azolla improved feed conversion efficiency by 20% and daily gain by ~16% in heifers (Roy et al., 2016). In Sahiwal calves (Bos indicus), substituting 15–30% of concentrate protein with Azolla pinnata significantly enhanced growth, particularly in winter (Bhatt et al., 2021). The substitution of groundnut cake nitrogen with Azolla in buffalo calves improved daily weight gain, while 25% protein replacement in Murrah bulls’ concentrate had no negative effect.
Studies on small ruminants confirm that Azolla can partially replace protein sources in their diets. In Black Bengal goat, replacing 50% of concentrate with sun-dried Azolla caused severe diarrhea, but up to 20% inclusion was tolerated without adverse effects (Tamang and Samanta, 1995). In Jalauni lambs, Azolla replaced 25% of mustard cake protein without impacting nutrient digestibility (Das et al., 2017). Similarly, in Mecheri lambs, 10% Azolla in concentrate feed had no effect on dry matter intake, average daily gain, or feed efficiency (Sankar et al., 2020). For Corriedale sheep, diets replacing 25% of linseed cake with 6% Azolla showed no negative impact on performance (Ahmed et al., 2016). In goats, up to 15% sun-dried Azolla could be included in concentrate feed without adverse effects (Sajjan Sihag et al., 2018). Goats supplemented with 15% Azolla maintained digestible crude protein and nutrient intake (Sajjan Sihag et al., 2018; El Naggar and El-Mesery, 2022).
In pigs, A. filiculoides partially replaced soybean protein at 15–30%, leading to reduced growth in the early phase but improved compensatory growth during finishing (Becerra et al., 1990). Optimal Azolla replacement rates were 10% in the growing phase and 20% in the finishing phase, with higher inclusion levels negatively affecting weight gain and feed conversion efficiency (Durán, 1994). Azolla pinnata inclusion up to 20% in pig diets reduced feed costs while maintaining weight gain (Cherryl et al., 2013).
In other monogastric animals, the beneficial effects of Azolla have been studied in horses and rabbits. In Marwari stallions, replacing 10% of concentrate protein with A. pinnata had no effect on body weight or nutrient digestibility, supporting its suitability as a protein supplement (Songara et al., 2018). Similarly, supplementing rabbit feed with 1.5–3% A. pinnata in place of wheat bran and lucerne meal maintained normal growth performance (Sireesha et al., 2017).
3.3.2 Azolla as poultry feed
Azolla is a sustainable poultry feed rich in protein, essential amino acids, vitamins, and bioactive compounds, enhancing growth performance and feed efficiency. A 5% Azolla inclusion enhanced broiler weight gain and feed efficiency (Parthasarathy et al., 2001), while 10% increased weight gain and reduced feed intake (Alalade and Iyayi, 2006). A 7.5% inclusion improved body weight by 2.6% (Prabina and Kumar, 2010), with optimal growth at 5–10%. Azolla supports digestion and gut microbiota in poultry, enhancing digestibility at 10–15% inclusion (Samad et al., 2020). Broilers fed 10% Azolla gained 1810 g versus 1270 g on conventional feed (Rai et al., 2012), with improved digestibility linked to increased duodenal thickness (Rana et al., 2017). A 5–7% Azolla diet with multivitamins and acidifiers lowered feed conversion ratio, mortality, and costs while boosting profit (Bolka, 2011; Islam and Nishibori, 2016). Previous studies reported that the Azolla fiber was more digestible than rice bran (Joysowal et al., 2018) and supported metabolism, immunity, and gut health in chickens and safety was confirmed up to 7% inclusion (Mishra et al., 2016). Broilers fed 10% Azolla showed higher Newcastle Disease antibody titers (Prabina and Kumar, 2010), while a 5.5% diet enhanced immune markers in turkeys (Bhattacharyya et al., 2016). Azolla supplementation (5–10%) boosted immunity, likely due to its carotenoids, minerals, and nitrogen-fixing Anabaena (Chichilichi et al., 2015; Mishra et al., 2016).
Previous studies confirmed that Azolla enhances egg production and quality without adverse effects up to 20% inclusion (Rathod et al., 2013; Chisembe et al., 2020; Alagawany et al., 2024). Layers fed 100 g/day produced more eggs at lower costs (Kannaiyan and Kumar, 2005). Ducks on a 10–20% Azolla diet showed increased egg weight and better feed conversion (Swain et al., 2022). A 5% inclusion improved egg production (53.2 vs. 49.9 on concentrate, 47 on forage) and body weight (Alebachew Chekol et al., 2024). Fresh Azolla supported growth in backyard poultry, while its carotenoids enhanced yolk pigmentation and egg yield (Ali and Leeson, 1995). Additionally, Azolla enhances meat quality and overall health outcomes in poultry. For example, a 5% Azolla diet significantly increased dressing percentage (Basak et al., 2002), while a 4.5% diet improved giblet yield and reduced serum cholesterol (Balaji et al., 2009). Broilers fed 5–10% Azolla exhibited better meat color and reduced cooking loss (Abdelatty et al., 2020). However, excessive supplementation may cause a greenish tint in meat.
3.3.3 Azolla in aquaculture and fish farming
Azolla has been extensively investigated as a potential feed supplement in aquaculture due to its high protein content, balanced amino acid profile, and natural pigments, which contribute to improved growth rates and enhanced coloration in fish species such as tilapia and carp (Marzouk et al., 2023) (Table 2). Additionally, the incorporation of Azolla in fish diets has been shown to enhance water quality by absorbing excess nutrients and mitigating algal blooms, thereby creating a more balanced aquatic environment (Kollah et al., 2016). Various freshwater fish species, including tilapia (Oreochromis niloticus), redbelly tilapia (Coptodon zillii), catfish, fringed-lipped carp (Labeo fimbriatus), calbasu (Labeo calbasu), and Thai silver barb, have been successfully fed Azolla-based diets in controlled experimental settings (Das et al., 2018; Yohana et al., 2023). Examples of previous studies assessing the impact of Azolla supplementation on fish growth and survival are provided in Table 2.Previous studies reported that Tilapia can tolerate up to 20% Azolla in their diet without growth impairment (Magouz et al., 2020; Alebachew Chekol et al., 2024), with a recommended daily intake of 100 g for juveniles and 200 g for adults (El-Sayed and Garling, 1988). Inclusion levels vary by species, with rohu tolerating up to 50%, Thai silver barb 25%, fringed-lipped carp 40%, and calbasu 30%. A 25% A. pinnata diet in Thai silver barb showed no significant differences in growth or survival compared to controls (Das et al., 2018; Yohana et al., 2023).
Moderate Azolla inclusion enhances feed conversion ratio, protein efficiency, and energy utilization, while excessive levels may impair digestion due to antinutritional factors like phytates and fibers (Yohana et al., 2023). Its bioactive compounds, including phenols and flavonoids, support antioxidant and immunostimulatory functions (Lumsangkul et al., 2022). Azolla supplementation also boosts goblet cell production, strengthening the mucosal barrier and enhancing disease resistance in fish (Lumsangkul et al., 2022). In biofloc systems, Nile tilapia fed 100 g/kg Azolla exhibited improved immune responses and growth performance. While Azolla is promising as an aquaculture feed, but its amino acid balance still needs improvement, and the negative effects of its anti-nutritional compounds need to be reduced (Lumsangkul et al., 2022; Yohana et al., 2023).
4 Azolla’s contribution to climate resilience
4.1 CO2 absorption potential
Azolla is an efficient natural sink for atmospheric CO2 due to its rapid growth, high biomass accumulation, and symbiosis with A. azollae, which enables continuous nitrogen fixation without external inputs ( (Vroom et al., 2024) (Kour et al., 2024). Its integration into wetlands and rice paddies enhances carbon cycling, soil carbon storage, and long-term ecosystem stability (Sadeghi et al., 2014).
Evidence shows that Azolla can sequester CO2 at rates comparable to, or greater than, terrestrial plants. The Eocene “Azolla bloom” contributed significantly to global cooling, highlighting its historic efficiency as a CO2 sink (Yuan et al., 2024). Modern studies estimate that a 1-ha Azolla Pond captures 21,266 kg CO2 annually, and that 1,018,023 km² (one-fifth the Amazon) could offset current global CO2 increases. Compared to terrestrial ecosystems, which absorb 20–30% of anthropogenic emissions, Azolla ponds remove CO2 18 times more efficiently than an equivalent Amazon forest area (Hamdan and Houri, 2022).
Practical applications extend beyond sequestration. As a biofertilizer, Azolla improves soil quality and reduces the need for inorganic fertilizers that contribute to GHG emissions (Hamdan and Houri, 2022). In poultry farming, replacing 50% of feed with Azolla reduced CO2 by 35%, N2O by 22.3%, and CH4 by 4.7%, corresponding to a 28.5% reduction in global warming potential per 1,000 birds (Espino and Bellotindos, 2020). Similarly, cultivation trials reported annual fixation of 1.86 t CO2 and 0.33 t N ha-¹, providing dual climate and agronomic benefits (Brinkhuis and Bijl, 2014). In Sri Lanka, expanded Azolla use in paddy fields could mitigate 509,422 t of CO2 annually (Surenthiran and Loganathan, 2012), while A. filiculoides sequesters 32.54 metric tons CO2 ha-¹ year-¹, surpassing grassland, forest, and algae (Dawson and Smith, 2007).
CO2 enrichment experiments (380–680 ppm) further demonstrated enhanced Azolla biomass, confirming its scalability as a mitigation strategy (Cheng et al., 2010). During the Azolla interval, mean sea surface temperatures dropped from 13°C to 10°C, demonstrating its historical role in climate regulation (Brinkhuis et al., 2006). Sensitivity analyses indicate that optimal Azolla cultivation could require sequestration areas between 763,518 and 1,527,036 km² to significantly counteract atmospheric CO2 rise. Given its historical impact on climate stabilization and its efficiency in CO2 capture, Azolla-based strategies could contribute significantly to mitigating global warming and enhancing carbon management in agroecosystems (Hamdan and Houri, 2022).
4.2 Impact on methane emissions in rice cultivation
Agriculture is a major contributor to greenhouse gas (GHG) emissions, particularly CO2, CH4, and N2O (Chataut et al., 2023). CO2 results from microbial decay and organic matter oxidation, while CH4, a potent GHG with 20–60 times the global warming potential of CO2, is produced under anaerobic conditions in flooded rice paddies, livestock digestion, and manure storage (Smith et al., 2008). N2O arises from nitrogen transformations in soil, particularly under excessive fertilization (Xiao et al., 2024). Rice paddies contribute ~20% of global CH4 emissions, necessitating mitigation strategies. Azolla offers a natural means of reducing CH4 emissions in rice systems. By releasing oxygen, absorbing excess nutrients, and altering soil redox potential, Azolla suppresses methanogenesis while maintaining or improving rice yields (Razavipour et al., 2018). Field studies consistently report 30–60% reductions in CH4 emissions when Azolla is used as green manure or a floating cover (Serag et al., 2000a). In a three-year double rice cropping study, Azolla integration lowered CH4 emissions, reduced nitrogen fertilizer requirements, and maintained yields, largely due to improved soil oxygenation (Xu et al., 2017).
Synergistic practices further enhance these benefits. In Japan, combining Azolla with poultry-litter biochar increased rice yields by 27–75% while cutting CH4 by ~25% and N2O by up to 98% (Kimani et al., 2020). In India, dual cropping with Azolla reduced CH4 flux by 40% compared to urea fertilization alone, confirming the role of oxygen release in lowering emissions (Bharati et al., 2000). Laboratory studies also show that soils treated with Azolla and urea exhibit higher CH4 oxidation than urea alone, due to oxygen supplied by cyanobacteria (Adhya et al., 2000; Sood et al., 2012). Comparisons with other organic amendments reveal that CH4 efflux per grain yield is lowest in Azolla+urea systems, outperforming Sesbania, farmyard manure, and urea alone (Adhya et al., 2000). Although some Chinese studies reported higher CH4 emissions under Azolla dual cropping (Ying et al., 2000), soil type and nutrient status strongly influence outcomes. For example, Indian soils tend to produce lower CH4 flux under similar management (Gollany et al., 2015). Alternative integrated models, such as rice–fish culture, also reduce CH4 by improving soil aeration, lowering emissions by ~35% compared to conventional paddies (Kollah et al., 2016). Combining Azolla with such climate-smart practices could provide scalable, site-specific solutions for mitigating CH4 emissions while enhancing rice productivity and sustainability.
4.3 Bioremediation and pollution control
Azolla is an efficient phytoremediator with the ability to absorb and accumulate heavy metals from contaminated water. Both living and dead biomass are effective in removing pollutants, owing to mechanisms that include passive adsorption onto cell walls and active metabolic uptake (Sood et al., 2012). Its rapid growth and high bioaccumulation potential enable the uptake of Pb, Cd, As, Cr, and Hg from industrial and agricultural effluents, with removal efficiencies reported up to 80% (Vroom et al., 2024). The mechanism of heavy metal uptake involves passive adsorption onto cell walls as well as active metabolic absorption, making Azolla a suitable candidate for the remediation of polluted wetlands, rivers, and agricultural runoff areas (Sadeghi et al., 2014).
Field studies show that A. pinnata removes 70–94% of heavy metals from effluents, with tissue concentrations up to 740 mg/kg (Rai, 2008). Azolla filiculoides efficiently absorbs Cr, Pb, Zn, Hg, Cu, Cd, Ag, and Ti from wetland environments, demonstrating its potential for metal removal in natural water bodies (Hassanzadeh et al., 2021). Hydroponic studies indicate species-specific differences: A. caroliniana accumulated up to 284 mg/kg of As, while A. filiculoides accumulated only 54 mg/kg (Zhang et al., 2008). Another study reported that A. caroliniana also bioaccumulates Hg and Cr (III and VI), with tissue concentrations between 71 and 964 mg/kg dry weight (Bennicelli et al., 2004).
The tolerance of Azolla to heavy metals varies among species. For example, A. filiculoides demonstrated the highest tolerance to Cr exposure, retaining 72% of its control biomass under contamination (Arora et al., 2006). Nonetheless, heavy metals reduce growth, chlorophyll, and protein content, with Cd and Pb showing the greatest toxicity (Sarkar and Jana, 1986; Guo-Xin et al., 2003; Sood et al., 2012). Stress responses include reduced photosynthesis, O2 evolution, and enzyme activity, while detoxification is supported by increased phenolics and PAL activity (Dai et al., 2006). Similarly, Hg toxicity in A. pinnata reduces chlorophyll a, protein, RNA, DNA, and nutrient uptake, further compromising growth and metabolic functions (Rai and Tripathi, 2009). Copper particularly affects photosystem II efficiency (Sanchez-Viveros et al., 2010).
Heavy metal exposure also induces ultrastructural damage in Azolla, affecting organelles at the cellular level. Structural disruptions include chloroplast swelling, mitochondrial deformation, chromatin condensation, and nuclear membrane disintegration (Sela et al., 1988, 1990; Sood et al., 2012). Copper accumulates preferentially in roots, whereas Cd is evenly distributed in plant tissues, forming detoxification aggregates with PO4 and Ca (Sela et al., 1988). Cadmium localizes in the epidermis, cortex, and bundle cell walls within 77 hours, leading to the formation of electron-dense granules (Sela et al., 1990). Lead precipitation in A. filiculoides was primarily localized in vacuoles, with higher accumulation in mature leaves (Benaroya et al., 2004). In A. pinnata, Pb exposure caused frond compactness, stomatal closure, and epicuticular wax deposition, though these effects were mitigated by Fe supplementation (Gaumat et al., 2008).
Beyond metal accumulation, Azolla has demonstrated potential for biosorption. Studies show that dead or pretreated Azolla biomass effectively removes Cs, Sr, Pb, Zn, Ni, Cu, Au, Cd, and Cr from contaminated water sources (Sood et al., 2012). The bioaccumulation capacity of Azolla is influenced by metal concentration and environmental conditions (Dai et al., 2006; Sood et al., 2012). Hydroponic experiments have shown that A. filiculoides can remove Cr6+ with a maximum adsorption capacity of 20.2 mg/g at pH 2 and 32°C, while Ni uptake reached 27.9 mg/g at 60% saturation (Zhao and Duncan, 1998). Pb removal efficiency by A. filiculoides remained at approximately 90% between 10 and 50°C, with minimal influence from biomass concentration (Sanyahumbi et al., 1998). Furthermore, A. filiculoides has demonstrated a 99.9% efficiency in gold biosorption at pH 2 (Sood et al., 2012).
Apart from heavy metal removal, Azolla effectively removes excess nutrients from wastewater, reducing eutrophication risks. Azolla filiculoides has been reported to extract up to 122 kg of phosphorus per hectare annually (Vroom et al., 2024). Sequential treatment using Landoltia punctata followed by A. filiculoides achieved complete NH4 and NO3 removal and a 93% reduction in PO4, significantly lowering wastewater toxicity (Miranda et al., 2020). In addition, Azolla plays a crucial role in domestic wastewater treatment by removing nitrogen and phosphorus, thereby improving water quality for irrigation (Muradov et al., 2014). Laboratory studies further indicate that A. filiculoides effectively removes textile dyes such as Congo Red, Acid Red 88, Acid Green 3, Acid Orange 7, and Basic Orange from industrial effluents (Tan et al., 2010; Sood et al., 2012). The biosorption potential of Azolla is enhanced by its rapid growth and high surface area, making it an eco-friendly alternative to conventional wastewater treatment methods (Sood et al., 2012).
5 Other uses of Azolla
Azolla is a promising and sustainable biofuel feedstock due to its rapid growth, high lipid content, and adaptability to various conversion processes (Arora et al., 2022; Ramesh and Rajendran, 2022). Pyrolysis of Azolla yields hydrocarbons, including straight-chain alkanes, making it a potential diesel substitute. However, its high moisture content requires drying, and heavy metal emissions must be managed. Activated carbon catalysts improve both bio-oil yield and quality.
Transesterification is another widely studied method for biodiesel production. Crude oil extracted from dried Azolla biomass can be processed through acid transesterification to produce fatty acid methyl esters with properties like conventional diesel. Efficiency depends on maintaining optimal reaction temperatures (47–60°C) and reactant ratios, which require further study (Arora et al., 2022; Prabakaran et al., 2022). Hydrothermal liquefaction and torrefaction convert Azolla into bio-crude oil under high temperature and pressure. Torrefaction reduces moisture content and enhances fuel stability. Ethanol production involves hydrolysis, yeast isolation, and fermentation: acid treatment breaks down the biomass, sugars are released, and microbial fermentation produces ethanol. Additionally, microbial fuel cells using Azolla biomass and pyrolyzed biochar as anodes have shown potential for bioelectricity generation while reducing chemical oxygen demand (Miranda et al., 2016; Arora et al., 2022; Prabakaran et al., 2022).
Azolla is also a promising feedstock for bioplastics due to its rapid growth, high biomass yield, and diverse biochemical composition (Kouchakinejad et al., 2024). Unlike corn and sugarcane, Azolla does not compete with food production, making it a more sustainable alternative. Its cellulose and hemicellulose support bioplastic synthesis through chemical and enzymatic hydrolysis, while protein and lipid fractions can produce protein-based and lipid-derived bioplastics, including polyhydroxyalkanoates (PHA).
Blending Azolla biomass with poly (lactic acid) or starch can improve biodegradability and mechanical performance. Microbial fermentation also enables PHA production, providing a renewable alternative to petroleum-based plastics. A biorefinery approach, integrating bioplastic production with biofuels and biofertilizers, maximizes resource efficiency. Given its rapid biomass doubling and adaptability to pond and bioreactor cultivation, Azolla offers a scalable and efficient pathway for sustainable bioplastic production (Supriya et al., 2023; Kouchakinejad et al., 2024).
6 Challenges and limitations in Azolla utilization
Despite its numerous benefits, large-scale cultivation of Azolla faces several constraints related to environmental requirements, ecological risks, preservation, and economic feasibility.
Environmental and agronomic constraints: Azolla thrives under specific conditions—temperatures of 20–30°C, high humidity, adequate sunlight, and still or slow-moving water (Watanabe et al., 1989). Fluctuations in climate, seasonal variations, and poor water quality reduce productivity in open systems (Sadeghi et al., 2013). Continuous nutrient uptake can deplete nitrogen and phosphorus, requiring supplementation to sustain biomass yields (Razavipour et al., 2018). Growth is also inhibited under highly acidic or alkaline pH (Madeira et al., 2013). Competition from algae and aquatic weeds, coupled with risks of stagnation, oxygen depletion, and biomass decay, further limit performance (Vroom et al., 2024).
Ecological risks and invasiveness: Certain species, such as A. filiculoides and A. pinnata, can proliferate rapidly, doubling biomass every 3–5 days under optimal conditions (Vroom et al., 2024). Unchecked growth forms dense mats that block light, lower dissolved oxygen, and disrupt aquatic ecosystems (Sadeghi et al., 2014). Invasive infestations in wetlands, irrigation canals, and lakes have displaced native vegetation and altered water chemistry (Madeira et al., 2013). Mitigation requires controlled cultivation, floating containment systems, routine harvesting, and ecological risk assessments before introduction into new environments.
Preservation and shelf-life limitations: Fresh Azolla decomposes within days, making storage and transport difficult for feed and biofertilizer use (Razavipour et al., 2018). Various preservation techniques, including sun-drying, freeze-drying, and ensiling, have been explored; however, these methods often result in nutrient degradation and reduced digestibility for livestock consumption (Sadeghi et al., 2014). Long-term storage demands airtight facilities to prevent fungal contamination. Cost-effective preservation strategies—such as optimized dehydration or fermentation-based methods—are still under development.
Safety and consistency concerns: Azolla’s ability to accumulate heavy metals, while valuable for phytoremediation, poses risks when biomass from polluted environments is used for feed or fertilizer (Madeira et al., 2013) Furthermore, nutrient composition varies with species, climate, and soil conditions, making it difficult to ensure consistent feed quality. Standardized cultivation protocols are required to deliver predictable nutritional value (Vroom et al., 2024).
Logistical and regulatory barriers: High water content makes transport of fresh biomass costly and inefficient, requiring drying or processing facilities for distribution. In some regions, Azolla is classified as an invasive species, restricting its cultivation and use (Razavipour et al., 2018). Clear regulatory frameworks and risk assessments are therefore essential to balance utilization with ecological safeguards.
Adoption and economic limitations: Despite proven agronomic benefits, adoption among farmers remains low due to limited awareness, technical expertise, and uncertainties about labor requirements (Watanabe et al., 1989). Initial investment in ponds, harvesting, and processing infrastructure also discourages small-scale farmers, even though long-term savings on fertilizers and feed are possible (Razavipour et al., 2018). High water content, short shelf life, and the need for preservation or processing facilities further increase production and transport costs, limiting economic feasibility. To overcome these barriers, farmer training, cost-sharing schemes, and targeted incentive programs—such as subsidies, integration into carbon credit markets, and inclusion in climate-smart agriculture policies—are needed to promote wider adoption and improve sustainability.”.
7 Future prospects and research directions
Azolla is a promising resource for sustainable agriculture, but realizing its large-scale potential requires advances in genetics, cultivation technologies, and commercial integration.
Genetic and biotechnological improvement: Selective breeding, molecular breeding, and gene editing hold potential to enhance biomass yield, nitrogen fixation efficiency, and tolerance to abiotic stresses such as drought, salinity, and temperature fluctuations (Madeira et al., 2013; Vroom et al., 2024). Future work should also explore microbial symbiosis optimization and metabolic engineering to develop high-yielding, stress-resilient strains with consistent nutrient composition for diverse environments.
Precision agriculture and digital tools: Integrating Azolla cultivation with remote sensing, drone-based monitoring, and modelling based analytics could improve nutrient management, predict biomass productivity, and optimize harvesting schedules. Automated water-quality sensors and modeling can further minimize risks of uncontrolled growth and invasiveness, enabling more efficient and scalable production systems.
Commercial applications and sustainability pathways: Beyond biofertilizers, Azolla offers opportunities in livestock feed, aquaculture, wastewater treatment, and carbon markets. Its high protein content supports poultry, cattle, swine, and fish diets, reducing dependence on conventional feeds. As a biofertilizer, it aligns with organic and regenerative farming systems, while its CO2 sequestration capacity creates potential for participation in carbon credit schemes. In parallel, its ability to absorb heavy metals makes it a valuable tool in industrial wastewater treatment and pollution control.
8 Conclusion
Azolla is a multifunctional resource that supports sustainable agriculture and climate resilience. Its symbiosis with A. azollae enables efficient nitrogen fixation, reducing dependence on synthetic fertilizers while enhancing soil health, conserving water, and suppressing weeds and pests. As a high-protein feed, Azolla improves livestock, poultry, and aquaculture performance, though anti-nutritional factors and compositional variability remain challenges. Beyond agriculture, Azolla contributes to climate change mitigation through rapid carbon sequestration and reduced methane emissions in rice systems, while also offering bioremediation potential for polluted waters. Its emerging applications as a feedstock for biofuels and bioplastics further highlight its industrial value. However, large-scale use faces barriers such as short shelf life, high water content, and ecological risks from invasiveness.
Overall, Azolla represents a low-cost, eco-friendly tool with wide-ranging benefits across food production, climate mitigation, and environmental management. Addressing preservation, standardization, and ecological safeguards, along with advances in genetics and precision cultivation, will be critical to unlocking its full potential as a cornerstone of climate-smart agriculture.
Author contributions
YoY: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. YaY: Conceptualization, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. SD: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. ZY: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the China Agriculture Research System Green Manure program (Grant No. CARS-22). The authors gratefully acknowledge this funding support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelatty, A., Mandouh, M., Al-Mokaddem, A., Mansour, H., Khalil, H., Elolimy, A., et al. (2020). Influence of level of inclusion of Azolla leaf meal on growth performance, meat quality and skeletal muscle p70S6 kinase α abundance in broiler chickens. Animal 14, 2423–2432. doi: 10.1017/S1751731120001421
Abdel-Tawwab, M. (2008). The preference of the Omnivorous–Macrophagous, Tilapia zillii (Gervais), to consume a natural free-floating fern, Azolla pinnata. J. World Aquaculture Soc. 39, 104–112. doi: 10.1111/j.1749-7345.2007.00131.x
Abou, Y., Aina, M. P., Fiogbé, E. D., and Micha, J.-C. (2013). Growth and fatty acid composition of Nile tilapia Oreochromis niloticus L. fed Azolla-diets, in tanks and in earthen ponds: a comparative study. Natural Sci. 5, 77–83. doi: 10.4236/ns.2013.51013
Abou, Y., Darchambeau, F., Fiogbé, E. D., and Micha, J.-C. (2012). Ecology of feeding nile tilapia under Azolla cover in earthen ponds: an assessment using structural equation modelling. Int. J. Biosci. 2, 105–111.
Abou, Y., Fiogbe, E., Aina, M., and Buldgen, A. (2010). Evaluation of nitrogen and phosphorus wastes produced by Nile tilapia (Oreochromis niloticus L.) fed Azolla-diets in concrete tanks. Int. J. Biol. Chem. Sci. 4, 41–50. doi: 10.4236/jep.2012.36060
Abou, Y., Fiogbé, E. D., and Micha, J. C. (2007). Effects of stocking density on growth, yield and profitability of farming Nile tilapia, Oreochromis niloticus L., fed Azolla diet, in earthen ponds. Aquaculture Res. 38, 595–604. doi: 10.1111/j.1365-2109.2007.01700.x
Adhikari, K., Bhandari, S., and Acharya, S. (2020). An overview of azolla in rice production: a review. Rev. Food Agric. 2, 04–08. doi: 10.26480/rfna.01.2021.04.08
Adhya, T., Bharati, K., Mohanty, S. R., Ramakrishnan, B., Rao, V. R., Sethunathan, N., et al. (2000). Methane emission from rice fields at Cuttack, India. Nutrient Cycling Agroecosystems 58, 95–105. doi: 10.1023/A:1009886317629
Ahluwalia, A., Pabby, A., and Dua, S. (2002). Azolla: A green gold mine with diversified applications. Indian Fern J. 19, 1–9.
Ahmed, H., Ganai, A., Beigh, Y., Sheikh, G., and Reshi, P. (2016). Performance of growing sheep on Azolla based diets. Indian J. Anim. Res. 50, 721–724. doi: 10.18805/ijar.9642
Alagawany, M., Elnesr, S. S., Saleh, A. A., El-Shall, N. A., Azzam, M. M., Dhama, K., et al. (2024). An updated review of Azolla in poultry diets. World’s Poultry Sci. J. 80, 155–170. doi: 10.1080/00439339.2023.2271886
Alalade, O. and Iyayi, E. (2006). Chemical composition and the feeding value of Azolla (Azolla pinnata) meal for egg-type chicks. Int. J. Poultry Sci. 5, 137–141. doi: 10.3923/ijps.2006.137.141
Alebachew Chekol, S., Nigussie, T. Z., and Fenta, B. A. (2024). Azolla as a beneficial macrophyte for livestock feed: a review. Cogent Food Agric. 10, 2367804. doi: 10.1080/23311932.2024.2367804
Ali, M. and Leeson, S. (1995). Nutritional value and aquatic weeds in the diet of poultry. World Poult. Sci. J. 50, 239–251.
Al-Sayed, H. M., Ali, A. M., Mohamed, M. A., and Ibrahim, M. F. (2022). Combined effect of prickly pear waste biochar and Azolla on soil fertility, growth, and yield of Roselle (Hibiscus sabdariffa L.) plants. J. Soil Sci. Plant Nutr. 22, 3541–3552. doi: 10.1007/s42729-022-00908-7
Ambrose, B. A. and Vasco, A. (2016). Bringing the multicellular fern meristem into focus. New Phytol. 210, 790–793. doi: 10.1111/nph.13825
Ameena, M., Deb, A., Umkhulzum, F., Kalyani, M., Sethulakshmi, V., Sreelakshmi, K., et al. (2024). Ecology and management of sedges in direct-seeded rice: A review. Agric. Rev. 10, 1–10. doi: 10.18805/ag.R-2717
Ansabayeva, A., Makhambetov, M., Rebouh, N. Y., Abdelkader, M., Saudy, H. S., Hassan, K. M., et al. (2025). Plant growth-promoting microbes for resilient farming systems: mitigating environmental stressors and boosting crops productivity—a review. Horticulturae 11, 260. doi: 10.3390/horticulturae11030260
Arora, A., Nandal, P., and Chaudhary, A. (2022). Critical evaluation of novel applications of aquatic weed Azolla as a sustainable feedstock for deriving bioenergy and feed supplement. Environ. Rev. 31, 195–205. doi: 10.1139/er-2022-0033
Arora, A., Saxena, S., and Sharma, D. K. (2006). Tolerance and phytoaccumulation of chromium by three Azolla species. World J. Microbiol. Biotechnol. 22, 97–100. doi: 10.1007/s11274-005-9000-9
Awodun, M. (2008). Effect of Azolla (Azolla species) on physiochemical properties of the soil. World J. Agric. Sci. 4, 157–160.
Bahadur, S., Verma, S., Prasad, S., Madane, A., Maurya, S., Gaurav, V., et al. (2015). Eco-friendly weed management for sustainable crop production-A review. J. Crop Weed 11, 181–189.
Balaji, K., Jalaludeen, A., Churchil, R. R., Peethambaran, P., and Senthilkumar, S. (2009). Effect of dietary inclusion of Azolla (Azolla pinnata) on production performance of broiler chicken. Indian J. Poultry Sci. 44, 195–198.
Barthakur, H. and Talukdar, H. (1983). Use of azolla and commercial nitrogen fertilizer in Jorhat, India. Plant Soil 62, 35–44.
Basak, B., Pramanik, M. A. H., Rahman, M. S., Tarafdar, S. U., and Roy, B. C. (2002). Azolla (Azolla pinnata) as a feed ingredient in broiler ration. Int. J. Poultry Sci. 1, 29–34. doi: 10.3923/ijps.2002.29.34
Becerra, M., Murgueitio, E., Reyes, G., and Preston, T. (1990). Azolla filiculoides as partial replacement for traditional protein supplements in diets for growing-fattening pigs based on sugar cane juice. Livestock Res. Rural Dev. 2, 15–22.
Benaroya, R. O., Tzin, V., Tel-Or, E., and Zamski, E. (2004). Lead accumulation in the aquatic fern Azolla filiculoides. Plant Physiol. Biochem. 42, 639–645. doi: 10.1016/j.plaphy.2004.03.010
Bennicelli, R., Stępniewska, Z., Banach, A., Szajnocha, K., and Ostrowski, J. (2004). The ability of Azolla caroliniana to remove heavy metals (Hg (II), Cr (III), Cr (VI)) from municipal waste water. Chemosphere 55, 141–146. doi: 10.1016/j.chemosphere.2003.11.015
Bharati, K., Mohanty, S., Singh, D., Rao, V., and Adhya, T. (2000). Influence of incorporation or dual cropping of Azolla on methane emission from a flooded alluvial soil planted to rice in eastern India. Agriculture Ecosyst. Environ. 79, 73–83. doi: 10.1016/S0167-8809(99)00148-6
Bhaskaran, S. K. and Kannapan, P. (2015). Nutritional composition of four different species of Azolla. Eur. J. Exp. Biol. 5, 6–12.
Bhatt, N., Tyagi, N., Chandra, R., Meena, D. C., and Prasad, C. K. (2021). Growth performance and nutrient digestibility of Azolla pinnata feeding in sahiwal calves (Bos indiens) by replacing protein content of concentrate with Azolla pinnata during winter season. Indian J. Anim. Res. 55, 663–668. doi: 10.18805/ijar.B-4004
Bhattacharyya, A., Shukla, P. K., Roy, D., and Shukla, M. (2016). Effect of Azolla supplementation on growth, immunocompetence and carcass characteristics of commercial broilers. J. Anim. Res. 6, 941–945. doi: 10.5958/2277-940X.2016.00122.4
Bhavyasree, K. (2015). Characterisation of soil and water of Palakkad eastern plains in relation to growth and nitrogenase content of Azolla spp. Kerala Agricultural University Vellanikkara, Thrissur, Kerala, India.
Bhuvaneshwari, K. and Kumar, A. (2013). Agronomic potential of the association Azolla-Anabaena. Sci. Res. Rep. 3, 78–82.
Bhuvaneshwari, K. and Singh, P. K. (2015). Response of nitrogen-fixing water fern Azolla biofertilization to rice crop. 3 Biotech. 5, 523–529. doi: 10.1007/s13205-014-0251-8
Bolka, P. (2011). Nutritional evaluation of azolla (Azolla pinnata) in broilers and layers. Karnataka Veterinary Animal And Fisheries Sciences University, Bidar, India.
Brinkhuis, H., Schouten, S., Collinson, M. E., Sluijs, A., Damsté, J. S. S., Dickens, G. R., et al. (2006). Episodic fresh surface waters in the Eocene Arctic Ocean. Nature 441, 606–609. doi: 10.1038/nature04692
Brouwer, P., Schluepmann, H., Nierop, K. G., Elderson, J., Bijl, P. K., van der Meer, I., et al. (2018). Growing Azolla to produce sustainable protein feed: the effect of differing species and CO2 concentrations on biomass productivity and chemical composition. J. Sci. Food Agric. 98, 4759–4768. doi: 10.1002/jsfa.9016
Bujak, A. and Bujak, J. (2022). “Azolla’s use as a biofertilizer and livestock feed,” in Ferns: Biotechnology, Propagation, Medicinal Uses and Environmental Regulation. Eds. Marimuthu, J., Fernández, H., and Kumar, A. (Singapore: Springer), 671–695.
Bujak, J. and Bujak, A. (2024). Origin and evolution of the Azolla superorganism. Plants 13, 2106. doi: 10.3390/plants13152106
Cagauan, A., Branckaert, R., and Van Hove, C. (2000). Integrating fish and azolla into rice-duck farming in Asia. Naga. ICLARM 23, 4–10.
Cagauan, A. and Pullin, R. (1994). “Azolla in aquaculture: past, present and future,” in Recent Advances in Aquaculture. Eds. Muir, J. and Roberts, R. J. (Blackwell Science, Oxford), 104–130.
Carrapiço, F. (2010). “Azolla as a superorganism. Its implication in symbiotic studies,” in Symbioses and stress: Joint ventures in biology. Eds. Seckbach, J. and Grube, M., (Dordrecht, Netherlands: Springer) 225–241.
Carrapiço, F. (2017). “The Azolla–Anabaena–Bacteria association: A case of symbiotic abduction?,” in Algal and Cyanobacteria Symbioses (Munich, Germany: World Scientific), 329–345.
Chataut, G., Bhatta, B., Joshi, D., Subedi, K., and Kafle, K. (2023). Greenhouse gases emission from agricultural soil: A review. J. Agric. Food Res. 11, 100533. doi: 10.1016/j.jafr.2023.100533
Chatterjee, A., Sharma, P., Ghosh, M., Mandal, M., and Roy, P. (2013). Utilization of Azolla microphylla as feed supplement for crossbred cattle. Int. J. Agric. Food Sci. Technol. 4, 207–214.
Cheng, W., Sakai, H., Matsushima, M., Yagi, K., and Hasegawa, T. (2010). Response of the floating aquatic fern Azolla filiculoides to elevated CO 2, temperature, and phosphorus levels. Hydrobiologia 656, 5–14. doi: 10.1007/s10750-010-0441-2
Cherryl, D., Prasad, R., and Jayalaxmi, P. (2013). A study on economics of inclusion of Azolla pinnata in swine rations. Int. J. Agric. Sci. Veterinary Med. 1, 50–56.
Chichilichi, B., Mohanty, G., Mishra, S., Pradhan, C., Behura, N., Das, A., et al. (2015). Effect of partial supplementation of sun-dried Azolla as a protein source on the immunity and antioxidant status of commercial broilers. Veterinary World 8, 1126–1130. doi: 10.14202/vetworld.2015.1126-1130
Chisembe, P., Banda, L. J., and Tanganyika, J. (2020). Effect of duck-rice-azolla integration on growth performance and carcass quality of native Malawian Muscovy ducks. Livestock Res. Rural Dev. 32, 110–112.
Cohen, M. F., Meziane, T., Tsuchiya, M., and Yamasaki, H. (2002). Feeding deterrence of Azolla in relation to deoxyanthocyanin and fatty acid composition. Aquat. Bot. 74, 181–187. doi: 10.1016/S0304-3770(02)00077-3
Dai, L. P., Xiong, Z. T., Huang, Y., and Li, M. J. (2006). Cadmium-induced changes in pigments, total phenolics, and phenylalanine ammonia-lyase activity in fronds of Azolla imbricata. Environ. Toxicol.: Int. J. 21, 505–512. doi: 10.1002/tox.20212
Das, M., Agarwal, R., Singh, J., Kumar, S., Singh, R. P., and Kumar, S. (2017). Nutrient intake and utilization in lambs fed Azolla microphylla meal as a partial replacement for mustard cake in concentrate mixture. Indian J. Anim. Nutr. 34, 45–49. doi: 10.5958/2231-6744.2017.00007.X
Das, M., Rahim, F. I., and Hossain, M. A. (2018). Evaluation of fresh Azolla pinnata as a low-cost supplemental feed for Thai Silver Barb Barbonymus gonionotus. Fishes 3, 15. doi: 10.3390/fishes3010015
Datta, S. N. (2011). Culture of Azolla and its efficacy in diet of Labeo rohita. Aquaculture 310, 376–379. doi: 10.1016/j.aquaculture.2010.11.008
Dawson, J. J. and Smith, P. (2007). Carbon losses from soil and its consequences for land-use management. Sci. Total Environ. 382, 165–190. doi: 10.1016/j.scitotenv.2007.03.023
Devaprakash, M., Thirumalaivasan, R., Sivakumar, N., and Shyamkumar, R. (2024). “Cyanobacterial interactions and symbiosis,” in Cyanobacteria Metabolisms to Molecules. Eds. Mishra, A. K. and Singh, S. S. (Mumbai, India: Elsevier), 425–489.
Dijkhuizen, L. W., Tabatabaei, B. E., Brouwer, P., Rijken, N., Mehta, R. S., Güngör, E., et al. (2021). Far-red light-induced Azolla filiculoides symbiosis sexual reproduction: responsive transcripts of symbiont Nostoc azollae encode transporters whilst those of the fern relate to the angiosperm floral transition. Front. Plant Sci. 12, 693039. doi: 10.3389/fpls.2021.693039
Durán, A. O. (1994). Raw palm oil as the energy source in pig fattening diets and Azolla filiculoides as a substitute for soya bean meal. Livestock Res. Rural Dev. 6, 1–8.
El Naggar, S. and El-Mesery, H. S. (2022). Azolla pinnata as unconventional feeds for ruminant feeding. Bull. Natl. Res. Centre 46, 66. doi: 10.1186/s42269-022-00752-w
El-Sayed, A. F. (1992). Effects of substituting fish meal with Azolla pinnata in practical diets for fingerling and adult Nile tilapia, Oreochromis niloticus (L.). Aquaculture Res. 23, 167–173. doi: 10.1111/j.1365-2109.1992.tb00607.x
El-Sayed, A.-F. M. and Garling, D. (1988). Carbohydrate-to-lipid ratios in diets for Tilapia zillii fingerlings. Aquaculture 73, 157–163. doi: 10.1016/0044-8486(88)90050-6
Espino, M. and Bellotindos, L. (2020). Reduction of greenhouse gas emissions of Azolla pinnata inclusion in backyard chicken production. Nat. Environ. pollut. Technol. 19, 18611869. doi: 10.46488/NEPT.2020.v19i05.010
Fao-Rome, I (1979). “China: Azolla propagation (water ferns) and small-scale biogas technology,” in Report on an FAO/UNDP study tour to the People’s Republic of China (Rome/ Italy: Food and Agriculture Organization of the United Nations), 81.
Fasakin, E., Balogun, A., and Fagbenro, O. (2001). Evaluation of Sun-dried water fern, Azolla africana and duckweed, Spirodela polyrrhiza in practical diets for Nile tilapia, Oreochromis niloticus fingerlings. J. Appl. Aquaculture 11, 83–92. doi: 10.1300/J028v11n04_09
Fiogbé, E., Micha, J. C., and Van Hove, C. (2004). Use of a natural aquatic fern, Azolla microphylla, as a main component in food for the omnivorous–phytoplanktonophagous tilapia, Oreochromis niloticus L. J. Appl. Ichthyol. 20, 517–520. doi: 10.1111/j.1439-0426.2004.00562.x
Gangadhar, B., Sridhar, N., Saurabh, S., Raghavendra, C., Hemaprasanth, K., Raghunath, M., et al. (2015). Effect of Azolla-incorporated diets on the growth and survival of Labeo fimbriatus during fry-to-fingerling rearing. Cogent Food Agric. 1, 1055539. doi: 10.1080/23311932.2015.1055539
Gaumat, S., Mishra, K., Rai, U. N., and Baipal, U. (2008). Ultramorphological variation in Azolla pinnata R. Br. under single and mixed metal treatment with lead and iron. Phytomorphology 58, 111–116.
Geetha, K., Bai, S. K., Pavan, A., Karthik, A., and Sindhu, K. (2022). Weed management in transplanted rice-A review. Mysore J. Agric. Sci. 56, 1–18. doi: 10.20546/ijcmas.2018.704.187
Gollany, H., Stott, D., Franzluebbers, A., and Johnson, J. (2015). Know your community: soil carbon and greenhouse gas emissions. Crops Soils Agron. News 60, 25. doi: 10.2134/csa2015-60-3-14
Guo-Xin, S., Qin-Song, X., Kai-Bin, X., Nan, X., Xiao-Lan, Z., Xiao-Min, Z., et al. (2003). Physiology and ultrastructure of Azolla imbricata as affected by Hg2+ and Cd2+ toxicity. J. Integr. Plant Biol. 45, 437.
Hamdan, H. Z. and Houri, A. F. (2022). CO 2 sequestration by propagation of the fast-growing Azolla spp. Environ. Sci. pollut. Res. 19, 16912–16924. doi: 10.1007/s11356-021-16986-6
Hassanzadeh, M., Zarkami, R., and Sadeghi, R. (2021). Uptake and accumulation of heavy metals by water body and Azolla filiculoides in the Anzali wetland. Appl. Water Sci. 11, 91. doi: 10.1007/s13201-021-01428-y
Herath, B., Karunarathna, S., Ishaq, M., Wariss, H., and Yapa, P. (2023). Azolla as the multifunctional fern in organic agriculture: prospects and challenges: a review article. Int. J. Agric. Technol. 19, 63–82.
Hundare, S., Pathan, D., and Ranadive, A. (2018). Use of fermented Azolla in diet of tilapia fry (Oreochromis niloticus). Int. J. Bio-resource Stress Manage. 9, 702–706. doi: 10.23910/IJBSM/2018.9.6.1925
Islam, M. and Nishibori, M. (2016). Use of multivitamin, acidifier and Azolla in the diet of broiler chickens. Asian-Australasian J. Anim. Sci. 30, 683–689. doi: 10.5713/ajas.16.0395
Jha, A. K., Rani, M., Padbhushan, R., Kumar, A., and Kumar, R. (2023). Combined application of azolla and inorganic potassium fertilizer influence the growth, yield and storability of onion in alluvial soil. Commun. Soil Sci. Plant Anal. 54, 1727–1740. doi: 10.1080/00103624.2023.2211091
Joysowal, M., Aziz, A., Mondal, A., Singh, S., Boda, S., Chirwatkar, B., et al. (2018). Effect of Azolla (Azolla pinnata) feed on the growth of broiler chicken. J. Entomol. Zool. Stud. 6, 391–393.
Kandel, S., Malla, R., Adhikary, B. H., and Vista, S. P. (2020). Effect of Azolla application on rice production at mid-hills condition of Nepal. Trop. Agroecosystems 1, 103–106. doi: 10.26480/taec.02.2020.103.106
Kannaiyan, S. and Kumar, K. (2005). Azolla biofertilizer for sustainable rice production (India: Daya Publishing House).
Katole, S. B., Lende, S. R., and Patil, S. (2017). A review on potential livestock feed: Azolla. Livestock Res. Int. 5, 1–9.
Khursheed, I., Masud, S., Khan, A., Khan, N., Kour, S., Dua, S., et al. (2019). Proximate evaluation of Azolla pinnata as sustainable feed supplement for poultry. J. Pharmacognosy Phytochem. 8, 3157–3160.
Kimani, S. M., Bimantara, P. O., Hattori, S., Tawaraya, K., Sudo, S., and Cheng, W. (2020). Azolla incorporation and dual cropping influences CH4 and N2O emissions from flooded paddy ecosystems. Soil Sci. Plant Nutr. 66, 152–162. doi: 10.1080/00380768.2019.1705736
Kimani, S. M., Bimantara, P. O., Kautsar, V., Torita, R., Hattori, S., Tawaraya, K., et al. (2022). Influence of Azolla incorporation and/or dual cropping on CH4 and N2O emission from a paddy field. Soil Sci. Plant Nutr. 68, 246–255. doi: 10.1080/00380768.2022.2047580
Kollah, B., Patra, A. K., and Mohanty, S. R. (2016). Aquatic microphylla Azolla: a perspective paradigm for sustainable agriculture, environment and global climate change. Environ. Sci. pollut. Res. 23, 4358–4369. doi: 10.1007/s11356-015-5857-9
Korsa, G., Alemu, D., and Ayele, A. (2024). Azolla plant production and their potential applications. Int. J. Agron. 2024, 1–12. doi: 10.1155/2024/1716440
Kouchakinejad, R., Lotfi, Z., and Golzary, A. (2024). Exploring Azolla as a sustainable feedstock for eco-friendly bioplastics: A review. Heliyon 10, e39252. doi: 10.1016/j.heliyon.2024.e39252
Kour, D., Ramniwas, S., Kumar, S., Rai, A. K., Singh, S., Rustagi, S., et al. (2024). Azolla for agro-environmental sustainability. Vegetos, 1–12. doi: 10.1007/s42535-024-01103-y
Kulasooriya, S. and De Silva, R. (1977). Effect of Azolla on yield of rice. Int. Rice Res. Newsl 2, 10.
Kumar, S., Sindhu, S. S., and Kumar, R. (2022). Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microbial. Sci. 3, 100094. doi: 10.1016/j.crmicr.2021.100094
Kumarasinghe, K. and Eskew, D. (1993). “Assessment of nitrogen fixation in Azolla using the 15N isotope dilution technique,” in Isotopic Studies of Azolla and Nitrogen Fertilization of Rice: Report of an FAO/IAEA/SIDA Co-ordinated Research Programme on Isotopic Studies of Nitrogen Fixation and Nitrogen Cycling by Blue-Green Algae and Azolla. Eds. Kumarasinghe, K. S. and Eskew, D. L. (Dordrecht, Netherland: Springer), 3–15.
Lechno-Yossef, S. and Nierzwicki-Bauer, S. A. (2002). “Azolla-Anabaena symbiosis,” in Cyanobacteria in symbiosis, eds Rai, A. N., Bergman, B., and Rasmussen, U. (Dordrecht, Netherland: Springer), 153–178.
Leonard, V., Breyne, C., Micha, J. C., and Larondelle, Y. (1998). Digestibility and transit time of Azolla filiculoides Lamarck in Oreochromis aureus (Steindachner). Aquaculture Res. 29, 159–165. doi: 10.1111/j.1365-2109.1998.tb01120.x
Li, F.-W., Brouwer, P., Carretero-Paulet, L., Cheng, S., De Vries, J., Delaux, P.-M., et al. (2018). Fern genomes elucidate land plant evolution and cyanobacterial symbioses. Nat. Plants 4, 460–472. doi: 10.1038/s41477-018-0188-8
Lu, X.-M., Lu, P.-Z., and Yang, K. (2017). Restoration using Azolla imbricata increases nitrogen functional bacterial groups and genes in soil. Appl. Microbiol. Biotechnol. 101, 3849–3859. doi: 10.1007/s00253-017-8108-9
Lumpkin, T. A. and Plucknett, D. L. (1980). Azolla: botany, physiology, and use as a green manure. Economic Bot. 34, 111–153. doi: 10.1007/BF02858627
Lumsangkul, C., Linh, N. V., Chaiwan, F., Abdel-Tawwab, M., Dawood, M. A., Faggio, C., et al. (2022). Dietary treatment of Nile tilapia (Oreochromis niloticus) with aquatic fern (Azolla caroliniana) improves growth performance, immunological response, and disease resistance against Streptococcus agalactiae cultured in bio-floc system. Aquaculture Rep. 24, 101114. doi: 10.1016/j.aqrep.2022.101114
Lydia, N., Basamba, T. A., Nyakoojo, C., Mustafa, A. S., Saidi, N., Mutumba, G. M., et al. (2023). Ecological distribution and genetic diversity of Azolla in Uganda. BMC Plant Biol. 23, 131. doi: 10.1186/s12870-023-04146-6
Madeira, P. T., Center, T. D., Coetzee, J. A., Pemberton, R. W., Purcell, M. F., and Hill, M. P. (2013). Identity and origins of introduced and native Azolla species in Florida. Aquat. Bot. 111, 9–15. doi: 10.1016/j.aquabot.2013.07.009
Madeira, P. T., Dray, F. A., Jr., and Tipping, P. W. (2019). Molecular identification of Azolla in the Yangtze River watershed, China. Aquat. Bot. 159, 103149. doi: 10.1016/j.aquabot.2019.103149
Madeira, P. T., Hill, M. P., Dray, F., Jr., Coetzee, J., Paterson, I., and Tipping, P. (2016). Molecular identification of Azolla invasions in Africa: The Azolla specialist, Stenopelmus rufinasus proves to be an excellent taxonomist. South Afr. J. Bot. 105, 299–305. doi: 10.1016/j.sajb.2016.03.007
Magouz, F. I., Dawood, M. A., Salem, M. F., and Mohamed, A. A. (2020). The effects of fish feed supplemented with Azolla meal on the growth performance, digestive enzyme activity, and health condition of genetically-improved farmed tilapia (Oreochromis niloticus). Ann. Anim. Sci. 20, 1029–1045. doi: 10.2478/aoas-2020-0016
Mahapatra, B. and Sharma, G. (1989). Integrated management of Sesbania, Azolla and urea nitrogen in lowland rice under a rice–wheat cropping system. J. Agric. Sci. 113, 203–206. doi: 10.1017/S0021859600086779
Mahmood, A. M., Khalil, M. I., and Darweesh, K. F. (2020). Morphological and molecular phylogenetic analyses reveal a new record to the flora of Iraq: Azolla filiculoides. Zanco J. Pure Appl. Sci. 32, 95–103. doi: 10.21271/ZJPAS.32.1.10
Maja, M. M. and Ayano, S. F. (2021). The impact of population growth on natural resources and farmers’ capacity to adapt to climate change in low-income countries. Earth Syst. Environ. 5, 271–283. doi: 10.1007/s41748-021-00209-6
Malyan, S. K., Singh, S., Bachheti, A., Chahar, M., Sah, M. K., Kumar, A., et al. (2020). “Cyanobacteria: a perspective paradigm for agriculture and environment,” in New and future developments in microbial biotechnology and bioengineering (Amsterdam, Netherlands: Elsevier), 215–224.
Marzouk, S. H., Tindwa, H. J., Amuri, N. A., Chande, H. H., and Semoka, J. M. (2024). Enhancing phosphorus use efficiency and soil quality indicators in lowland paddy ecosystem through Azolla, rice straw, and NPKS fertilizers. Front. Agron. 6, 1376110. doi: 10.3389/fagro.2024.1376110
Marzouk, S. H., Tindwa, H. J., Amuri, N. A., and Semoka, J. M. (2023). An overview of underutilized benefits derived from Azolla as a promising biofertilizer in lowland rice production. Heliyon 9, e13040. doi: 10.1016/j.heliyon.2023.e13040
Meena, G., Dhaka, B., Singh, B., Meena, R., and Meena, K. (2017). Effect of Azolla as feed supplement on milk yield in buffaloes. Int. J. Curr. Microbiol. Appl. Sci. 6, 3490–3494. doi: 10.20546/ijcmas.2017.612.406
Metzgar, J. S., Schneider, H., and Pryer, K. M. (2007). Phylogeny and divergence time estimates for the fern genus Azolla (Salviniaceae). Int. J. Plant Sci. 168, 1045–1053. doi: 10.1086/519007
Miranda, A. F., Biswas, B., Ramkumar, N., Singh, R., Kumar, J., James, A., et al. (2016). Aquatic plant Azolla as the universal feedstock for biofuel production. Biotechnol. Biofuels 9, 1–17. doi: 10.1186/s13068-016-0628-5
Miranda, A. F., Kumar, N. R., Spangenberg, G., Subudhi, S., Lal, B., and Mouradov, A. (2020). Aquatic plants, Landoltia punctata, and Azolla filiculoides as bio-converters of wastewater to biofuel. Plants 9, 437. doi: 10.3390/plants9040437
Mishra, D. B., Roy, D., Kumar, V., Bhattacharyya, A., Kumar, M., Kushwaha, R., et al. (2016). Effect of feeding azolla (Azolla pinnata) meal on the performance, nutrient utilization and carcass characteristics of Chabro chicken. Indian J. Poultry Sci. 51, 259–263. doi: 10.5958/0974-8180.2016.00060.X
Moore, A. W. (1969). Azolla: biology and agronomic significance. Bot. Rev. 35, 17–34. doi: 10.1007/BF02859886
Mosha, S. (2018). A review on significance of Azolla meal as a protein plant source in finfish culture. J. Aquaculture Res. Dev. 9, 533–544. doi: 10.4172/2155-9546.1000544
Muhie, S. H. (2022). Novel approaches and practices to sustainable agriculture. J. Agric. Food Res. 10, 100446. doi: 10.1016/j.jafr.2022.100446
Muradov, N., Taha, M., Miranda, A. F., Kadali, K., Gujar, A., Rochfort, S., et al. (2014). Dual application of duckweed and azolla plants for wastewater treatment and renewable fuels and petrochemicals production. Biotechnol. Biofuels 7, 1–17. doi: 10.1186/1754-6834-7-30
Muruganayaki, S., Jayachitra, A., and Jothimani, S. (2019). Soil fertility as influenced by K enriched Azolla. Asian Soil Res. J. 1, 1–8. doi: 10.9734/asrj/2018/v1i430040
Nasir, N., Kamaruddin, S. A., Zakarya, I. A., and Islam, A. K. M. A. (2022). Sustainable alternative animal feeds: Recent advances and future perspective of using azolla as animal feed in livestock, poultry and fish nutrition. Sustain. Chem. Pharm. 25, 100581. doi: 10.1016/j.scp.2021.100581
Nayak, S., Prasanna, R., Pabby, A., Dominic, T., and Singh, P. (2004). Effect of urea, blue green algae and Azolla on nitrogen fixation and chlorophyll accumulation in soil under rice. Biol. Fertility Soils 40, 67–72. doi: 10.1007/s00374-004-0738-2
Nyoni, S. S. (2011). The effect of rate and time of application of Azolla on rice (Oryza Sativa L.) grain yield. University of Zambia, Lusaka, Zambia.
Pabby, A., Prasanna, R., and Singh, P. (2003). Azolla-Anabaena symbiosis-from traditional agriculture to biotechnology. Indian J. Biotechnol. 2, 18–26.
Papaefthimiou, D., Hrouzek, P., Mugnai, M. A., Lukesova, A., Turicchia, S., Rasmussen, U., et al. (2008). Differential patterns of evolution and distribution of the symbiotic behaviour in nostocacean cyanobacteria. Int. J. Systematic Evolutionary Microbiol. 58, 553–564. doi: 10.1099/ijs.0.65312-0
Parthasarathy, R., Kadirvel, R., and Kathaperumal, V. (2001). Chemical evaluation of Azolla as poultry feed ingredient. Cheiron 30, 35–37.
Pereira, A. L., Bessa, L. J., Leão, P. N., Vasconcelos, V., and Martins Da Costa, P. (2015). Bioactivity of Azolla aqueous and organic extracts against bacteria and fungi. Symbiosis 65, 17–21. doi: 10.1007/s13199-015-0316-4
Pereira, A. L. and Vasconcelos, V. (2014). Classification and phylogeny of the cyanobiont Anabaena azollae Strasburger: an answered question? Int. J. Systematic Evolutionary Microbiol. 64, 1830–1840. doi: 10.1099/ijs.0.059238-0
Peters, G. A. (1978). Blue-green algae and algal associations. BioScience 28, 580–585. doi: 10.2307/1307514
Peters, G. and Meeks, J. (1989). The Azolla-Anabaena symbiosis: basic biology. Annu. Rev. Plant Biol. 40, 193–210. doi: 10.1146/annurev.pp.40.060189.001205
Phukon, P., Baruah, J., Sarmah, D. K., and Bhau, B. S. (2017). “Green Input in Agriculture: An Overview,” in Plant-Microbe Interactions in Agro-Ecological Perspectives. Eds. Singh, D. P., Singh, H. B., and Prabha, R. (Singapore: Springer), 279–305.
Pillai, K. (2001). Azolla-a sustainable feed substitute for livestock. SEDME (Small Enterprises Development Manage. Extension Journal) 28, 51–58.
Prabakaran, S., Mohanraj, T., Arumugam, A., and Sudalai, S. (2022). A state-of-the-art review on the environmental benefits and prospects of Azolla in biofuel, bioremediation and biofertilizer applications. Ind. Crops Products 183, 114942. doi: 10.1016/j.indcrop.2022.114942
Prabina, B. J. and Kumar, K. (2010). Dried Azolla as a nutritionally rich cost effective and immuno-modulatory feed supplement for broilers. Asian J. Anim. Sceinces 5, 20–22.
Prokopuk, M. (2016). New record of Azolla caroliniana in water bodies of Kiev. Hydrobiological J. 52, 54–58. doi: 10.1615/HydrobJ.v52.i2.60
Pujawati, S., Wildan, M. R., Betty, N. F., and Mieke, R. S. (2023). Azolla pinnata ammendment and P-solubilizing bacteria inoculation for improving Inceptisols fertility and rice (Oryza sativa L.) yield production. Int. J. Life Sci. Res. Arch. 5, 78–88. doi: 10.53771/ijlsra.2023.5.2.0104
Pushpanathan, K., Kumar, M. V., and Siddeswaran, K. (2004). Integrated use of various organic and inorganic nitrogen sources on growth, yield and quality of rice (Oryza sativa L.)–A review. Agric. Rev. 25, 298–303.
Qiu, Y.-L. and Yu, J. (2003). Azolla—a model organism for plant genomic studies. Genomics Proteomics Bioinf. 1, 15–25. doi: 10.1016/S1672-0229(03)01004-0
Rai, P. K. and Tripathi, B. (2009). Comparative assessment of Azolla pinnata and Vallisneria spiralis in Hg removal from GB Pant Sagar of Singrauli Industrial region, India. Environ. Monit. Assess. 148, 75–84. doi: 10.1007/s10661-007-0140-2
Rai, P. K. (2008). Phytoremediation of Hg and Cd from industrial effluents using an aquatic free floating macrophyte Azolla pinnata. Int. J. phytoremediation 10, 430–439. doi: 10.1080/15226510802100606
Rai, A. N., Bergman, B., and Rasmussen, U. (2002). Cyanobacteria in symbiosis (Dordrecht: Springer).
Rai, R., Dhama, K., Damodaran, T., Hamid Ali, H. A., Sweta Rai, S. R., Balvir Singh, B. S., et al. (2012). Evaluation of azolla (Azolla pinnata) as a poultry feed and its role in poverty alleviation among landless people in northern plains of India. Veterinary Practitioner 13, 250–254.
Raja, W., Rathaur, P., John, S. A., and Ramteke, P. W. (2012). Azolla: an aquatic pteridophyte with great potential. Int. J. Res. Biol. Sci. 2, 68–72.
Ramesh, P. and Rajendran, A. (2022). Green synthesis of nickel oxide nanoparticles for photodegradation analysis. Materials Today: Proc. 68, 367–372. doi: 10.1016/j.matpr.2022.06.226
Ramesh, P. and Rajendran, A. (2023). Photocatalytic dye degradation activities of green synthesis of cuprous oxide nanoparticles from Sargassum wightii extract. Chem. Phys. Impact 6, 100208. doi: 10.1016/j.chphi.2023.100208
Ran, L., Larsson, J., Vigil-Stenman, T., Nylander, J. A., Ininbergs, K., Zheng, W.-W., et al. (2010). Genome erosion in a nitrogen-fixing vertically transmitted endosymbiotic multicellular cyanobacterium. PloS One 5, e11486. doi: 10.1371/annotation/835c5766-5128-41c4-b636-adfe0c503103
Rana, D., Katoch, S., Mane, B. G., Rani, D., and Sankhyan, V. (2017). Biological evaluation of Azolla in ration of commercial chicken broiler. J. Anim. Res. 7, 601–607. doi: 10.5958/2277-940X.2017.00091.2
Rathod, G. R., Tyagi, P. K., Tyagi, P. K., Mandal, A., and Shinde, A. (2013). Feeding value of Azolla (Azolla pinnata) meal in growing Japanese quail. Indian J. Poultry Sci. 48, 154–158.
Razavipour, T., Moghaddam, S. S., Doaei, S., Noorhosseini, S. A., and Damalas, C. A. (2018). Azolla (Azolla filiculoides) compost improves grain yield of rice (Oryza sativa L.) under different irrigation regimes. Agric. Water Manage. 209, 1–10. doi: 10.1016/j.agwat.2018.05.020
Refaey, M. M., Mehrim, A. I., Zenhom, O. A., Areda, H. A., Ragaza, J. A., and Hassaan, M. S. (2023). Fresh Azolla, Azolla pinnata as a complementary feed for Oreochromis niloticus: growth, digestive enzymes, intestinal morphology, physiological responses, and flesh quality. Aquaculture Nutr. 2023, 1403704. doi: 10.1155/2023/1403704
Roy, D., Pakhira, M., and Bera, S. (2016). A review on biology, cultivation and utilization of Azolla. Adv. Life Sci. 5, 11–15.
Roy, P., Roy, A., Pathak, P., Mandal, N., Soren, S., and Kumar, S. (2018). Effect of Azolla supplementation on milk yield and economics under farmers’ field. J. Crop Weed 14, 77–80.
Sadeghi, R., Zarkami, R., Sabetraftar, K., and Van Damme, P. (2013). A review of some ecological factors affecting the growth of Azolla spp. Caspian J. Environ. Sci. 11, 65–76.
Sadeghi, R., Zarkami, R., and Van Damme, P. (2014). Modelling habitat preference of an alien aquatic fern, Azolla filiculoides (Lam.), in Anzali wetland (Iran) using data-driven methods. Ecol. Model. 284, 1–9. doi: 10.1016/j.ecolmodel.2014.04.003
Safriyani, E., Hasmeda, M., Munandar, Sulaiman, F., and Holidi (2020). Increasing the growth and production of irrigated rice through the integrated application of Rice–Duck–Azolla. Acta Agrobotanica 73, 1448–1517. doi: 10.5586/aa.7322
Said, D., Chrismadha, T., Mardiati, Y., and Mayasari, N. (2012). “Nutritional content and growth ability of aquatic plant Azolla pinnata on wastewater of catfish,” in IOP Conference Series: Earth and Environmental Science. 012012 (Bristol, England, United Kingdom: IOP Publishing).
Sajjan Sihag, S. S., Sihag, Z., Sushil Kumar, S. K., and Narender Singh, N. S. (2018). Effect of feeding Azolla (Azolla pinnata) based total mixed ration on growth performance and nutrients utilization in goats. Forage Res. 43, 314–318.
Samad, F. A., Idris, L. H., Abu Hassim, H., Goh, Y. M., and Loh, T. C. (2020). Effects of Azolla spp. as feed ingredient on the growth performance and nutrient digestibility of broiler chicken. J. Anim. Physiol. Anim. Nutr. 104, 1704–1711. doi: 10.1111/jpn.13345
Samal, K. C., Behera, L., and Sahoo, J. P. (2020). Azolla biofertilizer: the nature’s miracle gift for sustainable rice production. Res. Today 2, 971–973.
Samarajeewa, K., Kojima, N., Sakagami, J. I., and Chandanie, W. (2005). The effect of different timing of top dressing of nitrogen application under low light intensity on the yield of rice (Oryza sativa L.). J. Agron. Crop Sci. 191, 99–105. doi: 10.1111/j.1439-037X.2004.00132.x
Sanchez-Viveros, G., Gonzalez-Mendoza, D., Alarcon, A., and Ferrera-Cerrato, R. (2010). Copper effects on photosynthetic activity and membrane leakage of Azolla filiculoides and A. caroliniana. Int. J. Agric. Biol. 12, 365–368.
Sanginga, N. and Van Hove, C. (1989). Amino acid composition of Azolla as affected by strains and population density. Plant Soil 117, 263–267. doi: 10.1007/BF02220720
Sankar, V., Senthilkumar, P., Sribalaji, N., Nalini, P., Arun, L., and Muralidharan, J. (2020). Effect of feeding Azolla meal on growth performance of Mecheri sheep. Int. J. Curr. Microbiol. App Sci. 9, 1945–1949. doi: 10.20546/ijcmas.2020.905.222
Sanyahumbi, D., Duncan, J. R., Zhao, M., and Hille, R. V. (1998). Removal of lead from solution by the non-viable biomass of the water fern Azolla filiculoides. Biotechnol. Lett. 20, 745–747. doi: 10.1023/A:1005386703592
Sapcota, D. and Begum, K. (2022). “Integrated duck farming,” in Duck Production and Management Strategies, eds. Jalaludeen, A., Churchil, R. R., and Baéza, E. (Singapore: Springer), 247–264.
Sarkar, A. and Jana, S. (1986). Heavy metal pollutant tolerance of Azolla pinnata. Water Air Soil pollut. 27, 15–18. doi: 10.1007/BF00464765
Saunders, R. M. and Fowler, K. (1993). The supraspecific taxonomy and evolution of the fern genus Azolla (Azollaceae). Plant Systematics Evol. 184, 175–193. doi: 10.1007/BF00937434
Schluepmann, H., Bigot, I., Rijken, N., Grifoll, A. C., Gudde, P., Dijkhuizen, L., et al. (2022). “Domestication of the floating fern symbiosis Azolla,” in Ferns: Biotechnology, Propagation, Medicinal Uses and Environmental Regulation (Singapore: Springer), 149–180.
Sebastian, A., Deepa, P., and Prasad, M. N. V. (2021). “Azolla farming for sustainable environmental remediation,” in Handbook of assisted and amendment: Enhanced sustainable remediation technology. Ed. Prasad, M. N. V. (Hoboken, New Jersey, United State of America: John Wiley & Sons, Ltd), 517–533.
Sela, M., Fritz, E., Huttermann, A., and Tel-Or, E. (1990). Studies on cadmium localization in the water fern Azolla. Physiologia Plantarum 79, 547–553. doi: 10.1111/j.1399-3054.1990.tb02116.x
Sela, M., Tel-Or, E., Fritz, E., and Huttermann, A. (1988). Localization and toxic effects of cadmium, copper, and uranium in Azolla. Plant Physiol. 88, 30–36. doi: 10.1104/pp.88.1.30
Seleiman, M. F., Elshayb, O. M., Nada, A. M., El-Leithy, S. A., Baz, L., Alhammad, B. A., et al. (2022). Azolla compost as an approach for enhancing growth, productivity and nutrient uptake of Oryza sativa L. Agronomy 12, 416. doi: 10.3390/agronomy12020416
Serag, M. S., El-Hakeem, A., Badway, M., and Mousa, M. A. (2000a). On the ecology of Azolla filiculoides Lam. in Damietta district, Egypt. Limnologica-Ecol. Manage. Inland Waters 30, 73–81. doi: 10.1016/S0075-9511(00)80047-X
Serag, M. S., El-Hakeem, A., Badway, M., and Mousa, M. A. (2000b). On the ecology of Azolla filiculoides Lam. in the Damietta district, Egypt. Limnologica-Ecol. Manage. Inland Waters 30, 73–81. doi: 10.1016/S0075-9511(00)80047-X
Setiawati, M. R., Prayoga, M. K., Stöber, S., Adinata, K., and Simarmata, T. (2020). Performance of rice paddy varieties under various organic soil fertility strategies. Open Agric. 5, 509–515. doi: 10.1515/opag-2020-0050
Shaltout, K. H., El-Komi, T. M., and Eid, E. M. (2012). Seasonal variation in the phytomass, chemical composition and nutritional value of Azolla filiculoides Lam. along the water courses in the Nile Delta, Egypt. Feddes Repertorium 123, 37–49. doi: 10.1002/fedr.201200001
Shekhawat, K., Rathore, S. S., Babu, S., Raj, R., and Chauhan, B. S. (2022). Exploring alternatives for assessing and improving herbicide use in intensive agroecosystems of South Asia: A review. Adv. Weed Sci. 40, e0202200116. doi: 10.51694/AdvWeedSci/2022;40:seventy-five005
Shernazarov, S., Davronova, S., Tashpulatov, Y., Rakhmonov, V., Muminov, S., and Nurniyozov, A. (2024). “The importance of Azolla aquatic plants in creating a natural sustainable nutrient environment in the fisheries industry”, in E3S Web of Conferences (EDP Sciences), 02009.
Shiomi, N. and Kitoh, S. (2001). Culture of Azolla in a pond, nutrient composition, and use as fish feed. Soil Sci. Plant Nutr. 47, 27–34. doi: 10.1080/00380768.2001.10408365
Singh, P. (1977). Effect of Azolla on the yield of paddy with and without application of N fertilizer. Curr. Sci. 46, 642–644.
Singh, P. (1981). “Use of Azolla in rice production in India,” in Nitrogen and Rice (Los Baños, Philippines: International Rice Research Institute).
Singh, A. and Singh, P. (1987). Influence of Azolla management on the growth, yield of rice and soil fertility: II. N and P contents of plant and soil. Plant Soil 102, 49–54. doi: 10.1007/BF02370899
Singh, A. and Singh, P. (1990). Intercropping of Azolla biofertilizer with rice at different crop geometry. Trop. Agric. 67, 17–25.
Sireesha, A., Chakravarthi, M. K., Naveen, Z., Naik, B., and Babu, P. (2017). Carcass characteristics of New Zealand white rabbits fed with graded levels of Azolla (Azolla pinnata) in the basal diet. Int. J. Livestock Res. 7, 2277–1964. doi: 10.5455/ijlr.20170619042936
Sivakumar, C. and Solaimalai, A. (2003). Weed management in rice-fish-azolla farming system–A review. Agric. Rev. 24, 190–196.
Smith, P., Martino, D., Cai, Z., Gwary, D., Janzen, H., Kumar, P., et al. (2008). Greenhouse gas mitigation in agriculture. Philos. Trans. R. Soc. B: Biol. Sci. 363, 789–813. doi: 10.1098/rstb.2007.2184
Songara, M., Jhirwal, A. K., Goswami, S. C., Legha, R. A., Kumar, V., Singh, V., et al. (2018). Growth and digestibility of gross nutrients by azolla incorporation in Marwari stallion. Int. J. Chem. Stud. 6, 3215–3218.
Sood, A. and Ahluwalia, A. (2009). Cyanobacterial–plant symbioses with emphasis on Azolla-Anabaena symbiotic system. Indian Fern J. 26, 166–178.
Sood, A., Uniyal, P. L., Prasanna, R., and Ahluwalia, A. S. (2012). Phytoremediation potential of aquatic macrophyte, Azolla. Ambio 41, 122–137. doi: 10.1007/s13280-011-0159-z
Sow, S. and Ranjan, S. (2020). Integration of Azolla and Fish in Rice-Duck Farming System (India: Agriculture & Food: E-newsletter). B.a.U. Department of Agronomy, Sabour, Bhagalpur.
Subedi, P. and Shrestha, J. (2015). Improving soil fertility through Azolla application in low land rice: A review. Azarian J. Agric. 2, 35–39.
Sukumar, D., Subramaniyan, P., and Kannaiyan, S. (1988). Studies on the Azolla and nitrogen application on rice. Indian J. Agron. 33, 396–398.
Sun, Y., Wang, X., Yang, C., Xin, X., Zheng, J., Zong, T., et al. (2024). Effects of biochar on gaseous carbon and nitrogen emissions in paddy fields: A review. Agronomy 14, 1461. doi: 10.3390/agronomy14071461
Supriya, C. M., Kumar, B., and Basha, A. (2023). “Azolla: Nature’s Tiny Powerhouse Shaping Ecological System,” in Current Trends in Agricultural Sciences & Technology. Eds. Maheshwarareddy, C., Saikia, A. R., Bezboruah, M., Elumle, P., and Singh, S. S. (Vital Biotech Publication, Kota (Rajasthan, Indi), 165–175.
Surenthiran, S. and Loganathan, P. (2012). “Carbon sequestration of Azolla and soil nitrogen mineralization,” in Proceedings of International Forestry and Environment Symposium. (Jaffna, Sri Lanka: University of Jaffna), 17, 31–32.
Swain, B. K., Naik, P. K., and Beura, C. (2022). Nutritive value of Azolla as poultry feed-a review. Indian J. Anim. Nutr. 39, 1–11. doi: 10.5958/2231-6744.2022.00001.9
Tamang, Y. and Samanta, G. (1995). Feeding value of azolla (Azolla pinnata) in goats. Anim. Res. 44, 62–65. doi: 10.1051/animres:19950532
Tan, C.-Y., Li, G., Lu, X.-Q., and Chen, Z.-L. (2010). Biosorption of Basic Orange using dried A. filiculoides. Ecol. Eng. 36, 1333–1340. doi: 10.1016/j.ecoleng.2010.06.009
Tarif, S. T. (2021). Use of Azolla as a substitute poultry feed and its effect on growth and production: A review. Plant Arch. 21, 947–950.
Tekle-Haimanot, A. and Doku, E. (1995). Comparison of Azolla mexicana and N and P fertilization on paddy taro (Colocasia esculenta) yield. Trop. Agric. 72, 70–72.
Thapa, P. and Poudel, K. (2021). Azolla: Potential biofertilizer for increasing rice productivity, and government policy for implementation. J. Wastes Biomass Manag 3, 62–68. doi: 10.26480/jwbm.02.2021.62.68
Tharwat, A. (1999). Production evaluation of Nile tilapia Oreochromis niloticus utilized fresh and dried Azolla pinnata in semi-intensive fish culture. Egyptian J. Anim. Production 36, 71–82. doi: 10.21608/ejap.1999.110513
Van Hove, C. (1989). Azolla: and its multiple uses with emphasis on Africa (Italy: Food and Agriculture Organization).
Ventura, W., Watanabe, I., and Mascariña, G. B. (1992). Mineralization of Azolla N and its availability to wetland rice. Soil Sci. Plant Nutr. 38, 505–516. doi: 10.1080/00380768.1992.10415082
Verma, G., Prakriti, K., Babu, S., Singh, R., Gudade, B., Bhatt, M., et al. (2022). Implications and future prospects of Azolla as a low-cost organic input in agriculture. Agriculture 1, 1–7.
Vijayan, K., Deepthi, K., Reshma, C., and Menon, S. (2024). Exploring the multifaceted benefits of Azolla: A comprehensive review of an aquatic fern’s biological and practical contributions. Int. J. Ecol. Environ. Sci. 50, 661–672. doi: 10.55863/ijees.2024.0264
Vroom, R., Smolders, A., Van De Riet, B., Lamers, L., Güngör, E., Krosse, S., et al. (2024). Azolla cultivation enables phosphate extraction from inundated former agricultural soils. Water Res. 254, 121411. doi: 10.1016/j.watres.2024.121411
Wagner, G. M. (1997). Azolla: a review of its biology and utilization. Botanical Rev. 63, 1–26. doi: 10.1007/BF02857915
Watanabe, I. (1982). “Azolla—Anabaena symbiosis—its physiology and use in tropical agriculture,” in Microbiology of tropical soils and plant productivity, eds. Dommergues, Y. R. and Diem, H. G. (Dordrecht, Netherland: Springer), 169–185.
Watanabe, I. and Berja, N. S. (1983). The growth of four species of Azolla as affected by temperature. Aquat. Bot. 15, 175–185. doi: 10.1016/0304-3770(83)90027-X
Watanabe, I., Espinas, C., Berja, N., and Alimagno, B. (1977). “The utilization of the Azolla-Anabaena complex as a nitrogen fertilizer for rice,” in IRRI research paper series; no. 11 (International Rice Research Institute, Manila, Philippine).
Watanabe, I., Ventura, W., Mascarina, G., and Eskew, D. (1989). Fate of Azolla spp. and urea nitrogen applied to wetland rice (Oryza sativa L.). Biol. fertility soils 8, 102–110. doi: 10.1007/BF00257752
White, R. A. (1971). Experimental and developmental studies of the fern sporophyte. Botanical Rev. 37, 509–540. doi: 10.1007/BF02868687
Wijeysingha, I. S. and Amarasinghe, S. R. (2023). A review of Azolla spp. as a potential resource for sustainable agriculture in Sri Lanka: a new effort for the green agriculture among Sri Lankan farmers. Wijeysingha Amarasinghe Appl. Bio-Systems Technol. 3, 1–13.
Xiao, C., Zhang, F., Li, Y., Fan, J., Ji, Q., Jiang, F., et al. (2024). Optimizing drip irrigation and nitrogen fertilization regimes to reduce greenhouse gas emissions, increase net ecosystem carbon budget and reduce carbon footprint in saline cotton fields. Agriculture Ecosyst. Environ. 366, 108912. doi: 10.1016/j.agee.2024.108912
Xu, J., Li, Y., Zhang, M., and Zhang, S. (2024). Sustainable agriculture in the digital era: Past, present, and future trends by bibliometric analysis. Heliyon 10, e34612. doi: 10.1016/j.heliyon.2024.e34612
Xu, H., Zhu, B., Liu, J., Li, D., Yang, Y., Zhang, K., et al. (2017). Azolla planting reduces methane emission and nitrogen fertilizer application in double rice cropping system in southern China. Agron. Sustain. Dev. 37, 1–9. doi: 10.1007/s13593-017-0440-z
Yadav, R., Abraham, G., Singh, Y., and Singh, P. (2014). Advancements in the utilization of Azolla-Anabaena system in relation to sustainable agricultural practices. Proc. Indian Natl. Sci. Acad. 80, 301–316. doi: 10.16943/ptinsa/2014/v80i2/55108
Yao, Y., Zhang, M., Tian, Y., Zhao, M., Zeng, K., Zhang, B., et al. (2018). Azolla biofertilizer for improving low nitrogen use efficiency in an intensive rice cropping system. Field Crops Res. 216, 158–164. doi: 10.1016/j.fcr.2017.11.020
Ying, Z., Boeckx, P., Chen, G., and Van Cleemput, O. (2000). Influence of Azolla on CH4 emission from rice fields. Nutrient Cycling Agroecosystems 58, 321–326. doi: 10.1023/A:1009871308968
Yohana, M. A., Ray, G. W., Yang, Q., Tan, B., Chi, S., Asase, A., et al. (2023). A review on the use of Azolla meal as a feed ingredient in aquatic animals’ diets. Aquaculture Res. 2023, 2633412. doi: 10.1155/2023/2633412
Youssouf, A., Aliou, S., Daouda, M., Emile, D. F., and Jean-Claude, M. (2012). Evaluation of nitrogen and phosphorus wastes produced by Nile Tilapia (Oreochromis niloticus L.) fed Azolla-diets in earthen ponds. J. Environ. Prot. 3, 502–507. doi: 10.4236/jep.2012.36060
Youssouf, A., Emile Didier, F., Yves, B., and Jean-Claude, M. (2011). Approximate compositional values and tissue fatty acid profiles of Nile tilapia (Oreochromis niloticus L.) fed Azolla-diets in earthen ponds. Food Nutr. Sci. 2, 964–973. doi: 10.4236/fns.2011.29131
Yuan, W., Xia, J., Song, C., and Wang, Y.-P. (2024). Simulating the land carbon sink: Progresses and challenges of terrestrial ecosystem models. Agric. For. Meteorol. 358, 110264. doi: 10.1016/j.agrformet.2024.110264
Zhang, X., Lin, A.-J., Zhao, F.-J., Xu, G.-Z., Duan, G.-L., and Zhu, Y.-G. (2008). Arsenic accumulation by the aquatic fern Azolla: comparison of arsenate uptake, speciation and efflux by A. caroliniana and A. filiculoides. Environ. pollut. 156, 1149–1155. doi: 10.1016/j.envpol.2008.04.002
Keywords: Azolla, sustainable agriculture, biofertilizer, carbon sequestration, methane mitigation, climate resilience, biofuels, bioplastics
Citation: Yang Y, Yang Y, Deng S and Ying Z (2025) Role of Azolla in sustainable agriculture and climate resilience: a comprehensive review. Front. Plant Sci. 16:1661720. doi: 10.3389/fpls.2025.1661720
Received: 08 July 2025; Accepted: 01 September 2025;
Published: 14 October 2025.
Edited by:
Kai Huang, Jiangsu Academy of Agricultural Sciences (JAAS), ChinaReviewed by:
Balasubramanian Ramakrishnan, Indian Agricultural Research Institute (ICAR), IndiaAmrik Singh Ahluwalia, Eternal University, India
Ramesh P., Nehru Memorial College, India
Hmouni Driss, Ibn Tofail University, Morocco
Mehmet Tütüncü, Ondokuz Mayıs University, Türkiye
Copyright © 2025 Yang, Yang, Deng and Ying. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoyang Ying, eGlvbjA2M0BvdXRsb29rLmNvbQ==
 Youquan Yang
Youquan Yang Zhaoyang Ying
Zhaoyang Ying