- Department of Agriculture, Food, Natural Resources and Engineering (DAFNE), University of Foggia, Foggia, Italy
Introduction: Cakile maritima is a succulent halophyte from the Brassicaceae family, commonly found along sandy coasts. Understanding its response mechanisms to sodium excess is crucial for its exploitation under sustainable biosaline farming.
Methods: For the first time, this research investigated the pinnatifid C. maritima population from the Apulia region (Italy) grown under varying levels of NaCl (0 -T0, 100 -T100 and 400 -T400 mM NaCl).
Results: The T100 plants showed higher leaf area (LA) and specific leaf area (SLA) compared to T0, with a slight reduction in succulence index (SI). In T400 plants, a reduction in shoot and root fresh weight, water content (WC), leaf dry weight, LA, and SLA was observed, alongside an increase in SI and dry matter concentration. No changes were detected in leaf Na and Cl concentrations, whereas T400 stems accumulated Na. Leaf K, Mg, and Ca concentrations remained stable. The operating efficiency of PSII (ΦPSII) was similar across treatments. In salt-exposed plants, the decrease of Fv’/Fm’ was counteracted by an improvement of qP, with carotenoids and anthocyanins appearing to be involved in photoprotection. Salt-exposed plants maintained stomatal opening (gs), allowing a higher CO2 assimilation rate (An), especially in T100. Despite unimpaired An, T400 plants exhibited reduced canopy-level photosynthesis due to lower LA, leading to reduced shoot biomass. Among antioxidants, ascorbic acid and anthocyanins were effective in improving the antioxidative defence of T400 plants.
Discussion: The results indicate that C. maritima employs a complex protective strategy involving morphological adjustments, selective ion accumulation, efficient photoprotection, maintained gas exchange, and a potent antioxidant system to mitigate salinity stress, demonstrating its strong potential for biosaline agriculture.
1 Introduction
Globally, nearly 20% of cultivated land faces soil salinization, which is expected to increase to 50% by 2050 (Mann et al., 2023; Bazihizina et al., 2024). The impact of salinity stress on plant growth and development is substantial, affecting various aspects, including seed germination, plant anatomy and physiology, root structure, protein synthesis, and gene expression. This effect varies based on the level of salinity, the duration of exposure, and the specific plant species. With short-term exposure to salinity, the elongation rates of plant cells decrease. However, prolonged exposure to salinity reduces cell division, restricting plant growth and causing visible injuries, such as leaf death. High Na and Cl levels in soil limit the uptake of essential macronutrients, such as K, Ca, and Mg, thereby disrupting physiological functions. Salinity also negatively affects photosynthesis by damaging pigments, lowering stomatal density and conductance, and increasing mesophyll resistance, thus hampering the efficiency of photosystems I and II (Rahman et al., 2021). Salt stress also decreases the activity of RuBisCO, a crucial enzyme for CO2 assimilation, and leads to the production of reactive oxygen species (ROS) in cellular organelles, causing protein synthesis reduction, cell membrane damage, and instability in the photosynthetic apparatus (Rahman et al., 2021).
Halophytes represent a small group (fewer than 1% of all plant species) that exhibit a natural ability to resist or tolerate salinity levels ranging from 50 to 500 mM NaCl. These plants may require consistent saline conditions for optimal growth (obligate halophytes) or can endure certain levels of salinity (facultative halophytes), although they typically thrive better in non-saline or low-salinity environments (Rahman et al., 2021; Bazihizina et al., 2024).
The remarkable ability of halophytes to flourish in saline soils is mainly achieved through physiological mechanisms such as salt exclusion at the root level, salt excretion via specialized epidermal structures (e.g., salt glands and bladder cells), and salt inclusion. However, one or more of these features can be present in different halophytes to manage salinity (Rahman et al., 2021). The salt inclusion strategies aim to dilute absorbed salts within succulent leaves and stems using inherent mechanisms (ion compartmentalization, osmotic adjustments, antioxidative defense systems, redox homeostasis) (Mann et al., 2023).
Cakile marittima is an annual succulent halophyte belonging to the Brassicaceae family, abundantly found along the sandy coasts of the Atlantic and Mediterranean regions. Plants can potentially be used for food and industrial (high-healthy foods, pharmaceutical, oilseed extraction) purposes (Arbelet-Bonnin et al., 2019; Conversa et al., 2024), offering a valuable tool for sustainable biosaline farming or the exploitation of saline environments (Arbelet-Bonnin et al., 2019; Bazihizina et al., 2024). Additionally, it could be used as a plant model for salinity tolerance studies (Ben Hamed et al., 2016; Mir et al., 2024). Deciphering the response mechanisms in halophytes under conditions of sodium excess-induced tolerance and/or toxicity holds significant value for optimizing agricultural practices in saline environments, thereby maximizing both yield and the production of health-promoting secondary metabolites.
To the best of our knowledge, research on the salinity tolerance strategies of C. maritima has been conducted substantially on ecotypes of Tunisia. It highlighted that this species is a salt-including euhalophyte as its growth is improved by moderate soil salinity (50–100 mM NaCl) and it exhibits no adverse effects even at salinity levels as high as 200 mM NaCl (Debez et al., 2004, 2006, 2008, 2012; Arbelet-Bonnin et al., 2019; Ben Amor et al., 2020). In proportion to the external concentration, it can take up a large amount of Na and, to a lesser extent, of Cl. Sodium can be efficiently removed from the cytosol mainly through compartmentalization in vacuoles, since no epidermal salt excretion was observed (Debez et al., 2004, 2006). Na leaf accumulation has been positively correlated to the improved plant water status, leaf thickness and succulence (Debez et al., 2004, 2006; Ben Hamed et al., 2016). Moreover, despite a reduction of K, Ca, and Mg uptake (Debez et al., 2004, 2008) or no changes in K accumulation (Ben Hamed-Louati et al., 2016) have been observed, a selective K uptake has been suggested to regulate Na/K ratio. Photosynthesis (Debez et al., 2006) and chlorophyll fluorescence (Debez et al., 2008, 2012; Arbelet-Bonnin et al., 2020) parameters pointed out that carbon assimilation rate and PSII efficiency were improved under moderate NaCl concentration, while decreasing when plants experienced higher salinity. Similarly, both the enzymatic and non-enzymatic antioxidant systems were elicited depending on salinity pressure for leaf ROS detoxifying (Ben Amor et al., 2006, 2007; Ksouri et al., 2007; Ellouzi et al., 2011, 2014; Mansour et al., 2018). Among non-enzymatic antioxidants, ascorbate (Ben Amor et al., 2006, 2007; Ellouzi et al., 2014; Ben Hamed-Louati et al., 2016), phenols (Ksouri et al., 2007; Mansour et al., 2018) and carotenoids (Mansour et al., 2018) have a pivotal role in contrasting oxidative stress. The occurrence of these responses and their efficiency have been related to specific bioclimatic conditions of C. maritima ecotypes, with the arid-adapted populations being more tolerant to salinity exposure than the plants adapted to a humid habitat (Ben Amor et al., 2006; Ksouri et al., 2007; Megdiche et al., 2007).
In Italy, C. maritima subsp. maritima is spread along sandy coasts, with some studies exploring physiological (Gratani et al., 2009), morpho-functional (Ciccarelli et al., 2010) and biochemical (Conversa et al., 2024) adaptation in natural areas. Both in Tuscany (Ciccarelli et al., 2010) and Puglia (Conversa et al., 2024) regions, the diffusion of two morphotypes distinguished by entire or pinnatifid leaves has been ascertained (Ciccarelli et al., 2010) with the pinnatifid type appearing to be more adapted to the coastal climate of southern Italy (Conversa et al., 2024). Nevertheless, to date, no studies have been conducted specifically on their salinity tolerance/resistance. To gain knowledge on the salt response mechanisms of this species, the present work aims to explore the morpho-biophysiological traits, mineral nutrition and antioxidative characteristics of the pinnatifid population of C. maritima from the Apulia region grown at increasing NaCl levels.
2 Materials and methods
2.1 Experimental location and set-up
The experiment was conducted in an unheated glass greenhouse (model Tulip 6, Serra e Giardini Mirandola, MO, Italy) at the Department of Agriculture, Food, Natural Resources, and Engineering (DAFNE) (University of Foggia), located in Foggia, Puglia, southern Italy (latitude 41° 46′ N, longitude 15° 55′ E, 74 m above sea level). The experiment took place during February and March 2024. In the greenhouse, the mean minimum and maximum daily air temperature ranged from 10-11°C and 20-22°C, respectively. The relative humidity varied between 70 and 80%. Seeds of C. maritima were collected in September 2023 from Margherita di Savoia (BT), northern Puglia (latitude, 41.4° N; longitude, 16.0° E; altitude, 0–5 m a.s.l.). The area has a Mediterranean climate characterized by mild winters and hot, dry summers. Over the past thirty years, the average recorded temperatures have been a minimum of 9.8 ± 1.6°C and a maximum of 21.6 ± 2.1°C. The mean temperatures for the coldest and hottest months are 7.5°C in January and 25.0°C in July, respectively. Annual rainfall averages 497 mm, with 33% occurring from October to December, 25% from January to March, 22% in spring, and only 11% during July and August.
Seeds were sown in pots (0.72 L) filled with a standardized growth medium consisting of peat (Brill 3 TYPical, Gebr. Brill Substrate GmbH & Co. KG, Georgsdorf, NI, Germany), sieved sandy soil(collected from the same location as the seeds), and perlite (Agrilit3, Perlite Italiana s.r.l., Corsico, Milano, Italy). The physicochemical analysis of the sandy soil is reported in the Supplementary Table 1. The ratio of these components was 50:30:20. The pots were irrigated with tap water. Two-week-old seedlings, one per pot, were watered twice a week using a diluted (two-fold) Hoagland nutrient solution (NS). The chemical characteristics of the tap water used for irrigation and for NS production are reported in the Supplementary Table 2.
After a one-month pretreatment phase, plants with an average weight of 1.58 ± 0.48 g (height 8.4 ± 1.2 cm) were divided into three groups. The first group, which served as the control (T0), received a full-strength NS without sodium chloride (NaCl). The second group was irrigated with the same NS containing 100 mM NaCl, while the third group received the NS with 400 mM NaCl. To prevent osmotic shock, plants were top-irrigated for three weeks with NS at salt levels that were increased by 50 mM NaCl, until the desired salt concentration was achieved. This ensured a gradual transition to higher salinity. In total, each plant received 350 mL of T0, T100 and T400 NS. Treatments were arranged using a randomized block design with three replicates. The experimental unit consisted of 12 pots each containing one plant. Therefore, in total 36 plants (12 plants x 3 replications) were grown for each treatment. The plants were harvested three days after the last treatment application.
At the end of the trial, the electrical conductivity of the substrate was measured at 3.6 (± 0.07), 4.0 (± 0.03) and 6.9 (± 0.16) dS m-1, for treatments T0, T100 and T400, respectively.
2.2 Plant material, bio-physiological and chemical determinations
On 9 plants belonging to each experimental unit, the aerial biomass was distinguished in leaves, tender and coriaceous stems and roots. Number, area and fresh weight of leaves of each plant were determined. Leaf dry weight (DW) was determined by drying in a thermoventilated oven at 70°C until a constant mass was achieved. Fresh weight (FW) and dry weight (DW) for the stem and root were also measured. The dry matter (DM) content of leaves, stems, and roots was calculated as (DW/FW) × 100. Leaf area was measured by scanning leaves at 360 dpi using an Epson Perfection V750 PRO scanner (Epson Italia S.p.A., Cinisello Balsamo, MI, Italy). The scanned images were processed with the ImageJ software (National Institutes of Health, Bethesda, MD, USA). Specific leaf area (SLA) was determined as the ratio of leaf area to dry weight (LA/DW), while leaf mass per unit leaf area (LMA) was calculated as DW/LA. Furthermore, the succulence index (SI) was computed as the ratio of the difference between fresh and dry weight to leaf area, following Gratani et al. (2009). The water content (WC) of leaves, stems, and roots was determined as the ratio of the difference between fresh and dry weight to fresh weight. Six subsamples of leaves and stems per experimental unit were lyophilized (ScanVac CoolSafe 55–9 Pro; LaboGene ApS, Lynge, Denmark) for subsequent phytochemical analyses.
2.2.1 Chlorophyll fluorescence and leaf gas exchange
Leaf gas exchange and chlorophyll a fluorescence parameters were assessed on three plants per experimental unit. For each plant, two fully expanded leaves were selected for evaluation. Gas exchange parameters included net photosynthetic rate (An), intercellular CO2 concentration (Ci), stomatal conductance (gs), and transpiration rate (E). Chlorophyll fluorescence parameters included the effective quantum yield of photosystem II (ΦPSII), photochemical quenching (qP), and the maximum efficiency of PSII photochemistry in the light (Fv′/Fm′). All measurements were performed using an open gas exchange system equipped with a leaf chamber fluorometer and LED light source (LI-6400XT, LI-COR Inc., Lincoln, NE, USA). Preliminary light response curves were generated by exposing leaves to increasing photosynthetically active radiation (PAR) from 0 to 2,500 µmol m-2 s-1 (data not shown). Subsequent measurements were carried out under standardized saturating light conditions: PAR at 2,000 µmol m-2 s-1, leaf temperature maintained at 23 °C, ambient CO2 concentration set at 400 µmol mol-1, and an air flow rate of 300 cm³ s-1.
2.2.2 Inorganic ions and ascorbic acid
For the extraction of inorganic cations, 0.3 g of lyophilized samples of leaves and stems, previously ashed in a muffle furnace at 550°C for 6 hours, were subjected to acid digestion using 20 mL of 1 mol L-1 HCl, followed by boiling for 30 minutes. The digested solution was then filtered through a 0.22 µm Millipore filter, diluted, and analyzed by ion chromatography. This analysis was performed using a self-regenerating DRS-600 suppressor (4 mm), a Dionex IonPack CS12A analytical column (4 mm × 250 mm, 5 µm), and an eluent consisting of 20 mM methane-sulfonic acid, with a flow rate of 1 mL min-1. Inorganic anions were extracted from 0.5 g of lyophilized samples of leaves using 50 mL of eluent solution, followed by agitation in a shaking water bath at room temperature for 30 minutes. The resulting mixture was filtered twice through Whatman No. 2 filter paper, then further filtered through a 0.22 µm Millipore filter. The filtered extract was then injected into an ion chromatography system (ICS-3000, Dionex Corp., Milan, Italy), which was equipped with an isocratic pump, AS-DV autosampler, a self-regenerating ASR anion suppressor (4 mm), and a Dionex Ion-Pac AS23 analytical column (4 mm × 250 mm) along with a guard column (4 mm × 50 mm) maintained at 35°C. The mobile phase was composed of 3.5 mM sodium carbonate and 1 mM sodium bicarbonate, with a flow rate of 1 mL min-1. Cation and anion compounds were identified by comparing their retention times with those of standard solutions. Peak areas were analyzed using Dionex Chromeleon software (version 6.80, ThermoFisher Scientific, Waltham, MA, USA) (Cataldi et al., 2003). Leaf ascorbic acid content was measured with a reflectometer (RQflex 10 plus, Merck, Germany) using a 25–450 mg L−1 measuring range for ascorbic acid. The method consists of reducing yellow molybdophosphoric acid to molybdenum blue by the action of ascorbic acid (Balık et al., 2023).
2.2.3 Chlorophylls, carotenoids, and anthocyanins
Chlorophyll (Chl) (a, b, and total) and carotenoid concentrations were determined spectrophotometrically according to the method described by Sumanta et al. (2014), with slight modifications. Lyophilized leaf tissue (0.05 g) was extracted with 1.5 mL of 80% ethanol (ethanol:water, 80:20 v/v) containing 0.1% hydrochloric acid in 10 mL screw-cap tubes. Samples were subjected to ultrasonic extraction using an ultrasonic cleaner (DU-32 Digital; Argo Lab, Carpi, MO, Italy) at room temperature for 30 minutes. Following sonication, the samples were centrifuged at 4,000 × g for 15 minutes at 4 °C in a refrigerated centrifuge (Beckman Coulter Allegra™ 25, Fullerton, CA, USA). The supernatant was collected, and the extraction procedure was repeated twice. The combined supernatants were then filtered through 0.22 µm reinforced nylon membrane filters. The extract solution was then analyzed using a spectrophotometer (Evolution 201 UV-Visible Spectrophotometers, Thermo Scientific Waltham, MA, USA) at wavelengths of 664, 649 and 470 nm. The following equations were used for quantification: Chl a=13.36(A664)-5.19 (A649); Chl b=27.43(A649)-8.12(A664); Chl total=17.32(A649)+7.18(A664). Carotenoids were calculated using the equation: Carotenoids (1000(A470)-2.13Chl a-97.63Chl b)/209. Anthocyanin content was quantified using the same extract, following the method of Sims and Gamon (2002). Absorbance was measured at 525 nm and 650 nm, and chlorophyll interference was corrected using the equation: AA=A529-(0.288*A650), where AA represents the corrected anthocyanin absorbance. Total anthocyanin content was expressed as cyanidin-3-glucoside (c.g.) equivalents per unit weight, calculated using a molar extinction coefficient of 26,900 L mol-1 cm-1 at 525 nm (Murray and Hackett, 1991).
2.2.4 Total phenols and flavonoids
Phenols extraction was performed on lyophilized leaf samples (0.3 g) using 1 mL of an 80% methanol solution at room temperature in an ultrasonic cleaner bath (DU-32 Digital; Argo Lab, Carpi, MO, Italy) for 15 minutes. The mixture was then centrifuged at 14,000 rpm for 15 minutes at 4°C in a refrigerated centrifuge (ThermoFisher Scientific, Waltham, MA, USA), and the supernatant was collected, while the residual pellet underwent a second extraction. The extracts were stored at -20°C and analyzed within 24 hours. Total polyphenols (TP) were determined by spectrophotometric analysis according to the method of Singleton and Rossi (1965): 0.1 mL of the extracts were diluted with 3 mL of distilled water, after which 0.5 mL of Folin-Ciocalteu reagent was added and the mixture was kept at room temperature for 5 minutes. Then, 1.0 mL of 20% Na2CO3 was added to the mixture. After 45 minutes at 30°C, the absorbance of the resulting solutions was measured at 750 nm using a spectrophotometer (Shimatzu UV-1800, Shimadzu Scientific Instruments, North America, USA). The values were determined from a calibration curve (0–250 mg L-1; R² 0.998) prepared with standard gallic acid (g.a.) solutions, applying the same procedure used for the extracts. The results were expressed as gallic acid equivalents (g.a.e.) per unit weight. Flavonoids extraction was performed on lyophilized samples (0.3 g) using 1 mL of an 80% methanol solution at room temperature in an ultrasonic cleaner bath (DU-32 Digital; Argo Lab, Carpi, MO, Italy) for 15 minutes. The mixture was then centrifuged at 14,000 rpm for 15 minutes at 4°C in a refrigerated centrifuge (ThermoFisher Scientific, Waltham, MA, USA), and the supernatant was collected, while the residual pellet underwent a second extraction. The extracts were stored at -20°C and analyzed within 24 hours. The flavonoid content was quantified using the AlCl3 colorimetric method (Chandra et al., 2014). Briefly, 200 µL of the extract or diluted quercetin standard solutions were mixed with 40 µL of 10% AlCl3, 40 µL of 1 M potassium acetate, and 1,120 µL of distilled water. The mixture was incubated at room temperature for 30 minutes, and absorbance was measured at 415 nm using a UV-Vis spectrophotometer (Shimadzu UV-1800, Shimadzu Scientific Instruments, North America, USA). The total flavonoid content was expressed as quercetin equivalents (q.e.) per unit weight.
2.2.5 Antioxidant capacity
2.2.5.1 Radical scavenging capacity by the TEAC (ABTS) assay
Hydrophilic and lipophilic components were extracted from finely ground lyophilized leaf samples. To 0.3 g of each sample, 1 mL of 80% methanol was added, and the mixture was agitated for 15 minutes. The supernatant, containing the hydrophilic fraction, was recovered after centrifugation at 13,000 rpm for 10 minutes, while the pellet was re-extracted in the same manner. For the lipophilic fraction extraction, 1 mL of hexane was added to the pellet. The suspension was placed in an ultrasonic bath and subsequently centrifuged as previously described. The recovered supernatants were then combined. Both fractions’ extracts were stored at -20°C until analysis. The antioxidant activity of both the hydrophilic and lipophilic fractions was assessed using the TEAC (Trolox Equivalent Antioxidant Capacity) assay, as described by Re et al. (1999). Specifically, the ability of the antioxidants to reduce the ABTS•+ radical cation was measured spectrophotometrically at a wavelength of 734 nm, indicated by a decrease in absorbance. The results are expressed as Trolox equivalent antioxidant capacity, derived from the use of the Trolox reagent (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) to construct the calibration curve. The Trolox stock solution was 1,100 µM, and the concentration range of the calibration curve was 0-15 µM.
2.2.5.2 Radical scavenging capacity by the DPPH assay
The DPPH assay (2,2-diphenyl-1-picrylhydrazyl) was applied exclusively to hydrophilic antioxidants (only for methanolic extracts), following a modified method described by Lisanti et al. (2015). This method is based on the use of the free radical DPPH. The unpaired electron in the DPPH radical produces a characteristic absorbance peak at 517 nm. The DPPH radical was progressively solubilized in ethanol to achieve a concentration that produced an absorbance between 1.12 and 1.08 at 517 nm. The analytical solution was obtained by mixing 1.9 mL of diluted DPPH and 0.1 mL of the sample extract or standard Trolox solution. The Trolox stock solution was 1,000 µM and the concentration range of the calibration curve was 0-800 µM. Absorbance was measured at 517 nm after 30 minutes, with the sample kept in the dark. The values were determined from a calibration curve prepared with Trolox solutions, following the same procedure used for the sample extract. The results were expressed as Trolox Equivalent (T.E.) per unit weight.
2.2.5.3 Antioxidant activity assessed by the FRAP assay
The antioxidant activity was evaluated using a slightly modified version of the Ferric Reducing Antioxidant Power (FRAP) assay described by Lisanti et al. (2015), a method applied to both hydrophilic and lipophilic antioxidants. This assay is based on the ability of antioxidants to reduce Fe3+ ions to Fe2+ through an electron transfer mechanism in the presence of the TPTZ (2,4,6-tripyridyl-s-triazine) solution. The reaction creates a blue Fe2+–TPTZ complex that exhibits a maximum absorbance at 593 nm. This reaction is pH-dependent, with optimal performance occurring at pH 3.6. The decrease in absorbance is directly proportional to the concentration of antioxidant species present in the sample. The FRAP reagent was prepared by combining 2.5 mL of a 10 mM TPTZ solution in 40 mM HCl, 2.5 mL of a 20 mM FeCl3 solution, and 25 mL of 300 mM NaOAc buffer (pH 3.6). Next, 1.8 μL of the FRAP reagent was mixed with 200 μL of deionized water and 40 μL of the sample extracts. The reaction mixture was allowed to stand for 4 minutes at room temperature, after which the absorbance at 593 nm was measured. The values were determined from a calibration curve prepared using fixed concentrations of Trolox solutions, following the same procedure used for the sample extracts. The Trolox stock solution was 1,000 µM and the range of the calibration curve was 0-800 µM. Results were expressed as Trolox Equivalent (T.E.) per unit weight.
2.3 Statistical analysis
One-way ANOVA statistical analysis was performed with the Statistical Analysis System software using the General Linear Model (GLM Proc of the SAS Software; SAS 9.1; SAS Institute, Cary, NC, USA). The mean comparison was performed using the Tukey test (p=0.05).
3 Results
3.1 Plant bio-morphology
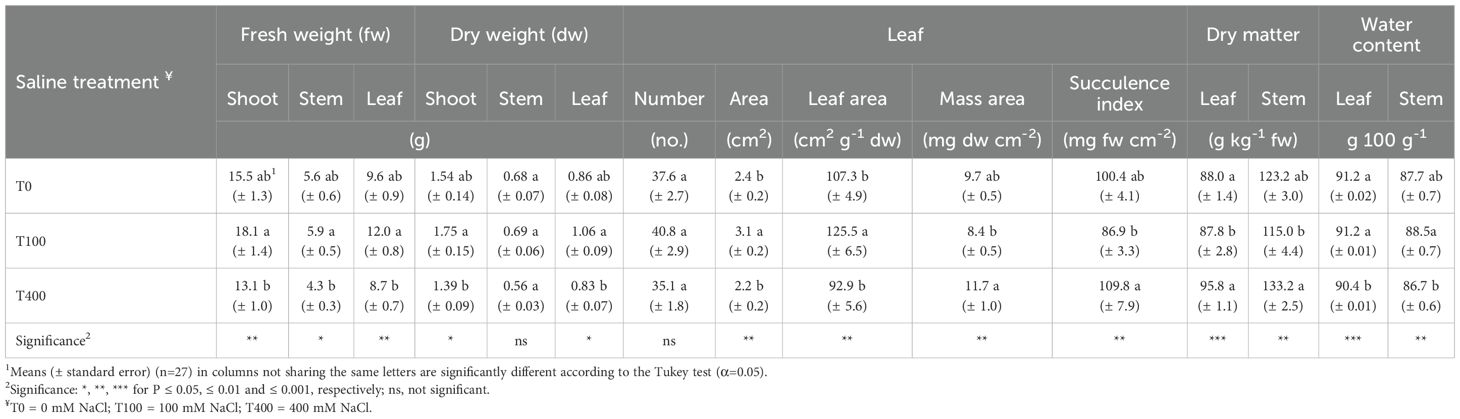
Plant height was the lowest in T0 plants (19.3 ± 3.4 cm) than in saline-treated plants (27.4 ± 3.1 and 25.3 ± 2.8 cm, respectively, in T100 and T400). The fresh and dry weights of shoots, stems, and leaves of C. maritima were significantly higher in the plants irrigated with a nutrient solution containing 100 mM NaCl (T100) than those irrigated with a solution containing 400 mM NaCl (T400). The only exception was the stem dry weight, which did not differ between salinity levels. Control plants (T0) exhibited intermediate values for these traits. Leaf number remained unchanged despite salinity levels; however, leaf area and specific leaf area (SLA) were highest in T100 and T0 plants. In contrast, leaf mass per area (LMA) and the succulence index were greatest in T400 plants, while T0 plants exhibited intermediate values. The leaf and stem dry matter (DM) were lowest in T100, whereas T400 plants had the lowest water content (Table 1).
The root apparatus of plants treated with 100 mM NaCl exhibited greater fresh weight, length, and volume, especially when compared to T400 plants. However, T100 plants had the lowest root DM and higher water content (WC). Root dry weight was similar across treatments, and no differences were observed in root diameter or shoot:root dry weight ratio (Table 2).
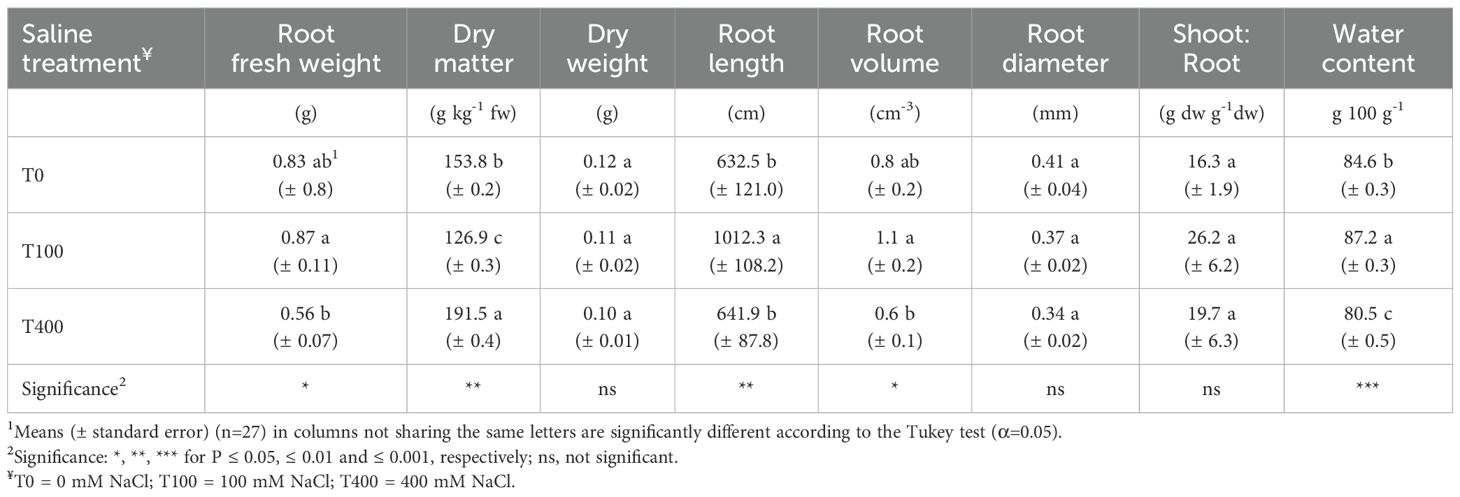
Table 2. Growth and bio-morphological traits of root apparatus of Cakile maritima as affected by salinity.
3.2 Mineral concentrations
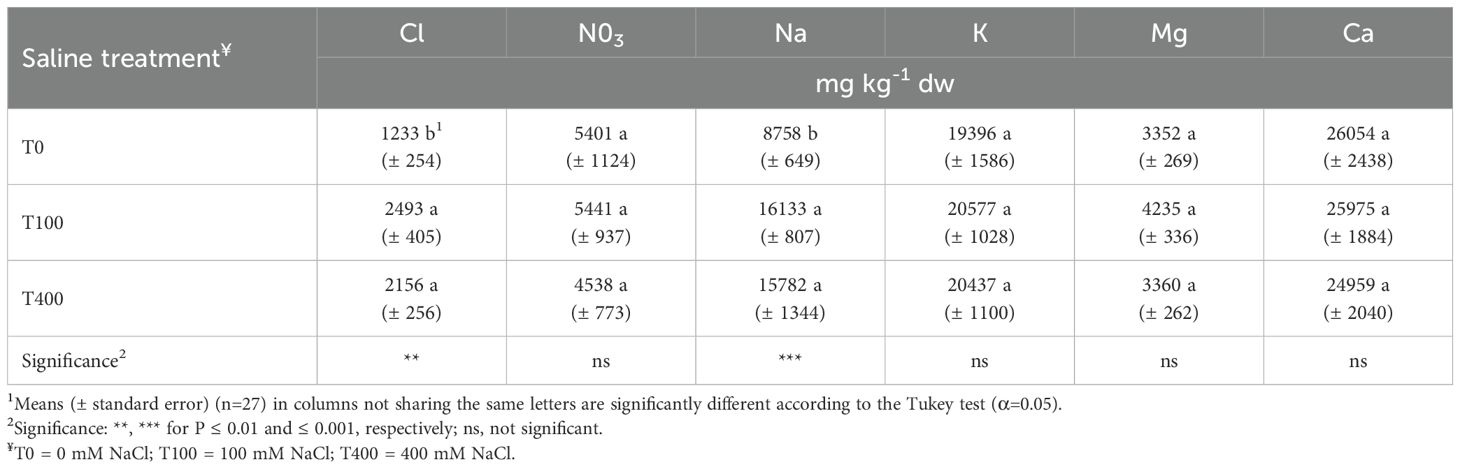
Leaf chloride, nitrate, potassium, calcium, and magnesium concentrations were not affected by the treatments. In contrast, sodium concentration was higher in the leaves of saline-treated plants, regardless of NaCl level (Table 3). In the stems, the T400 plants exhibited the highest sodium concentration, while the T0 plants showed the greatest calcium level (Table 4).
3.3 Photosynthetic gas exchange and chlorophyll fluorescence
Stomatal conductance (gs) and transpiration (E) were unaffected by salinity. However, intercellular CO2 concentration (Ci) decreased in T100 and T400 leaves, coinciding with a higher net CO2 assimilation rate (An), particularly in T100. The operating efficiency of PSII in the light (ΦPSII) did not differ between treatments, but the maximum PSII photochemical efficiency in the light (Fv’/Fm’) was highest in T0 leaves. Photochemical quenching (qP) was lower in T0, especially compared to T400 (Table 5).
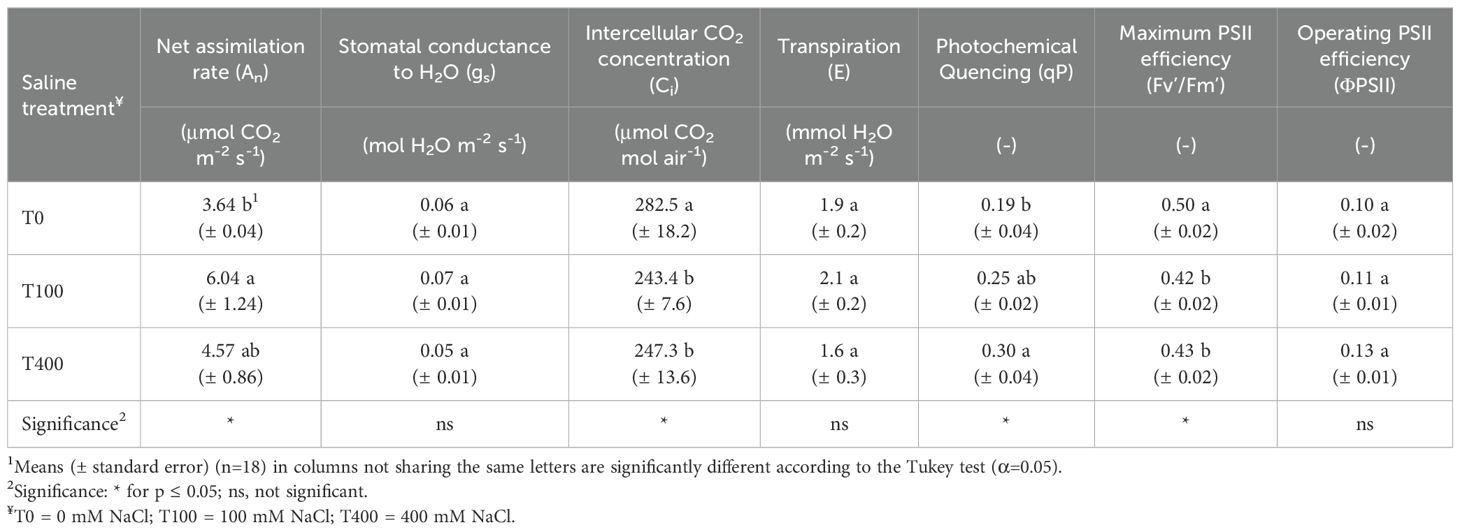
Table 5. Photosynthetic gas exchange and chlorophyll fluorescence of leaf of Cakile maritima as affected by salinity.
3.4 Pigment, bioactive compound concentrations and antioxidant capacity
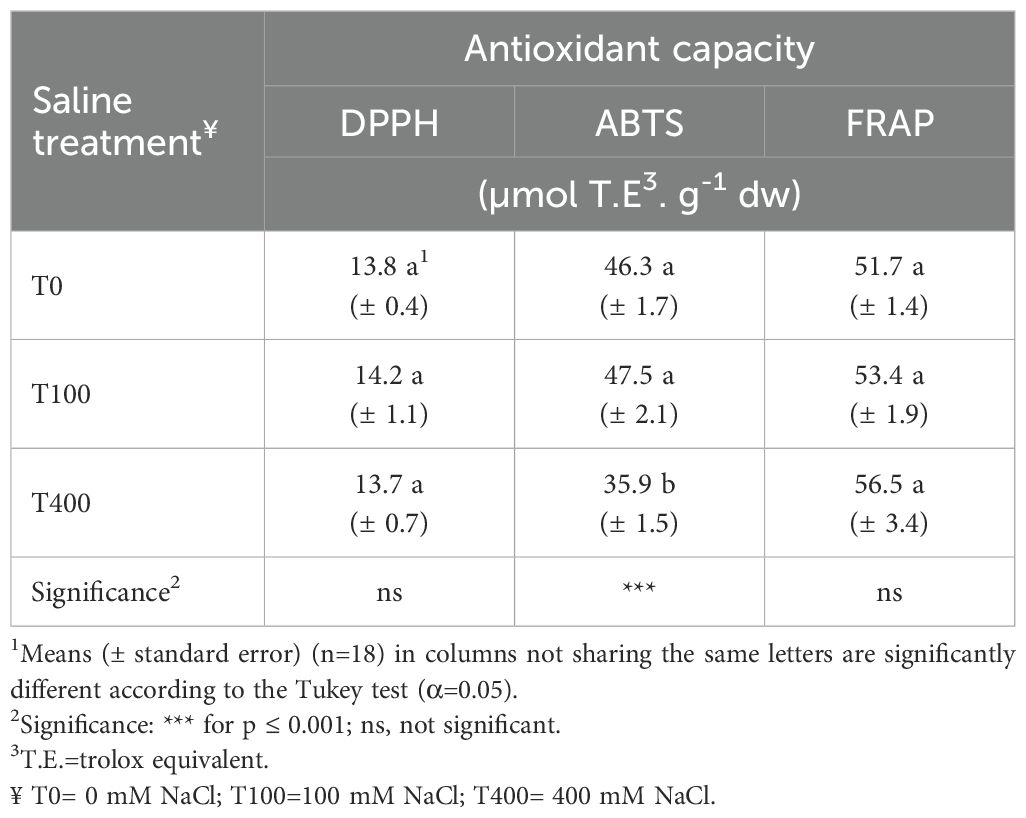
Chlorophyll a level decreased in T100 and T400 leaves, which also impacted the total concentration of these pigments. In contrast, chl b and carotenoid concentrations remained unchanged. Ascorbic acid, flavonoid, and anthocyanin levels were highest in T400 leaves, with T100 showing a significant decline in ascorbic acid (Table 6). Total phenols (TP) and antioxidant capacity (DPPH, ABTS, FRAP assays) were generally unaffected by treatments, except for lower TP and ABTS values in T400 leaves. Since lipophilic components were negligible, total antioxidant capacity was calculated as the sum of hydrophilic and lipophilic fractions (Table 7).
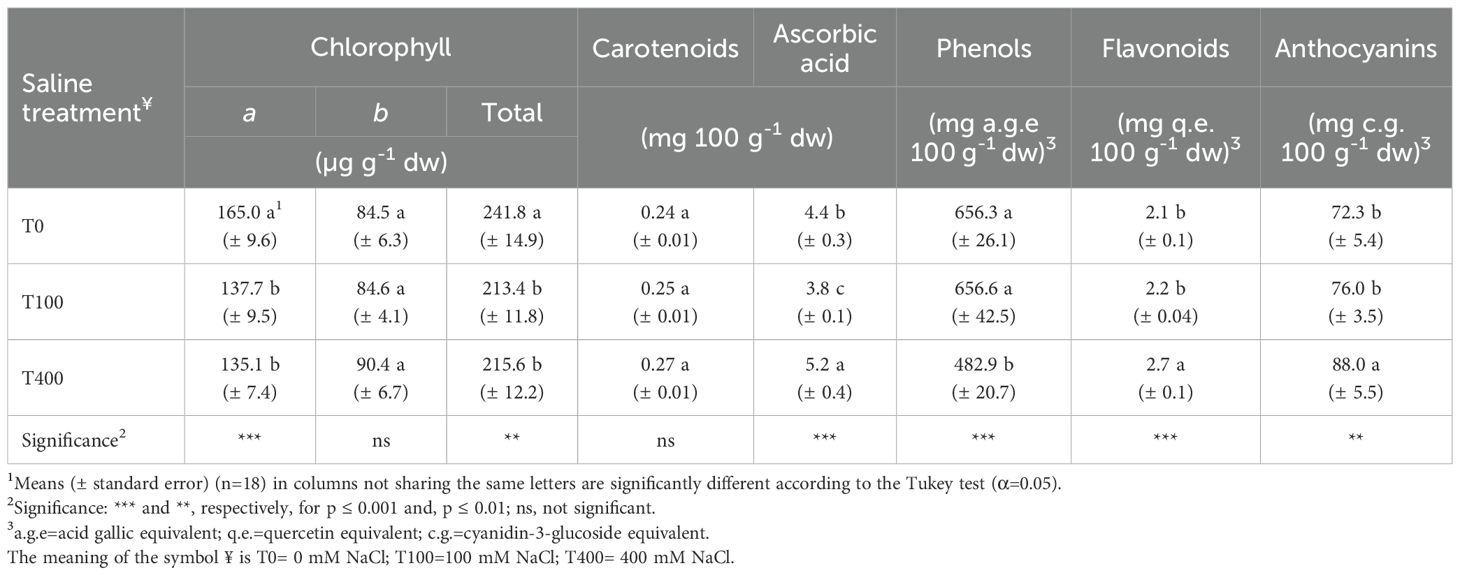
Table 6. Pigment and bioactive compound concentrations in leaves of Cakile maritima as affected by salinity.
4 Discussion
4.1 Growth and morpho-nutritional responses
In this study, the best growth was observed for T100 plants. The aerial part produced the highest fresh (+40%) and dry (+38%) biomass. The latter, primarily allocated to the leaves. The increase in leaf biomass was due to expansion rather than an increase in the number of leaves (Table 1). Plants grown at 100 mM NaCl also produced a root apparatus more developed with longer roots, but with lower dry matter (DM), suggesting a trade-off between growth and resource allocation. Although the shoot:root ratio trended higher in T100, the difference was not significant (Table 2).
Compared to control plants (T0), the most notable differences, rather than shoot and root dry and fresh biomass production, involved shoot height, leaf expansion and root length, ultimately leading to dry mass dilution in these organs. The shoot water content remained unchanged (leaves) or slightly increased (stems), and it even improved in roots (Tables 1, 2).
This research represents the first investigation into the saline responses of a C. maritima (pinnatifid) ecotype from Italy. To date, previous studies have focused solely on Tunisian C. maritima populations, reporting findings that are partially consistent with ours. When evaluating multiple NaCl concentrations, many authors (Debez et al., 2004, 2006, 2008, 2012; Yepes et al., 2018; Ben Amor et al., 2020) reported an improvement of dry biomass for plants nourished with 100 mM NaCl. However, others observed slight or no changes in plant growth (Ben Amor et al., 2006, 2007; Ben Hamed-Louati et al., 2016; Arbelet-Bonnin et al., 2020), with responses varying depending on the ecotype (Ben Amor et al., 2006; Ksouri et al., 2007; Megdiche et al., 2007). Moreover, these studies often found an enhancement of leaf number (Debez et al., 2004, 2008, 2012) rather than leaf area (Ksouri et al., 2007; Megdiche et al., 2007) passing from 0 to 100 mM NaCl.
Accounting for other morphological traits, the leaves of T100 plants tended to be thinner (exhibiting higher SLA and lower LMA) and less succulent than T0, despite having similar water content (Table 1). These bio-morphological traits were not strictly in line with previous research, which reported an improvement of plant water status along with succulence (Debez et al., 2004, 2006, 2012) and leaf thickness (Debez et al., 2006; Yepes et al., 2018). These changes occurred in response to increasing Na (and Cl) uptake and accumulation in leaves observed starting from a 50–100 mM NaCl concentration in the substrate.
Succulence mechanisms are the main strategy that salt-including halophytes (euhalophytes) adopt under saline conditions to maintain cell turgor pressure and prevent salt accumulation in the cytosol. It includes several biochemical responses such as ion homeostasis and osmotic adjustments, both involving Na, Cl and K regulation in the plant. The succulence of plants arises from solute and water storage in vacuoles, along with the corresponding osmolyte concentrations in the cytosol (Debez et al., 2012, 2013; Farhat et al., 2021; Ellouzi et al., 2011, 2014; Mir et al., 2024). Ultimately, this adaptive strategy results in tissues with enhanced water content, characterized by thick leaves and stems that exhibit large mesophyll cells and large vacuoles, ensuring Na and Cl dilution and maintaining photosynthetic efficiency (Rahman et al., 2021).
In C. maritima grown at up to 400 mM NaCl, the compartmentalization of excess sodium into vacuoles has been demonstrated to maintain cytosolic Na homeostasis (Debez et al., 2004, 2006; Ben Hamed-Louati et al., 2016). It was linked to the activity of vacuolar H+-ATPase, which creates a pH gradient between the cytoplasm and the vacuole, allowing Na transport against the electrochemical gradient (Debez et al., 2004), thus limiting the increase of Na/K ratio in the cytosol.
In the case of the Italian C. maritima ecotype, the accumulation of Na and Cl in leaves at T100 and the contemporary plant growth and balanced water status prove that these ions were excluded from the cytosol and likely were stored in the vacuoles, confirming the findings of other Authors (Debez et al., 2004, 2006; Ben Hamed-Louati et al., 2016). The different responses in terms of leaf thickness and succulence (Table 1) could be linked to the level of salt accumulation. In our research, the Na and Cl leaf concentrations increased by 84% and 102%, respectively, in comparison to T0 (Table 3); nevertheless, both Na (0.7 mmol g-1 DW) and Cl (0.03 mmol g-1 DW) were markedly lower than those reported for Tunisian populations (Debez et al., 2004, 2008, 2012; Ellouzi et al., 2011; Ben Hamed-Louati et al., 2016; Ben Amor et al., 2020) grown at the same saline treatment. Leaf Na and Cl concentrations similar to ours were reported only by Houmani et al. (2023), despite these authors not indicating ecotype provenience. Overall, these findings indicate that there are specific thresholds for inducing succulence that vary by ecotype.
Specifically, the reduction of leaf succulence was due to its expansion, which, along with root elongation, mainly contributed to the growth improvement in T100 plants (Tables 1, 2). Supporting this finding, a proteomic analysis of the Tunisian genotype of C. maritima, exposed to a salinity level of 100 mM NaCl, revealed an abundance of proteins potentially linked to cell growth (putative elongation chloroplast factor). The increase in the expression of this protein was associated with the greater leaf initiation and expansion compared to plants grown at 0 mM NaCl (Debez et al., 2012).
Differences in growth were significant when comparing T100 and T400 plants, with the latter not exhibiting visible damage. The T400 plants, despite having similar height, showed a reduction in shoot fresh (-28%) and dry (-21%) biomass, root fresh biomass (-36%) and length, as well as in leaf, stem, and root water content. In T400, leaves were thicker (higher LMA) and more succulent, exhibiting all typical morphological adaptations of halophytes to soil salinity (Tables 1, 2). Roots maintained stable dry biomass, suggesting that salts were not accumulated in the roots but could have been exported in the aerial parts, confirming the behavior observed in other ecotypes of C. maritima (Ben Hamed-Louati et al., 2016). It is noteworthy that the growth inhibition effect of 400 mM NaCl on C. maritima ecotype under study was milder than in other ecotypes (Debez et al., 2004, 2008; Ben Amor et al., 2006; Ksouri et al., 2007; Ben Hamed-Louati et al., 2016; Yepes et al., 2018; Arbelet-Bonnin et al., 2020).
No changes were detected in Na and Cl concentrations in T100 and T400 leaves (Table 3). If a Cl saturation at 50 mM NaCl level has been observed by Debez et al. (2004), the lack of increase in leaf sodium concentration with rising substrate salinity was unexpected, even though the T400 leaves typically exhibited succulence. Conversely, our results point out that Na accumulation occurred in the stems (green + mature), where it was enhanced by 83% in the T400 treatment. This suggests that stems serve as a primary Na sink in this ecotype (46.8 vs 13.5 mg g-1 DW, on average) (Table 4), diverging from prior studies that emphasize leaf accumulation (Debez et al., 2008).
Further investigation is needed to explore the overall low sodium content in the shoots of this ecotype of C. maritima and its partitioning in the different parts of the plant. Studies conducted on Italian morphotypes of C. maritima have indicated that salt-excreting structures are present in pinnatifid-leaf types (Ciccarelli et al., 2010). Previous research on the same pinnatifid morphotype of this study revealed a lower Na concentration in the shoots compared to the entire-leaf type (Conversa et al., 2024). Therefore, a more in-depth evaluation of leaf morphology is necessary to determine the presence of salt-excreting structures and their potential role in reducing Na content in the leaves. Additionally, along with the possible contribution of Na excretion, in future research, it is important to consider a Na-excluding mechanism at the root level. To deal with salt stress, the most significant signaling pathway is the salt overly sensitive (SOS), which requires the combined activities of three proteins, namely SOS3, SOS2, and SOS1 (a plasma membrane-localized Na+/H+ antiporter) expelling Na out of the cell (Shabala and Mackay, 2011; Ji et al., 2013; Shabala et al., 2015; Julkowska and Testerink, 2015). In C. maritima culture cells, the constitutive expression of the SOS has been determined, which could be involved at the root level not only in Na upload in the xylem leading to shoot accumulation, but also in salt exclusion from cells (Arbelet-Bonnin et al., 2018).
The stable concentration, from 0 up to 400 mM NaCl, of K and Mg in leaves and stems means that no impairment in uptake of these cations occurred at both saline levels. These findings seem to confirm the selective K uptake pointed out in Tunisian C. maritima ecotypes, counteracting the Na excess (Debez et al., 2004, 2006, 2008; Ben Hamed-Louati et al., 2016). Despite these authors (except Ben Hamed-Louati et al., 2016) reporting a reduction of K, Ca and Mg concentration, they never observed nutritional disruption in salt-exposed plants. The regulation of the uptake and transport of Na and K (and Mg) in the plant is essential in halophytes when facing salinity. This regulation helps maintain an optimal K/Na ratio in the cytosol since potassium is involved in various metabolic processes and osmotic adjustment. Specifically, while Na replaces K in the vacuoles to serve as an osmotic agent, potassium, together with compatible organic solutes, plays a vital role in the cytosol to maintain cell turgor pressure (Rahman et al., 2021).
Calcium was the most abundant cation, especially in the stems (see Tables 3, 4). A higher concentration of calcium compared to sodium in the leaves was noted by Yepes et al. (2018), although this finding contradicts other studies (Debez et al., 2004; Ellouzi et al., 2011). It remained unchanged in the leaves but showed a reduction in stems of salt-treated plants, irrespective of NaCl level. Exposure to NaCl triggers an increase in cytosolic Ca concentration, which is essential for activating plasma membrane H+-ATPases, ultimately leading to the stimulation of the SOS pathway (Ji et al., 2013; Shabala et al., 2015). Such a phenomenon has been observed in the cell suspension of C. maritima (Arbelet-Bonnin et al., 2018). Consequently, the decrease in calcium levels in accumulation sites (stems) when plants are exposed to salinity could indicate that leaves may act as a sink for this cation, playing a role in sodium regulation via the SOS pathway. On the other hand, a moderate calcium level has been proven to enhance the tolerance of C. maritima under salt stress by increasing several antioxidative enzyme activities (Ben Amor et al., 2010).
4.2 Photosynthetic responses
The plant growth in saline-tolerant genotypes, along with effective Na, Cl, and K regulation, is strictly dependent on efficient gas exchange and scavenging of reactive oxygen species (ROS) to ultimately preserve photosynthesis apparatus efficiency for producing plant dry biomass.
Salt stress typically impairs photosynthesis by both stomatal (e.g. CO2 uptake) and non-stomatal (e.g. chlorophyll, Rubisco) limitation, leading to excessive energy exposure of plants beyond their capacity to utilize it in photosynthesis. Therefore, one of the physiological adaptive mechanisms to salinity is the protection of the photosynthetic apparatus through the dissipation of energy excess and ROS detoxification (Qiu et al., 2003; Debez et al., 2008; Megdiche et al., 2008; Mann et al., 2023).
In the current study, the ΦPSII parameter (operating efficiency of PSII in the light), which indicates the fraction of the absorbed light actually used for photochemistry processes (Murchie and Lawson, 2013), remained stable across treatments. However, its components Fv’/Fm’ and qP parameters highlighted photosynthetic adjustments in plants treated with NaCl (Table 5). The maximum PSII photochemical efficiency in the light (Fv’/Fm’), representing the efficiency if all PSII centers were open, was the lowest in both saline-treated plants. Since any decrease of Fv’/Fm’ is inversely related to the dissipation of energy excess mainly as heat (non-photochemical quenching -NPQ) (Baker, 2008; Maxwell and Johnson, 2000), it can be argued that salt-treated plants exhibited higher NPQ as a photosynthetic apparatus protective strategy (Malnoë, 2018).
A reduction of Fv’/Fm’ was observed in C. maritima at 400 mM NaCl (Arbelet-Bonnin et al., 2020), while higher NPQ levels were also revealed in plants under saline conditions starting from 100 to 500 mM NaCl (Debez et al., 2008; Megdiche et al., 2008; Farhat et al., 2021). Photoprotection mechanisms acting under stressful conditions, such as salinity, involve various processes at the chloroplast level, including carotenoid and anthocyanin pigments (Megdiche et al., 2008). Carotenoids absorb short-wavelength light and transfer energy to chlorophylls, preventing the formation of chlorophyll triplets and reactive oxygen species (ROS). Anthocyanins effectively filter visible light, thus preventing the production of ROS that can be induced by excessive light (Ashikhmin et al., 2023).
In our study, carotenoids were not affected by salinity; however, the Car/Chl ratio improved in salt-treated plants due to chlorophyll reduction (Table 6). The higher Car/Chl was in line with results reported for a more salt-tolerant C. maritima Tunisian ecotype (Megdiche et al., 2008), confirming the role of carotenoids in avoiding the oxidative stress and photosynthesis impairment deriving from salt exposure. Reduction in chlophyll pigments was found due to a decrease in the Chl a (Table 6), which is reported to be more salt-sensitive than Chl b (Megdiche et al., 2008); however, it occurred only at moderate salinity levels. The rise in anthocyanins in T400 plants (Table 6) can be explained as an additional protective mechanism for chlorophyll and PSII. The enhancement of leaf anthocyanins in C. maritima under the highest salinity conditions has also been observed in many studies (Debez et al., 2008, 2012; Mansour et al., 2018; Farhat et al., 2021) as PSII protection related to a strong antioxidative response.
Despite Fv’/Fm’ reduction, the T100 and especially T400 plants showed a higher PSII efficiency factor (qP), which indicates the actual fraction of Fv’/Fm’ that is achieved. In other words, this factor is related to the proportion of open reaction centers, highlighting the level of photochemical quenching occurring in PSII. These findings suggest the capability of salt-exposed plants to maintain the efficiency of photosynthetic machinery, improving the ability to utilize light. In line with this study, unchanged qP has been reported in C. maritima from 0 to 400 mM NaCl (Megdiche et al., 2008). Additionally, improved qP was observed for plants grown at 200 mM NaCl compared with the control (Farhat et al., 2021).
In disagreement with our results, a reduction in ΦPSII was observed for C. maritima grown at salinity levels exceeding 200 mM NaCl (Debez et al., 2008) or 400 mM NaCl (Arbelet-Bonnin et al., 2020).
Whereas, comparing two ecotypes, ΦPSII decreased only in the less tolerant one at 400 mM NaCl (Megdiche et al., 2008). The ΦPSII impairment may be related to CO2 limitation due to stomatal closure as an adaptive response to salinity; CO2 limitation reduces the rate of ATP and NADPH consumption, and in turn, ΦPSII (Baker and Rosenqvist, 2004; Baker, 2008). In the case of the studied C. maritima ecotype, the steady ΦPSII could also be linked to unchanged stomatal conductance up to 400 mM NaCl (gs, E, Table 5).
The ΦPSII is linked to the linear electron flow through the reaction centers, which plays a role in CO2 assimilation (An). However, there is often a discrepancy between the efficiency of PSII and the rate of CO2 fixation. This gap is primarily attributed to the phenomenon of photorespiration which is essential for protecting PSII and sustaining photosynthesis (Maxwell and Johnson, 2000; Shi et al., 2022). Indeed, photorespiration can act as a sink for photosynthetically generated electrons, especially under stress conditions (Sunil et al., 2019; Voss et al., 2013).
In this study, after nearly one month of saline stress, a significant discrepancy in An and ΦPSII was detected only for the T0 plants (Table 5). Since no restriction on CO2 can be supposed to be caused by stomata closure, in control plants, the higher Ci suggests lower CO2 consumption and the occurrence of photorespiration as an electron sink, explaining their lower net CO2 assimilation (-40%) versus T100 (Table 5). This finding can help to explain the slight reduction in growth (shoot dry biomass) of the salt-free plants (Table 1), highlighting that they experienced stress more than moderate salt-exposed plants. Therefore, this investigation confirms that this species is an euhalophyte, revealing the role of Na as an essential nutrient.
Interestingly, the T400 plants showed a negligible decrease in An while exhibiting gas exchange (gs) and CO2 intercellular concentration (Ci) very similar to T100 (Table 5). Conversely, many Authors have highlighted that a drop in An generally occurs in C. maritima at salinity levels higher than 200 mM NaCl because of stomata closure to reduce water loss through transpiration (Debez et al., 2004, 2008; Megdiche et al., 2008; Ben Hamed-Louati et al., 2016; Arbelet-Bonnin et al., 2020). It is surprising to find unchanged stomatal conductance and transpiration (E) (Table 5). This phenomenon, observed at 100 mM NaCl, has been related to the replacement of K with Na in guard cells, which helps maintain stable turgor pressure (Debez et al., 2006). In our study, this mechanism could have occurred effectively even at salinity levels exceeding 100 mM NaCl, thereby preventing any restriction on CO2 uptake. Moreover, leaf anatomical features of a pinnatifid ecotype (large and outwardly turned epidermal vesicular cells, cell guards rich in essential oils) (Ciccarelli et al., 2010) have been reported to assure abiotic stress tolerance under natural habitat by sustaining higher transpiration rates (Conversa et al., 2024). They could also be involved in the salt responses. However, this aspect warrants further research to confirm and elucidate the unexpected salt-adaptive mechanisms of this C. maritima ecotype.
Although photosynthesis per unit leaf does not seem affected by higher salinity, the reduced leaf area of T400 plants leads to diminished photosynthesis at the canopy level, resulting in decreased shoot growth and dry biomass production. Likely this occurred for sustaining energy-requiring ion compartmentalization, osmoregulation, morphological adaptation, and antioxidative responses to salinity (Debez et al., 2006; Rahman et al., 2021; Bazihizina et al., 2024) observed in these plants. Additionally, an osmotic effect can be argued due to the enhancement of dry mass concentration and reduction in water content in leaves, stems and roots (Tables 1, 2).
4.3 Non-enzymatic antioxidative leaf status
Concerning antioxidant compounds, both carotenoids and anthocyanins may act as antioxidants directly scavenging ROS, along with other compounds such as ascorbate, phenols, flavonoids and glutathione (Rahman et al., 2021; Mann et al., 2023). Specifically, we found under supra-optimal salinity conditions an increase of flavonoids, anthocyanins and ascorbate (AsA) (Table 6). These results are consistent with prior reports for anthocyanins in this species. Contrarily, ascorbate reduction at increasing salinity is reported by many Authors (Ben Amor et al., 2006, 2007; Ben Hamed-Louati et al., 2016; Mansour et al., 2018) despite others having found that it was improved at 400 mM NaCl at the chloroplast level (Ben Amor et al., 2020). On the other hand, a higher AsA retention passing from 0 to 400 mM NaCl was observed in a more salt-tolerant C. maritima genotype (Ben Amor et al., 2006).
Ascorbate is the main biologically active form of vitamin C, as it interacts with ROS, undergoing oxidation to dehydroascorbic acid (DHAA); in plants, DHAA is either recycled to AsA by the dehydro-ascorbate reductase, using glutathione as the reductant, or irreversibly hydrolyzed. Efficient ascorbate recycling (high AsA/DHAA) may be critical to maintain ROS at a level that minimizes oxidative damage (Gallie, 2013). Although we did not detect DHAA for evaluating the changes of AsA/DHAA in salt-free and salt-treated plants, the highest AsA in T400 may suggest an improved antioxidative defense of these plants. An increase in AsA was found in halophytes C. maritima and Thellungiella salsuginea within 72 h to 400 mM NaCl. It was related to an earlier response to stress imposition compared to a glycophyte (Arabidopsis thaliana), with an efficient DHAA recycling (Ellouzi et al., 2014).
Among other antioxidant compounds, an increase in TP content in salt-exposed plants was expected, as it is involved in counteracting ROS resulting from abiotic stresses (Conversa et al., 2021). In our study, TP diminished at 400 mM NaCl with no changes between control and mild-saline treatment (Table 6). Partially in line with this study, in the more salt-tolerant sea rocket ecotype, TP accumulation drops from 100 to 400 mM NaCl, despite an enhancement occurring from 0 to 100 mM NaCl; in the less salt-tolerant sea rocket ecotype, TP accumulation was lower and unaffected by salinity (Ksouri et al., 2007). On the contrary, TP accumulation progressively increased under salinity constraints in another C. maritima Tunisian ecotype due to the activity of the key enzyme for their biosynthesis (Mansour et al., 2018).
In the euhalophytes Salicornia and Sarcocornia species, it has been demonstrated that, after an increase in TP at moderate salt levels (35–200 mM NaCl), plants lose the ability to produce these compounds, activating other protective mechanisms such as osmolyte synthesis and/or salt exclusion (Lima et al., 2020). Considering the morphological and physiological adaptation described above for the T400 plants, a shift of their resources towards other metabolic mechanisms for preventing ROS production or providing their detoxification could be plausible. Antioxidant capacity (AC) assays (FRAP, DPPH, ABTS) showed no salinity-linked trends (Table 7). Only ABTS revealed a relationship with TP changes, as expected (Conversa et al., 2019). There is limited and non-univocal published data regarding the link between antioxidant compounds and antioxidant capacity under saline treatments (Ksouri et al., 2007; Mansour et al., 2018). As a result, specific antioxidants and AC tests do not seem to fully capture the plant’s response to salinity. To obtain a comprehensive analysis, the enzymatic antioxidative system should be explored, as it is a strong indicator of oxidative stress (Ben Amor et al., 2006, 2007; Arbelet-Bonnin et al., 2019). Additionally, further research into bioactive compounds is needed, as their potential benefits to humans are significant. Extracts from whole wild C. maritima plants demonstrated high DPPH radical scavenging activity due to several bioactive compounds, which were positively correlated with their anti-proliferative effects on CaCo2 and HeLa carcinoma cell lines (Omer et al., 2019). The DPPH of the leaves and stems of C. maritima was associated with total phenolic content and exhibited anti-inflammatory, anti-bacterial, and anti-proliferative activities against multiple myeloma cells (Fuochi et al., 2019). These extracts showed inhibitory properties against key enzymes involved in human diseases (Placines et al., 2020).
5 Conclusions
Our investigation of the Apulian ecotype of C. maritima confirms its classification as a sodium-including halophyte, demonstrating optimal growth at moderate salinity levels of 100 mM NaCl. Under these conditions, we observed a significant sodium accumulation in leaves, exceeding 80% compared to non-saline controls. The leaf Na level optimal for growth remained consistent even at higher salt concentrations (400 mM NaCl), as the excess sodium was accumulated in the stems, proving the ability is this ecotype to maintain leaf sodium concentrations conducive to growth even at higher salt concentrations. At 400 mM NaCl, this adaptation allows plant growth, albeit at slightly reduced rates, with the plants showing characteristic morpho-physiological adjustments like increased leaf succulence while displaying no visible toxicity symptoms. The study reveals another noteworthy aspect regarding essential cations, with K, Mg, and Ca uptake levels remaining largely stable, indicating the presence of an efficient ion uptake regulation system that prevents nutritional imbalances under high salinity stress.
Although this represents an initial characterization of this specific ecotype, our findings clearly show these plants avoid severe growth limitations by maintaining effective stomatal regulation and preserving photosynthetic apparatus functionality under saline stress. To fully understand this ecotype’s potential, future research should explore the physiological mechanisms enabling water balance maintenance and sustained photosynthetic energy production, particularly during prolonged salinity exposure. Additional investigations could clarify the molecular basis of these adaptations and evaluate potential applications in marginal agriculture or soil salinization scenarios.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
GC: Data curation, Conceptualization, Writing – review & editing, Formal analysis, Funding acquisition, Writing – original draft, Project administration. LB: Investigation, Writing – original draft. CL: Writing – original draft, Investigation. AB: Writing – original draft, Data curation, Investigation. LD: Writing – original draft. AE: Writing – review & editing, Validation, Writing – original draft, Visualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by (1) the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR) -MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4 -D.D. 1032 17/06/2022, CN00000022); (2) Regione Puglia Project ‘Biodiversity of Apulian vegetable species (BiodiverSO Veg), Measure 10, Sub measure 10.2, Operation 1 “Program for the conservation and the valorization of the genetic resources in agriculture”.
Acknowledgments
We thank Dr. Ruggiero Piazzola for their assistance during the field activities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1662491/full#supplementary-material
References
Arbelet-Bonnin, D., Ben-Hamed-Louati, I., Laurenti, P., Abdelly, C., Ben-Hamed, K., and Bouteau, F. (2019). Cakile maritima, a promising model for halophyte studies and a putative cash crop for saline agriculture. Adv. Agron. 155, 45–78. doi: 10.1016/bs.agron.2019.01.003
Arbelet-Bonnin, D., Blasselle, C., Rose Palm, E., Redwan, M., Ponnaiah, M., Laurenti, P., et al. (2020). Metabolism regulation during salt exposure in the halophyte Cakile maritima. Environ. Exp. Bot. 177, 104075. doi: 10.1016/j.envexpbot.2020.104075
Arbelet-Bonnin, D., Ben Hamed-Laouti, I., Laurenti, P., Abdelly, C., Ben Hamed, K., and Bouteau, F. (2018). Cellular mechanisms to survive salt in the halophyte Cakile maritima. Plant Sci. 272, 173–178. doi: 10.1016/J.PLANTSCI.2018.04.018
Ashikhmin, A., Bolshakov, M., Pashkovskiy, P., Vereshchagin, M., Khudyakova, A., Shirshikova, G., et al. (2023). The Adaptive Role of Carotenoids and Anthocyanins in Solanum lycopersicum Pigment Mutants under High Irradiance. Cells 12, 1–17. doi: 10.3390/cells12212569
Baker, N. R. (2008). Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59, 89–113. doi: 10.1146/annurev.arplant.59.032607.092759
Baker, N. R. and Rosenqvist, E. (2004). Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 55, 1607–1621. doi: 10.1093/jxb/erh196
Balık, S., Kaya, T., and Aslantaş, R. (2023). Fruit quality parameters, sugars, vitamin C, antioxidant activity, organic acids, and phenolic compounds for a new endemic apple variety, “Long apple. Horticulturae 9, 1171. doi: 10.3390/horticulturae9111171
Bazihizina, N., Papenbrock, J., Aronsson, H., Ben Hamed, K., Elmaz, Ö., Dafku, Z., et al. (2024). The sustainable use of halophytes in salt-affected land: state-of-the-art and next steps in a saltier world. Plants 13 (16). doi: 10.3390/plants13162322
Ben Amor, N., Jiménez, A., Boudabbous, M., Sevilla, F., and Abdelly, C. (2020). Chloroplast implication in the tolerance to salinity of the halophyte cakile maritima. Russ. J. Plant Physiol. 67, 507–514. doi: 10.1134/S1021443720030048
Ben Amor, N., Jiménez, A., Megdiche, W., Lundqvist, M., Sevilla, F., and Abdelly, C. (2006). Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiol. Plant 126, 446–457. doi: 10.1111/j.1399-3054.2006.00620.x
Ben Amor, N., Jiménez, A., Megdiche, W., Lundqvist, M., Sevilla, F., and Abdelly, C. (2007). Kinetics of the anti-oxidant response to salinity in the halophyte Cakile maritima. J. Integr. Plant Biol. 49, 982–992. doi: 10.1111/j.1672-9072.2007.00491.x
Ben Amor, N., Megdiche, W., Jiménez, A., Sevilla, F., and Abdelly, C. (2010). The effect of calcium on the antioxidant systems in the halophyte Cakile maritima under salt stress. Acta Physiol. Plant 32, 453–461. doi: 10.1007/s11738-009-0420-2
Ben Hamed-Louati, I., Bouteau, F., Abdelly, C., and Ben Hamed, K. (2016). Impact of repetitive salt shocks on seedlings of the halophyte Cakile maritima. Environ. Control Biol. 54, 23–30. doi: 10.2525/ecb.54.23
Ben Hamed, K., Hamad, I. B., Bouteau, F., and Abdelly, C. (2016). “Insights into the Ecology and the Salt Tolerance of the Halophyte Cakile maritima Using Multidisciplinary Approaches”. in Halophytes for Food Security in Dry Lands, (Academic Press, San Diego), 197–211. doi: 10.1016/B978-0-12-801854-5.00012-1
Cataldi, T. R. I., Margiotta, G., Del Fiore, A., and Bufo, S. A. (2003). Ionic content in plant extracts determined by ion chromatography with conductivity detection. Phytochem. Anal. 14, 176–183. doi: 10.1002/pca.700
Chandra, S., Khan, S., Avula, B., Lata, H., Yang, M. H., Elsohly, M. A., et al. (2014). Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evidence-Based Complement. Altern. Med 2014, 253875. doi: 10.1155/2014/253875
Ciccarelli, D., Balestri, M., Pagni, A. M., and Forino, L. M. C. (2010). Morpho-functional adaptations in Cakile maritima Scop. subsp. maritima: Comparation of two different morphological types. Caryologia 63, 411–421. doi: 10.1080/00087114.2010.10589754
Conversa, G., Bonasia, A., Lazzizera, C., La Rotonda, P., and Elia, A. (2021). Reduction of nitrate content in baby-leaf lettuce and cichorium endivia through the soilless cultivation system, electrical conductivity and management of nutrient solution. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.645671
Conversa, G., Botticella, L., Lazzizera, C., Bonasia, A., and Elia, A. (2024). Ecophysiological and nutritional characterisation of two morphotypes of Cakile maritima subsp. maritima Scop. from Puglia region, Southern Italy. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1397852
Conversa, G., Lazzizera, C., Chiaravalle, A. E., Miedico, O., Bonasia, A., La Rotonda, P., et al. (2019). Selenium fern application and arbuscular mycorrhizal fungi soil inoculation enhance Se content and antioxidant properties of green asparagus (Asparagus officinalis L.) spears. Sci. Hortic. (Amsterdam). 252, 176-191. doi: 10.1016/j.scienta.2019.03.056
Debez, A., Ben Hamed, K., Grignon, C., and Abdelly, C. (2004). Salinity effects on germination, growth, and seed production of the halophyte Cakile maritima. Plant Soil 262, 179–189. doi: 10.1023/B:PLSO.0000037034.47247.67
Debez, A., Braun, H. P., Pich, A., Taamalli, W., Koyro, H. W., Abdelly, C., et al. (2012). Proteomic and physiological responses of the halophyte Cakile maritima to moderate salinity at the germinative and vegetative stages. J. Proteomics 75, 5667–5694. doi: 10.1016/j.jprot.2012.08.012
Debez, A., Koyro, H. W., Grignon, C., Abdelly, C., and Huchzermeyer, B. (2008). Relationship between the photosynthetic activity and the performance of Cakile maritima after long-term salt treatment. Physiol. Plant 133, 373–385. doi: 10.1111/j.1399-3054.2008.01086.x
Debez, A., Rejeb, K., Ghars, M. A., Gandour, M., Megdiche, W., Hamed, K.B., et al. (2013). Ecophysiological and genomic analysis of salt tolerance of cakile maritima. Environ. Exp. Bot. 92, 64–72. doi: 10.1016/j.envexpbot.2012.12.002
Debez, A., Saadaoui, D., Ramani, B., Ouerghi, Z., Koyro, H. W., Huchzermeyer, B., et al. (2006). Leaf H+-ATPase activity and photosynthetic capacity of Cakile maritima under increasing salinity. Environ. Exp. Bot. 57, 285–295. doi: 10.1016/j.envexpbot.2005.06.009
Ellouzi, H., Ben Hamed, K., Cela, J., Munné-Bosch, S., and Abdelly, C. (2011). Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol. Plant 142, 128–143. doi: 10.1111/j.1399-3054.2011.01450.x
Ellouzi, H., Hamed, K.B., Hernández, I., Cela, J., Müller, M., Magné, C., et al. (2014). A comparative study of the early osmotic, ionic, redox and hormonal signaling response in leaves and roots of two halophytes and a glycophyte to salinity. Planta 240, 1299–1317. doi: 10.1007/s00425-014-2154-7
Farhat, N., Kouas, W., Braun, H. P., and Debez, A. (2021). Stability of thylakoid protein complexes and preserving photosynthetic efficiency are crucial for the successful recovery of the halophyte Cakile maritima from high salinity. Plant Physiol. Biochem. 166, 177–190. doi: 10.1016/j.plaphy.2021.05.044
Fuochi, V., Barbagallo, I., Distefano, A., Puglisi, F., Palmeri, R., Rosa, M. D. I., et al. (2019). Biological properties of Cakile maritima Scop. (Brassicaceae) extracts. Eur. Rev. Med. Pharmacol. Sci. 23, 2280–2292. doi: 10.26355/eurrev_201903_17277
Gallie, D. R. (2013). The role of l-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 64, 433–443. doi: 10.1093/jxb/ers330
Gratani, L., Varone, L., and Crescente, M. F. (2009). Photosynthetic activity and water use efficiency of dune species: The influence of air temperature on functioning. Photosynthetica 47, 575–585. doi: 10.1007/s11099-009-0083-7
Houmani, H., Palma, J. M., and Corpas, F. J. (2023). High salinity stimulates the adaptive response to potassium deficiency through the antioxidant and the NADPH-generating systems in the roots and leaves of the halophyte cakile maritima. J. Plant Growth Regul. 42, 6286–6306. doi: 10.1007/s00344-022-10819-7
Ji, H., Pardo, J. M., Batelli, G., Van Oosten, M. J., Bressan, R. A., and Li, X. (2013). The salt overly sensitive (SOS) pathway: Established and emerging roles. Mol. Plant. Oxford University Press. 6, 275–286. doi: 10.1093/mp/sst017
Julkowska, M. M. and Testerink, C. (2015). Tuning plant signaling and growth to survive salt. Trends Plant Sci. 20, 586–594. doi: 10.1016/j.tplants.2015.06.008
Ksouri, R., Megdiche, W., Debez, A., Falleh, H., Grignon, C., and Abdelly, C. (2007). Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 45, 244–249. doi: 10.1016/j.plaphy.2007.02.001
Lima, A. R., Castañeda-Loaiza, V., Salazar, M., Nunes, C., Quintas, C., Gama, F., et al. (2020). Influence of cultivation salinity in the nutritional composition, antioxidant capacity and microbial quality of Salicornia ramosissima commercially produced in soilless systems. Food Chem. 333, 127525. doi: 10.1016/j.foodchem.2020.127525
Lisanti, A., Formica, V., Ianni, F., Albertini, B., Marinozzi, M., Sardella, R., et al. (2015). Antioxidant activity of phenolic extracts from different cultivars of Italian onion (Allium cepa) and relative human immune cell proliferative induction. Pharm. Biol. 54, 799–806. doi: 10.3109/13880209.2015.1080733
Malnoë, A. (2018). Photoinhibition or photoprotection of photosynthesis? Update on the (newly termed) sustained quenching component qH. Environ. Exp. Bot. 154, 123–133. doi: 10.1016/j.envexpbot.2018.05.005
Mann, A., Lata, C., Kumar, N., Kumar, A., Kumar, A., and Sheoran, P. (2023). Halophytes as new model plant species for salt tolerance strategies. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1137211
Mansour, R., Dakhlaouia, S., Msahli, W., Ksouri, R., and Ksouri, W. M. (2018). Differential Responses of Cakile maritima at Two Development Stages to Salinity: Changes on Phenolic Metabolites and Related Enzymes and Antioxidant Activity. Med. Chem. (Los. Angeles). 08, 100–108. doi: 10.4172/2161-0444.1000500
Maxwell, K. and Johnson, G. N. (2000). Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 51, 659–668. doi: 10.1093/jexbot/51.345.659
Megdiche, W., Amor, N., Debez, A., Hessini, K., Ksouri, R., Zuily-Fodil, Y., et al. (2007). Salt tolerance of the annual halophyte Cakile maritima as affected by the provenance and the developmental stage. Acta Physiol. Plant 29, 375–384. doi: 10.1007/s11738-007-0047-0
Megdiche, W., Hessini, K., Gharbi, F., Jaleel, C. A., Ksouri, R., and Abdelly, C. (2008). Photosynthesis and photosystem 2 efficiency of two salt-adapted halophytic seashore Cakile maritima ecotypes. Photosynthetica 46, 410–419. doi: 10.1007/s11099-008-0073-1
Mir, R., Mircea, D. M., Ruiz-González, M. X., Brocal-Rubio, P., Boscaiu, M., and Vicente, O. (2024). Cakile maritima: A halophyte model to study salt tolerance mechanisms and potential useful crop for sustainable saline agriculture in the context of climate change. Plants 13, 2880. doi: 10.3390/plants13202880
Murchie, E. H. and Lawson, T. (2013). Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 64, 3983–3998. doi: 10.1093/jxb/ert208
Murray, J. R. and Hackett, W. P. (1991). Dihydroflavonol reductase activity in relation to differential anthocyanin accumulation in juvenile and mature phase hedera helix L. Plant Physiol. 97, 343–351. doi: 10.1104/pp.97.1.343
Omer, E., Elshamy, A. I., Taher, R. F., El-Kashak, W. A., Shalom, J., White, A. A., et al. (2019). Cakile maritima scop. Extracts inhibit CaCo2 and heLa human carcinoma cell growth: GC-MS analysis of an anti-proliferative extract. Pharmacog J. 11, 258–266. doi: 10.5530/pj.2019.11.40258
Placines, C., Castañeda-Loaiza, V., Rodrigues, M. J., Pereira, C. G., Stefanucci, A., Mollica, A., et al. (2020). Phenolic profile, toxicity, enzyme inhibition, in silico studies, and antioxidant properties of Cakile maritima scop. (Brassicaceae) South. Portugal. Plants 9, 1–23. doi: 10.3390/plants9020142
Qiu, N., Lu, Q., and Lu, C. (2003). Photosynthesis, photosystem II efficiency and the xanthophyll cycle in the salt-adapted halophyte Atriplex centralasiatica. New Phytol. 159, 479–486. doi: 10.1046/j.1469-8137.2003.00825.x
Rahman, M. M., Mostofa, M. G., Keya, S. S., Siddiqui, M. N., Ansary, M. M. U., Das, A. K., et al. (2021). Adaptive mechanisms of halophytes and their potential in improving salinity tolerance in plants. Int. J. Mol. Sci. 22, 1–28. doi: 10.3390/ijms221910733
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237. doi: 10.1016/S0891-5849(98)00315-3
Shabala, S. and Mackay, A. (2011). “Ion transport in halophytes,” in Advances in Botanical Research (Academic Press), 151–199. doi: 10.1016/B978-0-12-387692-8.00005-9
Shabala, S., Wu, H., and Bose, J. (2015). Salt stress sensing and early signalling events in plant roots: Current knowledge and hypothesis. Plant Sci. 241, 109–119. doi: 10.1016/j.plantsci.2015.10.003
Shi, Q., Sun, H., Timm, S., Zhang, S., and Huang, W. (2022). Photorespiration alleviates photoinhibition of photosystem I under fluctuating light in tomato. Plants 11 (2). doi: 10.3390/plants11020195
Sims, D. A. and Gamon, J. A. (2002). Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 81, 337–354. doi: 10.1016/S0034-4257(02)00010-X
Singleton, V. L. and Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158. doi: 10.5344/ajev.1965.16.3.144
Sumanta, N., Haque, C. I., Nishika, J., and Suprakash, R. (2014). Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. Res. J. Chem. Sci. 4, 2231–2606. doi: 10.1055/s-0033-1340072
Sunil, B., Saini, D., Bapatla, R. B., Aswani, V., and Raghavendra, A. S. (2019). Photorespiration is complemented by cyclic electron flow and the alternative oxidase pathway to optimize photosynthesis and protect against abiotic stress. Photosynth. Res. 139, 67–79. doi: 10.1007/s11120-018-0577-x
Voss, I., Sunil, B., Scheibe, R., and Raghavendra, A. S. (2013). Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol. 15, 713–722. doi: 10.1111/j.1438-8677.2012.00710.x
Keywords: sea rocket, leaf gas exchanges, chlorophyll fluorescence, cations, chlorophylls, phenols, ascorbic acid, antioxidant capacity
Citation: Conversa G, Botticella L, Lazzizera C, Bonasia A, Duri LG and Elia A (2025) Salinity tolerance in the halophyte species Cakile maritima from the Apulia region, southern Italy. Front. Plant Sci. 16:1662491. doi: 10.3389/fpls.2025.1662491
Received: 09 July 2025; Accepted: 11 August 2025;
Published: 01 September 2025.
Edited by:
Raoudha Abdellaoui, Institut des Régions Arides, TunisiaReviewed by:
Mustapha Gorai, University of Gabes, TunisiaAleksandra Orzoł, University of Warmia and Mazury in Olsztyn, Poland
Copyright © 2025 Conversa, Botticella, Lazzizera, Bonasia, Duri and Elia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulia Conversa, Z2l1bGlhLmNvbnZlcnNhQHVuaWZnLml0
 Giulia Conversa
Giulia Conversa Lucia Botticella
Lucia Botticella Corrado Lazzizera
Corrado Lazzizera Anna Bonasia
Anna Bonasia Luigi Giuseppe Duri
Luigi Giuseppe Duri Antonio Elia
Antonio Elia