- 1School of Environmental and Chemical Engineering, Shanghai University, Shanghai, China
- 2Shanghai Key Laboratory of Bio-Energy Crops, Synthetic Biology Research Center, School of Life Sciences, Shanghai University, Shanghai, China
Diosgenin is a bioactive steroidal natural product extraced from plants and serves as an important precursor for the industrial production of steroidal hormone drugs. Despite its pharmacological significance, the biosynthetic and regulatory mechanisms underlying diosgenin production in the medicinal plant T. foenum-graecum remain poorly understood. In this study, we identified TfWRKY40, a WRKY transcription factor from T. foenum-graecum, whose expression strongly correlates with diosgenin accumulation. Using RNA interference and overexpression strategies combined with transcriptomic analysis and targeted metabolite quantification, we demonstrated that silencing of TfWRKY40 led to a 67.60% reduction in diosgenin content, which was accompanied by downregulation of key biosynthetic genes or transcript variants including ACAT1, HMGR1, PMK1, MVD, FPS, SQE2, CAS1, SMO3-1, SMO3-2, 8,7-SI, SMO4-3, CYP90B50, and CYP82J17 in the transgenic hairy roots. Conversely, overexpression of TfWRKY40 resulted in a 59.25% increase in diosgenin levels, along with upregulation of these biosynthetic genes or transcript variants. Taken together, these findings suggest that TfWRKY40 acts as a positive regulator of diosgenin biosynthesis in T. foenum-graecum, likely by activating the transcription of critical pathway genes, particularly CAS1, HMGR1, and CYP90B50. This work highlights TfWRKY40 as a promising target for metabolic engineering strategies aimed at enhancing diosgenin production and facilitating the development of diosgenin-derived steroidal therapeutics.
1 Introduction
Diosgenin, a steroidal sapogenin widely distributed in both monocotyledonous and dicotyledonous plants, including Dioscorea zingiberensis and Trigonella foenum-graecum, has emerged as a pharmacologically important plant-derived secondary metabolite with diverse therapeutic potential (Ge et al., 2015; Zhang et al., 2020; Katoch et al., 2024). This bioactive compound exhibits remarkable anti-cancer (He et al., 2014; Meng et al., 2019), anti-inflammatory (Wei et al., 2023), neuroprotective (Man et al., 2025), cardiovascular-protective (Katoch et al., 2024), and lipid-lowering (Xiang et al., 2024) properties, primarily through modulation of cell cycle progression and cellular signaling pathways. Owing to its structural versatility, many diosgenin-derived amino acid conjugates have been synthesized and investigated for their potential to treat pathological conditions such as cancer, diabetes, and neurodegenerative diseases (Huang et al., 2017; Cai et al., 2018; He et al., 2018; Mohseni-Moghaddam et al., 2023). Additionally, diosgenin serves as a critical precursor for the industrial synthesis of steroidal hormone drugs, including testosterone, progesterone, hydrocortisone, and estradiol, underscoring its significance in pharmaceutical applications (Huang et al., 2010; Zhang et al., 2016; Dong et al., 2018).
T. foenum-graecum is widely adopted as a model system for diosgenin studies, due to its short growth cycle and ease of cultivation (Ciura et al., 2017a, 2018). Previous studies have demonstrated that exogenous elicitors, including methyl jasmonate (MeJA), cholesterol, and squalene, significantly enhance diosgenin accumulation in T. foenum-graecum (Chaudhary et al., 2015; Ciura et al., 2017a, 2018). These efforts have led to two major breakthroughs: the identification of critical biosynthetic enzymes, especially members of the CYP72A family as essential components, in steroidal saponin biosynthesis (Chaudhary et al., 2015), and the characterization of diosgenin accumulation dynamics under various elicitor treatments (Ciura et al., 2017a, 2018). Diverse germplasm screening has also identified high-yielding lines, laying the foundation for future metabolic engineering and crop improvement initiatives (Dwivedi et al., 2017; Coban et al., 2025).
Diosgenin biosynthesis in plants originates from cholesterol (Joly et al., 1969a; 1969b; Stohs et al., 1969), which itself is synthesized from the C5 isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). Higher plants produce IPP and DMAPP primarily through the cytosolic mevalonate (MVA) pathway, which is derived from acetyl-CoA with the 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR) acting as a rate-limiting enzyme in this process. IPP is subsequently converted to 2,3-oxidosqualene. The biosynthetic cascade begins with the cyclization of 2,3-oxidosqualene to cycloartenol, catalyzed by cycloartenol synthase (CAS), the precursor to cholesterol formation. Multi-omics analyses in T. foenum-graecum leaves have confirmed that only CAS is expressed in leaves, the primary diosgenin-producing tissue, while lanosterol synthase (LAS) activity is absent, thereby validating cycloartenol as a key biosynthetic intermediate (Mohammadi et al., 2020). Stable isotope tracing has further substantiated cholesterol as the direct precursor of diosgenin and elucidated the complete nine-step enzymatic conversion from cycloartenol to cholesterol, including these enzymes such as CAS, sterol side chain reductase 2 (SSR2), C4-sterol methyl oxidase (SMO), cyclopropylsterol isomerase (CPI), CYP51, sterol C-14 reductase (C14-R), sterol 8,7 isomerase (8,7-SI), sterol C-5(6) desaturase 2 (C5-SD2) and 7-dehydrocholesterol reductase 2 (7-DR2) (Sonawane et al., 2017). Seminal studies by Sawai et al. (2014) identified SSR2 and C5-SD2 as critical genes in the cholesterol biosynthesis pathway in Solanaceae plants, catalyzing the conversion of cycloartenol to cycloartanol and cholesta-7-en-3β-ol to 7-dehydrocholesterol, respectively. In the process of converting cholesterol to diosgenin, cholesterol undergoes site-specific oxidations at the C-22, C-16, and C-26 positions (Christ et al., 2019; Zhou et al., 2022). Transcriptome analyses of methyl jasmonate-treated T. foenum-graecum seedlings identified cytochrome P450s (CYPs) and UDP-glycosyltransferases (UGTs) as key enzymes responsible for these modifications (Zhou et al., 2019). Two functional CYPs including TfCYP90B50 and TfCYP72A613/TfCYP82J17 have been characterized (Christ et al., 2019); however, due to the structural complexity of steroidal sapogenins, the complete biosynthetic and regulatory pathways remain unknown.
WRKY transcription factors, one of the ten major plant transcription factor families, are increasingly recognized as key regulators of secondary metabolite biosynthesis (Rushton et al., 2010; Schluttenhofer and Yuan, 2015). In Withania somnifera, for instance, WsWRKY1 regulates withanolide biosynthesis by modulating the expression of squalene synthase (SQS) and squalene epoxidase (SQE) genes (Singh et al., 2017). In Artemisia annua, AaWRKY1 promotes artemisinin production by directly regulating the transcription of the CYP71AV1 gene (Han et al., 2014). In Dioscorea composita, DcWRKY11 acts as a positive regulator in the biosynthesis of steroidal saponins (Yu et al., 2024). Similarly, PgWRKY4X from Panax ginseng binds to the W-box elements in the promoter of SQE gene, thereby enhancing ginsenoside biosynthesis (Yao et al., 2020). Other WRKY transcription factors, such as PqWRKY1 in P. quinquefolius (Sun et al., 2013), and CbWRKY24 in Conyza blinii (Sun et al., 2018), act as positive regulators in response to MeJA, upregulating key genes in the MVA pathway, thereby promoting the biosynthesis of triterpene ginsenoside and blinin, respectively. Additionally, the interactions between the WRKY transcription factors and miRNAs have been implicated in regulating triterpenoid saponin accumulation in Sapindus mukorossi (Xu et al., 2023). Collectively, these studies underscore the pivotal role of WRKY transcription factors in modulating complex secondary metabolic pathways, especially those associated with sterol-derived and saponin-type compounds.
In this study, we performed comparative transcriptome analysis of T. foenum-graecum seedlings treated with MeJA to identify transcriptional regulators involved in diosgenin biosynthesis. Through this approach, we isolated a WRKY transcription factor, designated TfWRKY40, from the MeJA-treated T. foenum-graecum seedlings. Phylogenetic tree analysis and Agrobacterium rhizogenes-mediated hairy root transformation were then used to functionally characterize TfWRKY40 and investigate its positive regulatory role in diosgenin biosynthesis. Moreover, comparative transcriptomic and metabolite analyses indicated that TfWRKY40 likely activates the expression of key biosynthetic genes or transcript variants, particularly HMGR1, CAS1, and CYP90B50, thereby regulating diosgenin biosynthesis. These findings not only identify TfWRKY40 as a positive regulator of diosgenin biosynthesis but also provide new insights into the regulatory mechanisms controlling cholesterol-derived secondary metabolism in plants. These results have potential applications in metabolic engineering strategies aimed at enhancing the biosynthesis of pharmacologically important steroidal compounds.
2 Materials and methods
2.1 Plant materials and growth conditions
T. foenum-graecum seeds used in this study were collected from Shandong Province, China, and Nicotiana benthamiana seeds were provided by the laboratory of Professor Xiangyang Hu at the Shanghai University of China. All plant materials were cultivated in a controlled climate chamber under the following conditions: temperature of 25°C ± 2°C, a photoperiod of 16 hours light/8 hours dark, and relative humidity of 60%-70%.
2.2 MeJA treatment of T. foenum-graecum seedlings
The T. foenum-graecum seeds with consistent size were carefully selected and initially soaked in 75% ethanol for 1 minute. Subsequently, the seeds were rinsed three times with sterile distilled water. Surface sterilization was then performed by immersing the seeds in a NaClO solution containing 5%~10% (w/v) available chlorine for 10 minutes, followed by three additional rinses with sterile distilled water to remove any residual disinfectant. The sterilized seeds were placed on 0.65% (w/v) water-agar medium and incubated in darkness at 25 ± 2°C for 42–44 hours.
MeJA was dissolved in absolute ethanol and then added to half-strength Murashige and Skoog (1/2 MS) liquid medium to obtain a final ethanol concentration of 0.01% (v/v). The control medium contained absolute ethanol at the same final concentration without MeJA. Both the MeJA-supplemented and control media were dispensed into sterile culture bottles (35 mL per bottle), which were then filled with sterile glass beads (approximately 0.5 cm in diameter) until the beads slightly protruded above the liquid surface.
Germinated seedlings were transferred into the MeJA-supplemented and control media, with their roots being gently secured by sterile glass beads (approximately 25 seedlings per bottle). The cultures were maintained under a 16-hour light/8-hour dark photoperiod at 25 ± 2°C. T. foenum-graecum seedlings were harvested at 6, 12, 24, 48, 72, and 120 hours following the MeJA treatment.
2.3 Extraction and quantitative analysis of diosgenin from T. foenum-graecum plants
T. foenum-graecum seedlings or hairy roots were ground to a fine powder under liquid nitrogen, and the resulting powder was dried to a constant weight at 37°C. Twenty milligrams of the dried powder were accurately weighed and extracted with 1 mL of methanol containing 20 μg of ursolic acid as an internal standard. Extraction was conducted using an ultrasonic bath and repeated three times. The pooled extracts were then evaporated to dryness under a gentle stream of nitrogen gas at ambient temperature. The residue was resuspended in distilled water, and concentrated sulfuric acid was added to achieve a final concentration of 1.8 M. Hydrolysis was performed by incubating the mixture in a 95°C water bath for 10–12 hours. After hydrolysis, liquid-liquid extraction was carried out by vigorous shaking twice with n-hexane, followed by a subsequent extraction with ethyl acetate. The organic layers were combined, evaporated to dryness under nitrogen, and the resulting residue was reconstituted in 100 μL methanol for subsequent HPLC analysis.
Diosgenin content was determined using a Shimadzu LC-20AT high-performance liquid chromatography (HPLC) system equipped with a Shim-pack GIST C18 column (250 mm × 4.6 mm, 5 μm particle size). The column temperature was maintained at 37°C. The mobile phase consisted of ultrapure water (aqueous phase) and HPLC-grade acetonitrile (organic phase) mixed at a ratio of 1:9 (v/v). Isocratic elution was performed at a flow rate of 0.8 mL/min with a total run time of 40 minutes. Detection was conducted using a UV detector set at 203 nm. The Shimadzu LabSolutions software (version 5.106 SP1) was used for the integration of targeted peaks and quantification of corresponding metabolites. Representative chromatograms of diosgenin and the internal standard ursolic acid in both chemical standards and sample extracts are provided in Supplementary Figure S1.
For the quantitative analysis, the following formula was used:
where is the correction factor; and represent the peak area and concentration of the internal standard (ursolic acid), respectively; and are the peak area and concentration of the reference standard (diosgenin); and refer to the peak area and concentration of diosgenin in the sample, respectively; is the peak area of the internal standard (ursolic acid) in the sample, and is the concentration of the internal standard added to the sample.
2.4 RNA extraction and quantitative real-time PCR
Total RNA (1 μg) was extracted using the EASYspin Plus Plant RNA Rapid Extraction Kit (Aidlab, Beijing, China), and the first-strand cDNA was synthesized using the TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (TransGen Biotech, China), following the manufacturers’ protocols. Quantitative real-time PCR (qRT-PCR) was performed using the Taq Pro Universal SYBR qPCR Master Mix (Vazyme Biotech, Nanjing, China) on a Bio-Rad CFX96™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s instructions.
The thermal cycling conditions were as follows: initial denaturation at 95°C for 10 minutes; followed by 40 cycles of denaturation at 95°C for 15 seconds, annealing at 58°C for 40 seconds, and extension at 72°C for 30 seconds. Following the amplification, quantification cycle (Cq) values were recorded and used for statistical analysis to determine the relative expression levels of the genes of interest. All qRT-PCR assays were performed with three biological replicates, each analyzed in three technical replicates. Relative gene expression levels were calculated using the 2−ΔΔCT method.
2.5 Phylogenetic tree construction and multiple sequence alignments
Sequence data were retrieved from the NCBI database (https://www.ncbi.nlm.nih.gov/). Phylogenetic trees were constructed employing the neighbor-joining method in MEGA software (version 7.0.26), with 2000 bootstrap replications for reliability. Sequence alignments were conducted using the DNAMAN software (version 9.0.1.116).
2.6 Subcellular localization
To analyze the subcellular localization of the TfWRKY40 protein, the full-length coding sequence excluding stop codon was amplified and cloned into the BamHI and SacI sites of the pHB-GFP (enhanced green fluorescent protein, EGFP) expression vector, generating the fusion construct pHB-TfWRKY40-GFP. The empty vector pHB-GFP was used as a negative control. The recombinant plasmids were co-transformed with the nuclear-localized marker pRI101-H2B-CFP (enhanced cyan fluorescent protein, ECFP) into A. tumefaciens strain GV3101. The resulting A. rhizogenes suspensions were infiltrated into the leaves of N. benthamiana.
Fluorescence signals were observed 48-72 hours post-infiltration using a ZEISS two-photon laser scanning confocal microscope. EGFP fluorescence was excited at 488 nm and ECFP at 458 nm. Subcellular localization of the TfWRKY40 protein was determined based on the co-localization patterns of EGFP and ECFP signals. The experiment was performed with three independent biological replicates, and at least 10 microscopic fields were examined for each sample.
2.7 Plant expression vector construction and A. tumefaciens-mediated transformation of T. foenum-graecum hairy roots
To construct the plant overexpression vector for TfWRKY40, the coding sequence of TfWRKY40 was amplified using the primers TfWRKY40-OE-F and TfWRKY40-OE-R. The amplified fragment was inserted into the BamHI and SacI sites of the plant expression vector pBI121. For gene silencing, the RNA interference (RNAi) vector pK7GWIWG2_II-RedRoot was used to suppress TfWRKY40 expression. A 273-bp fragment of the TfWRKY40 coding region was amplified by PCR using the primers TfWRKY40-RNAi-F and TfWRKY40-RNAi-R. The PCR product was subsequently introduced into the pDONR201 vector via BP recombination, followed by LR recombination into the pK7GWIWG2_II-RedRoot vector.
The resulting plant expression constructs, along with their corresponding empty vectors as negative controls, were transformed into A. rhizogenes strain Ar.Qual using a freeze-thaw method. Hairy roots were induced following the protocol described by (Garagounis et al., 2020), with minor modifications. In brief, T. foenum-graecum seeds were surface-sterilized by immersion in 70% ethanol for 1 min, followed by three washes with sterile distilled water. Seeds were then sterilized in a NaClO solution containing 5%~10% (w/v) available chlorine for 10 min, followed by three washes with sterile water. The sterilized seeds were germinated on 0.65% (w/v) agar plates in the dark at 24°C for 36 h. Germinated seedlings were then used for transformation. The radicles of the seedlings were excised, and the injured surfaces were immersed in the Agrobacterium slurry harboring the target constructs for 3–5 min. The inoculated seedlings were transferred onto large square petri dishes containing solid 1/2 MS medium. Plates were placed in a growth chamber at 22°C under a 16 h light/8 h dark photoperiod. Callus formation at the infected sites was observed approximately 2 weeks post-inoculation, and hairy root emergence occurred around 3 weeks post-inoculation. Approximately 40–50 days later, transgenic roots were confirmed by qRT-PCR and used for metabolite analysis. Each biological replicate consisted of pooled hairy roots from 30 infected plants. Data for each construct were collected from at least three independent biological replicates. All primers used in this study are listed in Supplementary Table S1.
2.8 RNA-seq library preparation and sequencing
Total RNA was extracted from the transformed T. foenum-graecum hairy roots, and submitted to Novogene Co., Ltd (Beijing, China) for high-throughput sequencing. RNA integrity and purity were first verified using Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA, USA) before library construction. Poly(A)+ mRNA was enriched from total RNA using oligo(dT)-coupled magnetic beads, followed by fragmentation under optimized conditions. Double-stranded cDNA was synthesized from the fragmented mRNA, subjected to end repair and A-tailing, and then ligated with Illumina sequencing adapters. The resulting libraries were size-selected (300–500 bp), PCR-amplified, and rigorously quality-checked before being sequenced on the Illumina HiSeq 2000 platform (San Diego, CA, USA). Raw sequencing reads were processed to generate high-quality clean reads by removing adapter sequences, the reads containing more than 10% ambiguous bases (N), and the low-quality reads in which more than 50% of the bases had Phred scores ≤ 20. Three biological replicates were used for RNA sequencing. For each biological replicate, total RNA was extracted from a pool of hairy roots derived from 30 independently infected plants.
2.9 Transcriptome analysis
Transcript assembly was performed using the Trinity software. To evaluate transcript abundance, clean reads were individually mapped to the assembled transcriptome, and transcript abundance for each unigene was normalized using the FPKM (fragments per kilobase per million mapped reads) method (Trapnell et al., 2010). The annotated unigenes were subsequently queried against the Gene Ontology (GO) database (http://www.geneontology.org/) and the Kyoto Encyclopedia of Genes and Genomes KEGG (http://www.genome.jp/kegg/). Functional annotation and enrichment analysis of differentially expressed genes (DEGs) were conducted using KEGG (Kanehisa and Goto, 2000). DEGs were identified using the DESeq2 R package, with a threshold of fold change (FC) ≥ 2 and False Discovery Rate (FDR)< 0.05 (Storey and Tibshirani, 2003; Anders and Huber, 2010; Love et al., 2014).
2.10 Statistical analysis
All experiments were performed with at least three biological replicates. Data were analyzed using GraphPad Prism 9 software (Version 9.4.1) and expressed as mean ± standard deviation (mean ± SD). Statistical significance was determined by a Student’s t-test, with ***p< 0.001, **p< 0.01, and *p< 0.05 being considered statistically significant.
3 Results
3.1 TfWRKY40 closely correlates to diosgenin biosynthesis in T. foenum-graecum
To assess the temporal effects of MeJA on diosgenin accumulation, T. foenum-graecum seedlings were treated with 0.01% (v/v) MeJA and harvested at 6, 12, 24, 48, 72, and 120 hours post-treatment for diosgenin extraction and quantification. Diosgenin content ranged from 0.22 to 0.64 mg/g dry weight (DW) across all time points, peaking at 12 hours post-treatment (0.64 mg/g DW) (Figure 1A). During the early phase (6–12 h), MeJA treatment significantly increased diosgenin levels, reaching 1.63- and 1.95-fold relative to their respective controls at 6 and 12 hours, respectively (Figure 1A). No significant induction was detected during the mid-phase (24–48 h), with a slight decrease observed in some samples. In the late phase (72–120 h), a modest upward trend was observed, although the changes were not statistically significant (Figure 1A). These results suggest that MeJA transiently but robustly activates diosgenin biosynthesis, with a maximal accumulation at 12 hours post-treatment.
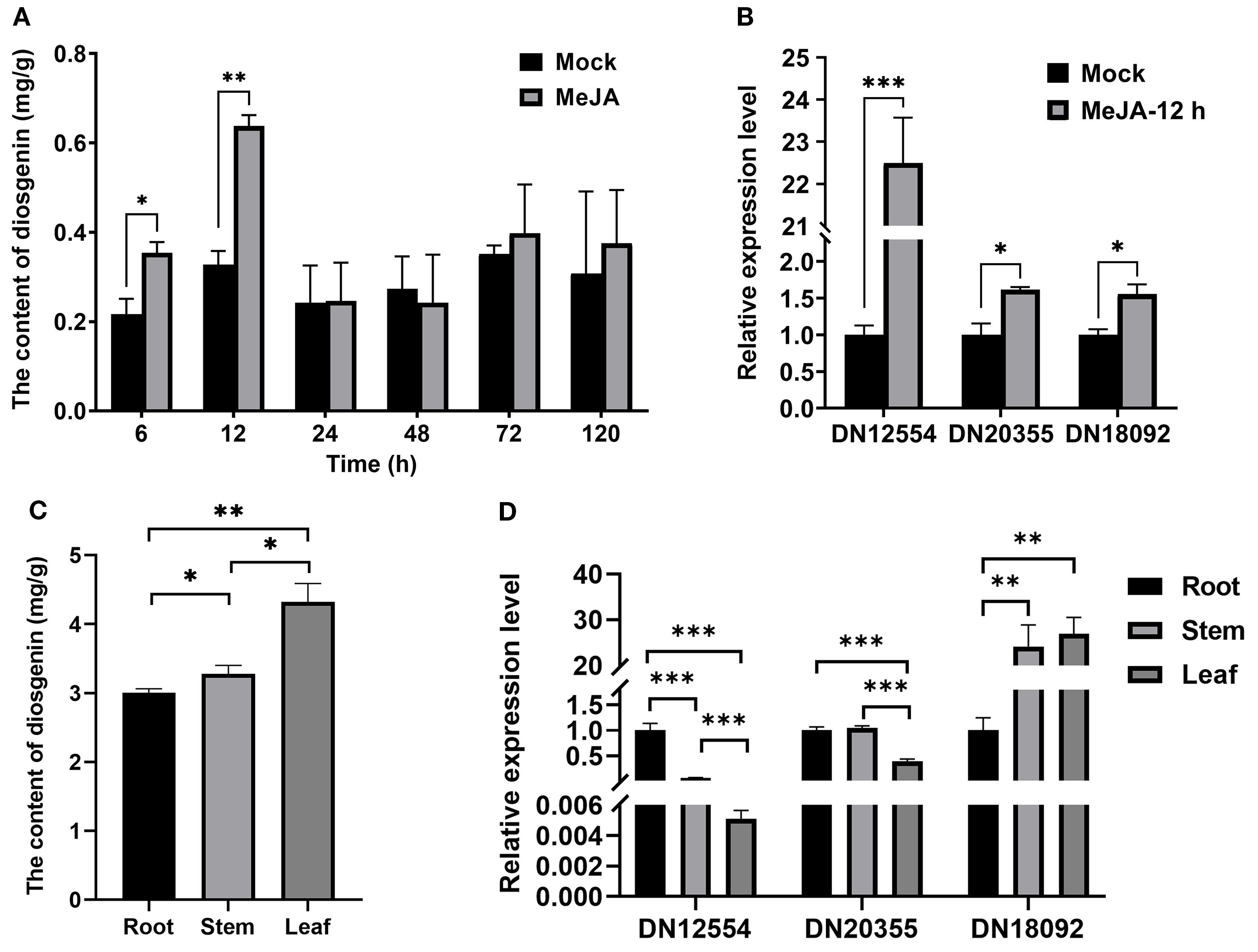
Figure 1. Correlation between DN18092 (TfWRKY40) expression and diosgenin biosynthesis in T. foenum-graecum. (A) Methyl jasmonate (MeJA) treatment significantly increased diosgenin accumulation at 6 h and 12 h compared to mock-treated controls. (B) Quantitative RT-PCR analysis showed that 12-h MeJA treatment markedly upregulated three transcription factor genes, identified from our previously published transcriptome data, which are implicated in diosgenin biosynthesis. (C) Tissue-specific distribution of diosgenin in T. foenum-graecum. Diosgenin content was quantified by HPLC in roots, stems, and leaves of 3-month-old plants. (D) Among the three transcription factors, only DN18092 expression levels showed a correlation with diosgenin accumulation in the above-mentioned tissues of T. foenum-graecum. Data represent mean ± SD (n=3 biological replicates). Statistical significance was determined by Student’s t-test (*p< 0.05, **p< 0.01, ***p< 0.001). Gene expression was normalized to TfActin and expressed as fold-change relative to controls.
To identify the transcriptional regulators involved in this response, we performed a combined analysis of our previously constructed MeJA-responsive transcriptome (Zhou et al., 2019) and the publicly available transcriptome data from different T. foenum-graecum tissues (Christ et al., 2019). From an initial set of 4,499 transcription factor-annotated unigenes, 16 transcription factors were considered as potential candidates, as their expression was consistent with diosgenin accumulation in a MeJA-induced or tissue-specific manner. Comparative transcriptomic analysis further narrowed these 16 candidates to three genes (DN18092_c0_g1, DN20355_c0_g1, and DN12554_c0_g1), hereafter referred to as DN18092, DN20355, and DN12554, respectively, based on putative functional annotation (Christ et al., 2019; Zhou et al., 2019). Importantly, DN18092 emerged as the most promising candidate, showing significant upregulation (log2 fold change > 2, p< 0.01) under MeJA treatment (Zhou et al., 2019).
qRT-PCR was performed to investigate the expression levels of DN18092, DN20355, and DN12554 in T. foenum-graecum seedlings treated with MeJA for 12 hours, corresponding to the peak of diosgenin accumulation. Results showed that the expression of all three genes was significantly upregulated compared to the mock controls (Figure 1B), which correlated with the increased diosgenin content in the same samples (Figure 1A). We then analyzed the tissue-specific expression of DN18092, DN20355, and DN12554 in T. foenum-graecum. Diosgenin was found to accumulate at significantly higher levels in above-ground organs (i.e., leaves and stems) than in roots (Figure 1C). Similarly, DN18092 transcript levels were higher in leaves and stems than in roots (Figure 1D), consistent with the tissue-specific distribution of diosgenin. In contrast, DN20355 and DN12554 showed relatively higher expression in roots, with the lowest expression detected in leaves. Taken together, DN18092 showed a positive correlation with diosgenin biosynthesis in both MeJA-induced and tissue-specific expression patterns, and was therefore selected for further investigation.
Phylogenetic analysis revealed that DN18092 shares high homology with the WRKY family members, clustering closest to MtWRKY40 from Medicago truncatula (Figure 2A). Protein sequence alignment confirmed the presence of the conserved WRKY domain and C2H2 zinc finger motif characteristic of this family (Figure 2B). Accordingly, DN18092 was named TfWRKY40. Subcellular localization analysis was performed by transient expression of TfWRKY40-GFP fusion protein in N. benthamiana leaves. Confocal microscopy revealed that TfWRKY40-GFP co-localized with the nuclear marker H2B-ECFP, while free GFP control exhibited diffuse fluorescence throughout the cell (Figure 3). These results confirm nuclear localization of TfWRKY40, consistent with its predicted function as a transcription factor.

Figure 2. Phylogenetic and sequence analysis of WRKY40 proteins. (A) A neighbor-joining phylogenetic tree of TfWRKY40 and its orthologs from Medicago truncatula, Lathyrus sativus, Pisum sativum, Gastrolobium bilobum, Mucuna pruriens, Phaseolus vulgaris, Abrus precatorius, Vigna angularis, Vigna umbellata and Glycine max was constructed using a MEGA software (version 7.0.26). The TfWRKY40 is highlighted in red. (B) Multiple sequence alignment of TfWRKY40 with its orthologs from the selected legume species was performed using a DNAMAN (version 9.0.1.116) software. The WRKY domain and the conserved WRKY sequence are indicated by a black line and a red box, respectively. The C2H2-type zinc finger motif is marked with a green triangle.

Figure 3. TfWRKY40 localizes to the nucleus in N. benthamiana leaf epidermal cells. The 35S::TfWRKY40-EGFP fusion construct and nuclear marker H2B-ECFP containing a chromatin-binding histone tag were co-transformed into leaf epidermal cells, with 35S::GFP as negative control. Fluorescence microscopy demonstrated the nuclear localization of TfWRKY40. Scale bar = 20 μm.
3.2 TfWRKY40 is a positive regulator in diosgenin biosynthesis
To investigate whether TfWRKY40 plays a regulatory role in diosgenin biosynthesis in T. foenum graecum, the overexpression and RNAi constructs targeting TfWRKY40, designated pBI121-TfWRKY40 (TfWRKY40-OE) and pK7GWIWG2D-Redroot-TfWRKY40 (TfWRKY40-RNAi), were prepared using the Gateway recombination method. These plant expression constructs, as well as their corresponding empty vectors, were introduced into T. foenum graecum hairy roots via Agrobacterium rhizogenes-mediated transformation. The transgenic hairy roots were then harvested for gene expression analysis and diosgenin content measurement.
qRT-PCR analysis revealed that the expression level of TfWRKY40 was upregulated by 6.15-fold in the TfWRKY40-OE transgenic hairy roots and downregulated by 71.24% in the TfWRKY40-RNAi lines, compared with their respective empty vector-transformed controls (Figure 4A). Overexpression of TfWRKY40 led to a diosgenin content of 0.063 mg/g in hairy roots, which was 59.25% higher than that of the control (Figure 4B). In contrast, silencing of TfWRKY40 resulted in a 67.60% reduction in diosgenin content in TfWRKY40-RNAi hairy roots compared to the control lines (Figure 4B). These results demonstrate that TfWRKY40 acts as a positive regulator of diosgenin biosynthesis in T. foenum-graecum. Together with prior evidence of its nuclear localization (Figure 3), these findings suggest that TfWRKY40 functions as a nuclear-localized transcriptional activator regulating diosgenin biosynthesis in T. foenum-graecum.

Figure 4. TfWRKY40 is a positive regulator for diosgenin biosynthesis in T. foenum-graecum. Analysis of TfWRKY40 expression (A) and diosgenin contents (B) in the transgenic T. foenum-graecum hairy roots. The hairy roots transformed with either pBI121-TfWRKY40 (TfWRKY40-OE) or pK7GWIWG2D-Redroot-TfWRKY40 (TfWRKY40-RNAi) were compared with their corresponding empty vector controls: pBI121 (OE-empty) and pK7GWIWG2D-Redroot (RNAi-empty). Data represent mean ± SD of three biological replicates. Statistical significance was determined by Student's t-test (*p < 0.05; ***p < 0.001).
3.3 TfWRKY40 transcriptionally regulates diosgenin biosynthesis-related gene expression
To further elucidate the molecular mechanism by which TfWRKY40 regulates diosgenin biosynthesis, RNA sequencing was performed on the TfWRKY40-OE and TfWRKY40-RNAi hairy roots, along with their corresponding empty vector controls. Differentially expressed genes were defined using a threshold of FC ≥ 2 and FDR< 0.05. A total of 662 DEGs were identified between the TfWRKY40-RNAi and its corresponding empty vector-transformed hairy roots, while 413 DEGs were found in the TfWRKY40-OE transgenic hairy roots compared to the empty vector controls. Among these, 41 DEGs were commonly responsive to both overexpression and silencing of TfWRKY40 in hairy roots (Supplementary Figure S2).
GO enrichment analysis revealed that the most significantly enriched molecular functions were “oxidoreductase activity” and “transition metal ion binding,” with each being represented by over 100 DEGs (Supplementary Figure S3). Both functions are directly involved in plant redox metabolism. Notably, in T. foenum-graecum, diosgenin is synthesized from cholesterol through multiple redox reactions (Christ et al., 2019; Zhou et al., 2022), suggesting TfWRKY40 may play an important role in the downstream pathway of converting cholesterol to diosgenin.
KEGG pathway analysis showed a predominant enrichment in phenylpropanoid biosynthesis and carbon fixation pathways in photosynthetic organisms, both of which are associated with secondary metabolite production and stress responses (Supplementary Figure S4). Diosgenin also functions as a defensive compound in response to environmental stresses, such as pathogen infections and attacks by pests or herbivores (Moses et al., 2014; Ebrahimibasabi et al., 2020; Cheng et al., 2021). These findings indicate that TfWRKY40 may enhance the ability of T. foenum-graecum to cope with environmental stresses through the regulation of diosgenin biosynthesis.
As previously established, the biosynthetic pathway from 2,3-oxidosqualene to cholesterol in plants has been well characterized, involving nine key enzymes, including CAS, SSR2, SMO, CPI, CYP51, C14-R, 8,7-SI, C5-SD2, and 7-DR2 (Figure 5). Although the complete biosynthetic pathway from cholesterol to diosgenin remains to be fully elucidated, several crucial P450-encoding genes (CYP90B50, CYP82J17, and CYP72A613) have been identified as crucial components in diosgenin biosynthesis (Christ et al., 2019; Zhou et al., 2022). Therefore, investigating the expression patterns of these key diosgenin biosynthetic genes through transcriptome analysis is pivotal for understanding the regulatory mechanism of TfWRKY40.

Figure 5. Comparative transcriptomic analysis of the genes involved in the diosgenin biosynthetic pathway. Heat maps display the expression profiles of diosgenin synthesis-related genes or transcript variants in OE-empty, TfWRKY40-OE, RNAi-empty, and TfWRKY40-RNAi T. foenum-graecum hairy roots. Gene expression levels (in FPKM) were log2-transformed after adding a pseudocount of 1 (log2(FPKM+1)) to stabilize variance and account for zero values. The data were then standardized as z-scores (mean-centered and scaled by standard deviation per gene) and visualized using a color gradient (pale green: low expression; red: high expression). Statistically significant differences compared to the corresponding control (OE-empty or RNAi-empty) are indicated by asterisks on the heatmap blocks: P< 0.05 (*) and P< 0.01 (**). Heat maps were generated using GraphPad Prism (version 9.4.1). Source data are provided in Supplementary Table S2, and the sequences for all the analyzed genes or transcripts are available in Supplementary Table S3. ACAT, Acetyl-CoA acetyltransferase; HMGS, hydroxymethylglutaryl-CoA synthase; HMGR, 3-hydroxy-3-methylglutaryl-coenzyme A (CoA); MVK, mevalonate kinase; PMK, phosphomevalonate kinase; MVD, mevalonate dipyrophosphate decarboxylase; FPS, farnesyl pyrophosphate synthase; SQS, squalene synthase; SQE, squalene epoxidase; CAS, cycloartenol synthase; SSR2, sterol side chain reductase 2; SMO, C4-sterol methyl oxidase; CPI, cyclopropylsterol isomerase; C14-R, sterol C-14 reductase; 8,7-SI, sterol 8,7 isomerase; C5-SD2, sterol C-5(6) desaturase 2; 7-DR2, 7-dehydrocholesterol reductase 2; HMG-CoA, 3-Hydroxy-3-methylglutaryl-CoA; MVA, mevalonate; IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; FPP, farnesyl pyrophosphate.
Gene expression was quantified using normalized FPKM values to assess the changes in the expression of diosgenin biosynthetic genes in both TfWRKY40-OE and -RNAi transgenic hairy roots, compared to their respective controls (Figure 5; Supplementary Table S2). Notably, thirteen transcripts exhibited coordinated transcript level changes in response to both up- and down-regulation of TfWRKY40 (Figure 5), suggesting they are regulated by this transcription factor. These include two transcripts from putative transcript variants or duplicated genes (ACAT1 and HMGR1) in the upstream MVA pathway, nine transcripts (PMK1, MVD, FPS, SQE2, CAS1, 8,7-SI, and SMO3-1, SMO3-2 and SMO4-3) involved in cholesterol biosynthesis, and two cytochrome P450 genes (CYP90B50 and CYP82J17) associated with cholesterol-to-diosgenin conversion. Among them, HMGR functions as the key rate-limiting enzyme in the MVA pathway (Venkateshwaran et al., 2015; İlgar et al., 2021; Onoda et al., 2023), and CYP90B50 is considered the first committed enzyme in the pathway from cholesterol to diosgenin (Christ et al., 2019; Zhou et al., 2022). In addition, CAS catalyzes the first committed step from 2,3-oxidosqualene to cholesterol in plants (Bach, 2017). Previous studies have shown that the treatments with MeJA or ethylene elevate CAS expression, which correlates with increased diosgenin biosynthesis (Diarra et al., 2013; Javan et al., 2024). Thus, these findings suggest that TfWRKY40 may regulate diosgenin biosynthesis primarily by transcriptionally modulating expression of HMGR1, CAS1 or CYP90B50.
4 Discussion
MeJA is a well-established signaling molecule commonly used to stimulate the production of plant secondary metabolites. Previous studies have demonstrated that MeJA treatment enhances diosgenin biosynthesis in T. foenum-graecum (Debjani and Bratati, 2011; Chaudhary et al., 2015; Ciura et al., 2017a, 2017b; Irankhah et al., 2020; Gonda et al., 2023). In our study, application of 0.015% (v/v) MeJA to T. foenum-graecum seedlings significantly increased diosgenin accumulation, in agreement with these prior findings (Zhou et al., 2019). During the early phase of induction (6–12 h), diosgenin levels rose markedly (Figure 1A), indicating rapid transcriptional activation of biosynthetic genes and efficient channeling of metabolic flux, hallmarks of a typical saponin-type defense response in plants. However, at 24–48 h post-treatment, no significant difference was observed between the MeJA-treated and control groups (Figure 1A), possibly reflecting a temporary metabolic adaptation or the resolution of the acute MeJA-induced response. Interestingly, by 72 h, diosgenin levels in treated seedlings again showed a slight yet consistent upward trend compared to controls, although the magnitude of this increase was notably lower than during the initial induction phase (Figure 1A). These temporal fluctuations are consistent with the findings previously described by Zhou et al. (2019), who also reported non-linear patterns of MeJA-induced diosgenin accumulation in T. foenum-graecum. Although the timing and extent of these fluctuations observed in this study differed from those reported by Zhou et al. (2019), likely due to variation in cultivars and growth conditions, the overall trend of transient enhancement followed by stabilization appears to be a common regulatory feature. This dynamic response may reflect a broader metabolic reprogramming during the treatment: early resource allocation favors rapid diosgenin production as a frontline stress response, while a longer-term adaptation to MeJA stress could involve a shift toward alternative defense mechanisms, leading to a reduced investment in diosgenin biosynthesis. Thus, although the MeJA-treated seedlings maintained higher diosgenin levels than the controls at 72 h, the increment in diosgenin content was attenuated compared to the increase observed at 6–12 h, suggesting a shift in physiological priorities as the induced defense response progressed.
In T. foenum-graecum, diosgenin primarily exists as a glycosylated form (e.g., dioscin), a steroidal saponin that, like most triterpenoid glycosides, accumulates predominantly in the leaves, with significantly lower concentrations detected in stems and roots (Figure 1C). This tissue-specific distribution pattern closely mirrors the organ-specific expression profile of TfWRKY40, suggesting a potential transcriptional regulatory relationship between TfWRKY40 and diosgenin biosynthesis (Brenac and Sauvaire, 1996; Ortuño et al., 1998; Oncina et al., 2000). In contrast, the expression patterns of two other candidate genes, DN12554 and DN20355, showed poor correlation with diosgenin accumulation (Figure 1D), further supporting TfWRKY40 (DN18092) as the most likely transcriptional regulator involved in the regulation of diosgenin biosynthesis in T. foenum-graecum. Nevertheless, it remains unclear whether diosgenin is synthesized exclusively in the leaves or also in other tissues and subsequently transported. If inter-tissue transport does occur, DN12554 and DN20355 may also play roles in regulating diosgenin biosynthesis elsewhere and thus warrant further investigation. Future studies involving isotope labeling or tissue-specific metabolic flux analysis will be essential to determine the spatial origin of diosgenin biosynthesis.
In planta experiments conducted in T. foenum-graecum hairy roots demonstrated that overexpression of TfWRKY40 significantly increased diosgenin accumulation, whereas its silencing via RNAi led to a marked reduction in diosgenin levels (Figure 4). This positive correlation between TfWRKY40 expression and diosgenin content strongly supports its role as a transcriptional activator in diosgenin biosynthesis. Transcriptomic analysis further revealed that overexpression and RNAi-mediated silencing of TfWRKY40 resulted in significant upregulation and downregulation of key enzymatic genes involved in diosgenin biosynthesis, notably HMGR (a rate-limiting enzyme in the MVA pathway), CAS (a critical enzyme in cholesterol biosynthesis) and CYP90B50 (a key rate-limiting enzyme in the biosynthetic pathway from cholesterol to diosgenin) (Figure 5), indicating that TfWRKY40 regulates diosgenin accumulation by transcriptionally activating cholesterol biosynthesis and conversion of cholesterol to diosgenin. This discovery suggests that TfWRKY40 modulates diosgenin biosynthesis by exerting transcriptional control through the pathways both upstream and downstream of cholesterol, likely by regulating cholesterol precursor supply and possibly by also influencing the downstream conversion of cholesterol to diosgenin. Given that intracellular cholesterol availability directly determines the metabolic flux toward diosgenin (Joly et al., 1969a; 1969b; Stohs et al., 1969; Christ et al., 2019; Zhou et al., 2022), TfWRKY40 appears to play a central role in coordinating this pathway, linking the upstream metabolic regulation with the downstream secondary metabolite production.
Previous studies have demonstrated that MeJA-induced accumulation of diosgenin in T. foenum-graecum is associated with the upregulation of HMGR and sterol-3-glucosyltransferase (STRL) (Chaudhary et al., 2015). More recently, transcriptomic analyses have shown that MeJA treatment also enhances the expression of other key enzymes in the diosgenin biosynthetic pathway, including SQS, SQE, and CAS (Javan et al., 2024). The central role of CAS in this pathway has been further corroborated by Ciura et al. (2017a); Ciura et al. (2018), who identified it as a crucial regulatory node. Supporting this, Diarra et al. (2013) reported that ethylene treatment specifically elevated CAS expression without affecting the expression of SQS or FPPS, which was accompanied by an increase of diosgenin, highlighting the unique and pivotal function of CAS in directing metabolic flux toward diosgenin biosynthesis.
The coordinated upregulation of TfWRKY40 expression during MeJA-induced diosgenin accumulation in T. foenum-graecum, together with the marked downregulation or upregulation of CAS1, HMGR1 or CYP90B50 in TfWRKY40-RNAi or TfWRKY40-OE transgenic hairy roots, strongly suggests that TfWRKY40 regulates diosgenin biosynthesis likely through direct transcriptional control of CAS1, HMGR1 and/or CYP90B50. Notably, only one of the three HMGR transcripts, HMGR1, showed a significant correlation with TfWRKY40 expression, whereas HMGR2 and HMGR3 did not display similar expression patterns. A comparable expression pattern was observed for SMO4, where only SMO4-3 exhibited consistent expression changes in response to TfWRKY40 manipulation, indicating potential transcript-level functional divergence among gene family members. Therefore, the observed correlations between TfWRKY40 and HMGR1/SMO4-3 warrant further isoform-specific functional validation to confirm these regulatory relationships. We hypothesize that TfWRKY40 binds to the promoter region of the CAS1, HMGR1 or CYP90B50 gene, thereby modulating its transcription and enhancing metabolic flux through the post-2,3-oxidosqualene steps of diosgenin biosynthetic pathway. To substantiate this proposed regulatory mechanism, it would be of particular interest to confirm these interactions experimentally using chromatin immunoprecipitation (ChIP) and electrophoretic mobility shift assay (EMSA) when the nuclear genome sequence of T. foenum-graecum becomes available.
5 Conclusion
In this study, we demonstrated that MeJA treatment significantly enhanced diosgenin production in Trigonella foenum-graecum seedlings, with a pronounced increase observed at 12 hours post-induction, thereby confirming the positive regulatory role of MeJA in diosgenin biosynthesis. Through a comparative transcriptome analysis, we identified TfWRKY40, a WRKY family transcription factor that showed expression pattern correlated to diosgenin accumulation. Utilizing the T. foenum-graecum hairy root transformation, we functionally validated TfWRKY40 as a key positive regulator of diosgenin biosynthesis in vivo. Transcriptomic analysis of the TfWRKY40-RNAi and TfWRKY40-OE transgenic hairy roots, along with their respective empty vector-transformed controls, revealed that TfWRKY40 likely promotes diosgenin biosynthesis by transcriptionally regulating expression of the key biosynthetic genes or transcript variants, particularly CAS1, HMGR1, and CYP90B50. These genes or transcript variants are essential for diosgenin biosynthesis from the upstream to downstream steps of cholesterol metabolism, with cholesterol serving as a critical precursor in the pathway.
Data availability statement
The transcriptome data have been deposited in the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA1321096. The nucleotide sequence of TfWRKY40 has been submitted to GenBank with accession number PX310700.
Author contributions
CX: Investigation, Formal analysis, Data curation, Visualization, Writing – original draft, Writing – review & editing. YX: Investigation, Formal analysis, Data curation, Visualization, Writing – review & editing. NT: Investigation, Writing – review & editing. CL: Investigation, Resources, Writing – review & editing. YZ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the grants from the National Natural Science Foundation of China (32270416 and 31670300), and the finding from the National Key Research and Development Program of China (2018YFC1706200) to YZ.
Acknowledgments
We thank Dr. Liangliang Yu from Shanghai University for generously providing the pHB-GFP vector, which was instrumental in our subcellular localization studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1666107/full#supplementary-material
References
Anders, S. and Huber, W. (2010). Differential expression analysis for sequence count data. Genome Biol. 11 (10), R106. doi: 10.1186/gb-2010-11-10-r106
Bach, T. J. (2017). Secondary metabolism: High cholesterol in tomato. Nat. Plants 3, 16213. doi: 10.1038/nplants.2016.213
Brenac, P. and Sauvaire, Y. (1996). Accumulation of sterols and steroidal sapogenins in developing fenugreek pods: Possible biosynthesis in situ. Phytochemistry 41, 415–422. doi: 10.1016/0031-9422(95)00658-3
Cai, B., Seong, K. J., Bae, S. W., Chun, C., Kim, W. J., and Jung, J. Y. (2018). A synthetic diosgenin primary amine derivative attenuates LPS-stimulated inflammation via inhibition of NF-κB and JNK MAPK signaling in microglial BV2 cells. Int. Immunopharmacol. 61, 204–214. doi: 10.1016/j.intimp.2018.05.021
Chaudhary, S., Chikara, S. K., Sharma, M. C., Chaudhary, A., Syed, B. A., Chaudhary, P. S., et al. (2015). Elicitation of diosgenin production in trigonella foenum-graecum (Fenugreek) seedlings by methyl jasmonate. Int. J. Mol. Sci. 16, 29889–29899. doi: 10.3390/ijms161226208
Cheng, J., Chen, J., Liu, X. N., Li, X. C., Zhang, W. X., Dai, Z. B., et al. (2021). The origin and evolution of the diosgenin biosynthetic pathway in yam. Plant Commun. 2, 100079. doi: 10.1016/j.xplc.2020.100079
Christ, B., Xu, C., Xu, M., Li, F.-S., Wada, N., Mitchell, A. J., et al. (2019). Repeated evolution of cytochrome P450-mediated spiroketal steroid biosynthesis in plants. Nat. Commun. 10, 3206. doi: 10.1038/s41467-019-11286-7
Ciura, J., Bocian, A., Kononiuk, A., Szeliga, M., Jaromin, M., and Tyrka, M. (2017a). Proteomic signature of fenugreek treated by methyl jasmonate and cholesterol. Acta Physiologiae Plantarum 39, 1–14. doi: 10.1007/s11738-017-2416-7
Ciura, J., Szeliga, M., Grzesik, M., and Tyrka, M. (2017b). Next-generation sequencing of representational difference analysis products for identification of genes involved in diosgenin biosynthesis in fenugreek (Trigonella foenum-graecum). Planta 245, 977–991. doi: 10.1007/s00425-017-2657-0
Ciura, J., Szeliga, M., Grzesik, M., and Tyrka, M. (2018). Changes in fenugreek transcriptome induced by methyl jasmonate and steroid precursors revealed by RNA-Seq. Genomics 110, 267–276. doi: 10.1016/j.ygeno.2017.10.006
Coban, F., Ozer, H., Yilmaz, B., and Lan, Y. Z. (2025). Characterization of bioactive compounds in fenugreek genotypes in varying environments: diosgenin, trigonelline, and 4-hydroxyisoleucine. Front. Plant Sci. 16. doi: 10.3389/fpls.2025.1562931
Debjani, D. and Bratati, D. (2011). Elicitation of diosgenin production in Trigonella foenum-graecum L. seedlings by heavy metals and signaling molecules. Acta Physiologiae Plantarum 33, 1585–1590. doi: 10.1007/s11738-010-0691-7
Diarra, S. T., He, J., Wang, J. B., and Li, J. R. (2013). Ethylene treatment improves diosgenin accumulation in in vitro cultures of Dioscorea zingiberensis via up-regulation of CAS and HMGR gene expression. Electronic J. Biotechnol. 16, 6–6. doi: 10.2225/vol16-issue5-fulltext-9
Dong, M., Meng, Z., Kuerban, K., Qi, F., Liu, J., Wei, Y., et al. (2018). Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota. Cell Death Dis. 9, 1039. doi: 10.1038/s41419-018-1099-3
Dwivedi, H., Singh, D., and Agrawal, S. (2017). Screening of fenugreek (Trigonella foenum-graecum L.) germplasm lines for diosgenin potential. Asian Res. J. Agric. 4, 1–7. doi: 10.9734/ARJA/2017/32527
Ebrahimibasabi, E., Ebrahimi, A., Momeni, M., and Amerian, M. R. (2020). Elevated expression of diosgenin-related genes and stimulation of the defense system in Trigonella foenum-graecum (Fenugreek) by cold plasma treatment. Scientia Hortic. 271, 109494. doi: 10.1016/j.scienta.2020.109494
Garagounis, C., Beritza, K., Georgopoulou, M.-E., Sonawane, P., Haralampidis, K., Goossens, A., et al. (2020). A hairy-root transformation protocol for Trigonella foenum-graecum L. as a tool for metabolic engineering and specialised metabolite pathway elucidation. Plant Physiol. Biochem. 154, 451–462. doi: 10.1016/j.plaphy.2020.06.011
Ge, L., Guo, L., Yang, K., Tao, K., Su, J., and Long, Y. (2015). Solubility of diosgenin in several imidazolium-based ionic liquids. J. Chem. Eng. Data 60, 11–15. doi: 10.1021/je5004324
Gonda, S., Szucs, Z., Plaszkó, T., Cziáky, Z., Kiss-Szikszai, A., Sinka, D., et al. (2023). Quality-controlled LC-ESI-MS food metabolomics of fenugreek (Trigonella foenum-graecum) sprouts: Insights into changes in primary and specialized metabolites. Food Res. Int. 164, 112347. doi: 10.1016/j.foodres.2022.112347
Han, J., Wang, H., Lundgren, A., and Brodelius, P. E. (2014). Effects of overexpression of AaWRKY1 on artemisinin biosynthesis in transgenic Artemisia annua plants. Phytochemistry 102, 89–96. doi: 10.1016/j.phytochem.2014.02.011
He, L., Jiang, Y., Liu, K., Gomez-Murcia, V., Ma, X., Torrecillas, A., et al. (2018). Insights into the impact of a membrane-anchoring moiety on the biological activities of bivalent compounds as potential neuroprotectants for alzheimer's disease. J. Medicinal Chem. 61, 777–790. doi: 10.1021/acs.jmedchem.7b01284
He, Z. M., Chen, H. Y., Li, G. F., Zhu, H. Y., Gao, Y. G., Zhang, L. X., et al. (2014). Diosgenin inhibits the migration of human breast cancer MDA-MB-231 cells by suppressing Vav2 activity. Phytomedicine 21, 871–876. doi: 10.1016/j.phymed.2014.02.002
Huang, C.-H., Liu, D.-Z., and Jan, T.-R. (2010). Diosgenin, a plant-derived sapogenin, enhances regulatory T-cell immunity in the intestine of mice with food allergy. J. Natural Products 73, 1033–1037. doi: 10.1021/np900690z
Huang, B.-Z., Xin, G., Ma, L.-M., Wei, Z.-L., Shen, Y., Zhang, R., et al. (2017). Synthesis, characterization, and biological studies of diosgenyl analogs. J. Asian Natural Products Res. 19, 272–298. doi: 10.1080/10286020.2016.1202240
İlgar, B. A., Özden, S., Çepni Yüzbaşıoğlu, F. E., Turgut Kara, N. J. P. C., and Tissue, and Culture, O. (2021). Diosgenin production in Trigonella foenum-graecum (Fenugreek) cell cultures in response to yeast extract elicitation. Plant Cell, Tissue Organ Culture. 146, 21–27. doi: 10.1007/s11240-021-02039-w
Irankhah, S., Chitarra, W., Nerva, L., Antoniou, C., Lumini, E., Volpe, V., et al. (2020). Impact of an arbuscular mycorrhizal fungal inoculum and exogenous MeJA on fenugreek secondary metabolite production under water deficit. Environ. Exp. Bot. 176, 104096. doi: 10.1016/j.envexpbot.2020.104096
Javan, S. L., Kashkooli, A. B., Shojaeiyan, A., and Majidian, S. (2024). Transcriptomic data reveals the dynamics of terpenoids biosynthetic pathway of fenugreek. BMC Genomics 25, 390. doi: 10.1186/s12864-024-10253-x
Joly, R. A., Bonner, J., Bennett, R. D., and Heftmann, E. (1969a). The biosynthesis of steroidal sapogenins in Dioscorea floribunda from doubly labelled cholesterol. Phytochemistry 8, 1709–1711. doi: 10.1016/S0031-9422(00)85958-0
Joly, R. A., Bonner, J., Bennett, R. D., and Heftmann, E. (1969b). Conversion of cholesterol to an open-chain saponin by Dioscorea floribunda. Phytochemistry 8, 857–859. doi: 10.1016/S0031-9422(00)85873-2
Katoch, R., Tripathi, A., Thakur, N., and Kiran (2024). “Nutraceuticals in legumes,” in Herbal nutraceuticals: products and processes. Eds. Upadhyay, S. K. and Singh, S. P. (Hoboken, NJ, USA: WILEY), 229–250.
Kanehisa, M. and Goto, S. (2000). KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28 (1), 27–30. doi: 10.1093/nar/28.1.27
Love, M. I., Huber, W., and Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 (12), 550. doi: 10.1186/s13059-014-0550-8
Man, S. L., Zhou, Y. X., Zhang, X. H., Tang, X. Q., Xie, L., Ma, L., et al. (2025). Diosgenin alleviates neuropathic pain based on the enhancement of autophagy and promotion of M2 polarization. Int. Immunopharmacol. 159, 114936. doi: 10.1016/j.intimp.2025.114936
Meng, X., Dong, H. H., Pan, Y. W., Ma, L., Liu, C. X., Man, S. L., et al. (2019). Diosgenyl saponin inducing endoplasmic reticulum stress and mitochondria-mediated apoptotic pathways in liver cancer cells. J. Agric. Food Chem. 67, 11428–11435. doi: 10.1021/acs.jafc.9b05131
Mohammadi, M., Mashayekh, T., Rashidi-Monfared, S., Ebrahimi, A., and Abedini, D. (2020). New insights into diosgenin biosynthesis pathway and its regulation in Trigonella foenum-graecum L. Phytochemical Anal. 31, 229–241. doi: 10.1002/pca.2887
Mohseni-Moghaddam, P., Khanmohammadi, M., and Roghani, M. (2023). Literature review on hepatoprotective effects of diosgenin: possible mechanisms of action. Front. Pharmacol. 14. doi: 10.3389/fphar.2023.1226548
Moses, T., Papadopoulou, K. K., and Osbourn, A. (2014). Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 49, 439–462. doi: 10.3109/10409238.2014.953628
Oncina, R., Botía, J. M., Del Río, J. A., and Ortuño, A. (2000). Bioproduction of diosgenin in callus cultures of Trigonella foenum-graecum L. Food Chem. 70, 489–492. doi: 10.1016/S0308-8146(00)00121-7
Onoda, K., Kato, M., Tsunematsu, Y., Eto, F., Sato, M., Yoshioka, Y., et al. (2023). Biosynthetic gene expression and tissue distribution of diosgenin in dioscorea japonica. J. Agric. Food Chem. 71, 4292–4297. doi: 10.1021/acs.jafc.2c08478
Ortuño, A., Oncina, R., Botía, J. M., and Del Río, J. A. (1998). Distribution and changes of diosgenin during development of Trigonella foenum-graecum plants. Modulation by benzylaminopurine. Food Chem. 63, 51–54. doi: 10.1016/S0308-8146(97)00233-1
Rushton, P. J., Somssich, I. E., Ringler, P., and Shen, Q. X.J. (2010). WRKY transcription factors. Trends Plant Sci. 15 (5), 247–258. doi: 10.1016/j.tplants.2010.02.006
Sawai, S., Ohyama, K., Yasumoto, S., Seki, H., Sakuma, T., Yamamoto, T., et al. (2014). Sterol side chain reductase 2 is a key enzyme in the biosynthesis of cholesterol, the common precursor of toxic steroidal glycoalkaloids in potato. Plant Cell 26, 3763–3774. doi: 10.1105/tpc.114.130096
Schluttenhofer, C. and Yuan, L. (2015). Regulation of Specialized Metabolism by WRKY Transcription Factors. Plant Physiol. 167 (2), 295–306. doi: 10.1104/pp.114.251769
Singh, A. K., Kumar, S. R., Dwivedi, V., Rai, A., Pal, S., Shasany, A. K., et al. (2017). A WRKY transcription factor from Withania somnifera regulates triterpenoid withanolide accumulation and biotic stress tolerance through modulation of phytosterol and defense pathways. New Phytol. 215, 1115–1131. doi: 10.1111/nph.14663
Sonawane, P. D., Pollier, J., Panda, S., Szymanski, J., Massalha, H., Yona, M., et al. (2017). Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nat. Plants 3, 16205. doi: 10.1038/nplants.2016.205
Stohs, S., Kaul, B., and Staba, E. (1969). The metabolism of 14C-cholesterol by Dioscorea deltoidea suspension cultures. Phytochemistry 8, 1679–1686. doi: 10.1016/S0031-9422(00)85954-3
Storey, J. D. and Tibshirani, R. (2003). Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 100 (16), 9440–9445. doi: 10.1073/pnas.1530509100
Sun, Y. Z., Niu, Y. Y., Xu, J., Li, Y., Luo, H. M., Zhu, Y. J., et al. (2013). Discovery of WRKY transcription factors through transcriptome analysis and characterization of a novel methyl jasmonate-inducible PqWRKY1 gene from Panax quinquefolius. Plant Cell Tissue Organ Culture 114, 269–277. doi: 10.1007/s11240-013-0323-1
Sun, W. J., Zhan, J. Y., Zheng, T. R., Sun, R., Wang, T., Tang, Z. Z., et al. (2018). The jasmonate-responsive transcription factor CbWRKY24 regulates terpenoid biosynthetic genes to promote saponin biosynthesis in Conyza blinii H. Lév. J. Genet. 97, 1379–1388. doi: 10.1007/s12041-018-1026-5
Trapnell, C., Williams, B. A., Pertea, G., Mortazavi, A., Kwan, G., Van Baren, M. J., et al. (2010). Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature Biotechnol. 28 (5), 511–515. doi: 10.1038/nbt.1621
Venkateshwaran, M., Jayaraman, D., Chabaud, M., Genre, A., Balloon, A. J., Maeda, J., et al. (2015). A role for the mevalonate pathway in early plant symbiotic signaling. Proc. Natl. Acad. Sci. United States America 112, 9781–9786. doi: 10.1073/pnas.1413762112
Wei, F., Nian, Q., Zhao, M. Y., Wen, Y. Q., Yang, Y., Wang, J. D., et al. (2023). Natural products and mitochondrial allies in colorectal cancer therapy. Biomedicine Pharmacotherapy 167, 115473. doi: 10.1016/j.biopha.2023.115473
Xiang, X., Xin, X., Hou, Y., Deng, Y., Liu, X., and Yu, W. (2024). Diosgenin alters LPS-induced macrophage polarization by activating PPARγ/NF-κB signaling pathway. Int. Immunopharmacol. 126, 111270. doi: 10.1016/j.intimp.2023.111270
Xu, Y., Liu, J., Ji, X., Zhao, G., Zhao, T., Wang, X., et al. (2023). Integrative analysis of microRNAs and mRNAs reveals the regulatory networks of triterpenoid saponin metabolism in Soapberry (Sapindus mukorossi Gaertn.). Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1037784
Yao, L., Wang, J., Sun, J. C., He, J. P., Paek, K. Y., Park, S. Y., et al. (2020). A WRKY transcription factor, PgWRKY4X, positively regulates ginsenoside biosynthesis by activating squalene epoxidase transcription in Panax ginseng. Ind. Crops Products 154, 112671. doi: 10.1016/j.indcrop.2020.112671
Yu, S. J., Zhang, J. N., Cao, Y. X., Zhong, C. M., and Xie, J. (2024). Comparative transcriptomic and metabolomic analyses reveal key regulatory gene for methyl jasmonate-induced steroidal saponins synthesis in Dioscorea composita. Int. J. Biol. Macromolecules 280, 135788. doi: 10.1016/j.ijbiomac.2024.135788
Zhang, X. X., Wang, X. B., Khurm, M., Zhan, G. Q., Zhang, H., Ito, Y., et al. (2020). Alterations of Brain Quantitative Proteomics Profiling Revealed the Molecular Mechanisms of Diosgenin against Cerebral lschemia Reperfusion Effects. J. Proteome Res. 19, 1154–1168. doi: 10.1021/acs.jproteome.9b00667
Zhang, R., Wen, L., Shen, Y., Shi, N., Xing, Z., Xia, Q., et al. (2016). One compound of saponins from Disocorea zingiberensis protected against experimental acute pancreatitis by preventing mitochondria-mediated necrosis. Sci. Rep. 6, 35965. doi: 10.1038/srep35965
Zhou, C., Li, X., Zhou, Z., Li, C., and Zhang, Y. (2019). Comparative transcriptome analysis identifies genes involved in diosgenin biosynthesis in trigonella foenum-graecum L. Molecules 24, 140. doi: 10.3390/molecules24010140
Keywords: TfWRKY40, transcription factor, positive regulator, diosgenin biosynthesis, Trigonella foenum-graecum
Citation: Xu C, Tang N, Xu Y, Li C and Zhang Y (2025) TfWRKY40 positively regulates diosgenin biosynthesis in Trigonella foenum-graecum L.. Front. Plant Sci. 16:1666107. doi: 10.3389/fpls.2025.1666107
Received: 15 July 2025; Accepted: 29 August 2025;
Published: 24 September 2025.
Edited by:
Ke Wang, Anhui Agricultural University, ChinaReviewed by:
Zhen Q. Wang, University at Buffalo, United StatesParul Tyagi, Babu Banarasi Das University, India
Copyright © 2025 Xu, Tang, Xu, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yansheng Zhang, emhhbmd5czFAc2h1LmVkdS5jbg==
†These authors have contributed equally to this work
 Chuanjia Xu
Chuanjia Xu Nan Tang2
Nan Tang2 Yansheng Zhang
Yansheng Zhang