- 1Department of Phytochemistry, Medicinal Plants and Drug Research Institute, Shahid Beheshti University, Evin, Tehran, Iran
- 2Animal Physiology and Neurobiology Section, Department of Biology, KU Leuven, Leuven, Belgium
- 3Leishmania Diagnostic & Drug Delivery Research Laboratory, University of Peshawar, Peshawar, Pakistan
- 4Department of Natural Sciences, Mid Sweden University, Sundsvall, Sweden
- 5Jiangxi Province Key Laboratory of Pharmacology of Traditional Chinese Medicine, National Engineering Research Center for Modernization of Traditional Chinese Medicine - Hakka Medical Resources Branch, School of Pharmacy, Gannan Medical University, Ganzhou, China
The genus Eryngium, belonging to the Umbelliferae family, comprises flowering plants with various pharmacological activities, including anti-inflammatory and antidiabetic properties. However, many of these activities lack scientific evaluation. This study aimed to characterize the metabolites and evaluate the antihelmintic, antibacterial, and antibiofilm activities of a methanolic extract derived from the aerial parts of Eryngium billardieri. Metabolite characterization was conducted using LC-MS combined with a computer-assisted structure elucidation method. The extract was tested against six fungi, six Gram-positive bacteria, and nine Gram-negative bacteria, and a non-parasitic nematode (Caenorhabditis elegans). A total of thirty-three compounds were identified, with the major constituents including isorhamnetin-3-O-glucoside, phytolaccagenin, terpinolene, 3,4-dimethoxybenzaldehyde, palmitic acid, isobornyl formate, isorhamnetin, and 1,4-dimethyl-7-(1-methylethenyl)-octahydroazulene. Across all tested concentrations, Gram-positive bacteria demonstrated greater sensitivity compared to Gram-negative bacteria, with Staphylococcus aureus and Micrococcus luteus showing the highest sensitivity (IC50 values of 57.47 µg/mL and 105.8 µg/mL, respectively). Among Gram-negative strains, only Brevundimonas diminuta exhibited sensitivity. In antifungal tests, six of seven yeast strains displayed sensitivity to the extract, with Candida parapsilosis and Candida albicans being particularly susceptible (IC50 values of 11.29 µg/mL and 63.29 µg/mL, respectively). The antibiofilm analysis demonstrated inhibitory effects within 24 hours after biofilm formation, with an IC50 of 6.3 µg/mL. Additionally, the antihelmintic assay revealed a mean inhibition rate of 97.7 ± 1.5 at 2.0 µg/mL. The results demonstrate that the extract effectively inhibited the tested bacteria, particularly against yeast strains. While the extract showed promising activity against a model nematode, further research is imperative to validate its anthelmintic efficacy against parasitic nematodes.
1 Introductions
Medicinal plants, renowned for their antimicrobial, anticancer, anti-inflammatory, and diverse pharmacological properties, have been employed globally for millennia (Perumal Samy and Gopalakrishnakone, 2010; Wang et al., 2014; Sadeghi et al., 2022). However, with the advent of antibiotics and synthetic chemical drugs in the 20th century, their use diminished, accompanied by a decline in scientific research into their effects. This shift has had significant repercussions for both human and animal health (Grenni et al., 2018). The inappropriate and widespread use of antibiotics has led to increasing resistance problems. In response, the European Union implemented regulations in 2006 to limit the use of antibiotics and other chemicals, aiming to curb the spread of antibiotic resistance among human pathogens. Consequently, efforts have intensified to explore plants or plant-derived extracts as natural alternatives (Alamgir, 2017).
The rise of resistance to synthetic drugs poses a significant challenge to public health (McEwen and Collignon, 2018). While various chemical drugs with distinct structures and mechanisms are available for treating helminthic, bacterial, and fungal infections, resistance often results in persistent, acute, or recurrent diseases (Manandhar et al., 2019). Prolonged drug usage has, in some cases, led to adverse side effects, restricting their therapeutic potential. Resistant microorganisms pose a substantial risk to human populations, animals, and plants (Manandhar et al., 2019; Saad et al., 2023). Presently, researchers are striving to optimize the use of chemical drugs, prevent diseases, and develop new, less toxic compounds with fewer side effects (Carracedo−Reboredo et al., 2021). Remarkably, although most drugs today are synthetic, at least one-third of these agents originate from plants or are derived from plant extracts. For millennia, plants have served as remedies, immune system enhancers, and agents against cancer and infections, and they remain invaluable sources in the quest for effective and safe therapeutic solutions (Romão et al., 2013; Mousavi et al., 2025).
Eryngium billardieri F. Delarche. (Figure 1), a member of the Umbelliferae family, is native to the Iran–Turonian floristic region, the species inhabits steppe ecosystems from plains to montane zones, favoring rocky, well-drained, nutrient-poor soils and full sun. It is intolerant to prolonged soil saturation and commonly colonizes disturbed habitats, including overgrazed rangelands, with populations often increasing after fire events (Bashari et al., 2025). E. billardieri has long been employed in traditional medicine to treat various inflammatory disorders (Osqueei et al., 2023). In Iranian traditional medicine, the aerial parts of E. billardieri have been used to address a wide range of conditions, including goiter (Kremer et al., 2021), lymphedema, inflammatory disorders (Daneshzadeh et al., 2020), rheumatism, hyperglycemia (Osqueei et al., 2023), urinary infections, and wound healing (Küpeli et al., 2006; Sepanlou et al., 2019). Notably, previous reports have highlighted the anti-inflammatory and anti-hyperglycemic effects of E. billardieri’s roots and aerial parts (Kremer et al., 2021). Furthermore, the cytotoxicity of E. billardieri extracts against PANC-1 cancer cells has been evaluated (Hasanbeiglu et al., 2022). Recent investigations have shown that both essential oil and solvent extracts of E. billardieri exhibit significant antibacterial activity. Hajian-Maleki and Shams-Bakhsh (2023) demonstrated that the plant’s essential oil exerts strong inhibitory effects against several Gram-positive and Gram-negative bacteria, producing inhibition zones of approximately 8–21 mm and minimum inhibitory concentrations ranging from 0.67 g L−1 to 34.17 g L−1. Gas chromatography–mass spectrometry (GC–MS) analysis identified 34 constituents accounting for over 95% of the total oil composition, with n-hexadecanoic acid, 2-pentadecanone, and cinnamyl tiglate among the major bioactive compounds (Hajian-Maleki and Shams-Bakhsh, 2023). Similarly, Farhan et al. (2012) reported antibacterial effects of crude E. billardieri extracts, with variations depending on solvent type and bacterial strain (Farhan et al., 2012). In another study, Allafchian et al. (2022) utilized E. billardieri extract in the green synthesis of silver nanoparticles and observed enhanced antimicrobial activity of the resulting nanocomposites against multiple bacterial species (Allafchian et al., 2022).
The growing resistance to current therapeutic agents for human and animal diseases underscores the urgent need for novel treatments (Küpeli et al., 2006; Rauf et al., 2021; Zargar Zarin et al., 2025). Plant-based drugs have garnered widespread attention (Lu et al., 2020; Shawky et al., 2021) due to their perceived safety and reliability as alternatives to expensive synthetic drugs (Ernst, 2007). Consequently, medicinal plants are subjected to extensive screening for potential biological activities (Romano et al., 2021). Although some plants have been extensively used by traditional healers as antiparasitic and antimicrobial agents, their efficacy under experimental conditions remains largely unverified (Zulhendri et al., 2021). Traditional Iranian medicinal plants, including E. billardieri, may offer more effective treatments for infections caused by parasites and microbes (Amiri et al., 2021; Rashidipour et al., 2022; Sadeghi et al., 2023; Tabefam et al., 2018). In continuing our research on Iranian medicinal plant, here in, E. billardieri is investigated for its antihelmintic, antibacterial, and antifungal properties, aiming to scientifically validate its traditional use by the local population of Iran.
2 Material and methods
2.1 Chemical reagents
Various chemical reagents and solvents, including acetonitrile, dimethyl sulfoxide (DMSO), ethyl acetate, formic acid, n-hexane, trifluoroacetic acid, and methanol, were purchased from Chem-Lab NV (Zedelgem, Belgium). Miconazole (200 mg/mL stock) served as the positive control for antifungal activity. Ciprofloxacin (100 μg/mL) and levamisole, both purchased from Sigma-Aldrich, were used as positive controls for antibacterial and anthelmintic activities. Resazurin salt was sourced from Acros Organics in Geel, Belgium. A Milli-Q system (Millipore, Bedford, MA, USA) was used to prepare deionized water.
2.2 Plant material and extraction
Eryngium billardieri F. Delarche. was collected in Shiraz, Fars Province, Iran, in May 2019. The plant was identified by Dr. Mojtaba Asadollahi, a botanist, and a voucher specimen (MPH-2698) was deposited at the herbarium of the Medicinal Plants and Drug Research Institute, Shahid Beheshti University, Tehran, Iran. The aerial parts (1.5 kg) were washed, dried in the laboratory away from direct sunlight, ground into powder, and subjected to methanol extraction three times (7 L×3, successively) at room temperature, with each cycle lasting 72 hours. The solvent was removed under reduced pressure using rotary evaporation at 40 °C. The drying process continued until a dry mass with a constant weight of 120 g was obtained. The extract was stored at 4 °C for further analysis.
2.3 Biological assay
2.3.1 Antimicrobial assay
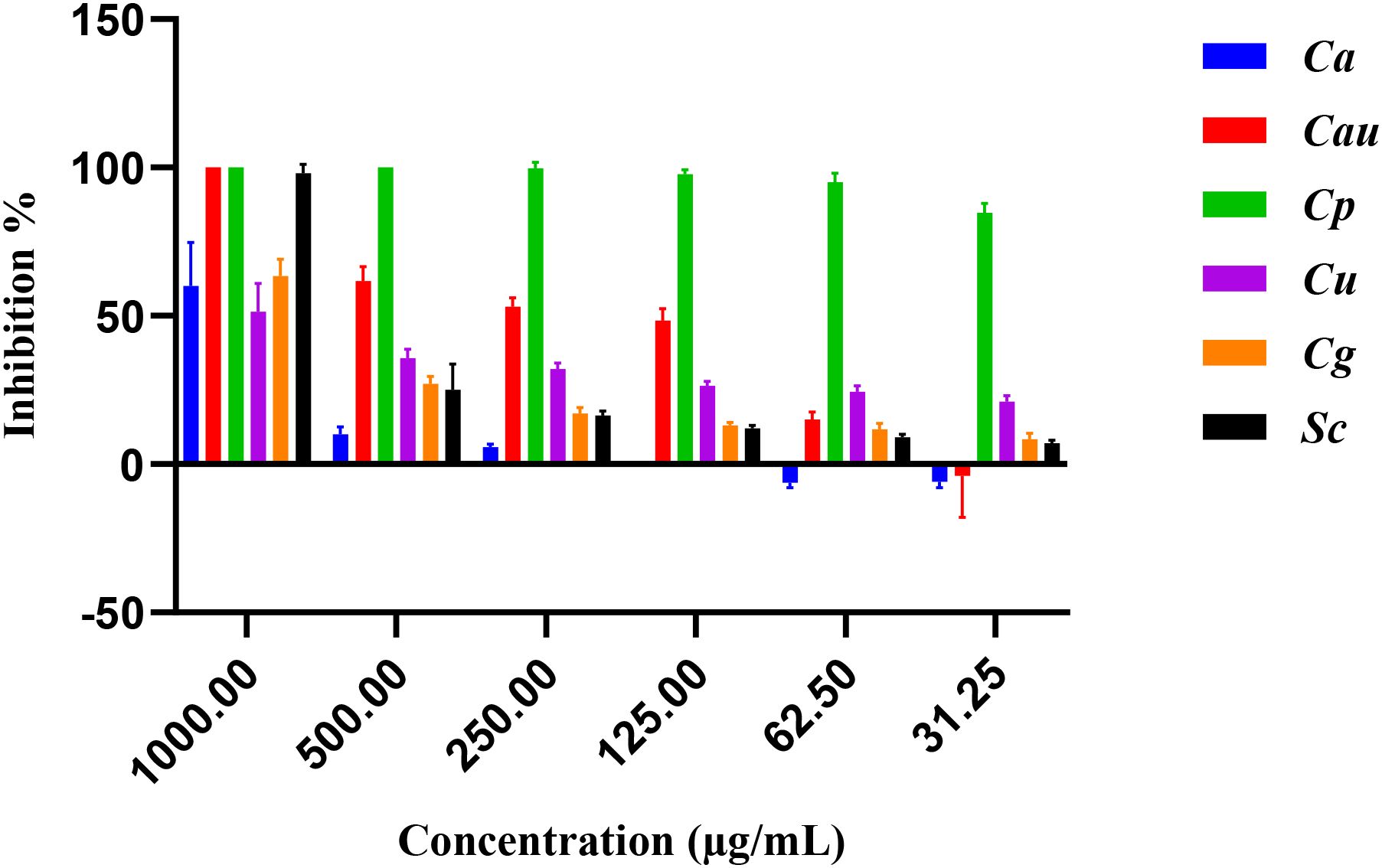
The extract was tested against six yeasts (Candida albicans, Candida auris, Candida parapsilosis, Candida utilis, Candida glabrata, Saccharomyces cerevisiae), six Gram-positive bacteria (Staphylococcus aureus, Micrococcus luteus, Enterococcus faecalis, Streptococcus faecalis, Staphylococcus epidermidis, Listeria innocua), and nine Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Aeromonas hydrophila, Shigella flexneri, Shigella sonnei, Acinobacter baumanii, Enterobacter aerogenes, Brevundimonas diminuta, Salmonella enteritidis). The test organisms used in this study were sourced from the American Type Culture Collection (ATCC) and are stored in our laboratory’s freezer/fridge for future use. The antimicrobial activity was assessed using a broth microdilution assay following the method described by Hu et al., 2023. Briefly, yeast cultures were grown in YPD medium (1% yeast extract, 2% peptone, and 2% dextrose), while bacterial cultures were grown in Mueller–Hinton (MH) medium (0.2% beef extract, 1.75% casamino acids, and 0.015% soluble starch). Under aseptic conditions, 5 μL of bacterial cultures (1×106 CFU/mL) and 10 μL of yeast cultures (1×105 CFU/mL) were inoculated in 96-well plates along with 10 μL of the test sample, solvent control (DMSO), and positive controls (miconazole at 200 μg/mL and ciprofloxacin at 100 μg/mL). The test organisms were adjusted to an optical density (OD) of 0.003 for bacteria and 0.001 for fungi and incubated at 37 °C for 20 hours. Miconazole (for fungi) and ciprofloxacin (for Gram-positive and Gram-negative bacteria) were included as positive controls (Hu et al., 2023). The inhibition values were calculated using the following Equation 1:
To ensure reliability, all experiments were conducted in replicate. The IC50 values were determined through nonlinear least-squares sigmoid regression curve fitting. Additionally, serial dilution agar tests were conducted to determine the minimum bactericidal concentration of the extract (Hu et al., 2022).
2.3.2 Antibiofilm test
The biofilm-forming strain was cultivated in Yeast Extract–Peptone (YPD) broth for Candida biofilms at 37 °C for 18–24 hours. The microorganism-containing culture was then centrifuged at 800 rpm for 2 minutes, and the supernatant was carefully discarded. One mL of RPMI-MOPS medium was added to the tube, and after gentle vortexing to ensure uniformity, the OD was measured and adjusted to 0.1 at 600 nm, corresponding approximately to 1 × 106 CFU/mL of Candida albicans cells. A 100 μL aliquot of the Candida suspension in RPMI-MOPS was transferred into a 96-well plate and incubated at 37 °C for 90 minutes in a stationary incubator to facilitate the initial adhesion phase of biofilm formation. Following incubation, the medium was removed, and each well was washed three times with 100 μL phosphate-buffered saline (PBS) to eliminate non-adherent cells. Test samples and YPD media were added to each well. Additionally, DMSO control and positive control wells were included, with one well left empty for the subsequent resazurin control during staining. The plate was further incubated at 37 °C for 24 hours in a stationary incubator. Following this incubating, the medium was removed, and the wells were washed twice with PBS. Biofilm staining was conducted using 100 μL of resazurin dye (40 μg/mL) pre well. After one hour of incubation at 37 °C, fluorescence was measured using a FlexStation II spectrofluorometer (Molecular Devices, USA) with excitation (λex) and emission (λem) wavelengths set at 535 nm and 590 nm, respectively. The following Equation 2 was used to calculate the surviving biofilm percentage:
2.3.3 Anthelmintic test
2.3.3.1 Culture, maintenance and synchronization of Caenorhabditis elegans
Caenorhabditis elegans (C. elegans) strains were cultured on Petri dishes containing a lawn of E. coli. Synchronized populations were prepared using a modified alkaline bleaching method. In brief, eggs and adult worms were washed with S-basal medium and treated with a bleaching solution composed of 1 mL bleach and 0.5 mL of 5 M NaOH. The resulting suspension was washed several times using S-basal medium and incubated for 24 hours to obtain L1 larvae. These L1 larvae were then transferred onto a nematode growth media plate with an E. coli lawn and incubated at 20 °C until they reached the L4 larval stage. This developmental stage was used for the anthelminthic assay.
2.3.3.2 Anthelmintic assay
The assay was performed following the method described by Cédric et al. (2023), with minor modifications. In summary, each well of a 96-well microplate was filled with 184 µL of E. coli culture (OD = 0.5 at 600 nm), followed by addition of synchronized C. elegans (L4 larvae) suspended in S-basal medium. Subsequently, 1 µL of plant extract was introduced into each well. Control wells contained 1 µL of DMSO as a solvent control and 50 µM levamisole as a positive control. The microplates were incubated at 20 °C for 16 hours in a WMicrotracker ONE system (Phylumtech), where worm movements were recorded every 30 minutes. The percentage inhibition of worm mobility was calculated using the following Equation 3:
2.4 LC-MS analysis
The MS sample was redissolved in acetonitrile (MeCN) at a concentration of 0.1 mg/mL. For MS detection, 5 µL of a 20-fold dilution was injected. A Shimadzu LCMS-2020 system was employed for analysis, operating in full scan mode with a mass range of 100–1500 m/z in both positive and negative ion modes. The MS data were processed using various software tools including Xcalibur 4.2, Freestyle™ 1.5, ACD/MS Workbook Suite 2021 with MS Fragmenter, and ChromGenius.
2.5 Data processing and analysis
The dose-response data were analyzed using GraphPad Prism version 8.0 for Windows (GraphPad Software Inc., San Diego, CA, USA). To assess the in vitro activity, one-way analysis of variance (ANOVA) and Tukey’s multiple comparison test were employed. The 50% inhibitory concentrations (IC50) were determined by plotting concentration-response curves, where the logarithm of the concentration was plotted against the percentage inhibition. All the tests were repeated three times to ensure reliable data.
3 Results
3.1 Antimicrobial activity of the extract
3.1.1 Effect of extract on gram-positive bacteria
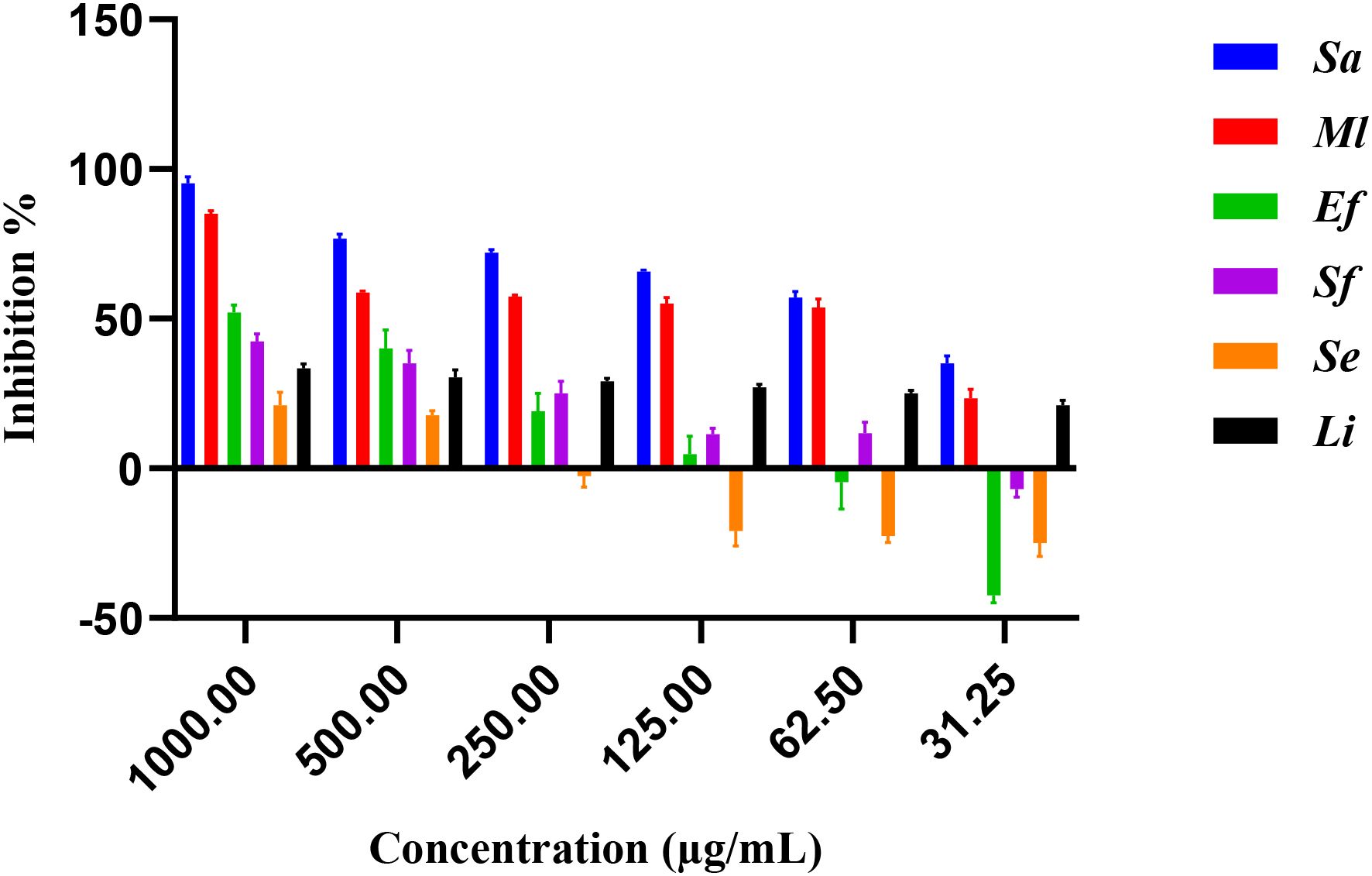
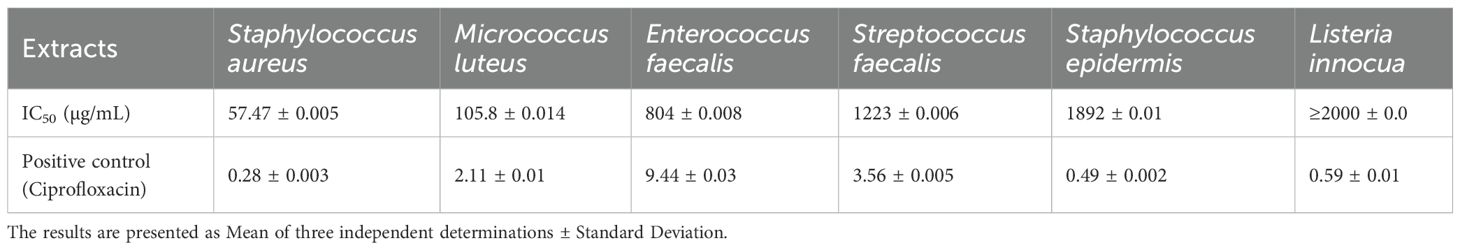
The antimicrobial activity of E. billardieri extract against Gram-positive bacteria is shown in Figure 2. The results indicate a concentration-dependent inhibition, with higher concentrations yielding greater effectiveness. Among the tested Gram-positive strains, Staphylococcus aureus and Micrococcus luteus demonstrated the highest sensitivity, with low IC50 values of 57.47 µg/mL and 105.8 µg/mL, respectively (Table 1). In contrast, Enterococcus faecalis, Streptococcus faecalis, and Staphylococcus epidermis showed relatively higher IC50 values (804 µg/mL, 1223 µg/mL, and 1892 µg/mL, respectively), indicating moderate resistance. Listeria innocua displayed the highest IC50 value (67.245 µg/mL), suggesting that it is the least susceptible Gram-positive species tested.
3.1.2 Effect of extract on gram-negative bacteria
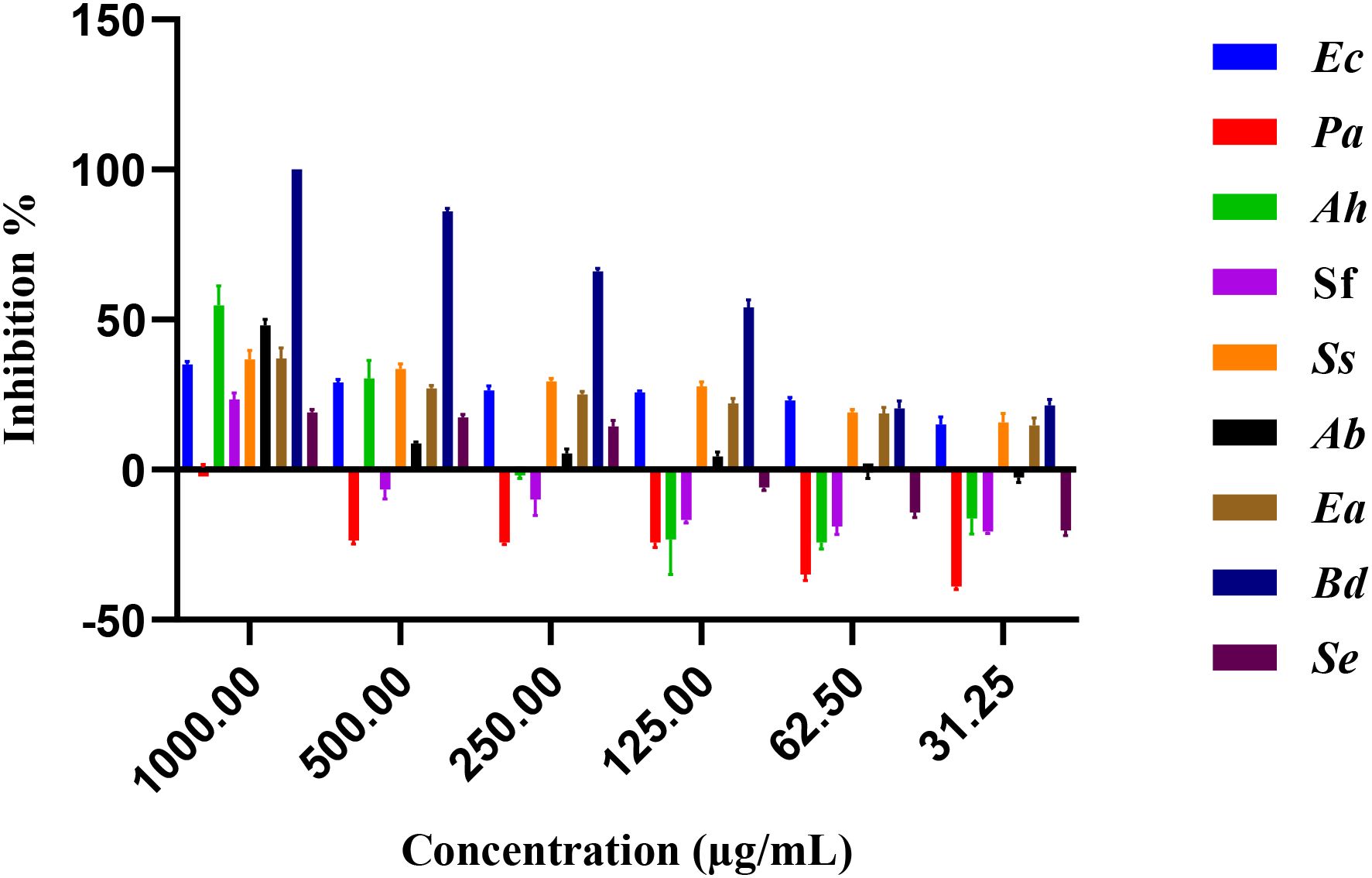
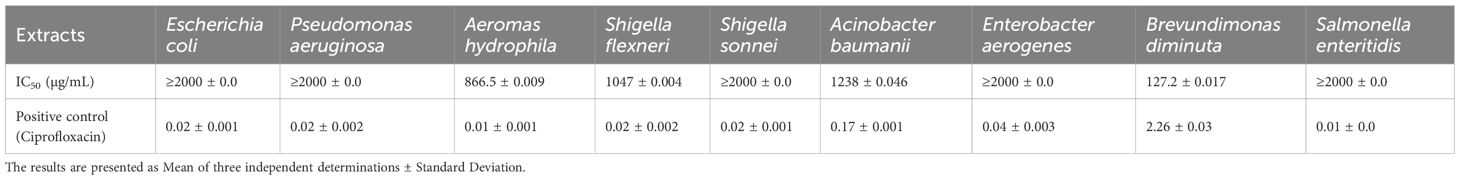
Figure 3 illustrates the inhibitory effects of E. billardieri extract on Gram-negative bacteria. The figure displays inhibition percentages across the experimental concentration range (31.25–1000 µg/mL). For strains that did not achieve 50% inhibition within this range, the IC50 values reported in Table 2 are listed as ≥2000 µg/mL, indicating that the actual IC50 exceeds the tested concentrations. Compared to Gram-positive species, Gram-negative bacteria exhibited higher IC50 values, suggesting lower susceptibility. Among the tested Gram-negative strains, Brevundimonas diminuta was the most susceptible, with an IC50 value of 127.2 µg/mL. In contrast, Escherichia coli and Pseudomonas aeruginosa showed the highest IC50 values (12,408 µg/mL and 759,196 µg/mL, respectively), indicating significant resistance (Table 2). Other species, such as Aeromonas hydrophila, Shigella flexneri, Shigella sonnei, Acinetobacter baumannii, and Enterobacter aerogenes, exhibited moderate levels of inhibition.
3.1.3 Effect of extract on yeasts
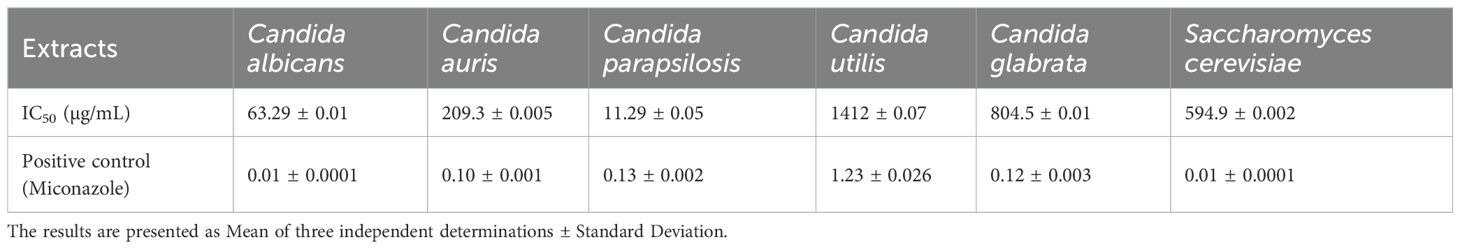
The antifungal activity of E. billardieri extract against yeast species is shown in Figure 4. The extract demonstrated substantial effects, with Candida parapsilosis and Candida albicans being the most susceptible strains, exhibiting IC50 values of 11.29 and 63.29 µg/mL, respectively (Table 3). Other yeasts, such as Candida auris and Saccharomyces cerevisiae, displayed moderate susceptibility, with IC50 values of 209.3 µg/mL and 594.9 µg/mL, respectively. In contrast, Candida glabrata, and Candida utilis exhibited relatively higher IC50 values (804.5 µg/mL, and 1412 µg/mL, respectively), indicating lower susceptibility.
3.1.4 Antibiofilm activity
The E. billardieri extract demonstrated an antibiofilm effect at sub-minimum inhibitory concentrations (sub-MICs). Within 24 hours of biofilm formation, the extract inhibited the growth of all tested strains, achieving an IC50 of 6.3 µg/mL (Figure 5). However, no significant antibiofilm activity was observed after 48 hours of biofilm formation. These findings suggest the potential use of the extract as an antibacterial agent to inhibit biofilm formation during the early stages.
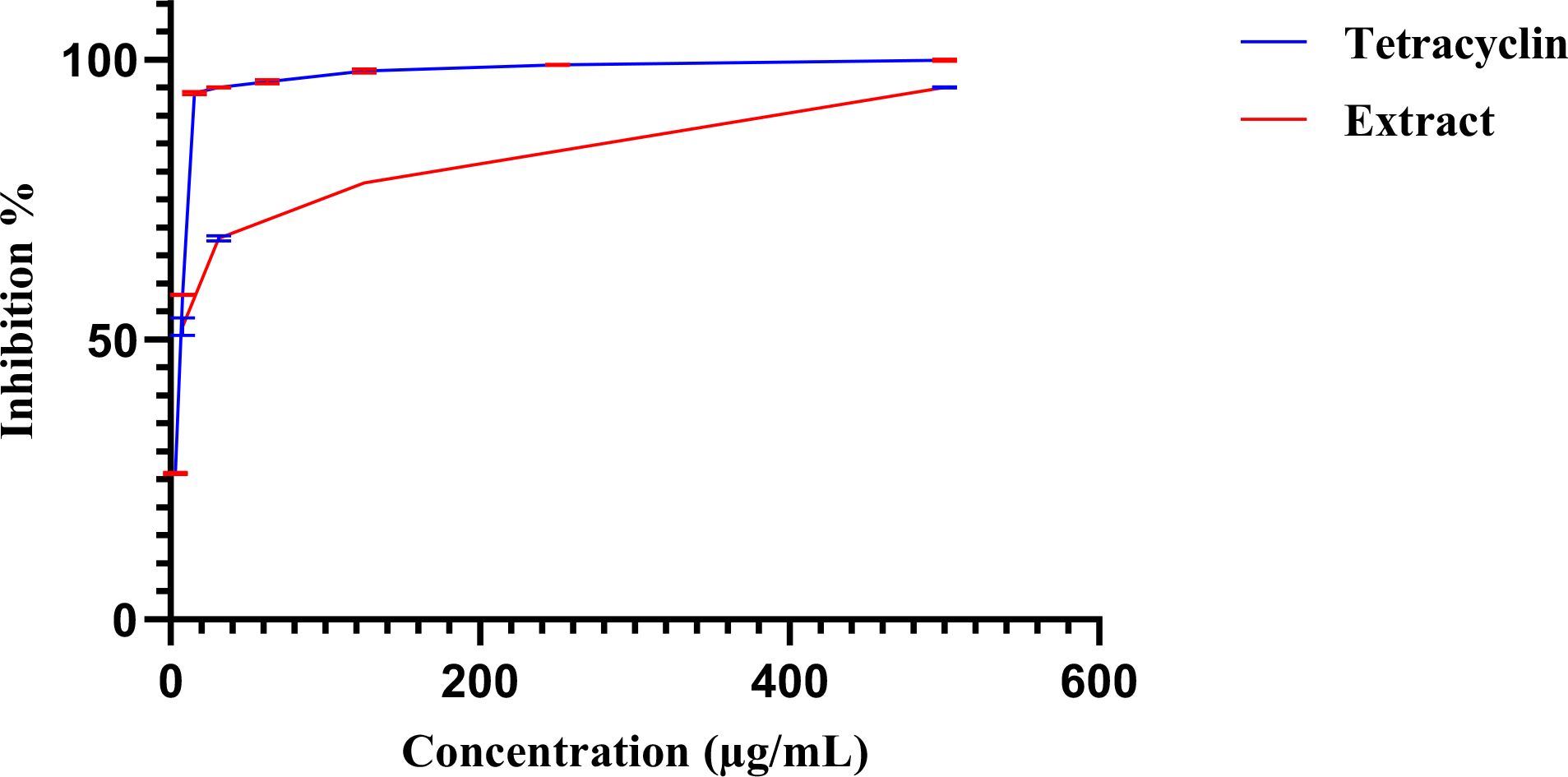
Figure 5. Dose–response curves of biofilm inhibition by the extract (red) and tetracycline (blue). Error bars, shown in the different color as each curve.
3.2 Anthelminthic activity
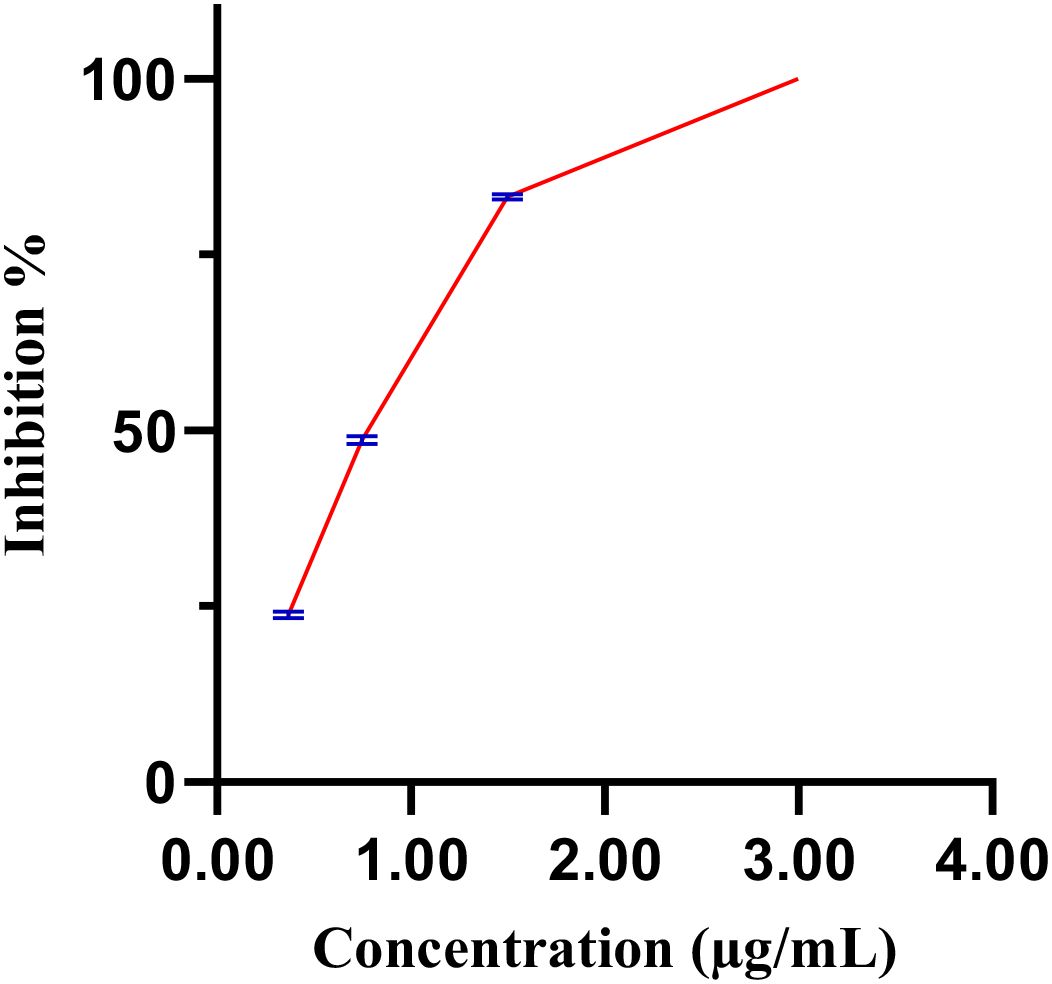
Figure 6 illustrates the inhibition percentages of C. elegance L4 larval mobility induces by the methanol extract over time. At a concentration of 2.0 µg/mL, the methanol extract completely inhibited larval mobility (97.7 ± 1.5%), closely mirroring the effect of the positive control, levamisole (99.1 ± 1.78%). In contrast, neither 1.5% DMSO nor distilled water (negative control) affected larval mobility. Decreasing the extract concentration from 3 µg/mL to 0.37 µg/mL resulted in a progressive reduction in inhibitory effects, with an estimated IC50 of 0.89 µg/mL (Figure 7).
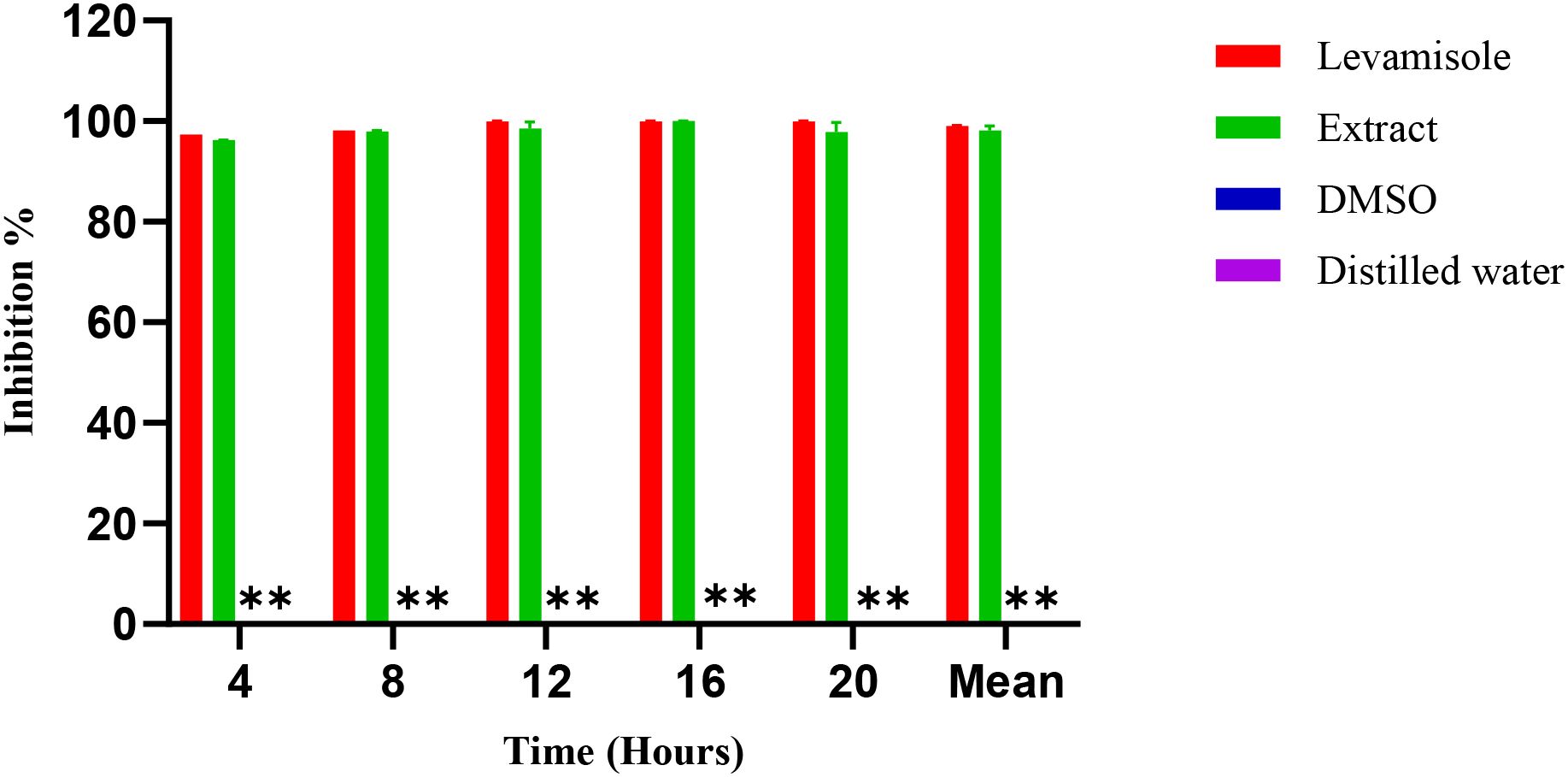
Figure 6. Inhibition (%) over time for Levamisole (positive control), Extract, DMSO, and Distilled water (negative controls). **: DMSO and distilled water show 0% inhibition at all time points and, therefore, appear as baseline bars.
3.3 Chemical profiling of E. billardieri
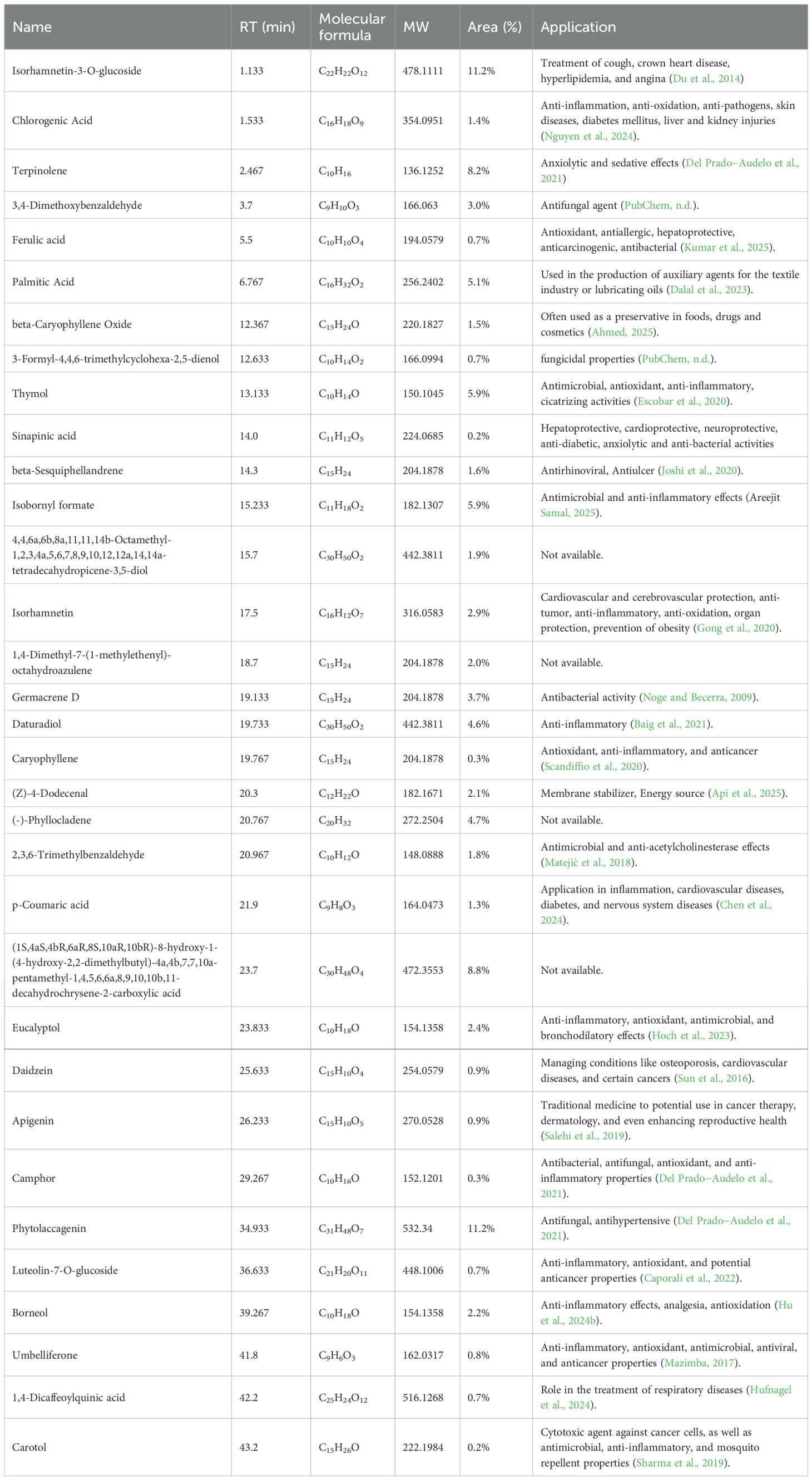
The MS data of the E. billardieri extract was analyzed and matched against a manually curated database using the ACD/MS Workbook Suite. The total ion chromatogram (TIC) was generated from matched ions, and extracted ion chromatograms (EICs) in both positive and negative ion modes are shown in Figure 8. The identified compounds and their fragmentations are listed in Table 4. A total of thirty-three components were tentatively identified, comprising seventeen terpenoids, one coumarin, five flavonoids, five phenolic acids, five fatty acids, and four aldehydes. Among these, isorhamnetin-3-O-glucoside and phytolaccagenin exhibited the highest relative contents, each accounting for 11.2% based on peak area comparison. Other major components with contents exceeding 2% included terpinolene, 3,4-dimethoxybenzaldehyde, palmitic acid, thymol, isobornyl formate, isorhamnetin, 1,4-dimethyl-7-(1-methylethenyl)-octahydroazulene, daturadiol, (-)-phyllocladene, eucalyptol, borneol, (1S,4aS,4bR,6aR,8S,10aR,10bR)-8-hydroxy-1-(4-hydroxy-2,2-dimethylbutyl)-4a,4b,7,7,10a-pentamethyl-1,4,5,6,6a,8,9,10,10b,11-decahydrochrysene-2-carboxylic acid.
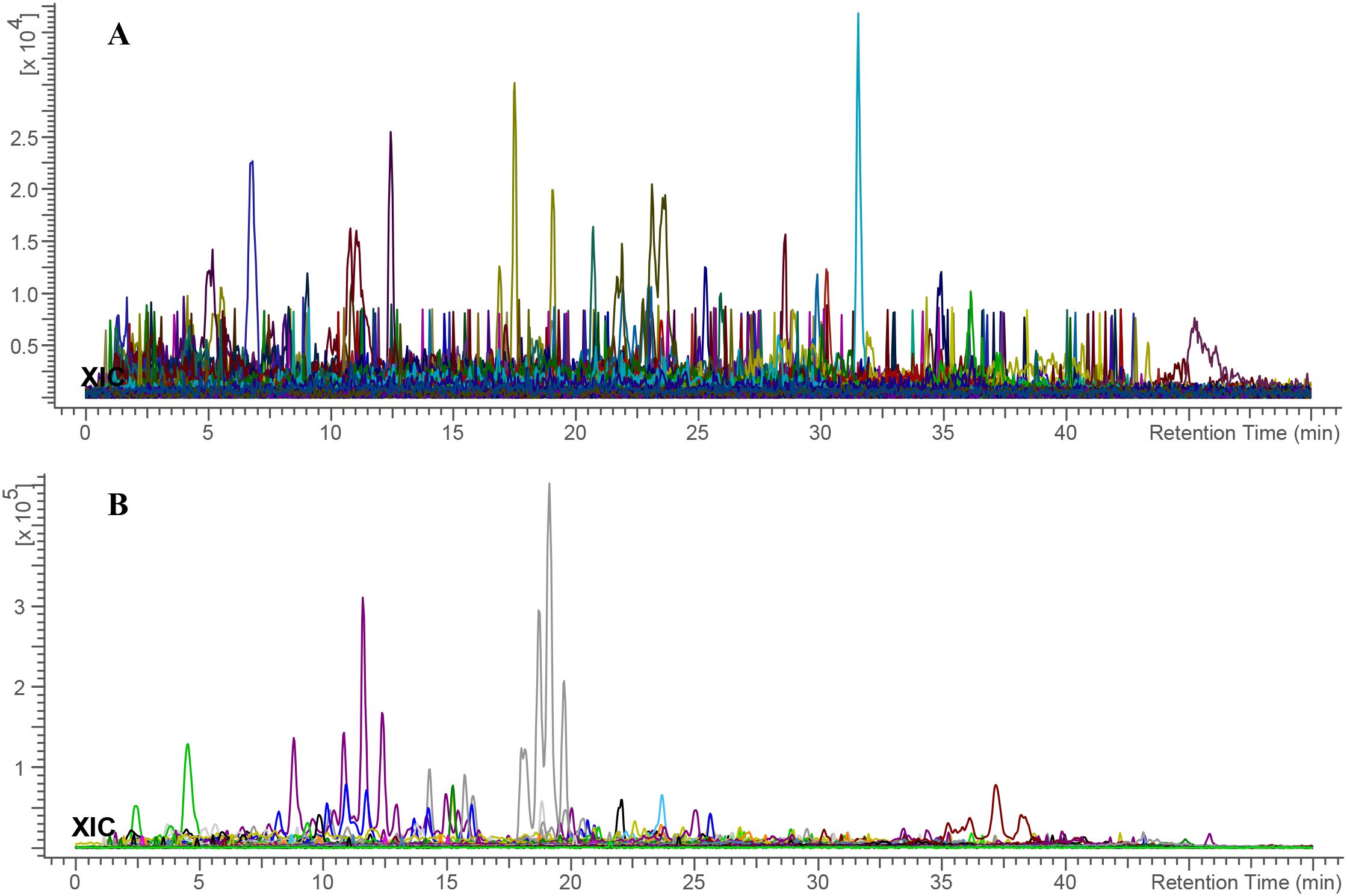
Figure 8. Total ion chromatogram of E. billardieri extract in (A) positive and (B) negative ionization modes. Each color indicates a distinct m/z signal.
3.3.1 Computer-assisted structure elucidation by MS
The chemical identification process for E. billardieri extract was conducted through UHPLC-MS analysis, following a multi-step approach (Hu et al., 2024a). Firstly, precursor ions were automatically calculated using the ACD/MS Workbook Suite. These ultrahigh-resolution mass data were then cross-referenced with chemical databases, including COCONUT (https://coconut.naturalproducts.net/) and a custom database of Eryngium plants. The custom database, which contains previously isolated and identified compounds from Eryngium species, provide a reliable foundation for correct identification. A variety of compounds were identified from the E. billardieri extract, some of which appeared as isomers. For instance, isorhamnetin-3-O-glucoside was identified based on its [M+H]+ precursor ion at m/z 479.118 and a retention time of 1.133 minutes. This mass corresponded to the chemical formula C22H22O12, confirming its identification. Experimental fragmentation spectra were then compared to predicted spectra generated by tools such as MS Fragmenter. This comparison refined the list of potential structures, retaining only those with fragmentation patterns closely aligned with the experimental data.
In cases where isomers could not be distinguished by fragmentation patterns alone, retention time calculations were employed. Using ChromGenius, expected retention times were computed and matched with the observed retention times under identical chromatographic conditions. For instance, the identification of chlorogenic acid was confirmed by its retention time of 1.533 minutes and its molecular formula C16H18O9.
The combination of fragmentation analysis and retention time prediction enabled the accurate identification of compounds in the Eryngium extract. Final confirmations were made by comparing results against reference standards, ensuring robust and reliable identifications. Key compounds identified included ferulic acid, palmitic acid, and beta-caryophyllene oxide. These findings underscore the diverse chemical composition of the E. billardieri extract, laying the groundwork for further studies and potential applications.
4 Discussions
In our study, we specifically investigated the biological activities of E. billardieri, focusing on its antihelmintic, antibacterial, and antifungal properties.
Previous studies have highlighted the significant antioxidant and antimicrobial activities of the ethanolic extract of E. billardieri aerial parts, as well as its high phenolic and flavonoid content (Daneshzadeh et al., 2020). Our results align with these findings, demonstrating the extract’s effectiveness against Gram-negative bacteria. Specifically, E. billardieri showed significant inhibition against Staphylococcus aureus and Micrococcus luteus, suggesting that the extract contains compounds active against Gram-positive bacteria. Conversely, the higher IC50 values against Escherichia coli and Pseudomonas aeruginosa indicate a less pronounced effect on Gram-negative bacteria. This could be attributed to the more complex cell wall structures of Gram-negative bacteria, which often hinder the penetration of antimicrobial agents. These findings suggest that E. billardieri may have selective antibacterial activity, which warrants further investigation for potential targeted applications.
Biofilm formation by microorganisms is a widespread phenomenon across various ecological niches, and both immunocompetent and immunocompromised individuals are susceptible to C. albicans infections. Given their inherent resistance to conventional antifungal treatments, Candida biofilms pose a significant clinical challenge (Gulati and Nobile, 2016). While our study did not identify the specific compound responsible for the extract’s antibiofilm activity against Candida, the observed IC50 value of 6.3 µg/mL is notably low for a crude extract. This is especially surprising given lack of activity against the planktonic form. The antibiofilm effect appears to be transient, as it was present at 24 hours after biofilm formation but diminished after 48 hours. This could be due to chemical instability of the bioactive compounds or their degradation by the tested fungus. Regardless, these results suggest that the extract exerts a fungistatic, rather than fungicidal, effect.
Helminthiasis is a debilitating condition that remains prevalent in many developing countries across Africa, often overlooked due to the majority of research funding being directed toward diseases such as HIV and tuberculosis (Fernandes et al., 2021). This neglected tropical disease (Hotez et al., 2008), caused by various helminths, has shown a concerning increase in drug resistance, highlighting the urgency of addressing this public health issue (Cédric et al., 2023). Our study is the first to demonstrate strong inhibition (IC50 of 0.89 µg/mL) in a model nematode, highlighting the potential of E. billardieri a natural antihelmintic agent.
Chemical profiling of the extract revealed the presence of several terpenoids, phenolic acids, and flavonoids, including isorhamnetin 3-O-glucoside and phytolaccagenin, which exhibited the highest relative content. These compounds, along with other constituents like terpinolene and thymol, may contribute to the extract’s observed biological activities. However, bioassay-guided purification is needed to pinpoint the specific compounds responsible for each effect.
Our study unveils the promising potential of E. billardieri as a source of bioactive compounds for antimicrobial and antihelmintic applications. Future studies should prioritize isolating and characterizing the specific active compounds, elucidating their mechanisms of action, and evaluating their safety and toxicity profiles. Continued research into plant-derived antimicrobials could provide valuable resources for developing novel treatments, especially in the light of the increasing resistance to conventional drugs.
5 Conclusion
The methanol extract of E. billardieri showed activity against pathogens such as Staphylococcus aureus, Micrococcus luteus, Candida albicans, and Candida parapsilosis, as well as Caenorhabditis elegans. Although activity was evident, the inhibitory concentrations required were relatively high, indicating a moderate level of potency compared to established antimicrobial agents. Chemical profiling identified diverse range of bioactive compounds, including terpenoids, flavonoids, and phenolic acids, which likely contribute to these observed effects. However, future research should focus on identifying specific components responsible for these activities, further enhancing our understanding of the plant’s therapeutic potential.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MY: Resources, Formal Analysis, Data curation, Visualization, Writing – original draft, Writing – review & editing, Conceptualization, Methodology. MM: Project administration, Conceptualization, Validation, Resources, Writing – review & editing, Supervision, Writing – original draft. AK: Writing – review & editing, Methodology. MA: Writing – review & editing, Resources. MO: Writing – original draft. WL: Validation, Writing – review & editing, Conceptualization, Writing – original draft, Supervision. HH: Conceptualization, Writing – original draft, Funding acquisition, Writing – review & editing, Software.
Funding
The author(s) declare financial support was received for the research, and/or publication of this article. Financial support by the Shahid Beheshti University Research Council and Iran National Science Foundation (INSF; Grant No. 97022126) is gratefully acknowledged. This work was also partially funded by GMU Talent Project (QD202305), Jiangxi Provincial Department of Education Project (JXJG231327), Jiangxi Provincial Natural Science Foundation (Grant No. 20252BAC240465) and Horizontal Project from Baishen Pharmacutial. CO. LTD. (HX202409/JXSTD409126659006).
Acknowledgments
Financial support by the Shahid Beheshti University Research Council is gratefully acknowledged.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, E. A. (2025). The potential therapeutic role of beta−caryophyllene as a chemosensitizer and an inhibitor of angiogenesis in cancer. Molecules 30, 1751. doi: 10.3390/molecules30081751
Alamgir, A. N. M. (2017). Therapeutic use of medicinal plants and their extracts: volume 1 (Cham: Springer).
Allafchian, A., Balali, F., Vahabi, M. R., and Jalali, S. A. H. (2022). Antibacterial and cytotoxic effects of silver nanoparticles fabricated by Eryngium billardieri Delar. extract. Chem. Phys. Lett. 791, 139385. doi: 10.1016/j.cplett.2022.139385
Amiri, M. S., Yazdi, M. E. T., and Rahnama, M. (2021). Medicinal plants and phytotherapy in Iran: glorious history, current status and future prospects. Plant Sci. Today 8, 95–111. doi: 10.14719/pst.2021.8.1.926
Api, A. M., Bartlett, A., Belsito, D., Botelho, D., Bruze, M., Bryant−Friedrich, A., et al. (2025). RIFM fragrance ingredient safety assessment, (Z)−4−dodecenal, CAS registry number 21944−98−9. Food Chem. Toxicol. 197, 115311. doi: 10.1016/j.fct.2025.115311
Baig, M. W., Fatima, H., Akhtar, N., Hussain, H., Okla, M. K., Al−Hashimi, A., et al. (2021). Anti−inflammatory potential of daturaolone from Datura innoxia Mill.: in silico, in vitro and in vivo studies. Pharmaceuticals 14, 1248. doi: 10.3390/ph14121248/S1
Bashari, H., Bazgir, F., and Vahabi, M. R. (2025). Prioritizing the risk of multiple invasive species in the semiarid rangelands of Iran: an ecological approach to multicriteria decision−making. Ecol. Evol. 15, e71287. doi: 10.1002/ece3.71287
Caporali, S., De Stefano, A., Calabrese, C., Giovannelli, A., Pieri, M., Savini, I., et al. (2022). Anti−inflammatory and active biological properties of the plant−derived bioactive compounds luteolin and luteolin 7−glucoside. Nutrients 14, 1155. doi: 10.3390/nu14061155
Carracedo−Reboredo, P., Liñares−Blanco, J., Rodríguez−Fernández, N., Cedrón, F., Novoa, F. J., Carballal, A., et al. (2021). A review on machine learning approaches and trends in drug discovery. Comput. Struct. Biotechnol. J. 19, 4538–4558. doi: 10.1016/j.csbj.2021.08.011
Cédric, Y., Christelle Nadia, N. A., Sylvain Raoul, S. N., Berinyuy, S., Azizi, M. A., Jemimah Sandra, T. N., et al. (2023). Antihelminthic activity of Lophira lanceolata on Heligmosomoides polygyrus using an automated high−throughput method. J. Trop. Med. 2023, 9504296. doi: 10.1155/2023/9504296
Chen, F., Zhang, X., Wang, J., Wang, F., and Mao, J. (2024). P−coumaric acid: advances in pharmacological research based on oxidative stress. Curr. Topics Medicinal Chem. 24, 416–436. doi: 10.2174/0115680266276823231230183519
Dalal, D., Kunte, S., Oblureddy, V. T., and Anjali, A. K. (2023). Comparative evaluation of antimicrobial efficacy of German chamomile extract, tea tree oil, and chlorhexidine as root canal irrigants against E. faecalis and Streptococcus mutans—an in vitro study. Int. J. Agric. Biosci. 12, 252–256. doi: 10.47278/journal.ijab/2023.072
Daneshzadeh, M. S., Abbaspour, H., Amjad, L., and Nafchi, A. M. (2020). An investigation on phytochemical, antioxidant and antibacterial properties of extract from Eryngium billardieri F. Delaroche. J. Food Measurement Characterization 14, 708–715. doi: 10.1007/s11694-019-00317-y
Del Prado−Audelo, M. L., Cortés, H., Caballero−Florán, I. H., González−Torres, M., Escutia−Guadarrama, L., Bernal−Chávez, S. A., et al. (2021). Therapeutic applications of terpenes on inflammatory diseases. Front. Pharmacol. 12. doi: 10.3389/fphar.2021.704197
Du, L. Y., Zhao, M., Xu, J., Qian, D. W., Jiang, S., Shang, E. X., et al. (2014). Analysis of the metabolites of isorhamnetin 3−O−glucoside produced by human intestinal flora in vitro by applying ultraperformance liquid chromatography/quadrupole time−of−flight mass spectrometry. J. Agric. Food Chem. 62, 2489–2495. doi: 10.1021/jf405261a
Ernst, E. (2007). Herbal medicines: balancing benefits and risks. Novartis Found Symp. 282, 154-167; discussion 167-172, 212-218. doi: 10.1002/9780470319444.ch11
Escobar, A., Pérez, M., Romanelli, G., and Blustein, G. (2020). Thymol bioactivity: a review focusing on practical applications. Arabian J. Chem. 13, 9243–9269. doi: 10.1016/j.arabjc.2020.11.009
Farhan, H., Malli, F., Rammal, H., Hijazi, A., Bassal, A., Ajouz, N., et al. (2012). Phytochemical screening and antioxidant activity of Lebanese Eryngium creticum L. Asian Pacific J. Trop. Biomedicine 2, S1217–S1220. doi: 10.1016/S2221-1691(12)60388-8
Fernandes, L. S., da Costa, Y. F. G., de Bessa, M. E., Ferreira, A. L. P., Corrêa, J. O.do A., Del−Vechio Vieira, G., et al. (2021). Metabolic profiling and antibacterial activity of Eryngium pristis Cham. & Schltdl. prospecting for its use in the treatment of bacterial infections. Arch. Pharm. Pharm. Sci. 5, 20–28. doi: 10.29328/journal.apps.1001027
Gong, G., Guan, Y. Y., Zhang, Z. L., Rahman, K., Wang, S. J., Zhou, S., et al. (2020). Isorhamnetin: a review of pharmacological effects. Biomedicine Pharmacotherapy 128, 110301. doi: 10.1016/j.biopha.2020.110301
Grenni, P., Ancona, V., and Caracciolo, A. B. (2018). Ecological effects of antibiotics on natural ecosystems: a review. Microchemical J. 136, 25–39. doi: 10.1016/j.microc.2017.02.006
Gulati, M. and Nobile, C. J. (2016). Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infection 18, 310–321. doi: 10.1016/j.micinf.2016.01.002
Hajian-Maleki, H. and Shams-Bakhsh, M. (2023). Identification of the chemical profile and evaluation of the antimicrobial effect of Eryngium billardieri Delar. essential oil components against bacterial species of agricultural and food interest. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1249780
Hasanbeiglu, S., Hosseini, K., Molavi, O., Asgharian, P., and Tarhriz, V. (2022). Eryngium billardieri extract and fractions induce apoptosis in cancerous cells. Anti−Cancer Agents Medicinal Chem. 22, 2189–2201. doi: 10.2174/1871520621666211201151736
Hoch, C. C., Petry, J., Griesbaum, L., Weiser, T., Werner, K., Ploch, M., et al. (2023). 1,8−cineole (eucalyptol): a versatile phytochemical with therapeutic applications across multiple diseases. Biomedicine Pharmacotherapy 167, 115467. doi: 10.1016/j.biopha.2023.115467
Hotez, P. J., Brindley, P. J., Bethony, J. M., King, C. H., Pearce, E. J., and Jacobson, J. (2008). Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 118, 1311–1321. doi: 10.1172/JCI34261
Hu, H., Hu, B., Hu, C., Zhu, Y., Zhang, R., Qiu, H., et al. (2024a). Picrasma quassioides leaves: insights from chemical profiling and bioactivity comparison with stems. Fitoterapia 177, 106108. doi: 10.1016/j.fitote.2024.106108
Hu, H., Tekin, V., Hu, B., Yaghoobi, M., Khan, A., Ghosh, A. K., et al. (2023). Metabolic profiling of Chimonanthus grammatus via UHPLC−HRMS−MS with computer−assisted structure elucidation and its antimicrobial activity. Front. Plant Sci. 14, 1138913. doi: 10.3389/fpls.2023.1138913
Hu, X., Yan, Y., Liu, W., Liu, J., Fan, T., Deng, H., et al. (2024b). Advances and perspectives on pharmacological activities and mechanisms of the monoterpene borneol. Phytomedicine 177, 106108. doi: 10.1016/j.phymed.2024.155848
Hu, H., Yang, Y., Aissa, A., Tekin, V., Li, J., Panda, S. K., et al. (2022). Ethnobotanical study of Hakka traditional medicine in Ganzhou, China and their antibacterial, antifungal, and cytotoxic assessments. BMC Complementary Med. Therapies 22, 244. doi: 10.1186/s12906-022-03712-z
Hufnagel, M., Rademaekers, A., Weisert, A., Häberlein, H., and Franken, S. (2024). Pharmacological profile of dicaffeoylquinic acids and their role in the treatment of respiratory diseases. Front. Pharmacol. 15. doi: 10.3389/fphar.2024.1371613
Joshi, A., Sunil Krishnan, G., and Kaushik, V. (2020). Molecular docking and simulation investigation: effect of beta−sesquiphellandrene with ionic integration on SARS−CoV−2 and SFTS viruses. J. Genet. Eng. Biotechnol. 18, 78. doi: 10.1186/s43141-020-00095-x
Kremer, D., Zovko Končić, M., Kosalec, I., Košir, I. J., Potočnik, T., Čerenak, A., et al. (2021). Phytochemical traits and biological activity of Eryngium amethystinum and E. alpinum (Apiaceae). Horticulturae 7, 364. doi: 10.3390/horticulturae7100364
Kumar, M., Kaushik, D., Shubham, S., Kumar, A., Kumar, V., Öz, E., et al. (2025). Ferulic acid: extraction, estimation, bioactivity and applications for human health and food. J. Sci. Food Agric. 105, 4168–4177. doi: 10.1002/jsfa.13931
Küpeli, E., Kartal, M., Aslan, S., and Yesilada, E. (2006). Comparative evaluation of the anti−inflammatory and antinociceptive activity of Turkish Eryngium species. J. Ethnopharmacology 107, 32–37. doi: 10.1016/j.jep.2006.02.005
Lu, R. M., Hwang, Y. C., Liu, I. J., Lee, C. C., Tsai, H. Z., Li, H. J., et al. (2020). Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 27, 1. doi: 10.1186/s12929-019-0592-z
Manandhar, S., Luitel, S., and Dahal, R. K. (2019). In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 1895340. doi: 10.1155/2019/1895340
Matejić, J. S., Stojanović−Radić, Z. Z., Ristić, M. S., Veselinović, J. B., Zlatković, B. K., Marin, P. D., et al. (2018). Chemical characterization, in vitro biological activity of essential oils and extracts of three Eryngium L. species and molecular docking of selected major compounds. J. Food Sci. Technol. 55, 2910. doi: 10.1007/s13197-018-3209-8
Mazimba, O. (2017). Umbelliferone: sources, chemistry and bioactivities review. Bull. Faculty Pharmacy Cairo Univ. 55, 223–232. doi: 10.1016/bfopcU.2017.05.001
McEwen, S. A. and Collignon, P. J. (2018). Antimicrobial Resistance: a One Health Perspective. Microbiol Spectr 6 (2), 521–547. doi: 10.1128/microbiolspec.arba-0009-2017
Mousavi, M., Moridi Farimani, M., Kashfi, K., and Ghasemi, A. (2025). Antidiabetic Potential of Sophora Species: Mechanisms, Bioactive Constituents, and Therapeutic Prospects. Planta Med. 91, 546–557. doi: 10.1055/a-2597-8133
Nguyen, V., Taine, E. G., Meng, D., Cui, T., and Tan, W. (2024). Chlorogenic acid: a systematic review on the biological functions, mechanistic actions, and therapeutic potentials. Nutrients 16, 924. doi: 10.3390/nu16070924
Noge, K. and Becerra, J. X. (2009). Germacrene D, a common sesquiterpene in the genus Bursera (Burseraceae). Molecules 14, 5289–5297. doi: 10.3390/molecules14125289
Osqueei, M. R., Mahmoudabadi, A. Z., Bahari, Z., Meftahi, G. H., Movahedi, M., Taghipour, R., et al. (2023). Eryngium billardieri extract affects cardiac gene expression of master regulators of cardiomyopathy in rats with high−fat−diet−induced insulin resistance. Clin. Nutr. ESPEN 56, 59–66. doi: 10.1016/j.clnesp.2023.04.016
Perumal Samy, R. and Gopalakrishnakone, P. (2010). Therapeutic potential of plants as anti−microbials for drug discovery. Evidence−Based Complementary Altern. Med. 7, 283–294. doi: 10.1093/ecam/nen036
PubChem Veratraldehyde. Available online at: https://pubchem.ncbi.nlm.nih.gov/compound/Veratraldehyde.
PubChem (Compound ID 12309880). Available online at: https://pubchem.ncbi.nlm.nih.gov/compound/12309880.
Rashidipour, M., Shakib, P., Goudarzi, G., Pournia, Y., Karimi, M., and Sarlak, M. (2022). Native Iranian medicinal plants with anti−vaginal infection properties: a systematic review. Infect. Disorders—Drug Targets 22, 41–47. doi: 10.2174/1871526522666220501171551
Rauf, A., Akram, M., Semwal, P., Mujawah, A. A. H., Muhammad, N., Riaz, Z., et al. (2021). Antispasmodic potential of medicinal plants: a comprehensive review. Oxid. Med. Cell. Longevity 2021, 4889719. doi: 10.1155/2021/4889719
Romano, B., Lucariello, G., and Capasso, R. (2021). Topical collection “pharmacology of medicinal plants. Biomolecules 11, 101. doi: 10.3390/biom11010101
Romão, V. C., Canhão, H., and Fonseca, J. E. (2013). Old drugs, old problems: where do we stand in prediction of rheumatoid arthritis responsiveness to methotrexate and other synthetic DMARDs? BMC Med. 11, 17. doi: 10.1186/1741-7015-11-17
Saad, M. J., Khashan, A. A., and Ibrahim, O. S. (2023). In vitro antibacterial activity of some plant essential oils against four different microbial strains. Biochem. Cell. Arch. 23, 9482. doi: 10.3390/app12199482
Sadeghi, Z., Yang, J.-L., Venditti, A., and Moridi Farimani, M. (2022). A review of the phytochemistry, ethnopharmacology and biological activities of Teucrium genus (Germander). Nat. Prod. Res. 36, 5647–5664. doi: 10.1080/14786419.2021.2022669
Sadeghi, Z., Rigi, F., Hosseini, S. H., Rahdari, R., Sahebkar, A., et al. (2023). Ethnopharmacological Survey of the Iranian Coastline of Makran Based on the Unani Medicine. Unani Med. doi: 10.2174/0122150838269638231027032542
Salehi, B., Venditti, A., Sharifi−Rad, M., Kręgiel, D., Sharifi−Rad, J., Durazzo, A., et al. (2019). The therapeutic potential of apigenin. Int. J. Mol. Sci. 20, 1305. doi: 10.3390/ijms20061305
Samal, A. (2025). sCentInDB | Database of essential oil chemical profiles of medicinal plants of India (Chennai, India: The Institute of Mathematical Sciences (IMSc). Available online at: https://cb.imsc.res.in/scentindb/chemicalsummary/EOCID002041 (Accessed October 28, 2025).
Scandiffio, R., Geddo, F., Cottone, E., Querio, G., Antoniotti, S., Gallo, M. P., et al. (2020). Protective effects of (E)−β−caryophyllene (BCP) in chronic inflammation. Nutrients 12, 3273. doi: 10.3390/nu12113273
Sepanlou, M. G., Ardakani, M. M., Hajimahmoodi, M., Sadrai, S., Amin, G. R., Sadeghi, N., et al. (2019). Ethnobotanical and traditional uses, phytochemical constituents and biological activities of Eryngium species growing in Iran. Traditional Med. Res. 4, 148. doi: 10.12032/TMR20190412114
Sharma, M., Chahal, K. K., Kaur, R., Singh, R., and Kataria, D. (2019). Antifungal potential and structure activity relationship of carrot seed constituents. J. Food Biochem. 43, e12971. doi: 10.1111/jfbc.12971
Shawky, M., Suleiman, W. B., and Farrag, A. A. (2021). Antibacterial resistance pattern in clinical and non−clinical bacteria by phenotypic and genotypic assessment. J. Pure Appl. Microbiol. 15, 2270–2279. doi: 10.22207/jpam.15.4.49
Sun, M. Y., Ye, Y., Xiao, L., Rahman, K., Xia, W., and Zhang, H. (2016). Daidzein: a review of pharmacological effects. Afr. J. Traditional Complementary Altern. Medicines 13, 117–132. doi: 10.4314/ajtcam.v13i3.15
Tabefam, M., Moridi Farimani, M., Danton, O., Ramseyer, J., Kaiser, M., Ebrahimi, S. N., et al (2018). Antiprotozoal Diterpenes from Perovskia abrotanoides. Planta Med. 84, 913–919. doi: 10.1055/a-0608-4946
Wang, C. Y., Bai, X. Y., and Wang, C. H. (2014). Traditional Chinese medicine: a treasured natural resource of anticancer drug research and development. Am. J. Chin. Med. 42, 543–559. doi: 10.1142/S0192415X14500359
Zargar Zarin, M. K., Moridi Farimani, M., Alilou, M., Azargoon, F., Kaiser, M., Gelbrich, T., et al (2025). Semisynthetic Derivatives of Perovskone: Development of a Promising Class of Antiprotozoal Lead Compounds. J. Nat. Prod. 88, 1041–1047. doi: 10.1021/acs.jnatprod.5c00138
Keywords: Eryngium billardieri, antimicrobial, methanol extract, antihelmintic, antibiofilm, antifungal
Citation: Yaghoobi M, Moridi Farimani M, Khan A, Asadollahi M, Omrani M, Luyten W and Hu H (2025) Investigation of phytochemical profiling and biological activities of methanol extract from Eryngium billardieri: antimicrobial, antibiofilm, and anthelmintic properties. Front. Plant Sci. 16:1667335. doi: 10.3389/fpls.2025.1667335
Received: 16 July 2025; Accepted: 15 October 2025;
Published: 06 November 2025.
Edited by:
Choon Hui Tan, UCSI University, MalaysiaReviewed by:
Habibeh Hajian Maleki, Tarbiat Modares University, IranStephanie La Blanche Guetchueng Tamdem, Institut de Recherches Médicales et d’Etudes des Plantes Médicinales, Cameroon
Copyright © 2025 Yaghoobi, Moridi Farimani, Khan, Asadollahi, Omrani, Luyten and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahdi Moridi Farimani, bV9tb3JpZGlAc2J1LmFjLmly; Haibo Hu, aGhiMjAxN0BnbXUuZWR1LmNu
 Mahdi Yaghoobi
Mahdi Yaghoobi Mahdi Moridi Farimani
Mahdi Moridi Farimani Ajmal Khan
Ajmal Khan Mojtaba Asadollahi
Mojtaba Asadollahi Marzieh Omrani1
Marzieh Omrani1 Walter Luyten
Walter Luyten Haibo Hu
Haibo Hu