Abstract
As a critical factor influencing crop productivity in agricultural ecosystems, phosphorus (P)-fertilizer application can significantly alter soil physicochemical properties. However, the relative efficiencies of different types of spatially stratified P fertilizers and their underlying biological mechanisms remain insufficiently elucidated. In this study, an 8-year field experiment was conducted in a black soil region of Northeast China to compare the effects of five P-fertilization regimes: CK (without P application), FP (100% as basal fertilizer), APP (20% as starter fertilization by ammonium polyphosphate), MAP (20% as starter fertilization by monoammonium phosphate), and CMP (20% as starter fertilization by calcium magnesium phosphate). We systematically investigated the effects of spatially stratified P fertilization on soil physical properties, nutrient accumulation, maize yield performance, and bacterial and fungal community structure. CMP demonstrated the best performance in improving soil aeration and enhancing water infiltration capacity. MAP significantly increased the soil total P content by 18.62% and the soil Olsen-P content by 81.46% compared to those of FP. Both MAP and CMP promoted P uptake in various parts of maize plants, including the roots, straw, and grains. All tested starter P fertilizers improved P use efficiency. Compared to that of FP, the soil P surplus was reduced by 7.52%, 14.74%, and 13.04% under APP, MAP, and CMP, respectively. MAP demonstrated the most pronounced yield-increasing effect. Based on amplicon sequencing (16S rRNA for bacteria, interspacer region for fungi) and microbiome profiling, this study confirms that fungi are more susceptible than bacteria to variations in fertilizer types and application methods. Furthermore, the relative abundance of Tausonia was most significantly influenced by MAP. By enhancing the relative abundance of P-cycling functional genes (gph, phoU), MAP modulated the abundance of dominant microbial taxa such as Acidobacteria and Proteobacteria, thereby significantly improving maize yield. Therefore, in maize cropping systems in the black soil region of Northeast China, optimized P fertilizer selection and application methods can effectively reduce soil P surplus and modulate microbial community structure and functional diversity while maintaining stable crop yields.
1 Introduction
In intensive agricultural systems, the continuous phosphorus (P)-fertilizer application employed to sustain high productivity leads to excessive soil P accumulation and associated environmental risks (MacDonald et al., 2011; Withers et al., 2014; Zhang et al., 2018; Zhu et al., 2018). Moreover, such practices may severely compromise terrestrial biodiversity and critical ecosystem functions (MacDonald et al., 2011; Withers et al., 2014; Zhang et al., 2018). Furthermore, as a finite and geographically concentrated resource, global phosphate rock reserves are projected to meet fertilizer demands only for several decades to centuries (MacDonald et al., 2011; Withers et al., 2014; Zhang et al., 2018). China exhibits significant potential to improve P use efficiency (PUE). Strategic optimization of P-fertilizer application can substantially reduce input requirements while simultaneously enhancing fertilizer utilization rates and crop productivity (Du et al., 2020). These approaches primarily include integrated organic-inorganic fertilization, fertigation systems, and localized nutrient placement techniques. However, most existing studies have narrowly focused on single-layer soil fertilization, overlooking the comprehensive impacts of fertilization practices on crop nutrient acquisition and utilization efficiency (Hansel et al., 2017; Wu et al., 2021).
Deep fertilization enhances nutrient acquisition efficiency by optimizing the soil nutrient distribution and balancing nutrient availability, thereby promoting crop root absorption of nitrogen (N), P, and potassium (K). This method induces the development of spatially efficient root architectures and establishes a synergistic relationship between soil nutrients and crops, representing a key strategy for improving the cultivated soil layer structure (Sun et al., 2024; Wang et al., 2022). Specifically, stratified fertilization not only stimulates root system development but also significantly enhances both crop yield and quality (Gao et al., 2024). Moreover, starter fertilizer is typically band-applied near the seeds to minimize P fixation through limited soil-fertilizer contact, thereby enhancing P availability for the seedlings during early germination. The efficacy of starter P in promoting the early growth vigor of maize is well-established, particularly in low-P soils (Preston et al., 2019; Vetsch and Randall, 2002). Therefore, systematic investigation of stratified fertilization techniques and P source selection is critical to identify the key contributors to maize yield. Moreover, optimizing P formulations that maximize yield gains under reduced input regimes can help to realize the ultimate goal of achieving both agricultural productivity and soil health sustainability.
Microorganisms constitute fundamental components of soil P-cycling, while playing pivotal roles in modulating plant AP. Previous studies have shown the important roles of soil microorganisms in mediating soil nutrient cycling and plant–soil interactions (Hinsinger et al., 2009). Notably, P-cycling microbial communities serve as key drivers of soil P transformation and P-fertilizer utilization efficiency (Lang et al., 2021). To date, research in this field has predominantly focused on elucidating the effects of P-fertilizer application rates on the soil microbial community structure (Lang et al., 2021; Liu et al., 2024a, b). Long-term P fertilization significantly affects soil fungal and bacterial communities (Beauregard et al., 2010). The abundance of most microbial taxa, including key bacterial and fungal guilds, exhibits dose-dependent responses to P input levels (Huang et al., 2016). Furthermore, soil microorganisms exert a critical regulatory role in driving nutrient mineralization and biogeochemical cycling throughout plant growth (Regehr et al., 2015). Notably, the dynamic fluctuations in rhizosphere microbial abundance and diversity across successive crop developmental stages significantly modulate soil nutrient availability (Su et al., 2024). Moreover, soil microbial communities demonstrate remarkable adaptive capacity, maintaining functional and structural stability across diverse soil conditions under 37 years of differential nutrient input regimes (Hamel et al., 2006). These previous findings demonstrate that the soil microbial community composition is shaped by complex and dynamic interactions among soil environmental factors, fertilization regimes, and crop species.
Certain keystone microbial taxa play a pivotal role in soil P cycling by mediating the transformation of both inorganic phosphorus (Pi) and organic phosphorus (Po) into plant-available forms (Khan et al., 2007; Sharma et al., 2013). The associated genes were classified into four functional categories according to their roles in soil P-cycling (Dai et al., 2020; Hartman et al., 2017; Mahmood et al., 2020), including P-starvation response (PSR) regulation, the P-uptake and transport system, Pi solubilization, and Po mineralization, and exhibited distinct distribution patterns across agricultural ecosystems (Bergkemper et al., 2016; Dai et al., 2020). For instance, genes encoding high-affinity transporters (e.g., pst and phn) and low-affinity transporters (e.g., pit) mediate Pi uptake under P-deficient and P-replete soil conditions, respectively (Hsieh and Wanner, 2010). Genes associated with PSR regulation, including phoU, phoR, phoP, and phoB, enable microorganisms to maintain a certain P content under P-limited conditions (Devine, 2018; Hsieh and Wanner, 2010; Tee et al., 2021). Several studies have found that different P input levels significantly affect functional genes related to soil P cycling; however, the potential influence of P-fertilizer types remains unclear.
To address this gap, this study focused on a maize cropping system in the black soil region of Northeast China. Based on an 8-year spatially stratified experiment, we investigated the response of soil microorganisms to stratified P application, with the following objectives: 1) to identify the optimal P-fertilizer type suitable for stratified application, 2) to investigate the effects of long-term stratified fertilization on soil microbial diversity and functionality, and 3) to uncover the microbial mechanisms underlying enhanced maize yield. Based on the experimental findings, we further aimed to establish a theoretical framework for optimizing fertilization practices and yield-enhancing strategies in sustainable agricultural systems.
2 Materials and methods
2.1 Site description
The experiment was carried out at the Experimental Station of Jilin University, Changchun, China (125°14′ E, 43°56′ N) since 2017. The site has a frost-free period of 140 days and an average annual effective accumulated temperature of 3442.3 °C. The soil type is classified as black calcic soil. The initial soil properties (0–20 cm) before the experiment were as follows: pH 5.81; organic matter content 2.99%; total nitrogen (TN) 0.67 g kg-1; total phosphorus (TP) 0.35 g kg-1; total potassium (TK) 9.75 g kg-1; available nitrogen 105.48 mg kg-1; available phosphorus (AP) 16.75 mg kg-1; available potassium 140.37 mg kg-1; bulk density 1.23 g cm-3; and soil temperature 14.8 °C.
2.2 Experimental design
The maize cultivar ‘Xianyu 335’ was used in the field experiment with a planting density of 75,000 plants per hectare. The planting arrangement followed uniform spacing with 65-cm row spacing. Each experimental plot had an area of 39 m² (3.9 m × 10 m). A balanced compound fertilizer (N-P-K = 15-15-15) was applied as the basal fertilizer (20 cm below the soil surface) at the sowing stage. Three different starter P fertilizers were selected for comparison, which were placed 5 cm laterally and below the seed as a band during sowing (Randall and Hoeft, 1988): ammonium polyphosphate (APP), monoammonium phosphate (MAP), and calcium magnesium phosphate (CMP). Preston et al. (2019) demonstrated that demonstrated that co-application of starter fertilizers with broadcast or deep-banded P fertilizer enhances phosphorus uptake and can increase corn yields, particularly when the starter-P proportion exceeds 20%. Additional urea and potassium sulfate were top-dressed at the jointing stage to achieve a uniform N application rate up to 200 kg N ha-1, while the total K application was maintained at 90 kg K2O ha-1. Accordingly, five P-fertilizer treatments were established for comparison as follows: CK (without P application), FP (75 kg P2O5 ha-1 as basal fertilizer), APP (60 kg P2O5 ha-1 as basal fertilizer + 15 kg P2O5 ha-1 starter P), MAP (60 kg P2O5 ha-1 as basal fertilizer + 15 kg P2O5 ha-1 starter P), and CMP (60 kg P2O5 ha-1 as basal fertilizer + 15 kg P2O5 ha-1 starter P) (Supplementary Table S1; Supplementary Figure S1). The experimental protocol followed an annual cycle with sowing in May and harvesting in October, during which both soil and maize plant samples were collected for further analysis.
2.3 Sample collection
From 2017 to 2024, mature maize plant samples were collected annually, including both aboveground and belowground parts. The yield data were standardized and converted to per-hectare values based on the harvested plot area. All plant samples were oven-dried at 65°C to constant weight for subsequent determination of plant nutritional components. Additionally, the following yield components were measured: ear length, ear diameter, kernel number per ear, ear tip length, and 100-kernel weight. The seed moisture content was determined in triplicate using a PM-8188 moisture meter (PM-8188-A, KETT, Tokyo, Japan). All final yield data were standardized to the 14% seed moisture content.
In October 2024, rhizosphere soil samples (0–20 cm depth) were collected from each experimental plot, with three replicate soil samples obtained per plot to ensure representative sampling. First, three maize plants with intact root systems were randomly selected from each plot. Subsequently, rhizosphere soil was collected by carefully removing the tightly adhering soil from root surfaces using sterile brushes (Riley and Barber, 1969). Each soil sample was passed through a 2-mm sieve to remove large root fragments, followed by division into two aliquots: one aliquot was preserved for microbial sequencing analysis and the other was used for physicochemical characterization.
2.4 DNA extraction and sequencing
The total DNA was extracted from each homogenized soil sample E.Z.N.A™ Mag-Bind Soil DNA Kit (Omega, M5635-02, USA). The V3-V4 region of bacterial 16S rRNA gene was amplified based on the primers 338F/806R (Caporaso et al., 2012), ITS primers ITS1 and ITS2 were used for fungal DNA amplification (Mueller et al., 2014). Samples were delivered to Sangon BioTech (Shanghai, China) for library construction using universal Illumina adaptor and index. Sequencing was performed using the Illumina MiSeq system (Illumina MiSeq, USA), according to the standard protocols. Then, the raw sequences were quality filtered, merged, and analyzed by standard methods. Finally, operational taxonomic units (OTUs) were clustered at 97% similarity cutoff using Usearch software (version 11.0.667). Bacterial and fungal OTU representative sequences were classified taxonomically by blasting against the RDP Database and UNITE fungal ITS Database, respectively.
2.5 Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 27.0.1 (IBM Corp., Armonk, NY, USA). One-way analysis of variance was conducted to assess the effects of different P fertilizers on soil physicochemical properties, plant nutrient contents, and crop yield parameters. Treatment means were compared using Fisher’s least-significant difference post-hoc test at a significance level of α = 0.05. Several agronomic indices were calculated using standard formulas.
PUE, based on the P-fertilizer application rate and aboveground P uptake, was calculated as follows (Margenot et al., 2025) (Equation 1):
where aboveground P uptake refers to the total P absorption in plant straw and grains.
The partial factor productivity of phosphorus (PFP, kg kg-1) index serves as a crucial indicator for evaluating phosphorus fertilizer use efficiency (Yang et al., 2022), reflecting the crop yield obtained per unit of P-fertilizer input, which was calculated with the following formula (Equation 2):
Agronomic efficiency of phosphorus (AEP) quantifies the incremental crop yield per unit of P fertilizer applied, reflecting the actual contribution of fertilization to yield enhancement (Hartina et al., 2025), which was calculated with the following formula (Equation 3):
Finally, the P surplus (kg ha-1) was calculated according to Equation 4 below (Guan et al., 2022), which refers to the portion of total P input exceeding the total output in an agricultural system, indicating either soil P accumulation or potential environmental risks. As a key indicator for evaluating the sustainability of phosphorus management, P surplus is widely used to assess the P-cycling status in agroecosystems.
Analyses of microbial α- and β-diversity were conducted using RStudio. After performing significance analysis on the 16S sequencing data, the P-values were corrected for multiple hypothesis testing using the FDR (False Discovery Rate) method. Principal coordinate analysis (PCoA) was employed to visually depict the compositional differences of rhizosphere bacterial and fungal communities across different P fertilizer treatments. Permutational multivariate analysis of variance (PERMANOVA) was further used to test for significant differences in community composition among treatments. To visualize associations between P fertilizer application and key rhizosphere microbial taxa, as well as soil functional genes, GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA) was utilized. STAMP (Statistical Analysis of Metagenomic Profiles) was applied to identify significantly differentially abundant species under varying water transport conditions and characterize their preferential environmental correlations.
Functional profiling was performed using default parameters in FUNGuild (Fungal Functional Guild), PICRUSt2, and the KEGG database. Genes associated with soil P-cycling were defined as soil functional genes following previously reported methods (Dai et al., 2020; Hsieh and Wanner, 2010; Liu et al., 2023b; Mahmood et al., 2020; McGrath et al., 2013). For metagenomic data processing, additional methodological details are provided in Appendix S2.
Finally, structural equation modeling (SEM) was used to quantify both direct and indirect effects of soil microbial communities, soil physicochemical properties, functional gene abundances, phosphorus fertilizer uptake/utilization efficiency, and stratified P fertilization on the sustainability of maize yield.
3 Results
3.1 Soil physical properties and plant nutrient content
APP resulted in the highest values of soil bulk density, water content, and electrical conductivity, as well as the greatest solid-to-liquid ratio among the five experimental groups. CMP exhibited the highest soil pH value among all treatments; APP and MAP treatments resulted in relatively lower soil pH values, rendering the soils acidic (Table 1). CMP demonstrated the highest air ratio (30.25%) among the four fertilization treatments, indicating improved soil aeration and higher porosity, which are conducive to plant root growth and soil microbial activity. CMP also resulted in the lowest bulk density value among the various fertilization conditions, demonstrating a significant improvement in soil aeration and water infiltration capacity compared to those of APP.
Table 1
| Items | CK | FP | APP | MAP | CMP |
|---|---|---|---|---|---|
| Soil Bulk Density (g cm-3) | 1.44 ± 0.1b | 1.46 ± 0.11ab | 1.58 ± 0.11a | 1.52 ± 0.12ab | 1.45 ± 0.14b |
| Soil Moisture (%) | 13.62 ± 0.91c | 14.7 ± 0.71ab | 15.21 ± 1.31a | 14.52 ± 0.79abc | 13.96 ± 0.96bc |
| Soil porosity (%) | 50.61 ± 3.76 | 50.36 ± 4.05 | 46.86 ± 5.36 | 47.9 ± 4.62 | 50.5 ± 4.52 |
| True Density (g cm-3) | 2.52 ± 0.06 | 2.52 ± 0.02 | 2.53 ± 0.10 | 2.5 ± 0.03 | 2.51 ± 0.02 |
| pH | 5.93 ± 0.10ab | 6.13 ± 0.16a | 5.48 ± 0.46c | 5.66 ± 0.37bc | 6.14 ± 0.12a |
| Electrical Conductivity (μS cm-1) | 53.83 ± 8.38b | 69.17 ± 7.25b | 119.17 ± 47.55a | 76.33 ± 53.63ab | 90.33 ± 14.84ab |
| Air Ratio (%) | 30.99 ± 6.03a | 28.9 ± 4.97ab | 22.83 ± 5.62b | 25.84 ± 5.79ab | 30.25 ± 7.23a |
| Solid Ratio (%) | 49.39 ± 3.76 | 49.64 ± 4.05 | 53.14 ± 5.36 | 52.1 ± 4.62 | 49.5 ± 4.52 |
| Liquid Ratio (%) | 19.62 ± 2.5c | 21.46 ± 1.29bc | 24.02 ± 1.91a | 22.06 ± 1.61ab | 20.25 ± 2.95bc |
| Void Ratio (%) | 15.78 ± 1.21 | 17.24 ± 0.97 | 17.96 ± 1.84 | 16.99 ± 1.08 | 16.24 ± 1.3 |
| Water-Solid-Ratio (%) | 0.4 ± 0.03b | 0.43 ± 0.03ab | 0.46 ± 0.07a | 0.43 ± 0.03ab | 0.41 ± 0.04b |
| Saturation Percentage (%) | 39.22 ± 7.27c | 43 ± 5.76bc | 51.89 ± 7.35a | 46.64 ± 7.17ab | 40.77 ± 8.82bc |
Effects of different P fertilizers on soil structure and physical properties. Different lowercase letters in each rows indicate significant differences (P < 0.05) among different P fertilizer treatments, the same below.
All P-fertilizer treatments except FP showed a significantly higher total P (TP) content compared to that of the CK group. Specifically, APP, MAP, and CMP increased the TP content by 3.60%, 18.62%, and 15.62%, respectively, relative to that of FP, while FP resulted in a 7.42% increase in TP over that of the CK treatment. Notably, MAP and CMP demonstrated the highest TP values among all treatments. The total N (TN) content under the MAP treatment was 11.53% higher than that under FP, indicating that MAP exhibited the most pronounced effect on enhancing the soil N and P contents. Although MAP showed the highest TN value among all treatments, the difference was not statistically significant (Figure 1A). APP had the highest stoichiometric N/P ratio of 2.35 among all treatments, although no significant differences were observed across the five treatment groups (Figure 1B). MAP demonstrated the most significant enhancement in soil AP content, showing an 81.46% increase (P < 0.0001) compared to that of the FP group. In contrast, both the APP and CMP applications resulted in decreased AP levels compared to that of the FP condition (Figure 1C). These results collectively indicate that MAP outperformed the other three fertilizer types in improving soil nutrient content, particularly with respect to the AP concentration.
Figure 1
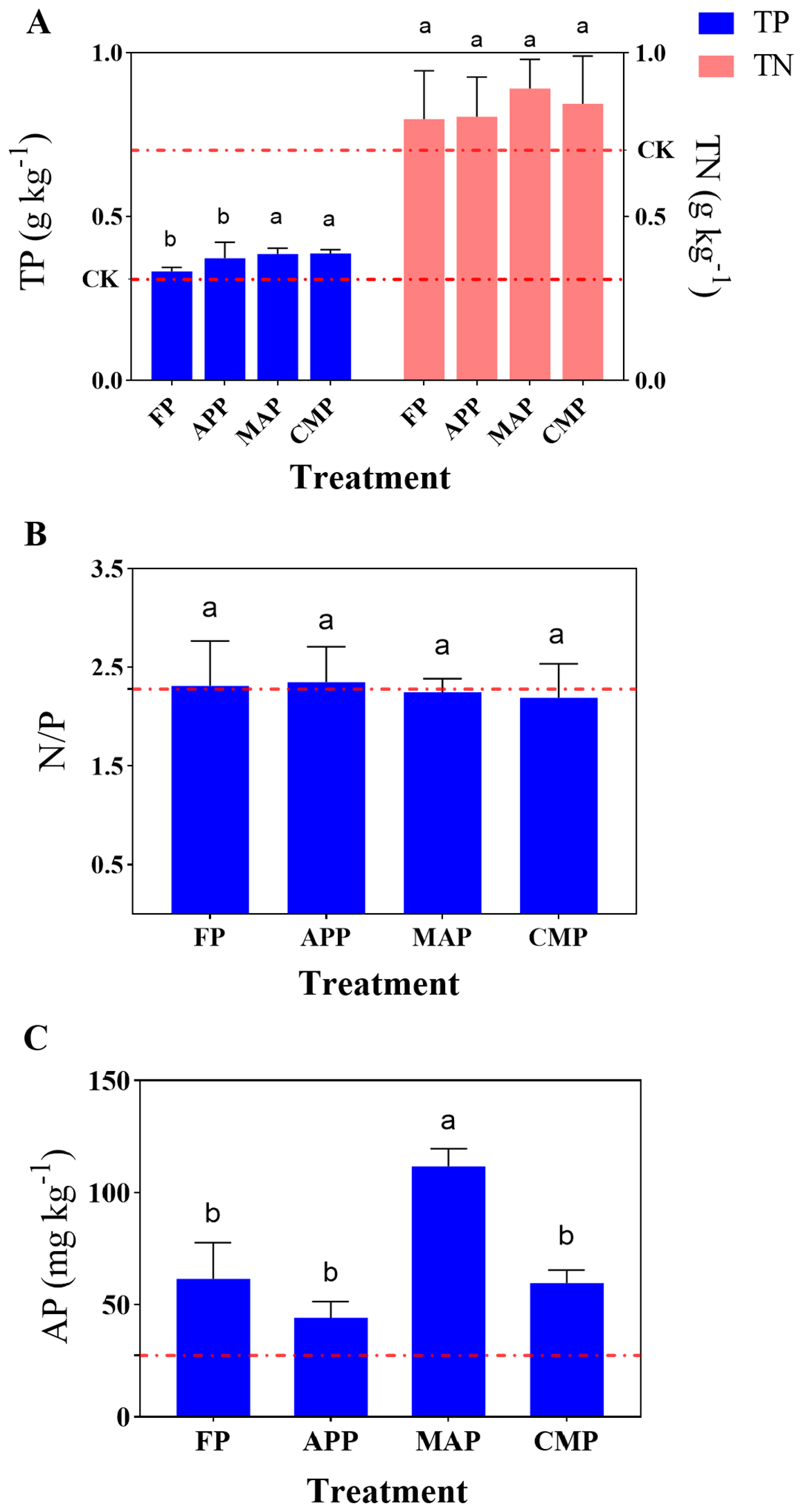
Effects of different P fertilizers on TN & TP (A), N/P ratio (B), and AP (C) content in soil. Error bars represent SD values of the means (n=3). Different lowercase letters indicate significant differences (P < 0.05) among different P fertilizer treatments; the control treatment was marked with a red dashed line, the same below.
The TP content in maize straw under the MAP treatment differed significantly from that of the FP, and CMP groups, with increases of 33.78% (P < 0.0001), and 16.47% (P = 0.0054), respectively. Additionally, the maize straw of the APP and CMP groups exhibited 24.32% and 14.86% higher TP levels than that of the FP group. The TP content in maize grains followed the order APP > CMP > MAP > FP. In the root systems, compared to that of FP, MAP increased the TP content by 47.5% (P = 0.0002), while CMP showed a 6.25% reduction. Notably, FP exhibited a 2.44% decrease in TP content relative to CK (Figure 2A). The TN content in maize straw showed a 12.61% increase under the MAP treatment compared to FP, while CMP demonstrated a 2.80% elevation relative to that of the FP treatment. In the root systems, FP exhibited a 55.58% higher TN content than that of the APP treatment (P < 0.0001) (Figure 2B). Comparative analysis of nutrient accumulation across different plant parts revealed a consistent pattern for both TN and TP: grains > straw > roots.
Figure 2
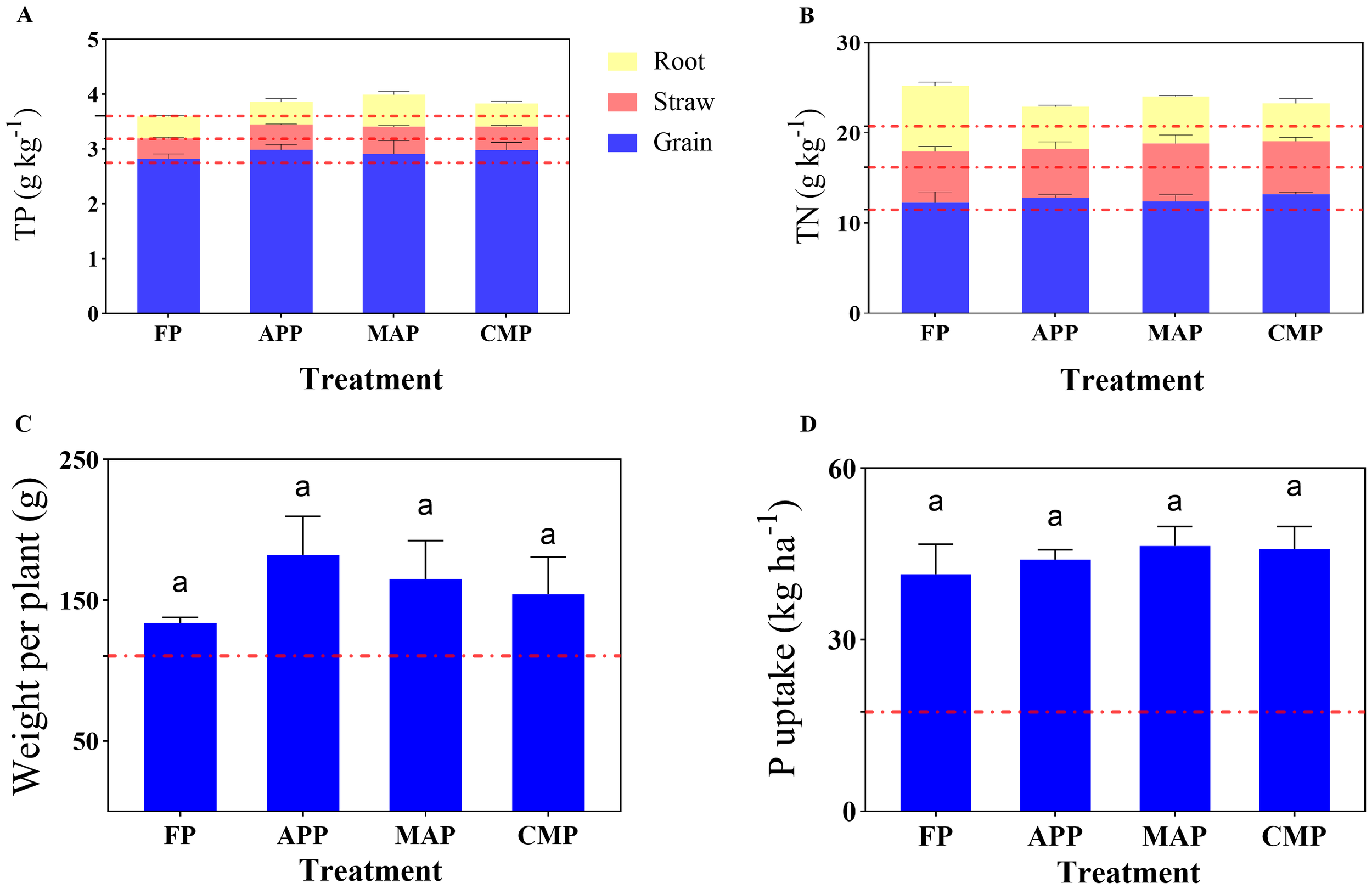
Effects of different P fertilizers on TP (A) and TN (B) contents in various maize plant parts, as well as shoot dry weight per plant (C) and P uptake per plant (D).
At maturity, although the APP treatment resulted in a 36.07% increase in dry weight compared to that under FP, the difference was not statistically significant. Similarly, MAP increased the dry weight by 23.23% relative to that of the FP condition, but neither difference reached statistical significance (Figure 2C). All four P-fertilizer treatments resulted in significantly higher total P uptake (calculated as the sum of P uptake in the grains and straw) compared to that of the CK control group. Specifically, APP, MAP, and CMP showed increases of 6.08%, 11.91%, and 10.54% in total P uptake, respectively, compared to that of the FP treatment (Figure 2D).
3.2 Maize yield and P-efficiency parameters
Integration of the 8-year yield data showed substantial interannual variability in maize production. The yield decline in 2019 was primarily attributed to typhoon-induced lodging. Due to the potential influence of the 2019 typhoon on yield measurements—which may have introduced anomalies—the comprehensive yield variability after excluding 2019 (presented in Supplementary Figure S2). Both the original and adjusted datasets exhibit identical trends. Annual yields clustered within the range of 8,000–12,000 kg ha-1 during 2017 and 2018, but reached higher values of 10,000–14,000 kg/ha in 2023 and 2024. Collectively, the dataset demonstrates a general upward yield trend across the observation period (Figure 3A). The mean annual yields across treatments ranked in descending order as follows: MAP (11,859 kg ha-1), CMP (11,691 kg ha-1), FP (11,110 kg ha-1), APP (11,086 kg ha-1), and CK (8,924 kg ha-1). All four P-fertilized treatments showed significantly higher yields than the non-fertilized treatment (CK) (P < 0.01). Specifically, MAP and CMP exhibited 6.74% and 5.23% increases relative to FP, respectively, although these differences were not statistically significant (Figure 3B).
Figure 3
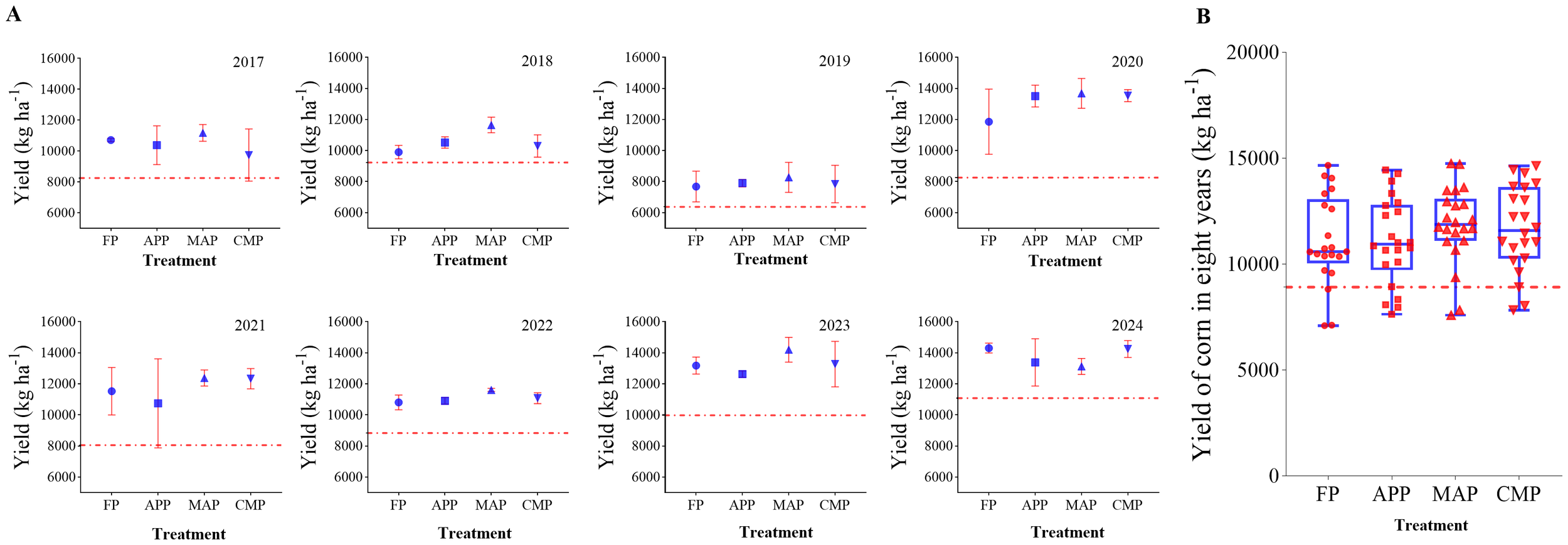
Yield differences among treatments during 2017-2024 (A) and average yield of all treatments (B).
The starter fertilizers demonstrated superior P-efficiency indices compared to FP. Specifically, MAP exhibited the highest PFP and AEP, showing 4.07% and 16% increases over those of FP, respectively. Furthermore, long-term application of starter fertilizers (APP, MAP, CMP) resulted in a lower soil P surplus than that of the FP treatment, with reductions of 7.52%, 14.74%, and 13.04%, respectively. Moreover, the PUE increased by 19.43%, 38.06%, and 33.68% in the APP, MAP, and CMP treatments compared to that of the FP group (Table 2). These results demonstrate that under equivalent P application rates, the addition of starter fertilizers enhances PUE and reduces the soil P surplus.
Table 2
| Items | FP | APP | MAP | CMP |
|---|---|---|---|---|
| PUE (%) | 17.34 ± 7.00 | 20.71 ± 2.38 | 23.94 ± 4.50 | 23.18 ± 5.31 |
| PFP (kg kg-1) | 178.41 ± 18.11 | 168.36 ± 2.00 | 185.68 ± 9.82 | 181.98 ± 16.29 |
| AEP(%) | 45.38 ± 18.11 | 35.32 ± 2.00 | 52.64 ± 9.82 | 48.94 ± 16.29 |
| P Surplus (kg ha-1) | 33.52 ± 5.25 | 31.00 ± 1.78 | 28.58 ± 3.38 | 29.15 ± 3.98 |
Response of P-nutrient efficiency indicators to P fertilizer types and application methods.
3.3 Microbial community structure and composition
Alpha-diversity analysis revealed significant differences among P-fertilization methods and types. In the bacterial communities of the phosphorus-amended treatments, the Shannon index of the MAP treatment was comparable to that of the CK control, whereas the FP treatment exhibited the lowest value. Specifically, the Shannon index of MAP was 2.27% higher than that of FP and 2.09% higher than APP. The CMP treatment showed a 1.05% increase over FP (Figure 4A). For the Chao index, all phosphorus-amended treatments were lower than the non-phosphorus control (CK). Among these, APP had the highest Chao index, exceeding MAP by 3.17% and FP by 3.02% (Figure 4B). In the fungal communities, MAP displayed the highest Shannon index, which was 27.62% greater than that of CMP and 12.56% higher than FP (Figure 4C). The Chao index of APP was 11.03% higher than CMP, while MAP exceeded CMP by 7.90% (Figure 4D).
Figure 4
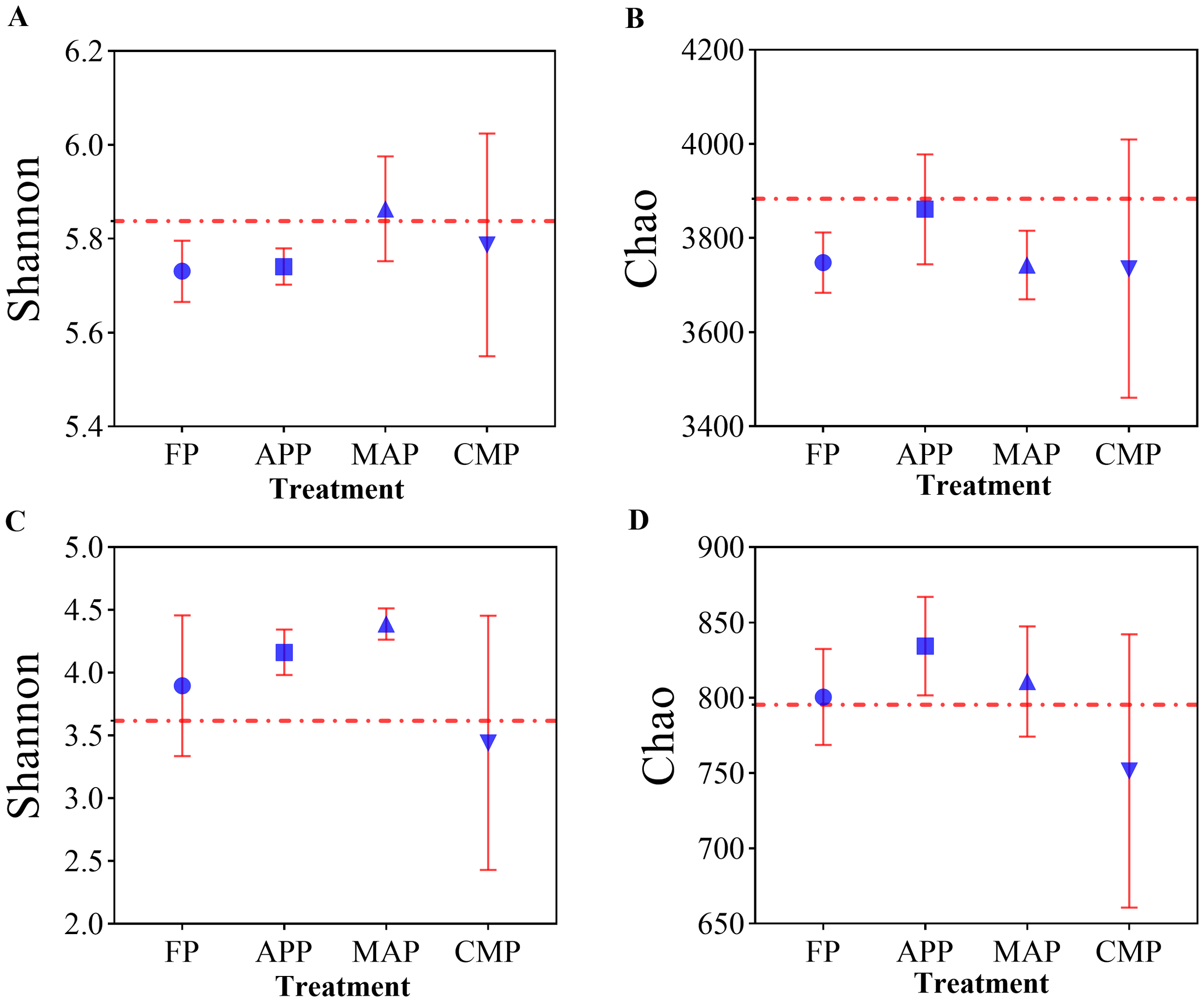
Effects of different P fertilizers on the diversity of soil bacterial (A, B) and fungal (C, D) communities.
Beta-diversity of soil fungal and bacterial communities was assessed by calculating Bray–Curtis dissimilarity matrices and the results were visualized using principal coordinate analysis (PCoA), with the first two axes (PCoA1 and PCoA2) explaining 26.33% and 20.5% (fungal communities, Figure 5A) or 38.04% and 19.55% (bacterial communities, Figure 5B) of the total variance, respectively. Fungal communities formed discrete clusters, reflecting their distinct compositional profiles, whereas bacterial communities exhibited pronounced clustering patterns, indicating partial community restructuring. PERMANOVA further revealed significant differences in β-diversity among treatments for fungal communities (F = 1.56, P = 0.017), with relatively independent distribution patterns across fertilization regimes. In contrast, bacterial communities showed no significant β-diversity variation among treatments (F = 0.84, P = 0.677).
Figure 5
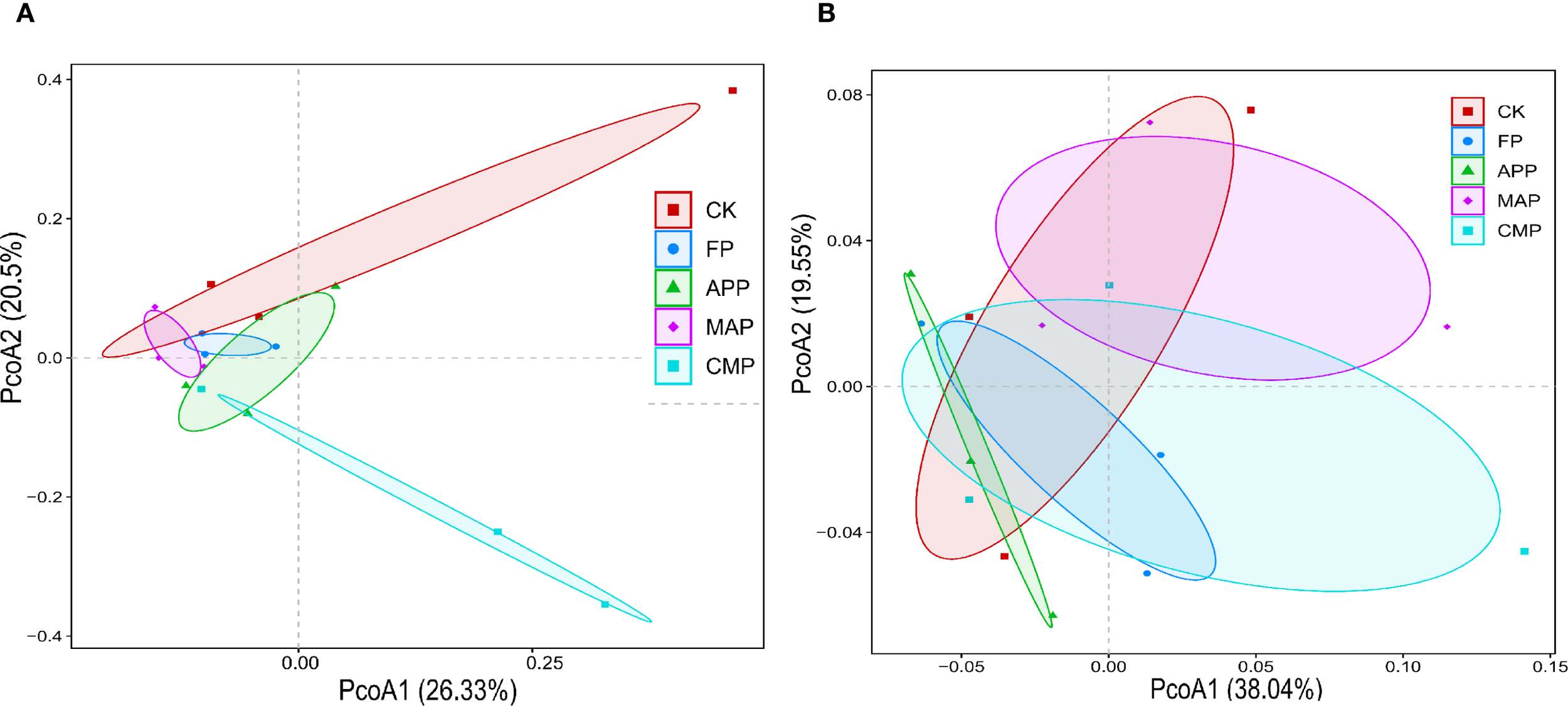
Effects of different treatments on the composition of soil fungal (A) and bacterial (B) communities.
Excluding unclassified phyla, the top 10 most abundant bacterial and fungal phyla across all soil samples were selected to investigate the impacts of different P application regimes on soil microbiological communities. In the FP, APP, MAP, and CMP treatments, the phyla Ascomycota, Basidiomycota, and Mortierellomycota accounted for 79.52%, 92.66%, 90.98%, and 84.27% of the total relative abundance, respectively. Both APP and MAP significantly enhanced the relative abundance of Ascomycota by 20.34% and 29.52%, respectively, compared to that detected under the FP treatment. Concurrently, APP and CMP also increased the relative abundance of Basidiomycota by 11.95% and 37.54%, respectively. The dominant bacterial phyla across all soil samples were Proteobacteria, Acidobacteriota, and Planctomycetota, collectively accounting for 71.85%, 70.67%, 70.06%, and 71.43% of the total relative abundance in the FP, APP, MAP, and CMP treatments, respectively. Notably, MAP significantly increased the relative abundance of Acidobacteriota by 16.82% compared to that under FP (P < 0.05), while CMP showed a 5.72% increase in Planctomycetota abundance relative to that of the FP treatment (Figure 6A).
Figure 6
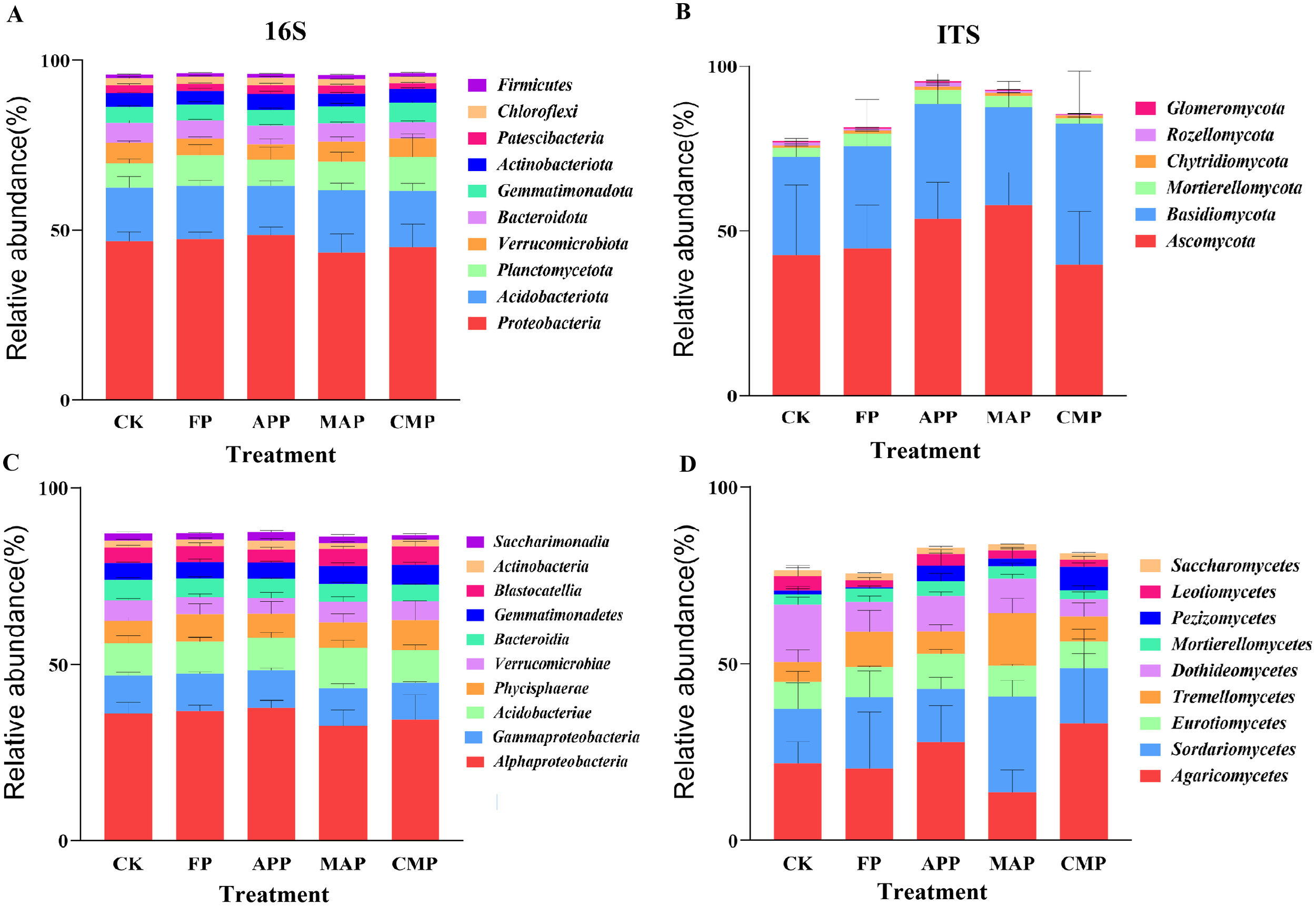
Relative abundance of dominant species at the phylum (A, B) and class (C, D) taxonomic level.
The dominant fungal classes across all soil samples were consistently Agaricomycetes, Sordariomycetes, and Eurotiomycetes, collectively representing 49.00%, 52.78%, 49.50%, and 56.20% of the total relative abundance in the FP, APP, MAP, and CMP treatments, respectively. Notably, compared to FP, MAP induced the greatest increases in relative abundance for both Sordariomycetes (34.53%) and Tremellomycetes (48.16%) (Figure 6D). Sordariomycetes, a phylogenetically significant fungal class within Ascomycota, plays dual ecological roles as key decomposers and potential pathogens. In the soil bacterial communities, Acidobacteriae exhibited a 23.13% increase, whereas Alphaproteobacteria showed an 11.22% reduction under the MAP treatment compared to FP. Integrated analysis of the relative abundance stacked plots revealed that different P-fertilization treatments exerted only a minimal influence on the composition of dominant microbial taxa at both the phylum and class levels. However, significant variations were observed in the relative abundance proportions of these taxa. Notably, fungal communities demonstrated greater sensitivity to P fertilizers and application methods than bacterial communities.
To investigate the interactions between P fertilizers and soil microbial communities, based on our observations of the relatively greater efficacy of the MAP treatment in enhancing maize yield, we conducted STAMP analysis to compare genus-level relative abundance differences between the MAP and CK treatments. Statistically significant differential taxa were identified through this analysis, along with their environmental preference trends. At the genus level, bacterial communities exhibited significant differences across all detected taxa between MAP and CK treatments (Figure 7A), whereas fungal communities showed limited differences in abundance between these two treatments. This demonstrates that MAP fertilization exerts stronger selective pressure on the bacterial community structure than on fungal assemblages. Notably, the fungal genus Tausonia exhibited significantly higher relative abundance in MAP soil compared to CK soil (P = 0.046) (Figure 7B).
Figure 7
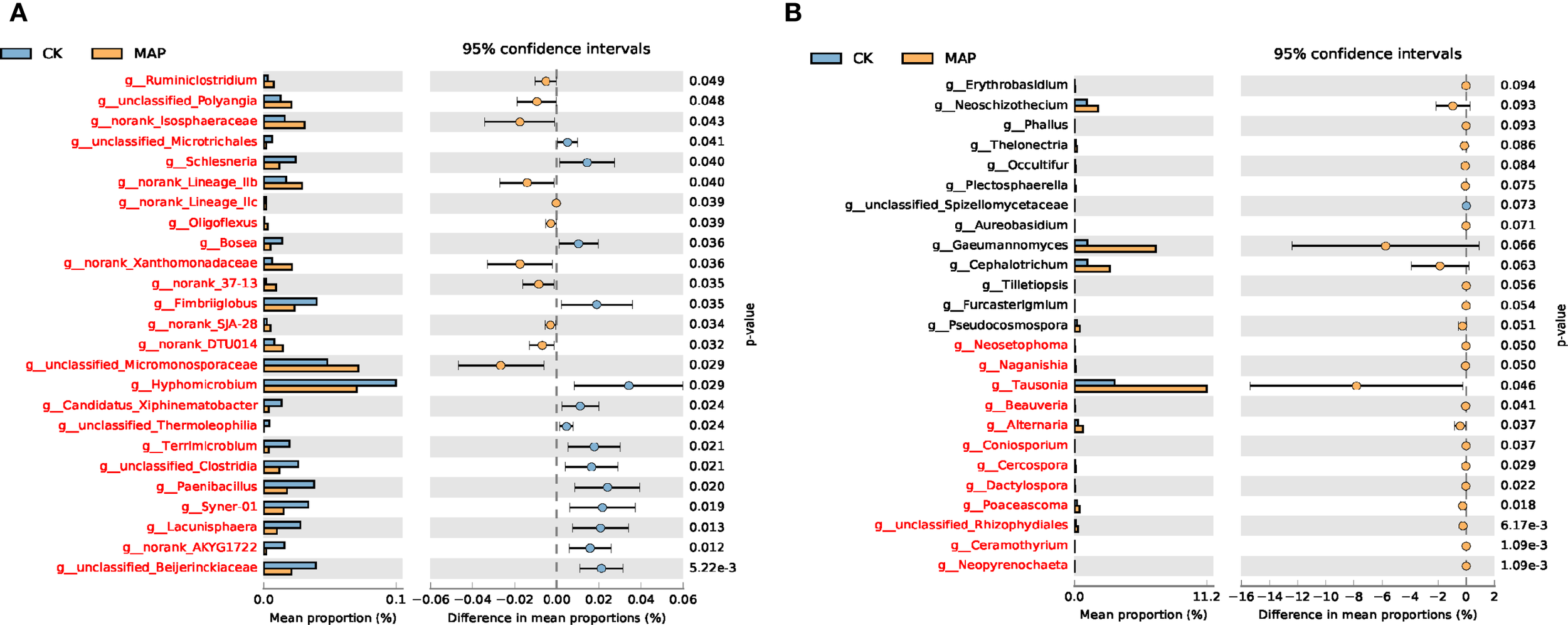
Comparative analysis of dominant genera-level taxa in bacterial (A) and fungal (B) communities based on relative abundance.
Correlation analysis between dominant soil microbial genera (bacteria and fungi) and soil/plant nutrient parameters revealed key functional relationships with crop yield. Tausonia exhibited significant positive correlations with AP (P = 0.003) and TP-R (P = 0.012); the fungal genus Deconica showed significant negative correlations with TN-S (P = 0.043), maize yield (P = 0.015), and P-uptake (P < 0.0001); the fungal genus Gaeumannomyces demonstrated a significant positive correlation with TP-R (P = 0.002); and the bacterial lineage norank_Acidobacteriales exhibited a significant positive correlation with TP-R (P = 0.006) (Figure 8)).
Figure 8
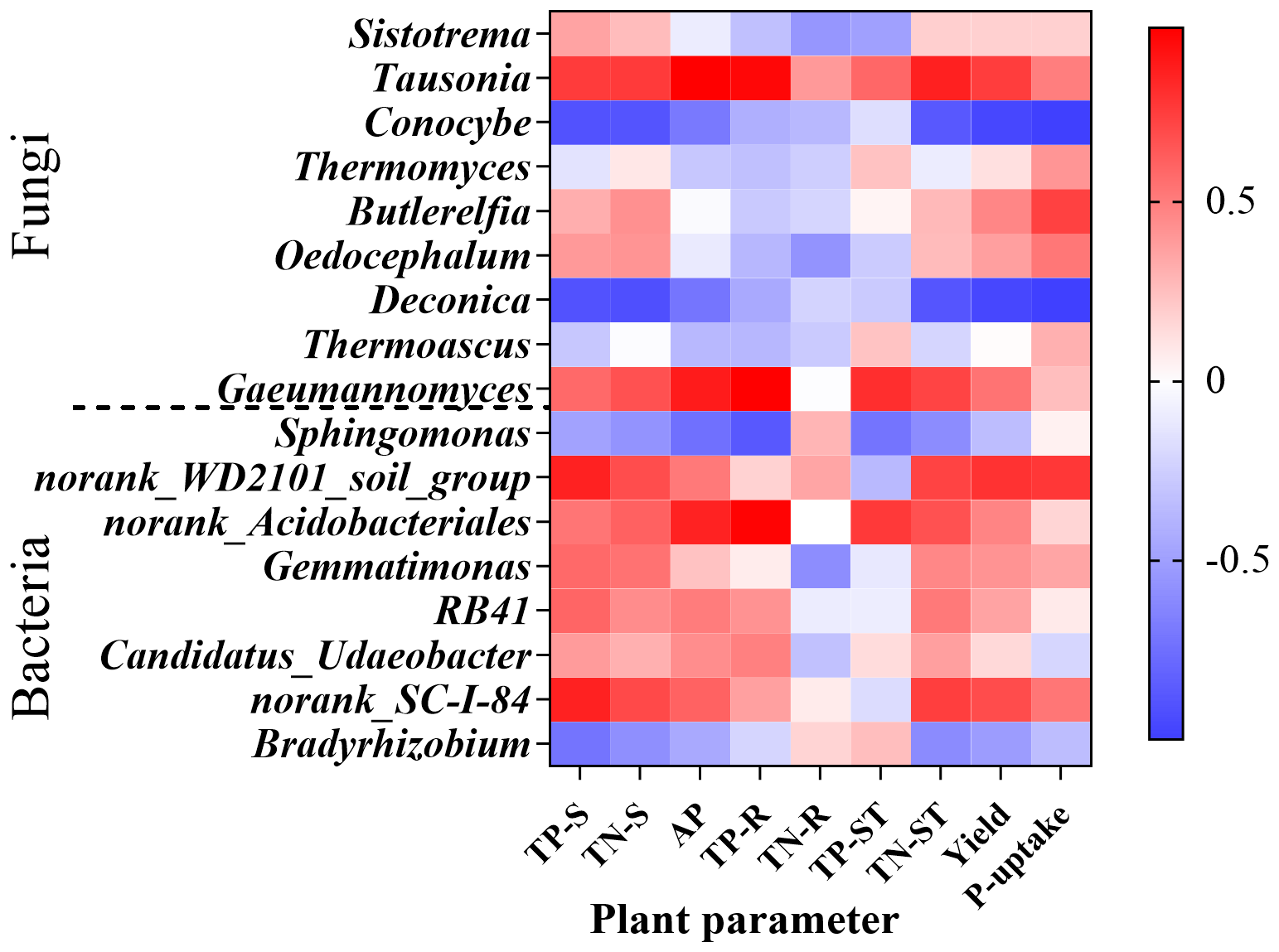
Heatmap of genus-level soil microbial correlations with edaphic and plant parameters. AP, soil Olsen-P content; TP-S, the total phosphorus of soil; TN-S, the total nitrogen of soil; TP-R, the total phosphorus of root; TN-R, the total nitrogen of root; TP-ST, the total phosphorus of straw; TN-ST, the total nitrogen of straw. The same below.
3.4 Soil microbial functional prediction and P-cycling functional genes
To identify distinct functional groups within rhizosphere soil fungal communities and link their relative abundances to different P-application methods and types, we classified fungal operational taxonomic units into specific trophic groups, which were then further subdivided into defined ecological guilds. Using the FUNGuild framework, soil fungal communities were primarily categorized into three major trophic modes: saprotrophy, symbiotrophy, and pathotrophy. Based on these trophic classifications, fungi were additionally assigned to multiple ecological guilds (e.g., animal pathogens, endophytes, mycorrhizal fungi).
Overall, the groups “Undefined Saprotrophs” and “Plant Pathogens” exhibited high relative abundances across all treatments. Among these, the MAP treatment displayed the highest values: Undefined Saprotrophs showed increases of 32.32% and 65.01% relative to the abundance of this group in the CK and FP conditions, respectively. In contrast, Plant Pathogens displayed a marked increase, with relative abundances rising by 440.78% and 432.43% relative to those under CK (P = 0.0406) and APP (P = 0.0412), respectively (Figure 9). These findings suggest that MAP strengthens the functional role of saprotrophic fungi within the fungal community, exhibiting superior efficiency in organic matter decomposition and nutrient cycling compared to other P fertilizers. However, this treatment also elevated the relative abundance of plant pathogens, posing a potential risk of plant disease.
Figure 9
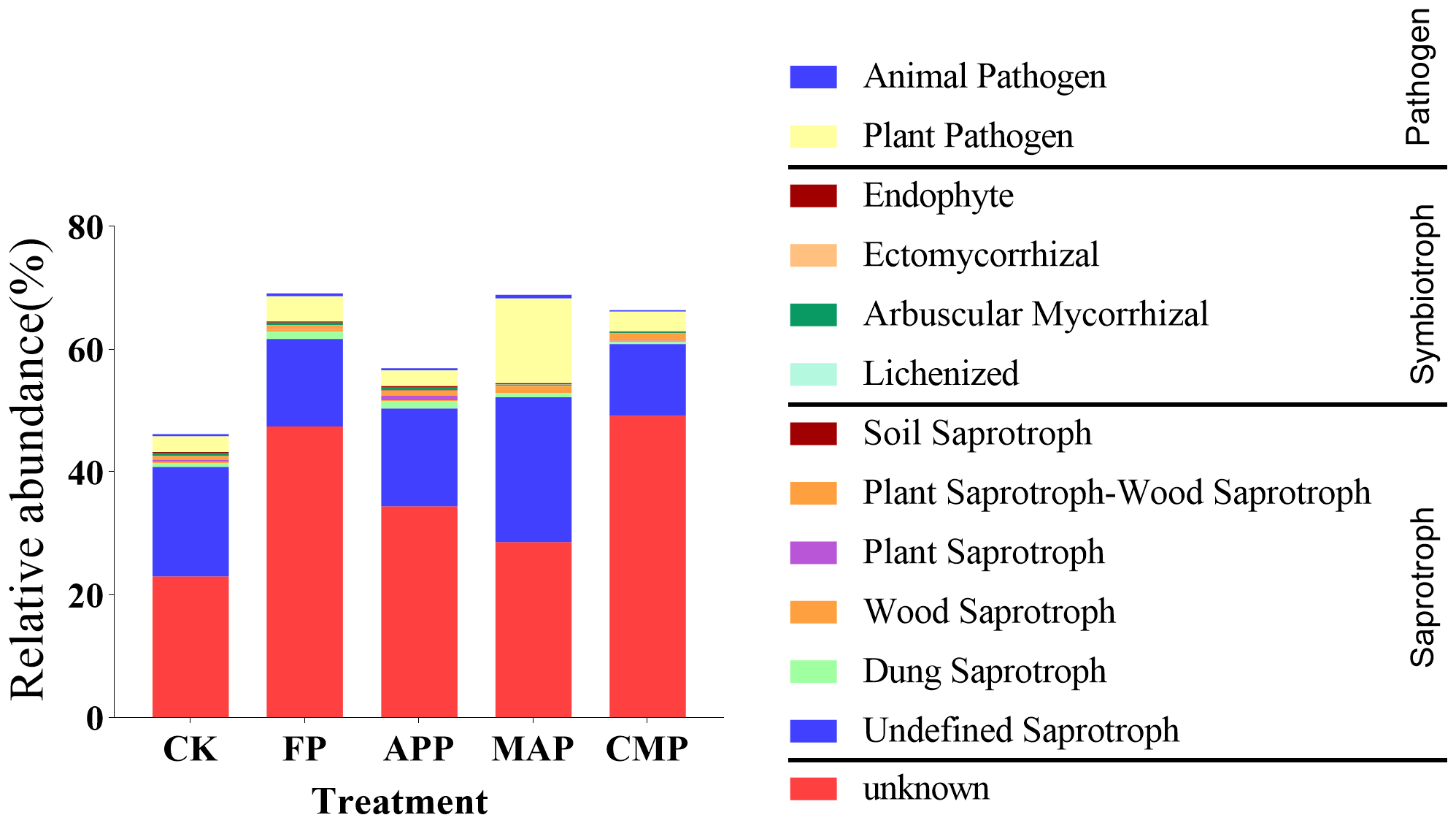
Functional classification of fungal communities under different P fertilization treatments.
The abundance of Undefined Saprotrophs showed a positive correlation with TP-ST (P = 0.045). The abundance of the Lichenized group exhibited positive correlations with TP-R (P = 0.037) and TP-ST (P = 0.024). Arbuscular Mycorrhizal fungi were negatively correlated with TN-S (P = 0.044) and TP-S (P = 0.037). Ectomycorrhizal fungi demonstrated a positive correlation with TP-R (P = 0.032), while Plant Pathogens were positively associated with AP (P = 0.026) and TP-R (P < 0.0001) (Figure 10).
Figure 10
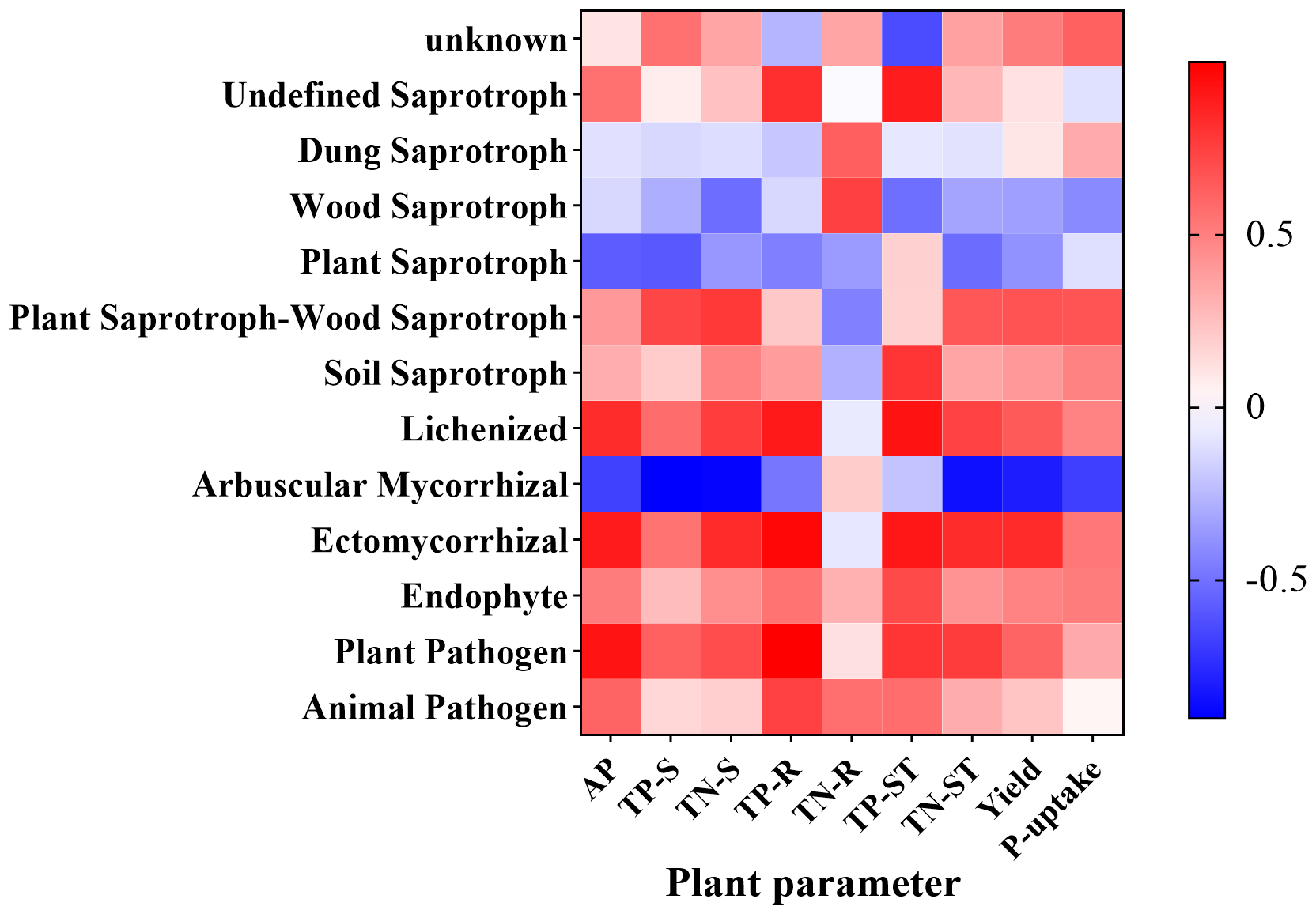
Heatmap illustrating the correlations between soil fungal functions and various indicators.
Based on previous studies, a total of 50 soil P cycling-related genes and their corresponding Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthology (KO) annotations were selected (Dai et al., 2019; Hsieh and Wanner, 2010; Liu et al., 2023b; Mahmood et al., 2020; McGrath et al., 2013). Following the functional aggregation of P cycling-related genes, our predictive data analysis revealed that long-term spatially stratified P inputs may exert a negligible impact on the total relative abundance of genes involved in the four functional categories. However, genes associated with the P-uptake and transport system consistently represented the highest proportion among all functional categories (Figure 11A). To further explore the differential abundances of P-cycling genes among different P-fertilizer treatments, our visualization analysis revealed that pstS, phoR, ppx, gph, and ugpQ displayed the highest relative abundances in soil samples. Relative to that of the CK treatment, it is plausible that MAP enhanced the relative abundance of phoB1—a gene involved in PSR regulation—by 6.98%, while reducing the abundance of phnA—which functions in Pi solubilization—by 5.71%. Compared to FP, MAP further resulted in a 1.35% increase in the relative abundance of phoU, a key constituent of the PSR regulatory system. MAP application may enhance the relative abundance of genes encoding enzymes involved in Po mineralization, including gph, appA, and phoD, with their relative abundances increasing by 2.67%, 10.98%, and 6.90%, respectively, compared to those under the FP condition (Figures 11B–D). The gene ugpQ, which also encodes enzymes involved in Po mineralization, displayed a 2.70% reduction in relative abundance under the FP treatment relative to CK. Compared to that in the FP treatment, our predictive data analysis suggests that MAP and CMP could elevate the relative abundance of ptsI by 3.12% and 9.38%, respectively (Figure 11D). Concerning genes associated with P uptake and transport, CMP may decrease the relative abundance of the low-affinity phosphate (Pi) transporter gene (pit) by 0.32% compared to that found in the FP group (Figure 11B).
Figure 11
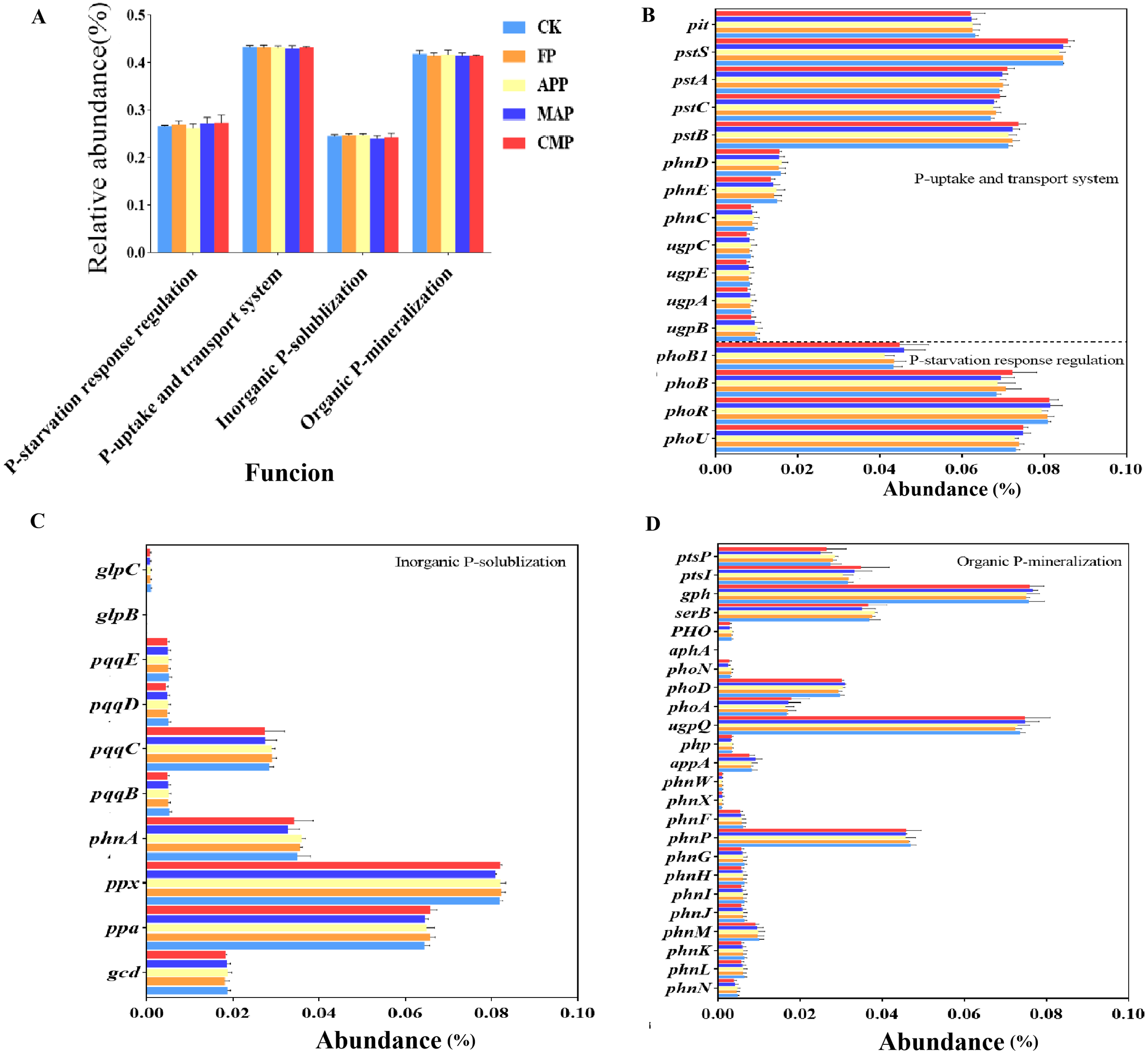
Relative abundance of representative P-cycling related genes in bacterial communities under different P fertilizer treatments.
At the phylum level of fungi, Mortierellomycota showed a significant negative correlation with pstS (P = 0.029), while Rozellomycota exhibited significant negative correlations with phoR (P = 0.046), pstS (P = 0.025), and phoA (P = 0.022). Glomeromycota demonstrated significant negative correlations with phoU (P = 0.049) and phnX (P = 0.022) but had significant positive correlations with pit (P = 0.025) and phnN (P = 0.012). At the bacterial phylum level, Proteobacteria displayed significant positive correlations with phnA (P = 0.002), php (P = 0.036), phoN (P = 0.001), and ptsP (P = 0.001) but exhibited significant negative correlations with phoU (P = 0.044), ugpQ (P = 0.048), and gph (P = 0.023). Acidobacteria showed significant negative correlations with phnA (P = 0.003), php (P = 0.009), phoN (P = 0.007), and ptsP (P = 0.006), whereas there was a significant positive correlation with gph (P = 0.033). Planctomycetota exhibited significant negative correlations with ugpB (P = 0.021), ugpA (P = 0.009), phnC (P = 0.004), pit (P = 0.045), and phnN (P = 0.034) but significant positive correlations with phoA (P = 0.042), pstA (P = 0.001), pstB (P = 0.005) and phoB (P = 0.003). Bacteroidota demonstrated significant positive correlations with ugpB (P = 0.017), ugpA (P = 0.011), phnC (P = 0.013), pit (P = 0.018), and phnN (P = 0.016) but significant negative correlations with pstA (P = 0.007), pstB (P = 0.005), and phoB (P = 0.02). Gemmatimonadota exhibited significant negative correlations with ugpA (P = 0.027), ugpB (P = 0.012), and phnN (P = 0.018), while showing significant positive correlations with pstB (P = 0.029), phoA (P = 0.026), and ptsI (P = 0.009). Actinobacteriota displayed a significant negative correlation with phoR (P = 0.01). Patescibacteria showed significant negative correlations with phoB (P = 0.023), pstB (P = 0.036), pstA (P = 0.028), pstS (P = 0.014), and phoA (P = 0.028). Firmicutes was significantly positively correlated with gcd (P = 0.038) (Figure 12).
Figure 12
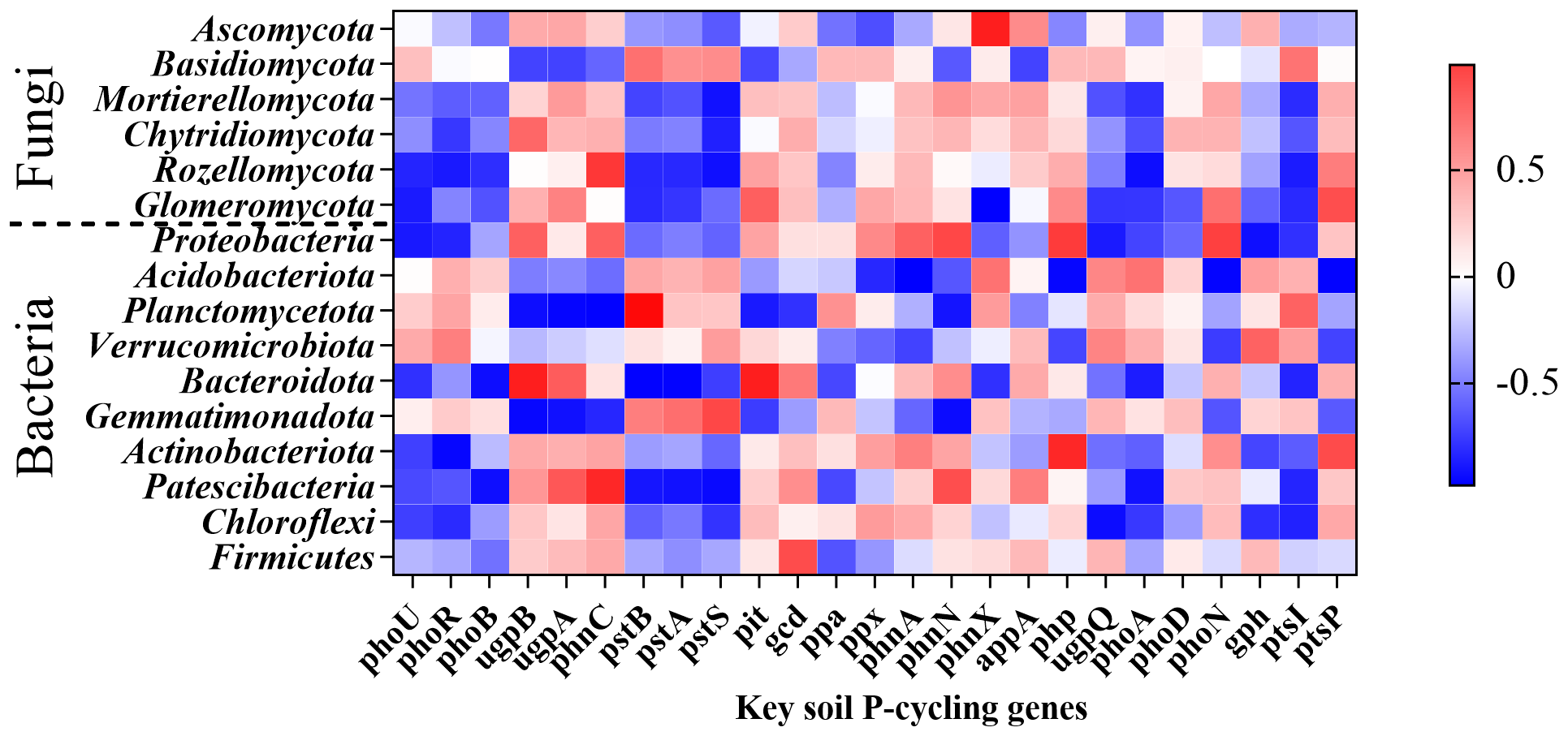
The relationship between major rhizosphere soil microbial taxa (bacteria, fungi) and the relative abundance of key soil functional genes (P-cycling genes).
3.5 Effects of P fertilization on soil properties, plant growth, rhizosphere microbial community structure and function, and maize yield
Partial least-squares path modeling analysis was applied to investigate both direct and indirect relationships among P-fertilizer types, microbial community structure, soil physical properties, AP, functional gene prediction, and maize yield, accounting for 76% of the total variation in maize yield (Figure 13). Long-term spatially stratified P fertilization significantly increased maize yield, primarily through its influence on the rhizosphere soil microbial community structure and AP content. Notably, the microbial community structure exerted a positive effect on yield, whereas functional gene prediction showed a non-significant negative association with yield.
Figure 13
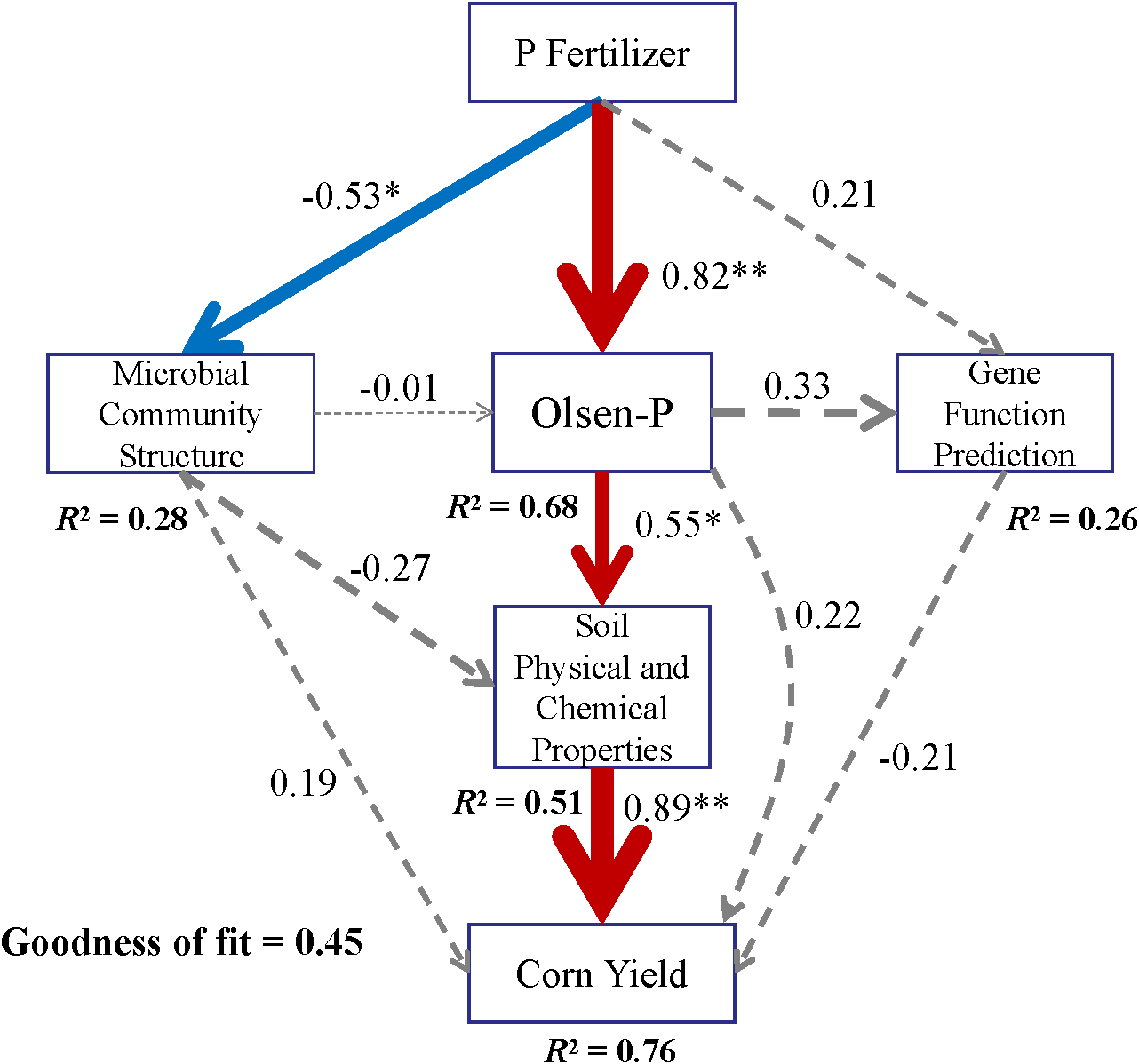
PLS-PM analysis showing the direct and indirect factors affecting maize yield under different P fertilizer applications. *P < 0.05, **P < 0.01.
4 Discussion
4.1 Impact of P-fertilizer application on soil microbial community structure and diversity
PCoA revealed significant differences in soil microbial β-diversity, demonstrating that the fungal community structure exhibited relative independence across different P-fertilizer treatments (P < 0.05), whereas bacterial communities showed no significant response to fertilizer type. This finding contrasts with several studies reporting significant structural changes in both bacterial and fungal communities under long-term P fertilization in grassland ecosystems (Leff et al., 2015). For example, long-term P-fertilizer application significantly altered the bacterial community structure in wheat field trials conducted in Shaanxi Province, China (Liu et al., 2023a). However, other studies have reported no significant changes in microbial community structure following P-fertilizer application (Hamel et al., 2006; Liu et al., 2012). The absence of significant differences in bacterial community structure may be attributed to either insufficient P supply levels to trigger microenvironmental changes or an inadequate duration of fertilizer application. Furthermore, this study specifically focused on the ultimate impact of long-term stratified fertilization on the microbial community, rather than tracking its temporal dynamics.
Long-term P fertilization exhibited minimal effects on the relative abundance of bacterial and fungal communities at both the phylum and class levels, although the dominant fungal phyla demonstrated greater responsiveness than their bacterial counterparts. This differential sensitivity stems from the inherently higher susceptibility of fungi to soil property alterations and P inputs compared to bacteria (He et al., 2008; Li et al., 2015). Fungal hyphae typically form extensive networks within soil systems, significantly increasing the surface area available for nutrient acquisition and thereby enhancing their capacity to access soil AP compared to other soil microbiota (Smith and Smith, 2011). P-fertilizer application induced significant restructuring of rhizosphere microbial communities (Figures 5, 7), particularly enhancing the complexity and stability of soil fungal assemblages. These modifications would in turn amplify plant–microbe interactions, demonstrating the indispensable role of rhizosphere microbiota in sustaining crop productivity.
4.2 Impact of P-fertilizer application on soil functional genes
Investigating the effects of long-term P fertilization on soil functional genes is critical, as C-, N-, and P-cycling genes typically dominate the rhizosphere soil microbiome and play pivotal roles in mediating plant traits and soil properties (Guo et al., 2021). Among agricultural practices, P-fertilizer application has been shown to significantly alter both the abundance and composition of P-cycling functional genes (Lang et al., 2021). Our results demonstrate that spatially stratified MAP application not only significantly increased the relative abundance of plant pathogens within soil fungal functional communities but also enhanced the relative abundance of undefined saprotrophs, indicating the capacity of MAP to improve the fungal community’s competence in litter decomposition and organic matter cycling. Correlation analyses further revealed significant positive relationships of plant pathogens with both AP and TP-R and of undefined saprotrophs with TP-ST. These mechanistic insights explain the superior maize yield enhancement observed under MAP compared to other treatments. Plant pathogens are a primary driver of global agricultural crop losses, as they infect a wide range of host plants and cause severe ecological and economic damage (De Silva et al., 2019; Jayawardena et al., 2021). Integrated management strategies are advocated to mitigate pathogen proliferation, including the application of novel bio-based fungicides (Serrano et al., 2025), crop rotation with non-host species, and optimized irrigation and so on. Notably, fertilization acts as a double-edged sword: while it enhances crop yields, it also elevates soil health risks.
Overall, P-fertilizer application suppressed the abundance of key P-cycling functional genes, including the Pi transporter (pit), P transport system permease protein (phnE), quinoprotein glucose dehydrogenase (gcd), P phosphodiesterase (phnP), and triphosphate diphosphatase (phnM) genes. This observed downregulation aligns with established patterns of reduced soil P-cycling gene abundance under long-term P supplementation (Bergkemper et al., 2016; Su et al., 2015). Furthermore, a significant positive correlation was observed between gph and Acidobacteria abundance (Figure 12). MAP exhibited the highest relative abundance of Acidobacteria at 18.38% (Figure 6). Notably, norank_Acidobacteriales, an Acidobacteria subgroup yet to be classified at the genus level, showed a significant positive correlation with TP-R (Figure 8). This is a particularly relevant finding, as the MAP treatment resulted in significantly higher TP-R compared to that under FP (Figure 2). These findings collectively suggest that gph enhances the root’s P uptake capacity. This interpretation is further supported by the 7.67% higher relative abundance of gph in MAP versus other treatments (Figure 11), providing mechanistic evidence for MAP’s promotion of root P accumulation through gph-mediated processes. Sphingomonas (affiliated with the phylum Proteobacteria) exhibited significant negative correlations with soil N, P, AP, TN-S, and TP-S (Figure 8). Concurrently, phoU showed a significant negative correlation with Proteobacteria (Figure 12). MAP demonstrated the lowest Proteobacteria relative abundance (43.20%, Figure 6), while exhibiting the highest phoU abundance among treatments (Figure 11). These findings collectively suggest that phoU plays a role in enhancing soil–plant nutrient translocation efficiency. However, the role of this gene in nutrient uptake rate remains unclear, and the specific mechanisms require further investigation. Similarly, phnA, a key gene encoding organophosphorus-degrading enzymes, displayed contrasting correlations: a significant positive correlation was found with Proteobacteria but a negative correlation was found with Acidobacteria (Figure 12). Notably, MAP treatment showed the lowest phnA abundance (3.27%, Figure 11), indicating that the nutrient enrichment mechanism of MAP involves suppression of phnA-mediated P mineralization pathways.
Nevertheless, the total abundance of bacterial functional genes showed little variation with fertilization regimes, which differs from previous reports indicating that P-fertilizer application reduced the abundance of P-cycling genes involved in PSR regulation (phoR and phoP) as well as P-uptake and transport (Dai et al., 2019; Hsieh and Wanner, 2010; Ikoyi et al., 2018). This discrepancy may be attributed to the fact that the applied P level in the present experiment was insufficient to significantly alter the relative abundance of functional genes. Alternatively, this discrepancy may arise from the inherent limitations of PICRUSt2 as a predictive tool for microbial metabolic functions, which suggests that metagenomic sequencing techniques are necessary to improve the accuracy and reliability of these findings (Niewiadomska et al., 2020).
4.3 The relationship between P-fertilizer application and functional microorganisms
Keystone taxa within microbial communities, irrespective of their relative abundance, play pivotal roles in shaping both community assembly and ecosystem functioning (Banerjee et al., 2018). Notably, Proteobacteriaand Actinobacteriaare key players in phosphorus solubilization and mineralization processes, which enhance the decomposition of soil organic matter and substantially contribute to terrestrial phosphorus cycling (Dai et al., 2020; Ragot et al., 2017; Sebastian and Ammerman, 2011). In our study, these two bacterial phyla exhibited significant positive and negative correlations with genes encoding Pi solubilization (phnA) and Po mineralization (phoN, gph, php, ptsP). Nevertheless, current research on phosphorus-associated fungal functional groups remains limited and warrants further refinement. Collectively, our findings align with previous studies (Fan et al., 2020; Ratliff and Fisk, 2016), confirming that dominant microbial groups in rhizosphere soil are intimately linked to soil functional gene networks.
Tausonia exhibited a significant positive correlation with AP. As a saprophytic ascomycete, it promotes plant nutrient uptake by decomposing organic matter (Shi et al., 2024), likely via the secretion of organic acids and phosphatases to solubilize insoluble Pi (e.g., calcium phosphate, iron phosphate) and mineralize Po (Sharma et al., 2013). This process may increase soil AP content and enhance plant P uptake, though this hypothesis remains to be validated and warrants further investigation into the underlying mechanisms. Additionally, STAMP analysis demonstrated that MAP significantly elevated the relative abundance of Tausonia. This finding further supports the notion that the MAP treatment’s notable increase in soil AP content underpins its superior performance in yield improvement.
Saprotrophs are organisms that derive energy and nutrients by decomposing dead organic matter via the secretion of extracellular enzymes, playing a pivotal role in nutrient cycling (Gil-Martínez et al., 2018). However, current research on P-associated fungal functional guilds remains limited, with taxonomic frameworks still incomplete. Nevertheless, our findings reveal that P fertilizer application enhances the abundance of Saprotrophic communities in soil, which not only strengthens soil nutrient cycling but also promotes crop root P uptake.
4.4 Integrated relationships among maize yield, soil physical properties, AP content, and microbial community structure
Excessive P application disrupts the soil nutrient balance. However, this study demonstrates that stratified fertilization not only helps maintain soil health-conducive microbial communities but also significantly enhances crop yield by increasing the soil AP content. By contrast, previous studies have reported a significant positive correlation between soil functional genes and plant productivity (Fan et al., 2020; Liu et al., 2023a). These discrepancies may stem from variations in cropping systems and soil environmental conditions. Alternatively, this could be attributed to differential mechanisms through which various P fertilizers promote yield increases, consequently leading to distinct effects on grain yield (Guo et al., 2025; Viceli et al., 2025). Furthermore, recent studies indicated that specific microbial taxa interact with soil physical properties and crop yield, which is inconsistent with the findings of this study. This discrepancy may stem from long-term fertilization-induced alterations in soil properties such as pH shifts and organic matter accumulation, which can significantly influence microbial community structure and function (Rousk et al., 2010). In conclusion, long-term reduced P application (commonly used P application rates in Northeast China are 100 kg P2O5 ha-1) can significantly modify the rhizosphere microbial community composition, effectively increase the soil AP content, and improve soil physical properties, ultimately achieving yield enhancement. These improvements are essential for enhancing soil fertility, promoting soil sustainability, and attaining high crop productivity.
4.5 Optimizing P-fertilizer management to coordinate soil microbial communities and functions
In our experimental results, the CK soil exhibited the highest bacterial and fungal diversity, while FP significantly reduced the bacterial species richness, indicating that improper P fertilization may disrupt microbial communities and functions (Lang et al., 2021). When comparing the relative abundance of microbial taxa across treatments, we observed that key taxa in the soil are not necessarily correlated with soil health. Although the spatially stratified MAP treatment did not significantly enhance microbial diversity, differential comparisons and correlation analysis revealed that the MAP-treated soil harbored distinct advantageous key taxa and functional compositions (e.g., Tausonia and Plant Pathogen). This resulted in a significantly higher Olsen-P content in this treatment compared to that detected for the other fertilizer groups, consequently demonstrating superior performance in increasing maize yield, improving P fertilizer agronomic efficiency, and reducing the soil P surplus relative to other P fertilizers. In contrast, conventional farmer fertilization practices (FP) at equivalent P application rates not only failed to maximize yield increases but also exacerbated P loss and compromised soil health. Previous studies have demonstrated that MAP contributes to improved crop yields. In no-till wheat-soybean cropping systems, the combined application of lime and MAP substantially enhanced crop yields. The AP content varies with different types of P fertilizer, and MAP typically outperforms other treatments in enhancing the soil AP content, which was confirmed in the present study. The MAP-treated soil exhibited an acidic pH (5.66), which is a key factor for enhancing P availability, leading to effective acidification of the rhizosphere environment (Ben Salah et al., 2022). In no-till wheat-soybean cropping systems, the combined application of lime and MAP significantly increased crop yield (Duart et al., 2024). MAP recruits specific microbial taxa to enhance microbial diversity, thereby improving plant biomass through pathways associated with nutrient cycling and energy metabolism. This mechanism further elucidates why MAP exhibits optimal yield-promoting effects under conditions of reduced phosphorus input.
5 Conclusion
This study provides the first comprehensive assessment of the systematic effects of long-term stratified P-fertilizer application on soil physical properties, plant nutrient content, microbial community structure, and functional genes in a maize cropping system. Stratified fertilization is an effective strategy for enhancing maize P uptake while minimizing soil P accumulation. Compared with the non-phosphorus treatment, MAP application alleviates fertilizer-induced disruptions to the soil microecological balance. This protective effect is primarily mediated through MAP’s regulation of P-cycling genes, including upregulating gph and phoU while downregulating phnA, which subsequently influences the abundance of dominant taxa such as Acidobacteria and Proteobacteria. These microbial community shifts enhance root nutrient acquisition, concurrently increasing crop yield, reducing the soil P surplus, and balancing P availability. Rational reduction of P input in soils with high Olsen-P levels can align crop P requirements for optimal growth with minimized soil P accumulation and conserved P resources, representing a promising intensive agricultural strategy for maize cropping systems. Furthermore, given the crucial role of soil microbial communities and functional diversity in sustaining maize productivity, greater attention should be directed toward understanding how P fertilizers influence soil microbiota and their ecological functions. These findings offer novel microbial perspectives to advance investigations into the mechanisms of soil–microbe interactions under P fertilization in the black soils of Northeast China, providing feasible approaches to enhance soil P transformation and improve PUE.
Statements
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: the NCBI Sequence Read Archive (SRA) repository (https://www.ncbi.nlm.nih.gov/) under accession numbers SAMN52820566, SAMN52820567, SAMN52820568, SAMN52820569, and SAMN52820570.4.
Author contributions
LH: Conceptualization, Funding acquisition, Supervision, Writing – original draft. BH: Data curation, Software, Writing – original draft. YW: Formal Analysis, Investigation, Writing – original draft. XW: Methodology, Resources, Writing – original draft. WS: Writing – review & editing. NC: Project administration, Visualization, Writing – review & editing. YZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the following funders, whose contributions enabled critical stages of the project: the Natural Science Foundation of Jilin Province (No. 20250102251JC) was used for article processing charges, the National Natural Science Foundation of China (No. 32272819) was applied to support study design, fieldwork, and data analysis, and the National Key Research and Development Program of China (No. 2023YFD15011014) was contributed for student research assistant stipends.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The authors verify and take full responsibility for the use of generative AI in the preparation of this manuscript. Generative AI was used to refine language clarity and consistency across technical descriptions (e.g., characterizing P-fertilizer regimes or microbial functional gene roles). All AI-generated content was rigorously reviewed, edited, and validated by the authors to ensure scientific accuracy, alignment with study objectives, and adherence to disciplinary standards. The authors retain exclusive responsibility for the final interpretation, conclusions, and integrity of the research presented.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1669876/full#supplementary-material
Supplementary Figure 1Schematic diagram of field experimental regimes.
Figure S2The average yield of all treatments from 2017 to 2024 (excluding data of 2019).
References
1
Banerjee S. Schlaeppi K. van der Heijden M. G. A. (2018). Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol.16, 567–576. doi: 10.1038/s41579-018-0024-1
2
Beauregard M. S. Hamel C. Atul N. St-Arnaud M. (2010). Long-term phosphorus fertilization impacts soil fungal and bacterial diversity but not AM fungal community in Alfalfa. Microb. Ecol.59, 379–389. doi: 10.1007/s00248-009-9583-z
3
Ben Salah Y. Oudadesse H. Lefeuvre B. Tounsi S. El Feki H. (2022). Purified monoammonium phosphate fertilizer promotes the yield and reduces heavy metals accumulation in tomato (Lycopersicon esculentum L.). Int. J. Environ. Sci. Technol.19, 1753–1764. doi: 10.1007/s13762-021-03223-3
4
Bergkemper F. Schöler A. Engel M. Lang F. Krüger J. Schloter M. et al . (2016). Phosphorus depletion in forest soils shapes bacterial communities towards phosphorus recycling systems. Environ. Microbiol.18, 1988–2000. doi: 10.1111/1462-2920.13188
5
Caporaso J. G. Lauber C. L. Walters W. A. Berg-Lyons D. Huntley J. Fierer N. et al . (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J.6, 1621–1624. doi: 10.1038/ismej.2012.8
6
Dai Z. M. Liu G. F. Chen H. H. Chen C. R. Wang J. K. Ai S. Y. et al . (2020). Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J.14, 757–770. doi: 10.1038/s41396-019-0567-9
7
De Silva N. I. Brooks S. Lumyong S. Hyde K. D. (2019). Use of endophytes as biocontrol agents. Fungal Biol. Rev.33, 133–148. doi: 10.1016/j.fbr.2018.10.001
8
Devine K. M. (2018). Activation of the PhoPR-mediated response to phosphate limitation is regulated by wall teichoic acid metabolism in Bacillus subtilis. Front. Microbiol.9. doi: 10.3389/fmicb.2018.02678
9
Du Y. D. Cui B. J. Zhang Q. A. Wang Z. Sun J. Niu W. Q. (2020). Effects of manure fertilizer on crop yield and soil properties in China: A meta-analysis. Catena193, 104617. doi: 10.1016/j.catena.2020.104617
10
Duart V. M. Caires E. F. Schornobai G. Spak M. A. S. Finkler V. G. (2024). Surface liming combined with the use of monoammonium phosphate and elemental sulphur in a no-till wheat–soybean cropping system. Soil Use Manage.40, e13132. doi: 10.1111/sum.13132
11
Fan K. K. Delgado-Baquerizo M. Zhu Y. G. Chu H. Y. (2020). Crop production correlates with soil multitrophic communities at the large spatial scale. Soil Biol. Biochem.151, 108047. doi: 10.1016/j.soilbio.2020.108047
12
Gao L. P. Wang C. Q. Wu A. Y. Chen H. Liao Q. X. Liao Y. T. (2024). Effect of layered fertilizer strategies on rapeseed (Brassica napus L.) productivity and soil macropore characteristics under mechanical direct-sowing. Sci. Rep.14, 25457. doi: 10.1038/s41598-024-76077-7
13
Gil-Martínez M. López-García Á. Domínguez M. T. Navarro-Fernández C. M. Kjøller R. Tibbett M. et al . (2018). Ectomycorrhizal fungal communities and their functional traits mediate plant–soil interactions in trace element contaminated soils. Front. Plant Sci.9. doi: 10.3389/fpls.2018.01682
14
Guan X. Liu C. Li Y. Wang X. Liu Y. Zou C. et al . (2022). Reducing the environmental risks related to phosphorus surplus resulting from greenhouse cucumber production in China. J. Clean. Prod.332, 130076. doi: 10.1016/j.jclepro.2021.130076
15
Guo X. He Z. Ren H. Ren B. Z. Zhang J. W. Liu P. et al . (2025). Long-term effects of coated fertilizers: Enhancing soil properties, optimizing microbial structures, and boosting soil fertility and crop productivity. Agr. Ecosyst. Environ.385, 109564. doi: 10.1016/j.agee.2025.109564
16
Guo J. J. Ling N. Li Y. Li K. S. Ning H. L. Shen Q. R. et al . (2021). Seed-borne, endospheric and rhizospheric core microbiota as predictors of plant functional traits across rice cultivars are dominated by deterministic processes. New Phytol.230, 2047–2060. doi: 10.1111/nph.17297
17
Hamel C. Hanson K. Selles F. Cruz A. F. Lemke R. McConkey B. et al . (2006). Seasonal and long-term resource-related variations in soil microbial communities in wheat-based rotations of the Canadian prairie. Soil Biol. Biochem.38, 2104–2116. doi: 10.1016/j.soilbio.2006.01.011
18
Hansel F. D. Amado T. J. C. Ruiz Diaz D. A. Rosso L. H. M. Nicoloso F. T. Schorr M. (2017). Phosphorus fertilizer placement and tillage affect soybean root growth and drought tolerance. Agron. J.109, 2936–2944. doi: 10.2134/agronj2017.04.0202
19
Hartina Monkham T. Vityakon P. Sukitprapanon T.-S. (2025). Coapplication of humic acid and gypsum affects soil chemical properties, rice yield, and phosphorus use efficiency in acidic paddy soils. Sci. Rep.15, 4350. doi: 10.1038/s41598-025-89132-8
20
Hartman W. H. Ye R. Horwath W. R. Tringe S. G. (2017). A genomic perspective on stoichiometric regulation of soil carbon cycling. ISME J.11, 2652–2665. doi: 10.1038/ismej.2017.115
21
He J. Z. Zheng Y. Chen C. R. He Y. Q. Zhang L. M. (2008). Microbial composition and diversity of an upland red soil under long-term fertilization treatments as revealed by culture-dependent and culture-independent approaches. J. Soil. Sediment.8, 349–358. doi: 10.1007/s11368-008-0025-1
22
Hinsinger P. Bengough A. G. Vetterlein D. Young I. M. (2009). Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil321, 117–152. doi: 10.1007/s11104-008-9885-9
23
Hsieh Y. J. Wanner B. L. (2010). Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol.13, 198–203. doi: 10.1016/j.mib.2010.01.014
24
Huang J. S. Hu B. Qi K. B. Chen W. J. Pang X. Y. Bao W. K. et al . (2016). Effects of phosphorus addition on soil microbial biomass and community composition in a subalpine spruce plantation. Eur. J. Soil Biol.72, 35–41. doi: 10.1016/j.ejsobi.2015.12.007
25
Ikoyi I. Fowler A. Schmalenberger A. (2018). One-time phosphate fertilizer application to grassland columns modifies the soil microbiota and limits its role in ecosystem services. Sci. Total Environ.630, 849–858. doi: 10.1016/j.scitotenv.2018.02.263
26
Jayawardena R. S. Hyde K. D. de Farias A. R. G. Bhunjun C. S. Ferdinandez H. S. Manamgoda D. S. et al . (2021). What is a species in fungal plant pathogens? Fungal Divers.109, 239–266. doi: 10.1007/s13225-021-00484-8
27
Khan M. S. Zaidi A. Wani P. A. (2007). Role of phosphate-solubilizing microorganisms in sustainable agriculture-A review. Agron. Sustain. Dev.27, 29–43. doi: 10.1051/agro:2006011
28
Lang M. Zou W. X. Chen X. X. Zou C. Q. Zhang W. Deng Y. et al . (2021). Soil microbial composition and phod gene abundance are sensitive to phosphorus level in a long-term wheat-maize crop system. Front. Microbiol.11. doi: 10.3389/fmicb.2020.605955
29
Leff J. W. Jones S. E. Prober S. M. Barberán A. Borer E. T. Firn J. L. et al . (2015). Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. P. Natl. A. Sci.112, 10967–10972. doi: 10.1073/pnas.1508382112
30
Li J. Li Z. A. Wang F. M. Zou B. Chen Y. Zhao J. et al . (2015). Effects of nitrogen and phosphorus addition on soil microbial community in a secondary tropical forest of China. Biol. Fert. Soils51, 207–215. doi: 10.1007/s00374-014-0964-1
31
Liu L. Gao Y. Gao Z. Y. Zhu L. Yan R. Yang W. J. et al . (2023a). The core microbiota as a predictor of soil functional traits promotes soil nutrient cycling and wheat production in dryland farming. Funct. Ecol.37, 2325–2337. doi: 10.1111/1365-2435.14388
32
Liu L. Gao Z. Y. Liu W. H. Li H. F. Wang Z. H. Liu J. S. (2024b). Phosphorus fertilizer input threshold shifts bacterial community structure and soil multifunctionality to maintain dryland wheat production. Soil Till. Res.243, 106174. doi: 10.1016/j.still.2024.106174
33
Liu L. Gao Z. Y. Yang Y. Gao Y. Mahmood M. Jiao H. J. et al . (2023b). Long-term high-P fertilizer input shifts soil P cycle genes and microorganism communities in dryland wheat production systems. Agr. Ecosyst. Environ.342, 108226. doi: 10.1016/j.agee.2022.108226
34
Liu L. Gao Y. Yang W. J. Liu J. S. Wang Z. H. (2024a). Community metagenomics reveals the processes of nutrient cycling regulated by microbial functions in soils with P fertilizer input. Plant Soil499, 139–154. doi: 10.1007/s11104-023-05875-1
35
Liu L. Gundersen P. Zhang T. Mo J. M. (2012). Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem.44, 31–38. doi: 10.1016/j.soilbio.2011.08.017
36
MacDonald G. K. Bennett E. M. Potter P. A. Ramankutty N. (2011). Agronomic phosphorus imbalances across the world’s croplands. P. Natl. A. Sci.108, 3086–3091. doi: 10.1073/pnas.1010808108
37
Mahmood M. Tian Y. Ma Q. X. Ahmed W. Mehmood S. Hui X. L. et al . (2020). Changes in phosphorus fractions and its availability status in relation to long term p fertilization in Loess Plateau of China. Agronomy. 10, 1818. doi: 10.3390/agronomy10111818
38
Margenot A. J. Chatterjee N. Li C. (2025). Radioisotopic labeling reveals inaccuracy of phosphorus use efficiency of ammonium phosphate fertilizers calculated by the difference method. Agr. Env. Lett.10, e70022. doi: 10.1002/ael2.70022
39
McGrath J. W. Chin J. P. Quinn J. P. (2013). Organophosphonates revealed: new insights into the microbial metabolism of ancient molecules. Nat. Rev. Microbiol.11, 412–419. doi: 10.1038/nrmicro3011
40
Mueller R. C. Paula F. S. Mirza B. S. Rodrigues J. L. M. Nüsslein K. Bohannan B. J. M. (2014). Links between plant and fungal communities across a deforestation chronosequence in the Amazon rainforest. ISME J.8, 1548–1550. doi: 10.1038/ismej.2013.253
41
Niewiadomska A. Majchrzak L. Borowiak K. Wolna-Maruwka A. Waraczewska Z. Budka A. et al . (2020). The influence of tillage and cover cropping on soil microbial parameters and spring wheat physiology. Agronomy10, 200. doi: 10.3390/AGRONOMY10020200
42
Preston C. L. Diaz D. A. R. Mengel D. B. (2019). Corn response to long-term phosphorus fertilizer application rate and placement with strip-tillage. Agron. J.111, 841–850. doi: 10.2134/agronj2017.07.0422
43
Ragot S. A. Kertesz M. A. Mészáros É. Frossard E. Bünemann E. K. (2017). Soil phoD and phoX alkaline phosphatase gene diversity responds to multiple environmental factors. FEMS Microbiol. Ecol.93, fiw212. doi: 10.1093/femsec/fiw212
44
Randall G. W. Hoeft R. G. (1988). Placement methods for improved efficiency of P and K Fertilizers: a review. J. Prod. Agric.1, 70–79. doi: 10.2134/jpa1988.0070
45
Ratliff T. J. Fisk M. C. (2016). Phosphatase activity is related to N availability but not P availability across hardwood forests in the northeastern United States. Soil Biol. Biochem.94, 61–69. doi: 10.1016/j.soilbio.2015.11.009
46
Regehr A. Oelbermann M. Videla C. Echarte L. (2015). Gross nitrogen mineralization and immobilization in temperate maize-soybean intercrops. Plant Soil391, 353–365. doi: 10.1007/s11104-015-2438-0
47
Riley D. Barber S. A. (1969). Bicarbonate accumulation and pH changes at the soybean (Glycine max (L.) Merr.) root-soil interface. Soil Sci. Soc Am. J.33, 905–908. doi: 10.2136/sssaj1969.03615995003300060031x
48
Rousk J. Baath E. Brookes P. C. Lauber C. L. Lozupone C. Caporaso J. G. et al . (2010). Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J.4, 1340–1351. doi: 10.1038/ismej.2010.58
49
Sebastian M. Ammerman J. W. (2011). Role of the phosphatase PhoX in the phosphorus metabolism of the marine bacterium Ruegeria pomeroyi DSS-3. Env. Microbiol. Rep.3, 535–542. doi: 10.1111/j.1758-2229.2011.00253.x
50
Serrano R. González-Menéndez V. Pérez-Victoria I. Sánchez I. Toro C. Mackenzie T. A. et al . (2025). Leaf litter associated fungi from South Africa to control fungal plant pathogens. Sci. Rep.15, 30219. doi: 10.1038/s41598-025-09693-6
51
Sharma S. B. Sayyed R. Z. Trivedi M. H. Gobi T. A. (2013). Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus2, 587. doi: 10.1186/2193-1801-2-587
52
Shi Y. He Y. Zheng Y. Liu X. Wang S. Xiong T.e. et al . (2024). Characteristics of the phyllosphere microbial community and its relationship with major aroma precursors during the tobacco maturation process. Front. Plant Sci.15. doi: 10.3389/fpls.2024.1346154
53
Smith S. E. Smith F. A. (2011). Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol.62, 227–250. doi: 10.1146/annurev-arplant-042110-103846
54
Su J. Q. Ding L. J. Xue K. Yao H. Y. Quensen J. Bai S. J. et al . (2015). Long-term balanced fertilization increases the soil microbial functional diversity in a phosphorus-limited paddy soil. Mol. Ecol.24, 136–150. doi: 10.1111/mec.13010
55
Su H. Lai H. L. Gao F. Y. Zhang R. P. Wu S. X. Ge F. R. et al . (2024). The proliferation of beneficial bacteria influences the soil C, N, and P cycling in the soybean–maize intercropping system. Environ. Sci. pollut. R.31, 25688–25705. doi: 10.1007/s11356-024-32851-8
56
Sun F. H. Xiao B. Tuller M. (2024). Multifaceted impacts of moss-dominated biocrusts on dryland soils: From soil pore structure to aeration and water infiltration. Catena236, 107755. doi: 10.1016/j.catena.2023.107755
57
Tee H. S. Waite D. Lear G. Handley K. M. (2021). Microbial river-to-sea continuum: gradients in benthic and planktonic diversity, osmoregulation and nutrient cycling. Microbiome9, 190. doi: 10.1186/s40168-021-01145-3
58
Vetsch J. A. Randall G. W. (2002). Corn production as affected by tillage system and starter fertilizer. Agron. J.94, 532–540. doi: 10.2134/agronj2002.5320
59
Viceli J. M. Acosta A. C. B. Pocojeski E. Casali C. A. Kessler N. C. H. Tessaro D. (2025). Influence of soil water content on chemical and microbiological characteristics of selected riparian forests in southern Brazil. Wetl. Ecol. Manage.33, 26. doi: 10.1007/s11273-025-10041-8
60
Wang Q. Qin Z. H. Zhang W. W. Chen Y. H. Zhu P. Peng C. et al . (2022). Effect of long-term fertilization on phosphorus fractions in different soil layers and their quantitative relationships with soil properties. J. Integr. Agr.21, 2720–2733. doi: 10.1016/j.jia.2022.07.018
61
Withers P. J. A. Neal C. Jarvie H. P. Doody D. G. (2014). Agriculture and eutrophication: where do we go from here? Sustainability6, 5853–5875. doi: 10.3390/su6095853
62
Wu P. Liu F. Li H. Cai T. Zhang P. Jia Z. K. (2021). Suitable fertilizer application depth can increase nitrogen use efficiency and maize yield by reducing gaseous nitrogen losses. Sci. Total Environ.781, 146787. doi: 10.1016/j.scitotenv.2021.146787
63
Yang Z. Zhang Y. Wang Y. Zhang H. Zhu Q. Yan B. et al . (2022). Intercropping regulation of soil phosphorus composition and microbially-driven dynamics facilitates maize phosphorus uptake and productivity improvement. Field Crop Res.287, 108666. doi: 10.1016/j.fcr.2022.108666
64
Zhang L. Ding X. Peng Y. George T. S. Feng G. (2018). Closing the loop on phosphorus loss from intensive agricultural soil: a microbial immobilization solution? Front. Microbiol.9. doi: 10.3389/fmicb.2018.00104
65
Zhu J. Li M. Whelan M. (2018). Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ.612, 522–537. doi: 10.1016/j.scitotenv.2017.08.095
Summary
Keywords
spatially stratified fertilization, phosphorus efficiency, maize yield, microbial community structure, functional genes
Citation
Hou L, Han B, Wang Y, Wang X, Shi W, Cao N and Zhang Y (2025) Microbial response to long-term spatially stratified phosphorus application in Northeast China. Front. Plant Sci. 16:1669876. doi: 10.3389/fpls.2025.1669876
Received
20 July 2025
Accepted
26 September 2025
Published
23 October 2025
Volume
16 - 2025
Edited by
Jie Zhou, Nanjing Agricultural University, China
Reviewed by
Tao Zhang, Northeast Normal University, China
Hong Wei Yu, Chinese Academy of Sciences (CAS), China
Updates
Copyright
© 2025 Hou, Han, Wang, Wang, Shi, Cao and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Cao, cao_ning@jlu.edu.cn; Yubin Zhang, ybzhang@jlu.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.