By Sato N (2025) Front. Plant Sci. 16:1603911. doi: 10.3389/fpls.2025.1603911
1 Introduction
Plastoquinone is the electron carrier in photosynthetic organisms, playing a crucial role in the photosynthetic electron transport chain. We previously reported the presence of two acylated plastoquinone species—acylplastoquinol (APQ) and plastoquinone-B (PQB)—in the unicellular cyanobacteria Synechocystis sp. PCC 6803 and Synechococcus sp. PCC 7002 (Kondo et al., 2023a, b). The gene slr2103 and its ortholog SYNPCC7002_A0918 encode a bifunctional acyltransferase responsible for the synthesis of both APQ and PQB in Synechocystis and Synechococcus, respectively (Kondo et al., 2023a, b). Orthologs of slr2103 are found in over 100 cyanobacterial genomes but are absent in eukaryotic photosynthetic organisms (Kondo et al., 2023b). Building on these studies, the article reported that both APQ and PQB are present in two filamentous cyanobacteria containing slr2103 orthologs, and also in a red alga, two green algae, a haptophyte, and a seed plant.
The commentary (Sato, 2025) on the article (Ito et al., 2025) raised issues regarding the identification methods of acylplastoquinone species and the research history of these compounds. Since the research history, including the initial identification of APQ in Synechocystis (Mori-Moriyama et al., 2023), is detailed in the commentary, this response will focus on the technical aspects.
2 Results and discussion
In the article, acylplastoquinone species were identified from total cellular lipids using profiling LC–MS analysis in ion-trap mode with information-dependent acquisition (IDA) MS² to analyze a broad range of compounds (retention time 2 to 18 min, m/z 300 to 1200; Ito et al., 2025). Identification was based on the m/z values of NH4+-adducted precursor ions, their retention times in the LC-MS chromatogram, and unique fragment ions in the MS² spectra (Supplementary Table S1; Kondo et al., 2023a, b). The relative contents of respective molecular species in APQ or PQB were estimated by measuring signal intensities of their corresponding lipid ions relative to total cellular lipid ions, revealing that APQ and PQB predominantly consist of saturated-acyl species. Importantly, this analytical approach allows for the detection of any substances that might interfere with the ionization of acylplastoquinone species (see below).
However, as noted in the commentary, fragment ions in the MS² spectra of acylplastoquinone species used for identification showed lower signal-to-noise (S/N) ratios (Ito et al., 2025) compared to those reported by Kondo et al. (2023b). The commentary suggested that low S/N ratios may result from low lipid content in the samples. Comparable total cellular lipid contents (based on cell mass, OD730·mL) routinely underwent LC-MS/IDA-MS² analysis in our studies, where similarly low S/N ratios were observed throughout the work by Kondo et al. (2023a, b). The difference likely arises from the MS analytical methods: unlike the profiling analysis by Ito et al. (2025), Kondo et al. (2023a, b) also performed targeted MS² analysis in ion-trap mode focusing on selected candidate ions of acylplastoquinone species, yielding improved S/N ratios.
Here, to evaluate our previous identification of acylplastoquinone species by profiling LC-MS/IDA-MS² analysis, we subjected samples identical to those used by Ito et al. (2025) to an alternative MS analysis. Using Supplementary Table S1, candidate acylplastoquinone species were searched via targeted LC-MS/MS analysis of total cellular lipids in multiple reaction monitoring (MRM) mode for enhanced selectivity and improved S/N ratios. Precursor ions of acylplastoquinone species were monitored under collision-induced dissociation at 50 eV, with transitions to m/z 153 for APQ and m/z 151 for PQB. Consequently, candidates of the same molecular species of APQ and PQB reported by Ito et al. (2025) were detected in their respective photosynthetic organisms. These candidates then underwent targeted MS² analysis in ion-trap mode with further optimization: collision-energy spread fine-tuning to confirm precursor and fragment ions, and dynamic fill time optimization to enhance overall ion signal intensity. The resulting MS² spectra showed diagnostic ions characteristic of acylplastoquinone species with high S/N ratios, consistent with those reported by Kondo et al. (2023a, b), strongly supporting our previous identification (Figure 1, Supplementary Figures S1-S8). Notably, MS² spectra for APQ were revised to include both de-prenylated and acyl-derived fragment ions (Figure 1, Supplementary Figures S1-S3), alongside a proposed model for generation of acyl-derived fragments within the chemical structure (Figure 1, Supplementary Figure S7), in response to the commentary. Moreover, both APQ and PQB included the precursor ions (Figure 1, Supplementary Figures S1-S6).
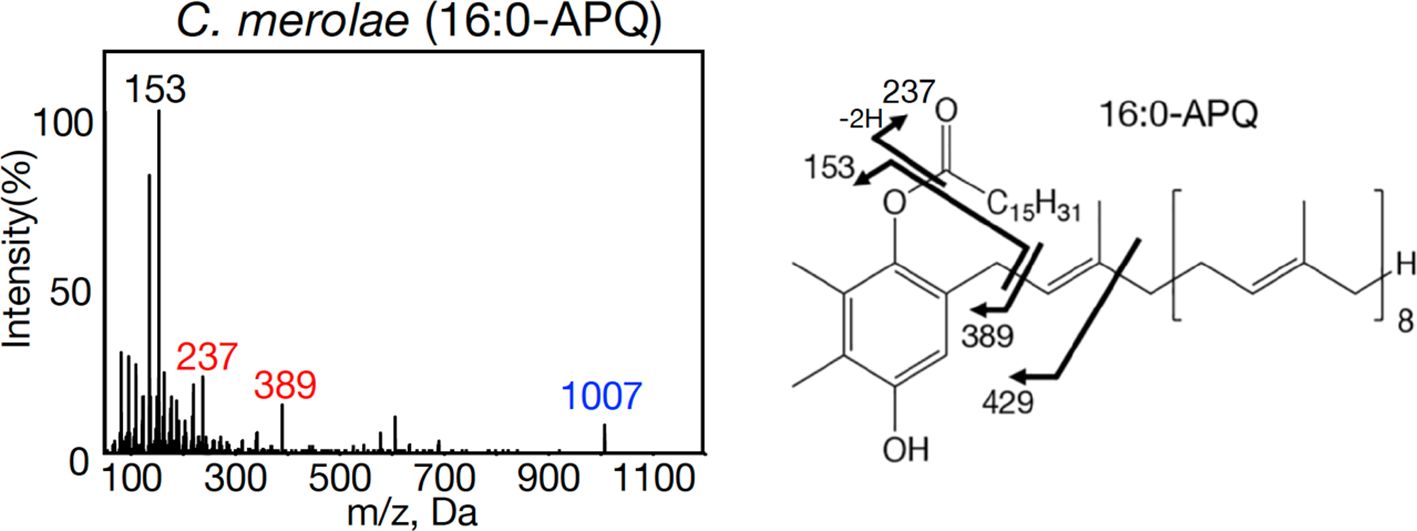
Figure 1. Representative MS2 spectra of 16:0-APQ with NH4+ adducts and its fragmentation patterns in the red alga Cyanidioschyzon merolae. Characteristic ions detected include m/z 153, m/z 237 (acyl-derived fragment), m/z 389 (de-prenylated fragment), and m/z 1007 (precursor).
Regarding Synechocystis, Tanikawa et al. (2025), unlike Ito et al. (2025), detected 18:1-APQ. The commentary suggested potential interference by other lipids in the Ito et al. study; however, we did not detect any lipid ions that could potentially interfere within the m/z range of 300 to 1200 at retention times matching or close to that of 18:1-APQ in the profiling LC-MS chromatogram. The presence of 18:1-APQ in Synechocystis may depend on culturing conditions, including light intensity, temperature, aeration, culture vessel type, or growth stage. Meanwhile, according to the commentary, APQ content decreases during lipid concentration steps, including evaporation of lipid extracts, likely due to its susceptibility to oxidation. We are currently preparing new total lipid extracts from cells with or without an antioxidant in lipid extraction, to investigate the effects of its addition on APQ molecular species content. Establishing a reliable method for quantitative APQ analysis, as discussed as essential in the commentary, will require gradual accumulation of such data.
In conclusion, this study reinforces the validity of our earlier findings (Ito et al., 2025): APQ and PQB have been evolutionarily conserved from cyanobacteria to eukaryotic photosynthetic organisms in the green and red lineages. Moreover, under the culturing conditions used by Ito et al. (2025), these organisms predominantly contain saturated APQ and PQB species.
Author contributions
RI: Validation, Data curation, Formal analysis, Writing – review & editing, Investigation. ME: Data curation, Investigation, Writing – review & editing, Validation, Formal analysis. MA: Formal analysis, Writing – review & editing, Methodology, Investigation, Data curation, Validation. SF: Investigation, Validation, Writing – review & editing. NS: Writing – review & editing, Supervision, Writing – original draft, Conceptualization, Investigation, Validation, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was financially supported by Grants-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (23K11481, NS).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interests.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1671717/full#supplementary-material
References
Ito, R., Endo, M., Aoki, M., Fujiwara, S., and Sato, N. (2025). Evolutionary conservation of acylplastoquinone species from cyanobacteria to eukaryotic photosynthetic organisms of green and red lineages. Front. Plant Sci. 16, 1569038. doi: 10.3389/fpls.2025.1569038
Kondo, M., Aoki, M., Hirai, K., Ito, R., Tsuzuki, M., and Sato, N. (2023a). Plastoquinone lipids: their synthesis via a bifunctional gene and physiological function in a euryhaline cyanobacterium, Synechococcus sp. PCC 7002. Microorganisms 11, 1177. doi: 10.3390/microorganisms1105117
Kondo, M., Aoki, M., Hirai, K., Sagami, T., Ito, R., Tsuzuki, M., et al. (2023b). slr2103, a homolog of type-2 diacylglycerol acyltransferase genes, for plastoquinone-related neutral lipid synthesis and NaCl-stress acclimatization in a cyanobacterium, Synechocystis sp. PCC 6803. Front. Plant Sci. 14, 1181180. doi: 10.3389/fpls.2023.1181180
Mori-Moriyama, N., Yoshitomi, T., and Sato, N. (2023). Acyl plastoquinol is a major cyanobacterial substance that co-migrates with triacylglycerol in thin-layer chromatography. Biochem. Biophys. Res. Commun. 641, 18–26. doi: 10.1016/j.bbrc.2022.12.003
Sato, N. (2025). Commentary: Evolutionary conservation of acylplastoquinone species from cyanobacteria to eukaryotic photosynthetic organisms of green and red lineages. Front. Plant Sci. 16, 1603911. doi: 10.3389/fpls.2025.1603911
Keywords: targeted LC-MS/MS analysis, LC-MS/IDA-MS² analysis, acylplastoquinol, plastoquinone-B, cyanobacteria, red and green algae, haptophyte, seed plants
Citation: Ito R, Endo M, Aoki M, Fujiwara S and Sato N (2025) Response: Commentary: Evolutionary conservation of acylplastoquinone species from cyanobacteria to eukaryotic photosynthetic organisms of green and red lineages. Front. Plant Sci. 16:1671717. doi: 10.3389/fpls.2025.1671717
Received: 23 July 2025; Accepted: 20 August 2025;
Published: 18 September 2025.
Edited by:
Michael Hippler, University of Münster, GermanyReviewed by:
Yuichiro Takahashi, Okayama University, JapanCopyright © 2025 Ito, Endo, Aoki, Fujiwara and Sato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Norihiro Sato, bnNhdG9AbHMudG95YWt1LmFjLmpw
†These authors have contributed equally to this work
 Ryo Ito†
Ryo Ito† Motohide Aoki
Motohide Aoki Shoko Fujiwara
Shoko Fujiwara Norihiro Sato
Norihiro Sato