- 1Department of Horticulture, Michigan State University, East Lansing, MI, United States
- 2Crop Physiology Laboratory, Utah State University, Logan, UT, United States
Blue (B; 400–499 nm) light, far-red (FR; 700–750 nm) light, and temperature are key regulators of plant growth and development, with responses varying by species. While the independent effects of these environmental signals are well established, their interactive effects are not clear. We postulated that the effects of FR light and temperature would depend on the photon flux density (PFD) of B light. To test this, we grew cold-tolerant lettuce and cold-sensitive basil at 19 and 24°C under lighting treatments with three FR fractions [FR-PFD divided by the sum of red (600–699 nm) and FR PFD; 0.01, 0.19, or 0.32] and two B-PFDs (40 or 100 µmol m−2 s−1). The total PFD (400–750 nm; 270 µmol m−2 s−1) and photoperiod (24 h d−1) were the same in all treatments. There were significant differences between species. As expected, increasing the FR fraction dramatically increased shoot expansion in lettuce and internode elongation in basil. The shoot expansion in lettuce was amplified by higher temperature but attenuated by higher B-PFD. Unlike lettuce, the FR effect on basil internodes did not interact with either temperature or B-PFD. The increased shoot expansion in lettuce decreased foliage coloration, but coloration was minimally altered in basil. These results reveal fundamentally different species responses to light and temperature that may have implications for shade-avoidant and shade-tolerant species. Overall, these findings demonstrate the complex integration of environmental signals in the regulation of growth.
1 Introduction
The photon spectrum (or spectral quality), the photon flux density (PFD), and temperature independently regulate plant growth, but their effects can interact and vary among species (Paradiso and Proietti, 2021; Jeong et al., 2024b). A high far-red fraction [far-red (FR; 700–750 nm) PFD/red (R; 600–699 nm)+FR PFD] and a low blue (B; 400–499 nm) PFD (e.g., B-PFDs lower than the ones of unfiltered sunlight) are environmental cues for shading from nearby vegetation. These photon signals induce morphological responses such as leaf, stem, and/or petiole elongation, increased apical dominance, and/or hyponasty (Franklin, 2008; Keuskamp et al., 2010; Keller et al., 2011; Casal, 2013; Gommers et al., 2013; Pedmale et al., 2016; Kusuma and Bugbee, 2021a; Shin and Runkle, 2025; Skabelund et al., 2025). Temperature also regulates the phenotypic and developmental responses of plants, which is known as thermomorphogenesis. Thermomorphogenesis mimics some morphological responses to a high FR fraction and a low B-PFD including stem elongation and leaf hyponasty, although specific responses vary among species (Erwin et al., 1989; Koini et al., 2009; Fukuda et al., 2011; Patel et al., 2013; Snowden et al., 2016; Skabelund et al., 2025).
Photon signals for morphological responses are perceived by plants through several classes of photoreceptors including R and FR photon-absorbing phytochromes and B photon-absorbing cryptochromes (Banerjee and Batschauer, 2005; Chaves et al., 2011; Zhang et al., 2013; Possart et al., 2014). Among several types of phytochrome (PHY), PHYB plays a dominant role in mediating the morphological responses to FR photons in light-grown plants (Sharrock and Clack, 2002). PHYB is homo- or hetero-dimetric chromoproteins that exist in three forms, PRPR, PRPFR, and PFRPFR. The PRPR is biologically inactive and is converted to PRPFR and PFRPFR upon R photon absorption, but is switched back to PRPR upon FR photon absorption (Burgie and Vierstra, 2014; Legris et al., 2016; Klose et al., 2020). The phytochrome photoequilibria [PPE; PFR/(PR+PFR)] integrates the effect of photons from 350 to 760 nm and, at least theoretically, provides an improved metric to predict PHY responses (Lagarias et al., 1987; Sager et al., 1988). When the PPE is low, extension growth is induced, and vice versa (Park and Runkle, 2017; Meng and Runkle, 2019). Internal PPE (iPPE), which accounts for spectral distortion within leaves of light-grown plants, has better predicted plant morphological responses than the more original metric of PPE (Kusuma and Bugbee, 2021b).
PHYB functions not only as photoreceptor but also as a thermo-sensor in plants (Jung et al., 2016; Legris et al., 2016; Qiu et al., 2019). An increase in temperature reverts photon-induced PFRPFR and PRPFR to PRPFR and PRPR, respectively, the rate of which increases exponentially with temperature (Klose et al., 2015, 2020). Therefore, FR fraction and temperature signals can converge at PHYB. Additionally, FR fraction and temperature signaling share downstream pathways (Proveniers and van Zanten, 2013; Qiu et al., 2019). For example, temperature can regulate the expression of the phytochrome-interacting factor (PIF) family, which are targets of active PHYB, through PHYB-independent mechanisms (Castillon et al., 2007; Leivar and Quail, 2011; Raschke et al., 2015; Chung et al., 2020; Jung et al., 2020; Casal and Fankhauser, 2023). Consistent with the signaling convergence, FR photons and temperature interacted to promote hypocotyl elongation in velvetleaf (Abutilon theophrasti) and Arabidopsis (Arabidopsis thaliana) seedlings (Weinig, 2000; Romero-Montepaone et al., 2020, 2021; Burko et al., 2022). However, given the diverse responsiveness to photon spectra and temperature among model species, whether this interaction between FR photons and temperature is transferable to horticultural crops remains largely unknown. Additionally, it is uncertain whether the responses in seedlings also occur in mature plants. Cryptochromes are flavoproteins that primarily perceive B photon signals (Cashmore et al., 1999). A low B-PFD, sensed by two types of cryptochrome [cryptochrome1 (CRY1) and cryptochrome2 (CRY2)], induces morphological responses including hypocotyl elongation (Ahmad et al., 1995). CRYs activated by B photons can suppress PIFs through physical interaction (Foreman et al., 2011; Ma et al., 2016; Pedmale et al., 2016). Therefore, the interaction between FR photons and temperature can be potentially modulated by B photons. However, this potential influence of B photons has not been considered and merits investigation.
Along with morphology, the photon spectrum and temperature can interact to regulate pigment accumulation, which influences foliage coloration. The biosynthesis of anthocyanins, which is a group of common red- and blue-colored phytopigments, can be triggered by ultraviolet (100–399 nm) and B photons. This process is positively regulated by transcription factors such as the LONG HYPOCOTYL5 (HY5), negatively regulated by CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1), and/or bidirectionally regulated by myeloblastosis (MYB) (Borevitz et al., 2000; Meng and Runkle, 2019; LaFountain and Yuan, 2021). In contrast, high temperature represses anthocyanin biosynthesis by degrading the HY5 protein in a COP1 activity-dependent manner and/or by down-regulating anthocyanin biosynthetic MYB genes (Kim et al., 2017; Rehman et al., 2017). Beyond biosynthesis repression, high temperatures can degrade accumulated anthocyanins (Rehman et al., 2017) and decrease foliage coloration (Tarr et al., 2023). The influence of FR fraction on coloration varies by growth stage, partly because dominant PHY differ by growth stages (Li and Kubota, 2009; Carvalho and Folta, 2014; Li et al., 2014; Liu et al., 2015; Shin and Runkle, 2024). For example, PHYA predominates during seedling de-etiolation, whereas PHYB is dominant in light-grown mature plants. PHY activated either by R or FR photons can suppress COP1 (Sheerin et al., 2015) and can trigger foliage coloration. Given the convergence of photon spectra and air temperature signaling, these environmental factors may interact to regulate the coloration of horticultural crops, particularly those with red- and purple-colored leaves.
The objective of this study was to explore interactions among photon spectra (specifically FR fraction and B-PFD) and air temperature in regulating biomass accumulation, morphological traits, and foliage coloration of two diverse horticultural species that are commonly grown in indoor agriculture, lettuce (Lactuca sativa) and basil (Ocimum basilicum). Lettuce is a cold-tolerant crop whereas basil is relatively cold sensitive. We hypothesized that FR fraction, B-PFD, and air temperature would interdependently control growth and foliage pigmentation, the magnitude of which would vary among species.
2 Materials and methods
2.1 The propagation phase
Red oakleaf lettuce ‘Rouxai’ and basil ‘Prospera’ were selected based on their diverse responses to photon spectra and temperature. Leaf expansion in lettuce is generally more sensitive to photon spectra than basil (Meng and Runkle, 2019; Shin and Runkle, 2024), but basil has a higher optimum temperature than lettuce (Walters and Currey, 2019; Tarr et al., 2023).
Seeds of lettuce ‘Rouxai’ (Rijk Zwaan, Salinas, CA, United States) and basil ‘Prospera’ (Johnny’s Selected Seeds, Winslow, ME, United States) were sown into 200-cell (individual plug dimensions 2.5 cm × 2.5 cm × 4.0 cm) Rockwool plugs (Grodan AO Plug 25/40; Grodan, Milton, ON, Canada), one seed per cell, on May 16, 2022, June 14, 2022, and August 7, 2024 for the first, second, and third replication (day 0), respectively. The plugs were presoaked with deionized water adjusted with diluted sulfuric acid (J.Y. Baker Inc., Phillipsburg, NJ, United States) to provide a pH = 4.4–4.5 and electrical conductivity (EC) = 0.03 mS cm−1 based on values measured with a pH and electrical conductivity meter (HI9814; Hanna Instruments, Woonsocket, RI, United States).
Germination (day 0–2) and seedling growth (day 3–7) were conducted in a walk-in room of the Controlled Environment Lighting Laboratory (Michigan State University, East Lansing, MI, United States). During the germination stage, seeds sprouted in plug trays covered with transparent plastic humidity domes under a photosynthetic PFD (PPFD; photon flux integral between 400 nm and 700 nm in µmol m−2 s−1) of 180 µmol m−2 s−1 for 24 h d−1 from warm-white (peak = 639 nm, correlated color temperature = 2700 K) light-emitting diodes (LEDs) (Phytofy RL; OSRAM Opto Semiconductors, Beverley, MA, United States). On day 3, the humidity domes were removed. We measured the air temperature near the middle of the room every 10 s by four thermocouples (0.13-mm type E; Omega Engineering, Inc., Stamford, CT, United States) connected to a data logger (CR1000; Campbell Scientific, Inc., Logan, UT, United States) and logged the hourly average. The air temperature was maintained at 23.3 ± 0.1°C, 23.4 ± 0.1°C, and 22.9 ± 0.3°C (mean ± standard deviation) for the first, second, and third replication, respectively. CO2 concentration was measured with a silicon-based nondispersive infrared CO2 concentration sensor (GMD20, Vaisala, Helsinki, Finland). The mean CO2 concentration was 408.3 ± 18.7 ppm, 402.6 ± 23.5 ppm, and 430.7 ± 35.3 ppm for the first, second, and third replication, respectively. Seedlings were sub-fertigated with deionized water supplemented with a water-soluble fertilizer (12N-4P-16K RO Hydro FeED; JR Peters, Inc., Allentown, PA, United States) and magnesium sulfate (Epsom salt, Pennington Seed, Inc., Madison, GA, United States) at a pH = 5.6 and EC = 1.6 mS cm−1. The nutrient solution contained the following (in mg L−1): 125 N, 42 P, 167 K, 73 Ca, 49 Mg, 39 S, 1.7 Fe, 0.52 Mn, 0.56 Zn, 0.13 B, 0.47 Cu, and 0.13 Mo.
2.2 The production phase
On day 8, when the first true leaf of lettuce was expanding and the roots of lettuce and basil reached the base of the plugs, 36 seedlings of each species were transplanted into each of twelve rafts (60.9 cm × 121.9 cm × 2.5 cm raft with 72 holes, each with a diameter of 1 cm; Beaver Plastics, Ltd., Acheson, AB, Canada) floating in deep-flow hydroponic systems in two climate rooms. The plants were spaced 10.0 cm horizontally and 14.1 cm diagonally apart in their respective centers, creating a planting density of 96.8 plants m−2 (Figure 1). We used the same nutrient solution in the recirculating hydroponic system for the production phase as that used for the propagation phase. Solutions were aerated at 70 L min−1 using a round flat air stone (20.3 cm × 2.5 cm; Active Aqua AS8RD; Hydrofarm). During the production phase, CO2 concentration remained near the ambient (Table 1). The air inside each climate room was continuously circulated to maintain environmental homogeneity.
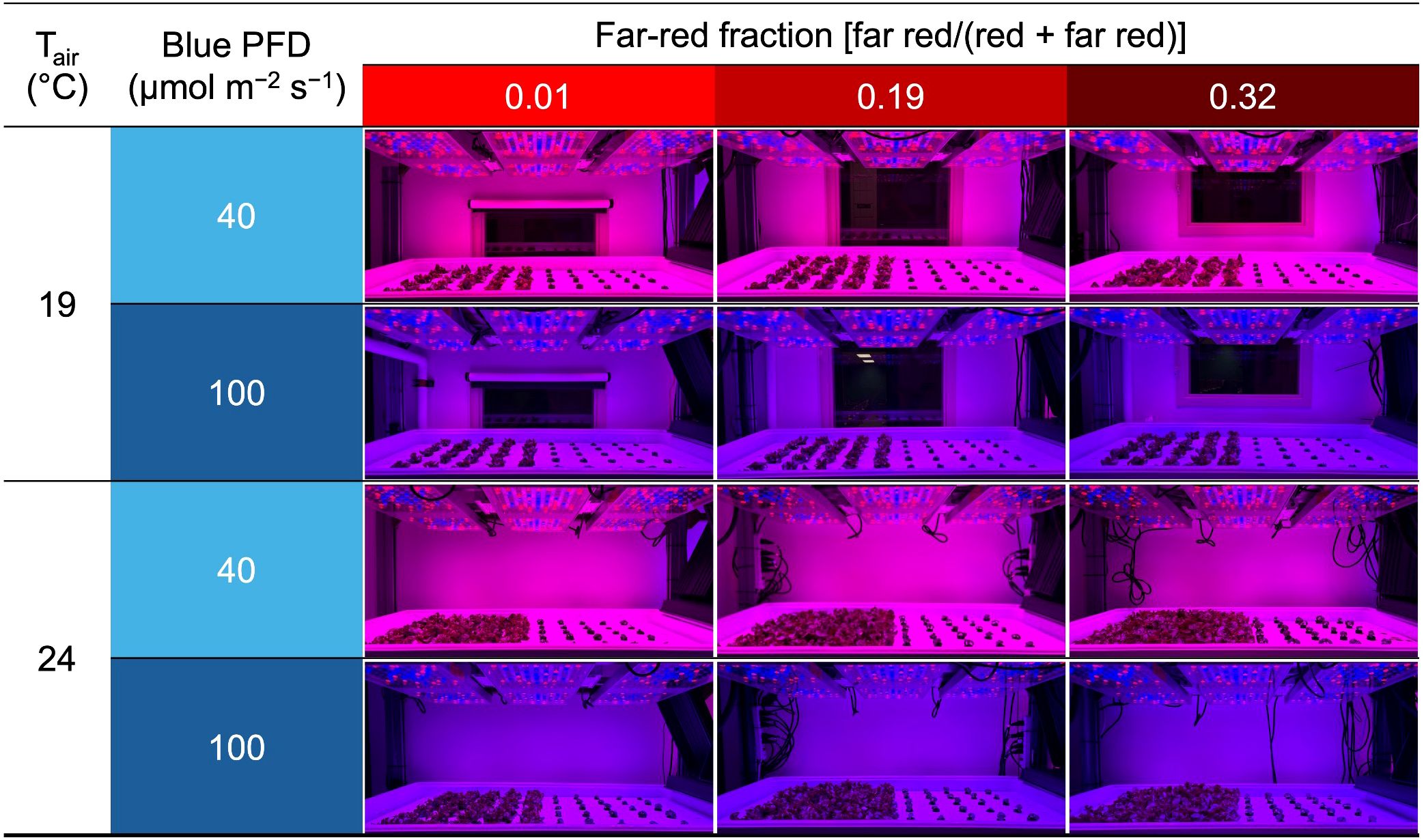
Figure 1. Photographs of the growing system on day 19 (the day of lettuce harvest). Tair refers to air temperature. Blue PFD and far-red fraction refer to the blue photon flux density (400–499 nm) and the fraction of the far-red (700–750 nm) photon flux density relative to the sum of the red (600–699 nm) and far-red photon flux density, respectively. Plants on the left and right sides are lettuce ‘Rouxai’ and basil ‘Prospera’, respectively.
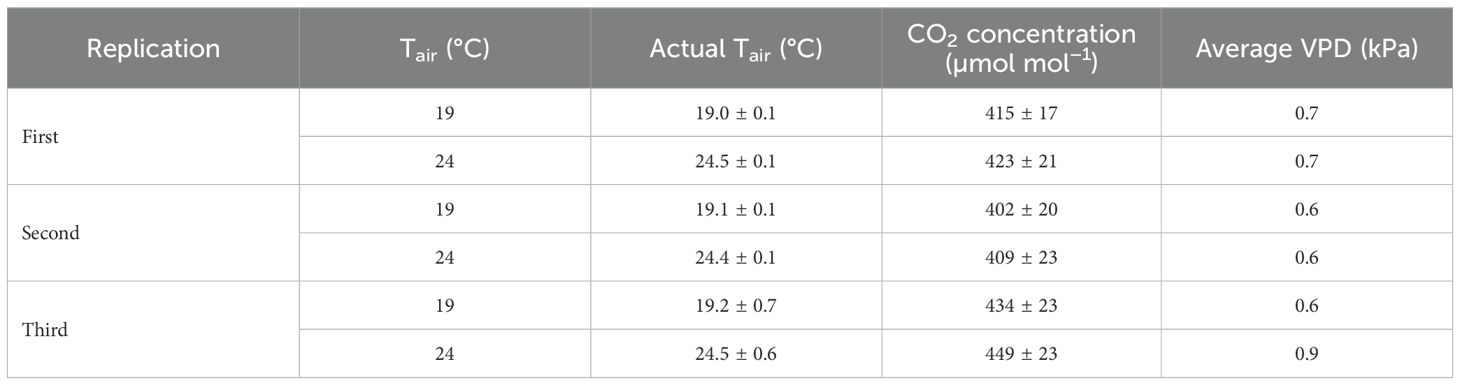
Table 1. Temperature set point (Tair), actual air temperature (actual Tair), actual CO2 concentration, and average vapor-pressure deficit (VPD) of two air temperature treatments for three experimental replications.
2.3 Temperature and photon spectra treatments
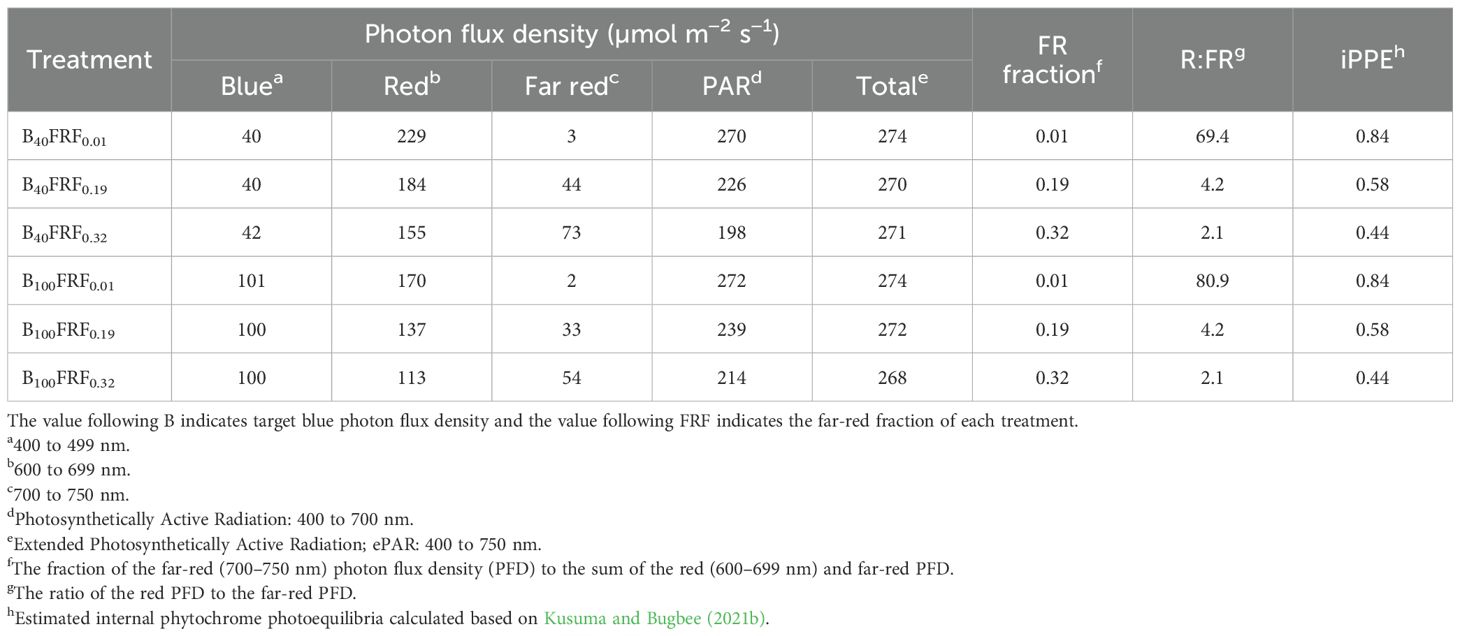
Plants were grown with twelve combinations of the B-PFD (40 or 100 µmol m−2 s−1), FR fraction (0.01, 0.19, or 0.32), and air temperature (19 or 24°C) (Tables 1, 2). The B-PFD and FR fraction treatments were delivered by the same fixtures as described previously using B, R, and FR narrowband LEDs. The fixtures have dimmable B, R, FR, and warm-white LEDs in the same unit. Photon spectra were measured at twelve locations on the floating raft for each photon spectra treatment using a portable spectroradiometer (LI-180, LI-COR Biosciences, Inc., Lincoln, NE, United States) at initial seedling height (Figure 2). As plants developed and grew closer to the LED fixtures, the total PFD (TPFD; photon flux integral between 400 nm and 750 nm in µmol m−2 s−1) increased by less than 7% (Supplementary Figure S1). For each photon spectra treatment, the PPFD, TPFD, R:FR, and estimated iPPE (Kusuma and Bugbee, 2021b) were calculated; the B photon fraction (the B-PFD divided by the TPFD) was 15 or 37%. The TPFD was maintained constant among treatments (270 µmol m−2 s−1) to minimize differences in photosynthetic photons among treatments. When plants acclimate to FR-enriched conditions, their photosynthetic rate per unit leaf area often decreases with increasing FR fraction under a constant TPFD (Jeong et al., 2024a). This is likely attributable to reduced leaf thickness (i.e., increased specific leaf area; Evans and Poorter, 2001), which can decrease area-based chlorophyll content (Jeong et al., 2024a), a common morphological response to FR photons (Gommers et al., 2013). Nevertheless, several recent studies have demonstrated that FR photons equivalently contribute to photosynthesis when combined with traditionally defined photosynthetically active radiation if the FR-PFD is <30 to 40% of the PPFD (Zhen and Bugbee, 2020; Zhen et al., 2021, 2022; Kelly and Runkle, 2024). This equivalence has been demonstrated both in short-term measurements of unit-area photosynthesis (without acclimation) and in long-term canopy photosynthesis. Consequently, delivering the same TPFD rather than the same PPFD has been increasingly adopted for evaluating the effects of FR photons on plant morphology and growth (Kusuma and Bugbee, 2023; Jeong et al., 2024a, 2024b; Shin and Runkle, 2025; Skabelund et al., 2025). We did not calculate yield photon flux density because it tends to underestimate the contribution of FR photons to photosynthesis (Sager et al., 1988). Lighting was continuous (24 h d−1).
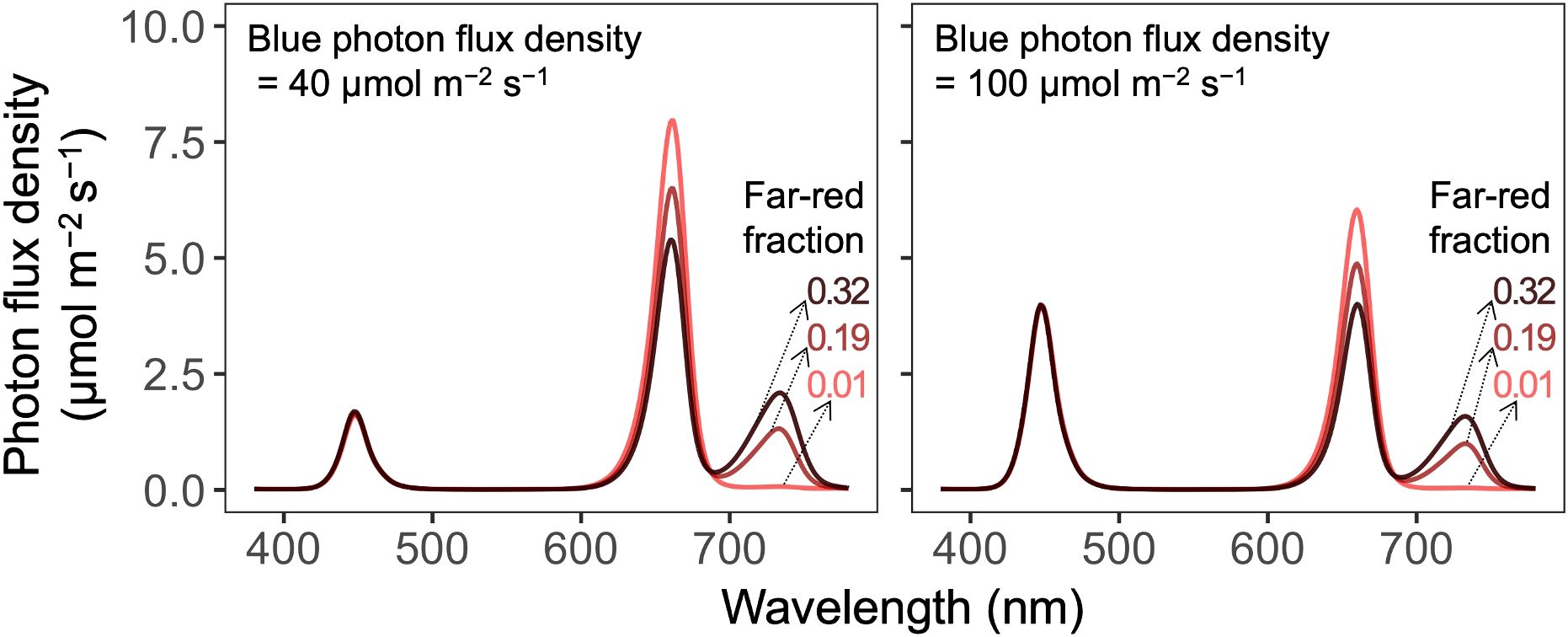
Figure 2. Spectral distributions of six photon spectra treatments with two blue photon flux densities (400–499 nm; in µmol m−2 s−1) and three far-red fractions [the fraction of far-red (700–750 nm) photon flux density to the sum of red (600–699 nm) and far-red photon flux density] delivered by blue (peak at 447 nm), red (peak at 661 nm), and far-red (peak at 733 nm) light-emitting diodes.
The air temperature of each climate room was measured near the middle of each room every 10 seconds by two thermocouples (0.13-mm type E; Omega Engineering, Inc.). The 24°C room was humidified to maintain an atmospheric vapor-pressure deficit (VPD) similar to the 19°C room (Table 1). Relative humidity was measured every 10 seconds by a relative humidity probe (HMP110; Vaisala, Inc., Louisville, CO, United States) connected to a data logger (CR1000; Campbell Scientific, Inc) and hourly averages were logged and used for VPD calculation.
2.4 Data collection and statistical analysis
Ten lettuce and basil plants per treatment were randomly selected after excluding a few outliers (plants with atypical growth) and were harvested on day 19 and day 32, respectively. Shoots were cut at the surface and dry mass was measured after drying them at 70°C for 5 days in a drying oven (Blue M, Blue Island, IL, United States). The leaf length (mm) of the most expanded leaf was measured. The maximum shoot diameter from a top-down view (for lettuce) and the length of the first internode (for basil) were also measured with a ruler. Area-based chlorophyll concentration was estimated by converting the SPAD values measured by a chlorophyll concentration meter (MC-100, Apogee Instruments, Logan, UT, United States) using generic conversion equations provided by Parry et al. (2014). SPAD values were measured at three locations on the most expanded leaf and were averaged.
To evaluate leaf coloration, the International Commission on Illumination (CIE) L*a*b* values, which indicate darkness-brightness (L*; ranges from 0 to 100), greenness-redness (a*; ranges from −128 to 127), and blueness-yellowness (b*; ranges from −128 to 127), were calculated based on RGB values extracted from images of plants taken overhead using ‘convertColor’ procedure in R statistical analysis software (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria). Photographs were taken under white fluorescent lamps at an exposure compensation of 0.0, a shutter speed of 1/40 second, and an ISO of 125.
The treatments were assigned in a complete randomized design with three replications in time (n = 3). Each response was the mean of ten individual plants per cultivar, but since these plants were in the same nutrient solution, they were not counted as true replicates. Statistical analysis was conducted using R statistical analysis software (version 4.1.1, R Core Team, 2021). The main effects and interaction effects of the treatments were evaluated by type III three-way analysis of variance test. Linear regression was conducted using the ‘lm’ function. Values of P < 0.05 were considered statistically significant.
3 Results
3.1 Shoot extension growth
Increasing the air temperature from 19°C to 24°C caused a 1.3- to 1.9-fold increase in the shoot diameter and leaf length of mature leaves in lettuce (Figures 3, 4, Table 3; Supplementary Figure S1). Similarly, increasing the FR fraction from 0.01 to 0.32, which decreased the iPPE from 0.84 to 0.44, increased the shoot diameter and the leaf length in lettuce by 1.1 to 1.5 times. The FR-mediated increase in shoot diameter of lettuce was more pronounced at 24°C than at 19°C. An increase in the B-PFD, which generally decreased the shoot diameter of lettuce, decreased the interaction between the FR fraction and air temperature in regulating the shoot diameter (a three-way interaction, p = 0.048, Table 3; Supplementary Table S1). The FR-mediated lettuce leaf elongation was not affected by air temperature.
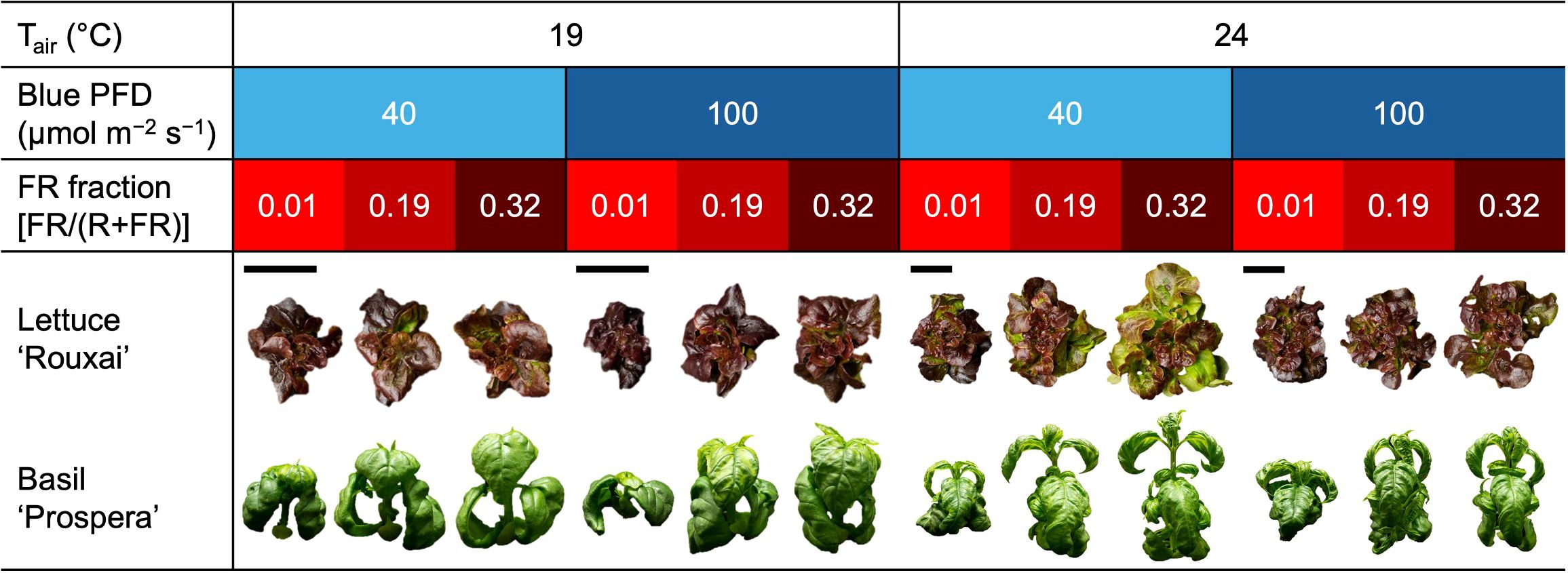
Figure 3. Representative photographs taken 19 or 32 days after seed sow of lettuce ‘Rouxai’ and basil ‘Prospera’, respectively. Plants were grown under six photon spectra treatments with two blue photon flux densities (Blue PFD; 400–499 nm) and three fractions of the far-red (FR; 700–750 nm) photon flux density relative to the sum of the red (R; 600–699 nm) and FR photon flux density (FR fraction) at two air temperatures (Tair). The size of photographs of plants grown at 19°C was increased by 80% relative to 24°C to improve visibility (bars represent the relative size of photographs).
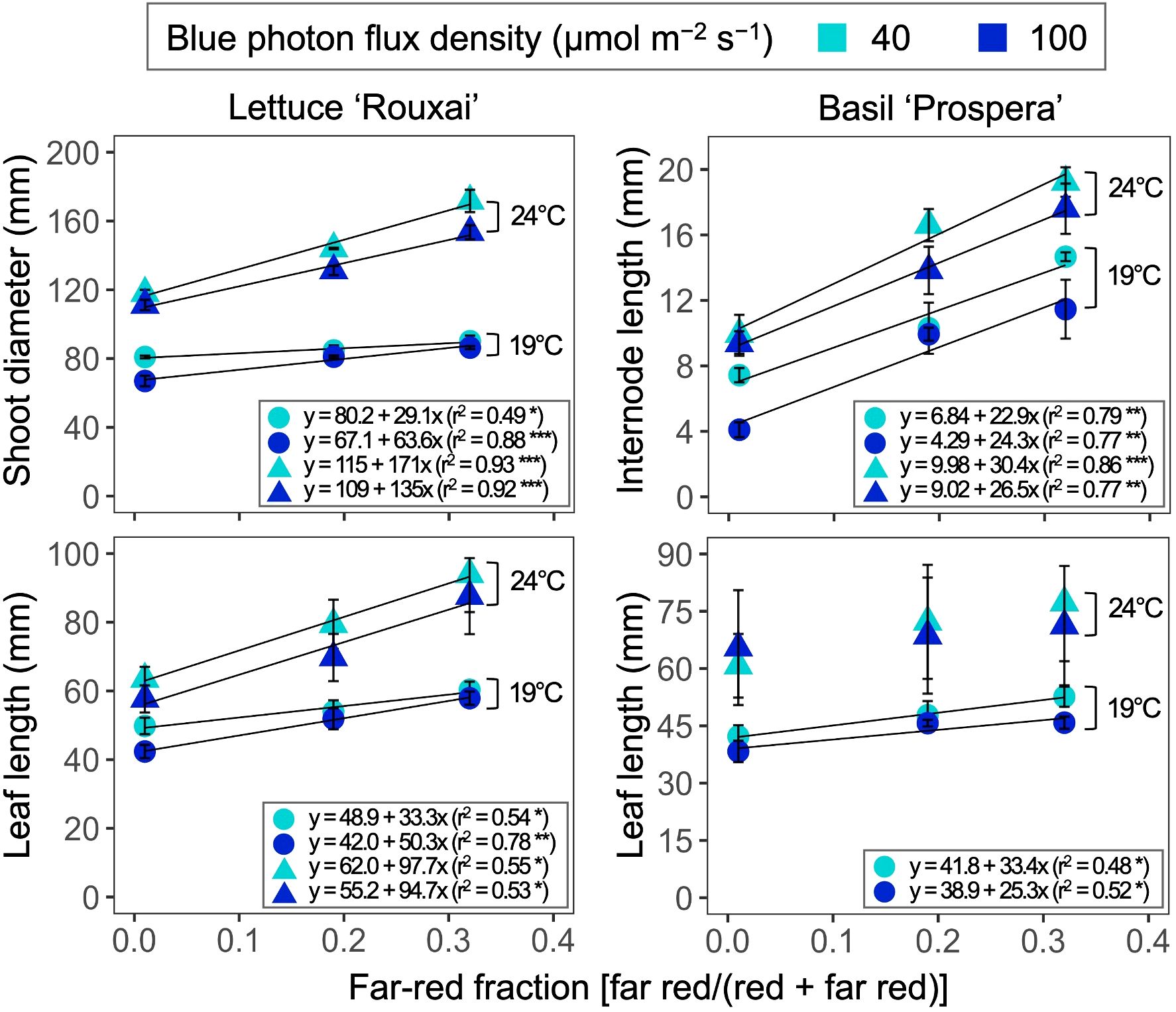
Figure 4. Influence of six photon spectra treatments at two air temperatures on the shoot diameter and leaf length of lettuce ‘Rouxai’ and the internode length and the leaf length of basil ‘Prospera’. The far-red fraction refers to the fraction of the far-red (FR; 700–750 nm) photon flux density relative to the sum of the red (R; 600–699 nm) and FR photon flux density. The unit for blue photon flux density in the legend is µmol m−2 s−1. Circle (19°C) and triangle (24°C) symbols represent air temperature. Each data point and error bar represents the mean and standard error for three replicates. Regression lines are presented when statistically significant (P < 0.05). The coefficient of determination (r2) and regression equations are presented for each air temperature and blue photon flux density. *, **, and *** indicate significance at P < 0.05, 0.01, or 0.001, respectively.
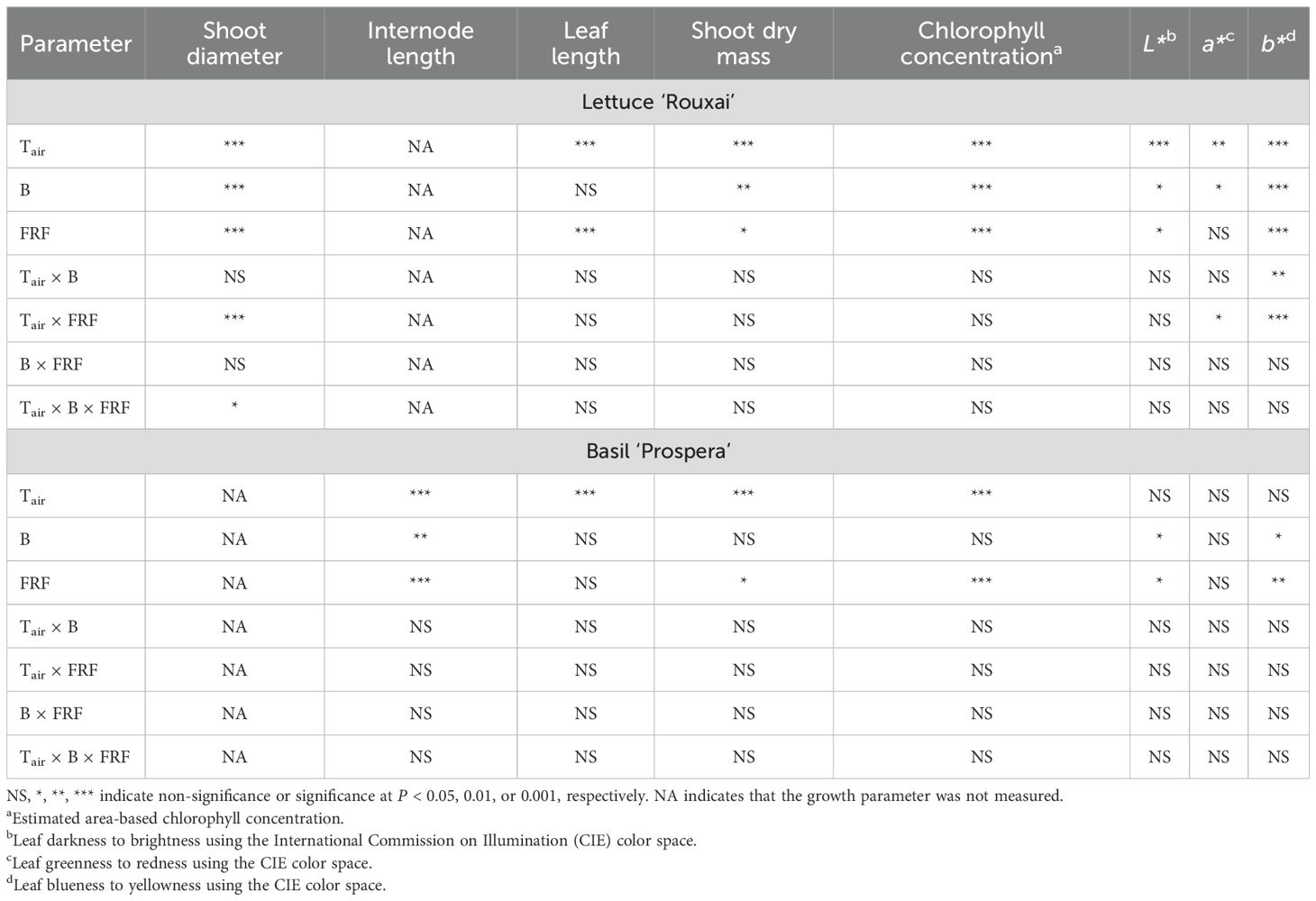
Table 3. Analysis of variance results for the effect of far-red fraction (FRF), blue (B; 400–499 nm) photon flux density, air temperature (Tair) and their interactions on plant growth and quality parameters of lettuce ‘Rouxai’ and basil ‘Prospera’.
Increasing the FR fraction increased the internode length by 1.9 to 2.8 times in basil, and the increased air temperature increased the internode length by 1.3 to 2.3 times. These combined effects caused a four-fold increase in internode length (from 4.1 to 17.6 mm). Decreasing the B-PFD caused a smaller but statistically significant increase in internode length (Figure 4, Table 3; Supplementary Table S2). Unlike lettuce, the influence of air temperature, FR fraction, and B-PFD were independent of each other in regulating basil internode length. The FR-fraction effect on basil leaf length was small and was only statistically significant at 19°C.
3.2 Shoot biomass and chlorophyll concentration
Increasing the air temperature from 19°C to 24°C increased the shoot dry mass of lettuce by 2.1 to 2.8 times, and the shoot dry mass of basil by 2.1 to 4.3 times (Tables 3; Supplementary Table S1, S2; Figure 5). Increasing the FR fraction from 0.01 to 0.32 significantly increased the shoot dry mass of lettuce only at 19°C and under a B-PFD of 100 µmol m−2 s−1. Overall, within the test range of 19–24°C, the influence of the FR fraction on the shoot dry mass of lettuce was not affected by air temperature. Similarly, increasing the FR fraction increased the shoot dry mass of basil only at 24°C and under the B-PFD of 40 µmol m−2 s−1; however, air temperature did not influence the effect of the FR fraction on the shoot dry mass. FR fraction did not interact with air temperature and B-PFD to regulate leaf number per plant (data not shown).
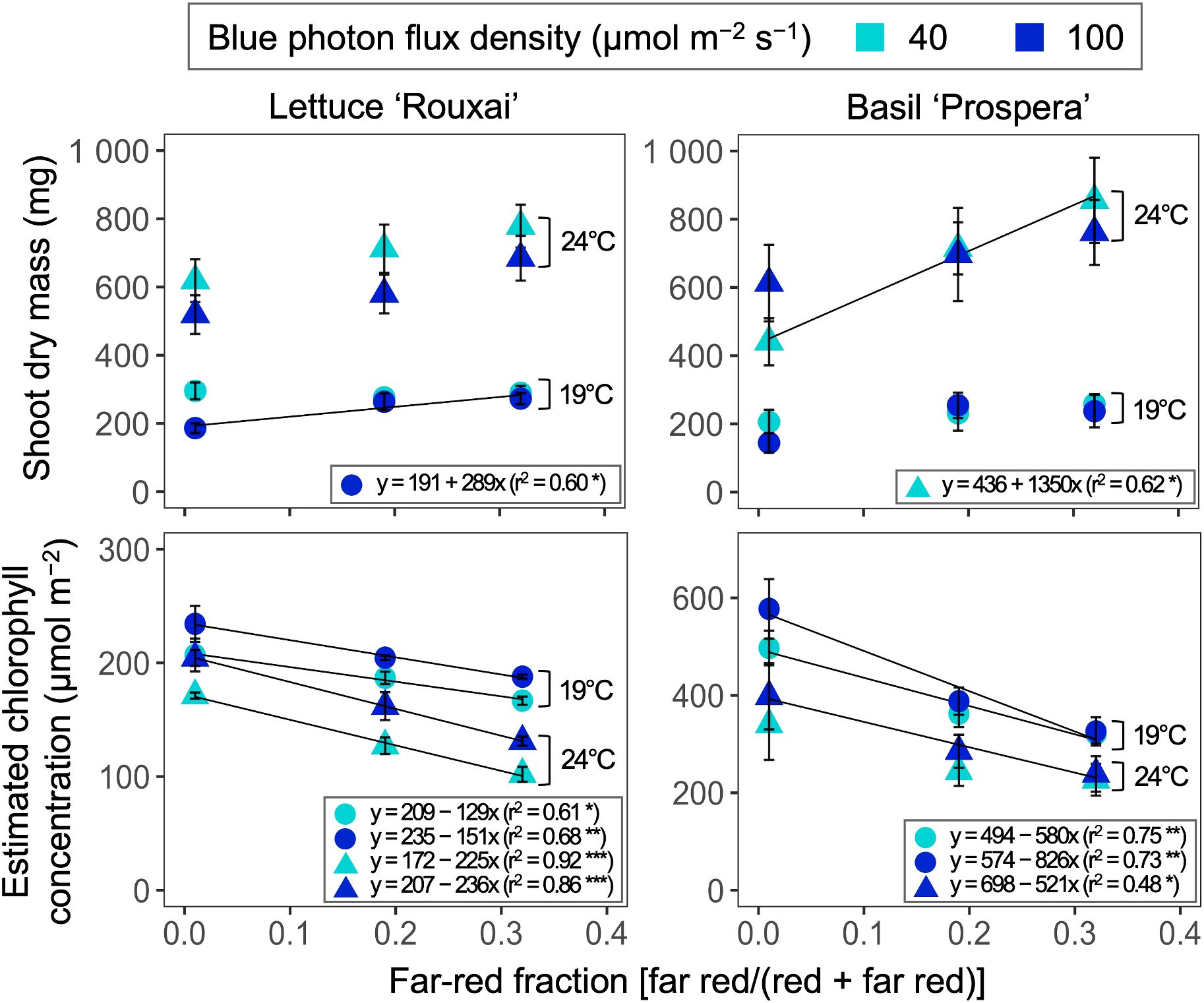
Figure 5. Influence of six photon spectra treatments at two air temperature treatments on the shoot dry mass and estimated area-based chlorophyll concentration of lettuce ‘Rouxai’ and basil ‘Prospera’. The far-red fraction refers to the fraction of the far-red (FR; 700–750 nm) photon flux density relative to the sum of the red (R; 600–699 nm) and FR photon flux density. The unit for blue photon flux density in the legend is µmol m−2 s−1. Circle (19°C) and triangle (24°C) symbols represent air temperature. Each data point and error bar represents the mean and standard error for three replicates. Regression lines are presented when statistically significant (P < 0.05). The coefficient of determination (r2) and regression equations are presented for each air temperature and blue photon flux density. *, **, and *** indicate significance at P < 0.05, 0.01, or 0.001, respectively.
Increasing the air temperature and FR fraction consistently decreased the estimated area-based chlorophyll concentration of both species, but B-PFD and air temperature did not influence the FR-mediated decrease in estimated chlorophyll concentration in either species.
3.3 Foliage coloration
Increasing the air temperature generally increased the leaf brightness (L*) in lettuce (Figures 2, 6, Table 3; Supplementary Table S1). Increasing the FR fraction from 0.01 to 0.32 significantly increased the brightness of lettuce leaves only at an air temperature of 24°C and under the B-PFD of 40 µmol m−2 s−1. However, overall, the influence of the FR fraction on the leaf brightness of lettuce was not affected by the air temperature and B-PFD. Compared to lettuce, the leaf brightness of basil was less or not responsive to the air temperature, FR fraction, and B-PFD.
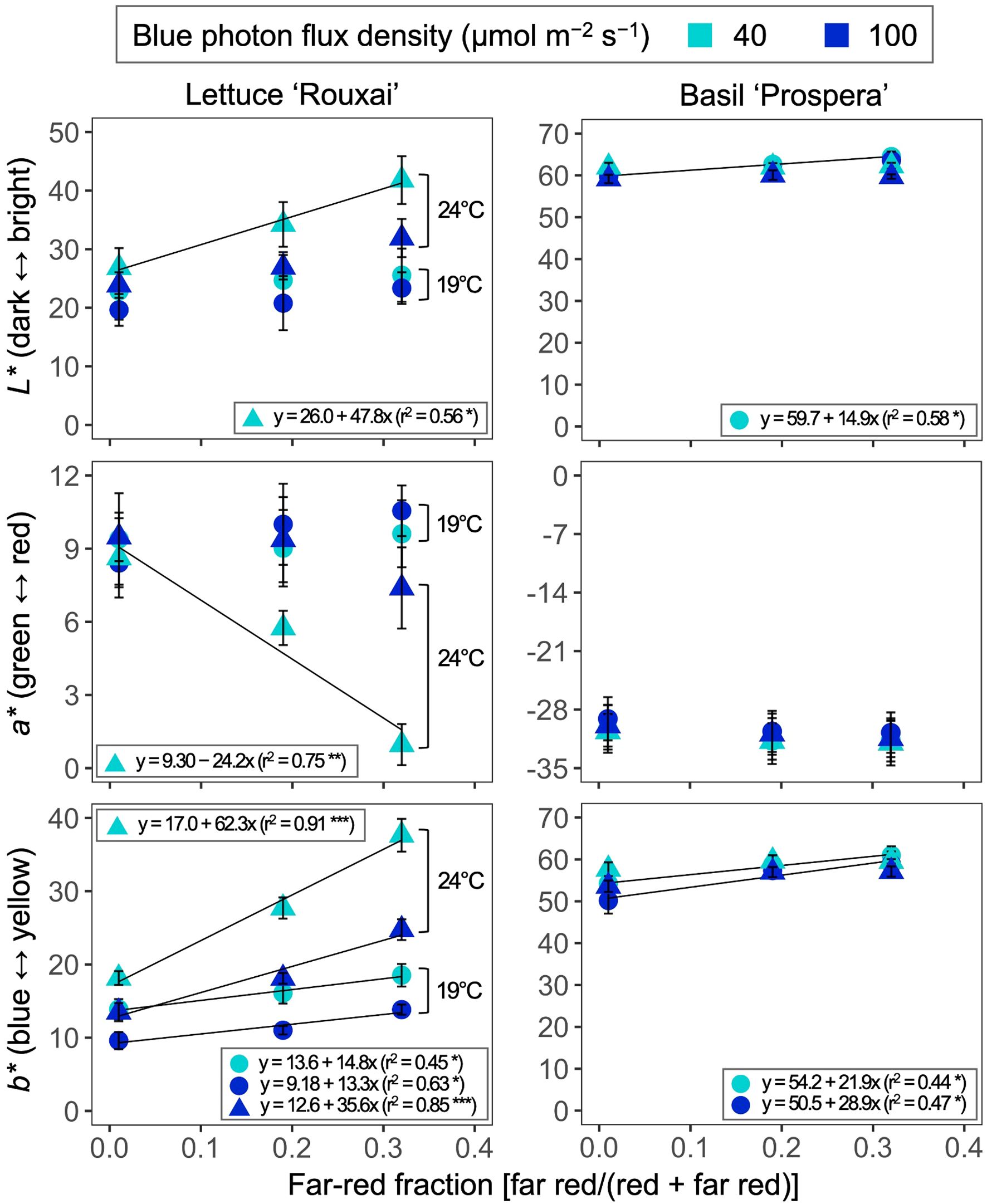
Figure 6. Influence of six photon spectra treatments at two air temperature treatments on CIE L*, a*, and b* of lettuce ‘Rouxai’ and basil ‘Prospera’. L* represents darkness-brightness (from 0 to 100), a* represents greenness-redness (from −128 to 127), and b* represents blueness-yellowness (from −128 to 127) based on the International Commission on Illumination. The far-red fraction refers to the fraction of the far-red (FR; 700–750 nm) photon flux density relative to the sum of the red (R; 600–699 nm) and FR photon flux density. The unit for blue photon flux density in the legend is µmol m−2 s−1. Circle (19°C) and triangle (24°C) symbols represent air temperature. Each data point and error bar represents the mean and standard error for three replicates. Regression lines are presented when statistically significant (P < 0.05). The coefficient of determination (r2) and regression equations are presented for each air temperature and blue photon flux density. *, **, and *** indicate significance at P < 0.05, 0.01, or 0.001, respectively.
Increasing the FR fraction usually did not influence the leaf redness (a*) of lettuce. However, the increase in the FR fraction decreased the redness of lettuce leaves at 24°C and under the low B-PFD. The air temperature, FR fraction, and B-PFD had little to no effect on the leaf redness of basil. The increase in the air temperature and the FR fraction and the decrease in the B-PFD generally increased the yellowness (b*) of lettuce. The FR-mediated increase in leaf yellowness was more pronounced at 24°C than at 19°C. Compared to lettuce, the leaf yellowness of basil was less or not responsive to the air temperature, FR fraction, and B-PFD.
4 Discussion
4.1 Mechanisms of the interaction between photon spectra and temperature
Lettuce shoot expansion elicited by an increase in the FR fraction was promoted by an increase in temperature. Similar to our results, the hypocotyl length of velvetleaf was more responsive to the FR fraction (or R:FR) at a day/night temperature of 26/20°C than at 18/16°C (Weinig, 2000). Also, hypocotyl elongation to the FR fraction was more pronounced at warmer temperatures in Arabidopsis (Romero-Montepaone et al., 2020; Burko et al., 2022). A high FR fraction reverts active PHYB to inactive PHYB, which increases the stability of PIFs (Sessa et al., 2005; Hersch et al., 2014). Similarly, high temperature decreases the activity of PHYB, which subsequently releases suppression on PIFs (Gray et al., 1998; Klose et al., 2015; Jung et al., 2016). In addition, high temperature reduces the suppression of PIFs in PHYB-independent manners. For example, high temperatures directly increase PIF7 expression (Chung et al., 2020; Fiorucci et al., 2020; Casal and Fankhauser, 2023). Also, high temperatures enhance PIF4 and PIF5 by downregulating EARLY FLOWERING 3, which is a negative regulator (Box et al., 2015; Raschke et al., 2015; Jung et al., 2020). Greater stability of PIF induced by a high FR fraction, combined with the increased PIF expression triggered by high temperatures, may amplify PIF effects (Romero-Montepaone et al., 2020; Burko et al., 2022; Casal and Fankhauser, 2023). Thus, FR-mediated auxin and gibberellin biosynthesis can be more pronounced at a high temperature, enhancing morphological responses to the FR fraction (Jones and Kaufman, 1983; Evans and Cleland, 1985).
An increase in the B-PFD diminished the interactive effects of the FR fraction and temperature on lettuce shoot expansion. To our knowledge, the modulation of the interaction between the FR fraction and temperature by B-PFD has not been previously reported. Cryptochromes can directly or indirectly suppress PIFs (Foreman et al., 2011; Ma et al., 2016; Pedmale et al., 2016), which is the convergence point of FR fraction and temperature signals (Romero-Montepaone et al., 2020; Burko et al., 2022; Casal and Fankhauser, 2023). Therefore, an increase in the B-PFD likely negates the interaction between the FR fraction and temperature (Castillon et al., 2007). This implies that the B-PFD can be a confounding factor when investigating and interpreting the interaction between the FR fraction and temperature in regulating plant morphology. However, although the three-way interaction among the FR fraction, temperature, and B-PFD was statistically significant in regulating the shoot expansion of lettuce, its biological significance was limited within the B-PFD range tested in our study.
4.2 Integrating spectra across multiple wavelengths: effects on internal phytochrome photoequilibria
The shoot diameter of lettuce and the leaf length of lettuce decreased as iPPE increased from 0.44 to 0.84 (i.e., the FR fraction decreased from 0.32 to 0.01) (Figure 7). The absolute slope of the regressed lines for the shoot diameter and leaf length of lettuce was greater when the air temperature was higher. This indicates a greater impact of iPPE on the shoot diameter and leaf length of lettuce at higher air temperatures, and vice versa. In contrast, while the increase in estimated iPPE also decreased the internode length of basil, the slope of the lines regressed for each temperature was similar. This indicates that the influence of iPPE on regulating the internode length of basil is not affected by temperature in the range tested in our study. These response differences between temperatures and plant species suggest that the versatility and power of iPPE in estimating the growth responses of plants can decrease when iPPE is used in a range of temperatures and/or plant species. Similar to these findings, the effects of PPE on the growth and morphology varied among plant species (Park and Runkle, 2017). Also, although specific responses differed, the effects of PPE on the growth and morphology of lettuce and basil varied by air temperature (Jeong et al., 2024a).
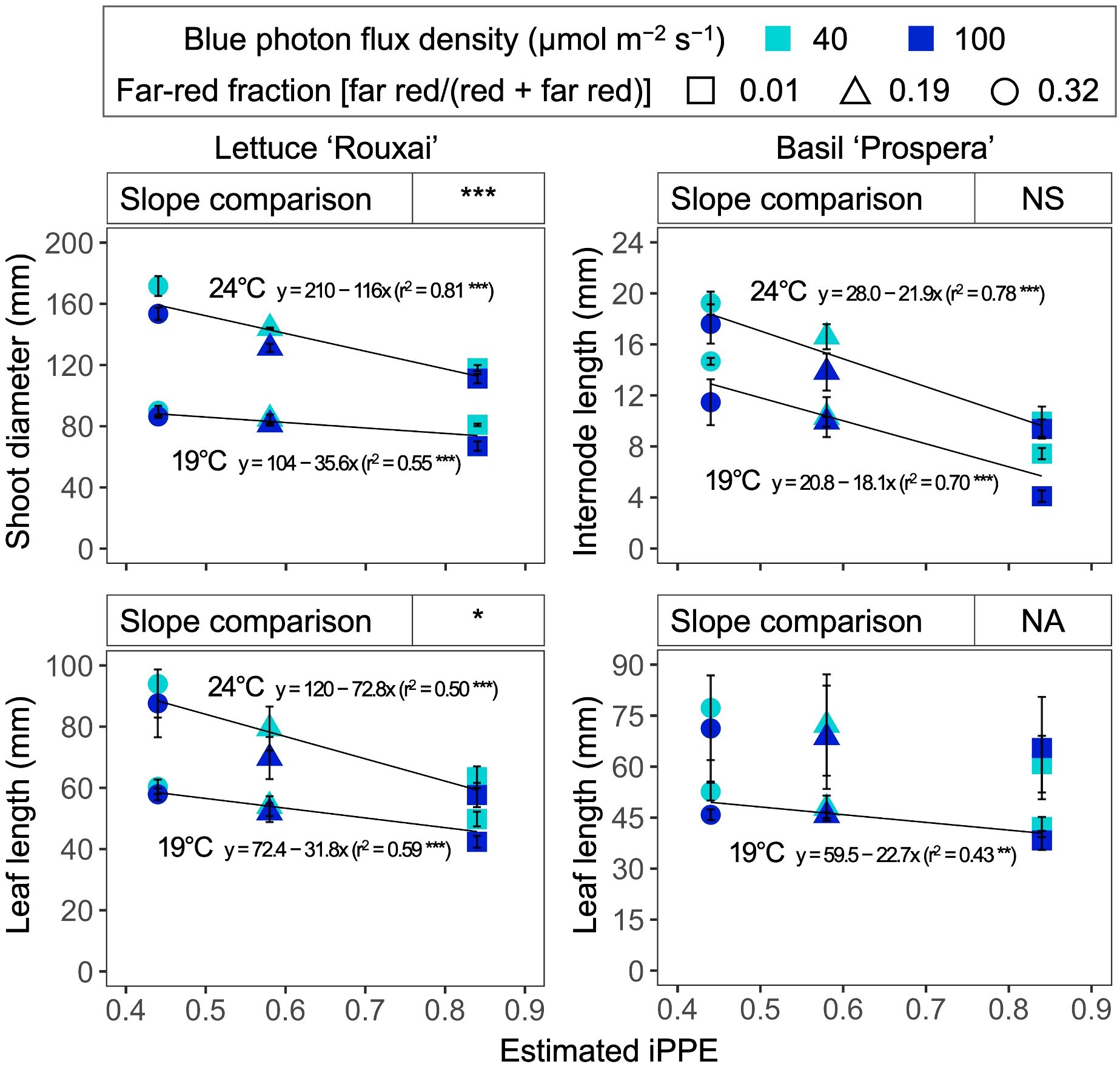
Figure 7. Influence of the estimated internal phytochrome photoequilibria (iPPE; Kusuma and Bugbee, 2021b) of six photon spectra treatments at two air temperature treatments on the shoot diameter and leaf length of lettuce ‘Rouxai’ and on the internode and leaf length of basil ‘Prospera’. The unit for blue (400–499 nm) photon flux density in the legend is µmol m−2 s−1. FR fraction in the legend refers to the fraction of the far-red (FR; 700–750 nm) photon flux density relative to the sum of the red (R; 600–699 nm) and FR photon flux density. Each data point and error bar represents the mean and standard error for three replicates. Regression lines are presented when statistically significant (P < 0.05). The coefficient of determination (r2) and regression equations are presented for each air temperature. The slope comparison at the top of each subfigure refers to the difference between the slope of the line regressed for each temperature. NA indicates that the slope comparison was not applicable. NS, *, or *** indicate non-significance or significance at P < 0.05 or 0.001, respectively.
4.3 The interaction between photon spectra and temperature on foliage coloration
The FR fraction and temperature interacted to regulate leaf redness (i.e., CIE a*) of lettuce ‘Rouxai’. Specifically, lettuce was the most green (least red) at the higher temperature, lower B-PFD, and highest FR fraction, but the effects of FR fraction were greatly diminished at the lower temperature and higher B-PFD. Considering that the responses of a* to the temperature, B-PFD, and FR fraction differed from those of the estimated area-based chlorophyll concentration, the decreases in a* are more likely from a decrease in leaf redness than an increase in leaf greenness. A high temperature represses anthocyanin biosynthesis and facilitates the degradation of accumulated anthocyanins by degrading the HY5 protein in a COP1 activity-dependent manner and by repressing anthocyanin biosynthetic MYB gene expression (Kim et al., 2017; Rehman et al., 2017). The regulation of anthocyanin biosynthesis induced by B photons is also mediated by HY5, COP1, and MYB transcription factors (Borevitz et al., 2000; Meng and Runkle, 2019; LaFountain and Yuan, 2021). Due to the overlap of the regulatory pathway, the high temperature in this study might have amplified the effects of a low B-PFD in decreasing the anthocyanin concentration and leaf redness. Also, amplified extension growth by a high FR fraction and a low B-PFD at the high air temperature might have diluted the anthocyanin content, thus decreasing the anthocyanin concentration and leaf coloration.
Although foliage coloration can provide proxies of pigment concentration, it does not always perfectly align with the concentration of individual pigments (Kelly and Runkle, 2023). This is partly because foliage color is determined by the combined effects of multiple pigments, which can mask one another. Thus, the interpretation of a* values in this study should be considered an indirect estimation of pigmentation. Similarly, as we estimated chlorophyll concentration through SPAD measurement (Parry et al., 2014), direct pigment extraction and measurement would be needed to confirm the interpretations.
4.4 Ecological implications of the interaction between spectral quality and temperature
An increase in temperature increased the responsiveness to FR light in cold-tolerant lettuce but not in cold-sensitive basil. Ecologically, the synergistic interaction between FR photons and temperature in inducing extension growth has been proposed as a morphological acclimation to enhance light capture under shaded conditions, particularly when respiratory demand is high (e.g., at elevated temperatures) (Romero-Montepaone et al., 2021; Casal and Fankhauser, 2023). This interaction between FR photons and temperature has been reported primarily in cold-tolerant plants (i.e., those with a low base temperature) such as Arabidopsis and lettuce (Jung et al., 2016; Romero-Montepaone et al., 2020, 2021; Burko et al., 2022; Jeong et al., 2024b) and is apparently uncommon in cold-sensitive plants (Weinig, 2000). Plants acclimated or adapted to high temperatures generally exhibit low respiration rates and thus have a higher optimum temperature for photosynthesis (Berry and Bjorkman, 1980; Hikosaka et al., 2006; Silim et al., 2010; Wataru et al., 2014). This suggests that cold-sensitive plants would exhibit a smaller increase in carbon demand in response to the same rise in temperature compared with cold-tolerant plants. Therefore, the interaction between FR photons and temperature may be less likely in cold-sensitive crops than in tolerant crops.
The contrasting responses of lettuce (leaf expansion) and basil (stem elongation) to simulated shade conditions (low B-PFD, high FR fraction, or both) and temperature may also be representative of shade-avoidant and shade-tolerant species. These response categories were recently reviewed by Xu et al. (2021) and Casal and Fankhauser (2023), and the implications for breeding high-density soybean genotypes were reviewed by Lyu et al. (2023). The difference might also be linked to the breeding history of lettuce, which focused on extending the harvest window and increasing yield through delaying bolting, during which notable stem elongation occurs (Han et al., 2021). Further investigation with additional cold-sensitive/cold-tolerant plants and shade-avoidant and shade-tolerant plants is needed to determine whether the differences between lettuce and basil in this study are fundamental temperature or shade responses or merely species-specific variation.
The lack of interaction between temperature and the FR fraction in inducing morphological changes in basil has been associated with reduced regulation of basil morphology by the photon spectrum (Meng and Runkle, 2019) relative to other species such as lettuce and Arabidopsis. The plasticity of responses to photon spectra (including PHYB activity and phenotype) can depend on population origin (Ikeda et al., 2021). Species endemic to lower latitudes (e.g., Cardamine nipponica) are sometimes less sensitive to R and FR photons than species of the same genus but endemic to higher latitudes (e.g., Cardamine bellidifolia) due to innate lower PFR stability of PHYB in the lower-latitude species. The shorter twilight period (when there is a meaningful R:FR change) at lower latitudes than at higher latitudes could have contributed to such adaptation of a PHYB response. Basil originates from sub-tropical and tropical regions (i.e., low latitudes), which have a relatively short twilight period and could have a lower PFR stability and functionality, which can decrease the sensitivity to the FR fraction. Considering that the synergism between the FR fraction and temperature can potentially occur due to the combination of FR-mediated PIF stability increase and temperature-mediated PIF expression increase (Romero-Montepaone et al., 2020; Burko et al., 2022; Casal and Fankhauser, 2023), the innate low sensitivity of basil to the FR fraction might have caused less of an FR-mediated PIF stability increase to make the synergism weaker. Similar to our results, the plant height increase elicited by the FR fraction was similar at 20°C and 24°C in basil ‘Genovese’ (Jeong et al., 2024a).
An elevated B-PFD mitigated the interactive effect between the FR fraction and temperature in regulating lettuce shoot expansion. An increase in B-PFD is an environmental cue indicating sun exposure and high carbon availability (Foreman et al., 2011; Ma et al., 2016; Pedmale et al., 2016). Therefore, our findings suggest that increased B-PFD can function to reduce excessive resource allocation towards extension growth caused by the interaction between the FR fraction and temperature in some cold-tolerant plants such as lettuce.
5 Conclusions
Photon spectra (FR fraction and B-PFD) and air temperature interactively regulated the morphology and foliage coloration of lettuce but not basil. Specifically, a high air temperature amplified the effects of a high FR fraction in inducing shoot expansion in lettuce. An increase in the B-PFD, which did not directly interact with air temperature in the range tested in our study, negated the interaction between the FR fraction and temperature. This suggests that the B-PFD can confound the interpretation of the synergism between the FR fraction and temperature in eliciting morphological responses. Such interaction between the FR fraction, B-PFD, and temperature did not occur in basil, which not surprisingly, indicates species-specific variation in morphological responses to the environment.
Data availability statement
Biometric and environmental data are available at https://doi.org/10.15482/USDA.ADC/28121552.
Author contributions
JS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. BB: Visualization, Writing – review & editing. ER: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work is supported by the Specialty Crops Research Initiative, project award no. 2019-51181-30017, and Hatch project award no. 192266 from the U.S. Department of Agriculture’s National Institute of Food and Agriculture.
Acknowledgments
We thank Dr. Chieri Kubota at the Ohio State University and Dr. Bert Cregg at Michigan State University for their critical review of this manuscript. We also thank Nathan DuRussel for technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1675087/full#supplementary-material
References
Ahmad, M., Lin, C., and Cashmore, A. R. (1995). Mutations throughout an Arabidopsis blue-light photoreceptor impair blue-light-responsive anthocyanin accumulation and inhibition of hypocotyl elongation. Plant J. 8, 653–658. doi: 10.1046/j.1365-313X.1995.08050653.x
Banerjee, R. and Batschauer, A. (2005). Plant blue-light receptors. Planta 220, 498–502. doi: 10.1007/s00425-004-1418-z
Berry, J. and Bjorkman, O. (1980). Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 31, 491–543. doi: 10.1146/annurev.pp.31.060180.002423
Borevitz, J. O., Xia, Y., Blount, J., Dixon, R. A., and Lamb, C. (2000). Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12, 2383–2393. doi: 10.1105/tpc.12.12.2383
Box, M. S., Huang, B. E., Domijan, M., Jaeger, K. E., Khattak, A. K., Yoo, S. J., et al. (2015). ELF3 controls thermoresponsive growth in Arabidopsis. Curr. Biol. 25, 194–199. doi: 10.1016/j.cub.2014.10.076
Burgie, E. S. and Vierstra, R. D. (2014). Phytochromes: an atomic perspective on photoactivation and signaling. Plant Cell 26, 4568–4583. doi: 10.1105/tpc.114.131623
Burko, Y., Willige, B. C., Seluzicki, A., Novák, O., Ljung, K., and Chory, J. (2022). PIF7 is a master regulator of thermomorphogenesis in shade. Nat. Commun. 13, 4942. doi: 10.1038/s41467-022-32585-6
Carvalho, S. D. and Folta, K. M. (2014). Sequential light programs shape kale (Brassica napus) sprout appearance and alter metabolic and nutrient content. Hortic. Res. 1, 8. doi: 10.1038/hortres.2014.8
Casal, J. J. (2013). Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 64, 403–427. doi: 10.1146/annurev-arplant-050312-120221
Casal, J. J. and Fankhauser, C. (2023). Shade avoidance in the context of climate change. Plant Physiol. 191, 1475–1491. doi: 10.1093/plphys/kiad004
Cashmore, A. R., Jarillo, J. A., Wu, Y. J., and Liu, D. (1999). Cryptochromes: blue light receptors for plants and animals. Science 284, 760–765. doi: 10.1126/science.284.5415.760
Castillon, A., Shen, H., and Huq, E. (2007). Phytochrome interacting factors: central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 12, 514–521. doi: 10.1016/j.tplants.2007.10.001
Chaves, I., Pokorny, R., Byrdin, M., Hoang, N., Ritz, T., Brettel, K., et al. (2011). The cryptochromes: blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 62, 335–364. doi: 10.1146/annurev-arplant-042110-103759
Chung, B. Y. W., Balcerowicz, M., Di Antonio, M., Jaeger, K. E., Geng, F., Franaszek, K., et al. (2020). An RNA thermoswitch regulates daytime growth in Arabidopsis. Nat. Plants 6, 522–532. doi: 10.1038/s41477-020-0633-3
Erwin, J. E., Heins, R. D., and Karlsson, M. G. (1989). Thermomorphogenesis in lilium longiflorum. Am. J. Bot. 76, 47–52. doi: 10.1002/j.1537-2197.1989.tb11283.x
Evans, M. L. and Cleland, R. E. (1985). The action of auxin on plant cell elongation. Crit. Rev. Plant Sci. 2, 317–365. doi: 10.1080/07352688509382200
Evans, J. R. and Poorter, H. (2001). Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 24, 755–767. doi: 10.1046/j.1365-3040.2001.00724.x
Fiorucci, A. S., Galvão, V. C., Ince, Y. Ç., Boccaccini, A., Goyal, A., Allenbach Petrolati, L., et al. (2020). PHYTOCHROME INTERACTING FACTOR 7 is important for early responses to elevated temperature in Arabidopsis seedlings. New Phytol. 226, 50–58. doi: 10.1111/nph.16316
Foreman, J., Johansson, H., Hornitschek, P., Josse, E. M., Fankhauser, C., and Halliday, K. J. (2011). Light receptor action is critical for maintaining plant biomass at warm ambient temperatures. Plant J. 65, 441–452. doi: 10.1111/j.1365-313X.2010.04434.x
Franklin, K. A. (2008). Shade avoidance. New Phytol. 179, 930–944. doi: 10.1111/j.1469-8137.2008.02507.x
Fukuda, M., Matsuo, S., Kikuchi, K., Kawazu, Y., Fujiyama, R., and Honda, I. (2011). Isolation and functional characterization of the FLOWERING LOCUS T homolog, the LsFT gene, in lettuce. J. Plant Physiol. 168, 1602–1607. doi: 10.1016/j.jplph.2011.02.004
Gommers, C. M., Visser, E. J., St Onge, K. R., Voesenek, L. A., and Pierik, R. (2013). Shade tolerance: when growing tall is not an option. Trends Plant Sci. 18, 65–71. doi: 10.1016/j.tplants.2012.09.008
Gray, W. M., Östin, A., Sandberg, G., Romano, C. P., and Estelle, M. (1998). High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc. Natl. Acad. Sci. 95, 7197–7202. doi: 10.1073/pnas.95.12.7197
Han, R., Truco, M. J., Lavelle, D. O., and Michelmore, R. W. (2021). A composite analysis of flowering time regulation in lettuce. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.632708
Hersch, M., Lorrain, S., de Wit, M., Trevisan, M., Ljung, K., Bergmann, S., et al. (2014). Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc. Natl. Acad. Sci. 111, 6515–6520. doi: 10.1073/pnas.1320355111
Hikosaka, K., Ishikawa, K., Borjigidai, A., Muller, O., and Onoda, Y. (2006). Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J. Exp. Bot. 57, 291–302. doi: 10.1093/jxb/erj049
Ikeda, H., Suzuki, T., Oka, Y., Gustafsson, A. L. S., Brochmann, C., Mochizuki, N., et al. (2021). Divergence in red light responses associated with thermal reversion of phytochrome B between high-and low-latitude species. New Phytol. 231, 75–84. doi: 10.1111/nph.17381
Jeong, S. J., Niu, G., and Zhen, S. (2024a). Far-red light and temperature interactively regulate plant growth and morphology of lettuce and basil. Environ. Exp. Bot.. 218, 105589. doi: 10.1016/j.envexpbot.2023.105589
Jeong, S. J., Zhang, Q., Niu, G., and Zhen, S. (2024b). The interactive effects between far-red light and temperature on lettuce growth and morphology diminish at high light intensity. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1497672
Jones, R. L. and Kaufman, P. B. (1983). The role of gibberellins in plant cell elongation. Crit. Rev. Plant Sci. 1, 23–47. doi: 10.1080/07352688309382170
Jung, J. H., Barbosa, A. D., Hutin, S., Kumita, J. R., Gao, M., Derwort, D., et al. (2020). A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Science 585, 256–260. doi: 10.1038/s41586-020-2644-7
Jung, J. H., Domijan, M., Klose, C., Biswas, S., Ezer, D., Gao, M., et al. (2016). Phytochromes function as thermosensors in Arabidopsis. Science 354, 886–889. doi: 10.1126/science.aaf6005
Keller, M. M., Jaillais, Y., Pedmale, U. V., Moreno, J. E., Chory, J., and Ballaré, C. L. (2011). Cryptochrome 1 and phytochrome B control shade-avoidance responses in Arabidopsis via partially independent hormonal cascades. Plant J. 67, 195–207. doi: 10.1111/j.1365-313X.2011.04598.x
Kelly, N. and Runkle, E. S. (2023). Ultraviolet A and blue light transiently regulate total phenolic and anthocyanin concentrations in indoor-grown red-leaf lettuce. HortScience 58, 1595–1602. doi: 10.21273/HORTSCI17395-23
Kelly, N. and Runkle, E. S. (2024). Dependence of far-red light on red and green light at increasing growth of lettuce. PloS One 19, e0313084. doi: 10.1371/journal.pone.0313084
Keuskamp, D. H., Sasidharan, R., and Pierik, R. (2010). Physiological regulation and functional significance of shade avoidance responses to neighbors. Plant Signal. Behav. 5, 655–662. doi: 10.4161/psb.5.6.11401
Kim, S., Hwang, G., Lee, S., Zhu, J. Y., Paik, I., Nguyen, T. T., et al. (2017). High ambient temperature represses anthocyanin biosynthesis through degradation of HY5. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01787
Klose, C., Nagy, F., and Schäfer, E. (2020). Thermal reversion of plant phytochromes. Mol. Plant 13, 386–397. doi: 10.1016/j.molp.2019.12.004
Klose, C., Venezia, F., Hussong, A., Kircher, S., Schäfer, E., and Fleck, C. (2015). Systematic analysis of how phytochrome B dimerization determines its specificity. Nat. Plants 1, 1–9. doi: 10.1038/NPLANTS.2015.90
Koini, M. A., Alvey, L., Allen, T., Tilley, C. A., Harberd, N. P., Whitelam, G. C., et al. (2009). High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr. Biol. 19, 408–413. doi: 10.1016/j.cub.2009.01.046
Kusuma, P. and Bugbee, B. (2021a). Far-red fraction: An improved metric for characterizing phytochrome effects on morphology. J. Am. Soc Hortic. Sci. 146, 3–13. doi: 10.21273/JASHS05002-20
Kusuma, P. and Bugbee, B. (2021b). Improving the predictive value of phytochrome photoequilibrium: consideration of spectral distortion within a leaf. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.596943
Kusuma, P. and Bugbee, B. (2023). On the contrasting morphological response to far-red at high and low photon fluxes. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1185622
LaFountain, A. M. and Yuan, Y. W. (2021). Repressors of anthocyanin biosynthesis. New Phytol. 231, 933–949. doi: 10.1111/nph.17397
Lagarias, J. C., Kelly, J. M., Cyr, K. L., and Smith, W. O., Jr. (1987). Comparative photochemical analysis of highly purified 124 kilodalton oat and rye phytochromes in vitro. Photochem. Photobiol 46, 5–13. doi: 10.1111/j.1751-1097.1987.tb04729.x
Legris, M., Klose, C., Burgie, E. S., Rojas, C. C. R., Neme, M., Hiltbrunner, A., et al. (2016). Phytochrome B integrates light and temperature signals in Arabidopsis. Science 354, 897–900. doi: 10.1126/science.aaf5656
Leivar, P. and Quail, P. H. (2011). PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 16, 19–28. doi: 10.1016/j.tplants.2010.08.003
Li, T., Jia, K. P., Lian, H. L., Yang, X., Li, L., and Yang, H. Q. (2014). Jasmonic acid enhancement of anthocyanin accumulation is dependent on phytochrome. A signaling pathway under far-red light in Arabidopsis. Biochem. Biophys. Res. Commun. 454, 78–83. doi: 10.1016/j.bbrc.2014.10.059
Li, Q. and Kubota, C. (2009). Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 67, 59–64. doi: 10.1016/j.envexpbot.2009.06.011
Liu, Z., Zhang, Y., Wang, J., Li, P., Zhao, C., Chen, Y., et al. (2015). Phytochrome-interacting factors PIF4 and PIF5 negatively regulate anthocyanin biosynthesis under red light in Arabidopsis seedlings. Plant Sci. 238, 64–72. doi: 10.1016/j.plantsci.2015.06.001
Lyu, X., Mu, R., and Liu, B. (2023). Shade avoidance syndrome in soybean and ideotype toward shade tolerance. Mol. Breed. 43, 31. doi: 10.1007/s11032-023-01375-3
Ma, D., Guo, Y., Chu, J., Fang, S., Yan, C., Noel, J. P., et al. (2016). Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc. Natl. Acad. Sci. 113, 224–229. doi: 10.1073/pnas.1511437113
Meng, Q. and Runkle, E. S. (2019). Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 255, 269–280. doi: 10.1016/j.scienta.2019.05.030
Paradiso, R. and Proietti, S. (2021). Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: the state of the art and the opportunities of modern LED systems. J. Plant Growth Regul., 1–39. doi: 10.1007/s00344-021-10337-y
Park, Y. and Runkle, E. S. (2017). Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 136, 41–49. doi: 10.1016/j.envexpbot.2016.12.013
Parry, C., Blonquist, J. M., Jr., and Bugbee, B. (2014). In situ measurement of leaf chlorophyll concentration: analysis of the optical/absolute relationship. Plant Cell Environ. 37, 2508–2520. doi: 10.1111/pce.12324
Patel, D., Basu, M., Hayes, S., Majlath, I., Hetherington, F. M., Tschaplinski, T. J., et al. (2013). Temperature-dependent shade avoidance involves the receptor-like kinase ERECTA. Plant J. 73, 980–992. doi: 10.1111/tpj.12088
Pedmale, U. V., Huang, S. S. C., Zander, M., Cole, B. J., Hetzel, J., Ljung, K., et al. (2016). Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell 164, 233–245. doi: 10.1016/j.cell.2015.12.018
Possart, A., Fleck, C., and Hiltbrunner, A. (2014). Shedding (far-red) light on phytochrome mechanisms and responses in land plants. Plant Sci. 217, 36–46. doi: 10.1016/j.plantsci.2013.11.013
Proveniers, M. C. and van Zanten, M. (2013). High temperature acclimation through PIF4 signaling. Trends Plant Sci. 18, 59–64. doi: 10.1016/j.tplants.2012.09.002
Qiu, Y., Li, M., Jean, R., Moore, C. M., and Chen, M. (2019). Daytime temperature is sensed by phytochrome B in Arabidopsis through a transcriptional activator HEMERA. Nat. Commun. 10, 1–13. doi: 10.1038/s41467-018-08059-z
Raschke, A., Ibañez, C., Ullrich, K. K., Anwer, M. U., Becker, S., Glöckner, A., et al. (2015). Natural variants of ELF3 affect thermomorphogenesis by transcriptionally modulating PIF4-dependent auxin response genes. BMC Plant Biol. 15, 1–10. doi: 10.1186/s12870-015-0566-6
R Core Team (2021). R: a language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available online at: https://www.R-project.org/ (Accessed July 1, 2025).
Rehman, R. N. U., You, Y., Zhang, L., Goudia, B. D., Khan, A. R., Li, P., et al. (2017). High temperature induced anthocyanin inhibition and active degradation in Malus profusion. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01401
Romero-Montepaone, S., Poodts, S., Fischbach, P., Sellaro, R., Zurbriggen, M. D., and Casal, J. J. (2020). Shade avoidance responses become more aggressive in warm environments. Plant Cell Environ. 43, 1625–1636. doi: 10.1111/pce.13720
Romero-Montepaone, S., Sellaro, R., Esteban Hernando, C., Costigliolo-Rojas, C., Bianchimano, L., Ploschuk, E. L., et al. (2021). Functional convergence of growth responses to shade and warmth in Arabidopsis. New Phytol. 231, 1890–1905. doi: 10.1111/nph.17430
Sager, J. C., Smith, W. O., Edwards, J. L., and Cyr, K. L. (1988). Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Transac. ASAE 31, 1882–1889. doi: 10.13031/2013.30952
Sessa, G., Carabelli, M., Sassi, M., Ciolfi, A., Possenti, M., Mittempergher, F., et al. (2005). A dynamic balance between gene activation and repression regulates the shade avoidance response in Arabidopsis. Genes Dev. 19, 2811–2815. doi: 10.1101/gad.364005
Sharrock, R. A. and Clack, T. (2002). Patterns of expression and normalized levels of the five Arabidopsis phytochromes. Plant Physiol. 130, 442–456. doi: 10.1104/pp.005389
Sheerin, D. J., Menon, C., zur Oven-Krockhaus, S., Enderle, B., Zhu, L., Johnen, P., et al. (2015). Light-activated phytochrome A and B interact with members of SPA family to promote photomorphogenesis in Arabidopsis by reorganinzing the COP1/SPA complex. Plant Cell 27 (1), 189–201. doi: 10.10.1105/tpc.114.134775
Shin, J. and Runkle, E. S. (2024). Considerations of utilizing far-red light in the production of leafy-green vegetables indoors. Eur. J. Hortic. Sci. 89 (3). doi: 10.17660/eJHS.2024/012
Shin, J. and Runkle, E. S. (2025). Plant morphology and a phytochrome B model reveal that the effects of far-red light on shade-avoidance-like responses persist under high light intensity. Plant Cell Environ. 48 (8), 5802–58918. doi: 10.10.1111/pce.15562
Silim, S. N., Ryan, N., and Kubien, D. S. (2010). Temperature response of photosynthesis and respiration in Populus balsamifera L.: acclimation versus adaptation. Photosynth. Res. 104, 19–30. doi: 10.1007/s11120-010-9527-y
Skabelund, H. A., Langenfeld, N. J., and Bugbee, B. (2025). On the lack of morphological response to far-red at high and low photon flux in spinach. HortScience 60, 415–418. doi: 10.21273/HORTSCI18410-24
Snowden, M. C., Cope, K. R., and Bugbee, B. (2016). Sensitivity of seven diverse species to blue and green light: interactions with photon flux. PloS One 11, e0163121. doi: 10.1371/journal.pone.0163121
Tarr, S. T., Valle de Souza, S., and Lopez, R. G. (2023). Influence of day and night temperature and radiation intensity on growth, quality, and economics of indoor green butterhead and red oakleaf lettuce production. Sustainability 15, 829. doi: 10.3390/su15010829
Walters, K. J. and Currey, C. J. (2019). Growth and development of basil species in response to temperature. HortScience 54, 1915–1920. doi: 10.21273/HORTSCI12976-18
Wataru, Y., Hikosaka, K., and Way, D. A. (2014). Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth. Res. 119, 101–117. doi: 10.1007/s11120-013-9874-6
Weinig, C. (2000). Limits of adaptive plasticity: temperature and photoperiod influence shade-avoidance responses. Am. J. Bot. 87, 1660–1668. doi: 10.2307/2656743
Xu, H., Chen, P., and Tao, Y. (2021). Understanding the shade tolerance responses through hints from phytochrome A-mediated negative feedback regulation in shade avoiding plants. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.813092
Zhang, J., Stankey, R. J., and Vierstra, R. D. (2013). Structure-guided engineering of plant phytochrome B with altered photochemistry and light signaling. Plant Physiol. 161, 1445–1457. doi: 10.1104/pp.112.208892
Zhen, S. and Bugbee, B. (2020). Far-red photons have equivalent efficiency to traditional photosynthetic photons: implications for redefining photosynthetically active radiation. Plant Cell Environ. 43, 1259–1272. doi: 10.1111/pce.13730
Zhen, S., van Iersel, M., and Bugbee, B. (2021). Why far-red photons should be included in the definition of photosynthetic photons and the measurement of horticultural fixture efficacy. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.693445
Keywords: photomorphogenesis, thermomorphogenesis, far-red light, blue light, phytochrome
Citation: Shin J, Bugbee B and Runkle ES (2025) Contrasting interactions between photon spectra and temperature in cold-sensitive basil and cold-tolerant lettuce. Front. Plant Sci. 16:1675087. doi: 10.3389/fpls.2025.1675087
Received: 28 July 2025; Accepted: 12 September 2025;
Published: 24 September 2025.
Edited by:
Xiuming Hao, Agriculture and Agri-Food Canada (AAFC), CanadaReviewed by:
Christopher P. Levine, The University of Tokyo, JapanJordan Van Brenk, Wageningen University and Research, Netherlands
Copyright © 2025 Shin, Bugbee and Runkle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiyong Shin, c2hpbmppeW9AbXN1LmVkdQ==
 Jiyong Shin
Jiyong Shin Bruce Bugbee
Bruce Bugbee Erik S. Runkle
Erik S. Runkle