- 1Engineering Research Center for Urban Modern Agriculture of Higher Education in Yunnan Province, School of Agriculture and Life Sciences, Kunming University, Kunming, Yunnan, China
- 2School of Mathematics Kunming University, Kunming University, Kunming, Yunnan, China
- 3School of Pharmacy, Chengdu University, Chengdu, Sichuan, China
- 4School of Mechanical and Electrical Engineering, Kunming University, Kunming, Yunnan, China
- 5Yunnan Hanzhe Technology Co., Ltd., Kunming, Yunnan, China
- 6Yulong County Jiuhe Xinxing Agricultural Development and Planting Co., Ltd., Lijiang, Yunnan, China
- 7Shangri-La Zangmei Agricultural Technology Co., Ltd., Diqing, Yunnan, China
Strawberry (Fragaria × ananassa) is a nutritionally valuable and widely popular fruit worldwide. Drought stress is a key factor affecting strawberry production; however, previous studies lacked in depth research on the physiological, biochemical, and molecular regulatory mechanism differences among various strawberry varieties. This study systematically examined the physiological and molecular responses of two cultivars, ‘Benihoppe’ and ‘Kaorino’, to drought stress. Under mild and severe drought conditions, significant changes were observed in the growth parameters, chlorophyll concentration, antioxidant enzyme activity, and proline accumulation of the two varieties. with ‘Kaorino’ exhibiting superior drought tolerance compared with ‘Benihoppe’. Transcriptomic analysis identified 34,168 differentially expressed genes, including 9,665 upregulated and 24,503 downregulated genes. Venn analysis revealed 229 genes associated with proline biosynthesis, MDA accumulation, and antioxidant enzyme regulation. Transcription factors(TFs) expression was profiled using cross-referenced databases. A total of 8,379 DEGs encoding TFs were identified and classified into 47 TF families, some of which (e.g., NAC and WRKY) are known to be involved in drought stress responses. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses suggest that drought tolerance in strawberry involves the coordinated activation of stress signaling pathways, metabolic reprogramming, hormonal regulation, and defense-related biosynthetic routes, with both shared and cultivar-specific features. Metabolomic analysis revealed dynamic shifts in metabolites associated with osmotic adjustment, antioxidant defense, and hormonal regulation. The integrated multiomics approach enabled the construction of a gene–metabolite regulatory network, clarifying the interactions between gene expression and metabolite accumulation. Key pathways implicated in the drought response included the glycerophospholipid metabolism and MAPK signaling cascade. Lysophosphatidylglycerol acyltransferase(LPGAT) and Sucrose non-fermenting 1-related protein kinase 2(SnRK2) may be key genes affecting the drought resistance differences between two strawberry varieties. These findings provide valuable insights into the physiological and molecular mechanisms underlying drought adaptation in strawberries, offering a theoretical basis for breeding drought-resistant cultivars.
1 Introduction
Strawberry (Fragaria × ananassa) is a nutritionally rich and economically important fruit crop, widely valued for its distinctive flavor and health promoting properties (Wang et al., 2024). It contains diverse phytonutrients, including phenolics, polyphenols, fiber, micronutrients, and vitamins, which contribute to anti-inflammatory, anti-carcinogenic, and cardioprotective effects (Charoenwoodhipong et al., 2024). These attributer, along with their delicious taste, enhance the appeal of strawberries to consumers, thereby driving a steady increase in global demand for the fruit (R. et al., 2023).
To adapt to the rapid growth of the strawberry market, determining how to effectively enhance the yield and quality of strawberries is a crucial issue for supporting the development of the strawberry industry. The key factors influencing strawberry production include water management, nutrient application, and pollination (Teresa et al., 2021; Xue et al., 2023), among which water plays a decisive role. Strawberries exhibit low water use efficiency. For instance, blueberries require approximately 9.0 × 103 m3 of irrigation water per hectare to achieve 60% irrigation efficiency. whereas strawberries require approximately 8.0 × 103 m3ha-1 to achieve only 40% irrigation efficiency (Gavilan et al., 2024). Moreover, strawberries are highly sensitive to water scarcity. Owing to their herbaceous nature and shallow root system, drought stress can severely impair strawberry growth, yield, and quality (Li et al., 2021; Thokchom and Hazarika, 2022). This challenge is further intensified by climate change and global warming, which exacerbate production difficulties in arid and semi-arid regions (Luis et al., 2022).
Strawberry’s response to drought stress are complex, involving morphological, physiological, biochemical, and molecular adjustments aimed at survival and adaptation (Toscano et al., 2019; Gulen et al., 2021; Bistgani et al., 2024).Under drought conditions, strawberries exhibit reduced water availability, which activates multiple defense mechanisms. Physiologically, drought stress causes a decline in net photosynthetic rate, stomatal conductance, and transpiration rate as stomata close to conserve water (Toscano et al., 2019; Juan et al., 2022; Weidong et al., 2023). Although this reduces water loss, it simultaneously limits carbon dioxide uptake, leading to impaired photosynthesis. Consequently, leaf water content and turgor pressure decline, which can cause wilting and growth inhibition (Toscano et al., 2019; Sakshi et al., 2024). Biochemically, drought induces the generation of reactive oxygen species (ROS), including superoxide anions (O2•−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH) (Sharma and Zheng, 2019). Excessive ROS accumulation causes oxidative damage to DNA, amino acids, and lipids, resulting in lipid peroxidation and protein dysfunction (Sharma and Zheng, 2019; Bistgani et al., 2024). Plants counteract this by activating antioxidative enzymes such as superoxide dismutase, catalase (CAT), and peroxidase (POD), as well as accumulating osmolytes including proline, glycine-betaine, and soluble sugars to maintain osmotic balance and protect cells (Sharma and Zheng, 2019; Guohua et al., 2021). However, under severe drought, the antioxidant system is compromised, contributing to elevated ROS levels and exacerbated oxidative damage (Sharma and Zheng, 2019). For example, in Sesuvium portulacastrum, a halophyte, chlorophyll content decreases under salt treatment, which is physiologically similar to drought stress (Guohua et al., 2021). Molecularly, drought stress regulates the expression of genes involved in stress responses, including those associated with abscisic acid biosynthesis, osmoprotectant production, and antioxidant defense (Songquan and Yanrong, 2002; Qiang et al., 2022; Gao et al., 2024). Abscisic acid(ABA) is a key regulator of stomatal closure and stress-responsive gene activation (Sharma and Zheng, 2019; Gao et al., 2024). In wild strawberry (Fragaria nilgerrensis), integrated transcriptomic and methylome analyses have identified DNA methylation as a mechanism contributing to drought stress memory (Qiang et al., 2022; Zi et al., 2024). The FvICE1 gene in Fragaria vesca has been shown to play an essential role in cold and drought tolerance as a transcription factor (TF) (Jiaxin et al., 2023). Moreover, the heterologous expression of an Arabidopsis thaliana gene that can enhance auxin (Indole-3-acetic acid IAA) accumulation and root growth has been reported to improve drought tolerance in strawberry (Li et al., 2021). Drought also affects fruit quality. While severe drought reduces yield, mild drought can enhance the levels of beneficial phytochemicals (e.g., phenolics, anthocyanins, and L-ascorbic acid), accompanied by stronger antioxidant activity (Perin et al., 2019; Nafiye and Volkan, 2023). This effect is linked to ABA-mediated physiological and metabolic processes( (Perin et al., 2019). In contrast, strawberry aroma, which is mainly determined by esters, is highly vulnerable to drought, thereby reducing the overall fruit quality (Arriaza et al., 2025).
Investigating the mechanisms underlying strawberry drought responses is of scientific interest and practical relevance to production. Despite numerous studies on various plant species, in-depth analyses of strawberries remain limited, particularly regarding their molecular mechanisms. In this study, the physiological responses and molecular mechanisms of two major cultivated strawberry varieties in China under drought stress were compared through the integration of transcriptomics and metabolomics. Transcriptome analysis identified differentially expressed genes (DEGs) involved in osmotic regulation, antioxidant defense, and hormone signaling. Metabolomics analysis revealed drought-induced fluctuations in osmolytes (e.g., proline and soluble sugars), antioxidants (e.g., glutathione and flavonoids), and hormones (e.g., ABA and IAA). Combined transcriptomic and metabolomic analyses enabled the construction of a gene-metabolite regulatory network, providing preliminary insights into the interplay between gene expression and metabolite accumulation. This study advances the understanding of the physiological and molecular basis of drought response in strawberry and offers a reference for breeding drought-tolerant cultivars.
2 Materials and methods
2.1 Plant material and growth conditions
The two strawberry cultivars used in this study, ‘Benihoppe’ and ‘Kaorino’, were commercial seedlings from Yunnan Shangri-La Zangmei Agricultural Technology Co., Ltd. (Shangri-La, elevation 3,400 m). ‘Benihoppe’ is an early maturing variety originating from Japan. Its adaptability, shallow dormancy, large fruit size, and excellent quality have rendered it as an important parental line and a frequent subject of biochemical studies (Song et al., 2024). After its introduction into China, it has been widely cultivated under the names ‘Hongyan’ or ‘Red Face’. In addition, ‘Kaorino’, referred to as ‘Xiangye’ or ‘Suizhu’ in different regions of China, is extensively planted because of its strong disease resistance (Kitamura et al., 2015). However, there are no previous reports on its drought tolerance, which is the basis for its selection in comparison with ‘Benihoppe’.
After 30 d of acclimation in a smart greenhouse (50 m2, Kunming University, 24°58′30.98″N, 102°47′57.98″E) equipped with an automated drip-irrigation system (Netafim, 2L h−1 per dripper), uniform seedlings (5–6 true leaves, height 12 ± 1 cm) were selected. The growth conditions were maintained at (25 ± 3) °C/(18 ± 3)°C (day/night), relative humidity (RH) 65% ± 5%, and CO2400 ± 20 ppm. Plants were potted individually in 1.2 L plastic pots (10.8 cm Ø × 13.1 cm height) containing a sterilized substrate (peat: composted earthworm castings, 2:1 v/v; pH 6.0 ± 0.2, EC 0.45 dS m−1).
2.2 Drought treatment and experimental design
The soil volumetric water content (θ, 0–10 cm depth) was monitored daily at 08:00 and 18:00 using a calibrated TZS-2X sensor Field capacity (FC) was determined gravimetrically after 24 h of free drainage (θFC = 42% v/v). Three irrigation regimes were applied for 18 d:
Each cultivar × regime combination included six biological replicates (one plant = one replicate), arranged in a randomized complete block design (total 108 pots). Pots were rotated every 3 d to minimize positional effects. θ was restored to the target levels within 30 min using automated drip irrigation controlled by a Netafim-brand PLC unit.
2.3 Sampling strategy
Root and fully expanded leaf samples (fourth leaf from apex) were harvested at 0, 6, 12, and 18 d after treatment initiation (DAT) between 09:00 and 10:00. Roots were gently rinsed with ice-cold 5 mM CaCl2, blotted dry, flash-frozen in liquid N2, and stored at −80°C until analysis. For each replicate, tissues from three plants were pooled to reduce individual variation while maintaining six independent replicates per treatment.
2.4 Physiological and biochemical assays
2.4.1 Photosynthetic pigments
Leaf tissue (0.1 g) was extracted in 5 mL of 80% acetone at 4°C in the dark, and absorbance was recorded at 470, 645, and 663 nm (UV-1800, Shimadzu). Chl a, chl b, and carotenoid concentrations were calculated according to Lichtenthaler (1987).
2.4.2 Malondialdehyde, proline, and antioxidant enzymes
Root or leaf tissue (0.2 g) was homogenized in 5 mL of ice-cold 50 mM phosphate buffer (pH 7.8, 1% PVP, 0.1 mM EDTA). MDA content was measured using the thiobarbituric acid method. Proline was measured using the acid-ninhydrin method, Superoxide dismutase (SOD) (NBT inhibition), POD (guaiacol oxidation), and CAT (H2O2 decomposition) activities were determined using commercial kits (Solarbio, Beijing; catalog numbers BC0020, BC0290, BC0170, BC0090, and BC0200) according to the manufacturer’s microplate protocols. Absorbance was recorded using a SpectraMax iD3 (Molecular Devices). Enzyme activities were expressed as U mg−1 protein, with soluble protein quantified using the Bradford assay (BSA as a standard).
2.5 RNA extraction and library construction
Total RNA was extracted from 100 mg of root tissue using TRIzol® Reagent (Invitrogen, 15596026) according the manufacturer’s instructions and purified using DNase I (Takara, 2270A). RNA integrity was verified on 1.2% agarose gels and with an Agilent 2100 Bioanalyzer (RIN ≥ 8.0). The concentration was quantified using a Qubit 4.0 fluorometer (Invitrogen). Only high-quality RNA sample [OD260/280 = 1.8~2.2,OD260/230≥2.0,RQN≥6.5, 28S:18S≥1.0, >1μgwas used to construct sequencing library. Strand-specific libraries were prepared using the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, E7770) following the 1 µg low-input protocol (insert size 300 ± 50 bp). Unique dual-index adapters (NEB, E7600) were ligated, and the libraries were pooled equimolarly.
2.6 RNA sequencing and data processing
Paired-end 150bp sequencing was performed on an Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA) at Shanghai Majorbio Bio-Pharm Technology Co., Ltd, yielding at least 6 Gb of clean data per sample. Raw reads were adapter and quality trimmed using Trimmomatic v0.39 (SLIDINGWINDOW:4:20, MINLEN:50). Clean reads were aligned to the Fragaria vesca reference genome v4.0 (https://www.rosaceae.org) using HISAT2 v2.2.1 (–dta, –rna-strandness RF). Gene-level counts were generated using FeatureCounts v2.0.1 (annotation: F. vesca v4.0.a2). Differential expression was analyzed in DESeq2 v1.34.0; genes with |log2 fold-change| ≥ 1 and FDR-adjusted P < 0.05 were defined as DEGs. Gene Ontology (GO) and KEGG enrichment analyses were performed using clusterProfiler v4.2.2 with a q-value threshold of 0.05.
2.7 Metabolite extraction and UHPLC-HRMS analysis
Metabolites were extracted from 50 mg of finely ground root powder with 1 mL of 80% methanol (pre-cooled at −20°C, containing 0.1 mg mL−1 lidocaine as an internal standard). Following vortexing (30s) and sonication (10 min, 4°C), the samples were centrifuged at 12,000 × g for10 min at 4°C). Supernatants were filtered through 0.22 µm PVDF membranes and analyzed on a Vanquish UHPLC system coupled to a Q Exactive HF-X Orbitrap MS (Thermo Fisher). Chromatographic separation was performed on a Hypersil GOLD C18 column (2.1 × 100 mm, 1.9 µm) at 40 °C. The mobile phases consisted of 0.1% formic acid in water (A) and acetonitrile (B), with the following gradient: 0–1 min, 5% B; 1–9 min, 5-95% B; 9–11 min, 95% B; 11-11.1 min, 95-5% B; 11.1–14 min, 5% B. The flow rate was 0.3 mL min−1. MS parameters included a full-scan range of 70-1,050 m/z, resolution of 120,000, spray voltages of +3.5/−2.8 kv, capillary temperature of 320 °C, and probe heater temperature of 350°C. Raw files were processed using Compound Discoverer 3.3 (Thermo) with a 5 ppm mass tolerance against HMDB, and METLIN and an in-house Fragaria spectral library. Peak areas were normalized to the internal standard and fresh weight. Differential metabolites were identified by OPLS-DA (SIMCA 16.0) with VIP > 1 and P < 0.05 (Student’s t-test, FDR-adjusted).
2.8 Integration of transcriptomic and metabolomic data
Pearson correlation coefficients were calculated between normalized metabolite abundances (log2- transformed) and DESeq2-normalized gene counts for each treatment × time combination. Gene-metabolite pairs with |r| ≥ 0.8 and P < 0.01 were retained. Pathway mapping was performed using KEGG Mapper v4.0, with F. vesca as the reference.
2.9 Statistical analyses
Physiological and biochemical data were presented as mean ± SD (n = 6). Normality (Shapiro-Wilk test) and homogeneity of variances (Levene test) were verified prior to one-way ANOVA. Significant differences among treatments were separated by Tukey’s HSD test at P < 0.05 using SPSS v26.0. Figures were prepared using GraphPad Prism v9.0 and Adobe Illustrator v26.0.
3 Results
3.1 Effects of drought stress on the morphology, physiology, and biochemistry of strawberries
The physiological and biochemical responses of two strawberry cultivars, (‘Benihoppe’ and ‘Kaorino’) were evaluated under drought stress. The treatments induced significant alterations in growth parameters, chlorophyll concentration, antioxidant enzyme activities, and proline accumulation in both cultivars. Marked morphological changes were observed in both cultivars under varying drought stress conditions. In the control group (CK), plants exhibited overall vigor, characterized by green leaves and robust growth (Figure 1A–BC, KC). Under mild drought stress, obvious leaf yellowing and partial withering occurred, leading to inhibited growth and a damaged state (Figure 1A–BM, KM). Under severe drought stress, leaf yellowing became widespread, and numerous leaves withered, indicating severe plant damage (Figure 1A–BS, KS). Overall, increasing drought severity resulted in a gradual transition of leaves from green to yellow, accompanied by growth inhibition and severe wilting. Under varying drought stress conditions, the chlorophyll concentration significantly decreased in both cultivars as drought stress intensified. Within each cultivar, reductions were greater under severe stress than under mild stress. Comparative analysis revealed that ‘Benihoppe’ initially exhibited higher chlorophyll content than ‘Kaorino’. However, under identical drought conditions, the reduction in chlorophyll was more pronounced in ‘Benihoppe’ than in ‘Kaorino’(Figure 1B). Proline levels significantly increased in both cultivars under drought stress. Within the same cultivar, the increase were greater under severe stress than under mild stress. The initial proline contents were similar between the cultivars, but under identical drought conditions, the increase in ‘Kaorino’ was significantly higher than that in ‘Benihoppe’ (Figure 1C). The baseline MDA was essentially identical in both cultivars, and both increased with increasing drought severity. However, the increase in MDA was slightly greater in ‘Benihoppe’ than in ‘Kaorino’(Figure 1D). For antioxidant enzyme activities, both cultivars exhibited upward trends in SOD, POD, and CAT with increasing drought severity. The increases in SOD and CAT activities were less pronounced in ‘Benihoppe’ than in ‘Kaorino’, whereas POD activity exhibited a more substantial increase in ‘Benihoppe’ than in ‘Kaorino’ (Figure 1E–G).
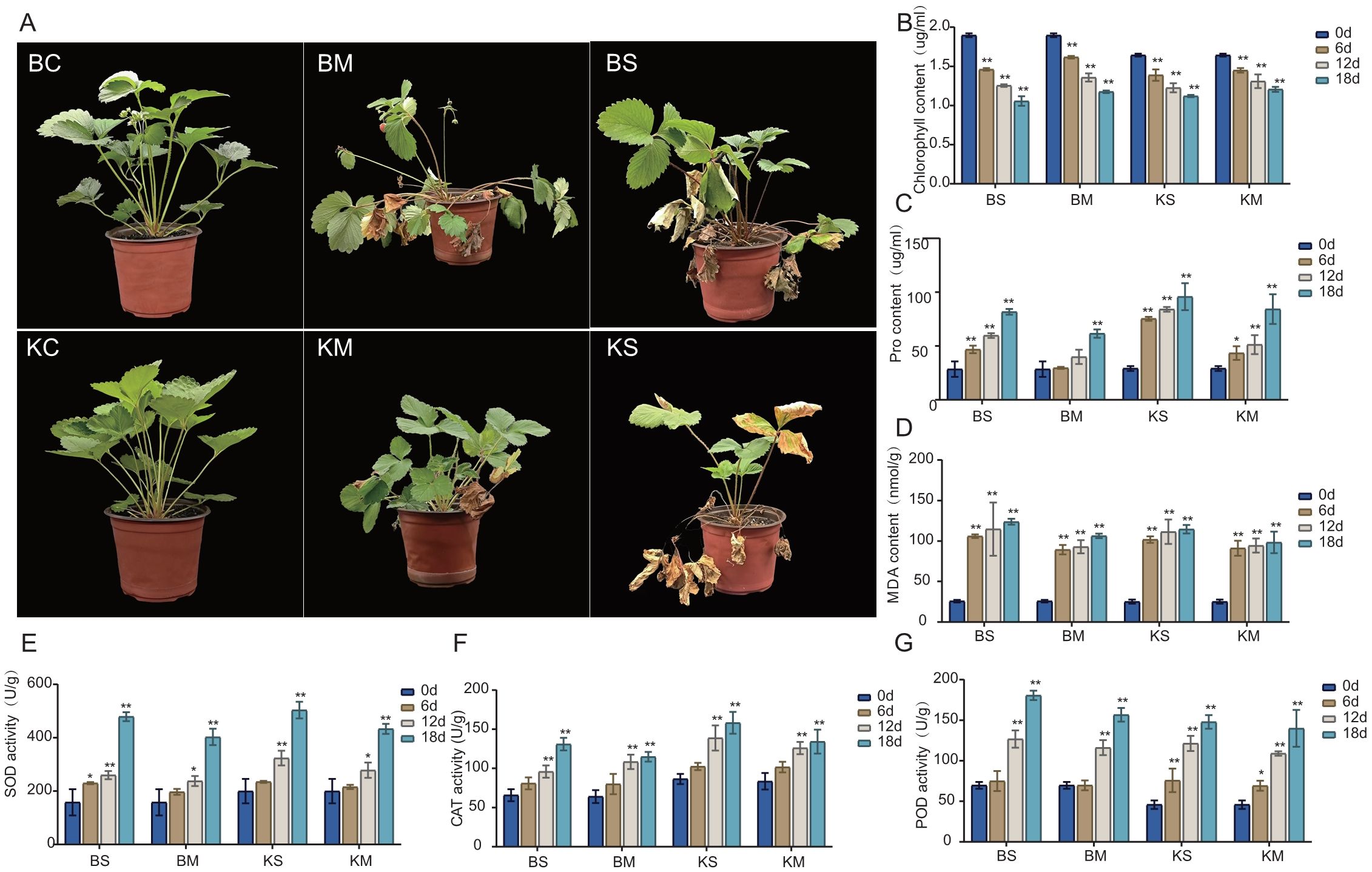
Figure 1. Effects of drought stress on strawberry physiology and morphology. (A) Phenotypic responses of strawberry plants under control, mild drought, and severe drought stress treatments; (BC: Benihoppe Root Control;BM: Benihoppe Root Mild Drought;BS;Benihoppe severe drought stress. KC, Kaorino Root Control; KM, Kaorino Root Mild Drought; KS, Kaorino Root Severe Dtought). (B–G) Changes in physiological and biochemical indices, including chlorophyll content, proline accumulation, and antioxidant enzyme activities, measured at different time points. Asterisks denote statistical significance: P < 0.05 (*), P < 0.01 (**).
3.2 Transcriptome sequencing results
In ‘Benihoppe’, the BC, BM, and BS groups generated 46.16 million, 44.70 million, and 43.40 million raw reads, respectively. Similarly, in ‘Kaorino’, the KC, KM, and KS groups produced 45.30 million, 40.67 million, and 44.14 million raw reads, respectively. Quality assessment confirmed that Q20 and Q30 values exceeded 95% in all libraries, indicating that the sequencing data satisfied the requirements for downstream bioinformatics analyses (Supplementary Table S1). Based on these findings, DEG analysis was performed to evaluate transcriptional changes under mild and severe drought conditions in both cultivars.
3.3 Differentially expressed genes under drought stress
To investigate the gene regulatory responses in the two strawberry cultivars, transcriptome analysis of root tissues under drought stress identified 34,168 DEGs, including 9,665 upregulated and 24,503 downregulated genes Across the four comparison groups, including BS vs. BC, BM vs. BC, KS vs. KC, and KM vs. KC, 4,980, 10,919, 9,891, and 8,378 DEGs were detected, with 1,426, 2,626, 2,362, and 3,251 genes upregulated, and 3,554, 8,293, 7,529, and 5,127 genes downregulated, respectively (Figure 2A). The predominance of downregulated DEGs suggests a potential adaptive strategy under drought stress involving the suppression of energy-intensive metabolic processes, growth modulation and activation of defense pathways. Venn diagram analysis (Figure 2B) identified 3,897 DEGs shared between mild and severe drought treatments in ‘Benihoppe’ (2,735 mild-specific and 503 severe-specific),and 5,521 in ‘Kaorino’ (1,327 mild-specific, and 2,295 severe-specific). A total of 2,247 DEGs were commonly regulated in both cultivars, indicating conserved transcriptional responses to drought stress. These findings highlight transcriptional suppression as a central mechanism of resource reallocation and stress adaptation. Venn analysis of the 2,247 shared DEGs (Figure 2C) revealed 229 genes associated with proline biosynthesis, MDA accumulation, and antioxidant enzyme regulation. Cluster heatmap analysis indicated consistent expression trends in both cultivars. Notably, the expression of the respiratory burst oxidase homolog (RBOH) gene increased significantly with drought severity (Figure 2D), consistent with its role in ROS-mediated signaling and oxidative stress defense (Nour et al., 2024). Similarly, the gene encoding Δ1-pyrroline-5-carboxylate synthase (P5CS), a key enzyme in proline biosynthesis, was significantly upregulated in both cultivars, consistent with enhanced proline accumulation under drought stress (Figure 2D) (Farooq et al., 2009).
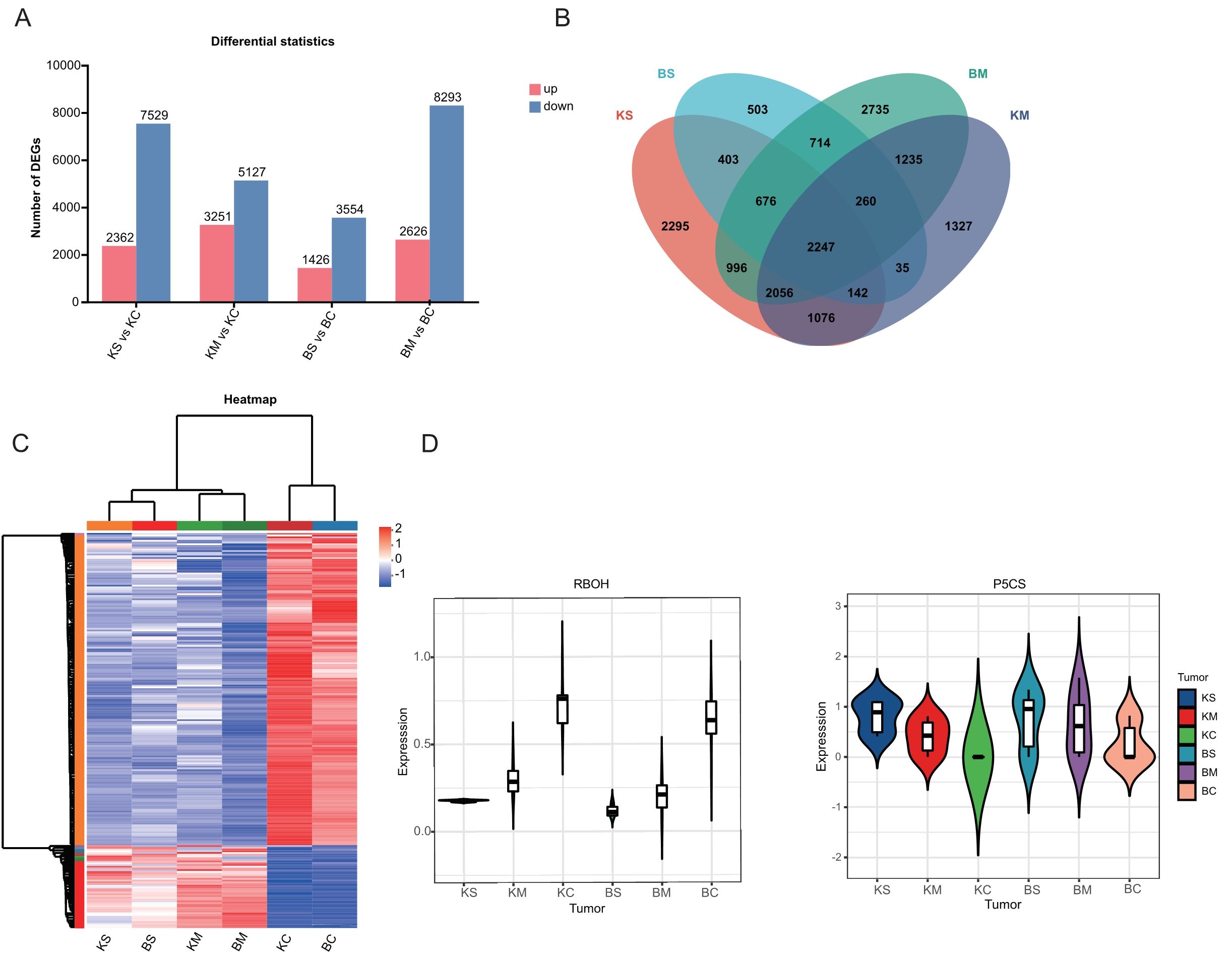
Figure 2. Identification and transcriptome analysis of DEGs. (A) Number of DEGs (upregulated and downregulated) under mild and severe drought stress. (B) Venn diagram showing shared and unique DEGs across drought treatments within each cultivar. (C) Heatmap displaying expression patterns of DEGs under various drought conditions. (D) Violin plots depicting expression levels of key drought-responsive genes (RBOH, P5CS).
3.4 Gene ontology enrichment analysis in response to drought stress
GO enrichment analysis was conducted to functionally characterize the DEGs identified under different drought stress conditions. GO terms were classified into three categories: biological process (BP), cellular component (CC), and molecular function (MF). Both ‘Benihoppe’ and ‘Kaorino’ exhibited similar GO enrichment patterns under mild and severe drought stress. In the BP category, DEGs were predominantly enriched in “cellular process”, “metabolic process”, and “response to stimulus”; In the CC category, “cellular anatomical entity” and “protein-containing complex” were the most represented. In the MF category, “catalytic activity” and “transporter activity” were significantly enriched (Figure 3). These results indicated that both cultivars activate conserved defense pathways in response to drought. Enhanced metabolic activity (BP) and catalytic function (MF) facilitate osmolytes (e.g., proline) and antioxidant biosynthesis to counteract oxidative damage. The enrichment of transporter activity (MF) supports ion homeostasis and solute transport, whereas the reorganization of protein complexes (CC) may reflect signal transduction mediated by receptor kinases and transcriptional regulators. The consistent enrichment of “response to stimulus” across different drought intensities suggests sustained stress perception and signaling. This may reflect a systemic shift from growth processes to protective mechanisms, including cell wall remodeling and metabolic reprogramming, which enhances drought tolerance.

Figure 3. Distribution and GO enrichment analysis of DEGs under different degrees of drought stress.
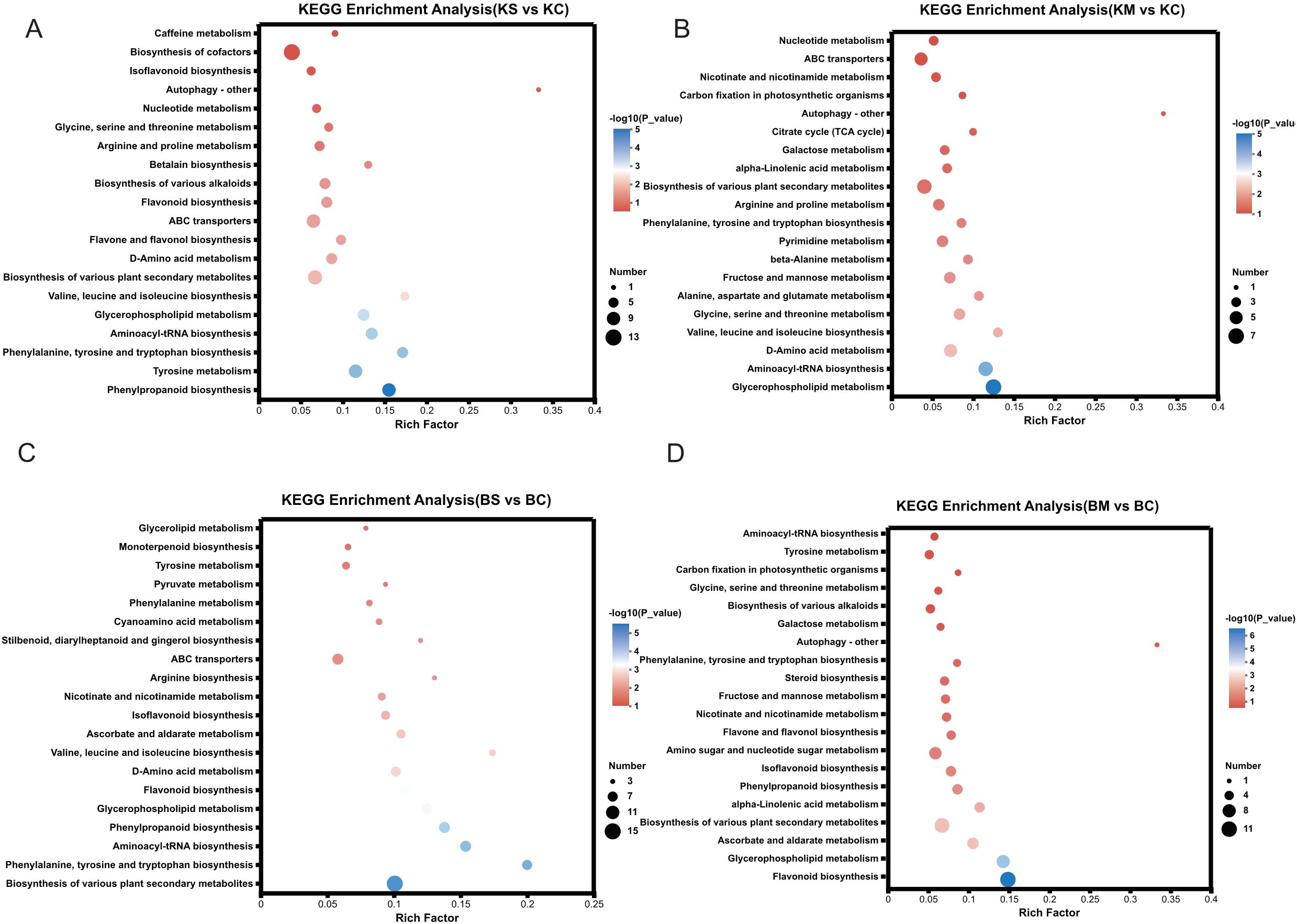
3.5 KEGG pathway enrichment analysis of DEGs under drought stress
To identify the major signaling and metabolic pathways underlying drought responses, KEGG pathway enrichment analysis was performed using DEGs from four comparisons: BS vs. BC, BM vs. BC, KS vs. KC, and KM vs. KC (Figure 4A). Commonly enriched pathways included plant-pathogen interaction (map04626), phenylpropanoid biosynthesis (map00940), and MAPK signaling pathway-plant (map04016), all of which are integral to stress perception, signaling cascades, and defense activation. Additionally, starch and sucrose metabolism (map00500) and terpenoid backbone biosynthesis (map00900) were significantly enriched, suggesting their roles in osmotic adjustment, energy redistribution, and secondary metabolite biosynthesis under drought conditions. In particular, plant hormone signal transduction (map04075) was significantly enriched in both ‘Benihoppe’ drought treatments (BS vs. BC and BM vs. BC), implying a prominent role for hormonal regulation, particularly ABA, IAA, and ethylene, in mediating drought responses. In contrast, diterpenoid biosynthesis (map00904), associated with secondary metabolites, was highly enriched in the KM vs. KC comparison, suggesting cultivar-specific activation of specialized pathways under severe drought. Collectively, these findings suggest that drought tolerance in strawberry involves the coordinated activation of stress signaling pathways, metabolic reprogramming, hormonal regulation, and defense-related biosynthetic routes, with both shared and cultivar-specific features.
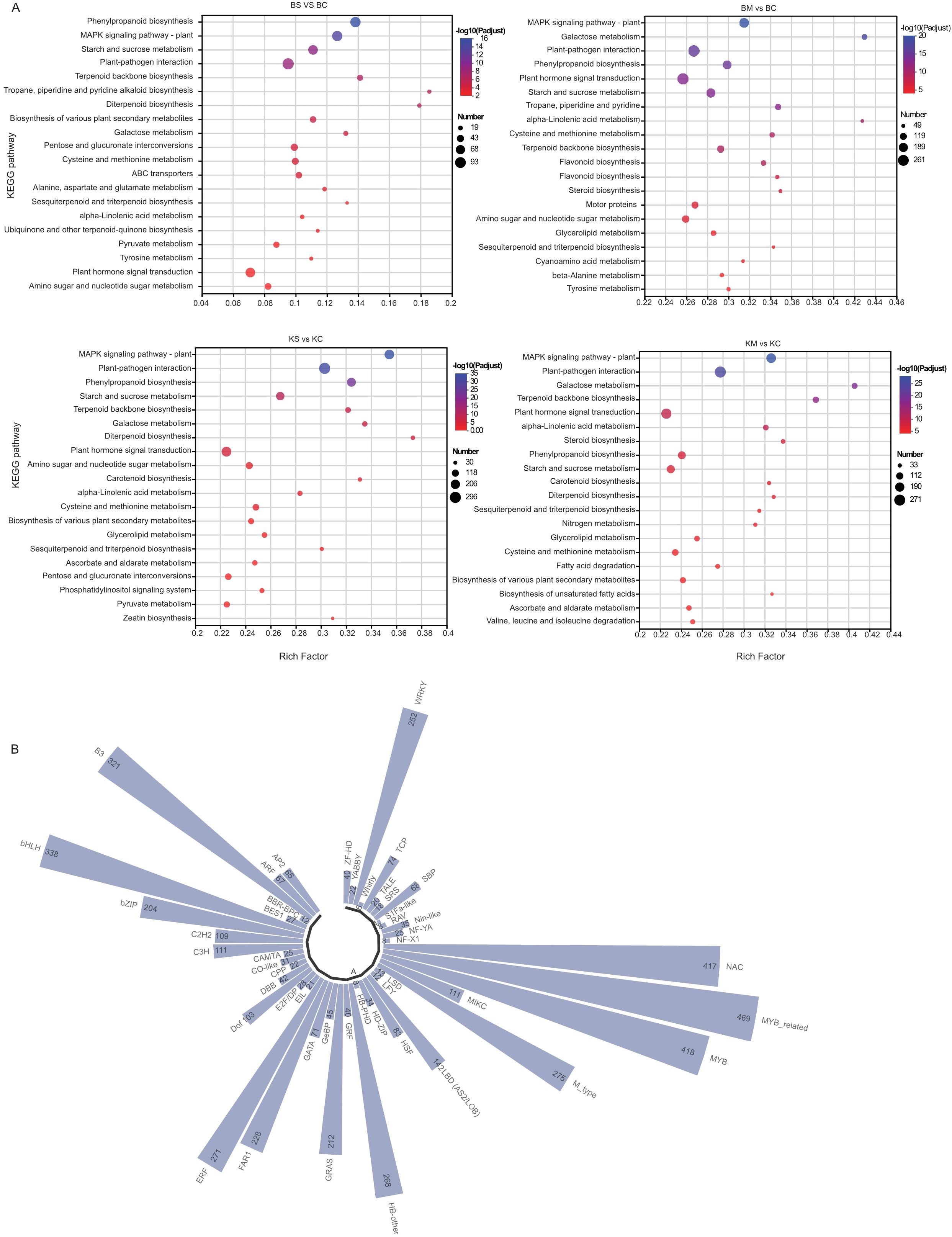
Figure 4. KEGG and TFs analysis of DEGs under drought stress. (A) KEGG pathway enrichment analysis of DEGs under different levels of drought stress; (B) Classification and distribution of differentially expressed TFs across treatments.
3.6 Analysis of differentially expressed transcription factors
TFs are key regulators of plant responses to drought stress, orchestrating complex molecular networks that enhance survival under water deficient conditions. To elucidate the regulatory mechanisms underlying drought responses in the two strawberry cultivars, TF expression was profiled using cross-referenced databases. A total of 8,379 DEGs encoding TFs were identified and classified into 47 TF families (Figure 4B). The top 10 TFs families by DEGs count were: MYB-related (n = 469), MYB (n = 418), NAC (n = 417), bHLH (n = 338), B3 (n = 321), M-type (n = 275), ERF (n = 271), HB-other (n = 268), WRKY (n = 252), and FAR1 (n = 228). These TFs families are associated with diverse biological functions, including stress perception, hormone signaling, secondary metabolite biosynthesis, and oxidative stress mitigation. The observed transcriptional changes emphasize the involvement of complex regulatory networks in cultivar-specific drought adaptation.
3.7 Identification of differentially accumulated metabolites under drought stress
To characterize metabolite-level responses to drought stress, comprehensive targeted metabolomic profiling was performed using UPLC-MS/MS. Orthogonal partial least squares-discriminant analysis (OPLS-DA) revealed clear metabolic distinctions between control and drought-stressed groups, confirming analytical reproducibility and method robustness (Figure 5A). Differentially accumulated metabolites (DAMs) were identified across stress gradients, and Venn diagram analysis revealed treatment and cultivar-specific metabolite profiles (Figure 5B). A subset of 96 DAMs was conserved across conditions, including key lipids and their derivatives—such as glycerophosphocholine and LysoPE (0:0/18:3(9Z,12Z,15Z))—and terpenoids like annuolide C. Comparative analysis revealed cultivar-specific metabolic responses. The ‘Kaorino’ cultivar exhibited 439 and 930 DAMs under mild and severe drought, respectively. In contrast, the ‘Benihoppe’ cultivar displayed 587 and 783 DAMs under the same stress conditions (Figure 5C). These findings suggest that metabolic reprogramming in response to drought is both stress intensity dependent and genotype specific, involving lipid remodeling, osmolyte accumulation, and secondary metabolite biosynthesis.
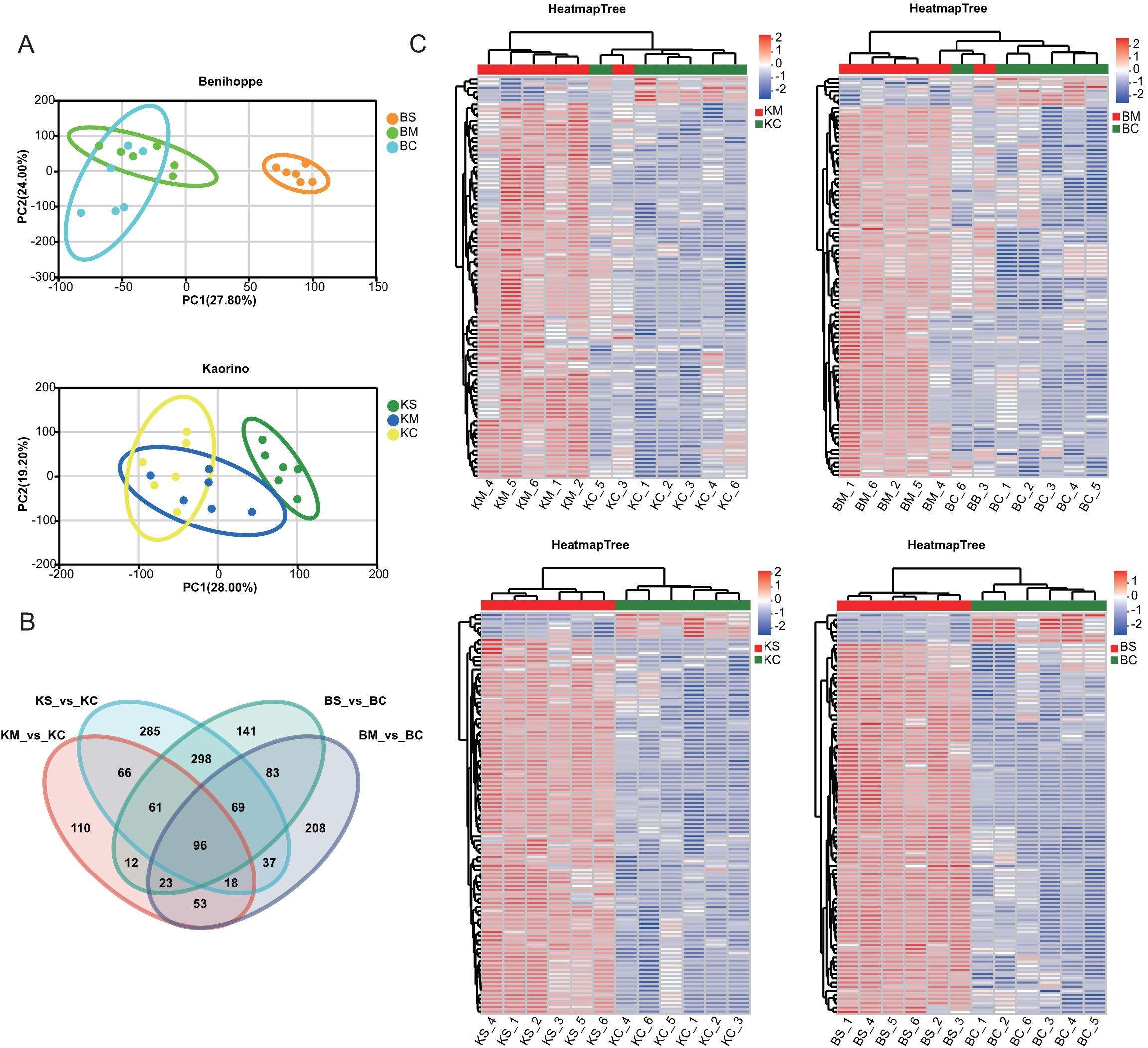
Figure 5. Extensive targeted metabolomic analyses of strawberry under different drought stress levels. (A) PCA showing sample separation under control, mild, and severe drought conditions; (B) Venn diagram of DAMs across drought treatments; (C) Heatmap clustering of DAMs compared to the control. Red and green represent high and low metabolite abundance, respectively.
3.8 KEGG enrichment analysis of differentially accumulated metabolites
To determine the metabolic pathways affected by drought stress, KEGG enrichment analysis was performed based on the number of significantly enriched metabolites in each treatment group. In ‘Benihoppe’, flavonoid biosynthesis and glycerophospholipid metabolism were significantly enriched in the BM vs. BC comparison, whereas the biosynthesis of various plant secondary metabolites and phenylalanine, tyrosine, and tryptophan biosynthesis were enriched in the BS vs. BC comparison (Figure 6A–B). In ‘Kaorino’, glycerophospholipid metabolism, aminoacyl tRNA biosynthesis, and D amino acid metabolism were enriched in KM vs KC, whereas phenylpropanoid biosynthesis and tyrosine metabolism were enriched in KS vs. KC (Figure 6C–D). These results demonstrate that drought stress induces both common and cultivar-specific changes in key metabolic pathways, particularly those involved in secondary metabolism, membrane lipid remodeling, and amino acid metabolism, thereby contributing to drought adaptation.
3.9 Integrated analysis of DEGs and DAMs
To identify the key regulatory components contributing to drought tolerance, we focused on pathways jointly enriched with DEGs and DAMs. Two major pathways emerged: glycerophospholipid metabolism and MAPK signaling pathway. In the glycerophospholipid metabolism pathway, significant transcriptional and metabolic changes were observed under drought stress (Figure 7). Phosphatidylglycerol(PG) biosynthesis genes were upregulated in both cultivars, whereas genes involved in phosphatidylcholine(PC) and phosphatidylethanolamine(PE) synthesis were downregulated. The expression of LPGAT genes varied between cultivars, being upregulated in ‘Kaorino’ and downregulated in ‘Benihoppe’, indicating genotype-specific regulation of membrane lipid remodeling. Furthermore, intermediate metabolites such as 1,2-diacyl-sn-glycerol-3-phosphate (1,2-DiAc1-sn-glycerol-3P) and sn-glycero-3-phosphocholine accumulated significantly under drought conditions. In contrast, the expression of genes such as Fx84Dg00479 (R01315), Fx94Bg00579 (R02053), and Fxa2Cg01240 (R02747), which encode enzymes responsible for PC and PE degradation, was downregulated. This repression may limit membrane lipid degradation, thereby enhancing structural stability during drought stress.
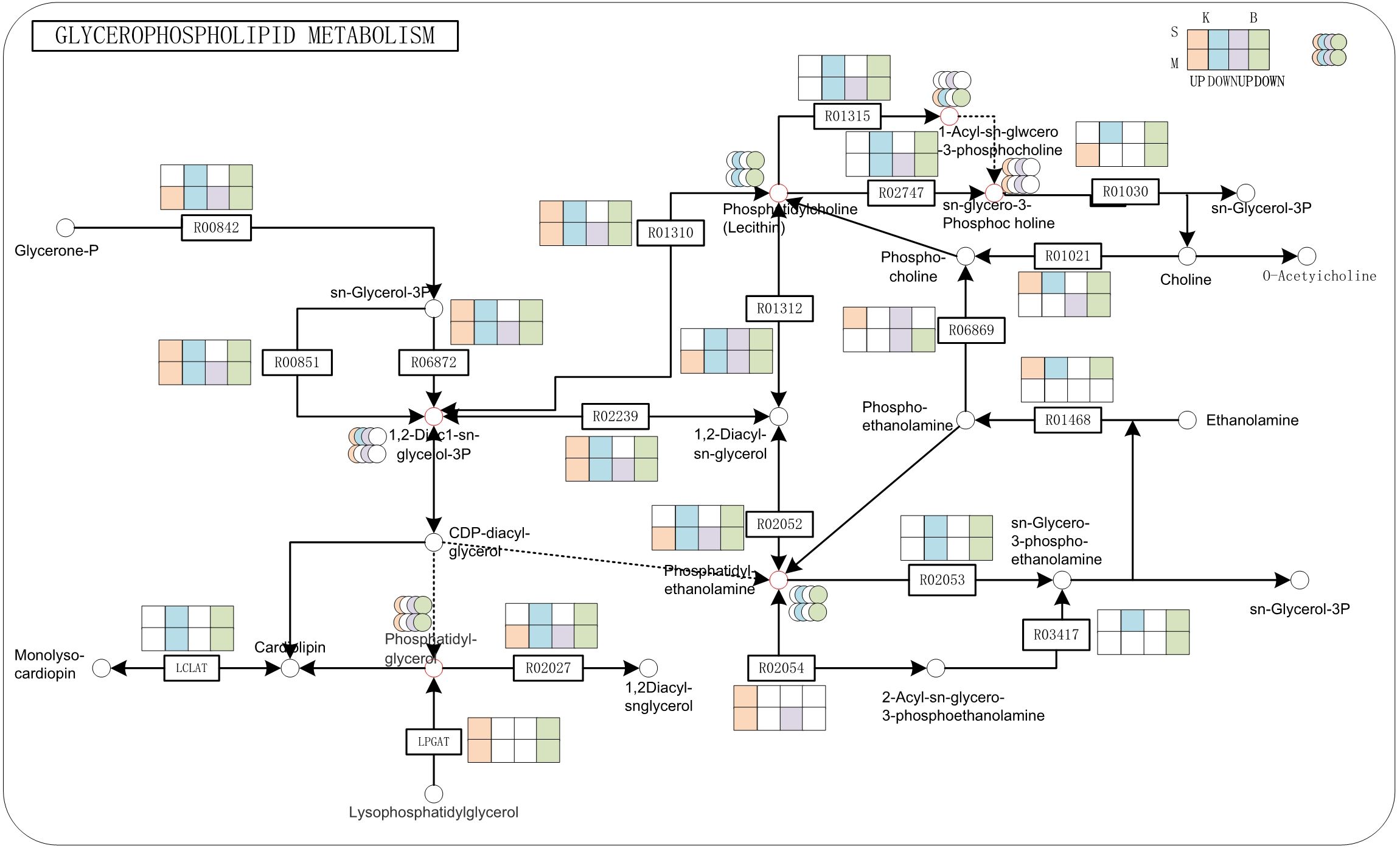
Figure 7. Glycerophospholipid metabolism pathway under moderate and severe drought in ‘Kaorino’. Red and purple circles represent up-regulated metabolites; green and blue circles represent down-regulated metabolites; red and purple rectangles indicate up-regulated genes; green and blue rectangles indicate down-regulated genes; B is for Benihoppe, K is for Kaorino, M is for Mild drought, S is for Severe drought.
The MAPK signaling pathway exhibited complex regulatory dynamics in response to drought stress (Figure 8). The expression of PYR/PYL receptor genes was downregulated, while PP2C phosphatase genes were upregulated, suggesting a negative feedback loop under prolonged stress. Interestingly, SnRK2 kinase genes displayed a mixed expression profile, with both upregulated and downregulated isoforms depending on the cultivar and drought severity, implying nuanced regulation of ABA signal transduction. Together, these findings from integrative transcriptomic and metabolomic analyses highlight glycerophospholipid metabolism and MAPK signaling as key pathways involved in drought tolerance. The observed cultivar-specific expression patterns further suggest that differential regulation of these pathways contributes to the contrasting drought resistance observed in ‘Benihoppe’ and ‘Kaorino’.
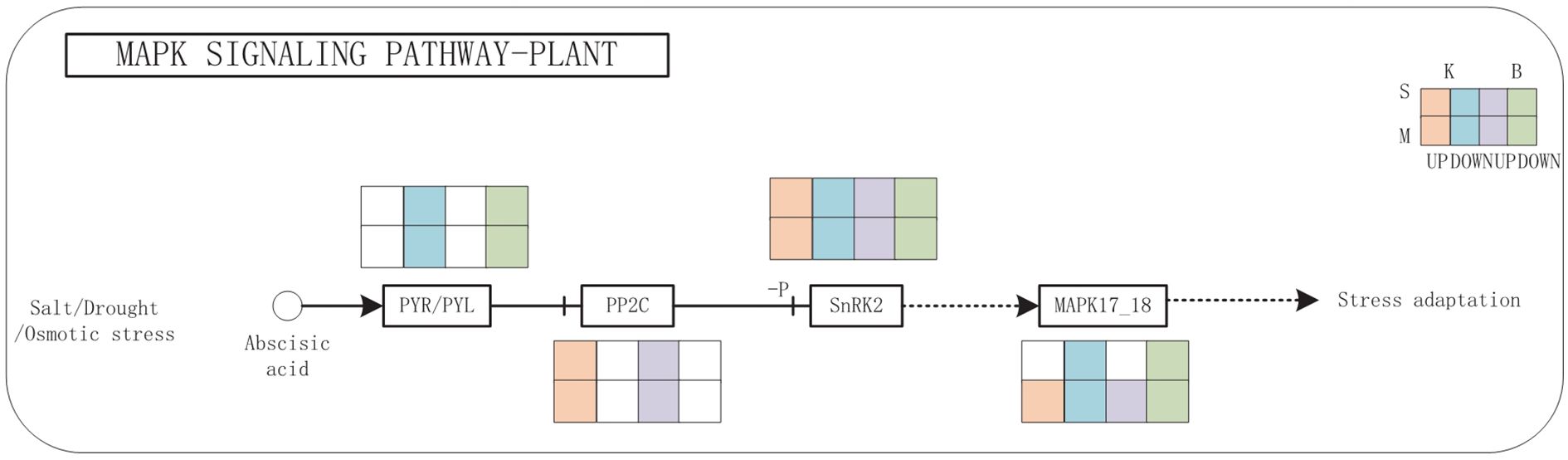
Figure 8. MAPK signaling pathways under drought stress. Red and purple circles represent up-regulated metabolites; green and blue circles represent down-regulated metabolites; red and purple rectangles indicate up-regulated genes; green and blue rectangles indicate down-regulated genes; B is for Benihoppe, K is for Kaorino, M is for Mild drought, S is for Severe drought.
4 Discussion
4.1 Physiological and biochemical responses to drought stress
Under varying drought stress intensities and durations, both cultivars exhibited significant morphological changes, including leaf yellowing, wilting, and withering, which are typical response of strawberries to drought conditions (Suresh et al., 2023). When evaluated solely based on morphological traits under identical conditions, ‘Benihoppe’ appeared to suffer less damage than ‘Kaorino’. However, further morphological analyses are required to determine the statistical significance of this difference.
Drought stress commonly reduces photosynthesis and transpiration rates because of the close correlation between chlorophyll content and the photosynthetic activity. Consistent with this process, chlorophyll levels decrease as a result of reduced precursor synthesis and enhanced degradation (Bistgani et al., 2024). The extent of chlorophyll decline varies across cultivars, with resistant varieties exhibiting a less pronounced reduction (Farooq et al., 2009). Under mild and severe drought stress, the chlorophyll loss in the chloroplasts of ‘Kaorino’ was less than that of ‘Benihoppe’, demonstrating a more effective chloroplast protection mechanism and stronger drought resistance in ‘Kaorino’.
Excessive drought stress can cause overproduction of ROS in plants (Sheng et al., 2022). The imbalance between ROS generation and antioxidant defense systems induces oxidative damage to cellular components, with lipid peroxidation of membranes serving as a major consequence (Yini et al., 2021). As a byproduct of polyunsaturated fatty acid peroxidation in membranes, MDA is widely regarded as a reliable marker of oxidative damage (Vardan et al., 2022). Under drought stress, the MDA levels in both cultivars increased significantly, with a greater increase in ‘Benihoppe’ than in ‘Kaorino’, indicating more severe oxidative damage in ‘Benihoppe’.
To counteract ROS, plants activate enzymatic antioxidant systems involving SOD, CAT, and POD (Hui et al., 2023). These enzymes function synergistically to detoxify ROS and maintain redox homeostasis under stress conditions (Vieira et al., 2024). SOD is the primary defense against ROS, as it converts superoxide radicals (O2−) into hydrogen peroxide (H2O2) and molecular oxygen (O2) (Qin et al., 2024). Because O2− is highly reactive and capable of initiating chain oxidative reactions (Yu et al., 2024), SOD activity generally increases as a direct response to elevated O2− production (Li et al., 2024). CAT decomposes H2O2 into H2O and O2 (Lin et al., 2024), mitigating the harmful effects of H2O2, which can cause oxidative damage if not efficiently scavenged (Parveen et al., 2019). Under drought conditions, CAT activity typically increases to cope with elevated H2O2 levels (Shen et al., 2024). POD is a broad group of enzymes that catalyze the oxidation of diverse substrates in H2O2 (Cheng et al., 2024). While SOD converts O2− into H2O2 and CAT subsequently decomposes H2O2 into H2O and O2, POD contributes to H2O2 detoxification and frequently plays a more prominent role in cell wall stiffening and lignification, processes essential for maintaining structural integrity under stress (Huangying et al., 2024). Drought stress is generally accompanied by an increase in POD activity (Khan et al., 2025).
Proline, an osmoprotective amino acid, is another critical marker of drought stress resilience in plants. Its accumulation mitigates osmotic imbalance, stabilizes cellular structures, and scavenges ROS under water-deficient conditions (Pál et al., 2018). In the present study, the proline levels increased in both cultivars with intensified drought, whereas the increase in ‘Benihoppe’ was less pronounced than that in ‘Kaorino’, suggesting that the drought resistance of ‘Benihoppe’s’ was weaker than that of ‘Kaorino’.
In summary, ‘Kaorino’ exhibited superior drought tolerance, characterized by higher chlorophyll retention, stronger antioxidant defenses, and greater proline accumulation. These physiological and biochemical traits indicate that inherent genetic differences contribute to more robust drought adaptation mechanisms in ‘Kaorino’.
4.2 Integration of transcriptomic and metabolomic insights: multi-pathway regulation of drought response
Differential expression analysis identified 47 TF families that responded to drought stress in both cultivars. Key families included MYB-related, MYB, NAC, bHLH, B3, WRKY, and FAR1, several of which are known to regulate stress responses in other plant species.
In this study, both NAC and WRKY families showed significant expression changes under drought stress, suggesting that strawberries activate these TFs to modulate growth, development, and defense processes. The upregulation of NAC genes may promote adaptive developmental changes, whereas WRKY expression changes likely contribute to enhanced defense signaling. Furthermore, the NAC family is a master regulator of stress in plants. NAC TFs bind to cis-elements (such as NACR) to activate genes involved in osmoprotectant synthesis, ROS clearance, and stomatal closure (Abdul et al., 2024; Zhang et al., 2024). The WRKY family is ABA-dependent, and WRKY TFs can modulate ABA biosynthesis and stomatal aperture via MAPK cascades (Kiranmai et al., 2018; Dingyue et al., 2023).Overexpression of MdWRKY56 in apple increased proline accumulation (2.5-fold) and reduced water loss by 30% via NCED3 (an ABA biosynthesis gene) activation (Dingyue et al., 2023). MYB is involved in metabolic reprogramming, with MYB TFs (e.g., MYB96) redirecting carbon toward triacylglycerol synthesis, thereby conserving energy during stress (Lee et al., 2019).
Our integrated analysis revealed that strawberries coordinate multiple signaling and metabolic pathways under drought stress, particularly the glycerophospholipid metabolism and MAPK signaling cascade —each contributing uniquely to drought tolerance. Glycerophospholipids, as major membrane constituents, are critical for maintaining membrane structure and signaling under stress (Matsuo et al., 1992). In our study, drought stress altered lipid profiles: PG levels increased, while PC and PE levels decreased. Genes promoting PG synthesis were upregulated, indicating active reinforcement of membrane integrity. In contrast, genes such as Fx84Dg00479 and Fx94Bg00579, involved in PC and PE degradation, were downregulated, suggesting suppressed membrane breakdown. Interestingly, expression of LPGAT, a gene involved in PG biosynthesis, was cultivar-dependent: upregulated in ‘Kaorino’ and downregulated in ‘Benihoppe’. These findings align with previous studies showing PG accumulation in stress-tolerant species. For example, Increased PG content in chloroplast membranes of cold-tolerant plants (Matsuo et al., 1992, and PG species critical for photosynthetic stability in salt-tolerant Dunaliella (Lynch and Thompson, 1984). MAPK signaling mediates environmental stress perception and signal transduction. The MAPK gene exhibited differential expression between mild and severe drought, showing upregulation under moderate stress and downregulation under severe stress. ABA is a core regulator of plant drought responses. Under drought conditions, ABA perception and signaling regulate stomatal closure, gene expression, and stress adaptation. In both cultivars, we observed downregulation of PYR/PYL ABA receptor genes, likely indicating negative feedback regulation to prevent overactivation of ABA signaling. Increased expression of PP2C genes, which act as negative regulators in the ABA pathway, further supports this feedback loop. Meanwhile, SnRK2 kinases showed variable expression, highlighting complex regulation by upstream signals, TFs, or cross-talk with other pathways.
In conclusion, our integrative analysis demonstrates that drought tolerance in strawberry involves a coordinated response across physiological, transcriptional, and metabolic levels. ‘Kaorino’ exhibits stronger drought tolerance than ‘Benihoppe’, likely due to superior regulation of photosynthesis, ROS detoxification, osmotic balance, and membrane stability. These traits are underpinned by the activation of ABA and MAPK signaling, lipid metabolism, and key transcription factor families.
5 Conclusions
‘Benihoppe’ and ‘Kaorino’ are the primary varieties of strawberries cultivated in China. In production, it has been found that ‘Kaorino’ has higher disease resistance than ‘Benihoppe’, but there is a lack of relevant research on drought resistance. In this study, through a comparative analysis under the same conditions, the physiological, biochemical, transcriptomic, and metabolomic responses of ‘Benihoppe’ and ‘Kaorino’ to drought stress were analyzed to explore their drought adaptation mechanisms and tolerance differences. The chlorophyll content decreased in both varieties under drought stress, whereas ‘Kaorino’ exhibited a smaller decline, suggesting more efficient chloroplast protection and better maintenance of photosynthesis. The activities of antioxidant enzymes (POD, SOD, and CAT) increased in both varieties, while ‘Kaorino’ showed a more significant enhancement and a smaller increase in MDA content, which demonstrated stronger oxidative stress resistance. The proline content also increased in both varieties, with ‘Kaorino’ presenting higher levels, indicating more efficient osmotic regulation. Transcriptome analysis identified 47 gene families, some of which (e.g., NAC and WRKY) are known to be involved in drought stress responses. The combined analysis of the transcriptome and metabolome revealed two key pathways associated with drought stress in strawberries: the glycerophospholipid metabolism pathway, which is related to cell membrane structure, and the MAPK signaling pathway, which is associated with environmental stress perception. The discovery of the LPGAT gene within the glycerophospholipid metabolism pathway may be a key factor in the differences in drought resistance observed between two varieties. In the MAPK signaling pathway, it was discovered that the SnRK2 gene displayed a mixed expression profile, with both upregulation and downregulation, in two varieties under different drought conditions. These results will be important directions for our future work. This study has uncovered the common molecular mechanisms of strawberries under drought stress and has also identified physiological, biochemical, and molecular evidence indicating that ‘Kaorino’ exhibits superior drought resistance compared to ‘Benihoppe’. These findings provide valuable insights into the genetic and metabolic regulation of drought tolerance in strawberries, offering a foundation for breeding more drought-resistant varieties.
Data availability statement
The original contributions presented in the study are publicly available. The transcriptomic data can be found in the NCBI Sequence Read Archive (SRA) under BioProject accession PRJNA1366720. The metabolomic data are accessible in MetaboLights (https://www.ebi.ac.uk/metabolights/) under the accession number MTBLS13366.
Author contributions
HQ: Investigation, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Software, Validation. TN: Conceptualization, Data curation, Formal Analysis, Investigation, Software, Validation, Writing – original draft, Writing – review & editing. HM: Writing – review & editing. WG: Data curation, Formal Analysis, Investigation, Software, Validation, Writing – original draft, Writing – review & editing. DL: Data curation, Formal Analysis, Software, Validation, Writing – original draft, Writing – review & editing, Conceptualization. YN: Writing – original draft. ZC: Writing – original draft. LJ: Writing – original draft. CH: Writing – original draft. YT: Writing – original draft. CD: Writing – original draft. MZ: Writing – original draft. XC: Writing – original draft. JL: Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (32260827), the Science and Technology Department of Yunnan Province (grant numbers 202001BA070001-137 and 202205AC160043), the Yunnan Xingdian Talent Support Program for Entrepreneurship Talents (XDYC-CYRC-2022-0002), the Spring City Plan Youth Top Talent Project Funding (201914005), and the Special Basic Cooperative Research Programs of Yunnan Provincial Undergraduate Universities’ Association (NO. 202401BA070001-004).
Conflict of interest
Author CD was employed by the company Yunnan Hanzhe Technology Co., Ltd. Author XC was employed by the company Yulong County Jiuhe Xinxing Agricultural Development and Planting Co., Ltd. Author JL was employed by the company Shangri-La Zangmei Agricultural Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1679472/full#supplementary-material
Abbreviations
ROS, Reactive oxygen species; ABA, Abscisic acid; IAA, Indole-3-acetic acid; BC, Benihoppe control group; BM, Benihoppe mild drought; BS, Benihoppe severe drought; KC, Kaorino control group; KM, Kaorino mild drought; KS, Kaorino severe drought; MDA, Malondialdehyde; SOD, Superoxide dismutase; POD, Peroxidase; CAT, Catalase; PG, phosphatidylglycerol; PC, Phosphatidylcholine; PE, Phosphatidylethanolamine; PI, Phosphatidylinositol; DEGs, Differentially expressed genes; DAMs, Differentially accumulated metabolites; BP, Biological process; CC, Cellular component; MF, Molecular function; RBOH, Respiratory burst oxidase homolog; KEGG, Kyoto encyclopedia of genes and genomes; TFs, Transcription factors; LPGAT, Lysophosphatidylglycerol acyltransferase; SnRK2:Sucrose non-fermenting 1-related protein kinase 2.
References
Abdul, R., Chunyan, T., Shoupu, H., Hongge, L., Shuai, L., Xiongming, D., et al. (2024). Transcriptome dynamics of Gossypium purpurascens in response to abiotic stresses by Iso-seq and RNA-seq data. Sci. Data 11, 477–477. doi: 10.1038/s41597-024-03334-9
Arriaza, F. R., Cortiella, M. G., Pollmann, S., Quintana, L. M., and Ramos, P. (2025). Modulation of volatile production in strawberries fruits by endophytic fungi: Insights into modulation of the ester's biosynthetic pathway under drought condition. Plant Physiol. Biochem. 219, 109347–109347. doi: 10.1016/j.Plaphy.2024.109347
Bistgani, Z. E., Barker, A. V., and Hashemi, M. (2024). Physiology of medicinal and aromatic plants under drought stress. Crop J. 12, 330–339. doi: 10.1016/j.Cj.2023.12.003
Charoenwoodhipong, P., Zuelch, M. L., Keen, C. L., Hackman, R. M., and Holt, R. R. (2024). Strawberry (Fragaria x Ananassa) intake on human health and disease outcomes: a comprehensive literature review. Crit. Rev. Food Sci. Nutr. 65, 31–31. doi: 10.1080/10408398.2024.2398634
Cheng, F., Kotha, S., Fu, M., Yang, Q., Wang, H., He, W., et al. (2024). Nanozyme enabled protective therapy for neurological diseases. Nano Today 54, 102142. doi: 10.1016/j.Nantod.2023.102142
Dingyue, D., Ran, Y., Yuli, M., Qinglong, D., Ke, M., and Fengwang, M. (2023). Apple WRKY transcription factor MdWRKY56 positively modulates drought stress tolerance. Environ. Exp. Bot. 212, 105400. doi: 10.1016/j.Envexpbot.2023.105400
Farooq, M., Wahid, A., Kobayashi, N., Fujita, D., and Basra, S. M. A. (2009). Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 29, 185–212. doi: 10.1051/agro:2008021
Gao, F., Li, J., Li, W., Shi, S., Song, S., Shen, Y., et al. (2024). Abscisic acid and polyamines coordinately regulate strawberry drought responses. Plant Stress 11, 100387. doi: 10.1016/j.Stress.2024.100387
Gavilan, P., Higueras, J. L., Lozano, D., and Ruiz, N. (2024). The Riego Berry mobile application: A powerful tool to improve on-farm irrigation performance in berry crops. Agric. Water Manage. 292, 108682. doi: 10.1016/j.Agwat.2024.108682
Gulen, H., Turhan, E., Eris, A., Husaini, A., and Neri, D. (2021). “Molecular and physiological responses of strawberry plants to abiotic stress,” in Husaini, A. M. and Neri, D. (Eds.), Strawberry: Growth, Development and Diseases. Wallingford, United Kingdom: CAB International.
Guohua, D., Qian, Y., Xueyu, R., Tingting, S., Boxuan, Y., Wenwei, Z., et al. (2021). Proteomics analysis of the effects for different salt ions in leaves of true halophyte Sesuvium portulacastrum. Plant Physiol. Biochem. 170, 234–248. doi: 10.1016/j.Plaphy.2021.12.009
Huangying, S., Kaijing, X., Xiangrui, L., Jiancheng, L., Ahsan, A. M., Huizhen, F., et al. (2024). Exogenous strigolactone enhanced the drought tolerance of pepper (Capsicum chinense) by mitigating oxidative damage and altering the antioxidant mechanism. Plant Cell Rep. 43, 106. doi: 10.1007/s00299-024-03196-w
Hui, H., Yixue, J., Yan, T., and Yuhua, W. (2023). Comparative analysis of drought-responsive biochemical and transcriptomic mechanisms in two Dendrobium officinale genotypes. Ind. Crops Prod 199, 116766. doi: 10.1016/j.Indcrop.2023.116766
Jiaxin, H., Xingguo, L., Wenhui, L., Qian, Y., Zhenghao, L., Zhi, C., et al. (2023). Isolation and preliminary functional analysis of FvICE1, involved in cold and drought tolerance in Fragaria vesca through overexpression and CRISPR/Cas9 technologies. Plant Physiol. Biochem. 196, 270–280. doi: 10.1016/j.Plaphy.2023.01.048
Juan, Z., Weidong, Y., Bao, D., Guanghua, Z., Jianxi, Z., Pengyu, Z., et al. (2022). Relationship among Electrical Signals, Chlorophyll Fluorescence, and Root Vitality of Strawberry Seedlings under Drought Stress. Agronomy 12, 1428. doi: 10.3390/agronomy12061428
Khan, P., Abdelbacki, A. M. M., Albaqami, M., Jan, R., and Kim, K. M. (2025). Proline promotes drought tolerance in maize. Biology 14, 41. doi: 10.3390/biology14010041
Kiranmai, K., Rao, G. L., Pandurangaiah, M., Nareshkumar, A., Reddy, V. A., Lokesh, U., et al. (2018). A Novel WRKY Transcription Factor, MuWRKY3 (Macrotyloma uniflorum Lam. Verdc.) Enhances Drought Stress Tolerance in Transgenic Groundnut (Arachis hypogaea L.) Plants. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00346
Kitamura, H., Mori, T., Kohori, J., Yamada, S., and Shimizu, H. (2015). Breeding and extension of the new strawberry cultivar ‘Kaorino’ with extremely early flowering and resistance to anthracnose. Hortic. Res. (Jpn) 14, 89–95. doi: 10.2503/hrj.14.89
Lee, H. G., Park, M.-E., Park, B. Y., Kim, H. U., and Seo, P. J. (2019). The arabidopsis MYB96 transcription factor mediates ABA-dependent triacylglycerol accumulation in vegetative tissues under drought stress conditions. Plants 8, 296. doi: 10.3390/plants8090296
Li, M., Yang, Y., Raza, A., Yin, S., Wang, H., Zhang, Y., et al. (2021). Heterologous expression of Arabidopsis thaliana rty gene in strawberry (Fragaria x ananassa Duch.) improves drought tolerance. BMC Plant Biol. 21, 57. doi: 10.1186/s12870-021-02839-4
Li, Z., Jiao, F., Sun, Z., Zhang, E., Song, X., Pei, Y., et al. (2024). ZmD11 gene regulates tobacco plant floral development under drought stress. Agronomy 14, 1381. doi: 10.3390/agronomy14071381
Lichtenthaler, H. K. (1987). “Chlorophylls and carotenoids: pigments of photosynthetic biomembranes,” in Methods Enzymology, Vol. 148, Orlando, FL: Academic Press, 350–382. doi: 10.1016/0076-6879(87)48036-1
Lin, L., Chen, Q., Yuan, K., Xing, C., Qiao, Q., Huang, X., et al. (2024). E3 ubiquitin ligase PbrATL18 is a positive factor in pear resistance to drought and Colletotrichum fructicola infection. Hortic. Plant J. 10, 698–712. doi: 10.1016/j.Hpj.2023.04.007
Luis, M. Q., Mario, M., Rómulo, S. M., Antonio, C. A., Claudia, R., Stephan, P., et al. (2022). Improvement in the physiological and biochemical performance of strawberries under drought stress through symbiosis with Antarctic fungal endophytes&13. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.939955
Lynch, D. V. and Thompson, G. A. (1984). Chloroplast phospholipid molecular species alterations during low temperature acclimation in dunaliella. Plant Physiol. 74, 198–203. doi: 10.1104/pp.74.2.198
Matsuo, T., Ide, S., and Shitida, M. (1992). Correlation between chilling sensitivity of plant tissues and fatty acid composition of phosphatidylglycerols. Phytochemistry 31, 2289–2293. doi: 10.1016/0031-9422(92)83266-2
Nafiye, Ü and Volkan, O. (2023). Effects of drought stress treatment on phytochemical contents of strawberry varieties. Scientia Hortic. 316, 112013. doi: 10.1016/j.Scienta.2023.112013
Nour, M. M., Aljabi, H. R., AL-Huqail, A. A., Horneburg, B., Mohammed, A. E., and Alotaibi, M. O. (2024). Drought responses and adaptation in plants differing in life-form. Front. Ecol. Evol. 12. doi: 10.3389/fevo.2024.1452427
Pál, M., Tajti, J., Szalai, G., Peeva, V., Végh, B., and Janda, T. (2018). Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci. Rep. 8, 12839. doi: 10.1038/s41598-018-31297-6
Parveen, A., Liu, W., Hussain, S., Asghar, J., Perveen, S., and Xiong, Y. (2019). Silicon priming regulates morpho-physiological growth and oxidative metabolism in maize under drought stress. Plants 8, 431. doi: 10.3390/plants8100431
Perin, E. C., Messias, R., Borowski, J. M., Crizel, R. L., Schott, I. B., Carvalho, I. R., et al. (2019). ABA-dependent salt and drought stress improve strawberry fruit quality. Food Chem. 271, 516–526. doi: 10.1016/j.foodchem.2018.07.213
Qiang, C., Lin, H., Jiamin, L., Peng, Q., Pang, T., C., C. M. J., et al. (2022). Integrated transcriptome and methylome analyses reveal the molecular regulation of drought stress in wild strawberry (Fragaria nilgerrensis). BMC Plant Biol. 22, 40. doi: 10.1186/s12870-022-04006-9
Qin, J., Guo, N., Yang, J., and Wei, J. (2024). Recent advances in metal oxide nanozyme-based optical biosensors for food safety assays. Food Chem. 447, 139019. doi: 10.1016/j.Foodchem.2024.139019
R., H.-M. N., Caroline, B., Daniel, W., and XXXR., S.-G. M. (2023). Current state and future perspectives of commercial strawberry production: A review. Scientia Hortic. 312, 111893. doi: 10.1016/j.Scienta.2023.111893
Sakshi, S., Priyanka, S., Jyoti, S., Pooja, P., and Asha, S. (2024). Drought stress in Lens culinaris: effects, tolerance mechanism, and its smart reprogramming by using modern biotechnological approaches. Physiol. Mol. Biol. Plants 30, 227–247. doi: 10.1007/s12298-024-01417-w
Sharma, A. and Zheng, B. (2019). Melatonin mediated regulation of drought stress: physiological and molecular aspects. Plants 8, 190. doi: 10.3390/plants8070190
Shen, S., Yan, W., Xie, S., Yu, J., Yao, G., Liu, Y., et al. (2024). Comparison of morphological, physiological, and related gene expression responses to drought stress in fiveCamellia VietnamensisCultivars. Agronomy 14, 915. doi: 10.3390/agronomy14050915
Sheng, F., Huiyi, Y., Guangwei, W., Tinghai, S., Zehua, W., Min, W., et al. (2022). Potassium application enhances drought tolerance in sesame by mitigating oxidative damage and regulating osmotic adjustment. Front. Plant Sci 13. doi: 10.3389/fpls.2022.1096606
Song, Y., Peng, Y., Liu, L., Li, G., Zhao, X., Wang, X., et al. (2024). Phased gap-free genome assembly of octoploid cultivated strawberry illustrates the genetic and epigenetic divergence among subgenomes. Horticult Res. 11, uhad252. doi: 10.1093/hr/uhad252
Songquan, S. and Yanrong, W. (2002). Molecular response of plant to drought stress. Ying yong sheng tai xue bao 13, 1037–1044. doi: 10.13287/j.1001-9332.2002.0240
Suresh, K., Karishma, S., and Trilochan, M. (2023). Biochemical and epigenetic modulations under drought: remembering the stress tolerance mechanism in rice. Life (Basel Switzerland) 13, 1156. doi: 10.3390/life13051156
Teresa, A. M., Luis, M., Antonio, G. J., Jesús, M. J., David, L., Pedro, G., et al. (2021). Yield and fruit quality of strawberry cultivars under different irrigation regimes. Agronomy 11, 261. doi: 10.3390/agronomy11020261
Thokchom, A. and Hazarika, B. N. (2022). Morpho-physiological and biochemical changes under drought stress in strawberry: A review. Agric. Rev. 45, 340–344. doi: 10.18805/ag.R-2295
Toscano, S., Ferrante, A., and Romano, D. (2019). Response of mediterranean ornamental plants to drought stress. Horticulturae 5, 6. doi: 10.3390/horticulturae5010006
Vardan, T., Armen, P., Michał, T., and Astghik, P. (2022). Evaluation of malondialdehyde levels, oxidative stress and host–bacteria interactions: escherichia coli and salmonella derby. Cells 11, 2989. doi: 10.3390/cells11192989
Vieira, Y., Mendes, E. F., Galhego, V., Bastos, G. A., Cordeiro, T. F., Ding, X., et al. (2024). Euphorbiaceae superoxide dismutase, catalase, and glutathione peroxidase as clues to better comprehend high drought tolerance in castor bean. Ind. Crops Prod 222, 119510. doi: 10.1016/j.Indcrop.2024.119510
Wang, J., Cheng, Y., Ma, C., Ni, Y., Yu, J., Gao, H., et al. (2024). Integrate analysis of metabolome and transcriptome of three Fragaria × ananassa cultivars to stablish the non-volatile compounds of strawberry flavor. LWT 198, 116043. doi: 10.1016/j.lwt.2024.116043
Weidong, Y., Juan, Z., Yu, Z., Tianran, D., Bao, D., and Ji, Q. (2023). Electrical and photosynthetic response of Rosa chinensis under drought stress. Biosyst. Eng. 236, 248–257. doi: 10.1016/j.Biosystemseng.2023.11.007
Xue, B., Kaili, L., Tiao, N., Changjun, D., Li, W., Diyan, L., et al. (2023). Effects of multiple N, P, and K fertilizer combinations on strawberry growth and the microbial community. PloS One 18, e0293088. doi: 10.1371/journal.Pone.0293088
Yini, Z., Qifu, L., Jingmin, J., and Yanjie, L. (2021). Prediction and utilization of malondialdehyde in exotic pine under drought stress using near-infrared spectroscopy&13. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.735275
Yu, C., Yao, A., Li, X., Li, W., Gao, R., Feng, Y., et al. (2024). Overexpression of a Malus baccata (L.) Borkh WRKY transcription factor gene MbWRKY65 increased the tolerance to cold and drought in transgenic tomato. In Vitro Cell. Dev. Biol. - Plant 60, 620–633. doi: 10.1007/s11627-024-10458-5
Zhang, S., Han, Y., Zeng, Q., Wang, C., Wang, H., Zhang, J., et al. (2024). Whole-Transcriptome Sequencing Reveals the Global Molecular Responses and NAC Transcription Factors Involved in Drought Stress inDendrobium catenatum. Antioxidants 13, 94. doi: 10.3390/antiox13010094
Keywords: drought stress, strawberry, transcriptomics, metabolomics, multi-omics integration
Citation: Qiu H, Ning T, Ma H, Gong W, Li D, Niu Y, Chen Z, Jin L, Han C, Tang Y, Deng C, Zhao M, Cui X and Li J (2025) Drought stress-induced physiological and molecular changes in strawberries: an integrated transcriptomic and metabolomic perspective. Front. Plant Sci. 16:1679472. doi: 10.3389/fpls.2025.1679472
Received: 04 August 2025; Accepted: 17 November 2025; Revised: 26 October 2025;
Published: 05 December 2025.
Edited by:
Maharajan Theivanayagam, Pohang University of Science and Technology, Republic of KoreaReviewed by:
Diaga Diouf, Cheikh Anta Diop University, SenegalGuo Jiaxuan, Beijing University of Agriculture, China
Copyright © 2025 Qiu, Ning, Ma, Gong, Li, Niu, Chen, Jin, Han, Tang, Deng, Zhao, Cui and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, bGlqaW5nQGttdS5lZHUuY24=
†These authors have contributed equally to this work
 Huimin Qiu1†
Huimin Qiu1† Weijun Gong
Weijun Gong Diyan Li
Diyan Li Lu Jin
Lu Jin Chengchou Han
Chengchou Han Jing Li
Jing Li