- 1Department of Agricultural, Food, Environmental and Forestry Sciences and Technologies (DAGRI), University of Florence, Sesto Fiorentino, Italy
- 2Council for Agricultural Research and Economics, Reasearch Centre for Olive, Fruit and Citrus Crop (CREA-OFA), Rende, Italy
- 3Department of Bioscience and Agro-Food and Environmental Technology, University of Teramo, Teramo, Italy
- 4Foundation for the Future of Cities, (FFC), Florence, Italy
- 5Department of Biology, Università di Firenze, Florence, Italy
Olea europaea L. subsp. europaea, var. europaea, plays a crucial role in cultural identity and economic prosperity across many regions of the Mediterranean Basin. The olive fruit fly, Bactrocera oleae, represents a major global challenge to olive and olive oil production. Its larvae feed exclusively on olive fruits, causing severe crop damage and substantial economic losses. In this study, we examined four olive cultivars differing in susceptibility to B. oleae, focusing on fruit morphology (weight, maturity index, penetration resistance), cuticle characteristics, and volatile organic compound (VOC) emissions. Confocal microscopy with Nile Red staining was used to analyze cuticle structure, while VOCs were measured using proton-transfer-reaction time-of-flight mass spectrometry (PTR-ToF-MS). Thicker cuticles were associated with reduced infestation, suggesting a mechanical barrier function against oviposition or larval penetration. PTR-ToF-MS analysis revealed cultivar- and ripening stage–specific VOC emission patterns, with certain compounds potentially acting as deterrents or attractants to the olive fly. These results indicate that fruit morphology, cuticle development, and VOC profiles act as interdependent determinants of cultivar-specific tolerance to B. oleae. The integration of these physical and chemical traits provides valuable markers for breeding programs and contributes to the development of sustainable, integrated pest management strategies in olive cultivation.
1 Introduction
Olea europaea (subsp. europaea, var. europaea) (Green, 2002) represents the foundation of both cultural heritage and economic development across Mediterranean and other olive-growing regions. Domestication and selection processes have resulted in more productive and adaptable trees, leading to a constantly increasing number of varieties, with more than 2,000 cultivars spread worldwide (Green, 2002). Italy stands out with the highest level of olive tree biodiversity, boasting approximately 600 described cultivars (Bartolini et al., 2014). The economic significance of olive cultivation is intrinsically linked to tree health and fruit production, which directly impacts both the yield and quality of olive oil.
A primary threat to global olive and oil production, however, is the olive fruit fly, Bactrocera oleae. Historically recognized as a major pest in Mediterranean olive-growing regions, this species has expanded its distribution due to globalization and climate change. The larvae of B. oleae feed exclusively on olive fruits, posing a severe threat to both intensive and traditional cultivation systems (Grasso et al., 2017). Under favorable climatic conditions, infestations cause substantial crop losses and economic damage to olive farmers.
However, the diverse genetic makeup of olive cultivars offers a potential solution, as some varieties exhibit natural tolerance to this pest. This genetic variability translates into different levels of resistance to B. oleae among cultivars (Grasso et al., 2017; Medjkouh et al., 2018). The degree of tolerance to infestation is largely determined by the cultivar’s genetic background, which regulates the activation of specific defense mechanisms. Additionally, B. oleae demonstrates a preference for laying eggs on certain olive cultivars over others (Malheiro et al., 2016). Fruit characteristics such as chemical and volatile compound profiles, morphological traits, and ripening stage significantly influence the insect’s behavior and infestation dynamics (Rodríguez et al., 2013; Giunti et al., 2020).
Interactions between the olive tree and its biotic stressors likely shape the compositional traits of the drupes, either reinforcing or diminishing certain features under complex genetic control. For example, the olive tree has developed physical defense barriers, such as a thicker and more elastic fruit skin, which can hinder fruit penetration and subsequent oviposition by pests (Corrado et al., 2023) and chemical defense mechanisms such as the emission of VOCs directly from the olive fruit, which affect the olive fly behavior (Kokkari et al., 2024).
Indeed, before laying eggs, female flies make exploratory punctures (sterile stings) in the olive fruit to determine if the fruit is suitable to host and nourish their developing larvae (Gonçalves et al., 2012). If this test proves favorable, the fly then creates an oviposition chamber by piercing the fruit and depositing an egg within a characteristic triangular incision measuring 1–1.5 mm in length (Rotondi et al., 2021). Daane and Johnson (2010) reported that different cultivars exposed to olive fruit fly showed difference in sterile punctures and larval infestation percentage. Thus, the female is guided both by visual and chemical stimuli in its search for suitable olives, showing preference for (1) large and unripe (green) fruits; (2) fruits with thinner skin (Gonçalves et al., 2012); and (3) cultivars with a specific chemical and volatile emission (Aluja and Mangan, 2008; Daane and Johnson, 2010; Malheiro et al., 2013). The varied capacity of different olive cultivars to cope with B. oleae highlights the need to evaluate olive genotypes under diverse environmental conditions and emphasizes the necessity for ongoing research to mitigate the damaging impact of this pest.
This study investigates three potentially interconnected mechanisms that contribute to olive fruit tolerance against B. oleae: cultivar morphological diversity, cuticle thickness, and volatile organic compound (VOC) profiling. Among the various traits distinguishing olive cultivars, fruit morphology and biochemical composition are central to determining their susceptibility or tolerance to pests. Specifically, the olive fruit cuticle plays an essential role in safeguarding the fruit from pathogens and pest attacks (Martin and Rose, 2014). Composed of a complex blend of biopolymers, this structure acts as a formidable physical barrier against water loss, pathogen invasion, and mechanical damage. Understanding the composition and dynamics of the olive fruit cuticle may offer new perspectives for pest and disease management strategies. In this context, the interaction between the cuticle and VOC emission remains underexplored. VOCs are lipophilic bioactive molecules involved in plant communication and defense, exerting direct deterrent or toxic effects on herbivores, or indirectly attracting their natural enemies or parasitoids (Heil, 2014; Wang and Erb, 2022). For example, studies on Petunia hybrida have shown that changes in cuticle thickness can influence VOC diffusion and internal redistribution (Liao et al., 2020). Similarly, the oxidation of unsaturated cuticular waxes observed in Populus trichocarpa, P. balsamifera, and Zea mays has been identified as a source of aldehyde volatiles that may trigger plant-to-plant signaling and defense responses (Chen et al., 2023). This research aims to contribute to a deeper scientific understanding of cultivated olives, contributing to the development of a more sustainable and resilient olive production system.
2 Materials and methods
Drupes from four olive cultivars (Arbequina, Canino, Coratina, and Nocellara messinese), were morphologically characterized, tested for their degree of susceptibility to B. oleae, subjected to VOCs analysis after stings simulation and to Nile Red staining. Fruit sampling was carried out by handpicking during the 2023 olive season at the CREA OFA’s International Olive World Germplasm bank (OWGB; Crosia, Italy, 39° 36’ 54.1’’, 16° 46’ 11.0’’). The study involved three trees per cultivar. Sixty drupes from each tree and cultivars were sampled at complete pit hardening (T1), when fruits become susceptible to B. oleae oviposition (Baratella et al., 2017), and at drupe veraison when olives are typically harvested (T2). The cultivars were chosen based on preliminary observations in the collection field that suggested varying levels of susceptibility to the olive fruit fly, these initial findings required further confirmation through this study.
2.1 Olives morphological aspects and B. oleae infestation level
A subsample of forty drupes/tree (120 drupes per cv and sampling date) was used to evaluate the ripening stage according to the Jaén index (IOC, 2011) and average drupe weight, as well as to assess the level of susceptibility to B. oleae. This assessment was based on the presence of sterile punctures, all preimaginal stages of the olive fruit fly (alive or dead), and abandoned tunnels.
Additionally, active infestation (presence of eggs, live first and second instar larvae) and harmful infestation (live third instar larvae, pupae, and abandoned tunnels) were evaluated after destructive analyses under a binocular microscope following Vizzarri et al. (2023) along with dead infestation (dead first, second, and third instar larvae, and pupae) and infestation limited to sterile punctures (dead first, second, and third instar larvae, and pupae) and infestation limited to sterile punctures. Furthermore, 15 drupes per tree, cultivar, and sampling date were collected to measure penetration resistance (PR) using a Fg-5000A electronic force gauge penetrometer (Lutron Electronic Enterprise Co., Ltd., Taipei, Taiwan) equipped with a 1-mm tip. Each fruit was pierced three times along the equatorial diameter.
2.2 Nile Red staining and tissue analysis by confocal microscopy
For the analysis of lipid accumulation within the olive fruit tissue, Nile Red staining was employed, followed by confocal microscopy. Nile Red (Sigma-Aldrich) was prepared as a stock solution at a concentration of 1 mg/mL in DMSO, and then aliquoted into 1.5 ml tubes to minimize thawing cycles and preserve its stability. A thin fruit slice was carefully obtained using a razor blade. Each slice was then incubated with the Nile Red solution (2ul of Nile Red at a concentration of 1 mg/mL and 998 ul of milliQ water) for 5 minutes.
After staining, samples were observed using a Leica SP5 confocal microscope equipped with a 40x objective (HCX PL APO OIL UV). For imaging the blue-shifted excitation of Nile Red, a 458 nm laser line was used, and the fluorescence emission signal was acquired between 475–495 nm. Three olive drupes for each cultivar were used and three experimental replicates were conducted on each of the two sampling dates to ensure the robustness and reliability of the results presented in this study.
2.3 Stings simulation and VOC analysis
Proton Transfer Reaction Time-of-Flight Mass Spectrometry (PTR-ToF-MS) was employed to monitor the emission of volatile organic compounds (VOCs) from the fruits of the four distinct olive cultivars. The analysis was conducted under controlled climatic conditions at 25 ± 1°C and 45–55% relative humidity using a PTR-MS 8000 instrument (Ionicon Analytik GmbH, Innsbruck, Austria). The tool was operated in its standard configuration with H3O+ as a reagent ion. The following settings were applied: drift tube voltage of 600 V, drift tube temperature 60°C and drift pressure 2.2 mbar, E/N of 145 Td, drift gas flow of 40 sccm, and a mass scan range of m/z 13–250. The internal calibration of the PTR-ToF spectra was performed offline using the following signals: m/z 29.9974 (NO), m/z 59.049 (C2H5O2), and m/z 180.937 (C6H4Cl3). To ensure high mass accuracy, we followed the procedure described by Cappellin et al. (2010), which employs a three-point calibration. This approach enabled high mass accuracy across the relevant mass range, sufficient for molecular formula identification. After mass peak selection and extraction, tentative compound identification was performed based on the molecular formulas provided by the analysis software and compared with data reported in the literature (Brilli et al., 2011; Rasulov et al., 2019).
With the aim of simulating the fly attacks, prior to analysis, fruits were stung using an Insulin pen with a 1.5 mm long and 0.3 mm diameter needle to simulate and replicate the ovipositor of Bactrocera oleae in both penetration depth and puncture diameter. Specifically, four stings were done at the opposite side of each fruit. For VOC collection, 10 stung fruits per cultivar were placed in 2/3 L glass bottles and incubated for 5 minutes. After incubation, headspace VOCs were analyzed following the experimental design and analytical methodology described by Taiti et al. (2022). Samples were analyzed in randomized order, with a 5-minute interval between runs to flush the inlet tubing and minimize memory effects. A Zero Air Generator (Peak Scientific, Inchinnan, UK) was used to purge the inlet line between measurements. In total, 16 PTR-ToF runs were conducted (4 cultivars x 2 sampling times x 2 replicates). Raw data (expressed as counts per second, cps) were acquired with TofDaq software v. 183 (Tofwerk AG, Innsbruck, Switzerland) using a dead time of 20 ns for the Poisson correction. Data were subsequently converted to parts per billion by volume (ppbv). After removal of NO+, O2+, and water clusters ions, a threshold of 0.5 ppbv was applied; all compounds below this threshold were excluded from further analysis. To ensure comparability of VOC profiles across cultivars despite differences in fruit mass, all data were normalized to a standard fruit weight of 100g. Headspace analyses were repeated for each sampling time using the same protocol.
2.4 Statistics
One-way ANOVA analysis was employed in conjunction with Tukey’s HSD post-tests to analyze both the morphological (fruit weight, penetration resistance and Jaén index) and B. oleae infestation data, of the tested cultivars at the two sampling dates. Capital letters A, B, C =P <0.01; lowercase letters a, b, c, =P <0.05. (Tables 1, 2) Pearson product-moment correlation coefficients between the 21 protonated masses emitted by the fruits and wounding punctures, sterile punctures, and sound olives, respectively, are calculated for each individual VOC. Computations were performed by Stat graphics Centurion 19.6.05. A Factor Analysis (FA) was applied to highlight the relationships among the 4 cultivar olive fruit samples collected at T1 and T2, respectively, considering as factors the three levels of infestation (sound olives, sterile punctures, and wounding punctures) and the protonated m/z having Pearson product-moment correlation coefficients (P) ≥-0.5 or ≥0.5, respectively. Computations were performed by XLSTAT 2024.4.1.1425. Cuticle thickness difference (graphs in Figure 1) where evaluated by One-way ANOVA, Sidak’s multiple comparison test. Using asterisks to better compare each couple of cvs. Asterisks denote significance in statistical analysis: n.s.: not significant; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
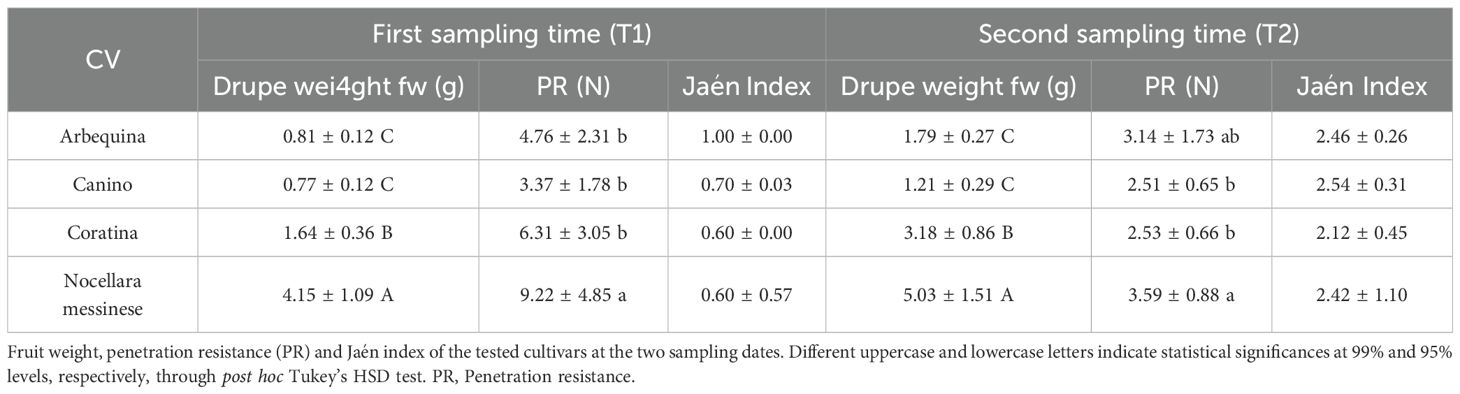
Table 1. Comparison of fruit weight, penetration resistance (PR), and Jaén index among cultivars at two sampling dates.
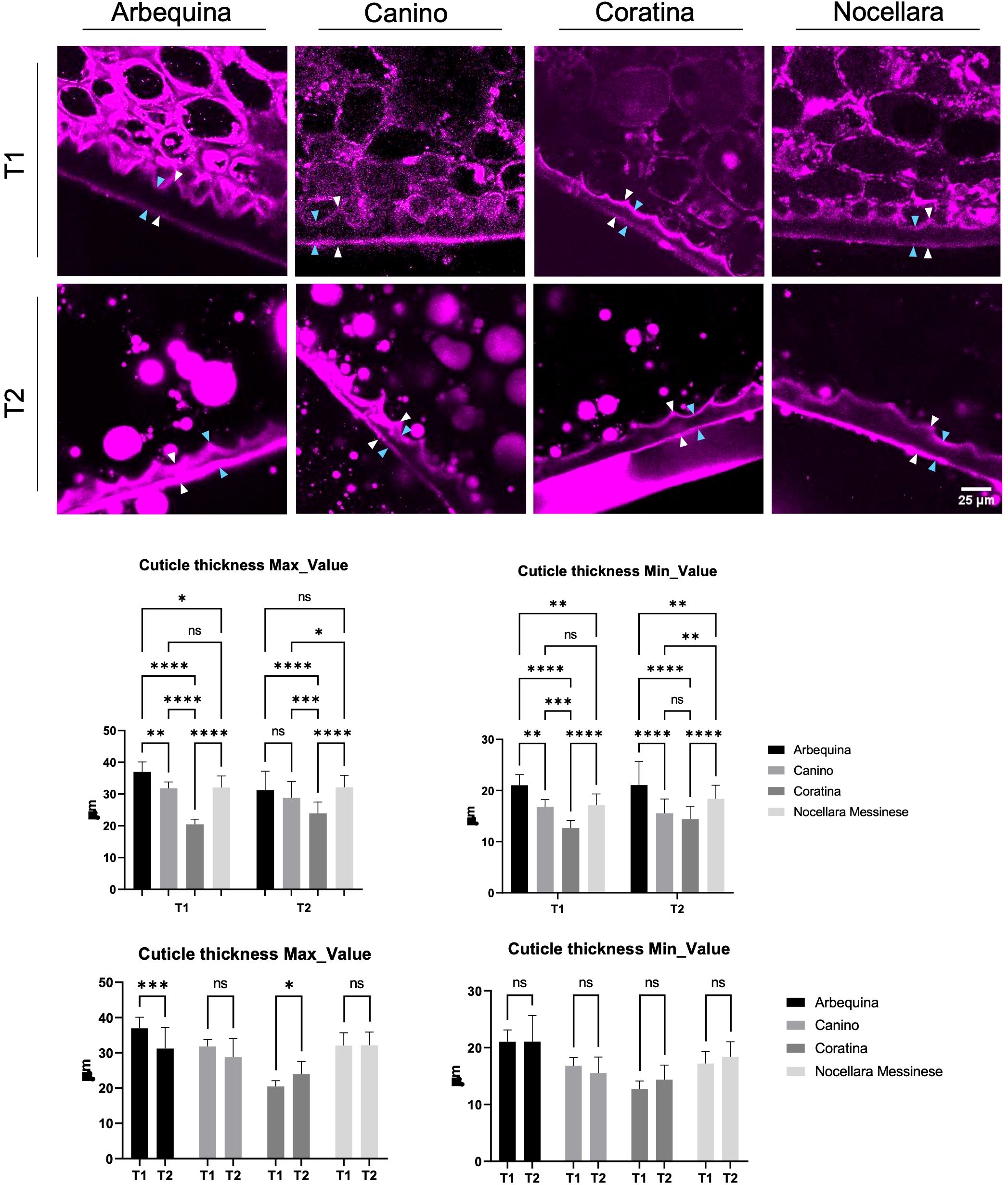
Figure 1. Confocal images of arbequina, canino, coratina and nocellara drupe cuticle labeled with Nile Red. White arrowheads indicate the points where the maximum cuticle thickness was measured. Cyan arrowheads indicate the points where the minimum cuticle thickness was measured. Minimum and maximum cuticle value for each sample monitored during two phases of fruit maturation (early and late in the development). n.s., not significant; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Error bars represent the S.E.M. Cuticular layer that enveloped the fruit surface at the two different developmental stages. n.s., not significant; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Error bars represent the SEM.
3 Results
3.1 Olives morphological aspects and B. oleae infestation level
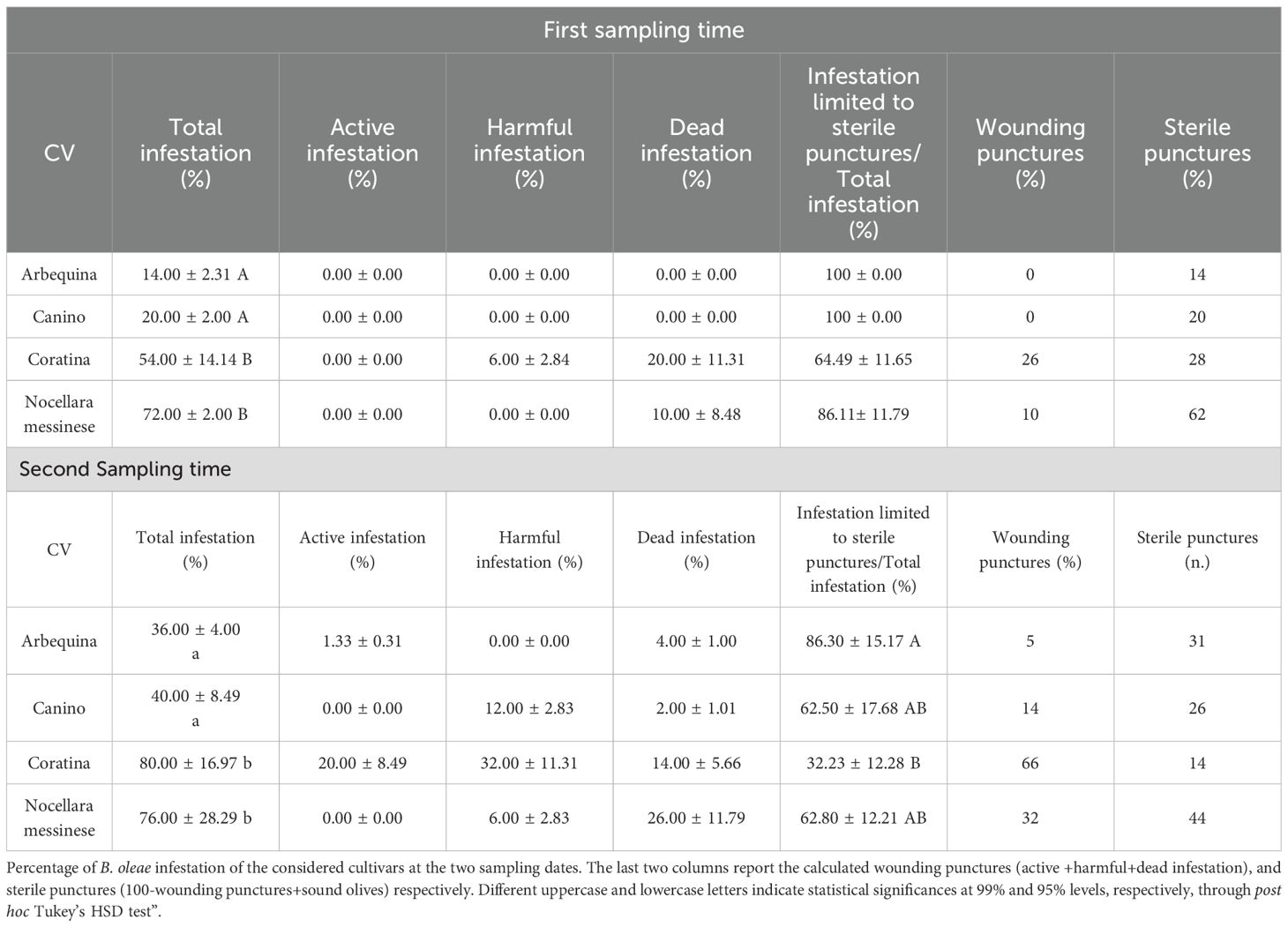
The results of the analyzed traits showed, in general, considerable variability (Table 1) over time among cultivars, as well as intra-cultivar variability in the case of PR (penetration resistance). A strong direct correlation (Pearson’s r=0.96) was found between drupe weight and PR at the early stage of maturation, with the cv Nocellara messinese presenting the statistically significant highest values at both sampling dates. Conversely, the values obtained from the fruits of Arbequina and Canino were overall very similar. The notable increase in weight recorded between the two sampling dates was counterbalanced by an equally remarkable reduction in PR. The cvs Arbequina and Coratina showed the highest weight gain (+120.99% and +93.90%, respectively; compared to +57.143% and +21.21% for the cvs Canino and Nocellara messinese), while drupe firmness was more pronouncedly reduced in cv Nocellara messinese (-61.150%) and Coratina (-59.89%). Nevertheless, the drupes of Nocellara messinese still displayed the highest PR at T2 with an average of 3.59 Newton. Drupe color ranged from green to yellowish green at T1 and from yellowish green to purple (veraisoned drupes) at T2.
Regarding B. oleae infestation level of the tested cultivars (Table 2), fruits of Arbequina and Canino resulted to be the less infested both at T1 (86 and 80% of non-infested drupes, respectively) and T2 (64 and 60%), while the drupes of Nocellara messinese and Coratina at the two sampling times recorded the highest statistically significant percentages of overall infestation (72 and 76% vs 54 and 80%, respectively). In particular, at T1, total infestation for the cv Arbequina and Canino was only represented by sterile punctures, whose percentages remained quite high even at T2 (86.30 and 62.50% of total infestation, respectively). The sum of sterile punctures (for the most part) and dead infestation made up 100% of total infestation in the drupes of the cv Nocellara messinese at T1 and 92% at T2. A further different trend was displayed by the drupes of Coratina which suffered the highest active and harmful infestation.
3.2 Nile red to screen for cuticle size in different cultivars during fruit maturation
Following Nile Red staining, olive drupe samples from all cultivars exhibited fluorescence due to the dye’s ability to bind to hydrophobic structures, such as the cuticle. This fluorescent dye has been previously utilized to investigate cuticle dynamics in plant tissues, demonstrating its effective absorption by the cuticle. Fluorescence microscopy revealed distinct staining patterns that corresponded to cuticle localization, allowing for the determination of minimum and maximum cuticle thickness values for each sample across two key phases of fruit maturation: early and late development. The intensity of fluorescence varied among cultivars (Figure 1), directly reflecting differences in cuticle thickness. Early in development, Arbequina, Canino, and Nocellara Messinese displayed the highest fluorescence signals for maximum cuticle thickness compared to Coratina (Figure 1). Similar differences were also observed for minimum cuticle thickness, except for Canino, which resembled Nocellara Messinese in this regard. This general profile was maintained at later developmental stages (Figure 1), and statistical analysis confirmed significant variations in cuticle size among the cultivars (Figure 1). To further visualize and monitor changes in cuticle size throughout fruit maturity, Nile Red staining was again applied to fruit sections of Arbequina, Canino, Coratina, and Nocellara Messinese. Fluorescence patterns associated with the cuticular layer dynamically changed across the two developmental phases, as determined by fluorescence microscopy. Notably, the fluorescence signal relative to the maximum cuticle thickness in Arbequina was observed to decrease during the maturation process. Conversely, in Coratina, the presence of a cuticular layer enveloping the fruit surface increased as the fruit underwent ripening. For the remaining cultivars, no significant difference was observed between the two distinct developmental stages (Figure 1). Similarly, contrasting trends in cuticle thickness during veraison and full ripening have been reported in nine olive cultivars in Spain (Diarte et al., 2019), with the ‘Arbequina’ cultivar showing a slight decrease under irrigated conditions and a more pronounced decrease in rainfed orchards (Diarte et al., 2023). Overall, our data fall within the range reported for other olive cultivars in studies employing scanning electron microscopy (SEM) (Lanza et al., 2015; Goldental-Cohen et al., 2019), as well as in studies where cuticle thickness was quantified using light microscopy combined with toluidine blue (Gomes et al., 2012) or Sudan IV staining (Diarte et al., 2023).
3.3 VOCS profile in olive fruits during early and late developmental stage
Using PTR-ToF-MS we identified different compounds emitted from olive fruits, all of which have been previously reported in other studies. The VOC data obtained are reported in Table 3. Qualitative differences in VOC composition were observed among the four olive cultivars and between the two sampling times. Comparison of the data (Table 3) revealed changes in VOC emissions from the artificial bite made on the olive fruits. Olives respond differently to internal physiological changes during fruit maturation: some volatile organic compounds (VOCs) like C5 and C6 decreased, while others, such as methanol and acetaldehyde, increased in release intensity across all cultivars and both sampling times. As suggested by White et al. (2016) in mango fruits, the increase in methanol emission could be due to the higher methyl esterification of pectins when fruits are immature compared to when they are ripe. Accordingly, the more marked increase in methanol release recorded with the progression of fruit maturation in the cvs Nocellara messinese (+152.01%) and Coratina (+141.09%) compared to Arbequina (+43.69%) and Canino (+15.43%) is accompanied by a specular reduction in drupe penetration resistance (-61.06 and -59.90% vs -34.03 and -25.52%, respectively). Acetaldehyde is a naturally occurring metabolite, resulting from pyruvate decarboxylation, which can be reduced to ethanol. As reported by Pesis (2005), both climacteric and non-climacteric fruit produce a lot of acetaldehydes mainly depending on their genetic characteristics. Acetaldehyde and ethanol which are precursors of natural aroma, are typically produced because of the normal ripening process (El Hadi et al., 2013). Methanol, ethanol, and acetaldehyde emissions increased throughout the maturation process, depending on the cultivar. Similarly, total VOC emissions increased for each cultivar between the two sampling points. These results align with findings by Beltrán et al. (2015) who reported variations in ethanol emissions among olive cultivars and at different maturation stages. For example, ethanol levels were lower in Arbequina and Canino, average in Coratina, and higher in Nocellara Messinese at both time points (see Table 3). Additionally, each cultivar exhibited different rates of increase in ethanol emissions depending on its ripening stage. Total VOC emission intensity also varied by cultivar and sampling time. Nocellara Messinese consistently showed the lowest overall emission intensity at both time points (Table 3). Moreover, an increase in dimethyl sulfide emissions was observed at T2 (approximately 10-fold in Nocellara Messinese, 6-fold in Coratina, and 4-fold in Arbequina). This increase could be related to the enzymatic activity of yeasts naturally present on the olive carposphere (Guerrini et al., 2019) and may also be linked to B. oleae preferences (Brkić Bubola et al., 2023). The rise in yeast and/or bacterial populations on the fruit surface may also contribute, at least in part, to the increased ethanol emissions observed in veraison-stage drupes. On the contrary, the emission intensity of most C5 and C6 compounds declined during ripening. Notably, signals detected at m/z 69.069 (Tentatively Identified as isoprene), 81.069 (TI C6 fragments), 99.080 (TI (E)-2-hexenal), and 101.059 (TI (Z)-3-hexen-1-ol/hexanal) all decreased between T1 and T2 across all cultivars. This trend is consistent with the known reduction in LOX (lipoxygenase) and hydroperoxide lyase (HPL) activities during fruit ripening (Yilmaz et al., 2002). An exception to this trend is the signal at m/z 87.080 (TI as pentanal/3-methylbutanal, aldehydes associated with fermentative processes), which tended to increase. At both sampling times, Nocellara Messinese and Coratina displayed the lowest emission of C5 and C6 compounds. This general C5/C6 decline (which imparts green-fruity notes), coupled with an increase in dimethyl sulfide, acetaldehyde, propanal, and acetates (which contribute to odor defects), represents an important factor when determining the optimal harvesting period (Yan et al., 2020; Taiti et al., 2022).
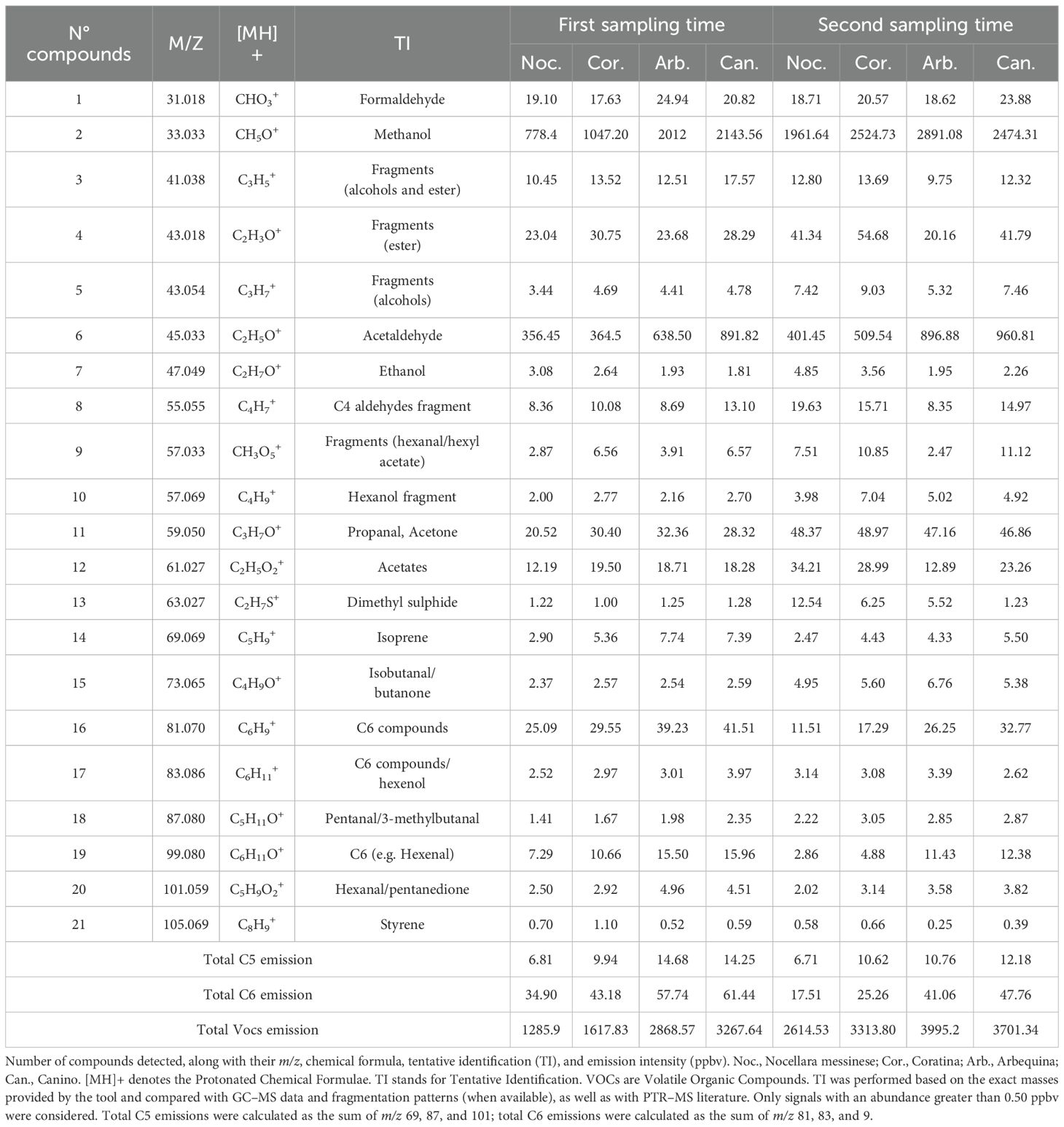
Table 3. Identification and emission intensity of volatile organic compounds (VOCs) detected in olive cultivars by PTR–MS.
4 Discussions
4.1 Morphological traits, cuticle thickness, and VOC emissions in relation to B. oleae infestation
Our results highlight the multifactorial nature of olive fruit tolerance to B. oleae infestation, demonstrating how morphological traits, cuticle structure, and VOCs emissions could collectively influence the susceptibility of different cultivars. Morphologically, a clear relationship was observed between olive drupe characteristics and infestation levels. Cultivars Arbequina and Canino consistently showed the lowest infestation and penetration resistance (PR) values, with minimal reductions in PR between sampling dates (-34.04% and -25.47%, respectively), likely due to the high prevalence of non-infested fruits within these cultivars. On the contrary, Nocellara Messinese exhibited the lowest percentage weight gain and highest percentage decrease in PR, consistent with its earlier fruiting stage at T1 and the physiological softening of fruit during ripening, driven by enzymatic degradation of cell wall polysaccharides (Jimenez et al., 2001; Cappelli et al., 2024). The higher PR values observed in Nocellara Messinese (9.22 N) and Coratina (6.22 N) support previous findings indicating B. oleae’s preference for large fruits with high pulp hardness (Gonçalves et al., 2012; Rizzo et al., 2012). It is important to contextualize the PR values reported here within the framework of ovipositor mechanics: the pressure exerted by the olive fly ovipositor ranges from 7.2 to 1.9 N mm-², applied over a minuscule surface (~1 × 10-4 mm²) (Figure 2).
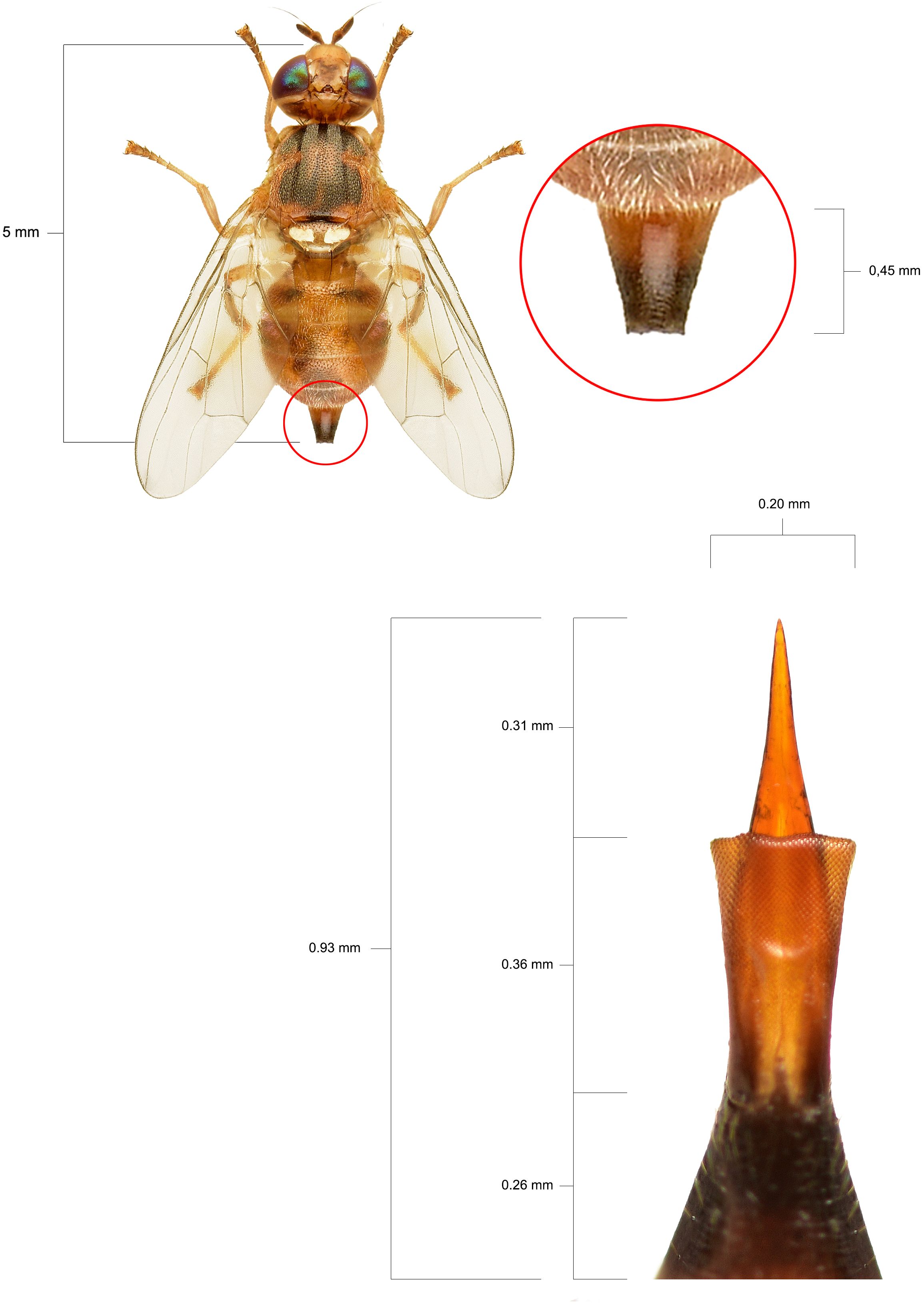
Figure 2. Typical dimensions of a female specimen of B. oleae and its ovipositor apparatus equipped with a sting.
This suggests that measured PR values are indicative rather than absolute resistance parameters. Notably, Coratina and Nocellara Messinese both with the highest total infestation, showed contrasting behaviors: Coratina experienced high levels of active and harmful infestation, whereas Nocellara Messinese displayed a significant prevalence of sterile punctures and dead infestation. This implies a post-puncture defense mechanism in Nocellara Messinese that either impedes successful egg laying or inhibits pre-imaginal development, a phenomenon aligned with previous studies demonstrating induced plant defenses in B. oleae-infested olives, including the production of VOCs, phytohormones, and defense proteins (Alagna et al., 2015; Bogka et al., 2023). Moreover, whole-plant VOC emissions are known to affect B. oleae mating and oviposition preferences (Malheiro et al., 2016; Kokkari et al., 2021). The slightly lower maturation index in Coratina drupes may also contribute to fly preference for greener fruits (Malheiro et al., 2019). The correlations between VOC profiles and infestation status (see VOC section) further support these defense mechanisms. Regarding cuticle thickness, Nile Red staining combined with confocal scanning microscopy (CSM) revealed significant variability among cultivars, enabling effective screening of this trait beyond laborious methods such as Transmission Electron Microscopy. The thickest cuticle was found in the less susceptible Arbequina cultivar, suggesting that a thicker cuticle serves as a physical barrier to B. oleae oviposition. The comparison between Nocellara Messinese (thicker cuticle, mainly sterile infestation) and Coratina (thinner cuticle, higher active infestation) reinforces this hypothesis. Such morphological barriers may reduce the likelihood of ovipositor penetration and successful infestation, indicating cuticle thickness as a promising trait for breeding resistant cultivars. These findings have direct implications for sustainable olive pest management, emphasizing cuticle morphology’s role in infestation susceptibility.
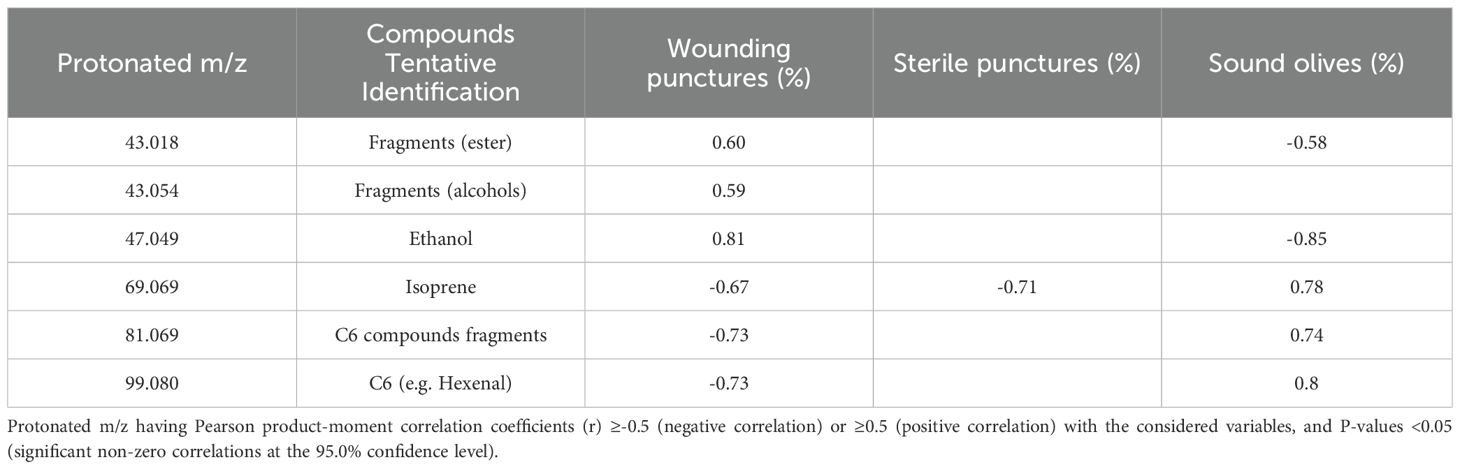
VOCs analysis further elucidated the biochemical interactions between olive fruits and B. oleae. By the VOC analysis emerged 21 protonated masses emitted by the fruits and their correlations with infestation levels were investigated. In particular, we focused on “wounding punctures,” which include active, harmful, and dead infestations; these punctures represent actual damage caused by larval development inside the fruit. VOC emissions were measured in four olive cultivars at two key ripening stages (T1 and T2). The detailed results of these correlations are presented in Figure 3 and summarized in Table 4.
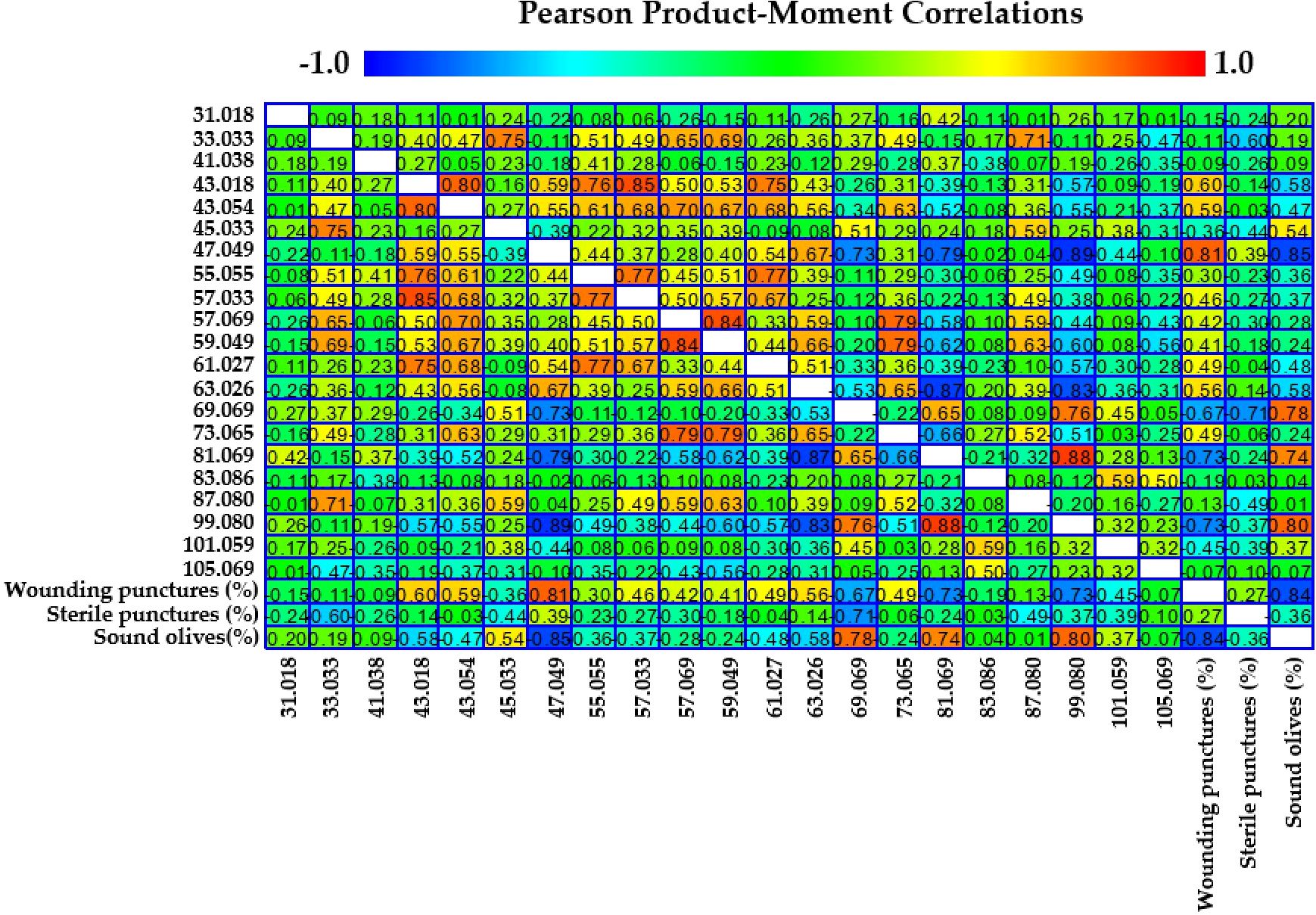
Figure 3. Pearson product-moment correlation coefficients between 21 protonated masses and wounding punctures, sterile punctures, and sound olives. Color is used to denote the magnitude of the correlations, which range from -1 to +1.
Moreover, the Factor Analysis plot (Figure 4) reveals that certain compounds, such as ethanol (m/z 47.049) and fragments corresponding to esters and alcohols (m/z 43.018, 43.054), were positively correlated with the percentage of wounding punctures at both sampling times, indicating their potential roles as attractants. Conversely, C5 and C6 compounds, which are early products of the lipoxygenase (LOX) cascade such as isoprene (m/z 69.070), C6 fragments (m/z 81.070), and hexenal (m/z 99.080) showed strong positive correlations with sound olives and negative correlations with wounded fruits, suggesting a deterrent effect on ovipositing females. These C6 compounds have been documented to disrupt insect behavior by acting as repellents or masking attractant signals (Germinara et al., 2024). Similarly, isoprene, produced via the mevalonic acid pathway, is a well-known general insect repellent emitted by vegetation (Kokkari et al., 2024). The classification of cultivars based on VOC emissions aligns with infestation susceptibility: Arbequina and Canino, which emit higher levels of these deterrent VOCs early in maturation, were classified as tolerant, while Coratina and Nocellara Messinese, with consistently lower levels, were classified as susceptible regardless of their ripening stage as assessed by the Jaén Index stage (Table 1). Therefore, Factor Analysis (Figure 5) highlights the association between specific VOCs and infestation categories, confirming the dual roles of attractant and deterrent compounds.
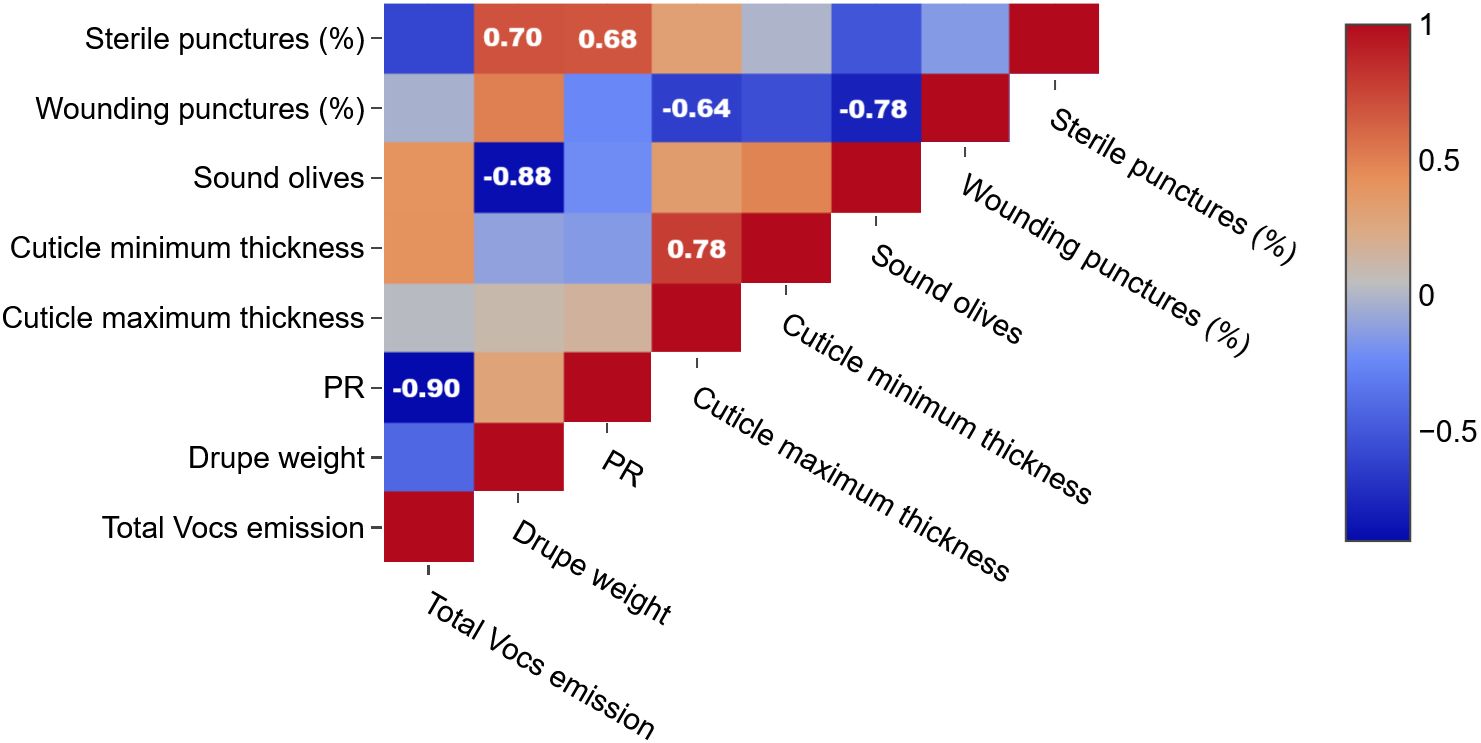
Figure 4. Pearson product-moment correlation coefficients between sterile punctures, wounding puncture e sound olives, cuticle minimum thickness, cuticle maximum thickness, PR, drupe weight, total VOCs emission. Color is used to denote the magnitude of the correlations, which range from -1 to +1. Reported values represent statistically significant (p<0.05) correlations.
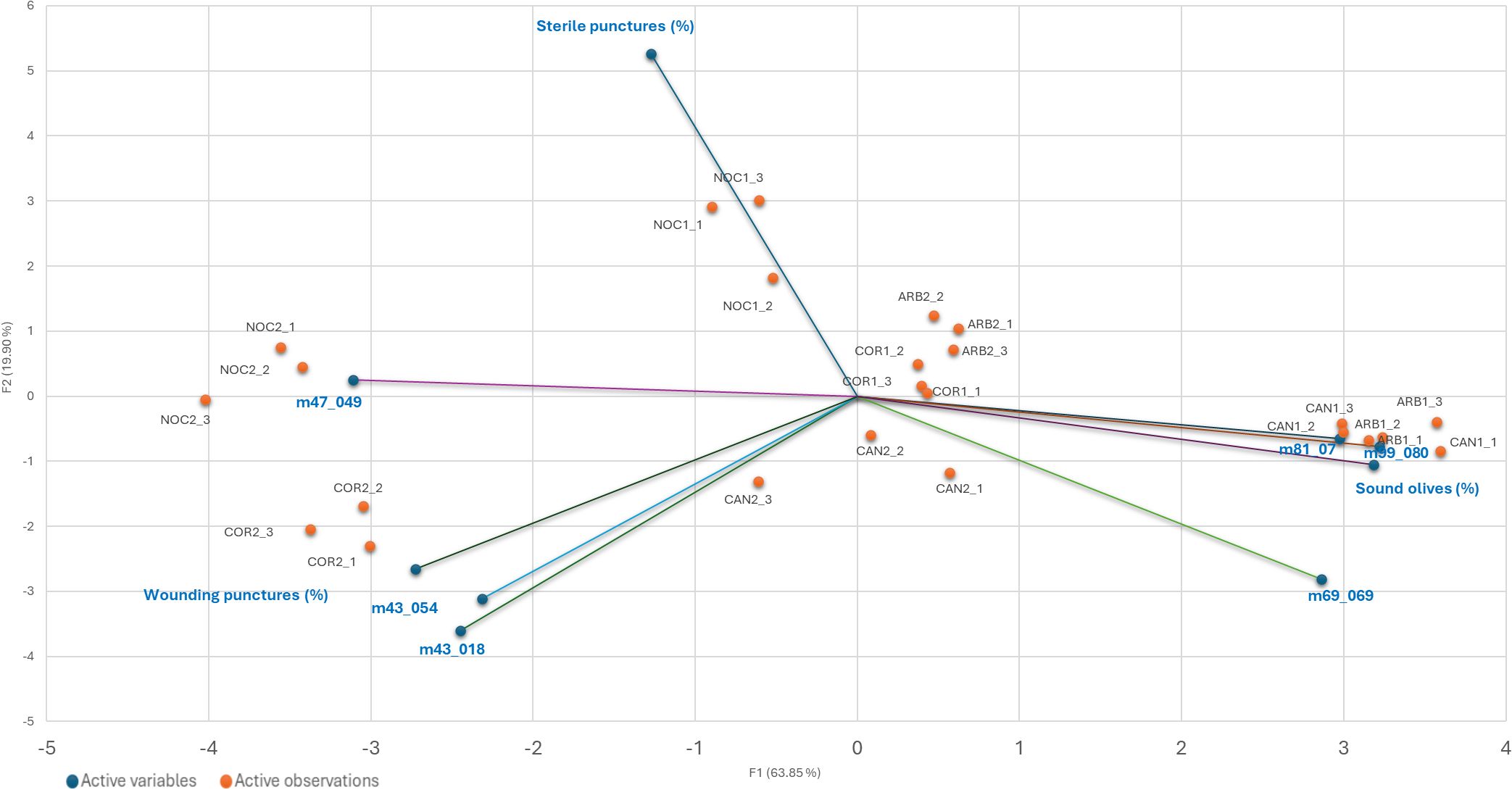
Figure 5. Biplot from Factor analysis. Relationships among the 4 cultivar olive fruit samples collected at T1 and T2, respectively, the three levels of infestation (sound olives, sterile punctures, and wounding punctures) and the protonated m/z having Pearson product-moment correlation coefficients (P) negative (≥-0.5) or positive (≥0.5).
Interestingly, no direct correlation was found between cuticle thickness and total VOC emissions (Figure 4). However, total VOC emission was negatively correlated with fruit firmness (Pearson’s r = –0.90), while cuticle thickness correlated negatively with harmful infestation levels (r = –0.62) and positively with the proportion of sterile punctures on total infestation (r = 0.68). These data indicate that morphological and biochemical defenses function through partially independent but complementary mechanisms to modulate B. oleae infestation dynamics.
5 Conclusions
This study provides a comprehensive analysis of the interplay between morphological traits, cuticle thickness, and VOC emissions in olive fruits from four cultivars with differing susceptibility levels to B. oleae. Our results highlight how cultivar-specific factors influence olive fruit fly infestation dynamics, with Arbequina and Canino consistently exhibiting lower levels of active infestation, likely due to a combination of higher cuticle thickness, lower pulp hardness, and VOC emission profiles dominated by deterrent compounds such as isoprene and C6 volatiles. Conversely, Nocellara Messinese and Coratina showed higher infestation rates, with Coratina particularly susceptible to active and harmful damage. Interestingly, while Nocellara Messinese exhibited the highest penetration resistance early in maturation, its fly damage was largely limited to sterile punctures and dead infestation, suggesting the presence of post-oviposition defense mechanisms. Cuticle thickness, was negatively associated with harmful infestation levels, supporting the role of this barrier in deterring successful oviposition. Moreover, dynamic changes in cuticle development during maturation varied among cultivars, with Coratina showing a late-stage increase in cuticle thickness possibly as an induced response to biotic stress. The VOC profiling identified methanol, ethanol, and acetaldehyde as key emissions associated with fruit ripening and could be a potential fly attraction, while C5 and C6 volatiles, including hexenal and isoprene, decreased with maturity and were positively associated with fly deterrence. The higher concentrations of VOCs such as isoprene and hexenal observed in tolerant cultivars may indicate a potential role in defense, possibly acting as natural repellents. Altogether, this multidisciplinary approach underscores the importance of integrating morphological, biochemical, and physiological traits to better understand host-pest interactions in olive orchards. These findings have practical implications for sustainable pest management, suggesting that VOC profiling and cuticle screening could serve as valuable tools for selecting or breeding olive cultivars with enhanced resistance to B. oleae. Future research should explore the genetic and molecular mechanisms underlying cuticle biosynthesis and VOC emission, and assess how environmental factors and microbial communities modulate these traits to inform effective, ecologically sound pest control strategies. Understanding the interplay of these factors is essential for developing targeted breeding programs and sustainable management strategies aimed at enhancing resistance against the olive fruit fly.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
CT: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. LL: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. GG: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. LR: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. EMas: Funding acquisition, Writing – review & editing. EMar: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. MB: Methodology, Data curation, Formal analysis, Investigation, Writing – review & editing. AI: Methodology, Data curation, Formal analysis, Investigation, Writing – review & editing. SZ: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. SR: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. EP: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. MR: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. PR: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. VV: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. GSp: Methodology, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. PF: Writing – review & editing. SM: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. GSt: Conceptualization, Methodology, Data curation, Resources, Funding acquisition, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported partly by the University of Florence and funded by the Ministero Università e Ricerca PRIN2022 award number 2022RYTHE3.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alagna, F., Kallenbach, M., Pompa, A., De Marchis, F., Rao, R., Baldwin, I. T., et al. (2015). Olive fruits infested with olive fly larvae respond with an ethylene burst and the emission of specific volatiles. J. Integr. Plant Biol. 58, 413–425. doi: 10.1111/jipb.12343
Aluja, M. and Mangan, R. L. (2008). Fruit fly (Diptera: Tephritidae) host status determination: critical conceptual, methodological, and regulatory considerations. Annu. Rev. Entomol 53, 473–502. doi: 10.1146/ANNUREV.ENTO.53.103106.093350
Baratella, V., Pucci, C., Paparatti, B., and Speranza, S. (2017). Response of Bactrocera oleae to different photoperiods and temperatures using a novel method for continuous laboratory rearing. 110, 79–88. doi: 10.1016/j.biocontrol.2017.04.010
Bartolini, S., Leccese, A., and Andreini, L. (2014). Influence of canopy fruit location on morphological, histochemical and biochemical changes in two oil olive cultivars. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology 148, 1221–1230. doi: 10.1080/11263504.2014.980360
Beltrán, G., Bejaoui, M. A., Jimenez, A., and Sanchez-Ortiz, A. (2015). Ethanol in olive fruit. Changes during ripening. J. Agric. Food Chem. 63, 5309–5312. doi: 10.1021/ACS.JAFC.5B01453/ASSET/IMAGES/LARGE/JF-2015-01453D_0002.JPEG
Bogka, G., Anastasaki, E., Milonas, P. G., Psoma, A., Kabourakis, E. M., Zwaan, B. J., et al. (2023). Chemical cues involved in the host foraging behavior of Psyttalia concolor wasps to locate the olive fruit fly Bactrocera oleae. Front. Ecol. Evol. 11. doi: 10.3389/FEVO.2023.1100983/FULL
Brilli, F., Ruuskanen, T. M., Schnitzhofer, R., Mü Ller, M., Breitenlechner, M., Bittner, V., et al. (2011). Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction “’Time-of-flight’. Mass Spectrometry (PTR-TOF) 6 (5), e20419. doi: 10.1371/journal.pone.0020419
Brkić Bubola, K., Lukić, I., Krapac, M., and Koprivnjak, O. (2023). Exploring the connection between the occurrence and intensity of “Grubby” Defect and volatile composition of olive oil. Foods 12, 4473. doi: 10.3390/FOODS12244473
Cappelli, A., Cividino, S., Redaelli, V., Tripodi, G., Aiello, G., Velotto, S., et al. (2024). Applying Spectroscopies, Imaging Analyses, and Other Non-Destructive Techniques to Olives and Extra Virgin Olive Oil: A Systematic Review of Current Knowledge and Future Applications. Agriculture 14, 1160. doi: 10.3390/agriculture14071160
Cappellin, L., Biasioli, F., Granitto, P. M., Schuhfried, E., Soukoulis, C., Costa, F., et al. (2010). On data analysis in PTR-TOF-MS: From raw spectra to data mining. Sensors Actuators B 155, 183–190. doi: 10.1016/j.snb.2010.11.044
Chen, J. Y., Kuruparan, A., Zamani-Babgohari, M., and Gonzales-Vigil, E. (2023). Dynamic changes to the plant cuticle include the production of volatile cuticular wax–derived compounds. Proc. Natl. Acad. Sci. U.S.A. 120, e2307012120. doi: 10.1073/PNAS.2307012120/-/DCSUPPLEMENTAL
Corrado, G., Mataffo, A., Garonna, A. P., Rao, R., and Basile, B. (2023). Investigation of fruit growth patterns, olive fly bactrocera oleae (Rossi) infestation, and genetic diversity in italian olive cultivars. Appl. Sci. (Switzerland) 13, 9929. doi: 10.3390/APP13179929
Daane, K. M. and Johnson, M. W. (2010). Olive fruit fly: managing an ancient pest in modern times. Annu. Rev. Entomol 55, 151–169. doi: 10.1146/ANNUREV.ENTO.54.110807.090553
Diarte, C., Iglesias, A., Graell, J., and Lara, I. (2023). Fruit Cuticle Composition in “Arbequina” Olive: Time-Course Changes along On-Tree Ripening under Irrigated and Rain-Fed Conditions. Horticulturae 9, 394. doi: 10.3390/horticulturae9030394
Diarte, C., Lai, P. H., Huang, H., Romero, A., Casero, T., Gatius, F., et al. (2019). Insights into olive fruit surface functions: A comparison of cuticular composition, water permeability, and surface topography in nine cultivars during maturation. Front. Plant Sci. 10. doi: 10.3389/FPLS.2019.01484
El Hadi, M. A. M., Zhang, F. J., Wu, F. F., Zhou, C. H., and Tao, J. (2013). Advances in fruit aroma volatile research. Molecules 18, 8200–8229. doi: 10.3390/MOLECULES18078200
Germinara, G. S., Pistillo, O. M., D’isita, I., Marta, A., Palma, D., Rotundo, G., et al. (2024). Inhibitory activity of some short-chain aliphatic aldehydes on pheromone and ammonium carbonate-mediated attraction in olive fruit fly, Bactrocera oleae. Pest Manag Sci. 80 (10), 5353–5363. doi: 10.1002/ps.8264
Giunti, G., Campolo, O., Laudani, F., Algeri, G. M., and Palmeri, V. (2020). Olive fruit volatiles route intraspecific interactions and chemotaxis in Bactrocera oleae (Rossi) (Diptera: Tephritidae) females. Sci. Rep. 2020 10:1 10, 1–10. doi: 10.1038/s41598-020-58379-8
Goldental-Cohen, S., Biton, I., Many, Y., Ben-Sason, S., Zemach, H., Avidan, B., et al. (2019). Green olive browning differ between cultivars. Front. Plant Sci. 10. doi: 10.3389/FPLS.2019.01260/FULL
Gomes, S., Bacelar, E., Martins-Lopes, P., Carvalho, T., and Guedes-Pinto, H. (2012). Infection process of olive fruits by colletotrichum acutatum and the protective role of the cuticle and epidermis. J. Agric. Sci. 4, 101–101. doi: 10.5539/jas.v4n2p101
Gonçalves, M. F., Malheiro, R., Casal, S., Torres, L., and Pereira, J. A. (2012). Influence of fruit traits on oviposition preference of the olive fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), on three Portuguese olive varieties (Cobrançosa, Madural and Verdeal Transmontana). Sci. Hortic. 145, 127–135. doi: 10.1016/J.SCIENTA.2012.08.002
Grasso, F., Coppola, M., Carbone, F., Baldoni, L., Alagna, F., Perrotta, G., et al. (2017). The transcriptional response to the olive fruit fly (Bactrocera oleae) reveals extended differences between tolerant and susceptible olive (Olea europaea L.) varieties. PloS One 12 (8), e0183050. doi: 10.1371/JOURNAL.PONE.0183050
Green, P. S. (2002). A revision of olea L. (Oleaceae). Source: Kew Bull. 57, 91–140. doi: 10.2307/4110824
Guerrini, S., Mari, E., Barbato, D., and Granchi, L. (2019). Extra virgin olive oil quality as affected by yeast species occurring in the extraction process. Foods 8 (10), 457. doi: 10.3390/FOODS8100457
Heil, M. (2014). Herbivore-induced plant volatiles: targets, perception and unanswered questions. New Phytol. 204, 297–306. doi: 10.1111/NPH.12977
IOC (2011). Guide for the determination of the characteristics of oil-olives. Available online at: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-OH-Doc.-1-2011-Eng.pdf
Jiménez, A., Rodríguez, R., Fernández-Caro, I., Guillén, R., Fernández-Bolaños, J., and Heredia, A. (2001). Olive fruit cell wall: degradation of cellulosic and hemicellulosic polysaccharides during ripening. J Agric Food Chem. 49, 2008–2013. doi: 10.1021/jf000809v
Kokkari, A., Kouloussis, N. A., Floros, G., and Koveos, D. S. (2024). Effect of Olive Fruit Volatiles on Landing, Egg Production, and Longevity of Bactrocera oleae Females under Different Temperatures. Insects 15 (9), 728. doi: 10.3390/insects15090728
Kokkari, A. I., Milonas, P. G., Anastasaki, E., Floros, G. D., Kouloussis, N. A., and Koveos, D. S. (2021). Determination of volatile substances in olives and their effect on reproduction of the olive fruit fly. J. Appl. Entomology 145, 841–855. doi: 10.1111/JEN.12929
Lanza, B., Gabriella, M., and Serio, D. (2015). SEM characterization of olive (Olea europaea L.) fruit epicuticular waxes and epicarp. Sci. Hortic. 191, 49–56. doi: 10.1016/j.scienta.2015.04.033
Liao, P., Ray, S., Boachon, B., Lynch, J. H., Deshpande, A., McAdam, S., et al. (2020). Cuticle thickness affects dynamics of volatile emission from petunia flowers. Nat. Chem. Biol. 2020 17, 138–145. doi: 10.1038/s41589-020-00670-w
Malheiro, R., Casal, S., Cunha, S. C., Baptista, P., and Pereira, J. A. (2016). Identification of leaf volatiles from olive (Olea europaea) and their possible role in the ovipositional preferences of olive fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae). Phytochemistry 121, 11–19. doi: 10.1016/J.PHYTOCHEM.2015.10.005
Malheiro, R., Casal, S., Pinheiro, L., Baptista, P., and Pereira, J. A. (2019). Olive cultivar and maturation process on the oviposition preference of Bactrocera oleae (Rossi) (Diptera: Tephritidae). Bull. Entomol Res. 109, 43–53. doi: 10.1017/S0007485318000135
Malheiro, R., Casal, S., Teixeira, H., Bento, A., and Pereira, J. A. (2013). Effect of olive leaves addition during the extraction process of overmature fruits on olive oil quality. Food Bioproc Tech 6, 509–521. doi: 10.1007/S11947-011-0719-Z/FIGURES/2
Martin, L. B. B. and Rose, J. K. C. (2014). There’s more than one way to skin a fruit: formation and functions of fruit cuticles. J. Exp. Bot. 65, 4639–4651. doi: 10.1093/jxb/eru301
Medjkouh, L., Costa, A., Tamendjari, A., Bekdouche, F., Bouarroudj, K., and Oliveira, M. B. P. P. (2018). Susceptibility of eight Algerian olive cultivars to Bactrocera oleae infestation – a pomological and nutritional quality perspective. Phytoparasitica 46, 595–605. doi: 10.1007/S12600-018-0697-Z/FULLTEXT.HTML
Pesis, E. (2005). The role of the anaerobic metabolites, acetaldehyde and ethanol, in fruit ripening, enhancement of fruit quality and fruit deterioration. Postharvest Biol. Technol. 37, 1–19. doi: 10.1016/J.POSTHARVBIO.2005.03.001
Rasulov, B., Talts, E., and Niinemets, Ü. (2019). A novel approach for real-time monitoring of leaf wounding responses demonstrates unprecedently fast and high emissions of volatiles from cut leaves. Plant Sci. 283, 256–265. doi: 10.1016/j.plantsci.2019.03.006
Rizzo, R., Caleca, V., and Lombardo, A. (2012). Relation of fruit color, elongation, hardness, and volume to the infestation of olive cultivars by the olive fruit fly, Bactrocera oleae. Entomol Exp. Appl. 145, 15–22. doi: 10.1111/J.1570-7458.2012.01311.X
Rodríguez, A., Alquézar, B., and Peña, L. (2013). Fruit aromas in mature fleshy fruits as signals of readiness for predation and seed dispersal. New Phytol. 197, 36–48. doi: 10.1111/J.1469-8137.2012.04382.X
Rotondi, A., Morrone, L., Facini, O., Faccini, B., Ferretti, G., Coltorti, M., et al. (2021). Distinct particle films impacts on olive leaf optical properties and plant physiology. Foods 10 (6), 1291. doi: 10.3390/foods10061291
Taiti, C., Masi, E., Cicaloni, V., Vinciguerra, V., Salvini, L., and Garzoli, S. (2022). SPME-GC-MS and PTR-toF-MS techniques for the profiling of the metabolomic pattern of VOCs and GC-MS for the determination of the cannabinoid content of three cultivars of cannabis sativa L. Pollen. Molecules 27 (24), 8739. doi: 10.3390/MOLECULES27248739
Vizzarri, V., Lombardo, L., Novellis, C., Rizzo, P., Pellegrino, M., Cruceli, G., et al. (2023). Testing the Single and Combined Effect of Kaolin and Spinosad against Bactrocera oleae and Its Natural Antagonist Insects in an Organic Olive Grove. Life 13, 607. doi: 10.3390/life13030607
Wang, L. and Erb, M. (2022). Volatile uptake, transport, perception, and signaling shape a plant’s nose. Essays Biochem. 66, 695–702. doi: 10.1042/EBC20210092
White, I. R., Blake, R. S., Taylor, A. J., and Monks, P. S. (2016). Metabolite profiling of the ripening of Mangoes Mangifera indica L. cv. 'Tommy Atkins' by real-time measurement of volatile organic compounds. Metabolomics 12, 57. doi: 10.1007/s11306-016-0973-1
Yan, J., Alewijn, M., and van Ruth, S. M. (2020). From extra virgin olive oil to refined products: intensity and balance shifts of the volatile compounds versus odor. Molecules 25 (11), 2469. doi: 10.3390/MOLECULES25112469
Keywords: Olea europaea cultivars, olive fly oviposition preference, VOCs, fruit cuticle thickness, Nile Red, Bactrocera oleae
Citation: Taiti C, Lombardo L, Godino G, Renna L, Masi E, Marone E, Beccaluva M, Ienco A, Zelasco S, Rizzo S, Perri E, Rizzo M, Rizzo P, Vizzarri V, Spinelli G, Fiorino P, Mancuso S and Stefano G (2025) Exploring morphological aspects, cuticle size and volatile compounds in the fruits of four olive cultivars as possibly interdependent components of Bactrocera oleae tolerance. Front. Plant Sci. 16:1681993. doi: 10.3389/fpls.2025.1681993
Received: 08 August 2025; Accepted: 30 October 2025;
Published: 28 November 2025.
Edited by:
Deepak Kasote, Qatar University, QatarReviewed by:
Jisun Lee, Chung-Ang University, Republic of KoreaAnastasia Kokkari, Aristotle University of Thessaloniki, Greece
Copyright © 2025 Taiti, Lombardo, Godino, Renna, Masi, Marone, Beccaluva, Ienco, Zelasco, Rizzo, Perri, Rizzo, Rizzo, Vizzarri, Spinelli, Fiorino, Mancuso and Stefano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Stefano, Z2lvdmFubmkuc3RlZmFub0B1bmlmaS5pdA==; Luca Lombardo, bHVjYS5sb21iYXJkb0BjcmVhLmdvdi5pdA==
 Cosimo Taiti
Cosimo Taiti Luca Lombardo
Luca Lombardo Gianluca Godino
Gianluca Godino Luciana Renna
Luciana Renna Elisa Masi
Elisa Masi Elettra Marone
Elettra Marone Marta Beccaluva
Marta Beccaluva Annamaria Ienco2
Annamaria Ienco2 Samanta Zelasco
Samanta Zelasco Santina Rizzo
Santina Rizzo Enzo Perri
Enzo Perri Marianna Rizzo
Marianna Rizzo Veronica Vizzarri
Veronica Vizzarri Giovanni Spinelli
Giovanni Spinelli Stefano Mancuso
Stefano Mancuso Giovanni Stefano
Giovanni Stefano