Abstract
Continuous cropping reduces the rooting rate and quality of chrysanthemum cuttings, negatively impacting the development of the chrysanthemum industry. This study provides the first evidence that Massilia consociata enhances root formation in chrysanthemum cuttings. Using pot experiments, combined with physiological indicator measurements and analysis of the endophytic microbial composition of the chrysanthemum cuttings, the mechanism promoting the rooting effect was investigated. After 106 CFU/mL of KC 009 fermentation was applied to chrysanthemum cuttings for 21 days, the rooting rate, root number, root length and root dry weight significantly increased by 28.97%-50% (p < 0.01). Some physiological indicators such as soluble protein, soluble sugar, chlorophyll and indole-3-acetic acid (IAA) were significantly enhanced. Correlation analysis between rooting and physiological indicators revealed that soluble protein was the most critical physiological factor contributing to root formation. The results of high-throughput sequencing of rhizosphere and endophytic microorganisms in chrysanthemum cuttings showed that KC 009 significantly reduced the richness and diversity of endophytic microorganisms. The dominant endophytic bacteria changed from Ochrobactrum to Chryseobacterium and Alcaligenes, which could produce IAA and enhance plant stress resistance. Spearman correlation analysis showed that Chryseobacterium was significantly positively correlated with soluble protein, starch, and chlorophyll, and Alcaligenes was positively correlated with PPO, POD, and soluble sugar. The abundance of Cladosporium, a potential pathogen in endophytic fungi, decreased by 16.70% (p < 0.05). Mantel test analysis indicated that soluble protein and starch were most closely related to the endophytic bacterial and fungal communities of chrysanthemum, respectively. Functional prediction of endophytic bacteria revealed that the abundance of 14 metabolic pathways related to plant growth was significantly increased. This study provides theoretical and practical references for promoting the rooting of chrysanthemum cuttings, holding significant importance for the development of the chrysanthemum industry.
1 Introduction
Chrysanthemum is one of the world’s four cut flowers along with rose, carnation, and gladiolus (Liu et al., 2023). Beyond its ornamental value, the chrysanthemum is rich in cultural connotation. It is honored as the “gentleman of flowers” in China. It is commonly used in celebrations and ceremonies, representing good fortune and longevity in many countries (Islam et al., 2021). Furthermore, chrysanthemum contains various bioactive compounds, including flavonoids, terpenoids, and polysaccharides, which contribute to its medicinal properties, such as an antihypertensive effect (Zhou et al., 2024), lipid-lowering, anti-inflammatory (Liang et al., 2017), and antibacterial activity (Wang et al., 2022). The wide range of application value makes the market demand for chrysanthemum continues to grow.
Chrysanthemum has diverse methods of propagation, including seed propagation, division, tissue culture, and cutting propagation. Cutting propagation is the most commonly used method for chrysanthemum cultivation due to its advantages, including the preservation of the genetic traits of the mother plant, low cost, simple operation, and a high propagation coefficient (Gao et al., 2023). However, with the increase in cultivation years, autotoxic substances such as coumaric acid gradually accumulate, and the microbial community also changes, which significantly inhibits the survival rate, rooting ability, and growth of chrysanthemum cuttings (Liao et al., 2024), greatly limiting the market supply of chrysanthemums. Currently, the improvement of rooting efficiency in chrysanthemum cuttings is primarily achieved through the application of chemicals, including growth regulators (Kaur and Jhanji, 2023), nutrient solutions (Quevedo et al., 2022), and phytohormones (Joel et al., 2023). However, long-term use and residual accumulation of these chemical substances may disrupt the balance and function of soil micro-ecosystems (Gupta et al., 2017). Therefore, it is necessary to explore green and low-risk alternative strategies to reduce dependence on chemical agents. Soil microorganisms are core members of the soil ecosystem and are closely associated with the healthy growth of plants. Plant Growth-Promoting Rhizobacteria (PGPR) can directly promote plant growth by producing growth hormones and activating other nutrients (Mohanty et al., 2021). They can also indirectly promote plant growth by suppressing plant pathogens, degrading autotoxic compounds from continuous cropping, and improving the rhizosphere microecological environment (Oleńska et al., 2020). These functions make PGPR a significant breakthrough in promoting the rooting and growth of plants. At present, the growth-promoting and disease-resistance effects of many PGPR have been widely verified (Shilpa et al., 2024).
The genus Massilia was first reported in 2000 (Kämpfer et al., 2011). Subsequent studies have shown that Massilia is widely distributed in the rhizosphere of various plants and exhibits plant growth-promoting traits such as nitrogen fixation, phosphate solubilization, production of plant hormones (Holochová et al., 2020), siderophore production (Diettrich et al., 2019), and ACC deaminase activity (Behrangi et al., 2024). Massilia niastensisi, which was isolated from potato roots, can generate indole-3-acetic acid (IAA) and degrade cellulose. The strain can significantly boost the tuber growth of Solanum tuberosum (Turnbull et al., 2012). Massilia sp. Sco-D23, isolated from the rhizosphere of Glycine max, was found to activate the glycolytic pathway in G. max, thereby promoting the accumulation of oil in soybean seeds (Han et al., 2024). Massilia can not only encourage the growth of plants alone but also synergistically enhance growth when combined with other microorganisms. Krishnamoorthy et al. (2016) inoculated Massilia aerilata together with Rhizophagus intraradices in maize, which enhanced maize growth under salt stress and improved the colonization and sporulation ability of arbuscular mycorrhizal fungi (AMF) at the roots of the maize plant. However, it remains unknown whether Massilia possesses root-promoting capabilities.
In our preliminary experiments, M. consociata KC 009 was isolated from Jiaozi Mountain in Kunming and identified as being able to promote the rooting of chrysanthemum cuttings. Although studies have reported mechanisms that promote rooting in chrysanthemum cuttings, the role of microorganisms remains unclear, particularly in terms of their effects on physiological indicators of cuttings and the functional dynamics of endophytic and rhizospheric microbial communities. This study employed pot experiments to evaluate the impact of KC 009 on chrysanthemum cutting rooting, aiming to elucidate the mechanism by which M. consociata promotes rooting and provide theoretical and practical insights for addressing low rooting rates and poor root quality in chrysanthemum propagation.
2 Materials and methods
2.1 Experimental materials
The chrysanthemum cuttings used in this study were from the cut-flower cultivar Chrysanthemum morifolium Ramat. (‘Jinba’). The cuttings, approximately 8 cm in length with 7–8 leaves, were taken from the healthy apical parts of mother plants, with the basal cut made at a 40-degree angle. Kunming Hongzhihua Horticulture Co., Ltd, provided all samples.
The experimental strain M. consociata KC 009 was isolated from the soil of Jiaozi Mountain in Kunming, Yunnan (24°48′N, 102°49′E). The isolation medium was starch-potassium nitrate agar (Seraman et al., 2010), consisting of the following ingredients: soluble starch, 20 g; KNO3, 1 g; K2HPO4·3H2O, 0.5 g; MgSO4·7H2O, 0.5 g; FeSO4·7H2O, 0.1 g; pH 7. KC 009 was cultured on ISP2 solid medium at 28 °C for 1 day, then transferred to ISP2 liquid medium (Astudillo et al., 2024) and incubated at 28 °C with shaking at 180 rpm for 5 days to obtain the fermentation broth. Some fermentation broth was used for determining the IAA content, while the rest was diluted 1,000-fold (106 CFU/mL) and used for irrigating the chrysanthemum cuttings.
The potting substrate was red soil collected from outside the greenhouse at Kunming University, with a pH of 7.04. The experiment was conducted inside the greenhouse at Kunming University (24°33′N, 102°27′E), Yunnan Province. The average annual temperature is about 15-25°C (Li et al., 2022), and the average sunshine duration is about 6 hours.
2.2 Determination of IAA content of KC 009
The fermentation broth of KC 009 was centrifuged at 3000 rpm for 10 min, and the supernatant was collected. The IAA content was determined using a microbial IAA ELISA kit (purchased from JiningBio) and measured at a wavelength of 450 nm with a microplate reader.
2.3 Pot experiment design
Stones and other impurities were removed from the red soil substrate, which was then crushed and filled into seedling trays with cells measuring 2 cm × 2 cm × 2 cm. Water the substrate thoroughly the day before planting. Remove 5–6 leaves from each chrysanthemum cutting and insert them into the seedling tray cells at a depth of 1–2 cm, with one cutting per cell. The cuttings were irrigated every three days with the KC 009 fermentation broth diluted 1000-fold. The cultivation period lasted for 21 days. The experiment was conducted at a temperature of 25-28°C, and the humidity ranged from 50% to 80%. Cuttings were sampled every 3 days, and each treatment had three repetitions. Ten chrysanthemum cuttings of each group were chosen at random and stored at 4°C to detect physiological indicators. The control group was watered with the blank medium dilution.
2.4 Measurement of chrysanthemum root phenotypes and plant physiological indicators
On the 15th day of cultivation, the rooting rate of cuttings was recorded. Cutting was considered rooted when the adventitious root reached 1 mm. The number of roots was counted, and root length was measured at 21 days. After thorough washing, the roots were washed and fixed at 105°C for 30 min, then dried at 80°C to a constant weight for dry weight determination.
The chlorophyll content of chrysanthemum cuttings was determined by spectrophotometric (Jabborova et al., 2024). The soluble protein content was determined using the Coomassie Brilliant Blue method (Wu and Shen, 2021). Soluble sugar content was determined using anthone colorimetric assay (Zhang et al., 2024), and soluble starch was determined by anthone method (Nayak et al., 2022); IAA and abscisic acid (ABA) contents were quantified with IAA and ABA ELISA kits purchased from Shanghai Youxuan Biotechnology Co., Ltd. Peroxidase (POD) activity was determined by guaiacol method (Nikerova et al., 2023), and polyphenol oxidase (PPO) was determined by catechol method (Song et al., 2023).
2.5 High-throughput sequencing of endophytic and rhizosphere microorganisms in chrysanthemum cuttings
After 21 days of cultivation, 0.5 g of rhizosphere soil and 0.5 mg of chrysanthemum cutting tissue, which had been disinfected using 75% alcohol, were collected and stored at –80°C for microbial diversity analysis. Genomic DNA of soil was extracted using the E.Z.N.A.® soil DNA kit (Omega Bio-tek, Norcross, GA, U.S.), In contrast, DNA of chrysanthemum root tissues was extracted using the CTAB method after grinding in liquid nitrogen (Xiao et al., 2024). Samples with DNA purity A260/A280 > 1.8 were used for amplification. The V3–V4 region of the bacterial 16S rRNA gene was amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) (Onyango et al., 2023). The fungal ITS1 region was amplified using primers ITS1F (5′-CTT GGT CAT TTA GAG GAA GTA A-3′) and ITS2R (5′-GCT GCG TTC TTC ATC GAT GC-3 (Liu et al., 2025). PCR reaction was performed in a 20 μL system containing 10 ng of template DNA, 4 μL 5 × Fast Pfu buffer, 2 μL 2.5 mM dNTPs, 0.8 μL each primer (5 μM), 0.4 μL Fast Pfu polymerase, and sterile water added to a final volume of 20 μL. The PCR amplification conditions were as follows: initial denaturation at 95°C for 3 min, followed by 27 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s, followed by a final extension at 72°C for 10 min. Three biological replicates were performed for each sample. After verification of the amplicons by 2% agarose gel electrophoresis, a sequencing library was prepared using the Illumina TruSeq® DNA PCR-Free Library Prep Kit. Paired-end sequencing (2 × 270 bp) was performed on the Illumina NovaSeq 6000 platform (Illumina, USA), generating approximately 51,555 high-quality reads per sample. The raw sequencing data were processed on the Majorbio Cloud Platform using Trimmomatic (v0.39) to remove low-quality sequences (Phred score < 20). Paired-end reads were assembled using FLASH (v1.2.11), and chimeric sequences were removed using UCHIME (v8.1). Species annotation was performed based on the bacterial SILVA database (http://www.arb-silva.de) and the fungal UNITE database (http://unite.ut.ee/) with a confidence threshold of 0.7.
2.6 Data processing
T-test and ANOVA were performed on experimental data using SPSS 21.0 to evaluate the statistical significance of differences (version 21.0; SPSS Inc, Chicago, IL, USA) (Zhang et al., 2020b). Root phenotypes, physiological indexes, community richness and diversity indices were visualized using Origin 2021 (Moberly et al., 2018). Correlation analysis among physiological indexes, root indexes, and endophytic community composition was performed using (Dag and Ilk, 2017). The analysis and plotting of the intra-endogenous microbial community at the phylum and genus levels were done using Source Tracker software (Rousseau et al., 2020). A diagram of the collinear network of endophytic microbial correlation was plotted with Network (Zhang et al., 2020a). LEfSe was used to analyze changes in endophytic microbial composition (Jin et al., 2022). PICRUSt2 (http://huttenhower.sph.harvard.edu/galaxy) and FUNGuild (http://www.funguild.org/) were used for predictive analysis and mapping of bacterial and fungal functions, respectively (Martínez et al., 2019; Peng et al., 2025).
3 Results
3.1 Determination of IAA production by KC 009
The microbial IAA ELISA kit was used to detect the fermentation broth of strain KC 009. The results showed that KC 009 could produce 15.995 nmol/L of IAA in ISP 2 liquid medium, indicating that the strain possesses the metabolic capability to synthesize IAA.
3.2 Effects of KC 009 on the root morphology of chrysanthemum cuttings
KC 009 application significantly promoted root growth in chrysanthemum cuttings (Figure 1A). The statistical analysis on the 15th day of cultivation showed that KC 009 significantly increased the rooting rate of cuttings by 50% (Figure 1B). By day 21, the number of roots, root length, and root dry weight of the cuttings increased significantly by 40.78%, 35.77%, and 54.65%, respectively (Figures 1C-E). These results indicated that KC 009 had the best effect on improving the rooting rate of chrysanthemum cuttings, followed by enhancements in root length and root dry weight.
Figure 1

Effect of KC 009 on root growth of chrysanthemum cuttings. (A) The root-promoting effect of KC 009 at 21 days. Up, blank treatment; Down, KC 009 treatment. (B) Rooting rate at 15 days. (C) Number of root at 21 days. (D) Root length at 21 days. (E) Root dry weight at 21 days. Left, blank treatment; Right, KC 009 treatment. The asterisk represented the significance level. **p < 0.01.
3.3 Effect of KC 009 on the physiological indexes of cutting chrysanthemums
After KC 009 treatment, the contents of soluble sugar and starch began to accumulate significantly from day 6, reaching peak increases between days 15 and 21, with significant increases of 11.55% and 6.71%, respectively (Figures 2A, B). The content of soluble protein began to rise significantly on day 3 after KC 009 treatment, with a maximum increase of 11.23% (Figure 2C). These results indicated that the application of KC 009 promoted the accumulation of carbon and nitrogen substances in chrysanthemum cuttings, and its regulation of different nutrients showed temporal specificity.
Figure 2
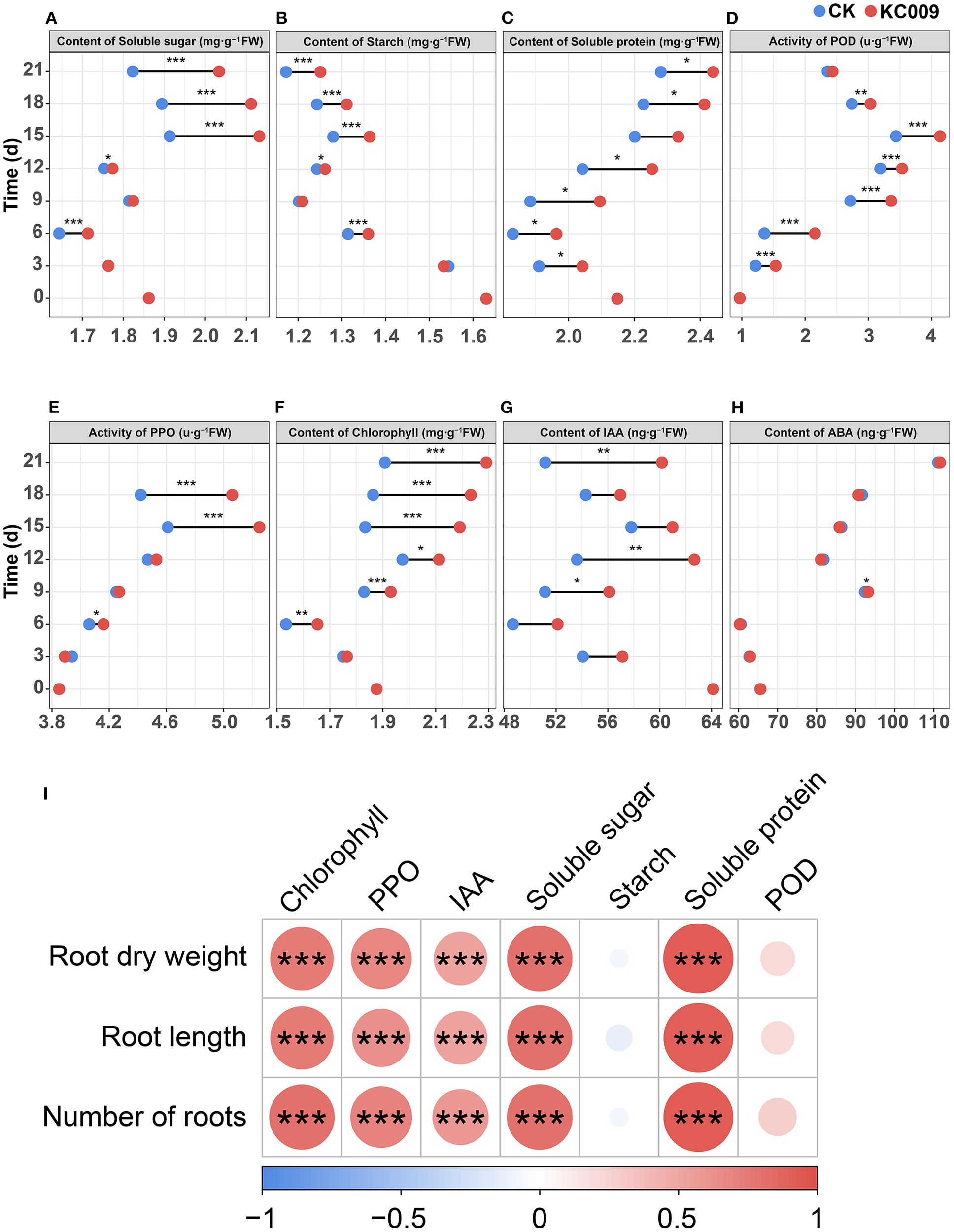
Effect of KC 009 on physiological indexes of chrysanthemum cuttings from 3 to 21 days and theirs correlation with root phenotypic indexes. (A) Content of soluble sugar. (B) Content of soluble sugar. (C) Content of soluble protein. (D) Activity of POD. (E) Activity of PPO. (F) Content of chlorophyll. (G) Content of IAA. (H) Content of ABA. (I) The correlation between physiological indexes and root phenotypic indexes. The asterisks in the graph indicated the significance level. *p < 0.05, **p < 0.01, ***p < 0.001.
The POD activity in chrysanthemum treated with KC 009 was significantly higher than the control group from the third day to the 21st day, with the highest increase occurring on the sixth day, rising by 59.01% (Figure 2D). The PPO activity began to increase significantly on the 15th day, with a maximum increase of 14.66% (Figure 2E). These results indicated that KC 009 significantly upregulated the POD and PPO activities involved in oxidative damage in chrysanthemum cuttings.
Starting from the 6th day after the application of KC 009, the chlorophyll content in the treatment group began to increase, exhibiting a sharp rise between days 15 and 21, with an increase of 19.44%–19.99% (Figure 2F). Regarding IAA, fermentation broth analysis of KC 009 confirmed its ability to produce IAA. From the 3rd day after KC 009 application, the IAA levels in chrysanthemum cuttings significantly increased, showing 16.89% and 17.60% higher levels than the control group on the 12th and 21st days, respectively (Figure 2G). This indicated that KC 009 not only synthesizes IAA in vitro but also significantly enhances endogenous IAA synthesis in chrysanthemum cuttings. KC 009 treatment had little effect on ABA levels in the cuttings (Figure 2H).
Spearman correlation analysis of physiological indexes and root phenotypes revealed (Figure 2I) that root dry weight, root length, and root number were significantly positively correlated with soluble protein, soluble sugar, chlorophyll content, and PPO activity, and IAA content, but not significantly correlated with soluble starch and POD activity. These results indicated that the increases of soluble protein, soluble sugar, chlorophyll, and PPO levels were the primary physiological factors contributing to root growth promoted by KC 009, followed by IAA content.
3.4 Effects of KC 009 on endophytic microbial diversity in chrysanthemum cuttings
3.4.1 Effects of KC009 on diversity of rhizospheric and endophytic microorganisms in chrysanthemum cuttings
Alpha diversity analysis of rhizospheric and endophytic microorganisms in chrysanthemum cuttings revealed that KC 009 had no significant impact on the composition or diversity of the rhizospheric microbial community. However, it reduced the richness and diversity of endophytic fungi, while significantly decreasing the richness and diversity of endophytic bacteria (Figure 3).
Figure 3
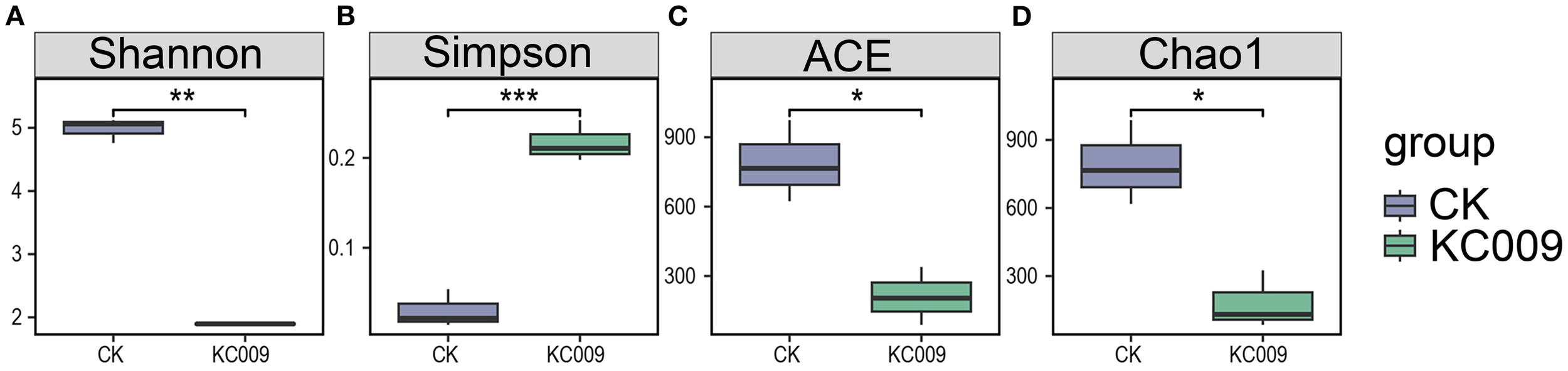
Effect of KC 009 on the diversity of endophytic bacteria in chrysanthemum cuttings. (A, B) Shannon and Simpson indices in species diversity. (C, D) ACE and Chao1 indices in species richness. The asterisk represented the significance level. *p < 0.05, **p < 0.01, ***p < 0.001.
3.4.2 Effect of KC 009 on microbial community composition in chrysanthemum cuttings
The analysis of endophytic bacterial communities at the phylum level in Chrysanthemum cuttings revealed that KC 009 treatment led to a 37.02% decrease in the relative abundance of Proteobacteria. The abundances of Bacteroidota and Actinobacteriota increased by 38.33% and 11.94%, respectively. Additionally, the Planctomycetota, Acidobacteriota, and Chloroflexi disappeared (Figure 4A). At the genus level, KC 009 significantly increased the relative abundances of Chryseobacterium, Alcaligenes, Rhodococcus, Microbacterium, and Acidovorax by 45.68%, 17.98%, 9.96%, 6.62%, and 6.01%, respectively (Figure 4C). LEfSe analysis (LDA score ≥ 4.0) showed that Chryseobacterium had the highest LDA score of 5.35, followed by Alcaligenes with a score of 4.90, indicating that these two genera are significant biomarkers distinguishing the KC 009 treatment group from the control group (Figure 4E). Co-occurrence network analysis of the top 30 most abundant endophytic bacteria revealed that the network density of bacterial associations increased from 0.35402 to 0.37566 after KC 009 treatment, suggesting that KC 009 enhanced the interactions and stability of the endophytic bacteria in chrysanthemum cuttings. Simultaneously, the significantly enriched Chryseobacterium showed positive correlations with beneficial plant growth-promoting genera such as Sanguibacter, Kocuria, and Microbacterium (Figures 4G, H).
Figure 4
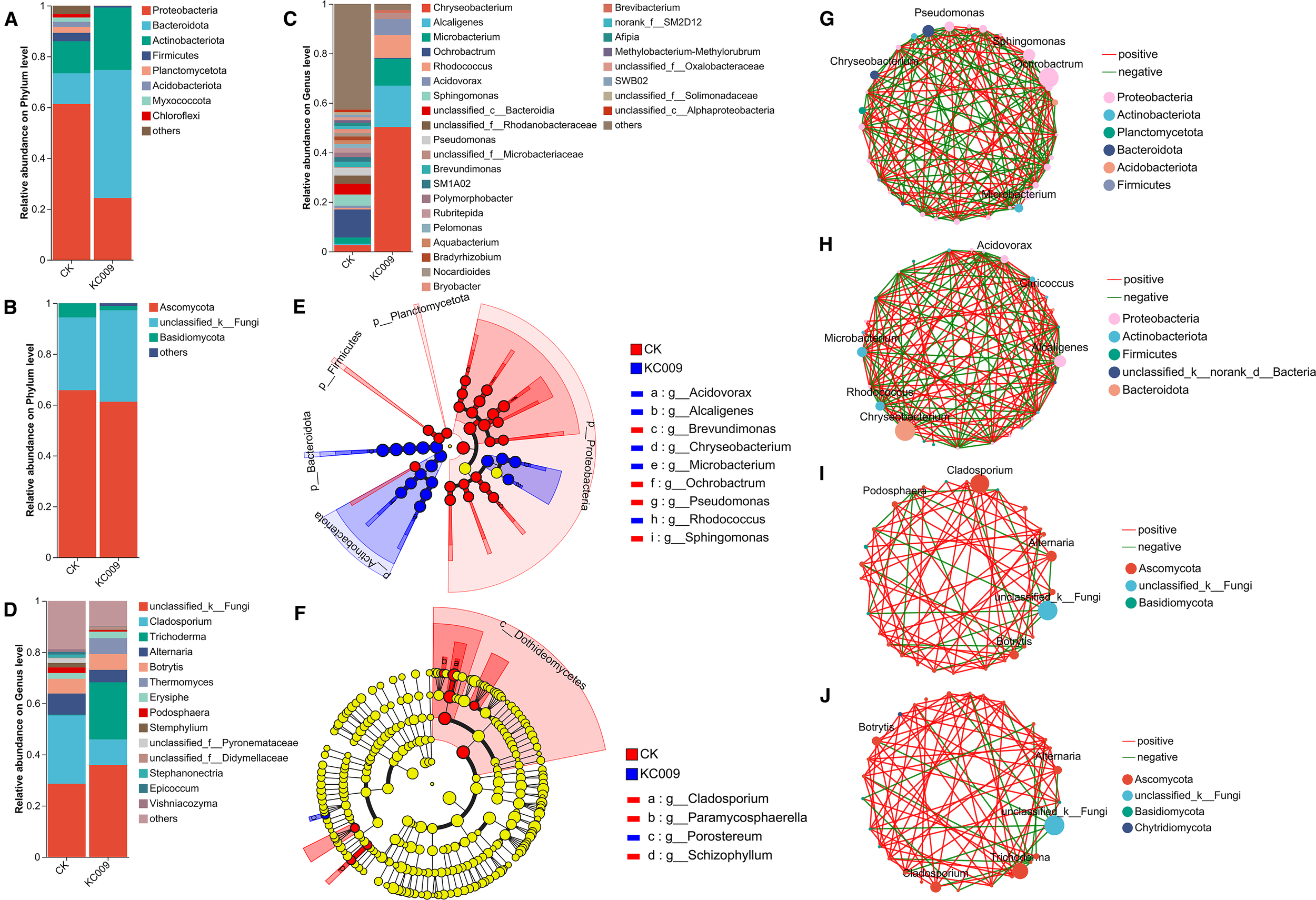
Effect of KC 009 on the endophytic microbial communities. (A, B) Community composition at the phylum level. (C, D) Community composition at the genus level. (E, F) LEfSe analyzed microorganisms with significant differences in abundance. (G, H) Co-occurrence network of bacteria. (I, J) Co-occurrence network of fungi. (A, C, E, G, I), Bacteria; (B, D, F, H, J), Fungi.
Regarding endophytic fungi, KC 009 treatment reduced the abundance of Ascomycota and Basidiomycota at the phylum level by 4.51% and 3.90%, respectively (Figure 4B). At the genus level, KC 009 led to a 26.67% increase in Trichoderma and a 9.77% increase in Thermomyces (Figure 4D), while reducing the relative abundances of potential pathogens Cladosporium and Alternaria by 16.70% and 3.35%, respectively. Furthermore, several fungal genera including Botrytis, Podosphaera, Stemphylium, Epicoccum, Acremonium, Meyerozyma, and Stephanonectria were completely eliminated following KC 009 treatment. LEfSe analysis revealed that the most significant biomarker shifted from Cladosporium to Proteosporum after KC 009 treatment (Figure 4F). Co-occurrence network analysis of the top 30 most abundant endophytic fungi revealed that the number of connections between network nodes increased from 152 to 168, and the network density rose from 0.18719 to 0.20689, suggesting that KC 009 enhanced the species richness and stability of the endophytic fungi in chrysanthemum cuttings. Furthermore, Cladosporium showed a positive correlation with potential pathogenic fungi such as Ramularia, Erysiphe, and Podosphaera in the control group (Figure 4I). In contrast, the abundance of Trichoderma increased and showed a positive correlation with the probiotic Acremonium in the KC 009 treatment group (Figure 4J).
In summary, KC 009 enhanced the interactions and stability of the endophytes in chrysanthemum cuttings. As the bacterial and fungal biomarkers, Chryseobacterium and Proteosporum contributed the most to the inter-group differences in the endophytic microbial community after KC 009 treatment, and both have the potential to promote plant growth.
3.4.3 Correlation analysis between endophytic microbial community and the physiological and root indexes of chrysanthemum cuttings
Mantel test analysis between endophyte composition and physiological indexes of chrysanthemum cuttings showed that the endophytic bacterial community was extremely significantly positively correlated with soluble protein, and significantly correlated with IAA, chlorophyll, and PPO (Figure 5A). In addition, the endophytic bacterial showed a significant positive correlation with root dry weight and root length (Figure 5E). Spearman analysis between dominant microbial taxa and physiological indexes revealed that Chryseobacterium, whose abundance increased significantly, was positively correlated with soluble protein, and correlated significantly with chlorophyll and starch. The enriched Microbacterium exhibited extremely significant positive correlations with chlorophyll, soluble protein, and starch, as well as a significant positive correlation with IAA (Figure 5C). Meantime, both Microbacterium and Chryseobacterium were significantly positively correlated with root length of chrysanthemum cuttings (Figure 5G). Alcaligenes with significantly increased abundance were positively correlated with physiological indicators, including PPO, POD, and soluble sugars. They were also positively correlated with root dry weight and the number of root.
Figure 5
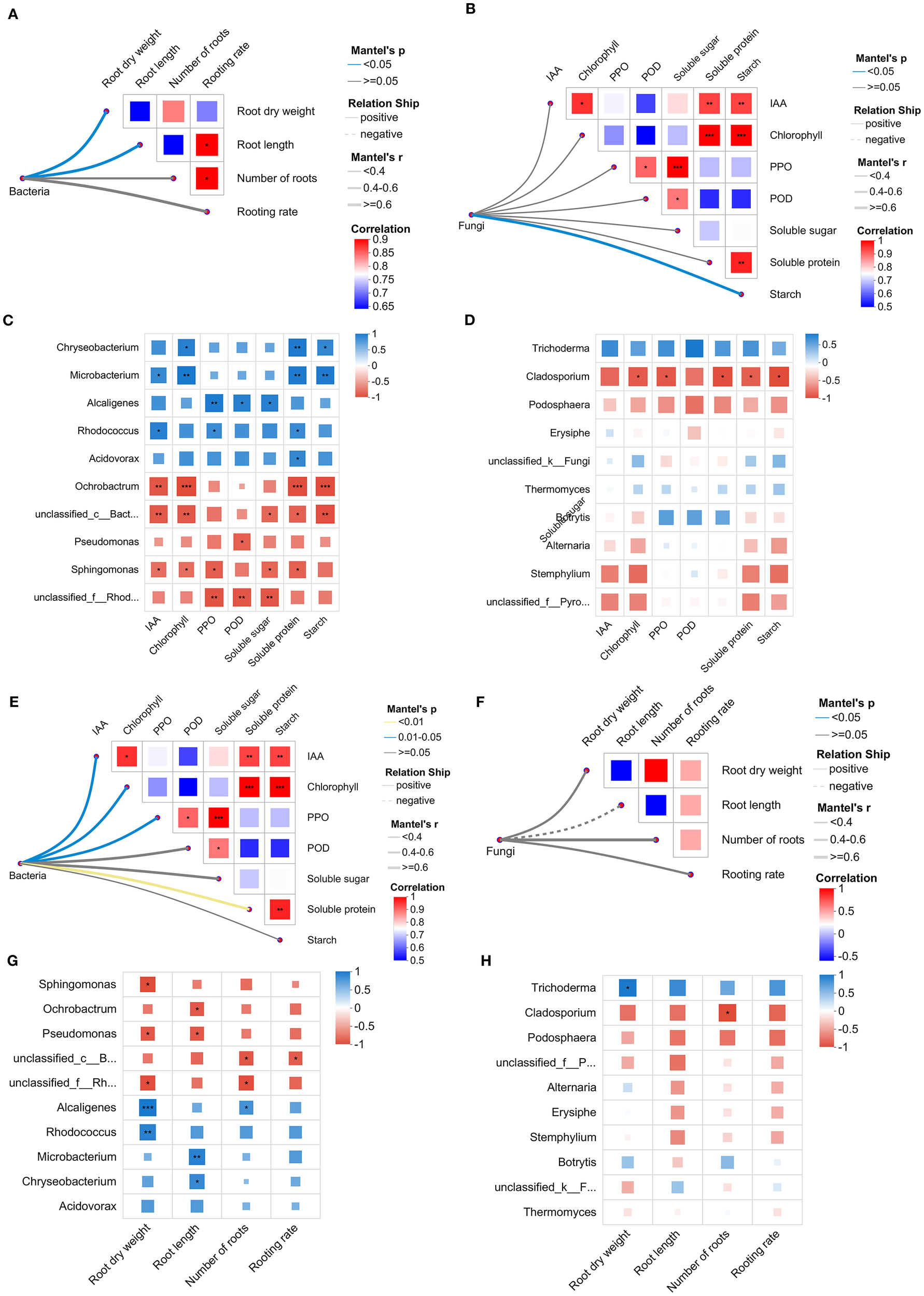
Spearman correlation analysis of endophytic microorganisms with physiological indicators (A-D) and root phenotype of chrysanthemums (E-H). (A, B) Endophytic microbial community and physiological indicators. (C, D) Dominant endophytic genera and physiological indicators. (E, F) Endophytic microbial community and root phenotype. (G, H) Dominant endophytic genus and root phenotype. Left, bacteria; Right, fungi. The asterisk represented the level of significance. *p < 0.05, **p < 0.01, ***p < 0.001.
In contrast, Ochrobactrum, whose abundance was extremely significantly reduced, showed highly significant negative correlations with IAA, chlorophyll, soluble protein, and starch. The reduced abundance of Sphingomonas showed a significant negative correlation with soluble sugar, soluble protein, IAA, chlorophyll, and PPO (Figure 5C). Both Ochrobactrum and Sphingomonas showed significant negative correlations with root dry weight and root length (Figure 5G). The decreased abundance of Pseudomonas showed a negative correlation with POD, root dry weight, and root length (Figures 5C, G).
For endophytic fungi, the whole community was significantly positively correlated with starch (Figure 5B). Among them, Cladosporium, which exhibited a markedly reduced abundance following KC 009 treatment, showed significant negative correlations with key physiological parameters, including chlorophyll content, PPO activity, soluble sugars, soluble protein, starch (Figure 5D), as well as root number. The increased abundance of Trichoderma was significantly positively correlated with root dry weight (Figure 5H).
3.5 Effects of KC 009 on the function and enzyme abundance of the endophytes in chrysanthemum cuttings
3.5.1 Effects of KC 009 on endophytic bacterial community function and enzyme abundance
PICRUSt 2 was used to predict the function of endophytic bacteria in cutting chrysanthemums. The results of KEGG level 2 functional predictive analysis revealed that after treatment with KC 009, the relative abundances of pathways related to amino acid metabolism, carbohydrate metabolism, and energy metabolism significantly increased. This suggested that KC 009 altered the metabolic regulation of amino acids and carbohydrates by endophytic microorganisms in cutting chrysanthemums. KEGG level 3 functional prediction showed that 14 metabolic pathways associated with plant growth were significantly enriched (Figure 6A). Among them, the abundance of the folate biosynthesis pathway, MAPK signaling pathway, and galactose metabolism pathway increased significantly by 149.49%, 39.88%, and 38.13%, respectively. The abundance of the C5-Branched dibasic acid metabolism pathway and riboflavin metabolism pathway showed significant increase of 30.44% and 13.56%, respectively.
Figure 6
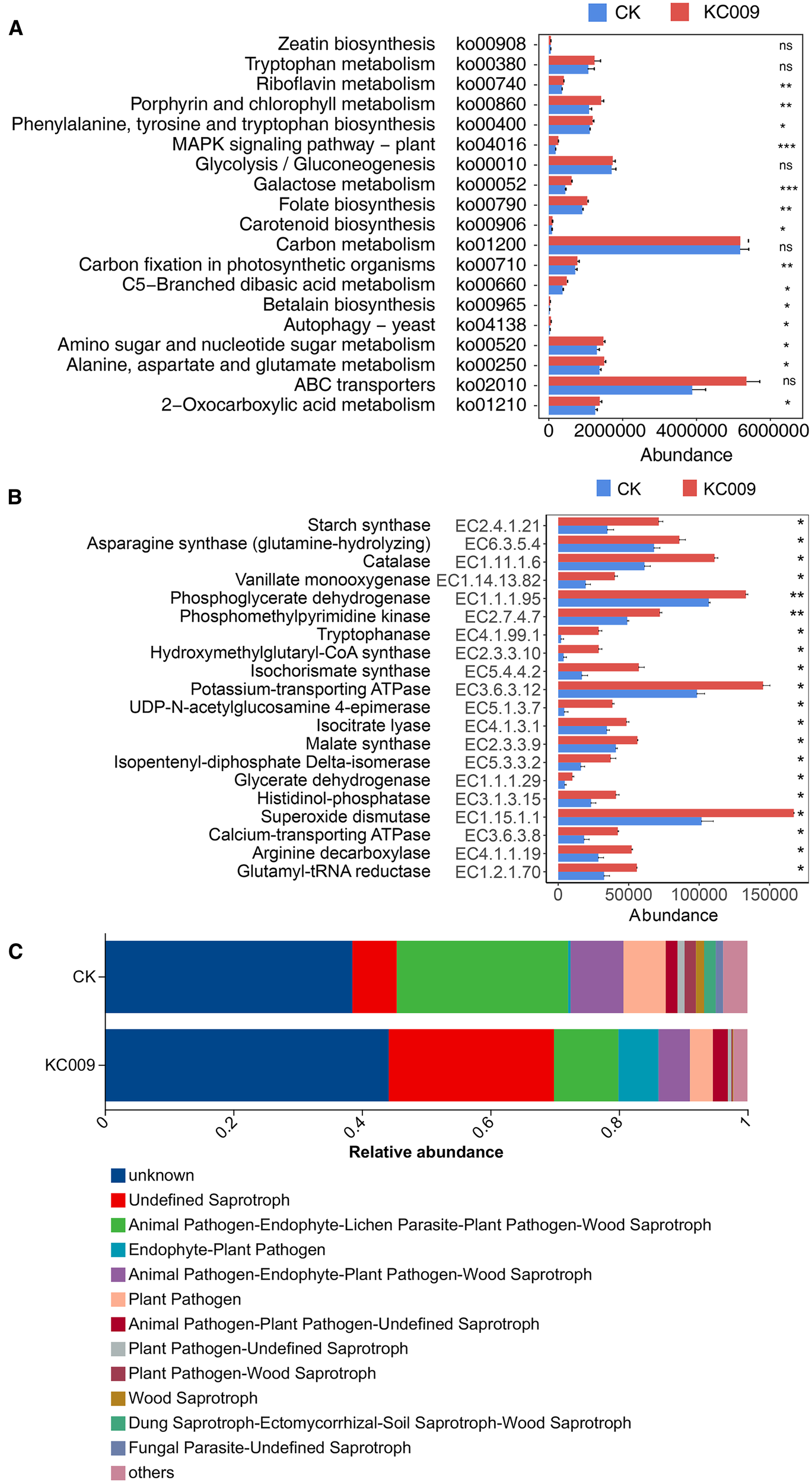
Effect of KC 009 on the function prediction of endophytic bacterial. (A) Bacterial function abundance at KEGG Level 3. (B) KEGG enzyme abundance of bacteria. (C) FUNGuild functional abundance of fungi. The asterisk in the figure represented the significance level, *p < 0.05, **p < 0.01, ***p < 0.001, ns: p >0.05.
KEGG Enzyme analysis revealed that 20 enzymes related to plant growth were significantly increased in the KC 009 treatment group (Figure 6B). Thereinto, the abundance of Tryptophanase (EC: 4.1.99.1) related to IAA synthesis increased by 129.71%, the abundance of Starch synthase (EC: 2.4.1.21) related to catalyzing starch synthesis significantly increased by 104.44%, and the abundance of Glutamyl-tRNA reductase (EC: 1.2.1.70) involved in plant chlorophyll synthesis increased by 71.34%. The abundance of Catalase (EC: 1.11.1.6), which plays a role in alleviating oxidative stress and enhancing stress resistance, increased significantly by 36.09%. Asparagine is a crucial protein in plants, playing a key role in nitrogen metabolism, energy balance, and stress response (Asif et al., 2022). KC 009 treatment significantly increased the abundance of Asparagine synthase (EC: 6.3.5.4) in endophytic bacteria of chrysanthemum cuttings by 26.37%.
3.5.2 Effects of KC 009 on the ecological function of the endophytic fungal community
Ecological functional analysis of endophytic fungi in cutting chrysanthemums using FUNGuild (Figure 6C) revealed that after KC 009 treatment, the abundances of the Animal Pathogen-Endophyte-Lichen Parasite-Plant Pathogen-Wood Saprotroph, Plant Pathogen, Animal Pathogen-Endophyte-Plant Pathogen-Wood Saprotroph were significantly reduced by 62.42%, 45.77% and 40.69%, respectively, and the main fungi of the three functional types were primarily plant pathogens. Additionally, the relative abundance of Undefined Saprotrophs increased by 366.47%, with the main groups being beneficial fungi known to promote plant growth, including Eurotiales, Hypocreales, Saccharomycetales, and Metarhizium.
4 Discussion
Cutting chrysanthemum is very important in the global floriculture industry, with strong export demand requiring rapid supply and stable quality. The root is a vital organ for nutrient uptake from the soil, and increasing the number and length of roots is essential to enhance nutrient absorption capacity and support the growth and development of the plant. The application of PGPR can not only stimulate root formation and growth in plants, but also alleviate the ecological risk caused by chemical applications. Massilia, as a type of PGPR, has not yet been reported to promote rooting in plant cuttings. This study is the first to demonstrate that applying M. consociata KC 009 can effectively promote rooting in chrysanthemum cuttings, indicating that Massilia can enhance rooting in plant cuttings. This study investigated the effect of M. consociata KC 009 on the rooting ability of cut chrysanthemum. It analyzed the potential growth-promoting mechanism of strain KC 009 based on physiological measurements and changes in endophytic and rhizosphere microbial communities. KC 009 promotes the root growth of chrysanthemum cuttings by reshaping the endophyte community and simultaneously enhancing physiological indicators, such as soluble proteins. The correlation analysis between physiological indicators and phenotypic indicators, as well as the correlation analysis between endophyte and phenotypic and physiological indicators of chrysanthemum roots, reveals the physiological indicators and endophytic microbial groups most closely related to chrysanthemum roots. Unlike previous studies that focused solely on a single aspect for promoting root formation, this study elucidates the mechanism by which KC 009 encourages root growth in chrysanthemum cuttings from multiple dimensions.
4.1 Significant effects of KC 009 on root development and physiological parameters in chrysanthemum
IAA and ABA play crucial roles in plant growth, development, and environmental adaptation (Feng et al., 2024; Zhang et al., 2019). Song et al. (2025) found that Bacillus sp. SYM-4 not only could produce IAA itself but also significantly increased the IAA content of Zea mays L. by 77.19% when inoculated. Massilla, a representative PGPR, is widely distributed in the rhizosphere soils of various plants. Yao et al. (2024) identified the IAA biosynthesis gene cluster trpEGDCFB in Massilia phyllosphaerae SCZ-792T isolated from Pennisetum sp. They confirmed that the strain can significantly increase primary root length and lateral root number in Oryza sativa L. In this study, fermentation of M. consociata KC 009 yielded 15.995 nmol/L IAA, and its application to chrysanthemum cuttings significantly increased their endogenous IAA levels by 17.60%. This indicated that KC 009 not only synthesizes IAA in vitro but also considerably enhances endogenous IAA synthesis in chrysanthemum cuttings. Furthermore, the rooting rate, root length, number of roots, and root dry weight increased by 50%, 35.77%, 40.78%, and 54.65% respectively.
Soluble sugars, soluble proteins, and soluble starch in plants play essential roles in regulating physiological and metabolic processes, providing nutritional support for growth and development, and enhancing stress resistance (Verma et al., 2024). The application of PGPR could modulate plant physiological activities, thereby promoting root development and growth. Mehrabi et al. (2024) inoculated Triticum aestivum with Bacillus velezensis UTB96 and found that the starch content of T. aestivum increased significantly by 17%-29%. Wyrwicka et al. (2019) inoculated Massilia niastensis p87 into Festuca arundinacea Schreb. and found that it significantly promoted shoot growth during the later stages of plant development, with a corresponding increase in soluble protein content in the leaves of up to 129%. KC 009 treatment systematically upregulated carbon and nitrogen nutrients, photosynthetic pigment content, and enzyme activity of chrysanthemum cuttings. PPO and POD are important enzymes involved in plant disease resistance defense and stress response. They work together to maintain cellular redox homeostasis and regulate the synthesis of secondary metabolites and strengthen the cell wall, thereby enhancing the plant’s resistance to pathogen invasion and oxidative damage (Zhao et al., 2024). Zhou et al. (2023) found that POD could reduce the adverse effects of oxidative stress on cutting root formation, accelerate wound healing, and promote rooting. In our study, the application of KC 009 significantly increased the POD and PPO content of chrysanthemum cuttings by 59.01% and 14.66%, respectively.
Spearman correlation analysis between root indexes and physiological parameters showed that root growth indexes were significantly positively correlated with increased levels of soluble protein, soluble sugar, chlorophyll, IAA, and PPO activity. These results confirm that the root-promoting effect of KC 009 on cutting chrysanthemum was driven by a coordinated regulatory mechanism involving carbon and nitrogen accumulation, photosynthetic assimilation, hormone signaling activation, and enhancement of related enzyme activities.
4.2 Regulatory effects of KC 009 on the rhizosphere and endophytic microbial community of cutting chrysanthemum
The addition of exogenous microorganisms can alter the rhizosphere or endophytic microbial community of plants. Ji et al. (2020) inoculated Enterobacter cloacae HG-1 into Wheat cv. Jimai 21, which significantly reduced the diversity and richness of rhizosphere microorganisms, yet significantly increased the root length and plant height. The application of KC 009 had a minimal effect on the rhizosphere microbial composition of chrysanthemum cuttings. Still, it significantly reduced the diversity and richness of the endophytic fungal community, resulting in a reconstructed endophytic bacterial composition. At the phylum level, the relative abundance of Bacteroidota and Actinobacteriota increased following KC 009 treatment, becoming the dominant bacteria in the endophytic community. Among them, Bacteroidota accounted for the most significant proportion in the KC 009 treatment, and its members not only inhibit pathogenic bacteria but also promote the phosphorus absorption and utilization (Lidbury et al., 2021). Actinobacteriota are known to facilitate nutrient uptake, thereby promoting plant growth (Oloumi et al., 2023). At the genus level, the abundance of plant growth-promoting bacteria such as Chryseobacterium, Alcaligenes, and Microbacterium significantly increased. Chryseobacterium, showing the most significant increase in abundance, was identified as the most important biomarker in the KC 009 treatment. It can secrete IAA, with the potential to stimulate root development. Kumar et al. (2023) found that treating Arabidopsis thaliana seeds with Chryseobacterium cucumeris PCH239 significantly improved germination rates and promoted primary root and root hair growth. Spearman correlation analysis revealed that Chryseobacterium was significantly positively correlated with physiological indicators, such as soluble protein, chlorophyll, and starch, and also positively correlated with beneficial genera, including Acidovorax and Microbacterium. Alcaligenes can promote plant growth by producing ACC deaminase and synthesizing siderophore (Das et al., 2022). Sapre et al. (2021) reported that inoculation with Alcaligenes faecalis IG27 significantly increased root length and root dry weight of Pisum sativum by 12.61% and 50%, respectively. Microbacterium can promote plant growth by alleviating stress and enhancing the utilization of trace element (Xiao et al., 2025). Zhao et al. (2024) reported that inoculation of Microbacterium testaceum M15 into Oryza sativa L. significantly increased chlorophyll content by over 30%. In summary, KC 009 promoted root development in chrysanthemum cuttings by enriching beneficial functional microorganisms, optimizing synergistic interactions among microbial communities, and reconstructing a low-diversity yet highly functional microbial community.
For endophytic fungi, Ascomycota and Basidiomycota were the dominant phyla in both the CK and the KC 009 treatment. Members of Ascomycota can promote plant development by regulating carbon and nitrogen cycling (Challacombe et al., 2019). At the genus level, the application of KC 009 significantly suppressed the growth of potential pathogens, such as Cladosporium and Alternaria, while markedly enhancing the growth-promoting Trichoderma. Trichoderma can strengthen root development and plant growth by promoting nutrient uptake and secreting growth hormones. Cladosporium with significantly reduced abundance often causes leaf spot (Robles et al., 2019) and tomato leaf mold (Yoshida et al., 2020), and is significantly negatively correlated with Chlorophyll, PPO, soluble sugars, soluble protein, and starch. Alternaria with reduced abundance not only produces phytotoxins such as Alternariol and AM-toxin to damage plant cell (Andersen et al., 2006), but also causes potato early blight (Patel and Gohel, 2023) and apple black spot (Han et al., 2023). Spearman analysis showed that it was positively correlated with other pathogens, including Gibberella, which causes American ginseng root rot disease (Tian et al., 2021), and Penicillium, which causes apple rot (Watpade et al., 2025). Co-occurrence network analysis further demonstrated that KC 009 indirectly suppressed the synergistic proliferation of other pathogens by reducing the abundance of Alternaria. In conclusion, KC 009 effectively promoted rooting of chrysanthemum through a regulatory effect of “suppressing harm and promoting benefits”. This provides a theoretical basis for developing microbial agents that integrate both plant growth promotion and disease control functions.
4.3 Effects of KC 009 on the function and enzyme abundance of endophyte in cutting chrysanthemum
KC 009 significantly enhanced carbon and nitrogen metabolism as well as stress resistance in cutting chrysanthemum by regulating the functional modules of endophytic bacteria. KEGG level 3 analysis showed that KC 009 increased carbohydrate storage in cutting chrysanthemum by upregulating starch synthase (EC: 2.4.1.21), and simultaneously enhancing the abundance of C5-branched dibasic acid metabolism and asparagine synthase (EC: 6.3.5.4) to promote nitrogen accumulation. These findings were consistent with the observed increases of 11.55%, 6.71%, and 11.23% in soluble sugar, starch, and soluble protein content, respectively, in the KC 009 treatment. The application of KC 009 also increased the abundance of glutamyl-tRNA reductase (EC: 1.2.1.70), as well as folate biosynthesis and riboflavin metabolism, thereby synergistically enhancing the synthesis of photosynthetic pigments and contributing to a 19.99% increase in chlorophyll content in chrysanthemum cuttings. The notable increase in tryptophanase (EC: 4.1.99.1) and MAPK signaling pathway-plant abundance was in line with the observed 17.60% rise in IAA content of KC 009 cuttings. A significant increase in catalase (EC: 1.11.1.6) abundance may help eliminate oxidative substances such as H2O2 during the early stages of callus formation in cutting chrysanthemum, thereby promoting cell division and callus development (Adil et al., 2019).
FUNGuild analysis of endophytic fungi revealed that KC 009 treatment significantly reduced the abundance of Animal Pathogen-Endophyte-Lichen Parasite-Plant Pathogen-Wood Saprotroph, which was predominantly composed of the pathogenic Cladosporium. The abundance of the Plant Pathogen also decreased significantly, mainly represented by Bipolaris which causing southern leaf blight and brown spot (Bhunjun et al., 2020), and Cylindrocladiella associating with root rot, seedling blight, and xylem-related diseases (Li et al., 2021). In addition, the abundance of the Animal Pathogen-Endophyte-Plant Pathogen-Wood Saprotroph, dominated by the pathogen Alternaria, was also reduced. These results suggest that KC 009 treatment has the potential to reduce the risk of fungal disease outbreaks in cutting chrysanthemum.
5 Conclusions
This study found that M. consociata KC 009 not only significantly enhanced physiological indicators, such as POD activity, chlorophyll content, and IAA levels, in cutting chrysanthemum but also reshaped the endophyte community. KC 009 induced a synergistic enhancement of protein and carbohydrate metabolism in endophytes, increased the abundance of starch synthase, chlorophyll synthase, and IAA synthase, and drove the carbon and nitrogen nutrients of chrysanthemum cuttings in the direction of energy storage and auxin synthesis, forming a microecological balance of “inhibiting pathogens and promoting benefit”, thereby significantly increasing rooting rate, root length, and root dry weight by 50%, 35.77%, and 35.33%, respectively. This study revealed the mechanism by which PGPR KC 009 promotes the rooting of chrysanthemum cuttings through a multidimensional synergistic strategy, providing theoretical support and practical reference for the development of microbial agents to regulate the rapid rooting of chrysanthemums. KC 009 can be directly applied to enhance the rooting efficiency of chrysanthemum cuttings, serving as a cost-effective and eco-friendly microbial agent that facilitates rapid rooting in ornamental seedlings during cutting propagation. However, this study is based solely on potted experiments and single chrysanthemum varieties, and it is necessary to supplement this with field verification and applicability tests using more diverse chrysanthemum varieties.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
CL: Conceptualization, Software, Visualization, Writing – original draft, Writing – review & editing. HD: Investigation, Writing – original draft. YJ: Methodology, Resources, Writing – original draft. MM: Writing – review & editing. YY: Data curation, Validation, Writing – original draft. JZ: Visualization, Writing – original draft. RL: Formal Analysis, Writing – original draft. YC: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by Joint Special Project of Universities in Yunnan Province (202401BA070001-003), the Applied Basic Research Foundation of Yunnan Province (202301AT070051), Scientific Research Foundation of the Education Department of Yunnan Provincial (2024Y732), a grant (no. 2024KF007) from State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan and Key Laboratory of Industrial Microbial Fermentation Engineering of Yunnan Province, Yunnan University and Ten Thousand Talent Plans for Young Top-notch Talents of Yunnan Province (YNWRQNBJ-2018-011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Adil M. Ren X. Jeong B. (2019). Light elicited growth, antioxidant enzymes activities and production of medicinal compounds in callus culture of Cnidium officinale Makino. J. Photoch Photobio B.196, 111509. doi: 10.1016/j.jphotobiol.2019.05.006
2
Andersen B. Smedsgaard J. Jørring I. Skouboe P. Pedersen L. (2006). Real-time PCR quantification of the AM-toxin gene and HPLC qualification of toxigenic metabolites from Alternaria species from apples. Int. J. Food Microbiol.111, 105–111. doi: 10.1016/j.ijfoodmicro.2006.04.021
3
Astudillo Á. Hormazábal E. Quiroz A. Rubilar O. Briceño G. Abdala R. et al . (2024). Recycling potato waste for the production of blue pigments by Streptomyces lydicus PM7 through submerged fermentation. Chem. Biol. Technol. Ag.11, 90–90. doi: 10.1186/s40538-024-00612-x
4
Behrangi S. Staňková E. Sedláček I. Šimoníková L. Souček P. Buršíková V. et al . (2024). A long-term study on the bactericidal effect of zrN-cu nanostructured coatings deposited by an industrial physical vapor deposition system. Nanomaterials (Basel).10, 14. doi: 10.3390/nano14060496
5
Bhunjun S. Dong Y. Jayawardena S. Jeewon R. Phukhamsakda C. Bundhun D. et al . (2020). A polyphasic approach to delineate species in Bipolaris. Fungal Divers.102, 225–256. doi: 10.1007/s13225-020-00446-6
6
Challacombe J. Hesse C. Bramer L. McCue L. Lipton M. Purvine S. et al . (2019). Genomes and secretomes of Ascomycota fungi reveal diverse functions in plant biomass decomposition and pathogenesis. BMC Genomics20, 976. doi: 10.1186/s12864-019-6358-x
7
Dag O. Ilk O. (2017). An algorithm for estimating Box–Cox transformation parameter in ANOVA. Commun. Stat-simul C.46, 6424–6435. doi: 10.1080/03610918.2016.1204458
8
Das S. Verma S. Choudhary P. Singh R. Saxena A. (2022). Deciphering the genetic and functional diversity of cultivable bacteria from chasmophytic pigweed (Chenopodium album) from Tsomoriri, Ladakh, India. 3 Biotech.12, 242. doi: 10.1007/s13205-022-03278-0
9
Diettrich J. Kage H. Nett M. (2019). Genomics-inspired discovery of massiliachelin, an agrochelin epimer from Massilia sp. NR 4-1. Beilstein J. Org. Chem.15, 1298–1303. doi: 10.3762/bjoc.15.128
10
Feng Y. Tian B. Xiong J. Lin G. Cheng L. Zhang T. et al . (2024). Exploring IAA biosynthesis and plant growth promotion mechanism for tomato root endophytes with incomplete IAA synthesis pathways. Chem. Biol. Technol. Ag.11, 187. doi: 10.1186/s40538-024-00712-8
11
Gao K. Chen Q. Pan B. Sun Y. Xu Y. Chen D. et al . (2023). Current achievements and future prospects in virus elimination technology for functional chrysanthemum. Viruses.20, 15. doi: 10.3390/v15081770
12
Gupta S. Kumar M. Kumar J. Ahmad V. Pandey R. Chauhan N. (2017). Systemic analysis of soil microbiome deciphers anthropogenic influence on soil ecology and ecosystem functioning. Int. J. Environ. Sci. Technol.14, 2229–2238. doi: 10.1007/s13762-017-1301-7
13
Han J. Fan Y. Li S. Jia B. Yang J. Shen A. et al . (2023). Nitric oxide induces defense responses in apple fruit against black spot disease caused by Alternaria alternata. S. Afr. J. Bot.158, 265–276. doi: 10.1016/j.sajb.2023.05.019
14
Han Q. Zhu G. Qiu H. Li M. Zhang J. Wu X. et al . (2024). Quality traits drive the enrichment of Massilia in the rhizosphere to improve soybean oil content. Microbiome.12, 224. doi: 10.1186/s40168-024-01933-7
15
Holochová P. Mašlaňová I. Sedláček I. Švec P. Králová S. Kovařovic V. et al . (2020). Description of Massilia rubra sp. nov., Massilia aquatica sp. nov., Massilia mucilaginosa sp. nov., Massilia frigida sp. nov., and one Massilia genomospecies isolated from Antarctic streams, lakes and regoliths. Syst. Appl. Microbiol.43, 126112. doi: 10.1016/j.syapm.2020.126112
16
Islam M. Yeng C. Chun K. Huey H. (2021). The influence of fibre orientation and chemical treatment on the performance of unsaturated polyester/chrysanthemum stem fibre composites. Polym. Compos.29, 1103–1113. doi: 10.1177/0967391120954067
17
Jabborova D. Davranov K. Jabbarov Z. Enakiev Y. Abdrakhmanov T. Datta R. et al . (2024). Impact of growth-promoting endophytic bacteria on ginger plant growth. Indian J. Microbiol.65, 1–10. doi: 10.1007/s12088-024-01379-3
18
Ji C. Liu Z. Hao L. Song X. Wang C. Liu Y. et al . (2020). Effects of enterobacter cloacae HG-1 on the nitrogen-fixing community structure of wheat rhizosphere soil and on salt tolerance. Front. Plant Sci.11. doi: 10.3389/fpls.2020.01094
19
Jin Q. Zhang Y. Ma Y. Sun H. Guan Y. Liu Z. et al . (2022). The composition and function of the soil microbial community and its driving factors before and after cultivation of Panax ginseng in farmland of different ages. Ecol. Indic.145, 109748. doi: 10.1016/j.ecolind.2022.109748
20
Joel P. Bala M. Kaur A. Dubey R. (2023). Efficacy of rooting hormones on propagation and rooting potential of Japanese chrysanthemums (Chrysanthemum morifolium Ramat.). Rhizosphere.27, 100740. doi: 10.1016/j.rhisph.2023.100740
21
Kämpfer P. Lodders N. Martin K. Falsen E. (2011). Revision of the genus Massilia La Scola et al., 2000, with an emended description of the genus and inclusion of all species of the genus Naxibacter as new combinations, and proposal of Massilia consociata sp. nov. Int. J. Syst. Evol. Micr.61, 1528–1533. doi: 10.1099/ijs.0.025585-0
22
Kaur G. Jhanji S. (2023). Plant growth regulators preserved the longevity of cut stems of Chrysanthemum morifolium by orchestrating physio-biochemical and anatomical responses. Plant Physiol. Bioch.196, 1098–1110. doi: 10.1016/j.plaphy.2023.02.044
23
Krishnamoorthy R. Kim K. Subramanian P. Senthilkumar M. Anandham R. Sa T. (2016). Arbuscular mycorrhizal fungi and associated bacteria isolated from salt-affected soil enhances the tolerance of maize to salinity in coastal reclamation soil. Agr Ecosyst. Environ.231, 233–239. doi: 10.1016/j.agee.2016.05.037
24
Kumar V. Patial V. Thakur V. Singh R. Singh D. (2023). Genomics assisted characterization of plant growth-promoting and metabolite producing psychrotolerant Himalayan Chryseobacterium cucumeris PCH239. Arch. Microbiol.205, 108. doi: 10.1007/s00203-023-03456-5
25
Li S. Yang N. Chen L. (2022). Paraffin section observation of flower bud differentiation of Chimonanthus praecox in Kunming and comparison of the differentiation processes in different regions, China. Hortic. Plant J.8, 221–229. doi: 10.1016/j.hpj.2021.11.001
26
Li X. Yu T. Jiang X. Zhang C. Zhao X. J. Wang J. (2021). First Report of Ear Rot of Chenopodium quinoa caused by Cladosporium cladosporioides in Shanxi Province, China. Plant Dis.5, 106. doi: 10.1094/pdis-11-20-2530-pdn
27
Liang Y. Niu H. Ma L. Du D. Wen L. Xia Q. et al . (2017). Eriodictyol 7−O−β−D glucopyranoside from Coreopsis tinctoria Nutt. ameliorates lipid disorders via protecting mitochondrial function and suppressing lipogenesis. Mol. Med. Rep.16, 1298–1306. doi: 10.3892/mmr.2017.6743
28
Liao Z. Chen Q. Li J. Wei L. Wu J. Wang X. et al . (2024). Influence of Chrysanthemum morifolium-maize intercropping pattern on yield, quality, soil condition, and rhizosphere soil microbial communities of C. morifolium. Front. Plant Sci.15. doi: 10.3389/fpls.2024.1383477
29
Lidbury I. Borsetto C. Murphy A. Bottrill A. Jones A. Bending G. et al . (2021). Niche-adaptation in plant-associated Bacteroidetes favours specialisation in organic phosphorus mineralisation. Isme J.15, 1040–1055. doi: 10.1038/s41396-020-00829-2
30
Liu H. Su Y. Ye C. Zuo D. Wang L. Mei X. et al . (2025). Nucleotides enriched under heat stress recruit beneficial rhizomicrobes to protect plants from heat and root-rot stresses. Microbiome.13, 160. doi: 10.1186/s40168-025-02126-6
31
Liu L. Xue Y. Luo J. Han M. Liu X. Jiang T. et al . (2023). Developing a UV-visible reporter-assisted CRISPR/Cas9 gene editing system to alter flowering time in Chrysanthemum indicum. Plant Biotechnol. J.21, 1519–1521. doi: 10.1111/pbi.14062
32
Martínez M. Andrés M. Bujanda R. Díaz E. Eichmeier A. Gramaje D. (2019). Soil-plant compartments affect fungal microbiome diversity and composition in grapevine. Fungal Ecology.41, 234–244. doi: 10.1016/j.funeco.2019.07.003
33
Mehrabi S. Sabokdast M. Bihamta M. Dedičová B. (2024). The coupling effects of PGPR inoculation and foliar spraying of strigolactone in mitigating the negative effect of salt stress in wheat plants: insights from phytochemical, growth, and yield attributes. Agriculture.14, 732. doi: 10.3390/agriculture14050732
34
Moberly J. Bernards M. Waynant K. (2018). Key features and updates for origin 2018. J. Cheminform.10, 5. doi: 10.1186/s13321-018-0259-x
35
Mohanty P. Singh P. Chakraborty D. Mishra S. Pattnaik R. (2021). Insight into the role of PGPR in sustainable agriculture and environment. Front. Sustain. Food Syst.5. doi: 10.3389/fsufs.2021.667150
36
Nayak D. Sahoo S. Barik S. Sanghamitra P. Sangeeta S. Pandit E. et al . (2022). Association mapping for protein, total soluble sugars, starch, amylose and chlorophyll content in rice. BMC Plant Biol.22, 620. doi: 10.1186/s12870-022-04015-8
37
Nikerova K. Galibina N. Sofronova I. Borodina M. Moshchenskaya Y. Tarelkina T. et al . (2023). An Indicating Role of Antioxidant System Enzymes at the Stage of Active Structural Anomalies Formation in Karelian Birch (Betula pendula Roth var. carelica (Mercl.) Hämet-Ahti). Protein Pept. Lett.30, 325–334. doi: 10.2174/0929866530666230228113430
38
Oleńska E. Małek W. Wójcik M. Swiecicka I. Thijs S. Vangronsveld J. (2020). Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ.743, 140682. doi: 10.1016/j.scitotenv.2020.140682
39
Oloumi H. Khaleghi M. Dalvand A. (2023). Isolation and identification of endophytic actinobacteria from Iris persica and Echium amoenum plants and investigation of their effects on germination and growth of wheat plant. Food Sci. Nutr.11, 5296–5303. doi: 10.1002/fsn3.3488
40
Onyango L. Ngonga F. Karanja E. Kuja J. Boga H. Cowan D. et al . (2023). The soil microbiomes of forest ecosystems in Kenya: their diversity and environmental drivers. Sci. Rep.13, 7156–7156. doi: 10.1038/s41598-023-33993-4
41
Patel J. Gohel N. (2023). Eco-safe management of potato early blight caused by alternaria solani (Ellis and martin) jones and grout. Potato Res.66, 1023–1032. doi: 10.1007/s11540-022-09612-6
42
Peng Z. Wu W. Chen R. Wang W. Liu A. Zhao Z. et al . (2025). PICRUSt2 function prediction analyzes the microbial metabolic pathway of “rock flavor substances Wuyi Rock Tea (Rougui).Food Biosci.64, 105870. doi: 10.1016/j.fbio.2025.105870
43
Quevedo B. Juárez M. Magdaleno J. Pérez M. Valdez E. Mejía J. (2022). Effect of the concentration of the nutrient solution on the nutrient content of chrysanthemum (Dendranthema grandiflorum (Ramat.). Agro Productividad.1341, 149–156. doi: 10.32854/agrop.v15i12.2467
44
Robles Y. Ayala E. Leyva M. Bernardi L. Camacho T. Tovar P. (2019). First report of Cladosporium cladosporioides causing leaf spot on tomato in Mexico. JPP.101, 759–759. doi: 10.1007/s42161-018-00218-x
45
Rousseau G. Cosmo R. Zacchiroli S. (2020). Software provenance tracking at the scale of public source code. Empir Softw Eng.25, 1–30. doi: 10.1007/s10664-020-09828-5
46
Sapre S. Mishra I. Tiwari S. (2021). Plant growth-promoting rhizobacteria ameliorates salinity stress in pea (Pisum sativum). J. Plant Growth Regul.41, 1–10. doi: 10.1007/s00344-021-10329-y
47
Seraman S. Rajendran A. Thangavelu V. (2010). Statistical optimization of anticholesterolemic drug lovastatin production by the red mold Monascus purpureus. Food Bioprod. Process.88, 266–276. doi: 10.1016/j.fbp.2010.01.006
48
Shilpa S. Chauhan M. Bijalwan P. (2024). Plant growth promoting rhizobacteria, organic manures, and chemical fertilizers: impact on crop productivity and soil health of capsicum (Capsicum annuum;L.) in North Western Himalayan region. J. Plant Nutr.47, 448–467. doi: 10.1080/01904167.2023.2280120
49
Song Y. Chen Q. Hua J. Zhang S. Luo S. (2025). The IAA-producing rhizobacterium bacillus sp. SYM-4 promotes maize growth and yield. Plants (Basel).14, 1587. doi: 10.3390/plants14111587
50
Song Z. Qiao J. Tian D. Guan Q. He Y. Liu P. et al . (2023). Glutamic acid can prevent the browning of fresh-cut potatoes by inhibiting PPO activity and regulating amino acid metabolism. LWT.180, 114735. doi: 10.1016/j.lwt.2023.114735
51
Tian L. Ou J. Sun X. Miao Y. Pei J. Zhao L. et al . (2021). The discovery of pivotal fungus and major determinant factor shaping soil microbial community composition associated with rot root of American ginseng. Plant Signaling Behav.16, 1952372. doi: 10.1080/15592324.2021.1952372
52
Turnbull A. Liu Y. Lazarovits G. (2012). Isolation of bacteria from the rhizosphere and rhizoplane of potato (Solanum tuberosum) grown in two distinct soils using semi selective media and characterization of their biological properties. Am. J. Potato Res.89, 294–305. doi: 10.1007/s12230-012-9253-4
53
Verma K. Verma S. Tamta P. Joshi R. Joshi N. (2024). Roasting induced phytoconstituents variability and effect of temperature on soluble proteins of buckwheat cultivars. J. Food Meas. Charact.5, 1–12. doi: 10.1007/s11694-024-02813-2
54
Wang Y. Li Y. Guo W. Yang X. Qu J. Gao M. et al . (2022). Comparison of the Chemical Components, Efficacy and Mechanisms of Action of Chrysanthemum morifolium Flower and Its Wild Relative Chrysanthemum indicum Flower against Liver-Fire Hyperactivity Syndrome of Hypertension via Integrative Analyses. Int. J. Mol. Sci.9, 23. doi: 10.3390/ijms232213767
55
Watpade S. Devi I. Bagul S. Khadke G. Kumar D. Kumari H. et al . (2025). Exploring eco-friendly strategies for the management of blue mold of apple caused by Penicillium crustosum thom. Postharvest Biol. Technol.227, 113581–113581. doi: 10.1016/j.postharvbio.2025.113581
56
Wu Y. Shen Y. (2021). Dormancy in Tilia miqueliana is attributab le to permeability barriers and mechanical constraints in the endosperm and seed coat. Braz. J. Bot.44, 725–740. doi: 10.1007/s40415-021-00749-1
57
Wyrwicka A. Urbaniak M. Siebielec G. Siebielec S. Chojak-Koźniewska J. Przybylski M. et al . (2019). The influence of bottom sediments and inoculation with rhizobacterial inoculants on the physiological state of plants used in urban plantings. Water.11, 1792. doi: 10.3390/w11091792
58
Xiao Q. Dai Y. Yu X. Wang K. Zhu X. Qin P. et al . (2025). Fumigant dazomet induces tobacco plant growth via changing the rhizosphere microbial community. Sci. Rep.15, 6673. doi: 10.1038/s41598-025-91432-y
59
Xiao S. Zhang Y. Tang X. Yang J. Zhong W. Zhang Y. et al . (2024). An improved Trizol method for extracting total RNA from Eleutherococcus senticosus (Rupr. & Maxim.) Maxim leaves. Phytochem. Anal.35, 1613–1619. doi: 10.1002/pca.3404
60
Yao L. Liu G. Zhang S. Peng G. Rensing C. Yang Q. et al . (2024). Genome-based taxonomy and functional prediction of Sphingomonas fuzhouensis sp. nov. and Massilia phyllosphaerae sp. nov. isolated from Pennisetum sp. with plant growth-promoting potential. Anton Leeuw Int. J. G.118, 6. doi: 10.1007/s10482-024-02017-0
61
Yoshida K. Asano S. Sushida H. Iida Y. (2020). Occurrence of tomato leaf mold caused by novel race 2.4.9 of Cladosporium fulvum in Japan. J. Gen. Plant Pathol.87, 35–38. doi: 10.1007/s10327-020-00963-x
62
Zhang Q. Chen Y. Ma L. Jiang Y. Chen J. Dong J. et al . (2020b). Study on the fingerprints and quality evaluation of angelica sinensis radix by HPLC coupled with chemometrics based on traditional decoction process of ACPTCM. Dose-response.18, 3. doi: 10.1177/1559325820951730
63
Zhang Y. Cheng W. Di H. Yang S. Tian Y. Tong Y. et al . (2024). Variation in nutritional components and antioxidant capacity of different cultivars and organs of basella alba. Plants (Basel).20, 13. doi: 10.3390/plants13060892
64
Zhang X. Goatley M. Wu W. Ervin E. Shang C. (2019). Drought-induced injury is associated with hormonal alteration in Kentucky bluegrass. Plant Signaling Behav.14, e1651607. doi: 10.1080/15592324.2019.1651607
65
Zhang L. Hu Y. Li X. Lu W. Li J. (2020a). Function prediction and network analysis to investigate the response of microbial communities to a single environmental factor. Freshw. Ecol.35, 271–289. doi: 10.1080/02705060.2020.1791269
66
Zhao J. Liu X. Hou L. Xu G. Guan F. Zhang W. et al . (2024). The seed endophytic microbe Microbacterium testaceum M15 enhances the cold tolerance and growth of rice (Oryza sativa L.). Microbiol. Res.289, 127908. doi: 10.1016/j.micres.2024.127908
67
Zhou X. Li R. Shen H. Yang L. (2023). Effect of exogenous plant growth regulators and rejuvenation measures on the endogenous hormone and enzyme activity responses of acer mono maxim in cuttage rooting. Int. J. Mol. Sci.25, 24. doi: 10.3390/ijms241511883
68
Zhou Y. Su J. Dong Y. He Z. Wang Y. Chen S. et al . (2024). Buddleoside-rich Chrysanthemum indicum L. extract modulates macrophage-mediated inflammation to prevent metabolic syndrome induced by unhealthy diet. BMC Complement Med. Ther.24, 315. doi: 10.1186/s12906-024-04583-2
Summary
Keywords
Massilia consociata , chrysanthemum cuttings rooting, soluble protein, endophytic bacteria, functional prediction
Citation
Lu C, Dong H, Jiao Y, Muhammad M, Yang Y, Zhang J, Liu R and Cao Y (2025) Study on the rooting promotion of chrysanthemum cuttings by Massilia consociata KC 009. Front. Plant Sci. 16:1685038. doi: 10.3389/fpls.2025.1685038
Received
13 August 2025
Accepted
22 September 2025
Published
08 October 2025
Volume
16 - 2025
Edited by
Ajay Kumar, Amity University, India
Reviewed by
Loekas – Soesanto, Jenderal Soedirman University, Indonesia
Zhibo Jiang, North Minzu University, China
Updates
Copyright
© 2025 Lu, Dong, Jiao, Muhammad, Yang, Zhang, Liu and Cao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanru Cao, yanrucao3@aliyun.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.