- 1Department of Agronomy, School of Agriculture, Lovely Professional University, Phagwara, Punjab, India
- 2Department of Soil Science, Indian Council of Agricultural Research (ICAR)-Directorate of Onion and Garlic Research Pune, Maharashtra, India
Introduction: Rainfall variability during the monsoon season poses a major challenge to onion production, especially due to waterlogging stress in clay loam soils. Saturated conditions reduce soil aeration, disrupt microbial activity and nutrient transformations, and impair nutrient uptake and crop performance.
Methods: To investigate these effects, a field experiment was conducted under a split-plot design with flatbed layout to assess changes in soil physical properties, microbial activity, nutrient availability, and their combined effects on nutrient uptake and bulb yield in eight onion genotypes (two tolerant and six sensitive).
Results and Discussion: Waterlogging increased bulk density by 5.30% and reduced infiltration rate by 76.5% compared to control. At 50 days after transplanting (DAT), microbial biomass carbon declined by 67.6%, while dehydrogenase, urease, acid phosphatase, and alkaline phosphatase activities declined by 55.8%, 33.9%, 33.9%, and 10.2%, respectively. Available macronutrients (N, P, K, S, Ca, Mg) and micronutrients (Fe, Cu, Mn, B) were significantly reduced at 55 DAT compared to 45 DAT. These changes led to reduced nutrient uptake and yield across genotypes. However, tolerant genotypes Accession 1666 and Bhima Dark Red (BDR) Selection exhibited better tolerance, with only 21.7% and 18.1% yield reductions, compared to 41.6–64.8% in sensitive types. Raised bed planting further improved performance of tolerant genotypes under waterlogged conditions.
Conclusion: These findings highlight genotypic selection and raised-bed cultivation as effective strategies to mitigate waterlogging stress in monsoonal onion systems.
Introduction
India contributes nearly 20% of global onion production, with the Deccan Plateau accounting for 50% of the national output. Onions are cultivated in three seasons—monsoon, late monsoon, and winter. Of these, monsoon and late monsoon crops are more severely affected by waterlogging compared to winter crops (Gedam et al., 2022; Thangasamy et al., 2023), resulting in market instability and frequent price fluctuations (Ghodke et al., 2018; Gedam et al., 2023). The increasing frequency and intensity of rainfall due to climate change have further exacerbated waterlogging issues, limiting onion productivity (Thangasamy et al., 2023). The shallow root system of onions makes them particularly susceptible to waterlogged conditions (Ghodke et al., 2018). Yield losses under moderate waterlogging range from 25–30%, while severe waterlogging can result in complete crop failure (Dubey et al., 2021). Gedam et al. (2022) reported that excessive rainfall could reduce bulb yield by 50–70%, posing a major challenge to onion cultivation during monsoon, especially with increasingly erratic monsoon patterns and unseasonal rains.
In India, floods affect an average of 7.5 million hectares of agricultural land annually (Sarkar, 2022), and from 2015 to 2021, extreme weather events such as floods have damaged approximately 33.9 million hectares of cropped area. Waterlogging has significant implications for soil health and nutrient dynamics (Pais et al., 2023). It increases bulk density and reduces total pore volume, thereby affecting water-holding capacity (Todisco et al., 2022). Saturated soil conditions reduce aeration, alter gas exchange and composition, and suppress microbial and plant growth (Wang et al., 2025). Additionally, particle dispersion under waterlogged conditions leads to surface crusting and reduced infiltration (Awedat et al., 2021). The resultant oxygen deficiency in the root zone triggers a shift from aerobic to anaerobic processes, thereby altering the soil’s redox potential and influencing nutrient transformation and availability (Ben-Noah and Friedman, 2018).
This anaerobic condition shift in soil conditions affects the solubility and availability of essential nutrients. Waterlogging increases the availability of iron (Fe) and manganese (Mn), potentially leading to toxicity, while reducing nitrogen (N) availability through denitrification and leaching (Tyagi et al., 2024). Anaerobic conditions can temporarily increase phosphorus (P) availability due to the reduction of Fe and Mn oxides (Salunkhe et al., 2022), while prolonged waterlogging often results in P deficiency due to restricted root growth and nutrient uptake (Yang et al., 2024).
Soil enzymatic activities serve as important indicators of soil fertility and health (Wang et al., 2023). Waterlogging significantly affects enzymes such as dehydrogenase, urease, alkaline phosphatase, and acid phosphatase (Gu et al., 2019), all of which play key roles in nutrient cycling and organic matter decomposition (Daunoras et al., 2024). Moreover, waterlogging leads to major shifts in the microbial community—declining aerobic populations and increasing anaerobic and facultative anaerobic organisms (Randle‐Boggis et al., 2018), with profound implications for soil nutrient cycling and overall health (Salunkhe et al., 2022). Oxygen deprivation in the root zone impairs root function, nutrient, and water uptake (Gedam et al., 2022), contributing to nutrient stress that ultimately affects onion bulb yield and quality (Salunkhe et al., 2022; Gedam et al., 2023).
Despite a general understanding of plant responses to waterlogging, limited studies have explored how waterlogging affects soil physical properties—particularly infiltration rate and bulk density—as well as nutrient availability and microbial activities in onion crops. Understanding these changes is essential for developing strategies to improve resilience under such conditions. Therefore, the present study was formulated with the hypotheses: 1. Waterlogging will increase bulk density while decreasing infiltration rate, microbial activity, nutrient transformation, and nutrient uptake. 2. Reduced nutrient availability and uptake will negatively affect plant growth, bulb size, and yield in onion genotypes. To test these hypotheses, the present study was undertaken to assess the effects of waterlogging on infiltration rate, bulk density, nutrient availability, and microbial activities. The study also evaluated how these changes influenced nutrient uptake, plant growth, and bulb yield in onion genotypes under waterlogged conditions. This study integrates assessments of soil physical properties, microbial activities, and nutrient availability to better understand the responses of onion genotypes to waterlogging stress.
Materials and methods
Experimental site
The field experiment was conducted at the Indian Council of Agricultural Research–Directorate of Onion and Garlic Research (ICAR–DOGR) experimental farm in Pune, Maharashtra, India (18.32° N, 73.51° E, 645 MSL) during the monsoon season (August to November 2023). The site experiences a tropical dry and humid climate. It receives an average annual rainfall of 820 mm, with approximately 99% occurring during the southwest monsoon season (June to October). The mean temperature during the experimental period ranged from 9.7 °C to 41.2 °C. The soil at the experimental site was classified as Typic Haplustepts, characterized as clay loam with a pH of 7.63, electrical conductivity of 0.25 dS m-¹, and soil organic carbon content of 8.6 mg kg-¹. The soil is low in N while high in P and K and contains adequate levels of S and micronutrients.
Experimental details
A split-plot design was adopted with eight onion genotypes evaluated under two conditions: control (well-watered) and waterlogged. The genotypes included four sensitive (Bhima Super, Bhima Shubra, Bhima Red, and Bhima Raj) and four tolerant (Accession 1666, Accession 1630, Bhima Dark red (BDR) Selection, and W 355) genotypes. Each treatment was replicated three times. Flat beds measuring 2 m × 3 m were prepared, and well-decomposed farmyard manure was incorporated at 5 t ha-¹. A basal dose comprising 16% of the recommended N, along with full doses of P, K, and S, was applied before transplanting. The nutrient sources included complex fertilizer (10:26:26), urea, muriate of potash, and bentonite sulfur. Pre-emergence herbicide oxyfluorfen (23% emulsifiable concentrates) was applied 15 minutes before transplanting, followed by irrigation to ensure uniform distribution. Forty-five-day-old seedlings were transplanted at 15 cm × 10 cm spacing, with a population of 400 plants per plot. Both control and waterlogged plots were irrigated uniformly until 45 days after transplanting (DAT). Waterlogging treatment was initiated at 45 DAT and continued for 10 days until 55 DAT. During this period, daily flooding in the morning was followed by sprinkler irrigation until 6:00 PM to simulate rainfall and maintain waterlogged conditions. After 55 DAT, normal irrigation resumed in all plots until harvest.
All agronomic practices and plant protection measures were followed as per ICAR–DOGR recommendations, particularly for managing anthracnose disease. Onion bulbs were harvested during the first week of November 2023. Equatorial and polar diameters of bulbs were measured using a vernier caliper and expressed in millimeters. Bulbs were separated from the foliage, and their weight was recorded and expressed in kg per plot. Plant height and number of leaves were recorded from ten tagged plants per plot at 45, 55, 75, and 90 DAT. Leaf area was measured using the third fully developed leaf from the top during the same intervals.
A parallel experiment using the same set of genotypes was conducted on raised beds, following identical agronomic practices as the flatbed system. During the waterlogging period, soil in the raised bed system remained saturated throughout the day without surface inundation. Yield parameters were recorded at harvest.
Soil physical properties
Infiltration rate and bulk density were measured in both the waterlogged and control plots after the onion harvest. The soil infiltration rate was determined using the double-ring infiltrometer method (Bouwer, 1986). Bulk density was measured using the core method (Blake and Hartge, 1986).
Soil enzymatic activity
Moist soil samples collected from each treatment were used to assess enzyme activities. Dehydrogenase activity was measured by the triphenyl tetrazolium chloride reduction method and expressed as µg triphenyl formazan (TPF) g-¹ dry soil h-¹ (Casida et al., 1964). Urease activity was determined by incubating 5 g of soil with 5 mL of 10 mg urea solution at 37 °C for 5 hours, followed by colorimetric estimation at 527 nm using diacetyl monoxime–thiosemicarbazide (DAM-TSC) reagents (Bremner and Douglas, 1971). Acid and alkaline phosphatase activities were determined by incubating 1 g of soil with p-nitrophenyl phosphate substrate in pH-adjusted buffers (pH 6.5 for acid and pH 11 for alkaline phosphatase) at 37 °C for 1 hour. Reactions were stopped with CaCl2-NaOH solution, and p-nitrophenol released was measured at 400 nm (Tabatabai and Bremner, 1969). Soil microbial biomass carbon (SMBC) was estimated using the chloroform fumigation-extraction method (Jenkinson and Powlson, 1976). Fresh soil (10 g) was fumigated with ethanol-free chloroform for 24 hours in a vacuum desiccator. Both fumigated and unfumigated samples were extracted with 0.5 M K2SO4, filtered, and analyzed for organic carbon via dichromate oxidation. Results were expressed as µg C g-¹ dry soil.
Soil sampling and analysis
Soil samples were collected from a 0–15 cm depth at five time points: pre-planting, 45, 55, and 75 days after transplanting (DAT), and at harvest, using a post-hole auger. Samples were collected between plant rows, air-dried, sieved (2 mm), and analyzed. Soil pH and electrical conductivity were measured using pH meter and conductivity bridge. Soil organic carbon (SOC) was estimated using the Walkley and Black (1934) wet oxidation method. Available N was analyzed using the alkaline permanganate method (Subbiah and Asija, 1956), P by the Olsen method (Olsen et al., 1954), and K using 1N ammonium acetate extraction (Jackson, 1967). Available S was extracted with 0.15% CaCl2 and analyzed using the turbidimetric method (Chesnin and Yien, 1951). Ca and Mg were determined by EDTA titration (Jackson, 1967), and micronutrients via atomic absorption spectrophotometry. DTPA extractable Fe, Mn, Zn, and Cu were extracted and analyzed in an atomic absorption spectrometer (Lindsay and Norvell, 1978). Available boron was extracted using hot water and determined in a spectrophotometer using the azomethine-H method (John et al., 1975).
Plant sampling and analysis
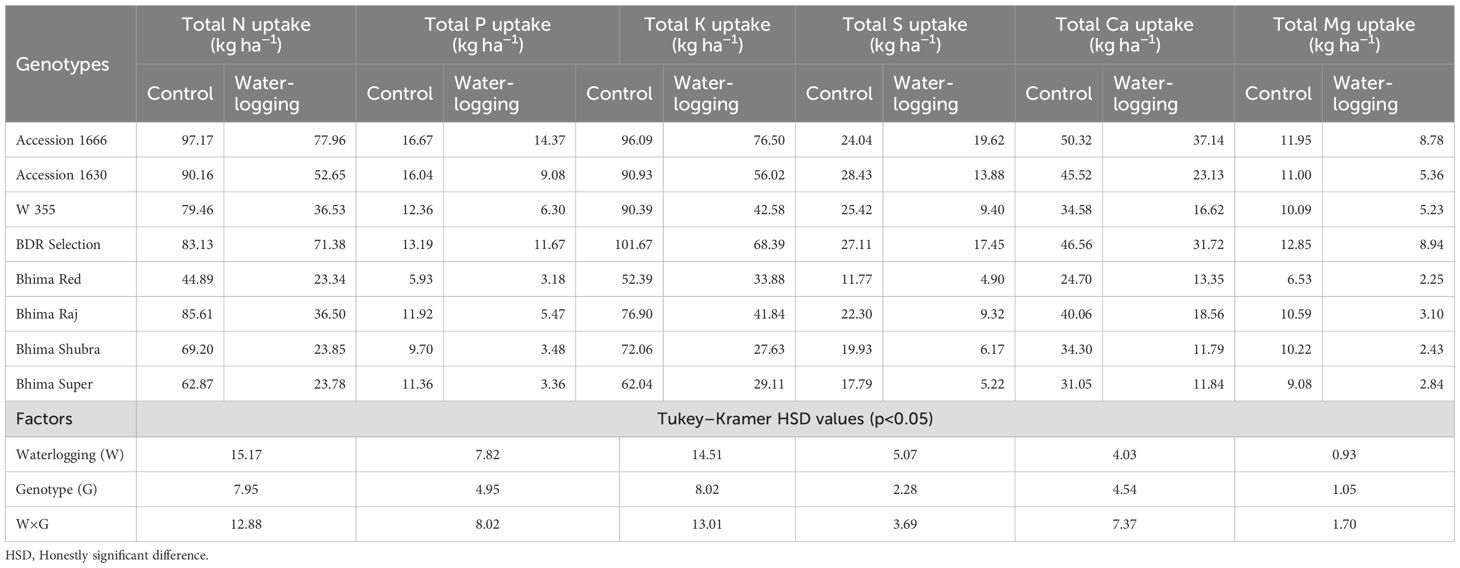
Whole plant samples (five per treatment) were collected at 45 DAT, 55 DAT, and at harvest. Samples were washed, rinsed with distilled water, and separated into bulbs and leaves. They were chopped, air-dried, and subsequently oven-dried at 60 °C until a constant weight was reached. The dry weight of bulbs and leaves was recorded, and the samples were ground and sieved (2.0 mm) for nutrient analysis. Total N was determined using the micro-Kjeldahl method. For P, K, and S estimation, 0.5 g of the ground sample was digested using a di-acid mixture, filtered through Whatman No. 40 paper, and analyzed. P was measured using the ammonium vanado-molybdate method, K with a flame photometer, and S via the turbidimetric method (Jackson, 1967). Ca and Mg concentrations were estimated using the modified EDTA titration method, while micronutrients (Fe, Zn, Mn, Cu) were analyzed using atomic absorption spectrophotometry (Lindsay and Norvell, 1978). Nutrient uptake was calculated by multiplying nutrient concentration with dry matter yield and expressed as kg ha-¹ for macronutrients and g ha-¹ for micronutrients.
Statistical analysis
The field experiment was conducted in a split-plot design with two treatments (control and waterlogging) as main plots and genotypes as sub-plots, replicated four times. Data were analyzed using Python (version 3.11; Python Software Foundation, 2023). Two-way ANOVA and mixed-model analyses were performed using the statsmodels library, considering treatment as a fixed factor and replication as a random effect. Treatment means were compared using the Tukey–Kramer Honestly Significant Difference (HSD) test at p ≤ 0.05 to identify significant differences among treatments and genotypes. Infiltration rate and bulk density data were analyzed using an independent two-sample t-test (p < 0.05, two-tailed).
Multivariate analyses were conducted to assess the interrelationships among soil physical, biological, and chemical parameters, nutrient uptake, and yield under control and waterlogged conditions. The first redundancy analysis (RDA) was performed using the scikit-learn library, incorporating soil physical properties (bulk density, infiltration rate), soil microbial activities (dehydrogenase enzyme, soil microbial biomass carbon, and urease activity), and available nutrient parameters as explanatory variables, with nutrient uptake and yield as response variables (n = 8). This analysis aimed to identify indicative relationships between soil conditions and crop responses under waterlogging, acknowledging the limitations associated with a small sample size. The second RDA was conducted using nutrient uptake and yield data (n = 64) to examine the direct associations between nutrient uptake efficiency and productivity under waterlogging stress. RDA biplots were generated using the matplotlib package in Python to illustrate the separation between control and waterlogged treatments.
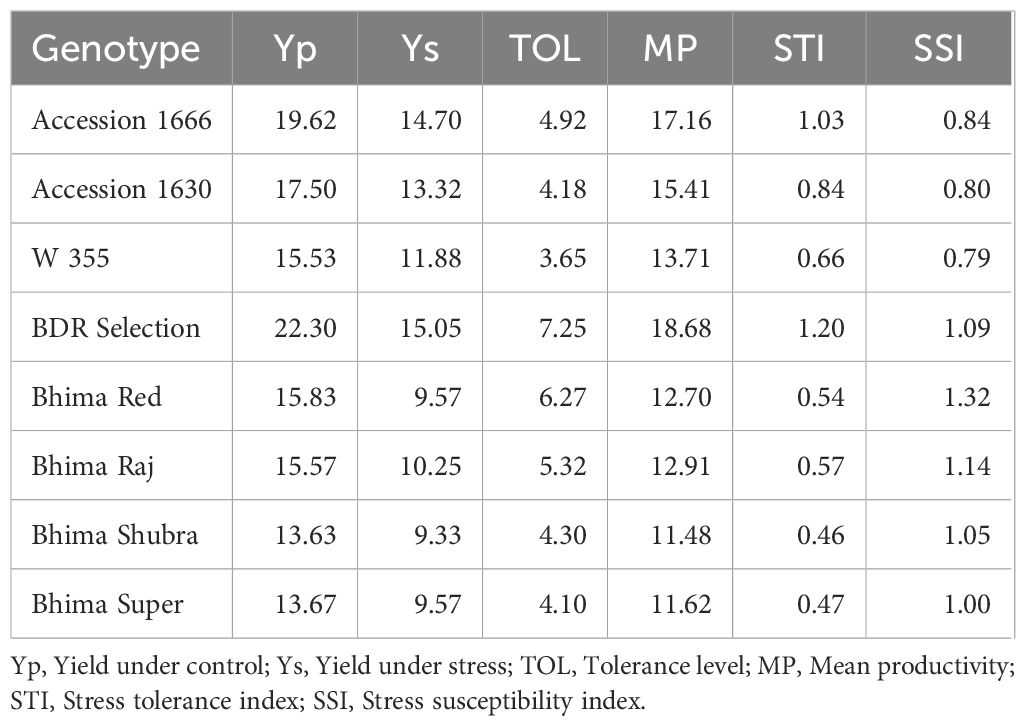
Pearson’s correlation analysis (two-tailed) was also performed to examine linear associations among soil, microbial, nutrient uptake, and yield traits. Additionally, tolerance level, stress tolerance index, and stress sustainability index were computed to quantify genotype performance under waterlogged and control conditions, using standard equations based on yield under waterlogging stress and the control.
Results
Soil physical and microbial properties
Infiltration rate and bulk density exhibited significant differences between the waterlogged and control plots. Waterlogging significantly increased soil bulk density from 1.32 Mg m-³ in the control to 1.39 Mg m-³ (p = 0.001), representing a 5.30% increase compared to the control plots (Figure 1). Waterlogging treatments recorded significantly lower infiltration rate and cumulative infiltration compared to the control (p < 0.0001). Cumulative infiltration under waterlogged conditions was reduced by 76.5% relative to the control (Figure 2). Moreover, infiltration rate stabilized within one hour in the waterlogged plots, whereas it continued to increase and stabilized only after four hours in the control (Figure 2). Additionally, waterlogging significantly influenced SMBC, dehydrogenase, urease, and both acid and alkaline phosphatase activities across growth stages (Table 1). At 50 DAT, waterlogged conditions led to reductions of 67.6% in SMBC, 55.8% in dehydrogenase, 33.9% in urease, 33.9% in acid phosphatase, and 10.2% in alkaline phosphatase compared to their respective values recorded at 45 DAT (pre-treatment). Post waterlogging stress at 55 DAT, SMBC, acid phosphatase, and alkaline phosphatase activities recovered slightly, showing reductions of 25.0%, 12.3%, and 12.3%, respectively, relative to their pre-treatment levels. Notably, dehydrogenase and urease activities at 55 DAT remained statistically similar to the levels observed at 50 DAT, indicating minimal recovery. In contrast, the control plots showed only minor changes in soil microbial biomass carbon, dehydrogenase, urease, and acid and alkaline phosphatase activities at 50 and 55 DAT compared to their respective pre-treatment values. Bulk density showed a significant negative correlation with soil microbial activities, infiltration rate, soil available nutrients, nutrient uptake, and bulb yield, whereas cumulative infiltration exhibited a significant positive correlation with these parameters (Supplementary Table 1). Soil enzyme activities—dehydrogenase, acid phosphatase, and urease—also showed significant positive correlations with nutrient availability, nutrient uptake, and bulb yield.
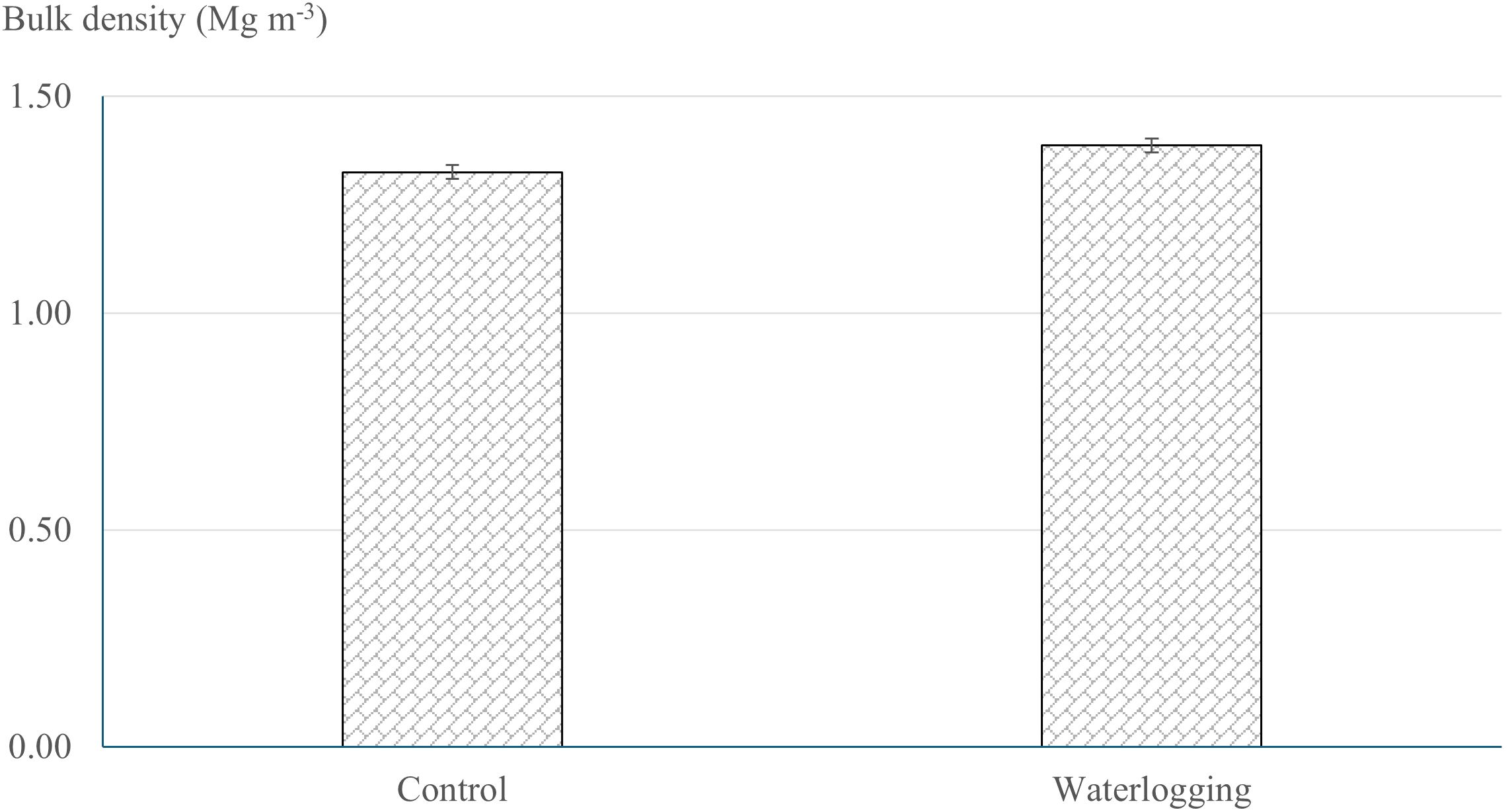
Figure 1. Effect of waterlogging on soil bulk density. Soil bulk density was measured at harvest under control and waterlogged conditions. Values represent mean ± SE.
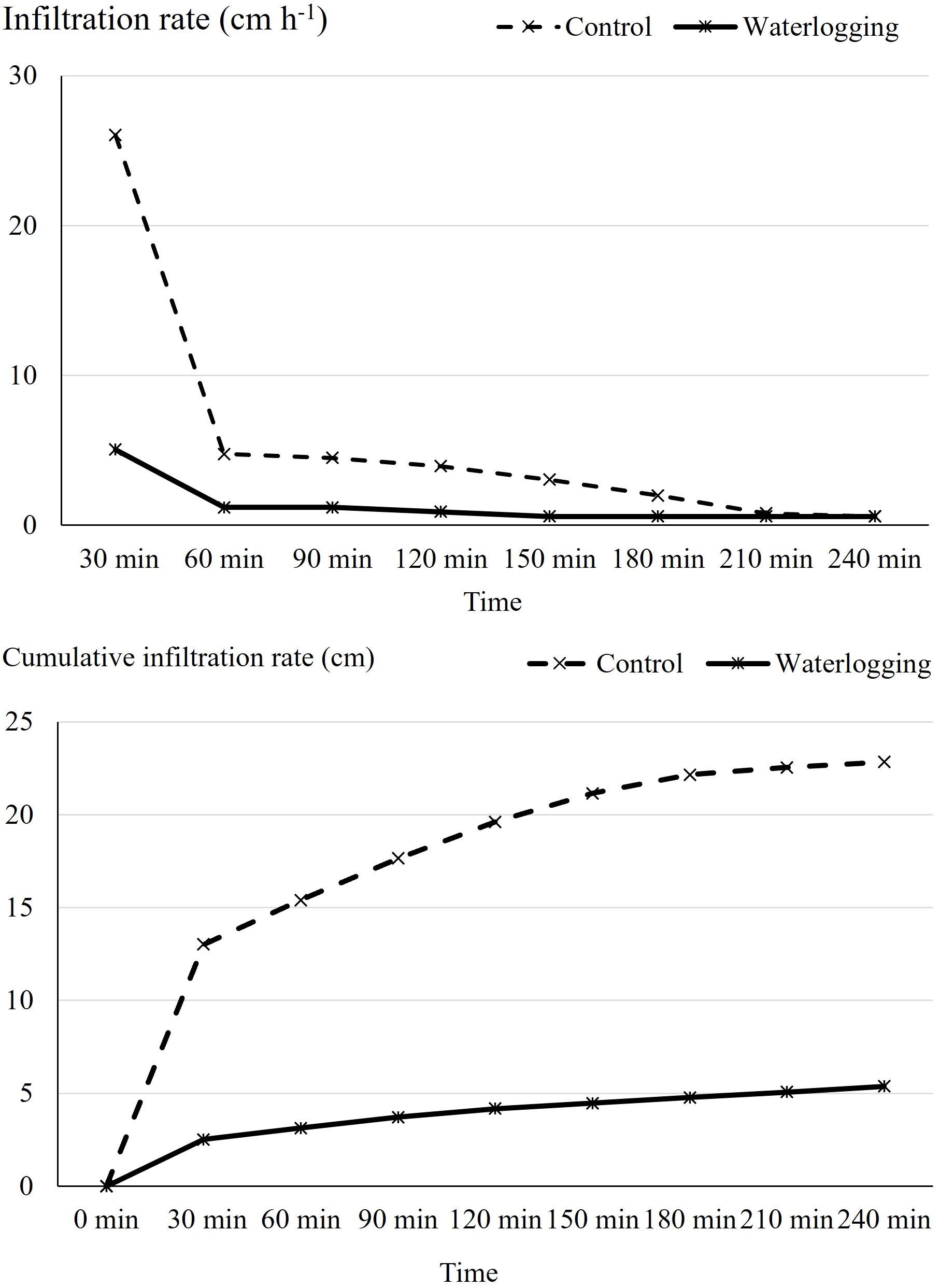
Figure 2. Effect of waterlogging on cumulative infiltration and infiltration rate. Cumulative infiltration and infiltration rate were assessed at harvest under control and waterlogged conditions. Values represent mean ± SE.
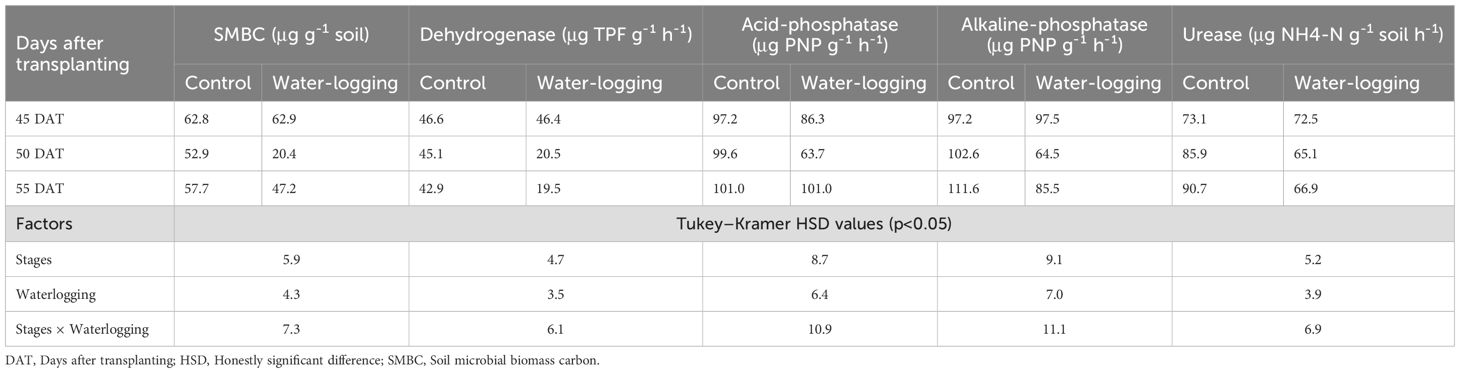
Table 1. Effect of waterlogging on SMBC, and soil enzymatic activity at pre- and post-waterlogging stages.
Soil nutrient available status
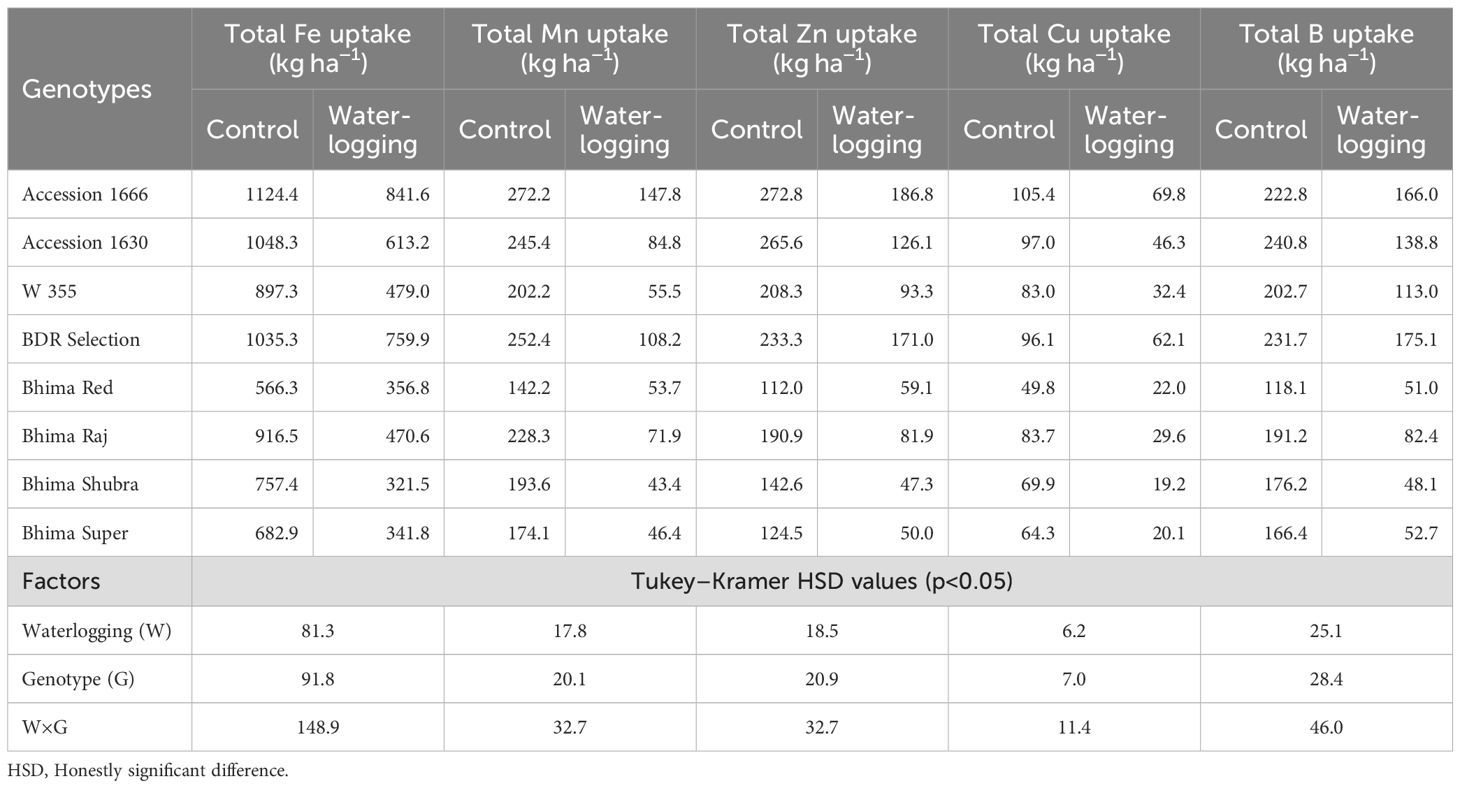
Treatments, waterlogging stages, and their interactions significantly affected soil-available macro- (Table 2) and micronutrient concentrations (Table 3). At 55 DAT, waterlogging markedly reduced the concentrations of available N, P, K, S, Ca, Mg, Fe, Cu, Mn, and B compared to their respective pre-treatment values recorded at 45 DAT. Specifically, the reductions were 33.4% for N, 27.8% for P, 16.5% for K, 22.7% for S, 12.5% for Ca, and 35.9% for Mg. Among the micronutrients, Fe, Mn, Cu, and B decreased by 31.8%, 32.9%, 22.1%, and 34.0%, respectively, compared to 45 DAT. Notably, the concentration of available Zn increased by 68.9% at 55 DAT relative to its pre-treatment level. During the recovery period at 75 DAT, nutrient availability improved compared to 55 DAT. Despite this, most nutrient concentrations remained substantially lower than the pre-treatment levels recorded at 45 DAT. In contrast, available Zn and B levels at 75 DAT were 65.6% and 27.7% higher, respectively, than their pre-treatment values. Meanwhile, the available nutrient concentrations in the control plots showed little change throughout the sampling period compared to the pre-treatment values. Waterlogging also led to reductions in soil pH, EC, and SOC by 7.9%, 8.0%, and 3.2%, respectively, compared to pre-treatment levels. By 75 DAT, soil pH, EC, and SOC had increased by 10.0%, 4.3%, and 30.6%, respectively, compared to their values at 55 DAT, indicating partial recovery of soil chemical properties. Soil available nutrients, both macro- and micronutrients (except zinc), showed significant positive correlations with nutrient uptake and bulb yield.
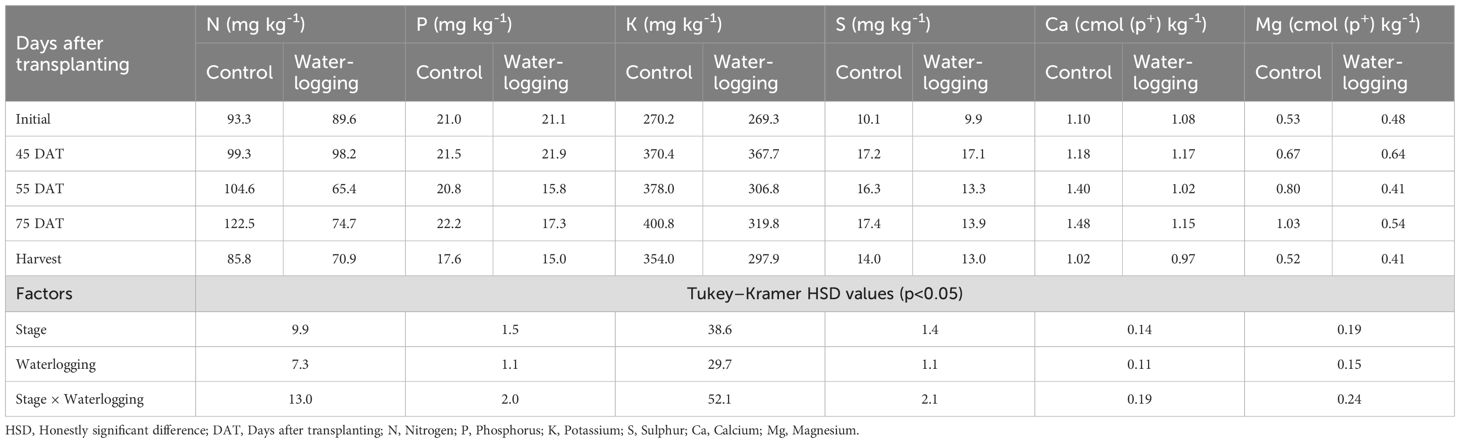
Table 2. Effect of waterlogging on soil available macronutrient availability at pre- and post-harvest stages.
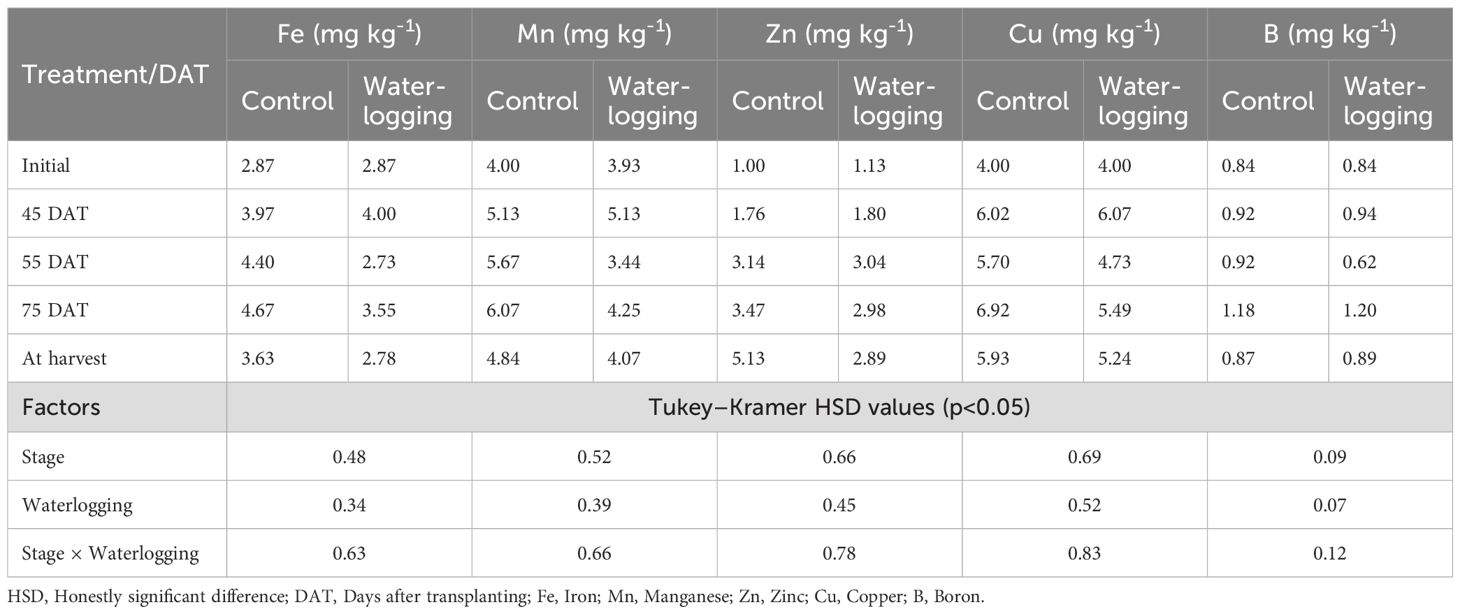
Table 3. Effect of waterlogging on soil micronutrient availability at pre- and post-waterlogging stages.
Total nutrient uptake
Genotypes, waterlogging, and their interaction significantly influenced both macro- (Table 4) and micronutrient uptake (Table 5). Under waterlogged conditions, Accession 1666 and BDR selection exhibited comparatively lower reductions in nutrient uptake than the other genotypes, relative to their respective control plants. Accession 1666 showed reductions of 19.8% in N, 13.8% in P, 20.4% in K, and 18.3% in S. For micronutrients, this genotype recorded decreases of 25.2% in Fe, 45.7% in Mn, 31.5% in Zn, 33.8% in Cu, and 25.5% in B. Similarly, BDR Selection exhibited reductions of 14.1% in N, 11.4% in P, 32.7% in K, and 35.4% in S. Its micronutrient uptake declined by 26.6% for Fe, 57.1% for Mn, 26.7% for Zn, 35.4% for Cu, and 24.4% for B. In contrast, the sensitive genotypes experienced markedly higher reductions in nutrient uptake under waterlogging stress. Their uptake of macronutrients decreased by 41.6%–65.5% for N, 43.1%–70.2% for P, 35.3%–61.7% for K, and 51.1%–70.8% for S. Micronutrient uptake in these genotypes declined by 41.5%–57.6% for Fe and 62.3%–77.6% for Mn. Additionally, Zn, Cu, and B uptake decreased by 47.2%–66.8%, 52.3%–72.7%, and 42.4%–72.7%, respectively, compared to their control plants.
Plant growth parameters
Waterlogging, genotypes, and their interaction significantly influenced plant growth parameters, including plant height (Supplementary Table 3), number of leaves (Supplementary Table 4), and total leaf area (Supplementary Table 5) across all growth stages. Under waterlogged conditions, Accession 1666 exhibited reductions of 7.2% in plant height, 22.7% in number of leaves, and 3.8% in total leaf area at 55 DAT compared to its control plants. BDR Selection showed slightly higher reductions, with plant height, number of leaves, and total leaf area declining by 22.2%, 21.9%, and 6.6%, respectively. Accession 1630 and W-355 also demonstrated relatively lesser reductions than those observed in the sensitive genotypes. In contrast, the sensitive genotypes under waterlogging stress showed significantly higher reductions, with plant height reduced by 31.4%–38.9%, number of leaves by 51.5%–58.3%, and total leaf area by 55.4%–60.8% compared to their respective control plants. All genotypes showed signs of recovery after the cessation of waterlogging, with improvements observed in plant height, leaf number, and leaf area by 75 DAT. By 90 DAT, tolerant genotypes (Accession 1666, BDR Selection, Accession 1630, and W-355) showed a slight decline in leaf number and area, indicating a slowdown in vegetative growth. By comparison, the sensitive genotypes continued to recover, with increases of 118.8%–122.6% in leaf number and 120.4%–147.7% in leaf area compared to 55 DAT.
Bulb size and yield
Both waterlogging and genotypes significantly affected bulb size, marketable bulb yield, and total bulb yield, whereas their interaction was not significant under either flatbed or raised bed conditions (Table 6). Tolerant genotypes such as Accession 1666 and BDR Selection experienced comparatively lower reductions in bulb size, yield, and dry matter under waterlogged conditions. Among these, Accession 1666 showed the least reduction, with a 21.7% decrease in bulb yield and minor reductions in equatorial (16.7%) and polar (4.8%) bulb diameters. BDR Selection also exhibited moderate losses in bulb yield (18.1%) and associated parameters. Accession 1666 showed a high stress tolerance index (STI) (1.03) and low stress susceptibility index (SSI) (<1), indicating strong stability under stress, whereas BDR Selection achieved the highest STI (1.20) and exhibited a slightly higher SSI (>1) (Table 6). Other genotypes recorded the lowest stressed yields and highest susceptibility indices, confirming their sensitivity to waterlogging under both flatbed and raised bed system. Additionally, a parallel experiment using a raised bed system with sprinkler irrigation to simulate rainfall resulted in higher marketable bulb yields in the tolerant genotypes, Accession 1666 (16.0 t/ha) and BDR Selection (18.0 t/ha) compared to the sensitive genotypes (Supplementary Table 6). Accession 1666 and BDR Selection exhibited yield reductions of 29.2% and 31.4%, respectively, under the raised bed system relative to their control plots. However, when compared to the flatbed system under waterlogged conditions, the raised bed system increased yields by 40.6% for Accession 1666 and 41.3% for BDR Selection. In contrast, the sensitive genotypes suffered substantial yield losses, with reductions ranging from 41.6% to 64.8% under flatbed waterlogged conditions. Even in the raised bed system, these genotypes showed significant yield declines of 46.4% to 52.1% compared to their respective controls.
Redundancy analysis
Exploratory RDA explained a total variation of 100%, with RDA1 accounting for 98.1% and RDA2 for 1.9% of the total variance. RDA1 distinctly separated waterlogged and control treatments, showing strong positive associations with infiltration rate and available macronutrients and micronutrients, as well as nutrient uptake and yield parameters, and a negative association with bulk density and acid phosphatase activity (Figure 3). RDA2 explained a smaller proportion of the variation, mainly associated with soil biological parameters. A separate RDA explaining 94.5% of the variation in nutrient uptake–yield relationships revealed that RDA1 (88.5%) clearly separated waterlogged and control treatments (Figure 4). Control plots showed strong association with N, P, K, Ca, Zn, and Cu uptake, along with higher bulb diameter and yield traits. RDA2 captured minor within-treatment variation, mainly linked to Mg and Mn uptake.
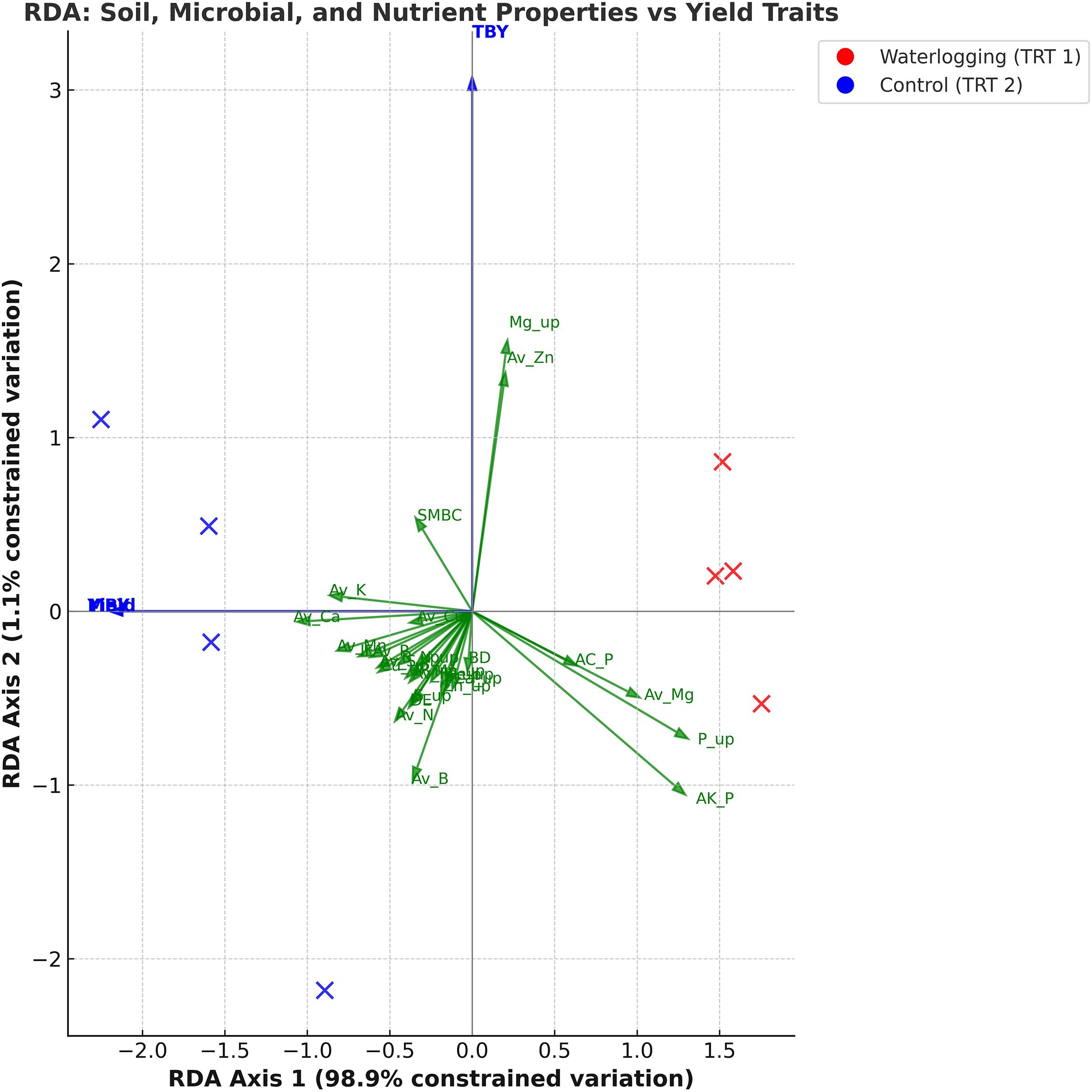
Figure 3. Redundancy analysis biplot illustrating the association of soil, microbial, and nutrient properties with onion yield under waterlogging and control conditions (n=8).
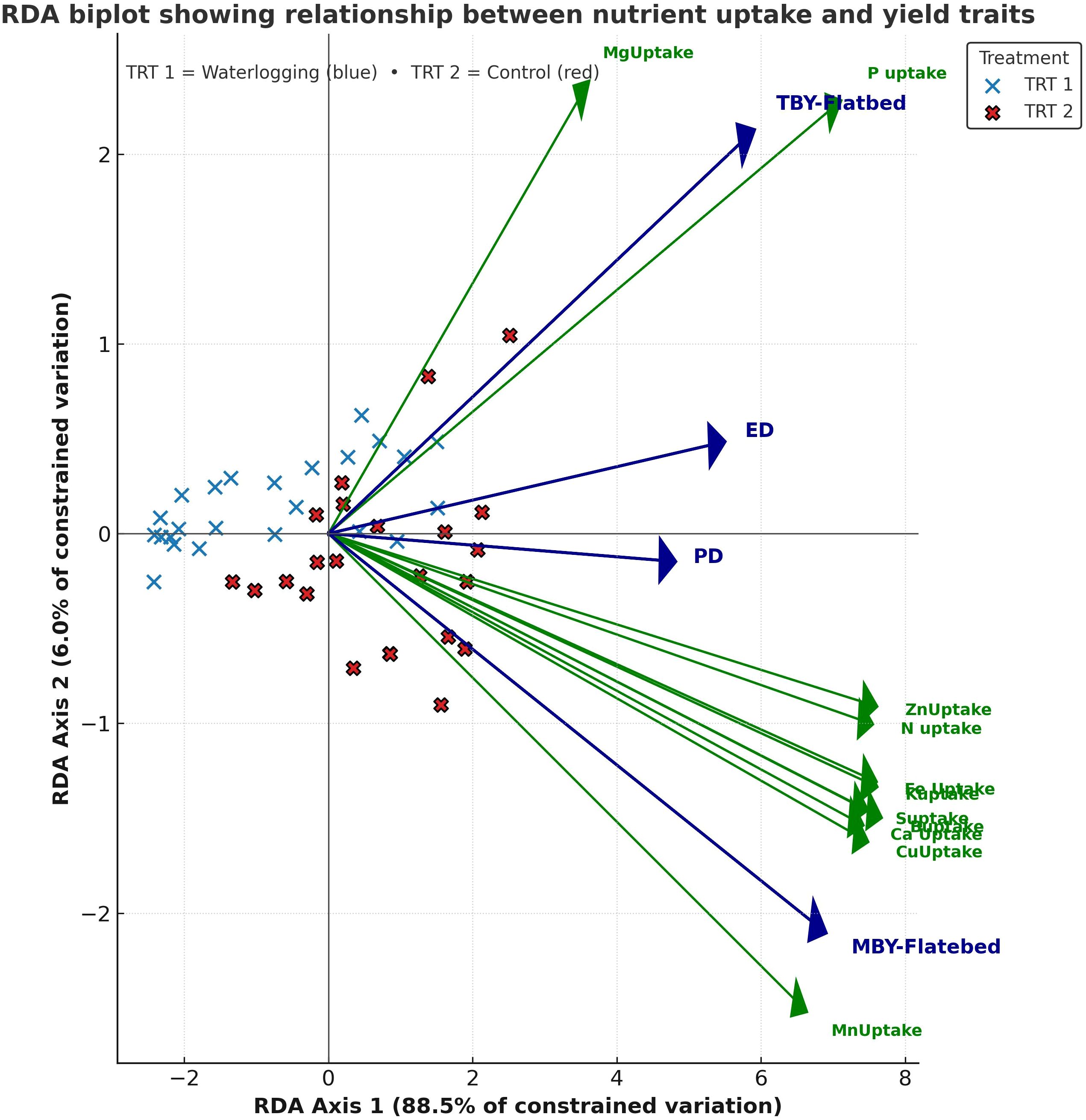
Figure 4. Redundancy analysis showing nutrient uptake–yield trait relationships under waterlogging and control conditions (n=64).
Discussion
Rainfall plays a vital role in maintaining adequate water supply for domestic and agricultural needs (Biswas et al., 2025). A normal distribution of rainfall is crucial for groundwater recharge, domestic use, and sustaining crop productivity (Woldearegay et al., 2024). However, rainfall deviations—either deficient or excessive—can severely affect agricultural yields, especially for sensitive crops like onion (Thimmegowda et al., 2025). Although drought stress is often manageable through supplemental irrigation (Thangasamy et al., 2023), waterlogging—especially during the monsoon season—remains a more severe and complex challenge (Wakchaure et al., 2023). Prolonged soil saturation creates anaerobic conditions that limit oxygen availability (Gedam et al., 2022), alter microbial communities, and disrupt nutrient transformations, thereby impairing nutrient availability and plant uptake (Gedam et al., 2023). To address these effects, this field experiment examined the effect of waterlogging on soil physical, chemical, and biological properties, as well as nutrient availability, uptake, growth, and yield performance of tolerant and sensitive onion genotypes under waterlogged conditions in clay loam soils.
Waterlogging effects on soil physical and microbial properties
Waterlogging increased bulk density and significantly reduced infiltration in the clay loam soil, primarily due to pore clogging by dispersed clay particles (Wu et al., 2017). A similar increase in bulk density, along with reduced infiltration rate, hydraulic conductivity, and permeability, as well as increased runoff, was observed by Kaur et al. (2019). The RDA biplot clearly separated control and waterlogged plots, with infiltration rate and soil available nutrients showing strong positive loadings and bulk density negative loadings, confirming that soil compaction and poor aeration were the principal factors influencing nutrient dynamics and yield variation.
Oxygen deficiency under waterlogging significantly affected soil biological properties (Manik et al., 2019). Reduced SMBC and dehydrogenase activity observed at 55 DAT in the present study indicated a shift in the microbial community from predominantly aerobic to anaerobic organisms (Tyagi et al., 2024), consistent with earlier findings by Ponnamperuma (1984) and Martínez-Arias et al. (2022). In this study, SMBC, enzyme activities, and nutrient availability together accounted for nearly all the explained variation, confirming the central role of microbes in nutrient transformation and mineralization processes. The strong positive correlation between microbial activity and nutrient availability under control conditions suggests that microbially mediated nutrient cycling was the key mechanism maintaining soil fertility. In contrast, reduced microbial efficiency under waterlogging led to declines in N and P mineralization and nutrient uptake, as also supported by the RDA results. These findings warrant further validation through multi-season and multi-location datasets to establish the robustness of these mechanisms.
Redox effect on nutrient availability
Unlike studies with continuous submergence, our experimental design involved daytime-only flooding (8 AM to 6 PM), resulting in fluctuating redox conditions. These periodic oxidizing phases during the night may have contributed to limited microbial Fe and Mn reduction, leading to the re-oxidation and precipitation of Fe²+ and Mn²+ into unavailable forms. Additionally, repeated flooding and drainage cycles may have facilitated leaching/run off of micronutrients from the surface soil (Sao et al., 2023; Rupngam and Messiga, 2024). This may partially explain the observed decline in available Fe, Mn, Cu, and B in the present study. In contrast, a unique increase in Zn availability was observed under waterlogged conditions, possibly due to organic matter–mediated chelation and transient redox mobilization—an observation not previously reported in onions.
During the recovery phase (55–75 DAT), partial restoration of microbial activity and nutrient availability indicated redox stabilization (Wang et al., 2017; Rupngam et al., 2024). The post-waterlogging increase in micronutrient concentrations may be attributed to adaptive chemical equilibria that regulate and stabilize micronutrient solubility (Sathi et al., 2022). However, these nutrient levels remained lower than in control soils, reflecting incomplete microbial and chemical recovery and restricted oxygen diffusion in the fine-textured clay loam (Rupngam et al., 2024). The positive association among microbial biomass, nutrient uptake, and yield further confirmed the critical role of root–soil–microbe interactions in stress adaptation (Tyagi et al., 2024; Muhammad et al., 2024).
Waterlogging effects on nutrient uptake, and plant growth and yield
Waterlogging significantly decreased both macro- and micronutrient uptake, as evidenced by the strong positive correlations observed between soil nutrient availability and plant uptake. This decline could be attributed to reduced soil aeration, altered nutrient solubility, and impaired root functions under waterlogged conditions (Zhang et al., 2025). However, tolerant genotypes like Accession 1666 and BDR Selection, which recorded higher stressed yields, exhibited higher STI and lower to moderate SSI values, indicating their superior performance and partial resilience under waterlogged conditions. Their relatively higher nutrient uptake under waterlogged conditions resulted in improved physiological adaptations such as enhanced aerenchyma formation, which enables internal oxygen transport to submerged roots (Pan et al., 2021; Zhang et al., 2025). The clear separation of treatments in the RDA biplot indicates that nutrient uptake and yield traits were strongly influenced by waterlogging. Under control conditions, enhanced uptake of N, P, K, S, and secondary and micronutrients (Ca, Mg, Fe, Zn, Cu, and B) contributed to improved metabolic processes, bulb size and yield, reflecting better root activity and translocation efficiency (Leon et al., 2021). In contrast, waterlogging markedly reduced nutrient uptake due to restricted root respiration and impaired active transport processes, leading to poor nutrient translocation and smaller bulbs. The strong positive associations of yield components (TBY, MBY, ED, and PD) with nutrient uptake confirm that nutrient uptake directly associated with bulb size and yield under normal control conditions. Elevated Ca, Mg, and S levels associated with bulb size indicate their role in maintaining bulb firmness, structural integrity, and quality under waterlogging stress. Although tolerant genotypes performed relatively better under waterlogged conditions, they still exhibited reduced growth and yield compared to controls.
Conversely, the weak or negative associations under waterlogging emphasize that oxygen deficiency disrupts rhizosphere nutrient dynamics, root metabolism, nutrient uptake and exhibited poor recovery (Ponnamperuma, 1984; Ma et al., 2022). This resulted in reduced leaf area, plant height, and biomass (Olorunwa et al., 2022). The decline in bulb size and yield across all genotypes confirmed waterlogging’s negative effects on onion growth and development (Thangasamy et al., 2023). However, the ability of tolerant genotypes to sustain dry matter and bulb yield through improved nutrient uptake and plant growth compared to sensitive genotypes in the present study highlight their suitability for waterlogged environments (Garcia et al., 2020). These findings highlight the potential of selecting genotypes with waterlogging stress tolerance mechanisms to support sustainable agricultural practices in areas increasingly affected by climate variability.
The flatbed system effectively simulated monsoonal waterlogging and enabled to identify onion genotypes capable of performing under waterlogged conditions. Since waterlogging is a major constraint during the monsoon, its negative effect can be effectively managed by integrating tolerant genotypes with raised bed planting methods (Thangasamy et al., 2023). In a parallel experiment, tolerant genotypes grown on raised beds produced higher yields than those on flatbeds under waterlogged conditions, indicating that this integrated approach has significant potential to enhance productivity in waterlogged environments (Manik et al., 2019). Although monsoon yields were lower overall, higher seasonal market prices and reduced labor requirements make monsoon onion cultivation economically viable when stress-tolerant genotypes are used.
Conclusion
This study demonstrated that monsoonal waterlogging significantly altered soil physical and biological properties by increasing bulk density and reducing infiltration and microbial activity. These changes impaired nutrient availability and uptake, resulting in reduced growth, bulb size, and yield in onion crops. However, tolerant genotypes such as Accession 1666 and BDR Selection maintained higher nutrient uptake, produced higher yield, and showed enhanced recovery and adaptability under waterlogging stress than sensitive genotypes. Moreover, these genotypes achieved higher yields under raised-bed planting systems than in flatbed systems, indicating that integrating tolerant genotypes with raised-bed cultivation effectively reduces yield loss and enhances productivity under monsoon conditions. These findings highlight the potential of combining genetic tolerance with adaptive management practices to strengthen climate resilience and sustainability in waterlogging-prone environments. Future research will focus on establishing mechanistic linkages among soil physical properties, microbial activity, nutrient availability, nutrient uptake, and yield using larger and more representative sample sets. It will also aim to elucidate how waterlogging alters soil structure, nutrient dynamics, root anatomy, and microbial community composition under both flatbed and raised-bed systems, to comprehensively define the integrated soil–microbe–root–plant interactions governing onion tolerance to waterlogging.
Data availability statement
The onion genotypes used in this study are maintained at the ICAR-Directorate of Onion and Garlic Research (ICAR-DOGR), Pune. All authors have institutional authorization to use these materials for research purposes. Accession 1666 (IC645764; INGR22082) is a registered germplasm with the ICAR-National Bureau of Plant Genetic Resources (ICAR-NBPGR), New Delhi. Seeds of this accession have been deposited at both ICAR-DOGR and ICAR-NBPGR to ensure long-term conservation and availability. Voucher specimens for Accession 1666 and BDR selection have been deposited at ICAR-DOGR, and the material is publicly accessible upon request as per ICAR-NBPGR guidelines. Taxonomic identification as Allium cepa L. was confirmed by subject matter experts at ICAR-DOGR. The genotypes used in the present study were identified by Gedam et al. (2022), and their registration has been published (Gedam et al., 2024).
Author contributions
AP: Conceptualization, Formal analysis, Data curation, Methodology, Writing – review & editing, Investigation, Writing – original draft, Software. SP: Supervision, Writing – review & editing, Formal analysis, Software, Methodology, Investigation, Data curation. MP: Formal analysis, Methodology, Writing – original draft, Data curation, Investigation. PM: Investigation, Formal analysis, Methodology, Writing – original draft. KG: Formal analysis, Writing – original draft, Investigation, Methodology. TA: Validation, Resources, Conceptualization, Data curation, Project administration, Formal analysis, Software, Methodology, Writing – review & editing, Funding acquisition, Supervision, Writing – original draft, Investigation. VM: Resources, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Research was supported by the Indian Council of Agricultural Research, Department of Agricultural Research and Education, Government of India.
Acknowledgments
The first author is grateful to Lovely Professional University, Punjab, for granting permission to conduct this research at ICAR-DOGR, Pune, and to the Director of ICAR-DOGR, Pune, for providing the necessary resources, facilities, and support throughout the course of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the authors used CHATGPT in order to improve readability and language of the work. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1692450/full#supplementary-material
References
Awedat, A. M., Zhu, Y., Bennett, J. M., and Raine, S. R. (2021). The impact of clay dispersion and migration on soil hydraulic conductivity and pore networks. Geoderma 404, 115297. doi: 10.1016/j.geoderma.2021.115297
Ben-Noah, I. and Friedman, S. P. (2018). Review and evaluation of root respiration and of natural and agricultural processes of soil aeration. Vadose Zone J. 17, 170119. doi: 10.2136/vzj2017.06.0119
Biswas, A., Sarkar, S., Das, S., Dutta, S., Choudhury, M. R., Giri, A., et al. (2025). Water scarcity: A global hindrance to sustainable development and agricultural production – A critical review of the impacts and adaptation strategies. Cambridge Prisms Water 3:e4. doi: 10.1017/wat.2024.16
Blake, G. R. and Hartge, K. H. (1986). “Bulk density,” in Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods, 2nd ed. Ed. Klute, A. (ASA and SSSA, Madison, WI), 363–375. doi: 10.2136/sssabookser5.1.2ed.c13
Bouwer, H. (1986). “Intake rate: Cylinder infiltrometer,” in Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods, 2nd ed. Ed. Klute, A. (ASA and SSSA, Madison, WI), 825–843.
Bremner, J. M. and Douglas, L. A. (1971). Inhibition of urease activity in soils. Soil Biol. Biochem. 3, 297–307. doi: 10.1016/0038-0717(71)90039-3
Casida, L. E., Klein, D. A., and Santoro, T. (1964). Soil dehydrogenase activity. Soil Sci. 98, 371–376. doi: 10.1097/00010694-196412000-00004
Chesnin, L. and Yien, C. H. (1951). Turbidimetric determination of available sulphur. Proc. Soil Sci. Soc. America 15, 149–151. doi: 10.2136/sssaj1951.036159950015000C0032x
Daunoras, J., Kačergius, A., and Gudiukaitė, R. (2024). Role of soil microbiota enzymes in soil health and activity changes depending on climate change and the type of soil ecosystem. Biology 13, 85. doi: 10.3390/biology13020085
Dubey, S., Kuruwanshi, V. B., Bhagat, K. P., and Ghodke, P. H. (2021). Impact of excess moisture in onion genotypes (Allium cepa L.) under climate change scenario. Int. J. Curr. Microbiol. Appl. Sci. 10, 166–175. doi: 10.20546/ijcmas.2021.1003.023
Garcia, N., da-Silva, C. J., Cocco, K. L. T., Pomagualli, D., de Oliveira, F. K., da Silva, J. V. L., et al. (2020). Waterlogging tolerance of five soybean genotypes through different physiological and biochemical mechanisms. Environ. Exp. Bot. 172, 103975. doi: 10.1016/j.envexpbot.2020.103975
Gedam, P. A., Khandagale, K., Shirsat, D., Thangasamy, A., Kulkarni, O., Kulkarni, A., et al. (2023). Elucidating the molecular responses to waterlogging stress in onion (Allium cepa L.) leaf by comparative transcriptome profiling. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1150909
Gedam, P. A., Shirsat, D. V., Thangasamy, A., Ghosh, S., Gawande, S. J., Mahajan, V., et al. (2022). Screening of onion (Allium cepa L.) genotypes for waterlogging tolerance. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.727262
Gedam, P. A., Thangasamy, A., Gupta, A. J., Mahajan, V., Khade, Y. P., Gawande, S. J., et al. (2024). ACC. 1666 (IC645764; INGR22082), an onion (Allium cepa) germplasm for water logging tolerance resulting in lower yield reduction (16.9%). Indian J. Plant Genet. Resour. 37, 536–537.
Ghodke, P. H., Shirsat, D. V., Thangasamy, A., Mahajan, V., Salunkhe, V. N., Khade, Y., et al. (2018). Effect of water logging stress at specific growth stages in onion crop. Int. J. Curr. Microbiol. Appl. Sci. 7, 3438–3448. doi: 10.20546/ijcmas.2018.701.405
Gu, C., Zhang, S., Han, P., Hu, X., Xie, L., Li, Y., et al. (2019). Soil enzyme activity in soils subjected to flooding and the effect on nitrogen and phosphorus uptake by oilseed rape. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00368
Jenkinson, D. S. and Powlson, D. S. (1976). The effects of biocidal treatments on metabolism in soil—I. Fumigation with chloroform. Soil Biol. Biochem. 8, 167–177. doi: 10.1016/0038-0717(76)90001-8
John, M. K., Chuah, H. H., and Neufeld, J. H. (1975). Application of improved Azomethine-H method to the determination of boron in soils and plants. Analytical Lett. 8, 559–568. doi: 10.1080/00032717508058240
Kaur, G., Singh, G., Motavalli, P. P., Nelson, K. A., Orlowski, J. M., and Golden, B. R. (2019). Impacts and management strategies for crop production in waterlogged or flooded soils: A review. Agron. J. 112, 147–162. doi: 10.1002/agj2.20093
León, J., Castillo, M. C., and Gayubas, B. (2021). The hypoxia–reoxygenation stress in plants. J. Exp. Bot. 72, 5841–5856. doi: 10.1093/jxb/eraa591
Lindsay, W. L. and Norvell, W. A. (1978). Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. America J. 42, 421–428. doi: 10.2136/sssaj1978.03615995004200030009x
Ma, J., Rukh, G., Ruan, Z., Xie, X., Ye, Z., and Liu, D. (2022). Effects of hypoxia stress on growth, root respiration, and metabolism of Phyllostachys praecox. Life (Basel) 12, 808. doi: 10.3390/life12060808
Manik, S. M. N., Pengilley, G., Dean, G., Field, B., Shabala, S., and Zhou, M. (2019). Soil and crop management practices to minimize the impact of waterlogging on crop productivity. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00140
Martínez-Arias, C., Witzell, J., Solla, A., Martin, J. A., and Rodríguez-Calcerrada, J. (2022). Beneficial and pathogenic plant–microbe interactions during flooding stress. Plant Cell Environ. 45, 2875–2897. doi: 10.1111/pce.14403
Muhammad, A., Kong, X., Zheng, S., Bai, N., Li, L., Khan, M. H. U., et al. (2024). Exploring plant–microbe interactions in adapting to abiotic stress under climate change: A review. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1482739
Olorunwa, O. J., Adhikari, B., Brazel, S., Popescu, S. C., Popescu, G. V., Shi, A., et al. (2022). Waterlogging during the reproductive growth stage causes physiological and biochemical modifications in the leaves of cowpea (Vigna unguiculata L.) genotypes with contrasting tolerance. Plant Physiol. Biochem. 190, 133–144. doi: 10.1016/j.plaphy.2022.08.018
Olsen, S. R., Cole, C. V., and Watanabe, F. S. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate (USDA Circular No. 939) (Washington, D.C: U.S. Government Printing Office).
Pais, I. P., Moreira, R., Semedo, J. N., Ramalho, J. C., Lidon, F. C., Coutinho, J., et al. (2023). Wheat crop under waterlogging: Potential soil and plant effects. Plants 12, 149. doi: 10.3390/plants12010149
Pan, J., Sharif, R., Xu, X., and Chen, X. (2021). Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.627331
Ponnamperuma, F. N. (1984). “Effects of flooding on soils,” in Flooding and plant growth. Ed. Kozlowski, T. T. (New York, USA: Academic Press), 9–45. doi: 10.1016/B978-0-12-424120-6.50007-9
Python Software Foundation (2023). Python Language Reference, version 3.11 (Wilmington, DE, USA: Python Software Foundation). Available online at: https://www.python.org/ (Accessed October 3, 2025).
Randle-Boggis, R. J., Ashton, P. D., and Helgason, T. (2018). Increasing flooding frequency alters soil microbial communities and functions under laboratory conditions. Microbiol. Open 7, e00548. doi: 10.1002/mbo3.548
Rupngam, T. and Messiga, A. J. (2024). Unraveling the interactions between flooding dynamics and agricultural productivity in a changing climate. Sustainability 16, 6141. doi: 10.3390/su16146141
Rupngam, T., Messiga, A. J., and Karam, A. (2024). Soil enzyme activities in heavily manured and waterlogged soil cultivated with ryegrass (Lolium multiflorum). Can. J. Soil Sci. 104, 146–155. doi: 10.1139/cjss-2023-0097
Salunkhe, V. N., Gedam, P., Pradhan, A., Gaikwad, B., Kale, R., and Gawande, S. (2022). Concurrent waterlogging and anthracnose-twister disease in rainy-season onions (Allium cepa): Impact and management. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1063472
Sao, S., Praise, S., and Watanabe, T. (2023). Effect of flood duration on water extractable dissolved organic matter in flood plain soils: A laboratory investigation. Geoderma 432, 116392. doi: 10.1016/j.geoderma.2023.116392
Sarkar, S. (2022). Drought and flood dynamics of Godavari basin, India: A geospatial perspective. Arabian J. Geosciences 15, 772. doi: 10.1007/s12517-022-10041-5
Sathi, K. S., Masud, A. A. C., Anee, T. I., and Hasanuzzaman, M. (2022). “Soybean plants under waterlogging stress: Responses and adaptation mechanisms,” in Managing plant production under changing environment. Ed. Hasanuzzaman, M. (Singapore: Springer), 97–123. doi: 10.1007/978-981-16-5059-8_5
Subbiah, B. V. and Asija, G. L. (1956). A rapid procedure for the estimation of available nitrogen in soils. Curr. Sci. 25, 259–260.
Tabatabai, M. A. and Bremner, J. M. (1969). Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1, 301–307. doi: 10.1016/0038-0717(69)90012-1
Thangasamy, A., Gedam, P. A., Soumia, P. S., Ghosh, S., Karuppaiah, V., Mahajan, V., et al. (2023). Inter-annual variability in monsoon rainfall and its distribution on plant growth and yield of kharif onion in tropical region of India. Curr. Sci. 124, 713–721. doi: 10.18520/cs/v124/i6/713-721
Thimmegowda, M. N., Manjunatha, M. H., Huggi, L., Bal, S. K., Chandran, M. A. S., Soumya, D. V., et al. (2025). Impact of rainfall variability on major crops using the deficient rainfall impact parameter (DRIP): A case study over Karnataka, India. Meteorological Appl. 32 (2), e70032. doi: 10.1002/met.70032
Todisco, F., Vergni, L., Vinci, A., and Torri, D. (2022). Infiltration and bulk density dynamics with simulated rainfall sequences. Catena 218, 106542. doi: 10.1016/j.catena.2022.106542
Tyagi, A., Ali, S., Mir, R. A., Sharma, S., Arpita, K., Almalki, M. A., et al. (2024). Uncovering the effect of waterlogging stress on plant microbiome and disease development: current knowledge and future perspectives. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1407789
Wakchaure, G. C., Minhas, P. S., Kumar, S., Khapte, P. S., Rane, J., and Reddy, K. S. (2023). Bulb productivity and quality of monsoon onion (Allium cepa L.) as affected by transient waterlogging at different growth stages and its alleviation with plant growth regulators. Agric. Water Manage. 278, 108136. doi: 10.1016/j.agwat.2023.108136
Walkley, A. and Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
Wang, C., Dor, M., Kravchenko, A., Guber, A., and Dorodnikov, M. (2025). Suppression of hydrolytic enzyme activities by short-term aeration of periodically anoxic soils: Evidence from upland ecosystems. Geoderma 457, 117278. doi: 10.1016/j.geoderma.2025.117278
Wang, L., Hamel, C., Lu, P., Wang, J., Sun, D., Wang, Y., et al. (2023). Using enzyme activities as an indicator of soil fertility in grassland—An academic dilemma. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1175946
Wang, Y., Uchida, Y., Shimomura, Y., Akiyama, H., and Masahito, H. (2017). Responses of denitrifying bacterial communities to short-term waterlogging of soils. Sci. Rep. 7, 803. doi: 10.1038/s41598-017-00953-8
Woldearegay, K., Grum, B., Hessel, R., van Steenbergen, F., Fleskens, L., Yazew, E., et al. (2024). Watershed management, groundwater recharge and drought resilience: An integrated approach to adapt to rainfall variability in northern Ethiopia. Int. Soil Water Conserv. Res. 12, 663–683. doi: 10.1016/j.iswcr.2023.08.009
Wu, X., Wei, Y., Wang, J., Cai, C., Deng, Y., and Xia, J. (2017). RUSLE erodibility of heavy-textured soils as affected by soil type, erosional degradation, and rainfall intensity: A field simulation. Land Degradation Dev. 29, 1021–1030. doi: 10.1002/ldr.2864
Yang, R., Wang, C., Yang, Y., Harrison, M. T., Zhou, M., and Liu, K. (2024). Implications of soil waterlogging for crop quality: A meta-analysis. Eur. J. Agron. 161, 127395. doi: 10.1016/j.eja.2024.127395
Keywords: monsoon onion, tolerant genotypes, Bhima Dark Red, Accession 1666, dehydrogenase, redundancy analysis
Citation: Pawar AR, Patil SS, Patil MB, Mahadule PA, Gade KA, Arunachalam T and Mahajan VB (2025) Effects of waterlogging on microbial activity, soil nutrient availability, nutrient uptake, and yield of tolerant and sensitive onion genotypes. Front. Plant Sci. 16:1692450. doi: 10.3389/fpls.2025.1692450
Received: 25 August 2025; Accepted: 20 October 2025;
Published: 13 November 2025.
Edited by:
Diaa Abd El Moneim, Arish University, EgyptReviewed by:
Kohtaro Iseki, Japan International Research Center for Agricultural Sciences (JIRCAS), JapanDr. Krishna Kaushik, Meerut Institute of Technology, India
Copyright © 2025 Pawar, Patil, Patil, Mahadule, Gade, Arunachalam and Mahajan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thangasamy Arunachalam, YXN0c2FteUB5YWhvby5jby5pbg==
 Amol R. Pawar
Amol R. Pawar Sushant Sukumar Patil1
Sushant Sukumar Patil1 Mayur B. Patil
Mayur B. Patil Payal A. Mahadule
Payal A. Mahadule Komal Anil Gade
Komal Anil Gade Thangasamy Arunachalam
Thangasamy Arunachalam Vijay B. Mahajan
Vijay B. Mahajan