- 1Department of Plant Production, College of Agriculture and Food, Qassim University, Buraydah, Qassim, Saudi Arabia
- 2Department of Environment and Natural Resources, College of Agriculture and Food, Qassim University, Buraydah, Saudi Arabia
Organic agriculture is recognized for its sustainability, although it typically yields less than conventional systems. This study evaluated seven elite wheat genotypes (Triticum aestivum L.) over five years in a randomized complete block design with three replications, under both organic and conventional fertilization conditions. Integrated analyses using the AMMI model and GGE biplot revealed the significant effects of genotype, environment, and their interactions. The AMMI analysis showed that genotype IC8 achieved the highest mean yield (1.868 t ha-1) and the lowest AMMI stability value (ASV=0.474). This low ASV suggests high stability, indicating broad adaptability, especially under organic conditions. In contrast, Sids_12 (mean = 1.492 t ha-1; ASV=2.017) and LOCAL (mean = 1.304 t ha-1) exhibited great instability and specific adaptation. GGE biplot analysis explained 75.46% of the total variation (PC1 = 57.09%, PC2 = 18.37%), further confirming IC8’s stable performance across both systems while identifying P5 and IC17 as particularly responsive under conventional fertilization. These findings provide a basis for selecting wheat genotypes that balance high yield and stability, informing breeding strategies for sustainable crop production in both organic and conventional systems.
Introduction
Organic agriculture is gaining popularity due to its sustainability and environmental benefits, such as improved soil health, lower chemical inputs, and increased biodiversity (Mäder et al., 2002; Reganold and Wachter, 2016). However, one of the most significant issues connected to organic farming is that it is generally less productive than conventional systems (Ponisio et al., 2015; Seufert et al., 2012). Wheat (Triticum aestivum L.) is a staple crop and the primary source of calories for a vast section of the world’s population. It is also critical to food security. As a result, increasing wheat yield while remaining sustainable, is crucial for fulfilling future food demands (FAO, 2020; Ritchie and Roser, 2022). Understanding genotype × environment (G×E) interactions is crucial for designing cultivars that thrive in various agricultural contexts (Kumar et al., 2021; Yan and Tinker, 2006). Recent improvements in statistical models, such as the additive main effects and multiplicative interaction (AMMI) model and the genotype and genotype-by-environment interaction (GGE) biplot analysis, have provided useful tools for understanding these complicated interactions. These methodologies allow breeders to not only discover high-yielding genotypes, but also to assess their stability under changing environmental conditions (Singh et al., 2019; Yan et al., 2007). Organic and inorganic fertilization approaches can influence Se and Zn concentrations, potentially affecting crop nutrition and human health (Alloway, 2008; Lyons et al., 2017). Abd-Elmoniem et al. (2025); Al-Ghumaiz et al. (2020) evaluated the impacts of fertilization methods on wheat mineral composition, focusing on the significance of fertilization strategies in increasing nutrient density.
Few studies have comprehensively evaluated the long-term stability of wheat (Triticum aestivum L.) genotypes under various fertilization regimes. Research assessing genotype performance across multiple years, particularly under conventional and organic fertilization systems, remains limited. Such studies are essential for stable, high-yielding cultivars capable of adapting to diverse agronomic conditions. The objectives of this study were to (i) evaluate the patterns and magnitude of G×E interactions affecting yield stability of seven elite wheat genotypes across five successive growing seasons (2019-2023) under conventional and organic fertilization systems, and (ii) identify wheat genotypes that combine highly productivity with stability across years and fertilization methods to support breeding programs for sustainable wheat production in arid and semi-arid environments.
Materials and methods
Experimental site
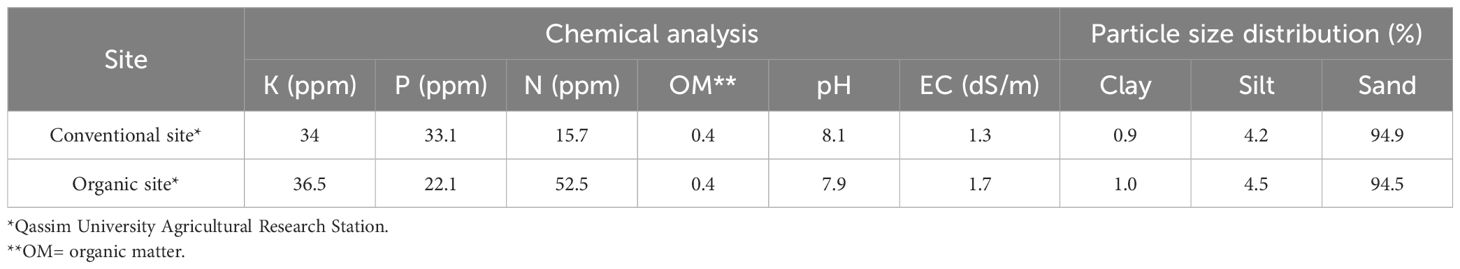
Field experiments were conducted over five consecutive growing seasons from 2019 to 2023 at the Agricultural Research and Experimental Station of Qassim University, Buraydah, Saudi Arabia (26°18′28″ N, 43°46′ E). The site is characterized by sand texture with low salinity (electrical conductivity, EC=1.5 dSm-1), low organic matter content (0.4%), and an alkaline pH of 8.1. The irrigation water used throughout the study had an EC of 1.7 dSm-1 and a pH of 7.8.
Experimental design and treatments
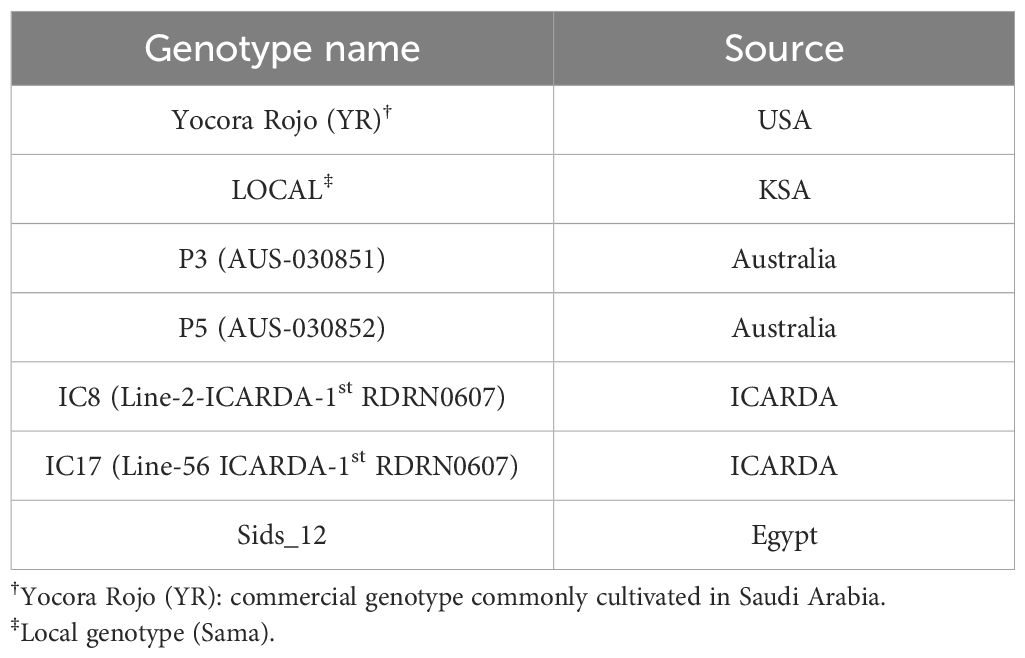
The study evaluated the performance of seven wheat (Triticum aestivum L.) genotypes (Table 1) under two fertilization regimes: organic (O) and inorganic (N). Thus, experimental factors were:
● Factor 1 (Genotypes): Seven wheat (Triticum aestivum L.) genotypes.
● Factor 2 (Fertilization): Two regimes-organic (O) and inorganic (N).
Trials were laid out in a randomized complete block design (RCBD) with three blocks. Each block contained 14 plots (7 genotypes × 2 fertilization regimes). Each plot measured 3 m² (1.5m × 2.0m) and comprised 10 rows spaced 25cm apart. Wheat seeds were sown at a seeding rate of 45kg ha-1.
The planting dates for the five seasons were as follows: December 2019, November 30, 2020, December 2021, December 2022, and December 2023.
Fertilizer application
Fertilizer application followed soil test recommendations. In the inorganic treatment, urea, diammonium phosphate (DAP), and potassium sulfate were applied at rates of 124kg N ha-1, 92kg P2O5 ha-1, and 57kg K2O ha-1, respectively. For the organic treatment, well-decomposed cow manure was applied at a rate of 10t ha-1 one month before sowing. The organic amendment was analyzed according to the methodology of (Sparks et al., 2020), revealing an N:P:K ratio of 0.5:0.21:0.5.
Environmental and soil conditions
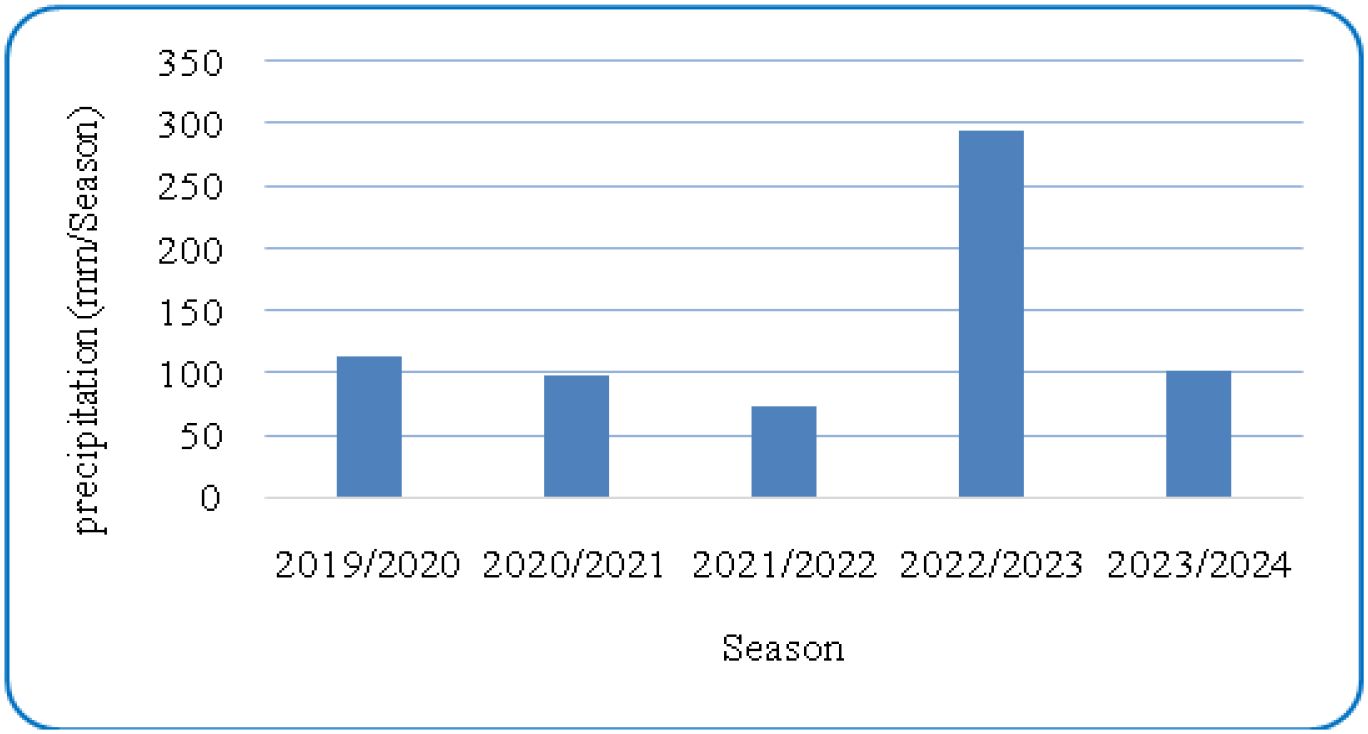
Figures 1 and 2 present the meteorological data for the study period (2019–2023), including maximum and minimum temperatures and total rainfall. A summary of environmental conditions across different years is provided in Table 2. Soil physical and chemical properties of the experimental site are detailed in Table 3.
Figure 2. Average of the maximum and minimum temperature from 2019 to 2023 in the experimental field consistently across environments.
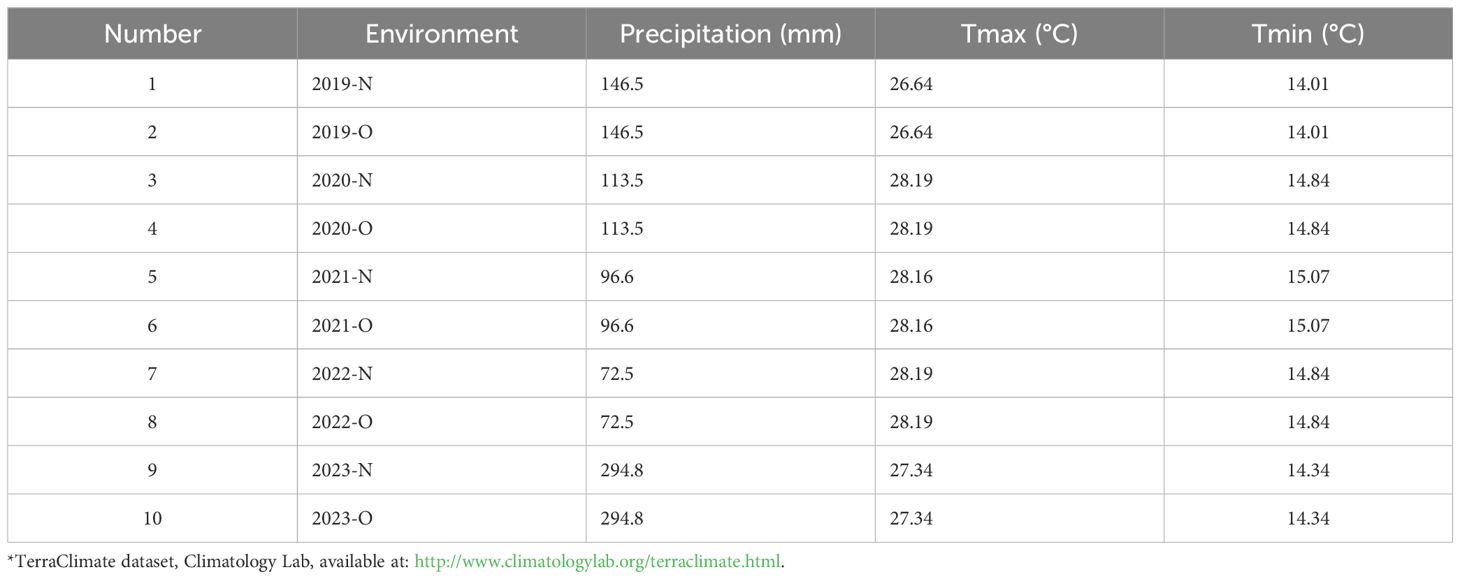
Table 2. Environmental conditions* across different years and fertilizations (organic (O) and inorganic (N) based on precipitation and temperature variables.
Statistical analysis
The AMMI model (Gauch, 1992, 2013) was used to calculate the yield stability of the elite spring wheat genotypes under organic and inorganic fertilizations. Principal component analysis (PCA) was used to determine the multiplicative effects of GEI after the AMMI model first fitted the additive effects for the primary influences of the genotypes (G) and environment (E). Biplot graphs were used to display the AMMI findings. According to (Nowosad et al., 2016), the AMMI model can be stated using the formula below:
where N is the number of PCA axes kept in the adjusted model; λn is the eigen value of the PCA axis, n; γgn is the genotype score for the PCA axis, n; δen is the score eigenvector for the PCA axis, n; yge is the trait mean of a genotype g in environment e; μ is the grand mean; αg is the mean genotype deviation; βe is the mean environment deviation; and Qge is the residual, including the AMMI noise and pooled experimental error.
AMMI analysis was carried out using the “AMMI” function from the GenStat statistical software, version 19 (GenStat, 2019). The AMMI stability value (ASV) coefficient (Purchase et al., 2000) was used to evaluate the genotype stability. The more stable the genotype in the conditions under study, the lower the ASV. Each genotype was assigned a genotype selection index (GSI), which is the total of the ASV and yield stability index (YSI) ranking positions (Farshadfar, 2008).
A GGE biplot was constructed using principal component analysis, wherein the scores of genotypes were multiplied by the corresponding environmental scores to produce a two-dimensional representation (Yan and Wu, 2008). This approach enabled a simultaneous assessment of both the main effects of genotypes and their interactions with the environment.
Results
Genotype stability based on AMMI analysis
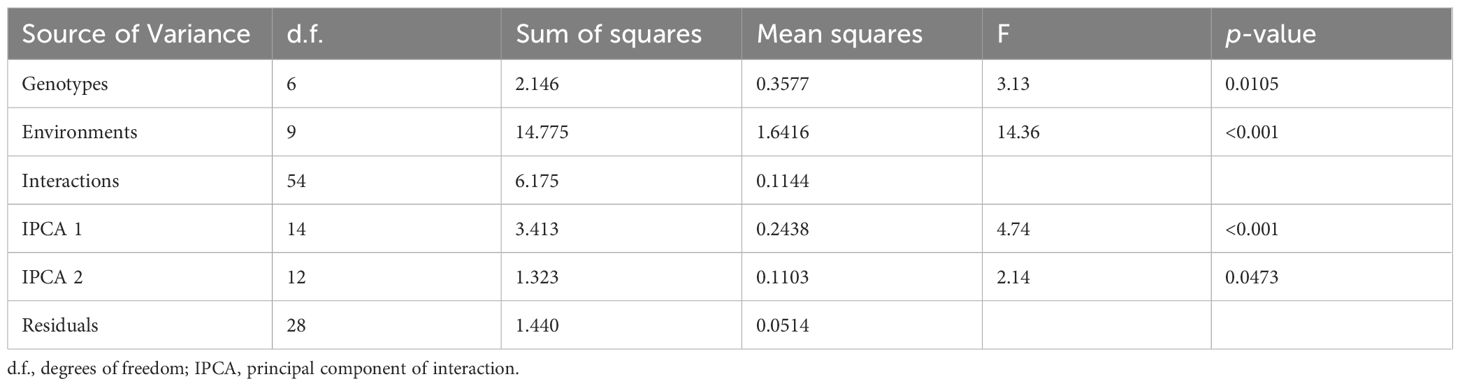
The ANOVA results (Table 4) obtained for the AMMI model clearly indicate the significant effects of both genotype and environment on the traits under consideration, supporting the hypothesis that performance varies across different conditions and genetic backgrounds. The interaction between genotypes and environments was also significant, with the first principal component (IPCA1) showing a high variance ratio (v.r. = 4.74; p< 0.001). The second principal component (IPCA2) was also significant (v.r. = 2.14; p<0.0473), indicating that specific genotype responses varied across environments.
Genotype means and scores
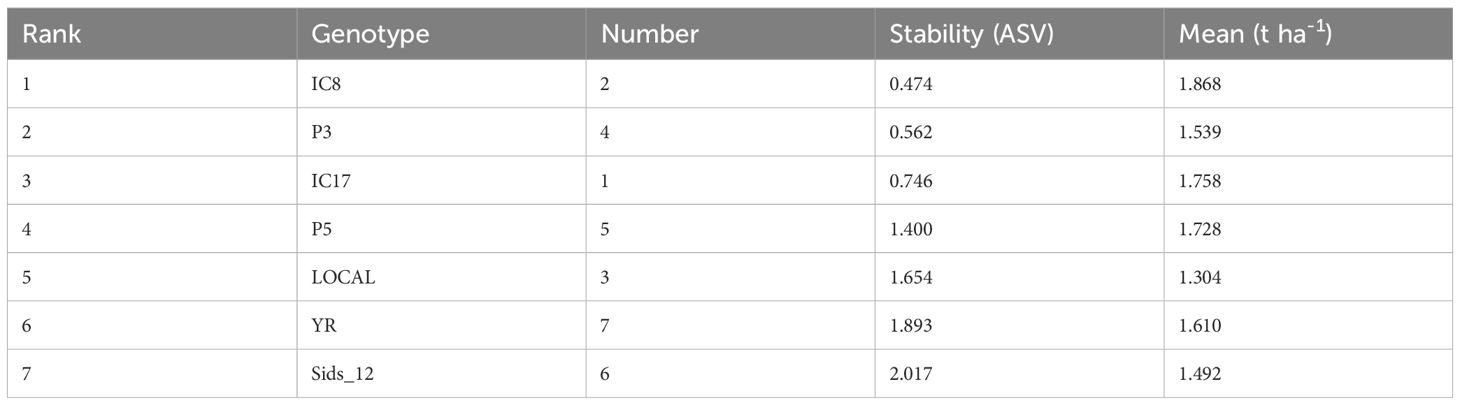
The mean performance and IPCA scores for each genotype revealed differences in adaptability and stability (Table 5). IC8 (mean = 1.868t ha-1) exhibited the highest mean performance among the tested genotypes and had a positive IPCA score in the first principal component, indicating favorable interactions with certain environments. IC17 (mean = 1.758t ha-1) also performed well, with a positive IPCAg1 score, suggesting its relative stability across environments. In contrast, LOCAL (mean = 1.304t ha-1) had the lowest mean performance and negative IPCA scores in both components, indicating its performance was less favorable across environments. Sids_12 had a mean of 1.492t ha-1, with a high positive IPCAg2 score, suggesting that it performed well under certain environmental conditions but may not be stable across all environments.
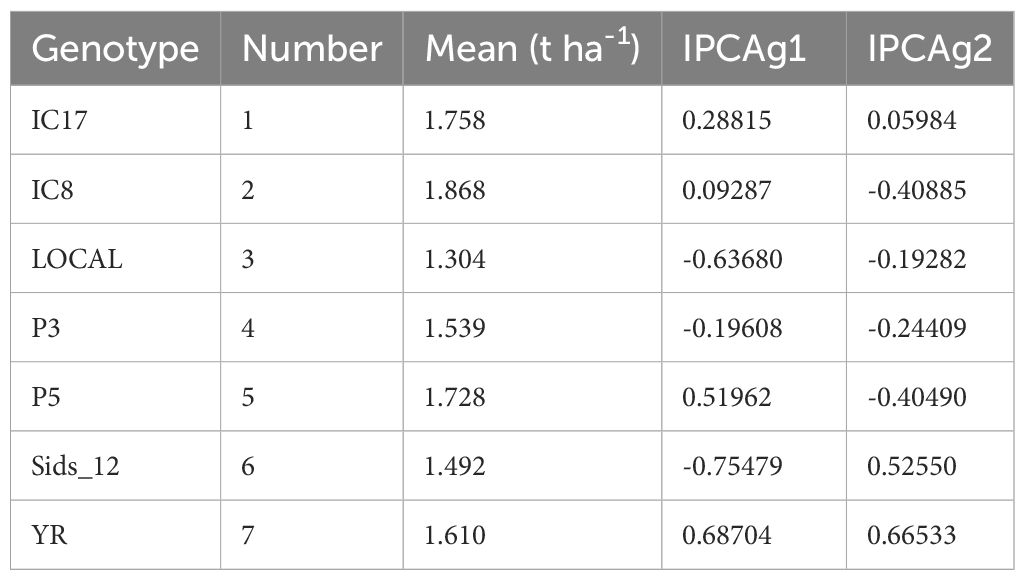
Table 5. Mean performance and principal component scores (IPCAg1 and IPCAg2) of wheat genotypes across all environments.
AMMI stability values
The ASVs provided insights into the performance and adaptability of genotypes across different environments (Table 6). Lower ASVs indicated greater stability, which means that the genotype is less affected by environmental changes. The AMMI analysis identified IC8 (ASV=0.474) as the most stable genotype with the highest mean yield (1.868t ha-1), indicating consistent performance across varying conditions. In contrast, Sids_12 (ASV=2.017) was ranked the least stable, suggesting it is more sensitive to environmental changes despite a mean yield of 1.492t ha-1. P3 and IC17 ranked second and third in stability, also showing relatively high mean yields of 1.539 and 1.758t ha-1, respectively. This balance between stability and yield performance is valuable for selecting genotypes for breeding programs. Conversely, LOCAL (ASV=1.654) and YR (ASV=1.893) exhibited high instability, indicating that they may perform well only under specific conditions but lack broad adaptability.
Identification of promising genotypes across environments
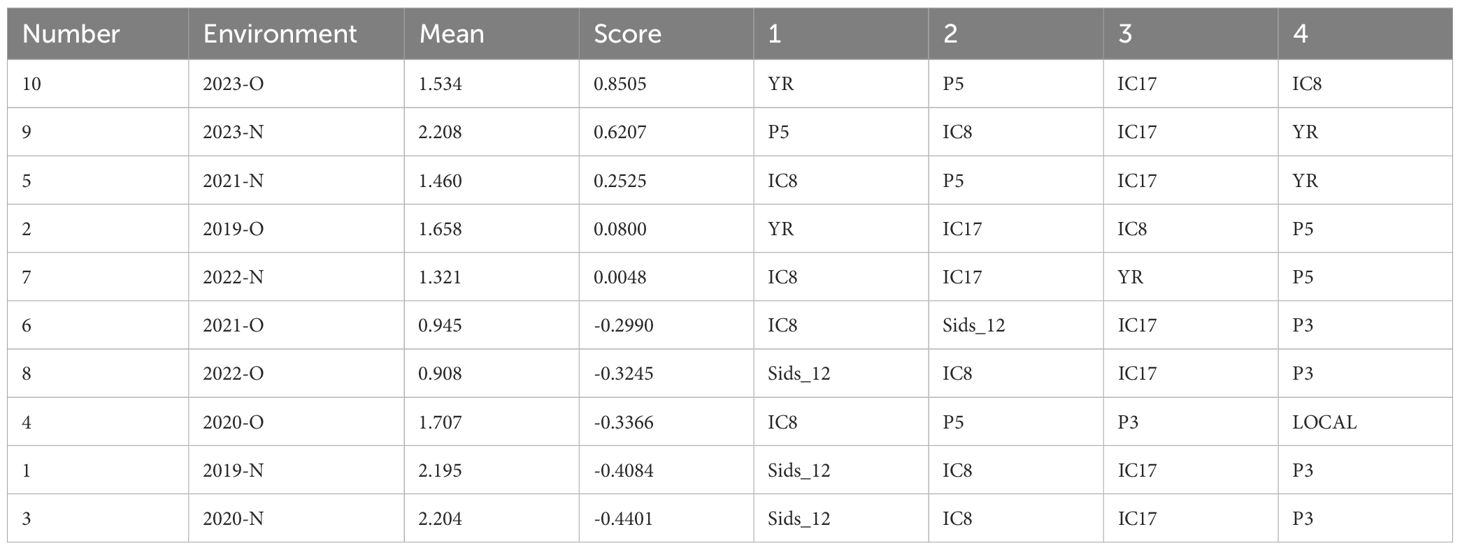
The AMMI analysis revealed the first four genotype selections for each environment based on their performance (Table 7). The highest mean yield was recorded in environment 2023-N (2.208t ha-1), followed closely by 2020-N (2.204t ha-1) and 2019-N (2.195t ha-1). These environments demonstrated favorable conditions for high-yielding genotypes. Conversely, environments such as 2022-O (0.908t ha-1) and 2021-O (0.945t ha-1) had the lowest mean performance, indicating suboptimal conditions for yield expression. Genotype IC8 emerged as the most frequently selected across environments, appearing in the top four rankings in nine out of ten environments. This highlights its broad adaptability and stability across varying conditions. Other frequently selected genotypes include IC17, P5, and YR, indicating their suitability for specific environments. Sids_12 was also prominent in environments such as 2022-O, 2020-N, and 2019-N, suggesting its potential for high performance in targeted conditions. The AMMI interaction scores varied significantly across environments. Environments such as 2023-O (0.8505) and 2023-N (0.6207) had high scores, demonstrating strong genotype-by-environment interaction effects. Lower interaction scores in environments such as 2022-N (0.0048) and 2021-O (-0.2990) suggest reduced genotype-by-environment interaction, emphasizing the role of stable environmental conditions.
Genotype stability based on GGE biplot analysis
The polygon view of the GGE biplot (Figure 3) provides a comprehensive visual representation of the “which-won-where” model, enabling the identification of wheat genotypes that perform optimally under specific environments (organic and conventional fertilization systems). The biplot presented in Figure 3 summarizes the principal component analysis (PCA) of the genotypic and environmental data, explaining 75.46% of the variation, with PC1 and PC2 contributing 57.09% and 18.37%, respectively. The environments are grouped into two distinct mega-environments based on their proximity to vertex genotypes: Mega-Environment 1 (Conventional Environments) includes environments 2023-N, 2021-N, 2022-N, and 2019-N. Genotypes located at the vertices of the polygon, such as P5 (AUS-030852) and IC17 (Line-56 ICARDA-1st RDRN0607), demonstrated superior adaptability and yield performance in their respective environments. These genotypes are likely the most suited to the environmental conditions and nutrient availability provided by the specific fertilization system. Mega-Environment 2 (Organic Environments: 2020-O, 2019-O, and 2023-O) was dominated by genotype IC8. Genotypes IC8, IC17, and P5 were the primary vertex genotypes, suggesting superior performance in their respective mega-environments. Other genotypes, such as YR, Sids_12, and LOCAL, were positioned further away from the polygon edges, indicating either lower interaction or suboptimal performance in specific environments. Genotypes close to the biplot origin (e.g., P3) demonstrated greater stability.
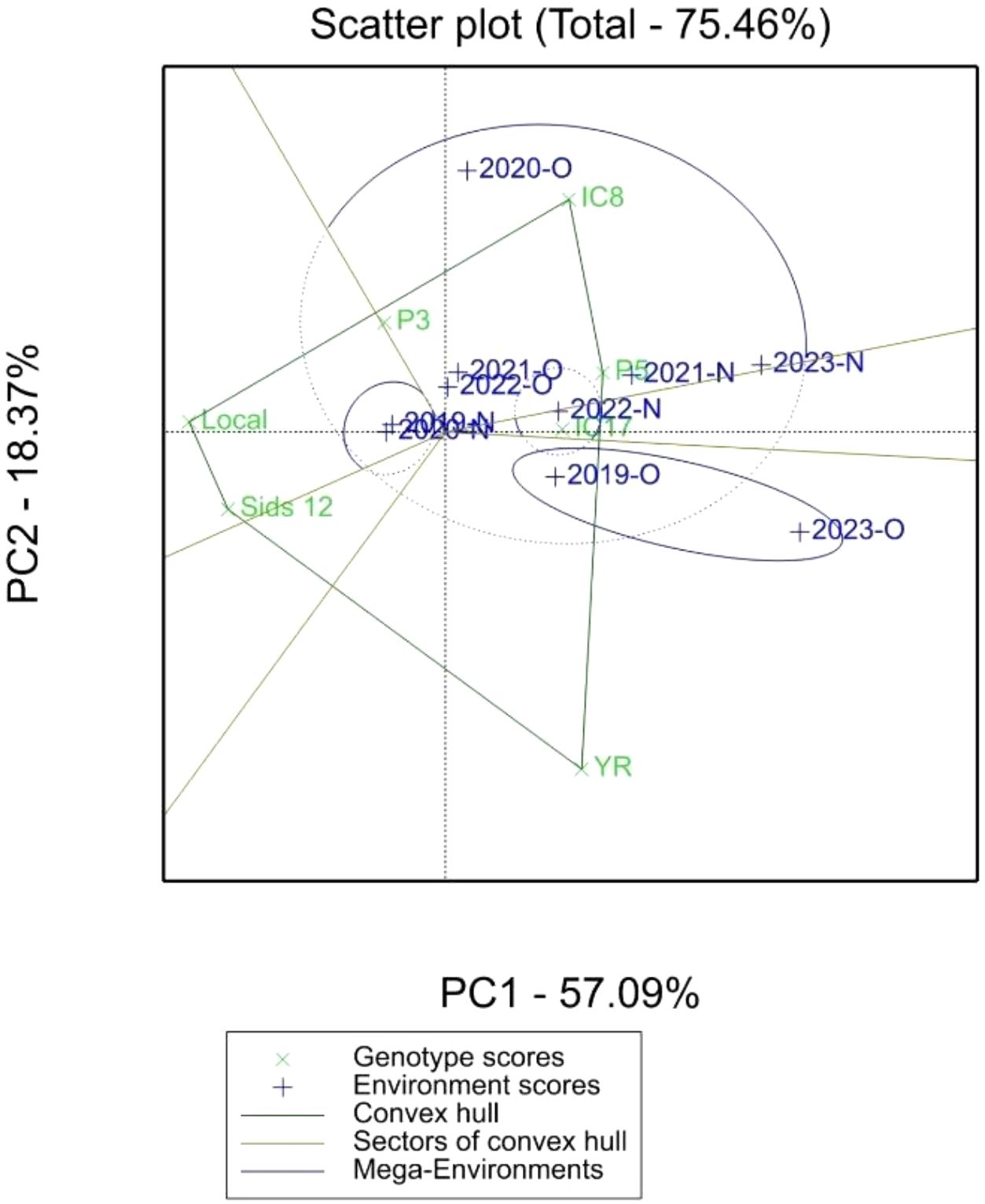
Figure 3. Polygon view of GGE biplot (which-won-where model) showing 7 elite wheat genotypes in organic (O) and conventional fertilization (N) environments.
Mean vs. stability
Figure 4 displays a ranking biplot that explains 75.46% of the total variation in genotype and environmental scores, with the first principal component (PC1) explaining 57.09% of the variance and the second component (PC2) explaining 18.37%. Genotypes are represented by green crosses, while environments are depicted by blue crosses with vectors. The average environment coordination (AEC) axis, shown as a blue line, indicates the average performance of genotypes across environments. The AEC circle helps visualize the distance of environments from the average performance. The genotypes “IC8,” “LOCAL,” and “P3” are spread close to the AEC, indicating stability in their performance across different environments. Conversely, “YR” and “IC8” are separated along PC1 and PC2, suggesting more specific adaptation to certain environments. Environments such as “2020-O” and “2023-O” are farther along the AEC axis, suggesting that they either posed challenges or provided advantages that influenced genotype performance.
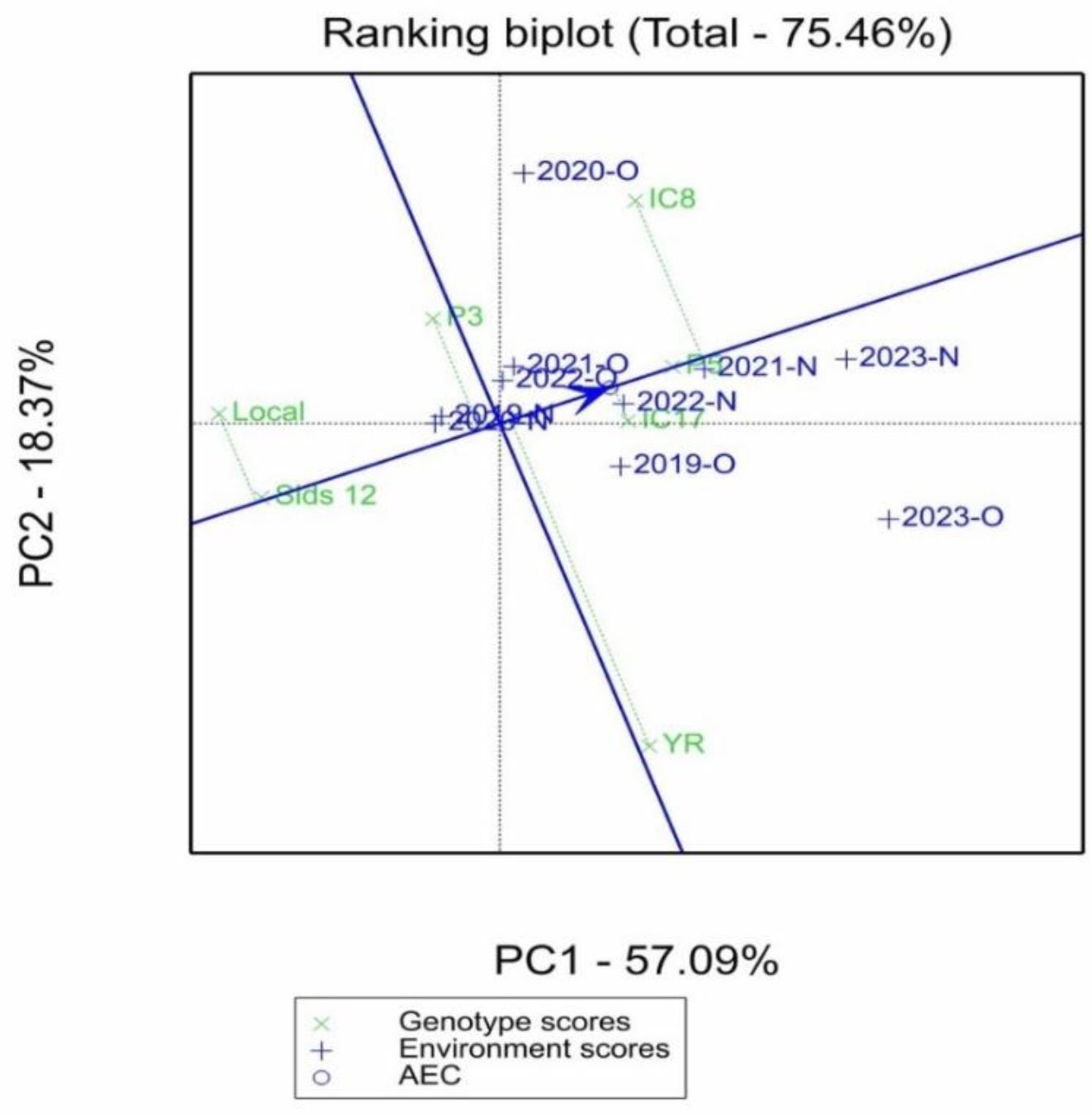
Figure 4. Mean vs. stability view of GGE biplot showing the mean performance and stability of seven elite wheat genotypes in organic (O) and conventional fertilization (N) environments.
Discussion
This study showed that the performance of wheat was influenced by both genotypic variances and environmental variables, with a strong G×E interaction. This finding is consistent with earlier research demonstrating that diversified genotype selection can increase total crop output (Zobel et al., 1988). The significant influence of environmental factors underscores the idea that genotype performance is extremely context-dependent, which is important in modern plant breeding practices (Kumar et al., 2021; Yan and Tinker, 2006). The significant interaction (F=4.74; p< 0.001) highlighted the need to consider both genetic and environmental factors when planning breeding programs to achieve consistent and high-yielding genotypes.
The AMMI model showed that the first two interaction principal component axes (IPCAs) account for the majority of variation in the G×E interactions. These findings support previous research showing the efficacy of the AMMI model in interpreting complicated relationships in multi-environment trials (Gauch, 1992, 2013). In our investigation, genotypes with low ASVs, such as IC8, P3, and IC17, produced high yields and performed consistently across conditions. In contrast, genotypes such as YR and Sids_12 had high ASVs and showed significant variability, indicating a preference for specific environmental circumstances over broad adaptability.
The ranking biplot contributed to our understanding by explaining 75.46% of the overall variation, with PC1 and PC2 accounting for 57.09% and 18.37%, respectively. The average environment coordination (AEC) axis represents average performance across situations. Genotypes along the AEC axis, such as IC8, LOCAL, and P3, indicated stability, but genotypes dispersed over both major components, such as YR, suggested particular adaptation to certain environmental conditions. The environmental conditions presented in Table 2 revealed considerable variation between years, particularly in terms of precipitation which ranged from 72.5mm in 2022 to a 294.8mm in 2023. These variations, in combination with the use of organic (O) and inorganic (N) fertilizers, resulted in various environments that influenced genotype performance. Interestingly, environments “2020-O” and “2023-O” were located further down the AEC axis, suggesting that they presented difficulties or benefits that had a large impact on genotype performance. This trend aligns with research showing how important environmental influences are in influencing genetic responses (Yan et al., 2007). Particularly in low-input systems where reliable performance is crucial, genotypes like IC8, P3, and IC17 that consistently performed well in a variety of environments are interesting candidates for widespread cultivation (Baresel et al., 2008; Singh et al., 2019). However, highly variable genotypes, such as YR and Sids_12, might be targeted for certain management techniques or settings where their distinct performance profiles can be best utilized (Annicchiarico, 2002). The clustering of organic environments around genotypes such as IC8 suggests enhanced nutrient-use efficiency and stress tolerance, traits that are highly desirable for sustainable organic agriculture (Baresel et al., 2008). Al-Ghumaiz et al. (2020) showed that the ICARDA genotypes (IC8 and IC17) had the greatest Se and Zn concentrations, respectively, under organic fertilization.
When yield performance is integrated with stability measurements, it becomes clear that selecting genotypes simply based on yield might overlook important performance consistency characteristics. Vassileva et al. (2023) reported that cultivating drought-resistant wheat genotypes and understanding stability determinants could markedly contribute to enhancing wheat production and ensuring stable yields under low-input and stress-pone environmental conditions. ASV index, a measure of yield stability that takes into account genotype-by-environment interactions (Purchase et al., 2000) was especially valuable in this investigation. IC8 was the most promising contender due to its high mean yield and low ASV, suggesting strong performance throughout a wide variety of environmental conditions. This is critical for creating genotypes that can maintain productivity in various environments (Ahakpaz et al., 2021). In contrast, despite being genetically distinct, IC17 showed a balance between stability and yield. Its moderate yield, coupled with consistent performance across environments, suggests that it may offer specific adaptive benefits that are not captured by yield alone. Conversely, genotypes such as YR and Sids_12 may require targeted management practices or further breeding interventions to enhance their performance, as they either did not yield as highly or exhibited greater variability. These findings highlight the significance of using a dual-criteria selection approach that incorporates both yield potential and stability. Such an integrated strategy ensures that selected genotypes are not only high-yielding but also resilient under diverse environmental conditions, a critical requirement for sustainable cultivation and breeding programs (Crossa et al., 2017).
Conclusion
The integration of AMMI analysis and GGE biplot visualization in this study provided a robust framework for evaluating the performance of genotypes under varying environmental conditions. Our results indicated that both yield and stability, as measured by ASV and mean yield, are crucial parameters in selecting genotypes for cultivation. Genotype IC8 emerged as the most promising candidate, combining high yield with stability, thereby offering broad adaptation to diverse environments. Similarly, IC17 also demonstrated a favorable balance between yield and stability, making it suitable for general cultivation. In contrast, genotypes such as YR and Sids_12, which displayed high variability, may be better suited for targeted environments where specific management practices can mitigate their instability. Future research should focus on integrating additional agronomic traits and exploring the molecular mechanisms underlying these interactions to further refine selection strategies for sustainable crop production.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Author contributions
NA: Resources, Conceptualization, Formal Analysis, Investigation, Writing – original draft. MM: Data curation, Writing – original draft, Methodology, Investigation, Validation, Writing – review & editing, Formal Analysis. AA: Data curation, Investigation, Writing – original draft, Methodology, Validation. SA: Investigation, Writing – original draft, Methodology, Data curation. AA: Formal Analysis, Investigation, Methodology, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The researchers would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this study (QU-APC-2025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd-Elmoniem, E. M., Al-Ghumaiz, N. S., Motawei, M. I., Al-Otayk, S., and Rabhi, M. (2025). Investigation of the magnesium content and productivity of wheat genotypes under organic and conventional inorganic fertilizer application. Life 15, 543. doi: 10.3390/life15040543
Ahakpaz, F., Abdi, H., Neyestani, E., Hesami, A., Mohammadi, B., Mahmoudi, K. N., et al. (2021). Genotype-by-environment interaction analysis for grain yield of barley genotypes under dryland conditions and the role of monthly rainfall. Agric. Water Manage. 245, 106665. doi: 10.1016/j.agwat.2020.106665
Al-Ghumaiz, N. S., Motawei, M. I., Abd-Elmoniem, E. M., and Al-Otayk, S. M. (2020). Selenium and zinc concentrations in spring wheat (Triticum aestivum) genotypes under organic and inorganic fertilization. J. Plant Nutr. 43, 1980–1987. doi: 10.1080/01904167.2020.1758135
Alloway, B. J. (2008). Zinc in soils and crop nutrition. 2nd (Brussels: International Zinc Association).
Annicchiarico, P. (2002). Genotype × environment interaction in plant breeding: Understanding and using the concept. Euphytica 128, 113–127.
Baresel, J., Zimmermann, G., and Reents, H. (2008). Effects of genotype and environment on N uptake and N partition in organically grown winter wheat (Triticum aestivum L.) in Germany. Euphytica 163, 347–354. doi: 10.1007/s10681-008-9718-1
Crossa, J., Pérez-Rodríguez, P., Cuevas, J., Montesinos-López, O., Jarquín, D., De Los Campos, G., et al. (2017). Genomic selection in plant breeding: methods, models, and perspectives. Trends Plant Sci. 22, 961–975. doi: 10.1016/j.tplants.2017.08.011
FAO (2020). The state of food security and nutrition in the world 2020: transforming food systems for affordable healthy diets Vol. 2020 (Rome, Italy: Food & Agriculture Org).
Farshadfar, E. (2008). Incorporation of AMMI stability value and grain yield in a single non-parametric index (GSI) in bread wheat. Pakistan J. Biol. Sci. 11, 1791–1796. doi: 10.3923/pjbs.2008.1791.1796
Gauch, H., Jr. (1992). Statistical analysis of regional yield trials: AMMI analysis of factorial designs (Amsterdam: Elsevier). doi: 10.2135/cropsci2013.04.0241
Gauch, J. H.G. (2013). A simple protocol for AMMI analysis of yield trials. Crop Sci. 53, 1860–1869. doi: 10.2135/cropsci2013.04.0241
Kumar, A., Chand, P., Thapa, R. S., and Singh, T. (2021). Assessment of stability performance and GXE interaction for yield and its attributing characters in bread wheat (Triticum aestivum L.). Electronic J. Plant Breed. 12, 235–241.
Lyons, S. M., Gudanis, D., Coyne, S. M., Gdaniec, Z., and Ivanov, P. (2017). Identification of functional tetramolecular RNA G-quadruplexes derived from transfer RNAs. Nat. Commun. 8, 1–11. doi: 10.1038/s41467-017-01278-w
Mäder, P., Fliessbach, A., Dubois, D., Gunst, L., Fried, P., and Niggli, U. (2002). Soil fertility and biodiversity in organic farming. Science 296, 1694–1697. doi: 10.1126/science.1071148
Nowosad, K., Liersch, A., Popławska, W., and Bocianowski, J. (2016). Genotype by environment interaction for seed yield in rapeseed (Brassica napus L.) using additive main effects and multiplicative interaction model. Euphytica 208, 187–194. doi: 10.1007/s10681-015-1620-z
Ponisio, L. C., M'Gonigle, L. K., Mace, K. C., Palomino, J., De Valpine, P., and Kremen, C. (2015). Diversification practices reduce organic to conventional yield gap. Proc. R. Soc. B: Biol. Sci. 282, 20141396. doi: 10.1098/rspb.2014.1396
Purchase, J., Hatting, H., and Van Deventer, C. (2000). Genotype× environment interaction of winter wheat (Triticum aestivum L.) in South Africa: II. Stability analysis of yield performance. South Afr. J. Plant Soil 17, 101–107. doi: 10.1080/02571862.2000.10634878
Reganold, J. P. and Wachter, J. M. (2016). Organic agriculture in the twenty-first century. Nat. Plants 2, 1–8. doi: 10.1038/nplants.2015.221
Ritchie, H. and Roser, M. (2022). Crop Yields (Our World in Data). Available online at: https://ourworldindata.org/crop-yields (Accessed August, 2025).
Seufert, V., Ramankutty, N., and Foley, J. A. (2012). Comparing the yields of organic and conventional agriculture. Nature 485, 229–232. doi: 10.1038/nature11069
Singh, C., Gupta, A., Gupta, V., Kumar, P., Sendhil, R., Tyagi, B., et al. (2019). Genotype x environment interaction analysis of multi-environment wheat trials in India using AMMI and GGE biplot models. Crop Breed. Appl. Biotechnol. 19, 309–318. doi: 10.1590/1984-70332019v19n3a43
Sparks, D. L., Page, A. L., Helmke, P. A., and Loeppert, R. H. (Eds.) (2020). Methods of soil analysis, part 3: Chemical methods (Hoboken, New Jersey, USA: John Wiley & Sons).
Vassileva, V., Georgieva, M., Zehirov, G., and Dimitrova, A. (2023). Exploring the genotype-dependent toolbox of wheat under drought stress. Agriculture 13, 1823. doi: 10.3390/agriculture13091823
Yan, W., Kang, M. S., Ma, B., Woods, S., and Cornelius, P. L. (2007). GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci. 47, 643–653. doi: 10.2135/cropsci2006.06.0374
Yan, W. and Tinker, N. A. (2006). Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 86, 623–645. doi: 10.4141/P05-169
Yan, W. and Wu, H. (2008). Application of GGE biplot analysis to evaluate Genotype (G), Environment (E), and G× E interaction on Pinus radiata: a case study. New Z. J. Forestry Sci. 38, 132–142.
Keywords: AMMI model, GGE biplot, genotype × environment interaction, wheat, stability, crop genetic diversity, organic and conventional fertilization
Citation: Al-Ghumaiz NS, Motawei MI, Aggag AM, Al-Otayk SM and Alzamil AA (2025) Phenotypic stability and adaptability of wheat genotypes under organic and conventional farming systems over five years using AMMI and GGE biplot analysis. Front. Plant Sci. 16:1693316. doi: 10.3389/fpls.2025.1693316
Received: 26 August 2025; Accepted: 09 October 2025;
Published: 23 October 2025.
Edited by:
Yuxiang Zeng, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Shiva Om Makaju, University of Georgia, United StatesArpit Gaur, Montana State University, United States
Copyright © 2025 Al-Ghumaiz, Motawei, Aggag, Al-Otayk and Alzamil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nasser S. Al-Ghumaiz, bmdtaWV6QHF1LmVkdS5zYQ==; Mohamad I. Motawei, bXRhb2FAcXUuZWR1LnNh
 Nasser S. Al-Ghumaiz1*
Nasser S. Al-Ghumaiz1* Mohamad I. Motawei
Mohamad I. Motawei Ahmed M. Aggag
Ahmed M. Aggag