- 1Department of Economic Entomology and Pesticides, Faculty of Agriculture, Cairo University, Giza, Egypt
- 2Department of Biology, College of Science and Humanities, Prince Sattam Bin Abdulziz University, Al-Kharj, Saudi Arabia
- 3Department of Biological Sciences, College of Science, King Faisal University, Al-Ahsa, Saudi Arabia
- 4Agricultural Biotechnology Department, College of Agriculture and Food Sciences, King Faisal University, Al-Ahsa, Saudi Arabia
- 5Department of Arid Land Agriculture, College of Agricultural and Food Sciences, King Faisal University, Al-Ahsa, Saudi Arabia
Spodoptera frugiperda (Lepidoptera: Noctuidae), commonly known as the fall armyworm, is a highly destructive migratory insect that poses a serious risk to global agricultural production, particularly maize crop. Targeting adult stages through ingestion-based control strategies offers a promising alternative to conventional broad-spectrum insecticide applications. In the current research, the lethal and sublethal impacts of five insecticides were evaluated against adult S. frugiperda, with a particular focus on chlorantraniliprole. Of the tested compounds, chlorantraniliprole revealed the highest toxicity (LC50 = 1.29 mg/L). Sublethal exposure to chlorantraniliprole significantly reduced larval and pupal development durations, decreased pupal weights, and shortened adult longevity in the offspring, without significantly affecting larval mortality, pupation rate, or emergence rate. Enzymatic assays showed a minimal induction of detoxification enzymes, suggesting a lower likelihood of rapid resistance emergence. This underscores the promise of chlorantraniliprole as an effective, environmentally favorable agent for integration into attract-and-kill strategies aimed at the sustainable control of S. frugiperda infestations.
1 Introduction
The fall armyworm, Spodoptera frugiperda (J.E. Smith, 1797) (Lepidoptera: Noctuidae), is a globally significant pest with an extensive host range, especially maize crops (Zea mays L.), and has become the most severe lepidopteran threat to maize production systems (Tepa-Yotto et al., 2023; Moustafa et al., 2024a). Owing to its nocturnal activity and post-emergence feeding behavior, S. frugiperda adults present an opportunity for pest control strategies that target them directly with toxicant mixtures. Like other migratory pests, the dissemination of S. frugiperda is greatly influenced by the adult’s ability to reproduce and disperse (Mao et al., 2023). Currently, S. frugiperda is documented from more than 100 countries, including Sub-Saharan, West, and Central Africa (Goergen et al., 2016; Day et al., 2017). In addition, it moves to the Asian continent in 2018 (Sharanabasappa et al., 2018), and in 2020 it reached Australia (Qi et al., 2021). Beyond life history traits, the flight capacity of this pest is a crucial concern for management efforts, especially given its highly migratory nature (Zhang et al., 2022).
Targeting bisexual adults using food attractants is one of the most effective approaches for area-wide integrated pest management (IPM) strategies against migrating pests (Wu and Guo, 2007; Zhang et al., 2022). Using the attract-and-kill strategy (Socorro et al., 2010; Gregg et al., 2018) instead of regular field spraying significantly reduces the environmental impact of pest management (Zhang et al., 2020a, b). Currently, chemical insecticides are commonly used and have lethal and sublethal consequences when used in real-world settings. Low amounts of pesticides can change essential features related to insects, including life duration, egg production, developmental stages, and mobility (Guedes et al., 2015; Lutz et al., 2018; Guedes et al., 2022). However, the repetitive application of high concentrations of insecticides often leads to resistance development (Daisley et al., 2018; Gressel, 2018). Insects respond to biological and abiotic stresses by modifying their migration habits, how they eat, reproduction, physiology, and metabolism (Sparks et al., 2021). Over time, insects have evolved multiple resistance mechanisms, such as target site, metabolic, behavioral, and penetration resistance (Khan et al., 2020). Metabolic resistance involves the rapid removal of insecticides by detoxifying chemicals using enzymes (Mokbel et al., 2024; Moustafa et al., 2024b). Therefore, it is essential to find new insecticides that can delay insects from developing resistance (Moustafa et al., 2023). In this regard, the anthranilic diamide class, developed by DuPont (Wilmington, France), exhibits unique features (Kandil et al., 2023). Chlorantraniliprole belongs to this group, and it results in the release of extra calcium, ultimately causing paralysis and death among the pests targeted (Akhtar et al., 2022). It is considered to be safe for mammals and exhibits selective action that preserves beneficial natural enemies including Chrysoperla externa Hagen, Eriopis connexa (Germar), Podisus nigrispinus (Dallas), and Orius insidiosus (Say) (MaChado et al., 2019). It works well against several species such as lepidopteran larvae, hemipteran, and coleopteran species (Lahm et al., 2009; Akhtar et al., 2022).
Various natural enzymes in insects are closely linked to biological processes and play critical roles in detoxification, including hydrolases, esterases, acetylcholinesterase, glutathione S-transferases (GSTs), carboxylesterases, and cytochrome P450 monooxygenases (Lin et al., 2015; Naik et al., 2018; Ismail, 2020; Clark et al., 2021; Yang et al., 2021; Ahmad et al., 2022; Siddiqui et al., 2022). There is evidence that S. frugiperda worldwide has developed resistance to roughly 47 chemical compounds (Mota-Sanchez and Wise, 2020). These include different insecticides such as organophosphates, carbamates, benzoylureas, diamides, spinosyns, pyrethroids, and Bacillus thuringiensis Berliner (Bt) toxins (Gutiérrez-Moreno et al., 2019; Kulye et al., 2021).
Nevertheless, the success of chemical applications may depend on understanding the sublethal effects of insecticides. Based on our knowledge, lethal and sublethal insecticidal effects have been recorded in the larval stage of several lepidopteran pests (Kandil et al., 2020; Awad et al., 2024; Moustafa et al., 2025). Thus, in the present work, the susceptibility of S. frugiperda moths to five common insecticides—lambda-cyhalothrin, indoxacarb, chlorantraniliprole, chlorfenapyr, and spinetoram—was evaluated. Additionally, chlorantraniliprole’s lethal and sublethal effects on bisexual adult development and enzymatic activity were assessed to determine its suitability for integration into food-attractant-based attract-and-kill control strategies.
2 Methodology
2.1 Insect
S. frugiperda eggs were acquired from laboratory colonies at Cairo University’s Faculty of Agriculture. The S. frugiperda larvae were reared on castor leaves oil (Ricinus communis L.) (Moustafa et al., 2024a, 2025) under laboratory conditions for over 12 consecutive generations without any exposure to insecticides (Moustafa et al., 2022) of 25 ± 1°C and 60 ± 5% relative humidity with 16:8-h light-to-dark cycle. The pupae were sorted by gender and placed in a glass jar (16.0cm height × 7.5cm diameter) until adult moths emerged. The newly emerged adult moths were used within 24h of emergence for the laboratory experiments.
2.2 Insecticides and chemicals
Five insecticides were utilized as technical-grade formulations (%w/v, as indicated), including lambda-cyhalothrin (95%), indoxacarb (95%), chlorantraniliprole (98%), chlorfenapyr (97%), and spinetoram (60%). All insecticides were provided by the Agricultural Research Center (Central Agricultural Pesticides Laboratory), Egypt. All chemicals including substrates and reagents that were used in the biochemical analysis were procured from Sigma Aldrich, Darmstadt, Germany.
2.3 Bioassay of S. frugiperda moths
A total of 100 mL of stock solution of the tested insecticides was prepared in N,N-dimethyl formamide and subsequently underwent dilution to five serial concentrations (ranging from 120 to 0.5 mg a.i., as shown in Supplementary Table S1) by adding a 10% v/v honey solution supplemented with 0.1% Triton X-100 (Zhang et al., 2022). The treated cotton ball by each concentration was placed inside a small plastic cup (2.5cm height × 4.5cm diameter), wrapped with cotton gauze (having a gap of 0.7mm) to minimize direct contact of the adult to the insecticide (Zhang et al., 2020), and put inside a glass jar (13.5cm height and 7.5cm diameter) with five S. frugiperda moths (24 days old) that were randomly chosen. It was exposed as one replicate (five replicates for each concentration). After 24h from exposure, the mortality was recorded to calculate the LC values (the moths have lost their ability to fly) (Wu and Guo, 2005).
2.4 Lethal and sublethal effects on reproduction
Five S. frugiperda moths (same sex) were transferred to a glass jar as described above (Section 2.3) and provided with a cotton ball saturated with sublethal and lethal concentrations (LC10 and LC50) of chlorantraniliprole or with 10% honey solution supplemented with 0.1% Triton X-100 for the control. A total of 40 replicates (200 female and 200 male moths) were used for the LC50 treatment, 20 replicates (100 female and 100 male) for the LC10 treatment, and 30 replicates (150 female and 150 male) for the control. After 24h from exposure, the surviving female and male moths from both treatments and control were paired. The pairing was done in the following order: LC10♀ + LC10 ♂, LC10♀ + CK♂, CK♀ + LC10♂; LC50♀ + LC50♂, LC50♀ + CK♂, CK♀ + LC50♂; CK♀ + CK♂. Each pair was transferred to a glass gar (11.5cm height × 5.5cm diameter) and fed with 10% honey solution only. Within each jar, the cotton ball was changed every day, and the number of eggs and egg hatching, respectively, were recorded. Three replicates (10 pairs/replicates) were performed for each treatment.
2.5 Lethal and sublethal effects on traits of offspring
Freshly hatched S. frugiperda offspring larvae were randomly selected from each treatment and placed into a plastic cup (9.0cm diameter × 5.5cm height) (one larvae per cup) with untreated castor leaves to assess chlorantraniliprole’s sublethal and lethal impacts on S. frugiperda growth. Three replicates (30 larvae per replicate) were performed for each treatment and kept under laboratory conditions (Moustafa et al., 2023). The insects were examined, and the castor leaves were changed daily. The development parameters were recorded using several key parameters, namely: larval and pupal durations, pupation (%), pupal weight, adult emergency (%), and female and male ratio (%).
2.6 Assay of enzymatic activity
2.6.1 Preparation of samples
Surviving adults (male and female) from the LC10, LC50, and control treatments were utilized in the enzyme activity assays (Zhang et al., 2020). After treatment with LC10 and LC50 of chlorantraniliprole, three of the individual adults (~250 mg) per sex were homogenized after removing the wings in 2 mL of 0.1 M phosphate buffer, pH 7. Then, the homogenates underwent 15-min centrifugation at 12,000 g and 4°C, and the supernatants were taken in new tubes as a source of enzyme. All treatments were performed three times per sex. The method of Bradford (1976) was employed to assess the total protein.
2.6.2 Carboxylesterases activity
Carboxylesterases (CarE) activity was performed following Van Asperen (1962) by using α-naphthyl acetate (α-NA) as the substrate. Then, 100 µL of α-NA (30mM) was incubated with 30 µL of enzyme source for 15min at 30°C. Next, 50 µL of a solution composed of a 2:5 mixture of 1% Fast Blue B and 5% sodium dodecyl sulfate (SDS) was dispensed into each sample. The optical density (OD) value was recorded at 600 nm, utilizing a Jenway Spectrophotometer-7205 UV/Vis., UK, considering α-naphthol as a reference.
2.6.3 Cytochrome P-450 activity
The activity of cytochrome P-450 was tested following the technique outlined in Hansen and Hodgson (1971). Then, 100 µL of 2 mM p-nitroanisole was incubated with 90 µL of enzyme solution for 2min at 27°C. Next, 9.6 mM of NADPH was added, and p-nitrophenol was considered as a standard. The OD value was recorded at 405 nm by using a microplate reader (Clindiag-MR-96, Steenberg, Belgium).
2.6.4 Glutathione S-transferase activity
Glutathione S-transferase (GST) activity was determined following Habig et al. (1974). Supernatant at 10 µL of was blended with 30 mM of CDNB (1-chloro,2,4-dinitrobenzene) and 50 mM of GSH. Optical density at 340 nm was at intervals of 1min for 5min, utilizing a Jenway Spectrophotometer-7205 UV/Vis., UK.
2.7 Data analysis
The data was analyzed with SPSS V.22, a statistical program. Data were analyzed for parametric test assumptions, and the normality of continuous variables was confirmed using the Shapiro–Wilk and Kolmogorov–Smirnov tests. The arcsine square root method was used to standardize the data. The mean and standard deviation of the biological, adult reproduction parameters and biochemical data were calculated using one-way ANOVA, with three replicates for each group. For the post-hoc analysis, Tukey’s pairwise comparison was utilized. Chi (χ2) was used to compare the actual and expected frequencies of the sex ratio utilizing MiniTab (V. 14). A P-value below 0.05 indicates a significant result. In addition, the correlation coefficient relationship between enzyme activities in both female and male S. frugiperda after exposure to the LC10 and LC50 concentrations of chlorantraniliprole was computed, whereas the analysis became available using SigmaPlot V12.0. Thus, data visualization (V. 2022.02.4) was performed using R studio.
3 Results
3.1 Moth bioassay
The bioassay results revealed that chlorantraniliprole had potent toxicity to S. frugiperda moths, with an LC50 of 1.29 mg/L, followed by indoxacarb (13.33 mg/L), lambda-cyhalothrin (19.39 mg/L), chlorfenapyr (41.68 mg/L), and spinetoram (45.30 mg/L) (Table 1). Based on these results, chlorantraniliprole was selected for further sublethal and lethal effect evaluations at LC10 and LC50 concentrations.
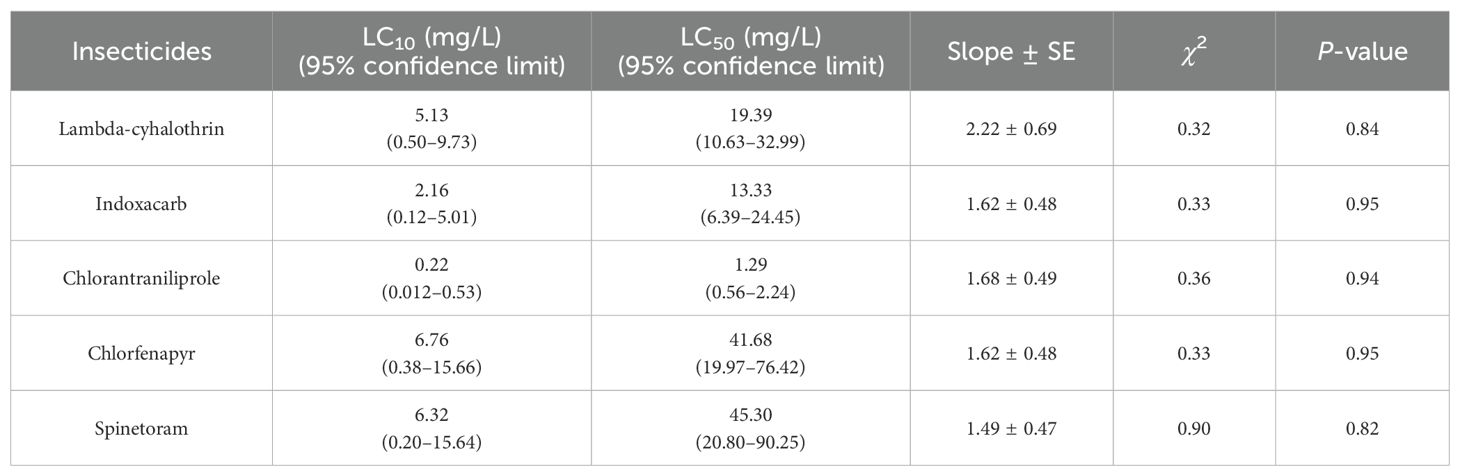
Table 1. Toxicity of lambda-cyhalothrin, indoxacarb, chlorantraniliprole, chlorfenapyr, and spinetoram to Spodoptera frugiperda moth.
3.2 Lethal and sublethal effects on offspring traits
Exposure of parental adults to lethal (LC50) and sublethal (LC10) levels of chlorantraniliprole significantly reduced the developmental durations of both larval [F6, 598 = 8.91, P=0.0001] and pupal stages [F6, 555 = 8.55, P=0.0001] in comparison with the control (Table 2). However, larval mortality [F6, 14 = 0.62, P=0.710], pupation percentage [F6, 14 = 0.77, P=0.605], and adult emergence percentage [F6, 14 = 0.39, P=0.874] were not significantly affected. The pupal weight of both male and female moths exhibited a significant reduction in the LC10 groups (Table 2). However, chi-square (χ²) tests revealed no treatment-related variations in sex ratio across the experimental groups (Table 3, Figure 1).
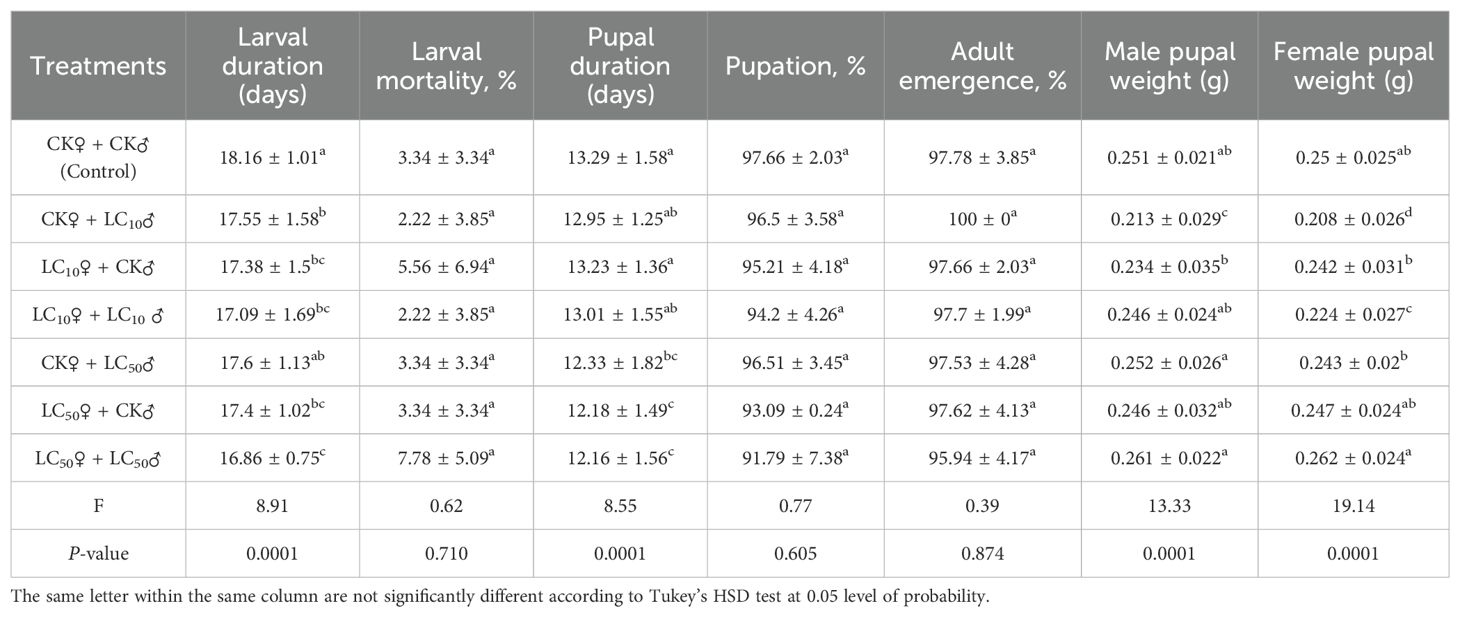
Table 2. Developmental parameters (mean ± SD) of S. frugiperda after exposure of adults to sublethal and lethal concentrations (LC10 and LC50) of chlorantraniliprole.
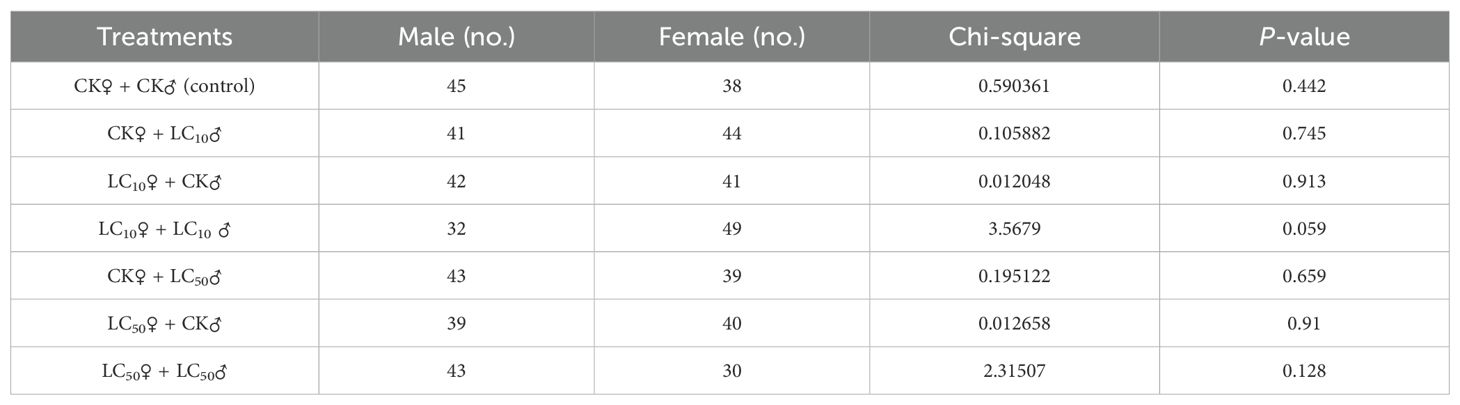
Table 3. Sex ratio (mean number) of the emerged adults of S. frugiperda after treating the adults with LC10 and LC50 of chlorantraniliprole.
Figure 1. Circle chart representing the sex ratio of the emerged adults of S. frugiperda after treating the adults with LC10 and LC50 of chlorantraniliprole.
3.3 Lethal and sublethal effects on reproduction and longevity
Chlorantraniliprole exposure at both LC10 and LC50 concentrations caused a reduction in egg production per female and the hatchability percentage; however, these reductions were insignificant relative to the control [number of eggs: F6, 14 = 1.13, P=0.396; hatchability: F6, 14 = 1.68, P=0.198] (Table 4). A significant decline was noted in the number of hatched larvae among all treatment groups [F6, 14 = 6.34, P=0.002] (Table 4). Moreover, female longevity was significantly shortened in the LC10♀ + LC10♂ and LC50♀ + LC50♂ groups (7.48 ± 0.16 and 7.07 ± 0.56 days, respectively) relative to the control (9.01 ± 0.21 days) [F=4.48, P=0.010]. Correspondingly, the survival rates declined to 70% and 66.67% in the LC10 and LC50 groups, respectively, relative to 90% in the control (Table 4).
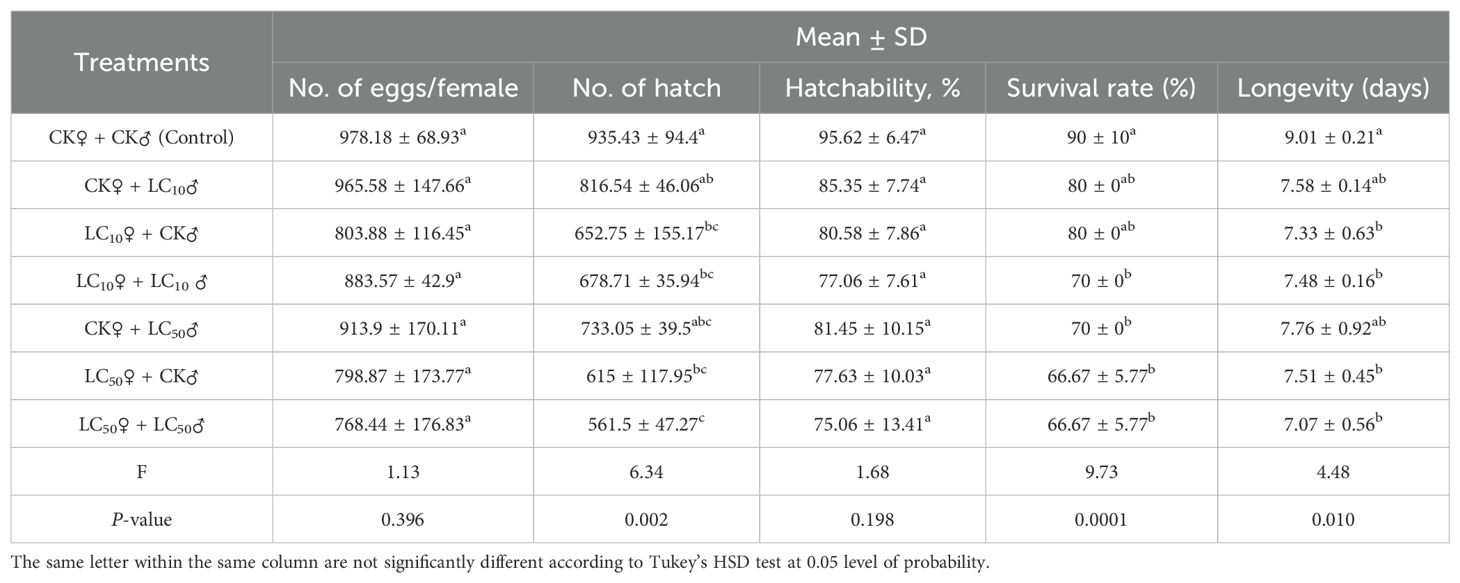
Table 4. Sublethal and lethal effects of chlorantraniliprole on the number of egg/female (fecundity), number of hatch, hatchability (%), survival rate, and longevity.
3.4 Enzyme activity
Following exposure to LC10 and LC50 concentrations, no significant differences were detected in the activities of cytochrome P450, α-esterase, and GST in female moths compared to the control [α-esterase: F2, 6 = 0.12, P=0.886; P450: F2, 6 = 1.38, P=0.321; GST: F2, 6 = 0.95, P=0.437] (Table 5). In male moths, GST activity significantly increased at LC50 [F2, 6 = 8.52, P=0.018], while α-esterase and cytochrome P450 activities remained statistically unchanged across treatments (Table 5).
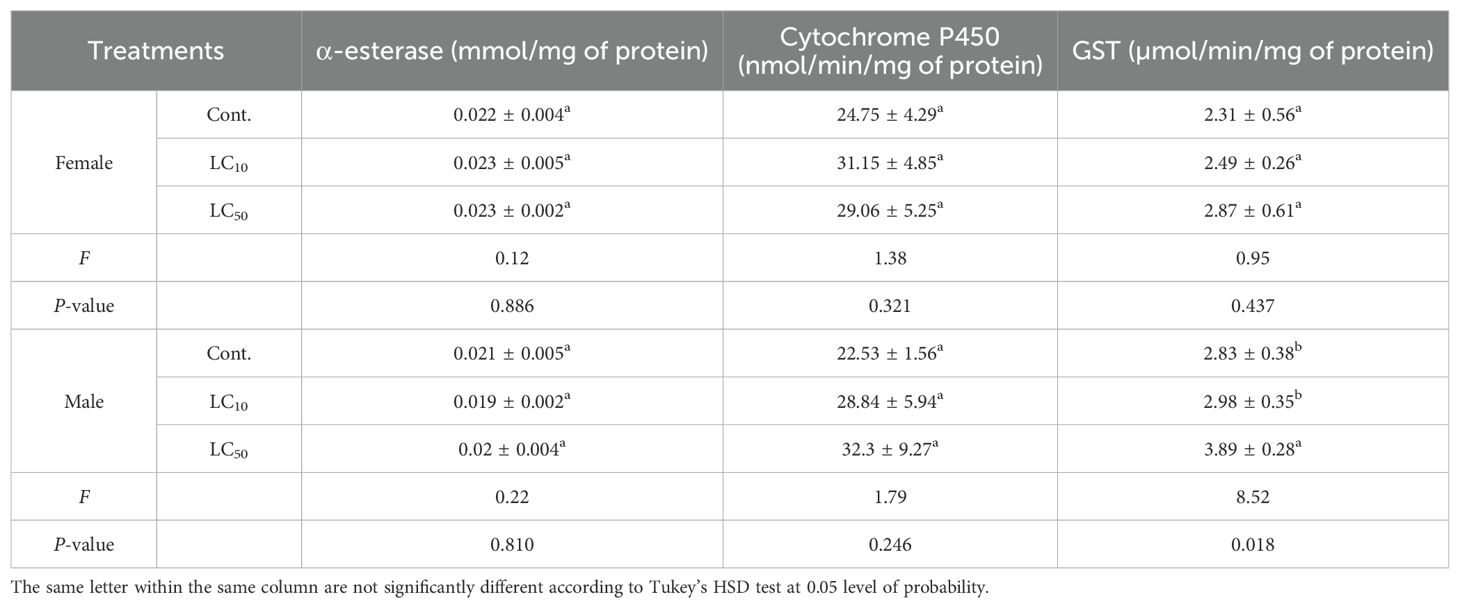
Table 5. Activities (mean ± SD) of detoxification enzymes in S. frugiperda adults following exposure to sublethal and lethal (LC10 and LC50) concentrations of chlorantraniliprole.
3.5 Correlation between enzyme activity in male and female individuals of S. frugiperda
A strong positive correlation was observed between α-esterase and cytochrome P450 activities in female adults (R=0.78, P=0.014), indicating that higher α-esterase levels were associated with increased cytochrome P450 activity (Figure 2). Moderate but non-significant positive correlations were also found between α-esterase and GST (R=0.58, P=0.103) and between cytochrome P450 and GST (R=0.60, P=0.090). In male adults, the correlation analysis revealed weak negative to moderate positive associations between enzyme activities, but none was statistically significant (Figure 2).
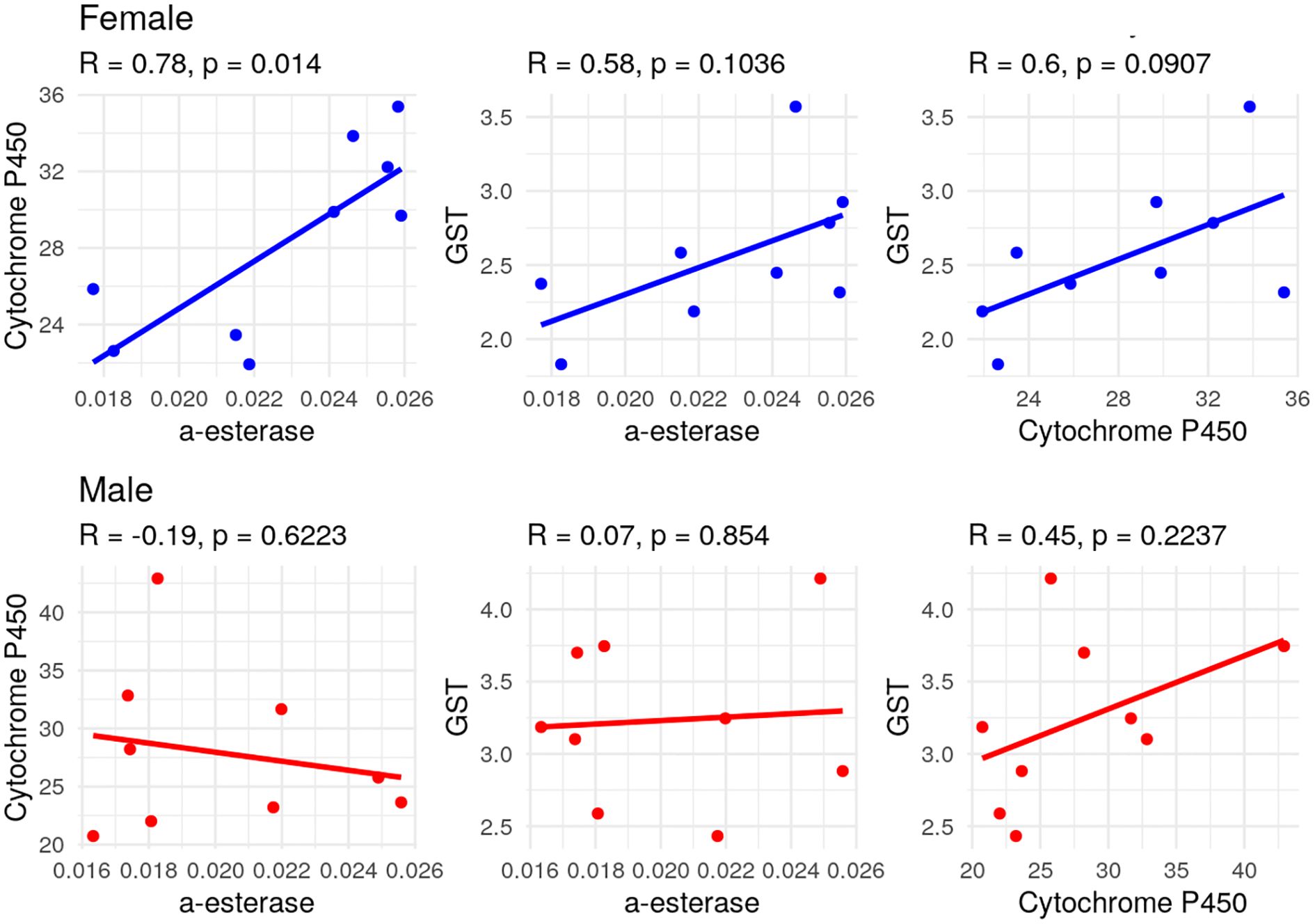
Figure 2. Matrix plot representing the correlation relationship between enzymatic activity in both female and male individuals of S. frugiperda after having been exposed to the LC10 and LC50 concentrations of chlorantraniliprole.
4 Discussion
Insect pests such as S. frugiperda impose significant threats to global agriculture, resulting in considerable economic losses and environmental costs (Gul et al., 2023). Although chemical insecticides remain widely employed for pest control (Moustafa et al., 2022, 2023), their effectiveness is often compromised by environmental degradation factors, including ultraviolet (UV) radiation, sunlight, and photolysis (Moustafa et al., 2018). Consequently, insects are frequently subjected to sublethal concentrations, which can significantly alter biological traits and enzymatic activities (Kandil et al., 2020).
Our research assessed the lethal and sublethal impacts of selected insecticides, with a focus on chlorantraniliprole, on S. frugiperda adults and their offspring. Within the studied compounds, chlorantraniliprole revealed the highest toxicity (LC50 = 1.29 mg/L). Generally, chlorantraniliprole has the potential to become one of the most promising compounds in pest management (Moustafa et al., 2023), including S. frugiperda (Zhang et al., 2020; Akhtar et al., 2022; Chen et al., 2023).
Exposure of parental adults to lethal (LC50) and sublethal (LC10) concentrations of chlorantraniliprole significantly reduced larval and pupal developmental durations, reduced pupal weights, and shortened adult longevity in their progeny, although larval mortality, pupation rate, and emergence rate were not significantly affected. Exposure to chlorantraniliprole seems to reduce the ability of S. frugiperda to reproduce and maintain a stable population (Akhtar et al., 2022; Abbas et al., 2025).
Previous findings indicated that chlorantraniliprole, a chemical compound related to anthranilic diamides, has been found to negatively influence the population of lepidopteran larvae (Lai and Su, 2011; Han et al., 2012; Guo et al., 2013; Zhang et al., 2013; Carneiro et al., 2016; Lutz et al., 2018). Importantly, chlorantraniliprole is considered highly selective with low toxicity toward natural enemies, making it an ideal candidate for integration into environmentally sustainable pest management programs (Brugger et al., 2010). Additional comparative studies reinforce the value of chlorantraniliprole. Zhang et al. (2022) demonstrated its high activity against Agrotis ipsilon (Hufnagel) and Agrotis segetum (Denis & Schiffermuller) adults, significantly reducing fecundity, egg hatchability, flight distance, and population growth. Similarly, Ismail (2020) found that chlorantraniliprole at low concentrations induced high mortality rates in A. ipsilon adults, with LC50 values varying across species. Our findings are consistent with these studies, confirming chlorantraniliprole’s high potency against adult moths, although species-specific sensitivity variations exist. Moreover, Liu et al. (2017) reported that chlorantraniliprole demonstrated greater toxicity against lepidopteran moths (A. ipsilon, Helicoverpa armigera Hubner, and Spodoptera litura Fabricius) and rapid efficacy compared to other insecticides such as methomyl, spinetoram, and emamectin benzoate, making it a preferable candidate for attract-and-kill systems. Although emamectin benzoate is toxic, its effects act too slowly to achieve fast control in strategies needed in adult-targeting strategies (Liu et al., 2017). Quickly rendering the insect incapable of movement reduces the chance of egg laying after being exposed to the insecticide.
Since chlorantraniliprole is both highly effective and environmentally safe, it can be safely used together with BioAttract, helping to attract and eliminate insects (attract-and-kill strategies).
As an obligate migratory pest, S. frugiperda’s high fecundity and strong flight capabilities contribute to its rapid regional spread and frequent outbreaks (Zhang et al., 2022). Trying to kill adult moths that feed on certain attractants can reduce their number and prevent them from migrating. Our findings indicate that controlling pests at the adult stage is very important (Zhang et al., 2022).
The larval stages of insects are found to be more sensitive to insecticides than the adult life stage (Xie et al., 2010; Kong et al., 2021). This observation aligns with findings that detoxification enzyme activity (e.g., CarE, GST, and MFOs) varies between larvae and adults (Sívori et al., 1997; Qiu and Zhang, 2001; Wang et al., 2008; Ou et al., 2012). Numerous studies have examined the functions of α-esterase, cytochrome P450, and GST, especially in relation to the insects’ exposure to insecticides (Moustafa et al., 2025). Because of their sensitivity in signaling chemical exposure, these detoxifying enzymes are powerful biological markers (Moustafa et al., 2023). Nevertheless, it has been noted that different insects’ responses to different chemicals exhibit varying degrees of activation and inhibition in their activity following insecticide exposure (Su and Xia, 2020). In our study, enzymatic assays indicated a minimal induction of detoxification pathways following chlorantraniliprole exposure, suggesting a lower risk of rapid resistance development at the adult stage.
The integration of chlorantraniliprole into attract-and-kill schemes, rather than conventional field sprays, could help delay resistance evolution and extend the effective lifespan of this active ingredient (Zhang et al., 2020).
5 Conclusions
Our study confirmed that chlorantraniliprole exerts significant lethal and sublethal impacts on Spodoptera frugiperda adults and their progeny. Exposure to sublethal concentrations accelerated larval and pupal development, decreased pupal weights, shortened adult longevity, and modestly impaired reproductive output, highlighting its potential to disrupt pest population dynamics even when direct mortality is limited. The minimal induction of detoxification enzyme activities in treated adults suggests a lower risk of rapid resistance development compared to other chemical classes. Furthermore, the sublethal impacts of chlorantraniliprole, comparable to those reported for the tested insecticides, reinforce its suitability for integration into attract-and-kill strategies targeting adult moths before migration and reproduction occur. Given its high efficacy, low non-target toxicity, and ingestion-based action, chlorantraniliprole represents a promising tool for the sustainable management of migratory lepidopteran pests such as S. frugiperda.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
NE: Conceptualization, Data curation, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. NA: Funding acquisition, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. BC: Data curation, Formal analysis, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HE: Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. ME: Funding acquisition, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MM: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant Number: KFU252702).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1694032/full#supplementary-material
References
Abbas, A., Hasnain, A., Hafeez, F., Chao, W. H., Hua, D. Y., Alam, A., et al. (2025). Cyantraniliprole-induced intergenerational sublethal effects on fall armyworm, Spodoptera frugiperda. Crop Prot. 190, 107116. doi: 10.1016/j.cropro.2025.107116
Ahmad, S., Bhatt, P., Ahmad, H. W., Cui, D., Guo, J., Zhong, G., et al. (2022). “Enzymes involved in the bioremediation of pesticides,” in Industrial applications of microbial enzymes. Ed. Bhatt, P. (CRC Press, Boca Raton, FL). doi: 10.1201/9781003202998-7
Akhtar, Z. R., Afzal, A., Idrees, A., Zia, K., Qadir, Z. A., Ali, S., et al. (2022). Lethal, sub-Lethal and trans-generational effects of chlorantraniliprole on biological parameters, demographic traits, and fitness costs of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 13, 881. doi: 10.3390/insects13100881
Awad, M., El Kenawy, A. H., Alfuhaid, N. A., Ibrahim, E.-D. S., Jósvai, J. K., Fónagy, A., et al. (2024). Lethal and sublethal effects of cyantraniliprole on the biology and metabolic enzyme activities of two lepidopteran pests, Spodoptera littoralis and Agrotis ipsilon, and a generalist predator, Chrysoperla carnea (Neuroptera: Chrysopidae). Insects 15, 450. doi: 10.3390/insects15060450
Bradford, M. M. A. (1976). Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1006/abio.1976.9999
Brugger, K. E., Cole, P. G., Newman, I. C., Parker, N., Scholz, B., Suvagia, P., et al. (2010). Selectivity of chlorantraniliprole to parasitoid wasps. Pest Manage. Sci. 66, 10751081. doi: 10.1002/ps.1977
Carneiro, E., Silva, L. B., Silva, A. F., Santos, V. B., Almeida, M. L. S., Carvalho, G. S., et al. (2016). Toxicity and sublethal effects of insecticides on Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). Afr. J. Agric. Res. 11, 1966–1972. doi: 10.5897/AJAR2015.10260
Chen, H.-L., Hasnain, A., Cheng, Q.-H., Xia, L.-J., Cai, Y.-H., Hu, R., et al. (2023). Resistance monitoring and mechanism in the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) for chlorantraniliprole from Sichuan Province, China. Front. Physiol. 14. doi: 10.3389/fphys.2023.1180655
Clark, M., Tepper, K., Petroll, K., Kumar, S., Sunna, A., and Maselko, M. (2021). Bioremediation of industrial pollutants by insects expressing a fungal laccase. ACS Synth. Biol. 11, 308–316. doi: 10.1021/acssynbio.1c00427
Daisley, B. A., Trinder, M., Mcdowell, T. W., Collins, S. L., Sumarah, M. W., and Reid, G. (2018). Microbiota-mediated modulation of organophosphate insecticide toxicity by species-dependent interactions with Lactobacilli in a Drosophila melanogaster insect model. Appl. Environ. Microbiol. 84, e02820–e02817. doi: 10.1128/AEM.02820-17
Day, R., Abrahams, P., Bateman, M., Beale, T., Clottey, V., Cock, M., et al. (2017). Fall armyworm: impacts and implications for Africa. Outlooks. Pest Manage. 28, 196–201. doi: 10.1564/v28_oct_02
Goergen, G., Kumar, P. L., Sankung, S. B., Togola, A., and Tamò, M. (2016). First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PloS One 11, e0165632. doi: 10.1371/journal.pone.0165632
Gregg, P. C., Del Socorro, A. P., and Landolt, P. J. (2018). Advances in attract-and-kill for agricultural pests: Beyond pheromones. Annu. Rev. Entomol. 63, 453–470. doi: 10.1146/annurev-ento-031616-035040
Gressel, J. (2018). Microbiome facilitated pest resistance: Potntial problems and uses. Pest Manage. Sci. 74, 511–515. doi: 10.1002/ps.4777
Guedes, R., Smagghe, G., Stark, J. D., and Desneux, N. (2015). Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 61, 43–62. doi: 10.1146/annurev-ento-010715-023646
Guedes, R. N. C., Benelli, G., and Agathokleous, E. (2022). Arthropod outbreaks, stressors, and sublethal stress. Curr. Opin. Environ. Sci. Health 28, 100371. doi: 10.1016/j.coesh.2022.100371
Gul, H., Gadratagi, B. G., Güncan, A., Tyagi, S., Ullah, F., Desneux, N., et al. (2023). Fitness costs of resistance to insecticides in insects. Front. Physiol. 14. doi: 10.3389/fphys.2023.1238111
Guo, L., Desneux, N., Sonoda, S., Liang, P., Han, P., and Gao, X. W. (2013). Sublethal and transgenerational effects of chlorantraniliprole on biological traits of the diamondback moth, Plutella xylostella L. Crop Prot. 48, 29–34. doi: 10.1016/j.cropro.2013.02.009\
Gutiérrez-Moreno, R., Mota-Sanchez, D., Blanco, C. A., Whalon, M. E., Terán Santofimio, H., Rodriguez-Maciel, J. C., et al. (2019). Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 112, 792–802. doi: 10.1093/jee/toy372
Habig, W. H., Pabst, M. J., and Jakoby, W. B. (1974). Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139. doi: 10.1016/S0021-9258(19)42083-8
Han, W. S., Zhang, S. F., Shen, F. Y., Liu, M., Ren, C. C., and Gao, X. W. (2012). Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae). Pest Manage. Sci. 68, 1184–1190. doi: 10.1002/ps.3282
Hansen, L. G. and Hodgson, E. (1971). Biochemical characteristics of insect microsomes: N- and O-demethylation. Biochem. Pharmacol. 20, 1569–1578. doi: 10.1016/0006-2952(71)90285-1
Ismail, S. M. (2020). Effect of sublethal doses of some insecticides and their role on detoxication enzymes and protein-content of Spodoptera littoralis (Boisd.)(Lepidoptera: Noctuidae). Bull. Nat. Res. Cent. 44, 1–6. doi: 10.1186/s42269-020-00294-z
Kandil, M. A., Abdel-kerim, R. N., and Moustafa, M. A. M. (2020). Lethal and sublethal effects of bio-and chemical insecticides on the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Egypt. J. Biol. Pest Cont. 30, 76. doi: 10.1186/s41938-020-00278-1
Kandil, M. A., Moustafa, M. A. M., Saleh, M. A., and Ateya, I. R. (2023). Dissipation kinetics and degradation products of cyantraniliprole in tomato plants and soil in the open field. Egypt. J. Chem. 66, 483–493. doi: 10.21608/EJCHEM.2023.195133.7625
Khan, H. A., Ali, N., Farooq, M. U., Asif, N., Gill, T. A., and Khalique, U. (2020). First authentic report of fall armyworm presence in Faisalabad Pakistan. J. Entomol. Zool. Stud. 8, 1512–1514. doi: 10.5958/0974-8172.2018.00238.9
Kong, F., Song, Y., Zhang, Q., Wang, Z., and Liu, Y. (2021). Sublethal effects of chlorantraniliprole on Spodoptera litura (Lepidoptera: Noctuidae) moth: implication for attract-and-kill strategy. Toxics 9, 20. doi: 10.3390/toxics9020020
Kulye, M., Mehlhorn, S., Boaventura, D., Godley, N., Venkatesh, S. K., Rudrappa, T., et al. (2021). Baseline susceptibility of Spodoptera frugiperda populations collected in India towards different chemical classes of insecticides. Insects 12, 758. doi: 10.3390/insects12080758
Lahm, G. P., Cordova, D., and Barry, J. D. (2009). New and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem. Lett. 17, 4127–4133. doi: 10.1016/j.bmc.2009.01.018
Lai, T. and Su, J. (2011). Effects of chlorantraniliprole on development and reproduction of beet armyworm, Spodoptera exigua (Hübner). J. Pest Sci. 84, 381–386. doi: 10.1007/s10340-011-0366-1
Lin, T., Cai, Z., and Wu, H. (2015). Transcriptome analysis of the Japanese pine sawyer beetle, Monochamus alternatus (Coleoptera: Cerambycidae) by highthroughput Illumina sequencing. J. Asia Pac. Entomol. 18, 439–445. doi: 10.1016/j.aspen.2015.04.011
Liu, Y. Q., Gao, Y., Liang, G. M., and Lu, Y. H. (2017). Chlorantraniliprole as a candidate pesticide used in combination with the attracticides for lepidopteran moths. PloS One 12, e0180255. doi: 10.1371/journal.pone.0180255
Lutz, A. L., Bertolaccini, I., Scotta, R. R., Curis, M. C., Favaro, M. A., Fernandez, L. N., et al. (2018). Lethal and sublethal effects of chlorantraniliprole on Spodoptera cosmioides (Lepidoptera: Noctuidae). Pest Manage. Sci. 74, 2817–2821. doi: 10.1002/ps.5070
MaChado, A. V. A., Potin, D. M., Torres, J. B., and Torres, C. S. A. (2019). Selective insecticides secure natural enemies action in cotton pest management. Ecotoxicol. Envirom. Saf. 184, 109669. doi: 10.1016/j.ecoenv.2019.109669
Mao, K. K., Li, H. R., Zhu, J. Y., Jin, M. H., Wang, P., Peng, Y., et al. (2023). Rapid test to detect insecticide resistance in field populations of Spodoptera frugiperda (Lepidoptera: Noctuidae). Front. Physiol. 14. doi: 10.3389/fphys.2023.1254765
Mokbel, E. M. S., Moustafa, M. A. M., Alfuhaid, N. A., and Fouad, E. A. (2024). Characterization of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae) resistance to indoxacarb: inheritance mode, realized heritability, and fitness costs. J. Econ. Entomol. 117, 619–628. doi: 10.1093/jee/toae024
Mota-Sanchez, D. and Wise, J. C. (2020). The Arthropod Pesticide Resistance Database (Michigan State University). Available online at: http://www.pesticideresistance.org (Accessed November 23, 2023).
Moustafa, M. A. M., Elmenofy, W. H., Osman, E. A., El-Said, N. A., and Awad, M. (2022). Biological impact, oxidative stress and adipokinetic hormone activities of Agrotis ipsilon in response to bioinsecticides. Plant Prot. Sci. 58, 326. doi: 10.17221/46/2022-PPS
Moustafa, M. A. M., El-Said, N. A., Ahmed, F. S., Amer, A., Awad, M., and Alfuhaid, N. A. (2025). In vitro and silico exploration of the insecticidal properties of Lavandula multifida L. essential oil and its binary combinations with cyantraniliprole and emamectin benzoate on Spodoptera frugiperda (Lepidoptera: Noctuidae). Crop Prot. 187, 106969. doi: 10.1016/j.cropro.2024.106969
Moustafa, M. A. M., El-Said, N. A., Alfuhaid, N. A., Abo-Elinin, F. M. A., Mohamed, R. M. B., and Aioub, A. A. A. (2024a). Monitoring and detection of insecticide resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae): Evidence for field-evolved resistance in Egypt. Insects 15, 705. doi: 10.3390/insects15090705
Moustafa, M. A. M., Fouad, E. A., Ibrahim, E., Erdei, A. L., Kárpáti, Z., and Fónagy, A. (2023). The Comparative toxicity, biochemical and physiological impacts of chlorantraniliprole and indoxacarb on Mamestra brassicae (Lepidoptera: Noctuidae). Toxics 11, 212. doi: 10.3390/toxics11030212
Moustafa, M. A. M., Osman, E. A., Mokbel, E. M. S., and Fouad, E. A. (2024b). Biochemical and molecular characterization of chlorantraniliprole resistance in Spodoptera littoralis (Lepidoptera: Noctuidae). Crop Prot. 177, 106533. doi: 10.1016/j.cropro.2023.106533
Moustafa, M. A. M., Saleh, M. A., Ateya, I. R., and Kandil, M. A. (2018). Influence of some environmental conditions on stability and activity of Bacillus thuringiensis formulations against the cotton leaf worm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Egypt. J. Biolog. Pest Cont. 61, 1–7. doi: 10.1186/s41938-018-0064-x
Naik, V. C., Kumbhare, S., Kranthi, S., Satija, U., and Kranthi, K. R. (2018). Field-evolved resistance of pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), to transgenic Bacillus thuringiensis (Bt) cotton expressing crystal 1Ac (Cry1Ac) and Cry2Ab in India. Pest Manage. Sci. 74, 2544–2554. doi: 10.1002/ps.5038
Ou, S. H., Liang, P., Song, D. L., Shi, X. Y., and Gao, X. W. (2012). Effects of sublethal dosage of chlorantraniliprole on development and detoxifying enzymes activity of Helicoverpa armigera. Plant Prot. 38, 1–8.
Qi, G. J., Ma, J., Wan, J., Ren, Y. L., McKirdy, S., Hu, G., et al. (2021). Source regions of the first immigration of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) invading Australia. Insects 12, 1104. doi: 10.3390/insects12121104
Qiu, L. H. and Zhang, W. J. (2001). Relationship between mixed function oxidases and the resistance to fenvalerate in Helicoverpa armigera. Acta Entomol. Sin. 44, 447–453.
Sharanabasappa, S. D., Kalleshwaraswamy, C. M., Asokan, R., Mahadevaswamy, H. M., Maruthi, M. S., Pavithra, H. B., et al. (2018). First report of the fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera, Noctuidae) an alien invasive pest on Maize in India. Pest Manage. Horticult. Ecosys. 24, 23–29.
Siddiqui, J. A., Khan, M. M., Bamisile, B. S., Hafeez, M., Qasim, M., Rasheed, M. T., et al. (2022). Role of insect gut microbiota in pesticide degradation: A review. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.870462
Sívori, J., Casabe, N., Zerba, E., and Wood, E. (1997). Induction of glutathione S-transferase activity in Triatoma infestans. Mem. Inst. Oswaldo. Cruz. 92, 797–802. doi: 10.1590/S0074-02761997000600013
Socorro, A. P. D., Gregg, P. C., Alter, D., and Moore, C. J. (2010). Development of a synthetic plant volatile-based attracticide for female noctuid moths. I. Potential sources of volatiles attractive to Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 49, 10–20. doi: 10.1111/j.1440-6055.2009.00733.x
Sparks, T. C., Storer, N., Porter, A., Slater, R., and Nauen, R. (2021). Insecticide resistance management and industry: the origins and evolution of the insecticide resistance action committee (IRAC) and the mode of action classifcation scheme. Pest Manage. Sci. 77, 2609–2619. doi: 10.1002/ps.6254
Su, C. and Xia, X. (2020). Sublethal effects of methylthio-diafenthiuron on the life table parameters and enzymatic properties of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Pestic. Biochem. Physiol. 162, 43–51. doi: 10.1016/j.pestbp.2019.08.011
Tepa-Yotto, G. T., Goergen, G., Chinwada, P., Rwomushana, I., and Subramanian, S. (2023). Integrated management of Spodoptera frugiperda 6 years post detection in Africa: a review. Curr. Opin. Insect Sci. 52, 100928. doi: 10.1016/j.cois.2022.100928
Van Asperen, K. (1962). A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol. 8, 401–416. doi: 10.1016/0022-1910(62)90074-4
Wang, D., Li, B., Guan, W. M., Zhao, H. Q., Chen, Y. H., and Shen, W. D. (2008). Cloning, sequence analysis and tissue expression of a carboxylesterase gene from Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Acta Entomol. Sin. 51, 979–985.
Wu, K. M. and Guo, Y. Y. (2005). The evolution of cotton pest management practices in China. Annu. Rev. Entomol. 50, 31–52. doi: 10.1146/annurev.ento.50.071803.130349
Wu, K. M. and Guo, Y. Y. (2007). Geotype differentiation and regional migratory regularity of Helicoverpa armigera in China. Plant Prot. 33, 6–11.
Xie, S. H., Liang, Y. P., Lin, Z. F., Li, H., and Ji, X. C. (2010). The toxicity and control efficiency of 9 insecticides to Spodoptera litura. Plant Prot. 36, 175177.
Yang, Y. X., Lin, R. H., Li, Z., Wang, A. Y., Xue, C., Duan, A. L., et al. (2021). Function analysis of P450 and GST genes to imidacloprid in Aphis craccivora (Koch). Front. Microbiol. 11. doi: 10.3389/fphys.2020.624287
Zhang, D. W., Dai, C. C., Ali, A., Liu, Y. Q., Pan, Y., Desneuxf, N., et al. (2022). Lethal and sublethal effects of chlorantraniliprole on the migratory moths Agrotis ipsilon and A. segetum: New perspectives for pest management strategies. Pest Manage. Sci. 78, 4105–4113. doi: 10.1002/ps.7029
Zhang, R. M., Dong, J. F., Chen, J. H., Ji, Q. E., and Cui, J. J. (2013). The sublethal effects of chlorantraniliprole on Helicoverpa armigera (Lepidoptera: Noctuidae). J. Integr. Agric. 12, 457–466. doi: 10.1016/S2095-3119(13)60246-4
Zhang, Q., Liu, Y., Wyckhuys, K. A. G., Liang, H., Desneux, N., and Lu, Y. (2020b). Lethal and sublethal effects of chlorantraniliprole on Helicoverpa armigera adults enhance the potential for use in ‘attract-and-kill’ control strategies. Entomol. Gen. XX, 1–10. doi: 10.1127/entomologia/2020/1104
Keywords: Spodoptera frugiperda, chlorantraniliprole, ingestion bioassay, fecundity suppression, detoxification enzymes
Citation: El-Said NA, Alfuhaid NA, Chellappan BV, El-Beltagi HS, El-Mogy MM and Moustafa MAM (2025) Lethal and sublethal effects of chemical and bio-insecticides on Spodoptera frugiperda adults: new perspectives for “attract-and-kill” control strategies. Front. Plant Sci. 16:1694032. doi: 10.3389/fpls.2025.1694032
Received: 27 August 2025; Accepted: 24 September 2025;
Published: 15 October 2025.
Edited by:
Jorge M. S. Faria, National Institute for Agricultural and Veterinary Research (INIAV), PortugalReviewed by:
Ali Hasnain, Nanjing Agricultural University, ChinaSharanabasappa Deshmukh, University of Agricultural and Horticultural Sciences, Shivamogga, India
Copyright © 2025 El-Said, Alfuhaid, Chellappan, El-Beltagi, El-Mogy and Moustafa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nawal AbdulAziz Alfuhaid, bi5hbGZ1aGFpZEBwc2F1LmVkdS5zYQ==; Mohamed M. El-Mogy, ZWxtb2d5QGtmdS5lZHUuc2E=
 Nourhan A. El-Said1
Nourhan A. El-Said1 Biju Vadakkemukadiyil Chellappan
Biju Vadakkemukadiyil Chellappan Hossam S. El-Beltagi
Hossam S. El-Beltagi Mohamed M. El-Mogy
Mohamed M. El-Mogy Moataz A. M. Moustafa
Moataz A. M. Moustafa