- 1Regional Center of Agricultural Research of Agadir, National Institute of Agricultural Research (INRA), Rabat, Morocco
- 2AgroBioSciences Department (AgBS), Mohammed VI Polytechnic University (UM6P), Ben Guerir, Morocco
- 3Research Team in Science and Technology, Higher School of Technology, Ibn Zohr University, Laayoune, Morocco
- 4Laboratory of Biotechnology and Valorization of Natural Resources, Faculty of Science, Ibn Zohr University, Agadir, Morocco
- 5Faculty of Applied Science, Ait Melloul, Ibn Zohr University, Agadir, Morocco
- 6Algal Biotechnology Center, MAScIR, Mohammed VI Polytechnic University (UM6P), Ben Guerir, Morocco
In this study, we investigated the chemical profile essential oils (EOs) extracted from Cymbopogon citratus, Salvia rosmarinus, and Lavandula dentata, as well as their antifungal activity against Botrytis cinerea in vitro and in vivo. GC–MS analysis showed that the EOs major components of C. citratus EOs were, Geranial (42.91%), Neral (34.11%), and β-Pinene (9.32%). While the S. rosmarinus major EOs components were Camphor (17.60%), α-Pinene (14.39%), and 1,8-cineol (14.13%). Contrariwise L. dentata EOs, Camphor (33.95%), 1,8-cineol (32.35%), and β-Pinene (5.23%) were the predominant compounds. Regarding the in vitro antifungal activity, the EOs of three plants inhibited the mycelial growth of B. cinerea in a dose-dependent manner. Moreover, at the concentration of 0.32 µL/mL air, all EOs demonstrated the inhibition of the mycelia growth of B. cinerea. In addition, the combination of EOs increased the antifungal activity of B. cinerea compared to their individual application. According to simplex-centroid design analysis, the most efficient antifungal of the mixture of EOs extracted from L. dentata, S. rosmarinus, and C. citratus was noted EOs at a ratio (1:1:1). This mixture inhibited the mycelial growth at 1.6 µL/mL, with IC50 and IC90 value of 0.46 µL/mL and 0.81 µL/mL, respectively. In addition, in vivo tests showed that this EOs mixture significantly reduced the decay of cherry tomatoes caused by B. cinerea with an average of 88.37%. Also, the disease severity value recorded for the plant treated with the EOs mixture was 19.29% compared to the control with an average of 88.57%. This study demonstrates that the mixture of L. dentata, S. rosmarinus, and C. citratus EOs is a promising natural antifungal agent for managing B. cinerea infections.
1 Introduction
EOs are complex mixtures of volatile, organic compounds generated by plants, and they are accountable for a plant’s distinctive flavor and fragrance (Tisserand and Young, 2014). EOs’ main compounds are categorized under two structural components: terpenoids and phenylpropane (Ni et al., 2021). For centuries, EOs were appreciated for their biological, antimicrobial, and other beneficial effects in medicine (Ni et al., 2021), and they’re currently applied in additional fields from cosmetics and food industries to agriculture (Moghaddam and Mehdizadeh, 2017). For example, EOs compounds can successfully inhibit the growth of microorganisms (Hammer et al., 1999; Koroch et al., 2007), which make them potential candidates for controlling plant pathogens (Isman, 2000; Berrada et al., 2012; Medina-Romero et al., 2021; Tagnaout et al., 2023). EOs and their components are also valued for their availability and their low cost (Mutlu-Ingok et al., 2020).
In recent years, studies have indicated efficiency against phytopathogenic fungi such as B. cinerea, the second most important phytopathogenic fungus worldwide (Dean et al., 2012) and the causal agent of grey mold, a standout post-harvest disease associated with tomato production (Lahmyed et al., 2021; Alkilayh et al., 2024). The losses caused by this pathogen can amount to 1 billion euros a year (Dean et al., 2012) and it’s a serious threat to many croups production in Morocco (Berrada et al., 2012; Qessaoui et al., 2024).
Based on the literature, EOs from various plant species indicated efficiency against this pathogen (Bouchra et al., 2003; Soylu et al., 2010; de Oliveira Filho et al., 2021; El Abdali et al., 2022; He et al., 2023; de Albuquerque Sousa et al., 2024; Ramos et al., 2024; Bouqellah et al., 2025). EOs lavender, lemongrass, mint and others were tested in vapor phase against B. cinerea and concluded ability to suppress the fungus (Tančinová et al., 2022). Syzygium aromaticum and Brassica nigra and their combination were found to exhibit inhibitory effect against the pathogen in vitro and in vivo (Aguilar-González et al., 2015). The monoterpenes carvacrol and thymol induced conidial death of B. cinerea (Pedroso et al., 2024), and EOs derived from Pelargonium roseum inhibited the mycelium growth and spore germination of B. cinerea in cherry tomato (Fuyan et al., 2025). EOs can also be applied in synergy with other EOs to increase their biological effect and reduce EOs doses (Targino de Souza Pedrosa et al., 2021; Milagres de Almeida et al., 2023).
The present study aims to investigate EOs extracted from the aerial parts of three plant species grown in south-eastern Morocco: L. dentata and S. rosmarinus from Lamiaceae family and C. citratus belonging to the Poaceae; and to test their synergistic effect in vitro and in vivo against B. cinerea. The findings intend to further knowledge on the synergism between different EOs and their multiple active compounds.
2 Materials and methods
2.1 Preparation of the pathogen
Botrytis cinerea (PX434395) was isolated from infested leaves of tomato in the Chouka Ait Baha region, Morocco. Isolate characterization was carried out at the Plant Protection Laboratory of INRA Agadir, Morocco. Identification of B. cinerea was conducted based on morphological, microscopic, and molecular characteristics (Gull and Trinci, 1971; Blancard, 2012). The fungus was maintained in potato dextrose agar (PDA) and stored at 4°C until use.
2.2 Extraction and gas chromatography–mass spectrometry analysis of EOs
Three plants (C. citratus, L. dentata, and S. rosmarinus) were collected at the flowering stage from the experimental farm of the National Institute for Agricultural Research (INRA), Agadir, Morocco (30°02′42.2”N 9°33′13.4”W) in May 2023. The plants were identified and confirmed by botanists from INRA in Agadir and Ibn Zohr University, Morocco. EOs were extracted from air-dried plant materials by hydro-distillation for 3 h using a Clevenger-type apparatus. Following extraction, the EOs were dried using anhydrous sodium sulfate and stored in sealed vials at temperature (4°C) until analysis (Soltanbeigi and Maral, 2025). The yields were calculated using the following formula:
The GC–MS analysis was performed using GCMS-TQ8040 SHIMADZU, JAPAN. Rtx®-5MS fused-bond column (30 m length, 0.25 mm internal diameter, and 0.25 µm film thickness, Restek, PA, USA) was installed. The starting temperature was 50°C (2min) to 300°C with a ramp of 5°C/min and an isotherm at 300°C for 3 min. The injector temperature was set at 250°C and helium with a flow rate of 1.5 mL/min was used as carrier gas. The conditions for the mass spectra were as follows: Ion source temp 200°C, Interface temp: 280°C, Mass range: 50–500 m/z, electron ionization (EI) at 70 eV. The samples were filtered using a syringe filter (0.45 µm). One μL of diluted samples (1:10 hexane, v/v) was injected in split mode. The analysis of compounds was identified with the database NIST 2017, and the retention indices were determined via n-alkanes standards (Mahmoud et al., 2023).
2.3 Optimization of the antifungal activity of the EOs using the methodology of mixture design
2.3.1 Determination of the in vitro antifungal activity of EOs
Antifungal activity of the three EOs and their mixtures (ratio 1:1:1) on mycelial growth of B. cinerea was determined by the volatile activity (VA) assay as described by Basaid et al. (2021) with some modifications. Sterilized Petri dishes (inner diameter 90 mm) were filled with 20 mL of PDA, and one mycelium plug (5-mm diameter) was placed on the PDA in the center of the Petri dishes. The EOs were pipetted onto sterile Whatman 1 filter paper discs (25-mm diameter) at the concentrations of (C1 = 0.02, C2 = 0.04, C3 = 0.08, C4 = 0.16, and C5 = 0.32 µL/mL air of Petri dish). The treated discs were attached to Petri dish lids, and the Petri dishes were sealed with parafilm and incubated at 24 ± 1°C for 7 days. The mycelial growth inhibition (MGI) rate was calculated using the formula described by Pandey et al. (1982):
where Dc and Dt represent mycelial growth diameter in control and treated Petri dishes, respectively. The treatments were replicated three times.
2.3.2 Experimental design
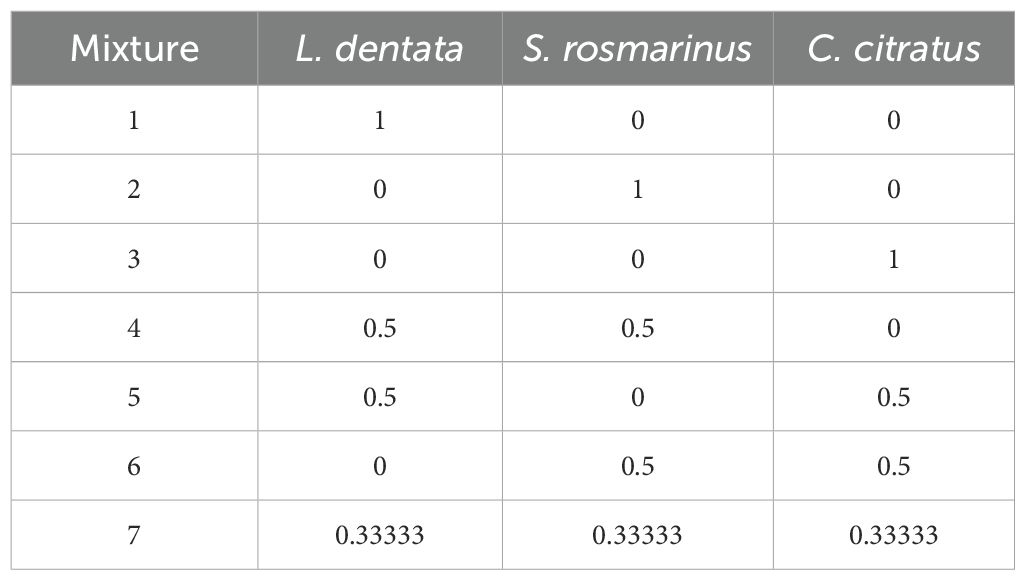
A simplex-centroid design was adopted to optimize the mixture of the EOs of L. dentata, S. rosmarinus, and C. citratus as outlined by Benkhaira et al. (2023). The components of the EOs system are detailed in Table 1. The proportion of each EO in the mixture can range in value from 0 to 1, with the total sum of the three components equaling 1. The antifungal activity of the EOs was evaluated using the inhibition rate and the half maximal inhibitory concentration (IC50).
2.3.3 Experimental matrix and mathematical model
Seven compositions are represented by an equilateral triangle (Figure 1). The triangle vertices (X1, X2, X3) correspond to the pure oils (100%), the binary mixtures (50%/50%) are conforming to midpoints of three sides of the triangle (X4, X5, X6), and equi-proportional mixture of the three oils (33.33%/33.33%/33.33%) is fixed in the center of the triangle (X7). The special cubic model was applied to assert responses based on the independent variables as represented by the following equation:
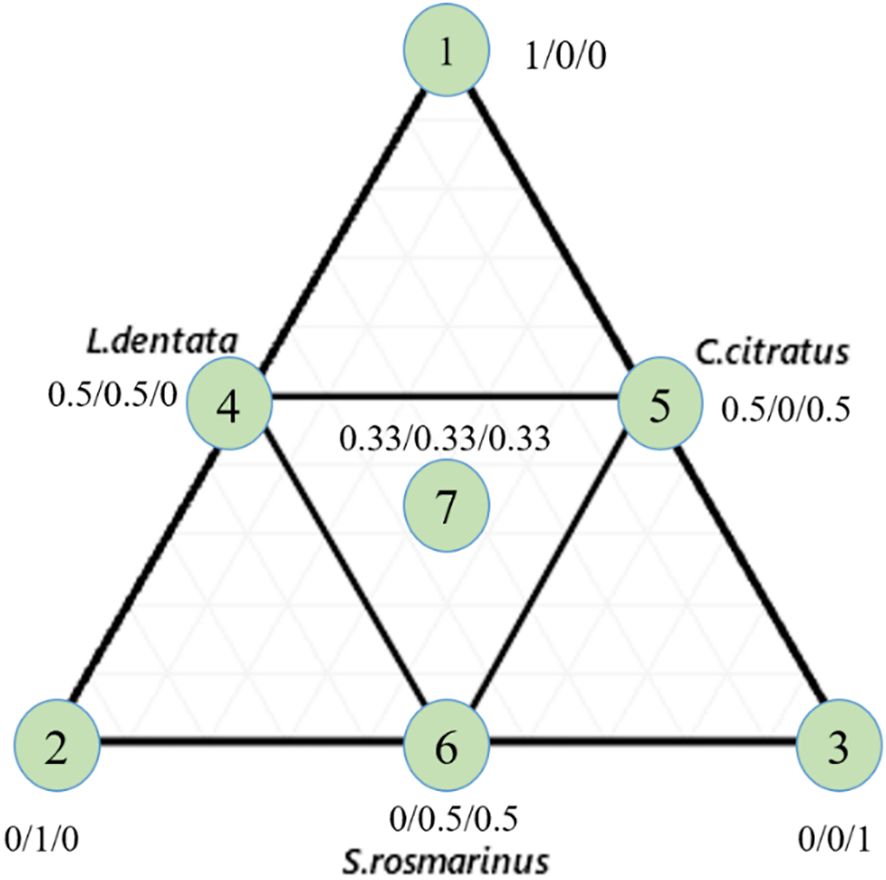
Figure 1. Equilateral triangle of the arrangement of mixtures using the simplex centroid design method. 1: L. dentata EO; 2: S. rosmarinus EO; 3: C. citratus EO.
where Y is the experimental response (IC50). The α1, α2, and α3 are linear regression coefficients, α12, α23 and α23 are binary regression coefficients, and α123 is the ternary regression coefficient, while is the regression error term (Benkhaira et al., 2023).
2.3.4 Mixture design analysis
The statistical significance of the mathematical model was assessed at a 95% confidence level using the F-ratio mean square regression/mean square residual, which compares the mean square regression to the mean square residual; higher F-values indicate greater variability in the results (EL Hachlafi et al., 2023). Additionally, the ratio of the mean square lack of fit to the mean square pure error was analyzed to evaluate the model’s adequacy, with higher values suggesting potential inadequacies. The coefficient of determination (R²) was calculated to assess model quality. The significance of estimated factors was evaluated using the Student’s t-test, while ANOVA’s F-test confirmed the overall model significance. Analyses were conducted using Design Expert software version 23.1 and Minitab® 19.2020.1. For optimization, contour plots and 3D surface plots illustrated trade-off areas among the studied components. The desirability tool was used to identify the optimal values, balancing factors for the best outcome. This tool adjusts the mathematical model within a range of 0 to 1, where 0 indicates an undesirable response and 1 signifies a highly desirable response.
2.4 Antifungal assay with the selected mixture
2.4.1 In vitro direct contact assay
The EOs mixture that showed a significant effect after the simplex-centroid design analysis was selected. Antifungal activity was evaluated by incorporating the EOs mixture into the culture medium. The EO was added to sterilized PDA to obtain the following range of concentrations: 0.2, 0.4, 0.8, 1.6, 3.2 μL/mL. The plates were inoculated with the fungus, using a 5 mm diameter agar disk. The agar plates were then incubated for 7 days at 24 ± 1°C. The antifungal activity was expressed by the percentage of mycelial growth inhibition and calculated according to the formula described in section 2.3.1. The treatments were replicated three times.
2.4.2 Minimum inhibitory concentration and minimum fungicidal concentration
MIC was determined by the broth microdilution method. One hundred μL of Sabouraud dextrose broth (SDB) was added to the 96-well plates, and a serial twofold dilution of the EOs mixture was prepared to obtain concentrations from 0.2 to 3.2 μL/mL. Then, 100 μL of spore suspension (105 spores/mL) was added to each well. The control was set up using SDB with spores. Plates were incubated at 24 ± 1°C for 24 h. The MIC is the lowest concentration that prevents B. cinerea growth. The MFC was determined by transferring 100 μL of the mixture which showed the lowest concentration without mycelial growth to PDA plates. The plates were incubated at 24 ± 1°C for 72 h. The MFC is the concentration at which B. cinerea showed no growth (Wiegand et al., 2008).
2.4.3 Spore germination assay
Botrytis cinerea spore suspension (105 spores/mL) was incorporated into a solution of Sabouraud dextrose broth and EOs mixture at different concentrations (0.2, 0.4, 0.8, 1.6, 3.2 μL/mL) at equal ratio (1:1, v/v). In the control treatment, the same volume of spores inoculated was used without the EOs. The tubes were incubated at 24 ± 1 °C for 24 h. Then the spore germination was visualized with a light microscope at 400 × magnification, and a minimum of 100 spores from each replicate were analyzed. Spores were considered germinated when the size of the germ tube surpassed the largest diameter of the spore. The inhibition of spore germination (IG) was calculated using the following formula:
Gc and Gt are, respectively, the averages of spores germinated in controls and treatment tubes. For each concentration, five replicates were assessed (Soylu et al., 2010).
2.4.4 In vivo assay on cherry tomato fruits
The effect of the EOs mixture on B. cinerea in vivo was assessed using a previously reported method by Yang et al. (2023) with some modifications. The fruits were disinfected with 0.01% sodium hypochlorite for 2 min and air-dried. Each fruit was punctured at two points (3 mm deep and 3 mm wide), and 10 μL of spore suspension (105 spores/mL) was pipetted into the wound. The fruits were air-dried for 1 h, then 10 μL of the mixture at 0.81 μL/mL (IC90) of EOs emulsified in Tween 80 (0.1%) solutions was added to each wound. Control samples were pipetted with 10 μL of sterile Tween 80 solution. The samples were stored in plastic containers at 24 ± 1°C for 7 days. Each treatment contained three replicates with 10 fruits per replicate. The proportion of rot lesions was calculated using the following formula:
Where, Dc is mycelial growth diameter in control, and Dt is mycelial growth diameter in treated fruits, while a and b represent the vertical and horizontal lesion ray (mm).
2.4.5 In vivo assay on tomato plants
IC90 of the EOs mixture was selected for the in vivo trial. The EOs were prepared by dissolving the requisite amounts in Tween 80 (0.1%) solution. Tomato plants were arranged in a split-plot design with three replicates of 6 plants per treatment. The plants were inoculated with a suspension of 105 spore/mL of B. cinerea. After 24 h, tomato plants were sprayed with the emulsion of EOs (10 mL for each plant) uniformly with a manually operated sprayer, while the control plants were sprayed with Tween 80 (0.1%) solution. Control and EOs-treated plots were assessed 3 weeks after treatment. The disease severity index of grey mold on the tomato leaves was rated on a scale of 0–5 (0 = no disease symptom, 1 = 0.1–20%, 2 = 20.1–40%, 3 = 40.1–60%, and 4 = 60.1–80%, 5 = 80.1-100%) as the percentage of diseased leaf area (Lee et al., 2006).
2.5 Statistical analysis
The data were analyzed using RStudio (Posit Software, PBC. 2024.04.2). The comparison of means was performed using the Tukey, Kruskal wallis and Mann-Whitney U tests (p<0.05). The IC50 and IC90 of EOs and their mixtures were calculated using GraphPad Prism 10 version 10.3.1 (509) 2024.
3 Results
3.1 Chemical composition of EOs
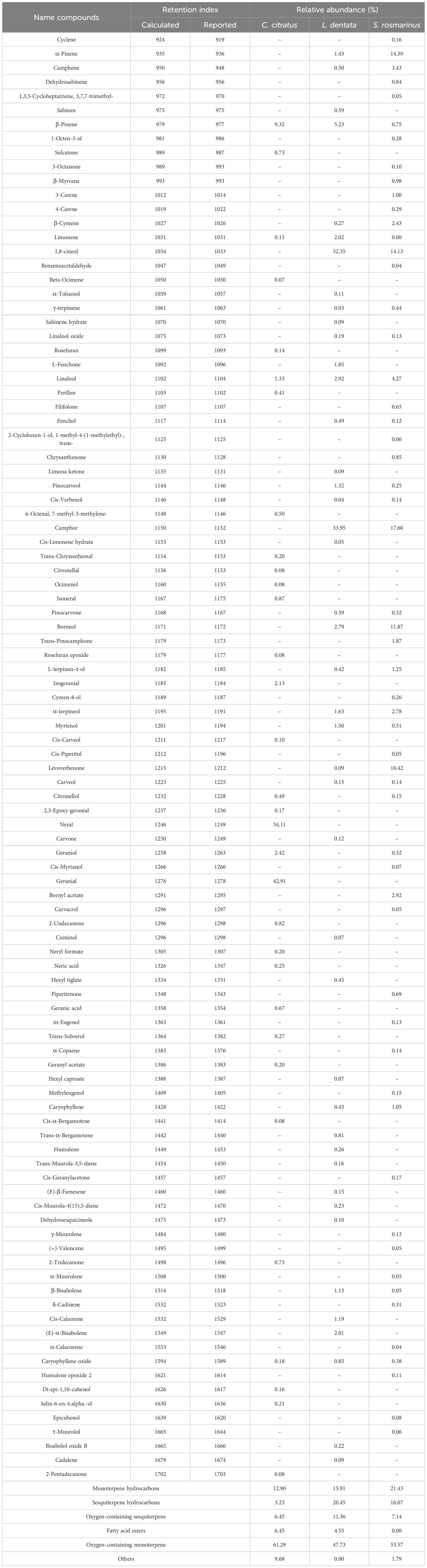
The EOs yields obtained by hydrodistillation for S. rosmarinus, L. dentata, and C. citratus were 0.97 ± 0.06%, 0.91 ± 0.04% and 1.02 ± 0.02%, respectively. The chemical profiles of the EOs of the three medicinal plant species are summarized in Table 2. A total of 102 volatile compounds were identified from the GC/MS analyses. Thirty-one volatile compounds were identified in the oil of C. citratus leaves, in which oxygen-containing monoterpene represented the most prevailing class, constituting 61.29% of the oil, followed by monoterpene hydrocarbons (12.90%). Geranial (42.91%), neral (34.11%), and β-pinene (9.32%) were the most abundant compounds. Furthermore, geraniol, isogeranial, and linalool were present at considerable quantities, representing 2.42, 2.13 and 1.33%, respectively.
Regarding L. dentata EOs, 44 volatile compounds were identified in which oxygen-containing monoterpene and sesquiterpene hydrocarbons constituted the major volatile compounds, representing 47.73 and 20.45% of the oil content, respectively. Camphor (33.95%), 1,8-cineol (32.35%), and β-pinene (5.23%) were the most abundant. Moreover, phytochemicals included linalool (2.92%), (E)-α-bisabolene (2.81%), α-terpineol (1.63%), borneol (2.79%), limonene (2.02%), α-pinene (1.43%).
Fifty-six compounds of S. rosmarinus EO were identified. Oxygen-containing monoterpene predominated with a value of 53.51%, followed by monoterpene hydrocarbons (21.43%) and sesquiterpene hydrocarbons (16.07%). The major components were camphor (17.60%), α-pinene (14.39%), and 1,8-cineol (14.13%), followed by borneol (11.87%), levoverbenone (10.42%), and linalool (4.27%). In addition, amphene (3.43%), bornyl acetate (2.92%), α-terpineol (2.78%), and β-cymene (2.43%).
3.2 Optimization of the antifungal activity of the EOs using the methodology of mixture design
3.2.1 Determination of the in vitro antifungal activity of EOs
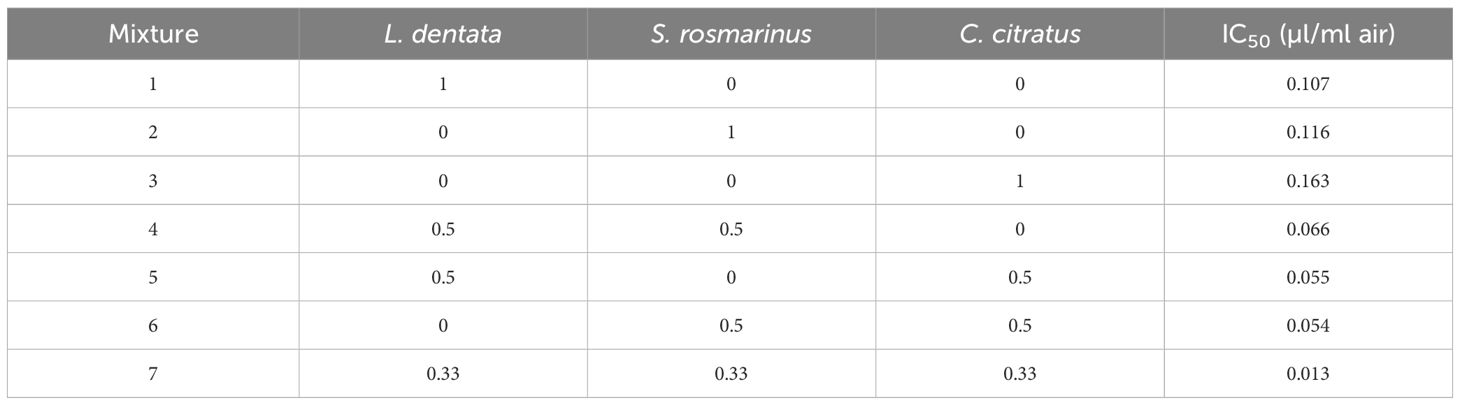
The results of in vitro antifungal activities of the EOs and their mixtures on B. cinerea are shown in Table 3. The application of all mixtures inhibited the mycelial growth of B. cinerea significantly in a dose-dependent manner (P< 0.001). Furthermore, all EOs mixtures showed higher inhibition compared to pure EOs. The concentration C5 in all EOs exhibited total inhibition of mycelial growth. The activity of EOs in the concentrations C4 and C3 was as follows: For S. rosmarinus, 80.69% and 55.30%, respectively; 72.05 and 46.79, respectively for C. citratus; and it reached 63.19% and 56.35%, respectively in the case of L. dentata. Concentration C1 demonstrates the lowest effect on fungal growth with an average of 8.74%, 18.20%, and 19.33% for S. rosmarinus, C. citratus, and L. dentata, respectively.
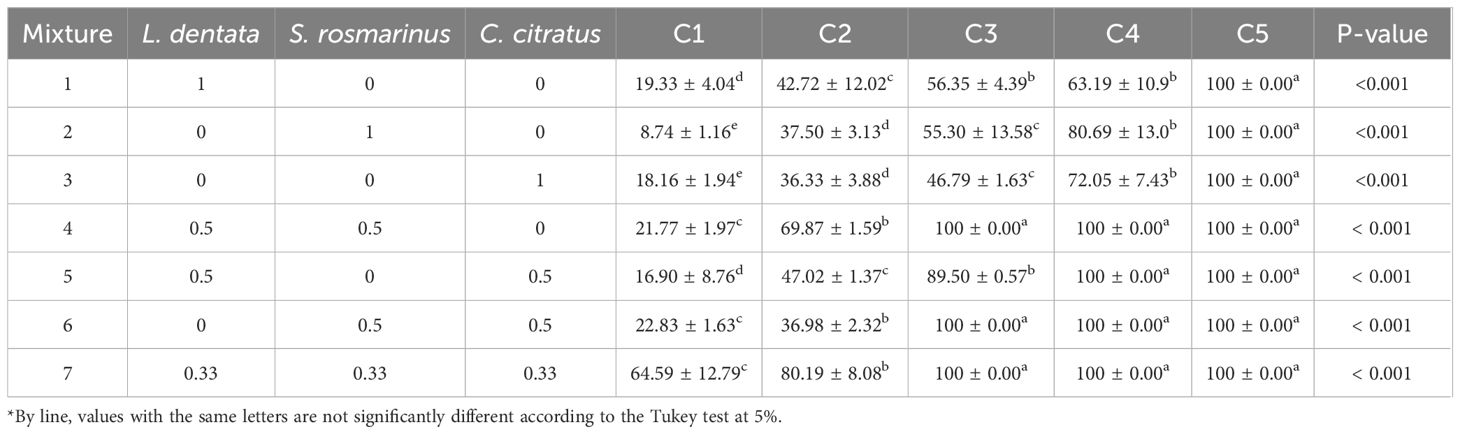
Table 3. Matrix of simplex centroid design and results for in vitro antifungal activity of EOs against mycelial growth of B. cinerea (Concentrations: C1-C5).
To examine the effect of synergism on the antifungal activity of the EOs, different ratios of mixtures were tested. The results indicate that the inhibition rate increased from 21.77% to 100% (Table 3). Mixture 7 (equal proportion of the three EOs) exhibited the highest inhibition rate compared to other treatments, showing an average of 64.59% in C1 followed by C2 with 80.19%, whereas 100% inhibition was obtained at C3 compared to individual treatments which showed this value only at a higher concentration (C5).
3.2.2 Simplex centroid design analysis
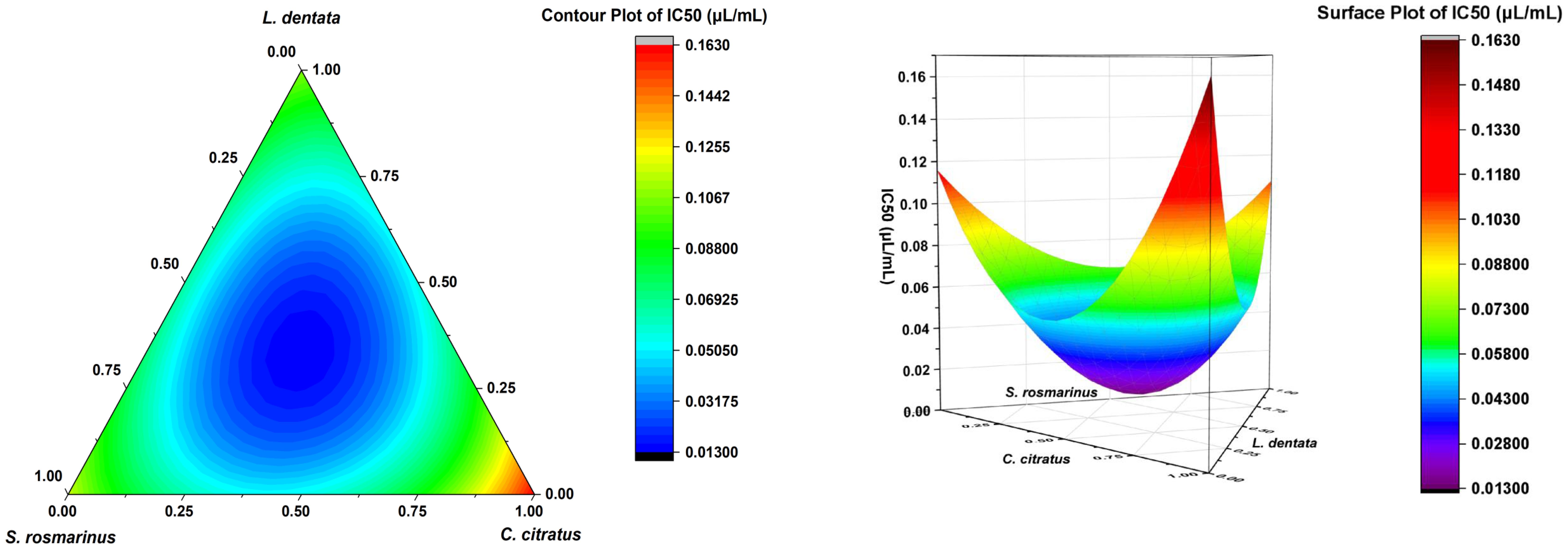
The matrix of simplex centroid design and results for IC50 are shown in Table 4. The IC50 was calculated based on mycelial growth inhibition. The results obtained showed that the lowest value (0.013 µL/mL) was obtained with mixture 7 containing the three EOs, while the highest value was obtained with C. citratus EOs (0.163 µL/mL).
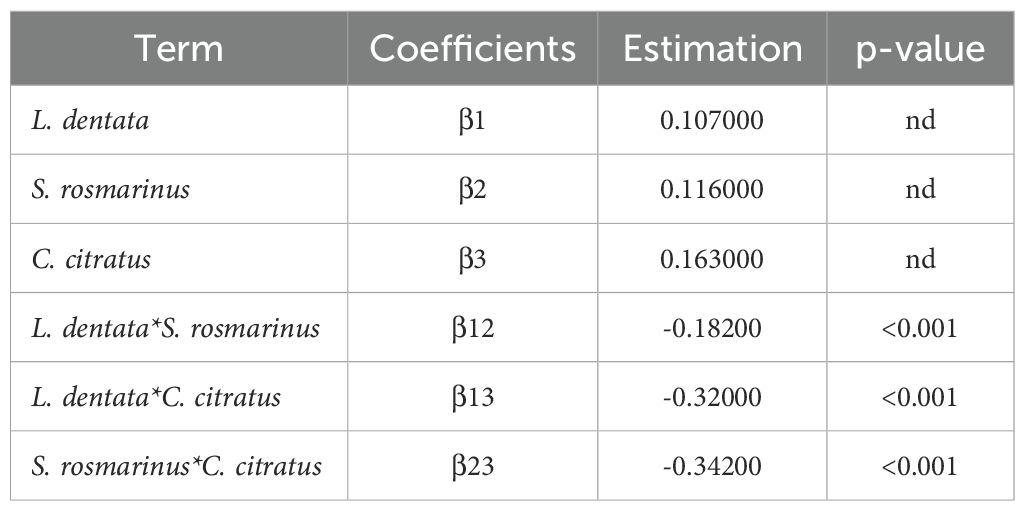
The regression coefficients for the special model have been computed and are presented in Table 5. The associations between the tested parameters and the obtained responses for IC50 were found using regression models with significant coefficients (p-values < 0.05). Due to the inherent dependency among the components (X1 + X2 + X3 = 1), individual p-values for the linear coefficients (β1, β2, β3) were not estimable. Among the interaction terms, the coefficients of the ternary (β123) and binary (β12, β13, β23) models demonstrate statistical significance regarding the IC50 response. These interactions have a negative impact. In fact, the interactions between the factors reduce the value of the response variable IC50. The term L. dentata * S. rosmarinus * C. citratus has the highest negative effect among the interactions. However, the coefficients of individual components (β1, β2, β3) are non-significant (p > 0.05) and do not demonstrate an effect on the IC50.
Equation 2 represents a mathematical model that describes the response as a function of the tested components.
The standard error is very small (S = 0.001), some p-values may not be calculated because there is not enough dispersion to detect significant differences (Case of individual mixtures). The Sum of Predicted Error Squares (0.0000315) is close to 0, indicating that all observations fit the model almost exactly. Moreover, R2 and R2Adjusted show higher values (99.97 and 99.96, respectively) suggest strong agreement between the modeled and observed data. Additionally, the ANOVA F-tests confirmed the validity of the models, with p-value<0.001 and F = 7436 (Table 6).
3.2.3 Mixture profile
The contour plot and 3D surface graph (illustrated as 2D and 3D mixture plots in Figure 2) demonstrate the optimal proportions of the three EOs mixture to maximize responses, thereby illustrating the relationship between responses and proportions of each EO. The creation of these plots was facilitated by Design-Expert software, which employs iso-response curves, thus ensuring optimal response values. The color scheme utilized in the plots offers a visual representation of the relationship between the responses and the proportion of each EO. Specifically, blue is indicative of lower IC50 values and higher antifungal activity, while the transition of colors from yellow to dark red signifies increasing IC50 values, thereby indicating reduced effectiveness.
3.3 Antifungal assay with the selected mixture
3.3.1 In vitro direct contact assay
Based on the results of simplex-centroid design analysis, the mixture 7 containing the three EOs was selected. As shown in Figure 3, the mycelial growth of B. cinerea was significantly reduced in all examined concentrations (P-value < 0.001). The highest inhibition rate was obtained with the concentrations 1.6 and 3.2 µL/mL reaching 100%, followed by the concentrations 0.8 and 0.4 µL/mL (88.65% and 38.28%, respectively). However, at the lowest concentration, the inhibition rate was 25.32%. Moreover, the IC50 and IC90 obtained were 0.46 and 0.81 µL/mL, respectively.
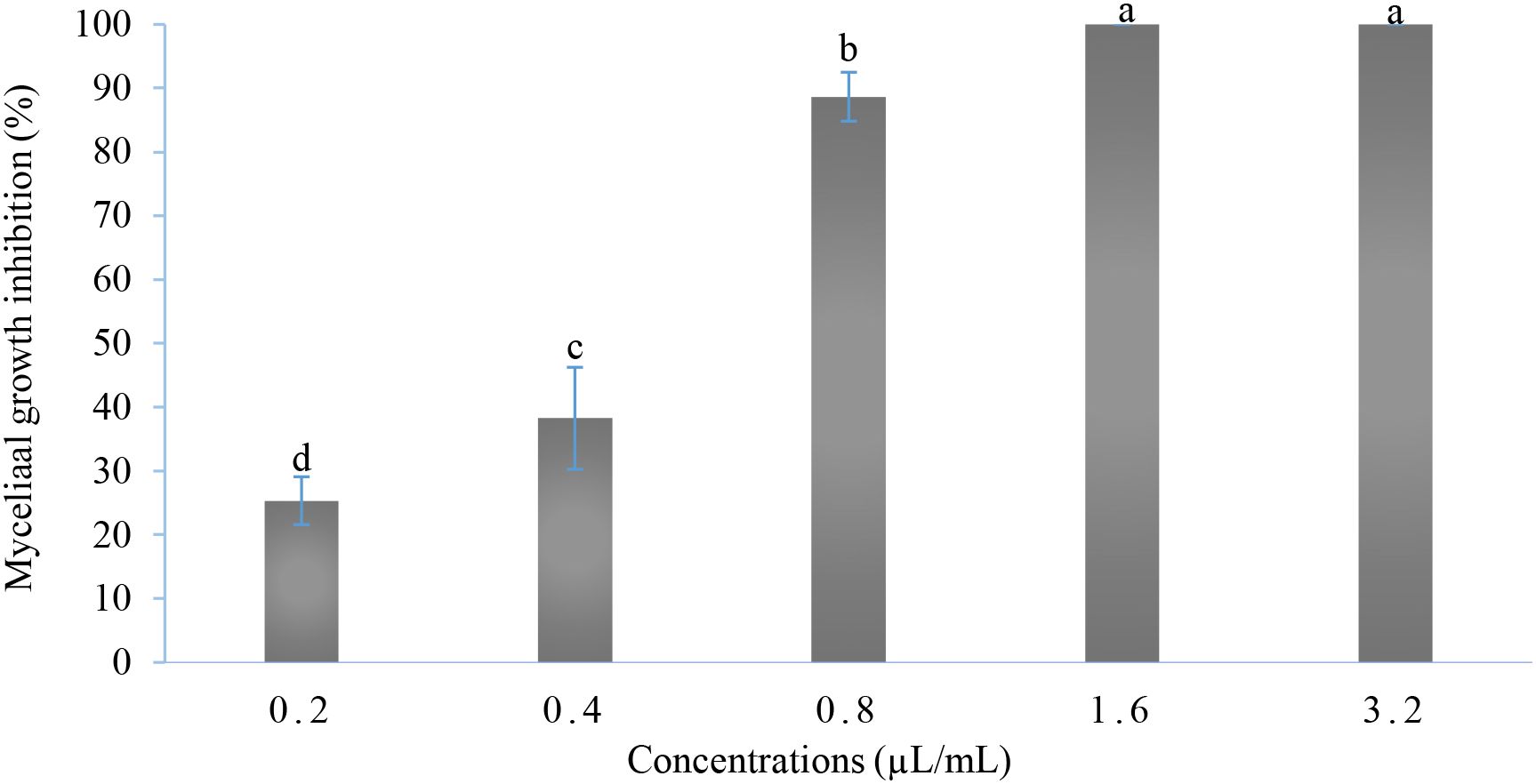
Figure 3. In vitro antifungal activity of the selected mixture. Values with the same letters are not significantly different according to the Kruskal-Wallis test followed by Dunn’s test at 5%.
3.3.2 MIC, MFC, and inhibition of spore germination
Effects of different concentrations of the EOs mixture on spore germination of B. cinerea are shown in Figure 4. According to the results obtained, the concentrations tested showed a significant difference in the inhibition of spore germination (P-value<0.001). The inhibition rate proportionally increased with concentrations ranging from 5.79% to 100%. On the other hand, the minimum inhibitory concentration was found to be 1.6 µL/mL, while the minimum fungicidal concentration was higher than 3.2 µL/mL (MFC > 3.2 µL/mL).
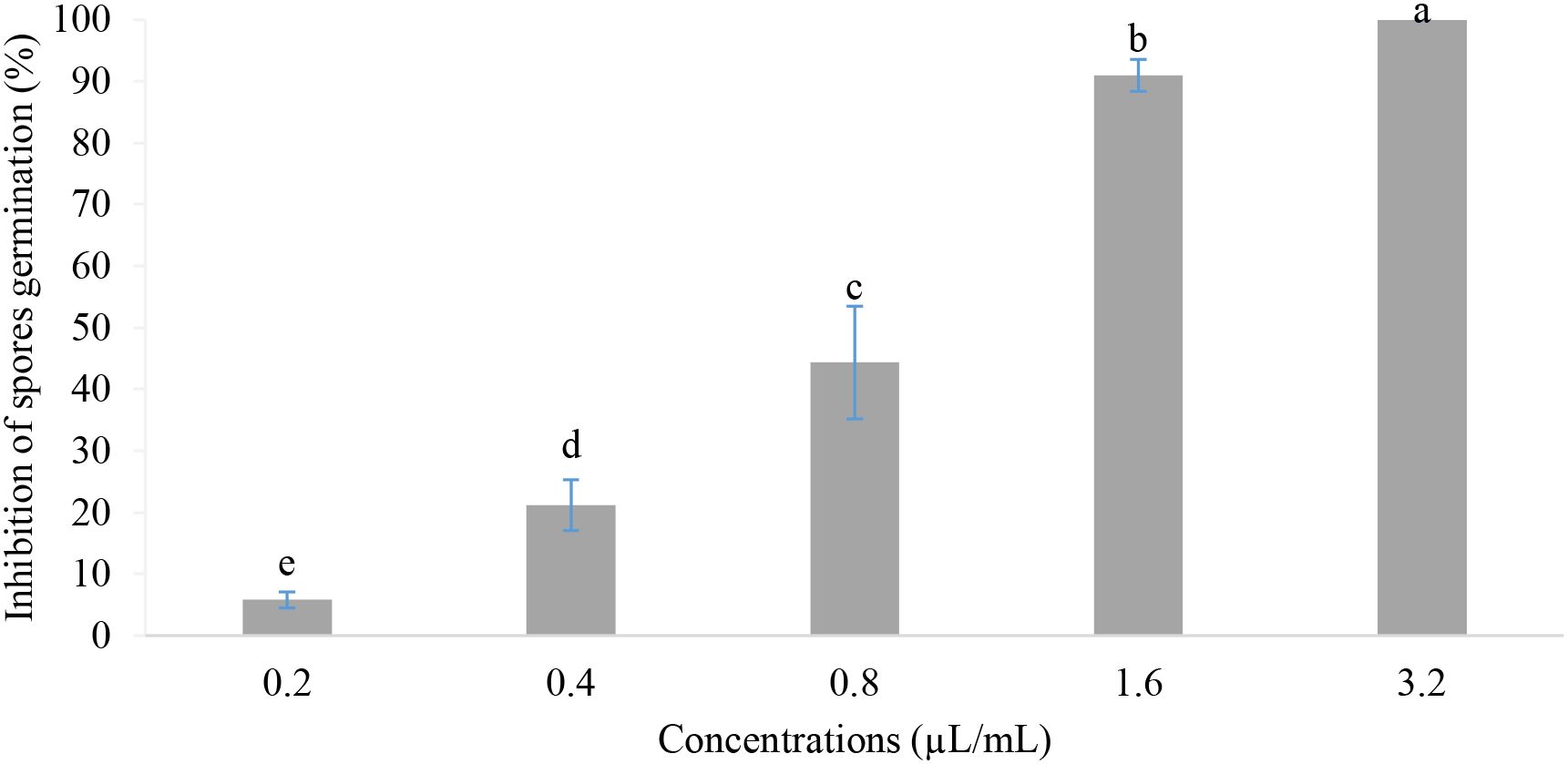
Figure 4. Spore germination inhibition in vitro. Values with the same letters are not significantly different according to the Kruskal-Wallis test followed by Dunn’s test at 5%.
3.3.3 In vivo assay of selected mixture on cherry tomato fruits
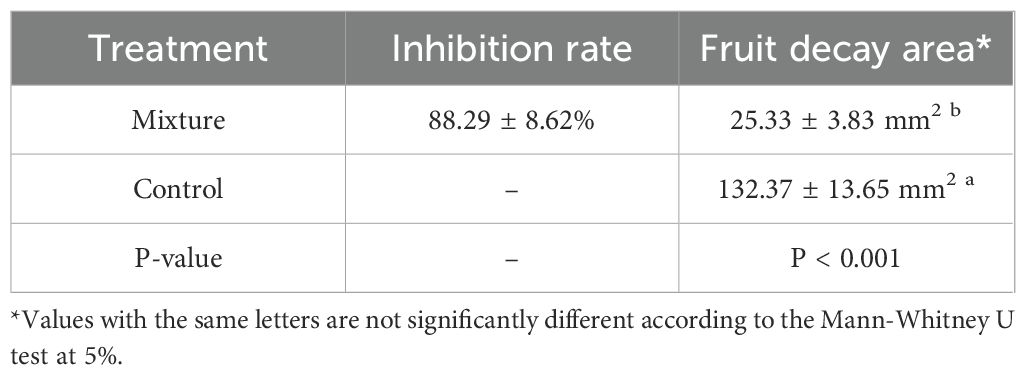
Table 7 summarizes the results of the antifungal activity in vivo of the selected mixture on cherry tomato inoculated with B. cinerea. IC90 of the mixture exhibited an antifungal activity in vivo with an average of 88.29%. Moreover, the fruit decay area for untreated fruits was 132.37 mm2, while for treated fruits, the fruit decay area was 25.33 mm2, revealing a range of reduction in fruit decay (P-value<0.001).
3.3.4 In vivo assay of selected mixture on tomato plants
The results of the in vivo antifungal activity of the selected mixture on tomato plants inoculated with B. cinerea are demonstrated in Figure 5. After three weeks, all plants showed the development of B. cinerea symptoms with an average disease incidence of 100 ± 0.00%. However, the disease severity was significantly lower in plants treated with the EOs (22.86 ± 4.26%), compared with the control plants (P < 0.05), with a recorded value of 75.71 ± 6.46% (Figure 6).
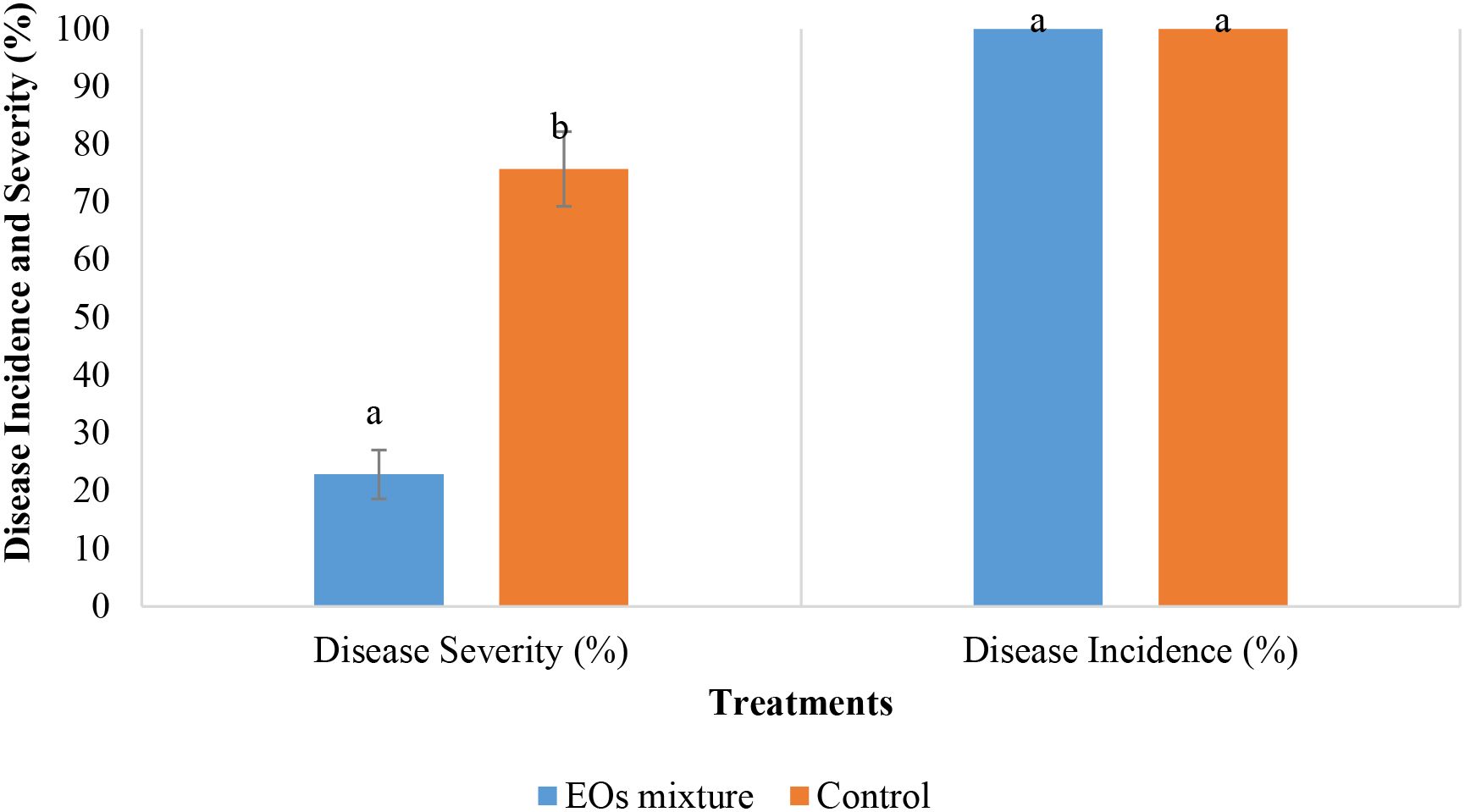
Figure 5. Effect of the mixture on the control of the grey mold caused by B. cinerea on tomato plants. Values with the same letters are not significantly different according to the Mann-Whitney U test at 5%.

Figure 6. Effect of EOs on B. cinerea infested tomato plants. (A) Control and (B) Treated plant with EOs.
4 Discussion
The present study explored the chemical profile and antifungal activity of EOs extracted from three medicinal and aromatic plants (C. citratus, S. rosmarinus, and L. dentata) against the phytopathogenic fungus B. cinerea. Through a combination of gas chromatography-mass spectrometry (GC-MS) profile analysis and in vitro antifungal assays, both individual and synergistic effects of the EOs were assessed. The results reveal not only the antifungal potential of these plant-derived oils but also demonstrate that their combination yields significantly improved inhibition, highlighting the value of synergistic interactions in botanical formulations.
GC-MS profiling of the tested EOs highlights both qualitative and quantitative differences in the major components, reflecting species-specific metabolic profiles. C. citratus EOs was predominantly composed of geranial, neral, and β-pinene. Our findings are consistent with the results obtained by other authors (He et al., 2023; Faria and Barbosa, 2024; Cherif et al., 2025; de Andrade Bomfim et al., 2025; Owolabi et al., 2025). Similar results were also found in a study previously conducted by Dangol et al. (2023), with a higher concentration of β-pinene compared to our results. S. rosmarinus was found rich in camphor, α-pinene, and 1,8-cineol; a comparable result to the previous study conducted by Cherif et al. (2025). However, the amount of 1.8-cineole was higher than the quantity found in our study (32.43%). Moreover, analogous results were described in other studies (Ait Bouzid et al., 2023; Annemer et al., 2023; Houzi et al., 2024; Ivanova et al., 2025; Xia et al., 2025). In contrast, Mozafari et al. (2024) found that the chemical composition of S. rosmarinus oils resulted in high concentration of 1,8-cineole, trans-caryophyllene, α and β-pinene; whereas Amiri et al. (2022) recorded camphene (33.8%) as the dominant compound in rosemary EO. Regarding L. dentata EOs, camphor, 1,8-cineol, and β-pinene were the most abundant compounds, in agreement with earlier reports (Cossetin et al., 2021; Ait Bouzid et al., 2023; Ait Elallem et al., 2024). The chemical analysis by Belcadi et al. (2024) revealed the same ranking, with a higher concentration of β-pinene. Different results were revealed by others. Akachoud et al. (2024) showed that camphor (28 %), terpinene-4-ol (12.14 %), and camphene hydrate (6.03 %) were the most abundant. Also, it was reported that 1,8-cineol, camphor, and fenchone were the major compounds of these EOs (Wagner et al., 2021; de Oliveira Xavier et al., 2024). This variability in the chemical profile of EOs may be attributed to several factors, including plant species, geographic origin, cultivation conditions, extraction method, and the developmental stage of the plant material (Mohamed Hanaa et al., 2012; Labhar et al., 2024; Jemai et al., 2025; Lahmar et al., 2025).
Furthermore, our findings revealed that the EOs of three plants exhibited varying levels of inhibitory activity of B. cinerea in a dose-dependent manner. This might be attributed to the mode of resistance behavior of the fungi against various substances present in EOs. For all plants, the concentration of 0.32 µL/mL air inhibited the mycelia growth of B. cinerea by 100%. These results corroborate those described by Aguilar-González et al. (2015), which showed that Syzygium aromaticum EO and Brassica nigra EO inhibited B. cinerea growth at a concentration of 92.56 μL/L air and 5.42 μL/L air, respectively. Amiri et al. (2022) found that Cuminum cyminum EO exhibited 100% inhibition in the vapor phase at 4 μL/mL, while EO of S. rosmarinus, Citrus limon and Eucalyptus globulus exhibited a complete inhibitory effect at 10 μL/mL, ranging between 41.1% and 40% inhibition rate for lemon and eucalyptus, respectively. In another study, the EOs of Cymbopogon martinii and Mentha spicata showed the greatest efficacy against B. cinerea, with an inhibition rate of 100% in the volume of 5 µL, while Cinnamomum camphora and Mentha×piperita revealed the same activity at 10 µL (de Oliveira Filho et al., 2021). Previous investigations reported that secondary metabolites act against fungi through diverse mechanisms such as the disruption of the fungal cell membrane, the inhibition of cell wall synthesis, the disruption of cell division by inhibiting mitosis, and the inhibition of DNA and RNA synthesis. In this sense, Pedroso et al. (2024) assessed the antifungal activity and mechanism of action of monoterpenes against B. cinerea. The findings showed that carvacrol, citral, citronellal, citronellol, geraniol, and thymol demonstrated significant antifungal activity. Moreover, thymol and carvacrol induced conidial death, resulting in the disruption of cell membrane integrity, increased intracellular ROS levels, and decreased mitochondrial membrane potential. Singh et al. (2024) also showed that 1,8-cineole significantly reduced the ergosterol biosynthesis and downregulated genes related to chitin synthase, ergosterol synthase, cellulase production, and glycosyl hydrolase. Furthermore, Kong et al. (2022) revealed that camphor inhibited the growth of Fusarium and caused cytomembrane destruction, enhancing its permeability and releasing intracellular macromolecules, such as nucleic acids and proteins. Also, Zheng et al. (2021) reported that citral inhibits ergosterol biosynthesis and damages membranes in filamentous fungi. In addition, it’s reported that citral downregulated ochratoxin biosynthetic genes, and reduced the level of enzymes associated with respiration, resulting in the disruption of energy metabolism (Wang et al., 2021).
Although many studies report the antifungal activity of individual EOs, fewer have addressed the effects of EO mixtures, confirming that the overall activity of botanical extracts is a result of mixtures of compounds with synergistic, additive, or antagonistic activity (de Oliveira Filho et al., 2021; Gonçalves et al., 2022; Faria et al., 2023; Ben Akacha et al., 2024; Elbouzidi et al., 2024; Kaya et al., 2025; Owolabi et al., 2025; Zhu et al., 2025). Similarly, our study confirmed that the combination of EOs increased the antifungal activity of B. cinerea. All evaluated mixtures showed total inhibition of mycelial growth with the concentration C3 (0.08 µL/mL air of Petri dish) except for mixture 5 (L. dentata EOs + C. citratus EOs), which inhibited the mycelial growth at C4 (0.16 µL/mL air of Petri dish). This increase in antifungal activity is hypothesized to result from the complementary mechanisms of action of the EOs compounds. For instance, while phenolic compounds such as carvacrol and eugenol have been shown to destabilize membranes (Hyldgaard et al., 2015; Bai et al., 2022), aldehydes such as citral have been demonstrated to interfere with intracellular proteins and enzymatic systems, thus amplifying the overall antifungal effect (Cai et al., 2019). Our findings are strongly supported by prior studies investigating EO synergies. In a study, 1,8-cineole showed synergistic interactions with α-pinene, increasing the activity against the yellow fever mosquito Aedes aegypti (Sarma et al., 2019). Similar results were found by Aguilar-González et al. (2015) which confirmed the effect of the combination of clove EO with mustard EO in amplifying the inhibition of B. cinerea compared to their individual application. Furthermore, studies reported that the binary mixtures of C. citratus with M. piperita and of Foeniculum vulgare with Satureja montana EOs against the Pinewood Nematode Bursaphelenchus xylophilus resulted in higher activity compared to the individual treatments (Gonçalves et al., 2022; Faria et al., 2023). Nevertheless, the combination of certain bioactive compounds may possess antagonistic interactions in comparison to each compound separately. Sarma et al. (2019) reported that 1,8-cineole showed mainly antagonistic interactions with other monoterpenes, e.g., carvone and limonene, while no interaction was noticed between α-pinene and other terpenes. In another study, Hossain et al. (2016) reported that the use of EOs of Citrus reticulate and Eucalyptus globulus combined with EOs of Thymus vulgaris and Origanum vulgare, respectively, generated no improvement in antifungal activity against Aspergillus niger, A. flavus, A. parasiticus and Penicillium chrysogenum. The same was observed by de Oliveira Filho et al. (2021) where the combination of EOs of M. piperita, C. martinii, C. camphora, and M. spicata as binary mixtures maintains a similar level of antifungal activity against B. cinerea compared to pure EOs.
Based on the simplex-centroid design analysis and the ranking of mixtures regarding their in vitro antifungal activity by the method of exposure to volatiles, the mixture 7 which contains L. dentata, S. rosmarinus, and C. citratus EOs at a ratio (1:1:1) was selected. After exposure by contact at different concentrations of this mixture, the mycelial growth was completely inhibited at 1.6 µL/mL and 3.2 µL/mL, while the IC50 and IC90 obtained were 0.46 µL/mL and 0.81 µL/mL, respectively. Moreover, the values of MIC and MFC were respectively 1.6 µL/mL and >3.2 µL/mL. Compared to other studies, de Oliveira Filho et al. (2021) showed that binary mixtures of M. piperita - C. martini and C. camphora - M. spicata inhibited the mycelial growth of B. cinerea at the concentrations of 500 and 750 µL/L. Many studies demonstrated the inhibition capability of EOs of postharvest fungi under in vitro conditions; however, few studies have been conducted on the in vivo efficacy of EOs. In this study, the optimal mixture of the three EOs showed a strong in vivo antifungal activity against B. cinerea. The decay of cherry tomatoes was inhibited with an average of 88.29%. Also, the disease severity value recorded for the plant treated with the EOs mixture was 22.86% compared to the control, with an average of 75.71%. Similarly, Brito et al. (2021) confirmed that the use of mixtures of EOs of Origanum vulgare, Thymus vulgaris, Citrus limon, and Citrus sinensis and their respective hydrolats enhanced the antifungal activity on B. cinerea inoculum on tomato fruits. In another study, Zhao et al. (2021) found a 70% reduction in the decay of cherry tomatoes with the use of O. vulgare EOs at 62.5 mg/L, compared to untreated samples, while at 250 mg/L, the reduction in the decay of fruits reached 96%. On the other hand, He et al. (2023) demonstrated the efficiency of C. citrates EOs at 125 mg/L, indicating the reduction of the decay area of cherry tomatoes by more than 90%. Regarding in vivo tests on plants, Soylu et al. (2010) evaluated the curative and protective effects of different concentrations of Origanum syriacum L. var. bevanii EO on the infection caused by B. cinerea in the greenhouse. The finding showed that the disease index at 75 mg/L of O. syriacum L. var. bevanii EOs was 77% and 33% in curative and protective effects, respectively.
To reiterate, the combination of EOs increased the in vitro antifungal activity compared to their individual application. Furthermore, the mixture containing the tested EOs has been shown to control infection of tomato fruits and plants by B. cinerea under greenhouse conditions. This interaction among the EOs not only enhances antifungal efficacy but also allows for reduced concentrations, offering both biological and economic advantages. These findings support the development of novel, plant-based fungicidal formulations and contribute to the growing body of research advocating for natural solutions in plant disease management.
5 Conclusion
The current study demonstrates the promising antifungal potential of EOs extracted from C. citratus, S. rosmarinus, and L. dentata, both alone and in combination, against B. cinerea, a major tomato pathogen. The positive effects observed when combining EO mixtures significantly increased antifungal efficacy, lowering the required effective doses and demonstrating their practical advantage over individual applications. The findings highlight the potential of these EO mixtures as a viable alternative to traditional fungicides. Furthermore, their efficacy at low concentrations may help to reduce the selective pressure for pathogen resistance development, thereby improving the long-term viability of crop protection strategies. The use of eco-friendly EO mixtures can have a significant impact on agroecological operations. These combinations are effective at increasing biodiversity, lowering chemical inputs, and addressing the environmental and health risks associated with synthetic pesticides. The ability of mixtures to function through numerous routes makes them an important tool in integrated disease management. Future studies should focus on formulations and their stability, as well as the cost sustainability of EO-based treatments in field settings. This ensures widespread use within agroecological and organic farming systems.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
SC: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. AO: Conceptualization, Methodology, Supervision, Writing – review & editing. RQ: Data curation, Formal Analysis, Methodology, Writing – review & editing. SE: Data curation, Formal Analysis, Writing – review & editing. MA: Data curation, Formal Analysis, Methodology, Writing – review & editing. HL: Data curation, Formal Analysis, Writing – review & editing. HE: Conceptualization, Project administration, Supervision, Writing – review & editing. RB: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the project Bioactive-Xpress funded by the Sustainable Agriculture, UM6P-INRA program.
Acknowledgments
The authors would like to thank Abderrahim Amarraque, and Yassine Imlil for their technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aguilar-González, A. E., Palou, E., and López-Malo, A. (2015). Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innov. Food Sci. Emerg. Technol. 32, 181–185. doi: 10.1016/J.IFSET.2015.09.003
Ait Bouzid, H., Oubannin, S., Ibourki, M., Bijla, L., Hamdouch, A., Sakar, E. H., et al. (2023). Comparative evaluation of chemical composition, antioxidant capacity, and some contaminants in six Moroccan medicinal and aromatic plants. Biocatal Agric. Biotechnol. 47, 102569. doi: 10.1016/J.BCAB.2022.102569
Ait Elallem, K., Ben Bakrim, W., Yasri, A., and Boularbah, A. (2024). Growth, biochemical traits, antioxidant enzymes, and essential oils of four aromatic and medicinal plants cultivated in phosphate-mine residues. Plants 13, 2656. doi: 10.3390/PLANTS13182656, PMID: 39339631
Akachoud, O., Bouamama, H., Laruelle, F., Facon, N., EL Broudi, S., Houssayni, S., et al. (2024). The developmental stage and arbuscular mycorrhizal symbiosis influence the essential oil yield, chemical profile, and biological activities in Thymus pallidus, T. satureioides, and Lavandula dentata. Ind. Crops Prod 220, 119188. doi: 10.1016/J.INDCROP.2024.119188
Alkilayh, O. A., Hamed, K. E., Sayyed, R. Z., Abdelaal, K., and Omar, A. F. (2024). Characterization of Botrytis cinerea, the causal agent of tomato grey mold, and its biocontrol using Bacillus subtilis. Physiol. Mol. Plant Pathol. 133, 102376. doi: 10.1016/J.PMPP.2024.102376
Amiri, A., Sourestani, M. M., Mortazavi, S. M. H., Kiasat, A. R., and Ramezani, Z. (2022). Efficiency of chemical composition of some essential oils against Botrytis cinerea, the pathogen of post-harvest strawberry fruits. J. Food Meas. Charact 16, 66–75. doi: 10.1007/S11694-021-01133-Z
Annemer, S., Ez-zoubi, A., Ez zoubi, Y., Satrani, B., Stambouli, H., Assouguem, A., et al. (2023). Optimization and antifungal efficacy against brown rot fungi of combined Salvia rosmarinus and Cedrus atlantica essential oils encapsulated in Gum Arabic. Sci. Rep. 13, 1–18. doi: 10.1038/s41598-023-46858-7, PMID: 37945688
Bai, X., Li, X., Liu, X., Xing, Z., Su, R., Wang, Y., et al. (2022). Antibacterial effect of eugenol on shigella flexneri and its mechanism. Foods 11, 2565. doi: 10.3390/FOODS11172565, PMID: 36076751
Basaid, K., Chebli, B., Bouharroud, R., Elaini, R., Alaoui, I. F., Kaoui, S., et al. (2021). Biocontrol potential of essential oil from Moroccan Ridolfia segetum (L.) Moris. J. Plant Dis. Prot 128, 1157–1166. doi: 10.1007/S41348-021-00489-0
Belcadi, H., Aknouch, A., El amrani, S., Chraka, A., Kassout, J., Lachkar, M., et al. (2024). Moroccan Lavandula dentata L. essential oil: γ-irradiation effect on the chemical composition and antibacterial activity. Sci. Afr 23, e02087. doi: 10.1016/J.SCIAF.2024.E02087
Ben Akacha, B., Michalak, M., Generalić Mekinić, I., Kačániová, M., Chaari, M., Brini, F., et al. (2024). Mixture design of α-pinene, α-terpineol, and 1,8-cineole: A multiobjective response followed by chemometric approaches to optimize the antibacterial effect against various bacteria and antioxidant activity. Food Sci. Nutr. 12, 574–589. doi: 10.1002/FSN3.3780, PMID: 38268912
Benkhaira, N., Zouine, N., Fadil, M., Ibnsouda Koraichi, S., El Hachlafi, N., Jeddi, M., et al. (2023). Application of mixture design for the optimum antibacterial action of chemically-analyzed essential oils and investigation of the antiadhesion ability of their optimal mixtures on 3D printing material. Bioprinting 34, e00299. doi: 10.1016/J.BPRINT.2023.E00299
Berrada, I., Benkhemmar, O., Swings, J., Bendaou, N., and Amar, M. (2012). Selection of halophilic bacteria for biological control of tomato gray mold caused by Botrytis cinerea. Phytopathol. Mediterr 51, 625–630. doi: 10.14601/Phytopathol_Mediterr-10627
Blancard, D. (2012). Tomato diseases. 2nd Edn (Academic Press, Cambridge, MA, USA: Elsevier). doi: 10.1016/C2010-0-66813-1
Bouchra, C., Achouri, M., Hassani, L. M. I., and Hmamouchi, M. (2003). Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J. Ethnopharmacol 89, 165–169. doi: 10.1016/S0378-8741(03)00275-7, PMID: 14522450
Bouqellah, N. A., Abdulmajeed, A. M., Rashed Alharbi, F. K., Mattar, E., Al-Sarraj, F., Abdulfattah, A. M., et al. (2025). Optimizing encapsulation of garlic and cinnamon essential oils in silver nanoparticles for enhanced antifungal activity against Botrytis cinerea pathogenic disease. Physiol. Mol. Plant Pathol. 136, 102522. doi: 10.1016/J.PMPP.2024.102522
Brito, C., Hansen, H., Espinoza, L., Faúndez, M., Olea, A. F., Pino, S., et al. (2021). Assessing the control of postharvest gray mold disease on tomato fruit using mixtures of essential oils and their respective hydrolates. Plants 10, 1719. doi: 10.3390/PLANTS10081719, PMID: 34451765
Cai, R., Hu, M., Zhang, Y., Niu, C., Yue, T., Yuan, Y., et al. (2019). Antifungal activity and mechanism of citral, limonene and eugenol against Zygosaccharomyces rouxii. LWT 106, 50–56. doi: 10.1016/J.LWT.2019.02.059
Cherif, A., Mansour, R., Ncibi, S., Hached, W., and Grissa-Lebdi, K. (2025). Chemical composition and fumigant toxicity of five essential oils toward Tuta absoluta and its mirid predator Macrolophus pygmaeus. J. Plant Dis. Prot 132, 1–11. doi: 10.1007/S41348-024-01030-9
Cossetin, L. F., Garlet, Q. I., Velho, M. C., Gündel, S., Ourique, A. F., Heinzmann, B. M., et al. (2021). Development of nanoemulsions containing Lavandula dentata or Myristica fragrans essential oils: Influence of temperature and storage period on physical-chemical properties and chemical stability. Ind. Crops Prod 160, 113115. doi: 10.1016/J.INDCROP.2020.113115
Dangol, S., Poudel, D. K., Ojha, P. K., Maharjan, S., Poudel, A., Satyal, R., et al. (2023). Essential oil composition analysis of cymbopogon species from eastern Nepal by GC-MS and chiral GC-MS, and antimicrobial activity of some major compounds. Molecules 28, 543. doi: 10.3390/MOLECULES28020543, PMID: 36677603
de Albuquerque Sousa, T. C., da Cunha, W. M., Rosas, A. L. G., Oppelt, C. Q., Gandra, E.Á., Rombaldi, C. V., et al. (2024). Essential oils as natural sources for the control of Botrytis cinerea: Chemical composition and antifungal effect. Food Biosci. 62, 105516. doi: 10.1016/J.FBIO.2024.105516
Dean, R., Van Kan, J. A. L., Pretorius, Z. A., Hammond-Kosack, K. E., Di Pietro, A., Spanu, P. D., et al. (2012). The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. doi: 10.1111/J.1364-3703.2011.00783.X, PMID: 22471698
de Andrade Bomfim, J. P., da Silva, N. N. P., da Silva, C. B., Amaral, J. C., das Graças Fernandes da Silva, M. F., Bonfim, F. P. G., et al. (2025). Compatibility of Cymbopogon citratus (DC.) Stapf (Poaceae) essential oil with egg parasitoids for the control of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Phytoparasitica 53, 1–11. doi: 10.1007/S12600-024-01223-W
de Oliveira Filho, J. G., da Cruz Silva, G., de Aguiar, A. C., Cipriano, L., de Azeredo, H. M. C., Bogusz Junior, S., et al. (2021). Chemical composition and antifungal activity of essential oils and their combinations against Botrytis cinerea in strawberries. J. Food Meas. Charact 15, 1815–1825. doi: 10.1007/S11694-020-00765-X
de Oliveira Xavier, C. M., Silva, E. H. A., de Siqueira, I. V. M., de Macedo, L. O., Bernardo, V. B., Goulart, H. F., et al. (2024). Chemical Composition and Acaricidal Activity of Essential Oil of Lavandula dentata L. @ on Engorged Females of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Acta Parasitol. 69, 1141–1147. doi: 10.1007/S11686-024-00835-W, PMID: 38568360
El Abdali, Y., Agour, A., Allali, A., Bourhia, M., El Moussaoui, A., Eloutassi, N., et al. (2022). Lavandula dentata L.: phytochemical analysis, antioxidant, antifungal and insecticidal activities of its essential oil. Plants 11, 311. doi: 10.3390/PLANTS11030311, PMID: 35161292
Elbouzidi, A., Taibi, M., El Hachlafi, N., Haddou, M., Jeddi, M., Baraich, A., et al. (2024). Formulation of a Three-Component Essential Oil Mixture from Lavandula dentata, Rosmarinus officinalis, and Myrtus communis for Improved Antioxidant Activity. Pharmaceuticals 17, 1071. doi: 10.3390/PH17081071, PMID: 39204175
EL Hachlafi, N., Benkhaira, N., Zouine, N., Fadil, M., Jeddi, M., Jeddi, S., et al. (2023). Exploration of Novel Antibacterial and Anti-adhesive Formulations from three Chemically Characterized Essential oils: Optimization using experimental design methodology. Sci. Afr 22, e01927. doi: 10.1016/J.SCIAF.2023.E01927
Faria, J. M. S. and Barbosa, P. (2024). Cymbopogon citratus Allelochemical Volatiles as Potential Biopesticides against the Pinewood Nematode. Plants 13, 2233. doi: 10.3390/PLANTS13162233, PMID: 39204667
Faria, J. M. S., Cavaco, T., Gonçalves, D., Barbosa, P., Teixeira, D. M., Moiteiro, C., et al. (2023). First Report on the Synergistic Interaction between Essential Oils against the Pinewood Nematode Bursaphelenchus xylophilus. Plants 12, 2438. doi: 10.3390/PLANTS12132438, PMID: 37446999
Fuyan, L., Runxia, Y., Yangcuiji, Z., Chongjun, Z., Liming, F., Fawu, S., et al. (2025). Antifungal Activity of Essential Oil Isolated from Pelargonium roseum against Grey Mold (Botrytis cinerea) of Cherry Tomato. Sci. Technol. Food 46, 143–151. doi: 10.13386/J.ISSN1002-0306.2024010250
Gonçalves, D., Cavaco, T., Pombo, A., Moiteiro, C., Teixeira, D. M., Inácio, M. L., et al. (2022). Synergistic Activity of Cymbopogon citratus and Mentha piperita Essential Oils against the Pinewood Nematode. Environ. Sci. Proc. 22, 21. doi: 10.3390/IECF2022-13054
Gull, K. and Trinci, A. P. J. (1971). Fine structure of spore germination in botrytis cinerea. Microbiology 68, 207–220. doi: 10.1099/00221287-68-2-207
Hammer, K. A., Carson, C. F., and Riley, T. V. (1999). Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 86, 985–990. doi: 10.1046/J.1365-2672.1999.00780.X, PMID: 10438227
He, L. L., Zhao, Y., Fan, L. M., Zhan, J. J., Tao, L. H., Yang, Y. H., et al. (2023). In vitro and in vivo antifungal activity of Cymbopogon citrates essential oils from different climate conditions against Botrytis cinerea. Sci. Hortic. 308, 111544. doi: 10.1016/J.SCIENTA.2022.111544
Hossain, F., Follett, P., Dang Vu, K., Harich, M., Salmieri, S., and Lacroix, M. (2016). Evidence for synergistic activity of plant-derived essential oils against fungal pathogens of food. Food Microbiol. 53, 24–30. doi: 10.1016/J.FM.2015.08.006, PMID: 26678126
Houzi, G., El Abdali, Y., Beniaich, G., Chebaibi, M., Taibi, M., Elbouzidi, A., et al. (2024). Antifungal, Insecticidal, and Repellent Activities of Rosmarinus officinalis Essential Oil and Molecular Docking of Its Constituents against Acetylcholinesterase and β-Tubulin. Scientifica (Cairo) 2024, 5558041. doi: 10.1155/2024/5558041, PMID: 39135848
Hyldgaard, M., Mygind, T., Piotrowska, R., Foss, M., and Meyer, R. L. (2015). Isoeugenol has a non-disruptive detergent-like mechanism of action. Front. Microbiol. 6. doi: 10.3389/FMICB.2015.00754, PMID: 26284043
Isman, M. B. (2000). Plant essential oils for pest and disease management. Crop Prot. 19, 603–608. doi: 10.1016/S0261-2194(00)00079-X
Ivanova, S., Nalbantova, V., Benbassat, N., Dzhoglova, V., Dzhakova, Z., Koleva, N., et al. (2025). Comparison between the chemical composition of essential oils isolated from biocultivated Salvia rosmarinus Spenn. (Rosmarinus officinalis L.) and some commercial products. Pharmacia 72, 1–13. doi: 10.3897/PHARMACIA.72.E140155
Jemai, N., Ben Mahmoud, K., Jedidi, E., Gargouri, S., Hachana, A., Jemmali, A., et al. (2025). Chemical Composition and Antifungal Activity of Essential Oils from Lavandula dentata L., Cymbopogon citratus DC. Stapf., and Salvia officinalis L. against Rhizoctonia solani in Potato Tubers. Potato Res., 68, 3179–3196. doi: 10.1007/S11540-025-09869-7
Kaya, O., Karakus, S., Bozkurt, A., Canturk, S., Yilmaz, T., and Hatterman-Valenti, H. (2025). Essential oil compounds and their impact on grape (Vitis vinifera L. cv. Narince) physiology under Botrytis cinerea infection. Physiol. Mol. Plant Pathol. 136, 102544. doi: 10.1016/J.PMPP.2024.102544
Kong, W., Huo, H., Gu, Y., Cao, Y., Wang, J., Liang, J., et al. (2022). Antifungal activity of camphor against four phytopathogens of Fusarium. S. Afr. J. Bot. 148, 437–445. doi: 10.1016/J.SAJB.2022.05.019
Koroch, A. R., Rodolfo Juliani, H., and Zygadlo, J. A. (2007). Bioactivity of Essential Oils and Their Components. In: Berger, R. G. (eds) Flavours and Fragrances. (Berlin: Springer Berlin Heidelberg). doi: 10.1007/978-3-540-49339-6_5
Labhar, A., El-Mernissi, Y., Ahidar, N., Zouhri, A., Benamari, O., Siddique, F., et al. (2024). Seasonal variations in the essential oil composition and biological activities of wild lavandula dentata L. Nat. Prod Commun. 19. doi: 10.1177/1934578X241230822
Lahmar, I., Chaieb, I., Yotova, L., and El Ayeb, N. (2025). Influence of harvesting period on essential oil: composition, bioactivity of Cymbopogon citratus (DC.) Stapf and insecticidal activity against Tribolium castaneum (Herbst 1797) in stored product. EuroMediterr J. Environ. Integr. 10, 3683–3695. doi: 10.1007/S41207-025-00756-8
Lahmyed, H., Bouharroud, R., Qessaoui, R., Ajerrar, A., Amarraque, A., Aboulhassan, M. A., et al. (2021). Actinomycete as biocontrol agents against tomato gray mold disease caused by Botrytis cinerea. Kuwait J. Sci. 48, 1–8. doi: 10.48129/KJS.V48I3.9200
Lee, J. P., Lee, S. W., Kim, C. S., Son, J. H., Song, J. H., Lee, K. Y., et al. (2006). Evaluation of formulations of Bacillus licheniformis for the biological control of tomato gray mold caused by Botrytis cinerea. Biol. Control 37, 329–337. doi: 10.1016/J.BIOCONTROL.2006.01.001
Mahmoud, M. F., Elrashidy, R. A., Mohammed, H. O., Drissi, B., Mahdi, I., and Sobeh, M. (2023). Essential oil and polyphenolics from Thymus satureioides Coss. counteract acrylamide-induced liver toxicity through suppression of NLRP3 inflammasome/NF-κB axis. J. Funct. Foods 107, 105641. doi: 10.1016/J.JFF.2023.105641
Medina-Romero, Y. M., Hernandez-Hernandez, A. B., Rodriguez-Monroy, M. A., and Canales-Martínez, M. M. (2021). Essential oils of Bursera morelensis and Lippia graveolens for the development of a new biopesticides in postharvest control. Sci. Rep. 11, 1–10. doi: 10.1038/s41598-021-99773-0, PMID: 34635777
Milagres de Almeida, J., Crippa, B. L., Martins Alencar de Souza, V. V., Perez Alonso, V. P., da Motta Santos Júnior, E., Siqueira Franco Picone, C., et al. (2023). Antimicrobial action of Oregano, Thyme, Clove, Cinnamon and Black pepper essential oils free and encapsulated against foodborne pathogens. Food Control 144, 109356. doi: 10.1016/J.FOODCONT.2022.109356
Moghaddam, M. and Mehdizadeh, L. (2017). “Chemistry of essential oils and factors influencing their constituents,” in Soft Chemistry and Food Fermentation (Academic Press, Cambridge, MA: Elsevier), 379–419. doi: 10.1016/B978-0-12-811412-4.00013-8
Mohamed Hanaa, A. R., Sallam, Y. I., El-Leithy, A. S., and Aly, S. E. (2012). Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods. Ann. Agric. Sci. 57, 113–116. doi: 10.1016/J.AOAS.2012.08.004
Mozafari, Z., Shams-Ghahfarokhi, M., Yahyazadeh, M., and Razzaghi-Abyaneh, M. (2024). Effects of Tripleurospermum caucasicum, Salvia rosmarinus and Tanacetum fruticulosum essential oils on aflatoxin B1 production and aflR gene expression in Aspergillus flavus. Int. J. Food Microbiol. 415, 110639. doi: 10.1016/J.IJFOODMICRO.2024.110639, PMID: 38417281
Mutlu-Ingok, A., Devecioglu, D., Dikmetas, D. N., Karbancioglu-Guler, F., and Capanoglu, E. (2020). Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: an updated review. Molecules 25, 4711. doi: 10.3390/MOLECULES25204711, PMID: 33066611
Ni, Z. J., Wang, X., Shen, Y., Thakur, K., Han, J., Zhang, J. G., et al. (2021). Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 110, 78–89. doi: 10.1016/J.TIFS.2021.01.070
Owolabi, T. A., Okubor, P. C., Danga, J., and Onimisi, I. (2025). Insecticidal effect of Essential Oils of Citrus limon, Cymbopogon citratus and Syzygium aromaticum and their Synergistic Combinations Against Anopheles Mosquitoes. Acta Biologica Slov. 68, 14–27. doi: 10.14720/ABS.68.2.19582
Pandey, D., Tripathi, N., Tripathi, R., and Dixit, S. (1982). Fungitoxic and phytotoxic properties of the essential oil of Hyptis suaveolens. J. Plant Dis. 89, 344–349. Available online at: https://www.jstor.org/stable/43214961 (Accessed May 1, 2025).
Pedroso, M. B., Scariot, F. J., Rocha, R. K. M., Echeverrigaray, S., and Delamare, A. P. L. (2024). Antifungal activity and mechanism of action of monoterpenes against Botrytis cinerea. Agric. Sci. 48, e018823. doi: 10.55779/NSB16111555
Qessaoui, R., Elaalaoui, M., Chafiki, S., Alouani, M., Chabbi, N., Ait Abd, N., et al (2024). Pseudomonas siderophores: production, spectrophotometry detection and Botrytis suppression. Not. Sci. Biol. 16, 11555. doi: 10.55779/NSB16111555
Ramos, E. G., de Queiroz, A. G., Veleirinho, M.B.D. R., Felipini, R. B., and Di Piero, R. M. (2024). Nanoformulations containing rosemary oil for gray mold control in strawberries. Sci. Hortic. 338, 113678. doi: 10.1016/J.SCIENTA.2024.113678
Sarma, R., Adhikari, K., Mahanta, S., and Khanikor, B. (2019). Combinations of Plant Essential Oil Based Terpene Compounds as Larvicidal and Adulticidal Agent against Aedes aEgypti (Diptera: Culicidae). Sci. Rep. 9, 1–12. doi: 10.1038/s41598-019-45908-3, PMID: 31263222
Singh, K., Deepa, N., Chauhan, S., Tandon, S., Verma, R. S., and Singh, A. (2024). Antifungal action of 1,8 cineole, a major component of Eucalyptus globulus essential oil against Alternaria tenuissima via overproduction of reactive oxygen species and downregulation of virulence and ergosterol biosynthetic genes. Ind. Crops Prod 214, 118580. doi: 10.1016/J.INDCROP.2024.118580
Soltanbeigi, E. and Maral, H. (2025). Volatile oil content and composition in fresh and dried Lavandula species: The impact of distillation time. Biochem. Syst. Ecol. 123, 105066. doi: 10.1016/J.BSE.2025.105066
Soylu, E. M., Kurt, Ş., and Soylu, S. (2010). In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mold disease agent Botrytis cinerea. Int. J. Food Microbiol. 143, 183–189. doi: 10.1016/J.IJFOODMICRO.2010.08.015, PMID: 20826038
Tagnaout, I., Zerkani, H., Bencheikh, N., Amalich, S., Bouhrim, M., Mothana, R. A., et al. (2023). Chemical composition, antioxidants, antibacterial, and insecticidal activities of origanum elongatum (Bonnet) emberger & Maire aerial part essential oil from Morocco. Antibiotics 12, 174. doi: 10.3390/ANTIBIOTICS12010174, PMID: 36671374
Tančinová, D., Mašková, Z., Mendelová, A., Foltinová, D., Barboráková, Z., and Medo, J. (2022). Antifungal Activities of Essential Oils in Vapor Phase against Botrytis cinerea and Their Potential to Control Postharvest Strawberry Gray Mold. Foods 11, 2945. doi: 10.3390/FOODS11192945, PMID: 36230021
Targino de Souza Pedrosa, G., Pimentel, T. C., Gavahian, M., Lucena de Medeiros, L., Pagán, R., and Magnani, M. (2021). The combined effect of essential oils and emerging technologies on food safety and quality. LWT 147, 111593. doi: 10.1016/J.LWT.2021.111593
Tisserand, R. and Young, R. (2014). Essential oil safety : a guide for health care professionals (Elsevier Limited). Available online at: https://books.google.com/books/about/Essential_Oil_Safety.html?hl=fr&id=DbEKAQAAQBA (Accessed April 18, 2025).
Wagner, L. S., Sequin, C. J., Foti, N., and Campos-Soldini, M. P. (2021). Insecticidal, fungicidal, phytotoxic activity and chemical composition of Lavandula dentata essential oil. Biocatal Agric. Biotechnol. 35, 102092. doi: 10.1016/J.BCAB.2021.102092
Wang, Y., Lin, W., Yan, H., Neng, J., Zheng, Y., Yang, K., et al. (2021). iTRAQ proteome analysis of the antifungal mechanism of citral on mycelial growth and OTA production in Aspergillus ochraceus. J. Sci. Food Agric. 101, 4969–4979. doi: 10.1002/JSFA.11140, PMID: 33543481
Wiegand, I., Hilpert, K., and Hancock, R. E. W. (2008). Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3, 163–175. doi: 10.1038/nprot.2007.521, PMID: 18274517
Xia, N., Wang, J., Jia, Y., Duan, J., Wang, X., Li, J., et al. (2025). Optimization of the process of extracting essential oil of rosemary by hydro distillation with different auxiliary methods. LWT 215, 117266. doi: 10.1016/J.LWT.2024.117266
Yang, D., Shi, H., Zhang, K., Liu, X., and Ma, L. (2023). The antifungal potential of the chelating agent EDTA against postharvest plant pathogen Botrytis cinerea. Int. J. Food Microbiol. 388, 110089. doi: 10.1016/J.IJFOODMICRO.2023.110089, PMID: 36682298
Zhao, Y., Yang, Y. H., Ye, M., Wang, K. B., Fan, L. M., and Su, F. W. (2021). Chemical composition and antifungal activity of essential oil from Origanum vulgare against Botrytis cinerea. Food Chem. 365, 130506. doi: 10.1016/J.FOODCHEM.2021.130506, PMID: 34237567
Zheng, Y., Shang, Y., Li, M., Li, Y., and Ouyang, W. (2021). Antifungal Activities of cis-trans Citral Isomers against Trichophyton rubrum with ERG6 as a Potential Target. Molecules 26, 4263. doi: 10.3390/MOLECULES26144263, PMID: 34299538
Keywords: antifungal activity, B. cinerea, essential oil, mixture, tomato
Citation: Chafiki S, Oukarroum A, Qessaoui R, El Assri S, Alouani M, Lahchimi H, El Arroussi H and Bouharroud R (2025) Study of Lavandula dentata, Salvia rosmarinus, and Cymbopogon citratus essential oils profile and antifungal activity of their mixture against the gray mold Botrytis cinerea. Front. Plant Sci. 16:1694585. doi: 10.3389/fpls.2025.1694585
Received: 28 August 2025; Accepted: 17 October 2025;
Published: 06 November 2025.
Edited by:
Nemesio Villa-Ruano, Meritorious Autonomous University of Puebla, MexicoReviewed by:
Adriane Silva, State University of Campinas, BrazilAarón Mendieta, National Polytechnic Institute, Mexico
Copyright © 2025 Chafiki, Oukarroum, Qessaoui, El Assri, Alouani, Lahchimi, El Arroussi and Bouharroud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachid Bouharroud, cmFjaGlkLmJvdWhhcnJvdWRAaW5yYS5tYQ==
 Salahddine Chafiki
Salahddine Chafiki Abdallah Oukarroum
Abdallah Oukarroum Redouan Qessaoui
Redouan Qessaoui Soumaya El Assri
Soumaya El Assri Mohamed Alouani
Mohamed Alouani Hasnaa Lahchimi2,6
Hasnaa Lahchimi2,6 Rachid Bouharroud
Rachid Bouharroud