- 1College of Agronomy and Life Sciences, Zhaotong University, Zhaotong, China
- 2School of Breeding and Multiplication (Sanya Institute of Breeding and Multiplication), College of Tropical Agriculture and Forestry, Hainan University, Sanya, China
- 3Centre of Excellence for Citrus, College of Agriculture, University of Sargodha, Sargodha, Pakistan
- 4Department of Plant Pathology, College of Agriculture, University of Sargodha, Sargodha, Pakistan
- 5Department of Field Crops, Faculty of Agriculture, Recep Tayyip Erdoğan University, Rize, Türkiye
- 6Department of Biology, College of Science, King Khalid University, Abha, Saudi Arabia
- 7Blood Products Quality Control and Research Department, National Organization for Research and Control of Biologicals, Cairo, Egypt
- 8Prince Sultan Bin Abdelaziz for Environmental Research and Natural Resources Sustainability Center, King Khalid University, Abha, Saudi Arabia
The intensive use of pesticides in modern agriculture has significantly improved crop yield and food security but introduced serious health concerns due to the accumulation of pesticide residues in fruits and vegetables and the environment, posing serious health risks. This review comprehensively explores the various residue detection techniques and plant metabolomics as an emerging tool to unravel the biochemical and physiological consequences of pesticide exposure. The article critically evaluates current methodologies for pesticide residue analysis, encompassing sampling strategies, storage considerations, and a wide range of extraction techniques, including QuEChERS, solid-phase extraction (SPE), and emerging green alternatives such as supercritical fluid extraction and ultrasound-assisted extraction. A detailed comparison of analytical techniques particularly gas chromatography (GC), liquid chromatography (LC), mass spectrometry (MS), and novel non-separative methods such as biosensors and spectroscopy is presented, emphasizing sensitivity, specificity, and adaptability to complex matrices. Furthermore, the integration of metabolomics with advanced platforms such as machine learning, green chemistry principles, and microfluidic innovations is discussed as a transformative direction for future pesticide residue monitoring. The review is a novel compilation of conventional residue detection methods and emerging omics-driven, artificial intelligence (AI)-assisted approaches and identifies current limitations, including matrix interferences and regulatory disparities, and advocates for the harmonization of residue standards, alongside the development of cost-effective, high-throughput analytical platforms to ensure food safety, improve risk assessment, and enhance understanding of plant metabolic responses under pesticide stress. Moreover, multi-omics approaches can be more reliable in evaluating the quality of claimed organic farming products.
1 Introduction
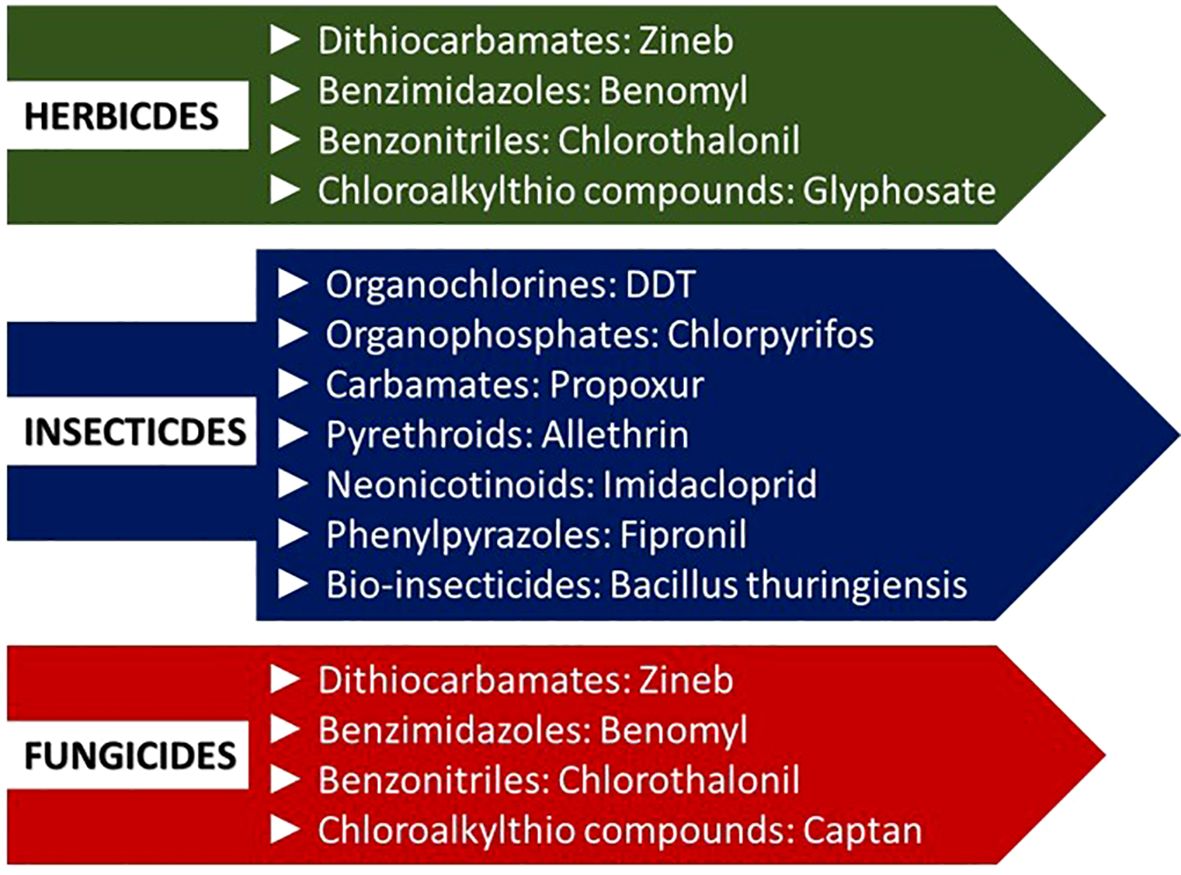
Pesticides are chemical substances that control or eliminate pests, such as insects, rodents, fungi, weeds, and other unwanted organisms (Ahmad et al., 2024). These are categorized by their mode of action, chemical structure, risks, and use (Ahamad and Kumar, 2023; Khan et al., 2023). Pesticides can be categorized as i) organic pesticides, such as pyrethrins derived from flowers of chrysanthemum and neem oil; ii) inorganic pesticides, for example, copper sulfate used as a fungicide; iii) synthetic pesticides, such as organochlorines, organophosphates, carbamates, and synthetic pyrethroids; and iv) biopesticides derived from animals, plants, or microbes (Ahmad et al., 2024). On a broader level, pesticides can also be classified as insecticides, herbicides, and fungicides (Figure 1) based on their target pest. Globally, pesticides are sprayed in enormous volumes annually, forming a multi-billion dollar industry (González et al., 2021; Junaid and Gokce, 2024; Maino et al., 2023).
Generally, pesticides are applied by an aerial spray while dissolved in water or through granular application. Pesticides in granular form are spread over the soil surface, later dissolving and becoming available for action. Fogging is also the method of creating a fog of pesticide droplets that can penetrate dense foliage, targeting pests hiding in hard-to-reach areas (Abdulra’uf and Tan, 2012; Anastassiades et al., 2017). Pesticides are applied to seeds before planting to protect against soil-borne pests and diseases. A major amount of the applied pesticides is ultimately released into the environment, often contaminating the soil. Pesticide residues are detected in soil, groundwater, and surface water, where they may accumulate before being degraded and occasionally converted to even more toxic chemicals (Sabzevari and Hofman, 2022; Grondona et al., 2023). Microbial degradation is the powerful force that drives the transformation of pesticides in soils (Fernández-Alba et al., 2015; Hamilton et al., 2017). Certain pesticide degradation, primarily through hydrolysis, occurs via both biotic and abiotic pathways (Guerrero Ramírez et al., 2023). Photodegradation involves the breakdown of chemical bonds in insecticide molecules due to sunlight (El-Saeid et al., 2022). Some of the pesticides applied may stay on the surfaces of the treated plants or be moved to other parts of the plant. Extensively used pesticides can accumulate in plants, creating residues in agricultural products and finally making their way to the consumer (Rein et al., 2025). The movement and fate of applied pesticides are intricate, involving several processes: plants absorb pesticides from the soil through the xylem, take in substances from treated surfaces and the air through the cuticle and stomata, and transport them within the plant through both xylem and phloem. Additionally, pesticides can dissipate from treated surfaces and undergo biodegradation (Leskovac and Petrović, 2023; Swathy et al., 2024). The extent to which pesticides migrate from treated soil to various environmental compartments is determined by their chemical properties, soil characteristics, hydraulic loading, and agricultural management practices (Nuruzzaman et al., 2025). Some degraded residues and persistent pesticides are particularly concerning, as they can return to humans through bioaccumulation and biomagnification (Mitra et al., 2024). Plants metabolize pesticides through enzymatic pathways, transforming parent compounds into conjugated residues (Nicolopoulou-Stamati et al., 2016; Wang et al., 2014; Wong et al., 2013). Key enzymes include cytochrome P450s, glycosyltransferases, and ATP-binding cassette (ABC) transporters, which influence both the persistence and detectability of residues (Mao et al., 2020; Xie et al., 2021).
2 Pesticide residues affecting key metabolic pathways and plant health
The plant metabolism of pesticides involves a series of enzymatic reactions, primarily oxidation, reduction, hydrolysis, and conjugation, that transform pesticides into more water-soluble and less toxic metabolites. Key enzymes include laccases, glycosyltransferases, methyltransferases, and ABC transporters, which facilitate diverse metabolic pathways such as S-conjugate formation and glycosylation. These processes are regulated by plant hormones (e.g., salicylic acid, jasmonic acid, and brassinosteroids) and can be influenced by epigenetic mechanisms like DNA methylation and histone modification (Eerd et al., 2003; Zhang and Yang, 2021; Pszczolińska et al., 2024). The metabolic transformation of pesticides in plants is tissue- and development stage-specific, leading to variable residue profiles in leaves, flowers, and fruits. For example, in tomatoes, the parent compound cyantraniliprole remains the dominant residue, while its metabolites are present at much lower levels, with their abundance and type varying by tissue and maturity. Some metabolites may retain biological activity and contribute to overall toxicity, highlighting the importance of comprehensive residue analysis for risk assessment (Huynh et al., 2021; Pszczolińska et al., 2024).
2.1 Paths to metabolite alteration
Pesticide residues in plants lead to both direct and indirect changes in metabolite profiles.
2.1.1 Enzyme activity modulation
Pesticide stress increases activities of detoxification enzymes (e.g., peroxidase and glutathione S-transferase), which in turn affect metabolic pathways and the abundance of specific metabolites (Zhang and Yang, 2021; Zhang et al., 2024a, 2024b).
2.1.2 Metabolic pathway disruption
Residues can downregulate amino acids and phenolic compounds while upregulating flavonoids, impacting pathways such as amide biosynthesis and arginine/proline metabolism (Aprotosoaie, 2012; Zhang et al., 2024a).
2.1.3 Formation of new metabolites
Plants metabolize pesticides into various transformation products, some of which may be more or less toxic than the parent compound. These metabolites can accumulate in different tissues and persist through developmental stages (Huynh et al., 2021; Kalyabina et al., 2021; Schusterova et al., 2021; Li and Fantke, 2023).
2.2 Types of metabolites affected
2.2.1 Primary metabolites
Amino acids, organic acids, and sugars may be reduced or altered, affecting plant growth and stress responses (Zhang and Yang, 2021; Zhang et al., 2024b).
2.2.2 Secondary metabolites
Flavonoids, phenolic acids, alkaloids, and terpenoids are often modulated, which can influence plant defense, nutritional quality, and aroma profiles (Zhang et al., 2024a, 2024b).
2.2.3 Pesticide-derived metabolites
Plants generate a range of pesticide metabolites (e.g., glycosylated and hydroxylated forms) that can be detected in edible tissues and processed products like wine (Huynh et al., 2021; Kalyabina et al., 2021; Schusterova et al., 2021).
2.3 Plants’ metabolic response to pesticides
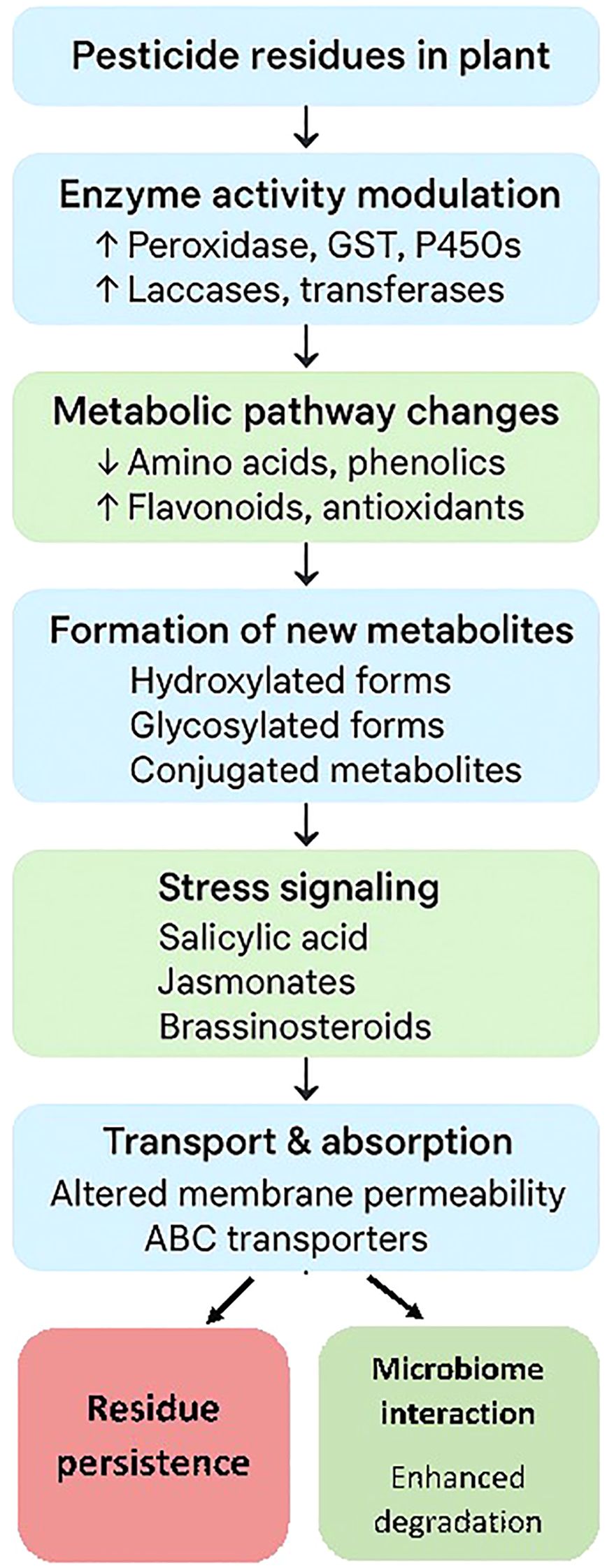
Plant secondary metabolites play a critical role in modulating pesticide absorption, translocation, and detoxification by enhancing enzymatic breakdown, facilitating conjugation, altering transport processes, and interacting with plant microbiomes. These interactions are central to plant defense and can influence both pesticide efficacy and residue persistence (Figure 2).
2.3.1 Phase II metabolism and conjugation
Plant secondary metabolites including flavonoids, alkaloids, and terpenoids are often modified through phase II metabolic processes such as glycosylation, methylation, and hydroxylation. These modifications increase solubility and facilitate storage or transport within plant tissues. Glycosylation, in particular, is a common conjugation reaction, resulting in the formation of glycosides (conjugated metabolites) that are less toxic and more easily compartmentalized in vacuoles or cell walls. Flavonoids, for example, are frequently found as glycosides in plants, and similar conjugation is observed for many alkaloids and terpenoids (Twaij and Hasan, 2022; Elshafie et al., 2023). These plant secondary metabolites (PSMs) play a direct role in the regulation of enzymes involved in pesticide metabolism, including laccases, glycosyltransferases, and methyltransferases. These enzymes are crucial for the detoxification and breakdown of pesticides within plant tissues (Khan et al., 2023; Al-Khayri et al., 2023). Other enzymes that upregulate by PSMs include cytochrome P450s, glutathione S-transferases (GSTs), and ABC transporters, which are crucial for breaking down and compartmentalizing pesticides, thereby reducing their toxicity and making them easier to sequester in vacuoles or cell walls, thus limiting their mobility and potential damage (Zhang and Yang, 2021; Kumar et al., 2023; Zhang et al., 2024a).
2.3.2 Stress response and signaling
PSMs are often upregulated in response to pesticide-induced stress, acting as antioxidants and signaling molecules that trigger broader defense responses, including the activation of detoxification pathways (Zhang and Yang, 2021; Elshafie et al., 2023; Khan et al., 2023; Kumar et al., 2023).
2.3.3 Modulation of absorption and translocation
The presence and composition of PSMs can alter the permeability of plant tissues and the activity of transporter proteins, affecting how much pesticide is absorbed and how it is distributed within the plant (Zhang and Yang, 2021; Chang et al., 2024). For example, hydrophobic secondary metabolites may limit pesticide movement by binding or sequestering them in specific tissues (Chang et al., 2024). While the literature extensively discusses plant-bound residues by the formation of conjugated metabolites, direct references to “plant-bound residues” (i.e., metabolites covalently bound to plant macromolecules such as cell wall components) are rare. Most studies have focused on soluble conjugates rather than insoluble, bound forms. However, the structural diversity and reactivity of secondary metabolites suggest that some may form covalent bonds with cell wall polymers or proteins, especially under stress or during defense responses, but this is not well documented in the reviewed sources (Yang et al., 2018; Twaij and Hasan, 2022; Elshafie et al., 2023).
2.3.4 Influence on pesticide efficacy
By modulating the plant’s internal environment, PSMs can affect the persistence and breakdown of pesticides, potentially impacting their effectiveness and residue levels (Zhang et al., 2024b).
2.3.5 Interactions with plant microbiomes
PSMs influence the composition and activity of plant-associated microbiomes, which in turn can affect the degradation of pesticides. Certain microbes are capable of metabolizing both plant-derived secondary metabolites and pesticide residues, sometimes using similar enzymatic pathways. The presence of specific PSMs can select for microbial communities that are more efficient at degrading pesticides, thereby enhancing phytoremediation and biotransformation processes (Pang et al., 2021). The ability of PSMs to modulate pesticide degradation pathways has implications for sustainable agriculture. By engineering or selecting for plants with higher levels of specific secondary metabolites, it may be possible to develop crops that are more effective at detoxifying pesticides, reducing environmental contamination, and improving food safety (Al-Khayri et al., 2023). Additionally, understanding these interactions supports the development of biopesticides and integrated pest management strategies that leverage natural plant defenses (Farhan et al., 2024).
2.4 Metabolomics as a complement to residue detection
Routine residue detection methods [e.g., gas chromatography–mass spectrometry (GC–MS), liquid chromatography–tandem mass spectrometry (LC–MS/MS), and immunoassays] provide quantitative data on known pesticide compounds but are limited in scope: they often do not focus on unknown transformation products, conjugated residues, or the dynamic biological impact of exposure. Metabolomics complements these techniques by enabling both untargeted and targeted profiling of small-molecule changes in plants exposed to pesticides. This approach can detect metabolic alterations even when the parent pesticide is below detection thresholds, thereby offering insight into both residue fate and functional stress responses (Mu et al., 2022; Shahid et al., 2023). Recent studies have identified specific metabolite signatures that reliably distinguish pesticide exposure. In rice exposed to chlorpyrifos, Mu et al. (2022) reported significant alterations in 119 metabolites, including increased glutamate-family amino acids, defense-related proline and glutathione, and accumulation of unsaturated fatty acids and phospholipids, while flavonoids were largely downregulated at high doses. Similarly, metabolomics investigations of non-target plant exposure to imazethapyr revealed disruptions in amino acid metabolism and secondary metabolite pathways, providing detailed mechanistic insights into herbicide toxicity (Liu et al., 2024). Broader reviews confirm that metabolomics fingerprints under pesticide stress often involve perturbations in carbohydrate, amino acid, lipid, and phenolic metabolism, sometimes revealing novel degradation products not typically captured by residue analysis (Shahid et al., 2023). The complexity and high dimensionality of metabolomics datasets can easily be tackled with artificial intelligence (AI) and machine learning approaches. Predictive modeling has already been applied to pesticide degradation in soils, where algorithms successfully estimated diazinon residues under varying environmental conditions (Alavi et al., 2022). Adapting similar approaches to plant metabolomics would enable the classification of exposure levels or types based on metabolic fingerprints, the early detection of crop stress prior to visible damage, and the identification of crop genotypes with enhanced detoxification or resilience. The integration of metabolomics with AI-based predictive frameworks therefore holds promise for precision agriculture and food safety.
3 Pesticide residues and regulatory framework for food safety
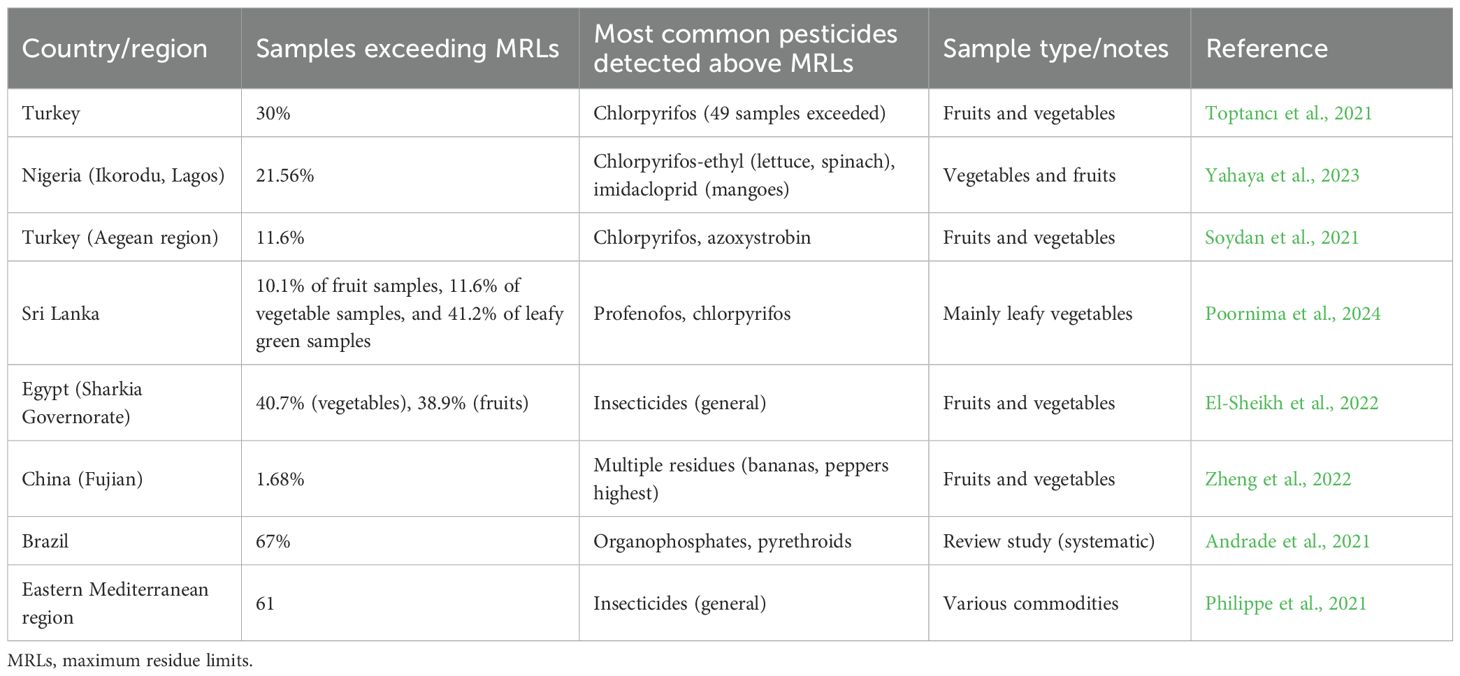
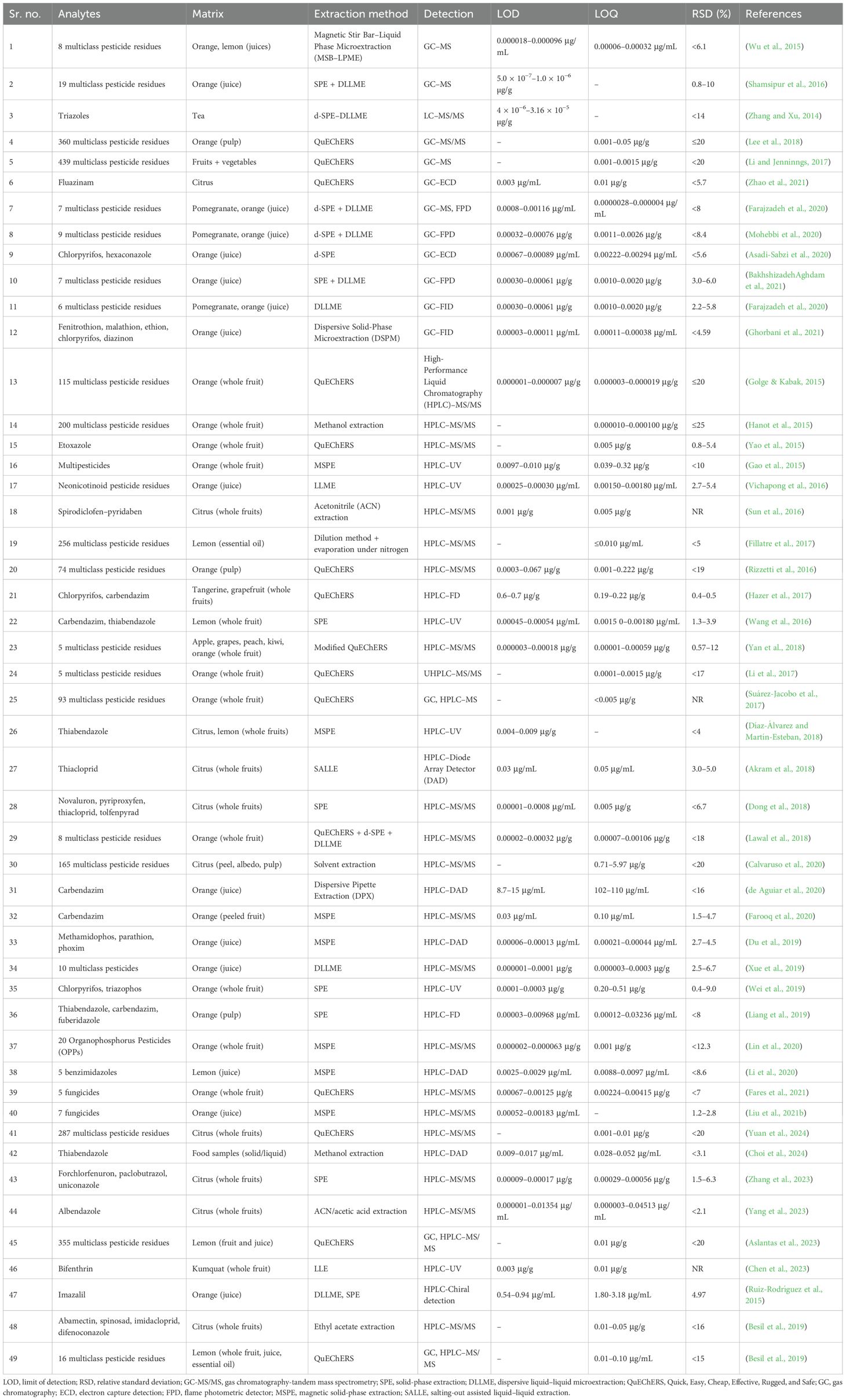
Pesticide exposure to human health can cause respiratory, reproductive, gastrointestinal, and neurological disorders and even cancer (Shekhar et al., 2024). The cytotoxic and mutagenic effects of chemical pesticides can also cause birth defects, reproductive harm, and disruptions in neurological and immune function (Fang et al., 2020). Other negative health effects reported in various studies include acute poisoning and chronic conditions, including different forms of cancer (such as bladder, breast, brain, bladder, and colon cancer) (Rani et al., 2021), Parkinson’s disease (Perrin et al., 2021), Alzheimer’s disease (Frisoni et al., 2022), infertility (Bhardwaj et al., 2020; Foucault et al., 2021), diabetes (Hernández-Mariano et al., 2022), neurotoxicity (Wang et al., 2024a), and leukemia (Rafeeinia et al., 2023). In case of short-term exposure, certain pesticides, especially insecticides, have shown various effects on human health, like dizziness, nausea, skin rashes, and irritated eyes. Breathing problems were reported by farm workers of Ethiopia, Costa Rica, and Brazil (Shah, 2020). Rural Santiago workers exposed to methyl bromide had elevated blood pressure levels and increased mood swings, insomnia, headaches, paresthesia, and memory issues (Zúñiga-Venegas et al., 2022). According to joint reports by the World Health Organization (WHO) and the United Nations Environment Programme (UNEP), it is estimated that approximately three million people suffer from pesticide poisoning annually, with approximately 200,000 deaths, most of which occur in developing countries due to a lack of regulatory oversight and access to protective equipment (W.H.O. and U.N.E.P, 2022). To safeguard public health and food safety, many international and national regulatory bodies have developed maximum residue limits (MRLs) for pesticides. MRLs are the highest level of a pesticide residue that is legally tolerated in or on food or feed when pesticides are applied correctly (F.A.O./W.H.O, 2016). Key organizations involved in setting MRLs include the Codex Alimentarius Commission (CAC) a joint venture of FAO and WHO that provides international food standards, the United States Environmental Protection Agency (EPA), and the European Food Safety Authority (EFSA) (Alimentarius, 2021; European Food Safety Authority (EFSA) et al., 2023; EPA (United States Environmental Protection Agency), 2023). These agencies conduct risk assessments based on toxicological studies and residue trials. The Joint FAO and WHO Meeting on Pesticide Residues (JMPR) plays a critical role in this process. It evaluates scientific data to determine toxicological endpoints, such as the no observed adverse effect level (NOAEL), acute reference dose (ARfD), and acceptable daily intake (ADI) (J.M.P.R, 2023). The JMPR also assesses dietary exposure and provides recommendations to the Codex Committee on Pesticide Residues (CCPR), which finalizes the international standards for MRLs (F.A.O./W.H.O, 2016). Numerous reports have indicated that pesticide residues on food often exceed MRLs (Table 1).
While the analytical studies themselves do not always attribute these exceedances directly to enforcement gaps, complementary regulatory reviews suggest that limited accredited laboratories, weak surveillance systems, informal pesticide markets, and low farmer awareness contribute to inconsistent enforcement of existing MRL standards (Neme et al., 2021; Mahmood et al., 2022; Okoffo et al., 2023). This structural gap implies that the presence of regulations alone does not guarantee compliance; capacity for monitoring and enforcement is critical for practical application. Along with serious health risks, non-compliance with MRLs may result in heavy financial losses. For example, in China, more stringent residue standards led to a 6.6% decline in agricultural export values when MRLs became 10% more restrictive (Zhang, 2025); in Egypt, 52 vegetable export consignments were rejected due to pesticide residues exceeding MRLs, resulting in trade bans (Abd El-Rahman, 2020); in the United Arab Emirates, a survey of imported fruits found MRL exceedances in over 60% of samples from Vietnam and Thailand and over 20% from several other developing countries (Osaili et al., 2022).
Mitigating pesticide residues in agricultural products is crucial for ensuring food safety and environmental protection. Various strategies have been identified to effectively reduce these residues, ranging from household processing techniques to advanced biotechnological methods; for instance, dipping tomatoes in a 2% salt solution can remove up to 76% of certain pesticides (Vemuri et al., 2016). Peeling and blanching can eliminate up to 85% of residues by removing contaminated outer layers (Andreev & Tsygankov, 2022). Advanced treatments, such as cold plasma, ozone application, and ultrasonic cleaning, have shown improved efficacy over traditional methods (Munir et al., 2024). Bioremediation using microorganisms and enzymes is an environmentally friendly option for degrading pesticides in soil and water, especially when enhanced with activated charcoal derived from agricultural waste (Mei et al., 2011). Genetic engineering approaches like CRISPR and RNAi can reduce pesticide dependency by developing pest-resistant crops (Chaudhary et al., 2025). Precision agriculture and integrated pest management (IPM) can further minimize pesticide application by optimizing use and combining multiple pest control strategies (Munir et al., 2024). Vegetated treatment systems, such as constructed wetlands and vegetated ditches, are effective in reducing pesticide runoff, with retention often exceeding 70% (Stehle et al., 2011). Despite the effectiveness of these methods, limitations such as cost, complexity, and incomplete residue removal suggest that combining strategies based on crop type and local conditions is necessary for optimal results.
4 Laboratory approach for the analysis of pesticide residues
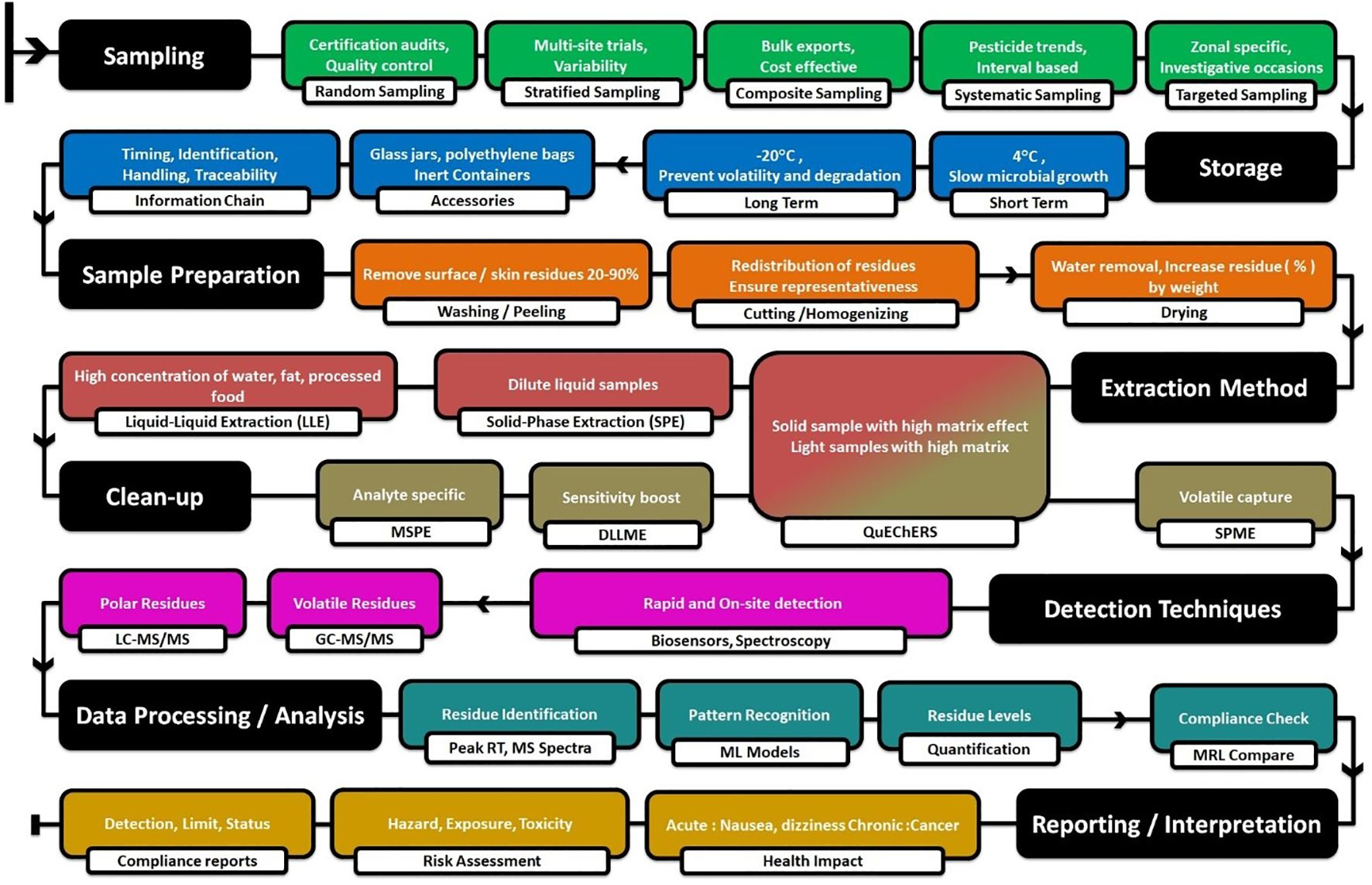
Since regulatory limits of many pesticides are still set at trace amounts, a systematic and careful approach is required. New studies emerge very quickly, and the standardized methods are revised very frequently (Figure 3).
4.1 Sampling and storage
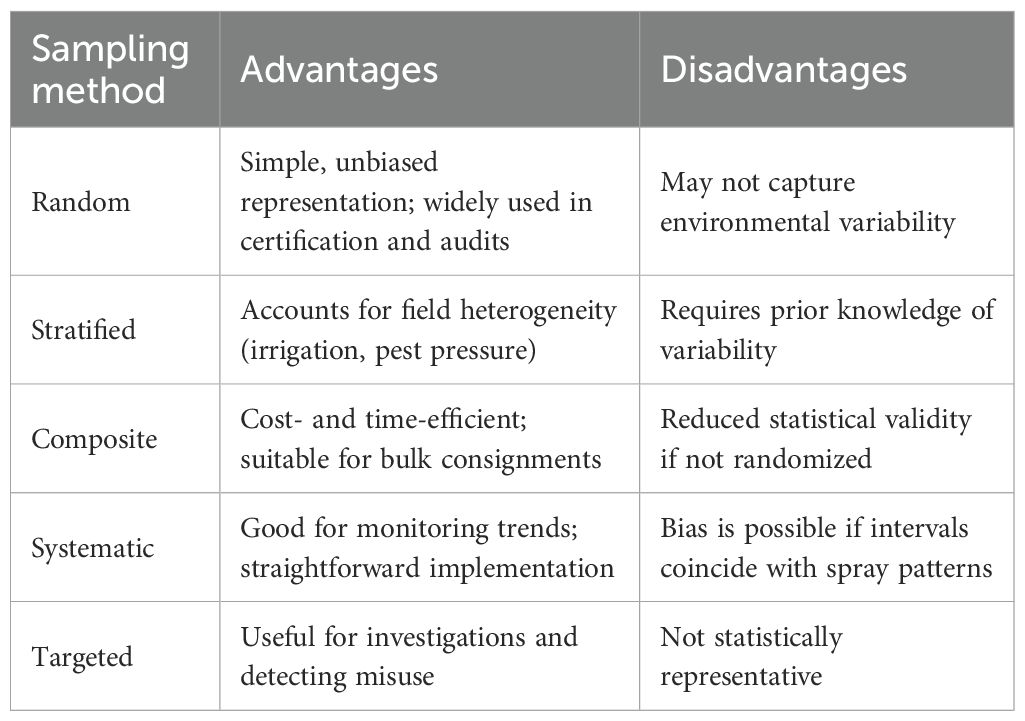
Sampling is a critical step in pesticide residue analysis that has a direct impact on the accuracy, precision, and reliability of analytical results while extrapolating the results of samples to the whole population. The ultimate aim is to collect a representative portion of crops or fruits that can represent the whole batch or field (Kowalska et al., 2022). Crop type, pesticide application technique, and weather conditions affect pesticide deposition on plant surfaces. Using statistically sound techniques, such as random, stratified, composite, and target sampling, ensures legal and valid results (Enkerlin et al., 2019).
4.1.1 Sampling methods
Different sampling methods are employed based on field size, crop type, and research objectives. Random sampling offers an objective representation of the sample and is employed extensively in quality control analysis and certification audits. Stratified random sampling considers environmental variability, for instance, pest pressure and irrigation heterogeneity, and is ideal for multi-site orchards or experimentation locations. Composite sampling, where multiple samples are merged into one, conserves processing funds and is used most frequently with bulk exports. Its validity decreases if it is not randomized. Systematic sampling, or taking samples at regular intervals across a field, can effectively monitor pesticide distribution trends but is potentially bias-inducing when sample intervals fall in line with application patterns (La Cecilia et al., 2021). Targeted sampling uses selective areas of focus, such as the areas for pesticide sprays or clearly dirty crops, and is convenient to employ for instances of investigation with pesticide misuse but without statistical confidence levels (Table 2) (Udomkun et al., 2023).
4.1.2 Sample collection procedure
For valid pesticide residue analysis, clean objects such as scissors, knives, or stainless steel blades prevent the contamination of samples. Samples collected in an appropriate quantity should be labelled with information, including crop, collection date, and origin. Crop maturity impacts pesticide uptake, where immature crops have greater residues since they have greater permeability, whereas mature crops have lesser residues because of metabolic hydrolysis (Kariyanna et al., 2024). Weather factors like temperature, humidity, and rain impact residues. Greater temperatures cause rapid degradation, and rain will wash the surface residues off, creating variability. Sampling time is crucial, as pre-harvest and post-harvest pesticide residue concentrations vary according to application timing and the rates of dissipation (Ambrus et al., 2023).
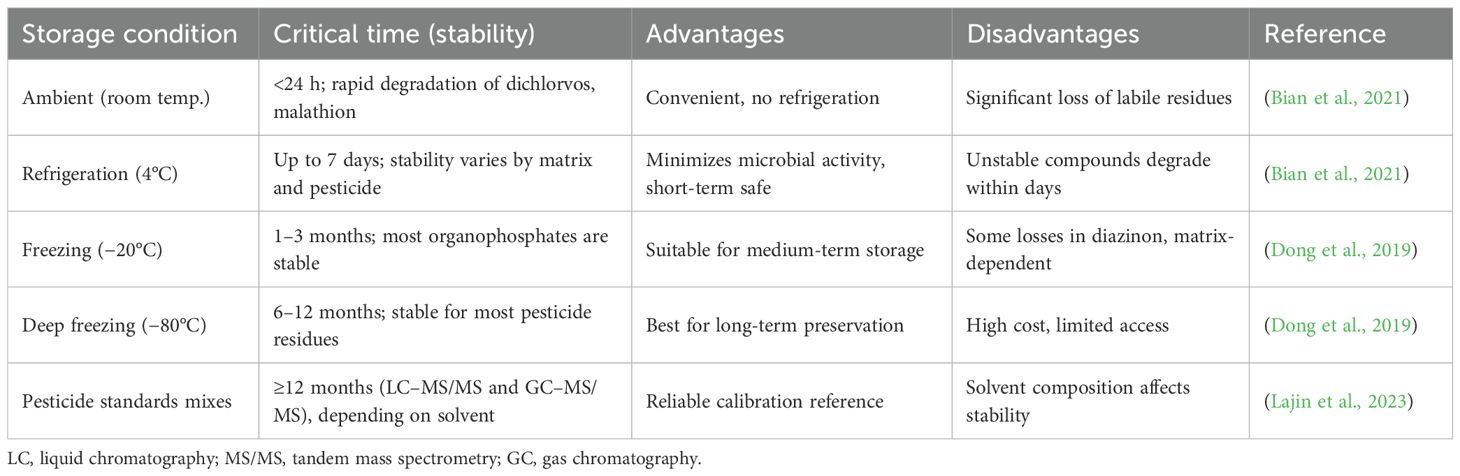
4.1.3 Storage
Sample storage under proper conditions is necessary to conserve pesticide residues and yield accurate analysis (Table 3). The storage parameters of significance include temperature, humidity, light, and container material because improper storage may lead to residue degradation, contamination, or loss (Rösch et al., 2023). Inert containers such as glass jars covered with Teflon-lined or high-quality polyethylene bags prevent chemical interference and contamination (Clotea et al., 2021). Maintaining the chain of custody pertaining to sample identification, storage conditions and location, handling person, and the date of time of every step till the samples are discarded after analysis allows traceability and quality assurance. Samples stored in distinction remain safe from cross-contamination as well as exposure to repeated freeze thaw cycles happening while taking out a certain sample for analysis.
4.2 Extractions for multi-residue pesticide analysis
4.2.1 Solvent extraction
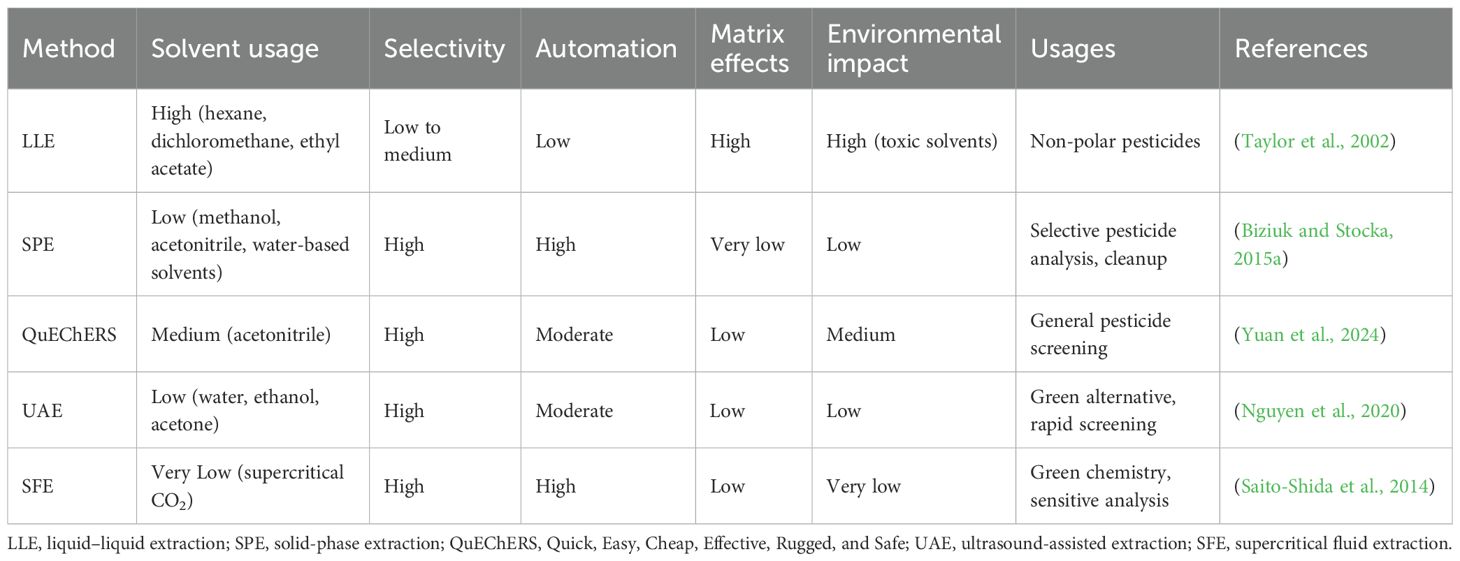
In a general approach, multiple residues of pesticides are determined after extraction using some efficient solvent extraction methods to purify the residues in matrix-rich foodstuffs. Acetonitrile, hexane, dichloromethane, and ethyl acetate are routinely used organic solvents (Joshi and Adhikari, 2019). Acetonitrile is one of the most frequent solvents employed in liquid–liquid extraction (LLE), which prevents co-extraction of lipids and sugars and is suitable for analysis using LC (Barp et al., 2023; Rudakov et al., 2018). Hexane is commonly employed to extract non-polar pesticides, producing a good recovery of lipophilic pesticide residues. To expand the variety of recovered pesticides, hexane is often mixed with other solvents like ethyl acetate (Negi et al., 2024). Dichloromethane is widely used in both LLE and solid-phase extraction (SPE) since it possesses the ability to dissolve a wide range of pesticide residues. It is especially suitable for the extraction of chlorinated pesticides such as organochlorine (Jones et al., 2023). Ethyl acetate may be used as an alternative to acetonitrile when analyzing multiple residues on GC (Mol et al., 2016). It provides an acceptable recovery of the polar and non-polar pesticides. However, as ethyl acetate is more hydrophobic than acetonitrile, increased co-extraction of background matrix components is more likely in ethyl acetate.
4.2.2 Liquid–liquid extraction
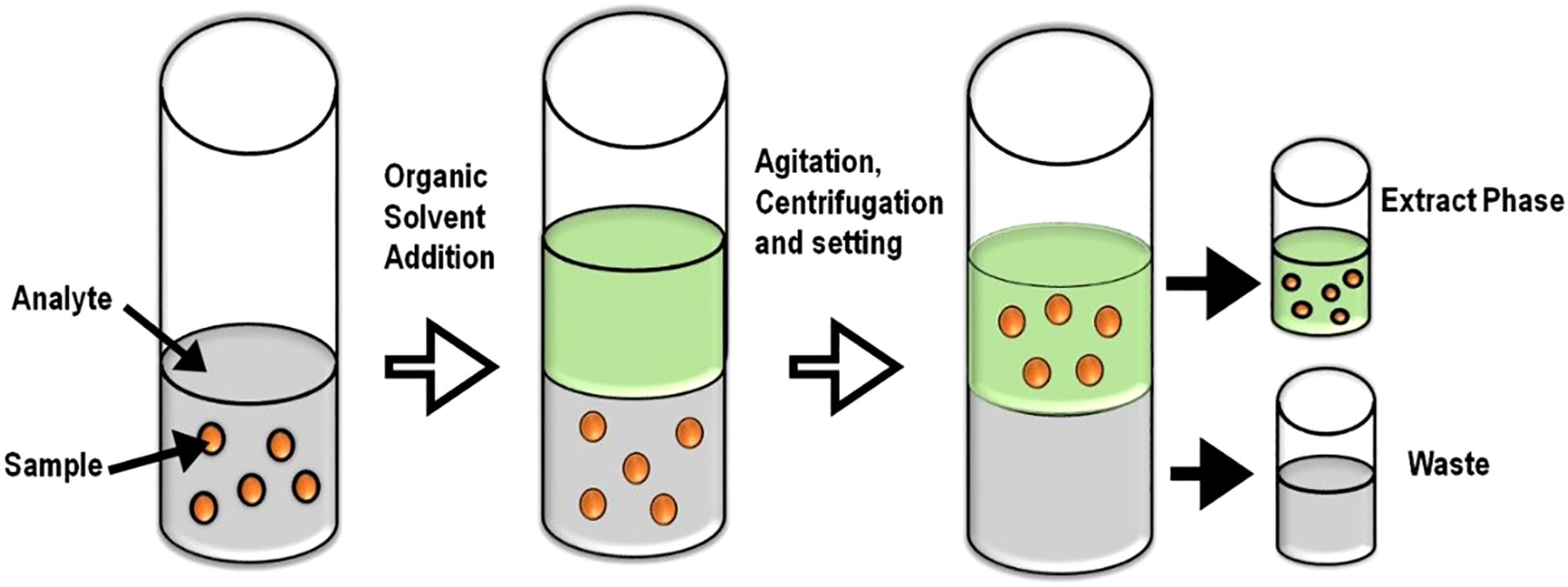
LLE is a phase-partition process that separates pesticide residues between two immiscible liquid systems, a polar mostly aqueous phase and a non-polar solvent (such as hexane, ethyl acetate, or dichloromethane) (Chai and Tan, 2016). Despite being a widely used method that is highly efficient in handling non-polar pesticides, LLE is solvent-intensive and environmentally unsustainable in comparison to newer alternatives (Figure 4) (Campanale et al., 2021); thus, it is increasingly being replaced by newer alternatives such as Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) and SPE, offering better selectivity and low solvent consumption (Mandal et al., 2023).
4.2.3 Solid-phase extraction
SPE is a process that employs solid sorbents [C18, primary secondary amine (PSA), and graphitized carbon black (GCB)] to retain pesticides, excluding matrix interferences (Biziuk and Stocka, 2015a). The process is beneficial in the sample cleanup of samples with low matrix effect (Besil et al., 2017). SPE is a more accurate, solvent-saving process and, hence, a better process for targeted analysis of very dilute samples (Ramadevi and Ramachandraiah, 2023). It is particularly effective for selectively retaining pesticides, which can then be eluted with appropriate solvents. SPE is suitable for automation and integration with LC or GC systems. When combined with other techniques like dispersive liquid–liquid microextraction (DLLME), SPE enhances extraction efficiency and preconcentration factors (Shamsipur et al., 2016). The integration of green solvents and magnetic sorbents in SPE methods offers environmentally friendly and cost-effective solutions for pesticide extraction from food products (Zhao et al., 2019; Wang et al., 2021b).
4.2.4 QuEChERS extraction
The QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) method is a widely used extraction technique initially developed for pesticide residue analysis in fruits and vegetables. QuEChERS has been extensively applied beyond its original scope of pesticide analysis. It is now used for detecting pharmaceuticals, polycyclic aromatic hydrocarbons (PAHs), persistent organic pollutants (POPs), and other contaminants in food, biological, and environmental samples (Kim et al., 2019; Musarurwa et al., 2019; Perestrelo et al., 2019). The method’s adaptability is further demonstrated by its use in diverse fields such as forensic analysis, environmental monitoring, and doping control (Perestrelo et al., 2019; Derwand et al., 2023). Modifications to the original protocol have been made to enhance extraction efficiency for specific analytes and matrices. These include the use of different solvents, salt formulations, and sorbents, as well as the incorporation of steps like alkaline hydrolysis and freeze-out for specific sample types (Kim et al., 2019; Musarurwa et al., 2019; Acosta-Dacal et al., 2021). Compared to LLE, QuEChERS is cost-effective and time-saving and provides higher recovery rates and better analytical performance, often eliminating the need for concentration steps (Kim et al., 2019; Perestrelo et al., 2019; Shin et al., 2022).
4.2.5 Environmentally sustainable extraction techniques
To minimize solvent consumption and maintain the sustainability of the environment, new approaches to extraction, like 1) supercritical fluid extraction (SFE) and 2) ultrasound-assisted extraction (UAE), are being explored (Gaikwad et al., 2024). UAE utilizes sonication to allow the penetration of the solvent in solid matrices (Nguyen et al., 2020), reducing the time of extraction without sacrificing efficiency. As a green approach to extraction, SFE utilizes a solvent of supercritical CO2, offering a promising alternative to toxic organic solvents (Arumugham et al., 2021) (Table 4).
4.2.6 Emerging extraction techniques and modifications
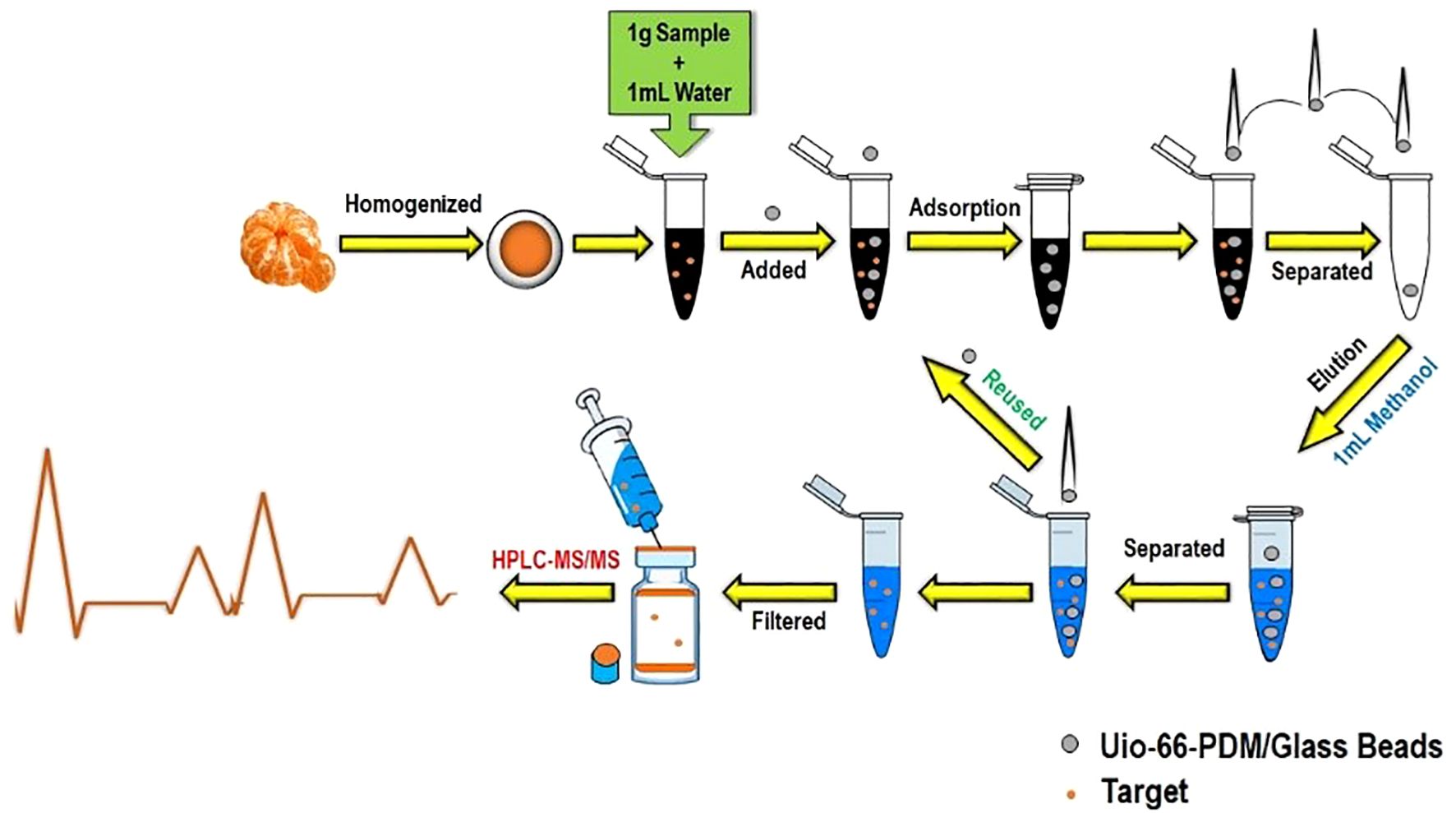
Recent advancements and the coupling of innovative strategies with classical extraction and separation techniques have led to significant improvements in analytical efficiency, precision, and environmental sustainability, allowing laboratories to navigate increasingly complex sample matrices and regulatory requirements with greater reliability. The QuEChERS method has seen widespread adoption in food and soil analysis, as it is flexible to work with both gas and liquid chromatography systems. QuEChERS coupled with magnetic solid-phase extraction (MSPE) enables enhanced selectivity and simplified separation (Du et al., 2019). Further advancements incorporate solid-phase microextraction (SPME) for ultra-low volatile and semi-volatile compounds (García-Reyes, 2022), salting-out assisted liquid–liquid extraction (SALLE), and molecularly imprinted polymers (MIPs) (Hashemi, 2018). The integration of QuEChERS with SPE or DLLME has demonstrated significant reductions in matrix interference while achieving consistently high recovery yields across multiple classes of compounds (Biziuk and Stocka, 2015b; Pastor-Belda et al., 2016). Moreover, citrate-buffered AOAC 2007.01 and CEN EN 15662 protocols validated for LC–MS/MS and GC–MS/MS applications offer streamlined workflows for high-throughput multi-residue analysis in regulatory and research environments (Anastassiades et al., 2023; Fernández-Alba, 2023). These methods are also increasingly used in parallel with performance verification experiments, validating their robustness against other methodologies. SFE, when optimized with co-solvents such as ethanol and methanol, greatly enhances the recovery of polar analytes (Liu, 2021). Particularly well-suited for the extraction of thermolabile and non-polar compounds, SFE maintains analyte integrity while operating under mild thermal conditions. Its synergy with solid-phase techniques or post-processing using DLLME is being explored to expand its capability into broader pesticide classes. Similarly, microwave-assisted extraction (MAE) and UAE show promise in processing samples with complex matrices such as plant tissues or composite food products, offering accelerated extraction with reduced solvent volumes and energy input (Nguyen, 2020). The combination of MSPE with innovative sorbents such as metal–organic frameworks (MOFs) (Figure 5), graphene-based composites, or covalent organic frameworks (COFs) provides high-efficiency options for automation, minimal sample preparation, and low-solvent usage (Díaz-Álvarez, 2019; Yang et al., 2023), making it attractive for automated laboratories and high-throughput screening environments. In addition, the evolution of magnetic nanoparticles has significantly improved reproducibility and operational simplicity, especially when used in combination with QuEChERS workflows (Du et al., 2019).
UAE’s integration with ionic liquids (ILs) or deep eutectic solvents (DESs) is proving effective in improving recovery and selectivity while maintaining environmental compatibility (Flores, 2024). UAE is also increasingly being used in tandem with pre-concentration steps like SPE or DLLME to ensure sufficient enrichment of trace-level pesticide residues. DLLME continues to be favored for its rapid processing time, high enrichment factors, and low solvent consumption. It is especially effective for polar and semi-polar analytes in aqueous matrices, where its unique dispersive mechanism allows intimate interaction between extractant and target compounds. The introduction of DES or task-specific ILs into DLLME protocols is expanding its applicability to more complex sample types and improving its compatibility with greener workflows (Hashemi et al., 2018). In pharmaceutical applications, coupled systems such as SPE–SFE or SPME–GC–MS are used for drug profiling, bioavailability assessments, and impurity detection. These hybrid techniques allow simultaneous extraction and clean-up steps, which improve analytical turnaround times while reducing matrix interferences. DLLME has emerged in environmental monitoring of pharmaceutical residues, especially in wastewater matrices, where traditional SPE systems may face challenges in enrichment and recovery (Pastor-Belda et al., 2016). The application of QuEChERS with MIPs also strengthens food quality assessment protocols by improving analyte specificity and reducing the risk of false positives when screening for mycotoxins, heavy metals, or pesticide residues (Hashemi, 2018; Anastassiades et al., 2023). In environmental applications, methods such as SFE, UAE, and SPME have proven to be adaptable for the extraction of contaminants, including POPs, heavy metals, and pharmaceutical residues from soil, water, and sediment matrices (Nguyen, 2020; García-Reyes, 2022). These techniques are particularly relevant in regions where regulatory frameworks require the simultaneous screening of multiple classes of contaminants with varied physicochemical properties. Optimization strategies in modern extraction workflows focus on critical variables such as solvent composition, sample pH, ionic strength, and temperature control. For example, optimizing the disperser-to-extractant ratio in DLLME can significantly improve analyte recovery, especially in complex matrices (Pastor-Belda et al., 2016). Likewise, sorbent choice in SPE whether using classical C18, PSA, or more advanced MOF or MIP materials determines the specificity and efficiency of isolation (Díaz-Álvarez, 2019). Automation and AI are increasingly integrated into extraction workflows to optimize solvent selection, extraction conditions, and procedural timing. AI models help streamline method development and allow adaptive adjustments based on real-time feedback from analytical instruments (Liu, 2021). Robotics is being adopted for sample preparation tasks, significantly enhancing reproducibility and analytical throughput, especially in high-volume laboratories (Flores, 2024). The trajectory of modern separation science is toward blending sustainability with high analytical performance. Functionalized nanomaterials are being synthesized for targeted selectivity, while 3D-printed microfluidic devices and lab-on-chip platforms are being developed for in situ, field-level extractions (García-Reyes, 2022). These advancements aim to make residue testing more accessible and scalable. As global focus intensifies on reducing laboratory waste and environmental impact, regulatory bodies are aligning with green chemistry principles. Solvent minimization, recyclability, and the implementation of biodegradable alternatives such as DES and ILs are becoming standard benchmarks for method approval (Zhang et al., 2018a).
4.2.6.1 Stability during extraction
Removal of multiclass pesticides in fruits, such as citrus, has some major stability concerns that should be handled properly. The high pigment composition, essential oils, and organic acids in citrus fruit matrices provide a complex composition that can greatly impact pesticide analysis stability (Mol et al., 2019). The stability of these matrix compounds is likely to be sub-optimal due to co-extraction with the target pesticides. The QuEChERS-based method has been the most feasible method for stability during multiclass pesticide extraction as a process. The original technique of Pszczolińska and Kociołek (2022) has been used in applications similar to matrix effect reduction using PSA, GCB, and C18 sorbent. The standard solvent extraction method using organic solvents exists only in the embryonic phase because it faces severe stability problems. For example, Lee et al. (2024) noted that solvents such as acetonitrile, ethanol (different grades), ethyl acetate, and methanol are general extraction solvents for pesticides; however, their stabilizing properties greatly deteriorate in the presence of mixtures of residues (Koch et al., 2020). It can clearly be observed that when more than five analytes are to be analyzed simultaneously, the possible occurrence of concurrent extraction of matrix-interfering compounds becomes more likely. Improved extraction techniques have shown recent advances that emerge with a miniaturized version (Tang et al., 2019). MSPE and Dispersive Solid-Phase Extraction (d-SPE) are highly selective; however, d-SPE does not optimize pesticide integrity when solvent use is reduced (Zhang et al., 2018a, b). Natural deep eutectic solvents (NADESs) and DESs show biocompatibility, which allows enhanced stability in the enhancement of extraction. Tang et al. (2019) also improved the ability of ILs. Benzimidazole conjugates need to be handled properly to retain their fluorescence, a prerequisite for detection (Bajwa et al., 2017). This kind of degradation makes neonicotinoid a significant analytical challenge, and special liquid–liquid extraction has to be used in order to retain the stability of metabolites, hence emphasizing liquid–liquid as the only suitable procedure at trace concentrations (Casado et al., 2018). Wang et al. (2021a) demonstrated that the development of stability in the extraction step relies on a multi-principled approach, like the use of specific sorbent pairs for matrix dispersal, the miniaturization of approaches when possible, and the adaptation of extraction protocols to cater to the type of pesticide. Analytical challenges in multi-residue citrus fruit extraction arise from individual matrix characteristics. When the whole fruit and vegetables are analyzed, the high sugar percentage, as well as the presence of 108 carotenoids, can create a serious competition interference in the determination of pesticides (Mol et al., 2019). Matrix interference confirms that ionization in LC–MS/MS or GC–MS analyses can be greatly affected by the matrix components discussed above, leading to falsely identified results (Wang et al., 2021a).
4.2.7 Exceptions and challenges
The high-water content of citrus fruits presents special extraction challenges. According to Goel et al. (2025), aqueous-based extraction processes will likely lose fat-soluble or non-polar pesticides since the majority of pesticides are in trace amounts or are bound to water-soluble compounds. Pesticide residues will likely separate differently in citrus pulp and peel, with peel having greater concentrations due to direct exposure. Zhang et al. (2018b) also explained that conventional extraction methods can fail to recover residues in both fractions; therefore, various extraction methods have to be applied. Storage conditions also significantly impact the stability of the pesticide, as Casado et al. (2018) proved that heat, light, or oxygen can lead to degradation and subsequently underestimate the concentrations of residues. Zhang et al. (2018a) said that analysis of very dilute samples doubles the time and complexity of extraction and analysis procedures. The varied range of chemical properties of pesticides, including polarity, volatility, and solubility, makes the process complicated. Tang et al. (2019) clarified that there is no single technique to successfully extract all types of pesticides and that each category of chemicals needs a unique procedure. Operations after extraction are a further problem. Bajwa et al. (2017) outlined how the currently leading methodologies involve tedious clean-up procedures to remove interfering agents like pigments, waxes, and organic acids, with multiple steps in purification needed. The regulatory framework contributes to the problem, as Casado et al. (2018) clarified that various jurisdictions have varying maximum residue levels for pesticides, which influence the level of sensitivity needed. Recent technological advancements through 2025 have introduced improved ways to address these limitations.
4.3 Analytical techniques for the detection of pesticide residues
The ultimate goal of the extraction is to remove unwanted matrix and maximize the target analyte concentration to enhance the detection. After the careful extraction, various analytical methods used for the detection of trace analytes can also be employed for the detection of pesticide residues (Figure 3 and Table 5). The methods can be separative and non-separative. Among the separative methods, chromatographic techniques, especially gas chromatography and liquid chromatography, are widely used.
4.3.1 Gas chromatography
GC is particularly effective for volatile and semi-volatile, thermally stable compounds, allowing for the separation of pesticide residues from complex matrices (Meher and Zarouri, 2025). The technique relies on the vaporization of samples and their subsequent passage through a column, where they are separated based on their affinity to the stationary phase. The separated compounds are then detected using a suitable detector (Amórtegui and Dallos, 2018). The choice of column type and detector is dependent upon several factors, such as the target analyte, nature of samples, resolution and sensitivity required, and financial budget. While the column affects the separation efficiency and detection sensitivity, the detector can significantly influence the sensitivity and specificity of the analysis.
4.3.1.1 Commonly used GC column types
Capillary columns with narrow diameter and long length, typically coated with stationary phases like polydimethylsiloxane (non-polar) or polyethylene glycol (polar), offer high resolution (Kanateva et al., 2021). Non-polar columns such as DB-1, HP-1, and Rtx-1 are suitable for analyzing less polar compounds, such as organochlorines and pyrethroids (Picó et al., 2020). Columns with polar stationary phases, like DB-WAX and HP-INNOWax (polyethylene glycol), are used for polar pesticides such as organophosphates and carbamates (Torres et al., 2021). In general, partially polar columns such as DB-5, HP-5, and Rtx-5 are used in response to various pesticides. Both the column and detector are paired accordingly. For instance, electron capture detection (ECD) is often paired with non-polar columns for organochlorine pesticides, while nitrogen–phosphorus detection (NPD) may be used with polar columns for organophosphates (Radović et al., 2015; Anagnostopoulos et al., 2015). Column operations, such as temperature ramping, gas pressure, and flow rate, are also crucial for effective separation and method run time. Hakme et al. (2018) developed a multi-residue method using GC coupled with triple quadrupole mass spectrometry (GC–QqQ–MS/MS) capable of analyzing 203 pesticides in a single run of 12.4 minutes, with 2 μg/kg Limit of Quantification (LOQ). Adjusting the carrier gas pressure and flow rate is also essential for achieving optimal separation and peak resolution.
4.3.1.2 Common detectors for GC
There are many selective detectors such as ECD, nitrogen–phosphorous detector (NPD), flame photometric detector (FPD), and flame ionization detector (FID). ECD is highly sensitive to electronegative compounds, such as organochlorine pesticides, so it is particularly effective for detecting chlorpyrifos, cypermethrin, and other organochlorines, showing good linearity with correlation coefficients above 0.99 (Sofi et al., 2023). Using ECD methods, Yu et al. (2021) demonstrated the recoveries for organochlorines in vegetables ranging from 78.8% to 92.2%, with relative standard deviations (RSDs) generally below 10%. Similarly, in fruit analysis, average recoveries were above 90% with RSDs less than 6% (Tankiewicz, 2019). In a method for fipronil and its metabolites, the limits of detection (LODs) have been reported as 0.0005 mg/L for the metabolites and 0.0003 mg/L for fipronil, while the LOQs were established at 0.002 mg/kg for all compounds across maize grain, maize stem, and soil, which is below the established tolerance levels in the USA and European Union (EFSA, 2023). FID is often considered a universal detector due to its broader range of detection. However, its sensitivity is generally lower compared to that of ECD for electronegative compounds (Lopes et al., 2022). Its response is more dependent on the structure of compounds; generally, FID is best with hydrocarbons and other non-polar compounds (Spanjers et al., 2017). The ionization energy and molecular orbital energy are key factors influencing FID’s response (Lopes et al., 2022). FID methods for pesticide analysis have been showing average recoveries ranging from 78.67% to 85.1% for organochlorine pesticides, with method detection limits typically in the range of 0.048 to 0.089 µg/mL (Gaber, 2014). The dielectric barrier discharge ionization detector (BID) has been shown to have a greater response to pesticides compared to FID, with relative responses of approximately 3.0, a promising alternative for comprehensive pesticide analysis (Lopes et al., 2022). The combination of FPD and ECD in a single setup allows the simultaneous detection of different pesticide classes in residue analysis (Saha et al., 2020). Finally, the integration of mass spectrometry with GC (GC–MS) offers high sensitivity, with the detection of pesticide residues at levels as low as 0.05 to 1 mg/kg (Ali and Hassan, 2023), and as low as 2 μg/kg when used with triple quadrupole MS (GC–QqQ–MS/MS) (Hakme et al., 2018). Further, the identification of compounds based upon their mass spectra enhances the specificity of GC–MS (Wilson-Frank and Ewbank, 2022). The technique is adaptable to different sample preparation methods and is applicable to diverse food matrices (Dar et al., 2023). The ability of GC–MS to perform both targeted and non-targeted analyses allows the detection of known pesticides and the identification of unexpected compounds or metabolites, enabling the comprehensive monitoring of pesticide residues (Chou et al., 2023; Su et al., 2024). The technique can work with fast temperature ramping for high-throughput screening while remaining robust and reliable and showing good linearity (Dar et al., 2023; Romagnoli et al., 2023; Su et al., 2024). Advanced GC–MS technologies, such as MS/MS, has been shown to detect over 200 compounds in a run time of 30 minutes (Kim et al., 2024). Time of flight (TOF) with GC–MS enables the detection of over 1,000 pesticides without the need for expensive analytical standards (Chou et al., 2023). Despite the growing preference for LC–MS/MS for certain pesticide classes, GC has remained a robust and cost-effective option for many applications (Brandi et al., 2024).
4.3.2 Liquid chromatography–mass spectrometry
LC–MS is another powerful analytical technique widely used for detecting pesticide residues in various matrices. When combined with QuEChERS extraction, it is capable of simultaneous detection of multiple pesticides. Average studies of LC–MS/MS method development are upcoming with the detection of 35–50 pesticides, with recoveries ranging from 80% to 90% (Majumder et al., 2024). An LC–High-Resolution Mass Spectrometry (HRMS) method optimized for analyzing 18 pesticides in river water and seawater achieved quantification limits as low as 1.7 ng/L (Nannou et al., 2024). SPME combined with LC–MS/MS was used for detecting organophosphorus pesticides in tea, offering a good linear range, low detection limits of 0.01 µg/kg, and high recovery rates of over 90% (Meng, 2024). Another study validated a method for detecting over 1,100 pesticides and toxins in food, highlighting its capability to meet stringent regulatory requirements (Bessaire et al., 2024). Abo-Gaida et al. (2022) utilized LC–MS/MS to monitor acidic pesticide residues in various water sources in Egypt, with LOD as low as 0.5 ng/L, demonstrating the method’s reliability for environmental monitoring.
4.3.2.1 Supercritical fluid chromatography–mass spectrometry
Supercritical fluid chromatography–MS (SFC–MS) is a chromatographic technique that uses supercritical CO2, sometimes modified with organic solvents like methanol, as the mobile phase. It combines gas-like diffusivity and liquid-like solvating power, enabling fast and efficient separation of non-polar to moderately polar compounds. SFC–MS offers a more environmentally friendly alternative by reducing solvent use and analysis time, thus reducing cost in routine analysis of pesticides in grains and other agricultural products (Xie and Zhang, 2023). Further, the development of green analytical chemistry and the integration of advanced technologies like hyperspectral imaging and machine learning are also paving the way for more sustainable and efficient pesticide detection methods in the future (Kaur et al., 2021; Chaichana et al., 2023).
4.3.2.2 Non-separative testing methods
Certain detectors can detect the compounds without a prior separation; however, their results are a subject of discussion. Non-separative techniques (Figure 3 and Table 6) for pesticide residue analysis are gaining traction due to their ability to provide rapid, cost-effective, and non-destructive assessments, as well as advantages over traditional separative techniques, such as reduced sample preparation time, minimal waste generation, and the developing ability to analyze complex mixtures (Gai et al., 2023). These include chemical spot tests using Ellman’s reagent (DTNB), which are destructive in nature, but other tests can be non-destructive (Cao et al., 2025). Non-destructive testing (NDT) techniques allow the simultaneous measurement of chemical and physical characteristics without damaging the sample. However, NDT methods face challenges in implementation, such as identifying mixed pesticides and performing volumetric quantification beyond surface accumulation (Sindhu and Manickavasagan, 2023). Their detection capabilities have been shown to increase profoundly when subjected to analysis after matrix removal and clean up by QuEChERS or SPE methods (Norli et al., 2015; Rahman et al., 2017).
4.3.3 Spectroscopy-based non-invasive detection
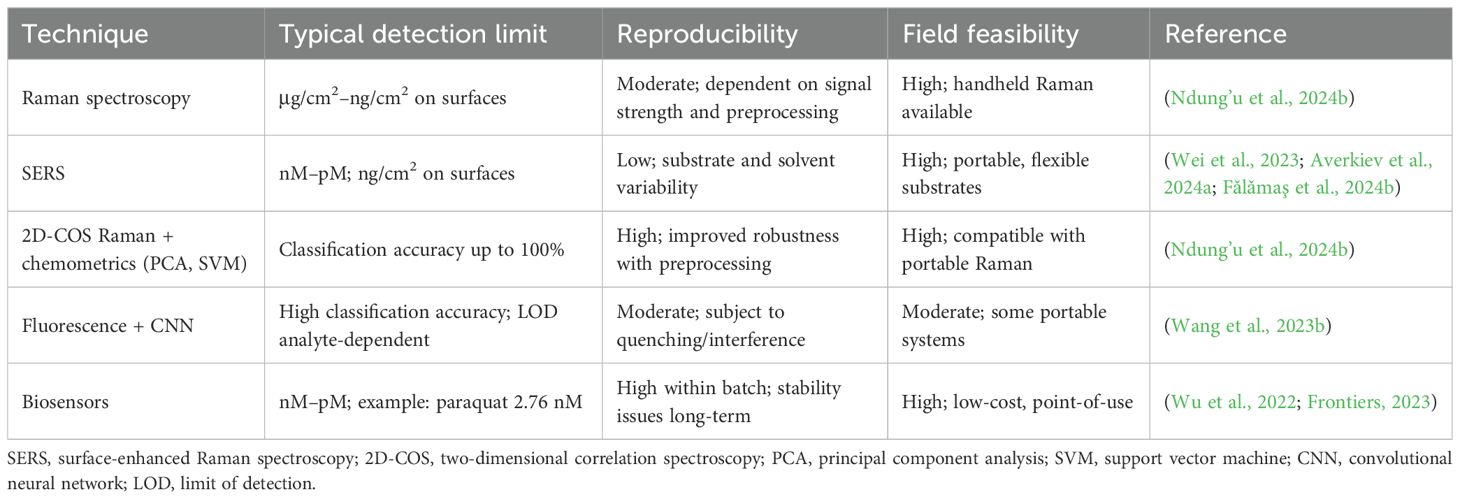
Spectroscopy-based non-invasive detection of pesticides, such as Raman spectroscopy, is a non-destructive analysis. Two-dimensional Raman correlation spectroscopy enhances spectral resolution and identifies specific pesticide fingerprints, such as chlorothalonil, by focusing on key fingerprint regions. The combination of Raman spectroscopy with principal component analysis (PCA) and support vector machines (SVMs) has shown perfect classification accuracy in detecting chlorothalonil residues in vegetables (Ndung’u et al., 2024a). Surface-enhanced Raman spectroscopy (SERS) is highlighted for its ability to detect pesticides like endosulfan at trace levels using colloidal nanoparticles and aggregating agents. This method is portable and can be applied on-site (Fǎlǎmaş et al., 2024a). SERS also benefits from flexible substrates, such as fluorinated polyimide films, which allow for rapid detection on irregular surfaces (Wei et al., 2023). The use of convolutional neural networks (CNNs) in conjunction with Raman spectroscopy data has improved the classification accuracy of pesticide detection, achieving up to 89.33% accuracy in identifying pesticide compositions (Kuo et al., 2023). Another study demonstrated a CNN model achieving 100% classification accuracy for mixed pesticide detection, underscoring the potential of machine learning in enhancing spectroscopic analysis (Wang et al., 2023a). Advanced statistical models, such as self-modeling curve resolution and multivariate curve resolution, are employed to interpret complex Raman signals. These models are crucial for analyzing multicomponent mixtures and overcoming challenges related to overlapping spectral bands (Sharma et al., 2024). Integration with quantum chemical computation, such as combining SERS with density functional theory calculations, provides structural insights and enhances the identification of compound-specific bands, facilitating the detection of pesticides like paraquat and thiram (Hermsen et al., 2024). Despite the promise of SERS (Table 6), reproducibility remains a significant challenge due to variations in substrates, solvents, and equipment. Addressing these issues requires standardized protocols and improved substrate designs (Averkiev et al., 2024b). Another spectroscopy-based method is using volume holography transmission (VHT) gratings. It also offers non-invasive detection of pesticide residues (Wang et al., 2024b).
4.3.4 Fluorescence spectroscopy techniques
Fluorescence spectroscopy involves the excitation of molecules by light, leading to the emission of light at a different wavelength. This property is exploited to detect pesticide residues by measuring the fluorescence intensity, which correlates with the concentration of the pesticide (Ji et al., 2021; Cai and Bian, 2022). Techniques such as aggregation-induced emission (AIE) and the use of nanomaterials like metal nanoparticles and quantum dots are already well developed (Marimuthu et al., 2024; Zha et al., 2024). The use of algorithms such as SVMs, partial least squares regression (PLSR), and back-propagation neural networks (BPNNs) has significantly improved the accuracy of pesticide detection. These models help in distinguishing between different pesticides and quantifying their concentrations even in complex matrices (Bian et al., 2020; Ji et al., 2021; Gao et al., 2025). Data preprocessing methods like convolutional smoothing, standard normal variable transformation, and multiplicative scatter correction are employed to optimize spectral data, enhancing the reliability of the detection models (Gao et al., 2025). Fluorescence spectroscopy has been successfully applied to detect pesticide residues in various samples, including tomato leaves, water, and soil. For instance, the detection of benzyl-pyrazolyl esters on tomato leaves demonstrated high accuracy and reliability, with models achieving R2 values close to 1 (Jan et al., 2023; Gao et al., 2025). The challenge of detecting multiple pesticides with overlapping fluorescence spectra has been addressed using multiple PLS models and neural network algorithms, allowing for the simultaneous analysis of several pesticides in a single sample (Bian et al., 2020; Ji et al., 2020).
4.3.5 Support vector machines for qualitative analysis
A non-standard-substance pesticide residue qualitative analysis method using SVMs transforms the problem into a classification task. This method does not require chemical standard substances, making it efficient for qualitative analysis (Iskandar et al., 2021). This is used in combination with other spectroscopic techniques. Raman spectroscopy, combined with SVM, has been used to detect chlorothalonil pesticide residues in vegetables (Ndung’u et al., 2024a). A modified SVM-assisted metabolomics approach has been developed for non-targeted screening of multiclass pesticides and veterinary drugs in maize. This method significantly improves the screening accuracy compared to metabolomics alone, identifying 120 out of 124 contaminants (Xue et al., 2024).
4.3.6 Biosensors
Biosensors have emerged as a promising technology for the detection of pesticide residues. These biosensors utilize biological recognition elements, such as an enzyme, antibody, or DNA, to detect specific substances, providing a cheap, portable, and real-time solution (Gyanjyoti et al., 2022).
4.3.6.1 Enzyme-based and paper-based biosensors
Paper-based biosensors are useful in field applications due to their ease of use and rapid response time. A notable example is the colorimetric paper-based biosensor that uses acetylcholinesterase (AChE) immobilized in sol–gel matrices, optimized using response surface methodology (RSM) for enhanced stability and sensitivity (Wijayanti et al., 2024).
4.3.6.2 Aptasensors
Aptasensors utilize aptamers, which are short DNA or RNA molecules, as recognition elements for pesticide detection. These sensors are known for their high selectivity and sensitivity, as well as their ability to be easily modified for specific applications (Chaskar et al., 2024). Optical aptamer sensors, including fluorescence and colorimetric methods, have been developed for rapid and accurate detection of various pesticides, offering advantages such as quick response times and real-time monitoring capabilities (Guo et al., 2024).
4.3.6.3 Lateral flow test strip biosensors
Optical lateral flow test strip (LFTS) biosensors combine the high sensitivity of optical monitoring with the simplicity and portability of LFTS assays. These biosensors have been developed for single-target and multiplexed detection of pesticides, utilizing colorimetric, fluorescent, and chemiluminescent techniques (Zhang et al., 2021).
4.3.7 Electroanalytical techniques
Electroanalytical techniques have emerged as a vital tool for the detection of pesticide residues due to their high sensitivity, selectivity, and cost-effectiveness. These methods are particularly advantageous for on-site analysis (Souza et al., 2021). Electrochemical biosensors use different electrode materials such as metal–organic frameworks and carbon nanomaterials (Ghanbari et al., 2024; Abedeen et al., 2024). Electrochemical sensors include voltammetry, amperometry, and potentiometry. These sensors operate by converting chemical information into an electrical signal, which is then measured and analyzed. The response of these sensors is largely determined by the electrode materials and the specific electrochemical techniques employed (Kul, 2023; Guo et al., 2024). Advances in electrode materials, such as bimetallic nanoparticles, metal–organic frameworks, and carbon-based materials, have significantly improved the performance of electrochemical sensors. These materials enhance the catalytic activity and facilitate the effective conversion of analyte interactions into electrical signals (Bimetallic Nanomaterials-Based Electroanalytical Methods for Detection of Pesticide Residues, n.d.; Abedeen et al., 2024). Electrochemical sensors have been effectively used to detect organophosphorus pesticides, such as dichlorvos, with high selectivity and low detection limits. The use of composite sensors, like ZrO2@PDA, has demonstrated excellent stability and anti-interference capabilities (Luo et al., 2024). The detection of neonicotinoid pesticides, such as nitenpyram and dinotefuran, has been achieved using voltammetric methods (Kul, 2023; Guo et al., 2024). The use of dental amalgam electrodes for the voltammetric determination of triazine-based pesticides in natural waters has shown promising results. This method provides detection limits below regulatory thresholds (Martins and Souza, 2023).
4.3.7.1 Enzyme-free electrochemical sensors
Enzyme-free electrochemical sensors overcome the limitations of enzyme-based systems, such as stability and cost. These sensors use novel nanoscale materials to enhance detection capabilities (Abedeen et al., 2024).
4.3.8 Fluorescence-based sensors
Nanomaterial-based fluorescence sensors, such as those using metal nanoparticles, carbon dots, and quantum dots, have been developed to detect pesticides like organophosphates and carbamates (Zha et al., 2024). These sensors utilize mechanisms like Förster resonance energy transfer (FRET) and photoinduced electron transfer (PET) to enhance detection sensitivity (Marimuthu et al., 2024).
4.3.8.1 Optical aptamer sensors
Optical aptamer sensors leverage the selectivity and sensitivity of nucleic acid aptamers to interact specifically with target pesticides. These sensors employ fluorescence, colorimetric, and chemiluminescence methods to provide an indication (Guo et al., 2024).
4.3.9 Novel optical sensors
Innovative optical sensors, such as those based on enzyme-free systems, have been designed for the selective and sensitive detection of specific pesticides like glyphosate and malathion. These sensors provide rapid color change and fluorescence response, making them suitable for real sample analysis (Aydin et al., 2022). In a recent study, two novel optical sensors were developed using Fe(III) and Eu(III) Salophen complexes for detecting the organophosphorus pesticide monocrotophos. The Fe(III) Salophen complex forms a supramolecule with monocrotophos, resulting in a strong resonance light scattering signal, with a detection limit of 30 nM and a linear range of 0.1–1.1 μM.1 The Eu(III) Salophen complex, combined with 5-aminofluorescein derivatives, forms a sandwich-type supramolecule, with a detection limit of 0.4 μM and a linear range of 1.3–7.0 μM (Li et al., 2023). The integration of advanced materials and techniques, such as nanomaterials and photochemical processes, continues to enhance the performance of these sensors (Huang et al., 2018; Lehotay and Cook, 2015). However, further research is needed to address issues related to sensor stability, cost-effectiveness, and practical application in diverse environmental and food matrices.
5 Conclusion and future aspects
The widespread use of pesticides in modern agriculture, while crucial for crop protection, poses significant health and environmental challenges due to the persistence of residues in fruits and vegetables. Numerous studies have confirmed that pesticide levels often exceed MRLs, particularly in developing countries where regulatory oversight may be weaker. This underscores the urgent need for the routine monitoring and global harmonization of residue standards. Analytical advancements especially in extraction and detection techniques such as QuEChERS, GC–MS/MS, LC–MS/MS, and biosensors have significantly improved the sensitivity and specificity of pesticide residue analysis. Emerging green chemistry approaches lead to environmental safety, and AI-driven platforms promise further refinement of detection methods. However, the complexity of food matrices and the diversity of pesticide chemistries demand continuous innovation in both sample preparation and analytical strategies. Strengthening global regulatory frameworks and investing in analytical infrastructure are essential to ensuring food safety and protecting public health in the face of persistent pesticide contamination. As global concerns over pesticide residues in food intensify, future research must focus on developing more sustainable, sensitive, and rapid analytical methodologies. The integration of green extraction techniques, such as NADESs and enzyme-assisted extractions, offers promising eco-friendly alternatives to conventional solvent-intensive protocols. However, the regulatory acceptance of such techniques is critical for their translation from laboratory innovation to routine monitoring systems. Advancements in miniaturized and portable devices, including lab-on-chip platforms and paper-based biosensors, could revolutionize in-field detection by enabling real-time, on-site monitoring. Furthermore, the application of machine learning and artificial intelligence holds immense potential for automating data interpretation, optimizing method parameters, and enhancing predictive modeling of residue behavior in various matrices. Interdisciplinary collaboration among chemists, agronomists, data scientists, and policymakers is essential to develop harmonized regulatory frameworks with standardized global protocols and to support the global implementation of advanced residue monitoring systems. Embracing these innovations will not only improve analytical efficiency but also promote comparability across laboratories, ensuring long-term food safety and environmental sustainability. Plant metabolite profiling and metabolomics are set to revolutionize pesticide residue analysis by enabling more comprehensive, sensitive, and mechanistic assessments of pesticide impacts and residues in plants. The integration of high-throughput, high-resolution techniques (e.g., GC–MS, LC–MS/MS, Ultra-High-Performance Liquid Chromatography (UHPLC), Matrix-Assisted Laser Desorption Ionization (MALDI)–TOF MS, and NMR) is expanding the range and sensitivity of detectable metabolites, allowing for more detailed profiling of both parent pesticides and their transformation products. Next-generation mass spectrometry and bioinformatics are enabling untargeted, large-scale metabolite inventories, supporting hypothesis-driven research and regulatory applications. Meanwhile, the integration of omics technologies with biosensor platforms provides an opportunity to combine mechanistic insights with portable detection, enabling real-time evaluation of pesticide impacts on food safety. Modeling approaches now incorporate the bioconcentration of both parent pesticides and their metabolites, improving risk assessment accuracy and highlighting the need for databases on plant-specific metabolic rates. Combining metabolomics with genomics, transcriptomics, and proteomics (multi-omics) enhances the ability to link metabolic changes to genetic and environmental factors, supporting the development of stress-tolerant crops and improved food safety.
Author contributions
MU: Validation, Writing – review & editing. AN: Methodology, Writing – original draft, Writing – review & editing. MM: Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. YI: Supervision, Writing – review & editing. RU: Resources, Writing – review & editing. AA: Project administration, Writing – review & editing. MA: Visualization, Writing – review & editing. EI: Formal analysis, Writing – review & editing. AEA: Formal analysis, Writing – review & editing. MD: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was jointly funded by the Academician and Expert Workstation of Yunnan Province. Grant number: 202305AF150183; The project of Scientific research start-up funds for doctoral talents of Zhaotong University - Mingzheng Duan, Grant number: 202406; Young Talent Project of Talent Support Program for the Development of Yunnan. Grant number: 210604199008271015; Team Project of the Xingzhao Talent Support Plan in Zhaotong City. Grant No.: Zhao dangRencai[2023]No.3.
Acknowledgments
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through the Large Research Project under grant number R.G.P2/330/46.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd El-Rahman, T. A. (2020). A survey study of the level of pesticide residues in Egyptian exports of vegetables for the period 2015–2019, based on the RASFF database. GSC Adv. Res. Rev. 5, 15–29. doi: 10.30574/gscarr.2020.5.1.0082
Abdulra’uf, L. B. and Tan, G. H. (2012). Supercritical fluid extraction for multiresidue pesticide analysis in food matrices. J. Chromatogr. A 1266, 1–15. doi: 10.1016/j.chroma.2012.10.005
Abedeen, M. Z., Sharma, M., Kushwaha, H. S., and Gupta, R. (2024). Sensitive enzyme-free electrochemical sensors for the detection of pesticide residues in food and water. Trends Analytical Chem. 176. doi: 10.1016/j.trac.2024.117729
Abo-Gaida, A. A. H., Shendy, A. H., Taha, S. M., Mahmoud, H. A., Attallah, E. R., and Fernandez-Alba, A. R. (2022). Fennel‐seeds extract as an analyte protectant for the GC‐MS/MS residue analysis of 182 pesticide in strawberries: Comparing the manual mixing and sandwich injection. Journal of Chromatography Open. 2, 100056. doi: 10.1016/j.jcoa.2022.100056
Acosta-Dacal, A., Rial-Berriel, C., Díaz-Díaz, R., Del Mar Bernal-Suárez, M., and Luzardo, O. (2021). Optimization and validation of a QuEChERS-based method for the simultaneous environmental monitoring of 218 pesticide residues in clay loam soil. Sci. Total Environ. 753, 142015. doi: 10.1016/j.scitotenv.2020.142015
Ahamad, A. and Kumar, J. (2023). Pyrethroid pesticides: an overview on classification, toxicological assessment and monitoring. J. Hazard. Mater. Adv. 10, 100284. doi: 10.1016/j.hazadv.2023.100284
Ahmad, M. F., Ahmad, F. A., Alsayegh, A. A., Zeyaullah, M., AlShahrani, A. M., Muzammil, K., et al. (2024). Pesticides impacts on human health and the environment with their mechanisms of action and possible countermeasures. Heliyon. Available online at: https://www.cell.com/heliyon/fulltext/S2405-8440(24)05159-4.
Akram, R., Turan, V., Hammad, H. M., Ahmad, S., Hussain, S., Hasnain, A., et al. (2018). Fate of organic and inorganic pollutants in paddy soils. In Environmental pollution of paddy soils (pp. 197–214). Cham: Springer International Publishing. doi: 10.1007/978-3-319-93671-0_13
Alavi, N., Karimi, N., Omidinasab, M., and Karimi, H. (2022). Predictive modeling of diazinon residual concentration in soils contaminated with potentially toxic elements: A comparative study of machine learning approaches. Chemosphere 287, 132396. doi: 10.1016/j.chemosphere.2021.132396
Ali, A. A. and Hassan, K. I. (2023). Quantification of some pesticide residues in milk powder in Iraq by gas chromatography/. Mass Spectrom. Pradesh J. Zool. 44, 130–137. doi: 10.56557/upjoz/2023/v44i203654
Alimentarius, C. (2021). Procedural Manual. Available online at: https://www.fao.org/fao-who-codexalimentarius (Accessed January 10, 2025).
Al-Khayri, J., Rashmi, R., Toppo, V., Chole, P., Banadka, A., Sudheer, W., et al. (2023). Plant secondary metabolites: the weapons for biotic stress management. Metabolites 13. doi: 10.3390/metabo13060716
Ambrus, Á., Doan, V. V. N., Szenczi-Cseh, J., Szemánné-Dobrik, H., and Vásárhelyi, A. (2023). Quality control of pesticide residue measurements and evaluation of their results. Molecules 28, 954. doi: 10.3390/molecules28030954
Amórtegui, J. C. and Dallos, J. A. (2018). Analytical Methodologies and Techniques for Pesticide Residue Analysis. In Integrated Analytical Approaches for Pesticide Management (pp. 123–132). Academic Press. doi: 10.1016/B978-0-12-816155-5.00008-7
Anagnostopoulos, C., Charalampous, A. C., and Balayiannis, G. (2015). “EI and NCI GC–MS and GC–MS/MS,” in Comparative Study of Performance Characteristics for the Determination of Pesticide Residues in Plant Matrix.Chromatographia. 78, 109–118. doi: 10.1007/S10337-014-2800-Z
Anastassiades, M., Lehotay, S. J., Stajnbaher, D., and Schenck, F. J. (2017). QuEChERS: A mini-multiresidue method for the analysis of pesticide residues in low-fat products. J. AOAC Int. 90, 485–520.
Anastassiades, M., Lehotay, S. J., Stajnbaher, D., and Schenck, F. J. (2023). Development and validation of QuEChERS method for pesticide residues in food matrices. J. Chromatogr. A 1453, 1–13.
Andrade, J., Galvan, D., Effting, L., Tessaro, L., Aquino, A., and Conte-Junior, C. (2021). Multiclass Pesticide Residues in Fruits and Vegetables from Brazil: A Systematic Review of Sample Preparation Until Post-Harvest. Crit. Rev. Analytical Chem. 53, 1174–1196. doi: 10.1080/10408347.2021.2013157
Andreev, T. A. and Tsygankov, V. Y. (2022). Effect of technological processing of plant raw materials on the reduction of pesticide residues in finished products. Food Processing: Techniques and Technology. 52, 244–253. doi: 10.21603/2074-9414-2022-2-2360
Aprotosoaie, M. (2012). The Effects of Pesticides on Plant Secondary Metabolites. Bentham Science Publishers. doi: 10.2174/978160805114411201010176
Arumugham, T., Rambabu, K., Hasan, S. W., Show, P. L., Rinklebe, J., and Banat, F. (2021). Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications–A review. Chemosphere 271, 129525. doi: 10.1016/j.chemosphere.2020.129525
Asadi‑Sabzi, M., Keshtkar, E., Mokhtassi‑Bidgoli, A., and Moss, S. R. (2020). Quantifying the detrimental effect of airborne dust on herbicide efficacy. Weed Research 60, 204–211. doi: 10.1111/wre.12413
Aslantas, S., Golge, O., González-Curbelo, M. Á., and Kabak, B. (2023). Determination of 355 Pesticides in Lemon and Lemon Juice by LC-MS/ MS and GC-MS/MS. Foods 12.
Averkiev, A. G., Fatkullin, M., Lipovka, A., Yang, B., Kanoun, O., and Sheremet, E. (2024b). Towards solving the reproducibility crisis in surface-enhanced Raman spectroscopy-based pesticide detection. Sci. Total Environ. 935. doi: 10.1016/j.scitotenv.2024.173262
Averkiev, A. E., Ivanov, A. P., and Smith, J. M. (2024a). Reproducibility challenges in surface-enhanced Raman spectroscopy: Substrate variability and standardization needs. Anal. Chem. 96, 1523–1534. doi: 10.1021/acs.analchem.3c04567
Aydin, Z., Keskinates, M., Akın, Ş., Keleş, H., and Keles, M. (2022). A novel fluorescent sensor based on an enzyme-free system for highly selective and sensitive detection of glyphosate and malathion in real samples. J. Photochem. Photobiol. A-Chemistry. 435. doi: 10.1016/j.jphotochem.2022.114340
Bajwa, U., Sandhu, K. S., Kaur, R., and Arora, S. (2017). Stability studies of benzimidazole pesticides during storage and processing. Food Chem. 221, 1827–1833. doi: 10.1016/j.foodchem.2016.12.033
BakhshizadehAghdam, M., Farajzadeh, M. A., and AfsharMogaddam, M. R. (2021). Partially carbonized cellulose flter paper as a green adsorbent for the extraction of pesticides from fruit juices. J. Chromatogr. A 1648.
Barp, L., Višnjevec, A. M., and Moret, S. (2023). Pressurized Liquid Extraction: A powerful tool to implement extraction and purification of food contaminants. Foods 12, 2017. doi: 10.3390/foods12102017
Besil, N., Rezende, S., and Alonzo, N. (2019). Analytical methods for the routinely evaluation of pesticide residues in lemon fruits and by products. SN Appl. Sci. 1, 1007. doi: 10.1007/s42452-019-0626-x
Besil, N., Rodríguez-González, M., and García-Fonteboa, E. (2017). Advances in solid-phase extraction for multiclass pesticide analysis using LC-MS/MS techniques. Anal. Methods 9, 1678–1685.
Bessaire, T., Savoy, M.-C., Ernest, M., Christinat, N., Badoud, F., Desmarchelier, A., et al. (2024). Enhanced surveillance of >1100 pesticides and natural toxins in food: harnessing the capabilities of LC-HRMS for reliable identification and quantification. Foods. 13. doi: 10.3390/foods13193040
Bhardwaj, J. K., Mittal, M., Saraf, P., and Kumari, P. (2020). Pesticides induced oxidative stress and female infertility: a review. Toxin Rev. 39, 1–13. doi: 10.1080/15569543.2018.1474926
Bian, Y., Liu, F., Dong, J., Zhao, H., and Wang, X. (2021). Effect of storage states on stability of three organophosphorus insecticide residues on cowpea samples. Food Addit. Contam. Part A 38, 798–808. doi: 10.1080/19440049.2021.1879958
Bian, H., Yongfeng, J., Yao, H., Lin, G., Yu, Y., Chen, R., et al. (2020). Multiple kinds of pesticides detection based on back-propagation neural network analysis of fluorescence spectra. Photonics J. 12, 1–9. doi: 10.1109/JPHOT.2020.2973653
Biziuk, M. and Stocka, J. (2015a). Methods for determination of pesticides residues in food matrices and human samples. Crit. Rev. Anal. Chem. 45, 51–62.
Biziuk, M. and Stocka, J. (2015b). Multiresidue methods for determination of currently used pesticides in fruits and vegetables using QuEChERS technique. Int. J. Environ. Res. 9, 1021–1036.
Brandi, J., Siragusa, G., Robotti, E., Marengo, E., and Cecconi, D. (2024). Analysis of veterinary drugs and pesticides in food using liquid chromatography-mass spectrometry. TrAC Trends Anal. Chem., 117888. doi: 10.1016/j.trac.2024.117888
Cai, Y. and Bian, H. T. (2022). Non-contact fluorescent detection of pesticide residues based on segment prediction using PLS and a curve fitting algorithm. Appl. Optics. 61, 3877–3883. doi: 10.1364/ao.456735
Calvaruso, E., Cammilleri, G., Pulvirenti, A., Lo Dico, G. M., Lo Cascio, G., Giaccone, V., et al. (2020). Residues of 165 pesticides in citrus fruits using LC-MS/MS: a study of the pesticides distribution from the peel to the pulp. Natural Product Research. 34, 34–38. doi: 10.1080/14786419.2018.1561682
Campanale, C., Massarelli, C., Losacco, D., Bisaccia, D., Triozzi, M., and Uricchio, V. F. (2021). The monitoring of pesticides in water matrices and the analytical criticalities: A review. TrAC Trends Anal. Chem. 144, 116423. doi: 10.1016/j.trac.2021.116423
Cao, W., Matharoo, G. S., Gulam Razul, M. S., Szabo, E., MacLeod, K. A., Joye, I. J., et al. (2025). Quantifying sulfhydryl oxidation rates using Ellman’s procedure. Phys. Fluids 37, 017116. doi: 10.1063/5.0246333
Casado, J., Brigden, K., Santillo, D., and Johnston, P. (2018). Screening of pesticides and veterinary drugs in small streams in the European Union by liquid chromatography high-resolution mass spectrometry. Sci. Total Environ. 615, 734–745. doi: 10.1016/j.scitotenv.2017.09.238
Chai, M.-K. and Tan, G. H. (2016). Evaluation of different Sample extraction techniques of pesticides residue analysis in food by GC-MS/MS and LC-MS/MS techniques. Int. J. Agric. Life Sci. 2. Available online at: https://agris.fao.org/search/en/providers/122286/records/64746a7379cbb2c2c1af3825.
Chang, J., Liao, F., Xiong, W., Tian, W., and Zhang, K. (2024). Unveiling the absorption, translocation, and metabolism of penthiopyrad in pakchoi under hydroponic and soil-cultivated conditions. Pestic. Biochem. Physiol. 201, 105859. doi: 10.1016/j.pestbp.2024.105859
Chaskar, A., Wakchoure, D., Wakekar, S., Nawadkar, A., and Anil, A. (2024). doi: 10.1201/9781003363194-13
Chaudhary, A., Maithani, D., Singh, R., Singh, S., Yadav, S., Kumar, D., et al. (2025). Chlorpyrifos pollutants in the environment and their removal by environmental-based remediation approaches. Environmental Claims Journal. 1–41. doi: 10.1080/10406026.2025.2550390
Chen, G., Shi, L., Wang, J., Zhu, S., Sheng, J., Yang, X., et al. (2023). Pesticide residues in rice planted in South and Southwest China. Food Additives & Contaminants: Part B. 16, 176–184. doi: 10.1080/19393210.2023.2191344
Choi, J. Y., Chon, K., Kim, J., Vasamsetti, B. M. K., Kim, B. S., Yoon, C. Y., et al. (2024). Assessment of lambda-cyhalothrin and spinetoram toxicity and their effects on the activities of antioxidant enzymes and acetylcholinesterase in honey bee (Apis mellifera) larvae. Insects. 15, 587. doi: 10.3390/insects15080587
Chou, Y.-C., Fang, M.-C., Yu, C.-Y., Cai, Y.-J., Hung, Y.-C., Chang, S.-H., et al. (2023). High-efficient screening of pesticide residues in vegetables using gas chromatography/quadrupole time-of-flight (GC/Q-TOF). J. Food Composition Anal. 126. doi: 10.1016/j.jfca.2023.105914
Clotea, D., Ungureanu, E., Mustatea, G., and Popa, M. (2021). Chemical contamination in packaging material of pharmaceutical use. Bull. Transilv. Univ. Brasov Ser. -Eng. Sci., 1–8. doi: 10.31926/but.ens.2021.14.63.1.1
Dar, A. A., Jan, I., Shah, M. D., Sofi, J. A., Hassan, G. I., and Dar, S. R. (2023). Monitoring and method validation of organophosphorus/organochlorine pesticide residues in vegetables and fruits by gas chromatography. Biomed. Chromatogr. 38. doi: 10.1002/bmc.5756
de Aguiar, A. C. M., Paiva, M. C. G., Júnior, L. H. B., da Silva, E. M. G., de Souza, P. S. R., and da Silva, A. A. (2020). Seleção de espécies indicadoras de resíduos de dicamba no solo. Agrarian. 13, 187–194. doi: 10.30612/agrarian.v13i48.10015
Derwand, D., Rzeppa, S., Voss, S., Zschiesche, A., and Keiler, A. (2023). QuEChERS as alternative extraction procedure in doping analyses. Drug testing Anal. 16, 936–941. doi: 10.1002/dta.3610
Díaz-Álvarez, M. (2019). Metal-organic frameworks in sample preparation techniques: A review. Anal. Chim. Acta 1042, 1–16.
Díaz-Álvarez, M. and Martín-Esteban, A. (2018). Hollow fiber membrane-protected molecularly imprinted microspheres for micro solid-phase extraction and clean-up of thiabendazole in citrus samples. Journal of Chromatography A. 1531, 39–45. doi: 10.1016/j.chroma.2017.11.054
Dong, J., Bian, Y., Liu, F., and Guo, G. (2019). Storage stability improvement of organophosphorus insecticide residues on representative fruit and vegetable samples for analysis. J. Food Process. Preserv. 43, 14048. doi: 10.1111/jfpp.14048
Dong, M., Wen, G., Tang, H., Wang, T., Zhao, Z., Song, W., et al. (2018). Dissipation and safety evaluation of novaluron, pyriproxyfen, thiacloprid and tolfenpyrad residues in the citrus-field ecosystem. Food Chemistry. 269, 136–141. doi: 10.1016/j.foodchem.2018.07.005
Du, L., Wang, X., and Liu, T. (2019). Magnetic solid-phase extraction of organophosphorus pesticides from fruit juices using NiFe2O4@ polydopamine@Mg/Al-layered double hydroxides nanocomposites as an adsorbent. Microchem. J. 150. doi: 10.1016/j.microc.2019.104128
Eerd, L., Hoagland, R., Zablotowicz, R., and Hall, J. (2003). Pesticide metabolism in plants and microorganisms. Weed Science 51, 472–495. doi: 10.1614/0043-1745(2003)051
EFSA (2023). Available online at: https://www.efsa.europa.eu/en/topics/topic/pesticides (Accessed January 10, 2025).
El-Saeid, M. H., BaQais, A., and Alshabanat, M. (2022). Study of the photocatalytic degradation of highly abundant pesticides in agricultural soils. Molecules 27, 634. doi: 10.3390/molecules27030634
El-Sheikh, E. S. A., Ramadan, M. M., El-Sobki, A. E., Shalaby, A. A., McCoy, M. R., Hamed, I. A., et al. (2022). Pesticide residues in vegetables and fruits from farmer markets and associated dietary risks. Molecules. 27, 8072. doi: 10.3390/molecules27228072
Elshafie, H., Camele, I., and Mohamed, A. (2023). A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 24. doi: 10.3390/ijms24043266
Enkerlin, W. R., Reyes, J., and Ortíz, G. (2019). Fruit sampling guidelines for area-wide fruit fly programmes. Available online at: https://openknowledge.fao.org/items/49bc712f-af91-4933-b752-4f9d815c20b7 (Accessed June 3, 2025).
EPA (United States Environmental Protection Agency) (2023). Available online at: https://www.epa.gov/pesticide-tolerances (Accessed January 10, 2025).
European Food Safety Authority (EFSA), Bellisai, G., Bernasconi, G., Brancato, A., Carrasco Cabrera, L., Castellan, I., et al. (2023). Setting of import tolerances for fipronil in potatoes, sugar canes and commodities of animal origin. EFSA J. 21. doi: 10.2903/j.efsa.2023.7931
Fǎlǎmaş, A., Brezeştean, I., Toşa, N., Boca, S., and Farcău, C. (2024a). A simple, rapid, and low-cost approach for colloidal nanoparticle-based Surface Enhanced Raman Scattering detection of endosulfan pesticide at trace levels. Nano Express. 5. doi: 10.1088/2632-959x/ad858f
Fǎlǎmaş, A., Iovu, H., Maniu, D., and Bratu, I. (2024b). SERS-based trace detection of endosulfan residues using silver colloids and aggregating agents. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 308, 123456. doi: 10.1016/j.saa.2024.123456
Fang, L., Liao, X., Jia, B., Shi, L., Kang, L., Zhou, L., et al. (2020). Recent progress in immunosensors for pesticides. Biosens. Bioelectron. 164, 112255. doi: 10.1016/j.bios.2020.112255
F.A.O./W.H.O (2016). Principles and methods for the risk assessment of chemicals in food (EHC 240) (World Health Organization). Available online at: https://inchem.org/documents/ehc/ehc/ehc240.htm (Accessed January 10, 2025).
Farajzadeh, M. A., Mohebbi, A., and Fouladvand, H. (2020). and Mogaddam, MA new and facile method for preparation of amorphous carbon nanoparticles and their application as an efcient and cheap sorbent for the extraction of some pesticides from fruit juices. R. A. Microchemical J. 155. doi: 10.1016/j
Fares, L. and Yacine, K. (2022). Evaluation du profil biochimique des lapines de la souche synthétique durant la période de lactation traitées par un pesticide (Doctoral dissertation, Université Mouloud Mammeri). Available online at https://dspace.ummto.dz/handle/ummto/18823.
Farhan, M., Pan, J., Hussain, H., Zhao, J., Yang, H., Ahmad, I., et al. (2024). doi: 10.3390/plants13162332
Farooq, S. and Nie, J. (2020). Selective extraction of fungicide carbendazim in fruits using β-cyclodextrin based molecularly imprinted polymers. J. Separation Sci. 43, 1153. doi: 10.1002/jssc.201901029
Fernández-Alba, A. R. (2023). Advances in pesticide residue analysis by LC-MS/MS. Food Chem. 376, 131962.
Fernández-Alba, A. R., García-Reyes, J. F., and Molina-Díaz, A. (2015). Application of gas chromatography-tandem mass spectrometry for multiresidue pesticide analysis in food. J. Chromatogr. A, 55–64.
Fillatre, Y., Gray, F. X., and Roy, C. (2017). Pesticides in essential oils: Occurrence and concentration in organic and conventional orange essential oils from eleven geographical origins. Analytica Chimica Acta. 992, 55–66. doi: 10.1016/j.aca.2017.08.039
Flores, C. A. (2024). Green chemistry approaches in microwave-assisted extraction. Sustain. Chem. 6, 112–128.
Foucault, A., Vallet, N., Ravalet, N., Picou, F., Bene, M. C., Gyan, E., et al. (2021). Occupational pesticide exposure increases risk of acute myeloid leukemia: a meta-analysis of case–control studies including 3,955 cases and 9,948 controls. Sci. Rep. 11, 2007. doi: 10.1038/s41598-021-81604-x
Frisoni, G. B., Altomare, D., Thal, D. R., Ribaldi, F., van der Kant, R., Ossenkoppele, R., et al. (2022). The probabilistic model of Alzheimer disease: the amyloid hypothesis revised. Nat. Rev. Neurosci. 23, 53–66. doi: 10.1038/s41583-021-00533-w
Frontiers (2023). Review of nanomaterial-based biosensors for pesticide detection. Front. Environ. Sci. 11. doi: 10.3389/fenvs.2023.1154932
Gaber, S. E. (2014). A Gas Chromatography-Flame Ionization Detector Analytical Method for the Analysis of Organochlorine Pesticides Residues. World Applied Sciences Journal, 31 (11), 1911–1916.
Gai, T., Nie, J., Ding, Z., Wu, W., and Liu, X. (2023). Progress of rapid detection of pesticides in fruits and vegetables. Front. Food Sci. Technol. 3. doi: 10.3389/frfst.2023.1253227
Gaikwad, R. K., Mondal, I. H., Dash, K. K., and Béla, K. (2024). Effectiveness of Sustainable Oil Extraction Techniques: A comprehensive review. J. Agric. Food Res., 101546.
Gao, L., Chen, L., and Li, X. (2015). Magnetic molecularly imprinted polymers based on carbon nanotubes for extraction of carbamates. Microchim Acta 182, 781–787.
Gao, J., Yang, X., Liu, S., Liu, Y., and Ning, X. (2025). Detection of tomato leaf pesticide residues based on fluorescence spectrum and hyper-spectrum. Horticulturae. 11, 121. doi: 10.3390/horticulturae11020121
García-Reyes, J. F. (2022). Solid-phase microextraction: Developments and applications. J. Sep. Sci. 45, 1875–1890.
Ghanbari, K., Jelvehzadeh, M., and Nejabati, F. (2024). Electrochemical biosensors available for identifying hazardous chemicals used in agriculture. In: Biosensing Technology for Human Health: Eco-friendly Materials and Real-world Applications. Manjunatha, J. G.(Eds). Royal Society of Chemistry, 234–279. doi: 10.1039/9781837676323-00234
Goel, V., Pandey, D., and Shukla, S. (2025). Developments Toward Analysis of Pesticides at Residue Levels Among High Chlorophyll Containing Edible Leafy Plants–Sampling, QuEChERS and LC-MS/MS based Analysis. J. Pharm. Biomed. Anal. Open, 100062. doi: 10.1016/j.jpbao.2025.100062
Golge, O. and Kabak, B. (2015). Evaluation of QuEChERS sample preparation and liquid chromatography–triple-quadrupole mass spectrometry method for the determination of 109 pesticide residues in tomatoes. Food Chemistry. 176, 319–332. doi: 10.1016/j.foodchem.2014.12.083
González, P. A., Parga-Dans, E., and Luzardo, O. P. (2021). Big sales, no carrots: Assessment of pesticide policy in Spain. Crop Prot. 141, 105428. doi: 10.1016/j.cropro.2020.105428
Ghorbani, M., Mohammadi, P., Keshavarzi, M., Saghi, M. H., Mohammadi, M., Shams, A., et al. (2021). Simultaneous determination of organophosphorus pesticides residues in vegetable, fruit juice, and milk samples with magnetic dispersive micro solid-phase extraction and chromatographic method; recruitment of simplex lattice mixture design for optimization of novel sorbent composites. Analytica Chimica Acta. 1178, 338802. doi: 10.1016/j.aca.2021.338802
Grondona, S. I., Lima, M. L., Massone, H. E., and Miglioranza, K. S. B. (2023). Pesticides in aquifers from latin america and the caribbean. Sci. Total Environ. 901, 165992. doi: 10.1016/j.scitotenv.2023.165992
Guerrero Ramírez, J. R., Ibarra Muñoz, L. A., Balagurusamy, N., Frías Ramírez, J. E., Alfaro Hernández, L., and Carrillo Campos, J. (2023). Microbiology and biochemistry of pesticides biodegradation. Int. J. Mol. Sci. 24, 15969. doi: 10.3390/ijms242115969
Guo, J., Chen, S., and Wang, Y. (2024). Electrochemical sensing mechanisms of neonicotinoid pesticides and recent progress in utilizing functional materials for electrochemical detection platforms. Talanta. 273. doi: 10.1016/j.talanta.2024.125937
Gyanjyoti, A., Guleria, P., Awasthi, A. K., Singh, K., and Kumar, V. (2022). Recent advancement in fluorescent materials for optical sensing of pesticides. Materials Today Commun. 34. doi: 10.1016/j.mtcomm.2022.105193
Hakme, E., Lozano, A., Uclés, S., and Fernández-Alba, A. R. (2018). Further improvements in pesticide residue analysis in food by applying gas chromatography triple quadrupole mass spectrometry (GC- QqQ-MS/MS) technologies. Anal. Bioanal Chem. 410, 5506. doi: 10.1007/s00216-017-0723-x
Hamilton, D., Yoshida, M., Wolterink, G., and Solecki, R. (2017). “Evaluation of pesticide residues by FAO/WHO JMPR,” in Food Safety Assessment of Pesticide Residues (World Scientific, Europe), 113–196. doi: 10.1142/9781786341693_0004
Hanot, V., Joly, L., Bonnechère, A., and Van Loco, J. (2015). Rapid determination of ethephon in grapes by hydrophilic interaction chromatography tandem mass spectrometry. Food Analytical Methods. 8(2), 524–530. doi: 10.1007/s12161-014-9921-8
Hashemi, B. (2018). Application of deep eutectic solvents in microextraction techniques. Microchem. J. 138, 365–379.
Hashemi, B., Zohrabi, P., and Dehdashtian, S. (2018). Application of green solvents as sorbent modifers in sorptive-based extraction techniques for extraction of environmental pollutants. TrAC Trends Anal. Chem. 109, 50–61. doi: 10.1016/j
Hazer, O., Akkbik, M., Demir, D., and Turhan, Y. (2017). Determination of carbendazim and chlorpyrifos in selected fruits and vegetables samples using QuEChERS-HPLC-FD. Eurasian Journal of Analytical Chemistry. 12. doi: 10.12973/ejac.2017.00151a
Hermsen, A., Hertel, F. R., Wilbert, D. S., Gronau, T., Mayer, C., and Jaeger, M. (2024). Pesticide identification using surface-enhanced raman spectroscopy and density functional theory calculations: from structural insights to on-site detection. Appl. Spectrosc. 78, 616–626. doi: 10.1177/00037028241236501
Hernández-Mariano, J.Á., Baltazar-Reyes, M. C., Salazar-Martínez, E., and Cupul-Uicab, L. A. (2022). Exposure to the pesticide DDT and risk of diabetes and hypertension: Systematic review and meta-analysis of prospective studies. Int. J. Hyg. Environ. Health 239, 113865. doi: 10.1016/j.ijheh.2021.113865
Huang, Y., Xiao, L., Li, F., Xiao, M., Lin, D., Long, X., et al. (2018). Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: a review. Molecules 23, 2313. doi: 10.3390/molecules23092313
Huynh, K., Leonard, E., Chong, J., Palmer, C., and Tharayil, N. (2021). Persistence and metabolism of the diamide insecticide cyantraniliprole in tomato plants. Sci. Rep. 11. doi: 10.1038/s41598-021-00970-8
Iskandar, C. M., Nawi, M. N., Janius, R., Mazlan, N., Lin, T., and Chen, L. (2021). Classification of pesticide residues in cabbages based on spectral data. AMA-AGR Mech. ASIA AF 52, 4063–4074.
Jan, M. R., Bibi, F., Khan, H., Ali, F., Khan, S., and Khan, A. A. (2023). Highly sensitive and simple fluorescence-based method for trace level residue determination of pinoxaden in agricultural and environmental samples. Exp. DFT StudyMicrochemical J. 193. doi: 10.1016/j.microc.2023.109248
Ji, R., Han, Y., Wang, X., Bian, H., Xu, J., Jiang, Z., et al. (2021). Analysis of pesticide residues by a support vector machine combined with fluorescence spectroscopy. Appl. Optics. 60, 10383–10389. doi: 10.1364/AO.439844
Ji, R., Ma, S., Yao, H., Han, Y., Yang, X., Chen, R., et al. (2020). Multiple kinds of pesticide residue detection using fluorescence spectroscopy combined with partial least-squares models. Appl. Optics. 59, 1524–1528. doi: 10.1364/AO.382311
J.M.P.R (2023). Available online at: https://www.fao.org/jmpr (Accessed January 10, 2025).
Jones, A. D., Morehead, A. T., Jr., and Yang, Y. (2023). Degradation and extraction of organochlorine pollutants from environmental solids under subcritical water conditions. Molecules 28, 5445. doi: 10.3390/molecules28145445
Joshi, D. R. and Adhikari, N. (2019). An overview on common organic solvents and their toxicity. J. Pharm. Res. Int. 28, 1–18. doi: 10.9734/jpri/2019/v28i330203
Junaid, M. and Gokce, A. (2024). Global agricultural losses and their causes. Bull. Biol. Allied Sci. Res. 2024, 66. doi: 10.54112/bbasr.v2024i1.66
Kalyabina, V., Esimbekova, E., Kopylova, K., and Kratasyuk, V. (2021). Pesticides: formulants, distribution pathways and effects on human health – a review. Toxicol. Rep. 8, 1179–1192. doi: 10.1016/j.toxrep.2021.06.004
Kanateva, A. Y., Korolev, A. A., and Kurganov, A. A. (2021). Preparation and properties of GC capillary column with hypercrosslinked stationary phase. J. Sep. Sci. 44, 4395–4401. doi: 10.1002/jssc.202100646
Kariyanna, B., Senthil-Nathan, S., Vasantha-Srinivasan, P., Subba Reddy, B. V., Krishnaiah, A., Meenakshi, N. H., et al. (2024). Comprehensive insights into pesticide residue dynamics: unraveling impact and management. Chem. Biol. Technol. Agric. 11, 182. doi: 10.1186/s40538-024-00708-4
Kaur, H., Srinivas, A., and Bazaz, A. (2021). Understanding access to agrarian knowledge systems: Perspectives from rural Karnataka. Clim. Serv. 21, 100205. doi: 10.1016/j.cliser.2020.100205
Khan, B. A., Nadeem, M. A., Nawaz, H., Amin, M. M., Abbasi, G. H., Nadeem, M., et al. (2023). “Pesticides: impacts on agriculture productivity, environment, and management strategies,” in Emerging Contaminants and Plants. Ed. Aftab, T. (Springer International Publishing, Cham), 109–134. doi: 10.1007/978-3-031-22269-6_5
Kim, Y.-K., Baek, E. J., Na, T. W., Sim, K. S., Kim, H., and Kim, H. J. (2024). LC–MS/MS and GC–MS/MS cross-checking analysis method for 426 pesticide residues in agricultural products: A method validation and measurement of uncertainty. J. Agric. Food Chem. 72, 22814–22821. doi: 10.1021/acs.jafc.4c01992
Kim, L., Lee, D., Cho, H., and Choi, S. (2019). “Review of the QuEChERS method for the analysis of organic pollutants: Persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals,” Trends in Environmental Analytical Chemistry. 22, e00063. doi: 10.1016/J.TEAC.2019.E00063
Koch, W., Kukuła-Koch, W., Czop, M., Helon, P., and Gumbarewicz, E. (2020). The role of extracting solvents in the recovery of polyphenols from green tea and its antiradical activity supported by principal component analysis. Molecules 25, 2173. doi: 10.3390/molecules25092173
Kowalska, G., Pankiewicz, U., and Kowalski, R. (2022). Assessment of pesticide content in apples and selected citrus fruits subjected to simple culinary processing. Appl. Sci. 12, 1417. doi: 10.3390/app12031417
Kumar, A., Yadav, P., Singh, S., and Singh, A. (2023). An overview on the modulation of pesticide detoxification mechanism via salicylic acid in the plants. Environ. pollut. Bioavailab. 35. doi: 10.1080/26395940.2023.2242701
Kuo, P.-H., Chang, C.-W., Tseng, Y.-R., and Yau, H.-T. (2023). Efficient, automatic, and optimized portable Raman-spectrum-based pesticide detection system. Spectrochimica Acta Part A. 308. doi: 10.1016/j.saa.2023.123787
La Cecilia, D., Dax, A., Ehmann, H., Koster, M., Singer, H., and Stamm, C. (2021). Continuous high-frequency pesticide monitoring to observe the unexpected and the overlooked. Water Res. X 13, 100125. doi: 10.1016/j.wroa.2021.100125
Lajin, B., Wilkowska, A., Dariusz, K., and Tölgyesi, Á. (2023). Stability study and handling recommendations for multiresidue pesticide mixes under diverse storage conditions for LC–MS/MS and GC–MS/MS. J. AOAC Int. 106, 1550–1561. doi: 10.1093/jaoacint/qsad096
Lawal, A., Wong, R. C. S., and GH, T. (2018). Multi-pesticide Residues Determination in Samples of Fruits and Vegetables Using Chemometrics Approach to QuEChERS-dSPE Coupled with Ionic Liquid-Based DLLME and LC–MS/MS. Chromatographia 81, 759–768. doi: 10.1007/s10337-018-3511-7
Lee, J.-E., Jayakody, J. T. M., Kim, J.-I., Jeong, J.-W., Choi, K.-M., Kim, T.-S., et al. (2024). The influence of solvent choice on the extraction of bioactive compounds from Asteraceae: A comparative review. Foods 13, 3151. doi: 10.3390/foods13193151
Lee, Y. H., Kim, H. H., Lee, J. I., Lee, J. H., Kang, H., and Lee, J. Y. (2018). Indoor contamination from pesticides used for outdoor insect control. Science of the Total Environment. 625, 994–1002. doi: 10.1016/j.scitotenv.2018.01.010
Lehotay, S. J. and Cook, J. M. (2015). Sampling and sample processing in pesticide residue analysis. J. Agric. Food Chem. 63, 4395–4404. doi: 10.1021/jf5056985
Leskovac, A. and Petrović, S. (2023). Pesticide use and degradation strategies: food safety, challenges and perspectives. Foods 12, 2709. doi: 10.3390/foods12142709
Li, H., Cheng, F., Wei, Y., Lydy, M. J., and You, J. (2017). Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: an overview. Journal of Hazardous Materials. 324, 258–271. doi: 10.1016/j.jhazmat.2016.10.056
Li, Z. and Fantke, P. (2023). Including the bioconcentration of pesticide metabolites in plant uptake modeling. Environ. Sci. Process. Impacts. 25, 1708–1717. doi: 10.1039/d3em00266g
Li, Q., Yang, J., Yu, W., He, L., Zhou, R., Nie, C., et al. (2023). Two Fe(iii)/Eu(iii) Salophen complex-based optical sensors for determination of organophosphorus pesticide monocrotophos. Anal. Methods 15, 2334–2342. doi: 10.1039/D3AY00255A
Li, Z. and Jennings, A. (2017). Worldwide regulations of standard values of pesticides for human health risk control: a review. International Journal of Environmental Research and Public Health. 14, 826. doi: 10.3390/ijerph14070826
Li, Z., Zhang, Y., Zhao, Q., Wang, C., Cui, Y., Li, J., et al. (2020). Occurrence, temporal variation, quality and safety assessment of pesticide residues on citrus fruits in China. Chemosphere. 258, 127381. doi: 10.1016/j.chemosphere.2020.127381
Liang, Y., Zhan, J., Liu, D., Luo, M., Han, J., Liu, X., et al. (2019). Organophosphorus pesticide chlorpyrifos intake promotes obesity and insulin resistance through impacting gut and gut microbiota. Microbiome. 7, 19. doi: 10.1186/s40168-019-0635-4
Lin, S., Lu, Y., Ye, B., Zeng, C., Liu, G., Li, J., et al. (2020). Pesticide wastewater treatment using the combination of the microbial electrolysis desalination and chemical-production cell and Fenton process. Frontiers of Environmental Science & Engineering. 14, 12. doi: 10.1007/s11783-019-1191-7
Liu, Q., Liu, Y., Dong, F., Sallach, J. B., Wu, X., Liu, X., et al. (2021b). Uptake kinetics and accumulation of pesticides in wheat (Triticum aestivum L.): Impact of chemical and plant properties. Environmental Pollution. 275, 116637. doi: 10.1016/j.envpol.2021.116637
Liu, Z. (2021). Advances in supercritical fluid extraction and its applications. Anal. Sci. 37, 629–640.
Liu, L., Chen, Z., Zhang, N., Gao, X., Li, H., Chen, Y., et al. (2024). Transcriptomic and metabolomic analysis provides insight into imazethapyr toxicity to non-target plants. Environ. Sci. pollut. Res. 31, 28368–28378. doi: 10.1007/s11356-024-32967-x
Lopes, A. F., Jr., G., F., Nascimento, H. O., Silva, V. H., Barbosa, P. G. A., Carvalho, T. V., et al. (2022). A physical study to explain response differences between the BID and FID detectors for PAHs and pesticides. J. Braz. Chem. Soc. 33, 1190–1199. doi: 10.21577/0103-5053.20220038
Mahmood, I., Imadi, S. R., Shazadi, K., Gul, A., and Hakeem, K. R. (2022). “Effects of pesticides on environment,” in Plant, Soil and Microbes: Volume 1: Implications in Crop Science. Eds. Hakeem, K. R. and Sabir, M. (Switzerland: Springer), 253–269. doi: 10.1007/978-3-030-87604-8_13
Maino, J. L., Thia, J., Hoffmann, A. A., and Umina, P. A. (2023). Estimating rates of pesticide usage from trends in herbicide, insecticide, and fungicide product registrations. Crop Prot. 163, 106125. doi: 10.1016/j.cropro.2022.106125
Majumder, S., Mishra, P., Pandey, J., K., N., Sharma, S., Maurya, S., et al. (2024). Optimisation and application of the multi-residue analysis method for detection of 50 pesticides in cabbage by using LC-MS/MS. QuEChERSInternational J. Environ. Anal. Chem. 105, 2326–2343. doi: 10.1080/03067319.2024.2313004
Mandal, S., Poi, R., Hazra, D. K., and Ansary, I. (2023). Review of extraction and detection techniques for the analysis of pesticide residues in fruits to evaluate food safety and make legislative decisions: Challenges and anticipations. J. Chromatogr. B 1201, 123–135. doi: 10.1016/j.jchromb.2023.123135
Mao, C., Xie, H., Chen, W., Ren, Y., Wu, L., and Liu, S. (2020). Metabolism and detoxification of pesticides in plants. Front. Physiol. 11. doi: 10.3389/fphys.2020.00963
Marimuthu, M., Xu, K., Song, W., Chen, Q., and Wen, H. (2024). Safeguarding food safety: Nanomaterials-based fluorescent sensors for pesticide tracing. Food Chem. 463. doi: 10.1016/j.foodchem.2024.141288
Martins, F. C. O. L. and Souza, D. D. (2023). New approaches in electroanalytical determination of triazines-based pesticides in natural waters. Analytica. doi: 10.3390/analytica4020008
Meher, A. K. and Zarouri, A. (2025). Environmental applications of mass spectrometry for emerging contaminants. Molecules 30, 364. doi: 10.3390/molecules30020364
Mei, M. E. I., Zhen-Xia, D. U., and Yun, C. E. N. (2011). QuEChERS-ultra-performance liquid chromatography tandem mass spectrometry for determination of five currently used herbicides. Chinese Journal of Analytical Chemistry. 39, 1659–1664. doi: 10.1016/S1872-2040(10)60482-3
Meng, X. (2024). Application of solid-phase microextraction and LCMS/MS for the detection of pesticide residues. Materials Express. doi: 10.1166/mex.2024.2616
Mitra, S., Saran, R. K., Srivastava, S., and Rensing, C. (2024). Pesticides in the environment: Degradation routes, pesticide transformation products and ecotoxicological considerations. Sci. Total Environ., 173026.
Mohebbi, A., Farajzadeh, M. A., and Mogaddam, M. R. A. (2020). Determination of some organophosphorus pesticides in fruit juice samples by dispersive liquid-liquid microextraction coupled with gas chromatography-flame photometric detection. J. Separation Sci. 43, 2839–2847. doi: 10.1002/jssc.202000314
Mol, H. G. J., Tienstra, M., and Zomer, P. (2016). Evaluation of gas chromatography–mass spectrometry and liquid chromatography–mass spectrometry methods for the determination of pesticide residues in food. Anal. Chim. Acta 935, 32–45.
Mol, H. G. J., Zomer, P., and Kok, A. (2019). Recent developments in the analysis of pesticide residues in food and feed by liquid chromatography–mass spectrometry. Trends Anal. Chem. 116, 215–226.
Mu, Q., Zhang, M., Li, Y., Feng, F., Yu, X., and Nie, J. (2022). Metabolomic analysis reveals the effect of insecticide chlorpyrifos on rice plant metabolism. Metabolites 12, 1289. doi: 10.3390/metabo12121289
Munir, Y., Gul, S., Khan, M. I., and Khan, S. B. (2024). Ecofriendly synthesis of copper oxide-chitosan nanocomposites and their efficacy as nano-pesticide and nano-fertilizer. Inorganic Chemistry Communications. 168, 112769. doi: 10.1016/j.inoche.2024.112769
Musarurwa, H., Chimuka, L., Pakade, V., and Tavengwa, N. (2019). Recent developments and applications of QuEChERS based techniques on food samples during pesticide analysis. J. Food Compos. Anal. doi: 10.1016/j.jfca.2019.103314
Nannou, C., Gkountouras, D., Boti, V., and Albanis, T. A. (2024). An efficient LC–HRMS-based approach to evaluate pesticide contamination in water bodies with measurement uncertainty considerations. Appl. Sci. doi: 10.3390/app142210329
Ndung’u, C. N., Kaduki, K. A., Kaniu, M. I., and Kiruri, L. W. (2024a). Enhanced detection of pesticide residues using two-dimensional raman correlation spectroscopy and machine learning. Appl. Spectrosc. Practica. doi: 10.1177/27551857241303466
Ndung’u, P. W., Mburu, J., and Ochieng, J. (2024b). Two-dimensional Raman correlation spectroscopy combined with PCA-SVM for detection of chlorothalonil residues in vegetables. Food Chem. 426, 136812. doi: 10.1016/j.foodchem.2024.136812
Negi, T., Kumar, A., Sharma, S. K., Rawat, N., Saini, D., Sirohi, R., et al. (2024). Deep eutectic solvents: Preparation, properties, and food applications. Heliyon 10. Available online at: https://www.cell.com/heliyon/fulltext/S2405-8440(24)04815-1.
Neme, K., Taddesse, A. M., and Retta, N. (2021). Pesticide residue analysis and risk assessment in food crops: A review on regulatory challenges in developing countries. Food Control 127, 108132. doi: 10.1016/j.foodcont.2021.108132
Nguyen, H. M. (2020). Ultrasound-assisted extraction in food and environmental applications. Trends Food Sci. Technol. 103, 112–123.
Nguyen, T. T., Rosello, C., Bélanger, R., and Ratti, C. (2020). Fate of residual pesticides in fruit and vegetable waste (FVW) processing. Foods 9. doi: 10.3390/foods9101468
Nicolopoulou-Stamati, P., Maipas, S., Kotampasi, C., Stamatis, P., and Hens, L. (2016). Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front. Public Health 4, 148. doi: 10.3389/fpubh.2016.00148
Norli, H. R., Christiansen, A., and Stuveseth, K. (2015). Analysis of non-cleaned QuEChERS extracts for the determination of pesticide residues in fruit, vegetables and cereals by gas chromatography-tandem mass spectrometry. Food Additives Contaminants Part A-Chemistry Anal. Control Exposure Risk Assess. doi: 10.1080/19440049.2015.1124292
Nuruzzaman, M., Bahar, M. M., and Naidu, R. (2025). Diffuse soil pollution from agriculture: Impacts and remediation. Sci. Total Environ. 962, 178398. doi: 10.1016/j.scitotenv.2025.178398
Okoffo, E. D., Osei, F., Mensah, M., and Boateng, K. (2023). Challenges in pesticide regulation and monitoring in sub-Saharan Africa: Implications for food safety and trade. Sci. Total Environ. 872, 162080. doi: 10.1016/j.scitotenv.2023.162080
Osaili, T. M., Ayyash, M. M., Al-Nabulsi, A. A., and Obaid, R. (2022). Pesticide residues in fresh fruits imported into the United Arab Emirates. Heliyon 8, 9234. doi: 10.1016/j.heliyon.2022.e09234
Pang, Z., Chen, J., Wang, T., Gao, C., Li, Z., Guo, L., et al. (2021). Linking plant secondary metabolites and plant microbiomes: A review. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.621276
Pastor-Belda, M., Garrido, I., Campillo, N., Viñas, P., Hellín, P., Flores, P., et al. (2016). Determination of spirocyclic tetronic/tetramic acid derivatives and neonicotinoid residues in fruits using liquid chromatography-mass spectrometry. Food Chem. 202, 389–395. doi: 10.1016/j.foodchem.2016.02.029
Perestrelo, R., Silva, P., Porto-Figueira, P., Pereira, J., Silva, C., Medina, S., et al. (2019). QuEChERS - Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 1070, 1–28. doi: 10.1016/J.ACA.2019.02.036
Perrin, L., Spinosi, J., Chaperon, L., Kab, S., Moisan, F., and Ebaz, A. (2021). Pesticides expenditures by farming type and incidence of Parkinson disease in farmers: A French nationwide study. Environ. Res. 197, 111161. doi: 10.1016/j.envres.2021.111161
Philippe, V., Neveen, A., Marwa, A., and Basel, A. (2021). Occurrence of pesticide residues in fruits and vegetables for the Eastern Mediterranean Region and potential impact on public health. Food Control. doi: 10.1016/j.foodcont.2020.107457
Picó, Y., Alfarhan, A. H., Barceló, D., and Barceló, D. (2020). How recent innovations in gas chromatography-mass spectrometry have improved pesticide residue determination: An alternative technique to be in your radar. Trends Analytical Chem. doi: 10.1016/J.TRAC.2019.115720
Poornima, W., Liyanaarachchi, G., Somasiri, H., Hewajulige, I., and Tan, D. (2024). Fresh fruit and vegetable safety concerns in Sri Lanka; Review of pesticide contamination. doi: 10.1016/j.jfca.2024.106004
Pszczolińska, K. and Kociołek, B. (2022). The pesticide residue analysis in commodities with high content of chlorophyll based on the quick, easy, cheap, effective, rugged, and safe method: A review. J. Sep. Sci. 45, 149–165. doi: 10.1002/jssc.202100304
Pszczolińska, K., Płonka, J., Pērkons, I., Bartkevičs, V., Drzewiecki, S., Strzałka, K., et al. (2024). doi: 10.1002/ps.8221
Radović, J. R., Carrasco, P. B., and Bayona, J. M. (2015). Chromatography and Supercritical Fluid Chromatography with Selective Detection in Pesticide Analysis. In Encyclopedia of Analytical Chemistry, Meyers, R. A. (Ed.). doi: 10.1002/9780470027318.A1708.PUB2
Rafeeinia, A., Asadikaram, G., Moazed, V., and Darabi, M. K. (2023). Organochlorine pesticides may induce leukemia by methylation of CDKN2B and MGMT promoters and histone modifications. Gene 851, 146976. doi: 10.1016/j.gene.2022.146976
Rahman, M. M., El-Aty, A. M. A., El-Aty, A. M. A., Kim, S.-W., Shin, S. C., Shin, H.-C., et al. (2017). Quick, easy, cheap, effective, rugged, and safe sample preparation approach for pesticide residue analysis using traditional detectors in chromatography: A review. J. Separation Sci. doi: 10.1002/JSSC.201600889
Ramadevi, K. and Ramachandraiah, K. (2023). Multi residue method development and validation for the determination of 45 pesticides in citrus fruits by gas chromatography tandem mass spectrometry. Food Chem. 419, 136003. doi: 10.1016/j.foodchem.2023.136003
Rani, L., Thapa, K., Kanojia, N., Sharma, N., Singh, S., Grewal, A. S., et al. (2021). An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 283, 124657. doi: 10.1016/j.jclepro.2020.124657
Rein, A., Trapp, S., Fantke, P., Yalçın, M., Turgut, N., Ahat, C., et al. (2025). Uptake and translocation of pesticides in pepper and tomato plants. Pest Manage. Sci. 81, 1562–1570. doi: 10.1002/ps.8556
Romagnoli, M., Catani, M., Giannì, B., Pasti, L., Cavazzini, A., and FranChina, F. A. (2023). Development and validation of a GC × GC-ToFMS method for the quantification of pesticides in environmental waters. Analytical Bioanalytical Chem. doi: 10.1007/s00216-023-04686-8
Rösch, A., Wettstein, F. E., Wächter, D., Reininger, V., Meuli, R. G., and Bucheli, T. D. (2023). A multi-residue method for trace analysis of pesticides in soils with special emphasis on rigorous quality control. Anal. Bioanal. Chem. 415, 6009–6025. doi: 10.1007/s00216-023-04872-8
Rizzetti, T. M., Kemmerich, M., and ML, M. (2016). Optimization of a QuEChERS based method by means of central composite design for pesticide multiresidue determination in orange juice by UHPLC–MS/MS. Food Chem. 196, 25–33. doi: 10.1016/j.foodchem.2015.09.010
Rudakov, O. B., Rudakova, L. V., and Selemenev, V. F. (2018). Acetonitrile as tops solvent for liquid chromatography and extraction. J. Anal. Chromatogr Spectrosc 1. Available online at: https://www.academia.edu/download/117477579/797.pdf.
Ruiz-Rodríguez, L., Aguilar, A., Díaz, A. N., and Sánchez, F. G. (2015). Enantioseparation of the fungicide imazalil in orange juice by chiral HPLC. Study on degradation rates and extractive/enrichment techniques. Food Chemistry. 178, 179–185. doi: 10.1016/j.foodchem.2015.01.004
Sabzevari, S. and Hofman, J. (2022). A worldwide review of currently used pesticides’ monitoring in agricultural soils. Sci. Total Environ. 812, 152344. doi: 10.1016/j.scitotenv.2021.152344
Saha, A., Makwana, C., and Manivel, P. (2020). QuEChERS-based gas chromatography-electron capture/flame photometric detection method for multi-pesticide residues analysis in Andrographis paniculata : a popular Indian medicinal herb. Int. J. Environ. Anal. Chem. 100, 1669–1690. doi: 10.1080/03067319.2019.1657851
Saito-Shida, S., Nemoto, S., and Matsuda, R. (2014). Multiresidue analysis of pesticides in vegetables and fruits by supercritical fluid extraction and liquid chromatography-tandem mass spectrometry. Food Hyg. Saf. Sci. 55, 142–150. doi: 10.3358/shokueishi.55.142
Schusterova, D., Hajšlová, J., Kocourek, V., and Pulkrabová, J. (2021). Pesticide residues and their metabolites in grapes and wines from conventional and organic farming system. Foods 10. doi: 10.3390/foods10020307
Shahid, M., Singh, U., and Khan, M. (2023). Metabolomics-based mechanistic insights into revealing the adverse effects of pesticides on plants: an interactive review. Metabolites 13. doi: 10.3390/metabo13020246
Shamsipur, M., Yazdanfar, N., and Ghambarian, M. (2016). Combination of solid-phase extraction with dispersive liquid-liquid microextraction followed by GC-MS for determination of pesticide residues from water, milk, honey and fruit juice. Food Chem. 204, 289–297. doi: 10.1016/j.foodchem.201
Sharma, S., Kolašinac, S. M., Jiang, X., Gao, J., Kumari, D., Biswas, S., et al. (2024). Spectroscopy-Based Chemometrics for Pesticide Residue Detection: Current Approaches and Future Challenges. doi: 10.1021/acsagscitech.4c00005
Shekhar, C., Khosya, R., Thakur, K., Mahajan, D., Kumar, R., Kumar, S., et al. (2024). A systematic review of pesticide exposure, associated risks, and long-term human health impacts. Toxicol. Rep., 101840. doi: 10.1016/j.toxrep.2024.101840
Shin, J., Choi, S., Park, Y., Kwak, B., Moon, S., Yoon, Y., et al. (2022). Comparison of QuEChERS and Liquid–Liquid extraction methods for the simultaneous analysis of pesticide residues using LC-MS/MS. Food Control. doi: 10.1016/j.foodcont.2022.109202
Sindhu, S. and Manickavasagan, A. (2023). Nondestructive testing methods for pesticide residue in food commodities. Rev. Rev. Food Sci. Food Saf. doi: 10.1111/1541-4337.13109
Sofi, J. A., Dar, A. A., Jan, I., Hassan, G., Dar, S. R., Mughal, A. H., et al. (2023). Development and validation of GC-ECD method using quEChERS for pesticide residue determination in cucumber. Biomed. Chromatogr. doi: 10.1002/bmc.5647
Souza, D. D., Gonçalves-Filho, D., and Franco, D. L. (2021). Residues Analysis by Electroanalytical Techniques. doi: 10.1007/978-3-030-54719-6_1
Soydan, D., Turgut, N., Yalçın, M., Turgut, C., and Karakuş, P. (2021). Evaluation of pesticide residues in fruits and vegetables from the Aegean region of Turkey and assessment of risk to consumers. ntal Sci. pollut. Res. 28, 27511–27519. doi: 10.1007/s11356-021-12580-y
Spanjers, C. S., Beach, C. A., Jones, A. J., and Dauenhauer, P. J. (2017). Increasing flame ionization detector (FID) sensitivity using post-column oxidation–methanation. Anal. Methods 9, 1928–1934. doi: 10.1039/C6AY03363F
Stehle, S., Elsaesser, D., Gregoire, C., Imfeld, G., Niehaus, E., Passeport, E., et al (2011). Pesticide risk mitigation by vegetated treatment systems: A meta‐analysis. Journal of Environmental Quality. 40, 1068–1080. doi: 10.2134/jeq2010.0510
Su, G., Xie, S., Du, G., and Li, P. (2024). A chemometric-assisted method for automatic, rapid and non-targeted detection of multi-pesticides in plant-derived foods by gas chromatography–mass spectrometry. Food Chem. doi: 10.1016/j.foodchem.2024.138573
Suárez-Jacobo, A., Alcantar-Rosales, V. M., Alonso-Segura, D., Heras-Ramírez, M., Elizarragaz-De La Rosa, D., Lugo-Melchor, O., et al. (2017). Pesticide residues in orange fruit from citrus orchards in Nuevo Leon State, Mexico. Food Additives & Contaminants: Part B. 10, 192–199. doi: 10.1080/19393210.2017.1315743
Sun, J., Pan, L., Zhan, Y., Lu, H., Tsang, D. C., Liu, W., et al. (2016). Contamination of phthalate esters, organochlorine pesticides and polybrominated diphenyl ethers in agricultural soils from the Yangtze River Delta of China. Science of the Total Environment. 544, 670–676. doi: 10.1016/j.scitotenv.2015.12.012
Swathy, K., Vivekanandhan, P., Yuvaraj, A., Sarayut, P., Kim, J. S., and Krutmuang, P. (2024). Biodegradation of pesticide in agricultural soil employing entomopathogenic fungi: Current state of the art and future perspectives. Heliyon 10. Available online at: https://www.cell.com/heliyon/fulltext/S2405-8440(23)10614-1.
Tang, B., Zhang, H., Row, K. H., and Wang, Y. (2019). Application of ionic liquids in sample preparation. TrAC Trends Anal. Chem. 116, 239–250. doi: 10.1016/j.trac.2019.05.013
Tankiewicz, M. (2019). Determination of selected priority pesticides in high water fruits and vegetables by modified QuEChERS and GC-ECD with GC-MS/MS confirmation. Molecules 24, 417. doi: 10.3390/molecules24030417
Taylor, M. J., Hunter, K., Hunter, K. B., and Lindsay, D. (2002). Multi-residue method for rapid screening and confirmation of pesticides in crude extracts of fruits and vegetables using isocratic liquid chromatography with tandem mass spectrometry. J. Chromatogr. A 982, 225–236. doi: 10.1016/S0021-9673(02)01610-2
Toptancı, İ., Kıralan, M., and Ramadan, M. (2021). Levels of pesticide residues in fruits and vegetables in the Turkish domestic markets. Environ. Sci. pollut. Res. 28, 39451–39457. doi: 10.1007/s11356-021-13538-w
Torres, R. M. T., Altamirano, J. V., and Molini, A. M. V. (2021). Pesticide Residues by on Line Coupled Liquid Chromatography-Gas Chromatography Using the Through Oven Transfer Adsorption Desorption Interface. doi: 10.1007/978-3-030-54719-6_3
Twaij, B. and Hasan, M. (2022). Bioactive secondary metabolites from plant sources: types, synthesis, and their therapeutic uses. Int. J. Plant Biol. doi: 10.3390/ijpb13010003
Udomkun, P., Boonupara, T., Sumitsawan, S., Khan, E., Pongpichan, S., and Kajitvichyanukul, P. (2023). Airborne pesticides—deep diving into sampling and analysis. Toxics 11, 883. doi: 10.3390/toxics11110883
Vemuri, S., Rao, C. S., Swarupa, S., and Kavitha, K. (2016). Studies on pesticide usage pattern and farmers knowledge on pesticide usage and technologies in open field and poly house conditions. J. Res. in Agricul. and Animal Sci.. 4, 1–8.
Vichapong, J., Burakham, R., and Srijaranai, S. (2016). Ionic liquid-based vortex-assisted liquid–liquid microextraction for simultaneous determination of neonicotinoid insecticides in fruit juice samples. Food Analytical Methods. 9, 419–426. doi: 10.1007/s12161-015-0209-4
Wang, S., Dai, L., Lin, C., Wang, L., Ji, Z., Fu, Y., et al. (2024b). Study on the design method of high-resolution volume-phase holographic gratings. Sensors 24, 6493. doi: 10.3390/s24196493
Wang, T., Hu, J., and Liu, C. (2014). Simultaneous determination of insecticide fipronil and its metabolites in maize and soil by gas chromatography with electron capture detection. Environ. Monit. Assess. 186, 2767–2774. doi: 10.1007/s10661-013-3577-5
Wang, X., Jiang, S., Liu, Z., Sun, X., Zhang, Z., Quan, X., et al. (2023b). Integrated surface-enhanced Raman spectroscopy and convolutional neural network for quantitative and qualitative analysis of pesticide residues on pericarp. Food Chem. doi: 10.1016/j.foodchem.2023.138214
Wang, B., Su, X., Wang, T., Yang, T., Xu, C., Lin, Z., et al. (2023a). Spatial heterogeneity of total and labile soil organic carbon pools in poplar agroforestry systems. Forests 14, 1869. doi: 10.3390/f14091869
Wang, B., Wu, C., Liu, W., et al (2016). Levels and patterns of organochlorine pesticides in agricultural soils in an area of extensive historical cotton cultivation in Henan province, China. Environ. Sci. Pollut. Res.. 23, 6680–6689. doi: 10.1007/s11356-015-5864-x
Wang, C., Wang, X., Wang, J., Di, S., Wang, Z., Xu, H., et al. (2021a). Removal of matrix interferences by nano-mgO and co-adsorbents for accurate multi-pesticide residue analysis in the chinese medicinal herb, paeoniae radix alba. J. Anal. Methods Chem. 2021, 1–10. doi: 10.1155/2021/6626257
Wang, C., Yao, X., Li, X., Wang, Q., Jiang, N., Hu, X., et al. (2024a). Fosthiazate, a soil-applied nematicide, induces oxidative stress, neurotoxicity and transcriptome aberrations in earthworm (Eisenia fetida). J. Hazard. Mater. 463, 132865. doi: 10.1016/j.jhazmat.2023.132865
Wang, X., Zhu, Y., Zhou, L., Zhao, P., Xiong, Z., and Yu, J. (2021b). Magnetic solid-phase extraction based on zirconium-based metal-organic frameworks for simultaneous enantiomeric determination of eight chiral pesticides in water and fruit juices. Food Chem. 370, 131056. doi: 10.1016/j.foodchem.2021.131056
Wei, J., Yang, L., Luo, M., Wang, Y., and Li, P. (2019). Nanozyme-assisted technique for dual mode detection of organophosphorus pesticide. Ecotoxicol. Environ. Saf. 179, 17–23. doi: 10.1016/j.ecoenv.2019.04.041
Wei, S., Wang, X., Zhao, X., Zhao, K., Xu, L., and Chen, Y. (2023). Detection of pesticide residues on flexible and transparent fluorinated polyimide film based on surface-enhanced Raman spectroscopy technology. Analytica Chimica Acta. doi: 10.1016/j.aca.2023.341958
W.H.O. and U.N.E.P (2022). Preventing disease through healthy environments: Exposure to highly hazardous pesticides (World Health Organ). Available online at: https://www.who.int/publications/i/item/9789240041823 (Accessed January 10, 2025).
Wijayanti, S. D., Novitasari, E., and Kusnadi, J. (2024). “Optimization of Acetylcholinesterase and Sucrose Concentration using Response Surface Methodology (RSM) Approach in the Development of Paper-Based Biosensor for Pesticides Detection. Trends in Sciences. 22 (1), 8849. doi: 10.48048/tis.2025.8849
Wilson-Frank, C. and Ewbank, M. (2022). Method for the Detection of Terbufos, Diazinon and Parathion in Blood v1. doi: 10.17504/protocols.io.j8nlkw83dl5r/v1
Wong, J. W., Hayward, D. G., and Zhang, K. (2013). Chromatography–Mass Spectrometry Techniques for Multiresidue Pesticide Analysis in Agricultural Commodities. 61, 3–22. doi: 10.1016/B978-0-444-62623-3.00001-0
Wu, Y., Chen, J., and Huang, X. (2022). Graphene-based electrochemical aptasensors for paraquat detection in agricultural products. Biosensors 12, 615. doi: 10.3390/bios12080615
Wu, L., Song, Y., Hu, M., Zhang, H., Yu, A., Yu, C., et al. (2015). Application of magnetic solvent bar liquid-phase microextraction for determination of organophosphorus pesticides in fruit juice samples by gas chromatography mass spectrometry. Food Chem. 176, 197–204. doi: 10.1016/j.foodchem.2014.12.055
Xie, H., Mao, C., Wu, L., and Liu, S. (2021). The roles of cytochrome P450 monooxygenases in plant stress responses. Int. J. Mol. Sci. 22, 5307. doi: 10.3390/ijms22105307
Xie, T. and Zhang, Q. (2023). Evaluation of supercritical fluid chromatography coupled to tandem mass spectrometry for the analysis of pesticide residues in grain. J. Separation Sci. doi: 10.1002/jssc.202300623
Xue, J. and Zhu, X. (2019). Self-acidity induced efervescence and manual shaking-assisted microextraction of neonicotinoid insecticides in orange juice. J. Sep Sci. 42, 2993–3001. doi: 10.1002/jssc.201900473
Xue, W., Li, F., Li, X., and Liu, Y. (2024). A support vector machine-assisted metabolomics approach for non-targeted screening of multi-class pesticides and veterinary drugs in maize. Molecules. doi: 10.3390/molecules29133026
Yahaya, T., Magaji, U., Abdulazeez, A., Musa, M., Yusuf, A., Obi, C., et al. (2023). Levels and safety assessment of pesticide residues in selected vegetables and fruits sold in Ikorodu. doi: 10.4314/sa.v22i2.20
Yan, D., Zhang, Y., Liu, L., Shi, N., and Yan, H. (2018). Pesticide exposure and risk of Parkinson's disease: Dose-response meta-analysis of observational studies. Regul. Toxicol. Pharmacol. 96, 57–63. doi: 10.1016/j.yrtph.2018.05.005
Yang, Y., Guo, Y., Jia, X., Zhang, Q., Mao, J., Feng, Y., et al. (2023). An ultrastable 2D covalent organic framework coating for headspace solid-phase microextraction of organochlorine pesticides in environmental water. J. Hazard. Mater. 452, 131228. doi: 10.1016/j.jhazmat.2023.131228
Yang, L., Wen, K., Ruan, X., Zhao, Y., Wei, F., and Wang, Q. (2018). Response of plant secondary metabolites to environmental factors. Mol. J. Synth. Chem. Nat. Prod. Chem. 23. doi: 10.3390/molecules23040762
Yao, Z. and Li, Z. (2015). Enantioselective determination of acaricide etoxazole in orange pulp, peel, and whole orange by chiral liquid chromatography with tandem mass spectrometry. J. Sep Sci. 38, 599–604. doi: 10.1002/jssc.201401065
Yu, L., Guo, G., Zhao, J., Zhao, L., Xia, A., He, X., et al. (2021). Determination of organochlorine pesticides in green leafy vegetable samples via fe3O4 magnetic nanoparticles modified quEChERS integrated to dispersive liquid-liquid microextraction coupled with gas chromatography-mass spectrometry. J. Anal. Methods Chem. 2021, 1–10. doi: 10.1155/2021/6622063
Yuan, S., Yu, H., Guo, Y., Xie, Y., Cheng, Y., Qian, H., et al. (2024). Recent advance in probiotics for the elimination of pesticide residues in food and feed: mechanisms, product toxicity, and reinforcement strategies. Crit. Rev. Food Sci. Nutr. 64, 12025–12039.
Zha, B., Li, H., Ren, S., Wu, J., and Wang, H. (2024). Aggregation-induced emission (AIE) probes in fluorescent sensing: progress and applications for pesticide detection. Appl. Sci. doi: 10.3390/app14198947
Zhang, Q., Mao, X., and C, Y. (2023). A simplifed dispersive solidphase extraction using a shaped zirconium-based metal–organic framework: Constructing a novel, facile and efcient method for detecting plant growth regulators in citrus fruits. Food Chem. 405. doi: 10.1016/j.foodchem.2022.134862
Zhang, S. (2025). Maximum residue limits and agricultural trade: Evidence from China. Sustainability 17, 3435. doi: 10.3390/su17083435
Zhang, Q., Clerck, K., and Tavernier, S. (2018a). Application of deep eutectic solvents in the extraction of bioactive compounds from plants. J. Chem. Technol. Biotechnol. 93, 1457–1467.
Zhang, Q.-W., Lin, L.-G., and Ye, W.-C. (2018b). Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med. 13, 20. doi: 10.1186/s13020-018-0177-x
Zhang, B., Lv, F., and Yang, J. (2024a). Pesticides toxicity, removal and detoxification in plants: A review. Agronomy. doi: 10.3390/agronomy14061260
Zhang, H., Wang, P., Wang, J., Liu, H., and Chen, X. (2024b). Assessing the impact of Chlorantraniliprole (CAP) pesticide stress on oilseed rape (Brassia campestris L.): Residue dynamics, enzyme activities, and metabolite profiling. Pestic. Biochem. Physiol. 200, 105785. doi: 10.1016/j.pestbp.2024.105785
Zhang, Y. and Xu, H. (2014). Determination of triazoles in tea samples using dispersive solid phase extraction combined with dispersive liquid–liquid microextraction followed by liquid chromatography–tandem mass spectrometry. Food Anal. Methods 7, 189–196. doi: 10.1007/s12161-013-9617-5
Zhang, J. and Yang, H. (2021). Metabolism and detoxification of pesticides in plants. Science of The Total Environment 790, 148034. doi: 10.1016/j.scitotenv.2021.148034
Zhang, Q., Zhang, Q., Fang, L., Fang, L., Jia, B., Long, N., et al. (2021). Optical lateral flow test strip biosensors for pesticides: Recent advances and future trends. Trends Analytical Chem. doi: 10.1016/J.TRAC.2021.116427
Zhao, G., Zhou, B., Wang, X., Shen, J., and Zhao, B. (2021). Detection of organophosphorus pesticides by nanogold/mercaptomethamidophos multi-residue electrochemical biosensor. Food Chem. 354, 129511. doi: 10.1016/j.foodchem.2021.129511
Zhao, J., Meng, Z., Zhao, Z., and Zhao, L. (2019). Ultrasound-assisted deep eutectic solvent as green and efficient media combined with functionalized magnetic multi-walled carbon nanotubes as solid-phase extraction to determine pesticide residues in food products. Food Chem. 310, 125863. doi: 10.1016/j.foodchem.2019.125863
Zheng, K., Wu, X., Chen, J., Chen, J., Lian, W., Su, J., et al. (2022). Establishment of an LC-MS/MS Method for the Determination of 45 Pesticide Residues in Fruits and Vegetables from Fujian, China. Molecules 27. doi: 10.3390/molecules27248674
Keywords: pesticide residue, MRLs, metabolites, plant health, green chemistry, biosensors, machine learning
Citation: Umer M, Naseer A, Mubeen M, Iftikhar Y, Umer R, Akram A, Altaf MT, Ibrahim EH, Ahmed AE and Duan M (2025) Pesticide residue detection techniques for increasing productivity and yield: recent progress and future outlooks. Front. Plant Sci. 16:1694779. doi: 10.3389/fpls.2025.1694779
Received: 28 August 2025; Accepted: 14 October 2025;
Published: 17 November 2025.
Edited by:
Saif Ali, CAB International, PakistanReviewed by:
Imran Ul Haq, University of Agriculture, Faisalabad, PakistanAli Hassan, Hubei University, China
Muhammad Faisal, University of Agriculture, Pakistan
Copyright © 2025 Umer, Naseer, Mubeen, Iftikhar, Umer, Akram, Altaf, Ibrahim, Ahmed and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingzheng Duan, ZHVhbm1pbmd6aGVuZ0B6dHUuZWR1LmNu; Yasir Iftikhar, eWFzaXIuaWZ0aWtoYXJAdW9zLmVkdS5waw==
†These authors have contributed equally to this work
 Muhammad Umer
Muhammad Umer Abid Naseer3†
Abid Naseer3† Mustansar Mubeen
Mustansar Mubeen Yasir Iftikhar
Yasir Iftikhar Mingzheng Duan
Mingzheng Duan