- 1Zhangzhou Affiliated Hospital of Fujian Medical University, Zhangzhou, Fujian, China
- 2International Centre of Insect Physiology and Ecology, Nairobi, Kenya
- 3Unit of Environmental Sciences and Management, North-West University, Potchefstroom, South Africa
- 4Fujian Academy of Agricultural Sciences Institute of Subtropical Agriculture, Zhangzhou Institute of Technology, Zhangzhou, Fujian, China
- 5College of Landscape Architecture and Art, Fujian Agriculture and Forestry University, Fujian, China
- 6Fujian Zhanglong Group Co., Ltd., Fujian, China
- 7College of Food Engineering, Zhangzhou Institute of Technology, Zhangzhou, Fujian, China
Introduction: Dust mites are a prevalent indoor allergen contributing to respiratory diseases like allergic rhinitis and asthma. Eucalyptus citriodora essential oil, known for its balsamic odor and repellent effects on various pests, has been scantily investigated for its impacts on dust mites.
Methods: The chemical composition of the essential oil and its head-space extracted from E. citriodora was determined using gas chromatography-mass spectrometry (GC-MS). The toxicity of the oil and its compounds were assessed through contact-fumigant and vapor-phase mortality bioassays. Repellent effects were evaluated using a fabric-contact assay. Data were analyzed using probit regression to determine LC50 values.
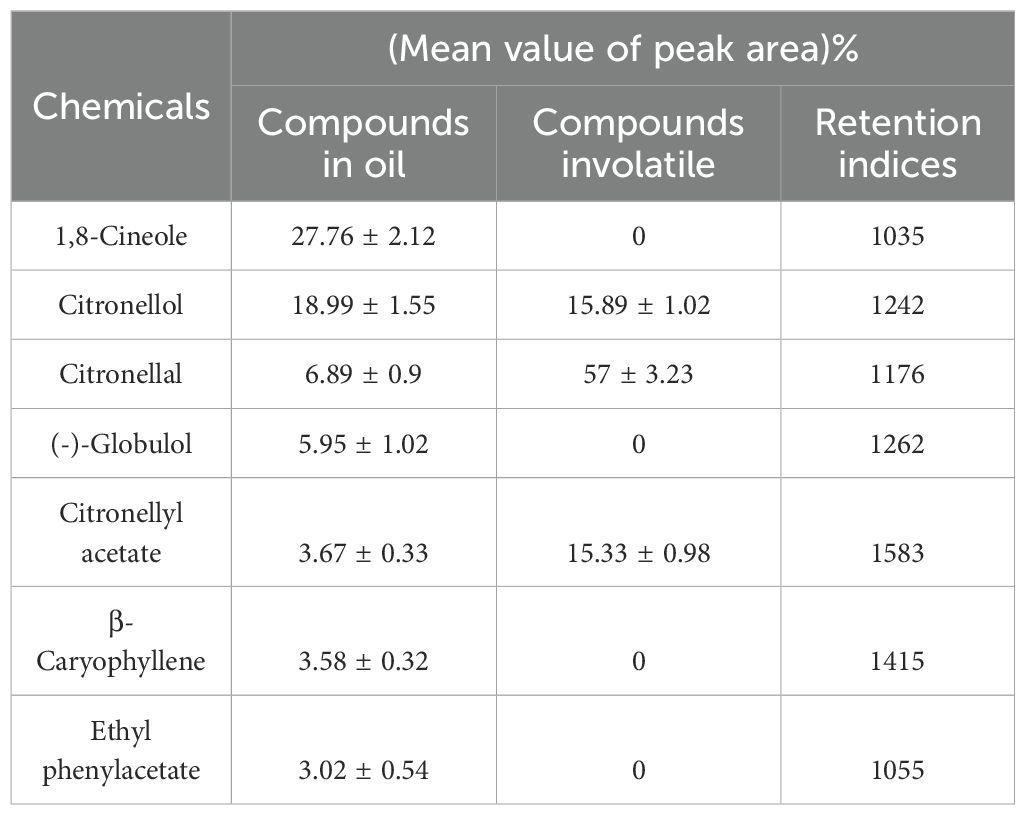
Results: The essential oil contained seven main compounds, and citronellal, citronellol and citronellyl acetate were the most abundant in the oil's volatile, accounting fora total of 88.22%. Citronellal exhibited the highest toxicity, and the essential oil itself showed strong toxicity with the LC50 of 63.94 and 84.53 μL/cm against Dermatophagoides pteronyssinus, 60.72 and 75.88 μL/cm against Dermatophagoides farinae, respectively. In vapor-phase assays, citronellal and ethyl phenylacetate caused 100% mortality.
Discussion: Citronellol had the highest repellent effect, and the essential oil, 1,8-cineole, and citronellyl acetate also showed significant repellency rates. E. citriodora essential oil and its compounds particularly citronellal and citronellol, showed high potential for effective dust mite control due to their natural origin, strong toxicity and repellency impacts. Thus, E. citriodora essential oil is a natural, eco-friendly alternative to synthetic acaricides, providing a scientific basis for the control of indoor dust mite allergies.
1 Introduction
Dust mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae) are a common indoor allergen, widely present in environments where humans live and work, such as homes, schools, and offices (Aggarwal and Senthilkumaran, 2024; Caraballo, 2024; Klain et al., 2024). They feed on human dander, proliferate rapidly, and release allergenic proteins such as Der p1 and Der p2 that are contained in fecal particles and carcass remains (Aggarwal and Senthilkumaran, 2024). The allergenic proteins that induce allergic reaction are significant contributors to respiratory diseases such as allergic rhinitis and asthma (Marko and Pawliczak, 2023; Rodriguez-Plata et al., 2023; Zheng et al., 2023; Turki et al., 2024). Dust mites thrive in warm, humid environments, and the adult stage is most harmful for spreading respiratory diseases due to its high production of allergenic feces and debris that become airborne and inhaled (Marko and Pawliczak, 2023; Zheng et al., 2023; Turki et al., 2024). In recent years, with the improvement of people’s living standards and living environments, the demand for dust mite control has significantly increased.
Considerable research has been conducted on dust mite control using chemicals and natural products (Maruoka et al., 2020; Gómez-Gutiérrez et al., 2023; Espinosa-Zaragoza et al., 2024). For chemical agents, some branched chain fatty acids (2-ethylhexanoic acid, 2-butyloctanoic acid, and isopalmitic acid) were found to be toxic to more than 50% dust mites in 90 min (Maruoka et al., 2020). Chemicals are also used to enhance the acaricidal activity of biological control agents, such as microbial strains or natural products against dust mites (Hussain and AlJabr, 2020). For natural products, three Serratia strains, S. ureilytica UTS2, UTS3, and UTS4 isolated from Mimosa pudica were found to hold acaricidal activity for controlling dust mite in vitro (Espinosa-Zaragoza et al., 2024). However, chemical methods may pose high risks to human health and the environment, while biological approaches are difficult to implement in household environments. Plant-derived essential oils have gained significant attention in pest management due to their natural origin, safety, environmental friendliness, and lack of harmful residues. It was found that cinnamon essential oil, hiba essential oil, and orange essential oil exhibited a strong acaricidal effect against house dust mites (Kim et al., 2015; Jung et al., 2020; Sabahi et al., 2020).
Eucalyptus citriodora Hook. (Myrtaceae), a medium-sized evergreen tree native to northeastern Australia with a slender trunk, lance-shaped glossy leaves (emitting a lemon scent when crushed), and resilience to drought and pollution, has long been used in folk medicine, which essential oil serves as an anti-inflammatory, antiseptic, and expectorant for respiratory issues. E. citriodora essential oil has been reported to have potential application as a natural pesticide against some key pests (El-Sakhawy et al., 2023). E. citriodora essential oil also showed antimicrobial potential against Staphylococcus aureus, Candida albicans and Hemileia vastatrix (Pinheiro et al., 2020; Caetano et al., 2022; Fayez et al., 2023). However, research on the repellent effect of E. citriodora essential oil on dust mites is relatively scarce, and existing studies lack depth and clarity on these aspects. Therefore, this study aims to further explore the repellent effect of E. citriodora essential oil on dust mites, providing a scientific basis for the development of novel, environmentally friendly dust mite control methods. The primary objective of this study is to (1) clarify the dose-response relationship of E. citriodora essential oil in repelling dust mites through bioassay experiments, (2) determine the chemical components and head-space of E. citriodora essential oil using Gas chromatography-mass spectrometry (GC-MS), and (3) assess the repellent effect and mechanisms of E. citriodora essential oil on dust mites.
2 Materials and methods
2.1 Mite colony and plant product
Dermatophagoides pteronyssinus and D. farinae were harvested using vacuum sampling from mattresses, carpets, and upholstered furniture in a residential bedroom in Zhangzhou, city (117.633919°E, 24.521705° N, China) and subsequently maintained for a period of six months without exposure to any known acaricides. Mite manipulation was performed under a dissecting microscope (×20–40 magnification) to ensure precision: adult mites (7–10 days old) were gently transferred using a fine brush (0.5 mm bristle diameter) to avoid physical damage. Mites with fine, fingerprint-like cuticular striations covering their body surfaces and a semi-transparent body are D. farinae, while mites with relatively sparse striations arranged in a wavy or longitudinal pattern and a milky yellow body color are D. pteronyssinus. These mites were reared within Petri dishes measuring 8.5 cm in diameter and 5.5 cm in depth, which were filled with a sterilized substrate composed of an equal parts mixture by weight of wheat bran and dried yeast. Previous studies demonstrated the physics of dust mites were regulated by the temperature and humidity obviously (Colloff, 1987; Vackova et al., 2023). The mites were kept in the incubator, maintaining at a temperature of 25 ± 1 °C and a relative humidity of 75%, under constant darkness. The dried yeast component of the diet was procured from Angel Yeast Co., Ltd., based in Yichang, China.
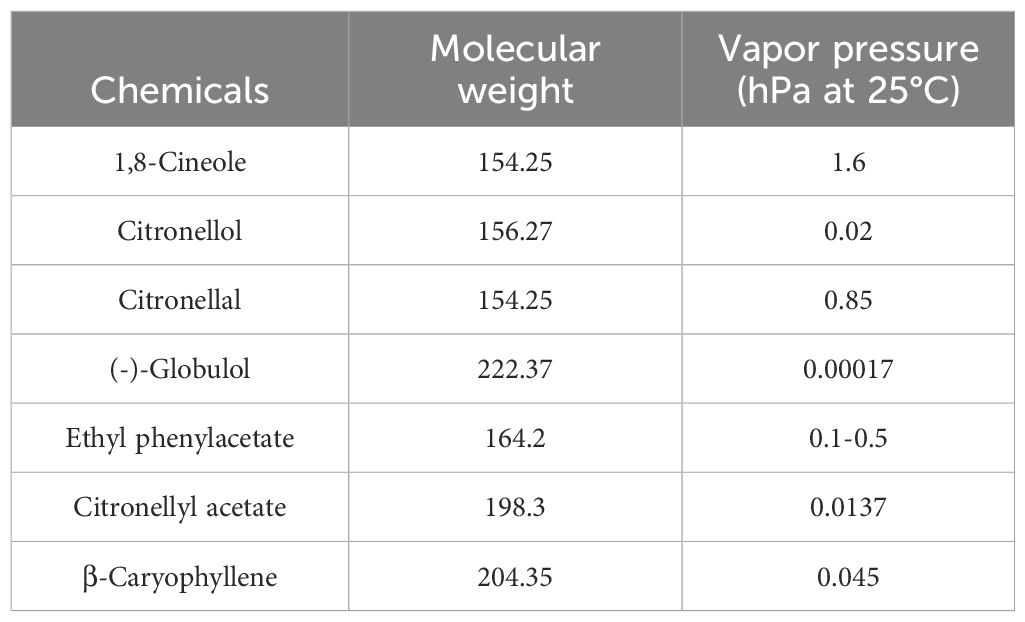
Essential oil of Eucalyptus citriodora Hook was purchased from Xiya Reagent, Shangdong, China. The seven artificial chemicals used in the study were listed in Table 1. For the quantitative structure-activity relationship (QSAR) analysis, the molecular weight (MW), vapor pressure (VP), and steric parameters of the test compounds were derived using ACD/Boiling Point and Vapor Pressure software (ACD/Labs Online, provided by Advanced Chemistry Development, Inc., Montreal, Canada). The calculated values are summarized in Table 1. All chemical reagents employed in the study were of analytical grade and were sourced from Xilong Scientific Co., Ltd., Shangxi, China.
2.2 Chromatographic analysis of the essential oil
Gas chromatography-mass spectrometry (GC-MS) analysis was conducted using a PerkinElmer Clarus 680 T gas chromatograph-mass spectrometer (Fort Belvoir, VA). The separation was achieved on an Agilent DB-5MS capillary column (30 m length, 0.25 mm internal diameter, Folsom, CA). The initial oven temperature was set at 50°C for 5 minutes, followed by a programmed ramp to 280°C at a rate of 5°C per minute, concluding with a 10-minute isothermal hold at 280°C. The helium carrier gas flow rate was maintained at 1.0 mL/min, mode split (50:1), injection volume was 1 μL. The ion source was operated at 250°C, while the interface temperature was set to 260°C. Electron ionization mass spectra were acquired at 70 eV, and the sector mass analyzer scanned a mass-to-charge (m/z) range from 35 to 550 every 0.2 seconds. Identification of the chemical constituents was achieved through spectral comparison with reference standards contained within a mass spectral library.
The volatile fraction of the essential oil was analyzed using a head-space solid-phase microextraction (HS-SPME) method coupled with GC-MS to isolate and identify volatile compounds. For volatile analysis, 1 g of the essential oil was placed in a 20 mL headspace vial, equilibrated at 60°C for 30 min, and then sampled using a 50 μm polydimethylsiloxane fiber (Supelco, Bellefonte, PA, USA) for 40 min. The fiber was desorbed in the GC inlet at 250°C for 5 min. The GC-MS conditions were identical to those described for the essential oil analysis.
2.3 Contact-fumigant mortality bioassay
A fabric-contact fumigant mortality bioassay was conducted to assess the biotoxicity of E. citriodora oil and seven selected compounds against adult dust mites, specifically D. pteronyssinus and D. farinae. Following preliminary testing, five concentrations of each test substance were prepared in 50 μL of ethanol and applied to black cotton fabric disks with a diameter of 5 cm. After application, the fabric disks were air-dried in a fume hood for one minute. Each disk was then positioned at the base of a disposable Petri dish (5 cm diameter × 1 cm height), and groups of 30 adult mites (both sexes, aged 7–10 days) were introduced onto the treated fabric. The Petri dishes were sealed with their original lids and further secured with Bemis Parafilm M (Neenah, WI). Control treatments were performed using disks treated with 50 μL of ethanol alone.
Both treated and control mites (exposed to ethanol only) were maintained under identical conditions consistent with those used for colony maintenance as described above. Mortality assessments were conducted 24 hours post-exposure using a dissecting microscope at a magnification of ×20. A mite was deemed dead if it failed to exhibit any movement in its body or appendages when stimulated with a fine wooden probe, as outlined in previous protocols (Kim et al., 2015). To account for the fact that all bioassays could not be executed simultaneously, treatments were organized into temporal blocks to control for time-dependent variability, each including a respective control group. For each block, freshly prepared solutions were utilized, and all treatments were replicated three times, with each replicate consisting of 30 adult mites.
2.4 Vapor-phase mortality bioassay
To ascertain whether the lethality of E. citriodora essential oil and the seven selected components against adult D. pteronyssinus and D. farinae was due to contact or fumigant action, a closed versus open container treatment protocol was employed. Groups of 30 adult mites (both sexes, aged 7–10 days) were placed on untreated cotton fabric disks positioned at the base of polystyrene containers (5 cm diameter × 2 cm height). Each container was sealed with a tight-fitting lid equipped with a fine wire mesh covering a central 4.5 cm diameter aperture. Approximately double concentration of the contact-fumigant LC50 values of each test material were applied to 5 cm diameter filter papers. These treated filter papers were then placed over the wire mesh, preventing direct contact between the mites and the test substances. Containers were sealed either with a solid lid (closed container treatment) or a lid with a 4.8 cm diameter central opening (open container treatment) to evaluate the vapor-phase toxicity of the substances. Control filter papers were treated with 50 μL of ethanol only. Mortality rates were assessed 24 hours after treatment, as previously detailed above. All bioassays were replicated three times, with each replicate containing 30 adult mites.
2.5 Repellent bioassay
For this assay, the LC50 concentration of the essential oil or chemicals effective against both dust mite species, D. pteronyssinus and D. farinae, was used. For each candidate chemical, a circular filter paper was divided into four equal quadrants with a central release zone, where two opposite quadrants were treated with 100 μL of test compounds and the other two served as 100 μL of solvent controls (Figure 1); mites were counted manually under a dissecting microscope by a trained observer at specified time points, with treatment labels blinded to minimize bias. A growth medium was placed on the filter paper for 5 minutes to allow its components to equilibrate with the test surface and maintain a stable microenvironment mimicking dust mite habitats, before being removed to isolate the treated area for behavioral observation. Subsequently, 30 adult mites were gently transferred using a brush to the compound-treated area of the cloth to avoid damage. Ethanol alone served as the control. Repellency was calculated using the following formula.
Ethanol alone served as the control. Both treated and control mites were maintained at 25 ± 1°C and 75% relative humidity (RH) in darkness. Instances where mites moved away from the treated area were recorded as avoidance behaviors. Repellency rates were evaluated after three hours. All treatments were replicated six times to ensure reliability and reproducibility of the results.
2.6 Data analysis
Mortality rates in the control groups were adjusted using Abbott’s formula. Concentration-mortality data were analyzed via probit regression to determine the lethal concentration for 50% of the tested subjects (LC50) for each experimental group. LC50 values were compared using non-overlapping 95% confidence intervals (CIs) to assess significant differences between compounds and mite species. For repellency data, mean repellency rates were calculated as described in Section 2.5, and mortality percentages and repellent rates were transformed using the arcsine square root transformation to stabilize variances and normalize the distribution prior to analysis of variance (ANOVA). Significant differences in repellency among treatments were identified using the Bonferroni multiple-comparison procedure, while a t-test was employed to evaluate differences between specific treatment pairs. Results are reported as means ± standard deviation (SD) based on the original, untransformed data.
3 Results
3.1 Main compounds of Eucalyptus citriodora essential oil
According to the GC-MS analysis, seven compounds (1,8-cineole, citronellol, citronellal, (-)-globulol, citronellyl acetate, β-caryophyllene, and ethyl phenylacetate) were identified in E. citriodora essential oil at the concentrations exceeding three percent (Table 2). Among these, three compounds (citronellol, citronellal, and citronellyl acetate) were present at different concentration levels greater than 10 percent in the volatile fraction of E. citriodora oil (Table 2). These seven compounds were designated as the primary constituents of both E. citriodora essential oil and its volatile components.
3.2 Contact-fumigant toxicity to dust mites
The toxicity data of E. citriodora essential oil and its seven main compounds against the two species of dust mites are presented in Tables 3 and 4. LC50 values were determined to assess the toxicity levels, where a lower value indicates higher toxicity. For D. pteronyssinus, citronellal exhibited the lowest LC50 value (63.94 μL/cm²) among all tested substances, while ethyl phenylacetate, 1,8-cineole, essential oil, and citronellyl acetate had LC50 values below 100 μL/cm². In contrast, β-caryophyllene, (-)-globulol, and citronellol demonstrated lower toxicity with LC50 values exceeding 100 μL/cm² (Table 3). For D farinae, citronellal, 1,8-cineole, ethyl phenylacetate, and essential oil showed higher toxicity with LC50 values ranging between 60.72-75.88 μL/cm²; whereas citronellyl acetate, citronellol, (-)-globulol, and β-caryophyllene displayed lower toxicity with LC50 values ranging between 104.08-208.77 μL/cm² (Table 4). Mortality rates for control treatments using only ethanol remained below 2%, which was used to adjust mortality rates in experimental treatments.
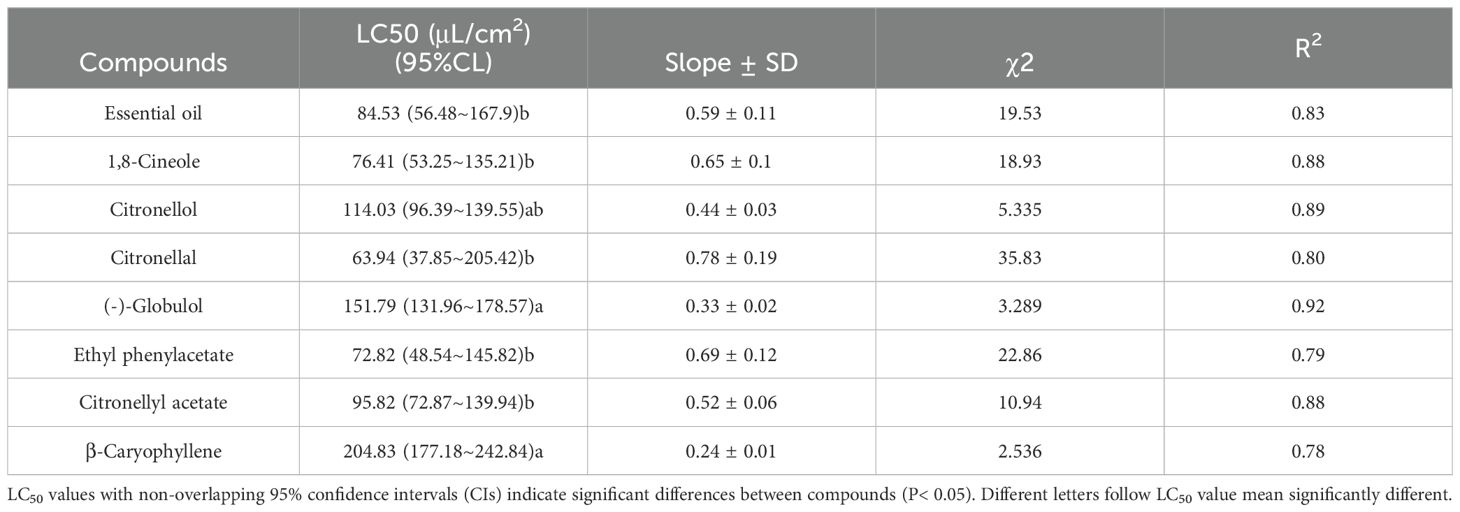
Table 3. Toxicity of Eucalyptus citriodora oil and the seven main compounds to Dermatophagoides pteronyssinus using a contact-fumigant mortality bioassay.
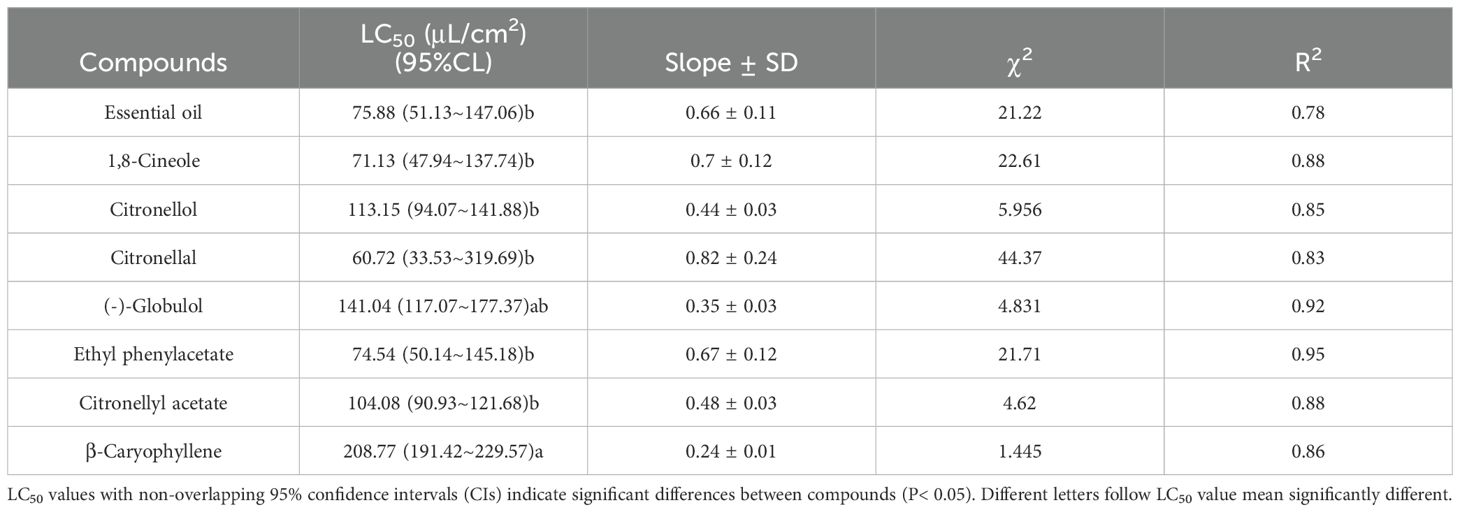
Table 4. Toxicity of Eucalyptus citriodora oil and the seven main compounds to Dermatophagoides farinae using a contact-fumigant mortality bioassay.
3.3 Vapor toxicity to dust mites
The fumigation efficacy of both the essential oil and its seven principal compounds was evaluated through vapor-phase bioassays conducted at twice the concentration of their respective LC50 values. Mortality rates were analyzed to determine fumigation effectiveness, where a positive correlation was observed between mortality rate and increased toxicity levels across treatments within closed versus open groups (P< 0.0001; Tables 5, 6). Both citronellal and ethyl phenylacetate achieved complete mortality against both Dermatophagoides species under closed conditions, even at the two lowest tested concentrations (127.88 μL/cm² for citronellal and 145.65 μL/cm² for ethyl phenylacetate, Table 5), highlighting their higher efficacy; additionally, the mortality effects associated with essential oils, 1,8-cineole, and citronellyl acetate exceeded 65% in closed containers (Tables 5, 6). This underscores the potential utility of citronellal, ethyl phenylacetate, essential oils, 1,8-cineole, and citronellyl acetate for controlling dust mites via fumigation.
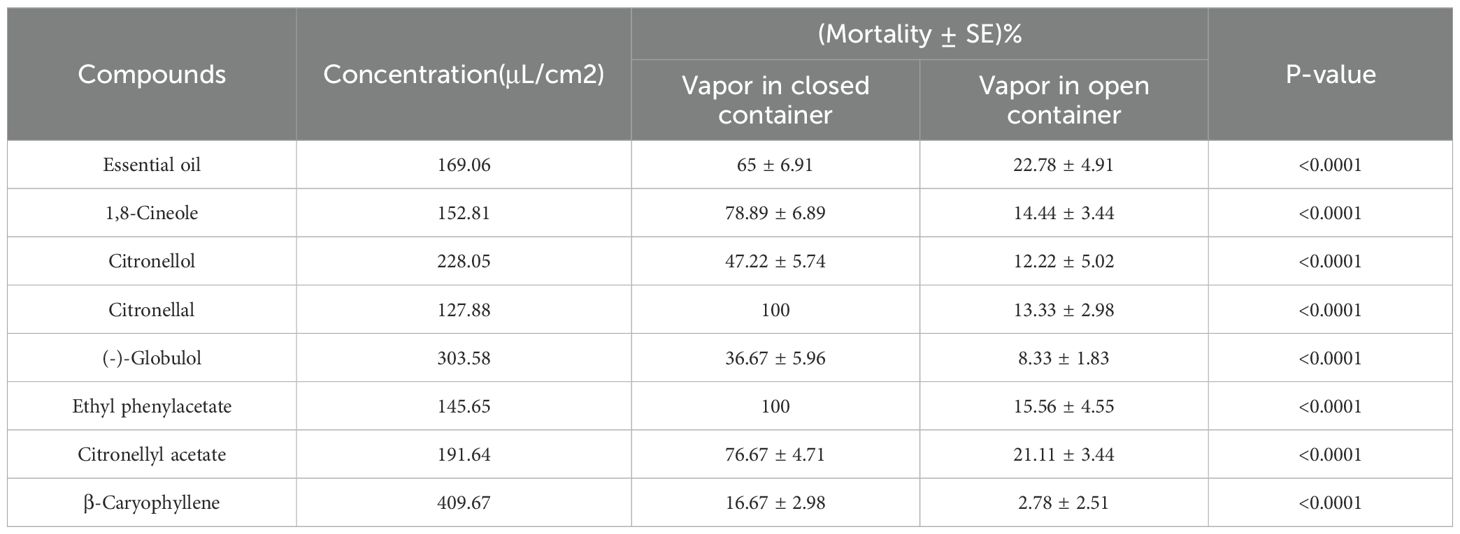
Table 5. Fumigant toxicity of Eucalyptus citriodora oil and the seven main compounds to adult Dermatophagoides pteronyssinus using a vapor-phase mortality bioassay after 24 h exposure.
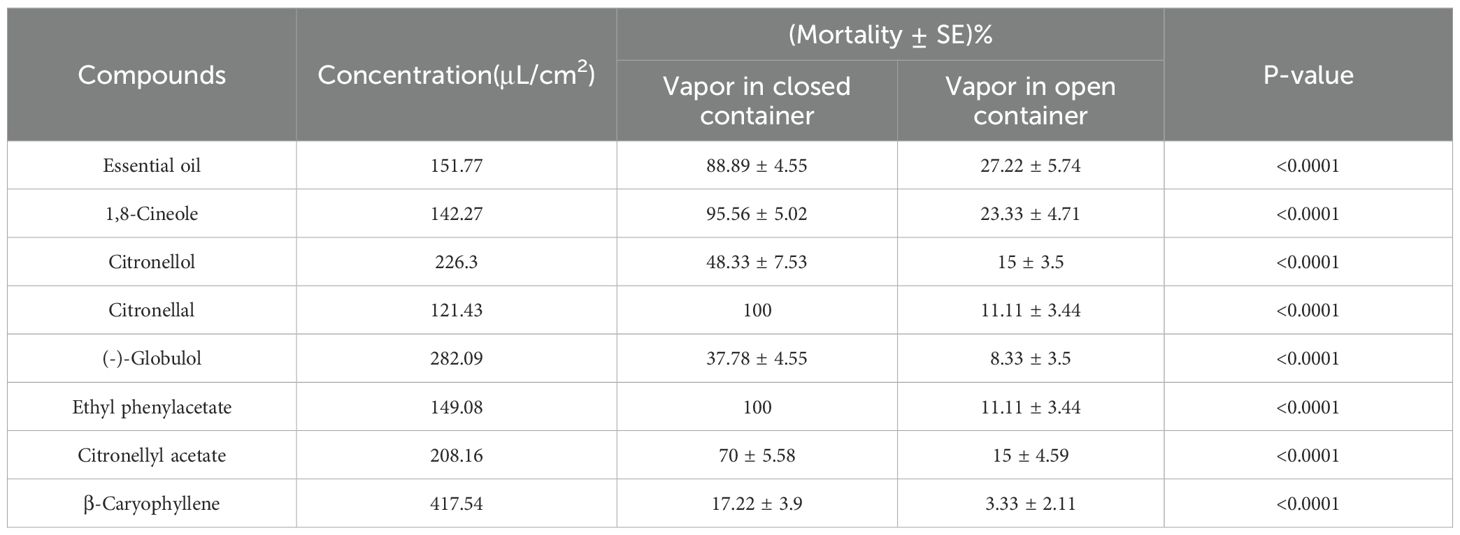
Table 6. Fumigant toxicity of Eucalyptus citriodora oil and the seven main compounds to adult Dermatophagoides farinae using a vapour-phase mortality bioassay after 24 h exposure.
3.4 Repellent effect
The repellent efficacy of E. citriodora essential oil along with its seven key compounds against dust mites is illustrated in Figure 2 and Figure 3. Due to the fumigant toxicity as well as the low concentration (LC50) of the essential oil and chemical compounds, both dead and alive (non escaping) mite data were considered when calculating the repellent rate. Citronellol demonstrated the highest repellent rate against both dust mite species D. pteronyssinus and D. farinae (78.33% and 85.56%, respectively), and significantly surpassing other tested compounds (P< 0.0001). The repellent rates of essential oil, 1, 8-cineole, and citronellyl acetate against both dust mite species exceeded 50%, which were also significantly higher than those of the remaining four compounds (P< 0.0001). Repellency rates were analyzed via ANOVA following arcsine square root transformation of mortality percentages to meet normality assumptions. Significant differences among compounds were identified using the Bonferroni multiple-comparison procedure (P< 0.001), with citronellol exhibiting the highest repellency (78.33-85.56%) and statistically distinct from all other tested substances. This indicates that citronellol, 1,8-cineole, and citronellyl acetate play crucial roles in the overall repellency effects of E. citriodora essential oil against both dust mite species. In our study, there was no indication that dead dust mites repelled living mites.
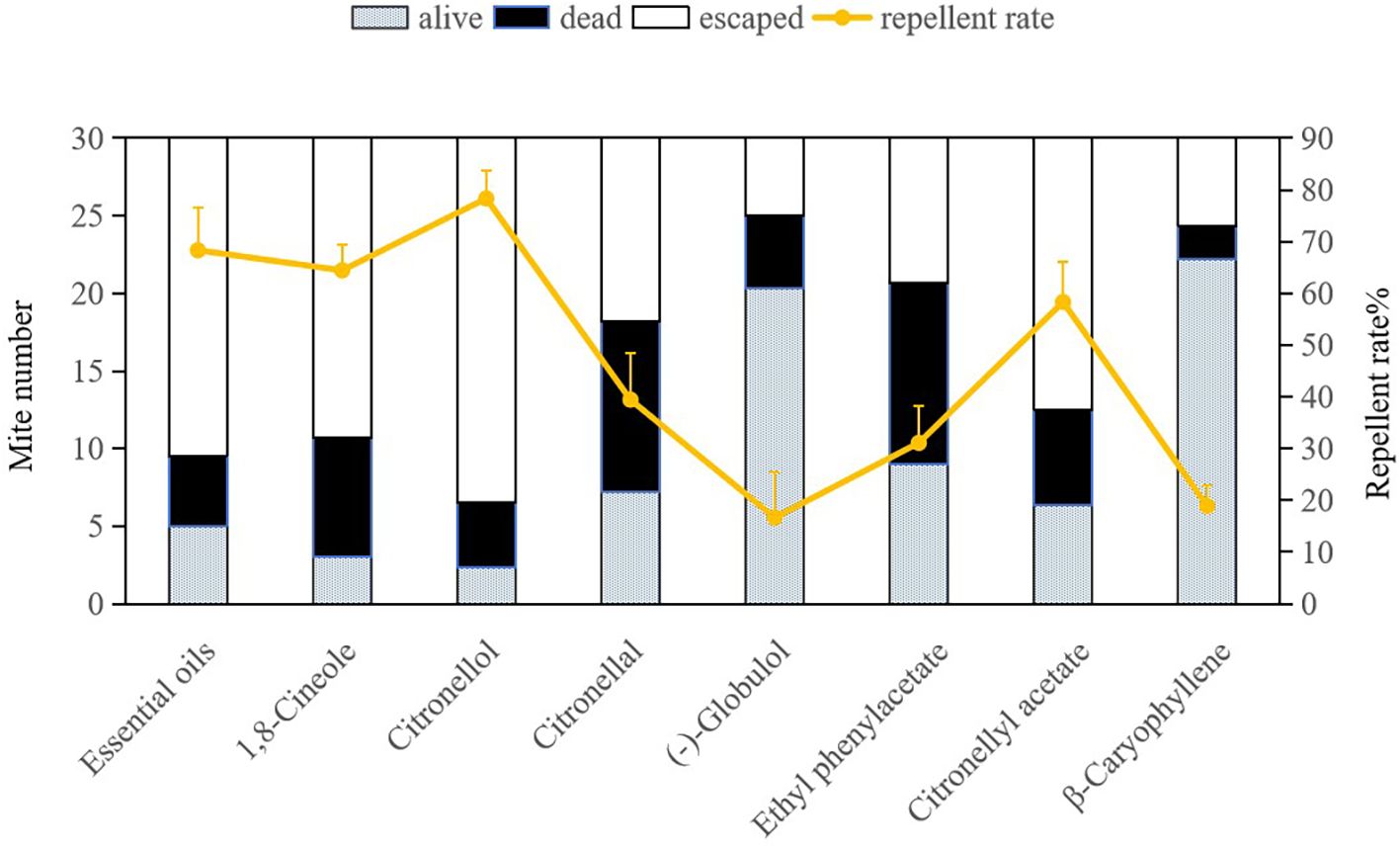
Figure 2. Repellent effects of Eucalyptus citriodora essential oil and its seven main compounds on Dermatophagoides pteronyssinus. Escaped means the mites were repelled from treat quadrant to control quadrant in the same patric dish. Data were analyzed using ANOVA followed by Bonferroni post-hoc tests; bars with different letters indicate significant differences (P< 0.05).
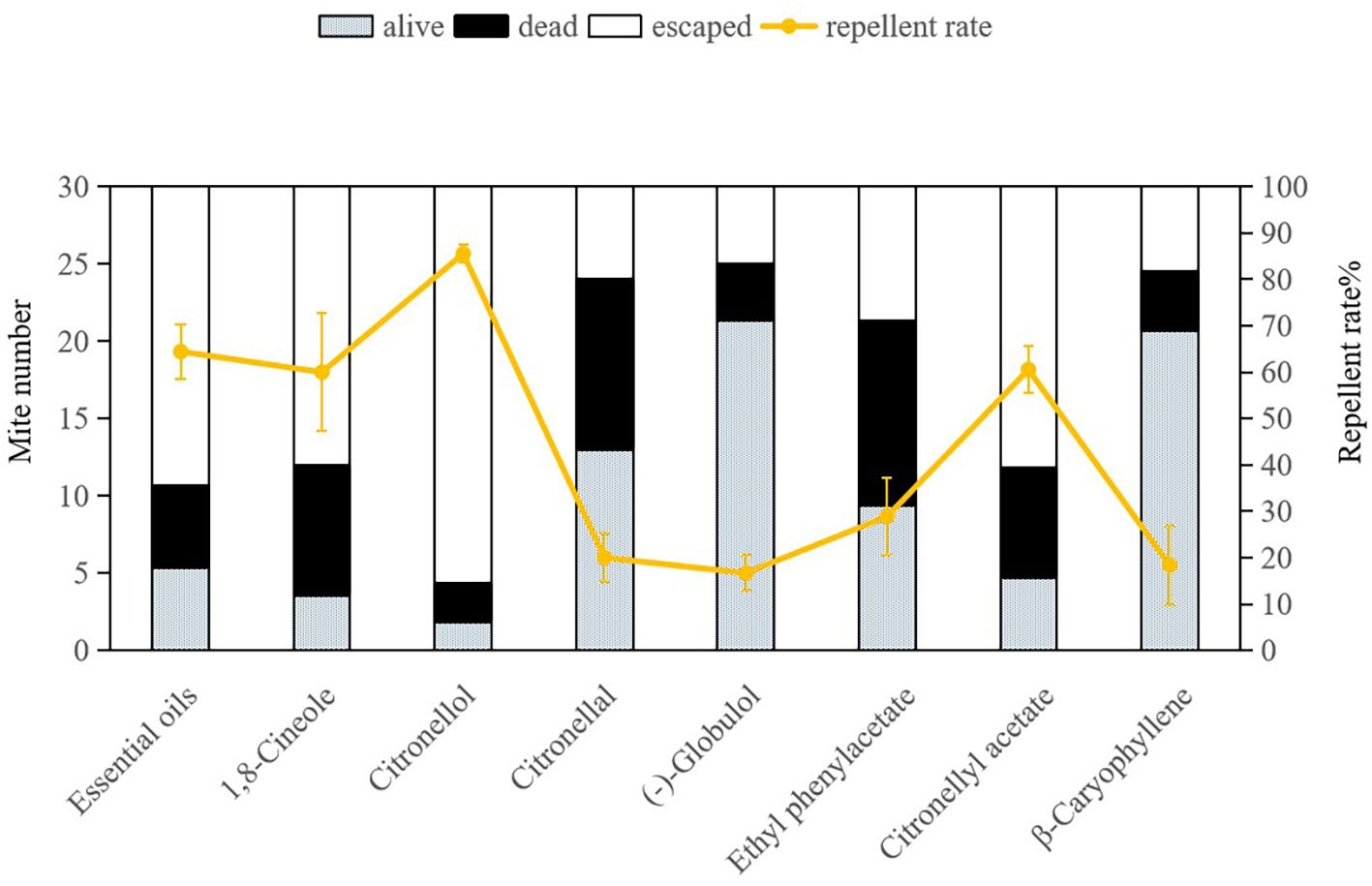
Figure 3. Repellent effects of Eucalyptus citriodora essential oil and its seven main compounds on Dermatophagoides farinae. Data were analyzed using ANOVA followed by Bonferroni post-hoc tests; bars with different letters indicate significant differences (P< 0.05).
4 Discussion
The present study aimed to evaluate the toxicity and repellent effects of E. citriodora essential oil and its main compounds against two common dust mite species, D. pteronyssinus and D. farinae. The results provide valuable insights into the potential use of this essential oil as a natural and environmentally friendly dust mite control agent.
The GC-MS analysis revealed that seven chemicals (1,8-cineole, citronellol, citronellal, (-)-globulol, citronellyl acetate, β-caryophyllene, and ethyl phenylacetate) were the main compounds of E. citriodora essential oil. Among these, citronellol, citronellal and citronellyl acetate were the predominant constituents in the volatile fraction. Citronellol and citronellal have been previously reported to exhibit insecticidal and repellent activities against various arthropod pests, including Aedes aegypti (Patel et al., 2023), Myzus persicae (Ahmed et al., 2022), and Musca domestica (Ahmadi et al., 2022; Pavela et al., 2022), which aligns with their demonstrated efficacy against dust mites in our study. Notably, our findings contrast and complement recent research on other botanical acaricides, Borneol essential oil (BEO) achieves >95% repellency at 0.5 mg/ml (Yu et al., 2022), comparable to the 85.56% repellency of citronellol in our study. Additionally, Abies sachalinensis oil inhibits dust mite allergen Der f 2 by reducing protein abundance (Lin et al., 2022), whereas E. citriodora acts directly via toxicity and repellency.
The contact and fumigant toxicity bioassays demonstrated that E. citriodora essential oil and several of its compounds exhibited significant toxicity against both dust mite species. Notably, citronellal, 1,8-cineole, and ethyl phenylacetate showed the lowest LC50 values, indicating the highest toxicity among the tested compounds. Meanwhile, the essential oil itself also showed strong toxicity, with an LC50 value below 85 μL/cm2 for both mite species, similar toxicity to above three singular constituents. It means that these three components of the E. citriodora essential oil (citronellal, 1,8-cineole, and ethyl phenylacetate) may be the key factors that enable the essential oil to kill the dust mites. This finding is in line with previous studies that have reported potent insecticidal activity of citronellal, ethyl phenylacetate, and 1,8-cineole (Sabio et al., 2024; Yan et al., 2024). The vapor-phase mortality bioassay further confirmed the biotoxicity of the essential oil and its compounds, with citronellal and ethyl phenylacetate showing 100% mortality in closed containers. This indicates that these compounds could effectively be used to control dust mites through vapor action alone, which is known to be a desirable property for a pest control agent, as it does not require direct contact with the target organism. Future studies should investigate that slow-release formulations of high-volatility compounds would be the important practice of dust mite IPM.
The repellent bioassay revealed that citronellol exhibited the highest repellent efficacy against D. pteronyssinus and D. farinae, with mean repellency rates of 85.56 ± 2.14% and 78.33 ± 1.89% at 60 min post-treatment, respectively. These values were significantly higher than those of other tested compounds. These findings indicated that citronellol is likely the principal contributor to the repellency of E. citriodora essential oil. The essential oil, 1,8-cineole, and citronellyl acetate also showed significant repellent effects, with repellency rates above 50%. Previous study also demonstrated that citronellol, 1,8-cineole, and citronellyl acetate repelled insects and mites (Han et al., 2011; Deletre et al., 2022; Ayllón-Gutiérrez et al., 2023; Kaneko et al., 2024; Kunnathattil et al., 2024; Yan et al., 2024).
The efficacy of citronellol and citronellal is closely linked to their physicochemical properties. Citronellal’s low molecular weight (154.23 g/mol) and high vapor pressure (0.85 hPa at 25°C) enable rapid vapor-phase diffusion, explaining its 100% mortality in closed-container assays even at low concentrations (127.88 μL/cm² for D. pteronyssinus). In contrast, β-caryophyllene’s larger molecular size (204.32 g/mol) and negligible volatility (0.045 hPa) correlate with its poor fumigant efficacy (<20% mortality), illustrating the critical role of vapor pressure in gaseous toxicity. Citronellol, despite lower volatility (0.02 hPa), exhibits strong repellency (85.56% against D. farinae), likely due to its alcohol functional group interacting with mite chemoreceptors, as hypothesized in insect repellency studies (Dawood and Snyder, 2020). This aligns with its structural similarity to known arthropod repellents, where hydroxyl moieties enhance binding to olfactory receptors (Zhang et al., 2019).
The findings of this study suggest that E. citriodora essential oil and its main compounds, particularly citronellal and citronellol, could serve as effective dust mite control bioagents. Their natural origin, strong toxicity, and repellent effects make them promising alternatives to traditional chemical control methods, which can have negative impacts on human health and the environment. To translate these findings into practical use, future work should explore slow-release formulations for citronellal/ethyl phenylacetate and fabric treatments with citronellol, plus optimize concentrations to balance efficacy and safety. Field testing of these methods in homes will also confirm real-world mite control and support household application.
5 Conclusions
This study demonstrates that Eucalyptus citriodora essential oil and its key components, particularly citronellal and citronellol, exhibit notable biotoxicity and repellent activity against Dermatophagoides pteronyssinus and D. farinae. Gas chromatography-mass spectrometry identified seven major compounds, with citronellal, citronellol, and citronellyl acetate dominating the volatile fraction. Contact and fumigant toxicity assays revealed that the essential oil and compounds like citronellal, 1,8-cineole, and ethyl phenylacetate primarily exert acaricidal effects via contact and fumigant actions while citronellol drives repellency likely through interactions with mite chemoreceptors. These findings underscore the potential of E. citriodora essential oil as a natural alternative to synthetic pesticides for dust mite control, while the proposed strategies provide actionable guidance for translating laboratory results into scalable indoor pest management solutions. Future research should focus on optimizing formulation stability, validating field efficacy in residential settings, and defining safety thresholds to support regulatory approval.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://doi.org/10.6084/m9.figshare.28632437.v1.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
HC: Writing – original draft, Formal Analysis, Data curation. PX: Project administration, Writing – original draft, Data curation, Methodology. XZ: Methodology, Writing – original draft, Software. ZL: Investigation, Writing – original draft, Methodology. ZX: Funding acquisition, Writing – original draft, Project administration, Methodology. SC: Writing – original draft, Data curation, Investigation, Resources. PR: Software, Data curation, Writing – original draft. KA: Writing – review & editing, Software. SL: Writing – original draft, Resources, Formal Analysis. HL: Writing – review & editing, Conceptualization. ZW: Writing – review & editing, Conceptualization. YL: Writing – original draft, Conceptualization, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Scientific Research and Innovation Team Fund of Zhangzhou Institute of Technology (170057), 2024 General Project of the Qihang Fund, Fujian Medical University (2024QH1371), and the Natural Science Foundation of Fujian Province (2025J011614).
Conflict of interest
Author ZW was employed by the company Fujian Zhanglong Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, P. and Senthilkumaran, S. (2024). “Dust mite allergy,” in StatPearls (StatPearls, Treasure Island (FL).
Ahmadi, E., Khajehali, J., Jonckheere, W., and Van Leeuwen, T. (2022). Biochemical and insecticidal effects of plant essential oils on insecticide resistant and susceptible populations of Musca domestica L. point to a potential cross-resistance risk. Pestic. Biochem. Physiol. 184, 105115. doi: 10.1016/j.pestbp.2022.105115
Ahmed, Q., Agarwal, M., Alobaidi, R., Zhang, H., and Ren, Y. (2022). Response of aphid parasitoids to volatile organic compounds from undamaged and infested Brassica oleracea with Myzus persicae. Molecules 27, 1522. doi: 10.3390/molecules27051522
Ayllón-Gutiérrez, R., López-Maldonado, E. A., Macías-Alonso, M., González Marrero, J., Díaz-Rubio, L., and Córdova-Guerrero, I. (2023). Evaluation of the stability of a 1,8-cineole nanoemulsion and its fumigant toxicity effect against the pests Tetranychus urticae, Rhopalosiphum maidis and Bemisia tabaci. Insects 14, 663. doi: 10.3390/insects14070663
Caetano, A. R. S., Cardoso, M. G., Resende, M. L. V., Chalfuon, S. M., Martins, M. A., Gomes, H. G., et al. (2022). Antifungal activity of poly(ϵ-caprolactone) nanoparticles incorporated with Eucalyptus essential oils against Hemileia vastatrix. Lett. Appl. Microbiol. 75, 1028–1041. doi: 10.1111/lam.13782
Caraballo, L. (2024). Exploring the relationship between house dust mites and asthma. Expert Rev. Clin. Immunol. 20, 1019–1022. doi: 10.1080/1744666x.2024.2346585
Colloff, M. J. (1987). Effects of temperature and relative humidity on development times and mortality of eggs from laboratory and wild populations of the European house-dust mite Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). Exp. Appl. Acarol. 3, 279–289. doi: 10.1007/bf01193165
Dawood, M. H. and Snyder, J. C. (2020). The alcohol and epoxy alcohol of zingiberene, produced in trichomes of wild tomato, are more repellent to spider mites than zingiberene. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00035
Deletre, E., Matu, F. K., Murungi, L. K., and Mohamed, S. (2022). Repellency potential of tomato herbivore-Induced volatiles against the greenhouse whitefly (Trialeurodes vaporariorum) (Hemiptera: Aleyrodidae). J. Econ. Entomol. 115, 565–572. doi: 10.1093/jee/toac015
El-Sakhawy, M. A., Soliman, G. A., El-Sheikh, H. H., and Ganaie, M. A. (2023). Anticandidal effect of Eucalyptus oil and three isolated compounds on cutaneous wound healing in rats. Eur. Rev. Med. Pharmacol. Sci. 27, 26–37. doi: 10.26355/eurrev_202301_30850
Espinosa-Zaragoza, S., Domínguez-Liévano, A., Gómez-Gutiérrez, J. A., Wong-Villarreal, A., Aguilar-Marcelino, L., Cerqueda-García, D., et al. (2024). In vitro acaricidal activity of serratia ureilytica against the dust mite tyrophagus putrescentiae and identification of genes related to biocontrol. Curr. Microbiol. 81, 199. doi: 10.1007/s00284-024-03728-3
Fayez, S., Gamal El-Din, M. I., Moghannem, S. A., Azam, F., El-Shazly, M., Korinek, M., et al. (2023). Eucalyptus-derived essential oils alleviate microbes and modulate inflammation by suppressing superoxide and elastase release. Front. Pharmacol. 14. doi: 10.3389/fphar.2023.1218315
Gómez-Gutiérrez, J. A., Wong-Villarreal, A., Aguilar-Marcelino, L., Yañez-Ocampo, G., Hernández-Nuñéz, E., Caspeta-Mandujano, J. M., et al. (2023). In vitro nematicidal and acaricidal effect of biosurfactants produced by Bacillus against the root-knot nematode Nacobbus aberrans and the dust mite Tyrophagus putrescentiae. Braz. J. Microbiol. 54, 1127–1136. doi: 10.1007/s42770-023-00981-9
Han, J., Kim, S. I., Choi, B. R., Lee, S. G., and Ahn, Y. J. (2011). Fumigant toxicity of lemon eucalyptus oil constituents to acaricide-susceptible and acaricide-resistant Tetranychus urticae. Pest Manag. Sci. 67, 1583–1588. doi: 10.1002/ps.2216
Hussain, A. and AlJabr, A. M. (2020). Potential synergy between spores of Metarhizium anisopliae and plant secondary metabolite, 1-chlorooctadecane for effective natural acaricide development. Molecules 25, 1900. doi: 10.3390/molecules25081900
Jung, Y., Yang, H., Lee, I. Y., Yong, T. S., and Lee, S. (2020). Core/Sheath-structured composite nanofibers containing cinnamon oil: Their antibacterial and antifungal properties and acaricidal effect against house dust mites. Polymers (Basel) 12, 243. doi: 10.3390/polym12010243
Kaneko, E., Matsui, K., Nakahara, R., and Arimura, G. I. (2024). Novel potential of rose essential oil as a powerful plant defense potentiator. J. Agric. Food Chem. 72, 6526–6532. doi: 10.1021/acs.jafc.3c08905
Kim, J. R., Perumalsamy, H., Kwon, M. J., Chae, S. U., and Ahn, Y. J. (2015). Toxicity of hiba oil constituents and spray formulations to American house dust mites and copra mites. Pest Manag. Sci. 71, 737–743. doi: 10.1002/ps.3843
Klain, A., Senatore, A. A., Licari, A., Galletta, F., Bettini, I., Tomei, L., et al. (2024). The prevention of house dust mite allergies in pediatric asthma. Children (Basel) 11, 469. doi: 10.3390/children11040469
Kunnathattil, M., Narayanankutty, A., Visakh, N. U., Pathrose, B., Punathil, T., and Kaimal, S. G. (2024). Phytochemical characterization, fumigant and contact toxicity activities of four essential oils against Eriophyid gall mite, Aceria pongamiae Keifer (Acarina: Eriophyidae). Chem. Biodivers. 21, e202401535. doi: 10.1002/cbdv.202401535
Lin, Y., Enyoh, C. E., Wang, Q., Lu, S., Zhang, W., Xiao, K., et al. (2022). Novel approaches for inhibiting the indoor allergen der f 2 excreted from house dust mites by todomatsu oil produced from woodland residues. Int. J. Environ. Res. Public Health 19, 10881. doi: 10.3390/ijerph191710881
Marko, M. and Pawliczak, R. (2023). Pharmacotherapy and immunotherapy of allergic rhinitis induced by house dust mite, grass, and birch pollen allergens: a meta-analysis of randomized clinical trials. Expert Rev. Respir. Med. 17, 607–621. doi: 10.1080/17476348.2023.2241364
Maruoka, A., Koda, T., and Morita, H. (2020). Mite control effect of branched chain fatty acid on the house dust mite Dermatophagoides pteronyssinus. Biocontrol Sci. 25, 63–71. doi: 10.4265/bio.25.63
Patel, K., Akbari, D., Pandya, R. V., Trivedi, J., Mevada, V., Wanale, S. G., et al. (2023). Larvicidal proficiency of volatile compounds present in Commiphora wightii gum extract against Aedes aEgypti (Linnaeus 1762). Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1220339
Pavela, R., Ferrati, M., Spinozzi, E., Maggi, F., Petrelli, R., Rakotosaona, R., et al. (2022). The essential oil from the resurrection plant Myrothamnus moschatus is effective against arthropods of agricultural and medical interest. Pharm. (Basel) 15, 1511. doi: 10.3390/ph15121511
Pinheiro, R. E. E., Chaves, T. P., Melo, E. S., Ali, S., Ali, S. W., Umer, M., et al. (2020). Modulatory-antibiotic activity of the essential oil from Eucalyptus citriodora against MDR bacterial strains. Cell Mol. Biol. (Noisy-le-grand) 66, 60–64. doi: 10.14715/cmb/2020.66.4.10
Rodriguez-Plata, E., Callero Viera, A., Ruiz-Garcia, M., Gomez-Cardenosa, A., Nieto, E., and García-Robaina, J. C. (2023). House dust mite subcutaneous immunotherapy has sustained long-term effectiveness on allergic rhinitis and asthma: A 10-year follow-up. Immun. Inflammation Dis. 11, e1004. doi: 10.1002/iid3.1004
Sabahi, Q., Morfin, N., Emsen, B., Gashout, H. A., Kelly, P. G., Otto, S., et al. (2020). Evaluation of dry and wet formulations of oxalic acid, thymol, and oregano oil for varroa mite (Acari: Varroidae) control in honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 113, 2588–2594. doi: 10.1093/jee/toaa218
Sabio, M. C., Alzogaray, R., and Fanara, J. J. (2024). Genetic architecture of the toxicological response to eucalyptol and citronellal in Drosophila melanogaster. Pestic. Biochem. Physiol. 202, 105938. doi: 10.1016/j.pestbp.2024.105938
Turki, S., Jbali, S., Hachicha, A., Chouchane, H., Sifaoui, A., and Charfi, M. R. (2024). Explanatory factors for the evolution of children’s allergic rhinitis. Tunis Med. 102, 303–309. doi: 10.62438/tunismed.v102i5.4463
Vackova, T., Pekar, S., Klimov, P. B., and Hubert, J. (2023). Population growth and respiration in the dust mite Dermatophagoides farinae under different temperature and humidity regimes. Exp. Appl. Acarol. 89, 157–169. doi: 10.1007/s10493-022-00775-y
Yan, B., Gong, C., Qian, Y., Zhang, Z., Liu, X., Yuan, H., et al. (2024). Preparation and insecticidal performance of 1,8-cineole/cellulose acetate electrospun fibrous membranes. Int. J. Biol. Macromol. 278, 134942. doi: 10.1016/j.ijbiomac.2024.134942
Yu, H., Ren, X., Yang, F., Xie, Y., Guo, Y., Cheng, Y., et al. (2022). Antimicrobial and anti-dust mite efficacy of Cinnamomum camphora chvar. Borneol essential oil using pilot-plant neutral cellulase-assisted steam distillation. Lett. Appl. Microbiol. 74, 258–267. doi: 10.1111/lam.13610
Zhang, Y.-X., Chen, X., Wang, J.-P., Zhang, Z.-Q., Wei, H., Yu, H.-Y., et al. (2019). Genomic insights into mite phylogeny, fitness, development, and reproduction. BMC Genomics 20, 954. doi: 10.1186/s12864-019-6281-1
Keywords: citronellol, Dermatophagoides pteronyssinus, Dermatophagoides farinae, toxicity, repellency, environmental control
Citation: Cai H, Xie P, Zhang X, Lin Z, Xu Z, Chen S, Ruan P, Akutse KS, Li S, Lin H, Wu Z and Lin Y (2025) Biotoxicity of essential oil of Eucalyptus citriodora Hook (Myrtaceae) to dust mites. Front. Plant Sci. 16:1708798. doi: 10.3389/fpls.2025.1708798
Received: 23 September 2025; Accepted: 17 November 2025; Revised: 12 November 2025;
Published: 04 December 2025.
Edited by:
Manuel Martinez, Polytechnic University of Madrid, SpainReviewed by:
Esraa A. Elhawary, Ain Shams University, EgyptAhmed Mshari, Southern Technical University, Iraq
Copyright © 2025 Cai, Xie, Zhang, Lin, Xu, Chen, Ruan, Akutse, Li, Lin, Wu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiquan Lin, bGluaHVpcXVhbkAxMjYuY29t; Ziyi Wu, NjY0OTY5NDgyQHFxLmNvbQ==; Yongwen Lin, bGlueW9uZ3dlbkBmanp6aXQuZWR1LmNu
†These authors have contributed equally to this work
 Haiming Cai1†
Haiming Cai1† Komivi S. Akutse
Komivi S. Akutse Yongwen Lin
Yongwen Lin