- 1School of tea and Coffee, Pu’er University, Puer, China
- 2Nanjing Agriculture University, College of Life Sciences, Nanjing, China
- 3School of Environment and Safety Engineering, Jiangsu University, Zhenjiang, China
- 4College of Tropical Crops, Hainan University, Haikou, China
Biochar amendment and halophyte intercropping are viable strategies for alleviating soil degradation in greenhouse systems, specifically the secondary salinization and autotoxicity induced by continuous cropping. Nevertheless, the potential synergistic effects of combining these practices remain poorly understood. This study investigated their synergistic effects on soil properties, microbial communities, and cucumber performance. A pot experiment was conducted with the following treatments: soil without amendment (CK), biochar (B), Paspalum vaginatum intercropping (S), and biochar combined with Paspalum vaginatum intercropping. The results showed that BS treatment led to the highest increases in soil organic carbon content, pH, total nitrogen content, available phosphorus content, and available potassium content compared to CK (p<0.05). Concurrently, BS significantly reduced available nitrogen, electrical conductivity, Na+, SO42-, and Cl- levels, while total phosphorus remained unaffected. Cucumber yield increased significantly by 11.50% and 27.12% under B and BS treatments, respectively, whereas S showed no significant effect. BS also achieved the highest fruit quality enhancement, followed by B and S. Notably, B and S treatments displayed the highest and lowest K+, Ca2+and Mg2+ accumulation, respectively, whereas the BS treatment led to K+ and Ca2+ concentrations that were significantly lower than those in the B treatment. Soil bacterial diversity was significantly enhanced under BS. The PLS-PM identified the alleviation of soil salinity and acidity, along with improved nutrient availability, as the primary drivers for enhanced crop performance, with soil bacterial diversity playing a secondary yet significant role. These findings suggest that biochar combined with intercropping (BS) effectively mitigates continuous cropping obstacles in greenhouse systems by synergistically improving soil health and microbial ecology.
1 Introduction
Greenhouse cultivation offers a stable environment that meets the global demand for vegetables, making it one of the most widely adopted agricultural systems worldwide (Li et al., 2019; Lopez-Marin et al., 2019). Its high profitability often leads to continuous cropping and excessive fertilization (Liu and Zhang, 2021; Lv et al., 2019). However, continuous monoculture and excessive fertilizer input often lead to a decline in soil quality (Wang et al., 2021b; Yang et al., 2016), secondary soil salinization (Hu et al., 2020), soil acidification (Song et al., 2016; Zhang et al., 2022b), autotoxicity (Xiao et al., 2020), and the accumulation of soil-borne pathogens (Meng et al., 2018), which limit the yield and quality of greenhouse crops (Sun et al., 2019). Hence, implementing effective soil management strategies is crucial to maintaining the yield and quality of greenhouse crops, given the system’s global importance and the numerous soil degradation processes it induces.
Owing to its strong adsorption capacity and alkaline nature, biochar is considered a promising amendment for soil health management (Shi et al., 2023; Spokas et al., 2012). It has been shown to mitigate several continuous cropping obstacles simultaneously, such as by adsorbing allelopathic phenolic compounds (Lin et al., 2023), enhancing nutrient availability (Han et al., 2023; Yan et al., 2022), and optimizing the microbial habitat (Wang et al., 2020). However, a critical but often overlooked risk is that biochar application may also introduce or exacerbate soil salinity, primarily due to the direct input of salt ions present in the biochar itself, especially when derived from high-salt feedstocks (Amini et al., 2016; Wang et al., 2023b). Additionally, the aging process can diminish biochar’s adsorption capacity, potentially causing the re-release of previously bound salt ions (Akhtar et al., 2015; Kong et al., 2014). This inherent limitation suggests that biochar alone might be insufficient or even risky for managing the saline conditions often associated with greenhouse continuous cropping.
Conversely, halophytes represent a low-cost phytoremediation strategy widely employed in agriculture to improve saline soil conditions (Jurado et al., 2024; Liang and Shi, 2021). For example, intercropping with salt-tolerant species, such as lawn grass (Paspalum vaginatum) (Hu et al., 2020), Portulaca oleracea (Simpson et al., 2018), or legumes (Zheng et al., 2023), has been demonstrated to alleviate salt stress, improve crop quality and yield, and enhance soil nutrient availability. This approach effectively reduces the adverse effects of salinity on crops (Aksoy et al., 2001). Although effective for salinity control, the capacity of halophyte intercropping alone to rapidly improve broader soil issues like severe acidification or nutrient immobilization may be limited.
Thus, we hypothesize that integrating biochar amendment with halophyte intercropping could create a synergistic solution for the multifaceted challenges of continuous cropping. Biochar’s ability to rapidly adjust pH, improve nutrient retention, and adsorb phenolics could establish a more favorable base soil condition. This improved environment might, in turn, enhance the establishment and salt-uptake efficiency of the intercropped halophyte. Meanwhile, the halophyte can continuously remove salts from the soil, mitigating the potential salinization risk from biochar and preventing salt rebound. While both biochar and intercropping individually enhance soil health (Liu et al., 2024; Wang et al., 2023a), their combined effects, particularly the potential synergy in reshaping the soil microbial community to foster a more resilient and beneficial microbiome under the complex stress of continuous cropping, remain poorly understood (Jin et al., 2024). This knowledge gap is critical given the crucial role of soil microorganisms in maintaining soil health and suppressing soil-borne diseases (Gu et al., 2023; Liu et al., 2023a).
Therefore, this study aimed to investigate the potential of combined biochar amendment and intercropping with the halophyte Paspalum vaginatum as an integrated strategy to concurrently address multiple soil constraints (acidity, salinity, nutrient imbalance, and autotoxicity) in a continuous cucumber system. The specific objectives were to: (1) assess the effects of biochar and/or Paspalum vaginatum intercropping on cucumber growth and fruit quality; (2) evaluate the changes in key soil physicochemical properties induced by these treatments; and (3) investigate their collective influence on soil bacterial communities to identify the key factors determining cucumber performance. We hypothesized that the combined application would synergistically ameliorate soil properties, reshape the microbial community structure towards a more beneficial state, and consequently lead to greater improvements in cucumber yield and quality compared to either practice alone.
2 Materials and methods
2.1 Experimental setup
A pot experiment was conducted in a greenhouse at the teaching and research base of Nanjing Agricultural University, Nanjing, China (118°51' E, 32°01' N). The experimental soil (sandy loam) was collected from the top layer (0–20 cm) of a greenhouse in Jurong City, Jiangsu Province, China (119°13' E, 31°47' N), which had been under continuous cucumber monoculture for 15 years. The soil properties were as follows: pH, 5.43; electrical conductivity (EC), 1500 µS cm-¹; soil organic carbon (SOC), 5.91 g kg-¹; total nitrogen (TN), 1.41 g kg-¹; total phosphorus (TP), 1.28 g kg-¹. Biochar, supplied by Zhenjiang Zedi Biotechnology Co., Ltd. (Zhenjiang, China), was produced by anaerobic pyrolysis of rice straw at 600 °C. Its physicochemical characteristics were: pH, 8.85; SOC, 620 g kg-1; TN 10.5 g kg-1; available phosphorus (AP), 1.13 g kg-1; available potassium (AK), 23.1 g kg-1; Brunauer-Emmett-Teller (BET) surface area, 675 m2 g-1;and elemental composition of carbon (C), hydrogen (H), nitrogen (N), and oxygen (O) at 62.0%, 2.5%, 2.2%, and 20.6%, respectively. Cucumber seeds (cv. Xinjin No. 4) were purchased from Luming Seed Co., Ltd. (Taian, China). The Paspalum vaginatum was selected for intercropping based on research reporting its salt tolerance and ability to effectively alleviate secondary salinization in greenhouse soils (Hu et al., 2020).
A completely randomized design with four treatments was implemented: soil without amendment (CK), intercropping with Paspalum vaginatum (S), soil amended with biochar at 2% (w/w) (B), and 2% (w/w) biochar amendment combined with P. vaginatum intercropping. The biochar was thoroughly mixed with the soil and then placed into plastic pots (total volume of 6.5 L). Each treatment consisted of three independent biological replicates. To ensure robust sampling, each biological replicate comprised three pots, which were treated as technical replicates. Cucumber seeds were germinated in seedling trays. At the two-true-leaf stage, uniform and vigorous seedlings were transplanted into the pots (one seedling per pot). Concurrently, stem cuttings of Paspalum vaginatum were planted at a density of ten cuttings per pot. Paspalum vaginatum was trimmed to 4 cm height at 25 and 40 days after transplanting, and all trimmings were removed from the pots. Throughout the experiment, only cucumbers and Paspalum vaginatum were retained; weeds were manually removed. No fertilizers, herbicides, or pesticides were applied. Pests were managed using insect-proof nets and yellow sticky traps. All pots were irrigated equally every three days.
2.2 Soil sampling and analysis
After harvest, rhizosphere soil was collected using the root-shaking method (Inderjit and Mallik, 1997). The composite soil sample from each replicate was divided into three parts: one was stored at -80 °C for microbial DNA sequencing; one was stored at 4 °C for the measurement of available·nitrogen (sum of NH4+-N and NO3--N) and the remainder air-dried for subsequent analysis of soil pH, EC, SOC, TN, TP, AP, AK, water-soluble ions, and phenolic compounds. For these analyses, measurements from the three technical pots within a biological replicate were averaged to yield a single value representing that replicate.
Soil pH and EC were measured in a 1:5 (w/v) soil-water suspension using a portable pH meter (FieldScout pH400, Spectrum Technologies Inc., USA) and a conductivity meter (DDSJ-308F, LeiCi, China), respectively. NH4+-N and NO3--N were extracted with 2 M KCl at a soil-to-solution ratio of 1:5 (w/v) and analyzed with a flow injection auto-analyzer (AutoAnalyzer 3, Seal Analytical, Norderstedt, Germany). SOC was determined by the potassium dichromate oxidation method using a Multi N/C 2100 analyzer (Analytik Jena, Germany), after removing inorganic carbon by fumigation with concentrated HCl. TN and TP were determined using a flow injection autoanalyzer (AutoAnalyzer 3, Seal Analytical, Germany) following digestion with H2SO4-HClO4. AP was extracted with 0.5 M NaHCO3 (1:5, w/v) and analyzed using the flow injection autoanalyzer. AK was extracted with 1 M ammonium acetate (1:10, w/v) and quantified using a flame photometer (BWB Technologies Ltd., UK). Water-soluble ions were extracted with deionized water at a 1:5 (w/v) ratio. Cation (Na+, K+, Ca²+, Mg²+) concentrations were determined by inductively coupled plasma optical emission spectrometry (ICP-OES; iCAP 6300, Thermo Fisher Scientific, USA), and anion (Cl-, SO4²-) concentrations were measured by ion chromatography (ICS-5000, Thermo Fisher Scientific, USA). Total phenolics, complex phenolics, and water-soluble phenolics were determined according to the Folin-Ciocalteu method (Zhou et al., 2021).
2.3 Plant growth and fruit quality analysis
Plant height was measured 30 days after transplanting. At harvest, three random plants per treatment were selected. The total yield per plant was determined by harvesting all fruits from each selected plant. The aboveground biomass and roots of the cucumber plants were separately oven-dried at 65 °C until a constant weight was achieved. The vitamin C content was determined using the 2,6-dichloroindophenol titration method. Nitrate content was measured using the salicylic acid method (Zhang et al., 2020). Soluble sugar content was determined by extraction with boiling water followed by analysis using the anthrone colorimetric method (Pasin et al., 2020; Zhao et al., 2017).
2.4 DNA extraction and illumina HiSeq sequencing
Genomic DNA was extracted from soil samples using the E.Z.N.A. Soil DNA Kit (Omega Bio-tek, Inc., USA) according to the manufacturer’s instructions. The quality and concentration of the extracted DNA were confirmed using a Nanodrop 2000 (ThermoFisher Scientific, Inc., USA). Bacterial 16S rRNA gene V3-V4 regions were amplified using the universal primers 38F and 806R (Caporaso et al., 2011). An 8-bp barcode sequence was added to the 5’ end of both the forward and reverse primers to distinguish different samples. The final barcoded primers were used for amplification on an ABI 9700 PCR instrument (Applied Biosystems, Inc., USA). Polymerase chain reaction (PCR) was performed as previously reported (Yang et al., 2019b). Two rounds of PCR amplification were conducted, and the size of the amplified target bands was detected using 1% agarose gel electrophoresis. PCR products were purified using the Agencourt AMPure XP (Beckman Coulter, Inc., USA) nucleic acid purification kit.
2.5 Microbial community analysis
The sequencing data were demultiplexed based on the barcode sequences. The software Pear (v0.9.6) was used to filter and assemble the sequencing data, with a minimum overlap of 10 bp during assembly (Zhang et al., 2014). After assembly, sequences shorter than 230 bp were removed using the Vsearch (v2.7.1) software (Rognes et al., 2016), and chimeric sequences were identified and removed using the uchime method against the Gold Database (Edgar et al., 2011). The uparse algorithm in Vsearch (v2.7.1) was used to cluster high-quality sequences into operational taxonomic units (OTUs) with a sequence similarity threshold of 97% (Edgar, 2013). The representative sequences of OTUs were aligned against the Silva138 database using the BLAST algorithm, with an e-value threshold set at 1e-5, to obtain the taxonomic information for each OTU (Johnson et al., 2008; Pruesse et al., 2007).
Based on the OTU and abundance results, alpha diversity indices were calculated using the QIIME (v1.8.0) software, and plots were generated using R (v3.6.0) software (Caporaso et al., 2010). Bacterial community diversity was analyzed based on the Bray-Curtis dissimilarity index, and principal coordinates analysis (PCoA) plots were generated (Caporaso et al., 2010). For community composition visualization, the top 10 most abundant bacterial phyla were plotted based on their relative abundance; all remaining phyla were grouped into an ‘Other’ category. Biomarker features in each group were screened by Metastats and LEfSe software. Functional profiling of the soil microbial metagenome was predicted from the 16S rRNA gene sequencing data using PICRUSt based on Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.
2.6 Statistical analyses
Given the nested design, data from the three technical pots per biological replicate were averaged, resulting in a sample size of n = 3 per treatment for all statistical tests. All data were statistically analyzed using SPSS 22.0 (SPSS, Inc., Chicago, IL, United States). Significant differences (p < 0.05) among treatments, based on one-way ANOVA followed by Duncan’s test, are indicated by different lowercase letters. All column charts were created using Origin 2024b (OriginLab Corporation, Northampton, MA, USA). Partial least squares path model (PLS-PM) was used to determine the direct and indirect effects of soil factors pH, available N, soil nutrients index (total P, available P, available K), phenolic index (total phenols, complex phenolic, and water-soluble phenolic), properties (SO42-, Na+) and soil bacteria on cucumber growth and quality. The reliability of the model was assessed using the Goodness of Fit (GoF) metric. A GoF value ≥ 0.36 indicates better model alignment (Wetzels et al., 2009).
3 Result
3.1 Effects of biochar and intercropping on cucumber growth
The application of biochar (B), intercropping with Paspalum vaginatum (S), and their combination promoted cucumber plant growth compared to CK (Figure 1). Plant height was significantly increased by treatments B and BS compared to CK, with the BS treatment achieving the greatest height among all treatments (Figure 1a). In contrast, above-ground biomass did not differ significantly among treatments (Figure 1b). Total root biomass increased by 11.19% (B), 10.92% (S), and 21.47% relative to CK (Figure 1c). Of these, only the increase in the BS treatment was statistically significant. Cucumber yield was also significantly increased by all treatments relative to CK, by 11.50% (B), 8.18% (S), and 27.12% (p < 0.05; Figure 1d).
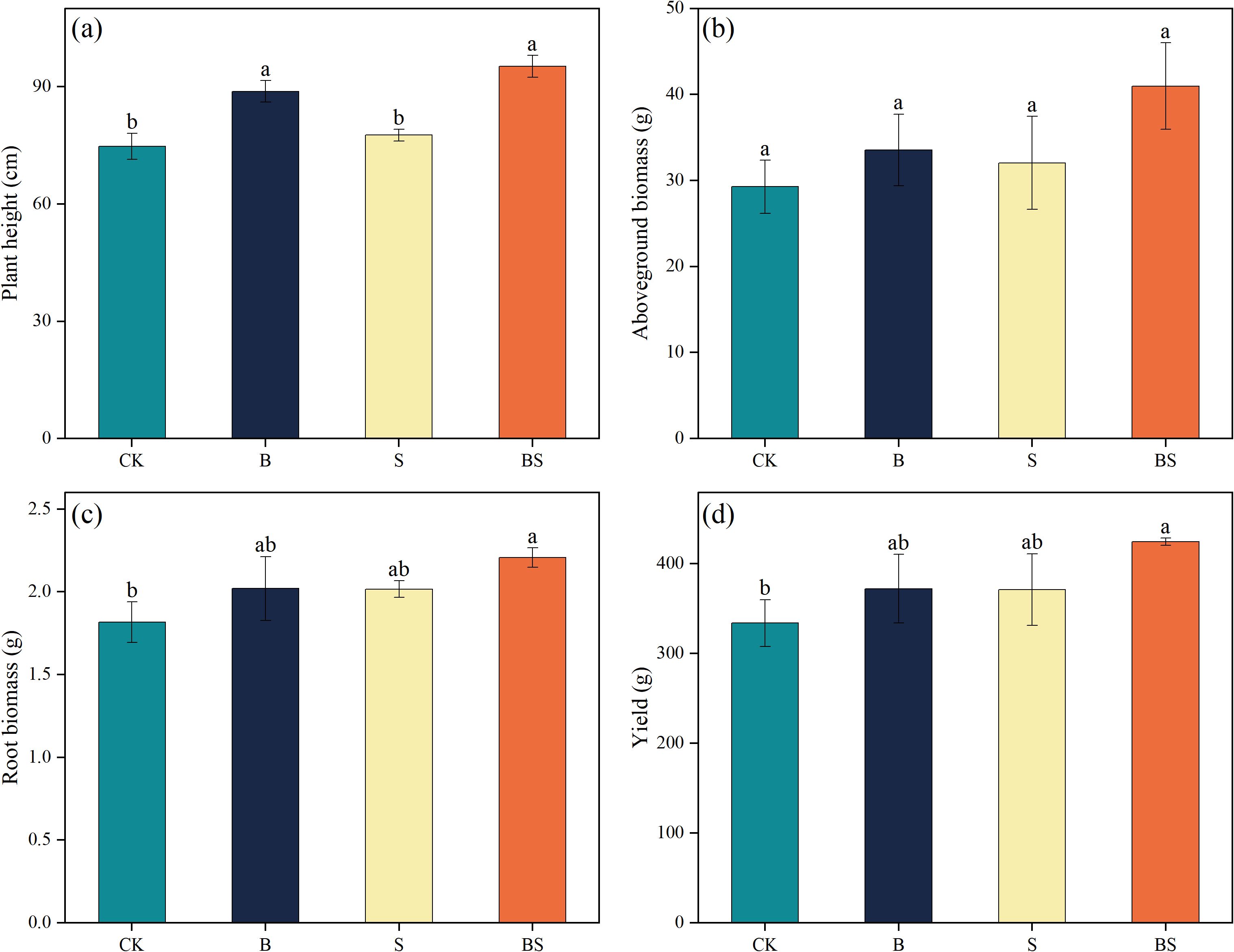
Figure 1. Effects of biochar and intercropping on plant height (a), above-ground biomass (b), below-ground biomass (c), and yield (d) of continuously cropped cucumbers. Each value represents three biological replicates (± SD) (n = 3). Different lowercase letters indicate significant differences among treatments at the p < 0.05 level.
3.2 Effects of biochar and intercropping on cucumber quality
Continuous cropping negatively affected cucumber fruit quality, an effect that was significantly ameliorated by biochar addition, intercropping, and particularly their combination (Figure 2). Compared to CK, the treatments B, S, and BS significantly increased the average content of soluble protein in cucumber fruits by 27.49%, 25.89%, and 37.73%, respectively (p < 0.05) (Figure 2a). No significant differences were observed among the three amendment treatments. Water-soluble sugar content exhibited a similar trend, with values ranking as S < B < BS (Figure 2b). The S and B treatments increased sugar content by 10.56% and 10.35%, respectively, compared to CK, but these increases were not statistically significant. In contrast, the BS treatment produced a significant 11.59% increase (Figure 2b). Vitamin C content was significantly increased by 15.25% in the BS treatment compared to CK (Figure 2c). All amendment treatments significantly reduced the fruit nitrate content relative to CK (145.45 mg kg-¹), with values decreasing to 83.65 (B), 93.32 (S), and 74.44 mg kg-¹ (p < 0.05; Figure 2d).
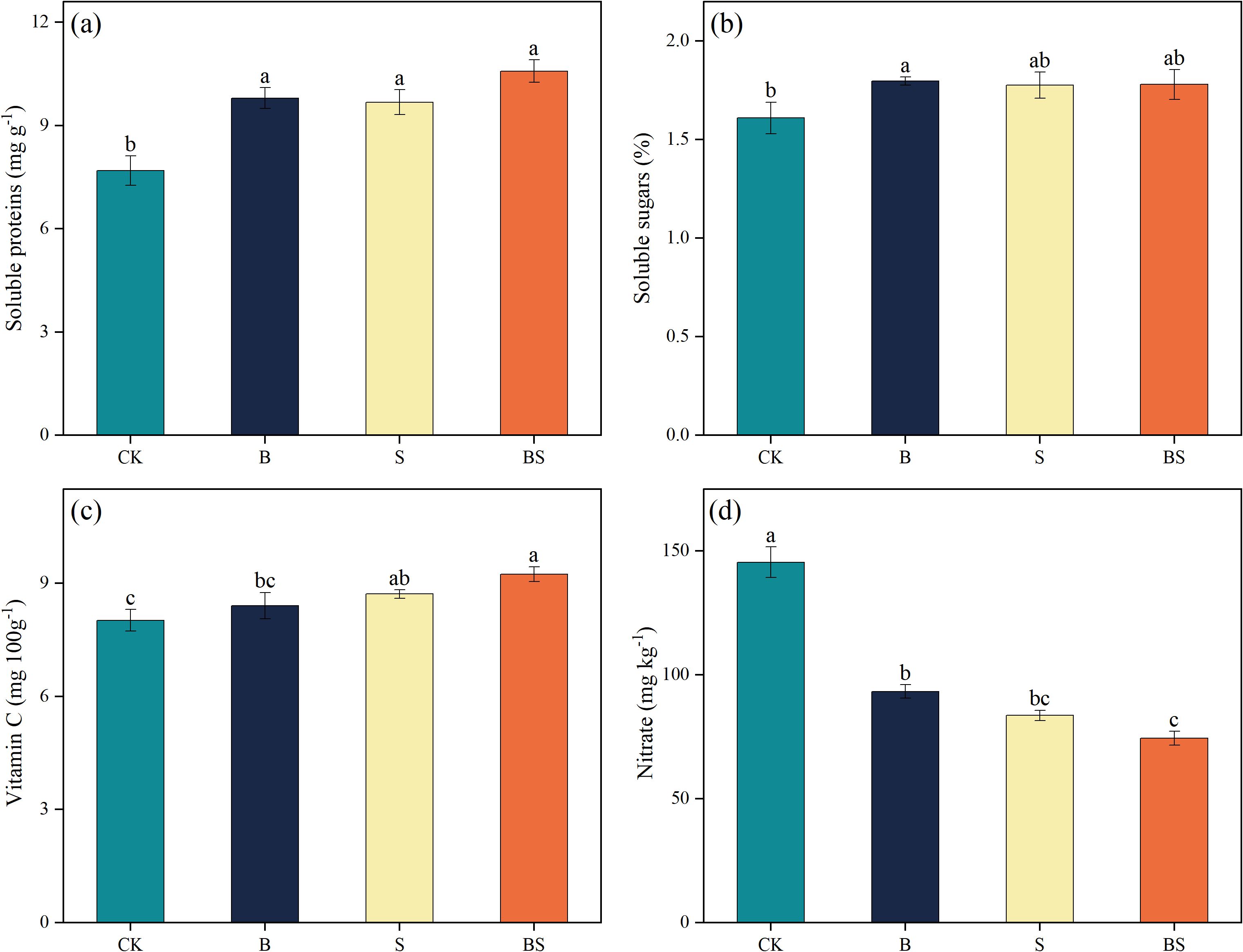
Figure 2. Effects of biochar and intercropping on the soluble protein (a), soluble sugar (b), vitamin C (c), and nitrate content (d) in fruits of continuously cropped cucumbers. Each value represents three biological replicates (± SD) (n = 3). Different lowercase letters indicate significant differences among treatments at the p < 0.05 level.
3.3 Effects of biochar and intercropping on soil phenolic
The application of biochar and intercropping significantly reduced the content of phenolic compounds in the soil (Figure 3). Total phenolic acid content in the cucumber rhizosphere soil was significantly decreased in all treatments relative to CK (p < 0.05; Figure 3a). The greatest reduction (29.66%) was observed in the BS treatment, although this value was not statistically different from that in the S treatment. The content of complex phenolics in cucumber roots was significantly reduced by 41.27% (B), 36.89% (S), and 45.73% compared to CK (Figure 3b). Similarly, the water-soluble phenolic content was significantly reduced by 17.10% (B), 11.90% (S), and 31.04% (Figure 3c).
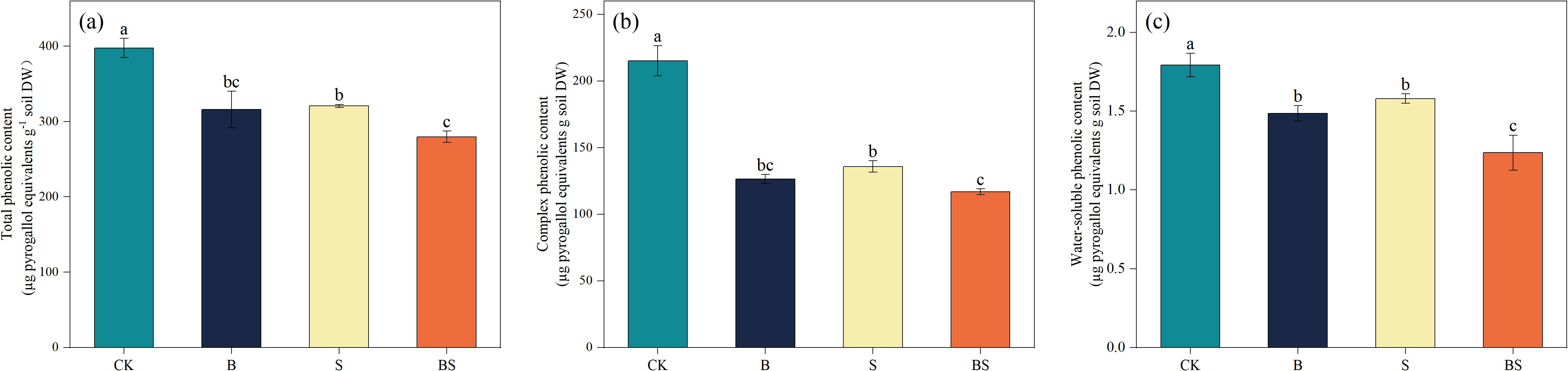
Figure 3. Effects of biochar and intercropping on total phenolics (a), complex phenolics (b), and water-soluble phenolics (c) in continuously cropped soils. Each value represents three biological replicates (± SD) (n = 3). Different lowercase letters indicate significant differences among treatments at the p < 0.05 level.
3.4 Effects of biochar and intercropping on soil salinity
All amended treatments significantly reduced the electrical conductivity (EC) of the cucumber rhizosphere soil compared to CK. The BS treatment resulted in the greatest reduction (14.09%), followed by B (13.41%) and S (8.86%). The concentrations of Na+, SO4²-, and Cl- were all significantly lowered by the amendments relative to CK (p < 0.05). The reduction in SO4²- content ranged from 25.64% to 45.45% across treatments.For Cl- and Na+, the BS treatment showed the greatest reductions (31.75% and 27.93%, respectively), followed by the B treatment (21.76% and 21.61%). The S treatment resulted in the smallest decreases (11.24% for Cl- and 16.23% for Na+). The reductions achieved by the B and S treatments were not significantly different from each other. In contrast, the effects on cation concentrations (Ca²+, K+, Mg²+) were more variable. The S treatment significantly decreased Ca²+ and K+ levels relative to CK, but had no significant effect on Mg²+. Conversely, the B treatment significantly increased the concentrations of all three cations. The BS combination treatment resulted in cation concentrations that were not significantly different from those in the CK soil.
3.5 Effects of biochar and intercropping on soil chemical properties
The application of biochar and intercropping significantly altered several soil chemical properties, including pH, SOC, TN, AP, NH4+ and NO3- (Table 1). The rhizosphere soil pH increased significantly in the B (to 7.07) and BS (to 6.85) treatments compared to the control (CK, 5.57), with increments of 1.50 and 1.28 units, respectively. In contrast, the increase observed in the S treatment (to 5.93, an increase of 0.36 units) was not statistically significant. The SOC content was significantly increased by the B and BS treatments compared to CK. In contrast, the S treatment had no significant effect on SOC. Interestingly, TN content was significantly increased by all treatments. Conversely, NO3- content was significantly reduced compared to CK (p < 0.05). Meanwhile, no significant differences were observed in the TP content among all treatments. AP increased significantly by 32.08%, 13.85%, and 34.03% respectively compared to CK. Similarly, the AK content was significantly higher than CK only in the B and BS treatments (p < 0.05).
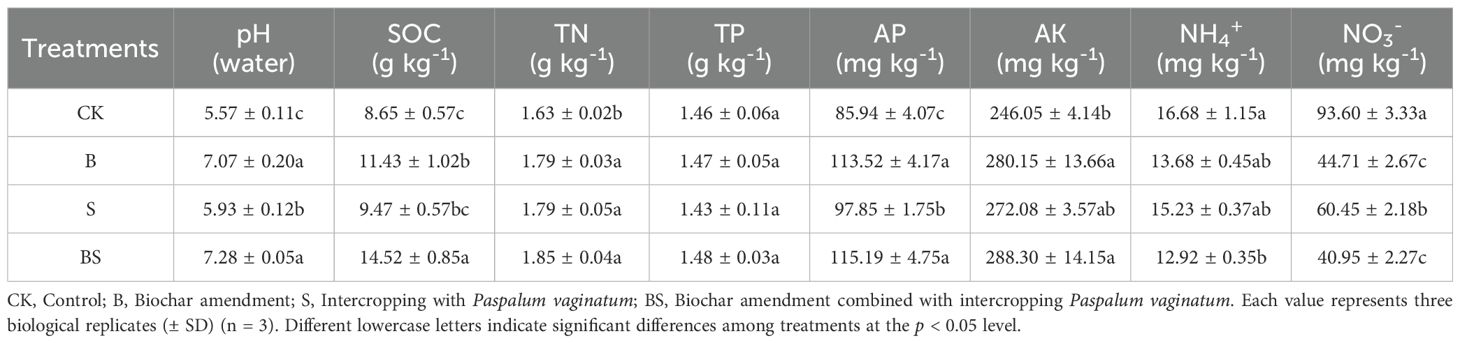
Table 1. Effects of biochar and intercropping on the chemical properties of continuously cropped soils.
3.6 Effects of biochar and intercropping on soil bacterial diversity and community structure
The CK treatment resulted in the lowest observed species richness, which was significantly lower than that in the B and BS treatments (p < 0.05; Figure 4a). A similar trend was observed for phylogenetic diversity (PD), with CK also being significantly lower than B and BS (Figure 4b). In contrast, the Chao1 index did not differ significantly among treatments (Figure 4c). The Shannon index, a measure of community diversity, was highest in the BS treatment and was significantly greater than in all other treatments (Figure 4d). Principal coordinates analysis (PCoA) based on OTU profiles (97% similarity) revealed clear separation in bacterial community structure among the treatments. The PCoA plot showed a clear separation along PC1, with the B and BS treatments clustering separately from the CK and S treatments (Figure 5).
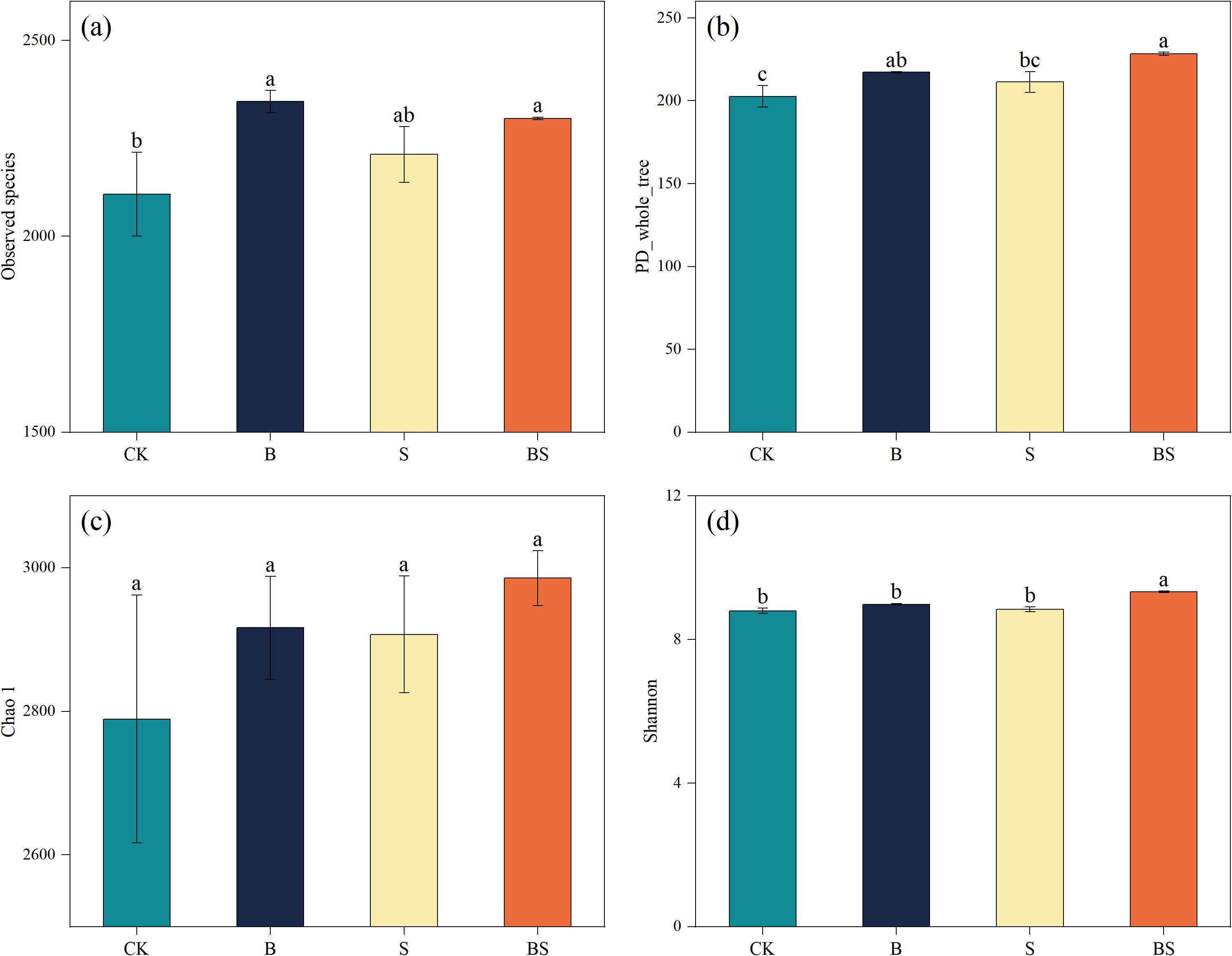
Figure 4. Effects of biochar and intercropping on soil bacterial observed species (a), PD whole tree (b), Chao1 (c), and Shannon index (d). Each value represents three biological replicates (± SD) (n = 3). Different lowercase letters indicate significant differences among treatments at the p < 0.05 level.
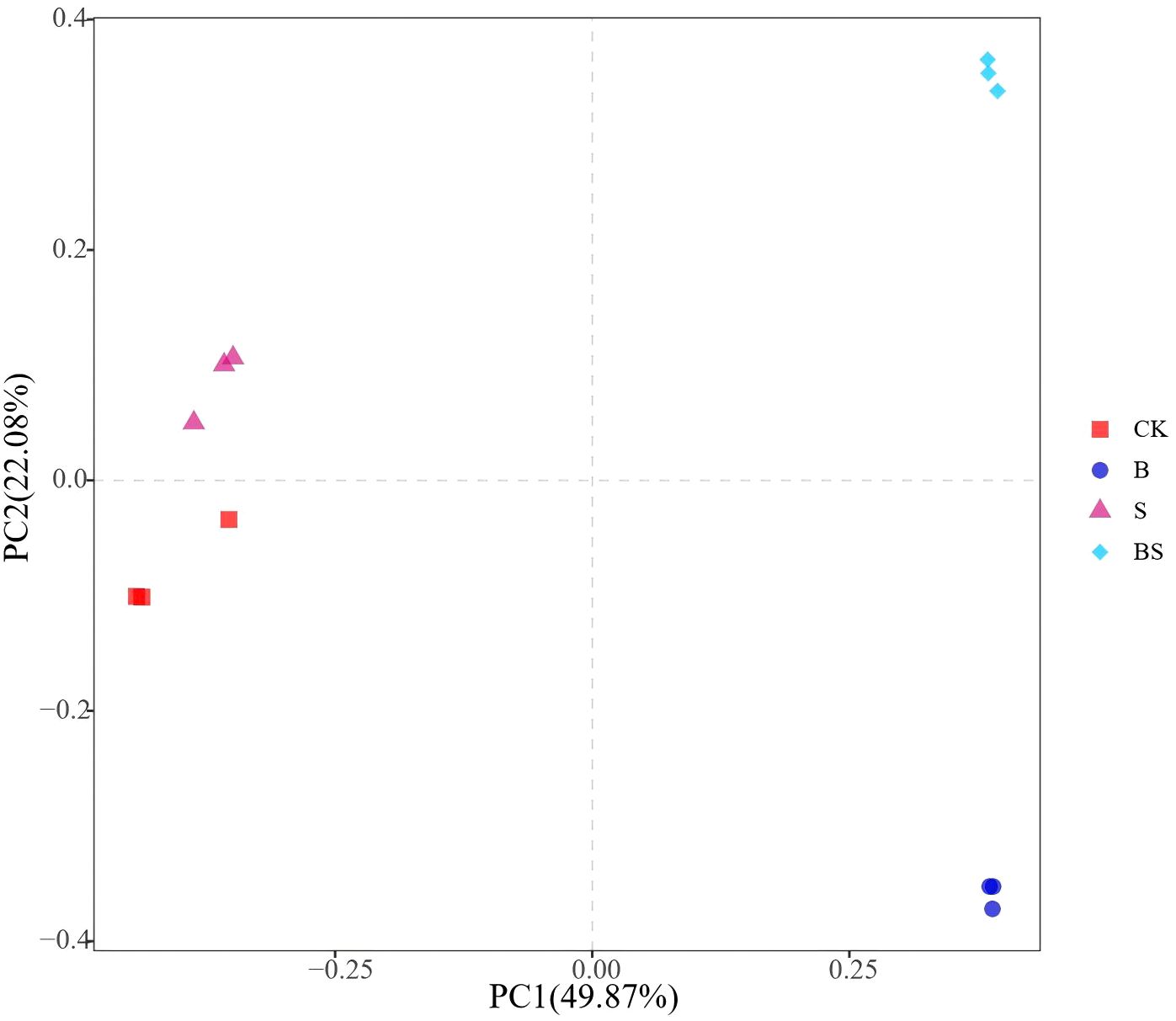
Figure 5. Structure of soil bacterial communities affected by biochar and intercropping. Bacterial community analysis based on Bray-Curtis dissimilarity. Different colored shapes represent different treatments. The distance between samples represents the degree of difference.
3.7 Effects of biochar and intercropping on the composition of soil bacterial communities
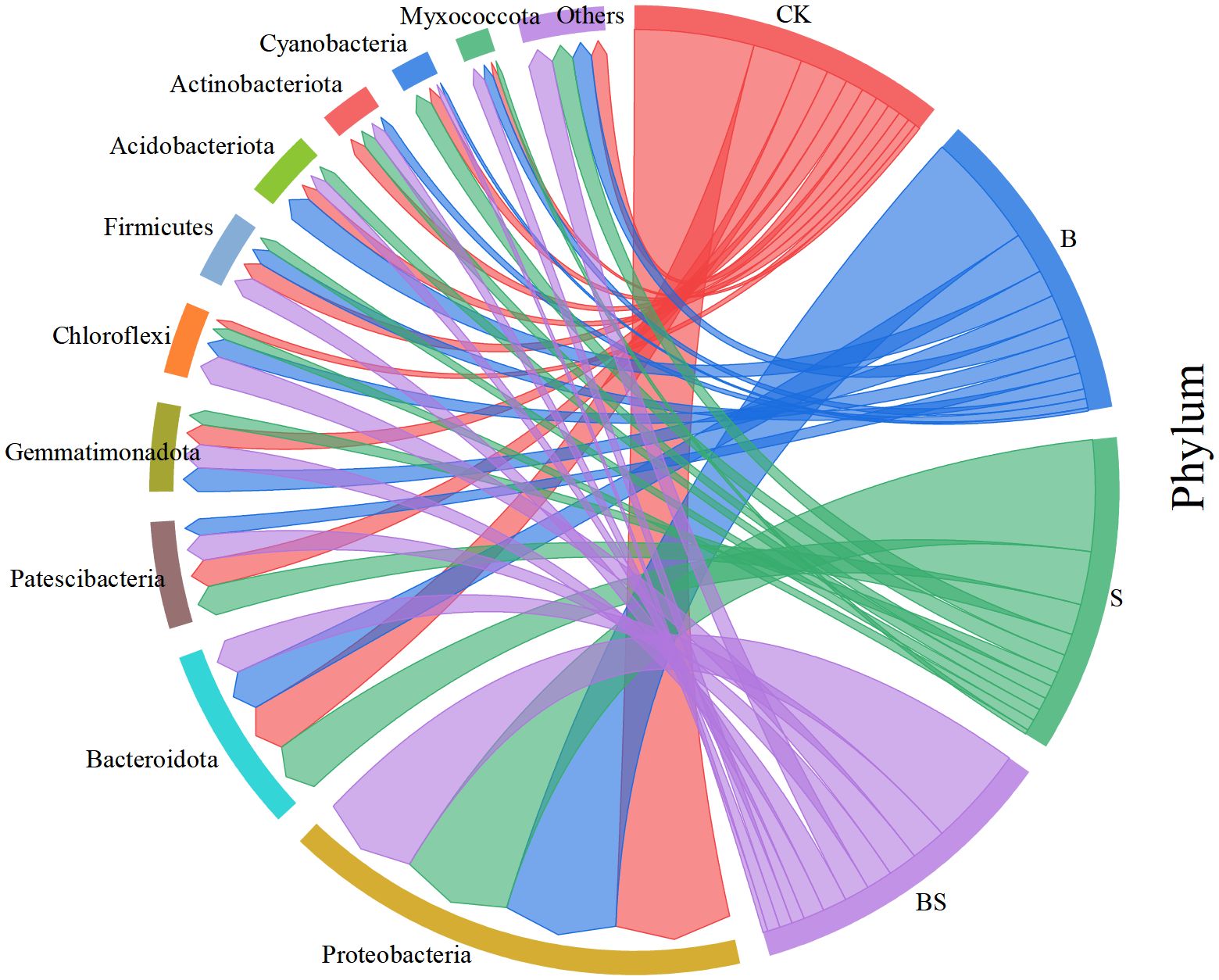
The composition of soil bacterial communities was significantly altered by the amendments at the phylum level (Figure 6). Despite treatment effects, the overall phylum-level profile was similar across all samples. The dominant phyla, collectively accounting for 92.87% of sequences, were Proteobacteria, Bacteroidota, Patescibacteria, Gemmatimonadota, Chloroflexi, Firmicutes, Acidobacteriota, Actinobacteriota, and Cyanobacteria. The relative abundance of Proteobacteria was significantly higher in the CK treatment than in the BS treatment. In contrast, the relative abundances of Gemmatimonadota, Bacteroidota, and Chloroflexi were lower in the CK treatment than in the amended treatments. The S treatment was associated with the highest relative abundances of Patescibacteria and Cyanobacteria. The relative abundance of Actinobacteriota was significantly lower in all amended treatments (B, S, BS) compared to the CK treatment.
3.8 Relationships between soil properties and cucumber yield and quality
The partial least-squares path model (PLS-PM) was used to explore the dominant factors affecting cucumber yield and quality (Figure 6). Cucumber yield was significantly affected by soil variables, with soil pH having a substantial positive impact, while available N, salt properties, and phenolic acids had significant negative effects (Figure 6a). In contrast, the influence of soil bacterial diversity on cucumber yield was relatively minor. Unlike yield, soil bacterial diversity and salt properties had direct and significant positive and negative effects on soluble sugar content, respectively (Figure 6b). Soil pH and nutrient availability indirectly enhanced the soluble sugar content of cucumbers, whereas available N had the opposite effect. Similar to soluble sugar, available N, salt properties, and phenolic acids were negatively correlated with soluble protein and vitamin C content in cucumbers, while pH had the opposite effect. Additionally, Vc content was significantly influenced by positive effects from microbial diversity and soil nutrient availability, and a negative effect from salt properties (Figures 6c, d). However, microbial diversity was not a primary driver of nitrate content in cucumbers. Instead, nitrate content in cucumbers was significantly and positively influenced by available N and phenolic acids (Figure 6e).
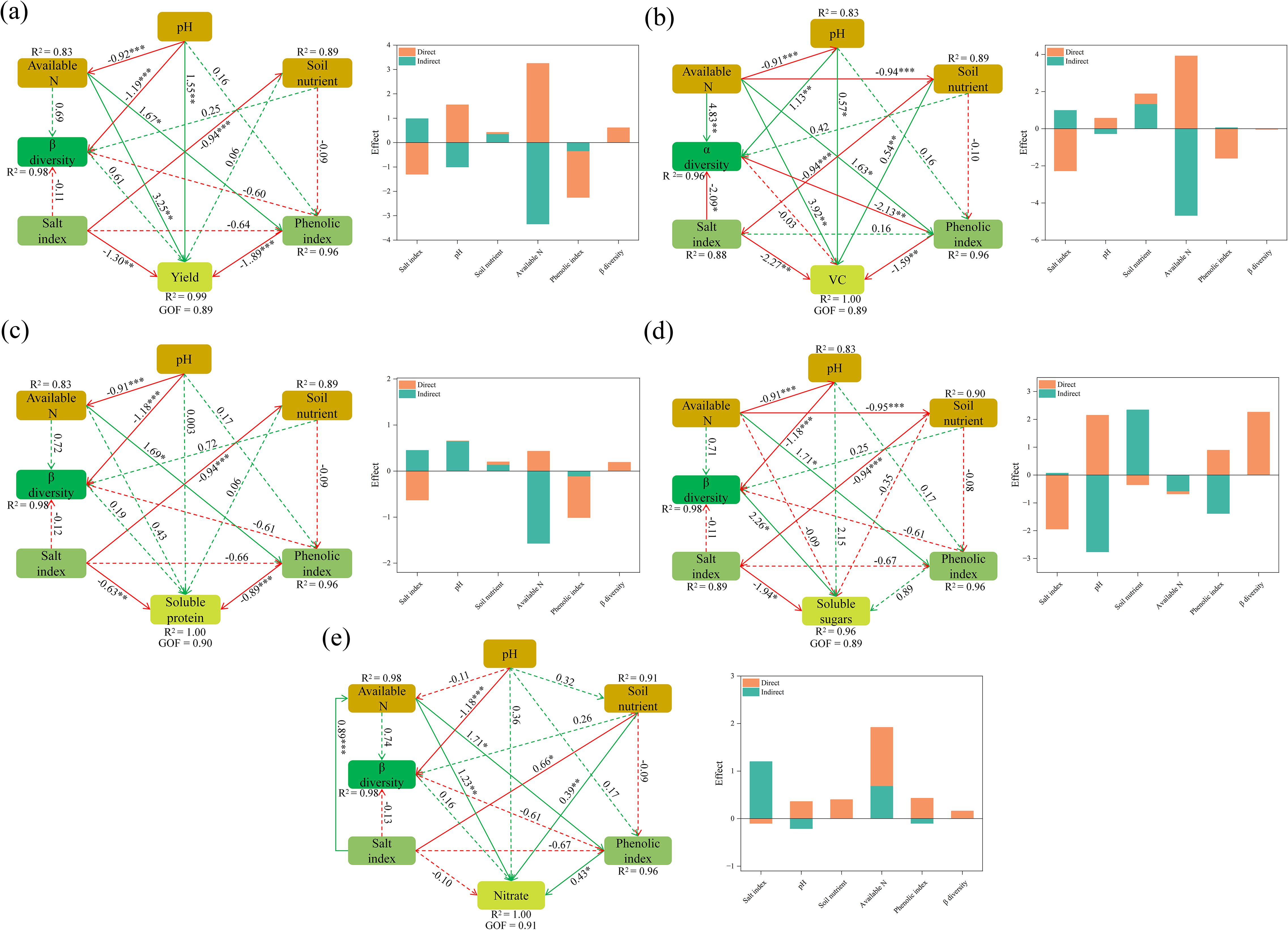
Figure 6. The partial least-squares path model (PLS-PM) showing the effects of biochar and intercropping on cucumber yield (a), soluble sugar (b), soluble protein (c), vitamin C (Vc) (d), and nitrate (e). Green and red solid lines represent significant positive and negative effects, respectively, while dashed lines indicate non-significant effects.
4 Discussion
To address the progressive degradation of greenhouse soil health caused by continuous cropping and to promote sustainable agricultural production, various techniques such as soil fumigation, soil replacement, and grafting have been proposed to mitigate the obstacles associated with continuous cropping (Ding et al., 2021). However, these methods may increase production costs, cause environmental damage, and reduce crop quality, whereas the use of biochar or intercropping avoids these disadvantages (Jesus et al., 2024; Wang et al., 2021a). Therefore, to reduce improvement costs and minimize environmental impact, we integrated biochar with intercropping to investigate the mechanisms through which this combination alleviates soil constraints in continuous cropping systems. In this study, the combined application of biochar and intercropping significantly alleviated the degradation of soil quality caused by continuous cropping obstacles. The application of biochar, both alone and in combination with intercropping, modified the soil’s biochemical properties, thereby directly or indirectly enhancing the yield and quality of cucumbers in continuously cropped soil.
4.1 Effect of biochar and intercropping on soil physicochemical properties
Long-term continuous cropping, coupled with excessive and imbalanced fertilizer inputs, leads to decreased soil pH, secondary salinization, and nutrient imbalances (Gong et al., 2024; Zheng et al., 2021). These phenomena are recognized as major factors contributing to continuous cropping obstacles in greenhouse production systems (Bai et al., 2020; Zhang et al., 2021). Consequently, persistent soil degradation markedly diminishes the productive capacity of agricultural land and threatens the sustainability of crop production (Li et al., 2024; Yan et al., 2024). In this study, owing to its inherent alkalinity and high organic carbon content, biochar application—both alone and combined with intercropping—effectively increased soil pH and SOC content (Table 1) (Liu et al., 2014; Zhang et al., 2010). Previous research has demonstrated a negative correlation between soil available N content and pH (Liu et al., 2023b). This relationship may be attributed to enhanced ammonification, which consumes H+ ions and consequently elevates soil pH (Xu et al., 2006). These processes contribute to creating a more favorable soil environment for plant growth. In continuous cropping systems, the availability of phosphorus (AP) and potassium (AK) often declines, whereas available N frequently accumulates, resulting in severe nutrient imbalances (Li et al., 2017; Liu et al., 2023b). Our findings showed that biochar amendment and intercropping can ameliorate soil quality by enhancing AP and AK availability, a phenomenon corroborated by previous studies (Table 1) (Wang et al., 2024c; Zhou et al., 2021). Interestingly, both biochar application and intercropping also significantly reduced the soil NO3- content (Table 1). The observed reduction in soil available N could potentially be attributed to the strong adsorption capacity of biochar, which may immobilize mineral nitrogen, coupled with the increased AN uptake by the intercropped plants, but may also alter the N-cycling mediated by microbes, such as ammonia-oxidizing bacteria (AOB) and archaea (AOA) (Lehmann et al., 2021; Xiang et al., 2024). Further research into microbe-involved nitrogen cycling processes is needed to reveal the mechanisms of N cycling mediated by biochar. The reduction in available N, particularly in the form of NO3-, might initially appear detrimental, excessively high levels of NO3- are in fact phytotoxic—a common scenario in greenhouse production systems (Zhang et al., 2021). The strong effect of biochar or intercropping in reducing available N content may be more effective when applied to greenhouse continuous cropping soils degraded by excessive nitrogen fertilizer application. Thus, both biochar and intercropping play crucial roles in rebalancing soil nutrient dynamics (Hauggaard-Nielsen and Jensen, 2005). Furthermore, compared to CK, the soil phenolic acid content decreased significantly across all amendment treatments, with the most substantial reduction observed in the BS treatment (Figure 3). This indicates that both biochar and intercropping directly or indirectly influence the soil micro-ecological environment, ultimately affecting cucumber yield and quality. The mitigation of autotoxic effects on cucumbers by biochar may be attributed to its high porosity, substantial adsorption capacity, and large specific surface area, which likely facilitate the adsorption of phenolic acids. Additionally, biochar-induced alterations in the soil microbial community might contribute to the degradation of these autotoxic substances (Yang et al., 2019a). Similarly, intercropping reduces phenolic acid levels,which may be due to the root exudates from Paspalum vaginatum that enhance nutrient availability and stimulate microbial activity, thus alleviating their autotoxic impact on cucumbers (Li et al., 2020). In conclusion, our results indicate that integrating biochar with intercropping may serve as a promising strategy for improving soil physicochemical properties and mitigating continuous cropping obstacles in intensive cultivation systems, warranting further validation under field conditions.
4.2 Effect of biochar and intercropping on soil salinity
Soil properties were influenced by the application of biochar and intercropping. As a halophyte, Paspalum vaginatum exhibits a high degree of salt tolerance, with certain ecotypes capable of withstanding salt concentrations equivalent to 80% of that in seawater (Lee et al., 2004). Consistent with its halophytic nature, intercropping with Paspalum vaginatum significantly reduced the levels of soluble salt ions and soil electrical conductivity (EC) (Table 2). This reduction is likely attributable to the pronounced capacity of Paspalum vaginatum to uptake and sequester salt ions, accumulating them in both root and shoot tissues (Guo et al., 2016; Hu et al., 2020). This mechanism is supported by similar findings for other species; for instance, the high salt-absorption potential of alfalfa has been shown to reduce soil salt accumulation and consequently lower EC (Su et al., 2024). Similarly, biochar ameliorates salinity through distinct mechanisms. Its surface, characterized by both positive and negative charges and a diversity of functional groups, facilitates the adsorption of various salt ions, thereby reducing soil salinity (Farhangi-Abriz and Ghassemi-Golezani, 2021; Rajapaksha et al., 2016). Our results showed that biochar application significantly reduced the concentrations of Na+, SO4²-, and Cl-, while concurrently increasing the levels of Ca²+ and Mg²+ (Table 1) (Jin et al., 2024; Zhang et al., 2022a). These observed ionic shifts are consistent with a potential cation exchange process, wherein the release of divalent cations (Ca2+ and Mg2+) from biochar could promote the displacement of Na+ from soil exchange sites into the soil solution, perhaps facilitating its leaching from the root zone (Agbna et al., 2017; Cui et al., 2021). Furthermore, the combined application of biochar and intercropping elicited a more pronounced reduction in key soil salinity indicators compared to biochar alone, pointing to a potential synergistic effect. This enhanced efficacy could be due to several interconnected factors. For instance, biochar-induced improvements in soil physical properties, such as porosity and moisture retention (Qian et al., 2020; Wang et al., 2022a), likely create a more favorable rhizosphere environment. This, in turn, may bolster the capacity of Paspalum vaginatum to absorb, sequester, or tolerate salts. Additionally, modifications to the microbial community by biochar or changes in root exudation patterns due to intercropping might also contribute to this synergistic salinity mitigation.
4.3 Effect of biochar and intercropping on the yield and quality of cucumbers
Continuous cropping obstacles inhibit crop growth and severely reduce crop yield and quality (Li and Cai, 2016; Liao et al., 2018). Previous studies have explored the potential of biochar application or intercropping to alleviate continuous cropping obstacles and thereby improve crop yield and quality (Hu et al., 2020; Zhang et al., 2023). However, it remains unclear whether the combined application of biochar and intercropping exerts a synergistic effect on improving crop growth under continuous cropping systems and on enhancing soil health. Enhancing crop yield and quality is a major objective in agricultural production (Souza et al., 2025; Wu et al., 2025). In this study, the combined application of biochar and intercropping significantly enhanced cucumber growth and increased yield (Figure 1). Studies have indicated that biochar can alleviate soil degradation induced by continuous cropping and excessive fertilization, while also enhancing crop yields (Cui et al., 2021; Zhang et al., 2023). Biochar is rich in mineral elements and possesses a large specific surface area and high adsorption capacity. These properties can reduce NO3- but increase AP and AK, thereby helping to balance soil nutrient status (Li et al., 2023). Moreover, biochar application can improve the soil microbial environment and enhance microbial activity, thereby promoting plant growth (Li et al., 2023; Lv et al., 2023). The positive effects of intercropping on crop yield can be attributed to multiple mechanisms. Studies have shown that intercropping can enhance the availability of soil nutrients, promote leaf photosynthesis, and facilitate fruit set and development (Wang et al., 2022b). Furthermore, intercropping with halophytes can ameliorate soil salinity conditions, thereby providing a more favorable low-salt environment for crop growth (Simpson et al., 2018). Additionally, our results showed that the combined application of biochar and intercropping increased the contents of soluble sugars, soluble proteins, and vitamin c in cucumber fruits, while significantly reducing fruit nitrate content (Figure 2). This indicates that both the organic amendment (biochar) and intercropping have positive effects on fruit quality. Due to its high stability, biochar allows for the slow and continuous release of nutrients, which promotes balanced nutrient uptake and utilization by crops, ultimately enhancing fruit quality (Naeem et al., 2018; Zhao et al., 2017). Previous studies have reported similar findings: in cucumber continuous cropping systems, biochar amendment improves the crop growth environment by mediating soil nutrient balance and reducing salinity, thereby promoting growth and increasing both yield and quality (Wang et al., 2021a; Zhou et al., 2021). Consistent with our findings, studies have shown that intercropping with halophytes also improves soil physicochemical properties and reduces soil salinity, thereby creating a more favorable growth environment for crops (Jurado et al., 2024; Slatni et al., 2024).
4.4 Effect of biochar and intercropping on soil bacterial communities
The application of biochar and intercropping significantly influenced soil microbial diversity and composition (Figures 4, 5), which are crucial for nutrient cycling and plant health in agricultural ecosystems. Soil acidification and salinization resulting from continuous cropping profoundly alter microbial community structure and suppress their metabolic activities (Ji et al., 2022; Marcheva et al., 2024). The community of microorganisms is primarily impacted by nutrients, pH, and salinity (Banda et al., 2021). In this study, the combined application of biochar and intercropping not only significantly increased soil microbial diversity (Figures 4, 5), but also induced a profound functional reshaping of the microbial community.
The LEfSe analysis provided tangible evidence for this structural shift, identifying specific bacterial taxa that were significantly enriched in the BS treatment (Supplementary Figure S1). These biomarkers included families within the Actinobacteriota, such as Micromonosporaceae and Nocardiopsaceae, which are renowned for their capacity as prolific producers of antibiotics and hydrolytic enzymes (Mitra et al., 2021). Crucially, this taxonomic shift was directly reflected in the functional potential predicted by PICRUSt2 (Supplementary Figure S2). The BS treatment exhibited a significant enhancement in the “Neomycin, kanamycin and gentamicin biosynthesis” pathway. This coherent finding strongly suggests that the BS combination does not merely increase microbial abundance but specifically enriches for keystone taxa within the actinobacterial community and upregulates their antibiotic synthesis potential, thereby constructing a more robust biological defense line against soil-borne pathogens—a core challenge in continuous cropping systems.
Concurrently, the PICRUSt2 results revealed a significant reduction in “Bacterial chemotaxis” under BS treatment (Supplementary Figure S2). This functional shift holds important ecological implications, as chemotaxis is an energy-costly behavior for microorganisms seeking resources in oligotrophic or stressful environments (Wadhams and Armitage, 2004). Its suppression likely indicates that the BS-amended soil offers a more favorable and less stressful microenvironment with improved nutrient accessibility (Tables 2, 1), allowing microbes to allocate more energy from motility to growth and beneficial metabolite production. This notion is further supported by the significant enrichment of “D-Alanine metabolism” and “D-Glutamine and D-glutamate metabolism” pathways, which are integral to bacterial cell wall synthesis and osmotic stress regulation (Cava et al., 2011; Waldemar et al., 2008), indicating a microbial community with enhanced growth activity and resilience under the BS regime. This finding aligns with previous studies showing that while salinity adversely affects bacterial community composition, the application of biochar or intercropping under salt stress mitigates these effects and exerts favorable effects on bacterial structure (Szoboszlay et al., 2019; Xie et al., 2022). These practices likely enhance microbial diversity by supplying nutrients and creating additional ecological niches for beneficial microorganisms (Fu et al., 2017). Furthermore, the increased diversity may enhance functional resilience, allowing the microbial community to maintain activity under a wider range of environmental conditions through niche adaptation and functional redundancy (Garcia-Garcia et al., 2019). Therefore, by enhancing microbial diversity, the biochar-intercropping combination fosters a more robust soil microenvironment and ensures the stability of ecosystem functions mediated by microbes. PCoA shows that the microbial communities of CK and BS treatments are clearly separated (Figure 5). This shift in community composition is consistent with previous findings that plant-soil interactions can greatly alter microbial community structures (Jin et al., 2020; Zhou et al., 2021). These community changes are functionally significant, as rhizosphere microbes are key agents in plant nutrient acquisition, soil structure formation, and the production of regulatory exometabolites (Chi et al., 2023; Tong et al., 2024). Thus, by reshaping the soil microbial composition, biochar and intercropping indirectly promote plant growth and health (Wang et al., 2024a). Specifically, the BS treatment significantly increased the relative abundance of several bacterial phyla, including Actinobacteriota, Gemmatimonadota, and Chloroflexi (Figure 7). The proliferation of these oligotrophic taxa may be driven by the moderated soil nitrogen availability (Wang et al., 2024b). These phyla are integral to soil nutrient cycling, facilitating the transformation of carbon, nitrogen, and phosphorus through the secretion of various enzymes and metabolites, thereby promoting plant growth (Morrissey et al., 2023; Mujakic et al., 2023; Rao et al., 2022). The overall improvement in soil nutrient supply capacity (Bandara et al., 2022) and the elevated pH are likely contributing factors to the success of these bacterial groups. It is noteworthy that Acidobacteriota, which are often considered acidophilic, dominated the CK treatment with lower pH (Table 1). Previous studies suggested that Acidobacteria played an important role in biogeochemical cycling of carbon and consequently might be adaptable to the environment of large variety of carbon sources present in biochar (Zhong et al., 2024). However, the soil showed a more alkaline environment after biochar amendment, which is not favorable for Acidobacteria, a phylum of bacteria usually being acidophilic (Lehmann et al., 2011). As a result, the abundance of Acidobacteria decreased with biochar addition. Notably, Actinobacteria not only produce antibiotics to suppress plant pathogens and decompose organic matter but also reduce bacterial wilt in plants. They exhibit accelerated growth in nutrient-rich environments (Lee et al., 2021), and the application of biochar improves soil conditions, thereby stimulating the proliferation of these beneficial microorganisms. Biochar itself provides a physical refuge and a slow-release carbon source for microorganisms, while the root exudates from the intercropped Paspalum vaginatum supply easily degradable carbon sources for specific microbial taxa. It is plausible that the release of phenolic compounds from the aromatic carbon structures of biochar, facilitated by Actinobacteria, may further stimulate the abundance and activity of microbial groups capable of decomposing phenolic acids (Kolton et al., 2017), thereby potentially alleviating their adverse effects on plants. Furthermore, the improved treatments reduced the relative abundance of potentially pathogenic Proteobacteria (Tan et al., 2017), while enriching for beneficial genera such as Gemmatimonas, which is known to suppress Fusarium wilt and promote plant growth (Shen et al., 2024; Zheng et al., 2024; Zhong et al., 2024). Furthermore, studies have shown that biochar and intercropping can alleviate the inhibitory effects of salt ions on microorganisms, promoting the growth and metabolism of dominant salt-tolerant bacteria such as Bacteroidota and Actinobacteriota (Wang et al., 2024d). This shift in the microbial balance, from a community dominated by potential pathogens and acidophiles (e.g., Acidobacteriota in low-pH CK) to one enriched with beneficial taxa identified by LEfSe (e.g., antibiotic-producing Actinobacteriota) and functionally equipped for nutrient cycling and stress resistance (as per PICRUSt2), likely contributed significantly to the observed enhancement in plant growth by simultaneously mitigating soil-borne diseases and improving soil fertility.
Our PLS-PM analysis identified the amelioration of soil physicochemical properties, not bacterial diversity, as the primary driver for enhanced cucumber yield. This can be attributed to several factors: First, the rapid alleviation of abiotic stressors (e.g., Al3+ toxicity, salinity) via increased pH and adsorption provided immediate physiological benefits, outweighing the more gradual effects of microbial shifts (Lehmann et al., 2011). Second, biochar and intercropping primarily improved the physicochemical environment, which subsequently steered the microbial community as a secondary effect (Lehmann et al., 2011). Third, under the severe abiotic stress of the degraded soil, directly mitigating these constraints was a prerequisite for plant growth, a role in which physicochemical amelioration is inherently faster and more direct than microbial mediation (Ranjbari et al., 2022). Consequently, the direct improvement of soil physicochemical properties emerged as the dominant mechanism in this short-term pot study.
To identify the key drivers influencing cucumber yield and quality, a partial least-squares path modeling (PLS-PM) analysis was performed (Figure 6). The results indicated that soil pH and soil nutrition play significant positive roles in enhancing cucumber yield and quality, while they are negatively correlated with soil properties, available N content, and phenolic acid concentrations. The PLS-PM results suggest that biochar amendment and intercropping likely enhance cucumber yield and quality primarily by mitigating these soil stressors (e.g., salinity, excessive AN, and phenolic acids). This finding is consistent with previous studies reporting that soil amendments and intercropping systems can alleviate soil salinity and phenolic acid toxicity, thereby promoting crop growth (Hu et al., 2020; Zhou et al., 2021). In contrast, microorganisms have a relatively minor impact on cucumber yield and fruit quality. Therefore, we propose that the enhancement of cucumber yield and quality is primarily a result of the direct improvement of soil physicochemical properties by biochar and intercropping, rather than being predominantly mediated through changes in the microbial community.
While this pot experiment provides compelling evidence for the synergistic benefits of combining biochar with halophyte intercropping under controlled conditions, it is important to acknowledge its limitations. The pot scale restricts the extrapolation of our findings directly to field-scale agricultural systems. Field environments are subject to greater heterogeneity in soil properties, climate variability, and larger-scale management practices, which could modulate the efficacy of the combined treatment. Therefore, our results should be interpreted as a proof-of-concept, demonstrating the strong potential of this integrated strategy for mitigating continuous cropping obstacles. The promising outcomes observed here justify and necessitate future long-term field trials to validate these findings, optimize application rates (e.g., biochar dosage, intercropping density), and assess the economic viability and large-scale practicality of this approach for sustainable greenhouse vegetable production.
5 Conclusions
The application of biochar, both alone and in combination with intercropping, effectively improved soil health, leading to enhanced yield and quality of cucumbers. Both biochar and intercropping significantly increased soil pH, SOC, TN, AP, and AK. In contrast, these treatments significantly reduced the soil NO3- content. Additionally, the concentrations of phenolic acids and soil salinity were significantly reduced. Among all treatments, the combined application of biochar and intercropping proved to be the most effective strategy for alleviating soil acidification and salinity, reducing phenolic acids, regulating nutrient balance, increasing SOC, enhancing bacterial diversity, and ultimately improving cucumber yield and quality. Partial least-squares path modeling (PLS-PM) results demonstrated that soil pH and nutrient availability had direct positive effects on cucumber yield and quality, whereas phenolic acids, salinity, and excessive available nitrogen exerted significant negative effects. In conclusion, our pot study provides evidence that the integrated application of biochar and intercropping holds promise as a sustainable strategy for mitigating continuous cropping obstacles in cucumber production. These findings, obtained under controlled conditions, offer a proof-of-concept demonstrating the potential of this combined approach to synergistically improve soil health and crop performance. To translate this potential into practical agricultural practice, the long-term efficacy and economic viability of this strategy must be validated through well-designed field trials.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, SAMN51751506.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
SS: Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. YX: Supervision, Writing – review & editing. ZL: Supervision, Writing – review & editing. YaL: Data curation, Supervision, Writing – review & editing. RW: Data curation, Supervision, Writing – review & editing. GL: Data curation, Formal Analysis, Investigation, Supervision, Writing – review & editing. YuL: Data curation, Formal Analysis, Funding acquisition, Investigation, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the Natural Science Foundation of Jiangsu Province (BK20150491).
Acknowledgments
This research was supported by the Natural Science Foundation of Jiangsu Province (BK20150491).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1711099/full#supplementary-material
References
Agbna, G. H. D., She, D., Liu, Z., Elshaikh, N. A., Shao, G., and Timm, L. C. (2017). Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci. Hortic. 222, 90–101. doi: 10.1016/j.scienta.2017.05.004
Akhtar, S. S., Andersen, M. N., and Liu, F. (2015). Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manage. 158, 61–68. doi: 10.1016/j.agwat.2015.04.010
Aksoy, U., Kayikçioglu, H., Kukul, Y. S., Hepaksoy, S., Can, H. Z., and Balci, B. (2001). An environmentally friendly technique to control salination: Salt removing crops. Int. symposium Sustain. Use Plant biodiversity to promote New opportunities Hortic. production development antalya Turkey 598, 137–142. doi: 10.17660/ActaHortic.2003.598.19
Amini, S., Ghadiri, H., Chen, C., and Marschner, P. (2016). Salt-affected soils, reclamation, carbon dynamics, and biochar: a review. J. Soils Sediments 16, 939–953. doi: 10.1007/s11368-015-1293-1
Bai, X., Gao, J., Wang, S., Cai, H., Chen, Z., and Zhou, J. (2020). Excessive nutrient balance surpluses in newly built solar greenhouses over five years leads to high nutrient accumulations in soil. Agr. Ecosyst. Environ. 288, 106717. doi: 10.1016/j.agee.2019.106717
Banda, J. F., Zhang, Q., Ma, L., Pei, L., and Dong, H. (2021). Both pH and salinity shape the microbial communities of the lakes in Badain Jaran Desert, NW China. Sci. Total Environ. 791, 148108. doi: 10.1016/j.scitotenv.2021.148108
Bandara, T., Krohn, C., Jin, J., Chathurika, J., Franks, A., Xu, J. M., et al. (2022). The effects of biochar aging on rhizosphere microbial communities in cadmium-contaminated acid soil. Chemosphere 303, 135153. doi: 10.1016/j.chemosphere.2022.135153
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Lozupone, C. A., Turnbaugh, P. J., et al. (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. PNAS 108, 4516–4522. doi: 10.1073/pnas.1000080107
Cava, F., Lam, H., Pedro, M. A. D., and Waldor, M. K. (2011). Emerging knowledge of regulatory roles of d-amino acids in bacteria. Cell. Mol. Life Sci. 68, 817–831. doi: 10.1007/s00018-010-0571-8
Chi, B., Liu, J., Dai, J., Li, Z., Zhang, D., Xu, S., et al. (2023). Alternate intercropping of cotton and peanut increases productivity by increasing canopy photosynthesis and nutrient uptake under the influence of rhizobacteria. Field Crops Res. 302, 109059. doi: 10.1016/j.fcr.2023.109059
Cui, Q., Xia, J., Yang, H., Liu, J., and Shao, P. (2021). Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 756, 103801. doi: 10.1016/j.scitotenv.2020.143801
Ding, S., Zhou, D., Wei, H., Wu, S., and Xie, B. (2021). Alleviating soil degradation caused by watermelon continuous cropping obstacle: Application of urban waste compost. Chemosphere 262, 128387. doi: 10.1016/j.chemosphere.2020.128387
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–99+. doi: 10.1038/nmeth.2604
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., and Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. doi: 10.1093/bioinformatics/btr381
Farhangi-Abriz, S. and Ghassemi-Golezani, K. (2021). Changes in soil properties and salt tolerance of safflower in response to biochar-based metal oxide nanocomposites of magnesium and manganese. Ecotoxicol.Environ. Saf. 211, 111904. doi: 10.1016/j.ecoenv.2021.111904
Fu, L., Penton, C. R., Ruan, Y., Shen, Z., Xue, C., Li, R., et al. (2017). Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol. Biochem. 104, 39–48. doi: 10.1016/j.soilbio.2016.10.008
Garcia-Garcia, N., Tamames, J., Linz, A. M., Pedros-Alio, C., and Puente-Sanchez, F. (2019). Microdiversity ensures the maintenance of functional microbial communities under changing environmental conditions. Isme J. 13, 2969–2983. doi: 10.1038/s41396-019-0487-8
Gong, B., He, Y., Luo, Z., Peng, H., Cai, H., Zhu, Y., et al. (2024). Response of rhizosphere soil physicochemical properties and microbial community structure to continuous cultivation of tobacco. Ann. Microbiol. 74, 1. doi: 10.1186/s13213-023-01748-1
Gu, Y., Liang, W., Li, Z., Liu, S., Liang, S., Lei, P., et al. (2023). The biocontrol agent Bacillus velezensis T-5 changes the soil bacterial community composition by affecting the tomato root exudate profile. Plant Soil 490, 669–680. doi: 10.1007/s11104-023-06114-3
Guo, H., Wang, Y., Li, D., Chen, J., Zong, J., Wang, Z., et al. (2016). Growth response and ion regulation of seashore paspalum accessions to increasing salinity. Environ. Exp. Bot. 131, 137–145. doi: 10.1016/j.envexpbot.2016.07.003
Han, S., Li, H., Rengel, Z., Du, Z., Hu, N., Wang, Y., et al. (2023). Biochar application promotes crops yield through regulating root development and the community structure of root endophytic fungi in wheat-maize rotation. Soil Till. Res. 234, 105827. doi: 10.1016/j.still.2023.105827
Hauggaard-Nielsen, H. and Jensen, E. S. (2005). Facilitative root interactions in intercrops. Plant Soil 274, 237–250. doi: 10.1007/s11104-004-1305-1
Hu, S., Liu, L., Zuo, S., Ali, M., and Wang, Z. (2020). Soil salinity control and cauliflower quality promotion by intercropping with five turfgrass species. J. Cleaner Prod. 266, 121991. doi: 10.1016/j.jclepro.2020.121991
Inderjit and Mallik, A. U. (1997). Effect of phenolic compounds on selected soil properties. For. Ecol. Manage. 92, 11–18. doi: 10.1016/S0378-1127(96)03957-6
Jesus, P. R. R. D., Leonel, M., Leonel, S., Candido, H. T., dos Ouros, L. F., Damatto Junior, E. R., et al. (2024). Variability assessment of banana cultivars and intercropping with lemongrass based on fruit quality indicators. Sci. Hortic. 10, 9. doi: 10.3390/horticulturae10090962
Ji, C., Ye, R., Yin, Y., Sun, X., Ma, H., and Gao, R. (2022). Reductive soil disinfestation with biochar amendment modified microbial community composition in soils under plastic greenhouse vegetable production. Soil Till. Res. 218, 105323. doi: 10.1016/j.still.2022.105323
Jin, F., Piao, J., Miao, S., Che, W., Li, X., Li, X., et al. (2024). Long-term effects of biochar one-off application on soil physicochemical properties, salt concentration, nutrient availability, enzyme activity, and rice yield of highly saline-alkali paddy soils: based on a 6-year field experiment. Biochar 6, 1. doi: 10.1007/s42773-024-00332-3
Jin, X., Shi, Y., Wu, F., Pan, K., and Zhou, X. (2020). Intercropping of wheat changed cucumber rhizosphere bacterial community composition and inhibited cucumber Fusarium wilt disease. Scientia Agricola 77, e20190005. doi: 10.1590/1678-992x-2019-0005
Johnson, M., Zaretskaya, I., Raytselis, Y., Merezhuk, Y., McGinnis, S., and Madden, T. L. (2008). NCBIBLAST: a better web interface. Nucleic Acids Res. 36, 5–9. doi: 10.1093/nar/gkn201
Jurado, C., Diaz-Vivancos, P., Gregorio, B.-E., Acosta-Motos, J. R., and Hernandez, J. A. (2024). Effect of halophyte-based management in physiological and biochemical responses of tomato plants under moderately saline greenhouse conditions. Plant Physiol. Biochem. 206, 108228. doi: 10.1016/j.plaphy.2023.108228
Kolton, M., Graber, E. R., Tsehansky, L., Elad, Y., and Cytryn, E. (2017). Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol. 213, 1393–1404. doi: 10.1111/nph.14253
Kong, L.-L., Liu, W.-T., and Zhou, Q.-X. (2014). Biochar: an effective amendment for remediating contaminated soil. Rev. Eenviron. Contam. T. 228, 83–99. doi: 10.1007/978-3-319-01619-1_4
Lee, G., Carrow, R. N., and Duncan, R. R. (2004). Photosynthetic responses to salinity stress of halophytic seashore paspalum ecotypes. Plant Sci. 166, 1417–1425. doi: 10.1016/j.plantsci.2003.12.029
Lee, S. M., Kong, H. G., Song, G. C., and Ryu, C. M. (2021). Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease. Isme J. 15, 330–347. doi: 10.1038/s41396-020-00785-x
Lehmann, J., Cowie, A., Masiello, C. A., Kammann, C., Woolf, D., Amonette, J. E., et al. (2021). Biochar in climate change mitigation. Nat. Geosci 14, 883–88+. doi: 10.1038/s41561-021-00852-8
Lehmann, J., Rillig, M. C., Thies, J., Masiello, C. A., Hockaday, W. C., and Crowley, D. (2011). Biochar effects on soil biota – A review. Soil Biol. Biochem. 43, 1812–1836. doi: 10.1016/j.soilbio.2011.04.022
Li, L.-A. and Cai, B.-Y. (2016). Advances in arbuscular mycorrhizal fungi alleviating continuous cropping obstacles. Chin. J. Ecol. 35, 1372–1377. doi: 10.13292/j.1000-4890.201605.014
Li, Q., Liu, P., Zhao, H., Song, X., Lin, H., Shen, Y., et al. (2020). Effects of maize root exudates on allelopathy of phenolic acids in soil of continuous cropping peanut. J. Agric. Sci. Technol. 22, 119–130. doi: 10.13304/j.nykjdb.2019.0482
Li, T., Liu, T., Zheng, C., Kang, C., Yang, Z., Yao, X., et al. (2017). Changes in soil bacterial community structure as a result of incorporation of Brassica plants compared with continuous planting eggplant and chemical disinfection in greenhouses. PloS One 12, e0173923. doi: 10.1371/journal.pone.0173923
Li, J. G., Wan, X., Liu, X. X., Chen, Y., Slaughter, L. C., Weindorf, D. C., et al. (2019). Changes in soil physical and chemical characteristics in intensively cultivated greenhouse vegetable fields in North China. Soil Till. Res. 195, 104366. doi: 10.1016/j.still.2019.104366
Li, X., Wu, D., Liu, X., Huang, Y., Cai, A., Xu, H., et al. (2024). A global dataset of biochar application effects on crop yield, soil properties, and greenhouse gas emissions. Sci. Data 11, 57. doi: 10.1038/s41597-023-02867-9
Li, C., Zhao, C., Zhao, X., Wang, Y., Lv, X., Zhu, X., et al. (2023). Beneficial effects of biochar application with nitrogen fertilizer on soil nitrogen retention, absorption and utilization in maize production. Agronomy-Basel 13, 113. doi: 10.3390/agronomy13010113
Liang, J. and Shi, W. (2021). Cotton/halophytes intercropping decreases salt accumulation and improves soil physicochemical properties and crop productivity in saline-alkali soils under mulched drip irrigation: A three-year field experiment. Field Crops Res. 262, 108207. doi: 10.1016/j.fcr.2020.108027
Liao, P., Liu, P., Wang, Y., Huang, C., Lan, L., Yang, Y., et al. (2018). Stereoscopic cultivation of Panax nowginseng: A new approach to overcome the continuous cropping obstacle. For. Ecol. Manage. 126, 38–47. doi: 10.1016/j.indcrop.2018.09.042
Lin, C. C., Liu, Y. T., Chang, P. H., Hsieh, Y. C., and Tzou, Y. M. (2023). Inhibition of continuous cropping obstacle of celery by chemically modified biochar: An efficient approach to decrease bioavailability of phenolic allelochemicals. J. Environ. Manage. 348, 119316. doi: 10.1016/j.jenvman.2023.119316
Liu, L., Chen, Z., Su, Z., Li, S., Ali, A., Cai, Z., et al. (2023a). Soil pH indirectly determines Ralstonia solanacearum colonization through its impacts on microbial networks and specific microbial groups. Plant Soil 482, 73–88. doi: 10.1007/s11104-022-05671-3
Liu, T. J., Cheng, Z. H., Meng, H. W., Ahmad, I., and Zhao, H. L. (2014). Growth, yield and quality of spring tomato and physicochemical properties of medium in a tomato/garlic intercropping system under plastic tunnel organic medium cultivation. Sci. Hortic. 170, 159–168. doi: 10.1016/j.scienta.2014.02.039
Liu, X., Ren, X., Tang, S., Zhang, Z., Huang, Y., Sun, Y., et al. (2023b). Effects of broccoli rotation on soil microbial community structure and physicochemical properties in continuous melon cropping. Agronomy-Basel 13, 2066. doi: 10.3390/agronomy13082066
Liu, M., Xue, R., Jin, S., Gu, K., Zhao, J., Guan, S., et al. (2024). Metabolomic and metagenomic analyses elucidate the role of intercropping in mitigating continuous cropping challenges in tobacco. Front. Plant Sci. 15, 1447225. doi: 10.3389/fpls.2024.1447225
Liu, X. and Zhang, Y. (2021). Exploring the communities of bacteria, fungi and ammonia oxidizers in rhizosphere of Fusarium -diseased greenhouse cucumber. Appl. Soil Ecol. 161, 103832. doi: 10.1016/j.apsoil.2020.103832
Lopez-Marin, J., Rodriguez, M., Del Amor, F. M., Galvez, A., and Brotons-Martinez, J. M. (2019). Cost-benefit analysis of tomato crops under different greenhouse covers. J. Agric. Sci. Technol. 21, 235–248.
Lv, H. F., Lin, S., Wang, Y. F., Lian, X. J., Zhao, Y. M., Li, Y. J., et al. (2019). Drip fertigation significantly reduces nitrogen leaching in solar greenhouse vegetable production system. Environ. pollut. 245, 694–701. doi: 10.1016/j.envpol.2018.11.042
Lv, Y., Xu, L., Guo, X., Liu, J., Zou, B., Guo, Y., et al. (2023). Effect of biochar on soil physiochemical properties and bacterial diversity in dry direct-seeded rice paddy fields. Agronomy-Basel 13, 4. doi: 10.3390/agronomy13010004
Marcheva, M., Petkova, M., Slavova, V., and Popov, V. (2024). Positive effect of camelina intercropping with legumes on soil microbial diversity by applying ngs analysis and mobile fluorescence spectroscopy. Appl. Sci. 14, 9046. doi: 10.3390/app14199046
Meng, T., Yang, Y., Cai, Z., and Ma, Y. (2018). The control of Fusarium oxysporum in soil treated with organic material under anaerobic condition is affected by liming and sulfate content. Biol. Fertil. Soils 54, 295–307. doi: 10.1007/s00374-017-1260-7
Mitra, D., Mondal, R., Khoshru, B., Senapati, A., and Mohapatra, P. D. (2021). Enhanced plant growth, nutrient acquisition, and crop protection by using Actinobacteria: advancements in soil, plant, and microbial multifactorial interactions. Pedosphere 32, 149–170. doi: 10.1016/S1002-0160(21)60042-5
Morrissey, E. M., Kane, J., Tripathi, B. M., Rion, M. S. I., Hungate, B. A., Franklin, R., et al. (2023). Carbon acquisition ecological strategies to connect soil microbial biodiversity and carbon cycling*. Soil Biol. Biochem. 177, 108893. doi: 10.1016/j.soilbio.2022.108893
Mujakic, I., Cabello-Yeves, P. J., Villena-Alemany, C., Piwosz, K., Rodriguez-Valera, F., Picazo, A., et al. (2023). Multi-environment ecogenomics analysis of the cosmopolitan phylum Gemmatimonadota. Microbiol. Spectr. 11, e01112–e01123. doi: 10.1128/spectrum.01112-23
Naeem, M. A., Khalid, M., Aon, M., Abbas, G., Amjad, M., Murtaza, B., et al. (2018). Combined application of biochar with compost and fertilizer improves soil properties and grain yield of maize. J. Plant Nutr. 41, 112–122. doi: 10.1080/01904167.2017.1381734
Pasin, T. M., Dos Anjos Moreira, E., de Lucas, R. C., Benassi, V. M., Ziotti, L. S., Cereia, M., et al. (2020). Novel amylase-producing fungus hydrolyzing wheat and brewing residues, Aspergillus carbonarius, discovered in tropical forest remnant. Folia Microbiol. 65, 173–184. doi: 10.1007/s12223-019-00720-4
Pruesse, E., Quast, C., Knittel, K., Fuchs, B. M., Ludwig, W., Peplies, J., et al. (2007). SILVA:: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35, 7188–7196. doi: 10.1093/nar/gkm864
Qian, Z., Tang, L., Zhuang, S., Zou, Y., Fu, D., and Chen, X. (2020). Effects of biochar amendments on soil water retention characteristics of red soil at south China. Biochar 2, 479–488. doi: 10.1007/s42773-020-00068-w
Rajapaksha, A. U., Chen, S. S., Tsang, D. C. W., Zhang, M., Vithanage, M., Mandal, S., et al. (2016). Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 148, 276–291. doi: 10.1016/j.chemosphere.2016.01.043
Rao, M. P. N., Luo, Z.-H., Dong, Z.-Y., Li, Q., Liu, B.-B., Guo, S.-X., et al. (2022). Metagenomic analysis further extends the role of Chloroflexi in fundamental biogeochemical cycles. Environ. Res. 209, 112888. doi: 10.1016/j.envres.2022.112888
Ranjbari, M., Esfandabadi, Z. S., Quatraro, F., Vatanparast, H., Lam, S. S., Aghbashlo, M., et al. (2022) Biomass and organic waste potentials towards implementing circular bioeconomy platforms: A systematic bibliometric analysis. Fuel 318, 123585. doi: 10.1016/j.fuel.2022.123585
Rognes, T., Flouri, T., Nichols, B., Quince, C., and Mahe, F. (2016). VSEARCH: a versatile open source tool for metagenomics. Peerj 4, e2584. doi: 10.7717/peerj.2584
Shen, C., Li, X., and Qin, J. (2024). Kiwifruit-Agaricus blazei intercropping effectively improved yield productivity, nutrient uptake, and rhizospheric bacterial community. Sci. Rep. 14, 18863. doi: 10.1038/s41598-024-69709-5
Shi, R.-Y., Ni, N., Wang, R.-H., Nkoh, J. N., Pan, X.-Y., Dong, G., et al. (2023). Dissolved biochar fractions and solid biochar particles inhibit soil acidification induced by nitrification through different mechanisms. Sci. Total Environ. 874, 162464. doi: 10.1016/j.scitotenv.2023.162464
Simpson, C. R., Franco, J. G., King, S. R., and Volder, A. (2018). Intercropping halophytes to mitigate salinity stress in watermelon. Sustainability 10, 681. doi: 10.3390/su10030681
Slatni, T., Selmi, A., Kalboussi, N., Zemni, H., EChadly, A., Espin, G. B., et al. (2024). Intercropping salt-sensitive Solanum lycopersicum L. and salt-tolerant Arthrocaulon macrostachyum in salt-affected agricultural soil under open field conditions: Physiological, hormonal, metabolic and agronomic responses. Environ. Exp. Bot. 228, 106013. doi: 10.1016/j.envexpbot.2024.106013
Song, H., Che, Z., Cao, W., Huang, T., Wang, J., and Dong, Z. (2016). Changing roles of ammonia-oxidizing bacteria and archaea in a continuously acidifying soil caused by over-fertilization with nitrogen. Environ. Sci. pollut. Res. 23, 11964–11974. doi: 10.1007/s11356-016-6396-8
Souza, V. S., Canisares, L. P., Schiebelbein, B. E., Santos, D. D. C., Menillo, R. B., Junior, C. R. P., et al. (2025). Cover crops enhance soil health, crop yield and resilience of tropical agroecosystem. Field Crops Res. 322, 109755. doi: 10.1016/j.fcr.2025.109755
Spokas, K. A., Cantrell, K. B., Novak, J. M., Archer, D. W., Ippolito, J. A., Collins, H. P., et al. (2012). Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 41, 973–989. doi: 10.2134/jeq2011.0069
Su, K., Mu, L., Zhou, T., Kamran, M., and Yang, H. (2024). Intercropped alfalfa and spring wheat reduces soil alkali-salinity in the arid area of northwestern China. Plant Soil 499, 275–292. doi: 10.1007/s11104-022-05846-y
Sun, H., Wei, C., Xu, W., Yang, J., Wang, X., and Qiu, Y. (2019). Characteristics of salt contents in soils under greenhouse conditions in China. Environ. Sci. pollut. Res. 26, 3882–3892. doi: 10.1007/s11356-018-3865-2
Szoboszlay, M., Nther, A., Liu, B., Carrillo, A., and Tebbe, C. C. (2019). Contrasting microbial community responses to salinization and straw amendment in a semiarid bare soil and its wheat rhizosphere. Sci. Rep. 9, 9795. doi: 10.1038/s41598-019-46070-6
Tan, Y., Cui, Y., Li, H., Kuang, A., Li, X., Wei, Y., et al. (2017). Diversity and composition of rhizospheric soil and root endogenous bacteria in Panax notoginseng during continuous cropping practices. J. Basic Microbiol. 57, 337–344. doi: 10.1002/jobm.201600464
Tong, C., Yu, R., Chen, S., Hu, A., Dong, Z., Tang, L., et al. (2024). Intercropping in coconut plantations regulate soil characteristics by microbial communities. Agriculture-Basel 14, 1564. doi: 10.3390/agriculture14091564
Wadhams, G. H. and Armitage, J. P. (2004). Making sense of it all: bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 5, 1024–1037. doi: 10.1038/nrm1524
Waldemar, V., Didier, B., and De, P. M. A. (2008). Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 32, 149–167. doi: 10.1111/j.1574-6976.2007.00094.x
Wang, X. J., Cao, B., Zou, J., Xu, A. Y., and Feng, X. R. (2022b). Intercropping Gramineae Herbage in Semiarid Jujube Cultivar 'LingwuChangzao' Ziziphus jujub Mill. cv. LingwuChangzao) Orchard Improves Productivity, Plant Nutritional Quality, and Soil Quality. Sci. Hortic. 8, 834. doi: 10.3390/horticulturae8090834
Wang, S., Gao, P., Zhang, Q., Shi, Y., Guo, X., Lv, Q., et al. (2023a). Biochar improves soil quality and wheat yield in saline-alkali soils beyond organic fertilizer in a 3-year field trial. Environ. Sci. pollut. Res. 30, 19097–19110. doi: 10.1007/s11356-022-23499-3
Wang, B., Huang, X. H., Chen, J. F., Fu, L. B., Chen, Y. Q., Gao, W. S., et al. (2024a). Intercropping with green manure regulates microbial community structure and improves tea quality by changing soil available nutrients under organic management. Land Degrad. Dev. 36, 1384–1397. doi: 10.1002/ldr.5437
Wang, X. F., Li, Y., Wang, H. R., Wang, Y. Z., Biswas, A., Chau, H. W., et al. (2022a). Targeted biochar application alters physical, chemical, hydrological and thermal properties of salt-affected soils under cotton-sugarbeet intercropping. Catena 216, 106414. doi: 10.1016/j.catena.2022.106414
Wang, Y., Lin, Q., Liu, Z., Liu, K., Wang, X., and Shang, J. (2023b). Salt-affected marginal lands: a solution for biochar production. Biochar 5, 21. doi: 10.1007/s42773-023-00219-9
Wang, S., Liu, J., Liu, Y., and Tian, C. (2024c). Enhanced soybean growth and the associated ion balance, nutrient accumulation, and rhizosphere bacterial community when intercropped with suaeda salsa in saline soils. Agronomy-Basel 14, 2181. doi: 10.3390/agronomy14102181
Wang, X., Riaz, M., Babar, S., Eldesouki, Z., Liu, B., Xia, H., et al. (2024d). Alterations in the composition and metabolite profiles of the saline-alkali soil microbial community through biochar application. Environ. Manage. 352, 120033. doi: 10.1016/j.jenvman.2024.120033
Wang, E., Sun, Y., Li, M., Ye, L., Yu, X., Yao, Z., et al. (2024b). Diversified cropping of grains and atractylodes lancea (Thunb.) DC. Enhances ecological benefits of agroecosystems. Agriculture-Basel 14, 2327. doi: 10.3390/agriculture14122327
Wang, F., Wang, X., and Song, N. (2021a). Biochar and vermicompost improve the soil properties and the yield and quality of cucumber (Cucumis sativus L.) grown in plastic shed soil continuously cropped for different years. Agr. Ecosyst. Environ. 315, 107425. doi: 10.1016/j.agee.2021.107425
Wang, W., Wang, Z., Yang, K., Wang, P., Wang, H., Guo, L., et al. (2020). Biochar application alleviated negative plant-soil feedback by modifying soil microbiome. Front. Microbiol. 11, 799. doi: 10.3389/fmicb.2020.00799
Wang, M., Xu, S., Yang, J., Xu, L., Yu, Q., Xie, X., et al. (2021b). The effect of organic and conventional management practices on soil macropore structure in greenhouse vegetable production. Eur. J. Soil Sci. 72, 2133–2149. doi: 10.1111/ejss.13106
Wetzels, M., Odekerken-Schröder, G., and van Oppen, C. (2009). Using PLS path modeling for assessing hierarchical construct models: guidelines and empirical illustration. MIS Quart. 33, 177–195. doi: 10.2307/20650284
Wu, Z., Fan, Y., Zhou, Z., Hao, X., and Kang, S. (2025). Interaction between biochar particle size and soil salinity levels on soil properties and tomato yield. Biochar 7, 30. doi: 10.1007/s42773-024-00417-z
Xiang, Q., Ma, T., Wang, X., Yang, Q., Lv, L., Wang, R., et al. (2024). Effects of different living grass mulching on soil carbon and nitrogen in an apple orchard on loess plateau. Agronomy-Basel 14, 1917. doi: 10.3390/agronomy14091917
Xiao, X., Lv, J., Xie, J., Feng, Z., Ma, N., Li, J., et al. (2020). Transcriptome analysis reveals the different response to toxic stress in rootstock grafted and non-grafted cucumber seedlings. Int. J. Mol. Sci. 21, 774. doi: 10.3390/ijms21030774
Xie, W. Z., Zhang, K., Wang, X. Y., Zou, X. X., Zhang, X. J., Yu, X. N., et al. (2022). Peanut and cotton intercropping increases productivity and economic returns through regulating plant nutrient accumulation and soil microbial communities. BMC Plant Biol. 22, 1–14. doi: 10.1186/s12870-022-03506-y
Xu, J. M., Tang, C., and Chen, Z. L. (2006). The role of plant residues in pH change of acid soils differing in initial pH. Soil Biol. Biochem. 38, 709–719. doi: 10.1016/j.soilbio.2005.06.022
Yan, B., Deng, T., and Shi, L. (2024). Towards sustainable productivity of greenhouse vegetable soils: limiting factors and mitigation strategies. Plants-Basel 13, 2885. doi: 10.3390/plants13202885
Yan, S., Zhang, S., Yan, P., and Aurangzeib, M. (2022). Effect of biochar application method and amount on the soil quality and maize yield in Mollisols of Northeast China. Biochar 4, 56. doi: 10.1007/s42773-022-00180-z
Yang, L., Huang, B., Mao, M., Yao, L., Niedermann, S., Yu, X. N., et al. (2016). Sustainability assessment of greenhouse vegetable farming practices from environmental, economic, and socio-institutional perspectives in China. Environ. Sci. pollut. Res. 23, 17287–17297. doi: 10.1007/s11356-016-6937-1
Yang, Y., Wang, P., and Zeng, Z. (2019b). Dynamics of bacterial communities in a 30-year fertilized paddy field under different organic-inorganic fertilization strategies. Agronomy-Basel 9, 14. doi: 10.3390/agronomy9010014
Yang, M., Yuan, Y., Huang, H., Ye, C., Guo, C., Xu, Y., et al. (2019a). Steaming combined with biochar application eliminates negative plant-soil feedback for sanqi cultivation. Soil Till. Res. 189, 189–198. doi: 10.1016/j.still.2019.02.006
Zhang, P., Bing, X., Jiao, L., Xiao, H., Li, B., and Sun, H. (2022a). Amelioration effects of coastal saline-alkali soil by ball-milled red phosphorus-loaded biochar. Chem. Eng. J. 431, 133904. doi: 10.1016/j.cej.2021.133904
Zhang, A., Cui, L., Pan, G., Li, L., Hussain, Q., Zhang, X., et al. (2010). Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agr. Ecosyst. Environ. 139, 469–475. doi: 10.1016/j.agee.2010.09.003
Zhang, J., Kobert, K., Flouri, T., and Stamatakis, A. (2014). PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30, 614–620. doi: 10.1093/bioinformatics/btt593
Zhang, M., Liu, Y., Wei, Q., Liu, L., Gu, X., Gou, J., et al. (2023). Effects of biochar and vermicompost on growth and economic benefits of continuous cropping pepper at karst yellow soil region in Southwest China. Front. Plant Sci. 14, 1238663. doi: 10.3389/fpls.2023.1238663
Zhang, Q., Pang, X., Chen, X., Ye, J., Lin, S., and Jia, X. (2020). Rain-shelter cultivation influence rhizosphere bacterial community structure in pear and its relationship with fruit quality of pear and soil chemical properties. Sci. Hortic. 269, 109419. doi: 10.1016/j.scienta.2020.109419
Zhang, X., Shan, X., Fu, H., and Sun, Z. (2022b). Effects of artificially-simulated acidification on potential soil nitrification activity and ammonia oxidizing microbial communities in greenhouse conditions. Peerj 10, e14088. doi: 10.7717/peerj.14088
Zhang, Z., Sun, D., Tang, Y., Zhu, R., Li, X., Gruda, N., et al. (2021). Plastic shed soil salinity in China: Current status and next steps. Clean. Prod. 296, 126453. doi: 10.1016/j.jclepro.2021.126453
Zhao, H.-T., Li, T.-P., Zhang, Y., Hu, J., Bai, Y.-C., Shan, Y.-H., et al. (2017). Effects of vermicompost amendment as a basal fertilizer on soil properties and cucumber yield and quality under continuous cropping conditions in a greenhouse. J. Soils Sediments 17, 2718–2730. doi: 10.1007/s11368-017-1744-y
Zheng, S., Bian, T., Wang, S., Zhang, X., Li, X., Zhang, Y., et al. (2021). Decoupling of P from C, N, and K elements in cucumber leaves caused by nutrient imbalance under a greenhouse continuous cropping system. Sci. Hortic. 7, 528. doi: 10.3390/horticulturae7120528
Zheng, Y., Cao, X., Zhou, Y., Li, Z., Yang, Y., Zhao, D., et al. (2023). Effect of planting salt-tolerant legumes on coastal saline soil nutrient availability and microbial communities. Environ. Manage. 345, 118574. doi: 10.1016/j.jenvman.2023.118574
Zheng, Y., Zhang, J., Wang, D., Yang, S., Cen, Z., and Dong, Y. (2024). Faba bean-wheat intercropping controls the occurrence of faba bean Fusarium wilt by improving the microecological environment of rhizosphere soil. Eur. J. Soil Biol. 123, 103685. doi: 10.1016/j.ejsobi.2024.103685
Zhong, L., Gu, Z., Sun, Y., Wang, R., Wang, H., Li, G., et al. (2024). Biochar increases pakchoi yield by regulating soil bacterial communities but reduces it through soil fungi in vegetable soil. J. Soils Sediments 24, 1348–1360. doi: 10.1007/s11368-024-03733-w
Keywords: biochar, phytoremediation, soil salinity, nutrient imbalance, bacterial community, cucumber, continuous cropping
Citation: Shen S, Xu Y, Liu Z, Luo Y, Wang R, Li G and Liu Y (2025) Combined application of biochar and halophyte intercropping enhances cucumber yield and quality by ameliorating soil properties in a continuous cropping system. Front. Plant Sci. 16:1711099. doi: 10.3389/fpls.2025.1711099
Received: 23 September 2025; Accepted: 27 October 2025;
Published: 20 November 2025.
Edited by:
Soumyadev Sarkar, Arizona State University, United StatesReviewed by:
Amr Adel Elkelish, Suez Canal University, EgyptTing Bian, Shenyang Agricultural University, China
Copyright © 2025 Shen, Xu, Liu, Luo, Wang, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanlin Li, bGlsaWd1YW5saW5AdWpzLmVkdS5jbg==; Yujing Liu, bGl1eXVqaW5nQG5qYXUuZWR1LmNu
 Songsong Shen
Songsong Shen Yusheng Xu
Yusheng Xu Zhongpeng Liu
Zhongpeng Liu Yating Luo1
Yating Luo1 Ruifang Wang
Ruifang Wang