- 1Shandong Provincial University Laboratory for Protected Horticulture, Weifang University of Science and Technology, Shouguang, China
- 2Division of Horticultural Science, College of Agriculture and Life Sciences, Gyeongsang National University, Jinju, Republic of Korea
- 3Jingzhi-Maoteng Agricultural Technology Limited Company in Qushui County, Lhasa, Tibet, China
Ammonium (NH4+) toxicity adversely curtails the growth and productivity of rapeseed plants. Current knowledge shows that blue (B) light is an alternative approach used to minimize or alleviate disturbances caused by various abiotic stresses. However, few studies have investigated NH4+-stressed rapeseed plants to illustrate the alleviatory role of blue light. Therefore, this study was conducted to determine whether blue light could reduce the degree of NH4+ toxicity in rapeseed and, at the same time, elucidate the underlying mechanism. To this end, rapeseed plants were cultured in a controlled environment (14 h light at 22°C and 10 h dark at 18°C) and treated with one of three NH4+:NO3− regimes (0:100, 50:50, and 100:0) with a constant nitrogen concentration of 13 me L−1, under white light-emitting diode (LED) light or blue LED light at 200 PPFD. Plants treated exclusively with NH4+ under white light exhibited decreased growth, disturbed photosynthesis, inhibited antioxidant defense systems, limited nitrogen (N) assimilation, and ultimately developed NH4+ toxicity symptoms (as characterized by chlorosis, necrosis, and stunted morphology). These traits and parameters were significantly mitigated by blue light treatment. Collectively, this study highlights the benefits of blue light on plants, particularly for NH4+-sensitive species such as rapeseed.
1 Introduction
Nitrogen (N) nutrition is of paramount importance for plant structural development and productivity, as it comprises many organic compounds, such as the amino acids, nucleic acids, and proteins (Bloom, 2015; Leghari et al., 2016). When limited, it severely reduces plant biomass. This fundamental element is absorbed mainly in forms of nitrate (NO3−) and ammonium (NH4+) (Song et al., 2022a). The availability, uptake, and metabolism of these two inorganic N sources by plants were found to differ markedly in their energetic, biological, and biochemical processes (Luo et al., 2013; Guo et al., 2007). Theoretically, the NH4+ can be readily assimilated by plants, whereas NO3− is energy-consuming (Bittsánszky et al., 2015). NH4+ is also less prone to leaching, conferring higher assimilation efficiency and lower environmental pollution. Additionally, many edible leafy vegetables were more prone to accumulate nitrite (NO2−), an intermediate product in the nitrogen assimilation pathway that is regarded as toxic to both human health and plant growth (Picetti et al., 2022).
Paradoxically, exclusively or unintentionally applied NH4+ nutrition can result in plant tissue acidification (Hachiya et al., 2021), and the plants ultimately manifest ammonium toxicity (or NH4+ toxicity). This phenomenon has been observed in many NH4+-sensitive plant species, such as cucumber (Roosta and Schjoerring, 2007), cabbage (Song et al., 2022a), salvia (Song et al., 2022b), and basil (Song et al., 2024a). During this period, several morphological and physiological dysfunctions can occur due to interrupted metabolism. Typically, plants exhibiting NH4+ toxicity show visual detrimental signs, including reduced growth, leaf chlorosis and necrosis, and stunted roots (Esteban et al., 2016). Meanwhile, certain integrated internal impacts may also be elicited: photosynthesis can be disturbed (Wang et al., 2019; Bittsánszky et al., 2015), oxidative stress in terms of reactive oxygen species (ROS) and corresponding scavenging enzymes can be increased (Song et al., 2022b; Esteban et al., 2016), and major enzymes in the NH4+ assimilation pathway can be significantly altered (Song et al., 2021; Bittsánszky et al., 2015; Cruz et al., 2006; Song et al., 2022a). However, the NH4+ tolerance in plants can be induced by improving the expression of glutamine synthetase (GS) and glutamate dehydrogenase (GDH); additionally, Xian et al. (2020) suggested that increasing GDH activity is an important strategy for NH4+ detoxification (Song et al., 2021, 2022).
Rapeseed (Brassica napus L.), known as oilseed rape, is an important and prolific oil crop that is not only used as a high-quality edible oil for humans but also serves as feed for animals and as a source of lubricants (Zhang and Flottmann, 2016). Its agro-industrial value has promoted large-scale cultivation worldwide; for example, the production area in China has exceeded 7 million hectares since 2019 (Lei et al., 2019). However, its certain types or varieties are sensitive to high NH4+ nutrition (Zhou et al., 2024; Li S et al., 2024). As a result, the yield and quality of rapeseed are often limited by inappropriate application of NH4+-containing fertilizers. Moreover, overuse of NH4+ fertilizers results not only in low nitrogen use efficiency (NUE) but also in significant environmental hazards. Therefore, agronomic practices or other horticultural strategies to increase plant NH4+ tolerances are recommended. Recently, researchers have improved plant tolerance to abiotic stresses by adjusting the photoenvironment, since light is an essential environmental signal determining plant growth and development (Ma et al., 2022; Morello et al., 2022; Ren et al., 2023).
Light quality, optical intensity, photoperiod, and light distribution are the predominant factors that impart a plethora of physiological effects on plants (Yamori et al., 2020; Trojak et al., 2022). The specific wavelength of light exerts precise influences on the quantum yield basis of photosynthesis. In other words, different light qualities carrying varying levels of energy affect plants by regulating a variety of biological processes, such as tissue differentiation and nutrient absorption (Lee et al., 2021). In particular, blue (B) light is regarded as the most efficient spectrum for photosynthesis, steering photomorphogenesis, cell division, leaf expansion, stomata opening, and pigment accumulation (Naznin et al., 2019). It has been reported that photosynthetic capacity significantly improves when the percentage of blue light increases in a red-light background (Hogewoning et al., 2010). Moreover, blue light treatment has been suggested to reinforce antioxidant capacity (Zhang et al., 2023; Ren et al., 2023), regulate nitrogen metabolism (Ren et al., 2023), and suppress symptoms of multiple abiotic stresses (Ren et al., 2023; Roeber et al., 2021). Recently, light-emitting diodes (LEDs) have emerged as a spectrally and energetically optimal alternative for maximum crop production in plant factories with artificial lighting (PFALs). A great deal of reports have also shown that manipulating LED-supplied blue light is a sustainable and powerful method for altering plant traits under environmental stresses (Kong and Zheng, 2023). However, studies on whether blue light treatment can reduce NH4+ toxicity in rapeseed (Brassica napus L.) are very scarce (Liu et al., 2020; Li S et al., 2021, 2024).
Therefore, the current study was undertaken to (1) characterize NH4+ toxicity in rapeseed by applying a high level of NH4+, (2) determine whether blue light can mitigate NH4+ toxicity in rapeseed, and (3) ascertain the alleviatory effects of blue light on rapeseed growth attributes, photosynthetic capacity, antioxidant defense system, and nitrogen metabolism machinery.
2 Materials and methods
2.1 Plant material and culture conditions
Rapeseed seeds “Qinyou” were selected as the plant material and purchased from Ronghua Agriculture Technology Co. Ltd. (Xian, Shanxi, China). Full-grain seeds of uniform size and without any mechanical damage were selected and sown in 128-cell plug trays filled with mini-K medium (Klasmann–Deilmann GmbH Company, Geeste, Germany). After sowing, the medium was carefully moistened with tap water and covered with cling film to preserve moisture until germination.
Rapeseed seeds were germinated 7 days after sowing (DAS) under an air-conditioned environment at 22 °C ± 2°C in darkness. The germinated rapeseed seeds were quickly transferred to a controlled alternating diurnal regime (14 h light at 22°C and 10 h dark at 18°C) and irrigated with multipurpose nutrient solution (MNS) according to our previous publications (Song et al., 2022a, 2022). The light environment was measured with a handheld spectrometer (PG200N Spectral PAR Meter, UPRtek, Miaoli County, Taiwan). Light condition was provided by white or blue LED light with an intensity of 200 µmol m−2 s−1 PPFD. The peak and dominant wavelengths of white LED light adopted were at 456 and 650 nm, respectively, while the peak wavelength of blue LED light was 456 nm (Figure 1).

Figure 1. The spectral distributions of (a) white LED light and (b) blue LED light supplied for the culture of rapeseed.
The rapeseed seedlings were allowed to develop for another 6 days until they entered the growing stage with two true leaves and one heart (13 DAS). They were then cultivated with distilled water for another 3 days to get all the nutrients leaching out (16 DAS). Healthy seedlings of uniform size and similar morphology, without mechanical flaws or disease, were screened, selected, and transplanted into a new 128-cell plug tray. The transplanted seedlings were simultaneously subjected to different treatments (17 DAS).
2.2 The experimental treatments and design
The treatment solutions were prepared with three different NH4+:NO3− ratios (0:100, 50:50, and 100:0), designated according to MNS with a constant N concentration at 13 me L−1. The detailed recipe and sourced chemicals are listed in Table 1. Transplanted seedlings under the three NH4+:NO3− regimes were equally divided into six parts and subjected to white LED light or blue LED light treatment (see the Graphical Abstract).
Overall, the three NH4+:NO3− ratios combined with two LED light qualities formed the experimental treatments in this study. The trial was arranged as a 2 × 3 factorial scheme in a completely randomized design, with three biological replicates. A total of 24 rapeseed seedlings were planted for one replicate per NH4+:NO3− ratio in this experiment.
2.3 Determination of plant growth parameters and destructive sampling
Subsequently, rapeseed plants subjected to different treatments were harvested (32 DAS) when they reached contrasting statuses and morphologies. The plants were first removed from the substrates and washed with distilled water. Plant roots were then surface-blotted with absorbent paper. Whole plant fresh biomass and dry weight (air-forced oven at 70°C for 48 h) were determined using an electronic balance. Shoot length, leaf length and width, and tap root length were measured with a metal ruler. Stem diameter was determined with a vernier caliper (CD-20CPX, Mitutoyo Korea Co., Gunpo, South Korea). Root volume, root surface area, and total root length were measured using a root analysis Microtek ScanWizard Pro system (MICROTEK, Shanghai, China). Leaf samples from different treatments were individually collected, immersed in liquid N2, and stored at − 80°C until further analysis.
2.4 Assessment of plant photosynthetic ability
Photosynthetic ability in this study was assessed using major parameters, including the net photosynthesis rate (Pn), stomatal conductance (gs), transpiration rate (Tr), chlorophylls (chlorophyll a and b), and carotenoids.
Specifically, the first three traits were measured with a portable photosynthesis measurement system (TARGAS-1, PP Systems, Amesbury, MA, USA). The three topmost fully expanded leaves were used for measurement, and each leaf was measured three times. During measurement, the leaf temperature was about 22°C, and the environment was identical to that previously set for culturing rapeseed. Photosynthesis-related pigments, including chlorophyll a, chlorophyll b, and carotenoids, were determined following a procedure by Sims and Gamon (2002): The absorbance of the extraction buffer (45% v/v acetone, 45% v/v ethanol, and 10% v/v H2O) was read at 645, 663, and 440 nm using a spectrophotometer (UV5100, Metash Instruments Co. Ltd., Shanghai, China), and the content was calculated using the following equations:
Here, “V” is 2 ml (the volume of the extraction buffer), and the chlorophyll content is expressed in milligrams per gram of leaf fresh weight (mg g−1 FW).
2.5 Calculations of the appearing ratio of ammonium toxicity (%)
Rapeseed plants developed ammonium toxicity symptoms in response to a 100% NH4+ supply. The appearance ratio of ammonium toxicity (%) per replicate was calculated using the following equation:
Where “24” represents the number of rapeseed plants per treatment per replicate.
2.6 Estimation of the antioxidant defense system
The antioxidant defense system was estimated in terms of the main antioxidant enzyme activities and ROS accumulation. The antioxidant enzymes mainly consisted of superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), guaiacol peroxidase (GPX), dehydroascorbate reductase (DHAR), and glutathione reductase (GR). The ROS level mainly included the superoxide (O2.−) and hydrogen peroxide (H2O2).
Specifically, about 100 mg of finely ground leaf powder samples were well mixed with an extraction buffer (1 mM EDTA, 50 mM PBS, 2% polyvinylpyrrolidone, and 0.05% Triton-X at pH 7.0). This mixture was centrifuged (12,000 rpm, 4°C, 20 min) to obtain the supernatant, which was subsequently used for the quantification of total protein content (Bradford, 1976) and antioxidant enzyme activities (Song et al., 2022b). SOD activity was determined based on the reduction of nitroblue tetrazolium (NBT) (Giannopolitis and Ries, 1977). APX activity was measured using a method based on ascorbate oxidation (Nakano and Asada, 1981). The decomposition of H2O2 was used to determine CAT concentration (Cakmak and Marschner, 1992). GPX activity was assessed using the guaiacol oxidation reaction (Amako et al., 1994). DHAR activity was measured following the approach proposed by Nakano and Asada (1981). GR activity was determined using a rapid and sensitive procedure described by Mavis and Stellwagen (1968). O2.− content was measured based on hydroxylamine oxidation by Wu and von Tiedemann (2002). H2O2 levels were colorimetrically determined following the protocol by Mukherjee and Choudhuri (1983).
2.7 Determination of NO3−, NO2−, and NH4+ content
Salicylic acid nitration was used to measure NO3− content according to Cataldo et al. (1975). A quick method based on the Griess reaction was adopted for the determination of NO2− concentration (Moshage et al., 1995). A colorimetric method based on the Berthelot reaction was employed to measure NH4+ content (Bräutigam et al., 2007). The specific steps can be found in Huang’s report (Huang et al., 2018).
2.8 Analysis of the key enzyme activities in the N-assimilation pathway
The key enzymes in the N-assimilation pathway mainly include nitrate reductase (NR), nitrite reductase (NIR), GS, glutamate synthetase (GOGAT), and Nicotinamide adenine dinucleotide (NADH)-dependent GDH, which were spectrophotometrically assayed using a spectrophotometer (UV3200, OptoSky, Xiamen, China) following our previous publications with minor modifications (Song et al., 2021, 2022). The NR activity was measured in vitro in accordance with a sensitive method by Högberg et al. (1986) and was expressed by the amount of nitrite formed, while the NIR concentration was determined by the reduction of NO2− during assay (Ogawa et al., 1999). Specifically, 0.5 g of finely ground leaf samples were homogenized in a 5-ml protein extraction medium containing Tris-HCl at 50 mM, MgSO4 at 2 mM, DTT-dithiothreitol at 2 mM, sucrose at 400 mM, and pH 8.0. This mixture was centrifuged (13,000 rpm, 4°C, 20 min) to obtain the supernatant, which was later used for the determination of the enzymes of GS, GOGAT, and NADH-GDH.
The GS activity was estimated following a method by Oaks et al. (1980): A total of 0.7 ml crude enzyme extract was mixed with 2.3 ml assay solution (0.1 M Tris-HCl, 80 mM Mg2+ and hydroxylamine hydrochloride, 2 mM EGTA, 20 mM sodium glutamate and cysteine, and 40 mM daily prepared ATP, pH 7.4) and was then subjected to incubation at 37°C for 30 min. To terminate the reaction, 1 ml of ferric chloride reagent (0.37 M FeCl3, 0.6 M HCl, and 0.2 M TCA) was added. The mixture was then vigorously shaken for 5 min and centrifuged (5,000×g, RT, 10 min) to obtain the supernatant, which was subsequently recorded spectrophotometrically at 540 nm. One unit of GS activity was defined as the synthesis of 1 μmol γ-glutamyl hydroxamate per hour per gram of fresh weight.
The GOGAT activity was determined based on an approach as presented by Lin and Kao (1996): 0.5 ml crude enzyme extract was added to a reaction medium (0.1 ml KCl at 10 mM, 0.2 ml NADH at 3 mM, 0.05 ml α-oxoglutarate at 0.1 M, and 0.4 ml l-glutamine at 20 mM, pH 7.6). The change of the absorbance of this mixture was spectrophotometrically read at 340 nm. The GOGAT activity was expressed as the change of absorbance at 0.001 per hour.
The NADH-GDH activity was assessed in accordance with a report by Kanamori et al. (1972): The reaction was triggered by adding 0.1 ml of crude enzyme extract to the 2.9-ml assay solution (distilled water at 0.3 ml, NH4Cl at 231 mM, α-ketoglutarate at 23.1 mM, Tris-HCl at 15.4 mM, 0.1 ml of CaCl2 at 30 mM, and 6 mM NADH). The absorbance of this mixture was immediately measured at 340 nm after a water bath at 37°C for 5 min. The NADH-GDH activity was characterized as the formation of nanomoles NAD+ per gram of fresh weight per minute.
2.9 Statistical analysis and graphs
The data displayed in this study were means ± SE from no less than three independent biological replicates (n ≥ 3). The statistical analysis of all data was performed with the SAS v8.0 program (SAS 8.2 Inst., Cary, NC, USA) by one-way analysis of variance (ANOVA) following Duncan’s multiple comparison range test at p = 0.05. The bar graphs were plotted using GraphPad Prism 8.0 software (GraphPad Software, Boston, MA, USA). The principal component analysis (PCA) was generated by the Origin 2023 procedure (Origin Lab Corp., Northampton, MA, USA) to visualize the interrelationships among the parameters regarding the antioxidant system and the N-assimilation pathway investigated in this study.
3 Results
3.1 The rapeseed plant growth and morphology
The rapeseed plants showed significantly different morphological appearances regarding shoot length, leaf area, and the root system (Figure 2). Setting light quality aside, it is clear that rapeseed plants treated with 50:50 NH4+:NO3− showed more vigorous and healthy growth compared with those cultured in the other two regimes. In contrast, solely NH4+-cultured plants displayed severely restricted growth and development, and solely NO3−-cultured plants exhibited better growth than those grown in the 100:0 NH4+:NO3−.

Figure 2. Morphological changes of rapeseed under white or blue light in response to three NH4+:NO3− ratios after several weeks of treatment. Ratios of 100:0, 50:50, and 0:100 NH4+:NO3− are shown from left to right, with each treatment applied to two rapeseed plant replicates of similar size.
However, blue light imparted greater growth ability compared with plants cultured under a white environment, regardless of the NH4+:NO3− ratio (Figure 2). In particular, for rapeseed plants solely supplied with 100% NH4+, blue light notably mitigated the reduced growth compared with that observed under white light (Figure 2).
3.2 The plant growth parameters
Indeed, the recorded responses of plant growth and morphology to different NH4+:NO3− treatments and light qualities were further supported by the investigated growth parameters (Figure 3; Table 2).

Figure 3. Rapeseed growth parameters: (a) whole plant fresh weight, (b) root volume, (c) shoot length, and (d) leaf width as affected by three NH4+:NO3− ratios under white or blue light. Data are means ± SE from six biological replicates (n = 6). Significant differences among treatments were determined by Duncan’s multiple comparison range test at p = 0.05 (one-way ANOVA) and are denoted by different lowercase letters above the bars.

Table 2. Rapeseed dry weight, stem diameter, tap root length, root surface area, total root length, and leaf length in response to three NH4+:NO3− ratios under white or blue light.
Across Figure 3, rapeseed plants grown in the 0:100 and 50:50 NH4+:NO3− regimes displayed similar trends, while 100% NH4+ nutrition conferred dramatically reduced growth, regardless of light quality. More importantly, compared with rapeseed plants grown under white light, blue light-treated plants significantly improved these traits by varying degrees, especially for the solely NH4+-treated plants. For example, for plants grown in the 100:0 NH4+:NO3− regime, blue light significantly increased shoot length by 60% relative to those cultivated under white light (Figure 3c).
Concomitantly, other important growth parameters such as dry weight, stem diameter, tap root length, root surface area, total root length, and leaf length also showed similar responses to the different NH4+:NO3− ratios and light qualities (Table 2). It is still worth noting that 100% NH4+ nutrition caused significant decreases in plant growth ability, whereas this phenomenon was markedly alleviated when plants were cultured under blue light.
3.3 Ammonium toxicity in solely NH4+-cultured rapeseed plants
Notably, the rapeseed plants treated with the 100:0 NH4+:NO3− solution eventually developed NH4+ toxicity symptoms, regardless of light quality. This phenomenon was characterized by chlorosis and visible foliage necrosis accompanied by burned tips, stunted roots, and inhibited growth (Figure 4a).

Figure 4. NH4+ toxicity-related parameters in rapeseed plants supplied solely with NH4+: (a) plant growth status comparison and (b) ratio of plants with NH4+ toxicity symptoms (%) under white or blue light. Red arrows in (a) indicate typical NH4+ toxicity symptoms in the foliage. Data in (b) are means ± SE from six biological replicates (n = 6). The significant differences between white light and blue light were determined according to a two-tailed Student’s t-test.
However, blue light-cultivated rapeseed plants significantly ameliorated the degree of NH4+ toxicity and improved plant growth. Indeed, the appearance ratio of plants with NH4+ toxicity symptoms under blue light drastically declined from 91.7% to 41.7%, compared with that under white light conditions (Figure 4b).
3.4 Photosynthetic ability
The photosynthesis of rapeseed plants was also distinctly altered in response to high NH4+ or NO3− supply under white light or blue light conditions. Thus, the photosynthetic ability regarding Pn, gs, Tr, chlorophyll a, chlorophyll b, and carotenoids was assessed (Figure 5).

Figure 5. Photosynthetic ability-related parameters: (a) net photosynthesis rate (Pn), (b) stomatal conductance (gs), (c) transpiration rates (Tr), (d) chlorophyll a, (e) chlorophyll b, and (f) carotenoids as affected by three NH4+:NO3− ratios and light quality. Data are means ± SE from six biological replicates (n = 6). Significant differences among treatments were denoted by different lowercase letters (one-way ANOVA following Duncan’s multiple comparison range test at p = 0.05).
Specifically, it is worth noting that plants treated with 100% NH4+ nutrition significantly decreased all the investigated parameters, regardless of light quality. Overall, irrespective of the light conditions considered, plants grown in the 0:100 NH4+:NO3− regime showed similar trends to those in the 50:50 NH4+:NO3− regime, with the exception of Pn (Figure 5a).
Blue light-treated plants significantly improved the photosynthetic ability compared with plants grown under white light, regardless of the NH4+:NO3− ratios (Figure 5). In particular, the disturbed photosynthetic ability of 100% NH4+-supplied plants under white light was notably mitigated when treated with blue light.
3.5 Antioxidant enzyme activity and ROS
The oxidative protective system is triggered when plants are subjected to external stresses. In this regard, antioxidant enzyme activities increase to enhance antioxidant capacity and reduce ROS accumulation.
Indeed, plants treated solely with NH4+ showed significantly increased accumulations of O2.− and H2O2 compared with those cultivated with 100% NO3− or a mixed NH4+ and NO3− (Figures 6A [a, b]). The antioxidant enzyme activities, however, did not improve as the external NH4+ supply increased from 50% to 100%. Notably, compared with plants cultured under white light, blue light-treated plants markedly increased major antioxidant enzyme activities and consequently reduced ROS accumulations (Figure 6A).

Figure 6. Analysis of the antioxidant defense system: (A) ROS content ((a) O2.− and (b) H2O2) major antioxidant enzyme activities ((c) SOD, (d) APX, (e) CAT, (f) GPX, (g) DHAR, (h) GR) and (B) multivariate data analysis by PCA. Data are means ± SE from no less than four biological replicates (n ≥ 4). Significant differences among treatments were denoted by different lowercase letters (one-way ANOVA following Duncan’s multiple comparison range test at p = 0.05).
Moreover, the impacts of three NH4+:NO3− solutions under white light or blue light on antioxidant enzymes and oxidative damage, along with the relationships among all treatments, were visualized using PCA. The PCA results along the first two principal dimensions (“PC1” = 80.3%, “PC2” = 5.6%) explained a total data variability of 85.9%. Overall, blue light-treated plants were mainly distributed on the right side of PC1, whereas white light-treated plants were primarily located on the left side of PC1 (Figure 6B). Additionally, blue light-treated plants exhibited higher antioxidant enzyme activities and lower ROS concentrations.
3.6 Key enzymes, activities, and chemical contents in the N-assimilation pathway
To investigate whether blue light affects the major N-assimilation enzymes and key chemical contents in the N-assimilation pathway during NH4+ toxicity alleviation, the activities of NR, NIR, GS, GOGAT, GDH, as well as the concentrations of NO3−, NO2−, and NH4+, were determined.
It is noteworthy that a high supply of NO3− or NH4+ results in correspondingly high levels of free NO3− or NH4+ in plants, respectively, regardless of light quality (Figures 7A [a, e]). Similarly, GS and GDH were significantly increased when external NH4+ nutrition was elevated from 0% to 50%, regardless of light quality (Figures 7A [f, g]). Conversely, the GOGAT activity gradually decreased in response to declining NH4+ nutrition supply (Figures 7A [h]).
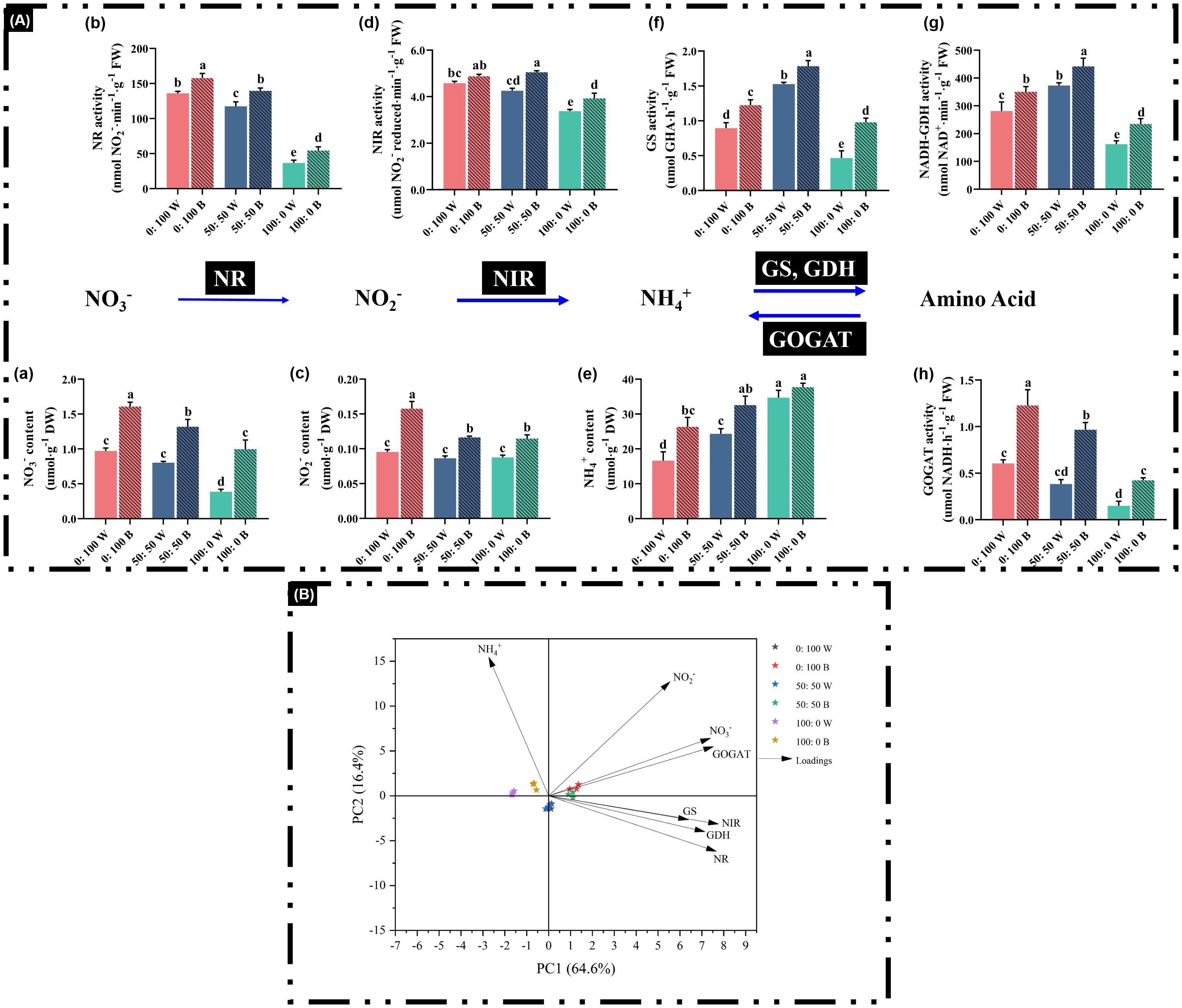
Figure 7. Analysis of main chemicals: (A) (a) NO3− content, (c) NO2− content, and (e) NH4+ content and major enzymes: (A) (b) NR activity, (d) NIR activity, (f) GS activity, (g) NADH-GDH activity, and (h) GOGAT activity in the N assimilation pathway, as well as (B) multivariate data analysis by PCA. Data are means ± SE from no less than four biological replicates (n ≥ 4). Significant differences among treatments were denoted by different lowercase letters (one-way ANOVA following Duncan’s multiple comparison range test at p = 0.05).
However, the blue light-treated plants not only dramatically increased the activities of enzymes in the N-assimilation pathway, particularly GS, GDH, and GOGAT (Figures 7A, [f–h]), but also notably increased the contents of free chemicals, such as NO3− (Figures 7A [a]). Importantly, 100% NH4+-treated plants under blue light showed marked enhancement of enzyme activities and chemical contents, except for free NH4+ concentrations (Figure 7A), compared to plants cultivated under white light. For instance, GS, GDH, and GOGAT activities in plants under the 100:0 NH4+:NO3− regime with blue light were significantly increased by 1.13-fold, 44.8%, and 1.83-fold, respectively (Figures 7A[f–h]).
All investigated parameters were analyzed through PCA to visualize how the NH4+:NO3− ratios and light quality affected the N-assimilation pathway. The first two principal components explained 81% (PC1 = 64.6%, PC2 = 16.4%) of the total variance (Figure 7B). On average, plants treated with blue light were mainly separated along PC2, while those treated with white light were located in the opposite direction, illustrating the contrasting traits between blue and white light treatments.
4 Discussion
A high or exclusive supply of NH4+ inevitably inhibits plant growth and development. In our trials, numerous morphological and physiological rapeseed traits were significantly altered by a high external NH4+ concentration, as shown by decreased plant weight, reduced plant shoot and leaf, and declined root-related parameters, including root volume and total root length (Figure 3; Table 2). The rapeseed plants in this study also developed NH4+ toxicity symptoms, characterized by severely restricted growth and development, chlorosis, necrosis, and stunted roots (Figure 4a). These detrimental effects of NH4+ toxicity are consistent with observations in other NH4+-sensitive plant species, such as basil (Song et al., 2024a), cabbage (Song et al., 2022a), and beans (Guo et al., 2002). These findings indicate that rapeseed is highly sensitive to high NH4+ supply and can be classified as an NH4+-sensitive plant.
Data to date on the alleviation of NH4+ toxicity in plants by blue light treatment are very limited, especially for NH4+-sensitive plant species (Bittsánszky et al., 2015; Esteban et al., 2016; Shilpha et al., 2023). However, blue light-induced alleviation of abiotic stresses has been reported in many plant species. For instance, blue light-treated pepper showed improved photosynthesis under UV light stress (Hoffmann et al., 2015), and it was suggested that drought resistance was increased through blue light regulation in melo (Li X et al., 2024). Several pioneering studies revealed that blue light application significantly promoted plant growth and development, ranging from agricultural crops to horticultural flowers or vegetables (Zheng and Van Labeke, 2017; Jin et al., 2023; Li et al., 2021). Our data also showed that rapeseed growth was notably improved under blue light, regardless of the NH4+:NO3− ratios (Figures 2, 3; Table 2), further demonstrating the beneficial effects of blue light on rapeseed plants.
Moreover, although growth parameters were severely inhibited in rapeseed plants grown under a 100% NH4+ regime with white light, blue light-spiked plants showed a significant alleviation of NH4+ toxicity, reducing these growth limitations (Figures 2, 3; Table 2). This finding further confirms the alleviatory role of blue light under this abiotic stress and contributes to sustainable crop production and higher productivity quality. In solely NH4+-cultured rapeseed plants, blue light treatment significantly reduced the occurrence of NH4+ toxicity (%) from 91.7% to 41.7%, compared to plants grown under white light (Figure 4). These results also demonstrated that NH4+ toxicity can be notably lessened in rapeseed after blue light treatment.
The photosynthesis of rapeseed plants was also severely limited when 100% NH4+ nutrition was applied, regardless of light quality, as indicated by the significantly reduced Pn, gs, Tr, chlorophylls, and carotenoids (Figure 5). This phenomenon was likely due to abiotic stresses impairing the performance of photosystems, chlorophyll biosynthesis, gas exchange parameters, and electron transport mechanisms (Sharma et al., 2020). In addition, overproduction of ROS caused oxidative damage, interfered with electron transport mechanisms, and even injured the chloroplast (Sharma et al., 2020; Guo et al., 2016). These damages to photosynthesis caused by high NH4+ nutrition are consistent with many previous reports, including those on cabbage (Song et al., 2021), salvia (Song et al., 2022b), and basil (Song et al., 2024b). As expected, the inhibition of photosynthesis by 100% NH4+ supply was markedly mitigated when rapeseed plants were grown under blue light (Figure 5). Blue light significantly improved the investigated photosynthesis-related parameters, further confirming its ability to alleviate NH4+ toxicity and enhance photosynthetic capacity. Hogewoning et al. (2010) reported that leaf photosynthesis increased quantitatively when the proportion of blue light reached a “qualitative” or “threshold”. Blue light has also been shown to promote stomatal opening more effectively than other wavelengths, resulting in higher photosynthetic rates and providing additional morphogenetic benefits (Wang et al., 2015). Overall, blue light treatment effectively attenuated the decreases in Pn, gs, Tr, and chlorophyll content, thereby maintaining photosynthetic capacity.
In this study, the blue light treatment was not 100% but was mixed with other light qualities (Figure 1b). Compared with white LED light, we increased the proportion of blue light and reduced other light qualities to achieve the blue light treatment. Previous studies found that physiological disorders in cucumber were eliminated after adding a small amount of blue light (Hogewoning et al., 2010). Hoffmann et al. (2015) demonstrated that higher amounts of blue light triggered better photosynthetic performance and greater pigment accumulation. Our data are consistent with these findings, showing that increasing the proportion of blue light promotes photosynthetic characteristics.
Usually, a dynamic equilibrium between the accumulation and scavenging of ROS is well maintained in plants under a normal environment (Gong et al., 2008; Song et al., 2022a). Meanwhile, the steady-state level of ROS is regulated in association with the antioxidant defense systems, mainly through stimulated antioxidative enzymes (Ali et al., 2019). For instance, as a prime candidate, SOD participates not only in the conversion of O2.− into H2O2 but also in the subsequent decomposition of H2O2 to H2O via APX, CAT, and GPX (Gong et al., 2008; Ali et al., 2019; Song et al., 2022a). Nevertheless, NH4+ toxicity causes imbalances and disturbances in redox signaling, as evidenced by increased accumulations of O2.− and H2O2 (Figures 6A [a, b]), suggesting that substantial externally spiked NH4+ obstructs the dynamic balance of ROS between production and elimination in rapeseed plants. In addition, antioxidant enzyme activities failed to increase in response to progressively higher NH4+ supply from 50% to 100%, regardless of light quality, further confirming that rapeseed plants in this experiment were extremely sensitive to high NH4+.
Many studies have explored the impacts of blue light on the regulation of the oxidative defense system, showing that blue light can induce tolerance against abiotic stresses (Sebastian and Prasad, 2014; Pech et al., 2024). In our investigation, blue light stimulated higher activities of antioxidant enzymes compared with those grown in white light, thereby neutralizing excessive O2.− and H2O2 (Figure 6A). This enhancement of antioxidant enzymes provided a protective role of blue light on the cell membrane (Shao et al., 2020). These findings are in line with previous publications showing that blue light treatment can enhance the antioxidant defense system and improve plant quality (Xu et al., 2014; Manivannan et al., 2015). NH4+ toxicity-caused excessive oxidative stress in rapeseed plants was clearly alleviated when plants were cultured under blue light conditions. Phototropins (PHOT) are one of three classes of receptors that modulate blue light responses. They are cytosolic and plasma membrane-associated photoreceptors that play important roles in adaptation to oxidative stresses (Chibani et al., 2025). Cryptochromes (Crys) are flavin-binding blue light receptors that regulate ROS generation. They absorbed blue light and act as key regulators in response to multiple abiotic stresses (El-Esawi et al., 2017). The mitigation of NH4+ toxicity-caused excessive oxidative stress by blue light in this study may be partially attributed to these blue light receptors.
In higher plants, the N-use pathway is conserved and involved in many biological and biochemical processes (i.e., uptake, assimilation, and translocation) (Masclaux-Daubresse et al., 2010). Plants were not to assimilate NO3− directly, but reduce it to NO2− via NR. NO2− is further converted to NH4+ via NIR, and NH4+ is finally incorporated, catalyzed, and assimilated through the GS/GOGAT pathway or alternatively taken up by GDH (Figure 7A) (Cruz et al., 2006; Bittsánszky et al., 2015; Song et al., 2021). A positive correlation between NR activity and free NO3− content was observed, and similarly, high NH4+ supply led to high free NH4+ accumulation in plants (Figures 7A [a, b, e]), consistent with previous reports (Cruz et al., 2006; Horchani et al., 2010; Liu et al., 2017; Song et al., 2021). Unlike NR, NIR activity decreased significantly only when external NH4+ supply increased rapidly from 50% to 100%, regardless of light quality, in agreement with Song et al. (2021). Regulation of NR and NIR under high NO3− or NH4+ supply could inevitably affect the activities of downstream GS, GOGAT, and GDH (Song et al., 2021). We observed that the enhancement of GS and NADH-GDH under external NH4+ supply increased from 0% to 50%, illustrating a regulatory mechanism in rapeseed in response to high environmental NH4+. By contrast, an opposite regulatory pattern of GOGAT compared with GS and NADH-GDH was observed (Figures 7A [h]).
Most importantly, blue light treatments on rapeseed significantly improved the N assimilation pathway-related enzymes and chemicals mentioned above compared with plants cultured under white light (Figure 7A). Numerous pioneering studies have shown that NH4+ tolerance is improved by progressive and sustained upregulations of NH4+ assimilation enzyme activities (Horchani et al., 2010; Esteban et al., 2016; Song et al., 2021, 2022, 2022, 2022). Blue light-treated rapeseed plants exhibited enhanced N assimilation enzymes and chemicals in the N assimilation pathway, suggesting positive effects conferred by blue light due to better growth performance under a blue light environment (Figures 2, 3; Table 2). Notably, blue light-treated plants showed increased activities of NR, NIR, GS, GOGAT, and NADH-GDH and markedly reduced NH4+ toxicity in plants treated with 100% NH4+ (Figures 4a, 7A). Additionally, boosting or maintaining high GS and GDH levels is considered an important strategy for NH4+ tolerance; in other words, GS and GDH can underpin NH4+ tolerance to some extent in plant species (Song et al., 2021, 2022; Xian et al., 2020). As a consequence, the improved NR/NIR route, GS/GOGAT cycle, and GDH/GOGAT cycle contribute to enhanced NUE and NH4+ tolerance, thereby reducing NH4+ toxicity (Esteban et al., 2016; Peng et al., 2023). Blue light-treated plants also showed higher free contents of NO3−, NO2−, and NH4+ compared with those cultured under white light (Figure 7A), indicating that a higher uptake rate of these chemicals was associated with improved NUE under blue light. Accordingly, NH4+ toxicity in rapeseed plants can be alleviated by blue light treatment through improvement of the N assimilation pathway, including GS and GDH activities.
Interestingly, a similar study by Sakuraba and Yanagisawa (2018) revealed that phytochromes (Phys) and Crys perceive blue light signals and play an important role in nitrate assimilation and the regulation of NR activity. In cabbage, certain phytochrome-related genes (PHYA, PHYC, and PHYE) and cryptochrome-related genes (CRY2a and CRY2b) were significantly upregulated under a blue light regime compared to white light, resulting in higher concentrations of NR, NIR, and GS (Fan et al., 2022). These blue light receptors may also participate in the N metabolism pathway in plants.
5 Conclusion
In summary, this work demonstrates that NH4+ toxicity in rapeseed plants is significantly alleviated under blue LED light treatment. This amelioration effect in NH4+-stressed rapeseed plants involves multiple aspects, mainly including ameliorated growth, enhanced photosynthesis, strengthened antioxidative machinery, and a more efficient N assimilation pathway. These findings suggest that blue light is highly effective in reinforcing the NH4+ tolerance and promoting the growth and quality of rapeseed plants.
The current endeavor not only provides new avenues for applying blue light to enhance NH4+ tolerance but also offers considerable practical value in PFALs for increasing rapeseed productivity. Concomitantly, plant yield and safety could be ensured under this lighting regime, and a precise manipulation of blue light in practical agriculture could alter plant morphological plasticity, thereby promoting desirable commercial value. Further study of the alleviatory role of blue light in NH4+ toxicity at the molecular level is warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WL: Software, Investigation, Writing – review & editing, Writing – original draft, Methodology. JS: Validation, Resources, Conceptualization, Software, Supervision, Writing – review & editing, Funding acquisition, Formal analysis, Writing – original draft, Project administration, Visualization, Methodology, Data curation. QS: Methodology, Software, Investigation, Writing – review & editing. JY: Resources, Investigation, Writing – review & editing. JZ: Resources, Writing – review & editing. HX: Resources, Writing – review & editing. DP: Resources, Writing – review & editing. MS: Resources, Writing – review & editing. BJ: Conceptualization, Writing – review & editing, Resources.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was financially supported by the young researcher project (KJRC2023018) from Weifang University of Science and Technology.
Acknowledgments
The authors thank Mengxuan Zhu (Department of Jiasixie Agriculture, Weifang University of Science and Technology) and Na Li (Department of Jiasixie Agriculture, Weifang University of Science and Technology) for their assistances in investigating the activities of enzymes activities and chemicals in the N assimilation pathway.
Conflict of interest
Author MS was employed by the company Jingzhi-Maoteng Agricultural Technology Limited Company in Qushui County.
The remaining authors declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ali, M., Cheng, Z.-H., Hayat, S., Ahmad, H., Ghani, M. I., and Liu, T. (2019). Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L.). J. Int. Agr. 18, 1001–1013. doi: 10.1016/S2095-3119(18)62129-X
Amako, K., Chen, G.-X., and Asada, K. (1994). Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 35, 497–504. doi: 10.1093/oxfordjournals.pcp.a078621
Bittsánszky, A., Pilinszky, K., Gyulai, G., and Komives, T. (2015). Overcoming ammonium toxicity. Plant Sci. 231, 184–190. doi: 10.1016/j.plantsci.2014.12.005
Bloom, A. J. (2015). The increasing importance of distinguishing among plant nitrogen sources. Curr. Opin. Plant Biol. 25, 10–16. doi: 10.1016/j.pbi.2015.03.002
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Bräutigam, A., Gagneul, D., and Weber, A. P. (2007). High-throughput colorimetric method for the parallel assay of glyoxylic acid and ammonium in a single extract. Anal. Biochem. 362, 151–153. doi: 10.1016/j.ab.2006.12.033
Cakmak, I. and Marschner, H. (1992). Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 98, 1222–1227. doi: 10.1104/pp.98.4.1222
Cataldo, D. A., Maroon, M., Schrader, L. E., and Youngs, V. L. (1975). Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plan. 6, 71–80. doi: 10.1080/00103627509366547
Chibani, K., Gherli, H., and Fan, M. (2025). The role of blue light in plant stress responses: modulation through photoreceptors and antioxidant mechanisms. Front. Plant Sci. 16. doi: 10.3389/fpls.2025.1554281
Cruz, C., Bio, A., Domínguez-Valdivia, M., Aparicio-Tejo, P. M., Lamsfus, C., and Martins-Louçao, M. A. (2006). How does glutamine synthetase activity determine plant tolerance to ammonium? Planta 223, 1068–1080. doi: 10.1007/s00425-005-0155-2
El-Esawi, M., Arthaut, L. D., Jourdan, N., d’Harlingue, A., Link, J., Martino, C. F., et al. (2017). Blue-light induced biosynthesis of ROS contributes to the signaling mechanism of Arabidopsis cryptochrome. Sci. Rep. 7, 13875. doi: 10.1038/s41598-017-13832-z
Esteban, R., Ariz, I., Cruz, C., and Moran, J. F. (2016). Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 248, 92–101. doi: 10.1016/j.plantsci.2016.04.008
Fan, X. X., Bian, Z. H., Song, B., and Xu, H. (2022). Transcriptome analysis reveals the differential regulatory effects of red and blue light on nitrate metabolism in pakchoi (Brassica campestris L.). J. Integr. Agric. 21, 1015–1027. doi: 10.1016/S2095-3119(21)63784-X
Giannopolitis, C. N. and Ries, S. K. (1977). Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 59, 309–314. doi: 10.1104/pp.59.2.309
Gong, H., Chen, K., Zhao, Z., Chen, G., and Zhou, W. (2008). Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biol. Plantarum 52, 592–596. doi: 10.1007/s10535-008-0118-0
Guo, H., Hong, C., Chen, X., Xu, Y., Liu, Y., Jiang, D., et al. (2016). Different growth and physiological responses to cadmium of the three Miscanthus species. PloS One 11, e0153475. doi: 10.1371/journal.pone.0153475
Guo, S., Brück, H., and Sattelmacher, B. (2002). Effects of supplied nitrogen form on growth and water uptake of French bean (Phaseolus vulgaris L.) plants. Plant Soil 239, 267–275. doi: 10.1023/A:1015014417018
Guo, S., Zhou, Y., Shen, Q., and Zhang, F. (2007). Effect of ammonium and nitrate nutrition on some physiological processes in higher plants-growth, photosynthesis, photorespiration, and water relations. Plant Biol. 9, 21–29. doi: 10.1055/s-2006-924541
Hachiya, T., Inaba, J., Wakazaki, M., Sato, M., Toyooka, K., Miyagi, A., et al. (2021). Excessive ammonium assimilation by plastidic glutamine synthetase causes ammonium toxicity in Arabidopsis thaliana. Nat. Commun. 12, 4944. doi: 10.1038/s41467-021-25238-7
Hoffmann, A. M., Noga, G., and Hunsche, M. (2015). High blue light improves acclimation and photosynthetic recovery of pepper plants exposed to UV stress. Environ. Exp. Bot. 109, 254–263. doi: 10.1016/j.envexpbot.2014.06.017
Högberg, P., Granström, A., Johansson, T., Lundmark-Thelin, A., and Näsholm, T. (1986). Plant nitrate reductase activity as an indicator of availability of nitrate in forest soils. Can. J. For. Res. 16, 1165–1169. doi: 10.1139/x86-207
Hogewoning, S. W., Trouwborst, G., Maljaars, H., Poorter, H., van Ieperen, W., and Harbinson, J. (2010). Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 61, 3107–3117. doi: 10.1093/jxb/erq132
Horchani, F., Hajri, R., and Aschi-Smiti, S. (2010). Effect of ammonium or nitrate nutrition on photosynthesis, growth, and nitrogen assimilation in tomato plants. J. Plant Nutr. Soil Sc. 173, 610–617. doi: 10.1002/jpln.201000055
Huang, L., Li, M., Zhou, K., Sun, T., Hu, L., Li, C., et al. (2018). Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol. Bioch. 127, 185–193. doi: 10.1016/j.plaphy.2018.03.031
Jin, D., Su, X., Li, Y., Shi, M., Yang, B., Wan, W., et al. (2023). Effect of red and blue light on cucumber seedlings grown in a plant factory. Horticulturae 9, 124. doi: 10.3390/horticulturae9020124
Kanamori, T., Konishi, S., and Takahashi, E. (1972). Inducible formation of glutamate dehydrogenase in rice plant roots by the addition of ammonia to the media. Physiol. Plant 26, 1–6. doi: 10.1111/j.1399-3054.1972.tb03536.x
Kong, Y. and Zheng, Y. (2023). Magic blue light: A versatile mediator of plant elongation. Plants 13, 115. doi: 10.3390/plants13010115
Lee, H., Han, G., and Cheong, E. J. (2021). Effect of different treatments and light quality on Ulmus pumila L. germination and seedling growth. For. Sci. Technol. 17, 162–168. doi: 10.1080/21580103.2021.1968960
Leghari, S. J., Wahocho, N. A., Laghari, G. M., HafeezLaghari, A., MustafaBhabhan, G., HussainTalpur, K., et al. (2016). Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 10, 209–219.
Lei, Y., Shah, T., Cheng, Y., Yan, L., and Zhang, X.-K. (2019). Physiological and molecular responses to cold stress in rapeseed (Brassica napus L.). J. Integr. Agr. 18, 2742–2752. doi: 10.1016/S2095-3119(18)62147-1
Li, Y., Liu, C., Shi, Q., Yang, F., and Wei, M. (2021). Mixed red and blue light promotes ripening and improves quality of tomato fruit by influencing melatonin content. Environ. Exp. Bot. 185, 104407. doi: 10.1016/j.envexpbot.2021.104407
Li, S., Yan, L., Riaz, M., White, P. J., Yi, C., Wang, S., et al. (2021). Integrated transcriptome and metabolome analysis reveals the physiological and molecular responses of allotetraploid rapeseed to ammonium toxicity. Environ. Exp. Bot. 189, 104550. doi: 10.1016/j.envexpbot.2021.104550
Li, S., Yan, L., Zhang, W., Yi, C., Haider, S., Wang, C., et al. (2024). Nitrate alleviates ammonium toxicity in Brassica napus by coordinating rhizosphere and cell pH and ammonium assimilation. Plant J. 117, 786–804. doi: 10.1111/tpj.16529
Li, X., Zhao, S., Cao, Q., Qiu, C., Yang, Y., Zhang, G., et al. (2024). Effect of Green Light Replacing Some Red and Blue Light on Cucumis melo under Drought Stress. Int. J. Mol. Sci. 25, 7561. doi: 10.3390/ijms25147561
Lin, C. C. and Kao, C. H. (1996). Disturbed ammonium assimilation is associated with growth inhibition of roots in rice seedlings caused by NaCl. Plant Growth Regul. 18, 233–238. doi: 10.1007/BF00024387
Liu, X., Chen, Z., Jahan, M. S., Wen, Y., Yao, X., Ding, H., et al. (2020). RNA-Seq analysis reveals the growth and photosynthetic responses of rapeseed (Brassica napus L.) under red and blue LEDs with supplemental yellow, green, or white light. Hortic. Res. 7. doi: 10.1038/s41438-020-00429-3
Liu, G., Du, Q., and Li, J. (2017). Interactive effects of nitrate-ammonium ratios and temperatures on growth, photosynthesis, and nitrogen metabolism of tomato seedlings. Sci. Hortic. 214, 41–50. doi: 10.1016/j.scienta.2016.09.006
Luo, J., Li, H., Liu, T., Polle, A., Peng, C., and Luo, Z.-B. (2013). Nitrogen metabolism of two contrasting poplar species during acclimation to limiting nitrogen availability. J. Exp. Bot. 64, 4207–4224. doi: 10.1093/jxb/ert234
Ma, Y., Hu, L., Wu, Y., Tang, Z., Xiao, X., Lyu, J., et al. (2022). Green light partial replacement of red and blue light improved drought tolerance by regulating water use efficiency in cucumber seedlings. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.878932
Manivannan, A., Soundararajan, P., Halimah, N., Ko, C. H., and Jeong, B. R. (2015). Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 56, 105–113. doi: 10.1007/s13580-015-0114-1
Masclaux-Daubresse, C., Daniel-Vedele, F., Dechorgnat, J., Chardon, F., Gaufichon, L., and Suzuki, A. (2010). Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann. Bot. 105, 1141–1157. doi: 10.1093/aob/mcq028
Mavis, R. D. and Stellwagen, E. (1968). Purification and subunit structure of glutathione reductase from bakers’ yeast. J. Biol. Chem. 243, 809–814. doi: 10.1016/S0021-9258(19)81737-4
Morello, V., Brousseau, V. D., Wu, N., Wu, B.-S., MacPherson, S., and Lefsrud, M. (2022). Light quality impacts vertical growth rate, phytochemical yield and cannabinoid production efficiency in Cannabis sativa. Plants 11, 2982. doi: 10.3390/plants11212982
Moshage, H., Kok, B., Huizenga, J. R., and Jansen, P. (1995). Nitrite and nitrate determinations in plasma: a critical evaluation. Clin. Chem. 41, 892–896. doi: 10.1093/clinchem/41.6.892
Mukherjee, S. and Choudhuri, M. (1983). Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant 58, 166–170. doi: 10.1111/j.1399-3054.1983.tb04162.x
Nakano, Y. and Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880. doi: 10.1093/OXFORDJOURNALS.PCP.A076232
Naznin, M. T., Lefsrud, M., Gravel, V., and Azad, M. O. K. (2019). Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 8, 93. doi: 10.3390/plants8040093
Oaks, A., Stulen, I., Jones, K., Winspear, M. J., Misra, S., and Boesel, I. L. (1980). Enzymes of nitrogen assimilation in maize roots. Planta 148, 477–484. doi: 10.1007/BF02395318
Ogawa, T., Fukuoka, H., Yano, H., and Ohkawa, Y. (1999). Relationships between nitrite reductase activity and genotype-dependent callus growth in rice cell cultures. Plant Cell Rep. 18, 576–581. doi: 10.1007/s002990050625
Pech, R., Volná, A., Špunda, V., and Nezval, J. (2024). Blue light as an important factor increasing plant tolerance to acute photooxidative stress. Environ. Exp. Bot. 226, 105923. doi: 10.1016/j.envexpbot.2024.105923
Peng, W., ZHANG, Y., and Yi, S. (2023). Increasing nitrogen absorption and assimilation ability under mixed NO3– and NH4+ supply is a driver to promote growth of maize seedlings. J. Integr. Agr. 22, 1896–1908. doi: 10.1016/j.jia.2023.04.037
Picetti, R., Deeney, M., Pastorino, S., Miller, M. R., Shah, A., Leon, D. A., et al. (2022). Nitrate and nitrite contamination in drinking water and cancer risk: A systematic review with meta-analysis. Environ. Res. 210, 112988. doi: 10.1016/j.envres.2022.112988
Ren, M., Liu, S., Mao, G., Tang, C., Gai, P., Guo, X., et al. (2023). Simultaneous application of red and blue light regulate carbon and nitrogen metabolism, induces antioxidant defense system and promote growth in rice seedlings under low light stress. Int. J. Mol. Sci. 24, 10706. doi: 10.3390/ijms241310706
Roeber, V. M., Bajaj, I., Rohde, M., Schmülling, T., and Cortleven, A. (2021). Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 44, 645–664. doi: 10.1111/pce.13948
Roosta, H. R. and Schjoerring, J. K. (2007). Effects of ammonium toxicity on nitrogen metabolism and elemental profile of cucumber plants. J. Plant Nutr. 30, 1933–1951. doi: 10.1080/01904160701629211
Sakuraba, Y. and Yanagisawa, S. (2018). “Light signalling-induced regulation of nutrient acquisition and utilisation in plants,” in Seminars in cell & developmental biology (Amsterdam, Netherlands: Elsevier), 123–132. doi: 10.1016/j.semcdb.2017.12.014
Sebastian, A. and Prasad, M. (2014). Red and blue lights induced oxidative stress tolerance promote cadmium rhizocomplexation in Oryza sativa. J. Photoch. Photobio. B 137, 135–143. doi: 10.1016/j.jphotobiol.2013.12.011
Shao, M., Liu, W., Zha, L., Zhou, C., Zhang, Y., and Li, B. (2020). Differential effects of high light duration on growth, nutritional quality, and oxidative stress of hydroponic lettuce under red and blue LED irradiation. Sci. Hortic. 268, 109366. doi: 10.1016/j.scienta.2020.109366
Sharma, A., Kumar, V., Shahzad, B., Ramakrishnan, M., Singh Sidhu, G. P., Bali, A. S., et al. (2020). Photosynthetic response of plants under different abiotic stresses: a review. J. Plant Growth Regul. 39, 509–531. doi: 10.1007/s00344-019-10018-x
Shilpha, J., Song, J., and Jeong, B. R. (2023). Ammonium phytotoxicity and tolerance: An insight into ammonium nutrition to improve crop productivity. Agronomy 13, 1487. doi: 10.3390/agronomy13061487
Sims, D. A. and Gamon, J. A. (2002). Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 81, 337–354. doi: 10.1016/S0034-4257(02)00010-X
Song, J., Yang, J., Dong, X., Zhang, H., and Jeong, B. (2024a). Supplemented biochar mitigates the ammonium toxicity in basil (Ocimum basilicum L.) plants. Russ. J. Plant Physl. 71, 189. doi: 10.1134/S1021443724605512
Song, J., Yang, J., and Jeong, B. R. (2021). Growth, quality, and nitrogen assimilation in response to high ammonium or nitrate supply in cabbage (Brassica campestris L.) and lettuce (Lactuca sativa L.). Agronomy 11, 2556. doi: 10.3390/agronomy11122556
Song, J., Yang, J., and Jeong, B. R. (2022a). Silicon mitigates ammonium toxicity in cabbage (Brassica campestris L. ssp. pekinensis) ‘Ssamchu’. Front. Sustain. Food Syst. 6. doi: 10.3389/fsufs.2022.922666
Song, J., Yang, J., and Jeong, B. R. (2022b). Alleviation of ammonium toxicity in salvia splendens ‘Vista Red’ with silicon supplementation. Toxics 10, 446. doi: 10.3390/toxics10080446
Song, J., Yang, J., and Jeong, B. R. (2022c). Root GS and NADH-GDH play important roles in enhancing the ammonium tolerance in three bedding plants. Int. J. Mol. Sci. 23, 1061. doi: 10.3390/ijms23031061
Song, J., Yang, J., and Jeong, B. R. (2024). Characterization of physiology, photosynthesis, and nutrition based on induced deficiencies of macro-and micronutrients in basil (Ocimum basilicum L.). Agronomy 14, 208. doi: 10.3390/agronomy14010208
Trojak, M., Skowron, E., Sobala, T., Kocurek, M., and Pałyga, J. (2022). Effects of partial replacement of red by green light in the growth spectrum on photomorphogenesis and photosynthesis in tomato plants. Photosynthesis Res. 151, 295–312. doi: 10.1007/s11120-021-00879-3
Wang, X., Xu, X., and Cui, J. (2015). The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 53, 213–222. doi: 10.1007/s11099-015-0083-8
Wang, J., Zhou, W., Chen, H., Zhan, J., He, C., and Wang, Q. (2019). Ammonium nitrogen tolerant Chlorella strain screening and its damaging effects on photosynthesis. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.03250
Wu, Y.-x. and von Tiedemann, A. (2002). Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ. pollut. 116, 37–47. doi: 10.1016/S0269-7491(01)00174-9
Xian, L., Zhang, Y., Cao, Y., Wan, T., Gong, Y., Dai, C., et al. (2020). Glutamate dehydrogenase plays an important role in ammonium detoxification by submerged macrophytes. Sci. Total Environ. 722, 137859. doi: 10.1016/j.scitotenv.2020.137859
Xu, F., Shi, L., Chen, W., Cao, S., Su, X., and Yang, Z. (2014). Effect of blue light treatment on fruit quality, antioxidant enzymes and radical-scavenging activity in strawberry fruit. Sci. Hortic. 175, 181–186. doi: 10.1016/j.scienta.2014.06.012
Yamori, W., Kusumi, K., Iba, K., and Terashima, I. (2020). Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant Cell Environ. 43, 1230–1240. doi: 10.1111/pce.13725
Zhang, H. and Flottmann, S. (2016). Seed yield of canola (Brassica napus L.) is determined primarily by biomass in a high-yielding environment. Crop Pasture Sci. 67, 369–380. doi: 10.1071/CP15236
Zhang, R., Yang, W., Pan, Q., Zeng, Q., Yan, C., Bai, X., et al. (2023). Effects of long-term blue light irradiation on carotenoid biosynthesis and antioxidant activities in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Food Res. Int. 174, 113661. doi: 10.1016/j.foodres.2023.113661
Zheng, L. and Van Labeke, M.-C. (2017). Long-term effects of red-and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00917
Keywords: antioxidant enzymes, nitrogen assimilation pathway, nitrogen nutrition, photosynthetic ability, rapeseed
Citation: Li W, Song J, Sun Q, Yang J, Zhang J, Xu H, Peng D, Sang M and Jeong BR (2025) LED-supplied blue light mitigates ammonium toxicity in rapeseed (Brassica napus L.). Front. Plant Sci. 16:1724504. doi: 10.3389/fpls.2025.1724504
Received: 14 October 2025; Accepted: 27 November 2025; Revised: 18 November 2025;
Published: 16 December 2025.
Edited by:
Hafiz Hassan Javed, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Tatjana Shibaeva, Institute of Biology of Karelian Research Centre (RAS), RussiaFrank H., Sichuan Agricultural University, China
Copyright © 2025 Li, Song, Sun, Yang, Zhang, Xu, Peng, Sang and Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinnan Song, amlubmFuc29uZzkzQGdtYWlsLmNvbQ==
†ORCID: Jinnan Song, orcid.org/0000-0002-8821-2678
 Wenjing Li1
Wenjing Li1 Jinnan Song
Jinnan Song Byoung Ryong Jeong
Byoung Ryong Jeong