- 1College of Rehabilitation Medicine, Fujian University of Traditional Chinese Medicine, Fuzhou, China
- 2Provincial Clinical Medical College, Fujian Medical University, Fuzhou, China
- 3Rehabilitation Medicine Center, Fujian Provincial Hospital, Fuzhou, China
- 4Rehabilitation Medicine Center, Fuzhou University Affiliated Provincial Hospital, Fuzhou, China
- 5College of Acupuncture and Tuina, Fujian University of Traditional Chinese Medicine, Fuzhou, China
- 6Department of Rehabilitation Medicine, Quanzhou First Hospital Affiliated to Fujian Medical University, Quanzhou, China
Background and objective: Despite the extensive utilization of proprioceptive exercise in the management of knee osteoarthritis (KOA), the therapeutic efficacy of this approach remains inconclusive. The present study sought to systematically evaluate the effects of proprioceptive exercise on symptoms and functional outcomes in patients with KOA, with a particular focus on balance performance.
Methods: Following PRISMA guidelines, a comprehensive search was conducted across six electronic databases from the establishment of the database to January 21, 2025. The inclusion criteria were randomized controlled trials investigating proprioceptive exercise interventions for KOA. The primary outcome measures encompassed balance function assessment (Timed Up and Go test), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) total score and its pain, stiffness, and function subscales, and pain intensity (Numerical Rating Scale and Visual Analog Scale). Subgroup analyses were stratified by intervention duration (≤8 weeks vs. >8 weeks).
Results: A comprehensive analysis of 22 randomized controlled trials revealed that proprioceptive exercise significantly improved performance of the Timed Up and Go test [MD = 1.53, 95% CI (1.09, 1.97), I2 = 0%, P < 0.00001]. Additionally, a significant improvement in WOMAC-total scores was observed [MD = 3.37, 95% CI (1.58, 5.16), I2 = 44%, P = 0.0002]. However, individual WOMAC subscales for pain (P = 0.11, I2 = 85%), stiffness (P = 0.97, I2 = 0%), and function (P = 0.16, I2 = 86%) showed no significant improvements. For pain assessment, Numerical Rating Scale scores showed a significant improvement [MD = 0.85, 95% CI (0.56, 1.15), I2 = 46%, P < 0.00001]. Notably, Visual Analog Scale scores exhibited a significant reduction, but only in the short-term intervention subgroup (≤8 weeks) [MD = 0.27, 95% CI (0.11, 0.42), I2 = 0%, P = 0.0008], whereas longer interventions (>8 weeks) showed no significant benefit [MD = −0.49, 95% CI (−1.10, 0.11), I2 = 0%, P = 0.11].
Conclusion: Based on low-certainty evidence, proprioceptive exercise has been demonstrated to be efficacious in improving balance function and overall clinical status in patients with KOA. Optimal benefits have been observed during short-term intervention periods.
1 Introduction
Knee osteoarthritis (KOA) is a common degenerative joint disease (1), with global epidemiological data indicating a prevalence of approximately 16% (2), which continues to increase with the aging population. Research has indicated that patients with KOA demonstrate a considerably elevated risk of falling, with approximately 50%–60% of patients with KOA over the age of 60 reporting at least one fall per year (3). This increased risk not only compromises quality of life but also imposes substantial economic burdens on patients, families, and healthcare systems (4, 5).
A substantial body of research has identified a strong correlation between falls and balance dysfunction (6, 7). Patients with KOA suffer from proprioceptive deficits due to structural changes in the joints and decreased mechanoreceptor function, which means that the body's ability to sense its own position, movement, and strength is impaired (8–10). The proprioceptive system relies primarily on mechanoreceptors located in joints, muscles, and ligaments to maintain normal motor control by transmitting information about joint angles, muscle tension, and speed of movement (11). As KOA progresses, impaired proprioceptive function results in a significant reduction in the accuracy of joint position and motion perception, which in turn weakens the effectiveness of the joint stability control system. When proprioceptive inputs are insufficient or inaccurate, the central nervous system is unable to accurately assess the body's position and motion status in space, resulting in temporal delays and inappropriate magnitude of compensatory postural adjustments. This condition manifests specifically as a decrease in anticipatory postural adjustment and a decrease in the efficiency of reactive balance control, and the patient walks with abnormalities such as shortened stride length, gait asymmetry, and increased gait variability, and the risk of falling is significantly increased, especially in complex environments such as turning corners, crossing an obstacle, or coping with uneven surfaces (12, 13). In summary, impaired proprioception weakens the function of the stability control system and ultimately increases the risk of falling.
Patients with KOA suffer from proprioceptive deficits due to structural changes in the joints and decreased mechanoreceptor function, which means that the body's ability to sense its own position, movement, and strength is impaired. The proprioceptive system relies primarily on mechanoreceptors located in joints, muscles, and ligaments to maintain normal motor control by transmitting information about joint angles, muscle tension, and speed of movement. As KOA progresses, impaired proprioceptive function results in a significant reduction in the accuracy of joint position and motion perception, which in turn weakens the effectiveness of the joint stability control system. When proprioceptive inputs are insufficient or inaccurate, the central nervous system is unable to accurately assess the body's position and motion status in space, resulting in temporal delays and inappropriate magnitude of compensatory postural adjustments. This condition manifests specifically as a decrease in anticipatory postural adjustment and a decrease in the efficiency of reactive balance control, and the patient walks with abnormalities such as shortened stride length, gait asymmetry, and increased gait variability, and the risk of falling is significantly increased, especially in complex environments such as turning corners, crossing an obstacle, or coping with uneven surfaces. In summary, impaired proprioception weakens the function of the stability control system and ultimately increases the risk of falling.
Currently, the predominant clinical intervention for proprioceptive dysfunction is proprioceptive exercise (14), which enhances neuromuscular control by means of the stimulation of the mechanoreceptors of the joints and muscles, thereby improving joint position sense and balance. The training modality of proprioceptive exercise is versatile and can be performed in a number of ways. These include joint position perception training (15, 16) or training with the aid of equipment such as wobble boards (17, 18). However, there is considerable heterogeneity in the implementation protocols for proprioceptive exercise, and the current research is divided in its evaluation of the efficacy of this intervention. Some studies have found proprioceptive exercise to be effective in improving pain and function in patients (19–21), while others have shown that the effects may be limited (22, 23). The findings from previous meta-analyses also demonstrate significant heterogeneity, with the study by Jeong et al. indicating that the effect of proprioceptive training on improving pain and function was in the lower range of the smallest clinically significant difference (24), whereas the study by Smith et al. suggests that it may be advantageous in terms of functional improvement (25). In a recent study, Wang et al. conducted a meta-analysis and found that proprioceptive training significantly improved pain, stiffness, physical function and proprioception in patients with KOA (26). However, these previous meta-analyses had notable limitations. Jeong et al.'s review was restricted to only seven randomized controlled trials with a limited cumulative sample size of 558 participants, while Smith et al. included only seven studies with a total of 560 participants. Neither study adequately explored the sources of the high heterogeneity observed in their analyses, despite reporting substantial heterogeneity in several outcomes (I2 reaching 65% for pain and 86% for function in the study by Jeong et al, and up to 88% for function in the study by Smith et al). Furthermore, their primary focus remained on outcomes such as pain, joint function and proprioception, while balance function, a crucial clinical indicator in patients with KOA, was inadequately addressed.
Based on the limitations, we conducted this systematic review and meta-analysis to comprehensively evaluate the effects of proprioceptive exercise on knee joint symptoms and function in patients with KOA, especially attention to its role in improving balance ability. Additionally, given that previous studies have indicated that adaptive changes in the neuromuscular system typically occur within 4–8 weeks (27–29), intervention duration may be an important factor affecting therapeutic efficacy. Consequently, this study investigated the possible influence of intervention duration on clinical outcomes. Through these analyses, we aimed to provide more comprehensive evidence-based support for clinical practice.
2 Methods
We conducted this study following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (30) and registered the protocol with PROSPERO (registration number: CRD42025642296).
2.1 Search strategy
A comprehensive literature search was conducted in six electronic databases, including PubMed, Embase, Cochrane Library, Web of Science, CINAHL Complete, and Scopus. All searches were performed from the establishment of the database to January 21, 2025. The search strategy employed a combination of medical subject headings (MeSH) and free terms, including “knee osteoarthritis”, “proprioception”, and “randomized controlled trial”. The search strategy employed for the PubMed database is shown in Table 1, and other databases used a similar search strategy, adapted to the characteristics of each database (Supplementary S1). Additionally, manual screening of reference lists from included studies and relevant systematic reviews was conducted to identify any potentially eligible studies that may have been missed during the electronic database search.
2.2 Eligibility criteria
Two investigators independently reviewed titles and abstracts and evaluated the full texts of potentially eligible studies according to predetermined inclusion and exclusion criteria. Any disagreements were resolved through discussion or consultation with a third investigator.
Inclusion criteria encompassed: (a) randomized controlled trials (RCT); (b) participants with clinical or radiographic diagnosis of KOA according to established criteria; (c) proprioceptive exercise interventions, including proprioceptive training, apparatus-assisted exercise (e.g., wobble boards, unstable platforms), balance training, neuromuscular training, or sensorimotor training. In this review, proprioceptive exercise was operationally defined as structured physical interventions designed to enhance joint position sense, neuromuscular control, and balance function through targeted stimulation of mechanoreceptors in joints and muscles. To qualify for inclusion, interventions needed to meet the following minimum criteria: intervention duration of at least 2 weeks; for short-term interventions (≤8 weeks), a frequency of at least once a week on average; for long-term interventions (>8 weeks), a frequency of at least once every two weeks on average; (d) comparator groups comprising either a blank control or other non-surgical treatment methods (intervention measures can be implemented in clinical, family, or collaborative environments, potentially involving personalized or group training under professional supervision); and (e) published by English.
Exclusion criteria encompassed: (a) research not using an RCT design; (b) irrelevant research content; (c) insufficient data reported; and (d) redundant publications (multiple reports of the same study with the same participant population).
2.3 Data extraction
Two investigators independently extracted data using a standardized form. The extracted information included: (a) basic study characteristics (authors, publication year, country/region); (b) sample demographics and group allocation details; (c) comparator intervention; (d) experimental intervention; (e) frequency and intervention duration; (f) blinding (Non-Blinded, single-blinded, double-blinded, or patrial blinding); and (g) outcome measurements, including: Timed Up and Go (TUG) test results measured in seconds, with lower values indicating better mobility; Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) total scores (ranging from 0 to 96 points, with lower scores indicating better outcomes) and its subscales including pain (0–20 points), stiffness (0–8 points), and physical function (0–68 points); and pain assessment scores using the Numerical Rating Scale (NRS, 0–10 points) and Visual Analog Scale (VAS, 0–10 cm), with higher scores indicating greater pain intensity.
2.4 Assessment of study quality
The methodological quality of all included studies was independently assessed by two investigators. The Cochrane risk-of-bias tool (ROB 2) (31, 32) was utilized to evaluate the randomization process, deviation from expected interventions, completeness of outcome data, measurement of outcomes, and selectivity in reporting outcomes. For each domain, specific signaling questions were answered as “yes”, “probably yes”, “no information”, “probably no”, or “no” based on the information provided in the studies. Following the ROB 2 algorithm, each study was ultimately classified as having “low risk”, “some concerns”, or “high risk” for each domain and overall. The detailed assessment criteria and decision-making process are provided in the Supplementary S2. The certainty of evidence for each outcome was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, considering risk of bias, inconsistency, indirectness, imprecision, and publication bias. Any disagreements were resolved through discussion or consultation with a third investigator.
2.5 Data analysis
Statistical analyses were performed using RevMan 5.4 and Stata 17.0 software. For missing standard deviations, conversions were made using reported data. For continuous variables, mean differences (MD) and 95% confidence intervals (CI) were calculated. Positive values indicate favorable outcomes for the experimental group. Heterogeneity was assessed using the I2 statistic, with I2 ≤ 50% indicating no significant heterogeneity or low heterogeneity, suggesting possible common effect sizes, and thus a fixed-effects model was used, and I2 > 50% indicating high heterogeneity, suggesting possible different true effects and the need to account for between-study differences, and thus a random-effects model was used (33, 34). Separate meta-analyses were conducted for each outcome measure. Pre-specified subgroup analyses were performed for all outcome measures according to intervention duration (≤8 weeks vs. >8 weeks). We also conducted subgroup analyses by publication year, intervention type (single vs. combined), and study region (Asian vs. non-Asian) to explore sources of heterogeneity (the results of intervention type and study region are presented in the Supplementary Materials). Sensitivity analyses were conducted by removing individual studies one by one, with an effect estimate change greater than 20% after exclusion regarded as an unstable result (detailed data presented in Supplementary S3). Publication bias for each outcome measure was evaluated using Egger's test. Funnel plots were used to assess publication bias for outcome measures that included more than 10 studies. Statistical significance was set at P < 0.05.
3 Results
3.1 Study selection results and characteristics
A comprehensive database search yielded 2,882 potentially relevant articles across six electronic databases, with 1,436 remaining after duplicate removal. Title and abstract screening identified 198 articles for full-text assessment. We subsequently excluded 115 articles that failed to meet eligibility criteria: 32 employed non-RCT designs (including observational studies, case series, quasi-experimental designs, and uncontrolled trials), 54 addressed topics outside our research focus (including non-KOA populations, surgical intervention studies, primarily pharmacological treatment investigations, and intervention without proprioceptive exercise), and seven lacked sufficient outcome data. The final analysis included 22 randomized controlled trials (21, 23, 35–54) (Figure 1).
The included studies spanned from 2008 to 2024, representing nine countries and regions. Geographic distribution analysis revealed predominance from India (7/22), the United States (3/22), and Taiwan, China (3/22) (Table 2). Proprioceptive interventions varied methodologically, encompassing proprioceptive training, dynamic stabilization exercises, sensorimotor training, and neuromuscular training. These approaches typically incorporated balance and coordination components and were administered either as primary interventions or as adjuncts to conventional therapy (Table 2). Intervention duration was predominantly short-term, with 17 of 22 studies (21, 23, 35–37, 41–47, 49, 50, 52–54) implementing protocols of 8 weeks or less, while the most extended intervention lasted 12 months (Table 2).
3.2 Risk of bias
The 22 studies assessed for risk of bias revealed variable levels of quality across different domains (Figure 2). Ten studies demonstrated low risk of bias in the randomization process, eight studies exhibited some concern, and four studies were classified as high risk due to inadequate randomization details. In terms of deviation from the intended intervention, 19 studies were deemed low risk, while one study exhibited some concerns and two studies were considered high risk. With regard to the completeness of outcome data, 20 studies were classified as low risk, while only two studies were deemed high risk due to incomplete follow-up data. In terms of outcome measures, nine studies were rated as low risk, 12 studies exhibited some concern, and one study was categorized as high risk. Regarding the choice of reporting outcomes, 18 studies were considered low risk, while four studies were assigned some concern due to the absence of a pre-specified analysis plan. The overall assessment indicated that the majority of studies (21, 23, 35, 37, 42, 44, 45, 47, 49, 50, 52, 54) exhibited some concern regarding risk of bias, while seven studies (36, 40, 41, 46, 48, 51, 53) were categorized as high risk and only three studies (38, 39, 43) achieved low risk status.
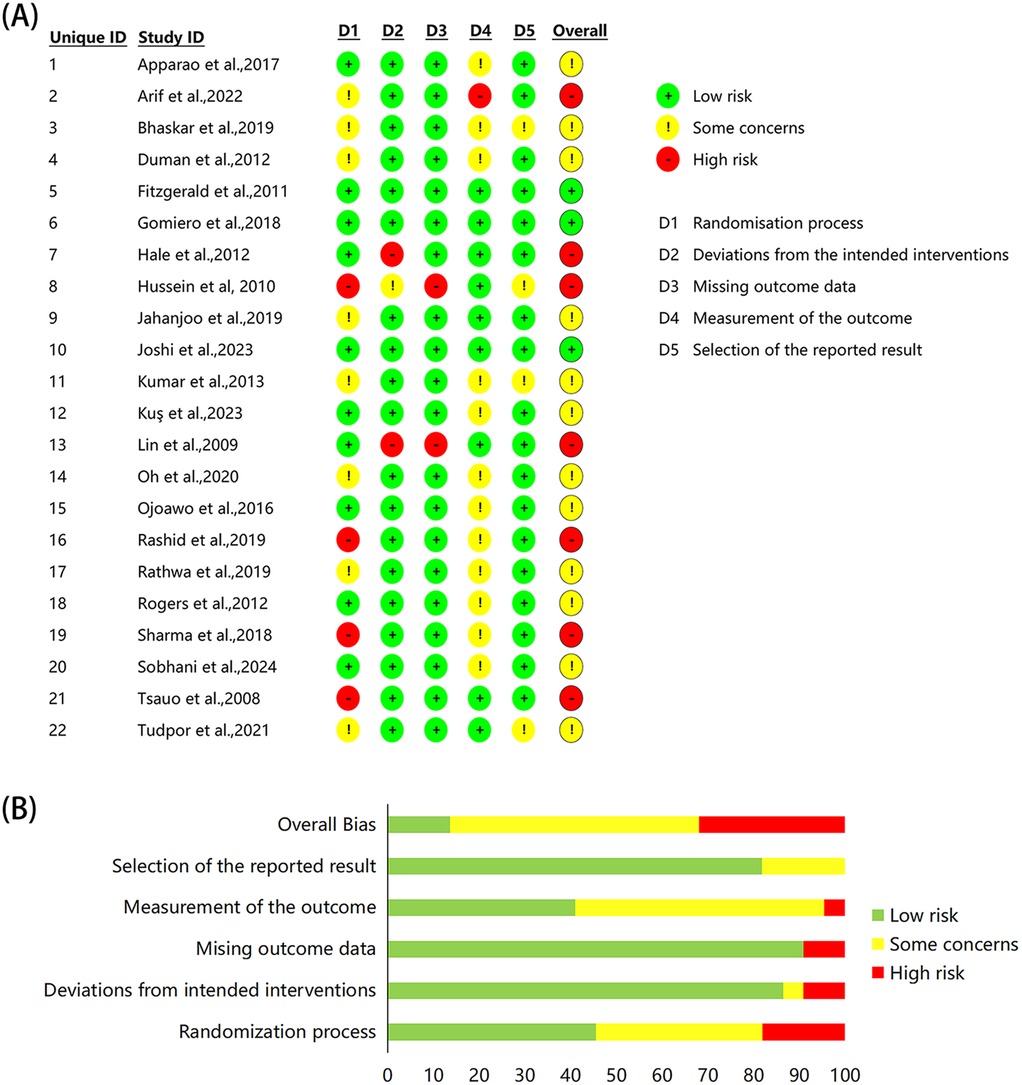
Figure 2. Risk of bias assessment of the included studies. (A) Individual study assessment, (B) overall proportions across domains.
3.3 TUG
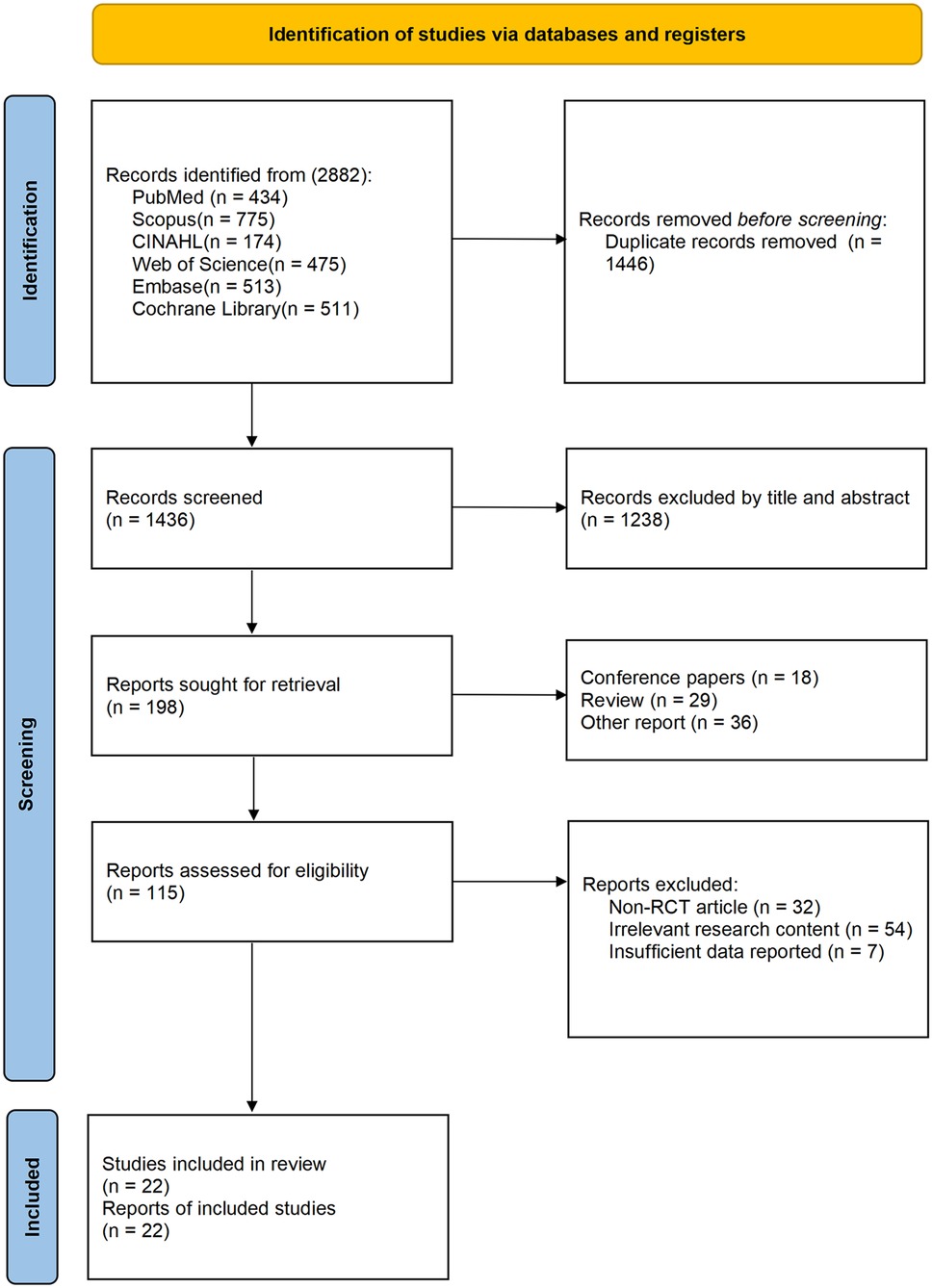
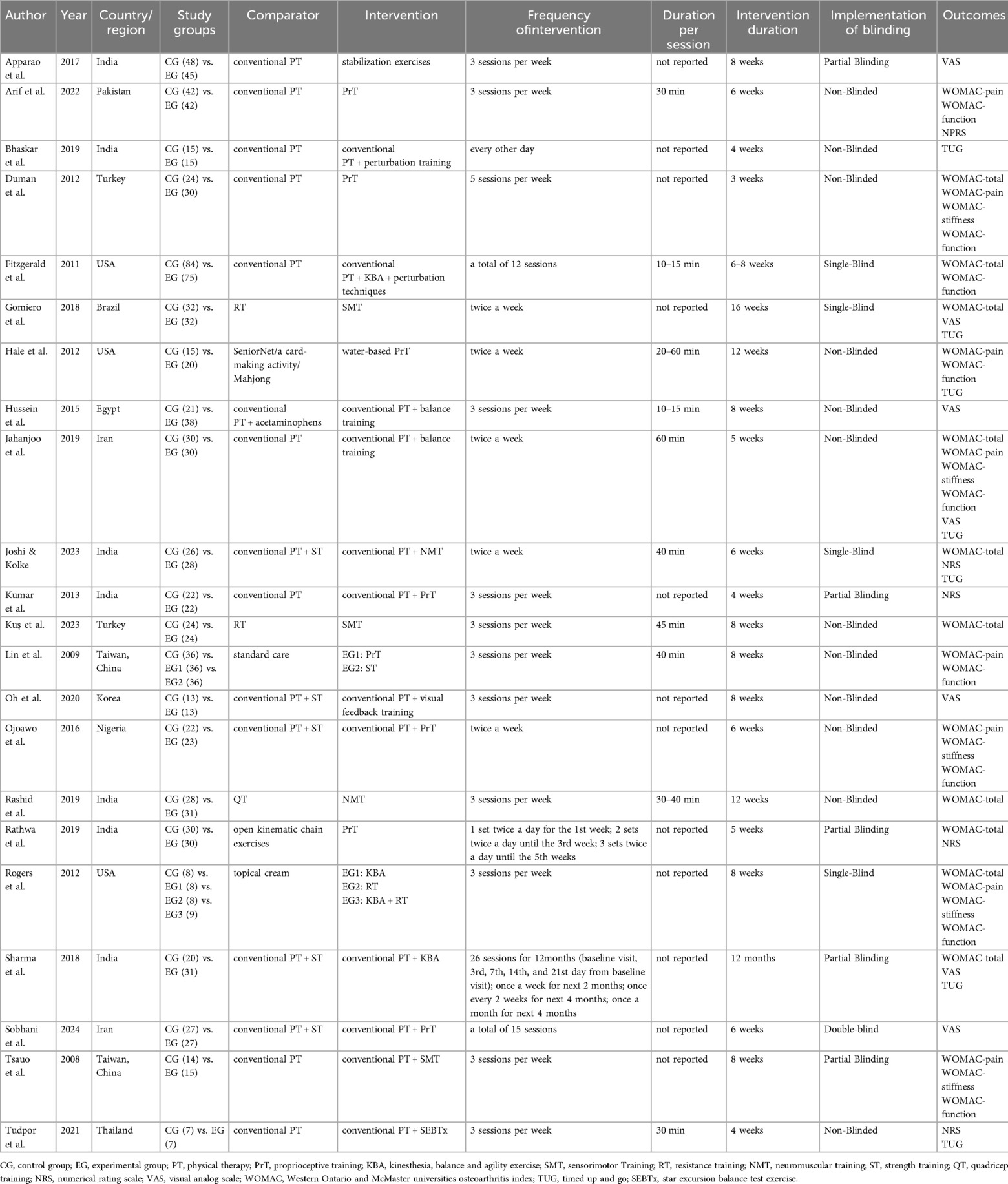
Seven studies (37, 39, 40, 42, 43, 51, 54) reported TUG results in the outcome metrics, involving 308 participants. The experimental group demonstrated a significant improvement in performance on the TUG test compared with the control group [MD = 1.53, 95% CI (1.09, 1.97), I2 = 0%, P < 0.00001, Figure 3]. This improvement exceeded the established minimal clinically important difference (MCID) threshold of 1.3 s (55). The pre-set subgroup analyses revealed that the group receiving interventions duration of ≤8 weeks exhibited a significant improvement [MD = 1.81, 95% CI (1.27, 2.34), I2 = 0%, P < 0.00001, Figure 3], while group with an intervention duration of >8 weeks also demonstrated a statistically significant improvement [MD = 0.95, 95% CI (0.17, 1.73), I2 = 0%, P = 0.02, Figure 3]. Sensitivity analyses indicated good robustness of the results (Supplementary Figure S1A). When assessing the possibility of publication bias, the Egger's test results showed no significant bias (P = 0.740 > 0.05, Supplementary Figure S1B).
3.4 WOMAC
3.4.1 WOMAC-total
Twelve studies (23, 38–40, 42, 43, 45, 48–51) reported WOMAC-total in the outcome metrics, involving 669 participants. The experimental group demonstrated a significant reduction in WOMAC-total scores compared with the control group [MD = 3.37, 95% CI (1.58, 5.16), I2 = 44%, P = 0.0002, Figure 4A]. However, this improvement did not reach the established MCID threshold of 4.9 points (56). The pre-set subgroup analyses revealed that the group receiving interventions duration of ≤8 weeks showed a significant improvement [MD = 3.30, 95% CI (1.13, 5.47), I2 = 55%, P = 0.003, Figure 4A], while the group with an intervention duration of >8 weeks also demonstrated a statistically significant improvement [MD = 3.52, 95% CI (0.34, 6.70), I2 = 27%, P = 0.03, Figure 4A]. Sensitivity analyses indicated good robustness of the results (Supplementary Figure S2A). When assessing the possibility of publication bias, both funnel plot analysis (Figure 4B) and Egger's test results showed no significant bias (P = 0.252 > 0.05, Supplementary Figure S2B).
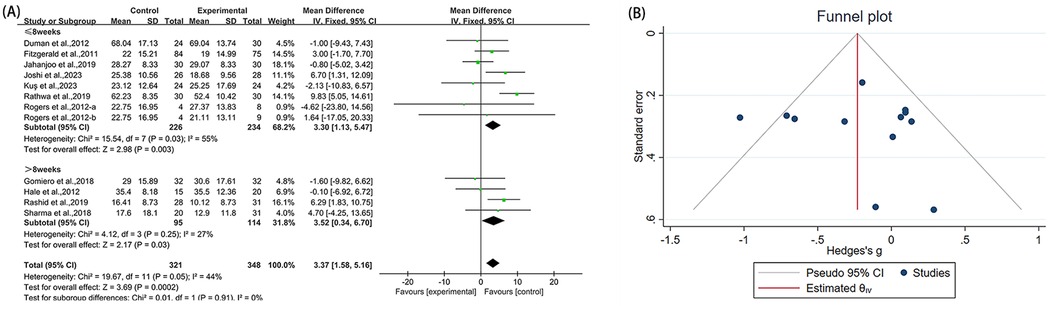
Figure 4. Forest plot and funnel plot of WOMAC-total comparing experimental group with control group, (A) forest plot, (B) funnel plot.
3.4.2 WOMAC-pain
Ten studies (21, 23, 36, 40, 42, 46, 50, 53) reported WOMAC-pain in the outcome metrics, involving 440 participants. The experimental group did not demonstrate statistically significant alterations in WOMAC-pain scores compared with the control group [MD = 1.12, 95% CI (−0.24, 2.49), I2 = 85%, P = 0.11, Figure 5A]. We explored sources of high heterogeneity, and subgroup analyses based on publication years found that studies of the 2000s [MD = 0.95, 95% CI (−0.88, 2.78), I2 = 82%, P = 0.31, Figure 5A] and the 2010s showed no significant improvement [MD = 0.42, 95% CI (−0.53, 1.37), I2 = 27%, P = 0.39, Figure 5A]. The 2020s group included only one study that showed significant improvement [MD = 5.23, 95% CI (3.94, 6.52), Figure 5A]. Subgroup analyses based on study region (Asian/non-Asian) and intervention type (single/combined) are presented in the Supplementary Figures S8A, S9A. Sensitivity analyses indicated good robustness of the results (Supplementary Figure S3A). When assessing the possibility of publication bias, both funnel plot analysis (Figure 5B) and Egger's test results showed no significant bias (P = 0.785 > 0.05, Supplementary Figure S3B).
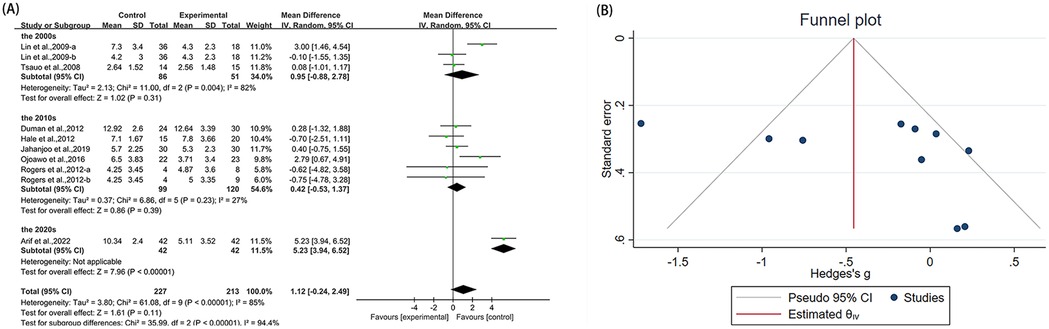
Figure 5. Forest plot and funnel plot of WOMAC-pain comparing experimental group with control group, (A) forest plot, (B) funnel plot.
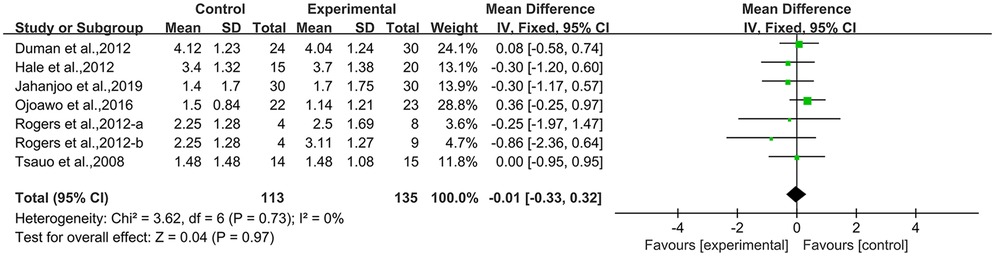
3.4.3 WOMAC-stiffness
Seven studies (21, 23, 40, 42, 50, 53) employed WOMAC-stiffness as an outcome measure, involving 248 participants. The experimental group did not demonstrate statistically significant alterations in WOMAC-stiffness scores compared with the control group [MD = −0.01, 95% CI (−0.33, 0.32), I2 = 0%, P = 0.97, Figure 6A]. Sensitivity analyses demonstrated that the overall effect estimate remained stable after sequential exclusion of individual studies, supporting the robustness of the conclusion (Supplementary Figure S4A). Assessment of publication bias revealed potential risks through Egger's test (P = 0.041 < 0.05, Supplementary Figure S4B).
3.4.4 WOMAC-function
Eleven studies (21, 23, 36, 38, 40, 42, 46, 50, 53) utilized WOMAC-function as an outcome measure, involving 599 participants. The experimental group did not demonstrate statistically significant alterations in WOMAC-function scores compared with the control group [MD = 3.35, 95% CI (−1.28, 7.99), I2 = 86%, P = 0.16, Figure 7A]. We explored sources of high heterogeneity, and subgroup analyses based on publication years found that studies of the 2000s [MD = 1.22, 95% CI (−7.66, 10.10), I2 = 87%, P = 0.79, Figure 7A] and the 2010s [MD = 1.38, 95% CI (−0.88, 3.64), I2 = 17%, P = 0.23, Figure 7A] showed no significant improvement. The 2020s group included only one study that showed significant improvement [MD = 22.87, 95% CI (17.20, 28.54), Figure 7A]. Subgroup analyses based on study region (Asian/non-Asian) and intervention type (single/combined) are presented in the Supplementary Figures S8B, S9B. Sensitivity analyses confirmed minimal changes to effect estimates upon study exclusion, reinforcing result stability (Supplementary Figure S5A). Both funnel plot symmetry (Figure 7B) and Egger's test (P = 0.790 > 0.05, Supplementary Figure S5B) showed no evidence of publication bias.
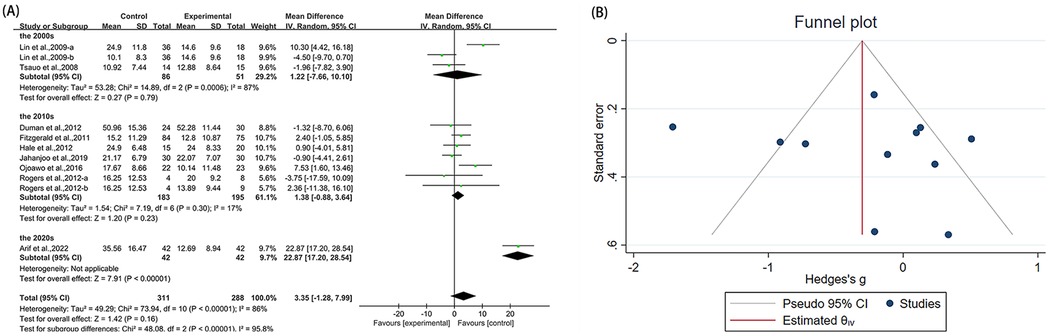
Figure 7. Forest plot and funnel plot of WOMAC-function comparing experimental group with control group, (A) forest plot, (B) funnel plot.
3.5 Pain score
3.5.1 NRS
Five studies (36, 38, 43, 44, 54) reported NRS in the outcome metrics, involving 355 participants. The experimental group demonstrated a significant reduction in NRS scores compared with the control group [MD = 0.85, 95% CI (0.56, 1.15), I2 = 46%, P < 0.00001, Figure 8A]. However, this improvement did not reach the established MCID of 1.65 points for NRS scores (57). Given that all studies implemented interventions with a duration of ≤8 weeks, the pre-specified subgroup analyses by duration were not performed. Sensitivity analyses indicated high robustness of the results (Supplementary Figure S6A). When assessing the possibility of publication bias, Egger's test results showed no significant bias (P = 0.066 > 0.05, Supplementary Figure S6B).
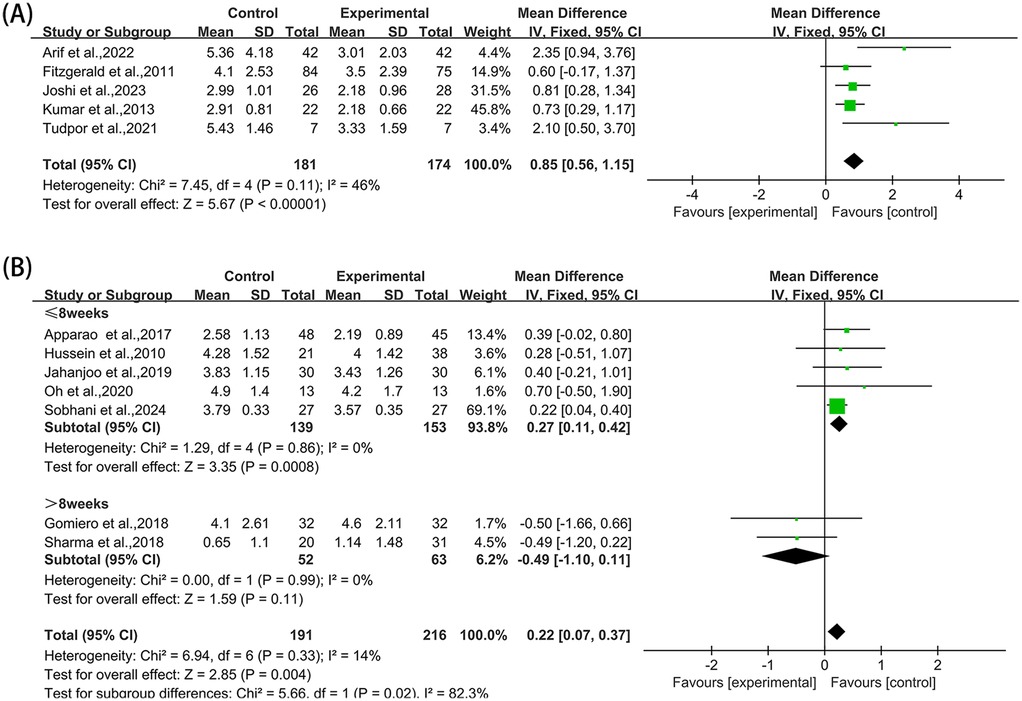
Figure 8. Forest plot of NRS and VAS comparing experimental group with control group, (A) NRS, (B) VAS.
3.5.2 VAS
Seven studies (35, 39, 41, 42, 47, 51, 52) reported VAS in the outcome metrics, involving 407 participants. A statistically significant decrease in pain scores was observed in the experimental group compared with the control group [MD = 0.22, 95% CI (0.07, 0.37), I2 = 14%, P = 0.004, Figure 8B]. Nevertheless, this reduction fell substantially below the MCID threshold of 1.80 cm for VAS scores (58). The pre-set subgroup analysis showed that the intervention group receiving intervention duration of ≤ 8 weeks exhibited better improvement [MD = 0.27, 95% CI (0.11, 0.42), I2 = 0%, P = 0.0008, Figure 8B], while the intervention group receiving intervention duration of >8 weeks had no statistically significant improvement [MD = −0.49, 95% CI (−1.10, 0.11), I2 = 0%, P = 0.11, Figure 8B]. Sensitivity analyses substantiated the robustness of these conclusions (Supplementary Figure S7A). No significant publication bias was identified through Egger's test (P = 0.671 > 0.05, Supplementary Figure S7B).
3.6 Certainty of evidence
The certainty of evidence was assessed using the GRADE approach. Overall, the quality of evidence was rated as low due to concerns regarding risk of bias across multiple domains and potential publication bias detected for WOMAC-stiffness outcomes, resulting in a downgrade of two levels from the initial high quality rating.
4 Discussion
This meta-analysis demonstrates that proprioceptive exercise provides beneficial effects for patients with KOA. The most pronounced improvement was observed in balance function as measured by the TUG test, reaching clinically meaningful levels. While statistically significant reductions were observed in overall WOMAC-total scores and pain intensity measures (NRS and VAS), these improvements fell below established minimal clinically important difference thresholds. No significant improvements were found for individual WOMAC subscales (pain, stiffness, or function). Proprioceptive exercise has increasingly become a focus of scholarly investigation in KOA management. Prior systematic reviews and meta-analyses have demonstrated that proprioceptive exercises may enhance joint position sense, reduce pain intensity, and offer some other therapeutic benefits for patients with KOA (24–26). Nevertheless, empirical evidence specifically addressing balance function outcomes has remained insufficiently examined in the literature. In addressing this gap in the literature, our research has sought to contribute to the body of knowledge by including a more comprehensive array of randomized controlled trials. These trials have enabled the investigation of the effects of proprioceptive exercise on pain, overall symptoms, and function, as well as its association with improved balance capabilities.
This study is the first meta-analysis to assess the impact of proprioceptive exercises on balance capabilities in patients with KOA. This clinical parameter has received scant attention in previous research. The TUG test improvements demonstrated both statistical significance and clinical meaningfulness, with effect sizes exceeding the established MCID threshold, indicating that the changes translate into meaningful functional benefits for patients. These improvements translate into increased confidence and efficiency during activities of daily living, including sit-stand transitions, walking, and turning. They also provide increased stability during orientation changes (59, 60). The underlying neural mechanisms extend beyond peripheral improvements to include significant central nervous system adaptations. Research has demonstrated that proprioceptive exercise modifies spinal reflex circuits, as evidenced by changes in H-reflex amplitudes, indicating altered processing of afferent inputs at the spinal level (61). At supraspinal levels, studies utilizing transcranial magnetic stimulation have revealed reduced corticospinal excitability and enhanced intracortical inhibition following proprioceptive training, particularly during postural responses. These cortical adaptations exhibit a strong correlation with improvements in postural stability, suggesting that optimized sensory processing in sensorimotor cortical areas plays a critical role in balance enhancement (62). Collectively, these central adaptations facilitate more efficient integration of proprioceptive information and refinement of motor output, improving the body's ability to maintain balance during functional activities (63). Subgroup analysis based on intervention duration revealed significant TUG improvements for both short-term (≤8 weeks) and long-term (>8 weeks) interventions. However, short-term interventions demonstrated larger effect sizes and stronger statistical significance. These findings imply that proprioceptive exercises hold promise in enhancing balance function in patients with KOA, particularly among those with existing balance impairments and elevated fall risks. Moreover, maintaining exercise within an 8-week period may be associated with superior outcomes.
While balance function showed robust improvements, the effects on other clinical outcomes presented a more complex pattern. With regard to the evaluation of KOA symptoms, the analysis of WOMAC-total results indicates that proprioceptive exercises significantly improved the overall condition of patients with KOA, irrespective of intervention duration (P = 0.0002). However, the mean difference (MD = 3.37) did not reach the MCID threshold, suggesting that while the improvement was statistically significant, its clinical relevance may be limited. This finding indicates that proprioceptive exercise shows promise for patients with KOA, but modifications may be needed to achieve clinically meaningful outcomes. This finding is consistent with the results of previous research (25, 26). However, pain-related outcome analyses revealed notable variability across different measurement tools. While WOMAC-pain assessments did not reach statistical significance (P = 0.11), VAS (P = 0.004) and NRS (P < 0.00001) demonstrated statistically significant improvements that fell short of MCID thresholds, suggesting that these tools may have different dimensional focuses and sensitivities (64). These findings partially align with Wang et al.'s study though the clinical meaningfulness of such improvements requires careful interpretation considering MCID thresholds (26). VAS and NRS primarily evaluate overall perceived pain intensity, emphasizing immediate, general pain perception (65), whereas WOMAC-pain, as a multidimensional assessment tool, evaluates pain experiences across several daily activities (66). This distinction aligns closely with the characteristic symptom pattern in patients with KOA, where pain typically worsens during activity and diminishes during rest (67, 68). The observed pattern suggests that proprioceptive exercise may have modest effects on general pain perception while potentially having limited impact on activity-specific pain experiences. These findings suggest that multiple pain assessment tools should be considered when evaluating intervention efficacy, with recognition that proprioceptive exercise may primarily benefit balance and functional outcomes rather than serving as a primary pain management strategy.
Subgroup analysis of VAS scores revealed statistical significance for short-term interventions (P = 0.0008), but not for long-term interventions (P = 0.11). This phenomenon may be attributed to several factors. These include rapid neural adaptations that occur during the early stages of proprioceptive exercise (69). Typically, adherence and motivation of patients exhibit higher levels during initial intervention periods but may gradually diminish with extended intervention duration, potentially influencing the observed outcomes in longer interventions. This phenomenon was potentially exemplified in the study by Sharma et al., which reported relatively high dropout rates, possibly attributable to their extended intervention duration of 12 months (51). Additionally, potential plateau effects where initial benefits stabilize after reaching maximum potential (70). Despite this finding in VAS measurements, it is important to emphasize that other outcome measures, including WOMAC-total and TUG, continued to show benefits beyond 8 weeks, underscoring the efficacy of proprioceptive exercises as a long-term intervention strategy.
The findings indicate a lack of statistical significance in WOMAC-stiffness (P = 0.97) and WOMAC-function (P = 0.16) analyses, contrasting with the significant improvement observed in WOMAC-total. This phenomenon suggests that proprioceptive exercises may produce broad but modest cumulative effects on the overall condition of patients with KOA. While improvements in individual symptom dimensions may not reach significant thresholds, composite scores appear more sensitive in capturing these multidimensional, incremental improvements.
We employed comprehensive analytical approaches to investigate the sources of heterogeneity. Subgroup analyses based on intervention duration and methodology (single vs. combined interventions) did not adequately explain the high heterogeneity observed in WOMAC-pain results. However, regional subgroup analysis (Asian vs. non-Asian) for WOMAC-function revealed low heterogeneity (I2 = 0%) and statistical significance (P = 0.03) in the non-Asian group, suggesting that geographic factors may significantly influence intervention consistency. The analysis of other potential classification methods, including patient demographics (age, gender, BMI), K-L grading, and specific proprioceptive exercise protocols, was hindered by inconsistent reporting and a lack of standardized information across studies. Future research should standardize reporting of patient demographics, disease severity, and intervention parameters to enable more targeted analyses of which patient subgroups benefit most from proprioceptive exercises.
After extensive exploration, we employed publication era-based subgroup analysis, revealing a meaningful temporal trend. Studies of the 2000s generally exhibited high heterogeneity, with WOMAC-pain (I2 = 82%) and WOMAC-function (I2 = 86%) demonstrating significant inter-study differences. Conversely, studies of the 2010s showed improved homogeneity, with heterogeneity substantially reduced to 27% for WOMAC-pain and 17% for WOMAC-function. This heterogeneity trend may signify the field's advancement towards a more consolidated understanding of proprioceptive exercise applications in KOA management, with intervention designs progressively centered on specific mechanisms, thereby yielding more consistent therapeutic effects.
This study is subject to several limitations. Methodologically, the 22 randomized controlled trials included in this analysis demonstrated moderate overall quality, with only thre studies achieving a low risk of bias classification. There was limited implementation of robust blinding procedures across studies, with only one study utilizing a double-blind design, which may introduce potential detection and performance bias, particularly for subjective outcomes such as pain assessment. This limitation could potentially inflate effect sizes for patient-reported measures like VAS and NRS scores, warranting cautious interpretation of these findings. Four studies showed weaknesses in the randomization process due to inadequate reporting of sequence generation or allocation concealment. The heterogeneity of intervention protocols presents another important consideration, as the included studies varied in training content, duration, and methodology. Notably, none of the included studies provided detailed reporting of exercise intensity parameters such as percent of target heart rate or metabolic equivalent (MET) values, which represents a significant gap in standardization of interventions. This variability makes it challenging to formulate specific recommendations for standardized proprioceptive exercise protocols in clinical settings. The inclusion of both no-treatment and active therapy controls in our analysis may have contributed to this pattern of statistical significance without clinical meaningfulness across multiple measures. Additionally, the inconsistent reporting of patient characteristics across studies limits our ability to determine which patient subgroups might benefit most from these interventions. Furthermore, the study's inclusion of English-language publications may introduce a language bias, as it overlooks relevant research published in other languages. While Egger's tests for most outcome measures showed no significant bias, the results for WOMAC-stiffness (P = 0.041 < 0.05) indicated potential publication bias. These limitations underscore the necessity for future research to develop standardized proprioceptive exercise protocols through high-quality longitudinal studies. The investigation of individualized intervention approaches, tailored to patient characteristics and disease staging, would be a valuable avenue for future research.
Collectively, these findings indicate that the incorporation of proprioceptive exercises into the rehabilitation of KOA is a crucial component, particularly for patients experiencing knee pain and balance dysfunction. Evidence suggests that the implementation of proprioceptive exercises within an 8-week timeframe may yield optimal therapeutic effects, especially for improving balance function and alleviating pain. Clinical practice should incorporate comprehensive assessment approaches that combine subjective and objective measurements to track therapeutic progress and optimize patient outcomes. These insights provide more comprehensive evidence-based guidance for clinical KOA management practices.
5 Conclusion
Based on low-certainty evidence, proprioceptive exercises may improve balance function and overall clinical status in patients with KOA, though further high-quality research is needed to strengthen confidence in these findings.The analysis revealed that short-term interventions yield particularly significant effects. However, none of the WOMAC subscales reached statistical significance individually. Based on these findings, we recommend incorporating proprioceptive exercises into clinical rehabilitation protocols for patients with KOA, especially for those suffering from balance dysfunction.
Author contributions
YL: Writing – original draft, Conceptualization, Formal analysis, Investigation, Visualization, Writing – review & editing. DY: Formal analysis, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XC: Investigation, Visualization, Writing – original draft. PC: Data curation, Investigation, Writing – original draft. NC: Data curation, Investigation, Writing – original draft. BS: Investigation, Validation, Writing – original draft. QL: Formal analysis, Methodology, Writing – original draft. FW: Conceptualization, Writing – review & editing, Funding acquisition, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant number: 2024Y9052).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fresc.2025.1596966/full#supplementary-material
References
1. Lespasio MJ, Piuzzi NS, Husni ME, Muschler GF, Guarino A, Mont MA. Knee osteoarthritis: a primer. Perm J. (2017) 21:16–183. doi: 10.7812/TPP/16-183
2. Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. (2020) 29-30:100587. doi: 10.1016/j.eclinm.2020.100587
3. Levinger P, Menz HB, Wee E, Feller JA, Bartlett JR, Bergman NR. Physiological risk factors for falls in people with knee osteoarthritis before and early after knee replacement surgery. Knee Surg Sports Traumatol Arthrosc. (2011) 19(7):1082–89. doi: 10.1007/s00167-010-1325-8
4. Kamsan SS, Singh DKA, Tan MP, Kumar S. The knowledge and self-management educational needs of older adults with knee osteoarthritis: a qualitative study. PLoS One. (2020) 15(3):e230318. doi: 10.1371/journal.pone.0230318
5. McDonough CM, Jette AM. The contribution of osteoarthritis to functional limitations and disability. Clin Geriatr Med. (2010) 26(3):387–99. doi: 10.1016/j.cger.2010.04.001
6. Mirdamadi JL, Ting LH, Borich MR. Distinct cortical correlates of perception and motor function in balance control. J Neurosci. (2024) 44(15):e1520232024. doi: 10.1523/JNEUROSCI.1520-23.2024
7. Cuevas-Trisan R. Balance problems and fall risks in the elderly. Clin Geriatr Med. (2019) 35(2):173–83. doi: 10.1016/j.cger.2019.01.008
8. Zeng Z, Shan J, Zhang Y, Wang Y, Li C, Li J, et al. Asymmetries and relationships between muscle strength, proprioception, biomechanics, and postural stability in patients with unilateral knee osteoarthritis. Front Bioeng Biotechnol. (2022) 10:922832. doi: 10.3389/fbioe.2022.922832
9. Knoop J, Steultjens MPM, van der Leeden M, van der Esch M, Thorstensson CA, Roorda LD, et al. Proprioception in knee osteoarthritis: a narrative review. Osteoarthritis Cartilage. (2011) 19(4):381–88. doi: 10.1016/j.joca.2011.01.003
10. McNair PJ, Marshall RN, Maguire K, Brown C. Knee joint effusion and proprioception. Arch Phys Med Rehabil. (1995) 76(6):566–68. doi: 10.1016/s0003-9993(95)80512-5
11. Heroux ME, Butler AA, Robertson LS, Fisher G, Gandevia SC. Proprioception: a new look at an old concept. J Appl Physiol (1985). (2022) 132(3):811–14. doi: 10.1152/japplphysiol.00809.2021
12. Mohapatra S, Krishnan V, Aruin AS. Postural control in response to an external perturbation: effect of altered proprioceptive information. Exp Brain Res. (2012) 217(2):197–208. doi: 10.1007/s00221-011-2986-3
13. Khalaj N, Abu Osman NA, Mokhtar AH, Mehdikhani M, Wan Abas WAB. Balance and risk of fall in individuals with bilateral mild and moderate knee osteoarthritis. PLoS One. (2014) 9(3):e92270. doi: 10.1371/journal.pone.0092270
14. Roijezon U, Clark NC, Treleaven J. Proprioception in musculoskeletal rehabilitation. Part 1: basic science and principles of assessment and clinical interventions. Man Ther. (2015) 20(3):368–77. doi: 10.1016/j.math.2015.01.008
15. Psenicnik Sluga S, Kozinc Z. Sensorimotor and proprioceptive exercise programs to improve balance in older adults: a systematic review with meta-analysis. Eur J Transl Myol. (2024) 34(1):12010. doi: 10.4081/ejtm.2024.12010
16. Espejo-Antunez L, Perez-Marmol JM, Cardero-Duran MDLA, Toledo-Marhuenda JV, Albornoz-Cabello M. The effect of proprioceptive exercises on balance and physical function in institutionalized older adults: a randomized controlled trial. Arch Phys Med Rehabil. (2020) 101(10):1780–88. doi: 10.1016/j.apmr.2020.06.010
17. Goncalves C, Bezerra P, Clemente FM, Vila-Cha C, Leao C, Brandao A, et al. Effects of bodyweight neuromuscular training with and without instability on balance control in active universitarians. Res Sports Med. (2022) 30(2):128–44. doi: 10.1080/15438627.2020.1853544
18. Verhagen E, van der Beek A, Twisk J, Bouter L, Bahr R, van Mechelen W. The effect of a proprioceptive balance board training program for the prevention of ankle sprains: a prospective controlled trial. Am J Sports Med. (2004) 32(6):1385–93. doi: 10.1177/0363546503262177
19. Ince B, Goksel Karatepe A, Akcay S, Kaya T. The efficacy of balance and proprioception exercises in female patients with knee osteoarthritis: a randomized controlled study. Clin Rehabil. (2023) 37(1):60–71. doi: 10.1177/02692155221111929
20. Aljehani MS, Crenshaw JR, Rubano JJ, Dellose SM, Zeni JAJ. Falling risk in patients with end-stage knee osteoarthritis. Clin Rheumatol. (2021) 40(1):3–09. doi: 10.1007/s10067-020-05165-6
21. Ojoawo AO, Olaogun MOB, Hassan MA. Comparative effects of proprioceptive and isometric exercises on pain intensity and difficulty in patients with knee osteoarthritis: a randomised control study. Technol Health Care. (2016) 24(6):853–63. doi: 10.3233/THC-161234
22. Tunay VB, Baltaci G, Atay AO. Hospital-based versus home-based proprioceptive and strengthening exercise programs in knee osteoarthritis. Acta Orthop Traumatol Turc. (2010) 44(4):270–77. doi: 10.3944/AOTT.2010.2306
23. Duman I, Taskaynatan MA, Mohur H, Tan AK. Assessment of the impact of proprioceptive exercises on balance and proprioception in patients with advanced knee osteoarthritis. Rheumatol Int. (2012) 32(12):3793–98. doi: 10.1007/s00296-011-2272-5
24. Jeong HS, Lee S, Jee H, Song JB, Chang HS, Lee SY. Proprioceptive training and outcomes of patients with knee osteoarthritis: a meta-analysis of randomized controlled trials. J Athl Train. (2019) 54(4):418–28. doi: 10.4085/1062-6050-329-17
25. Smith TO, King JJ, Hing CB. The effectiveness of proprioceptive-based exercise for osteoarthritis of the knee: a systematic review and meta-analysis. Rheumatol Int. (2012) 32(11):3339–51. doi: 10.1007/s00296-012-2480-7
26. Wang Y, Wu Z, Chen Z, Ye X, Chen G, Yang J, et al. Proprioceptive training for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Front Med. (2021) 8:699921. doi: 10.3389/fmed.2021.699921
27. Enoka RM. Neural adaptations with chronic physical activity. J Biomech. (1997) 30(5):447–55. doi: 10.1016/s0021-9290(96)00170-4
28. Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. (2006) 36(2):133–49. doi: 10.2165/00007256-200636020-00004
29. Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med. (2007) 37(2):145–68. doi: 10.2165/00007256-200737020-00004
30. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
31. Crocker TF, Lam N, Jordao M, Brundle C, Prescott M, Forster A, et al. Risk-of-bias assessment using Cochrane’s revised tool for randomized trials (RoB 2) was useful but challenging and resource-intensive: observations from a systematic review. J Clin Epidemiol. (2023) 161:39–45. doi: 10.1016/j.jclinepi.2023.06.015
32. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
33. Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical primer: heterogeneity, random- or fixed-effects model analyses? Interact Cardiovasc Thorac Surg. (2018) 27(3):317–21. doi: 10.1093/icvts/ivy163
34. Chen W, Li M, Li H, Lin Y, Feng Z. Tai Chi for fall prevention and balance improvement in older adults: a systematic review and meta-analysis of randomized controlled trials. Front Public Health. (2023) 11:1236050. doi: 10.3389/fpubh.2023.1236050
35. Apparao P, Sandeep G, Sudhakar S, Ch GS, Sg S, Satyaprakash T, et al. Effectiveness of stabilization exercises and conventional physiotherapy in subjects with knee osteoarthritis. Int J Res Pharm Sci. (2017) 8:542–48. Available at: https://ijrps.com/home/article/view/4617
36. Arif H, Arif N, Kanwal N, Tahir H, Akmal I, Munawar SH, et al. Screening of therapeutic potentials of proprioceptive exercises and topical glucosamine sulfate on pain and functional disability in knee osteoarthritis. Trop J Pharm Res. (2023) 21(11):2447–52. doi: 10.4314/tjpr.v21i11.25
37. Bhaskar B, JimshadT U, Solomen S. Efficacy of perturbation training in improving balance and function in the management of knee osteoarthritis. Int J Physiother. (2019) 8(4):542–8. doi: 10.15621/ijphy/2019/v6i4/185414
38. Fitzgerald GK, Piva SR, Gil AB, Wisniewski SR, Oddis CV, Irrgang JJ. Agility and perturbation training techniques in exercise therapy for reducing pain and improving function in people with knee osteoarthritis: a randomized clinical trial. Phys Ther. (2011) 91(4):452–69. doi: 10.2522/ptj.20100188
39. Gomiero AB, Kayo A, Abraão M, Peccin MS, Grande AJ, Trevisani VF. Sensory-motor training versus resistance training among patients with knee osteoarthritis: randomized single-blind controlled trial. Sao Paulo Med J. (2018) 136(1):44–50. doi: 10.1590/1516-3180.2017.0174100917
40. Hale LA, Waters D, Herbison P. A randomized controlled trial to investigate the effects of Water-Based exercise to improve falls risk and physical function in older adults with Lower-Extremity osteoarthritis. Arch Phys Med Rehabil. (2012) 93(1):27–34. doi: 10.1016/j.apmr.2011.08.004
41. Hussein NA, Saad M, Sawey NAE. Effect of combined balance and isotonic resistive exercises versus isotonic resistive exercise alone on proprioception and StabilizingReactions of quadriceps and hamstrings and functional capacity of KneeOsteoarthritis patients. J Nov Physiother. (2015) 2015:1–08. doi: 10.4172/2165-7025.1000273
42. Jahanjoo F, Eftekharsadat B, Bihamta A, Babaei-Ghazani A. Efficacy of balance training in combination with physical therapy in rehabilitation of knee osteoarthritis: a randomized clinical trial. Crescent J Med Biol Sci. (2019) 3:325–34. Available at: https://www.cjmb.org/text.php?id=238
43. Joshi S, Kolke S. Effects of progressive neuromuscular training on pain, function, and balance in patients with knee osteoarthritis: a randomised controlled trial. Eur J Physiother. (2023) 25(4):179–86. doi: 10.1080/21679169.2022.2052178
44. Kumar S, Kumar A, Kumar R. Proprioceptive training as an adjunct in osteoarthritis of knee. J Musculoskelet Res. (2013) 16(01):1350002. doi: 10.1142/S0218957713500024
45. Kuş G, Tarakçı E, Razak Ozdincler A, Erçin E. Sensory-motor training versus resistance training in the treatment of knee osteoarthritis: a randomized controlled trial. Clin Rehabil. (2023) 37(5):636–50. doi: 10.1177/02692155221137642
46. Lin D, Lin CJ, Lin Y, Jan M. Efficacy of 2 non-weight-bearing interventions, proprioception training versus strength training, for patients with knee osteoarthritis: a randomized clinical trial. J Orthop Sports Phys Ther. (2009) 39(6):450. doi: 10.2519/jospt.2009.2923
47. Oh H, Lee S, Lee K, Choi J. The effects of visual feedback balance training on the pain and physical function of patients with chronic degenerative knee arthritis. J Phys Ther Sci. (2020) 32(9):563–65. doi: 10.1589/jpts.32.563
48. Rashid SA, Moiz JA, Sharma S, Raza S, Rashid SM, Hussain ME. Comparisons of neuromuscular training versus quadriceps training on gait and WOMAC index in patients with knee osteoarthritis and varus malalignment. J Chiropr Med. (2019) 18(1):1–08. doi: 10.1016/j.jcm.2018.07.003
49. Rathwa A, Prajapati N, Deepak AN. To compare the effectiveness of proprioceptive circuit exercises versus open kinematics chain exercises on pain and improve muscle strength and physical function in osteoarthritis knee patients. Indian J Physiother Occup Therapy Int J. (2019) 13(1):88. doi: 10.5958/0973-5674.2019.00017.0
50. Rogers MW, Tamulevicius N, Semple SJ, Krkeljas Z. Efficacy of home-based kinesthesia, balance & agility exercise training among persons with symptomatic knee osteoarthritis. J Sports Sci Med. (2012) 11(4):751–58. Available at: https://www.jssm.org/jssm-11-751.xml%3EFulltext24150088
51. Sharma M, Singh A, Dhillon M, Kaur S. Comparative impact of nonpharmacological interventions on pain of knee osteoarthritis patients reporting at a tertiary care institution: a randomized controlled trial. Indian J Palliat Care. (2018) 24(4):478. doi: 10.4103/IJPC.IJPC_14_18
52. Sobhani V, Hashemi SE, Mir SM, Ghorbanpour A. Impact of proprioceptive exercises on pain, balance, and fall risk in the elderly with knee osteoarthritis: a randomized clinical trial. Cureus. (2024) 16(10):e70885. doi: 10.7759/cureus.70885
53. Tsauo J, Cheng P, Yang R. The effects of sensorimotor training on knee proprioception and function for patients with knee osteoarthritis: a preliminary report. Clin Rehabil. (2008) 22(5):448–57. doi: 10.1177/0269215507084597
54. Tudpor K, Kanjanawanishkul K, Kam-Ard S, Intarak T, Traithip W, Sombateyotha K, et al. Star excursion balance test as an exercise to improve static and dynamic balance in community-dwelling persons with unilateral osteoarthritis of knee. Indian J Physiother Occup Therapy Int J. (2021) 15:30–6. doi: 10.37506/ijpot.v15i2.14509
55. Yagi M, Ohne H, Kaneko S, Machida M, Yato Y, Asazuma T. Does corrective spine surgery improve the standing balance in patients with adult spinal deformity? Spine J. (2018) 18(1):36–43. doi: 10.1016/j.spinee.2017.05.023
56. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. (2001) 45(4):384–91. doi: 10.1002/1529-0131(200108)45:4%3C384::AID-ART352%3E3.0.CO;2-0
57. Bahreini M, Safaie A, Mirfazaelian H, Jalili M. How much change in pain score does really matter to patients? Am J Emerg Med. (2020) 38(8):1641–46. doi: 10.1016/j.ajem.2019.158489
58. Guo J, Peng C, Hu Z, Guo L, Dai R, Li Y. Effect of Wu Qin Xi exercises on pain and function in people with knee osteoarthritis: a systematic review and meta-analysis. Front Med. (2022) 9:979207. doi: 10.3389/fmed.2022.979207
59. Moon KM, Kim J, Seong Y, Suh B, Kang K, Choe HK, et al. Proprioception, the regulator of motor function. BMB Rep. (2021) 54(8):393–402. doi: 10.5483/BMBRep.2021.54.8.052
60. Woo MT, Davids K, Chow JY, Jaakkola T. Acute effects of wearing compression knee-length socks on ankle joint position sense in community-dwelling older adults. PLoS One. (2021) 16(2):e245979. doi: 10.1371/journal.pone.0245979
61. Sun Y, Hurd CL, Barnes MM, Yang JF. Neural plasticity in spinal and corticospinal pathways induced by balance training in neurologically intact adults: a systematic review. Front Hum Neurosci. (2022) 16:921490. doi: 10.3389/fnhum.2022.921490
62. Taube W, Gruber M, Beck S, Faist M, Gollhofer A, Schubert M. Cortical and spinal adaptations induced by balance training: correlation between stance stability and corticospinal activation. Acta Physiol. (2007) 189(4):347–58. doi: 10.1111/j.1748-1716.2007.01665.x
63. Zemkova E, Hamar D. Physiological mechanisms of post-exercise balance impairment. Sports Med. (2014) 44(4):437–48. doi: 10.1007/s40279-013-0129-7
64. Da Costa BR, Saadat P, Basciani R, Agarwal A, Johnston BC, Juni P. Visual analogue scale has higher assay sensitivity than WOMAC pain in detecting between-group differences in treatment effects: a meta-epidemiological study. Osteoarthritis Cartilage. (2021) 29(3):304–12. doi: 10.1016/j.joca.2020.10.004
65. Alghadir AH, Anwer S, Iqbal A, Iqbal ZA. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res. (2018) 11:851–56. doi: 10.2147/JPR.S158847
66. Woolacott NF, Corbett MS, Rice SJC. The use and reporting of WOMAC in the assessment of the benefit of physical therapies for the pain of osteoarthritis of the knee: findings from a systematic review of clinical trials. Rheumatology. (2012) 51(8):1440–46. doi: 10.1093/rheumatology/kes043
67. Pulling BW, Braithwaite FA, Mignone J, Butler DS, Caneiro JP, Lipp OV, et al. People with painful knee osteoarthritis hold negative implicit attitudes towards activity. Pain. (2024) 165(9):2024–34. doi: 10.1097/j.pain.0000000000003210
68. Vitaloni M, Botto-van Bemden A, Sciortino Contreras RM, Scotton D, Bibas M, Quintero M, et al. Global management of patients with knee osteoarthritis begins with quality of life assessment: a systematic review. BMC Musculoskelet Disord. (2019) 20(1):493. doi: 10.1186/s12891-019-2895-3
69. Xu Z, An N, Wang Z. Exercise-induced hypoalgesia following proprioceptive neuromuscular facilitation and resistance training among individuals with shoulder myofascial pain: randomized controlled trial. JMIRx Med. (2022) 3(4):e40747. doi: 10.2196/40747
Keywords: knee osteoarthritis, proprioceptive exercise, pain, balance function, rehabilitation, meta-analysis
Citation: Lin Y, Yu D, Chen X, Chen P, Chen N, Shao B, Lin Q and Wu F (2025) Effects of proprioceptive exercise for knee osteoarthritis: a systematic review and meta-analysis. Front. Rehabil. Sci. 6:1596966. doi: 10.3389/fresc.2025.1596966
Received: 26 March 2025; Accepted: 10 June 2025;
Published: 24 June 2025.
Edited by:
Pantelis Theodoros Nikolaidis, University of West Attica, GreeceReviewed by:
Shu Xie, Shanghai University of Sport, ChinaXiao z, China Institute of Sport Science, China
Copyright: © 2025 Lin, Yu, Chen, Chen, Chen, Shao, Lin and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuchun Wu, aG9wZXNmbHlpbmdAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Yaoyu Lin
Yaoyu Lin Debiao Yu
Debiao Yu Xiaoting Chen
Xiaoting Chen Peng Chen
Peng Chen Nan Chen
Nan Chen Bin Shao
Bin Shao Qiuxiang Lin
Qiuxiang Lin Fuchun Wu
Fuchun Wu
![A forest plot shows the mean differences between control and experimental groups from various studies regarding a treatment effect. Studies are divided into two subgroups: ≤8 weeks and >8 weeks. For the ≤8 weeks subgroup, the combined mean difference is 1.81 with confidence interval [1.27, 2.34]. For the >8 weeks subgroup, it is 0.95 [0.17, 1.73]. The overall total mean difference is 1.53 [1.09, 1.97]. Diamonds represent overall effects; squares represent individual studies. The plot visually indicates that the treatment generally favors the experimental group.](https://www.frontiersin.org/files/Articles/1596966/fresc-06-1596966-HTML/image_m/fresc-06-1596966-g003.jpg)