- 1The Second School of Clinical Medicine, Southern Medical University, Guangzhou, Guangdong, China
- 2Department of Obstetrics, The Affiliated Foshan Women and Children Hospital, Guangdong Medical University, Foshan, Guangdong, China
- 3Foshan Fetal Medicine Research Institute, the Affiliated Foshan Women and Children Hospital, Guangdong Medical University, Foshan, Guangdong, China
- 4Foshan Key Laboratory of Stem Cell and Maternal-Child Health, Foshan Women and Children Hospital, Foshan, Guangdong, China
- 5Stem Cell Clinical Research Center, Foshan Women and Children Hospital, Foshan, Guangdong, China
- 6Department of Gynecology, Maternal and Child Health Center of Anhui Medical University, The Fifth Affiliated Clinical College of Anhui Medical University, Hefei, Anhui, China
Introduction: Placenta accreta spectrum with antepartum hemorrhage is closely related to maternal and fetal morbidity and mortality. It is of utmost importance to predict the possibility of antepartum hemorrhage using perinatal factors before delivery in women with placenta accreta spectrum. The aim of this study is to identify the risk factors for antepartum hemorrhage in women with placenta accreta spectrum.
Methods: This retrospective cohort study evaluated pregnant women with placenta accreta spectrum. Multivariate logistic regression was used to identify the independent variables associated with antepartum hemorrhage and a nomogram was developed to predict the possibility of antepartum hemorrhage. An Excel form computer interface was constructed to use the prediction model.
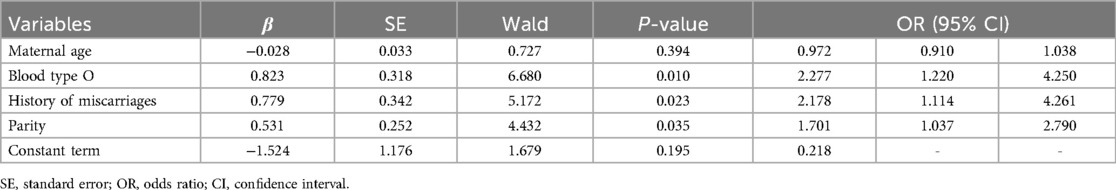
Results: This retrospective cohort study included 188 participants (74 with antepartum hemorrhage). According to multivariate logistic regression analysis, blood type O [odds ratio (OR) 2.277, confidence interval (CI) 1.220–4.250], history of miscarriage (2.178, 95% CI 1.114–4.261), and parity (1.701, 1.037–2.790) were independent risk factors for antepartum hemorrhage in women with placenta accreta spectrum.
Discussion: This results revealed that women with placenta accreta spectrum with blood type O, history of miscarriage, and multiparity may have a significant risk of experiencing antepartum hemorrhage.
Introduction
Placenta accreta spectrum (PAS) is defined as abnormal placental trophoblast adherence with a varying range of myometrial invasion (1, 2). It is encompassed placenta accreta (chorionic villi attached to the myometrium), increta (villi present in the myometrium to the serosa), and percreta (villi penetrating the myometrium beyond the serosa) (3, 4). PAS is a serious pregnancy complication that can lead to severe postpartum hemorrhage, which may necessitate hysterectomy and can even cause maternal death (5–7). We previously reported that the prevalence of placenta accreta spectrum was about 0.2% among pregnant Chinese women (8). Due to the rise in global cesarean delivery rates, uterine surgery, advanced maternal age, and assisted reproductive technology, the incidence of placenta accreta spectrum has increased dramatically in recent years (9, 10).
Major obstetric hemorrhage in the antepartum setting can be life threatening and is a leading cause of intensive care unit admissions of pregnant women worldwide (11). Antepartum hemorrhage is bleeding in late pregnancy and can cause maternal and fetal complications, such as uteroplacental insufficiency, preterm birth, life-threatening maternal hemorrhage, and even perinatal mortality (12–14). In addition, antepartum bleeding is also associated with a greater risk of postpartum hemorrhage (15). Potential causes of severe antepartum hemorrhage include placental abruption, placenta previa, placenta accreta spectrum disorders, and vasa previa (11, 16). Previous studies have found that antepartum hemorrhage has an incidence of about 35% in women with placenta accreta spectrum (17, 18). In addition to well-known causes such as placental abruption and placenta previa along with their associated risk factors (9, 11), the aetiology and pathogenesis of antepartum hemorrhage in women with placenta accreta spectrum have not been clearly identified yet, and clinical studies focusing on placenta accreta spectrum in women with antepartum hemorrhage are rare.
Considering the dangers of antepartum hemorrhage resulting from placenta accreta spectrum, it is of utmost importance to prepare for the possibility of antepartum hemorrhage in women with placenta accreta spectrum based on perinatal factors. Unfortunately, a strategy to identify women at risk of antepartum hemorrhage has not been defined yet. This retrospective cohort aimed to investigate the risk factors for with antepartum hemorrhage among women with placenta accreta spectrum to enable the early prediction and timely management of antepartum hemorrhage in women with placenta accreta spectrum.
Methods
This was a retrospective cohort analysis of participants who presented with placenta accreta spectrum at a tertiary care medical center between January 2012 and December 2022. The Foshan Women and Children Hospital Ethics Committee approved (FSFY-Med-2019044) access to participant information via electronic medical records and waived the requirement for informed consent.
The inclusion criteria were as follows: (1) gestational age between 28 and 42 weeks; (2) underwent prenatal imaging (ultrasound, MRI or cystoscopy) that raised suspicious of PAS; (3) and confirmed diagnosis of PAS after delivery (14, 19–21). Prenatal suspicion of PAS was based on clinical and imaging findings during pregnancy. The main ultrasound criteria used to suspect PAS included loss of the retroplacental sonolucent zone, presence of lacunae within the placenta, increased vascularity at the uterine-bladder interface, and abnormal placental location (e.g., low-lying or covering a previous cesarean scar) (14, 22). When ultrasound findings were inconclusive, MRI was used as an adjunctive tool, with T2-weighted images typically showing dark intraplacental bands, focal or irregular myometrial thinning, and marked heterogeneous placental enhancement (21). Cystoscopy was also occasionally performed in selected cases to rule out bladder invasion (14). The diagnosis of PAS after delivery was confirmed based on histopathological examination of the placental tissue combined with intraoperative findings (23, 24). Specifically, the presence of abnormal placental adherence to the myometrium without a clear plane of separation, or invasion beyond, was considered diagnostic of PAS (19, 20). The exclusion criteria were fetal malformation, intrauterine fetal death, coagulation dysfunction, and incomplete antenatal records. The participants were divided into placenta accreta spectrum with antepartum hemorrhage (PAS-APH) and placenta accreta spectrum without antepartum hemorrhage (PAS-non-APH) groups. Antepartum hemorrhage was defined as cumulative vaginal bleeding greater than 20 ml from 28 gestational weeks to prior to delivery (12, 13, 25).
Maternal characteristics, including maternal age at delivery, parity, preexisting diseases before pregnancy, prior dilatation and curettage, in vitro fertilization, regular antenatal care, gestational age, birth weight, sex of fetus, Apgar score at 5 min, risk factors associated with antepartum hemorrhage before 28 weeks of gestational age, and pregnancy complications, were analyzed. In this study, multiparity was defined as having had two or more previous deliveries beyond 28 weeks of gestation, whether those deliveries resulted in live births or stillbirths. Information of all participants obtained from the electronic medical records was anonymized prior to analysis in the form of an Excel file.
Statistical analysis
Continuous data are reported as mean ± standard deviation, and count data are reported as rates (%). Multivariate logistic regression was used to determine the association of perinatal factors with risk of antepartum hemorrhage. Statistical analyses were performed using the R version 4.3.1 and SPSS 20. A P-value less than 0.05 was considered statistically significant. An Excel form computer interface was constructed to use the models.
Results
After applying the exclusion criteria, 188 participants were included in this study. According to the definition of antepartum hemorrhage, 74 participants (74/188, 39.4%) were included in the PAS-APH group, and 114 participants (114/188, 60.6%) were included in the PAS-non-APH group. Table 1 shows the baseline characteristics of the participants. The PAS-APH group had a higher proportion of participants with blood type O (52.7% vs. 30.7%, p = 0.003), history of miscarriage (75.7% vs. 57.0%, p = 0.009), and male fetus (66.2% vs. 50.9%, p = 0.038) compared to the PAS-non-APH group. On performing multivariate logistic regression analysis, three perinatal factors were found to be independent predictors of PAS-APH: blood type O [odds ratio (OR) 2.277, confidence interval (CI) 1.220–4.250], history of miscarriage (2.178, 95% CI 1.114–4.261), and parity (1.701, 1.037–2.790) (Table 2).
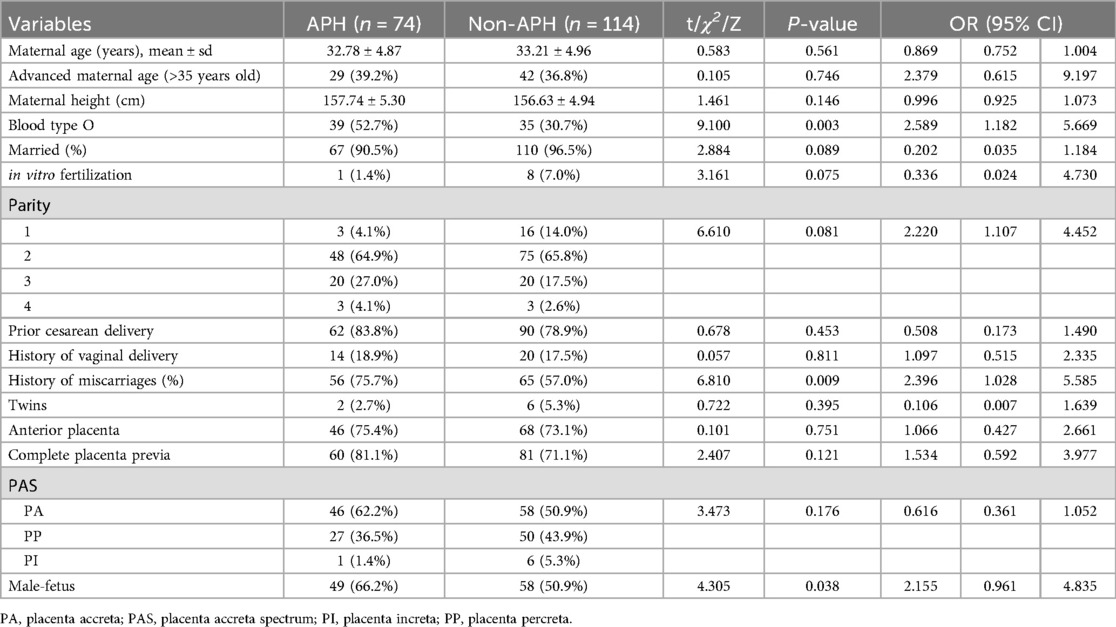
Table 1. General characteristics of participants and multivariate logistic regression analyses for screening predictors.
We then constructed a nomogram by comabining the factors obtained from the logistic regression analysis (blood type O, history of miscarriage, parity) and maternal age (Figure 1). Each factor was assigned a point value, and the total score matched the predicted probability according to their contribution to the overall antepartum hemorrhage risk in women with placenta accreta spectrum. The bias-corrected curve was statistically close to the ideal cureve, and the nomogram calibration curves are shown in Figure 2. The receiver operating characteristic (ROC) cure of the nomogram is shown in Figure 3. It had an area under the curve (AUC) value of 0.826 (95% CI: 0.767–0.884). Additionally, we constructed an Excel form computer interface to calculate the likelihood of antepartum hemorrhage in pregnant women with placenta accreta spectrum using the aforementioned prediction model. The interactive interface generated based on Excel can be viewed in the attachment. An example of this interface is shown in Figure 4.
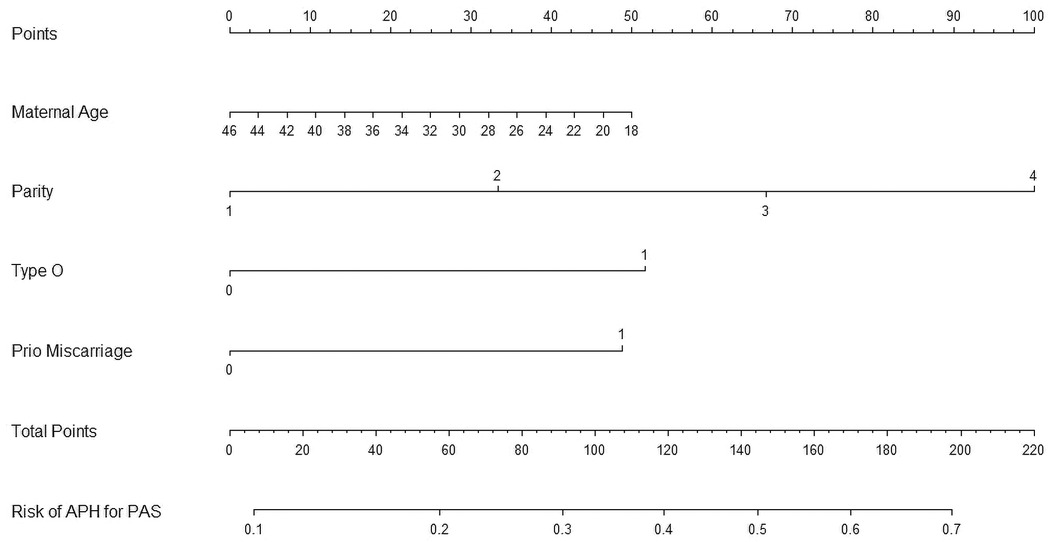
Figure 1. Nomogram for the antepartum hemorrhage for placenta accreta spectrum women. APH, antepartum hemorrhage; PAS, placenta accreta spectrum.
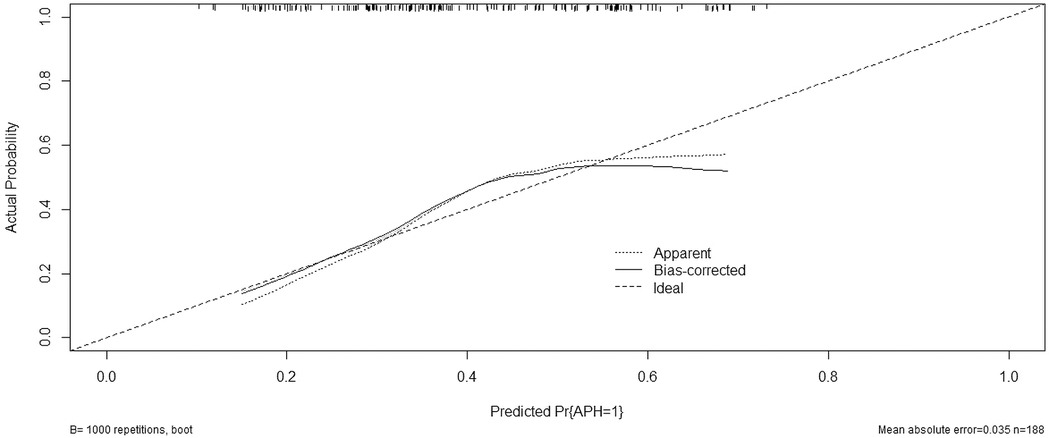
Figure 2. Calibration curve for predicting probability of antepartum hemorrhage for placenta accreta spectrum women. APH, antepartum hemorrhage; PP, placenta accreta spectrum.
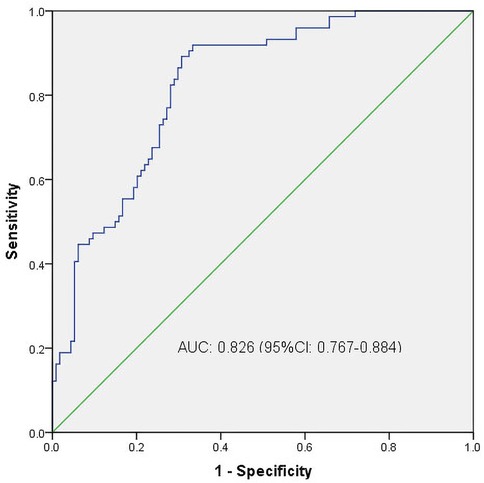
Figure 3. ROC curves. ROC, receiver operating characteristic; AUC, area under the ROC curve; CI, confidence interval.
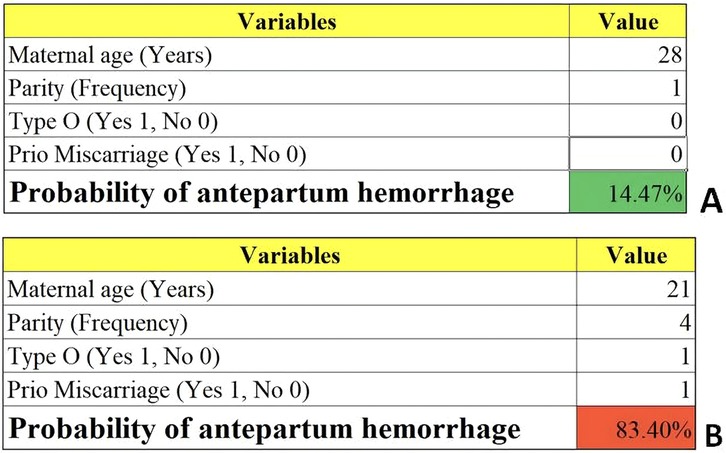
Figure 4. Examples of the computer interface (excel form). Two examples of placenta accreta spectrum women with low (A) and high risk (B) of antepartum hemorrhage.
Discussion
In this study, we found that blood type O, history of miscarriage, and parity increased the risk of antepartum hemorrhage in women with placenta accreta spectrum. We also developed and validated a nomogram based on four perinatal factors (blood type O, history of miscarriage, parity, and maternal age) that accurately predicted the probability of antepartum hemorrhage in women with placenta accreta spectrum.
ABO blood types are the most important blood groups in clinical practice and their relevance to reproductive well-being has been studied (26–29). Franchini et al. described an association between blood type O and venous thromboembolism in pregnant women (30). A retrospective study demonstrated that women with blood type B had an increased risk of gestational diabetes mellitus compared to women with blood type O during singleton pregnancies, while blood type A was associated with a higher risk of placenta previa during twin pregnancies (31). Our recently study on women with placenta previa found that the incidence of antepartum hemorrhage, predelivery anemia, and preterm birth was higher in women with blood type AB, and the incidence of neonatal asphyxia was higher in those with blood type O (32). In this study, we found that the risk of antepartum hemorrhage was increased by 2.2-fold in women with placenta accreta spectrum women with blood type O compared to that in women with blood type non-O blood types. As an independent predictor, blood type O increases the likelihood of antepartum hemorrhage in women with placenta accreta spectrum according to the results of our study.
While our study did not directly explore these mechanisms, existing literature provides some clues that might explain this association. Firstly, individuals with blood type O have lower levels of von Willebrand factor (vWF) and factor VIII compared to other blood types, which could contribute to impaired hemostasis and thus increase the risk of bleeding disorders, including conditions like APH in pregnant women with PAS (28, 32). Secondly, ABO blood group antigens are expressed on the surface of various cells, including endothelial cells, potentially influencing cellular interactions and adhesion processes that are crucial for placentation (29, 31). In the context of PAS, where abnormal placental attachment is a hallmark, differences in how blood type O affects these processes could lead to variations in the risk of developing severe bleeding complications. Lastly, there is evidence suggesting that blood type O is associated with elevated levels of soluble fms-like tyrosine kinase-1 (sFlt-1), a protein linked to the pathophysiology of preeclampsia and other placental-related disorders (27, 30). Elevated sFlt-1 levels could disrupt angiogenic balance, potentially contributing to abnormal placental development and increasing the risk of APH in PAS cases.
A history of miscarriage is associated with various maternal and neonatal complications, which have been reported in the literature. The most common complications consistently linked to a history of miscarriage are preterm birth, preeclampsia, low birth weight, and increased perinatal mortality (33). A systematic review and meta-analysis showed that women with a history of miscarriage are at increased risk of preterm birth in subsequent pregnancies (34). Similarly, a retrospective cohort study conducted over 10 years found that there was an increased risk of adverse obstetric and perinatal outcomes, including placental dysfunction disorder (preterm preeclampsia and preterm birth), and abnormal placentation (placenta previa and placenta accrete), in a subsequent pregnancy among women with a history of miscarriage (35). The present study demonstrates an association between a history of miscarriage and increased risk of antepartum hemorrhage in women with placenta accreta spectrum. This finding may be useful in guiding the clinical management of placenta accreta spectrum in women with a history of miscarriage.
Although parity has been reported to be associated with maternal and neonatal outcomes, the results were inconsistent. A study has claimed that the prevalence of postpartum bleeding, postpartum anemia, gestational diabetes, and preterm pregnancy is related to multiparity (36). In contrast, other studies have reported that multiparous women have similar risks of maternal and neonatal complications as the other parity groups (37). We previously showed a positive correlation between multiparity and the prevalence of antepartum hemorrhage in a meta-analysis that included 29 articles (25). However, Nur Azurah et al. Revealed that there was no difference in the incidence of antepartum hemorrhage between 56 primigravida and 187 non-primigravida women (38). Other factors, such as socioeconomic and sociocultural factors, might play a major role in determining pregnancy outcomes. Because multiparity is more likely to occur in older women, the risks might also be attributed to advanced maternal age in addition to high parity. Interestingly, we found that multiparity was a significant risk factors for antepartum hemorrhage in women with placenta accreta spectrum after adjusting for maternal age.
Maternal age at birth is increasing globally, and is associated with perinatal outcomes (39). A prospective multicenter cohort study found that maternal age is nonlinearly associated with a greater risk of placenta accreta spectrum, placenta previa, gestational diabetes mellitus, hypertensive disorders of pregnancy, preeclampsia, cesarean delivery, preterm birth, large for gestational age, macrosomia, and fetal congenital anomaly, with inflection points around 35.6–40.4 years, but is not related to postpartum hemorrhage or small for gestational age (40). Kuribayashi et al. showed that maternal age below 30 years is more likely to be associated with antepartum hemorrhage in an individual pregnant woman with placenta previa (41). However, studies on the risk of antepartum hemorrhage in women with placenta accreta spectrum women are sparse and equivocal. We did not identify an association between maternal age and antepartum hemorrhage in women with placenta accreta spectrum using multivariate logistic regression analysis. Considering the effects of maternal age on perinatal outcomes, maternal age was considered when constructing the nomogram.
While maternal age did not emerge as a significant factor in this specific context, we believe its inclusion in the nomogram remains justified for several reasons. Firstly, maternal age is widely recognized for its broad impact on perinatal outcomes, making it a clinically relevant factor even if not statistically significant in our model. Secondly, the nomogram serves as a practical tool designed to integrate multiple risk factors, some of which may influence risk through complex interactions or subtle effects that are not fully captured by statistical significance alone. Additionally, maternal age might interact with other variables such as parity or prior cesarean sections, potentially influencing APH risk in ways not fully explored in our initial analysis. Lastly, including maternal age enhances the interpretability and educational value of the nomogram for clinical practitioners who routinely consider maternal age when assessing obstetric risks.
We evaluated the prenatal risk factors for antepartum hemorrhage in women with placenta accreta spectrum and developed a likelihood prediction model for the early identification and management of antepartum hemorrhage in women with placenta accreta spectrum. Our model demonstrated good accuracy and conformity. As a visual and individualized model, a nomogram is an easy-to-use and understandable tool for realistic predictions in clinical practice. However, it needs to be emphasized is that while nomogram is an advantageous and valuable tool for making decisions, clinical judgment remains central to decision-making. While we fully recognize that PAS is associated with a high baseline risk of bleeding, our objective in identifying additional perinatal factors was not to replace current management protocols, but rather to explore potential variables associated with antepartum hemorrhage in this population. We believe such findings may support more refined risk stratification, enhance antenatal counseling, and inform individualized care strategies.
To the best of our knowledge, this is the first study to propose and verify a straight forward technique for estimating the risk of antepartum hemorrhage in women with placenta accreta spectrum in a large group of individuals. However, this study has several limitations that need to be noted. First, recall bias was inevitable, and may have influenced the results because of the retrospective nature of the analysis. Second, owing to the samll sample size and lack of data, several potentially meaningful predictors, including cervical length during pregnancy (before an attack of antepartum bleeding), history of bleeding in the first trimester, previous lower segment cesarean section and placenta previa, weight gain during pregnancy, and maternal occupation, were not evaluated in this study (42, 43). Last, although our study was conducted at a single tertiary care center, the diverse patient population and standardized clinical protocols suggest that our findings may be applicable to similar urban medical centers managing complex obstetric cases. However, differences in healthcare practices, resource availability, and prenatal care access across regions may affect the generalizability of our results. Future prospective multi-center large-sample studies are still needed to further verify the stability and representativeness of the results of this research. Additional potential indicators need to be evaluated in conjunction with clinical variables in a subsequent study to establish a more precise forecasting algorithm for antepartum hemorrhage with the aim of reducing maternal and newborn mortality.
Conclusions
Overall, this study revealed that women with placenta accreta spectrum with blood type O, history of miscarriage, and multiparity may have a significant risk of experiencing antepartum hemorrhage. We also developed the first perinatal prediction nomogram based on several perinatal factors for the early prediction of antepartum hemorrhage in women with placenta accreta spectrum. Our prediction score may prove to be a valuable resource for doctors, midwives, and obstetric nurses, and the nomogram may provide assistance in clinical decision-making.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by This study has been performed in accordance with the Declaration of Helsinki. This study was approved by the Ethic Committee of the Foshan Women and Children Hospital (FSFY-Med-2019044). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
PH: Data curation, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. SY: Data curation, Funding acquisition, Methodology, Supervision, Writing – review & editing. SZ: Investigation, Methodology, Project administration, Resources, Writing – review & editing. XG: Conceptualization, Project administration, Supervision, Validation, Visualization, Writing – review & editing. DF: Conceptualization, Formal analysis, Funding acquisition, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Foshan Health Bureau Medical Scientific Research Project (No. 20230814A010028, No. 20240720A010564, and No. 202532031004), 2022 Foshan self-funded Science and Technology Innovation Project (No. 2220001004010), Anhui Province Clinical Medical Research Translation Special Program (202204295107020048), and Special Project for Clinical and Basic Sci&Tech Innovation of Guangdong Medical University (GDMULCJC2024137).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2025.1588498/full#supplementary-material
Supplementary File 1 | Computer interface (Excel form) of antepartum hemorrhage for placenta previa women.
References
1. Jauniaux E, Hussein AM, Thabet MM, Elbarmelgy RM, Elbarmelgy RA, Jurkovic D. The role of transvaginal ultrasound in the third-trimester evaluation of patients at high risk of placenta accreta spectrum at birth. Am J Obstet Gynecol. (2023) 229:445.e1–445.e11. doi: 10.1016/j.ajog.2023.05.004
2. Nieto-Calvache AJ, Palacios-Jaraquemada JM, Hussein AM, Jauniaux E, Milani Coutinho C, Rijken M. Management of placenta accreta spectrum in low- and middle-income countries. Best Pract Res Clin Obstet Gynaecol. (2024) 94:102475. doi: 10.1016/j.bpobgyn.2024.102475
3. Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, Fox KA, Collins S. FIGO placenta accreta diagnosis and management expert consensus panel. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. (2019) 146:20–4. doi: 10.1002/ijgo.12761
4. Jauniaux E, Chantraine F, Silver RM, Langhoff-Roos J. FIGO placenta accreta diagnosis and management expert consensus panel. FIGO consensus guidelines on placenta accreta spectrum disorders: epidemiology. Int J Gynaecol Obstet. (2018) 140:265–73. doi: 10.1002/ijgo.12407
5. You H, Wang Y, Han R, Gu J, Zeng L, Zhao Y. Risk factors for placenta accreta spectrum without prior cesarean section: a case-control study in China. Int J Gynaecol Obstet. (2024) 166:1092–9. doi: 10.1002/ijgo.15493
6. Fujita T, Yoshizato T, Mitao H, Shimomura T, Kuramoto T, Obara H, et al. Risk factors for placenta accreta spectrum in pregnancies conceived after frozen-thawed embryo transfer in a hormone replacement cycle. Eur J Obstet Gynecol Reprod Biol. (2024) 296:194–9. doi: 10.1016/j.ejogrb.2024.02.040
7. Wang W, Fan D, Wang J, Wu S, Lu Y, He Y, et al. Association between hypertensive disorders complicating pregnancy and risk of placenta accreta: a meta-analysis and systematic review. Hypertens Pregnancy. (2018) 37:168–74. doi: 10.1080/10641955.2018.1498880
8. Fan D, Li S, Wu S, Wang W, Ye S, Xia Q, et al. Prevalence of abnormally invasive placenta among deliveries in mainland China: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). (2017) 96:e6636. doi: 10.1097/MD.0000000000006636
9. Fan D, Lin D, Rao J, Li P, Chen G, Zhou Z, et al. Factors and outcomes for placental anomalies: an umbrella review of systematic reviews and meta-analyses. J Glob Health. (2024) 14:04013. doi: 10.7189/jogh.14.04013
10. Fan D, Liu Y, Hu P, Lin D, Rao J, Sun L, et al. Prevalence of placenta previa among deliveries: an update systematic review and meta-analysis after the introduction of the two-child policy in Mainland China. J Glob Health. (2024) 14:04108. doi: 10.7189/jogh.14.04108
11. Melamud K, Wahab SA, Smereka PN, Dighe MK, Glanc P, Kamath A, et al. Imaging of antepartum and postpartum hemorrhage. Radiographics. (2024) 44:e230164. doi: 10.1148/rg.230164
12. Coomarasamy A, Devall AJ, Cheed V, Harb H, Middleton LJ, Gallos ID, et al. A randomized trial of progesterone in women with bleeding in early pregnancy. N Engl J Med. (2019) 380:1815–24. doi: 10.1056/NEJMoa1813730
13. Gelan M, Bekela T, Angasu K, Ebisa M. Adverse perinatal and maternal outcomes and associated factors among women with antepartum hemorrhage in Jimma university medical center, Southwest Ethiopia, 2020. Obstet Gynecol Int. (2022) 2022:4594136. doi: 10.1155/2022/4594136
14. Liu Y, Fan D, Fu Y, Wu S, Wang W, Ye S, et al. Diagnostic accuracy of cystoscopy and ultrasonography in the prenatal diagnosis of abnormally invasive placenta. Medicine (Baltimore). (2018) 97:e0438. doi: 10.1097/MD.0000000000010438
15. Fan D, Xia Q, Liu L, Wu S, Tian G, Wang W, et al. The incidence of postpartum hemorrhage in pregnant women with placenta previa: a systematic review and meta-analysis. PLoS One. (2017) 12:e0170194. doi: 10.1371/journal.pone.0170194
16. Fan D, Wu S, Ye S, Wang W, Wang L, Fu Y, et al. Random placenta margin incision for control hemorrhage during cesarean delivery complicated by complete placenta previa: a prospective cohort study. J Matern Fetal Neonatal Med. (2019) 32:3054–61. doi: 10.1080/14767058.2018.1457638
17. Fan D, Rao J, Lin D, Zhang H, Zhou Z, Chen G, et al. Anesthetic management in cesarean delivery of women with placenta previa: a retrospective cohort study. BMC Anesthesiol. (2021) 21:247. doi: 10.1186/s12871-021-01472-w
18. Fan D, Zhang H, Rao J, Lin D, Wu S, Li P, et al. Maternal and neonatal outcomes in transverse and vertical skin incision for placenta previa : skin incision for placenta previa. BMC Pregnancy Childbirth. (2021) 21:441. doi: 10.1186/s12884-021-03923-1
19. Liu Z, Fan D, Lin D, Zhang H, Rao J, Wang W, et al. Double-uterine-incision in the management of placenta previa complicated by placenta accreta spectrum. Am J Transl Res. (2021) 13:13017–23.34956519
20. Feng J, Fan DZ, Lin D, Zhang H, Rao J, Liu Z. Sandwich excision in patients with placenta percreta involving maternal bladder. Eur Rev Med Pharmacol Sci. (2022) 26:4252–7. doi: 10.26355/eurrev_202206_29062
21. Chen F, Fan D, Chen R, Zhang Y, Tian G, Zhou D, et al. Grading magnetic resonance imaging signs for diagnosing invasive placenta accreta spectrum disorders. Placenta. (2025) 165:62–72. doi: 10.1016/j.placenta.2025.04.004
22. Jauniaux E, D’Antonio F, Bhide A, Prefumo F, Silver RM, Hussein AM, et al. Modified delphi study of ultrasound signs associated with placenta accreta spectrum. Ultrasound Obstet Gynecol. (2023) 61:518–25. doi: 10.1002/uog.26155
23. Afshar Y, Yin O, Jeong A, Martinez G, Kim J, Ma F, et al. Placenta accreta spectrum disorder at single-cell resolution: a loss of boundary limits in the decidua and endothelium. Am J Obstet Gynecol. (2024) 230:443.e1–443.e18. doi: 10.1016/j.ajog.2023.10.001
24. Premkumar A, Huysman B, Cheng C, Einerson BD, Moayedi G. Placenta accreta spectrum in the second trimester: a clinical conundrum in procedural abortion care. Am J Obstet Gynecol. (2025) 232:92–101. doi: 10.1016/j.ajog.2024.07.045
25. Fan D, Wu S, Liu L, Xia Q, Wang W, Guo X, et al. Prevalence of antepartum hemorrhage in women with placenta previa: a systematic review and meta-analysis. Sci Rep. (2017) 7:40320. doi: 10.1038/srep40320
26. Fan D, Hu P, Rao J, Lin D, Yang J, Liu Z, et al. Prediction nomogram for antepartum hemorrhage in placenta previa women. Ther Adv Reprod Health. (2025) 19:26334941251315127. doi: 10.1177/26334941251315127
27. Burgess A, Johnson TS, Simanek A, Bell T, Founds S. Maternal ABO blood type and factors associated with preeclampsia subtype. Biol Res Nurs. (2019) 21:264–71. doi: 10.1177/1099800419833782
28. Burd JE, Quist-Nelson JA, Edwards SE, Suhag A, Berghella VP, Nijjar JB. Blood type and postpartum hemorrhage by mode of delivery: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. (2021) 256:348–53. doi: 10.1016/j.ejogrb.2020.11.041
29. Seyfizadeh N, Seyfizadeh N, Yousefi B, Borzoueisileh S, Majidinia M, Shanehbandi D, et al. Is there association between ABO blood group and the risk factors of unfavorable outcomes of pregnancy? J Matern Fetal Neonatal Med. (2015) 28:578–82. doi: 10.3109/14767058.2014.927424
30. Franchini M, Mengoli C, Lippi G. Relationship between ABO blood group and pregnancy complications: a systematic literature analysis. Blood Transfus. (2016) 14:441–8. doi: 10.2450/2016.0313-15
31. Chen D, Mao X, Zhang J, Wu L. The impact of maternal ABO blood type on obstetric and perinatal outcomes after frozen embryo transfer. Reprod Biomed Online. (2023) 46:767–77. doi: 10.1016/j.rbmo.2023.01.004
32. Fan D, Rao J, Zhang H, Lin D, Guo X, Liu Z. Blood type and outcomes in pregnant women with placenta previa. Oxid Med Cell Longev. (2023) 2023:4725064. doi: 10.1155/2023/4725064
33. Patel K, Pirie D, Heazell AEP, Morgan B, Woolner A. Subsequent pregnancy outcomes after second trimester miscarriage or termination for medical/fetal reason: a systematic review and meta-analysis of observational studies. Acta Obstet Gynecol Scand. (2024) 103:413–22. doi: 10.1111/aogs.14731
34. Wu CQ, Nichols K, Carwana M, Cormier N, Maratta C. Preterm birth after recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. (2022) 117:811–9. doi: 10.1016/j.fertnstert.2022.01.004
35. Zhang J, Liu X, Rao L, Ma R, Wu W, Chen C, et al. Adverse obstetric and perinatal outcomes of patients with history of recurrent miscarriage: a retrospective cohort study. Fertil Steril. (2023) 120(3):626–34. doi: 10.1016/j.fertnstert.2023.04.028
36. Başkiran Y, Uçkan K, Çeleğen I. Effect of grand multiparity on maternal, obstetric, fetal and neonatal results. Eur Rev Med Pharmacol Sci. (2023) 27:10979–84. doi: 10.26355/eurrev_202311_34466
37. Al-Shaikh GK, Ibrahim GH, Fayed AA, Al-Mandeel H. Grand multiparity and the possible risk of adverse maternal and neonatal outcomes: a dilemma to be deciphered. BMC Pregnancy Childbirth. (2017) 17:310. doi: 10.1186/s12884-017-1508-0
38. Nur Azurah AG, Wan Zainol Z, Lim PS, Shafiee MN, Kampan N, Mohsin WS, et al. Factors associated with placenta praevia in primigravidas and its pregnancy outcome. ScientificWorldJournal. (2014) 2014:270120. doi: 10.1155/2014/270120
39. Fan D, Wu S, Wang W, Xin L, Tian G, Liu L, et al. Prevalence of placenta previa among deliveries in Mainland China: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). (2016) 95:e5107. doi: 10.1097/MD.0000000000005107
40. Zhou Y, Yin S, Sheng Q, Yang J, Liu J, Li H, et al. Association of maternal age with adverse pregnancy outcomes: a prospective multicenter cohort study in China. J Glob Health. (2023) 13:04161. doi: 10.7189/jogh.13.04161
41. Kuribayashi M, Tsuda H, Ito Y, Tezuka A, Ando T, Tamakoshi K, et al. Evaluation of the risk factors for antepartum hemorrhage in cases of placenta previa: a retrospective cohort study. J Int Med Res. (2021) 49:3000605211054706. doi: 10.1177/03000605211054706
42. Wang L, Fan D, Guo H, Chen B, Liu Z. Weight gain during pregnancy and placenta morphology: a prospective cohort study. Eur Rev Med Pharmacol Sci. (2022) 26:1978–83. doi: 10.26355/eurrev_202203_28346
Keywords: placenta accreta spectrum, antepartum hemorrhage, placenta previa, risk factor, nomogram
Citation: Hu P, Ye S, Zhou S, Guo X and Fan D (2025) Perinatal factors for antepartum hemorrhage in women with placenta accreta spectrum. Front. Surg. 12:1588498. doi: 10.3389/fsurg.2025.1588498
Received: 6 March 2025; Accepted: 11 June 2025;
Published: 25 June 2025.
Edited by:
Marco La Verde, Università degli Studi della Campania "Luigi Vanvitelli", ItalyReviewed by:
Petra Hanulikova, Institute for the Care of Mother and Child, CzechiaNaeema Mahmood, Arabian Gulf University, Bahrain
Nesrine Souayeh, Tunis El Manar University, Tunisia
Copyright: © 2025 Hu, Ye, Zhou, Guo and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoling Guo, ZnNndW94bEAxNjMuY29t; Dazhi Fan, ZmFuZGF6aGlnd0AxNjMuY29t
 Pengzhen Hu1,2
Pengzhen Hu1,2 Shuguang Zhou
Shuguang Zhou Xiaoling Guo
Xiaoling Guo Dazhi Fan
Dazhi Fan