- 1Clinic of Family Medicine, Faculty of Medicine, Vilnius University, Vilnius, Lithuania
- 2Department of Radiology, Nuclear Medicine and Medical Physics, Institute of Biomedical Sciences, Faculty of Medicine, Vilnius University, Vilnius, Lithuania
- 3Clinic of Gastroenterology, Nephrourology, and Surgery, Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Vilnius, Lithuania
Variations in hepatic arterial anatomy are highly prevalent, occurring in 45% to 75% of individuals. Understanding hepatic arterial anatomy is critical in optimizing surgical outcomes in hepatopancreatobiliary surgery, transplantation, and interventional radiology. Aberrant arteries are prone to accidental injury during surgery, which may lead to life threatening complications, such as hemorrhage or ischemia. Therefore, a clear understanding of hepatic arterial anatomy is essential for reducing intraoperative risk and improving surgical outcomes. To address this need, the present review provides an overview of hepatic arterial variants according to Michel's classification, aiming to enhance anatomical awareness and contribute to improved procedural planning across disciplines.
Introduction
Atypical hepatic arterial variants are observed in approximately 20%–45% of individuals, with some surgical cohorts reporting even higher rates of up to 60% (1–7). These variations are clinically relevant in hepatopancreatobiliary surgery, transplantation, and interventional radiology, where, if unrecognized, arterial anomalies increase the risk of complications (8–11). Visualizing hepatic arterial anatomy is critical for planning interventions involving the liver and surrounding structures (9). High-quality imaging not only facilitates precise surgical navigation but is also integral in minimizing the risk of post-operative complications (1, 3, 10, 12, 13). Despite the advanced imaging possibilities, preoperative scans may miss up to one-third of hepatic arterial variants, which remain unidentified until surgery, leaving patients at risk for hemorrhage, ischemia, or incomplete tumor resection (5, 8, 9, 11, 14). This highlights the need for accurate mapping and informed surgical planning to reduce preventable morbidity.
While several classification systems have been proposed to categorize hepatic arterial variants, Michel's classification is the most widely recognized system, offering a structured description of the 10 most common anatomical variants (6). In this mini-review, we describe hepatic arterial variants according to Michel's classification, emphasizing key anatomical features and clinical relevance.
Normal hepatic arterial anatomy
In a classical hepatic arterial anatomy (Figure 1), blood reaches the liver through the two main vessels: the portal vein carrying nutrient-rich blood, and the hepatic artery which carries oxygenated blood (15). The common hepatic artery [CHA (2), a. hepatica communis] arises from the celiac trunk [CT (1); truncus celiacus]. The CT also gives rise to the left gastric artery [LGA (3); a. gastrica sinistra] and the splenic artery [SA (4); a. lienalis]. The CHA, LGA and SA form a trifurcation and are sometimes still referred to as Tripus Halleri, first described by the Swiss anatomist and physiologist von Haller (12). The LGA divides into anterior and posterior branches, which course toward the lesser curvature of the stomach, where they form anastomosis with the right gastric artery [RGA (9); a. gastrica dextra], arising from the CHA (3). After originating from the CT, the CHA travels along the superior edge of the pancreas, travelling through the Winslow's canal, also known as epiploic foramen. Then it bifurcates, forming a “Y” shape, giving rise to the proper hepatic artery [PHA (6); a. hepatica propria] and the gastroduodenal artery [GDA (10); a. gastroduodenalis] (8, 9). The PHA divides into anterior and posterior branches, which supply the anterior and posterior parts of the right liver lobe, respectively. The LHA [8] also gives rise to smaller vessels which supply segments II, III, and IV of the liver. The caudate lobe is vascularized by the branches that arise from both the left and right hepatic arteries. Segment IV of the liver is supplied by the middle hepatic artery (MHA; a. hepatica media) which always arises from the CHA, either directly or indirectly (12).
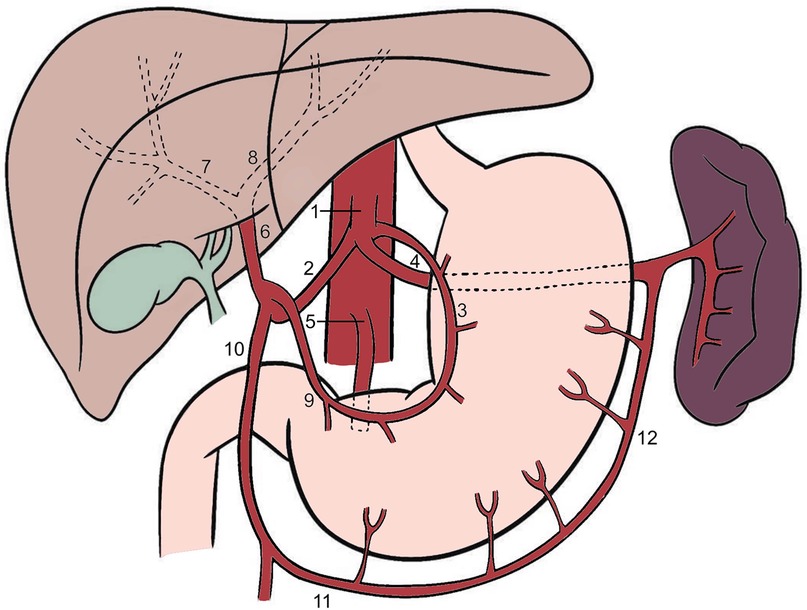
Figure 1. Classical anatomy of the celiac trunk and hepatic arteries. 1—celiac trunk, 2—common hepatic artery, 3—left gastric artery, 4—splenic artery, 5—superior mesenteric artery, 6—proper hepatic artery, 7—right hepatic artery, 8—left hepatic artery, 9—right gastric artery, 10—gastroduodenal artery, 11—right gastroepiploic artery, 12—left gastroepiploic artery.
Comparative overview of hepatic artery classification systems
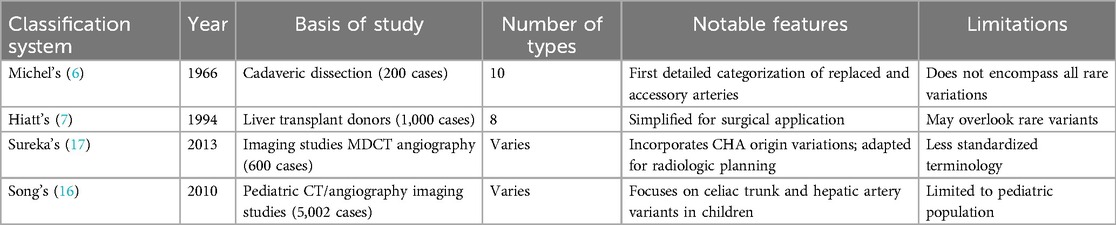
Several classification systems have been created to describe hepatic arterial anatomy (Table 1). The most significant contribution was the now standard classification system published in 1966 by American anatomist Neil A. Michel (Table 2). Based on the analysis of 200 cadaver dissections, it describes the 10 most common arterial variants. Although the sample size may be considered moderate, the classification remains widely used in academic and clinical settings because of its anatomical clarity and surgical relevance (6). In 1994 Hiatt et al. proposed a simplified classification based on 1,000 liver transplant donor cases. Hiatt system presented 6 categories instead of 10, which made the system easier to apply clinically, though at the cost of reduced anatomical detail. While easier to use, it did not capture the complexity of arterial branching, and thus, offered limited insight for surgical planning (7).
More recent imaging-based systems were proposed by Song et al. and Sureka et al. in 2010 and 2013, respectively. Song's system focuses on pediatric imaging. It highlights developmental anomalies, thus its use is mostly restricted to the pediatric population. Song's system provides insight into early vascular development, which aids in diagnosing and treating pediatric hepatobiliary conditions; however, it has limited applicability in adult surgical practice (16). Sureka's classification is based on multidetector CT angiography and is specifically designed for preoperative evaluation. It incorporates common hepatic artery variations and complements Michel's anatomical framework by including additional variants involving the origin of the common hepatic artery (CHA), which Michel's system does not fully adress (17, 18).
Variations of hepatic arterial anatomy according to Michel’s classification
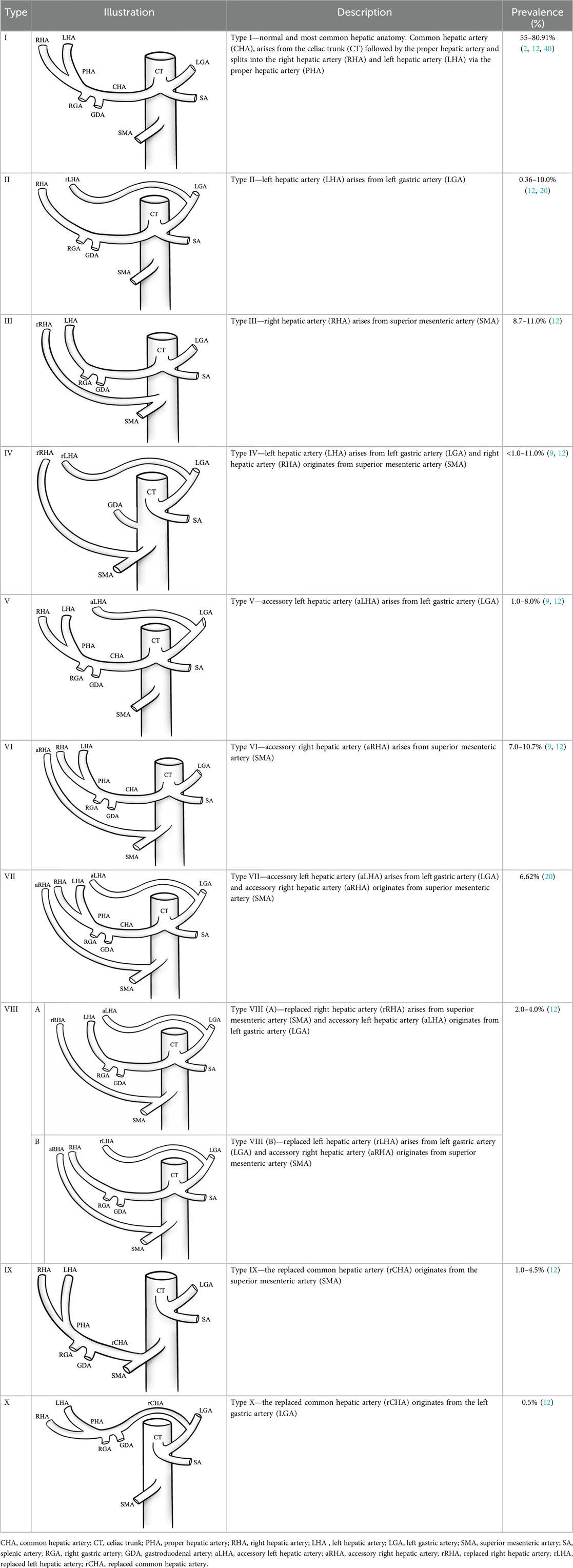
According to Michel's classification, more than half of the population has one of the atypical hepatic arterial variants (12, 19). Within this classical framework, three key terms are used in the classification to describe arterial positioning:
Accessory artery provides additional blood supply for the tissues, together with the already existing arteries of a regular anatomic course (6, 20).
Replaced artery is a vessel that originates from a location different from the usual anatomical position. In the context of hepatic circulation, a replaced artery supplies blood to the liver segments that would normally be served by standard artery, which is absent in this case.
Aberrant artery is an umbrella term for accessory and replaced arteries. In short, an aberrant artery is one that, compared to the normal anatomical variant, branches atypically. This term indicates that the course of the artery is altered, but it does not specify whether the artery provides additional blood flow alongside other arteries supplying the same area or if it is the only artery providing blood supply.
Imaging of hepatic arterial variations
Several imaging modalities are available for evaluating hepatic arterial anatomy, namely computed tomography angiography (CTA), magnetic resonance angiography (MRA), and digital subtraction angiography (DSA). The first choice for imaging is CTA, which effectively visualizes arterial branching, stenosis, and occlusions (19, 21). The main advantage of CTA is its ability to obtain high-quality imaging data of a broad anatomical region. Moreover, it offers shorter acquisition time compared to MRA (22). Three-dimensional vascular CTA reconstructions are particularly valuable for preoperative assessment of arterial branching and spatial relationships with adjacent structures. CTA enables precise visualization of small-calibre and short arterial segments, which is achieved through maximum intensity projection and automated volume rendering techniques (3).
Alternative imaging modalities, such as MRA and DSA, have limited clinical applicability regarding hepatic arterial anatomy visualization. Although MRA does not involve ionizing radiation, it requires longer imaging time and offers lower spatial resolution. MRA is mainly indicated for the assessment of aneurysms, dissection, coarctation, and arterial anomalies, and therefore, has limited relevance in mapping hepatic arterial anatomy (23, 24). DSA is primarily reserved for therapeutic interventions (thrombectomies, stenting, embolization) rather than routine arterial imaging because of its invasive nature (25–27).
Clinical significance of variations in hepatic arterial anatomy
A detailed assessment of hepatic arterial anatomy plays a pivotal role in the preoperative phase of liver surgeries, particularly resections and transplantations, which are among the most common surgeries requiring arterial imaging (28, 29). Research has demonstrated that atypical arterial anatomy is associated with an increased incidence of hepatic artery thrombosis (HAT) in liver transplantation (30). A study analyzing 836 liver transplantations reported that patients with arterial reconstructions due to variant anatomy had a higher risk of developing early HAT, with arterial reconstruction identified as an independent risk factor [adjusted hazard ratio (aHR) = 3.72] (31).
Identifying aberrant arteries is pivotal in oncologic hepatobiliary surgeries, especially in pancreaticoduodenectomy (the Whipple procedure). One of the variants particularly vulnerable to injuries is Michel's type III, where a replaced RHA arises from the SMA and often courses through or close to the pancreatic head, making it susceptible to intraoperative injury and potentially compromising oncologic resection margins (32, 33). Moreover, a 2022 study of 207 patients undergoing pancreatic head resection confirmed the presence of an accessory RHA as an independent predictor of hepatic recurrence, with 61.8% in patients with an aberrant RHA vs. 25.3% of those without an atypical arterial variant (P < 0.0001) (34). Michel's type II (replaced LHA from LGA) variation poses a risk of left hepatic lobe ischemia in distal pancreatectomy with celiac axis ligation, as the arterial supply to the left liver lobe may be interrupted by inadvertent ligation of the LGA. Michel's type IX (replaced CHA from SMA) places the liver and bile duct at risk of ischemia as dissection near SMA may damage a single arterial source that is responsible for multiple organ blood supply (35).
During laparoscopic cholecystectomy, RHA is the most commonly injured vessel (21, 36, 37). Michel's type III (replaced RHA from SMA), may increase the risk of intraoperative bleeding and bile duct injury. Extensive knowledge of anatomical variations improves dissection and minimizes the likelihood of adverse surgical events, especially while preoperative arterial imaging is not usually performed before cholecystectomy (21). For example, if surgeons are not aware of RHA variations, such as in the case of unclear Calot's triangle, RHA could be accidentally injured or mistaken for cystic artery and cut off (21). Recent evidence confirms that hepatic arterial variations (like a replaced or accessory RHA) are clinically significant risk factors for complications in cholecystectomy, for example intraoperative hemorrhage occurred in 16 out of 95 patients with anatomical variants (16.8%) vs. 4 out of 203 in the normal group (1.9%) (P < 0.001) ( (38).
In interventional radiology, radioembolization presents a particular challenge, as inaccurately assessed aberrant arteries can compromise procedural effectiveness and lead to suboptimal distribution of radioactive microspheres (4). In chemoembolization and intra-arterial liver chemotherapy, poor knowledge of the anatomical variations may lead to ineffective treatment or potential damage to the hepatic parenchyma or the adjacent soft tissue structures (9). For example, navigating Michel's type III (replaced RHA from SMA), may require an altered catheterization strategy due to its retroportal course, which results in more complicated catheter access (39). Therefore, meticulous vascular mapping should be considered standard practice in interventional hepatic procedures involving arterial access.
Conclusions
Anatomical variations of hepatic arteries are highly prevalent and clinically significant. Detailed understanding and accurate reporting of these variants are essential for reducing procedural risks in hepatopancreatobiliary surgery, liver transplantation, and interventional radiology. Increased awareness of hepatic arterial variants should be encouraged in clinical practice and education to ensure more effective patient care.
Author contributions
AS: Visualization, Writing – review & editing, Writing – original draft. RL-L: Supervision, Writing – review & editing, Writing – original draft, Resources, Methodology. MK: Writing – original draft, Supervision, Resources, Methodology, Conceptualization, Writing – review & editing, Project administration.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Türkyılmaz Z, Kula O, Çelik AO, Demirel T, Günay B. Evaluation of celiac artery and common hepatic artery variations by CT-angiography and new classification model. Surg Radiol Anat SRA. (2023) 45(8):1037–47. doi: 10.1007/s00276-023-03180-1
2. Zaki SM, Abdelmaksoud AHK, Khaled BEA, Kader IAA. Anatomical variations of hepatic artery using the multidetector computed tomography angiography. Folia Morphol. (2020) 79(2):247–54. doi: 10.5603/FM.a2019.0090
3. Araujo Neto SA, de Mello Júnior CF, Franca HA, Duarte CMA, Borges RF, de Magalhães AGX. Multidetector computed tomography angiography of the celiac trunk and hepatic arterial system: normal anatomy and main variants. Radiol Bras. (2016) 49:49–52. doi: 10.1590/0100-3984.2014.0041
4. van den Hoven AF, Smits MLJ, de Keizer B, van Leeuwen MS, van den Bosch MAAJ, Lam MGEH. Identifying aberrant hepatic arteries prior to intra-arterial radioembolization. Cardiovasc Intervent Radiol. (2014) 37(6):1482–93. doi: 10.1007/s00270-014-0845-x
5. Walker BS, Sutton TL, Eil RL, Korngold EK, Kolbeck KJ, Billingsley KG, et al. Conventional hepatic arterial anatomy? Novel findings and insights of a multi-disciplinary hepatic arterial infusion pump program. Am J Surg. (2021) 221(6):1188–94. doi: 10.1016/j.amjsurg.2021.02.021
6. Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. (1966) 112(3):337–47. doi: 10.1016/0002-9610(66)90201-7
7. Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg. (1994) 220(1):50–2. doi: 10.1097/00000658-199407000-00008
8. Malviya KK, Verma A. Importance of anatomical variation of the hepatic artery for complicated liver and pancreatic surgeries: a review emphasizing origin and branching. Diagnostics. (2023) 13(7):1233. doi: 10.3390/diagnostics13071233
9. Favelier S, Germain T, Genson PY, Cercueil JP, Denys A, Krausé D, et al. Anatomy of liver arteries for interventional radiology. Diagn Interv Imaging. (2015) 96(6):537–46. doi: 10.1016/j.diii.2013.12.001
10. Karakoyun R, Romano A, Yao M, Dlugosz R, Ericzon BG, Nowak G. Impact of hepatic artery variations and reconstructions on the outcome of orthotopic liver transplantation. World J Surg. (2020) 44(6):1. doi: 10.1007/s00268-020-05406-4
11. Balzan SMP, Gava VG, Pedrotti S, Magalhães MA, Schwengber A, Dotto ML, et al. Prevalence of hepatic arterial variations with implications in pancreatoduodenectomy. Arq Bras Cir Dig. (2019) 32(3):e1455. doi: 10.1590/0102-672020190001e1455
12. Mathew RP, Venkatesh SK. Liver vascular anatomy: a refresher. Abdom Radiol. (2018) 43(8):1886–95. doi: 10.1007/s00261-018-1623-z
13. Bodzin AS, Baker TB. Liver transplantation today: where we are now and where we are going. Liver Transpl. (2018) 24(10):1470–5. doi: 10.1002/lt.25320
14. Samuilis A. Anatomical Variants of the Hepatic Arteries and Their Influence on Superior Mesenteric Artery Hemodynamics [dissertation]. Vilnius: Vilnius University (2011). Available online at: https://epublications.vu.lt/object/elaba:2062219/ (accessed March 11, 2025).
15. Geller DA, Goss JA, Busuttil RW, Tsung A. Chapter 31: Liver. In: Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Kao LS, Hunter JG, et al. editors. Schwartz’s Principles of Surgery. 11th ed. New York, NY: McGraw-Hill Education (2019). https://accessmedicine.mhmedical.com/content.aspx?bookid=2576§ionid=216215448
16. Song SY, Chung JW, Yin YH, Jae HJ, Kim HC, Jeon UB, et al. Celiac axis and common hepatic artery variations in 5002 patients: systematic analysis with spiral CT and DSA. Radiology. (2010) 255(1):278–88. doi: 10.1148/radiol.09090389
17. Sureka B, Mittal MK, Mittal A, Sinha M, Bhambri NK, Thukral BB. Variations of celiac axis, common hepatic artery and its branches in 600 patients. Indian J Radiol Imaging. (2013) 23(3):223–33. doi: 10.4103/0971-3026.120273
18. Ugurel MS, Battal B, Bozlar U, Nural MS, Tasar M, Ors F, et al. Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography. Br J Radiol. (2010) 83(992):661–7. doi: 10.1259/bjr/21236482
19. Garg S, Kumar KH, Sahni D, Yadav TD, Aggarwal A, Gupta T. Anatomy of the hepatic arteries and their extrahepatic branches in the human liver: a cadaveric study. Ann Anat Anat Anz Off Organ Anat Ges. (2020) 227:151409. doi: 10.1016/j.aanat.2019.07.010
20. Cirocchi R, D’Andrea V, Amato B, Renzi C, Henry BM, Tomaszewski KA, et al. Aberrant left hepatic arteries arising from left gastric arteries and their clinical importance. Surg J R Coll Surg Edinb Irel. (2020) 18(2):100–12. doi: 10.1016/j.surge.2019.06.002
21. Pesce A, Fabbri N, Feo CV. Vascular injury during laparoscopic cholecystectomy: an often-overlooked complication. World J Gastrointest Surg. (2023) 15(3):338–45. doi: 10.4240/wjgs.v15.i3.338
22. Osborn AG, Digre KB. Introduction to imaging. In: Osborn AG, Digre KB, editors. Imaging in Neurology. 1st ed. Philadelphia: Elsevier (2016). p. 2–7. Available at: https://www.sciencedirect.com/science/article/pii/B9780323447812500085 (Accessed March 11, 2025).
23. Caserta MP, Chaudhry F, Bechtold RE. Chapter 11: Liver, biliary tract, and pancreas. In: Chen MYM, Pope TL, Ott DJ, editors. Basic Radiology. 2nd ed. New York, NY: McGraw-Hill Companies (2011). Available at: https://accessmedicine.mhmedical.com/content.aspx?aid=6671146 (Accessed March 11, 2025).
24. Aguet J, Gill N, Tassos VP, Chavhan GB, Lam CZ. Contrast-enhanced body magnetic resonance angiography: how we do it. Pediatr Radiol. (2022) 52(2):262–70. doi: 10.1007/s00247-021-05020-z
25. Aburto-Murrieta Y, Méndez B, Marquez-Romero JM. Extended time window mechanical thrombectomy for pediatric acute ischemic stroke. J Cent Nerv Syst Dis. (2022) 14:11795735221098140. doi: 10.1177/11795735221098140
26. Park S, Jeong B, Shin JH, Jang EH, Hwang JH, Kim JH. Transarterial embolisation for gastroduodenal bleeding following endoscopic resection. Br J Radiol. (2021) 94(1122):20210062. doi: 10.1259/bjr.20210062
27. Wang HY, Zhang R, Dou K, Huang Y, Xie L, Qiao Z, et al. Left main bifurcation stenting: impact of residual ischaemia on cardiovascular mortality. Eur Heart J. (2023) 44(41):4324–36. doi: 10.1093/eurheartj/ehad318
28. Goud Kurelli SC, Wang J, Abbas S, Kanduri HK, Liu W. Hepatobiliary and hepatic vascular anatomy evaluated with computed tomography and magnetic resonance imaging: the current status. Radiol Infect Dis. (2020) 7(1):1–6. doi: 10.1016/j.jrid.2020.02.005
29. Schroering JR, Kubal CA, Hathaway TJ, Robinson RC, Mangus RS. Impact of variant donor hepatic arterial anatomy on clinical graft outcomes in liver transplantation. Liver Transpl. (2018) 24(10):1481–4. doi: 10.1002/lt.25316
30. Bekker J, Ploem S, Jong KPD. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. (2009) 9(4):746–57. doi: 10.1111/j.1600-6143.2008.02541.x
31. Minciuna I, De Jonge J, Den Hoed C, Maan R, Polak WG, Porte RJ, et al. Antiplatelet prophylaxis reduces the risk of early hepatic artery thrombosis following liver transplantation in high-risk patients. Transpl Int. (2024) 37:13440. doi: 10.3389/ti.2024.13440
32. Swami A, Yadav T, Varshney VK, Sreesanth KS, Dixit SG. Hepatic arterial variations and its implication during pancreatic cancer surgeries. J Gastrointest Cancer. (2021) 52(2):462–70. doi: 10.1007/s12029-021-00598-x
33. Li T, Dong L, Zhang D, Han J, Dai M, Guo J, et al. Evaluating the surgical and oncological outcomes of hepatic artery variations in minimally invasive pancreaticoduodenectomy: insights from 2023 data at a high-volume pancreatic center. World J Surg Oncol. (2025) 23:44. doi: 10.1186/s12957-025-03704-6
34. Mangieri CW, Clark CJ. ASO Author reflections: aberrant right hepatic artery anatomy, an independent prognostic factor for hepatic recurrence of pancreatic cancer. Ann Surg Oncol. (2022) 29(5):3229–30. doi: 10.1245/s10434-022-11406-6
35. Xu YC, Yang F, Fu DL. Clinical significance of variant hepatic artery in pancreatic resection: a comprehensive review. World J Gastroenterol. (2022) 28(19):2057–75. doi: 10.3748/wjg.v28.i19.2057
36. Kazi IA, Siddiqui MA, Thimmappa ND, Abdelaziz A, Gaballah AH, Davis R, et al. Post-operative complications of cholecystectomy: what the radiologist needs to know. Abdom Radiol. (2025) 50(1):109–30. doi: 10.1007/s00261-024-04387-5
37. Blecha MJ, Frank AR, Worley TA, Podbielski FJ. Aberrant right hepatic artery in laparoscopic cholecystectomy. JSLS. (2006) 10(4):511–3.17575769
38. Gupta R, Kumar A, Hariprasad CP, Kumar M. Anatomical variations of cystic artery, cystic duct, and gall bladder and their associated intraoperative and postoperative complications: an observational study. Ann Med Surg (Lond). (2023 Aug) 85(8):3880. doi: 10.1097/MS9.0000000000001079
39. Choi TW, Chung JW, Kim HC, Lee M, Choi JW, HJ J, et al. Anatomic variations of the hepatic artery in 5625 patients. Radiol Cardiothorac Imaging. (2021) 3(4):e210007. doi: 10.1148/ryct.2021210007
Keywords: hepatic arterial variations, hepatic vasculature, hepatopancreatobiliary surgery, interventional radiology, preoperative imaging
Citation: Samuolyte A, Luksaite-Lukste R and Kvietkauskas M (2025) Anatomical variations of hepatic arteries: implications for clinical practice. Front. Surg. 12:1593800. doi: 10.3389/fsurg.2025.1593800
Received: 14 March 2025; Accepted: 23 May 2025;
Published: 18 June 2025.
Edited by:
Lovenish Bains, University of Delhi, IndiaReviewed by:
Ece Meram, University of Minnesota Medical Center, United StatesMikayle Holm, Medtronic (United States), United States
Copyright: © 2025 Samuolyte, Luksaite-Lukste and Kvietkauskas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Austeja Samuolyte, YXVzdGVqYS5zYW1AZ21haWwuY29t
 Austeja Samuolyte
Austeja Samuolyte Raminta Luksaite-Lukste
Raminta Luksaite-Lukste Mindaugas Kvietkauskas
Mindaugas Kvietkauskas