- 1Department of Biomedical Sciences, Veterinary Faculty, Institute of Biomedicine (IBIOMED), University of Leon, Leon, Spain
- 2Applied Mathematics, Department of Mathematics, University of Leon, Leon, Spain
- 3Department of Veterinary Medicine, Surgery and Anatomy. Director of the Veterinary Teaching Hospital (HVULE), Veterinary Faculty, University of Leon, Leon, Spain
Cancer is one of the most common causes of death for companion animals. The study aimed to describe the characteristics of the clinical cases of pets attending at the Veterinary Teaching Hospital (University of Leon, Spain) and diagnosed with tumors. A retrospective study was carried out between 2015 and 2024. A total of 123 animals comprising 107 dogs and 16 cats were obtained from the clinical records. A mean annual incidence risk of 530 of 100,000 animals was calculated. Most animals were dogs (87.0%), females (62.6%), purebred (77.2%) and aged (78.9%). Tumors were mainly malignant (87.8%), they were of epithelial origin (40.7%), and mostly located in mammary glands (27.6%) or skin/mucosa (26.8%). Carcinoma (35.8%) and lymphoma (19.5%) were the major histological types. Almost half of the animals underwent surgical treatment (42.3%). Chemotherapy was administered to 37.4% of the animals, mostly by the oral route. QL01E (protein kinase inhibitors) was the main pharmacological group employed. Concomitant treatments and dietary supplements were also used. Euthanasia was applied to 26.8% of the animals.
1 Introduction
Cancer is a leading cause of morbidity and death in pets, as a consequence of the recent extension of their lifespan (1–5). According to the Veterinary Cancer Society, one out of four dogs will be diagnosed with cancer in their lifetime (6). Prevention, early detection and treatment of diseases in companion animals, mainly dogs and cats, have contributed to increasing their life expectancy beyond the age established by nature. Medical and social advances and nutrition improvements achieved in recent decades, from which animals also benefit, are also factors that may help to explain this increase (7). Moreover, the unique relationship established between owners and pets, which are often considered “a member of the family” (8, 9), has made owners more inclined to take care for these animals and spend more on them and on those treatments they may need for various diseases (9), cancer among them.
Although this disease is not reportable in these animals, several studies have determined cancer incidence in pets (10–12), and different sources have been used to obtain case records. The incidence rate for malignant cancers in pets ranges from 142.8 (10) to 852/100,000 dogs (11), and from 63 (10) to 412/100,000 cats (12). It should be noted that the recording of tumors in dogs and cats are not completely standardized, and different tools such as VetCompass (13), networks like VetOncoNet (14), national or regional tumor registries (15, 16) or data from referral hospitals (17, 18) have been used to calculate cancer incidence. In other cases the incidence of a particular type of tumor, such as lymphoma (13, 18), cutaneous (19) or mammary gland tumors (17, 20), or salivary neoplasia (21) has been documented.
Regarding treatment possibilities, although guidelines have been established in companion animals (22), there is no information available on their use in field conditions. As sick animals are brought by people of the surrounding areas, Veterinary Teaching Hospitals may be a reliable source of information of animal diseases. They are also referrals for primary care professionals, although information on clinical cases is often used only for accounting purposes, to demonstrate that they have reached a minimum number of clinical cases to be seen by the students regarding the different animal species or clinical services offered. In Great Britain or Australia, databases such as UK VetCompass and Australia VetCompass are available, indexing clinical records around these countries as a way to improve knowledge on identification, prevention, and treatment of diseases in pets (23, 24), sharing this information among practitioners. In other countries little evidence-based data are available to veterinarians to improve animal health and welfare, and only some prescription surveys have been conducted (25, 26). However, it is necessary to have practical and real information about the diseases diagnosed in pets and those treatments carried out on a daily basis. In addition, in the case of cancer, although some veterinary drugs have recently been approved, most of the medicines used are still of human use. Therefore, in this study we have retrospectively evaluated a population of pets diagnosed with cancer in a Spanish Veterinary Teaching Hospital over a 10-year period. The objective was to characterize the population affected, the types of tumor diagnosed, and the modalities of treatment followed.
2 Materials and methods
A retrospective review of medical records from the Veterinary Teaching Hospital (HVULE) (Faculty of Veterinary Medicine, University of Leon, Spain), from January 2015 to December 2024, was performed. The Veterinary Teaching Hospital works as a referral hospital for other practitioners in the surrounding area, and carries out diagnostic, clinical and preventive practices. Clinical services in the HVULE are organized according to two main groups of target species: small and large animals, both equipped with emergency facilities. All the teaching staff were qualified veterinarians. The HVULE offers specialty services for oncology diagnosis and treatment such as Diagnostic Imaging, General Surgery, Clinical Pathology or Oncology, among others. The hospital also has a veterinarian responsible for Oncology and Internal Medicine, with part-time collaboration of an external practitioner accredited in Oncology by AVEPA (Spanish Small Animals Veterinary Association). Regarding Surgery in companion animals, three trained veterinarians performed surgeries at the HVULE, and they were accredited by AVEPA in Soft Tissue Surgery, and also recognized at European level. In this study, animals attending at the Small Animals Service were evaluated.
Medical records stored in the veterinary hospital database (GestorVet, Las Palmas de Gran Canaria, Spain) were reviewed and searched for pet patients with a diagnosis of cancer. Potential cases of cancer from the electronic database were identified by searching for different Spanish search terms: cancer, tumor, carcin*, adenocarc*, mastocitoma, sarcoma, *oma, tocera*, carbopl*, ciclof*, cloram*, doxorub*, lomust*, mitoxant*, vincr*, vinbl*, CHOP, COP, *LOP, and UW within 1st January 2015 to 31st December 2024. The retrieved clinical records of these potential cases were then assessed by a veterinarian, and confirmed after having checked diagnosis and treatment. Patients whose medical record lacked essential information (presumptive diagnosis, or absence of diagnosis or treatment) were excluded.
Information collected from medical records included data on signalment, such as age at diagnosis, purebred or not (if purebred, which breed), sex, neuter status, and weight, as well as those characteristics related to tumor diagnosis (malignancy, type of tumor, histological origin of cells, and location). For these latter characteristics data recorded was based on the results of the cytology and/or biopsy, and the advice of a pathologist who usually diagnoses tumors in the HVULE was required. Regarding histogenesis classification, the type of the tumor cells were grouped as epithelial, mesenchymal, or round cells (27). Tumors were coded according to the Vet-ICD-O-canine system (Veterinary International Classification of Diseases for Oncology canine neoplasms, first edition) (28). In this case and to simplify data presentation, tumors were grouped into 7 anatomical sites or locations and 6 histotypes.
Treatment information encompassed those modalities of treatment followed (surgery or chemotherapy) or if the animal was euthanized. Supportive medical treatments were also collected. As for chemotherapy treatment, information included drugs, dosages, administration route, and outcome of treatment (death, cure, disease progression, disease recurrence, unknown). Adverse events were also recorded according to the Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) (29). Personal data such as pet names or information on the owners was not accessed. The age of cases was calculated from their date of birth, and the date at which the first tumor diagnosis was also obtained. Dogs were described as young (< 2 years), mature (2–6 years), senior (7–11 years) and geriatric (> 12 years) (30), whereas cats were grouped as kitten (< 7 months), junior (7 months to 2 years), prime (3–6 years), mature (7–10 years), senior (11–14 years) and geriatric (> 15 years) (31). Weight and breed were used to define the size of the animals. For adult dogs, they were classified as toy and small (< 10 kg), medium (10 to < 20 kg), large (20 to > 40 kg), and giant (≥ 40 kg). Cats were grouped as small (< 2.5 kg) or medium (2.5–6 kg).
The Strengthening the Reporting of Observational Studies in Epidemiology-Veterinary Extension (STROBE-Vet) Statement was used to report data (32).
2.1 Statistical analysis
Descriptive statistics (mean, standard deviation, ranges, and frequencies with 95% confidence intervals) were used to characterize this specific pet population. Annual incidence risk with 95% confidence intervals (CI) was estimated by calculating the proportion of incident cases within the total number of dogs and cats under veterinary care at the HVULE from 2015 to 2024 (n = 22,987 animals), and the same was made with the animal attendances. The odds ratio (OR) was calculated with their respective 95% CI. Multivariate forward-step logistic regression analysis was also performed to identify those variables potentially associated with the use of surgery as treatment. Model calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test. The statistical package IBM SPSS Statistics 26 (IBM Corporation, Armonk, NY, United States) was used to perform data analysis. A p value of 0.05 was always considered as significant.
3 Results
The study population consisted of 22,987 companion animals (17,307 dogs and 5,680 cats) under care at the HVULE between 2015 and 2024, attending a total of 63,608 times at the hospital over this period of time. A total of 123 animals were diagnosed with cancer, treated and cared at the HVULE in the 10-year period studied. Mean annual incidence risk was estimated as 530 of 100,000 animals attending at the HVULE over the 10 years studied (range 437–625 of 100,000 animals), 618 of 100,000 dogs (range 501–735 of 100,000 animals), and 264 of 100,000 cats (range 131–398 of 100,000 animals). The annual incidences are also shown in Supplementary Table S1.
Table 1 describes the demographic characteristics of the animals attending at the hospital. Dogs (107 animals) were almost 7 times more represented in the sample than cats (16 patients), and females encompassed 62.6% of the overall cases. In dogs, females (65.4%) almost double the number of males, whereas cats were more homogeneously distributed between males and females. Mean weight at diagnosis was 24.0 ± 13.2 kg in dogs (range 2.9–65.5 kg) and 3.9 ± 1.1 kg in cats (range = 1.5–5.8 kg). Cats tended to be slightly older (9.9 ± 3.8 years) than dogs (9.5 ± 2.7 years), ranging the age of the animals from 2 to 15 years old for dogs, and from 3 to 17 years old for cats. A higher frequency of tumors was seen for senior and geriatric animals, mounting up 78.9% of all tumor cases.
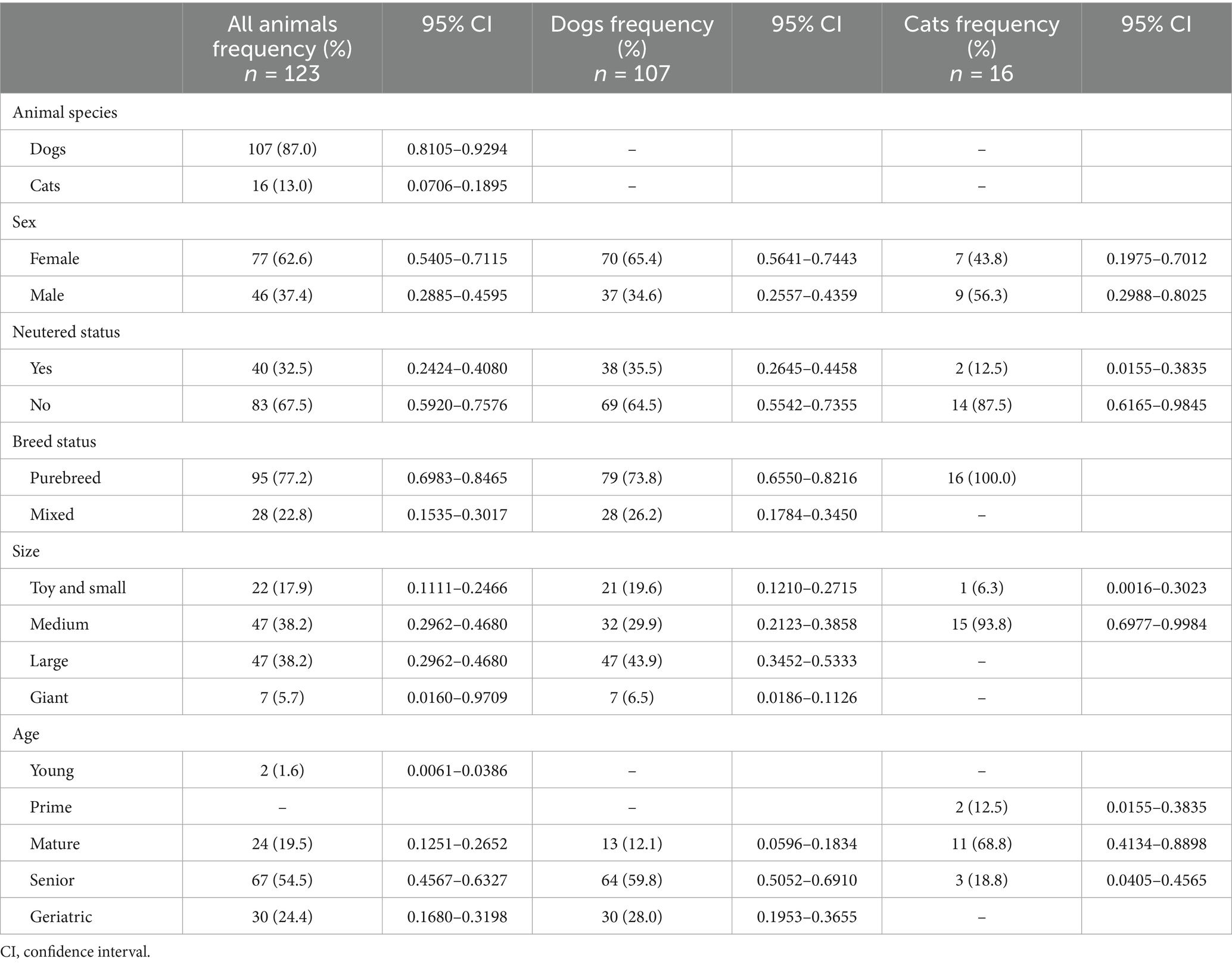
Table 1. Demographic characteristics of the animals attending to the HVULE (Spain) and diagnosed with cancer from 2015 to 2024.
The majority of the animals were medium to large in size. Dogs were mostly large-giant (43.9%) or medium-sized animals (38.2%), whereas practically all cats were of medium size (93.8%). Purebred animals were in the majority in both species, accounting for 3 quarters of dogs (73.8%) and all cats. With regard to animal breeds, there was a greater variety of breeds among dogs, with a number of animals ranging in many of them from 1 to 6 individuals. Tumors were most frequently diagnosed in Golden Retriever and Boxer breeds (6.5% each) followed by German shepherds and Labrador Retriever (5.6%). As for cats, although with a much smaller number of animals, more than three quarters of them belonged to the European shorthair cat, and the other 3 animals were each of a different breed (Maine Coon, Bombay and Siamese).
Table 2 summarizes the characteristics of the tumors diagnosed in these animals. Moreover, the classification according to the Vet-ICD-O-canine system is presented in Supplementary Table S2. Benign tumors were found in only 12.2% of patients. Mean age was nearly similar in those dogs diagnosed for benign (9.8 ± 2.5 years old) and malignant tumors (9.5 ± 2.7 years). Something similar occurred in cats, as the only animal with a benign tumor was 10 years old, and the age in feline animals with malignant tumors was 10.0 ± 3.8 years old. Epithelium was the predominant tissue of origin (40.7%), followed by those that had their origin in round cells (30.1%). The most frequent histotype was carcinoma (35.8%), followed by lymphoma (19.5%), and mast cell tumors (10.6%). In this characteristic, the group “other” accounts for 22.0%, and includes different typologies with lower frequencies. Of the carcinomas, 59.1% were present in the mammary gland. As for topography, the three most affected systems were mammary glands (27.6%), skin/mucosa (26.8%), and the hematolymphoid system (17.9%), although proportions changed from one species to another. All the animals with mammary tumors were female. Regarding carcinoma, female dogs were 4 times more likely to developed this last histotype than males (OR = 3.936; 95% CI = 1.476–10.501; p = 0.006); and it was also 3 times more likely for mixed than purebreed dogs (OR = 2.959; 95% CI = 1.159–7.555; p = 0.023). In the case of lymphoma, no differences were found according to sex, breed or age but was higher in purebreed (83.3%), females (61.1%) and senior (44.4%) dogs.
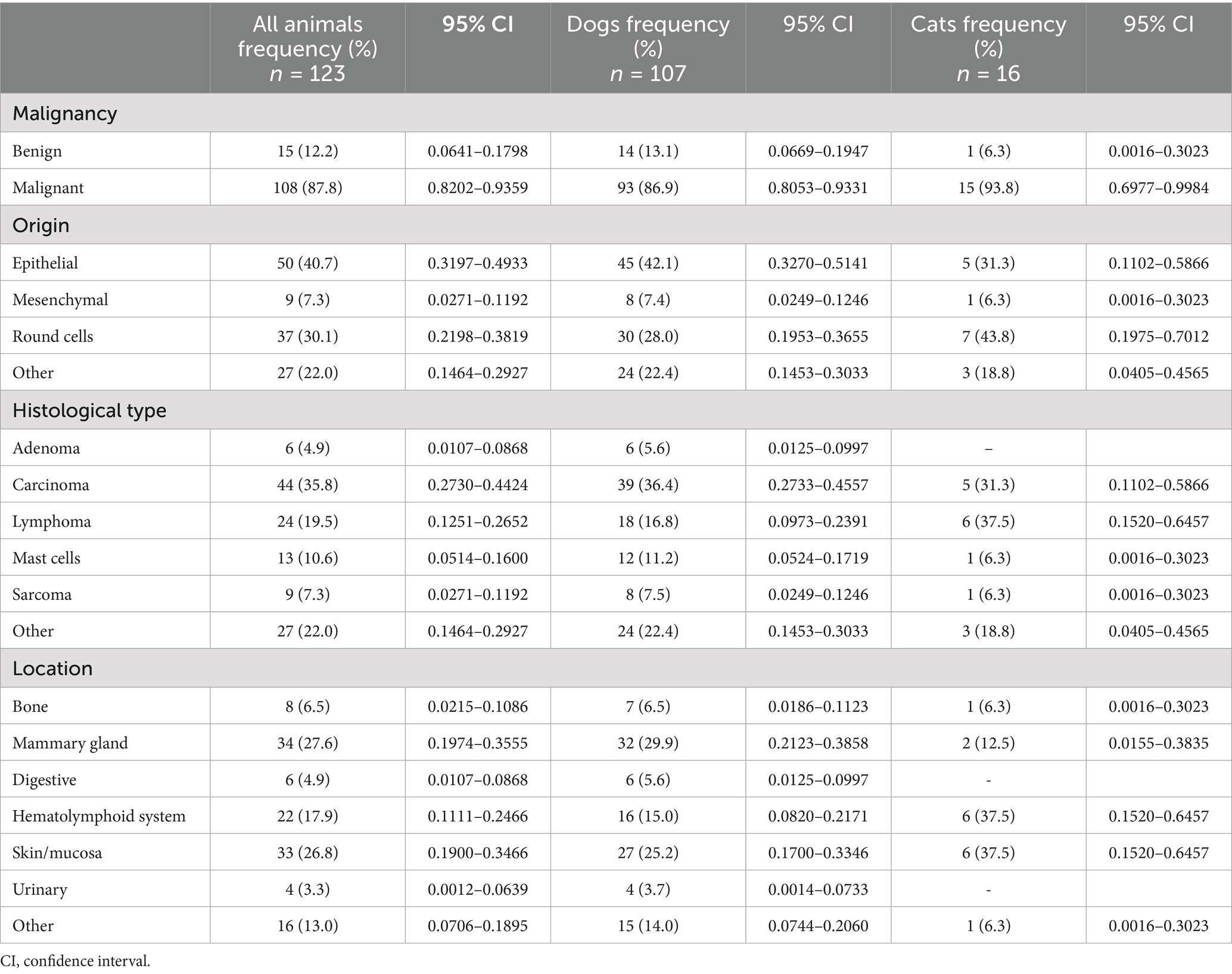
Table 2. Characteristics of the tumors diagnosed in pets attending to the HVULE (Spain) from 2015 to 2024.
The clinical characteristics of the animals diagnosed with a tumor are shown in Table 3. They attended 318 times (range 1–21 visits) at the HVULE during the 10 years assessed, most of them only once (25.8%). The frequency of diagnosis was not constant throughout the study period, being concentrated between 2017 and 2019 (19.5; 21.1 and 16.3%, respectively). Approximately half of the animals (42.3%) underwent surgical treatment. For those who were hospitalized (40.7%), the length of the stay was 2.9 ± 3.5 days (range 1–24 days; median 2). Two thirds of those hospitalized had undergone surgery (68.0%). Euthanasia was carried out in 26.8% of the animals, and almost half of them (42.4%) did not receive any treatment at all. Table 4 shows the logistic analysis performed to identify those variables associated with surgery as therapeutic treatment in those animals diagnosed with tumors. Logistic regression was performed with all the animals diagnosed and only with dogs. Model calibration was good in both models (all animals and only dogs), as shown by the Hosmer-Leweshow goodness-of-fit test (χ2 = 7.503, p = 0.277 when all animals were considered; and χ2 = 9.470, p = 0.149 if only dogs were included). Moreover, the observed:expected ratio was 77% for the model defined with all the animals, and 78.5% when only dogs were considered. When the animals were considered as a whole, the likelihood of following surgical treatment was significantly 4.4 times higher in female animals, 6.4 times higher if a benign tumor was detected, 15.0 times higher if the animal had been hospitalized, and 6.3 times higher if no chemotherapy was used to treat the disorder. Furthermore, a negative correlation was obtained with the year of the first attendance at HVULE, as over the years the number of cases treated with surgery was decreasing. If only dogs were included in the logistic analysis, the probability of being treated with surgery was 3.8 times higher in female animals, 22.2 times higher if animals had been hospitalized, and 11.3 times higher in those animals in which chemotherapy was not employed as treatment modality. Again, a negative correlation was observed with the year of the first attendance to HVULE.
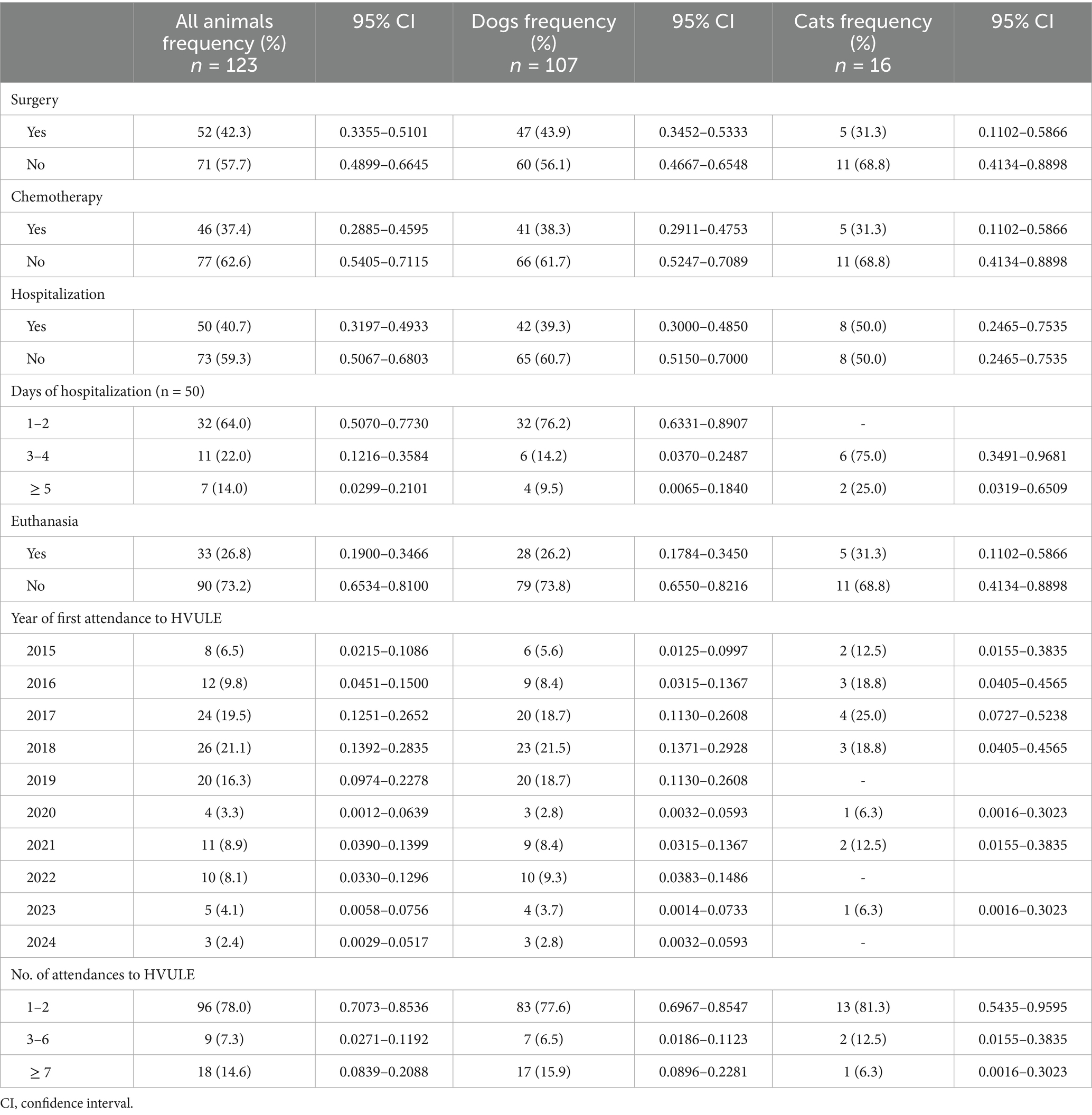
Table 3. Clinical characteristics and treatment modalities administered to pets attending to the HVULE (Spain) from 2015 to 2024 and diagnosed with tumors.
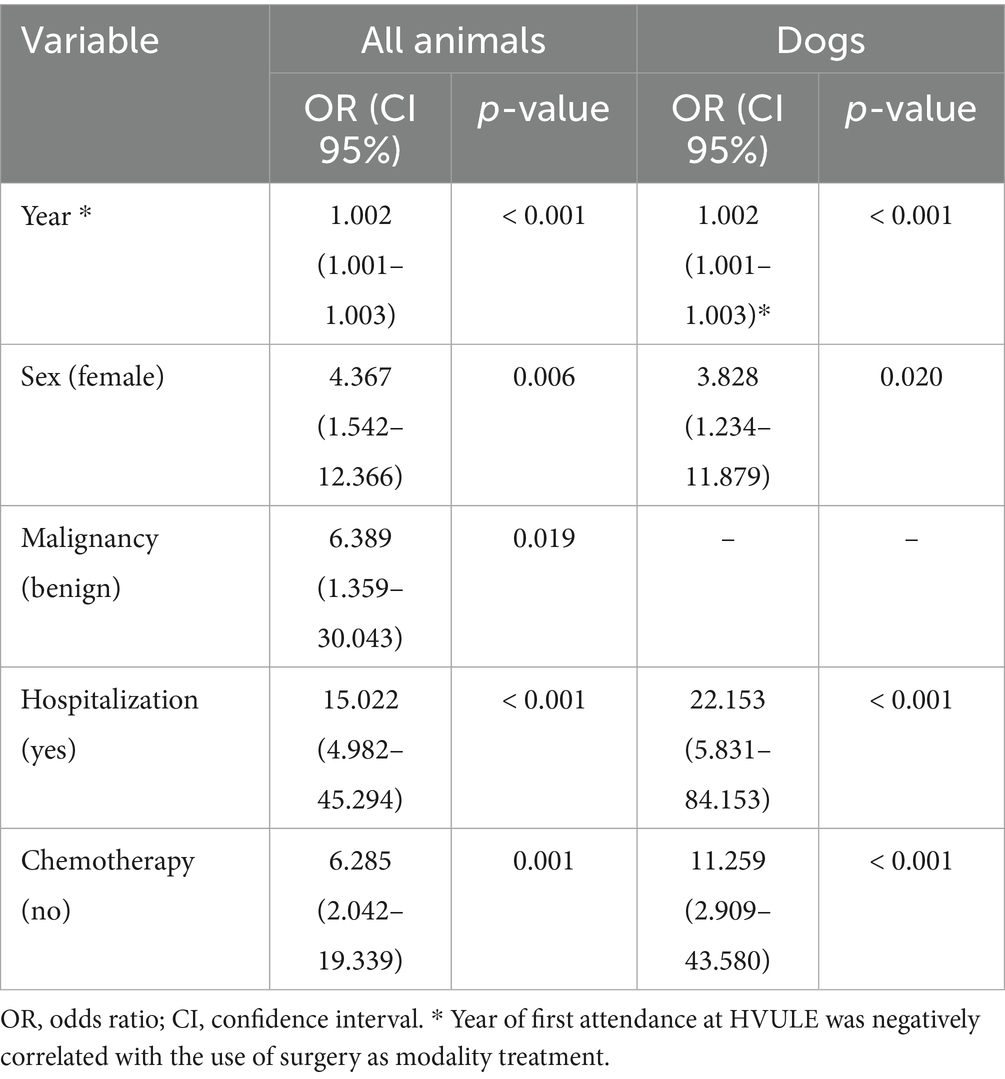
Table 4. Multivariate logistic regression analysis of demographic and clinical characteristics relevant to the selection of surgery as treatment.
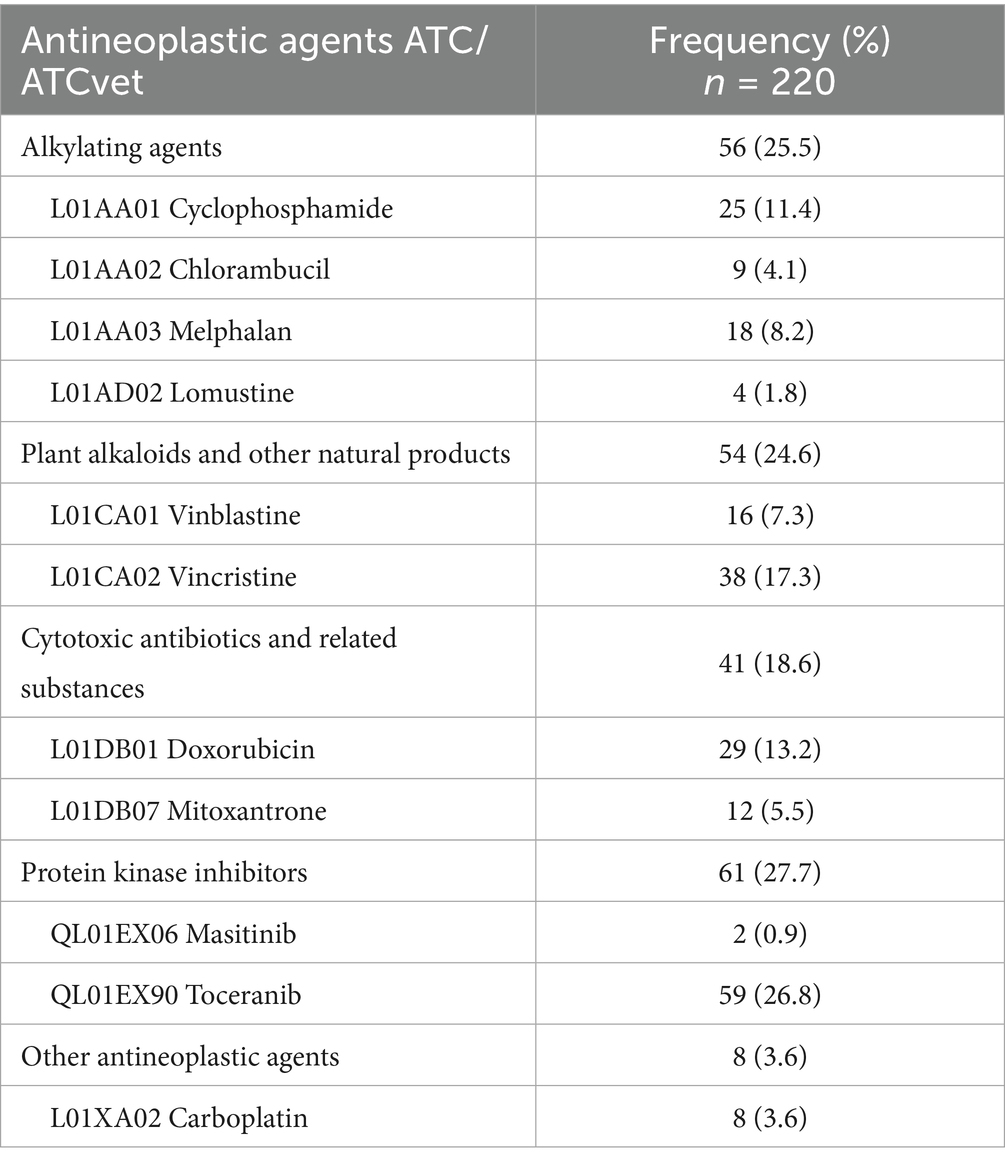
Chemotherapy was prescribed to 46 animals (37.4%), with only 5 cats treated with this modality. Both treatments (surgery and chemotherapy) were combined in 10 animals (8.1%). Table 5 listed cytotoxic agents administered to pets, according to ATC and ATCvet classifications (33, 34). A total of 74 different chemotherapeutic treatments were prescribed to animals (median = 1; range 1–19 treatments/animal), and they included 11 different drugs. Single-agent chemotherapy was used in more than two thirds of the animals (69.6%). Most of these treatments were administered orally (53.2%), and the rest by the intravenous route. In dogs, intravenous treatments included mitoxantrone, doxorubicine, vincristine, vinblastine and carboplatin. Among oral medications, the predominant drug was toceranib, but other active ingredients such as cyclophosphamide, lomustine, chlorambucil, melphalan and masitinib were also used. In cats, intravenous treatments were based on carboplatin and vincristine, and oral ones in chlorambucil, cyclophosphamide and toceranib. Moreover, metronomic chemotherapy was administered to 4 animals (8.7%): 3 dogs (2 received toceranib and 1 animal cyclophosphamide) and 1 cat (toceranib). Medicinal products approved for human use were mostly prescribed (72.3%). Regarding chemotherapeutic protocols, they were very varied. In fact, up to 24 different protocols were used. These protocols were administered either to only one (36.9%) or two animals (26.1%). Only toceranib was administered to 17 patients (37.0%), usually in neoplasias other than the approved indication (non-resectable canine mast cell tumors). In most of these animals, this drug was given in alternating days with an NSAID except for 4 animals, which received only toceranib.
Regarding concomitant treatments (Table 6), the most commonly used drugs were antiemetics (23.5%), followed by corticosteroids for systemic use (21.9%), antibacterials for systemic use (16.2%), and antiinflammatory and antirheumatic products (13.2%). Unlike in the case of cytotoxic drugs, veterinary medicines were mostly employed (66.3%). Moreover, dietary supplements were recommended to owners during and after chemotherapeutic treatment (41.3%) to protect gastric mucosa (89.5%), liver (36.8%), or as a probiotic (10.5%).
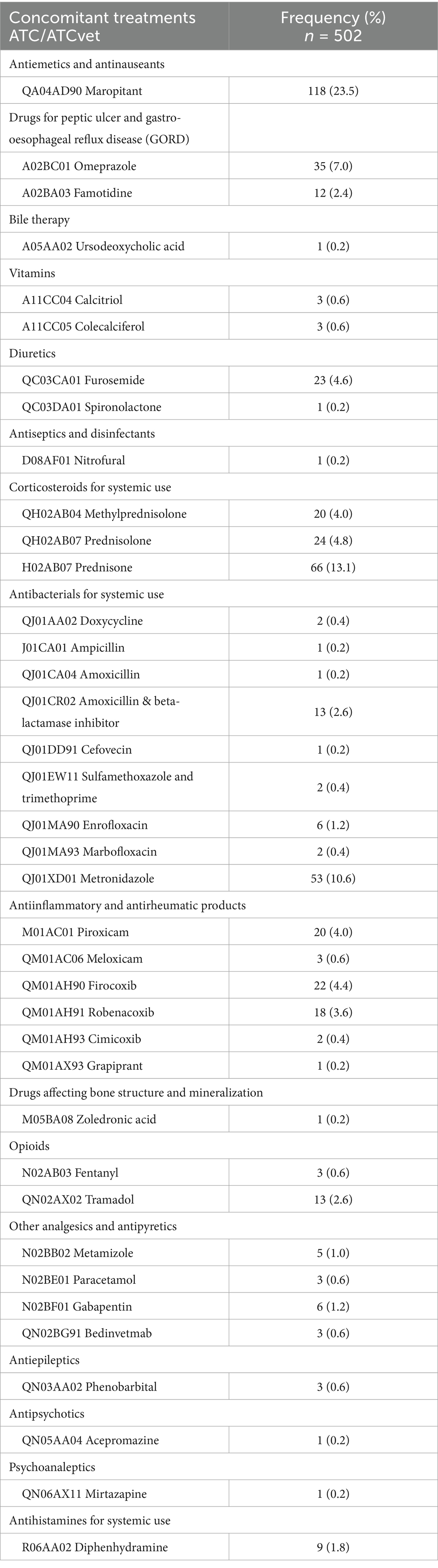
Table 6. Concomitant treatments prescribed to pets treated with antineoplastic at HVULE (Spain) from 2015 to 2024.
As for the adverse events caused by antineoplastic agents and classified according to VCOG-CTCAE v2 (29), they were recorded in 20 (1 cat and 19 dogs) of those 46 animals following chemotherapeutic treatment (43.5%), with neutropenia (70%) and vomiting (55%) as the most frequent, followed by colitis (35%), anemia, diarrhoea and lethargy (each one 30%). Grade 5 neutropenia was reported in 2 dogs, both treated with lomustine and euthanized. Of the 20 animals developing adverse events, 55% were treated in polytherapy and the rest in monotherapy (Supplementary Table S3). An overdose of toceranib was also detected in one dog, leading to discontinuation of treatment.
Disease outcome after chemotherapy was grouped into 5 categories. In almost 4 out of 10 animals the outcome of treatment was unknown (18 animals; 2 cats). Mortality was also high, as 13 animals (28.2%; 2 cats) unfortunately died, whereas a progression of the disease was observed in 12 animals (26.1%; 1 cat). Finally, disease recurrence was recorded in 2 cases (4.3%), and only 1 animal appeared to be cured, as no further information on the treated tumor was included in its subsequent medical record. The last 3 animals (recurrence or cure) underwent both surgical and chemotherapeutic treatments.
4 Discussion
Up to the knowledge of the authors, this is the first study aimed at investigating the characteristics of tumors diagnosed in companion animals over a long period of time (10 years), with a special focus on the pattern of treatments for this disease. The availability of similar studies in literature is really scarce, and they have carried out partial studies of either the characteristics of tumors in dogs or cats, or the description of the prescribed cytotoxic agents. A University clinical setting was used due to the ease of access to clinical information, but also because it is considered a referral center in the geographical area considered.
Cancer has become a major disease in companion animals and one of the leading causes of pet mortality (10). Studies such ours may contribute to a better knowledge of its epidemiology in small animals and the actual treatment options carried out by practitioners. The low number of cases reported in this paper may be partly attributed to the hospital’s location, in a medium-sized city. Other authors have also obtained a low ratio of clinical cases focused on animals diagnosed with cancer and treated against this disease (26).
Tumors were mostly diagnosed in aged animals, which is consistent with data reported by other authors (28, 35, 36). On the other hand, in our study a clearly much smaller number of tumors has been diagnosed in cats, which could be related to the less often attendance to veterinary hospitals of cat’s owners (28), although in Spain the number of cats registered as pets is slightly more than half the number of dogs (37).
The higher incidence in purebred dogs observed is consistent with the literature (16, 38–40), which would be associated to a genetic predisposition in purebred animals, and heritable risks associated with this disease due to inbreeding (7). As for the size of the animals, other studies have indicated that small dogs live longer than large animals and developed lower cancer rates (41, 42). In our study, tumor frequency in large/giant-sized animals was not higher than in small and medium ones, although this fact may be related to the owner preferences for certain breeds in the geographical area where the HVULE is located.
In agreement with data reported elsewhere (36), malignant neoplasms were the most prevalent in our study, being the predominant tissue of origin the epithelial one. Regarding tumor location, other authors have indicated that the most common affected organs/systems were also the mammary gland and the skin (15, 16, 38, 43, 44), which is consistent with our results. Specifically in female dogs, we have observed a high incidence of mammary tumors, which is in agreement with other researchers (10, 16, 45). Mammary gland tumors are the most common neoplasms in intact female dogs, accounting for over 40% of all tumors (46). As stated by Pinello et al. (14), female dogs are at higher risk of developing tumors than male animals, but the same does not occur with cats. A high proportion of lymphomas has also been evidenced in cats, more prone to this type of tumor (28), although in our study the number of cats is very low, and these data should be interpreted with caution.
Treatment choice was based on case-by-case clinical judgement. Nevertheless, the final decision was always made on a shared basis with the owners. As expected, surgery was the most common modality of cancer treatment implemented at the HVULE. In veterinary medicine, it is considered the most important therapeutic option in pets to improve their quality of life (47). As for chemotherapy, this treatment has been prescribed in slightly more than one third of patients, with a lower proportion than surgery. On the other hand, a quarter of the animals were euthanized. Of those, euthanasia was recommended for 14 animals, which did not receive any treatment; surgery was previously carried out in 9; chemotherapy was administered to 10 animals, and 2 received both surgical and cytotoxic treatments before euthanasia was applied.
Regarding chemotherapy, antineoplastic agents have been used to treat pet neoplasias for more than 60 years. Although the use of chemotherapy is more and more common in veterinary medicine, it is not without debate, as it is a form of palliative care to prolong the animal life, and it may have potential severe side effects (3). Cave et al. pointed out that the use of cytotoxic drugs was infrequent among British veterinarians, with a median frequency of at least once every 3 months (48), which would be consistent with our results.
Unlike in our study, other authors reported a higher use of intravenous cytotoxic treatments in pets in comparison with oral treatments (26, 48). It should be noted that in recent years more oral antitumor veterinary drugs have become available (toceranib, and masitinib). In the case of toceranib, although this tyrosine kinase inhibitor is licensed for mast cell tumors, it has also been used by veterinary oncologists for multiple neoplastic diseases (49), as occurred in our study, which would explain its increasing importance in pet treatments. Intravenous medications were always used off-label. Regarding the molecules employed, in a survey carried out in the UK, the most widely prescribed antineoplastic agents were cyclophosphamide and vincristine (48), which may reflect their use against lymphomas, according to the opinion of these authors. Tanaka et al. (26) observed that carboplatin, vincristine and doxorubicine were the most administered drugs in two Japanese Veterinary Teaching Hospitals, pointing out that both doxorubicine and vincristine were also indicated for the treatment of lymphomas. These results are consistent with ours.
Respecting concomitant medications, the administration of antiemetics is common in these treatments, and has allowed to improve the quality of life of pet patients, as well as to better withstand the effects of antineoplastic therapy. Substance P (Neurokinin-1 receptor) antagonist maropitant has become the antiemetic of choice in veterinary patients for the prevention of chemotherapy-induced vomiting (50). NSAIDs and glucocorticoids are common adjuvant treatments for different cancers in veterinary medicine as well. Glucocorticoids are part of several protocols (CHOP, CLOP, COP and LOP), and sometimes are also used as a chemotherapy single agent, due to their ability to inhibit DNA synthesis (22). As for NSAIDs, apart from their usual indications, they are also used when metronomic chemotherapy is performed, and due to their ability to inhibit cyclooxygenase isoform-2 (COX-2), whose expression is considered a negative prognostic factor in various types of canine and feline tumors (51). This inhibitory effect compromises endothelial cell tube formation and VEGF expression, preventing tumor progression (52). Regarding antibacterials, metronidazole was the most prescribed, specifically against diarrhea (53). However, for other authors the used of metronidazole as first-line treatment would not be justified in dogs with chemotherapy-induced diarrhea (54), as this drug significantly reduces in dogs bacterial diversity indices, alters the microbiome composition, and may increase the risk of occurrence of nosocomial or opportunistic infections with microbial resistance (55). Metronidazole and chemotherapy affect Clostridium cluster IV and XIVa, which are known to positively affect the gut health through improved nutrient absorption, production of short chain fatty acids with antiinflammatory properties and epithelial maturation (55). These treatments were also consistent with the adverse reactions observed in the animals. Veterinary medicines were mostly employed as concomitant treatments, which is in agreement with European regulations (56).
Regarding disease outcome after chemotherapeutic treatment, almost a third of the animals died (13 cases). These animals had a wide variety of tumors, and only 2 were underwent surgery. So, chemotherapy treatment in these cases should be considered as palliative, which would improve the quality and length of life of the patient, but ultimately most animals will relapse and die. On the other hand, for a significant proportion of the animals (39.1%) the information was missing. It should be taken into account in these latter cases that these animals may have been followed up in another veterinary clinic, which makes it impossible to know the final result of the treatment, or the animals may have had a fatal outcome, not reported by the owners.
The study is prone to have limitations associated with its retrospective nature. First, some medical records were excluded because of missing information. Second, data were obtained from a veterinary hospital located in a defined geographical area, and may limit the possibility of providing a broader picture of the actual situation regarding the characteristics and treatment of cancer in pets. In this sense, it should be noted that the number of feline cases was very small, which may affect the statistical results in this species and, therefore, the generalizability of our findings. Additionally, the worst moment of COVID-19 pandemic occurred in 2020, and may have had an impact on hospital visits. On the other hand, clinical decisions may be influenced by various factors such as the severity of the disease or the age of the animals. The socioeconomic status of the owners should be also considered, due to the cost of diagnostic and therapeutic procedures. However, and despite these limitations, the current study provides information not previously evaluated in veterinary medicine, and may be a background for further studies.
5 Conclusion
This is the first study that describes the pattern of diagnosis and treatment of cancer in pets at a Veterinary Teaching Hospital. We have observed that most tumors have been described in dogs, aged, purebred and female animals. Tumors were usually malignant, and were mostly located in the mammary glands or the skin, being the main histological type carcinoma. The study also provides an actual insight into the treatment modalities mainly followed in pet patients, with surgery as the major therapeutic option employed. Regarding cytotoxic drugs, just over half of the treatments were administered orally (mainly toceranib), whereas intravenous treatments were used off-label. Finally, slightly more than a quarter of the animals were euthanized.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval were waived for this study as the research did not involve any regulated animals, and no scientific procedures of any kind were performed on animals. For this reason, formal approval by an ethics committee was not necessary under the provisions of the Spanish regulations.
Author contributions
BR: Methodology, Writing – review & editing. JS: Conceptualization, Formal Analysis, Writing – review & editing. RD: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. EMV: Methodology, Writing – review & editing. CL: Methodology, Writing – review & editing. RP: Methodology, Writing – review & editing. MJD: Conceptualization, Formal Analysis, Writing – review & editing. NF: Data curation, Writing – review & editing. JMR-A: Resources, Writing – review & editing. AMS: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1588840/full#supplementary-material
References
1. Inoue, M, Hasegawa, A, Hosoi, Y, and Sugiura, K. Breed, gender and age pattern of diagnosis for veterinary care in insured dogs in Japan during fiscal year 2010. Prev Vet Med. (2015) 119:54–60. doi: 10.1016/j.prevetmed.2015.02.010
2. Perrin, T. The business of urban animals survey: the facts and statistics on companion animals in Canada. Can Vet J. (2009) 50:48–52.
3. Stephens, T. The use of chemotherapy to prolong the life of dogs suffering from cancer: the ethical dilemma. Animals. (2019) 9:441. doi: 10.3390/ani9070441
4. Urfer, SR, Kaeberlein, M, Promislow, DEL, and Creevy, KE. Lifespan of companion dogs seen in three independent primary care veterinary clinics in the United States. Canine Med Genet. (2020) 7:7. doi: 10.1186/s40575-020-00086-8
5. Withthrow, S. “Introduction: why worry about Cancer in pets?” In Withrow & MacEwen’s Small Animal Clinical Oncology. 4th ed, editors S. Withrow, DM. Vail. (St. Louis, MO: WB Saunders) (2007) xv–xvii. doi: 10.1016/B978-072160558-6.50003-4
6. Veterinary Cancer Society. Pet owners resources. Available online at: https://vetcancersociety.org/resources/pet-owners/pet-owner-resources/ (Accessed February 15, 2025).
7. Sarver, AL, Makielski, KM, DePauw, TA, Schulte, AJ, and Modiano, JF. Increased risk of cancer in dogs and humans: a consequence of recent extension of lifespan beyond evolutionarily determined limitations? Aging Cancer. (2022) 3:3–19. doi: 10.1002/aac2.12046
9. Walsh, F. Human-animal bonds I: the relational significance of companion animals. Fam Process. (2009) 48:462–80. doi: 10.1111/j.1545-5300.2009.01296.x
10. Vascellari, M, Baioni, E, Ru, G, Carminato, A, and Mutinelli, F. Animal tumour registry of two provinces in northern Italy: incidence of spontaneous tumours in dogs and cats. BMC Vet Res. (2009) 5:39. doi: 10.1186/1746-6148-5-39
11. Reid-Smith, R. The incidence of neoplasia in the canine and feline patient populations of private veterinary practices in southern Ontario University of Ghelph (2000). Available online at: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20073108562#:~:text=The%20incidence%20rates%20of%20benign,per%20100%2C000%20cat%20years%20respectively (Accessed March 1, 2025).
12. Mac Vean, DW, Monlux, AW, Anderson, PS, Silberg, SL, and Roszel, JF. Frequency of canine and feline tumors in a defined population. Vet Pathol. (1978) 15:700–15. doi: 10.1177/030098587801500602
13. Pittaway, C, Schofield, I, Dobson, J, O’Neill, DG, and Brodbelt, DC. Incidence and risk factors for the diagnosis of lymphoma in dogs in UK primary-care practice. J Small Anim Pract. (2019) 60:581–8. doi: 10.1111/jsap.13054
14. Pinello, K, Amorim, I, Pires, I, Canadas-Sousa, A, Catarino, J, Faísca, P, et al. Vet-OncoNet: malignancy analysis of neoplasms in dogs and cats. Vet Sci. (2022) 9:535. doi: 10.3390/vetsci9100535
15. Grüntzig, K, Graf, R, Hässig, M, Welle, M, Meier, D, Lott, G, et al. The Swiss canine cancer registry: a retrospective study on the occurrence of tumours in dogs in Switzerland from 1955 to 2008. J Comp Pathol. (2015) 152:161–71. doi: 10.1016/j.jcpa.2015.02.005
16. Baioni, E, Scanziani, E, Vincenti, MC, Leschiera, M, Bozzetta, E, Pezzolato, M, et al. Estimating canine cancer incidence: findings from a population-based tumour registry in northwestern Italy. BMC Vet Res. (2017) 13:203. doi: 10.1186/s12917-017-1126-0
17. Rodríguez, J, Santana, Á, Herráez, P, Killick, DR, and de los Monteros, AE. Epidemiology of canine mammary tumours on the canary archipelago in Spain. BMC Vet Res. (2022) 18:268. doi: 10.1186/s12917-022-03363-9
18. Bennett, PF, Taylor, R, and Williamson, P. Demographic risk factors for lymphoma in Australian dogs: 6201 cases. J Vet Intern Med. (2018) 32:2054–60. doi: 10.1111/jvim.15306
19. Kok, MK, Chambers, JK, Tsuboi, M, Nishimura, R, Tsujimoto, H, Uchida, K, et al. Retrospective study of canine cutaneous tumors in Japan, 2008–2017. J Vet Med Sci. (2019) 81:1133–43. doi: 10.1292/jvms.19-0248
20. Moon, C-H, Kim, D-H, Yun, S-H, Lee, H-B, and Jeong, S-M. Assessment of prognostic factors in dogs with mammary gland tumors: 60 cases (2014-2020). Korean J Vet Res. (2022) 62:e9. doi: 10.14405/kjvr.20210046
21. Cray, M, Selmic, LE, and Ruple, A. Salivary neoplasia in dogs and cats: 1996–2017. Vet Med Sci. (2020) 6:259–64. doi: 10.1002/vms3.228
22. Biller, B, Berg, J, Garrett, L, Ruslander, D, Wearing, R, Abbott, B, et al. 2016 AAHA oncology guidelines for dogs and cats. J Am Anim Hosp Assoc. (2016) 52:181–204. doi: 10.5326/JAAHA-MS-6570
23. VetCompass Australia. Vet Compass Australia. (2024). Available online at: https://www.vetcompass.com.au/ (Accessed March 2, 2025).
24. Royal Veterinary College. VetCompass. (2024). Available online at: https://www.rvc.ac.uk/vetcompass (Accessed March 1, 2025).
25. Tanaka, N, Takizawa, T, Miyamoto, N, Funayama, S, Tanaka, R, Okano, S, et al. Real world data of a veterinary teaching hospital in Japan: a pilot survey of prescribed medicines. Vet Rec Open. (2017) 4:e000218. doi: 10.1136/vetreco-2016-000218
26. Tanaka, N, Takizawa, T, Tanaka, R, Okano, S, Funayama, S, and Iwasaki, T. Pilot prescription survey of antineoplastic agents: real-world data from veterinary teaching hospitals in Japan. Vet Med Sci. (2019) 5:297–306. doi: 10.1002/vms3.173
27. Kamstock, D, Russell, D, and Powers, B. The pathology of neoplasia In: D Vail, D Thamm, and J Liptak, editors. Withrow and Mac Ewen’s small animal clinical oncology. St. Louis: Elsevier (2019). 61–80.
28. Pinello, K, Pires, I, Castro, AF, Carvalho, PT, Santos, A, de Matos, A, et al. Cross species analysis and comparison of tumors in dogs and cats, by age, sex, topography and main morphologies. Data from vet-OncoNet. Vet Sci. (2022) 9:167. doi: 10.3390/vetsci9040167
29. LeBlanc, AK, Atherton, M, Bentley, RT, Boudreau, CE, Burton, JH, Curran, KM, et al. Veterinary cooperative oncology group—common terminology criteria for adverse events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet Comp Oncol. (2021) 19:311–52. doi: 10.1111/vco.12677
30. Harvey, ND. How old is my dog? Identification of rational age groupings in pet dogs based upon normative age-lnked processes. Front Vet Sci. (2021) 8:643085. doi: 10.3389/fvets.2021.643085
31. Vogt, A, Rodan, I, Brown, M, and Brown, S. AAFP–AAHA feline life stage guidelines background and goals. J Am Anim Hosp Assoc. (2010) 12:70–85. doi: 10.1016/j.jfms.2009.12.006
32. O’Connor, AM, Sargeant, JM, Dohoo, IR, Erb, HN, Cevallos, M, Egger, M, et al. Explanation and elaboration document for the STROBE-vet statement: strengthening the reporting of observational studies in epidemiology—veterinary extension. J Vet Intern Med. (2016) 30:1896–928. doi: 10.1111/jvim.14592
33. World Health Organization Collaborating Centre for Drug Statistics Methodology. ATCvet index 2025. (2025). Available online at: https://atcddd.fhi.no/atcvet/atcvet_index/ (Accessed February 14, 2025).
34. World Health Organization Collaborating Centre for Drug Statistics Methodology. ATC/DDD index 2025. (2025) Available online at: https://atcddd.fhi.no/atc_ddd_index/ (Accessed February 14, 2025).
35. Rafalko, JM, Kruglyak, KM, McCleary-Wheeler, AL, Goyal, V, Phelps-Dunn, A, Wong, LK, et al. Age at cancer diagnosis by breed, weight, sex, and cancer type in a cohort of more than 3,000 dogs: determining the optimal age to initiate cancer screening in canine patients. PLoS One. (2023) 18:e0280795. doi: 10.1371/journal.pone.0280795
36. Pérez-Enriquez, JM, Romero-Romero, L, Alonso-Morales, RA, and Fuentes-Pananá, EM. Tumor prevalence in cats: experience from a reference diagnostic center in Mexico City (2006-2018). Vet México OA. (2020) 7. doi: 10.22201/fmvz.24486760e.2020.4.837
37. National Association of Companion Animal Feed Manufacturers (ANFAAC) Pet census in Spain (2025). Available online at: https://www.anfaac.org/datos-sectoriales/ (Accessed March 12, 2025).
38. Brønden, LB, Nielsen, SS, Toft, N, and Kristensen, AT. Data from the Danish veterinary Cancer registry on the occurrence and distribution of neoplasms in dogs in Denmark. Vet Rec. (2010) 166:586–90. doi: 10.1136/vr.b4808
39. Ostrander, EA, Dreger, DL, and Evans, JM. Canine cancer genomics: lessons for canine and human health. Annu Rev Anim Biosci. (2019) 7:449–72. doi: 10.1146/annurev-animal-030117-014523
40. Dobson, JM. Breed-predispositions to cancer in pedigree dogs. ISRN Vet Sci. (2013) 2013:1–23. doi: 10.1155/2013/941275
41. Nunney, L. Resolving Peto’s paradox: modeling the potential effects of size-related metabolic changes, and of the evolution of immune policing and cancer suppression. Evol Appl. (2020) 13:1581–92. doi: 10.1111/eva.12993
42. Yordy, J, Kraus, C, Hayward, JJ, White, ME, Shannon, LM, Creevy, KE, et al. Body size, inbreeding, and lifespan in domestic dogs. Conserv Genet. (2020) 21:137–48. doi: 10.1007/s10592-019-01240-x
43. Jitpean, S, Hagman, R, Ström Holst, B, Höglund, O, Pettersson, A, and Egenvall, A. Breed variations in the incidence of pyometra and mammary tumours in Swedish dogs. Reprod Domest Anim. (2012) 47:347–50. doi: 10.1111/rda.12103
44. Komazawa, S, Sakai, H, Itoh, Y, Kawabe, M, Murakami, M, Mori, T, et al. Canine tumor development and crude incidence of tumors by breed based on domestic dogs in Gifu prefecture. J Vet Med Sci. (2016) 78:1269–75. doi: 10.1292/jvms.15-0584
45. Egenvall, A, Bonnett, BN, Öhagen, P, Olson, P, Hedhammar, Å, and von Euler, H. Incidence of and survival after mammary tumors in a population of over 80,000 insured female dogs in Sweden from 1995 to 2002. Prev Vet Med. (2005) 69:109–27. doi: 10.1016/j.prevetmed.2005.01.014
46. Sorenmo, K, Worley, D, and Zappulli, V. Tumors of the mammary gland In: D Vail, D Thamm, and J Liptack, editors. Withrow and MacEwen’s small animal clinical oncology. St. Louis: Elsevier (2019). 604–25.
47. Liptak, JM. The principals of surgical oncology: diagnosis and staging. Compend Contin Educ Vet. (2009) 31:E1–E12. quiz E13
48. Cave, TA, Norman, P, and Mellor, D. Cytotoxic drug use in treatment of dogs and cats with cancer by UK veterinary practices (2003 to 2004). J Small Anim Pract. (2007) 48:371–7. doi: 10.1111/j.1748-5827.2007.00343.x
49. Frezoulis, P, and Harper, A. The role of toceranib phosphate in dogs with non-mast cell neoplasia: a systematic review. Vet Comp Oncol. (2022) 20:362–71. doi: 10.1111/vco.12799
50. Sharun, K, Jambagi, K, Arya, M, Aakanksha,, Chaithra, SN, Patel, PK, et al. Clinical applications of substance P (neurokinin-1 receptor) antagonist in canine medicine. Arch Razi Inst. (2021) 76:1175–82. doi: 10.22092/ari.2021.356171.1797
51. Gregório, H, Magalhães, TR, Pires, I, Prada, J, Carvalho, MI, and Queiroga, FL. The role of COX expression in the prognostication of overall survival of canine and feline cancer: a systematic review. Vet Med Sci. (2021) 7:1107–19. doi: 10.1002/vms3.460
52. London, CA, Gardner, HL, Mathie, T, Stingle, N, Portela, R, Pennell, ML, et al. Impact of toceranib/piroxicam/cyclophosphamide maintenance therapy on outcome of dogs with appendicular osteosarcoma following amputation and carboplatin chemotherapy: a multi-institutional study. PLoS One. (2015) 10:e0124889. doi: 10.1371/journal.pone.0124889
53. Langlois, DK, Koenigshof, AM, and Mani, R. Metronidazole treatment of acute diarrhea in dogs: a randomized double blinded placebo-controlled clinical trial. J Vet Intern Med. (2020) 34:98–104. doi: 10.1111/jvim.15664
54. Fournier, Q, Serra, J, Williams, C, and Bavcar, S. Chemotherapy-induced diarrhoea in dogs and its management with smectite: results of a monocentric open-label randomized clinical trial. Vet Comp Oncol. (2021) 19:25–33. doi: 10.1111/vco.12631
55. Igarashi, H, Maeda, S, Ohno, K, Horigome, A, Odamaki, T, and Tsujimoto, H. Effect of oral administration of metronidazole or prednisolone on fecal microbiota in dogs. PLoS One. (2014) 9:e107909. doi: 10.1371/journal.pone.0107909
56. European Parliament and The Council of the European Union. Regulation (EU) 2019/6 of the European Parliament and of The Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC. Off J Eur Union. (2019) L4:43–167. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R0006 (Accessed February 25, 2025).
Keywords: antineoplastic agent, cancer, chemotherapy, pet, prescription, surgery, veterinary teaching hospital
Citation: Romero B, Susperregui J, Díez R, Vazquez EM, Lopez C, de la Puente R, Diez MJ, Fernandez N, Rodriguez-Altonaga JM and Sahagun AM (2025) Pet cancer cases and patterns of treatment at a Spanish veterinary teaching hospital: a retrospective study from 2015 to 2024. Front. Vet. Sci. 12:1588840. doi: 10.3389/fvets.2025.1588840
Edited by:
Arturo Anadón, Complutense University of Madrid, SpainReviewed by:
Antonella Perillo, University of Bari Aldo Moro, ItalyJosé A. G. Agúndez, University of Extremadura, Spain
Mónica García Domingo, University of Salamanca, Spain
Copyright © 2025 Romero, Susperregui, Díez, Vazquez, Lopez, de la Puente, Diez, Fernandez, Rodriguez-Altonaga and Sahagun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raquel Díez, cmRpZWx6QHVuaWxlb24uZXM=
 Beatriz Romero
Beatriz Romero Julen Susperregui
Julen Susperregui Raquel Díez
Raquel Díez E. Milena Vazquez
E. Milena Vazquez Cristina Lopez
Cristina Lopez Raúl de la Puente
Raúl de la Puente M. Jose Diez1
M. Jose Diez1 Nelida Fernandez
Nelida Fernandez Ana M. Sahagun
Ana M. Sahagun