Abstract
The moss Physcomitrella patens is unique among plants in that homologous recombination can be used to knock out genes, just like in yeast. Furthermore, transformed plasmids can be rescued from Physcomitrella back into Escherichia coli, similar to yeast. In the present study, we have tested if a third important tool from yeast molecular genetics, auxotrophic selection markers, can be used in Physcomitrella. Two auxotrophic moss strains were made by knocking out the PpHIS3 gene encoding imidazoleglycerol-phosphate dehydratase, and the PpTRP1 gene encoding phosphoribosylanthranilate isomerase, disrupting the biosynthesis of histidine and tryptophan, respectively. The resulting PpHIS3Δ and PpTRP1Δ knockout strains were unable to grow on medium lacking histidine or tryptophan. The PpHIS3Δ strain was used to test selection of transformants by complementation of an auxotrophic marker. We found that the PpHIS3Δ strain could be complemented by transformation with a plasmid expressing the PpHIS3 gene from the CaMV 35S promoter, allowing the strain to grow on medium lacking histidine. Both linearized plasmids and circular supercoiled plasmids could complement the auxotrophic marker, and plasmids from both types of transformants could be rescued back into E. coli. Plasmids rescued from circular transformants were identical to the original plasmid, whereas plasmids rescued from linearized transformants had deletions generated by recombination between micro-homologies in the plasmids. Our results show that cloning by complementation of an auxotrophic marker works in Physcomitrella, which opens the door for using auxotrophic selection markers in moss molecular genetics. This will facilitate the adaptation of shuttle plasmid dependent methods from yeast molecular genetics for use in Physcomitrella.
Introduction
Much of our knowledge about basic functions of the eukaryotic cell such as the cell cycle, intracellular transport, and gene expression was obtained by research in the budding yeast Saccharomyces cerevisiae. The success of yeast as a model organism can be attributed to the sophisticated tools and methods that have long been available in yeast. First and foremost comes gene targeting by homologous recombination, a method that was invented in yeast, thus making reverse genetics possible (Orr-Weaver et al., 1981). Gene targeting also works in some multicellular eukaryotes, but with a much reduced efficiency as compared to yeast. The moss Physcomitrella patens is the only multicellular organism that has gene targeting whose efficiency is comparable to that in yeast (Schaefer and Zrÿd, 1997), a finding that helped to establish Physcomitrella patens as a model organism (Schaefer, 2002; Cove, 2005; Lang et al., 2008; Rensing et al., 2008).
A second important method in yeast molecular genetics is the use of shuttle plasmids that are able to replicate both in yeast and in bacteria (Beggs, 1978; Stinchcomb et al., 1979). Shuttle plasmids make it possible to clone genes from plasmid libraries by complementation of yeast mutants, and also enable more advanced methods such as dosage suppressor screens (Rine, 1991). Gene targeting and dosage suppression complement each other in that gene targeting looks at what happens when a gene function is lost, whereas dosage suppressor screens look at what happens when a gene is overexpressed. By using both methods much can therefore be elucidated about the function of a given gene. The dosage suppressor screen is a particularly useful tool, since new suppressor genes are cloned in the screen and are thus directly available for further studies, and since a suppressor screen can be carried out in a relatively short time. Theoretically it is possible to identify all proteins in a given signaling or metabolic pathway in one single dosage suppressor screen.
Several attempts have been made to develop shuttle plasmids for use in plants and animals, but they have been frustrated by the fact that plasmids transformed into plant or animal cells tend to rearrange and randomly integrate into the host chromosomes, making it difficult to recover (rescue) the original plasmid from transformed cells (Van Craenenbroeck et al., 2000). However, transformed DNA can replicate episomally in Physcomitrella (Schaefer et al., 1991; Knight, 1994; Schaefer, 1994; Ashton et al., 2000) and we showed in a previous study that plasmids transformed into Physcomitrella can be rescued back into Escherichia coli without having undergone deletions or rearrangements (Murén et al., 2009). This opens up the possibility of using shuttle plasmid-based methods in Physcomitrella.
A third important tool in yeast molecular genetics is the use of multiply auxotrophic yeast strains. These are strains that carry mutations in biosynthetic genes, typically genes needed for amino acid or nucleotide biosynthesis. Auxotrophic strains are therefore unable to grow unless the medium is supplemented with the necessary amino acid or nucleotide. With such strains, it is possible to select tranformants by using plasmids that carry a wild type copy of the mutated gene, provided that the reversion frequency of the mutation is lower than the transformation frequency. The latter condition is ensured by using non-reversible knockout mutations. The advantages of using auxotrophic selection markers is that they do not require the use of antibiotics that may have undesired side effects on the organism under study, and that there are many more auxotrophic markers than drug resistance markers, which facilitates experiments with several plasmids and/or gene knockouts in the same strain.
Previous work in Phsycomitrella (Ashton and Cove, 1977) and Arabidopsis (Last and Fink, 1988; Last et al., 1991; Muralla et al., 2007) has shown that it is possible to find auxotrophic mutant plants that require a given nutrient in classical genetic screens. In the present study, we use gene targeting to create stable auxotrophic knockout mutants in Physcomitrella that are suitable for reverse genetics and work with shuttle plasmids. This was done by knocking out the moss ortholog of the yeast phosphoribosylanthranilate isomerase gene, ScTRP1, needed for biosynthesis of tryptophan, and the moss ortholog of the yeast imidazoleglycerol-phosphate dehydratase, ScHIS3, needed for biosynthesis of histidine. As a proof of principle, we further show that the PpHIS3Δ knockout strain can be complemented by a plasmid carrying the wild type PpHIS3 gene expressed from the CaMV35S promoter, and that such transformants can be selected directly on histidine-less medium. Finally we show that the original plasmid can be rescued from moss transformants back into E. coli. This shows that cloning by complementation is possible in Physcomitrella and that auxotrophic selection markers can be used for molecular genetics work in moss.
Materials and Methods
Plant Material and Growth Conditions
Physcomitrella patens of the Gransden Wood ecotype (Ashton and Cove, 1977) was grown on 0.8% agar plates containing BCD medium (Nishiyama et al., 2000), i.e., 1 mM MgSO4, 1.85 mM KH2PO4, 10 mM KNO3, 45 μM FeSO4, 1 mM CaCl2, and trace elements, supplemented with 5 mM ammonium tartrate. The PpHIS3Δ knockout strain was grown on the same medium but with 250–500 μM of added histidine and the PpTRP1Δ knockout strain was grown on medium with 75–200 μM of added tryptophan. Moss protonemal tissue was grown on plates covered with cellophane. The growth conditions were 25°C in a Sanyo MLR-350 light chamber under constant light at about 30 μmol m-2.
Construction of the PpHIS3Δ and PpTRP1Δ Knockouts
To clone the flanking sequences of PpTRP1, primers MU59 (containing an HindIII site) and MU47 TRP1-5R (containing an XhoI site) (Supplementary Table S1) were used to amplify a 901 bp fragment that was cloned into the TA-cloning vector pCR®2.1-TOPO, thus producing pTRP1-5′. Primers MU60 (containing a NotI site) and MU49 TRP1-3R (containing a BglII and a HindIII site) (Supplementary Table S1) were used to amplify a 548 bp fragment that was cloned into the TA-cloning vector pCR®2.1-TOPO, thus producing pTRP1-3′. To clone the flanking sequences of PpHIS3, primers MU64 situated upstream of a native HindIII site and MU65 (containing an XhoI site) (Supplementary Table S1) were used to amplify a 1424 bp fragment that was cloned into the TA-cloning vector pCR®2.1-TOPO, thus producing pHIS3-5′. Primers MU63 (containing a NotI site) and MU57 HIS3-3R (containing a BglII site) (Supplementary Table S1) were used to amplify an 893 bp fragment that was cloned into pCR®2.1-TOPO, thus producing pHIS3-3′.
The plasmids pHIS3-5′ and pTRP1-5′ were cut with HindIII and XhoI. The fragments containing the Physcomitrella 5′ upstream sequence of PpTRP1 and PpHIS3 were then cloned into pBHRF (Thelander et al., 2007; Schaefer et al., 2010) using the HindIII and XhoI sites in the vector, producing plasmids pBHRF-HIS3-5′ and pBHRF-TRP1-5′, respectively. Plasmids pHIS3-3′ and pTRP1-3′ were cut with NotI and BglII. The Physcomitrella 3′ downstream sequence of PpHIS3 from pHIS3-3′ was then cloned into pBHRF-HIS3-5′ and the Physcomitrella 3′ downstream sequence of PpTRP1 from pTRP1-3′ was cloned into pBHRF-TRP1-5′ using the NotI and BglII sites in these plasmids, thus producing pBHRF-HIS3-5′-3′ and pBHRF-TRP1-5′-3′, respectively.
For the gene targeting, pBHRF-HIS3-5′-3′ was cut with HindIII and SpeI and pBHRF-TRP1-5′-3′ was cut with HindIII. The linearized plasmids were transformed into moss protoplasts using the polyethylene glycol method (Schaefer et al., 1991), but with 500 μM of histidine or 100 μM of tryptophan added to all solutions and plates. pBHRF-HIS3-5′-3′ transformants were selected in the presence of 30 μg/L hygromycin and 500 μM histidine. pBHRF-TRP1-5′-3′ transformants were selected on plates containing 5–30 μg/L hygromycin and 25–100 μM tryptophan. The concentrations of the supplemented amino acids proved to be important. Initially a concentration of 500 μM was used for both tryptophan and histidine. This was, however, growth inhibitory, particularly in the case of tryptophan, which also showed a negative interaction with hygromycin. We therefore lowered both the concentration of tryptophan and hygromycin in the selection plates. The resulting stable transformants were then screened for inability to grow on histidine-less or tryptophan-less media.
Verification of Gene Disruptions
Disruptions were verified by PCR using the Advantage 2 PCR kit from Clontech (Mountain View, CA, United States). For PpHIS3Δ, primers MU70 HIS3-5F and 35S-R (Supplementary Table S1) were used to verify 5′-end integration of the knockout construct, and primers MU71 HIS3-3R and Hyg-Ter-F (Supplementary Table S1) were used to verify 3′-end integration. To verify that the PpHIS3 gene had been removed from the moss genome, a PCR on the gene was performed using primers MU72his and MU73his (Supplementary Table S1). For PpTRP1Δ, primers MU66 TRP1-5F and 35S-R (Supplementary Table S1) were used to verify 5′-end integration of the knockout construct, and primers MU67 TRP1-3R and Hyg-Ter-F (Supplementary Table S1) were used to verify 3′-end integration. To verify that the PpTRP1 gene had been removed from the moss genome, a PCR on the gene was performed using primers MU76trp and MU77trp (Supplementary Table S1). All PCR experiments used the ThermoFisher Scientific GeneRuler 1 kb DNA Ladder with fragment sizes of 10000, 8000, 6000, 5000, 4000, 3500, 3000, 2500, 2000, 1500, 1000, 750, 500, and 250bp.
Construction of the Moss cDNA Expression Vector pMJ1
pMJ1 is a cDNA expression vector that was designed to either replicate episomally in moss, or integrate by homologous recombination into the non-essential moss BS213 locus (Schaefer and Zrÿd, 1997) after digestion with NotI. pMJ1 was made in several steps. First, a 754 bp fragment of pCMAK1 (Hiwatashi et al., 2008) carrying the 3′ part of the BS213 locus was amplified by PCR, adding an EcoRI site at the 3′ end of the fragment and a NotI site followed by an EcoRI site at the 5′ end. Similarly, a 696 bp fragment of pCMAK1 carrying the 5′ part of the BS213 locus was amplified by PCR, adding a HindIII site followed by a NotI site at the 5′ end and a HindIII site at the 3′ end. The 5′ and 3′ PCR products were digested with HindIII and EcoRI, respectively, and cloned one after the other into the HindIII and EcoRI sites of the pUC119 polylinker (Vieira and Messing, 1987). The resulting plasmid, pUC119-5′-3′-BS213, is a targeting construct that will integrate anything inserted into the SmaI site between the 5′ and 3′ BS213 fragments into the BS213 locus by homologous recombination, provided that the pUC119 backbone is released by cutting with NotI prior to transformation into moss. pUC119-5′-3′-BS213 was linearized with SmaI and ligated to a 5431 bp PmeI-EcoR147I fragment of the Gateway vector pGWB2 (Nakagawa et al., 2007) containing the nptII kanamycin resistance gene expressed from the NOS1 promoter and with the NOS1 terminator, followed by a modified CaMV35S promoter in front of a Gateway cassette followed by the NOS1 terminator. The resulting plasmid pMJ1 was used to make a moss cDNA expression library, which is why it has a Gateway cassette. The cassette is of no relevance in the present work since it was removed when the PpHIS3 cDNA was cloned into pMJ1 (see below).
Complementation of the PpHIS3Δ Strain by a Plasmid Expressing PpHIS3
The sequence of PpHIS3 was used to search the PHYSCObase EST data base (Nishiyama et al., 2003). The cDNA clone (pdp05102) derived from PpHIS3 was ordered from the RIKEN Bioresource Center (Ibaraki, Japan) and cloned into the TA-cloning vector pCR®2.1-TOPO, thus producing pTOPO-HIS3. pTOPO-HIS3 was cleaved with XhoI to release the PpHIS3 insert, which was then cloned between the XhoI and Eco47III sites of pMJ1, producing pHGZ404. pHGZ404 was either left uncut or cut with NotI to remove the pUC119 backbone and produce a linear plasmid intended to target the integration to the non-essential BS213 locus (Schaefer and Zrÿd, 1997). Circular and linearized plasmids were transformed into the PpHIS3Δ moss strain using the same method as described for the generation of PpHIS3 knockouts, but using selection for histidine prototrophy instead of drug resistance.
PCR Verification of Recombination and Integration of pHGZ404 into the Genome
To verified the presence of in planta recombination between the NOS1 terminator PCR using the Advantage 2 PCR kit was performed on DNA from the moss transformed with pHGZ404 using primers MU83 and MU84 (Supplementary Table S1). To check if linearized pHGZ404 plasmids had integrated into the BS213 locus through its homologous flanking sequences, a PCR was performed using primers MU BS213 5′F and MU BS213 5′R (Supplementary Table S1) to check 5′-end integration and primers MU BS213 3′F and MU BS213 3′R (Supplementary Table S1) to check 3′-end integration. To check that the PpHIS3Δ mutant transformed with pHGZ404 plasmid contained the PpHIS3 cDNA expressed from the CaMV35S promoter that was present on the pHGZ404 plasmid, a PCR with the primers HGZ46 and HGZ58 (Supplementary Table S1) was carried out. A PCR to check if the BS213 locus was still intact or had been disrupted was done with primers BS213 5 new2 and BS213 3 new2 (Supplementary Table S1). To check for integration into the genome by recombination between the CaMV35S promoter sequences present both on the plasmid and in front of the hygromycin resistance gene already present in the auxotrophic knockouts, a PCR was done using primers HGZ65-f and HGZ66-r (1334 bp) and also the primer pair MuHis3-start and MuBHRF-BS213 5 (897 bp) (Supplementary Table S1).
Rescue of Plasmids from Moss Transformants Back into E. coli
Genomic DNA was extracted from moss transformants using the CTAB method (Dellaporta et al., 1983), using 1 g of chloronemal tissue grown on cellophane covered histidine-less selection plates. E. coli strain SF8 (thi-1 thr-l leu-6 lacY tonA21 supE44 recBC lop-11) was transformed with 0.02–1 μg of moss DNA and spread on to LB plates containing 150 μg/ml of ampicillin. Rescued plasmids were sequenced at Eurofins MWG Operon (Ebersberg, Germany).
Concatemer Formation of Transformed Constructs
To check if the pBHRF-HIS3-5′-3′ knockout construct hade formed concatemers at the targeted PpHIS3 locus, PCRs were done on genomic DNA from the PpHIS3Δ strains B8 and J7 using primers MU78 and MU79 (Supplementary Table S1). To check if the pBHRF-TRP1-5′-3′ knockout construct hade formed concatemers at the targeted PpTRP1 locus, PCRs were done on genomic DNA from the PpTRP1Δ strains B4, N4 and N11 using primers MU107 and MU108 (Supplementary Table S1). Similarly, to check for concatemer formation in the pHGZ404 transformants, PCRs were done on genomic DNA from the transformants using primers MU83 and MU84 (Supplementary Table S1).
Verification of the Presence of the nptII Marker in the pHGZ404 Transformants
To assay G418 resistance, the pHGZ404 transformants and the wild type and P5 controls were grown on 15, 20, and 30 μg/ml G418 plates for 16 days after which the results were documented. PCRs were also performed on all strains using primers NptII-F and NptII-R (Supplementary Table S1) that amplify a 786 bp fragment of the nptII gene.
Plasmid Loss Experiment
To check for loss of episomally replicating DNA in the pHGZ404 moss transformants, protonemal tissue of the PpHIS3 knockout strain transformed with either circular or linearized pHGZ404 plasmid was homogenized in a Mini Beadbeater 8 (Biospec). The homogenized material was diluted in water and plated onto BCD plates containing 5 mM ammonium tartrate (MM plates) covered with cellophane at a dilution estimated to generate approximately 25 moss colonies per plate. The plates were incubated for 2 weeks in a light chamber as described above after which the cellophane together with the moss colonies were moved to selection plates containing 50 μg/ml G418 and incubated for another week. The total number of surviving and dead moss colonies were then counted, and representative photographs were taken of each transformant.
Results
Construction of Histidine and Tryptophan Auxotrophic Moss Strains
We searched the Physcomitrella genome for orthologs of several auxotrophic markers used in yeast. Many moss genes are duplicated, but we found that the moss orthologs of the yeast TRP1 (Pp3s17_23550) and HIS3 genes (Pp3c13_21930) are present in only one copy in the moss genome, making them suitable knockout targets. These genes will subsequently be referred to as PpTRP1 and PpHIS3. The constructs used to knock out PpHIS3 and PpTRP1 are shown in Figure 1A. The constructs were designed to completely remove the open reading frames and replace them with the hph hygromycin resistance gene expressed from the CaMV35S promoter. Transformants were selected in the presence of hygromycin B on medium supplemented with the appropriate amino acid for 2 weeks, after which they were moved to non-selection plates and grown for an additional 2 weeks. The first step kills untransformed cells, and the second step allows loss of the plasmid in transient transformants. The transformants were then moved back to selection medium to identify stable transformants. Stable transformants were screened by growth on medium with and without the supplemented amino acid, to verify that they had become auxotrophic.
FIGURE 1
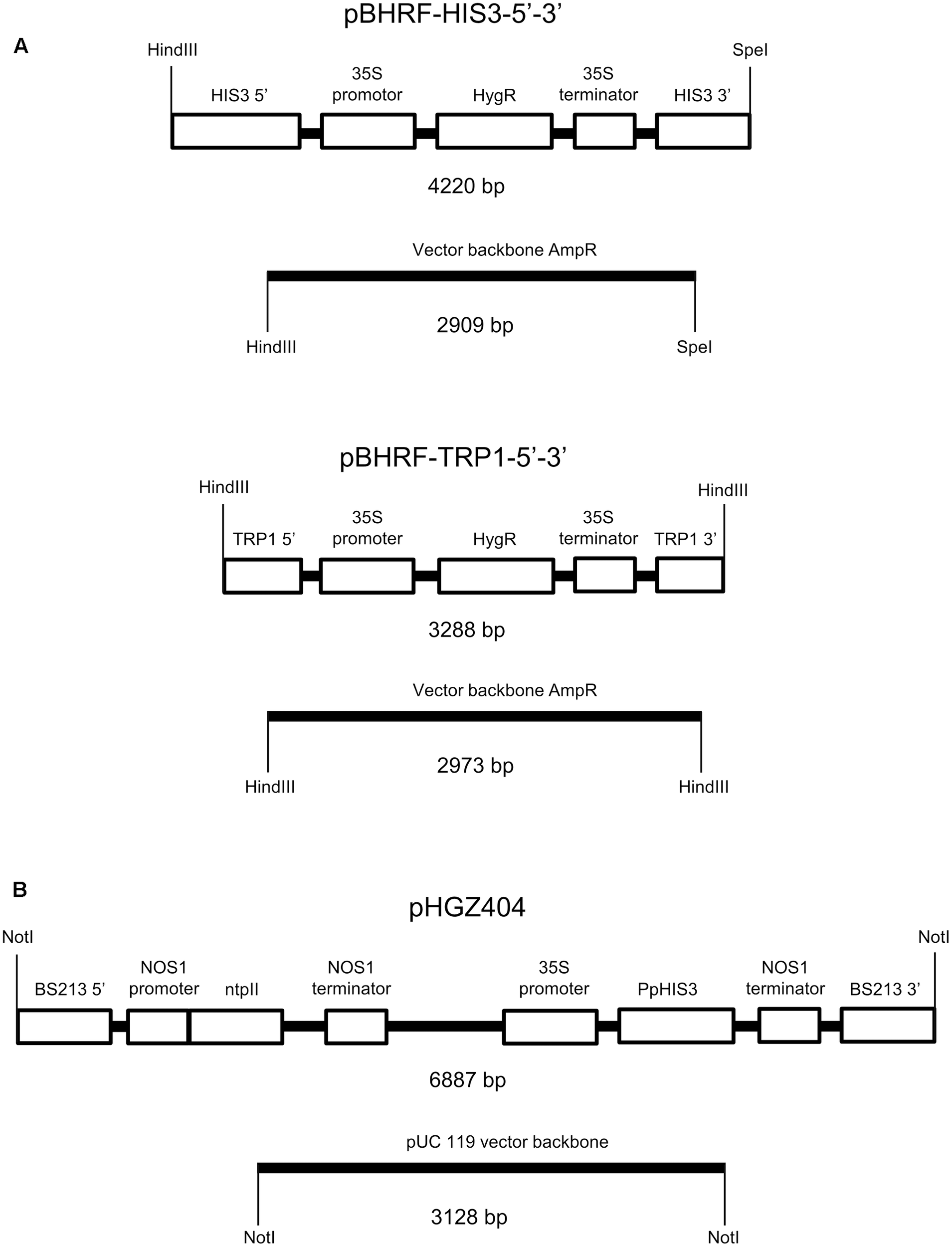
Plasmids used to make the auxotrophic PpHIS3Δ and PpTRP1Δ knockout strains and to transform the PpHIS3Δ strain. (A) The knockout plasmid pBHRF-HIS3-5′-3′ used to generate the PpHIS3Δ knockout strain and knockout plasmid pBHRF-TRP1-5′-3′ used to generate the PpTRP1Δ knockout strain. (B) Plasmid pHGZ404 used to transform the PpHIS3Δ knockout strain. Both circular (supercoiled) and linearized pHGZ404 was used to transform the moss. The linearized plasmid comprising two fragments is shown in the figure.
Characterization of the Auxotrophic Moss Strains
To confirm the targeted genes had been correctly knocked out we used PCR with primers located inside the PpHIS3 and PpTRP1 gene, respectively. Since gene targeting in moss sometimes can involve homologous recombination at one end and non-homologous recombination at the other end, PCR was also performed on the 5′-flanking and 3′-flanking region for both types of transformants with one primer located inside the knockout construct and the other primer located in the flanking region in the genome. We found four lines that were auxotrophic for histidine but only two of them (B8 and J7) had correctly integrated the HygR cassette by homologous recombination at both the 5′ and 3′ end of the PpHIS3 locus (Figure 2). We isolated three tryptophan auxotrophs (B4, N4, and N11), all of which had the HygR cassette integrated by recombination at both ends of the PpTRP1 gene (Figure 2). We found no auxotrophic moss colonies that did not show an alteration within the targeted locus. Gene targeting in Physcomitrella usually results in integration of multiple tandem copies of the knockout construct at the targeted locus (Schaefer and Zrÿd, 1997; Schaefer, 2002; Cove, 2005). Consistent with this, PCR experiments with divergent primers at each end of the knockout constructs confirmed the presence of tandem repeats of the knockout constructs in all five knockout strains (Figure 3).
FIGURE 2
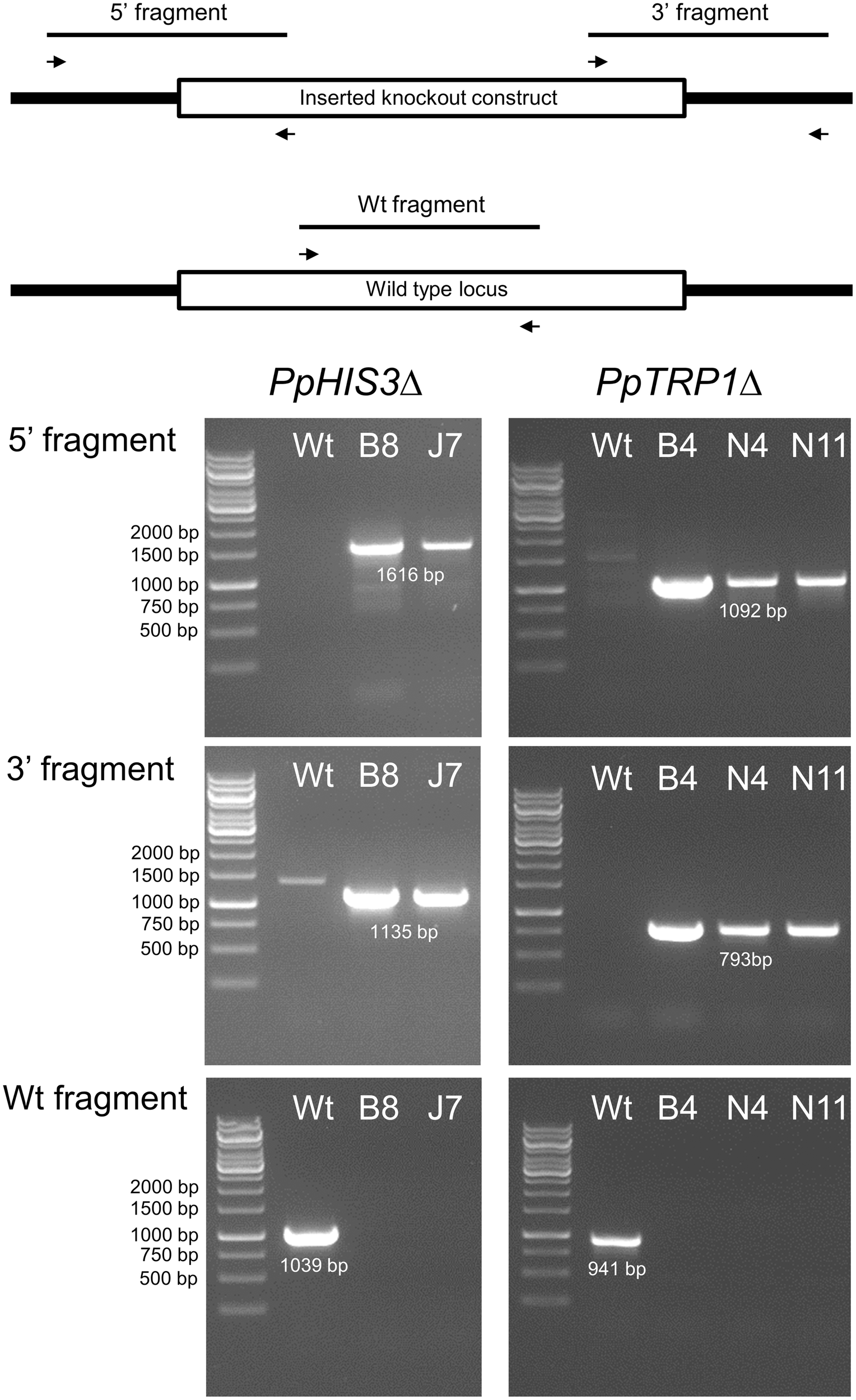
Polymerase chain reaction verification of the PpHIS3Δ and PpTRP1Δ knockouts. PCRs on genomic DNA from the PpHIS3Δ, PpTRP1Δ, and wild type (Wt) moss strains. PCRs amplifying the 5′-end region (top) and the 3′-end region (middle) are shown, in which one primer was located inside the knockout construct the other primer outside. Also shown is PCRs using two internal primers from the targeted locus (bottom) which verify that the targeted gene has been deleted in the knockout strains.
FIGURE 3
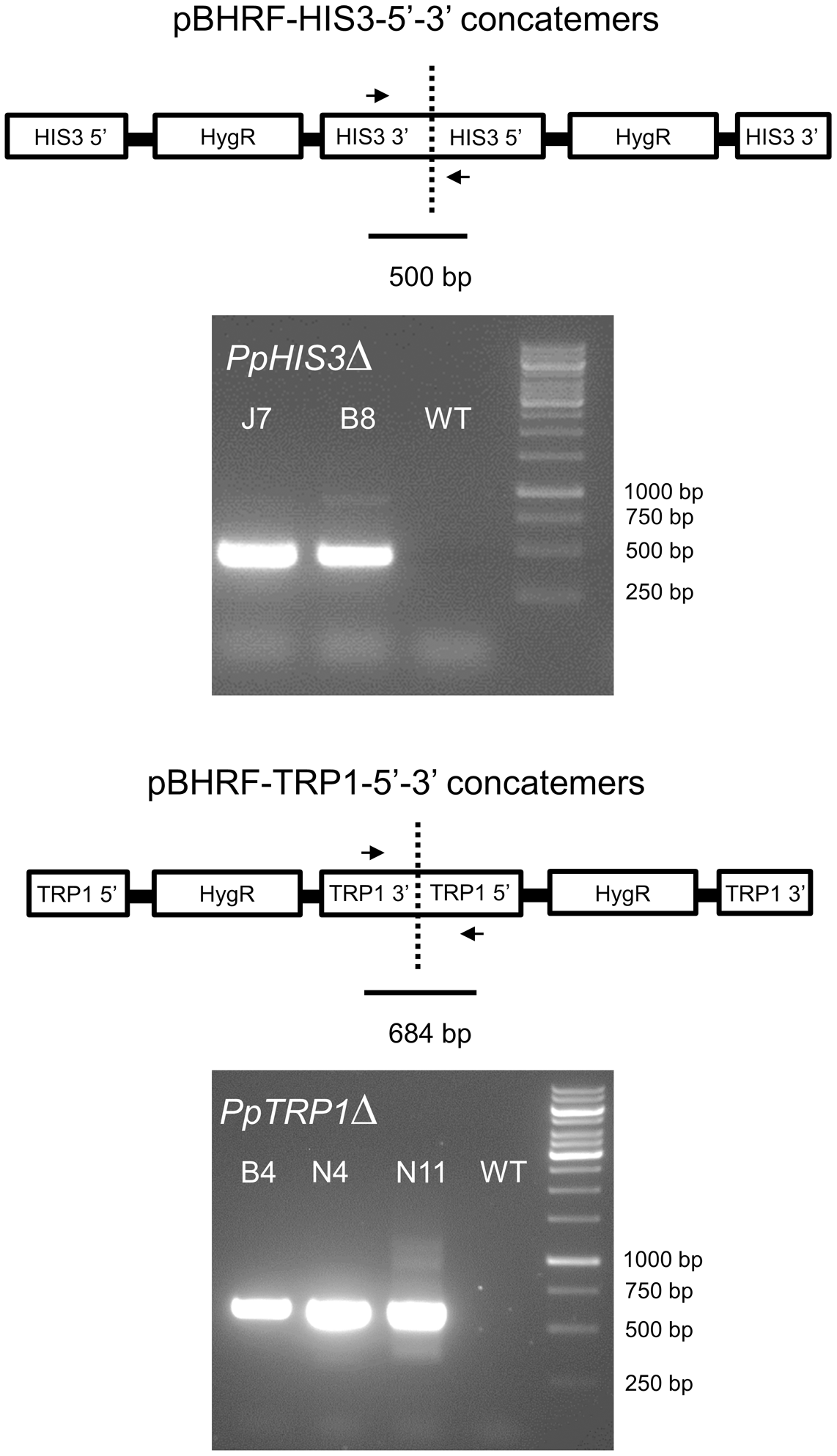
Polymerase chain reactions on genomic DNA from the PpHIS3Δ and PpTRP1Δ knockout strains to show that the knockout construct has formed concatemers. The divergent PCR primers were located in the 3′ and 5′ flanking regions of each gene that were used for targeting, in order to obtain PCR fragments that would span the concatemer junctions.
While high concentrations of histidine, and in particular tryptophan had an inhibitory effect on growth, the PpHIS3Δ knockouts grown on medium supplemented with 250 μM histidine had a growth rate comparable to that of the wild type. The PpTRP1Δ knockouts grew reasonably well when supplemented with 100 μM tryptophan even though their growth rate was much slower than that of the wild type. Without the supplemented amino acids the knockout strains were unable to grow (Figures 4, 5). We further observed that the PpTRP1Δ strains grew best when the moss colonies were frequently moved to fresh plates. It is possible that this could due to degradation of the supplemented tryptophan, which is photosensitive and thus less stable than other amino acids. It also seemed that the PpTRP1Δ strains did not generate protonemal tissue to the same extent as the wild type, producing more gametophores instead. In contrast, the PpHIS3Δ strains grew well as long as they were supplemented with 250 μM histidine, and behaved phenotypically like the wild type. For this reason we choose to use on of the histidine auxotrophs, the PpHIS3Δ strain B8, in our shuttle plasmid experiments.
FIGURE 4
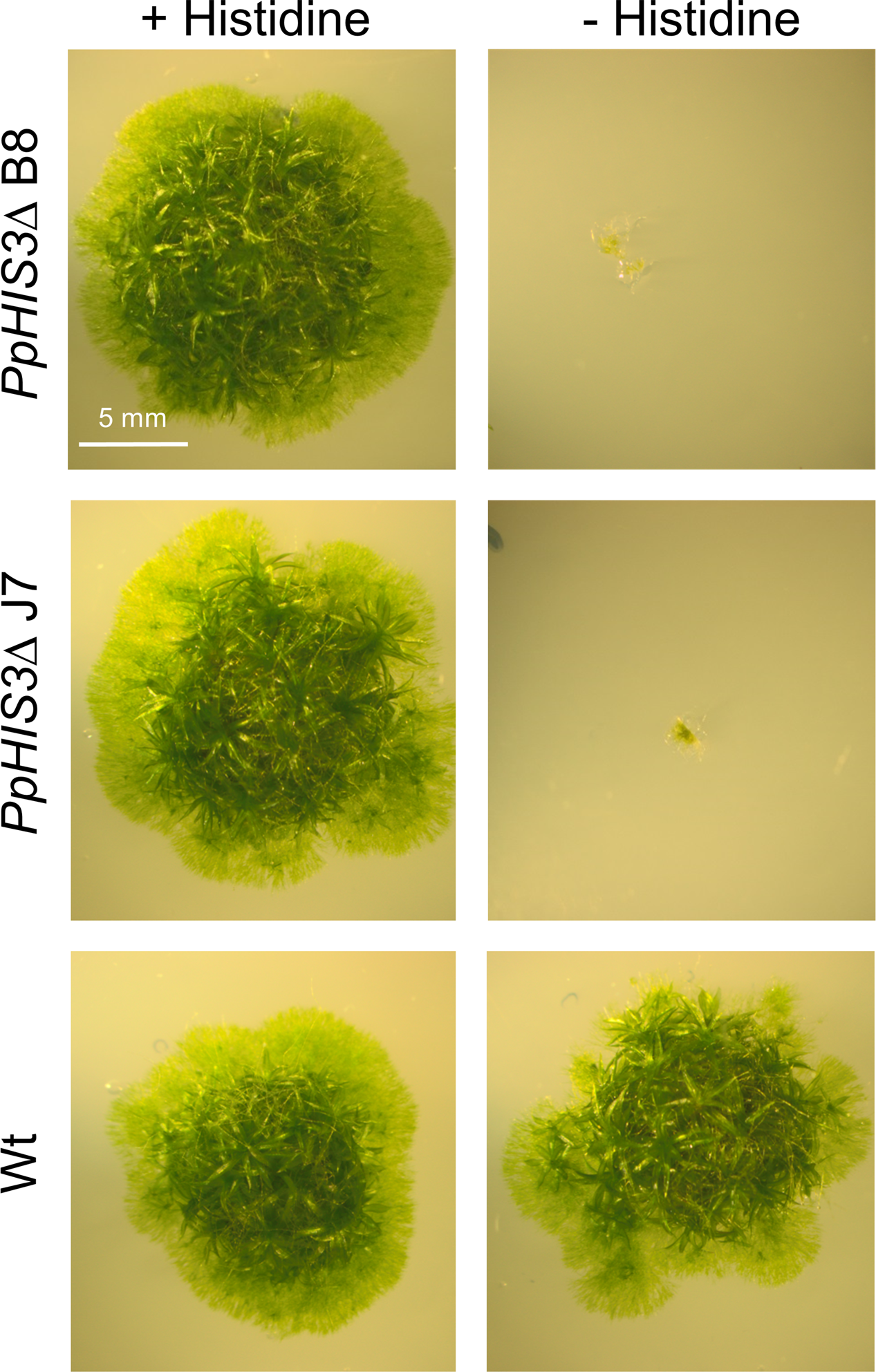
Phenotypes of PpHIS3Δ knockout strains. The PpHIS3Δ strains B8 and J7 and the Wt were grown with or without 250 μM histidine in the medium.
FIGURE 5

Phenotypes of PpTRP1Δ knockout strains. The PpTRP1Δ strains (B4, N4, and N11) and the Wt were grown with or without 100 μM tryptophan in the medium.
Selection of Prototrophic Transformants of the PpHIS3Δ Moss Strain by Using the PpHISS3 Gene on a Plasmid
We next wanted to test if the auxotrophic PpHIS3Δ strain could be complemented by a wild type PpHIS3 gene and in particular if transformants could be selected by using the auxotrophic marker instead of a drug resistance marker. For this, we used the pHGZ404 plasmid containing the PpHIS3 gene expressed from the CaMV35S promoter (Figure 1B). The plasmid also contains the G418 resistance gene nptII and sequences from the Physcomitrella BS213 locus that can be used for targeted integration into the genome (Schaefer and Zrÿd, 1997). The latter requires that the plasmid is cleaved with NotI prior to transformation in order to release the gene targeting fragment, with the BS213 5′ and 3′ sequences at either end, from the pUC119 backbone (see “Materials and Methods).
In our previous study with plasmids carrying a drug resistance marker we found that circular and linearized plasmids behaved differently when transformed into moss (Murén et al., 2009). Thus, circular plasmids mostly produced unstable transformants indicating that the DNA replicated episomally, and plasmids rescued from these transformants were always identical to the original plasmid. In contrast, linearized plasmids either produced stable chromosomal integrants or highly unstable episomal transformants in which the plasmid DNA was present in a very high copy number but still lost at a high frequency (Murén et al., 2009). Moreover, plasmids rescued from moss transformed with linearized DNA differed from those rescued from circular DNA transformants in that they always contained deletions indicative of concatemer formation in planta (Murén et al., 2009).
In the present study, we wanted to test if these findings would hold true also for transformants obtained by selection for complementation of an auxotrophic marker. The PpHIS3Δ strain was therefore transformed with both circular supercoiled plasmid and plasmid linearized with NotI. A conceptual difference from our first study was that the linearized plasmids were expected to target the BS213 locus instead of integrating at random points in the genome, making the molecular analysis of these events easier. Furthermore, we reasoned that stable integration into a selectively neutral locus (BS213) could provide an interesting alternative to episomal replication in cases where it is desirable to maintain a low copy number. As discussed below, we did see targeted integration of the linearized plasmid, but, unexpectedly, into the PpHIS3Δ knockout allele instead of the BS213 locus.
The transformed cells were plated on medium lacking histidine and incubated for 3 weeks to select prototrophic transformants. As shown in Figure 6A, we obtained transformants with both circular supercoiled and linearized plasmid DNA. In contrast, mock transformations with no added plasmid produced no colonies. In order to eliminate transient transformants that fail to replicate the PpHIS3 marker, individual transformants were picked to fresh histidine-less media and incubated for 2 weeks. This procedure was repeated three times. At the end, we found that 25 of 314 picked colonies from the transformation with circular plasmid DNA survived, whereas 169 of 174 picked colonies from the transformation with linearized plasmid DNA survived. This is consistent with our previous results using an antibiotic resistance marker (Murén et al., 2009), where we found that the circular plasmid initially produced more transformants than the linearized plasmid, but many of these early transformants were unstable transients that fail to replicate the plasmid DNA and eventually lose it.
FIGURE 6
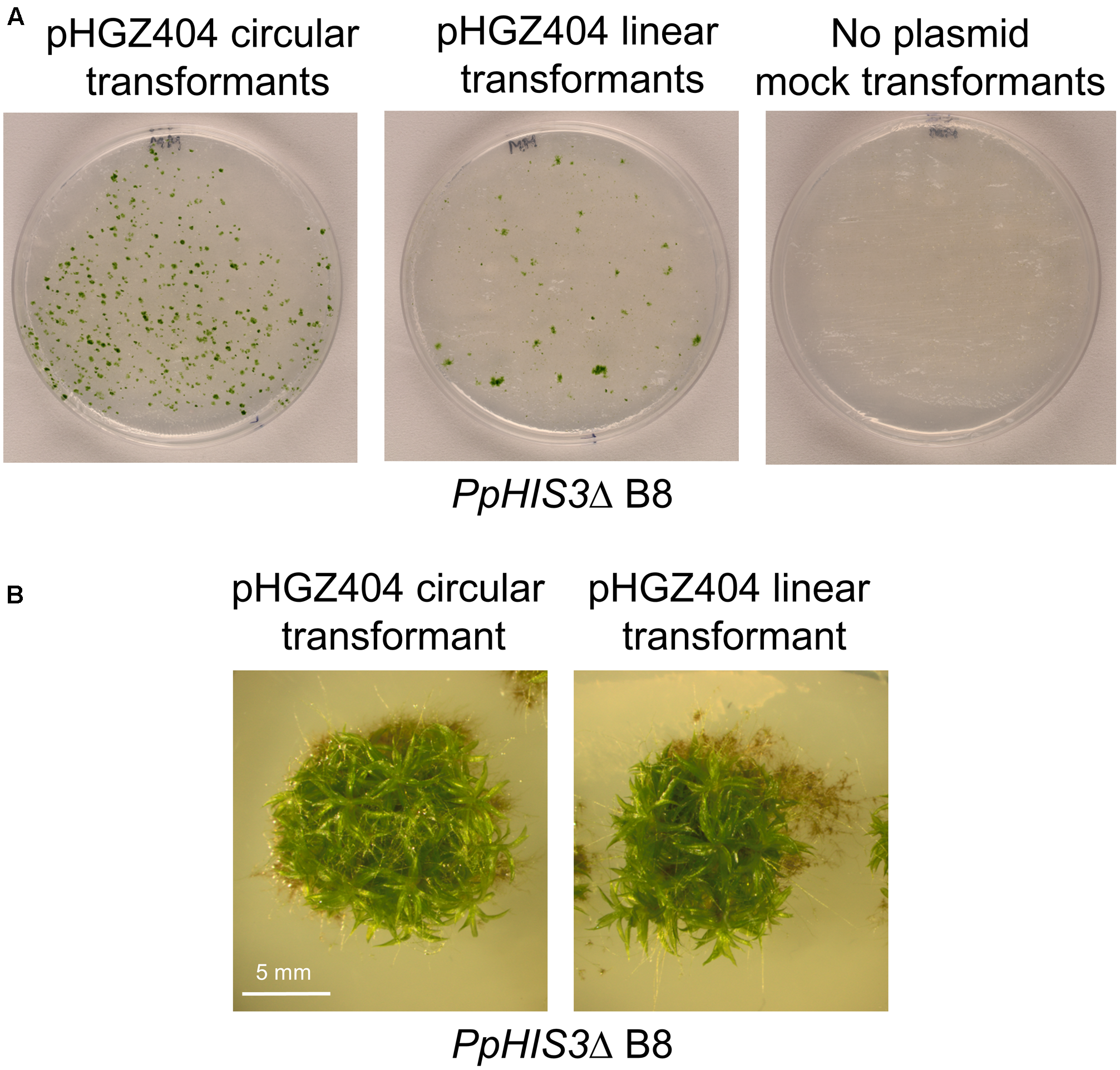
Transformation of the PpHIS3Δ knockout strain with circular or linearized pHGZ404 plasmid. (A) Agar plates with colonies obtained from the PpHIS3Δ strain when transformed with circular and linearized pHGZ404, as well as a mock transformation with no added DNA. Transformants were selected on histidine-less plates. (B) Gametophore containing colonies of the PpHIS3Δ knockout strain complemented with either circular or linearized pHGZ404.
We picked 16 Physcomitrella transformants for further characterization, eight of which were obtained by transformation with circular supercoiled plasmid and eight by transformation with the linearized plasmid. To prove that the transformants had acquired the PpHIS3 gene, we conducted PCR experiments with DNA from these transformants. All 16 transformants were positive for the presence of a fragment of the expected size (Figure 7). The fragment was cloned and sequenced to confirm that it was derived from the transformed CaMV35S-PpHIS3 marker.
FIGURE 7
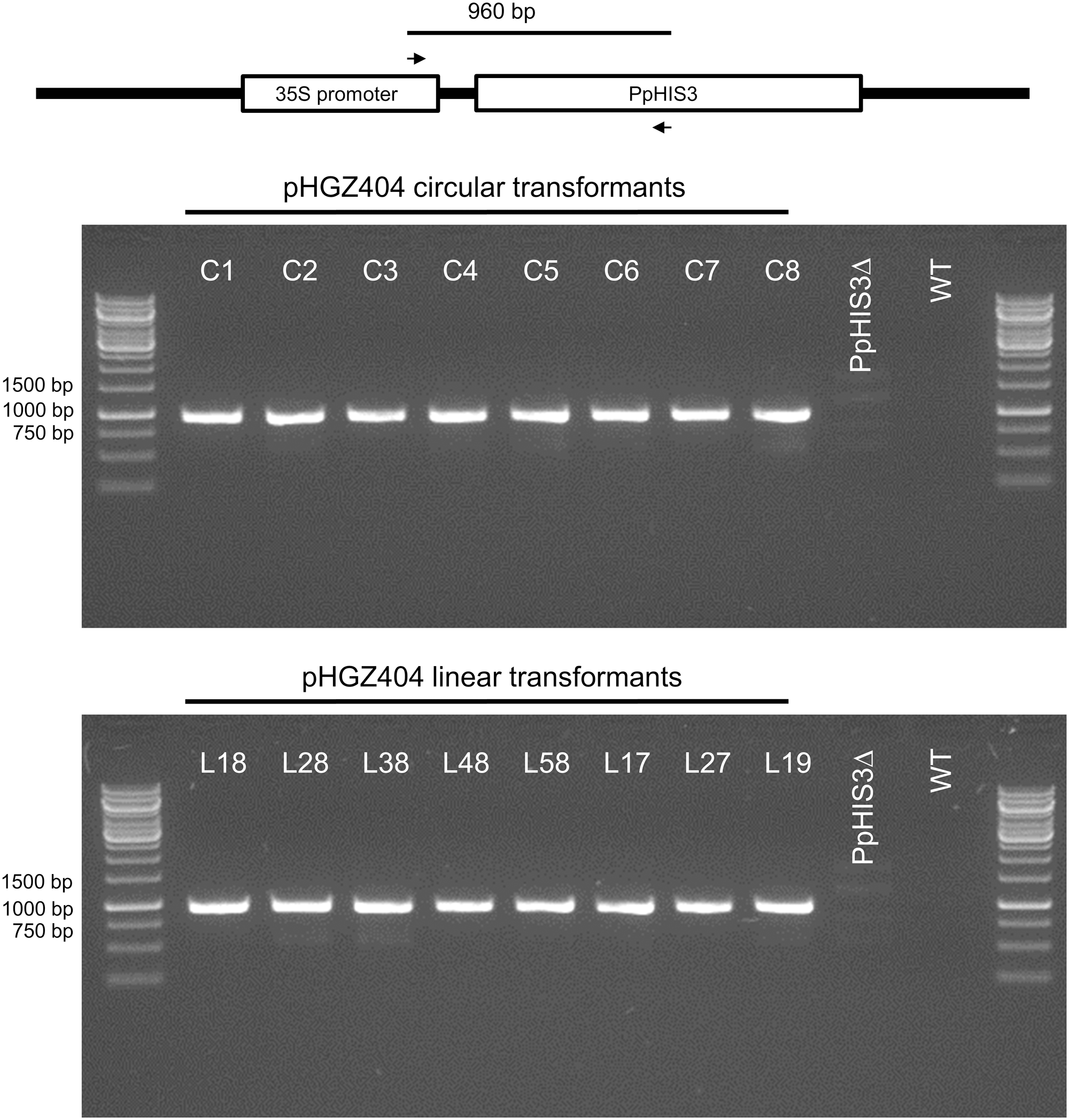
Polymerase chain reaction verification of the presence of the plasmid PpHIS3 marker in pHGZ404 transformants. Genomic DNA from the PpHIS3Δ knockout strain transformed with circular or linearized pHGZ404 were checked for the presence of PpHIS3 by amplification of a 960 bp internal fragment of PpHIS3 expression cassette.
Formation of Concatemers in Planta by Recombination between the Two NOS1 Terminators on the Plasmid
Plasmids transformed into moss are known to frequently form concatemers, i.e., arrays of tandem repeats of transformed DNA. These arrays can comprise the entire plasmid or shorter deletion products where parts of the plasmid have been lost. We previously found that transformation with linearized DNA is particularly prone to result in the formation of large concatemers that are highly unstable, indicating that they replicate episomally (Murén et al., 2009).
We proceeded to test for the presence of concatemers using pairs of PCR primers that were designed to span across junctions in concatemers of linearized plasmid, thus amplifying a fragment that would be indicative of concatemer formation. However, we were unable to detect any such PCR products until we used internal primers located between the two NOS1 terminators present on the pHGZ404 plasmid (Figure 1B). The binding site of the first primer, MU84, was placed after the NOS1 terminator of the nptII gene, causing the polymerase to move upstream across the first NOS1 terminator. The binding site of the second primer, MU83, was located within the PpHIS3 gene, causing the polymerase to move downstream across the second NOS1 terminator. Our rationale for testing this pair of primers was the assumption that recombination between the two NOS1 terminators in the plasmid would occur during concatemer formation, resulting in the deletion of flanking parts of the linearized plasmid but retaining the internal fragment containing the PpHIS3 marker that was being selected for.
The PCR results shown in Figure 8 confirm this assumption. Thus, among the circular DNA transformants all but C4, C5, and C7 showed bands indicative of recombination between the two NOS1 terminators in adjacent repeats. In the linear transformants, all but L18 contained concatemer junctions created by NOS1 terminator recombination. Recombination between the NOS1 terminators is not the only possible mechanism for concatemer formation, and it is likely that different concatemers of pHGZ404 may exist within the transformants. That being said, the evidence of NOS1-mediated recombination in most of the transformants and our failure to detect junction fragments from concatemers of full-length plasmids indicates that NOS1-mediated recombination is a major pathway for concatemer formation in cells transformed with pHGZ404. This is consistent with our previous finding that concatemer formation in moss frequently involves recombination between repeated sequences within the transformed DNA, and not just end to end ligation of linearized plasmids (Murén et al., 2009).
FIGURE 8
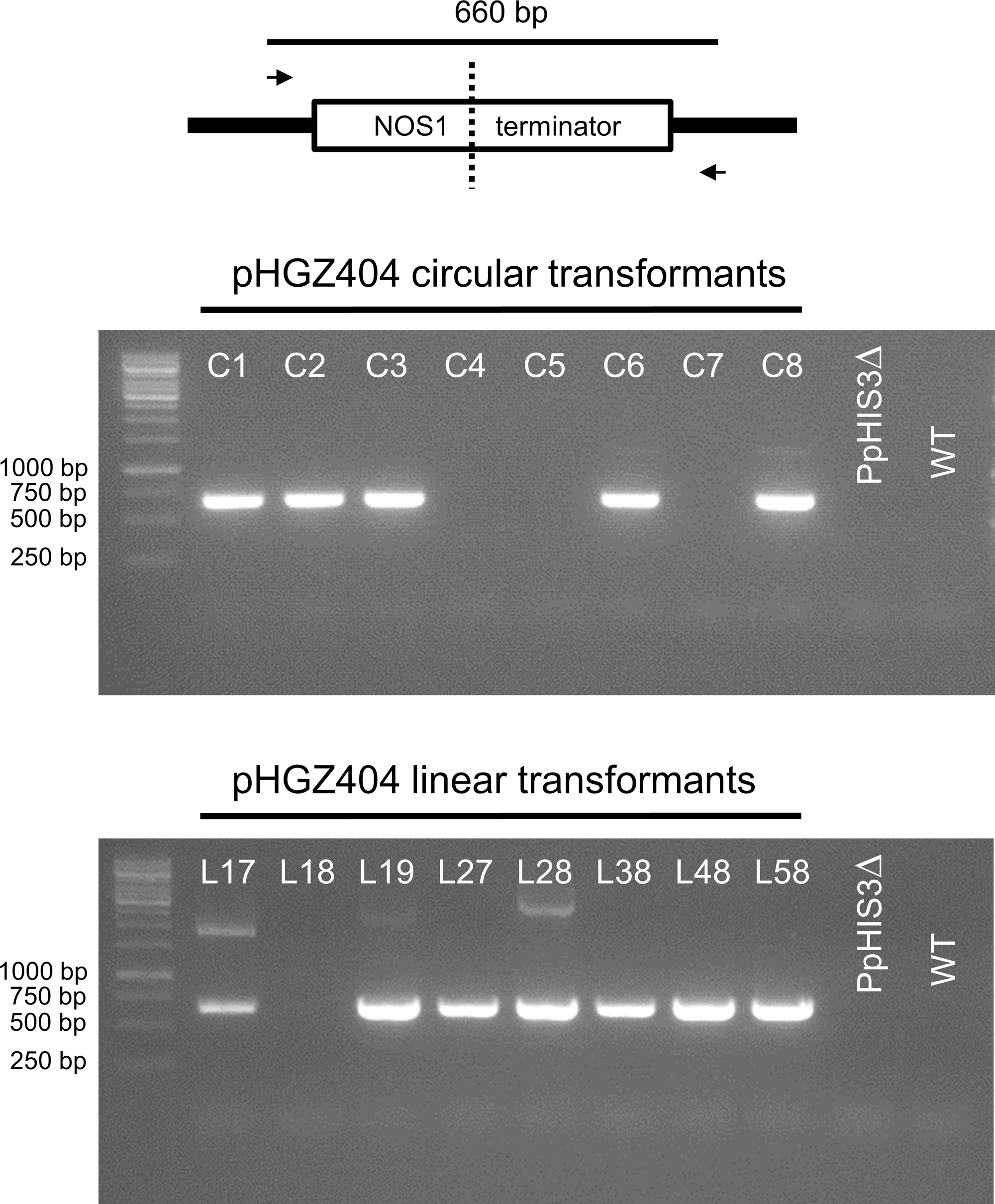
Polymerase chain reaction to confirm concatemer formation of pHGZ404 plasmids in transformants by homologous recombination between the two NOS1 terminators. PCRs were performed on genomic DNA from the eight circular and eight linearized pHGZ404 moss transformants. The primers were located down-stream of the first NOS1 terminator and up-stream of the second NOS1 terminator on the pHGZ404 plasmid.
Integration of Linearized Plasmid at the Disrupted PpHIS3 Locus
We proceeded to test if the BS213 locus, to which the linearized plasmids would be targeted for integration, was disrupted in any of the transformants. We expected this to be true for linearized DNA transformants, but not for transformants with circular DNA. Surprisingly, we found that the BS213 locus was intact in all 16 transformants, including those obtained with linearized DNA (Figure 9). Several other PCR experiments were done to check if the linearized plasmids had integrated into the BS213 locus, but we were unable to detect any such events (data not shown). We conclude that none of the linearized plasmid transformants tested had plasmid DNA integrated into the BS213 locus.
FIGURE 9
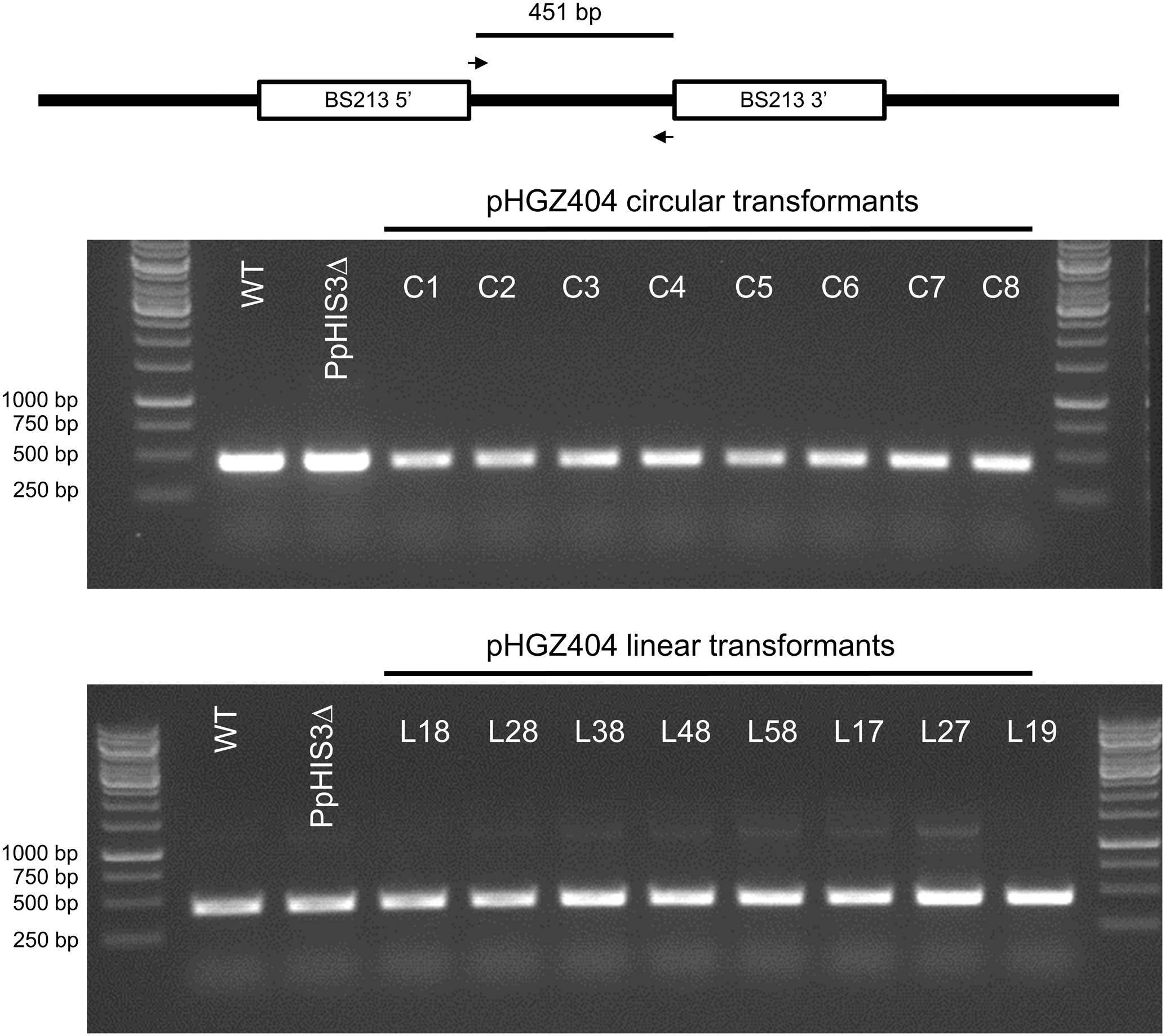
Polymerase chain reaction to check for integration at the BS213 locus. PCRs were performed on genomic DNA from stable transformants obtained with either circular or linearized plasmids. The PCR amplifies an internal fragment of the BS213 locus which unexpectedly was present in all transformants.
We therefore considered the possibility that the linearized plasmids could have integrated at some other location in the genome. Ectopic integration of transformed DNA by non-homologous recombination is less common in Physcomitrella (Ashton et al., 2000; Kamisugi et al., 2005, 2006), and it therefore seemed unlikely that this would have happened in all eight linear transformants tested. However, gene targeting by homologous recombination, which is a much more efficient process, could occur between the CaMV35S promoter in front of the PpHIS3 gene on the plasmid and the CaMV35S promoter in front of the hph hygromycin resistance gene integrated at the disrupted PpHIS3 locus. As previously noted, the knockout construct including the CaMV35S promoter is itself present as a concatemer at the PpHIS3 locus (Figure 3), which would increase the likelihood of a recombination event, particularly if the incoming plasmid DNA also is a concatemer with multiple copies of the CaMV35S promoter (Figure 10A).
FIGURE 10
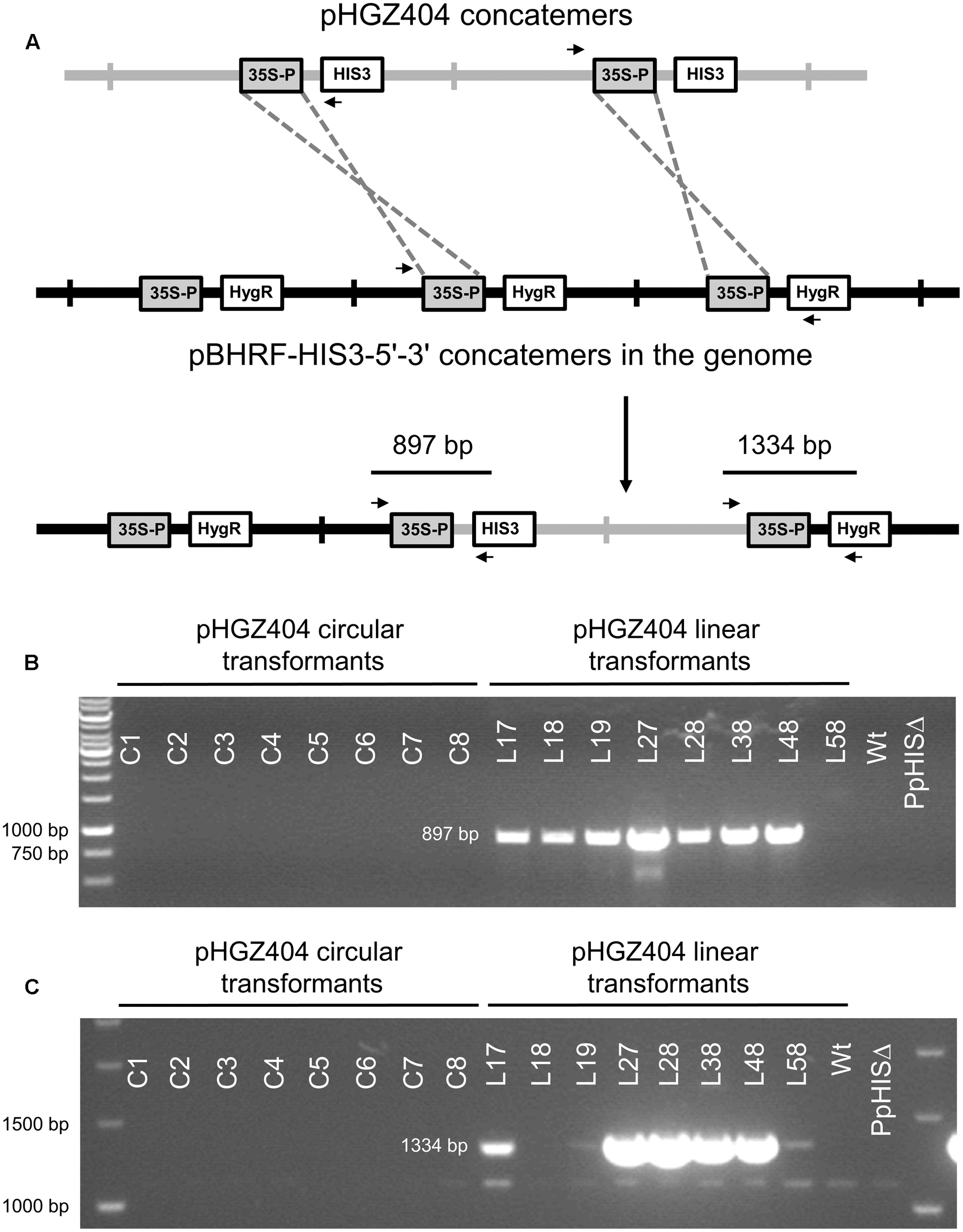
Integration of pHGZ404 concatemers into the disrupted PpHIS3Δ locus by homologous recombination between CaMV35S promoter sequences. (A) Proposed model for concatemer formation. A concatemer of the linearized pHGZ404 plasmid (gray) integrates into the concatemer at the disrupted PpHIS3Δ locus (black) by homologous recombination between CaMV35S promoter sequences on the plasmid and at the disrupted PpHIS3Δ locus. PCR primers used to check for this type of integration events are shown as arrows. PCRs were performed on genomic DNA from eight circular and eight linearized moss transformants with pHGZ404 in the PpHIS3Δ strain. 5′-end (B) and 3′-end (C) integration events were detected using one primer located within pHGZ404 and one primer located in the pBHRF-HIS3-5′-3′ knockout construct.
To test this possibility, we used pairs of primers with one primer positioned in pHGZ404 and the other in the knockout construct at the disrupted PpHIS3 locus (Figure 10A). PCR with these primers showed that seven of the eight linear transformants had undergone a CaMV35S promoter-mediated integration event at the 5′ end of pHGZ404 (Figure 10B) and five of these transformants also showed evidence of a 3′ integration event (Figure 10C). PCR fragments from both the 5′ and 3′ integration events were cloned and sequenced to verify that integration had occurred at the disrupted PpHIS3 locus. Only one linearized transformant, L58, showed no evidence of CaMV35S promoter mediated integration at the PpHIS3 locus. In contrast, none of the eight circular transformants had any sign of integration via recombination in the CaMV35S promoter. We conclude that gene targeting by homologous recombination occurred with high frequency in the linearized transformants, though with a different target than intended. The possibility remains that pHGZ404 also integrated at other, unknown locations in some of the transformants, but the intended target BS213 and the observed target PpHIS3 are the only regions of homology to pHGZ404 in the Physcomitrella genome, and as noted above integration of transformed DNA by non-homologous recombination is less common in moss (Ashton et al., 2000; Kamisugi et al., 2005, 2006).
We also considered the possibility that the junction fragments detected in Figure 10 might be formed during the PCRs by template switch recombination (Hommelsheim et al., 2014) between NOS1 or CaMV35S elements on different molecules. However, this is a very unlikely explanation since these fragments were reproducibly seen in some transformants but not in others, even though all transformants contained the same plasmid DNA and the same knockout construct at the PpHIS3Δ locus. Thus, junction fragments consistent with integration of plasmid concatemers at the PpHIS3Δ locus were reproducibly seen in all linearized plasmid transformants except L58, but in none of the circular transformants on the multiple occasions that we repeated this PCR. Similarly, a junction fragment consistent with concatemer formation by recombination between the NOS1 elements was always seen in all linearized plasmid transformants except L18, and in circular transformants C1, C2, C3, C6, and C8, but never in C4, C5, and C7. It is unlikely that this was due to failure of the PCR reactions in some samples since the PCRs in Figures 7 and 9 did work for the same samples. These reproducible patterns, which were seen with several different primer pairs, strongly suggest that the junction fragments are present in some strains but not in others, and are not formed by template switches during the PCR.
Episomal Replication of the Unselected nptII Marker
An important question is to what extent the overall structure of the plasmid is maintained after transformation into moss, since this will affect the feasibility of plasmid rescue back into E. coli, for which the bacterial origin of replication and the AmpR marker must be retained. In particular, NOS1-mediated recombination would tend to unlink the PpHIS3 marker, which is selected for, from the plasmid backbone and the nptII marker which confers resistance to G418 in moss (Figure 1A). To assess retention of the nptII marker in transformants selected for histidine prototrophy, we tested eight circular and eight linear transformants for their ability to grow on plates containing different concentrations of G418 (Figure 11). As a positive control, we included a knockout strain (P5) that has the nptII marker integrated into the PpHXK1 gene (Olsson et al., 2003). The presence or absence of the nptII marker was also analyzed for each transformant by PCR of an nptII internal fragment, as shown in Figure 12.
FIGURE 11
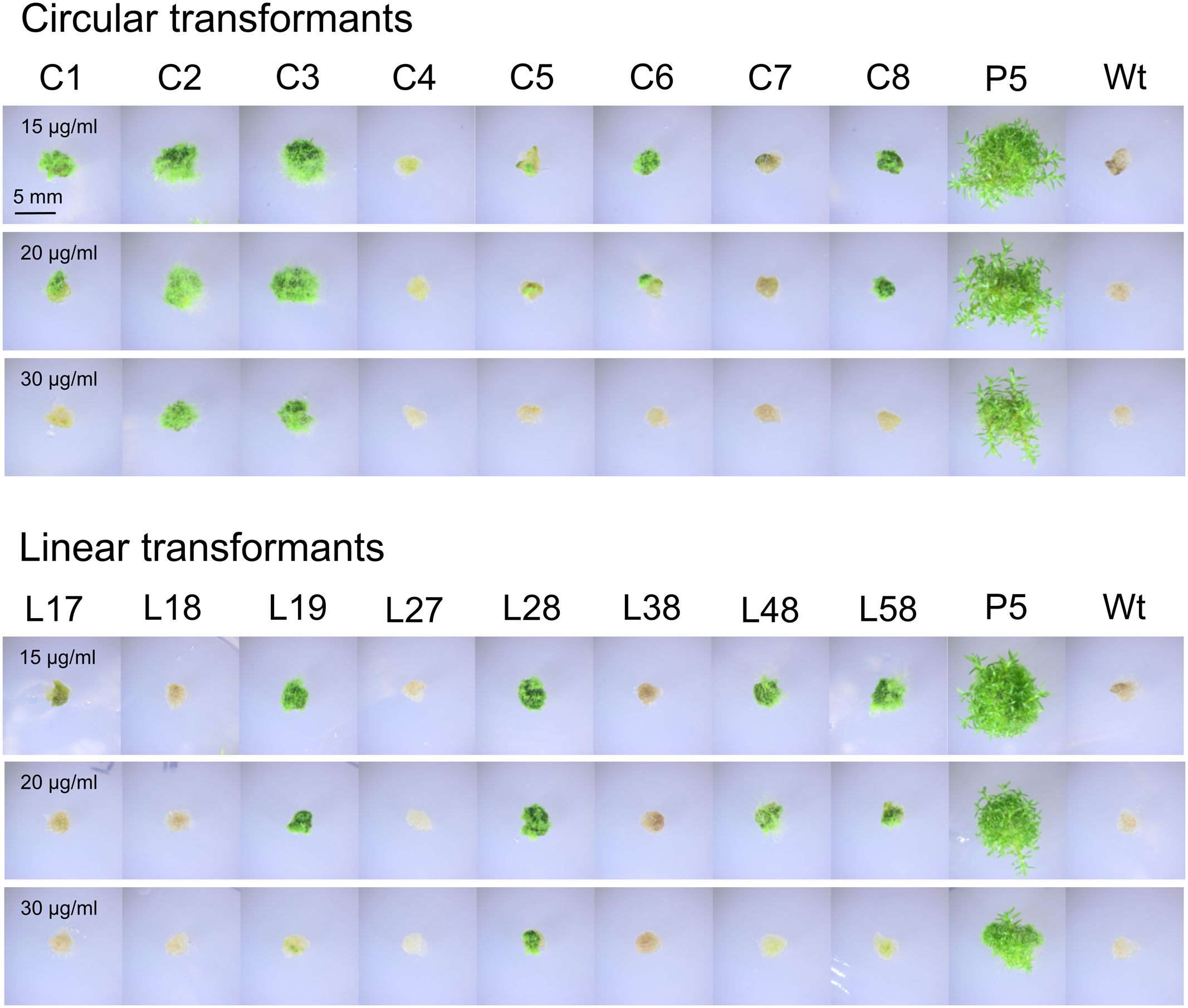
Assay for G418 resistance in different transformants of the PpHIS3Δ strain with plasmid pHGZ404. Eight linearized (L17–L58) and eight circular (C1–C8) pHGZ404 transformants were grown on G418-containing plates to established to what extent they retained the nptII drug resistance marker. Also shown as controls are the drug sensitive Wt and the stably drug resistant P5 strain, which carries chromosomally integrated copies of the nptII marker at the PpHXK1 locus.
FIGURE 12
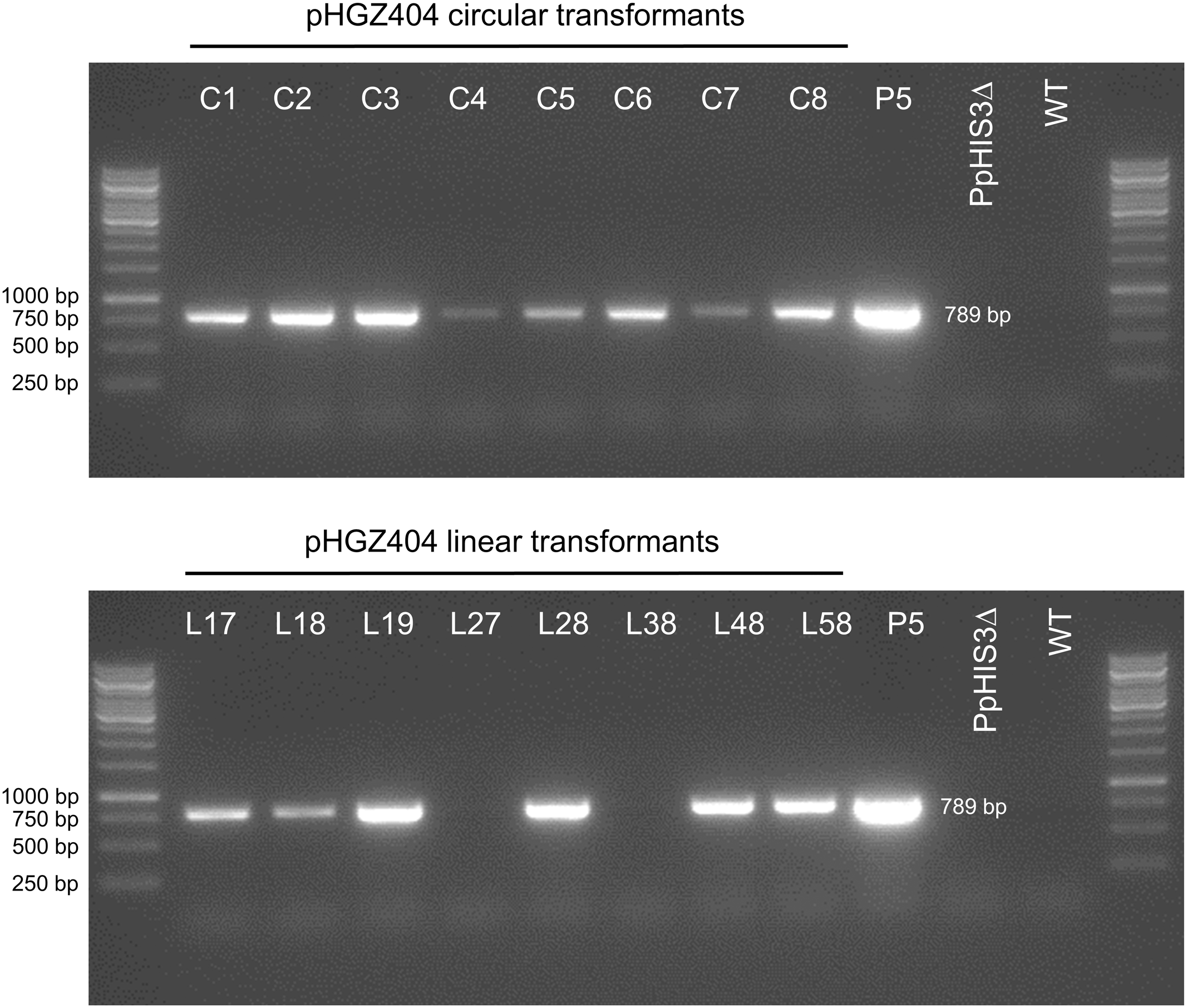
Polymerase chain reaction experiment to check for the presence of the nptII marker in different transformants of the PpHIS3Δ strain with plasmid pHGZ404. Eight linearized (L17–L58) and eight circular (C1–C8) pHGZ404 transformants were checked by PCR for the presence of the nptII marker. Also shown as controls are the drug sensitive Wt, the untransformed PpHIS3Δ strain, and the stably drug resistant P5 strain, which carries chromosomally integrated copies of the nptII marker at the PpHXK1 locus.
First, we note that there was a strong correlation between resistance to G418 (Figure 11) and the amount of PCR product (Figure 12). While copy numbers cannot be accurately estimated from standard PCRs, the correlation suggests that the degree of drug resistance depends on the copy number of the nptII marker. In particular, we note that only two linear transformants (L27 and L38) lacked the PCR band, and they failed to grow at all three concentrations of G418. One transformant with a weak PCR band (L17) also failed to grow in the presence of G418. All other transformants showed varying degrees of resistance to G418 which correlated with the amount of PCR product. Some transformants with a weak PCR band, such as C5 and C6, showed papillation on G418, indicating that only some cells contained the nptII marker. This is the expected result for an episomal plasmid that is lost at high frequency (Murén et al., 2009) and suggests that at least some of the circular transformants carry such plasmids. We further note that none of the transformants grew as well as the P5 strain on G418. A possible explanation is that the latter carries multiple tandem copies of the nptII marker at the PpHXK1 locus which may have been further amplified by selection on G418 plates. In contrast, our transformants were selected for histidine prototrophy and not G418 resistance.
Significantly, two linearized plasmid transformants, L27 and L38, had no trace of the nptII marker (Figure 12) even though they retained the PpHIS3 marker (Figure 6). This shows that loss of unselected parts of the plasmid does occur in linearized transformants, presumably by NOS1-terminator mediated recombination during concatemer formation. In contrast, all eight circular plasmid transformants retained the nptII DNA, though in variable amounts (Figure 12). This is consistent with our previous finding (Murén et al., 2009) that intact plasmids can be rescued form circular but not linearized plasmid transformants (see also below).
In order to confirm that transformed DNA carrying the nptII marker replicated episomally, we carried out a plasmid loss experiment (Murén et al., 2009). Protonemal tissue from the different transformants was homogenized, diluted to generate an estimated 25 colonies per plate, and plated on four non-selective cellophane covered plates for each strain. After 2 weeks, the cellophane sheets were moved to G418-containing plates to select for the nptII marker and incubated for another week. The total numbers of surviving (green) and dead (white) colonies (Figure 13) were then counted for each strain.
FIGURE 13

Plasmid loss experiment to check for retention of G418 resistance in different transformants of the PpHIS3Δ strain with plasmid pHGZ404. Representative photographs of eight linearized (L17–L58) and eight circular (C1–C8) pHGZ404 transformants after growth on G418-containing plates are shown. Colonies that survived due to retention of the episomally replicating nptII marker are green, and those that lost the marker and died are white. Also shown as controls are the drug sensitive Wt and the stably drug resistant P5 strain, which carries chromosomally integrated copies of the nptII marker at the PpHXK1 locus.
As seen in Table 1, we found that all moss transformants that carried the nptII marker lost it at variable frequencies when grown on non-selective plates, indicating that it had not integrated into the moss genome but instead replicated episomally in these transformants. In contrast, the control Pphxk1::nptII knockout strain P5 (Olsson et al., 2003) retained the marker in all colonies, as expected. The frequency of marker loss varied, being lowest (7 and 19%) in transformants C3 and C2, the two transformants that grew best under selection (Figure 11). We conclude from these experiments that even though parts of the original plasmid including the PpHIS3 marker integrated into the genome in some transformants, other parts including the nptII marker were maintained in an episomal state. In fact, none of the transformants contained a stably integrated nptII marker, which suggests that episomal replication is the default fate for transformed DNA in the absence of targeted integration by homologous recombination. This is consistent with the observation that episomally replicating DNA that carried a selectable marker could be maintained for several years in moss transformants without integration into the genome (Ashton et al., 2000).
Table 1
| Moss strain | Total colonies plated | G418-resistant colonies | Loss frequency |
|---|---|---|---|
| C1 | 69 | 7 | 90% |
| C2 | 31 | 25 | 19% |
| C3 | 45 | 42 | 7% |
| C4 | 76 | 0 | 100% |
| C5 | 75 | 2 | 97% |
| C6 | 82 | 5 | 94% |
| C7 | 68 | 0 | 100% |
| C8 | 36 | 5 | 86% |
| L17 | 70 | 0 | 100% |
| L18 | 70 | 2 | 97% |
| L19 | 105 | 9 | 91% |
| L27 | 71 | 0 | 100% |
| L28 | 35 | 2 | 94% |
| L38 | 120 | 0 | 100% |
| L48 | 64 | 19 | 70% |
| L58 | 52 | 2 | 96% |
| P5 | 94 | 94 | 0% |
| Wt | 50 | 0 | No marker |
Plasmid loss experiment.
For each moss strain, the total number of plated colonies and the surviving number after transfer to G418 plates is shown. The loss frequency is the fraction of non-surviving colonies.
Rescue of Plasmids from Moss Transformants Back into E. coli
Finally, we wanted to show not only that complementation of an auxotrophic moss strain from a plasmid works, but that such plasmids can be rescued from moss transformants back into E. coli, thus completing the shuttle plasmid circle. We therefore extracted DNA from the transformants and transformed this DNA into E. coli, selecting for ampicillin resistance. The yield was lower than previously observed (Murén et al., 2009), but three plasmids could be rescued from two circular transformants, C1 and C2, and all three plasmids were identical to the original pHGZ404. This is in agreement with our previous finding that plasmid rescued from moss transformated with circular plasmids usually are identical to the original plasmid (Murén et al., 2009). It provides further support for the notion that shuttle plasmid-based methods from yeast genetics can be adapted for use in Physcomitrella.
We were also able to rescue three plasmids from one of the linearized DNA transformants, L48. This was one of the five linearized DNA transformants that had both a 5′ and a 3′ integration event at the disrupted PpHIS3 locus, as determined by PCR (Figure 10). In contrast to the plasmids rescued from the circular plasmid transformants, the plasmids rescued from the linearized plasmid transformant all had deletions spanning one or both of the NotI sites used to cleave the plasmid prior to transformation into moss. One plasmid had a single deletion spanning the second NotI site. The other two plasmids, which were identical to each other, had the same deletion, but also a small deletion spanning the first NotI site and a big deletion that removed half of the plasmid including the PpHIS3 marker (Figure 14). An examination of the sequences revealed that all three deletion events involved direct repeat recombination between short micro-homologies on the plasmid (Figure 14). This is consistent with previous findings when transforming moss with linearized plasmids, where deletions due to direct repeat recombination between micro-homologies frequently were observed (Kamisugi et al., 2006; Murén et al., 2009). Finally, we note that both deletions spanning a NotI site had the left endpoint just a few bp from the NotI site whereas the other endpoint was much farther away (Figure 14). This suggests that the deletion may have been generated by one free double-stranded end invading the other end and scanning it until a micro-homology was found. The alternative mechanism where both free ends are degraded by exonuclease until matching micro-homologies are exposed would have generated more symmetric deletions centered on the NotI sites.
FIGURE 14
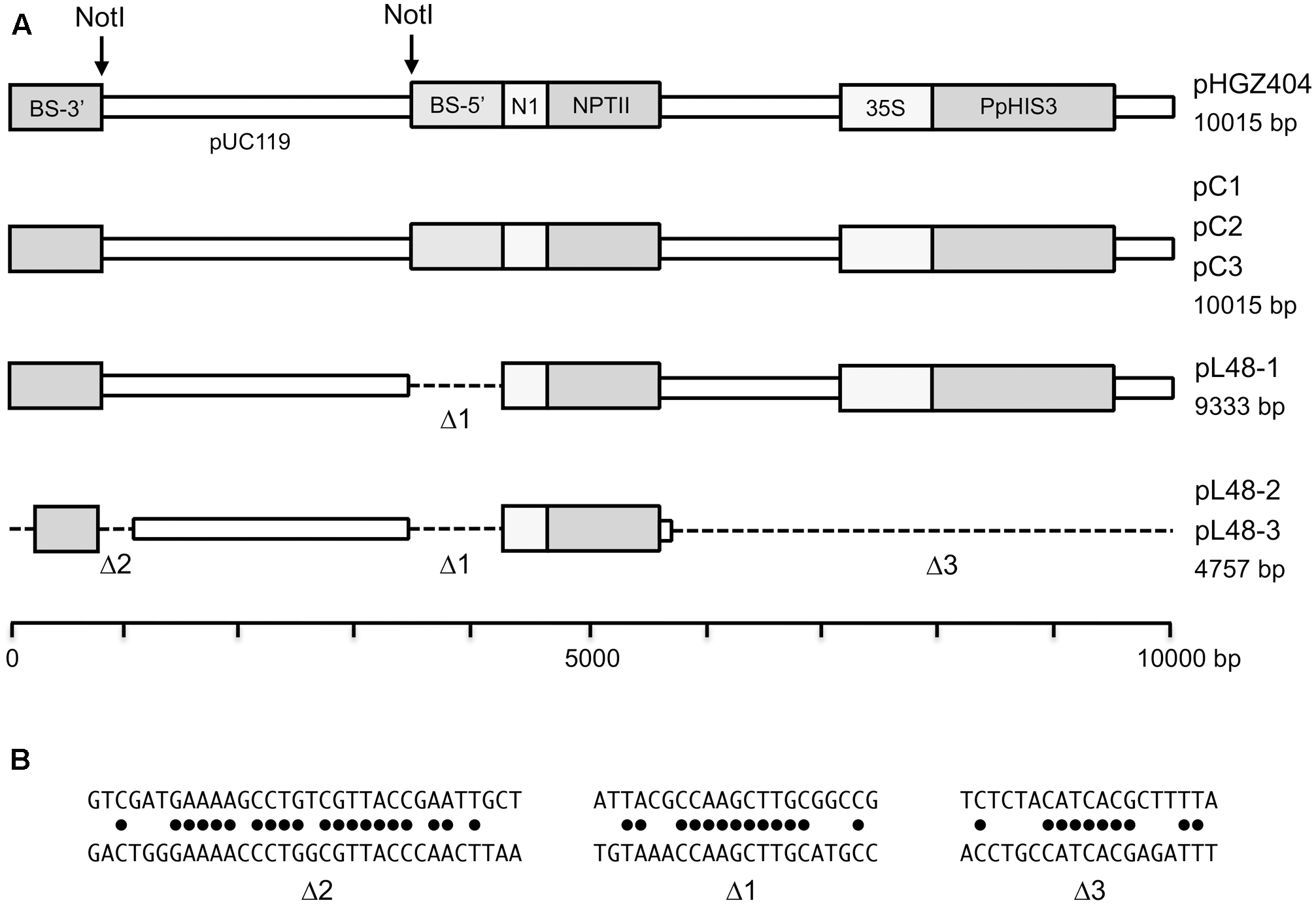
Structures of plasmids that were rescued from moss transformants back into Escherichia coli.(A) The map of the original pHGZ404 plasmid is shown at the top, and the plasmids that were rescued from moss transformants below. (B) The micro-homologies that were involved in the three internal deletion events.
Discussion
We here report the construction of a histidine auxotrophic Physcomitrella strain in which the ortholog of the yeast ScHIS3 gene, PpHIS3, was knocked out. This histidine auxotrophic strain could not survive without histidine in the growth medium, but with histidine the PpHIS3Δ mutant grows and behaves like wild type Physcomitrella. We further show that it is possible to complement this PpHIS3Δ knockout with a plasmid expressing a PpHIS3 cDNA, and that such transformants can be selected on histidine-less medium, without the use of drug resistance markers. Given that it was also possible to create a tryptophan auxotroph by knocking out the PpTRP1 gene it is reasonable to assume that other biosynthetic genes could be knocked out to create more auxotrophic markers and that these too could be complemented by plasmids expressing the relevant cDNAs. Using multiply auxotrophic strains would significantly increase the number of selection markers available in Physcomitrella. Today, there are only four selection markers that can be readily used in Physcomitrella, which confer resistance to hygromycin B, geneticine (G418), zeocin, and blasticidin S, respectively (Schaefer et al., 1991; Kasahara et al., 2004; Ishikawa et al., 2011). An additional advantage of using auxotrophic markers is that unwanted side effects of antibiotics can be avoided. It should be noted that in order to make multiply auxotrophic moss strains, it will be necessary to re-use the drug resistance markers that are used to knock out the biosynthetic genes. Furthermore, some candidate auxotrophic markers such as the Physcomitrella ortholog of the yeast URA3 gene are present in two copies in the genome. Fortunately, this is now possible since the Cre-Lox system works in Physcomitrella (Wu et al., 2011). One could therefore make multiply auxotrophic strains with clean deletions instead of insertions of drug resistance markers at the biosynthetic loci.
Phenotype of the PpTRP1Δ Knockout Mutant
Whereas the PpHIS3Δ mutant had no discernible phenotype except histidine auxotrophy, the PpTRP1Δ mutant shows both an altered phenotype and slower growth. Thus, when we sub-cultured the PpTRP1Δ mutant by spreading sheared moss tissue onto new agar plates it did not produce as much protonema as the wild type but instead grew slowly into small colonies containing gametophores. It is possible that the poor growth of the PpTRP1Δ mutant even in the presence of tryptophan is due to inefficient uptake of this amino acid from the medium. Alternatively, loss of PpTRP1 could have an effect on the metabolism that is not remedied by the addition of tryptophan. A third possibility is that the observed phenotype, in particular the shift from protonema tissue production in favor of producing gametophores, is an effect of the added tryptophan in the plate. Tryptophan is a precursor to IAA, the most common naturally occurring variant of the plant hormone auxin (Sherwin and Purves, 1969; Zhao et al., 2002). Excess tryptophan might therefore affect the IAA level in moss. Auxin promotes the shift from chloronema to caulonema, and also stimulates the formation of buds on caulonema that eventually give rise to gametophores (Ashton et al., 1979; Jang and Dolan, 2011). The slow growth and altered phenotype of the PpTRP1Δ mutant would make PpTRP1 less suitable as an auxotrophic marker, but it might still be used in cases where these effects do not matter.
Different Fates of Circular and Linearized DNA in Planta
We found in our previous study using selection for a drug resistance marker that circular and linearized DNA behave differently when transformed into moss (Murén et al., 2009). Thus, circular plasmids that replicate episomally are always recovered in their original form when rescued back into E. coli. In contrast, linearized plasmids tend to form concatemers that either replicate episomally or integrate into the moss genome. Furthermore, linearized plasmids that are rescued back into E. coli always contain deletions resulting from recombination between micro-homologies within the plasmid.
The present study which used selection for an auxotrophic marker confirms and extends these findings, and provides further insights into the mechanisms of concatemer formation. Thus, we found that concatemer formation preferentially occurred by homologous recombination between the two copies of the NOS1 terminator that are present on the plasmid (Figure 9). In fact, we were unable to detect concatemers resulting from simple end to end ligation of the linearized plasmid, indicating that such event are rare or non-existent in planta. Interestingly, we saw NOS1-mediated concatemer formation also in five of eight circular transformants, so this process is not limited to linearized DNA. However, it should be noted that plasmids rescued from circular transformants both in the previous (Murén et al., 2009) and the present study showed no evidence of the internal deletions associated with concatemer formation. This suggests but does not prove that intact plasmids may propagate in planta independently of concatemer formation.
Unexpected Targeting of Linearized Plasmid into the PpHIS3Δ Locus
Surprisingly, we found that the linearized plasmid integrated into the disrupted PpHIS3Δ locus (Figure 10) instead of into the intended target, the BS213 locus (Figure 9). A likely explanation for this is that the construct used to knock out the PpHIS3 gene is present in the genome as a concatemer with multiple copies of the CaMV35S promoter in front of the HygR gene, and that the pHGZ404 plasmid also forms a concatemer prior to integration (Figure 10A). Accordingly, the multiple CaMV35S promoter fragments at the disrupted PpHIS3 locus would present a much larger and therefore more likely target for homologous recombination than the single copy of the BS213 target locus. This finding is consistent with our previous observations suggesting that when different sequence homologies are available, homologous recombination in Physcomitrella almost always proceeds by using the most extensive sequence homology. Thus, we found that when a plasmid carries two copies of a 17 bp sequence, deletions within the linearized plasmid always occurs by recombination between these two sequences, even though shorter micro-homologies also are available (Murén et al., 2009). Similarly, as discussed above, concatemer formation in the present study occurs largely or exclusively by recombination between the two NOS1 terminators on the plasmid. It should be noted that this was true even for those circular plasmid transformants in which concatemer formation could be detected. We conclude from these findings that it is important to design shuttle plasmids and targeting constructs for use in moss in such a way that undesired recombination events are minimized. In particular, repeated sequences on plasmids should be avoided, and a different promoter such as the NOS1 promoter should be used for expression of the auxotrophic marker. Alternatively, the above discussed use of the Cre-Lox system to generate multiply auxotrophic moss strains would prevent undesired targeting since it would leave no CaMV35S promoter sequences in the genome.
Plasmid Rescue from Circular and Linearized Plasmid Transformants
All three plasmids rescued from circular plasmid transformants were identical to the original pHGZ404 plasmid. This confirms our previous finding that the original plasmid can be rescued from moss transformed with a circular plasmid (Murén et al., 2009), and suggests that this is a general result that is not dependent on the marker used for selection, which will facilitate further work with shuttle plasmids in moss. In contrast, the three plasmids rescued from one of the linearized plasmid transformants all had internal deletions, which had been generated by recombination between micro-homologies within the plasmid (Figure 14). This is also consistent with our previous finding that linearized plasmids are re-ligated in planta by direct repeat recombination between short micro-homologies on the plasmid (Murén et al., 2009).
The frequency of plasmid rescue was lower than previously observed (Murén et al., 2009) for both types of transformants. A possible reason is the presence of the two NOS1 terminators in pHGZ404, which recombine during concatemer formation in planta. Such recombination will unlink the PpHIS3 marker, which is being selected for, from the plasmid backbone that carries the bacterial origin of replication and the ampicillin resistance gene. Consistent with this, we found that the nptII marker was present in variable amounts, and was completely lost in some transformants (Figure 12). We note that none of the plasmids rescued from the linearized plasmid transformants consisted of just the re-ligated plasmid backbone. This suggests that end-to-end ligation is rare in planta, and that recombination and concatemer formation is the major pathway for processing of linearized DNA in moss. However, we rescued only three plasmids from the linear transformants, and more plasmids need to be examined before this type of event can be ruled out.
It should be emphasized that our ability to rescue circular plasmids identical to the original plasmid back into E. coli does not necessarily mean that the plasmid replicated as a single copy circular plasmid in planta. As previously discussed (Murén et al., 2009) it is conceivable that the transformed DNA replicates as a concatemer from which single copy plasmids pop out by homologous recombination. What is important is not how the transformed plasmid replicates but that it can be rescued back into E. coli without rearrangements or deletions since this makes it possible to use molecular genetics methods based on shuttle plasmids in moss.
Use of Shuttle Plasmids in Moss Molecular Genetics
The fact that we could complement the PpHIS3Δ knockout mutant with PpHIS3 expressed from a plasmid implies that cloning of genes and cDNAs by complementation from plasmid libraries is possible in Physcomitrella. This is very interesting since it would not just enable cloning of novel genes by complementation, but may also make dosage suppressor screens with plasmid libraries possible, just as in yeast. By transforming a Physcomitrella mutant with a cDNA library and screening for plasmids capable of suppressing the phenotype of the mutant, one might thus isolate functionally related genes. This is a powerful molecular genetic tool that is used extensively in yeast genetics (Rine, 1991), but its use in other organisms has been hampered by the lack of suitable shuttle plasmids. Dosage suppressor screens in Physcomitrella would make it possible to study plant specific genes and functions, such as photosynthesis, that are impossible to study in yeast.
There are also other methods from yeast molecular genetics that could be adapted for use in moss. One such method is plasmid shuffling, in which a plasmid with an essential wild type gene is replaced by a plasmid carrying a conditional mutant allele of the same gene. Plasmid shuffling enables easy selection of new temperature-sensitive mutations and other conditional mutations in essential genes. This method relies on the ability to select against a marker on the first plasmid, so that its loss is forced. The most commonly used negative selection marker in yeast is the URA3 gene, which can be selected both for, on uracil-less medium, and against, on 5-fluoroorotic acid containing medium. In the Physcomitrella genome there are two URA3 orthologs. It would therefore be interesting to make a double knockout of the two moss URA3 orthologs, and test if a PpURA3 gene on a plasmid can be used for both positive and negative selection. Other possible auxotrophic markers are the orthologs of the yeast ADE2 and LEU2 genes, both of which exist in single copies in Physcomitrella.
Conclusion
Adapting methods and tools from yeast to the model plant Physcomitrella holds great promise. With the successful complementation of the PpHIS3Δ knockout strain we are one step closer to a working moss shuttle vector system. This will facilitate the adaption of additional tools and methods from yeast to moss and thus expand the boundaries for what is currently possible in plant research. Finally, we note that these methods also could be used in other plants where homologous recombination is efficient, such as the moss Ceratodon purpureus (Brücker et al., 2005; Trouiller et al., 2009).
Statements
Author contributions
MU and HR conceived the study, designed the experiments, and wrote the manuscript. MU, G-ZH, and MJ did the experimental work. All four authors read and approved the manuscript.
Funding
This work was supported by grants to HR from the Swedish Research Council VR (621-2011-6004) and the Knut and Alice Wallenberg Foundation (KAW 2015.0056).
Acknowledgments
We thank Mitsuyasu Hasebe for providing us with the pCMAK1 plasmid.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2017.01850/full#supplementary-material
Abbreviations
- IAA
indole-3-acetic acid
- PCR
polymerase chain reaction
- Pp
Physcomitrella patens
- Sc
Saccharomyces cerevisiae
References
1
AshtonN. W.ChampagneC. E. M.WellerT.VerkoczyL. K. (2000). The bryophyte Physcomitrella patens replicates extrachromosomal transgenic elements.New Phytol.146391–402. 10.1046/j.1469-8137.2000.00671.x
2
AshtonN. W.CoveD. J. (1977). The isolation and preliminary characterisation of auxotrophic and analogue resistant mutants of the moss, Physcomitrella patens.Mol. Gen. Genet.15487–95. 10.1007/BF00265581
3
AshtonN. W.GrimsleyN. H.CoveD. J. (1979). Analysis of gametophytic development in the moss, Physcomitrella patens, using auxin and cytokinin resistant mutants.Planta144427–435. 10.1007/BF00380118
4
BeggsJ. D. (1978). Transformation of yeast by a replicating hybrid plasmid.Nature275104–109. 10.1038/275104a0
5
BrückerG.MittmannF.HartmannE.LamparterT. (2005). Targeted site-directed mutagenesis of a heme oxygenase locus by gene replacement in the moss Ceratodon purpureus.Planta220864–874. 10.1007/s00425-004-1411-6
6
CoveD. (2005). The moss Physcomitrella patens.Annu. Rev. Genet.39339–358. 10.1146/annurev.genet.39.073003.110214
7
DellaportaS. L.WoodJ.HicksJ. B. (1983). A plant DNA minipreparation: version II.Plant Mol. Biol. Rep.119–21. 10.1007/BF02712670
8
HiwatashiY.ObaraM.SatoY.FujitaT.MurataT.HasebeM. (2008). Kinesins are indispensable for interdigitation of phragmoplast microtubules in the moss Physcomitrella patens.Plant Cell203094–3106. 10.1105/tpc.108.061705
9
HommelsheimC. M.FrantzeskakisL.HuangM.ÜlkerB. (2014). PCR amplification of repetitive DNA: a limitation to genome editing technologies and many other applications.Sci. Rep.4:5052. 10.1038/srep05052
10
IshikawaM.MurataT.SatoY.NishiyamaT.HiwatashiY.ImaiA.et al (2011). Physcomitrella cyclin-dependent kinase A links cell cycle reactivation to other cellular changes during reprogramming of leaf cells.Plant Cell232924–2938. 10.1105/tpc.111.088005
11
JangG.DolanL. (2011). Auxin promotes the transition from chloronema to caulonema in moss protonema by positively regulating PpRSL1 and PpRSL2 in Physcomitrella patens.New Phytol.192319–327. 10.1111/j.1469-8137.2011.03805.x
12
KamisugiY.CumingA. C.CoveD. J. (2005). Parameters determining the efficiency of gene targeting in the moss Physcomitrella patens.Nucleic Acids Res.33e173. 10.1093/nar/gni172
13
KamisugiY.SchlinkK.RensingS. A.SchweenG.von StackelbergM.CumingA. C.et al (2006). The mechanism of gene targeting in Physcomitrella patens: homologous recombination, concatenation and multiple integration.Nucleic Acids Res.346205–6214. 10.1093/nar/gkl832
14
KasaharaM.KagawaT.SatoY.KiyosueT.WadaM. (2004). Phototropins mediate blue and red light-induced chloroplast movements in Physcomitrella patens.Plant Physiol.1351388–1397. 10.1104/pp.104.042705
15
KnightC. D. (1994). Studying plant development in mosses: the transgenic route.Plant Cell Environ.17669–674. 10.1111/j.1365-3040.1994.tb00158.x
16
LangD.ZimmerA. D.RensingS. A.ReskiR. (2008). Exploring plant biodiversity: the Physcomitrella genome and beyond.Trends Plant Sci.13542–549. 10.1016/j.tplants.2008.07.002
17
LastR. L.BissingerP. H.MahoneyD. J.RadwanskiE. R.FinkG. R. (1991). Tryptophan mutants in Arabidopsis: the consequences of duplicated tryptophan synthase beta genes.Plant Cell3345–358. 10.1105/tpc.3.4.345
18
LastR. L.FinkG. R. (1988). Tryptophan-requiring mutants of the plant Arabidopsis thaliana.Science240305–310. 10.1126/science.240.4850.305
19
MurallaR.SweeneyC.StepanskyA.LeustekT.MeinkeD. (2007). Genetic dissection of histidine biosynthesis in Arabidopsis.Plant Physiol.144890–903. 10.1104/pp.107.096511
20
MurénE.NilssonA.UlfstedtM.JohanssonM.RonneH. (2009). Rescue and characterization of episomally replicating DNA from the moss Physcomitrella.Proc. Natl. Acad. Sci. U.S.A.10619444–19449. 10.1073/pnas.0908037106
21
NakagawaT.KuroseT.HinoT.TanakaK.KawamukaiK.NiwaY.et al (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation.J. Biosci. Bioeng.10434–41. 10.1263/jbb.104.34
22
NishiyamaT.FujitaT.Shin-IT.SekiM.NishideH.UchiyamaI.et al (2003). Comparative genomics of Physcomitrella patens gametophytic transcriptome and Arabidopsis thaliana: implication for land plant evolution.Proc. Natl. Acad. Sci. U.S.A.1008007–8012. 10.1073/pnas.0932694100
23
NishiyamaT.HiwatashiY.SakakibaraK.KatoM.HasebeM. (2000). Tagged mutagenesis and gene-trap in the moss Physcomitrella patens by shuttle mutagenesis.DNA Res.79–17. 10.1093/dnares/7.1.9
24
OlssonT.ThelanderM.RonneH. (2003). A novel type of chloroplast stromal hexokinase is the major glucose-phosphorylating enzyme in the moss Physcomitrella patens.J. Biol. Chem.27844439–44447. 10.1074/jbc.M306265200
25
Orr-WeaverT. L.SzostakJ. W.RothsteinR. J. (1981). Yeast transformation: a model system for the study of recombination.Proc. Natl. Acad. Sci. U.S.A.786354–6358.
26
RensingS. A.LangD.ZimmerA. D.TerryA.SalamovA.ShapiroH.et al (2008). The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants.Science31964–69. 1126/science.1150646
27
RineJ. (1991). Gene overexpression in studies of Saccharomyces cerevisiae.Methods Enzymol.194239–251.
28
SchaeferD. G. (1994). Molecular Genetic Approaches to the Biology of the Moss Physcomitrella Patens.Ph.D. thesis, Université de Lausanne, Lausanne.
29
SchaeferD. G. (2002). A new moss genetics: targeted mutagenesis in Physcomitrella patens.Annu. Rev. Plant Biol.53477–501. 10.1146/annurev.arplant.53.100301.135202
30
SchaeferD. G.DelacoteF.CharlotF.VrielynckN.Guyon-DebastA.Le GuinS.et al (2010). RAD51 loss of function abolishes gene targeting and de-represses illegitimate integration in the moss Physcomitrella patens.DNA Repair9526–533. 10.1016/j.dnarep.2010.02.001
31
SchaeferD. G.ZrÿdJ.-P. (1997). Efficient gene targeting in the moss Physcomitrella patens.Plant J.111195–1206. 10.1046/j.1365-313X.1997.11061195.x
32
SchaeferD. G.ZrÿdJ.-P.KnightC.CoveD. (1991). Stable transformation of the moss Physcomitrella patens.Mol. Gen. Genet.226418–424. 10.1007/BF00260654
33
SherwinJ. E.PurvesW. K. (1969). Tryptophan as an auxin precursor in cucumber seedlings.Plant Physiol.441303–1309. 10.1104/pp.44.9.1303
34
StinchcombD. T.StruhlK.DavisR. W. (1979). Isolation and characterisation of a yeast chromosomal replicator.Nature28239–43. 10.1038/282039a0
35
ThelanderM.NilssonA.OlssonT.JohanssonM.GirodP.-A.SchaeferD. G.et al (2007). The moss genes PpSKI1 and PpSKI2 encode nuclear SnRK1 interacting proteins with homologues in vascular plants.Plant Mol. Biol.64559–573. 10.1007/s11103-007-9176-5
36
TrouillerB.CharlotF.ChoinardS.SchaeferD. G.NoguéF. (2009). Comparison of gene targeting efficiencies in two mosses suggests that it is a conserved feature of Bryophyte transformation.Biotechnol. Lett.291591–1598. 10.1007/s10529-007-9423-5
37
Van CraenenbroeckK.VanhoenackerP.HaegemanG. (2000). Episomal vectors for gene expression in mammalian cells.Eur. J. Biochem.267k5665–5678. 10.1046/j.1432-1327.2000.01645.x
38
VieiraJ.MessingJ. (1987). Production of single-stranded plasmid DNA.Methods Enzymol.1533–11. 10.1016/0076-6879(87)53044-0
39
WuS. Z.RitchieJ. A.PanA. H.QatranoR. S.BezanillaM. (2011). Myosin VIII regulates protonemal patterning and developmental timing in the moss Physcomitrella patens.Mol. Plant.4909–921. 10.1093/mp/ssr068
40
ZhaoY.HullA. K.GuptaN. R.GossK. A.AlonsoJ.EckerJ. R.et al (2002). Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3.Genes Dev.163100–3112. 10.1101/gad.1035402
Summary
Keywords
auxotrophic selection markers, complementation, imidazoleglycerol-phosphate dehydratase, phosphoribosylanthranilate isomerase, Physcomitrella patens, plasmid rescue, shuttle plasmid
Citation
Ulfstedt M, Hu G-Z, Johansson M and Ronne H (2017) Testing of Auxotrophic Selection Markers for Use in the Moss Physcomitrella Provides New Insights into the Mechanisms of Targeted Recombination. Front. Plant Sci. 8:1850. doi: 10.3389/fpls.2017.01850
Received
11 July 2017
Accepted
11 October 2017
Published
03 November 2017
Volume
8 - 2017
Edited by
Abd El-Latif Hesham, Assiut University, Egypt
Reviewed by
Andrew Wood, Southern Illinois University Carbondale, United States; Keiko Sakakibara, Rikkyo University, Japan; Mingfeng Cao, Iowa State University, United States
Updates
Copyright
© 2017 Ulfstedt, Hu, Johansson and Ronne.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hans Ronne, hans.ronne@slu.se
This article was submitted to Technical Advances in Plant Science, a section of the journal Frontiers in Plant Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.