- 1The New Zealand Institute for Plant and Food Research Limited, Palmerston North, New Zealand
- 2Institute of Fundamental Sciences, Massey University, Palmerston North, New Zealand
- 3The New Zealand Institute for Plant and Food Research Limited, Hamilton, New Zealand
The signal that initiates the age-regulated senescence program in flowers is still unknown. Here we propose for the ephemeral Arabidopsis thaliana flower that it dies because of continued expression of the MADS-box transcription factor AGAMOUS (AG). AG is necessary for specifying the reproductive structures of the flower. Flowers of ag-1, which lack AG, exhibited delayed sepal senescence and abscission. The flowers also had reduced jasmonic acid (JA) content. Other anther-defective sterile mutants deficient in JA, defective in anther dehiscence 1 (dad1) and delayed dehiscence 2 (dde2), exhibited delayed sepal senescence and abscission as well. Manually pollinated dad1 flowers produced siliques but still had delayed senescence, demonstrating that absence of pollination does not cause delayed senescence. When ag-1, dad1 and dde2 flowers were sprayed with 100 μM methyl jasmonate, the sepal senescence and abscission phenotypes were rescued, suggesting that JA has a role in these processes. Our study uncovers a novel role for AG in determining the timing of death of the flower it helps develop and highlights a role for JA in sepal senescence.
Introduction
Flower longevity varies widely among species. Some flowers last only a few hours before dying, whereas others may persist for several months before they senesce (Stead, 1992). It is still unknown how senescence is initiated in flowers (van Doorn and Woltering, 2008). In some flowers ethylene is important (Woltering and van Doorn, 1988), with the hormone being produced in the gynoecium following pollination (Hunter et al., 2004; van Doorn and Woltering, 2008). Other flowers senesce independently of pollination and an associated ethylene burst (van Doorn, 1997). For ephemeral flowers (flowers lasting less than a day), no clear effect of pollination on longevity has been found (van Doorn, 1997).
MADS-box proteins are transcription factors, which have been integral to the evolution of complexity and reproductive fitness in plants (Smaczniak et al., 2012). Phylogenetic analyses of MADS-box genes in green algae suggested that they first appeared ∼500 million years ago in branched filamentous charophycean algae, from which land plants evolved (Tanabe et al., 2005). They bind to promoter DNA sequences, having high similarity with the motif CC(A/T)6GG known as the CArG-box (Theissen, 2001). MADS-box proteins regulate many plant-developmental processes including flower senescence, floral organ specification, gametophyte, embryo and seed development (Smaczniak et al., 2012; Chen et al., 2015). They also influence two age-related maturity transitions in plant tissues that are essential for the plant to complete its life cycle successfully: the juvenile-to-adult (vegetative phase) and adult-to-reproductive (flowering phase) transitions (Deng et al., 2011; Huijser and Schmid, 2011).
The MADS-box family of proteins are perhaps best known for their role in specifying floral identity in the ABCDE model of flower development. In this model floral homeotic genes are classified into five functional classes (A-E). Class A genes are APETALA1 and APETALA2, B are APETALA3 and PISTILLATA, C is AGAMOUS, D are FLORAL-BINDING PROTEIN 7 and 11, and E are SEPALLATA1-4 (Pelaz et al., 2000; Honma and Goto, 2001; Smaczniak et al., 2012). The identities of the four whorls of the flower are governed by the overlapping expression of the above-mentioned MADS-box genes. Mutations in these genes result in floral homeotic abnormalities (Irish and Sussex, 1990). For example, sepals are transformed into leaf-like organs when A class activity of APETALA1 and APETALA2 is lost.
Results from ectopic expression studies of MADS box genes suggest that this family of transcription factors can also control the timing of flower senescence. For example, ectopically expressed AGAMOUS-LIKE 15 (AGL15) and AGL18 delay sepal senescence and perianth abscission in Arabidopsis thaliana (Arabidopsis) (Fernandez et al., 2000; Adamczyk et al., 2007). Similarly, when AGL42 was constitutively expressed in Arabidopsis, floral senescence was delayed through suppression of components in the ethylene signaling pathway (Chen et al., 2011, 2015). Thus, MADS-box proteins are not only involved in specifying the complex architecture of plants, but may also be involved in controlling their eventual fate.
The MADS-box gene AG was identified by Yanofsky et al. (1990) after a T-DNA insertion mutant was found to display a similar phenotype to the ethyl methanesulfonate mutant ag-1, first described by Koornneef et al. (1983). In addition to its well-described role in specifying stamen organ identity in floral primordia, it is now known that AG continues to be expressed in stamens and directly activate the jasmonic acid (JA) biosynthesis gene, DEFECTIVE IN ANTHER DEHISCENCE 1 (DAD1) (Ito et al., 2007). Ishiguro et al. (2001) suggested that JA drives anther development and floral petal and sepal expansion by regulating water transport in stamens and petals.
Senescence functions to ensure efficient removal of nutrients from dying parts of the plant to the flowers, fruit and grains (Gan and Amasino, 1997). Better understanding of the key regulators of senescence will help to maximize crop yield and improve quality of fruits, vegetables and cut flowers. The role of endogenous JA in controlling age-related senescence is not clear (Jibran et al., 2013). For although senescence of detached leaves in many plant species is accelerated by exogenously applied methyl jasmonate (MeJA) or JA, leaf senescence is not delayed in JA biosynthesis mutants such as allene oxide synthase (aos)/delayed dehiscence 2 (dde2) (Park et al., 2002) and oxophytodienoate reductase 3 (opr3) (Schommer et al., 2008). Perianth abscission does, however, appear to be regulated by JA in Arabidopsis (Kim et al., 2013).
Here we propose an additional role for AGAMOUS in flower development, suggesting that it is key for controlling lifespan of the flower. In our model, AGAMOUS specifies stamen and carpel identity and later controls senescence of the flower by directly activating DAD1 expression in maturing stamens to produce JA.
Materials and Methods
General Plant Growth Conditions
Seeds were stratified at 4°C in the dark for 3–4 days. Mutants and wild type seeds of Ler-0 and Col-0 were sown in wet seed Raising MixTM (Oderings, New Zealand) in a temperature-controlled growth chamber (20–22°C, 16-h/8-h light/dark cycle, white light ∼180 μE and 60% relative humidity, unless otherwise stated). The T-DNA insertion mutants dad1 (SALK_025432.28.25.X), transposon insertion dde2 (CS65993), and EMS mutant ag-1 (CS19985) were purchased from The Arabidopsis Information Resource1.
Phenotypic Analysis
Sepal senescence and abscission phenotypes of the mutant plants were compared with their respective wild types. To study senescence and abscission events, flower positions were defined according to the numbering system of Bleecker and Patterson (1997), which identifies flower position 1 as the top-most flower with visible white petals. In addition, sepal senescence and abscission was described using the developmental stages of flowers according to Alvarez-Buylla et al. (2010).
To quantify the onset of sepal senescence for Figures (Figures 4B, 5B and Supplementary Figures S1–S5) the flowers of a single inflorescence were placed on a black background and photographed. The photograph was subsequently analyzed using Image J software. In each photograph, all buds/flowers were placed linearly in order from youngest to oldest. Five contiguous flowers were identified in this ordered set defined by the sepals of the first flower (Flo1) being completely green and the sepals of the last flower (Flo5) being completely yellow. The onset of senescence in the five flowers was then determined by the following method, for those flowers exhibiting yellowing during the time period the experiment was performed.
The color intensity of the sepals from each of the five candidate florets was quantified by recording mode values in Image J. The process for obtaining mode values for each sepal was as follows: (1) The photograph containing the five flowers was imported into Image J and converted into a red spectrum channel/ multi-channel composite image. (2) The sepal area of each flower was selected using polygon selection. (3) The color intensity of this selected area was analyzed with the histogram function. The peak of the histogram represented the intensity of the color present in the area. The mode value of the histogram peak was used as a quantitative measure of the color for sepals of each flower.
Once the mode values were acquired, the colour difference ratio (CDR) of the sepals of adjacent flowers was measured, i.e., this was a measure of the change in color between the developmental stages of adjacent flowers. For example, CDR1 = Mode value of Flo1 sepal/Mode value of Flo2 sepal. For a set of five flowers, four different CDR values were generated from which the maximum CDR value was considered to be the point of onset of sepal senescence, i.e., the maximum change in color from green to yellow.
Jasmonic Acid Treatment
Six and eight-week-old plants were grown in long-day conditions (16-h light and 8-h dark) in the plant house and sprayed with 100 μM MeJA (Sigma–Aldrich NZ), dissolved in 1% (v/v) ethanol, each day for 3 days. Sepal senescence and abscission events were visually noted for the flowers from primary inflorescences of developmental stage 13, when the petals become visible between the sepals and continue to elongate rapidly (Alvarez-Buylla et al., 2010). This stage is before flower developmental stage 14 when the flower opens after pollination. For determining the effect of MeJA treatment on sepal senescence and abscission, flowers of 6-week-old plants at stage 13 were tagged with a cotton thread at day 0 of treatment and these tagged flowers were designated as ‘T’. The change in color from green to yellow of the T flowers and the flowers adjacent to them was analyzed for sepal senescence and abscission phenotypes 3 days following the MeJA or mock treatments [1% (v/v) ethanol]. The adjacent flowers were designated either positive or negative depending on their position relative to that of the tagged flower ‘T’, i.e., flower T-1 was the younger flower adjacent to flower T and was located above the T flower on a stalk, while flower T+1 was the older flower adjacent to flower T and was located below the T flower.
Ethylene Measurement
Ethylene was quantified with a laser-based ETD-300 ethylene detector (Sensor Sense BV, Nijmegen, The Netherlands). The stop and flow method was used to measure ethylene. In this method, selected cuvettes (2 mL screw-capped closed tubes) were first flushed with air for 30 min and then the detached primary inflorescences (∼100 mg), from 8-week-old Ler-0 and ag-1 plants, placed in the tubes. Two needles (serving as inlet and outlet) were inserted into the top of the microfuge tube and instrument tubing fitted to the needles. Air that had passed through a catalyser to remove hydrocarbons entered the cuvette (through the inlet) and then carried the ethylene produced by the tissue to a scrubber (to remove water and CO2) and then onto the ethylene detector.
Hormone Measurements
A 1000 μg mL-1 stock solution of JA, abscisic acid (ABA), salicylic acid (SA), and 1-aminocyclopropane-1-carboxylic acid (ACC) was prepared in methanol. A 10 μg mL-1 labeled internal standard stock solution of the isotopically labeled analogs, [2H5]JA, [2H4]SA and [2H6]ABA, was also prepared in methanol. A set of 0.1, 1, 10, 100, and 1000 ng mL-1 calibration standards of JA, SA, ABA, and ACC were made by diluting each stock solution with 1:1 methanol:water. The labeled internal standard solution mix (25 ng) was aliquoted into 400 μL of each calibration standard. Twenty five ng of the labeled internal standard solution mix and 400 μL of the extraction solvent (20:79:1 methanol:isopropanol:acetic acid) was added to 110 mg FW of freeze-dried tissue kept on ice. Each of the samples were then shaken for 30 min at 4°C and stored overnight -20°C. Next morning the samples were centrifuged at 13,000 × g for 10 min at 4°C and then 100 μL of the supernatant diluted 10-fold with water and analyzed by Liquid Chromatography Mass Spectrometry (LC-MS).
LC-MS analysis was performed using a 5500 QTrap triple quadrupole/linear ion trap (QqLIT) mass spectrometer fitted with a TurboIon-SprayTM interface (AB Sciex, Concord, ON, Canada) and Ultimate 3000 UHPLC (Dionex, Sunnyvale, CA, United States). A kinetex column (Phenomenex, Torrance, CA, United States) at 60°C was used for the chromatographic separation. Water +1% formic acid (A) and acetonitrile +0.1% formic acid (B) were used as solvents at flow rate of 600 μL min-1. To identify JA, SA, and ABA, the initial mobile phase 15% B was ramped linearly to 50% B at 8 min, then ramped to 100% B at 8.1 min before being held for 2 min and resetting to the original conditions. An injection volume of 50 μL was used. Data was collected in the negative mode by using a multiple reaction monitoring (MRM) method. To analyze ACC, the initial mobile phase 10% B was ramped linearly to 50% B at 10 min before being held for 2 min and resetting to the original conditions. Data was collected in the positive mode using a multiple reaction monitoring (MRM) method. The Q1 and Q3 transitions monitored together with their optimized parameters (declustering potential (DP), entrance potential (EP), collision energy (CE) and collision cell exit potential (CXP) are listed in the Supplementary Table S1.
Additional operating parameters were: dwell time of 60 ms, ionspray voltage of -4500 V for negative mode and 4500 V for positive mode, temperature at 600°C, curtain gas of 45 psi, ion source gas 1 of 60 psi, ion source gas 2 of 60 psi. JA, SA, and ABA was quantified by the internal standard ratio method and ACC by external calibration.
Chlorophyll Measurement
The chlorophyll concentration of detached uncrushed inflorescences and sepals was measured by holding the tissue in 96% (v/v) ethanol (10 mg of tissue per 30 μL of ethanol) for 4 days in the dark at 4°C. An aliquot of 2 μL of the supernatant was measured with a Nanodrop ND-1000 spectrophotometer using absorbances at wavelengths 649 and 665 nm and calculations were performed according to Wintermans and De Mots (1965).
The chlorophyll content of each line or treatment from day 0 to the other days was compared with that of wild type. Student’s t-test was applied for statistical analysis of the regression parameter estimates.
Results
ag-1 Flowers Show Delayed Sepal Yellowing and Abscission
Flower opening of wild type Ler-0 Arabidopsis plants was first initiated after ∼ 5 weeks of growth that is almost similar to the time of first flower opening in Col-0 as described by Boyes et al. (2001). After 6 weeks, wild type Ler-0 plants displayed many siliques with senesced and abscised floral organs, whereas none of the ag-1 flowers (defective in AGAMOUS) showed senescence or floral organ abscission (Supplementary Figures S1, S2). Furthermore, it was clear that opening of ag-1 flowers was delayed with petals not clearly expanding out of bud until their pedicels were well clear of the compressed inflorescence (Supplementary Figure S1). This was consistent with what has been previously reported by Ishiguro et al. (2001). However, by 8 weeks, the flowers of the ag-1 inflorescences did not appear to be delayed in opening as the plants had many opened flowers within the compressed inflorescence (Figure 1B).
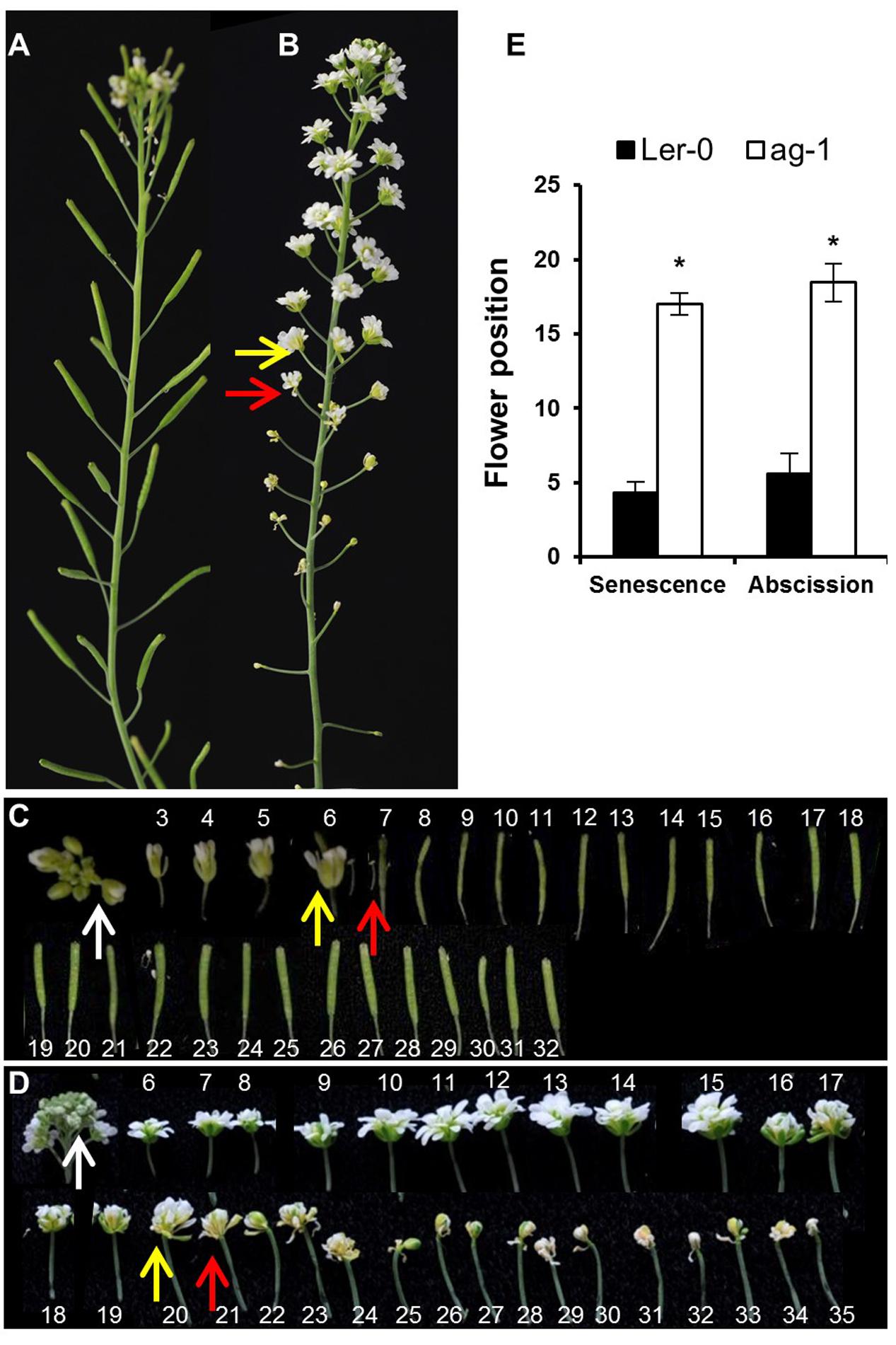
FIGURE 1. Yellowing and abscission of sepals in Ler-0 and ag-1 plants. (A) 8 week-old Ler-0 plant. (B) 8 week-old ag-1 plant. (C) Ler-0 flowers detached from 8 week-old plant. (D) ag-1 flowers detached from 8 week-old plant. (E) Position of flowers showing yellowing and abscission in 8 week-old Ler-0 and ag-1 plants. Plants were grown under long-day conditions for 8 weeks in a growth room (20–22°C, 16-h light and 8-h dark cycle, white light ∼180 μE and 60% relative humidity) and flowers were detached and subsequently photographed. The yellow and red arrows indicate flowers with senesced and abscised sepals, respectively. The white arrows indicate the flowers used for quantifying delayed sepal senescence for Figure 2D. The flowers are numbered according to the numbering system of Bleecker and Patterson (1997). The numbers in (C,D) indicate the positions of the flowers/siliques along the flowering stalk: flower 1 is the first opened flower present at the top, whereas the high numbers are the flowers/siliques present at the bottom of the stalk. The ag-1 flower is composed of a repeating series of sepal-petal-petal and when whorls of petals abscises, the inner sepal whorl, green in color, appears. This can be seen by reduction in the ag-1 flower size after flower position 20 in (D). The photographs shown are representative of six to eight biological replicates. The error bars indicate standard errors of the mean of eight biological replicates. ∗Indicates statistically significant (p < 0.05) between Ler-0 and ag-1 using Student’s t-test.
Arabidopsis flowers of 8-week-old ag-1 plants (grown in long-day conditions) exhibited a dramatic delay in sepal yellowing and abscission compared with the wild type Ler-0 (Figure 1). Sepal yellowing occurred at flower positions 3 to 7 in the wild type (Figures 1A,C,E) and position 11 to 24 in ag-1 (Figures 1B,D,E) according to the numbering system of Bleecker and Patterson (1997), which defines flower position 1 as the top-most flower with visible white petals. The ag-1 sepals did not abscise until flower positions 12–22 compared with flower positions 4 to 8 of the wild type (Figures 1A,C,D). Thus, lack of AG activity caused delayed sepal yellowing and abscission.
To quantify the difference in yellowing between ag-1 and wild type and determine if the differences can be seen at the early stages of flower development, chlorophyll concentration was measured in the sepals taken from wild type and ag-1 newly opened flowers (flower position 2, indicated by the white arrows in Figures 1C,D) and comparing it to the chlorophyll concentration of sepals from the outermost buds of unopened inflorescences (indicated by white arrows in Figures 2A,B). Sepal chlorophyll concentration of the ag-1 unopened outermost bud was not significantly different from that of wild type (Figure 2C). However, ag-1 sepals from opened flowers showed a reduced loss in chlorophyll (∼6%) compared with the wild type (∼40%) (Figure 2D). This suggested that lack of AGAMOUS activity in ag-1 affected chlorophyll degradation but not accumulation or synthesis.
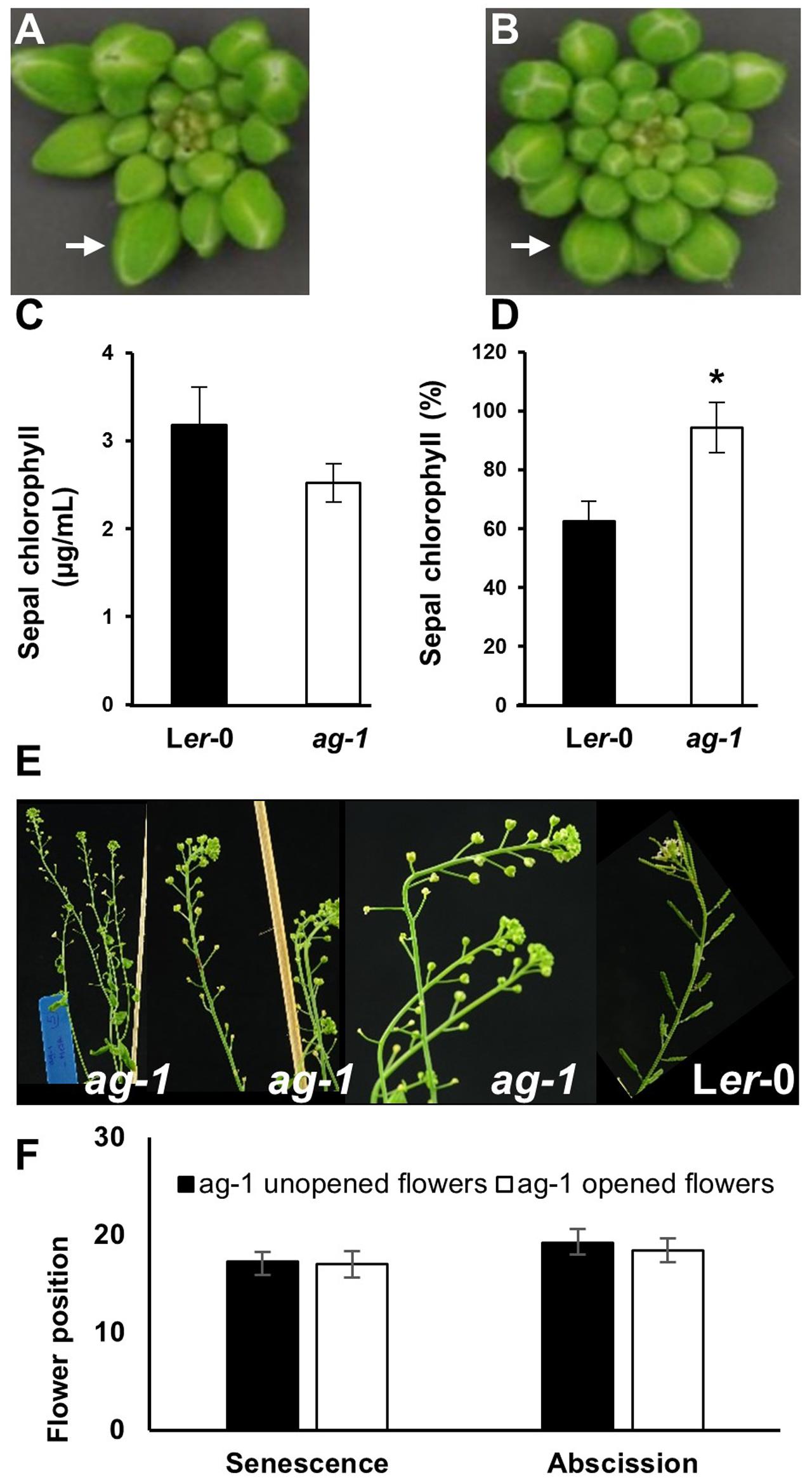
FIGURE 2. Sepal Yellowing of ag-1 and Ler-0 flowers. (A) Ler-0 inflorescence. (B) ag-1 inflorescence. (C) Chlorophyll concentration of Ler-0 and ag-1 sepals obtained from outermost buds. (D) Percentage chlorophyll of Ler-0 and ag-1 sepals at flower position 2 (indicated by white arrows in Figures 1C,D) compared with that in sepals from unopened flowers of the outer whorl shown here in (A,B), indicated by white arrows. Sepal chlorophyll percentage was calculated by comparing the chlorophyll concentration of sepals from flowers at position 2 to that in sepals from unopened flowers. Plants were grown under long-day conditions for 8 weeks as described in Section Materials and methods. (E) 8-week-old ag-1 plants with un-opened flowers. (F) Position of flowers showing yellowing and abscission in 8 week-old-ag-1 plants with opened and un-opened flowers. 8-week-old ag-1 plants with un-opened flowers were grown in a growth chamber with16-h-light/8-h-dark cycle, 100 μmol m-2 s-1 white light, and 60% relative humidity. The flowers are numbered according to the numbering system of Bleecker and Patterson (1997). The error bars indicate standard error of the means of six biological replicates. ∗Indicates statistically significant (p < 0.05) between the wild type (Ler-0) and ag-1 using Student’s t-test.
When ag-1 plants were grown in a different environment at a different time, the flowers of older plants failed to open completely (Figures 2E,F). The reason for this is unclear, but despite this, the sepals of unopened ag-1 flowers yellowed and abscised at similar flower positions (Figures 2E,F) as observed for sepals of ag-1 flowers that opened (Figures 1B,D,E). Thus flower opening is not a prerequisite for sepal yellowing and abscission of ag-1 flowers; rather flower opening and sepal senescence and abscission appeared to be independently regulated.
ag-1 Inflorescences Have Less JA than the Wild Type
To determine whether changes in plant hormones could be responsible for the delayed sepal senescence phenotype of ag-1 plants, we measured JA, ACC (a precursor of ethylene), ABA, and SA concentrations in Ler-0 and ag-1 inflorescences (Figures 3A,B). In contrast with the wild type, JA concentration was significantly lower in ag-1 inflorescences (Figure 3A). Concentrations of ACC, ABA and SA in ag-1 inflorescences were not different from those in the wild type. Furthermore, the amount of ethylene evolved into the headspace from detached ag-1 inflorescences was not significantly different from that of wild type inflorescences (Figure 3C). This was consistent with lowered JA concentration in the inflorescences causing the delayed senescence and abscission of the ag-1 sepals.
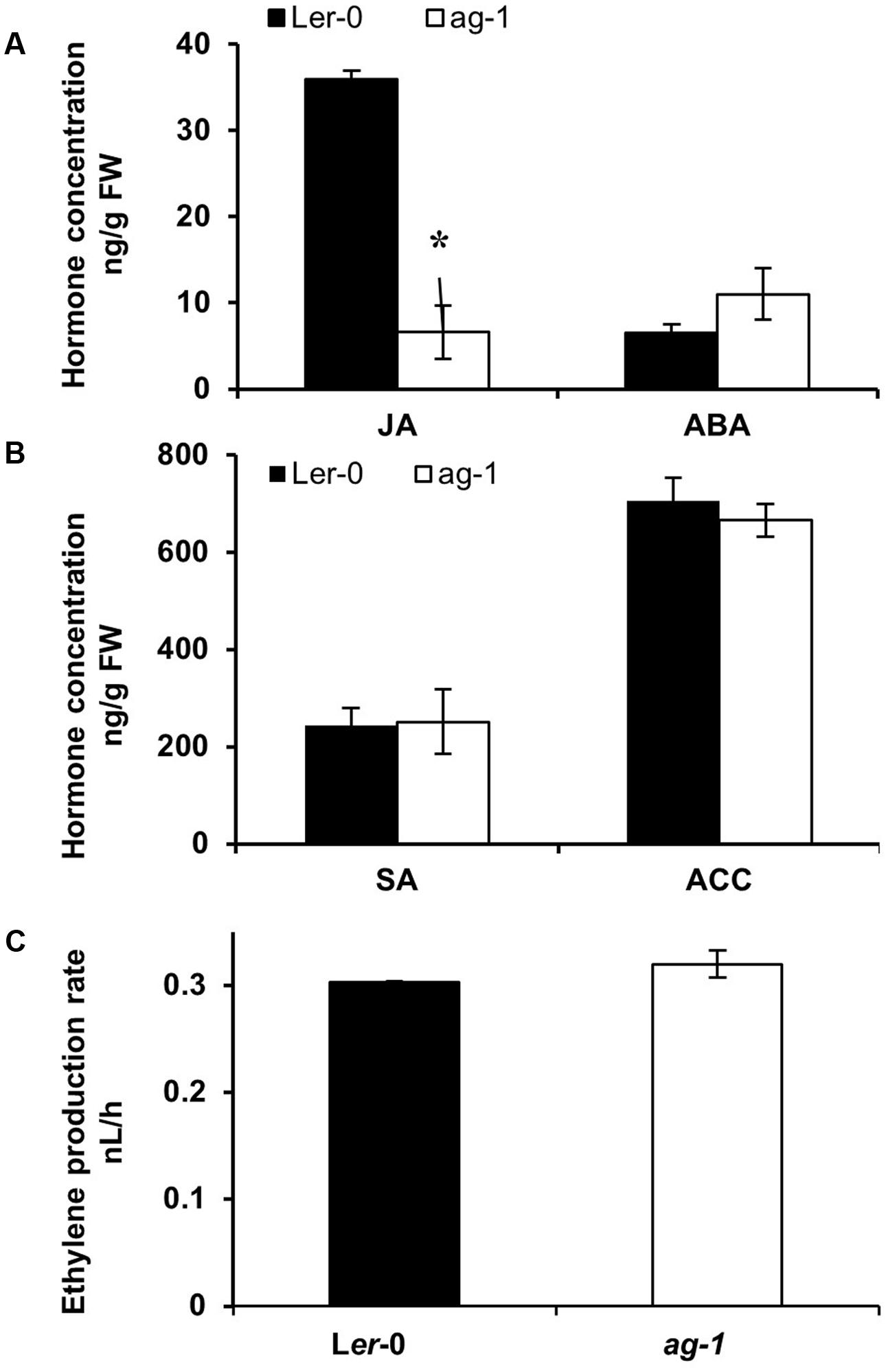
FIGURE 3. Endogenous hormone concentrations in Ler-0 and ag-1 inflorescences. (A,B) Endogenous hormone concentrations in Ler-0 and ag-1 inflorescences. (C) Ethylene evolution in detached inflorescences. Plants were grown under long-day conditions for 8 weeks and flowers were detached and sepals were snap frozen for hormone analyses. Jasmonic acid (JA), 1-Aminocyclopropane-1-carboxylic acid (ACC), Abscisic acid (ABA), and Salicylic acid (SA) concentrations were measured as described in the Section “Materials and Methods.” Ethylene evolution was measured from detached inflorescences as described in the Section “Materials and Methods.” The error bars represent the standard error of two biological replicates. ∗Indicates statistically significant (p < 0.006) between the Ler-0 and ag-1 inflorescences using Student’s t-test. FW, Fresh Weight. Student’s t-test at p < 0.05 showed that ethylene evolution between Ler-0 and ag-1 inflorescences was not significant.
Methyl Jasmonate Treatment of ag-1 Flowers Induces Sepal Yellowing and Abscission
To further investigate whether lack of JA was the cause of the ag-1 delayed sepal yellowing and abscission we tested whether exogenously applied MeJA would accelerate sepal yellowing in planta. To do this, we used 6-week-old plants because of the ability to clearly stage flowers of ag-1 and wild type. To describe the developmental stages of flowers in the following experiments we made use of the system described by Alvarez-Buylla et al. (2010). Six-week-old ag-1 and Ler-0 flowers of developmental stage 13 were tagged with cotton threads. At this stage white petals were visible among the sepals (Alvarez-Buylla et al., 2010). Plants were then sprayed with mock or MeJA solutions each day for 3 days. Three days post-treatment, sepal senescence and abscission phenotypes of the tagged flower and the four flowers surrounding the tagged flower were compared between the mock- and MeJA-treated plants of each genotype. The cotton-thread tagged flowers were designated as ‘T’ (for tagged) flowers, while the flanking two younger flowers were called T-1 and T-2 and the flanking two older flowers T+1 and T+2 (Figure 4).
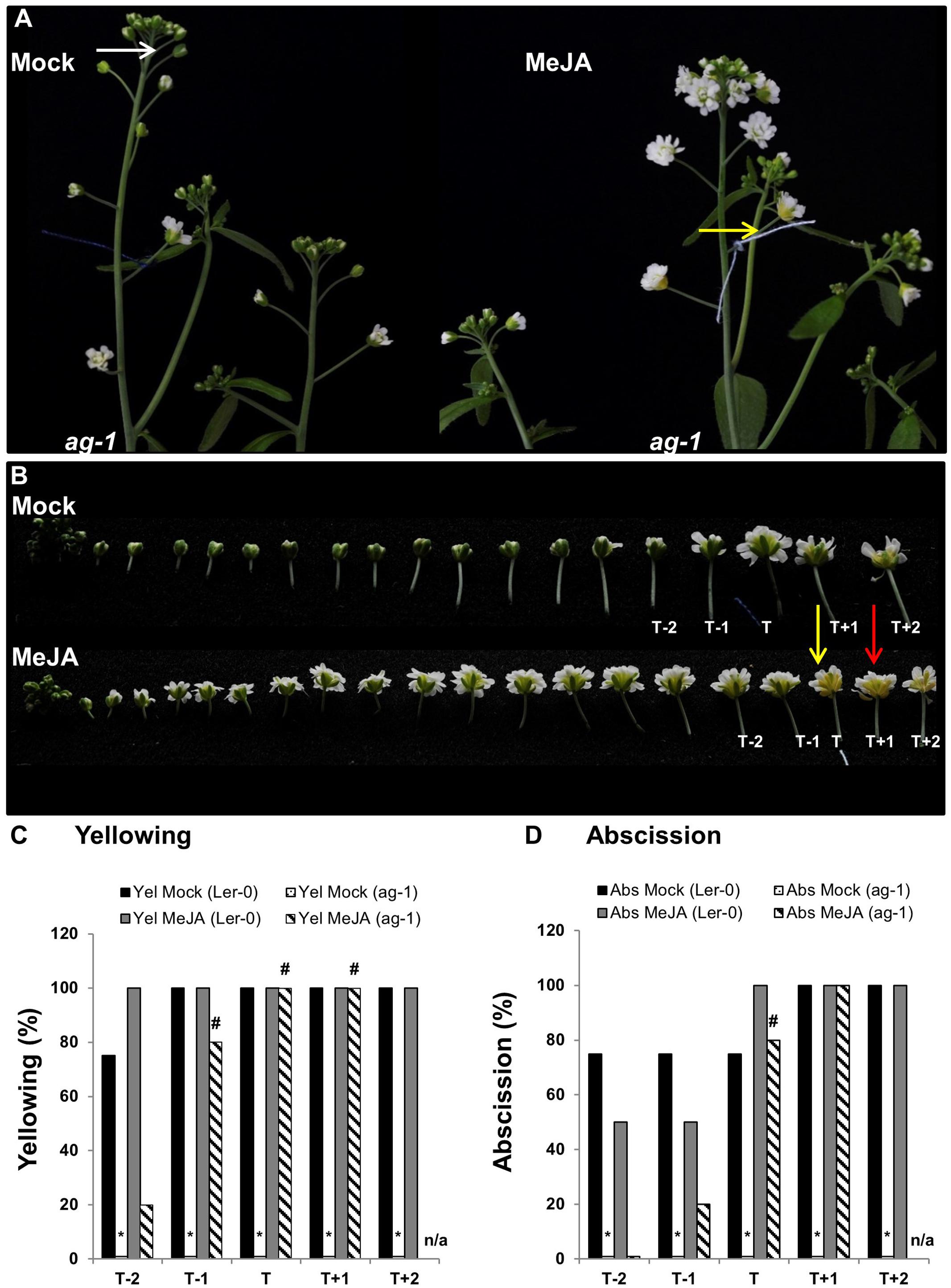
FIGURE 4. Exogenous application of MeJA to ag-1 flowers. (A) Mock- and 100 μM MeJA-treated ag-1 plants. Plants were grown under long-day conditions for 6 weeks and sprayed once a day with mock or MeJA solutions for 3 days. Flower buds with visible white petals were tagged with a cotton thread at day 0 of the MeJA or mock treatments and inflorescences were photographed after 3 days of treatment. (B) Flowers of mock- and 100 μM MeJA-treated ag-1 inflorescences were detached and photographed. (C) Percentage sepal yellowing of Mock- and MeJA-treated ag-1 and Ler-0 flowers. (D) Percentage sepal abscission of Mock- and MeJA-treated ag-1 and Ler-0 flowers. White arrow indicates floral bud at stage 13 (Alvarez-Buylla et al., 2010), yellow arrows indicate flowers with senesced sepals, and red arrow indicates a flower with abscised sepals. T represents the tagged flower, T-1 and T-2 represent flowers younger than T, T+1 and T+2 represent flowers older than T. 0% datapoints are placed slightly above the X-axis. Statistical differences between mock treated wild type and mutants (indicated by ∗) and mock- and MeJA-treated mutant plants (indicated by #) were calculated according to the Wilson score confidence limits for a percentage as calculated in Agresti and Coull, 1998 (Supplementary Table S2). The photographs shown are representatives of three (C,D; MeJA treated T+1 and T+2) or four to six biological replicates. Sen, Senescence; Abs, Abscission, n/a, not applicable.
The data showed that 100% of the T flowers on the MeJA-treated ag-1 plants showed sepal yellowing compared to 0% for the T flowers of the mock-treated ag-1 plants (Figure 4C). This difference is significant according to the Wilson score confidence limits for a percentage as calculated in Agresti and Coull (1998) (Supplementary Table S2). In addition, 20% of T-1 and 80% of the T flowers of MeJA-treated ag-1 plants also displayed sepal abscission (Figure 4D and Supplementary Figure S1). All T+1 flowers of the MeJA-treated ag-1 plants showed yellowing and abscission (Figures 4C,D). Eighty and twenty percent of the T-1 and T-2 flowers of the MeJA-treated ag-1 plants showed sepal yellowing, respectively. In comparison, none of the equivalent flowers of the mock-treated ag-1 plants had yellow or abscised sepals.
In contrast to the ag-1 flowers, no significant differences were observed in the sepal yellowing and abscission phenotypes of the T-2 to T+2 flowers of the mock- and MeJA-treated Ler-0 plants (Figures 4C,D and Supplementary Figure S2).
The data shows that exogenously applied MeJA complemented the delayed sepal yellowing and abscission phenotypes in the ag-1 plants, further supporting the suggestion that decreased JA concentrations in ag-1 sepals are responsible for the delayed sepal yellowing and abscission.
JA Deficient Mutants Show Delayed Sepal Yellowing and Abscission
To provide further evidence that the lack of JA was likely responsible for the delayed yellowing and abscission phenotypes of ag-1 flowers, we examined the timing of sepal yellowing in the JA-deficient mutants dad1 and dde2. DAD1/CHLOROPLASTIC LIPASE A1 produces linolenic acid, which is used as a substrate for JA production. DDE2/ALLENE OXIDE SYNTHASE controls the conversion of 13-HPOT, the first dedicated step of JA biosynthesis pathway, to unstable allene oxide (Creelman and Mullet, 1997; Mueller, 1997; Schaller and Weiler, 1997; Schaller et al., 1998). Sepals of flowers from 8-week-old wild type Col-0 plants yellowed at positions 7–8 and abscised at positions 9–11 whereas dad1 sepals senesced at positions 13–16, and abscised at positions 15–17 (Figure 5A). Furthermore, the sepals of dde2 were always more delayed in senescence than dad1 and appeared to senesce just prior to abscission at positions 15–17 (Figures 5A,C). The delayed sepal senescence and abscission phenotypes of these JA deficient mutants suggest that the observed mutant phenotypes were caused by lack of JA. To test this further, we sprayed 6 week-old Col-0, dad1 and dde2 plants with mock and MeJA solutions. Before treatment, flowers of developmental stage 13 were tagged and after treatment sepal senescence and abscission phenotypes were noted for the tagged and adjacent flowers as described in the previous section (Figures 5B–D and Supplementary Figures S3–S5). All flowers of MeJA-treated dad1 and dde2 plants present at the positions T-2 to T+2 showed sepal yellowing (Figure 5C). Sepals of MeJA-treated T-2 and T-1 dad1 and dde2 flowers showed no or 20% abscission while 60% or more abscission was found in T to T+2 flowers (Figures 5B,D and Supplementary Figures S4, S5). In contrast to MeJA-treated flowers, mock-treated T-2 dad1 and dde2 flowers did not show sepal yellowing and abscission. Of the mock-treated dad1 flowers, 20% of T-1 flowers showed sepal yellowing and this increased to 100% in T+2 flowers. Sepal abscission was first found in T+1 mock-tretated dad1 flowers. Unexpectedly, we did not observe any yellowing in mock-treated dde2 flowers of 6-week-old plants, even though abscission started from T+1 flowers (Figures 5C,D and Supplementary Figures S4, S5).
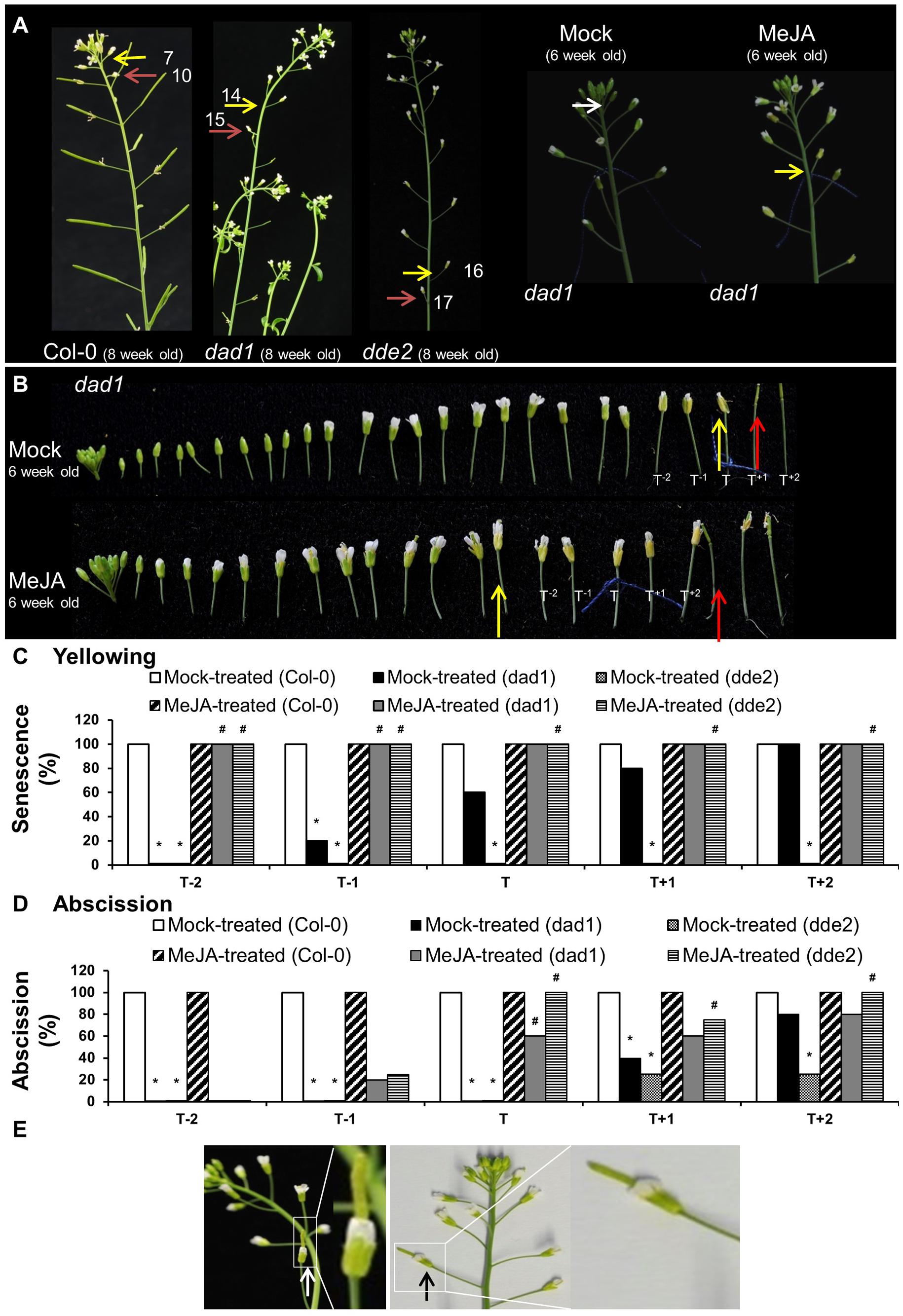
FIGURE 5. Exogenous application of MeJA to Col-0, dad1 and dde2 flowers. (A) Col-0, dad1 and dde2 plants were grown for 8 weeks and photographed. Mock and MeJA treated dad1 plants were grown for 6 weeks and flower buds with visible white petals were tagged with a cotton thread at day 0 of MeJA or mock treatment. Plants were sprayed once a day with a mock solution or MeJA for 3 days. Inflorescences were photographed after 3 days of treatment. (B) Detached flowers of Mock- and MeJA-treated dad1 inflorescences. (C) Percentage sepal yellowing of Mock- and MeJA-treated Col-0, dad1 and dde2 flowers. (D) Percentage sepal abscission of Mock- and MeJA-treated Col-0, dad1 and dde2 flowers. (E) dad1 inflorescences showing hand-pollinated flowers developing into siliques (white and black arrows). The white arrow in (A) indicate a floral bud at stage 13 according to Alvarez-Buylla et al. (2010), yellow arrows indicate flowers with senesced sepals, red arrows indicate flowers with abscised sepals. T represents the tagged flower, T-1 and T-2 represent flowers younger than T, T+1 and T+2 represent flowers older than T. The numbers in (A) represent the flowering position according to the numbering system of Bleecker and Patterson, 1997. The photographs shown are representatives of four to six biological replicates. Sen, Senescence; Abs, Abscission, 0% datapoints are placed slightly above the X-axis. Statistical differences between mock treated wild type and mutants (indicated by ∗) and mock- and MeJA-treated mutant plants (indicated by #) were calculated according to the Wilson score confidence limits for a percentage as calculated in Agresti and Coull (1998) (Supplementary Table S2).
Regardless of the treatment, all the sepals of the T-2 to T+2 flowers of Col-0 plants were abscised (Figure 5D and Supplementary Figure S3). Closer inspection of T-10 to T-3 Col-0 flowers suggested that sepal yellowing and abscission was not enhanced upon the MeJA treatment (Supplementary Figure S3). This data suggests that MeJA treatment enhances sepal senescence and abscission in both dad1 and dde2 mutants, but not in Col-0 plants. The results from these JA defective mutants is consistent with the lack of JA being the cause of delayed sepal senescence and abscission in the ag-1 mutant.
Arabidopsis Sepal Yellowing and Abscission Does Not Dependent on Pollination
The MeJA treatment, in addition to complementing the delayed sepal yellowing and abscission phenotypes of the JA-deficient dad1 mutant, also corrected defects in anther dehiscence, enabling pollination and silique development (Supplementary Figure S4B). Because silique development is closely associated with sepal yellowing and petal shatter, we investigated the role of reproductive development in accelerating sepal yellowing. For this purpose, we pollinated dad1 flowers with the pollen from Col-0 flowers. Hand pollination of these mutants resulted in silique development and seed set, however, their sepals remained green and attached (Figure 5E), although ultimately they did yellow and abscise. This shows that sepal yellowing and abscission in these Arabidopsis flowers was not dependent on any pollination-mediated effects and suggests that that delayed sepal yellowing and abscission of ag-1 is not due to lack of pollination.
Discussion
Our study has highlighted a novel role for AGAMOUS in controlling senescence of the flower it helps specify. Researchers have for many years demonstrated through physiological studies that flower senescence is regulated by plant hormones such as ethylene, ABA and cytokinins, and by source-sink relationships (Rogers, 2013). However, the key genetic regulators that are critical for initiating age-dependent flower senescence have remained elusive. Here we suggest that for the ephemeral flower of Arabidopsis, the MADS box transcription factor, AG, plays a role in the regulation of sepal senescence by inducing JA production in the stamens.
MADS Box Genes Regulate Flower Longevity in Arabidopsis
Control of flower longevity by MADS box transcription factors has been found by multiple studies. Ectopically expressed AGL15, AGL18, and AGL42 delayed sepal yellowing and perianth abscission in Arabidopsis (Fernandez et al., 2000; Adamczyk et al., 2007; Chen et al., 2011; Deng et al., 2011). Chen et al. (2011) found that ectopically expressed AGL42 reduced transcript abundance of the repressor-encoding genes ETHYLENE RESPONSE DNA-BINDING FACTOR 1 (EDF1) and EDF2. The authors subsequently revealed that ectopic AGL42 expression delayed floral senescence by increasing transcript abundance of an ETHYLENE RESPONSE FACTOR (AtERF21), whose product activated a mechanism that suppressed transcript induction of the EDF genes (Chen et al., 2015). This led them to propose that flower senescence in Arabidopsis is initiated during flower aging because of AGL42 transcript abundance declining. This results in lowered transcript accumulation of AtERF21/FUF1, preventing its product from activating a suppressor of EDF transcription. The increased EDF transcription then results in lowered transcript abundance of a suppressor of senescence. In addition, the authors found that perianth senescence and abscission was further delayed when the EDFs ectopic expression occurred in the ethylene response 1 (etr1) and ethylene-insensitive 2 (ein2) backgrounds. This suggests that the programs are under the regulation of an additional component other than ethylene.
Fernandez et al. (2000) proposed an ethylene-independent role of AGL15 in regulating Arabidopsis perianth senescence and abscission. The extent in the delay in flower organ abscission in the JA receptor (dab4) and biosynthesis mutant (aos) was greater than the delay observed in etr1-1 or ein2-1 mutants, suggesting that JA plays a major role in perianth abscission (reviewed by Kim, 2014). Kim et al. (2013) further found an increased transcript abundance of AGL15 in the JA non-responsive coi1 mutant, which exhibits delayed floral organ abscission. A recent study by Patharkar and Walker (2015) showed that AGL15 overexpression resulted in altered expression of genes involved in abscission. Notably, this included the reduced expression of receptor-like protein kinase HAESA that has been shown to play a role in floral organ abscission. At the time of flower opening a transient increase in AGL15 transcripts was found to be enough for delaying flower senescence and abscission (Fang and Fernandez, 2002). Kim et al. (2013) also speculated that delayed senescence and abscission in AGL15 overexpression mutants was due to altered levels of senescence regulating hormones such as cytokinin and auxin.
Thus, a reduced JA presence and response correlates with increased AGL15 transcript abundance, while increased AGL15 levels correlate with delayed perianth senescence and abscission. Therefore, it is conceivable that AGAMOUS may regulate sepal senescence and abscission by controlling JA production and AGL15 expression. Lower JA quantities in ag-1 flowers may result in increased AGL15 transcript and subsequently delayed flower development. Interestingly AGL15 transcript abundance was found to be increased after 7 days of AG activation in AG-GR transgenic plants where AG expression was under the external control of glucocorticoids (Gomez-Mena et al., 2005). Fang and Fernandez (2002) demonstrated that siliques of plants overexpressing AGL15 remained green compared to the wild-types. The AG-mediated increased AGL15 activity may have a role in ensuring proper seed maturation in developing siliques so that siliques do not dehisce until the fruit has matured. Thus we suggest that AG regulates both positive and negative regulators of flower maturity to ensure a timely flower development and death.
Role of JA in Sepal Senescence and Abscission
Many studies have demonstrated the critical roles of ethylene and ABA in regulating senescence and abscission of leaves and flowers (Abeles and Rubinstein, 1964; Jackson and Osborne, 1970; Brown, 1997; Rungruchkanont, 2011). By contrast, the evidence for JA regulating age-related senescence processes has been ambiguous (Jibran et al., 2013). On the one hand, JA concentrations increase in senescing Arabidopsis leaves (Seltmann et al., 2010), exogenously applied JA accelerates senescence of attached leaves (He et al., 2002), JA treatment increases expression of chlorophyll catabolic genes (Reinbothe et al., 2009) and JA represses RUBISCO ACTIVASE, an enzyme involved in carbon fixation, in a COI1-dependent manner (Shan et al., 2011). However, on the other hand, JA-deficient mutants such as opr3 and dde2/aos do not show delayed leaf senescence (Stintzi and Browse, 2000; Park et al., 2002). Thus the role of JA as a regulator of developmental senescence has remained questionable (Jibran et al., 2013). Kim et al. (2013) have, however, recently demonstrated a role for JA in petal abscission and here we provide further evidence in support of a role for JA during senescence, by using Arabidopsis sepals as a model.
The sepals of dde2 flowers showed a greater delay in senescence compared to dad1 sepals (Figure 5A). This could be because DAD1 specifically contributes to JA production in the flower and does not affect wound-induced and basal JA production (Ishiguro et al., 2001; Hyun et al., 2008), whereas dde2 plants are completely devoid of JA (Stintzi and Browse, 2000). Thus it is possible that in dad1 mutants JA translocation from other plant parts may have caused the earlier sepal senescence and abscission. The function of JA signaling components such as COI1, JAZ proteins, and JA-Ile in the regulation of flower senescence is still unclear and research in this area deserves attention.
MeJA treatment opened many flowers along the stalk rapidly, but only the older unopened florets at time of treatment yellowed. This strongly suggests presence of an age-related component to senescence of the flower, which involves the flowers developing an increased competence to senesce in response to JA. By contrast, the oldermost florets of the mock treated ag-1 flowers were not yellow after the 3 days. This suggests that these florets had developed a competence to senesce in response to JA, but had not done so because of the lack of JA production in their flowers. This clearly reveals an age-related increase in competence to respond to this hormone.
Arabidopsis Sepal Senescence Does Not Depend on Pollination or a Fertilization-Associated Ethylene Burst
Pollination and fertilization in many plants, including petunia, daffodil, tobacco and carnation, cause a burst in ethylene production that induces the different flower organs to wilt and/or abscise (Hunter et al., 2004; Jones et al., 2007; van Doorn and Woltering, 2008). The rise in ethylene is caused mainly by the reproductive organs, particularly following pollination. By contrast, the sepals produce low amounts of the hormone and only after production by the reproductive organs has started. Ethylene produced by the reproductive organs may be what increases sepal sensitivity to ethylene and may induce sepal yellowing (Tian et al., 1994). Since ag-1 flowers do not have reproductive organs, the absence of an ethylene burst may be the reason for the delayed sepal yellowing. Indeed, it has been found in various studies that the removal of reproductive organs can delay senescence, presumably due to the absence of sink tissue or decreased ethylene production (Hensel et al., 1994; Tian et al., 1994; Nooden and Penney, 2001). Here we showed that fertilization and silique development occurred in hand-pollinated, jasmonate-deficient flowers. However, sepal senescence was still delayed, suggesting that pollination-based signals, that typically involve ethylene, do not affect longevity of the Arabidopsis flower or have only a minor effect. This suggestion is consistent with the small delay in abscission seen for the ethylene-insensitive ein2-1 mutant of Arabidopsis (Kim et al., 2013) and contrasts with the larger delay in senescence and abscission observed in the jasmonate-deficient flowers of mutants ag-1, dde2 and dad1 (Figures 4, 5; Kim et al., 2013).
Our results are further supported by Azumi et al. (2002) who identified a meiotic mutant called solo dancer (sds) that is both male and female sterile but otherwise normal in plant and flower development. The sds mutant flowers do not show delayed sepal senescence phenotypes (Figures 1A–C, Azumi et al., 2002). Similarly, Huang et al. (2016) showed in their Figures 5A–C that the sepals of the sterile SDS::SDS-BARNASE lines senesced and abscised at a similar time to wild type. Further, Wang et al. (2008) reported that the mpk3+/-mpk6-/- sterile mutants were somewhat delayed in senescence, but not as much as the ag-1 mutant shown here. Moreover, mpk6-/- mutants have a delayed leaf senescence phenotype (Zhou et al., 2009) which may affect sepal senescence as well, although this was not studied. Therefore, from these studies we conclude that infertility in Arabidopsis is not tightly linked with delayed sepal and petal senescence and abscission in Arabidopsis.
Functions for AGAMOUS in Flower Development
In conclusion, we propose that AG has a central role in initiation, development and senescence of the flower it helps specify. The model in Figure 6 describes that (1) AG activity initially specifies stamen and carpel identity in the floral primordia (reviewed in Wellmer et al., 2014); (2) its continued expression drives late-stage stamen development by binding to the DAD1 promoter to induce jasmonate biosynthesis (Ito et al., 2007); (3) jasmonate then drives petal and sepal expansion possibly by regulating AGL15 expression levels and water transport, resulting in flower opening, stamen maturation and dehiscence (Ishiguro et al., 2001). We further propose that JA signaling initiates sepal yellowing and perianth abscission through suppressing transcript accumulation of AGL15 (Kim et al., 2013; our data), independently of its effects on pollination and fertilization (Figure 6).
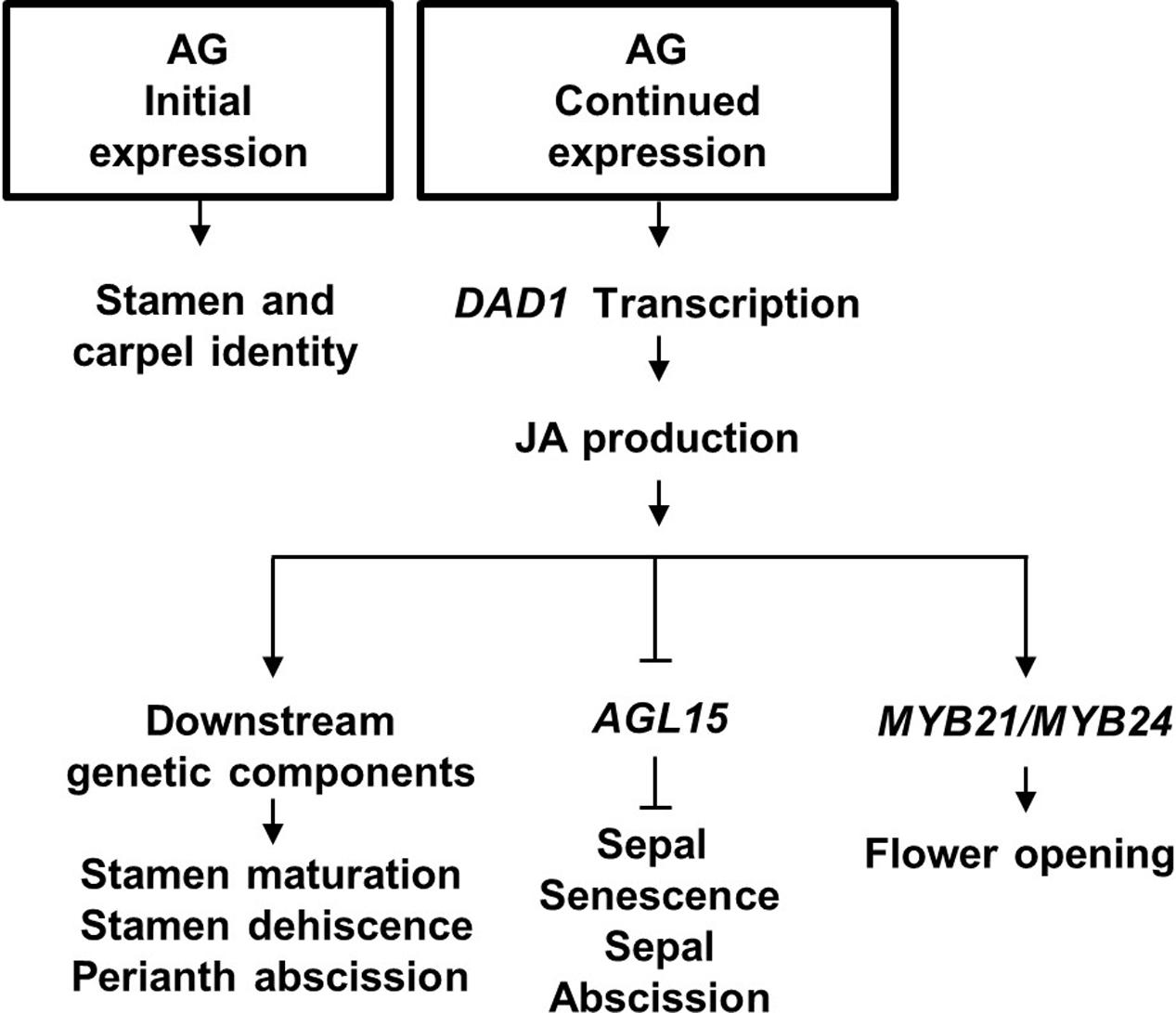
FIGURE 6. Proposed model for Arabidopsis flower development. Initial AGAMOUS (AG) expression is required for stamen and carpel identity of the innermost whorls of the flower, whereas its late expression causes Jasmonic acid (JA) synthesis in the mature stamens by directly binding to the JA production DAD1 gene. JA in the flower ensures stamen maturation, and stamen dehiscence. JA is proposed to derepress sepal senescence and abscission by inhibiting AGL15 expression.
This model predicts that the timing of AG expression is important for the regulation of sepal senescence and conditional AG expression may help establish temporal expression effects. Ectopic overexpression of AG in a 35S:AG-GR line caused sepals to develop carpelloid features (Sun et al., 2009) and therefore this approach may not yield answers. An amiRNAi knock-down approach may be more suitable and Ó’Maoiléidigh et al. (2013) showed that inducible amiRNAi flowers had sepals that were more green than the control flowers. Another avenue for future research would be to establish the roles of B class genes such as APETALA3 and PISTILLATA in sepal senescence. The mutant unusual floral organs (ufo) is deficient in APETALA3 and PISTILATA and has flowers in which the second and the third whorls are composed of a mosaic combination of sepals, petals, stamens and carpels. Nevertheless, sepals of the first whorl appear normal (Levin and Meyerowitz, 1995). The floral phenotype of the ufo mutant suggests that ufo sepals were not delayed in degreening, although the inflorescence phenotype is altered (Levin and Meyerowitz, 1995) and therefore not that easy to use for sepal senescence studies. Regardless, this study is consistent with our hypothesis that flowers with functional stamens will senesce in a timely manner.
Author Contributions
RJ, DH, and PD jointly conceived the study, acquired and interpreted the data, wrote the manuscript and assisted with manuscript revision. JC and JT acquired and analyzed data and commented on the manuscript. All the authors have read and approved the final version of the manuscript.
Funding
This work was supported by the Plant and Food Research Future Vegetables contract C02X0701 from the New Zealand Ministry of Business, Innovation and Employment, and by Plant and Food Research Core funding, and the Massey Doctoral Research fund.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Sue Nicholson from Massey University for help with using the ETD-300 ethylene detector, and Plant and Food Research staff Ian King, Julie Ryan and Andrew McLachlan for their help with plant maintenance and statistical analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2017.02101/full#supplementary-material
Footnotes
References
Abeles, F. B., and Rubinstein, B. (1964). Regulation of ethylene evolution and leaf abscission by Auxin. Plant Physiol. 39, 963–969. doi: 10.1104/pp.39.6.963
Adamczyk, B. J., Lehti-Shiu, M. D., and Fernandez, D. E. (2007). The MADS domain factors AGL15 and AGL18 act redundantly as repressors of the floral transition in Arabidopsis. Plant J. 50, 1007–1019. doi: 10.1111/j.1365-313X.2007.03105.x
Agresti, A., and Coull, B. A. (1998). Approximate is better than “exact” for interval estimation of binomial proportions. Am. Stat. 52, 119–126.
Alvarez-Buylla, E. R., Benitez, M., Corvera-Poire, A., Chaos Cador, A., de Folter, S., Gamboa de Buen, A., et al. (2010). Flower development. Arabidopsis Book 8:e0127. doi: 10.1199/tab.0127
Azumi, Y., Liu, D., Zhao, D., Li, W., Wang, G., Hu, Y., et al. (2002). Homolog interaction during meiotic prophase I in Arabidopsis requires the SOLO DANCERS gene encoding a novel cyclin-like protein. EMBO J. 21, 3081–3095. doi: 10.1093/emboj/cdf285
Bleecker, A. B., and Patterson, S. E. (1997). Last exit: senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell 9, 1169–1179. doi: 10.1105/tpc.9.7.1169
Boyes, D. C., Zayed, A. M., Ascenzi, R., McCaskill, A. J., Hoffman, N. E., Davis, K. R., et al. (2001). Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13, 1499–1510. doi: 10.1105/tpc.13.7.1499
Brown, K. M. (1997). Ethylene and abscission. Physiol. Plant. 100, 567–576. doi: 10.1111/j.1399-3054.1997.tb03062.x
Chen, M. K., Hsu, W. H., Lee, P. F., Thiruvengadam, M., Chen, H. I., and Yang, C. H. (2011). The MADS box gene, FOREVER YOUNG FLOWER, acts as a repressor controlling floral organ senescence and abscission in Arabidopsis. Plant J. 68, 168–185. doi: 10.1111/j.1365-313X.2011.04677.x
Chen, W. H., Li, P. F., Chen, M. K., Lee, Y. I., and Yang, C. H. (2015). FOREVER YOUNG FLOWER negatively regulates ethylene response DNA-binding factors by activating an ethylene-responsive factor to control Arabidopsis floral organ senescence and abscission. Plant Physiol. 168, 1666–1683. doi: 10.1104/pp.15.00433
Creelman, R. A., and Mullet, J. E. (1997). Biosynthesis and action of Jasmonates in plants. Annu. Rev. Plant Biol. 48, 355–381. doi: 10.1146/annurev.arplant.48.1.355
Deng, W., Ying, H., Helliwell, C. A., Taylor, J. M., Peacock, W. J., and Dennis, E. S. (2011). FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 108, 6680–6685. doi: 10.1073/pnas.1103175108
Fang, S. C., and Fernandez, D. E. (2002). Effect of regulated overexpression of the MADS domain factor AGL15 on flower senescence and fruit maturation. Plant Physiol. 130, 78–89. doi: 10.1104/pp.004721
Fernandez, D. E., Heck, G. R., Perry, S. E., Patterson, S. E., Bleecker, A. B., and Fang, S. C. (2000). The embryo MADS domain factor AGL15 acts postembryonically. Inhibition of perianth senescence and abscission via constitutive expression. Plant Cell 12, 183–198. doi: 10.1105/tpc.12.2.183
Gan, S., and Amasino, R. M. (1997). Making sense of senescence (Molecular genetic regulation and manipulation of leaf senescence). Plant Physiol. 113, 313–319. doi: 10.1104/pp.113.2.313
Gomez-Mena, C., de Folter, S., Costa, M. M. R., Angenent, G. C., and Sablowski, R. (2005). Transcriptional program controlled by the floral homeotic gene AGAMOUS during early organogenesis. Development 132, 429–438. doi: 10.1242/dev.01600
He, Y. H., Fukushige, H., Hildebrand, D. F., and Gan, S. S. (2002). Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 128, 876–884. doi: 10.1104/pp.010843
Hensel, L. L., Nelson, M. A., Richmond, T. A., and Bleecker, A. B. (1994). The fate of inflorescence meristems is controlled by developing fruits in Arabidopsis. Plant Physiol. 106, 863–876. doi: 10.1104/pp.106.3.863
Honma, T., and Goto, K. (2001). Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409, 525–529. doi: 10.1038/35054083
Huang, J., Smith, R. A., Zhang, T., and Zhao, D. (2016). Creating completely both male and female sterile plants by specifically ablating microspore and megaspore mother cells. Front. Plant Sci. 7:30. doi: 10.3389/fpls.2016.00030
Huijser, P., and Schmid, M. (2011). The control of developmental phase transitions in plants. Development 138, 4117–4129. doi: 10.1242/dev.063511
Hunter, D. A., Yi, M. F., Xu, X. J., and Reid, M. S. (2004). Role of ethylene in perianth senescence of daffodil (Narcissus pseudonarcissus L. ‘Dutch Master’). Postharvest Biol. Technol. 32, 269–280. doi: 10.1016/j.postharvbio.2003.11.013
Hyun, Y., Choi, S., Hwang, H. J., Yu, J., Nam, S. J., Ko, J., et al. (2008). Cooperation and functional diversification of two closely related galactolipase genes for jasmonate biosynthesis. Dev. Cell 14, 183–192. doi: 10.1016/j.devcel.2007.11.010
Irish, V. F., and Sussex, I. M. (1990). Function of the APETALA-1 gene during Arabidopsis floral development. Plant Cell 2, 741–753. doi: 10.1105/tpc.2.8.741
Ishiguro, S., Kawai-Oda, A., Ueda, J., Nishida, I., and Okada, K. (2001). The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel a phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13, 2191–2209. doi: 10.1105/tpc.13.10.2191
Ito, T., Ng, K. H., Lim, T. S., Yu, H., and Meyerowitz, E. M. (2007). The homeotic protein AGAMOUS controls late stamen development by regulating a jasmonate biosynthetic gene in Arabidopsis. Plant Cell 19, 3516–3529. doi: 10.1105/tpc.107.055467
Jackson, M. B., and Osborne, D. J. (1970). Ethylene, the natural regulator of leaf abscission. Nature 225, 1019–1022. doi: 10.1038/2251019a0
Jibran, R., Hunter, D. A., and Dijkwel, P. P. (2013). Hormonal regulation of leaf senescence through integration of developmental and stress signals. Plant Mol. Biol. 82, 547–561. doi: 10.1007/s11103-013-0043-2
Jones, M. L., Bai, S., Willard, B., Stead, A., and Kinter, M. (2007). “Proteomic analysis of pollination-induced senescence in Petunia flowers,” in Advances in Plant Ethylene Research, eds A. Ramina, C. Chang, J. Giovannoni, H. Klee, P. Perata, and E. Woltering (Dordrecht: Springer), 279–284.
Kim, J. (2014). Four shades of detachment: regulation of floral organ abscission. Plant Signal. Behav. 9:e976154. doi: 10.4161/15592324.2014.976154
Kim, J., Dotson, B., Rey, C., Lindsey, J., Bleecker, A. B., Binder, B. M., et al. (2013). New clothes for the jasmonic acid receptor COI1: delayed abscission, meristem arrest and apical dominance. PLOS ONE 8:e60505. doi: 10.1371/journal.pone.0060505
Koornneef, M., Vaneden, J., Hanhart, C. J., Stam, P., Braaksma, F. J., and Feenstra, W. J. (1983). Linkage map of Arabidopsis thaliana. J. Hered. 74, 265–272. doi: 10.1093/oxfordjournals.jhered.a109781
Levin, J. Z., and Meyerowitz, E. M. (1995). UFO: an Arabidopsis gene involved in both floral meristem and floral organ development. Plant Cell 7, 529–548. doi: 10.1105/tpc.7.5.529
Mueller, M. J. (1997). Enzymes involved in jasmonic acid biosynthesis. Physiol Plant. 100, 653–663. doi: 10.1111/j.1399-3054.1997.tb03072.x
Nooden, L. D., and Penney, J. P. (2001). Correlative controls of senescence and plant death in Arabidopsis thaliana (Brassicaceae). J. Exp. Bot. 52, 2151–2159. doi: 10.1093/jexbot/52.364.2151
Ó’Maoiléidigh, D. S., Wuest, S. E., Rae, L., Raganelli, A., Ryan, P. T., Kwasniewska, K., et al. (2013). Control of reproductive floral organ identity specification in Arabidopsis by the C function regulator AGAMOUS. Plant Cell 25, 2482–2503. doi: 10.1105/tpc.113.113209
Park, J. H., Halitschke, R., Kim, H. B., Baldwin, I. T., Feldmann, K. A., and Feyereisen, R. (2002). A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J. 31, 1–12. doi: 10.1046/j.1365-313X.2002.01328.x
Patharkar, O. R., and Walker, J. C. (2015). Floral organ abscission is regulated by a positive feedback loop. Proc. Natl. Acad. Sci. U.S.A. 112, 2906–2911. doi: 10.1073/pnas.1423595112
Pelaz, S., Ditta, G. S., Baumann, E., Wisman, E., and Yanofsky, M. F. (2000). B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405, 200–203. doi: 10.1038/35012103
Reinbothe, C., Springer, A., Samol, I., and Reinbothe, S. (2009). Plant oxylipins: role of jasmonic acid during programmed cell death, defence and leaf senescence. FEBS J. 276, 4666–4681. doi: 10.1111/j.1742-4658.2009.07193.x
Rogers, H. J. (2013). From models to ornamentals: how is flower senescence regulated. Plant Mol. Biol. 82, 563–574. doi: 10.1007/s11103-012-9968-0
Rungruchkanont, K. (2011). Auxins and cytokinins regulate abscission and physiological changes of flowers in cut Dendrobium cv Eiskul inflorescences. Sci. J. UBU 2, 1–11.
Schaller, F., Hennig, P., and Weiler, E. W. (1998). 12-oxophytodienoate-10,11-reductase: occurrence of two isoenzymes of different specificity against stereoisomers of 12-oxophytodienoic acid. Plant Physiol. 118, 1345–1351. doi: 10.1104/pp.118.4.1345
Schaller, F., and Weiler, E. W. (1997). Molecular cloning and characterization of 12-oxophytodienoate reductase, an enzyme of the octadecanoid signaling pathway from Arabidopsis thaliana - Structural and functional relationship to yeast old yellow enzyme. J. Biol. Chem. 272, 28066–28072. doi: 10.1074/jbc.272.44.28066
Schommer, C., Palatnik, J. F., Aggarwal, P., Chetelat, A., Cubas, P., Farmer, E. E., et al. (2008). Control of jasmonate biosynthesis and senescence by miR319 targets. PLOS Biol. 6:e230. doi: 10.1371/journal.pbio.0060230
Seltmann, M. A., Hussels, W., and Berger, S. (2010). Jasmonates during senescence: signals or products of metabolism? Plant Signal. Behav. 5, 1493–1496. doi: 10.4161/psb.5.11.13644
Shan, X., Wang, J., Chua, L., Jiang, D., Peng, W., and Xie, D. (2011). The role of Arabidopsis Rubisco activase in jasmonate-induced leaf senescence. Plant Physiol. 155, 751–764. doi: 10.1104/pp.110.166595
Smaczniak, C., Immink, R. G., Angenent, G. C., and Kaufmann, K. (2012). Developmental and evolutionary diversity of plant MADS-domain factors: insights from recent studies. Development 139, 3081–3098. doi: 10.1242/dev.074674
Stead, A. D. (1992). Pollination-induced flower senescence - a review. Plant Growth Regul. 11, 13–20. doi: 10.1007/BF00024427
Stintzi, A., and Browse, J. (2000). The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. U.S.A. 97, 10625–10630. doi: 10.1073/pnas.190264497
Sun, B., Xu, Y., Ng, K. H., and Ito, T. (2009). A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Genes Dev. 23, 1791–1804. doi: 10.1101/gad.1800409
Tanabe, Y., Hasebe, M., Sekimoto, H., Nishiyama, T., Kitani, M., Henschel, K., et al. (2005). Characterization of MADS-box genes in charophycean green algae and its implication for the evolution of MADS-box genes. Proc. Natl. Acad. Sci. U.S.A. 102, 2436–2441. doi: 10.1073/pnas.0409860102
Theissen, G. (2001). Development of floral organ identity: stories from the MADS house. Curr. Opin. Plant Biol. 4, 75–85. doi: 10.1016/S1369-5266(00)00139-4
Tian, M. S., Downs, C. G., Lill, R. E., and King, G. A. (1994). A role for ethylene in the yellowing of Broccoli after harvest. J. Am. Soc. Hortic. Sci. 119, 276–281.
van Doorn, W. G. (1997). Effects of pollination on floral attraction and longevity. J. Exp. Bot. 48, 1615–1622. doi: 10.1093/jxb/48.9.1615
van Doorn, W. G., and Woltering, E. J. (2008). Physiology and molecular biology of petal senescence. J. Exp. Bot. 59, 453–480. doi: 10.1093/jxb/erm356
Wang, H., Liu, Y., Bruffett, K., Lee, J., Hause, G., Walker, J. C., et al. (2008). Haplo-insufficiency of MPK3 in MPK6 mutant background uncovers a novel function of these two MAPKs in Arabidopsis ovule development. Plant Cell 20, 602–613. doi: 10.1105/tpc.108.058032
Wellmer, F., Graciet, E., and Riechmann, J. L. (2014). Specification of floral organs in Arabidopsis. J. Exp. Bot. 65, 1–9. doi: 10.1093/jxb/ert385
Wintermans, J., and De Mots, A. (1965). Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 109, 448–453. doi: 10.1016/0926-6585(65)90170-6
Woltering, E. J., and van Doorn, W. G. (1988). Role of ethylene in senescence of petals - morphological and taxonomical relationships. J. Exp. Bot. 39, k1605–1616. doi: 10.1093/jxb/39.11.1605
Yanofsky, M. F., Ma, H., Bowman, J. L., Drews, G. N., Feldmann, K. A., and Meyerowitz, E. M. (1990). The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346, 35–39. doi: 10.1038/346035a0
Keywords: abscission, AGAMOUS, ag-1, delayed dehiscence 2 (dde2), defective in anther dehiscence 1 (dad1), flower, jasmonate, senescence
Citation: Jibran R, Tahir J, Cooney J, Hunter DA and Dijkwel PP (2017) Arabidopsis AGAMOUS Regulates Sepal Senescence by Driving Jasmonate Production. Front. Plant Sci. 8:2101. doi: 10.3389/fpls.2017.02101
Received: 26 May 2017; Accepted: 27 November 2017;
Published: 11 December 2017.
Edited by:
Stefan de Folter, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (CINVESTAV-IPN), MexicoReviewed by:
Frank Wellmer, Trinity College, Dublin, IrelandAstrid Wingler, University College Cork, Ireland
Copyright © 2017 Jibran, Tahir, Cooney, Hunter and Dijkwel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul P. Dijkwel, cC5kaWprd2VsQG1hc3NleS5hYy5ueg== Donald A. Hunter, ZG9uYWxkLmh1bnRlckBwbGFudGFuZGZvb2QuY28ubno=
 Rubina Jibran
Rubina Jibran Jibran Tahir
Jibran Tahir Janine Cooney
Janine Cooney Donald A. Hunter
Donald A. Hunter Paul P. Dijkwel
Paul P. Dijkwel