- 1State Key Laboratory of Plant Physiology and Biochemistry, College of Life Sciences, Zhejiang University, Hangzhou, China
- 2Institute of Horticulture, Zhejiang Academy of Agricultural Sciences, Hangzhou, China
- 3Institute of Crop Science, Anhui Academy of Agricultural Sciences, Hefei, China
- 4College of Resources and Environment, Huazhong Agricultural University, Wuhan, China
Aquaporins play an essential role in water uptake and transport in vascular plants. The soybean genome contains a total of 22 plasma membrane intrinsic protein (PIP) genes. To identify candidate PIPs important for soybean yield and stress tolerance, we studied the transcript levels of all 22 soybean PIPs. We found that a GmPIP2 subfamily member, GmPIP2;9, was predominately expressed in roots and developing seeds. Here, we show that GmPIP2;9 localized to the plasma membrane and had high water channel activity when expressed in Xenopus oocytes. Using transgenic soybean plants expressing a native GmPIP2;9 promoter driving a GUS-reporter gene, it was found high GUS expression in the roots, in particular, in the endoderm, pericycle, and vascular tissues of the roots of transgenic plants. In addition, GmPIP2;9 was also highly expressed in developing pods. GmPIP2;9 expression significantly increased in short term of polyethylene glycol (PEG)-mediated drought stress treatment. GmPIP2;9 overexpression increased tolerance to drought stress in both solution cultures and soil plots. Drought stress in combination with GmPIP2;9 overexpression increased net CO2 assimilation of photosynthesis, stomata conductance, and transpiration rate, suggesting that GmPIP2;9-overexpressing transgenic plants were less stressed than wild-type (WT) plants. Furthermore, field experiments showed that GmPIP2;9-overexpressing plants had significantly more pod numbers and larger seed sizes than WT plants. In summary, the study demonstrated that GmPIP2;9 has water transport activity. Its relative high expression levels in roots and developing pods are in agreement with the phenotypes of GmPIP2;9-overexpressing plants in drought stress tolerance and seed development.
Introduction
Aquaporins (AQPs) are integral membrane proteins that increase membrane permeability to water and other small molecules (Uehlein et al., 2003; Kaldenhoff and Fischer, 2006; Sade et al., 2010). AQPs play key roles in plant water balance and efficient water use. Plant AQPs are classified into four subfamilies: plasma membrane (PM) intrinsic proteins (PIPs), tonoplast membrane intrinsic proteins (TIPs), nodulin 26-like intrinsic proteins (NIPs), and small basic intrinsic proteins (SIPs)(Luu and Maurel, 2005). PIPs are further divided into two subclasses, PIP1s and PIP2s, based on the length of the N terminal in the proteins. It was shown that PIP2s function as water channels when expressed in Xenopus oocytes, whereas PIP1s generally have much lower or no water channel activity (Daniels et al., 1994; Kammerloher et al., 1994; Weig et al., 1997; Chaumont et al., 2000; Moshelion et al., 2002). The water permeability of PIP1s requires co-expression of PIP2s to form hetero-tetramers (Chaumont et al., 2000).
Expression of AQPs can be regulated in tissues by hormone treatments and abiotic stresses (Tyerman et al., 2002; Jang et al., 2004; Alexandersson et al., 2005; Chaumont et al., 2005; Bienert et al., 2006; Aroca et al., 2012). In Arabidopsis, the expression patterns of AQPs have been evaluated in different organs and under drought stress using microarrays and/or quantitative reverse transcription polymerase chain reaction (qRT-PCR; Jang et al., 2004; Alexandersson et al., 2005, 2010). It was shown that many AQPs are predominantly expressed in either roots or flowers, whereas no AQPs have leaf-specific expression (Alexandersson et al., 2005). With gradual soil drought stress, the abundance of Arabidopsis PIPs (AtPIPs) transcripts in leaves is generally downregulated, with the exceptions of AtPIP1;4 and AtPIP2;5, which are upregulated, and AtPIP2;6, which is unaffected (Alexandersson et al., 2005). Bioinformatic analysis showed strong co-expression of many AtPIPs and AtTIPs that were downregulated upon drought, whereas AtPIP1;4, AtPIP2;5, and AtPIP2;6 are not co-expressed (Alexandersson et al., 2010). Under osmotic stress conditions created by addition of mannitol, many AtPIPs are downregulated in aerial parts of the plant, while the transcript levels of AtPIP1;3, AtPIP1;4, AtPIP2;1, and AtPIP2;5 are upregulated in both root and aerial parts of the mannitol-treated plants (Jang et al., 2004). Studies of rice PIPs (OsPIPs) showed that transcription levels of OsPIP2;1, OsPIP2;5, and OsPIP2;6 are suppressed in leaves upon polyethylene glycol (PEG)-mediated drought treatment, while OsPIP1;2 and OsPIP2;4 are not affected (Guo et al., 2006). In contrast, almost all the OsPIPs, especially OsPIP1;1, OsPIP2;5, and OsPIP2;7, were upregulated with short-term PEG treatment in roots.
In addition to water transport in roots, a variety of AQPs are expressed in the coats of developing seeds (Schuurmans et al., 2003). Nutrient and water transport across PMs in seed coats is highly coordinated by regulatory mechanisms and integrates the activities of many nutrient transporters and facilitators (Walker et al., 1995; Zhou et al., 2007a). Thus, it is expected that PIPs that are specifically expressed in native PMs of seed coats are important for seed filling (Zhou et al., 2007a).
Drought is considered one of the most devastating abiotic stress factors that adversely affect crop growth and productivity (Manavalan et al., 2009; Tran and Mochida, 2010). To cope with drought stress, higher plants have evolved sophisticated responses, including stomata closure, increased root/shoot ratio, accumulation of protective solutes and proteins, and production and scavenging of reactive oxygen species (Verslues et al., 2006). Root water uptake can be enhanced or reduced by the overexpression or loss of one or more PIP genes, respectively (Aharon et al., 2003; Javot, 2003; Zhou et al., 2014). These findings suggest that alteration of the expression of certain PIPs in transgenic crops can improve the tolerance to drought stress.
A recent study showed that altered plant transpiration led to rapid changes in root expression of soybean PIP1;6 (GmPIP1;6) that correlated with changes in root hydraulic conductance (Vandeleur et al., 2014). Thus, GmPIP1;6 is proposed to play a role in regulating root hydraulic conductance. Consistent with this idea, our previous study showed that under salt stress, overexpression of GmPIP1;6 enhanced root water transport, photosynthesis, and seed filling (Zhou et al., 2014). The soybean genome contains a total of 22 PIP genes (Sakurai et al., 2005). To identify candidate PIPs important for soybean yield and stress tolerance, we studied the transcript levels of all 22 soybean PIPs. We found that a GmPIP2 subfamily member, GmPIP2;9, was predominately expressed in roots and developing seeds. In this study, we developed and characterized transgenic soybean plants that overexpressed GmPIP2;9. Our results showed that GmPIP2;9 overexpression conferred enhanced drought stress tolerance and improved seed setting and filling.
Materials and Methods
Plant Materials, Growth Conditions, and Treatments
We used soybean cultivar Williams 82 for all physiological experiments and as the recipient for transformations. For physiological experiments, we germinated seeds in filter papers for 4 days prior to transferring into half-strength Hoagland’s solution (Hoagland and Arnon, 1950) or soil pots. After germination, we grew soybean seedlings in growth chambers with a 12-h photoperiod (200 μmol photons m-2 s-1) and a day/night temperature of 30/22°C.
For PEG-mediated drought treatments, we transferred 10-day-old seedlings into half-strength Hoagland’s solution with or without addition of 20% PEG 8000. For the soil pot drought tolerance test, soybean seeds were planted in a soil pot for 2 weeks. The soil pots in the drought treatment were not watered for 12 days, followed by re-watering for 3 days.
In a different soil pot experiment, 40-day-old plants were not watered for 21 days and then re-watered for 7 days. The transgenic and wild-type (WT) plants were grown together in pots to minimize experimental error. We used five pots for each treatment group, including the control. Soil water content during the treatment was recorded daily (Supplementary Figure S1).
Quantitative and Semi-quantitative RT-PCR
We measured GmPIP2;9 expression in different tissues and during drought treatment with qRT-PCR. Samples for tissue-specific expression analysis were collected from three different plants as three biological replicates. RNA extraction and reverse transcription were performed using TRIzol reagent and SuperScript II reverse transcriptase, respectively (Invitrogen, Carlsbad, CA, United States). We performed qRT-PCR performed using SYBR Green I dye as a fluorescent signal. We calculated relative expression levels for three biological replicates using the 2-ΔΔCt method. The primer pair for the qRT-PCR of GmPIP2;9 was 5′-TCACTTGGCAACCATCCCAG-3′ and 5′-CAAGAGCCTTAGCAGCACCT-3′. The primer pair used for the housekeeping gene GmACTIN was 5′-CAGAGAAAGTGCCCAAATCATGT-3′ and 5′-TTGCATACAAGGAGAGAACAGCTT-3′.
Construction of Vectors and Soybean Transformation
We constructed binary vectors for overexpression of GmPIP2;9 and expression of GmPIP2;9 promoter-fused β-glucuronidase (GUS)-reporter (PPIP2;9::GUS) as follows: we amplified full-length 858 bp GmPIP2;9 cDNA (Phytozome No. Glyma.02g073600) by RT-PCR, using the primers 5′-GCTCTAGAATGGCTAAGCATGATGTTGAG-3′ and 5′-CGGGATCCTCAAATAGTGGGGTTGCTCCT-3′. Then, we cloned the amplified fragment into the pTF101.1-derived vector pLM-B001 (Paz et al., 2004) under control of the 35S promoter of the cauliflower mosaic virus. For the PPIP2;9::GUS construct, we amplified the promoter region of a 2-kb fragment upstream of the ATG start codon from Williams 82 genomic DNA, using the primers 5′-CGGGATCCGTGTTTTATCACATATACACACATTTT-3′ and 5′-GCTCTAGATGCAATTTGCAACTACCCTTT-3′. The PCR product was cloned into the pTF101.1-derived vector PTF101-GUS. The resulting vectors were confirmed by sequencing and transfected into Agrobacterium tumefaciens strain EHA101. We performed transformations of vectors into soybean as previously described (Song et al., 2013).
GUS Histochemical Analysis
We took samples of leaves, roots, flowers, and pods from PPIP2;9::GUS transgenic soybean plants and stained them with GUS histochemical staining buffer, containing 100 mM sodium phosphate buffer (pH 7.0), 1 mM 5-bromo-4-chloro-3-indolyl-b-D-glucuronidase (X-Gluc), 1 mM K4[Fe(CN)6], 1 mM K3[Fe(CN)6], 0.5% (v/v) Triton X-100, and 20% (v/v) methanol. To prepare the root sections, we embedded soybean roots using 5% (w/v) low-melting-point agarose (Sigma, St Louis, MO, United States) and cut sections with a vibrating blade microtome (Leica VT1000S; Heidelberger, Germany). We examined all samples under a bright-field a microscope (Nikon, Tokyo, Japan).
Subcellular Localization of GmPIP2;9 in Plant Cells
We amplified full-length GmPIP2;9 cDNA without a stop code, using the primers 5′-TGTCGGAGCTCGGTACCCATGGCTAAGCATGATGTTGAGGGTG-3′ and 5′-TCGCCCTTGCTCACCATGTCCAATAGTGGGGTTGCTCCTGAAT-3′. The PCR product was fused to the 5′ end of the green fluorescent protein (GFP)-encoding gene under control of the CaMV 35S promoter in the pCAMBIA1302 vector1. We used a construct carrying AtPIP2A::mCherry as a marker for PM localization (Nelson et al., 2007). We transiently co-expressed the two constructs in onion epidermal cells, as previously described (Zhou et al., 2014).
Subcellular Localization and Water-Permeability Assay of GmPIP2;9 in Xenopus Oocytes
To analyze the water transport activity of GmPIP2;9, we ligated the amplified fragment of the GmPIP2;9 cDNA or nYFP-fused GmPIP2;9 sequence into the Xenopus oocyte expression vector pGEMHE (Liman et al., 1992). The pGEMHE::nYFP-GmPIP2;9 was injected into oocytes and incubated for 48 h in BS before imaging with confocal laser scanning microscopy to determine the subcellular localization of GmPIP2;9 in oocytes. The pGEMHE::GmPIP2;9 vector was transcribed into complementary RNA (cRNA), as previously described (Vandeleur et al., 2009). We injected 23 ng of cRNA into oocyte cells of Xenopus laevis using a Nanoject microinjector (Drummond Scientific Co., Broomall, PA, United States). Xenopus oocytes were incubated in Ca-Ringer’s solution with horse serum and antibiotics at 18°C for 1 day and then transferred the oocytes into a 5× diluted ND96 solution. We measured and analyzed oocyte volume, as previously described (Fetter et al., 2004; Vandeleur et al., 2009). Osmotic water permeability (Pos) was calculated using the equation Pos = Jw/Vw × A × ΔOsm, where Jw = the initial rate of change of relative cell volume, Vw = the partial molar volume of water, A = the area of the oocyte, and ΔOsm = the change in osmolarity.
Measurement of Net CO2 Assimilation (AN), Stomatal Conductance (gs), and Transpiration Rate (Tr)
We grew soybean plants in soil pots with a normal water and nutrient supply for 40 days in a greenhouse prior to drought treatment. We recorded the AN, gs, and Tr of fully expanded leaves using an Li-6400 portable gas-exchange system (LI-COR, NE, United States) on day 11 of the drought treatment. We performed all measurements between 8:00 AM and 2:00 PM. The photosynthetic photon flux density was 1200 μmolm-2 s-1, the leaf surface temperature was 30°C, and the CO2 concentration was 400 μmol mol-1. To minimize experimental error, we planted GmPIP2;9-Oe plants and WT plants together as shown in Figure 6.
Agronomic Performance of WT and GmPIP2;9-Oe Transgenic Plants Grown in the Field
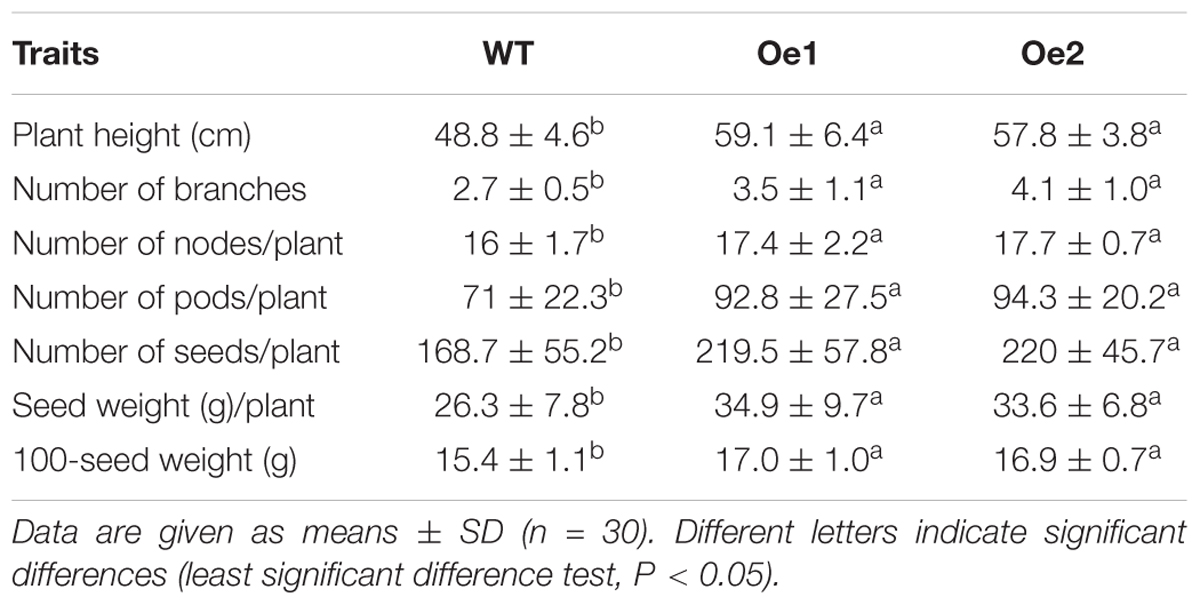
We measured agronomic characteristics, including plant height, numbers of branches, nodes, pods, and seeds per plant, 100-seed weight, and seed weight per plant using samples from a 2015 field experiment at the Anhui Academy of Agricultural Sciences. The field experiment was arranged in a triplicate randomized block design with 10 m2 for each plot. For each replication, we sampled 10 plants from each of two transgenic (GmPIP2;9-Oe1 and Oe2) lines and the WT line.
Statistical Analysis
Statistical analysis of the data was performed using the Data Processing System (DPS version 7.05, Zhejiang, China). We used Student’s t-tests to determine significant differences between the WT and transgenic lines for each treatment. We used least significant difference tests for pairwise comparisons between samples.
Results
GmPIP2;9 Localizes to the PM
GmPIP2;9 is predicted to localize to the PM (Zhou et al., 2013). To obtain direct experimental evidence, we transiently co-expressed a 35S-GmPIP2;9::GFP construct and a mCherry-fused PM marker CD3-1007 in onion epidermal cells. As shown in Figure 1, 35S-GmPIP2;9::GFP fluorescence was confined to the PM and co-localized with CD3-1007 red fluorescence. In contrast, the green fluorescent signal from the control construct with GFP alone was distributed throughout the nucleus and cytoplasm.
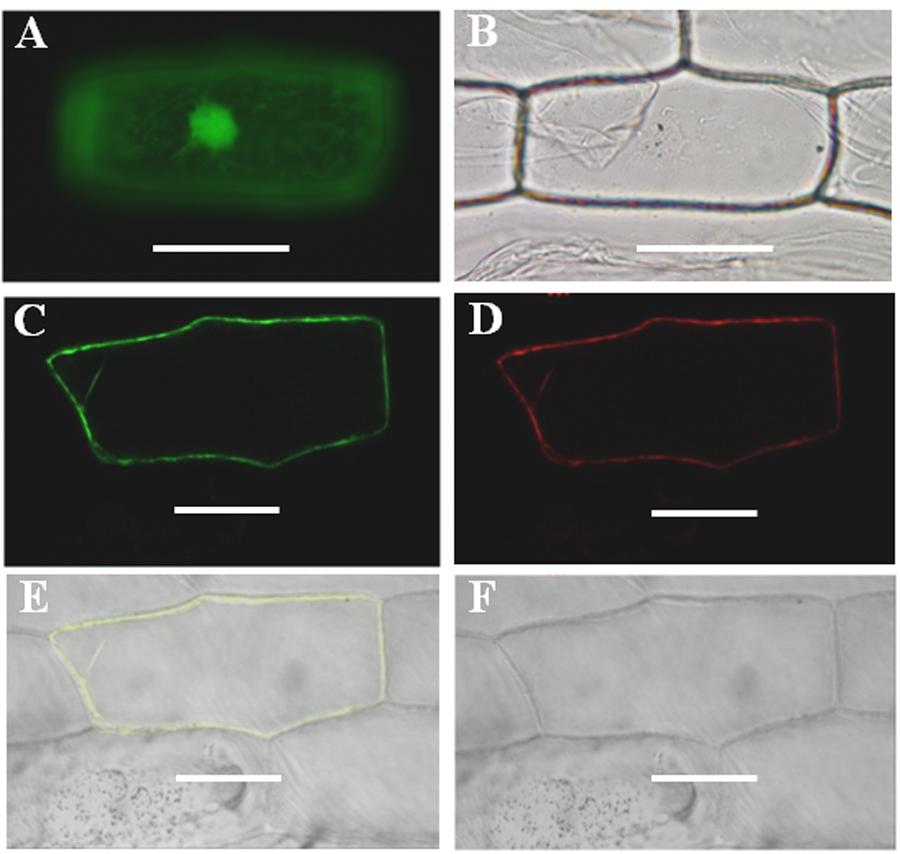
FIGURE 1. Subcellular localization of GmPIP2;9. (A,B) Onion epidermal cells expressing green fluorescent protein (GFP). (A) GFP signals in the nucleus, cytoplasm, and plasma membrane (PM). (B) Bright-field light image. (C) Onion epidermal cells expressing GmPIP2;9-GFP with GFP signals in the PM. (D) Onion epidermal cells expressing red fluorescent signal from the CD3-1007 PM marker. (E,F) Merged fluorescent and bright-field images of an epidermal cell expressing the GmPIP2;9-sGFP fusion protein and the CD3-1007 PM marker. Bars = 100 μm.
GmPIP2;9 Has High Water Channel Activity When Expressed in Xenopus Oocytes
Plant PIP2 proteins that have been examined so far have high water channel activity when expressed in Xenopus oocytes (Fetter et al., 2004; Verdoucq et al., 2008). To confirm whether GmPIP2;9 has water channel activity, we first investigated the localization of GmPIP2;9 in Xenopus oocytes. As shown in Figure 2A, expression of pGEMHE::nYFP-GmPIP2;9 construct into Xenopus oocytes resulted in PM localization of the YFP-GmPIP2;9 fusion protein. The osmotic water permeability coefficient (Pf) was measured by injected with GmPIP2;9 cRNA. We used human AQP cRNA (AQP) and water as the positive and negative controls, respectively. We found a significantly higher Pf value in oocytes injected with GmPIP2;9 than in oocytes injected with the water control (Figure 2B), suggesting that GmPIP2;9 is a functional AQP with high water channel activity in Xenopus oocytes. The high water channel activity of GmPIP2;9 was confirmed in a separate oocyte expression experiment that compared the Pf values of nYFP-GmPIP1;6 (Zhou et al., 2014), nYFP-GmPIP2;9, and nYFP-GmPIP1;6+GmPIP2;9 cRNA (Supplementary Figure S2). While nYFP-GmPIP1;6 had no water channel activity, nYFP-GmPIP2;9 showed a similar Pf value to GmPIP2;9 (Supplementary Figure S2).
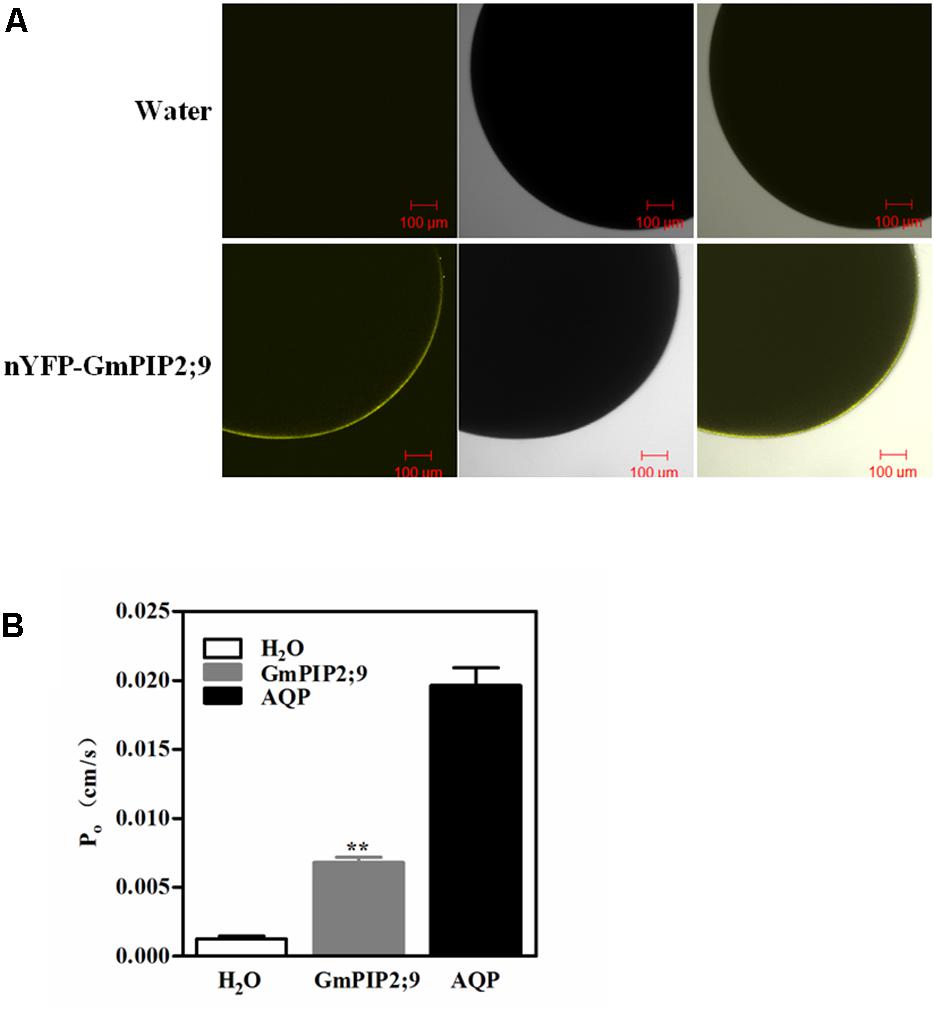
FIGURE 2. Expression of functional GmPIP2;9 in Xenopus oocytes. (A) YFP localization of nYFP-GmPIP2;9 in Xenopus oocytes. The oocytes were observed 2 days after injection as described in Section “Methods.” Bars = 100 μ m. Fluorescence (left), bright-field (middle), and merged images (right) showing that nYFP-GmPIP2;9 fusion protein is expressed in plasma membrane of oocytes. (B) Oocytes were injected with 23 ng GmPIP2;9 cRNA or water. The osmotic water permeability coefficients (Pf) of oocytes were determined from swelling kinetics. Data represent the mean ± SD measurements from 10 oocytes. ∗∗P < 0.01.
Expression Patterns of GmPIP2;9 in Different Tissues and in Response to Osmotic Stress
We used qRT-PCR to examine the expression of GmPIP2;9 in different soybean tissues and found that GmPIP2;9 was expressed in all examined tissues, with the highest expression in roots (Figure 3A). Furthermore, using transgenic soybean plants expressing a native GmPIP2;9 promoter driving a GUS-reporter gene, we confirmed that GmPIP2;9 was expressed in all examined tissues, including roots, leaves, stems, flowers, and pods during the seed development stage (Figure 3B). Consistent with qRT-PCR results, we observed high GUS expression in the roots of transgenic plants (Figure 3B). Cross sections of root tissues showed that GmPIP2;9 was predominantly expressed in the endoderm, pericycle, and vascular tissues (Figure 3B). Notably, we observed high GUS expression in the pods and the seed hilum, which assimilate and transport water.
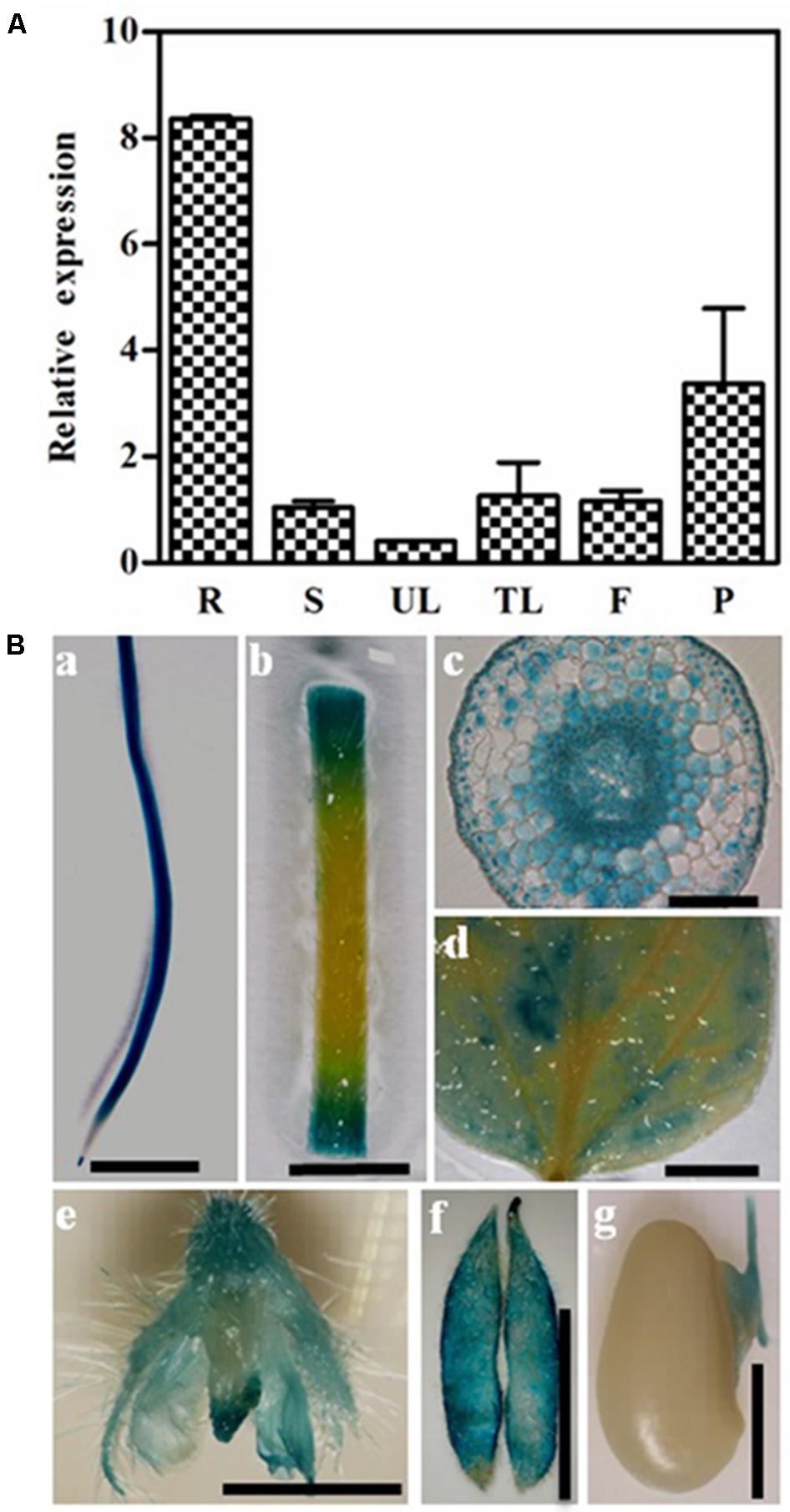
FIGURE 3. Expression patterns of GmPIP2;9. (A) Relative expression levels of GmPIP2;9 in root (R), stem (S), unifoliate leaf (UL), trifoliolate leaf (TL), flower (F), and pod (P). All data are means of three biological replicates with error bars indicating SD. (B) β-glucuronidase (GUS) staining of root (a), stem (b), cross section of root (c), leaf (d), flower (e), pod (f), and developing seeds (g). Bar = 1 cm in a, b, d, e, and f. Bar = 1 mm in c and g.
We also investigated the effect of osmotic stress on GmPIP2;9 expression, using a 20% PEG drought treatment in 13-day-old seedlings. We found increased GmPIP2;9 expression in roots after 6 h of PEG treatment and expression was highest after 12 h of the treatment (Figure 4B). In roots, GmPIP2;9 expression levels returned to baseline within 24 h, although the osmotic stress was not removed (Figure 4B). In leaves, GmPIP2;9 expression increased within 2 h of PEG treatment, which was faster than in roots (Figure 4A). Expression returned to baseline within 1 day also without recovery (Figure 4A). These results suggest that GmPIP2;9 responds quickly to osmotic stress.
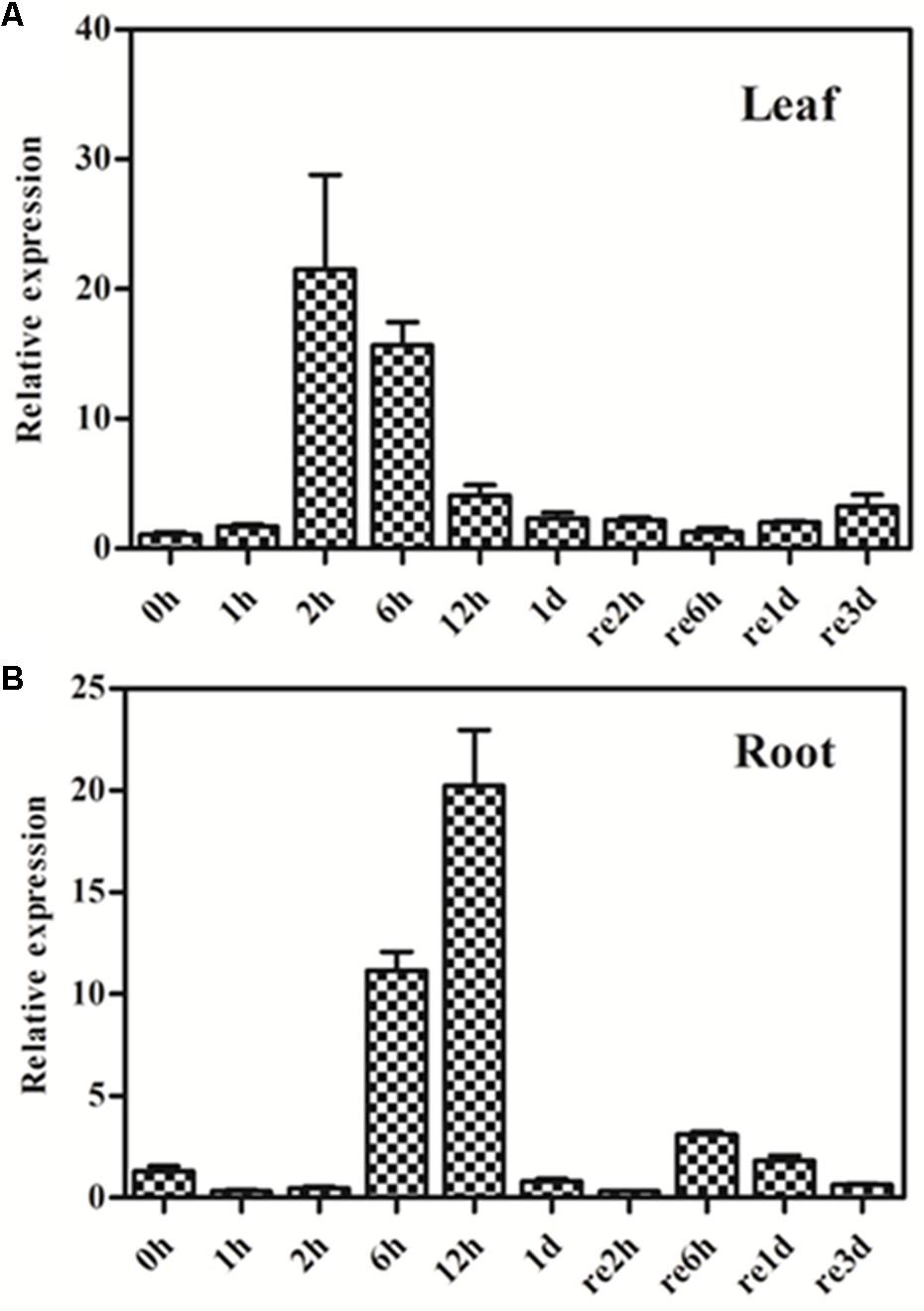
FIGURE 4. Expression pattern of GmPIP2;9 in soybean plants under normal and drought treatments. Thirteen-day-old soybean seedlings were treated with or without 20% polyethylene glycol (PEG) in nutrient solution. Total RNA was extracted from (A) leaves and (B) roots of these seedlings at 1 h, 2 h, 6 h, 12 h, and 1 day (1–12h and 1d) of PEG treatment, and 2 h, 6 h, 1 day, and 3 days of the recovery (re2h, re6h, re1d, and re3d). Data represent the means ± SD. Three replicates were used. Expression levels of treated plants are relative to control plants at each time point.
Generation of Transgenic Soybean Plants Overexpressing GmPIP2;9
To further investigate the function of GmPIP2;9 in drought stress tolerance, we generated transgenic soybean plants that overexpressed GmPIP2;9 (GmPIP2;9-Oe). The T-DNA region of the binary vector used for soybean transformation is shown in Supplementary Figure S3A. We obtained five independent transgenic events and verified these by semi-quantitative RT-PCR (Supplementary Figure S3B). We found that the abundance of GmPIP2;9 transcripts in the leaves and seeds of the T1 plants from the Oe1 and Oe2 events was more than 20-fold higher than in WT leaves and seeds (Supplementary Figure S3C). Thus, we used these two events (GmPIP2;9-Oe1 and GmPIP2;9-Oe2) for further experiments.
Overexpression of GmPIP2;9 Enhances Drought Tolerance
To investigate the role of GmPIP2;9 in drought tolerance, we treated 13-day-old GmPIP2;9-Oe1, GmPIP2;9-Oe2, and WT plants with 20% PEG in hydroponic solution cultures for 2 days, followed by a 5-day recovery. As shown in Figure 5A, PEG treatment suppressed the growth of both WT and transgenic plants. Although the stress-treated plants were significantly smaller than the non-stressed plants for both the WT and transgenic plants, the fresh weights of aerial parts and roots of PEG-treated GmPIP2;9-Oe1 and -Oe2 plants were significantly higher than that of the WT plants (Figures 5B, 6C). These results indicate that overexpression of GmPIP2;9 in soybean enhances tolerance to osmotic stress.
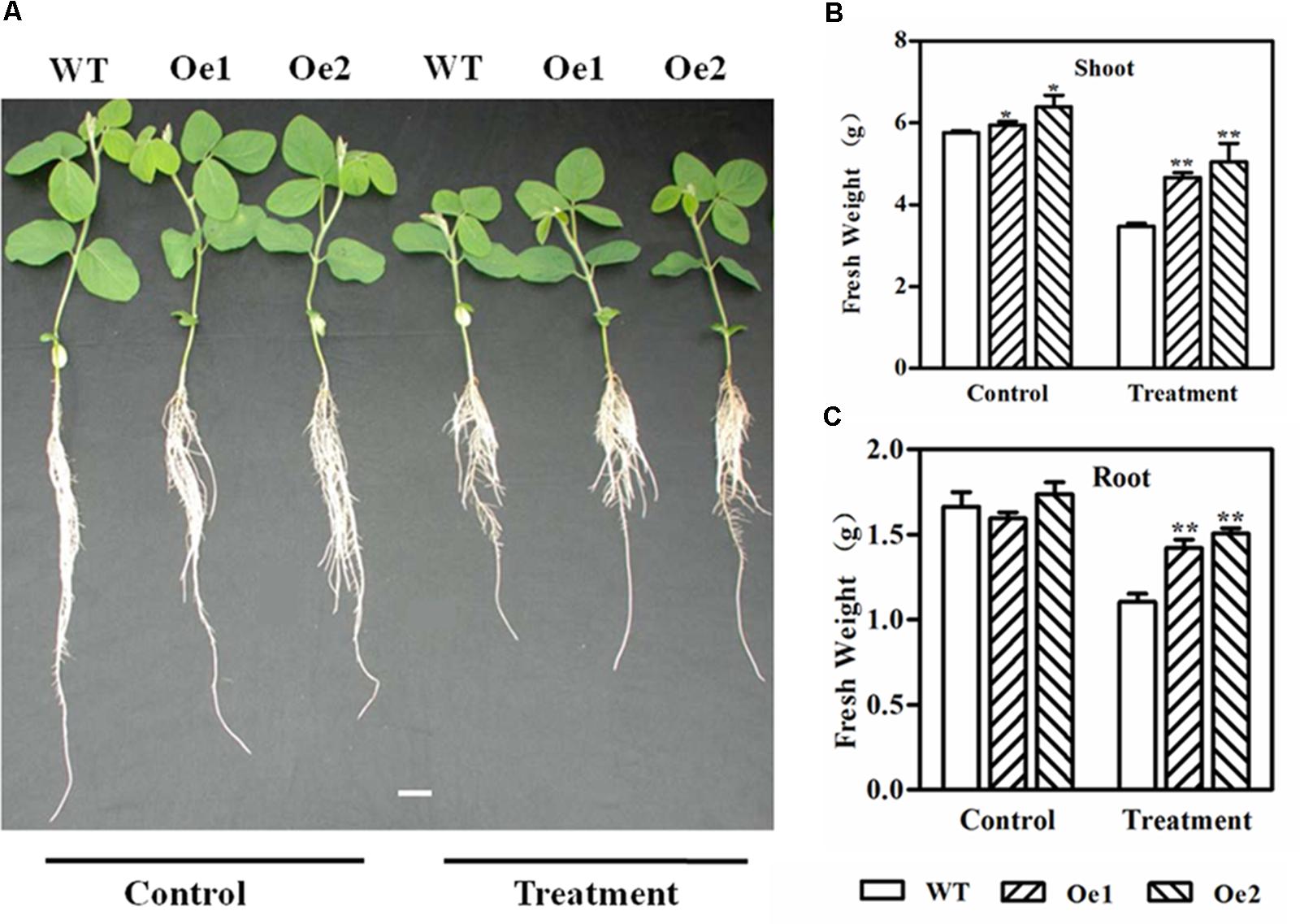
FIGURE 5. Growth performance of wild-type (WT) and GmPIP2;9-overexpression (Oe) plants under normal and polyethylene glycol (PEG)-mediated drought conditions. (A) Thirteen-day-old WT and GmPIP2;9-Oe plants were treated with or without 20% PEG in culture media for 2 days, followed by a 5-day recovery. (B) Fresh shoot weight. (C) Fresh root weight. Data are means ± SD (n = 3). ∗P < 0.05 and ∗∗P < 0.01.
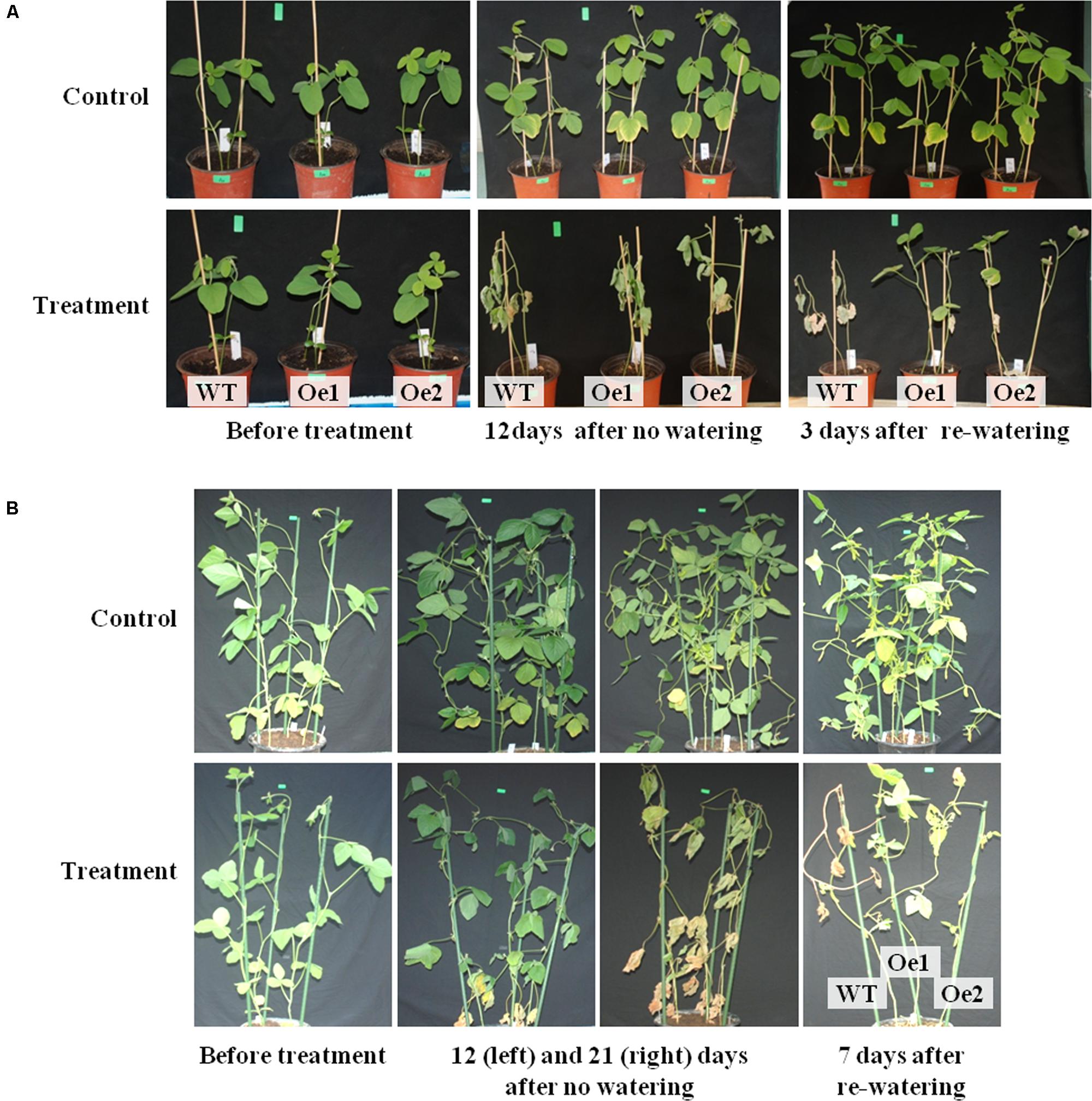
FIGURE 6. Performance of wild-type (WT) and GmPIP2;9-overexpression (Oe) plants under drought stress conditions. (A) Drought stress treatment was applied to 2-week-old seedlings of WT, GmPIP2;9-Oe1, and -Oe2 lines by withholding water for 12 days and then re-watering for 3 days. (B) Water was withheld from 40-day-old WT and GmPIP2;9-Oe plants for 21 days, and then plants were re-watered for 7 days.
To further assess the effect of GmPIP2;9 overexpression on drought tolerance, we withheld water from 2-week-old plants in soil pots to apply drought stress. The drought treatment was applied for 12 days, after which the stressed plants were completely wilted. When the wilted plants were re-watered, the GmPIP2;9-Oe plants revived, but WT plants did not recover (Figure 6A).
In an additional drought tolerance experiment, we withheld water from 40-day-old, pot-grown GmPIP2;9-Oe1, GmPIP2;9-Oe2, and WT plants for 3 weeks, followed by re-watering for 1 week. After 12 days without watering, the leaves of WT and GmPIP2;9-Oe plants began to wilt (Figure 6B). After 3 weeks without watering, the leaves of the WT and GmPIP2;9-Oe lines were completely wilted (Figure 6B). Re-watering revived the two transgenic plant lines, but WT plants did not recover (Figure 6B).
GmPIP2;9-Oe Plants Maintain High Photosynthesis Rates Under Drought Stress
We measured photosynthetic processes, including net CO2 assimilation (AN), stomatal conductance (gs), and transpiration rate (Tr), in the leaves of GmPIP2;9-Oe1, GmPIP2;9-Oe2, and WT plants after 11 days of drought treatment (Figure 7). Although drought stress significantly suppressed these photosynthetic processes in all plants, the GmPIP2;9-Oe1 and -Oe2 plants maintained significantly higher photosynthetic rates than WT plants (Figures 7A–C). These results further suggest that GmPIP2;9 plays a role in drought tolerance.
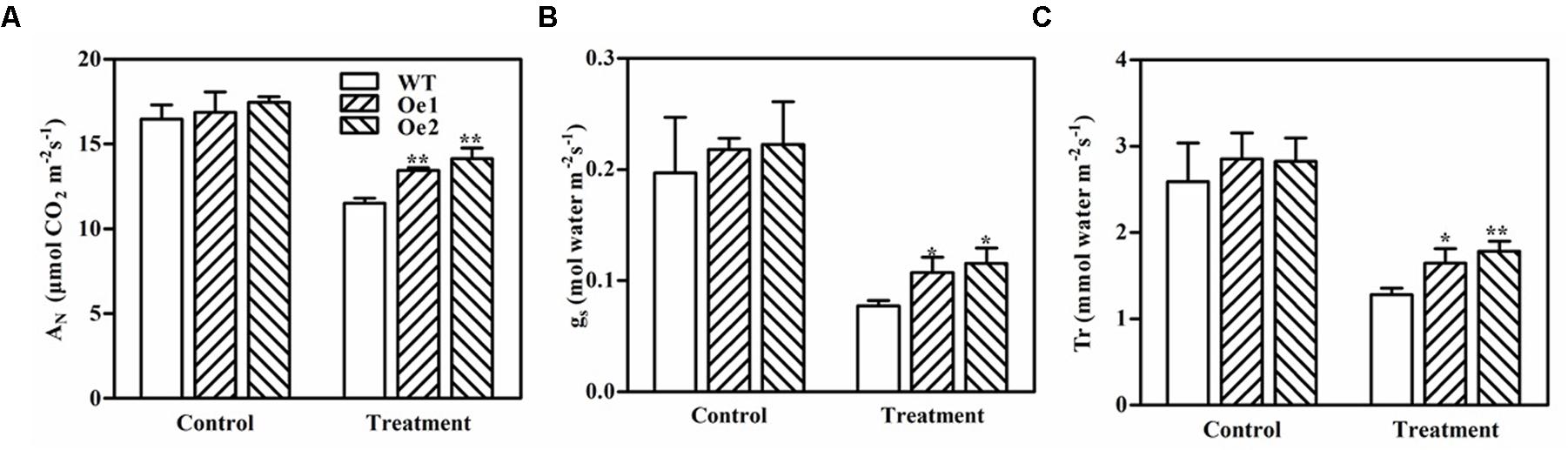
FIGURE 7. Daily (A) net CO2 assimilation (AN), (B) stomatal conductance (gs), and (C) transpiration rate (Tr) of wild-type (WT) and GmPIP2;9-overexpression (Oe) transgenic soybean plants under normal and drought stress conditions. Water was withheld from 40-day-old WT and GmPIP2;9-Oe transgenic soybean plants for 11 days. An LI-6400 was used to measure AN, gs, and Tr between 8 AM and 2 PM on day 11 of drought treatment. The parameters were measured in four different plants per treatment. Data are the means ± SD (n = 4). ∗P < 0.05 and ∗∗P < 0.01.
Overexpression of GmPIP2;9 Results in Increased Seed Number and Seed Size in the Field
We grew WT plants and homozygous transgenic T3 plants from two independent GmPIP2;9-Oe transformation events and evaluated their agronomic traits under field conditions. We found a significantly higher pod number, seed number, and seed weight per plant in GmPIP2;9-Oe plants than in WT plants (Table 1 and Supplementary Figure S4).
Discussion
Regulation of water transport is crucial for drought tolerance in crop plants, such as soybeans. PIPs are involved in water transport and predicted to localize to the PM (Zhou et al., 2013). There are eight PIP1 and 14 PIP2 genes in the soybean genome; however, the specific functions of individual PIP proteins are largely unknown. Thus, we have a limited understanding of how water transport is regulated within plants and from the outside environment. Here, we confirmed that GmPIP2;9 has functional water channel activity when expressed in Xenopus oocytes. We also developed transgenic soybean lines that overexpress GmPIP2;9. GmPIP2;9 overexpression significantly increased drought stress tolerance (Figures 5, 6) and the transgenic plants had significantly increased seed number and seed size, indicating enhanced yields (Table 1 and Supplementary Figure S4).
Analysis of transgenic plants expressing a PPIP2;9::GUS reporter construct revealed that GmPIP2;9 is predominantly expressed in roots (Figure 3Ba–g). High expression in roots suggests that GmPIP2;9 plays an important role in water transport in soybean roots. Given this finding, we expected that GmPIP2;9 overexpression would increase water flow across roots and enhance tolerance to drought stress. This hypothesis was supported by the finding that the GmPIP2;9-Oe1 and GmPIP2;9-Oe2 transgenic plants were less susceptible to drought stress and recovered from drought stress when WT plants did not (Figure 6).
Developing legume seeds import nutrients by mass flow through the phloem (Zhou et al., 2007a,b). To maintain water balance, the import of nutrients via phloem and the transport of water through pod walls and seed coats are maintained at similar levels (Zhou et al., 2007a,b). The high expression of GmPIP2;9 in the developing pod and seed hilum, which we observed in GmPIP2;9 promoter::GUS transgenic plants (Figure 3), suggests that GmPIP2;9 may facilitate water transport through pod walls and from seed coats to developing seeds. Consequently, seed abortion rates in GmPIP2;9-Oe plants were reduced, as evidenced by the increased seed numbers per plant. Furthermore, we saw that GmPIP2;9-Oe seeds were more filled than WT seeds, as evidenced by increased seed weights (Table 1). Additionally, strong GUS expression in pods and hilum of the developing seeds in GmPIP2;9 promoter::GUS transgenic plants supports our hypothesis.
This study, combined with previous work, has indicated several avenues for additional investigation to further define the role of GmPIP2;9 in water transport. It was reported that the expression and the transport activity of many plant AQPs are regulated at transcriptional and post-translational level (Liu et al., 2013). Studies of GmPIP2;9 expression in response to hormones, such as GA3, ABA, and brassinolide, should bring us information about what signaling pathways GmPIP2;9 is associated with. Furthermore, developing antibody to directly examine the levels of GmPIP2;9 proteins in different soybean tissues and under stress conditions may clarify the exact physiological mechanism by which overexpression of GmPIP2;9 increased the tolerance to drought stress. Finally, comparison of water transport activities in native PMs of soybean seed coats from GmPIP2;9-Oe transgenic and WT plants, and development of GmPIP2;9 knocking out materials using the CRISPR/CAS9 system will bring additional insights to understand the roles of GmPIP2;9.
Conclusion
We developed transgenic soybean plants that expressed 35S-GmPIP2;9 or GmPIP2;9 promoter::GUS reporter constructs. We demonstrated that: (1) GmPIP2;9-Oe plants have increased drought tolerance and this enhanced drought tolerance is consistent with the predominant expression of the gene in roots and (2) GmPIP2;9-Oe plants have increased pod number, seed number, and seed weight, indicating higher yields. It may associate with the relative high expression of GmPIP2;9 in developing seeds, or by enhanced water uptake from the roots which indirectly improved photosynthetic efficiency.
Author Contributions
HS and CW designed the experiments. LL, CD, RL, BZ, and CW conducted the experiments and analyzed the data. LL, CD, and HS wrote the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31572189 and 31771689), the Natural Science Foundation of Zhejiang Province (LZ16C150001 and LY17C130002), the Ministry of Science and Technology of China (2016YFD0100703 and 2016ZX08004001), and the Ministry of Education of China (B14027).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JY and handling Editor declared their shared affiliation.
Acknowledgments
The authors would like to thank Prof. Stephen Tyerman of the University of Adelaide for providing experimental support and productive discussion regarding the water-permeability assay in Xenopus oocytes and Prof. Lei Zhang of the Anhui Academy of Agricultural Sciences for technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00530/full#supplementary-material
Footnotes
References
Aharon, R., Shahak, Y., Wininger, S., Bendov, R., Kapulnik, Y., and Galili G. (2003). Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell 15, 439–447. doi: 10.1105/tpc.009225
Alexandersson, E., Danielson, J. A., Rade, J., Moparthi, V. K., Fontes, M., Kjellbom, P., et al. (2010). Transcriptional regulation of aquaporins in accessions of Arabidopsis in response to drought stress. Plant J. 61, 650–660. doi: 10.1111/j.1365-313X.2009.04087.x
Alexandersson, E., Fraysse, L., Sjovall-Larsen, S., Gustavsson, S., Fellert, M., Karlsson, M., et al. (2005). Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol. 59, 469–484. doi: 10.1007/s11103-005-0352-351
Aroca, R., Porcel, R., and Ruiz-Lozano, J. M. (2012). Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot. 63, 43–57. doi: 10.1093/jxb/err266
Bienert, G. P., Schjoerring, J. K., and Jahn, T. P. (2006). Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 1758, 994–1003. doi: 10.1016/j.bbamem.2006.02.015
Chaumont, F., Barrieu, F., Jung, R., and Chrispeels, M. J. (2000). Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 122, 1025–1034. doi: 10.1104/pp.122.4.1025
Chaumont, F., Moshelion, M., and Daniels, M. J. (2005). Regulation of plant aquaporin activity. Biol. Cell 97, 749–764. doi: 10.1042/BC20040133
Daniels, M. J., Mirkov, T. E., and Chrispeels, M. J. (1994). The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol. 106, 1325–1333. doi: 10.1104/pp.106.4.1325
Fetter, K., Van Wilder, V., Moshelion, M., and Chaumont, F. (2004). Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 16, 215–228. doi: 10.1105/tpc.017194
Guo, L., Wang, Z. Y., Lin, H., Cui, W. E., Chen, J., Liu, M., et al. (2006). Expression and functional analysis of the rice plasma-membrane intrinsic protein gene family. Cell Res. 16, 277–286. doi: 10.1038/sj.cr.7310035
Hoagland, D. R., and Arnon, D. I. (1950). The Water-Culture Method for Growing Plants Without Soil. Berkeley, CA: The College of Agriculture.
Jang, J. Y., Kim, D. G., Kim, Y. O., Kim, J. S., and Kang, H. (2004). An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol. Biol. 54, 713–725. doi: 10.1023/B:PLAN.0000040900.61345.a6
Javot, H. (2003). Role of a single aquaporin isoform in root water uptake. Plant Cell 15, 509–522. doi: 10.1105/tpc.008888
Kaldenhoff, R., and Fischer, M. (2006). Aquaporins in plants. Acta. Physiol. (Oxf) 187, 169–176. doi: 10.1111/j.1748-1716.2006.01563.x
Kammerloher, W., Fischer, U., Piechottka, G. P., and Schaffner, A. R. (1994). Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 6, 187–199. doi: 10.1046/j.1365-313X.1994.6020187.x
Liman, E. R., Tytgat, J., and Hess, P. (1992). Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9, 861–871. doi: 10.1016/0896-6273(92)90239-A
Liu, C., Li, C., Liang, D., Ma, F., Wang, S., Wang, P., et al. (2013). Aquaporin expression in response to water-deficit stress in two Malus species: relationship with physiological status and drought tolerance. Plant Growth Regul. 70, 187–197. doi: 10.1007/s10725-013-9791-x
Luu, D. T., and Maurel, C. (2005). Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant Cell Environ. 28, 85–96. doi: 10.1111/j.1365-3040.2004.01295.x
Manavalan, L. P., Guttikonda, S. K., Tran, L. S., and Nguyen, H. T. (2009). Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 50, 1260–1276. doi: 10.1093/pcp/pcp082
Moshelion, M., Becker, D., Biela, A., Uehlein, N., Hedrich, R., Otto, B., et al. (2002). Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. Plant Cell 14, 727–739. doi: 10.1105/tpc.010351
Nelson, B. K., Cai, X., and Nebenfuhr, A. (2007). A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51, 1126–1136. doi: 10.1111/j.1365-313X.2007.03212.x
Paz, M. M., Shou, H., Guo, Z., Zhang, Z., Banerjee, A. K., and Wang, K. (2004). Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica 136, 167–179. doi: 10.1023/B:EUPH.0000030669.75809.dc
Sade, N., Gebretsadik, M., Seligmann, R., Schwartz, A., Wallach, R., and Moshelion, M. (2010). The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol. 152, 245–254. doi: 10.1104/pp.109.145854
Sakurai, J., Ishikawa, F., Yamaguchi, T., Uemura, M., and Maeshima, M. (2005). Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 46, 1568–1577. doi: 10.1093/pcp/pci172
Schuurmans, J. A., van Dongen, J. T., Rutjens, B. P., Boonman, A., Pieterse, C. M., and Borstlap, A. C. (2003). Members of the aquaporin family in the developing pea seed coat include representatives of the PIP, TIP, and NIP subfamilies. Plant Mol. Biol. 53, 633–645. doi: 10.1023/B:PLAN.0000019070.60954.77
Song, Z. Y., Tian, J. L., Fu, W. Z., Li, L., Lu, L. H., Zhou, L., et al. (2013). Screening Chinese soybean genotypes for Agrobacterium-mediated genetic transformation suitability. J. Zhejiang Univ. Sci. B. 14, 289–298. doi: 10.1631/jzus.B1200278
Tran, L. S., and Mochida, K. (2010). Functional genomics of soybean for improvement of productivity in adverse conditions. Funct. Integr. Genomics 10, 447–462. doi: 10.1007/s10142-010-0178-z
Tyerman, S. D., Niemietz, C. M., and Bramley, H. (2002). Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ. 25, 173–194. doi: 10.1046/j.0016-8025.2001.00791.x
Uehlein, N., Lovisolo, C., Siefritz, F., and Kaldenhoff, R. (2003). The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425, 734–737. doi: 10.1038/nature02027
Vandeleur, R. K., Mayo, G., Shelden, M. C., Gilliham, M., Kaiser, B. N., and Tyerman, S. D. (2009). The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 149, 445–460. doi: 10.1104/pp.108.128645
Vandeleur, R. K., Sullivan, W., Athman, A., Jordans, C., Gilliham, M., Kaiser, B. N., et al. (2014). Rapid shoot-to-root signalling regulates root hydraulic conductance via aquaporins. Plant Cell Environ. 37, 520–538. doi: 10.1111/pce.12175
Verdoucq, L., Grondin, A., and Maurel, C. (2008). Structure-function analysis of plant aquaporin AtPIP2;1 gating by divalent cations and protons. Biochem. J. 415, 409–416. doi: 10.1042/BJ20080275
Verslues, P. E., Agarwal, M., Katiyar-Agarwal, S., Zhu, J., and Zhu, J. K. (2006). Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 45, 523–539. doi: 10.1111/j.1365-313X.2005.02593.x
Walker, N. A., Patrick, J. W., Zhang, W. H., and Fieuw, S. (1995). Efflux of photosynthate and acid from developing seed coats of Phaseolus vulgaris L.: a chemiosmotic analysis of pump-driven efflux. J. Exp. Bot. 46, 539–549. doi: 10.1093/jxb/46.5.539
Weig, A., Deswarte, C., and Chrispeels, M. J. (1997). The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiol. 114, 1347–1357. doi: 10.1104/pp.114.4.1347
Zhou, D., Zhang, D. Y., Ali, Z., Wang, C. B., Xu, L., Yi, J. X., et al. (2013). Genome-wide sequence characterization and expression analysis of major intrinsic proteins in soybean (Glycine max L.). PLoS One 8:e56312. doi: 10.1371/journal.pone.0056312
Zhou, L., Wang, C., Liu, R., Han, Q., Vandeleur, R. K., Du, J., et al. (2014). Constitutive overexpression of soybean plasma membrane intrinsic protein GmPIP1;6 confers salt tolerance. BMC Plant Biol. 14:181. doi: 10.1186/1471-2229-14-181
Zhou, Y., Qu, H., Dibley, K. E., Offler, C. E., and Patrick, J. W. (2007a). A suite of sucrose transporters expressed in coats of developing legume seeds includes novel pH-independent facilitators. Plant J. 49, 750–764. doi: 10.1111/j.1365-313X.2006.03000.x
Keywords: aquaporin, drought tolerance, transgenic, soybean, overexpression
Citation: Lu L, Dong C, Liu R, Zhou B, Wang C and Shou H (2018) Roles of Soybean Plasma Membrane Intrinsic Protein GmPIP2;9 in Drought Tolerance and Seed Development. Front. Plant Sci. 9:530. doi: 10.3389/fpls.2018.00530
Received: 06 December 2017; Accepted: 05 April 2018;
Published: 26 April 2018.
Edited by:
Kendal Hirschi, Baylor College of Medicine, United StatesReviewed by:
Toshiro Shigaki, The University of Tokyo, JapanJian Yang, Baylor College of Medicine, United States
Copyright © 2018 Lu, Dong, Liu, Zhou, Wang and Shou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huixia Shou, aHVpeGlhQHpqdS5lZHUuY24=
†These authors have contributed equally to this work.
 Linghong Lu
Linghong Lu Changhe Dong1†
Changhe Dong1† Chuang Wang
Chuang Wang Huixia Shou
Huixia Shou